| Comparison | Name of test | Sensitivity | Specificity |
| Cancerous versus benign or precancerous | EUS‐FNA
(cytology) | 0.79 (95% CI 0.60 to 0.91) | 1.00 (95% CI 0.85 to 1.00) |
| Cancerous versus benign or precancerous | EUS‐FNA
(CEA > 500 ng/mL) | 0.93 (95% CI 0.70 to 0.99) | 0.33 (95% CI 0.12 to 0.65) |
| Cancerous versus benign or precancerous | PET
(criteria unspecified) | 0.85 (95% CI 0.73 to 0.92) | 0.91 (95% CI 0.72 to 0.97) |
| Cancerous versus benign | EUS | 0.95 (95% CI 0.84 to 0.99) | 0.53 (95% CI 0.31 to 0.74) |
| Cancerous versus benign | EUS‐FNA
(cytology) | 0.79 (95% CI 0.07 to 1.00) | 1.00 (95% CI 0.91 to 1.00) |
| Cancerous versus benign | PET (criteria unspecified) | 0.92 (95% CI 0.80 to 0.97) | 0.65 (95% CI 0.39 to 0.85) |
| Cancerous versus benign | PET (SUVmax > 3.5) | 0.96 (95% CI 0.87 to 0.99) | 0.62 (95% CI 0.43 to 0.78) |
| Cancerous versus benign | CT | 0.98 (95% CI 0.00 to 1.00) | 0.76 (95% CI 0.02 to 1.00) |
| Cancerous versus benign | MRI | 0.80 (95% CI 0.58 to 0.92) | 0.89 (95% CI 0.57 to 0.98) |
| Precancerous or cancerous versus benign | EUS | 0.92 (95% CI 0.74 to 0.98) | 0.60 (95% CI 0.31 to 0.83) |
| Precancerous or cancerous versus benign | EUS‐FNA (cytology) | 0.73 (95% CI 0.01 to 1.00) | 0.94 (95% CI 0.15 to 1.00) |
| Precancerous or cancerous versus benign | EUS‐FNA
(CEA > 50 ng/mL) | 0.29 (95% CI 0.08 to 0.64) | 0.25 (95% CI 0.05 to 0.70) |
| Precancerous or cancerous versus benign | PET
(SUVmax 2.4) | 0.94 (95% CI 0.74 to 0.99) | 0.93 (95% CI 0.69 to 0.99) |
| Precancerous or cancerous versus benign | CT | 0.62 (95% CI 0.45 to 0.76) | 0.64 (95% CI 0.39 to 0.84) |
| Precancerous or cancerous versus benign | MRI | 0.93 (95% CI 0.69 to 0.99) | 0.85 (95% CI 0.58 to 0.96) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | EUS | 0.78 (95% CI 0.45 to 0.94) | 0.91 (95% CI 0.61 to 0.98) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | EUS‐FNA
(cytology) | 0.66 (95% CI 0.03 to 0.99) | 0.92 (95% CI 0.73 to 0.98) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | EUS‐FNA
(CEA > 200 ng/mL) | 1.00 (95% CI 0.57 to 1.00) | 0.64 (95% CI 0.48 to 0.78) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | CT | 0.72 (95% CI 0.50 to 0.87) | 0.92 (95% CI 0.81 to 0.97) |
| Cancerous (invasive carcinoma) versus precancerous (dysplasia) | MRI | 0.75 (95% CI 0.30 to 0.95) | 0.93 (95% CI 0.77 to 0.98) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS | 0.86 (95% CI 0.74 to 0.92) | 0.91 (95% CI 0.83 to 0.96) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA
(cytology) | 0.47 (95% CI 0.24 to 0.70) | 0.91 (95% CI 0.32 to 1.00) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA
(CEA > 200 ng/mL) | 0.58 (95% CI 0.28 to 0.83) | 0.51 (95% CI 0.19 to 0.81) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA
(CA 19‐9 > 1000 U/mL) | 0.90 (95% CI 0.60 to 0.98) | 0.42 (95% CI 0.26 to 0.59) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | EUS‐FNA
(CEA > 692.8 ng/mL) | 0.80 (95% CI 0.49 to 0.94) | 0.90 (95% CI 0.60 to 0.98) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | PET
(SUVmax > 2 to 2.5) | 0.90 (95% CI 0.79 to 0.96) | 0.94 (95% CI 0.81 to 0.99) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | CT | 0.87 (95% CI 0.00 to 1.00) | 0.96 (95% CI 0.00 to 1.00) |
| Cancerous (high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐ or intermediate‐grade dysplasia) | MRI | 0.69 (95% CI 0.44 to 0.86) | 0.93 (95% CI 0.43 to 1.00) |
| Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) | EUS | 0.77 (95% CI 0.50 to 0.92) | 0.89 (95% CI 0.76 to 0.96) |
| Cancerous (invasive carcinoma) versus precancerous (low‐grade dysplasia) | CT | 0.50 (95% CI 0.22 to 0.78) | 0.95 (95% CI 0.83 to 0.99) |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) | CT | 0.83 (95% CI 0.68 to 0.92) | 0.83 (95% CI 0.64 to 0.93) |
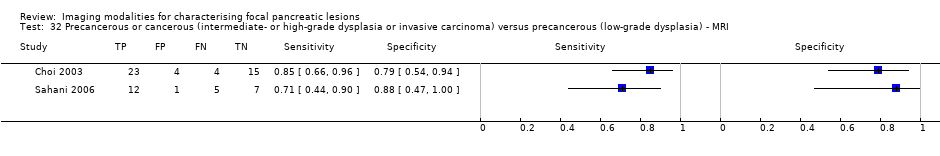
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) | MRI | 0.80 (95% CI 0.58 to 0.92) | 0.81 (95% CI 0.53 to 0.95) |
| Precancerous or cancerous (intermediate‐ or high‐grade dysplasia or invasive carcinoma) versus precancerous (low‐grade dysplasia) or benign | EUS | 0.97 (95% CI 0.83 to 0.99) | 0.40 (95% CI 0.26 to 0.55) |
| Cystic lesion subgroup analysis | Cancerous versus benign ‐ EUS‐FNA (cytology) | 0.50 (95% CI 0.19 to 0.81) | 1.00 (95% CI 0.84 to 1.00) |
| Cystic lesion subgroup analysis | Cancerous versus benign ‐ PET
(SUVmax > 3.5) | 0.96 (95% CI 0.87 to 0.99) | 0.62 (95% CI 0.43 to 0.78) |
| Cystic lesion subgroup analysis | Precancerous or cancerous versus benign ‐ EUS‐FNA
(cytology) | 0.43 (95% CI 0.19 to 0.71) | 1.00 (95% CI 0.74 to 1.00) |