Psihološke terapije za posttraumatski stresni poremećaj i istovremeni poremećaj zlouporabe droga
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Randomised effectiveness trial | |
| Participants | Setting: Participants were military veterans recruited from a VA outpatient substance use disorder clinic. Treatment was delivered on an outpatient basis. Inclusion criteria: male veteran status and VA health care eligibility; a diagnosis of any current alcohol or drug use disorder; completion of an intake for outpatient SUD treatment at the participating mental health clinic; and meeting partial (i.e. defined as meeting criteria for 2 out of 3 PTSD symptom clusters, or at least 1 symptom in each symptom cluster) or full PTSD in clinical evaluation using CAPS. Exclusion criteria: current participation in other day or inpatient mental health treatment; any contraindications communicated by the person’s primary clinician; and acute psychosis, mania, dementia, or suicidal intent. Sample size: 125 individuals were assessed for eligibility; 117 were randomised; 8 participants were withdrawn after randomisation because they were found to meet 1 or more exclusion criteria; 98 attended at least 1 treatment session and were included in the analyses. PTSD diagnosis: 90.8% participants met full diagnosis for PTSD as measured by the CAPS; 9.8% met subthreshold diagnosis for PTSD. SUD type and diagnosis: All participants met diagnosis for SUD. Participants were polydrug users. Mean age: Seeking safety 55.1 (SD = 9.2) years; treatment as usual 52.9 (SD = 10.0) Gender: 100% male Ethnicity: 60.2% African American; 19.4% white; 7.1% Hispanic; 2% Native American; 5.1% other. Country: USA | |
| Interventions | Group 1: Group‐based Seeking Safety plus treatment as usual: n = 54. Seeking Safety is a present‐focused, manualised, cognitive behavioural integrated treatment for PTSD and SUD, designed for both genders. Its primary goal is to reduce both PTSD and SUD by focusing on safe coping skills addressed through cognitive, behavioural, interpersonal, and case management domains. Participants were also able to access the treatment‐as‐usual interventions (described below). However, participants in this arm substituted SS groups and case management for the clinic’s core substance use‐focused group therapy and case management sessions. Groups were held twice weekly. Case management was based on the SS manual. Group 2: Group‐based treatment as usual: n = 55. Treatment as usual involved participants entering twice‐weekly "recovery" groups, focusing on building abstinence and, after approximately 90 days of therapy, on maintaining abstinence. Participants attended additional groups on smoking cessation, sobriety support, cocaine recovery, alcohol recovery, dual‐diagnosis recovery, family therapy, anger management, cognitive behavioural therapy, fitness, relaxation, health education, hepatitis education, and developing outside activities as needed. All participants were assigned a case manager, and case management and individual therapy were available as deemed appropriate. Participants made use of clinic services as indicated by their treating clinician or as desired. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: IES‐R SUD: ASI drug and alcohol composite scores for the previous 30 days. Treatment acceptability: Data are reported for the mean number of treatment sessions attended; participant satisfaction at 3‐month assessment based on the Client Satisfaction Questionnaire. Other: Coping Responses Inventory Follow‐up: End of treatment and at 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation sequences were generated by the study statistician and implemented by use of sequentially numbered containers |
| Allocation concealment (selection bias) | Low risk | Random allocation sequences were generated by the study statistician and implemented by use of sequentially numbered containers |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Research staff who enrolled participants and conducted outcomes assessment were blind to treatment assignment. To maintain blinding, staff conducting outcomes assessment were password‐restricted from accessing data with information regarding treatment assignment, and participants were warned not to divulge information that might compromise blinding during interviews |
| Incomplete outcome data (attrition bias) | Unclear risk | Treatment dropouts and withdrawals were clearly reported. Analysis of treatment outcomes was based on those participants available to follow‐up |
| Selective reporting (reporting bias) | Low risk | Primary outcomes are specified in the protocol registered with ClinicalTrials.gov |
| Other bias | Unclear risk | Insufficient information available to be able to assess |
| Methods | Design: RCT ‐ described as a laboratory‐based experiment. The authors tested the hypothesis that alcohol craving elicited by a trauma cue might be attenuated if trauma‐elicited negative emotion was reduced following trauma‐focused imaginal exposure | |
| Participants | Setting: Participants were recruited from 2 outpatient substance use treatment programmes. Inclusion criteria: Participants needed to meet current diagnosis for alcohol dependence and PTSD. All participants were involved in alcohol treatment. Exclusion criteria: Individuals were excluded if they met current diagnostic criteria for a psychotic disorder or were currently experiencing a manic episode. Although current major depression was not an exclusion criterion, severe major depression was. Individuals were also excluded if their PTSD diagnosis stemmed from combat or if they were currently or had ever engaged in exposure‐based PTSD treatment. Sample size: The number of individuals assessed for eligibility is not reported. 43 individuals were invited to take part in the study and were randomised and 31 (who attended at least 1 treatment session) were included in the analyses. PTSD diagnosis: 43 (100%) of participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: 43 (100%) of participants met diagnosis for alcohol dependence. Mean age: 37.5 (SD = 8.0) years Gender: 29 (67%) female Ethnicity: 65% African American; 28% white; 5% Native American; 2% other Country: USA | |
| Interventions | Group 1: Individual imaginal exposure: n= 16. Participants assigned to the exposure condition took part in six 60‐min sessions of imaginal exposure targeting an index traumatic event. Participants were instructed to tell the story of their trauma by describing the event in the present tense from the first‐person perspective. Participants were encouraged to include emotions and cognitions in their verbal description of the event. Participants described their trauma repeatedly and continuously over the course of the six 60‐min clinical sessions. SUDS ratings were collected approximately every 5 min during each session. Each session was audiotaped, and participants were instructed to listen to the tape daily. Group 2: Imagery‐based relaxation: n= 15. Participants assigned to the relaxation condition listened to an imagery‐based relaxation audiotape for the 60‐min session. As in the exposure condition, SUDS ratings were collected approximately every 5 min during each session. Participants were instructed to listen to the relaxation tape daily. Experimental intervention modality: Concurrent | |
| Outcomes | PTSD: IES‐R SUD: A cue reactivity paradigm was used to assess alcohol craving prior to, and after completion of, the 6 clinical sessions. Treatment acceptability: Attendance of 1, at least 4, and all 6 clinical sessions is described. Other: The PANAS; emotional distress as measured by SUDS Follow‐up: End of treatment. | |
| Notes | 43 participants were assessed and randomised. Outcome data is reported for 17 responders of 31 participants who attended 1 or more intervention session | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | The research assistant who conducted the assessment session and both laboratory sessions was unaware of the experimental condition to which participants had been randomly assigned. The research staff involved in the clinical sessions did not participate in the assessment session or either laboratory session |
| Incomplete outcome data (attrition bias) | High risk | Outcome data is reported for 17 responders of 31 participants who attended 1 or more intervention session |
| Selective reporting (reporting bias) | High risk | Data related to craving response was based on a subset of 12 participants who reported a craving response to the cues at the first or the second laboratory session. This appears to have been based on a post‐hoc decision |
| Other bias | Unclear risk | High attrition rates |
| Methods | Design: RCT | |
| Participants | Setting: Participants were recruited from an unlocked residential SUD treatment facility. Inclusion criteria: DSM‐IV diagnosis of both PTSD related to a non‐combat trauma and alcohol dependence; 1 heavy drinking day in the past 60 days, as defined by consumption of 4 standard drinks for women and 5 standard drinks for men; and age between 18 and 60. Exclusion criteria: The presence of an acute psychotic disorder, bipolar disorder with an active manic episode (but not the presence of bipolar disorder, per se), imminent risk for suicide, prescription of craving‐reducing medications (e.g. naltrexone) or medications to reduce alcohol use (e.g. disulphiram), self reported use, or urine drug screen indicating use, of a benzodiazapine, judged to have a medical condition that might limit co‐operation or compromise the integrity of the data (e.g. organic brain syndrome, dementia, head injury, neuropathy, etc.), illiteracy in English, and being in an ongoing abusive relationship that resulted in a PTSD Criterion A event (but not a history of intimate partner violence, per se). Sample size: 222 individuals were assessed for eligibility; 148 were randomised, but 28 were subsequently excluded. Reasons for exclusion included cognitive impairment, psychosis, medical issues, drug screening, moved away, refusal to participate, and in one case for unknown reasons. The remaining 120 participants attended at least 1 treatment session and were included in analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met diagnosis for alcohol dependence and 98.3% met criteria for other drug dependence, as measured by the CDIS‐IV. Mean age: 33.72 (SD = 10.25) years Gender: 64 (53.3%) male; 56 (46.7%) female Ethnicity: 18.3% African American; 80.0% white; 0.8% other Country: USA | |
| Interventions | Group 1: Trauma‐focused exposure therapy (EXP) + TAU: n = 82. EXP is a well‐described cognitive behavioural therapy that utilises imaginal and in vivo exposure techniques, either singly or in combination, to reduce the symptoms of PTSD resulting from a range of traumas. In addition to imaginal and in vivo exposure techniques, in the current study participants were provided psycho‐education about PTSD, a rationale for EXP, and were taught breathing retraining as a method to manage arousal associated with PTSD. The imaginal exposures were audiotaped, and participants were instructed to listen to the tapes daily. 9 sessions of exposure were offered initially; if PTSD symptom severity did not decrease by at least 70%, an additional 3 sessions of EXP were offered. A number of adaptations were made to conventional exposure. Traditionally, exposure sessions are 90 minutes. The current study utilised 60‐min EXP sessions. Additionally, protocol contained added psycho‐education about the relationship between trauma and SUD symptoms and weekly check‐ins about SUD treatment progress. Finally, the protocol provides integration of care at the team level, rather than the individual provider level. All participants received standard TAU for substance abuse. TAU consisted of daily group therapy for approximately 3 hours each day, daily recreation therapy, AA and NA meetings, individual drug counselling sessions, and completion of drug counselling homework. The 6‐week TAU was provided by drug and alcohol counsellors unaffiliated with the current study. Group 2: Healthy Lifestyles Sessions (HLS) + TAU: n = 38. HLS is a structured 9‐ to 12‐session intervention that provides education about a variety of health‐related topics. HLS was designed to involve a similar amount of therapist contact and between‐session homework as exposure. Topics covered included an introduction to treatment; sleep hygiene; progressive muscle relaxation; starting/maintaining an exercise programme; personal role identification; healthy eating and nutrition (2 sessions); diabetes (prevention or diabetes treatment adherence, depending on diabetes status); monitoring goals and values; cancer (a focus on breast cancer for women and colon cancer for men); HIV (reducing HIV risk or adhering to HIV treatment, depending on HIV status); and a final review session. Sessions included the provision of information, discussing participants’ understanding of information, and answering questions about the information provided. Experimental intervention modality: Combined | |
| Outcomes | PTSD: CAPS; IES‐R SUD: TLFB, the primary outcome was per cent days abstinent; ACQ‐Now Treatment acceptability: Number completing at least 8 treatment sessions Other: BDI; BAI Follow‐up: End of treatment; 3 and 6 months post‐treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was undertaken by urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors were blind to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was used to analyse data based on participants who attended at least 1 treatment session. A number of other participants were also excluded from the study postrandomisation, and it was unclear if some of these exclusions might have been due to intervention‐related factors |
| Selective reporting (reporting bias) | Unclear risk | All outcomes that were described were reported, but we were not able to identify a previously published protocol |
| Other bias | Unclear risk | 28 individuals who were randomised were removed from the study. It is unclear whether this may have caused additional bias. Approximately half of the participants receiving the experimental intervention were also provided a session of motivational enhancement therapy for PTSD prior to beginning intervention. The other half of the experimental group and all of the HLS participants were provided a 60‐minute relaxation session prior to the first scheduled treatment session. No significant differences were found between the 2 experimental groups, and they were therefore collapsed into a single experimental condition |
| Methods | Design: RCT | |
| Participants | Setting: Treatment‐seeking individuals were recruited through advertisements and professional referrals and treated on an outpatient basis. Inclusion criteria: Current PTSD and alcohol dependence according to DSM‐IV; clinically significant trauma‐related symptoms, as indicated by a score of at least 15 on the PSS‐I; and heavy drinking in the past 30 days, defined as an average of more than 12 standard alcohol drinks per week with at least 1 day of 4 or more drinks determined by the TFBI. Exclusion criteria: Current substance dependence other than nicotine or cannabis; current psychotic disorder (e.g. schizophrenia, bipolar disorder); clinically significant suicidal or homicidal ideation; opiate use in the month prior to study entry; medical illnesses that could interfere with treatment (e.g. AIDS, active hepatitis); or pregnancy or nursing. Sample size: 657 individuals were assessed for eligibility; 165 were randomised, and all were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met full diagnosis for alcohol dependence. Mean age: prolonged exposure + naltrexone 40.1 (95% CI 36.7 to 43.5); prolonged exposure + placebo 44.7 (95% CI 41.8 to 47.7); supportive counselling + naltrexone 44.9 (95% CI 41.8 to 47.9); supportive counselling + placebo 41.2 (95% CI 38.6 to 43.9) Gender: 108 (65.5%) male; 57 (34.5%) female Ethnicity: 63% African American; 30% white; 4.2% Hispanic; 0.6% other Country: USA | |
| Interventions | Before randomisation to treatment, participants completed outpatient medical detoxification (at least 3 consecutive days of alcohol abstinence) with oxazepam as required to manage alcohol withdrawal symptoms. Group 1: Prolonged exposure + naltrexone + supportive counselling: n = 40. Prolonged exposure therapy consisted of 12 weekly 90‐minute sessions followed by 6 biweekly sessions and included repeated imaginal exposure (i.e. revisiting and recounting traumatic memories) and processing the memory (i.e. discussing thoughts and feelings related to revisiting the memory). The target dose of naltrexone was 100 mg/d, starting with 50 mg/d for a minimum of 3 days and titrating up within 1 week. Supportive counselling was available as described below. Group 2: Prolonged exposure + placebo + supportive counselling: n = 40. Participants received PE as described above. Supportive counselling was available as described below. Group 3: Supportive counselling + naltrexone: n = 42. Supportive counselling was based on the BRENDA model, which combines medication management with compliance enhancement techniques based on motivational interviewing. Supportive counselling sessions were administered by a study nurse and lasted 30 to 45 minutes. Input included dispensing medication, monitoring compliance, assessing and providing education about alcoholism, and offering support and advice concerning drinking. Visits were weekly during the first 3 months and biweekly during the remaining 3 months. Group 4: Supportive counselling + placebo: n = 43. Supportive counselling was as described above. Experimental intervention modality: Combined | |
| Outcomes | PTSD: PSS‐I SUD: TLFB; PACS Treatment acceptability: Reported in terms of the mean number of sessions attended for PE. Other: ‐ Follow‐up: End of treatment and 6 months' post‐treatment | |
| Notes | For the purpose of the review, we were interested in the comparison between prolonged exposure plus supportive counselling and supportive counselling | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not be blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Evaluators were blind to treatment group assignment |
| Incomplete outcome data (attrition bias) | Low risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was employed using hierarchical linear and non‐linear modelling, which took into account dropouts and missing data |
| Selective reporting (reporting bias) | Low risk | Key outcomes are as specified in the protocol registered with ClinicalTrials.gov |
| Other bias | Low risk | There was no significant difference between the treatment groups on any demographic or baseline diagnostic characteristics. We identified no other potential biases |
| Methods | Design: RCT | |
| Participants | Setting: Participants were recruited from amongst outpatients at 3 participating substance use disorder clinics. Inclusion criteria: (a) a history of trauma that fulfilled the conditions for DSM‐IV PTSD criterion A, (b) a substance use disorder, and (c) DSM criteria for one of the following: PTSD, DESNOS plus at least 1 or more DSM‐IV Axis I disorders, or a diagnosis of major depressive disorder, dysthymic disorder, or dissociative disorder. Exclusion criteria: Not specified. Sample size: 274 individuals were assessed for eligibility; 213 were randomised PTSD diagnosis: 202 (94.8%) of participants met full diagnosis for PTSD as measured by the CAPS. Of these, 72 (33.8%) met criteria for PTSD with DESNOS. 7 (3.3%) met criteria for DESNOS without PTSD, and 4 (1.9%) met criteria for other disorders, such as dissociative disorder and major depression. SUD type and diagnosis: Participants met diagnosis for substance abuse and substance dependence. Participants were polydrug users. Mean age: Intervention group: 37.84 (SD = 8.42) years; control group: 36.85 (SD = 8.44) years Gender: 130 (61%) female Ethnicity: 24.4% African American; 56.3% white; 10.3% Hispanic; 8.92% other Country: USA | |
| Interventions | Group 1: Group‐based trauma‐sensitive usual care plus Trauma Adaptive Recovery Group Education and Therapy (TARGET): n = 141 Participants randomised to TARGET treatment were offered 8 or 9 weeks of manualised group treatment. TARGET provided psycho‐education about the impact of traumatic exposure and PTSD on the body’s stress response system and the brain using the strength‐based concept of an adaptive psychobiological “alarm reaction”. Participants were taught 7 core skills: focusing, recognising stress triggers, emotion identification, evaluating cognitions, defining personal goals, making choices with options grounded in personal strengths, and making a contribution to restore a sense of hope, faith, and purpose in the wake of trauma and PTSD. Experiential exercises were used to teach, model, role‐play, and integrate skills and to use them to develop a coherent memory narrative of the client’s life that incorporates a range of experiences including but not limited to traumatic stress. To enhance retention in the groups, small incentives that also reinforced aspects of the TARGET model (e.g. pens, key chains) were handed out on 3 occasions during the group. Group 2: Trauma‐sensitive usual care: n = 72 Participants received regular substance abuse treatment sessions. Counsellors providing this intervention received training on trauma‐sensitive care. Training workshops included information about the effect of traumatic events and disorders that trauma may cause or exacerbate. The counsellors also learned about the typical problems of trauma survivors and some of the ways in which past trauma can interfere with substance abuse recovery. Counsellors received literature about trauma, post‐traumatic stress, and substance abuse recovery that could be shared with clients. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: GAIN‐traumatic stress symptoms; PTCI SUD: GAIN subscales for substance use frequency, per cent drinking to intoxication, per cent using any drugs, and per cent abusing drugs or alcohol were used to assess changes in substance use and abuse. Treatment acceptability: Mean number of sessions attended for the active intervention group and mean number of standard‐care sessions attended for both groups. Other: GAIN for depressive symptoms, anxiety symptoms, and self efficacy Follow‐up: 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By cohort using a random number program |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to be able to assess |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | A trained research assistant conducted face‐to‐face interviews. No further information was available |
| Incomplete outcome data (attrition bias) | Low risk | Attrition and drop‐out are well described. Hierarchical linear modelling was used to enable estimation where data were unavailable |
| Selective reporting (reporting bias) | Unclear risk | Traumatic stress symptoms as indexed by GAIN were described in the methodology as a primary outcome, along with a number of other outcomes. However, this outcome is not reported, although other prespecified outcomes are reported |
| Other bias | High risk | The process of allocation was modified partway through the study, as delays in starting groups early on "meant that many participants received an insufficient dose". It was unclear as to why the number of participants randomised to the active intervention group was nearly double the number randomised to the control group. There was a high drop‐out rate. Counsellors to participants in the control condition could not be prevented from formally referring to the FREEDOM steps or using the handouts or other tangible materials from TARGET in non‐TARGET groups. This led study authors to conclude that there was "contamination of the comparison group treatment with TARGET principles and techniques". |
| Methods | Design: Partial RCT described as a "quasi‐experimental clinical trial" | |
| Participants | Setting: Participants were recruited through advertisements and referred through substance use treatment programmes. Participants were treated on an outpatient basis. Inclusion criteria: Current or subthreshold PTSD (defined as DSM‐IV criteria A, B, and E and the presence of either C or D) and current DSM‐IV substance dependence; if they reported using substances at least 3 times a week on the Substance Use Inventory; Mini‐Mental State Examination score greater than 21; age 18 to 55 years; female; and English‐speaking. Exclusion criteria: Advanced‐stage medical disease (e.g. AIDS, tuberculosis) as indicated by global physical deterioration and incapacitation, organic mental syndrome (associated with chronic drug abuse), and psychiatric exclusions (current active suicidality; current Axis I diagnoses other than atypical bipolar, depressive, or anxiety disorders; and history of psychosis). Sample size: 207 individuals were assessed for eligibility; 128 met full study eligibility criteria, 115 agreed to participate, and 96 of these were randomised. 32 of the 128 women became a non‐randomised community care comparison group. 75 of the 96 women who were randomised attended at least 1 treatment session and were included in the ITT analyses. PTSD diagnosis: 88% of women met full diagnosis for PTSD as measured by the CAPS. The other 12% met criteria for subthreshold PTSD. SUD type and diagnosis: Women were included on the basis that they met criteria for substance dependence. Women were polydrug users. Mean age: 38.2 (SD = 9.1) for Seeking Safety; 33.8 (SD = 8.3) for relapse prevention. The difference in age was statistically significant. Gender: 128 (100%) female Ethnicity: 42.7% African American; 36% white; 20% Hispanic; 13.3% multiracial; 1.3% other Country: USA | |
| Interventions | Group 1: Individual‐based Seeking Safety plus standard care: n = 41. The intervention is not fully described but is introduced as a short‐term, manualised cognitive behavioural treatment that simultaneously addresses trauma and substance abuse. Women were offered two 1‐hour treatment sessions weekly over 12 weeks. Group 2: Individual‐based relapse prevention plus standard care: n = 34. The intervention is not fully described but is introduced as an empirically validated cognitive behavioural therapy focusing on the identification of triggers and coping strategies for managing substance cravings and relapse. Group 3: Standard community treatment: n = 32. The intervention is not described. Women in this arm were not randomised. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: The primary PTSD outcome was a composite score from scores on the CAPS, IES‐R, and CGI Scale. SUD: The primary SUD outcome was a composite score from scores on the Substance Use Inventory and CGI Scale. Treatment acceptability: Data are reported for number of participants attending at least 25% of treatment sessions. Other: Items from CGI Scale were used to assess global severity of psychiatric symptoms; Global Assessment Scale and the HDRS were also used. Follow‐up: 3, 6, and 9 months postbaseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to be able to assess |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to be able to assess |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to be able to assess |
| Incomplete outcome data (attrition bias) | Unclear risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was used, using last observation carried forward. However, ITT was based on participants attending at least 1 treatment session. It was unclear if those who were randomised but who did not attend any sessions were aware of their allocation (Fergusson 2002) |
| Selective reporting (reporting bias) | Unclear risk | Composite outcome scores for both PTSD and SUD were used as primary outcomes. The authors state that this was done to reduce the possibility of Type I error. It is unclear how composite scores were generated. Raw scores are reported for PTSD measures but not for SUD measures |
| Other bias | Unclear risk | Insufficient information to be able to assess |
| Methods | Design: RCT | |
| Participants | Setting: Participants were enrolled in 7 community‐based substance abuse treatment programs (CTPs) across the USA. Inclusion criteria: To be eligible, individuals needed to have had at least 1 traumatic event in their lifetime and to meet DSM–IV–TR criteria for either full or subthreshold PTSD (where they did not meet criteria for either category C (avoidance and numbing symptoms) or category D (symptoms of increased arousal) but met all other criteria). Other inclusion criteria were between 18 and 65 years of age, had used alcohol or an illicit substance within the past 6 months, had a current diagnosis of drug or alcohol abuse or dependence, and were capable of giving informed consent. Exclusion criteria: Individuals were excluded if they had an advanced stage medical disease as indicated by global physical deterioration, impaired cognition, significant risk of suicidal/homicidal intent or behaviour, a history of schizophrenia‐spectrum diagnosis, a history of active (past 2 months) psychosis, or involvement in litigation related to PTSD. Individuals were also excluded if they did not speak English or if they refused to be video‐ or audiotaped. Sample size: 1963 individuals were assessed for eligibility; 353 were randomised, and all were included in the analyses. PTSD diagnosis: 80.4% of women met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: Women met diagnosis for substance abuse and substance dependence. Women were polydrug users. Mean age: 39.2 (SD = 9.2) years Gender: 353 (100%) female Ethnicity: 34% African American; 45.6% white; 6.5% Latina; 13.3% multiracial; 0.6% other Country: USA | |
| Interventions | Group 1: Group‐based Seeking Safety plus treatment as usual: n = 176. Seeking Safety is a structured cognitive behavioural treatment with both safety/trauma and substance use components integrated into each session. All sessions have the same structure: (a) check‐in, including reports of any unsafe behaviours and use of coping skills, (b) session quotation, a brief point of inspiration to affectively engage women and link to the session topic, (c) relating the material to the women’s lives, in which handouts are used to facilitate discussion and structured skill practice, and (d) check‐out, including a commitment to specific between‐session skills practice. Each session covered a different topic as follows: safety, taking back power from PTSD, when substances are in control, honesty, setting boundaries in relationships, compassion, healing from anger, creating meaning, integrating the split self, taking good care of oneself, red and green flags, and detaching from emotional pain (grounding). Seeking Safety treatment was abbreviated from 25 to 12 core sessions (75 to 90 minutes) delivered over 6 weeks to fit within a feasible time frame for community‐based outpatient treatment programs. However, because 2 women needed to be present to conduct the group, many women took longer than 6 weeks to complete the interventions. All study participants were enrolled in 1 of the participating community treatment programs and were asked to attend treatment as usual at the program during the 6‐week treatment phase of the study. Treatment as usual was not kept constant across sites but was allowed to vary. Outpatient treatment differed across sites in frequency and length of sessions per week, although most offered intensive outpatient services of 3 days per week or more. The treatment orientation of the programs also varied, but none of the programs provided trauma‐focused treatment to women during the study. Group 2: Group‐based Women's Health Education plus treatment as usual: n = 177. Women’s Health Education (WHE) was intended to control for therapeutic time and attention. WHE is a psycho‐educational, manualised health curriculum focused on topics such as understanding the female body, human sexual behaviour, pregnancy and childbirth, sexually transmitted diseases, HIV, and AIDS. WHE was designed to provide equivalent therapeutic attention, expectancy of benefit, and an issue‐oriented focus, but without theory‐driven techniques (i.e. those of Seeking Safety) or any explicit focus on or psycho‐education specific to substance abuse or trauma. All WHE sessions followed a common format: (a) introduction of topic, (b) review of group rules and between‐session assignment, (c) topic presentation, (d) a video, storytelling, and/or text readings, and (e) topic exercises in a variety of formats to facilitate group discussion and application of session materials, and (f) setting between‐session goals. Treatment as usual for the WHE group was as described above. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: CAPS total score; PSS‐SR total score SUD: Substance use diagnosis as measured by the Composite International Diagnostic Interview for DSM–IV; quantity and frequency of substance use as measured by the Substance Use Inventory; biologically confirmed abstinence from drugs of abuse was obtained by use of the SureStep urine drug screen card; recent alcohol use was tested with the Alco Screen‐Saliva Alcohol Test. Treatment acceptability: Data are reported for number of women attending at least 1 group treatment session and number attending at least 6 treatment sessions. Other: None Follow‐up: 1 week, 3, 6, and 12 months. The PSS‐SR and SUI were administered weekly during the treatment phase as well as at all other time points. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician generated 1 blocked randomisation list (block size known only to the statistician) for the entire study |
| Allocation concealment (selection bias) | Low risk | Each participating community‐based substance abuse treatment program received sets of 60 sealed, tamper evident security envelopes, containing 1 randomisation number and the corresponding treatment assignment |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Independent assessors who remained unaware of randomisation assignment performed all baseline and post‐treatment assessments |
| Incomplete outcome data (attrition bias) | Low risk | Treatment dropouts and withdrawals were clearly reported. An ITT approach was employed using generalised estimating equations |
| Selective reporting (reporting bias) | Unclear risk | Follow‐up outcomes were obtained at 1 week and 3, 6, and 12 months. The summary of outcomes table reports average outcome scores for the follow‐up period of 3 to 12 months. Use of averaged outcome scores was not specified in the methodology. It is unclear how data from these time points were used in analyses. Some analyses are reported for the 12‐month follow‐up point. It is unclear if data were reported in this way at the request of the publishing journal or by decision of the research group |
| Other bias | Low risk | There was no significant difference between the 2 treatment groups on any demographic or baseline diagnostic characteristics. We identified no other potential biases |
| Methods | Design: RCT | |
| Participants | Setting: Participants were new admissions to a community addiction treatment program and recruited from 1 of 7 participating community intensive outpatient or methadone maintenance programs. Inclusion criteria: Participants were at least 18 years of age, were actively enrolled in outpatient addiction services, and met criteria for any substance use disorder. Participants were also required to have a diagnosis of PTSD verified by the CAPS with total symptom score equal to or greater than 44. Exclusion criteria: Acute psychotic symptoms (people with a psychotic disorder were eligible if their symptoms were stable and they were receiving appropriate mental health services); psychiatric hospitalisation or suicide attempt in the past month, unless the hospitalisation or attempt was directly related to substance intoxication or detoxification and the person was currently stable; or unstable medical and legal situations such that ability to participate in the full duration of the study seemed unlikely. Sample size: 77 individuals were assessed for eligibility; 53 were randomised, and 36 attended at least 1 treatment session. 53 were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met diagnosis for a substance use disorder. Type of substance use and the number meeting substance dependence were not specified. Mean age: Integrated CBT plus standard care group: 39.09 (SD = 11.32) years; individual addiction counselling plus standard care group: 35.48 (SD = 9.44) years Gender: 23 (43.4%) male; 30 (56.6%) female Ethnicity: 90.6% white; other ethnicities were not described Country: USA | |
| Interventions | Group 1: Integrated cognitive behavioural therapy (ICBT) plus standard care: n = 32. ICBT is a manual‐guided individual‐based therapy focusing on PTSD symptoms and substance use. It was designed for integration into routine community addiction treatment programming. Participants were required to be active in either intensive outpatient or methadone maintenance services. ICBT consisted of 8 modules: introduction to treatment, crisis and relapse prevention planning, breathing retraining, psycho‐education about PTSD primary symptoms, psycho‐education about additional associated symptoms, two cognitive restructuring modules, and generalisation training. ICBT was delivered in an individual format, within a weekly 45‐ to 50‐minute session, over approximately 12 to 14 sessions. A client workbook was to be used in conjunction with the therapist manual with practice handout items for homework in between treatment sessions. Standard care occurred in either methadone maintenance or intensive outpatient clinics. 2 of the 7 recruiting programs were methadone maintenance, and 5 were intensive outpatient programs. Group 2: Individual addiction counselling (IAC) plus standard care: n = 21. IAC is a manual‐guided individual‐based therapy designed to be integrated into an addiction treatment or methadone maintenance program. IAC targeted substance use only and was considered complementary to a typical community addiction treatment program. IAC consisted of 5 modules: treatment initiation, early abstinence, maintaining abstinence, recovery, and termination. IAC was delivered in 10 to 12 weekly sessions. As with ICBT, individual addiction counselling had participant practice handouts for homework in between treatment sessions. Standard care was as described above. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: CAPS SUD: ASI alcohol and drug composites; toxicology: recent alcohol intake and drug metabolites for amphetamine, benzodiazepines, cannabis, cocaine, methamphetamine, and opiates were screened for using urine and breath samples gathered at each assessment period. Treatment acceptability: This was described in terms of initiation (number of participants attending at least 1 treatment session), engagement (number completing at least 2 sessions), and completion (number attending at least 75% of sessions). Other: BDI Follow‐up: 3 and 6 months postbaseline | |
| Notes | It is unclear as to why there is such sizeable difference between the numbers of participants randomised to the 2 conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported. It is reported that research interviewers were blinded to treatment assignment at randomisation, but other information about the randomisation process is not provided |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | High risk | Research interviewers were not blinded to treatment assignment at the follow‐up assessments |
| Incomplete outcome data (attrition bias) | Unclear risk | Analysis was undertaken using generalised estimating equations analyses, which allowed analyses without excluding participants based on missing data points or drop‐out. However, the study only achieved a follow‐up rate of 53%, and as the authors acknowledge, this "reduces the power and the ability to detect differences between treatment conditions". |
| Selective reporting (reporting bias) | Low risk | Primary outcomes are specified in the protocol registered with ClinicalTrials.gov |
| Other bias | High risk | There were a number of minor differences between the 2 groups at baseline (e.g. PTSD severity). The effects of these differences are unclear. The number of treatment sessions provided to the intervention (12 to 14) was longer than that provided to the control intervention (10 to 12) |
| Methods | Design: RCT | |
| Participants | Setting: Participants were seen on an outpatient basis and were recruited from substance use treatment services, media advertisements, and practitioner referrals. Inclusion criteria: Past‐month DSM‐IV‐TR diagnoses of PTSD and substance dependence, age 18 years or older, and fluency in English. Exclusion criteria: Individuals were excluded from participating if they were currently suicidal (expressed suicidal ideation accompanied by a plan and intent), had a recent history of self harm (past 6 months), had current active symptoms of psychosis, or experienced cognitive impairment severe enough to impede treatment. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. Sample size: 334 individuals were assessed for eligibility; 103 were randomised, and all were included in the analyses. SUD type and diagnosis: All participants were reported to be substance dependent. Participants were polydrug users and had used a mean of 4 drug classes in the previous month. Mean age: 33.7 (SD = 7.9) years Gender: 64 (62.1%) female Ethnicity: Australian born: 87 (84.5%); 6 (5.8%) Aboriginal or Torres Strait Islander Country: Australia | |
| Interventions | Group 1: Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE): n = 55. COPE is a modified version of Concurrent Treatment of PTSD and Cocaine Dependence. The model represents an integration of existing evidence‐based manualised CBT interventions for PTSD and substance dependence. Intervention consists of 13 individual‐based 90‐minute sessions (i.e. 19.5 hours) delivered by a clinical psychologist. Although designed to be delivered weekly, flexibility was permitted. Treatment components include motivational enhancement and CBT for substance use; psycho‐education relating to both disorders and their interaction; in vivo exposure; imaginal exposure; and cognitive therapy for PTSD. The final session was dedicated to providing a review of the treatment, devising an aftercare plan, and termination of therapy. Group 2: Usual treatment: n = 48. Both the treatment and the control group were able to engage in usual treatment for substance dependence. As such, participants could access any type of substance use treatment currently available in the community, including outpatient counselling, inpatient or outpatient detoxification, residential rehabilitation, and pharmacotherapies (e.g. methadone, buprenorphine, buprenorphine plus naloxone, naltrexone). Experimental intervention modality: Combined | |
| Outcomes | PTSD: CAPS SUD: CIDI Treatment acceptability: Data are reported for number of participants attending at least 1 treatment session; number attending at least 1 imaginal exposure session; and number attending all sessions. Other: BDI, STAI, IPDE Follow‐up: 6 weeks, 3 and 9 months postbaseline | |
| Notes | The study concluded with a lower sample size than planned due to a low recruitment rate. It is unclear why there is such sizeable difference between the numbers of participants randomised to the 2 conditions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization was conducted in groups of 10, stratified according to sex ... " |
| Allocation concealment (selection bias) | Low risk | " ... by a person independent of the research." |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Interviews were administered by 2 trained research officers blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were analysed on an ITT basis. Missing data were imputed using multiple imputation |
| Selective reporting (reporting bias) | Low risk | Key outcomes are as specified in the protocol registered with the World Health Organization's trials portal |
| Other bias | High risk | Participants in the control group were more likely to have reported a history of childhood sexual abuse. This was controlled for in analyses. There was considerable variability in the time taken to complete treatment, with some participants continuing treatment well beyond the planned treatment period of 13 weeks and at least 1 receiving treatment around the final follow‐up point |
| Methods | Design: RCT | |
| Participants | Setting: Individuals with severe mental illness were recruited from community mental health centres. Inclusion criteria: Minimum age 18 years; designation by the states of New Hampshire or Vermont as having a severe mental illness, defined as a DSM–IV Axis I disorder and persistent impairment in the areas of work, school, or ability to care for oneself; DSM–IV diagnosis of major depression, bipolar disorder, schizoaffective disorder, or schizophrenia; current DSM–IV diagnosis of PTSD; and legal ability and willingness to provide informed consent to participate in the study. Exclusion criteria: Psychiatric hospitalisation or suicide attempt within the past 3 months; current DSM–IV substance dependence. Sample size: 270 individuals were assessed for eligibility; 108 were randomised, and all were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: Nature of substance abuse was not specified. 44 (40.7%) of participants met diagnosis for substance use disorder. Outcome data are available for this subgroup. Mean age: 44.21 (SD = 10.64) years Gender: 35/44 (79.5%) female Ethnicity: 38 (86.4%) white; (4.5%) African American; (4.5%) American Indian/Alaska Native; (2.3%) Hispanic; (2.3%) Asian‐Pacific Islander Country: USA | |
| Interventions | Group 1: Individual CBT for PTSD: n = 17. Sessions included an introduction to the programme; crisis plan review; psycho‐education on core and associated symptoms of PTSD; cognitive restructuring; generalisation training; and termination. Participants were offered 12 to 16 sessions over 4 to 6 months. Group 2: Treatment as usual: n = 27. Participants assigned to TAU continued to receive the usual services they had been receiving before enrolment in the program. None of the mental health centres offered either cognitive restructuring or exposure therapy treatments for PTSD, although supportive counselling for trauma‐related problems was available. Experimental intervention modality: Treatment of PTSD only | |
| Outcomes | PTSD: CAPS SUD: ‐ Treatment acceptability: Data are reported for number of participants in the experimental condition attending at least 6 treatment sessions. Other: PTCI; PTSD Knowledge Test; Brief Psychiatric Rating Scale; BDI‐II; BAI; 12‐Item Short Form Health Survey; client version of the Working Alliance Inventory Follow‐up: End of treatment and at 3 and 6 months' post‐treatment | |
| Notes | This study did not specifically aim to treat individuals meeting diagnosis for SUD. A subset of participants met SUD diagnosis, and study authors provided data for these individuals. These participants only met criteria for substance abuse; individuals meeting diagnosis for substance dependence were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by a computer‐based randomisation program |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted at a central location in a research centre. Assignments were not known in advance by either clinical or research staff |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Assessments were conducted by blinded interviewers at all assessment points. Participants were instructed at the beginning of interviews not to talk about any treatments for trauma‐related problems they may have received. Interviewers were requested to inform the project co‐ordinator if the client broke the blind during an interview. Interviewers were not asked to guess clients’ treatment assignments, to avoid directly encouraging them to formulate hypotheses about how treatment may have affected clients’ symptoms, which could have influenced subsequent ratings. No specific instances of blind breaking were noted in the study |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals are thoroughly described. ITT analysis was conducted to determine the effects of primary outcomes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were reported as specified in the methodology section, but we were not able to identify a previously published protocol |
| Other bias | Low risk | There were no differences between the groups on any demographic, diagnostic, or baseline measures or in the rates of follow‐up assessments |
| Methods | Design: RCT | |
| Participants | Setting: Participants were adolescent girls who were treated on an outpatient basis and were recruited through posted fliers and active recruitment from local clinics, hospitals, schools, and clinicians. Inclusion criteria: Participants met current DSM‐IV criteria for both PTSD and SUD. They also had to report active substance use within the past 60 days. Exclusion criteria: Potential participants were excluded if they had a history of bipolar I disorder (mania), psychotic disorder, were mandated to treatment, or had characteristics that would interfere with treatment completion (mental retardation, homelessness, impending incarceration, or a life‐threatening illness). Sample size: The number of individuals assessed for eligibility was not specified; 33 were randomised, and all were included in the analyses. PTSD diagnosis: All participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: Most participants (n = 31, 93.9%) met diagnosis for substance dependence. Participants were polydrug users. Mean age: 16.06 (1.22) Gender: 100% female Ethnicity: 78.8% white; 12.8% Asian‐Pacific Islander; 3% Hispanic; 3% African American; 3% multiethnic Country: USA | |
| Interventions | Group 1: Individual‐adapted Seeking Safety plus treatment as usual: n = 18. This coping skills therapy targets current PTSD and SUD. The treatment manual has 25 topics representing cognitive, behavioural, and interpersonal domains. Each topic offers a safe coping skill relevant to both disorders, such as Asking for Help, Compassion, Setting Boundaries in Relationships, and Honesty. The treatment has five principles: (1) safety as the priority; (2) integrated treatment of both disorders; (3) a focus on ideals; (4) four content areas: cognitive, behavioural, interpersonal, and case management; and (5) attention to therapist processes. The original manual was modified to take account of the developmental level of adolescents. Participants were offered 25 50‐minute sessions over 3 months. Group 2: Treatment as usual: n = 15. All participants were allowed to attend any concurrent treatments they naturalistically sought (e.g. Alcoholics Anonymous, psychotropic medication, and other individual and group psychotherapies). Experimental intervention modality: Integrated | |
| Outcomes | PTSD: Trauma Symptom Checklist for Children SUD: Personal Experiences Inventory Treatment acceptability: For the Seeking Safety group, the mean number of all treatment sessions attended and the number of Seeking Safety sessions attended were reported. Data were also reported for client satisfaction (see below). Other: Beliefs About Substance Use; World Assumptions Scale; Adolescent Psychopathology Scale; CSQ; HAQ; Teen Treatment Services Review interview Follow‐up: End of treatment and at 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information about sequence generation and allocation concealment were provided by the lead study author. A statistician independent of the study generated the randomisation list prior to the first randomisation using a random number generator |
| Allocation concealment (selection bias) | Low risk | The list was administered "lock‐step", and the principal investigator and therapists were unable to influence randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | The process of outcome assessment was not described |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts were well reported. Authors employed a full intent‐to‐treat analysis using a random effects model to analyse data |
| Selective reporting (reporting bias) | High risk | The Personal Experiences Inventory was identified as the primary measure. This measure was described as having 2 subsections (chemical involvement problem severity and psychosocial problems), each with multiple subscales. More specific information about whether a total score, subsection score, or subscale score was the primary outcome was not given. Other outcome measures also had multiple subscales. Outcomes were not clearly reported. A table describing outcomes at intake, end of treatment, and 3 months' follow‐up only shows data for outcomes that were significant |
| Other bias | High risk | The TAU group had a higher level of psychopathology as measured at baseline. There was no attempt to control for this. The study describes a number of outcome measures, with a large number of subscales. We estimated 40 outcomes. It appears there were no attempts to correct for the use of multiple statistical testing |
| Methods | Design: RCT | |
| Participants | Setting: Participants were female victims of interpersonal violence. They were recruited through flyers posted in community agencies that serve IPV victims and in primary care and psychiatry clinics. Inclusion criteria: Female interpersonal violence victims over the age of 18, with at least 1 month out of the abusive relationship, met DSM‐IV criteria for PTSD and an alcohol use disorder, literate in English, had not changed psychotropic medications or dosages within the previous 2 months and agreed not to during the active phase (first 12 weeks) of the intervention, and had an identified primary care physician. Exclusion criteria: Moderate or severe cognitive impairment as measured by a Mini‐Mental State Examination score less than or equal to 18, history of psychosis (women with histories of psychosis or mania were only included if their symptoms had been well managed by pharmacotherapy for the most recent 6‐month period). Sample size: 78 individuals were assessed for eligibility, 35 were randomised, and 29 received at least 1 session of treatment or remained in contact with the research group and were included in analysis. PTSD diagnosis: 25 (86.2%) of women met full diagnosis for PTSD as measured by the CAPS; other women met subthreshold diagnosis for PTSD. SUD type and diagnosis: All women met diagnosis for alcohol use disorder. Mean age: Seeking Safety: 45.27 (SD = 8.44); facilitated 12‐step: 37.38 (SD = 9.13) Gender: 100% female Ethnicity: 65.5% white; 6.9% African American; 24.1% Hispanic; 3.4% American Indian Country: USA | |
| Interventions | Group 1: Adapted group‐based Seeking Safety plus treatment as usual: n = 20. Seeking Safety plus Cognitive Trauma Therapy for Battered Women with PTSD (CTT‐BW) (Kubany 2004).The intervention was a 24‐session group treatment protocol delivered over 12 weeks, incorporating the following interventions: psycho‐education regarding PTSD and alcohol use disorders, skills to reduce self harm behaviours, behavioural activation, exposure to trauma, identifying and managing triggers, building social support, coping skills, assertive communication, managing affect, problem solving, grounding, and cognitive restructuring. Group 2: 12‐step supportive group: n = 9. Therapist‐led supportive group using a 12‐step model where women were encouraged to discuss issues related to domestic violence and abstinence from alcohol. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: CAPS, PCL‐C SUD: TLFB, Conceptual Cues/Coping Questionnaire Treatment acceptability: ‐ Other: Adult Attachment Interview, Motivation/Self Esteem Scale, Anxiety Sensitivity Index, BDI, CD‐RISC‐10, Negative Mood Regulation Expectancies Scale, Self Compassion Scale, Coping Skills Follow‐up: End of treatment and at 3 and 6 months' post‐treatment | |
| Notes | This study had originally intended to recruit 100 individuals but were unable to achieve this within the period that the study was funded. Investigators had great difficulty gathering data after the initial follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation was handled by the study co‐ordinator |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | High risk | Assessors were not blind to allocation |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was performed on women who attended at least 1 treatment session. Women who were randomised but did not attend any intervention sessions were unaware of their allocation (Fergusson 2002) |
| Selective reporting (reporting bias) | Low risk | Key outcomes are as specified in the protocol registered with ClinicalTrials.gov |
| Other bias | High risk | There was a significant difference in the average age of the two groups. It is unclear why there is such sizeable difference in the numbers of women randomised to the 2 conditions. There was also a sizeable, though non‐significant, difference in alcohol consumption at baseline, and notable differences in ethnic makeup between the 2 groups |
| Methods | Design: RCT | |
| Participants | Setting: Participants were recruited from a range of services in metropolitan Sydney, Australia and seen on an outpatient basis. Inclusion criteria: Individuals were eligible if they were 18 years of age or older, consumed alcohol at hazardous levels (men 29 or more and women 15 or more 10 g ethanol drinks per week) and met DSM‐IV diagnostic criteria for PTSD, determined by the CAPS. AUD diagnosis was determined by the Structured Clinical Interview for DSM‐IV. Individuals on stable doses (for 2 months or longer) of pharmacotherapy for depression or alcohol dependence were eligible, as were individuals who needed and completed alcohol withdrawal. Exclusion criteria: People were excluded if they were 17 years or younger, had current psychosis, severe suicide risk, significant cognitive impairment, limited English comprehension, or severe substance dependence. Sample size: 154 individuals were screened and 90 assessed for eligibility; 62 were randomised, and all were included in the analyses. PTSD diagnosis: 58 (94%) of participants met full diagnosis for PTSD as measured by the CAPS. SUD type and diagnosis: All participants met criteria for AUD. Mean age: 41.18 (SD = 11.91) years Gender: 33 (53%) female Ethnicity: Not reported Country: Australia | |
| Interventions | Group 1: Integrated CBT for PTSD and AUD: n = 33. Participants in both conditions received the same treatment targeting AUD. This consisted of motivational interviewing, intervention focused on coping with cravings, cognitive intervention related to drinking and management of negative moods. Participants in this arm also received cognitive behavioural intervention for PTSD, based on a prolonged exposure model with cognitive restructuring. Treatment in both the experimental and control condition was manualised, and consisted of 12, once‐weekly 90‐minute individual sessions with structured daily homework tasks. Group 2: CBT for AUD and supportive counselling: n = 29. In addition to the shared components described above, this group also received supportive counselling. Treatment in this arm targeted AUD symptoms only, not PTSD symptoms. Experimental intervention modality: Combined | |
| Outcomes | PTSD: CAPS, PDS SUD: TLFB, SADQ Treatment acceptability: Median sessions attended, number attending 1 or more sessions, 6 or more sessions, 9 or more sessions. Other: Short Inventory of Problems, BDI, STAI Follow‐up: Post‐treatment and 5 and 9 months' post‐treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted according to a random number system by a person independent of the study ... " |
| Allocation concealment (selection bias) | Low risk | " ... and treatment was concealed." |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Follow‐up assessments were conducted by independent clinicians who were unaware of the participants’ treatment condition and did not have access to participant clinical or supervision notes or treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | All analyses were based on intent‐to‐treat, including all participants who entered the trial |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as specified in the methodology section |
| Other bias | Low risk | There were no differences between the groups on any demographic, diagnostic, or baseline measures or in the rates of follow‐up assessments |
| Methods | Design: RCT | |
| Participants | Setting: Treatment was conducted on the minimum‐security wing of a female prison. Participants were recruited from a voluntary residential substance abuse treatment program for women requesting intensive substance abuse treatment. Inclusion criteria: DSM‐IV criteria for current PTSD or subthreshold PTSD (i.e. had at least 1 symptom from all 3 clusters that were associated with impairment/distress) within the previous month as determined by the CAPS; and DSM‐IV criteria for substance dependence 1 month prior to entering prison as determined by the Structured Clinical Interview for DSM‐IV. Exclusion criteria: Women were excluded if they were actively psychotic at the time of recruitment, did not know English well enough to be able to understand the consent form or measures, or were diagnosed with organic brain impairment. Sample size: 92 women were assessed for eligibility; 49 were randomised, and 44 were included in the analyses. PTSD diagnosis: 83.5% of women met full diagnosis for PTSD as measured by the CAPS, and 16.5% met the subthreshold definition. SUD type and diagnosis: Women were polydrug users. 87.8% met criteria for alcohol dependence prior to imprisonment, with another 4.1% meeting criteria for lifetime alcohol abuse. The percentages of women who had ever used a single substance at a level typically indicating dependence (10 or more times in 1 month) were 93.9% for cocaine, 75.5% for cannabis, 59.2% for heroin or other opioids, 38.8% for sedatives/hypnotics/anxiolytics, 30.6% for hallucinogens/PCP, and 26.5% for stimulants. Mean age: 34.6 (SD = 7.4) years Gender: 100% female Ethnicity: 23 (46.9%) white; 16 (32.7%) African American; 7 (14.2%) Hispanic; and 3 (6.1%) other races/ethnicities Country: USA | |
| Interventions | Group 1: Group‐based Seeking Safety plus treatment as usual: n = 27. Seeking Safety is a present‐focused therapy to help people attain safety from trauma/PTSD and substance abuse. The treatment was designed for flexible use. SS is based on a number of key principles: safety, integrated treatment of both PTSD and substance abuse at the same time, a focus on ideals, and attention to clinician processes. Interventions are in the domain of cognitive, behavioural, interpersonal, and case management. Seeking Safety consists of 25 topics that can be conducted in any order: Introduction/Case Management, Safety, PTSD: Taking Back Your Power, When Substances Control You, Honesty, Asking for Help, Setting Boundaries in Relationships, Getting Others to Support Your Recovery, Healthy Relationships, Community Resources, Compassion, Creating Meaning, Discovery, Integrating the Split Self, Recovery Thinking, Taking Good Care of Yourself, Commitment, Respecting Your Time, Coping with Triggers, Self‐Nurturing, Red and Green Flags, Detaching from Emotional Pain (Grounding), Life Choices, and Termination. SS was conducted in group modality for 90 min, typically 3 times a week for 6 to 8 weeks while the women were in prison, with 3 to 5 women per group. After release from prison, each woman in SS was offered weekly individual 60‐min “booster” sessions for 12 weeks to reinforce material from the group sessions. Group 2: Treatment as usual: n = 22. All women in this study were enrolled in a 28‐bed residential substance use treatment program in the minimum‐security wing (approximately 30 hours per week). Women typically attend this program for 3 to 6 months, depending on the length of their sentences. Substance use treatment was abstinence‐oriented, focused on the 12‐step model, and took place in a psycho‐educational large‐group format, with weekly individual case management and drug counselling. Psycho‐educational groups included attention to women's health, domestic violence, affect management, relapse prevention, career exploration, anger management, and parenting, conducted by the same clinicians who conducted the SS treatment. This program did not offer any treatment specifically for trauma. Prior to prison release, the women received case management services, although this discontinued once the women were released from prison. All women leaving prison were referred for further substance use treatment. Experimental intervention modality: Integrated | |
| Outcomes | PTSD: CAPS; Trauma Symptom Checklist 40 SUD: ASI; TLFB Treatment acceptability: Treatment utilisation; CSQ; mean number of sessions attended Other: Brief Symptom Inventory; legal composite score of the ASI (for criminal activity) Follow‐up: 12 weeks after the start of the program and 3 and 6 months following release from prison | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded to allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | The study included data only from those who were available to follow‐up. The number of women who did not provide data at follow‐up was fairly small but slightly disproportionate to the SS group. We did not feel this difference was sufficient to have a clinically relevant impact on observed effect sizes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were reported as specified in the methodology section, but we were not able to identify a previously published protocol |
| Other bias | High risk | The authors acknowledge potential contamination of treatment and control conditions in the closed communal setting of a prison wing. Postrelease follow‐up dose was not equivalent. Women in the SS group were offered up to 12 booster sessions on release from prison. Women in the control group were referred for further substance use treatment |
AA: Alcoholics Anonymous
ACQ‐Now: Alcohol Craving Questionnaire‐Now
ASI: Addiction Severity Index
AUD: alcohol use disorder
BAI: Beck Anxiety Inventory
BDI: Beck Depression Inventory
CAPS: Clinician Administered PTSD Scale
CBT: cognitive behavioural therapy
CDIS‐IV: Computerized Diagnostic Interview Schedule
CD‐RISC‐10: 10‐item Connor‐Davidson Resilience Scale
CGI: Clinical Global Impressions
CI: confidence interval
CIDI: Composite International Diagnostic Interview
CSQ: Client Satisfaction Questionnaire
DESNOS: Disorders of Extreme Stress Not Otherwise Specified
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition
DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Text Revision
FREEDOM: self‐regulation via Focusing (SOS: Slow down, Orient, Self‐check); processing current traumatic stress reactions via Recognizing current triggers, Emotions, and cognitive Evaluations, and, strength‐based reintegration by Defining core goals, identifying currently effective responses (Options), and affirming core values by Making positive contributions
GAIN: Global Appraisal of Individual Needs
HAQ: Helping Alliance Questionnaire
HDRS: Hamilton Depression Rating Scale
HLS: Healthy Lifestyles Sessions
IES‐R: Impact of Events Scale‐Revised
IPDE: International Personality Disorder Examination
IPV: interpersonal violence
ITT: intention‐to‐treat
HIV: human immunodeficiency virus
NA: Narcotics Anonymous
PACS: Penn Alcohol Craving Scale
PANAS: Positive and Negative Affect Schedule
PCL‐C: PTSD Checklist‐Civilian Version
PDS: Post‐traumatic Stress Diagnostic Scale
PE: prolonged exposure
PSS‐I: PTSD Symptom Scale‐Interview
PSS‐SR: PTSD Symptom Scale–Self‐Report
PTCI: Post‐traumatic Cognitions Inventory
PTSD: post‐traumatic stress disorder
RCT: randomised controlled trial
SADQ: Severity of Alcohol Dependence Questionnaire
SD: standard deviation
SS: Seeking Safety
STAI: State‐Trait Anxiety Inventory
SUD: substance use disorder
SUDS: Subjective Units of Distress
SUI: Substance Use Inventory
TAU: treatment as usual
TLFB: Timeline Followback Interview
VA: Veterans Affairs
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument | |
| Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument | |
| Types of participants: Less than 80% had SUD at baseline. We did obtain outcome data for a subgroup of participants in this study but decided that we could not include the study as participants who were identified as having an alcohol use disorder were not diagnosed through a clinician‐administered assessment | |
| Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument | |
| Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument | |
| Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument | |
| Types of assessment: PTSD was established in a subset of participants at 3 years' follow‐up. PTSD was not established at baseline | |
| Type of intervention: The experimental intervention was pharmacological | |
| Types of studies: Not a randomised controlled trial | |
| Types of participants: Participants were included on the basis of screening for hazardous drinking. Alcohol use disorder was not diagnosed through a clinician‐administered assessment | |
| Types of participants: Less than 80% had PTSD at baseline. We were unable to obtain outcome data for the subset who did have PTSD | |
| Types of assessment: PTSD was not established through a formal and reliable means but through use of a self report instrument | |
| Type of intervention: Not a psychological therapy | |
| Types of studies: Not a randomised controlled trial | |
| Types of participants: Less than 80% had PTSD at baseline. We were unable to obtain outcome data for the subset who did have PTSD | |
| A full report of this study is not yet available | |
| Types of participants: Less than 80% of participants were diagnosed as having PTSD/SUD at baseline, and we were unable to obtain subset data |
PTSD: post‐traumatic stress disorder
SUD: substance use disorder
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | Male prisoners with PTSD and comorbid substance use |
| Interventions | (Seeking Safety + TAU) vs TAU (alone) |
| Outcomes | SUDs, PTSD (acceptability, feasibility, and preliminary efficacy of Seeking Safety among male Australian prisoners) |
| Notes | Does not meet 80% PTSD threshold but does meet other criteria. I will approach the author for subset data |
| Methods | Randomised controlled trial |
| Participants | Outpatients in addiction services with PTSD and SUDs |
| Interventions | Cognitive behavioural therapy vs individual addiction counselling vs TAU |
| Outcomes | Primary outcomes: PTSD symptom severity (CAPS score); Drug and alcohol symptom severity (ASI‐Self Administered); Frequency of substance use (TLFB Interview); Positive toxicology screens (urine drug screen and breathalyser). |
| Notes | Study reports not retrieved in March 2015 search results |
| Methods | Randomised controlled trial |
| Participants | A subset of 204 participants with PTSD were included in a cohort of 553 randomised participants with co‐occurring addiction and mental disorder |
| Interventions | Integrated chronic disease management vs primary care intervention |
| Outcomes | Abstinence, depression, anxiety |
| Notes | Conference abstract only (full study report not retrieved in March 2015 search results, added to CCDANCTR 13 April 2015, via PsycINFO OVID alert) |
| Methods | Randomised controlled trial |
| Participants | Treatment of SUD in 7 women with SUD and PTSD comorbidity |
| Interventions | Effects of EMDR associated with ST 3‐phase protocol: 1. Eight EMDR sessions focused on reprocessing traumatic memory; 2. Eight EMDR sessions (traumatic memory) associated with ST (traumatic attachment) 3. Eight EMDR sessions (addictive memory) associated with ST |
| Outcomes | PTSD, SUDs, and attachment disorder |
| Notes | Conference abstract only, full study report not yet available |
| Methods | Randomised controlled trial |
| Participants | 84 individuals with current PTSD and alcohol dependence were randomised |
| Interventions | Experiential acceptance vs cognitive restructuring vs attention placebo |
| Outcomes | 71 participants completed the study (84.5%); 13 of the 84 were lost to follow‐up. PTSD and alcohol‐related outcomes were not reported in the conference abstract. |
| Notes | Report of primary outcomes identified later (Stappenbeck 2015), added to CCDANCTR 13 April 2015, via a PsycINFO OVID alert dated 25 March 2015) Contact with trialists Tracey Simpson and Cindy Stappenbeck, confirmed this was the same trial as NCT00760994 |
| Methods | Randomised controlled trial |
| Participants | 145 individuals with co‐occurring alcohol or substance dependence, depression, and trauma exposure were randomised |
| Interventions | Integrated cognitive behavioural therapy (ICBT) for co‐occurring depression and addiction plus cognitive processing therapy vs ICBT for co‐occurring depression and addiction plus continuation of ICBT |
| Outcomes | Group assignment (ICBT vs CPT) was not related to attendance. PTSD and alcohol‐related outcomes were not reported in abstract |
| Notes | Conference abstract, full study report not yet available |
| Methods | Randomised and non‐randomised participants |
| Participants | Incarcerated men with PTSD and SUDs, housed at a high‐security prison operated by the Pennsylvania Department of Corrections |
| Interventions | Integrated group therapy: Seeking Safety vs Men’s Trauma Recovery and Empowerment Model vs wait‐list control |
| Outcomes | Primary outcomes: |
| Notes | This study meets most of our inclusion criteria. However, it includes both randomised and non‐randomised participants. We would need to determine whether we could obtain data for randomised participants only |
ASI: Addiction Severity Index
CAPS: Clinician Administered PTSD Scale
EMDR: eye movement desensitisation and reprocessing
PTSD: post‐traumatic stress disorder
ST: schema therapy
SUD: substance use disorder
TAU: treatment as usual
TLFB: Timeline Followback Interview
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Cognitive‐behavioral treatment for female patients with PTSD and SUD |
| Methods | Multicentre randomised controlled trial |
| Participants | 342 females with PTSD and SUD |
| Interventions | Seeking Safety (14 sessions) vs structured relapse prevention vs TAU |
| Outcomes | PTSD symptoms, substance use |
| Starting date | October 2012 |
| Contact information | Ingo Schäfer: [email protected] |
| Notes |
| Trial name or title | Couple‐based treatment for alcohol use disorders and post‐traumatic stress disorder (CTAP) |
| Methods | Randomised controlled trial |
| Participants | Veterans meeting current DSM‐IV diagnosis for alcohol abuse or dependence and PTSD |
| Interventions | TAU vs couple‐based treatment for alcohol use disorders and PTSD |
| Outcomes | Number of days drinking or using drugs; problems related to drinking or using drugs; PTSD; couple relationship adjustment; number of days of heavy drinking or using drugs (outcome measures not specified) |
| Starting date | August 2010 |
| Contact information | Jeremiah A Schumm: [email protected] |
| Notes |
| Trial name or title | Multicomponent cognitive behavioral therapy (CBT) for posttraumatic stress disorder (PTSD) and substance abuse (PTSD/SUD) |
| Methods | Pilot randomised controlled trial |
| Participants | Participants will be 50 volunteer adults with PTSD, SUD, and serious mental illness who are receiving services at the Freedom House Recovery Center, served through the Orange Person Chatham Area Program |
| Interventions | Group and individual CBT and exposure therapy for PTSD |
| Outcomes | Clinician Administered PTSD Scale, Addiction Severity Index |
| Starting date | August 2009 |
| Contact information | Karen Cusack: [email protected] |
| Notes |
| Trial name or title | Post‐traumatic stress disorder (PTSD), addiction, and virtual reality |
| Methods | Randomised controlled trial |
| Participants | Military veterans, National Guardsmen, and reservists with PTSD and problems with addiction |
| Interventions | Prolonged exposure vs prolonged exposure plus virtual reality‐based exposure to cues for marijuana, cocaine, heroin, cigarette, and/or alcohol use, and phone‐based reminders of learning (extinction reminders) to virtual reality exposure |
| Outcomes | Acceptibility, change in PTSD symptoms, change in substance use |
| Starting date | December 2008 |
| Contact information | Zachary Rosenthal, Duke University |
| Notes |
| Trial name or title | Integrated vs sequential treatment for PTSD and addiction among OEF/OIF veterans |
| Methods | Randomised controlled trial |
| Participants | Male or female Persian Gulf‐era veterans (18 to 65 years old). Current diagnosis of PTSD (symptom duration > 3 months) with clinically significant trauma‐related symptoms, as indicated by a score of at least 50 on the PTSD Checklist. Current abuse or dependence on alcohol, stimulants such as cocaine, opioids, including prescription opioids or benzodiazepines. |
| Interventions | Prolonged exposure vs motivational enhancement therapy |
| Outcomes | Substance use and PTSD symptoms |
| Starting date | February 2011 |
| Contact information | David W. Oslin: [email protected] |
| Notes |
| Trial name or title | Pilot study of an integrated exposure‐based model for posttraumatic stress disorder and substance use disorder |
| Methods | Randomised controlled trial |
| Participants | Individuals meeting DSM‐IV criteria for current PTSD and current SUD |
| Interventions | Integrated psychotherapy for PTSD/SUD ("Creating Change") vs modified TAU |
| Outcomes | Change in substance use from baseline to 3 months' post‐treatment measured via urine drug screens and the Addiction Severity Index composite scores; change in PTSD symptoms from baseline to 3 months' post‐treatment assessed using the PTSD Checklist and the Clinician Administered PTSD Scale |
| Starting date | January 2011 |
| Contact information | Lisa Najavits: [email protected] |
| Notes |
| Trial name or title | Integrated treatment of Operation Enduring Freedom/Operation Iraqi Freedom veterans with post‐traumatic stress disorder and substance use disorders |
| Methods | Randomised controlled trial |
| Participants | Adult male and female active‐duty Operation Iraqi Freedom (OIF)/Operation Enduring Freedom (OEF) military personnel and separated OIF/OEF veterans aged 18 to 65 years |
| Interventions | Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE) vs TAU |
| Outcomes | Clinician Administered PTSD Scale; reduction of substance use or abstinence |
| Starting date | April 2011 |
| Contact information | Sudie E. Back: [email protected] |
| Notes |
| Trial name or title | Cognitive behavioral therapy (CBT) for PTSD in veterans with co‐occurring SUDs |
| Methods | Randomised controlled trial |
| Participants | Veterans with a current SUD diagnosis, scoring at least 45 on the Clinician Administered PTSD Scale |
| Interventions | CBT plus TAU vs TAU |
| Outcomes | PTSD symptom severity will be measured by the Clinician Administered PTSD Scale. Other measures not provided |
| Starting date | October 2012 |
| Contact information | Liz Forshay: [email protected] |
| Notes |
| Trial name or title | Concurrent treatment for substance dependent individuals with post‐traumatic stress disorder (PTSD) |
| Methods | Randomised controlled trial |
| Participants | Participants must meet DSM‐IV criteria for current or past substance dependence and current PTSD |
| Interventions | Concurrent treatment of PTSD and substance use disorders using prolonged exposure (COPE) and relapse prevention therapy vs a delayed‐treatment control group |
| Outcomes | PTSD symptom severity, substance use severity, global psychiatric symptom severity, treatment retention and compliance |
| Starting date | September 2008 |
| Contact information | Teresa Lopez‐Castro: [email protected]; Lesia Ruglass: [email protected] |
| Notes |
| Trial name or title | Integrated cognitive behavioral therapy for co‐occurring PTSD and substance use disorders |
| Methods | Randomised controlled trial |
| Participants | OEF/OIF/OND Veteran status with a diagnosis of PTSD (confirmed by the Clinician Administered PTSD Scale with a total symptom score of 44 or more) and a diagnosis of a SUD (abuse or dependence) (confirmed by the Structured Clinical Interview for DSM‐IV Section E) |
| Interventions | Integrated cognitive behavioural therapy vs TAU |
| Outcomes | Decrease from baseline in Clinician Administered PTSD Scale score (PTSD symptom severity) at 3 and 6 months. Reduction from baseline in substance use severity (Addiction Severity Index) at 3 and 6 months. |
| Starting date | February 2011 |
| Contact information | Mark McGovern: [email protected] |
| Notes |
| Trial name or title | Evaluation and treatment of substance abuse in veterans with PTSD disability claims |
| Methods | Randomised controlled trial |
| Participants | Veteran of OEF or OIF enrolling for compensation and pension for PTSD |
| Interventions | Screening, brief intervention, and referral to treatment (SBIRT) vs no additional treatment |
| Outcomes | Treatment attendance, substance use, days of alcohol use, PTSD |
| Starting date | March 2013 |
| Contact information | Marc Rosen: [email protected] |
| Notes |
| Trial name or title | Sequence of symptom change during AUD (alcohol use or dependence) or PTSD (posttraumatic stress disorder) treatment for comorbid PTSD/AUD |
| Methods | Randomised controlled trial |
| Participants | Adults ≥ 18 years of age with a current DSM‐V diagnosis of alcohol abuse/dependence and PTSD |
| Interventions | Cognitive processing therapy vs relapse prevention therapy vs assessment only |
| Outcomes | Primary outcomes: Reduction in PTSD symptom severity (Clinician Administered PTSD Scale) Reduction in alcohol consumption (Form 90 (Alcohol Consumption)) |
| Starting date | March 2013 |
| Contact information | Debra Kaysen: [email protected] |
| Notes |
| Trial name or title | Contingency outcomes in prolonged exposure (COPE) |
| Methods | Randomised controlled trial |
| Participants | Participants must meet DSM‐IV criteria for SUD and current PTSD |
| Interventions | Prolonged exposure with contingency management vs prolonged exposure |
| Outcomes | Prolonged exposure attendance, PTSD symptoms, drug use |
| Starting date | September 2012 |
| Contact information | Jessica Peirce, Johns Hopkins University |
| Notes |
| Trial name or title | Cognitive processing intervention for HIV/STI and substance use among native women |
| Methods | Randomised controlled trial |
| Participants | Sexually active women with current substance use and PTSD (score 30 or hire on the PTSD Checklist) |
| Interventions | Cognitive processing therapy vs wait‐list control |
| Outcomes | Primary outcome: PTSD Symptom Scale‐Interview |
| Starting date | October 2013 |
| Contact information | Cynthia Pearson: [email protected] |
| Notes |
| Trial name or title | Patient‐centered trauma treatment (PaCTT) |
| Methods | Randomised controlled trial |
| Participants | Meet DSM‐IV diagnostic criteria for lifetime and current full or subthreshold PTSD and DSM‐IV diagnostic criteria for current substance abuse or dependence |
| Interventions | Peer‐led Seeking Safety group vs clinician‐led Seeking Safety group |
| Outcomes | PTSD severity as indexed by the PTSD Checklist, change in substance use as indexed by the Addiction Severity Index |
| Starting date | October 2013 |
| Contact information | Annette Crisanti: [email protected] |
| Notes |
| Trial name or title | A policy relevant US trauma care system pragmatic trial for PTSD and comorbidity pilot (TSOS 6) |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: Inpatient/emergency admission for traumatic injury (The goal of this pilot study is to develop and implement a larger‐scale, multisite, stepped collaborative care trial that targets injured patients with presentations of PTSD and related comorbidity) |
| Interventions | The intervention aims to prevent the development of chronic PTSD and depressive symptoms, alcohol use problems, and enduring physical disability in survivors of both traumatic brain and non‐traumatic brain injuries |
| Outcomes | Primary outcomes: PTSD Checklist‐Civilian Version at 1 month; Alcohol Use Disorders Identification Test at 1 month; Patient Health Questionnaire 9‐item Depression Scale at 1 month |
| Starting date | February 2015 |
| Contact information | Douglas Zatzick: [email protected] |
| Notes |
| Trial name or title | A study on the effectiveness of the cognitive behavioral therapy "Seeking Safety" in reducing trauma and addiction related symptoms in a Dutch substance‐use disorder population |
| Methods | Randomised controlled trial |
| Participants | Outpatients from substance misuse services with active symptoms of PTSD in the past 6 months |
| Interventions | Seeking Safety vs TAU |
| Outcomes | The primary outcome measure will be substance use severity. Secondary outcome measures are PTSD and trauma symptoms, coping skills, functioning, and cognitions |
| Starting date | January 2012 |
| Contact information | Tim Kok: [email protected] |
| Notes |
AUD: alcohol use disorder
CBT: cognitive behavioural therapy
DSM: Diagnostic and Statistical Manual of Mental Disorders
OEF/OIF/OND: Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn
PTSD: post‐traumatic stress disorder
SUD: substance use disorder
TAU: treatment as usual
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||
| 1 PTSD severity following treatment completion Show forest plot | 4 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.10] | ||||||||||||||||||||||||
| Analysis 1.1 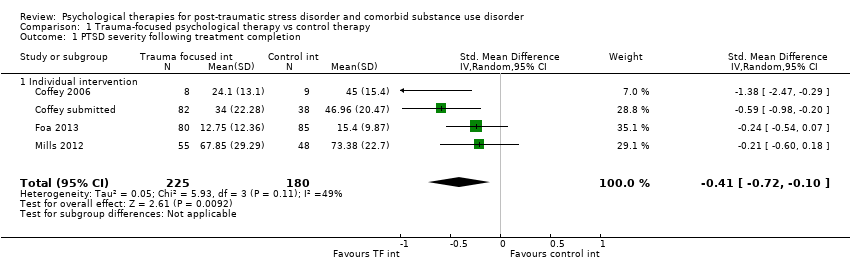 Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 1 PTSD severity following treatment completion. | ||||||||||||||||||||||||||||
| 1.1 Individual intervention | 4 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.10] | ||||||||||||||||||||||||
| 2 PTSD severity 3‐4 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 1.2  Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 2 PTSD severity 3‐4 months following treatment completion. | ||||||||||||||||||||||||||||
| 2.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 3 PTSD severity 5‐7 months following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] | ||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 3 PTSD severity 5‐7 months following treatment completion. | ||||||||||||||||||||||||||||
| 3.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] | ||||||||||||||||||||||||
| 4 Drug or alcohol use, or both following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] | ||||||||||||||||||||||||
| Analysis 1.4 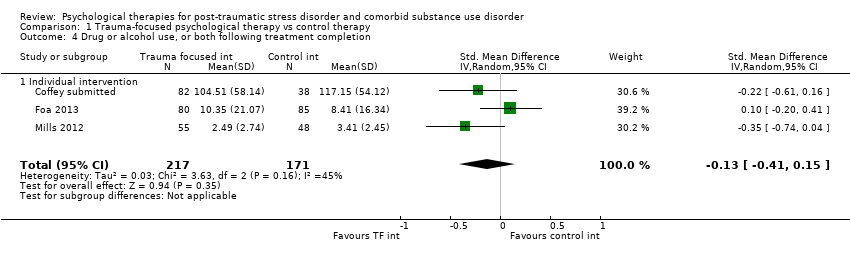 Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 4 Drug or alcohol use, or both following treatment completion. | ||||||||||||||||||||||||||||
| 4.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] | ||||||||||||||||||||||||
| 5 Drug or alcohol use, or both 3‐4 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 1.5 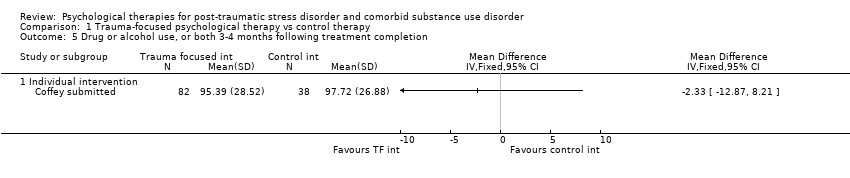 Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 5 Drug or alcohol use, or both 3‐4 months following treatment completion. | ||||||||||||||||||||||||||||
| 5.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 6 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] | ||||||||||||||||||||||||
| Analysis 1.6  Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 6 Drug or alcohol use, or both 5‐7 months following treatment completion. | ||||||||||||||||||||||||||||
| 6.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] | ||||||||||||||||||||||||
| 7 Treatment completers Show forest plot | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.96] | ||||||||||||||||||||||||
| Analysis 1.7 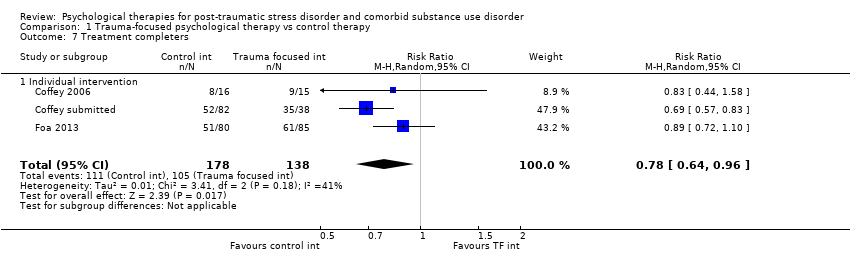 Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 7 Treatment completers. | ||||||||||||||||||||||||||||
| 7.1 Individual intervention | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.96] | ||||||||||||||||||||||||
| 8 PTSD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 1.8 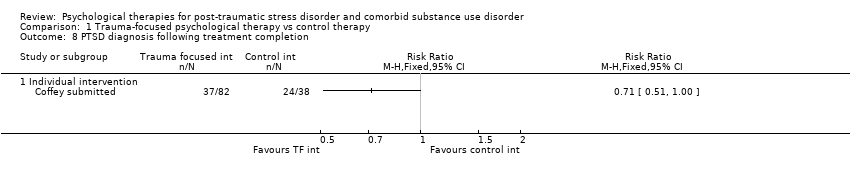 Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 8 PTSD diagnosis following treatment completion. | ||||||||||||||||||||||||||||
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 9 Adverse events Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 1.9
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 9 Adverse events. | ||||||||||||||||||||||||||||
| 9.1 Individual intervention | Other data | No numeric data | ||||||||||||||||||||||||||
| 10 Adverse events Show forest plot | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.90] | ||||||||||||||||||||||||
| Analysis 1.10  Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 10 Adverse events. | ||||||||||||||||||||||||||||
| 10.1 Individual intervention | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.90] | ||||||||||||||||||||||||
| 11 Mean number of sessions attended for intervention group Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 1.11
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 11 Mean number of sessions attended for intervention group. | ||||||||||||||||||||||||||||
| 11.1 Studies including intervention for SUD | Other data | No numeric data | ||||||||||||||||||||||||||
| 12 Sensitivity analysis: PTSD severity following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.10] | ||||||||||||||||||||||||
| Analysis 1.12 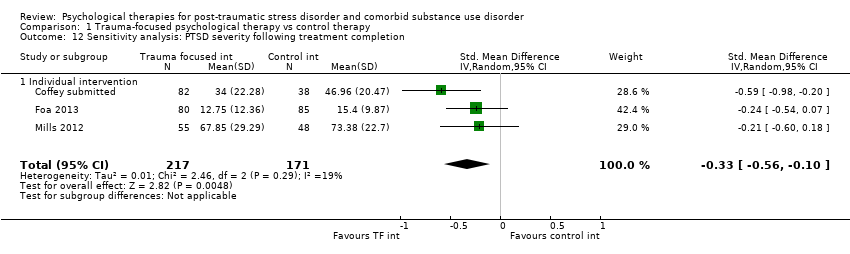 Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 12 Sensitivity analysis: PTSD severity following treatment completion. | ||||||||||||||||||||||||||||
| 12.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.10] | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PTSD severity following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 1 PTSD severity following treatment completion. | ||||
| 1.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PTSD severity 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 2 PTSD severity 5‐7 months following treatment completion. | ||||
| 2.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PTSD severity 8‐10 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 3 PTSD severity 8‐10 months following treatment completion. | ||||
| 3.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Drug or alcohol use, or both following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4 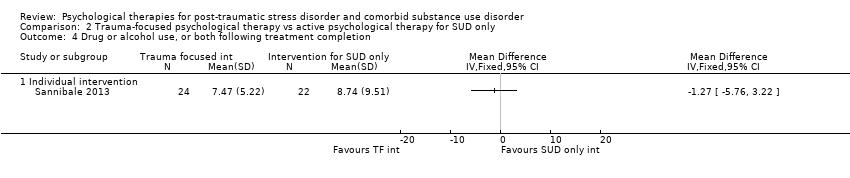 Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 4 Drug or alcohol use, or both following treatment completion. | ||||
| 4.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 5 Drug or alcohol use, or both 5‐7 months following treatment completion. | ||||
| 5.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Drug or alcohol use, or both 8‐10 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 6 Drug or alcohol use, or both 8‐10 months following treatment completion. | ||||
| 6.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment completers Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 7 Treatment completers. | ||||
| 7.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 PTSD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 8 PTSD diagnosis following treatment completion. | ||||
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 SUD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.9 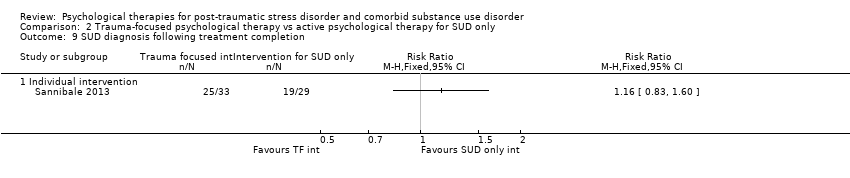 Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 9 SUD diagnosis following treatment completion. | ||||
| 9.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 PTSD severity following treatment completion Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.1  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 1 PTSD severity following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.83, 0.39] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Group intervention | 4 | 513 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.16] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 PTSD severity 3‐4 months following treatment completion Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.2  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 2 PTSD severity 3‐4 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.86, 0.36] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 Group intervention | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.17, 0.18] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 PTSD severity 5‐7 months following treatment completion Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.3  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 3 PTSD severity 5‐7 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.81, 0.41] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Group intervention | 4 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.31, 0.03] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 PTSD severity 12 months following treatment completion Show forest plot | 2 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.10] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.4  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 4 PTSD severity 12 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 Group intervention | 2 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.10] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Drug or alcohol use, or both following treatment completion Show forest plot | 3 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.5  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 5 Drug or alcohol use, or both following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 Group intervention | 3 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Drug or alcohol use, or both 3‐4 months following treatment completion Show forest plot | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.23] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.6  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 6 Drug or alcohol use, or both 3‐4 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 Group intervention | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.23] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 4 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.7  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 7 Drug or alcohol use, or both 5‐7 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 Group intervention | 4 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Drug or alcohol use, or both 12 months following treatment completion Show forest plot | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.20] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.8  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 8 Drug or alcohol use, or both 12 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 Group intervention | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.20] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Treatment completers Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.9
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 9 Treatment completers. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Individual intervention | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.2 Group intervention | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Treatment completers Show forest plot | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.45] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.10 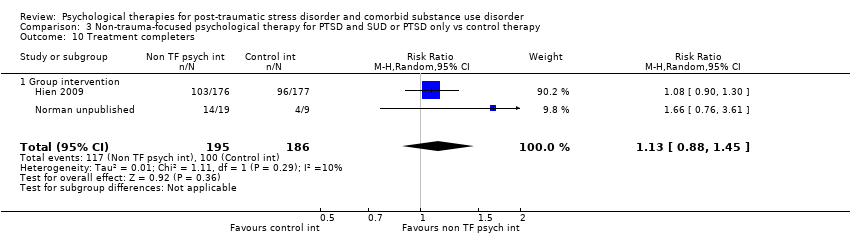 Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 10 Treatment completers. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 Group intervention | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.45] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 PTSD diagnosis following treatment completion Show forest plot | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.11 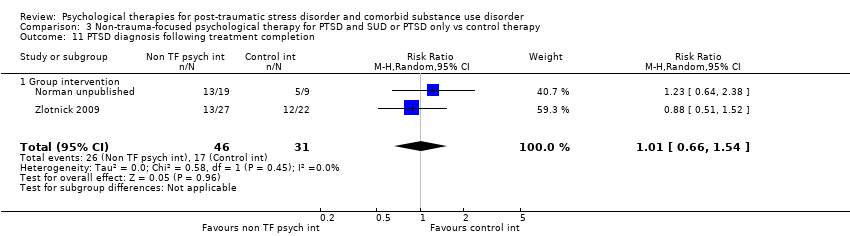 Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 11 PTSD diagnosis following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.1 Group intervention | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Adverse events Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.12
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 12 Adverse events. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.1 Group intervention | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13 Study‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.13  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 13 Study‐related adverse events. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.1 Group intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14 Mean number of sessions attended for intervention group Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.14
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 14 Mean number of sessions attended for intervention group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.1 Group intervention | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 Mean number of sessions attended Show forest plot | 2 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.15  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 15 Mean number of sessions attended. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.1 Group intervention | 2 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 Sensitivity analysis: PTSD severity 5‐7 months following treatment completion Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.16 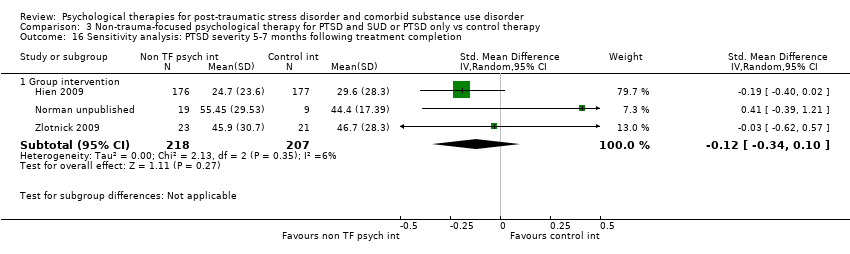 Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 16 Sensitivity analysis: PTSD severity 5‐7 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.1 Group intervention | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.34, 0.10] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 Sensitivity analysis: PTSD severity 12 months following treatment completion Show forest plot | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.17 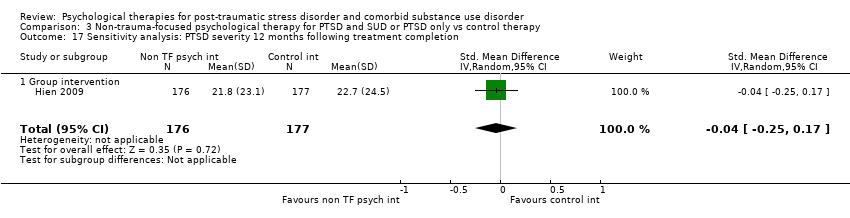 Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 17 Sensitivity analysis: PTSD severity 12 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.1 Group intervention | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 Sensitivity analysis: drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.18  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 18 Sensitivity analysis: drug or alcohol use, or both 5‐7 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18.1 Group intervention | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 Sensitivity analysis: drug or alcohol use, or both 12 months following treatment completion Show forest plot | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 3.19  Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 19 Sensitivity analysis: drug or alcohol use, or both 12 months following treatment completion. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19.1 Group intervention | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PTSD severity following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.29, 0.77] |
| Analysis 4.1  Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 1 PTSD severity following treatment completion. | ||||
| 1.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.29, 0.77] |
| 2 PTSD severity 3‐4 months following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.31, 0.55] |
| Analysis 4.2  Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 2 PTSD severity 3‐4 months following treatment completion. | ||||
| 2.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.31, 0.55] |
| 3 PTSD severity 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 3 PTSD severity 5‐7 months following treatment completion. | ||||
| 3.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Drug or alcohol use, or both following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.13, 0.57] |
| Analysis 4.4 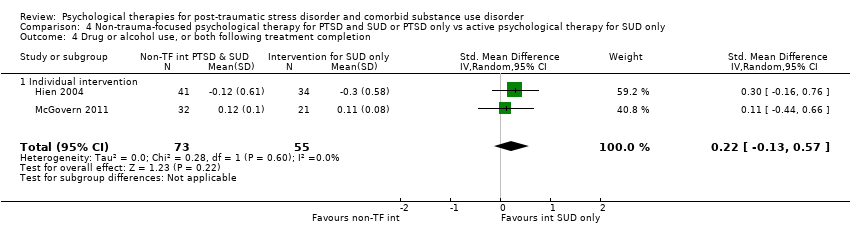 Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 4 Drug or alcohol use, or both following treatment completion. | ||||
| 4.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.13, 0.57] |
| 5 Drug or alcohol use, or both 3‐4 months following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.53] |
| Analysis 4.5  Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 5 Drug or alcohol use, or both 3‐4 months following treatment completion. | ||||
| 5.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.53] |
| 6 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 6 Drug or alcohol use, or both 5‐7 months following treatment completion. | ||||
| 6.1 Individual intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment completers Show forest plot | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.20] |
| Analysis 4.7 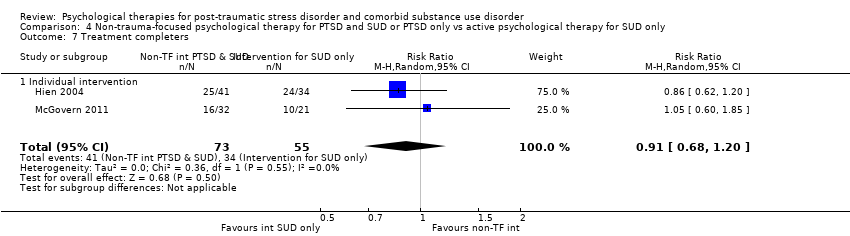 Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 7 Treatment completers. | ||||
| 7.1 Individual intervention | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.20] |
| 8 PTSD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.8 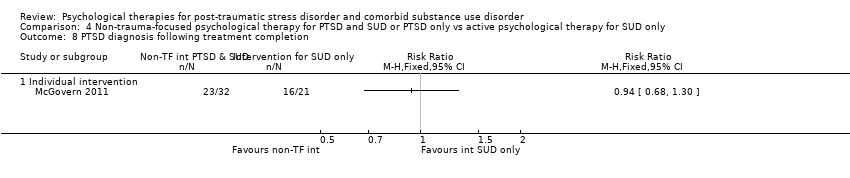 Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 8 PTSD diagnosis following treatment completion. | ||||
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Mean number of sessions attended Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.9  Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 9 Mean number of sessions attended. | ||||
| 9.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
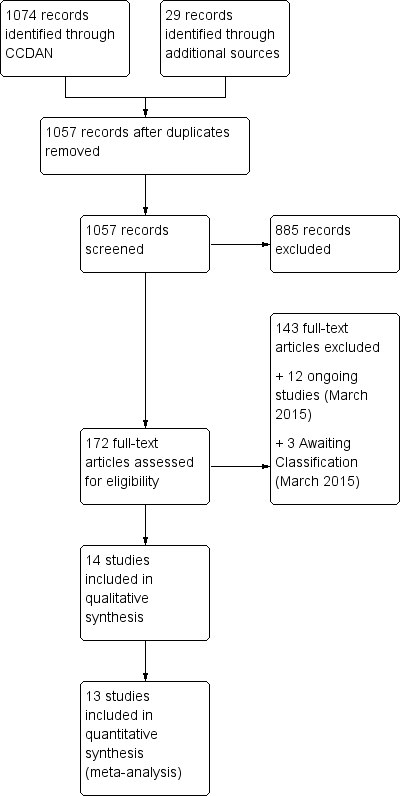
Study flow diagram.
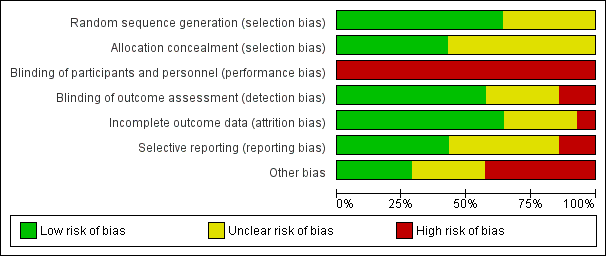
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
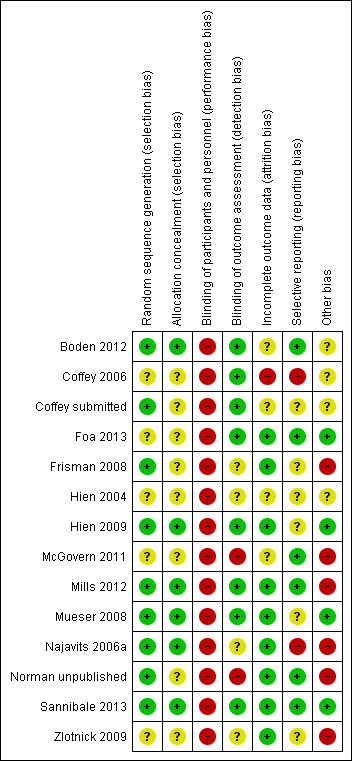
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
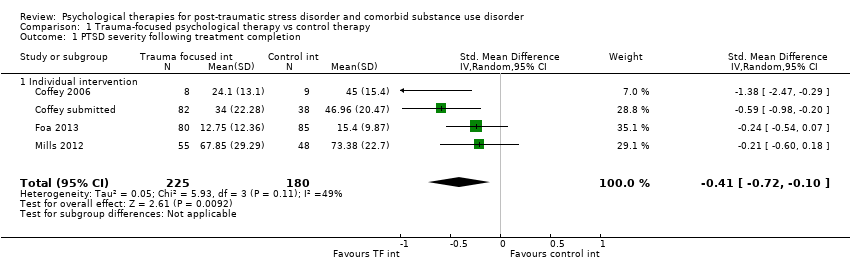
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 1 PTSD severity following treatment completion.

Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 2 PTSD severity 3‐4 months following treatment completion.
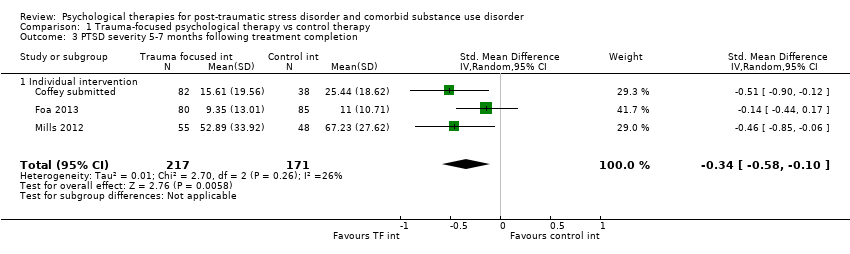
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 3 PTSD severity 5‐7 months following treatment completion.
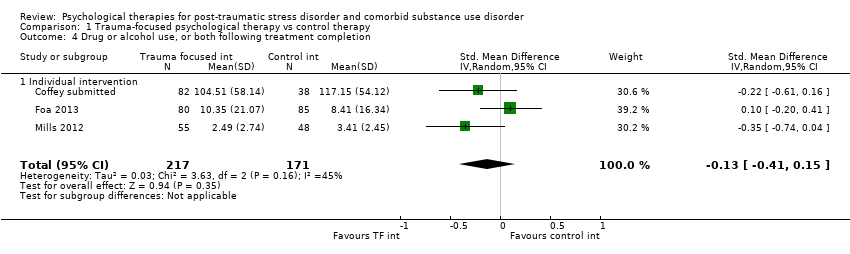
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 4 Drug or alcohol use, or both following treatment completion.

Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 5 Drug or alcohol use, or both 3‐4 months following treatment completion.
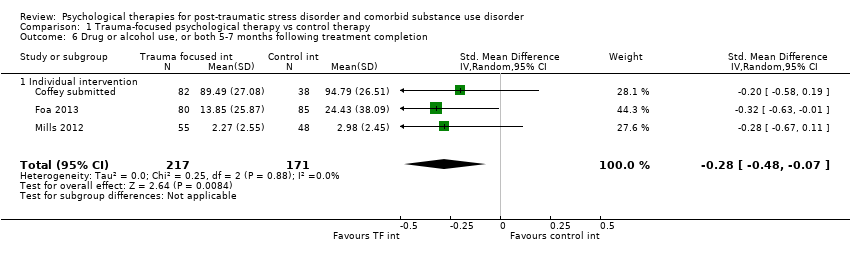
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 6 Drug or alcohol use, or both 5‐7 months following treatment completion.
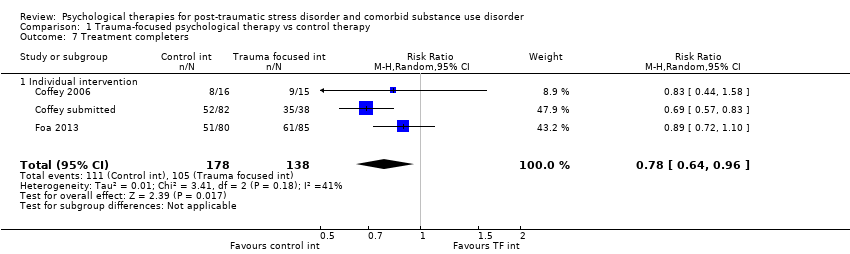
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 7 Treatment completers.
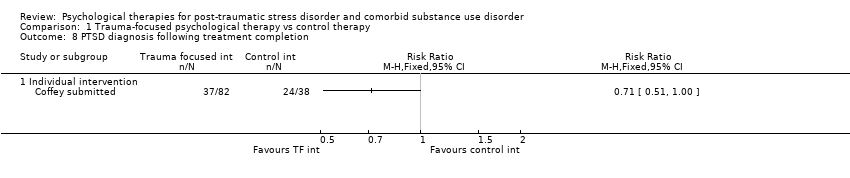
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 8 PTSD diagnosis following treatment completion.
| Study | |
| Individual intervention | |
| Coffey 2006 | Not reported |
| Coffey submitted | Not reported |
| Foa 2013 | Twelve participants were removed from the study because of serious adverse events (serious suicidal ideation, n = 7; serious medical illness, n = 3; psychotic symptoms, n = 1; death, n = 1; however, none of these events was determined to be related to the study). |
| Mills 2012 | Two participants from the treatment group (3.6%) and 5 participants from the control group (10.4%) attempted suicide during the study (OR, 0.32 [95% CI, 0.06‐1.76]). Although it is possible that these attempts were related to participation in the study, all 7 individuals reported that this was not the case and elected to remain involved with the study. Additionally, 1 participant from the treatment group (1.8%) died as a result of a preexisting medical condition. |
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 9 Adverse events.

Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 10 Adverse events.
| Study | Mean number sessions attended by intervention group (& SD) | Number sessions available | Percentage attended |
| Studies including intervention for SUD | |||
| Coffey submitted | 8.16 (3.26) approximated | 12 | 68.0% |
| Foa 2013 | 6.33 (5.31) | 18 | 35.2% |
| Mills 2012 | 5.83 (4.94) | 13 | 44.9% |
Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 11 Mean number of sessions attended for intervention group.

Comparison 1 Trauma‐focused psychological therapy vs control therapy, Outcome 12 Sensitivity analysis: PTSD severity following treatment completion.

Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 1 PTSD severity following treatment completion.

Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 2 PTSD severity 5‐7 months following treatment completion.

Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 3 PTSD severity 8‐10 months following treatment completion.
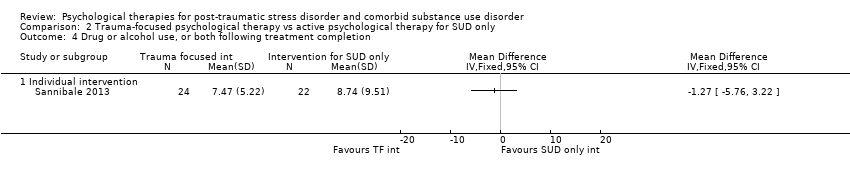
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 4 Drug or alcohol use, or both following treatment completion.
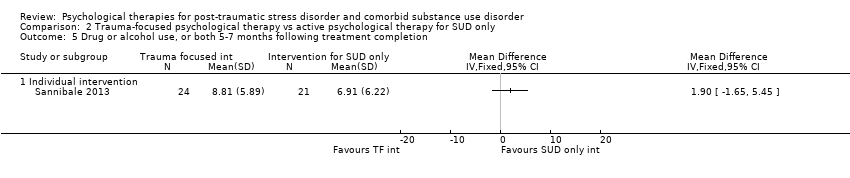
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 5 Drug or alcohol use, or both 5‐7 months following treatment completion.

Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 6 Drug or alcohol use, or both 8‐10 months following treatment completion.

Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 7 Treatment completers.

Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 8 PTSD diagnosis following treatment completion.
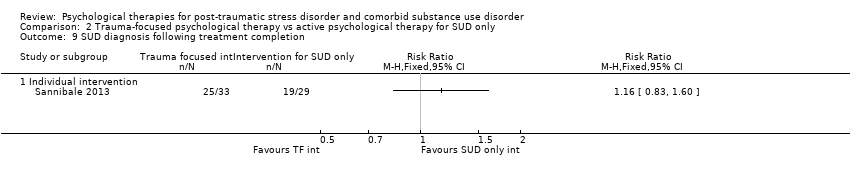
Comparison 2 Trauma‐focused psychological therapy vs active psychological therapy for SUD only, Outcome 9 SUD diagnosis following treatment completion.
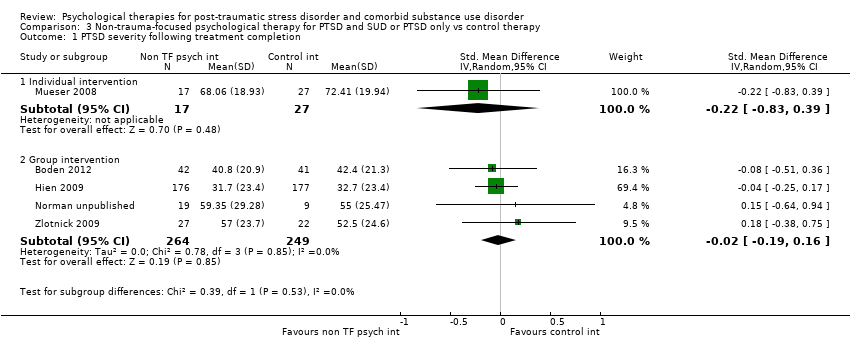
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 1 PTSD severity following treatment completion.
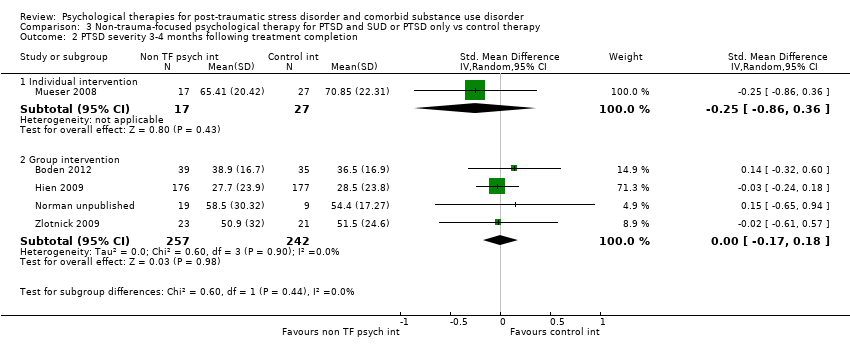
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 2 PTSD severity 3‐4 months following treatment completion.
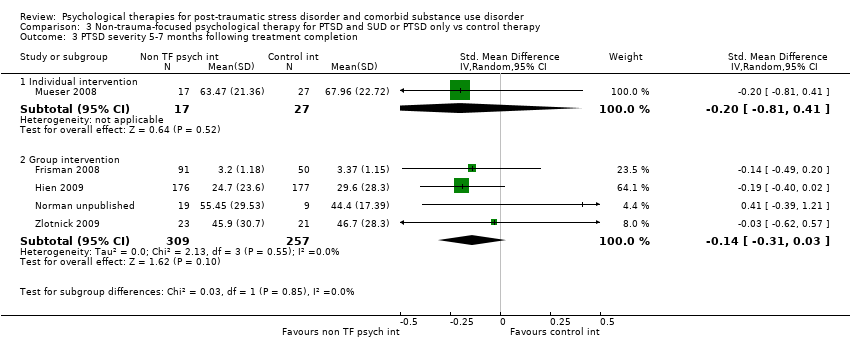
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 3 PTSD severity 5‐7 months following treatment completion.
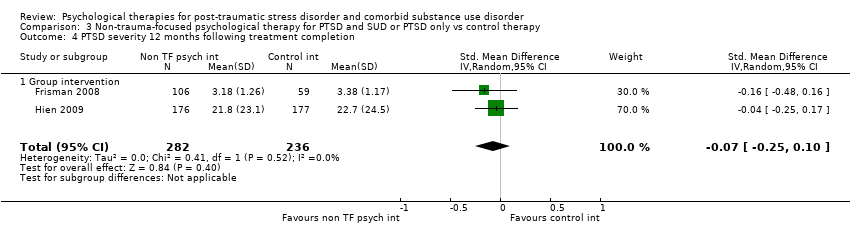
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 4 PTSD severity 12 months following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 5 Drug or alcohol use, or both following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 6 Drug or alcohol use, or both 3‐4 months following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 7 Drug or alcohol use, or both 5‐7 months following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 8 Drug or alcohol use, or both 12 months following treatment completion.
| Study | |
| Individual intervention | |
| Mueser 2008 | 12/16 (70.6%) |
| Group intervention | |
| Frisman 2008 | 39/141 (28%) |
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 9 Treatment completers.
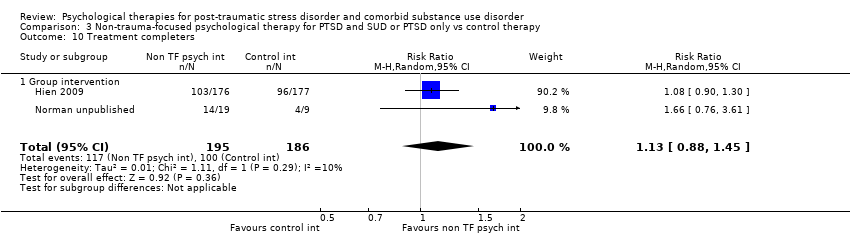
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 10 Treatment completers.
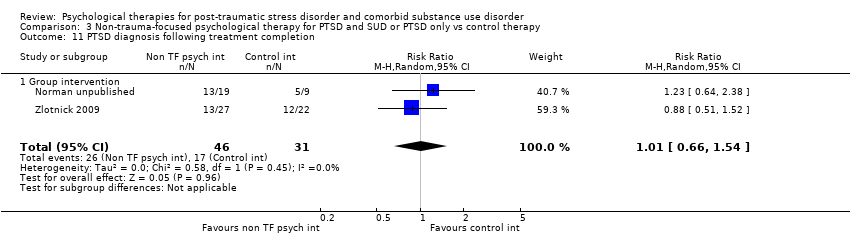
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 11 PTSD diagnosis following treatment completion.
| Study | |
| Group intervention | |
| Boden 2012 | No harmful or unintended effects were observed during the trial. |
| Frisman 2008 | Not reported |
| Hien 2009 | 83 study related adverse events were identified (Killeen 2008). Of these 61 were rated as moderate to severe: 28 for the experimental condition; 33 for the control condition. |
| Najavits 2006a | Not reported |
| Norman unpublished | No adverse events occurred during the study. |
| Zlotnick 2009 | Not reported |
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 12 Adverse events.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 13 Study‐related adverse events.
| Study | Mean number treatment condition sessions attended by intervention group (& SD) | Number sessions available | Percentage active intervention sessions attended | Mean number sessions attended by control group (& SD) | Percentage attended |
| Group intervention | |||||
| Boden 2012 | Not reported | Not reported | |||
| Frisman 2008 | 3.41 (3.38) active intervention sessions + 30.67 (37.38) TAU sessions | 9 active intervention sessions plus TAU sessions | 37.9% | 39.0 (69.62) TAU sessions | |
| Hien 2009 | 6.2 (4.5) | 12 | 51.7% | 6.9 (4.3) | 57.5% |
| Najavits 2006a | 9.67(5.05) active intervention session (11.78 (6.25) active intervention +TAU sessions) | 25 active intervention sessions plus TAU sessions | 38.7% | Not reported | |
| Norman unpublished | 12.5 (8.77) | 24 | 52.1% | 7.78 (5.78) | 32.4% |
| Zlotnick 2009 | 15.6 (6.2) | 25 | 62.4% | Not reported | |
Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 14 Mean number of sessions attended for intervention group.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 15 Mean number of sessions attended.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 16 Sensitivity analysis: PTSD severity 5‐7 months following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 17 Sensitivity analysis: PTSD severity 12 months following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 18 Sensitivity analysis: drug or alcohol use, or both 5‐7 months following treatment completion.

Comparison 3 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs control therapy, Outcome 19 Sensitivity analysis: drug or alcohol use, or both 12 months following treatment completion.

Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 1 PTSD severity following treatment completion.
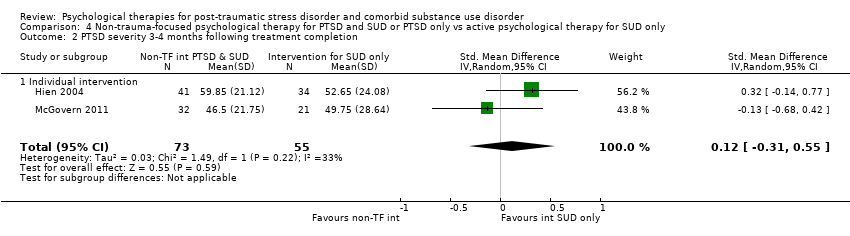
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 2 PTSD severity 3‐4 months following treatment completion.

Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 3 PTSD severity 5‐7 months following treatment completion.

Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 4 Drug or alcohol use, or both following treatment completion.
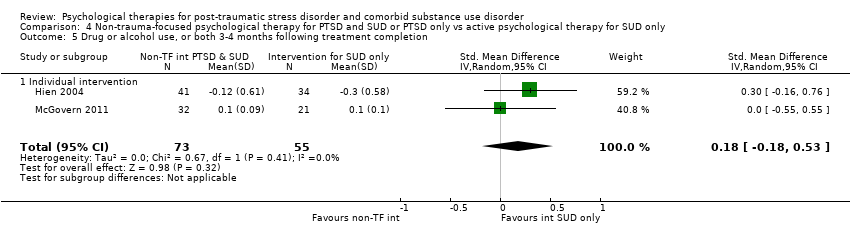
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 5 Drug or alcohol use, or both 3‐4 months following treatment completion.
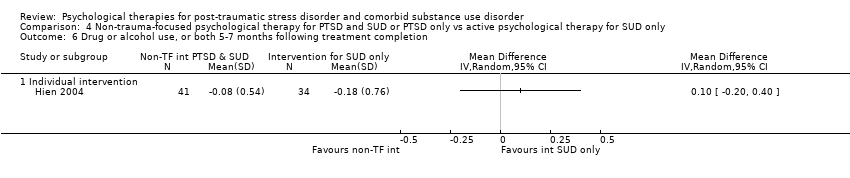
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 6 Drug or alcohol use, or both 5‐7 months following treatment completion.

Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 7 Treatment completers.
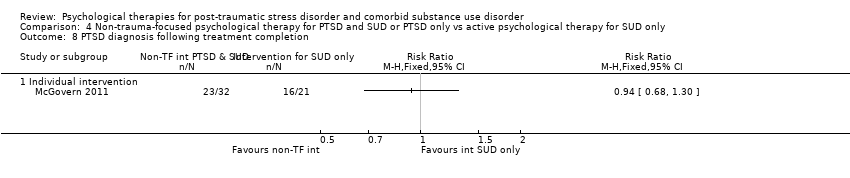
Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 8 PTSD diagnosis following treatment completion.

Comparison 4 Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only vs active psychological therapy for SUD only, Outcome 9 Mean number of sessions attended.
| Trauma‐focused psychological therapy compared to control intervention | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU/ minimal intervention | Individual‐based psychological therapyincluding a trauma‐focused component | |||||
| PTSD severity following treatment completion As assessed by the CAPS, PSS‐I, or IES‐R. High scores indicate greater symptom severity | ‐ | The mean PTSD severity following treatment completion in the intervention groups was | ‐ | 405 | ⊕⊝⊝⊝ | SMD ‐0.41 (‐0.72 to ‐0.1) Effect sizes of the range 0.2 to 0.5 indicate a small treatment effect |
| Drug or alcohol use, or both following treatment completion As assessed by the TLFB or CIDI. High scores indicate greater symptom severity | ‐ | The mean drug/alcohol use following treatment completion in the intervention groups was | ‐ | 388 | ⊕⊝⊝⊝ | SMD ‐0.13 (‐0.41 to 0.15) Not significant |
| Treatment completers | Study population | RR 0.80 | 316 | ⊕⊕⊝⊝ | Indicates higher drop‐out in the intervention group | |
| 761 per 1000 | 609 per 1000 | |||||
| Moderate | ||||||
| 718 per 1000 | 574 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality of evidence downgraded by one point because the risk of bias in most trials was high or unclear in several domains. SUD based adjunctive therapy was not a formal part of either the experimental or control condition in one study (Coffey 2006). However, participants were recruited through an SUD based service and it is likely that they would have had access to adjunctive SUD‐ based therapy on an informal basis. All other studies in this comparison included formal access SUD‐based adjunctive therapy. | ||||||
| Trauma‐focused psychological therapy compared to active psychological therapy for SUD only | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Active psychological therapyfor SUD only | Individual‐based psychological therapyincluding a trauma‐focused component | |||||
| PTSD severity following treatment completion As assessed by the CAPS. High scores indicate greater symptom severity | ‐ | The mean PTSD severity following treatment completion in the intervention groups was | ‐ | 46 | ⊕⊕⊝⊝ | Not significant |
| Drug or alcohol use, or both following treatment completion As assessed by the TLFB. High scores indicate greater symptom severity | ‐ | The mean drug/alcohol use following treatment completion in the intervention groups was | ‐ | 46 | ⊕⊕⊝⊝ | Not significant |
| Treatment completers | Study population | RR 1 | 62 | ⊕⊕⊝⊝ | Not significant | |
| 724 per 1000 | 724 per 1000 | |||||
| Moderate | ||||||
| 724 per 1000 | 724 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality of evidence downgraded by two points because findings were based on outcomes from one study with a small sample size. SUD based adjunctive therapy was not a formal part of either the experimental or control condition in the study contributing to this comparison. | ||||||
| Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only compared to control intervention | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| TAU/minimal intervention | Group or Indvidual based non‐trauma‐focused psychological therapy | |||||
| PTSD severity following treatment completion ‐ Individual‐based intervention As assessed by the CAPS. High scores indicate greater symptom severity | ‐ | The mean PTSD severity following treatment completion in the intervention groups was | ‐ | 44 | ⊕⊕⊝⊝ | SMD ‐0.22 (‐0.83 to 0.39) |
| PTSD severity following treatment completion ‐ Group‐based intervention As assessed by the CAPS or IES‐R. High scores indicate greater symptom severity | ‐ | The mean PTSD severity following treatment completion in the intervention groups was | ‐ | 513 | ⊕⊕⊝⊝ | SMD ‐0.02 (‐0.19 to 0.16) |
| Drug or alcohol use, or both following treatment completion ‐ Individual‐based intervention | ‐ | No data | ‐ | ‐ | ‐ | Not estimable |
| Drug or alcohol use, or both following treatment completion ‐ Group‐based intervention As assessed by the ASI, TLFB or CIDI. High scores indicate greater symptom severity | ‐ | The mean drug/alcohol use following treatment completion in the intervention groups was | ‐ | 464 | ⊕⊝⊝⊝ | SMD ‐0.41 (‐0.97 to 0.14) Not significant |
| Treatment completers ‐ Individual‐based intervention | ‐ | No data | ‐ | ‐ | ‐ | Not estimable |
| Treatment completers ‐ Group‐based intervention | Study population | RR 1.13 | 381 | ⊕⊕⊝⊝ | ‐ | |
| 538 per 1000 | 608 per 1000 | |||||
| Moderate | ||||||
| 493 per 1000 | 557 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality of evidence downgraded by two points because findings were based on outcomes from one study with a small sample size. The individual‐based study (Mueser 2008) in this comparison did not include access to SUD based adjunctive therapy. Participants in all other studies were able to access SUD‐based adjunctive therapy. | ||||||
| Non‐trauma‐focused psychological therapy for PTSD and SUD or PTSD only compared to active psychological therapy for SUD only | ||||||
| Patient or population: Individuals with post‐traumatic stress disorder and comorbid substance use disorder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Active psychological therapyfor SUD only | Individual‐based combined non‐trauma‐focused psychological therapy | |||||
| PTSD severity following treatment completion As assessed by the CAPS. High scores indicate greater symptom severity | ‐ | The mean PTSD severity following treatment completion in the intervention groups was | ‐ | 128 | ⊕⊝⊝⊝ | SMD ‐0.26 (‐1.29 to 0.77) Not significant |
| Drug or alcohol use, or both following treatment completion As assessed by the SUI or ASI. High scores indicate greater symptom severity | ‐ | The mean drug/alcohol use following treatment completion in the intervention groups was | ‐ | 128 | ⊕⊕⊝⊝ | SMD 0.22 (‐0.13 to 0.57) Not significant |
| Treatment completers | Study population | RR 0.91 | 128 | ⊕⊝⊝⊝ | Not significant | |
| 618 per 1000 | 563 per 1000 | |||||
| Moderate | ||||||
| 591 per 1000 | 538 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Quality of evidence downgraded by one point because the risk of bias in most trials was high or unclear in several domains. Both studies in this comparison involved access to adjunctive SUD‐based therapy. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PTSD severity following treatment completion Show forest plot | 4 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.10] |
| 1.1 Individual intervention | 4 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.72, ‐0.10] |
| 2 PTSD severity 3‐4 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PTSD severity 5‐7 months following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 3.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.58, ‐0.10] |
| 4 Drug or alcohol use, or both following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 4.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 5 Drug or alcohol use, or both 3‐4 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] |
| 6.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] |
| 7 Treatment completers Show forest plot | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.96] |
| 7.1 Individual intervention | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.64, 0.96] |
| 8 PTSD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events Show forest plot | Other data | No numeric data | ||
| 9.1 Individual intervention | Other data | No numeric data | ||
| 10 Adverse events Show forest plot | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.90] |
| 10.1 Individual intervention | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.90] |
| 11 Mean number of sessions attended for intervention group Show forest plot | Other data | No numeric data | ||
| 11.1 Studies including intervention for SUD | Other data | No numeric data | ||
| 12 Sensitivity analysis: PTSD severity following treatment completion Show forest plot | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.10] |
| 12.1 Individual intervention | 3 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.56, ‐0.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PTSD severity following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PTSD severity 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 PTSD severity 8‐10 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Drug or alcohol use, or both following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Drug or alcohol use, or both 8‐10 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment completers Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 PTSD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 SUD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PTSD severity following treatment completion Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.83, 0.39] |
| 1.2 Group intervention | 4 | 513 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.16] |
| 2 PTSD severity 3‐4 months following treatment completion Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.86, 0.36] |
| 2.2 Group intervention | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.17, 0.18] |
| 3 PTSD severity 5‐7 months following treatment completion Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Individual intervention | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.81, 0.41] |
| 3.2 Group intervention | 4 | 566 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.31, 0.03] |
| 4 PTSD severity 12 months following treatment completion Show forest plot | 2 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.10] |
| 4.1 Group intervention | 2 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.25, 0.10] |
| 5 Drug or alcohol use, or both following treatment completion Show forest plot | 3 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] |
| 5.1 Group intervention | 3 | 464 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] |
| 6 Drug or alcohol use, or both 3‐4 months following treatment completion Show forest plot | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.23] |
| 6.1 Group intervention | 4 | 499 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.40, 0.23] |
| 7 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 4 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| 7.1 Group intervention | 4 | 572 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| 8 Drug or alcohol use, or both 12 months following treatment completion Show forest plot | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.20] |
| 8.1 Group intervention | 2 | 528 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.20] |
| 9 Treatment completers Show forest plot | Other data | No numeric data | ||
| 9.1 Individual intervention | Other data | No numeric data | ||
| 9.2 Group intervention | Other data | No numeric data | ||
| 10 Treatment completers Show forest plot | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.45] |
| 10.1 Group intervention | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.88, 1.45] |
| 11 PTSD diagnosis following treatment completion Show forest plot | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 11.1 Group intervention | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.54] |
| 12 Adverse events Show forest plot | Other data | No numeric data | ||
| 12.1 Group intervention | Other data | No numeric data | ||
| 13 Study‐related adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Group intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Mean number of sessions attended for intervention group Show forest plot | Other data | No numeric data | ||
| 14.1 Group intervention | Other data | No numeric data | ||
| 15 Mean number of sessions attended Show forest plot | 2 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] |
| 15.1 Group intervention | 2 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.59, 0.79] |
| 16 Sensitivity analysis: PTSD severity 5‐7 months following treatment completion Show forest plot | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Group intervention | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.34, 0.10] |
| 17 Sensitivity analysis: PTSD severity 12 months following treatment completion Show forest plot | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 17.1 Group intervention | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.25, 0.17] |
| 18 Sensitivity analysis: drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 18.1 Group intervention | 3 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 19 Sensitivity analysis: drug or alcohol use, or both 12 months following treatment completion Show forest plot | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
| 19.1 Group intervention | 1 | 353 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 PTSD severity following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.29, 0.77] |
| 1.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.29, 0.77] |
| 2 PTSD severity 3‐4 months following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.31, 0.55] |
| 2.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.31, 0.55] |
| 3 PTSD severity 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Drug or alcohol use, or both following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.13, 0.57] |
| 4.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.13, 0.57] |
| 5 Drug or alcohol use, or both 3‐4 months following treatment completion Show forest plot | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.53] |
| 5.1 Individual intervention | 2 | 128 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.18, 0.53] |
| 6 Drug or alcohol use, or both 5‐7 months following treatment completion Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Individual intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Treatment completers Show forest plot | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.20] |
| 7.1 Individual intervention | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.68, 1.20] |
| 8 PTSD diagnosis following treatment completion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Individual intervention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Mean number of sessions attended Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Individual intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

