Interventions for promoting habitual exercise in people living with and beyond cancer
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Both groups had access to standard care, which consisted of a holistic nurse‐led colorectal cancer follow‐up service | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by an independent researcher via code numbers using nQuery statistical software |
| Allocation concealment (selection bias) | Low risk | Randomization was undertaken by a senior academic who was not directly involved in the recruitment or assessment of participants |
| Blinding of outcome assessment (detection bias) | Low risk | All outcomes were assessed by an experienced exercise physiologist, who was blind to the group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis was used to compare participants in the groups to which they were randomly assigned, with data carried over from previous visits in |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Low recruitment rate (18/180) could represent a biased sample |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Men randomly assigned to standard care were followed up in the urology clinic as normal and were seen by an oncology nurse specialist and a urologist | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out remotely, using nQuery statistical software (nQuery Advisor 6.01; Statistical Solutions) |
| Allocation concealment (selection bias) | Low risk | Randomization was undertaken by a senior academic who was not directly involved in the recruitment or assessment of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Physiological and functional fitness outcomes were assessed by a trained technician blinded to group allocation. Responses on the self‐administered questionnaires were checked for completeness by the researcher in the presence of the respondent |
| Incomplete outcome data (attrition bias) | High risk | 44% attrition at six‐month postintervention follow‐up |
| Selective reporting (reporting bias) | Low risk | None; all outcomes reported |
| Other bias | Low risk | None |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
Adherence:
| |
| Description of usual care | Unclear | |
| Notes | Only YES trial included in the review because of the requirement that participants must be sedentary at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme randomly assigned each YES study participant with equal probability to the exercise group or the usual care group |
| Allocation concealment (selection bias) | Low risk | The randomisation code for each participant was obtained by the principal investigator (who was not involved in recruitment or data collection) only after baseline measures for that individual had been completed and staff conducting clinic visits did not have access to the randomisation programme |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | Low risk | Analyses were conducted according to the intention‐to‐treat principle. Baseline QOL values were carried forward for the five IMPACT study participants (three exercisers and two controls) and 10 YES study participants (five exercisers and five controls) for whom six‐month data were unavailable |
| Selective reporting (reporting bias) | Low risk | None, all outcomes reported |
| Other bias | Low risk | None |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | All participants continue to receive usual care from their health team | |
| Notes | Mean and SD data for aerobic exercise tolerance at 8 and 24 weeks provided by authors in response to email request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A telephone randomisation service was provided by an independent trials unit. Randomisation to the three treatment arms was done on a 1:1:1 ratio and was performed using stratified random permuted blocks (with block size of six). Stratification factors were chemotherapy (yes/no) and tamoxifen (yes/no) |
| Allocation concealment (selection bias) | Low risk | Randomisation service was provided by an independent trials unit telephone service |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessors were not blinded to participants’ group allocation |
| Incomplete outcome data (attrition bias) | Low risk | Little’s D test indicated that missing data were missing completely at random (2 88.2; df 1290; P = 0.99). Data were analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Each participant was treated with external beam radiation five days per week for seven weeks. The affected breast and regional lymph nodes received a 4500 to 5000 cGy dose in 200c Gy fractions with a boost of 1000 to 1600 cGy delivered to the primary tumour bed. Treatment dosages were similar between groups | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | Low risk | 2 of 23 participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | Low risk | None |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
Qualitative quotes:
| |
| Description of usual care | Physiotherapy, massage, compression, lymphatic drainage or laser therapy for lymphoedema | |
| Notes | Resistance aspect of this intervention will be excluded from analysis because of unclear exercise metrics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated using a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Low risk | All measures were assessed pre‐intervention (time 1; T1), immediately postintervention (time 2; T2) and at 12‐week follow‐up (time 3; T3) and were conducted by the same assessor, who was blinded to participant group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All participants (n = 32) participated in T1 and T2, whereas data were unavailable for two participants (one in the IG and one in the CG) at T3. To ensure that missing data did not contribute to the results found, data analysis was repeated with these two participants excluded, and no differences in results were observed (data not shown) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Adherence data on home‐based aspect of the intervention not clear |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Unclear | |
| Notes | Resistance aspect of this intervention will be excluded from analysis because of unclear exercise metrics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Method of measuring exercise behaviour and adherence not reported |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Usual cancer care included general information on the benefits of exercise but did not include specific instructions or further guidance for exercise. Seventy‐eight per cent of women had Stage I and Stage II breast cancer, and chemotherapy was the most common type of adjuvant therapy (48.8%), followed by radiotherapy (34.1%) and a combination of chemotherapy and radiotherapy (17.1%). Regimens of adjuvant therapy most often consisted of adriamycin 60 mg/m2 and cytoxan 600 mg/m2 every 2 to 3 weeks for 3 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | High risk | Data on only 41 of 74 randomly assigned participants reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Women randomly assigned but excluded had higher BMI and more advanced stages of cancer |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Unclear | |
| Notes | Resistance aspect of this intervention will be excluded from analysis because of unclear exercise metrics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Adherence to prescribed exercise not reported |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation table maintained by office staff in the clinical research office |
| Blinding of outcome assessment (detection bias) | Low risk | Physical fitness testing was performed at a hospital‐based fitness centre. The same research assistant, blinded to participant group allocation, performed these measurements at pre‐intervention and postintervention measurement time points |
| Incomplete outcome data (attrition bias) | High risk | Thirteen women (24%) did not complete their assigned 12‐week programme |
| Selective reporting (reporting bias) | High risk | Waist, upper and mid and lower arm circumference measures not reported |
| Other bias | High risk |
|
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by cancer stage and were randomly assigned to groups |
| Allocation concealment (selection bias) | Low risk | Participant assignment to groups at enrolment was concealed from the project director |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians monitoring graded exercise tests were blinded to participant group assignment. Similarly, a physical therapist or an exercise physiologist, blinded to participant assignment, performed strength assessments |
| Incomplete outcome data (attrition bias) | Low risk | Intent‐to‐treat analysis done and multiple imputation used |
| Selective reporting (reporting bias) | Low risk | None |
| Other bias | High risk | Numbers randomly assigned to intervention and control groups are unclear, as are numbers completing in each arm |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Unclear | |
| Notes | *We estimated trial recruitment rate on the basis of numbers randomly assigned of those approached and eligible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | High risk | Exercise tolerance test performed but no control group comparison data reported. 38% lost to follow‐up |
| Selective reporting (reporting bias) | High risk | None of the physiological assessments were performed for the control group at 12 weeks |
| Other bias | High risk | A statistically significant difference was noted between groups for body esteem at baseline (weight concerns and physical |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Unclear | |
| Notes | *Data from baseline questionnaires indicated that two participants in the intervention group were active at baseline (i.e. a discrepancy was noted between telephone screening and assessment). However, the author has advised that outliers were removed during data analysis of trial outcomes. Author advised that accelerometer data should have been reported as kcal/h) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat approach used and low attrition reported (5%) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Significantly more control group participants were receiving hormone treatment: 49% versus 74% in the intervention and control groups, respectively (P = 0.015). Ojective accelerometer data do not support the self‐reported physical activity behaviour |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Process measures |
| |
| Compliance |
| |
| Description of usual care | Unclear | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit a "low" or "high" risk judgement |
| Incomplete outcome data (attrition bias) | Low risk | < 10% attrition reported |
| Selective reporting (reporting bias) | High risk | Accelerometer data not reported |
| Other bias | High risk | Accelerometer correlation with self‐report questionnaires is weak at follow‐up points when significant differences between groups in physical activity are reported (i.e. r = 0.32 at 3 months). Substantial contamination in the control group |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Sedentary status at baseline is unclear | |
| Exercise prescription metrics are unclear | |
| Sedentary status at baseline is unclear | |
| Author advised that baseline sedentary status was not assessed. | |
| Author advised that baseline sedentary status was not assessed. | |
| Linked to Battaglini 2007 | |
| Unclear if participants were meeting the baseline moderate exercise sedentary criteria. | |
| Intervention exercise prescription metrics unclear | |
| Linked to Cantarero‐Villanueva 2011 | |
| Linked to Carmack Taylor 2006 | |
| Exercise prescription metrics are unclear | |
| Linked to Carmack Taylor 2006 | |
| Author advised that baseline sedentary status was not assessed | |
| Sedentary status at baseline is unclear | |
| Exercise prescription metrics are unclear | |
| Exercise prescription metrics are unclear | |
| Sedentary status at baseline is unclear | |
| Linked to Ornish 2005 | |
| Sedentary status at baseline is unclear | |
| START trial includes non sedentary participants | |
| Author advised that cohort was not sedentary at baseline. | |
| Linked to Mutrie 2007 | |
| Intervention exercise prescription metrics unclear | |
| Linked to Ornish 2005 | |
| Sedentary status at baseline is unclear | |
| Linked to Galvao 2010 | |
| Cohort not sedentary at baseline | |
| Sedentary status at baseline is unclear | |
| Author advised that baseline sedentary status was not assessed. | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Author advised that exercise intensity was not clear | |
| Linked to Ligibel 2008 | |
| Sedentary status at baseline is unclear | |
| Exercise prescription metrics are unclear | |
| Sedentary status at baseline is unclear | |
| Linked to Waltman 2010 | |
| Author advised that cohort was not sedentary | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Author advised that baseline sedentary status was not assessed | |
| Exercise prescription metrics are unclear | |
| Author advised that cohort was not sedentary at baseline | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Linked to Schmitz 2005 | |
| Sedentary status at baseline is unclear | |
| Linked to Ornish 2005 | |
| Linked to Ornish 2005 | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Author not able to confirm sedentary status | |
| Author advised that cohort was not sedentary at baseline | |
| Author advised that cohort was not sedentary at baseline | |
| Sedentary status at baseline is unclear | |
| Author advised intensity not assessed | |
| Author advised intensity not assessed | |
| Author advised exercise behavior not formally assessed at baseline | |
| Author advised exercise behavior not formally assessed at baseline | |
| Author advised exercise behavior not formally assessed at baseline | |
| Author advised that cohort was not sedentary at baseline | |
| Linked to von Gruengien 2008 | |
| Author advised that cohort was not sedentary | |
| Author advised that cohort was not sedentary | |
| Sedentary status at baseline is unclear | |
| Sedentary status at baseline is unclear | |
| Author not able to clarify exercise metrics | |
| Author advised that cohort was not sedentary at baseline |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Bai S‐M, Ma C, Liu Y‐M, Xue W‐P, Luo M, Ou Z‐H. Effects of cognitive behavior intervention and cinesiateics on the quality of life of patients with nasopharyngeal carcinoma after radiotherapy. Chinese Journal of Clinical Rehabilitation 2004;8(29):6312–3. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Chen J, Luo A, He Y. Influence of postoperative rehabilitation exercises on functional recovery of ill limb of breast cancer patients. Chinese Nursing Research 2010;24(4A):875–7. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Cho OH. Effects of a comprehensive rehabilitation programme for mastectomy patients. Taehan Kanho Hakhoe Chi 2004;34(5):809–19. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Dong HY, Wang ZF, Cai L. Correlation between quality of life and rehabilitative guidance education in the postoperative patients with breast cancer. Chinese Journal of Clinical Rehabilitation 2006; 10(42), 28‐30. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Guo Y‐M. Effects of moderate strength and endurance exercise on emotion and quality of sleep in patients with malignant tumor. Chinese Journal of Clinical Rehabilitation 2004;8(35):7896–7. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Le Vu B, Dumortier A, Guillaume MV, Mouriesse H, Barreau‐Pouhaer L. Efficacy of massage and mobilization of the upper limb after surgical treatment of breast cancer. Bulletin du Cancer 1997;80(10):957–61. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Oliveira MM, Souza GA, Miranda Mde S, Okubo MA, Amaral MT, Silva MP, Gurgel MS. Upper limb exercises during radiotherapy for breast cancer and quality of life. Revista Brasileira de Ginecologia e Obstetrícia 2010;32(3):133‐8. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Park HS, Cho GY, Park KY. The effects of a rehabilitation program on physical health, physiological indicator and quality of life in breast cancer mastectomy patients. Taehan Kanho Hakhoe Chi 2006;36(2):310‐20. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Wang Y;Yao J‐F;Yang J‐Y. Effect of rehabilitation exercises on the recovery outcomes of lung function in postoperative patients with lung cancer. Zhongguo Linchuang Kangfu (Chinese Journal of Clinical Rehabilitation) 2005; 9(39):14‐16. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting translation: Zhang T, Chang XM, He YG, Huang HX, Fan KS. Effects of rehabilitation therapy in relieving pain and improving quality of life in patients with advanced cancer. Zhongguo Linchuang Kangfu (Chinese Journal of Clinical Rehabilitation) 2005;40:59‐61. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) Show forest plot | 7 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.51, 0.95] |
| Analysis 1.1  Comparison 1 Aerobic exercise tolerance, Outcome 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up). | ||||
| 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) Show forest plot | 3 | 154 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.51, 1.17] |
| Analysis 1.2  Comparison 1 Aerobic exercise tolerance, Outcome 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis). | ||||
| 3 Aerobic exercise tolerance (all cancers: 6 months) Show forest plot | 5 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.45, 0.94] |
| Analysis 1.3  Comparison 1 Aerobic exercise tolerance, Outcome 3 Aerobic exercise tolerance (all cancers: 6 months). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Strength tests Show forest plot | 3 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.09, 0.93] |
| Analysis 2.1  Comparison 2 Strength tests (all cancers), Outcome 1 Strength tests. | ||||
| 2 Strength tests (all cancers: sensitivity analysis) Show forest plot | 2 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐0.01, 0.96] |
| Analysis 2.2  Comparison 2 Strength tests (all cancers), Outcome 2 Strength tests (all cancers: sensitivity analysis). | ||||

PRISMA flow diagram.
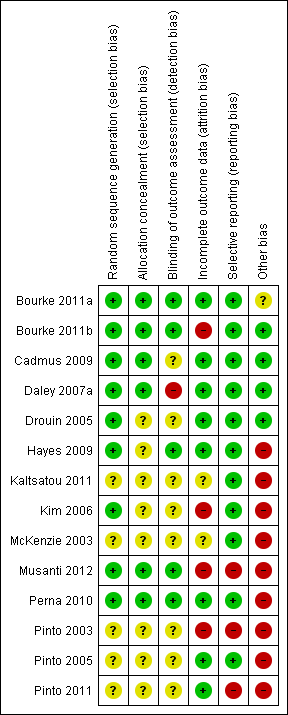
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
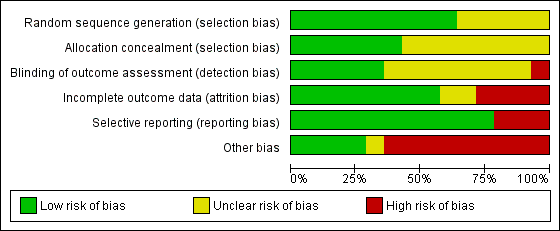
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Aerobic exercise tolerance, Outcome 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up).

Comparison 1 Aerobic exercise tolerance, Outcome 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis).
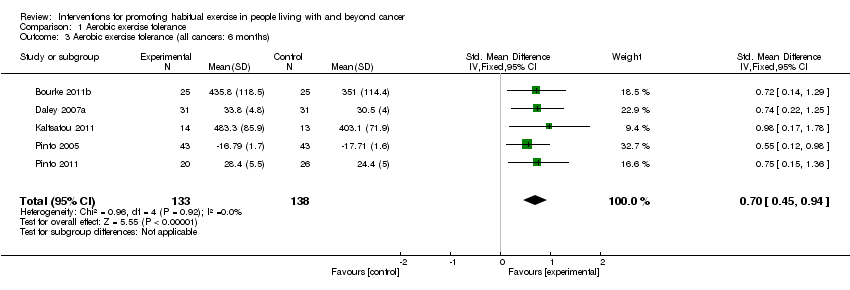
Comparison 1 Aerobic exercise tolerance, Outcome 3 Aerobic exercise tolerance (all cancers: 6 months).
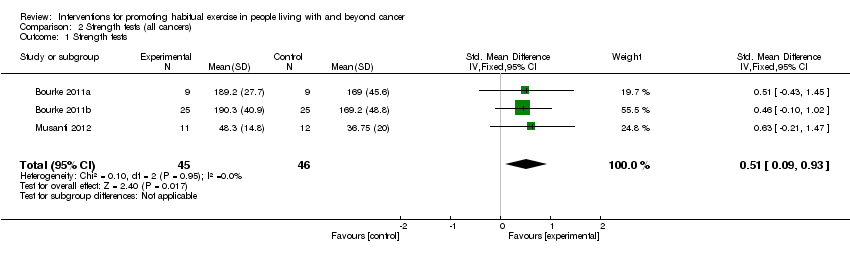
Comparison 2 Strength tests (all cancers), Outcome 1 Strength tests.

Comparison 2 Strength tests (all cancers), Outcome 2 Strength tests (all cancers: sensitivity analysis).
| Study | Exercise components | n | Meets Rock et al guidelines? | Adherence summary | At least 75% adherence? | High risk of bias? | Change in AET reported? | Adverse effects |
| Aerobic | 37, 38 (intervention vs control) | 33% reported 150 minutes/wk of moderate intensity aerobic exercise at an average of 76% HR, for six months | 75% of women were doing between 90 and 119 minutes of moderate intensity aerobic activity per week at six months | Yes; for up to 119 minutes per week | No | No | Five of the 37 women randomly assigned to exercise experienced an adverse effect; two were related to the study (plantar fasciitis) | |
| Aerobic | 34, 36, 38 (intervention, sham, control, respectively) | No | 77% of the exercise therapy; attended 70% (at least 17 of 24 sessions) or more of sessions | Unclear | Yes; outcome assessors were not blinded to participants’ group allocation | Yes | Three withdrawals in the intervention group: unclear as to why this occurred. Some withdrawals because of medical complications in placebo and control arms but unclear whether study related | |
| Aerobic | 13 intervention, 8 placebo stretching controls | Unclear | Participants in the intervention group averaged 3.6 days per week of aerobic exercise over an 8‐week period | Unclear | No | Yes | None reported | |
| Aerobic | 14, 13 (intervention vs control) | Unclear | Not reported | Not reported | Yes; method of measuring exercise and adherence not reported | No | None reported | |
| Aerobic | 22,19 (intervention vs control). | No | Average weekly frequency of exercise was 2.4 ± 0.6 sessions, and average duration of exercise within prescribed target HR was 27.8 ± 8.1 minutes per session. Overall adherence was 78.3% ± 20.1% | Yes | Yes; data missing for 45% of the cohort | Yes | Reasons for withdrawal included personal problems (n = 2), problems at home (n = 2), problems related to chemotherapy (n = 3), thrombophlebitis in the lower leg (n = 2), non-exercise‐related injuries (n = 1), and death (n = 1). Unclear to which arm of the trial these date relate | |
| Aerobic | 12, 12 (intervention vs control) | Unclear | Participants attended a mean of 88% of the 36‐session supervised exercise programme | Yes | Yes; 38% lost to follow‐up. Exercise tolerance test was performed but no control group comparison data were reported | Yes | None reported; however, it is unclear why the six controls dropped out | |
| Aerobic | 43, 43 (intervention vs control) | Unclear | At week 12, intervention participants reported a mean of 128.53 minutes/wk of moderate intensity exercise. However, no changes were reported in the accelerometer data in the intervention group (change score = ‐0.33 kcal/h) | Less than 75% of the intervention group was meeting the prescribed goal after week 4 | Yes; significantly more control group participants were receiving hormone treatment. Accelerometer data do not support the self‐reported physical activity behaviour | Yes | Not clear whether chest pain was related to exercise in dropout whose participation was terminated | |
| Aerobic | 20, 26 (intervention vs control) | Three‐day PAR questionnaire indicates that 64.7% of the intervention group and 40.9% of the control group were achieving the guidelines at three months | Correlation between self‐reported moderate intensity exercise and accelerometer data at three‐month follow‐up, when the only significant between‐group change is reported: r = 0.32 | No | Yes; accelerometer data were not reported; also, cited correlation is weak (0.32). Further, substantial contamination was noted in the control group | Yes | One cancer recurrence in the control group at three months | |
| Aerobic and resistance | 9, 9 (intervention vs control) | Six weeks of resistance exercise twice a week | 90% attendance at the supervised sessions. 94% of independent exercise sessions were completed | Yes | No | Yes | One stroke in the intervention group, unrelated to the exercise programme | |
| Aerobic and resistance | 25, 25 (intervention vs control) | Six weeks of resistance exercise twice a week | 95% attendance at supervised exercise sessions. Compliance with self‐directed exercise aspect of the lifestyle intervention was 87% | Yes | Yes; high dropout rate at postintervention six‐month follow‐up assessment | Yes | Two men in the intervention arm were discontinued because of cardiac complications before the 12‐week assessments. Two more reported musculoskeletal complaints before the six‐month assessment. Five men reported various health problems in the control group that prohibited them from attending the six‐month assessment | |
| Aerobic and resistance | 16, 16 (intervention vs control) | Unclear | Most women (88%) allocated to the intervention group participated in 70% or more of scheduled supervised exercise sessions | Unclear | Yes; adherence data on unsupervised aspect of the intervention are not clear | No | None reported | |
| Aerobic and resistance | 7,7 (intervention vs control) | No | Unclear | Unclear | Yes; adherence to exercise not reported | No | None reported | |
| Aerobic and resistance | Flexibility group (n = 13), aerobic group (n = 12), resistance group (n = 17), aerobic and resistance group (n = 13) | 12 weeks of resistance exercise two or three times per week | Mean percentages of adherence were as follows: flexibility = 85%, aerobic = 81%, resistance = 91% and aerobic plus resistance = 86% | Unclear | Yes; a significant number of dropouts belonged to the resistance exercise group (n = 8/13). Only 50% of activity logs were returned | Yes | Adverse effects were reported in two women during the study. In both cases, the women developed tendonitis: one in the shoulder and the other in the foot. Both had a history of tendonitis, and both received standard treatment | |
| Aerobic and resistance | 51 participants in total. Numbers randomly assigned to each arm are unclear | Three months of resistance exercise three times per week | Women assigned to the structured intervention completed an average of 83% of their scheduled hospital‐based exercise sessions (only 4 weeks in duration), and 76.9% completed all 12 sessions. Home‐based component (8 weeks in duration) | Unclear | Yes; numbers randomly assigned to intervention and control groups are unclear, as are numbers completing in each arm | No | Unclear | |
| AET = aerobic exercise tolerance. | ||||||||
| Behaviour change technique | YALE | |||||||||||||
| Theory | TTM | EXSEM | TTM | TTM | TTM SCT | |||||||||
| 1. Provide Info on consequences of behaviour in general | X | X | X | X | ||||||||||
| 2. Provide Info on consequences of behaviour to the individual | ||||||||||||||
| 3. Provide Info about others' approval | ||||||||||||||
| 4. Provide normative info about others' behaviour | ||||||||||||||
| Programme set goal | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 5. Goal setting (behaviour) | X | X | X | X | X | X | ||||||||
| 6. Goal setting (outcome) | ||||||||||||||
| 7. Action planning | ||||||||||||||
| 8. Barrier identification/Problem solving | X | X | X | X | X | X | ||||||||
| 9. Setting of graded tasks | X | X | X | X | X | X | X | X | X | |||||
| 10. Prompt review of behavioural goals | X | X | ||||||||||||
| 11. Prompt review of outcome goals | ||||||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | X | X | X | |||||||||||
| 13. Provide rewards contingent on successful behaviour | X | |||||||||||||
| 14. Shaping | ||||||||||||||
| 15. Prompt generalisation of a target behaviour | X | X | X | X | X | |||||||||
| 16. Prompt self‐monitoring of behaviour | X | X | X | X | X | X | X | X | X | X | ||||
| 17. Prompt self‐monitoring of behavioural outcome | X | X | X | X | X | X | ||||||||
| 18. Prompt focus on past success | X | |||||||||||||
| 19. Feedback on performance provided | X | X | X | X | ||||||||||
| 20. Information provided on where and when to perform behaviour | X | X | ||||||||||||
| 21. Instruction provided on how to perform the behaviour | X | X | X | X | X | X | X | X | X | X | ||||
| 22. Modelling/Demonstration of behaviour | X | X | X | |||||||||||
| 23. Teaching to use prompts/cues | X | X | X | |||||||||||
| 24. Environmental restructuring | X | X | ||||||||||||
| 25. Agreement on behavioural contract | X | |||||||||||||
| 26. Prompt practise | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 27. Use of follow‐up prompts | X | X | ||||||||||||
| 28. Facilitating social comparison | ||||||||||||||
| 29. Planning social support/social change | X | X | X | |||||||||||
| 30. Prompt identification as role model/position advocate | ||||||||||||||
| 31. Prompt anticipated regret | ||||||||||||||
| 32. Fear arousal | ||||||||||||||
| 33. Prompt self‐talk | ||||||||||||||
| 34. Prompt use of imagery | ||||||||||||||
| 35. Relapse prevention/coping planning | X | X | ||||||||||||
| 36. Stress management/emotional control training | X | |||||||||||||
| 37. Motivational interviewing | ||||||||||||||
| 38. Time management | ||||||||||||||
| 39. General communication skills training | ||||||||||||||
| 40. Stimulation of anticipation of future rewards | ||||||||||||||
| EXSEM = exercise self‐esteem model; SCT = social cognitive theory; TTM = transtheroretical model. | ||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) Show forest plot | 7 | 330 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.73 [0.51, 0.95] |
| 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) Show forest plot | 3 | 154 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.84 [0.51, 1.17] |
| 3 Aerobic exercise tolerance (all cancers: 6 months) Show forest plot | 5 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.45, 0.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Strength tests Show forest plot | 3 | 91 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [0.09, 0.93] |
| 2 Strength tests (all cancers: sensitivity analysis) Show forest plot | 2 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐0.01, 0.96] |

