Intervenciones para la promoción del ejercicio habitual en pacientes con cáncer o que han presentado la enfermedad
Resumen
Antecedentes
Esta es una versión actualizada de la revisión Cochrane original publicada en la Cochrane Library 2013, número 9. A pesar de que existe evidencia de buena calidad de los efectos beneficiosos para la salud del ejercicio regular en los pacientes con cáncer o que han presentado la enfermedad, no se comprende tan bien cómo promover un cambio sostenible en la conducta respecto al ejercicio en los supervivientes de cáncer sedentarios, en particular a largo plazo. La gran mayoría de los pacientes con cáncer o que se recuperan de un cáncer no cumplen las recomendaciones de ejercitarse. Por consiguiente, es importante examinar la evidencia sobre la forma de promover y mantener la conducta respecto al ejercicio para comprender las estrategias más eficaces para asegurar un efecto beneficioso en la población de pacientes e identificar las carencias de la investigación.
Objetivos
Evaluar los efectos de las intervenciones para promover la conducta respecto al ejercicio en pacientes con cáncer o que han presentado la enfermedad y abordar la siguiente pregunta: ¿Qué intervenciones son las más eficaces para mejorar la capacidad aeróbica y la fuerza y la resistencia musculoesquelética? ¿Qué intervenciones son las más eficaces para mejorar la conducta respecto al ejercicio entre los pacientes con cánceres diferentes? ¿Qué intervenciones son más probable que promuevan la conducta respecto al ejercicio a largo plazo (12 meses o más)? ¿Qué frecuencia de contacto con entrenadores profesionales o profesionales sanitarios se asocia con el aumento de la conducta respecto al ejercicio? ¿Qué base teórica se asocia más a menudo con mejores resultados conductuales? ¿Qué técnicas de modificación de la conducta (TMC) se asocian con mayor frecuencia con un aumento en la conducta respecto al ejercicio? ¿Qué efectos adversos se atribuyen a las diferentes intervenciones con ejercicios?
Métodos de búsqueda
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane. La revisión sistemática Cochrane de 2013 se actualizó con búsquedas en las siguientes bases de datos electrónicas: Registro Cochrane Central de Ensayos Controlados (CENTRAL) en The Cochrane Library, MEDLINE, Embase, AMED, CINAHL, PsycLIT/PsycINFO, SportDiscus y PEDro hasta mayo de 2018. También se buscó en la literatura gris, en los registros de ensayos, se escribió a los principales expertos en la materia y se buscaron las listas de referencias de los estudios incluidos y otras revisiones sistemáticas recientes relacionadas.
Criterios de selección
Solamente se incluyeron ensayos controlados aleatorizados (ECA) que compararon una intervención con ejercicios con un enfoque de atención habitual en pacientes sedentarios mayores de 18 años con un diagnóstico homogéneo de cáncer primario.
Obtención y análisis de los datos
Dos autores de la revisión que trabajaron de forma independiente examinaron todos los títulos y resúmenes para identificar los estudios que podrían cumplir los criterios de inclusión, o que no fue posible excluir de manera segura sin evaluar el texto completo (p.ej., cuando no hubo resúmenes disponibles). Se extrajeron los datos de todos los trabajos elegibles con al menos dos miembros del equipo de autores trabajando de forma independiente (RT, LS y RG). Las TMC se codificaron de acuerdo con la taxonomía CALO‐RE. El riesgo de sesgo se evaluó mediante la herramienta de Cochrane para este fin. Cuando fue posible y apropiado se realizó un metanálisis de efectos fijos de los resultados de los estudios. De observarse heterogeneidad estadística, se realizaría un metanálisis con un modelo de efectos aleatorios. Para los resultados continuos (p.ej., capacidad cardiorrespiratoria), se extrajo el valor final, la desviación estándar del resultado de interés y el número de participantes evaluados al seguimiento en cada brazo de tratamiento, para calcular la diferencia de medias estandarizada (DME) entre los brazos de tratamiento. Se utilizó la DME porque los investigadores utilizaron métodos heterogéneos para evaluar los resultados individuales. Si no fue posible ni apropiado realizar un metanálisis, se realizó una síntesis narrativa. La calidad de la evidencia se evaluó mediante el enfoque GRADE con el perfilador GRADE.
Resultados principales
En esta revisión se incluyeron 23 estudios con un total de 1372 participantes (se agregaron diez estudios, 724 participantes a la revisión original); se revisaron 227 textos completos en la actualización y 377 textos completos en la revisión original, lo que dejó 35 publicaciones de un total de 23 estudios únicos incluidos en la revisión. Se planificó incluir todos los tipos de cáncer, pero sólo cumplieron los criterios de inclusión estudios sobre cáncer de mama, próstata, colorrectal y pulmón. Trece estudios incorporaron un nivel objetivo de ejercicio que podría cumplir las recomendaciones actuales de ejercicio aeróbico de intensidad moderada (es decir, 150 minutos por semana); o ejercicio de resistencia (es decir, ejercicios de entrenamiento de fuerza al menos dos días por semana).
La adherencia a las intervenciones de ejercicio, que es fundamental para comprender la dosis de tratamiento, todavía se informa de manera inconsistente. Ocho estudios informaron de una adherencia con la intervención del 75% o más, para una prescripción de ejercicio que cumplía las guías actuales. Todos estos estudios incluyeron un componente de supervisión: en el análisis de las TMC estos estudios se designaron como "ensayos de nivel 1". Seis estudios informaron de una adherencia a la intervención del 75% o más, para un objetivo de ejercicio aeróbico que era menor que las recomendaciones de la guía actual: en el análisis de las TMC estos estudios se designaron como "ensayos de nivel 2". Se desarrolló una jerarquía de las TMC para los ensayos de nivel 1 y nivel 2, y entre las técnicas de modificación de la conducta más frecuentes se encuentran el programa de establecimiento de objetivos, la configuración de tareas escalonadas y la instrucción sobre cómo implementar la conducta. A pesar de la incertidumbre alrededor de la adherencia en algunos de los estudios incluidos, las intervenciones dieron lugar a mejorías en la tolerancia al ejercicio aeróbico a las ocho a 12 semanas (DME 0,54; IC del 95%: 0,37 a 0,70; 604 participantes, diez estudios; evidencia de calidad baja) en comparación con la atención habitual. A los seis meses también mejoró la tolerancia al ejercicio aeróbico (DME 0,56; IC del 95%: 0,39 a 0,72; 591 participantes; siete estudios; evidencia de calidad baja).
Conclusiones de los autores
Desde la última versión de esta revisión, ninguno de los nuevos estudios relevantes ha proporcionado información adicional para modificar las conclusiones. Se ha encontrado una cierta mejoría en la comprensión de cómo animar a los supervivientes de cáncer anteriormente inactivos a lograr las guías internacionales de actividad física. El establecimiento de objetivos, la configuración de tareas escalonadas y la instrucción sobre cómo implementar la conducta, figuran entre las intervenciones que cumplen los objetivos de las recomendaciones e informan una adherencia del 75% o más. Sin embargo, los datos de seguimiento a largo plazo son todavía limitados, y la mayoría de los estudios se realizaron en mujeres blancas con cáncer de mama. Todavía hay un número considerable de estudios publicados con numerosos y variados problemas relacionados con el alto riesgo de sesgo y estándares de informe deficientes. Además, a menudo los metanálisis se consideraron consistentes con evidencia de certeza baja a muy baja. Se informó de un número muy pequeño de efectos adversos graves entre los estudios, lo que proporciona tranquilidad con respecto a la seguridad del ejercicio en esta población.
PICO
Resumen en términos sencillos
Intervenciones para la promoción del ejercicio habitual en pacientes con cáncer o que han presentado la enfermedad
El problema
Mantenerse regularmente activo puede aportar una serie de efectos beneficiosos para la salud de los pacientes con cáncer o que han presentado la enfermedad, que incluyen una mejor calidad de vida y una mejor función física. La actividad física también puede reducir el riesgo de recidiva del cáncer y de morir por cáncer. Debido a que la mayoría de los sobrevivientes de cáncer no son físicamente activos de manera habitual, es necesario entender cómo promover y mantener la actividad física en esta población.
Objetivo de la revisión
Comprender cuáles son las maneras más eficaces de mejorar y mantener la conducta respecto al ejercicio en pacientes con cáncer o que han presentado la enfermedad.
Características de los estudios
Sólo se incluyeron los estudios que compararon una intervención de ejercicios con una comparación de atención habitual o un control en "lista de espera". Solo fueron elegibles los estudios que incluyeron pacientes sedentarios mayores de 18 años con el mismo diagnóstico de cáncer. Los participantes se deben haber asignado al azar a ejercicio o a atención habitual. La evidencia se buscó en las bases de datos de estudios de investigación desde 1946 hasta mayo de 2018.
¿Cuáles son los principales hallazgos?
Se incluyeron 23 estudios con 1372 participantes. La evidencia indica que los estudios de ejercicios que incorporan un elemento de supervisión pueden ayudar a los supervivientes de cáncer. Sin embargo, todavía no se sabe cómo promover el ejercicio a largo plazo (más de seis meses). Existe cierta preocupación por el hecho de que los estudios de investigación no se informan con la claridad que debieran. Se determinó que establecer objetivos, graduar las tareas de actividad física y proporcionar instrucciones sobre cómo realizar los ejercicios podría ayudar a los pacientes a hacer cantidades beneficiosas de ejercicio. Además, se encontró alguna evidencia de que los pacientes que cumplen con los niveles de ejercicio recomendados, se ponen en forma en el transcurso de seis meses.
Calidad de la evidencia
Los problemas principales que se encontraron con respecto a la calidad de los estudios en esta revisión incluyeron no saber cómo los investigadores de los estudios realizaron la asignación al azar en los ensayos, ni si los investigadores que hicieron las evaluaciones en los ensayos conocían a qué grupo se había asignado al azar el paciente que evaluaban. Se encontró que la calidad de la evidencia de estos estudios fue baja debido a que la mayoría de los ensayos a menudo contenían un escaso número de participantes.
¿Cuáles son las conclusiones?
Las principales conclusiones de esta revisión son que el ejercicio es generalmente seguro para los sobrevivientes de cáncer. Existe una mejor comprensión sobre cómo animar a los supervivientes de cáncer a cumplir con las recomendaciones de ejercicios actuales. Sin embargo, todavía hay falta de evidencia sobre cómo fomentar el ejercicio en los supervivientes de cáncer durante seis meses.
Authors' conclusions
Summary of findings
| Exercise interventions compared to usual care for promoting habitual exercise in people living with and beyond cancer to improve aerobic exercise tolerance | ||||
| Outcomes | № of participants | Certainty of the evidence | Anticipated absolute effects* (95% CI) | |
| Risk with usual care | Risk difference with exercise interventions | |||
| Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) | 604 | ⊕⊕⊝⊝ | The mean aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) was 0 | SMD 0.54 higher |
| Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) | 201 | ⊕⊕⊝⊝ | The mean aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) was 0 | SMD 0.85 higher |
| Aerobic exercise tolerance (all cancers: 6 months) | 591 | ⊕⊕⊝⊝ | The mean aerobic exercise tolerance (all cancers: 6 months) was 0 | SMD 0.56 higher |
| Aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) | 441 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) was 0 | SMD 0.57 higher |
| Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) | 357 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 0.53 higher |
| Aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) | 155 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 0.70 higher |
| Aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) | 92 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 1.07 higher |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1 Some concerns with high number of participants lost to follow‐up, selective reporting of data and other risks of bias | ||||
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2013, Issue 9) Bourke 2013.
Description of the condition
Cancer is a major public health issue. In 2015, there were 17.5 million cases of cancer globally, 8.7 million deaths and the disease is estimated to be responsible for 208 million disability adjusted life years (Global Burden of Disease Cancer Collaboration 2017). Age‐standardised cancer mortality rates are decreasing (in the Western hemisphere), which is encouraging progress (Hashim 2016). However, although increasing numbers of cancer survivors live longer, this does not equate to living well. Survivors face a multitude of unique, debilitating health problems, even after treatment with curative intent. These range from an increased risk of recurrent cancers (Low 2014), persistent symptoms such as fatigue (Low 2014), ongoing poor health and well‐being (Elliott 2011), and mental health comorbidity (Nakash 2014). The burden of these problems can lead to negative impacts on health‐related quality of life (HRQoL) (Corner 2013). Throughout this review, the term we define as 'cancer survivor' is synonymous with someone 'living with and beyond cancer', in accordance with the Macmillan Cancer Support definition (Macmillan Cancer Support 2011).
Description of the intervention
The goal of any exercise intervention is to offer a sustained physiological challenge that, over time, will induce a spectrum of beneficial cardiovascular, respiratory, musculoskeletal, neurological, metabolic adaptations as well as bringing a host of psychosocial benefits. In the context of living with and beyond cancer, such adaptations underpin improvements in cancer‐related fatigue, HRQoL and physical function (Mishra 2014; Stout 2017). The UK Chief Medical Officer recommends that in adults, weekly activity should add up to at least 150 minutes of moderate intensity aerobic activity, performed in bouts of 10 minutes or longer (Department of Health 2011), with similar international recommendations for cancer survivors (Rock 2012). For example, this could translate to 30 minutes of aerobic activity that raises heart rate and breathing rate, five times per week. Alternatively, 75 minutes of vigorous intensity aerobic activity spread across the week has been suggested to confer similar benefit (Schmitz 2010a).
We have deliberately chosen the term 'habitual' over 'regular' to reflect the intention to assess which interventions could both A) improve and B) sustain exercise behaviour. 'Regular exercise' can be applied to both short‐term and long‐term contexts, where as a 'habitual' exerciser indicates a sustained and regular pattern of behaviour. Whilst 'habitual' refers to the process of behavioural 'habit forming' and an automaticity of behaviour (Gardner 2011; Verplanken 2009), we recognise there are other theoretical principals underpinning physical activity behaviour (Kwasnica 2016).
How the intervention might work
Encouraging people to participate in regular exercise from a background of an inactive lifestyle is difficult, requiring attention to important psychosocial and behavioural influences (Kampshoff 2014; Ormel 2017). A major challenge is to provide a support structure for physical activity until it becomes a pattern of sustained healthy behaviour. Randomised controlled trials (RCTs) in cancer survivors have assessed a number of exercise interventions, with the aim of promoting short‐ and long‐term habitual exercise. A wide range of approaches have been investigated; including supervised exercise and home‐based exercise (Bourke 2014), and inclusive of group counselling sessions, (Rogers 2015). Tailored exercise interventions commonly comprise aerobic exercise training, strength training or a combination of both, with or without behaviour change support. Behaviour change theory within exercise interventions is often viewed as essential, with the UK Medical Research Council (MRC) recommending the use of theory in intervention development for complex interventions to help improve behaviour change (Craig 2008). However, the application of behaviour change theory or specific behaviour change techniques is often generally poor, unclear and not clearly examined for impact of effectiveness.
Why it is important to do this review
The majority of people living with and beyond cancer are not regularly active, with estimates ranging from less than 10% to 20% to 30% of cancer survivors meeting the physical activity guidelines (Garcia 2014). There are a number of important beneficial effects of exercise participation in cancer survivors reported from RCTs including improved HRQoL, reduced fatigue and improved physical function, (Bourke 2014; Dittus 2017; Meneses‐Echavez 2015; Mishra 2012a; Mishra 2012b; Stout 2017). However, the original review (Bourke 2013) found that most of the current evidence comes from studies with short‐term interventions and follow‐up. Understanding which interventions are most efficacious in supporting the maintenance of long‐term exercise behaviour is critical not just because of the HRQoL benefits (Bourke 2012a), but multiple observational reports link being regularly active to reduced chances of dying from cancer after diagnosis (Li 2016).
The original review showed that there is a poor understanding of how to encourage people living with and beyond cancer to meet current exercise recommendations (Bourke 2013). Poor study reporting standards was a pervasive issue e.g. failure to report adherence data. However, there were some useful data regarding the use of behaviour change techniques (BCTs). An updated review can firstly, offer insight as to whether interventions being tested in contemporary studies are mapping to the existing international recommendations i.e. the American Cancer Society (ACS) guidance (i.e. provided by Rock 2012). Secondly, this will allow us to evaluate if there have been any improvements in the quality of intervention reporting around specifics of set prescriptions (i.e. frequency, intensity, duration etc). Thirdly, and critically, we can use a larger data set from our updated searches to assess if both the quality of reporting of exercise adherence has improved and if there is more to learn about how to promote and sustain better adherence to exercise behaviour interventions in previously inactive cancer survivors.
In the UK, the Independent Cancer Taskforce strategy document sets out a number of initiatives to achieve world class outcomes in cancer; ensuring survivors have the best possible quality of life and improving rates of mortality. Promoting habitual exercise participation could help to accomplish these high priority agendas within the UK.
Objectives
Primary objective
To assess the effects of interventions designed to promote exercise behaviour in sedentary people living with and beyond cancer.
Secondary objectives
To address the following questions.
-
Which interventions are most effective in improving aerobic fitness and skeletal muscle strength and endurance?
-
What adverse effects are attributed to different exercise interventions?
-
Which interventions are most effective in improving exercise behaviour amongst patients with different cancers?
-
Which interventions are most likely to promote long‐term (12 months or longer) exercise behaviour?
-
What frequency of contact with exercise professionals and/or healthcare professionals is associated with increased exercise behaviour?
-
What theoretical basis is most often associated with increased exercise behaviour?
-
What behaviour change techniques are most often associated with increased exercise behaviour?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that allocated participants or clusters of participants by a random method to an exercise‐promoting intervention compared with usual care or 'waiting list' control. We included studies conducted both during and after primary treatment or during active monitoring. Only interventions that included a component targeted at increasing aerobic exercise and/or resistance exercise behaviour were included in this review. We did not include studies of heterogeneous cancer cohorts (i.e. participants with different primary cancer sites). We did not include studies in 'at risk' populations (i.e. studies involving individuals who have risk factors for cancer but who have not yet been diagnosed with the disease) that addressed primary prevention research questions.
Types of participants
We included only studies involving adults (18 years of age or older) who had a sedentary lifestyle or physically inactive at baseline (i.e. not undertaking 30 minutes or more of exercise of at least moderate intensity, three days per week, or 90 minutes in total of moderate intensity exercise per week). Participants must have been histologically or clinically diagnosed with cancer regardless of sex, tumour site, tumour type, tumour stage and type of anticancer treatment received. We excluded studies directed specifically at end‐of‐life‐care patients and individuals who were currently hospital inpatients.
Types of interventions
For the purposes of this review, the phrases 'exercise' and 'physical activity' were used interchangeably. Definitions of exercise, related terms and nomenclature that describe the performance of exercise must adhere to principles of science and must satisfy the Système International d'Unités (SI), which was adopted universally in 1960. Hence, we referred to the appropriate, combined definition that applies to all situations: 'A potential disruption to homeostasis by muscle activity that is either exclusively or in combination, concentric, eccentric or isometric' (Winter 2009). Investigators must have reported the frequency, duration and intensity of aerobic exercise behaviour or frequency, intensity, type, sets and repetitions of resistance exercise behaviour that was prescribed in the intervention.
We acknowledge that the maximal aerobic capacity (V̇O2max)/peak is often the most informative metric for setting aerobic exercise intensity; however, given the nature of the population involved (elderly, potentially with multiple comorbidities), it is often difficult to conduct maximal testing protocols to prescribe intensity on the basis of this measures because of the requirements for medically qualified staff to be present during assessment. As such, for reasons of pragmatism, we accepted that exercise intensity is more frequently reported in cancer the cohorts in terms of age‐predicted maximum heart rate(HRmax) or Borg Rating of Perceived Exertion (RPE) (Borg 1982). The interventions in this review were categorised as achieving a mild (less than 60% HRmax/10 RPE or less), moderate (60% to 84% HRmax/11 to 14 RPE) or vigorous (85% HRmax or more/15 RPE or more) exercise intensity.
Types of outcome measures
Primary outcomes
Aerobic exercise behaviour as measured by:
-
exercise frequency (number of bouts per week);
-
exercise duration (total minutes of exercise achieved);
-
exercise intensity (e.g. % HRmax, RPE);
-
estimated energy expenditure from free‐living physical activity (e.g. from accelerometer readings (where available));
-
adherence to the exercise intervention (% of exercise sessions completed/attended); total duration of intervention when ≥75% adherence is achieved (in weeks);
-
total duration of sustained exercise behaviour meeting American Cancer Society guidelines for exercise in people living with and beyond cancer (Rock 2012; i.e. aim to exercise at least 150 minutes per week, with at least two days per week of strength training).
Resistance exercise behaviour as measured by:
-
exercise frequency (number of bouts per week);
-
exercise intensity (e.g. % of 1 repetition max or % of body mass);
-
type of exercise (e.g. free weights, body weight exercise);
-
repetitions;
-
sets.
Secondary outcomes
-
Change in aerobic fitness or exercise tolerance (maximal or submaximal when measured directly or by a standard field test).
-
Change in skeletal muscle strength and endurance.
-
Adverse effects.
-
study recruitment rate.
-
Intervention attrition rate.
Interventions were judged as successful in achieving exercise goals if investigators reported at least 75% adherence over a given follow‐up period as done in the original review (Bourke 2013). Data on compliance with the intervention were quantified in terms of number of prescribed exercise sessions completed as a proportion of the total set. The intervention must have included at least six weeks of follow‐up. Interventions were described according to whether they reported being based on a behaviour change theory e.g. control theory, social cognitive theory; (Bandura 2000; Bandura 2002; Carver 1982. This relates to the National Institute for Health and Clinical Excellence (NICE) guidance for behaviour change, which recommends that clinicians should be explicit about the theoretical constructs on which interventions are based (NICE 2007). Interventions were also coded using the ‘Coventry, Aberdeen & London-Refined’ (CALO‐RE) taxonomy (Michie 2011). This is a validated taxonomy of behaviour change techniques (BCTs) that can be used to help people change their exercise behaviour. Coding interventions according to this taxonomy allows for a better understanding of which techniques are employed by current interventions and how they are related to short‐ and longer‐term exercise behaviour change.
Search methods for identification of studies
Electronic searches
The searches were run for the original review from inception to August 2012. The subsequent searches from the following electronic databases were run from August 2012 up to 3 May 2018. We carried out the following searches:
-
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 5) in The Cochrane Library;
-
MEDLINE via OVID August 2012 to April week 4 2018;
-
Embase via OVID August 2012 to 2018 week 18;
-
AMED (Allied and Alternative Medicine Database; covers occupational therapy, physiotherapy and complementary medicine) August 2012 to May 2018;
-
CINAHL (Cumulative Index to Nursing and Allied Health Literature) August 2012 to May 2018;
-
PsycINFO (Database of the American Psychological Association) August 2012 to May 2018;
-
SportDiscus (Sports Evidence Database) August 2012 to April 2017;
-
PEDro (Physiotherapy Evidence Database) August 2012 to April 2017.
The search strategies are presented in the Appendices, with both the 2018 updated strategy and previous 2012 strategy reported. CENTRAL search strategy is presented in Appendix 1 and the MEDLINE search strategy in Appendix 2. For databases other than MEDLINE, we adapted the search strategy accordingly: Embase (Appendix 3), AMED (Appendix 4), CINAHL (Appendix 5) PsycINFO (Appendix 6) PEDro (Appendix 7) SportsDiscus (Appendix 8).
The search strategies were developed with the Cochrane Gynaecological Cancer Group Information Specialist and included MeSH and text word terms as appropriate.
Searching other resources
We used snowballing, by searching reference lists of retrieved articles and published reviews on the topic.
We expanded the database search by identifying additional relevant studies for this review, including unpublished studies and references in the grey literature. This was done by searching the OpenGrey database (www.opengrey.eu/), which includes technical or research reports, doctoral dissertations, conference papers and other types of grey literature. We also searched the following clinical trials web pages.
-
World Health Organisation apps.who.int/trialsearch/Default.aspx
-
National cancer institute www.cancer.gov/about‐cancer/treatment/clinical‐trials/search
Furthermore, we wrote to Cancer Research UK (CRUK), Macmillan Cancer Support, the World Cancer Research Fund (WCRF), Worldwide Cancer Research , the American Association for Cancer Research (AACR), the American Cancer Society (ACS) and the American Society of Clinical Oncology (ASCO) to enquire about relevant unpublished papers.
Data collection and analysis
Since publication of the previous version of this review, we have included the use of the GRADE assessment to assess the quality of the evidence and produced a summary of findings Table for the main comparison.
Selection of studies
We imported results from each database into the reference management software package Endnote, from which we removed duplicates. After training on the first 100 references retrieved from two different databases to ensure a consistent approach, two review authors (RT and HQ) worked independently to screen all titles and abstracts to identify studies that met the inclusion criteria, or that could not be safely excluded without assessment of the full text (e.g. when no abstract was available). Disagreements were resolved by discussion with another review author (LB). Full texts were retrieved for these articles.
After training was provided to ensure a consistent approach to study assessment and data abstraction, two review authors worked independently to assess the retrieved full texts (RT and HQ). We linked together multiple publications and reports on the same study. Studies that appeared to be relevant but were excluded at this stage are listed in the 'Characteristics of excluded studies' table. We resolved disagreements by discussion with other group members. We attempted to contact study corresponding authors if we could not access a full text (e.g. if only an abstract was available), if we required more information to determine whether a study could be included (e.g. to determine baseline exercise behaviour of a cohort), or if we required supplementary information about an already eligible study (please also see Excluded studies).
Data extraction and management
Review authors (RT and LB) extracted the following data using the same data extraction form used in the original review and entered data into RevMan 5.3 (Review Manager 2014).
-
Study details: author; year; title; journal; research question/study aim; country where the research was carried out; funding source; recruitment source (e.g. consecutive sampling from outpatient appointments; advertising in the community; convenient sample from support groups); inclusion and exclusion criteria; study design (cluster RCT, non‐cluster RCT, single centre or multi‐centre); sample size; number of participants per arm; length of follow‐up; description of usual care.
-
Intervention details: categorisation of intervention (e.g. supervised, independent, educational); setting (e.g. dedicated exercise facility, community, home); exercise prescription components (e.g. aerobic exercise, resistance exercise, stretching); theoretical basis, behaviour change techniques (using CALO‐RE taxonomy), frequency of contact with an exercise professional and or healthcare professional; instructions to controls.
-
Participant characteristics: primary cancer diagnosis; any cancer treatment currently undertaken; metastatic disease status; age; sex; body mass index (BMI); ethnicity; reported comorbidities.
-
Resulting exercise behaviour: method of measuring exercise (e.g. self‐report questionnaire). Numbers of participants randomly assigned and assessed at specified follow‐up points. Frequency, duration, intensity of aerobic exercise achieved; frequency, intensity, type, sets and repetitions of resistance exercise achieved; total duration of the intervention; total duration of sustained meaningful exercise behaviour as a result of the intervention and whether the Rock 2012 guidelines were met, adherence to the intervention; rate of attrition and adverse effects reported.
-
Resulting change in other outcomes: changes in aerobic fitness and estimated energy expenditure from free‐living physical activity.
Three members of the group worked independently (RT, RG and LS) to extract data from all eligible papers using the data collection form. Data were entered into the Cochrane's statistical software, Review Manager 2014, by one review author and checked by a second review author.
Assessment of risk of bias in included studies
Risk of bias and methodological quality were assessed in accordance with Cochrane's tool for assessing risk of bias (Higgins 2011). The tool includes the following seven domains:
-
sequence generation (method of randomisation);
-
allocation concealment (selection bias);
-
blinding (masking) of participants and personnel (detections bias);
-
blinding (masking) of outcome assessors (detection bias);
-
incomplete outcome data;
-
selective outcome reporting;
-
other sources of bias.
However, we did not include blinding to group allocation, as it is not possible (e.g. in a supervised exercise setting) to blind participants to an intervention while promoting exercise behaviour. Two review authors (RT and RG) independently applied the 'Risk of bias' tool, and differences were resolved by discussion with a third review author (LB). We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias. We contacted study authors to ask for additional information or for further clarification of study methods if any doubt surrounded potential sources of bias. Individual 'Risk of bias' items can be seen in Appendix 9.
Measures of treatment effect
For the purposes of this review, all exercise behaviour was synthesised as specified in the primary outcomes. For comparison of measures of change in fitness levels or estimated energy expenditure from free‐living physical activity, please see the section on 'Continuous data' in Data synthesis.
Unit of analysis issues
We did not include any cross‐over trials in this review because of the high risk of contamination. It can be very difficult to “wash out” exercise behaviour. Cancer survivors in particular can be a highly‐motivated cohort, and significant contamination has been reported even in conventional RCT settings (Courneya 2003; Mock 2005). Hence this learning effect distorts results. Furthermore, asking individuals to revert to sedentary behaviour could be considered unethical (Das 2012). Therefore, any cross‐over trials identified were rejected at the title and abstract screening stage.
Dealing with missing data
We assessed missing data and dropout rates for each of the included studies and reported the numbers of participants included in the final analysis as a proportion of all participants included in the study. We assessed the extent to which studies conformed to an intention‐to‐treat analysis.
Assessment of heterogeneity
Consistency of results was assessed visually and through examination of the I2 statistic, a quantity that describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error.An I2 greater than or equal to 50% was considered significant heterogeneity. We addressed this by performing a sensitivity analysis that excluded any heterogeneous trials. We supplemented this with a test of homogeneity to determine the strength of evidence that the heterogeneity was genuine. When significant statistical heterogeneity was detected, differences in characteristics of the studies or other factors were explored as possible sources of explanation. Any differences were summarised in a narrative synthesis.
Assessment of reporting biases
Publication bias
We intended to examine funnel plots corresponding to meta‐analysis of the primary outcomes to assess the potential for small study effects such as publication bias if a sufficient number of studies (i.e. more than 10) was identified. However, this was not the case; therefore this step was not included in the analysis.
Data synthesis
Continuous data
For continuous outcomes (e.g. cardiorespiratory fitness), we extracted the final value, the standard deviation (SD) of the outcome of interest and the number of participants assessed at endpoint for each treatment arm at the end of follow‐up, to estimate standardised mean differences (SMD) between treatment arms.
Dichotomous outcomes
For dichotomous outcomes (e.g. adverse effects, deaths), if it was not possible to use a hazard ratio (HR), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, to estimate a risk ratio (RR).
Meta‐analysis
When possible, and if appropriate, we performed a meta‐analysis of review outcomes. If statistical heterogeneity was noted, a meta‐analysis was performed using a random‐effects model. We planned to use a fixed‐effect model if no significant statistical heterogeneity was observed.
When possible, all data extracted were those relevant to an intention‐to‐treat analysis in which participants were analysed in groups to which they were assigned. We noted the time points at which outcomes were collected and reported.
Subgroup analysis and investigation of heterogeneity
If a sufficient number of studies were identified, we performed subgroup analyses for the following.
-
Cancer site.
-
Type of intervention (i.e. supervised, home‐based, etc).
-
Age of individuals (i.e. elderly versus non‐elderly).
-
Current treatment (currently undergoing treatment versus not currently undergoing treatment).
-
Participants with metastatic disease (metastatic cohort versus non‐metastatic cohort).
-
Accordance with behaviour change theory.
-
Interventions in obese individuals (mean body mass index (BMI) of intervention group > 30 kg/m2 versus mean BMI of intervention group < 30 kg/m2).
Sensitivity analysis
Methodological strength was judged using Cochrane's tool for assessing risk of bias to identify studies of high and low quality (Higgins 2011). Sensitivity analyses were performed with the studies of low quality excluded.
Summary of findings
To assess the overall quality of the evidence for each outcome of the meta‐analysis, we employed the GRADE approach. The GRADE profile (https://gradepro.org) enabled us to import data directly from Review Manager 5.3 to create summary of findings Table for the main comparison. These tables provide outcome‐specific information concerning the overall certainty of the evidence from studies included in the meta‐analysis. Risk of bias, inconsistency of the data, the preciseness of the data publication bias and the indirectness of the data were all considered in assessing the quality of the data.
We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation.
-
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
The following outcomes were included in the 'Summary of findings' table.
-
Aerobic exercise tolerance (all cancers: eight to 12 weeks of follow‐up)
-
Aerobic exercise tolerance (all cancers: eight to 12 weeks of follow‐up sensitivity analysis)
-
Aerobic exercise tolerance (all cancers: six months of follow‐up)
-
Aerobic exercise tolerance (breast cancer: eight to 12 weeks of follow‐up)
-
Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: eight to 12 weeks of follow‐up)
-
Aerobic exercise tolerance (all cancers: supervised exercise: eight to 12 weeks of follow‐up)
-
Aerobic exercise tolerance (all cancers: home‐based exercise: eight to 12 weeks of follow‐up)
Results
Description of studies
Please see Table 1, 'Summary of included studies'. See 'Characteristics of included studies'; 'Characteristics of excluded studies'; 'Characteristics of studies awaiting classification'; and 'Characteristics of ongoing studies'.
| Study | Exercise components | n | Meets Rock et al guidelines? | Adherence summary | At least 75% adherence? | High risk of bias? | Change in AET reported? | Adverse effects |
| Aerobic | 37, 38 (intervention vs control) | 33% reported 150 minutes/week of moderate intensity aerobic exercise at an average of 76% HR, for six months | 75% of women were doing between 90 and 119 minutes of moderate intensity aerobic activity per week at six months | Yes; for up to 119 minutes per week | No | Not reported | Five of the 37 women randomly assigned to exercise experienced an adverse effect; two were related to the study (plantar fasciitis) | |
| Aerobic | 34, 36, 38 (intervention, sham, control, respectively) | No | 77% of the exercise therapy; attended 70% (at least 17 of 24 sessions) or more of sessions | Unclear | Yes; outcome assessors were not blinded to participants’ group allocation | Yes | Three withdrawals in the intervention group: unclear as to why this occurred. Some withdrawals because of medical complications in placebo and control arms but unclear whether study related | |
| Aerobic | 13 intervention, 8 placebo stretching controls | Unclear | Participants in the intervention group averaged 3.6 days per week of aerobic exercise over an 8‐week period | Unclear | No | Yes | None reported | |
| Aerobic | 14, 13 (intervention vs control) | Unclear | Not reported | Not reported | Yes; method of measuring exercise and adherence not reported | Not reported | None reported | |
| Aerobic | 22,19 (intervention vs control). | No | Average weekly frequency of exercise was 2.4 ± 0.6 sessions, and average duration of exercise within prescribed target HR was 27.8 ± 8.1 minutes per session. Overall adherence was 78.3% ± 20.1% | Yes | Yes; data missing for 45% of the cohort | Yes | Reasons for withdrawal included personal problems (n = 2), problems at home (n = 2), problems related to chemotherapy (n = 3), thrombophlebitis in the lower leg (n = 2), non-exercise‐related injuries (n = 1), and death (n = 1). Unclear to which arm of the study these date relate | |
| Aerobic | 12, 12 (intervention vs control) | Unclear | Participants attended a mean of 88% of the 36‐session supervised exercise programme | Yes | Yes; 38% lost to follow‐up. Exercise tolerance test was performed but no control group comparison data were reported | Yes | None reported; however, it is unclear why the six controls dropped out | |
| Aerobic | 43, 43 (intervention vs control) | Unclear | At week 12, intervention participants reported a mean of 128.53 minutes/week of moderate intensity exercise. However, no changes were reported in the accelerometer data in the intervention group (change score = ‐0.33 kcal/hour) | Less than 75% of the intervention group was meeting the prescribed goal after week 4 | Yes; significantly more control group participants were receiving hormone treatment. Accelerometer data do not support the self‐reported physical activity behaviour | Yes | Not clear whether chest pain was related to exercise in dropout whose participation was terminated | |
| Aerobic | 20, 26 (intervention vs control) | Three‐day PAR questionnaire indicates that 64.7% of the intervention group and 40.9% of the control group were achieving the guidelines at three months | Correlation between self‐reported moderate intensity exercise and accelerometer data at three‐month follow‐up, when the only significant between‐group change is reported: r = 0.32 | No | Yes; accelerometer data were not reported; also, cited correlation is weak (0.32). Further, substantial contamination was noted in the control group | Yes | One cancer recurrence in the control group at three months | |
| Aerobic and resistance | 9, 9 (intervention vs control) | Six weeks of resistance exercise twice a week | 90% attendance at the supervised sessions. 94% of independent exercise sessions were completed | Yes | No | Yes | One stroke in the intervention group, unrelated to the exercise programme | |
| Aerobic and resistance | 16, 16 (intervention vs control) | Unclear | Most women (88%) allocated to the intervention group participated in 70% or more of scheduled supervised exercise sessions | Unclear | Yes; adherence data on unsupervised aspect of the intervention are not clear | No | None reported | |
| Aerobic and resistance | 7,7 (intervention vs control) | No | Unclear | Unclear | Yes; adherence to exercise not reported | Not reported | None reported | |
| Aerobic and resistance | Flexibility group (n = 13), aerobic group (n = 12), resistance group (n = 17), aerobic and resistance group (n = 13) | 12 weeks of resistance exercise two or three times per week | Mean percentages of adherence were as follows: flexibility = 85%, aerobic = 81%, resistance = 91% and aerobic plus resistance = 86% | Unclear | Yes; a significant number of dropouts belonged to the resistance exercise group (n = 8/13). Only 50% of activity logs were returned | Yes | Adverse effects were reported in two women during the study. In both cases, the women developed tendonitis: one in the shoulder and the other in the foot. Both had a history of tendonitis, and both received standard treatment | |
| Aerobic and resistance | 51 participants in total. Numbers randomly assigned to each arm are unclear | Three months of resistance exercise three times per week | Women assigned to the structured intervention completed an average of 83% of their scheduled hospital‐based exercise sessions (only 4 weeks in duration), and 76.9% completed all 12 sessions. Home‐based component (8 weeks in duration) | Unclear | Yes; numbers randomly assigned to intervention and control groups are unclear, as are numbers completing in each arm | Not reported | Unclear | |
| Aerobic | 7,7 (intervention vs control) | No | Adherence to per‐protocol exercise sessions was very high, ranging between 95% and 97%. | Yes | No | Yes | None reported | |
| Aerobic and resistance | 25,25 (intervention vs control) | Yes; 6 weeks of resistance exercise | Adherence was 94% for the supervised and 82% of the prescribed independent exercise sessions over the first 12 week. | Yes | Yes incomplete outcome data at 6 months. | Yes | None reported | |
| Aerobic | 10 in exercise intervention, 9 in delayed exercise control | 150 minutess per week of moderate‐vigorous aerobic exercise for 24 weeks. | Participants attended 88% of supervised gym sessions (mean 1.8 sessions/ week and 87.5 minutes/week), and participants met 82% of the prescribed exercise targets (mean intensity 74.5% HRR). Home session completion was 87% (mean 2.4 sessions/week and 101.5 minutes/week), and participants met 87% of the prescribed exercise targets (mean intensity 73.5% HRR) | Yes | Yes; Low study recruitment rate. | Yes | None reported | |
| Aerobic | 33,33 (intervention vs control) | Three sixty minute sessions per week for 8 weeks. | All intervention group completed more than 85% of the 24 water exercise sessions, showing a high adherence rate to the program. | Yes | No | Not reported | One participant in the intervention dropped out due to a recurrence of breast cancer during the program. Three women reported a transient increase of oedema, and four women noted an increase in fatigue immediately after the beginning of the first session, which improved in the next few days. These women did not dropout of the study. No other adverse effects were reported. | |
| Aerobic and resistance | 9, 8 (intervention vs control) | Yes; six weeks of resistance exercise. | Nine of the participants randomised to the EG, four (44%) adhered to exercise training by completing 15 or more training sessions (i.e.,≥60%). | No | Yes; missing patient data in both arms with no reasons given. | Yes | One participant completed four sessions and another completed six sessions. Both stopped training as they felt unwell. They completed some of the post‐intervention assessments and were later diagnosed with a primary cancer other than lung cancer. | |
| Aerobic and resistance | 15, 15 (intervention vs control) | Three sixty minute sessions per week for 12 weeks. | Vague statement: Two participants did not fulfil the required exercise | Unclear | Yes; Age differences between groups in baseline demographics were present. Adherence data is vague. | Not reported | None reported | |
| Aerobic | 15, 15 (intervention vs control) | No | Unclear | Unclear | Yes; No adherence data. | Unclear | Unclear | |
| Aerobic | 110, 112 (intervention vs control) | Yes | Adherence to the intervention was 98 % for supervised exercise sessions, 96 % for update sessions, and 91 % for discussion group sessions. | Yes | Yes; differences in objective and subjective measures of physical activity reported | Yes | Related expected adverse events in the intervention group included back or lower extremity musculoskeletal pain or injury (n = 14), heart rate monitor rash (n = 1), fall while walking (n = 1), breast reconstruction (n = 3), and chest pain during treadmill fitness test (n = 1) | |
| Aerobic and resistance | 47, 43 (intervention vs control) | Yes, six weeks of resistance exercise. | Adherence for the intervention group was 80% | Yes | No | Yes | None reported. | |
| Aerobic | 35, 30 (intervention vs control) | Yes | The exercise goal was 150 minutes/week of moderate intensity aerobic exercise; 33% of women | No | Yes; not all outcomes were reported and low recruitment rate. | Not reported | None reported. | |
| Aerobic and resistance | 61, 60 (intervention vs control) | Yes | Women randomly assigned to exercise also reported their exercise prospectively in daily activity logs and reported an average 119 minutes per week of aerobic exercise, with an average of 70% of strength‐training sessions completed. Women randomly assigned to exercise increased their physical activity by an average 159 minutes per week, compared with 49 minutes per week in the usual‐care group. | No | No | Yes | 5 participants had to discontinue the use of Atromatise inhibitors. |
AET = aerobic exercise tolerance.
Results of the search
Figure 1 illustrates the process of the literature search and study selection for the review. The updated search identified 5442 unique records from databases searched. In addition, we identified 2750 records from grey literature and 'snowballing' techniques for this update. Given that the details of prescribed exercise are rarely reported in manuscript abstracts (e.g. frequency, intensity, duration of exercise prescription), this led to evaluation of a large number of manuscripts at full text stage (n = 227). From these full‐text articles, 212 manuscripts were excluded, leaving 15 publications from 10 unique studies included in the review (total unique studies = 23). Reasons for excluding these 212 publications and a subset of the original review total (n = 377) are covered in the Excluded studies section below.

PRISMA flow diagram.
Included studies
This update identified 15 publications from 10 new studies, which when combined with the studies from the original review equates to 40 publications in total from 23 studies. (al‐Majid 2015; Bourke 2014; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Drouin 2005; Hayes 2009; Irwin 2015; Kaltsatou 2011; Kim 2006; Kim 2017; McKenzie 2003; Mohamady 2017; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). One study (Bourke 2014) was a efficacy study, following on from a previous feasibility study (Bourke 2011a) from the original review.
For the 2018 update, we sent an additional 112 emails to request unpublished information for manuscripts that were unclear in reporting relative to our inclusion and exclusion criteria. We were able to include an additional seven published manuscripts and to exclude an additional eight published manuscripts on the basis of information received in correspondence from authors.
Only randomised controlled trials (RCTs) were included in the review. All included studies used a parallel‐group design with baseline assessment and follow‐up of 12 months maximum. All included studies were conducted using participant‐level randomisation. The format of reporting precluded data extraction for meta‐analytical combination in two studies (Drouin 2005; Pinto 2003). Sample size ranged from 14 to 222, with a total of 1372 participants included in this review (mean age range 51 to 72).
Participants
Twenty of the included trials were on breast cancer survivors (al‐Majid 2015; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Daley 2007a; Drouin 2005; Hayes 2009; Irwin 2015; Kaltsatou 2011; Kim 2006; Kim 2017; McKenzie 2003; Mohamady 2017; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2005; Rogers 2015; Scott 2013; Thomas 2013); only two studies involved colorectal cancer (Bourke 2011a; Pinto 2011), one prostate cancer (Bourke 2014,) and one lung cancer (Cavalheri 2017). Of these studies, 12 included participants who were currently undergoing active treatment inclusive of hormone‐based therapy (al‐Majid 2015; Bourke 2014; Cadmus 2009; Daley 2007a; Drouin 2005; Irwin 2015; Kim 2006; Mohamady 2017; Musanti 2012; Perna 2010; Pinto 2005; Scott 2013). We found only one study that reported data from participants with metastatic disease (Bourke 2014), and six studies that were conducted in obese cohorts (i.e. mean BMI > 30 kg/m2; (Cadmus 2009; Drouin 2005; Mohamady 2017; Rogers 2015; Scott 2013; Thomas 2013). The majority of participants were white, and only five studies reported data from an ethnically diverse sample (al‐Majid 2015; Irwin 2015; Perna 2010; Rogers 2015; Thomas 2013). Comorbidities at baseline were largely unclear or unreported.
Interventions
Type of exercise
Fourteen studies prescribed exclusively aerobic exercise (al‐Majid 2015; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Daley 2007a; Drouin 2005; Kaltsatou 2011; Kim 2006; Mohamady 2017; Pinto 2003; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013); the remaining RCTs used a mix of aerobic and resistance training (no exclusively resistance training studies met our inclusion criteria). Ten studies used a combination of supervised and home‐based exercise (Bourke 2011a; Bourke 2014; Cadmus 2009; Campbell 2017; Hayes 2009; Irwin 2015; Kim 2006; Perna 2010; Pinto 2003; Rogers 2015), four studies opted to use an exclusively home‐based design (Drouin 2005; Musanti 2012; Pinto 2005; Pinto 2011), and 10 studies were exclusively supervised studies (al‐Majid 2015; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Kaltsatou 2011; Kim 2017; McKenzie 2003; Mohamady 2017; Scott 2013; Thomas 2013).
Exercise sessions and the role of exercise professionals and healthcare professionals
Contact with exercise professionals or study researchers ranged from two to three weekly supervised sessions (Rogers 2015), to weekly phone calls after an initial one‐to‐one exercise consultation (Pinto 2005; Pinto 2011). Most commonly however, supervised sessions were offered two to three times per week. Of note, seven studies (Drouin 2005; Kaltsatou 2011; Kim 2006; McKenzie 2003; Pinto 2003; Pinto 2005; Pinto 2011), placed restrictions on the control group regarding exercise behaviour during the course of the study, usually taking the form of direct instruction to refrain from changing exercise behaviour. However, the 2018 update found no additional studies that placed restrictions on the control group, usual activities were encouraged. Contact with healthcare professionals was not frequent amongst the studies, with three studies having healthcare professionals carry out medical assessments for eligibility (Cantarero‐Villanueva 2012b; Kim 2017; Mohamady 2017), two studies having oncologists refer the participants onto the study, but it was not stated explicitly if they delivered any aspects of the intervention (al‐Majid 2015; Campbell 2017).
Level of exercise and adherence
Thirteen studies incorporated prescriptions that would meet the Rock 2012 recommendations for aerobic exercise (i.e.150 minutes per week); (Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Pinto 2011; Rogers 2015) or resistance exercise (i.e. resistance training strength training exercises at least two days per week); (Bourke 2011a; Bourke 2014; Cavalheri 2017; Irwin 2015; Kim 2017; Musanti 2012; Perna 2010; Scott 2013). However, only eight of these studies reported 75% adherence to these guidelines, (Bourke 2011a; Bourke 2014; Campbell 2017; Cantarero‐Villanueva 2012b; Irwin 2015; Kim 2017; Rogers 2015; Scott 2013).
Theoretical basis
Of the interventions provided, only six were explicitly based on a theoretical model (Daley 2007a; Musanti 2012; Perna 2010; Pinto 2005; Pinto 2011; Rogers 2015); the trans‐theoretical model was most common, followed by social cognitive theory and exercise, and self‐esteem theory. Only one intervention from the 2018 update was found to be based on a theoretical model (Rogers 2015).
Behaviour change techniques (BCT) and adherence
Full details of intervention BCT coding according to the CALO‐RE taxonomy for the previous review (Bourke 2013) and the 2018 update can be seen in Table 2 and Table 3 (respectively). In the previous review, there was a lack of identified studies that met the Rock 2012 guidelines. For this updated version of the review, our searches found more instances of studies (eight in total) that meet the 150 minutes per week or two strength sessions per week Rock 2012 target. Also, there were other studies with lower exercise targets but good adherence (i.e. over 75%). Hence, we presented BCTs in a hierarchy format: Tier 1 and Tier 2. Tier 1 BCTs are presented from interventions that set prescriptions which meet the Rock 2012 target and achieved 75% or more adherence. Tier 2 BCTs are presented from interventions that reported good adherence (i.e. 75% or more) but set prescriptions that are below the 150 minutes per week Rock 2012 target. BCTs reported in the eight Tier 1 trials are presented in Table 4. It is notable that four of these studies incorporated both a supervised and an independent exercise component as part of their intervention and four were exclusively supervised, with all placing no restrictions on the control group in terms of exercise behaviour. Six studies were included in Tier 2 BCTs and reported adherence of 75% or greater to a specified exercise aerobic prescription which was lower than the targets set in the Rock 2012 guidelines (al‐Majid 2015; Bourke 2011a; Bourke 2014; Cadmus 2009; Kim 2017; Scott 2013). BCTs reported in Tier 2 studies are presented in Table 5.
| Behaviour change technique | YALE | ||||||||||||
| Theory | TTM | EXSEM | TTM | TTM | TTM SCT | ||||||||
| 1. Provide Info on consequences of behaviour in general | X | X | X | X | |||||||||
| 2. Provide Info on consequences of behaviour to the individual | |||||||||||||
| 3. Provide Info about others' approval | |||||||||||||
| 4. Provide normative info about others' behaviour | |||||||||||||
| Programme set goal | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 5. Goal setting (behaviour) | X | X | X | X | X | X | |||||||
| 6. Goal setting (outcome) | |||||||||||||
| 7. Action planning | |||||||||||||
| 8. Barrier identification/Problem solving | X | X | X | X | X | ||||||||
| 9. Setting of graded tasks | X | X | X | X | X | X | X | X | X | ||||
| 10. Prompt review of behavioural goals | X | X | |||||||||||
| 11. Prompt review of outcome goals | |||||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | X | X | X | ||||||||||
| 13. Provide rewards contingent on successful behaviour | X | ||||||||||||
| 14. Shaping | |||||||||||||
| 15. Prompt generalisation of a target behaviour | X | X | X | X | |||||||||
| 16. Prompt self‐monitoring of behaviour | X | X | X | X | X | X | X | X | X | ||||
| 17. Prompt self‐monitoring of behavioural outcome | X | X | X | X | X | X | |||||||
| 18. Prompt focus on past success | X | ||||||||||||
| 19. Feedback on performance provided | X | X | X | X | |||||||||
| 20. Information provided on where and when to perform behaviour | X | X | |||||||||||
| 21. Instruction provided on how to perform the behaviour | X | X | X | X | X | X | X | X | X | ||||
| 22. Modelling/Demonstration of behaviour | X | X | X | ||||||||||
| 23. Teaching to use prompts/cues | X | X | X | ||||||||||
| 24. Environmental restructuring | X | X | |||||||||||
| 25. Agreement on behavioural contract | X | ||||||||||||
| 26. Prompt practise | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 27. Use of follow‐up prompts | X | ||||||||||||
| 28. Facilitating social comparison | |||||||||||||
| 29. Planning social support/social change | X | X | X | ||||||||||
| 30. Prompt identification as role model/position advocate | |||||||||||||
| 31. Prompt anticipated regret | |||||||||||||
| 32. Fear arousal | |||||||||||||
| 33. Prompt self‐talk | |||||||||||||
| 34. Prompt use of imagery | |||||||||||||
| 35. Relapse prevention/coping planning | X | X | |||||||||||
| 36. Stress management/emotional control training | X | ||||||||||||
| 37. Motivational interviewing | |||||||||||||
| 38. Time management | |||||||||||||
| 39. General communication skills training | |||||||||||||
| 40. Stimulation of anticipation of future rewards |
EXSEM = exercise self‐esteem model; SCT = social cognitive theory; TTM = trans‐theoretical model.
| Behaviour change technique | |||||||||||
| Theory | SCT | ||||||||||
| 1. Provide Info on consequences of behaviour in general | x | x | |||||||||
| 2. Provide Info on consequences of behaviour to the individual | |||||||||||
| 3. Provide Info about others' approval | |||||||||||
| 4. Provide normative info about others' behaviour | |||||||||||
| Programme set goal | x | x | x | x | x | x | x | x | x | x | x |
| 5. Goal setting (behaviour) | x | x | |||||||||
| 6. Goal setting (outcome) | |||||||||||
| 7. Action planning | |||||||||||
| 8. Barrier identification/Problem solving | x | x | |||||||||
| 9. Setting of graded tasks | x | x | x (implicit) | x | x | x | x | x | x | x | |
| 10. Prompt review of behavioural goals | x | ||||||||||
| 11. Prompt review of outcome goals | |||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | x | ||||||||||
| 13. Provide rewards contingent on successful behaviour | |||||||||||
| 14. Shaping | |||||||||||
| 15. Prompt generalisation of a target behaviour | x (from linked paper Gilbert 2016* | x | |||||||||
| 16. Prompt self‐monitoring of behaviour | |||||||||||
| 17. Prompt self‐monitoring of behavioural outcome | x | x | x | ||||||||
| 18. Prompt focus on past success | |||||||||||
| 19. Feedback on performance provided | x | ||||||||||
| 20. Information provided on where and when to perform behaviour | x | x | |||||||||
| 21. Instruction provided on how to perform the behaviour | x | x | x | ||||||||
| 22. Modelling/Demonstration of behaviour | x | ||||||||||
| 23. Teaching to use prompts/cues | |||||||||||
| 24. Environmental restructuring | |||||||||||
| 25. Agreement on behavioural contract | |||||||||||
| 26. Prompt practise | x | x | |||||||||
| 27. Use of follow‐up prompts | |||||||||||
| 28. Facilitating social comparison | |||||||||||
| 29. Planning social support/social change | x | ||||||||||
| 30. Prompt identification as role model/position advocate | |||||||||||
| 31. Prompt anticipated regret | |||||||||||
| 32. Fear arousal | |||||||||||
| 33. Prompt self‐talk | |||||||||||
| 34. Prompt use of imagery | |||||||||||
| 35. Relapse prevention/coping planning | x | ||||||||||
| 36. Stress management/emotional control training | |||||||||||
| 37. Motivational interviewing | |||||||||||
| 38. Time management | |||||||||||
| 39. General communication skills training | |||||||||||
| 40. Stimulation of anticipation of future rewards |
| BCT | |||||||||
| Resistance | Aerobic | Aerobic | Resistance | Aerobic | Resistance | Resistance | Aerobic | Frequency of BCTs | |
| Programme set goal | x | x | x | x | x | x | x | x | 8 |
| 9. Setting of graded tasks | x | x | x | x | x | x | x | 7 | |
| 21. Instruction provided on how to perform behaviour | x | x | x | 3 | |||||
| 26. Prompt practise | x | x | 2 | ||||||
| 5. Goal setting (outcome) | x | x | 2 | ||||||
| 8. Barrier identification/problem solving | x | x | 2 | ||||||
| 1. Provide information on consequences of behaviour in general | x | x | 2 | ||||||
| 15. Prompt generalisation of a target behaviour | x | x | 2 | ||||||
| 17. Prompt self‐monitoring of behavioural outcome | x | x | 2 | ||||||
| 20. Information provided on where and when to perform behaviour | x | x | 2 | ||||||
| 19. Feedback on performance provided | x | 1 | |||||||
| 22. Modelling/demonstration of behaviour | x | 1 | |||||||
| 12. Prompt rewards contingent on effort or progress towards goal | x | 1 | |||||||
| 29. Planning social support/social | x | 1 | |||||||
| 35. Relapse prevention/coping planning | x | 1 |
BCTs: behaviour change techniques
| BCT | |||||||
| Frequency of BCTs | |||||||
| Programme set goal | x | x | x | x | x | x | 6 |
| 9. Setting of graded tasks | x | x | x | x | x | 5 | |
| 21. Instruction provided on how to perform behaviour | x | x | x | 3 | |||
| 1. Provide information on consequences of behaviour in general | x | x | 2 | ||||
| 26. Prompt practise | x | x | 2 | ||||
| 8. Barrier identification/problem solving | x | x | 2 | ||||
| 15. Prompt generalisation of a target behaviour | x | x | 2 | ||||
| 5. Goal setting (outcome) | x | 1 | |||||
| 16. Prompt self‐monitoring of behaviour | x | 1 | |||||
| 17. Prompt self‐monitoring of behavioural outcome | x | 1 | |||||
| 20. Information provided on where and when to perform behaviour | x | 1 | |||||
| 29. Planning social support/social | x | 1 | |||||
| 27. Use of follow‐up prompts | x | 1 |
BCTs: behaviour change techniques
Few interventions (Bourke 2014; Cadmus 2009; Daley 2007a; Kim 2006; Perna 2010; Rogers 2015) reported providing information on the consequences of behaviour (BCT #1). All interventions had programme set goals, which we have highlighted as being different for the purpose of this review to goal setting (behaviour) and goal setting (outcome). Only seven studies set exercise goals in conjunction with participants (BCT # 5) (Bourke 2014; Cadmus 2009; Daley 2007a; Perna 2010; Pinto 2005; Pinto 2011; Rogers 2015). These same seven studies also reported problem‐solving with barriers identified (BCT #8) and solutions facilitated. Three interventions (Daley 2007a; Perna 2010; Rogers 2015) which participants had some input into setting of goals were these reviewed (BCT #10). When monitoring did occur (BCT #16) or monitoring of outcome behaviour occurred (BCT #17), feedback on performance (BCT #19) was provided in only five out of 10 (Cadmus 2009; Perna 2010; Pinto 2005; Pinto 2011; Rogers 2015), which is important to note. Fourteen studies (Bourke 2011a; Bourke 2014; Cadmus 2009; Daley 2007a; Drouin 2005; Hayes 2009; Kaltsatou 2011; Kim 2006; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2011; Rogers 2015; Scott 2013) reported providing instruction on how to perform the behaviour (BCT #21), although it may be anticipated that this did occur but just was not reported. In addition, 15 studies prompted practise of the behaviour (BCT #26) (Bourke 2011a; Bourke 2014; Cadmus 2009; Daley 2007a; Drouin 2005; Hayes 2009; Kaltsatou 2011; Kim 2006; McKenzie 2003; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2005; Pinto 2011; Rogers 2015), Only four studies used techniques to increase social support (BCT #29); (Bourke 2014; Cadmus 2009; Daley 2007a; Perna 2010). Other common BCTs included setting of graded tasks (i.e. increased exercise duration or intensity over time) and self‐monitoring of behaviour (exercise) and outcomes of behaviour (e.g. heart rate), although it is not clear for all interventions whether this was done primarily for data collection or as a mechanism of behaviour change.. Only three studies reported relapse prevention (BCT #35) (Daley 2007a; Perna 2010; Rogers 2015).
Measurement of exercise behaviour
Ten studies were identified that attempted to objectively validate independent exercise behaviour with accelerometers or heart rate monitoring (al‐Majid 2015; Bourke 2014; Cadmus 2009; Irwin 2015; Mohamady 2017; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). Seven of these studies attempted to validate self‐reported independent exercise behaviour by using accelerometers or heart rate monitors (al‐Majid 2015; Bourke 2014; Irwin 2015; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013), however in three studies (Pinto 2005; Pinto 2011; Rogers 2015), data either were not supportive of exercise behaviour recorded by participants or were not reported in their entirety.
Excluded studies
Reasons for excluding published studies included the following.
-
Non‐RCTs (e.g. review manuscripts, comment/editorial articles).
-
Mixed cancer cohorts or cohorts that included non‐cancer populations.
-
Studies that failed to describe essential metrics of exercise prescription used in the intervention (e.g. frequency, intensity, duration).
-
Studies involving active participants at baseline.
-
Studies involving hospital inpatients.
-
Interventions that provided follow‐up of less than 6 weeks.
-
Studies involving participants younger than 18 years of age.
All excluded studies (N = 180) for the 2018 update, are presented in the Characteristics of excluded studies. However for the original review only a subset of excluded studies could be included in the 'Characteristics of excluded studies' section. This is a result of the large volume of studies that had to be full text screened (N = 402) and the high proportion (around 90%) that were excluded. In accordance with editorial advice, we divided this large number (N = 365) into initially unclear studies that required further investigation (N = 76) and those that clearly were not eligible after full text had been retrieved (N = 289). This approach is analogous to the approach adopted in recent reviews (Galway 2012), and is detailed in the existing PRISMA diagram (Figure 1).
For the 2018 update, we sent an additional 101 emails to corresponding authors to request additional information (regarding included studies, excluded studies and studies that we could not access) to determine eligibility and to supplement published data for this review.
Risk of bias in included studies
Only seven studies were judged not to include a high risk of bias (al‐Majid 2015; Bourke 2011a; Cadmus 2009; Cantarero‐Villanueva 2012b; Drouin 2005; Irwin 2015; Scott 2013). Full results of the methodological quality assessment for allocation bias, blinding, incomplete data outcome and selective reporting (along with justifications) are covered in the 'Risk of bias' tables for each study and are illustrated in Figure 2; Figure 3. Twelve studies stated that an intention‐to‐treat analysis was used (Bourke 2011a; Bourke 2014; Cadmus 2009; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Irwin 2015; Perna 2010; Pinto 2005; Rogers 2015; Scott 2013; Thomas 2013).

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Eleven studies had an unclear risk in their description of concealment in randomisation allocation. However, no study was judged to have a high risk of bias in this respect.
Blinding
Eleven studies had undertaken the blinding of study assessors (Bourke 2011a; Bourke 2014; Cantarero‐Villanueva 2012b; Cavalheri 2017; Hayes 2009; Kim 2017; Musanti 2012; Perna 2010; Rogers 2015; Scott 2013; Thomas 2013). The remaining studies did not include enough information for the review authors to make a definitive judgement on this criterion.
Incomplete outcome data
Five studies were judged to have been subject to incomplete data outcome bias: Kim 2006 reported data from only 41 of 74 participants randomly assigned; Musanti 2012 reported that 13 women (24%) did not complete their assigned 12‐week programme; and Pinto 2003 did not report control group data for the exercise tolerance test. Bourke 2014 had incomplete outcome data at six months follow‐up. Cavalheri 2017 reported missing patient data in both arms with no reasons given.
Selective reporting
Most studies reported all listed outcomes; however, four studies were judged to omit outcomes from their results reporting. Musanti 2012 did not report waist and upper, mid and lower arm circumference outcomes; Pinto 2003 reported none of the physiological assessments in the control group at 12 weeks of follow‐up; Pinto 2011 did not report data derived from the use of accelerometers; and Thomas 2013 did not report on body fat or lean mass values and no data were given from food frequency questionnaire.
Other potential sources of bias
Other sources of bias found in the included studies that are worth highlighting include adherence data missing or not clear (Cavalheri 2017; Hayes 2009; Kaltsatou 2011; Kim 2017; McKenzie 2003; Mohamady 2017; Musanti 2012); high attrition at follow‐up (Pinto 2003); low recruitment rate (Bourke 2011a; Campbell 2017; Thomas 2013); Significant differences in participants excluded from study analysis/dropouts (Kim 2006; Musanti 2012; Pinto 2003); numbers randomly assigned to study arms with study completion rate unclear (Perna 2010); significant differences in cohorts at baseline (Kim 2017; Musanti 2012; Pinto 2003; Pinto 2005); and inconsistencies between objective and subjective measures of exercise behaviour (Pinto 2005; Pinto 2011; Rogers 2015). Insufficient information was reported to permit a judgement about any single element of bias because of lack of data (al‐Majid 2015; Bourke 2011a; Cadmus 2009; Campbell 2017; Drouin 2005; Hayes 2009; Irwin 2015; Kaltsatou 2011; Kim 2006; McKenzie 2003; Mohamady 2017; Pinto 2003; Pinto 2005; Pinto 2011).
Effects of interventions
Primary outcome
To assess the effects of interventions designed to promote exercise behaviour in sedentary people living with and beyond cancer
Please see Table 1, 'Summary of included studies'. As it is not meaningful to interpret individually the component metrics of aerobic (frequency, intensity and duration) or resistance exercise (frequency, intensity, type of exercise, sets and repetitions) behaviour, these primary outcomes are presented in the narrative synthesis below of interventions achieving 75% or greater adherence.
In Rogers 2015, adherence to planned intervention components was 98% for supervised exercise sessions, 96% for update sessions, and 91% for discussion group sessions. Only five participants did not receive the allocated intervention (i.e. did not complete 75 % of all intervention components combined). With the intervention group reporting an average of 169 minutes of moderate intensity exercise per week at 12 weeks, and 137 minutes of moderate intensity exercise per week at six months. Although, there is a high risk of bias around how 'in‐active' the recruited participants were at baseline, as baseline accelerometer recordings are incongruent with the inclusion criteria.
Four of the studies included in this review reported that 75% or more of the intervention group met the (Rock 2012) aerobic exercise guidelines at any given follow‐up (Campbell 2017; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015). Four studies reported that 75% or more of the intervention group met the (Rock 2012) resistance exercise guidelines. (Bourke 2011a; Bourke 2014; Kim 2017; Scott 2013). Behaviour change techniques (BCTs) reported in these eight studies are presented in Table 4. Of these studies, only one study explicitly stated it had theoretical basis (Rogers 2015).
Due to of unclear reporting it was not possible to make a judgement on whether some trials achieved adherence of 75% or greater . Reasons for an unclear judgement or unsuccessful adherence are detailed below.
-
Daley 2007a: judgement unclear; adherence reported as a proportion of participants attending a proportion of set exercise sessions (i.e. 77% of the intervention group attending 70% of sessions).
-
Drouin 2005: judgement unclear; adherence reported as mean number of days per week when exercise was undertaken, relative to a range within the prescription (i.e. 3.6 days per week, when the prescription was for three to five days per week).
-
Kaltsatou 2011: judgement unclear; no adherence data reported.
-
Kim 2006: judgement unclear; high adherence was reported (78%), but in tandem with substantial attrition (i.e. data missing for 45% of the cohort).
-
Pinto 2003: judgement unclear; high adherence was reported (88%) but in tandem with substantial attrition (i.e. 25% of the intervention group dropped out over the intervention period).
-
Pinto 2005: judgement unsuccessful; 75% adherence threshold was not met after week four.
-
Pinto 2011: judgement unsuccessful; three‐day Physical Activity Recall (PAR) questionnaire indicates that 64.7% of the intervention group and 40.9% of controls were adhering to the exercise guidelines at three months.
-
Hayes 2009: judgement unclear; adherence reported as a proportion of participants attending a proportion of set exercise sessions (i.e. 88% allocated to the intervention group participated in 70% or more of scheduled supervised exercise sessions). Further, adherence from the unsupervised aspect is not reported.
-
McKenzie 2003: judgement unclear; no adherence data reported.
-
Musanti 2012: judgement unclear; high adherence reported but only 50% of activity logs returned.
-
Perna 2010: judgment unclear; women assigned to the structured intervention completed an average of 83% of their scheduled hospital‐based exercise sessions (four weeks in total). Home‐based adherence is not clear.
-
Mohamady 2017: judgement unclear; no adherence data reported.
-
Thomas 2013: judgement unsuccessful; the goal of the intervention was for participants to achieve 150 minutes of moderate intensity exercise per week; 33% of the intervention group achieved 150 minutes per week, 56% of the intervention group achieved 120 minutes per week and 75% achieved 90 minutes per week.
-
Irwin 2015: judgement unsuccessful; resistance exercise ‐ an average of 70% of strength‐training sessions completed.
-
Cavalheri 2017: judgement unsuccessful; of the nine participants randomised to the exercise intervention, four (44%) adhered to exercise training by completing 15 or more training sessions (i.e.≥60%).
Ideally, a meta‐analysis of objectively verified (e.g. using accelerometers or heart rate monitoring) minutes per week of moderate intensity aerobic exercise achieved in an intervention group, compared with controls, for whom the exercise prescription adherence is at least 75%, would be most informative. However, due to variation in measurement and reporting amongst included studies, this was not possible. Insufficient data were available for a synthesis of evidence to be conducted around free‐living energy expenditure.
Secondary outcomes
Aerobic exercise tolerance
1.1 All cancers: eight to 12 weeks of follow‐up
A meta‐analysis of change in aerobic exercise tolerance was carried out on 10 studies that reported these outcomes and also reported means for final value scores. Standardised mean differences (SMDs) were used to produce effect estimates as variation in how studies assessed this outcome was evident. Standard deviations (SDs) were calculated from 95% confidence intervals (CIs) using the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (i.e. SD = √N * (upper limit‐lower limit)/(t distribution *2), and from standard errors (SEs) using SD = SE*√N, when they were not reported. Length of follow‐up ranged from eight (Cavalheri 2017; Daley 2007a; Kim 2006), to 12 weeks (al‐Majid 2015; Bourke 2011a; Bourke 2014; Musanti 2012; Pinto 2005; Pinto 2011; Rogers 2015) . Aerobic exercise tolerance was significantly better in intervention versus control groups in 604 participants: (SMD 0.54, 95% CI 0.37 to 0.70; 10 studies, 604 participants: low‐certainty evidence; Analysis 1.1). Results were analysed using a fixed‐effect model. Certainty of the evidence assessed using GRADE and was graded as low. As there was some serious concerns over risk of bias and concerns over the number of small studies with positive results (please see summary of findings Table for the main comparison).
1.2 All cancers: eight to 12 weeks of follow‐up (sensitivity analysis)
We then removed studies with a high risk of bias relative to this outcome and repeated the analysis with the four remaining studies (al‐Majid 2015; Bourke 2011a; Bourke 2014; Pinto 2005), and aerobic exercise tolerance was better in intervention versus control groups (SMD 0.85, 95% CI 0.56 to 1.14; 4 studies, 201 participants; low‐certainty evidence; Analysis 1.2). The certainty of the evidence was graded as low using GRADE as there were concerns imprecision as there were variations in effect sizes and concerns over the low number of participants in the studies (please see summary of findings Table for the main comparison).
1.3 All cancers: six months of follow‐up
Seven studies included data from a follow‐up of six months (Bourke 2014; Daley 2007a; Kaltsatou 2011; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013) showing that aerobic exercise tolerance was significantly better at six months in intervention versus control groups (SMD 0.56, 95% CI 0.39 to 0.72; 7 studies; 591 participants: low‐certainty evidence; Analysis 1.3). It should be highlighted that six of these studies have a high risk of bias, which could affect this outcome at six months; specifically, Bourke 2014 had high attrition at six months follow‐up; no adherence data in the Kaltsatou 2011 study; substantial contamination among controls in the Pinto 2011 study; Rogers 2015 objective and subjective measures of exercise results varied greatly and non‐blinded assessors in the Daley 2007a study. Note that in all meta‐analyses, data from Pinto 2005 have been multiplied by ‐1 to control for direction of effect (i.e. lower values in a timed test indicate a better outcome). Brief narrative descriptions of studies not suitable for meta‐analyses include the following: Drouin 2005 VO2 peak data are reported as medians and interquartile ranges; for Pinto 2003, no control group data are presented for the exercise tests; for Campbell 2017, no means or SDs present at baseline and follow‐up. For grading of data please see summary of findings Table for the main comparison.
1.4 Breast cancers: eight to 12 weeks of follow‐up
We were able to carry out one subgroup analysis in breast cancer patients This was a meta‐analysis of change in aerobic exercise tolerance carried out on six studies (al‐Majid 2015; Daley 2007a; Kim 2006; Musanti 2012; Pinto 2005; Rogers 2015), showing that aerobic exercise tolerance was significant (SMD 0.57, 95% CI 0.22 to 0.93; 6 studies, 441 participants; very low‐certainty evidence; Analysis 1.4). However, it should be noted that four of the studies were considered to have high risk of bias (Daley 2007a; Kim 2006; Musanti 2012; Rogers 2015). The certainty of the evidence was graded as very low as there was high risk of bias, concerns over the precision of the data as the confidence intervals were wide and there were serious concerns over the heterogeneity of the data (please see summary of findings Table for the main comparison).
1.5 All cancers: combination of supervised and home‐based exercise: eight to 12 weeks of follow‐up
A meta‐analysis of aerobic exercise tolerance was carried out in the following subgroups: supervised exercise interventions, home‐based interventions, and a combination of both. In a combination of home‐based and supervised exercise interventions (Bourke 2014; Bourke 2011a; Kim 2006; Rogers 2015), aerobic exercise tolerance was better in the intervention than the control : (SMD 0.53, 95% CI 0.01 to 1.04; 4 studies, 357 participants; very low‐certainty evidence; Analysis 1.5). The certainty of the evidence was graded as very low as there were concerns over the precision of the data as the confidence intervals were wide and there were very serious concerns over inconsistency due to the heterogeneity of the data and variations in effect sizes (please see summary of findings Table for the main comparison).
1.6 All cancers: home‐based exercise: eight to 12 weeks of follow‐up
In home‐based interventions (Musanti 2012; Pinto 2005; Pinto 2011), aerobic exercise tolerance was better in the intervention than the control (SMD 0.70, 95% CI 0.37 to 1.03: 3 studies, 155 participants; very low‐certainty evidence; Analysis 1.6). The certainty of the evidence was graded as very low due to high risk of bias, low number of participants within the studies and wide confidence intervals (please see summary of findings Table for the main comparison).
1.7 All cancers: supervised exercise: eight to 12 weeks of follow‐up
In supervised interventions (al‐Majid 2015; Cavalheri 2017; Daley 2007a), aerobic exercise tolerance was better in the intervention group versus control (SMD 1.07, 95% CI 0.26 to 1.89; 3 studies, 92 participants; very low‐certainty evidence; Analysis 1.7). Serious concerns with inconsistency and imprecision were presented due to wide variations in effect sizes and wide confidence intervals. Therefore the certainty of evidence was classed as very low (please see summary of findings Table for the main comparison).
1.8 All cancers: undergoing active treatment: eight to 12 weeks of follow‐up
A meta‐analysis of active treatment and no current treatment for aerobic exercise tolerance was carried out. For participants undergoing active treatment in six studies, (al‐Majid 2015; Bourke 2014; Daley 2007a; Kim 2006; Musanti 2012; Pinto 2005), demonstrated aerobic exercise tolerance was better in the intervention than the control (SMD 0.72, 95% CI 0.49 to 0.95; 6 studies, 313 participants; Analysis 1.8). However, five of these studies had a high risk of bias so interpretation of these results should be done with caution.
1.9 All cancers: no active treatment: eight to 12 weeks of follow‐up
A meta‐analysis of aerobic exercise tolerance in participants not undergoing active treatment was carried out in four studies (Bourke 2011a; Cavalheri 2017; Pinto 2011; Rogers 2015), showing that aerobic exercise tolerance was better in the intervention than the control (SMD 0.61, 95% CI 0.10 to 1.12; 4 studies, 291 participants: Analysis 1.9).
Sketal muscle strength
2.1 All cancers: eight to 12 weeks of follow‐up
Four studies that used resistance exercise as a component of the intervention reported changes in lower‐ (Bourke 2011a; Rogers 2015 ) and upper limb (Musanti 2012;Kim 2017) strength. All four studies had reported strength changes at 12 weeks of follow‐up. No significant improvement in strength was found (SMD 0.20, 95% CI ‐0.03 to 0.44; 4 studies, 278 participants: Analysis 2.1).
2.2 All cancers: eight to 12 weeks of follow‐up
After two studies was removed for high risk of bias (Kim 2017; Musanti 2012), effect estimates remained non‐significant (SMD 0.17, 95% CI ‐0.09 to 0.43; 2 studies, 231 participants; Analysis 2.2).
Planned subgroup analysis was not possible according to participant age, presence of metastatic disease, theoretical underpinning of interventions or participant body mass index (BMI).
Adverse effects
Thirteen studies reported adverse effects (Bourke 2011a; Bourke 2014; Cadmus 2009; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Irwin 2015; Kim 2006; Musanti 2012; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013); these ranged from minor (e.g. musculoskeletal problems; Musanti 2012; Rogers 2015), to major events (e.g. death; Kim 2006). However, only five studies (Cadmus 2009; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015; Thomas 2013) were explicit as to which of these adverse effects were caused by inclusion of the participant in the intervention group (two instances of plantar fasciitis).
Study recruitment rate
Study recruitment rate ranged from 9.5% (Thomas 2013) to 94% (Thomas 2013, Cantarero‐Villanueva 2012b, respectively). Eleven studies reported a priori sample size estimates (Bourke 2014; Cadmus 2009; Campbell 2017; Daley 2007a; Hayes 2009; Kaltsatou 2011; Musanti 2012; Perna 2010; Pinto 2003; Pinto 2011; Scott 2013), and seven (Bourke 2014; Cadmus 2009; Cantarero‐Villanueva 2012b; Hayes 2009; Perna 2010; Rogers 2015; Scott 2013) met their recruitment target.
Intervention attrition rate
Fifteen studies produced CONSORT diagrams (al‐Majid 2015; Bourke 2011a; Bourke 2014; Cadmus 2009; Campbell 2017; Cantarero‐Villanueva 2012b; Cavalheri 2017; Daley 2007a; Irwin 2015; Kim 2017; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). Intervention attrition rates from the included studies ranged from 0% to 25% (Campbell 2017, Pinto 2003) ,respectively, with seven studies not clearly reporting attrition in the intervention arm (Cavalheri 2017; Kaltsatou 2011; Kim 2006; McKenzie 2003; Mohamady 2017; Musanti 2012; Perna 2010).
Discussion
Summary of main results
In this review update we have found more evidence that there are interventions that meet the Rock 2012 guidelines for aerobic (Campbell 2017; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015) and resistance (Bourke 2011a; Bourke 2014; Kim 2017; Scott 2013) exercise with 75% adherence in previously inactive cancer cohorts. We have identified a hierarchy of the most common behaviour change techniques (BCTs) that feature in these studies (Table 4). The most frequent of these interventions were setting of graded tasks (#BCT 9), programme set goal and instruction of how to perform behaviour (#21). These studies were predominantly exclusively supervised studies or a combination of supervised and home‐based studies. Supervision usually consisted of contact with the exercise professional or research team at least twice weekly. However, from our review of studies at full‐text screening stage, it is still true that adherence to exercise interventions, which is crucial for understanding treatment dose, is frequently either poorly reported or not reported at all in randomised controlled trials (RCTs).
Despite the uncertainty surrounding adherence in many of the included studies, interventions caused improvement in aerobic exercise tolerance at eight to 12 weeks (Analysis 1.1) in intervention participants compared with controls. There is also evidence that this can be sustained at six months of follow‐up, but owing to potential high risk of bias, this should be viewed with caution (Analysis 1.3). We were able to carry out one cancer subgroup analysis in breast cancer patients, showing that aerobic exercise tolerance was significantly improved up to 12 weeks (Analysis 1.4), however caution is again warranted when interpreting the result, due to frequent high risk of bias.
Adverse effects these ranged from minor e.g. musculoskeletal problems ( Musanti 2012; Rogers 2015) to major events e.g. death (Kim 2006). However, only five studies were explicit as to which of these adverse effects were caused by inclusion of the participant in the intervention group (two instances of plantar fasciitis) (Cadmus 2009; Cantarero‐Villanueva 2012b; Irwin 2015; Rogers 2015; Thomas 2013).
Overall completeness and applicability of evidence
We included 23 studies in this systematic review, all of which were RCTs. These studies randomly assigned 1372 participants to exercise or comparison groups. A large majority of these studies included women with breast cancer, two involved colorectal cancer survivors, one involved men with advanced prostate cancer, and one involved lung cancer survivors. As found in the original review (Bourke 2013), although these four primary cancers account for most of the population living with and beyond cancer, other common cancers such as lymphoma do not appear at all in this review and less common cancers also are not represented in the evidence base.
Furthermore, an overwhelming majority of participants were white, and only five studies included an ethnically diverse population. As such, other ethnicities are still substantially underrepresented, as found previously (Bourke 2013). Comorbidities were rarely reported at baseline and only six studies were carried out in obese cohorts. Although we set a limit in this review of 90 minutes per week of moderate‐intensity exercise at baseline as the criterion for categorising participants as 'sedentary' or 'physically inactive', we did not specify any threshold for vigorous exercisers. It is possible that we could have included individuals who were performing as much as 90 minutes per week of vigorous intensity exercise. However, it is important to note that baseline reporting of behaviour in terms of how much 'vigorous' exercise these cohorts were undertaking was rare.
Nineteen of the included studies were conducted in Northern America or Western Europe, and two studies were completed in Australia, one in Egypt and one in North Korea. The majority all are considered high‐income nations according to the World Health Organization (WHO) taxonomy. Very little evidence was derived from developing countries, and it is uncertain whether the resources, infrastructure or both required for some of the interventions included in this review would be applicable in these parts of the world.
Although no single tool for measuring physical activity is infallible (Warren 2010), when possible it is desirable to have self‐reported exercise behaviour supported by objective measurements such as accelerometers or heart rate data. Ten studies were identified that attempted to objectively validate independent exercise behaviour with accelerometers or heart rate monitoring (al‐Majid 2015; Bourke 2014; Cadmus 2009; Irwin 2015; Mohamady 2017; Pinto 2005; Pinto 2011; Rogers 2015; Scott 2013; Thomas 2013). Seven studies of these studies attempted to validate self‐reported independent exercise behaviour by using accelerometers or heart rate monitors (al‐Majid 2015; Bourke 2014; Irwin 2015; Pinto 2005; Pinto 2011; Rogers 2015; Thomas 2013), however in three studies, data either were not supportive of exercise behaviour recorded by participants or were not reported in their entirety (Pinto 2005; Pinto 2011; Rogers 2015). Still a number of studies evaluated non‐supervised exercise behaviour by using self‐report logs or seven‐day physical activity questionnaires. Whilst these tools are relatively non‐complex and affordable for implementation in study design, they are prone to well‐established bias, including difficulties in ascertaining the frequency, duration and intensity of physical activity; social desirability bias; the cognitive demands of recall and overestimation of behaviour, particularly when such data are used to extrapolate MET/hours of exercise per week performed, or kcal/week of energy expenditure.
Analysis by behaviour change techniques as it relates to any given outcome (e.g. aerobic exercise tolerance) was not possible given that few studies stated a theoretical basis for their intervention, and only one study in this update being based on theory i.e. the trans theoretical model in Rogers 2015. It is worthy of note, however, that interventions frequently consisted of little more than telling people how to exercise and providing opportunities for this to occur, with little consideration of the psychological aspects of changing behaviour. A number of interventions were excluded at full‐text screening stage, that had a theoretical basis but did not meet our inclusion criteria. Whilst, the use of theory is variable amongst behaviour change interventions generally, the lack of interventions based on a theoretical model in this review is a concern.
It is also acknowledged that although coding of BCTs was done primarily on the basis of study reports, it is possible that some BCTs may have been implemented but not reported. To overcome this possibility and enhance understanding of the techniques important for changing behaviour in cancer patients, adoption of the CALO‐RE taxonomy or the broader BCT v1 taxonomy is recommended.
We acknowledge that in this review, we have undertaken a synthesis of RCTs that represent a combination of exercise efficacy and behaviour change studies (Courneya 2010), we recognise the distinction and this is reflected in the literature. During the screening process, there were a number of behaviour change studies based on theory that we excluded as they did not meet our inclusion criteria. However, it should be noted by the reader that all eight studies that we judged as successful (i.e. reported 75% or greater adherence over the intervention period to the Rock 2012 guidelines) incorporated intervention elements that were designed to promote independent exercise behaviour and did not place any restrictions on the control group in terms of the exercise they were permitted to undertake during the study.
Finally, we stated in the justification for this review that a better understanding of the types of interventions that could promote long‐term, habitual physical activity (i.e. 12 months or longer) in people living with and beyond cancer was a valuable addition to our knowledge due to the original review not being able to address this issue. Unfortunately, because of limitations in the evidence that we identified, we have not been able to address this issue. As such, this is an area of uncertainty that represents an important research gap. Whilst there is more research available of how to promote exercise behaviour at around eight to 12 weeks up until six months, there is still a lack of long‐term follow‐up of anything beyond this amongst these studies.
Quality of the evidence
Most of the uncertainty in judging study bias came from lack of clarity around randomisation procedures, allocation concealment and blinding of study outcome assessors. Most of the studies in this review were judged to include at least one element of high risk of non‐standard bias, as described in the 'Other potential sources of bias outcome. Of note, we chose to refrain from judging studies according to the performance bias criterion because we considered it not possible to realistically blind intervention participants to 'sham' conditions. Public health guidelines (e.g. the UK CMO report) for aerobic and resistance exercise (which are identical to the Rock 2012 recommendations) are freely available to the public, and given their ease of access via the Internet, the validity of a 'sham' condition is highly dubious. The summary of findings Table for the main comparison and 'Risk of bias' tables and Figure 2 and Figure 3 provide a summary of the certainty of evidence. Reporting of adherence of exercise behaviour within the studies was infrequent, which impacted upon the certainty of evidence.
We found the certainty of evidence assessed using the GRADE methodology for the majority of the outcomes to be low to very low; this was mainly due to high risk of bias, inconsistency of the results and imprecise results. One of the main reasons for a very low‐certainty of evidence grading was due to the high number of studies presenting a low number of participants in their study. Concerns over inconsistency were present due to variations in effect sizes and heterogeneity. Additionally, the serious concerns were present with the imprecision of the data due to wide confidence intervals and overall low numbers of participants in each study.
Additionally, attrition ranged from 0% to 25%, some studies with longer term follow‐up (post six months) demonstrated poorer attrition rates, but reasons for this were seldom explained. Ensuring reasons for dropout is reported in future studies is important.
Potential biases in the review process
We were not able to translate all non‐English language studies identified through our database, grey literature and snowballing searches, due to not having access to or resources for translation services. However, a huge effort was made to identify all relevant RCTs in this field. To the review authors' knowledge, we have identified and evaluated more RCTs involving exercise interventions in sedentary people living with or beyond cancer than any other systematic review in this field. More than 190 papers were screened at full‐text stage for eligibility for this update, in addition to the 400 papers screen at full text for the original review. For this 2018 update, we sent 112 emails in addition to the 116 emails to request data to inform the screening and data extraction process, so that the conclusions of the review would be as accurate and informative as possible. Dual data extraction was used throughout the review, except for study characteristics.
Agreements and disagreements with other studies or reviews
To the review authors' knowledge, this is still the most comprehensive systematic review of exercise behaviour interventions in sedentary cancer cohorts. A recent systematic review of predictors of adherence to exercise in people living with and beyond cancer found that the trans‐theoretical model of behaviour change and the theory of planned behaviour were significantly associated with better exercise adherence (Husebø 2013). The current review does not explicitly support such conclusions: mainly due to reporting in the included studies.
Howlett 2018 conducted a systematic review and meta‐analysis that aimed to evaluate physical activity interventions for healthy inactive adults. The BCT analysis found that interventions that included 'biofeedback', 'demonstration of the behaviour', 'behaviour practice/rehearsal' and 'setting of graded tasks' showed larger effect sizes for physical activity outcomes than studies that did not include these BCTs. Our review also found 'setting of graded tasks' to be common amongst the studies with higher adherence rates. Additionally, this review reported a number of studies that were judged as having high risk of bias or were judged as being unclear due to the lack of clear reporting. A suggestion was for future studies to use the TIDieR (Template for Intervention Description and Rreplication) framework (Hoffman 2014), particularly around the description of intervention content. Our review findings support this recommendation, as a number of exercise studies appear to display problems with reporting and are often judged with high risk of bias.
Ormel 2017 carried out a recent systematic review of predictors of adherence and identified the issues with low adherence to exercise interventions for people with cancer. Home‐based interventions were found to possibly address the issue of time and travelling to a location, which was identified as a potential barrier. Our review found that interventions that incorporated an element of supervision have better adherence rates to the set exercise target than home‐based interventions. However, as previously stated adherence was infrequently reported amongst the studies.
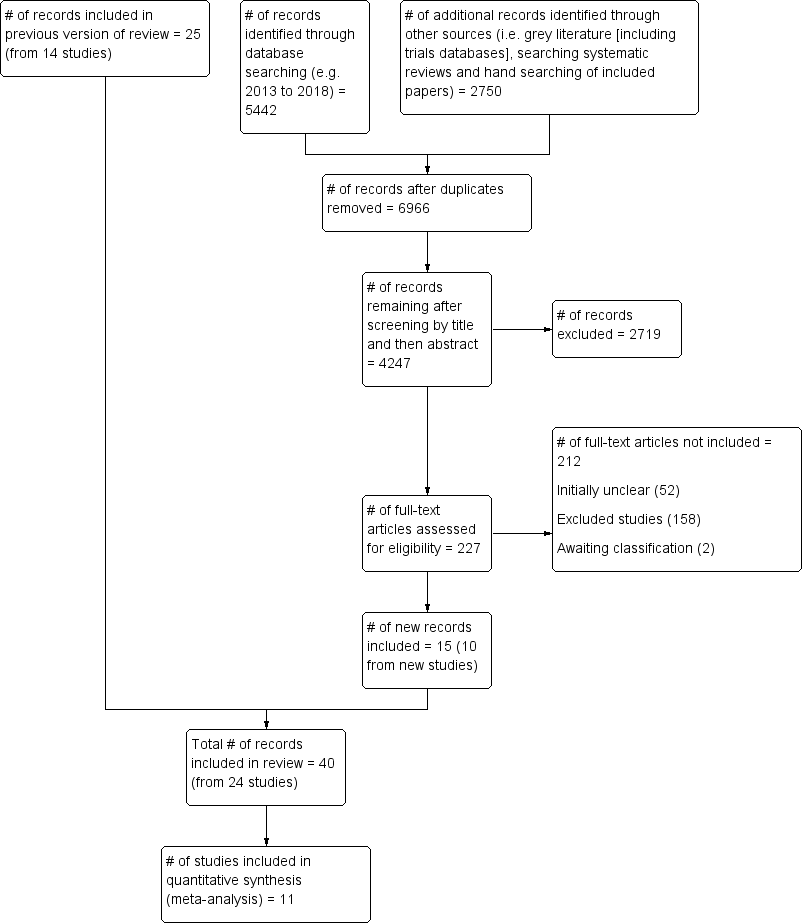
PRISMA flow diagram.
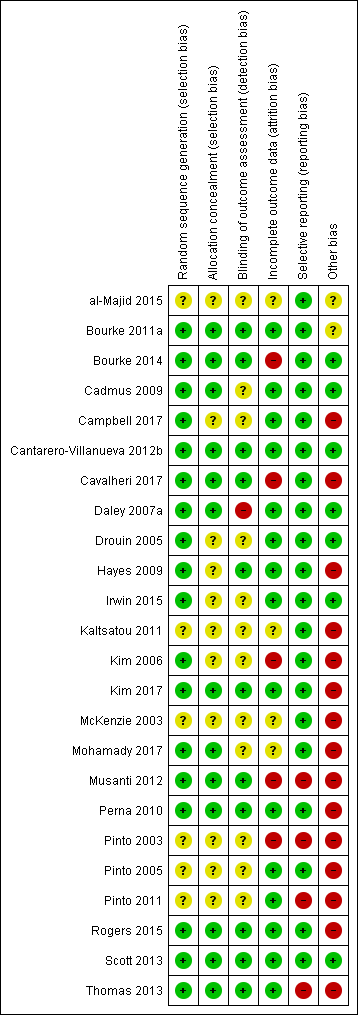
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
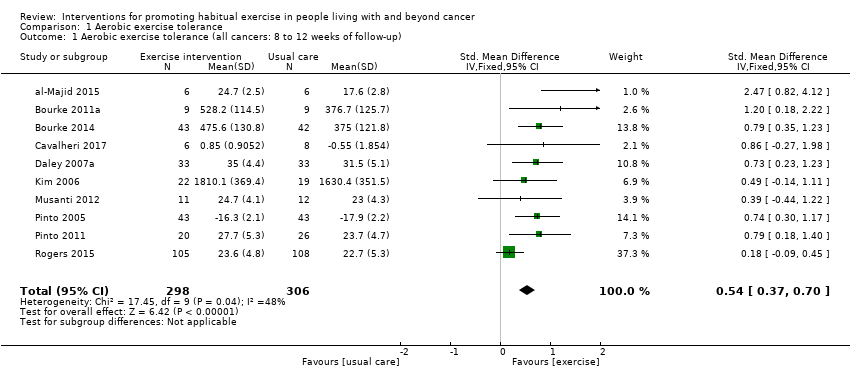
Comparison 1 Aerobic exercise tolerance, Outcome 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up).
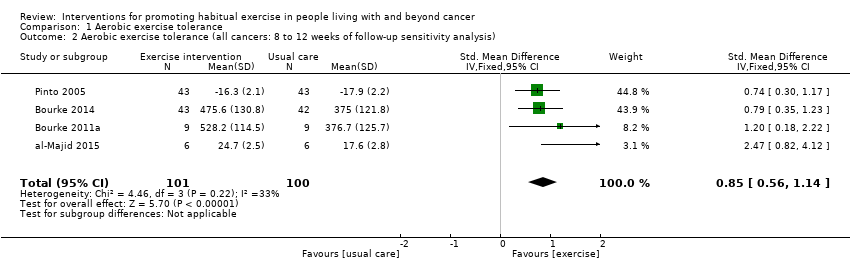
Comparison 1 Aerobic exercise tolerance, Outcome 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis).
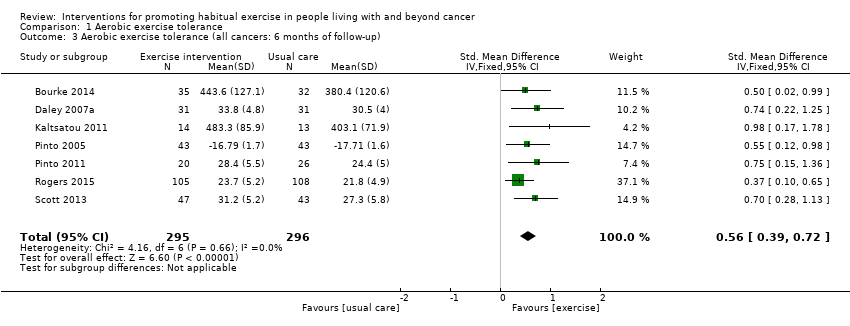
Comparison 1 Aerobic exercise tolerance, Outcome 3 Aerobic exercise tolerance (all cancers: 6 months of follow‐up).

Comparison 1 Aerobic exercise tolerance, Outcome 4 Aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up).
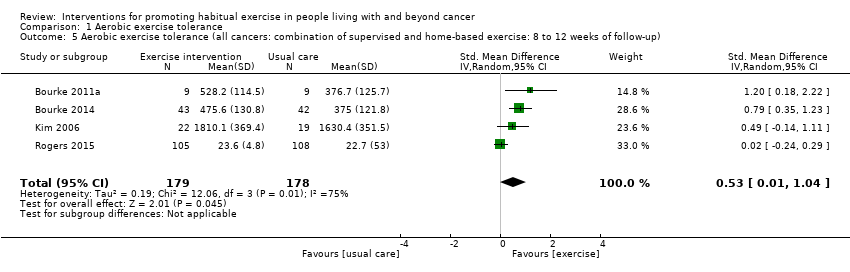
Comparison 1 Aerobic exercise tolerance, Outcome 5 Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up).

Comparison 1 Aerobic exercise tolerance, Outcome 6 Aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up).
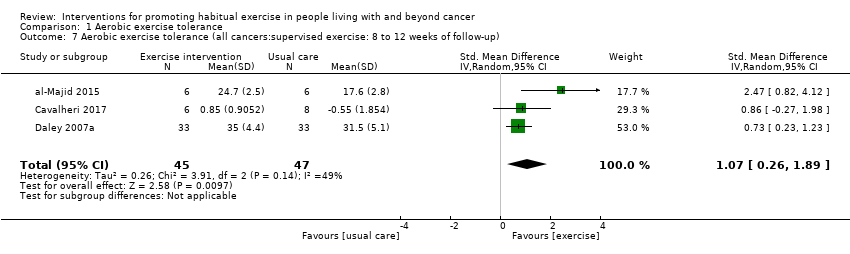
Comparison 1 Aerobic exercise tolerance, Outcome 7 Aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up).

Comparison 1 Aerobic exercise tolerance, Outcome 8 Aerobic exercise tolerance (all cancers: undergoing active treatment: 8 to 12 weeks follow‐up).
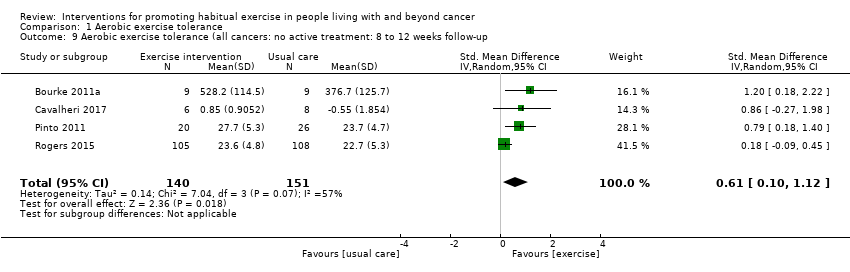
Comparison 1 Aerobic exercise tolerance, Outcome 9 Aerobic exercise tolerance (all cancers: no active treatment: 8 to 12 weeks follow‐up.
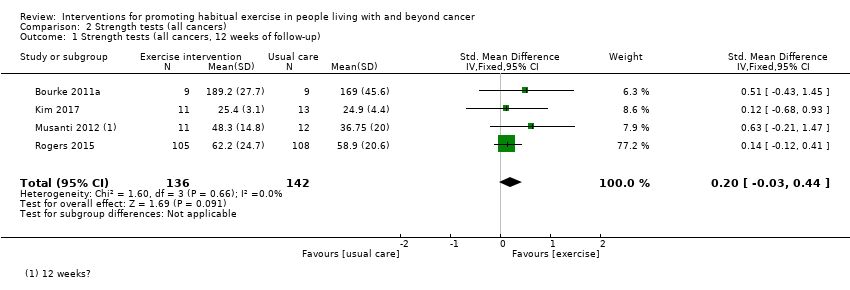
Comparison 2 Strength tests (all cancers), Outcome 1 Strength tests (all cancers, 12 weeks of follow‐up).

Comparison 2 Strength tests (all cancers), Outcome 2 Strength tests (all cancers: 12 weeks of follow‐up: sensitivity analysis).
| Exercise interventions compared to usual care for promoting habitual exercise in people living with and beyond cancer to improve aerobic exercise tolerance | ||||
| Outcomes | № of participants | Certainty of the evidence | Anticipated absolute effects* (95% CI) | |
| Risk with usual care | Risk difference with exercise interventions | |||
| Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) | 604 | ⊕⊕⊝⊝ | The mean aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) was 0 | SMD 0.54 higher |
| Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) | 201 | ⊕⊕⊝⊝ | The mean aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) was 0 | SMD 0.85 higher |
| Aerobic exercise tolerance (all cancers: 6 months) | 591 | ⊕⊕⊝⊝ | The mean aerobic exercise tolerance (all cancers: 6 months) was 0 | SMD 0.56 higher |
| Aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) | 441 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) was 0 | SMD 0.57 higher |
| Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) | 357 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 0.53 higher |
| Aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) | 155 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 0.70 higher |
| Aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) | 92 | ⊕⊝⊝⊝ | The mean aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) was 0 | SMD 1.07 higher |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||
| GRADE Working Group grades of evidence | ||||
| 1 Some concerns with high number of participants lost to follow‐up, selective reporting of data and other risks of bias | ||||
| Study | Exercise components | n | Meets Rock et al guidelines? | Adherence summary | At least 75% adherence? | High risk of bias? | Change in AET reported? | Adverse effects |
| Aerobic | 37, 38 (intervention vs control) | 33% reported 150 minutes/week of moderate intensity aerobic exercise at an average of 76% HR, for six months | 75% of women were doing between 90 and 119 minutes of moderate intensity aerobic activity per week at six months | Yes; for up to 119 minutes per week | No | Not reported | Five of the 37 women randomly assigned to exercise experienced an adverse effect; two were related to the study (plantar fasciitis) | |
| Aerobic | 34, 36, 38 (intervention, sham, control, respectively) | No | 77% of the exercise therapy; attended 70% (at least 17 of 24 sessions) or more of sessions | Unclear | Yes; outcome assessors were not blinded to participants’ group allocation | Yes | Three withdrawals in the intervention group: unclear as to why this occurred. Some withdrawals because of medical complications in placebo and control arms but unclear whether study related | |
| Aerobic | 13 intervention, 8 placebo stretching controls | Unclear | Participants in the intervention group averaged 3.6 days per week of aerobic exercise over an 8‐week period | Unclear | No | Yes | None reported | |
| Aerobic | 14, 13 (intervention vs control) | Unclear | Not reported | Not reported | Yes; method of measuring exercise and adherence not reported | Not reported | None reported | |
| Aerobic | 22,19 (intervention vs control). | No | Average weekly frequency of exercise was 2.4 ± 0.6 sessions, and average duration of exercise within prescribed target HR was 27.8 ± 8.1 minutes per session. Overall adherence was 78.3% ± 20.1% | Yes | Yes; data missing for 45% of the cohort | Yes | Reasons for withdrawal included personal problems (n = 2), problems at home (n = 2), problems related to chemotherapy (n = 3), thrombophlebitis in the lower leg (n = 2), non-exercise‐related injuries (n = 1), and death (n = 1). Unclear to which arm of the study these date relate | |
| Aerobic | 12, 12 (intervention vs control) | Unclear | Participants attended a mean of 88% of the 36‐session supervised exercise programme | Yes | Yes; 38% lost to follow‐up. Exercise tolerance test was performed but no control group comparison data were reported | Yes | None reported; however, it is unclear why the six controls dropped out | |
| Aerobic | 43, 43 (intervention vs control) | Unclear | At week 12, intervention participants reported a mean of 128.53 minutes/week of moderate intensity exercise. However, no changes were reported in the accelerometer data in the intervention group (change score = ‐0.33 kcal/hour) | Less than 75% of the intervention group was meeting the prescribed goal after week 4 | Yes; significantly more control group participants were receiving hormone treatment. Accelerometer data do not support the self‐reported physical activity behaviour | Yes | Not clear whether chest pain was related to exercise in dropout whose participation was terminated | |
| Aerobic | 20, 26 (intervention vs control) | Three‐day PAR questionnaire indicates that 64.7% of the intervention group and 40.9% of the control group were achieving the guidelines at three months | Correlation between self‐reported moderate intensity exercise and accelerometer data at three‐month follow‐up, when the only significant between‐group change is reported: r = 0.32 | No | Yes; accelerometer data were not reported; also, cited correlation is weak (0.32). Further, substantial contamination was noted in the control group | Yes | One cancer recurrence in the control group at three months | |
| Aerobic and resistance | 9, 9 (intervention vs control) | Six weeks of resistance exercise twice a week | 90% attendance at the supervised sessions. 94% of independent exercise sessions were completed | Yes | No | Yes | One stroke in the intervention group, unrelated to the exercise programme | |
| Aerobic and resistance | 16, 16 (intervention vs control) | Unclear | Most women (88%) allocated to the intervention group participated in 70% or more of scheduled supervised exercise sessions | Unclear | Yes; adherence data on unsupervised aspect of the intervention are not clear | No | None reported | |
| Aerobic and resistance | 7,7 (intervention vs control) | No | Unclear | Unclear | Yes; adherence to exercise not reported | Not reported | None reported | |
| Aerobic and resistance | Flexibility group (n = 13), aerobic group (n = 12), resistance group (n = 17), aerobic and resistance group (n = 13) | 12 weeks of resistance exercise two or three times per week | Mean percentages of adherence were as follows: flexibility = 85%, aerobic = 81%, resistance = 91% and aerobic plus resistance = 86% | Unclear | Yes; a significant number of dropouts belonged to the resistance exercise group (n = 8/13). Only 50% of activity logs were returned | Yes | Adverse effects were reported in two women during the study. In both cases, the women developed tendonitis: one in the shoulder and the other in the foot. Both had a history of tendonitis, and both received standard treatment | |
| Aerobic and resistance | 51 participants in total. Numbers randomly assigned to each arm are unclear | Three months of resistance exercise three times per week | Women assigned to the structured intervention completed an average of 83% of their scheduled hospital‐based exercise sessions (only 4 weeks in duration), and 76.9% completed all 12 sessions. Home‐based component (8 weeks in duration) | Unclear | Yes; numbers randomly assigned to intervention and control groups are unclear, as are numbers completing in each arm | Not reported | Unclear | |
| Aerobic | 7,7 (intervention vs control) | No | Adherence to per‐protocol exercise sessions was very high, ranging between 95% and 97%. | Yes | No | Yes | None reported | |
| Aerobic and resistance | 25,25 (intervention vs control) | Yes; 6 weeks of resistance exercise | Adherence was 94% for the supervised and 82% of the prescribed independent exercise sessions over the first 12 week. | Yes | Yes incomplete outcome data at 6 months. | Yes | None reported | |
| Aerobic | 10 in exercise intervention, 9 in delayed exercise control | 150 minutess per week of moderate‐vigorous aerobic exercise for 24 weeks. | Participants attended 88% of supervised gym sessions (mean 1.8 sessions/ week and 87.5 minutes/week), and participants met 82% of the prescribed exercise targets (mean intensity 74.5% HRR). Home session completion was 87% (mean 2.4 sessions/week and 101.5 minutes/week), and participants met 87% of the prescribed exercise targets (mean intensity 73.5% HRR) | Yes | Yes; Low study recruitment rate. | Yes | None reported | |
| Aerobic | 33,33 (intervention vs control) | Three sixty minute sessions per week for 8 weeks. | All intervention group completed more than 85% of the 24 water exercise sessions, showing a high adherence rate to the program. | Yes | No | Not reported | One participant in the intervention dropped out due to a recurrence of breast cancer during the program. Three women reported a transient increase of oedema, and four women noted an increase in fatigue immediately after the beginning of the first session, which improved in the next few days. These women did not dropout of the study. No other adverse effects were reported. | |
| Aerobic and resistance | 9, 8 (intervention vs control) | Yes; six weeks of resistance exercise. | Nine of the participants randomised to the EG, four (44%) adhered to exercise training by completing 15 or more training sessions (i.e.,≥60%). | No | Yes; missing patient data in both arms with no reasons given. | Yes | One participant completed four sessions and another completed six sessions. Both stopped training as they felt unwell. They completed some of the post‐intervention assessments and were later diagnosed with a primary cancer other than lung cancer. | |
| Aerobic and resistance | 15, 15 (intervention vs control) | Three sixty minute sessions per week for 12 weeks. | Vague statement: Two participants did not fulfil the required exercise | Unclear | Yes; Age differences between groups in baseline demographics were present. Adherence data is vague. | Not reported | None reported | |
| Aerobic | 15, 15 (intervention vs control) | No | Unclear | Unclear | Yes; No adherence data. | Unclear | Unclear | |
| Aerobic | 110, 112 (intervention vs control) | Yes | Adherence to the intervention was 98 % for supervised exercise sessions, 96 % for update sessions, and 91 % for discussion group sessions. | Yes | Yes; differences in objective and subjective measures of physical activity reported | Yes | Related expected adverse events in the intervention group included back or lower extremity musculoskeletal pain or injury (n = 14), heart rate monitor rash (n = 1), fall while walking (n = 1), breast reconstruction (n = 3), and chest pain during treadmill fitness test (n = 1) | |
| Aerobic and resistance | 47, 43 (intervention vs control) | Yes, six weeks of resistance exercise. | Adherence for the intervention group was 80% | Yes | No | Yes | None reported. | |
| Aerobic | 35, 30 (intervention vs control) | Yes | The exercise goal was 150 minutes/week of moderate intensity aerobic exercise; 33% of women | No | Yes; not all outcomes were reported and low recruitment rate. | Not reported | None reported. | |
| Aerobic and resistance | 61, 60 (intervention vs control) | Yes | Women randomly assigned to exercise also reported their exercise prospectively in daily activity logs and reported an average 119 minutes per week of aerobic exercise, with an average of 70% of strength‐training sessions completed. Women randomly assigned to exercise increased their physical activity by an average 159 minutes per week, compared with 49 minutes per week in the usual‐care group. | No | No | Yes | 5 participants had to discontinue the use of Atromatise inhibitors. | |
| AET = aerobic exercise tolerance. | ||||||||
| Behaviour change technique | YALE | ||||||||||||
| Theory | TTM | EXSEM | TTM | TTM | TTM SCT | ||||||||
| 1. Provide Info on consequences of behaviour in general | X | X | X | X | |||||||||
| 2. Provide Info on consequences of behaviour to the individual | |||||||||||||
| 3. Provide Info about others' approval | |||||||||||||
| 4. Provide normative info about others' behaviour | |||||||||||||
| Programme set goal | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 5. Goal setting (behaviour) | X | X | X | X | X | X | |||||||
| 6. Goal setting (outcome) | |||||||||||||
| 7. Action planning | |||||||||||||
| 8. Barrier identification/Problem solving | X | X | X | X | X | ||||||||
| 9. Setting of graded tasks | X | X | X | X | X | X | X | X | X | ||||
| 10. Prompt review of behavioural goals | X | X | |||||||||||
| 11. Prompt review of outcome goals | |||||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | X | X | X | ||||||||||
| 13. Provide rewards contingent on successful behaviour | X | ||||||||||||
| 14. Shaping | |||||||||||||
| 15. Prompt generalisation of a target behaviour | X | X | X | X | |||||||||
| 16. Prompt self‐monitoring of behaviour | X | X | X | X | X | X | X | X | X | ||||
| 17. Prompt self‐monitoring of behavioural outcome | X | X | X | X | X | X | |||||||
| 18. Prompt focus on past success | X | ||||||||||||
| 19. Feedback on performance provided | X | X | X | X | |||||||||
| 20. Information provided on where and when to perform behaviour | X | X | |||||||||||
| 21. Instruction provided on how to perform the behaviour | X | X | X | X | X | X | X | X | X | ||||
| 22. Modelling/Demonstration of behaviour | X | X | X | ||||||||||
| 23. Teaching to use prompts/cues | X | X | X | ||||||||||
| 24. Environmental restructuring | X | X | |||||||||||
| 25. Agreement on behavioural contract | X | ||||||||||||
| 26. Prompt practise | X | X | X | X | X | X | X | X | X | X | X | X | X |
| 27. Use of follow‐up prompts | X | ||||||||||||
| 28. Facilitating social comparison | |||||||||||||
| 29. Planning social support/social change | X | X | X | ||||||||||
| 30. Prompt identification as role model/position advocate | |||||||||||||
| 31. Prompt anticipated regret | |||||||||||||
| 32. Fear arousal | |||||||||||||
| 33. Prompt self‐talk | |||||||||||||
| 34. Prompt use of imagery | |||||||||||||
| 35. Relapse prevention/coping planning | X | X | |||||||||||
| 36. Stress management/emotional control training | X | ||||||||||||
| 37. Motivational interviewing | |||||||||||||
| 38. Time management | |||||||||||||
| 39. General communication skills training | |||||||||||||
| 40. Stimulation of anticipation of future rewards | |||||||||||||
| EXSEM = exercise self‐esteem model; SCT = social cognitive theory; TTM = trans‐theoretical model. | |||||||||||||
| Behaviour change technique | |||||||||||
| Theory | SCT | ||||||||||
| 1. Provide Info on consequences of behaviour in general | x | x | |||||||||
| 2. Provide Info on consequences of behaviour to the individual | |||||||||||
| 3. Provide Info about others' approval | |||||||||||
| 4. Provide normative info about others' behaviour | |||||||||||
| Programme set goal | x | x | x | x | x | x | x | x | x | x | x |
| 5. Goal setting (behaviour) | x | x | |||||||||
| 6. Goal setting (outcome) | |||||||||||
| 7. Action planning | |||||||||||
| 8. Barrier identification/Problem solving | x | x | |||||||||
| 9. Setting of graded tasks | x | x | x (implicit) | x | x | x | x | x | x | x | |
| 10. Prompt review of behavioural goals | x | ||||||||||
| 11. Prompt review of outcome goals | |||||||||||
| 12. Prompt rewards contingent on effort or progress towards goal | x | ||||||||||
| 13. Provide rewards contingent on successful behaviour | |||||||||||
| 14. Shaping | |||||||||||
| 15. Prompt generalisation of a target behaviour | x (from linked paper Gilbert 2016* | x | |||||||||
| 16. Prompt self‐monitoring of behaviour | |||||||||||
| 17. Prompt self‐monitoring of behavioural outcome | x | x | x | ||||||||
| 18. Prompt focus on past success | |||||||||||
| 19. Feedback on performance provided | x | ||||||||||
| 20. Information provided on where and when to perform behaviour | x | x | |||||||||
| 21. Instruction provided on how to perform the behaviour | x | x | x | ||||||||
| 22. Modelling/Demonstration of behaviour | x | ||||||||||
| 23. Teaching to use prompts/cues | |||||||||||
| 24. Environmental restructuring | |||||||||||
| 25. Agreement on behavioural contract | |||||||||||
| 26. Prompt practise | x | x | |||||||||
| 27. Use of follow‐up prompts | |||||||||||
| 28. Facilitating social comparison | |||||||||||
| 29. Planning social support/social change | x | ||||||||||
| 30. Prompt identification as role model/position advocate | |||||||||||
| 31. Prompt anticipated regret | |||||||||||
| 32. Fear arousal | |||||||||||
| 33. Prompt self‐talk | |||||||||||
| 34. Prompt use of imagery | |||||||||||
| 35. Relapse prevention/coping planning | x | ||||||||||
| 36. Stress management/emotional control training | |||||||||||
| 37. Motivational interviewing | |||||||||||
| 38. Time management | |||||||||||
| 39. General communication skills training | |||||||||||
| 40. Stimulation of anticipation of future rewards |
| BCT | |||||||||
| Resistance | Aerobic | Aerobic | Resistance | Aerobic | Resistance | Resistance | Aerobic | Frequency of BCTs | |
| Programme set goal | x | x | x | x | x | x | x | x | 8 |
| 9. Setting of graded tasks | x | x | x | x | x | x | x | 7 | |
| 21. Instruction provided on how to perform behaviour | x | x | x | 3 | |||||
| 26. Prompt practise | x | x | 2 | ||||||
| 5. Goal setting (outcome) | x | x | 2 | ||||||
| 8. Barrier identification/problem solving | x | x | 2 | ||||||
| 1. Provide information on consequences of behaviour in general | x | x | 2 | ||||||
| 15. Prompt generalisation of a target behaviour | x | x | 2 | ||||||
| 17. Prompt self‐monitoring of behavioural outcome | x | x | 2 | ||||||
| 20. Information provided on where and when to perform behaviour | x | x | 2 | ||||||
| 19. Feedback on performance provided | x | 1 | |||||||
| 22. Modelling/demonstration of behaviour | x | 1 | |||||||
| 12. Prompt rewards contingent on effort or progress towards goal | x | 1 | |||||||
| 29. Planning social support/social | x | 1 | |||||||
| 35. Relapse prevention/coping planning | x | 1 | |||||||
| BCTs: behaviour change techniques | |||||||||
| BCT | |||||||
| Frequency of BCTs | |||||||
| Programme set goal | x | x | x | x | x | x | 6 |
| 9. Setting of graded tasks | x | x | x | x | x | 5 | |
| 21. Instruction provided on how to perform behaviour | x | x | x | 3 | |||
| 1. Provide information on consequences of behaviour in general | x | x | 2 | ||||
| 26. Prompt practise | x | x | 2 | ||||
| 8. Barrier identification/problem solving | x | x | 2 | ||||
| 15. Prompt generalisation of a target behaviour | x | x | 2 | ||||
| 5. Goal setting (outcome) | x | 1 | |||||
| 16. Prompt self‐monitoring of behaviour | x | 1 | |||||
| 17. Prompt self‐monitoring of behavioural outcome | x | 1 | |||||
| 20. Information provided on where and when to perform behaviour | x | 1 | |||||
| 29. Planning social support/social | x | 1 | |||||
| 27. Use of follow‐up prompts | x | 1 | |||||
| BCTs: behaviour change techniques | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up) Show forest plot | 10 | 604 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.54 [0.37, 0.70] |
| 2 Aerobic exercise tolerance (all cancers: 8 to 12 weeks of follow‐up sensitivity analysis) Show forest plot | 4 | 201 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.56, 1.14] |
| 3 Aerobic exercise tolerance (all cancers: 6 months of follow‐up) Show forest plot | 7 | 591 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.39, 0.72] |
| 4 Aerobic exercise tolerance (breast cancer: 8‐12 weeks of follow‐up) Show forest plot | 6 | 441 | Std. Mean Difference (IV, Random, 95% CI) | 0.57 [0.22, 0.93] |
| 5 Aerobic exercise tolerance (all cancers: combination of supervised and home‐based exercise: 8 to 12 weeks of follow‐up) Show forest plot | 4 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.01, 1.04] |
| 6 Aerobic exercise tolerance (all cancers: home‐based exercise: 8 to 12 weeks of follow‐up) Show forest plot | 3 | 155 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.37, 1.03] |
| 7 Aerobic exercise tolerance (all cancers:supervised exercise: 8 to 12 weeks of follow‐up) Show forest plot | 3 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 1.07 [0.26, 1.89] |
| 8 Aerobic exercise tolerance (all cancers: undergoing active treatment: 8 to 12 weeks follow‐up) Show forest plot | 6 | 313 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.49, 0.95] |
| 9 Aerobic exercise tolerance (all cancers: no active treatment: 8 to 12 weeks follow‐up Show forest plot | 4 | 291 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.10, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Strength tests (all cancers, 12 weeks of follow‐up) Show forest plot | 4 | 278 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.03, 0.44] |
| 2 Strength tests (all cancers: 12 weeks of follow‐up: sensitivity analysis) Show forest plot | 2 | 231 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.09, 0.43] |

