دسترسی به شریان فمورال از راه پوست بهطور کامل در برابر جراحی کاتدان برای ترمیم انتخابی اندوواسکولار آنوریسم آئورت شکمی دوشاخه شده
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع مطالعات در حال انجام
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT | |
| Participants | Country: USA No: 151 Ages: 70 ± 6.6, 74 ± 11, 73 ± 8.8 for PG, PS and femoral cut down (cut‐down femoral artery access) groups respectively Sex: 136 male, 15 female Inclusion criteria:
Exclusion criteria:
| |
| Interventions | All aneurysms were repaired using the Endologix 21F profile based sheath system (Endologix, Inc, Irvine, California). | |
| Outcomes | Primary endpoint: 'treatment success'; a composite endpoint comprising procedural technical success and absence of major adverse events or vascular complications at 30 days. | |
| Notes | It should be noted that one study author served on the Scientific Advisory Board for Endologix, Inc, and three authors are on the Endologix, Inc speaker’s bureau. In addition, the trial was funded by Endologix, Inc, with financial contribution by Abbott Vascular, Inc. and all Abbott Vascular, Inc ipsilateral closure devices provided free of charge. This is unlikely to have influenced the primary outcomes as there was good follow up to 30 days and the protocol was adhered to. However had the results shown inferiority of percutaneous access publication bias may have been introduced. One further manuscript (Krajcer 2013), presented the 30‐day results of the same trial. The data extracted from Krajcer 2013 was the same as Nelson 2014, and was not considered as a separate data set; data were cross checked to ensure reporting consistency. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by study site, using two block sizes (3 or 6) with random choice of block size order. Sealed randomisation envelopes were used. 2:1 randomisation (PEVAR:FE) was used, with equal allocation to the two PEVAR groups (PG, PS). |
| Allocation concealment (selection bias) | Low risk | Sealed randomisation envelopes were used: on screening eligibility confirmation the next sequential randomisation envelope was opened and assignment immediately allocated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not possible due to due nature of surgical interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Unlikely relating to the primary endpoint and subgroup analysis as they are objective in nature |
| Incomplete outcome data (attrition bias) | Low risk | All participants reported with no loss to follow‐up at 30 days. There was 9% loss to follow‐up at 6 months. This only affects 1 secondary outcome within the review (long‐term complications). Reasons for loss to follow‐up were given for each group. |
| Selective reporting (reporting bias) | Low risk | Primary endpoint, secondary outcomes and subgroup analysis clearly defined and reported |
| Other bias | Low risk | See 'notes' section in table above |
| Methods | Study design: RCT Method of randomisation: not reported Concealment of allocation: unblinded | |
| Participants | Country: Germany No: 30 Age: 51‐90 years (mean 72.9 ± 9.9 years) Sex: 29 male, 1 female Inclusion criteria:
Exclusion criteria:
| |
| Interventions | Aneurysms were repaired using either the Zenith graft (Cook, Bloomington, Ind), n = 16, or using the Talent endovascular graft (Medtronic, Sunrise, Fla), n = 14 Percutaneous access, n = 15. In these participants a 10F Prostar XL percutaneous vascular surgery device (Perclose, Redwood City, Calif) was used in the pre‐close technique for closure of the access site. Surgical cut‐down (femoral artery access), n = 15. In these participants a transverse groin incision was made to expose the common femoral artery for direct needle puncture. All participants received 5000 IU of heparin after sheath insertion. Duplex ultrasound scanning was performed before and after the procedure. | |
| Outcomes | Outcomes: operative success (the successful closure of the femoral artery following insertion of graft); in‐hospital mortality and major complications; wound complications; re‐intervention rate; blood loss; operative time and time to ambulation; total operative cost. | |
| Notes | No conflicts of interest reported. Attempt made to contact authors via e‐mail on 21/12/2015 with no reply received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported and no details given |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not possible due to nature of surgical interventions |
| Blinding of outcome assessment (detection bias) | Low risk | Unlikely given the outcome measures used being objective in nature |
| Incomplete outcome data (attrition bias) | Low risk | All patients fully reported with no loss to follow‐up due to small numbers and short time scale |
| Selective reporting (reporting bias) | Unclear risk | Selected outcomes not outlined making the paper more prone to selective reporting, however most major outcomes appear to have been reported |
| Other bias | Low risk | None identified |
AAA: abdominal aortic aneurysm
BMI: body mass index
EVAR: endovascular aneurysm repair
FE: femoral access
ICU: intensive care unit
PEVAR percutaneous endovascular aneurysm repair
PG: 8F Perclose ProGlide device
PS: 10F Prostar XL device
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Did not include primary intervention. Study only examined percutaneous closure devices not aneurysm repairs | |
| Non‐randomised | |
| Non‐randomised | |
| Non‐randomised | |
| Non‐randomised |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Economic comparison of percutaneous (pEVAR) vs. open access in EVAR (EVAccess) |
| Methods | Study design: RCT |
| Participants | Country: Austria Ages eligible for study: 18‐90 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | pEVAR: percutaneous femoral access using a suture‐mediated closure system |
| Outcomes | Primary outcome measures: overall costs of each different access Overall costs of each different access way including used material and duration of the procedure (shorter duration = less costs of running operation theatre) |
| Starting date | April 2016 |
| Contact information | Miriam Uhlmann, MD +43 49150 4107 [email protected] Fadi Taher, MD +43 49150 4107 [email protected] |
| Notes | Estimated study completion date: April 2017 |
| Trial name or title | A comparison of Percutaneous femoral access in Endovascular Repair versus Open femoral access (PiERO) |
| Methods | Study design: RCT |
| Participants | Country: Netherlands Inclusion criteria:
Exclusion criteria:
|
| Interventions | Aneurysm repair (graft type not stated) with bilateral groin access obtained in all participants by open femoral access (surgical access) in one groin, and a percutaneous device (either the Prostar XL or Proglide, both Abbott Vasc, Inc, Redwood City, California) in the contralateral groin. Participants will serve as their own controls. |
| Outcomes | Primary: number of surgical site infections in the groin 30 days and at 1 year postoperatively, evaluated by the Southampton Wound Assessment Score and with wound culture confirming diagnosis. Wound control will be performed at 1 day, 2 weeks, 6 weeks postoperatively, with final evaluation at 1 year. |
| Starting date | Registered 10th November 2013 |
| Contact information | Principal Investigator BP Vierhout [email protected] |
| Notes | Contacted 21/12/2015, responded with trial still recruiting participants in Dec 2015 |
CFA: common femoral artery
EVAR: endovascular aneurysm repair
pEVAR: percutaneous endovascular aneurysm repair
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality rate (30‐day or in‐hospital) Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 36.18] |
| Analysis 1.1  Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 1 Short‐term mortality rate (30‐day or in‐hospital). | ||||
| 2 Aneurysm exclusion Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.02] |
| Analysis 1.2  Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 2 Aneurysm exclusion. | ||||
| 3 Major complications Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.68] |
| Analysis 1.3  Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 3 Major complications. | ||||
| 4 Major complications (6 months) Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.34, 3.15] |
| Analysis 1.4  Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 4 Major complications (6 months). | ||||
| 5 Bleeding complications Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.82] |
| Analysis 1.5  Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 5 Bleeding complications. | ||||
| 6 Operating time (minutes) Show forest plot | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐31.46 [‐47.51, ‐15.42] |
| Analysis 1.6  Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 6 Operating time (minutes). | ||||

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
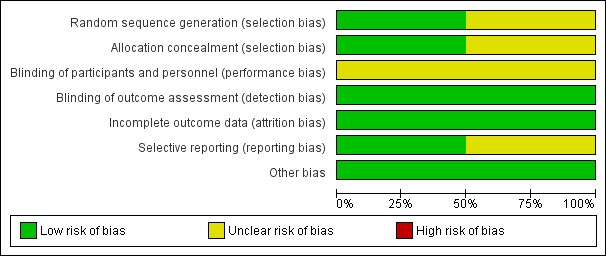
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 1 Short‐term mortality rate (30‐day or in‐hospital).

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 2 Aneurysm exclusion.

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 3 Major complications.

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 4 Major complications (6 months).
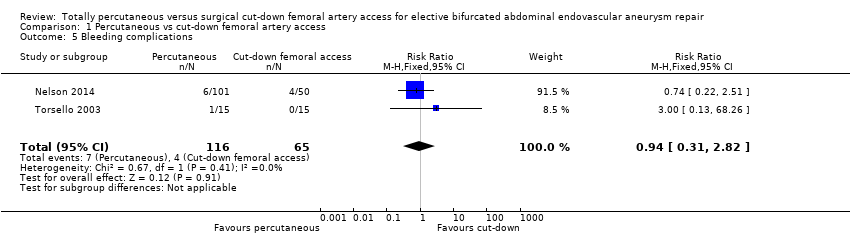
Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 5 Bleeding complications.

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 6 Operating time (minutes).
| Totally percutaneous compared to cut‐down femoral artery access for elective bifurcated abdominal endovascular aneurysm repair | ||||||
| Patient or population: people undergoing elective bifurcated abdominal endovascular aneurysm repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with cut‐down femoral artery access | Risk with totally percutaneous | |||||
| Short‐term mortality rate (30‐day or in‐hospital) | See comment | See comment | RR 1.50 | 181 | ⊕⊕⊕⊝ | It was not possible to calculate risk as only one event occurred. Note that although 2 RCTs included, only one contributes to effect estimate (no events in Torsello 2003) |
| Failure of aneurysm exclusion | Study population | RR 0.17 (0.01 to 4.02) | 151 | ⊕⊕⊕⊝ | ||
| 20 per 1000 | 3 per 1000 | |||||
| Wound infection rate (30‐day or in‐hospital) | See comment | See comment | not estimable | 181 | ⊕⊕⊕⊝ | Risk and relative effect were not estimable as no events occurred |
| Major complications (30‐day or in‐hospital) | Study population | RR 0.91 | 181 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 182 per 1000 | |||||
| Long term complications | Study population | RR 1.03 | 134 | ⊕⊕⊕⊝ | ||
| 95 per 1000 | 98 per 1000 | |||||
| Bleeding complications and haematoma (30‐day or in‐hospital) | Study population | RR 0.94 | 181 | ⊕⊕⊕⊕ | ||
| 62 per 1000 | 58 per 1000 | |||||
| Operating time (minutes) | The mean operating time was 99 minutes | The mean operating time in the intervention group was 31.46 minutes lower (47.51 lower to 15.42 lower) | ‐ | 181 | ⊕⊕⊕⊝ | |
| * The basis for the assumed risk for 'Study population' was the average risk in the comparison group (i.e. total number of participants with events divided by the total number of participants in the comparison group included in the meta‐analysis. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) and calculated where possible from the data provided in the studies. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded by one level due to the low number of events and imprecision (wide confidence intervals include both harm and benefit) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality rate (30‐day or in‐hospital) Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 36.18] |
| 2 Aneurysm exclusion Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.02] |
| 3 Major complications Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.68] |
| 4 Major complications (6 months) Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.34, 3.15] |
| 5 Bleeding complications Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.82] |
| 6 Operating time (minutes) Show forest plot | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐31.46 [‐47.51, ‐15.42] |

