Abord entièrement percutané versus abord standard de l'artère fémorale pour la réparation endovasculaire bifurquée élective d'anévrismes abdominaux
Résumé scientifique
Contexte
Les anévrismes de l'aorte abdominale (AAA) sont une affection vasculaire aux risques significatifs, en particulier en cas de rupture. Il est donc essentiel de les identifier et les réparer par une procédure élective avant leur rupture nécessitant une intervention chirurgicale d'urgence. La réparation s'est traditionnellement faite par une technique de chirurgie ouverte nécessitant une grande incision en travers de l'abdomen. Plus récemment, la réparation endovasculaire de l'anévrisme (EVAR) est devenue une alternative courante. Dans cette procédure, l'artère fémorale commune est exposée par dissection veineuse et un endoprothèse est introduit dans l'anévrisme par ce biais. Cette revue examine une approche entièrement percutanée de l'EVAR. Cette technique offre une approche mini‐invasive pour l'abord de l'artère fémorale qui pourrait réduire les taux de complication de la plaie à l'aine et améliorer le temps de récupération. Cette technique peut cependant être moins applicable chez les patients avec une cicatrisation à l'aine, par exemple, ou une calcification artérielle.
Objectifs
Cette revue vise à comparer les résultats cliniques de l'abord percutané avec l'abord standard de l'artère fémorale dans la réparation endovasculaire bifurquée élective d'anévrismes abdominaux (EVAR).
Stratégie de recherche documentaire
Le co‐ordinateur de la recherche d'essais du groupe Cochrane sur les maladies vasculaires périphériques a effectué des recherches dans son registre spécialisé (dernière recherche en juillet 2013), CENTRAL (2013, numéro 6) et les bases de données d'essais cliniques. Les références bibliographiques des articles trouvés ont été examinées.
Critères de sélection
Seuls les essais contrôlés randomisés ont été pris en compte. L'intervention principale était une réparation endovasculaire totalement percutanée. Tous types de dispositifs ont été pris en compte. Cette intervention était comparée à la réparation endovasculaire standard par l'artère fémorale. Seules les études examinant des réparations électives ont été pris en compte. Les études portant sur la chirurgie d'urgence pour une rupture de l'anévrisme de l'aorte abdominale (RAAA) et sur la réparation aorto‐uni‐iliaque ont été exclues.
Recueil et analyse des données
Toutes les données ont été recueillies de manière indépendante par deux auteurs de la revue. En raison du faible nombre d'essais identifiés, aucune évaluation formelle des analyses d'hétérogénéité ou de sensibilité n'a été effectuée.
Résultats principaux
Un seul essai remplissait les critères d'inclusion, portant sur un total de 30 participants, 15 subissant la technique percutanéeet et 15 l'approche standard de dissection de l'artère fémorale. Il n'y avait aucune différence significative entre les deux groupes au départ.
Aucune mortalité ni échec de l'exclusion de l'anévrisme n'a été observé dans aucun des deux groupes. Trois infections de la plaie sont survenues dans le groupe de dissection standard de l'artère fémorale, alors qu'aucune n'a été observée dans le groupe percutané. Cela n'était pas statistiquement significatif. Une seule complication majeure a été observée dans l'étude, une conversion à la technique de dissection veineuse dans le groupe d'abord percutané. Aucun critère de jugement à long terme n'était rapporté. Un épisode de complication hémorragique a été signalé dans le groupe percutané. Des différences significatives ont été observées dans la durée de l'opération (technique percutanée 86,7 ± 27 minutes versus conventionnelle 107,8 ± 38,5 minutes ; P < 0,05).
L'étude incluse avait un petit effectif et ne rendait pas compte de manière approppriée de la méthode de randomisation, de l'assignation secrète et des critères de jugement pré‐sélectionnés.
Conclusions des auteurs
Une seule petite étude a été identifiée, qui n'a pas fourni de preuves adéquates pour déterminer l'efficacité et l'innocuité de l'approche percutanée par rapport à la réparation endovasculaire standard de l'anévrisme. Cette revue a identifié un besoin évident pour d'autres recherches sur cette technique potentiellement bénéfique. Une étude en cours a été identifiée par la recherche, qui pourrait fournir de meilleures preuves à l'avenir.
PICO
Résumé simplifié
Ponction cutanée comparée à l'exposition de l'artère fémorale pour la réparation mini‐invasive des anévrismes de l'aorte abdominale
L'anévrisme aortique abdominal est un ballonnement du plus grand vaisseau sanguin de l'abdomen, l'aorte abdominale, en raison d'un affaiblissement de la paroi du vaisseau. Ce ballonnement peut entraîner une rupture engageant le pronostic vital. La réparation de l'anévrisme est recommandée si le risque qu'il pose est considéré significatif. Toute réparation implique la pose d'un greffon artificiel, un tube en tissu, pour aider à renforcer la paroi artérielle. Il existe deux méthodes principales pour la réparation. La première est une technique ouverte dans laquelle l'abdomen entier est ouvert et le greffon est placé à l'intérieur du vaisseau sanguin. L'autre technique est la réparation endovasculaire de l'anévrisme. Avec cette technique, le greffon est introduit dans l'aorte abdominale par une artère située dans l'aine (l'artère fémorale), permettant d'éviter la grande incision abdominale. Cette revue a examiné une méthode alternative pour l'introduction du greffon dans l'artère fémorale, l'accès percutané. Au lieu d'une incision dans l'aine pour exposer l'artère fémorale (dissection veineuse), un petit orifice est pratiqué dans la peau et ensuite une aiguille avec un tube en plastique par‐dessus est introduite dans l'artère fémorale. Après avoir été introduite, l'aiguille peut être retirée par le tube, laissant le tube en place dans l'artère. Le greffon et tous les autres matériaux peuvent ensuite être introduits dans l'artère via le tube en plastique. Une fois la procédure complétée, le tube peut être retiré. L'incision en surface peut généralement être refermée avec juste un point de suture.
Cette revue n'a trouvé qu'une étude comparant la technique de dissection veineuse et la technique percutanée dans la réparation endovasculaire. L'étude portait sur 30 participants, dont 15 ont subi la technique de dissection veineuse et 15 autres la technique percutanée. Personne n'est décédée pendant l'étude et la procédure a été efficace chez tous les participants. Cependant, pour un participant la chirurgie par accès percutané a dû être remplacée par la technique de dissection veineuse, et pour un participant subissant la chirurgie par voie percutanée, l'artère a dû être réparée chirurgicalement en raison d'un saignement continu. La chirurgie a pris moins de temps dans le groupe percutané par rapport au groupe de dissection (87 minutes contre 108 minutes). L'étude n'a pas mis en évidence d'autre différence significative.
L'étude incluse n'a pas rendu compte des méthodes de randomisation ou d'assignation sacrète, ni des critères de jugement pré‐sélectionnés.
Cette étude ne fournissait pas suffisamment de données et aucune conclusion définitive n'a pu en être tirée. Des études à plus grande échelle sont nécessaires pour fournir des données supplémentaires. La revue indique que la nouvelle technique pourrait être une bonne option pour certains patients, mais des recherches supplémentaires sont nécessaires.
Authors' conclusions
Summary of findings
| Totally percutaneous compared to cut‐down femoral artery access for elective bifurcated abdominal endovascular aneurysm repair | ||||||
| Patient or population: people undergoing elective bifurcated abdominal endovascular aneurysm repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with cut‐down femoral artery access | Risk with totally percutaneous | |||||
| Short‐term mortality rate (30‐day or in‐hospital) | See comment | See comment | RR 1.50 | 181 | ⊕⊕⊕⊝ | It was not possible to calculate risk as only one event occurred. Note that although 2 RCTs included, only one contributes to effect estimate (no events in Torsello 2003) |
| Failure of aneurysm exclusion | Study population | RR 0.17 (0.01 to 4.02) | 151 | ⊕⊕⊕⊝ | ||
| 20 per 1000 | 3 per 1000 | |||||
| Wound infection rate (30‐day or in‐hospital) | See comment | See comment | not estimable | 181 | ⊕⊕⊕⊝ | Risk and relative effect were not estimable as no events occurred |
| Major complications (30‐day or in‐hospital) | Study population | RR 0.91 | 181 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 182 per 1000 | |||||
| Long term complications | Study population | RR 1.03 | 134 | ⊕⊕⊕⊝ | ||
| 95 per 1000 | 98 per 1000 | |||||
| Bleeding complications and haematoma (30‐day or in‐hospital) | Study population | RR 0.94 | 181 | ⊕⊕⊕⊕ | ||
| 62 per 1000 | 58 per 1000 | |||||
| Operating time (minutes) | The mean operating time was 99 minutes | The mean operating time in the intervention group was 31.46 minutes lower (47.51 lower to 15.42 lower) | ‐ | 181 | ⊕⊕⊕⊝ | |
| * The basis for the assumed risk for 'Study population' was the average risk in the comparison group (i.e. total number of participants with events divided by the total number of participants in the comparison group included in the meta‐analysis. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) and calculated where possible from the data provided in the studies. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded by one level due to the low number of events and imprecision (wide confidence intervals include both harm and benefit) | ||||||
Background
Description of the condition
Abdominal aortic aneurysms (AAAs) are a vascular condition whereby the abdominal aorta increases in diameter. This enlargement is defined as an aneurysm of the abdominal aorta when the enlargement is 1.5 times greater than the normal diameter, or over 3 cm. The enlargement carries significant risk: if left untreated there is a high risk of rupture, which carries an approximately 80% risk of mortality (Chambers 2009). It is therefore critical to identify and repair these aneurysms as an elective procedure before they rupture and require emergency surgery.
The prevalence of AAAs in men in the UK has been estimated to range from 1.3% to 8.4% (Lucarotti 1993), depending on the criteria used to define an AAA. Meanwhile studies have consistently shown women to have a far lower prevalence (Fowkes 1989; Scott 1995). In Scott 1995 1.3% of women were found to have AAAs compared to 7.6% of men. The incidence according to hospital records in Scotland, between 1971 and 1984, was 63.6 per 100,000 people per year (Naylor 1988).
Description of the intervention
Repair has traditionally been an open surgical repair, which required a large incision across the abdomen to allow clear access to the aorta so that the damaged section could be repaired by the insertion of a prosthetic graft. However, in 1991 Parodi and colleagues reported their initial experiences with what they described as a transfemoral intraluminal graft (Parodi 1991). This rapidly developed into what we now refer to as endovascular abdominal aortic aneurysm repair (EVAR).
In this procedure the common femoral arteries are exposed via incisions in each groin, and guidewires and access sheaths are introduced. The components of the endovascular graft are then advanced over the guidewires and deployed under radiographic control. Angiography is used to confirm satisfactory deployment of the prosthesis and exclusion of the aneurysm sac, following which all catheters, sheaths and guidewires are removed. The defects in the femoral arteries are surgically repaired and the cut‐down incisions closed (Rutherford 2005).
EVARs and open repairs are now the two primary forms of treatment for AAAs. EVAR has grown rapidly in popularity and in the USA, since 2003, has accounted for the majority of procedures taking place (Schwarze 2009). In the UK, the National Vascular Registry reported that EVAR is currently used in the majority (66%) of elective infra‐renal AAA repairs with 2779 elective EVAR repairs conducted between 2012 and 2014 (Waton 2015). It was also used in 20% of ruptured AAA repairs in the same time period (Waton 2015).
More recently, further advances have taken place allowing for a wholly percutaneous approach. This approach was reported as early as 1999 (Papazoglou 1999) and has grown in popularity with a recent review of NSQIP (National Surgical Quality Improvement Program) data showing almost half of elective EVAR cases reviewed used a percutaneous approach (Kauvar 2016).
The approach has developed with the use of several different suture‐mediated closure devices being reported in the literature including the Prostar XL (Abbott) (Malkawi 2010) and Proglide system (Abbott) for closure (Dosluoglu 2007). The adoption may also have been influenced by the rise of low‐profile stent grafts, which are more malleable, easier to introduce and navigate, and have been shown to compare favourably in the mid‐term in those with challenging anatomy (Sobocinski 2015).
The most common technique employed is the pre‐close technique. Access is gained to the common femoral artery and the initial sheath is then replaced by a percutaneous closure device over a guidewire. The major difference in this technique from percutaneous techniques used in other procedures is the deployment of a suture‐mediated closure device at the start of the procedure. This allows the arteriotomy to be enlarged, enabling the insertion of sheaths of a larger size and allowing the EVAR to be conducted normally. At the conclusion of the repair the sheath is then removed with the use of a knot pusher, aiding closure. The final wound is closed using a single suture or tape. Some variations of this technique exist at different centres and with the use of different devices (Lee 2008; Malkawi 2010; Watelet 2006).
How the intervention might work
The stent‐grafts used and the manner in which they achieve aneurysm exclusion are identical to the already widely used EVAR techniques. As such, many of the long‐term complications are similar.
The key differences are the initial arterial access, wound closure and the use of a percutaneous suture‐mediated closure device during surgery. One concern that has been identified with EVAR procedures is the continued problem of wound closure, which a number of studies have examined (Dalainas 2004; Faries 2002). As the percutaneous approach is less invasive, and results in a smaller final wound closure, it may help to address this problem. Wound infection rates with the use of percutaneous closure devices have been reported to be as low as 0.2% (Watelet 2006), compared with 2% in cut‐down femoral artery access (Slappy 2003).
Some patients may be at risk of problems with arterial access using this intervention. These include people with calcification of the femoral artery, groin scarring or an inguinal arterial prosthesis, morbid obesity and small or tortuous arterial iliac arteries (Traul 2000; Watelet 2006). This makes patient selection key to the initial success of the procedure. In addition, the percutaneous technique may not always achieve adequate haemostasis (Torsello 2003), and there may be increased risk of bleeding and haematoma formation with a need to convert to a conventional cut‐down approach.
Why it is important to do this review
It has been suggested that a totally percutaneous approach to EVAR offers a less invasive treatment with faster recovery and reduced wound infection rates than with cut‐down femoral artery access. This hypothesis must be tested by a robust systematic review as this would make percutaneous access a preferable choice for many surgical interventions. In the review we aim to demonstrate the efficacy and safety of percutaneous access as an alternative procedure and highlight any shortcomings in the technique. This review is important to allow clinicians to make a more informed decision when deciding on the appropriate technique for each individual patient.
This review was originally conducted in 2014, with one included and one ongoing trial identified that met the inclusion criteria. It is good practice to formally update the review to include any newly available data.
Objectives
This review aims to compare the clinical outcomes of percutaneous access with cut‐down femoral artery access in elective bifurcated abdominal endovascular aneurysm repair (EVAR).
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised controlled trials (RCTs) for evaluation. We would have included cross‐over trials, with only the first phase results considered, but we did not identify any.
Types of participants
We included all patients diagnosed with an abdominal aortic aneurysm (AAA), which had been visualised using either computed tomography (CT) or ultrasonography, in the review.
We considered only people undergoing elective repairs. People undergoing emergency surgery for a ruptured abdominal aortic aneurysm (rAAA) and those undergoing aorto‐uni‐iliac (also known as aorto‐mono‐iliac) repairs were excluded.
Types of interventions
The primary intervention was a totally percutaneous endovascular repair. We considered all device types. We compared this against cut‐down femoral artery access for endovascular repair.
Types of outcome measures
Primary outcomes
-
Short‐term mortality rates (30‐day or in‐hospital, i.e. procedure related)
-
Aneurysm exclusion (no flow into the abdominal aortic aneurysm (AAA) sac on follow‐up imaging) 30 days after the procedure
-
Wound infection rate (30‐day or in‐hospital)
Secondary outcomes
-
Major complications, for example, myocardial infarction, stroke, renal failure, respiratory failure, lower limb ischaemia, pneumonia, technical failure, or conversion to alternative technique (30‐day or in‐hospital)
-
Long‐term (12 months) complications and mortality (re‐intervention rates, device‐related complications, cause of death)
-
Bleeding complications and haematoma
-
Operating time, duration of intensive treatment unit (ITU) and hospital stay
With regard to the primary outcomes mortality and aneurysm exclusion, at least equivalence but not necessarily superiority over surgical cut‐down had to be demonstrated to allow a recommendation as percutaneous interventions may still be preferable if there are fewer wound problems and length of hospital stay is reduced.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
-
The Cochrane Vascular Specialised Register (October 2016);
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) via The Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trials registries (October 2016) for details of ongoing and unpublished studies using the terms percutaneous and aneurysm:
-
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch/);
-
ClinicalTrials.gov (clinicaltrials.gov/);
-
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of the search strategies.
Searching other resources
We did not impose any restriction on language of publication or publication status. We checked reference lists of retrieved articles.
Data collection and analysis
We conducted data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
One review author (MG) scanned the titles and abstracts of retrieved articles, excluding irrelevant studies. Those studies deemed relevant were retrieved in full. Two review authors (MG, AJ), then independently assessed the full‐text articles for suitability for inclusion. All disagreements were successfully resolved by consensus.
Data extraction and management
Two review authors (MG, AJ) extracted data from the included studies independently using a data extraction form. We found no discrepancies but had there been any, we would have resolved them by consensus. If necessary, we would have contacted study authors for clarification.
Assessment of risk of bias in included studies
We assessed included studies for risk of bias using Cochrane's 'Risk of bias' tool (Higgins 2011b). This tool covers sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. We assessed each of these domains as either high, low or unclear risk of bias and we provided support for each judgement. We presented our conclusions in a 'Risk of bias' table.
Measures of treatment effect
We measured the treatment effect according to the statistical guidelines recommended by Cochrane Vascular. For dichotomous data, we calculated risk ratios (RRs) with 95% confidence intervals (CI) as the measure of effect. We analysed continuous data in a continuous form by mean difference (MD). If similar outcomes were reported on different scales, we planned to calculate standardised mean difference (SMD).
Unit of analysis issues
The primary analysis was per individual randomised. Our search did not identify any cross‐over trials but if it had, we would only have included the first phase data.
Dealing with missing data
No data relating to primary outcomes were found to be missing. However, had data been missing we would have contacted the study authors to request missing data as the first step. If this was unsuccessful we would assess whether the data were missing at random. If this was found to be the case we would have excluded the missing data from the review. If the data were found not to be missing at random then we would have undertaken imputation of individual values and explored its impact in the final discussion.
Assessment of heterogeneity
Heterogeneity was measured by the I2 statistic (Higgins 2003). Moderate heterogeneity, defined as over 30%, would have been discussed and reasons identified (Deeks 2011).
Assessment of reporting biases
The single best step undertaken to minimise reporting bias was a full and comprehensive search encompassing both published and grey literature, whilst being aware of duplication of data. Had more than 10 studies been included within the final analysis we would have used a funnel plot to explore small study effects.
Data synthesis
We synthesised available data using Review Manager 5 (RevMan) (RevMan 2014). We investigated pooled estimates of the effects of treatment using a fixed‐effect model to calculate RR with 95% CIs for dichotomous outcomes and MD for continuous outcomes. If it was not possible to pool data we described the results reported by the studies in the text.
Subgroup analysis and investigation of heterogeneity
We did not plan or perform any subgroup analyses owing to the small number of studies included. We did not identify any significant heterogeneity (above 30%, moderate), therefore we conducted no further investigation into heterogeneity. If significant heterogeneity had been identified, we would have identified the clinical causes and conducted a sensitivity analysis.
Sensitivity analysis
We intended to carry out sensitivity analyses to explore the robustness of the conclusions reached. These would have examined a number of factors, including study quality and risk of bias, data source (published or unpublished), and any other analyses which might have appeared significant upon completion of the review. Given the low number of trials (two) and low risk of bias, a sensitivity analysis was not deemed necessary.
Summary of findings
We produced a 'Summary of findings' table (Schünemann 2011a) for the comparison of totally percutaneous access versus cut‐down artery access for people undergoing elective bifurcated abdominal endovascular aneurysm repair; using the GRADEpro GDT software (gradepro.org/). We used the GRADE approach to assess the quality of the evidence for the review outcomes as described in Types of outcome measures. We downgraded the evidence from 'high quality' for serious or very serious study limitations (risk of bias, indirectness and inconsistency of evidence, imprecision of effect estimates or potential publication bias) according to the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b) and the GRADE Working Group (GRADE Working Group 2004).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See Figure 1.

Study flow diagram
Included studies
One new study (two reports) was included for this update (Nelson 2014). Two independent studies therefore met the inclusion criteria: Torsello 2003 and Nelson 2014.
The Torsello 2003 study was conducted in Germany and was a small randomised pilot study. It consisted of 30 participants (29 male, one female) aged 51 to 90 years (mean 72.9 ± 9.9 years). Anyone presenting with an aortic aneurysm was considered, including those with calcification of the femoral artery, scars in the groin or obesity. The exclusion criteria included people with psychiatric conditions, those undergoing the implantation of an aorto‐mono‐iliac graft, and people with an aneurysm of the femoral artery. Aneurysms were repaired using either the Zenith graft (Cook, Bloomington, Indiana) (n = 16) or using the Talent endovascular graft (Medtronic, Sunrise, Florida) (n = 14), although the paper did not describe how these different grafts were distributed among the treatment arms.
In participants undergoing the percutaneous procedure (n = 15), a 10F Prostar XL percutaneous vascular surgery device (Perclose, Redwood City, California) was used in the 'pre‐close' technique for closure of the access site. For participants undergoing cut‐down femoral artery access (n = 15), a transverse groin incision was made to expose the common femoral artery for direct needle puncture. All participants received 5000 IU of heparin after sheath insertion. Duplex ultrasound scanning was performed before and after the procedure. The paper did not explicitly state which outcomes had been selected but reported a wide range of outcomes, including time of surgery, mortality and major complications.
The Nelson 2014 study presented the PEVAR (totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair) trial. This was a multicentre randomised controlled trial conducted in 18 centres in the USA. All centres had prior experience in EVAR using both cut‐down femoral artery access and percutaneous techniques. All investigators (surgeons and interventionalists) were required to provide evidence of experience in the use of closure devices, complete the Endologix EVAR training programme and perform 20 PEVAR preclose cases before commencing the trial.
The trial consisted of 151 participants (136 male, 15 female). Anyone over the age of 18 presenting with an AAA that was 5 cm or more, or rapidly expanding was considered. Participants had to understand and sign an informed consent form, and agree to all follow‐up visits. Anatomical eligibility was determined by computed tomography of the thoracic and abdominal aorta. Additional assessments of common femoral artery characteristics were conducted by an experienced, independent vascular surgeon. Participants were only included if they had an absence of the following: prior groin incision, haematoma or significant scarring at the ipsilateral arterial access site, clip‐based vascular closure device placement ever, collagen‐based vascular closure device placement in either arterial access site within 90 days, femoral artery needle puncture in either arterial access site within 30 days, active groin infection, traumatic vascular injury, femoral artery aneurysm or pseudoaneurysm, or arteriovenous fistula.
Participants had to have a life expectancy of more than one year, serum creatinine 1.7 mg/dL or less, BMI less than 40 kg/m2, have no planned major interventions or surgery within 30 days after the study procedure, and be free from myocardial infarction and cerebrovascular accident within three months of enrolment.
Of 179 screened participants, 153 were identified as suitable, and 151 were enrolled in the trial. Participants were randomised by a 2:1 design to percutaneous access/closure (n = 101) or cut‐down femoral artery access (termed open femoral exposure) (n = 50). The percutaneous access/closure procedure group was divided equally between two suture‐mediated pre‐closure device types: the 8F Perclose ProGlide (PG, n = 50) and the 10F Prostar XL (PS, n = 51) (both Abbott Vasc, Inc, Redwood City, California). All endovascular AAA repairs were performed using the Endologix 21F profile sheath‐based system (Endologix, Inc, Irvine, California). Post deployment graft position/aneurysm exclusion was confirmed on angiography.
The primary endpoint was identified as 'treatment success', a composite endpoint comprising 1) procedural technical success and 2) absence of major adverse events and vascular complications at 30 days. Six‐month follow‐up was also conducted. Subgroup analysis of major ipsilateral access‐related complications (vascular injury, lower extremity ischaemia, bleeding/transfusion, nerve injury) of PS and PG versus femoral exposure at 30 days was conducted. The following secondary procedural and in‐hospital outcomes were recorded: activated clotting time, contrast volume, fluoroscopy time, estimated blood loss, blood transfusion, procedure time, ipsilateral time to haemostasis, time to ambulation, time to normal diet, ICU length of stay, medication for groin pain and time to hospital discharge.
Excluded studies
One new study was excluded in this update (Ichihashi 2016). In total we excluded five studies. One trial did not include the primary intervention (Hattab 2012). Four trials were non‐randomised (Ichihashi 2016; Jean‐Baptiste 2008; Krajcer 2010; Xiong 2012).
Ongoing studies
We identified two ongoing studies (NCT02822560; Vierhout 2015). See Characteristics of ongoing studies for details. Future updates of this review will include any data available at that time.
Risk of bias in included studies

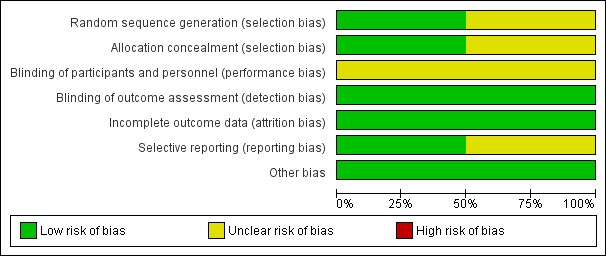
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Nelson 2014 reported randomisation by study site, using two block sizes (3 or 6) with random choice of block size order. The study used 2:1 randomisation (PEVAR:FE), with equal allocation to the two PEVAR groups (PG, PS). Nelson 2014 provided centres with a set of sealed randomised envelopes. Upon a participant meeting eligibility screening criteria, the next sequential envelope was opened and the assignment immediately allocated. Nelson 2014 was therefore assessed to be at low risk of selection bias. Torsello 2003 did not report on either random sequence allocation or allocation concealment. This made it impossible to make a formal judgement on the risk of bias generated and was judged to be at unclear risk of selection bias.
Blinding
Both Nelson 2014 and Torsello 2003 did not report on blinding of trial participants and outcome assessments, and it is impossible to blind personnel performing the procedure. We judged both studies to be at an unclear risk of performance bias. This is unlikely to affect the risk of bias as the outcomes selected by the authors of both papers, and those analysed by this review, were not susceptible to bias from a lack of blinding, so both studies were assessed as being at low risk of detection bias.
Incomplete outcome data
Both Nelson 2014 and Torsello 2003 had complete follow‐up of all participants at 30 days or in‐hospital, and were at low risk of attrition bias. Nelson 2014 had 9% loss to follow‐up at six months, which introduced attrition bias for this isolated outcome. Loss to follow‐up was higher in the cut‐down femoral artery access group (7/50, 14%) than the totally percutaneous group (8/100, 8%), however we did not find this difference to be statistically significant (P = 0.53). Reasons for loss to follow up were given for each group. In the totally percutaneous group six participants withdrew, and two participants were missed. In the cut‐down femoral artery access group, three participants withdrew, two participants were missed, and two refused six‐month follow‐up. The same proportion of participants in each group were withdrawn (6%). Twice as many participants were missed in the cut‐down femoral artery access group (2/50, 4%) compared with the totally percutaneous group (2/100, 2%) and a further two participants (4%) of the cut‐down femoral artery access group refused six‐month follow‐up.
Selective reporting
Nelson 2014 clearly stated its primary and secondary outcomes and reported on these fully with raw data presented, and was assessed as being at low risk of selective reporting bias. Torsello 2003 did not outline its selected outcomes and therefore it was prone to a higher risk of bias due to selective reporting of the results. However, major outcomes identified by this review appeared to have been reported so we have assessed it as being at an unclear risk of reporting bias.
Other potential sources of bias
No other sources of bias were identified within Nelson 2014 or Torsello 2003.
Effects of interventions
See summary of findings Table for the main comparison
Primary outcomes
Short‐term mortality rates (30‐day or in‐hospital, i.e. procedure‐related)
Torsello 2003 recorded no short‐term or operative mortality in either group. Nelson 2014 reported one cardiac death on day 28 in the percutaneous group. See Analysis 1.1 (RR 1.50, 95% CI 0.06 to 36.18; participants = 181; studies = 2; P = 0.8). Note that although Analysis 1.1 considered two studies, Torsello 2003 reported no mortality and therefore did not contribute to the effect estimate in this analysis.
Aneurysm exclusion (no flow into the abdominal aortic aneurysm (AAA) sac on follow‐up imaging) 30 days after the procedure
Torsello 2003 did not provide data on aneurysm exclusion post procedure.
Nelson 2014 conducted follow up CT scans at 28 days, with one participant in the cut‐down femoral artery access group (n = 50) requiring a type 1a endoleak repair within one month post‐procedure.
Wound infection rate (30‐day or in‐hospital)
Both Torsello 2003 and Nelson 2014 reported that there were no wound infections in either group.
Secondary outcomes
Major complications (30 day or in‐hospital)
Torsello 2003 reported two major complications in the cut‐down femoral artery access group and four major complications in the percutaneous group. In one case in the percutaneous group, the Prostar device was unsuccessful in closing the arterial entry site, and conversion to an open groin incision was necessary. In a further two cases surgical repair of the access artery was necessary due to bleeding in one, and arterial thrombosis in the other. Two participants (one in each group) developed post‐operative femoral artery occlusion requiring surgical thrombectomy. One participant in the cut‐down femoral artery access group required surgical repair of the access artery due to arterial thrombosis.
Nelson 2014 reported "unsuccessful treatment" due to procedural technical failure, a major adverse event or a vascular complication in 17 endovascular participants and 11 cut‐down femoral artery access participants. The number of major complications exceeded the number of participants suffering major complications, indicating some participants suffered more than one major complication.
In the cut‐down femoral artery access group 14 major complications occurred (11 participants): neurological complication (3), renal failure (1), respiratory complication (1), secondary procedure (2), femoral neuropathy (1), thrombosis (3), vascular injury (2). There was one endovascular device failure which required switching to a lower‐profile Endologix system for EVAR and external iliac artery stenting. In the percutaneous group 26 major complications occurred (17 participants): cardiac morbidity (2), renal failure (3), respiratory complication (3), secondary procedure (1), thrombosis (4), vascular injury (4). Nine procedural technical failures were reported; eight ipsilateral pre‐close failures requiring surgical cut down/return to operating theatre and one thrombectomy/iliac stenting for thrombosis.
When the numbers of participants experiencing adverse events were combined, there was no difference in major complications between the percutaneous and cut‐down femoral artery access groups (RR 0.91, 95% CI 0.5 to 1.68; participants = 181; studies = 2; P = 0.77; see Analysis 1.3). Little heterogeneity was detected between the studies (I2 = 20%).
Long term complications (12 months) and mortality
Torsello 2003 only included in‐hospital data and did not report any long‐term complications or mortality.
Nelson 2014 did not report 12 month data, however did report trial data at 6 months. Between 31 and 210 days, nine major adverse events or vascular complications were reported in percutaneous participants, and four events were reported in cut‐down femoral artery access participants. In the endovascular group the following occurred: renal failure (1), ABI decrease (not requiring intervention) (3), cancer death (1), critical renal artery stenosis requiring stenting (1), and endoleak requiring secondary procedure (3). In the cut‐down femoral artery access group the following occurred: cancer death (1), endoleak requiring secondary procedure (2), lymphocele (1). This was assessed as not statistically significant by the study authors (the P value was not reported by the study authors but was calculated by the review authors as P = 0.96. See Analysis 1.4 (RR 1.03, 95% CI 0.34 to 3.15; participants = 134; studies = 1)).
In Nelson 2014 loss to follow‐up at six months was 9%. Loss to follow‐up was higher in the cut‐down femoral artery access group (7/50, 14%) than the totally percutaneous group (8/100, 8% ‐ note only 100 participants as one participant died in hospital and was therefore not included in the six‐month follow‐up). Loss to follow‐up was broken down into the following reasons in the totally percutaneous group: participant withdrawn (6), participant missed (2). In the cut‐down femoral artery access group loss to follow up was due to: participant withdrawn (3), participant missed (2), participant refused follow‐up (2). The same proportion of participants in each group were withdrawn (6%). Twice as many participants were missed in the cut‐down femoral artery access group (2/50, 4%) compared with the totally percutaneous group (2/100, 2%) and a further two participants (4%) of the cut‐down femoral artery access group refused six‐month follow‐up.
Bleeding complications and haematoma
Torsello 2003 reported one bleeding‐related complication requiring surgical repair of the access artery in the percutaneous group. There was no significant difference between groups regarding blood loss (haemoglobin or haematocrit). Nelson 2014 reported no haematomas in either group. Six cases of bleeding were reported in the percutaneous group, and four cases of bleeding were reported in the cut‐down femoral artery access group. There was no significant difference in estimated blood loss or blood transfusion requirements between groups.
When the studies were combined, there was no difference in bleeding‐related complications between the percutaneous and cut‐down femoral artery access groups (RR 0.94, 95% CI 0.31 to 2.82; participants = 181; studies = 2; P = 0.91; see Analysis 1.5). No heterogeneity was detected between the studies (I2 = 0%).
Operating time
Torsello 2003 reported significant differences in operating time (percutaneous 86.7 ± 27 minutes versus cut‐down femoral artery access 107.8 ± 38.5 minutes; P < 0.05). Nelson 2014 reported a significant difference in operating time (percutaneous group PG 107 ± 45 minutes versus cut‐down femoral artery access 141 ± 73 minutes, P < 0.01, and percutaneous group PS 95 ± 35 minutes versus cut‐down femoral artery access 141 ± 73 minutes, P < 0.01).
To facilitate meta‐analysis, the results of the two different percutaneous groups (PS, PG) were combined using the methods described in the Cochrane Handbook (Deeks 2011).
When the two studies were combined, there was a difference in operating time between the percutaneous and cut‐down femoral artery access groups (MD ‐31.46 minutes; 95% CI ‐47.51 to ‐15.42 minutes; participants = 181; studies = 2; P = 0.0001; see Analysis 1.6) favouring percutaneous access. No significant heterogeneity was detected between the studies (I2 = 25%).
Duration of ITU stay
Torsello 2003 did not report on duration of ITU stay.
Nelson 2014 reported no difference in length of ITU stay (hours) between groups (cut‐down femoral artery access 35 ± 38, percutaneous PG 26 ± 9, percutaneous PS 31 ± 15; percutaneous PG versus cut‐down femoral artery access P = 0.269, percutaneous PS versus cut‐down femoral artery access P = 0.61).
Duration of hospital stay.
Torsello 2003 did not report on duration of hospital stay.
Nelson 2014 reported no difference in time to hospital discharge (days) between groups (cut‐down femoral artery access 1.8 ± 2.4, percutaneous PG 1.3 ± 0.7, percutaneous PS 1.4 ± 0.9; percutaneous PG versus cut‐down femoral artery access P = 0.135, percutaneous PS versus cut‐down femoral artery access P = 0.213).
Discussion
Summary of main results
This review aimed to compare the clinical outcomes of percutaneous access with cut‐down femoral artery access in elective bifurcated abdominal endovascular aneurysm repair (EVAR). With regard to the primary outcomes mortality and aneurysm exclusion, at least equivalence, but not necessarily superiority, over cut‐down EVAR had to be demonstrated to allow a recommendation, as percutaneous interventions may still be preferable if there are fewer wound problems and reduced length of hospital stay.
The two included studies, Torsello 2003 and Nelson 2014, recorded only one operative mortality and one endoleak requiring repair in the 30‐day follow‐up. Wound infection rates were zero across both studies. The results of the meta‐analysis indicated no significant difference in 30‐day major complication rate or bleeding across the studies. Six‐month data from one study (Nelson 2014), continued to show no significant difference in major complications and mortality between percutaneous and cut‐down femoral artery access groups.
We detected a difference in operating time, with percutaneous access taking less time than cut‐down femoral artery access, with a significantly shorter operating time representing a potential benefit.
The Nelson 2014 study reported no significant difference in ITU stay between groups. Nelson 2014 also reported time from procedure to hospital discharge (days). The study authors found no difference between percutaneous and cut‐down femoral artery access groups (percutaneous PG vs cut‐down femoral artery access P = 0.135, percutaneous PS vs cut‐down femoral artery access P = 0.213). This was not one of the outcomes selected by this review and we cannot confirm its validity given the age of the study. However, this is in contrast to previous hypotheses that percutaneous access may reduce length of hospital stay by proxy of fewer wound complications.
Torsello 2003 also reported on the costs of the percutaneous and cut‐down femoral artery access interventions. The study investigators identified a higher cost of percutaneous interventions (percutaneous EUR 474.7 ± 109.7) versus cut‐down femoral artery access (EUR 375.5 ± 153.3; P < 0.01), which was, in their view, related to the cost of closure devices.
Overall completeness and applicability of evidence
The single biggest limitation of this review is that only two studies, one study with a small sample size, were included. This gave a pool of 181 participants to examine, 151 from one study and 30 from the other. Clearly for a systematic review this is a low number of studies and limited pool of participants, and limits the widespread applicability of the results. There was a significant difference in the participant inclusion criteria between the studies. The Torsello 2003 study included participants with calcification of the femoral artery, scars in the groin or obesity, which are considered risk factors for wound complications. By contrast, the Nelson 2014 study was more selective of its participants, specifically excluding the morbidly obese and those with significant scarring at the arterial access site. Not enough data regarding the co‐morbidities of the Torsello 2003 participants were reported to examine this further. This may have an influence on the outcomes included, however overall this was not deemed significant as all other aspects of the trial conduction were comparable. Also of note were the differing sheath diameters used in the studies. Torsello 2003 reported using multiple sheath sizes, while Nelson 2014 reported use of only 21F sheaths. This again may influence outcomes and may not be reflective of modern practice, where lower profile sheaths can be found.
This review did not identify any studies, meeting inclusion criteria, considering the fascia suture technique, which has been reported as a potential alternative to closure devices for percutaneous access (Larzon 2006; Larzon 2015).
The review has identified two ongoing studies (NCT02822560; Vierhout 2015), which may be suitable for inclusion in future reviews and could greatly increase the pool of data available for analysis.
Quality of the evidence
Of the two studies, the Nelson 2014 study was methodologically stronger, had a large sample size, good follow‐up and reported pre‐defined outcome data with minimal risk of bias. The Torsello 2003 study had more limitations, namely a failure to adequately report the method of randomisation, allocation concealment and the pre‐selected outcomes. Although in general it was a study of moderate quality with complete follow‐up of all included participants, it remains hampered by its small sample size as mentioned above.
We used the GRADEpro GDT software (http://gradepro.org/) to assess the overall quality of evidence in relation to each review outcome. We graded bleeding complications and haematoma, and operating time, as high‐quality evidence. Bleeding complications and haematoma, were well documented in both studies, with no significant bias or confounding. Operating time was documented in both studies with no significant bias identified. Short‐term mortality, failure of aneurysm exclusion, wound infection rate, and major complications we graded as moderate in quality. We downgraded short‐term mortality by one evidence level due to a low number of events and wide confidence interval. We downgraded failure of aneurysm exclusion by one evidence level due to having data from one study and a low event rate (Nelson 2014). We downgraded wound infection rate by one evidence level as no wound infections were reported. We graded long‐term complications as moderate quality as it involved only the Nelson 2014 study. Although losses to follow‐up were present at six months, these were well described and there was no significant difference in the rate of loss between the percutaneous and cut‐down access groups. We downgraded operating time to moderate quality, as the included studies reported this as a secondary outcome and were not adequately powered for this outcome.
Potential biases in the review process
The bias within the review process was minimised as far as possible. The most important aspect of this was a comprehensive literature search, encompassing grey literature as well as published sources. The review is limited by the number of studies included and as such no firm conclusions can be drawn. In the future, a further review should be conducted when a bigger pool of data from multiple larger studies can be identified. Hospital duration should be included alongside ITU stay, as this is a major determinant of overall procedure cost. Neither included study reported one‐year outcomes. It may be beneficial to examine medium‐term outcomes at less than one year, for example six months, if suitable outcomes are reported at the same time across studies.
Agreements and disagreements with other studies or reviews
A previous review (Malkawi 2010) examined 22 papers (one randomised, 10 non‐randomised and 11 retrospective studies). The review concluded that the percutaneous approach appeared safe and effective with low access‐related complications, but that further research was required to identify suitable candidates for the percutaneous approach. This broadly matches with the findings of the present review with regard to safety and efficacy. However, due to the nature of this review (only RCTs were included) the pool of data for this review was substantially smaller and, as such, no firm conclusions were reached. No significant changes in the conclusion have been drawn since the first version of this review.

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 1 Short‐term mortality rate (30‐day or in‐hospital).

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 2 Aneurysm exclusion.

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 3 Major complications.

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 4 Major complications (6 months).
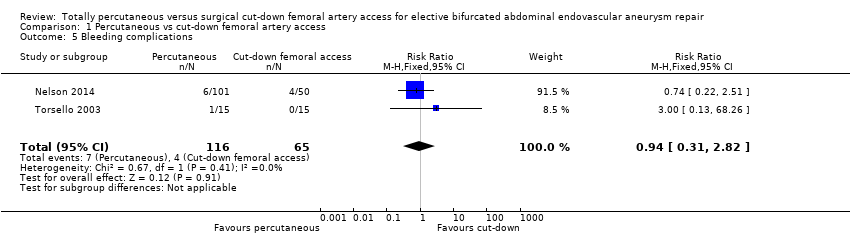
Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 5 Bleeding complications.

Comparison 1 Percutaneous vs cut‐down femoral artery access, Outcome 6 Operating time (minutes).
| Totally percutaneous compared to cut‐down femoral artery access for elective bifurcated abdominal endovascular aneurysm repair | ||||||
| Patient or population: people undergoing elective bifurcated abdominal endovascular aneurysm repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with cut‐down femoral artery access | Risk with totally percutaneous | |||||
| Short‐term mortality rate (30‐day or in‐hospital) | See comment | See comment | RR 1.50 | 181 | ⊕⊕⊕⊝ | It was not possible to calculate risk as only one event occurred. Note that although 2 RCTs included, only one contributes to effect estimate (no events in Torsello 2003) |
| Failure of aneurysm exclusion | Study population | RR 0.17 (0.01 to 4.02) | 151 | ⊕⊕⊕⊝ | ||
| 20 per 1000 | 3 per 1000 | |||||
| Wound infection rate (30‐day or in‐hospital) | See comment | See comment | not estimable | 181 | ⊕⊕⊕⊝ | Risk and relative effect were not estimable as no events occurred |
| Major complications (30‐day or in‐hospital) | Study population | RR 0.91 | 181 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 182 per 1000 | |||||
| Long term complications | Study population | RR 1.03 | 134 | ⊕⊕⊕⊝ | ||
| 95 per 1000 | 98 per 1000 | |||||
| Bleeding complications and haematoma (30‐day or in‐hospital) | Study population | RR 0.94 | 181 | ⊕⊕⊕⊕ | ||
| 62 per 1000 | 58 per 1000 | |||||
| Operating time (minutes) | The mean operating time was 99 minutes | The mean operating time in the intervention group was 31.46 minutes lower (47.51 lower to 15.42 lower) | ‐ | 181 | ⊕⊕⊕⊝ | |
| * The basis for the assumed risk for 'Study population' was the average risk in the comparison group (i.e. total number of participants with events divided by the total number of participants in the comparison group included in the meta‐analysis. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) and calculated where possible from the data provided in the studies. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded by one level due to the low number of events and imprecision (wide confidence intervals include both harm and benefit) | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Short‐term mortality rate (30‐day or in‐hospital) Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.06, 36.18] |
| 2 Aneurysm exclusion Show forest plot | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.02] |
| 3 Major complications Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.68] |
| 4 Major complications (6 months) Show forest plot | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.34, 3.15] |
| 5 Bleeding complications Show forest plot | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.31, 2.82] |
| 6 Operating time (minutes) Show forest plot | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐31.46 [‐47.51, ‐15.42] |

