Drenaje abdominal para la prevención del absceso intraperitoneal después de una apendicectomía abierta por una apendicitis complicada
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010168.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 09 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Colorrectal
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Li Z: drafted the final review, extracted data from the trials, entered data into RevMan, and carried out the analysis.
Zhao L: extracted data from the trials and entered data into RevMan.
Cheng Y: drafted the protocol, selected which trials to include, and assessed the risk of bias of the trials.
Cheng N: selected which trials to include and assessed the risk of bias of the trials.
Deng Y: revised the final review and secured funding for the review.
Li Z, Zhao L, and Cheng Y: contributed equally to developing the review.
Declarations of interest
Li Z: None known
Zhao L: None known
Cheng Y: None known
Cheng N: None known
Deng Y: None known
Acknowledgements
We would like to thank Cochrane Colorectal Cancer editorial office, Dr. Henning Keinke Andersen and Dr. Anne Sofie Christensen, who assisted in the development of the review, and Dr. Sys Johnsen and Dr. Sara Hallum, who developed the search strategy and ran the literature search. We would also like to thank editors and peer referees for valuable comments to this updated review
Finally, we would like to thank the contribution of authors of the previous version of this review: including Dr. Shiyi Zhou, Dr. Rongxing Zhou, Dr. Jiong Lu, Dr. Sijia Wu, Dr. Xianze Xiong, Dr. Hui Ye, Dr. Yixin Lin and Dr. Taixiang Wu.
Version history
| Published | Title | Stage | Authors | Version |
| 2021 Aug 17 | Abdominal drainage to prevent intra‐peritoneal abscess after appendectomy for complicated appendicitis | Review | Zhuyin Li, Zhe Li, Longshuan Zhao, Yao Cheng, Nansheng Cheng, Yilei Deng | |
| 2018 May 09 | Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | Review | Zhe Li, Longshuan Zhao, Yao Cheng, Nansheng Cheng, Yilei Deng | |
| 2015 Feb 07 | Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | Review | Yao Cheng, Shiyi Zhou, Rongxing Zhou, Jiong Lu, Sijia Wu, Xianze Xiong, Hui Ye, Yixin Lin, Taixiang Wu, Nansheng Cheng | |
| 2012 Oct 17 | Abdominal drainage after appendectomy for complicated appendicitis | Protocol | Yao Cheng, Rongxing Zhou, Sijia Wu, Jiong Lu, Xianze Xiong, Yixin Lin, Taixiang Wu, Hui Ye | |
Differences between protocol and review
We changed the title to reflect the primary outcome and open appendectomy because none of the trials included patients undergoing laparoscopic appendectomy. We included two quasi‐RCTs and performed a sensitivity analysis by excluding the two trials according to the suggestions of the editors and reviewers. None of the included RCTs compared open drain with closed drain (tubes), or early drain removal with late drain removal. Thus, we did not consider these two types of interventions. We added that all patients received similar antibiotic regimens after open appendectomy in the intervention; studies that included participants who did not receive prophylactic antibiotics were excluded, because the use of antibiotic regimens after open appendectomy was found to have a positive effect on clinically relevant outcomes by another Cochrane Review (Andersen 2005). The trials did not report infection at 14 days, therefore the data for 30 days were reported as these data were still considered to be clinically relevant.
We also performed trial sequential analysis (TSA) for the primary outcome intra‐peritoneal abscess. TSA aims to reduce the risk of random error in the setting of repetitive testing of accumulating data, thereby improving the reliability of conclusions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Abdominal Abscess [*prevention & control];
- Appendectomy [*adverse effects];
- Appendicitis [complications, *surgery];
- Drainage [*methods];
- Emergencies;
- Length of Stay;
- Peritoneal Diseases [*prevention & control];
- Postoperative Complications [*prevention & control];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Humans;
PICO
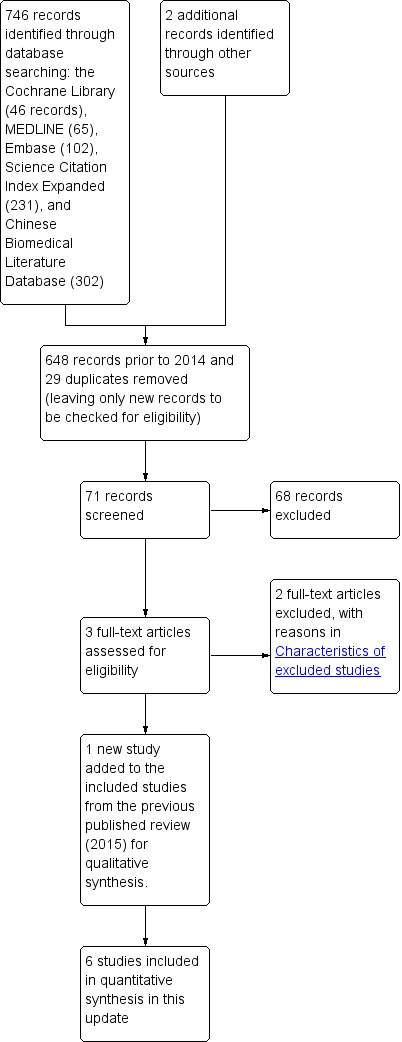
Study flow diagram.
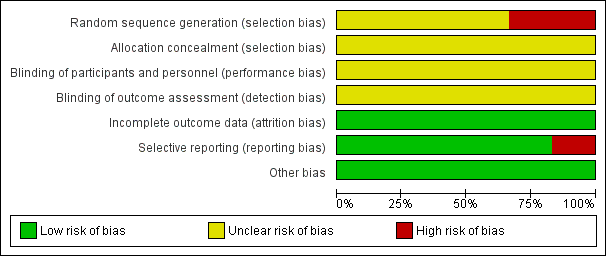
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Trial sequential analysis of drain use versus no drain use for intra‐peritoneal abscess. Analysis was performed with an event rate of 10.7% (Pc) in the control group, a risk ratio reduction of 20%, alpha 5%, beta 20%, and observed diversity 63%. The cumulative Z‐curve did not cross the trial sequential boundaries (inward sloping etched lines). The results showed that the observed diversity‐adjusted required information size was 2,570 participants, corresponding to 20.3% of the total sample size in the included trials. Accordingly, the meta‐analysis did not support or refute an intervention effect as data were too few.

Comparison 1 Drain use versus no drain use, Outcome 1 Intra‐peritoneal abscess.

Comparison 1 Drain use versus no drain use, Outcome 2 Wound infection.

Comparison 1 Drain use versus no drain use, Outcome 3 Morbidity.

Comparison 1 Drain use versus no drain use, Outcome 4 Mortality.

Comparison 1 Drain use versus no drain use, Outcome 5 Hospital stay.

Comparison 2 Drain use versus no drain use (sensitivity analyses by excluding quasi‐randomised trials), Outcome 1 Intra‐peritoneal abscess.
| Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | ||||||
| Patient or population: people undergoing emergency open appendectomy for complicated appendicitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with no drain use | Risk with drain use | |||||
| Intra‐peritoneal abscess Follow‐up: 14 days | 107 per 1000 | 131 per 1000 | RR 1.23 | 453 | ⊕⊝⊝⊝ | |
| Wound infection Follow‐up: 30 days | 254 per 1000 | 511 per 1000 | RR 2.01 | 478 | ⊕⊝⊝⊝ | |
| Morbidity Follow‐up: 30 days | 67 per 1000 | 445 per 1000 | RR 6.67 | 90 | ⊕⊝⊝⊝ | |
| Mortality Follow‐up: 30 days month | 6 per 1000 | 27 per 1000 | Peto OR 4.88 | 363 | ⊕⊕⊕⊝ | |
| Hospital stay (days) | The mean hospital stay in the control groups was 4.60 days | The mean hospital stay in the intervention groups was | MD 2.17 days higher | 298 | ⊕⊝⊝⊝ | |
| Hospital cost | Not reported | |||||
| Pain | Not reported | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk is the mean comparison group proportion in the studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded two levels for very serious risk of bias. b Downgraded one level for severe inconsistency (substantial heterogeneity as indicated by the I2 statistic). c Downgraded one level for serious imprecision. For abscess, morbidity and infection, the confidence interval includes appreciable benefit and harm, and the sample size is small. For mortality, there are few events (8 deaths in total) d Downgraded one level for serious imprecision (small sample size). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intra‐peritoneal abscess Show forest plot | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.47, 3.21] |
| 2 Wound infection Show forest plot | 5 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.88, 4.56] |
| 3 Morbidity Show forest plot | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 6.67 [2.13, 20.87] |
| 4 Mortality Show forest plot | 4 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.88 [1.18, 20.09] |
| 5 Hospital stay Show forest plot | 3 | 298 | Mean Difference (IV, Random, 95% CI) | 2.17 [1.76, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intra‐peritoneal abscess Show forest plot | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.28, 2.02] |

