تاثیر استفاده از درناژ شکمی برای پیشگیری از آبسههای داخل صفاقی پس از آپاندکتومی باز در آپاندیسیتهای عارضهدار
چکیده
پیشینه
آپاندکتومی (appendectomy)، جراحی برداشتن آپاندیس، سابقا برای آپاندیسیتهای حاد انجام میشد. بیمارانی که تحت جراحی آپاندکتومی برای آپاندیسیتهای عارضهدار قرار میگیرند، که تحت عنوان آپاندیسیتهای گانگرن یا سوراخ شده (gangrenous or perforated appendicitis) تعریف میشوند، به احتمال زیاد از عوارض پس از جراحی رنج میبرند. استفاده روزمره از درناژ شکمی (abdominal drainage) برای کاهش عوارض پس از جراحی آپاندکتومی در آپاندیسیتهای عارضهدار، قابل بحث است.
این یک نسخه بهروز شده از مروری است که اولین بار در سال 2015 منتشر شده است.
اهداف
ارزیابی ایمنی و اثربخشی درناژ شکمی برای پیشگیری از ایجاد آبسههای داخل صفاقی (intra‐peritoneal abscess) پس از جراحی باز آپاندکتومی در آپاندیسیتهای عارضهدار.
روشهای جستوجو
پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL؛ کتابخانه کاکرین 2017، شماره 6)، Ovid MEDLINE (از 1946 تا 30 جون 2017)، Ovid Embase (از 1974 تا 30 جون 2017)، و Science Citation Index Expanded (از 1900 تا 30 جون 2017)، پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (30 جون 2017)؛ ClinicalTrials.gov (30 جون 2017) و پایگاه اطلاعاتی منابع علمی بیومدیکال چین (CBM) (از 1978 تا 30 جون 2017) را جستوجو کردیم.
معیارهای انتخاب
تمام کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) را وارد کردیم که درناژ شکمی را با عدم درناژ در افرادی که تحت عمل آپاندکتومی اورژانسی باز برای درمان آپاندیسیتهای عارضهدار قرار گرفته بودند، مقایسه کردهاند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم، کارآزماییهایی را برای ورود شناسایی کرده، دادهها را جمعآوری، و خطر سوگیری (bias) را ارزیابی کردند. با استفاده از Review Manager 5 متاآنالیز را انجام دادیم. برای پیامدهای دو‐حالتی خطر نسبی (RR) (یا نسب شانس پتو (Peto) برای پیامدهای خیلی نادر)، و برای پیامدهای پیوسته، تفاوت میانگین (MD) را با 95% فواصل اطمینان (CI) محاسبه کردیم. برای رتبهبندی کیفیت شواهد از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) استفاده کردیم.
نتایج اصلی
ما شش RCT (521 شرکتکننده) را وارد مرور کردیم که درناژ شکمی را با عدم انجام درناژ در بیمارانی که تحت عمل آپاندکتومی اورژانسی باز برای درمان آپاندیسیتهای عارضهدار قرار گرفته بودند، مقایسه کردند. مطالعات در آمریکای شمالی، آسیا و آفریقا انجام شدند. عمده شرکتکنندگان آپاندیس سوراخ شده (perforated appendiciti) با التهاب صفاق (peritonitis) موضعی یا کلی داشتند. تمام شرکتکنندگان رژیمهای آنتیبیوتیکی را پس از جراحی باز آپاندکتومی دریافت کردند. هیچ یک از کارآزماییها خطر پائین سوگیری نداشتند.
شواهد کافی برای تعیین تاثیر درناژ شکمی و عدم درناژ بر آبسههای داخل صفاقی در 14 روز (RR: 1.23؛ 95% CI؛ 0.47 تا 3.21؛ 5 RCT؛ 453 شرکتکننده؛ شواهد با کیفیت بسیار پائین) یا برای عفونت زخم (wound infection) در 14 روز (RR: 2.01؛ 95% CI؛ 0.88 تا 4.56؛ 5 RCT؛ 478 شرکتکننده؛ شواهد با کیفیت بسیار پائین) وجود نداشت. افزایش خطر 30 روزه در نرخ عوارض کلی (موربیدیتی) در گروه درناژ با شواهد با کیفیت بسیار پائین ارزیابی شد (RR: 6.67؛ 95% CI؛ 2.13 تا 20.87؛ 1 RCT؛ 90 شرکتکننده). تعداد هفت مورد مرگومیر در گروه درناژ (تعداد: 183 نفر) در مقایسه با افرادی که در گروه عدم درناژ قرار داشتند (تعداد: 180 نفر) اتفاق افتاد، معادل افزایش خطر مرگومیر 30 روزه از 0.6% تا 2.7% (نسبت شانس (OR) پتو: 4.88؛ 95% CI؛ 1.18 تا 20.09؛ 4 RCT؛ 363 شرکتکننده؛ شواهد با کیفیت متوسط). شواهدی با کیفیت بسیار پائین وجود دارد که نشان میدهند درناژ در مقایسه با گروه عدم درناژ، مدت بستری را در بیمارستان تا 2.17 روز افزایش میدهد (95% CI؛ 1.76 تا 2.58؛ 3 RCT؛ 298 شرکتکننده).
سایر پیامدهای مشخص، هزینههای بیمارستانی، درد، و کیفیت زندگی، در هیچ کدام از مطالعات وارد شده، گزارش نشده بودند.
نتیجهگیریهای نویسندگان
کیفیت شواهد موجود بسیار پائین است. تاثیر درناژ شکمی بر پیشگیری از آبسههای داخل صفاقی یا عفونت زخم پس از جراحی باز آپاندکتومی برای بیماران مبتلا به آپاندیسیت عارضهدار، نامطمئن است. افزایش نرخ عوارض کلی و مدت بستری در بیمارستان برای گروه درناژ در مقایسه با گروه عدم درناژ، همچنان موضوعی با عدم قطعیت بسیار است. بنابراین، هیچ شواهدی برای هر گونه بهبود بالینی با استفاده از درناژ شکمی در بیماران تحت عمل باز آپاندکتومی برای آپاندیسیتهای عارضهدار، وجود ندارد. افزایش خطر مرگومیر با درناژ از هشت مورد مرگومیر مشاهده شده در تنها 400 فردی که مورد مطالعه قرار گرفتهاند، ناشی میشود. مطالعات بیشتری برای تعیین تاثیر درناژ بر پیامدهای قابل اطمینانتر موربیدیتی و مورتالیتی لازم است.
PICOs
خلاصه به زبان ساده
استفاده از درناژ پس از جراحی باز آپاندکتومی برای درمان آپاندیسیتهای عارضهدار
سوال ما
آیا درناژ میتواند بروز آبسههای داخل صفاقی (تجمع موضعی چرک در شکم یا لگن) را پس از جراحی باز آپاندکتومی (appendectomy) (برداشتن آپاندیس از طریق یک برش بزرگ در پائین شکم، معروف به لاپاروتومی) برای آپاندیسیتهای عارضهدار کاهش دهد.
پیشینه
آپاندیسیت (appendicitis) به التهاب آپاندیس برمیگردد. آپاندکتومی، جراحی برای برداشتن آپاندیس، اغلب در افرادی که آپاندیسیست حاد دارند انجام میشود. افرادی که تحت عمل آپاندکتومی برای آپاندیسیتهای عارضهدار قرار میگیرند، که تحت عنوان آپاندیسیت گانگرن (gangrenous) (بافت نرم مرده) یا سوراخ ده (ترکیده) شناخته میشود، بیشتر از عوارض پس از جراحی رنج میبرند. انجام روتین درناژ جراحی برای پیشگیری از تشکیل آبسههای داخل صفاقی پس از آپاندکتومی برای آپاندیسیتهای عارضهدار، موضوع بحثبرانگیزی است که مورد سوال قرار گرفته است.
ویژگیهای مطالعه
تمام مطالعات مرتبط را تا 30 جون 2017 جستوجو کردیم.
شش مطالعه بالینی را شامل مجموع 521 شرکتکننده شناسایی کردیم. تمام شش مطالعه استفاده از درناژ را در برابر عدم استفاده از آن در افرادی که عمل اورژانسی باز آپاندکتومی برای آپاندیسیت پیچیده داشتند، مقایسه کرده بود. مطالعات در امریکا، هند، کنیا، پاکستان و ترکیه انجام شده بودند. سن افراد در کارآزماییهای بین 0 تا 82 سال بود.
نتایج کلیدی
تجزیهوتحلیلها نتوانستند تفاوت را در تعداد افراد مبتلا به آبسههای داخل صفاقی یا عفونت زخم هنگام استفاده یا عدم استفاده از درناژ نشان دهند. نرخ مرگومیر در گروه درناژ بالاتر از گروه بدون درناژ بود. مدت زمان بستری در بیمارستان (تقریبا دو روز با 43.5% افزایش در «میانگین» زمان بستری) در گروه درناژ طولانیتر از گروه بدون درناژ بود. هیچ یک از مطالعات هزینهها، درد، و کیفیت زندگی را گزارش نکردند. بهطور کلی، هیج شواهدی برای هر گونه بهبود بالینی با استفاده از درناژ شکمی در افراد تحت عمل باز آپاندکتومی برای آپاندیسیتهای عارضهدار وجود ندارد.
کیفیت شواهد
تمام مطالعات وارد شده نواقصی را در زمینه کیفیت روششناسی یا گزارشدهی پیامدها داشتند. بهطور کلی، کیفیت شواهد موجود بسیار پائین است.
Authors' conclusions
Summary of findings
| Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | ||||||
| Patient or population: people undergoing emergency open appendectomy for complicated appendicitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with no drain use | Risk with drain use | |||||
| Intra‐peritoneal abscess Follow‐up: 14 days | 107 per 1000 | 131 per 1000 | RR 1.23 | 453 | ⊕⊝⊝⊝ | |
| Wound infection Follow‐up: 30 days | 254 per 1000 | 511 per 1000 | RR 2.01 | 478 | ⊕⊝⊝⊝ | |
| Morbidity Follow‐up: 30 days | 67 per 1000 | 445 per 1000 | RR 6.67 | 90 | ⊕⊝⊝⊝ | |
| Mortality Follow‐up: 30 days month | 6 per 1000 | 27 per 1000 | Peto OR 4.88 | 363 | ⊕⊕⊕⊝ | |
| Hospital stay (days) | The mean hospital stay in the control groups was 4.60 days | The mean hospital stay in the intervention groups was | MD 2.17 days higher | 298 | ⊕⊝⊝⊝ | |
| Hospital cost | Not reported | |||||
| Pain | Not reported | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk is the mean comparison group proportion in the studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded two levels for very serious risk of bias. b Downgraded one level for severe inconsistency (substantial heterogeneity as indicated by the I2 statistic). c Downgraded one level for serious imprecision. For abscess, morbidity and infection, the confidence interval includes appreciable benefit and harm, and the sample size is small. For mortality, there are few events (8 deaths in total) d Downgraded one level for serious imprecision (small sample size). | ||||||
Background
Description of the condition
Appendicitis refers to inflammation of the appendix. Appendectomy, the surgical removal of the appendix, is performed primarily as an emergency procedure to treat acute appendicitis (Andersen 2005).
Acute appendicitis is the most common cause of acute abdominal pain (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011). The overall incidence of acute appendicitis varies between 76 and 227 cases per 100,000 population per year in different countries (Addiss 1990; Andersson 1994; Andreu‐Ballester 2009; Buckius 2012; Ferris 2017; Körner 1997; Lee 2010; Pieper 1982; Williams 1998). The overall lifetime risk for acute appendicitis is approximately 7% to 8% in the USA, but as high as 16% in South Korea (Addiss 1990; Lee 2010). It affects all age groups, with the highest incidence in individuals 10 to 20 years of age (Addiss 1990; Wilms 2011).
The cause of acute appendicitis is an issue of considerable debate (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011). Acute appendicitis may be associated with obstruction of the appendix lumen (the inside space of the appendix), which could result in increased intraluminal pressure with transmural tissue necrosis (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011). Tissue necrosis is followed by bacterial invasion, which leads to inflammation of the appendix (Andersen 2005; Rehman 2011; Sauerland 2010; Wilms 2011).
Acute appendicitis can be divided into two subgroups: simple appendicitis (e.g. early appendicitis, uncomplicated appendicitis) and complicated appendicitis (e.g. gangrenous appendicitis, perforated appendix without phlegmon or abscess, perforated appendicitis with phlegmon or abscess) (Andersen 2005; Cheng 2017; Simillis 2010). The proportion of complicated appendicitis varies between 15% and 35% in different case series (Andersson 1994; Boomer 2010; Cheng 2017; Cueto 2006; Körner 1997; Livingston 2007; Oliak 2000).
Description of the intervention
Patients with complicated appendicitis usually require appendectomy to relieve symptoms and avoid complications (Andersson 1994; Santacroce 2017). Appendectomy is one of the most common emergency surgical procedures worldwide (Andersen 2005; Rehman 2011; Santacroce 2017; Sauerland 2010; Wilms 2011). There are two types of appendectomy: open appendectomy (removal of the appendix by laparotomy) and laparoscopic appendectomy (removal of the appendix by key‐hole surgery) (Cheng 2012b; Cheng 2015; Yu 2017; Santacroce 2017; Sauerland 2010). Approximately 300,000 appendectomies are performed each year in the USA alone (Hall 2010).
The prognosis of complicated appendicitis is good (Santacroce 2017). The overall mortality rate of complicated appendicitis following appendectomy is less than 1% (Markides 2010; Santacroce 2017). The most common complication after an appendectomy for complicated appendicitis is surgical site infection (e.g. wound infection, intra‐peritoneal abscess) (Andersen 2005; Cueto 2006). Patients with complicated appendicitis are more likely to suffer from surgical site infections than those with simple appendicitis (Markides 2010). Recent published reviews have reported an approximately 10% incidence of surgical site infection (Markides 2010; Santacroce 2017). Patients with surgical site infections usually present with a mild fever, abdominal pain, and bowel dysfunction (e.g. diarrhoea, constipation) (Santacroce 2017). Surgical site infections are associated with increased hospital stays and costs (Horan 1992; Mangram 1999).
Various methods for the prevention of surgical site infections have been suggested, including antibiotic regimens, delayed wound closure, and the use of laparoscopic appendectomy (Andersen 2005; Duttaroy 2009; Markides 2010; Sauerland 2010). One of the most common and convenient interventions might be the application of surgical drains after appendectomy for patients with complicated appendicitis (Petrowsky 2004).
Surgical drains are used to remove blood, pus, and other body fluids from wounds (Durai 2009). There are two primary types of surgical drains: open and closed. An open drain is not air tight (Durai 2009; Gurusamy 2007a; Samraj 2007; Wang 2015). A closed drain consists of a tube that drains into a bag or bottle, the contents of which is air tight (Durai 2009; Gurusamy 2007a; Samraj 2007; Wang 2015).
How the intervention might work
The primary reasons for placing an abdominal drain after an appendectomy are as follows: (i) drainage of established intra‐peritoneal collection; (ii) prevention of further fluid accumulation; (iii) identification and drainage of faecal fistula (Allemann 2011; Gurusamy 2007a; Jani 2011).
The use of abdominal drainage can avoid the accumulation of intra‐peritoneal dirty collections, thereby reducing bacterial contamination of the surgical site (Greenall 1978; Jani 2011; Stone 1978; Tander 2003). Theoretically, abdominal drainage has the potential to reduce the rate of surgical site infection (Greenall 1978; Jani 2011; Stone 1978; Tander 2003).
However, abdominal drainage may fail to prevent intra‐peritoneal abscess because a drain may become blocked and thus ineffective within a few hours after appendectomy (Greenall 1978; Haller 1973; Jani 2011; Magarey 1971). Additionally, the drain itself may act as a foreign body, which interferes with wound healing and increases the risk of surgical site infection (Jani 2011; Stone 1978; Magarey 1971). The use of a drain may also increase the length of the patient's hospital stay (Allemann 2011; Jani 2011; Stone 1978; Tander 2003).
Why it is important to do this review
The use of abdominal drainage after open appendectomy for complicated appendicitis is controversial (Narci 2007; Petrowsky 2004; Piper 2011). It may potentially decrease the risk of surgical site infection following open appendectomy, but it is also possible that it may have no therapeutic benefit and may be associated with negative outcomes (Jani 2011; Mustafa 2016; Petrowsky 2004).
The first version of this review was published in 2015 (Cheng 2015). Further randomised controlled trials (RCTs) evaluating the role of abdominal drainage after open appendectomy for complicated appendicitis have been published since the review, and these studies have now been assessed for inclusion and presented in this update.
Objectives
To assess the safety and efficacy of abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) (irrespective of sample size, language, or publication status) that compared drain use and no drain use in patients undergoing open appendectomy for complicated appendicitis. Quasi‐randomised controlled trials (in which the allocation was performed on the basis of a pseudo‐random sequence, e.g. odd/even hospital number or date of birth, alternation) were also included (Chapter 16 in Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011a).
Types of participants
We included all persons (irrespective of age, sex, or race) who underwent emergency open appendectomy for complicated appendicitis (irrespective of gangrenous appendicitis, perforated appendix without phlegmon or abscess, or perforated appendicitis with phlegmon or abscess), and receiving antibiotic regimens after open appendectomy.
Types of interventions
Use of drain (irrespective of type or material) versus no drain.
Types of outcome measures
Primary outcomes
-
Intra‐peritoneal abscess (e.g. intra‐abdominal abscess, pelvic abscess) (14 days).
Secondary outcomes
-
Wound infection (14 days).
-
Morbidity (overall complication rate; graded by the Clavien‐Dindo complications classification system) (30 days).
-
Mortality (30 days).
-
Hospital stay (days).
-
Hospital costs.
-
Pain (30 days, any validated score).
-
Quality of life (30 days, any validated score).
Morbidity was defined by review authors and graded according to the Clavien‐Dindo classification of surgical complications (Clavien 2009). Surgical site infection has been defined and classified as superficial incisional, deep incisional, and organ/space surgical site infection by the Centers for Disease Control and Prevention (CDC) (Anderson 2014; Horan 1992; Mangram 1999). We included intra‐peritoneal abscess (organ/space surgical site infection) as the primary outcome. Wound infection (either superficial or deep incisional surgical site infection) as defined by the study authors.
Search methods for identification of studies
We designed the search strategy with the help of Sys Johnsen (Cochrane Information Specialist of the Cochrane Colorectal Cancer Group). Searches were conducted 30 June 2017 irrespective of language, year, or publication status.
Electronic searches
We searched the following electronic databases with no language or date of publication restrictions:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library) (2017, Issue 6) (Appendix 1);
-
MEDLINE (Ovid) (1946 to 30 June 2017) (Appendix 2);
-
Embase (Ovid) (1974 to 30 June 2017) (Appendix 3);
-
Science Citation Index Expanded (Web of Science) (1900 to 30 June 2017) (Appendix 4);
-
World Health Organization International Trials Registry Platform search portal (apps.who.int/trialsearch/) (30 June 2017);
-
ClinicalTrials.gov (www.clinicaltrials.gov/) (30 June 2017);
-
Chinese Biomedical Literature Database (CBM) (1978 to 30 June 2017).
Searching other resources
Furthermore, we also searched the following databases 30 June 2017:
-
Current Controlled Trials (www.controlled‐trials.com/);
-
Chinese Clinical Trial Register (www.chictr.org/);
-
EU Clinical Trials Register (www.clinicaltrialsregister.eu/).
We also searched the reference lists of identified studies and meeting abstracts via the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) (www.sages.org/) and Conference Proceedings Citation Index to explore further relevant clinical trials. We planned to communicate with the authors of RCTs that were included for further information in the review.
Data collection and analysis
We conducted this systematic review according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and the Cochrane Colorectal Cancer Group Module (Andersen 2016).
Selection of studies
After completing the searches, we merged the search results using the software package Endnote X7 (reference management software) and removed duplicate records. Two review authors (CY, CN) independently scanned the title and abstract of every record identified by the search for inclusion. We retrieved the full text for further assessment if the inclusion criteria were unclear from the abstract. We included eligible studies irrespective of whether they reported the measured outcome data. We detected duplicate publications by identifying common authors, centres, details of the interventions, numbers of participants, and baseline data (Chapter 7, the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011c). We excluded papers that did not meet the inclusion criteria and listed the reasons for their exclusion. A third review author (DY) resolved any discrepancy between the two review authors by discussion.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data, which had been piloted on at least one study in the review. Two review authors (LZ, ZL) extracted the following study characteristics from included studies:
-
methods: study design, total duration study and run in, number of study centres and location, study setting, withdrawals, date of study;
-
participants: number of participants, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria, exclusion criteria;
-
interventions: intervention, comparison;
-
outcomes: primary and secondary outcomes specified and collected, time points reported;
-
notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (LZ, ZL) independently extracted outcome data from included studies. We resolved disagreements by consensus or by involving a third review author (DY). One review author (LZ) copied across the data from the data collection form into Review Manager 5 (version 5.3, RevMan 2014). We double‐checked that the data were entered correctly by comparing the study reports with how the data were presented in the systematic review.
A second review author (ZL) cross‐checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
Two review authors (CY, CN) independently assessed the risk of bias in the included trials, using the Cochrane 'Risk of bias' tool (Chapter 8, the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011d). We assessed risk of bias for the following domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective reporting bias;
-
other sources of bias (baseline imbalances).
We judged each domain as low risk, high risk, or unclear risk of bias according to the criteria used in the Cochrane 'Risk of bias' tool (see Appendix 5) (Chapter 8.5.d, Higgins 2011d). We considered a trial to be at low risk of bias if we assessed the trial as at low risk of bias across all domains. Otherwise, we considered trials at unclear risk of bias or at high risk of bias regarding one or more domains as at high risk of bias. We resolved any difference in opinion by discussion. In case of disagreements, consensus was reached by discussion with a third review author (DY).
We presented the results of the risk of bias in two figures (a 'Risk of bias' graph and a 'Risk of bias' summary) generated by Review Manager 5 (RevMan 2014).
Measures of treatment effect
We performed the meta‐analyses using the software package Review Manager 5.3 (RevMan 2014). For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence interval (Deeks 2011). In the case of rare events (e.g. mortality), we calculated the Peto odds ratio (Peto OR) (Deeks 2011). For continuous outcomes, we calculated the mean difference (MD) with 95% confidence interval (Deeks 2011).
Unit of analysis issues
The unit of analysis was the individual patient. We did not encounter any cluster‐randomised trials.
Dealing with missing data
We contacted the original investigators to request further information in case of missing data. However, there was no reply. Thus, we used only the available data in the analyses.
Assessment of heterogeneity
We described heterogeneity in the data using the Chi2 test (Deeks 2011). We considered a P value less than 0.05 to be statistically significant heterogeneity (Deeks 2011). We also used the I2 statistic to measure the quantity of heterogeneity. In case of statistical heterogeneity or clinical heterogeneity (or both), we performed the meta‐analysis but interpreted the result cautiously and planned to investigate potential sources of the heterogeneity.
Assessment of reporting biases
We planned to perform and examine a funnel plot to explore possible publication biases. However, as the number of trials included was less than 10, we did not produce any funnel plots as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 10 (Sterne 2011).
Data synthesis
We performed the meta‐analyses using the Review Manager 5 software provided by Cochrane (version 5.3, RevMan 2014). For all analyses, we employed the random‐effects model for conservative estimation, except for the Peto OR which only has a fixed‐effect method. We considered a P value less than 0.05 to be statistically significant.
Subgroup analysis and investigation of heterogeneity
We did not perform any planned subgroup analysis because too few trials were included in this review.
Sensitivity analysis
We performed a sensitivity analysis for the primary outcome Intra‐peritoneal abscess by excluding quasi‐randomised controlled trials to determine whether the conclusions were robust to the decisions made during the review process.
Trial sequential analysis
We performed trial sequential analysis (TSA) for the primary outcome if possible. TSA aims to reduce the risk of random error in the setting of repetitive testing of accumulating data, thereby improving the reliability of conclusions (Brok 2008; Wetterslev 2008; Wetterslev 2009). The required information size was calculated on the basis of a risk ratio reduction (RRR) of 20% (Brok 2008; Wetterslev 2008; Wetterslev 2009). The results of the trials were presented as a cumulative Z‐curve. The trial sequential monitoring boundaries were constructed and the diversity‐adjusted required information size calculated with a type 1 error of 5% and a type 2 error of 20% (Brok 2008; Wetterslev 2008; Wetterslev 2009). The results were presented as a graph with the cumulative meta‐analysis results entered. The TSA shows firm evidence of intervention effects (or no intervention effects) if the cumulative Z‐curve crosses the monitoring boundaries; it also shows that additional trials may be needed if the boundaries are not crossed (Brok 2008; Wetterslev 2008; Wetterslev 2009). TSA was performed using Trial Sequential Analysis software (TSA 2011).
'Summary of findings' tables
We evaluated the quality of evidence using the GRADE (Schünemann 2009) approach for each outcome, including any subgroup analysis or sensitivity analysis.
We presented the quality of evidence in 'Summary of finding' table for the following comparison:
-
drainage versus no drainage.
We justified judgements about the quality of the evidence (high, moderate, low, or very low), documented, and incorporated into the reporting of results for each outcome. The quality of evidence could be downgraded by one level (serious concern) or two levels (very serious concerns) applying to each of the following five reasons listed: risk of bias; inconsistency (unexplained heterogeneity, inconsistency of results); indirectness (indirect population, intervention, control, outcomes); imprecision (wide CIs, single trials); and publication bias.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Date of search 30 June 2017.
In this updated review, we identified 746 records through the electronic searches of the Cochrane Library (46 records), MEDLINE (Ovid) (65 records), Embase (Ovid) (102 records), Science Citation Index Expanded (Web of Science) (231 records), and Chinese Biomedical Literature Database (CBM) (302 records). We identified two records through scanning reference lists of the identified randomised controlled trials (Haller 1973; Johnson 1993). Of the 748 records, 677 records had already been assessed for the first version of this updated review (648 records prior to 2014 and 29 duplicates). Of the remaining 71 records, we excluded 68 clearly irrelevant records after reading titles and abstracts. The remaining three records were retrieved in full for further assessment (Mustafa 2016; Beek 2015; Song 2015). Two of these, Beek 2015 and Song 2015, were excluded being non‐randomised studies. The study by Mustafa 2016 was included in this updated review.
We identified six trials ( with a total of 521 participants) comparing drainage with no drainage for persons undergoing appendectomy for complicated appendicitis (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003). Two hundred and sixty‐two persons were randomised to the drainage group and 259 persons to the no drainage group. With so few participants, all of the analyses were underpowered.The study flow diagram is shown in Figure 1.

Study flow diagram.
Included studies
In the first published version of this review from 2015 (Cheng 2015), we included five trials, published between 1973 and 2003 (Dandapat 1992; Haller 1973; Jani 2011; Stone 1978; Tander 2003). In this update, we identified one recent trial (Mustafa 2016), to a total of six included trials (including 521 participants). All the six trials were completed trials and all of these provided data for the analyses. Details of the trials are shown in the table "Characteristics of included studies". Four trials were randomised controlled trials (Dandapat 1992; Jani 2011; Mustafa 2016; Tander 2003), and two trials were quasi‐randomised controlled trials (Haller 1973; Stone 1978). All six trials compared drain use with no drain use for patients undergoing open appendectomy. Studies were conducted in the USA (Haller 1973; Stone 1978), India (Dandapat 1992), Kenya (Jani 2011), Pakistan (Mustafa 2016), and Turkey (Tander 2003). The age of the individuals in the trials varied between 0 years and 82 years. The mean proportion of females varied between 19% and 44%. There was no difference in the characteristics of patients in the intervention group or control in any of the trials. Overall, 32 (6.1%) participants had gangrenous appendicitis, 11 (2.1%) participants had appendiceal abscess, and 478 (91.8%) participants had perforated appendicitis in the trials. All of the participants received antibiotic regimens after open appendectomy. The outcomes measured were intra‐peritoneal abscess, wound infection, morbidity, mortality, and hospital stay.
Excluded studies
One randomised controlled trial was excluded because it focused on extraperitoneal wound drainage (Everson 1977). Another randomised controlled trial was excluded because it compared peritoneal lavage with abdominal drainage (Toki 1995). Two randomised controlled trials were excluded because the antibiotic regimens were used in a non‐random manner (several participants received antibiotic regimens after appendectomy, whereas other participants did not) (Greenall 1978; Magarey 1971). None of the other excluded studies were randomised controlled trials (Allemann 2011; Al‐Shahwany 2012; Beek 2015; Ezer 2010; Johnson 1993; Narci 2007; Piper 2011; Song 2015).
Risk of bias in included studies
The risk of bias of the included studies is shown in Figure 2 and Figure 3. None of the included trials were judged to be of low risk of bias.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was at unclear risk of bias in four trials (Dandapat 1992; Jani 2011; Mustafa 2016; Tander 2003) and high risk of bias in two trials where participants were randomised using pseudo‐random sequences (odd/even hospital number) (Haller 1973; Stone 1978). Allocation concealment was at an unclear risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Blinding
Blinding of participants and personnel was at an unclear risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003). Blinding of outcome assessment was also of unclear risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Incomplete outcome data
There was a low risk of bias for incomplete outcome data in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Selective reporting
The trial protocols were not available for any of the included trials. Five trials reported the primary outcomes of this review (Dandapat 1992; Haller 1973; Jani 2011; Stone 1978; Tander 2003). There may have been some selective outcome reporting in the secondary outcomes (secondary outcomes of this review were not reported), but the review authors considered these five trials to be free of selective reporting for the primary outcomes. One trial was at high risk of selective reporting bias as the primary outcome of the review was not reported (Mustafa 2016).
Other potential sources of bias
No baseline imbalances were observed, therefore this was at a low risk of bias in all six trials (Dandapat 1992; Haller 1973; Jani 2011; Mustafa 2016; Stone 1978; Tander 2003).
Effects of interventions
Primary outcome
Intra‐peritoneal abscess
We identified five trials (453 participants) reporting intra‐peritoneal abscess. The rate of intra‐peritoneal abscess was 15.8% in the drainage group and 10.7% in the no drainage group. There was no evidence of a difference in the rate of intra‐peritoneal abscess (including intra‐abdominal abscess and pelvic abscess) between the groups (risk ratio (RR) 1.23, 95% confidence interval (CI) 0.47 to 3.21; P = 0.67; Analysis 1.1). We downgraded the quality of the evidence to very low due to risk of bias, serious imprecision, and serious inconsistency.
The Trials Sequential Analysis graph showed that the cumulative Z‐curve did not cross the trial sequential boundaries (Figure 4). The analysis showed a diversity‐adjusted required information size of 2570 participants (the number of participants needed to reach firm evidence of an intervention effect of 20% RRR). The number of participants included corresponded to only a small fraction (20.3%) of the diversity‐adjusted required information size. Accordingly, we lack evidence to support or refute an intervention effect as data were too few.

Trial sequential analysis of drain use versus no drain use for intra‐peritoneal abscess. Analysis was performed with an event rate of 10.7% (Pc) in the control group, a risk ratio reduction of 20%, alpha 5%, beta 20%, and observed diversity 63%. The cumulative Z‐curve did not cross the trial sequential boundaries (inward sloping etched lines). The results showed that the observed diversity‐adjusted required information size was 2,570 participants, corresponding to 20.3% of the total sample size in the included trials. Accordingly, the meta‐analysis did not support or refute an intervention effect as data were too few.
Secondary outcomes
Wound infection
We identified 5 trials (478 participants) reporting wound infection. The rate of wound infection was 37.0% in the drainage group and 25.4% in the no drainage group. There was no evidence of a difference in the wound infection rate between the groups (RR 2.01, 95% CI 0.88 to 4.56; P = 0.10; Analysis 1.2). We downgraded the quality of the evidence to very low due to risk of bias, serious imprecision, and serious inconsistency.
Morbidity
We identified one trial (90 participants) reporting overall complication rate (morbidity). The overall complication rate defined according to the Clavien‐Dindo classification was 44.4% in the drainage group and 6.7% in the no drainage group. The overall morbidity was higher in the drainage group than in the no drainage group (RR 6.67, 95% CI 2.13 to 20.87; P = 0.001; Analysis 1.3). We downgraded the quality of the evidence to very low due to risk of bias and serious imprecision.
Mortality
We identified four trials (363 participants) reporting mortality. The death rate was 3.8% in the drainage group and 0.6% in the no drainage group. Mortality was higher in the drainage group than in the no drainage group (Peto odds ratio (OR) 4.88, 95% CI 1.18 to 20.09; P = 0.03; Analysis 1.4). We downgraded the quality of the evidence to moderate due to serious imprecision.
Hospital stay
We identified three trials (298 participants) reporting hospital stay. The mean length of hospital stay was 6.6 days in the drainage group and 4.6 days in the no drainage group. The hospital stay was longer in the drainage group than in the no drainage group [mean difference (MD) 2.17 days (43.5% increase of an 'average' hospital stay); 95% CI 1.76 to 2.58; P < 0.001; Analysis 1.5). We downgraded the quality of the evidence to very low due to risk of bias and serious imprecision.
Hospital costs
None of the trials reported this outcome.
Pain
None of the trials reported this outcome.
Quality of life
None of the trials reported this outcome.
Reporting biases
We did not create funnel plots to assess reporting biases because the number of trials included was fewer than 10 (Sterne 2011).
Sensitivity analysis
We observed no change in the intra‐peritoneal abscess outcome by excluding two quasi‐randomised controlled trials (Haller 1973; Stone 1978) (Analysis 2.1).
Discussion
Summary of main results
Six studies with 521 people contributed data to the primary outcome of this review, and showed no evidence of difference in the incidence of intra‐peritoneal abscess between the drainage group and the no drainage group. Very low‐quality trials furthermore showed that abdominal drainage increased rate of wound infection, overall morbidity, mortality, and length of hospital stay.
The primary reason for the placement of a drain after appendectomy is to prevent intra‐peritoneal abscess (e.g. intra‐abdominal abscess, pelvic abscess). We found that the routine use of abdominal drainage after complicated appendectomy did not reduce the incidence of intra‐peritoneal abscess. The possible reasons for the failure to decrease the incidence of intra‐peritoneal abscess are as follows. First, surgical drains may become blocked by blood or fibrin clots (Schein 2008; Yates 1905). Additionally, surgical drains cannot drain the entire abdominal cavity. Abdominal collections or abscesses can occur despite abdominal drainage (Schein 2008; Yates 1905). Moreover, this review included six trials with only 262 participants undergoing abdominal drainage. This review may not have the statistical power to detect the any clinically meaningful difference between abdominal drainage and no drainage for the prevention of intra‐peritoneal abscess even if such a difference was present. In addition, where differences were detected, confidence in these results is low, as the small numbers means they could be spurious results, and the impact of a bias can be exacerbated in underpowered analyses. Thus, it is not clear whether routine abdominal drainage has any effect on the prevention of intra‐peritoneal abscess in patients undergoing open appendectomy for complicated appendicitis.
Overall completeness and applicability of evidence
All of the trials included patients undergoing emergency open appendectomy for complicated appendicitis (e.g. gangrenous appendicitis and perforated appendicitis). The majority (91.8%) of the patients had perforated appendicitis with local or general peritonitis. Thus, the results of this review are applicable to patients undergoing emergency open appendectomy for perforated appendicitis.
Quality of the evidence
None of the trials were at a low risk of bias. The trials included under each comparison were too few to assess inconsistency and publication bias. Direct comparisons of different types of drain were not available, only comparisons for each type of drain with no drain. In theory, these trials could allow indirect comparisons of the effect of different types of drain, however, small sample sizes and methodological flaws of the trials preclude such indirect comparison. The confidence intervals for the majority of outcomes were wide, indicating that the estimates of effect obtained are imprecise. Overall, we considered the quality of the evidence to be very low (summary of findings Table for the main comparison).
Potential biases in the review process
There were several potential biases of note in the review process. First, there were only six trials with 521 patients included in the review; thus, there was a lack of data on this topic to date. Second, we did not create funnel plots to assess potential publication bias due to the small number of included trials. Third, we did not perform any planned subgroup analyses to assess heterogeneity because of the small number of trials included for each outcome. Additionally, patient selection processes and blinding were unclear for most of the studies. We contacted the original investigators to request further information. However, we did not receive any replies. Moreover, an important source of bias in the included studies was the use of antibiotic regimens (Andersen 2005). For example, the type and length of antibiotic therapy were important confounding factors for various outcomes (e.g. intra‐peritoneal abscess, wound infection) (Andersen 2005). However, the type and length of antibiotic therapy varied a great deal in different trials. It was difficult to perform subgroup analyses to assess the heterogeneity. A final point is that the imputation of the standard deviation for hospital stay from the range might also introduce bias into this review.
Agreements and disagreements with other studies or reviews
There is increasing evidence in Cochrane Reviews that routine abdominal drainage after various abdominal operations is not essential (Cheng 2016; de Jesus 2004; Gurusamy 2007a; Gurusamy 2013; Gurusamy 2007b; Wang 2015). The routine use of surgical drains has been questioned in other areas, including thyroid, gynaecological, and orthopaedic surgeries (Charoenkwan 2017; Gates 2013; Parker 2007; Samraj 2007).
The systematic review by Petrowsky and colleagues (Petrowsky 2004) included five trials comparing drain use with no drain use in patients undergoing appendectomy for complicated appendicitis (Dandapat 1992; Greenall 1978; Haller 1973; Magarey 1971; Stone 1978). Two of these five trials, in which antibiotic regimens were used in a non‐random manner (some participants received antibiotic regimens after appendectomy, whereas other participants did not), were not included in this review (Greenall 1978; Magarey 1971). Petrowsky and colleagues concluded that abdominal drainage did not reduce postoperative complications and appeared harmful with respect to the development of faecal fistula (Petrowsky 2004). Thus, these authors recommended that abdominal drainage should be avoided at any stage of appendicitis (Petrowsky 2004). This review has not reached definitive conclusions, in part because the number of participants included is low for detecting a benefit on intra‐peritoneal abscess. A sample size of 2570 would be required to detect an absolute reduction in the intra‐peritoneal abscess rate of 2% (from 10% to 8%) at 80% power and an alpha‐error set at 0.05.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Trial sequential analysis of drain use versus no drain use for intra‐peritoneal abscess. Analysis was performed with an event rate of 10.7% (Pc) in the control group, a risk ratio reduction of 20%, alpha 5%, beta 20%, and observed diversity 63%. The cumulative Z‐curve did not cross the trial sequential boundaries (inward sloping etched lines). The results showed that the observed diversity‐adjusted required information size was 2,570 participants, corresponding to 20.3% of the total sample size in the included trials. Accordingly, the meta‐analysis did not support or refute an intervention effect as data were too few.
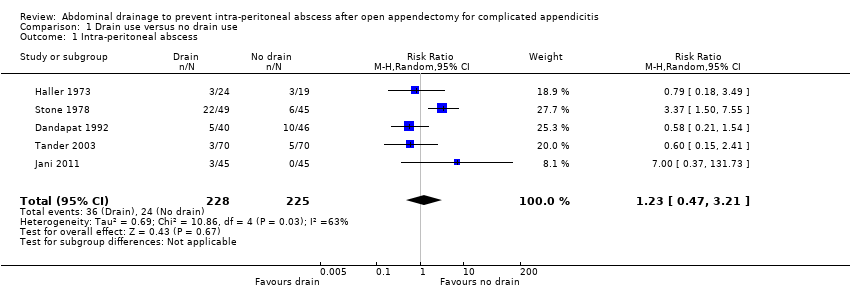
Comparison 1 Drain use versus no drain use, Outcome 1 Intra‐peritoneal abscess.

Comparison 1 Drain use versus no drain use, Outcome 2 Wound infection.

Comparison 1 Drain use versus no drain use, Outcome 3 Morbidity.

Comparison 1 Drain use versus no drain use, Outcome 4 Mortality.
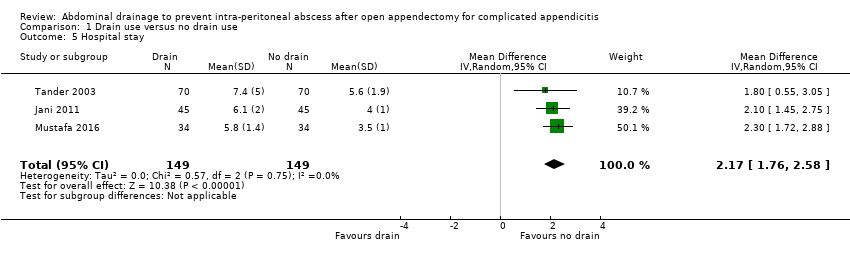
Comparison 1 Drain use versus no drain use, Outcome 5 Hospital stay.

Comparison 2 Drain use versus no drain use (sensitivity analyses by excluding quasi‐randomised trials), Outcome 1 Intra‐peritoneal abscess.
| Abdominal drainage to prevent intra‐peritoneal abscess after open appendectomy for complicated appendicitis | ||||||
| Patient or population: people undergoing emergency open appendectomy for complicated appendicitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with no drain use | Risk with drain use | |||||
| Intra‐peritoneal abscess Follow‐up: 14 days | 107 per 1000 | 131 per 1000 | RR 1.23 | 453 | ⊕⊝⊝⊝ | |
| Wound infection Follow‐up: 30 days | 254 per 1000 | 511 per 1000 | RR 2.01 | 478 | ⊕⊝⊝⊝ | |
| Morbidity Follow‐up: 30 days | 67 per 1000 | 445 per 1000 | RR 6.67 | 90 | ⊕⊝⊝⊝ | |
| Mortality Follow‐up: 30 days month | 6 per 1000 | 27 per 1000 | Peto OR 4.88 | 363 | ⊕⊕⊕⊝ | |
| Hospital stay (days) | The mean hospital stay in the control groups was 4.60 days | The mean hospital stay in the intervention groups was | MD 2.17 days higher | 298 | ⊕⊝⊝⊝ | |
| Hospital cost | Not reported | |||||
| Pain | Not reported | |||||
| Quality of life | Not reported | |||||
| *The basis for the assumed risk is the mean comparison group proportion in the studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded two levels for very serious risk of bias. b Downgraded one level for severe inconsistency (substantial heterogeneity as indicated by the I2 statistic). c Downgraded one level for serious imprecision. For abscess, morbidity and infection, the confidence interval includes appreciable benefit and harm, and the sample size is small. For mortality, there are few events (8 deaths in total) d Downgraded one level for serious imprecision (small sample size). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intra‐peritoneal abscess Show forest plot | 5 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.47, 3.21] |
| 2 Wound infection Show forest plot | 5 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [0.88, 4.56] |
| 3 Morbidity Show forest plot | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 6.67 [2.13, 20.87] |
| 4 Mortality Show forest plot | 4 | 363 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.88 [1.18, 20.09] |
| 5 Hospital stay Show forest plot | 3 | 298 | Mean Difference (IV, Random, 95% CI) | 2.17 [1.76, 2.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Intra‐peritoneal abscess Show forest plot | 3 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.28, 2.02] |

