Cirugía por la contractura de Dupuytren de los dedos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre UK study Randomised controlled trial Recruitment between June 2000 and March 2001 | |
| Participants | 31 participants 28:3 male/female ratio Mean age: 61 years Inclusion criteria: not specified Exclusion criteria: not specified | |
| Interventions | Automated staple device closure vs Polybutester nonabsorbable suture closure | |
| Outcomes |
| |
| Notes | Length of follow‐up: 2 weeks Low‐quality evidence due to risk of bias regarding allocation concealment and imprecision No funding sources acknowledged; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Not discussed in paper; use of unsecured random numbers table assumed |
| Incomplete outcome data (attrition bias) | Low risk | 4 of 5 endpoints reported with complete data; 1 of 5 not reported (wound appearance category at 2 weeks) |
| Selective reporting (reporting bias) | Low risk | 4 of 5 endpoints reported with complete data; 1 of 5 not reported (wound appearance category at 2 weeks) |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | Blinding not possible |
| Blinding of outcome assessment (detection bias) | High risk | 3 of 5 assessments performed by surgeon or reported by participant; 1 other |
| Methods | Single‐centre UK study Randomised controlled trial Recruitment dates not specified | |
| Participants | 15 participants All male Mean age: 61 years Inclusion criteria: yes: age < 70 years, Luck involutional stage, ≥ 2 rays on hand affected Exclusion criteria: not specified | |
| Interventions | Intraoperative wound bath in 5‐FU vs Wound bath in normal saline | |
| Outcomes |
| |
| Notes | Length of follow‐up: 18 months Low‐quality evidence due to risk of bias and imprecision Funded by the RAFT Institute of Plastic Surgery; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unmarked envelopes; unclear whether sealed or opaque |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | Surgeon administering treatment not blinded; study described as double‐blinded: participant and assessor |
| Blinding of outcome assessment (detection bias) | Low risk | Measurements performed by blinded therapist |
| Methods | Single‐centre French study Randomised controlled clinical trial Recruitment between 2007 and 2010 | |
| Participants | 68 participants 54 male:10 female; 4 excluded after randomisation not described Mean age: 61.4 (SD 8.8) years for intervention group; 66.0 (SD 7.7) years for control group Inclusion criteria: yes: "healthy individuals older than 18 years without any comorbidity who had been scheduled for elective McCash (open palm) surgery for Dupuytren disease" Exclusion criteria: yes: "Patients allergic to one of the dressing’s components, with diabetes mellitus type 1, who were undergoing cancer treatment, who were pregnant, or who were unable to participate in follow‐up visits were excluded" | |
| Interventions | Leucocyte‐ and platelet‐rich fibrin platelet concentrate applied to wound vs Petroleum jelly mesh applied to wound | |
| Outcomes |
| |
| Notes | Length of follow‐up: 60 days Low‐quality evidence due to risk of bias and imprecision Funding source: academic grant from the French Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing randomisation from a "predefined randomization list, constructed through random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | "sealed, sequentially numbered, opaque envelopes containing treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | 5% loss to follow‐up; split between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Blocked randomisation employed in single‐blinded study (blinding of outcome assessment only) |
| Blinding of participants and personnel (performance bias) | High risk | Randomisation performed before procedure; surgeon probably unblinded throughout operative procedure, including fasciectomy component of procedure |
| Blinding of outcome assessment (detection bias) | Low risk | All assessors blinded |
| Methods | Single‐centre UK study Pseudorandomised controlled clinical trial Recruitment between 1996 and 2000 | |
| Participants | 30 participants 24 male:6 female Mean age (at diagnosis): 67 years for treatment group; 66 years for control group Inclusion criteria: yes: "Dupuytren’s contracture of a single ray confined to the palm and affecting only the MCPJ, a single cord of Dupuytren’s tissue, no previous surgery for Dupuytren’s disease in that ray, agreement to surgery" Exclusion criteria: not specified | |
| Interventions | Longitudinal incision closed with z‐plasty vs Transverse incision | |
| Outcomes |
| |
| Notes | Mean length of follow‐up: 2 years (range 2.0 to 3.5) Low‐quality evidence, as pseudorandomised and at high risk of bias and imprecision No funding sources acknowledged; no conflicts of interest declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation rather than randomisation |
| Allocation concealment (selection bias) | High risk | Alternation rather than randomisation |
| Incomplete outcome data (attrition bias) | Low risk | 10% loss to follow‐up but balanced between groups |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported |
| Other bias | Unclear risk | Unblinded study with alternation rather than randomisation. |
| Blinding of participants and personnel (performance bias) | High risk | Surgeon unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Multiple unblinded assessors throughout study |
| Methods | Single‐centre UK study Randomised controlled trial Recruitment between February 1998 and August 2002 | |
| Participants | 100 participants 63 male:16 female (21 incomplete) Mean age: 65 (SD 10) years Inclusion criteria: yes: Dupuytren’s disease in 1 ray only and any degree of resultant contracture Exclusion criteria: yes: bleeding diathesis, recurrent disease | |
| Interventions | Bruner incision closed with Y‐V plasties vs Longitudinal incision closed with z‐plasties | |
| Outcomes |
| |
| Notes | Length of follow‐up: 2 years Low‐quality evidence, as high risk of performance bias, which may have influenced outcomes and imprecision No funding sources acknowledged; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers in envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed sequentially numbered envelopes |
| Incomplete outcome data (attrition bias) | Low risk | Attrition unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | Surgeon unblinded; participant possibly unblinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Independent observer, but unclear whether blinded |
| Methods | Single‐centre New Zealand study Randomised controlled clinical trial Study period: 2010 to 2011 | |
| Participants | 56 participants 45 male:11 female Mean age: 68 (SD 8) years in intervention group; 67 (SD 9) years in control group Inclusion criteria: yes: "Patients of all ages and surgery types were included, provided they attended their first postoperative hand therapy appointment within 14 days after surgery" Exclusion criteria: yes: "K‐wiring of the proximal interphalangeal joint during surgery or inability to comply with hand therapy" | |
| Interventions | Night extension orthosis plus standard hand therapy vs Hand therapy alone (apart from participants in this group who had a net loss of ≥ 20 degrees at the PIPJ and/or a net loss of ≥ 30 degrees at the MCPJ of the operated fingers, in which case a splint was given) | |
| Outcomes |
| |
| Notes | Length of follow‐up: 3 months Low‐quality evidence, as inadequate detail provided on study design and high risk of performance bias and imprecision Funding source: A grant was received through the Clinical Centre for Research and Effective Practice (CCREP) Innovation Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate detail: "participant selecting a tag from an envelope with group allocation concealed" |
| Allocation concealment (selection bias) | Unclear risk | Inadequate detail: "participant selecting a tag from an envelope with group allocation concealed" |
| Incomplete outcome data (attrition bias) | Low risk | Attrition described: split between groups; attrition unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Inadequate details of randomisation in paper |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Methods | Single‐centre Belgian study Randomised controlled clinical trial Study period not stated | |
| Participants | 30 participants 26 male:4 female Mean age: 63.5 (SD 8) years Inclusion criteria: Adult patients scheduled for subtotal fasciectomy to treat Dupuytren's disease were eligible for inclusion if they had a D score > 4 Exclusion criteria: patients undergoing a reintervention for recurrent contractures; patients with a need for skin grafts or flaps; premenopausal women; patients using anti‐inflammatory drugs; patients with a history of malignancy; patients with a known allergy to tamoxifen | |
| Interventions | Segmental fasciectomy with 80 mg oral tamoxifen daily for 6 weeks before surgery continuing until 12 weeks after surgery vs Segmental fasciectomy with 80 mg oral placebo daily for 6 weeks before surgery continuing until 12 weeks after surgery | |
| Outcomes |
| |
| Notes | Length of follow‐up: 24 months Low‐quality evidence, as inadequate details on study design and imprecision Funding source: Belgian Orthopaedic Society | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate detail: not described |
| Allocation concealment (selection bias) | Unclear risk | Inadequate detail: boxes used to store allocation, but opacity not described; second copies of allocations stored in envelopes with inadequate details provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition described; systematic differences between groups unlikely |
| Selective reporting (reporting bias) | Unclear risk | No data presented for hand function (DASH) at 3 months |
| Other bias | Low risk | Blinded study; hence blocked randomisation not problematic |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded outcome measurements |
| Methods | Single‐centre UK study Randomised controlled trial Recruitment dates not specified | |
| Participants | 62 participants Gender ratio not presented Age data not presented Inclusion criteria: not specified Exclusion criteria: not specified | |
| Interventions | Absorbable polyglactin suture closure vs Non‐absorbable polypropylene suture closure | |
| Outcomes |
| |
| Notes | Length of follow‐up: primary outcome assessed at 10 to 14 days Low‐quality evidence, as review group was concerned that exclusion of outliers despite use of non‐parametric statistics in analysis of primary outcome created significant differences and imprecision No funding sources acknowledged; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Unclear from paper |
| Incomplete outcome data (attrition bias) | Low risk | 3 of 62 missing |
| Selective reporting (reporting bias) | High risk | Protocol for exclusion of outliers not given; non‐parametric statistics used after test of normality described; unclear why outliers excluded; outlier exclusion may have influenced outcome of study |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded until start of procedure |
| Blinding of outcome assessment (detection bias) | High risk | Assessor unblinded |
| Methods | Multi‐centre (5‐centre) UK study Randomised controlled trial Recruitment between October 2007 and January 2009 | |
| Participants | 154 participants 120 male:34 female Mean age: 67.2 years in splint group; 67.5 years in no splint group Inclusion criteria: yes: Patients with Dupuytren’s disease affecting ≥ 1 digit of either hand and requiring fasciectomy or dermofasciectomy were invited to participate. Patients had to be over 18 years of age and competent to give fully informed written consent Exclusion criteria: yes: contracture of the thumb or first webspace | |
| Interventions | Static splint for 3 months postop vs No splint (apart from participants in this group who had a net loss ≥ 15 degrees at the PIPJ and/or a net loss ≥ 20 degrees at the MCPJ of the operated fingers, in which case a splint was given) | |
| Outcomes |
| |
| Notes | Length of follow‐up: 12 months Low‐quality evidence due to risk of bias and imprecision Funded by Action Medical Research Charity; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process at central site not explained |
| Allocation concealment (selection bias) | Low risk | Central telephone cluster randomisation |
| Incomplete outcome data (attrition bias) | Low risk | Attrition recorded and explained |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Cluster randomisation used in an unblinded study, but unclear it if would have introduced bias |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Patient‐reported outcome measure unblinded but unclear whether likely to be biased; independent observer measured range of motion but unclear whether blinded |
| Methods | 2‐Centre Dutch study Randomised controlled clinical trial Recruitment between 2007 and 2008 | |
| Participants | 54 participants 46 male:8 female Mean age: 63 (SD 9) years in intervention group; 64 (SD 11) years in control group Inclusion criteria: yes: "DD (Dupuytren's disease) and a proximal inter‐phalangeal (PIP) joint flexion contracture of at least 30°" Exclusion criteria: yes: "below 18 years of age, had undergone partial amputation or arthrodesis of a digit or were patients with insufficient knowledge of the Dutch language" | |
| Interventions | 3‐Month splinting protocol together with hand therapy vs Hand therapy alone | |
| Outcomes |
| |
| Notes | Length of follow‐up: 1 year Low‐quality evidence due to risk of bias and imprecision No specific funding sources declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not specified; allocation concealed from outcome assessor but allocation concealment not clear |
| Incomplete outcome data (attrition bias) | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | Surgeon blinded but therapist and participant not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | All assessments by "same independent third party, a resident who had no part in the operative procedure or postoperative treatment", although blinding status not specified |
| Methods | Single‐centre Canadian study Randomised controlled trial Recruitment dates not specified | |
| Participants | 47 participants 41 male:6 female Mean age: 61.2 years Inclusion criteria: yes: "at least one joint contracture of at least 20°" Exclusion criteria: yes: "diabetes mellitus and those who had previously had hand surgery, including PNA, on the affected hand for any reason" | |
| Interventions | Steroid injection at end of percutaneous needle fasciotomy, repeated at 6 weeks and 3 months, vs no steroid injection | |
| Outcomes |
| |
| Notes | Length of follow‐up: 6 months Low‐quality evidence due to risk of bias and imprecision Funded by the Canadian Society of Plastic Surgeons; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions after randomisation |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | No blinding; no sham injection for control group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Methods | Single‐centre UK study Randomised controlled trial Recruitment dates not specified | |
| Participants | 79 participants 65 male:14 female Mean age: 62.9 (range 27 to 85) years Inclusion criteria: yes: "primary Dupuytren’s contracture greater than 30° of the PIP joint of a finger" Exclusion criteria: yes: "receiving anticoagulation treatment or were unable to complete questionnaires, give consent or attend for follow‐up" | |
| Interventions | Firebreak full‐thickness skin graft closure vs z‐plasty closure | |
| Outcomes |
| |
| Notes | Length of follow‐up: 36 months Low‐quality evidence due to risk of bias and imprecision No sources of funding acknowledged; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via sequential envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed sequential envelopes but unclear whether opaque |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not described |
| Selective reporting (reporting bias) | Unclear risk | 2‐Week postoperative data not presented; some data presented only graphically |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | Low risk | Randomisation not performed until after contracture excised |
| Blinding of outcome assessment (detection bias) | High risk | Independent observer unlikely to be blinded |
| Methods | Single‐centre Dutch study Randomised controlled trial Recruitment between August 2002 and January 2005 | |
| Participants | 125 hands in 121 participants 94 male:19 female; 8 incomplete Mean age: 63 years Inclusion criteria: yes: "(1) a flexion contracture of at least 30° in the MCP, PIP, or DIP joints; (2) a clearly defined pathologic cord in the palmar fascia; and (3) willingness to participate in this trial" Exclusion criteria: yes: "(1) patients with postsurgical recurrence or extension of the disease, (2) patients who were not allowed to stop taking their anticoagulants, (3) patients generally unfit to have surgery, and (4) patients who were not willing to participate in this study or had a specific treatment wish" | |
| Interventions | Percutaneous needle fasciotomy vs Limited fasciectomy | |
| Outcomes |
| |
| Notes | Length of follow‐up: 6 weeks Low‐quality evidence due to risk of bias and imprecision No sources of funding acknowledged; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via sealed sequential envelopes |
| Allocation concealment (selection bias) | Unclear risk | Sealed sequential envelopes but unclear whether opaque |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up not different between groups |
| Selective reporting (reporting bias) | Low risk | All data reported |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Methods | (as per van Rijssen 2006) Single‐centre Dutch study Randomised controlled trial Recruitment between August 2002 and January 2005 | |
| Participants | 93 participants (out of 113 patients studied in van Rijssen 2006) 76 male:17 female Mean age 62.8 years for needle fasciotomy; 63.1 years for limited fasciectomy Inclusion criteria: yes: "(1) a flexion contracture of at least 30° in the MCP, PIP, or DIP joints; (2) a clearly defined pathologic cord in the palmar fascia; and (3) willingness to participate in this trial" Exclusion criteria: yes: "(1) patients with postsurgical recurrence or extension of the disease, (2) patients who were not allowed to stop taking their anticoagulants, (3) patients generally unfit to have surgery, and (4) patients who were not willing to participate in this study or had a specific treatment wish" | |
| Interventions | Percutaneous needle fasciotomy vs Limited fasciectomy | |
| Outcomes |
| |
| Notes | Length of follow‐up: 5 years Low‐quality evidence due to risk of bias and imprecision No sources of funding acknowledged; no conflicts of interest declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether envelopes were numbered but were numbered in van Rijssen 2006, in which early outcomes of same study were reported; unclear whether opaque |
| Incomplete outcome data (attrition bias) | High risk | Significantly different attrition between cohorts possibly because of differences in true outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes presented |
| Other bias | Low risk | No blocked randomisation in an unblinded study |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded |
Abbreviations:
5‐FU: 5‐Fluorouracil.
DASH: Disabilities of the Arm, Shoulder and Hand Scale.
DIPJ: Distal interphalangeal joint.
MCPJ: Metacarpophalangeal joint.
PIPJ: Proximal interphalangeal joint.
PROM: Patient‐reported outcome measure.
TAED: Total active extension deficit.
VAS: Visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial | |
| Excluded on the basis of question 5 of Appendix 3; no concurrent control and intervention groups | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 5 of Appendix 3; retrospective service evaluation of participants treated with a splint and those treated without a splint based on clinical grounds rather than randomisation/pseudorandomisation | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 3 of Appendix 3; publication reports the protocol of a study; final study is described in Jerosch‐Herold 2011, which is among the Included studies | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 5 of Appendix 3; no concurrent control and intervention groups | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 5 of Appendix 3; not a randomised or pseudorandomised trial | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions | |
| Excluded on the basis of question 4 of Appendix 3; no comparison of an intervention vs control and no comparison of 2 interventions |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unclear |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Single‐centre UK study Unclear study design Recruitment dates not specified |
| Participants | 39 participants 17 male:4 female; 18 incomplete Mean age: not presented, range 46 to 76 years Inclusion criteria: not specified Exclusion criteria: not specified |
| Interventions | Intermittent pneumatic postoperative compression vs Boxing glove dressing and roller towel elevation |
| Outcomes |
|
| Notes | Length of follow‐up: 7 days ‐ "one follow‐up appointment" Low‐quality evidence, as extremely limited details of study design provided Funded by the Department of Health and Social Security; no conflicts of interest declared |
| Methods | Unclear |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Single‐centre UK study Unclear study design Recruitment dates not specified |
| Participants | 20 participants 13 male:7 female Mean age: not reported (range 42 to 81 years) Inclusion criteria: yes: "previously untreated simple Dupuytren's disease" Exclusion criteria: yes: "any illness or medication that might influence fluid retention" |
| Interventions | Elevated hand table for surgery vs Intraoperative tourniquet |
| Outcomes |
|
| Notes | Length of follow‐up: 28 days Low‐quality evidence No funding sources acknowledged; no conflicts of interest declared |
| Methods | Unclear |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH Show forest plot | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.74, 4.74] |
| Analysis 1.1  Comparison 1 Preoperative measurements, Outcome 1 DASH. | ||||
| 2 Total active extension Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐7.72, 3.94] |
| Analysis 1.2 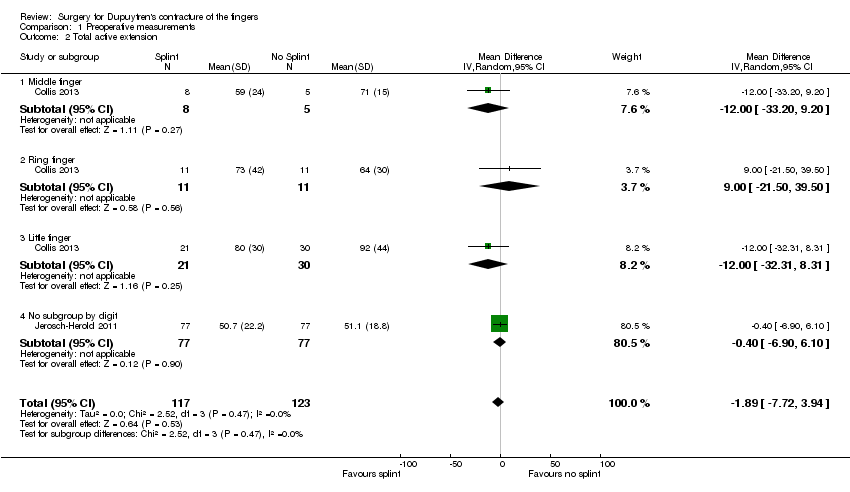 Comparison 1 Preoperative measurements, Outcome 2 Total active extension. | ||||
| 2.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐33.20, 9.20] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐21.50, 39.50] |
| 2.3 Little finger | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐32.31, 8.31] |
| 2.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.90, 6.10] |
| 3 Total active flexion Show forest plot | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 2.42 [‐4.98, 9.83] |
| Analysis 1.3  Comparison 1 Preoperative measurements, Outcome 3 Total active flexion. | ||||
| 3.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐11.52, 23.52] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 11.00 [‐0.47, 22.47] |
| 3.3 Little finger | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐21.26, 3.26] |
| 3.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐3.35, 8.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score at 3 months Show forest plot | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐2.32, 4.62] |
| Analysis 2.1  Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 1 DASH score at 3 months. | ||||
| 2 Total active extension at 3 months Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐8.01, 3.59] |
| Analysis 2.2  Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 2 Total active extension at 3 months. | ||||
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐30.26, 38.26] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐23.24, 15.24] |
| 2.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐27.35, 17.35] |
| 2.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐8.43, 4.43] |
| 3 Total active flexion at 3 months Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | 12.36 [1.21, 23.50] |
| Analysis 2.3 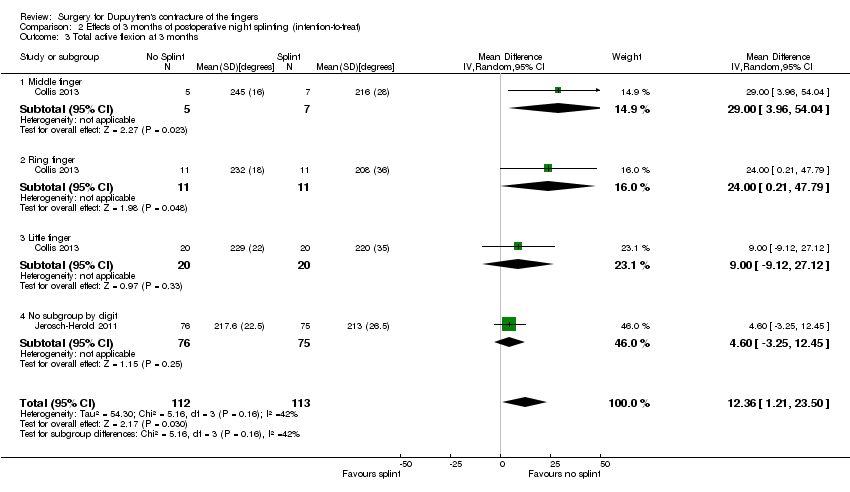 Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 3 Total active flexion at 3 months. | ||||
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 29.00 [3.96, 54.04] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 24.0 [0.21, 47.79] |
| 3.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐9.12, 27.12] |
| 3.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐3.25, 12.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score at 3 months Show forest plot | 2 | 184 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐2.85, 4.86] |
| Analysis 3.1 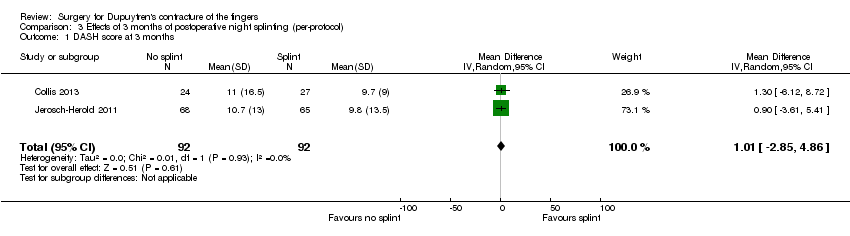 Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 1 DASH score at 3 months. | ||||
| 2 Total active extension at 3 months [degrees] Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐21.14, 2.15] |
| Analysis 3.2 ![Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees].](/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-CMP-003-02.png) Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees]. | ||||
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐29.81, 37.61] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐33.79, ‐0.01] |
| 2.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐22.20 [‐41.05, ‐3.35] |
| 2.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐8.77, 4.97] |
| 3 Total active flexion at 3 months [degrees] Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 12.64 [3.68, 21.60] |
| Analysis 3.3 ![Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees].](/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-CMP-003-03.png) Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees]. | ||||
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 28.60 [3.79, 53.41] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 21.70 [‐0.80, 44.20] |
| 3.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 13.10 [‐4.61, 30.81] |
| 3.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐1.42, 15.02] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
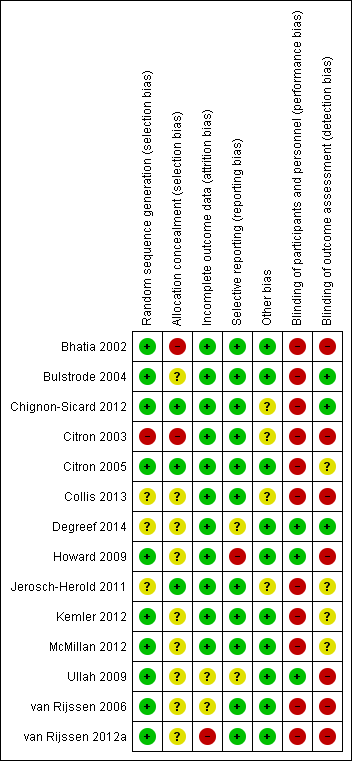
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
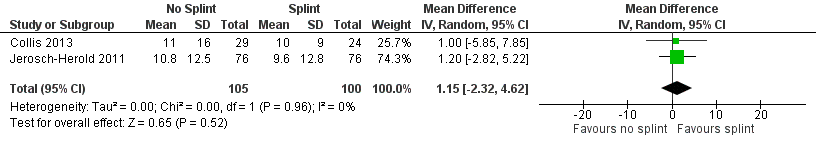
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.1 DASH score at 3 months.
![Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.2 Total active extension at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-AFig-FIG05.png)
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.2 Total active extension at 3 months [degrees].
![Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.3 Total active flexion at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-AFig-FIG06.png)
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.3 Total active flexion at 3 months [degrees].

Comparison 1 Preoperative measurements, Outcome 1 DASH.

Comparison 1 Preoperative measurements, Outcome 2 Total active extension.
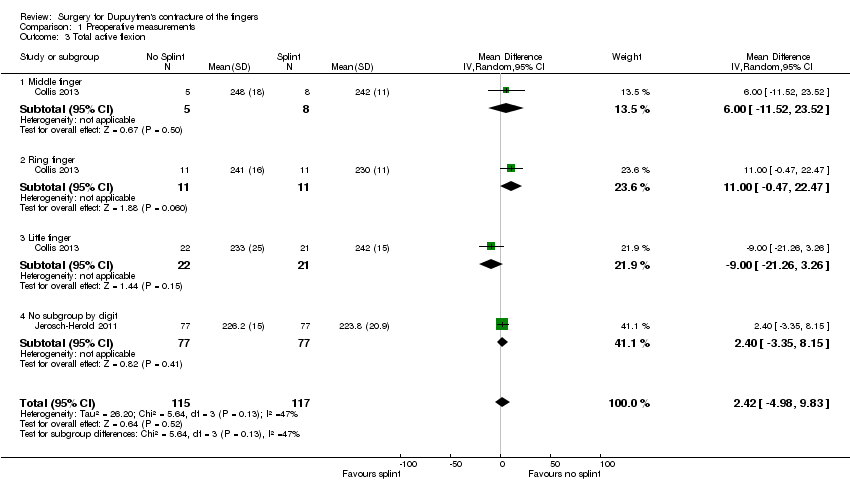
Comparison 1 Preoperative measurements, Outcome 3 Total active flexion.

Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 1 DASH score at 3 months.
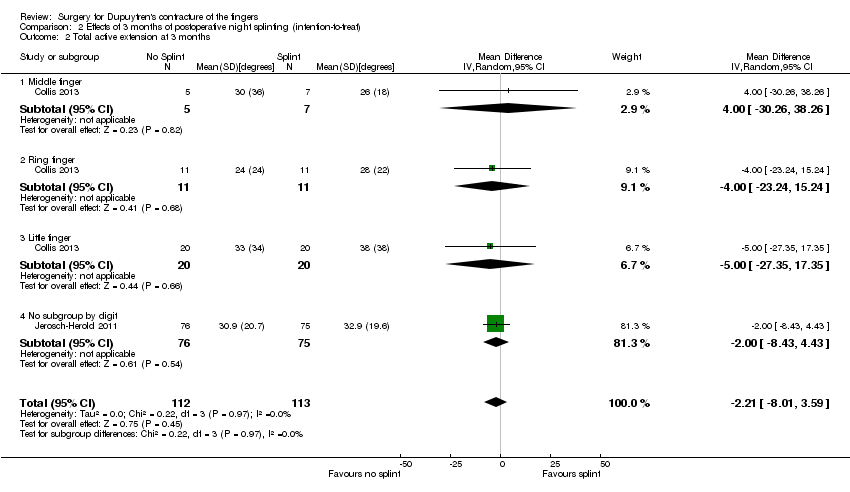
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 2 Total active extension at 3 months.
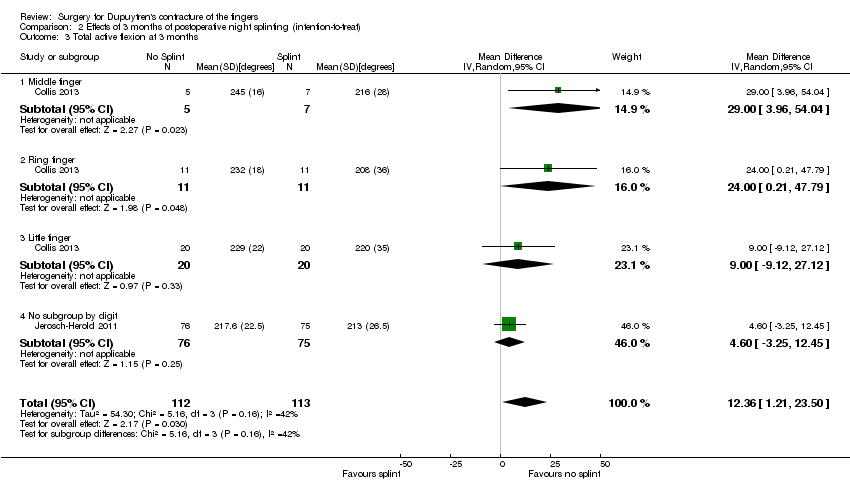
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 3 Total active flexion at 3 months.

Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 1 DASH score at 3 months.
![Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-CMP-003-02.png)
Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees].
![Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-CMP-003-03.png)
Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees].
| Comparison of operation types: early results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease | |||||
| Patient or population: 125 hands in 121 participants with Dupuytren's disease of the fingers for early outcomes (van Rijssen 2006) Settings: single‐centre Dutch study Intervention: needle fasciotomy Comparison: limited fasciectomy | |||||
| Outcomesa | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed riskb | Corresponding risk | ||||
| Limited fasciectomy | Needle fasciotomy | ||||
| DASH hand function score at 5 weeks Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) | Mean DASH hand function score in the fasciectomy group was 16 | DASH hand function score in the fasciotomy group was 5 lower than in the fasciectomy group | 97 | ⊕⊕⊝⊝ | P value = 0.017 as quoted in van Rijssen 2006 24/121 participants in the study did not adequately complete the DASH PROM tools Insufficient detail in article to allow calculation of 95% CI (standard deviations not provided) Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Patient satisfaction at 6 weeks Major outcome group 2 (other PROM) (scores from "0 (no/very negative) to 10 (yes/very positive)") | See comment | See comment | 121 | ⊕⊕⊝⊝ | Data not described in van Rijssen 2006. Only level of significance provided P value = 0.002 as quoted in van Rijssen 2006 |
| Early angular outcome at 6 weeks for Tubiana grade I disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 0 to 45 degrees) Early angular outcome at 6 weeks for Tubiana grade II disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 45 to 90 degrees) Early angular outcome at 6 weeks for Tubiana grade III disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 90 to 135 degrees) Early angular outcome at 6 weeks for Tubiana grade IV disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED > 135 degrees) Major outcome group 3 (early objective measurement) | For Tubiana grade I disease, mean percentage reduction in TPED in the fasciectomy group was 82% For Tubiana grade II disease, mean percentage reduction in TPED in the fasciectomy group was 78% For Tubiana grade III disease, mean percentage reduction in TPED in the fasciectomy group was 75% For Tubiana grade IV disease, mean percentage reduction in TPED in the fasciectomy group was 79% | For Tubiana grade I disease, mean percentage reduction in TPED in the fasciotomy group was 11% lower than in the fasciectomy group For Tubiana grade II disease, mean percentage reduction in TPED in the fasciotomy group was 11% lower than in the fasciectomy group For Tubiana grade III disease, mean percentage reduction in TPED in the fasciotomy group was 29% lower than in the fasciectomy group For Tubiana grade IV disease, mean percentage reduction in TPED in the fasciotomy group was 32% lower than in the fasciectomy group | For grade I disease, 57 (1 study) For grade II disease, 70 (1 study) For grade III disease, 27 (1 study) For grade IV disease, 10 (1 study) | ⊕⊕⊝⊝ | For grade I disease, P value = 0.329 in van Rijssen 2006 For grade II disease, P value = 0.071 in van Rijssen 2006 For grade III disease, P value = 0.000 in van Rijssen 2006 For grade IV disease, P value = 0.004 in van Rijssen 2006 |
| Major outcome group 4 (recurrence) | See comment | See comment | See comment | See comment | Not studied in van Rijssen 2006 |
| Paraesthesia at 1 week Major outcome group 5 (adverse effects) Defined as "tingling sensations at any part of the treated digit without objective disturbance of sensation at the tip of the digit" per hand | 228 per 1000 | 67 per 1000 | 117 | ⊕⊕⊝⊝ | P value = 0.013 in van Rijssen 2006 Relative effect not calculated as only study available |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aRecurrence was not studied in van Rijssen 2006, as this article considered early outcomes only. Recurrence is a late effect, and recurrence in this trial is considered in the next 'Summary of findings' table. bAll assumed risks are based on mean values for limited fasciectomy as reported in van Rijssen 2006. cEvidence downgraded from high to low for DASH at 5 weeks because of significant attrition. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. dEvidence downgraded from high to low for patient satisfaction at 6 weeks, as scale used was not validated. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. eEvidence downgraded from high to low for early angular outcomes in grade I disease at 6 weeks, as van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. fParaesthesia at 6 weeks downgraded from high to low, as scale was not validated. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. | |||||
| Comparison of operation types: late results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease | |||||
| Patient or population: 93 participants (van Rijssen 2012a) Settings: single‐centre Dutch study Intervention: needle fasciotomy Comparison: limited fasciectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Limited fasciectomy | Needle fasciotomy | ||||
| DASH hand function score at 5 years Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) | See comment | See comment | See comment | See comment | Not studied in van Rijssen 2012a |
| Patient satisfaction at 5 years Major outcome group 2 (other PROM) (scores between "1 (not at all), 10 (excellent)") | Mean satisfaction score in fasciectomy group was 8.3 | Mean satisfaction score in fasciotomy group was 2.1 lower than in fasciectomy group | 93 | ⊕⊕⊝⊝ | P value < 0.001 as quoted in van Rijssen 2012a Likelihood of selecting treatment again significantly higher after fasciectomy (P value = 0.008) Insufficient detail in article to allow calculation of 95% CI (standard deviations not provided) |
| Major outcome group 3 (early angular outcome)b | See comment | See comment | See comment | See comment | This major outcome group is not relevant to a late outcome comparison |
| Recurrence at 5 years Major outcome group 4 (recurrence) Defined as reoperation or progressive angular deformity of 20 degrees in a successfully treated joint | 209 per 1000 | 849 per 1000 | 93 | ⊕⊕⊝⊝ | Progressive angular deformity defined in van Rijssen 2006 as an increase in TPED ≥ 30 degrees. In van Rijssen 2012a, different definitions used (increase of 20 degrees in a successfully treated joint) in other studies of Dupuytren's disease, such as Hurst 2009, acknowledged and applied P value < 0.001 in van Rijssen 2012a Relative effect not calculated, as only study available Recurrence rate influenced by the definition of recurrence used, and by length of follow‐up period |
| Major outcome group 5 (adverse effects)d | see comment | see comment | see comment | see comment | Not discussed in van Rijssen 2012a; analysed in van Rijssen 2006 |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aQuality of evidence for patient satisfaction at 5 years downgraded from high to low because of significant risks of bias in van Rijssen 2012a, and as the result of imprecision. cQuality of evidence for recurrence at 5 years downgraded from high to low because of significant risks of bias in van Rijssen 2012a, and as the result of imprecision. | |||||
| Comparison of operation types: firebreak skin grafting vs z‐plasty closure of fasciectomy for Dupuytren's disease | |||||
| Patient or population: 79 participants (Ullah 2009) Settings: single‐centre UK study Intervention: firebreak skin grafting to close incision Comparison: z‐plasty closure of incision | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| z‐plasty | Firebreak skin grafting | ||||
| PEM hand function score at 3 years Major outcome group 1 (hand function) (scores between 0 and 77, where 0 represents no impairment in hand function and 77 represents maximum impairment in hand function) | See comment | See comment | 79 (1 study) | ⊕⊕⊝⊝ | Data represented graphically only; differences between groups described as not statistically significant; no P value provided |
| Major outcome group 2 (patient satisfaction and other PROM) | See comment | See comment | See comment | See comment | Not studied in Ullah 2009 |
| Correction of MCPJ and PIPJ deformities at Major outcome group 3 (early angular outcomes) | All MCPJs fully corrected Mean PIPJ correction 6 degrees in the z‐plasty group | All MCPJs also fully corrected Mean PIPJ correction no different (also 6 degrees) in the skin graft group from the z‐plasty group | 79 (1 study) | ⊕⊕⊝⊝ | |
| Progressive contracture by 3 years Major outcome group 4 (recurrence) | 109 per 1000 | 136 per 1000 | 79 (1 study) | ⊕⊕⊝⊝ | P value = 0.17 in Ullah 2009 Rates assessed per finger (90 fingers treated among 79 participants) |
| Hypoaesthesia Major outcome group 5 (adverse effects) | Radial digital nerve territory: 217 per 1000 Ulnar digital nerve territory: 217 per 1000 | Radial digital nerve territory: 341 per 1000 Ulnar digital nerve territory: 455 per 1000 | 79 (1 study) | ⊕⊕⊝⊝ | P value = 0.2 for radial digital nerve territory in Ullah 2009 P value = 0.03 for ulnar digital nerve territory in Ullah 2009 |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aQuality of evidence for PEM hand function score at 3 years downgraded from high to low, as neither data nor P value was provided to support statement, and as the result of imprecision. b,c,dQuality of evidence downgraded from high to low because of risks of bias and imprecision. | |||||
| Refining rehabilitation: three months of postoperative night splinting with hand therapy vs hand therapy alone for rehabilitation following surgery for Dupuytren's disease | |||||
| Patient or population: 210 participants with Dupuytren's disease of the fingers in 2 studies (225 digits reported across all studies) (Collis 2013; Jerosch‐Herold 2011) Settings: multi‐centre UK RCT and single‐centre New Zealand RCT Intervention: three months of night splinting in extension in addition to hand therapy ("splint") Comparison: hand therapy alone ("no splint") | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| No splint | Splint | ||||
| DASH hand function score at 3 months Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) | Mean DASH ranged across 'no splint' groups from | Mean DASH in 'splint' groups was 1.15 lower (95% CI ‐2.32 to 4.62) than in 'no splint' groups | 205 participants | ⊕⊕⊝⊝ | Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Major outcome group 2 (patient satisfaction) | See comment | See comment | See comment | See comment | Not assessed in these studies |
| Total active extension at 3 months Major outcome group 3 (early objective measurement) Total active extension (TAE) of MCPJ, PIPJ and DIPJ; higher value indicates loss of extension and a worse outcome | Mean TAE ranged across 'no splint' groups from | Mean TAE in 'splint' groups was 2.21 degrees higher (95% CI ‐3.59 to 8.01) than in 'no splint' groups | 225 digits | ⊕⊕⊝⊝ | Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Major outcome group 4 (recurrence) | See comment | See comment | See comment | See comment | Not assessed in these studies |
| Total active flexion at three months Major outcome group 5 (adverse effects) Total active flexion (TAF) of MCPJ, PIPJ and DIPJ; lower value indicates loss of flexion and a worse outcome | Mean TAF ranged across 'no splint' groups from | Mean TAF in 'splint' groups was 8.42 degrees lower (95% CI 1.78 to 15.07) than in 'no splint' groups | 225 digits | ⊕⊕⊝⊝ | Conflicting findings from subgroups Unclear whether this is the most appropriate time point for study of 'early' outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| a,b,cQuality of evidence was downgraded from high to low because of risks of bias and imprecision. | |||||
| Article | Aspect of care studied | Length of follow‐up, months | Outcomes measured | ||||||||
| Recurrence | Extension deficit | Flexion deficit | Total motion | PROM | Time | Complications as an outcome measure | Hand volume | Other | |||
| Technical refinement | 0.5 | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | Wound appearance | |
| Technical refinement | 18 | ‐ | + | ‐ | + | ‐ | + | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 2 | ‐ | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | |
| Technical refinement | 24 | + | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Technical refinement | 24 | + | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 3 | ‐ | + | + | ‐ | + | ‐ | ‐ | ‐ | Grip strength, composite flexion | |
| Technical refinement | 24 | + | + | ‐ | ‐ | + | ‐ | + | ‐ | ‐ | |
| Technical refinement | 0.5 | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 12 | ‐ | + | + | + | + | ‐ | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 12 | ‐ | + | ‐ | ‐ | + | ‐ | + | ‐ | ‐ | |
| Technical refinement | 6 | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Procedure type | 36 | + | + | ‐ | + | + | + | ‐ | ‐ | Grip strength | |
| Procedure type | 1.5 | ‐ | + | + | ‐ | + | ‐ | + | ‐ | ‐ | |
| Procedure type | 60 | + | + | ‐ | ‐ | + | ‐ | ‐ | ‐ | ‐ |
| Tubiana stage preop | % improvement in TPED for needle fasciotomy | % improvement in TPED for fasciectomy | Significance of differences between procedures |
| I | 71 | 82 | 0.329 |
| II | 67 | 78 | 0.071 |
| III | 46 | 75 | 0.000 |
| IV | 47 | 79 | 0.004 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH Show forest plot | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.74, 4.74] |
| 2 Total active extension Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐7.72, 3.94] |
| 2.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐33.20, 9.20] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐21.50, 39.50] |
| 2.3 Little finger | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐32.31, 8.31] |
| 2.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.90, 6.10] |
| 3 Total active flexion Show forest plot | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 2.42 [‐4.98, 9.83] |
| 3.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐11.52, 23.52] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 11.00 [‐0.47, 22.47] |
| 3.3 Little finger | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐21.26, 3.26] |
| 3.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐3.35, 8.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score at 3 months Show forest plot | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐2.32, 4.62] |
| 2 Total active extension at 3 months Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐8.01, 3.59] |
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐30.26, 38.26] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐23.24, 15.24] |
| 2.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐27.35, 17.35] |
| 2.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐8.43, 4.43] |
| 3 Total active flexion at 3 months Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | 12.36 [1.21, 23.50] |
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 29.00 [3.96, 54.04] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 24.0 [0.21, 47.79] |
| 3.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐9.12, 27.12] |
| 3.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐3.25, 12.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score at 3 months Show forest plot | 2 | 184 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐2.85, 4.86] |
| 2 Total active extension at 3 months [degrees] Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐21.14, 2.15] |
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐29.81, 37.61] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐33.79, ‐0.01] |
| 2.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐22.20 [‐41.05, ‐3.35] |
| 2.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐8.77, 4.97] |
| 3 Total active flexion at 3 months [degrees] Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 12.64 [3.68, 21.60] |
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 28.60 [3.79, 53.41] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 21.70 [‐0.80, 44.20] |
| 3.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 13.10 [‐4.61, 30.81] |
| 3.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐1.42, 15.02] |

