Kirurški zahvat za liječenje Dupuytrenove kontrakture prstiju
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dupuytren Contracture] explode all trees 36
#2 MeSH descriptor: [Fibroma] explode all trees 4
#3 Dupuytren*:ti,ab,kw (Word variations have been searched) 49
#4 Fibromatosis 12
#5 MeSH descriptor: [Fascia] explode all trees 142
#6 palmar fibromatosis 1
#7 viking disease 1
#8 palmar fascia 7
#9 MeSH descriptor: [Fibroblasts] explode all trees 161
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 357
Appendix 2. MEDLINE search strategy: Ovid (numbers of results from original search in 2012)
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1948 to Present>Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp Dupuytren Contracture/ (2121)
2 exp Fibroma/ (11134)
3 Fibromatosis.tw. (2482)
4 exp Fascia/ (7945)
5 Fibroblasts/ (93904)
6 (palmar adj3 fascia).tw. (212)
7 Dupuytren*.tw. (2087)
8 (palmar adj3 fibromatosis).tw. (65)
9 (viking adj3 disease).tw. (1)
10 or/1‐9 (115275)
11 randomized controlled trial.pt. (336898)
12 controlled clinical trial.pt. (85168)
13 randomized.ab. (252166)
14 placebo.ab. (139534)
15 clinical trials as topic.sh. (162410)
16 randomly.ab. (184567)
17 trial.ti. (108505)
18 or/11‐17 (807835)
19 exp animals/ not humans.sh. (3780560)
20 18 not 19 (746667)
21 10 and 20 (767)
Appendix 3. EMBASE search strategy
EMBASE Classic+EMBASE <1947 to 2012 September 17>
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Dupuytren contracture/ (3303)
2 fibroma/ (12928)
3 Fibromatosis.tw. (3239)
4 fascia/ (9898)
5 *fibroblast/ (30576)
6 (palmar adj3 fascia).tw. (309)
7 Dupuytren*.tw. (3062)
8 (palmar adj3 fibromatosis).tw. (84)
9 (viking adj3 disease).tw. (1)
10 or/1‐9 (59116)
11 random$.tw. (776280)
12 factorial$.tw. (20394)
13 crossover$.tw. (45803)
14 cross over.tw. (20874)
15 cross‐over.tw. (20874)
16 placebo$.tw. (189116)
17 (doubl$ adj blind$).tw. (140770)
18 (singl$ adj blind$).tw. (12915)
19 assign$.tw. (216631)
20 allocat$.tw. (72946)
21 volunteer$.tw. (171262)
22 crossover procedure/ (35355)
23 double blind procedure/ (115561)
24 randomized controlled trial/ (331643)
25 single blind procedure/ (16422)
26 or/11‐25 (1294294)
27 10 and 26 (1139)
Appendix 4. CINAHL search strategy
CINAHL via Ebscohost ‐ 1985‐2012
| S18 | S5 and S17 |
| S17 | S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 |
| S16 | TX allocat* random* |
| S15 | (MH "Quantitative Studies") |
| S14 | (MH "Placebos") |
| S13 | TX placebo* |
| S12 | TX random* allocat* |
| S11 | (MH "Random Assignment") |
| S10 | TX randomi* control* trial* |
| S9 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) |
| S8 | TX clinic* n1 trial* |
| S7 | PT Clinical trial |
| S6 | (MH "Clinical Trials+") |
| S5 | S1 or S2 or S3 or S4 |
| S4 | "Dupuytren" |
| S3 | (MH "Fascia") |
| S2 | "Fibromatosis" |
| S1 | (MM "Dupuytren's Contracture") |
Appendix 5. LILACS search strategy
LILACS (Latin American and Caribbean Health Sciences) ‐ 1982 to current date
Search terms: dupuytren's contracture
Appendix 6. ProQuest Dissertations & Theses search strategy
ProQuest Dissertations & Theses (PQDT) (all years)
| Set# | Searched for | Databases | Results |
| S1 | dupuytren's contracture | ProQuest Dissertations & Theses (PQDT) | 18 |
| S2 | Fibroma | ProQuest Dissertations & Theses (PQDT) | 256 |
| S3 | Fibromatosis | ProQuest Dissertations & Theses (PQDT) | 118 |
| S4 | palmar fibromatosis | ProQuest Dissertations & Theses (PQDT) | 2 |
| S6 | palmar fascia | ProQuest Dissertations & Theses (PQDT) | 86 |
| S7 | "viking disease" | ProQuest Dissertations & Theses (PQDT) | 0 |
| S8 | ab(viking disease) | ProQuest Dissertations & Theses (PQDT) | 1 |
| S9 | S1 OR S2 OR S3 OR S4 OR S6 OR S7 OR S8 | ProQuest Dissertations & Theses (PQDT) | 462 |
Appendix 7. ISI Web of Science search strategy
ISI Web of Science via Thomson Web of Knowledge Conference Proceedings Citation Index ‐ Science (CPCI‐S) ‐‐ 1990‐present
Topic=(dupuytren contracture)
Refined by: Web of Science Categories=(SURGERY ) AND Document Types=( PROCEEDINGS PAPER OR MEETING ABSTRACT )
Databases=CPCI‐S Timespan=All Years
Lemmatization=On
Appendix 8. Clinicaltrials.gov search strategy
(advanced search screen)
Conditions: Dupuytren's contracture
Appendix 9. Study eligibility form
| Study eligibility form ‐ Surgery for Dupuytren's disease | |||
| Authors _______________________________
Title _______________________________
_______________________________ | Journal __________________________
Date of publication _______________
| ||
| Q1. Does the paper report the outcome of a clinical study? (i.e. not a review article or just a paper describing an operative technique description)? | Yes
Next question | No
Exclude | Unclear
Refer |
| Q2. Participant Have participants had a surgical intervention for Dupuytren's contracture of a finger? | Yes
Next question | No
Exclude | Unclear
Refer |
| Q3. Outcomes Did the study report short‐term or long‐term outcomes (recurrence) of surgery? | Yes
Next question | No
Exclude | Unclear
Refer |
| Q4. Intervention Did participants receive an intervention compared with a control group, or were at least 2 interventions compared? | Yes
Next question | No
Exclude | Unclear
Refer |
| Q5. Type of study Was the study randomised or quasi‐randomised? | Yes
Include | No
Exclude | Unclear
Refer |
Appendix 10. Assessment of potential for bias in report (tool of The Cochrane Collaboration for assessing risk of bias)
| Paper title
| ||
| Paper authors
| ||
| Reviewer
| ||
| Domain | Support for judgement | Review authors' judgement |
| Selection bias | Insert description, preferably a direct quote from report or correspondence, and add comment | One of : "Low risk", "High risk", "Unclear risk" |
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups | Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment |
| Performance bias |
|
|
| Blinding of participants and personnel Assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information related to whether intended blinding was effective | Performance bias due to knowledge of allocated interventions by participants and personnel during the study |
| Detection bias |
|
|
| Blinding of outcome assessmentAssessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information related to whether intended blinding was effective | Detection bias due to knowledge of allocated interventions by outcome assessors |
| Attrition bias |
|
|
| Incomplete outcome dataAssessments should be made for each main outcome (or class of outcomes) | Describe completeness of outcome data for each main outcome, including attrition and exclusions from analysis. State whether attrition and exclusions were reported, numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions when reported and any re‐inclusions in analyses performed by review authors | Attrition bias due to amount, nature or handling of incomplete outcome data |
| Reporting bias |
|
|
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what they found | Reporting bias due to selective outcome reporting |
| Other bias |
|
|
| Other sources of bias | State any important concerns about bias not addressed in other domains of the tool If particular questions/entries were pre‐specified in the review protocol, responses should be provided for each question/entry | Bias due to problems not covered elsewhere in the table |
Appendix 11. Paper assessment form
| Surgery for Dupuytren’s disease ‐ Study checklist | ||||||
| Authors and year |
| |||||
| Title |
| |||||
| Journal/Source if not published |
| |||||
| Study ID (Revman) |
| Date of extraction |
| |||
| Study design | ||||||
| Single/Multi‐centre |
| Study setting (country) |
| |||
| Number of participants |
| Mean (SD:range) age |
| |||
| Male:Female |
|
|
| |||
| Inclusion criteria |
| Exclusion criteria |
| |||
| Randomisation technique
|
| Concealment of allocation sequence? | Yes (envelopes, etc.) No Unclear | |||
| Blinding of participant | Yes No Unclear | Incomplete outcome data (%FU) | Yes No Unclear | |||
| Interventions | ||||||
| Intervention |
| Control intervention |
| |||
| Length of follow‐up (mean, SD, median, min, max) |
| Withdrawals (and why) |
| |||
| Number lost to follow‐up |
|
|
| |||
| Outcomes assessed | ||||||
| Recurrence |
| Recurrence | No. Rec. | Total | ||
|
| Treatment |
|
|
| ||
|
| Control treatment |
|
|
| ||
| Pre‐op contracture (mean/SD) | MCP PIP Combined | |||||
| Imm post‐op contracture | MCP PIP Combined | |||||
| Final contracture | MCP PIP Combined | |||||
| Complications of surgery | Infection (definition) Digital nerve injury Tendon injury Other | |||||
| Other outcomes |
| |||||
| Quality of evidence |
| |||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
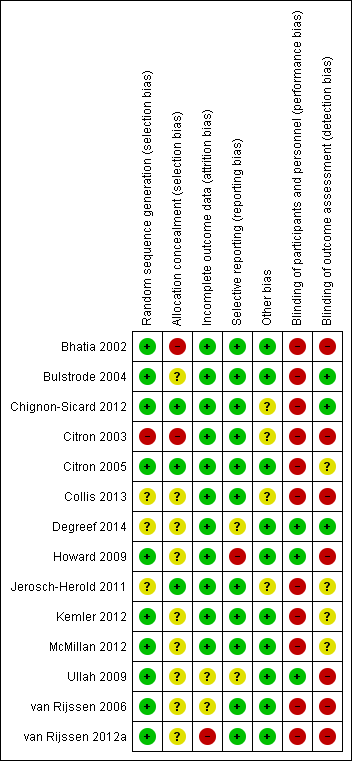
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
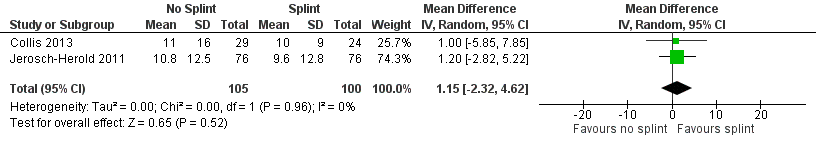
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.1 DASH score at 3 months.
![Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.2 Total active extension at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-AFig-FIG05.png)
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.2 Total active extension at 3 months [degrees].
![Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.3 Total active flexion at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-AFig-FIG06.png)
Forest plot of comparison: 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), outcome: 2.3 Total active flexion at 3 months [degrees].

Comparison 1 Preoperative measurements, Outcome 1 DASH.

Comparison 1 Preoperative measurements, Outcome 2 Total active extension.
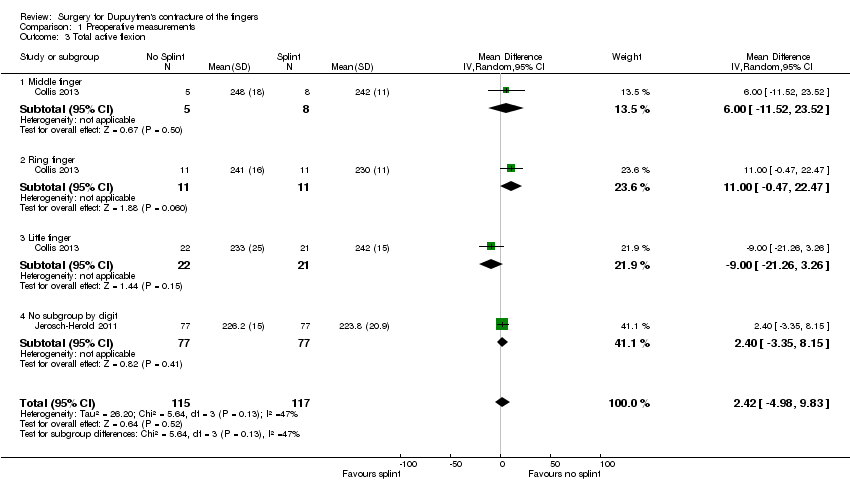
Comparison 1 Preoperative measurements, Outcome 3 Total active flexion.

Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 1 DASH score at 3 months.
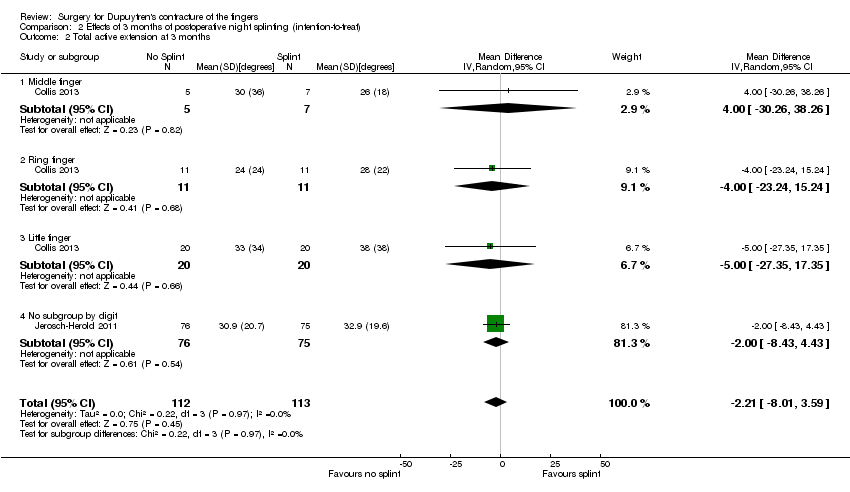
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 2 Total active extension at 3 months.
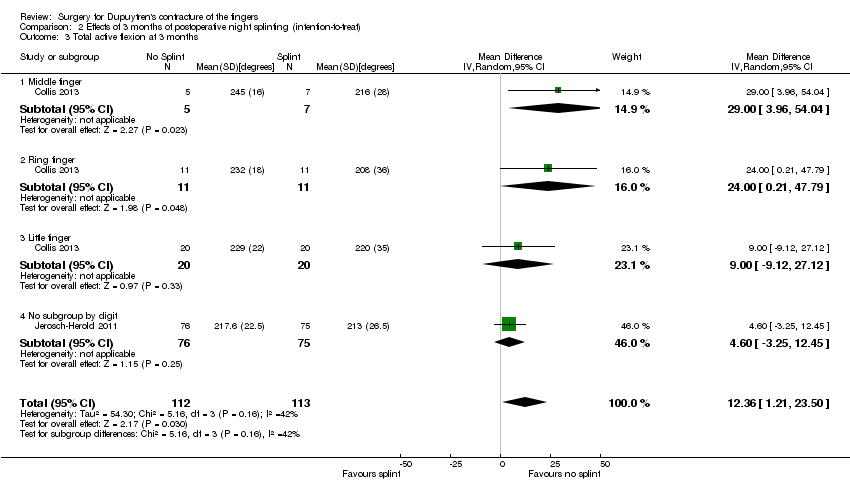
Comparison 2 Effects of 3 months of postoperative night splinting (intention‐to‐treat), Outcome 3 Total active flexion at 3 months.

Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 1 DASH score at 3 months.
![Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-CMP-003-02.png)
Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 2 Total active extension at 3 months [degrees].
![Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees].](/es/cdsr/doi/10.1002/14651858.CD010143.pub2/media/CDSR/CD010143/image_n/nCD010143-CMP-003-03.png)
Comparison 3 Effects of 3 months of postoperative night splinting (per‐protocol), Outcome 3 Total active flexion at 3 months [degrees].
| Comparison of operation types: early results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease | |||||
| Patient or population: 125 hands in 121 participants with Dupuytren's disease of the fingers for early outcomes (van Rijssen 2006) Settings: single‐centre Dutch study Intervention: needle fasciotomy Comparison: limited fasciectomy | |||||
| Outcomesa | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed riskb | Corresponding risk | ||||
| Limited fasciectomy | Needle fasciotomy | ||||
| DASH hand function score at 5 weeks Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) | Mean DASH hand function score in the fasciectomy group was 16 | DASH hand function score in the fasciotomy group was 5 lower than in the fasciectomy group | 97 | ⊕⊕⊝⊝ | P value = 0.017 as quoted in van Rijssen 2006 24/121 participants in the study did not adequately complete the DASH PROM tools Insufficient detail in article to allow calculation of 95% CI (standard deviations not provided) Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Patient satisfaction at 6 weeks Major outcome group 2 (other PROM) (scores from "0 (no/very negative) to 10 (yes/very positive)") | See comment | See comment | 121 | ⊕⊕⊝⊝ | Data not described in van Rijssen 2006. Only level of significance provided P value = 0.002 as quoted in van Rijssen 2006 |
| Early angular outcome at 6 weeks for Tubiana grade I disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 0 to 45 degrees) Early angular outcome at 6 weeks for Tubiana grade II disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 45 to 90 degrees) Early angular outcome at 6 weeks for Tubiana grade III disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED of 90 to 135 degrees) Early angular outcome at 6 weeks for Tubiana grade IV disease (total passive extension deficit (TPED) of the MCPJ, PIPJ and DIPJ for preoperative contractures with a TPED > 135 degrees) Major outcome group 3 (early objective measurement) | For Tubiana grade I disease, mean percentage reduction in TPED in the fasciectomy group was 82% For Tubiana grade II disease, mean percentage reduction in TPED in the fasciectomy group was 78% For Tubiana grade III disease, mean percentage reduction in TPED in the fasciectomy group was 75% For Tubiana grade IV disease, mean percentage reduction in TPED in the fasciectomy group was 79% | For Tubiana grade I disease, mean percentage reduction in TPED in the fasciotomy group was 11% lower than in the fasciectomy group For Tubiana grade II disease, mean percentage reduction in TPED in the fasciotomy group was 11% lower than in the fasciectomy group For Tubiana grade III disease, mean percentage reduction in TPED in the fasciotomy group was 29% lower than in the fasciectomy group For Tubiana grade IV disease, mean percentage reduction in TPED in the fasciotomy group was 32% lower than in the fasciectomy group | For grade I disease, 57 (1 study) For grade II disease, 70 (1 study) For grade III disease, 27 (1 study) For grade IV disease, 10 (1 study) | ⊕⊕⊝⊝ | For grade I disease, P value = 0.329 in van Rijssen 2006 For grade II disease, P value = 0.071 in van Rijssen 2006 For grade III disease, P value = 0.000 in van Rijssen 2006 For grade IV disease, P value = 0.004 in van Rijssen 2006 |
| Major outcome group 4 (recurrence) | See comment | See comment | See comment | See comment | Not studied in van Rijssen 2006 |
| Paraesthesia at 1 week Major outcome group 5 (adverse effects) Defined as "tingling sensations at any part of the treated digit without objective disturbance of sensation at the tip of the digit" per hand | 228 per 1000 | 67 per 1000 | 117 | ⊕⊕⊝⊝ | P value = 0.013 in van Rijssen 2006 Relative effect not calculated as only study available |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aRecurrence was not studied in van Rijssen 2006, as this article considered early outcomes only. Recurrence is a late effect, and recurrence in this trial is considered in the next 'Summary of findings' table. bAll assumed risks are based on mean values for limited fasciectomy as reported in van Rijssen 2006. cEvidence downgraded from high to low for DASH at 5 weeks because of significant attrition. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. dEvidence downgraded from high to low for patient satisfaction at 6 weeks, as scale used was not validated. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. eEvidence downgraded from high to low for early angular outcomes in grade I disease at 6 weeks, as van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. fParaesthesia at 6 weeks downgraded from high to low, as scale was not validated. van Rijssen 2006 had significant risk of performance and detection biases, and imprecision. | |||||
| Comparison of operation types: late results of needle fasciotomy vs limited fasciectomy for Dupuytren's disease | |||||
| Patient or population: 93 participants (van Rijssen 2012a) Settings: single‐centre Dutch study Intervention: needle fasciotomy Comparison: limited fasciectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Limited fasciectomy | Needle fasciotomy | ||||
| DASH hand function score at 5 years Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) | See comment | See comment | See comment | See comment | Not studied in van Rijssen 2012a |
| Patient satisfaction at 5 years Major outcome group 2 (other PROM) (scores between "1 (not at all), 10 (excellent)") | Mean satisfaction score in fasciectomy group was 8.3 | Mean satisfaction score in fasciotomy group was 2.1 lower than in fasciectomy group | 93 | ⊕⊕⊝⊝ | P value < 0.001 as quoted in van Rijssen 2012a Likelihood of selecting treatment again significantly higher after fasciectomy (P value = 0.008) Insufficient detail in article to allow calculation of 95% CI (standard deviations not provided) |
| Major outcome group 3 (early angular outcome)b | See comment | See comment | See comment | See comment | This major outcome group is not relevant to a late outcome comparison |
| Recurrence at 5 years Major outcome group 4 (recurrence) Defined as reoperation or progressive angular deformity of 20 degrees in a successfully treated joint | 209 per 1000 | 849 per 1000 | 93 | ⊕⊕⊝⊝ | Progressive angular deformity defined in van Rijssen 2006 as an increase in TPED ≥ 30 degrees. In van Rijssen 2012a, different definitions used (increase of 20 degrees in a successfully treated joint) in other studies of Dupuytren's disease, such as Hurst 2009, acknowledged and applied P value < 0.001 in van Rijssen 2012a Relative effect not calculated, as only study available Recurrence rate influenced by the definition of recurrence used, and by length of follow‐up period |
| Major outcome group 5 (adverse effects)d | see comment | see comment | see comment | see comment | Not discussed in van Rijssen 2012a; analysed in van Rijssen 2006 |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aQuality of evidence for patient satisfaction at 5 years downgraded from high to low because of significant risks of bias in van Rijssen 2012a, and as the result of imprecision. cQuality of evidence for recurrence at 5 years downgraded from high to low because of significant risks of bias in van Rijssen 2012a, and as the result of imprecision. | |||||
| Comparison of operation types: firebreak skin grafting vs z‐plasty closure of fasciectomy for Dupuytren's disease | |||||
| Patient or population: 79 participants (Ullah 2009) Settings: single‐centre UK study Intervention: firebreak skin grafting to close incision Comparison: z‐plasty closure of incision | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| z‐plasty | Firebreak skin grafting | ||||
| PEM hand function score at 3 years Major outcome group 1 (hand function) (scores between 0 and 77, where 0 represents no impairment in hand function and 77 represents maximum impairment in hand function) | See comment | See comment | 79 (1 study) | ⊕⊕⊝⊝ | Data represented graphically only; differences between groups described as not statistically significant; no P value provided |
| Major outcome group 2 (patient satisfaction and other PROM) | See comment | See comment | See comment | See comment | Not studied in Ullah 2009 |
| Correction of MCPJ and PIPJ deformities at Major outcome group 3 (early angular outcomes) | All MCPJs fully corrected Mean PIPJ correction 6 degrees in the z‐plasty group | All MCPJs also fully corrected Mean PIPJ correction no different (also 6 degrees) in the skin graft group from the z‐plasty group | 79 (1 study) | ⊕⊕⊝⊝ | |
| Progressive contracture by 3 years Major outcome group 4 (recurrence) | 109 per 1000 | 136 per 1000 | 79 (1 study) | ⊕⊕⊝⊝ | P value = 0.17 in Ullah 2009 Rates assessed per finger (90 fingers treated among 79 participants) |
| Hypoaesthesia Major outcome group 5 (adverse effects) | Radial digital nerve territory: 217 per 1000 Ulnar digital nerve territory: 217 per 1000 | Radial digital nerve territory: 341 per 1000 Ulnar digital nerve territory: 455 per 1000 | 79 (1 study) | ⊕⊕⊝⊝ | P value = 0.2 for radial digital nerve territory in Ullah 2009 P value = 0.03 for ulnar digital nerve territory in Ullah 2009 |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aQuality of evidence for PEM hand function score at 3 years downgraded from high to low, as neither data nor P value was provided to support statement, and as the result of imprecision. b,c,dQuality of evidence downgraded from high to low because of risks of bias and imprecision. | |||||
| Refining rehabilitation: three months of postoperative night splinting with hand therapy vs hand therapy alone for rehabilitation following surgery for Dupuytren's disease | |||||
| Patient or population: 210 participants with Dupuytren's disease of the fingers in 2 studies (225 digits reported across all studies) (Collis 2013; Jerosch‐Herold 2011) Settings: multi‐centre UK RCT and single‐centre New Zealand RCT Intervention: three months of night splinting in extension in addition to hand therapy ("splint") Comparison: hand therapy alone ("no splint") | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| No splint | Splint | ||||
| DASH hand function score at 3 months Major outcome group 1 (hand function) (scores between 0 and 100, where 0 represents no impairment in hand function and 100 represents maximum impairment in hand function) | Mean DASH ranged across 'no splint' groups from | Mean DASH in 'splint' groups was 1.15 lower (95% CI ‐2.32 to 4.62) than in 'no splint' groups | 205 participants | ⊕⊕⊝⊝ | Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Major outcome group 2 (patient satisfaction) | See comment | See comment | See comment | See comment | Not assessed in these studies |
| Total active extension at 3 months Major outcome group 3 (early objective measurement) Total active extension (TAE) of MCPJ, PIPJ and DIPJ; higher value indicates loss of extension and a worse outcome | Mean TAE ranged across 'no splint' groups from | Mean TAE in 'splint' groups was 2.21 degrees higher (95% CI ‐3.59 to 8.01) than in 'no splint' groups | 225 digits | ⊕⊕⊝⊝ | Unclear whether this is the most appropriate time point for study of 'early' outcome |
| Major outcome group 4 (recurrence) | See comment | See comment | See comment | See comment | Not assessed in these studies |
| Total active flexion at three months Major outcome group 5 (adverse effects) Total active flexion (TAF) of MCPJ, PIPJ and DIPJ; lower value indicates loss of flexion and a worse outcome | Mean TAF ranged across 'no splint' groups from | Mean TAF in 'splint' groups was 8.42 degrees lower (95% CI 1.78 to 15.07) than in 'no splint' groups | 225 digits | ⊕⊕⊝⊝ | Conflicting findings from subgroups Unclear whether this is the most appropriate time point for study of 'early' outcome |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| a,b,cQuality of evidence was downgraded from high to low because of risks of bias and imprecision. | |||||
| Article | Aspect of care studied | Length of follow‐up, months | Outcomes measured | ||||||||
| Recurrence | Extension deficit | Flexion deficit | Total motion | PROM | Time | Complications as an outcome measure | Hand volume | Other | |||
| Technical refinement | 0.5 | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | Wound appearance | |
| Technical refinement | 18 | ‐ | + | ‐ | + | ‐ | + | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 2 | ‐ | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | |
| Technical refinement | 24 | + | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Technical refinement | 24 | + | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 3 | ‐ | + | + | ‐ | + | ‐ | ‐ | ‐ | Grip strength, composite flexion | |
| Technical refinement | 24 | + | + | ‐ | ‐ | + | ‐ | + | ‐ | ‐ | |
| Technical refinement | 0.5 | ‐ | ‐ | ‐ | ‐ | + | + | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 12 | ‐ | + | + | + | + | ‐ | ‐ | ‐ | ‐ | |
| Rehabilitation adjunct | 12 | ‐ | + | ‐ | ‐ | + | ‐ | + | ‐ | ‐ | |
| Technical refinement | 6 | ‐ | + | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Procedure type | 36 | + | + | ‐ | + | + | + | ‐ | ‐ | Grip strength | |
| Procedure type | 1.5 | ‐ | + | + | ‐ | + | ‐ | + | ‐ | ‐ | |
| Procedure type | 60 | + | + | ‐ | ‐ | + | ‐ | ‐ | ‐ | ‐ |
| Tubiana stage preop | % improvement in TPED for needle fasciotomy | % improvement in TPED for fasciectomy | Significance of differences between procedures |
| I | 71 | 82 | 0.329 |
| II | 67 | 78 | 0.071 |
| III | 46 | 75 | 0.000 |
| IV | 47 | 79 | 0.004 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH Show forest plot | 2 | 210 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐2.74, 4.74] |
| 2 Total active extension Show forest plot | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐7.72, 3.94] |
| 2.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐33.20, 9.20] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐21.50, 39.50] |
| 2.3 Little finger | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐32.31, 8.31] |
| 2.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.90, 6.10] |
| 3 Total active flexion Show forest plot | 2 | 232 | Mean Difference (IV, Random, 95% CI) | 2.42 [‐4.98, 9.83] |
| 3.1 Middle finger | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐11.52, 23.52] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 11.00 [‐0.47, 22.47] |
| 3.3 Little finger | 1 | 43 | Mean Difference (IV, Random, 95% CI) | ‐9.0 [‐21.26, 3.26] |
| 3.4 No subgroup by digit | 1 | 154 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐3.35, 8.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score at 3 months Show forest plot | 2 | 205 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐2.32, 4.62] |
| 2 Total active extension at 3 months Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐8.01, 3.59] |
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐30.26, 38.26] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐23.24, 15.24] |
| 2.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐27.35, 17.35] |
| 2.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐8.43, 4.43] |
| 3 Total active flexion at 3 months Show forest plot | 2 | 225 | Mean Difference (IV, Random, 95% CI) | 12.36 [1.21, 23.50] |
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 29.00 [3.96, 54.04] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 24.0 [0.21, 47.79] |
| 3.3 Little finger | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.0 [‐9.12, 27.12] |
| 3.4 No subgroup by digit | 1 | 151 | Mean Difference (IV, Random, 95% CI) | 4.60 [‐3.25, 12.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score at 3 months Show forest plot | 2 | 184 | Mean Difference (IV, Random, 95% CI) | 1.01 [‐2.85, 4.86] |
| 2 Total active extension at 3 months [degrees] Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | ‐9.50 [‐21.14, 2.15] |
| 2.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 3.90 [‐29.81, 37.61] |
| 2.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐16.9 [‐33.79, ‐0.01] |
| 2.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐22.20 [‐41.05, ‐3.35] |
| 2.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐8.77, 4.97] |
| 3 Total active flexion at 3 months [degrees] Show forest plot | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 12.64 [3.68, 21.60] |
| 3.1 Middle finger | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 28.60 [3.79, 53.41] |
| 3.2 Ring finger | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 21.70 [‐0.80, 44.20] |
| 3.3 Little finger | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 13.10 [‐4.61, 30.81] |
| 3.4 No subgroup by digit | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐1.42, 15.02] |

