اثر مواد ضد‐عفونی کننده پوست در کاهش عفونتهای ناشی از کاتتر ورید مرکزی
Appendices
Appendix 1. Glossary of terms (lay definitions in the context of this review only)
Colonisation: occupation by bacteria or other micro‐organisms in a specific body part or a device in the body without causing infection
Erythema: redness
Induration: a term usually used to describe the hardening of a small area of the skin
Infusates: liquid that is being infused through a device, such as a line, from the source (such as the fluid bag) to the patient
Nosocomial infection: also known as a hospital‐acquired infection or HAI, an infection whose development is favoured by a hospital environment, such as one acquired by a patient during a hospital visit or one developed among hospital staff. Such infections include fungal and bacterial infections and are aggravated by the reduced resistance of individual patients.
Pathogenesis: the chain of events leading to the appearance of a disease or a medical problem, described scientifically in detail
Placebo: a simulated or 'sham' treatment that is designed to be indistinguishable from the actual treatment in all aspects except for the active component tested
Plasmapheresis: a medical procedure in which a person's blood is channeled out of his body to a special 'filtering machine' and then returned to the body after the removal of the unwanted substance. It is used to treat a variety of medical problems in which unwanted substances, usually in the form of harmful antibodies, are produced
Purulence: the state where pus appears at or around a lesion such as a wound
Regimen: a systematic plan of single or multiple measures designed to improve the health of a patient
Single agent: the use of only one antiseptic agent
A combination of agents: the use of more than one antiseptic agent together
Transient flora: bacteria that occupy a specific place in the body or a device for a short‐term period
Subclavian vein: large blood vessels on each the side of the neck; commonly used as a site for inserting a central venous catheter.
Appendix 2. Definitions of infections linked to vascular access
Table 1. Definitions of infections linked to vascular access (Pagani 2008)
| Type of infection | Criteria |
| Catheter colonisation | A significant growth of a micro‐organism (> 15 CFU) from the catheter tip, subcutaneous segment or catheter hub in the absence of clinical signs of infection |
| Exit‐site/insertion site infection | Microbiologically documented: exudates at catheter exit site yield a micro‐organism with or without concomitant bloodstream infection. Clinically documented: erythema or induration within 2 cm of the catheter insertion site in the absence of associated bloodstream infection and without concomitant purulence |
| Positive blood culture | Micro‐organism, potentially pathogenic, cultured from one or more blood culture |
| Bloodstream infection | Positive blood culture with a clinical sepsis (see below) |
| Primary bloodstream infection | Laboratory‐confirmed bloodstream infection or clinical sepsis occurring without documented infection |
| Secondary bloodstream infection | Laboratory‐confirmed bloodstream infection secondary to another documented infection |
| Clinical sepsis | Requires one of the following with no other recognised cause: fever (> 38° C), hypotension (SBP < 90 mmHg), oliguria (< 20 ml/h); and all of the following: blood culture not performed or no organism detected in blood, no apparent infection at another body site and clinical response to therapy following catheter removal or change |
| Catheter‐associated bloodstream infection | Primary bloodstream infection or clinical sepsis in the presence of an intravascular device |
| Catheter‐related bloodstream infection | Laboratory‐confirmed bloodstream infection in the presence of an intravascular access: at least 1 positive blood culture obtained from a peripheral vein, clinical manifestation of infection and no apparent source of the bloodstream infection except the vascular access, and with 1 of the microbiological methods: a positive result of semi‐quantitative (> 15 CFUs per catheter segment) or quantitative culture (> 103 CFU/catheter segment) with the same organism, paired quantitative blood cultures with a > 5:1 ratio device versus peripheral, differential time to positivity (blood culture obtained from a CVC is positive at least 2 h earlier than a peripheral blood culture) |
| CFU: colony‐forming units; CVC: central venous catheter; S BP: systolic blood pressure. | |
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Catheterization, Central Venous] explode all trees
#2 central next venous next catheter*:ti,ab,kw
#3 central next venous next line*:ti,ab,kw
#4 {or #1‐#3}
#5 MeSH descriptor: [Antisepsis] explode all trees
#6 antisepsis:ti,ab,kw
#7 MeSH descriptor: [Hand Hygiene] explode all trees
#8 (handwash* or hand wash* or "hand hygiene"):ti,ab,kw
#9 aseptic next technique*:ti,ab,kw
#10 barrier next precaution*:ti,ab,kw
#11 MeSH descriptor: [Anti‐Infective Agents, Local] explode all trees
#12 MeSH descriptor: [Chlorhexidine] explode all trees
#13 MeSH descriptor: [Iodine] explode all trees
#14 MeSH descriptor: [Povidone] explode all trees
#15 MeSH descriptor: [Triclosan] explode all trees
#16 MeSH descriptor: [Hexachlorophene] explode all trees
#17 MeSH descriptor: [Cetrimonium Compounds] explode all trees
#18 MeSH descriptor: [Phenol] explode all trees
#19 MeSH descriptor: [Hydrogen Peroxide] explode all trees
#20 MeSH descriptor: [Alcohols] explode all trees
#21 MeSH descriptor: [Soaps] explode all trees
#22 (iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or "hydrogen peroxide" or alcohol or alcohols or antiseptic* or soap*):ti,ab,kw
#23 skin near/3 disinfect*:ti,ab,kw
#24 {or #5‐#23}
#25 {and #4, #24} in Trials
Appendix 4. Ovid MEDLINE search strategy
1 exp Catheterization, Central Venous/
2 central venous catheter*.tw.
3 central venous line*.tw.
4 or/1‐3
5 exp Antisepsis/
6 antisepsis.tw.
7 exp Hand Hygiene/
8 (handwash* or hand wash* or hand hygiene).tw.
9 aseptic technique*.tw.
10 barrier precaution*.tw.
11 exp Anti‐Infective Agents, Local/
12 exp Chlorhexidine/
13 exp Iodine/
14 exp Povidone/
15 exp Triclosan/
16 exp Hexachlorophene/
17 exp Cetrimonium Compounds/
18 exp Phenol/
19 exp Hydrogen Peroxide/
20 exp Alcohols/
21 exp Soaps/
22 (iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap*).tw.
23 (skin adj3 disinfect*).tw.
24 or/5‐23
25 4 and 24
26 randomized controlled trial.pt.
27 controlled clinical trial.pt.
28 randomi?ed.ab.
29 placebo.ab.
30 clinical trials as topic.sh.
31 randomly.ab.
32 trial.ti.
33 or/26‐32
34 exp animals/ not humans.sh.
35 33 not 34
36 and/25,35
Appendix 5. Ovid EMBASE search strategy
1 exp central venous catheter/
2 central venous catheter*.tw.
3 central venous line*.tw.
4 or/1‐3
5 exp antisepsis/
6 antisepsis.tw.
7 exp hand washing/
8 (handwash* or hand wash* or hand hygiene).tw.
9 aseptic technique*.tw.
10 barrier precaution*.tw.
11 exp topical antiinfective agent/
12 exp chlorhexidine/
13 exp iodine/
14 exp povidone/
15 exp povidone iodine/
16 exp triclosan/
17 exp hexachlorophene/
18 exp cetrimide/
19 exp benzalkonium/
20 exp octenidine/
21 exp phenol/
22 exp hydrogen peroxide/
23 exp alcohol/
24 exp soap/
25 (iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap*).tw.
26 (skin adj3 disinfect*).tw.
27 or/5‐26
28 4 and 27
29 Randomized controlled trials/
30 Single‐Blind Method/
31 Double‐Blind Method/
32 Crossover Procedure/
33 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab.
34 (doubl$ adj blind$).ti,ab.
35 (singl$ adj blind$).ti,ab.
36 or/29‐35
37 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
38 human/ or human cell/
39 and/37‐38
40 37 not 39
41 36 not 40
42 28 and 41
Appendix 6. EBSCO CINAHL Plus search strategy
S1 (MH "Central Venous Catheters+")
S2 (MH "Catheterization, Central Venous+")
S3 TI central venous catheter* or AB central venous catheter*
S4 TI central venous line* or AB central venous line*
S5 S1 or S2 or S3 or S4
S6 TI antisepsis or AB antisepsis
S7 (MH "Handwashing+")
S8 TI ( handwash* or hand wash* or hand hygiene ) or AB ( handwash* or hand wash* or hand hygiene )
S9 TI aseptic technique* or AB aseptic technique*
S10 TI barrier precaution* or AB barrier precaution*
S11 (MH "Antiinfective Agents, Local+")
S12 (MH "Chlorhexidine")
S13 (MH "Iodine")
S14 (MH "Povidone‐Iodine")
S15 (MH "Hexachlorophene")
S16 (MH "Benzalkonium Compounds")
S17 (MH "Phenols")
S18 (MH "Hydrogen Peroxide")
S19 (MH "Alcohols+")
S20 (MH "Soaps")
S21 TI iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap*
S22 AB iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap*
S23 TI skin N3 disinfect* or AB skin N3 disinfect*
S24 S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23
S25 S5 and S24
S26 MH "Clinical Trials+"
S27 PT Clinical trial
S28 TI clinic* N1 trial* or AB clinic* N1 trial*
S29 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S30 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S31 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S32 MH "Random Assignment"
S33 TI random* allocat* or AB random* allocat*
S34 MH "Placebos"
S35 TI placebo* or AB placebo*
S36 MH "Quantitative Studies"
S37 S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36
S38 S25 and S37
Appendix 7. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
-
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
-
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
-
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
-
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following:
-
Insufficient information to permit judgement of low or high risk of bias.
-
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
-
No missing outcome data.
-
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
-
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
-
Missing data have been imputed using appropriate methods.
High risk of bias
Either of the following:
-
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
-
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
-
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following:
-
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
-
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
-
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
-
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
-
Not all of the study’s prespecified primary outcomes have been reported.
-
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
-
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
-
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
-
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
-
had a potential source of bias related to the specific study design used;
-
had extreme baseline imbalance;
-
has been claimed to have been fraudulent; or
-
had some other problem.
Unclear
There may be a risk of bias, but there is either:
-
insufficient information to assess whether an important risk of bias exists; or
-
insufficient rationale or evidence that an identified problem will introduce bias.
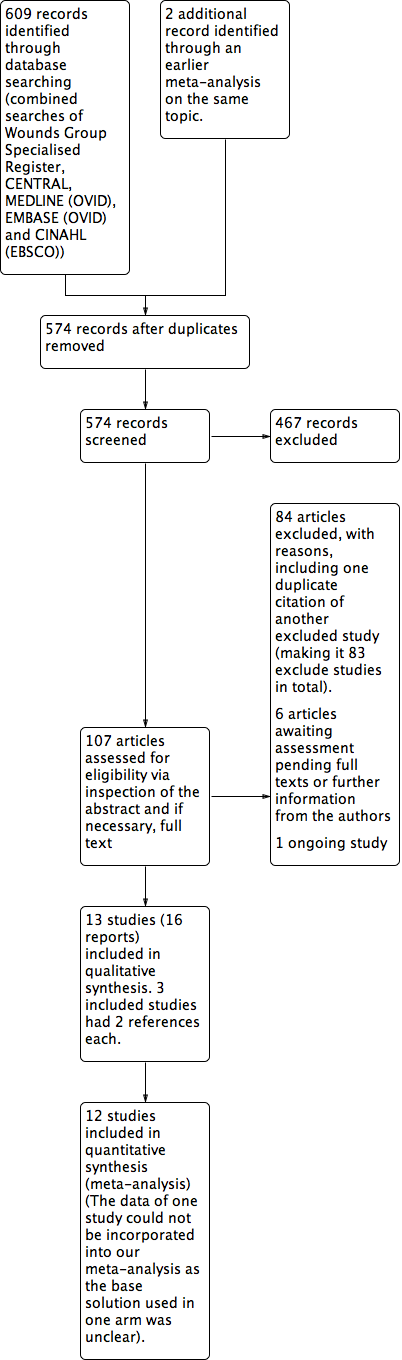
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
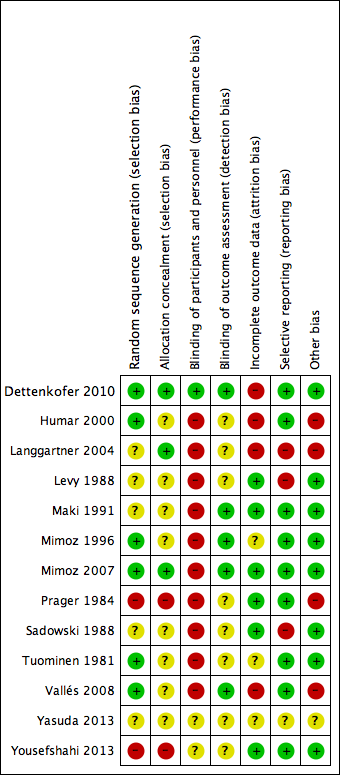
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.1 Catheter‐related BSI.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.3 All‐cause mortality.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.4 Catheter colonisation.

Comparison 1 Povidone‐iodine (in aqueous solution) versus no skin antisepsis, Outcome 1 Catheter‐related BSI.
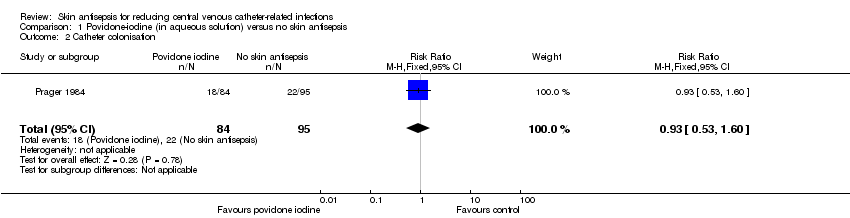
Comparison 1 Povidone‐iodine (in aqueous solution) versus no skin antisepsis, Outcome 2 Catheter colonisation.

Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 1 Septicaemia.
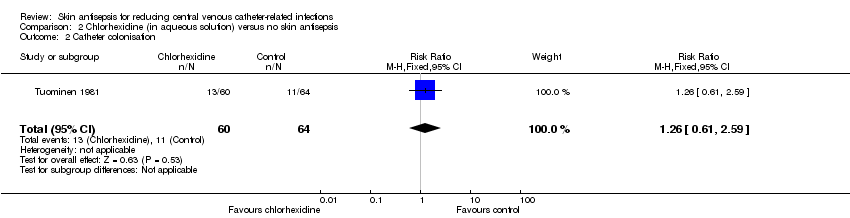
Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 2 Catheter colonisation.

Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 3 Number of patients who required antibiotics during in‐dwelling period of catheter.

Comparison 3 Alcohol versus no skin antisepsis, Outcome 1 Catheter colonisation.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 1 Catheter‐related BSI.
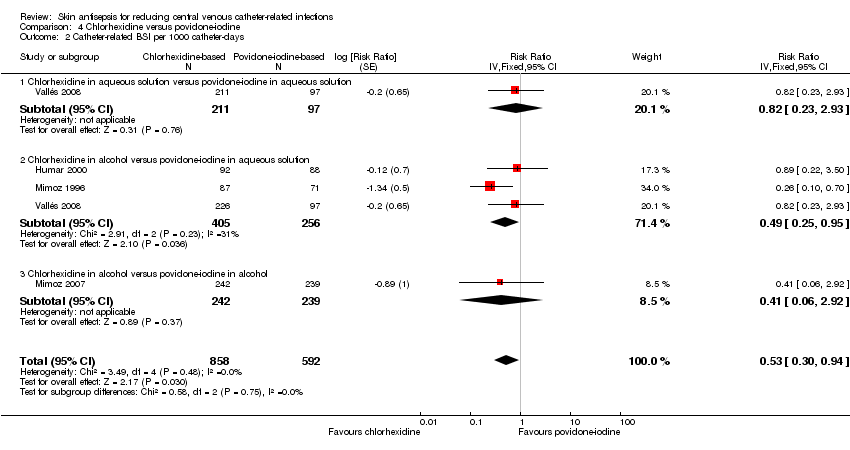
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 2 Catheter‐related BSI per 1000 catheter‐days.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 3 All‐cause mortality.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 4 Catheter colonisation.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 5 Catheter colonisation per 1000 catheter‐days.
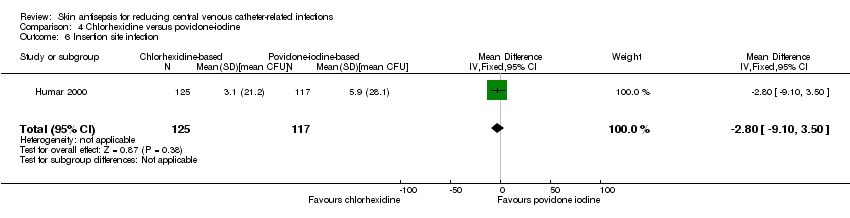
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 6 Insertion site infection.
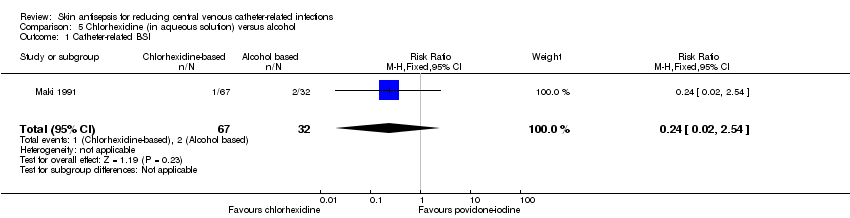
Comparison 5 Chlorhexidine (in aqueous solution) versus alcohol, Outcome 1 Catheter‐related BSI.
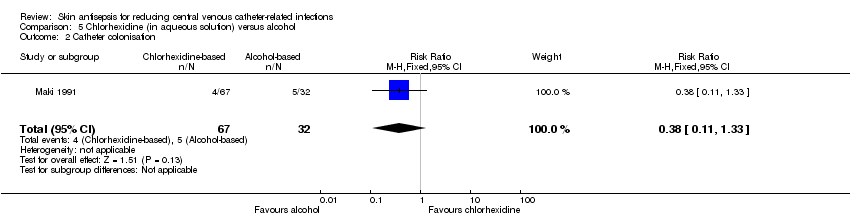
Comparison 5 Chlorhexidine (in aqueous solution) versus alcohol, Outcome 2 Catheter colonisation.

Comparison 6 Povidone‐iodine (in aqueous solution) versus alcohol, Outcome 1 Catheter‐related BSI.

Comparison 6 Povidone‐iodine (in aqueous solution) versus alcohol, Outcome 2 Catheter colonisation.
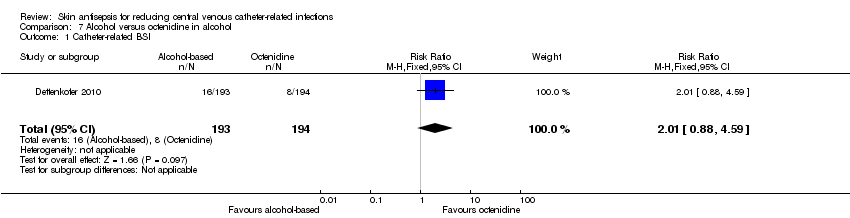
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 1 Catheter‐related BSI.

Comparison 7 Alcohol versus octenidine in alcohol, Outcome 2 Catheter‐related BSI per 1000 catheter‐days.
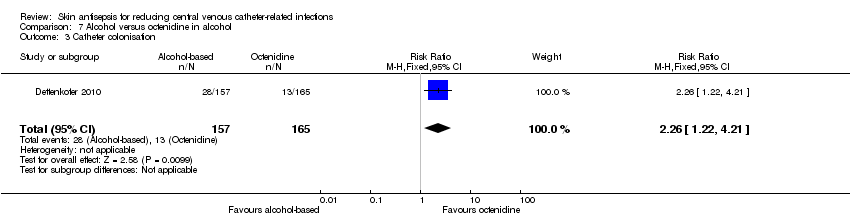
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 3 Catheter colonisation.
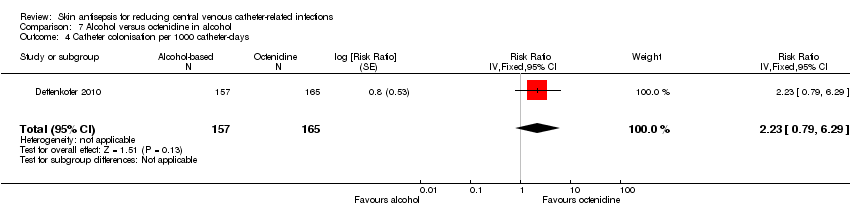
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 4 Catheter colonisation per 1000 catheter‐days.

Comparison 7 Alcohol versus octenidine in alcohol, Outcome 5 Skin colonisation.

Comparison 7 Alcohol versus octenidine in alcohol, Outcome 6 Adverse effects.
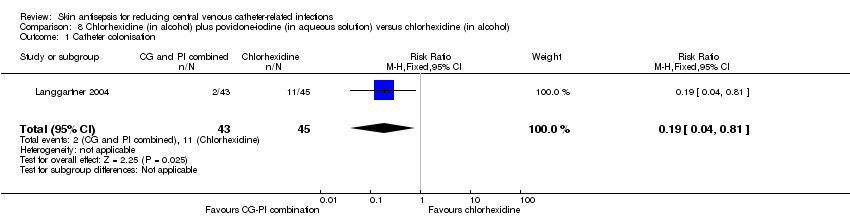
Comparison 8 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol), Outcome 1 Catheter colonisation.
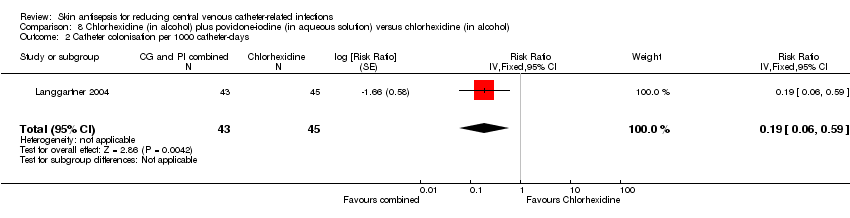
Comparison 8 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol), Outcome 2 Catheter colonisation per 1000 catheter‐days.

Comparison 9 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution), Outcome 1 Catheter colonisation.

Comparison 9 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution), Outcome 2 Catheter colonisation per 1000 catheter‐days.

Comparison 10 Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine aqueous 10% scrub, Outcome 1 Catheter colonisation.
| Chlorhexidine compared to povidone‐iodine for patients with a central venous catheter | |||||
| Patient or population: patients with a central venous catheter | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Povidone‐iodine | Chlorhexidine | ||||
| Catheter‐related BSI ‐ overall comparison between chlorhexidine and povidone‐iodine (during in‐patient stay) | Study population | RR 0.64 | 1436 | ⊕⊝⊝⊝ | |
| 64 per 1000 | 41 per 1000 | ||||
| Moderatea | |||||
| 46 per 1000 | 29 per 1000 | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | Study population | RR 0.64 | 452 | ⊕⊝⊝⊝ | |
| 86 per 1000 | 55 per 1000 | ||||
| Moderate | |||||
| 84 per 1000 | 54 per 1000 | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | Study population | RR 0.77 | 503 | ⊕⊝⊝⊝ | |
| 70 per 1000 | 54 per 1000 | ||||
| Moderate | |||||
| 69 per 1000 | 53 per 1000 | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in alcohol versus povidone‐iodine in alcohol | Study population | RR 0.4 | 481 | ⊕⊕⊕⊝ | |
| 42 per 1000 | 17 per 1000 | ||||
| Moderate | |||||
| 42 per 1000 | 17 per 1000 | ||||
| Primary BSI or clinical sepsis | No studies under this comparison assessed this outcome. | ||||
| All‐cause mortality ‐ Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | Study population | RR 1.15 | 213 | ⊕⊕⊝⊝ | |
| 236 per 1000 | 271 per 1000 | ||||
| Moderate | |||||
| 236 per 1000 | 271 per 1000 | ||||
| All‐cause mortality ‐ Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | Study population | RR 0.8 | 222 | ⊕⊕⊝⊝ | |
| 236 per 1000 | 189 per 1000 | ||||
| Moderate | |||||
| 236 per 1000 | 189 per 1000 | ||||
| Mortality attributable the CVC‐related infections. | No studies under this comparison assessed this outcome. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a'Moderate risk' was calculated from the median control event rate for each outcome. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.37, 2.61] |
| 2 Catheter colonisation Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Septicaemia Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.31, 27.31] |
| 2 Catheter colonisation Show forest plot | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.61, 2.59] |
| 3 Number of patients who required antibiotics during in‐dwelling period of catheter Show forest plot | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.85] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 4 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.99] |
| 1.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 2 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.28] |
| 1.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.39, 1.53] |
| 1.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.13, 1.24] |
| 2 Catheter‐related BSI per 1000 catheter‐days Show forest plot | 4 | 1450 | Risk Ratio (Fixed, 95% CI) | 0.53 [0.30, 0.94] |
| 2.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 308 | Risk Ratio (Fixed, 95% CI) | 0.82 [0.23, 2.93] |
| 2.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 3 | 661 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.25, 0.95] |
| 2.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (Fixed, 95% CI) | 0.41 [0.06, 2.92] |
| 3 All‐cause mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.72, 1.83] |
| 3.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.48, 1.34] |
| 4 Catheter colonisation Show forest plot | 5 | 1533 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.12, ‐0.03] |
| 4.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 2 | 452 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.17, ‐0.02] |
| 4.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 3 | 600 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 4.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.17, ‐0.04] |
| 5 Catheter colonisation per 1000 catheter‐days Show forest plot | 5 | 1547 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.50, 0.81] |
| 5.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 308 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 5.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 4 | 758 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.48, 0.85] |
| 5.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (Fixed, 95% CI) | 0.53 [0.24, 1.17] |
| 6 Insertion site infection Show forest plot | 1 | 242 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐9.10, 3.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 2.54] |
| 2 Catheter colonisation Show forest plot | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.08] |
| 2 Catheter colonisation Show forest plot | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2.1 Povidone‐iodine in aqueous solution versus alcohol | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.14] |
| 2.2 Povidone‐iodine‐impregnated adherent film versus alcohol | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.51, 160.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐related BSI Show forest plot | 1 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.88, 4.59] |
| 2 Catheter‐related BSI per 1000 catheter‐days Show forest plot | 1 | 387 | Risk Ratio (Fixed, 95% CI) | 2.18 [0.54, 8.77] |
| 3 Catheter colonisation Show forest plot | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.22, 4.21] |
| 4 Catheter colonisation per 1000 catheter‐days Show forest plot | 1 | 322 | Risk Ratio (Fixed, 95% CI) | 2.23 [0.79, 6.29] |
| 5 Skin colonisation Show forest plot | 1 | 365 | Mean Difference (IV, Fixed, 95% CI) | 79.00 [32.76, 125.24] |
| 6 Adverse effects Show forest plot | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.81] |
| 2 Catheter colonisation per 1000 catheter‐days Show forest plot | 1 | 88 | Risk Ratio (Fixed, 95% CI) | 0.19 [0.06, 0.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 2 Catheter colonisation per 1000 catheter‐days Show forest plot | 1 | 95 | Risk Ratio (Fixed, 95% CI) | 0.17 [0.05, 0.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter colonisation Show forest plot | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.72] |

