نقش تک‐دوز ایبوپروفن خوراکی به همراه کدئین در درمان درد حاد پس از جراحی در بزرگسالان
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010107.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 febrero 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All authors contributed to writing the protocol.
For the original review, SMK and SD carried out searches, assessed studies for inclusion, and extracted data. RAM acted as arbitrator. All authors were involved in writing the review.
For the update RAM and SD carried out searches and revised the text where necessary. All authors read the final review.
RAM will be responsible for updating the review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
External sources
-
No sources of support supplied
Declarations of interest
RAM and SD have received research support from charities, government and industry sources at various times, but none related to this review. RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. SK has no interests to declare.
Acknowledgements
This review received infrastructure support from the Oxford Pain Relief Trust.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service, or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Feb 05 | Single dose oral ibuprofen plus codeine for acute postoperative pain in adults | Review | Sheena Derry, Samuel M Karlin, R Andrew Moore | |
| 2013 Mar 28 | Single dose oral ibuprofen plus codeine for acute postoperative pain in adults | Review | Sheena Derry, Samuel M Karlin, R Andrew Moore | |
| 2012 Sep 12 | Single dose oral ibuprofen plus codeine for acute postoperative pain in adults | Protocol | Sheena Derry, Samuel M Karlin, R Andrew Moore | |
Differences between protocol and review
We have included additional information relating to the criteria used to assess risk of bias in this updated review.
Notes
A new search is not likely to identify any potentially relevant studies likely to change the conclusions and this review has been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: ibuprofen 400 mg + high dose codeine (ibu/cod) versus placebo, outcome: 2.1 Participants with at least 50% pain relief.

Studies comparing ibuprofen plus codeine with placebo, with the outcome of at least 50% maximum pain relief over 4 to 6 hours. Colour code: white ‐ ibuprofen 200 mg + codeine 15 mg; yellow ‐ ibuprofen 200 mg + codeine 30 mg; light pink ‐ ibuprofen 400 mg + codeine 25.6 mg; medium pink ‐ ibuprofen 400 mg + codeine 30 mg; red ‐ ibuprofen 400 mg + codeine 60 mg; blue ‐ ibuprofen 800 mg + codeine 60 mg.

Studies comparing ibuprofen plus codeine with same dose of ibuprofen, with the outcome of at least 50% maximum pain relief over 4 to 6 hours. Colour code: darker yellow ‐ ibuprofen 200 mg + codeine 20 mg versus ibuprofen 200 mg; lighter yellow ‐ ibuprofen 400 mg + codeine 60 mg versus ibuprofen 400 mg.
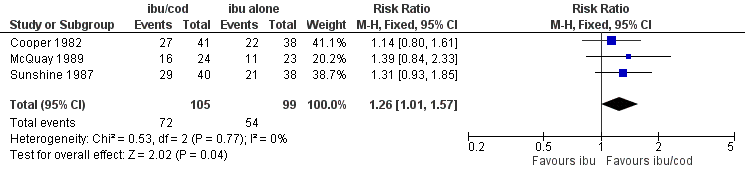
Forest plot of comparison: ibuprofen + codeine (all doses; ibu/cod) versus same dose of ibuprofen alone (ibu), outcome: 3.1 Participants with at least 50% pain relief.

Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 1 Participants with ≥ 50% pain relief.
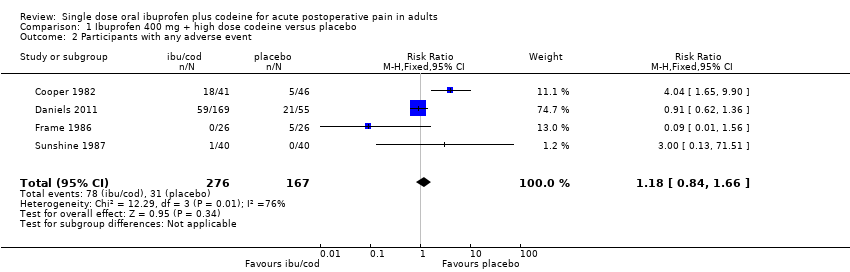
Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 2 Participants with any adverse event.

Comparison 2 Ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, Outcome 1 Participants with ≥ 50% pain relief.
| Ibuprofen plus codeine compared with placebo for acute postoperative pain | ||||||
| Population: adults with moderate or severe acute postoperative pain Settings: community or hospital Intervention: ibuprofen 400 mg + codeine 25.6 mg to 60 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with | Relative effect and NNT or NNH (95% CI) | Number of studies, events | Quality of the evidence | Comments | |
| Comparator | Intervention | |||||
| At least 50% of maximum pain relief over 4 to 6 h | 180 in 1000 | 640 in 1000 | RR 4.1 (2.8 to 5.9) NNT 2.2 (1.8 to 2.6) | 4 studies 443 participants 208 events | High | Adequate numbers of studies, participants and events. Consistency across studies |
| Participants with at least 1 adverse event | 180 in 1000 | 280 in 1000 | RR 1.2 (0.84 to 1.7) NNH not calculated | 4 studies 443 participants 109 events | Moderate | Adequate numbers of studies, participants, but moderate number of events. Consistency across studies. Single dose studies may not reflect clinical practice |
| Participants with a serious adverse event | No serious adverse events | Low | Studies underpowered to detect rare events | |||
| Deaths | No deaths | Low | Studies underpowered to detect rare events | |||
| CI: confidence interval; NNH: number needed to treat to harm; NNT: number needed to treat for benefit; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [2.82, 5.93] |
| 2 Participants with any adverse event Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.01, 1.57] |

