Prise unique d'ibuprofène associé à la codéine par voie orale pour le traitement de la douleur postopératoire aiguë chez l'adulte
Résumé scientifique
Contexte
Il existe des preuves probantes que la combinaison de deux analgésiques différents à des doses fixes dans un comprimé unique peut permettre de soulager plus efficacement la douleur en cas de douleur aiguë et de céphalées que l'un ou l'autre des deux médicaments en monothérapie, et que les effets médicamenteux spécifiques sont essentiellement additifs. Cela semble être vrai surtout pour la douleur postopératoire et la migraine sur une gamme de combinaisons médicamenteuses différentes et quand elles sont testées dans les mêmes essais et des essais différents. Certaines combinaisons d'ibuprofène et de codéine sont disponibles sans prescription (mais normalement en pharmacie uniquement) quand la dose de codéine est plus faible, et sur prescription quand la dose de codéine est plus élevée.
Objectifs
Évaluer l'efficacité analgésique et les effets indésirables d'une prise unique d'ibuprofène associé à la codéine par voie orale pour le traitement de la douleur postopératoire modérée à aiguë. Nous avons comparé l'ibuprofène associé à la codéine à un placebo et à la même dose d'ibuprofène en monothérapie.
Stratégie de recherche documentaire
Nous avons effectué une recherche dans le registre Cochrane des essais contrôlés (CENTRAL), MEDLINE, EMBASE, l'Oxford Pain Database, ClinicalTrials.gov, et les listes bibliographiques des articles. La recherche la plus récente a été réalisée le 30.09.12.
Critères de sélection
Les essais cliniques avec contrôle actif ou placebo, randomisés, en double aveugle portant sur la prise unique d'ibuprofène associé à la codéine par voie orale pour le traitement de la douleur postopératoire aiguë chez l'adulte.
Recueil et analyse des données
Deux auteurs de la revue ont sélectionné les essais à inclure, évalué leur qualité et extrait les données de façon indépendante. Nous avons utilisé l'aire sous la courbe du soulagement de la douleur sur le temps pour déduire la proportion de participants ayant reçu l'ibuprofène associé à la codéine, un placebo, ou la même dose d'ibuprofène en monothérapie avec au moins 50 % de soulagement de la douleur sur six heures, en utilisant des équations validées. Nous avons calculé le risque relatif (RR) et le nombre de sujets à traiter (NST) pour observer un bénéfice du traitement. Nous avons utilisé les informations sur l'usage d'un médicament de secours pour calculer la proportion de participants nécessitant un médicament de secours et la moyenne pondérée du délai moyen avant usage. Nous avons aussi recueilli des informations sur les effets indésirables. Des analyses ont été prévues pour différentes doses d'ibuprofène et de codéine, mais surtout pour la codéine dans lesquelles nous avons défini des critères pour les doses faibles (< 10 mg), moyennes (10 à 20 mg), et élevées (> 20 mg).
Résultats principaux
Les informations étaient disponibles dans six études totalisant 1 342 participants, avec différentes doses d'ibuprofène et de codéine. Dans quatre études (443 participants) portant sur l'ibuprofène 400 mg associé à la codéine 25,6 à 60 mg (dose élevée de codéine), 64 % des participants ont obtenu au moins 50 % de soulagement maximum de la douleur avec la combinaison par rapport à 18 % avec le placebo. Le NST était de 2,2 (IC à 95 % 1,8 à 2,6). Dans trois études (204 participants), l'ibuprofène associé à la codéine (n'importe quelle dose) ont été plus efficaces que la même dose d'ibuprofène (69 % contre 55 %), mais le résultat était à peine significatif avec un bénéfice relatif de 1,3 (IC à 95 % 1,01 à 1,6). Dans deux études (159 participants), l'ibuprofène associé à la codéine semblaient être plus efficaces que la même dose de codéine en monothérapie (69 % contre 33 %), mais aucune analyse n'a été effectuée. Aucune différence n'a été constatée entre la combinaison et le placebo dans les comptes‐rendus d'événements indésirables dans ces études portant sur la douleur aiguë.
Conclusions des auteurs
La combinaison d'ibuprofène 400 mg associé à la codéine 25,6 à 60 mg révèle une efficacité analgésique satisfaisante. Des données très limitées suggèrent que la combinaison est plus efficace que la même dose de l'un ou l'autre des deux médicaments en monothérapie. L'usage d'analgésiques en combinaison contenant de la codéine soulève certaines inquiétudes en raison du mésusage des préparations sans ordonnance.
PICO
Résumé simplifié
Prise unique d'ibuprofène associé à la codéine par voie orale pour le traitement de la douleur postopératoire aiguë chez l'adulte
L'ibuprofène et la codéine sont tous deux des analgésiques, mais ils ont des mécanismes d'action différents. Nous savons que dans certaines circonstances, la combinaison d'analgésiques différents dans le même comprimé permet de bien soulager la douleur chez davantage de personnes que l'un des deux analgésiques en monothérapie, à la même dose. Cette revue a examiné l'efficacité obtenue de la combinaison d'ibuprofène et de codéine pour soulager une douleur modérée ou sévère après une intervention chirurgicale. L'ibuprofène 400 mg associé à des doses élevées de codéine (25,6 mg à 60 mg) a permis de soulager efficacement la douleur chez plus de 6 participants sur 10 (64 %), comparés à un peu moins de 2 participants sur 10 (18 %) avec le placebo. Des événements indésirables sont survenus à des taux similaires avec les combinaisons et le placebo dans ces études portant sur une prise unique, et aucun événement indésirable ou abandon dû à des événements indésirables n'a eu lieu avec la combinaison.
Authors' conclusions
Background
Description of the condition
Acute pain occurs as a result of tissue damage either accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury and/or nerve injury. The management of postoperative pain and inflammation is a critical component of patient care.
This is one of a series of reviews whose aim is to increase awareness of the range of analgesics that are potentially available, and present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level. The series covers all analgesics licensed for acute postoperative pain in the UK, and dipyrone, which is commonly used in Spain, Portugal, and Latin‐American countries. The results have been examined in an overview (Moore 2011a), and important individual reviews include ibuprofen (Derry 2009), codeine (Derry 2010), paracetamol (Toms 2008), and etoricoxib (Clarke 2012).
Description of the intervention
Acute pain trials
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants are small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working, it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double‐blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following four to six hours for shorter‐acting drugs, and up to 12 or 24 hours for longer‐acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to six hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first six hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Knowing the relative efficacy of different analgesic drugs at various doses can be helpful.
Ibuprofen
Ibuprofen is a non‐steroidal anti‐inflammatory drug (NSAID). It was developed in the 1960s and is used extensively throughout the world for relief of pain and inflammation in both acute and chronic conditions. It is available over the counter in most countries, usually as 200 mg tablets, with 1200 mg as the recommended maximum daily dose for adults. Under medical supervision, up to 3200 mg daily may be taken, divided into three doses. Soluble salts of ibuprofen have better efficacy (Derry 2009).
A major concern regarding the use of conventional NSAIDs postoperatively is the possibility of bleeding from both the operative site (because of the inhibition of platelet aggregation) (Forrest 2002) and from the upper gastrointestinal tract, (especially in patients stressed by surgery, the elderly, frail, or dehydrated). Other potentially serious adverse events include acute liver injury, acute renal injury, heart failure, and adverse reproductive outcomes (Hernandez‐Diaz 2001). However, such complications are more likely to occur with chronic use and NSAIDs generally present fewer risks if used in the short term, as in the treatment of postoperative pain (Rapoport 1999).
Codeine
Codeine is an opioid. Patients’ response to opioids varies considerably, so that dose frequently needs to be adjusted individually, but the usual dose by mouth for adults is 30 mg to 60 mg every four hours, to a maximum of 240 mg daily. As with other opioids, repeated administration of codeine in the absence of pain can cause dependence and tolerance, but long term use for pain relief, or use of high doses, tends to be limited by adverse effects, in particular constipation and drowsiness. In severe or persistent pain, or both, for which large amounts of codeine are required, smaller doses of stronger opioids are thought to be better tolerated. Respiratory depression is dose‐related and may have serious consequences in people without previous experience of opioid use, those who are “extensive metabolizers”, and the elderly in whom reduced renal function leads to accumulation of active metabolites. Misuse of codeine combination products has resulted in serious morbidity from gastrointestinal bleeding and/or dependence associated with exceptionally high doses (mean daily doses of 435 to 602 mg of codeine phosphate and 6800 to 9400 mg ibuprofen) (Frei 2010).
How the intervention might work
Clinicians prescribe NSAIDs on a routine basis for a range of causes of mild‐to‐moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (FitzGerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. Ibuprofen, like most NSAIDs, causes reversible inhibition of the cyclooxygenases, which interferes with thromboxane and prostaglandin synthesis, and increases production of anti‐inflammatory lipoxins.
Codeine is an opioid. Its analgesic effects are attributed to its metabolism in the liver to the active compounds morphine and morphine‐6‐glucuronide. Normally, between 5% and 10% is converted to morphine, and a dose of about 30 mg codeine phosphate is considered equivalent to 3 mg morphine. The capacity to metabolise codeine to its active metabolites varies between individuals, however, with up to 10% of Caucasians, 2% of Asians and 1% of Arabs being “poor metabolizers” (Cascarbi 2003). In these individuals codeine is a relatively ineffective analgesic. A few individuals are “extensive metabolizers” and are able to convert more of the codeine to morphine, putting them at increased risk of toxicity from standard doses. Various medications interfere with the enzymes that catalyse the metabolism of codeine, increasing or decreasing the extent of conversion and hence the analgesic effect. For example, the selective serotonin reuptake inhibitors fluoxetine and paroxetine reduce conversion, while rifampicin and dexamethasone increase it.
Combination analgesics
We now have convincing evidence that combining two analgesics can provide additional levels of analgesia in acute pain and headache (Moore 2011b; Moore 2012), and that the drug‐specific effects are essentially additive. Results confirm that the assumption that the efficacy of combination analgesics in postoperative pain and migraine headache is the sum of the efficacies of the individual analgesic components is broadly true across a range of different drug combinations, and when tested in the same and different trials (Moore 2012). There is no convincing evidence for combination analgesics in chronic pain, however (Chaparro 2012).
Why it is important to do this review
Ibuprofen is a widely available and inexpensive NSAID with proven efficacy in relief of acute postoperative pain (Derry 2009). Codeine is also widely available and relatively inexpensive, and although on its own in single doses it has not shown good efficacy in acute postoperative pain (Derry 2010), in combination it has been shown to significantly enhance the efficacy of paracetamol (Toms 2009) and other drugs (Moore 2012). The two drugs are used in combination in clinical practice and are available as a fixed dose combination tablet over‐the‐counter in some countries (in the UK there are no combination analgesic preparations that contain a higher dose of codeine than 12.8 mg codeine phosphate (equivalent to 10 mg codeine base per tablet) available without prescription). It is important to know how this combination compares with other analgesics when assessed in the same way (Moore 2011a).
Objectives
To assess the efficacy and adverse effects of single dose oral ibuprofen plus codeine for acute postoperative pain using methods that permit comparison with other analgesics evaluated in standardised trials using almost identical methods and outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included studies of double‐blind trials with at least 10 participants randomly allocated to each treatment group of single dose oral ibuprofen plus codeine compared with placebo, or the same dose of ibuprofen alone, for the treatment of moderate to severe postoperative pain in adults. We included multiple dose studies if appropriate data from the first dose were available, and cross‐over studies provided that data from the first arm were presented separately.
We excluded the following:
-
review articles, case reports, and clinical observations;
-
studies of experimental pain;
-
studies where pain relief was assessed only by clinicians, nurses, or carers (i.e. not patient‐reported);
-
studies of less than four hours duration or studies that fail to present data over four to six hours post dose.
For postpartum pain, we included studies if the pain investigated was due to episiotomy or Caesarean section irrespective of the presence of uterine cramps; we excluded studies investigating pain due to uterine cramps alone.
Types of participants
We included studies of adult participants (> 15 years) with established postoperative pain of moderate to severe intensity following day surgery or in‐patient surgery. For studies using a visual analogue scale (VAS), we considered that pain intensity of greater than 30 mm equates to pain of at least moderate intensity (Collins 1997).
Types of interventions
Ibuprofen plus codeine, administered as a single oral dose, compared with matched placebo or the same dose of ibuprofen alone for postoperative pain. The ibuprofen and codeine could be administered as separate tablets taken together, or in a combined tablet. We included all dose combinations.
Types of outcome measures
We collected the following data where available:
-
participant characteristics;
-
patient reported pain at baseline (physician, nurse, or carer‐reported pain was not be included in the analysis);
-
patient reported pain relief expressed at least hourly over four to six hours using validated pain scales (pain intensity or pain relief in the form of VAS or categorical scales, or both);
-
patient global assessment of efficacy (PGE), using a standard categorical scale;
-
time to use of rescue medication;
-
number of participants using rescue medication;
-
number of participants with one or more adverse events;
-
number of participants with serious adverse events;
-
number of withdrawals (all‐cause, adverse events).
Primary outcomes
Participants achieving at least 50% pain relief over four to six hours.
Secondary outcomes
-
Median (or mean) time to use of rescue medication.
-
Participants using rescue medication.
-
Participants with: any adverse event; any serious adverse event (as reported in the study); withdrawal due to an adverse event.
-
Other withdrawals: withdrawals for reasons other than lack of efficacy (participants using rescue medication)
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (30 September 2012);
-
MEDLINE (via Ovid) (to 30th September 2012);
-
EMBASE (via Ovid) (to 30th September 2012);
-
Oxford Pain Relief Database (Jadad 1996a).
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, and Appendix 3 for the CENTRAL search strategy. We did not limit the searches by language.
Searching other resources
We searched for additional studies in reference lists of retrieved articles and reviews, and in clinicaltrials.gov. The manufacturers of the combination formulation (Reckitt Benckiser) had already been asked for information on published and unpublished studies, but did not know of any additional studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies to be included in the review. Disagreements would have been resolved by consensus or referral to a third review author but this was not necessary.
Data extraction and management
Two review authors extracted data and recorded them on a standard data extraction form. One review author entered data suitable for pooling into RevMan 2011.
Assessment of risk of bias in included studies
Two review authors independently assessed each study for methodological quality using a three‐item, five‐point scale (Jadad 1996b), and agreed a consensus score.
The scale used is as follows.
-
Is the study randomised? If yes ‐ one point.
-
Is the randomisation procedure reported and is it appropriate? If yes add one point, if no deduct one point.
-
Is the study double‐blind? If yes add one point.
-
Is the double‐blind method reported and is it appropriate? If yes add one point, if no deduct one point.
-
Are the reasons for patient withdrawals and dropouts described? If yes add one point.
We also completed a 'Risk of bias' table using methods adapted from those described by the Cochrane Pregnancy and Childbirth Group. Two authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) with any disagreements resolved by discussion. The following were assessed for each study:
-
Random sequence generation (checking for possible selection bias). The method used to generate the allocation sequence was assessed as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) ‐ these studies would be excluded; unclear risk of bias.
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. The methods were assessed as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth) ‐ these studies would be excluded; unclear risk of bias.
-
Blinding of outcome assessment (checking for possible detection bias). The methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received were assessed. Studies were considered to be at low risk of bias if they stated that they were blinded and described the method used to achieve blinding (e.g. identical tablets; matched in appearance and smell), or at unknown risk if they stated that they were blinded, but did not provide an adequate description of how it was achieved. Single blind and open studies would be excluded.
-
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably due to methodological weaknesses (Nüesch 2010). Studies were considered to be at low risk of bias if they had ≥ 200 participants, at unclear risk if they had 50 to 200 participants, and at high risk if they had < 50 participants.
Measures of treatment effect
We used relative risk (or 'risk ratio', RR) to establish statistical difference, and numbers needed to treat to benefit (NNT) and pooled percentages as absolute measures of benefit or harm.
We use the following terms to describe adverse outcomes in terms of harm or prevention of harm:
-
When significantly fewer adverse outcomes occur with treatment than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occur with treatment compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted only randomisation to the individual patient.
Dealing with missing data
The only likely issue with missing data in these studies was from imputation using last observation carried forward when a patient requested rescue medication. We have previously shown that this does not affect results for up to six hours after taking study medication (Barden 2004).
Assessment of heterogeneity
We examined heterogeneity visually using L'Abbé plots (L'Abbé 1987), a visual method for assessing differences in results of individual studies.
Data synthesis
We followed QUOROM guidelines (Moher 1999). For efficacy analyses we used the number of participants in each treatment group who were randomised, received medication, and provided at least one post‐baseline assessment. For safety analyses we used the number of participants randomised to each treatment group who took the study medication. Results for different doses were analysed separately.
For each study we converted the mean total pain relief (TOTPAR), summed pain intensity difference (SPID), VAS TOTPAR, or VAS SPID (Appendix 4) values for the active and placebo groups to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). We then calculated the proportion of participants in each treatment group who achieved at least 50%maxTOTPAR using verified equations (Moore 1996; Moore 1997a; Moore 1997b). We converted these proportions into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. We used this information on the number of participants with at least 50%maxTOTPAR for active and placebo groups to calculate relative benefit or relative risk and NNT.
We accepted the following pain measures for the calculation of TOTPAR or SPID (in order of priority):
-
five‐point categorical pain relief (PR) scales with comparable wording to 'none, slight, moderate, good or complete';
-
four‐point categorical pain intensity (PI) scales with comparable wording to 'none, mild, moderate, severe';
-
VAS for pain relief;
-
VAS for pain intensity.
If none of these measures was available, we used the number of participants reporting 'very good or excellent' on a five‐point categorical global scale with the wording 'poor, fair, good, very good, excellent' for the number of participants achieving at least 50% pain relief (Collins 2001).
For each treatment group we extracted the number of participants reporting treatment‐emergent adverse effects, and calculated relative benefit and risk estimates with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). We calculated NNT and NNH with 95% CIs using the pooled number of events using the method devised by Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the relative risk or relative benefit did not include the number one.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to determine the effect of dose and presenting condition (pain model: dental versus other postoperative pain). In particular, there are issues around the dose of codeine; if there were sufficient data we would analyse the findings according to low (< 10 mg), medium (10 to 20 mg), and high (> 20 mg) doses of codeine. A minimum of two studies and 200 participants had to be available in any subgroup or sensitivity analysis (Moore 1998), which was restricted to the primary outcome (50% pain relief over four to six hours) and the dose with the greatest amount of data. We would determine significant differences between NNT, NNTp, or NNH for different groups in subgroup and sensitivity analyses using the z test (Tramèr 1997).
Sensitivity analysis
We planned sensitivity analyses for quality score (two versus three or more) and trial size (39 or fewer versus 40 or more per treatment arm).
Results
Description of studies
Included studies
We identified six studies, with 1342 participants, that fulfilled the inclusion criteria (Cooper 1982; Daniels 2011; Frame 1986; McQuay 1989; Petersen 1993; Sunshine 1987). Details of individual studies are in the Characteristics of included studies table.
All of the included studies recruited participants aged 16 years or older (mean ages ranged from 20 to 26 years) and the majority of participants were female (50% to 100% in individual studies). One study, (Sunshine 1987) with 195 participants, included women who had undergone episiotomy, Caesarian section or other gynaecologic surgery, and the remaining five included men and women who had undergone surgical extraction of 1 to 4 impacted third molars, at least one of which had to be mandibular. Participants were required to be in good general health, and were excluded if they had a history of gastrointestinal disturbance, renal or hepatic disease, psychiatric disorder, or required medication that might interfere with the study results. In all studies participants took their medication when baseline pain reached a moderate or severe intensity. Pain intensity and pain relief were measured at set time intervals after dosing on standard 4‐ and 5‐point scales respectively, and/or 100 mm VAS, with the exception of Frame 1986, in which a non‐standard scale was used for pain intensity. Three studies (Cooper 1982; Daniels 2011; McQuay 1989) also carried out a patient global evaluation of treatment at the end of treatment using a standard 5‐point scale.
Four studies (Cooper 1982; Daniels 2011; Frame 1986; Sunshine 1987) used only a single dose, and two (McQuay 1989; Petersen 1993) used multiple doses, but provided results for the first dose for some outcomes.
Studies used placebo and/or active comparators. The following treatments were administered:
-
Ibuprofen 200 mg + codeine 15 mg (Frame 1986), n = 32;
-
Ibuprofen 200 mg + codeine 30 mg (Sunshine 1987), n = 40;
-
Ibuprofen 400 mg + codeine 20 mg (McQuay 1989), n = 24;
-
Ibuprofen 400 mg + codeine 26.5 or 30 mg (Daniels 2011; Frame 1986), n = 195;
-
Ibuprofen 400 mg + codeine 60 mg (Cooper 1982; Petersen 1993; Sunshine 1987), n = 110;
-
Ibuprofen 800 mg + codeine 60 mg (Frame 1986), n = 26;
-
Ibuprofen 400 mg (Cooper 1982; McQuay 1989; Petersen 1993; Sunshine 1987), n = 132;
-
Codeine 60 mg (Cooper 1982; Sunshine 1987), n = 78;
-
Placebo (Cooper 1982; Daniels 2011; Frame 1986; Sunshine 1987), n = 167;
-
Aspirin 600 or 650 mg (Cooper 1982; Frame 1986), n = 63;
-
Aspirin 650 mg + codeine 60 mg (Cooper 1982), n = 45;
-
Paracetamol 1000 mg + codeine 60 mg (Daniels 2011), n = 113;
-
Ibuprofen 200 mg + paracetamol 500 mg (Daniels 2011), n = 173;
-
Ibuprofen 400 mg + paracetamol 1000 mg (Daniels 2011), n = 168.
Using our predefined categories, no treatment arms used low dose codeine (< 10 mg), two (Frame 1986; McQuay 1989) used medium dose codeine (10 mg to 20 mg), and the remainder used high dose codeine (> 20 mg; range 26.5 mg to 60 mg).
Excluded studies
We excluded eight studies after reading the full papers (Carlos 1989; Cater 1985; Giles 1985; Giles 1986; Hellman 1992; McQuay 1992; Norman 1985; Walton 1990). Details of the reasons for exclusion are in the Characteristics of excluded studies table.
Risk of bias in included studies
All included studies were randomised and double‐blind; one study (Daniels 2011) scored 5/5 on the Oxford Quality Score, and the remaining five scored 4/5 due to failure to report the method used to generate the randomisation schedule. It is likely that this was a failure of reporting rather than a flaw in the methods.
Risk of bias was also assessed using the 'Risk of bias' tool (Figure 1; Figure 2).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Details for each study are in the Characteristics of included studies table.
Allocation
All studies reported that they were randomised, but only one properly described the method used to generate the schedule. Two studies (Cooper 1982; Frame 1986) described the methods used to conceal the random allocation, while in the other studies this was not described.
Blinding
All studies were double‐blind and adequately described how this was achieved.
Other potential sources of bias
Treatment group size was an issue. Small studies are thought to be at increased risk of bias, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised. None of the treatment groups in this review was large enough to be confident that bias would be avoided; five of the six studies had treatment group sizes that put them at high risk of bias.
Effects of interventions
The small numbers of participants in most of these treatment groups meant that analysis was severely limited. Results for individual studies are provided in Appendix 5 (analgesia and use of rescue medication) and Appendix 6 (adverse events and withdrawals).
Participants with at least 50% pain relief
One study, (Petersen 1993) with 60 participants, did not report data in a way that could be used to calculate the primary outcome of this review. It reported that the mean percentage pain reduction over 10 hours following the first dose (using last observation carried forward for participants who remedicated) was 63% (SD 25) for the ibuprofen 400 mg + codeine 60 mg group, and 50% (SD 31) for the ibuprofen 400 mg group (P = 0.12), but last observation carried forward is known to be an inappropriate method in acute pain studies (Moore 2005).
Ibuprofen 200 mg + codeine versus placebo
One study (Frame 1986) included a comparison of ibuprofen 200 mg + codeine 15 mg (medium dose) with placebo; 18/32 participants experienced at least 50% pain relief with the combination, and 10/26 with placebo.
One study (Sunshine 1987) included a comparison of ibuprofen 200 mg + codeine 30 mg (high dose) with placebo; 23/40 participants experienced at least 50% pain relief with the combination, and 13/40 with placebo.
Ibuprofen 400 mg + codeine versus placebo
Four studies (Cooper 1982; Daniels 2011; Frame 1986; Sunshine 1987) included comparisons of ibuprofen 400 mg + codeine 25.6 mg to 60 mg (high dose) with placebo.
-
The proportion of participants with ≥ 50% pain relief with ibuprofen + codeine was 64% (178/276, range 62% to 73%);
-
The proportion of participants with ≥ 50% pain relief with placebo was 18% (30/167, range 4% to 38%);
-
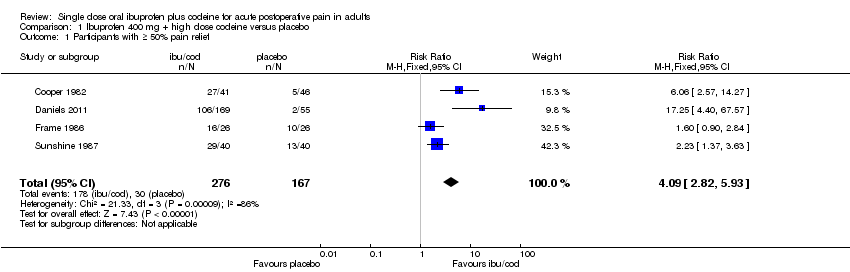
The relative benefit of treatment compared with placebo was 4.1 (95% CI 2.8 to 5.9); the NNT was 2.2 (95% CI 1.8 to 2.6). (Analysis 1.1; Figure 3).
Figure 3
Forest plot of comparison: ibuprofen 400 mg + high dose codeine versus placebo, outcome: 2.1 Participants with ≥ 50% pain relief.
Ibuprofen 800 mg + codeine versus placebo
One study (Frame 1986) included a comparison of ibuprofen 400 mg + codeine 60 mg (high dose) with placebo; 18/26 participants experienced at least 50% pain relief with the combination, and 10/26 with placebo.
Figure 4 shows the distribution of results for all combinations of ibuprofen and codeine compared with placebo; the dose of codeine made little obvious difference to the proportion of patients benefiting with the combination.

Studies comparing ibuprofen plus codeine with placebo, with the outcome of at least 50% maximum pain relief over 4‐6 hours. Colour code: white ‐ ibuprofen 200 mg + codeine 15 mg; yellow ‐ ibuprofen 200 mg + codeine 30 mg; light pink ‐ ibuprofen 400 mg + codeine 25.6 mg; medium pink ‐ ibuprofen 400 mg + codeine 30 mg; red ‐ ibuprofen 400 mg + codeine 60 mg; blue ‐ ibuprofen 800 mg + codeine 60 mg.
Ibuprofen 400 mg + codeine versus same dose of ibuprofen alone
Figure 5 shows the distribution of results for all doses of ibuprofen and codeine compared with the same dose of ibuprofen. One study (McQuay 1989) compared ibuprofen 400 mg + codeine 20 mg (medium dose) with ibuprofen 400 mg alone; 16/24 (67%) participants experienced at least 50% pain relief with the combination, and 11/23 (48%) with ibuprofen alone. There were too few data for analysis. Two studies (Cooper 1982; Sunshine 1987) compared ibuprofen 400 mg + codeine 60 mg (high dose) with ibuprofen 400 mg alone; 56/81 participants experienced at least 50% pain relief with the combination, and 43/76 with ibuprofen alone. For these three studies (Figure 5) ibuprofen plus codeine was better than ibuprofen alone, although the difference only just reached statistical significance.
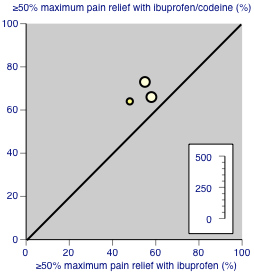
Studies comparing ibuprofen plus codeine with same dose of ibuprofen, with the outcome of at least 50% maximum pain relief over 4‐6 hours. Colour code: darker yellow ‐ ibuprofen 200 mg + codeine 20 mg versus ibuprofen 200 mg; lighter yellow ‐ ibuprofen 400 mg + codeine 60 mg versus ibuprofen 400 mg.
-
The proportion of participants with ≥ 50% pain relief with ibuprofen 400 mg + high dose codeine was 69% (72/105, range 66% to 73%);
-
The proportion of participants with ≥ 50% pain relief with ibuprofen alone was 55% (54/99, range 48% to 58%);
-
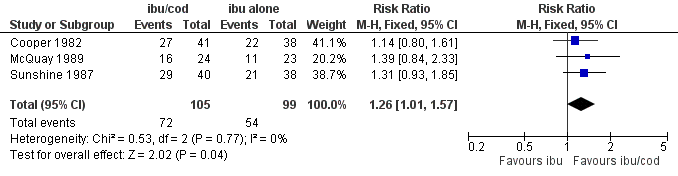
The relative benefit of treatment compared with placebo was 1.4 (95% CI 1.01 to 1.6); the NNT was 7.1 (95% CI 3.7 to 126). (Analysis 2.1; Figure 6).
Figure 6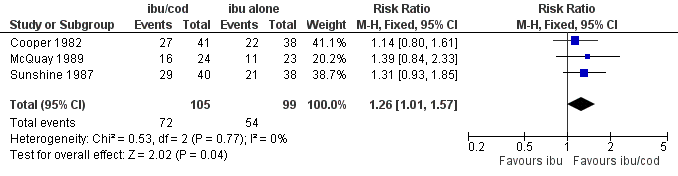
Forest plot of comparison: ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, outcome: 3.1 Participants with ≥ 50% pain relief.
Ibuprofen 400 mg + codeine versus same dose of codeine alone
Two studies (Cooper 1982; Sunshine 1987) compared ibuprofen 400 mg + codeine 60 mg (high dose) with codeine 60 mg alone; 56/81 (69%) participants experienced at least 50% pain relief with the combination, and 26/78 (33%) with codeine alone. There were too few data for analysis.
Subgroup analysis
The main analysis has been carried out according to dose of both ibuprofen and codeine. There were insufficient data to allow any subgroup analysis by pain condition; one study (Sunshine 1987) enrolled participants following gynaecological surgery and the others following dental surgery.
Sensitivity analysis
There were too few data for sensitivity analysis based on quality score (and all studies scored ≥ 3/5).
In the analysis of ibuprofen 400 mg + high dose codeine versus placebo, only one study had > 50 participants in both treatment arms. Removing this study from the analysis did not significantly change the result (RR 2.7, 95% CI 1.9 to 3.7); NNT 2.4, 95% CI 1.8 to 3.3).
Time to use of rescue medication
One study (Daniels 2011) reported the median time to use of rescue medication, which was 8.1 hours for ibuprofen 400 mg + codeine 26.5 mg compared with 1.7 hours for placebo (data from 224 participants).
One study (Cooper 1982) reported the mean time to use of rescue medication, which was 3.7 hours for ibuprofen 400 mg + codeine 60 mg, 3.8 hours for ibuprofen 400 mg alone, and 2.4 hours for placebo (data from 125 participants).
Participants using rescue medication
Two studies (Frame 1986; Sunshine 1987) reported the number of participants using rescue medication within 4 to 5 hours. In both studies the number using rescue medication was greater in the placebo group than the combination group for all doses, by a factor of two or more (data from 230 participants).
Adverse events
All studies reported the number of participants experiencing any adverse event, but the two studies using multiple doses (McQuay 1989; Petersen 1993) did not provide data for the first dose only.
Event rates in studies using a single dose varied considerably, in both the active and control treatment arms. Four studies (Cooper 1982; Daniels 2011; Frame 1986; Sunshine 1987) had treatment arms using ibuprofen 400 mg + high dose codeine (25.6 mg to 60 mg).
-
The proportion of participants experiencing any adverse event with ibuprofen 400 mg + high dose codeine was 28% (78/276, range 0% to 35%);
-
The proportion of participants experiencing any adverse event with placebo was 18% (31/167, range 0% to 38%);
-
The relative risk of treatment compared with placebo was 1.2 (95% CI 0.84 to 1.7); the NNH was not calculated (Analysis 1.2).
Only one study provided single dose data for the combination compared with the same dose of ibuprofen alone (Sunshine 1987); 6/40 participants experienced adverse events with ibuprofen 200 mg + codeine 30 mg, 2/40 with ibuprofen 400 mg + codeine 60 mg, and 4/38 with ibuprofen 400 mg alone.
Serious adverse events
All studies reported that there were no serious adverse events.
Withdrawals
Withdrawals due to lack of efficacy have been considered under 'Use of rescue medication' (above), and were not consistently reported.
There were no adverse event withdrawals.
Discussion
The background to this review is a knowledge that combinations of different analgesics provide additive effects in acute pain and migraine (Moore 2011b; Moore 2012). The main thrust of this review is to assess the analgesic efficacy of ibuprofen and codeine combination analgesics because they are widely available to the public without prescription, and used to some extent in treating acute pain in hospital or in primary care. The differentiating factor is usually the dose of codeine, with lower doses of codeine in non‐prescription medicines, and higher doses of codeine in prescription medicines; typically doses of codeine above 30 mg are associated with prescription medicines. The review therefore sought evidence according to the dose of codeine used: low (< 10 mg), medium (10 to 20 mg), or high (> 20 mg) doses.
In the UK there are no combination analgesic preparations available without prescription that contain a higher dose of codeine than 12.8 mg codeine phosphate (equivalent to 10 mg codeine base per tablet). To obtain higher codeine doses, as used in some of the studies identified, it would require the prescription of the individual components rather than a combination preparation.
Summary of main results
No data could be found relating to low dose codeine, limited data were available relating to medium dose codeine, and most data related to high dose codeine (25.6 to 60 mg). The combination of ibuprofen plus high dose codeine produced high rates of patients with good pain relief, in the range of 62% to 73% compared with 4% to 38% with placebo, though the actual dose of codeine did not appear to greatly influence the overall benefits of the combination (Figure 4) in the limited data set available. The absolute difference of 46% produced an NNT of 2.2 (95% CI 1.8 to 2.6), which is one of the lower (better) NNTs obtained from comparable data in an overview of acute pain studies (Moore 2011a). Based on limited data, ibuprofen plus codeine was better than the same dose of either drug alone.
Overall completeness and applicability of evidence
The main limitation of the review is the small number of studies and participants for some combinations. However, the general results are in accord with those known for ibuprofen and codeine (Derry 2009; Derry 2010) and for combination drugs in acute pain (Moore 2011b; Moore 2012).
The limited number of studies and participants did not allow for any sensible assessment of common or rare adverse events, though both ingredients are widely studied drugs. Serious morbidity, mainly gastrointestinal haemorrhage and opioid dependence, have been reported with ibuprofen‐codeine combination products (Frei 2010). These patients were taking mean daily doses of 435 to 602 mg of codeine phosphate and 6800 to 9400 mg of ibuprofen. Most of these patients had no previous history of substance use disorder, though similar issues have been reported for a small group with previous history of alcohol dependence (Robinson 2010).
The nature of the review, evaluating single dose effectiveness, means that issues of misuse of codeine combination products cannot be dealt with directly. There clearly is a problem with this in some parts of the world, particularly in Australasia (Frei 2010; McAvoy 2011).
Quality of the evidence
The studies themselves were of high quality but sample sizes were somewhat limited.
Potential biases in the review process
We carried out extensive searches to identify relevant studies but there always remains the possibility of unidentified studies. We calculated that for ibuprofen 400 mg plus codeine 25.6 to 60 mg, an additional 1168 participants would have to have been involved in unpublished trials with zero treatment effects for the NNT for at least 50% pain relief to increase above 8, a level we consider to be the limit of clinical utility for this outcome (Moore 2008). It is unlikely that this amount of unidentified information exists.
There are no other known potential biases in the review process.
Agreements and disagreements with other studies or reviews
Only one previous systematic review of ibuprofen plus codeine was found (Li Wan Po 1998). That review included eight studies comparing ibuprofen plus codeine versus placebo, several of which we excluded because of methodological issues (Cater 1985; Giles 1985; Norman 1985). It also included five studies comparing ibuprofen plus codeine versus ibuprofen. The present review included one large study not available in 1998 (Daniels 2011). Despite some differences in approach, the findings of the two reviews were remarkably similar in terms of treatment efficacy for ibuprofen plus codeine versus placebo, and for ibuprofen plus codeine versus ibuprofen.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
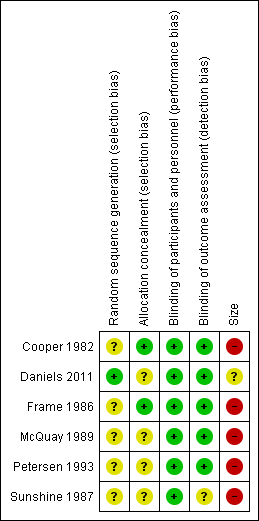
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: ibuprofen 400 mg + high dose codeine versus placebo, outcome: 2.1 Participants with ≥ 50% pain relief.
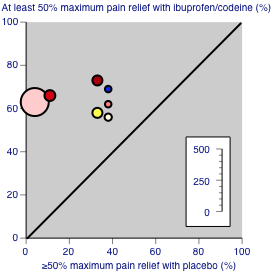
Studies comparing ibuprofen plus codeine with placebo, with the outcome of at least 50% maximum pain relief over 4‐6 hours. Colour code: white ‐ ibuprofen 200 mg + codeine 15 mg; yellow ‐ ibuprofen 200 mg + codeine 30 mg; light pink ‐ ibuprofen 400 mg + codeine 25.6 mg; medium pink ‐ ibuprofen 400 mg + codeine 30 mg; red ‐ ibuprofen 400 mg + codeine 60 mg; blue ‐ ibuprofen 800 mg + codeine 60 mg.

Studies comparing ibuprofen plus codeine with same dose of ibuprofen, with the outcome of at least 50% maximum pain relief over 4‐6 hours. Colour code: darker yellow ‐ ibuprofen 200 mg + codeine 20 mg versus ibuprofen 200 mg; lighter yellow ‐ ibuprofen 400 mg + codeine 60 mg versus ibuprofen 400 mg.
Forest plot of comparison: ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, outcome: 3.1 Participants with ≥ 50% pain relief.
Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 1 Participants with ≥ 50% pain relief.

Comparison 1 Ibuprofen 400 mg + high dose codeine versus placebo, Outcome 2 Participants with any adverse event.

Comparison 2 Ibuprofen + codeine (all doses) versus same dose of ibuprofen alone, Outcome 1 Participants with ≥ 50% pain relief.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [2.82, 5.93] |
| 2 Participants with any adverse event Show forest plot | 4 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.01, 1.57] |

