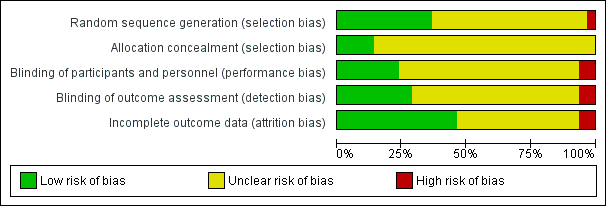
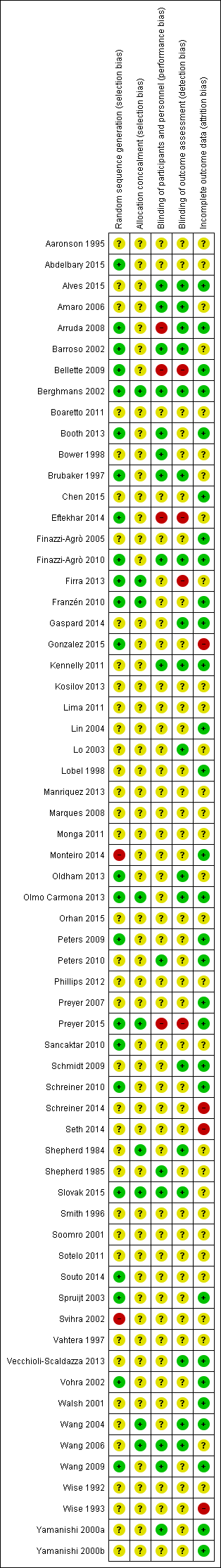
| Study | Current | Current intensity | Pulse shape & duration | Frequency (Hz) | Duty cycle | Electrodes | Treatment duration/supervision |
| Aaronson 1995 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Unclear |
| Abdelbary 2015 | | 30‐60 mA according to patient tolerance (mean 43 mA) | 320 ms | 20 | Unclear | Intravaginal | Two 30‐min sessions per week for 12 weeks |
| Alves 2015 | Unclear | "Sensory threshold, activating superficial cutaneous nerve fibers with larger diameter" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks |
| Alves 2015 | Unclear | "Motor threshold, non‐painful contraction is induced and the stimulation can simply make pain relief in the same way that sensory stimulation level (blocking activation of the peripheral or cental inhibition)" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks |
| Amaro 2006 | Bipolar | 0‐100 mA according to participant tolerance | Bipolar square wave 0.1 µs | 4 | 2 s on, 4 s off | Intravaginal | Three 20‐min sessions per week on alternate days for 7 weeks |
| Arruda 2008 | Biphasic | 10‐100 mA according to participant tolerance | 1 ms intermittent | 10 | Unclear | Intravaginal | Two 20‐min sessions per week for 12 weeks |
| Barroso 2002 | Biphasic | 0‐100 mA | Asymmetric, 1 s rise time, sustained for 5 s and resting for 5 s | 20 | 1 s rise time, sustained for 5s and resting for 5 s | Intravaginal | Home use: two 20‐min sessions per day for 12 weeks |
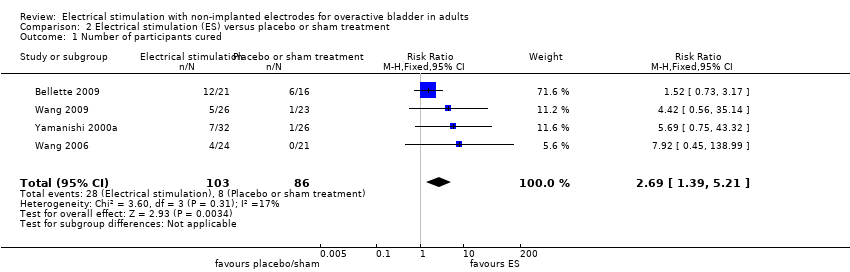
| Bellette 2009 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve | Two 30‐min sessions per week for 4 weeks |
| Berghmans 2002 | Biphasic | 0‐100 mA | Rectangular 200 µs stochastic variation | 4‐10 | Unclear | Intravaginal | Unclear |
| Boaretto 2011 | Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous posterior tibial nerve | Twelve 30‐min sessions |
| Boaretto 2011 | Unclear | Unclear | 500 µs | 10 | Unclear | Intravaginal | Twelve 30‐min sessions |
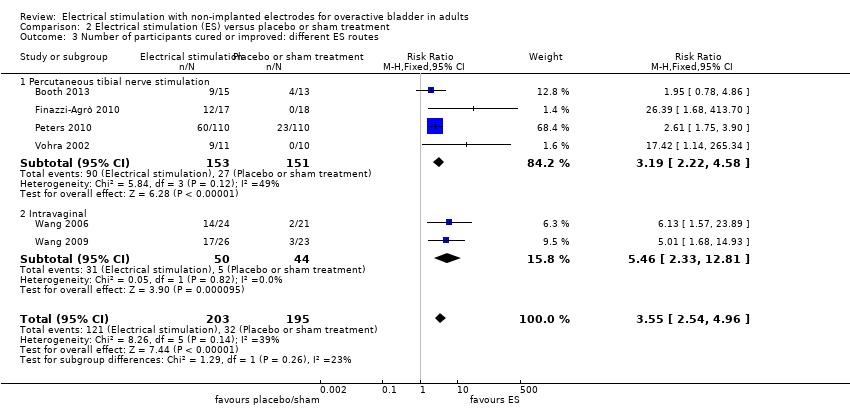
| Booth 2013 | Unclear | 0‐50 mA | 200 µs | 10 | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks |
| Bower 1998 | Unclear | Unclear | 200 µs | 150 | Unclear | Transcutaneous electrical nerve stimulation – suprapubic placement | Unclear |
| Bower 1998 | Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation – sacral placement | Unclear |
| Brubaker 1997 | Bipolar | 0‐100 mA | Bipolar square wave 0.1 µs | 20 | 2 s on ‐ 4 s off | Intravaginal | 20 minutes daily for 8 weeks |
| Olmo Carmona 2013 | Unclear | 0‐10 mA | Square wave 320 µs | 20 | unclear | Percutaneous posterior tibial nerve stimulation | 30 min once a week for 12 weeks |
| Chen 2015 | Bipolar | According to participant tolerance | Continuous bipolar square wave 200 µs | 20 | Unclear | Percutaneous posterior tibial nerve stimulation ‐ adhesive skin electrodes | Unclear |
| Eftekhar 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation ‐ "34 gauge needle placed 5 cm near internal malleolus" | 30‐min sessions |
| Finazzi‐Agrò 2010 | Unclear | 0‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Percutaneous tibial nerve stimulation | Three 30‐min sessions per week for 4 weeks |
| Firra 2013 | Unclear | Unclear current, intensity according to participant tolerance | Unclear | 12.5 | 5 s on, 10 s off | Intravaginal | Fourteen 30‐min sessions |
| Franzén 2010 | Unclear | According to participant tolerance | Unclear | 5‐10 | | Intravaginal/transanal | 10 sessions: 1‐2 20‐min sessions per week for 5‐7 weeks |
| Gaspard 2014 | Biphasic | Unclear | Biphasic rectangular 220 µs | 10 | 20 s on, 4 s off | Transcutaneous posterior tibial nerve stimulation: external electrode 5 cm above medial malleolus, 1 cm behind the tibia. The other electrode on dorsum of foot | One 30‐min session per week for 9 weeks |
| Gonzalez 2015 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twice a week for 6 weeks, performed by either physiotherapist or continence midwife |
| Kennelly 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | VERV electrode patches, placed by the participant ‐ exact placement unclear | One patch per week for 12 weeks |
| Kosilov 2013 | Diadynamic | 20–40 mA, 50%‐75% intensity | Unclear | 20 | Unclear | Active electrode (50 cm2 to 70 cm2) above the pubis, and a passive electrode (150 cm2) in lumbosacral area | 15 procedures every other day |
| Lima 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Twelve 30‐min sessions |
| Lima 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twelve 30‐min sessions |
| Lin 2004 | Unclear | 8‐70 mA | Unclear | Unclear | Unclear | Vaginal/anorectal | 20‐30 20‐min sessions |
| Lo 2003 | Unclear | According to participant tolerance | Unclear | 0‐100 | Unclear | Interferential therapy. 2 anterior flat electrodes placed over obturator foramen 1.5 cm to 2 cm lateral to symphysis, two posterior electrodes placed medial to ischial tuberosities either side of anus | 12 sessions: first session 15 min, all others 30 min |
| Lobel 1998 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Once per week |
| Lobel 1998 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Twice per week |
| Manriquez 2013 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous tibial nerve stimulation | Twice a week with at least 48 hour intervals for 12 weeks |
| Marques 2008 | Biphasic | Immediately below motor threshold | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation through 1 channel and 2 electrodes | Two 30‐min sessions per week for 4 weeks |
| Monga 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal amplitude‐modulated signal through a patch applied to the skin, controlled by wireless handheld remote control | Patch worn for 4 weeks |
| Monteiro 2014 | Unclear | Below the threshold that causes motor contraction | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation with surface electrodes. Negative electrode on medial malleolus, and the positive electrode 10 cm above negative electrode, also on the medial side. Rhythmic flexion of the second toe during the stimulation determined the correct position of the negative electrode | 30‐min twice weekly over 12 sessions (45 days) |
| Oldham 2013 | Unclear | Pre‐programmed to increase intensity over 24 s to reach therapeutic level and switch off automatically after 30 min. All devices same level of stimulation (average intensity considered comfortable and capable of producing contractions of pelvic floor muscles) | Unclear | During the 10 s ‘‘on time’’ the device delivers 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation | 10 s on, 10 s off | Intravaginal, single‐use tampon‐like Pelviva device | One disposable device per day for 12 weeks except during menstruation |
| Orhan 2015 | Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | Unclear |
| Peters 2009 | Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle slightly cephalad to medial malleolus | One 30‐min session per week for 12 weeks |
| Peters 2010 | Unclear | 0.5‐9 mA | Unclear | 20 | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle inserted at 60º angle 5 cm cephalad to medial malleolus, slightly posterior to tibia. Surface electrode placed on ipsilateral calcaneous | One 30‐min session per week for 12 weeks |
| Phillips 2012 | Unclear | Unclear | Unclear | Unclear | Unclear | Participant‐managed neuromodulation system patch | Subject placement versus investigator placement |
| Preyer 2007 | Unclear | Unclear | Unclear | Unclear | Unclear | Peripheral tibial neurostimulation | One 30‐min session per week for 12 weeks |
| Preyer 2015 | Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 3 months |
| Sancaktar 2010 | Unclear | 0.5‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Stoller afferent neurostimulation: 34‐gauge needle inserted at 30° angle 2 cm to 3 cm superior‐medial aspect of tibial medial malleolus along posterior tibial nerve trace | One 30‐min session per week for 12 weeks |
| Schmidt 2009 | Biphasic | Controlled by participant according to tolerance | 300 µs | Asymmetrical, 50 | Unclear | Intravaginal: probe with two 26 mm rings 40 mm apart | Unclear |
| Schreiner 2010 | Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous tibial nerve stimulation | One 30 min session per week for 12 weeks |
| Schreiner 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Unclear |
| Seth 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30 min session per day for 12 weeks |
| Seth 2014 | Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30‐min session per week for 12 weeks |
| Shepherd 1984 | Unclear | Up to 40 v | Unclear | 10‐50 | Unclear | Maximum perineal stimulation: Scott electrode in vagina, large indifferent electrode under buttocks | Single 20‐min session |
| Shepherd 1985 | Unclear | Unclear | Unclear | 10 | Unclear | Intravaginal cushion attached to stimulator worn around waist | Cushion worn for 8 out of 24 h, day or night according to participant preference |
| Slovak 2015 | Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Unilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on the right ankle | Unclear |
| Slovak 2015 | Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Bilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on both ankles | Unclear |
| Smith 1996 | Unclear | 5‐25 mA | Unclear | Device uses 2 programmes simultaneously: 12.5 Hz and 50 Hz | 5 s impulse | Intravaginal | Twice daily for 4 months. Length of session increased monthly: 15, 30, 45, 60 minutes |
| Soomro 2001 | Unclear | Participants asked to control stimulation to achieve tickling sensation | 200 µs | 20 | Continuous | Transcutaneous. 2 self‐adhesive pads applied bilaterally over the perianal region (S2‐S3 dermatome) | Up to 6 hours daily for 6 weeks |
| Sotelo 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. Horizontal placement of electrode patch near sacral nerve | Patch worn continuously for 7 days |
| Sotelo 2011 | Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. 30° angle placement of electrode patch near sacral nerve | Patch worn continuously for 7 days |
| Souto 2014 | Unclear | According to participant tolerance | 250 µs | 10 | Unclear | Posterior tibial nerve stimulation. Surface electrode placed behind media malleolus and another placed 10 cm above first electrode | Two 30 min sessions per week for 12 weeks |
| Spruijt 2003 | Biphasic | 0‐100 mA, according to participant tolerance | 100 µs | 20 | 2 s contraction time, duty cycle 1–2 s | Intravaginal | Three 30‐min sessions per week for 8 weeks. 5 min rest between each 15 min |
| Svihra 2002 | Square | 25 mA. 70% of intensity of maximal amplitude of registered response from abductor hallucis muscle | Square impulse 100 µs | 1 | Unclear | Stoller afferent neurostimulation. Electrodes placed behind medial ankle of left lower extremity, cathode placed proximally and anode distally | One 30 min session per week for 5 weeks |
| Vahtera 1997 | Unclear | According to participant tolerance | Unclear | 10 min of each frequency, 3 min: 5‐10 Hz, 10‐50 Hz, 50 Hz | 7 s on, 25 s off | Intravaginal/transanal | 6 sessions over two weeks |
| Vecchioli‐Scaldazza 2013 | Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks |
| Vohra 2002 | Unclear | 0‐10 mA | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 12 weeks |
| Walsh 2001 | Unclear | Unclear | 200 ms | 10 | Unclear | Transcutaneous neurostimulation. Electrode pads affixed bilaterally to the skin overlying S3 dermatomes (junction of buttock and upper thigh) | Single session |
| Wang 2004 | Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks |
| Wang 2006 | Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks |
| Wang 2009 | Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks |
| Wise 1992 | Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | One session per day (at home) for 6 weeks |
| Wise 1993 | Unclear | 0‐90 mA, according to participant tolerance | Unclear | 20 | Unclear | Intravaginal | One session per day (at home) for 6 weeks |
| Yamanishi 2000 | Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed electrodes | Two 15‐min sessions per day for 4 weeks |
| Yamanishi 2000 | Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed
electrodes | Single session |