Rangsangan elektrik dengan elektrod yang tidak diimplan untuk pundi kencing yang terlalu aktif pada orang dewasa
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: RCT Period: October 1992‐January 1994 | |
| Participants | N: 47 randomised and analysed. Age: 24‐82 years Sex: women Inclusion criteria: genuine stress urinary incontinence (GSUI) or detrusor instability (DI) Exclusion criteria: not reported | |
| Interventions | For detrusor overactivity incontinence women only (DO) A (n = x): probanthine B (n = x): ES 2nd RCT in people with GSUI C (n = x): PFMT D (n = x): ES | |
| Outcomes | Cure ‐ defined as cessation of incontinence. A: not reported B: not reported Improvement defined as reduction in frequency of voids per 24 hours by ≥ 50%, or ≤ 10 voids per 24 hours, or decrease number of pads per 24 hours by ≥ 50%. Cured or improved: A (n = x): unclear (50% ‘responded well’), B (n = x) 69%, C (n = x) 44%, D (n = x) 66% | |
| Notes | No useable data Study authors contacted for further data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Setting: Egypt Follow‐up: 6 weeks’ treatment, 6 months’ follow‐up | |
| Participants | N: 315 randomised, 300 analysed Mean (SD) age: A, 49.7 (6.0); B, 47.7 (6.0); C, 48.0 (6.0) Sex: women Inclusion criteria: ≥ 40 years, no evidence of urinary tract infection, no SUI, no previous history of anti‐incontinence or pelvic surgery or anti‐incontinence drugs (within 3 months), and no history of bladder malignancy. Exclusion criteria: not reported | |
| Interventions | A: (n = 105) vaginal ES twice weekly for 12 sessions B: (n = 105) local vaginal oestrogen 0.625 mg/g (Premarin), 2 g daily for 6 weeks C: (n = ) ES plus local vaginal oestrogen | |
| Outcomes | Voids per day (mean, SD, N) End of treatment: A 4.7 (0.8), 105. B 5.0 (0.9), 105. C 5 (0.8), 105 3 months: A 5.0 (1.0), 105. B 5.3 (0.9), 105. C 5 (0.8), 105 6 months: A 6.6 (1.5), 105. B 5.0 (0.8), 105. C 5 (0.8), 105 Voids per night (mean SD, N): End of treatment: A 0.9 (0.7), 105. B 1.4 (0.8), 105. C 0.5 (0.5), 105 3 months: A 1.1 (0.9), 105. B 1.5 (0.8), 105. C 1 (0.9), 105 6 months: A 2.2 (0.9), 105. B 5.0 (0.8), 105. C .5 (0.8), 105 Incontinence episodes (mean SD, N) End of treatment: A 0.1 (0.3), 105. B 0.4 (0.6), 105. C 0.07 (0.25), 105 3 months: A 0.1 (0.3), 105. B 0.5 (0.6), 105. C 0.09 (0.28), 105 6 months: A 0.4 (0.6), 105. B 0.4 (0.6), 105. C 0.09 (0.28), 105 Urgency episodes (mean SD, N) End of treatment: A 2 (0.7), 105. B 4 (1.3), 105. C 1.4 (0.7), 105 3 months: A 2.7 (1.0), 105. B 4.5 (1.5), 105. C 1.6 (0.9), 105 6 months: A 4.7 (1.3), 105. B 4 (1.3), 105. C 2 (0.8), 105 QoL score (higher score = greater severity, instrument not reported) (mean SD, N) End of treatment: A 2.8 (2), 105. B 5 (1.8), 105. C 2.9 (2.2), 105 3 months: A 4 (1.7), 105. B 6 (2), 105. C 3.7 (2.5), 105 6 months: A 7.6 (3), 105. B 6 (2), 105. C 4.8 (1.9), 105 Functional bladder capacity (ml) (mean SD, N) End of treatment: A 343.8 (46), 105. B 310 (40.6), 105. C 361 (40), 105 Detrusor overactivity (mean SD, N) End of treatment: A 27/105. B 32/105. C 12/105 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated random numeric table" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No differential withdrawal, no explanation for withdrawals, no indication on how missing data were dealt with in analysis |
| Methods | Study design: RCT Setting: Brazil Follow‐up: 4 weeks' treatment | |
| Participants | N: 28 randomised Sex: women Inclusion criteria: female, ≥ 60 years with likely urinary dysfunction, identified by a score ≥ 8 points on OAB‐V8 questionnaire Exclusion criteria: urinary infection, identified by urine test, history of treatment for OAB and hormone replacement therapy in the last six months, prior surgery to treat UI, neurological diseases base, genital‐urinary cancer history, complaints of pain in the lower abdomen for more than six months, prior pelvic irradiation, genital prolapse above third degree of Baden and Walker scale, use of cardiac pacemakers, metal implants in foot and right ankle region, inability to respond to questionnaires properly and abstentions to treatment | |
| Interventions | A: (n = 15) tibial nerve stimulation (TNS). 8 sessions (2 x 30‐minute sessions per week)F = 10 Hz, T = 200 μs. Sensory threshold, activating superficial cutaneous nerve fibres with larger diameter B: (n = 13) TNS 8 sessions (2 x 30‐minute sessions per week). F = 10 Hz, T = 200 μs. Motor threshold, non‐painful contraction was induced and "the stimulation can simply make pain relief in the same way that sensory stimulation level (blocking activation of the peripheral or central inhibition." | |
| Outcomes | All scores are higher score = greater severity ICIQ‐OAB score (mean SD, N) A 4.46 (2.66), 15. B 4.53 (3.07), 13 Bother of daytime frequency (mean SD, N) A 3.20 (2.59), 15. B 3.38 (3.17), 13 Bother of nocturia (mean SD, N) A 3.40 (3.26), 15. B 1.84 (2.51), 13 Bother of urgency (mean SD, N) A 4.00 (2.59), 15. B 3.53 (3.59), 13 Bother of urgency incontinence (mean SD, N) A 2.73 (3.65), 15. B 4.38 (4.29) Micturitions per 24 h (mean SD N) A 8.33 (2.52), 15. B 7.89 (2.64), 13 Nocturia episodes (mean SD, N) A 1.26 (1.21), 15. B 1.05 (1.01), 13 Urgency episodes (mean SD, N) A 0.79 (0.96), 15. B 0.58 (0.65), 13 Urgency incontinence episodes A 0.33 (0.57), 15. B 0.84 (1.39), 13 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomisation of two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "blind assessment and comparison between groups" |
| Blinding of outcome assessment (detection bias) | Low risk | "blind assessment and comparison between groups" |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals reported |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Botucatu Medical School, Unesp ‐ Univ Estadual Paulista, Brazil Period: January 2001‐February 2002. Sample size: "Based on outcome measurements with no numerical variable…the statistical test sample size had previously been established as at least 40 women." Follow‐up: 7‐week treatment period, follow‐up appointments one month after end of treatment | |
| Participants | N: 40 randomised Mean age: A: 49.0 (range 41‐79) B: 47.0 (range 40‐78) Sex: women Inclusion criteria: symptoms of predominant urge incontinence Exclusion criteria: vaginal prolapse greater than grade II (Baden), retention complaint or obstruction diagnosis during USD, urinary infection, changes in cutaneous sensitivity, metal implants, and neurological complaints | |
| Interventions | A: (n = 20): electrostimulation. 3 x 20‐min sessions per week on alternate days over a 7‐week period, performed using Dualpex Uro996. Frequency at 4 Hz, a 2‐to 4‐s work rest cycle and a 0.1 us pulse width. The bipolar square wave could be delivered over a range of 0‐100 mA. Intensity was controlled according to participant discomfort level feedback B: (n = 20): sham. Same type of vaginal probe with wires disconnected so no electrical energy was supplied | |
| Outcomes | Number of micturitions per 24 h (mean, SD*, N): A: 7.0 (1.78), 20; B: 7.5 (1.78), 20 P = 0.38 1 hour PAD test (g): A: 1.05; B: 1.13 Number of participants with UUI: A: 3/20 (15%), B: 6/20 (31.5%) Number of participants ‘satisfied’: A: 16/20 (80%), B: 13/20 (65%) Reduction in "analog wetness sensation": A: 31.5%. B: 26.9% Reduction in "analog discomfort sensation": A: 39.7%; B: 24.5% Pelvic floor muscle strength measured with portable perineometer (Dynamed) (cmH2O) (mean, SD, N): A: 53.8 (18.6), 20; B: 46.8 (12.5), 20 Vaginal cone weight test (g) (mean, SD, N): A: 4.0 (1.3), 20; B: 2.0 (1.1), 20 | |
| Notes | No SDs reported (except for 2 outcomes). *SD calculated by FS using means and P value No evidence of source of data in review Information received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In the Randomization the participants in each groups were raffled" (from correspondence with author) |
| Allocation concealment (selection bias) | Unclear risk | "the allocations were concealed because a nurse, at each session, was responsible for carrying out the random assignment of patients" (from correspondence with author) |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded. ES sessions carried out by physiotherapist and outcome assessment carried out by different personnel (from correspondence with author) |
| Blinding of outcome assessment (detection bias) | Low risk | ES sessions carried out by physiotherapist and outcome assessment carried out by different personnel (from correspondence with author) |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals reported, % given without denominators, unclear if all participants present for follow‐up |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Department of Uroginecology, Federal University of São Paulo, Brazil Period: August 2001‐September 2005 Sample size: justified (a power calculation was performed based upon a predicted minimum difference of eight episodes of urinary leakage, with a significance level of 0.05, yielding a power estimate of 90% for a sample size of 20 women per each group) Follow‐up: 12 weeks' treatment, 1‐year follow‐up | |
| Participants | N: 77 randomised, 64 analysed Mean age (SD): A 51.9 (13,4); B 51.5 (11.4); C 54.1 (11.6) Sex: women Inclusion criteria: OAB and DO Exclusion criteria: persistent urinary tract infection, inability to comply with regular follow‐up visits, current pregnancy, postvoid residual volume greater than 100 mL, contraindications to anticholinergic therapy, cardiac pacemaker, type III stress urinary incontinence, uncontrolled metabolic conditions or indwelling catheterisation, using medications including anticholinergic drugs, calcium antagonists, β agonists, dopamine agonists, striated muscle relaxants, or oestrogens | |
| Interventions | A: (n = 26): oxybutynin immediate release 5 mg twice daily for 12 weeks B: (n = 25): ES. Ambulatory stimulation applied vaginally by a physiotherapist, twice a week, for 20 min at each session using 1 ms of intermittent biphasic waves, frequency 10 Hz. Current intensity ranged from 10‐100 mA, according to participant tolerance to the procedure. C: (n = 26): exercises (PFMT), performed twice a week in orthostatic, sitting, and supine positions. Each session had a total duration of 45 minutes. A total of 40 fast (2 and 5 s) and 20 sustained (10 s) contractions with an equal period of relaxation between them were administered by a physiotherapist in the outpatient setting. | |
| Outcomes | Participants with urgency symptoms (subjective) A 8/22. B 10/21. C 9/21 Participants not satisfied (subjective) 12 weeks: A 5/22. B 10/21. C 5/21 1 year: A 12/22. B 17/21. C 12/21 Participants not cured (objective evaluation: urodynamics) A 14/22. B 9/21. C 10/21. Number of leakage episodes per 24 hours (mean, SD, N) A 7 (10.6), 22. B 7.9 (13.7), 21. C 7.8 (15.3), 21 Number of micturitions per 24 hours (mean, SD, N) A 6.4 (1.6), 22. B 7.9 (2.63), 21. C 7.1 (2.1), 21 Number of nocturia episodes per night (mean, SD, N) A 0.9 (0.8), 22. B 1.2 (1.3), 21. C 1.0 (1.1), 21 Number of pads used per 24 hours (mean, SD, N) A 0.9 (1.5), 22. B 0.9 (1.7), 21. C 0.8 (1.3), 21 Post micturition residual volume, mL (mean, SD, N) A 4.8 (9.4), 22. B 1.1 (2.5), 21. C 2.1 (3.5), 21 Maximum cystometric capacity, mL (mean, SD, N) A 517.3 (191.7), 22. B 436.7 (178.7), 21. C 489.0 (141.3), 21 Volume at FDV (mean, SD, N) A 157.3 (63.8), 22. B 123.8 (59.0), 21. C 137.6 (76.7), 21 *Involuntary detrusor contraction volume, mL (mean, SD, N) A 188.6 (183.2), 22. B 173.3 (112.4), 21. C 114.3 (154.2), 21 Involuntary detrusor contraction maximal pressure, mmH2O (mean, SD, N) A 19.6 (20.9), 22. B 22.4 (6.6), 21. C 17.2 (25.5), 21 Adverse effects Dry mouth: A 16/22. B, C not reported Difficulty on micturition: A 2/22. B, C not reported Dizziness: A 1/22. B, C not reported Blurred vision: A 1/22. B, C not reported Constipation: A 1/22. B, C not reported | |
| Notes | *Value for group B reported in paper as 73.3; queried with author and correct value is 173.3. We contacted the main study author to clarify methodological aspects of the study and request further information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "blindly randomized to one of the three treatment groups" Additional information from study author correspondence: "Patients were randomised using a table of random numbers generated by a statistical program on a computer" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | Additional information from author correspondence: "patients and researchers knew to which group the patients belonged" |
| Blinding of outcome assessment (detection bias) | Low risk | Additional information from author correspondence: "Data were analysed by a statistician who did not know which group the patients belonged to." |
| Incomplete outcome data (attrition bias) | Low risk | No differential withdrawal. Adequate explanation for withdrawals |
| Methods | Study design: RCT Setting: Department of Gynecology and Obstetrics Hospital das Clínicas de Porto Alegre, Rio Grande do Sul, Brazil Period: March 2000‐August 2001 Sample size: 36 participants for a power of 80% and a 2:1 ratio Follow‐up: 6 months | |
| Participants | N: 36 Sex: women Mean (SD) age: A: 54 (9.5); B: 56 (12.2) Inclusion criteria: SUI, UUI or MUI, understanding and signing a letter of informed consent Exclusion criteria: prolapse or first degree urogenital prolapse, intrinsic sphincter deficiency, cardiac pacemaker, pregnancy or in the puerperal period, post‐menopausal climacteric's symptoms and signs of urogenital atrophy, genitourinary surgery during the previous 6 months, previous ES of the pelvic floor, medication chronically known to possibly change voiding function, change in the dose or if they had begun to use a new medication in the last 3 months, or during treatment with ES, reflex urinary incontinence, paradoxical urinary incontinence, urinary incontinence of intravesical obstructive factor, urinary incontinence caused by overflow, characterised by the presence of a large urinary residual volume, urgency incontinence treated with medication during last 3 months, or during treatment with ES; reflex urinary incontinence (clear presence of neurological lesions); paradoxical urinary incontinence (presence of intravesical obstructive factor); urinary incontinence caused by the presence of a large urinary residual volume; people with urge incontinence who had treatment with medication during last 3 months. | |
| Interventions | A: transvaginal ES (n = 24). Battery‐powered, portable device, 20 or 50 Hz, a pulse width of 300 ms, with asymmetrical biphasic pulses, an adjustable current intensity (0–100 mA), a 1 s rise time, sustained for 5 s and resting for 5 s. A time‐of‐use counter allowed a check on patient compliance with treatment, because it stored in the microcontroller memory the total time of use, corresponding to the time during which current actually circulated through the electrodes. Two 20‐min sessions per day while recumbent, for 12 weeks. UUI or MUI: equipment programmed for 20 Hz Stress urinary incontinence: equipment programmed for 50 Hz. UUI or MUI: equipment programmed for 20 Hz SUI: equipment programmed for 50 Hz B: sham (n = 12). Identical equipment and regimen but without electrical stimulus All participants requested to complete 3‐day voiding diary at beginning of study and again at 12 weeks’ follow‐up. | |
| Outcomes | Number of participants cured/improved at 12 weeks A: 21 (88%) B: not reported Number of voids per 24 hours (mean (SD) N) A: 7.5 (2.0) 24; B: 10.5 (2.8) 12 Number of nocturia episodes (mean, SD, N) A: 1.1 (0.5), 24; B: 2.3 (0.9), 12. Number of incontinence episodes per 24 h (mean, SD): A: 1.3 (1.0) 24; B: 3.0 (0.9) 12 Number of uninhibited contractions per 24 h (mean, SD): A: 2 (8), 24; B: 4 (not reported) Maximum bladder capacity (mean, SD, N) A: 425.0 mL (97.8), 24; B: 316.7 mL (71.8), 12 | |
| Notes | Compliance: 60 h of equipment use was expected. A: 46 hours B: 40 hours We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The participants were randomized before the study by drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "The participants were randomized before the study by drawing lots, with no participation by the examiner who, at the start of the treatment of each patient, was already receiving the group determined by randomization (study or control). Likewise the patients did not know into which group they had been placed (active or placebo). The patients in the control group were evaluated at different times from the study group, to avoid any exchange of information among them" |
| Blinding of outcome assessment (detection bias) | Low risk | Urodynamic evaluations carried out by examiner unaware of the study. Participants also unaware of intervention allocated |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals reported |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Female Urology Clinic of the Hospital das Clínicas at Campinas (HC/UNICAMP), Brazil Follow‐up: 4 weeks | |
| Participants | N: 37 randomised and analysed Mean age: 47.73 (10.90) Sex: women Inclusion criteria: 18‐85 years, symptoms of OAB for > 6 months, voiding frequency > 8 micturitions daily, episodes of nocturia and/or urgency Exclusion criteria: pregnancy, neurological problems, accentuated dystopias (stages II and III in the definitions of ICS), urinary tract infection and urinary stress incontinence | |
| Interventions | A: (n = 21): ES. Transcutaneous posterior tibial nerve stimulation. 8 sessions with Dualpex device 961, 30 min twice a week B (n = 16) sham. Electrodes placed without electricity | |
| Outcomes | Participants with urgency A 9/21. B 10/16. Frequency of micturitions (mean, N)* A 8.29, 21 B 10.55, 16 Decrease in frequency and urgency A 62.5%. B 42.8% (P < 0.05) OAB‐Q severity score A 31.72 (18.25), 21. B 51.21 (32.11), 16 OAB‐Q total score A 83.99 (16.99), 21. B 66.63 (25.06), 16 Nocturia episodes A 1.14 (1), 21. B 2.06 (1.2), 16 | |
| Notes | *Contacted study author to ask for SDs, no reply. Estimated SD used in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization process was made by the FCM's statistics department" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | "The evaluations were carried out by the investigator or the physiotherapist, and treatment was performed by the same person who evaluated the patient, thus creating a bond with the physiotherapist." |
| Blinding of outcome assessment (detection bias) | High risk | "The evaluations were carried out by the investigator or the physiotherapist, and treatment was performed by the same person who evaluated the patient, thus creating a bond with the physiotherapist." |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants included in analysis. "All women were submitted to eight sessions of therapy, all the questionnaires were completed and none of the women failed to attend the sessions more than 3 times. The reasons for missing sessions were very variable, but did not alter the results of the study." |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: hospital and private clinic (University Hospital Maastricht, Department of Urology, the Netherlands) Sample size: a level of significance of 95%, a power of 80%, an expected dropout rate of 10%, and an expected improvement of bladder overactivity status of treatment groups in comparison with non‐treatment group, expressed as a decrease of approximately 30% in the Detrusor Activity Index (DAI), 20 participants in each of the 4 groups had to be recruited. Therefore, the intended sample size was set on 80 people. Follow‐up: unclear (9 weeks?) | |
| Participants | N: 80 randomised, 68 participated and analysed (12 excluded as randomised ‘erroneously’) Mean (SD) age: A: 50.5 (11.8) B: 55.6 (14.8) C: 61.9 (13.5) D: 52.3 (15.4) Sex: women Inclusion criteria: Detrusor Activity Index 0.5 or greater; > 18 years, female, drug‐free interval of at least 4 weeks before start of the study for the following drugs: anticholinergic, beta sympathicomimetic, alpha‐blocker and psychopharmacological agents. Exclusion criteria: mechanical intravesical obstruction, urinary calculus, repetitive symptomatic UTI (> 3 x per year), colpitis, clinical evidence of disordered action of heart (Lown III), pacemaker, pregnancy of lactating period, inability to comply with follow‐up, treatment with physical therapies within 3 months before start of therapy, neurogenic or congenital disorders resulting in urinary incontinence (e.g. spina bifida), psychological disorders, irritation of the vagina (consult with the general practitioner and participant), poor adjustable diabetes mellitus: last HbA1C > 10, contra‐indication for the use of an intravaginal or anal electrode, not able to understand Dutch, not able to travel | |
| Interventions | A: controls (n = 14) B: Lower Urinary Tract Exercises (LUTE) (n = 18). 1 session per week for 9 weeks. Patient information and education; bladder training; specific PFMT aiming at detrusor inhibition reflex (DIR); toilet behaviour aiming at the aspects of the micturition process itself C: FES (n = 17). FES was applied vaginally through plug‐mounted electrodes. The maximum level of the ES was 100 mA (Ieff = 6 mA), participant was instructed to use. The maximal characteristics were (frequency modulation of 0.1 s trains of rectangular biphasic 200 µs long pulses which varied stochastically between 4 and 10 Hz). Duration of treatment unclear D: FES + LUTE group (n = 19). Same LUTE programme plus an additional weekly FES session (for 9 weeks) Dropouts: A ?0, B 5, C 3, D 2 | |
| Outcomes | Detrusor Activity Index (DAI): urodynamic variables of ambulatory cystometry combined with data from micturition diary (i.e. condition‐specific measure; 0‐1 scale where higher = worse) (mean, SD): A 0.80 (0.26) 14, B 0.62 (0.33) 18, C 0.57 (0.33) 17, D 0.84 (0.27) 19 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done in blocks of four using opaque and sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "Randomization was done in blocks of four using opaque and sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Besides the participant and the physical therapist all others, involved in randomisation, registration and evaluation were blinded for group allocation. |
| Blinding of outcome assessment (detection bias) | Low risk | Besides the participant and the physical therapist all others, involved in randomisation, registration and evaluation were blinded for group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout A total number of 10 women dropped out of the trial. 1 woman stopped before start of therapy, because she considered the burden of investigation too high. During the treatment period, 5 women stopped because of illness (2 in group II and 2 in group III or allegedly reasons of too much burden felt (1 in group IV). "Missing data in the set of post‐treatment DAI‐scores were substituted by post‐treatment means of the empirical data according to the intention‐to‐treat principle." |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Brazil Period: August 2008‐2010 Sample size: not reported Follow‐up: 4 weeks | |
| Participants | N: 73 randomised, unclear how many included in analysis Mean (SD) age: 61.3 (not reported) Sex: women Inclusion criteria: women with OAB Exclusion criteria: not reported | |
| Interventions | A: (n = 22) PFMT. 12 sessions. Group exercises performed in sitting, standing and supine positions with 20 contractions of 2 s, 10 contractions of 5 s and 5 contractions every 10 s. B: (n = 22) ES, pulse width 200 ms. Transcutaneous posterior tibial nerve stimulation (TPTNS). Frequency 10 Hz. 12 x 30‐min sessions C: (n = 16) functional ES with vaginal electrode, pulse width 500 microseconds. Frequency 10 Hz.12 30‐minute sessions. D: (n = 13) oxybutynin. 5 mg immediate release twice daily for 12 weeks | |
| Outcomes | Satisfaction A 91% (20/22). B 77% (17/22). C 69% (11/16). D 61.5% (8/13) (not satisfied: A 2/22. B 5/22. C 5/16. D 5/13.) | |
| Notes | Data presented for urinary frequency, nocturia, urgency and urgency incontinence but not usable Unable to find contact details for study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized into four treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals reported. Outcome data presented without denominators or SDs |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Sample size: not reported Follow‐up: 6 weeks | |
| Participants | N: 30 randomised, 28 analysed Sex: men and women Mean age: 84.2 (10.0) Inclusion criteria: men and women > 65 in residential care home settings or sheltered accommodation with bothersome LUTS, urinary incontinence, faecal incontinence, or constipation; capacity to provide ongoing informed consent to participate. Exclusion criteria: pacemaker in situ, leg ulcers or broken skin on lower limb, peripheral vascular disease, reduced/absent sensation at the electrode sites, moderate or severe cognitive impairment or learning difficulties, UTI on assessment, or clinical diagnosis of only SUI | |
| Interventions | A: (n = 15) PTNS. 2 x 30‐min sessions per week for 6 weeks. Frequency 10 Hz and pulse width 200 ms in continuous modeThe intensity level of the stimulation current range (0‐50 mA). B: (n = 13) Sham. Same procedure with stimulation current reduced to 2 mA | |
| Outcomes | Number of participants with no improvement in incomplete bladder emptying A 7/15, B 12/13 Number of participants with no improvement in voiding frequency A 4/15, B 7/13 Number of participants with no improvement in urgency A 4/15, B 9/13 Number of participants with no improvement in nocturia A 8/15, B 10/13 Number of participants with no improvement in weak urinary stream A 6/15, B 12/13 Number of participants with no improvement in intermittency A 10/15, B 11/13 Number of participants with no improvement in urinary straining A 9/15, B 12/13 Number of participants with no improvement in frequency of UI episodes A 8/15. B 11/13. Number of participants with no improvement in amount of urine leaked A 7/15. B 11/13. Number of participants with no improvement in interference with everyday life A 6/15. B 7/13. Number of participants with no improvement in constipation A 14/15, B 6/13 Number of participants with no improvement in bowel urgency A 11/15, B 12/13 Number of participants with no improvement in faecal leakage A 8/15, B 10/13 Reduction in AUASI score (median, IQR, N): A ‐7 (‐8 to ‐3), 15. B 1 (‐1 to 4), 13. (P < 0.001, Mann‐Whitney U 16.5000, Z ‐3.742) Reduction in ICIQ‐SF score (median, IQR, N): A 2 (0 to ‐6), 15. B 0 (‐3 to 3), 13. (P = 0.132) Number of participants with no improvement in ICIQ‐SF score A 5/15. B 7/13 | |
| Notes | Two participants had predominantly faecal incontinence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "online randomization service" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded. "Staff were blind to the group allocation." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | "Fidelity to the protocol was high and 28 of the 30 participants completed the 12 session course, with two discontinued at session five because they developed infections" |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Australia Period: January 1996–February 1997 Sample size: 40% volume increase and 35% decrease in maximum detrusor pressure 16 participants would be required per group for an 80% chance of detecting significant change Follow‐up: immediately following single ES session | |
| Participants | DO group: 48 randomised Urgency group: 31 randomised Mean (SD) age: overall 55.4 (16.8). DO group: 56.5 (16.8). Urgency group: 56.3 (16.9) Sex: women Inclusion criteria: DO or urgency Exclusion criteria: UTI, pregnancy, cardiac pacemaker, impaired cognition, neurogenic bladder dysfunction or cystocele beyond the introitus | |
| Interventions | DO group A1 (n = 16) TENS – suprapubic placementFrequency 150 Hz, 200 ms pulse width B1 (n = 16) TENS – sacral placementFrequency 10 Hz, 200 ms pulse width C1 (n = 15) sham ES Urgency group A2 (n = ?) TENS – suprapubic placementFrequency 150 Hz, 200 microsecond pulse width B2 (n = ?) TENS – sacral placement Frequency 10 Hz, 200 mspulse width C3 (n = ?) sham ES | |
| Outcomes | Vol. at FDV (mean, SD, N) A1 208.5 (132), 16. B1 154 (61), 16. C1 186 (77), 15 A2 180 (51). B2 111 (37). C2 138 (51) (n not reported) Max. cystometric capacity (mean, SD, N) A1 352 (144), 16. B1 305 (146), 16. C 313.5 (81), 15 A2 291 (51). B2 241 (53). C2 285 (45) (n not reported) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized to 3 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Both the supervising urogynaecologist and the patient were blind to group allocation" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Data for urgency group not presented with numbers of participants, unclear how many in urgency group were randomised to each intervention |
| Methods | Study design: RCT Multicentre or single‐centre: 4 centres Setting: Rush‐Presbyterian‐St.Luke's Medical Center, Chicago; Methodist Hospital, Indianopolis; Greater Baltimore Medical Center; and the Oregon Health Science University, Portland, USA Period: not reported Sample size: not reported Follow‐up: 8 weeks | |
| Participants | N: 148 enrolled, 121 randomised and analysed Mean (SD) age for all participants (not stratified by GSUI/DO): A 56 (11.9); B 57.7 (12.4) Sex: women Inclusion criteria: women with symptoms or urodynamic evidence of genuine stress incontinence or detrusor instability Exclusion criteria: urinary incontinence other than genuine stress incontinence, detrusor instability, or mixed incontinence. Age < 25 years, leakage episodes ≤ 3/weeks, inadequate cognitive ability (investigator judgment), infected urine, anatomic defect that precluded use of device, postvoid residual > 100 mL, implanted electric device, genitourinary surgery < 6 months previously, medication alteration ≤ 3 months previously, anticipated geographic relocation during study | |
| Interventions | For DO and mixed women only (n = 61): A (n = 33) transvaginal electric stimulation. Device: InCare Microgyn II. 20 Hz frequency, 2‐second/4‐second work‐rest cycle, pulse width 0.1‐us. Bipolar square wave could be delivered over a range of 0‐100 mA. 20 min daily B (n = 28) sham. Identical device with disconnected wire so no electricity supplied. 20 min daily | |
| Outcomes | Definition of cure: absence of abnormality as measured objectively by urodynamics Number of participants with DO: A 14/32, B 23/28 UI frequency 2.2 No improvement 2.3 Compliance 2.4 | |
| Notes | We contacted the main author of the study to request further information about further 3 publications of the same study. The study authors replied with information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, and used for stratified randomisation |
| Allocation concealment (selection bias) | Unclear risk | The study nurse at each site was responsible for carrying out the random assignment of participants in accordance with the randomisation scheme. |
| Blinding of participants and personnel (performance bias) | Low risk | The study nurse at each site was aware of the difference in probes, however the physician investigators were masked as to the type of vaginal probe provided to each participant. |
| Blinding of outcome assessment (detection bias) | Low risk | Data sent to centralised data manager |
| Incomplete outcome data (attrition bias) | Unclear risk | "A total of 148 women were enrolled, 18% of whom withdrew from the study, leaving of a total 121 participants who completed the study. There was no statistically significant difference between the treatment groups with respect to withdrawal rates: 21% for the sham group and 14% for the stimulation group." No explanation reported for withdrawals |
| Methods | Study design: RCT Setting: China Follow‐up: 4 weeks’ treatment | |
| Participants | N: 100 randomised Inclusion criteria: neurogenic DO secondary to spinal cord injury Exclusion criteria: urinary tract infection, tumour of the urinary system, urinary calculus, vesicoureteral reflux confirmed by video urodynamics, bladder compliance > 10 mL/cmH2O | |
| Interventions | A (n = 50) PTNS using adhesive skin surface electrodes. Continuous, bi‐polar square wave form with pulse duration of 200 μs and stimulation frequency of 20 Hz. "The stimulator was controlled to determine the minimal current needed to induce a toe twitch. The intensity was then increased to the highest level tolerated by the participant who cannot induce lower limb muscle spasm in complete SCI patients and uncomfortable feeling on stimulating sites in incomplete SCI patients" B (n = 50) solifenacin succinate 5 mg per day | |
| Outcomes | Leakage volume per day (ml) (mean SD, N) A 541.4 (47.5), 50. B 449.1 (89.2), 48 I‐QoL (mean, SD, N) A 25.2 (1.0), 50. B 24.2 (1.0), 48 Adverse effects: A 0/50 B 5/50 (all dry mouth) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the patients were randomized into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No differential withdrawal, adequate explanation for withdrawals |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Iran Follow‐up: 12 weeks’ treatment | |
| Participants | N: randomised and analysed Sex: women Inclusion criteria: women with neurologic OAB confirmed by urodynamic diagnosis Exclusion criteria: not reported | |
| Interventions | A: PTNS. 34‐gauge needle placed 5 cm near internal malleolus. Sessions lasted 30 min B: 4 mg tolterodine daily for 3 months | |
| Outcomes | Sexual function Subjective assessment of pelvic disorders | |
| Notes | No useable data. Contacted study author 21‐04‐2016 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | "nor patients nor the physician were blinded to the patient’s group" |
| Blinding of outcome assessment (detection bias) | High risk | "nor patients nor the physician were blinded to the patient’s group" |
| Incomplete outcome data (attrition bias) | Unclear risk | Before study began, 2 in PTNS group and 8 in the control group withdrew. No explanation reported |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Rome, Italy Period: not reported Sample size: not reported Follow up: not reported | |
| Participants | N: 35 randomised and analysed Mean (SD) age: not reported Sex: 28 women, 7 men Inclusion criteria: OAB not responding to antimuscarinic therapy Exclusion criteria: not reported | |
| Interventions | Says all cases treated in the same way as detailed in Stoller 1999. A (n = 17, 14 F, 3 M) weekly PTNS B (n = 18, 14 F, 4 M ) 3 times per week PTNS – every 2 days | |
| Outcomes | Success = > 50% reduction in micturitions/24 hours OR If incontinent, > 50% reduction in UI episodes/24 hours A 11/17 (4/11 incontinent participants). B 12/18 (5/11 incontinent participants) Subjective improvement after 6‐8 sessions A 17/17. B 18/18 Adverse effects A 0/17. B 0/18 Adverse effects: “None of the patients discontinued the treatment and all considered it tolerable and painless” Incontinence episodes per 24 hours (median, range, N) A 1 (0‐3), 11. B 1 (0‐3), 11 Micturitions per 24 hours (median, range, N) A 8 (5‐15), 17. B 8 (6‐18), 18 SF‐36 (median, range, N) A 62 (24‐81), 17. B 62 (25‐80), 18 I‐QoL (median, range, N) A 77 (35‐100), 17. B 78 (33‐100), 18 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All participants randomised seem to be included in analysis |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Tor Vergata University Hospital in Rome, Italy Period: February 2007‐February 2009 Sample size: with a sample size of 15 in each group this study had a power of 82.3% to yield a statistically significant result assuming that the difference in proportions was 0.45 (specifically 0.05 vs 0.50). This effect was selected because the magnitude was reasonable according to previously published findings. To account for a dropout rate of 10% the number of participants to be recruited was set at 17 for each group, 34 total Follow‐up: 4 weeks | |
| Participants | N: 35 randomised, 32 analysed Mean age (no SD reported): A 44.9; B 45.5 Sex: women Inclusion criteria: female, urgency incontinence and urodynamically diagnosed detrusor overactivity incontinence, unresponsive to behavioural and rehabilitation therapy or antimuscarinics, able to give written, informed consent, 18 years of age or older, mentally competent and able to understand all study requirements, able to understand the procedures, advantages and possible side effects, willing and able to complete a 3‐day voiding diary and I‐QoL questionnaire, bladder capacity 100 mL or greater, no signs of neurologic abnormalities at objective examination; no history of neurologic pathology, no pharmacological treatment or pharmacological treatment unchanged for 30 days before beginning the study. Exclusion criteria: pregnancy or intention to become pregnant during the study, active UTI or recurrent UTI (more than 4 per year), presence of urinary fistula, bladder or kidney stones, interstitial cystitis, cystoscopic abnormalities that could be malignant, diabetes mellitus, cardiac pacemaker or implanted defibrillator | |
| Interventions | A (n = 18) PTNS. 12 sessions, 30 min, 3 times a week for 4 weeks. 34‐gauge needle inserted percutaneously approx 5 cm cephalad to the medial malleolus of right or left ankle; surface electrode placed on medial aspect of ipsilateral calcaneous. Stimulation current (0‐10 mA) with a fixed frequency of 20 Hz and a pulse width of 200 ms was increased until flexion of the big toe or fanning of all toes became noticeable. The current was set at the highest level that was tolerable to the participant. B (n = 17) sham. Same schedule as PTNS group with stimulator briefly activated for approximately 30 seconds so the participant felt a minor electrical sensation in the skin. | |
| Outcomes | Number of participants with < 50% reduction in urgency incontinence episodes: A 5/17. B 18/18 Number of incontinence episodes per 24 hours (mean, range, N): A 1.8 (1.2‐2.2), 17. B 3.8 (3.0‐4.5), 15 Number of micturitions per 24 hours (mean, range, N): A 9.5 (8.4‐10.7), 17. B 13.9 (11.3‐16.5), 15 Voided volume mL (mean, range, N): A 150.5 (126.8‐174.3) 17. B 150.4 (125.8‐175.1), 15 I‐QoL score (mean, range, N): A 69.9 (65.8‐73.3), 17. B 70.6 (62.2‐79.1), 15 | |
| Notes | Contacted study author asking for SDs 27‐11‐14 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization list." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | To verify participant blindness with respect to the assigned treatment after 3 sessions participants were asked which procedure they believed they received. |
| Blinding of outcome assessment (detection bias) | Low risk | The results of the 2 groups were collected by 2 physicians, and analysed by a third physician and a statistician, both of whom were blinded regarding the procedure used in any single participant. |
| Incomplete outcome data (attrition bias) | Low risk | In the PTNS group 1 participant and in the placebo group 2 did not complete the study for personal reasons not related to the used technique. There remained 17 participants in the PTNS group and 15 in the placebo group. There was a loss of less than 20% so considered at low risk of bias. |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: USA Period: not reported Sample size: "to achieve a power of 0.80 with an estimated conventional large effect size (f = 0.40), we sought a sample size of 66 women (33 with urge UI and 33 with stress UI) with 11 participants per treatment by diagnosis group." Follow‐up: 8 weeks | |
| Participants | N: 63 randomised, 48 analysed Mean (SD) age: UUI overall 61.0 (12.4), A 57.3 (12.5) B 66.5 (12.4) C 63.0 (14.5) SUI overall 55.1 (14.4), D 52.7 (15.0) E 63.6 (13.3) F 48.2 (16.2) Sex: women Inclusion criteria: SUI or UUI diagnosed by urodynamics or Medical, Epidemiological and Social Aspects of Aging (MESA) questionnaire, parous or nulliparous women 21 years or older, manual dexterity to dial the Liberty Electrical Stimulation Unit, fluent English, ≥ 3 incontinent episodes in 3 days. Women on HRT to maintain same oestrogen intake throughout study, women not taking hormones were asked not start an oestrogen regimen during study. Exclusion criteria: zero score on Oxford pelvic floor muscle strength scale, denervation injury to the sphincters, anti‐incontinence surgery, vaginal extent to extent that middle finger could not be inserted into vagina, BMI > 50, stage III/IV prolapse, pregnancy, neurologic conditions, any potentially confounding prescriptions drugs | |
| Interventions | UUI A (n = 7) intravaginal ES plus PFMT. 14 sessions of 60 min PFMT exercises, then 30 min (12.5 Hz) at highest tolerable intensity Tampon‐shaped Liberty ES device B (n = 8) PFMT alone. 60 minutes twice a week for 8 weeks C (n = 7) no active treatment SUI D (n = 14) as per group A E (n = 15) as per group B F (n = 12) as per group C | |
| Outcomes | York Incontinence Perception Scale (YIPS) score (higher score is better) (mean, SD, N): UUI: A 41.2 (10.2), 6. B 47.0 (5.5), 6. C 28.8 (2.9), 6 SUI: D 46.4 (7.2), 9. E SUI 44.8 (6.3), 12. F 29.9 (2.2), 9 % change in YIPS score (mean, N): UUI: A 38.7%, 6. B 78.7%, 6. C ‐2.4%, 6 SUI: D 57.8%, 9. E SUI 37.0%, 12. F 2.0%, 9 Pelvic floor muscle strength, cm H2O (mean, SD, N): UUI: A 27.0 (16.0), 6. B 47.2 (22.7), 6. C 34.3 (25.5), 6 SUI: D 36.7 (14.1), 9. E 32.5 (18.5), 12. F 26.1 (18.6), 9 % change in pelvic floor muscle strength, cm H2O: UUI: A 8.9%, 6. B 155.1%, 6. C 1.2%, 6 SUI: D 119.8%, 9. E 49.8%, 12. F 5.2%, 9 Incontinence episodes in 3 days (mean, SD, N): UUI: A 3.0 (4.4), 6. B 2.3 (2.9), 6. C 7.8 (5.9), 6 SUI: D 1.4 (1.6), 9. E 4.1 (4.2), 12. F 8.0 (5.6), 9 *incontinence episodes per day (mean, SD, N): A 1.0 (1.47), 6. B 0.8 (0.97), 6. C 2.6 (1.97), 6 D 0.5 (0.53), 9. E 1.4 (1.4), 12. F 2.7 (1.87), 9 % change in incontinence episodes in 3 days (mean, N): UUI: A ‐78.1%, 6. B ‐70.5%, 6. C ‐4.0%, 6 SUI: D SUI ‐83.7%, 9. E SUI ‐66.9%, 12. F SUI 50.9%, 9 Frequency of micturitions in 3 days (mean, SD, N): UUI: A 25.7 (9.4), 6. B 23.5 (5.9), 6. C 24.2 (10.4), 6 SUI: D 24.1 (10.4), 9. E 22.8 (8.3), 12. F 24.6 (8.9), 9 *frequency of micturitions per day (mean, SD, N): A 8.6 (3.13), 6. B 7.8 (1.97), 6. C 8.1 (3.47), 6 D 8.0 (3.47), 9. E 7.6 (2.77), 12. F 8.2 (2.97), 9 % change in frequency of micturitions in 3 days (mean, N): A ‐19.2%, 6. B ‐16.7%, 6. C 27.4%, 6 D ‐6.6%, 9. E ‐8.8%, 12. F ‐14.9%, 9 | |
| Notes | Different numbers of participants reported in thesis and journal article. *Mean (SD) per day calculated from 3‐day data: mean and SD divided by 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "2 containers were prepared representing diagnosis groups (urge or stress incontinence). Each container held 33 slips of paper with 11 reading “e‐stim,” 11 reading “therapeutic exercise” and 11 reading “control.” The office assistant offered the correct diagnostic container to the participant on the second visit.” |
| Allocation concealment (selection bias) | Low risk | "2 containers were prepared representing diagnosis groups (urge or stress incontinence). Each container held 33 slips of paper with 11 reading “e‐stim,” 11 reading “therapeutic exercise” and 11 reading “control.” The office assistant offered the correct diagnostic container to the participant on the second visit.” |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The primary researcher performed the outcome measures and administered the exercise programs. She was blinded to the participants’ diagnosis as determined by the MESA but was not blinded to group allocation." |
| Blinding of outcome assessment (detection bias) | High risk | "The primary researcher performed the outcome measures and administered the exercise programs. She was blinded to the participants’ diagnosis as determined by the MESA but was not blinded to group allocation." |
| Incomplete outcome data (attrition bias) | Unclear risk | Some differential attrition: "of those who dropped out after randomization most (11/16) were in the exercise and stimulation group...there was no indication that discomfort was a factor." |
| Methods | Study design: RCT Multicentre or single‐centre: 3 centres in Sweden Period: September 2001 and December 2005 Sample size: the power analysis was calculated on the basis of the primary outcome measure, reduction of micturitions per 24 h. The minimal patient‐perceivable improvement has been found to be a mean reduction of micturitions per 24 h equivalent to 20%. A reduction smaller than 20% would thereby not be of any significant clinical importance. There is a large uncertainty regarding the efficacy that can be expected for both ES treatment and drug treatment being 30% to 50%. Under the assumption that ES treatment would give a 70% reduction of symptoms and drug treatment (tolterodine) a 50% reduction and thereby give a difference between treatments of 20%, a Chi2 test with a 2‐sided significance level of 5% yielded a power of 80% for a sample size of 103 participants in each group. If the assumption was even bigger difference in efficacy, 70% for ES treatment vs. 40% for tolterodine, the sample size with an additional 10% to compensate for dropouts would be 55 participants in each group. Follow‐up: 24 months | |
| Participants | N: 72 randomised and 61 analysed at 6 months, 52 analysed at 12 months, 46 at 24 months Sex: Women Mean (SD) age: A 55 (11); B 61 (12) Inclusion criteria: urgency incontinence symptoms for ≥ 3 months, increased frequency of micturition (≥ 8 micturitions per 24 hours), mean volume of urine voided per micturition ≤ 200 mL, total urine volume per 24 hours of < 3000 mL during a 48‐hour bladder diary Exclusion criteria: Persistent UTI, post‐void volume greater than 150 mL, history of neurological disease or dementia, pregnancy, contraindications to anticholinergic therapy, and a cardiac pacemaker. Participants were also excluded if they had used tolterodine or any other anticholinergic drugs in order to treat urgency/urge incontinence during the last 2 months or had received ES treatment within the last 3 years. | |
| Interventions | A (n = 33). ES vaginally and/or transanally with the MS‐310 Device, MIC Rehab AB. Over 5‐7 weeks, 10 stimulation treatments 1‐2 times per week for 20 min with a frequency of 5‐10 Hz. The maximum ES was done with maximum tolerable intensity, which was adjusted up to the level of tolerable discomfort. B (n =31) tolterodine SR 4 mg orally once daily for 6 months, with dose reduction allowed to tolterodine SR 2 mg daily if intolerable side effects occurred | |
| Outcomes | Number of participants with moderate or severe urgency symptoms: A 10/33, B 12/31 Number of participants with no improvement in urgency symptoms: A 9/33, B 9/31 Change in frequency of micturition (mean, 95%CI (SD)*, N): 6 months: A ‐2.8 (‐3.6 to ‐2.2 (1.96)), 30. B −3.2 (−4.1 to −2.4 (2.41)), 31. 12 months: A −3.1 (95% CI, −4.0 to −2.1 (2.65)), n = 30. B −3.1 (95% CI, −4.3 to −1.9 (3.41)) n = 31 24 months: A −3.4 (−4.6 to −2.2 (3.35), n = 30. B −3.7 (−4.8 to −2.6 (3.12)), n = 31 Change in mean urine volume (mL) (mean, 95%CI (SD)*, N): A 54 (28‐80 (72.66)), 30. B 55 (36‐74 (53.97)), 31 Side effects: A 0/33 B** 9/30 dry mouth, 1/30 muscular pain KHQ: see Table 3. Various outcomes reported | |
| Notes | *SD calculated by FS, using 95% CI **based on information received from study author 6‐month data used in analysis because treatment was given for 6 months. Most other included studies provided data for end of treatment period N per treatment group at 12 and 24 months not given, assumed same as 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization sequence was developed centrally, using a computer random number generator." |
| Allocation concealment (selection bias) | Low risk | "Assignment was enclosed in sequentially numbered opaque sealed envelopes by a person not involved in the study. Patients were included into the study and allocated to treatment group by the clinical staff responsible for the study at each participating center, by opening the lowest numbered envelope" |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Blinding of study personnel and participants to treatment assignment for the duration of the study was not possible due to the nature of the interventions." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout. Adequate explanation for withdrawals |
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Belgium Period: November 2010 – November 2012 Sample size: 15 per group required for 80% power to detect between‐group difference Follow‐up: 9 weeks’ treatment, 6 months’ follow‐up | |
| Participants | N: 31 randomised and analysed Mean (SD) age: A 43.5 (14.0). B 40.5 (9.5) Sex: women and men Inclusion criteria: EDSS score < 7 and, urgency symptoms, nocturia, urgency incontinence, urinary retention and/or weak stream, post‐voiding symptoms such as incomplete bladder emptying sensation Exclusion criteria: acute MS episodes during the study, UTI, pelvic‐perineal treatment in the past 6 months, pregnancy | |
| Interventions | A (n = 16) PFME with biofeedback. One 30‐min session per week for 8 weeks B (n = 15) ES + PFME. As per group A plus transcutaneous posterior tibial nerve stimulation. Frequency 10 Hz, 220 µs pulse width. One 30‐min session per week for 9 weeks. Rectangular biphasic pulse. An external electrode was located 5 cm above the medial malleolus and 1 cm behind the tibia. The other electrode was positioned on the dorsum of the foot. 20 s on, 4 s off | |
| Outcomes | Number of participants not satisfied: A 1/16. B 4/15 SF‐Qualiveen total score (higher score = greater severity) (median, IQR, N): 9 weeks: A 1.000 (0.656, 1.719), 16. B 1.375 (0.625, 2.188), 15 6 months: A 1.313 (0.687, 1.625), 16. B 1.500 (0.344, 2.094), 15 *mean, SD, N 9 weeks: A 1.07 (0.65), 16. B 1.51 (0.83), 15. 6 months: A 1.21 (0.74), 16. B 1.39 (0.91), 15 Bladder hyperactivity score (median, IQR, N): 9 weeks: 5.00 (1.50, 8.00), 16. B 6.00 (2.5, 9.25), 15 6 months: 7.00 (3.50, 9.50), 16. B 5.00 (4.25, 7.75), 15 *mean, SD, N 9 weeks: A 5.4 (3.67), 16. B 6.75 (3.91), 15 6 months: A 6.42 (3.9), 16. B 6.5 (3.45), 15 Daily urgency episodes (median, IQR, N): 9 weeks: A 1.2 (0.3, 5.0), 16. B 0.7 (0.2, 4.3), 15 6 months: A 2.0 (0.3, 2.7), 15. B 1.4 (0.0, 2.0), 15 *mean, SD, N 9 weeks: A 2.69 (3.02), 16. B 2.63 (3.08), 15 6 months: A 2.25 (2.53), 16. B 1.67 (1.64), 15 Adverse effects: A 0/16. B 0/15 | |
| Notes | Subcategories of Qualiveen scores available in paper Emailed study authors asking for means (SDs) 2 April 2015. Replied with data marked * | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants not possible. Other blinding not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "Data analysis was blinded" |
| Incomplete outcome data (attrition bias) | Low risk | No differential withdrawal. Adequate explanations for withdrawal not reported. Intention‐to‐treat analysis carried out |
| Methods | Study design: randomised cross‐over trial Setting: Chile Follow‐up: switch modalities at 3 months, follow‐up at 6 months | |
| Participants | N: 82 randomised Sex: not reported Inclusion criteria: OAB symptoms Exclusion criteria: unable to comply with follow‐up or had a history of neurological disease | |
| Interventions | A (n = 40 randomised and 31 analysed): transcutaneous posterior tibial nerve stimulation and behavioural therapy. Twice a week for 6 weeks B (n = 42 randomised and 37 analysed): behavioural therapy. One‐to‐one interview and assessment with a continence physiotherapist and written information After 3 months both groups switched treatment modalities for another 3 months | |
| Outcomes | After 3 months’ treatment: Visual analogue scale (VAS) (higher score = greater severity) (mean SD, N): A 5.81 (2.89), 31. B 7.50 (2.50), 37 Incontinence severity index (ISI) (higher score = greater severity) (mean, SD, N): A 5.15 (3.23), 31. B 7.38 (4.00), 37. Patient’s Global improvement (PGI‐I): A 85.7%. B 60.9% OAB‐Q (higher score = greater severity) (mean SD, N): A 100.81 (41.50), 31. B 127.71 (40.64), 37 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated sequence" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Withdrawals: A 9/40, B 5/42. No explanations for withdrawal |
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: USA Period: June 2011–December 2013 Sample size: the sample size calculation was determined using the 2‐sided Chi2test with a significance level of 5% and 80% power based upon the following assumptions: (1) proportion of responders at end of 12 weeks of treatment would be 50% in the active (test) group and 25% in the inactive (control) group; (2) a responder was defined as a subject who experienced decrease of ≥ 50% in mean UUI episodes (leaks) between baseline and week 12 of the study; (3) 20% dropout rate Follow‐up: 12 weeks | |
| Participants | N: 163 randomised Mean age (SD): A 60.8 (14.3); B 62.4 (13.8) Sex: 138 women, 25 men Inclusion criteria: men and women, at least 18 years of age. Failure on primary OAB treatment, such as behaviour modification or fluid/diet management, AND at least 1 anti‐cholinergic drug (unless participant was contra‐indicated for anti‐cholinergic use). Symptoms of OAB for at least 6 months Exclusion criteria: Dysfunctional voiding symptoms unrelated to OAB, such as clinically significant bladder outlet obstruction, and urinary retention (pvr > 100 cc). Morbidly obese, defined as having BMI > 40 kg/m2. Stress predominant MUI. Neurological disease affecting urinary bladder function, including but not limited to Parkinson's disease, multiple sclerosis, stroke, spinal cord injury and uncontrolled epilepsy. Pelvic surgery (such as sub‐urethral sling, pelvic floor repair) within the past 6 months. Intravesical or urethral sphincter Botulinum Toxin Type A injections within the past 12 months. Any neuromodulation therapy for OAB within the past 3 months. Failure to respond to previous neuromodulation therapy for OAB. Leading edge of any vaginal prolapse beyond hymenel ring. Prior peri‐urethral or transurethral bulking agent injections for bladder problems within the past 12 months. Any skin conditions affecting treatment or assessment of the treatment sites. History of lower back surgery or injury that could impact placement of the patch, or where underlying scar tissue or nerve damage may impact treatment. Presence of an implanted electro‐medical device (e.g. pacemaker, defibrillator, InterStim®, etc.), or any metallic implant in the lower back. Pregnant, nursing, suspected to be pregnant (by urine pregnancy method), or plans to become pregnant during the course of the study. Known latex allergies, or allergies or hypersensitivity to patch materials that will be in contact with the body (e.g. hydrogel, acrylic‐based adhesive, polyurethane). Uncontrolled diabetes and/or diabetes with peripheral neuropathy. Current UTI or history of recurrent UTIs (> 3 UTIs in the past year). History of lower tract genitourinary malignancies within the last 6 months or any previous pelvic radiation. Any clinically significant systemic disease or condition that in the opinion of the Investigator would make the patient unsuitable for the study | |
| Interventions | A (n = 80) 1 VERV electrode patch worn per week for 12 weeks B (n = 83) 1 sham electrode patch worn per week for 12 weeks | |
| Outcomes | Change in urgency (urinary) incontinence episodes per day (median (IQR), N): A ‐3.7 (‐4.7 to ‐1.0), 68. B ‐1.7 (‐3.3 to ‐1.0), 75. P = 0.2191) Change in urinary frequency per day (median (IQR), N): A ‐1.0 (‐2.7 to 0.3), 80. B ‐1.3 (‐3.0 to ‐0.3), 83. P = 0.2893 Change in volume per void (mL) (median (IQR), N): A 1.0 (‐26.6 to 23.5), 80. B 8.8 (‐24.3 to 33.3), 83. P = 0.3387 Change in urgency episodes (median (IQR), N): A ‐1.7 (‐3.3 to 0.3), 80. B ‐1.7 (‐3.3 to 0.3). P = 0.6557 Change in OAB‐symptom composite score (median (IQR), N): A ‐5.8 (‐14.7 to 1.3), 80. B ‐8.0 (‐15.3 to 0.3), 83. P = 0.4354 Change in OAB‐Q score (median (IQR), N): A 8.8 (1.6 to 20.0), 56. B 9.2 (‐0.8 to 27.2), 66. P = 0.9918 Percentage of participants with improvement in severity according to Patient Perception of Bladder Condition scale: A 53.7% of 80 (43/80). B 44.2% of 83 (37/83) Percentage of participants with overall improvement according to Treatment Benefit Scale: A 55.4% of 56 (31/56). B 42.4% of 66 (28/66) Percentage of participants with Improvement as measured by Overactive Bladder Satisfaction With Treatment Questionnaire: A 65.3% of 32 (21/32). B 57.6% of 34 (20/34) Percentage of participants improved as measured by clinicians using Clinical Global Impressions: A 23.2% of 80 (19/80). B 24.2% of 83 (20/83) Participants with adverse effects: A 30/80. B 29/82 | |
| Notes | Emailed study author asking for means (SDs) 6 January 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Allocation: randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Masking: Double Blind (Subject, Investigator)" |
| Blinding of outcome assessment (detection bias) | Low risk | "Masking: Double Blind (Subject, Investigator)" |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout. Adequate explanation for withdrawals |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Russia Period: 2008‐2010 Details of sample size calculation: not reported Follow‐up: 1‐month's treatment, 12 months’ follow‐up | |
| Participants | N: 229 randomised, 208 analysed at 12 months Mean (SD) age: 66.3 (range 65‐77) Sex: women Inclusion criteria: elderly women with urodynamic impairments and clinically confirmed OAB Exclusion criteria: not reported | |
| Interventions | All groups: trospium 60 mg + solifenacin 40 mg for 6 weeks then one of the following, beginning 2.5 months after end of drug treatment: A (n = 59) drugs: trospium 60 mg + solifenacin 40 mg for a month B (n = 51) detrusor ES: an active electrode (50‐70 cm2) above the pubis, and a passive electrode (150 cm2) in lumbosacral area, diadynamic current, frequency 20 Hz, modulation depth 50%‐75%, intensity 20–40 mA, exposure 15 min, a course consisting of 15 procedures every other day C (n = 63) conservative treatment: laseropuncture by helium‐neon laser (632.8 nm) at acupuncture points RP 6, RP 9, VC 2 within 1‐1.5 min for each point every day, light guide output power, 2 mW, 25 procedures D (n = 56) placebo | |
| Outcomes | Daily urinary incontinence episodes (mean, SD, N) 6 months: A 1.1 (0.7), 59. B 2.2 (0.9), 51. C 3.8 (0.8), 63. D 2.7 (1.1), 56 12 months: A 1.5 (0.9), 59. B 3.7 (1.3), 51. C 5.5 (1.4), 63. D 4.8 (2.4), 56 Volume at FDV, mL (mean, SD, N): 6 months: A 289.3 (37.6), 59. B 297.0 (45.3), 51. C 254.5 (49.1), 63. D 279.7 (54.8), 56 12 months: A 257.5 (28.9), 59. B 210.9 (28.7), 51. C 199.3 (49.4), 63. D 192.9 (28.9), 56 Volume at maximal desire to urinate, mL (mean, SD, N): 6 months: A 313.7 (47.1), 59. B 334.8 (38.3), 51. C 286.0 (36.6), 63. D 311.5 (51.7), 56 12 months: A 279.9 (33.8), 59. B 251.9 (42.9), 51. C 178.9 (29.0), 63. D 206.3 (SD missing), 56 Maximum bladder pressure, cmH2O (mean, SD, N): 6 months: A 32.8 (6.0), 59. B 35.4 (9.3), 51. C 38.9 (7.8), 63. D 31.0 (7.9), 56 12 months: A 28.8 (4.7), 59. B 30.9 (4.9), 51. C 29.8 (6.3), 63. D 23.9 (5.4), 56 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we randomized 229 women" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 14 participants withdrew due to side effects, 2 discontinued due to the lack of an immediate positive effect; and 2 withdrew for reasons unrelated to the treatment course. Numbers of withdrawals not reported per treatment group. |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: not reported Period: not reported Sample size: not reported Follow‐up: not reported | |
| Participants | N: 45 Sex: women Mean age: not reported Inclusion criteria: women with OAB symptoms Exclusion criteria: not reported | |
| Interventions | A (n = 16) PFMT B (n = 14) Intravaginal ES. Twelve 30‐min sessions C (n = 15) Transcutaneous posterior tibial nerve stimulation. Twelve 30‐minsessions | |
| Outcomes | Symptoms of urgency incontinence, defined as "absence, a little, more or less and much" | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Setting: China Follow‐up: 4 weeks’ maximum treatment | |
| Participants | N: 60 randomised Sex: not reported | |
| Interventions | A (n = 35) vaginal/anorectal ES, 8‐70 mA, 20 min, 20‐30 sessions B (n = 25) 2 mg tolterodine daily, 2‐4 weeks | |
| Outcomes | Cure rate: A 13/35. B 10/25 Improved: A 13/35. B 9/25 Satisfied or fairly satisfied: A 19/35. B 20/25 Side effects: Dry mouth: A 1/35. B 20/25 Uroschesis: A 0/35. B 2/25 Constipation: A 1/35. B 6/25 Blurred vision: A 0/35. B 1/25 | |
| Notes | Only partial translation available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants, other blinding unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No differential withdrawal |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Department of a Regional Hospital in Perth, Western Australia Period: not reported Sample size: 50 participants in each group would be sufficient to give 0.8 power at the 0.05 alpha level for two‐sided alternative. Calculation of sample size was performed using the PASS statistical software (NCSS, Kaysville, Utah, USA). Parameters used in the calculations were derived from Jundt et al and Lamhut. Follow‐up: 4 weeks | |
| Participants | N: 24 randomised and analysed Sex: women Mean age (SD): A (n =12) 52.1 (17.5) B (n = 12) 55.1 (15.1) Inclusion criteria: women, aged 20 years or older, with stress or UUI Exclusion criteria: altered mental state, urinary incontinence caused by problems other than stress or urge, transient incontinence, or severe disability requiring full assistance with all acts of daily living | |
| Interventions | A (n = 12) PFMT. 12 sessions (3 per week for 4 weeks): 10 sets of 5 contractions with 30‐s rest between each set. Then repeated after an hour. B (n = 12) ITT plus PFMT. 12 sessions (3 per week for 4 weeks) of 50 pelvic floor contractions followed by ITT with Nemectrodyne 5 stimulator then another 50 contractions. 2 anterior flat electrodes placed over obturator foramen 1.5cm to 2 cm lateral to symphysis, 2 posterior electrodes placed medial to ischial tuberosities either side of anus. ITT was at highest tolerable frequency between 0‐100 Hz for 15 min (session 1), then 30 min for sessions 2‐12 | |
| Outcomes | Pelvic floor muscle strength measured with perineometer (mean, SD, N): A 9.55 (3.50), 12. B 8.08 (4.83), 12 Pad test (g) (mean, SD, N): A 1.25 (1.76), 12. B 9.00 (29.3), 12 Frequency (number of micturitions per day) (mean, SD, N): A 6.29 (2.2), 12. B 7.24 (2.62), 12 Nocturia (number of nocturia episodes per night) (mean, SD, N): A 0.45 (0.86), 12. B 0.99 (1.04), 12 Change in pelvic floor muscle strength (mean, SD, N): A 2.03 (2.10), 12. B 2.04 (2.47), 12. (P = 0.253) Change in pad test (g) (mean, SD, N): A ‐4.33 (8.37), 12. B ‐85.1 (150), 12. (P = 0.101) Change in frequency (mean, SD, N): A ‐0.07 (1.76), 12. B ‐1.81 (1.62), 12. (P = 0.006) Change in nocturia (mean, SD, N): A ‐0.49 (0.89), 12. B 0.86 (1.14), 12. (P = 0.199) No improvement in stop/start test, defined as change from unable to stop to being able to slow, or change from able to slow to able to stop: A 9/12. B 6/12 (P = 0.2) No improvement in urgency (not defined): A 8/12. B 4/12 | |
| Notes | We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply No useable data. Not stratified by stress/urgency incontinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly allocated as soon as they gave written consent, using the sealed envelope method". |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants were not blinded due to the nature of the interventions but unclear if this would have effect on outcomes |
| Blinding of outcome assessment (detection bias) | Low risk | "Only the assessor but not the patients could be blinded." |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: USA Period: not reported Sample size: not reported Follow‐up: 5 weeks’ treatment then another 5 weeks’ treatment if improvement observed after first 5 weeks, then follow‐up six months after end of 10 weeks’ treatment | |
| Participants | N: 42 recruited, 37 randomised and analysed Mean (SD) age: 61 (17) Sex: women Inclusion criteria: DO Exclusion criteria: not reported | |
| Interventions | A (n = 18) ES once a week for 5 weeks B (n = 19) ES twice a week for 5 weeks Medicon MS‐210 with vaginal and anal probes | |
| Outcomes | Incontinence episodes after 5 weeks (mean, N): 12 (37) Participants not improved after 5 weeks (N): 0 Participants satisfied enough to request no further treatment: 25% (9) Adverse effects: Discomfort: 16% (6/37) Leg tremor: 8% (3/37) UTI: 8% (3/37) | |
| Notes | Data not presented by treatment – not useable | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized into two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants not possible. Other blinding not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | 5/42 participants withdrew before treatment; no explanation reported. All participants treated included in analysis. No withdrawals due to adverse effects |
| Methods | Study design: RCT Setting: Chile Follow‐up: 12 weeks’ treatment | |
| Participants | N: 56 randomised Sex: women Age: not reported Inclusion criteria: OAB according to ICI 2002 definition Exclusion criteria: not reported | |
| Interventions | A (n = 28?) transcutaneal tibial nerve stimulation, twice a week with at least 48 h intervals for 12 weeks B (n = 28?) long release oxybutynin 10 mg | |
| Outcomes | Frequency (mean? range, N): A 4 (2‐7), 28. B 8 (1‐13), 28 Urgency (mean? range, N): A 4 (1‐6), 28. B 7 (4‐15), 28 Urgency incontinence (mean? range, N): A 2 (0‐3), 28. B 6 (1‐11), 28 Daily pads (mean? range, N): A 0 (0‐3), 28. B 4 (3‐6), 28 | |
| Notes | Numbers randomised to each group not reported, assume equal numbers Table does not state if means or medians are reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the randomization was made by permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Brazil Period: not reported Sample size: not reported Follow‐up: 4 weeks | |
| Participants | N: 43 randomised Mean (SD) age: not reported Sex: women Inclusion criteria: OAB Exclusion criteria: not reported | |
| Interventions | A (n = ?) ES 30 min, twice per week for 4 weeks TENS, biphasic with 200 ms pulse duration, 10 Hz frequency, variation of intensity and frequency through one channel and two electrodes. B (n = ?) unclear if sham or no active treatment: "same protocol but without electrical stimulation." | |
| Outcomes | Daytime frequency: difference between groups P = 0.0001 (in favour of intervention) Nocturia: difference between groups P = 0.0186 (in favour of intervention) Improvement in SUI: difference between groups P = 0.0273 (in favour of intervention) Urgency symptoms: difference between groups P = not significant Participants with no involuntary detrusor contraction: A 4/?. B 5/? | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized’ ‘divided into two different groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many participants included in analysis |
| Methods | Study design: RCT Multicentre or single‐centre: multi‐centre Setting: UK Period: not reported Sample size: not reported Follow‐up: 4 weeks | |
| Participants | N: 74 randomised, 64 analysed Mean (SD) age: not reported Sex: men and women Inclusion criteria: ≥ 18 years, OAB symptoms ≥ 6 months, failure of OAB therapies such as behaviour modification and failure of ≥ anti‐cholinergic drug for OAB. Exclusion criteria: not reported | |
| Interventions | Patient‐managed neuromodulation system (PMNS): transdermal amplitude‐modulated signal through a patch applied to the skin, controlled by wireless handheld remote control. Patch worn for 4 weeks, placed by investigator initially. A (n = 30) Investigator placement group. Participants returned every 7 days for patch removal and placement of a new patch on contra‐lateral side. B (n = 34) Subject placement group. Participants returned on day 7 for investigator observation of patch self‐placement and replaced patch at home for the remaining 2 weeks. | |
| Outcomes | UUUI episodes (mean, SD, N): 2.2 (2.5), 64. % change from baseline in UUI episodes (mean, SD, N): ‐2.7% (3.1), 64. Change from baseline in UUI episodes (mean, SD, N): ‐47.8 (60.6), 64. Voiding frequency (mean, SD, N): 9.4 (2.7), 64. % change from baseline in voiding frequency (mean, SD, N): ‐1.9% (2.5), 64. Change from baseline in voiding frequency (mean, SD, N): ‐15.0 (19.1) Volume per void (mean, SD, N): 187.6 (75.0), 64. % change from baseline in volume per void (mean, SD, N): 8.2% (46.7), 64. Change from baseline in volume per void (mean, SD, N): 7.5 (26.4), 64. Urgency episodes (mean, SD, N): 7.8 (3.3), 64. % change from baseline in urgency episodes (mean, SD, N): ‐2.2 (2.8), 64. Change from baseline in urgency episodes (mean, SD, N): ‐21.2 (28.6), 64. | |
| Notes | Not useable – results not presented per treatment group Contacted study author requesting data per group 17 February 2015. Author responded "The device has been withdrawn. Probably doesn’t need to be in the review." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No explanation reported for withdrawals. Data not presented per treatment group. |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Brazil Period: February–June 2008 Sample size: "Pocock formula, with 47% of neurogenic OAB prevalence and decrease of 30% after treatment" Follow‐up: 45 days’ treatment, 12 months’ follow‐up | |
| Participants | N: 24 randomised and analysed Mean (SD) age: A 65.1 (3.6). B 56.1 (10.9) Sex: men Inclusion criteria: ≥ 18 years with neurogenic OAB, with stroke occurring between 6 months and 3 years before recruitment Exclusion criteria: implanted cardiac pacemaker, UTI, bladder cancer, pre‐existing urinary incontinence before stroke, or surgery in the urogenital region | |
| Interventions | A (n = 12) ES of posterior tibialis nerve. Negative electrode was placed on the medial malleolus, and the positive electrode was placed 10 cm above the negative electrode, also on the medial side. The rhythmic flexion of the second toe during the stimulation determined the correct position of the negative electrode. The intensity level was set below the threshold that causes motor contraction because the participant should be comfortable and no pain should occur during the procedure. ES of the posterior tibialis nerve was performed for 30 minutes twice weekly over 12 sessions (45 days), with a frequency of 10 Hz and a pulse width of 200 µs in continuous mode. B (n = 12) no active treatment for OAB. 12 stretching sessions of the lower limbs | |
| Outcomes | Participants with no improvement in OAB symptoms: 12 months: A 0/12. B 9/12 Participants with urinary urgency: 45 days: A 7/12. B 10/12 12 months: A 6/12. B 9/12 Participants with UUI: 45 days: A 8/12. B 9/12 12 months: A 7/12. B 8/12 Participants with nocturnal enuresis: 45 days: A 0/12. B 2/12 12 months: A 0/12. B 2/12 Participants with nocturia: 45 days: A 5/12. B 9/12 12 months: A 1/12 B 6/12 Participants with increased daytime frequency: 45 days: A 3/12. B 11/12 12 months: A 0/12. B 9/12 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | All participants were numbered sequentially from 1‐24 and divided into 2 groups of 12 assigned to the treatment group |
| Allocation concealment (selection bias) | Unclear risk | All participants were numbered sequentially from 1‐24 and divided into 2 groups of 12 assigned to the treatment group |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. Impossible to blind participants |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants included in analysis. One dropout. "One patient in the placebo group died after treatment, but was analyzed as if improved." |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Sample size: the study was powered to detect a 3‐point (common standard deviation of 6) between‐group difference on the ICIQ‐UI (scale of 0‐21) with 80% power at a 5% level of significance. Follow‐up: 12 weeks | |
| Participants | N: 124 randomised, 97 analysed Mean (SD) age: A 47.9 (8.9). B 48.2 (8.6) Sex: women Inclusion criteria: women, 18–65 years with self‐reported SUI, UUI, or MUI Exclusion criteria: Pregnancy or a baby in the last 3 months. Recent abdominal surgery and previous or current active therapy for pelvic malignancy. Implanted pacemaker. Manual dexterity insufficient to place the device. Previous treatment for incontinence (including supervised PFME. Presence of a neurological condition such as multiple sclerosis or Parkinson’s disease | |
| Interventions | A (n = 64) ES. Pelviva device inserted like a tampon into the vagina. The stimulation programme was delivered using a duty cycle of 10‐sstimulation followed by 10‐s rest that runs for a period of 30 min, pre‐programmed to automatically gradually ramp‐up the intensity of stimulation over a 24‐s period to reach a therapeutic level and switch off automatically after 30 min. During the 10 seconds 'on time' the device delivered 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation. Plus standardised advice about how and when to undertake PFME. These included 10 slow and controlled squeezing and lifting contractions and 10 quick contractions each repeated 3‐4 times a day B (n = 60) unsupervised conservative treatment (no active treatment). Standardised advice about how and when to undertake PFME. These included 10 slow and controlled squeezing and lifting contractions and 10 quick contractions each repeated 3–4 times a day. | |
| Outcomes | Participants with no improvement in symptoms (i.e. same or worse ICIQ score): A 9/49. B 14/46 *A UUI 5/50. B 6/47. *A MUI 8/50. B 19/47. *A UUI+MUI 13/50. B 25/47 Participants with SUI, UUI or MUI A 94% (i.e. 46/49) B 100% (i.e. 46/46) International Consultation on Incontinence Questionnaire – Urinary Incontinence (ICIQ‐UI) score (higher score is increased severity) (median, range, N): A 6 (0‐17), 49. B 9 (3‐18), 46 Leak frequency (0‐5 scale, higher score is more leaks) (median, range, N): A 1 (0‐4), 49. B 2 (1‐4), 46 Leak interference (0‐10 scale, higher score is more interference) (median, range, N): A 3 (0‐10), 49. B 4 (0‐10), 46 Leak amount (0‐6 scale, higher score is greater amount) (median, range, N): A 2 (0‐6), 49. B 2 (2‐4), 46 Adverse effects: A 0/49. B 0/46 | |
| Notes | *Outcome data not separated by SUI/UUI/MUI – contacted study author 3 February 2015, replied with supplementary data. Femeda, the company responsible for developing and producing the Pelviva device was the trial sponsor. The sponsor was responsible for developing the Pelviva device, was the funder of the study, and was engaged in the development of the trial design. The sponsor has provided full access to the data and is fully informed of this publication process. The primary author (J.O.) takes full responsibility for the integrity of the data and accuracy of the data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "subjects were assigned by a simple computer generated AB randomization list to either the exercise or Pelviva group." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Participants could not be blinded to the treatment group and were aware of the study hypothesis. Every care was taken to ensure the assessor remained blind to treatment allocation and participants were advised not to discuss their treatment with them." |
| Blinding of outcome assessment (detection bias) | Low risk | "the assessor remained blind to treatment allocation" |
| Incomplete outcome data (attrition bias) | Unclear risk | No differential dropout. No explanations for withdrawals |
| Methods | Study design: RCT Multicentre or single‐centre: single Setting: Spain Period: not reported Details of sample size calculation: no previous data available for power calculation Follow up: 12 weeks | |
| Participants | N: 24 randomised, 22 analysed Mean (SD) age: 60 (14.4) Sex: women Inclusion criteria: urgency incontinence, either men or women, 45‐75 years, moderate‐severe on ICIQ‐SF and CACV, previous conservative treatment, at least 1 year of incontinence, willing to participate Exclusion criteria: neurological damage to tibial nerve, diseases of central nervous system, previous incontinence surgery, pacemaker, not well‐controlled cardiac disease, pregnancy, important venous disease in the lower limbs, skin problems in lower limbs that would impede acupuncture, treatment with oral anticoagulants, acute infectious processes, psychiatric or cognitive impairments | |
| Interventions | AWQ‐104L Digital. 20 Hz, 320 μs. Square wave, current 0‐10 mA. 30 mm x 1.5” needle A (n = 12) electrostimulation with SP 6 Sanyinjiao B (n = 12) percutaneous tibial nerve stimulation | |
| Outcomes | Micturitions per day (mean (SD) N) A 7.73 (1.67), 11. B 8 (1.73), 11 Nocturia episodes (mean (SD), N) A 2.09 (1.92), 11. B 1.09 (1.51), 11 Urgency episodes per 24 h (mean (SD) N) A 5.09 (3.42), 11. B 3.09 (2.21), 11 Incontinence episodes per 24 h (mean (SD), N) A 4.55 (4.03), 11. B 1.64 (1.91), 11 B‐SAQ score score (mean, SD, N) Symptoms: A 7.82 (1.83), 11. B 5.09 (2.17), 11 Complaints/problems: A 7.27 (2.24), 11. B 5.18 (2.56), 11 ICIQ‐SF score (mean (SD), N) A 7.27 (2.24), 11. B 5.18 (2.56), 11 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Low risk | Allocation carried out centrally by member of research team not involved in the intervention |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants can’t be blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded – had no involvement in carrying out intervention |
| Incomplete outcome data (attrition bias) | Low risk | No differential attrition |
| Methods | Study design: RCT Period: January 2010 and April 2011 Setting: not reported Sample size: not reported Follow‐up: 12 weeks’ treatment | |
| Participants | N: 30 randomised Sex: not reported Age: not reported Inclusion criteria: people OAB in whom all conventional therapies had failed Exclusion criteria: not reported | |
| Interventions | A: percutaneous posterior tibial nerve stimulation B: anticholinergic agent C: PTNS plus anticholinergic agent | |
| Outcomes | A (n = not reported) percutaneous posterior tibial nerve stimulation B (n = not reported) anticholinergic agent C (n = not reported) PTNS plus anticholinergic agent | |
| Notes | Urinary Distress Inventory (UDI‐6) Incontinence Impact Questionnaire (IIQ‐7) Over Active Bladder symptom scores (OABSS) "there was a statistically significantly higher improvement in PTNS and PTNS + ACA groups when compared to group 2" (B: anticholinergic agent alone) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly divided into 3 groups." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Multicentre or single‐centre: 11 centres in the USA Setting: not reported Period: June 2006‐September 2008 Sample size: the sample size used to support this analysis was based on the assumptions of significance level of 5%, power of 80%, and expected mean reduction in voids of 1.8 for tolterodine and 3.6 for PTNS based on previously published efficacy data. Secondary end points were analysed using 2‐sided t tests with 95% CI. An independent biostatistician performed all analyses using SAS® Version 9.2. All voiding diary data were sent to the biostatistician for compilation and analysis. Follow‐up: 12 weeks | |
| Participants | N: 100 randomised, 85 analysed Mean (SD) age: A 57.5 (15.2); B 58.2 (11.3) Sex: 94 women, 6 men Inclusion criteria: adults with OAB symptoms, with or without a history of previous anticholinergic drug use, with at least 8 voids per 24 h documented by history and physical and voiding diary. Exclusion criteria: OAB pharmacotherapy within the previous month, primary complaint of SUI, demonstrated sensitivity to tolterodine or its ingredients, pacemakers or implantable defibrillators, excessive bleeding, urinary or gastric retention, nerve damage or neuropathy, uncontrolled narrow angle glaucoma, positive urinalysis for infection or pregnancy, or current pregnancy or planning to become pregnant during the trial | |
| Interventions | A (n = 50) PTNS. 1 session per week for 12 weeks (no details reported on frequency, make/model of stimulator etc) B (n = 50) tolterodine. Extended‐release 4 mg daily for 90 days (decreased to 2 mg if intolerability was experienced – 2 participants reduced to 2 mg) | |
| Outcomes | Number of participants not cured or improved (subject assessment): A 9/44. B 19/42 Number of participants not cured or improved (investigator assessment): A 9/44. B 17/42 Number of voids per 24 hours (mean, SD, N): A 9.8 (3.0), 41. B 9.9 (3.8), 43 Number of nocturia episodes (mean, SD, N): A 1.7 (1.1), 41. B 1.9 (1.6), 43 Number of urgency incontinence episodes per 24 hours (mean, SD, N): A 1.2 (1.6), 41. B 1.8 (2.5), 43 Number of moderate to severe urgency episodes per 24 hours (mean, SD, N): A 3.9 (2.8), 41. B 4.5 (3.6), 43 Volume voided per 24 hours (cc) (mean, SD, N): A 185.5 (81.1), 41. B 158.7 (99.8), 43 Change in number voids per 24 hours (mean, SD, N): A ‐2.4 (4.0), 41. B ‐2.5 (3.9), 43 Change in number of nocturia episodes per 24 hours (mean, SD, N): A ‐0.7 (1.0), 41. B ‐0.6 (1.7), 43 Change in number of urgency incontinence episodes per 24 hours (mean, SD, N): A ‐1.0 (2.2), 41. B ‐1.7 (3.8), 43 Change in number of moderate to severe urgency episodes per 24 hours (mean, SD, N): A ‐2.2 (4.3), 41. B ‐2.9 (4.8), 43 Change in volume voided per 24 hours (cc) (mean, SD, N): A 32.8 (61.3), 41. B 17.6 (58.4), 43 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random blocks design stratified by investigational site |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawn prior to 12 week follow‐up: withdrew consent n = 5; lost to follow‐up n = 1; withdrew consent n = 3; treatment unsuccessful n = 3; others n = 1 |
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: USA Period: September 2008‐January 2009 Sample size: "A sample size estimate of approximately 214 participants, 107 per study arm, calculated using a 2‐sided Fisher’s exact binomial test based on an estimated 60% responder rate in the PTNS group and a 40% responder rate in the sham group with a 5% significance level and 80% power." Follow‐up: 13 weeks | |
| Participants | N: 220 randomised (174 women, 46 men), 208 analysed Mean age (no SD): A 62.5; B 60.2 Sex: men and women Inclusion criteria: > 18 years of age, score of > 4 on the OAB‐Q short form for urgency, average urinary frequency of > 10 voids per day, self‐reported bladder symptoms > 3 months, self‐reported failed conservative care, discontinued all antimuscarinics for > 2 weeks, capable of giving informed consent, ambulatory and able to use toilet independently without difficulty, capable and willing to follow all study‐related procedures Exclusion criteria: pregnant or planning to become pregnant during study duration, neurogenic bladder, Botox® use in bladder or pelvic floor muscles within past year, pacemakers or implantable defibrillators, current UTI, current vaginal infection, Use of Interstim®, use of Bion®, Current use of TENS in pelvic region, back or legs, previous PTNS treatment, use of investigational drug/device therapy within past 4 weeks, participation in any clinical investigation involving or impacting gynaecologic, urinary or renal function within past 4 weeks | |
| Interventions | A (n = 110) PTNS. One 30‐minute session per week for 12 weeks. 34‐gauge needle electrode inserted at a 60º angle approximately 5 cm cephalad to the medial malleolus, slightly posterior to the tibia. PTNS surface electrode placed on the ipsilateral calcaneus and 2 inactive sham surface electrodes, 1 under the little toe and 1 on the top of the foot. Current level of 0.5‐9 mA at 20 Hz was selected based on each participant’s foot and plantar motor and sensory responses. B (n = 110) sham PTNS. One 30‐minute session per week for 12 weeks. Streitberger placebo needle was used to simulate the location and sensation of PTNS needle electrode insertion. An inactive PTNS surface electrode was placed on the ipsilateral calcaneus. Two active TENS surface electrodes were placed, 1 under the little toe and 1 on the top of the foot. | |
| Outcomes | "responder was defined as reporting bladder symptoms as moderately or markedly improved on a 7‐level GRA at week 13" Moderate or marked improvement on global response assessment: A 60/110. B 23/110 No improvement in OAB symptoms: A 50/110. B 87/110 No improvement in urinary urgency: A 59/103. B 81/105 No improvement in urinary frequency: A 54/103. B 82/105 No improvement in urgency incontinence: A 64/103. B 81/104 Frequency of voiding per 24 hours (mean, SD, N): A 9.8 (2.8), 103. B 11.0 (3.1), 105 Frequency of nocturia (mean, SD, N): A 2.1 (1.4), 103. B 2.6 (1.6), 105 Mean voided vol (cc) (mean, SD, N): A 183.0 (75.6), 103. B 172.6 (90.6), 102 Adverse effects: A 6/110. B 0/110 Change in OAB‐Q symptom score (mean, SD, N) (lower score is better): A ‐36.7 (21.5), 101. B ‐29.2 (20.0), 102 Change in SF‐36 score (mean, SD, N) (higher score is better): A 34.2 (21.3), 103. B 20.6 (20.6), 105 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All subjects were randomized 1:1 at the first intervention visit to PTNS or sham using a random block design stratified by investigational site." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Subjects and study coordinators were blinded to the intervention" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout. ITT analysis carried out for primary outcome |
| Methods | Study design: RCT Setting: USA Follow‐up: 4 weeks | |
| Participants | N: 74 randomised Sex: men and women Age: not reported Inclusion criteria: symptoms OAB with UUI for at least 6 months, other therapies previously failed, including ≥ anticholinergic drug Exclusion criteria: not reported | |
| Interventions | A (n = 34 patient‐managed neuromodulation system (PMNS) patch – subject placement B (n = 30) patient‐managed neuromodulation system (PMNS) patch – investigator placement | |
| Outcomes | % reduction in UUI episodes OAB‐Q score Adverse effects | |
| Notes | No useable data. Numbers per group not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized between two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: not reported Period: June 2004 and July 2006 Sample size: not reported Follow‐up: 12 weeks | |
| Participants | N: 31 randomised (n analysed unclear) Sex: women Mean (SD) age: 59.4 (10.9) Inclusion criteria: adults with urgency incontinence and urge symptoms Exclusion criteria: contraindications against anticholinergics, pregnancy, tolterodine before | |
| Interventions | A (n = 16) PTNS, one 30‐min session per week for 12 weeks B (n =15) tolterodine 2 mg daily for 12 weeks. | |
| Outcomes | Change in number of micturitions per 24 h (mean, 95%CI (SD)*, N): A ‐0.1 (‐3.3 to 3.6 (7.04)), 16. B ‐0.7 (‐2.3 to 3.7 (5.93)), 15. (P = 0.77) Change in number of incontinence episodes per 24 hours (mean, 95%CI (SD)*, N): A ‐1.3 (0.6 to 3.2 (2.65)), 16. B ‐2.6 (0.1 to 5.3 (5.14)), 15 Change in number of urgency episodes per 24 hours (mean, 95%CI (SD)*, N): A ‐9.3 (7.0 to 11.7 (4.80)), 16. B ‐9.5 (6.3 to 12.7 (6.32)), 15 Side effects: A 1/16. B 6/15 Change in QoL (instrument used not reported) (mean, 95%CI (SD)*, N): A 4.4 (1.7 to 7.1 (5.51)), 16. 4.6 (2.1 to 7.0 (4.84)), 15. | |
| Notes | *SD calculated by FS Dropouts: A 3. B 2. Unclear if these participants included in analysis We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants (10.3%) in the PTNS group and; 2 (6.9%) in the drug group (tolterodine) |
| Methods | Study design: RCT Multicentre or single‐centre: multicentre Setting: 3 centres in Austria and Germany Period: not reported Details of sample size calculation: "A provisional power calculation based on an exaggerated difference of 20% was performed for this pilot study. A reduction from a mean micturition per 24 h after a 3 months treatment with tolterodine of 13–10.4 under PTNS (assuming a common standard deviation of 2.7) could have been detected with 80% power and a two‐sided significance level of 5% with 18 patients per group" Follow up: 3 months’ treatment | |
| Participants | N: 36 randomised and 32 analysed Mean (SD) age:not reported Sex: women Inclusion criteria: female; minimum age of 18 years; complaints of OAB dry or wet consistent with the IUGA/ICS criteria; no prior treatment with PTNS or anticholinergics Exclusion criteria: pregnancy or intention to become pregnant during the study period; active or recurrent UTIs (more than 4 per year); residual urine of more than 100 ml; history of urinary fistula, bladder or kidney stones, interstitial cystitis; history of cystoscopic abnormalities or possible malignancy, diabetes mellitus, cardiac pacemaker or implanted defibrillator; history of anatomic or post traumatic malformations of the lower limbs; immobility; contraindications for anticholinergics or PTNS; disability to understand the study requirements and procedures, advantages and possible side effects | |
| Interventions | A (n = 18 randomized and 16 analysed) PTNS. One 30 min session per week for 3 months. "PTNS was performed as described by Stoller et al. (Stoller 1999) and Vandoninck et al. (Vandoninck 2003) (Urgent PC1 device by UroplastyTM" B (n = 18 randomised and 16 analysed) tolterodine 2 mg twice daily | |
| Outcomes | Micturitions per 24 h (mean, SD, N): A 10.4 (4.1), 16. B 9.1 (3.6), 16 QoL measured by VAS (higher score = greater severity) (median, range, N): A 1.9 (0‐8), 16. B 2.7 (0‐8.5), 16 Incontinence episodes in 24 h (median, range, N): A 0 (0‐6), 16. B 1 (0‐5), 16 Adverse effects: A 3/18 (pain at puncture site). B 9/18 (dry mouth and dizziness) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was centralised by telephone and the random allocation sequence was generated by computer assistance using a method of adaptive randomisation" "Stratification for randomisation was done for micturitions per 24 h (0–8, 9–12, 13–24, 25), incontinence episodes in 24 h (0–2, 3–10, 11–18, 19–24, 25), age (18–44, 45–55, 56–65, 66 years), and smoking." |
| Allocation concealment (selection bias) | Low risk | "the random allocation sequence was generated by computer assistance using a method of adaptive randomisation" |
| Blinding of participants and personnel (performance bias) | High risk | "The patients and assessors were not blinded" |
| Blinding of outcome assessment (detection bias) | High risk | "The patients and assessors were not blinded" |
| Incomplete outcome data (attrition bias) | Low risk | No differential withdrawal. Adequate reasons for withdrawals (not related to interventions) |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Turkey Period: not reported Sample size: not reported Follow‐up: 12 weeks | |
| Participants | N: 40 randomised Sex: women Mean age (range): overall 46.4 (33 to 61); mean (SD): A 45.4 (8.7). B 47.4 (10.1) Inclusion criteria: severe OAB symptoms defined as median 6 urgency incontinence episodes per 48 hours Exclusion criteria: stress incontinence, genital prolapse higher than Stage II on POP‐Q system, ocular, cardiological, neurological or metabolic disease, history of pelvic surgery ultrasonographic evidence of postvoidal retention more than 100 mL and bladder capacity less than 200 mL, menopausal symptoms indicating significant decrease in QoL, presence of UTI, prior treatment for OAB | |
| Interventions | A (n = 20) tolterodine 4 mg daily for 12 weeks B (n = 20) Stoller Afferent Neuro‐stimulation (SANS) plus tolterodine 4 mg daily for 12 weeks. One 30‐min session per week for 12 weeks. 34‐G acupuncture needle inserted at 30º angle into 2‐3 cm superior‐medial aspect of tibial medial malleolus along posterior tibial nerve trace. 20 Hz frequency, 0.2 ms duration, amplitude of stimulus adjusted according to participant toleration | |
| Outcomes | Frequency per 24 hours (mean, SD, N): A 6.4 (0.6), 18. B 4.5 (0 [sic]), 20. (P < 0.05) Urgency episodes per 24 hours (mean, SD, N): A 7.6 (0.9), 18. B 5.7 (0.6), 20. (P < 0.05) Incontinence episodes per week (mean, SD, N): A 12.3 (0.8), 18. B 6.4 (0.5), 20. (P < 0.001) IIQ‐7 score (mean, SD, N) (higher score is worse incontinence): A 11.2 (2.7), 18. B 9.0 (0.8), 20. Adverse events: Severe dry mouth: A 3/18. B 2/20 Severe constipation: A 2/18. B 2/20 Headache: A 1/18. B. 0/20 Local irritation on puncture site: A N/A. B 1 > 1 adverse event: A 2/18. B 1/20 | |
| Notes | We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was obtained using a list of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 withdrawals from tolterodine alone group; no reason reported |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Hosptial das Clínicas de Porto Alegre (HCPA), Brazil Period: January 2006‐May 2007 Sample size: to detect a difference of one standard deviation in the study variables after 12 weeks of treatment, the sample size was established as 11 participants per group. This sample size assumes a significance level of 5% power of 90% and a correlation between measurements at the 2 different points of 0.5. Follow‐up: 12 weeks' treatment, 6 months' follow‐up | |
| Participants | N: 32 randomised Sex: Women Age mean (SD): A 54.7 (6.94); B 49.18 (6.06); C 52.09 (13.78) Inclusion criteria: women were older than 30 years of age; SUI or MUI; had not received any clinical or surgical treatment during the previous 6 months; were free of significant genital prolapse (below stage 2 on the pelvic organ prolapse quantification system); and had no urethral sphincter involvement (leak point pressure less than 60 cmH20). The criteria for prolapse classification were defined in accordance with International Continence Society (ICS) guidelines. Exclusion criteria: not reported | |
| Interventions | All participants received identical specially designed equipment, providing real‐time information on the contraction waveform and information or guidance. Vaginal probe transducer for monitoring pelvic muscle contraction pressure during exercises. Programmable for either PFMT plus biofeedback, PFMT plus ES or PFMT without feedback All participants same exercise programme: supine position with rapid contractions (2 seconds contraction, 4 seconds rest) then slow contractions (4 seconds contraction, 4 seconds of rest), repeated 3 times with rest interval. A (n = 10) PFMT plus biofeedback for 12 weeks. Device displays information on contraction intensity B (n = 11) PFMT plus ES for 12 weeks. Frequency 50 Hz and pulse duration of 300 μs C (n = 11) PFMT alone for 12 weeks. Participants received no information from device on contraction intensity | |
| Outcomes | Subjective self‐evaluation at 12 weeks: Cure or significant improvement: 71.9% (23/32) Partial improvement: 18.8% (6/32) Poor response: 9.4% (3/32) Perineometric intensity (pelvic floor muscle strength) (IC cm H2O) (mean, SD, N): 12 weeks: A 57.93 (26.15), 10. B 49.7 (25.87), 11. C 47.67 (25.26), 11 6 months: A 51.12 (28.69), 10. B 41.85 (26.1), 11. C 48.88 (19.25), 11 Number of daytime micturitions (median, IQR, N): 12 weeks: A 7 (4‐8.25), 10. B 5 (5‐6), 11. C 7 (5‐10), 11 6 months: A 7.5 (6‐9.25), 10. B 4.5 (4‐6), 11. C 1.5 (0‐3), 11 Number of nocturia episodes (median, IQR, N): 12 weeks: A 1 (1‐2), 10. B 0 (0‐1), 11. C 2 (1‐2), 11 6 months: A 1.5 (0‐3), 10. B 1 (0.75‐2.25), 11. C, 1 (0.75‐2.25), 11 Number of SUI episodes (median, IQR, N): 12 weeks: A 1 (0‐2), 10. B 0 (0‐1), 11. C 2 (0‐3), 11 6 months: A 1 (0.75‐2.25), 10. B 0.5 (0‐1.25), 11. C 0 (0‐5.25), 11 Number of UUI episodes (median, IQR, N): 12 weeks: A 0 (0‐1.25), 10. B 0 (0‐0), 11. C 1 (0‐2), 11 6 months: A 0.5 (0‐1), 10. B 0 (0‐0), 11. C 2 (1‐3), 11 KHQ scores (mean, SD, N): 12 weeks: A 44.25 (9.11), 10. B 33.12 (19.54), 11. C 48.7 (22.21), 11 6 months: A 41.12 (15.44), 10. B 28.25 (11), 11. C 49.3 (24.96), 11 | |
| Notes | No useable data because SUI and MUI participants not separated. Cure/significant improvement not stratified by treatment group Emailed study author 19/12/2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported. Blinding of participants not possible |
| Blinding of outcome assessment (detection bias) | Low risk | "The examiner who performed perineometry was blinded to the patients [sic] group." |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants included in the analysis. No dropouts reported |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Urogynecology Section of the Gynecology Department in São Lucas Hospital of Pontificia Universidade Católica do Rio Grande do Sul, Brazil Period: February 2008‐October 2008 Sample size: not reported Follow‐up: 12 weeks' treatment, 2 years' follow‐up | |
| Participants | N: 52 randomised, 51 analysed Mean (SD) age: overall: 68.3 (5.3); A 67.6 (5.2); B 68.9 (5.4) Sex: women Inclusion criteria: UUI and age of 60 years of more Exclusion criteria: the presence of urinary infection during the recruitment process, prior surgery for urinary incontinence, history of genito‐urinary cancer, prior pelvic irradiation, pure SUI, genital prolapse above the second degree of Baden Walker, and inability to perform the Kegel exercises | |
| Interventions | All participants: PFMT (Kegel exercises); 15 contractions 3 times per day for 12 weeks A (n = 25) transcutaneous tibial nerve stimulation. One 30‐minute session per week for 12 weeks. Pulse duration 200 ms, frequency 10 Hz B (n = 26) PFMT only | |
| Outcomes | Daytime frequency (mean, SD, N): A 5.9 (1.4), 25. B 6.8 (1.9), 26 Change in daytime frequency (mean, SD, N): A ‐1.4 (2), 25. B ‐0.2 (0.9), 26 Number of nocturia episodes (mean, SD, N): A 1.3 (1.5), 25. B 2.4 (1.3), 26 Change in nocturia (mean, SD, N): A ‐1.6 (1.1), 25. B ‐0.4 (1.1), 26 Number of SUI episodes (mean, SD, N): A 2.4 (3.4), 25. B 4.0 (6.0), 26 Change in SUI episodes (mean, SD, N): A ‐1.1 (4.9), 25. B ‐1.9 (3.1), 26 Number of UUI episodes (mean, SD, N): A 1.8 (2.7), 25. B 4.6 (3.7), 26 Change in UUI episodes (mean, SD, N): A ‐6.3 (5.3), 25. B ‐1.3 (1.6), 26 Number of participants with > 50% reduction in UUI episodes: A 76.0% (19/25). B 26.9% (7/26) (P = 0.001) Subjective global satisfaction: 12 weeks: A 68.0% (17/25). B 34.6% (9/26) (P = 0.017) 2 years: A 64.7%. B not reported Number of participants with UUI: A 44.0% (11/25). B 80.8% (20/26) ICIQ‐SF score (mean, SD, N): A 7.9 (4.5), 25. B 10.6 (4.4), 26 Adverse effects: A 0. B 0 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly divided (through simple random number generator) into two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | "One patient from group 1 (with electrical stimulation of the tibial nerve) left the study due to health problems unrelated to the therapy |
| Methods | Study design: RCT Setting: Brazil Follow‐up: 3 months’ treatment, 12 months’ follow‐up | |
| Participants | N: 106 randomised Sex: women Inclusion criteria: elderly women (> 60 years) with UUI Exclusion criteria: not reported | |
| Interventions | A (n = 50) conservative treatment. 12 weeks of bladder retraining and PFME B (n = 51) transcutaneous tibial nerve ES | |
| Outcomes | ICIQ‐SF: "there was a greater improvement in the group treated with ES in all parameters." Recurrence of incontinence within 12 months: A not reported. B 16/34 Satisfaction at end of treatment: A 32.0% (16/50). B 66.7% (34/51) | |
| Notes | 71% had associated stress incontinence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the study design was a randomized clinical trial, parallel group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants, other blinding not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 12‐month data reported only for proportion of ES participants satisfied at end of treatment, no 12‐month data for bladder training group |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: UK Period: not reported Sample size: not reported Follow‐up: 12 weeks | |
| Participants | N: 48 randomised and 35 analysed Mean (SD) age: not reported Sex: not reported Inclusion criteria: either multiple sclerosis or idiopathic OAB Exclusion criteria: not reported | |
| Interventions | A (n = 24*) 30 min stimulation once per day for 12 weeks with Geko device B (n = 24*) 30 min stimulation once per week for 12 weeks with Geko device | |
| Outcomes | Improvement in ICIQOAB score: ‐10.2 (‐13.5 to ‐6.9, P = 0.001) Improvement in ICIQLUTS‐QOL score: ‐40.8 (‐57.4 to ‐24.3, P = 0.000) *Responders: 18/34 | |
| Notes | N randomised per group not reported. Outcome data not presented per group. Contacted study author for more information 5 February 2014 – replied with data marked * | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 48 randomised, 35 completed study (differential attrition: 20 with MS, 15 with idiopathic OAB). Unclear how many withdrew from each group. Unclear if all randomised participants were included in analysis |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: UK Period: not reported Sample size: not reported Follow‐up: 12 weeks | |
| Participants | N: 107 randomised, 94 analysed SUI 42 UUI 26 MUI 39 Mean (SD) age: not reported Sex: women Inclusion criteria: SUI, UUI or MUI Exclusion criteria: not reported | |
| Interventions | A (n = 53) ES under general anaesthesia. Single session. Scott electrode in vagina, large indifferent electrode under buttocks. Current up to 40 v, 10‐50 Hz for 20 min B (n = 54) sham treatment. Single session. Vaginal electrode but no current | |
| Outcomes | Participants with no improvement in frequency of incontinence: A 16/45. B 18/49 Participants not dry: A 37/45. B 43/49 Participants with no improvement in pad changes: A 27/45. B 31/49 Participants with no improvement in objectively measured pelvic floor control: A 23/45. B 23/49 Participants with no improvement in incontinence: A 18/45. B 16/49 | |
| Notes | Not useable because data not presented by SUI/UUI/MUI groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Allocated at random into trial and control groups." |
| Allocation concealment (selection bias) | Low risk | "a sealed envelope was opened stating which group the patient was in" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants blinded. Other blinding not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "Patients’ subjective statements were recorded by a single observer who was unaware of the treatment allocation" |
| Incomplete outcome data (attrition bias) | Unclear risk | No differential dropout. No explanation reported for withdrawals |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Sample size: not reported Follow‐up: 6 months | |
| Participants | N: 40 randomised, 15 analysed Mean (SD) age: not reported Sex: women Inclusion criteria: genuine stress incontinence or DO Exclusion criteria: not reported | |
| Interventions | A (n = 6 SUI, 4 DO) ES. Intra‐vaginal cushion attached to stimulator worn around the waist. Cushion worn for 8 h per 24, night or day according to participant preference. Stimulation: 50 Hz (SUI participants), 10 Hz (DO participants) B (n = 3 SUI, 2 DO) sham ES. Identical device to Group A but not activated | |
| Outcomes | Subjective and objective improvement in symptoms | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | All participants given identical devices but unaware which were activated. "The code was held by the manufacturer and only broken when the trial was completed." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawal per group not reported. Substantial withdrawal overall: 15/40 completed trial |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: June 2013 and December 2014 Sample size: not reported Follow up: 4 weeks’ treatment, then 4 weeks’ follow‐up | |
| Participants | N: 22 randomised, 19 analysed Mean (SD) age: 59 (7.9) Sex: 9 men, 10 women Inclusion criteria: people with idiopathic OAB symptoms who had not responded or could not tolerate (due to side effects) conventional drug therapy, Exclusion criteria: not reported | |
| Interventions | A (n = 7 analysed) ES with unilateral PTNS with conventional TENS* machine using a pair of adhesive surface electrodes and a stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles. Electrodes placed above and below the medial malleolus on the right ankle B (n = 6 analysed) ES with bilateral PTNS. Electrodes placed in same position as unilateral stimulation group but on both ankles. C (n = 6 analysed) sham stimulation, electrodes placed on the anterior aspect of the left shoulder | |
| Outcomes | Decrease in micturitions per 24 h (mean, 95%CI, N): A 1.7 (‐9 to 3.7), 7. B 2.8 (‐6.7 to 1.1), 6. C 0.7 (‐2.1 to 6.3) Decrease in urgency episodes (mean, 95%CI, N): A 1.3 (‐5.0 to 2.2), 7. B 3.2 (‐8.5 to 2.1), 6. C 0.7 (‐5.0 to 3.7) Number of responders (defined as > 30% reduction in daily micturitions and/or urgency episodes, and self‐reported subjective improvement: A 3/7. B 2/6. C 1/6 | |
| Notes | *no explanation given for TENS abbreviation "Initial effects were reported after the first week of the therapy in all responders. In the majority of responders the effects ceased at the follow‐up visit, four weeks after therapy had finished." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated" |
| Allocation concealment (selection bias) | Low risk | "opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded. “The participants were unaware that one of the stimulation groups was considered as a placebo group.” "The researcher who provided the training to participants was not blinded…data were recorded only by the participants" |
| Blinding of outcome assessment (detection bias) | Low risk | "the research team did not interact with participant’s outcome questionnaires and bladder diary, and data were recorded only by the participants" |
| Incomplete outcome data (attrition bias) | Unclear risk | Per‐protocol analysis. No reasons given for participant withdrawal |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Department of Urology, Lahey Clinic, Burlington, Massachusetts, USA Period: October 1992‐ January 1994 Sample size: not reported Follow‐up: 16 weeks | |
| Participants | N: 57 randomised in total. 38 with DI randomised and analysed Mean age (range): A 65 (45‐82) B 60 (44‐73) Sex: Women Inclusion criteria: genuine SUI or DI Exclusion criteria: type 3 SUI, pregnancy, history of prolonged urinary retention, vaginal vault prolapse, diminished sensory perception or cardiac pacemaker | |
| Interventions | A (n = 20) propantheline bromide 7.5 mg to 45 mg 2‐3 times daily ("or until side effects prevented its continuance") for at least 4 months B (n = 18) ES. 5‐s impulse time, duty cycle 1‐2, increasing monthly treatment time from 15, 30, 45 and 60 min. Amplitude started at 5 mA and did not exceed 25 mA. Twice daily for 4 months | |
| Outcomes | Number of participants cured (defined as cessation of incontinence and no longer requiring pads): A 3/20. B 4/18 Number of participants with objective improvement (defined as reduction of ≥ 50% in episodes and pads, and ≤ 10 voiding episodes per 24 hours): A 7/20. B 9/18 Number of participants with no improvement: A 10/20. B 5/18 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomized to 1 of 2 treatment arms" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants. Blinding of others not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: cross‐over RCT Multicentre or single‐centre: single‐centre Setting: University of New South Wales, New South Wales, Australia Period: not reported Sample size: the study was designed to obtain a type 1 error of 5% and a power of 85% which gave a sample size of 35 Follow‐up: 6 weeks | |
| Participants | N: 43 randomised and analysed Mean (SD) age: 50 (15) Sex: 13 men, 30 women Inclusion criteria: history of frequency, urgency and urge incontinence with no previous treatment for at least 6 months Exclusion criteria: not reported | |
| Interventions | A (n = 43) oxybutynin 2.5 mg twice daily, titrated to 5 mg 3 times daily by day 7 B (n = 43) TENS 20 Hz, pulse width 0.2 ms on a continuous mode up to 6 hours daily for 6 weeks All participants had washout period of 2 weeks then 6 weeks of the other treatment. | |
| Outcomes | Number of daily voids (mean, SD, N): A 9 (5), 43. B 9 (4), 43 Number of participants with no subjective improvement: A 30/40. B 29/38 Total bladder capacity (mL) (mean, SD, N): A 303.3 (142.5), 43. B 222.1 (99.2), 43 Volume at first desire to void (mL) (mean, SD, N): A 191.8 (130.1), 43. B 117.4 (84.7), 43 Residual volume (mL) (mean, SD, N): A 81.3 (81.3), 43. B 38.9 (55.03), 43 Volume at instability (mL) (mean, SD, N): A 180.9 (92.8), 43. B 96.3 (55.9), 43 Number of participants with > 25% improvement in bladder capacity: A 6/43. B 2/43 Number of participants with > 25% improvement in daily voids: A 21/43. B 24/43 Number of participants with side effects (N unclear): Dry mouth: A 87.2% (37/43). B 6.2% (3/43) Blurred vision: A 52.6% (23/43). B 6.2% (3/43) Dry skin: A 29.7% (13/43). B 6.2% (3/43) Skin irritation: A 25.6% (11/43). B 28.1% (12/43) Cost per participant: A oxybutynin £15.00 for 6 weeks B ES, including consumables, £60 for 6 weeks | |
| Notes | N assumed to be 43 unless otherwise stated Data not useable. Cross‐over design requires paired difference and SD for each outcome but paper reports insufficient data for analysis Contacted study author asking for further data 26 January 2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized to initial treatment with either transcutaneous electrical nerve stimulation or oxybutynin. After a washout period of 2 weeks, patients were started on the second arm of treatment" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants. Blinding of others not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if data available for all participants. Also risk of carry‐over effect is unclear |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: USA Period: not reported Sample size: not reported Follow‐up: 8 days | |
| Participants | N: 50 randomised and analysed Mean (SD) age: 57 Sex: not reported Inclusion criteria: OAB Exclusion criteria: not reported | |
| Interventions | A (n = 15) ES, no tub bathing or exercise. Horizontal placement of electrode patch near sacral nerve B (n = 15) ES, no tub bathing or exercise. 30º angle placement of electrode patch near sacral nerve C (n = 5) ES, with daily tub bathing or swimming. Horizontal placement of electrode patch near sacral nerve D (n = 5) ES, with daily tub bathing or swimming. 30º angle placement of electrode patch near sacral nerve E (n = 5) ES, with daily 30‐min exercise regimen. Horizontal placement of electrode patch near sacral nerve F (n = 5) ES, with daily 30‐min exercise regimen. 30‐degree angle placement of electrode patch near sacral nerve | |
| Outcomes | Adverse effects: 1 participant (not reported by group) Patch awareness, discomfort, bother, 1‐10 VAS (mean, SD), N): A + B: 1.4 (1.1), 30. C + D: 1.2 (0.9), 10. E + F: 1.3 (1.0), 10 | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized to one of two sacral placement angles" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Data not reported per group |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Brazil Period: August 2008–May 2010 Details of sample size calculation: "a prior power calculation…even after dropout, 80% sample power was kept (post hoc analysis)" Follow‐up: 12 weeks' treatment, 6 months' follow‐up | |
| Participants | N: 75 randomised, 58 analysed Mean (range) age: A 56.9 (33‐77). B 57.7 (34‐79). C 60.1 (33‐77) Sex: women Inclusion criteria: clinical complaints of OAB: urinary frequency, nocturia, and/or urgency incontinence with negative urinalysis and urine culture Exclusion criteria: previous treatment, residual urine, cognitive and psychiatric deficits, pregnancy, glaucoma, SUI, any pelvic organ prolapse quantification system (POPQ) C grade II, neurogenic OAB, those using anticholinergic drugs, calcium antagonists, b‐antagonists, and dopamine antagonists | |
| Interventions | A (n = 25) ES of posterior tibial nerve using Neurodyn Portable. 10 Hz frequency, pulse width of 250 µs. Two 30‐minute sessions per week for 12 weeks. B (n = 25) slow release oxybutynin 10 mg, once daily for 12 weeks C (n = 25) multimodal treatment, A + B | |
| Outcomes | Frequency (mean*, N): 12 weeks: A 8, 18. B 7.9, 19. C 7.6, 21. (P = 0.75) 24 weeks: A 7.9, 18. B 9.2, 19. C 7.8, 21 (P = 0.51) Participants with urinary incontinence: 12 weeks: A 11% (2/18). B 31% (6/19). C 19% (4/21) 24 weeks: A 14% (3/18). B 34% (6/19). C 18% (4/21) Participants with nocturia: 12 weeks: A 11% (2/18). B 5% (1/19). C 14% (3/21). (P = 0.24) 24 weeks: A 13% (2/18). B 15% (3/19). C 14% (3/21). (P = 0.51) International Consultation on Incontinence Questionnaire (ICIQ‐SF) score (mean*, range, N): 12 weeks: A 7.2 (0‐18), 18. B 9.8 (0‐18), 19. C 7.9 (0‐14), 21 24 weeks: A 8.3 (0‐20), 18. B 13.3 (8‐20), 19. C 7.4 (0‐14), 21 ICIQ‐OAB (mean*, range, N): 12 weeks: A 5.9 (1‐11), 18. B 4.6 (0‐10), 19. C 2.9 (0‐5), 21 24 weeks: A 6.1 (1.‐12), 18. B 9.2 (4‐13), 19. C 3.0 (0‐5), 21 Bother: 0‐10 analogue scale (mean, range, N): 12 weeks: A 3.9 (0‐8), 18. B 3.4 (0‐9), 19. C 1.7 (0‐4), 21 24 weeks: A 4.2 (0‐8), 18. B 7.0 (2‐10), 19. C 1.6 (0‐4), 21 | |
| Notes | *SD not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were divided randomly into three groups using online randomization" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible to blind participants. Personnel not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | "Patients who failed to comply with the 12 weeks of treatment (Week 12) and/or did not attend the reassessment after treatment (Week 24) at 6 months follow‐up were excluded from analysis." No differential withdrawal. No reasons given for withdrawals |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre. Setting: Vrije University Medical Center Amsterdam, the Netherlands Period: January 1996 and May 1998 Sample size: 75 participants for this study (alpha = 5%, beta 10%, estimated difference = 10%) Follow‐up: 8 weeks | |
| Participants | N: 72 enrolled, 37 randomised, 35 analysed Sex: women Median age (range): A 72 (65‐92); B 74 (66‐86) Inclusion criteria: women ≥ 65 with symptoms of SUI, UUI or MUI for ≥ 3 months, urinary leakage of 10 cc or more per 24 h Exclusion criteria: persistent UTI (positive urine culture after antibiotic treatment), recurrent UTI (within 4 weeks after treatment), bladder pathology or dysfunction because of fistula, tumour, pelvic irradiation, neurological or other chronic conditions (diabetes mellitus, Parkinson's disease), any incontinence treatment during the past 6 months, genital prolapse to, or beyond, the introitus, having a pacemaker, and insufficient mental condition/cognition | |
| Interventions | A (n = 25) ES. Three 30‐min sessions, with 5 min rest between each 15 min of treatment, per week for 8 weeks. Frequency 50 Hz for predominant SUI and 20 Hz for predominant UUI. 2‐s contraction time and duty cycle of 1–2 s, stimulation intensity gradually increasing up to the level of tolerable discomfort (0‐100 mA) B (n = 12) PFMT. Verbal instructions on performing Kegel exercises at home for 8 weeks | |
| Outcomes | Urinary leakage per day (mg) (mean, range, N): A 65 (0‐489), 24. B 26 (4‐157), 11 Number of participants with no objective improvement: A 17/24. B 7/11 Pelvic muscle strength (mean, range, N): A 15.375 (1.75–40.00), 24. B 10.00 (3.25–23.00) Number of participants with DI defined as spontaneous detrusor contraction(s) of 15 cm H2O or more on (ambulant) urodynamic registration (ICS standard): A 14/24. B 5/11 Number of participants with no subjective improvement (measured with PRAFAB score): A 13/24. B 6/11 | |
| Notes | No useable data ‐ not presented by SUI/UUI/MUI participants Study authors contacted for data 09‐02‐2015 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation according to Pocock’s method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | There was one participant in each group lost to follow‐up. |
| Methods | Study design: quasi‐RCT Multicentre or single‐centre: not reported Setting: Slovakia Period: 2001 Sample size: not reported Follow‐up: 5 weeks | |
| Participants | N: 28 Sex: women Mean age (range): 54 (45‐63) Inclusion criteria: OAB without bladder outlet obstruction confirmed by urodynamic examination Exclusion criteria: not reported | |
| Interventions | A (n = 9) SANS ES (Stoller Afferent Neuro Stimulation). One 30‐min session per week for 5 weeks. Frequency 1 Hz, square impulse duration 0.1 ms, intensity 25 mA B (n = 10) oxybutynin 3 mg 3 times per day C (n = 9) no active treatment | |
| Outcomes | IPSS (mean, SD, N) A 6 (4), 9. B not reported. C not reported Incontinence Quality of Life Questionnaire (I‐QoL) score (mean, SD, N): A 68 (20), 9. B not reported. C not reported Behavioural Urge Score (BUS) (mean, SD, N): A 0.43 (0.16), 9. B not reported. C not reported Change in IPSS (mean, N): A 60%, 9. B 80%, 10. C 20%, 9 Change in I‐QoL (mean, N): A 100%, 9. B 90%, 10. C 25%, 9 Change in BUS (mean, N): A 30%, 9. B 30%, 10. C 5%, 9 Number of participants with no significant improvement in IPSS, IQoL, BUS: A 4/9. B not reported. C 9/9 Number of participants with adverse effects: A 0/9. B 2/10 (dry mouth). C not reported | |
| Notes | Only adverse events data were useable We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Nine randomly chosen females formed the group with SANS stimulation, ten females formed the oxybutynin group and nine females the group without treatment." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Finland Period: not reported Details of sample size calculation: not reported Follow‐up: 2 weeks’ treatment then 6 months’ follow‐up | |
| Participants | N: 80 randomised, unclear how many analysed Mean (SD) age: A women 42.2 (8.9). A men 45.3 (6.3). B women 45.7 (10.7). B men 41.8 (11.8) Sex: 50 women, 30 men Inclusion criteria: stable phase of MS, baseline Expanded Disability Score ≤ 6.5, LUTS, postvoid residual volume < 100 mL Exclusion criteria: pregnancy, cardiac pacemaker or any metallic implant near the treated area, history of pelvic malignancy, dementia or any nervous system disorder other than MS | |
| Interventions | A (n = 40) ES. 6 sessions over two weeks. Intravaginal electrodes for women, intra‐anal for men. 10 minutes of each frequency: 5‐10 Hz, 10‐50 Hz, 50 Hz (7 s pulse, 25 s pause), with 3 min rest in between. Currents at maximal tolerated intensity. After 6 ES sessions biofeedback used to teach PFME, participants advised to continue PFME 3‐5 times per week for ≤ 6 months B (n = 40 no active treatment | |
| Outcomes | Urgency, urine leakage, volume of urine loss, voiding need during daytime, slow urine flow, sensation of incomplete bladder emptying, need of assistance in emptying bladder | |
| Notes | No useable data: no outcomes reported by treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Men and women were separately randomized into a treatment group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No outcomes reported for control group |
| Methods | Study design: cross‐over RCT Multicentre or single‐centre: not reported Setting: Italy Period: June 2010‐October 2011 Sample size: not reported Follow‐up: approximately 6 months (40 days’ drug treatment, 6 weeks ES, with 3‐month washout period in between) | |
| Participants | N: 40 randomised, 30 analysed Sex: women Mean age (range): 62 (35‐81) Inclusion criteria: women with OAB syndrome Exclusion criteria: stress incontinence, UTI, neurological disease, bladder lithiasis, genital prolapse higher than stage II on POP‐Q system, uncontrolled narrow angle glaucoma, pelvic tumours, postvoid residual urine ≥ 100 mL, or previously treated with pelvic surgery, radiation therapy or antimuscarinic agents | |
| Interventions | A (n = 20) solifenacin succinate, 5 mg daily for 40 days. 3‐month washout period then percutaneous tibial nerve stimulation, 30‐min session twice a week for 6 weeks B (n = 20) reverse of group A | |
| Outcomes | Number of voids per 24 hours (mean, SD, N): Post‐SS: A 10 (2.1), 14. B 10.4 (1.8), 16 Post‐ES: A 8.5 (2.3), 14. B 9.4 (1.9), 16 Number of nocturia episodes: Post‐SS: A 1.9 (1.4), 14. B 2.1 (1.4), 16 Post‐ES: A 1.6 (1.3), 14. B 1.7 (0.9), 16 Number of urgency incontinence episodes: Post‐SS: A 2.6 (1.6), 14. B 2.7 (1.6), 16 Post‐ES: A 1.7 (1.3), 14. B 1.7 (1.5), 16 Voided volume (cc?) (mean, SD, N): Post‐SS: A 147.4 (27.5), 14. B 145.5 (29.6), 16 Post‐ES: A 157.5 (25.5), 14. B 156.1 (18.4), 16 QoL measured with Overactive Bladder Questionnaire Short Form (6 item OAB‐Q SF score (mean, (SD), N)) (lower score is better): Post‐SS: A 3.2 (1.1), 14. B 3.5 (1.2), 16 Post‐ES: A 2.7 (1.0), 14. B 3.0 (1.0), 16 QoL measured with Overactive Bladder Questionnaire Short Form (13 item OAB‐Q SF score (mean, (SD), N)) (lower score is better): Post‐SS: A 3.1 (1.1), 14. B 3.4 (1.2), 16 Post‐ES: A 2.9 (0.9), 14. B 2.9 (1.1), 16 Urgency measured with Patient Perception of Intensity of Urgency Scale (PPIUS score (mean, (SD), N)) (lower score is better): Post‐SS: A 2.7 (1.2), 14. B 2.7 (1.3), 16 Post‐ES: A 2.1 (0.9), 14. B 2.2 (1.1), 16 Improvement measured with Patient Global Impression of Improvement questionnaire (PGI‐I score [mean, SD, N]) (lower score is more improvement) Post‐SS: A 2.9 (1.1), 14. B 3.1 (1), 16 Post‐ES: A 2.1 (0.7), 14. B 2.3 (0.7), 16 | |
| Notes | We included data from first period of randomisation only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Follow‐up was performed by a physician who was not involved in the study |
| Incomplete outcome data (attrition bias) | Low risk | A: 2 participants withdrew due to side effects, 2 withdrew after SS due to improved symptoms, 2 refused to undergo further therapy B: 3 withdrew due to improved symptoms, 1 refused to undergo further therapy |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Bedford, UK Period: not reported Sample size: not reported Follow‐up: 12 weeks | |
| Participants | N: 22 randomised, 21 analysed Sex: not reported Mean age (range): 52.6 (28‐78) Inclusion criteria: symptoms of at least six months duration, clinical diagnosis of urgency, frequency syndrome and urodynamic findings of DO Exclusion criteria: not reported | |
| Interventions | A (n = 11) Stoller Afferent Nerve Stimulation (SANS) one 30‐minsession per week for 12 weeks. Stimulation of posterior tibial nerve with percutaneous needle, current up to 10 mA B (n = 10) sham treatment without nerve stimulation | |
| Outcomes | Number of participants with no improvement: A 2/11. B 10/10 | |
| Notes | ‐‐‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were computer randomised to either the treatment arm or as controls" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Only one participant discontinued the treatment. |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: not reported Period: not reported Sample size: not reported Follow‐up: not reported | |
| Participants | N: 146 randomised and analysed Mean age (range): 47 (17‐79) Sex: 35 men /111 women Inclusion criteria: urgency incontinence; idiopathic DI, SU, or DH secondary to either spinal injury, myelomeningocele, or multiple sclerosis Exclusion criteria: not reported | |
| Interventions | A (n = 74) transcutaneous neurostimulation. One session: electrode pads of a transcutaneous neurostimulator (Coba 208 neurostimulator unit, Tenscare Ltd., Surrey, UK) were affixed bilaterally to the skin overlying the S3 dermatomes (situated at the junction of buttock and upper thigh) in all participants. Standard urodynamic filling cystometry was performed via a dual‐lumen 7‐Ch fluid filled catheter system at a 50 mL/minute fill rate B (n = 72) sham treatment. Standard urodynamic filling cystometry was performed via a dual‐lumen 7‐Ch fluid filled catheter system at a 50 mL/minute fill rate. Electrode pads in place but without applying current | |
| Outcomes | Infused bladder volume (mL) at FDV (mean, SD, N): A 167.2 (11.3), 74. B 114.2 (10.7), 72 Detrusor pressure at FDV (mean, SD, N): A 8.4 (1.3), 74. B 9.4 (1.5), 72 Infused bladder volume (mL) at SDV (mean, SD, N): A 247.4 (12.8), 74. B 193.7 (18.4), 72 Detrusor pressure at SDV (mean, SD, N): A 10.9 (3.1), 74. B 10.6 (1.8), 72 Infused bladder volume (mL) at sensation of urgency (Urge) (mean, SD, N): A 331.5 (15.9), 74. B 255.4 (11.4), 72 Detrusor pressure at Urge (mean, SD, N): A 18.6 (3.2), 74. B 22.6 (5.3), 72 Maximum infused cystometric capacity (mL) (CMax) (mean, SD, N): A 404.2 (26.7), 74. B 315.9 (22.9), 72 Detrusor pressure at CMax (mean, SD, N): A 20.5 (3.2), 74. B 25.9 (3.5), 72 | |
| Notes | We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized into age‐ and gender‐matched control and study groups." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All participants completed the study and were included in the analysis |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Taiwan Period: July 2001‐December 2002 Sample size: on the basis of the outcome measures (including QOL assessment, bladder diary, participant perception of improvement and satisfaction with treatment, and the improvement rate of ES, PFMT, and BAPFMT, which was 49%, 82.39%, and 80.7%, respectively), the authors conducted a test with a significance level of 0.05 and power of 0.9 and anticipated that groups of equal size were required. The total sample size required was at least 109.5. Follow‐up: 12 weeks | |
| Participants | N: 120 randomised, 103 analysed Mean age: A 50.09; B 52.32; C 55.74 Sex: women Inclusion criteria: OAB symptoms for ≥ 6 months, 16‐75 years old, frequency of voiding ≥ 8 times per day, ≥ 1 urgency incontinence episode per day Exclusion criteria: pregnancy, deafness, neurologic disorders, diabetes mellitus, pacemaker or intrauterine device use, genital prolapse greater than Stage II of the International Continence Society grading system, residual urine greater than 100 mL, and UTI | |
| Interventions | A (n = 40) PFMT. At least 3 times daily, performed according to PERFECT scheme (power/endurance/repetition//fast contraction), B (n = 38) BAPFMT. Intravaginal electromyogram probe (Periform, Neen Health‐Care) twice per week, participants contracted or relaxed pelvic floor muscles according to visual EMG signals. Also encouraged to perform PFMT at home according to PERFECT scheme C (n = 42) ES. Two 20‐min sessions per week with intravaginal electrode (Periform, Neen HealthCare); biphasic, symmetric, pulsed current with frequency of 10 Hz, pulse width 400 µs, duty cycle of 10 s on, 5 s off, and intensity varying with patient tolerance (minimum 20‐63 mA, maximum 40‐72 mA) | |
| Outcomes | Number of participants with urgency incontinence (no improvement): A 21/34. B 17/34. C 17/35 Number of participants with no improvement in OAB: A 21/34. B 17/34. C 17/35 KHQ total score (mean, SD, N) (lower score is better): A 50.27 (171.42), 34. B 185.86 (176.57), 34. C 180.08 (176.03), 35 Data for all 9 KHQ domains available: see Table II | |
| Notes | Gives data for incontinence episodes per day but then states "We decided not to use this parameter as an outcome measure because of the large number of incomplete records, which could have resulted in a statistical bias." We contacted the main author of the study to clarify methodological aspects of the study and request further information. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "The allocation of the three study groups was undertaken by sequentially opening a sealed envelope, prepared by the Biostatistics Center for Chang Gung Medical College in blocks of 6" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants could not be blinded. "The physiotherapist conducted the regimens while unaware of the progress and outcomes of the interventions." |
| Blinding of outcome assessment (detection bias) | Low risk | "The principal investigator was not involved in any of the interventions and was unaware of the group allocation." |
| Incomplete outcome data (attrition bias) | Low risk | No differential dropout. Adequate explanation for dropouts |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Taiwan Period: July 2004‐November 2005 Sample size: on the basis of the reduction rate of urge incontinence after ES, oxybutynin, and placebo (51%, 7; 76%, 5; and 19%, 8 respectively), we conducted a test with a significance level of 0.05 and power of 0.95 and anticipated that groups of equal size were required. We concluded that at least 72 women were required Follow‐up: 12 weeks | |
| Participants | N: 74 randomised, 68 analysed Sex: women Mean age (SD): not reported Inclusion criteria: OAB ≥ 6 months, age 16‐80, in particular urinary urgency 4 times or more per day Exclusion criteria: pregnancy, neurologic disorders, diabetes mellitus, demand cardiac pacemaker or intrauterine device use, genital prolapse greater than Stage II of the International Continence Society grading system, a postvoid residual urine volume greater than 100 mL, overt SUI, a history of anti‐incontinence surgery, and UTI. | |
| Interventions | A (n = 25) ES. Two 20‐min sessions per week. Biphasic, symmetric, pulsed current with a frequency of 10 Hz, pulse width of 400 ms, duty cycle of 10 son and 5 s off, and intensity varying with participant tolerance (minimum 20‐63 mA and maximum 40‐72 mA) B (n =26) oxybutynin 2.5 mg, 3 times per day for 12 weeks C (n = 23) placebo tablets identical to oxybutynin, 3 times per day for 12 weeks | |
| Outcomes | No improvement in urgency: A 10/24. B 14/23. C 19/21 Daily voided volume (mL) (median, range, N): A 2270 (1210‐3106), 24. B 2100 (1619‐3200), 23. C 2305 (1351‐3221) 21 Pad count (median, range, N): A 0 (0‐2), 24. B 0 (0‐2.5), 23. C 1 (0‐3), 21 Urgency episodes per 24 h (median, range, N): A 1.0 (0.0‐12.3), 24. B 6 (0.5‐13), 23. C 7.4 (3.9‐13.4), 21 Frequency per 24 h (median, range, N): 7.8 (1.8‐13.0), 24. B 7.4 (2‐14), 23. C 10 (6.6‐16.3), 21 Nocturia episodes per night (median, range, N): A 0 (0‐3.0), 24. B 0 (0‐2.0), 23. C 1 (0‐3.6), 21 Urgency incontinence episodes per 24 h (median, range, N): A 0.5 (0‐2), 24. B 0 (0‐2), 23. C 1 (0‐2), 21 Change in daily voided volume (mL) (median, range, N): A 70 (‐216 to 1190), 24. B 10.5 (‐1031 to 962), 23. C ‐14.5 (‐590 to 413), 21 Change in pad count (median, range, N): A ‐0.9 (‐2.1 to 2), 24. B 0 (‐1 to 2), 23. C 0 (‐4 to 3), 21 Change in urgency episodes per 24 h (median, range, N): A ‐3 (‐14 to 0.5), 24. B ‐3 (‐12 to ‐0.1), 23. C ‐1.3 (‐10.5 to 2) Change in frequency per 24 h (median, range, N): A ‐3.0 (‐14 to 0.5), 24. B ‐2.15 (‐12.8 to 2.3), 23. C ‐0.75 (‐6.5 to 2.3) Change in nocturia episodes per night (median, range, N): A ‐0.8 (‐6.5 to 0.4), 24. B 0 (‐2 to 1), 23. C 0 (‐1.5 to 2) Change in urgency incontinence episodes per 24 h (median, range, N): A 0 (‐2 to 2), 24 B 0 (‐1 to 1), 23. C 0 (‐2 to 1), 21 | |
| Notes | Contacted study author December 2014 to clarify if this is different study from Wang 2009. Awaiting reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "The allocation of the three study groups was undertaken by sequentially opening a sealed envelope, prepared by the Biostatistics Center for Chang Gung Medical College in blocks of six for each patient" |
| Blinding of participants and personnel (performance bias) | Low risk | "For the pharmacotherapy groups, the patients and all investigators were unaware of the regimen they received from the central pharmacy of our hospital." Not possible to blind ES group |
| Blinding of outcome assessment (detection bias) | Low risk | "The principal investigator was not involved in any of the interventions and was unaware of the group allocation." "For the pharmacotherapy groups, the patients and all investigators were unaware of the regimen they received from the central pharmacy of our hospital." |
| Incomplete outcome data (attrition bias) | Unclear risk | "One woman in the ES group withdrew because of fear of the electricity. Three women in the oxybutynin group withdrew, all because of intolerable dry mouth. Two women in the placebo group withdrew because they felt no response." |
| Methods | Study design: RCT Multicentre or single‐centre: single‐centre Setting: Taiwan Period: July 2006‐November 2007 Sample size: calculations for the treatment and placebo groups were based on the assumption that participants in the treatment groups had a 0.76 probability and others in the placebo group had a 0.36 probability of achieving a better outcome (increased UFI). To achieve 0.80 power with 0.05 significance level, it required at least 24 participants in each group. Follow‐up: 12 weeks | |
| Participants | N: 73 randomised, 73 analysed Sex: women Mean age (SD): overall 53.14 (9.98); A 51.46 (9.92); B 54.92 (9.83); C 53.17 (10.30) Inclusion criteria: OAB for ≥ 6 months (symptom of urgency ≥ 3 times daily) Exclusion criteria: pregnancy, neurologic disorders, diabetes mellitus, demand cardiac pacemaker or intrauterine device use, genital prolapse greater than the ICS grading system stage II, overt SUI, a history of anti‐incontinence surgery, UTI and participants receiving any OAB treatment during the 14‐day washout/run‐in period preceding randomisation | |
| Interventions | A (n = 26) ES. Two 20‐min sessions per week for 12 weeks with intravaginal electrode (Periform, Neen HealthCare). Biphasic, symmetric, pulsed current with varying intensity B (n = 24) Oxybutynin. Three 2.5 mg per day for 12 weeks C (n = 23) placebo. 1 tablet identical to oxybutynin, 3 times per day for 12 weeks | |
| Outcomes | No improvement in urgency: A 9/26. B 12/24. C 20/23 Number of micturitions per 24 hours (median, range, N): A 7.05 (2.7, 12), 26. B 5.35 (1, 13.1), 24. C 8.8 (4.1, 13), 23 Number of incontinence episodes (median, range, N): A 0.85 (0, 2.8), 26. B 0.3 (0, 2.1), 24. C 0.8 (0, 4.3), 23 Number of urgency episodes (median, range, N): A 2.4 (0, 6.9), 26. B 3.05 (1, 8.1), 24. C 7.2 (3.5, 10.2), 23 Number of nocturia episodes per night (median, range, N): A 1.65 (0, 4.3), 26. 1.45 (0, 5.4), 24. C 3 (0.1, 4.1), 23 Change in number of micturitions per 24 hours (median, range, N): A 3.6 (−2.1, 7.2), 26. B 5.3 (−3.5, 10.9), 24. C 1.6 (−5.2, 7.7), 23 Change in number of incontinence episodes (median, range, N): A 0 (−2.8, 3.3), 26. B 0.4 (−0.3, 3.2), 24. C 0.2 (−2.5, 2.2), 23 Change in number of nocturia episodes per night (median, range, N): A 2.8 (−2.7, 7.8), 26. B. 2.35 (−3.1, 6.2), 24. C −0.3 (−6.2, 4.7), 23 Change in number of nocturia episodes per night (median, range, N): A 0 (−3.2, 3.5), 26. B 0.45 (−5.4, 3), 24. C 0 (−4.1, 2.7), 23 KHQtotal score (median, range, N): A 142.25 (‐11.5, 432.4), 26. B 104.75 (‐49.9, 383.8), 24. C 36.7 (‐137.2, 525), 23 All nine KHQ domains available: see Table 5 | |
| Notes | Contacted author to clarify if this study is study is separate from Wang 2006. Awaiting reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Predetermined computer‐generated randomization code’ was used. Participants were ‘assigned randomly in sequential order." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "The principal investigator was not involved in any of the interventions and was unaware of the group allocation." "For the pharmacotherapy groups, [groups B and C] the subjects and all investigators were unaware of the regimen they received from the central pharmacy of our hospital." Group C received "a placebo looking exactly the same as Oxybutynin." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Three patients in the ES and four each in the oxybutynin and placebo groups withdrew after randomisation, leaving 23 in the ES, 20 in the oxybutynin, and 19 in the placebo group who completed the study. Reasons for withdrawal not reported. ITT analysis carried out "based on the data obtained from initially randomized 73 subjects." |
| Methods | Study design: comparative (unclear if randomised) Multicentre or single‐centre: single‐centre Setting: UK Period: not reported Details of sample size calculation: not reported Follow‐up. not reported | |
| Participants | N: 40 recruited Mean (SD) age: not reported Sex: women Inclusion criteria: urodynamically proven idiopathic DO Exclusion criteria: not reported | |
| Interventions | A (n = ?) ES. Daily session at home for 6 weeks with intravaginal maximal electrical stimulator B (n = ?) terodiline 25 mg daily for 6 weeks | |
| Outcomes | Reduction in symptoms: urgency, frequency, urgency incontinence, stress incontinence | |
| Notes | No data reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: UK Period: not reported Sample size: not reported Follow‐up: 6 weeks | |
| Participants | N: 60 randomised Sex: women Mean age: not reported Inclusion criteria: urodynamically proved DI Exclusion criteria: not reported | |
| Interventions | A (n = 32) oxybutynin hydrochloride 5 mg B (n = 28) ES. 20‐min sessions. Participants taught to insert vaginal electrodes and gradually increase stimulus to just below level of discomfort. Frequency 20 Hz, current 0‐90 mA | |
| Outcomes | Adverse effects: A 7/32. B 0/28 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Sixty women were recruited and randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | Differential dropout: "Nine patients in the oxybutynin group failed to complete the full treatment period. In seven cases this was due to unacceptable drug side effects. All patients in the MES group completed six weeks therapy and all found the method of treatment acceptable." |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Japan Period: not reported Sample size: not reported Follow‐up: After 4‐week treatment, participants who were cured or improved were followed up monthly on the basis of the records in the frequency/volume chart to evaluate post‐stimulation effects. If the participant relapsed, the stimulation was repeated periodically in the same way using the same device until continence was regained. | |
| Participants | N: 68 randomised, 58 analysed Sex: 29 men and 39 women Mean age (range): 70 (35‐87) Inclusion criteria: urinary incontinence due to DO Exclusion criteria: not reported | |
| Interventions | A (n = 37) ES. Two 15‐min sessions per day for 4 weeksAlternating pulses of 10‐Hz square waves of 1‐ms pulse duration and a maximum output current of 60 mA, stimulation up to maximum tolerable level B (n = 31) sham device identical to active device but with no stimulus output | |
| Outcomes | Number of daytime voids (mean, N (SD not reported)): A 8, 32. B 7.5, 26 Number of nighttime voids (mean, N (SD not reported)): A 2, 32. B 2.3, 26 Number of leaks (mean, N (SD not reported)): A 1.2, 32. B 2.4, 26 Bladder capacity at first desire to void (mL) (mean, SD, N): A 174.2 (83.1), 32. B 130.0 (69.9), 26 Maximum cystometric capacity (mL) (mean, SD, N): A 285.0 (143.4), 32. B 182.9 (99.0), 26 Detrusor pressure at maximum sensation (cm H2O) (mean, SD, N): A 34.6 (12.5), 32. B 50.9 (29.8). 26 Number of pad changes per 24 hours (mean, SD, N): A 0.8 (1.2), 37. B 1.1 (2.0), 31 Urgency score (0‐3 scale: from 0 = none to 3 = very much) (mean, SD, N): A 1.7 (0.7), 37. B 2.0 (0.8), 31 Quality of life score (0‐3 scale: from 0 = delighted to 3 = mostly dissatisfied) (mean, SD, N): A 1.6 (0.7), 37. B 2.2 (0.9), 31 Number with DO: A 24/32, B 24/26 Number of participants with no improvement in DO: A 4/32 (FS1) . B 17/26 (FS2) Subjective impressions (very good or good, fair or not good): number of participants with fair or not good (i.e. not satisfied): A 13/32. B 17/26 Not cured (cure defined as "no incontinence on the frequency/volume chart and no detrusor overactivity according to cystometry") i.e. number of participants with UUI: A 25/32. B 25/26 Not improved (improvement defined as "if the frequency of the incontinence decreased by more than 50% compared with the baseline level or the cystometric capacity increased by more than 50 mL") i.e. number of participants with no improvement in UUI: A 6/26. B 19/28. (FS3) Adverse effects: A 2/37. B 2/31 | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to either the active or the sham device." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "The sham device was identical to the active device in appearance but with no stimulus output." 'Neither doctors, nurses, nor patients knew which device was active or sham." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No differential attrition. "Four patients (three in the active group and one in the sham group) did not return after the first visit, and four patients (two at both groups) discontinued because of disagreeable feelings or vaginal pain" |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: Japan Period: not reported Details of sample size calculation: not reported Follow‐up: single session | |
| Participants | N: 32 randomised and analysed Mean (SD) age: A 66.8 (11.4). B 57.1 (20.1). Overall 62.3 (16.6) Sex: 15 men, 17 women Inclusion criteria: DO Exclusion criteria: not reported | |
| Interventions | A (n = 17) functional ES. Alternating pulses of 10‐Hz square waves 1 ms duration, maximum output current 60 mA. Stimulation up to maximum tolerable level. Device designed for home use. Surface electrodes for men (dorsal part of penis), vaginal plug for women B (n = 15) functional magnetic stimulation. Magnetic coil on armchair seat; perineum positioned to feel highest contraction of vaginal/anal sphincter. Intensity gradually increased up to tolerable limit, continuous eddy current 10 Hz, maximum output at the 100% setting of at least 270 J | |
| Outcomes | Participants with DO: A 17/17. B 12/15 Bladder capacity at first desire to void, mL (mean, SD, N): A 220.4 (110.9), 17. B 225.1 (123.7), 15 Maximum cystometric capacity, mL (mean, SD, N): A 266.9 (151.0), 17. B 290.5 (146.3), 15 Detrusor pressure at maximum capacity, cmH20 (mean, SD, N): A 15.4 (10.5), 17. B 13.9 (15.4), 15 Amplitude of detrusor overactive contraction, cmH20 (mean, SD, N): A 51.3 (36.9), 17. B 51.5 (48.2), 15 Bladder compliance at maximum sensation, mL/ cmH20 (mean, SD, N): A 24.3 (18.3), 17. B 32.7 (25.6), 15 Adverse effects: A 0/17. B 0/15 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | "using envelopes containing a card indicating FES or FMS" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants included in analysis. No withdrawals reported |
AUASI: Americal Urological Association Symptom Index
BAPFMT: biofeedback‐assisted pelvic floor muscle training
BMI: body mass index
B‐SAQ: Bladder Self‐assessment questionnaire
CI: confidence interval
DH: detrusor hyperreflexia
DI: detrusor instability
DO: detrusor overactivity
ES: electrical stimulation
FDV: volume at first desire
FES: functional electrical stimulation
GSUI: stress urinary incontinence
HRT: hormone replacement therapy
ICIQ: International Consultation on Incontinence questionnaire (SF: short form)
ICS: International Continence Society
ITT: interferential therapy
ITT analysis: intention‐to‐treat analysis
IQR: interquartile range
KHQ: King's Health Questionnaire
LUTS: lower urinary tract symptoms
MS: multiple sclerosis
MUI: mixed urinary incontinence
OAB: overactive bladder
PFME: pelvic floor muscle exercises
PFMT: Pelvic floor muscle training
QoL: quality of life
RCT: randomised controlled trial
SANS: Stoller Afferent Neuro‐stimulation
SD: standard deviation
SDV: strong desire to void
SU: sensory urge
SUI: stress urinary incontinence
TENS: transcutaneous electrical nerve stimulation
UI: urinary incontinence
UTI: urinary tract infection
UUI: urgency urinary incontinence
VAS: visual analogue score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not non‐implanted device | |
| Not OAB | |
| Not OAB | |
| Not non‐implanted device | |
| Not OAB | |
| Not RCT | |
| Not non‐implanted device | |
| Not OAB | |
| Not RCT | |
| Not electrical stimulation | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not non‐implanted device | |
| Not RCT | |
| Not OAB | |
| Not RCT | |
| Not electrical stimulation | |
| Not electrical stimulation | |
| Not OAB | |
| Not RCT | |
| Not RCT | |
| Not OAB | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not electrical stimulation | |
| Not RCT | |
| Ineligible intervention | |
| Not RCT | |
| Not RCT | |
| Not non‐implanted device | |
| Not OAB | |
| RCT of PFMT + ES + oestrogen versus ‘wait’ group. Women have SUI or undefined UI, but no definite diagnosis of OAB. | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Ineligible comparison | |
| Ineligible intervention | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not OAB | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not OAB | |
| Not OAB | |
| Not OAB | |
| Not non‐implanted device | |
| Not RCT | |
| Not RCT | |
| Not electrical stimulation | |
| Not electrical stimulation | |
| Not OAB | |
| Not non‐implanted device | |
| Withdrawn prior to enrolment | |
| Not non‐implantable device | |
| Not non‐implanted device | |
| Not OAB | |
| Not RCT | |
| Not OAB | |
| Ineligible comparator | |
| Ineligible comparator | |
| Not OAB | |
| Not OAB | |
| Not OAB | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Ineligible comparator | |
| Not OAB | |
| Not RCT | |
| Not OAB | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not OAB | |
| Not RCT | |
| Not non‐implanted device | |
| Not non‐implanted device | |
| Not electrical stimulation | |
| Not RCT | |
| Not OAB | |
| Unclear if RCT/OAB | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not electrical stimulation | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Not RCT | |
| Ineligible intervention and comparator is urodynamic evaluation only | |
| Not electrical stimulation | |
| Ineligible intervention | |
| Not non‐implanted device | |
| Not RCT | |
| Not electrical stimulation | |
| Not electrical stimulation | |
| Not electrical stimulation | |
| Not RCT | |
| Not RCT | |
| Not OAB | |
| Not RCT | |
| Not RCT | |
| Not RCT |
ES: electrical stimulation
OAB: overactive bladder
PFMT: pelvic floor muscle training
RCT: randomised controlled trial
SUI: stress urinary incontinence
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Electromagnetic Stimulation for the treatment of urge urinary incontinence and overactive bladder (ELEC STIM) |
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: USA Follow‐up: unclear |
| Participants | N: 130 Sex: women Inclusion criteria: age 18 +, UUI, urinary frequency Exclusion criteria: primary complaint of stress incontinence, neurogenic bladder, overflow incontinence, functional incontinence |
| Interventions | A: Electrical field stimulation device B: Sham nerve stimulation device |
| Outcomes | Reduction of incontinence episodes Serious adverse events or unanticipated adverse device effects |
| Starting date | October 2011 |
| Contact information | |
| Notes | Study terminated. Contacted manufacturer 20 February 2015 |
| Trial name or title | Peripheral Electrical Stimulation for the Treatment of Overactive Bladder (PESTOB) |
| Methods | Study design: RCT Multicentre or single‐centre: Setting: Follow‐up: 4 weeks |
| Participants | N: 36 Sex: men and women Inclusion criteria: at least 18 years of age, documented symptoms of idiopathic OAB for at least 3 months, failure of primary OAB treatment, such as behaviour modification or fluid/diet management, participants can remain on stable medication, willing and capable of understanding and complying with all requirements of the protocol Exclusion criteria: urinary retention or post voiding residual greater than 100 mL, clinically significant bladder outlet obstruction, stress predominant MUI, neurological disease affecting urinary bladder function, including but not limited to Parkinson's disease, multiple sclerosis, stroke, spinal cord injury, pelvic surgery (such as sub‐urethral sling, pelvic floor repair) within the past 6 months, de novo OAB following pelvic surgery, sub‐urethral sling, intravesical or urethral sphincter. Botulinum Toxin Type A injections within the past 6 months, PTNS therapy for overactive bladder within the past 6 months, any form of ES to the pelvis or lower limbs within 4 weeks, vaginal prolapse greater than Stage II in the anterior compartment of the vagina using ICS Pelvic Organ Prolapse Quantification (POPQ) criteria, prior periurethral or transurethral bulking agent injections for bladder problems within the past 12 months, history of pelvic radiation therapy, any skin conditions affecting treatment sites, lacking dexterity to properly utilise the components of the stimulator system, presence of an implanted electro‐medical device (e.g. pacemaker, defibrillator, InterStim®, etc), pregnant, nursing, suspected to be pregnant (by urine pregnancy method), or plans to become pregnant during the course of the study, recurrent UTI (> 3 UTI's in the past year), history of, or current, lower tract genitourinary malignancies, any clinically significant systemic disease or condition that in the opinion of the Investigator would make the patient unsuitable for the study, any other clinical trial within 6 months |
| Interventions | A: Unilateral PTNS. 40 min every day for a duration of 4 weeks. The participant places the cathode electrode above, and the anode electrode behind the medial malleolus, over the posterior tibial nerve and sets the stimulation intensity to a comfortable level. B: Bilateral PTNS. 40 min every day for a duration of 4 weeks. The participant places the cathode electrode above, and the anode electrode behind the medial malleolus, over the posterior tibial nerve on both legs and sets the stimulation intensity to a comfortable level. C: Shoulder stimulation. 40 min every day for a duration of 4 weeks. The participant places the cathode and the anode electrodes on the lateral side of the left shoulder. |
| Outcomes | Change in frequency of voiding Change in Patient Perception of Bladder Condition (PPBC) Changes in symptom severity score and health‐related quality of life score (HRQL) based on OAB‐Q Changes in the mental/physical scores of RAND36 Change in urinary symptoms score and bother symptom score based on the ICIQ‐OAB questionnaire |
| Starting date | March 2013 |
| Contact information | Martin Slovak [email protected] |
| Notes | Contacted February 2015. Manuscript due for submission shortly. |
| Trial name or title | Comparison of posterior tibial nerve electrical stimulation protocols for overactive bladder syndrome |
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: Brazil |
| Participants | N: 145 Sex: women Inclusion criteria: age 18 +, cognitive level adequate for understanding orientations during treatment; clinical diagnosis of OAB syndrome for at least six months prior to the study Exclusion criteria: pregnant women or women who wish to get pregnant; neurological disease; urinary infection; nephrolithiasis; SUI; MUI; women in pharmacological treatment for OAB; women undergoing hormone replacement therapy in the last 6 months; peripheral neuropathy; cystocoele stage two or higher |
| Interventions | A: Placebo: electrodes will be fixed to one leg and sessions will be held once a week B: ES on 1 leg once a week C: ES on 1 leg twice a week D: ES on 2 legs once a week E: ES on 2 legs once a week F: ES on 2 legs twice a week |
| Outcomes | Change in urinary frequency in 12 sessions Number of micturitions per day Change in nocturia in 12 sessions Number of micturitions per night, interrupting sleep Change in urinary urgency in 12 sessions Number of urgent micturitions per day Change in urinary urge‐incontinence in 12 sessions Number of leaks per day |
| Starting date | March 2012 |
| Contact information | Nanci Valeis [email protected] PI Munick L Pierre |
| Notes | Currently recruiting participants |
| Trial name or title | Electrical nerve stimulation for overactive bladder a comparison of treatments |
| Methods | Study design: RCT Multicentre or single‐centre: unclear Setting: USA |
| Participants | N: 114 Sex: women Inclusion criteria: Female age >18 years, predominant complaint urge urinary incontinence (3 or more episodes per week) OR overactive bladder (8 or more voids per day, and/or 2 or more voids per night), failed trial of conservative therapy (bladder training, fluid modification, diet modification, caffeine restriction, pelvic floor training), failed trial of anticholinergic either due to inability to take the medication, adverse reaction to medication, or no improvement on medication, willing and mentally competent to participate in study, willing to complete study questionnaires, no contraindications to undergoing percutaneous tibial nerve stimulation or TENS therapy. Exclusion criteria: Age < 18 years, presence of urinary fistula, recurrent or current urinary tract infection (5 or more infections in the last 12 months), bladder stones, bladder cancer or suspected bladder cancer, haematuria, pregnancy or planning to become pregnant during the study (urine pregnancy test will be administered to those who are premenopausal and who have not had a hysterectomy), central or peripheral neurologic disorders such as multiple sclerosis, Parkinson's disease, spina bifida, or other spinal cord lesion, metal implants such as pacemaker, implantable defibrillator, or metal implants where percutaneous tibial nerve stimulation or TENS device needs to be placed (sacrum or ankle/leg), uncontrolled diabetes, diabetes with peripheral nerve involvement, anticoagulants, current use of anticholinergics or use within the last 4 weeks, current use of botulinum toxin bladder injections or bladder botulinum toxin injection within the last year, current use of InterStim® therapy or currently implanted InterStim® device or leads, bladder outlet obstruction, urinary retention or gastric retention, painful bladder syndrome/interstitial cystitis |
| Interventions | A: PTNS once weekly for 30 min for 12 weeks. If at 12 weeks participants are considered to have a positive response to therapy, they will continue maintenance therapy in a tapered fashion: participants will come in every 2 weeks for the next 8 weeks for 30‐mintreatments (4 visits total), then every 3‐4 weeks for 30‐min treatments for the remaining 32 weeks of the year (8‐10 visits) B: TENS. Home TENS device (EMPI TENS Select) and for self‐treatment daily for 2 h per day (1 h in the morning and 1 h in the evening) for 12 weeks. If considered to have a positive response with TENS treatment at 12 weeks, participants will continue by weaning use over a 3‐month time period, beginning with 3 x per week for 1 month, then 2 x per week for 1 month, then 1 x per week for 1 month, all at 2 h per day |
| Outcomes | Success at 1 year, defined as a 50% or more reduction in the total number of incontinence episodes, or a 25% or more reduction in number of daily or nightly voids AND that the participant continues to use the therapy at 1 year. Therefore primary response is: 50% reduction in incontinence, OR 25% reduction in nightly voids AND continued use of therapy at 1 year. Participant compliance defined as 75% adherence to the recommended use for each device Changes in the OAB‐Q Changes in urodynamic studies |
| Starting date | October 2013 |
| Contact information | PI Mary E McVearry Shannon Lamb, Physician, Walter Reed National Military Medical Cente |
| Notes | Due to complete December 2016 |
| Trial name or title | |
| Methods | RCT Setting: Israel Follow‐up: 12 weeks |
| Participants | Estimated enrolment: 40 Inclusion criteria
Exclusion criteria: children, people who were unable to or did not sign an informed consent or do not understand the study design and the treatment, implanted electric devices (e.g. cardiac stimulators etc.), post voiding residual more than 100 mL, neuropathic OAB or pelvic ongoing malignancy or prior pelvic radiation, treated in the last 6 months with SNM, posterior tibial nerve stimulation or intravesical Botox injections, de novo OAB after recent implantation of tension‐free vaginal tape (TVT) procedure, SUI predominant complaints in people with MUI, significant pelvic organ prolapse in women or an evidence of significant bladder outlet obstruction in male patients, history of recurrent UTIs during the last 2 years, any medical condition that involves skin on the lower extremity, bilateral leg amputation, any medical condition that in the investigator's opinion could have an adverse impact on the participant during the study, participation in a clinical study at the last 6 months |
| Interventions | TENS at posterior tibial nerve area Sham comparator: TENS at shoulder area |
| Outcomes | Day and night‐time frequency of micturitions OAB‐Q Participant perception of bladder condition (PPBC) Participant perception of global improvement (PPGI) Quality of life 5 dimensions (EQ5D) |
| Starting date | April 2014 May 2015 ‐ study withdrawn prior to enrolment |
| Contact information | Michael Vainrib, M.D. [email protected] |
| Notes | Estimated Study Completion Date: |
| Trial name or title | |
| Methods | RCT Setting: China Follow‐up: 4 weeks' treatment, 1 year follow‐up |
| Participants | N = 80 Inclusion criteria
Exclusion criteria
|
| Interventions | Electrical pudendal nerve stimulation at a frequency of 2.0 Hz and a moderate intensity (25˜35 mA); 60 min 3 times a week for a total of 4 weeks Transvaginal ES at a current intensity of < 60 mA (as high as possible to get a contraction) and frequencies of 15 Hz and 85 Hz (alternate 3‐min periods of stimulation); 20 min 3 times a week for a total of 4 weeks |
| Outcomes | Severity of UUI symptoms 24‐hour urine leakage amount |
| Starting date | December 2014 |
| Contact information | Xiaoming Feng, Ph.D [email protected] |
| Notes | Estimated Study Completion Date: Jan 2016 |
| Trial name or title | |
| Methods | RCT Setting: UK Follow‐up: 6 months |
| Participants | N = 24 Inclusion criteria
Exclusion criteria
|
| Interventions | Percutaneus Stimulation PTNS performed bilaterally every 4 weeks within the Physiotherapy Department. Transcutaneous Stimulation TPTNS applied bilaterally, using two surface, self‐adhesive, round electrodes (3 cm in diameter) in each leg at least 3 times per week |
| Outcomes | Symptom severity measured by OAB‐Q Changes in 24‐hour micturition frequency Mean number of micturition episodes recorded in 3‐day bladder chart |
| Starting date | February 2014 |
| Contact information | Louise Hardman [email protected] |
| Notes | Due to complete: Feb 2016 |
| Trial name or title | |
| Methods | RCT Setting: Brazil Follow‐up: 8 weeks' treatment, 3 months' follow‐up |
| Participants | N = 30 Inclusion criteria ‐ women with UUI or MUI older than 18 years Exclusion criteria
|
| Interventions | Transcutaneous electrical stimulation of the tibial nerve at home Development of an innovative portable equipment, with domestic technology for home application of the posterior tibial nerve stimulation technique using the type SSP surface electrodes (Silver Spike Point). Frequency: 20 Hz, Pulse width: 200 us; duration: 15 minutes daily Active Comparator: "Pelvic Floor Exercises": This group will do pelvic muscle training 3 times a day . In dorsal decubitus posture, legs flexed and abductee. Perform pelvic floor contractions keeping 2 seconds and relaxing 4 seconds for 10 times, and contractions keeping 4 seconds and relaxing 8 seconds for 10 times |
| Outcomes | Number of participants with UUI |
| Starting date | January 2014 |
| Contact information | Magda Ms Aranchip [email protected] Luciana Dr Paiva [email protected] |
| Notes | Due to complete August 2015 |
| Trial name or title | |
| Methods | RCT Setting: Brazil Follow‐up: unclear |
| Participants | N = 12 Inclusion criteria
Exclusion Criteria
|
| Interventions | A: TENS: 2 self‐adhesive electrodes, one immediately behind the medial malleolus and the other 10 cm above will be used. Through a chain of 1 Hz, the aim is to correctly identify the tibial nerve. This position is confirmed with the rhythmic movement of the finger flexion. The frequency is then changed to 10 Hz, the pulse width set at 200 "microseconds" and adjusted according to the intensity threshold for each participant, below motor threshold. This current generator also has a device, the VIF (variation in intensity and frequency) that aims to ease the accommodation of sensory receptors and enhance its effects. The application time is 30 min B: Placebo: active current for 15 seconds by means of an apparatus also IBRAMED brand externally similar to that used in A. Two self‐adhesive electrodes, one immediately behind the medial malleolus and the other 10 cm above will be used. The application time 30 min |
| Outcomes | Parasympathetic and sympathetic system values obtained from heart rate variability (HRV) after TENS application |
| Starting date | March 2014 |
| Contact information | None given |
| Notes | Due to complete August 2014 |
| Trial name or title | |
| Methods | RCT Setting: Canada Follow‐up: 12 weeks |
| Participants | N = 60 Inclusion criteria
Exclusion criteria
|
| Interventions | Sham transcutaneous tibial nerve stimulation: transcutaneous stimulation in a location and with settings not related to the bladder nerves, 3 x/week for 30 min for 12 weeks. Patch electrodes applied posterior to the lateral malleolus, and 5‐10 cm above the lateral malleolus of the same leg. Bipolar stimulation setting will be used, with a frequency of 10 Hz, 200 ms pulse, and the amplitude will be set a 1 mA. This will be done by the participants at home 3 x/week for 30 min, over 12 weeks Transcutaneous tibial nerve stimulation. Patch electrodes applied posterior to the medial malleolus, and 5‐10 cm above the medial malleolus of the same leg, just behind the medial tibial edge. Bipolar stimulation setting will be used, with a frequency of 10 Hz, 200 ms pulse, and the amplitude will be titrated up to participant's maximum nonpainful tolerance (between 0.5‐10 mA). This will be done by the participants at home 3 x/week for 30 min, over 12 weeks |
| Outcomes | OAB‐Q SF Voiding diary 24‐hour pad weights Physician assessment of treatment benefit |
| Starting date | November 2015 |
| Contact information | None given |
| Notes | Due to complete Nov 2017 |
| Trial name or title | |
| Methods | RCT Setting: Canada Follow‐up: 3 months |
| Participants | N = 60 Inclusion criteria
Exclusion criteria
|
| Interventions | Sham tibial nerve stimulation Use of peripheral nerve stimulator in a location that will not actively stimulate the tibial nerve. Tibial nerve stimulation Transcutaneous peripheral nerve stimulator in a location that will actively stimulate the tibial nerve. Device: EV‐906 Digital Transcutaneous electrical nerve stimulation (TENS) machine Percutaneous patch electrodes are used to deliver low level electrical currents. |
| Outcomes | Neurogenic bladder symptom score questionnaire Qualiveen‐Short Form Questionnaire Participant‐reported urinary frequency, urgency, incontinence episodes 24‐hour incontinence pad weights Physician assessment of participant benefit |
| Starting date | December 2015 |
| Contact information | Mary McKibbon [email protected] |
| Notes | Due to complete Dec 2016 |
| Trial name or title | |
| Methods | RCT Setting: Brazil Follow‐up: 12 weeks |
| Participants | N = 30 Inclusion criteria
Exclusion criteria
|
| Interventions | Back Tibial Nerve Electrostimulation The BTNE will be made with electrodes Silver Spike Point (SSP) set in an ankle with the negative pole positioned on the inner malleolus and the positive approximately 0.5 cm below the previous, and connected to a portable stimulator powered by rechargeable battery developed by the Biomedical Engineering Department of the HCPA. Placebo ES |
| Outcomes | Hoehn and Yahr Disability Stage of scale |
| Starting date | July 2014 |
| Contact information | Tatiane Gomes de Araujo [email protected] |
| Notes | Due to complete December 2017 |
| Trial name or title | |
| Methods | Study design: RCT Multicentre or single‐centre: not reported Setting: the Netherlands Period: planned to be April 2009‐October 2010 Details of sample size calculation: not reported Follow‐up: 12 weeks |
| Participants | Inclusion criteria: OAB, defined as urgency and frequency (more than 8 voids per 24 h and the sudden urge to void can hardly be suppressed); urgency incontinence (urgency leading to urinary leakage occurring at least 3 times weekly); age > 18 years Exclusion criteria: symptoms existing for less than 6 months; pregnancy; active UTI or recurrent UTI; severe cardiopulmonary disease; diabetes with peripheral nerve involvement; neurological disorders; flowmetry < 15 mm/s; previous treatment for OAB |
| Interventions | 1. PTNS 2. Bladder training |
| Outcomes | Primary outcomes: ICIQ‐UI‐SF scores; percentage of 70% improvement on the ICIQ‐UI‐SF scores. Secondary outcomes: incontinence episodes per week; frequency of micturition per 24 h |
| Starting date | |
| Contact information | |
| Notes | Contacted study author asking for data 06 January 2015 |
DO: detrusor overactivity
ES: electrical stimulation
ICIQ: International Consultation on Incontinence Questionnaire
ICIQ‐UI SF: International Consultation on Incontinence Questionnaire‐Urinary Incontinence‐Short Form
MUI: mixed urinary incontinence
OAB: overactive bladder
RCT: randomised controlled trial
SUI: stress urinary incontinence
PTNS: posterior tibial nerve stimulation
TPTNS: transcutaneous posterior tibial nerve stimulation
TEVS: transcutaneous electrical nerve stimulation
UUI: urgency urinary incontinence
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured or improved Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.34, 2.55] |
| Analysis 1.1  Comparison 1 Electrical stimulation (ES) versus no active treatment, Outcome 1 Number of participants cured or improved. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured Show forest plot | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.39, 5.21] |
| Analysis 2.1  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 1 Number of participants cured. | ||||
| 2 Number of participants cured or improved Show forest plot | 10 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.85, 2.77] |
| Analysis 2.2  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 2 Number of participants cured or improved. | ||||
| 3 Number of participants cured or improved: different ES routes Show forest plot | 6 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [2.54, 4.96] |
| Analysis 2.3  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 3 Number of participants cured or improved: different ES routes. | ||||
| 3.1 Percutaneous tibial nerve stimulation | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [2.22, 4.58] |
| 3.2 Intravaginal | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.46 [2.33, 12.81] |
| 4 Number of participants satisfied Show forest plot | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.02, 2.04] |
| Analysis 2.4  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 4 Number of participants satisfied. | ||||
| 5 Number of participants with improvement in urgency urinary incontinence Show forest plot | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 5.03 [0.28, 89.88] |
| Analysis 2.5  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 5 Number of participants with improvement in urgency urinary incontinence. | ||||
| 6 Number of participants with improvement in urinary frequency Show forest plot | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.43, 2.92] |
| Analysis 2.6  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 6 Number of participants with improvement in urinary frequency. | ||||
| 7 Number of incontinence episodes per 24 h Show forest plot | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐1.92, ‐0.95] |
| Analysis 2.7  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 7 Number of incontinence episodes per 24 h. | ||||
| 8 Number of nocturia episodes Show forest plot | 2 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| Analysis 2.8  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 8 Number of nocturia episodes. | ||||
| 9 Number of micturitions per 24 h Show forest plot | 3 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.70, ‐0.47] |
| Analysis 2.9  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 9 Number of micturitions per 24 h. | ||||
| 10 Number of participants with adverse effects Show forest plot | 3 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.84, 1.83] |
| Analysis 2.10  Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 10 Number of participants with adverse effects. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured or improved Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.19, 2.14] |
| Analysis 3.1  Comparison 3 Electrical stimulation (ES) versus pelvic floor muscle training (PFMT), Outcome 1 Number of participants cured or improved. | ||||
| 2 Number of participants satisfied Show forest plot | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| Analysis 3.2  Comparison 3 Electrical stimulation (ES) versus pelvic floor muscle training (PFMT), Outcome 2 Number of participants satisfied. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinence episodes per 24 h Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐2.33, ‐1.35] |
| Analysis 4.1  Comparison 4 Electrical stimulation (ES) versus laseropuncture/electro‐acupuncture, Outcome 1 Number of incontinence episodes per 24 h. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured Show forest plot | 7 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.41] |
| Analysis 5.1  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 1 Number of participants cured. | ||||
| 1.1 ES versus tolterodine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.47] |
| 1.2 ES versus oxybutynin | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.72] |
| 1.3 ES versus propantheline bromide | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.38, 5.74] |
| 2 Number of participants cured or improved Show forest plot | 8 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.04, 1.38] |
| Analysis 5.2  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 2 Number of participants cured or improved. | ||||
| 2.1 ES versus tolterodine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.41] |
| 2.2 ES versus oxybutynin | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.91, 1.52] |
| 2.3 ES versus propantheline bromide | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.86, 2.44] |
| 3 Number of participants cured or improved: routes of ES Show forest plot | 5 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.04, 1.54] |
| Analysis 5.3  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 3 Number of participants cured or improved: routes of ES. | ||||
| 3.1 Transcutaneous posterior tibial nerve stimulation | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.92] |
| 3.2 Intravaginal/transanal | 4 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 4 Number of participants satisfied Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| Analysis 5.4  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 4 Number of participants satisfied. | ||||
| 4.1 ES versus oxybutynin | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 5 Number of incontinence episodes per 24 h Show forest plot | 5 | 477 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.11, 1.60] |
| Analysis 5.5  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 5 Number of incontinence episodes per 24 h. | ||||
| 5.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.49, 0.29] |
| 5.2 ES versus oxybutynin | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐6.45, 8.25] |
| 5.3 ES versus trospium + solifenacin | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 2.2 [1.78, 2.62] |
| 5.4 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 5.5 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| 6 Number of urgency episodes per 24h Show forest plot | 2 | 294 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.28, 0.96] |
| Analysis 5.6  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 6 Number of urgency episodes per 24h. | ||||
| 6.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.98, 0.78] |
| 6.2 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.35, 1.05] |
| 7 Number of micturitions per 24 h Show forest plot | 6 | 646 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.15, 0.52] |
| Analysis 5.7  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 7 Number of micturitions per 24 h. | ||||
| 7.1 ES versus tolterodine | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐1.06, 1.50] |
| 7.2 ES versus oxybutynin | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐0.18, 1.91] |
| 7.3 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.04, 0.84] |
| 7.4 ES versus oestrogen cream | 1 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.15, 0.52] |
| 8 Number of nocturia episodes per night Show forest plot | 4 | 367 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐2.27, ‐1.88] |
| Analysis 5.8  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 8 Number of nocturia episodes per night. | ||||
| 8.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 8.2 ES versus oxybutynin | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.35, 0.95] |
| 8.3 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.06, 0.66] |
| 8.4 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.8 [‐3.03, ‐2.57] |
| 9 Number of participants with adverse effects Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.9  Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 9 Number of participants with adverse effects. | ||||
| 9.1 ES versus oxybutynin | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.84] |
| 9.2 ES versus tolterodine | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.27] |
| 9.3 ES versus solifenacin succinate | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants satisfied Show forest plot | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.13, 2.20] |
| Analysis 6.1  Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 1 Number of participants satisfied. | ||||
| 2 Number of incontinence episodes per 24h Show forest plot | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.84, 0.64] |
| Analysis 6.2  Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 2 Number of incontinence episodes per 24h. | ||||
| 3 Number of urgency episodes per 24 h Show forest plot | 2 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐2.74, ‐2.24] |
| Analysis 6.3  Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 3 Number of urgency episodes per 24 h. | ||||
| 4 Number of micturitions per 24 h Show forest plot | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.62, 0.12] |
| Analysis 6.4  Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 4 Number of micturitions per 24 h. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 2 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.72, 0.72] |
| Analysis 7.1  Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 1 Quality of life. | ||||
| 2 Number of incontinence episodes per 24h Show forest plot | 2 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.63, ‐0.43] |
| Analysis 7.2  Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 2 Number of incontinence episodes per 24h. | ||||
| 3 Number of urgency episodes per 24 hours Show forest plot | 2 | 248 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.11, ‐1.54] |
| Analysis 7.3  Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 3 Number of urgency episodes per 24 hours. | ||||
| 4 Number of micturitions per 24 hours Show forest plot | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.22, 0.21] |
| Analysis 7.4  Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 4 Number of micturitions per 24 hours. | ||||

PRISMA study flow diagram
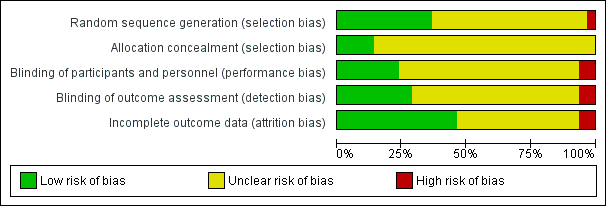
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Electrical stimulation (ES) versus no active treatment, Outcome 1 Number of participants cured or improved.
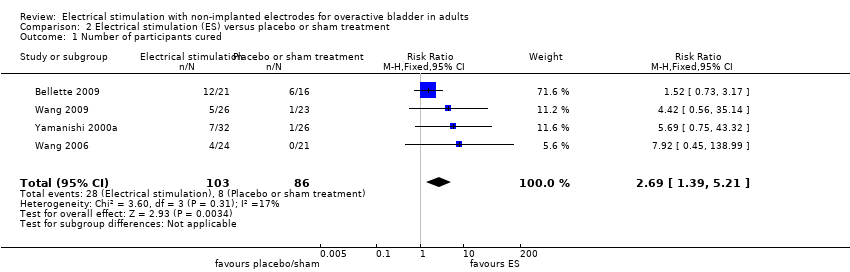
Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 1 Number of participants cured.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 2 Number of participants cured or improved.
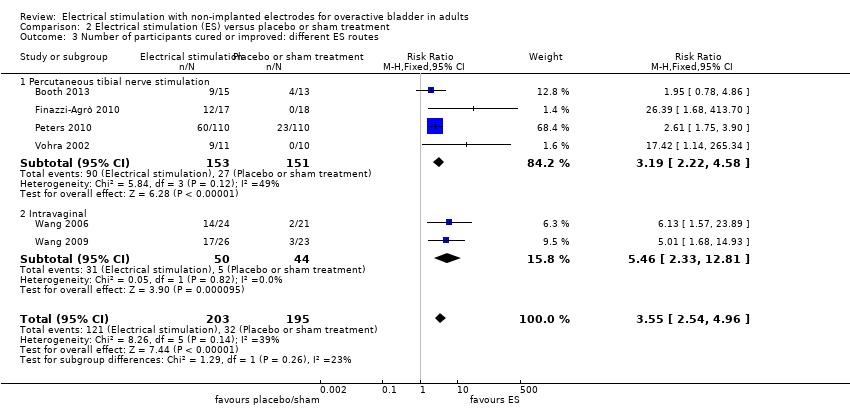
Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 3 Number of participants cured or improved: different ES routes.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 4 Number of participants satisfied.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 5 Number of participants with improvement in urgency urinary incontinence.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 6 Number of participants with improvement in urinary frequency.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 7 Number of incontinence episodes per 24 h.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 8 Number of nocturia episodes.
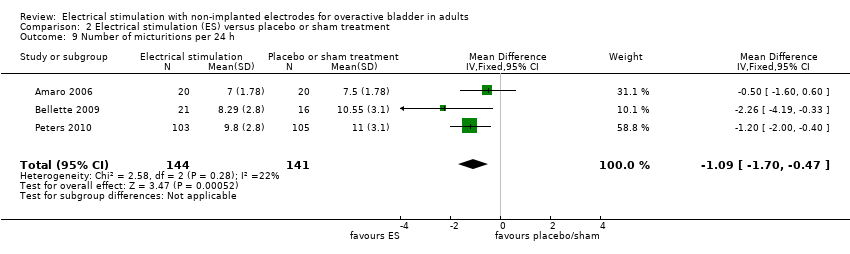
Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 9 Number of micturitions per 24 h.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 10 Number of participants with adverse effects.

Comparison 3 Electrical stimulation (ES) versus pelvic floor muscle training (PFMT), Outcome 1 Number of participants cured or improved.

Comparison 3 Electrical stimulation (ES) versus pelvic floor muscle training (PFMT), Outcome 2 Number of participants satisfied.
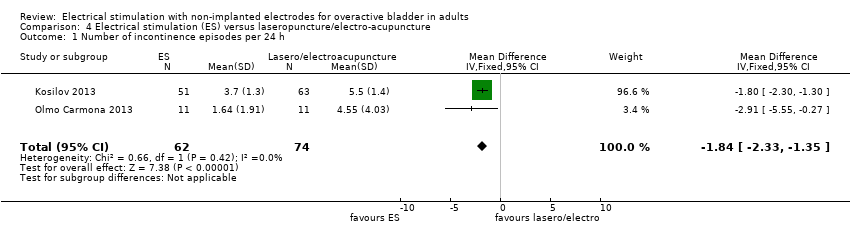
Comparison 4 Electrical stimulation (ES) versus laseropuncture/electro‐acupuncture, Outcome 1 Number of incontinence episodes per 24 h.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 1 Number of participants cured.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 2 Number of participants cured or improved.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 3 Number of participants cured or improved: routes of ES.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 4 Number of participants satisfied.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 5 Number of incontinence episodes per 24 h.
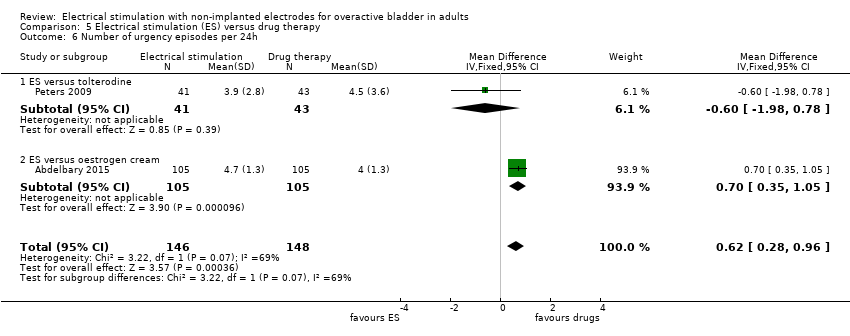
Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 6 Number of urgency episodes per 24h.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 7 Number of micturitions per 24 h.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 8 Number of nocturia episodes per night.
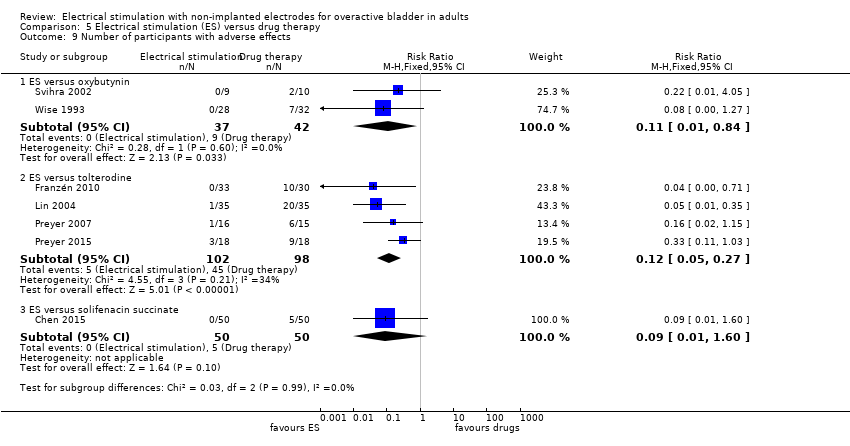
Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 9 Number of participants with adverse effects.
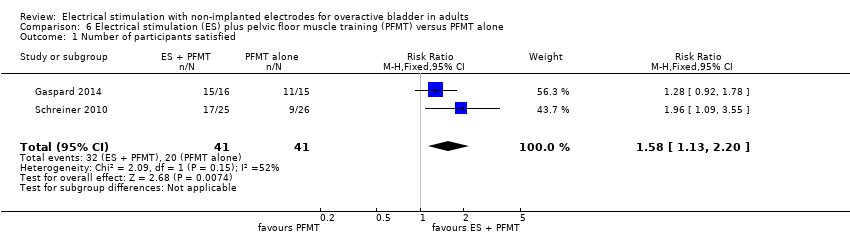
Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 1 Number of participants satisfied.

Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 2 Number of incontinence episodes per 24h.

Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 3 Number of urgency episodes per 24 h.

Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 4 Number of micturitions per 24 h.

Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 1 Quality of life.
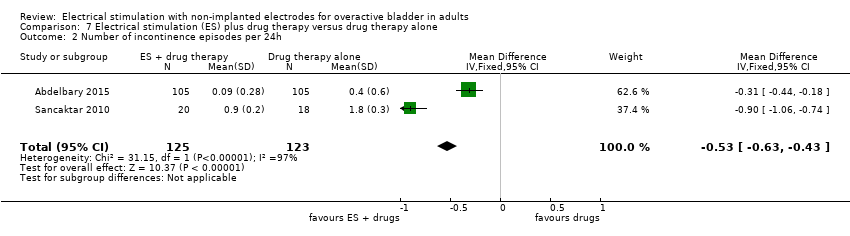
Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 2 Number of incontinence episodes per 24h.

Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 3 Number of urgency episodes per 24 hours.
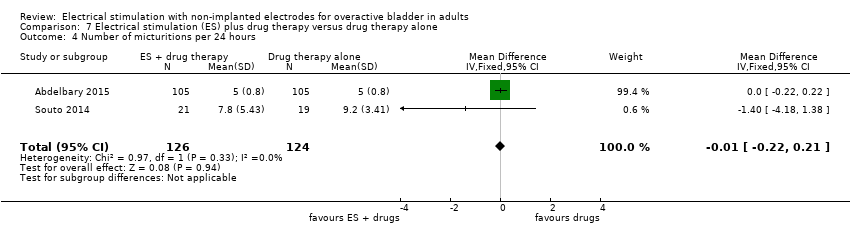
Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 4 Number of micturitions per 24 hours.
| Electrical stimulation versus no active treatment | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no active treatment | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 12 weeks to 12 months | Study population | RR 1.85 | 121 | ⊕⊕⊝⊝ | ||
| 424 per 1000 | 784 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life (higher score indicates better quality of life) Follow‐up: range 5 weeks to 12 weeks | In one trial participants in the intervention group had lower ICI‐Q scores (unclear if this was an important difference). In another no evidence of a difference was found between groups in of improvement in a range of QoL scores. | ‐ | 148 (2 RCT) | ⊕⊕⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high likelihood of selection bias). | ||||||
| Electrical stimulation versus placebo or sham treatment | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or sham treatment | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 4 weeks to 12 weeks | Study population | RR 2.26 | 677 | ⊕⊕⊕⊝ | ||
| 262 per 1000 | 593 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence Follow‐up: range 4 weeks to 13 weeks | Study population | RR 5.03 (0.28 to 89.88) | 242 | ⊕⊕⊝⊝ | ||
| 189 per 1000 | 948 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 4 weeks to 13 weeks | 3/7 trials reported significantly higher quality of life in the intervention groups. Others reported no evidence of a difference between groups. | ‐ | 627 (7 RCTs) | ⊕⊕⊝⊝ | ||
| Adverse effects Follow‐up: median 12 weeks | Study population | RR 1.24 | 450 | ⊕⊕⊝⊝ | ||
| 139 per 1000 | 172 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high risk of performance and detection bias in one trial; unclear risk of bias in many domains in other trials) | ||||||
| Electrical stimulation versus PFMT | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: median 12 months | Study population | RR 1.60 | 195 | ⊕⊕⊕⊝ | ||
| 390 per 1000 | 625 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence Follow‐up: 6 weeks | Study population | RR 1.62 | 52 | ⊕⊝⊝⊝ | ||
| 382 per 1000 | 619 per 1000 | |||||
| OAB‐related quality of life Follow‐up: 6 weeks | The mean OAB‐related quality of life in the intervention group was 129.81 higher (47.83 higher to 211.79 higher) | ‐ | 49 | ⊕⊝⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Dowgraded one level due to serious risk of bias (some risk of performance and attrition bias) | ||||||
| Electrical stimulation versus PFMT plus biofeedback | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT plus biofeedback | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence Follow‐up: 6 weeks | Study population | RR 1.06 | 51 | ⊕⊕⊝⊝ | ||
| 500 per 1000 | 530 per 1000 | |||||
| OAB‐related quality of life (lower scores indicate better quality of life) Follow‐up: 6 weeks | The mean OAB‐related quality of life in the intervention group was 5.78 lower (88.99 lower to 77.43 higher) | ‐ | 51 | ⊕⊕⊝⊝ | No evidence of a difference between groups in quality of life scores | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of selection and performance bias) | ||||||
| Electrical stimulation versus magnetic stimulation | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with magnetic stimulation | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Adverse effects Follow‐up: 4 weeks | Not estimable | 32 | ⊕⊝⊝⊝ | No events reported in either group | ||
| 0 per 1,00 | 0 per 1,00 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious imprecision (single trial, small sample size) | ||||||
| Electrical stimulation versus laseropuncture/electro‐acupuncture | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with laseropuncture/electro‐acupuncture | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Assessed with: Bladder Self‐Assessment Questionnaire (lower scores indicate better quality of life) Follow‐up: 12 weeks | The mean OAB‐related quality of life in the intervention group was 2.09 lower (4.1 lower to 0.08 lower) | ‐ | 22 | ⊕⊕⊝⊝ | Significantly greater quality of life in intervention group | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious imprecision (single trial, small sample size) | ||||||
| Electrical stimulation versus drug therapy | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with drugs | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 4 weeks to 2 years | Study population | RR 1.20 | 439 | ⊕⊕⊕⊝ | ||
| 585 per 1000 | 702 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 4 weeks to 6 months | One trial used OAB‐Q, PGII and PPIUS and found a significant result only in the PGII, which was in favour of ES. Another trial found no evidence of a difference between groups in I‐QoL scores. A third trial found higher QoL scores in the ES group at the end of treatment and at 3 months' follow‐up but no evidence of a difference at 6 months' follow‐up. | ‐ | 336 (3 RCTs) | ⊕⊕⊝⊝ | ||
| Adverse effects ‐ ES versus oxybutynin Follow‐up: 5 weeks | Study population | RR 0.11 | 79 | ⊕⊕⊝⊝ | ||
| 214 per 1000 | 24 per 1000 | |||||
| Adverse effects ‐ ES versus tolterodine Follow‐up: range 4 weeks to 2 years | Study population | RR 0.12 | 200 | ⊕⊕⊕⊝ | ||
| 459 per 1000 | 55 per 1000 | |||||
| Adverse effects ‐ ES versus solifenacin succinate Follow‐up: 4 weeks | Study population | RR 0.09 | 100 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Electrical stimulation plus PFMT versus PFMT alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT alone | Risk with electrical stimulation plus PFMT | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence Follow‐up: 12 weeks | Study population | RR 2.82 | 51 | ⊕⊕⊝⊝ | ||
| 269 per 1000 | 759 per 1000 | |||||
| Adverse effects Follow‐up: 12 weeks | Study population | Not estimable | 51 | ⊕⊝⊝⊝ | No events reported in treatment groups | |
| 0 per 1000 | 0 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 8 weeks to 6 months | One trial found greater quality of life in the intervention group (measured with ICIQ‐SF). Two other trials found no evidence of a difference between groups (measured with SF‐Qualiveen and York Incontinence Perception Scale) | ‐ | 201 (3 RCTs) | ⊕⊕⊝⊝ | ||
| Cost‐effectiveness | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Electrical stimulation plus behavioural therapy versus behavioural therapy alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with behavioural therapy alone | Risk with electrical stimulation plus behavioural therapy | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Follow‐up: 3 months | Intervention group reported significantly better quality of life measured with OAB‐Q and Incontinence Severity Index | ‐ | 82 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high risk of attrition bias, low risk of selection bias and unclear in other domains) | ||||||
| Electrical stimulation plus drug therapy versus drug therapy alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with drug therapy alone | Risk with electrical stimulation plus drug therapy | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life (lower scores indicate greater quality of life) Follow‐up: range 12 weeks to 6 months | The mean OAB‐related quality of life in the intervention group was 1.50 lower (3.72 lower to 0.72 higher) | ‐ | 248 | ⊕⊕⊝⊝ | ||
| Adverse effects Follow‐up: 12 weeks | Study population | RR 0.45 | 38 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 50 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| ES once a week versus ES twice a week | |||
| Patient or population: Adults with overactive bladder (OAB) | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Participants cured or improved Follow‐up: 6 months | 100% (37/37) of participants in both groups reported improvement in symptoms but only 9/37 were satisfied enough to request no further treatment | 37 (1 RCT) | ⊕⊝⊝⊝ |
| Participants with improvement in urgency urinary incontinence | Not reported | (0 studies) | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | |||
| ES once a week versus ES three times a week | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with ES 3 times a week | Risk with ES once a week | |||||
| Participants cured or improved (follow‐up not reported) | Study population | RR 0.97 | 35 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 647 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence (follow‐up not reported) | Study population | RR 0.80 | 22 | ⊕⊝⊝⊝ | ||
| 455 per 1000 | 364 per 1000 | |||||
| OAB‐related quality of life (follow‐up not reported) | I‐QoL scores very similar in the 2 groups (median (range) N): once a week: 77 (35‐100), 17. 3 times per week: 78 (33‐100), 18 | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse effects (follow‐up not reported) | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 35 (1 studies) | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Sensory threshold ES versus motor threshold ES | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with motor threshold ES | Risk with sensory threshold ES | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Follow‐up: 4 weeks | The mean OAB‐related quality of life in the intervention group was 0.07 lower (2.21 lower to 2.07 higher) | ‐ | 28 | ⊕⊝⊝⊝ | No evidence of a difference between groups | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (low risk of performance, detection and attrition bias but unclear risk of selection bias) | ||||||
| Study | Current | Current intensity | Pulse shape & duration | Frequency (Hz) | Duty cycle | Electrodes | Treatment duration/supervision |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Unclear | |
| 30‐60 mA according to patient tolerance (mean 43 mA) | 320 ms | 20 | Unclear | Intravaginal | Two 30‐min sessions per week for 12 weeks | ||
| Unclear | "Sensory threshold, activating superficial cutaneous nerve fibers with larger diameter" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks | |
| Unclear | "Motor threshold, non‐painful contraction is induced and the stimulation can simply make pain relief in the same way that sensory stimulation level (blocking activation of the peripheral or cental inhibition)" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks | |
| Bipolar | 0‐100 mA according to participant tolerance | Bipolar square wave 0.1 µs | 4 | 2 s on, 4 s off | Intravaginal | Three 20‐min sessions per week on alternate days for 7 weeks | |
| Biphasic | 10‐100 mA according to participant tolerance | 1 ms intermittent | 10 | Unclear | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | 0‐100 mA | Asymmetric, 1 s rise time, sustained for 5 s and resting for 5 s | 20 | 1 s rise time, sustained for 5s and resting for 5 s | Intravaginal | Home use: two 20‐min sessions per day for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve | Two 30‐min sessions per week for 4 weeks | |
| Biphasic | 0‐100 mA | Rectangular 200 µs stochastic variation | 4‐10 | Unclear | Intravaginal | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous posterior tibial nerve | Twelve 30‐min sessions | |
| Unclear | Unclear | 500 µs | 10 | Unclear | Intravaginal | Twelve 30‐min sessions | |
| Unclear | 0‐50 mA | 200 µs | 10 | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks | |
| Unclear | Unclear | 200 µs | 150 | Unclear | Transcutaneous electrical nerve stimulation – suprapubic placement | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation – sacral placement | Unclear | |
| Bipolar | 0‐100 mA | Bipolar square wave 0.1 µs | 20 | 2 s on ‐ 4 s off | Intravaginal | 20 minutes daily for 8 weeks | |
| Unclear | 0‐10 mA | Square wave 320 µs | 20 | unclear | Percutaneous posterior tibial nerve stimulation | 30 min once a week for 12 weeks | |
| Bipolar | According to participant tolerance | Continuous bipolar square wave 200 µs | 20 | Unclear | Percutaneous posterior tibial nerve stimulation ‐ adhesive skin electrodes | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation ‐ "34 gauge needle placed 5 cm near internal malleolus" | 30‐min sessions | |
| Unclear | 0‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Percutaneous tibial nerve stimulation | Three 30‐min sessions per week for 4 weeks | |
| Unclear | Unclear current, intensity according to participant tolerance | Unclear | 12.5 | 5 s on, 10 s off | Intravaginal | Fourteen 30‐min sessions | |
| Unclear | According to participant tolerance | Unclear | 5‐10 | Intravaginal/transanal | 10 sessions: 1‐2 20‐min sessions per week for 5‐7 weeks | ||
| Biphasic | Unclear | Biphasic rectangular 220 µs | 10 | 20 s on, 4 s off | Transcutaneous posterior tibial nerve stimulation: external electrode 5 cm above medial malleolus, 1 cm behind the tibia. The other electrode on dorsum of foot | One 30‐min session per week for 9 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twice a week for 6 weeks, performed by either physiotherapist or continence midwife | |
| Unclear | Unclear | Unclear | Unclear | Unclear | VERV electrode patches, placed by the participant ‐ exact placement unclear | One patch per week for 12 weeks | |
| Diadynamic | 20–40 mA, 50%‐75% intensity | Unclear | 20 | Unclear | Active electrode (50 cm2 to 70 cm2) above the pubis, and a passive electrode (150 cm2) in lumbosacral area | 15 procedures every other day | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Twelve 30‐min sessions | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twelve 30‐min sessions | |
| Unclear | 8‐70 mA | Unclear | Unclear | Unclear | Vaginal/anorectal | 20‐30 20‐min sessions | |
| Unclear | According to participant tolerance | Unclear | 0‐100 | Unclear | Interferential therapy. 2 anterior flat electrodes placed over obturator foramen 1.5 cm to 2 cm lateral to symphysis, two posterior electrodes placed medial to ischial tuberosities either side of anus | 12 sessions: first session 15 min, all others 30 min | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Once per week | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Twice per week | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous tibial nerve stimulation | Twice a week with at least 48 hour intervals for 12 weeks | |
| Biphasic | Immediately below motor threshold | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation through 1 channel and 2 electrodes | Two 30‐min sessions per week for 4 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal amplitude‐modulated signal through a patch applied to the skin, controlled by wireless handheld remote control | Patch worn for 4 weeks | |
| Unclear | Below the threshold that causes motor contraction | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation with surface electrodes. Negative electrode on medial malleolus, and the positive electrode 10 cm above negative electrode, also on the medial side. Rhythmic flexion of the second toe during the stimulation determined the correct position of the negative electrode | 30‐min twice weekly over 12 sessions (45 days) | |
| Unclear | Pre‐programmed to increase intensity over 24 s to reach therapeutic level and switch off automatically after 30 min. All devices same level of stimulation (average intensity considered comfortable and capable of producing contractions of pelvic floor muscles) | Unclear | During the 10 s ‘‘on time’’ the device delivers 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation | 10 s on, 10 s off | Intravaginal, single‐use tampon‐like Pelviva device | One disposable device per day for 12 weeks except during menstruation | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle slightly cephalad to medial malleolus | One 30‐min session per week for 12 weeks | |
| Unclear | 0.5‐9 mA | Unclear | 20 | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle inserted at 60º angle 5 cm cephalad to medial malleolus, slightly posterior to tibia. Surface electrode placed on ipsilateral calcaneous | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Participant‐managed neuromodulation system patch | Subject placement versus investigator placement | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Peripheral tibial neurostimulation | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 3 months | |
| Unclear | 0.5‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Stoller afferent neurostimulation: 34‐gauge needle inserted at 30° angle 2 cm to 3 cm superior‐medial aspect of tibial medial malleolus along posterior tibial nerve trace | One 30‐min session per week for 12 weeks | |
| Biphasic | Controlled by participant according to tolerance | 300 µs | Asymmetrical, 50 | Unclear | Intravaginal: probe with two 26 mm rings 40 mm apart | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous tibial nerve stimulation | One 30 min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30 min session per day for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30‐min session per week for 12 weeks | |
| Unclear | Up to 40 v | Unclear | 10‐50 | Unclear | Maximum perineal stimulation: Scott electrode in vagina, large indifferent electrode under buttocks | Single 20‐min session | |
| Unclear | Unclear | Unclear | 10 | Unclear | Intravaginal cushion attached to stimulator worn around waist | Cushion worn for 8 out of 24 h, day or night according to participant preference | |
| Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Unilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on the right ankle | Unclear | |
| Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Bilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on both ankles | Unclear | |
| Unclear | 5‐25 mA | Unclear | Device uses 2 programmes simultaneously: 12.5 Hz and 50 Hz | 5 s impulse | Intravaginal | Twice daily for 4 months. Length of session increased monthly: 15, 30, 45, 60 minutes | |
| Unclear | Participants asked to control stimulation to achieve tickling sensation | 200 µs | 20 | Continuous | Transcutaneous. 2 self‐adhesive pads applied bilaterally over the perianal region (S2‐S3 dermatome) | Up to 6 hours daily for 6 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. Horizontal placement of electrode patch near sacral nerve | Patch worn continuously for 7 days | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. 30° angle placement of electrode patch near sacral nerve | Patch worn continuously for 7 days | |
| Unclear | According to participant tolerance | 250 µs | 10 | Unclear | Posterior tibial nerve stimulation. Surface electrode placed behind media malleolus and another placed 10 cm above first electrode | Two 30 min sessions per week for 12 weeks | |
| Biphasic | 0‐100 mA, according to participant tolerance | 100 µs | 20 | 2 s contraction time, duty cycle 1–2 s | Intravaginal | Three 30‐min sessions per week for 8 weeks. 5 min rest between each 15 min | |
| Square | 25 mA. 70% of intensity of maximal amplitude of registered response from abductor hallucis muscle | Square impulse 100 µs | 1 | Unclear | Stoller afferent neurostimulation. Electrodes placed behind medial ankle of left lower extremity, cathode placed proximally and anode distally | One 30 min session per week for 5 weeks | |
| Unclear | According to participant tolerance | Unclear | 10 min of each frequency, 3 min: 5‐10 Hz, 10‐50 Hz, 50 Hz | 7 s on, 25 s off | Intravaginal/transanal | 6 sessions over two weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks | |
| Unclear | 0‐10 mA | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | 200 ms | 10 | Unclear | Transcutaneous neurostimulation. Electrode pads affixed bilaterally to the skin overlying S3 dermatomes (junction of buttock and upper thigh) | Single session | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | One session per day (at home) for 6 weeks | |
| Unclear | 0‐90 mA, according to participant tolerance | Unclear | 20 | Unclear | Intravaginal | One session per day (at home) for 6 weeks | |
| Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed electrodes | Two 15‐min sessions per day for 4 weeks | |
| Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed | Single session |
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | No active treatment (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Improvement in QoL measured by Incontinence Quality of Life Questionnaire, Behavioural Urge Score and International Prostate Symptom Score | 5/9 | 0/9 | RR 11.00 (95% CI 0.70 to 173.66) | |
| ICI‐Q score1 | Median (range), N: 6 (0‐17), 64 | Median (range), N: 9 (3‐18), 60 | Not estimable | |
| Secondary outcomes | ||||
| Daytime frequency | NR | NR | Favours ES P = 0.0001 | |
| Nocturia | NR | NR | Favours ES P = 0.0186 | |
| Participants with nocturnal enuresis | 45 days' treatment: 0/12 | 45 days' treatment: 2/12 | Favours ES RR 5.00 (95% CI 1.63 to 15.31) | |
| 12 months' follow‐up: 0/12 | 12 months' follow‐up: 2/12 | |||
| Participants with nocturia | 45 days' treatment: 5/12 | 45 days' treatment: 9/12 | RR 2.33 (95% CI 0.78 to 6.94) | |
| 12 months' follow‐up: 1/12 | 12 months' follow‐up: 6/12 | Favours ES RR 0.17 (95% CI 0.02 to 1.18) | ||
| Participants with increased daytime frequency | 45 days' treatment: 3/12 | 45 days' treatment: 11/12 | Favours ES RR 0.27 (95% CI 0.10 to 0.74) | |
| 12 months' follow‐up: 0/12 | 12 months' follow‐up: 9/12 | Favours ES RR 0.05 (95% CI 0.00 to 0.81) | ||
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Placebo or sham treatment (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| ICIQ‐SF score | Median (IQR), N: 2 (0 to ‐6), 15 | 0 (‐3 to 3), 13 | P = 0.132 | |
| Participants with improvement in ICIQ‐SF score | 10/15 | 6/13 | RR 1.44 (95% CI 0.73 to 2.87) | |
| OAB‐Q total score1 | 83.99 (16.99), 21 | 66.63 (25.06), 16 | Favours ES MD 17.36 (95% CI 3.09 to 31.63) | |
| I‐QoL score1 | 69.9 (65.8‐73.3), 17 | 70.6 (62.2‐79.1), 15 | No evidence of a difference | |
| Change in OAB‐Q score | Median (IQR), N: 8.8 (1.6 to 20.0), 80 | Median (IQR), N: 9.2 (‐0.8 to 27.2), 83 | P = 0.9918 | |
| Change in OAB‐Q score | 36.7 (21.5), 101 | 29.2 (20.0), 102 | Favours ES MD 7.50 (1.79, 13.21) | |
| QoL score2 | 1.6 (0.7), 37 | 2.2 (0.9), 31 | Favours ES MD ‐0.60 (95% CI ‐0.99 to ‐0.21) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Number of pads per day | 0.8 (1.2), 37 | 1.1 (2.0), 31 | MD ‐0.30 (95% CI ‐1.10 to 0.50) | |
| Other outcomes | ||||
| Participants with reduction in analogue discomfort sensation | 8/20 | 5/20 | RR 1.60 (95% CI 0.63 to 4.05) | |
| Participants with reduction in analogue wetness sensation | 6/20 | 5/20 | RR 1.20 (95% CI 0.44 to 3.30) | |
| Pelvic floor muscle strength (cmH2O) | 53.8 (18.6), 20 | 46.8 (12.5), 20 | MD 7.00 (95% CI ‐2.82 to 16.82) | |
| Urgency score2 | 1.7 (0.7), 37 | sham ES: 2 (0.8), 31 | MD ‐0.30 (95 CI ‐0.66 to 0.06) | |
| Results in bold are statistically significant 1Lower score = greater severity | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | PFMT (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants cured | 14/21 | 12/21 | RR 1.17 (95% CI 0.72 to 1.88) | |
| Participants with improvement in UUI | 9/18 | 13/34 | RR 1.62 (95% CI 0.51 to 5.12) | |
| King's Health Questionnaire score1 | 180.08 (176.03), 35 | 50.27 (171.42), 34 | Favours ES MD 129.81 (95% CI 47.83 to 211.79) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Incontinence episodes per 24 hours | 7.9 (13.7), 21 | 7.8 (15.3), 21 | MD 0.10 (95% CI ‐8.68 to 8.88) | |
| Micturitions per 24 hours | 7.9 (2.3), 21 | 71. (2.1), 21 | MD 0.80 (95% CI ‐0.53 to 2.13) | |
| Nocturia episodes per night | 1.2 (1.3), 21 | 1 (1.1), 21 | MD 0.20 (95% CI ‐0.53 to 0.93) | |
| Number of pads per day | 0.9 (1.7), 21 | 0.8 (1.3), 21 | MD 0.10 (95% CI ‐0.82 to 1.02) | |
| 1Higher score = greater QoL | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | PFMT plus biofeedback (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants with improvement in UUI | 9/17 | 17/34 | RR 1.06 (95% CI 0.60 to 1.85) | |
| King's Health Questionnaire score1 | 180.08 (176.03), 35 | 185.86 (176.57), 34 | MD ‐5.78 (95% CI ‐88.99 to 77.43) | |
| 1Higher score = greater QoL | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Laseropuncture/electro‐acupuncture (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Bladder Self‐Assessment Questionnaire score | 5.18 (2.56), 11 | 7.27 (2.24), 11 | Favours ES MD ‐2.09 (95% CI ‐4.10 to ‐0.08) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Micturitions per day | 8 (1.73), 11 | 7.73 (1.67), 11 | MD 0.27 (95% CI‐1.15 to 1.69) | |
| Nocturia episodes per night | 1.09 (1.51), 11 | 2.09 (1.92), 11 | MD ‐1.00 (95% CI ‐2.44 to 0.44) | |
| 1Higher score = greater severity | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Comparator (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants cured or improved | 69% (N not reported) | Probanthine 50% (N not reported) | Not estimable | |
| I‐QoL score1 | 25.2 (1.0), 50 | Solifenacin succinate: 24.2 (1.0), 48 | MD 1.00 (95% CI 0.60 to 1.40) | |
| OAB‐Q score2 | 2.9 (0.9), 14 | Solifenacin succinate: 3.1 (1.1), 14 | MD ‐0.20 (95% CI ‐0.94 to 0.54) | |
| Patient Global Impression of Improvement score2 | 2.1 (0.7), 14 | Solifenacin succinate: 2.9 (1.1), 14 | Favours ES MD ‐0.80 (95% CI ‐1.48 to ‐0.12) | |
| Participant Perception of Intensity of Urgency Scale score2 | 2.1 (0.9), 14 | Solifenacin succinate: 2.7 (1.2), 14 | MD ‐0.60 (95% CI ‐1.39 to 0.19) | |
| ES | Oestrogen cream | Favours ES MD ‐2.20 (95% CI ‐2.71 to ‐1.69) Favours ES MD ‐2.00 (95% CI ‐2.50 to ‐1.50) MD 1.60 [0.91, 2.29] | ||
| QoL score2 (instrument not reported) | End of treatment: 2.8 (2), 105 3 months: 4 (1.7), 105 6 months: 7.6 (3), 105 | End of treatment: 5 (1.8), 105 3 months: 6 (2), 105 6 months: 6 (2), 105 | ||
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| ES | Oestrogen cream | |||
| Voids per 24 hours | End of treatment: 4.7 (0.8), 105 3 months: 5.0 (1.0), 105 6 months: 6.6 (1.5), 105 | End of treatment: 5.0 (0.9), 105 3 months: 5.3 (0.9), 105 6 months: 5.0 (0.8), 105 | Favours ES MD (‐0.30 (95% CI ‐0.56 to ‐0.04) Favours ES MD ‐0.30 (95% CI ‐0.53 to ‐0.07) Favours oestrogen cream MD 1.60 (95% CI 1.27 to 1.93) | |
| Nocturia episodes per night | End of treatment: 0.9 (0.7), 105 3 months: 1.1 (0.9), 105 6 months: 2.2 (0.9), 105 | End of treatment: 1.4 (0.8), 105 3 months: 1.5 (0.8), 105 6 months: 5.0 (0.8), 105 | Favours ES MD ‐0.50 (95% CI ‐0.70 to ‐0.30) Favours ES MD ‐0.40 (95% CI ‐0.63 to ‐0.17) MD ‐2.80 (95% CI ‐3.03 to ‐2.57) | |
| Incontinence episodes | End of treatment: 0.1 (0.3), 105 3 months: 0.1 (0.3), 105 6 months: 0.4 (0.6), 105 | End of treatment: 0.4 (0.6), 105 3 months: 0.5 (0.6), 105 6 months: 0.4 (0.6), 105 | Favours ES MD ‐0.30 (95% CI ‐0.43 to ‐0.17) Favours ES ‐0.40 (95% CI ‐0.53 to ‐0.27) 0.00 [‐0.16, 0.16] | |
| Urgency episodes | End of treatment: 2 (0.7), 105 3 months: 2.7 (1.0), 105 6 months: 4.7 (1.3), 105 | End of treatment: 4 (1.3), 105 3 months: 4.5 (1.5), 105 6 months: 4 (1.3), 105 | ‐3.00 [‐3.28, ‐2.72] ‐1.80 [‐2.14, ‐1.46]0.70 [0.35, 1.05] | |
| Number of pads per day | 0.9 (1.8), 21 | Oxybutynin: 0.9 (1.5), 22 | MD 0.00 (95% CI ‐0.96 to 0.96) | |
| Participants with nocturia | 2/18 | Oxybutynin: 3/19 | RR 0.70 (95% CI 0.13 to 3.73) | |
| Results in bold are statistically significant 1Lower score = greater severity | ||||
| Study | Outcome | ES plus PFMT (mean (SD/range), N or n/N; if available) | PFMT (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| SF‐Qualiveen1 | Median (IQR), N: 9 weeks: 1.000 (0.656, 1.719), 16 6 months: 1.313 (0.687, 1.625), 16. | Median (IQR), N: 9 weeks: 1.375 (0.625, 2.188) 6 months: 1.500 (0.344, 2.094), 15 | Not estimable | |
| York Incontinence Perception Scale2 | 41.2 (10.2), 6 | 47 (5.5), 6 | MD ‐0.65 (95% CI ‐1.83 to 0.52) | |
| Participants with improvement in UUI | 19/25 | 7/26 | Favours ES plus PFMT RR 2.82 (95 CI 1.44 to 5.52) | |
| ICIQ‐SF score1 | 7.9 (4.5), 25 | 10.6 (4.4), 26 | Favours ES plus PFMT MD ‐2.70 (95% CI ‐5.14 to ‐0.26) | |
| Secondary outcomes | ||||
| Nocturia episodes per night | 1.3 (1.5), 25 | 2.4 (1.3), 26 | Favours ES plus PFMT MD ‐1.10 (95% CI ‐1.87 to ‐0.33) | |
| Adverse effects | 0/25 | 0/26 | Not estimable | |
| Other outcomes | ||||
| Pelvic floor muscle strength (cmH2O) | 27 (16), 6 | 47.2 (22.7), 6 | MD ‐20.20 (95% CI ‐42.42 to 2.02) | |
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES plus behavioural therapy (mean (SD/range), N or n/N; if available) | Behavioural therapy (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| OAB‐Q score1 | 100.81 (41.5), 31 | 127.71 (40.64), 37 | Favours ES plus behavioural therapy MD ‐26.90 (95% CI ‐46.52 to ‐7.28) | |
| Incontinence Severity Index score1 | 5.15 (3.23), 31 | 7.38 (4.00), 37 | Favours ES plus behavioural therapy MD ‐26.90, 95% CI ‐46.52 to ‐7.28 | |
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES plus drugs (mean (SD/range), N or n/N; if available) | Drugs (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| IIQ‐7 score1 | ES plus tolterodine: 9.0 (0.8), 20 | Tolterodine: 11.2 (2.7), 18. | Favours ES plus tolterodine MD ‐2.20 (95% CI ‐3.50 to ‐0.90 | |
| ES plus oestrogen cream: | Oestrogen cream: | |||
| QoL score1 (instrument not reported) | End of treatment: 2.9 (2.2), 105. 3 months: 1.6 (0.9), 105. 6 months: 2 (0.8), 105. | End of treatment: 5 (1.8), 105 3 months: 6 (2), 105 6 months: 6 (2), 105 | MD ‐2.10 (95% CI ‐2.64, ‐1.56] MD ‐4.40 (95% CI ‐4.82 to ‐3.98) MD ‐4.00 (95% CI ‐4.41 to ‐3.59) | |
| Secondary outcomes | ||||
| ES plus oestrogen cream: | Oestrogen cream: | |||
| Voids per day | End of treatment: 5 (0.8), 105. 3 months: 5 (0.8), 105. 6 months: 5 (0.8), 105. | End of treatment: 5.0 (0.9), 105 3 months: 5.3 (0.9), 105 6 months: 5.0 (0.8), 105 | MD 0.00 (95% CI ‐0.23 to 0.23) MD ‐0.30 (95% CI ‐0.53 to ‐0.07) MD 0.00 (95% CI ‐0.22 to 0.22) | |
| Nocturia episodes per night | End of treatment: 0.5 (0.5), 105 3 months: 1 (0.9), 105 6 months: 1.5 (0.8), 105 | End of treatment: 1.4 (0.8), 105 3 months: 1.5 (0.5), 105 6 months: 5 (0.8), 105 | MD ‐0.90 (95% CO ‐1.08 to ‐0.72) MD ‐0.50 (95% CI ‐0.70 to ‐0.30) MD ‐3.50 (95% CI ‐3.72 to ‐3.28) | |
| Incontinence episodes per 24 hours | End of treatment: 1.4 (0.7), 105 3 months: 0.09 (0.28), 105. 6 months: 0.09 (0.28), 105. | End of treatment: 0.4 (0.6), 105 3 months: 0.5 (0.6), 105 6 months: 0.4 (0.6), 105 | MD 1.00 (95% CI 0.82 to 1.18) MD ‐0.41 (95% CI ‐0.54 to ‐0.28) MD ‐0.31 (95% CI ‐0.44 to ‐0.18) | |
| Urgency episodes per 24 hours | End of treatment: 1.4 (0.7), 105 3 months: 1.6 (0.9), 105 6 months: 2 (0.8), 105 | End of treatment: 4 (1.3), 105 3 months: 4.5 (1.5), 105 6 months: 4 (1.3), 105 | MD ‐2.60 (95% CI ‐2.88 to ‐2.32) MD ‐2.90 (95% CI ‐3.23 to ‐2.57) MD ‐2.00 (95% CI ‐2.29 to ‐1.71) | |
| Adverse effects | ES plus tolterodine: 1/20 | Tolterodine: 2/18 | RR 0.45 (95% CI 0.04 to 4.55) | |
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES A (mean (SD/range), N or n/N; if available) | ES B (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| ICIQ‐OAB score1 | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 4.46 (2.66), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 4.53 (3.07), 13 | MD ‐0.07 (95% CI ‐2.21 to 2.07) | |
| Success = > 50% reduction in micturitions/24 hours OR If incontinent, success > 50% reduction in UI episodes/24 hours | ES once a week: 11/17 (4/11 incontinent participants) | ES 3 times per week: 12/18 (5/11 incontinent participants) | RR 0.97 (95% CI 0.60 to 1.57) Incontinence participants: RR 0.80 (95% CI 0.29 to 2.21) | |
| I‐QoL score2 | ES once a week (median, range, N): 77 (35‐100), 17 | ES 3 times a week (median, range, N): 78 (33‐100), 18 | Not estimable | |
| Participants with improvement in symptoms | ES once a week: 100% | ES twice a week: 100% | Not estimable | |
| Participants satisfied enough to request no further treatment | 24% (9/37) | Not estimable, not reported per treatment group | ||
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Adverse effects | ES once a week: 0/17 | ES 3 times per week: 0/18 | Not estimable | |
| Subjective improvement after 6‐8 sessions | ES once a week: 17/17 | ES 3 times a week: 18/18 | Not estimable | |
| Incontinence episodes per 24 hours | ES once a week (median, range, N): 1 (0‐3), 11 | ES 3 times a week (median, range, N): 1 (0‐3), 11 | Not estimable | |
| Micuturitions per 24 hours | ES once a week (median, range, N): 8 (5‐15), 17 | ES 3 times a week (median, range, N): 8 (6‐18), 18 | Not estimable | |
| SF‐36 score | ES once a week (median, range, N): 62 (24‐81), 17 | ES 3 times per week (median, range, N): 62 (25‐80), 18 | Not estimable | |
| UUI episodes per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 0.33 (0.57), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 0.84 (1.39), 13 | MD ‐0.51 (95% CI ‐1.32 to 0.30) | |
| Urgency episodes per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 0.79 (0.97), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 0.58 (0.65), 13 | MD 0.21 (95% CI ‐0.39 to 0.81) | |
| Micturitions per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 8.33 (2.52), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 7.89 (2.64), 13 | MD 0.44 (95% CI ‐1.48 to 2.36) | |
| Nocturia episodes per night | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 1.26 (1.21), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 1.05 (1.01), 13 | MD 0.21 (95% CI ‐0.61 to 1.03) | |
| Maximum cystometric capacity | 150 Hz: 351 (144), 16 | 10 Hz: 305 (146), 16 | MD 46.00 (95% CI ‐54.48 to 146.48) | |
| Volume at first desire to void | 150 Hz: 208.5 (132), 16 | 10 Hz: 154 (61), 16 | MD 54.50 (95% CI ‐16.75 to 125.75) | |
| Other outcomes | ||||
| Participants satisfied | 200 µs pulse width: 17/22 | 500 µs pulse width: 11/16 | RR 1.12 (95% CI 0.75 to 1.68) | |
| 1Higher score = greater severity | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured or improved Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.34, 2.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured Show forest plot | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.39, 5.21] |
| 2 Number of participants cured or improved Show forest plot | 10 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.85, 2.77] |
| 3 Number of participants cured or improved: different ES routes Show forest plot | 6 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [2.54, 4.96] |
| 3.1 Percutaneous tibial nerve stimulation | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [2.22, 4.58] |
| 3.2 Intravaginal | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.46 [2.33, 12.81] |
| 4 Number of participants satisfied Show forest plot | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.02, 2.04] |
| 5 Number of participants with improvement in urgency urinary incontinence Show forest plot | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 5.03 [0.28, 89.88] |
| 6 Number of participants with improvement in urinary frequency Show forest plot | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.43, 2.92] |
| 7 Number of incontinence episodes per 24 h Show forest plot | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐1.92, ‐0.95] |
| 8 Number of nocturia episodes Show forest plot | 2 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 9 Number of micturitions per 24 h Show forest plot | 3 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.70, ‐0.47] |
| 10 Number of participants with adverse effects Show forest plot | 3 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.84, 1.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured or improved Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.19, 2.14] |
| 2 Number of participants satisfied Show forest plot | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinence episodes per 24 h Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐2.33, ‐1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured Show forest plot | 7 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.41] |
| 1.1 ES versus tolterodine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.47] |
| 1.2 ES versus oxybutynin | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.72] |
| 1.3 ES versus propantheline bromide | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.38, 5.74] |
| 2 Number of participants cured or improved Show forest plot | 8 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.04, 1.38] |
| 2.1 ES versus tolterodine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.41] |
| 2.2 ES versus oxybutynin | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.91, 1.52] |
| 2.3 ES versus propantheline bromide | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.86, 2.44] |
| 3 Number of participants cured or improved: routes of ES Show forest plot | 5 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.04, 1.54] |
| 3.1 Transcutaneous posterior tibial nerve stimulation | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.92] |
| 3.2 Intravaginal/transanal | 4 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 4 Number of participants satisfied Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 4.1 ES versus oxybutynin | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 5 Number of incontinence episodes per 24 h Show forest plot | 5 | 477 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.11, 1.60] |
| 5.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.49, 0.29] |
| 5.2 ES versus oxybutynin | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐6.45, 8.25] |
| 5.3 ES versus trospium + solifenacin | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 2.2 [1.78, 2.62] |
| 5.4 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 5.5 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| 6 Number of urgency episodes per 24h Show forest plot | 2 | 294 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.28, 0.96] |
| 6.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.98, 0.78] |
| 6.2 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.35, 1.05] |
| 7 Number of micturitions per 24 h Show forest plot | 6 | 646 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.15, 0.52] |
| 7.1 ES versus tolterodine | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐1.06, 1.50] |
| 7.2 ES versus oxybutynin | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐0.18, 1.91] |
| 7.3 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.04, 0.84] |
| 7.4 ES versus oestrogen cream | 1 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.15, 0.52] |
| 8 Number of nocturia episodes per night Show forest plot | 4 | 367 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐2.27, ‐1.88] |
| 8.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 8.2 ES versus oxybutynin | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.35, 0.95] |
| 8.3 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.06, 0.66] |
| 8.4 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.8 [‐3.03, ‐2.57] |
| 9 Number of participants with adverse effects Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 ES versus oxybutynin | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.84] |
| 9.2 ES versus tolterodine | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.27] |
| 9.3 ES versus solifenacin succinate | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants satisfied Show forest plot | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.13, 2.20] |
| 2 Number of incontinence episodes per 24h Show forest plot | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.84, 0.64] |
| 3 Number of urgency episodes per 24 h Show forest plot | 2 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐2.74, ‐2.24] |
| 4 Number of micturitions per 24 h Show forest plot | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.62, 0.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 2 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.72, 0.72] |
| 2 Number of incontinence episodes per 24h Show forest plot | 2 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.63, ‐0.43] |
| 3 Number of urgency episodes per 24 hours Show forest plot | 2 | 248 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.11, ‐1.54] |
| 4 Number of micturitions per 24 hours Show forest plot | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.22, 0.21] |

