成人の過活動膀胱に対する非植え込み型電極を用いた電気刺激
アブストラクト
背景
過活動膀胱(OAB)の管理に関しては、非植え込み型デバイスによる電気刺激(ES)、保存療法、医薬品などの、幾つかの選択肢がある。非植え込み型デバイスによる電気刺激は、排尿筋の収縮を阻止し、排尿の頻度と切迫感を減じる可能性がある。
目的
切迫性尿失禁の有無に拘わらず、以下のことと比較して、OAB用非植え込み型デバイスによるESの効果を評価すること:プラセボあるいはその他の実薬治療;その他の単独介入と比較した他の介入に追加されたES;互いに比較したESの種々の方法。
検索戦略
Cochrane Central Register of Controlled Trials (CENTRAL) 、MEDLINE,、MEDLINE In‐Process、ClinicalTrials.gov, WHO ICTRP、雑誌のハンドサーチおよび会議の議事録(2015年12月10日検索)から特定した試験を含む Cochrane Incontinence Specialised Registerを検索した。関連論文の参考文献リストを検索し、その分野での専門家と連絡をとった。言語の制限は設けなかった。
選択基準
成人のOABのための他の治療法と比較して、非植え込み型デバイスを用いたESのランダム化および準ランダム化比較試験を組み込んだ。適格な試験には、切迫性尿失禁(UUI)の有無にかかわらずOABの成人が含まれる。参加者が腹圧性尿失禁(SUI)を有する試験は除外した。
データ収集と分析
2名のレビュー著者はそれぞれ検索結果をスクリーニングし、データを適格な試験から抽出し、そしCochrane 'Risk of bias' tool.を用いてバイアスのリスクを評価した。
主な結果
63件の適格な試験(4424例の無作為化された試験)を確認した。44件の試験ではOABの治癒や改善の認識という主要アウトカムは報告されていない。大多数の試験は、選択バイアスおよび脱落バイアスの低度または不明瞭なリスク、実行バイアスそして検出バイアスの不明瞭なリスクがあると思われる。バイアスのリスクに関する明確さの欠如は主として報告不良に依る。
OAB症状改善の自覚に関して、中等度の質のエビデンスが、ESが骨盤底筋体操(PFMT)(リスク比(RR)1.60、95%信頼区間(CI)1.19-2.14;n=195)、薬物治療(RR 1.20、95%1.04-1.38、n=439)、プラセボもしくは疑似治療(RR 2.26、95%CI 1.85-2.77、n=677)に比較してより優れる事を示した。然しこれは切迫性尿失禁(UUI)(RR5.03、95%CI0.28-89.88、n=242)に関して、ESがプラセボ/疑似治療よりも効果が高い場合不明瞭であった。その試験に含まれる薬物治療はエストロゲンクリーム、オキシブチニン、臭化プロパンセリン、プロバンサイン、コハク酸ソリフェナジン、テロジリン、トルテロジン、塩化トロスピウムであった。
バイオフィードバックの有無に拘わらず、ESがPFMTと比較された場合、低いまたは非常に低い質のエビデンスにより切迫性尿失禁(UUI)改善の自覚における差のエビデンスは示唆されなかった。
OAB症状はESを用いた方が実薬治療を行わない場合(RR1.85、95%信頼区間1.34~2.55、n=121)よりも改善する可能性があることが低い質のエビデンスにより示唆されている。
ESに加えてPFMTも受けている参加者が、PFMTだけを受けている参加者に比較して、UUIの改善を報告する可能性が2倍を超える(RR2.82、95%信頼区間1.44~5.52、n=51)ことが低い質のエビデンスによって示唆された。
OAB関連のQOLに関して、ESを実薬治療無し、プラセボ/疑似治療またはバイオフィードバックを用いたPFMTと比較した場合、あるいはPFMT単独と比較してESにPFMTを追加した場合、低いまたは非常に低い質のいずれかである不確定のエビデンスが得られた。OAB関連のQOLに関してESはPFMTよりも望ましいことを示唆するために単回の試験からの非常に低い質のエビデンスが得られた。
トルテロジン(RR 0.12、95%信頼区間005-0.27、n=200)(中等度の質のエビデンス)およびオキシブチニン(RR0.11、95%信頼区間0.01-0.84、n=79)(低い質のエビデンス)に比較してESの副作用のリスクはより低い。
入手可能な非常に低い質のエビデンスにより、ESの副作用はプラセボ/疑似治療、磁気刺激、またはコハク酸ソリフェナシンに比較してより少ないかどうか確信できなかった。ESを、PFMTに加えるか、あるいは薬物治療に加えることによって、PFMTまたは薬物治療単独に比較して副作用がより少なくなるかどうかも全く確信できなかった。それぞれ違った型のES間に副作用リスクのなんらかの差があるかどうかも分らなかった。
1つの型のESが別のものに比較して効果的かどうか、またはESの有益性が実薬治療期間が終了した後も持続するかどうか確認するためにはエビデンスが不十分である。
著者の結論
OAB治療における電気刺激(ES)は、実薬治療、プラセボ/疑似治療、PFMT、および薬物治療に比較して有望であることを立証した。PFMTの様な他の治療にESを加える事は有益性があるという可能性がある。然し、低い質のエビデンスにより、主観的アウトカムと副作用を評価する十分に検出力のある試験を実施するまでは、これ等の結果に十分な確信がもてない。
PICO
一般語訳
成人過活動膀胱に対する非侵襲的電気刺激
背景
過活動膀胱(OAB)の患者には頻繁で抑制しがたい尿意が有り、それはQOLに多大な影響を及ぼす。多くのOABの患者には尿失禁もある。世界の人口の約17%がOABに罹患し、特に高齢者に良く見られる。OAB治療には骨盤底筋体操、薬物治療、電気刺激がある。
非侵襲的電気刺激は、膣または肛門のプローブを経由して、あるいは足首周囲の脛骨神経に挿入した細い針を通して、膀胱筋に電流を流す事で奏効する。その電流は排尿筋(尿を絞り出す膀胱筋)の収縮を減じ(妨げ)るように意図されている;これはヒトが排尿する必要がある回数をへらすはずである。侵襲的電気刺激は身体に電極を植え込み、外科的処置を要する
目的
我々は全くの未治療またはOABに関する実行可能な他のあらゆる治療法に比較して電気刺激が優れているか否かを検討したどの型の電気刺激がOABにとってより望ましいか、電気刺激が安全であるか否かについても我々は検討した。
結果
電気刺激法を未治療または他の可能な治療法と比較した63件の試験(全体で4424例の患者)を確認した。電気刺激はおそらく疑似電気刺激または骨盤底筋体操に比較してOABの主症状をより軽減するであろう事を確認した。
電気刺激は、実薬治療無しまたは薬物治療に比較してOAB症状をより軽減するが、入手可能なエビデンスは信頼性がより低いため、これ等の結果について我々は確信を持っていない。
同様に、OAB症状軽減に役立つ骨盤底筋体操または薬物治療に電気刺激を加える場合十分なエビデンスは得られていない。どの型の電気刺激が良いかを見分ける事も出来ない。
電気刺激が他の治療法に比較してより安全であるかどうか、または一つの電気刺激の型が他よりも安全であるとkどうかを判断するのに十分な情報は確認されなかった。
その治療法がOAB症状を改善したかどうか、または何らかの治療法によって引き起された副作用があるかどうか、我々が確認した多くの試験では報告されなかった。
最終的に一連の電気刺激を中止した後、電気刺激継続の有益性があるかどうかをエビデンスから見分けることはできなかった。
エビエンスは2015年12月現在のものである。
Authors' conclusions
Summary of findings
| Electrical stimulation versus no active treatment | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no active treatment | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 12 weeks to 12 months | Study population | RR 1.85 | 121 | ⊕⊕⊝⊝ | ||
| 424 per 1000 | 784 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life (higher score indicates better quality of life) Follow‐up: range 5 weeks to 12 weeks | In one trial participants in the intervention group had lower ICI‐Q scores (unclear if this was an important difference). In another no evidence of a difference was found between groups in of improvement in a range of QoL scores. | ‐ | 148 (2 RCT) | ⊕⊕⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high likelihood of selection bias). | ||||||
| Electrical stimulation versus placebo or sham treatment | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or sham treatment | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 4 weeks to 12 weeks | Study population | RR 2.26 | 677 | ⊕⊕⊕⊝ | ||
| 262 per 1000 | 593 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence Follow‐up: range 4 weeks to 13 weeks | Study population | RR 5.03 (0.28 to 89.88) | 242 | ⊕⊕⊝⊝ | ||
| 189 per 1000 | 948 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 4 weeks to 13 weeks | 3/7 trials reported significantly higher quality of life in the intervention groups. Others reported no evidence of a difference between groups. | ‐ | 627 (7 RCTs) | ⊕⊕⊝⊝ | ||
| Adverse effects Follow‐up: median 12 weeks | Study population | RR 1.24 | 450 | ⊕⊕⊝⊝ | ||
| 139 per 1000 | 172 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high risk of performance and detection bias in one trial; unclear risk of bias in many domains in other trials) | ||||||
| Electrical stimulation versus PFMT | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: median 12 months | Study population | RR 1.60 | 195 | ⊕⊕⊕⊝ | ||
| 390 per 1000 | 625 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence Follow‐up: 6 weeks | Study population | RR 1.62 | 52 | ⊕⊝⊝⊝ | ||
| 382 per 1000 | 619 per 1000 | |||||
| OAB‐related quality of life Follow‐up: 6 weeks | The mean OAB‐related quality of life in the intervention group was 129.81 higher (47.83 higher to 211.79 higher) | ‐ | 49 | ⊕⊝⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Dowgraded one level due to serious risk of bias (some risk of performance and attrition bias) | ||||||
| Electrical stimulation versus PFMT plus biofeedback | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT plus biofeedback | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence Follow‐up: 6 weeks | Study population | RR 1.06 | 51 | ⊕⊕⊝⊝ | ||
| 500 per 1000 | 530 per 1000 | |||||
| OAB‐related quality of life (lower scores indicate better quality of life) Follow‐up: 6 weeks | The mean OAB‐related quality of life in the intervention group was 5.78 lower (88.99 lower to 77.43 higher) | ‐ | 51 | ⊕⊕⊝⊝ | No evidence of a difference between groups in quality of life scores | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of selection and performance bias) | ||||||
| Electrical stimulation versus magnetic stimulation | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with magnetic stimulation | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Adverse effects Follow‐up: 4 weeks | Not estimable | 32 | ⊕⊝⊝⊝ | No events reported in either group | ||
| 0 per 1,00 | 0 per 1,00 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious imprecision (single trial, small sample size) | ||||||
| Electrical stimulation versus laseropuncture/electro‐acupuncture | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with laseropuncture/electro‐acupuncture | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Assessed with: Bladder Self‐Assessment Questionnaire (lower scores indicate better quality of life) Follow‐up: 12 weeks | The mean OAB‐related quality of life in the intervention group was 2.09 lower (4.1 lower to 0.08 lower) | ‐ | 22 | ⊕⊕⊝⊝ | Significantly greater quality of life in intervention group | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious imprecision (single trial, small sample size) | ||||||
| Electrical stimulation versus drug therapy | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with drugs | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 4 weeks to 2 years | Study population | RR 1.20 | 439 | ⊕⊕⊕⊝ | ||
| 585 per 1000 | 702 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 4 weeks to 6 months | One trial used OAB‐Q, PGII and PPIUS and found a significant result only in the PGII, which was in favour of ES. Another trial found no evidence of a difference between groups in I‐QoL scores. A third trial found higher QoL scores in the ES group at the end of treatment and at 3 months' follow‐up but no evidence of a difference at 6 months' follow‐up. | ‐ | 336 (3 RCTs) | ⊕⊕⊝⊝ | ||
| Adverse effects ‐ ES versus oxybutynin Follow‐up: 5 weeks | Study population | RR 0.11 | 79 | ⊕⊕⊝⊝ | ||
| 214 per 1000 | 24 per 1000 | |||||
| Adverse effects ‐ ES versus tolterodine Follow‐up: range 4 weeks to 2 years | Study population | RR 0.12 | 200 | ⊕⊕⊕⊝ | ||
| 459 per 1000 | 55 per 1000 | |||||
| Adverse effects ‐ ES versus solifenacin succinate Follow‐up: 4 weeks | Study population | RR 0.09 | 100 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Electrical stimulation plus PFMT versus PFMT alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT alone | Risk with electrical stimulation plus PFMT | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence Follow‐up: 12 weeks | Study population | RR 2.82 | 51 | ⊕⊕⊝⊝ | ||
| 269 per 1000 | 759 per 1000 | |||||
| Adverse effects Follow‐up: 12 weeks | Study population | Not estimable | 51 | ⊕⊝⊝⊝ | No events reported in treatment groups | |
| 0 per 1000 | 0 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 8 weeks to 6 months | One trial found greater quality of life in the intervention group (measured with ICIQ‐SF). Two other trials found no evidence of a difference between groups (measured with SF‐Qualiveen and York Incontinence Perception Scale) | ‐ | 201 (3 RCTs) | ⊕⊕⊝⊝ | ||
| Cost‐effectiveness | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Electrical stimulation plus behavioural therapy versus behavioural therapy alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with behavioural therapy alone | Risk with electrical stimulation plus behavioural therapy | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Follow‐up: 3 months | Intervention group reported significantly better quality of life measured with OAB‐Q and Incontinence Severity Index | ‐ | 82 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high risk of attrition bias, low risk of selection bias and unclear in other domains) | ||||||
| Electrical stimulation plus drug therapy versus drug therapy alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with drug therapy alone | Risk with electrical stimulation plus drug therapy | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life (lower scores indicate greater quality of life) Follow‐up: range 12 weeks to 6 months | The mean OAB‐related quality of life in the intervention group was 1.50 lower (3.72 lower to 0.72 higher) | ‐ | 248 | ⊕⊕⊝⊝ | ||
| Adverse effects Follow‐up: 12 weeks | Study population | RR 0.45 | 38 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 50 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| ES once a week versus ES twice a week | |||
| Patient or population: Adults with overactive bladder (OAB) | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Participants cured or improved Follow‐up: 6 months | 100% (37/37) of participants in both groups reported improvement in symptoms but only 9/37 were satisfied enough to request no further treatment | 37 (1 RCT) | ⊕⊝⊝⊝ |
| Participants with improvement in urgency urinary incontinence | Not reported | (0 studies) | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | |||
| ES once a week versus ES three times a week | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with ES 3 times a week | Risk with ES once a week | |||||
| Participants cured or improved (follow‐up not reported) | Study population | RR 0.97 | 35 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 647 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence (follow‐up not reported) | Study population | RR 0.80 | 22 | ⊕⊝⊝⊝ | ||
| 455 per 1000 | 364 per 1000 | |||||
| OAB‐related quality of life (follow‐up not reported) | I‐QoL scores very similar in the 2 groups (median (range) N): once a week: 77 (35‐100), 17. 3 times per week: 78 (33‐100), 18 | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse effects (follow‐up not reported) | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 35 (1 studies) | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Sensory threshold ES versus motor threshold ES | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with motor threshold ES | Risk with sensory threshold ES | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Follow‐up: 4 weeks | The mean OAB‐related quality of life in the intervention group was 0.07 lower (2.21 lower to 2.07 higher) | ‐ | 28 | ⊕⊝⊝⊝ | No evidence of a difference between groups | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (low risk of performance, detection and attrition bias but unclear risk of selection bias) | ||||||
Background
Description of the condition
Overactive bladder (OAB) is a chronic disorder with an overall prevalence in the adult population of over 10%, but that may exceed 40% in elderly groups (Irwin 2006). According to the International Continence Society, OAB is characterised by symptoms of urinary urgency (a strong compelling desire to urinate that is difficult to overcome), with or without urinary incontinence. If there is urinary incontinence accompanied by urgency, the leakage is called urgency urinary incontinence (UUI). Overactive bladder is usually accompanied by daytime frequency (increased need to urinate) and nocturia (waking during the night to urinate), but without urinary infection or other bladder pathologies (Abrams 2003). Overactive bladder with urinary incontinence is known as 'overactive bladder wet'; OAB without incontinence is known as 'overactive bladder dry'.
Overactive bladder has many potential causes, such as urinary tract infections, neurogenic diseases and pelvic organ prolapse. Urgency symptoms are often associated with involuntary contractions of the detrusor muscle in the bladder: this is termed detrusor overactivity if it is diagnosed using urodynamics. This overactivity can be related to neurogenic, myogenic, or idiopathic origins (Shaw 2011). However, currently its aetiology is unclear.
Urinary incontinence has many psychosocial implications. It appears that OAB has a greater psychological impact than stress urinary incontinence (SUI), with 60% of people with OAB reporting a history of depression compared with 14% of people with SUI (Zorn 1999).
Additionally, the financial impact of OAB can be substantial. Costs to health services and to patients are likely to be considerable given the relatively high prevalence of OAB, particularly in elderly people. The overall annual economic burden of OAB in the US in 2007 was estimated to be USD 65.9 billion, with the average annual per capita costs estimated to be USD 1925 (Gantz 2010). WIth the worldwide problems of increasingly constrained budgets and an aging population, it is imperative to ensure the efficient allocation of available resources; therefore value for money in OAB treatments must be considered.
Description of the intervention
Conservative management, such as bladder training (Wallace 2004) or pelvic floor muscle training, has been recommended as a first‐line treatment for OAB (Abrams 2003).
The main type of medical treatment for OAB is pharmacotherapy with anticholinergics, which have proven to be effective in several randomised controlled trials (RCTs) (Madhuvrata 2012). However, common side effects such as dry mouth and constipation limit long‐term compliance, with discontinuation rates of 70% to 90% within one year (D'Souza 2008). Intravesical botulinum toxin injections may be an effective and safe option to treat refractory OAB (Duthie 2011); in the UK, bladder wall injections with botulinum toxin A are recommended for women with OAB caused by proven detrusor overactivity if conservative or drug treatments have failed (NICE 2013). This is considered to be a surgical intervention in this review.
In people for whom conservative or drug treatment is not sufficient, neuromodulation is an alternative. It is thought that neuromodulation with electrical stimulation (ES) can target specific nerves in the sacral plexus that control pelvic floor function.
ES can be used to treat OAB via different routes, such as implantable or internal (sacral neuromodulation) and non‐implantable external electrodes. Stimulation with non‐implanted electrodes can be delivered invasively (percutaneous stimulation), semi‐invasively (typically vaginal or anal probes) or non‐invasively (transcutaneous stimulation).
ES can be used on its own or in association with pelvic floor muscle training, often indicated in SUI and OAB. There is currently little consensus regarding the optimum treatment regimen, the number and duration of sessions and the parameters used, such as electrical frequency and pulse width.
This review includes non‐implanted electrodes only; implanted devices are included in another Cochrane systematic review (Herbison 2009).
Routes of administration
Intravaginal electrical stimulation
Intravaginal ES for treating urinary incontinence was first reported in the literature in the 1960s (Cadwell 1963). Subsequently, it has been shown to achieve satisfactory results with frequencies below 12 Hertz (Hz) stimulating the pudendal nerve, which is thought to inhibit the detrusor muscle, reduce involuntary contractions and, consequently, reduce the number of micturitions (Messelink 1999). ES also works in a passive way, helping people with OAB become conscious of their perineal (pelvic floor) muscle contractions and this may, in turn, help to inhibit involuntary detrusor contractions (Amaro 2003).
The contraindications to intravaginal ES are pregnancy, vaginal infection or lesion, a reduced perception of vaginal sensation, menstruation, and metallic implants (Richardson 1996).
Rectal (anal) electrical stimulation
Transcutaneous electrical nerve stimulation (TENS) delivers an electrical current through an electrode placed in the ischiorectal area. Electrodes inserted in the rectal canal may inhibit detrusor contractions through contact with the pudendal nerve afferent fibres and thus may be effective in the treatment of UUI and OAB.
Posterior tibial nerve stimulation
Percutaneous tibial nerve stimulation is a form of neuromodulation that delivers retrograde stimulation to the sacral nerve plexus via a needle electrode inserted into the ankle, cephalad to the medial malleolus, an anatomical area recognised as the bladder centre. Transcutaneous tibial nerve stimulation is less invasive than percutaneous stimulation and can be delivered over the peroneal region of the ankle through surface electrodes (ICI 2013).
How the intervention might work
ES is thought to inhibit detrusor contractions, thus decreasing the number of micturitions and potentially increasing bladder capacity (Wang 2006). Electrodes can be located in the vaginal or rectal canals in such a way as to obtain direct contact with a significant quantity of afferent nerve fibres of the pudendal nerve. This stimulation of the pudendal nerve activates the skeletal pelvic floor muscles and inhibits detrusor contraction. Partial or total innervation of the pudendal nerve is necessary so that nerve stimulation can occur (Messelink 1999). The anal electrode can be used for men to stimulate the pudendal nerve, or in women where the vaginal approach is contraindicated.
There are two main mechanisms whereby ES is thought to work.
-
ES in the form of neurostimulation aims to stimulate motor efferent fibres of the pudendal nerve, which elicits a direct response from the effector organ, for instance a contraction of the pelvic floor muscles (Fall 1991; Scheepens 2003).
-
ES in the form of neuromodulation aims to remodel reflex loops, for instance the detrusor inhibition reflex, by stimulating afferent nerve fibres of the pudendal nerve that influence these reflex loops via the spinal cord (Vodusek 1986; Weil 2000).
The different sites for non‐implanted ES, for instance direct intravaginal stimulation or peripheral transcutaneous tibial nerve stimulation, may involve different mechanisms and therefore may have different degrees of effectiveness.
Why it is important to do this review
Numerous treatment options exist for OAB, including behavioural therapies such as pelvic floor muscle rehabilitation, bladder training, and dietary modification, as well as pharmacological therapy and neuromodulation. Overall, behavioural therapies are considered the mainstay of treatment for urinary incontinence. It is known that OAB can be improved through behavioural therapy or drug treatment, but it is not known whether non‐invasive ES achieves better clinical outcomes. This review aims to present an overview of current evidence related to ES in the treatment of OAB.
This systematic review aims to investigate the effects of non‐implanted ES in people with OAB with or without urgency incontinence. It also aims to compare specific subgroups to investigate whether ES might be more beneficial for some populations than for others.
Objectives
To assess the effects of electrical stimulation (ES) with non‐implanted electrodes for OAB, with or without urgency urinary incontinence (UUI), compared with: placebo or any other active treatment; ES added to another intervention compared with the other intervention alone; different methods of ES compared with each other.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs (RCTs in which allocation to treatment was based on methods such as alternate medical records, date of birth, or other predictable methods) and randomised cross‐over trials.
Types of participants
Eligible studies included adults (≥18 years old, or according to study authors' definitions of adult) with either of the following:
-
symptomatic diagnosis of overactive bladder (OAB), urgency urinary incontinence (UUI), or mixed urinary incontinence;
-
urodynamic diagnosis of detrusor overactivity in addition to OAB symptoms (urgency, frequency or episodes of urgency incontinence).
Studies including participants with stress urinary incontinence (SUI), with or without OAB symptoms were included if data were reported separately for SUI and participants with OAB, or if the majority (> 50%) of the population had OAB/UUI‐predominant symptoms.
Types of interventions
Eligible comparators were any intervention intended to decrease urinary frequency and included placebo, sham treatment, conservative treatment (including complementary therapies), drugs and surgery. We also included studies comparing different electrical stimulation (ES) methods with each other. There were no restrictions by type of device, stimulation parameters (such as continuous, interrupted, or duration of stimulation), duration of treatment, route of administration (e.g. vaginal, rectal, skin, pretibial area), or other similar factors. We excluded trials of different combinations of treatments even if one of those was ES, where it was not possible to identify the effect of this treatment alone (e.g. ES plus another treatment versus ES plus other combined treatments).
We investigated the following comparisons:
-
ES versus no active treatment
-
ES versus placebo or sham treatment
-
ES versus other conservative treatments (e.g. bladder training, pelvic floor muscle training, biofeedback, magnetic stimulation)
-
ES versus drug therapy (e.g. anticholinergics)
-
ES versus surgery (including botulinum toxin)
-
ES plus another treatment versus other treatment alone
-
One type of electrical stimulation versus another.
Types of outcome measures
We considered the following outcomes. Where outcome data were reported at more than one follow‐up point, we extracted the data from the end of treatment and from the longest available follow‐up period.
Primary outcomes
-
Perception of cure (number of participants without OAB symptoms; number of participants without self‐reported UUI)
-
Perception of improvement (number of participants with improvement in OAB symptoms; number of participants with improvement in self‐reported UUI)
-
Condition‐related quality‐of‐life measures (however defined by authors or by any validated measurement scales such as the International Consultation on Incontinence Questionnaire (ICIQ))
Secondary outcomes
-
Quantification of symptoms
-
Number of incontinence episodes (per 24 hours)
-
Number of urgency episodes (per 24 hours)
-
Number of micturitions (per 24 hours)
-
Number of nocturia episodes (per night)
-
Number of pads used per 24 hours
-
-
Economic data
-
Costs of interventions
-
Cost‐effectiveness of interventions
-
Resource implications
-
-
Procedure outcome measures
-
Duration of procedure
-
Length of hospital stay
-
Time to return to normal activity level
-
-
Adverse effects
-
Skin damage
-
Pain or discomfort
-
Vascular, visceral or nerve injury
-
Voiding dysfunction
-
Other complications
-
We also included other outcomes that were not pre‐specified but were deemed important during the course of data analysis.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) outcomes
We included the following outcomes in 'Summary of findings' tables (Guyatt 2008).
-
Number of participants with improvement in OAB symptoms or urgency symptoms
-
Number of participants with improvement in self‐reported UUI
-
OAB‐related quality of life
-
Number of participants with adverse effects (pain or discomfort due to treatment)
-
Cost‐effectiveness of interventions
Search methods for identification of studies
We did not impose any restrictions, for example language or publication status, on the searches described below.
Electronic searches
This review drew on the search strategy developed for Cochrane Incontinence. We identified relevant trials from the Cochrane Incontinence Specialised Trials Register. For more details of the search methods used to build the Specialised Register please see the Group's module in the Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (WHO ICTRP), UK Clinical Research Network Portfolio and handsearching of journals and conference proceedings. Most of the trials in the Cochrane Incontinence Specialised Register are also contained in CENTRAL. The date of the last search was 10 December 2015. The terms used to search the Cochrane Incontinence Specialised Register are given in Appendix 1.
Some of the review authors (OLFG, RE, MOG, AK, JLA) also searched the following databases; the search terms used are given in Appendix 1
-
PubMed (inception to December 2015) was searched on 12 December 2015;
-
CENTRAL (The Cochrane Library 2015, Issue 12 ) was searched on 12 December 2015;
-
Embase on OvidSP (covering from 1980 onwards) and the Latin‐American and Caribbean Center on Health Sciencies Information (LILACS) (on the Virtual Health Library/Bireme) (covering from 1982 to December 2015) were both searched on 12 December 2015. The highly sensitive Embase and LILACS strategies for identification of RCTs (Castro 1997; Castro 1999; Lefebvre 2011) were combined with search terms relating to the condition and interventions;
-
Information about ongoing clinical trials was sought by searching the clinical trials registration sites ClinicalTrials.gov and WHO ICTRP on 12 December 2015.
Searching other resources
Reference lists
The review authors scrutinised the reference lists of the identified relevant studies for additional citations.
Personal contact
We consulted clinical specialists and contacted authors of included trials where appropriate to obtain unpublished data.
Data collection and analysis
Selection of studies
Two review authors independently screened the trials identified by the literature search. We resolved any disagreements by consulting a third review author.
Data extraction and management
One review author extracted data, which was checked by a second reviewer, with discrepancies resolved by discussion. We used a pre‐standardised data extraction form to extract data pertaining to study characteristics (design, methods of randomisation), participants, interventions and outcomes.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias in included trials using the Cochrane tool for assessing risk of bias (Higgins 2011), considering the following four domains: random sequence generation, allocation concealment, blinding, and incomplete outcome data. We resolved any disagreements by consulting a third review author.
Measures of treatment effect
We analysed included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Binary outcomes
For dichotomous data, we calculated risk ratios (RRs) with 95% confidence intervals (CIs).
Continuous outcomes
For continuous data, we have presented mean differences (MDs) with 95% CIs.
Unit of analysis issues
The unit of analysis is each participant recruited into the trials.
We analysed studies with non‐standard designs as described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). We analysed studies with multiple treatment groups by treating each pair of arms as a separate comparison, as appropriate. For randomised cross‐over studies we used data from the first period of treatment only.
Dealing with missing data
We analysed data on an intention‐to‐treat (ITT) basis, as far as possible, whereby all participants must be analysed according to the groups to which they were randomised. Where participants were excluded after allocation or withdrew from the trial, we have reported any details provided in full. Where data from randomised cross‐over trials were incomplete we have included data from the first period of randomisation only.
We made all reasonable attempts to contact study authors for clarification of missing data. Where trials reported mean values without standard deviations (SDs) but with P values or 95% CIs, we used Review Manager's (RevMan) calculator to estimate the SDs (RevMan 2014). Where trials reported mean values only, we assumed the outcome to have a SD equal to the highest SD from the other trials within the same analysis.
Assessment of heterogeneity
We assessed clinical heterogeneity by examination of the study details and tested for statistical heterogeneity between trial results using the Chi2 test (Deeks 2011) and the I2 statistic (Higgins 2003), using the following I2 values:
-
less than 30% heterogeneity may not be important;
-
30% to 50% may represent moderate heterogeneity;
-
more than 50% may represent substantial or considerable heterogeneity.
Assessment of reporting biases
We intended to assess the likelihood of potential publication bias using funnel plots but insufficient data were available.
Data synthesis
We used Cochrane's statistical software, Review Manager 5 (RevMan) (RevMan 2014), for data analysis. We used the fixed‐effect model to analyse data. Where we identified significant heterogeneity (for example I2 higher than 50%), we computed pooled estimates of the treatment effect for each outcome under a random‐effects model (with two or more studies).
Where outcomes were reported which were similar to, but not precisely the same, as pre‐specified ones, we used 'surrogate' outcomes to substitute for missing data. For example, if a trial reported episodes of urinary incontinence without specifying the type of incontinence (e.g. SUI or UUI), we used the data as a substitute for UUI. Similarly, we used 'improvement in urgency symptoms' as a substitute for 'improvement in OAB symptoms'. Finally, if a subjective outcome (such as OAB symptoms) was reported as combined with an objective outcome (such as detrusor overactivity) without reporting them separately, we used that outcome as a surrogate for the subjective outcome.
In comparing ES to drug therapy we have presented subgroups for each drug but this is for presentation purposes only and is not intended to act as an indirect comparison between drugs. When comparing ES to drug therapy, in terms of adverse effects, we did not use a pooled estimate of effect because of the variation between drugs in the range of possible side effects.
Subgroup analysis and investigation of heterogeneity
In the case of substantial heterogeneity (I2 > 50%), we investigated the causes of heterogeneity and, where data permitted, carried out the following subgroup analyses:
-
participants with idiopathic OAB versus those with neurogenic OAB;
-
approaches of electrodes (transcutaneous (e.g. perineal skin, sacral, posterior pretibial nerve), endocavitary (vaginal, rectal, urethral), and percutaneous (posterior pretibial nerve).
In some cases, we have presented forest plots with subgroups for illustrative purposes only, for instance in comparison 2 (electrical stimulation compared to other conservative treatments), we wanted to demonstrate the various comparators in the trials so we conveyed this information in the names of the subgroups. Similarly, we used the same approach in comparison 4 (electrical stimulation plus another treatment compared to the other treatment alone), to demonstrate the various other treatments.
Sensitivity analysis
We intended to perform a sensitivity analysis comparing trials with low risk of selection bias to those with high risk of bias but there were insufficient numbers of eligible trials.
'Summary of findings' tables
We applied the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (perception of cure, perception of improvement and OAB‐related quality of life) (Guyatt 2008). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias. We constructed 'Summary of findings' tables using the GRADEpro GDT software (GRADEpro GDT 2015).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search strategy identified 3862 records; after removal of duplicate references there was a total of 3428 titles and abstracts to screen. Following assessment of 230 full‐text articles, we considered 84 reports of 63 studies that met the minimal methodological requirements for inclusion in this review. Figure 1 details the screening process.

PRISMA study flow diagram
Thirteen reports of 13 ongoing studies were identified and have been added to the list of ongoing studies (NTR2192; NCT01783392; NCT02456441; NCT02583529; NCT02377765; NCT01940367; NCT02582151; NCT01464372; NCT01912885; NCT02452593; NCT02110680; NCT02311634; NCT02511717) (see: Characteristics of ongoing studies).
Included studies
The individual trials are described in the Characteristics of included studies table.
Sixty‐three trials (84 reports) met the inclusion criteria and were included in this review. A total of 4224 participants were randomised across the included trials.
Design
All but five of the included studies were reported as RCTs. We included three randomised cross‐over trials (Gonzalez 2015; Soomro 2001; Vecchioli‐Scaldazza 2013) and two quasi‐RCTs (Svihra 2002; Wise 1992).
Sample size
Thirty‐seven of the included studies did not report any details relating to sample size calculation. Sample sizes ranged from 22 to 315 (median 51).
Setting
The trials took place in a variety of countries:
-
12 trials took place in Brazil (Alves 2015; Amaro 2006; Arruda 2008; Barroso 2002; Boaretto 2011; Bellette 2009; Marques 2008; Monteiro 2014; Schmidt 2009; Schreiner 2010; Schreiner 2014; Souto 2014);
-
10 in the UK (Booth 2013; Monga 2011; Oldham 2013; Seth 2014; Shepherd 1984; Shepherd 1985; Slovak 2015; Vohra 2002; Wise 1992; Wise 1993);
-
nine in the USA (Brubaker 1997; Firra 2013; Kennelly 2011; Lobel 1998; Peters 2009; Peters 2010; Phillips 2012; Smith 1996; Sotelo 2011);
-
three each in Australia (Bower 1998; Lo 2003; Soomro 2001), Italy (Finazzi‐Agrò 2005; Finazzi‐Agrò 2010; Vecchioli‐Scaldazza 2013) and Taiwan (Wang 2004; Wang 2006; Wang 2009);
-
two each in Chile (Gonzalez 2015; Manriquez 2013), China (Chen 2015; Lin 2004), Japan (Yamanishi 2000a; Yamanishi 2000b) the Netherlands (Berghmans 2002; Spruijt 2003);
-
one each in Belgium (Gaspard 2014), Egypt (Abdelbary 2015), Finland (Vahtera 1997), Iran (Eftekhar 2014), Russia (Kosilov 2013), Slovakia (Svihra 2002), Spain (Olmo Carmona 2013) and Sweden (Franzén 2010); and Turkey (Sancaktar 2010)
-
one in Austria and Germany (Preyer 2015).
Five studies did not report the country or any details on study setting (Aaronson 1995; Lima 2011; Orhan 2015; Preyer 2007; Walsh 2001).
Very few details were reported regarding study settings; exceptions were one trial carried out in residential care homes and sheltered accommodation (Booth 2013) and trials investigating types of ES suitable for home or portable use (Barroso 2002; Kennelly 2011; Monga 2011; Oldham 2013; Phillips 2012; Seth 2014; Shepherd 1985; Soomro 2001; Sotelo 2011; Wise 1992; Wise 1993).
Participants
The trials included a variety of participant groups.
Sex
Fourteen trials were open to men and women (Booth 2013; Olmo Carmona 2013; Gaspard 2014; Kennelly 2011; Monga 2011; Peters 2009; Peters 2010; Phillips 2012; Slovak 2015; Soomro 2001; Vahtera 1997; Walsh 2001;Yamanishi 2000a; Yamanishi 2000b), one was open only to men (Monteiro 2014), and six did not report the participants' sex (Gonzalez 2015; Lin 2004; Orhan 2015; Seth 2014; Sotelo 2011; Vohra 2002). All other trials were open to women only.
Age
One trial included only participants over 65 years (Booth 2013). Two trials included only participants over 60 years (Alves 2015; Schreiner 2014) and another imposed a lower age limit of 40 (Abdelbary 2015). The Olmo Carmona 2013 trial included participants aged 45 to 75 (mean 60 years). Fourteen trials did not report participants' mean age (Alves 2015; Lima 2011; Manriquez 2013; Marques 2008; Monga 2011; Orhan 2015; Phillips 2012; Preyer 2015; Seth 2014; Shepherd 1984; Shepherd 1985; Wang 2006; Wise 1992; Wise 1993). Across the remaining trials, the mean age of participants in the trials ranged from 46 to 70 years.
Diagnosis
The participants had a variety of diagnoses of the causes of their overactive bladder (OAB).
-
Fourteen trials based their inclusion criteria on urodynamic diagnosis (Aaronson 1995; Arruda 2008; Berghmans 2002; Bower 1998; Brubaker 1997; Finazzi‐Agrò 2010; Lobel 1998; Shepherd 1985; Smith 1996; Walsh 2001; Wise 1992; Wise 1993; Yamanishi 2000a; Yamanishi 2000b).
-
Six trials included only participants with neurogenic OAB or detrusor overactivity (Chen 2015; Eftekhar 2014; Gaspard 2014; Monteiro 2014; Seth 2014; Vahtera 1997).
-
All other trials reported inclusion criteria based on symptomatic diagnosis of OAB, urgency urinary incontinence (UUI), or any kind of incontinence or bladder dysfunction.
Eleven trials included participants with mixed urinary incontinence (MUI and stress urinary incontinence (SUI)) (Barroso 2002; Booth 2013; Brubaker 1997; Firra 2013; Lo 2003; Oldham 2013; Schmidt 2009; Shepherd 1984; Shepherd 1985; Smith 1996; Spruijt 2003). All other trials included participants with OAB and UUI only.
Duration of trials
Treatment duration ranged from a single one‐off session to four months. Fifteen trials followed up participants beyond the end of the treatment period (Abdelbary 2015; Amaro 2006; Arruda 2008; Barroso 2002; Gaspard 2014; Kosilov 2013; Lobel 1998; Monteiro 2014; Peters 2010; Schmidt 2009; Schreiner 2010; Slovak 2015; Souto 2014; Vahtera 1997; Vecchioli‐Scaldazza 2013). The duration of post‐treatment follow‐up ranged from one month to two years. Four trials did not report treatment duration or follow‐up.
Types of interventions
The parameters and components of the active electrical stimulation (ES) interventions varied widely and are summarised in Table 1.
| Study | Current | Current intensity | Pulse shape & duration | Frequency (Hz) | Duty cycle | Electrodes | Treatment duration/supervision |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Unclear | |
| 30‐60 mA according to patient tolerance (mean 43 mA) | 320 ms | 20 | Unclear | Intravaginal | Two 30‐min sessions per week for 12 weeks | ||
| Unclear | "Sensory threshold, activating superficial cutaneous nerve fibers with larger diameter" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks | |
| Unclear | "Motor threshold, non‐painful contraction is induced and the stimulation can simply make pain relief in the same way that sensory stimulation level (blocking activation of the peripheral or cental inhibition)" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks | |
| Bipolar | 0‐100 mA according to participant tolerance | Bipolar square wave 0.1 µs | 4 | 2 s on, 4 s off | Intravaginal | Three 20‐min sessions per week on alternate days for 7 weeks | |
| Biphasic | 10‐100 mA according to participant tolerance | 1 ms intermittent | 10 | Unclear | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | 0‐100 mA | Asymmetric, 1 s rise time, sustained for 5 s and resting for 5 s | 20 | 1 s rise time, sustained for 5s and resting for 5 s | Intravaginal | Home use: two 20‐min sessions per day for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve | Two 30‐min sessions per week for 4 weeks | |
| Biphasic | 0‐100 mA | Rectangular 200 µs stochastic variation | 4‐10 | Unclear | Intravaginal | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous posterior tibial nerve | Twelve 30‐min sessions | |
| Unclear | Unclear | 500 µs | 10 | Unclear | Intravaginal | Twelve 30‐min sessions | |
| Unclear | 0‐50 mA | 200 µs | 10 | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks | |
| Unclear | Unclear | 200 µs | 150 | Unclear | Transcutaneous electrical nerve stimulation – suprapubic placement | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation – sacral placement | Unclear | |
| Bipolar | 0‐100 mA | Bipolar square wave 0.1 µs | 20 | 2 s on ‐ 4 s off | Intravaginal | 20 minutes daily for 8 weeks | |
| Unclear | 0‐10 mA | Square wave 320 µs | 20 | unclear | Percutaneous posterior tibial nerve stimulation | 30 min once a week for 12 weeks | |
| Bipolar | According to participant tolerance | Continuous bipolar square wave 200 µs | 20 | Unclear | Percutaneous posterior tibial nerve stimulation ‐ adhesive skin electrodes | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation ‐ "34 gauge needle placed 5 cm near internal malleolus" | 30‐min sessions | |
| Unclear | 0‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Percutaneous tibial nerve stimulation | Three 30‐min sessions per week for 4 weeks | |
| Unclear | Unclear current, intensity according to participant tolerance | Unclear | 12.5 | 5 s on, 10 s off | Intravaginal | Fourteen 30‐min sessions | |
| Unclear | According to participant tolerance | Unclear | 5‐10 | Intravaginal/transanal | 10 sessions: 1‐2 20‐min sessions per week for 5‐7 weeks | ||
| Biphasic | Unclear | Biphasic rectangular 220 µs | 10 | 20 s on, 4 s off | Transcutaneous posterior tibial nerve stimulation: external electrode 5 cm above medial malleolus, 1 cm behind the tibia. The other electrode on dorsum of foot | One 30‐min session per week for 9 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twice a week for 6 weeks, performed by either physiotherapist or continence midwife | |
| Unclear | Unclear | Unclear | Unclear | Unclear | VERV electrode patches, placed by the participant ‐ exact placement unclear | One patch per week for 12 weeks | |
| Diadynamic | 20–40 mA, 50%‐75% intensity | Unclear | 20 | Unclear | Active electrode (50 cm2 to 70 cm2) above the pubis, and a passive electrode (150 cm2) in lumbosacral area | 15 procedures every other day | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Twelve 30‐min sessions | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twelve 30‐min sessions | |
| Unclear | 8‐70 mA | Unclear | Unclear | Unclear | Vaginal/anorectal | 20‐30 20‐min sessions | |
| Unclear | According to participant tolerance | Unclear | 0‐100 | Unclear | Interferential therapy. 2 anterior flat electrodes placed over obturator foramen 1.5 cm to 2 cm lateral to symphysis, two posterior electrodes placed medial to ischial tuberosities either side of anus | 12 sessions: first session 15 min, all others 30 min | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Once per week | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Twice per week | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous tibial nerve stimulation | Twice a week with at least 48 hour intervals for 12 weeks | |
| Biphasic | Immediately below motor threshold | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation through 1 channel and 2 electrodes | Two 30‐min sessions per week for 4 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal amplitude‐modulated signal through a patch applied to the skin, controlled by wireless handheld remote control | Patch worn for 4 weeks | |
| Unclear | Below the threshold that causes motor contraction | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation with surface electrodes. Negative electrode on medial malleolus, and the positive electrode 10 cm above negative electrode, also on the medial side. Rhythmic flexion of the second toe during the stimulation determined the correct position of the negative electrode | 30‐min twice weekly over 12 sessions (45 days) | |
| Unclear | Pre‐programmed to increase intensity over 24 s to reach therapeutic level and switch off automatically after 30 min. All devices same level of stimulation (average intensity considered comfortable and capable of producing contractions of pelvic floor muscles) | Unclear | During the 10 s ‘‘on time’’ the device delivers 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation | 10 s on, 10 s off | Intravaginal, single‐use tampon‐like Pelviva device | One disposable device per day for 12 weeks except during menstruation | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle slightly cephalad to medial malleolus | One 30‐min session per week for 12 weeks | |
| Unclear | 0.5‐9 mA | Unclear | 20 | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle inserted at 60º angle 5 cm cephalad to medial malleolus, slightly posterior to tibia. Surface electrode placed on ipsilateral calcaneous | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Participant‐managed neuromodulation system patch | Subject placement versus investigator placement | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Peripheral tibial neurostimulation | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 3 months | |
| Unclear | 0.5‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Stoller afferent neurostimulation: 34‐gauge needle inserted at 30° angle 2 cm to 3 cm superior‐medial aspect of tibial medial malleolus along posterior tibial nerve trace | One 30‐min session per week for 12 weeks | |
| Biphasic | Controlled by participant according to tolerance | 300 µs | Asymmetrical, 50 | Unclear | Intravaginal: probe with two 26 mm rings 40 mm apart | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous tibial nerve stimulation | One 30 min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30 min session per day for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30‐min session per week for 12 weeks | |
| Unclear | Up to 40 v | Unclear | 10‐50 | Unclear | Maximum perineal stimulation: Scott electrode in vagina, large indifferent electrode under buttocks | Single 20‐min session | |
| Unclear | Unclear | Unclear | 10 | Unclear | Intravaginal cushion attached to stimulator worn around waist | Cushion worn for 8 out of 24 h, day or night according to participant preference | |
| Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Unilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on the right ankle | Unclear | |
| Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Bilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on both ankles | Unclear | |
| Unclear | 5‐25 mA | Unclear | Device uses 2 programmes simultaneously: 12.5 Hz and 50 Hz | 5 s impulse | Intravaginal | Twice daily for 4 months. Length of session increased monthly: 15, 30, 45, 60 minutes | |
| Unclear | Participants asked to control stimulation to achieve tickling sensation | 200 µs | 20 | Continuous | Transcutaneous. 2 self‐adhesive pads applied bilaterally over the perianal region (S2‐S3 dermatome) | Up to 6 hours daily for 6 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. Horizontal placement of electrode patch near sacral nerve | Patch worn continuously for 7 days | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. 30° angle placement of electrode patch near sacral nerve | Patch worn continuously for 7 days | |
| Unclear | According to participant tolerance | 250 µs | 10 | Unclear | Posterior tibial nerve stimulation. Surface electrode placed behind media malleolus and another placed 10 cm above first electrode | Two 30 min sessions per week for 12 weeks | |
| Biphasic | 0‐100 mA, according to participant tolerance | 100 µs | 20 | 2 s contraction time, duty cycle 1–2 s | Intravaginal | Three 30‐min sessions per week for 8 weeks. 5 min rest between each 15 min | |
| Square | 25 mA. 70% of intensity of maximal amplitude of registered response from abductor hallucis muscle | Square impulse 100 µs | 1 | Unclear | Stoller afferent neurostimulation. Electrodes placed behind medial ankle of left lower extremity, cathode placed proximally and anode distally | One 30 min session per week for 5 weeks | |
| Unclear | According to participant tolerance | Unclear | 10 min of each frequency, 3 min: 5‐10 Hz, 10‐50 Hz, 50 Hz | 7 s on, 25 s off | Intravaginal/transanal | 6 sessions over two weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks | |
| Unclear | 0‐10 mA | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | 200 ms | 10 | Unclear | Transcutaneous neurostimulation. Electrode pads affixed bilaterally to the skin overlying S3 dermatomes (junction of buttock and upper thigh) | Single session | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | One session per day (at home) for 6 weeks | |
| Unclear | 0‐90 mA, according to participant tolerance | Unclear | 20 | Unclear | Intravaginal | One session per day (at home) for 6 weeks | |
| Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed electrodes | Two 15‐min sessions per day for 4 weeks | |
| Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed | Single session |
Control/comparator interventions included the following.
-
No active treatment (Berghmans 2002; Marques 2008; Monteiro 2014; Oldham 2013; Slovak 2015: Svihra 2002; Vahtera 1997)
-
Sham ES (Amaro 2006; Barroso 2002; Bellette 2009; Booth 2013; Bower 1998; Brubaker 1997; Finazzi‐Agrò 2010; Kennelly 2011; Peters 2010; Shepherd 1984; Shepherd 1985; Vohra 2002; Walsh 2001; Yamanishi 2000a)
-
Placebo (Kosilov 2013; Wang 2006; Wang 2009)
-
Pelvic floor muscle training (PFMT) (Arruda 2008; Berghmans 2002; Boaretto 2011; Firra 2013; Gaspard 2014; Lima 2011; Lo 2003; Schmidt 2009; Schreiner 2010; Spruijt 2003; Wang 2004)
-
PFMT plus biofeedback (Gaspard 2014; Schmidt 2009; Wang 2004)
-
Bladder training and PFMT (Schreiner 2014)
-
Behavioural therapy (Gonzalez 2015)
-
Electro‐acupunture (Olmo Carmona 2013)
-
Laseropuncture (Kosilov 2013)
-
Functional magnetic stimulation (Yamanishi 2000b)
-
Drug treatment (oestrogen cream, oxybutynin, propantheline bromide, probanthine, solifenacin succinate, terodiline, tolterodine and trospium chloride) (Aaronson 1995; Abdelbary 2015; Arruda 2008; Boaretto 2011; Chen 2015; Franzén 2010; Kosilov 2013; Lin 2004; Manriquez 2013; Orhan 2015; Peters 2009; Preyer 2007; Preyer 2015Sancaktar 2010; Smith 1996; Soomro 2001; Souto 2014; Svihra 2002; Vecchioli‐Scaldazza 2013; Wang 2006; Wang 2006; Wang 2009; Wise 1992; Wise 1993)
-
Different ES regimens (Alves 2015; Lobel 1998; Monga 2011; Phillips 2012; Seth 2014; Slovak 2015; Sotelo 2011)
In one trial (Marques 2008) it was unclear whether the comparator was no active treatment or sham treatment; the description was "the same protocol but without electrical stimulation."
Types of outcomes
Nineteen trials reported the primary outcomes of perception of cure or improvement of OAB symptoms (Aaronson 1995; Bellette 2009; Booth 2013; Kennelly 2011; Lin 2004; Lo 2003; Lobel 1998; Monteiro 2014; Peters 2009; Peters 2010; Schmidt 2009; Shepherd 1985; Smith 1996; Soomro 2001; Spruijt 2003; Vohra 2002; Wang 2004; Wang 2006; Wang 2009).
A validated measure of quality of life (QoL) was reported in 22 trials (Alves 2015; Bellette 2009; Olmo Carmona 2013; Chen 2015; Finazzi‐Agrò 2010; Firra 2013; Gaspard 2014; Gonzalez 2015; Oldham 2013; Orhan 2015; Peters 2010; Phillips 2012; Sancaktar 2010; Schmidt 2009; Schreiner 2010; Schreiner 2014; Seth 2014; Souto 2014; Svihra 2002; Vecchioli‐Scaldazza 2013; Wang 2004; Wang 2009). Two trials reported QoL, but did not state the instrument used (Abdelbary 2015; Preyer 2007), and another trial used an in‐house QoL instrument (Yamanishi 2000a).
Thirteen trials did not report any of the primary outcomes (Berghmans 2002; Bower 1998; Eftekhar 2014; Kosilov 2013; Manriquez 2013; Monga 2011; Preyer 2015; Sancaktar 2010; Slovak 2015; Sotelo 2011; Vahtera 1997; Wise 1993; Yamanishi 2000b).
Five trials reported urodynamic outcomes only (Berghmans 2002; Bower 1998; Vahtera 1997; Walsh 2001; Yamanishi 2000b).
Twenty trials reported data relating to adverse effects (Chen 2015; Finazzi‐Agrò 2005; Franzén 2010; Gaspard 2014; Kennelly 2011; Lin 2004; Lobel 1998; Oldham 2013; Peters 2010; Phillips 2012; Preyer 2007; Preyer 2015; Sancaktar 2010; Schreiner 2010; Soomro 2001; Sotelo 2011; Svihra 2002; Wise 1993; Yamanishi 2000a; Yamanishi 2000b).
None of the trials reported any data relating to procedure outcome measures.
Excluded studies
After full‐text screening, we excluded 132 reports of 128 studies from the review. The main reasons for exclusion were ineligible study design (non‐RCTs), ineligible population (participants did not have OAB or UUI), and ineligible interventions such as sacral neuromodulation with implanted devices or magnetic stimulation.
See the table 'Characteristics of excluded studies' for full details of the excluded studies.
Studies awaiting classification
One report of one study is awaiting translation (Zhao 2000).
Ongoing studies
We identified 13 reports of 13 ongoing trials that met our inclusion criteria (Characteristics of ongoing studies).
The following comparisons are being investigated in the ongoing trials.
-
ES versus sham ES (NCT02456441; NCT02583529; NCT01464372; NCT02582151; NCT02110680; NCT02511717)
-
ES versus conservative treatment (bladder training: NTR2192; PFMT: NCT02452593)
-
Different types of ES (NCT01783392; NCT02377765; NCT01940367; NCT01912885; NCT02311634)
Risk of bias in included studies

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Two trials were judged to be at high risk of bias for random sequence generation (Monteiro 2014; Svihra 2002) because their methods of sequence generation did not appear to be truly random. Twenty‐three trials were judged to be at low risk of bias for randomisation (Abdelbary 2015; Arruda 2008; Barroso 2002; Bellette 2009; Berghmans 2002; Booth 2013; Brubaker 1997; Olmo Carmona 2013; Eftekhar 2014; Finazzi‐Agrò 2010; Firra 2013; Franzén 2010; Gonzalez 2015; Oldham 2013; Peters 2009; Preyer 2015; Sancaktar 2010; Schreiner 2010; Slovak 2015; Souto 2014; Spruijt 2003; Vohra 2002; Wang 2009). The remaining trials did not report their methods in sufficient detail to judge whether allocation to groups was fully randomised and therefore were at unclear risk of bias.
Allocation concealment
Nine trials reported adequate methods of concealment of allocation and so were at low risk of bias (Berghmans 2002; Olmo Carmona 2013; Firra 2013; Franzén 2010; Preyer 2015, Shepherd 1984; Slovak 2015; Wang 2004; Wang 2006), none were judged to be at high risk and the remainder did not report sufficient detail regarding their methods of allocation concealment and we therefore judged them to have an unclear risk of bias.
Blinding
Blinding of participants and personnel (performance bias)
Four trials (Arruda 2008; Bellette 2009; Eftekhar 2014; Preyer 2015) were judged to be at high risk of performance bias because treatment was carried out by personnel who were aware of treatment group allocation, which may have influenced their treatment methods.
Fifteen trials had adequate blinding methods to be judged at low risk of performance bias (Alves 2015; Amaro 2006; Barroso 2002; Berghmans 2002; Booth 2013; Bower 1998; Brubaker 1997; Finazzi‐Agrò 2010; Kennelly 2011; Peters 2010; Shepherd 1985; Slovak 2015; Wang 2006; Wang 2009; Yamanishi 2000a) and the remainder were unclear.
For some comparisons, blinding of participants would not be possible, for instance ES versus drug treatment, versus surgery or versus conservative treatment. Trials investigating those comparisons were judged to be at unclear risk of performance bias because knowledge of the treatment received may have had an influence on self‐reported outcomes but there was no means of avoiding it.
Blinding of outcome assessment (detection bias)
Four trials (Firra 2013; Bellette 2009; Eftekhar 2014; Preyer 2015) were at high risk of detection bias because the outcome assessors were not blinded to group allocation.
Eighteen trials were judged to be at low risk of detection bias (Alves 2015; Amaro 2006; Arruda 2008; Barroso 2002; Berghmans 2002; Brubaker 1997; Olmo Carmona 2013; Finazzi‐Agrò 2010; Gaspard 2014; Kennelly 2011; Lo 2003; Oldham 2013; Schmidt 2009; Shepherd 1984; Slovak 2015; Vecchioli‐Scaldazza 2013; Wang 2004; Wang 2006) and the remainder were unclear.
Incomplete outcome data
Four trials were at high risk of attrition bias.
-
Gonzalez 2015 and Seth 2014 reported differential attrition with no adequate explanation and did not report whether the analysis included all participants who were randomised.
-
Schreiner 2014 reported 12 month follow‐up data for a proportion of the intervention group and no 12 month data for the comparator group.
-
Wise 1993 experienced differential withdrawal for reasons attributable to the comparator.
Twenty‐eight trials were judged to be at low risk of attrition bias (Alves 2015; Arruda 2008; Bellette 2009; Berghmans 2002; Booth 2013; Olmo Carmona 2013; Chen 2015; Finazzi‐Agrò 2010; Franzén 2010; Gaspard 2014; Kennelly 2011; Lin 2004; Lobel 1998; Monteiro 2014; Peters 2009; Peters 2010; Preyer 2007; Preyer 2015; Schmidt 2009; Schreiner 2010; Spruijt 2003; Vecchioli‐Scaldazza 2013; Vohra 2002; Walsh 2001; Wang 2004; Wang 2009; Yamanishi 2000a; Yamanishi 2000b) and the remainder were unclear.
Effects of interventions
See: Summary of findings for the main comparison Electrical stimulation versus no active treatment; Summary of findings 2 Electrical stimulation versus placebo or sham treatment; Summary of findings 3 Electrical stimulation versus pelvic floor muscle training (PFMT); Summary of findings 4 Electrical stimulation versus pelvic floor muscle training (PFMT) plus biofeedback; Summary of findings 5 Electrical stimulation versus magnetic stimulation; Summary of findings 6 Electrical stimulation versus laseropuncture/electro‐acupuncture; Summary of findings 7 Electrical stimulation versus drug therapy; Summary of findings 8 Electrical stimulation plus pelvic floor muscle training (PFMT) versus PFMT alone; Summary of findings 9 Electrical stimulation plus behavioural therapy versus behavioural therapy alone; Summary of findings 10 Electrical stimulation plus drug therapy versus drug therapy alone; Summary of findings 11 Electrical stimulation (ES) once a week versus ES twice a week; Summary of findings 12 Electrical stimulation (ES) once a week versus ES three times a week; Summary of findings 13 Sensory threshold electrical stimulation (ES) versus motor threshold ES
1. Electrical stimulation versus no active treatment
Five trials with 336 participants compared ES with no active treatment (Berghmans 2002; Monteiro 2014; Oldham 2013; Svihra 2002; Vahtera 1997).
Primary outcomes
Perception of cure or improvement of OAB symptoms
Two trials reported subjective cure or improvement (Monteiro 2014; Oldham 2013). Low‐quality evidence indicated that participants receiving ES were more likely to report cure or improvement in symptoms than those receiving no active treatment (RR 1.85, 95% CI 1.34 to 2.55; n = 121) (Analysis 1.1; summary of findings Table for the main comparison).
Number of participants satisfied with treatment
Not reported
Improvement in urgency urinary incontinence (UUI)
Not reported
OAB‐related quality of life
Two trials (Oldham 2013, Svihra 2002) reported QoL measured by the following instruments:
-
International Consultation on Incontinence Questionnaire (ICI‐Q);
-
Incontinence Quality of Life Questionnaire (I‐QoL);
-
Behavioural Urge Score (BUS); and
-
International Prostate Symptom Score (IPSS)
Low quality evidence indicated no evidence of a difference in quality of life between those undergoing ES and those who received no active treatment (summary of findings Table for the main comparison; Table 2).
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | No active treatment (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Improvement in QoL measured by Incontinence Quality of Life Questionnaire, Behavioural Urge Score and International Prostate Symptom Score | 5/9 | 0/9 | RR 11.00 (95% CI 0.70 to 173.66) | |
| ICI‐Q score1 | Median (range), N: 6 (0‐17), 64 | Median (range), N: 9 (3‐18), 60 | Not estimable | |
| Secondary outcomes | ||||
| Daytime frequency | NR | NR | Favours ES P = 0.0001 | |
| Nocturia | NR | NR | Favours ES P = 0.0186 | |
| Participants with nocturnal enuresis | 45 days' treatment: 0/12 | 45 days' treatment: 2/12 | Favours ES RR 5.00 (95% CI 1.63 to 15.31) | |
| 12 months' follow‐up: 0/12 | 12 months' follow‐up: 2/12 | |||
| Participants with nocturia | 45 days' treatment: 5/12 | 45 days' treatment: 9/12 | RR 2.33 (95% CI 0.78 to 6.94) | |
| 12 months' follow‐up: 1/12 | 12 months' follow‐up: 6/12 | Favours ES RR 0.17 (95% CI 0.02 to 1.18) | ||
| Participants with increased daytime frequency | 45 days' treatment: 3/12 | 45 days' treatment: 11/12 | Favours ES RR 0.27 (95% CI 0.10 to 0.74) | |
| 12 months' follow‐up: 0/12 | 12 months' follow‐up: 9/12 | Favours ES RR 0.05 (95% CI 0.00 to 0.81) | ||
Results in bold are statistically significant
1Higher score = greater severity
Secondary outcomes
Quantification of symptoms
One trial reported a statistically significant effect in favour of ES in terms of nocturia and daytime frequency (Marques 2008) but without giving any raw data (Table 2).
One trial reported symptom outcomes at two different time points (Monteiro 2014), which suggested that the effectiveness of ES did not diminish over time (Table 2).
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Not reported
2. Electrical stimulation versus placebo or sham treatment
Eighteen trials with 1569 participants compared ES to placebo or sham treatment: drug placebo: Kosilov 2013; Wang 2006; Wang 2009; and sham ES: Amaro 2006; Barroso 2002; Bellette 2009; Booth 2013; Bower 1998; Brubaker 1997; Finazzi‐Agrò 2010; Kennelly 2011; Peters 2010; Shepherd 1984; Shepherd 1985; Slovak 2015; Vohra 2002; Walsh 2001; Yamanishi 2000a.
Primary outcomes
Perception of cure or improvement of OAB symptoms
Based on four trials (Bellette 2009; Wang 2006; Wang 2009; Yamanishi 2000a), participants receiving ES were almost three times more likely than those in the placebo or sham treatment groups to be cured, according to subjective assessment (RR 2.69, 95% CI 1.39 to 5.21; n = 189) (Analysis 2.1).
Moderate‐quality evidence, based on 10 trials, suggested that participants receiving ES were more than twice as likely as those in the placebo or sham treatment groups to report cure or improvement of OAB symptoms (RR 2.26, 95% CI 1.85 to 2.77; n = 677) (Analysis 2.2; summary of findings Table 2) (Bellette 2009; Booth 2013; Finazzi‐Agrò 2010; Kennelly 2011; Peters 2010; Slovak 2015; Vohra 2002; Wang 2006; Wang 2009; Yamanishi 2000a). Heterogeneity was high (I2 = 66%) but the estimate of effect remained statistically significant with a random‐effects model (RR 2.46, 95% CI 1.60 to 3.80).
Moderate‐quality evidence relating to subjective cure or improvement showed that percutaneous tibial nerve stimulation was more effective than sham or placebo treatment (RR 3.19, 95% CI 2.22 to 4.58; n = 304) (Booth 2013; Finazzi‐Agrò 2010; Peters 2010; Vohra 2002), while intravaginal ES showed an even greater effect (RR 5.46, 95% CI 2.33 to 12.81; n = 94) (Wang 2006; Wang 2009) (Analysis 2.3).
Number of participants satisfied with treatment
Two small trials (Amaro 2006; Yamanishi 2000a) showed that participants undergoing ES were more likely to report satisfaction with treatment than those receiving sham ES (RR 1.44, 95% CI 1.02 to 2.04; n = 98) (Analysis 2.4).
Improvement in urgency urinary incontinence
Moderate‐quality evidence supported the use of ES in terms of improvement in UUI when compared to placebo or sham treatment (RR 2.23, 95% CI 1.46 to 3.40), however, heterogeneity was high (I2 = 78%), probably due to the large differences in effect sizes between the trials (Finazzi‐Agrò 2010; Peters 2010). A random‐effects model still favoured ES but the result was no longer statistically significant (RR 5.03, 95% CI 0.28 to 89.88; n = 242) (Analysis 2.5; summary of findings Table 2).
OAB‐related quality of life
Seven trials reported a measure of QoL related to OAB or incontinence. One trial used an instrument that was not validated (Yamanishi 2000a); the other instruments used were:
-
International Consultation on Incontinence Questionnaire – Urinary Incontinence (ICIQ‐UI) (Booth 2013; Oldham 2013);
-
Overactive Bladder Questionnaire (OAB‐Q) (Bellette 2009; Peters 2010); and
-
Incontinence Quality of Life (I‐QOL) (Finazzi‐Agrò 2010; Svihra 2002).
Three trials reported statistically significant differences in favour of ES in QoL scores (Bellette 2009; Peters 2010; Yamanishi 2000a) but the other trials found no evidence of a difference (Table 3); these results were based on very low‐quality evidence (summary of findings Table 2).
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Placebo or sham treatment (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| ICIQ‐SF score | Median (IQR), N: 2 (0 to ‐6), 15 | 0 (‐3 to 3), 13 | P = 0.132 | |
| Participants with improvement in ICIQ‐SF score | 10/15 | 6/13 | RR 1.44 (95% CI 0.73 to 2.87) | |
| OAB‐Q total score1 | 83.99 (16.99), 21 | 66.63 (25.06), 16 | Favours ES MD 17.36 (95% CI 3.09 to 31.63) | |
| I‐QoL score1 | 69.9 (65.8‐73.3), 17 | 70.6 (62.2‐79.1), 15 | No evidence of a difference | |
| Change in OAB‐Q score | Median (IQR), N: 8.8 (1.6 to 20.0), 80 | Median (IQR), N: 9.2 (‐0.8 to 27.2), 83 | P = 0.9918 | |
| Change in OAB‐Q score | 36.7 (21.5), 101 | 29.2 (20.0), 102 | Favours ES MD 7.50 (1.79, 13.21) | |
| QoL score2 | 1.6 (0.7), 37 | 2.2 (0.9), 31 | Favours ES MD ‐0.60 (95% CI ‐0.99 to ‐0.21) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Number of pads per day | 0.8 (1.2), 37 | 1.1 (2.0), 31 | MD ‐0.30 (95% CI ‐1.10 to 0.50) | |
| Other outcomes | ||||
| Participants with reduction in analogue discomfort sensation | 8/20 | 5/20 | RR 1.60 (95% CI 0.63 to 4.05) | |
| Participants with reduction in analogue wetness sensation | 6/20 | 5/20 | RR 1.20 (95% CI 0.44 to 3.30) | |
| Pelvic floor muscle strength (cmH2O) | 53.8 (18.6), 20 | 46.8 (12.5), 20 | MD 7.00 (95% CI ‐2.82 to 16.82) | |
| Urgency score2 | 1.7 (0.7), 37 | sham ES: 2 (0.8), 31 | MD ‐0.30 (95 CI ‐0.66 to 0.06) | |
Results in bold are statistically significant
1Lower score = greater severity
2Higher score = greater severity
Secondary outcomes
Quantification of symptoms
ES was found to be more effective than placebo or sham treatment for the following outcomes.
-
Incontinence episodes (per 24 hours): MD ‐1.43, 95% CI ‐1.92 to 0.95; n = 143) (Analysis 2.7) (Barroso 2002; Kosilov 2013).
-
Nocturia episodes (per night): MD ‐0.37, 95% CI ‐0.73 to ‐0.02; n = 245 (Analysis 2.8) (Bellette 2009; Peters 2010).
-
Micturitions (per 24 hours): MD ‐1.09, 95% CI ‐1.70 TO ‐0.47; n = 285 (Analysis 2.9) (Amaro 2006; Bellette 2009; Peters 2010).
One trial (Kosilov 2013) measured the number of incontinence episodes at two time points. At the end of six months’ treatment there was no evidence of a difference between ES and placebo (MD ‐0.50, 95% CI ‐1.18 to 0.18) but at 12 months after baseline there were significantly fewer incontinence episodes in the ES group than the placebo group (MD ‐1.10, 95% CI‐1.82 to ‐0.38; n = 107). The pooled estimate of effect reported above used the 12‐month data from Kosilov 2013 but the result did not change substantially if the six‐month data were used (pooled MD ‐1.13, 95% CI ‐1.59 to ‐0.66).
One trial (Yamanishi 2000a) found no evidence of a difference between groups in the number of pads used per 24 hours (Table 3).
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Low‐quality evidence indicated no evidence of a difference between ES and placebo or sham treatment in the number of adverse effects (RR 1.24, 95% CI 0.84 to 1.83; n = 450) (Analysis 2.10) (Kennelly 2011; Peters 2010; Yamanishi 2000a) (summary of findings Table 2). Adverse effects reported by participants included skin irritation, urinary tract infection, vaginal pain, discomfort and tingling.
3. Electrical stimulation versus other conservative treatments
Eleven trials with 882 participants compared ES to other conservative treatments (Arruda 2008; Berghmans 2002; Boaretto 2011; Olmo Carmona 2013; Kosilov 2013; Lima 2011; Schreiner 2010; Schreiner 2014; Spruijt 2003; Wang 2004; Yamanishi 2000b).
i) ES versus pelvic floor muscle training (PFMT)
Seven trials (n = 519) compared ES to PFMT (Arruda 2008; Berghmans 2002; Boaretto 2011; Lima 2011; Schreiner 2014; Spruijt 2003; Wang 2004).
Primary outcomes
Perception of cure or improvement of OAB symptoms
One small trial (n = 22) reported the number of participants cured and found no significant difference between ES and PFMT (Table 4) (Arruda 2008).
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | PFMT (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants cured | 14/21 | 12/21 | RR 1.17 (95% CI 0.72 to 1.88) | |
| Participants with improvement in UUI | 9/18 | 13/34 | RR 1.62 (95% CI 0.51 to 5.12) | |
| King's Health Questionnaire score1 | 180.08 (176.03), 35 | 50.27 (171.42), 34 | Favours ES MD 129.81 (95% CI 47.83 to 211.79) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Incontinence episodes per 24 hours | 7.9 (13.7), 21 | 7.8 (15.3), 21 | MD 0.10 (95% CI ‐8.68 to 8.88) | |
| Micturitions per 24 hours | 7.9 (2.3), 21 | 71. (2.1), 21 | MD 0.80 (95% CI ‐0.53 to 2.13) | |
| Nocturia episodes per night | 1.2 (1.3), 21 | 1 (1.1), 21 | MD 0.20 (95% CI ‐0.53 to 0.93) | |
| Number of pads per day | 0.9 (1.7), 21 | 0.8 (1.3), 21 | MD 0.10 (95% CI ‐0.82 to 1.02) | |
1Higher score = greater QoL
Based on three trials, moderate‐quality evidence indicated that ES was better than PFMT in terms of cure or improvement of OAB symptoms (RR 1.60, 95% CI 1.19 to 2.14; n = 195) (Analysis 3.1) (Arruda 2008; Schreiner 2014; Wang 2004) (summary of findings Table 3).
Number of participants satisfied with treatment
Data from two trials, one of which was a three‐arm trial with two different ES groups, suggested that participants were significantly more likely to be satisfied with PFMT treatment with ES (RR 0.76, 95% CI 0.60 to 0.96; n = 102) (Analysis 3.2) (Arruda 2008; Boaretto 2011).
Improvement in urgency urinary incontinence (UUI)
Very low‐quality evidence from a single trial (Wang 2004) found no evidence of a difference between ES and PFMT in terms of improvement in UUI (RR 1.62, 95% CI 0.51 to 5.12) (Table 4, summary of findings Table 3).
OAB‐related quality of life (QoL)
Very low‐quality evidence, from a single trial, suggested better QoL in the ES group than the PFMT group (Wang 2004) (summary of findings Table 3).
Secondary outcomes
Quantification of symptoms
One small trial (Arruda 2008; n = 22) found no evidence of a difference between ES and PFMT in incontinence episodes, daily micturitions, pads per day or nocturia episodes (Table 4).
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Not reported
ii) ES versus PFMT plus biofeedback
One trial (n = 120) compared ES to biofeedback‐assisted PFMT (Wang 2004).
Primary outcomes
Perception of cure or improvement of OAB symptoms
Low‐quality evidence from one trial (Wang 2004) found no evidence of a difference between ES and PFMT plus biofeedback in terms of improvement in UUI (RR 1.06, 95% CI 0.60 to 1.85) (summary of findings Table 4; Table 5).
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | PFMT plus biofeedback (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants with improvement in UUI | 9/17 | 17/34 | RR 1.06 (95% CI 0.60 to 1.85) | |
| King's Health Questionnaire score1 | 180.08 (176.03), 35 | 185.86 (176.57), 34 | MD ‐5.78 (95% CI ‐88.99 to 77.43) | |
1Higher score = greater QoL
Number of participants satisfied with treatment
Not reported
OAB‐related quality of life (QoL)
Low‐quality evidence from the same trial (Wang 2004) suggested no evidence of a difference in OAB‐related QoL measured by the King's Health Questionnaire (MD ‐5.78 (95% CI ‐88.99 to 77.43) (summary of findings Table 4; Table 5).
Secondary outcomes
None of the secondary outcomes were reported.
iii) ES versus PFMT plus behavioural therapy
One trial compared ES to PFMT plus behavioural therapy (Berghmans 2002) but none of the outcomes of interest were reported.
iv) ES versus magnetic stimulation
Primary outcomes
One trial (Yamanishi 2000b) compared ES to magnetic stimulation but did not report any of our primary outcomes.
Secondary outcomes
Quantification of symptoms
Not reported
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Very low‐quality evidence from one trial (n = 32) indicated no evidence of a difference between ES and magnetic stimulation in the numbers of participants with adverse effects (no adverse effects in either group) (Yamanishi 2000b) (summary of findings Table 5).
v) ES versus laseropuncture/electro‐acupuncture
One trial compared ES to laseropuncture (Kosilov 2013; n = 229) and another compared ES to electro‐acupuncture (Olmo Carmona 2013; n = 22).
Primary outcomes
Perception of cure or improvement of OAB symptoms
Not reported
Number of participants satisfied with treatment
Not reported
OAB‐related quality of life
Moderate‐quality evidence, from one trial, reported significantly better QoL scores (Olmo Carmona 2013;) in the ES group than in the electro‐acupuncture group (summary of findings Table 6) (Table 6).
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Laseropuncture/electro‐acupuncture (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Bladder Self‐Assessment Questionnaire score | 5.18 (2.56), 11 | 7.27 (2.24), 11 | Favours ES MD ‐2.09 (95% CI ‐4.10 to ‐0.08) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Micturitions per day | 8 (1.73), 11 | 7.73 (1.67), 11 | MD 0.27 (95% CI‐1.15 to 1.69) | |
| Nocturia episodes per night | 1.09 (1.51), 11 | 2.09 (1.92), 11 | MD ‐1.00 (95% CI ‐2.44 to 0.44) | |
1Higher score = greater severity
Secondary outcomes
Quantification of symptoms
Based on two trials, there were significantly fewer incontinence episodes in the ES groups than in those receiving laseropuncture or electro‐acupuncture (MD ‐1.84, 95% CI ‐2.33 to ‐1.35; n = 136) (Analysis 4.1 ) (Olmo Carmona 2013; Kosilov 2013). Kosilov 2013 (n = 114) reported the number of incontinence episodes at two time points; after six months’ treatment there were significantly fewer incontinence episodes in the ES group (MD – 1.60, 95% CI ‐1.92 to ‐1.28) and after nine months’ follow‐up the difference increased to ‐1.80 (95% CI ‐2.30 to 1.30). The pooled results reported above included the nine‐month follow‐up data from this trial; replacing it with the six‐month data changed the result to MD ‐1.62 (95% CI ‐1.93 to ‐1.30). Additionally, the other trial (Olmo Carmona 2013; n = 22) reported mean numbers of micturitions and nocturia episodes but found no evidence of a difference in number of micturitions or nocturia episodes (Table 6).
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Not reported
4. Electrical stimulation versus drug therapy
Twenty‐three trials with 1756 participants compared ES to the following drug treatments.
-
Oestrogen cream (Abdelbary 2015)
-
Oxybutynin: immediate release (Arruda 2008; Boaretto 2011); extended‐release (Manriquez 2013); not reported if extended or immediate release (Soomro 2001; Souto 2014; Svihra 2002; Wang 2006; Wang 2009; Wise 1993)
-
Probantheline bromide (Smith 1996)
-
Probanthine (Aaronson 1995)
-
Solifenacin succinate (Chen 2015; Vecchioli‐Scaldazza 2013)
-
Terodiline (Wise 1992)
-
Tolterodine, extended‐release (Peters 2009)
-
Tolterodine (not reported if extended or immediate release (Franzén 2010; Lin 2004; Preyer 2007; Preyer 2015; Sancaktar 2010)
-
Trospium and solifenacin (Kosilov 2013)
-
Unspecified anticholinergic agent (Orhan 2015)
Primary outcome
Perception of cure or improvement of OAB symptoms
Overall, there was no evidence of a difference between ES and drug treatment in curing OAB (RR 0.98, 95% CI 0.69 to 1.41; n = 388). Nor was there any evidence of a difference between ES and individual drugs (tolterodine (Franzén 2010; Lin 2004; Peters 2009), oxybutynin (Arruda 2008; Wang 2006; Wang 2009), propantheline bromide (Smith 1996)) (Analysis 5.1).
When measuring cure or improvement together, moderate‐quality evidence suggested that ES was more effective than drug treatment overall (RR 1.20, 95% 1.04 to 1.38; n = 439) but no evidence of a difference was found when comparing ES to individual drugs (tolterodine (Franzén 2010; Lin 2004; Peters 2009), oxybutynin (Arruda 2008; Wang 2006; Wang 2009), propantheline bromide (Smith 1996)) (Analysis 5.2). Another trial (Aaronson 1995) reported data not suitable for meta‐analysis but found that 69% of participants receiving ES were cured or improved compared to 50% of participants taking probanthine (summary of findings Table 7).
With regard to cure or improvement of OAB symptoms, a subgroup analysis based on low‐quality evidence found that ES delivered through intravaginal or transanal routes was more effective than drug treatment (RR 1.28, 95% CI 1.03 to 1.59; n = 199) (Analysis 5.3), but there was no evidence of a difference in cure or improvement between transcutaneous posterior tibial nerve stimulation and drug treatment (RR 0.51, 95% CI 0.23 to 1.13; n = 64).
There was no evidence of a difference in the number of people satisfied with ES or drug therapy with oxybutynin (RR 0.90, 95% CI 0.72 to 1.14; n = 125) (Arruda 2008; Boaretto 2011) (Analysis 5.4).
Number of participants satisfied with treatment
Not reported
Improvement in urgency urinary incontinence
None of the trials comparing ES to drug treatment reported improvement in UUI.
OAB‐related quality of life
Based on low‐quality evidence from two trials comparing ES to solifenacin succinate (Chen 2015; Vecchioli‐Scaldazza 2013) and another trial comparing ES to vaginal oestrogen cream (Abdelbary 2015), there was no evidence of a difference between the groups in terms of I‐Qol, OAB‐Q and PPIUS scores. However, statistically significant differences in favour of ES over drug therapy were reported, measured by an unspecified QoL instrument and the Patient Globe Impression of Improvement tool (summary of findings Table 7; Table 7).
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Comparator (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants cured or improved | 69% (N not reported) | Probanthine 50% (N not reported) | Not estimable | |
| I‐QoL score1 | 25.2 (1.0), 50 | Solifenacin succinate: 24.2 (1.0), 48 | MD 1.00 (95% CI 0.60 to 1.40) | |
| OAB‐Q score2 | 2.9 (0.9), 14 | Solifenacin succinate: 3.1 (1.1), 14 | MD ‐0.20 (95% CI ‐0.94 to 0.54) | |
| Patient Global Impression of Improvement score2 | 2.1 (0.7), 14 | Solifenacin succinate: 2.9 (1.1), 14 | Favours ES MD ‐0.80 (95% CI ‐1.48 to ‐0.12) | |
| Participant Perception of Intensity of Urgency Scale score2 | 2.1 (0.9), 14 | Solifenacin succinate: 2.7 (1.2), 14 | MD ‐0.60 (95% CI ‐1.39 to 0.19) | |
| ES | Oestrogen cream | Favours ES MD ‐2.20 (95% CI ‐2.71 to ‐1.69) Favours ES MD ‐2.00 (95% CI ‐2.50 to ‐1.50) MD 1.60 [0.91, 2.29] | ||
| QoL score2 (instrument not reported) | End of treatment: 2.8 (2), 105 3 months: 4 (1.7), 105 6 months: 7.6 (3), 105 | End of treatment: 5 (1.8), 105 3 months: 6 (2), 105 6 months: 6 (2), 105 | ||
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| ES | Oestrogen cream | |||
| Voids per 24 hours | End of treatment: 4.7 (0.8), 105 3 months: 5.0 (1.0), 105 6 months: 6.6 (1.5), 105 | End of treatment: 5.0 (0.9), 105 3 months: 5.3 (0.9), 105 6 months: 5.0 (0.8), 105 | Favours ES MD (‐0.30 (95% CI ‐0.56 to ‐0.04) Favours ES MD ‐0.30 (95% CI ‐0.53 to ‐0.07) Favours oestrogen cream MD 1.60 (95% CI 1.27 to 1.93) | |
| Nocturia episodes per night | End of treatment: 0.9 (0.7), 105 3 months: 1.1 (0.9), 105 6 months: 2.2 (0.9), 105 | End of treatment: 1.4 (0.8), 105 3 months: 1.5 (0.8), 105 6 months: 5.0 (0.8), 105 | Favours ES MD ‐0.50 (95% CI ‐0.70 to ‐0.30) Favours ES MD ‐0.40 (95% CI ‐0.63 to ‐0.17) MD ‐2.80 (95% CI ‐3.03 to ‐2.57) | |
| Incontinence episodes | End of treatment: 0.1 (0.3), 105 3 months: 0.1 (0.3), 105 6 months: 0.4 (0.6), 105 | End of treatment: 0.4 (0.6), 105 3 months: 0.5 (0.6), 105 6 months: 0.4 (0.6), 105 | Favours ES MD ‐0.30 (95% CI ‐0.43 to ‐0.17) Favours ES ‐0.40 (95% CI ‐0.53 to ‐0.27) 0.00 [‐0.16, 0.16] | |
| Urgency episodes | End of treatment: 2 (0.7), 105 3 months: 2.7 (1.0), 105 6 months: 4.7 (1.3), 105 | End of treatment: 4 (1.3), 105 3 months: 4.5 (1.5), 105 6 months: 4 (1.3), 105 | ‐3.00 [‐3.28, ‐2.72] ‐1.80 [‐2.14, ‐1.46]0.70 [0.35, 1.05] | |
| Number of pads per day | 0.9 (1.8), 21 | Oxybutynin: 0.9 (1.5), 22 | MD 0.00 (95% CI ‐0.96 to 0.96) | |
| Participants with nocturia | 2/18 | Oxybutynin: 3/19 | RR 0.70 (95% CI 0.13 to 3.73) | |
Results in bold are statistically significant
1Lower score = greater severity
2Higher score = greater severity
The Orhan 2015 trial reported that they found a statistically significantly higher improvement in the ES group than in the anticholinergic group according to three QoL measures; Urinary Distress Inventory (UDI‐6), Incontinence Impact Questionnaire (IIQ‐7), Over Active Bladder symptom scores (OABSS). However, no raw data were reported.
One trial reported QoL measured at three different time points; at the end of treatment and at three and six months' follow‐up (Abdelbary 2015). The data suggested better QoL of life in the ES group initially but at six months there was no evidence of a difference between ES and vaginal oestrogen cream.
Secondary outcomes
Quantification of symptoms
Overall, ES was more effective than drug treatment in terms of incontinence episodes per 24 hours (MD 0.24, 95% CI 0.09 to 0.38; n = 477); however, heterogeneity was high (I2 = 96%) and the result was no longer statistically significant in a random‐effects model (MD 0.25, 95% CI ‐1.11 to 1.60) (Analysis 5.5) (Abdelbary 2015; Arruda 2008; Kosilov 2013; Peters 2009; Vecchioli‐Scaldazza 2013).
Comparing ES to individual drugs, one trial reported significantly more incontinence episodes in the ES group than the trospium plus solifenacin group (MD 2.20, 95% CI 1.78 to 2.62; n = 110) (Kosilov 2013) but there was no evidence of a difference between ES and tolterodine (Peters 2009), oxybutynin (Arruda 2008) or oestrogen cream (Abdelbary 2015) in incontinence episodes.
There was insufficient evidence of a difference between ES and drug treatment for the following outcomes (Analysis 5.6; Analysis 5.7; Analysis 5.8 and Table 7).
-
Urgency episodes
-
Number of micturitions per 24 hours
-
Nocturia episodes
-
Number of people with nocturia
-
Pads used per day
Economic data
One cost‐effectiveness study (Chen 2012) found that percutaneous tibial nerve stimulation (PTNS) was not cost‐effective compared to extended release tolterodine (incremental cost‐effectiveness ratio (ICER) of USD 70,754 per quality‐adjusted life year, USD 20,754 above the USD 50,000 acceptable threshold). The probability of cost‐effectiveness at the USD 50,000 threshold was 21%.
Procedure outcomes
Not reported
Adverse effects
The reported adverse effects included dry mouth, constipation, headache, skin irritation, blurred vision, muscular pain, indigestion, nausea and dizziness. Due to the variety of adverse effects associated with different drugs, we did not pool the data to obtain one overall estimate effect, as this may have led to a misleading result.
Comparing ES to individual drugs, low‐quality evidence suggested fewer adverse effects with ES than with oxybutynin (RR 0.11, 95% CI 0.01 to 0.84; n = 79) (Svihra 2002; Wise 1993). Moderate‐quality evidence indicated fewer adverse effects with ES than with tolterodine (RR 0.12, 95% CI 0.05 to 0.27; n = 200) (Franzén 2010; Lin 2004; Preyer 2007; Preyer 2015) but there was no evidence of a difference in adverse effects between ES and solifenacin succinate (Chen 2015) (very low‐quality evidence) (Analysis 5.9) (Table 7).
5. Electrical stimulation versus surgery
No studies were identified that compared ES with surgery. However, one economic evaluation was identified (Robinson 2010), which found that PTNS was more cost‐effective than botulinum toxin (ICER GBP 50,133 and GBP 111,953 respectively), although neither treatment would be considered cost‐effective according to the thresholds used by the UK’s National Institute for Health and Care Excellence.
6. Electrical stimulation plus another treatment versus another treatment alone
i) ES plus PFMT versus PFMT alone
Five trials (203 participants) compared ES plus PFMT to PFMT alone (Firra 2013; Gaspard 2014; Lo 2003; Schmidt 2009; Schreiner 2010).
Primary outcome
Perception of cure or improvement of OAB symptoms
None of the trials comparing ES plus another treatment versus another treatment alone reported cure or improvement.
Based on two small trials (Gaspard 2014; Schreiner 2010), significantly more participants reported satisfaction with ES plus PFMT than PFMT alone (RR 1.58, 95% CI 1.13 to 2.20; n = 82) (Analysis 6.1).
Number of participants satisfied with treatment
Not reported
Improvement in urgency urinary incontinence
Low‐quality evidence from one small trial (Schreiner 2010) found that participants receiving ES plus PFMT were more than twice as likely to report improvement in UUI (RR 2.82, 95% CI 1.44 to 5.52; n = 51) (Table 8; summary of findings Table 8)
| Study | Outcome | ES plus PFMT (mean (SD/range), N or n/N; if available) | PFMT (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| SF‐Qualiveen1 | Median (IQR), N: 9 weeks: 1.000 (0.656, 1.719), 16 6 months: 1.313 (0.687, 1.625), 16. | Median (IQR), N: 9 weeks: 1.375 (0.625, 2.188) 6 months: 1.500 (0.344, 2.094), 15 | Not estimable | |
| York Incontinence Perception Scale2 | 41.2 (10.2), 6 | 47 (5.5), 6 | MD ‐0.65 (95% CI ‐1.83 to 0.52) | |
| Participants with improvement in UUI | 19/25 | 7/26 | Favours ES plus PFMT RR 2.82 (95 CI 1.44 to 5.52) | |
| ICIQ‐SF score1 | 7.9 (4.5), 25 | 10.6 (4.4), 26 | Favours ES plus PFMT MD ‐2.70 (95% CI ‐5.14 to ‐0.26) | |
| Secondary outcomes | ||||
| Nocturia episodes per night | 1.3 (1.5), 25 | 2.4 (1.3), 26 | Favours ES plus PFMT MD ‐1.10 (95% CI ‐1.87 to ‐0.33) | |
| Adverse effects | 0/25 | 0/26 | Not estimable | |
| Other outcomes | ||||
| Pelvic floor muscle strength (cmH2O) | 27 (16), 6 | 47.2 (22.7), 6 | MD ‐20.20 (95% CI ‐42.42 to 2.02) | |
Results in bold are statistically significant
1Higher score = greater severity
2Higher score = less severity
OAB‐related quality of life
Low‐quality evidence from three trials suggested no evidence of a difference between groups in QoL scores when measured with SF‐Qualiveen and York Incontinence Perception Scale but there was better QoL in the ES plus PFMT group in one trial reporting ICIQ‐SF scores (summary of findings Table 8; Table 8).
Secondary outcomes
Quantification of symptoms
Data from two trials suggested that adding ES to PFMT was more effective than PFMT alone in terms of incontinence episodes (MD ‐0.83, 95% CI ‐1.47 to ‐0.19; n = 119) (Firra 2013; Gaspard 2014). However, heterogeneity was high (I2 = 61%), probably due to the differences between trials in direction of effect. A random‐effects analysis altered the result so that it was no longer statistically significant (MD ‐0.60, 95% CI ‐1.84 to 0.64) (Analysis 6.2). In terms of urgency episodes, two trials found that ES with PFMT was better than PFMT alone (MD ‐2.49 (‐2.74 to ‐2.24) but there was unexplained heterogeneity (I2 = 87%) and a random‐effects analysis maintained a statistically significant result but with wider confidence intervals (MD ‐2.33, 95 CI ‐3.11 to ‐1.54; n = 248) (Analysis 6.3) (Firra 2013; Gaspard 2014). Data from two trials showed no evidence of a difference in micturitions per day between ES plus PFMT and PFMT alone. One trial found that adding ES to PFMT was more effective than PFMT alone in terms of number of nocturia episodes (Schreiner 2010) (Table 8).
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Very low‐quality evidence from a single trial found no evidence of a difference between ES plus PFMT and PFMT only in the number of people with adverse effects (summary of findings Table 8; Table 8).
Other outcomes
Further data from a trial comparing ES plus PFMT to PFMT alone (Firra 2013) are presented in Table 8. These data relate to pelvic floor muscle strength and are inconclusive.
ii) ES plus behavioural therapy versus behavioural therapy alone
One trial (Gonzalez 2015; n = 82) compared ES plus behavioural therapy to behavioural therapy alone.
Primary outcome
Perception of cure or improvement of OAB symptoms
Not reported
Number of participants satisfied with treatment
Not reported
Improvement in urgency urinary incontinence
Not reported
OAB‐related quality of life
Very low‐quality evidence from a single trial (Gonzalez 2015) suggested higher QoL when ES was added to behavioural therapy (summary of findings Table 9; Table 9).
| Study | Outcome | ES plus behavioural therapy (mean (SD/range), N or n/N; if available) | Behavioural therapy (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| OAB‐Q score1 | 100.81 (41.5), 31 | 127.71 (40.64), 37 | Favours ES plus behavioural therapy MD ‐26.90 (95% CI ‐46.52 to ‐7.28) | |
| Incontinence Severity Index score1 | 5.15 (3.23), 31 | 7.38 (4.00), 37 | Favours ES plus behavioural therapy MD ‐26.90, 95% CI ‐46.52 to ‐7.28 | |
Results in bold are statistically significant
1Higher score = greater severity
Secondary outcomes
Not reported
iii) ES plus drug therapy versus drug therapy alone
Three trials compared ES plus drug therapy to drug therapy alone (Abdelbary 2015; Orhan 2015; Souto 2014)
Primary outcome
Perception of cure or improvement of OAB symptoms
Not reported
Number of participants satisfied with treatment
Not reported
Improvement in urgency urinary incontinence
Not reported
OAB‐related quality of life
Low‐quality evidence, from two trials, suggested there may be no difference in QoL when ES was added to drug therapy (tolterodine or vaginal oestrogen cream) (SMD ‐1.50 (95% CI ‐3.72 to 0.72; n = 248) (Analysis 7.1) (summary of findings Table 10) (Abdelbary 2015; Sancaktar 2010)
The trial by Abdelbary 2015 measured QoL at three different time points; at the end of treatment and at three and six months' follow‐up. There was a statistically significant difference in favour of ES plus oestrogen cream at all time points (Table 10).
| Study | Outcome | ES plus drugs (mean (SD/range), N or n/N; if available) | Drugs (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| IIQ‐7 score1 | ES plus tolterodine: 9.0 (0.8), 20 | Tolterodine: 11.2 (2.7), 18. | Favours ES plus tolterodine MD ‐2.20 (95% CI ‐3.50 to ‐0.90 | |
| ES plus oestrogen cream: | Oestrogen cream: | |||
| QoL score1 (instrument not reported) | End of treatment: 2.9 (2.2), 105. 3 months: 1.6 (0.9), 105. 6 months: 2 (0.8), 105. | End of treatment: 5 (1.8), 105 3 months: 6 (2), 105 6 months: 6 (2), 105 | MD ‐2.10 (95% CI ‐2.64, ‐1.56] MD ‐4.40 (95% CI ‐4.82 to ‐3.98) MD ‐4.00 (95% CI ‐4.41 to ‐3.59) | |
| Secondary outcomes | ||||
| ES plus oestrogen cream: | Oestrogen cream: | |||
| Voids per day | End of treatment: 5 (0.8), 105. 3 months: 5 (0.8), 105. 6 months: 5 (0.8), 105. | End of treatment: 5.0 (0.9), 105 3 months: 5.3 (0.9), 105 6 months: 5.0 (0.8), 105 | MD 0.00 (95% CI ‐0.23 to 0.23) MD ‐0.30 (95% CI ‐0.53 to ‐0.07) MD 0.00 (95% CI ‐0.22 to 0.22) | |
| Nocturia episodes per night | End of treatment: 0.5 (0.5), 105 3 months: 1 (0.9), 105 6 months: 1.5 (0.8), 105 | End of treatment: 1.4 (0.8), 105 3 months: 1.5 (0.5), 105 6 months: 5 (0.8), 105 | MD ‐0.90 (95% CO ‐1.08 to ‐0.72) MD ‐0.50 (95% CI ‐0.70 to ‐0.30) MD ‐3.50 (95% CI ‐3.72 to ‐3.28) | |
| Incontinence episodes per 24 hours | End of treatment: 1.4 (0.7), 105 3 months: 0.09 (0.28), 105. 6 months: 0.09 (0.28), 105. | End of treatment: 0.4 (0.6), 105 3 months: 0.5 (0.6), 105 6 months: 0.4 (0.6), 105 | MD 1.00 (95% CI 0.82 to 1.18) MD ‐0.41 (95% CI ‐0.54 to ‐0.28) MD ‐0.31 (95% CI ‐0.44 to ‐0.18) | |
| Urgency episodes per 24 hours | End of treatment: 1.4 (0.7), 105 3 months: 1.6 (0.9), 105 6 months: 2 (0.8), 105 | End of treatment: 4 (1.3), 105 3 months: 4.5 (1.5), 105 6 months: 4 (1.3), 105 | MD ‐2.60 (95% CI ‐2.88 to ‐2.32) MD ‐2.90 (95% CI ‐3.23 to ‐2.57) MD ‐2.00 (95% CI ‐2.29 to ‐1.71) | |
| Adverse effects | ES plus tolterodine: 1/20 | Tolterodine: 2/18 | RR 0.45 (95% CI 0.04 to 4.55) | |
Results in bold are statistically significant
1Higher score = greater severity
Secondary outcomes
Quantification of symptoms
Data from two trials suggested that adding ES to drug therapy (tolterodine or oestrogen cream) resulted in significantly fewer incontinence episodes than drug therapy alone (MD ‐0.53, 95% CI ‐0.63 to ‐0.43; n = 248) (Abdelbary 2015; Sancaktar 2010) (Analysis 7.2). However, heterogeneity was very high (I2 = 97%), probably due to considerable differences in sample sizes. A random‐effects analysis altered the result slightly but it remained statistically significant (MD ‐0.60, 95% CI ‐1.18 to ‐0.02).
ES added to drug therapy (tolterodine or oestrogen cream) also resulted in significantly fewer urgency episodes (MD ‐2.49, 95% CI ‐2.74 to ‐2.24; 248) (Abdelbary 2015; Sancaktar 2010) (Analysis 7.3). Again, heterogeneity was high (I2 = 87%) and a random‐effects analysis altered the result only slightly (MD ‐2.33, 95% CI ‐3.11 to ‐1.54).
However, no evidence of a difference was found in the following outcomes when comparing ES plus drug therapy to drug therapy alone micturitions per 24 hours (Abdelbary 2015; Souto 2014; n = 250) (tolterodine or oxybutynin) (Analysis 7.4)
The trial by Abdelbary 2015 (n = 210) measured symptoms at three different time points; at the end of treatment and at three and six months' follow‐up. In almost all cases, the result suggested adding ES to oestrogen cream was more effective than oestrogen cream alone.
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
Very low‐quality evidence from a single trial indicated no evidence of a difference in adverse effects when ES was added to tolterodine compared to tolterodine alone (Sancaktar 2010; n = 38) (summary of findings Table 10; Table 10). The reported adverse effects included constipation, dry mouth, headache and skin irritation.
7. One type of electrical stimulation versus another type of electrical stimulation
Ten trials with 533 participants compared one type of ES with another (Alves 2015; Boaretto 2011; Bower 1998; Finazzi‐Agrò 2005; Lobel 1998; Monga 2011; Phillips 2012; Seth 2014; Slovak 2015; Sotelo 2011).
Primary outcome
Perception of cure or improvement of OAB symptoms
Very low‐quality evidence from a single trial (Lobel 1998; n = 37), comparing ES once a week versus ES twice a week, found that all participants were improved after five weeks of treatment and that 24% (9/37) were satisfied enough to request no further treatment. However, these data were not reported separately for the two treatment groups (summary of findings Table 11); Table 11).
| Study | Outcome | ES A (mean (SD/range), N or n/N; if available) | ES B (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| ICIQ‐OAB score1 | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 4.46 (2.66), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 4.53 (3.07), 13 | MD ‐0.07 (95% CI ‐2.21 to 2.07) | |
| Success = > 50% reduction in micturitions/24 hours OR If incontinent, success > 50% reduction in UI episodes/24 hours | ES once a week: 11/17 (4/11 incontinent participants) | ES 3 times per week: 12/18 (5/11 incontinent participants) | RR 0.97 (95% CI 0.60 to 1.57) Incontinence participants: RR 0.80 (95% CI 0.29 to 2.21) | |
| I‐QoL score2 | ES once a week (median, range, N): 77 (35‐100), 17 | ES 3 times a week (median, range, N): 78 (33‐100), 18 | Not estimable | |
| Participants with improvement in symptoms | ES once a week: 100% | ES twice a week: 100% | Not estimable | |
| Participants satisfied enough to request no further treatment | 24% (9/37) | Not estimable, not reported per treatment group | ||
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Adverse effects | ES once a week: 0/17 | ES 3 times per week: 0/18 | Not estimable | |
| Subjective improvement after 6‐8 sessions | ES once a week: 17/17 | ES 3 times a week: 18/18 | Not estimable | |
| Incontinence episodes per 24 hours | ES once a week (median, range, N): 1 (0‐3), 11 | ES 3 times a week (median, range, N): 1 (0‐3), 11 | Not estimable | |
| Micuturitions per 24 hours | ES once a week (median, range, N): 8 (5‐15), 17 | ES 3 times a week (median, range, N): 8 (6‐18), 18 | Not estimable | |
| SF‐36 score | ES once a week (median, range, N): 62 (24‐81), 17 | ES 3 times per week (median, range, N): 62 (25‐80), 18 | Not estimable | |
| UUI episodes per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 0.33 (0.57), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 0.84 (1.39), 13 | MD ‐0.51 (95% CI ‐1.32 to 0.30) | |
| Urgency episodes per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 0.79 (0.97), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 0.58 (0.65), 13 | MD 0.21 (95% CI ‐0.39 to 0.81) | |
| Micturitions per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 8.33 (2.52), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 7.89 (2.64), 13 | MD 0.44 (95% CI ‐1.48 to 2.36) | |
| Nocturia episodes per night | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 1.26 (1.21), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 1.05 (1.01), 13 | MD 0.21 (95% CI ‐0.61 to 1.03) | |
| Maximum cystometric capacity | 150 Hz: 351 (144), 16 | 10 Hz: 305 (146), 16 | MD 46.00 (95% CI ‐54.48 to 146.48) | |
| Volume at first desire to void | 150 Hz: 208.5 (132), 16 | 10 Hz: 154 (61), 16 | MD 54.50 (95% CI ‐16.75 to 125.75) | |
| Other outcomes | ||||
| Participants satisfied | 200 µs pulse width: 17/22 | 500 µs pulse width: 11/16 | RR 1.12 (95% CI 0.75 to 1.68) | |
1Higher score = greater severity
2Lower score = greater severity
Finazzi‐Agrò 2005 (n = 35), which compared one session of percutaneous ES per week to three sessions per week, found little evidence of a difference between the groups in terms of successful treatment. Success was defined as greater than 50% reduction in micturitions per 24 hours, or as greater than 50% reduction in UUI episodes in participants who had UUI at baseline (summary of findings Table 12; Table 11). Again, the quality of evidence was very low.
Number of participants satisfied with treatment
Not reported
OAB‐related quality of life
Very low‐quality evidence, from a single trial (Alves 2015; n = 28) comparing sensory threshold ES to motor threshold ES, suggested there was no evidence of a difference in QoL measured with ICIQ‐OAB (summary of findings Table 13; Table 11). Similarly, very low‐quality evidence from another trial (Finazzi‐Agrò 2005) suggested little evidence of a difference in I‐QoL scores when once a week ES was compared to three times per week (summary of findings Table 12).
Secondary outcomes
Quantification of symptoms
One trial (Monga 2011; n = 74), comparing ES patches placed by investigators versus patches placed by participants, reported various outcomes relating to quantification of symptoms but did not separate the data according to treatment group.
Another small trial (Alves 2015; n = 28), comparing two different kinds of tibial nerve stimulation found no evidence of a difference between treatments in the number of UUI episodes, urgency episodes, micturitions or nocturia episodes (Table 11). Similarly, the Finazzi‐Agrò 2005 trial (n = 35) comparing ES delivered once a week to ES three times per week, reported little evidence of a difference between the groups in incontinence episodes and micturitions per 24 hours (Table 11).
No other outcomes relating to quantification of symptoms were reported by any of the identified trials.
Economic data
Not reported
Procedure outcomes
Not reported
Adverse effects
One trial (n = 37; Lobel 1998), comparing ES once a week versus ES twice a week, reported the following adverse effects across all participants but not separated by treatment group.
-
Discomfort: 16% (6/37)
-
Leg tremor: 8% (3/37)
-
Urinary tract infection: 8% (3/37)
Another trial (n = 50; Sotelo 2011), comparing different ES patch placements, reported one participant experiencing adverse effects but did not report to which treatment group the participant belonged. Very low‐quality evidence from Finazzi‐Agrò 2005, comparing one ES session per week to three sessions per week, reported no adverse effects in either group (summary of findings Table 12).
Other outcomes
One trial (Boaretto 2011) compared two different pulse widths (200 microseconds and 500 microseconds) and reported similar satisfaction in both groups (RR for number of people not satisfied: 0.73, 95% CI 0.25 to 2.10; n = 38) (Table 11).
Discussion
Summary of main results
To the best of our knowledge, this is the first systematic review to synthesise all available data from randomised controlled trials (RCTs) relating to the effectiveness of electrical stimulation (ES) with non‐implanted devices compared with any other treatment for overactive bladder (OAB). The results of the review suggest that ES shows promise in treating OAB.
Improvement of OAB symptoms
ES is likely to be more effective than placebo/sham treatment, PFMT or drug therapy (summary of findings Table 2; summary of findings Table 3; summary of findings Table 7) in improving OAB symptoms. Specifically considering urgency urinary incontinence (UUI), ES may be more effective than placebo or sham treatment (summary of findings Table 2) but we are very uncertain that ES is better for UUI than PFMT, (summary of findings Table 3), nor can we be certain that adding ES to PFMT leads to improvement in UUI (summary of findings Table 8). The conclusions regarding improvement in UUI should be interpreted with caution due to the lack of clarity in the trials' reporting of rates of urgency incontinence at baseline.
Furthermore, it appears that while both intravaginal ES and percutaneous tibial nerve stimulation are likely to lead to greater improvement in symptoms than sham/placebo, intravaginal ES is likely to have a larger effect.
For OAB symptoms, low‐quality evidence indicates that ES may be more effective than no active treatment (summary of findings Table for the main comparison). This is supported by evidence from symptom quantification, such as the number of people with nocturia or increased frequency. Additionally, low‐quality evidence suggests that ES may be more effective than biofeedback‐assisted PFMT (summary of findings Table 4) but this is based on a single trial and we did not identify any secondary outcome data to support or refute this finding.
OAB‐related quality of life
It is difficult to state with certainty that ES is likely to improve OAB‐related quality of life more than treatment with PFMT or electro‐acupuncture; notwithstanding the moderate quality of the evidence identified, these findings are based on single small trials and are therefore not conclusive (summary of findings Table 3; summary of findings Table 6).
Low‐quality evidence suggests that ES may lead to improved OAB‐related quality of life compared to no active treatment (summary of findings Table for the main comparison). We cannot be certain there is any difference in OAB‐related QoL between ES and drug therapy summary of findings Table 7), nor when ES is added to PFMT or drug therapy, compared to PFMT or drug therapy alone (summary of findings Table 8; summary of findings Table 10).
It is possible that ES improves OAB‐related QoL more than placebo/sham treatment, and that adding ES to behavioural therapy is better than behavioural therapy alone in terms of OAB‐related QoL, but the very low quality of the evidence means that we cannot draw these conclusions with any certainty (summary of findings Table 2; summary of findings Table 9).
Adverse effects
Low‐quality evidence suggests that there may be a lower risk of adverse effects with ES than with oxybutynin or tolterodine ( summary of findings Table 7).
Due to the very low‐quality evidence available, we cannot be certain whether there are fewer adverse effects with ES compared to placebo/sham treatment, magnetic stimulation, electro/laseropuncture or solifenacin succinate (summary of findings Table 2; summary of findings Table 5; summary of findings Table 6; summary of findings Table 7). We are also very uncertain whether adding ES to PFMT or to drug therapy results in fewer adverse effects than PFMT or drug therapy alone (summary of findings Table 8; summary of findings Table 10). Nor can we tell if there is any difference in the risk of adverse effects between different types of ES (summary of findings Table 11; summary of findings Table 12; summary of findings Table 13).
Effectiveness of ES over time
Based on the small number of trials reporting outcomes at the end of treatment as well as after a longer follow‐up period, it appears that the effect of ES diminishes after the end of treatment. However, this was also the case for most other interventions and is likely to be due to the nature of the condition. Where ES was found to be more effective than a comparator intervention at the first measurement point, this trend was generally found to be maintained at the longer‐term follow‐up. Nonetheless, this evidence should be considered in the context that the outcomes measured at multiple time points in this small set of trials tended to be objective measures rather than the more reliable and meaningful subjective report of symptoms.
Overall completeness and applicability of evidence
The included studies do not address all of the objectives of the review because many of them did not report data in a usable way or did not measure the primary outcomes, that is, a subjective report of symptoms. Five trials reported urodynamic outcomes only (Berghmans 2002; Bower 1998; Vahtera 1997; Walsh 2001; Yamanishi 2000b), which was of limited use because subjective, patient‐reported cure or improvement should take precedence over objective, clinician‐observed outcomes; for instance, a patient may still have OAB according to objective measurements but if their subjective assessment is that of no bothersome symptoms, then usually no further treatment will be required. Future trials should ensure appropriate subjective outcomes are measured and reported.
Of particular note is the absence of data on subjective cure or improvement from the trials comparing ES plus drug therapy to drug therapy alone. Furthermore, the paucity of data in many of the included trials meant that we could not draw any conclusions regarding adverse effects between ES and placebo/sham treatment or other conservative treatments. Nor can we tell if adding ES to another treatment increases the risk of adverse effects.
Another key outcome, QoL associated with OAB or incontinence, was inadequately addressed by the included studies. While 22 of the 64 trials incorporated a validated measure of QoL, it was difficult to discern a clear picture regarding clinically meaningful results. Two trials included definitions of clinical significance relating to the QoL instruments used (Oldham 2013; Svihra 2002); the QoL findings of those trials were not clinically meaningful. The remaining trials that measured QoL were unclear about the clinical significance of their QoL instruments (Abdelbary 2015; Alves 2015; Bellette 2009; Chen 2015; Finazzi‐Agrò 2010; Firra 2013; Gaspard 2014; Gonzalez 2015; Orhan 2015; Peters 2010; Phillips 2012; Sancaktar 2010; Schmidt 2009; Schreiner 2010; Schreiner 2014; Seth 2014; Souto 2014; Vecchioli‐Scaldazza 2013; Wang 2004; Wang 2009). It is therefore difficult to form any conclusions regarding the potential for ES to improve QoL in relation to OAB.
Nevertheless, the findings presented here are based on evidence from trial populations that were reasonably representative of OAB in clinical practice, including people with both OAB‐wet and OAB‐dry.
Economic commentary
To supplement the main systematic review of effects, we sought to identify economic evaluations which have compared electrical stimulation with non‐implanted electrodes to other treatments. Only one economic evaluation (Chen 2012) was identified. This study was a cost‐utility analysis, conducted using the framework of a decision model, comparing percutaneous tibial nerve stimulation (PTNS) with extended release tolterodine. The model was based on direct medical costs, in 2010 USD, during a one year time horizon and the analysis was conducted from a societal perspective.
The authors concluded that PTNS was not cost‐effective compared to tolterodine (incremental cost‐effectiveness ratio (ICER) of USD 70,754 per quality‐adjusted life year, USD 20,754 above the USD 50,000 acceptable threshold). Furthermore, sensitivity analyses indicated that the ICER was above the acceptable threshold in nine of eleven possible scenarios. The authors noted that their findings were limited by the quality of the literature.
However, there was a degree of ambiguity in the study. Firstly, it was unclear whether a Markov model or a simple decision tree model was used. Secondly, a societal perspective would generally be expected to incorporate more than direct medical costs so it may be more accurate to consider this analysis to be have been conducted from a healthcare payer perspective. Finally, it appears that the authors have inaccurately interpreted the eleven scenarios presented in the sensitivity analyses and therefore their conclusions may be misleading.
We did not subject this economic evaluation to critical appraisal and we do not attempt to draw any firm or general conclusions regarding the relative costs or efficiency. The apparent scarcity of relevant economic evaluations indicates that economic evidence regarding is currently lacking.
Quality of the evidence
Despite the large number of identified trials (64), the amount and quality of evidence is insufficient to reach a robust conclusion regarding the effectiveness of ES compared to other active treatments. The sample sizes for individual outcomes were small, which led to downgrading the quality of evidence in some instances because underpowered trials are likely to have a greater degree of imprecision. Small sample sizes in individual trials can also lead to under‐powered meta‐analyses, which then give inconclusive overall estimates of effect.
Assessing the risk of bias and methodological quality of the included trials was limited by the extent to which adequate details were provided in reports of trials. Future trials should adhere to CONSORT guidelines to ensure clarity and completeness in the reporting of methods (Schulz 2010). Risk of selection bias through randomisation and allocation concealment was generally unclear because of insufficient reporting. The risk of performance bias was also relatively unclear because of a lack of information to judge whether or not participants, healthcare providers and outcome assessors were adequately blinded. In many trials, it would not have been possible to blind participants; however, an element of risk of bias remains where participants were not blinded, because self‐reported, subjective outcomes could have been affected by participants' perception of the intervention received, leading to uncertainty regarding the extent to which the estimate of effect was truly attributable to the intervention.
Potential biases in the review process
Every attempt was made to reduce the risk of bias in the review process, with broad inclusion criteria and a comprehensive search strategy to identify eligible trials. There were no language restrictions and we obtained translations of non‐English trials wherever possible. The risk of bias was further minimised by two review authors undertaking independent screening of search results and independent data extraction.
However, unclear reporting of trial methods and data, and subsequent problems obtaining clarifications from trial authors limited the extent to which we could meaningfully compare all of the relevant data from the identified trials.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review of RCTs to investigate the effectiveness of ES with non‐implanted devices compared to any other treatment for OAB. A systematic review focusing on ES of the pelvic floor found evidence in favour of ES for urinary incontinence, with or without OAB symptoms (Jerez‐Roig 2013). Similarly, a systematic review investigating ES for any kind of urinary incontinence in women (Schreiner 2013) found evidence suggesting that ES was more effective than other treatments for UUI, but that the evidence for stress urinary incontinence (SUI) or mixed urinary incontinence (MUI) was much less clear. As UUI is one of the key symptoms of OAB, our findings with regard to OAB can be taken together with the reviews by Schreiner and colleagues and Jerez‐Roig and colleagues to indicate that ES is effective in treating OAB symptoms. Additionally, our findings are in accord with Berghmans 2013, whose systematic review of ES for any kind of urinary incontinence in men found limited evidence that ES was more effective than sham treatment and that ES enhanced the effectiveness of pelvic floor muscle training (PFMT) in the short term.
Schreiner and colleagues' findings regarding different types of ES were similar to ours in that the heterogeneity of ES interventions in the identified trials was such that no conclusions could be drawn on which types of ES may be more effective than others.
The findings of our review lend further weight to another systematic review (Rai 2012) comparing drug treatment with other active treatments for OAB, which found limited evidence that ES was more effective than drugs in improving OAB symptoms and that there were fewer adverse effects associated with ES than with drug treatment. Our review identified eight additional trials not included by Rai 2012; consequently, the conclusions of our review add strength to the evidence base for ES compared to drug treatment.

PRISMA study flow diagram
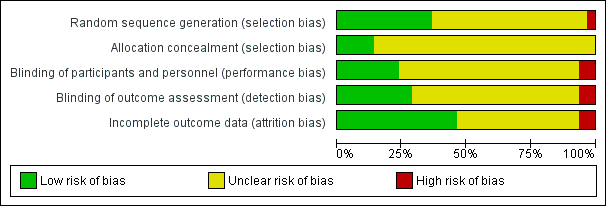
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Electrical stimulation (ES) versus no active treatment, Outcome 1 Number of participants cured or improved.
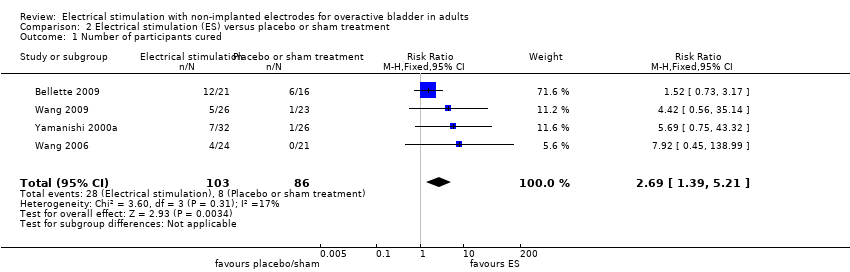
Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 1 Number of participants cured.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 2 Number of participants cured or improved.
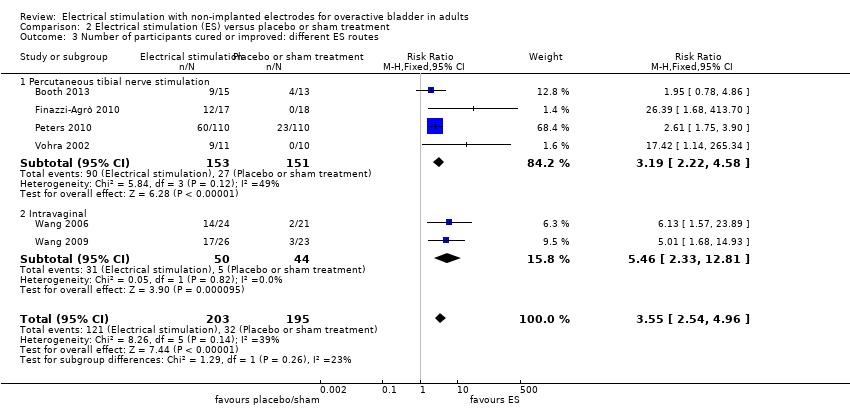
Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 3 Number of participants cured or improved: different ES routes.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 4 Number of participants satisfied.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 5 Number of participants with improvement in urgency urinary incontinence.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 6 Number of participants with improvement in urinary frequency.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 7 Number of incontinence episodes per 24 h.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 8 Number of nocturia episodes.
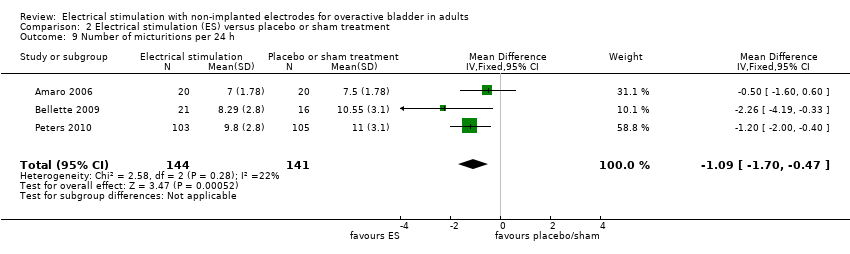
Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 9 Number of micturitions per 24 h.

Comparison 2 Electrical stimulation (ES) versus placebo or sham treatment, Outcome 10 Number of participants with adverse effects.

Comparison 3 Electrical stimulation (ES) versus pelvic floor muscle training (PFMT), Outcome 1 Number of participants cured or improved.

Comparison 3 Electrical stimulation (ES) versus pelvic floor muscle training (PFMT), Outcome 2 Number of participants satisfied.
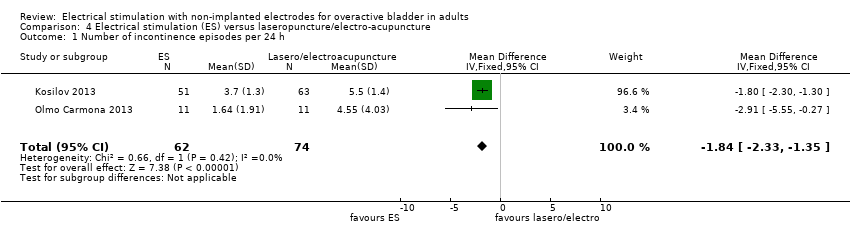
Comparison 4 Electrical stimulation (ES) versus laseropuncture/electro‐acupuncture, Outcome 1 Number of incontinence episodes per 24 h.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 1 Number of participants cured.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 2 Number of participants cured or improved.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 3 Number of participants cured or improved: routes of ES.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 4 Number of participants satisfied.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 5 Number of incontinence episodes per 24 h.
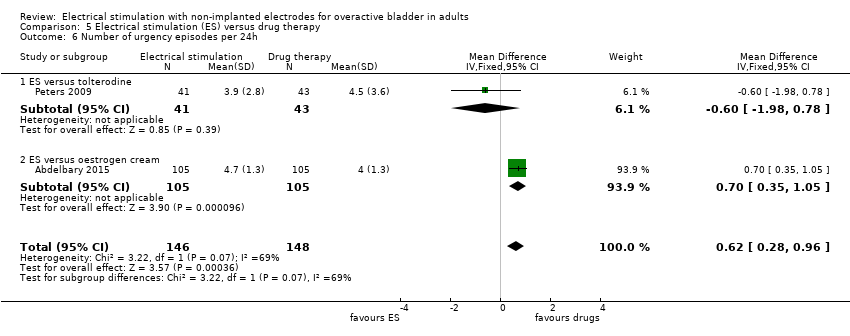
Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 6 Number of urgency episodes per 24h.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 7 Number of micturitions per 24 h.

Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 8 Number of nocturia episodes per night.
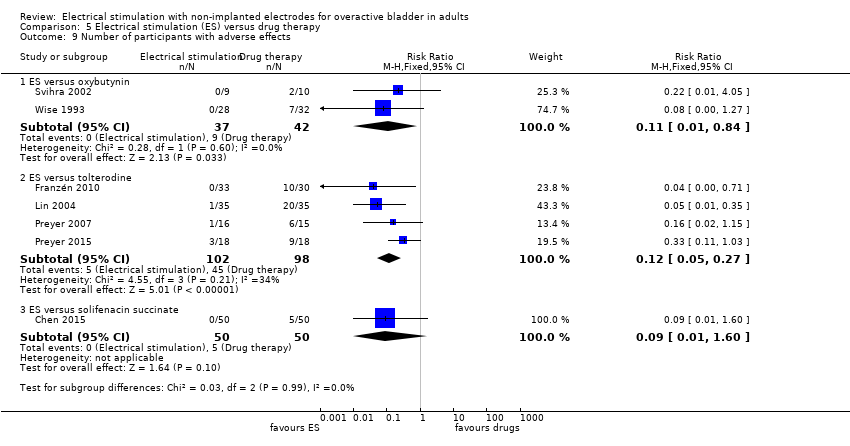
Comparison 5 Electrical stimulation (ES) versus drug therapy, Outcome 9 Number of participants with adverse effects.
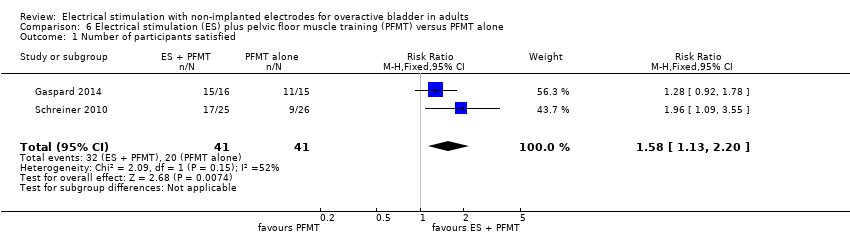
Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 1 Number of participants satisfied.

Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 2 Number of incontinence episodes per 24h.

Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 3 Number of urgency episodes per 24 h.

Comparison 6 Electrical stimulation (ES) plus pelvic floor muscle training (PFMT) versus PFMT alone, Outcome 4 Number of micturitions per 24 h.

Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 1 Quality of life.
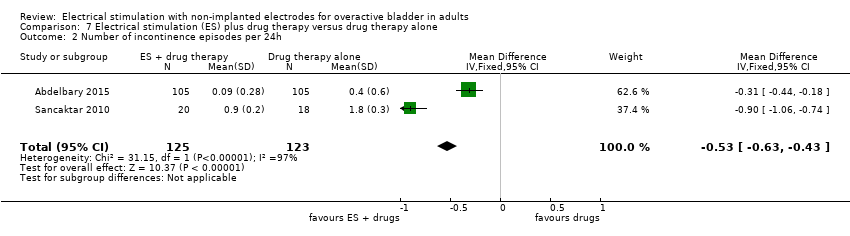
Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 2 Number of incontinence episodes per 24h.

Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 3 Number of urgency episodes per 24 hours.
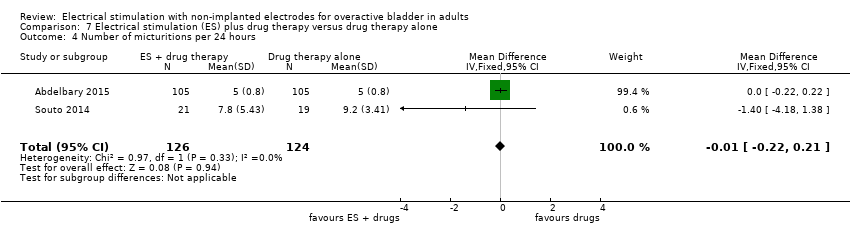
Comparison 7 Electrical stimulation (ES) plus drug therapy versus drug therapy alone, Outcome 4 Number of micturitions per 24 hours.
| Electrical stimulation versus no active treatment | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no active treatment | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 12 weeks to 12 months | Study population | RR 1.85 | 121 | ⊕⊕⊝⊝ | ||
| 424 per 1000 | 784 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life (higher score indicates better quality of life) Follow‐up: range 5 weeks to 12 weeks | In one trial participants in the intervention group had lower ICI‐Q scores (unclear if this was an important difference). In another no evidence of a difference was found between groups in of improvement in a range of QoL scores. | ‐ | 148 (2 RCT) | ⊕⊕⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high likelihood of selection bias). | ||||||
| Electrical stimulation versus placebo or sham treatment | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or sham treatment | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 4 weeks to 12 weeks | Study population | RR 2.26 | 677 | ⊕⊕⊕⊝ | ||
| 262 per 1000 | 593 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence Follow‐up: range 4 weeks to 13 weeks | Study population | RR 5.03 (0.28 to 89.88) | 242 | ⊕⊕⊝⊝ | ||
| 189 per 1000 | 948 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 4 weeks to 13 weeks | 3/7 trials reported significantly higher quality of life in the intervention groups. Others reported no evidence of a difference between groups. | ‐ | 627 (7 RCTs) | ⊕⊕⊝⊝ | ||
| Adverse effects Follow‐up: median 12 weeks | Study population | RR 1.24 | 450 | ⊕⊕⊝⊝ | ||
| 139 per 1000 | 172 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high risk of performance and detection bias in one trial; unclear risk of bias in many domains in other trials) | ||||||
| Electrical stimulation versus PFMT | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: median 12 months | Study population | RR 1.60 | 195 | ⊕⊕⊕⊝ | ||
| 390 per 1000 | 625 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence Follow‐up: 6 weeks | Study population | RR 1.62 | 52 | ⊕⊝⊝⊝ | ||
| 382 per 1000 | 619 per 1000 | |||||
| OAB‐related quality of life Follow‐up: 6 weeks | The mean OAB‐related quality of life in the intervention group was 129.81 higher (47.83 higher to 211.79 higher) | ‐ | 49 | ⊕⊝⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Dowgraded one level due to serious risk of bias (some risk of performance and attrition bias) | ||||||
| Electrical stimulation versus PFMT plus biofeedback | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT plus biofeedback | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence Follow‐up: 6 weeks | Study population | RR 1.06 | 51 | ⊕⊕⊝⊝ | ||
| 500 per 1000 | 530 per 1000 | |||||
| OAB‐related quality of life (lower scores indicate better quality of life) Follow‐up: 6 weeks | The mean OAB‐related quality of life in the intervention group was 5.78 lower (88.99 lower to 77.43 higher) | ‐ | 51 | ⊕⊕⊝⊝ | No evidence of a difference between groups in quality of life scores | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of selection and performance bias) | ||||||
| Electrical stimulation versus magnetic stimulation | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with magnetic stimulation | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Adverse effects Follow‐up: 4 weeks | Not estimable | 32 | ⊕⊝⊝⊝ | No events reported in either group | ||
| 0 per 1,00 | 0 per 1,00 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious imprecision (single trial, small sample size) | ||||||
| Electrical stimulation versus laseropuncture/electro‐acupuncture | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with laseropuncture/electro‐acupuncture | Risk with electrical stimulation | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Assessed with: Bladder Self‐Assessment Questionnaire (lower scores indicate better quality of life) Follow‐up: 12 weeks | The mean OAB‐related quality of life in the intervention group was 2.09 lower (4.1 lower to 0.08 lower) | ‐ | 22 | ⊕⊕⊝⊝ | Significantly greater quality of life in intervention group | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious imprecision (single trial, small sample size) | ||||||
| Electrical stimulation versus drug therapy | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with drugs | Risk with electrical stimulation | |||||
| Participants cured or improved Follow‐up: range 4 weeks to 2 years | Study population | RR 1.20 | 439 | ⊕⊕⊕⊝ | ||
| 585 per 1000 | 702 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 4 weeks to 6 months | One trial used OAB‐Q, PGII and PPIUS and found a significant result only in the PGII, which was in favour of ES. Another trial found no evidence of a difference between groups in I‐QoL scores. A third trial found higher QoL scores in the ES group at the end of treatment and at 3 months' follow‐up but no evidence of a difference at 6 months' follow‐up. | ‐ | 336 (3 RCTs) | ⊕⊕⊝⊝ | ||
| Adverse effects ‐ ES versus oxybutynin Follow‐up: 5 weeks | Study population | RR 0.11 | 79 | ⊕⊕⊝⊝ | ||
| 214 per 1000 | 24 per 1000 | |||||
| Adverse effects ‐ ES versus tolterodine Follow‐up: range 4 weeks to 2 years | Study population | RR 0.12 | 200 | ⊕⊕⊕⊝ | ||
| 459 per 1000 | 55 per 1000 | |||||
| Adverse effects ‐ ES versus solifenacin succinate Follow‐up: 4 weeks | Study population | RR 0.09 | 100 | ⊕⊝⊝⊝ | ||
| 100 per 1000 | 9 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Electrical stimulation plus PFMT versus PFMT alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with PFMT alone | Risk with electrical stimulation plus PFMT | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence Follow‐up: 12 weeks | Study population | RR 2.82 | 51 | ⊕⊕⊝⊝ | ||
| 269 per 1000 | 759 per 1000 | |||||
| Adverse effects Follow‐up: 12 weeks | Study population | Not estimable | 51 | ⊕⊝⊝⊝ | No events reported in treatment groups | |
| 0 per 1000 | 0 per 1000 | |||||
| OAB‐related quality of life Follow‐up: range 8 weeks to 6 months | One trial found greater quality of life in the intervention group (measured with ICIQ‐SF). Two other trials found no evidence of a difference between groups (measured with SF‐Qualiveen and York Incontinence Perception Scale) | ‐ | 201 (3 RCTs) | ⊕⊕⊝⊝ | ||
| Cost‐effectiveness | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Electrical stimulation plus behavioural therapy versus behavioural therapy alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with behavioural therapy alone | Risk with electrical stimulation plus behavioural therapy | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Follow‐up: 3 months | Intervention group reported significantly better quality of life measured with OAB‐Q and Incontinence Severity Index | ‐ | 82 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (high risk of attrition bias, low risk of selection bias and unclear in other domains) | ||||||
| Electrical stimulation plus drug therapy versus drug therapy alone | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with drug therapy alone | Risk with electrical stimulation plus drug therapy | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life (lower scores indicate greater quality of life) Follow‐up: range 12 weeks to 6 months | The mean OAB‐related quality of life in the intervention group was 1.50 lower (3.72 lower to 0.72 higher) | ‐ | 248 | ⊕⊕⊝⊝ | ||
| Adverse effects Follow‐up: 12 weeks | Study population | RR 0.45 | 38 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 50 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| ES once a week versus ES twice a week | |||
| Patient or population: Adults with overactive bladder (OAB) | |||
| Outcomes | Impact | № of participants | Quality of the evidence |
| Participants cured or improved Follow‐up: 6 months | 100% (37/37) of participants in both groups reported improvement in symptoms but only 9/37 were satisfied enough to request no further treatment | 37 (1 RCT) | ⊕⊝⊝⊝ |
| Participants with improvement in urgency urinary incontinence | Not reported | (0 studies) | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | |||
| ES once a week versus ES three times a week | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with ES 3 times a week | Risk with ES once a week | |||||
| Participants cured or improved (follow‐up not reported) | Study population | RR 0.97 | 35 | ⊕⊝⊝⊝ | ||
| 667 per 1000 | 647 per 1000 | |||||
| Participants with improvement in urgency urinary incontinence (follow‐up not reported) | Study population | RR 0.80 | 22 | ⊕⊝⊝⊝ | ||
| 455 per 1000 | 364 per 1000 | |||||
| OAB‐related quality of life (follow‐up not reported) | I‐QoL scores very similar in the 2 groups (median (range) N): once a week: 77 (35‐100), 17. 3 times per week: 78 (33‐100), 18 | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ | ||
| Adverse effects (follow‐up not reported) | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 35 (1 studies) | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (unclear risk of bias in most domains) | ||||||
| Sensory threshold ES versus motor threshold ES | ||||||
| Patient or population: Adults with overactive bladder (OAB) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with motor threshold ES | Risk with sensory threshold ES | |||||
| Participants cured or improved | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| Participants with improvement in urgency urinary incontinence | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| OAB‐related quality of life Follow‐up: 4 weeks | The mean OAB‐related quality of life in the intervention group was 0.07 lower (2.21 lower to 2.07 higher) | ‐ | 28 | ⊕⊝⊝⊝ | No evidence of a difference between groups | |
| Adverse effects | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias (low risk of performance, detection and attrition bias but unclear risk of selection bias) | ||||||
| Study | Current | Current intensity | Pulse shape & duration | Frequency (Hz) | Duty cycle | Electrodes | Treatment duration/supervision |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Unclear | |
| 30‐60 mA according to patient tolerance (mean 43 mA) | 320 ms | 20 | Unclear | Intravaginal | Two 30‐min sessions per week for 12 weeks | ||
| Unclear | "Sensory threshold, activating superficial cutaneous nerve fibers with larger diameter" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks | |
| Unclear | "Motor threshold, non‐painful contraction is induced and the stimulation can simply make pain relief in the same way that sensory stimulation level (blocking activation of the peripheral or cental inhibition)" | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation | Two 30‐min sessions per week for 12 weeks | |
| Bipolar | 0‐100 mA according to participant tolerance | Bipolar square wave 0.1 µs | 4 | 2 s on, 4 s off | Intravaginal | Three 20‐min sessions per week on alternate days for 7 weeks | |
| Biphasic | 10‐100 mA according to participant tolerance | 1 ms intermittent | 10 | Unclear | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | 0‐100 mA | Asymmetric, 1 s rise time, sustained for 5 s and resting for 5 s | 20 | 1 s rise time, sustained for 5s and resting for 5 s | Intravaginal | Home use: two 20‐min sessions per day for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve | Two 30‐min sessions per week for 4 weeks | |
| Biphasic | 0‐100 mA | Rectangular 200 µs stochastic variation | 4‐10 | Unclear | Intravaginal | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous posterior tibial nerve | Twelve 30‐min sessions | |
| Unclear | Unclear | 500 µs | 10 | Unclear | Intravaginal | Twelve 30‐min sessions | |
| Unclear | 0‐50 mA | 200 µs | 10 | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks | |
| Unclear | Unclear | 200 µs | 150 | Unclear | Transcutaneous electrical nerve stimulation – suprapubic placement | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation – sacral placement | Unclear | |
| Bipolar | 0‐100 mA | Bipolar square wave 0.1 µs | 20 | 2 s on ‐ 4 s off | Intravaginal | 20 minutes daily for 8 weeks | |
| Unclear | 0‐10 mA | Square wave 320 µs | 20 | unclear | Percutaneous posterior tibial nerve stimulation | 30 min once a week for 12 weeks | |
| Bipolar | According to participant tolerance | Continuous bipolar square wave 200 µs | 20 | Unclear | Percutaneous posterior tibial nerve stimulation ‐ adhesive skin electrodes | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation ‐ "34 gauge needle placed 5 cm near internal malleolus" | 30‐min sessions | |
| Unclear | 0‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Percutaneous tibial nerve stimulation | Three 30‐min sessions per week for 4 weeks | |
| Unclear | Unclear current, intensity according to participant tolerance | Unclear | 12.5 | 5 s on, 10 s off | Intravaginal | Fourteen 30‐min sessions | |
| Unclear | According to participant tolerance | Unclear | 5‐10 | Intravaginal/transanal | 10 sessions: 1‐2 20‐min sessions per week for 5‐7 weeks | ||
| Biphasic | Unclear | Biphasic rectangular 220 µs | 10 | 20 s on, 4 s off | Transcutaneous posterior tibial nerve stimulation: external electrode 5 cm above medial malleolus, 1 cm behind the tibia. The other electrode on dorsum of foot | One 30‐min session per week for 9 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twice a week for 6 weeks, performed by either physiotherapist or continence midwife | |
| Unclear | Unclear | Unclear | Unclear | Unclear | VERV electrode patches, placed by the participant ‐ exact placement unclear | One patch per week for 12 weeks | |
| Diadynamic | 20–40 mA, 50%‐75% intensity | Unclear | 20 | Unclear | Active electrode (50 cm2 to 70 cm2) above the pubis, and a passive electrode (150 cm2) in lumbosacral area | 15 procedures every other day | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | Twelve 30‐min sessions | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Twelve 30‐min sessions | |
| Unclear | 8‐70 mA | Unclear | Unclear | Unclear | Vaginal/anorectal | 20‐30 20‐min sessions | |
| Unclear | According to participant tolerance | Unclear | 0‐100 | Unclear | Interferential therapy. 2 anterior flat electrodes placed over obturator foramen 1.5 cm to 2 cm lateral to symphysis, two posterior electrodes placed medial to ischial tuberosities either side of anus | 12 sessions: first session 15 min, all others 30 min | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Once per week | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal/transanal | Twice per week | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous tibial nerve stimulation | Twice a week with at least 48 hour intervals for 12 weeks | |
| Biphasic | Immediately below motor threshold | 200 µs | 10 | Unclear | Transcutaneous electrical nerve stimulation through 1 channel and 2 electrodes | Two 30‐min sessions per week for 4 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal amplitude‐modulated signal through a patch applied to the skin, controlled by wireless handheld remote control | Patch worn for 4 weeks | |
| Unclear | Below the threshold that causes motor contraction | 200 µs | 10 | Unclear | Posterior tibial nerve stimulation with surface electrodes. Negative electrode on medial malleolus, and the positive electrode 10 cm above negative electrode, also on the medial side. Rhythmic flexion of the second toe during the stimulation determined the correct position of the negative electrode | 30‐min twice weekly over 12 sessions (45 days) | |
| Unclear | Pre‐programmed to increase intensity over 24 s to reach therapeutic level and switch off automatically after 30 min. All devices same level of stimulation (average intensity considered comfortable and capable of producing contractions of pelvic floor muscles) | Unclear | During the 10 s ‘‘on time’’ the device delivers 10 repeats of a short high intensity burst of 50 Hz stimulation immediately preceded by a doublet (125 Hz), superimposed on continuous low frequency 2 Hz stimulation | 10 s on, 10 s off | Intravaginal, single‐use tampon‐like Pelviva device | One disposable device per day for 12 weeks except during menstruation | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle slightly cephalad to medial malleolus | One 30‐min session per week for 12 weeks | |
| Unclear | 0.5‐9 mA | Unclear | 20 | Unclear | Percutaneous tibial nerve stimulation: 34‐gauge needle inserted at 60º angle 5 cm cephalad to medial malleolus, slightly posterior to tibia. Surface electrode placed on ipsilateral calcaneous | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Participant‐managed neuromodulation system patch | Subject placement versus investigator placement | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Peripheral tibial neurostimulation | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 3 months | |
| Unclear | 0.5‐10 mA, according to participant tolerance | 200 µs | 20 | Unclear | Stoller afferent neurostimulation: 34‐gauge needle inserted at 30° angle 2 cm to 3 cm superior‐medial aspect of tibial medial malleolus along posterior tibial nerve trace | One 30‐min session per week for 12 weeks | |
| Biphasic | Controlled by participant according to tolerance | 300 µs | Asymmetrical, 50 | Unclear | Intravaginal: probe with two 26 mm rings 40 mm apart | Unclear | |
| Unclear | Unclear | 200 µs | 10 | Unclear | Transcutaneous tibial nerve stimulation | One 30 min session per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous posterior tibial nerve stimulation | Unclear | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30 min session per day for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transcutaneous: discrete [sic], self‐contained, portable device adhesive to the skin | One 30‐min session per week for 12 weeks | |
| Unclear | Up to 40 v | Unclear | 10‐50 | Unclear | Maximum perineal stimulation: Scott electrode in vagina, large indifferent electrode under buttocks | Single 20‐min session | |
| Unclear | Unclear | Unclear | 10 | Unclear | Intravaginal cushion attached to stimulator worn around waist | Cushion worn for 8 out of 24 h, day or night according to participant preference | |
| Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Unilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on the right ankle | Unclear | |
| Unclear | Stimulus intensity just below that which would cause a motor contraction of toes/shoulder muscles | Unclear | Unclear | Unclear | Bilateral posterior tibial nerve stimulation with conventional TENS machine ‐ electrodes placed above and below the medial malleolus on both ankles | Unclear | |
| Unclear | 5‐25 mA | Unclear | Device uses 2 programmes simultaneously: 12.5 Hz and 50 Hz | 5 s impulse | Intravaginal | Twice daily for 4 months. Length of session increased monthly: 15, 30, 45, 60 minutes | |
| Unclear | Participants asked to control stimulation to achieve tickling sensation | 200 µs | 20 | Continuous | Transcutaneous. 2 self‐adhesive pads applied bilaterally over the perianal region (S2‐S3 dermatome) | Up to 6 hours daily for 6 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. Horizontal placement of electrode patch near sacral nerve | Patch worn continuously for 7 days | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Transdermal. Carrier signal and pulse envelope through patch applied on skin over spinal nerves in lower back. 30° angle placement of electrode patch near sacral nerve | Patch worn continuously for 7 days | |
| Unclear | According to participant tolerance | 250 µs | 10 | Unclear | Posterior tibial nerve stimulation. Surface electrode placed behind media malleolus and another placed 10 cm above first electrode | Two 30 min sessions per week for 12 weeks | |
| Biphasic | 0‐100 mA, according to participant tolerance | 100 µs | 20 | 2 s contraction time, duty cycle 1–2 s | Intravaginal | Three 30‐min sessions per week for 8 weeks. 5 min rest between each 15 min | |
| Square | 25 mA. 70% of intensity of maximal amplitude of registered response from abductor hallucis muscle | Square impulse 100 µs | 1 | Unclear | Stoller afferent neurostimulation. Electrodes placed behind medial ankle of left lower extremity, cathode placed proximally and anode distally | One 30 min session per week for 5 weeks | |
| Unclear | According to participant tolerance | Unclear | 10 min of each frequency, 3 min: 5‐10 Hz, 10‐50 Hz, 50 Hz | 7 s on, 25 s off | Intravaginal/transanal | 6 sessions over two weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Percutaneous tibial nerve stimulation | Two 30‐min sessions per week for 6 weeks | |
| Unclear | 0‐10 mA | Unclear | Unclear | Unclear | Percutaneous posterior tibial nerve stimulation | One 30‐min session per week for 12 weeks | |
| Unclear | Unclear | 200 ms | 10 | Unclear | Transcutaneous neurostimulation. Electrode pads affixed bilaterally to the skin overlying S3 dermatomes (junction of buttock and upper thigh) | Single session | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Biphasic | Minimum 20‐63 mA, maximum 40‐72 mA, according to participant tolerance | Biphasic symmetrical 400 µs | 10 | 10 s on, 5 s off | Intravaginal | Two 20‐min sessions per week for 12 weeks | |
| Unclear | Unclear | Unclear | Unclear | Unclear | Intravaginal | One session per day (at home) for 6 weeks | |
| Unclear | 0‐90 mA, according to participant tolerance | Unclear | 20 | Unclear | Intravaginal | One session per day (at home) for 6 weeks | |
| Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed electrodes | Two 15‐min sessions per day for 4 weeks | |
| Square | 0‐60 mA, according to participant tolerance | Square, 1 ms | 10 | Unclear | Intravaginal (women), surface electrode or anal plug (men) Surface electrode placed on dorsal part of penis. Anal electrode bullet‐shaped, vaginal plug cylinder‐formed with ring‐formed | Single session |
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | No active treatment (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Improvement in QoL measured by Incontinence Quality of Life Questionnaire, Behavioural Urge Score and International Prostate Symptom Score | 5/9 | 0/9 | RR 11.00 (95% CI 0.70 to 173.66) | |
| ICI‐Q score1 | Median (range), N: 6 (0‐17), 64 | Median (range), N: 9 (3‐18), 60 | Not estimable | |
| Secondary outcomes | ||||
| Daytime frequency | NR | NR | Favours ES P = 0.0001 | |
| Nocturia | NR | NR | Favours ES P = 0.0186 | |
| Participants with nocturnal enuresis | 45 days' treatment: 0/12 | 45 days' treatment: 2/12 | Favours ES RR 5.00 (95% CI 1.63 to 15.31) | |
| 12 months' follow‐up: 0/12 | 12 months' follow‐up: 2/12 | |||
| Participants with nocturia | 45 days' treatment: 5/12 | 45 days' treatment: 9/12 | RR 2.33 (95% CI 0.78 to 6.94) | |
| 12 months' follow‐up: 1/12 | 12 months' follow‐up: 6/12 | Favours ES RR 0.17 (95% CI 0.02 to 1.18) | ||
| Participants with increased daytime frequency | 45 days' treatment: 3/12 | 45 days' treatment: 11/12 | Favours ES RR 0.27 (95% CI 0.10 to 0.74) | |
| 12 months' follow‐up: 0/12 | 12 months' follow‐up: 9/12 | Favours ES RR 0.05 (95% CI 0.00 to 0.81) | ||
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Placebo or sham treatment (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| ICIQ‐SF score | Median (IQR), N: 2 (0 to ‐6), 15 | 0 (‐3 to 3), 13 | P = 0.132 | |
| Participants with improvement in ICIQ‐SF score | 10/15 | 6/13 | RR 1.44 (95% CI 0.73 to 2.87) | |
| OAB‐Q total score1 | 83.99 (16.99), 21 | 66.63 (25.06), 16 | Favours ES MD 17.36 (95% CI 3.09 to 31.63) | |
| I‐QoL score1 | 69.9 (65.8‐73.3), 17 | 70.6 (62.2‐79.1), 15 | No evidence of a difference | |
| Change in OAB‐Q score | Median (IQR), N: 8.8 (1.6 to 20.0), 80 | Median (IQR), N: 9.2 (‐0.8 to 27.2), 83 | P = 0.9918 | |
| Change in OAB‐Q score | 36.7 (21.5), 101 | 29.2 (20.0), 102 | Favours ES MD 7.50 (1.79, 13.21) | |
| QoL score2 | 1.6 (0.7), 37 | 2.2 (0.9), 31 | Favours ES MD ‐0.60 (95% CI ‐0.99 to ‐0.21) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Number of pads per day | 0.8 (1.2), 37 | 1.1 (2.0), 31 | MD ‐0.30 (95% CI ‐1.10 to 0.50) | |
| Other outcomes | ||||
| Participants with reduction in analogue discomfort sensation | 8/20 | 5/20 | RR 1.60 (95% CI 0.63 to 4.05) | |
| Participants with reduction in analogue wetness sensation | 6/20 | 5/20 | RR 1.20 (95% CI 0.44 to 3.30) | |
| Pelvic floor muscle strength (cmH2O) | 53.8 (18.6), 20 | 46.8 (12.5), 20 | MD 7.00 (95% CI ‐2.82 to 16.82) | |
| Urgency score2 | 1.7 (0.7), 37 | sham ES: 2 (0.8), 31 | MD ‐0.30 (95 CI ‐0.66 to 0.06) | |
| Results in bold are statistically significant 1Lower score = greater severity | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | PFMT (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants cured | 14/21 | 12/21 | RR 1.17 (95% CI 0.72 to 1.88) | |
| Participants with improvement in UUI | 9/18 | 13/34 | RR 1.62 (95% CI 0.51 to 5.12) | |
| King's Health Questionnaire score1 | 180.08 (176.03), 35 | 50.27 (171.42), 34 | Favours ES MD 129.81 (95% CI 47.83 to 211.79) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Incontinence episodes per 24 hours | 7.9 (13.7), 21 | 7.8 (15.3), 21 | MD 0.10 (95% CI ‐8.68 to 8.88) | |
| Micturitions per 24 hours | 7.9 (2.3), 21 | 71. (2.1), 21 | MD 0.80 (95% CI ‐0.53 to 2.13) | |
| Nocturia episodes per night | 1.2 (1.3), 21 | 1 (1.1), 21 | MD 0.20 (95% CI ‐0.53 to 0.93) | |
| Number of pads per day | 0.9 (1.7), 21 | 0.8 (1.3), 21 | MD 0.10 (95% CI ‐0.82 to 1.02) | |
| 1Higher score = greater QoL | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | PFMT plus biofeedback (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants with improvement in UUI | 9/17 | 17/34 | RR 1.06 (95% CI 0.60 to 1.85) | |
| King's Health Questionnaire score1 | 180.08 (176.03), 35 | 185.86 (176.57), 34 | MD ‐5.78 (95% CI ‐88.99 to 77.43) | |
| 1Higher score = greater QoL | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Laseropuncture/electro‐acupuncture (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Bladder Self‐Assessment Questionnaire score | 5.18 (2.56), 11 | 7.27 (2.24), 11 | Favours ES MD ‐2.09 (95% CI ‐4.10 to ‐0.08) | |
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Micturitions per day | 8 (1.73), 11 | 7.73 (1.67), 11 | MD 0.27 (95% CI‐1.15 to 1.69) | |
| Nocturia episodes per night | 1.09 (1.51), 11 | 2.09 (1.92), 11 | MD ‐1.00 (95% CI ‐2.44 to 0.44) | |
| 1Higher score = greater severity | ||||
| Study | Outcome | ES (mean (SD/range), N or n/N; if available) | Comparator (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| Participants cured or improved | 69% (N not reported) | Probanthine 50% (N not reported) | Not estimable | |
| I‐QoL score1 | 25.2 (1.0), 50 | Solifenacin succinate: 24.2 (1.0), 48 | MD 1.00 (95% CI 0.60 to 1.40) | |
| OAB‐Q score2 | 2.9 (0.9), 14 | Solifenacin succinate: 3.1 (1.1), 14 | MD ‐0.20 (95% CI ‐0.94 to 0.54) | |
| Patient Global Impression of Improvement score2 | 2.1 (0.7), 14 | Solifenacin succinate: 2.9 (1.1), 14 | Favours ES MD ‐0.80 (95% CI ‐1.48 to ‐0.12) | |
| Participant Perception of Intensity of Urgency Scale score2 | 2.1 (0.9), 14 | Solifenacin succinate: 2.7 (1.2), 14 | MD ‐0.60 (95% CI ‐1.39 to 0.19) | |
| ES | Oestrogen cream | Favours ES MD ‐2.20 (95% CI ‐2.71 to ‐1.69) Favours ES MD ‐2.00 (95% CI ‐2.50 to ‐1.50) MD 1.60 [0.91, 2.29] | ||
| QoL score2 (instrument not reported) | End of treatment: 2.8 (2), 105 3 months: 4 (1.7), 105 6 months: 7.6 (3), 105 | End of treatment: 5 (1.8), 105 3 months: 6 (2), 105 6 months: 6 (2), 105 | ||
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| ES | Oestrogen cream | |||
| Voids per 24 hours | End of treatment: 4.7 (0.8), 105 3 months: 5.0 (1.0), 105 6 months: 6.6 (1.5), 105 | End of treatment: 5.0 (0.9), 105 3 months: 5.3 (0.9), 105 6 months: 5.0 (0.8), 105 | Favours ES MD (‐0.30 (95% CI ‐0.56 to ‐0.04) Favours ES MD ‐0.30 (95% CI ‐0.53 to ‐0.07) Favours oestrogen cream MD 1.60 (95% CI 1.27 to 1.93) | |
| Nocturia episodes per night | End of treatment: 0.9 (0.7), 105 3 months: 1.1 (0.9), 105 6 months: 2.2 (0.9), 105 | End of treatment: 1.4 (0.8), 105 3 months: 1.5 (0.8), 105 6 months: 5.0 (0.8), 105 | Favours ES MD ‐0.50 (95% CI ‐0.70 to ‐0.30) Favours ES MD ‐0.40 (95% CI ‐0.63 to ‐0.17) MD ‐2.80 (95% CI ‐3.03 to ‐2.57) | |
| Incontinence episodes | End of treatment: 0.1 (0.3), 105 3 months: 0.1 (0.3), 105 6 months: 0.4 (0.6), 105 | End of treatment: 0.4 (0.6), 105 3 months: 0.5 (0.6), 105 6 months: 0.4 (0.6), 105 | Favours ES MD ‐0.30 (95% CI ‐0.43 to ‐0.17) Favours ES ‐0.40 (95% CI ‐0.53 to ‐0.27) 0.00 [‐0.16, 0.16] | |
| Urgency episodes | End of treatment: 2 (0.7), 105 3 months: 2.7 (1.0), 105 6 months: 4.7 (1.3), 105 | End of treatment: 4 (1.3), 105 3 months: 4.5 (1.5), 105 6 months: 4 (1.3), 105 | ‐3.00 [‐3.28, ‐2.72] ‐1.80 [‐2.14, ‐1.46]0.70 [0.35, 1.05] | |
| Number of pads per day | 0.9 (1.8), 21 | Oxybutynin: 0.9 (1.5), 22 | MD 0.00 (95% CI ‐0.96 to 0.96) | |
| Participants with nocturia | 2/18 | Oxybutynin: 3/19 | RR 0.70 (95% CI 0.13 to 3.73) | |
| Results in bold are statistically significant 1Lower score = greater severity | ||||
| Study | Outcome | ES plus PFMT (mean (SD/range), N or n/N; if available) | PFMT (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| SF‐Qualiveen1 | Median (IQR), N: 9 weeks: 1.000 (0.656, 1.719), 16 6 months: 1.313 (0.687, 1.625), 16. | Median (IQR), N: 9 weeks: 1.375 (0.625, 2.188) 6 months: 1.500 (0.344, 2.094), 15 | Not estimable | |
| York Incontinence Perception Scale2 | 41.2 (10.2), 6 | 47 (5.5), 6 | MD ‐0.65 (95% CI ‐1.83 to 0.52) | |
| Participants with improvement in UUI | 19/25 | 7/26 | Favours ES plus PFMT RR 2.82 (95 CI 1.44 to 5.52) | |
| ICIQ‐SF score1 | 7.9 (4.5), 25 | 10.6 (4.4), 26 | Favours ES plus PFMT MD ‐2.70 (95% CI ‐5.14 to ‐0.26) | |
| Secondary outcomes | ||||
| Nocturia episodes per night | 1.3 (1.5), 25 | 2.4 (1.3), 26 | Favours ES plus PFMT MD ‐1.10 (95% CI ‐1.87 to ‐0.33) | |
| Adverse effects | 0/25 | 0/26 | Not estimable | |
| Other outcomes | ||||
| Pelvic floor muscle strength (cmH2O) | 27 (16), 6 | 47.2 (22.7), 6 | MD ‐20.20 (95% CI ‐42.42 to 2.02) | |
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES plus behavioural therapy (mean (SD/range), N or n/N; if available) | Behavioural therapy (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| OAB‐Q score1 | 100.81 (41.5), 31 | 127.71 (40.64), 37 | Favours ES plus behavioural therapy MD ‐26.90 (95% CI ‐46.52 to ‐7.28) | |
| Incontinence Severity Index score1 | 5.15 (3.23), 31 | 7.38 (4.00), 37 | Favours ES plus behavioural therapy MD ‐26.90, 95% CI ‐46.52 to ‐7.28 | |
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES plus drugs (mean (SD/range), N or n/N; if available) | Drugs (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| IIQ‐7 score1 | ES plus tolterodine: 9.0 (0.8), 20 | Tolterodine: 11.2 (2.7), 18. | Favours ES plus tolterodine MD ‐2.20 (95% CI ‐3.50 to ‐0.90 | |
| ES plus oestrogen cream: | Oestrogen cream: | |||
| QoL score1 (instrument not reported) | End of treatment: 2.9 (2.2), 105. 3 months: 1.6 (0.9), 105. 6 months: 2 (0.8), 105. | End of treatment: 5 (1.8), 105 3 months: 6 (2), 105 6 months: 6 (2), 105 | MD ‐2.10 (95% CI ‐2.64, ‐1.56] MD ‐4.40 (95% CI ‐4.82 to ‐3.98) MD ‐4.00 (95% CI ‐4.41 to ‐3.59) | |
| Secondary outcomes | ||||
| ES plus oestrogen cream: | Oestrogen cream: | |||
| Voids per day | End of treatment: 5 (0.8), 105. 3 months: 5 (0.8), 105. 6 months: 5 (0.8), 105. | End of treatment: 5.0 (0.9), 105 3 months: 5.3 (0.9), 105 6 months: 5.0 (0.8), 105 | MD 0.00 (95% CI ‐0.23 to 0.23) MD ‐0.30 (95% CI ‐0.53 to ‐0.07) MD 0.00 (95% CI ‐0.22 to 0.22) | |
| Nocturia episodes per night | End of treatment: 0.5 (0.5), 105 3 months: 1 (0.9), 105 6 months: 1.5 (0.8), 105 | End of treatment: 1.4 (0.8), 105 3 months: 1.5 (0.5), 105 6 months: 5 (0.8), 105 | MD ‐0.90 (95% CO ‐1.08 to ‐0.72) MD ‐0.50 (95% CI ‐0.70 to ‐0.30) MD ‐3.50 (95% CI ‐3.72 to ‐3.28) | |
| Incontinence episodes per 24 hours | End of treatment: 1.4 (0.7), 105 3 months: 0.09 (0.28), 105. 6 months: 0.09 (0.28), 105. | End of treatment: 0.4 (0.6), 105 3 months: 0.5 (0.6), 105 6 months: 0.4 (0.6), 105 | MD 1.00 (95% CI 0.82 to 1.18) MD ‐0.41 (95% CI ‐0.54 to ‐0.28) MD ‐0.31 (95% CI ‐0.44 to ‐0.18) | |
| Urgency episodes per 24 hours | End of treatment: 1.4 (0.7), 105 3 months: 1.6 (0.9), 105 6 months: 2 (0.8), 105 | End of treatment: 4 (1.3), 105 3 months: 4.5 (1.5), 105 6 months: 4 (1.3), 105 | MD ‐2.60 (95% CI ‐2.88 to ‐2.32) MD ‐2.90 (95% CI ‐3.23 to ‐2.57) MD ‐2.00 (95% CI ‐2.29 to ‐1.71) | |
| Adverse effects | ES plus tolterodine: 1/20 | Tolterodine: 2/18 | RR 0.45 (95% CI 0.04 to 4.55) | |
| Results in bold are statistically significant 1Higher score = greater severity | ||||
| Study | Outcome | ES A (mean (SD/range), N or n/N; if available) | ES B (mean (SD), N or n/N; if available) | Result |
| Primary outcomes: cure/improvement of OAB symptoms; OAB‐related quality of life | ||||
| ICIQ‐OAB score1 | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 4.46 (2.66), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 4.53 (3.07), 13 | MD ‐0.07 (95% CI ‐2.21 to 2.07) | |
| Success = > 50% reduction in micturitions/24 hours OR If incontinent, success > 50% reduction in UI episodes/24 hours | ES once a week: 11/17 (4/11 incontinent participants) | ES 3 times per week: 12/18 (5/11 incontinent participants) | RR 0.97 (95% CI 0.60 to 1.57) Incontinence participants: RR 0.80 (95% CI 0.29 to 2.21) | |
| I‐QoL score2 | ES once a week (median, range, N): 77 (35‐100), 17 | ES 3 times a week (median, range, N): 78 (33‐100), 18 | Not estimable | |
| Participants with improvement in symptoms | ES once a week: 100% | ES twice a week: 100% | Not estimable | |
| Participants satisfied enough to request no further treatment | 24% (9/37) | Not estimable, not reported per treatment group | ||
| Secondary outcomes: clinicians' observations and other quantification of symptoms | ||||
| Adverse effects | ES once a week: 0/17 | ES 3 times per week: 0/18 | Not estimable | |
| Subjective improvement after 6‐8 sessions | ES once a week: 17/17 | ES 3 times a week: 18/18 | Not estimable | |
| Incontinence episodes per 24 hours | ES once a week (median, range, N): 1 (0‐3), 11 | ES 3 times a week (median, range, N): 1 (0‐3), 11 | Not estimable | |
| Micuturitions per 24 hours | ES once a week (median, range, N): 8 (5‐15), 17 | ES 3 times a week (median, range, N): 8 (6‐18), 18 | Not estimable | |
| SF‐36 score | ES once a week (median, range, N): 62 (24‐81), 17 | ES 3 times per week (median, range, N): 62 (25‐80), 18 | Not estimable | |
| UUI episodes per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 0.33 (0.57), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 0.84 (1.39), 13 | MD ‐0.51 (95% CI ‐1.32 to 0.30) | |
| Urgency episodes per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 0.79 (0.97), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 0.58 (0.65), 13 | MD 0.21 (95% CI ‐0.39 to 0.81) | |
| Micturitions per 24 hours | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 8.33 (2.52), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 7.89 (2.64), 13 | MD 0.44 (95% CI ‐1.48 to 2.36) | |
| Nocturia episodes per night | Tibial nerve stimulation: sensory threshold activating superficial cutaneous nerve fibres with larger diameter: 1.26 (1.21), 15 | Tibial nerve stimulation: motor threshold, non‐painful contraction is induced: 1.05 (1.01), 13 | MD 0.21 (95% CI ‐0.61 to 1.03) | |
| Maximum cystometric capacity | 150 Hz: 351 (144), 16 | 10 Hz: 305 (146), 16 | MD 46.00 (95% CI ‐54.48 to 146.48) | |
| Volume at first desire to void | 150 Hz: 208.5 (132), 16 | 10 Hz: 154 (61), 16 | MD 54.50 (95% CI ‐16.75 to 125.75) | |
| Other outcomes | ||||
| Participants satisfied | 200 µs pulse width: 17/22 | 500 µs pulse width: 11/16 | RR 1.12 (95% CI 0.75 to 1.68) | |
| 1Higher score = greater severity | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured or improved Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.34, 2.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured Show forest plot | 4 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.39, 5.21] |
| 2 Number of participants cured or improved Show forest plot | 10 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.85, 2.77] |
| 3 Number of participants cured or improved: different ES routes Show forest plot | 6 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [2.54, 4.96] |
| 3.1 Percutaneous tibial nerve stimulation | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [2.22, 4.58] |
| 3.2 Intravaginal | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.46 [2.33, 12.81] |
| 4 Number of participants satisfied Show forest plot | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.02, 2.04] |
| 5 Number of participants with improvement in urgency urinary incontinence Show forest plot | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 5.03 [0.28, 89.88] |
| 6 Number of participants with improvement in urinary frequency Show forest plot | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.43, 2.92] |
| 7 Number of incontinence episodes per 24 h Show forest plot | 2 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐1.43 [‐1.92, ‐0.95] |
| 8 Number of nocturia episodes Show forest plot | 2 | 245 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.73, ‐0.02] |
| 9 Number of micturitions per 24 h Show forest plot | 3 | 285 | Mean Difference (IV, Fixed, 95% CI) | ‐1.09 [‐1.70, ‐0.47] |
| 10 Number of participants with adverse effects Show forest plot | 3 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.84, 1.83] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured or improved Show forest plot | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.19, 2.14] |
| 2 Number of participants satisfied Show forest plot | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of incontinence episodes per 24 h Show forest plot | 2 | 136 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐2.33, ‐1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants cured Show forest plot | 7 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.41] |
| 1.1 ES versus tolterodine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.47] |
| 1.2 ES versus oxybutynin | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.65, 1.72] |
| 1.3 ES versus propantheline bromide | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.38, 5.74] |
| 2 Number of participants cured or improved Show forest plot | 8 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.04, 1.38] |
| 2.1 ES versus tolterodine | 3 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.00, 1.41] |
| 2.2 ES versus oxybutynin | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.91, 1.52] |
| 2.3 ES versus propantheline bromide | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.86, 2.44] |
| 3 Number of participants cured or improved: routes of ES Show forest plot | 5 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.04, 1.54] |
| 3.1 Transcutaneous posterior tibial nerve stimulation | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.74, 1.92] |
| 3.2 Intravaginal/transanal | 4 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 4 Number of participants satisfied Show forest plot | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 4.1 ES versus oxybutynin | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 5 Number of incontinence episodes per 24 h Show forest plot | 5 | 477 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.11, 1.60] |
| 5.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.49, 0.29] |
| 5.2 ES versus oxybutynin | 1 | 43 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐6.45, 8.25] |
| 5.3 ES versus trospium + solifenacin | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 2.2 [1.78, 2.62] |
| 5.4 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.16, 0.16] |
| 5.5 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.01, 0.21] |
| 6 Number of urgency episodes per 24h Show forest plot | 2 | 294 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [0.28, 0.96] |
| 6.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.98, 0.78] |
| 6.2 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.35, 1.05] |
| 7 Number of micturitions per 24 h Show forest plot | 6 | 646 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.15, 0.52] |
| 7.1 ES versus tolterodine | 2 | 116 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐1.06, 1.50] |
| 7.2 ES versus oxybutynin | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.87 [‐0.18, 1.91] |
| 7.3 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.04, 0.84] |
| 7.4 ES versus oestrogen cream | 1 | 420 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.15, 0.52] |
| 8 Number of nocturia episodes per night Show forest plot | 4 | 367 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐2.27, ‐1.88] |
| 8.1 ES versus tolterodine | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 8.2 ES versus oxybutynin | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.35, 0.95] |
| 8.3 ES versus solifenacin succinate | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.06, 0.66] |
| 8.4 ES versus oestrogen cream | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐2.8 [‐3.03, ‐2.57] |
| 9 Number of participants with adverse effects Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 ES versus oxybutynin | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.84] |
| 9.2 ES versus tolterodine | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.27] |
| 9.3 ES versus solifenacin succinate | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants satisfied Show forest plot | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.13, 2.20] |
| 2 Number of incontinence episodes per 24h Show forest plot | 2 | 119 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.84, 0.64] |
| 3 Number of urgency episodes per 24 h Show forest plot | 2 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐2.49 [‐2.74, ‐2.24] |
| 4 Number of micturitions per 24 h Show forest plot | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.62, 0.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life Show forest plot | 2 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.72, 0.72] |
| 2 Number of incontinence episodes per 24h Show forest plot | 2 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐0.63, ‐0.43] |
| 3 Number of urgency episodes per 24 hours Show forest plot | 2 | 248 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.11, ‐1.54] |
| 4 Number of micturitions per 24 hours Show forest plot | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.22, 0.21] |

