درمان با ید رادیواکتیو در مقابل داروهای ضد تیروئید در مدیریت بالینی بیماری گریوز
Appendices
Appendix 1. Search strategies
| Cochrane Library |
| #1 [mh "Graves disease"] |
| MEDLINE (Ovid SP) |
| 1. exp Graves Disease/ |
| EMBASE (Ovid SP) |
| 1. exp Graves disease/ 3. (graves* adj6 (orbitopath* or ophthalmopath*)).tw. |
| ICTRP Search Portal (Standard search) |
| [searched as one string] grave* AND radiotherap* OR |
| ClinicalTrials.gov (Advanced search) |
| Search Terms: (grave OR graves OR basedow OR basedows) AND (radiotherapy OR "radio therapy" OR radioiodine OR "radio iodine" OR "131" OR "131‐I" OR "RAI") |
Appendix 2. Description of interventions
| Intervention(s) [route, frequency, total dose/day] | Comparator(s) [route, frequency, total dose/day] | |
| Traisk 2009 | A single oral activity of 131I was administered using the following formula: activity (MBq) = 23.4 x thyroid mass (grams) x 120 (Gy)/estimated uptake (0 h; %) x effective half‐life (days) | Methimazole was given as 15 mg twice daily; at day 14, 50 μg L‐thyroxine was added daily; methimazole was discontinued after 18 months with an additional month of L‐thyroxine substitution of 100 μg daily, which thereafter was discontinued |
| Tallstedt 1992a | A single oral activity of 131I was administered, based on thyroid size, 24‐hour 131I uptake, and the measured effective half‐life of the isotope in the thyroid | Oral administration of 10 mg methimazole 4 times daily (40 mg/day). 3 to 5 weeks later thyroxine was added in doses between 0.1 and 0.3 mg/day (mean dose 0.17 mg/day). Methimazole and thyroxine were discontinued simultaneously after 18 months. Propranolol at a dose of 20 to 60 mg 3 to 4 times daily or 50 to 300 mg metoprolol daily in divided doses was given initially until the participants became euthyroid. In cases of adverse reactions to methimazole, the drug was exchanged with propylthiouracil (100 mg 4 times daily) |
| aThe participants who were between 20 and 34 years old were randomly assigned to treatment with antithyroid drug plus thyroxine (young medical group) or subtotal thyroidectomy (young surgical group); the participants who were between 35 and 55 years old were randomly assigned to antithyroid drug plus thyroxine (old medical group), subtotal thyroidectomy (old surgical group) or to iodine‐131 (iodine‐131 group) 131I: iodine‐131 (radioiodine) | ||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of intervention | Description of participants | Trial period | Country | Setting | Ethnic groups | Diagnostic criteria for Graves' disease | |
| Traisk 2009 | I: radioiodine | 1 day (4 years) | Patients aged 35 to 69 years; symptomatic Graves' hyperthyroidism | Started in May 1996 and the trial was closed in 2003. With the 4‐year clinical follow‐up, the trial was terminated by the end of 2007 | Sweden | Outpatients | ‐ | Serum TSH ≤ 0.1 mIU/L and elevated triiodothyronine and/or free thyroxine, thyroid uptake of iodine‐131, and radionuclide scans compatible with Graves' disease, i.e. an even distribution of radionuclide |
| C: methimazole | 18 months (4 years) | |||||||
| Tallstedt 1992 | I: radioiodine | 1 day (24 monthsa) | Participants aged 20 to 55 years with hyperthyroidism caused by Graves' disease | 1991 (most participants had been followed for at least 24 months) | Sweden | Outpatients | ‐ | Presence of symptoms and signs of hyperthyroidism, a diffuse goitre, elevated total and free thyroxine and total triiodothyronine concentrations in serum, detectable serum concentrations of thyrotropin‐receptor antibodies, increased uptake of iodine‐131 by the thyroid, and thyroid radionuclide scans showing a diffuse pattern of isotope uptake |
| C: methimazole | 18 months (24 monthsa) | |||||||
| ‐ denotes not reported C: comparator; I: intervention; TSH: thyroid‐stimulating hormone aDifferent follow‐up times for various outcome measures. | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and | Sex | Age | Duration of disease | Co‐medications/Co‐interventions | Comorbidities | |
| Traisk 2009 | I: radioiodine | 87 | 51 (8) | 5 (4) | Beta‐blockers were used as pretreatment to radioiodine; L‐thyroxine substitution was administered at day 14 after radioiodine treatment | Current smokers: 36 |
| C: methimazole | 91 | 50 (8) | 6 (5) | At day 14, 50 μg of L‐thyroxine was added and increased to 100 μg 2 weeks later; at week 6, the dose of L‐thyroxine was adjusted to normalise the levels of serum T3 and free T4 and to bring TSH levels to less than 0.4 mIU/L; a slightly elevated serum free T4 was accepted up to 20% above the upper normal limit; beta‐blockers were used for symptomatic treatment | Current smokers: 42 | |
| Tallstedt 1992 | I: radioiodine | 85 | 45 (5) | ‐ | Unless contraindicated, propanolol or metoprolol was given to all participants; 18 participants were given more than one dose of iodine‐131 from 10 weeks to 23 months; all participants eventually had hypothyroidism and received thyroxine 0.1 ‐ 0.3 mg daily | Smoker: 56 |
| C: methimazole | 85 | 28 (4) & 45 (6) | ‐ | Thyroxine 0.1 ‐ 0.3 mg daily was added after 3 ‐ 5 weeks; propanolol 60 ‐ 240 mg daily or metoprolol 50 ‐ 300 mg daily was given initially for a few weeks; methimazole and thyroxine were discontinued simultaneously | Smoker: 42 | |
| ‐ denotes not reported BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; SD: standard deviation; T3: triiodothyronine; T4: thyroxine; TSH: thyroid‐stimulating hormone | ||||||
Appendix 5. Matrix of trial endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) | Trial results/ | Endpoints quoted in publication(s)b,c | Endpoints quoted in abstract of publication(s)b,c | |
| Traisk 2009 | N/T | Primary outcome measure(s): difference in the proportion of participants with worsening or development of thyroid‐associated ophthalmopathy during a 4‐year follow‐up | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): recurrence of hyperthyroidism (relapse), adverse events, thyroid hormone levels, corticosteroid treatment for thyroid‐associated ophthalmopathy, health‐related quality of life | Other outcome measure(s): worsening or development of thyroid‐associated ophthalmopathy; smoking as a risk factor | |||
| Tallstedt 1992 | N/T | Primary outcome measure(s): occurrence of ophthalmopathy within 2 years after the initiation of therapye | Primary outcome measure(s): ‐ | |
| Secondary outcome measure(s): ‐ | Secondary outcome measure(s): ‐ | |||
| Other outcome measure(s): recurrent hyperthyroidism; predictive factors of ophthalmopathy; participants assessment of the treatment; sick leave; adverse effects; euthyroidism; health‐related quality of life; costs; laboratory assessments; TSH‐receptor autoimmunityf | Other outcome measure(s): development or worsening of Graves' ophthalmopathy | |||
| ‐ denotes not reported aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers). fRefers to outcome measures from all of the six publications of Tallstedt 1992. EMA: European Medicines Agency; FDA: Food and Drug Administration (US); mo: month(s); N/T: no trial document available; TSH: thyroid‐stimulating hormone | ||||
Appendix 6. High risk of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias | High risk of bias | High risk of bias | High risk of bias | |
| Traisk 2009 | N/A | ||||
| Tallstedt 1992 | N/A | ||||
| aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant. bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported. N/A: not applicable; ORBIT: Outcome Reporting Bias In Trials | |||||
Appendix 7. Definition of endpoint measurement
| All‐cause mortality | Health‐related quality of life | Adverse events other than development and worsening of Graves' ophthalmopathy [hypothyroidism] | Recurrence of hyperthyroidism [relapse] | Development and worsening of Graves' disease | Individuals in euthyroid state | Bone mineral density | Socioeconomic effects | |
| Traisk 2009 | ‐ | Short‐Form Health Status Survey (SF‐36) | TSH was elevated and/or FT3, FT4 or T3 was below the lower limit of the reference range | ‐ | The worsening or development and improvement of ophthalmopathy met 2 of the following 4 decisive factors compared with baseline data: 1) change in exophthalmometry readings of 2 mm or more; 2) improvement or deterioration of the patient's eye movements between the 4 scoring levels (no impairment, clearly impaired, diplopia in the primary position, fixation of the globe); 3) changes of visual acuity caused by optic neuropathy; and 4) changes in 2 of the 3 ophthalmopathy activity measures (chemosis, eyelid oedema, and conjunctival redness) | ‐ | N/I | N/I |
| Tallstedt 1992 | ‐ | Short‐Form Health Status Survey (SF‐36) and quality of life 2004 | TSH was elevated and/or FT3, FT4 or T3 were below the lower limit of the reference range | TSH was decreased and/or FT3, FT4 or T3 were above the lower limit of the reference range | The eye examination by the study ophthalmologist included testing of visual acuity, tonometry, Hertel exophthalmometry, slitlamp examination and tests of extraocular‐muscle function with Lee's screen; the results of the examination were summarised as an ophthalmopathy‐index score, which was based on a modification of the American Thyroid Association's classification of ocular changes in Graves' disease; 4 categories of findings (soft tissue involvement, exophthalmos, extraocular muscle involvement and sight loss) were scored from 1 to 3 according to their severity | TSH (0.1 ‐ 4.5 mu/L), T4 (75 ‐ 150 nmol/L) and T3 (1.1 ‐ 2.5) nmol/L levels within the normal range | N/I | The costs were assessed by analysing the official hospital reimbursement system for both outpatient and inpatient costs for a period of 2 years from the day of randomisation; sick leave: number of days the participants stayed at home from work due to Graves' disease or other diseases |
| "‐" denotes not reported FT3: free triiodothyronine; FT4: free thyroxin; N/I: not investigated; T3: triiodothyronine; T4: thyroxine; TSH: thyroid‐stimulating hormone | ||||||||
Appendix 8. Adverse events except development/worsening of Graves' opthalmopathy (I)
| Intervention(s) and comparator(s) | Participants included in analysis | Deaths | Deaths | Participants with at least one adverse event | Participants with at least one adverse event | Participants with at least one severe/serious adverse event | Participants with at least one severe/serious adverse event | |
| Traisk 2009 | I: radioiodine | 163 | ‐ | ‐ | 0 | 0 | ‐ | ‐ |
| C: methimazole | 150 | ‐ | ‐ | 12 | 8 | ‐ | ‐ | |
| Tallstedt 1992a | I: radioiodine | 39 | ‐ | ‐ | 39b | 100 | ‐ | ‐ |
| C: methimazole | 68 | ‐ | ‐ | 11 | 16 | ‐ | ‐ | |
| ‐ denotes not reported aData from up to 48 months follow‐up. C: comparator; I: intervention; T4: thyroxine | ||||||||
Appendix 9. Adverse events except development/worsening of Graves' opthalmopathy (II)
| Intervention(s) and comparator(s) | Participants included in analysis | Participants discontinuing trial due to an adverse event | Participants discontinuing trial due to an adverse event | |
| Traisk 2009 | I: radioiodine | 163 | ‐ | ‐ |
| C: methimazole | 150 | ‐ | ‐ | |
| Tallstedt 1992 | I: radioiodine | 39 | ‐ | ‐ |
| C: methimazole | 68 | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention | ||||
Appendix 10. Health‐related quality of life: instruments
| Instrument | Dimensions (subscales) | Validated instrument | Answer options | Scores | Minimum score Maximum score | Weighting | Direction | Minimal important difference | |
| SF‐36 (G) | Physical functioning (PF) (10) Role‐physical (RP) (4) Bodily pain (BP) (2) General health (GH) (5) Vitality (VT) (4) Social functioning (SF) (2) Role‐emotional (RE) (3) Mental health (MH) (5) Reported health transition (RHT) (1) | Yes | 3‐, 5‐ and 6‐point Likert‐scale | Scores for dimensions Mental component summary (MCS) | Minimum scores: Maximum scores: | No | Higher values | PCS: 2 to 3 points MCS: 3 points Dimensions: PF/BT/VT: 2 points, if score < 40; 3 points, if score ≥ 40 RP: 2 points SF/MH: 3 points RE: 4 points | |
| Traisk 2009 | Quote from the publication: "Patients who had experienced development or worsening of eye problems at any time point during the 4‐year study period (TAO group) had lower QoL estimated by the SF‐36 questionnaire when no attention was paid to the mode of treatment ... throughout the whole study period, the TAO group had lower MCS and PCS for the first 3 years ... There were no differences in the results of the SF‐36 scores between the two treatment groups ... Already after 3 months, the physical component score reached the average for the Swedish reference population (score 50) and remained at this level throughout the study period (48 months). The mental component score showed some delay in both the radioiodine and medically treated groups to reach the average score of 50 not until 12 months. Thereafter, the mental component score remained rather constant for 3 years or at least until 48 months ... Comparisons between the two groups of patients without TAO showed no significant differences in QoL regardless whether they had been treated with radioiodine or ATDs ... The study also showed that QoL was rather equal in both treatment groups when no attention was paid to the possible influence of TAO ... Patients with TAO generally had significantly lower QoL scores as compared with patients without TAO at several time points, but no consistent treatment‐ or time‐related pattern could be found ... The result of this analysis showed that the SF‐36 due to its generic properties is not an optimal instrument for measuring QoL‐related issues in this population ... Although the SF‐36 is used extensively, it has not been specifically evaluated in a population of GD patients, but neither have other disease‐specific instruments." | ||||||||
| Tallstedt 1992a | Quote from publication: "The Physical Component Summary and Mental Component Summary were not significantly different among the three old‐age groups. Also the sub‐component Summaries of the Physical and Mental Component Summary were similar .... Compared to the Swedish reference population at 50‐74 years, we observed that the Vitality score was lower for all three treatment groups (p < 0.05). The medical group had a significantly lower Mental Component Summary than the reference population ... The radioiodine‐treated group had a significantly lower General Health score (p < 0.05)" | ||||||||
| a145/179 participants with evaluated questionnaires (56 on surgery, 55 on antithyroid drugs, 34 on radioiodine); only figures are provided (Abraham‐Nordling 2005 under Tallstedt 1992). ATD: antithyroid drug; G: generic; GD: Graves' disease; QoL: quality of life; S: specific; SF: short‐form health survey | |||||||||
Appendix 11. Survey of trial investigators providing information on included trials
| Trial author contacted | Trial author replied | Trial author asked for additional information | Trial author provided data | |
| Traisk 2009 | Yes (2012) | No | No | No |
| Tallstedt 1992 | Yes (2012) | Yes (2012) | Yes (2012) | Yes (2012); trial author provided the exact number of participants in different thyroid status after treatment for hyperthyroidism |
Appendix 12. Checklist to aid consistency and reproducibility of GRADE assessments
| (1) Health‐related quality of life | (2) Development and worsening of Graves' ophthalmopathy | (3) Individuals in euthyroid state | (4) Recurrence of hyperthyroidism (relapse) | (5) Adverse events other than development and worsening of Graves' ophthalmopathy (hypothyroidism, adverse drug effects) | (6) All‐cause mortality | (7) Socioeconomic effects | ||
| Trial limitations | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Unclear | Unclear | Unclear | N/A | Unclear |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes/Unclear | Yes/Unclear | Yes/Unclear | Yes/Unclear | Yes/Unclear | N/A | Yes | |
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | No (↓) | Yes/No (↓) | Yes | Yes | Yes | N/A | Yes | |
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes/No (↓) | Yes/No (↓) | Yes | Yes | Yes | N/A | Yes | |
| Was an objective outcome used? | Yes | Yes | Yes | Yes | Yes/No (↓) | N/A | Yes | |
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Did the trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Inconsistencyb | Point estimates did not vary widely? | N/A | Yes | N/A | No (↓) | N/A | N/A | N/A |
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | N/A | Some | N/A | No (↓) | N/A | N/A | N/A | |
| Was the direction of effect consistent? | N/A | Yes | N/A | Yes | N/A | N/A | N/A | |
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² 40% to 60%), high I² > 60%)? | N/A | Low | N/A | High (↓) | N/A | N/A | N/A | |
| Was the test for heterogeneity statistically significant (P value < 0.1)? | N/A | Not statistically significant | N/A | Statistically significant (↓) | N/A | N/A | N/A | |
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | N/A | Highly applicable |
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | Highly applicable | Highly applicable | N/A | Highly applicable | |
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | Yes | Yes/No (↓) | N/A | Yes | |
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Sufficient | Sufficient | N/A | Sufficient | |
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | Yes | N/A | No (↓) | N/A | N/A | N/A |
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Intermediate | Intermediate | Intermediate | Intermediate | Intermediate | N/A | Low (↓) | |
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | Small (↓) | Small (↓) | N/A | Small (↓) | |
| Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | Yes | N/A | Yes | N/A | N/A | N/A | |
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | N/A | Yes |
| Was grey literature searched? | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | N/A | No (↓) | |
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | N/A | Yes | |
| There was no evidence of funnel plot asymmetry? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| There was no discrepancy in findings between published and unpublished trials? | Unclear | Unclear | Unclear | Unclear | Unclear | N/A | Unclear | |
| aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual trials. cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable | ||||||||
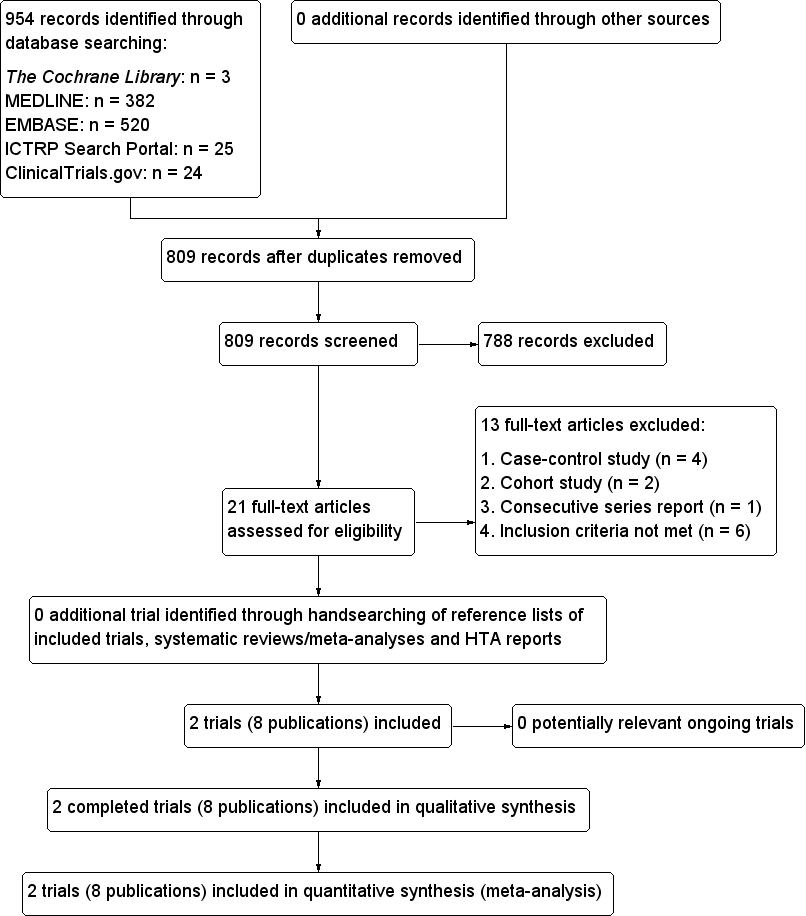
Study flow diagram.
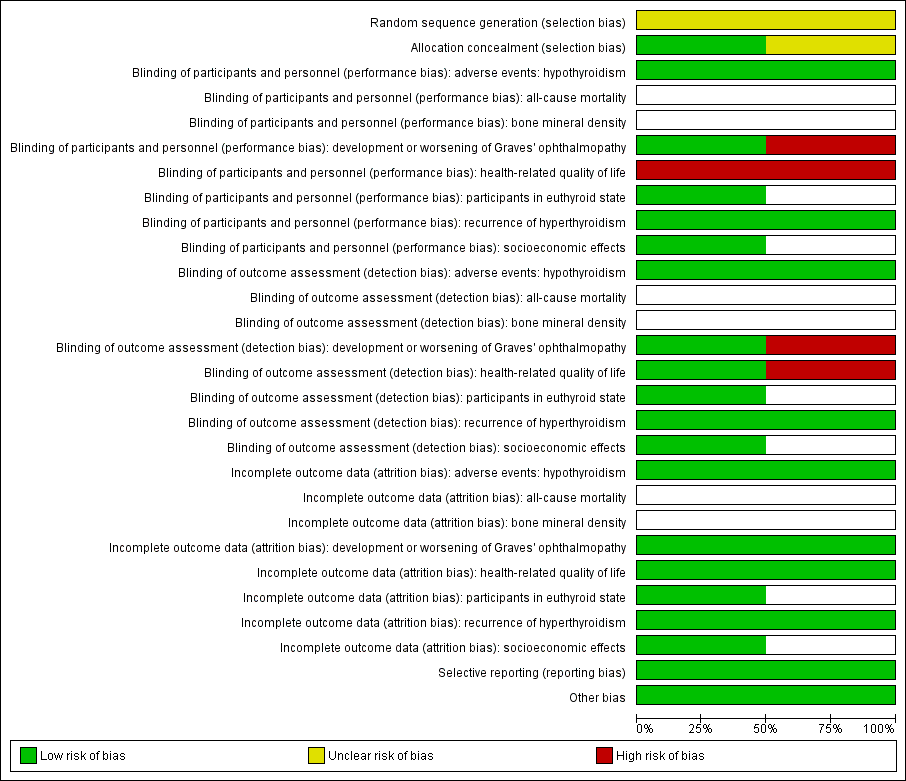
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial (blank cells indicate that the trial did not measure that particular outcome).
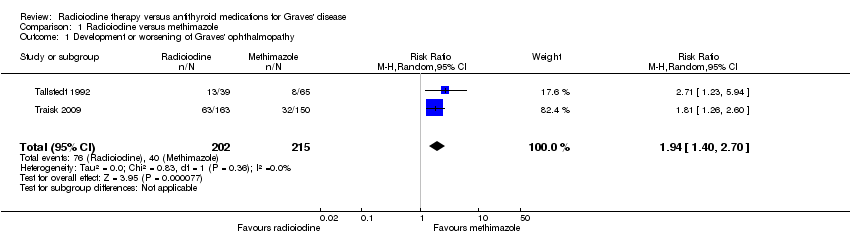
Comparison 1 Radioiodine versus methimazole, Outcome 1 Development or worsening of Graves' ophthalmopathy.

Comparison 1 Radioiodine versus methimazole, Outcome 2 Adverse events: hypothyroidism.
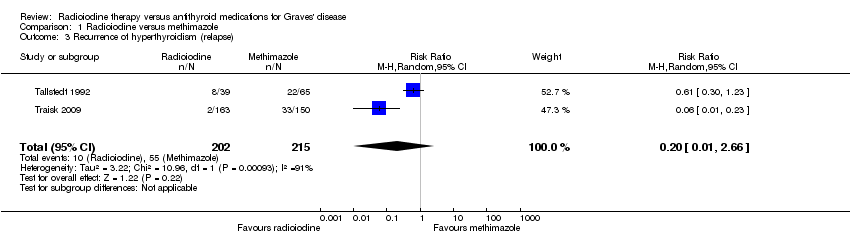
Comparison 1 Radioiodine versus methimazole, Outcome 3 Recurrence of hyperthyroidism (relapse).

Comparison 1 Radioiodine versus methimazole, Outcome 4 Participants in euthyroid state.
| Radioiodine therapy compared with antithyroid medications for Graves' disease | ||||||
| Patient: participants with Graves' disease Settings: outpatients Intervention: radioiodine Comparison: methimazole | ||||||
| Outcomes | Assumed risk | Corresponding risk | Relative effect | No of participants | Quality of the evidence | Comments |
| Methimazole | Radioiodine | |||||
| Health‐related quality of life Follow‐up: 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | 2 trials assessed this outcome but no quantitative data for comparisons between intervention groups were provided; trial authors in 1 trial reported that there were no differences in the results of the SF‐36 scores between the 2 treatment groups |
| Development and worsening of Graves' ophthalmopathy | 186 per 1000 | 361 of 1000 (260 to 502) | RR 1.94 (1.40 to 2.70) | 417 (2) | ⊕⊕⊝⊝ | ‐ |
| Individuals in euthyroid state Follow‐up: at least 4 years | See comment | See comment | See comment | 112 (1) | See comment | No participant who underwent radioiodine treatment achieved an euthyroid state compared to 4/68 participants not becoming euthyroid treated by methimazole (Tallstedt 1992); however, in this trial thyroxine therapy was not introduced early in both treatment arms to avoid hypothyroidism |
| Recurrence of hyperthyroidism (relapse) Follow‐up: at least 4 years | 256 per 1000 | 51 of 1000 (3 to 680) | RR 0.20 (0.01 to 2.66) | 417 (2) | ⊕⊝⊝⊝ | ‐ |
| Adverse events other than development or worsening of Graves' disease (a) Hypothyroidism [measured by TSH and/or thyroid hormones] (b) Drug reactions Follow‐up: (a) at least 2 years (b) at least 4 years | See comment | See comment | See comment | (a) 104 (1) (b) 215 (2) | a) ⊕⊝⊝⊝ b) ⊕⊝⊝⊝ | (a) 39 of 41 participants developed hypothyroidism after radioiodine treatment for Graves' disease, compared with 0 of 65 participants receiving methimazole (Tallstedt 1992); however, in this trial thyroxine was not introduced early in both treatment groups in order to avoid hypothyroidism (b) 23 of 215 participants (11%) reported adverse effects likely related to methimazole treatment |
| All‐cause mortality Follow‐up: at least 4 years and 14 to 21 years | See comment | See comment | See comment | 425 (2) | See comment | No quantitative data for all‐cause mortality were reported |
| Socioeconomic effects Follow‐up: 2 years | See comment | See comment | See comment | 112 (1) | See comment | Costs for patients without relapse and methimazole treatment were USD 1126/USD 1164 (young/older methimazole group) and for radioiodine treatment USD 1862 Costs for patients with relapse and methimazole treatment were USD 2284/1972 (young/older methimazole group) and for radioiodine treatment USD 2760 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Assumed risk was derived from the event rates in the comparator groups. | ||||||
| Intervention(s) and | Sample sizea | Screened/eligible | Randomised | ITT | Analysed | Finishing trial | Randomised finishing trial | Follow‐up | |
| Traisk 2009 | Radioiodine | Primary endpoint was the difference in the proportion of participants with worsening or development of thyroid‐associated ophthalmopathy during a 4‐year follow‐up in the 2 groups (intention‐to‐treat analysis). A comparison (0.05, two‐tailed test) of the binomial proportions between 2 groups of 300 patients each would give more than 90% probability (power) to detect a true difference of 10% | 482/333 | ‐ | 163 | 163 | 163 | ‐ | 24 months (4 years) |
| Methimazole | ‐ | 150 | 150 | 150 | ‐ | ||||
| total: | 333 | 313 | 313 | 313 | 94 | ||||
| Tallstedt 1992e | Radioiodine | ‐ | 179 | 41 | ‐ | 39f | 39 | 95 | At least 24 months (at least 48 months; 3 years; 5 years; 14‐21 years)g |
| Methimazole | 71 | 65f | 64 | 90 | |||||
| total: | 112 | 104 | 103 | 92 | |||||
| Grand total | All radioiodine‐treated participants | 204 | |||||||
| All participants treated with methimazole | 221 | ||||||||
| All interventions | 425 | ||||||||
| "‐" denotes not reported ITT: intention‐to‐treat | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Development or worsening of Graves' ophthalmopathy Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [1.40, 2.70] |
| 2 Adverse events: hypothyroidism Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Recurrence of hyperthyroidism (relapse) Show forest plot | 2 | 417 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 2.66] |
| 4 Participants in euthyroid state Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

