واکسنهای آنفلوآنزا برای پیشگیری از وقوع اوتیت میانی حاد در نوزادان و کودکان
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Prospective, randomised, double‐blind, placebo‐controlled and multicentre trial | |
| Participants | Healthy children aged 15 to 71 months at the time of initial vaccination in year 1 Year 1: 1602 (vaccine group: 1070, placebo group: 532) Year 2: 1358 (vaccine group: 917, placebo group: 441) Exclusion criteria: history of clinically significant hypersensitivity to eggs or children with underlying chronic illnesses Setting: healthcare setting | |
| Interventions | Participants were randomised in a 2:1 ratio to receive vaccine or placebo and followed through the subsequent 2 influenza seasons. In year 1, participants at 8 of the 10 centres primarily received 2 doses of vaccine or placebo and a single dose at the other 2 centres. In year 2, participants received a single dose of vaccine or placebo. Intervention: CAIV‐T was administered. Duration of follow‐up: for year 1, initial vaccination was given during the period of September to November 1997 and revaccination from November to March 1998. Follow‐up was done during the influenza season, i.e. November to March 1998. Hence, the follow‐up period postvaccination was 7 months (September to March). | |
| Outcomes |
The case definition of febrile OM was any healthcare provider diagnosis of OM associated with fever (either thermometer‐documented or not). | |
| Notes | Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective, randomised, double‐blind, placebo‐controlled, and multicentre trial |
| Allocation concealment (selection bias) | Unclear risk | Prospective, randomised, double‐blind, placebo‐controlled, and multicentre trial |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "... subjects and staff remained blinded throughout the study" |
| Blinding of outcome assessment (detection bias) | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 153 children (14.6%) from the vaccine group and 91 children (17.1%) from the placebo group dropped out, the reasons for which were not explained. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Placebo‐controlled, multicentre study conducted during the 2001 and 2002 influenza seasons at 35 sites in South Africa, Brazil, and Argentina | |
| Participants | 2821 children aged 6 to 36 months Exclusion criteria: serious chronic disease, immunosuppression or presence of an immunocompromised household member, receipt of any commercial or investigational influenza vaccine before enrolment, a documented history of hypersensitivity to any component of LAIV or placebo Year 1: mean (SD) age (months): 2 doses vaccine group (20.4/8.5); 1 dose vaccine and 1 dose saline placebo group (20.1/8.6); 2 doses excipient placebo group (20.6/8.3); 2 doses saline placebo group (20.1/8.3) Setting: healthcare setting | |
| Interventions | In year 1: children were randomised to 1 of 4 regimens of 2 doses LAIV, a single‐dose vaccine, excipient placebo, or saline placebo. In year 2: vaccine recipients were to receive 1 of vaccine, and placebo recipients were to receive saline placebo. Year 1: 2821 (2 doses vaccine: 944; 1 dose vaccine and 1 dose saline placebo: 935; 2 doses excipient placebo: 468; 2 doses saline placebo: 474) Year 2: 2054 (1 dose vaccine: 339 + 690 + 346; 1 dose vaccine: 935; 1 dose saline placebo: 337 + 342) The total volume of vaccine and both placebos was 0.2 mL administered intranasally (approximately 0.1 mL into each nostril). Duration of follow‐up: 11 days after treatment in year 1 and 28 days after treatment in year 2 | |
| Outcomes |
| |
| Notes | The number of children with episodes of AOM was reported only for those receiving 2 doses or a single dose of vaccine during year 1, and a single dose of vaccine during year 2. The number of children with episodes of AOM receiving excipient placebo or saline placebo was not reported. We therefore did not include the findings for the primary outcome in the meta‐analysis due to lack of data for comparison with the control group. Only the secondary outcomes of adverse events were included. Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... randomised (2:2:1:1) to one of four study groups according to a preprinted randomisation allocation list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Because of a treatment allocation coding and labelling error in the second season ..." Comment: Allocation concealment was stated although not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects and personnel evaluating vaccine efficacy and safety remained blinded throughout the entire study period" |
| Blinding of outcome assessment (detection bias) | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | 2821 children (88.2%) completed year 1 without major protocol violations, and 2202 children continued in year 2. Due to an unintended treatment allocation error, 1 treatment group randomised to LAIV–LAIV/LAIV received placebo rather than LAIV (LAIV–LAIV/placebo), and 1 treatment group randomised to placebo–placebo/placebo received LAIV rather than placebo (placebo–placebo/LAIV). As a result, the overall year 2 per‐protocol population included 1364 children (42.6%). |
| Selective reporting (reporting bias) | Low risk | Part of the results were available as online Supplemental Digital Content. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Mentioned as a prospective cohort study. However, this study is better described as a randomised controlled trial because (1) intervention is given, and (2) randomisation is present. | |
| Participants | 186 children aged 6 months to 5 years from day‐care centres Exclusion criteria: no known or suspected acute illness, cancer, or impairment of immunologic function; and had not received any medications known to suppress the immune system in the previous 2 months Setting: day‐care centres in North Carolina, USA | |
| Interventions | Group 1 (N = 94) received 1 (children from previous year) or 2 doses of 0.25 mL trivalent subvirion influenza virus vaccine Group 2 (N = 11) received 3rd dose of hepatitis B vaccine (0.25 mL) Group 3 (N = 55) received ear examination by parents' request Group 4 (N = 26) received ear examination Sex (male/female): vaccine group (42/52), control groups (47/45) Duration of follow‐up: mid‐November to December 1993 (period 1), January to mid‐February 1993 (period 2: influenza period), mid‐February to mid‐March 1994 (period 3: end of observation period). Hence, the follow‐up period was 5 months (November to March) | |
| Outcomes | The ear examination was coded as SOM if fluid only was seen on visual otoscopic examination. The ear examination was coded as AOM if redness and fluid were seen in the same ear on either side, if pus alone was seen on either side, and if both SOM and AOM were present.
| |
| Notes | Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... children were randomised "; "New participants could be randomised to receive (3:1) the flu shot or nothing, or ear examinations only" Comments: Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Children in the previous study continued in the study in the same group (blinded) if they were ..." Comment: Children were initially randomised to receive influenza vaccine or hepatitis B vaccine. However, following the Advisory Committee on Immunization Practice, the recommendation to receive hepatitis B vaccine was changed. Children in the previous group therefore continued to be blinded, and new participants were randomised to receive either the influenza vaccine or ear examination. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The examiners were blinded to the category of the participants" |
| Incomplete outcome data (attrition bias) | Low risk | All children completed the study. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | 182 healthy children aged 6 to 18 months of age Exclusion criteria: not reported Setting: vaccine evaluation units at Baylor College of Medicine, St Louis University, University of Rochester, Vanderbilt University, and University of Maryland, USA | |
| Interventions | Vaccine groups (N = 136 + 2) consisted of H1N1 (N = 44), H3N2 (N = 45), bivalent (N = 47). Control group (N = 44) Live attenuated, cold‐adapted monovalent and bivalent influenza or placebo was given by nose drops as a 0.5 mL dose. Duration of follow‐up: recruitment and vaccination was done in the autumn of 1991 and followed up in winter 1991 to 1992. Hence, the follow‐up period postvaccination was around 3 months. | |
| Outcomes |
| |
| Notes | The primary outcome was observed within 10 days postvaccination, hence results were not suitable to be included in meta‐analysis. Only outcomes of adverse events were included. Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double‐blind, placebo‐controlled trial |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind, placebo‐controlled trial |
| Blinding of participants and personnel (performance bias) | Low risk | Researchers were blinded to the groups. Quote: "Subjects were randomised in a double‐blind fashion to receive a single dose of cold adapted monovalent influenza vaccine, bivalent influenza vaccine or placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Data were missing for 2 children (1.4%) from the vaccine group within 10 days of vaccination because the diary information was not available. Otherwise data were complete. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Randomised, parallel‐group trial | |
| Participants | 786 children (411 in first cohort and 375 in second cohort) aged 6 to 24 months stratified into (1) prone to otitis media (2) attend day care First cohort: sex (male/female): vaccine group (128/145), control group (75/63) Second cohort: sex (male/female): vaccine group (139/113), control group (53/123) Exclusion criteria: premature or had a craniofacial abnormality; had or were living with persons at high risk of influenza; neurologic disorder, history of tympanostomy tube insertion, hypersensitivity to egg protein or thimerosal; febrile illness or severe respiratory illness within the preceding 48 hours Setting: Children's Hospital of Pittsburgh, Pennsylvania, USA | |
| Interventions | Intervention group (N = 525) received inactivated trivalent subvirion influenza vaccine intramuscularly. 513 received 2 doses (0.25 mL each) 4 weeks apart, and 12 received 1 dose. Control group (N = 261) received placebo intramuscularly. 252 received 2 doses (0.25 mL each) 4 weeks apart, and 9 received 1 dose. Duration of follow‐up: for the first cohort, recruitment and vaccination were given during the period of October to November 1999 and followed up until March to November. Hence, the duration of follow‐up postvaccination was between 6 months and 1 year. For the second cohort, recruitment and vaccination were given during the period of September to December 2000 and followed up until March 2001. Hence, the follow‐up period postvaccination was 6 months. | |
| Outcomes | Acute otitis media is defined as presence of purulent otorrhoea of recent onset not due to otitis externa or of middle ear effusion accompanied by 1 or more of the following: ear pain, marked redness of the tympanic membrane, and substantial bulging of the tympanic membrane.
| |
| Notes | Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned the children in blocks of nine, using a computer generated list. ... in a 2:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Randomised, parallel‐group trial |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Assignments to treatment groups were not revealed to parents, investigators, research personnel conducting clinical follow‐up, or non‐study health care providers, all of whom remained blinded throughout the study" "Administration was performed by non‐blinded research nurses who were not involved in subsequent clinical follow‐up of the children." "Randomization lists were kept in locked files not accessible to blinded personnel" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "In the subjects in which otoscopic and tympanometric findings presented ambivalency or inconsistency, visual otoscopy was re‐performed by another physician (any of the authors of this study) in a blind manner, and then tympanometry was repeated" |
| Incomplete outcome data (attrition bias) | Low risk | 27 children (5.1%) from the vaccine group and 11 children (4.2%) from the placebo group were excluded from the study. Reasons were provided. Intention‐to‐treat analysis was applied. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Randomised, placebo‐controlled, observer‐blinded study | |
| Participants | 838 children (6 months to 17 years at the time of first vaccination) Exclusion criteria: previous receipt of H5N1 vaccine; receipt of seasonal influenza vaccine within 14 days (inactivated vaccine) or 30 days (live vaccine); receipt of any vaccine not foreseen by the protocol up to 42 days from baseline; receipt of any investigational or non‐registered product from 30 days before to 42 days after study vaccination; any significant acute or chronic uncontrolled illness; temperature of ≥ 38°C (≥ 100.4°F) at baseline assessment; cancer diagnosis within previous 3 years; immunosuppressive or immunodeficient conditions; receipt of glucocorticoids within 1 month of the start of and throughout the study; receipt of cytotoxic, immunosuppressive drugs within 6 months of the start of and throughout the study; receipt of immunoglobulins within 3 months of the start of and throughout the study; and history of allergy to influenza vaccine Setting: USA (N = 450), Canada (N = 96), and Thailand (N = 292) | |
| Interventions | Randomisation ratio was 8:3 for vaccine to placebo, with equal allocation between 3 age strata.
Assessment of immunogenicity: at days 0, 21, and 42; for half of the children in each age strata at day 182, and for the other half at day 385 Assessment of reactogenicity: at day 7 postvaccination Assessment safety: up to 1 year after vaccination Intervention group (N = 607) received 2 doses of H5N1 influenza vaccine (AS03B‐adjuvanted H5N1 A/Indonesia/5/2005 with antigen produced in Quebec). Control group (N = 231) received 2 doses of placebo (0.25 mL of saline). Vaccine or placebo injections were administered in the non‐dominant (dose 1) and dominant arm (dose 2) 21 days apart. Duration of follow‐up: 1 year | |
| Outcomes | Immunogenicity objectives were to assess:
Reactogenicity objectives were to assess:
Safety objectives were to assess:
| |
| Notes | Prospective registration: ClinicalTrials.gov identifier: NCT01310413 (first received 24 February 2011) We contacted the authors and obtained further information from the clinical trials register via Clinical Study Data Request. We have reported only the results of general symptoms, namely febrile illness, drowsiness, irritability/fussiness, and loss of appetite, for all children aged less than 6 years (N = 294 vaccine group, N = 122 control group, N = 416 total). Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a blocking scheme ..." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... treatment allocation at study sites was done using an Internet‐based system" Note: Not described. If the recruiting personnel understood the blocking scheme used, it might be possible to predict the next patient. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Participants and study personnel involved in the collection and analysis of data were blinded to treatment." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "... study personnel involved in the collection and analysis of data were blinded to treatment." |
| Incomplete outcome data (attrition bias) | Low risk | Data were missing for 42 participants (565/607, 93.1%) from the vaccine group and for 14 participants (217/231, 93.9%) from the control group at day 385. Missing data were evenly balanced across the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Protocol available. All prespecified outcomes reported in final report. All expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Prospective, randomised, double‐blind, placebo‐controlled, multicentre, phase III study conducted over an influenza season at 32 sites in 13 countries (Bangladesh, Belgium, Finland, Germany, Hong Kong, Lithuania, Malaysia, Mexico, the Philippines, Poland, Singapore, South Korea, and Thailand) | |
| Participants | 1120 healthy children aged 11 to less than 24 months Mean (SD) age (months): vaccine group (14.4/3.0), placebo group (14.4/3.2) Sex (male/female): vaccine group (383/364), placebo group (175/189) Exclusion criteria: serious chronic disease, including progressive neurologic disease, Down syndrome or other cytogenetic disorder or known or suspected disease of the immune system, received aspirin or aspirin‐containing products 2 weeks before immunisation, and documented history of hypersensitivity to egg or egg protein Surveillance for influenza‐like illness and the decision to obtain a nasal swab sample were based on information obtained through weekly telephone contacts, clinic visits, or home visits. Setting: healthcare setting | |
| Interventions | 2 intranasal doses of trivalent LAIV or placebo were given 35 ± 7 days apart. LAIV + combined measles, mumps, and rubella vaccine, live, attenuated (Priorix): 747 children Placebo + Priorix: 373 children Duration of follow‐up: recruitment and vaccination were done during a 3‐week period from 4 October 2002 and followed up until 31 May 2003. Follow‐up began on the 11th day after receipt of the first dose of study treatment and continued for around 8 months over 1 full influenza season (until 31 May 2003). | |
| Outcomes |
Acute otitis media was defined by a visually abnormal tympanic membrane concomitant with at least 1 of the following: fever, earache, irritability, diarrhoea, vomiting, acute otorrhoea not caused by external otitis or other symptoms of respiratory infection. An episode of AOM was diagnosed as a new episode if at least 30 days had elapsed since the previous episode, regardless of aetiology. | |
| Notes | Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised 2:1 (LAIV:placebo) to receive 2 doses of LAIV or placebo 35 ± 7 days apart using a randomisation schedule generated by Wyeth Vaccines Research. Participants were assigned a treatment using an interactive voice recognition system" |
| Allocation concealment (selection bias) | Low risk | Quote: "All subjects received open‐label Priorix administered concomitantly with the first dose of LAIV or placebo. Study subjects, their parents / legal guardians and study clinical personnel were not aware of whether LAIV or placebo was co administered with Priorix" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Study subjects, their parents / legal guardians and study clinical personnel were not aware of whether LAIV or placebo was co administered with Priorix. There were no instances in which subjects were unblinded until after the completion of the study" |
| Blinding of outcome assessment (detection bias) | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 72 children (8.8%) from the vaccine group and 41 children (9.9%) from the placebo group were excluded from the study. Reasons were provided. Intention‐to‐treat analysis was applied. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Randomised, parallel‐group trial | |
| Participants | 133 children aged 1 to 5 years with a history of recurrent AOM (defined as ≥ 3 episodes in the preceding 6 months or ≥ 4 episodes in the preceding 12 months, with the most recent episode of AOM in the previous 2 to 8 weeks) Mean (SD) age (months): vaccine group (32.6/14.6), control group (36.2/15.9) Sex (male/female): vaccine group (38/29), control group (42/24) Exclusion criteria: acute febrile illness, severe atopy, any previous influenza vaccination, acquired or congenital immunodeficiency, recent administration of blood products, cleft palate, chronically ruptured eardrum, obstructive adenoids, sleep apnoea syndrome, and placement of tympanostomy tubes Setting: healthcare setting, Italy | |
| Interventions | Intervention group (N = 67) received 2 doses of intranasal, inactivated, virosomal subunit influenza vaccine on day 1 and day 8. Control group (N = 66) received no treatment. Duration of follow‐up: every 4 to 6 weeks for 25 weeks | |
| Outcomes | Acute otitis media was based on the presence of any combination of: fever, earache, irritability, and hyperaemia or opacity accompanied by bulging or immobility of the tympanic membrane. Otitis media with effusion was based on the presence of impaired mobility, opacification, fullness, or retraction of the eardrum associated with a tympanogram with a flat tracing, and the absence of signs and symptoms of acute infection.
| |
| Notes | Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... assigned randomly" Comments: Method of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "... assignment and vaccine administration were performed by two investigators" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "... the parents were instructed not to discuss group assignment with the investigator responsible for the clinical and ontological follow‐up, who remained blinded to group assignment until the end of the follow‐up period" Comment: We judged this to be of low risk for all outcomes except participant‐reported adverse effects. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "... the investigator responsible for the clinical and ontological follow‐up, who remained blinded to group assignment until the end of the follow‐up period" |
| Incomplete outcome data (attrition bias) | Low risk | 2 children (3.0%) from the vaccine group and 5 children (7.6%) from the placebo group did not complete the study. Reasons were provided. Intention‐to‐treat analysis was carried out. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Randomised controlled trial | |
| Participants | 22 healthy children aged 2 to 22 months Setting: not reported, USA | |
| Interventions | Intervention group (N = 17) received 3 doses (0.5 mL each) of CAIV intranasally 60 days apart. Control group (N = 5) received placebo. Exclusion criteria: not reported Duration of follow‐up: clinical observation was followed up for 11 days, and serum for antibody determinations was obtained 30 to 60 days postvaccination. | |
| Outcomes | Immunogenicity | |
| Notes | Primary outcome was not included in the meta‐analysis due to short follow‐up period. Only outcomes for adverse events were included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised to receive vaccine or placebo in a double‐blinded way. One of every three or four children received placebo" |
| Allocation concealment (selection bias) | Unclear risk | Randomised controlled trial |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Subjects were randomised to receive vaccine or placebo in a double‐blinded way. One of every three or four children received placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | Data were not reported for 2 children (11.8%) from the vaccine group and 2 children (40.0%) from the placebo group at day 60, and 2 children (23.5%) from the vaccine group and 4 children (80.0%) from the placebo group at day 120. The reasons were not provided and the group losses were > 20%. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Prospective, randomised, double‐blind, placebo‐controlled, multicentre, cross‐over trial conducted during 2 consecutive years at 16 sites in 8 regions (China, Hong Kong, India, Malaysia, the Philippines, Singapore, Taiwan, and Thailand) | |
| Participants | Healthy children aged 12 to 36 months Mean (SD) age (months): vaccine group (23.6/7.4), placebo group (23.4/7.3) Year 1: sex (male/female): vaccine group (880/773), placebo group (588/523) (per‐protocol population) Exclusion criteria: serious chronic disease, including progressive neurologic disease; Down syndrome or other cytogenetic disorder, or known or suspected disease of the immune system; and those with documented history of hypersensitivity to egg or egg protein Setting: healthcare setting | |
| Interventions | In year 1, children were randomised 3:2 (CAIV‐T:placebo) to receive 2 doses of CAIV‐T or 2 doses of placebo. In year 2, children were re‐randomised in a 1:1 ratio to receive a single dose of CAIV‐T or placebo without consideration of their group assignment in the first year. Year 1 (N = 3174): intervention group (N = 1900) received 2 doses (0.2 mL each) of CAIV‐T 28 days apart. Control group (N = 1274) received saline placebo. Year 2 (N = 2947): intervention group (N = 1900) received 1 dose of CAIV‐T. Control group (N = 1274) received saline placebo. Duration of follow‐up: follow‐up began on the 11th day after receipt of the first dose of study treatment and continued for 2 years. | |
| Outcomes |
| |
| Notes | Acute otitis media cases were too few, and hence were not reported in the study. Additionally, the duration of follow‐up is less than 6 months. Only outcomes of adverse events were included. Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomised 3:2 (CAIV‐T:placebo) to receive two doses of CAIV‐T or two doses of placebo at least 28 days apart using a randomisation schedule generated by Wyeth. In year 2, subjects were re‐randomised in a 1:1 ratio to receive a single dose of CAIV‐T or placebo without consideration of their group assignment in the first year." Quote: "The randomisation schedule for each year was generated by Wyeth Vaccines Research ... using an interactive voice response system ... numbered according to a predetermined randomisation list" |
| Allocation concealment (selection bias) | Low risk | Quote: "Both CAIV‐T and placebo were supplied in identically packaged sprayers; neither the study subjects, their parents/guardians, or the clinical personnel were aware of the treatment being administered" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Both CAIV‐T and placebo were supplied in identically packaged sprayers; neither the study subjects, their parents/guardians, or the clinical personnel were aware of the treatment being administered" |
| Blinding of outcome assessment (detection bias) | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | In year 1, 247 children (13.0%) from the vaccine group and 163 children (12.8%) from the control group, and in year 2, 203 children (13.7%) from the vaccine group and 217 children (14.8%) from the control group dropped out. Reasons were provided. Intention‐to‐treat analysis was applied in both years. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
| Methods | Prospective, randomised, double‐blind, placebo‐controlled, multicentre trial conducted over 2 consecutive influenza seasons at 70 clinical centres located in Belgium, Finland, Israel, Spain, and the UK between 2 October 2000 and 31 May 2002 Setting: healthcare setting | |
| Participants | 1616 children aged 6 to 36 months who attended day care Exclusion criteria: serious chronic disease, Down syndrome or other cytogenetic disorders, documented history of hypersensitivity to egg or egg protein, immunosuppression or a household member with immunosuppression, received immunoglobulin in the past 6 months or investigational agent 1 month before enrolment; influenza treatment or aspirin or clinically confirmed respiratory illness or wheezing 2 weeks before enrolment Year 1: 1616 children (vaccine: 951, placebo: 665 children) Mean (SD) age (months): vaccine group (23.3/8.0), placebo group (23.5/7.8) Sex (male/female): vaccine group (496/455), placebo group (337/328) Year 2: 1090 children (vaccine: 640, placebo: 450 children) Mean (SD) age (months): vaccine group (23.5/7.9), placebo group (23.7/7.8) Sex (male/female): vaccine group (341/299), placebo group (219/231) | |
| Interventions | The total single‐dose volume of 0.2 mL (0.1 mL into each nostril) of LAIV or placebo was administered intranasally with the spray applicator. Placebo consisted of sterile physiologic saline solution. The first dose of the primary series was administered on day 0. Duration of follow‐up: in year 1, study treatment was administered by December 2000. Follow‐up began on the 11th day after receipt of the first dose of study treatment and continued until May 2001. In year 2, study treatment was administered by December 2001. Follow‐up began on the 11th day after receipt of the first dose of study treatment and continued until May 2002. Hence, the follow‐up period postvaccination for both year 1 and year 2 was 6 months. | |
| Outcomes | Acute otitis media was defined as a visually abnormal tympanic membrane (with regard to colour, position, and/or mobility) suggesting an effusion in the middle ear cavity, concomitant with 1 of the following signs and/or symptoms of acute infection: fever (rectal temperature of 38°C or axillary temperature of 37.5°C), earache, irritability, diarrhoea, vomiting, acute otorrhoea not caused by external otitis, or other symptoms of respiratory infection. Influenza‐associated AOM was defined as an episode of AOM in a child with a positive culture for influenza virus that occurred ≥ 15 days after receipt of the first dose of vaccine or placebo, during the period in which influenza virus was isolated in each country.
| |
| Notes | Declared funding from vaccine manufacturer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were assigned randomly to receive a primary series of two doses of either CAIV‐T or placebo, in a 3:2 ratio ... In year 2, all participants received a single dose of either CAIV‐T or placebo according to their year 1 treatment assignments" Quote: "The randomisation schedule was generated by Wyeth Vaccines Research. Study product for year 1 was labelled with 1 of five letter codes, namely, A, H, or M (CAIV‐T) or B or K (placebo). Each subject was assigned the next sequential number by the study site investigator and received study product for the treatment assigned to that subject number, according to a preprinted randomisation allocation list provided to the study site by Wyeth Vaccines Research" |
| Allocation concealment (selection bias) | Low risk | Quote: "... study subjects, their parents or guardians, and the clinical personnel were unaware of the treatment being administered. CAIV‐T and placebo were supplied in single‐dose, identically packaged sprayers labelled with the codes to which subjects were assigned" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The randomisation schedule was generated by Wyeth Vaccines Research. Study product for year 1 was labelled with 1 of five letter codes, namely, A, H, or M (CAIV‐T) or B or K (placebo). Each subject was assigned the next sequential number by the study site investigator and received study product for the treatment assigned to that subject number, according to a preprinted randomisation allocation list provided to the study site by Wyeth Vaccines Research" |
| Blinding of outcome assessment (detection bias) | Low risk | The assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 1735 children (97.3%) completed year 1; of the 49 children (2.7%) who withdrew during year 1, most did so at parental request (1.2%) or were lost to follow‐up monitoring (1.0%). 4 children (2 in the CAIV‐T group and 2 in the placebo group) withdrew during year 1 because of adverse effects. In year 2, 1119 children who completed year 1 successfully (i.e. received both doses of study vaccine according to the protocol) received a single dose of the same treatment they had received in year 1. An additional 22 children (17 in the CAIV‐T group and 5 in the placebo group) were excluded from the efficacy analysis in year 2 because of major protocol violations. Intention‐to‐treat analysis was applied. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but all expected outcomes were reported. |
| Other bias | Low risk | We identified no other biases. |
AOM: acute otitis media
CAIV: cold‐adapted influenza vaccine
CAIV‐T: trivalent cold‐adapted influenza vaccine
ELISA: enzyme‐linked immunosorbent assay
HAI: haemagglutination antibody inhibition
LAIV: live attenuated influenza vaccine
OM: otitis media
OME: otitis media with effusion
SD: standard deviation
SOM: serous otitis media
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Compares efficacy and safety of cold‐adapted influenza vaccine, trivalent with trivalent inactivated influenza vaccine. Involved 2187 children aged 6 to 71 months old. No placebo or intervention control group included. | |
| Compares efficacy of vaccine and placebo based on viral cultures for influenza. Involved 1602 children aged 15 to 71 months old. No related primary or secondary outcome measures | |
| Compares efficacy of cold‐adapted trivalent live attenuated influenza vaccine and trivalent inactivated vaccine. Involved 8352 children aged 6 to 59 months old. No placebo or intervention control group included. No related primary or secondary outcome measures | |
| Compares safety of single‐dose cold‐adapted influenza vaccine and placebo. Involved 5637 children aged 1 to 8 years old. The medical adverse events were reported for otitis media, pharyngitis, and febrile illness. However, data for the number or proportion of children aged less than 6 years were not available after contacting the authors. | |
| Compares safety and immunogenicity between trivalent inactivated influenza vaccine and the US‐licensed influenza vaccine. There was no placebo group. | |
| Compares monovalent influenza vaccine and unvaccinated control groups. Involved 92 children aged 6 to 60 months old. Excluded for quasi‐randomisation. Reported primary outcome but follow‐up for 4 to 8 weeks only. | |
| Compares respiratory‐related morbidity between inactivated, trivalent, virosome‐formulated subunit influenza vaccine and placebo. Involved 127 children aged 6 months to 9 years. No related primary or secondary outcome measures | |
| Compares cell‐mediated immunity responses between 3 dose levels of vaccine and saline placebo. Involved 2172 children aged 6 to less than 36 months old. No related primary or secondary outcome measures | |
| Compares serologic responses between cold‐adapted influenza vaccine and placebo. Involved 1126 children aged 2 to 36 months old. No related primary or secondary outcome measures | |
| Compares immunogenicity and safety between single dose and two doses of influenza vaccine. There was no placebo group. | |
| Compares incidence of acute otitis media between vaccine and control. Involved 374 children aged 1 to 3 years old. Excluded for quasi‐randomisation. Reported primary outcome but follow‐up for 6 weeks only. | |
| Compares the potential increases in reactogenicity and allergic events of Northern Hemisphere 2014/2015 formulation of the inactivated split‐virion intramuscular trivalent influenza vaccine (Vaxigrip) and historical data. No placebo or intervention control group included. | |
| Compares immunogenicity and reactogenicity between quadrivalent and trivalent influenza vaccine. Involved 601 children aged 6 to 35 months old. There was no placebo group. | |
| Compares effectiveness of vaccination between intranasal live attenuated influenza vaccine and inactivated influenza vaccine based on reverse transcriptase polymerase chain reaction‐confirmed influenza A or B virus. Involved 4611 children and adolescents aged 36 months to 15 years old. There was no placebo group. | |
| Compares efficacy between cold‐adapted influenza vaccine and placebo based on viral cultures for influenza. Involved 1601 children aged 15 to 71 months old. No related primary or secondary outcome measures | |
| Compares the prophylactic effect between inactivated influenza vaccine and control. Involved 346 children aged 6 to 24 months. No related primary or secondary outcome measures | |
| Compares socioeconomic impact of vaccine and control. Involved 303 children aged 6 months to 5 years old. No related primary or secondary outcome measures | |
| Compares nasopharyngeal bacterial colonisation between live attenuated influenza vaccine and control. Involved 151 children. No related primary or secondary outcome measures | |
| Compares the efficacy of trivalent inactivated influenza vaccine with or without the presence of an oil‐in‐water emulsion of adjuvant MF59. Involved 4707 children aged 6 to 72 months old. No related primary or secondary outcome measures |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
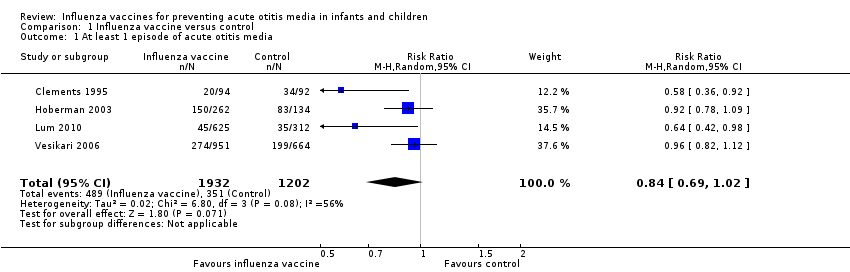
| 1 At least 1 episode of acute otitis media Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| Analysis 1.1  Comparison 1 Influenza vaccine versus control, Outcome 1 At least 1 episode of acute otitis media. | ||||
| 2 Acute otitis media by courses Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Influenza vaccine versus control, Outcome 2 Acute otitis media by courses. | ||||
| 2.1 First course (1 or 2 doses) | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2.2 Second course (1 dose) | 2 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 3 Acute otitis media by type of vaccine Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| Analysis 1.3  Comparison 1 Influenza vaccine versus control, Outcome 3 Acute otitis media by type of vaccine. | ||||
| 3.1 Trivalent cold‐adapted influenza vaccine | 2 | 2552 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.21] |
| 3.2 Trivalent sub virion influenza vaccine | 2 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.23] |
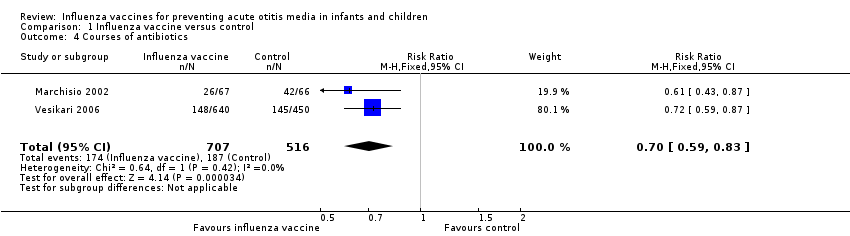
| 4 Courses of antibiotics Show forest plot | 2 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| Analysis 1.4 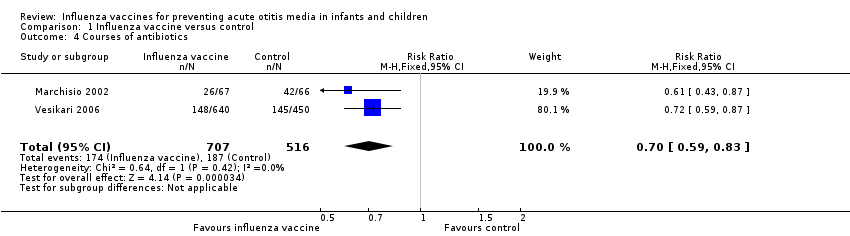 Comparison 1 Influenza vaccine versus control, Outcome 4 Courses of antibiotics. | ||||
| 5 Fever Show forest plot | 7 | 10615 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.24] |
| Analysis 1.5 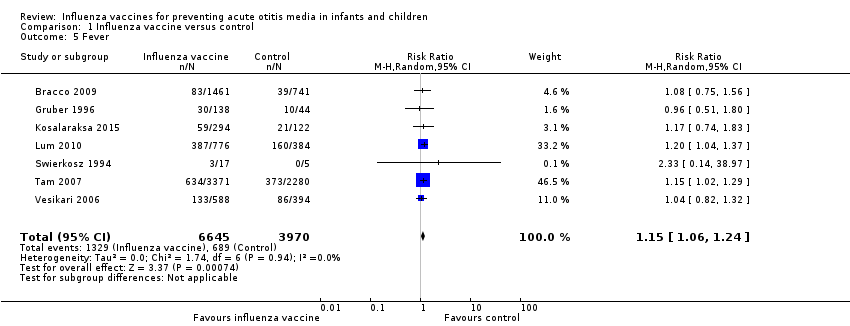 Comparison 1 Influenza vaccine versus control, Outcome 5 Fever. | ||||
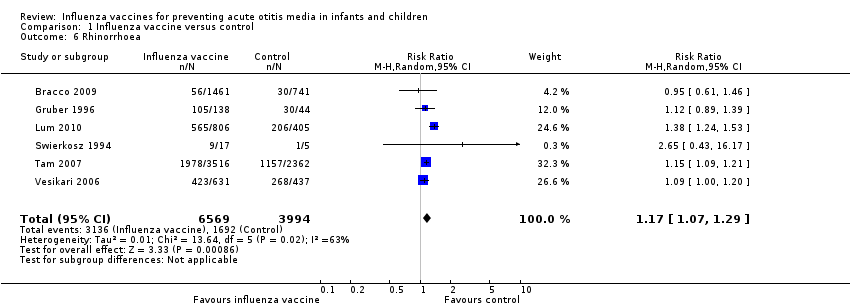
| 6 Rhinorrhoea Show forest plot | 6 | 10563 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.07, 1.29] |
| Analysis 1.6  Comparison 1 Influenza vaccine versus control, Outcome 6 Rhinorrhoea. | ||||
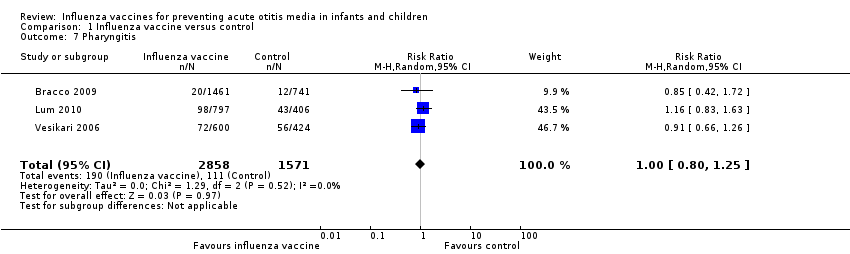
| 7 Pharyngitis Show forest plot | 3 | 4429 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.25] |
| Analysis 1.7  Comparison 1 Influenza vaccine versus control, Outcome 7 Pharyngitis. | ||||

Study flow diagram.
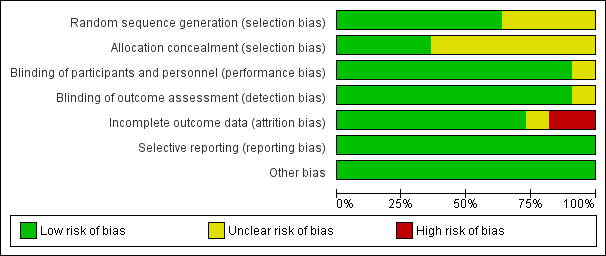
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
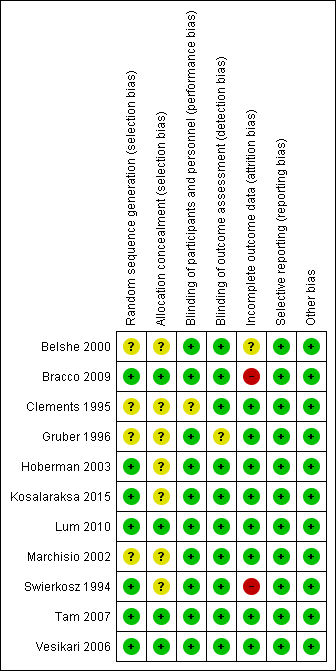
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Influenza vaccine versus control, Outcome 1 At least 1 episode of acute otitis media.

Comparison 1 Influenza vaccine versus control, Outcome 2 Acute otitis media by courses.

Comparison 1 Influenza vaccine versus control, Outcome 3 Acute otitis media by type of vaccine.
Comparison 1 Influenza vaccine versus control, Outcome 4 Courses of antibiotics.

Comparison 1 Influenza vaccine versus control, Outcome 5 Fever.
Comparison 1 Influenza vaccine versus control, Outcome 6 Rhinorrhoea.
Comparison 1 Influenza vaccine versus control, Outcome 7 Pharyngitis.
| Influenza vaccine compared to control for preventing acute otitis media in infants and children | ||||||
| Patient or population: infants and children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with influenza vaccine | |||||
| At least 1 episode of acute otitis media | Study population | RR 0.84 | 3134 | ⊕⊕⊝⊝ | The basis for the assumed risk is the mean risk in the control group across the included studies. There was moderate unexplained inconsistency across the studies. We downgraded for inconsistency. The effect estimates were all in the same direction, but there was uncertainty in the confidence of the effect estimate. | |
| 292 per 1000 | 245 per 1000 | |||||
| Courses of antibiotics | Study population | RR 0.70 | 1223 | ⊕⊕⊕⊝ | Assumed risk calculated from the mean risk across the control groups of the 2 included studies | |
| 362 per 1000 | 254 per 1000 | |||||
| Fever | Study population | RR 1.15 | 10,615 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 7 included studies | |
| 174 per 1000 | 200 per 1000 | |||||
| Rhinorrhoea | Study population | RR 1.17 | 10,563 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 6 included studies | |
| 424 per 1000 | 496 per 1000 | |||||
| Pharyngitis | Study population | RR 1.00 | 4429 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 3 included studies | |
| 71 per 1000 | 71 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Includes trial(s) at high risk of publication bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 1 episode of acute otitis media Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2 Acute otitis media by courses Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 First course (1 or 2 doses) | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2.2 Second course (1 dose) | 2 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 3 Acute otitis media by type of vaccine Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 3.1 Trivalent cold‐adapted influenza vaccine | 2 | 2552 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.21] |
| 3.2 Trivalent sub virion influenza vaccine | 2 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.23] |
| 4 Courses of antibiotics Show forest plot | 2 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 5 Fever Show forest plot | 7 | 10615 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.24] |
| 6 Rhinorrhoea Show forest plot | 6 | 10563 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.07, 1.29] |
| 7 Pharyngitis Show forest plot | 3 | 4429 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.25] |

