Vacuna contra la gripe para la prevención de la otitis media aguda en lactantes y niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD010089.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 17 octubre 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Infecciones respiratorias agudas
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Designing the review: Mohd N Norhayati (MNN), Mohd Y Azman (MYA), and Jacqueline J Ho (JJH).
Co‐ordinating the review: MNN.
Literature search: MNN, MYA.
Quality assessment: MNN, MYA. For this update MNN, JJH.
Entering data into Review Manager 5: MNN.
Data analysis: JJH, MNN.
Data interpretation: JJH, MNN.
Writing the review: MNN, JJH, MYA.
Sources of support
Internal sources
-
Universiti Sains Malaysia, Malaysia.
-
Penang Medical College, Malaysia.
External sources
-
No sources of support supplied
Declarations of interest
Mohd N Norhayati: none known.
Jacqueline J Ho: none known.
Mohd Y Azman: none known.
Acknowledgements
We wish to acknowledge the authors of a previous protocol, Elspeth Kay, Kwong Ng, Allison Salmon, and Chris Del Mar (Kay 2005). The new authors would like to thank Elizabeth Dooley, Managing Editor of the Cochrane Acute Respiratory Infections (ARI) Group, for the comments and feedback during the preparation of this review. We would also like to thank Justin Clark, Information Specialist of the ARI Group, for his assistance with this review update.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Oct 17 | Influenza vaccines for preventing acute otitis media in infants and children | Review | Mohd N Norhayati, Jacqueline J Ho, Mohd Y Azman | |
| 2015 Mar 24 | Influenza vaccines for preventing acute otitis media in infants and children | Review | Mohd N Norhayati, Jacqueline J Ho, Mohd Y Azman | |
| 2012 Sep 12 | Influenza vaccines for preventing acute otitis media in infants and children | Protocol | Mohd N Norhayati, Mohd Y Azman, Jacqueline J Ho | |
Differences between protocol and review
We included a subgroup analysis for otitis media episodes by season, as we think there might be a difference in the outcome for vaccine administered during the influenza and the broader respiratory season. We removed the types of influenza vaccine from our secondary outcomes, deciding this was better included as a subgroup analysis. Neither of these subgroup analyses were stated in the protocol. For this update, we did not perform the subgroup analyses for trial setting and season due to the small number of trials in the subgroups. We removed Belshe 2000 from the analyses of primary outcomes (courses of vaccine and types of vaccine) because it reported episodes per person, and there was no measure of dispersion. We did not perform a subgroup analysis for utilisation of healthcare due to the limited number of trials involved. We did not do a subgroup analysis by type of adverse event. We have reported each type of adverse event as a separate analysis.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Child, Preschool; Humans; Infant;
PICO

Study flow diagram.
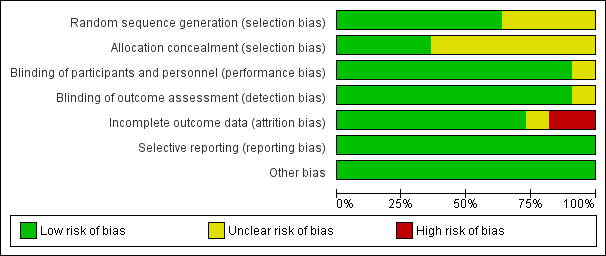
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
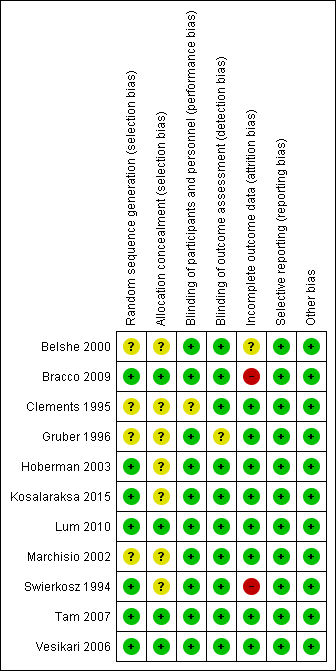
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
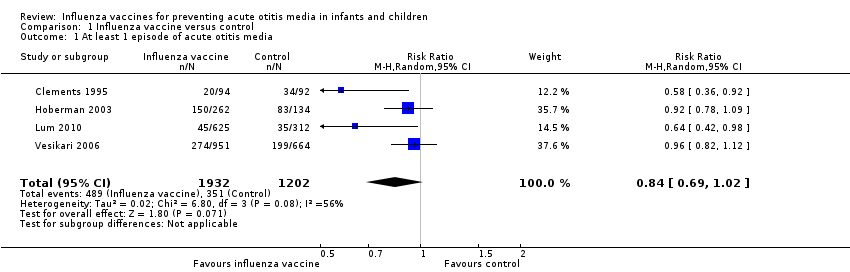
Comparison 1 Influenza vaccine versus control, Outcome 1 At least 1 episode of acute otitis media.

Comparison 1 Influenza vaccine versus control, Outcome 2 Acute otitis media by courses.

Comparison 1 Influenza vaccine versus control, Outcome 3 Acute otitis media by type of vaccine.
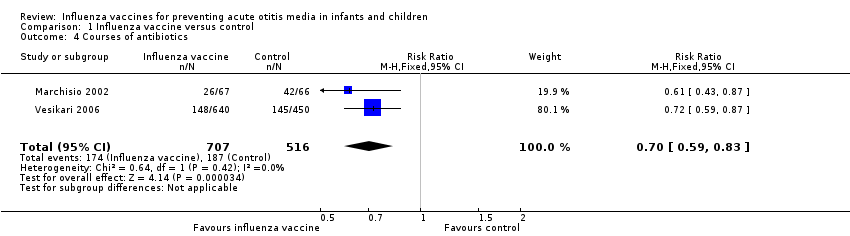
Comparison 1 Influenza vaccine versus control, Outcome 4 Courses of antibiotics.

Comparison 1 Influenza vaccine versus control, Outcome 5 Fever.
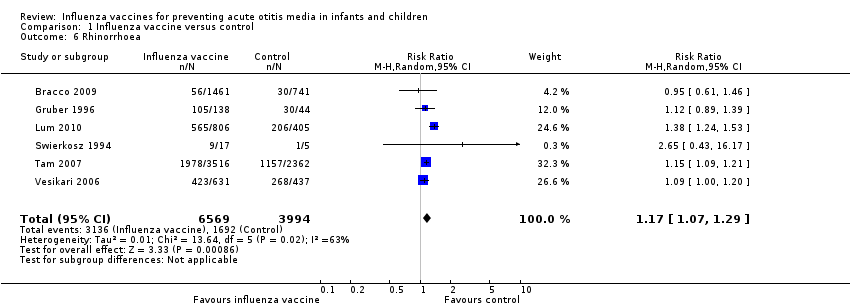
Comparison 1 Influenza vaccine versus control, Outcome 6 Rhinorrhoea.
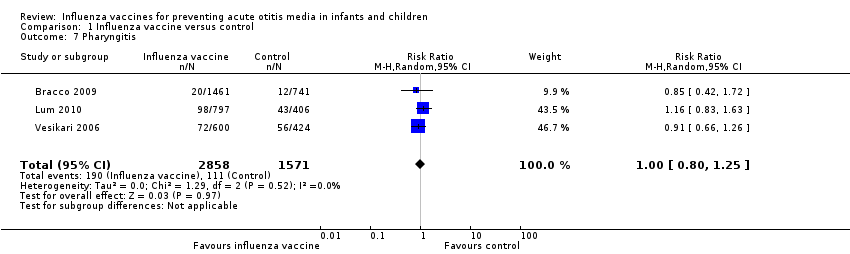
Comparison 1 Influenza vaccine versus control, Outcome 7 Pharyngitis.
| Influenza vaccine compared to control for preventing acute otitis media in infants and children | ||||||
| Patient or population: infants and children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with influenza vaccine | |||||
| At least 1 episode of acute otitis media | Study population | RR 0.84 | 3134 | ⊕⊕⊝⊝ | The basis for the assumed risk is the mean risk in the control group across the included studies. There was moderate unexplained inconsistency across the studies. We downgraded for inconsistency. The effect estimates were all in the same direction, but there was uncertainty in the confidence of the effect estimate. | |
| 292 per 1000 | 245 per 1000 | |||||
| Courses of antibiotics | Study population | RR 0.70 | 1223 | ⊕⊕⊕⊝ | Assumed risk calculated from the mean risk across the control groups of the 2 included studies | |
| 362 per 1000 | 254 per 1000 | |||||
| Fever | Study population | RR 1.15 | 10,615 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 7 included studies | |
| 174 per 1000 | 200 per 1000 | |||||
| Rhinorrhoea | Study population | RR 1.17 | 10,563 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 6 included studies | |
| 424 per 1000 | 496 per 1000 | |||||
| Pharyngitis | Study population | RR 1.00 | 4429 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 3 included studies | |
| 71 per 1000 | 71 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Includes trial(s) at high risk of publication bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 1 episode of acute otitis media Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2 Acute otitis media by courses Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 First course (1 or 2 doses) | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2.2 Second course (1 dose) | 2 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 3 Acute otitis media by type of vaccine Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 3.1 Trivalent cold‐adapted influenza vaccine | 2 | 2552 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.21] |
| 3.2 Trivalent sub virion influenza vaccine | 2 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.23] |
| 4 Courses of antibiotics Show forest plot | 2 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 5 Fever Show forest plot | 7 | 10615 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.24] |
| 6 Rhinorrhoea Show forest plot | 6 | 10563 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.07, 1.29] |
| 7 Pharyngitis Show forest plot | 3 | 4429 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.25] |

