Vacuna contra la gripe para la prevención de la otitis media aguda en lactantes y niños
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 exp Otitis Media/
2 otitis media.tw.
3 (OM or OME or AOM or CSOM).tw.
4 glue ear*.tw.
5 (middle ear* adj5 (infect* or inflam*)).tw.
6 or/1‐5
7 exp influenzavirus a/ or exp influenzavirus b/ or influenzavirus c/
8 Influenza, Human/
9 (influenza* or flu).tw.
10 or/7‐9
11 exp Vaccines/
12 exp Vaccination/
13 (laiv or tiv).tw.
14 exp Immunization/
15 (vaccin* or immuni* or innocul*).tw.
16 or/11‐15
17 10 and 16
18 Viral Vaccines/
19 Influenza Vaccines/
20 or/17‐19
21 6 and 20
Appendix 2. Embase (Elsevier) search strategy
#27 #18 AND #26
#26 #21 NOT #25
#25 #22 NOT #24
#24 #22 AND #23
#23 'human'/de
#22 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de
#21 #19 OR #20
#20 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti
#19 'randomised controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp
#18 #5 AND #17
#17 #15 OR #16
#16 'virus vaccine'/de OR 'influenza vaccine'/de
#15 #9 AND #14
#14 #10 OR #11 OR #12 OR #13
#13 laiv:ab,ti OR tiv:ab,ti
#12 vaccin*:ab,ti OR immuni*:ab,ti OR inocul*:ab,ti
#11 'immunization'/exp
#10 'vaccine'/exp
#9 #6 OR #7 OR #8
#8 influenza*:ab,ti OR flu:ab,ti
#7 'influenza'/exp
#6 'influenza virus'/de OR 'influenza virus a'/exp OR 'influenza virus b'/de OR 'influenza virus c'/de
#5 #1 OR #2 OR #3 OR #4
#4 ('middle ear' NEAR/5 (infect* OR inflam*)):ab,ti OR ('middle ears' NEAR/5 (infect* OR inflam*)):ab,ti
#3 'glue ear':ab,ti OR 'glue ears':ab,ti
#2 'otitis media':ab,ti OR om:ab,ti OR ome:ab,ti OR aom:ab,ti OR csom:ab,ti
#1 'otitis media'/exp
Appendix 3. CINAHL (EBSCO) search strategy
S32 S22 and S31
S31 S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30
S30 (MH "Quantitative Studies")
S29 TI placebo* OR AB placebo*
S28 (MH "Placebos")
S27 TI random* OR AB random*
S26 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*))
S25 TI clinic* trial* OR AB clinic* trial*
S24 PT clinical trial
S23 (MH "Clinical Trials+")
S22 S6 and S21
S21 S18 or S19 or S20
S20 (MH "Influenza Vaccine")
S19 (MH "Viral Vaccines")
S18 S12 and S17
S17 S13 or S14 or S15 or S16
S16 TI (laiv or tiv) OR AB (laiv or tiv)
S15 TI (vaccin* or immuni* or inocul*) OR AB (vaccin* or immuni* or inocul*)
S14 (MH "Immunization+")
S13 (MH "Vaccines+")
S12 S7 or S8 or S9 or S10 or S11
S11 TI (influenza* or flu) OR AB (influenza* or flu)
S10 (MH "Influenza+")
S9 (MH "Influenzavirus C")
S8 (MH "Influenzavirus B+")
S7 (MH "Influenzavirus A+")
S6 S1 or S2 or S3 or S4 or S5
S5 TI (middle ear* N5 (infect* or inflam*)) OR AB (middle ear* N5 (infect* or inflam*))
S4 TI glue ear* OR AB glue ear*
S3 TI (OM or OME or AOM or CSOM) OR AB (OM or OME or AOM or CSOM)
S2 TI otitis media OR AB otitis media
S1 (MH "Otitis Media+")
Appendix 4. LILACS (BIREME) search strategy
> Search > (MH:"Otitis Media" OR MH:C09.218.705.663$ OR "otitis media" OR "Otite Média" OR "glue ear" OR "glue ears" OR OME OR AOM OR CSOM OR OM OR "middle ear infection" OR "middle ear infections" OR "middle ear inflammation") AND (MH:"Influenza Vaccines" OR MH:D20.215.894.899.302 OR "Vacunas contra la Influenza" OR "Vacinas contra Influenza" OR "Vacunas contra Gripe" OR "Vacunas Antigripales" OR "Vacinas Antigripais" OR MH:"Viral Vaccines" OR MH:D20.215.894.899$ OR "Vacunas Virales" OR "Vacinas Virais" OR (MH:Vaccines OR Vacunas OR Vacinas OR MH:D20.215.894$ OR MH:Immunization OR Inmunización OR Imunização OR MH:E02.095.465.425.400$ OR MH:E05.478.550$ OR MH:N02.421.726.758.310$ OR MH:N06.850.780.200.425$ OR MH:N06.850.780.680.310$ OR MH:SP2.026.182.113$ OR SP4.001.002.015.049$ OR SP8.946.819.838$ OR "Estimulación Inmunológica" OR Inmunoestimulación OR "Sensibilización Inmunológica" OR Variolación OR Variolización OR Inmunizaciones OR "Estimulação Imunológica" OR Imunoestimulação "Sensibilização Imunológica" OR Variolação OR Imunizações OR vaccin$ OR immuni$ OR inocul$ OR laiv OR tiv) AND (MH:"Influenza, Human" OR Gripe OR Grippe OR influenza$ OR flu OR MH:"Influenzavirus A" OR MH:B04.820.545.405$ OR MH:B04.909.777.545.405$ OR MH:"Influenzavirus B" OR MH:B04.820.545.407$ OR MH:B04.909.777.545.407$ OR MH:"Influenzavirus C")) > clinical_trials
Appendix 5. Web of Science (Thomson Reuters) search strategy
| # 5 | #4 AND #3 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On | |
|
| ||
| # 4 | Title=(trial) OR Topic=(random* or placebo* or ((singl* or doubl*) NEAR/1 blind*) or rct or "clinical trial") Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On | |
|
| ||
| # 3 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On | |
|
| ||
| # 2 | Topic=(influenza* or flu) AND Topic=(vaccin* or immuni* or inocul* or laiv or tiv) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On | |
|
| ||
| # 1 | Topic=("otitis media" OR "glue ear" OR "glue ears" OR ("middle ear" NEAR/5 (infect* or inflam*))) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On | |

Study flow diagram.
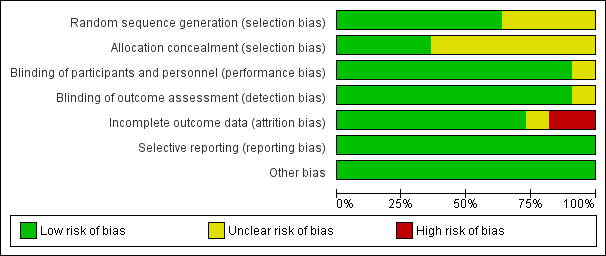
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
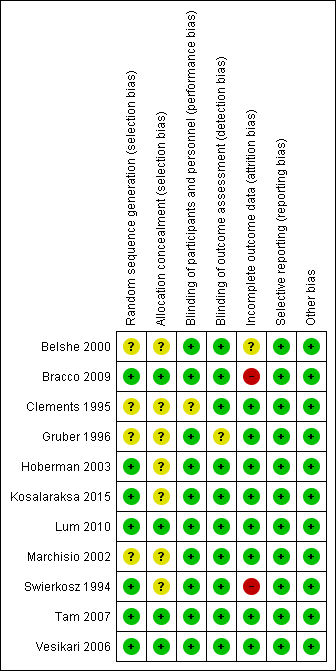
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
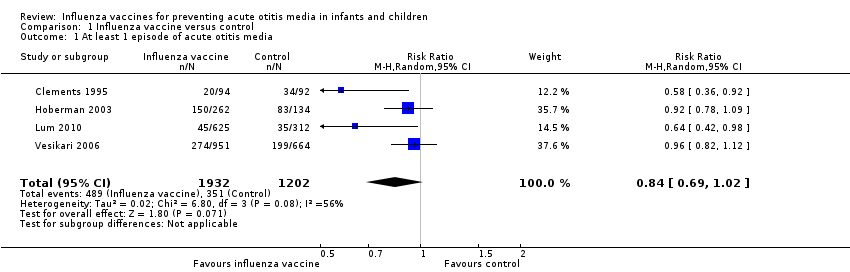
Comparison 1 Influenza vaccine versus control, Outcome 1 At least 1 episode of acute otitis media.

Comparison 1 Influenza vaccine versus control, Outcome 2 Acute otitis media by courses.

Comparison 1 Influenza vaccine versus control, Outcome 3 Acute otitis media by type of vaccine.
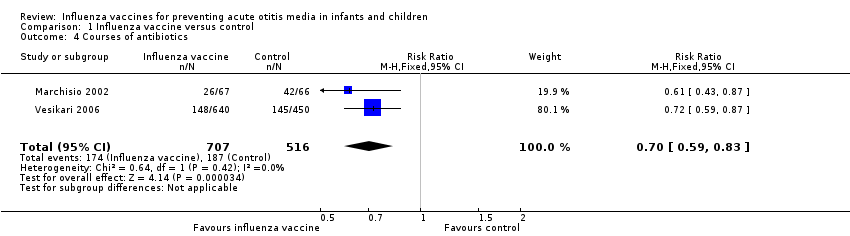
Comparison 1 Influenza vaccine versus control, Outcome 4 Courses of antibiotics.

Comparison 1 Influenza vaccine versus control, Outcome 5 Fever.
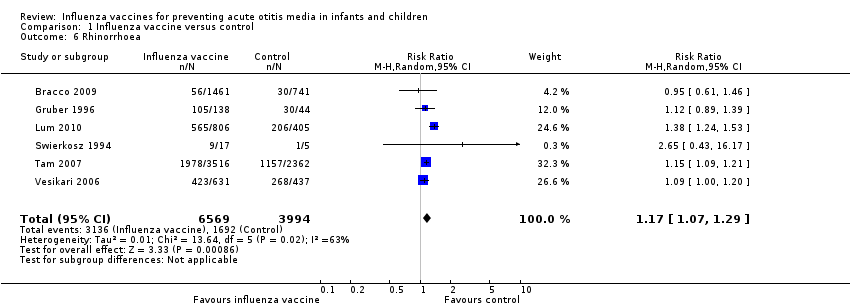
Comparison 1 Influenza vaccine versus control, Outcome 6 Rhinorrhoea.
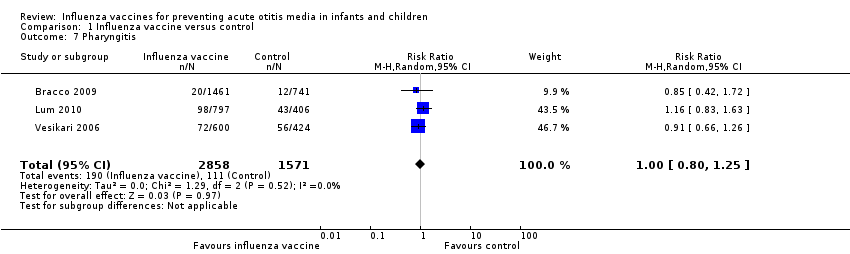
Comparison 1 Influenza vaccine versus control, Outcome 7 Pharyngitis.
| Influenza vaccine compared to control for preventing acute otitis media in infants and children | ||||||
| Patient or population: infants and children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with influenza vaccine | |||||
| At least 1 episode of acute otitis media | Study population | RR 0.84 | 3134 | ⊕⊕⊝⊝ | The basis for the assumed risk is the mean risk in the control group across the included studies. There was moderate unexplained inconsistency across the studies. We downgraded for inconsistency. The effect estimates were all in the same direction, but there was uncertainty in the confidence of the effect estimate. | |
| 292 per 1000 | 245 per 1000 | |||||
| Courses of antibiotics | Study population | RR 0.70 | 1223 | ⊕⊕⊕⊝ | Assumed risk calculated from the mean risk across the control groups of the 2 included studies | |
| 362 per 1000 | 254 per 1000 | |||||
| Fever | Study population | RR 1.15 | 10,615 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 7 included studies | |
| 174 per 1000 | 200 per 1000 | |||||
| Rhinorrhoea | Study population | RR 1.17 | 10,563 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 6 included studies | |
| 424 per 1000 | 496 per 1000 | |||||
| Pharyngitis | Study population | RR 1.00 | 4429 | ⊕⊕⊝⊝ | Assumed risk calculated from the mean risk across the control groups of the 3 included studies | |
| 71 per 1000 | 71 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Includes trial(s) at high risk of publication bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 1 episode of acute otitis media Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2 Acute otitis media by courses Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 First course (1 or 2 doses) | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 2.2 Second course (1 dose) | 2 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.94, 1.34] |
| 3 Acute otitis media by type of vaccine Show forest plot | 4 | 3134 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.69, 1.02] |
| 3.1 Trivalent cold‐adapted influenza vaccine | 2 | 2552 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.56, 1.21] |
| 3.2 Trivalent sub virion influenza vaccine | 2 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.23] |
| 4 Courses of antibiotics Show forest plot | 2 | 1223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.83] |
| 5 Fever Show forest plot | 7 | 10615 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.24] |
| 6 Rhinorrhoea Show forest plot | 6 | 10563 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.07, 1.29] |
| 7 Pharyngitis Show forest plot | 3 | 4429 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.80, 1.25] |

