Tratamientos ricos en plaquetas para lesiones de partes blandas musculoesqueléticas
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial: allocation concealment by computer‐generated randomisation. Participants were followed for 6 months Trial conducted: Sao Paulo University Medical School, Brazil; recruitment November 2008‐February 2010 | |
| Participants | Participants: 27 undergoing ACL reconstruction Included participants: patients with ACL injuries, bone maturity and aged < 45 years Excluded participants: complex ligament lesions, osteoarthritis, previous surgeries at the same joint, post operative infection, arthrofibrosis, reoperation, inadequate follow‐up and thrombocytopenia Age: Gender: PRT group (number of participants men:women): 10:2 Sports activity: not available | |
| Interventions | All participants underwent ACL reconstruction with bone‐patellar tendon bone graft 1. PRT (number of participants = 12). Single and intraoperative intervention: 450 mL blood, resulted in 30‐50 mL PRP. Remaining blood was returned to the participant. To generate PRP gel, CaCl2 and autologous thrombin was added. PRP gel applied in patellar tendon harvest site PRT preparation: kit: Haemonetics MCS+/ 995‐E 2. No PRT (number of participants = 15): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | VAS MRI (to assess the patellar tendon harvest site healing: gap area of the patellar tendon harvest site, cross‐sectional area of the patellar tendon, patellar height by the Insall‐Salvati index) Lysholm Questionnaire IKDC Kujala Questionnaire Tegner Questionnaire Isokinetic strength measurements | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: available | |
| Notes | The authors provided extra information after request (academic thesis): measures of dispersion (standard deviation) for VAS, Lysholm, IKDC, Kujala and Tegner scores The authors provided the study protocol / trial registration details, ID: NCT01111747 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Computer‐generated sequence was used |
| Allocation concealment | Unclear risk | Not reported |
| Blinding | Unclear risk | Probably not blinded |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is available and one primary outcome (pain) was measured only within the first 24 hours after surgery, which was preplanned in the study's protocol. In addition, the clinical follow‐up period is short for participants who underwent ACL surgery |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled pilot trial: computer‐generated randomisation performed and kept in opaque envelopes Participants were followed for 2 years. Follow‐up assessors were blinded to the outcomes Trial conducted: Hospital Universitario La Paz, Madrid, Spain; recruitment: May 2007‐June 2009 | |
| Participants | Participants: 28 undergoing arthroscopic repair of rotator cuff tears Included participants: adults with massive rotator cuff tears (postero‐superior rotator cuff, 2 tendons, > 5 cm) that failed conservative treatment. Diagnosis performed by clinical examinations and MRI. Participant final eligibility occurred after intraoperative visual inspection Excluded participants: evidence of anterosuperior tears that affected the subscapularis; previous surgery on the affected shoulder; major joint trauma to the shoulder; radiographic osteoarthritis; major medical condition that affects quality of life; workers' compensation claims and unwillingness to be followed for the duration of the study. Participants with haematological abnormalities were also excluded Age: mean (range): 65 years (53‐77) Gender (number of men:women): 22:6 PRT group: not available Sports activity: not available | |
| Interventions | All participants underwent arthroscopic repair of rotator cuff tears with absorbable anchors PRT (number of participants = 14):Single, intraoperative intervention, as an augmentation therapy: 120 mL blood resulted in 6 mL PRF applied over the repair site, under endoscopic visualisation PRT preparation: kit: Vivostat PRF (Alleroed, Denmark) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 14): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Constant score DASH VAS MRI (with regard to integrity of repair) | |
| Other quality issues | Sample size: the authors did not calculate the sample size. Authors report that their sample is underpowered Validation of PRT: PRT concentration/validation was not reported | |
| Notes | Pilot trial. The authors provided extra information upon request: measures of dispersion (standard deviation) for VAS and Constant scores. In addition, there was insufficient information about whether baseline was balanced. The authors have provided the study protocol/trial registration details, ID: NCT01612845 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Used computer‐generated sequence |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes. The envelope was only opened following intraoperative inspection of the shoulder |
| Blinding | Low risk | The surgeon was not blinded to the treatment allocation, but the research assistant performing follow‐up evaluations and the radiologist were blinded |
| Incomplete outcome data addressed | Low risk | No missing outcome data |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: participants followed for at least 16 months. It is not clear if clinical assessors and participants were not blinded to the procedure. MRI assessors were blinded to the procedure Trial conducted: Department of Orthopaedic and Trauma Surgery, Ospedale Civile, Jesi, Italy; recruitment: from January 2007‐April 2008 | |
| Participants | Participants: 88 undergoing arthroscopic repair of rotator cuff tears Included participants: participants with repairable small or medium rotator cuff tears (supraspinatus), as assessed in the operative procedure Excluded participants: presence of inflammatory joint disease; irreparable or partial lesions; acromioclavicular arthritis; rotator cuff arthropathy; subscapularis tendon abnormalities; workers' compensation claims; prior surgery on the affected shoulder Age: Gender: PRT group(number of men:women):23:22 Sports activity: not available | |
| Interventions | All patients underwent arthroscopic repair with double row fixation. PRT was applied as an augmentation procedure PRT (number of participants = 43): single platelet‐rich fibrin matrix ‐ 9 mL blood centrifuged for 6 minutes PRP separated and CaCl2 was added for a 2‐phase centrifugation PRT preparation: kit: Cascade Autologous Platelet System Quantification of platelet concentrates after preparation: not assessed No PRT (number of participants = 45): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Constant Score MRI (integrity of the rotator cuff repair, retear) | |
| Other quality issues | Sample size: adequate power for Constant Validation of PRT: PRT concentration/validation was not reported | |
| Notes | The authors have provided the study protocol/trial registration details, ID: ISRCTN49643328 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The authors used a random numbers table to allocate study participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Unclear risk | Clinical assessors and participants were probably not blinded to the procedure, but MRI assessors were blinded to the procedure |
| Incomplete outcome data addressed | Low risk | No missing outcome data |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: 2 blocks of 20 participants that were randomly selected by an external researcher. It is not clear how the allocation blocks were created. Participants followed for 12 months. Participants and radiologists were blinded to the intervention Trial conducted: Department of Sports Traumatology and Arthroscopic Surgery of the Galeazzi | |
| Participants | Participants: 40 undergoing arthroscopic ACL reconstruction Included participants: adults requiring ACL reconstruction Excluded participants: associated ligament damage; associated immune‐rheumatologic pathologies; chondropathies (Outerbrigde > III); pre‐existing anterior knee pain; femoropatellar pathologies and previous surgery on the same knee Age: Gender: PRT group: not available Sports activity: included patients were in "high level" of sports activity | |
| Interventions | All patients underwent ACL reconstruction with bone‐patellar tendon graft PRT (number of participants = 20): single, intra operative intervention, 54 mL blood plus 6 mL citrate anticoagulant, 15 minutes centrifugation. Buffy coat containing PRP was centrifuged with participant's thrombin (from another venous puncture) and applied after jellified. PRP gel was applied in the patellar and tendon bone plug harvest site and fixed with peritenon suture PRT preparation: kit: Gravitional Platelet Separation (GPS II). Addiction of CaCl2 and autologous thrombin Quantification of platelet concentrates after preparation: not assessed No PRT (number of participants = 20): no platelet‐rich therapy controls Co‐Interventions: same rehabilitation protocol | |
| Outcomes | VISA VAS MRI (assessment of new bone formation in the graft site; gap filling > 70% considered as satisfactory) | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: PRT concentration or its validation was not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding | Unclear risk | Participants and radiologists were blinded to the intervention |
| Incomplete outcome data addressed | Low risk | No missing outcome data |
| Free of selective reporting | Unclear risk | The study protocol was not available. Relevant outcomes were reported |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: randomisation held in sealed envelopes. Not clear how the allocation sequence was generated. Participants followed for 6 months. Participants were blinded to the procedure. Assessors were independent Trial conducted: no details available; recruitment: no details available | |
| Participants | Participants: 150 with elbow tendinopathy Included participants: adults with elbow tendinopathy (< 6 months' duration) that had failed to respond to physical therapy exercises Exluded participants: previous injection therapies (e.g. Corticoid) Age: Gender: PRT group (number of men:women): 46:34 Sports activity: not available | |
| Interventions | All participants underwent 2 injections (at 0 and 1 month) with previous local anaesthesia (2 mL bupivacaine). Injections performed by ultrasound guidance by an musculoskeletal radiologist PRT (number of participants = 80): 8.5 mL blood sample, tube with citrate anticoagulant PRT preparation: no kit. Preparation: 15 minutes of centrifugation, 1.5 mL platelet‐rich plasma siphoned from buffy coat layer Quantification of platelet concentrates after preparation: 10 random samples of blood demonstrated a 2.8‐fold (CI 2.3‐3.5) elevation from baseline for the platelet concentration No PRT (number of participants = 70): autologous blood injections ‐ details not reported Co‐interventions: same rehabilitation protocol for both groups | |
| Outcomes | PRTEE | |
| Other quality issues | Sample size: powered for PRTEE Validation of PRT: quantification reported | |
| Notes | Participants who did not improve with the proposed intervention (failure) had the option to undergo surgical treatment. This study was included using an inclusion criterion that differed from the published protocol: autologous whole blood was considered as a control intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | Participants and outcomes assessors were blinded |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups. In addition, intention‐to‐treat analyses were performed |
| Free of selective reporting | High risk | The study protocol is not available and the authors evaluated only 1 primary outcome (PRTEE). In addition, the clinical follow‐up period was short for participants who underwent elbow tendinopathy treatment |
| Free of other bias | Low risk | Participants were permitted to receive other treatments. However, authors performed analysis as intention‐to‐treat |
| Methods | Randomised controlled trial: block randomisation (12 participants per block). Randomisation was made by sealed blank envelopes. Participants were pre‐stratified according to whether pre‐injury activity levels were high‐ or low‐level, based on a score that assesses ankle‐related activity. Participants were followed for 24 months (researcher was not blinded) and 52 months (researcher was blinded). Researchers divided the study protocol into 2 reports Trial conducted: The Hague Medical Center Antoniushove, Leidschendam, the Netherlands; recruitment: 28 August 2008‐29 January 2009 | |
| Participants | Participants: 54 with chronic Achilles tendinopathy, participants were contacted by email or telephone for the first consultation Included participants: presence of midportion achilles tendinopathy (2‐7 cm proximal to the insertion on the calcaneous), and aged between 18‐70 years. Diagnosis based on clinical findings (painful and thickened tendon in relation to activity and on palpation) Excluded participants: clinical suspicion of other musculoskeletal (insertional disorders and tendon rupture) injuries; inflammatory internal disorders or use of specific medications that can cause tendinopathy (fluoroquinolones); previous performance of a complete heavy load eccentric exercise program or inability to perform it or previous injection with PRP Age: Gender: PRT group (number of men:women): 13:14 Sports activity (active, PRT:no PRT): 22:24 | |
| Interventions | All participants received a single injection. Previous local anaesthesia (2 mL bupivacaine (Marcaine)). All injections performed by ultrasonographic guidance by an experienced sports physician at 3 different locations proximal to the Achilles tendon insertion PRT (number of participants = 27): blood sample (54 mL) resulted in 4 mL PRP. Additional 6 mL citrate was added Preparation: 15 minutes centrifugation with the addition of 0.3 mL sodium bicarbonate (bicarbonate was added to match tissue pH. 4 mL was collected for infiltration) PRT preparation (number of participants = 27): kit: Recover Platelet separation kit (Gravitational Platelet Separation ‐ GPS III). No addition of CaCl2 or thrombin Quantification of platelet concentrates after preparation: no No PRT: saline injection Co‐interventions: same rehabilitation protocol both groups. Paracetamol (acetaminophen) was used as rescue medication in both groups | |
| Outcomes | VISA‐A score Participant satisfaction (good or excellent reported satisfaction was considered as satisfied) Return to sports activity (cut‐off: return to desired sport on a pre‐injury level) Sonographic evaluation (tendon structure and neovascularisation) | |
| Other quality issues | Sample size: powered for VISA‐A Validation of PRT: PRT concentration/validation was not reported | |
| Notes | JAMA 2010 ‐ Premilinary communication; AJSM 2011; BJSM 2011 ‐ Final reports The authors provided the study protocol/trial registration details, ID: NCT00761423 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | A block randomisation was performed with a block size of 12 participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | Personnel, participants and outcomes assessors were blinded |
| Incomplete outcome data addressed | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | Low risk | The study protocol is available and all expected outcomes were assessed |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: participants were allocated after randomisation derived from sealed envelopes. It is not clear how the randomisation sequence was generated. Participants and assessors were blinded to the intervention. Trial conducted: no details available; recruitment: no details available | |
| Participants | Participants: 40 undergoing open surgery for shoulder impingement syndrome Included participants: impingement syndrome (stage II), diagnosed at least 6‐months preoperatively. Participants with typical anterior shoulder pain during elevation, loss of active and passive shoulder motion and positive response to 3 subacromial infiltrations (local anaesthetics and corticoids) performed in a 6‐month period Excluded participants: presence of rotator cuff injury; frozen shoulder; acromioclavicular joint disorder; glenohumeral joint degenerative arthritis; shoulder instability; shoulder and elbow disorders; hand disorders; post‐traumatic disorder; participants with diseases that would affect post‐operative wound healing or who were treated for acute shoulder dysfunction Age: Gender: PRT group (number of men:women): 7:8 Sports activity: not available | |
| Interventions | All participants underwent open subacromial decompression PRT (number of participants = 20): single intraoperative platelet‐leucocyte gel application. From 52 mL blood, 12 mL used to prepare intervention. Citrate dextrose and autologous thrombin were used for gel formation PRT preparation: kit: Magellan Autologous Platelet Separator System (MAPS) Quantification of platelet concentrates after preparation: 1183 SD 396/109/L, 5.7‐fold increase from baseline No PRT (number of participants = 20): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol both groups | |
| Outcomes | ASES (American Shoulder and Elbow Surgeons scoring system) VAS ADL Shoulder range of motion Use of pain medication | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: quantification reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Drew random numbers |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | Participants and assessors were blinded to the intervention |
| Incomplete outcome data addressed | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | Unclear risk | The study protocol was not available. It appears that the study’s prespecified primary and secondary outcomes that are of interest in the review have been reported in the prespecified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: allocation concealment derived from randomisation (sealed envelopes). Participants were followed for a mean of 13 months Trial conducted: Orthopaedic Clinic, University of Rome 'Sapienza', Rome, Italy; recruitment: from June‐December 2009 | |
| Participants | Participants: 80 undergoing arthroscopic repair of rotator cuff tears Included participants: reparable large full‐thickness posterosuperior rotator cuff tears Excluded participants: partial‐thickness tear; small or massive full‐thickness tear; traumatic tear; biceps instability; labral pathology amenable to surgical treatment; os acromiale; degenerative arthritis of the glenohumeral joint; autoimmune or rheumatologic disease; previous surgery in the same shoulder and Workers' compensation claims Age: Gender: PRT group (number of men:women): 20:19 Sports activity: not available | |
| Interventions | All participants underwent arthroscopic rotator cuff repair PRT (number of participants = 40): single, intraoperative intervention (platelet‐leukocyte membrane), 10 mL blood was centrifuged for 10 minutes at 120 x g. The product was added to gluconate and batroxobin, for 20‐30 minutes (product is a platelet‐leukocyte membrane) PRT preparation: kit: RegenKit, Regen Lab, Le Mont‐Sur‐Lausanne, Switzerland) Quantification of platelet concentrates after preparation: white blood cells (7 x 103/mm3), platelet (> 400 x 103/mm3), 1.7 times greater than the normal level in whole blood. No PRT (number of participants = 40): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol for both groups | |
| Outcomes | Constant scores Simple Shoulder Test MRI (repair integrity): Sugaya classification | |
| Other quality issues | Sample size: a priori power calculations not available Validation of PRT: quantification reported | |
| Notes | In the intervention group, 1 membrane was used for each repair anchor 4 follow‐up losses (1 in the PRT group), reasons not known The authors provided the study protocol/trial registration details, ID: ISRCTN93082180 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation reporting was unclear |
| Allocation concealment | High risk | The envelope was opened 3 days prior to surgery rather than during surgery |
| Blinding | Unclear risk | The study was probably not blinded |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: endpoint assessors and participants were blinded to the procedure. Allocation sequence controlled by randomisation performed as blocks of 6 participants. Study's outcomes were measured at 3 months Trial conducted: Diagnostic Centre, Region Hospital Silkeborg, Silkeborg, Denmark; recruitment: from January 2009‐July 2010 | |
| Participants | Participants: 40 with elbow lateral epicondylitis Included participants: participants with symptoms for more than 3 months Excluded participants: participants < 18 years old; treated with glucocorticoid injection in previous 3 months; previous tennis elbow surgery; inflammatory diseases; neck pain on the ipsilateral side and chronic pain syndromes Lateral epicondylitis defined as pain on the lateral side of the elbow for at least 3 months, pain at the lateral epicondyle on direct palpation and during resisted dorsiflexion of the wrist. Ultrasonography was also performed at the origin of the extensor tendon; required a definite sign of tendinopathy with colour Doppler flow of at least grade 2 at baseline Age: Gender: PRT group (number of men:women): 9:11 Sports activity: not available | |
| Interventions | All participants underwent platelet‐rich plasma or glucocorticoid or saline ultrasound‐guided single injection. A blood sample was collected from all participants, and all interventions were prepared out of the reach of the participant PRT (number of participants = 20): PRP: 3.0‐3.5 mL PRP derived from 27 mL blood. Blood was centrifuged at 3200 rpm for 15 minutes, before the addition of 3 mL citrate. Bicarbonate was added to the PRP to achieve physiological pH. PRT preparation: Recover GPS II system (Biomet Biologics Inc, Warsaw, Indiana) Quantification of platelet concentrates after preparation: 8‐fold (compared with whole blood) No PRT (number of participants = 20): saline (3 mL of 0.9%) Co‐interventions: same rehabilitation protocol for both groups | |
| Outcomes | Pain section of the PRTEE questionnaire Functional disability of the PRTEE questionnaire Safety (adverse events) Injection‐related pain Ultrasound assessment: colour doppler changes and tendon thickness | |
| Other quality issues | Sample size: the authors calculated the sample size based on the PRTEE pain domain at 12 months (we expect that this based on another population) Validation of PRT: quantification reported | |
| Notes | We excluded all the analyses relating to glucocorticoid intervention (not considered as placebo) The authors provided the study protocol/trial registration details, ID: NCT 01109446 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Used permuted blocks of 6 participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | The participant and outcome assessors were blinded to the treatment, but the treating physician was not |
| Incomplete outcome data addressed | High risk | Only 13 out of 40 participants in the 2 groups completed 12 months' follow‐up |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent elbow tendinopathy treatment |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: randomisation performed by coin toss and concealment was kept in sealed, opaque envelopes. participants and outcome assessors (clinical and imaging) were blinded to the procedure. Participants were followed for 12 months Trial conducted: Sao Paulo University Medical School, Brazil; recruited: September 2008‐December 2013 | |
| Participants | Participants: 54 undergoing arthroscopic repair of rotator cuff tears Inclusion criteria: skeletally‐mature participants with no previous affected shoulder surgery. Complete supraspinatus tear, assessed by MRI, with small tendon retraction (< 3 cm). Pain and disability for > 3 months, not improving by standard non operative care. Absence of: other rotator cuff tears, anatomical abnormalities such as cyst that could potentially jeopardise the repair; rotator cuff fatty degeneration (Grades 2, 3 and 4), osteoarthritis (glenohumeral and acromioclavicular), or other conditions that could influence the results (mental and rheumatic disorders, pregnancy, infection) Age: Gender: PRT group (number of men:women): 8:19 Sports activity: not available | |
| Interventions | All participants had arthroscopic supraspinatus repair with anchors PRP (number of participants = 27): single intraoperative application. 400 mL whole blood provided 30 mL PRP. After PRP separation, blood was returned by the apheresis device. Sodium citrate and autologous thrombin were added Quantification of platelet concentrates after preparation: 8‐fold (compared with whole blood) PRT preparation: kit: Haemonetics MCS+ 9000® and 994‐CFE (Haemonetics Corporation MA, USA) No PRP (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol both groups | |
| Outcomes | Constant score UCLA VAS Frequency of rerupture (assessed by MRI) | |
| Other quality issues | Sample size: the authors set an a priori calculation of sample size for the primary endpoint Validation of PRT: the authors quantified the concentration of platelet concentrate | |
| Notes | Sample size was calculated for Constant scores as primary endpoint. The authors provided extra information after request (academic thesis): measures of dispersion (standard deviation) for VAS, Constant and UCLA scores. The authors provided the study protocol/trial registration details, ID: NCT01029574 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Sequence generated by internet‐based coin toss |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | Assessors and participants were blinded to the procedure |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | Most of the outcomes were reported, but with discrepancies among primary and secondary outcomes |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Quasi‐randomised controlled trial: participants were allocated to an intervention consecutively, following a predefined sequence. Outcomes were measured at 3 and 6 months Trial conducted: Departamento de Traumatología, Hospital Militar de Santiago,Chile; recruitment:from January 2005‐December 2006 | |
| Participants | Participants: 53 undergoing ACL reconstruction Inclusion criteria: mature skeleton, clinical instability, MRI showing total rupture of the ACL and voluntary acceptance of participation in the study Exclusion criteria: capsulo‐ligamentous injuries Age mean (range): 30 years (15‐57) Gender (number of men:women): 99:17 PRT group (number of men:women): not available Sports activity: not available | |
| Interventions | 4‐arm intervention: 1. Standard semitendinosus‐gracilis graft ACL reconstruction 2. Standard semitendinosus‐gracilis graft ACL reconstruction augmentation with platelet concentrate 3. Standard semitendinosus‐gracilis graft ACL reconstruction with bone plug association 4. Standard semitendinosus‐gracilis graft ACL reconstruction and platelet concentrate and bone plug association PRT (number of participants = 26): single PRP application, 67 mL blood produced 10 mL PRP. Blood centrifuged for 10 minutes and clothing derived from participants' thrombin (obtained after a 10‐minute centrifugation). CaCl2 was added to the PRP product. A 2‐step application was performed: the graft was immersed in the PRP clot and PRP was injected in the bone femoral tunnel PRT preparation: kit: Biomet GPS II ( Warsaw, Indiana) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | MRI assessments: maturation of the graft (graft signal intensity, osteo ligamentous interface, tunnel widening) IKDC | |
| Other quality issues | Sample size: the authors calculated the sample size; however, it is not clear if for the main endpoint Validation of PRT: PRT concentration or its validation was not reported | |
| Notes | For this review's purposes, data from interventions numbered as 3 and 4 were excluded (not considered as placebo) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Quasi‐randomised clinical trial |
| Allocation concealment | High risk | Quasi‐randomised clinical trial |
| Blinding | High risk | Only the MRI assessor was blinded |
| Incomplete outcome data addressed | Unclear risk | Missing outcome data were probably balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent to ACL surgery |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: participants were randomised utilising block procedure. Participants had final follow‐up at 24 months Trial conducted: Department of Scienze Medico Chirurgiche, University of Milano, IRCCS Policlinico San Donato, Milano, Italy; recruitment: from April 2007‐January 2008 | |
| Participants | Participants: 53 undergoing arthroscopic repair of rotator cuff tears Inclusion criteria: a complete rotator cuff tear confirmed intraoperatively; agreed to wear a dedicated brace for 4 weeks postoperatively; had a preoperative platelet count > 150,000; minimum preoperative haemoglobin of 11.0 g/dL; no infectious diseases or diseases that may have limited follow‐up; BMI < 33 Exclusion criteria: previous rotator cuff repair; active infection; osteomyelitis or sepsis, or distant infections; osteomalacia or other metabolic bone disorders; unco‐operative or had disorders that made them incapable of following directions, or who were unwilling to return for follow‐up examinations; vascular insufficiency, muscular atrophy, or neuromuscular diseases of the affected arm; cigarette smokers; had received steroid injection(s) in the affected shoulder Age: Gender: PRT group (number of men:women): 8:19 Sports activity: not available | |
| Interventions | Participants were submitted to arthroscopic rotator cuff repair (single row repair, absorbable anchors) by a single surgeon. Acromioplasty was performed in all cases PRT (number of participants = 26): single, intraoperative injection. 54 mL blood mixed with 6 mL citrate as an anticoagulant. The product was centrifuged for 15 minutes at 3200 rpm. PRP was separated and centrifuged (2 minutes) to increase fibrinogen concentration and mixed with PRP. A final 6 mL PRP was applied through the arthroscopic portals PRT preparation: kit: GPS II, Biomet Biologics (Warsaw, IN) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Constant score SST UCLA score VAS Strength in external rotation Rate of retear | |
| Other quality issues | Sample size: the authors calculated the sample size Validation of PRT: the exact composition of the PRP was unknown | |
| Notes | Authors had 8 follow‐up losses (4 in each group). Pain was measured in short intervals in the early postoperative period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | A block randomised procedure was used to generate a randomisation list |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | The participants and outcome assessors were blinded to the treatment |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | Unclear risk | The study protocol is not available but all expected outcomes were assessed |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial Trial conducted: Sports Medicine and Shoulder Service, The Hospital for Special Surgery, New York, New York, USA; recruitment: no details available Participants were followed for 24 months | |
| Participants | Participants: 80 undergoing arthroscopic repair of rotator cuff tears Inclusion criteria: participants ≥ 40 years of age for whom non operative treatment had failed Exclusion criteria: people undergoing revision, mini‐open, or open procedures; people with concomitant labral tears. Age: Gender: PRT group (number of men:women): 23:17 Sports activity: not available | |
| Interventions | All participants underwent arthroscopic rotator cuff repair with bone anchors PRT (number of participants = 40): single intraoperative application, PRFM, 9 mL blood produced a PRFM product. Fibrin matrix was produced after a second centrifugation step, by the addition of CaCl2 PRT preparation: kit: Cascade Autologous Platelet System, Musculoskeletal Transplant Foundation, Edision, New Jersey, USA) Quantification of platelet concentrates after preparation: not stated No PRT (number of participants = 40): no platelet‐rich therapy controls Quantification of platelet concentrates after preparation: not reported Co‐interventions: same rehabilitation protocol | |
| Outcomes | Ultrasound assessment (tendon healing) ASES Score L' Insalata score Shoulder strength | |
| Other quality issues | Sample size: author stopped trial as it had detected no benefit (target: 65 participants per group) Validation of PRT: the exact composition of the PRP was unknown | |
| Notes | Participants lost to follow‐up: n = 5 (PRT), n = 7 (no PRT) The authors provided the study protocol/trial registration details, ID: NCT01029574 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | The participants and outcome assessors were blinded to the treatment |
| Incomplete outcome data addressed | High risk | Reasons for missing outcome data were not reported and there was imbalance in numbers across intervention groups |
| Free of selective reporting | Low risk | The study protocol was available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review were reported in the pre‐specified way |
| Free of other bias | Low risk | The study appears to be free of other sources of bias |
| Methods | Randomised controlled trial: randomisation occurred as blocks of 6 participants, assignment kept in sealed envelopes. Allocation concealment was kept until the operative time. Participants and outcome assessors were blind to the intervention. Participants were followed for 1 year Trial conducted: Linköping University, Linköping, Sweden; recruitment: September 2007‐April 2008 | |
| Participants | Participants: 30 undergoing open repair of acute achilles tendon rupture Inclusion criteria: participants aged 18‐60 years, with an acute (< 3 days) rupture of Achilles tendon Exclusion criteria: diabetes mellitus; a history of cancer or lung or heart diseases; or diseases that could compromise the locomotor system Age: Gender: PRT group (number of men:women): 13:3 Sports activity: All participants were recreational athletes injured during sports or sports‐related activities | |
| Interventions | All participants underwent open repair of acute Achilles tendon injuries, with implantation of tantalum beads to aid in image analyses PRT (number of participants = 16): 450 mL blood derived a mean volume of 21 mL PRP. PRP was prepared and stored, with constant rotation, up to 20 hours before use. Platelet viability was assessed, and found to have been maintained in all cases PRT preparation: no dedicated kit. Authors stated that they utilised a credited procedure (Europe 2007) Quantification of platelet concentrates after preparation: 3673 (SD 1051) x 109 platelets per mL No PRT (number of participants = 14): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Tendon strain per load: distance between the tantalum beads (roentgen stereophotogrammetric analysis (RSA)) while participants resisted different dorsal flexion moment over the ankle joint Estimate of elasticity modulus (using callus dimensions from computed tomography) Functional outcome: heel‐raise index and Achilles tendon Total Rupture Score | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: the exact composition of the PRP is unknown | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Used permuted blocks of 6 participants |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | The participants and outcome assessors were blinded to the treatment |
| Incomplete outcome data addressed | High risk | Missing outcome data were not balanced in numbers across intervention groups; more participants in the PRP group were lost to follow‐up (4/16 (25%) PRP versus 0/14 (0%) control) |
| Free of selective reporting | Unclear risk | The study protocol is not available, but all expected outcomes were assessed |
| Free of other bias | Low risk | The study appears to be free of other bias |
| Methods | Randomised controlled trial. Blocks were randomised from a sequence derived from an Internet‐based program Participants were followed for 6 months. Only the outcome assessors were blinded to the procedure Trial conducted: Department of Orthopaedic Surgery, | |
| Participants | Participants: 29 with elbow lateral epicondylitis Inclusion criteria: clinically diagnosed lateral epicondylitis (based on symptoms, site of tenderness, and pain elicited with resisted active extension of the wrist in pronation and elbow extension); no history of trauma; duration ≥ 3 months; no previous local injection treatment of any kind; no medical history of rheumatic disorder; and no signs of posterior interosseous nerve entrapment Exclusion criteria: recent onset of symptoms (< 3 months); history of trauma; medical comorbidities such as rheumatoid arthritis; previous local injections (e.g. cortisone); and suspicion of nerve involvement Age: Gender: PRT group (number of men:women): 5:10 Sports activity: not available | |
| Interventions | All participants received 1 ultrasound‐guided injection for lateral epicondylitis at the origin of wrist extensors with a peppering technique (single skin insertion, deep peripheral multiple sites of injection) PRT (number of participants = 14): 55 mL blood produced 3‐6 mL PRP. Used 3 mL anticoagulant, but no activator, since authors stated that in vivo contact with collagen is responsible for activation Quantification of platelet concentrates after preparation: 235,000/mL to 1,292,500/mL (5.5 times, on average).An average ratio for white blood cells was reported as: 111/1 (platelets/leukocytes) PRT preparation: kit: GPS III, Biomet Biologics (Warsaw, IN) No PRT (number of participants = 15): 3 mL autologous peripheral whole blood, deep at the origin of wrist extensors with a peppering technique (single skin insertion, deep peripheral multiple sites of injection) under aseptic technique with the assistance of ultrasound guidance Co‐interventions: same rehabilitation protocol. Painkiller and ice therapy were prescribed in both groups | |
| Outcomes | Pain (VAS) Liverpool elbow score | |
| Other quality issues | Sample size: the authors calculated the sample size. However they did not provide the estimate of the effect that they intended to identify in group comparisons Validation of PRT: the exact composition of the PRP is unknown | |
| Notes | This study was included using an inclusion criterion that differed from the published protocol: autologous whole blood was considered as a control intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The sequence generation was performed by a computer random number generator |
| Allocation concealment | Unclear risk | Not reported |
| Blinding | High risk | Only outcomes assessors were blinded |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent to elbow tendinopathy treatment |
| Free of other bias | Low risk | The study appears to be free of other bias |
| Methods | Randomised controlled trial. Sequence generation and allocation methodology were not reported. Participants were followed for a mean of 14.7 months Trial conducted: no details available; recruitment: no details available | |
| Participants | Participants: 40 undergoing ACL reconstruction Inclusion criteria: participants with chronic instability (> 30 days of trauma) Exclusion criteria: age > 50 years; concomitant medial or lateral collateral ligament injuries; degenerative joint disease or chondral damage (MRI or radiographic examinations) Age mean (range): 34.5 years (18‐48) Gender: all were men PRT group (number of men:women): 20:0 Sports activity: not available | |
| Interventions | All patients underwent arthroscopic ACL reconstruction with hamstring graft PRT (number of participants = 20): single intraoperative application. PRP was applied in the femoral and tibial tunnel. 10 mL blood was centrifuged, thrombin and calcium gluconate added few minutes before its application in order to obtain a thick and adhesive gel PRT preparation: kit: PRP Fast Biotech kit (MyCells PPT‐Platelet Preparation Tube) Quantification of platelet concentrates after preparation: not reported No PRT (number of participants = 20): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Tunnel enlargement (assessed by CT) Tegner activity score Lysholm score IKDC score | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: PRP preparation methodology was not clear and there are some inconsistencies between sections of the manuscript | |
| Notes | The authors described different quantities for PRP preparation and application | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding | Low risk | Outcome assessors were blinded |
| Incomplete outcome data addressed | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | High risk | The study protocol is not available and the authors did not report outcomes at each time point |
| Free of other bias | Low risk | The study appears to be free of other bias |
| Methods | Randomised controlled trial: participants were randomised by a computer‐generated sequence. MRI assessors were blinded to the intervention. Participants' last assessment performed at 12 months. Trial conducted: Clínica Universitaria of Navarra, Pamplona, Spain; recruitment: no details available | |
| Participants | Participants: 100 undergoing ACL reconstruction Inclusion criteria: ACL disruption stabilised by an orthopaedic surgeon; positive Lachman e pivot‐shift test and MRI; no prior knee surgery and normal contra‐lateral knee Exclusion criteria: previous knee pathology or symptoms before ACL rupture Age: Gender: PRT group (number of men:women): 40:10 Sports activity: not available | |
| Interventions | ACL reconstruction with patellar tendon allograft fixed by cross‐pin fixation (proximal) and interference screws (distal) PRT (number of participants = 50): 40 mL blood provided 4 mL platelet‐enriched gel Quantification of platelet concentrates after preparation: 3‐5 fold increase in platelet concentration over baseline PRT preparation: no dedicated kit. Authors stated that they used a modified reported method (Sonnleitner 2000). No PRT (number of participants = 50): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Pain (VAS) Anterior Laxity (KT‐1000) IKDC Protein‐C MRI (graft status, tunnel placement, graft position) Radiographs (graft healing) | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The sequence generation was performed by a computer random number generator |
| Allocation concealment | Unclear risk | Not reported |
| Blinding | High risk | Only the MRI assessors were blinded |
| Incomplete outcome data addressed | Low risk | No participants were lost to follow‐up |
| Free of selective reporting | Unclear risk | The study protocol is not available but all expected outcomes were assessed. Complications were not assessed |
| Free of other bias | Low risk | The study appears to be free of other bias |
| Methods | Quasi‐randomised controlled trial: sequence generated by the presence of odd or even numbers. Participants followed for 6 months after the procedure Trial conducted: Department of Orthopedic Surgery, University Hospital Maribor, Maribor, Slovenia; recruitment: February‐June 2008 | |
| Participants | Participants: 55 undergoing ACL reconstruction Inclusion criteria: participants with unstable knee resulting from ACL rupture; aged 18‐50 years Exclusion criteria: inflammatory diseases; diabetes mellitus; developed knee osteoarthrosis; malignant diseases; allergy to contrast media, renal diseases and thrombocytopenia Age: Gender: PRT group (number of men:women): 13:9 Sports activity: not available | |
| Interventions | Arthroscopic ACL reconstruction with semitendinosus and gracilis tendons (fixed with 2 cross pins in the femur and 1 interference screw in the tibia) PRT (number of participants = 28): single, intraoperative application in the bone tunnels after graft placement. 52 mL blood mixed with 8 mL calcium citrate as anticoagulant. The authors pre‐defined the PRP volume as 6 mL, and the process resulted in 6 mL of PRP. The product was activated with human thrombin and applied in the surgical site PRT preparation: kit: Magellan autologous platelet separator (Medtronic Biologic Therapeutics and Diagnostics, Minneapolis, MN, USA) Quantification of platelet concentrates after preparation: 962 (552‐1326) g/L; participants' average blood platelet concentration:192 g/L No PRT (number of participants = 27): no platelet‐rich therapy controls Co‐interventions: same rehabilitation protocol | |
| Outcomes | Knee stability (KT‐ 2000) Tegner activity score Lysholm score IKDC score | |
| Other quality issues | Sample size: the authors did not calculate the sample size Validation of PRT: quantification reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | High risk | Sequence generated by odd or even date ‐ quasi‐randomised |
| Allocation concealment | High risk | Quasi‐randomised clinical trial |
| Blinding | High risk | The participants and outcome assessors were not blinded to the treatment |
| Incomplete outcome data addressed | Low risk | Missing outcome data were balanced in numbers across intervention groups |
| Free of selective reporting | High risk | The study protocol is not available and the clinical follow‐up period was short for participants who underwent to ACL surgery |
| Free of other bias | Low risk | The study appears to be free of other bias |
| Methods | Randomised controlled trial: randomisation sequence was generated by coin toss. Allocation concealment was kept in opaque envelopes that were opened on the day of the intervention. Orthopaedic surgeon and assessors were blinded to the procedure until 26‐weeks follow‐up, except for those for whom the procedure failed. Participants followed for 6 months Trial conducted: Stanford University School of Medicine, California USA; recruitment: October 2009‐June 2012 | |
| Participants | Participants: 23 with patellar tendinopathy Inclusion criteria: > 18 years old; diagnosed patellar tendinopathy; persistence of symptoms after 6 weeks of physical therapy with eccentric exercise Exclusion criteria: previous injection or surgery in the affected knee; inability to complete participant‐reported outcomes Age: Gender: PRT group (number of men:women): 8:1 Sports activity: not available | |
| Interventions | Patellar tendon ultrasound‐guided treatment: single dry needling or PRP with the aid of a board‐certified radiologist For both groups, tendinopathy area was penetrated 10 times PRT (number of participants = 10): 55 mL blood resulted in 6 mL leukocyte‐rich PRP, injected into the patellar tendon during the dry needling procedure Quantification of platelet concentrates after preparation: not reported PRT preparation: kit: GPS III (Biomet Inc, Warsaw, IN, USA) No PRT (number of participants = 13): dry needling, as described above, and the 55 ml of blood that had been drawn was discarded Co‐interventions: same post procedure interventions, same rehabilitation protocol | |
| Outcomes | VISA Tegner VAS Lysholm SF‐12 | |
| Other quality issues | Sample size: small sample size was powered for VISA, assuming an 13‐point effect size Validation of PRT: quantification not reported | |
| Notes | Participants who were not satisfied with the procedure were allowed to receive other treatments. Analyses were performed on an intention‐to‐treat basis The authors provided the study protocol/trial registration details, ID: NCT01406821 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Sequence generated by coin toss |
| Allocation concealment | Low risk | Used sequentially‐numbered, opaque and sealed envelopes |
| Blinding | Low risk | Participants and assessors were blinded |
| Incomplete outcome data addressed | Low risk | Separate analysis were performed for participants who failed the allocated intervention, as a per protocol analysis and an intention to treat analysis |
| Free of selective reporting | Low risk | Data reported as depicted in the study protocol. Short follow‐up |
| Free of other bias | Low risk | Patients were permitted to receive other treatments. However, authors performed analysis as intention‐to‐treat |
Abbreviations
> = greater/more than
< = less/fewer than
≥ = greater/more than or equal to
ACL = anterior cruciate ligament
ADL = activities of daily living
AJSM = the American Journal of Sports Medicine
ASES = American Shoulder and Elbow Surgeons' scoring system
BMI = body:mass index
BJSM = the British Journal of Sports Medicine
CT = computed tomography
DASH = Disabilities of the Arm Shoulder and Hand questionnaire
IKDC = International Knee Documentation Committee
JAMA = Journal of the American Medical Association
MRI = magnetic resonance imaging
PRF = platelet‐rich fibrin
PRFM = platelet‐rich fibrin matrix
PRP = platelet‐rich plasma
PRT = platelet‐rich therapy
PRTEE = Patient‐Related Tennis Elbow Evaluation
SF‐12 = the Short Form health survey
SST = Simple Shoulder Test
UCLA = University of California, Los Angeles score
VAS = visual analogue scale
VISA = Victorian Institute Sports Assessment
VISA‐A = Victorian Institute of Sports Assessment ‐ Achilles questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This was not a randomised study | |
| This was not a randomised study | |
| This was not a randomised study | |
| This was not a randomised study |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Northland, New Zealand Musculoskeletal Group Study on the effectiveness of platelet‐rich plasma for the treatment of greater trochanteric pain syndrome |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: platelet‐rich plasma given with local anaesthetic as a single once‐off injection into the focal area of pain and tenderness over the outer hip (details not reported) Controls or placebo or no intervention (standard care): placebo (saline and local anaesthetic) |
| Outcomes | Primary outcomes: pain according to NCS Timing of outcomes measurement: 6 months |
| Starting date | Main ID: ACTRN12612000982819 Last refreshed on: not reported |
| Contact information | Name: Dr Grant Thompson Telephone: +64 9 4594400 |
| Notes |
| Trial name or title | Pilot randomised trial to assess the safety and potential efficacy of platelet rich plasma tenotomy for the treatment of chronic epicondylitis |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: PRP injection (details were not reported) Controls or placebo or no intervention (standard care): lidocaine wet tenotomy |
| Outcomes | Primary outcomes: DASH Timing of outcomes measurement: baseline, 6th week, and 3, 6 and 12 months |
| Starting date | Main ID: EUCTR201300047832ES Last refreshed on: August 2013 |
| Contact information | Name: Isabel Andi Ortiz |
| Notes |
| Trial name or title | A randomised controlled trial: comparing the effectiveness of ultrasound guided injection of platelet rich plasma and shoulder physiotherapy on pain and function of patients with partial thickness rotator cuff tears |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: ultrasound guided, 3 mL PRP and 2 mL lidocaine injected directly into rotator cuff at the site of tear. Preparation must contain a platelet count of 100,000 per unit or be 5 times the basal level of the normal platelet count Controls or placebo or no intervention (standard care): 15 sessions of shoulder physiotherapy |
| Outcomes | Primary outcomes: Constant score; VAS |
| Starting date | Main ID: IRCT201011205214N1 Last refreshed on: July 2013 |
| Contact information | Name: Dr Ramin Kordi |
| Notes |
| Trial name or title | A randomised controlled trial to assess the effectiveness of treating subacromial impingement and partial thickness rotator cuff tears with the administration of platelet rich plasma during arthroscopic decompression surgery |
| Methods | Study design: randomised trial |
| Participants | Location: unknown Contraindications to PRP: history of diabetes mellitus; platelet abnormality or platelet count < 100 x 109/L; haematological disorder; serum haemoglobin < 11 g/dL; use of systemic cortisone; use of any anticoagulant; evidence of gangrene/ulcers or peripheral vascular disease; history of hepatic or renal impairment or dialysis; person is known to have a psychological, developmental, physical, emotional or social disorder that may interfere with compliance with study requirements; history of alcohol or drug abuse; person has a religious or cultural conflict with the use of platelet gel treatment or blood products; has inadequate venous access for blood draw; is currently receiving or has received radiation or chemotherapy within the last 3 months prior to the study; pregnant women, or women who are lactating or planning pregnancy during the course of the study; any other significant disease or disorder that, in the opinion of the Investigator, may either put the participants at risk because of participation in the study, or may influence the result of the study, or the participant's ability to participate in the study |
| Interventions | PRP: subacromial decompression plus an autologous PRP concentrate injection into the rotator cuff tendon (gel sprayed directly to the decompression area) Controls or placebo or no intervention (standard care): subacromial decompression (alone) |
| Outcomes | Primary outcomes: Oxford Shoulder Score |
| Starting date | Main ID: ISRCTN10464365 Last refreshed on: June 2013 |
| Contact information | Name: Andrew Carr |
| Notes |
| Trial name or title | Achilles Tendinopathy Management: a randomised controlled trial comparing platelet rich plasma with an eccentric loading programme |
| Methods | Study design: randomised trial |
| Participants | Location: |
| Interventions | PRP: injected into the Achilles tendinopathy, PRP preparation protocol available Controls or placebo or no intervention (standard care): eccentric loading programme |
| Outcomes | Primary outcomes: VISA‐ A Timing of outcomes measurement: at 6, 12, 24, 30, 36 and 52 weeks |
| Starting date | Main ID: ISRCTN95369715 Last refreshed on: February 2010 |
| Contact information | Name: Matthew Costa |
| Notes |
| Trial name or title | Impact of autologous platelet rich plasma on enhancing repair of rotator cuff tendons: a multicentre randomised controlled trial |
| Methods | Study design: randomised controlled trial |
| Participants | Location: |
| Interventions | PRP: PRP will be applied to the surgical site after completion of the repair (methods not reported) Standard‐of‐care: arthroscopic repair |
| Outcomes | Primary outcomes: visual analogue pain scale |
| Starting date | Main ID: NCT01000935 Last refreshed on: August 2013 |
| Contact information | Name: Richard Holtby, MD |
| Notes |
| Trial name or title | Arthroscopic surgery and platelet rich plasma In rotator cuff tear evaluation (ASPIRE): the use of platelet rich plasma following arthroscopic repair of rotator cuff tears, a pilot study |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: ACP ‐ details not reported Controls or placebo or no intervention (standard care): saline ‐ details not reported |
| Outcomes | Primary outcomes: pain score |
| Starting date | Main ID: NCT01170312 Last refreshed on: November 2012 |
| Contact information | Name: Mohit Bhandari |
| Notes |
| Trial name or title | Multicenter double blind, with evaluator blinding, parallel, randomised clinical trial, to assess the efficacy of platelet rich plasma for treatment of muscle rupture with haematoma |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: autologous PRP injection (details not reported) Controls or placebo or no intervention (standard care): evacuation of haematoma |
| Outcomes | Primary outcomes: time to complete recovery from muscular lesions |
| Starting date | Main ID: NCT01440725 Last refreshed on: January 2013 |
| Contact information | Name: Mª José Martínez Zapata, |
| Notes |
| Trial name or title | Treatment of plantar fasciitis with injection of platelet rich plasma Into the origin of the plantar fascia: a prospective, randomised and double blinded study |
| Methods | Study design: randomised trial |
| Participants | Location: |
| Interventions | PRP: plasma (3 mL plasma injected once into the plantar fascia) Controls or placebo or no intervention (standard care): 2 arms ‐ saline (3 mL saline injected once into the plantar fascia) and physiotherapy (3 times a day for 8 weeks) plus heel cap |
| Outcomes | Primary outcomes: pain (VAS score) Timing of outcomes measurement: at inclusion and after 1, 2, 3, 6 and 12 months |
| Starting date | Main ID: NCT01509274 Last refreshed on: 16 January 2012 |
| Contact information | Name: Bjørn Nedergaard |
| Notes |
| Trial name or title | A double blind, randomised, placebo controlled study evaluating the use of platelet rich plasma therapy for acute ankle sprains in the Emergency Department |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: platelet rich plasma injection (details not reported) Controls or placebo or no intervention (standard care): standard care |
| Outcomes | Primary outcomes: LEFS |
| Starting date | Main ID: NCT01518335 Last refreshed on: February 2013 |
| Contact information | Name: Adam Rowden |
| Notes |
| Trial name or title | A prospective comparison of ultrasound guided percutaneous platelet rich plasma injection versus tenotomy for treatment of gluteus minimus and medius tendinosis |
| Methods | Study design: randomised trial |
| Participants | Location: University of Michigan Hospital |
| Interventions | PRP: ultrasound‐guided percutaneous PRP injection (methods not reported) Controls or placebo or no intervention (standard care): tenotomy (alone) |
| Outcomes | Primary outcomes: pain Timing of outcomes measurement: 15 days, and 30 days after intervention |
| Starting date | Main ID: NCT01600326 Last refreshed on: 17 July 2013 |
| Contact information | Name: Jon Jacobson, MD |
| Notes |
| Trial name or title | Impact of platelet rich plasma over alternative therapies in patients with lateral epicondylitis |
| Methods | Study design: randomised trial |
| Participants | Location: multicentre |
| Interventions | PRP: Arthrex ACP system Controls or no intervention (standard care): whole blood injection, dry needle fenestration |
| Outcomes | Primary outcomes: rate of recruitment; ability to recruit 60 participants within a 12‐month period; adherence to study protocol |
| Starting date | Main ID: NCT01668953 Last refreshed on: 26 July 2013 |
| Contact information | Name: Meg Chiavaras, PhD, MD |
| Notes |
| Trial name or title | Effect of intraoperative application of autologous PRP on post operative morbidity in ACL reconstruction |
| Methods | Study design: randomised trial |
| Participants | Location: not reported |
| Interventions | PRP: ACL reconstruction bone patellar tendon bone autograft, PRP to be added to the participant's bone graft chips and placed into the donor site at the end of the case Controls or placebo or no intervention (standard care): ACL reconstruction bone patellar tendon bone autograft (standard care) |
| Outcomes | Primary outcomes: anterior knee pain |
| Starting date | Main ID: NCT01765712 Last refreshed on: 8 January 2013 |
| Contact information | Name: Brian Walters |
| Notes |
| Trial name or title | Use of platelet rich plasma in the management of acute hamstring muscle strain injury |
| Methods | Study design: randomised trial |
| Participants | Location: |
| Interventions | PRP: complex growth factor preparations (PRP) in combination with exercise therapy Controls or placebo or no intervention (standard care): 2 groups: 1) PPP injections in combination with exercise therapy (control injection and usual care) and 2) exercise therapy (usual care) |
| Outcomes | Primary outcomes: time to return to play |
| Starting date | Main ID: NCT01812564 Last refreshed on: 13 March 2013 |
| Contact information | Name: Johannes Tol, MD PhD |
| Notes |
| Trial name or title | Percutaneous needle tenotomy (PNT) versus platelet rich plasma (PRP) with PNT in the treatment of chronic tendinosis |
| Methods | Study design: randomised trial |
| Participants | Location: Icahn School of Medicine at Mount Sinai |
| Interventions | PRP: percutaneous needle tenotomy with peritendinous platelet rich plasma injection Controls or placebo or no intervention (standard care): percutaneous needle tenotomy (alone) |
| Outcomes | Primary outcomes: pain Timing of outcomes measurement: 2, 4, 6, 8, 12 weeks |
| Starting date | Main ID: NCT01833598 Last refreshed on: July 2013 |
| Contact information | Name: Alexandra Voigt |
| Notes |
| Trial name or title | The effect of platelet rich plasma on lateral epicondylitis the treatment of lateral epicondylitis: the effect of platelet rich plasma on healing ‐ a randomised controlled double blinded trial |
| Methods | Study design: randomised controlled trial |
| Participants | Location: University of Tampere |
| Interventions | PRP: 9 mL autologous venous blood centrifuged using the Arthrex ACP® Double Syringe System and 2 mL PRP injected to the proximal insertion of the extensor carpi radialis brevis muscle Controls or placebo or no intervention (standard care): saline injections (1 arm) and whole blood injections (1 arm) |
| Outcomes | Primary outcomes: pain (VAS scale) and DASH score |
| Starting date | Main ID: NCT01851044 Last refreshed on: May 2013 |
| Contact information | Name: Olli Leppänen |
| Notes |
Abbreviations
> = greater/more than
< = less/fewer than
≥ = greater/more than or equal to
≤ = less/fewer than or equal to
ACL = anterior cruciate ligament
ACP = autologous conditioned plasma
ASES = American Shoulder and Elbow Surgeons' scoring system
ASPETAR = Qatar's Orthopaedic and Sports Medicine Hospital
BMI = body:mass index
CM = Constant‐Murley score
DASH = Disabilities of the Arm Shoulder and Hand questionnaire
EQ‐5D = Euroqol 5D a standardised instrument for measuring quality of life
HADS = Hospital Anxiety and Depression Scale
Hgb = haemoglobin
LEFS = Lower extremity Function Scale
MRI = magnetic resonance imaging
NCS = numeric rating scale
NSAIDs = non‐steroidal anti‐inflammatories
PPP = platelet poor plasma
PRP = platelet‐rich plasma
SF‐12 = the Short Form health survey
SLAP = Superior Labral Anterior and Posterior lesions
US = ultrasound
VAS = visual analogue scale
VISA‐A = Victorian Institute of Sports Assessment ‐ Achilles questionnaire
WORC = Western Ontario Rotator Cuff outcome measure
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (all scores/instruments): short term (up to 3 months follow‐up) Show forest plot | 5 | 273 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.07, 0.56] |
| Analysis 1.1  Comparison 1 PRT versus control: all conditions, Outcome 1 Function (all scores/instruments): short term (up to 3 months follow‐up). | ||||
| 1.1 Rotator cuff tear (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Shoulder impingement syndrome (surgery) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.25] |
| 1.3 Elbow epicondylitis | 3 | 179 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.60] |
| 1.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 ACL reconstruction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Patellar tendinopathy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 1.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up) Show forest plot | 6 | 262 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.39, 0.51] |
| Analysis 1.2  Comparison 1 PRT versus control: all conditions, Outcome 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up). | ||||
| 2.1 Rotator cuff tear (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Elbow epicondylitis | 2 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.25, 0.94] |
| 2.4 ACL reconstruction (patellar tendon graft donor site) | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.76, 0.84] |
| 2.5 ACL reconstruction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Patellar tendinopathy | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.95, 0.09] |
| 2.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.57, 0.49] |
| 2.8 Achilles tendon rupture (surgical repair) | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.21, 0.29] |
| 3 Functional (all scores/instruments): long term (1 year or more follow‐up) Show forest plot | 10 | 484 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.07, 0.57] |
| Analysis 1.3  Comparison 1 PRT versus control: all conditions, Outcome 3 Functional (all scores/instruments): long term (1 year or more follow‐up). | ||||
| 3.1 Rotator cuff tear (surgical repair) | 6 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.51] |
| 3.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Elbow epicondylitis | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 ACL reconstruction (patellar tendon graft donor site) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.53 [0.82, 2.24] |
| 3.5 ACL reconstruction | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.31, 0.94] |
| 3.6 Patellar tendinopathy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.56] |
| 3.8 Achilles tendon rupture (surgical repair) | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.69, 0.85] |
| 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up) Show forest plot | 4 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.41, ‐0.48] |
| Analysis 1.4  Comparison 1 PRT versus control: all conditions, Outcome 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up). | ||||
| 4.1 Rotator cuff tears (surgical repair) | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.64, 0.25] |
| 4.2 Shoulder impingement syndrome (surgery) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.36, ‐0.44] |
| 4.3 Elbow epicondylitis | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.51, ‐0.21] |
| 4.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Patellar tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 PRT versus control: all conditions, Outcome 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up). | ||||
| 5.1 Rotator cuff tear (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Elbow epicondylitis | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.57, 0.07] |
| 5.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Patellar tendinopathy | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.32, 2.48] |
| 5.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 PRT versus control: all conditions, Outcome 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up). | ||||
| 6.1 Rotator cuff tear (surgical repair) | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.02, 0.44] |
| 6.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Elbow epicondylitis | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Patellar tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adverse effects (any of PRT or placebo application) Show forest plot | 11 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.48, 3.59] |
| Analysis 1.7  Comparison 1 PRT versus control: all conditions, Outcome 7 Adverse effects (any of PRT or placebo application). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (all scores/instruments): short term (up to 3 months follow‐up) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 1 Function (all scores/instruments): short term (up to 3 months follow‐up). | ||||
| 1.1 Tendinopathies | 4 | 233 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.17, 0.50] |
| 1.2 Augmentation procedures | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.25] |
| 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up) Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up). | ||||
| 2.1 Tendinopathies | 4 | 209 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.40, 0.75] |
| 2.2 Augmentation procedures | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.77, 0.32] |
| 3 Functional (all scores/instruments): long term (1 year or more follow‐up) Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 3 Functional (all scores/instruments): long term (1 year or more follow‐up). | ||||
| 3.1 Tendinopathies | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.56] |
| 3.2 Augmentation procedures | 9 | 430 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.08, 0.64] |
| 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up) Show forest plot | 4 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.41, ‐0.48] |
| Analysis 2.4  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up). | ||||
| 4.1 Tendinopathies | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.51, ‐0.21] |
| 4.2 Augmentation procedures | 3 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.71, ‐0.37] |
| 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up. | ||||
| 5.1 Tendinopathies | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Augmentation procedures | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up). | ||||
| 6.1 Tendinopathies | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Augmentation procedures | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse effects (any of PRT pr placebo application Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 7 Adverse effects (any of PRT pr placebo application. | ||||
| 7.1 Tendinopathies | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 7.2 Augmentation procedures | 9 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.29, 5.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (Constant score): long term (1 year follow‐up) Show forest plot | 5 | 290 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [0.68, 4.26] |
| Analysis 3.1  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 1 Function (Constant score): long term (1 year follow‐up). | ||||
| 2 Function (Constant score): long term (2 year follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 2 Function (Constant score): long term (2 year follow‐up). | ||||
| 3 Function (UCLA score): long term (1 year follow‐up) Show forest plot | 2 | 98 | Mean Difference (IV, Fixed, 95% CI) | 1.56 [‐0.19, 3.31] |
| Analysis 3.3  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 3 Function (UCLA score): long term (1 year follow‐up). | ||||
| 4 Function (Simple Shoulder Test (SST)): long term (1 year follow‐up) Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.07, 0.78] |
| Analysis 3.4  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 4 Function (Simple Shoulder Test (SST)): long term (1 year follow‐up). | ||||
| 5 Function (DASH score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 5 Function (DASH score): long term (1 year follow‐up). | ||||
| 6 Function (DASH score): long term (2 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 6 Function (DASH score): long term (2 year follow‐up). | ||||
| 7 Function (L'Insalata score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 7 Function (L'Insalata score): long term (1 year follow‐up). | ||||
| 8 Function (ASES score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 8 Function (ASES score): long term (1 year follow‐up). | ||||
| 9 Function (all scores/instruments): long term (1 year follow‐up) Show forest plot | 6 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.51] |
| Analysis 3.9  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 9 Function (all scores/instruments): long term (1 year follow‐up). | ||||
| 10 Pain (Analogue Scale): short term (7 day follow‐up) Show forest plot | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.44, ‐0.36] |
| Analysis 3.10  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 10 Pain (Analogue Scale): short term (7 day follow‐up). | ||||
| 11 Pain (Analogue Scale): long term (2 year follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.11  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 11 Pain (Analogue Scale): long term (2 year follow‐up). | ||||
| 12 Pain (Analogue Scale): long term (1 year follow‐up) Show forest plot | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.61] |
| Analysis 3.12  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 12 Pain (Analogue Scale): long term (1 year follow‐up). | ||||
| 13 Pain (Analogue Scale): short term (30 day follow‐up) Show forest plot | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.64, 0.25] |
| Analysis 3.13  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 13 Pain (Analogue Scale): short term (30 day follow‐up). | ||||
| 14 Rate of retear: long term (1 year follow‐up) Show forest plot | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| Analysis 3.14  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 14 Rate of retear: long term (1 year follow‐up). | ||||
| 15 Rate of retear: long term (2 year follow‐up) Show forest plot | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.32] |
| Analysis 3.15  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 15 Rate of retear: long term (2 year follow‐up). | ||||
| 16 Patient satisfaction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.16  Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 16 Patient satisfaction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional (self‐evaluation instability score: short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 1 Functional (self‐evaluation instability score: short term (6 week follow‐up). | ||||
| 2 Functional instability after surgery: 6 week follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 2 Functional instability after surgery: 6 week follow‐up. | ||||
| 3 Pain (VAS): short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 3 Pain (VAS): short term (6 week follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (PRTEE score): short term (3 month follow‐up) Show forest plot | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | 4.67 [‐1.25, 10.59] |
| Analysis 5.1  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 1 Function (PRTEE score): short term (3 month follow‐up). | ||||
| 2 Function (PRTEE scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 2 Function (PRTEE scores): medium term (6 month follow‐up). | ||||
| 3 Function (Liverpool elbow score): short term (3 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 3 Function (Liverpool elbow score): short term (3 month follow‐up). | ||||
| 4 Function (Liverpool elbow score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 4 Function (Liverpool elbow score): medium term (6 month follow‐up). | ||||
| 5 Function (all scores/instruments): short term (3 months or less follow‐up) Show forest plot | 3 | 179 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.60] |
| Analysis 5.5  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 5 Function (all scores/instruments): short term (3 months or less follow‐up). | ||||
| 6 Function (all scores/instruments): medium term (6 month follow‐up) Show forest plot | 2 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.25, 0.94] |
| Analysis 5.6  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 6 Function (all scores/instruments): medium term (6 month follow‐up). | ||||
| 7 Pain (VAS): short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.7  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 7 Pain (VAS): short term (6 week follow‐up). | ||||
| 8 Pain (VAS): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.8  Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 8 Pain (VAS): medium term (6 month follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (Tegner scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 1 Function (Tegner scores): medium term (6 month follow‐up). | ||||
| 2 Function (Lysholm score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 2 Function (Lysholm score): medium term (6 month follow‐up). | ||||
| 3 Function (VISA score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 3 Function (VISA score): long term (1 year follow‐up). | ||||
| 4 Pain (VAS): first post‐op day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 4 Pain (VAS): first post‐op day. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (IKDC scores): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 PRT versus control: ACL reconstruction, Outcome 1 Function (IKDC scores): long term (1 year follow‐up). | ||||
| 2 Function (IKDC categories A & B: normal/nearly normal): medium and long term follow‐up Show forest plot | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| Analysis 7.2  Comparison 7 PRT versus control: ACL reconstruction, Outcome 2 Function (IKDC categories A & B: normal/nearly normal): medium and long term follow‐up. | ||||
| 3 Function (Lysholm score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 PRT versus control: ACL reconstruction, Outcome 3 Function (Lysholm score): long term (1 year follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (VISA scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 1 Function (VISA scores): medium term (6 month follow‐up). | ||||
| 2 Function (Tegner scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.2  Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 2 Function (Tegner scores): medium term (6 month follow‐up). | ||||
| 3 Function (Lysholm score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.3  Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 3 Function (Lysholm score): medium term (6 month follow‐up). | ||||
| 4 Pain (VAS): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.4  Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 4 Pain (VAS): medium term (6 month follow‐up). | ||||
| 5 Quality of Life (SF‐12 score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.5  Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 5 Quality of Life (SF‐12 score): medium term (6 month follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (VISA‐A scores): short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 1 Function (VISA‐A scores): short term (6 week follow‐up). | ||||
| 2 Function (VISA‐A score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 2 Function (VISA‐A score): medium term (6 month follow‐up). | ||||
| 3 Function (VISA‐A scores): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.3  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 3 Function (VISA‐A scores): long term (1 year follow‐up). | ||||
| 4 Satisfied patients: medium term (6 month follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.4  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 4 Satisfied patients: medium term (6 month follow‐up). | ||||
| 5 Satisfied patients: long term (1 year follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.5  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 5 Satisfied patients: long term (1 year follow‐up). | ||||
| 6 Return to desired sports: medium term (6 month follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.6  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 6 Return to desired sports: medium term (6 month follow‐up). | ||||
| 7 Return to desired sports: long term (1 year follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.7  Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 7 Return to desired sports: long term (1 year follow‐up). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (heel‐raise index): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 1 Function (heel‐raise index): medium term (6 month follow‐up). | ||||
| 2 Function (heel‐raise index): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.2  Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 2 Function (heel‐raise index): long term (1 year follow‐up). | ||||
| 3 Complications Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.3  Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 3 Complications. | ||||

Study flow diagram
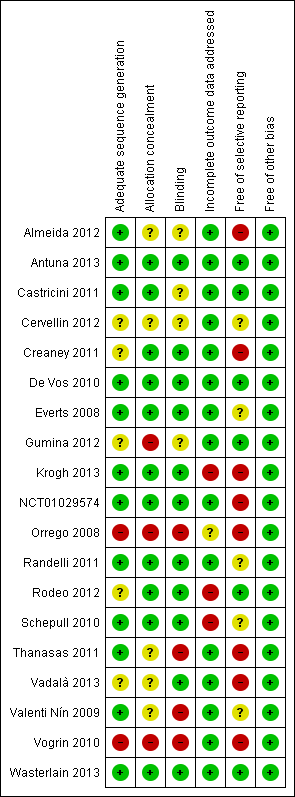
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
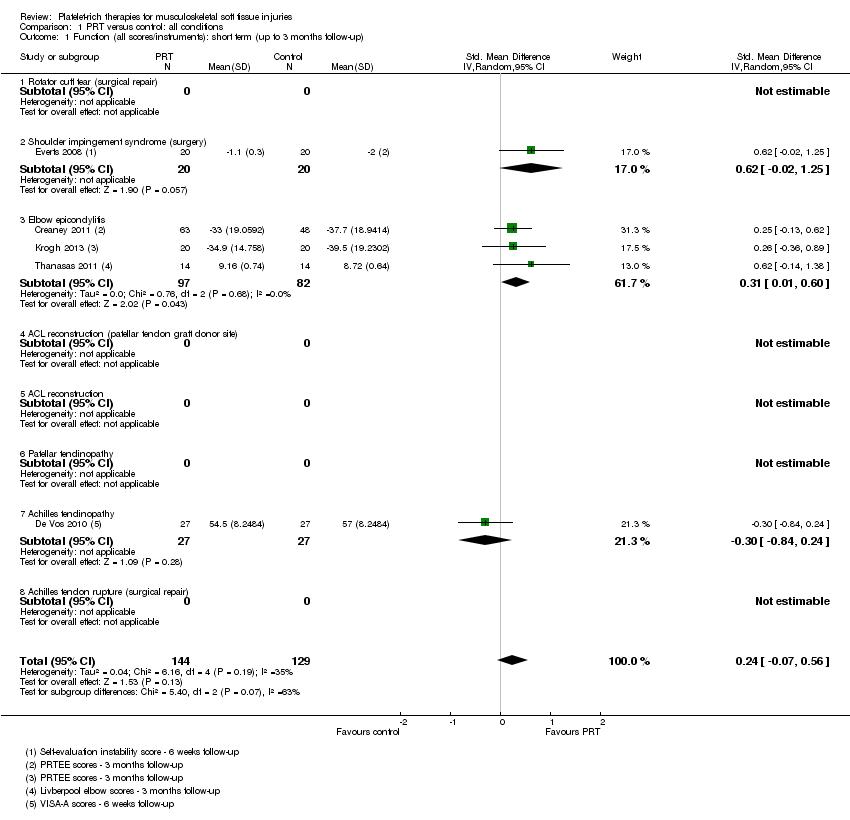
Comparison 1 PRT versus control: all conditions, Outcome 1 Function (all scores/instruments): short term (up to 3 months follow‐up).

Comparison 1 PRT versus control: all conditions, Outcome 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up).

Comparison 1 PRT versus control: all conditions, Outcome 3 Functional (all scores/instruments): long term (1 year or more follow‐up).
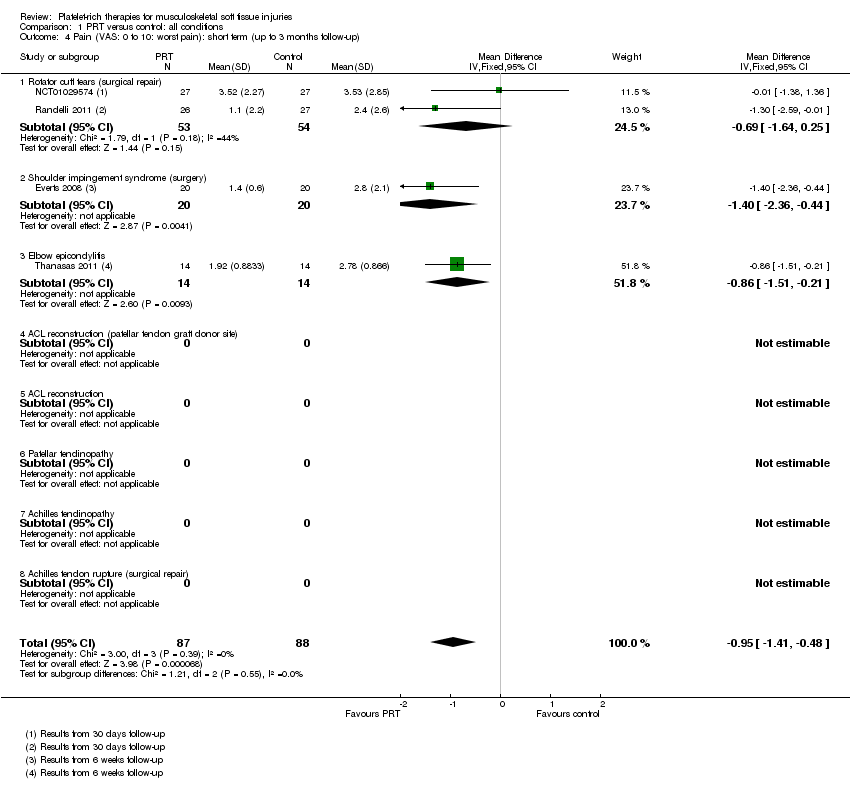
Comparison 1 PRT versus control: all conditions, Outcome 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up).
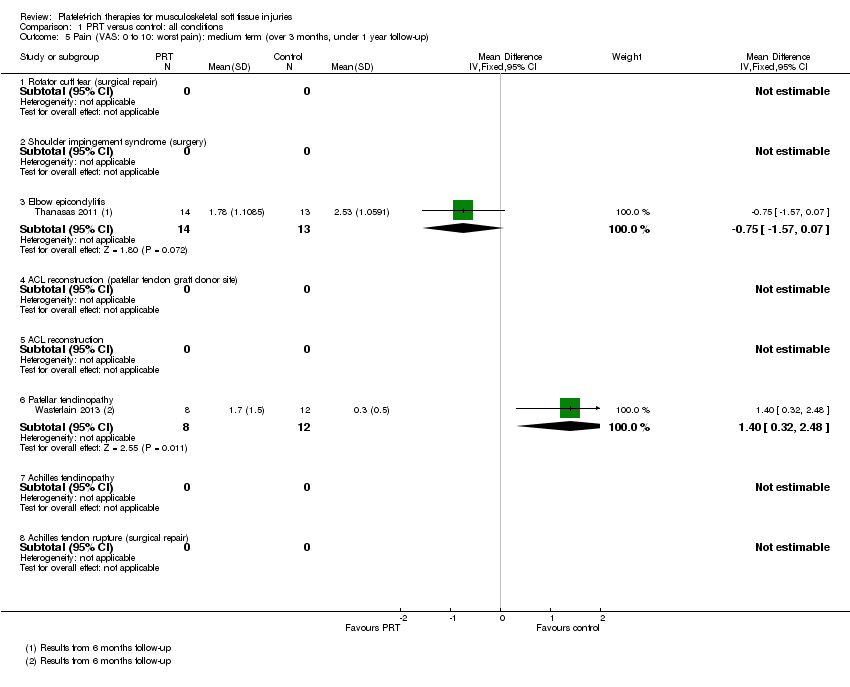
Comparison 1 PRT versus control: all conditions, Outcome 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up).

Comparison 1 PRT versus control: all conditions, Outcome 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up).

Comparison 1 PRT versus control: all conditions, Outcome 7 Adverse effects (any of PRT or placebo application).
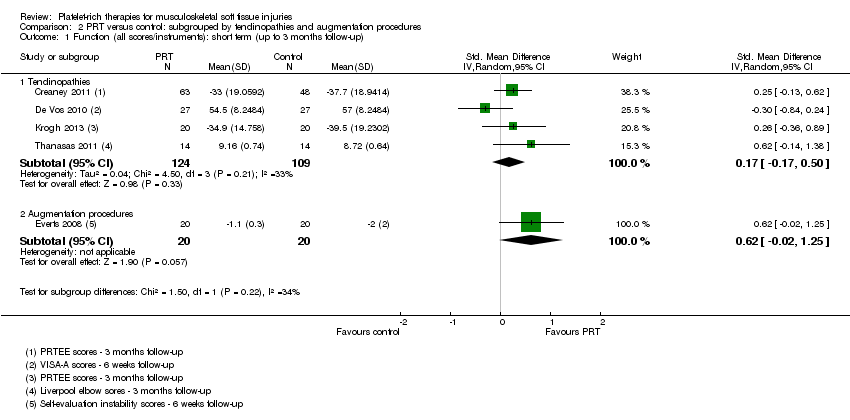
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 1 Function (all scores/instruments): short term (up to 3 months follow‐up).

Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up).

Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 3 Functional (all scores/instruments): long term (1 year or more follow‐up).

Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up).
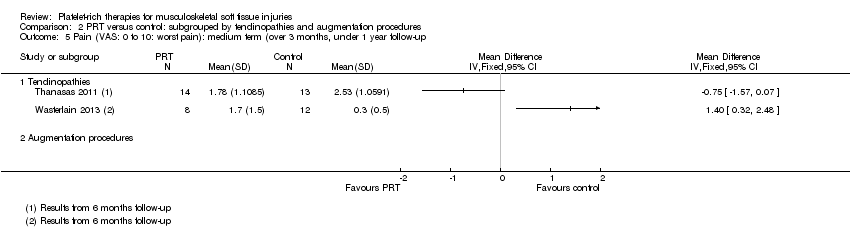
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up.
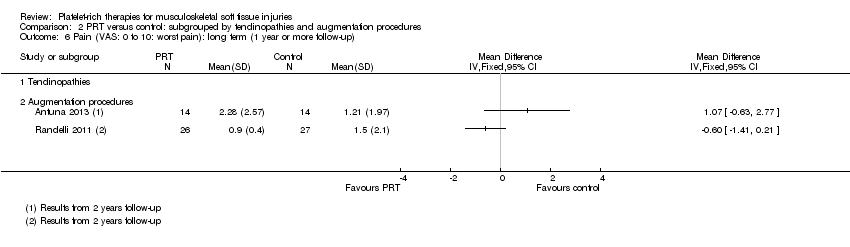
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up).
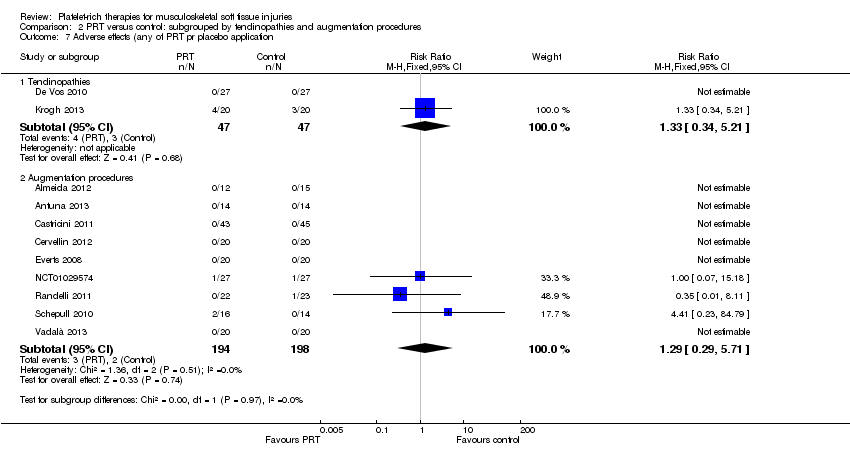
Comparison 2 PRT versus control: subgrouped by tendinopathies and augmentation procedures, Outcome 7 Adverse effects (any of PRT pr placebo application.
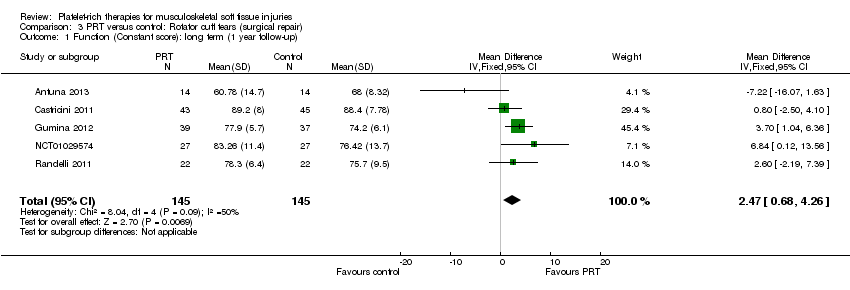
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 1 Function (Constant score): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 2 Function (Constant score): long term (2 year follow‐up).
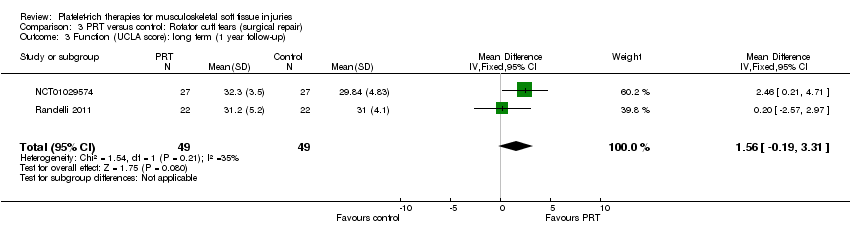
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 3 Function (UCLA score): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 4 Function (Simple Shoulder Test (SST)): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 5 Function (DASH score): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 6 Function (DASH score): long term (2 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 7 Function (L'Insalata score): long term (1 year follow‐up).
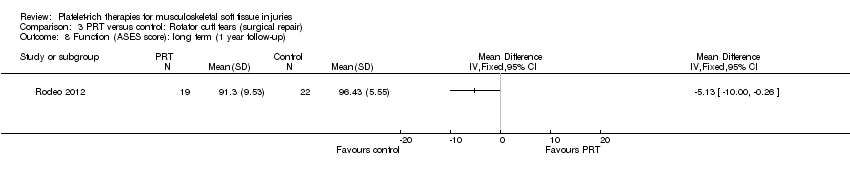
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 8 Function (ASES score): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 9 Function (all scores/instruments): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 10 Pain (Analogue Scale): short term (7 day follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 11 Pain (Analogue Scale): long term (2 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 12 Pain (Analogue Scale): long term (1 year follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 13 Pain (Analogue Scale): short term (30 day follow‐up).

Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 14 Rate of retear: long term (1 year follow‐up).
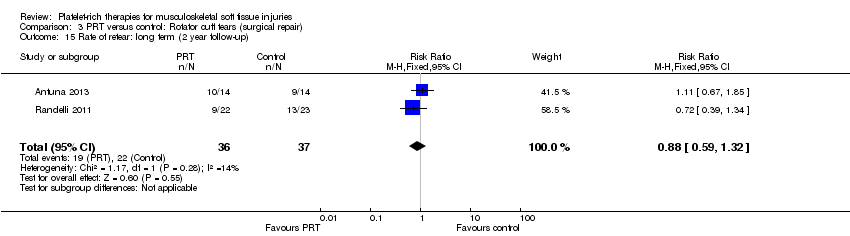
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 15 Rate of retear: long term (2 year follow‐up).
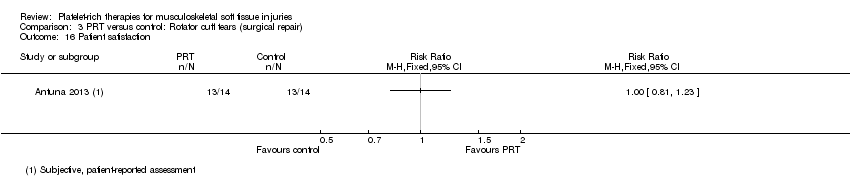
Comparison 3 PRT versus control: Rotator cuff tears (surgical repair), Outcome 16 Patient satisfaction.
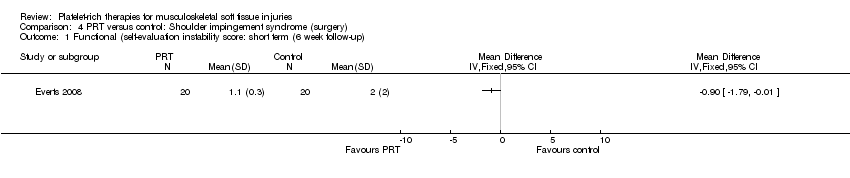
Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 1 Functional (self‐evaluation instability score: short term (6 week follow‐up).

Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 2 Functional instability after surgery: 6 week follow‐up.

Comparison 4 PRT versus control: Shoulder impingement syndrome (surgery), Outcome 3 Pain (VAS): short term (6 week follow‐up).
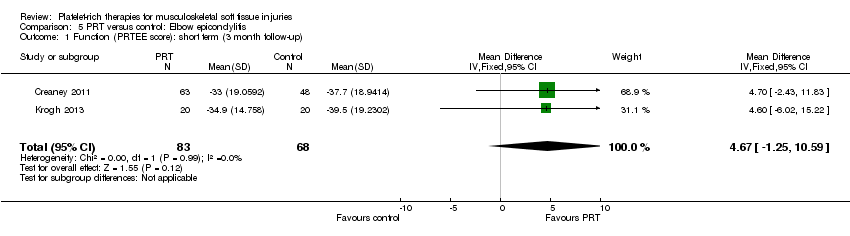
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 1 Function (PRTEE score): short term (3 month follow‐up).

Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 2 Function (PRTEE scores): medium term (6 month follow‐up).

Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 3 Function (Liverpool elbow score): short term (3 month follow‐up).

Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 4 Function (Liverpool elbow score): medium term (6 month follow‐up).

Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 5 Function (all scores/instruments): short term (3 months or less follow‐up).

Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 6 Function (all scores/instruments): medium term (6 month follow‐up).
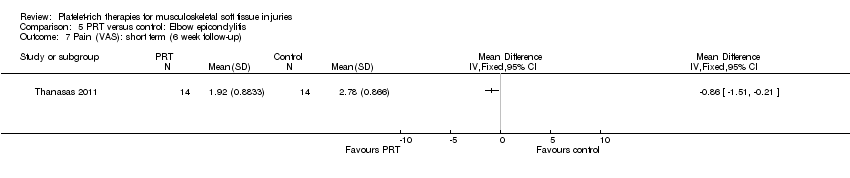
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 7 Pain (VAS): short term (6 week follow‐up).
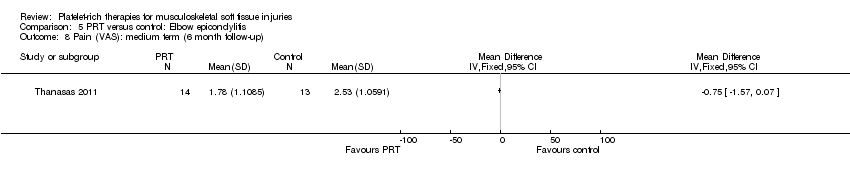
Comparison 5 PRT versus control: Elbow epicondylitis, Outcome 8 Pain (VAS): medium term (6 month follow‐up).

Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 1 Function (Tegner scores): medium term (6 month follow‐up).

Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 2 Function (Lysholm score): medium term (6 month follow‐up).
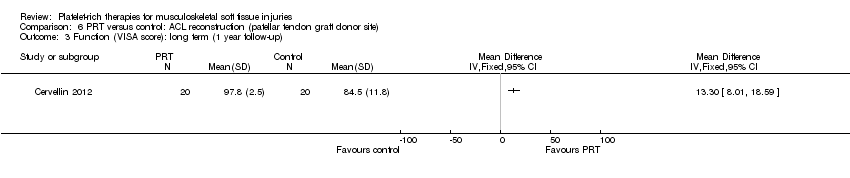
Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 3 Function (VISA score): long term (1 year follow‐up).

Comparison 6 PRT versus control: ACL reconstruction (patellar tendon graft donor site), Outcome 4 Pain (VAS): first post‐op day.

Comparison 7 PRT versus control: ACL reconstruction, Outcome 1 Function (IKDC scores): long term (1 year follow‐up).

Comparison 7 PRT versus control: ACL reconstruction, Outcome 2 Function (IKDC categories A & B: normal/nearly normal): medium and long term follow‐up.
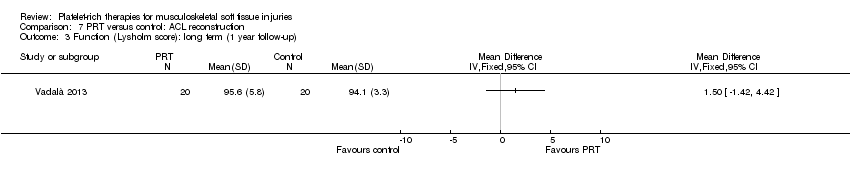
Comparison 7 PRT versus control: ACL reconstruction, Outcome 3 Function (Lysholm score): long term (1 year follow‐up).

Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 1 Function (VISA scores): medium term (6 month follow‐up).

Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 2 Function (Tegner scores): medium term (6 month follow‐up).
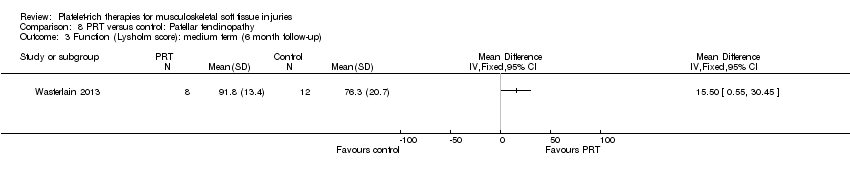
Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 3 Function (Lysholm score): medium term (6 month follow‐up).

Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 4 Pain (VAS): medium term (6 month follow‐up).

Comparison 8 PRT versus control: Patellar tendinopathy, Outcome 5 Quality of Life (SF‐12 score): medium term (6 month follow‐up).

Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 1 Function (VISA‐A scores): short term (6 week follow‐up).
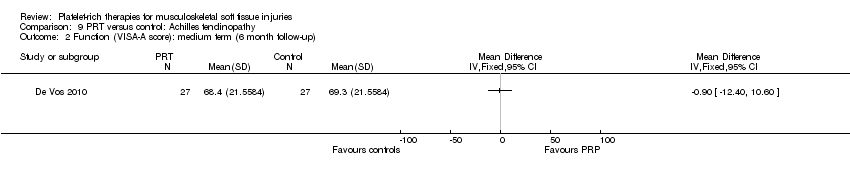
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 2 Function (VISA‐A score): medium term (6 month follow‐up).

Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 3 Function (VISA‐A scores): long term (1 year follow‐up).
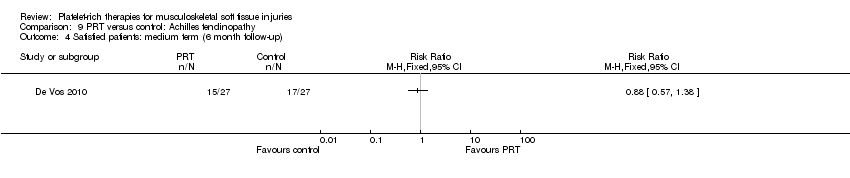
Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 4 Satisfied patients: medium term (6 month follow‐up).

Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 5 Satisfied patients: long term (1 year follow‐up).

Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 6 Return to desired sports: medium term (6 month follow‐up).

Comparison 9 PRT versus control: Achilles tendinopathy, Outcome 7 Return to desired sports: long term (1 year follow‐up).
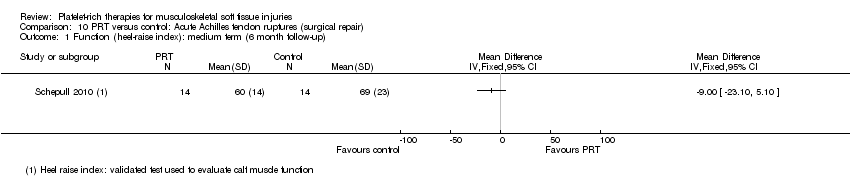
Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 1 Function (heel‐raise index): medium term (6 month follow‐up).
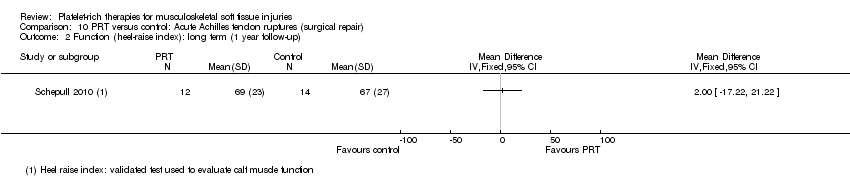
Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 2 Function (heel‐raise index): long term (1 year follow‐up).

Comparison 10 PRT versus control: Acute Achilles tendon ruptures (surgical repair), Outcome 3 Complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (all scores/instruments): short term (up to 3 months follow‐up) Show forest plot | 5 | 273 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.07, 0.56] |
| 1.1 Rotator cuff tear (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Shoulder impingement syndrome (surgery) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.25] |
| 1.3 Elbow epicondylitis | 3 | 179 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.60] |
| 1.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 ACL reconstruction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Patellar tendinopathy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.84, 0.24] |
| 1.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up) Show forest plot | 6 | 262 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.39, 0.51] |
| 2.1 Rotator cuff tear (surgical repair) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Elbow epicondylitis | 2 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.25, 0.94] |
| 2.4 ACL reconstruction (patellar tendon graft donor site) | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.76, 0.84] |
| 2.5 ACL reconstruction | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Patellar tendinopathy | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.95, 0.09] |
| 2.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.57, 0.49] |
| 2.8 Achilles tendon rupture (surgical repair) | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.21, 0.29] |
| 3 Functional (all scores/instruments): long term (1 year or more follow‐up) Show forest plot | 10 | 484 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.07, 0.57] |
| 3.1 Rotator cuff tear (surgical repair) | 6 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.51] |
| 3.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Elbow epicondylitis | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 ACL reconstruction (patellar tendon graft donor site) | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.53 [0.82, 2.24] |
| 3.5 ACL reconstruction | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.31, 0.94] |
| 3.6 Patellar tendinopathy | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.7 Achilles tendinopathy | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.56] |
| 3.8 Achilles tendon rupture (surgical repair) | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.69, 0.85] |
| 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up) Show forest plot | 4 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.41, ‐0.48] |
| 4.1 Rotator cuff tears (surgical repair) | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.64, 0.25] |
| 4.2 Shoulder impingement syndrome (surgery) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.36, ‐0.44] |
| 4.3 Elbow epicondylitis | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.51, ‐0.21] |
| 4.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Patellar tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Rotator cuff tear (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Elbow epicondylitis | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.57, 0.07] |
| 5.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Patellar tendinopathy | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.32, 2.48] |
| 5.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Rotator cuff tear (surgical repair) | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.02, 0.44] |
| 6.2 Shoulder impingement syndrome (surgery) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Elbow epicondylitis | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.4 ACL reconstruction (patellar tendon graft donor site) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 ACL reconstruction | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 Patellar tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 Achilles tendinopathy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Achilles tendon rupture (surgical repair) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adverse effects (any of PRT or placebo application) Show forest plot | 11 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.48, 3.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (all scores/instruments): short term (up to 3 months follow‐up) Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Tendinopathies | 4 | 233 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.17, 0.50] |
| 1.2 Augmentation procedures | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.02, 1.25] |
| 2 Function (all scores/instruments): medium term (over 3 months, under 1 year follow‐up) Show forest plot | 6 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Tendinopathies | 4 | 209 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.40, 0.75] |
| 2.2 Augmentation procedures | 2 | 53 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.77, 0.32] |
| 3 Functional (all scores/instruments): long term (1 year or more follow‐up) Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Tendinopathies | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.51, 0.56] |
| 3.2 Augmentation procedures | 9 | 430 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.08, 0.64] |
| 4 Pain (VAS: 0 to 10: worst pain): short term (up to 3 months follow‐up) Show forest plot | 4 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.41, ‐0.48] |
| 4.1 Tendinopathies | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.51, ‐0.21] |
| 4.2 Augmentation procedures | 3 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.71, ‐0.37] |
| 5 Pain (VAS: 0 to 10: worst pain): medium term (over 3 months, under 1 year follow‐up Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Tendinopathies | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Augmentation procedures | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pain (VAS: 0 to 10: worst pain): long term (1 year or more follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Tendinopathies | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Augmentation procedures | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse effects (any of PRT pr placebo application Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Tendinopathies | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| 7.2 Augmentation procedures | 9 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.29, 5.71] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (Constant score): long term (1 year follow‐up) Show forest plot | 5 | 290 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [0.68, 4.26] |
| 2 Function (Constant score): long term (2 year follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (UCLA score): long term (1 year follow‐up) Show forest plot | 2 | 98 | Mean Difference (IV, Fixed, 95% CI) | 1.56 [‐0.19, 3.31] |
| 4 Function (Simple Shoulder Test (SST)): long term (1 year follow‐up) Show forest plot | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.07, 0.78] |
| 5 Function (DASH score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Function (DASH score): long term (2 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Function (L'Insalata score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Function (ASES score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Function (all scores/instruments): long term (1 year follow‐up) Show forest plot | 6 | 324 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.24, 0.51] |
| 10 Pain (Analogue Scale): short term (7 day follow‐up) Show forest plot | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐2.44, ‐0.36] |
| 11 Pain (Analogue Scale): long term (2 year follow‐up) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Pain (Analogue Scale): long term (1 year follow‐up) Show forest plot | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.20, 0.61] |
| 13 Pain (Analogue Scale): short term (30 day follow‐up) Show forest plot | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.64, 0.25] |
| 14 Rate of retear: long term (1 year follow‐up) Show forest plot | 3 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 15 Rate of retear: long term (2 year follow‐up) Show forest plot | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.32] |
| 16 Patient satisfaction Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional (self‐evaluation instability score: short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional instability after surgery: 6 week follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pain (VAS): short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (PRTEE score): short term (3 month follow‐up) Show forest plot | 2 | 151 | Mean Difference (IV, Fixed, 95% CI) | 4.67 [‐1.25, 10.59] |
| 2 Function (PRTEE scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (Liverpool elbow score): short term (3 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Function (Liverpool elbow score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Function (all scores/instruments): short term (3 months or less follow‐up) Show forest plot | 3 | 179 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.60] |
| 6 Function (all scores/instruments): medium term (6 month follow‐up) Show forest plot | 2 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.25, 0.94] |
| 7 Pain (VAS): short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Pain (VAS): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (Tegner scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (Lysholm score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (VISA score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (VAS): first post‐op day Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (IKDC scores): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (IKDC categories A & B: normal/nearly normal): medium and long term follow‐up Show forest plot | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 3 Function (Lysholm score): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (VISA scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (Tegner scores): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (Lysholm score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain (VAS): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Quality of Life (SF‐12 score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (VISA‐A scores): short term (6 week follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (VISA‐A score): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Function (VISA‐A scores): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Satisfied patients: medium term (6 month follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Satisfied patients: long term (1 year follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Return to desired sports: medium term (6 month follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Return to desired sports: long term (1 year follow‐up) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Function (heel‐raise index): medium term (6 month follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Function (heel‐raise index): long term (1 year follow‐up) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Complications Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

