Asid lemak omega‐3 untuk mencegah atau melambatkan proses degenerasi makular disebabkan usia
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group RCT, 2 x 2 factorial design Both eyes included in the trial, both eyes received same treatment, adjustment made for within person correlation | |
| Participants | Country: USA Setting: community Number of participants: 2080, 55% women Average age: 74 years Age range: 50 to 85 years Inclusion criteria:
Exclusion criteria:
Approximately 90% of participants were taking an additional multivitamin supplement | |
| Interventions |
Omega 3 fatty acids were DHA (350 mg per day) and EPA (650 mg per day). Composition of placebo not specified All participants were asked to take the original AREDS formulation (vitamin C 500 mg, vitamin E 400 IU, beta‐carotene 15 mg, zinc oxide 80 mg, cupric oxide 2 mg). Those who agreed to take AREDS and consented to a second randomisation were assigned as follows
The participants who did not agree to a secondary randomisation largely took the AREDS formula: omega 3 fatty acids group n = 305 (28.6%); placebo group n = 265 (26.2%) Participants who were current smokers or former smokers who had stopped smoking within the year before enrolment were randomly assigned to 1 of the 2 arms without beta‐carotene Duration: 5 years | |
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up: annually | |
| Dates participants recruited | 10/2006 to 09/2008 | |
| Declaration of interest | Yes ‐ reported in paper. Including patent for AREDS formula | |
| Sources of funding | This study was supported by the intramural program funds and contracts from the National Eye Institute (NEI), National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, Maryland (contract HHS‐N‐260‐2005‐00007‐C; ADB contract N01‐EY‐5‐0007). Funds were contributed by the following NIH institutes: Office of Dietary Supplements; National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung, and Blood Institute; and National Institute of Neurological Disorders and Stroke. The study medications and raw materials were provided by Alcon, Bausch & Lomb, DSM, and Pfizer | |
| Notes | In the primary randomisation 84% of participants took 75% of the study medications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A random block design was implemented using the AREDS2 Advantage Electronic Data Capture system by the AREDS2 Coordinating Center (The EMMES Corporation, Rockville, MD) and stratified by clinical center and AMD status (large drusen both eyes or large drusen in 1 eye and advanced AMD in the fellow eye) to ensure approximate balance across centers over time.” Page 2285 of protocol paper |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled study “Participants and study personnel were masked to treatment assignment in both randomizations.” Page E2 of main study report |
| Blinding of participants and personnel (performance bias) | Low risk | “Participants and study personnel were masked to treatment assignment in both randomizations.” Page E2 of main study report |
| Blinding of participants and personnel (performance bias) | Low risk | “Participants and study personnel were masked to treatment assignment in both randomizations.” Page E2 of main study report |
| Blinding of outcome assessment (detection bias) | Low risk | “Participants and study personnel were masked to treatment assignment in both randomizations.” Page E2 of main study report |
| Blinding of outcome assessment (detection bias) | Low risk | “Participants and study personnel were masked to treatment assignment in both randomizations.” Page E2 of main study report CNV was determined by masked readers from stereoscopic fundus photographs |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up was high and balanced across groups
|
| Selective reporting (reporting bias) | Low risk | Not detected |
| Methods | Parallel‐group RCT One eye only included, study eye was selected on the basis of early AMD with neovascular AMD (CNV) in the fellow eye | |
| Participants | Country: France Setting: community Number of participants: 300, 65% women Average age: 74 years Age range: 55 to 85 years Inclusion criteria:
Exclusion criteria:
| |
| Interventions |
Omega 3 fatty acids were 3 fish oil capsules, each capsule contained: DHA (280 mg), EPA (90 mg) and vitamin E (2 mg) (Reti‐Nat, provided by Bausch & Lomb, Montpellier, France) Placebo contained 602 mg of olive oil Duration: 3 years | |
| Outcomes | Primary outcome:
Secondary outcome:
Follow‐up: annually | |
| Dates participants recruited | 12/2003 to 10/2005 | |
| Declaration of interest | Eric H Souied: Consultant and lecturer—Laboratoire Bausch & Lomb Chauvin Cécile Delcourt: Consultant and financial support—Laboratoire Bausch & Lomb Chauvin; Consultant and financial support—Laboratoires Théa; Consultant—Novartis | |
| Sources of funding | Sponsored by Laboratoire Bausch & Lomb Chauvin, Montpellier | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "QL‐Ranclin software (Qualilab, Olivet, France) was used to generate the randomization list before enrolment." Souied et al 2013 p3 |
| Allocation concealment (selection bias) | Low risk | "The patients and the study personnel both were blinded to the treatment assignment." Souied et al 2013 p3 |
| Blinding of participants and personnel (performance bias) | Low risk | Not well reported. However, Qualilab provides an independent trial auditing service (not clear if this was the case here). No information provided regarding the outcome assessors (?study personnel), however it is likely that they remained masked as to the allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Not well reported. However, Qualilab provides an independent trial auditing service (not clear if this was the case here). No information provided regarding the outcome assessors (?study personal), however it is likely that they remained masked as to the allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Not well reported. However, Qualilab provides an independent trial auditing service (not clear if this was the case here). No information provided regarding the outcome assessors (?study personal), however it is likely that they remained masked as to the allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Not well reported. However, Qualilab provides an independent trial auditing service (not clear if this was the case here). No information provided regarding the outcome assessors (?study personal), however it is likely that they remained masked as to the allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Used a per protocol analysis. Main reason for protocol deviation was premature withdrawal which occurred at a similar rate in DHA and placebo groups. Other protocol deviations included ‘non‐compliance with study medication or use of non‐permitted medication’; 263 of the original 300 patients randomised were included in the analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary outcomes reported. All secondary outcomes (with the exception of mERG listed in trial protocol) were reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Omega 3 fatty acids combined with carotenoids. Not able to isolate the effect of omega 3 treatment | |
| A total of 106 participants with early AMD were randomised to receive a supplement containing acetyl‐L‐carnitine, co‐enzyme Q10 and omega 3 fatty acids or placebo for 12 months. The outcomes measured were change in visual field defect, visual acuity and AMD grading from baseline. This study was excluded since it was not possible to isolate the specific effects of the omega 3 treatment | |
| Omega 3 fatty acids combined with carotenoids. Not able to isolate the effect of omega 3 treatment | |
| This was a bioavailability study for the AREDS2 trial; 40 participants with AMD were randomly assigned to receive lutein and zeaxanthin, DHA/EPA or placebo for 6 months. Serum levels of lutein, zeaxanthin, DHA and EPA were measured in addition to macular pigment optical density. This study was excluded since no data on AMD outcomes were reported | |
| A total of 49 participants recruited from the general population were randomly assigned to placebo, lutein or lutein plus DHA. Supplements were taken for 4 months. The outcomes were serum levels of lutein and DHA and macular pigment optical density. This study was excluded since no data on AMD outcomes were reported | |
| The ‘Short Term Ocular Safety Assessment of High Dose Omega 3 Supplementation for Age‐Related Macular Degeneration’ study was a RCT that used multifocal ERGs to establish the safety of omega LCPUFA supplements. The trialists confirmed that no AMD outcomes were collected and safety data or quality of life data were not available | |
| The ‘Older People And n‐3 Long‐chain polyunsaturated fatty acids’ study was a randomised trial to assess the effects of oral supplementation with omega 3 LCPUFAs on cognitive decline. The effect of the supplement on visual function was also investigated in a sub‐set of participants by assessing rod photoreceptor response to light and visual‐cortical integration. Trialists confirmed that no AMD outcomes were collected and the study was therefore excluded. Reporting of minor adverse events was similar between trial arms | |
| A total of 35 participants with bilateral late AMD were randomly assigned to either receive (20 patients) or not receive (15 patients) a supplement containing vitamin E and polyunsaturated fatty acid for 60 days after photodynamic therapy. The outcomes measured were visual acuity (logMAR) and retinal metabolic function. This study was excluded since it was not possible to isolate the specific effects of the omega 3 treatment | |
| Commentary |
AMD: age‐related macular degeneration
DHA: docosahexaenoic acid
EPA: eicosapentaenoic acid
ERG: electroretinogram
LCPUFA: long‐chain polyunsaturated fatty acids
logMAR: logarithm of the Minimum Angle of Resolution
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Drusen morphology changes in non‐exudative age‐related degeneration after oral antioxidants supplementation |
| Methods | Randomised unmasked controlled trial of oral supplementation with a preparation containing antioxidants and omega 3 fatty acids for one year compared to no supplement (observation) |
| Participants | People with AREDS category 2 and 3 AMD |
| Interventions | Daily dose of a AREDS‐like supplement containing lutein (12 mg), zeaxanthin (2 mg), astaxanthin (8 mg), omega 3 fatty acids (DHA 540 mg and EPA 360 mg), vitamin C (40 mg), vitamin E (20 mg), zinc (16 mg) and copper (2 mg) |
| Outcomes | Changes in drusen morphology (volume and area) measured automatically with Topcon 3D OCT‐2000 at baseline and 12 months |
| Starting date | January 2013. Final data collection date for primary outcome measure July 2014 |
| Contact information | See ClinicalTrials.gov website for details |
| Notes |
| Trial name or title | Ancillary AMD study to the vitamin D and omega 3 trial (VITAL) |
| Methods | VITAL is a randomised, double‐masked, placebo‐controlled, 2 x 2 factorial trial of vitamin D and marine omega 3 fatty acid (eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) supplements in the primary prevention of cancer and cardiovascular disease |
| Participants | The study planned to enrol 20,000 male or female participants aged 50 years without cancer or cardiovascular disease at baseline |
| Interventions | The intervention groups received oral supplementation with vitamin D (cholecalciferol; 2000 IU) or marine omega 3 fatty acid (EPA + DHA, 1 g/d) supplements. Control groups received an inactive placebo |
| Outcomes | The AMD endpoint will be based on medical record‐confirmed self reports and retinal photographs |
| Starting date | July 2010 |
| Contact information | See ClinicalTrials.gov website for details |
| Notes |
AMD: age‐related macular degeneration
LCPUFA: long‐chain polyunsaturated fatty acids
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression of AMD Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.84, 1.10] | |
| Analysis 1.1 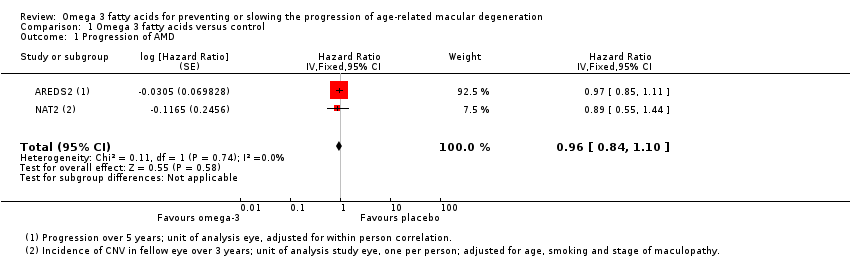 Comparison 1 Omega 3 fatty acids versus control, Outcome 1 Progression of AMD. | ||||
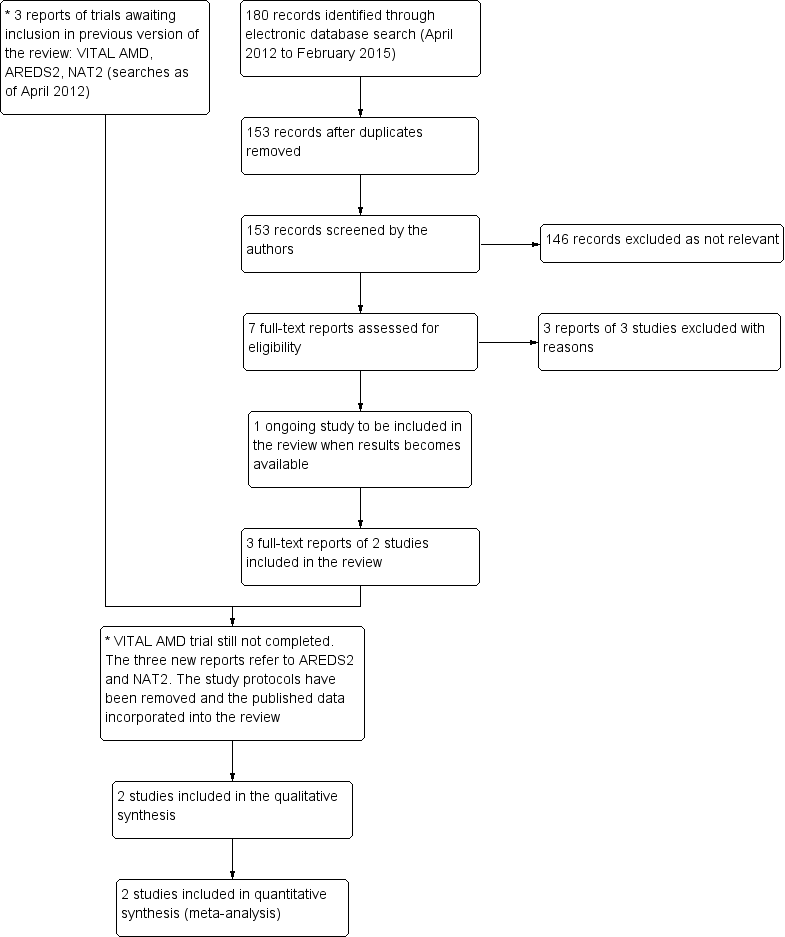
Results from searching for studies for inclusion in the review.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
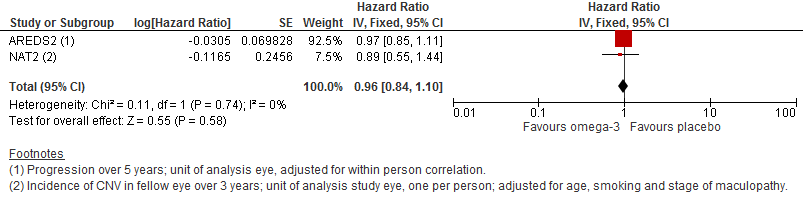
Forest plot of comparison (Analysis 1.1): 1 Omega 3 fatty acids versus control, outcome: 1.10 Progression of AMD.
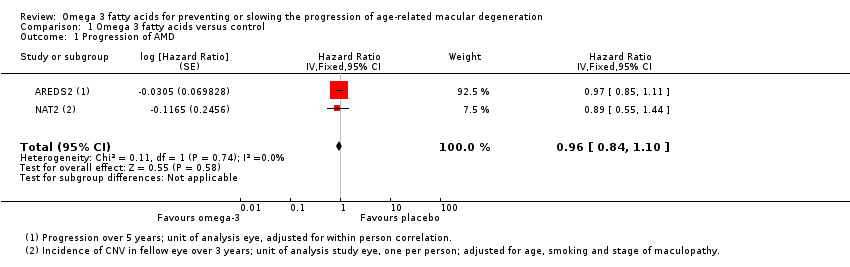
Comparison 1 Omega 3 fatty acids versus control, Outcome 1 Progression of AMD.
| Omega 3 fatty acids compared to placebo for slowing the progression of age‐related macular degeneration | ||||||
| Patient or population: people with AMD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No omega 3 fatty acids (placebo) | Omega 3 fatty acids | |||||
| Loss of 3 or more lines of VA at 24 months | 100 per 1000 | 114 per 1000 | RR 1.14, 95% CI 0.53 to 2.45 | 236 | ⊕⊕⊕⊝ | |
| Loss of 3 or more lines of VA at 36 months | 150 per 1000 | 187 per 1000 | RR 1.25, 95% CI 0.69 to 2.26) | 230 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 24 months | 100 per 1000 | 106 per 1000 | RR 1.06, 95% CI 0.47 to 2.40 | 224 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 36 months | 150 per 1000 | 168 per 1000 | RR 1.12, 95% CI 0.53 to 2.38 | 195 | ⊕⊕⊕⊝ | |
| Progression of AMD over 5 years | 300 per 1000 | 290 per 1000 | HR 0.96 | 2343 | ⊕⊕⊕⊕ | |
| Adverse effects | 500 per 1000 | 505 per 1000 | RR 1.01, 95% CI 0.94 to 1.09 | 2343 | ⊕⊕⊕⊕ | AREDS2 reported participants with one or more serious adverse events (AE). NAT‐2 reported total AE including treatment emergent and serious non‐ocular events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision | ||||||
| Adverse effects | Omega 3 N (%) | Placebo N (%) |
| AREDS 2 | ||
| Total number of participants | N = 1068 | N = 1012 |
| Participants with ≥ 1 adverse event · Cardiac disorders · Gastrointestinal disorders · Infections · Neoplasms · Nervous system disorders · Respiratory and chest disorders | 505 (47.3) 119 (11.1) 58 (5.4) 103 (9.6) 83 (7.8) 72 (6.7) 37 (3.5) | 479 (47.3) 96 (9.5) 76 (7.5) 90 (8.9) 80 (7.9) 66 (6.5) 44 (4.3) |
| NAT‐2 | ||
| Total number of participants | N = 134 | N = 129 |
| Total adverse events · Treatment emergent adverse events* · Ocular · Serious non‐ocular | 125 (83.3) 5 (4.7) 88 (58.4) 31 (23.1) | 115 (77.7) 2 (1.6) 74 (50.0) 30 (23.6) |
| * As defined by the study authors (including gastrointestinal disorders, allergic dermatitis and breath odour) | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression of AMD Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.84, 1.10] | |

