| Inclusion criteria | | | |
| Review title | Studies | Participants | Intervention | Comparison | Primary outcome measures | Date of search | No. included studies (all) | No. included studies (children only) |
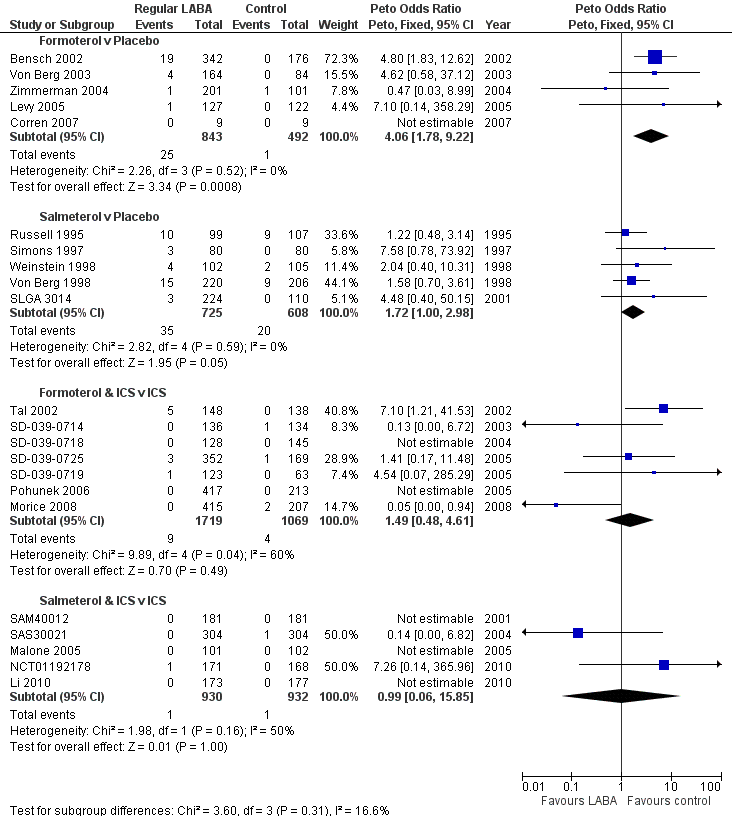
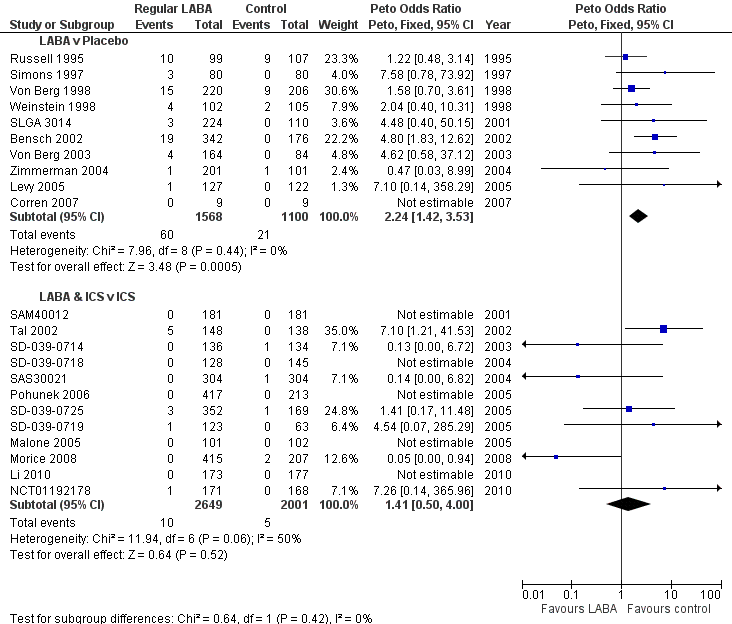
| 1. Regular treatment with formoterol for chronic asthma: serious adverse events Cates 2012a | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled formoterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or short‐acting beta2‐agonists | All‐cause mortality All‐cause non‐fatal SAEs | January 2012 | 22 | 5 |
| 2. Regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2008 | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled salmeterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or short‐acting beta2‐agonists | All‐cause mortality All‐cause non‐fatal SAEs | August 2011 | 32 | 5 |
| 3. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events Cates 2009b | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled corticosteroids and formoterol once or twice/day; at least least 12 weeks duration; any dose; any single or separate device | Same dose and type of inhaled corticosteroids | All‐cause mortality All‐cause non‐fatal SAEs | October 2008 | 21 | 7 |
| 4. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events Cates 2009a | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled corticosteroids and salmeterol once or twice/day; at least least 12 weeks duration; any dose; any single or separate device | Same dose and type of inhaled corticosteroids | All‐cause mortality All‐cause non‐fatal SAEs | October 2008 | 33 | 3 (2 additional studies identified by updated search) |
| 5. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2012b | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled formoterol; at least 12 weeks duration; not randomised with inhaled corticosteroids | Inhaled salmeterol; at least 12 weeks duration; not randomised with inhaled corticosteroids | All‐cause mortality All‐cause non‐fatal SAEs | January 2012 | 4 | 1 |
| 6. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events Cates 2010 | Randomised controlled trials | Diagnosis of asthma; any age group | Inhaled formoterol with an inhaled steroid; at least 12 weeks duration; any dose; any single or separate delivery device | Inhaled salmeterol with an inhaled steroid; at least 12 weeks duration; any dose; any single or separate delivery device | All‐cause mortality All‐cause non‐fatal SAEs | August 2011 | 7 | 0 |