抗生素预防宫颈转化区切除术后感染
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Antibiotic Prophylaxis, this term only
#2 MeSH descriptor Anti‐Infective Agents, this term only
#3 MeSH descriptor Cephalosporins explode all trees
#4 MeSH descriptor Penicillins explode all trees
#5 MeSH descriptor Macrolides explode all trees
#6 MeSH descriptor Fluoroquinolones explode all trees
#7 MeSH descriptor Sulfonamides explode all trees
#8 MeSH descriptor Tetracyclines explode all trees
#9 MeSH descriptor Aminoglycosides explode all trees
#10 MeSH descriptor Glycopeptides explode all trees
#11 MeSH descriptor Antiprotozoal Agents explode all trees
#12 (antibiotic* or antimicrob* or anti‐microb* or antibacteria* or anti‐bacteria* or antiinfect* or anti‐infect*)
#13 ((prevent* or prophyla*) near/5 (bacteria* or microb* or infect*))
#14 (cef* or ceph* or loracarbef* or penicillin* or amoxicillin or erythromycin or clarithromycin or azithromycin or metronidazole or ciprofloxacin or levofloxacin or ofloxacin or co‐trimoxazole or cotrimoxazole or trimethoprim or tetracycline or doxycycline or gentamycin or gentamicin or vancomycin or augmentin)
#15 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
#16 MeSH descriptor Uterine Cervical Neoplasms, this term only
#17 MeSH descriptor Cervical Intraepithelial Neoplasia, this term only
#18 MeSH descriptor Cervix Uteri, this term only
#19 cervi*
#20 (#16 OR #17 OR #18 OR #19)
#21 Any MeSH descriptor with qualifier: SU
#22 (surg* or excis* or laser* or conization)
#23 MeSH descriptor Gynecologic Surgical Procedures explode all trees
#24 (LEEP or LLETZ or LC or CKC)
#25 (#21 OR #22 OR #23 OR #24)
#26 (#15 AND #20 AND #25)
Appendix 2. MEDLINE search strategy
Medline (Ovid)
1 Antibiotic Prophylaxis/
2 exp Anti‐Infective Agents/
3 exp Cephalosporins/
4 exp Penicillins/
5 exp Macrolides/
6 exp Fluoroquinolones/
7 exp Sulfonamides/
8 exp Tetracyclines/
9 exp Aminoglycosides/
10 exp Glycopeptides/
11 exp Antiprotozoal Agents/
12 (antibiotic* or antimicrob* or anti‐microb* or antibacteria* or anti‐bacteria* or antiinfect* or anti‐infect*).mp.
13 ((prevent* or prophyla*) adj5 (bacteria* or microb* or infect*)).mp.
14 (cef* or ceph* or loracarbef* or penicillin* or amoxicillin or erythromycin or clarithromycin or azithromycin or metronidazole or ciprofloxacin or levofloxacin or ofloxacin or co‐trimoxazole or cotrimoxazole or trimethoprim or tetracycline or doxycycline or gentamycin or gentamicin or vancomycin or augmentin).mp.
15 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 Uterine Cervical Neoplasms/
17 Cervical Intraepithelial Neoplasia/
18 Cervix Uteri/
19 cervi*.mp.
20 16 or 17 or 18 or 19
21 surgery.fs.
22 (surg* or excis* or laser* or conization).mp.
23 exp Gynecologic Surgical Procedures/
24 (LEEP or LLETZ or LC or CKC).mp.
25 21 or 22 or 23 or 24
26 15 and 20 and 25
27 randomized controlled trial.pt.
28 controlled clinical trial.pt.
29 randomized.ab.
30 placebo.ab.
31 drug therapy.fs.
32 randomly.ab.
33 trial.ab.
34 groups.ab.
35 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
36 26 and 35
37 exp animals/ not humans.sh.
38 36 not 37
key:
mp=title, original title, abstract, name of substance word, subject heading word, unique identifier
pt=publication type
fs=floating subheading
sh=subject heading
Appendix 3. Embase search strategy
Embase (Ovid)
1 antibiotic prophylaxis/
2 exp antiinfective agent/
3 exp cephalosporin derivative/
4 exp penicillin derivative/
5 exp macrolide/
6 exp quinolone derivative/
7 exp sulfonamide/
8 exp tetracycline derivative/
9 exp aminoglycoside/
10 exp glycopeptide/
11 exp antiprotozoal agent/
12 (antibiotic* or antimicrob* or anti‐microb* or antibacteria* or anti‐bacteria* or antiinfect* or anti‐infect*).mp.
13 ((prevent* or prophyla*) adj5 (bacteria* or microb* or infect*)).mp.
14 (cef* or ceph* or loracarbef* or penicillin* or amoxicillin or erythromycin or clarithromycin or azithromycin or metronidazole or ciprofloxacin or levofloxacin or ofloxacin or co‐trimoxazole or cotrimoxazole or trimethoprim or tetracycline or doxycycline or gentamycin or gentamicin or vancomycin or augmentin).mp.
15 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
16 exp uterine cervix tumor/
17 uterine cervix carcinoma in situ/
18 exp uterine cervix/
19 cervi*.mp.
20 16 or 17 or 18 or 19
21 su.fs.
22 (surg* or excis* or laser* or conization).mp.
23 exp gynecologic surgery/
24 (LEEP or LLETZ or LC or CKC).mp.
25 21 or 22 or 23 or 24
26 15 and 20 and 25
27 crossover procedure/
28 double‐blind procedure/
29 randomized controlled trial/
30 single‐blind procedure/
31 random*.mp.
32 factorial*.mp.
33 (crossover* or cross over* or cross‐over*).mp.
34 placebo*.mp.
35 (double* adj blind*).mp.
36 (singl* adj blind*).mp.
37 assign*.mp.
38 allocat*.mp.
39 volunteer*.mp.
40 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39
41 26 and 40
42 (exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/
43 41 not 42
key:
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. LILACS search strategy
1 Antibiotic OR Anti‐bacterial agents/
2 cephalosporins
3 penicillins
4 macrolides
5 fluoroquinolones
6 sulfonamides
7 aminogylocosides
8 glycopeptides
9 antiprotozoals
10 erythromycin
11 clarithromycin
12 metronidazole
13 azithromycin
14 methronidazole
15 ciprofloxacin
16 levofloxacin
17 ofloxacin
18 trimethoprim
19 tetracycycline
20 doxycycline
21 vancomycin
22 augmentin
23 #1 or # 2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
24 infection or prevention
25 cervical intraepithelial surgery or cervix uteri surgery
26 randomized or clinical trials or controlled or trial
27 #23 and #24 and #25 and #26
Appendix 5. 'Risk of bias' assessment
Risk of bias assessment based on chapter 8 of Higgins 2011;
• Random sequence generation
i) Low risk of bias e.g. participants assigned to treatments on the basis of a computer‐generated random sequence or a table of
random numbers
ii) High risk of bias e.g. participants assigned to treatments on the basis of date of birth, clinic id‐number or surname, or no
attempt to randomise participants
iii) Unclear risk of bias e.g. not reported, information not available
• Allocation concealment
i) Low risk of bias e.g. where the allocation sequence could not be foretold
ii) High risk of bias e.g. allocation sequence could be foretold by patients, investigators or treatment providers
iii) Unclear risk of bias e.g. not reported.
• Blinding of participants and personnel
i) Low risk of bias if participants and personnel were adequately blinded
ii) High risk of bias if participants were not blinded to the intervention that the participant received
iii) Unclear risk of bias if this was not reported or unclear
• Blinding of outcomes assessors
i) Low risk of bias if outcome assessors were adequately blinded
ii) High risk of bias if outcome assessors were not blinded to the intervention that the participant received
iii) Unclear risk of bias if this was not reported or is unclear
• Incomplete outcome data: we will record the proportion of participants whose outcomes were not reported at the end of the
study. We will code a satisfactory level of loss to follow‐up for each outcome as:
i) Low risk of bias e.g.if fewer than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar
in both treatment arms
ii) High risk of bias e.g. if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed
between treatment arms
iii) Unclear risk of bias e.g. if loss to follow‐up was not reported
• Selective reporting of outcomes
i) Low risk of bias e.g. review reports all outcomes specified in the protocol
ii) High risk of bias e.g. the study is suspected that outcomes have been selectively reported
iii) Unclear risk of bias e.g. it is unclear whether outcomes have been selectively reported
• Other bias
i) Low risk of bias e.g. the review authors do not suspect any other source of bias and the trial appears to be methodologically
sound
ii) High risk of bias e.g. the review authors suspect that the trial was prone to an additional bias
iii) Unclear risk of bias e.g. the review authors are uncertain whether an additional bias may have been present

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Prolonged vaginal discharge, Outcome 1 Number of participants who experienced prolonged vaginal discharge.

Comparison 2 Excessive vaginal bleeding, Outcome 1 Number of participants who had to be admitted for postoperative bleeding.

Comparison 3 Fever, Outcome 1 Number of participants who developed fever.

Comparison 4 Lower abdominal pain, Outcome 1 Number of participants who experienced lower abdominal pain.

Comparison 5 Adverse effects, Outcome 1 Number of participants who experienced any adverse effects related to antibiotics.
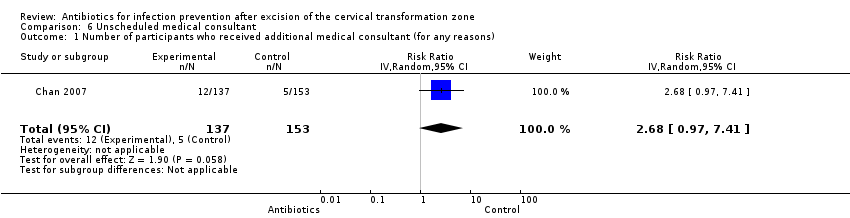
Comparison 6 Unscheduled medical consultant, Outcome 1 Number of participants who received additional medical consultant (for any reasons).

Comparison 7 Additional self‐medication, Outcome 1 Number of participants who had additional self‐medication.
| Antibiotics compared with placebo or no treatment for infection prevention after excision of the cervical transformation zone | ||||||
| Patient or population: Women undergoing excision of the cervical transformation zone for cervical neoplasia Settings: Outpatients setting, the colposcopy clinic Intervention: Prophylactic antibiotics Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| [Placebo or no treatment] | [Antibiotics] | |||||
| Number of participants who experienced prolonged vaginal discharge Follow‐up period: 2 weeks after the procedure | 103 per 1000 | 133 per 1000 | RR 1.29 (0.72 to 2.31) | 348 | ⊕⊕⊝⊝ | |
| Number of participants who had to be admitted for post‐procedure bleeding Follow‐up period: 2‐3 weeks after the procedure | 31 per 1000 | 38 per 1000 | RR 1.21 (0.52 to 2.82) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who developed fever Follow‐up period: 3 weeks after the procedure | 7 per 1000 | 16 per 1000 | RR 2.23 (0.20 to 24.36) | 290 (1 study) | ⊕⊝⊝⊝ | |
| Number of participants who experienced lower abdominal pain Follow‐up period: 3 weeks after the procedure | 163 per 1000 | 168 per 1000 | RR 1.03 (0.61 to 1.72) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who experienced any adverse effects related to antibiotics Follow‐up period: 2‐3 weeks after the procedure | 37 per 1000 | 63 per 1000 | RR 1.69 (0.85 to 3.34) | 638 (2 studies) | ⊕⊝⊝⊝ | |
| Number of participants who received additional medical consultant (for any reasons) Follow‐up period: 3 weeks after the procedure | 33 per 1000 | 88 per 1000 | RR 2.68 (0.97 to 7.41) | 290 (1 study) | ⊕⊕⊝⊝ | |
| Number of participants who had additional self‐medication Follow‐up period: 3 weeks after the procedure | 72 per 1000 | 88 per 1000 | RR 1.22 (0.56 to 2.67) | 290 (1 study) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Based on the high risk of attrition bias (high rate of incomplete participants' record charts) | ||||||
| Study | Antibiotic group | Placebo or no treatment |
| Number of participants who experienced prolonged vaginal discharge | ||
| 23/173 (13.3%) | 18/175 (10.3%) | |
| Number of participants who had to be admitted for post‐procedure bleeding | ||
| 2/173 (1.2%) | 3/175 (1.7%) | |
| 9/137 (6.6%) | 7/153 (4.6%) | |
| Number of participants who experienced lower abdominal pain | ||
| 23/137 (16.8%%) | 25/153 (16.3%) | |
| Number of participants who developed fever | ||
| 2/137 (1.5%) | 1/153 (0.7%) | |
| Number of participants who experienced adverse events | ||
| 20/173 (11.6%) | 12/175 (6.9%) | |
| 0/137 (0%) | 0/153 (0%) | |
| Number of participants who required unscheduled medical consultation | ||
| 12/137 (8.8%) | 5/153 (3.3%) | |
| Number of participants reported to have additional self‐medication | ||
| 12/137 (8.8%) | 11/153 (7.2%) |
| Outcomes reported in Gornall 1999 | Sultrin (sample size = n) | Control (sample size = 77‐n) | 95% confidence interval | P value |
| Mean duration of bleeding (days) | 15.2 | 11.2 | ‐7.7 to ‐0.2 | 0.04 |
| Mean duration of discharge (days) | 16.4 | 13.1 | ‐7.2 to 0.7 | 0.11 |
| Mean duration of pain (days) | 7.7 | 5.7 | ‐5.4 to 1.5 | 0.26 |
| Number of participants who received additional antibiotic therapy | 2 | 7 | Not reported | Not reported |
| Number of participants who had to be admitted for postoperative bleeding | 0 | 2 | Not reported | Not reported |
| Numbers of the participant in each comparison group were not reported. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced prolonged vaginal discharge Show forest plot | 1 | 348 | Risk Ratio (IV, Random, 95% CI) | 1.29 [0.72, 2.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had to be admitted for postoperative bleeding Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.21 [0.52, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who developed fever Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.23 [0.20, 24.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced lower abdominal pain Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.61, 1.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who experienced any adverse effects related to antibiotics Show forest plot | 2 | 638 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.85, 3.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who received additional medical consultant (for any reasons) Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 2.68 [0.97, 7.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who had additional self‐medication Show forest plot | 1 | 290 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.56, 2.67] |

