Терапия упражнениями при усталости у больных рассеянным склерозом
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: random assignment to aerobic treadmill training (AT), yoga (Y) or a wait‐list control (NEX) Setting: physiotherapy clinic, Iran Categorization: AT = endurance, Y = other | |
| Participants | n = 31; AT = 10, Y = 11, NEX = 10 Inclusion criteria: physician diagnosis MS, self reported EDSS 1 >< 4.0, able to walk a treadmill > 5 minutes at stable speed, no physical activity in the last 3 months Exclusion criteria: cardiovascular disease, liver or kidney failure, lung disease, diabetes, thyroid disorder, gout or orthopaedic limitations, pregnant, addicted (i.e. smoking) Type MS: ? Disease duration (yr) ± SD: AT = 5.6 ± 3.3, Y = 4.7 ± 5.6, NEX = 5.0 ± 3.1 Mean age (yr) ± SD: AT = 36.8 ± 9.2, Y = 32.3 ± 8.7, NEX = 36.7 ± 9.3 % Female: 100% Mean EDSS ± SD: AT = 2.4 ± 1.2, Y = 2.0 ± 1.1, NEX = 2.3 ± 1.3 | |
| Interventions | AT: 8 weeks, 3x per week, 30 minutes physiotherapist‐led treadmill training at 40‐75% of predicted maximal heart rate + 20 minutes of stretching. Comfortable walking speed as starting point, progression directed by participant Y: 8 weeks, 3x per week, 60‐70 minutes Hatha yoga led by a physiotherapist and neurologist. Hatha yoga included stretching, postures, breathing, and meditation NEX: wait list control | |
| Outcomes | FSS, BDI, BAI, BBS, 10MWT, 2MWT (walking speed, walking endurance) | |
| Notes | Drop‐outs No drop‐outs reported Measurements Baseline, 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to supervised hospital‐based callisthenic exercise (EX) OR home‐based callisthenic exercise (Home‐EX) Setting: outpatient clinic, Iran Categorization: EX = muscle power, Home‐EX = muscle power | |
| Participants | n = 40; EX = 20, Home‐EX = 20 Inclusion criteria: RRMS, EDSS < 4.5, aged 18‐50 years Exclusion criteria: acute exacerbation; Ashworth spasticity score > 2.0; thyroid disorder; history of serious psychiatric disorders, alcohol abuse, or other chronic diseases Type MS: RRMS Disease duration (yr) ± SD: EX = 6.43 ± 2.78, Home‐EX = 7.40 ± 3.43 Mean age (yr) ± SD: EX = 32.62 ± 3.15, Home‐EX = 33.00 ± 4.06 % Female (n/n group): EX = 9/16, Home‐EX = 11/20 Mean EDSS ± SD: EX = 3.6 ± 1.3, Home‐EX = 3.4 ± 2.1 | |
| Interventions | EX: 12 weeks, 5x per week, 60 minutes hospital‐based exercise supervised by a physiatrist. 3 out 5 session/week were callisthenic exercise (60 minutes), 2 out of 5 sessions were relaxation exercises (20 minutes). Callisthenic exercises were focused on the large muscles and were applied rhythmically and in combination with breathing exercises. The callisthenic training sessions consisted of 15 minutes' warming up, 20 minutes' intensive training, 10 minutes' cooling down, and 15 minutes' relaxation Home‐EX: same as EX yet participants were asked to perform the exercise at home and their exercise schedule was followed by telephone every day | |
| Outcomes | FSS, 10MWT, BBS, MusiQoL, HADS | |
| Notes | Drop‐outs EX: 2 failure to adapt to exercise, 2 transportation problems Home‐EX: no drop‐outs Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Concealed allocation procedure |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out rate of 10% in the exercise condition, 0 drop‐outs reported in the control condition |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to Ergometer Land Group (ELG) or Ergometer Water Group (EWG) Setting: Department of Sports Therapy, Rehabilitationsklinik Valens, Valens, Switzerland Categorization: ELG = endurance, EWG = endurance | |
| Participants | n = 60; ELG = 30, EWG = 30 Inclusion criteria: EDSS 1.0‐6.5 Exclusion criteria: incontinent, persistent infections, cardiovascular and pulmonary diseases, or immunosuppressive therapy Type MS: ? Disease duration (yr) ± SD: ? Mean age (yr) ± SD: ELG = 52 ± 14.2, EWG = 50 ± 14.2 % Female (n/n group): ELG = 18/28, EWG = 17/25 Mean EDSS ± SD: ELG = 4.7 ± 1.5, EWG = 4.6 ± 1.4 | |
| Interventions | ELG: 3 weeks, 5x per week; 30‐minute physiotherapist‐led overland ergometry training at 60% of peak oxygen uptake/70% maximal heart rate EWG: 3 weeks, 5x per week; 30‐minute physiotherapist‐led aquatic training at 70% maximal heart rate corrected for water temperature (‐7 beats per minute). Water temperature at 28°C | |
| Outcomes | FSMC, neurotrophin, cytokines | |
| Notes | Drop‐outs ELG: 2 immunosuppressive medication during training EWG: 3 immunosuppressive medication during training, 3 unable to comply with daily time schedule Measurements Baseline, 3 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | Unclear risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate < 15%, without indications of differences in reasons |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to Nintendo® Wii® balance training (EX) or control (NEX) Setting: Italian Multiple Sclerosis Foundation, Genoa, Italy Categorization: EX = other | |
| Participants | n = 36; EX = 18, NEX = 18 Inclusion criteria: no relapse < 6 months, EDSS ≤ 6.0, Ambulation Index ≤ 4 Exclusion criteria: psychiatric disorder, blurred vision, severe cognitive impairment Type MS: ? Disease duration (yr) ± SD: EX = 11.2 ± 6.4, NEX = 12.3 ± 7.2 Mean age (yr) ± SD: EX = 40.7 ± 11.5, NEX = 43.2 ± 10.6 % Female (n/n group): EX = 10/18, NEX = 12/18 Mean EDSS ± SD: EX = 3.9 ± 1.6, NEX = 4.3 ± 1.6 | |
| Interventions | EX: 4 weeks, 3x per week; 1 hour Nintendo® Wii® balance board training. Special focus on soccer heading, skiing, table tilt, snowboarding, tight rope walking, and zazen (relaxation) NEX: 4 weeks, 3x per week; 1 hour static and dynamic lower body exercise using single and double leg stances with or without an equilibrium board | |
| Outcomes | MFIS, BBS, open and closed eye stabilometry | |
| Notes | Drop‐outs No drop‐outs reported Measurements Baseline, 4 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Biased‐coin randomization procedure |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to arm‐cycling (ARM), rowing (ROW), bicycling (BIC), and a control (NEX) Setting: MS outpatient clinic, Germany Categorization: ARM = endurance, ROW = endurance, BIC = endurance | |
| Participants | n = 47; ARM = 12, ROW = 12, BIC = 12, NEX = 11 Inclusion criteria: definite secondary‐progressive or primary‐progressive MS, EDSS 4.0‐6.0 Exclusion criteria: contraindications for exercise, start immunomodulatory treatment < 6 months, steroid treatment < 4 weeks, MS relapse < 12 months, abnormal liver or kidney function, immunodeficiency, other serious medical illness or psychiatric, developmental or neurological disorder Type MS: SPMS and PPMS Disease duration (yr) ± SD: ARM = 17.1 ± 7.2, ROW = 14.1 ± 6.1, BIC = 13.3 ± 5.4, NEX = 18.9 ± 9.8 Mean age (yr) ± SD: ARM = 49.1 ± 8.5, ROW = 50.9 ± 9.2, BIC = 48.8 ± 6.8, NEX = 50.4 ± 7.6 % Female (n/n group): ARM = 5/10, ROW = 7/11 , BIC = 6/11 , NEX = 6/10 Mean EDSS ± SD: ARM = 5.2 ± 0.9, ROW = 4.7 ± 0.8, BIC = 5.0 ± 0.8, NEX = 4.9 ± 0.9 | |
| Interventions | ARM, ROW, and BIC: 8‐10 weeks, 2 or 3 times per week, 15‐45 minutes aerobic training led by licensed physiotherapists at the individual determined aerobic threshold (AT), 120%AT and 130%AT. Aim was to perform 20 training sessions in which the time spent at the higher levels was increased. Only the mode of exercise (i.e. ARM, ROW, or BIC) different between groups NEX: wait list control | |
| Outcomes | MFIS, VO2peak, 6MWT, SDMT, VLMT, TAP, LPS, RWT, IDS ‐ SR30 | |
| Notes | Drop‐outs ARM: 1, logistics + 1 did not receive allocated intervention ROW: 1, unrelated injury BIC: 1, fatigued NEX: 1, lost to follow‐up Measurements Baseline, 8‐10 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated biased coin procedure |
| Allocation concealment (selection bias) | Low risk | Randomization after determining eligibility |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 10%, equally balanced across groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: Random assignment to Tai Chi (EX) or treatment as usual (NEX) Setting: Department of neurology, Bayreuth, Germany Categorization: EX = other | |
| Participants | n = 38; EX = 21, NEX = 17 Inclusion criteria: diagnosis of any type of MS, able to walk without a walking aid, EDSS < 5, relapse‐free < 4 weeks Exclusion criteria: severe cognitive impairment Type MS: RRMS, SPMS, and clinically isolated syndrome (n = 1) Disease duration (yr) ± SD: EX = 6.0 ± 4.7, NEX = 7.8 ± 6.8 Mean age (yr) ± SD: EX = 42.6 ± 9.4, NEX = 43.6 ± 8.0 %Female (n/n group): EX = 10/15, NEX = 12/17 Median EDSS (range): EX = 2 (1‐4), NEX = 4 (1‐4.5) | |
| Interventions | EX: 6 months, 1x per week, 90 minutes of centre‐based, structured, compact Tai Chi. The Tai Chi programme was based on the Yang‐style 10‐form. Exercises were structured so that during each session of the course, the same essential elements were repeated NEX: treatment as usual | |
| Outcomes | FSMC, balance, co‐ordination, CES‐D, QLS | |
| Notes | Drop‐outs EX: 5 time constraints, 1 health problems Measurements Baseline, 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assignment to EX or NEX was based on participants' weekday preference |
| Allocation concealment (selection bias) | High risk | Unconcealed allocation |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Drop‐out rate in EX group was 29%, no drop‐outs reported in the control condition. In the EX group, adherence varied between 15 and 44 out of 50 classes offered |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Unclear risk | There was an apparent (yet non‐significant) difference in the disease severity and baseline fatigue scores between the EX and NEX group |
| Methods | Design: random assignment to progressive resistance training group (PRT), home‐based resistance training (EX), or control (NEX) Setting: rehabilitation clinic, Ankara, Turkey Categorization: PRT = muscle power, EX = muscle power | |
| Participants | n = 45; PRT = 15, EX = 15, NEX = 15 Inclusion criteria: definite RRMS or SPMS, EDSS ≤ 6.0 stand upright > 4 seconds, no steroid or immunosuppressive therapy (or both) < 1 month Exclusion criteria: severe MS, acute exacerbation, active physical therapy or regular exercise training programme < 1 month, unable to cycle a static bike, visual involvement or diplopia, high level of spasticity, persistent severe fatigue or depression Type MS: RRMS or SPMS Disease duration (yr) ± SD: PRT = 9.2 ± 5.0, EX = 6.2 ± 2.2, NEX = 6.6 ± 2.4 Mean age (yr) ± SD: PRT = 36.4 ± 10.5, EX = 43.0 ± 10.2, NEX = 35.5 ± 10.9 %Female (n/n group): PRT = 9/14, EX = 8/10, NEX = 6/9 Mean EDSS ± SD: ? | |
| Interventions | PRT: 2x per week for 8 weeks; 30 minutes' cycling progressive resistance training, 5 minutes' walking/stretching and 20‐25 minutes' balance exercises supervised by a physiatrist EX: 2x per week for 8 weeks; home‐based exercise programme consisting of 5 minutes' walking/stretching and 20‐25 minutes' balance exercises NEX: wait list | |
| Outcomes | FSS, number of relapses, duration of exercise, Wmax, TUG, DGI, FR, FES, 10MWT, BDI, SF‐36 | |
| Notes | Drop‐outs PRT: 1 acute exacerbation EX : 2 work‐related, 2 acute exacerbation, 1 unknown NEX: 3 acute exacerbation, 3 unknown Measurements Baseline, 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were used |
| Allocation concealment (selection bias) | Unclear risk | Unclear who performed the randomization procedure |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate was 27%, with a relative higher drop‐out rate in the control (40%) versus the exercise group (combined 20%). However, no indications for differences in reasons between groups |
| Selective reporting (reporting bias) | Low risk | All introduced outcomes were reported |
| Other bias | High risk | The PRT intervention included group‐based components |
| Methods | Design: random assignment to pragmatic exercise intervention + usual care (EX) or usual care (NEX) Setting: MS clinic, UK Categorization: EX = mixed | |
| Participants | n = 120; EX = 60, NEX = 60 Inclusion criteria: clinical diagnosis MS, EDSS 1‐6.5, able to walk 10 metres, aged 18‐65 years, clinically stable, and stable medical treatment < 3 months Exclusion criteria: structured exercise ≥ 3x per week, ≥ 30 minutes; living ≥ 20 minutes travelling from study centre, co‐morbid conditions precluding exercise participation Type MS: RRMS, SPMS, and PPMS Disease duration (yr) ± SD: EX = 8.4 ± 7.4, NEX = 9.2 ± 7.9 Mean age (yr) ± SD: EX = 45.7 ± 9.1, NEX = 46.8 ± 8.4 % Female (n/n group): EX = 43/60, NEX = 43/60 Mean EDSS ± SD: EX = 3.8 ± 1.5, NEX = 3.8 ± 1.5 | |
| Interventions | EX: 12 weeks, 3x per week; partly home based with more supervised sessions in the first 6 weeks of the intervention and vice‐versa in the second 6 weeks. Short bouts (5 x 3 minutes) of low to moderate intensity aerobic exercise at 50‐69% of predicted maximal heart rate or 12‐14 on the BORG scale. Participants were encouraged during the intervention period to increase the duration of the bouts or reduce the resting time in between bouts. When appropriate, participants could also perform 6 different resistance exercises using body resistance, light weights, or Therabands. Generally, 1‐3 sets of 20 repetitions based on the participant's level of disability, strength, and stage of the programme. In case of balance or control problems, also balance board and exercise ball work was included. The supervised exercise sessions included also cognitive behavioural techniques to promote long‐term physical activity behaviour NEX: usual care | |
| Outcomes | MFIS, GLTEQ, Accelerometer, MSQoL‐54, MSFC, 6MWT, EDSS | |
| Notes | Drop‐outs EX: 2 relapse, 2 ill health, 1 poor adherence, 6 unable to contact NEX: 1 relapse, 2 ill health, 1 work commitments, 1 no reason give, 5 unable to contact Measurements Baseline, 12 weeks, 6 months' follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Distant randomization service; minimization according to gender and EDSS. Allocation not disclosed until after baseline measurement |
| Allocation concealment (selection bias) | Low risk | Independent and off‐site randomization service |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rate in the combined exercise group was 17%, in the control group 18%. No indication for differences in reasons |
| Selective reporting (reporting bias) | Low risk | Study protocol published |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to Ai‐Chi aquatic exercise programme (EX) or control group (NEX) Setting: Multiple Sclerosis Association of Almeria, Almeria, Spain Categorization: EX = other | |
| Participants | n = 73; EX = 36, NEX = 37 Inclusion criteria: diagnosis MS, aged 18‐75 years, VAS pain score > 4 for at least two months, EDSS ≤ 7.5 Exclusion criteria: treatment with other complementary and alternative medicine ≤ 3 months, relapse requiring hospitalization or steroid treatment ≤ 2 months Type MS: ? Disease duration (yr) ± SD: EX = 10.7 ± 9.1, NEX = 11.9 ± 8.7 Age (yr): EX = 46 ± 9.97, NEX = 50 ± 12.31 % Female (n/n group): EX = 26/36, NEX = 24/37 Mean EDSS ± SD: EX = 6.3 ± 0.8, NEX = 5.9 ± 0.9 | |
| Interventions | EX: Ai‐Chi exercise programme (20 weeks, 2x per week, 60 minutes per session) in shoulder‐depth 36°C water; consisting of 16 different slow and broad movements of the arms, legs, and torso to work on balance, strength, relaxation, flexibility, and breathing. Each physiotherapist‐led session began and ended with 10 minutes of relaxation. During the course of each session relaxing tai‐chi music was played NEX: 20 weeks, 2x per week, 60 minutes per session led by a physiotherapist identical to the 10‐minute relaxation (breathing, contraction ‐ relaxation) periods of the intervention group yet in a therapy room (26°C), on an exercise mat without ambient music | |
| Outcomes | FSS, MFIS, number of relapses, pain (VAS), MPQ‐PRI, MPQ‐PPI, RMDQ, spasm (VAS), MSIS‐29, BDI, BI | |
| Notes | Drop‐outs NEX: 2 relapsed Measurements Baseline, 20 weeks, 24 weeks, 30 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned by a blinded researcher |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rate was 3% |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Unclear risk | Large proportion of participants with unknown disease course despite 'a definite diagnosis of MS' as inclusion criterion |
| Methods | Design: random assignment to continuous (CON), intermittent (INT), or mixed endurance training (CB) Setting: Oxford Brookes University and at 4 community leisure centres, London, UK Categorization: CON = endurance, INT = endurance, CB = endurance | |
| Participants | n = 61; CON = 20, INT = 18, CB = 20 Inclusion criteria: none Exclusion criteria: medical condition precluding safe exercise, unable to walk 2 minutes, unable to sit 60 seconds on cycling ergometer and pedal 60 seconds with no resistance Type MS: RRMS, SPMS, PPMS and unknown Disease duration (yr) ± SD: CON = 15 ± 8, INT = 11 ± 7, CB = 12 ± 11 Mean age (yr) ± SD: CON = 52 ± 8, INT = 50 ± 10, CB = 55 ± 10 % Female (n/n group): CON = 16/20, INT = 14/18, CB = 9/17 Mean EDSS ± SD: ? | |
| Interventions | CON: 12 weeks, 2x per week; 20 minutes supervised continuous endurance training at 45% of maximal power INT: 12 weeks, 2x per week; 20 minutes of supervised intermittent endurance training consisting of 30 seconds at 90% maximal power, 30 seconds rest CB: 12 weeks, 2x per week; 20 minutes of supervised intermittent (10 minutes) endurance training followed by continuous endurance training (10 minutes) | |
| Outcomes | FSS, 2MWT, TUG, leg extensor power, BI, SF‐36 | |
| Notes | Drop‐outs CON: no drop‐outs INT: 1 loss of consciousness during intervention, 1 exacerbation of symptoms, 1 knee pain during cycling, 1 leg pain during cycling CB: 1 exacerbation of knee injury, 1 leg pain during cycling Measurements Baseline, 6 weeks, 12 weeks (post‐intervention), 24 weeks (follow‐up) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | Central randomization by statistician |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate 10%, unequally balanced in favour of the continuous exercise protocol versus the intermittent and combined protocol |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to progressive resistance training (PRT) or progressive resistance training augmented by neuromuscular electrical stimulation (NMES) Setting: MS Society, Ireland Categorization: PRT = muscle power, NMES = muscle power | |
| Participants | n = 37; PRT = 18, NMES = 19 Inclusion criteria: definite MS, use of walking aid most of the time, and could walk at least 10 metres unaided Exclusion criteria: contraindications to electrical stimulation, had participated in an exercise programme < 1 month, relapse or steroid treatment < 3 months Type MS: RRMS, PPMS, SPMS, benign, unknown Disease duration (yr) ± SD: PRT = 12.2 ± 4, NMES = 11.8 ± 5.5 Mean age (yr) ± SD: PRT = 51.8 ± 12.1, NMES = 51.8 ± 12.6 % Female (n/n group): PRT = 6/10, NMES = 11/15 Mean EDSS ± SD: ? | |
| Interventions | PRT: 12 weeks, 2x per week for weeks 1‐6, 3x per week for week 7‐12. The programme consisted of 6 lower limb exercises performed in the home environment. Participants progressed from 1 set of 12 repetitions to 3 sets of 12 repetitions. Once 3 sets of 12 repetitions could be completed, free‐weights in the hands, around the ankle, or in a backpack were added in increments of 0.5 or 1 kg as advised during weekly telephone calls. Rest period of 2‐3 minutes between sets were advised NMES: participants in the NMES group followed the same PRT programme while wearing The Kneehab®. The Kneehab® is a synthetic garment that consists of 4 electrodes strategically placed to activate the quadriceps muscle through a novel Multipath® system. It is placed on the thigh and attached using Velcro fastenings. The pre‐set programme parameters used were a frequency of 50 Hz, on/off time 5/10 seconds, ramp up/down of 1/0.5 seconds. Participants were encouraged to use the highest tolerable intensity | |
| Outcomes | MFIS, muscle strength and endurance, VAS lower limb spasticity, TUG, MSWS‐12, BBS, MSIS29v2 | |
| Notes | Drop‐outs PRT: 3 relapse, 2 musculoskeletal injury, 2 MS‐related fatigue, 1 non‐compliance NMES: 2 muscle spasm with device use, 1 non‐compliance, 1 medical problem Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 60 randomizations were generated by repeatedly drawing cards from a pool of 3 PRT and 3 NMES cards |
| Allocation concealment (selection bias) | Low risk | 'The sequence of allocation was concealed from all study personnel' |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate was 32%, with indications for differences in reasons |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other bias identified |
| Methods | Design: random assignment to progressive resistance training (EX) or control (NEX) stratified by gender Setting: outpatients MS Clinic, Aarhus University Hospital, Aarhus, Denmark Categorization: EX = muscle power | |
| Participants | n = 38; EX = 19, NEX = 19 Inclusion criteria: definite RRMS according to McDonald criteria, EDSS 3.0 ≥ ≤ 5.5; pyramid function score ≥ 2.0, ability to walk ≥ 100 metres, no help with transportation to training facility, aged > 18 years, acceptation of diagnosis and treatment Exclusion criteria: serious medical co‐morbidities, MS attack < 2 months, pregnant, systematic resistance training < 3 months Type MS: RRMS Disease duration (yr) ± SD: EX = 6.6 ± 5.9, NEX = 8.1 ± 6.0 Mean age (yr) ± SD: EX = 47.7 ± 10.4 NEX = 49.1 ± 8.4 % Female (n/n group): EX = 10/15, NEX = 10/16 Mean EDSS ± SD: EX = 3.7 ± 0.9, NEX = 3.9 ± 0.9 | |
| Interventions | EX: progressive resistance training (PRT) programme (12 weeks, 2x per week). 5 minutes warm‐up on stationary bike followed by leg press, knee extension, hip flexion, hamstring curl, and hip extension exercises. Progression was achieved by decreasing the repetition maximum (15RM ‐ 8RM) and varying the number of sets (3 or 4) and repetitions (8‐12). Between sets and exercises 2‐3 minutes of rest was allowed. All sessions were supervised and in groups of 2‐4 participants NEX: no training; training programme offered at completion of trial | |
| Outcomes | FSS, MFI‐20 (sub‐scales), MDI, SF‐36 (PCS, MCS), muscle strength, functional capacity | |
| Notes | Drop‐outs EX: 1 lower back problems, 1 sick relative, 1 personal problems, 1 lack of time NEX: 1 personal problems, 1 broken arm, 1 problems with psoriasis Measurements Baseline, 12 weeks, 24 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation provided |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 18%, equally balanced across the exercise group (21%) and control group (16%). No indication for differences in reasons or reasons related to the exercise condition. Adherence to exercise regimen > 80% |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Unclear risk | Imbalance in attention between NEX and EX |
| Methods | Design: random assignment to endurance training (EX) and control treatment (NEX) Setting: inpatient rehabilitation unit, Schmieder Konstanz clinic, Konstanz, Germany Categorization: EX = endurance | |
| Participants | n = 30; EX = 16 (see drop‐outs), NEX = 15 Inclusion criteria: mild to moderate MS (EDSS < 4.5), complained of fatigue, walking distance < 2500 metres Exclusion criteria: serious leg weakness (degree of paresis < 4), ataxia at rest, spasticity at rest, relapse < 3 months, corticosteroid treatment < 3 months, prominent cognitive deficits, major depression, insufficient motivation for additional training Type MS: RRMS, SPMS, PPMS Disease duration (yr) ± SD: EX = 8.0 ± 5.9, NEX = 6.1 ± 4.3 Mean age (yr) ± SD: EX = 45.8 ± 7.9, NEX = 39.7 ± 9.1 % Female (n/n group): EX = 10/15, NEX = 11/15 Mean EDSS ± SD: EX = 2.6 ± 1.2, NEX = 2.8 ± 0.7 | |
| Interventions | EX: 3 weeks, 3x per week, 45 minutes per session. Warming up, mild strength training, repetitive endurance exercise followed by relaxation and feedback. All sessions were supervised and playful elements were introduced to camouflage training difficulties NEX: 3 weeks, 3x per week, 45 minutes per session. Warming up, sensory training, stretching, balance, co‐ordination training, and periods of relaxation | |
| Outcomes | MFIS, FSMC, maximal walking distance, relative walking ability, BDI, HAQUAMS | |
| Notes | Drop‐outs EX: 1 training too demanding; replaced by a newly recruited 16th participant Measurements Baseline, 3 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random order list |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | 6% drop‐out rate in the exercise condition. However, only 19 of 30 forms completed at discharge (63%) |
| Selective reporting (reporting bias) | High risk | No point and variance measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to group progressive resistance training programme (EX) or usual care in combination with a social programme (NEX); stratified by ambulation index score Setting: MS outpatient clinic, Victoria, Australia Categorization: EX = muscle power | |
| Participants | n = 76; EX = 39, NEX = 37 Inclusion criteria: aged ≥ 18 years, confirmed RRMS, Ambulation index score of 2, 3, or 4, medical clearance to participate Exclusion criteria: acute exacerbation < 2 months, benign or progressive/relapsing types of MS, serious unstable medical condition, any concurrent condition, progressive resistance training < 6 months Type MS: RRMS Disease duration (yr) ± SD: ? Mean age (yr) ± SD: EX = 47.7 ± 10.8, NEX = 50.4 ± 9.6 % Female (n/n group): EX = 26/36, NEX = 26/35 Mean EDSS ± SD: ? | |
| Interventions | EX: group PRT programme (10 weeks, 2x per week). Exercise targeted the key lower limb muscles for supporting body weight and for generating and absorbing power during walking (leg press, knee extension, calf raise, leg curl, and reverse leg press). Training intensity was 10‐12 repetitions at 10‐12 repetition maximum. Load was increased if 2 sets of 12 repetitions were completed. 2 minutes rest between each set NEX: usual care + social programme 1 hour per week | |
| Outcomes | MFIS, MSSS‐88, adverse events, number of relapses, 2MWT, 1RM seated leg press, 1RM reversed leg press, WHOQoL‐BREF | |
| Notes | Drop‐outs EX: 3 withdrew after allocation (2 experienced relapse but did attend assessment) NEX: 2 withdrew after allocation, 3 experienced relapse, 1 did not attend at follow‐up Measurements Baseline, 10 weeks, 22 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Separate randomization procedure for each stratum using permuted blocks |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes made by research co‐ordinator |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate 12%. Not equally balanced across the exercise (8%) and control group (16%); however, no indication for differences in reasons taken into account the 2 participants who did have a relapse but attended the assessment |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: internet‐based home training (IBT) versus hippotherapy (HT) Setting: regional MS groups, Germany Categorization: IBT = other, HT = other | |
| Participants | n = 18; IBT = 9, HT = 9 Inclusion criteria: definite MS, EDSS 2‐6, aged 18‐60 years, clinically stable < 4 weeks Exclusion criteria: internal or orthopaedic diseases unrelated to MS, allergy or aversion to horses, previous hippotherapy post‐diagnosis Type MS: RRMS and SPMS Disease duration (yr) ± SD: IBT = 16.1 ± 11.3, HT = 22.3 ± 8.3 Mean age (yr) ± SD: IBT = 44.3 ± 8.1, HT = 46.9 ± 7.6 % Female (n/n group): IBT = 7/9, HT = 8/9 Mean EDSS ± SD: IBT = 3.8 ± 1.5, HT = 3.8 ± 1.1 | |
| Interventions | IBT: 12 weeks, 2x per week, 45 minutes' balance and strength e‐training; exercise performed on unstable surface in eyes open or closed condition. 5‐8 different exercise, 2‐3 sets, 8‐15 repetitions. BORG 11‐14. Perceived exertion monitored by physical therapist HT: 12 weeks, 12x per week, 30 minutes' balance exercises by means of horse riding exercises and riding patterns/movements | |
| Outcomes | MFIS, FSS, BBS, DGI, isometric muscle strength, TUG, 2MWT, HAQUAMS | |
| Notes | Drop‐outs IBT: 1 unable to work with computer HT: 1 MS relapse Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing lots |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate 11% equally balanced across groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to home‐based inspiratory muscle training (EX) or control (NEX) Setting: Physical Therapy Department, University of Michigan‐Flint, Flint, Michigan, USA Categorization: EX = other | |
| Participants | n = 46; EX = 23, NEX = 23 Inclusion criteria: ambulatory, aged ≥ 18 years Exclusion criteria: acute respiratory infection, oral temperature > 100°F (37.8°C), smoking, cardiac of musculoskeletal condition unrelated to MS Type MS: RRMS, SPMS, PPMS or primary relapsing MS Disease duration (yr): ? Mean age (yr) ± SD: EX = 50 ± 9.1, NEX = 46.2 ± 9.4 % Female (n/n group): EX = 21/23, NEX = 17/23 Mean EDSS ± SD: EX = 3.96 ± 1.80, NEX = 3.36 ± 1.47 | |
| Interventions | EX: 10 weeks, daily; 3x 15 repetitions of home‐based Inspiratory muscle training using a Threshold Inspiratory Muscle Trainer. Resistance was adjusted to match a perceived exertion of 15, 16 on the BORG scale NEX: no intervention; informative telephone call 4, 8, and 10 weeks | |
| Outcomes | FSS, 6MWT, PCI, RPE | |
| Notes | Drop‐outs 2 due to illness unrelated to a neurological exacerbation, 3 no longer wished to participate Measurements Baseline, 10 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized by date of enrolment |
| Allocation concealment (selection bias) | High risk | Researcher could foresee date of enrolment |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall drop‐out rate 11%. No information provided on the distribution between groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to robot‐assisted gait training (RAGT) or sensory integration balance training (SIBT) Setting: outpatient clinic, Neurological Rehabilitation Unit, Italy Categorization: RAGT = other, SIBT = other | |
| Participants | n = 26; RAGT = 14, SIBT = 12 Inclusion criteria: aged 30‐60 years, EDSS 1.5‐6.5, MMSE > 23, ability to maintain standing position without aids for at least 1 minute and ability to walk independently for at least 15 metres, absence of concomitant neurological or orthopaedic conditions that may interfere with ambulation Exclusion criteria: any rehabilitation treatment < 1 month, MS relapse < 3 months, pharmacological treatment not well defined or changed during study, presence of paroxysmal vertigo, lower limb botulinum toxin injections < 12 weeks Type MS: RRMS or SPMS Disease duration (yr): RAGT = 13.5 ± 7.6, SIBT = 14.9 ± 8.68 Mean age (yr) ± SD: RAGT = 50.83 ± 8.42, SIBT = 50.1 ± 6.29 % Female (n/n group): RAGT = 7/12, SIBT = 9/10 Mean EDSS ± SD: RAGT = 3.96 ± 0.75, SIBT = 4.35 ± 0.67 | |
| Interventions | RAGT: 6 weeks, 2x per week, 50‐minute individual sessions of RAGT. The RAGT group was treated by means of the electromechanical Gati Trainer GT1 (Reha‐stim, Berlin, Germany). Participants received 40 minutes of RAGT followed by 10 passive lower limb joint mobilizations and stretching exercises. Each training consisted of 2 x 15‐minute sessions separated by 5 minutes of rest if required. The first session was performed at 20% supported body‐weight and 1.3 km/hour speed; the second sessions at 10% supported body weight and 1.6 km/hour speed SIBT: 6 weeks, 2x per week, 50‐minute individual sensory integration balance training. Each session consisted of exercises fitting to 3 different levels of difficulty and repeated under 3 different sensory conditions (free vision, wearing a mask, and wearing a helmet). Level I included tasks that induced external destabilization of the centre‐of‐body mass (CoP). Level II included exercises of self destabilization of the CoP. Level III consisted of exercises of external destabilization and exercise of self destabilization of CoP while standing on different types of compliant surfaces. During each treatment session, a total of 10 exercises (3 from level I, 3 from level II and 4 from level III) were repeated 2‐5 times with a 5‐minute period | |
| Outcomes | FSS, spatiotemporal gait parameters, BBS, SOT, stabilometric assessment, MSQoL‐54, ABC | |
| Notes | Drop‐outs RAGT: 2 difficulties in transportation SIBT: 2 medical complications Measurements Baseline, 6 weeks, 1‐month follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Simple software‐generated randomisation scheme' |
| Allocation concealment (selection bias) | Low risk | Randomization by an independent blinded collaborator not involved in the treatment or care of participants |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate 15.4% equally balanced across groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to physiotherapists‐led exercise (PT), yoga (YG), fitness‐instructor led exercise (FI), or control (NEX) Setting: Clinical Therapies Department, University of Limerick, Limerick, Ireland Categorization: PT = mixed, YG = other, FI = mixed | |
| Participants | n = 314; PT = 80, YG = 77, FI = 86, NEX = 71 Inclusion criteria: 0‐2 on the GNDR mobility sub‐scale, aged > 18 years, diagnosis of MS Exclusion criteria: relapse/steroid treatment < 3 months, pregnant, serious co‐morbidity Type MS: RRMS, SPMS, PPMS, benign, or unknown Disease duration (yr): PT = 9.8 ± 7.0, YG = 11.6 ± 8.0, PI = 10.5 ± 6.9, NEX = 10.6 ± 8.2 Mean age (yr) ± SD: PT = 51.7 ± 10.0, YG = 49.6 ± 10.0, PI = 50.3 ± 10.0, NEX = 48.8 ± 11.0 % Female (n/n group): PT = 50/63, YG = 44/63, PI = 45/77, NEX = 43/49 Mean EDSS ± SD: ? | |
| Interventions | PT: 10 weeks, 1x per week; 1‐hour circuit style ‐ body weight/free weight resisted group (6‐8 participants) training. Exercises consisted of, for example: sit to stand, bridging, shoulder flexion, walking, cycling. Classes were supervised by a trained physiotherapist. Difficulty of the exercises was aimed to fail at the 12th repetition. In addition, participants were advised to do aerobic training of choice, 2x per week at 65% HRmax YG: 10 weeks, 1x per week; 1‐hour sessions led by YG instructors. The YG sessions were not pre‐defined; but consisted of breathing exercise, relaxation, range of motion exercise, and dynamic weight‐bearing poses FI: 10 weeks, 1x per week; 1‐hour community fitness led by local fitness instructors. The content of the intervention was not pre‐defined NEX: no change in exercise habits for 10 weeks | |
| Outcomes | MFIS, MSIS‐29v2, 6MWT | |
| Notes | Drop‐outs PT: 7 health condition, of which 2 relapse, 1 personal factors, 2 environmental factor, 7 unknown YG: 5 health condition, of which 2 relapse, 3 personal factors, 1 environmental factor, 5 unknown FI: 7 health condition, of which 3 relapse, 5 personal factors, 7 unknown NEX: 11 health condition, of which 6 relapse, 6 personal factors, 1 environmental factor, 4 unknown Measurements Baseline, 12 weeks (post intervention), 24 weeks follow‐up for intervention groups only A secondary analysis to predict the response of each therapy on gait function was also published (Kehoe 2014). The results of a second study strand, in more severely disabled people with MS, has been included as a separate study (Hogan 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization per geographical region |
| Allocation concealment (selection bias) | Low risk | Randomization performed by central co‐ordinator |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate 23% equally balanced across the different exercise conditions. |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to home walking programme (EX) and no training (NEX) Setting: Department of Physical Therapy, The George Washington University, Washington, USA Categorization: EX = task‐oriented | |
| Participants | n = 15; EX = 9, NEX = 6 Inclusion criteria: aged 18‐65 years, diagnosis MS > 1 year, no exacerbation < 6 months, no participation in regular aerobic exercise < 6 months, EDSS ≤ 6.0 Exclusion criteria: cardiovascular, pulmonary, or orthopaedic conditions Type MS: ? Disease duration (yr): EX: 3‐30 (information provided for 5 participants), NEX = ? Age range (yr): EX = 40‐64, NEX = 22‐50 % Female (n/n group): EX = 6/8, NEX = 3/4 Mean EDSS ± SD: ? | |
| Interventions | EX: individualized home walking programme (12 weeks, 3x per week). Training heart rate (THR) range was determined based on 6MWT using the Karvonen formula. Each session consisted of 5 minutes below THR, 15 minutes in THR, and cooling down below THR. Time within THR increased gradually during training programme NEX: no training, walking programme offered following completion of trial | |
| Outcomes | FSS, 6MWT, PCI, RPE | |
| Notes | Drop‐outs EX: 1 poor compliance and failure to show at post‐test assessment NEX: 2 poor compliance and failure to show at post‐test assessment Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by coin toss |
| Allocation concealment (selection bias) | Unclear risk | Unclear who performed coin toss |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 20%, equally balanced across groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | High risk | Small and unevenly distributed sample |
| Methods | Design: random assignment to standard exercise programme plus supplementary resistance training (EX) or standard exercise programme (STD) Setting: Department of Physical Therapy, University of Utah, Salt Lake City, USA Categorization: EX = mixed, STD = endurance | |
| Participants | n = 22; EX = 11, STD = 11 Inclusion criteria: definite MS, no exacerbation < 3 months, aged 18‐65 years, ambulatory, impaired gait pattern, no lower extremity joint problems Exclusion criteria: participation in regular strength training Type MS: ? Disease duration (yr) ± SD: EX = 12.5 ± 11.2, STD = 11.8 ± 7.3 Mean age (yr) ± SD: EX = 48.0 ± 11.9, STD = 49.7 ± 10.98 % Female (n/n group): EX = 5/9, STD = 6/10 Mean EDSS ± SD: EX = 5.3 ± 1.0, STD = 5.2 ± 1.0 | |
| Interventions | EX: supplementary lower extremity eccentric ergometric resistance training (12 weeks, 3x per week). During the course of the training programme resistance was increased based on perceived exertion (BORG 7/20 ‐ BORG 13/20) STD: aerobic training, lower extremity stretching, upper extremity strength training, and balance exercises (12 weeks, 3x per week) | |
| Outcomes | FSS, voluntary maximal isometric strength (flexion/extension of knee, hip, dorsi, and sum), TUG, 6MWT, stair test, 10MWT, BBS | |
| Notes | Drop‐outs EX: 1 difficulty committing to 3x per week schedule STD: 1 during pre‐testing, 1 discontinued intervention Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on randomization procedure |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 14% equally balanced across the exercise groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other risk of bias identified |
| Methods | Design: random assignment to vestibular rehabilitation (VES), exercise control (EX), or wait‐list control group (NEX); stratified by yes/no involvement of brain stem/cerebellar Setting: Department of Physical Medicine and Rehabilitation, and Department of Neurology, University of Colorado, Aurora, USA. Medical Campus Categorization: VES = other, EX = endurance | |
| Participants | n = 38; VES = 12, EX = 13, NEX = 13 Inclusion criteria: definite MS, aged 18‐65 years, able to walk 100 metres with at most a single‐sided device, score ≥ 45/85 on the MFIS, score < 72 on SOT; limited standing balance Exclusion criteria: unable to walk, use of pharmacological agent that control or cause fatigue, change in MS medication < 3 months, MS‐related relapse < 6 months, other conditions that cause fatigue, impaired or limited upright postural control, participation in a vestibular or endurance exercise programme < 2 months Type MS: RRMS or SPMS Disease duration (yr) ± SD: VES = 6.5 ± 5.6, EX = 5.1 ± 3.2, NEX = 9.1 ± 7.3 Mean age (yr) ± SD: VES = 46.8 ± 10.5, EX = 42.6 ± 10.4, NEX = 50.2 ± 9.2 % Female (n/n group): VES = 9/12, EX = 11/13, NEX = 11/13 Mean EDSS ± SD: ? | |
| Interventions | VES: standardized vestibular rehabilitation programme (6 weeks, 2x per week, 55 minutes). In addition, individual exercises were selected and constituted an individualized home training programme (postural control, eye movement). Also 5 minutes of fatigue management education was provided EX: endurance and stretching exercises (6 weeks, 2x per week); 5 minutes warming up, 2 x 15 minutes at 65‐75% HRpeak, 2‐5 minutes cooling down. In addition, an individualized home training programme (endurance and stretching) at a similar intensity as the supervised programme and 5 minutes of fatigue management education were provided NEX: wait list control | |
| Outcomes | MFIS, SOT, 6MWT, DHI, BDI | |
| Notes | Drop‐outs NEX: 1 refused continuation after wait‐list control allocation Measurements Baseline (weeks 1, 2, 4), intervention phase (weeks 6, 8, 10), follow‐up phase (weeks 12, 14); not all measures assessed at each occasion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Low risk | Randomization performed by a clinician not involved in the trial |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rate was 3% |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to group physiotherapy (GP), individual physiotherapy (IP), yoga (Y), or control (C) Setting: Clinical Therapies Department, University of Limerick, Limerick, Ireland Categorization: GP = muscle power, IP = muscle power, Y = other | |
| Participants | n (drop‐outs) = 146; GP = 66, IP = 45, Y = 16, C = 19 Inclusion criteria: confirmed diagnosis of MS, GNDS score of 3 or 4 Exclusion criteria: relapse or steroid treatment < 12 weeks, pregnant, aged < 18 years Type MS: RRMS, SPMS, PPMS, unknown Disease duration (yr) ± SD: GP = 18 ± 9, IP = 13 ± 8, Y = 15 ± 8, C = 10 ± 3 Mean age (yr) ± SD: GP = 57 ± 10, IP = 52 ± 11, Y = 58 ± 8, C = 49 ± 6 % Female (n/n group): GP = 30/48, IP = 20/35, Y = 8/13, C = 13/15 Mean EDSS ± SD: ? | |
| Interventions | GP: 10 weeks, 1x per week, 1‐hour group physiotherapy. GP was a self paced circuit style class of exercises (sit to stand, squat, heel raises, step‐ups, side stepping, and tandem) that targeted strength and balance with the aim of increasing balance and mobility. The aim was to perform 1 set of 12 repetitions and according to the progression was increased to 3 sets of 12 repetitions IP: 10 weeks, 1x per week, 1‐hour individual physiotherapy with the same content as the GP Y: 10 weeks, 1x per week, 1‐hour yoga classes by yoga instructors of the Yoga Federation of Ireland. The content of each yoga class was kept in a diary C: usual care | |
| Outcomes | MFIS, BBS, 6MWT, MSIS29v2 | |
| Notes | Drop‐outs GP: 1 relapse, 5 unwell at day of measurement, 3 unwell and discontinued intervention, 1 steroids for lower back pain, 2 moved to different study strand, 1 on holiday, 1 requested 1 : 1 treatment, 1 fall, 3 unknown IP: 2 relapse, 1 unwell at day of measurement, 1 inclement weather, 2 on holidays, 3 moved to different study strand, 1 unknown Y: 1 discontinued intervention by choice, 2 unknown C: 2 relapse, 1 rapidly progressing MS, 1 conflicting appointments Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization per geographical region |
| Allocation concealment (selection bias) | Low risk | Randomization performed by central co‐ordinator |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | The overall drop‐out rate was 24%, equally balanced across exercise (24%) and control (21%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Unclear risk | Unequal group sizes |
| Methods | Design: random assignment to 8‐week aquatic exercise programme (EX) or no training/wait list (NEX) Setting: Faculty of Physical Education and Sport Sciences, University of Isfahan, Isfahan, Iran Categorization: EX = endurance | |
| Participants | n = 32; EX = 16, NEX = 16 Inclusion criteria: definite MS, > 2 years after diagnosis, no relapse < 1 month, ability to participate regular exercise sessions Exclusion criteria: relapse during intervention, disease preventing participation Type MS: RRMS Disease duration (yr) ± SD: EX = 4.9 ± 2.3, NEX = 4.6 ± 1.9 Mean age (yr) ± SD: EX = 33.7 ± 8.6, NEX = 31.6 ± 7.7 % Female (n/n group): EX = 10/10, NEX = 11/11 Mean EDSS ± SD: EX = 2.9 ± 0.9, NEX = 3.0 ± 0.7 | |
| Interventions | EX: 8‐week aquatic exercise (3x per week, 60 minutes); 10‐minute warm‐up, 40‐minute exercise, 10‐minute cool‐down at 50‐75% heart rate reserve. Exercises included activities focused on joint mobility, flexor and extensor muscle strengths, balance movements, posture, functional activities, and intermittent walking NEX: no training/maintain current habits | |
| Outcomes | MFIS, MSQoL‐54 | |
| Notes | Drop‐outs EX: 6, no reasons specified NEX: 5, no reasons specified Measurements Baseline, 4 weeks, 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffled envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall drop‐out rate 34%, equally balanced across groups. Reasons for drop‐out not specified |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | High risk | A large increase in fatigue in the control condition |
| Methods | Design: random assignment to 10 weeks' inspiratory muscle training (EX) or control group (NEX) Setting: 3 rehabilitation outpatient clinics in Stockholm, Sweden Categorization: EX = other | |
| Participants | n = 15; EX = 7, NEX = 8 Inclusion criteria: progressive MS according to Poser criteria, EDSS 6.5‐9.5 Exclusion criteria: EDSS < 6.0, chronic obstructive airways, asthma, emphysema, cystic fibrosis, heart insufficiency, another diagnosis/disorder Type MS: progressive MS Disease duration (yr); range: EX = 12 (3‐19), NEX = 20 (12‐35) Mean age (yr); range: EX = 46 (37‐49), NEX = 52.5 (38‐61) % Female (n/n group): EX = 1/7, NEX = 5/8 Mean EDSS; range: EX = 7.5 (6.5‐8.0), NEX = 8.0 (6.5‐9.0) | |
| Interventions | EX: inspiratory muscle training (10 weeks, 70 sessions). Each session consisted of 3x 10 loaded inspirations with 1‐minute rest in between at 40‐60% of maximal inspiratory pressure NEX: no additional training besides regular physiotherapy | |
| Outcomes | FSS, maximal inspiratory ‐ expiratory pressure, FVC, FEV, VC, RPE after morning bathing/dressing | |
| Notes | Drop‐outs No drop‐outs reported Measurements Baseline, 10 weeks, 14 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to leisure centre‐based exercise group (EX) or control group (NEX); separate randomization for 2 different treatment sites Setting: Multiple Sclerosis Service, Scotland, UK Categorization: EX = mixed | |
| Participants | n = 32; EX = 20, NEX = 12 Inclusion criteria: confirmed diagnosis MS, EDSS 5‐6.5, stable rehabilitation and drug therapy < 1 month, cognitive score > 24 on the MMSE Exclusion criteria: exacerbation < 3 months, medical condition that may preclude participants from exercise intervention Type MS: ? Disease duration (yr) ± SD: EX = 13.4 ± 6.4, NEX = 12.6 ± 8.1 Mean age (yr) ± SD: EX = 51.4 ± 8.06, NEX = 51.8 ± 8.0 % Female (n/n group): EX = 15/20, NEX = 8/12 Mean EDSS ± SD: EX = 6.14 ± 0.36, NEX = 5.82 ± 0.51 | |
| Interventions | EX: leisure centre‐based exercise group (12 weeks, 2x per week). 10‐minute warm‐up of aerobic and stretching components; 30‐40 minutes of circuit exercises (8‐12) designed for aerobic endurance, resistance, and balance NEX: usual routine, avoid new exercise regimen for the 12‐week study period | |
| Outcomes | FSS, T25FW, 6MWT, BBS, TUG, QPW, BMI, PF, ABC, HADS, LMSQoL | |
| Notes | Drop‐outs EX: 1 family commitments, 1 participate in other study, 1 increased work commitments, 1 influenza‐like symptoms, 1 suspected trigeminal neuralgia NEX: 1 unable to commit time for assessments, 1 unable to attend due to weather conditions Measurements Baseline, 8 weeks, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization procedure |
| Allocation concealment (selection bias) | High risk | Randomization list was generated beforehand to include a potential 20 participants |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate 22%, without indication of differences in reasons |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to 12‐week group circuit training (EX) or control group (NEX) Setting: Physiotherapy Gym, Dublin, Ireland Categorization: EX = mixed | |
| Participants | n = 30; EX = 17, NEX = 13 Inclusion criteria: definite MS, independently mobile without use of aids, able to attend twice weekly + exercise independently at home Exclusion criteria: relapse < 3 months; history of cardiac conditions that would limit exercise capacity; cognitive or psychosocial condition that would limit class participation Type MS: RRMS and SPMS Disease duration (yr) ± SD: EX = 5.4 ± 4.35, NEX = 5 ± 3.52 Mean age (yr) ± SD: EX = 40.5 ± 12.68, NEX = 33.58 ± 6.1 % Female (n/n group): EX = 14/17, NEX = 10/13 Mean EDSS ± SD: ? | |
| Interventions | EX: leisure centre‐based group classes (12 weeks, 2x per week) 5‐minute warm‐up, 40‐minute exercises. 4 different stations (10 minutes each): treadmill walking/running, cycling, stair‐master training, arm‐strengthening exercises, volleyball, and outdoor walking. In addition, participants were required to do a single type home exercise programme of choice for 40‐60 minutes at RPE 11‐13 NEX: normal activity + physiotherapy once monthly | |
| Outcomes | MFIS, FAMS, MSIS‐29, exercise capacity (HRmax, RPE) | |
| Notes | Drop‐outs EX: 2 relapses, 1 inconvenient timing of classes, 1 pregnancy, 1 personal reasons NEX: 1 moved home Measurements Baseline, 3 months, 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by blinded withdrawal of labelled slips from box |
| Allocation concealment (selection bias) | Unclear risk | Only 2 slips were present in the box during the allocation of each participant |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate 20%, unequally balanced across exercise (29%), and control (8%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | High risk | After 17 people had been allocated to the experimental group the remaining people were allocated to the control group to maintain equal group sizes |
| Methods | Design: random assignment to transcranial magnetic stimulation (TMS), exercise group (EX), or control (NEX); group allocation was counter‐balanced by EDSS score Setting: outpatient clinical service of the University of Tor Vergata, Rome, Italy Categorization: TMS = mixed, EX = mixed | |
| Participants | n = 30; TMS = 10, EX = 10, NEX = 10 Inclusion criteria: RRMS according to McDonald criteria, remitting phase of disease, EDSS 2.0‐6.0, lower limb spasticity Exclusion criteria: gadolinium‐induced cerebral or spinal lesion enhancement 2‐30 days before beginning of trial Type MS: RRMS Disease duration (yr) ± SD: ? Mean age (yr) ± SD: TMS = 39.1 ± 10.7, EX = 37.7 ± 12.3, NEX = 38.3 ± 11.9 % Female (n/n group): TMS = 3/10, EX = 4/10, NEX = 5/10 Mean EDSS ± SD: TMS = 3.6 ± 1.2, EX = 3.8 ± 1.6, NEX = 3.5 ± 1.0 | |
| Interventions | TMS: intermittent theta burst stimulation of the soleus muscle at 80% of the active motor threshold for a total of 600 stimuli + exercise therapy (2 weeks, 5x per week); 2‐hour daily sessions. 1 hour on land, 1 hour aquatic, 2 or 3 sets per exercise type, 15 repetitions per set. Exercises were scheduled in order to improve range of motion, muscular flexibility, equilibrium reactions, motor co‐ordination, postural passages and transfers, and ambulation. Intensity was reduced with increasing disability EX: exercise therapy as for TMS group however with sham TMS NEX: TMS only | |
| Outcomes | FSS, MAS, MSSS‐88, BI, EDSS, MSQoL‐54 (physical and mental composite) | |
| Notes | Drop‐outs No drop‐outs, training adherence unknown Measurements Baseline, 2 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment of patients (EXMS) and healthy controls (EXH) to short‐term exercise programme or control (patients: NEXMS, healthy controls: NEXH); healthy people were matched with respect to gender, age, and level of physical activity Setting: Rehabilitation Centre, Valens, Switzerland Categorization: EX = endurance | |
| Participants | n = 52; EXMS = 20, NEXMS = 18, EXH = 13, NEXH = 13 Inclusion criteria: definite MS; able to pedal on free‐standing ergometer; no history of cardiovascular, respiratory, orthopaedic, metabolic diseases, or other medical conditions; no acute exacerbation < 2 months Exclusion criteria: ? Type MS: RRMS, PPMS, and SPMS Disease duration (yr) ± SD: EXMS = 11.2 ± 8.5, NEXMS = 12.6 ± 8.1 Mean age (yr) ± SD: EXMS = 45.23 ± 8.66, NEXMS = 43.92 ± 13.90, EXH = 44.7 ± 10.0, NEXH = 41.69 ± 11.15 % Female (n/n group): EXMS = 10/13, NEXMS = 11/13, EXH = 10/13, NEXH = 11/13 Mean EDSS ± SD: EXMS = 4.6 ± 1.2, NEXMS = 4.5 ± 1.9 | |
| Interventions | EXMS: short‐term inpatient exercise programme (3‐4 weeks, 5x per week). Consisted of 30 minutes' bicycle exercise training at individually determined anaerobic threshold NEXMS: normal inpatient physiotherapy yet not increase physical activity level EXH: identical training programme as people with MS NEXH: no intervention, no change increase in physical activity level | |
| Outcomes | FSS, VO2max, anaerobic threshold, FVC, MVV, BAECKE, SF‐36 | |
| Notes | Drop‐outs EXMS: 3 quit after allocation to exercise group, 2 excluded based on ST segment change during exercise electrocardiogram (ECG), 2 had elevated spasticity of the lower extremities following exercise testing NEXMS: 3 motivational problems to sustain intervention programme, 2 symptom exacerbations Measurements Baseline, 4 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate was 32% equally balanced across groups, but with some indication of differences in reasons |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to massage therapy (M), exercise therapy (EX), massage + exercise therapy (MEX), or a control (C); the massage therapy group was excluded from this review Setting: Musculoskeletal Rehabilitation Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran Categorization: EX = mixed, MEX = mixed | |
| Participants | n = 48; M = 12, EX = 12, MEX = 12, C = 12 Inclusion criteria: RRMS or SPMS, EDSS 2‐6, walk 10 metres, stand unassisted for > 60 seconds Exclusion criteria: severe relapse < 1 month, participation in an physiotherapy programme before start of trial, cardiovascular disease, diabetes, or lower limb arthritis Type MS: RRMS or SPMS Disease duration (months) ± SD: M = 148.7 ± 97.11, EX = 102 ± 81.06, MEX = 115.3 ± 78.28, C = 86.58 ± 34.33 Mean age (yr) ± SD: M = 36.33 ± 7.62, EX = 36.67 ± 6.69, MEX = 36.67 ± 7.63, C = 36.83 ± 8.74 % Female (n/n group): M = 10/12, EX = 10/12, MEX = 10/12, C = 10/12 Mean EDSS ± SD: M = 3.75 ± 1.37, EX = 3.50 ± 1.13, MEX = 3.75 ± 1.43, C = 3.83 ± 1.39 | |
| Interventions | M: 5 weeks, 5x per week, 30‐minute standard Swedish massage by a trained massage therapist. The following techniques were applied to the bilateral quadriceps femoris, hip adductors, peroneal, and calf muscles: petrissage, effleurage, and friction. Participants were asked to lie in a supine position on a treatment table and a 7‐minute massage was applied on the quadriceps femoris and hip adductors. In addition, a 4‐minute massage was applied both on the peroneal muscles and the calf muscles EX: 5 weeks, 3x per week, 30‐minute combined set of strength, stretch, endurance, and balance exercises. Exercise included leg raising, forward lunge, hip adductor, and calf muscle stretching, and walking on a treadmill MEX: 5 weeks, 3x per week, 15‐minute exercise therapy (EX) + 15 minutes of passive massage therapy (M) C: participants in the control group were asked to continue their standard medical care | |
| Outcomes | FSS, VAS scale for pain, MAS, BBS, TUG, 10MWT, 2MWT, MSQoL‐54 | |
| Notes | Drop‐outs No drop‐outs reported Measurements Baseline, 5 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear who had access to table of random numbers |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs or adverse events reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: Random assignment to Iyengar yoga classes plus home programme (YOGA), weekly bicycle exercise classes along with home exercise (EX) or wait‐list control group (NEX) using a minimization procedure. Setting: Oregon Health & Science University, Portland, USA Categorization: YOGA = other, EX = endurance | |
| Participants | n = 69; YOGA = 26, EX = 21, NEX = 22 Inclusion criteria: EDSS ≤ 6.0 Exclusion criteria: insulin‐independent diabetes, uncontrolled hypertension, liver or kidney failure, symptomatic lung disease, alcoholism/drug abuse, ischaemic heart disease or signs of congestive heart failure, visual acuity worse than 20/50 binocularly, yoga/tai‐chi < 6 months, regular aerobic exercise > 30 minutes/day Type MS: ? Disease duration (yr) ± SD: ? Mean age (yr) ± SD: YOGA = 49.8 ± 7.4, EX = 48.8 ± 10.4, NEX = 48.4 ± 9.8 % Female (n/n group): YOGA = 20/22, EX = 13/15, NEX = 20/20 Mean EDSS ± SD: YOGA = 3.2 ± 1.7, EX = 2.9 ± 1.7, NEX = 3.1 ± 2.1 | |
| Interventions | YOGA: 90‐minute Iyengar yoga classes, 1x per week for 6 months; all poses were supported by chair, wall, or floor. Each pose was held 10‐30 seconds with rest periods of 30‐60 seconds. Emphasis on breathing and relaxation. Each session ended with 10 minutes' deep relaxation, supine on the floor. Participants were encouraged to practice yoga at home by means of a demonstration booklet EX: bicycle exercise classes 1x per week ‐ up to 60 minutes for 6 months. Each class began and ended with 5 minutes' stretching. Primary mode of exercise was cycling at modified BORG 2‐3. Each participant was provided with a home bicycle and encouraged to continue cycling at home or do any other mode of exercise NEX: wait‐list control | |
| Outcomes | POMS fatigue sub‐scale, MFI, SSS, POMS, SF‐36, STAI, CES‐D‐10, MSFC including 25‐foot walk, 9HPT, Stroop colour‐word interference, Electroencephalography median power frequency | |
| Notes | Drop‐outs YOGA: 1 exacerbation EX: 1 exacerbation Other drop‐outs not specified to a single trial arm: 3 unrelated surgeries, 1 low back pain related to car accident, 6 not able to attend classes for various reasons Measurements Baseline, 3 months, 6 months. Data from 3 months concerned only limited outcomes and was not presented in the report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimization procedure |
| Allocation concealment (selection bias) | Low risk | By independent statistician |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate 17%, equally balanced across groups. Drop‐outs not specified to study arm unlikely to be related to treatment allocation |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to 15 weeks' aerobic training programme (EX) or control group (NEX) Setting: University of Utah, Salt Lake City, USA Categorization: EX = endurance | |
| Participants | n = 54; EX = 27, NEX = 27 Inclusion criteria: clinically definite MS; EDSS ≤ 6.0; no history of cardiovascular respiratory, orthopaedic, metabolic, or other medical condition that would preclude participation in the training programme; no regular physical activity < 6 months Exclusion criteria: ? Type MS: ? Disease duration (yr) ± SD: EX = 9.3 ± 1.6, NEX = 6.2 ± 1.1 Mean age (yr) ± SD: EX = 41.1 ± 2.0, NEX = 39.0 ± 1.7 % Female (n/n group): EX = 15/21, NEX = 16/25 Mean EDSS ± SD: EX = 3.8 ± 0.3, NEX = 2.9 ± 0.3 | |
| Interventions | EX: aerobic training (15 weeks, 3x per week) under supervision. 5‐minute warm‐up 30% VO2max, 30 minutes at 60% VO2max, 5‐minute cool‐down. 5‐10 minutes of stretching. Workload was updated at week 5 and 10 NEX: control group instructed to not change activity pattern; programme was offered after completion of trial | |
| Outcomes | POMS fatigue sub‐scale, FSS, number of relapses, EDSS, ISS, VO2max, maximum voluntary contractions of five upper and lower extremity muscle groups, skin‐fold thickness, % body fat, blood lipids, POMS, SIP | |
| Notes | Drop‐outs EX: 4 relapses NEX: 3 relapses Measurements Baseline, 5 weeks, 10 weeks, 15 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate, equally balanced across exercise (15%) versus control (11%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to individualized physical rehabilitation (IPR) or a group wellness intervention (GWI) Setting: Department of Physical Medicine & Rehabilitation, University of Minnesota, Minneapolis, USA Categorization: IPR = mixed | |
| Participants | n = 50; IPR = 22, GWI = 20 Inclusion criteria: physician‐confirmed diagnosis MS, ability to walk with or without an assistive device Exclusion criteria: pregnancy, cardiovascular disease, more than 2 falls in past month, inability to understand trial Type MS: ? Disease duration (yr) ± SD: ? Mean age (yr) ± SD: IPR = 48.5 ± 12.3, GWI = 48.5 ± 9.1 % Female (n/n group): IPR = 17/20, GWI = 16/20 Mean EDSS ± SD: ? | |
| Interventions | IPR: individualized home‐exercise programme (8 weeks, 5x per week). 3x per week indoor cycling and stretching. 2x per week strength and balance exercises. Additional physical therapy session every other week GWI: home‐exercise programme without individual adjustment but with additional energy conservation modules based on Packer 1995. (8 weeks, 2 hours/week) | |
| Outcomes | MFIS, MHI, SF‐36, HPLP‐II physical activity sub‐scale, BMI, % body fat, waist circumference, blood pressure, resting heart rate, VO2max (YMCA protocol), bench‐press (repetitions/1 minute), military‐press (repetitions/1 minute), sit‐to‐stand (repetitions/45 seconds), hamstring flexibility, balance | |
| Notes | Drop‐outs 8 participants excluded from analyses, as they did not receive intervention (reason not provided). An additional 4 participants excluded from analyses due to missing values of primary outcome (GWI = ‐3, IPR = ‐1) Measurements 6 weeks pre‐intervention, immediately pre‐intervention, post‐intervention, 8 weeks post‐intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate 29% with a larger proportion in the GWI (35%) versus the IPR (23%). Reasons for not receiving intervention were not provided |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design (cross‐over): random assignment to aerobic training (EX) first or neurorehabilitation programme (NRP) first Setting: MS outpatient clinics at Parma University Hospital and Piacenza Hospital, Italy Categorization: EX = endurance | |
| Participants | n = 19; EX = 8, NRP = 11 Inclusion criteria: diagnosis MS according to Poser criteria, EDSS ≤ 6.0, aged 20‐55 years Exclusion criteria: relapse < 1 month; history of cardiac, pulmonary, orthopaedic, metabolic, or other medical condition precluding participation; receiving steroid treatment < 2 months Type MS: ? Disease duration (yr) ± SD: EX + NRP = 6 ± 4 Mean age (yr) ± SD: EX + NRP = 41 ± 8 % Female (n/n group): EX + NRP = 14/11; 4 more drop‐outs after cross‐over Mean EDSS (range): EX + NRP = 3.5 (1‐6) | |
| Interventions | EX: aerobic training (8 weeks, 3x per week) under supervision. 5‐minute warm‐up 30% VO2max, 30 minutes at 60% VO2max, 5‐minute cool‐down. 5‐10 minutes of stretching. Similar to the trial by Petajan 1996 NRP: neurorehabilitation programme (8 weeks, 3x per week). 60 minutes of exercise aimed at improving respiratory‐motor synergies and stretching | |
| Outcomes | MFIS, EDSS, MSQoL‐54, pulmonary function, 6MWT, walking speed, cost of walking, Wmax, VO2max, VO2/HR | |
| Notes | Drop‐outs EX: 1 relapse, 1 did not adhere to protocol; after cross‐over, 3 more drop‐outs in EX due to relapse (1) and not adhering to protocol (2) NRP: 1 relapse, 1 did not adhere to protocol; after cross‐over, 1 more drop‐out due to relapse Measurements Baseline, 8 weeks, 16 weeks, 24 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization procedure |
| Allocation concealment (selection bias) | Unclear risk | Unclear who performed randomization procedure |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | High risk | Overall drop‐out rate 42% for which high risk. However, a large proportion dropped out during the wash‐out phase. No imbalances in drop‐outs during intervention phase |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Unclear risk | No data on 'success' of wash‐out phase presented |
| Methods | Design (cross‐over): random assignment to endurance training (ET) first or resistance training (RT) with 8‐week wash‐out period between interventions Setting: Queensland Health, Queensland, Australia Categorization: ET = endurance, RT = muscle power | |
| Participants | n = 21; ET = 6, RT = 15 Inclusion criteria: ambulant (with or without aid) Exclusion criteria: ? Type MS: RRMS, PPMS, SPMS Disease duration (yr) ± SD: ET + RT = 10 ± 10 Mean age (yr) ± SD: ET + RT = 55 ± 7 % Female (n/n group): ET + RT = 12/16 Mean EDSS ± SD: ? | |
| Interventions | ET: endurance training (8 weeks, 2x per week); circuit of 8 exercise stations (step‐ups, arm cranking, upright cycling, arm cranking, recumbent cycling, cross‐trainer, treadmill walking, arm cranking (5 minutes each). Intensity was increased based on RPE (3‐5 on BORG 10 scale) RT: resistance training (8 weeks, 2x per week); 3 upper, 3 lower body exercises, 1 core strength, 1 stability. 2‐3 sets of 6‐10 repetitions | |
| Outcomes | MFIS, grip strength, functional reach test, four step square test, TUG, 6MWT, MSIS‐29, SF‐36 | |
| Notes | Drop‐outs ET: no drop‐outs during or directly following endurance training RT: 1 for time reasons; following the wash‐out period, 2 participants drop‐out due to time constrains, 1 moved house and 1 became ill dependent Measurements Baseline, directly following 8 weeks' intervention 1, after an 8‐week wash‐out period and following 8 weeks' intervention 2 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | High risk | A large proportion of the participants randomized to resistance training first |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 24%. No imbalances in the number of drop‐outs during endurance or resistance training |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to a combination exercise therapy (EX) and a control (NEX) Setting: physiotherapy clinic, Tehran, Iran Categorization: EX = mixed | |
| Participants | n = 72; EX = 42, NEX = 30 Inclusion criteria: RRMS, aged 18‐50 years, no MS attack < 3 months, EDSS 0‐4.0 Exclusion criteria: ? Type MS: RRMS Disease duration (yr) ± SD: ? Mean age (yr) ± SD: EX = 33.05 ± 7.68, NEX = 32.05 ± 6.35 % Female (n/n group): EX = 24/39, NEX = 15/22 Mean EDSS: EX = 1.7, NEX = 1.96 | |
| Interventions | EX: 10 weeks, 3x per week, 90 minutes of combination exercises. 7‐10 minutes of stretching exercises including movements for spine, neck, upper, and lower limbs. 20‐40 minutes of aerobic exercise on cycle or treadmill of which the duration progressively increased based on the fatigue perception. Intensity started at 40% of HRmax and was increased to 70% of HRmax. Subsequently, 10‐20 minutes of strength and balance exercises NEX: usual care | |
| Outcomes | FSS, EDSS, BBS, 6MWT, MQoL, PQoL | |
| Notes | Drop‐outs EX = 1 lack of time, 1 muscular pain and stiffness, 1 MS attack NEX = 5 performed exercise or rehabilitation, 1 MS attack, 2 unable to contact Measurements Baseline, 10 weeks ('after all exercise sessions') | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done before invitation to participate |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 15%. Unequal distribution between exercise (7%) and control (27%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Unclear risk | There was no clarity regarding the reported means, mean differences, and time points; unequal group‐sizes |
| Methods | Design: random assignment to aerobic training (EX) or control (NEX) Setting: University Hospital Eppendorf, Hamburg, Germany Categorization: EX = endurance | |
| Participants | n = 28; EX = 15, NEX = 13 Inclusion criteria: diagnosis MS according to Poser criteria, EDSS < 5.0, without steroid or immunosuppressive therapy < 4 weeks Exclusion criteria: no clear diagnosis, acute relapse, severe cognitive deficits, signs of any psychiatric disease Type MS: RRMS (19 participants), SPMS (5 participants), PPMS (2 participants), not reported (2 participants) Disease duration (yr) ± SD: ? Mean age (yr) ± SD: EX = 39 ± 9, NEX = 40 ± 11 % Female (n/n group): EX = 11/15, NEX = 8/13 Mean EDSS (range): EX = 2.0 ± 1.4, NEX = 2.5 ± 0.8 | |
| Interventions | EX: aerobic interval training (8 weeks, 2x per week) under supervision. Maximal intensity at 75% Wmax from ergometry test NEX: wait list | |
| Outcomes | MFIS, fitness parameters, immune‐ and neurotrophic factor, HAQUAMS | |
| Notes | Drop‐outs No drop‐outs, adherence unknown; 18 participants withdrawn from initial 46 included due to inability to cycle 30 minutes at 100 Watt Measurements Baseline, 8 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | High risk | 18 participants withdrawn from 46 included due to inability to complete exercise test |
| Methods | Design: random assignment to endurance training (EX) or control (NEX) Setting: Danish MS Hospital, Ry, Denmark Categorization: EX = endurance | |
| Participants | n = 11; EX = 6, NEX = 5 Inclusion criteria: EDSS 6.5‐8.0, aged > 18 years, SPMS or PPMS, no serious comorbidity Exclusion criteria: n.a. Type MS: SPMS or PPMS Disease duration (yr) ± SD: ? Mean age (yr) ± SD: EX = 62.0 ± 5.9, NEX = 55.2 ± 8.2 % Female (n/n group): ? Mean EDSS (range): ? | |
| Interventions | EX: 4 weeks' inpatient rehabilitation + 10 supervised upper‐body exercise sessions. Exercise consisted of 5 minutes' warming‐up followed by 6x 3‐minute intervals at a target heart rate corresponding to 65‐75% of VO2peak. To ensure physical exhaustion, short 30‐ to 60‐second sprints at the highest possible intensity were performed at the end of each interval. The inpatient rehabilitation programme was provided by a highly specialized multidisciplinary team consisting of difference active treatments ranging from self management education to various exercise therapies depending on the person's goals and needs NEX: 4 weeks' inpatient rehabilitation similar to the programme in the exercise group | |
| Outcomes | FSMC, VO2peak, peak HR, 9HPT, hand grip power, Box and Blocks, 6‐minute wheelchair, MDI, MSIS‐29 | |
| Notes | Drop‐outs EX: 1 due to hospitalization unrelated to the intervention Measurements Baseline, 4 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure unknown |
| Allocation concealment (selection bias) | Low risk | It was stated that the randomization occurred while 'applying concealed allocation (with respect to gender)' |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | 1 drop‐out reported in the exercise condition, unrelated to the intervention. 96% of planned exercise sessions completed |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to Norway‐based physiotherapy programme (NOR) first or Spain‐based physiotherapy programme (ESP) first; wash‐out phase of 1 year Setting: Departments of Neurology at Haukeland University Hospital and Akershus University Hospital, Norway (recruitment); treatment was provided in Tenerife, Spain and Hakadal, Norway Categorization: NOR = other, ESP = other | |
| Participants | n = 60; NOR = 30, ESP = 30 Inclusion criteria: gait problems, EDSS 4.0‐6.5, aged 18‐60, no relapse < 1 month Exclusion criteria: heat intolerance or excessive fatigue, pregnancy, breastfeeding, severe cognitive dysfunction, other co‐morbidities that could influence participation Type MS: RRMS, PPMS, SPMS Disease duration (yr) ± SD: ? (age at onset NOR = 33.0 ± 9.2, ESP = 29.6 ± 9.8) Mean age (yr) ± SD: NOR = 49.9 ± 8.0, ESP = 47.0 ± 9.8 % Female (n/n): NOR = 16/30, ESP = 20/30 Mean EDSS ± IQR: NOR = 4.5 ± 1.5, ESP = 4.75 ± 1.5 | |
| Interventions | NOR and ESP both comprehended the same physiotherapy programme (4 weeks, 5x per week, 60 minutes) based on the Bobath concept aimed to improve physical functioning through motor learning | |
| Outcomes | FSS, 6MWT (+ RPE), TUG, 10MWT, BBS, TIS, MSIS‐29, MHAQ, pain, balance, gait | |
| Notes | Drop‐outs Not specified to intervention; 2 relapses, 1 pregnancy, 1 college studies, 1 accident with bone fracture, 4 personal reasons Measurements Baseline, 4 weeks, 3 months' follow‐up, 6 months' follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 15%. Distribution across exercise conditions unknown; however, reasons for drop‐out unlikely related to exercise intervention. Training adherence was 99% |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random allocation to task‐oriented circuit training + home exercise (EX) or control (NEX) Setting: outpatient clinic of the Physical Medicine and Rehabilitation Department, Ferrara, Italy Categorization: EX = task‐oriented | |
| Participants | n: 24; EX = 12, NEX = 12 Inclusion criteria: aged 18‐75 years, definite diagnosis MS, EDSS 4‐5.5, no relapse < 3 months Exclusion criteria: other conditions that affect motor function, MMSE < 24 Type MS: RRMS, PPMS, and SPMS Disease duration (yr) ± SD: ? Mean age (yr) ± SD: EX = 49.92 ± 7.51, NEX = 55.25 ± 13.82 % Female (n/n): EX = 7/12, NEX = 10/12 Mean EDSS ± SD: EX = 4.95 ± 0.61, NEX = 4.83 ± 0.49 | |
| Interventions | EX: 2 weeks, 5x per week, 1 hour' task‐oriented circuit training + 30 minutes' treadmill training (2 hours including rest) followed by 3 months, 3x per week, 60 minutes' home‐based exercise. The task‐oriented circuit training consisted of 6 workstations in which participants exercised for 5 minutes in each (3 minutes of exercise + 2 minutes of rest). 2 laps of each workstation were done with 10 minutes of rest after each lap. Subsequently, walking endurance was trained for 30 minutes on the treadmill. Each session included up to 3 participants. A home‐based exercise brochure was provided so that they could independently train for the following 3 months. The brochure included similar exercises as for the task‐oriented circuit training NEX: usual care; exercise in non‐rehabilitative contexts was allowed | |
| Outcomes | FSS, 10MWT, 6MWT, TUG, DGI, MSWS‐12, MSIS‐29 | |
| Notes | Drop‐outs NEX = 3 (denial) Measurements Baseline, 2 weeks (post task‐oriented training), 3 months (post home‐based exercise) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | www.randomization.com |
| Allocation concealment (selection bias) | Low risk | www.randomization.com |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rate was 13% in the control condition |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other bias identified |
| Methods | Design: random assignment to 3‐week inpatient rehabilitation programme followed by 23 weeks of home‐based exercise (EX) or no exercise (NEX) Setting: Masku Neurological Rehabilitation Centre, Masku, Finland Categorization: EX = mixed | |
| Participants | n = 95; EX = 47, NEX = 48 Inclusion criteria: aged 30‐54 years, EDSS 1.0‐5.5 Exclusion criteria: cardiovascular disease, musculoskeletal disorder, relapse < 1 month, intensive exercise (5x per week, 30 minutes) < 3 months, medical or psychological or other reasons indicating potential drop‐out Type MS: RRMS, PPMS, SPMS Disease duration (yr) ± SD: EX men = 6 ± 7, EX women = 6 ± 6, NEX men = 5 ± 6, NEX women = 6 ± 7 Mean age (yr) ± SD: EX men = 45 ± 6, EX women = 43 ± 6, NEX men = 44 ± 7, NEX women = 44 ± 7 % Female (n/n): EX = 30/47, NEX = 31/48 Mean EDSS ± SD: EX men = 2.9 ± 1.2, EX women = 2.0 ± 0.8, NEX men = 3.1 ± 1.2, NEX women = 2.5 ± 1.0 | |
| Interventions | EX: 3 weeks' inpatient rehabilitation course of 5 resistance and 5 aquatic aerobic exercise (65‐70% HRmax) sessions followed by 23 weeks of individualized home‐based exercise; 4‐5x weekly. Home exercise consisted of 8 progressive resistance exercises using elastic bands NEX: no participation in any exercise programme; asked to continue normal living | |
| Outcomes | FSS, leg flexor/extensor torque, motor fatigue, ambulatory fatigue index | |
| Notes | Drop‐outs EX: 2 refused measurement, 1 traffic accident NEX: 2 refused, 1 data conversion failed, 1 knee pain Measurements Baseline, 3 weeks, 26 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization procedure |
| Allocation concealment (selection bias) | Low risk | Randomization by project statistician |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Drop‐out rate was 8% equally balanced across groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to aquatic aerobic training (EX) or control (NEX). Matched by age and gender Setting: Victoria University, Melbourne, Australia Categorization: EX = endurance | |
| Participants | n = 22; EX = 11, NEX = 11 Inclusion criteria: EDSS ≤ 5.0, no exercise programme < 6 months Exclusion criteria: cardiovascular disease, musculoskeletal disorder, relapse < 1 month, intensive exercise (5x per week, 30 minutes) < 3 months, medical or psychological or other reasons indicating potential drop‐out Type MS: ? Disease duration (yr) ± SD: EX = 7.00 ± 5.59, NEX = 6.18 ± 3.63 Mean age (yr) ± SD: EX = 47.18 ± 4.75, NEX = 45.45 ± 5.05 % Female (n/n): EX = 6/11, NEX = 6/11 Mean EDSS ± SD: EX men = 2.9 ± 1.2, EX women = 2.0 ± 0.8, NEX men = 3.1 ± 1.2, NEX women = 2.5 ± 1.0 | |
| Interventions | EX: supervised aerobic aquatic training programme for 10 weeks, 3x per week, 45 minutes. Weeks 5 and 6 were land‐based weight resistance training. Aquatic exercise consisted of water jogging and deep water running NEX: control group was requested to not change their exercise habits | |
| Outcomes | POMS‐SF (including fatigue sub‐scale), MSPSS, MSQoL‐54 | |
| Notes | Drop‐outs No drop‐outs; mean adherence 27/30 training sessions Measurements Baseline, 8 weeks (2 weeks prior to end of intervention) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to group exercise training (EX) or control (NEX) Setting: Division of Physiotherapy and Rehabilitation, Istanbul University, Istanbul, Turkey Categorization: EX = mixed | |
| Participants | n = 110; EX = 55, NEX = 55 Inclusion criteria: definite MS, EDSS 2.0‐6.5, no relapse < 30 days, stable medication, no transport difficulties Exclusion criteria: other central nervous system disease, pregnant, medical condition precluding exercise participation, regular exercise < 3 months Type MS: RRMS, PPMS, and SPMS Disease duration (yr) ± SD: EX = 9.00 ± 4.71, NEX = 8.42 ± 5.38 Mean age (yr) ± SD: EX = 49.49 ± 9.37, NEX = 39.6 ± 11.18 % Female (n/n group): EX = 34/51, NEX = 30/48 Mean EDSS ± SD: EX = 4.38 ± 1.37, NEX = 4.21 ± 1.44 | |
| Interventions | EX: 12 weeks, 3x per week; 60‐minute group exercise programme (up to 7 participants per group) including flexibility, range of motion, strengthening with/without Theraband for lower extremity, core stabilization, balance, and co‐ordination exercises. Perceived exertion < 14 (6‐20 scale) NEX: waiting list | |
| Outcomes | FSS, BBS, 10MWT, 10‐stair climbing test, MAS, MusiQoL | |
| Notes | Drop‐outs EX: 1 exacerbation, 1 personal problems, 2 < 80% participation NEX: 1 participation in exercise programme, 3 exacerbation, 3 not coming to assessment Measurements Baseline, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | High risk | Complete randomization outcome known prior to trial |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Overall drop‐out rate was 10%. Somewhat imbalanced across exercise (7%) and control (13%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design (cross‐over): random assignment to immediate or delayed (EX) supervised treadmill training; the follow‐up phase of the immediate group and the phase prior to training in the delayed group served as control (NEX) Setting: School of Health Sciences, University of Birmingham, Birmingham, UK Categorization: EX = endurance | |
| Participants | n = 19; EX = 10, NEX = 9 Inclusion criteria: confirmed clinical diagnosis of MS, ability to follow training instructions, walk 10 metres < 60 seconds without hands on support, using an aid if necessary, able to walk on treadmill with or without hands on support Exclusion criteria: significant relapse < 2 months, serious medical condition that might impair ability to walk on a treadmill and participate in aerobic exercise Type MS: ? Disease duration (yr) ± SD: ? Age (yr): 30‐65 % Female (n/n group): 14/17 Mean EDSS ± SD: ? | |
| Interventions | EX: supervised treadmill training 3x per week/4 weeks. Walking duration increased up to 30 minutes, subsequently walking speed increased; target HR 55‐85% age predicted HRmax NEX: wait list or follow‐up phase | |
| Outcomes | FSS, 10MWT, 2MWT, GDNS, RMI, heart rate walking | |
| Notes | Drop‐outs EX: 2 unknown reason NEX: 1 unknown reason Measurements Baseline, 7 weeks, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐randomization using computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall drop‐out rate 17%; reasons for drop‐out not provided. |
| Selective reporting (reporting bias) | Unclear risk | Outcome on Rivermead Mobility Index was not reported |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to sports climbing (SC) or Hatha Yoga (HY) Setting: Multiple Sclerosis Center at the Division of Neurology, University Medical Center, Ljubljana, Slovenia Categorization: SC = other, HY = other | |
| Participants | n = 20; SC = 10, HY = 10 Inclusion criteria: RRMS, PPMS, or SPMS; EDSS ≤ 6.0; EDSS pyramidal functions score > 2.0; aged 26‐50 Exclusion criteria: none reported Type MS: RRMS, PPMS, and SPMS Disease duration (yr) ± SD: ? Median age (yr): SC = 42, HY = 41 % Female (n): ? Median EDSS: SC = 4, HY = 4.2 | |
| Interventions | SC: 10 weeks, 1x per week sports climbing on a 5‐metre wall, 90° inclination adjusted for participants by increasing the size and number of holds HY: 10 weeks, 1x per week Hatha yoga; stretching and strengthening exercises, breathing exercises | |
| Outcomes | MFIS, EDSS, MAS, executive function, selective attention, CES‐D | |
| Notes | Drop‐outs ? Measurements Baseline, 10 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure unknown |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of drop‐outs unknown |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Design: random assignment to robot‐assisted treadmill training first (R‐T) versus conventional body weight supported treadmill training first (T‐R) Setting: Department of Community Health, Brown University, Rhode Island, USA Categorization: R‐T = endurance, T‐R = endurance | |
| Participants | n = 13; R‐T = 6, T‐R = 7 Inclusion criteria: clinical diagnosis of MS by McDonald criteria, EDSS 4‐6 Exclusion criteria: myocardial infarction; uncontrolled hypertension or diabetes; symptomatic orthostasis; or body weight, joint, or lower‐limb musculoskeletal injuries that limited the range of motion necessary for safe use of the Lokomat Type MS: RRMS, PPMS, SPMS Disease duration (yr) ± SD: ? Mean age (yr) ± SD: 49.8 ± 11.1 % Female (n): 6 Mean EDSS ± SD: 4.9 ± 1.2 | |
| Interventions | R: robot (Lokomat) assisted, body weight supported treadmill training (2x per week, 3 weeks) T: conventional body weight supported treadmill training (2x per week, 3 weeks); In random order, including 6 weeks' wash‐out | |
| Outcomes | MFIS, MSQLI, FSS, SF‐36, PES, SSS, Bladder Control Scale, Bowel Control Scale, IVIS, PDQ‐5, MHI‐5, and MSSS‐5 | |
| Notes | Drop‐outs 0 Measurements Baseline, 6 weeks, 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | High risk | Unclear who performed coin toss |
| Blinding of participants and personnel (performance bias) | High risk | Due to the nature of the intervention, neither participants nor personnel could be blinded |
| Blinding of outcome assessment (detection bias) | High risk | Fatigue was self reported and therefore outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | No selective reporting identified |
| Other bias | Low risk | No other sources of bias identified |
For an overview of abbreviations, see Appendix 5.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The assessment of fatigue was not part of the study design | |
| Not an RCT | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| Not an RCT | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| No exercise intervention | |
| No exercise intervention | |
| Not an RCT | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| No exercise intervention | |
| Not an RCT | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| Not an RCT | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| The assessment of fatigue was not part of the study design | |
| Unable to translate | |
| The assessment of fatigue was not part of the study design | |
| No exercise intervention |
RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: random assignment to stretching + aerobic exercise (SA), aerobic exercise (A), or control (C) Setting: MS Society of Tehran, Iran |
| Participants | n = 120 Inclusion criteria: clinical definite MS, EDSS 1‐5.5 Exclusion criteria: unknown |
| Interventions | SA: stretching + aerobic exercise A: Aerobic exercise C: Control |
| Outcomes | Some demographic variables, disease variables, FSS |
| Notes | No English full‐text of this study was available during the conduction of this review. The above information was derived from the abstract |
| Methods | Design: random assignment to home‐based endurance and resistance training (EX) versus a control (NEX) Setting: community; mid‐Willamette Valley, Oregon, USA |
| Participants | n = 33; EX =17, NEX = 16 Inclusion criteria: healthy females with MS; ability to walk (with or without assistive device) for at least 20 metres without rest; not participated in a community‐based resistance training programme for at least 2 months prior to the study Exclusion criteria: ‐ Type MS: RRMS, PPMS, SPMS Disease duration (yr) ± SD: EX = 12.3 ± 10.2, NEX = 14.9 ± 11.7 Mean age (yr) ± SD: EX = 51.4 ± 7.7, NEX = 49.5 ± 9.5 % Female (n): all female Mean EDSS ± SD: EX = 3.7 ± 4.2, NEX = 3.4 ± 3.5 |
| Interventions | EX: 6 supervised, regional‐based, instructional sessions on warm‐up, stretching, resistance exercises, and cool‐down. Followed by 6 weeks' home‐based functional resistance training. 5 different exercises: chair raises, forward lunges, step‐ups, toe‐raises, and hamstring curls. Weighted vests were included to increase intensity NEX: Unknown |
| Outcomes | VAS‐Fatigue (daily), VAS‐Physical Activity (daily), EDSS, Health and History Questionnaire, CES‐D‐10, MAS, lower extremity power, TUG |
| Notes | Drop‐outs EX: 1 MS relapse NEX: 1 MS relapse, 2 conflicting schedules Measurements Baseline, 8 weeks (at both time points the actual measurement was preceded by a familiarization measurement on the day prior the actual one) |
For an overview of abbreviations, see Appendix 5.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 36 | 1603 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.57, ‐0.13] |
| Analysis 1.1  Comparison 1 Overall analysis, Outcome 1 Fatigue. | ||||
| 1.1 Exercise versus non‐exercise control | 27 | 1325 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.81, ‐0.34] |
| 1.2 Exercise versus exercise | 9 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.00, 0.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| Analysis 2.1  Comparison 2 Sensitivity analysis (Intervention contrast), Outcome 1 Fatigue. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| Analysis 3.1 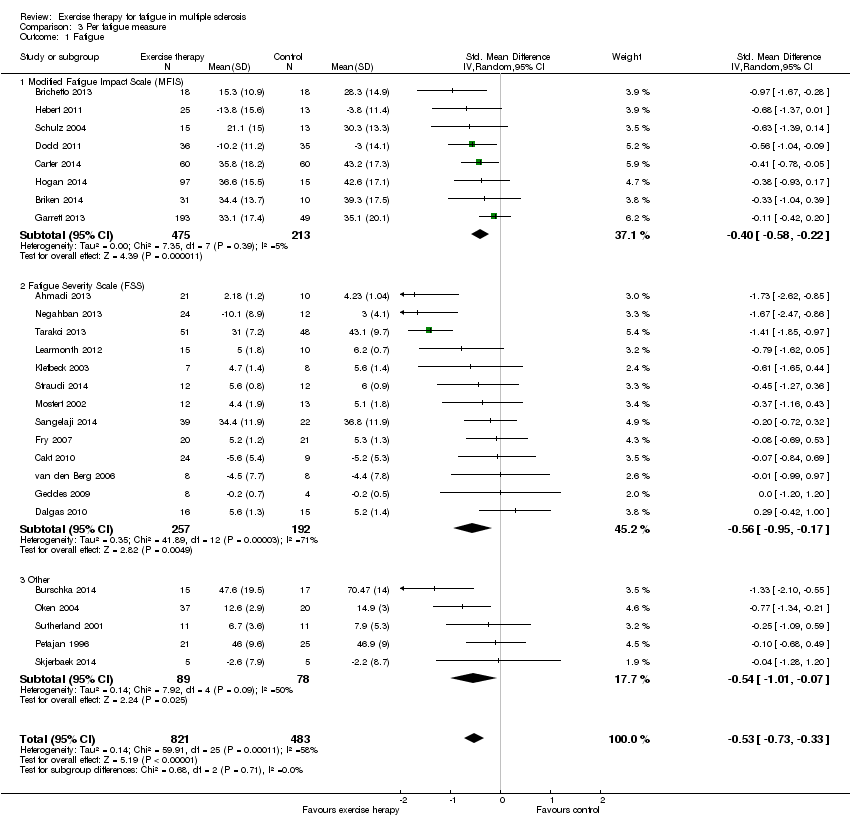 Comparison 3 Per fatigue measure, Outcome 1 Fatigue. | ||||
| 1.1 Modified Fatigue Impact Scale (MFIS) | 8 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] |
| 1.2 Fatigue Severity Scale (FSS) | 13 | 449 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.95, ‐0.17] |
| 1.3 Other | 5 | 167 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.01, ‐0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1299 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.72, ‐0.32] |
| Analysis 4.1  Comparison 4 Per exercise group (random‐effects model), Outcome 1 Fatigue. | ||||
| 1.1 Endurance | 11 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.78, ‐0.15] |
| 1.2 Muscle power | 4 | 207 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.65, 0.71] |
| 1.3 Task‐oriented | 2 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.02, 0.33] |
| 1.4 Mixed | 6 | 495 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.23, ‐0.23] |
| 1.5 Other | 9 | 295 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [1.00, ‐0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1299 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.62, ‐0.37] |
| Analysis 5.1  Comparison 5 Per exercise group (fixed‐effect model), Outcome 1 Fatigue. | ||||
| 1.1 Endurance | 11 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.69, ‐0.17] |
| 1.2 Muscle power | 4 | 207 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.53, 0.15] |
| 1.3 Task‐oriented | 2 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.02, 0.33] |
| 1.4 Mixed | 6 | 495 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.83, ‐0.43] |
| 1.5 Other | 9 | 295 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.79, ‐0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 14 | 801 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.95, ‐0.32] |
| Analysis 6.1  Comparison 6 Sensitivity analysis (methodological quality), Outcome 1 Fatigue. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.

Funnel plot of trials comparing exercise versus a non‐exercise control condition.
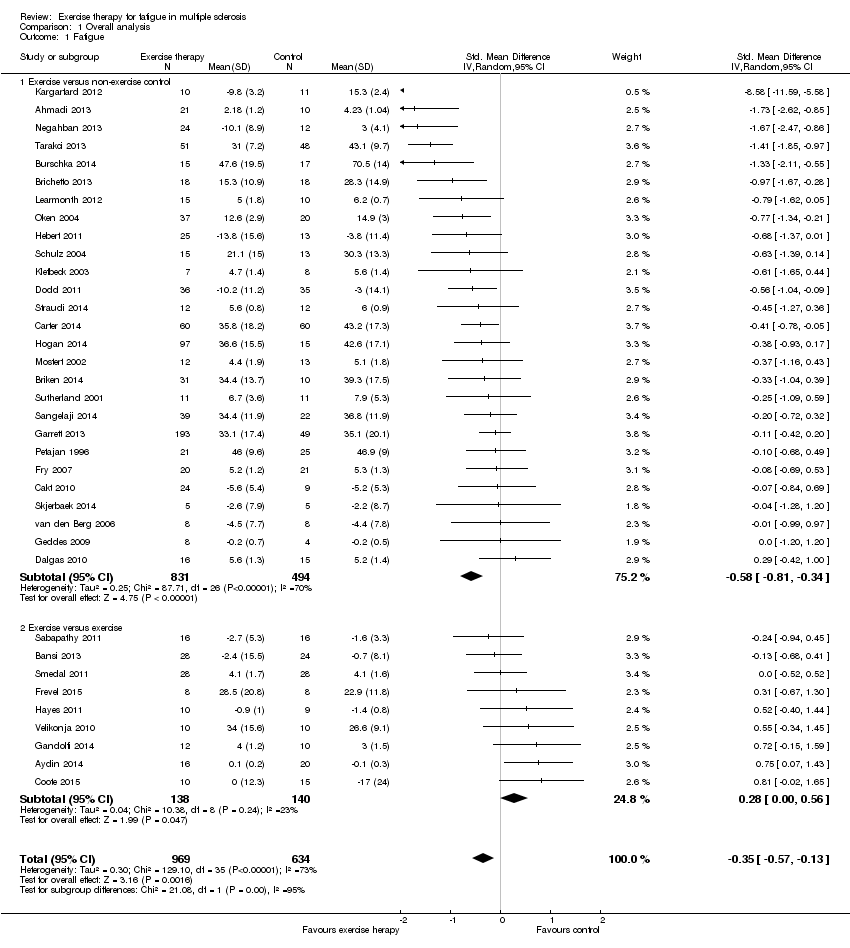
Comparison 1 Overall analysis, Outcome 1 Fatigue.

Comparison 2 Sensitivity analysis (Intervention contrast), Outcome 1 Fatigue.

Comparison 3 Per fatigue measure, Outcome 1 Fatigue.
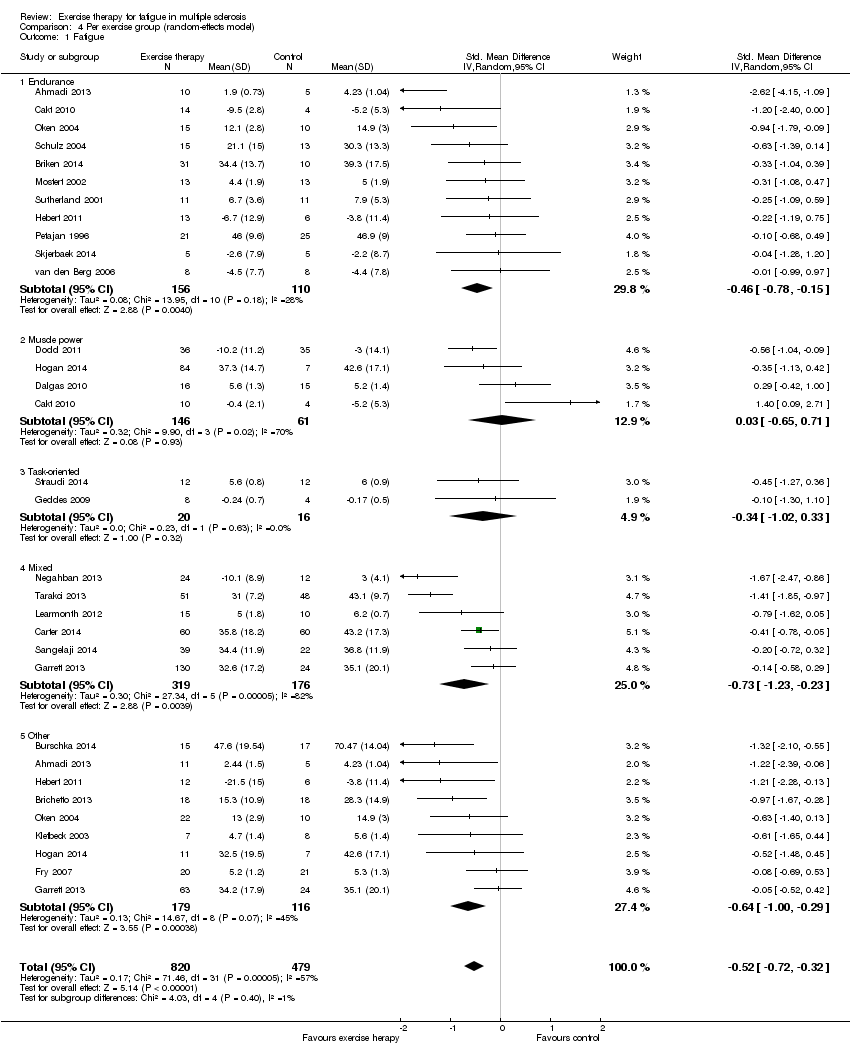
Comparison 4 Per exercise group (random‐effects model), Outcome 1 Fatigue.

Comparison 5 Per exercise group (fixed‐effect model), Outcome 1 Fatigue.

Comparison 6 Sensitivity analysis (methodological quality), Outcome 1 Fatigue.
| Effect of exercise therapy for fatigue in multiple sclerosis ‐ overall analysis | |||||
| Patient or population: people with multiple sclerosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Overall analysis | ||||
| Fatigue | No risk assumed | The mean fatigue outcome in the intervention groups was | 1603 | ⊕⊕⊕⊝ | Indirectness (‐1) |
| Exercise versus no‐exercise control | No risk assumed | The mean fatigue outcome in the intervention groups was 0.58 standard deviations lower (0.81 to 0.34 lower) compared to a no‐exercise control group | 1325 | ⊕⊕⊕⊝ | Indirectness (‐1) |
| Exercise versus exercise | No risk assumed | The mean fatigue outcome in the intervention groups was 0.28 standard deviations higher (0 to 0.56 higher) compared to an exercise control group | 278 | ⊕⊕⊝⊝ | Indirectness (‐1) Imprecision (‐1) |
| *The argumentation for downgrading the grades of evidence is provided in the footnotes. CI: confidence interval. | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The presence of fatigue, beyond a pre‐defined level, was most often not an inclusion criterion. In addition, fatigue was not a primary outcome. | |||||
| Effect of exercise therapy for fatigue in multiple sclerosis ‐ analysis per fatigue measure | |||||
| Patient or population: people with multiple sclerosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Per fatigue measure | ||||
| Modified Fatigue Impact Scale | No risk assumed | The mean fatigue, on the Modified Fatigue Impact Scale, in the intervention groups was 0.40 standard deviations lower (0.58 to 0.22 lower) | 688 | ⊕⊕⊕⊝ | Indirectness (‐1) |
| Fatigue Severity Scale | No risk assumed | The mean fatigue, on the Fatigue Severity Scale, in the intervention groups was 0.56 standard deviations lower (0.95 to 17 lower) | 449 | ⊕⊕⊕⊝ | Indirectness (‐1) |
| Other | No risk assumed | The mean fatigue, on the 'other' included fatigue measures, in the intervention groups was 0.54 standard deviations lower (1.01 to 0.07 lower) | 167 | ⊕⊕⊝⊝ | Indirectness (‐1) Imprecision (‐1) |
| *The argumentation for downgrading the grades of evidence is provided in the footnotes. CI: confidence interval. | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The presence of fatigue, beyond a pre‐defined level, was most often not an inclusion criterion. In addition, fatigue was not a primary outcome. | |||||
| Effect of exercise therapy for fatigue in multiple sclerosis ‐ analysis per exercise modality | |||||
| Patient or population: people with multiple sclerosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | ||||
| Control | Per exercise group | ||||
| Endurance training | No risk assumed | The mean fatigue outcome in the intervention groups applying endurance training was 0.43 standard deviations lower (0.69 to 0.17 lower) | 266 | ⊕⊕⊕⊝ | Indirectness (‐1) |
| Muscle power training | No risk assumed | The mean fatigue outcome in the intervention groups applying muscle power training was 0.03 standard deviations higher (0.65 lower to 0.71 higher) | 207 | ⊕⊕⊝⊝ | Indirectness (‐1) Imprecision (‐1) |
| Task‐oriented training | No risk assumed | The mean fatigue outcome in the intervention groups applying task‐oriented training was 0.34 standard deviations lower (1.02 lower to 0.33 higher) | 36 | ⊕⊝⊝⊝ | Indirectness (‐1) Imprecision (‐2) |
| Mixed training | No risk assumed | The mean fatigue outcome in the intervention groups applying mixed training was 0.73 standard deviations lower (1.23 to 0.23 lower) | 495 | ⊕⊕⊕⊝ | Indirectness (‐1) |
| 'Other' training | No risk assumed | The mean fatigue outcome in the intervention groups applying 'Other' types of training was 0.54 standard deviations lower (0.79 to 0.29 lower) | 295 | ⊕⊕⊝⊝ | Indirectness (‐1) Imprecision (‐1) |
| *The argumentation for downgrading the grades of evidence is provided in the footnotes. The data is some sub‐groups was heterogeneous, and in some homogeneous. Hence, data for this 'Summary of findings' table is extracted from Analysis 4.1 and 5.1. CI: confidence interval. | |||||
| GRADE Working Group grades of evidence | |||||
| 1 The presence of fatigue, beyond a pre‐defined level, was most often not an inclusion criterion. In addition, fatigue was not a primary outcome. | |||||
| Study ID | Fatigue scale | ITT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total score |
| Ahmadi 2013 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Aydin 2014 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Bansi 2013 | FSMC | no | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Brichetto 2013 | MFIS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Briken 2014 | MFIS | no | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Burschka 2014 | FSMC | no | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Cakt 2010 | FSS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 5 |
| Carter 2014 | MFIS | yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Castro‐Sanchez 2012 | MFIS, FSS | no | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 |
| Collett 2011 | FSS | yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Coote 2014 | MFIS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Dalgas 2010 | FSS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Dettmers 2009 | MFIS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 |
| Dodd 2011 | MFIS | yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Frevel 2014 | MFIS, FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Fry 2007 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 5 |
| Gandolfi 2014 | FSS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Garrett 2013 | MFIS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Geddes 2009 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Hayes 2011 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Hebert 2011 | MFIS | yes | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Hogan 2014 | MFIS | no | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Kargarfard 2012 | MFIS | yes | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| Klefbeck 2003 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5 |
| Learmonth 2011 | FSS | yes | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 6 |
| McCullagh 2008 | MFIS | no | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |
| Mori 2011 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 4 |
| Mostert 2002 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Negahban 2013 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Oken 2004 | MFI (general) | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 4 |
| Petajan 1996 | POMS fatigue, FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 4 |
| Plow 2009 | MFIS | no | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| Rampello 2007 | MFIS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Sabapathy 2010 | MFIS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Sangelaji 2014 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
| Schulz 2004 | MFIS | no | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| Smedal 2011 | FSS | yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Skjerbaek 2014 | FSMC | no | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Straudi 2014 | FSS | yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Surakka 2004 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Sutherland 2001 | POMS fatigue | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
| Tarakci 2013 | FSS | no | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| van den Berg 2006 | FSS | no | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 7 |
| Velikonja 2010 | MFIS | no | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 4 |
| Wier 2011 | FSS | no | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| % of trials | 96% | 42% | 87% | 0% | 0% | 0% | 53% | 80% | 80% | 89% | |||
| Risk of bias assessment based on the PEDro scale; see Appendix 5 for abbreviations. 1: Random allocation. 2: Concealed allocation. 3: Groups similar at baseline on disease severity, fatigue, and depression (if reported). 4: Blinding of all participants (zero per definition). 5: Blinding of all therapists. 6: Blinding of assessors. 7: Measures of key outcome (fatigue) > 85% of participants initially allocated to group (rated for fatigue outcome). 8: All participants of whom outcome is available received treatment or control; if not, intention‐to‐treat (ITT) analysis was performed. 9: Between‐group statistics of fatigue outcome reported. 10: Point measures and measures of variability for fatigue provided. | |||||||||||||
| Study | Time (i.e. duration of intervention) | Fatigue scale | Effect | Other outcomes | Effect |
| Ahmadi 2013 Compared aerobic training vs. control | 8 weeks | FSS | ‐ | BBS Walk time Walk distance BDI BAI | + ‐ + ns ns |
| Ahmadi 2013 Compared yoga vs. control | 8 weeks | FSS | ‐ | BBS Walk time Walk distance BDI BAI | + ns + ‐ ‐ |
| Brichetto 2013 Compared Nintendo® Wii® balance training vs. control | 4 weeks | MFIS | ns | BBS Open‐eye stabilometry Closed‐eye stabilometry | + ‐ ‐ |
| Briken 2014 Compared arm‐ergometry vs. control | 10 weeks | MFIS | ‐ | VO2peak 6MWT SDMT VLMT TAP (alertness) TAP (shift of attention) LPS RWT IDS ‐ SR30 | ns + ns + ns ‐ ns ns ‐ |
| Briken 2014 Compared rowing vs. control | 10 weeks | MFIS | ns | VO2peak 6MWT SDMT VLMT TAP (alertness) TAP (shift of attention) LPS RWT IDS ‐ SR30 | ns ns ns + ns ns ns ns ns |
| Briken 2014 Compared bicycling vs. control | 10 weeks | MFIS | ns | VO2peak 6MWT SDMT VLMT TAP (alertness) TAP (shift of attention) LPS RWT IDS ‐ SR30 | + + ns + ‐ ‐ ns ns ‐ |
| Burschka 2014 Compared Tai‐Chi yoga vs. control * no change of fatigue in experimental group, increase in fatigue in control group | 24 weeks | FSMC* | ‐ | Balance Co‐ordination CES‐D QLS | + + ‐ + |
| Cakt 2010 Compared progressive resistance training vs. control | 8 weeks | FSS | ‐ | Duration of exercise Wmax TUG DGI FR FES 10MWT BDI SF‐36 | + + ‐ + + ‐ ‐ ‐ unk |
| Cakt 2010 Compared home‐based exercise vs. control | 8 weeks | FSS | ns | Duration of exercise Wmax TUG DGI FR FES 10MWT BDI SF‐36 | ns ns ns ns ns ns ns ns unk |
| Carter 2014 Compared a pragmatic exercise intervention vs. control | 12 weeks | MFIS | ‐ | GLTEQ Accelerometer MSQoL‐54 MSFC 6MWT EDSS | + + + ns ns ns |
| Castro‐Sanchez 2012 Compared Ai‐Chi aquatic programme vs. control | 20 weeks | FSS MFIS ‐ physical ‐ cognitive ‐ psychosocial | ns ‐ ns ns | Pain MPQ‐PRI MPQ‐PPI RMDQ Spasm ‐ physical ‐ psychological BDI BI | ‐ ‐ ns ‐ ‐ ‐ ‐ ‐ ns |
| Dalgas 2010 Compared progressive resistance training vs. control | 12 weeks | FSS MFI‐20 ‐ General fatigue ‐ Physical fatigue ‐ Reduced activity ‐ Reduced motivation ‐ Mental fatigue | ‐ ‐ ns ns ns ns | MDI SF‐36 ‐ PCS ‐ MCS MVC (knee extensor) FS (%) | ‐ ‐ ns + + |
| Dodd 2011 Compared progressive resistance training vs. control | 10 weeks | MFIS ‐ physical ‐ cognitive ‐ psychosocial | ‐ ‐ ns ns | MSIS‐88 muscle stiffness MSIS‐88 muscle spasms 2MWT Walking speed Leg press endurance (repetitions) Reversed leg press endurance (repetitions) 1RM leg press (kg) 1RM reversed leg press (kg) WHOQoL‐BREF overall quality of life WHOQoL‐BREF overall health WHOQoL‐BREF physical health | ns ns ns ns ns + ns ns ns ns + |
| Fry 2007 Compared inspiratory muscle training vs. control | 10 weeks | No interaction effects reported | |||
| Garrett 2013 Compared physiotherapist‐led exercise vs. control | 10 weeks | MFIS ‐ physical ‐ cognitive | ‐ ‐ ns | MSIS‐29 physical component MSIS‐29 cognitive component 6MWT | ‐ ‐ + |
| Garrett 2013 Compared fitness instructor‐led exercise vs. control | 10 weeks | MFIS ‐ physical ‐ cognitive | ‐ ‐ ns | MSIS‐29 physical component MSIS‐29 cognitive component 6MWT | ‐ ‐ + |
| Garrett 2013 Compared yoga vs. control | 10 weeks | MFIS ‐ physical ‐ cognitive | ‐ ‐ ns | MSIS‐29 physical component MSIS‐29 cognitive component 6MWT | ns ‐ ns |
| Geddes 2009 Compared home walking vs. control | 12 weeks | FSS | ns | 6MWT PCI RPE | ns ns ns |
| Hebert 2011 Compared vestibular rehabilitation vs. no exercise control | 6 weeks | MFIS | ‐ | SOT DHI 6MWT | + ‐ ns |
| Hebert 2011 Compared exercise control vs. no exercise control | 6 weeks | MFIS | ns | SOT DHI 6MWT | ns ns ns |
| Hogan 2014 Compared group physiotherapy vs. control | 10 weeks | MFIS | ns | BBS 6MWT MSIS29v2 | + ns ns |
| Hogan 2014 Compared individual physiotherapy vs. control | 10 weeks | MFIS | ns | BBS 6MWT MSIS29v2 | + ns ns |
| Hogan 2014 Compared yoga vs. control | 10 weeks | MFIS | ns | BBS 6MWT MSIS29v2 | + ns ns |
| Kargarfard 2012 Compared aquatic training vs. control | 8 weeks | MFIS ‐ physical ‐ psychosocial ‐ cognitive | ‐ ‐ ‐ ‐ | MSQoL‐54 ‐ Physical ‐ Mental | + + |
| Klefbeck 2003 Compared inspiratory muscle training vs. control | 10 weeks | No interaction effects reported | |||
| Learmonth 2011 Compared leisure centre‐based exercise group vs. control | 12 weeks | FSS | ns | T25FW 6MWT BBS TUG QPW BMI PF ABC HADS LMSQoL | ns ns ns ns ns ns + ns ns ns |
| McCullagh 2008 Compared group circuit training vs. control | 12 weeks | No interaction effects reported | |||
| Mori 2011 Compared transcranial magnetic stimulation (TMS) vs. control | 2 weeks | No interaction effects reported | |||
| Mori 2011 Compared exercise control vs. control | 2 weeks | No interaction effects reported | |||
| Mostert 2002 Compared short‐term exercise vs. control | 4 weeks | No interaction effects reported | |||
| Negahban 2013 Compared exercise therapy vs. control | 5 weeks | FSS | ‐ | VAS scale for pain MAS BBS TUG 10MWT 2MWT MSQoL‐54 | ns ‐ + ‐ ‐ + ns |
| Negahban 2013 Compared massage + exercise therapy vs. control | 5 weeks | FSS | ‐ | VAS scale for pain MAS BBS TUG 10MWT 2MWT MSQoL‐54 | ‐ ns + ‐ ‐ + ns |
| Oken 2004 Compared Iyengar yoga classes plus home programme vs. control | 24 weeks | No interaction effects reported | |||
| Oken 2004 Compared weekly bicycle exercise classes along with home exercise vs. control | 24 weeks | No interaction effects reported | |||
| Petajan 1996 Compared aerobic training vs. control | 15 weeks | FSS POMS ‐ fatigue | ns ns | EDSS ISS VO2max PWC HRmax Upper extremity strength Lower extremity strength POMS SIP | ns ns + + ns + + ns ns |
| Sangelaji 2014 Compared combination exercise therapy vs. control | 10 weeks | FSS | ‐ | EDSS BBS 6MWT MSQoL | ns + + + |
| Schulz 2004 Compared aerobic interval training vs. control | 8 weeks | MFIS ‐ physical ‐ cognitive ‐ social | ns ns ns ns | Wmax VO2max HRmax W endurance Lactate change Immune and neurotrophic factors IL‐6 (rest) IL‐6 (AUC) sIL‐6R (rest) sIL‐6R (AUC) BDNF (rest) BDNF (AUC) NGF (rest) NGF (AUC) HAQUAMS ‐ fatigue/thinking ‐ mobility lower ‐ mobility upper ‐ social function ‐ mood | ns ns ns ns ‐ ns ns ns ‐ ns ns ns ns ‐ ns ns ns ‐ ‐ |
| Skjerbaek 2014 Compared endurance training vs. control | 4 weeks | FSMC | ns | VO2peak HRpeak 9HPT Hand grip power Box and blocks 6‐minute wheelchair MDI MSIS‐29 | ns ns ns ns ns ns ns ns |
| Straudi 2014 Compared task‐oriented circuit training vs. control | 2 weeks | FSS | ns | 10MWT 6MWT TUG DGI MSWS‐12 MSIS‐29 ‐ physical ‐ psychosocial ‐ psychological | ns + ns ns + + + ‐ |
| Surakka 2004 Compared inpatient rehabilitation plus home‐based exercise vs. control | 26 weeks | FSS | ns | Leg flexor/extensor torque Motor fatigue Ambulatory fatigue index | ns ns ns |
| Sutherland 2001 Compared aerobic aquatic training vs. control | 10 weeks | No interaction effects reported | |||
| Tarakci 2013 Compared group exercise programme vs. control | 12 weeks | FSS | ‐ | BBS 10MWT R Hip flexors MAS R Hamstring MAS R Achilles MAS MusiQoL | + ‐ ‐ ‐ ‐ ‐ ‐ ‐ ‐ + |
| van den Berg 2006 Compared treadmill exercise vs. control (cross‐over) | 4 weeks | No interaction effects reported | |||
| ns, non‐significant; '+', a significant group‐by‐time effect in favour of the exercise group versus the non‐exercise control group; '‐' , a significant negative group‐by‐time effect in the exercise group versus the non‐exercise control group. For an overview of abbreviations, see Appendix 5. | |||||
| Study | Time (i.e. duration of intervention) | Fatigue scale | Effect | Other outcomes | Effect |
| Ahmadi 2013 Compared aerobic training vs. yoga | 8 weeks | FSS | ns | BBS Walk time Walk distance BDI BAI | ns ns ns ns ‐ |
| Aydin 2014 Compared hospital‐based callisthenic exercise vs. home‐based callisthenic exercise | 12 weeks | FSS | ns | 10MWT BBS MusiQoL HADS depression HADS anxiety | ns + ns + ns |
| Bansi 2013 Compared overland endurance training vs. aquatic endurance training | 3 weeks | FSMC ‐ motor ‐ cognitive | ns ns ns | Loadmax VO2peak HRpeak BORG | ns ns ns ns |
| Briken 2014 Compared arm‐ergometry vs. rowing | 10 weeks | No interaction effects reported | |||
| Briken 2014 Compared rowing vs. bicycling ergometry | 10 weeks | No interaction effects reported | |||
| Briken 2014 Compare arm‐ergometry vs. bicycling ergometry | 10 weeks | No interaction effects reported | |||
| Cakt 2010 Compared progressive resistance training vs. home‐based exercise | 8 weeks | FSS | ‐ | Duration of exercise Wmax TUG DGI FR FES 10MWT BDI SF‐36 | + + ‐ + + ‐ ns ‐ unk |
| Collett 2011 Compared endurance training vs. intermittent endurance training | 12 weeks | No interaction effects reported | |||
| Collett 2011 Compared intermittent training vs. mixed endurance training | 12 weeks | No interaction effects reported | |||
| Collett 2011 Compared endurance training vs. mixed endurance training | 12 weeks | No interaction effects reported | |||
| Coote 2014 Compared progressive resistance training vs. progressive resistance training augmented by neuromuscular electrical stimulation | 12 weeks | MFIS | ‐ | Quadriceps strength Hip strength Quadriceps endurance VAS lower limb spasticity TUG MSWS‐12 BBS MSIS29v2 | ns ns ns ns ns ns ns ns |
| Dettmers 2009 Compared endurance training vs. control treatment | 3 weeks | MFIS | ns | FSMC Maximal walking distance rWa BDI HAQUAMS | ns + + ns ns |
| Frevel 2014 Compare Internet home‐based training vs. hippotherapy | 12 weeks | MFIS FSS | ns ns | BBS DGI Isometric muscle strength TUG 2MWT HAQUAMS | ns ns ns ns ns ns |
| Gandolfi 2014 Compared robot‐assisted gait training vs. sensory integration balance training | 6 weeks | FSS | ns | Gait analysis BBS SOT Stabilometric assessment MSQoL‐54 | ns ns ns ns ns |
| Garrett 2013 Compared physiotherapist‐led exercise vs. fitness instructor‐led exercise | 10 weeks | No interaction effects reported | |||
| Garrett 2013 Compared fitness instructor‐led exercise vs. yoga | 10 weeks | No interaction effects reported | |||
| Garrett 2013 Compared physiotherapist‐led exercise vs. yoga | 10 weeks | No interaction effects reported | |||
| Hayes 2011 Compared a resistance training programme supplementary to a standard exercise programme vs. standard exercise programme | 12 weeks | FSS | ns | Isometric strength 6MWT TUG Stair ascent Stair descent 10MWT self paced 10MWT max paced BBS | ns ns ns + + ns ns ‐ |
| Hebert 2011 Compared vestibular rehabilitation vs. exercise control | 6 weeks | MFIS | ‐ | SOT DHI 6MWT | + ‐ ns |
| Hogan 2014 Compared group physiotherapy vs. individual physiotherapy | 10 weeks | MFIS | unk | BBS 6MWT MSIS29v2 | ns unk unk |
| Hogan 2014 Compared individual physiotherapy vs. yoga | 10 weeks | No interaction effects reported | |||
| Hogan 2014 Compared group physiotherapy vs. yoga | 10 weeks | No interaction effects reported | |||
| Mori 2011 Compared transcranial magnetic stimulation (TMS) vs. exercise control | 2 weeks | No interaction effects reported | |||
| Negahban 2013 Compared exercise therapy vs. exercise therapy + massage | 5 weeks | FSS | ns | VAS scale for pain MAS BBS TUG 10MWT 2MWT MSQoL‐54 | ‐ ns ns ns ns ns ns |
| Oken 2004 Compared Iyengar yoga classes vs. weekly bicycle exercise classes | 24 weeks | No interaction effects reported | |||
| Plow 2009 Compared individualized physical rehabilitation vs. group wellness intervention | 8 weeks | No interaction effects reported | |||
| Rampello 2007 Compared aerobic training vs. neurorehabilitation programme (cross‐over) | 8 weeks | No interaction effects reported | |||
| Sabapathy 2011 Compared resistance training vs. endurance training (cross‐over) | 8 weeks | No interaction effects reported | |||
| Smedal 2011 Compared warm vs. cold climate physiotherapy | 4 weeks | FSS | ns | 6MWT RPE TUG 10MWT BBS TIS MSIS‐29 physical MSIS‐29 psychosocial MHAQ Pain Balance Gait | + ‐ ns ns ns ns ‐ ‐ ‐ ‐ ‐ ‐ |
| Velikonja 2010 Compared sports climbing vs. yoga | 10 weeks | No interaction effects reported | |||
| Wier 2011 Compared robot‐assisted treadmill training vs. body‐weight supported treadmill training | 3 weeks | No interaction effects reported | |||
| ns, non‐significant; '+', a significant group‐by‐time effect in favour of the experimental exercise condition versus the exercise control condition; '‐' , a significant negative group‐by‐time effect in the experimental exercise condition versus the exercise control condition; unk, unknown. For an overview of abbreviations, see Appendix 5. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 36 | 1603 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.57, ‐0.13] |
| 1.1 Exercise versus non‐exercise control | 27 | 1325 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.81, ‐0.34] |
| 1.2 Exercise versus exercise | 9 | 278 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.00, 0.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.73, ‐0.33] |
| 1.1 Modified Fatigue Impact Scale (MFIS) | 8 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] |
| 1.2 Fatigue Severity Scale (FSS) | 13 | 449 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.95, ‐0.17] |
| 1.3 Other | 5 | 167 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.01, ‐0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1299 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.72, ‐0.32] |
| 1.1 Endurance | 11 | 266 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.78, ‐0.15] |
| 1.2 Muscle power | 4 | 207 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.65, 0.71] |
| 1.3 Task‐oriented | 2 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.02, 0.33] |
| 1.4 Mixed | 6 | 495 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.23, ‐0.23] |
| 1.5 Other | 9 | 295 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [1.00, ‐0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 26 | 1299 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐0.62, ‐0.37] |
| 1.1 Endurance | 11 | 266 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.69, ‐0.17] |
| 1.2 Muscle power | 4 | 207 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.53, 0.15] |
| 1.3 Task‐oriented | 2 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐1.02, 0.33] |
| 1.4 Mixed | 6 | 495 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.63 [‐0.83, ‐0.43] |
| 1.5 Other | 9 | 295 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.79, ‐0.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fatigue Show forest plot | 14 | 801 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.95, ‐0.32] |

