Techniki deeskalacyjne a agresja lub pobudzenie o podłożu psychotycznym
Abstract
Background
Aggression is a disposition, a willingness to inflict harm, regardless of whether this is behaviourally or verbally expressed and regardless of whether physical harm is sustained.
De‐escalation is a psychosocial intervention for managing people with disturbed or aggressive behaviour. Secondary management strategies such as rapid tranquillisation, physical intervention and seclusion should only be considered once de‐escalation and other strategies have failed to calm the service user.
Objectives
To investigate the effects of de‐escalation techniques in the short‐term management of aggression or agitation thought or likely to be due to psychosis.
Search methods
We searched Cochrane Schizophrenia Group's Study‐Based Register of Trials (latest search 7 April, 2016).
Selection criteria
Randomised controlled trials using de‐escalation techniques for the short‐term management of aggressive or agitated behaviour. We planned to include trials involving adults (at least 18 years) with a potential for aggressive behaviour due to psychosis, from those in a psychiatric setting to those possibly under the influence of alcohol or drugs and/or as part of an acute setting as well. We planned to include trials meeting our inclusion criteria that provided useful data.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Two review authors inspected all abstracts of studies identified by the search process. As we were unable to include any studies, we could not perform data extraction and analysis.
Main results
Of the 345 citations that were identified using the search strategies, we found only one reference to be potentially suitable for further inspection. However, after viewing the full text, it was excluded as it was not a randomised controlled trial.
Authors' conclusions
Using de‐escalation techniques for people with psychosis induced aggression or agitation appears to be accepted as good clinical practice but is not supported by evidence from randomised trials. It is unclear why it has remained such an under‐researched area. Conducting trials in this area could be influenced by funding flow, ethical concerns ‐ justified or not ‐ anticipated pace of recruitment as well the difficulty in accurately quantifying the effects of de‐escalation itself. With supportive funders and ethics committees, imaginative trialists, clinicians and service‐user groups and wide collaboration this dearth of randomised research could be addressed.
PICOs
Streszczenie prostym językiem
Techniki deeskalacyjne a agresja lub pobudzenie o podłożu psychotycznym
Pytanie przeglądu
Czy techniki deeskalacyjne są skuteczne w opanowaniu agresji lub pobudzenia wywołanego psychozą?
Wprowadzenie
Agresja to chęć wyrządzenia szkody, niezależnie od tego, czy będzie wyrażona słownie czy na poziomie zachowania i niezależnie od tego, czy występuje krzywda fizyczna.
Deeskalacja jest interwencją psychospołeczną mającą na celu opanowanie agresywnego lub pobudzonego zachowania. Obejmuje techniki, które pomagają osobie agresywnej lub pobudzonej w samodzielnej kontroli swoich emocji tak, aby zahamować eskalację zachowań agresywnych.
Wyszukiwanie
Przeprowadzono elektroniczne wyszukiwanie (ostatnie wyszukiwanie: kwiecień 2016) badań z randomizacją, w których osoby z psychozą przejawiające zachowania agresywne lub pobudzone otrzymywały leczenie obejmujące techniki deeskalacyjne, standardową opiekę lub inną interwencję w celu opanowania agresji. Wyszukano 345 badań, które zostały sprawdzone przez autorów przeglądu.
Wyniki
Nie znaleziono badań spełniających kryteriów przeglądu. Obecnie nie ma dostępnych danych opartych na badaniach naukowych, które oceniają skuteczność technik deeskalacyjnych w radzeniu sobie z agresją lub pobudzeniem.
Wnioski
Nie jest jasne, dlaczego nie istnieją żadne badania z randomizacją dotyczące tego tematu. Kilka kwestii, takich jak koszty badania, zagadnienia etyczne, trudności z rekrutacją osób do badań, a także możliwość właściwej oceny efektów technik deeskalacyjnych, może mieć znaczenie. Tymczasem techniki te są obecnie stosowane bez żadnych naukowych dowodów potwierdzających ich skuteczność.
Authors' conclusions
Summary of findings
| Patient or population: people who are aggressive secondary to serious mental illness | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care control | De‐escalation technique | |||||
| Clinically important changes in global state | We identified no relevant studies. | |||||
| Aggression ‐ Improved to an important extent | ||||||
| Aggression ‐ deterioration: incidence of violence to self or others | ||||||
| Aggression ‐ changes in aggression as recorded by any other outcomes | ||||||
| Adverse effects ‐ physical adverse effects | ||||||
| Adverse effects ‐ death, suicide or natural causes | ||||||
| Adverse effects ‐ psychological adverse effects | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Aggression is a range of behaviours or actions to inflict harm, hurt or injury to anther person regardless of whether this is behaviourally or verbally expressed and regardless of whether physical harm is sustained or intention is clear (NICE 2015). Aggression can present in a variety of settings including emergency rooms, inpatient unit, community and primary care settings and some times in police cells. Untreated, this can quickly escalate risks to the patient, healthcare professionals and family members who are often victims of violence (Bourget 2002; Maguire 2007).
The incidence of aggression is more frequent in emergency departments (25%) and inpatient psychiatric hospitals (32.4%) (Bowers 2011; NHS Protect 2013). The relationship between psychosis and violence is uncertain. There is some evidence to suggest that individuals with a mental disorder/severe mental disorder are more likely to be violent than the general population (Fazel 2006; van Dorn 2012); however others have argued that it is substance misuse, instead of a mental health problem itself that increases the risk for violence (Elbogen 2009). A recently published guideline indicated that a mental disorder is probably only a predictive factor of violence, and that substance misuse is more likely related to the incidence of violence (NICE 2015). Swift containment of the situation is essential and preventive de‐escalation techniques, such as restraint, should be utilised prior to interventional measures for the management of aggression (NICE 2015).
Description of the intervention
De‐escalation is an intervention using emotional regulation or self‐management techniques to avert aggressive or violent behaviour (NICE 2015). 'The assault cycle' typically includes a trigger phase, escalation phase, crisis phase, recovery phase and depression phase (Kaplan 1983). De‐escalation is a complex range of skills designed to abort the assault cycle during the escalation phase, and this includes both verbal and non‐verbal communication skills (CRAG 1996).
De‐escalation is part of the process of managing aggression and is usually recommended as either a preventative measure or an early step to prevent deterioration in the patient's condition. Secondary management strategies such as rapid tranquillisation, physical intervention and seclusion should only be considered once de‐escalation and other strategies have failed to calm the service user (NICE 2015). Regrettably, these recommendations are impractical in most emergency medical settings in developing countries, where resources are strained by heavy patient loads and understaffing, and where a sleeping patient is better than one who needs constant observation to assess the need for tranquillisation. From the patient's perspective, being asleep is also less traumatic than being physically restrained (Andrade 2007).
How the intervention might work
There are competing theoretical approaches to de‐escalation, including verbal de‐escalation. Some approaches make use of communication theory (Paterson 1997), others of situational analysis (Rix 2001). All approaches emphasise the need to observe for signs and symptoms of anger and agitation, approaching the person in a calm controlled manner, giving choices and maintaining the service user's dignity. Some approaches suggest mirroring the patient's mood.
De‐escalation methods help by establishing a positive therapeutic alliance with the patient, and the active collaboration of the patient in the treatment process with behavioural expectations and prescribed treatments it makes it easier to manage patient‐staff conflicts and de‐escalate aggressive episodes (Levenson 2004).
Why it is important to do this review
Guidelines recommend de‐escalation as an intervention in their algorithms for managing patients with acute aggression (NICE 2015). A recent report notes that, despite the emphasis that is often placed on the importance of de‐escalation, little research has been carried out into the effectiveness of any given approach, leaving nurses to contend with conflicting advice and theories. Also, a survey of guidelines suggests that recommendations based on NICE were of (grade D) and there were no trials or systematic reviews in this area. There is also lack of evidence regarding effectiveness of rapid tranquillisation, physical intervention and seclusion (Yeung 2009).
There is no standard approach to de‐escalation (Paterson 1997). There has been little research conducted into the effectiveness of different approaches to de‐escalation, or, for that matter, into the effectiveness of training in any given approach. There is thus an urgent need to systematically review the available evidence evaluating the effectiveness of de‐escalation in managing patients with acute aggression.
This is one of a series of related Cochrane reviews (Table 1).
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis‐induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| Droperidol for acute psychosis | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Clozapine for people with schizophrenia and recurrent physical aggression | |
| De‐escalation techniques for managing aggression | |
| Haloperidol for long‐term aggression in psychosis | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression or agitation | |
| Risperidone for psychosis‐induced aggression or agitation | |
Objectives
To investigate the effects of de‐escalation techniques in the short‐term management of aggression or agitation thought or likely to be due to psychosis.
Methods
Criteria for considering studies for this review
Types of studies
We planned to select all relevant randomised controlled trials using de‐escalation techniques for the management of aggressive behaviour. If a trial was described as 'double blind' but implied randomisation, we would have included such trials in a sensitivity analysis (see Sensitivity analysis). We planned to exclude quasi‐randomised studies, such as those allocating by alternate days of the week. For trials where people were given additional treatments, we would have included the data if the adjunct treatment was evenly distributed between groups and it was only the de‐escalation that was randomised.
Types of participants
We would have included adults (at least 18 years) with a potential for aggressive behaviour due to psychosis, from those in a psychiatric setting to those possibly under the influence of alcohol or drugs and/or as part of an acute setting as well.
Types of aggressive behaviours can include the following.
-
Verbal abuse
-
Non‐verbal abuse
-
Physical violence to self/others
-
Threatening behaviours
-
Assault
Types of interventions
1. De‐escalation:
NICE 2015 defines de‑escalation as the use of techniques (including verbal and non‐verbal communication skills) aimed at defusing anger and averting aggression. Prescibed 'as required' ('pro re nata' or p.r.n.) medication can be used as part of a de‐escalation strategy but, used alone is not de‐escalation.
There are various techniques that can be used to defuse an aggressive situation.
-
Verbal communication techniques
-
Use of body language
-
Prevention and recognition strategies (risk assessment tools)
-
Staff attitudes, knowledge and skills
-
Setting of limits for patients to follow
-
Environmental controls (such as minimising light, noise, conversations and so on) used for the management of aggression
De‐escalation does not involve restraint, medication used alone or seclusion.
We planned to include trials that used the above techniques specifically during, or prior to, aggressive or agitated behaviour.
Types of outcome measures
The effects of de‐escalation techniques for managing aggression would usually be immediate (15 minutes) to short term (one to two hours).
Primary outcomes
1. Clinically important change in global state: as defined by studies
2. Aggression
2.1 Improved to an important extent
2.2 Deterioration: incidence of violence to self or others (harm)
2.3 Changes in aggression as recorded by any other outcomes
3. Adverse effects
3.1 Physical adverse effects
3.2 Death, suicide or natural causes
3.3 Psycological adverse effects
Secondary outcomes
1. Global state
1.1 Relapse: as defined by individual studies
1.2 Any change in global state
2. Mental state
2.1 Changes to mental state deemed clinically significant by study
2.2 Continuous measures of mental state
2.3 Psychiatric symptoms
3. Leaving the study early
4. Service use
4.1 Hospital admission
4.2 Length of stay in hospital
4.3 Changes to hospital status
5. Social functioning
5.1 Continuous measures in social functioning
6 Satisfaction
6.1 Patient satisfaction
6.2 Carer satisfaction
6.3 Changes to quality of life defined as significant by individual studies
7. Economic outcomes
8. Aggression/behaviour
8.1 Changes in verbal aggression
8.2 Changes in violence towards objects
8.3 Other changes in behaviour
'Summary of findings' table
We intended to use the GRADE approach to interpret findings (Schünemann 2008) and to use the GRADE profiler (GRADEPRO) to import data from RevMan 5 (Review Manager) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rate as important to patient‐care and decision making. We intended to select the following main outcomes for inclusion in the 'Summary of findings' table.
1. Clinically important changes in global state (short‐term outcomes)
2. Aggression
2.1 Improved to an important extent
2.2 Deterioration: incidence of violence to self or others (harm)
2.3 Changes in aggression as recorded by any other outcomes
3. Adverse effects
3.1 Physical adverse effects
3.2 Death, suicide or natural causes
3.3 Psycological adverse effects
Search methods for identification of studies
No language or publication status restriction was applied within the limitations of the search tools.
Electronic searches
Cochrane Schizophrenia Group's Study‐based Register of Trials
On 7 April, 2016, the Information Specialist searched the register using the following search strategy which has been developed based on literature review and consulting with the authors of the review:
((*Aggress* OR *Agitat* OR *Violen*) in Title OR Abstract of REFERENCE OR Intervention of STUDY) AND ((*De‐Escalat* OR *Deescalat* OR *De‐Stimulat* OR *Destimulat* OR *De‐Fus* OR *Defus* OR *One‐To‐One* OR *One To One* OR *Diffuse* OR *Calming* OR *Non Aversive* OR *Non‐Aversive* OR *Non Confrontat* OR *Non‐Confrontat* OR *Psycho Social* OR *Psycho‐Social* OR *Verbal* OR *Talk* OR *Nurse* Role*) in Title OR Abstract of REFERENCES)
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics.
This register is compiled by systematic searches of major resources (including MEDLINE, Embase, AMED, BIOSIS, CINAHL, PsycINFO, PubMed, and registries of clinical trials), and their monthly updates, handsearches, grey literature, and conference proceedings (see Group’s Module). There is no language, date, document type, or publication status limitations for inclusion of records into the register.
For previous searches, please see Appendix 1.
Searching other resources
1. Reference searching
We would have inspected the references of all included studies for further relevant studies.
2. Personal contact
We would have contacted the first author of each included study for information regarding unpublished trials.
Data collection and analysis
Selection of studies
Review authors WY and HR inspected all abstracts of studies identified as above for potentially relevant reports. In addition, to ensure reliability, review author MBJ inspected a random sample of these abstracts, comprising 20% of the total. Where disagreement occurred, this was resolved by discussion, or where there was still doubt, we obtained the full article for further inspection. We acquired the full article of the relevant report for reassessment and carefully inspected for a final decision on inclusion(see Criteria for considering studies for this review). Once the full article was obtained, in turn MD and WS inspected the full report and independently decided whether it met inclusion criteria. MD and WS were not blinded to the names of the authors, institutions or journal of publication.
Data extraction and management
1. Extraction
Review author MD and SY would have extracted data from all included studies. In addition, to ensure reliability, if trials had been found, XW would have independently extracted data from a random sample of these studies, comprising 20% of the total. Again, we would have discussed any disagreement, documented decisions and, if necessary, contacted authors of studies for clarification. With remaining problems, XW would have helped clarify issues and we would have documented these final decisions. We would have extracted data presented only in graphs and figures whenever possible, but included the data only if two review authors independently had the same result. We would have attempted to contact authors through an open‐ended request in order to obtain missing information or for clarification whenever necessary. If studies were multi‐centre, where possible, we would have extracted data relevant to each component centre separately.
2. Management
2.1 Forms
We planned to extract data onto standard, simple forms.
2.2 Scale‐derived data
We would have included continuous data from rating scales only if:
a. the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and
b. the measuring instrument had not been written or modified by one of the trialists for that particular trial.
Ideally, the measuring instrument should either be i. a self‐report or ii. completed by an independent rater or relative (not the therapist). We realise that this is not often reported clearly, and we would have noted in the 'Description of studies' if this was the case or not.
c) the instrument should be a global assessment of an area of functioning and not sub‐scores which are not, in themselves, validated or shown to be reliable. However there are exceptions, and we would have included sub‐scores from mental state scales measuring positive and negative symptoms of schizophrenia.
2.3 Endpoint versus change data
There are advantages of both endpoint and change data. Change data can remove a component of between‐person variability from the analysis. On the other hand, calculation of change needs two assessments (baseline and endpoint) which can be difficult in unstable and difficult to measure conditions such as schizophrenia. We decided primarily to use endpoint data, and only use change data if the former were not available. We would have combined endpoint and change data in the analysis as we would have preferred to use mean differences (MD) rather than standardised mean differences (SMD) throughout (Higgins 2011).
2.4 Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we planned to apply the following standards to all data before inclusion:
a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors;
b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996);
c) if a scale started from a positive value (such as the Positive and Negative Syndrome Scale (PANSS, Kay 1986), which can have values from 30 to 210), we planned to modify the calculation described above to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score.
Endpoint scores on scales often have a finite start and end point and these rules can be applied. We would have presented skewed endpoint data from studies of less than 200 participants as other data within the data analyses section rather than in analyses. Skewed data pose less of a problem when looking at means if the sample size is large (> 200) and we would have entered these into the syntheses.
When continuous data are presented on a scale that include a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We would have entered change data, regardless of sample size, into the analyses.
2.5 Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
2.6 Conversion of continuous to binary
Where possible, we would have made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). If data based on these thresholds were not available, we would have used the primary cut‐off presented by the original authors.
2.7 Direction of graphs
Where possible, we would have entered data in such a way that the area to the left of the line of no effect indicates a favourable outcome for de‐escalation. Where keeping to this would have made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'Not improved'), we would have reported data where the left of the line indicates an unfavourable outcome. This would have been noted in the relevant graphs.
Assessment of risk of bias in included studies
Again, review authors MD and WS would have worked independently to assess risk of bias using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess trial quality. This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias of the article such as sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting.
If the raters disagreed, the final rating would have been made by consensus, with the involvement of another member (SZ) of the review group. If inadequate details of randomisation and other characteristics of trials had been provided, we would have contacted the authors of the studies in order to obtain further information. We would have reported non‐concurrence in quality assessment, but if disputes had arisen as to which category a trial was to be allocated, again, resolution would have been made by discussion.
We would have noted the level of risk of bias in both the text of the review and in the 'Summary of findings' table.
Measures of treatment effect
1. Binary data
For binary outcomes, we would have calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000).
2. Continuous data
For continuous outcomes, we would have estimated the MD between groups. We prefer not to calculate effect size measures (SMD). However, if scales of very considerable similarity had been used, we would have presumed there was a small difference in measurement, and we would have calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster‐randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra‐class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We would have sought to contact first authors of such studies to obtain intra‐class correlation coefficients (ICCs) for their clustered data and adjusted for this by using accepted methods (Gulliford 1999). Where clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We were advised that we needed to reduce the size of each trial to its 'effective sample size' (Rao 1992). The effective sample size of a single intervention group in a cluster‐randomised trial is its original sample size divided by a quantity called the 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC [Design effect = 1+(m‐1)*ICC] (Donner 2002). If the ICC was not reported, we would have assumed it to be 0.1 (Ukomunne 1999). A common design effect is usually assumed across intervention groups. For dichotomous data, both the number of participants and the number experiencing the event are divided by the same design effect. Since the resulting data must be rounded to whole numbers for entry into RevMan, this approach may be unsuitable for small trials. For continuous data, only the sample size needs to be reduced; means and SDs should remain unchanged.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase, the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in severe mental illness, we would have only used data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we would have presented the additional treatment arms in comparisons. If data were binary, we simply would have added and combined them within the two‐by‐two table. If data were continuous, we would have combined data following the formula in section 7.7.3.8 (Combining groups) of the Cochrane Handbook for Systematic Reviews of Interventions . Where the additional treatment arms were not relevant, we would not present these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose that, for any particular outcome, should more than 50% of data be unaccounted for, we would not present these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we would have marked such data with (*) to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we would have presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early would all be assumed to have the same rates of negative outcome as those who completed, with the exception of the outcomes of death and adverse effects. For these outcomes, the rate of those who stayed in the study ‐ in that particular arm of the trial ‐ would be used for those who did not. We would have undertaken a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared with the intention‐to‐treat analysis using the above assumptions.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50% and data only from people who completed the study to that point were reported, we would have reproduced these.
3.2 Standard deviations (SDs)
If SDs were not reported, we would have first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals available for group means, and either 'P' value or 't' value available for differences in mean, we would have calculated them according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When only the SE is reported, SDs can be calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 (Higgins 2011) present detailed formulae for estimating SDs from P values, t or F values, confidence intervals, ranges or other statistics. If these formulae did not apply, we would have calculated the SDs according to a validated imputation method, based on the SDs of other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study's outcome and thus to lose information. We nevertheless would have examined the validity of the imputations in a sensitivity analysis excluding imputed values.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would have been employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, where LOCF data had been used in the trial, if less than 50% of the data had been assumed, we would have reproduced these data and indicated that they were the product of LOCF assumptions.
Assessment of heterogeneity
1. Clinical heterogeneity
We planned to consider all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We would have simply inspected all studies for clearly outlying people or situations which we had not predicted would arise. If such situations or participant groups had been noted, these would have been fully discussed.
2. Methodological heterogeneity
We would have considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We would have simply inspected all studies for clearly outlying methods which we had not predicted would arise. If such methodological outliers had been noted, these would have been fully discussed.
3. Statistical heterogeneity
3.1 Visual inspection
We would have visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I2 statistic
We intended to investigate heterogeneity between studies by considering the I2 method alongside the Chi2 'P' value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. 'P' value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic, is interpreted as evidence of substantial levels of heterogeneity (Section 9.5.2 ‐ Higgins 2011). If substantial levels of heterogeneity had been found in the primary outcome, we would have explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We would not have used funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In other cases, where funnel plots were possible, we would have sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies even if there is no statistically significant heterogeneity.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
We anticipated one subgroup analysis to test the hypothesis that de‐escalation is most effective for those with acute aggression. We hoped to present data for this subgroup for the primary outcomes.
2. Investigation of heterogeneity
If inconsistency was high, we would have reported this. First, we would have investigated whether data had been entered correctly. Second, if data had been entered correctly, we would have visually inspected the graph and successively removed outlying studies to see if homogeneity was restored. For this review, we had decided that should this occur with data contributing to the summary finding of no more than around 10% of the total weighting, we would present the data. If not, we would not pool data but discuss the issues. We know of no supporting research for this 10% cut‐off but considered investigating the use of prediction intervals as an alternative to this unsatisfactory state. If unanticipated clinical or methodological heterogeneity were obvious, we would have simply stated hypotheses regarding these for future reviews or versions of this review. We would not have anticipated undertaking analyses relating to these.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way to imply randomisation. For the primary outcomes, we aimed to include these studies and if their inclusion did not result in a substantive difference, they would have remained in the analyses. If their inclusion resulted in important clinically significant, but not necessarily statistically significant differences, we would not have added the data from these lower quality studies to the results of the better trials, but presented such data within a subcategory.
2. Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we planned to compare the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. If there was a substantial difference, we planned to report results and to discuss them, but would have continued to employ our assumption.
Where assumptions had to be made regarding missing SDs data (see Dealing with missing data), we would have compared the findings of the primary outcomes when we used our assumption/s and when we used data only from people who completed the study to that point. A sensitivity analysis would have been undertaken to test how prone results were to change when completer‐only data only were compared with the imputed data using the above assumption. If there was a substantial difference, we would report results and discuss them but would continue to employ our assumption.
Results
Description of studies
Results of the search
Over 300 records were identified using the search criteria. No record met the inclusion criteria (please refer to Figure 1 for study screening flow diagram).
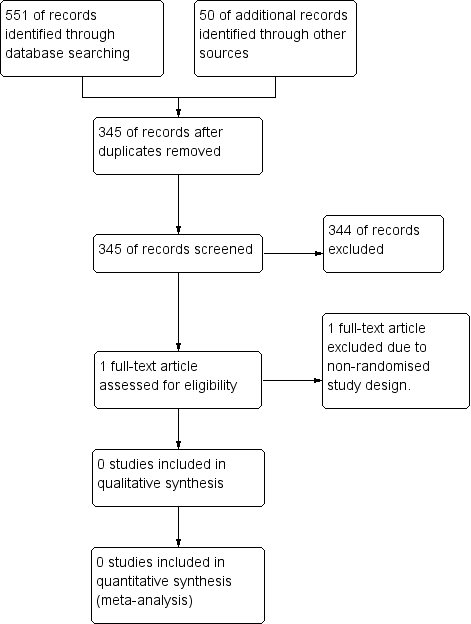
Study flow diagram.
Included studies
We were unable to include any study in this review.
Excluded studies
We assessed Nijman 1997, but we excluded this trial as it was not randomised. We identified no registered clinical ongoing studies. No studies are awaiting assessment.
Risk of bias in included studies
Allocation
There were no included studies to assess risk of bias.
Blinding
There were no included studies to assess risk of bias.
Incomplete outcome data
There were no included studies to assess risk of bias.
Selective reporting
There were no included studies to assess risk of bias.
Other potential sources of bias
There were no included studies to assess risk of bias.
Effects of interventions
There were no randomised controlled trials identified comparing the effect of interventions with de‐escalation techniques in managing psychosis‐induced aggression.
Discussion
Summary of main results
Of the 345 studies identified from the search, none were found suitable to include in the review. In summary, no trial‐based evidence is available for any of the key outcomes of clinical relevance (summary of findings Table for the main comparison).
Overall completeness and applicability of evidence
There were no randomised controlled trials relevant to the review and hence, we are unable to make any inferences about applicability. Because of the broad scope of what could be considered 'de‐escalation', it is possible that very wide‐ranging and less precise searching may find occasional trials of some relevance to this area. However, we think it unlikely that we have missed large relevant studies.
Quality of the evidence
There were no randomised controlled trials identified relevant to this review
Potential biases in the review process
We searched for published and unpublished trials and two review authors screened the potential studies. It is possible that our English language searching failed to identify trials, but important studies such as would be expected for this review are probably likely to be published in journals indexed by the major databases we had searched.
Agreements and disagreements with other studies or reviews
Hockenhull 2012 revealed extensive literature that does exist in this field. Unfortunately the studies included in this review were low quality. Much of the research is opportunistic by practitioners on the basis of what is possible within their own clinical setting. Although this is laudable as a contribution to the principle of evidence‐based practice, without adequate resources to improve study design, the cumulative evidence base may never allow conclusions to be made that are strong enough to direct policy. We know of no robust quantification of the effects of de‐escalation techniques.
| Methods | Allocation: cluster‐randomised, clearly described, with researched and recorded intra‐class correlation coefficient (ICC) reported. Blinding: none. Duration: 2 weeks. Setting: any psychiatric ward with high rate of aggression. |
| Participants | Diagnosis: any. History: people admitted or, or getting admitted to psychiatric ward. N =*. Age: adult. Sex: men or women. Exclude: those already randomised. |
| Interventions | 1. De‐escalation technique training. 2. Waiting list for training. The de‐escalation technique training could involve refining of: a. Verbal communication techniques; b. Use of body language; c. Prevention and recognition strategies (risk assessment tools); d. Staff attitudes, knowledge and skills; e. Setting of limits for patients to follow; f. Environmental controls (such as minimising light, noise, conversations and so on) used for the management of aggression ‐ or any combination of these. |
| Outcomes | Primarily routinely‐recorded binary outcomes. 1. Clinically important changes in global state (short‐term outcomes) 2. Aggression 2.1 Improved to an important extent 2.4 Recurrance of aggression 3. Adverse effects 3.1 Physical adverse effects 4. Service outcomes 4.1 Time in hospital 5. Acceptability 5.1 To staff 5.2 To patients 6. Cost |
| Notes | * We are unclear of power calculations at this point. It is likely that the sample of people will have to total at least 300 to gain sufficient power to find clear outcomes that are likely to effect clinical practice, but this figure would have to be modified depending on a well‐researched (not imputed) ICC. |
| Patient or population: people who are aggressive secondary to serious mental illness | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care control | De‐escalation technique | |||||
| Clinically important changes in global state | We identified no relevant studies. | |||||
| Aggression ‐ Improved to an important extent | ||||||
| Aggression ‐ deterioration: incidence of violence to self or others | ||||||
| Aggression ‐ changes in aggression as recorded by any other outcomes | ||||||
| Adverse effects ‐ physical adverse effects | ||||||
| Adverse effects ‐ death, suicide or natural causes | ||||||
| Adverse effects ‐ psychological adverse effects | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Focus of review | Reference |
| Completed and maintained reviews | |
| 'As required' medication regimens for seriously mentally ill people in hospital | |
| Benzodiazepines for psychosis‐induced aggression or agitation | |
| Chlorpromazine for psychosis‐induced aggression or agitation | |
| Clotiapine for acute psychotic illnesses | |
| Containment strategies for people with serious mental illness | |
| Droperidol for acute psychosis | |
| Haloperidol for psychosis‐induced aggression or agitation (rapid tranquillisation) | |
| Haloperidol plus promethazine for psychosis‐induced aggression | |
| Olanzapine IM or velotab for acutely disturbed/agitated people with suspected serious mental illnesses | |
| Seclusion and restraint for serious mental illnesses | |
| Zuclopenthixol acetate for acute schizophrenia and similar serious mental illnesses | |
| Reviews in the process of being completed | |
| Clozapine for people with schizophrenia and recurrent physical aggression | |
| De‐escalation techniques for managing aggression | |
| Haloperidol for long‐term aggression in psychosis | |
| Loxapine inhaler for psychosis‐induced aggression | |
| Quetiapine for psychosis‐induced aggression or agitation | |
| Risperidone for psychosis‐induced aggression or agitation | |

