予防医療および長期疾患管理を目的とした自動電話通信システム
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Aim: to determine whether use of Personal Health Partner (PHP) was associated with significant differences in parental report of primary care visit content. Additional goals included evaluating the intervention effect on medication management, asthma care, and parent and clinician satisfaction Study design: RCT; recruitment: primary care (mail) Study duration: 25 months; study type: prevention; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: children aged 4 months to 11 years (and their parents) who had a routine healthcare maintenance or well‐child visit. Parents and children had to speak English and could not be planning to move away from the Boston area within 3 months Sample size: 475; mean age: 5 years (child) 35 years (parent);sex: women ‐ 48% (child), 93% (parent); men ‐ 52% (child) 7% (parent); ethnicity: African‐Americana 67% (child); 47% (parent); other 33% (child); 53% (parent) Country: USA | |
| Interventions | Personal Health Partner (PHP) tailors call content based on the participant's age and prescription of asthma medication. Call content was based on American Academy of Pediatrics Bright Futures topics reflected in the electronic health record (EHR) templates at the study site as well as Medicaid‐recommended health risk questions for routine healthcare maintenance (RHCM), asthma symptoms, and medication safety. When available, PHP scripts were based on validated tools. RHCM areas include general health supervision, developmental screening, diet and physical activity, tuberculosis risk assessment, smoking risk assessment, and maternal depression screening. Each call also addressed medication safety, examining what medications on the EHR medication list the child was actually taking, age‐appropriate medication use, and proper use of asthma controller and reliever medication if applicable. The day before each scheduled visit, PHP data were transferred to the EHR. PHP questions yielding actionable data generate an "Alert" displayed within the "Alerts" section of the "Patient Entered Data Review" form. Control group completed a single automated call, but the content was limited to the 18‐question Framingham Safety Survey. At the completion of the call, parents in the control group received tailored advice related to unsafe behaviours reported during the call. Because the Framingham Safety Survey was not part of routine primary care at Boston Medical Center, data from these calls were not shared with the EHR. | |
| Outcomes | Comprehensiveness of screening and counselling (primary), assessment of medications and their management, and parent and clinician satisfaction (secondary) | |
| Funding | Agency for Healthcare Research and Quality, grant R18HS017248 | |
| Declaration of conflict of interest | No potential conflicts of interest disclosed | |
| Power calculations for sample size | No | |
| Notes | The authors have been contacted for results from the Medication Adherence Scale with no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children were randomly assigned to groups at the start of each call". Comment: insufficient information to judge whether random sequence generation was ensured |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Study staff members were not aware of allocation group at the time of interviews" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | No results from the Medication Adherence Scale have been reported. Comment: insufficient information to judge whether this introduced bias |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to compare motivational interviewing (MI) HealthCall to MI‐only to reduce non‐injection drug use (NIDU) in urban HIV primary care patients Study design: RCT; recruitment: primary care (health professional referral) Study duration: 2 months; study type: prevention; subtype: substance abuse | |
| Participants | Inclusion criteria: HIV‐positive, English‐ or Spanish‐speaking, aged 18 years, enrolled in a New York City hospital‐affiliated HIV primary care clinic, using drugs ≥ 4 days during the prior 30 days (including illicit non‐injection drugs or prescription drugs taken without prescription or more than prescribed) Sample size: 33; mean age: 46 years; sex: men ‐ 76%; women ‐ 24%; ethnicity: African American 64%, Hispanic 21%, Caucasian (understood to be white) 15% Country: USA | |
| Interventions | MI + HealthCall: participants call HealthCall daily via a toll‐free number to report on the targeted health behaviour and potentially related moods, behaviours, and situations that occurred in the prior 24 h. HealthCall menu for NIDU included a short set of prerecorded questions in English or Spanish about the previous day covering use of primary drug, dollar amount spent on the drug used, use of other drugs, HIV medication adherence, and feelings of wellness, stress, and overall quality of the day. Participants responded by pressing numbers on the telephone keypad. After the practice call, counsellors helped participants identify an accessible telephone and convenient time for daily calls and set the watch alarm to this time as a reminder to call. Counselors were bilingual (English/Spanish) and from the same race/ethnic groups as most of the participants. HealthCall data were automatically uploaded to a database and used to provide personalised feedback to participants about their drug use in a single‐page form that included a computer‐generated graph of participants' drug use as called into the IVR and a set of summary statistics during the 30‐ and 60‐day visits. The personalised graph contained the participant's goal set in the baseline MI interview with the counsellor (NIDU Goal), with diamond‐shaped dots representing the dollar amount of drugs used on the days that the participant called HealthCall Participants in MI‐only arm (control) received a 20–25 min MI at baseline, using standard MI techniques, e.g. dialogue about health consequences of NIDU, exploring ambivalence, barriers to change, developing a change plan, including (for those who chose) a specific NIDU‐reduction goal (reflected in USD amounts) for the next 30 days. Participants then received a digital alarm watch which they were told they could use as a medication reminder. At 30 and 60 days, counsellor and participant met for 10–15 min to review overall drug use and set or re‐set a drug reduction goal for the next 30 days. | |
| Outcomes | Days used primary drug in last 30 days (primary); patient satisfaction (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was done via 10‐block standard ABAB design" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were blinded to their random assignment until after the MI session" |
| Blinding of participants and personnel (performance bias) | High risk | Counsellors were not blinded to their random assignment |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Treatment groups did not differ on attrition (p = 0.10) and thus attrition is not likely to be a source of bias in our results." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | No significant baseline differences |
| Methods | Aims: to study if there is a difference in effect between automated interventions delivered by IVR and over the Internet Study design: RCT; recruitment: other ‐ university (web‐based survey) Study duration: 6 weeks; study type: management; subtype: alcohol consumption | |
| Participants | Inclusion criteria: Swedish university students having an AUDIT score above cutoff (8 and 6) Sample size: 1423; mean age: *; sex: *; ethnicity: * Country: Sweden | |
| Interventions | Single IVR call of less than 500 words, one week after the baseline assessment, consisting of feedback on the baseline assessment and instructions on how to obtain a recommended Blood Alcohol Concentration (BAC) below 0.6‰ (0.06 percentages) Single Internet‐delivered intervention given one week after baseline Repeated IVR call Repeated Internet‐delivered intervention given 1 and 2 weeks after intervention No intervention (controls) | |
| Outcomes | Alcohol Use Disorders Identification Test (primary) | |
| Funding | Swedish National Institute of Public Health and Edwin Berger Foundation | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | In the present review we report a comparison between single IVR call and no intervention. Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to determine whether a multifaceted intervention increases adherence to annual faecal occult blood testing compared with usual care Study design: RCT; recruitment: community centre (organisation referral) Study duration: 12 months; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: age 51 to 75 years; preferred language listed as English or Spanish; and a negative faecal occult blood testing result obtained between 1 March 2011, and 28 February 2012 Sample size: 450; mean age: 60 years; sex: men ‐ 28%; women ‐ 72%; ethnicity: Latino ‐ 87%, other ‐ 13% Country: USA | |
| Interventions | The multimodal intervention group received (1) a mailed reminder letter, a free faecal immunochemical test with low‐literacy instructions, and a postage‐paid return envelope; (2) an automated telephone and text message reminding them that they were due for screening and that a faecal immunochemical test was being mailed to them; (3) an automated telephone and text reminder 2 weeks later for those who did not return the faecal immunochemical test; and (4) personal telephone outreach by a colorectal cancer screening navigator after 3 months in addition to UC which included computerised reminders, standing orders for medical assistants to give participants home faecal immunochemical tests, and clinician feedback on colorectal cancer screening rates. Usual care (control group) at participating health centres included computerised reminders, standing orders for medical assistants to give participants home faecal immunochemical tests, and clinician feedback on colorectal cancer screening rates. | |
| Outcomes | Completion of faecal occult blood testing within 6 months of the date the participant was due for annual screening (primary) Costs (secondary) | |
| Funding | Grant P01 HS021141‐ the Agency for Healthcare Research and Quality | |
| Declaration of conflict of interest | None reported | |
| Power calculations for sample size | To detect a 10% difference (45% vs 35%) with 80% power (2‐tailed alpha = 0.05), we would need 752 participants (376 in each arm).This is less than the 800 participants that we estimated will be eligible for the study | |
| Notes | The estimated cost of the outreach intervention was USD 34.59 per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators were blinded to the outcomes in the control group |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Using only EHR data for outcome assessment is conceptually similar to blinded outcome assessment" |
| Incomplete outcome data (attrition bias) | Low risk | All 450 participants were included in the analyses |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest reported |
| Other bias | Low risk | Groups were comparable at baseline |
| Methods | Aim: to test the effectiveness of a theory‐based IVR intervention to improve adherence to controller medications among adults with asthma Study design: RCT; recruitment: community (advert in newspaper) Study duration: 10 weeks; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: no significant disease or disorder (chronic health disorders, current substance abuse or dependence, mental retardation, or psychiatric disorder); and lack of participation in any other asthma‐related research or clinical trial Sample size: 50; mean age:42 years;sex: women ‐ 59%; men ‐ 41% Ethnicity: white ‐ 58%, African American ‐ 20%, Hispanic ‐ 18%, Asian ‐ 4% Country: USA | |
| Interventions | In the IVR group, each participant received ≥ 2 calls separated by 1 month. Calls were programmed to reach out at several time points throughout the day and evening until the participant answered. If an answering machine was reached, a toll‐free number was provided, which the participant could use to call back. When a call connection was completed, the IVR call identified itself as coming from the Denver Interactive Asthma Learning System program and verified that the correct person had been called. Content of the call then included an explanation of how the call works followed by 3 questions inquiring whether during the previous week the participant had been awakened at night, had limited their activities, or had used their rescue inhaler more than twice because of asthma symptoms (symptom module). Participants who responded affirmatively to any of the 3 questions were told that daily use of their controller medication should help prevent such symptoms and were advised to discuss the symptoms with their physician. All participants also listened to a short module about the benefits of their asthma medication and were asked about whether they were filling and using their medication, with IVR responses tailored to specific participant responses (refill module). Finally, participants were informed about the Lung Line, a free telephone service staffed by nurses capable of answering most questions about asthma, and about the Colorado Quit Line, offering free telephone based tobacco cessation intervention (resources module) Participants in the control group received no calls. | |
| Outcomes | Medication adherence (primary); Asthma Control Test, Asthma Quality of Life Questionnaire, Beliefs about Medications Questionnaire (secondary) | |
| Funding | Astra Zeneca | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | Power and sample size calculations indicated that 25 participants in each group would provide 75% power to detect a group difference of 36% | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation table generated before study initiation determined group assignment by order of entry into the study" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigators remained blind to treatment until the final data set was completed" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Participants were comparable at baseline |
| Methods | Aim: to improve adherence in paediatric asthma Study design: RCT; recruitment: secondary care (*) Study duration: 24 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: children, ages 3‐12 years, treated for persistent asthma at Kaiser Permanente of Colorado Sample size: 1187; mean age: *sex: * ethnicity: * Country: USA | |
| Interventions | Parents in the IVR group received a call reminding them that inhaled corticosteroid fill was overdue, and assisted with automated mail order refills or transfer to a Kaiser Permanente of Colorado pharmacy or asthma nurse specialist. Telephone calls in this group pulled information from the electronic health record (EHR) enabling the automated call to provide personalised participant and medication information. Parents in the control group received usual care | |
| Outcomes | Medication adherence (primary); utilisation of care (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to evaluate the effectiveness of a behavioural intervention that emphasised weight loss and hypertension medication adherence among primary care patients in the community health centre setting Study design: RCT; recruitment: community centre (telephone) Study duration: 24 months; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: BMI 30‐50 kg/m2 (and weighing < 181.4 kg (400 pounds)), undergoing treatment for hypertension, aged ≥ 21 years, and enrolled participant at one of the participating community health centres (CHC). Additionally, participants had to read and speak English or Spanish, provide informed consent, and be willing to change diet, physical activity, and weight Sample size: 365; mean age: 55 years; sex: men ‐ 31%; women ‐ 69%; ethnicity: non‐Hispanic black ‐ 71%, Hispanic ‐ 13%, non‐Hispanic white ‐ 4%, other – 12% Country: USA | |
| Interventions | Be Fit, Be Well: participants can choose to use either the Internet or print + IVR as a mode of delivery of the intervention. In print + IVR condition, participants track their behavioural goals daily on a paper log and then enter this information weekly using the telephone keypad during their IVR telephone call. The goals are divided into 3 categories: dietary, physical activity, and lifestyle goals. For the first 13 weeks, participants work on 3 goals; for the rest of the intervention period, they work on 4 goals simultaneously. Participants pick new behaviour change goals every 13 weeks. 2 goals ("Walk 10,000 steps per day" and "Take your blood pressure medicine the right way every day") remain constant throughout the intervention period. Skill training materials, in print, provide instruction in behavioural strategies to facilitate achieving their behavioural goals. The also provide additional dietary, physical activity, and lifestyle goals that may need additional contextualisation. Participants monitor their behavioural goals over the telephone using IVR. After entering data on their behaviour, participants receive immediate feedback on their progress compared to the previous entry. Participants receive social support via telephone coaches administered by community health educators (CHE) and group support sessions. CHE call the participants once a month in the 1st year and then bimonthly in the following year, during which they discuss progress, barriers, strategies to overcome barriers,self‐monitoring, and social support. Each call lasts for 15‐20 min. Group sessions include an interactive skill training and a physical activity component. The intervention materials include information on community resources such as public parks, local walking groups, and farmers' markets that can aid participants in their behaviour change efforts. All participants receive a walking kit that includes a pedometer and maps of the local community with associated step counts.Participants receive a personalised, tailored behaviour change "prescription" (generated from the baseline data) with the doctor's signature included electronically. This "prescription" presents recommendations for making changes in the targeted risk behaviours, and lets patients know that their doctor considers these recommendations to be important to their health Participants in the control group received usual care (self‐help booklet) | |
| Outcomes | Change in body weight and BMI (primary); change in blood pressure; medication adherence; adverse‐events (secondary) | |
| Funding | National Heart, Lung, and Blood Institute; National Cancer Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | The trial was designed to provide 80% power to detect a mean weight change in 24 months of 2.75 kg in the intervention arm, assuming no weight change in usual care | |
| Notes | All participants are diagnosed with hypertension. In addition, 36% are diagnosed with hypercholesterolaemia, and 20% with type 2 diabetes mellitus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocations were performed, blocked by clinic and sex |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The trial design precluded blinding either patients or interventionists to treatment assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 365 participants are included in the primary outcomes analysis, including 15 participants (4.1%) who had only a baseline assessment." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Both the groups were balanced in all other characteristics at baseline |
| Methods | Aims: to compare changes in weight and cardiometabolic risk during a 12‐month period among black women randomised to a primary care–based behavioural weight gain prevention intervention or to usual care Study design: RCT; recruitment: community centre (mail) Study duration: 18 months; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: aged 25‐44 years, BMI of 25‐34.9 kg/m2, ≥ 1 visit to a Piedmont Health Center in the previous 24 months, North Carolina residency, and self‐reported English fluency Sample size: 194; mean age: 35 years; sex: women ‐ 100%; ethnicity: black ‐ 100% Country: USA | |
| Interventions | The multimodal intervention (the Shape Program) contained 5 components: obesogenic behaviour change goals; self‐monitoring via IVR phone calls; tailored skills training materials; 12 interpersonal counselling calls; and a 12‐month YMCA membership Participants in the control group received usual care: study staff made no attempts to influence the medical treatment provided to those in the usual care arm. Every 6 months, we sent usual‐care participants newsletters that covered general wellness topics but did not discuss weight, nutrition, or physical activity | |
| Outcomes | Change in body weight and BMI (primary); maintenance of change at 18 months; adverse‐events (secondary) | |
| Funding | R01DK078798 from the National Institute for Diabetes and Digestive and Kidney Diseases; and K05CA124415 from the National Cancer Institute | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | This trial was designed to have 80% power to detect significant BMI differences of 1.03 kg/m2 between treatment groups 12 months after baseline | |
| Notes | 6 serious adverse events were reported among participants in the intervention arm, including gynaecological surgery in 2 participants and knee replacement, breast abscess, musculoskeletal injury, and cancer diagnosis in 1 participant each; all participants except the one with the cancer diagnosis required hospitalisation. The authors of the study could not conclusively determine whether reported events resulted from study participation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After completing baseline assessments, research staff initiated a computer‐generated randomisation algorithm to allocate participants equally (1:1) across the two treatment arms (intervention and usual care); those in the intervention arm were further randomised to one of two interventionists." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study design precluded blinding patients and interventionists to treatment assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data balanced in numbers across groups (low attrition). ITT analysis was used to include all participants who received the intervention or usual care in the analysis. ITT analyses were based on the mean difference in weight and BMI between treatment arms at 12 months after adjustment for health centre. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | The groups were well‐balanced at baseline. |
| Methods | Aims: to assess the ability of automated reminders to improve adherence with once‐daily glaucoma medications Study design: RCT; recruitment: primary care (*) Study duration: 6 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: patients non‐adherent with their medications after 3 months of electronic monitoring (prospective cohort study phase) Sample size: 70; mean age: 66 years; sex: men ‐ 49%, women ‐ 51%; ethnicity: African American ‐ 58%, European ‐ 32%, Asian ‐ 6%, Hispanic ‐ 3%, Middle Eastern ‐ 1% Country: USA | |
| Interventions | Automated reminders (by telephone or text message) informed each participant in the intervention group that it was time to take his or her medication. The IVR system also allowed participants to reset the reminder and receive it again in 1 hour: "Hello, this is your automated reminder to take your eye drop. Press 1 if you have or are about to take your drop. If you are not able to take your eye drop right now and would like a second reminder in 1 hour, please press 2 now." Participants in the control group received usual care | |
| Outcomes | Medication adherence | |
| Funding | Microsoft BeWell Fund | |
| Declaration of conflict of interest | None reported | |
| Power calculations for sample size | No | |
| Notes | Communication with the author: "there was only one person (1.42% of the sample) who specified SMS (text) reminders in the study, however" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study participants were then assigned to a control or intervention group using assignments randomised equally in blocks of 10 and placed in envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "Study participants were then assigned to a control or intervention group using assignments randomised equally in blocks of 10 and placed in envelopes." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Large percentage of participants lost to and unavailable for follow‐up, however ITT analysis was used in addition to real efficacy approach" |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were reported |
| Other bias | High risk | Quote: "At baseline, there were statistically significant differences between the two groups with regard to age, educational level, and Mini‐Mental State Examination (MMSE) score" |
| Methods | Aims: to compare the effectiveness of an Internet and telephone‐based telemedicine communication system to usual care from a primary care provider in managing patients with hypertension Study design: RCT; recruitment: primary care (advert in clinic) Study duration: 6 months; study type: management; study subtype: hypertension | |
| Participants | Inclusion criteria: systolic blood pressure > 140 mmHg Sample size: 241; mean age: 60 years;sex: women ‐ 79%; men ‐ 21%; ethnicity: African American ‐ 81%, white‐ 15%, Hispanic ‐ 3%, other ‐ 1% Country: USA | |
| Interventions | Participants in the multimodal intervention group reported their weight, blood pressure, steps/day, cigarettes/day, at least twice weekly via an Internet or IVR phone system to the clinical centre. If the systolic blood pressure was < 140 mmHg, thetelemedicine system automatically sent a short message to the participant stating that the measures were acceptable, a short message on health care, and instructions to continue with the scheduled transmission of data. Monthly blood pressure summaries were sent to all subjects and to their primary care providers Participants in the control group received usual care by their physicians | |
| Outcomes | Blood pressure control at 6 months (primary) | |
| Funding | The Agency for Healthcare Quality and Research | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | To achieve a power of 0.8 with α value of 0.05, the authors aimed to recruit 252 subjects to accommodate a dropout rate of 20% and an expected 30% incidence of diabetes | |
| Notes | The telemedicine (intervention group) subjects used telephone communication 65% of the time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Consecutive patients were assigned a random number from a random number list. Patients assigned odd numbers were placed in the control group, and patients assigned even numbers were placed in the telemedicine group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data balanced in numbers across groups (low attrition) |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐defined outcomes have been reported |
| Other bias | Low risk | Participants were comparable at baseline |
| Methods | Aims:To assess the long‐term efficacy of a fully automated digital multimedia smoking cessation intervention Study design: RCT; recruitment: community (banner advertisements in Internet newspapers) Study duration: 12 months; study type: management; study subtype: smoking | |
| Participants | Inclusion criteria: people who were willing to make an attempt to quit smoking, were aged ≥ 18 years, smoked ≥ 10 cigarettes daily and had access to the Internet, email and a cellphone on a daily basis Sample size: 396; mean age: 36 years; sex: men ‐ 50%, women ‐ 50%; ethnicity: * Country: Norway | |
| Interventions | Multimodal intervention (Happy Ending (HE)). The IVR programme lasted for 6 weeks, with participants receiving 2 messages per day, delivered through mobile phones. In the morning when the participants logged on to the HE, they received IVR message. They received automated reminders if failed to log in. In the evening, participants received an automated call that asked about their smoking behaviour during the day. If they had smoked, they were directed to the tailored relapse prevention therapy. Craving helpline was available 24 h from day 15 onwards and participants were able to choose to hear therapeutic problem solving message related to emotion regulation, motivation boost, or stress regulation. Participants were encouraged to call the helpline each time they felt tempted to have a cigarette. Until week 11, the intervention had multiple daily contact points and was highly intensive. HE recommended the use of nicotine replacement therapy and they could choose between gum (2 mg or 4 mg) and patches (15 mg/16 h). HE also offered an 11‐month follow‐up phase. During this phase, the log‐off procedure continued daily for another 4 weeks, twice a week for another 2 weeks, and then once a week for the remaining follow‐up period. All the features provided in the active phase remained functional including craving helpline and supportive IVR messages. Participants in the control group received self‐help intervention (booklet) | |
| Outcomes | Repeated point abstinence at 1, 3, 6 and 12 months post‐cessation (primary); nicotine replacement therapy adherence, self‐efficacy and nicotine dependence (secondary) | |
| Funding | University of Oslo, Happy Ending AS and the Norwegian Research Council. Pfizer Norway provided a free supply of nicotine replacement therapy | |
| Declaration of conflict of interest | The second author has a financial interest in the intervention, as a shareholder of Happy Ending AS | |
| Power calculations for sample size | The report confirms that power analysis was performed. 396 were required | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Quote: "The names and identities of the subjects, however, were concealed to the experimenter during randomization." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods; ITT analysis was used to include all participants who received the intervention or usual care in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are of interest in the review have been reported. |
| Other bias | Low risk | Quote: "At baseline, there were no variables on which treatment and control subjects differed significantly" |
| Methods | Aims: to evaluate the effectiveness of comprehensive home telemonitoring service (TMS) in participants discharged from a Heart Failure Unit Study design: RCT; recruitment: primary care (organisation referral) Study duration:12 months; study type: management; subtype: heart failure | |
| Participants | Inclusion criteria: patients with chronic heart failure Sample size: 133; mean age: 57 years; sex: men ‐ 88%, women ‐ 12%; ethnicity: * Country: Italy | |
| Interventions | TMS: participants called a toll‐free number. After entering the unique identification code, the IVR system asked a series of question about vital signs and symptoms such as weight, systolic blood pressure, heart rate, dyspnoea, asthenia, oedema, therapy changes, blood urea nitrogen, creatinine, sodium, potassium, and bilirubin. Participants answered by using the touchpad of their home or mobile phone. If advice or help was needed, participants could leave a message to contact the medical staff. Those who failed to call the system for > 2 days were personally contacted by phone. Similarly, those with abnormal readings were flagged up and received a phone call from the medical team. Participants in the control group received usual community care. At discharge, participants were referred to their community primary care physician and cardiologist or cardiology department. During follow‐up the process of care was governed by different providers which managed the participant's needs with a heterogeneous range of strategies: emergency room management, hospital admission and outpatient access | |
| Outcomes | All‐cause mortality; re‐hospitalisations; emergency room use (composite primary); and adherence to the treatment (secondary) | |
| Funding | Ministero della Salute funds | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | All participants received educational materials, including cardiac failure book, telemonitoring service booklet, daily computerised medications plan, pillboxes with scheduling time, summary sheets of domestic and physical activities. Participants received an individualised personal care plan designed by the physician | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | All assigned participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are of interest in the review have been reported. |
| Other bias | Low risk | Quote: "No significant clinical or instrumental differences were observed between two groups" |
| Methods | Aims: to test the efficacy of IVR in recycling low‐income smokers who had previously used Quitline (QL) support back to QL support for a new quit attempt Study design: RCT; recruitment: primary care (mail) Study duration: 4 months; study type: management; study subtype: smoking | |
| Participants | Inclusion criteria: previous Quitline callers and current smokers. Sample size: 521; mean age: 40 years;sex: women ‐ 62.50%; men ‐ 37.50%; ethnicity: white, non‐Hispanic ‐ 81%, African American ‐ 6%, other – 5%, Hispanic or Latino ‐ 4%, Native American or Pacific Islander – 3%, Asian ‐ 1% Country: USA | |
| Interventions | The ATCS Plus intervention utilised in this trial was developed in 2 steps. The first step focused on creating the content of the IVR messages: 4 prototype IVR messages about possible barriers to re‐engagement in QL support for quitting smoking were developed, based on previous work with low income ethnic/racial minority smokers. These prototype messages were tested and changed according to feedback received through individual telephone interviews with fifteen Medicaid insured and uninsured smokers who had previously used a QL and agreed to be contacted further. The messages aimed to redefine relapse as a learning opportunity and not as a failure; motivate new quit attempts by reminding smokers about benefits in quitting (e.g. personal health and well being, financial savings, concern for family members); educate smokers about the different offerings of QL support services; reiterate how QL support can increase the chances of quitting; and inform smokers of their eligibility to re‐enrol in QL services The control group received only the first 2 components of the ATCS intervention (greeting and screening of smoking status), followed by a message thanking them for the information | |
| Outcomes | Re‐enrollment into Quitline support line (primary) | |
| Funding | National Cancer Institute grants: R21CA141568 and 1R25‐CA117865 | |
| Declaration of conflict of interest | No competing interests | |
| Power calculations for sample size | No | |
| Notes | ClinicalTrials.gov Identifier: NCT01260597 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "Eligible participants were randomised to the intervention |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐defined outcomes have been reported |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to determine the effect of automated symptom and self‐reported weight monitoring compared with usual care on the combined endpoint of all cause hospitalisation and mortality in patients recently hospitalised for heart failure Study design: RCT; recruitment: secondary care (organisational referral) Study duration: 6 months; study type: management; study subtype: heart failure | |
| Participants | Inclusion criteria: patients recently discharged from a heart failure hospitalisation Sample size: 1653; median age: 61 years;sex: women ‐ 42%; men ‐ 58%; ethnicity: white ‐ 49%, black ‐ 39%, other – 12% (inclusive of Hispanic or Latino – 3%) Country: USA | |
| Interventions | Tele‐HF: an automated, daily symptom and self‐reported weight monitoring intervention. During each call, participants heard a series of questions about general health and heart‐failure symptoms, and they enter responses using the telephone keypad. Information from the telemonitoring system was downloaded daily to a secure Internet site and was reviewed every weekday (except on holidays) by site coordinators. Any variance in any of the information are flagged up for clinician's attention who would then offer advice to the participant (e.g. modify diet, increase diuretic dose or adhere to medications); consult with the physicians in their practice site; advise an urgent clinic or emergency department visit; or refer the participant to another specialist, as appropriate. Participants in the control group received usual care (educational materials) | |
| Outcomes | Readmission for any reason or death from any cause (primary); hospitalisation for heart failure, number of days in the hospital, number of hospitalisations, and adverse events (secondary) | |
| Funding | National Heart, Lung, and Blood Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | With an alpha error of 0.05 and a power of 90%, for a 25% relative risk reduction, 1640 participants were needed (820 in each group), with a follow‐up period of 6 months | |
| Notes | Adherence in the telemonitoring group was defined as placement of ≥ 3 calls a week to the telemonitoring system (a cutoff point representing approximately half the expected usage). A total of 85.6% of participants in the telemonitoring group made ≥ 1 call; among these participants, adherence to the intervention was highest, at 90.2%, during the first week of the study period and decreased to 55.1% by week 26. A total of 29,163 variances were generated during the study period, with a median of 21 (interquartile range, 5 to 54) per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: sequence of computer‐generated random numbers, with stratification on the basis of the study site |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was centralized and performed by telephone. Randomization is stratified by study site, and force randomised within each study site in blocks of 20 (10 intervention, 10 control), to ensure a balance across study arms within each site. The randomisation sequence is developed by the coordinating centre using a computer random‐number generator. The sequence is unknown to the attending cardiologists and nurses" |
| Blinding of participants and personnel (performance bias) | Low risk | Study investigators and personnel (except for members of the data and safety monitoring board) were unaware of the treatment‐group results until endpoint data had been finalised for all the participants |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An independent Events Review Committee will assess and classify the primary and secondary end point events in a centralized and blinded manner . . . A committee of physicians, all of whom were unaware of the treatment‐group assignments, adjudicated each potential readmission to ensure that the event qualified as a readmission." |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Low risk | Quote: "Baseline characteristics of the patients were similar between the two groups" |
| Methods | Aims: to examine whether at‐home symptom monitoring plus feedback to clinicians about severe symptoms contributes to more effective postoperative symptom control Study design: RCT; recruitment: primary care (advert in clinic) Study duration: 1 month; study type: management; study subtype: cancer | |
| Participants | Inclusion criteria: men and women scheduled for thoracic surgery for primary lung cancer or lung metastases; ≥ 18 years old, able to understand English and the study requirements, and willing and able to respond to a repeated IVR‐administered symptom rating scale Sample size: 79; mean age:60 years;sex: women ‐ 47%; men ‐ 53%; ethnicity: white, non‐Hispanic ‐ 85%, other ‐ 15% Country: USA | |
| Interventions | In the intervention group, the IVR screened the 5 targeted symptoms. On the occurrence of ≥ 1 symptom threshold events for a participant, the IVR system immediately generated an email alert to the surgical team's advanced practice nurse (APN). The email provided the participant's name, phone number(s), and case history number, along with the severity of each symptom that had generated a symptom. If a participant missed a scheduled call, the IVR system initiated up to 2 more calls, spaced 45 min apart. If a participant in the intervention group had ≥ 1 symptom threshold events, the staff member initiated an alert email to the participant's surgical team. Participants in the control group received only automated monitoring and usual symptom care. | |
| Outcomes | Symptom threshold events, cumulative distribution of symptom threshold events, differences in mean symptom severity (primary) | |
| Funding | RSGPB‐03‐244‐01‐BBP from the American Cancer Society, and Grant No. R01 CA026582 from the National Cancer Institute | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | 59 participants per arm would be needed to detect a medium effect size difference in postoperative symptom severity between groups, using a 2 tailed alpha = 0.05 and 80% power | |
| Notes | 2 different types of ATCS were compared against each other | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment was completed electronically by MD Anderson's protocol management system." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All 79 patients completed the 4‐week study" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to understand whether IVR could be effective to engage individuals overdue for colorectal cancer screening in community practice settings and to determine if the effect would persist over time Study design: RCT; recruitment: primary care (telephone) Study duration: 12 months; study type: prevention; study subtype: screening | |
| Participants | Inclusion criteria: men and women aged 50–81 years who were not adherent to colorectal cancer screening Sample size: 11,010; mean age: 61 years;sex: women ‐ 46%; men ‐ 54%; ethnicity: white ‐ 86%, other – 14% Country: USA | |
| Interventions | The intervention was a single IVR telephone call (average length = 5 min) to the primary telephone number listed in the participant's records. The call included the following features: assessment of prior colorectal cancer screening; information about the benefits of screening and elicitation of the barriers to screening; and offer of a faecal occult blood testing kit mailed to the participant's home. The IVR call mentioned both faecal occult blood testing and colonoscopy as recommended screening tests. If the IVR system left a message, only 1 additional message was sent. When there was no answer or a busy signal at the telephone number, up to 6 total attempts were made to reach the participant Participants in the control group received usual care, defined as a personalised outreach letter, mailed annually to all Group Health members before their birthday, informing them of upcoming preventive service needs, including cancer screening | |
| Outcomes | The receipt of any recommended colorectal cancer screening (primary) | |
| Funding | NA | |
| Declaration of conflict of interest | One author (DCG) is a shareholder in Group Health Physicians, which contracts exclusively with Group Health Cooperative to provide medical services. The remaining authors declared no conflicts of interest | |
| Power calculations for sample size | No | |
| Notes | Participants in both the intervention and usual care could have received the outreach letter at any point during the 12‐month follow‐up period near their birthday | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We randomised 10,000 individuals to the intervention and 3279 individuals to usual care. Because the intervention was originally implemented as a pilot quality improvement initiative, the decision was made to maximize the number of individuals who could receive the IVR intervention with the available resources." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | There were significantly more men in the control group (P < 0.001), but there is insufficient evidence that this imbalance has introduced bias. |
| Methods | Aims: to assess the efficacy of an IVR brief intervention in increasing cervical screening rates in 1 Australian region; to determine the cost per additional cervical screen; to compare the cost per additional cervical screen to other cervical screening interventions Study design: RCT; recruitment: primary care (mail) Study duration: 6 months; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: women, aged 18–69 years who had not had a hysterectomy Sample size: 75,532;Mean age: * ; sex: women ‐ 100% Country: Australia | |
| Interventions | Brief advice IVR cervical screening intervention was provided by Generalized Electronic Interviewing System (GEIS) software. The GEIS software explained the nature of the call; identified if women aged 18–69 years were present; selected 1 eligible woman; determined her screening status; delivered a message that either congratulated her on being correctly screened, a message of encouragement if she was under‐screened, or another message appropriate to her status; offered additional messages to counter common barriers to screening; offered additional information on cervical screening and cancer; offered to readout contact sources where she could obtain more information; offered to have someone ring her back if she still had questions; and offered to record any question she may wished answered. GEIS could reschedule the call and participants could request call backs. GEIS generated an email to advise a local staff member responsible for cervical screening promotion in the Hunter region along with any question the woman had recorded. The script contain domains concerned with Pap status determination, cervical screening barrier messages, demographic items, information items, and contact numbers Participants in the control group received no calls | |
| Outcomes | Cervical cancer screening status at 6 months (primary); costs (secondary) | |
| Funding | Hunter Medical Research Institute and the University of Newcastle | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "To obtain a screening rate increase equal to 1.0% of the adult female population, an additional 75,532 (0.01/2) = 378 women would be needed to be screened in the intervention postcodes." | |
| Notes | The cost per additional screening obtained in this study is favourable compared to the other studies, which suggests that the IVR method could be used to target identified individuals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "A brief advice IVR cervical screening intervention was delivered to 17,008 randomly selected households in the Hunter region in New South Wales (NSW) between April and July 2001 in 15 randomly selected postcodes. The change in screening rates before and after the intervention was compared to another 15 randomly selected control postcodes" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aim: to test whether a speech recognition (SR) reminder system would improve adherence to an ICS in a large unselected population of paediatric asthma patients Study design: RCT; recruitment: * Study duration: 12 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: children, aged 3‐12 years with persistent asthma Sample size: 1393; mean age:*sex: * ethnicity: * Country: USA | |
| Interventions | The intervention group received up to 3 tailored SR reminder calls when they were due to refill their inhaled corticosteroids. The calls provided information about asthma, facilitated a rapid inhaled corticosteroids refill, and offered an opportunity to receive a call back from an asthma nurse specialist Control group (no further information) | |
| Outcomes | Medication adherence (refill rate) (primary); acceptability/satisfaction (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no statistically significant differences between the intervention and control groups in age, sex, co‐morbidities, and length of HMO enrolment." |
| Methods | Aims: to conduct a feasibility study of self‐monitoring with a pedometer administered through an IVR system and mobile phones; to examine the added benefit of a human coach Study design: RCT; recruitment: community (advert elsewhere ‐ radio, television, newsletter) Study duration: 3 months; study type: prevention; study subtype: physical activity | |
| Participants | Inclusion criteria: BMI of 25–40 kg/m2, postmenopausal status, access to a mobile phone during the intervention and willingness to walk ≥ 30 min per day Sample size: 71; mean age: 57 years;sex: women ‐ 100%; ethnicity: white ‐ 93%, other ‐ 7% Country: USA | |
| Interventions | Coach group: participants assigned to the coach condition were introduced to the coach by the study facilitator. The coach was trained by the study team to offer a lifestyle intervention. She explained the intervention and offered the steps goal for the first week after reviewing the participant's baseline physical activity and time taken to complete the 1‐mile walk. Then the coach trained the participant to use the pedometer and the IVR system and identified herself as the person who would offer support during the intervention. To receive help from the coach, participants were asked to call the IVR system and leave a message for her. After the baseline visit, the participants interacted only via the telephone and IVR system. 2 daily telephone interactions with the IVR system were scheduled. The IVR system called the participant's mobile phone between 07:00 and 17:00, during a 2‐hour period identified by the participant. To minimise disruption during working hours, this call was limited to 3 questions: an assessment of whether the participant had walked or planned to walk that day, the participant's self‐efficacy to achieve the steps goal for the day and a general enquiry about whether the participant was having a good or bad day. In addition, participants called the IVR system every evening to enter their daily step count from the pedometer and receive an intervention message. During the call, they provided an assessment of self‐efficacy for walking the following day, an assessment of the present day and satisfaction with their walking plan for that day. Participants could use their mobile phone or a land‐line for the evening call The no‐coach (control) group received similar Instructions and training to the coach condition and were offered by the same individual, but with 2 exceptions: the individual did not identify herself as the coach, and participants were not informed that they had access to a coach. Participants had also access to the same technical support for problems with the IVR system or the pedometer. Thus the subjects in the no‐coach condition interacted only with the IVR system. | |
| Outcomes | 1‐mile walk after the intervention (primary); body weight; BMI; waist and hip circumference; self‐efficacy (secondary) | |
| Funding | National Center for Research Resources: UL1RR025755 | |
| Declaration of conflict of interest | Not mentioned | |
| Power calculations for sample size | No | |
| Notes | Delivery of the intervention was both via mobile and landline; first call was initiated by the system (IVR); and the second one by participants themselves | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the end of this visit, participants were stratified by BMI and randomized to the coach or no‐coach condition." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Withdrawal, attrition and retention rates were not significantly different between treatment arms." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Participants in the no‐coach group had higher BMI at baseline (P = 0.29), but unclear whether this has introduced bias. |
| Methods | Aims: to test the ability of an automated, interactive, culturally adapted telephone exercise coach to increase physical activity and lower blood pressure in urban African Americans with poorly controlled hypertension Study design: RCT; Recruitment: primary care (mail) Study duration: 3 months; Study type: management; Study subtype: hypertension | |
| Participants | Inclusion criteria: sedentary, hypertensive, adults in primary care Sample size: 253; Mean age: 58 years;sex: women ‐ 73%; men ‐ 27% Ethnicity: African American ‐ 100% Country: USA | |
| Interventions | Participants in the intervention group received Telephone‐Linked Care for Physical Activity (TLC‐PA); computerised system that 'converses' with participants by telephone using pre‐recorded human speech Participants in the control group received usual primary care and an educational brochure on hypertension | |
| Outcomes | Change in minutes of moderate or greater physical activity from baseline to 3 months; and change in systolic blood pressure from baseline to 3 months (primary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Participants in the control group had higher blood pressure at baseline; but unclear whether this has introduced bias. |
| Methods | Aims: to compare the efficacy of 3 types of reminders in promoting annual repeat mammography screening Study design: RCT; recruitment: other ‐ health plan (mail and telephone) Study duration: 42 months; study type: prevention; study subtype: screening | |
| Participants | Inclusion criteria: women residents of North Carolina aged 40–75 years; were enrolled with the State Health Plan for 2 years; had their last screening mammograms (enrolment mammograms) between September 2003 and September 2004, and had only 1 mammogram in the designated timeframe (to exclude those who had diagnostic mammograms) Sample size: 3547; mean age: > 40 years; sex: women ‐ 100%; ethnicity: white ‐ 88%, black ‐ 11%, Asian, Native Hawaiian/Pacific Islander, American Indian/Alaskan Native or other ‐ 1% Country: USA | |
| Interventions | Participants in the intervention group received automated telephone calls by TeleVox Software, Inc, consisting of reminders 3 months prior to mammography due dates. The message was 69 seconds long and consisted of 224 words. Those who listened to ≥ 20 seconds were considered as successful contact as key message content (due for a mammogram) was delivered during this time. In total, they received 3 reminders. Call attempts were terminated after a 2‐week call window or 10 unsuccessful call attempts to reach intended recipients. Message contents included: dates of women's last mammograms; information about benefits of mammography; recommended guidelines; contact information for the National Cancer Institute's Cancer Information Service; and State Health Plan coverage The second arm received enhanced letter reminders (the same information as the other 2 reminders with several additions; additional text, informed by the Health Belief Model, about the severity of breast cancer and breast cancer susceptibility, names and telephone numbers for the facility where recipients had their last mammograms, and stickers to remind women to make and keep their mammogram appointments) The enhanced usual care group received reminders (mailed letters, included dates of women's last mammograms; information about benefits of mammography; recommended guidelines; contact information for the National Cancer Institute's Cancer Information Service; and State Health Plan coverage) | |
| Outcomes | Mammography adherence (primary) | |
| Funding | National Cancer Institute | |
| Declaration of conflict of interest | None | |
| Power calculations for sample size | To provide 80% power to detect a 6% difference in effect among intervention arms, with alpha 0.05, the sample size required was 3545 participants | |
| Notes | This is a comparison between automated telephone reminder and enhanced usual care reminders | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Prior to study recruitment, women were assigned randomly to one of three reminder groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Analyses were intent‐to‐treat and included all study participants (n= 3547)" |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes were reported |
| Other bias | Low risk | Comment: groups were comparable across all baseline characteristics |
| Methods | Aims: to investigate the effectiveness of totally automated telephone technology in improving adherence to prescribed continuous positive airway pressure (CPAP) therapy Study design: RCT; recruitment: other ‐ home care company (telephone) Study duration: 2 months; study type: management; study subtype: obstructive sleep apnoea syndrome (OSAS) | |
| Participants | Inclusion criteria: English‐speaking adults, having a physician diagnosis of OSAS, and polysomnography demonstrating 15 episodes of apnoea or hypopnoea per hour of sleep Sample size: 30; mean age: 46 years; sex: * ; ethnicity: * Country: USA | |
| Interventions | Telephone‐linked communications technology (TLC) CPAP is based on patterns of CPAP adherence and side‐effect profiles. After receiving salutation, participants enter personal password for maintaining security and confidentiality. TLC assessed participants' frequency and duration of CPAP use during the previous week (except for the first call, in which 3 days' use were collected). In case of non‐use of the CPAP, or use for fewer than 4 h per night (on nights they used it) or fewer than 5 nights per week (or fewer than 2 nights in the case of the 3‐day call), the system proceeded to ask a series of questions aimed at identifying the cause of CPAP non‐adherence (side effects, difficulty using CPAP, lack of perceived benefit, machine malfunction). The severity of each side effect was also ascertained. For those with good adherence, TLC reinforces this behaviour. The call is initiated by participants 3 days after starting CPAP therapy (3‐day call) and thereafter weekly (1‐week call) for a total of 2 months. Calls could be made at any time of day that was convenient for the user. If participant failed to call TLC on a scheduled day, TLC called that person the next day, repeating calls periodically during a time period set with the user. If 2 days elapsed from the day of the scheduled call, the system administrator was notified automatically and informed the research assistants working on the project, who then would follow up with the participant to determine why the call was not made. TLC ascertains the severity of OSAS‐related symptoms, including snoring, breathing pauses, and daytime sleepiness. Those with OSAS symptoms, TLC recommends follow‐up with their physician as well as provide a brief counselling dialogue, focusing on appropriate CPAP use, expected benefits, correct CPAP operating technique, and potential side effects and their treatment. Reinforcement of the need for regular CPAP use was provided , stressing that regular use would reduce daytime sleepiness and could also have the additional benefit of reducing the risk of cardiovascular disease. Continuous reports including frequency and duration of CPAP use, side effects, and OSAS symptoms was sent to the physicians, biweekly or on a need basis. Participants in the control group received usual care alone. | |
| Outcomes | CPAP use (primary); sleep symptoms checklist; functional outcomes of sleep questionnaire (secondary) | |
| Funding | VA Health Services Research and Development Service | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the conclusion of a baseline examination . . . eligible participants were randomised to either TLC and usual medical care or usual medical care alone." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "At baseline, intervention and usual‐care participants had similar characteristics; there were no differences at P < 0.05 level" |
| Methods | Aims: to evaluate the effectiveness of automated systems to prompt patients with diabetes mellitus to obtain overdue laboratory tests Study design: RCT; recruitment: other ‐ health plan (mail and telephone) Study duration: 6 months; study type: management; study subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: health plan members with diabetes were passively enrolled if they met the following criteria: (1) age older than 18 years; (2) no HbA1C, low‐density lipoproteins, and urinary microalbumin tests in more than 365 days; and (3) a birthday within the next 3 months Sample size: 13,057; mean age: 51 years; sex: men ‐ 54%; women ‐ 46%; ethnicity: other or unknown – 48%, white ‐ 23%, Hispanic ‐ 14%, black ‐ 10%, Asian ‐ 5% Country: USA | |
| Interventions | Thetelephone call group received a single call beginning with a standard greeting saying that the message to follow was from Kaiser Permanente. The message was in English and informed the recipient to call a toll‐free number to receive a message from his or her health plan. Members who called in used an interactive menu to select English or Spanish and retrieved the message by inputting their medical record number. Message content: "Telephone calls began with a standard greeting saying that the message to follow was from Kaiser Permanente. The message was in English and informed the recipient to call a toll‐free number to receive a message from his or her health plan. Members who called in used an interactive menu to select English or Spanish and retrieved the message by inputting their medical record number." The member was informed that he or she may have diabetes and was due for laboratory tests that had already been ordered. The tests were named, and the member was directed to go to his or her local health plan laboratory for the tests.The message duration was 40 s long and consisted of 100 words. Letter group received a single letter. Letter + call group received a letter followed by a telephone call at 4 weeks for non‐response. Call + letter group received a telephone call followed by a letter at 4 weeks for non‐response. Letter + call + letter group received a letter that is followed by a telephone call at 4 weeks for non‐response, followed by a second letter at 8 weeks for continued non‐response. Control group received no intervention. | |
| Outcomes | Adherence to all 3 laboratory tests (glycated haemoglobin, low‐density lipoproteins, and urinary microalbumin) by 12 weeks (primary) | |
| Funding | Merck Health Management Services | |
| Declaration of conflict of interest | None | |
| Power calculations for sample size | Aimed for 90% power to detect a difference between 35% (call group) and 40% (call + letter group), which required 2008 participants per group | |
| Notes | This is a comparison between telephone call group and control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computerised random number generator was used |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All subjects' data were analysed according to initial randomisation whether the subject was successfully contacted or was lost to follow‐up" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "Randomization resulted in small but statistically significant (P = .002) differences in the distribution of race/ethnicity across study arms. There were no significant (P < .05) differences in the distribution of other subject characteristics across study arms." |
| Methods | Aim: to evaluate an automated system to decrease primary non‐adherence to statins for lowering cholesterol Study design: RCT; recruitment: other ‐ health plan (organisational referral) Study duration: 10 weeks; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: ≥ 1 years of membership from the prescription date and no gap in enrolment more than 30 days during the past year; 24 years and older at the time of the prescription; no record of the statin prescription being filled at a health plan pharmacy after 1 to 2 weeks. Sample size: 5216; mean age: 56 years;sex: women ‐ 51%; men ‐ 49% ethnicity: white ‐ 28%, black ‐ 10%, Hispanic ‐ 30%, Asian and Pacific Islander ‐ 7%, other ‐ 2%, unknown ‐ 23% Country: USA | |
| Interventions | ATCS Plus: participants were contacted 1 to 2 weeks after the prescription date by an automated telephone call to retrieve a personalised message from the health plan. If no one answered, messages were left on answering machines directing participants to call a toll‐free number to retrieve their message. Busy signals resulted in up to 2 more attempts to make telephone contact on subsequent days. Calls were made between 10 am and 8 pm. 1 week after the initiation of calls, participants who still did not fill their prescription were sent a letter. The letters were expected to arrive 9 to 11 days after the first outreach contact by telephone. More than 95% of all health plan members have a telephone number on record, and more than 99% have an address. Telephone calls began with a standard greeting saying that the message was from Kaiser Permanente. The message could be retrieved through interactive messaging during the call or by dialling a toll‐free number. The personalised message conveyed that a statin drug was prescribed by their clinician and there was no record of the drug being dispensed by health plan pharmacies. The potential importance of the medication was described, and participants were encouraged to either have the prescription filled or contact the prescribing physician. The contact number of the local health plan pharmacy was provided. The telephone message was accessed in either English or Spanish and was approximately 40 seconds in duration. The letter was printed on one side in English and the other side in Spanish, and the text occupied approximately half a page. Letters were signed using the prescribing physician's name, a standard outreach practice in the health plan Control group received usual care (no calls) | |
| Outcomes | Medication (statins) adherence (primary) | |
| Funding | Merck Sharp & Dohme Corp, a subsidiary of Merck & Co Inc, Whitehouse Station, New Jersey | |
| Declaration of conflict of interest | Ms Marrett is an employee of Merck. Dr Tunceli is an employee of Merck and owns stock in the company | |
| Power calculations for sample size | We aimed for sufficient power to detect a 5% difference in adherence between the study arms based on a response rate of 20% in the control arm. Use of a significance level of 0.05, 90% power, a 2‐sided test of proportions, and equal‐sized groups required 1504 participants per group | |
| Notes | Quote: "Although a detailed cost analysis was not attempted, the marginal costs of the telephone calls and mailings were approximately USD 1.70 per person" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A study programmer used computer‐generated random numbers to sort participants into the intervention and control groups in equal proportion (day 0)." |
| Allocation concealment (selection bias) | Low risk | Quote:"Assignment was concealed from study investigators and analysts. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All participants' data were analysed according to initial randomisation (intent‐to‐treat) whether or not the participant was successfully contacted." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | No statistically significant differences were noted between groups at baseline |
| Methods | Aims: to evaluate the effectiveness of computer generated telephone reminder calls in increasing kept appointment rates in a public health setting Study design: quasi‐RCT; recruitment: primary care (organisational referral) Study duration: 1 month; study type: either; subtype: appointment reminder | |
| Participants | Inclusion criteria: all clients with scheduled appointments for any of 4 public health programmes (immunisation, well child, or family planning) at the health clinic were eligible for participation Sample size: 517; mean age: * sex: * ethnicity: * Country: USA | |
| Interventions | Computer‐generated telephone reminder: households of clients received 1 of 4 automated telephone messages specific to the programme for which the clients had an appointment. The messages were delivered between 6 pm and 9 pm on the evening preceding the scheduled appointments. Up to 9 attempts was made in order to get a successful contact Participants in the control group did not receive reminders (no intervention) | |
| Outcomes | Appointment adherence (primary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | The cost per additional appointment kept was USD 5.20 during the first full year of operation and USD 1.04 for subsequent years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Clients with last names beginning with the letters A through L were as signed to receive a computer‐generated telephone reminder message during the evening prior to their scheduled appointment. Clients with last names beginning with the letters M through were designated as controls and did not receive a reminder message." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | All participants were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to assess the sustained impact of computer generated messages on immunisation coverage during the first 2 years of life Study design: RCT; recruitment: primary care (organisational referral) Study duration: 36 months; study type: prevention; study subtype: immunisation | |
| Participants | Inclusion criteria: all children who were 60 to 90 days of age, who had received the first dose of diphtheria‐tetanus‐pertussis or poliovirus (PV) vaccines, and who had telephone numbers listed in the pre‐existing computerised health department database Sample size: 1227 mean age: * sex: * ethnicity: * Country: USA | |
| Interventions | Telephone messages alone received 1 telephone reminder message prior to the scheduled immunisation date and up to 4 telephone recall messages (1/week) over the 4‐week period following the due date. Contacts were made during weekday evening hours between 6:00 pm and 9:00 pm and on Saturdays from noon to 8:00 pm (up to 5 messages) Telephone messages + letters (up to 5 messages and/or letters) Letters only (up to 5 letters) No notification control | |
| Outcomes | Immunisation series completion at 24 months of age (primary); acceptability and costs (secondary) | |
| Funding | National Immunisation Programme, CDC | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | Target sample size was 1200 | |
| Notes | Costs per month (and per year) were as follows: telephone messages alone, USD 139 (USD 1672); telephone messages + letter, USD 126 (USD 1518); and letters only, USD 66 (USD 796). There were no cost‐effectiveness data available for no notification control group. This is a comparison between the telephone messages alone and control groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Children enrolled in the evaluation were randomised to receive telephone messages followed by letters (Group A); telephone messages alone (Group B); letters only (Group C); or no notification (Group D)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "No significant differences were noted between groups with regard to sex (p = 0.12), number of children in the household (p = 0.69), or whether children were insured by Medicaid (p = 0.72). However, significant ethnic and language differences were noted between groups." Insufficient evidence that this imbalance has introduced bias. |
| Methods | Aims: to test a hypothesis that participants who received telephone follow‐up nurse counselling would report greater adherence to the walking goals than participants who received no follow‐up calls, and those who received personal calls would report greater adherence than participants receiving a mixture of personal and automated calls Study design: RCT; recruitment: primary care (mail) Study duration: 12 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: 60–80 years of age, enrolled in primary care clinic, non‐institutionalised and independent in activities of daily living, stable health, willing to increase walking for exercise and attend research clinic visits, and satisfactory performance on a 6‐minute walking test Sample size: 181; mean age: 69 years; sex: men ‐ 99%; women ‐ 1%; ethnicity: * Country: USA | |
| Interventions | 20 personal phone calls delivered by a nurse Multimodal intervention received 10 personal phone calls from the nurse interspersed randomly with 10 automated phone calls (P&AC) that delivered a message recorded by the nurse. Automated calls were phased in beginning with month 2. The schedule of calls was not predictable. Automated calls, designed to maintain contact and cue walking in an inexpensive and efficient manner, delivered a brief message recorded by the nurse such as, "This is your STEPS nurse reminding you to keep up your walking . . . the weather is hot now so be sure to drink plenty of water." These were delivered by a Phone Tree (Personal Communication Systems, Winston‐Salem, NC). Control received no phone calls. | |
| Outcomes | Self‐reported (diary) walking adherence (primary); quality of life (secondary) | |
| Funding | Department of Veterans Affairs Health Services Research and Development Service | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between the multimodal intervention and control. There was no evidence of a pattern of increased risk associated with increased walking (the intervention effect). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the intervention components common to all participants were completed, they were randomised to one of the three groups for different telephone follow‐up interventions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The data collector was blinded to intervention group assignment and at the end of the trial was unable to guess individual patient group assignment better than what would be predicted by chance. The nurse was blinded to walking diary adherence data and other self‐report follow‐up data" |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition rate; missing outcome data balanced in numbers, with similar reasons for missing data across groups. Quote: "Only 31 (15%) of the 212 randomised participants failed to complete the 12‐month trial." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "BL participant characteristics were not different between any of the three treatment groups"; however participants in complex intervention arm had lower educational status; were living in rural area; and smoked more cigarettes than participants in the other 2 groups. There is insufficient evidence that this imbalance has introduced bias. |
| Methods | Aims: to develop a methodology that stratifies members by likelihood of completing a colorectal cancer screening Study design: RCT; recruitment: other ‐ insurance company (organisational referral) Study duration: 3 months; study type: prevention; study subtype: screening | |
| Participants | Inclusion criteria: members of an insurance plan from Horizon Blue Cross Blue Shield of New Jersey eligible for colorectal cancer screening Sample size: 47,097; mean age: 58 years;sex: women ‐ 53%; men ‐ 47%; ethnicity:* Country: USA | |
| Interventions | Participants in the IVR group received 1 call with varying messaging. Depending on the number of non‐adherent members and the segments' health descriptions, an outreach segment may contain ≥ 1 model segments Participants in the control group received no calls | |
| Outcomes | Receipt of colorectal cancer screening at 3 months (primary); costs (secondary) | |
| Funding | Silverlink Communications | |
| Declaration of conflict of interest | Drs Durant and Newsom are employees of Silverlink Communications, have attended meetings and conferences for the company, and own stock options. Dr Berger is an employee of Silverlink Communications. Dr Pomerantz is an employee of Horizon Blue Cross Blue Shield of New Jersey. Ms Rubin has no financial interests to disclose. | |
| Power calculations for sample size | A power analysis was performed before the launch of the intervention to determine the minimal size needed for each segment, given an estimated effect size of 2% increase for each graded segment and an α level = .05 | |
| Notes | Authors of this study were contacted for unpublished analyses on 14 June 2015. The authors were seeking approval to share data. Communication cost per screening was USD 14.84. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Another 400 members per outreach segment were randomly assigned to a control group and received no communication." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Quote: "Given that the sizes of the segmented groups were determined by the insurer's nonadherent population and not via a recruitment method, it was determined at the launch of the communication that the comparison of the completion rate of segment 4 and segment 5 was underpowered given the segment sizes and the estimated effect size" |
| Methods | Aims: to develop and evaluate cost‐effective intervention strategies for pregnant smokers with diverse demographic and smoking related characteristics Study design: RCT; recruitment: other ‐ health plan (telephone) Study duration: 34 weeks; study type: management; study subtype: smoking | |
| Participants | Inclusion criteria: English‐speaking women 18 years of age or older who self‐reported to be active smokers at their initial prenatal appointment Sample size: 332; mean age: 30 years; sex: women ‐ 100%; ethnicity: white ‐ 61%, black ‐ 16%, Hispanic ‐ 15%, other – 8% Country: USA | |
| Interventions | IVR. Women assigned to this group were sent Living Smoke‐Free and had access to a computerised interactive telephone support system developed with InfoMedics. Subjects were mailed an informational brochure and provided a unique identification number and password to gain access to the system. A subsequent 10‐minute telephone call from a health educator answered questions and provided further details on use of the system. Using a touch‐tone telephone, subjects could access the IVR programme with a toll‐free number 7 days a week, 24 h a day. Upon calling the system, subjects were asked a series of questions about their smoking behaviour, beliefs, and readiness to change. Users provided answers through their touch‐tone telephone keypad. In response, the programme provided stage‐appropriate customised messages recorded by a professional voice model. With stored data from previous calls, the programme automatically reinforced any positive changes made by a smoker over time (e.g. a reduction of > 25% in number of cigarettes smoked per day, a decision to set a quit date). Each call was designed to be approximately 5 min in length and included stage relevant interactive exercises, a summary and reinforcement of key messages and goal commitments, and advice to review Living Smoke‐Free Motivational interviewing (MI). Women assigned to the MI group were sent Living Smoke‐Free and were provided telephone counselling by nurse educators trained in the techniques of MI. MI has been defined as a "directive, client‐centred counselling style for helping clients explore and resolve ambivalence about behaviour change." It emerged as an alternative to direct persuasion in counselling people with addictive problems. MI conceptualises motivation as a state that fluctuates from time to time or situation to situation, rather than as an inherent character trait. Thus, motivation is perceived as open to therapeutic intervention. The dangers of prenatal smoking have been widely disseminated and pregnant women report strong belief in that harm. MI attempts to highlight and help resolve ambivalence resulting from the discrepancy between beliefs and behaviour through reflection, advice, and support. Investigators trained 17 preterm nurse educators experienced in telephone‐based patient counselling in the principles and strategies of MI. The training consisted of a 6‐hour session led by nationally‐known experts, a 2‐hour small‐group meeting, and an 85‐page reference manual with salary support for up to 8 h of self‐study. Booklet only. Women assigned to this group only received Living Smoke‐Free. The multicolour, 32‐page booklet was developed by the investigators in collaboration with Krames Communications, a Division of the StayWell Co. Targeted to the lifestyle of pregnant smokers, it is printed in clear type and written at an eighth‐grade reading level. Multiracial/ethnic illustrations of smokers were designed to appeal to a wide audience of pregnant women. Visual and written messages tailored to stage of readiness to change are presented through 4 different characters, each representing a different stage. The booklet includes advice about preparing to quit, setting a quit date, methods for quitting, obtaining social support, and relapse prevention strategies. Advice about exercise, diet, and stress management are also included. | |
| Outcomes | Smoking abstinence (biochemically confirmed); satisfaction with the intervention (secondary) | |
| Funding | Robert Wood Johnson Foundation | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | At alpha set at 0.05 and power at 0.80 (1‐tailed test), 125 participants per group were needed to detect the 13% difference in quit rates projected for the booklet‐only versus IVR comparison | |
| Notes | This is a comparison between IVR arm and booklet arm only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After random assignment to one of the three intervention groups . . . subjects were mailed a copy of the self‐help smoking cessation booklet, Living Smoke‐Free—A Healthier Start for You and Your Baby." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Providers were blind to study participation and group assignment" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | Only participants who remained in the intervention were included in the final analysis. Although the reasons for attrition such as abortion/miscarriage (n = 31), disenrollment from the health plan prior to delivery (n = 22), and delivery prior to the 32nd week of pregnancy (n = 5) were reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "No statistically significant differences were observed for any baseline measures" |
| Methods | Aims: to determine the feasibility and effectiveness of automated telephone support calls targeting physical activity and healthful eating as strategies for weight loss for patients with pre‐diabetes. Study design: RCT; recruitment: other ‐ community (in‐person during diabetes prevention classes) Study duration: 12 weeks; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: adults participating in diabetes prevention class, English‐speaking, not pregnant during the study period, had access to a telephone, and were not concurrently enrolled in another research study involving diabetes management or weight management. Sample size: 77; mean age: 59 years; sex: men ‐ 39%, women ‐ 71%; ethnicity: white ‐ 68%, Hispanic ‐ 18%, other or unknown ‐ 7%, black ‐ 4%, Asian ‐ 3% Country: USA | |
| Interventions | Participants in the intervention group received IVR calls that were designed to address and reinforce the messages delivered in the pre‐diabetes class and the content of the participant action plans. Participants had the option to choose to listen to messages related to either nutrition or physical activity, followed by behaviour change techniques between goal‐setting and self‐monitoring. Received 7 counselling calls lasting 5‐10 min while 5 calls provided either physical activity or nutrition tip, that lasted for a minute. Participants in the control group did not receive calls (no intervention). | |
| Outcomes | Physical activity; dietary habits; weight (percent lost) (primary); satisfaction (secondary) | |
| Funding | Department of Prevention at Kaiser Permanente Colorado | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | ClinicalTrials.gov Identifier: NCT00384488 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization occurred at the class‐level and was completed by a research assistant who chose a slip of paper with study group assignment from a hat" |
| Allocation concealment (selection bias) | Low risk | Study participants were not informed of the study arm until they completed the informed consent as to not influence the decision to participate |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "While research staff was unblinded to study arm designation, study participants were not informed of the study arm until they completed the informed consent as to not influence the decision to participate". |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | There were no differences in dropout rate between study conditions. |
| Selective reporting (reporting bias) | Low risk | The protocol was available, and all outcomes of interest were reported. |
| Other bias | Low risk | Groups were balanced at baseline. |
| Methods | Aims: to determine the effectiveness of automated telephone counselling to support parents of overweight or at‐risk children to change the home environment to foster more healthful child eating and activity behaviours, thereby reducing child BMI and BMI z‐scores Study design: RCT; recruitment: * (telephone) Study duration: 12 months; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: children aged 8–12 years with a BMI of 85th percentile for their age who received care from Kaiser Permanente Colorado Sample size: 220; mean age: 11 years; sex: boys ‐ 54%; girls ‐ 46%; ethnicity: white ‐ 63%, Hispanic ‐ 26%, other – 11% Country: USA | |
| Interventions | The Family Connections (FC) IVR group received 10 calls, 1st call a week after the group session, the call contents were tailored to participants responses using logic branching method. Calls can be initiated by either the system or the participant. At each call, the goals set in the previous week are assessed, and participants hear related tips and then select specific messages. Calls concluded with a goal setting procedure. The 6th IVR‐counselling call provided parents with instruction on a family goal‐setting procedure related to physical activity and eating based on the 5A's model. Calls 7–10 reinforced the information delivered in the initial 6 calls. FC workbook. A 61‐page workbook was developed to promote increased physical activity and the consumption of fruits and vegetables in concert with decreased sugared‐drink consumption and television viewing/recreational computer time. The workbook included 2 distinct sections. Part 1 targeted 3 days of intervention, and part 2 targeted 2 days, each with specific homework assignments. The workbook encouraged parents to complete 5 days of intervention across a single week. Homework assignments were intended to encourage lasting changes in the families. All parents randomly assigned to this intervention received the workbook from study research assistants FC group. This intervention consisted of 2 small‐group sessions (2 h each, spaced 1 week apart) held at a local clinic and delivered by a dietitian. Each session included 10–15 parents representing distinct children and utilised the Family Connections workbook. The first session focused on parents' behavioural health skills and knowledge of weight, nutrition, and physical activity. It also identified key parenting skills: limit setting, effective communication, and role modelling. This session concluded with role playing, problem‐solving, and the development of an action plan. Session 2 integrated the knowledge acquired in Session 1, the experiences associated with the action plan, and strategies for restructuring the home environment. The session again concluded with parents' completing an action plan for parental behaviours, role modelling, and changes to the home environment that would facilitate healthy eating and physical activity | |
| Outcomes | BMI z‐score, physical activity; sedentary behaviour; dietary habits (primary) | |
| Funding | Garfield Memorial Fund, Kaiser Permanente Colorado Weight Management Program | |
| Declaration of conflict of interest | None | |
| Power calculations for sample size | Sample size calculations were completed, varying the detectable effect sizes from small to medium with a power of 0.8. The result was a need for 42 participants per intervention to detect a medium effect and 64 participants to detect a small effect | |
| Notes | This is a comparison between FC IVR group and FC group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Through a random‐numbers table, participants were assigned randomly . . . to the FC‐workbook, the FC‐group, or the FC‐IVR intervention." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across groups. ITT analysis was used to include all participants who received the intervention or FC workbook in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were balanced at baseline. Quote: "The intervention conditions did not differ on any demographic variables." |
| Methods | Aims: to test the feasibility and impact of an automated workplace mental health assessment and intervention Study design: RCT; Recruitment: primary care (advert in clinic) Study duration: 6 months; study type: management; subtype: mental health | |
| Participants | Inclusion criteria: ability to speak and understand conversational English, 18 years of age or older, access to a touch‐tone telephone, not undergoing mental health treatment or currently taking a medication prescribed for mental health treatment, and experiencing some type of emotional distress as indicated by scoring positive on the WHO‐5 Well‐being Index and the Functional Impairment question Sample size: 164; mean age: 39 years; sex: men ‐ 24%, women ‐ 76%; ethnicity: white ‐ 56%, black/African American ‐ 32%, other – 12% Country: USA | |
| Interventions | Telephone‐Linked Communications (TLC) detect system is an automated mental health screening and counselling programme that employees could access from any phone. The assessment is made in a hierarchical manner. Those testing positive proceed to 2nd level of more disorder‐specific and in‐depth screening by additional screening instruments. This provides extensive information about user's mental health problem, including its symptoms, natural history, and available treatments. It also directs the user to the referral sub‐module providing disorder‐specific information on both self‐management and professional help appropriate to the level of its severity as determined by the system's assessment. Follow‐up calls were used to check user's adherence to the system's advice and to check if they had sought professional assistance or engaged in self‐help. For those who did not adhere, an intervention follow‐up module provided tailored educational materials, including description of the disorder and providing treatment options. Both intervention and follow‐up calls provided an option to spread out the information into multiple sessions to reduce the time burden. This also included a validation function that checked whether the health care providers agreed with the system's assessment. Each call lasted between 30‐90 min. The calls used digitised voice of a female voice actor who received coaching to deliver the message appropriately Participants in the control group received advice only (via IVR) | |
| Outcomes | Quality of life (physical health scale and mental health scale), total depression, perceived stress levels/score, total well‐being (WHO‐5) (primary); acceptability of service/satisfaction (secondary) | |
| Funding | CDC | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After eligibility screening, baseline data were collected from study participants, who were subsequently randomised and connected to the automated program to receive assessment for mental health disorders (all subjects) and intervention (only experimental subjects)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used to include all participants who received the intervention or control group in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no significant demographic differences between the two study groups at baseline" |
| Methods | Aims: to evaluate interventions to improve laboratory monitoring at initiation of medication therapy Study design: cluster RCT with 15 clusters; recruitment: other ‐ health plan (organisational referral) Study duration: 25 days study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: adults aged 18 years & above; spoke English; had continuous HMO membership for ≥ 12 months, a pharmacy benefit,and a telephone number; had received a new prescription of a study medication from their PCP; and had not had recommended baseline laboratory monitoring within 5 days after the medication dispensing Sample size: 961; mean age: 59 years; sex: men ‐ 47%; women ‐ 53 %; ethnicity:* Country: USA | |
| Interventions | Automated telephone voice message (AVM): AVM prompted participants to seek preordered laboratory tests. A personalised message retrieved after entering a health record number and year of birth stated that the medication the participant had been dispensed required laboratory monitoring; messages referenced the actual drug dispensed and the monitoring tests required. The participant was advised that the testing had been ordered and could be completed at any health maintenance organisations laboratory The EMR intervention consisted of a participant‐specific electronic message to the PCP from the chair of the participant safety committee. The message stated that computer records indicated that the participant had been dispensed a new medication, laboratory monitoring was recommended, and the participant had not received the test(s) between 6 months before and 5 days after the dispensing. The message referenced internal and external guideline resources, recommended specific tests, and provided a sample letter the PCP could send to the participant to request that he or she go to the laboratory. Pharmacy Team Outreach Intervention began with a telephone call from a nurse in the pharmacy department to the participant to encourage laboratory testing. If the nurse successfully contacted the participant, a follow‐up letter reminded the participant to obtain the laboratory test(s). If telephone contact was not successful, the nurse sent a letter suggesting that the participant go in for testing. If participants had questions or concerns about their medication during the contacts, a pharmacist was available for consultation. Usual care (controls) | |
| Outcomes | Completion of all recommended baseline laboratory tests (primary) | |
| Funding | This project was supported by Kaiser Permanente's Garfield Memorial Fund and cooperative agreement U18 HS010391 from the Agency for Healthcare Research and Quality | |
| Declaration of conflict of interest | None reported | |
| Power calculations for sample size | "Using retrospective data, we estimated that 25% of the UC group would receive laboratory testing by 30 days after a new medication was dispensed. With 200 participants per group, we determined that we could detect a difference of approximately 13% between the groups with a probability of 0.80." | |
| Notes | This is a comparison between the AVM arm versus usual care. 3 clusters (267 participants) were allocated to AVM and 4 clusters (237 participants) to usual care; remaining clusters (n = 8) were arms not considered in this review. Note that analysis did not appear to adjust for clustering; therefore a unit of analysis error exists that may result in overly precise effect estimates for this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence was generated by a computerized random‐number generator" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All 15 clinics were randomised at one time; therefore, allocation concealment was not an issue." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Patient participants were masked from the nature of the study. Because of the nature of the intervention, the study nurse conducting the interventions was not blinded to group assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Primary outcomes were obtained entirely from electronic records, and the study analyst was blinded to study group assignment before ascertainment of outcomes." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "No patients were lost to follow‐up." |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest reported |
| Other bias | Unclear risk | There was a small baseline imbalance, but it is unlikely that this influenced the results. Quote: "The other characteristics of the study groups were also similar except that the AVM group had a smaller proportion of female PCPs". There was insufficient information to judge whether selective recruitment of cluster participants may have occurred. |
| Methods | Aim: to examine the impact of a multimodal intervention on mammography and colorectal cancer screening rates in a safety‐net practice caring for underserved patients Study design: RCT; recruitment: primary care (health professional referral) Study duration: 12 months; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: registered patient at the practice; (≥ 1 visit to the practice in the past 2 years (to ensure participants were actively receiving care at the practice); aged 40‐75 years for mammography screening, and 50‐75 years for colorectal cancer screening; past due for annual mammography or colorectal cancer screening (recommended intervals are 10 years for those screened through colonoscopy, 5 years for those screened with sigmoidoscopy and/or barium enema, and annually for those screened through faecal occult blood tests). Sample size: 469; mean age: *; sex: women ‐ 56%; men ‐ 44% (for colorectal cancer); ethnicity: white ‐ 61%; black/African Amercian ‐ 28%; Hispanic ‐ 5%; Asian ‐ 5% Country: USA | |
| Interventions | Multimodal intervention: outreach to unscreened participants consisted of 2 personalised letters and up to 4 automated telephone reminder (ATR) calls. The automated telephone reminders were scripted, pre‐recorded messages that include the participant's first name. The message identified the callers and the practices; it then informed the participants they were past due and the phone number to call to schedule a screening (mammography) or an appointment (to discuss colorectal cancer). The first letter was sent within the first week of enrolment. This was followed by 2 completed ATRs at week 2 and 6. For participants who remain unscreened, a second letter was mailed out at week 12 followed by a third ATR at week 14. For participants past due for colorectal cancer screening, the letter included a testing kit for faecal immunochemical testing for home use. A final ATR was made at week 26. Both the letters and ATRs provided the phone number of the outreach worker if help is needed. Using a 3‐way call option, the outreach worker could link participants with mammography schedulers or with the National Breast and Cervical Cancer Early Detection Program (NBCCEP), which provides free screening for the uninsured. The intervention also included participant and physicians prompts. Participants in the control group received usual care (chart review). | |
| Outcomes | Chart documentation of breast cancer screening, colorectal cancer screening, or both (primary) | |
| Funding | RSGT‐08‐077‐01‐CPHPS American Cancer Society | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | 80% power to detect a difference of 18% in the mammography group and 13% in the colorectal screening group using 95% confidence intervals has been calculated | |
| Notes | Clinicaltrials.gov Identifier: NCT00818857 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by screening type (mammography or colorectal cancer) to ensure that comparable groups of patients are randomised to each arm." |
| Allocation concealment (selection bias) | Low risk | Quote: "Unique ID numbers were assigned to patients that identify their intervention group." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The statistician maintained the key; all other study personnel were blinded to the intervention group assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Baseline and follow‐up measures were taken by a research assistant who is blinded to group assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Quuote: "We adopted an intention‐to‐treat analysis. That is, all patients originally assigned to a group were analysed." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all outcomes of interest have been reported in the pre‐specified way. |
| Other bias | Unclear risk | Quote: "There was no statistically significant baseline difference between the intervention and control groups for the mammography intervention. Race was the only characteristic that differed between participants at baseline between those in the intervention and control groups in the colorectal cancer group" |
| Methods | Aims: to assess the relative impact of various components of the reminder, recall, and outreach (RRO) model on breast cancer and colorectal cancer screening rates within a safety net practice Study design: RCT; recruitment: primary care (mail) Study duration: 12 months; study type: prevention; study subtype: screening | |
| Participants | Inclusion criteria: being a registered patient at the study clinic; being an active patient at the practice (having ≥ 1 visit to the practice in the last 2 years); women aged 40–74 for breast cancer screening; aged 50 to 74 for colorectal cancer screening; past due for breast cancer or colorectal cancer screening Sample size: 1008; mean age: inestimable;sex: women ‐ 55%; men ‐ 45% ethnicity: non‐Hispanic white ‐ 48%, non‐Hispanic black ‐ 37%, other (including Hispanic) – 15% Country: USA | |
| Interventions | Letter and automated telephone message (letter + autodial) group received the letter plus a series of up to 5 automated telephone calls. Investigators used the participants' most current available telephone numbers from the medical record. Telephone calls were attempted for up to 2 weeks at varying times throughout the day/evening until a person or an answering machine responded. The automated message contained similar information to the letter, with instructions to call the outreach worker or the practice to arrange for screening or with questions. These calls were delivered to participants on weeks 2 and 8 following randomisation. Until there was documented screening, chart reviews were performed on weeks 12 and 26. Automated telephone messages were repeated on weeks 14, 28, and 38 for participants remaining unscreened at these time periods. Letter + autodial + prompt group received the same intervention as above plus paper prompts delivered at the time of a participant‐initiated visit. We used paper prompts because this enabled us to deliver similar prompts to participants and clinicians simultaneously, and because of doubts regarding effects of electronic prompts on clinician screening. Research staff reviewed scheduling modules weekly to check for planned acute and preventive visits by participants in this group. Prompts were delivered to the treating clinician at the point of care to remind the participant and provider about overdue screening. Prompts were provided at both acute and preventive visits. The back of each colorectal cancer prompt sheet summarised advantages and limitations for colorectal cancer screening modalities as a way of facilitating clinician–participant discussion. The prompt addressed both colonoscopy and faecal immunochemical tests. Letter + personal call group received the letter plus a personal telephone call from a trained outreach worker. These telephone calls were attempted up to 3 times, at varying times of the day and varying days of the week, with a 1‐week period between attempts. When/if the participant was reached, the outreach worker explained that she was calling on behalf of the practice to remind the participant that s/he was overdue for cancer screening. She used motivational interviewing principles to encourage screening and offered assistance with scheduling an appointment, as well as relevant telephone numbers and logistical assistance, including referral(s) for free mammography and faecal immunochemical test for the uninsured. Participants that did not want to undergo a colonoscopy were offered a mailed faecal immunochemical test as an alternative method of colorectal cancer screening. If a participant refused to have any screening tests done for breast cancer or colorectal cancer, it was indicated in the patient registry and interventions were stopped Reminder letter. A single letter from the practice using the participant's most current available home address from the medical record. The letter, with a personalised salutation, indicated to the participant that s/he was overdue for screening and included information regarding the importance of screening and how to schedule screening. The letter provided the name and telephone number of the outreach worker available to provide assistance with scheduling mammography or arranging colonoscopy referrals. The letter also indicated that free screening for uninsured/underinsured participants was available through a state sponsored programme. Letters were available in English and Spanish. | |
| Outcomes | Electronic medical records documentation of mammography screening at 52 weeks (primary) | |
| Funding | American Cancer Society ‐ RSGT‐08‐077‐01‐CPHPS | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | No | |
| Notes | This is a comparison between letter + autodial group versus letter only (control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computerised random number generator (random number algorithm, stratified by the type of screening(s) was used |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed … An offsite study statistician, who was blinded to the identity of the patient, assigned participants equally into one of the four intervention groups" |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of study personnel was ensured. Quote: "Healthcare personnel and study staff were unaware of group assignment" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "All subjects were analysed in the originally assigned study group, based on intention‐to‐treat" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were balanced at baseline. Quote: "There were no significant differences in participants at baseline between the four intervention groups" |
| Methods | Aims: to measure the efficacy of reminder/recall systems (manual postcard or a computer generated phone message) in private provider offices through collection of return visits and vaccine delivery rates Study design: cluster RCT with 6 clusters; recruitment: primary care (organisational referral) Study duration: *; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: children < 12 months of age and eligible for first, second, or third diphtheria‐tetanus‐pertussis vaccine Sample size: 1138; mean age: *; sex: *; ethnicity: * Country: USA | |
| Interventions | Autodialer: participants received an automated reminder message about their upcoming visits for immunisation 7 days prior to the appointment The mailing arm received a postcard reminder 7 days prior to the appointment. No calls (control) | |
| Outcomes | Immunisation status; cost‐effectiveness (both primary) | |
| Funding | Association of Teachers of Preventive Medicine, National Centers for Disease Control, National Immunisation Program | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between Autodialer and control; 295 participants in mailing arm were not included in the review. Note that analysis did not appear to adjust for clustering; therefore a unit of analysis error exists that may result in overly precise effect estimates for this study. The average cost per child in the Autodialer (intervention) group was USD 15.46 and in the control the average cost per child was USD 11.46. These do not include start‐up costs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sites were randomly assigned to one of three arms of the study: mail, Autodialer, or control" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | There was insufficient information reported to allow an assessment of whether cluster participants were selectively recruited. Baseline imbalances may have existed, quote, "With the exception of age, demographic characteristics of the sites were not uniform." |
| Methods | Aims: to assess the impact of telecommunication system on antihypertensive medication adherence and blood pressure control Study design: RCT; recruitment: community centres (mail and telephone) Study duration: 6 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: aged 60 years and above, be under the care of a physician for hypertension, and be prescribed antihypertensive medication Sample size: 267; mean age: 76 years; sex: men ‐ 23%, women ‐ 77%; ethnicity: other – 89%, black ‐ 11% | |
| Interventions | The Telephone‐Linked Computer (TLC) system is an interactive computer‐based telecommunications system that is totally automated and carries out telephone conversations with hypertension patients in their homes for the purpose of monitoring their blood pressure and treatment, and counselling them to be adherent to their medication regimens. TLC speaks to participants over the telephone using computer‐controlled speech while the participants communicate using the touch‐tone keypad on their telephones. TLC applications promoted self‐efficacy by setting small incremental goals and by providing positive feedback and reinforcement regarding the users' actions. During the conversation, participants reported their blood pressure, their understanding of their prescribed antihypertensive medication regimen (medication names, dosages, and frequency of administration), their adherence to the medication regimen, and whether they had symptoms known to be side effects of their antihypertensive medications. TLC provided education and motivational counselling to improve medication adherence. At the end of the conversation, the information provided by the participant was stored in a database and was transmitted to the participant's physician on a printed report in which data was displayed over time and clinically significant information was highlighted. Calls can be initiated by either TLC or the user, and are made once weekly, each lasting for 4 min. Participants also received training to use TLC and an automated sphygmomanometer. Participants in this group continued to receive usual care Participants in the control group received usual care alone | |
| Outcomes | Change in antihypertensive medication adherence (primary); systolic blood pressure and diastolic blood pressure during 6 months (primary); satisfaction (participants and physicians); cost‐effectiveness (both secondary) | |
| Funding | National Heart, Lung and Blood Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | The system was cost‐effective, especially for non‐adherent participant users ‐ USD 3.69 per 1 mmHg improvement in diastolic blood pressure at 80% baseline adherence to USD 0.87 per 1 mmHg improvement at 50% adherence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "During the home visit a trained field technician confirmed final eligibility and completed baseline measurements, after which participants were randomly assigned to either the TLC or usual care groups using a paired randomisation protocol." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Blindig of personnel ensured. Quote: "All participants received a final home visit 6 months after entry into the study when all study measurements were re‐administered by technicians blinded to the study assignments." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers, with similar reasons for missing data across groups. Quote: "There were no significant differences in the characteristics of TLC users and nonusers who dropped out of the study" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | There was no statistically significant difference in any characteristic between individuals randomised to TLC or to usual care. |
| Methods | Aims: to determine the efficacy of an automated, interactive, telephone‐based health communication intervention for improving glaucoma treatment adherence among patients in 2 hospital‐based eye clinics Study design: RCT; recruitment: secondary care (mail and telephone) Study duration:12 months; study type: management; study subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: treatment for their eye condition at 1 of the 2 participating eye clinics; aged 18‐80 years; white or black/African American; have a home or cellular telephone; speak and understand English; be diagnosed with glaucoma or ocular hypertension for ≥ 1 year; be prescribed daily doses of topical glaucoma treatments for at least the past year; no eye surgery within the past 3 months; have better than 20/200 vision in at least 1 eye; and be able to read or have someone who can help them with reading printed materials. Participants also had to acknowledge non‐adherence, in the past year, with medication taking, obtaining refills or clinic appointments in a screening interview Sample size: 312; mean age: 63 years;sex: women ‐ 37.5%; men ‐ 62.5% ethnicity: white ‐ 9%; black/African American ‐ 91%. Country: USA | |
| Interventions | Automated, interactive, telephone‐based health communication intervention and accompanying printed materials. The telephone intervention consisted of 12 educational telephone calls over a 9‐month period: a call every 2 weeks during months 1 and 2; a call every 3 weeks during months 3, 4, and 5; and a call every 4 weeks during months 6, 7, 8, and 9. The objectives of the calls were to provide individually tailored messages to encourage adherence with medication taking, appointment keeping, and refills; provide information about glaucoma; and intervene on barriers to adherence. The telephone‐based health communication intervention utilised interactive voice recognition technology to facilitate interest, participation, and interaction with call recipients and to standardise the content and delivery of the calls. Participants had the option to respond orally or use a telephone keypad. Telephone calls were primarily outbound, but participants had the option to call into the system if they missed a call. After 5 days of unsuccessful attempts to deliver a call, a reminder card was sent requesting that the participant call in to receive his or her message. Each call was structured to include a salutation; a medication regimen review; the core conversation, with tips to address barriers to adherence; general glaucoma information; and a closing. Participants in the control group received usual care. | |
| Outcomes | Self‐reported medication adherence; self‐reported refill adherence (primary) | |
| Funding | National Institutes of Health grant R01 EY016997 and National Eye Institute Core Grant for Vision Research P30 EY 006360 | |
| Declaration of conflict of interest | None reported | |
| Power calculations for sample size | Using a 2‐group design and a planned sample size of 300 participants, there was adequate power (80%) to detect a 15‐20% percentage point difference in adherence with glaucoma treatment at 12‐month follow‐up. Investigators used software programme Power and Precision by Borenstein et al. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random number generator was used in Excel (Microsoft), and participants were randomised in blocks of 10." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Medical providers were masked to assignment because they were not directly involved in the trial" |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Research interviewers were not masked to assignment because it was necessary to determine treatment group participants' preferences for intervention delivery" |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition rate (intervention = 7, control = 5). Missing outcome data balanced in numbers across groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were balanced at baseline |
| Methods | Aims: to determine if home‐centred monitoring through telemedicine has an impact on clinical characteristics, metabolic profile and quality of life in overweight and obese participants Study design: RCT; recruitment: secondary care (organisational referral) Study duration: 6 months; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: adults aged ≥ 18 years; BMI > 25 kg/m2, and could operate regular phones and electronic microdevices. Participants were also not on any obesity pharmaceutical treatment in the past year. Sample size: 122 ; mean age: 44 years; sex: men ‐ 12%; women ‐ 88 %; ethnicity:* Country: Greece | |
| Interventions | All participants of intervention group (in addition to care as usual) were supplied with an electronic blood pressure monitor (Card Guard CG800BP) and an electronic weight scale (Rowenta). They were given a treatment plan, where they had to measure and transmit 3 times a week, for 6 months, their blood pressure and weight and answer 2 life style questions: 'Did you follow your diet plan during the last 2 days?' and 'Did you follow your exercise plan during the last 2 days?'. The participants chose the type of data transmission they preferred among 3 options: Automated Call Centre through a regular phone, Wireless Application Protocol (WAP) server through a cellular phone and World Wide Web (Internet) server through a personal computer. All of them chose the Automated Call Centre Participants in the control group received usual care, which included a regular, hospital‐based, obesity treatment programme on an outpatient basis consisted of diet and physical activity guidelines | |
| Outcomes | Clinical parameters (body weight, BMI, systolic blood pressure, diastolic blood pressure); laboratory parameters (plasma glucose, serum triglycerides, serum high‐density lipoprotein‐cholesterol and total serum cholesterol), obesity assessment (primary); Health Related Quality of Life, European Quality of Life (5 Dimensions) (secondary) | |
| Funding | European Commission: distance Information Technologies for Home Care for Citizens' Health System (CHS), IST‐1999‐13352 | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "Power calculation indicated that a minimum sample size of N = 100 was required, assuming 0.10 level of significance and 80 percent statistical power" | |
| Notes | During the study, intervention group and control group participants engaged in a hospital‐based, obesity treatment programme based on diet and physical activity guidelines. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into intervention and control groups with a proportion of 1:2. Upon meeting the eligibility criteria and signing the consent form, all patients were allocated using central computerized randomizations. The random numbers were generated in blocks of six. Patients who received an odd number formed the intervention group, whereas patients who received an even number served as the control group" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "all patients were allocated using central computerized randomisation" |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of key study personnel was ensured. Quote: "Both physicians and dieticians were blinded to the treatment arm of the patient" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Data were analysed in an intention‐to‐treat way using the LOFC procedure (last observation carried forward)." |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported |
| Other bias | Low risk | There were no baseline differences between the groups |
| Methods | Aims: to determine the impact of a daily, automated telephone intervention on glycated haemoglobin levels; self‐monitoring blood glucose (SMBG) frequency; self‐reported beliefs regarding severity of diabetes, susceptibility to complications of diabetes, and the benefits of and barriers to self‐management of diabetes compared with standard care in adults with type 2 diabetes mellitus Study design: RCT; recruitment: primary care (health professional referral) Study duration:12 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: aged ≥ 50 years with a diagnosis of type 2 diabetes mellitus documented in the medical record for ≥ 12 months, glycated haemoglobin levels equal to or greater than 7.0% within the past month, speak and understand English, access to either a landline or cellular phone, ability to hear and orally respond to automated telephone voice commands, responsible for own self‐care, access to reliable glucose meter that has 3‐month storage capacity, and self‐care regimen that includes SMBG at least daily Sample size: 119; mean age: 62 years; sex: men ‐ 55%, women ‐ 45%; ethnicity: white ‐ 77%; non‐white – 23% Country: USA | |
| Interventions | In addition to care as usual, the intervention group received daily, automated, prerecorded voice message lasting less than a minute related to type 2 diabetes mellitus. A trained actor playing "Alice," a 60‐year‐old woman with type 2 diabetes mellitus recorded the scripted messages in a professional recording studio. The messages changed every day during the 90‐day intervention period. Messages focused on the American Association of Diabetes Educators' AADE7 Self‐care Behaviours including healthy eating, being active, monitoring (i.e. SMBG), taking medication, problem‐solving, reducing risks, and healthy coping. The messages also focused on changing attitudes and beliefs regarding the susceptibility and severity of type 2 diabetes mellitus and reduction of barriers related to performing self‐care behaviours. Participants chose the time of day they wanted to receive the automated calls and the telephone number they wanted the system to call. The system delivered up to 3 calls each day. If there was no answer or if an answering machine picked up the first call, the system called back an additional 2 times at 15‐minute intervals. If the call was not received by the participant after the third attempt, the system called back the next day at the previously agreed time. No messages were left. Participants were asked to answer and respond to as many calls as possible throughout the study. After listening to the prerecorded message, participants responded to Alice's questions regarding SMBG. The responses are relayed to a website that the investigators have access to. The system was programmed to send an email alert to the investigator when a participant reported a blood glucose level equal to or greater than 400 mg/dL, equal to less than 60 mg/dL, or an answer of 'yes' to either of the final questions. The investigator followed up with a telephone call to the participant and to the participant's clinic if necessary. Participants in the control group received usual care. | |
| Outcomes | Glycated haemoglobin (primary); self‐monitoring of blood glucose frequency (secondary) | |
| Funding | Novo Nordisk | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | An effect size of −0.6 glycated haemoglobin percentage points ± 1.2 percentage points was used for the power calculation. These calculations assumed a sample size of 60 per group, 80% power, and a 2‐sided t‐test with type 1 error set at 0.05 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A predetermined randomisation schedule from a series of permuted blocks was employed for each stratum" |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque randomisation envelopes that contained the randomisation assignment were labelled with participants' study numbers by a third party prior to initiation of the study." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Blinding of participants and the investigator was not possible because of the nature of the intervention. An attempt was made to avoid drawing attention to the randomisation assignment when providers were present" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Laboratory personnel who ran the HbA1c assays were unaware of the patients' study status." |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition (intervention n = 2, control n = 4). Missing outcome data balanced in numbers, with similar reasons for missing data across groups. Quote: "One participant in the study died shortly after being allocated to the treatment group and another participant in that group did not comply with study follow‐up procedures. 2 participants in the comparison group were lost to follow‐up, and 2 participants did not comply with study follow‐up procedures" |
| Selective reporting (reporting bias) | Low risk | Outcomes of interest reported |
| Other bias | Low risk | Groups were balanced at baseline; no significant differences were found (P < 0.05) |
| Methods | Aims: to evaluate the effectiveness of an automated telephone system reminding participants with hypertension to obtain overdue antihypertensive medication refills Study design: RCT; recruitment: other ‐ health plan (*) Study duration: *; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: participants aged ≥ 18 years with hypertension identified from a case‐identification database Sample size: 8306; mean age: *; sex: *; ethnicity: * Country: USA | |
| Interventions | Intervention group: the outreach consisted of an automated telephone call that instructed the member to order a refill for their overdue prescription by calling the number on their medication bottle or by using the Kaiser Permanente online refill system. Participants in the control group received usual care. | |
| Outcomes | Refill rate at 2 weeks (primary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to compare the value of computer‐guided behaviour therapy value with that of a clinician‐guided behaviour therapy and systematic relaxation as a control treatment Study design: RCT; recruitment: primary care (adverts in radio, newspapers and articles, health professional referrals) Study duration: 3 months; study type: management; subtype: mental health | |
| Participants | Inclusion criteria: participants aged ≥ 14 years with a primary diagnosis of obsessive compulsive disorder for ≥ 2 years on the Structured Clinical Interview for DSM‐IV Sample size: 218; mean age: 39 years; sex: men ‐ 58%; women ‐ 42%; ethnicity: white ‐ 93%, other – 7% Country: USA | |
| Interventions | Computer‐based behaviour therapy. BT STEPS is a 9‐step, computer‐driven IVR system that allows participants with obsessive compulsive disorder to telephone from home and progress through a self‐paced workbook. Clinician‐guided behaviour therapy consisted of 11 weekly 1‐hour (or longer) sessions to negotiate self‐exposure homework to be done for ≥ 1 hour daily between sessions and recorded in daily diaries. Sessions were audiotaped and rated blindly by an expert behaviour therapist for quality of instructions Relaxation therapy. Participants receiving relaxation therapy were asked to perform progressive relaxation exercises for ≥ 1 hour daily and to keep daily relaxation diaries for 10 weeks | |
| Outcomes | Yale‐Brown obsessive compulsive scale (primary); Clinical and Patient's Global Impressions; depression (Hamilton Rating for Depression Scale); satisfaction (secondary) | |
| Funding | Pfizer, Inc | |
| Declaration of conflict of interest | Drs Greist and Kobak, Mr Wenzel, and Ms Hirsch are employees of Healthcare Technology Systems (HTS), Madison, Wisconson. Ms Mantle was employed at HTS during this study and is currently self‐employed in Boise, Idaho. Mr Wenzel and Ms Hirsch own stock in HTS. Drs Marks and Baer receive royalties from BT STEPS. BT STEPS is a trademark of HTS. Dr Clary is an employee of Pfizer, Inc. | |
| Power calculations for sample size | "Sample size aimed for a power of 0.90, using estimates of means and standard deviations from a meta‐analysis of multicenter obsessive compulsive disorder trials." | |
| Notes | This is a comparison between computer‐based behaviour therapy and relaxation therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After screening by a clinician, patients were randomly assigned to 10 weeks of behavior therapy treatment guided by (1) a computer accessed by telephone and a user workbook or (2) a behavior therapist or (3) systematic relaxation guided by an audiotape and manual." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Sessions [clinician‐guided therapy] were audiotaped and rated blindly by an expert behaviour therapist for quality of instructions." Comment: insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "In an intent‐to‐treat analysis, the last available post randomisation rating was input to endpoint for subjects who stopped prematurely." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to assess the equivalence of theory‐based phone messages and education provided by an IVR system and by nurse‐delivered calls (NDCs) in promoting appointment attendance and adherence to preparation instructions for flexible sigmoidoscopy (FS) and colonoscopy, to compare the effect of the timing of IVR messages delivered 3 days versus 7 days before the scheduled appointment, and to evaluate any differences in patient satisfaction between IVR messages and NDCs Study design: RCT; recruitment: primary care (organisational referral) Study duration: 6 weeks; study type: either; subtype: appointment reminders | |
| Participants | Inclusion criteria: patients with upcoming FS or colonoscopy appointments scheduled in 2 gastrointestinal (GI) endoscopic procedure clinics at the Minneapolis Veterans Affairs Medical Center. Participants included those being screened and those having follow‐up appointments after receipt of abnormal test results Sample size: 3610; mean age: 63 years; sex: men ‐ 95% , women ‐ 5%; ethnicity: white ‐ 83%, non‐white ‐ 3%, other or > 1 race or unknown ‐ 14% Country: USA | |
| Interventions | Arm a: IVR‐3 Arm b: IVR‐7 Participants in the IVR study arms (IVR7 and IVR3) were mailed appointment information and preparation instructions and materials identical to those mailed in the NDC arm. Phone calls were programmed to start in the morning. If an answering machine picked up on the initial call, the IVR system left a general message about the purpose of the call. The system was programmed to call again in the afternoon and then again in the evening until the participant answered. Messages were left only on the first attempt. If the IVR call was not completed that day, the process was repeated the following day. Participants who answered the call had the option to have the system call back at a later time. An IVR call was considered complete if the participant answered and confirmed his or her appointment. The IVR system allowed participants to verify and confirm their appointment, respond to instructions about logistics, request additional preparation materials, answer queries about their current health, listen to preparation instructions, have any information repeated, ask for a summary of instructions, or leave a message for a nurse who would call back within 24 h. Embedded in these messages was the educational information about susceptibility and severity of colorectal cancer, as well as motivational messages that addressed risks, benefits, barriers, and self‐efficacy associated with preparation and procedures. At any time during the call, the participant could request to be transferred to the clinic to leave a message for a nurse Nurse delivered calls (arm c). A recovery room nurse attempted to call to remind participants of the appointment and review preparation instructions 7 days before the appointment | |
| Outcomes | Appointment non‐attendance and preparation non‐adherence for FS (primary); perceptions about the call (secondary) | |
| Funding | Department of Veterans Affairs, Veterans Health Administration | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | "Using an equivalence boundary of 0.10, a sample size of 743 subjects per group provided 90% power for the study with a level of .05 divided by 3 and an underlying 65% baseline completion rate." | |
| Notes | ClinicalTrials.gov Identifier: NCT00310362. Non‐attendance was defined as cancelling the appointment or not attending the appointment. Appointments cancelled by the clinic were not considered as non‐attendance. Preparation non‐adherence assessed whether participants had adequately prepared to complete the procedure. Procedure notes was used to determine if the participant was adequately prepared or if the physician was unable to evaluate the quality of the preparation, attitudes and beliefs. This is a comparison between IVR‐3 and NDC 7 days before the procedure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote:" Clinic procedure nurses and physicians were blinded to the randomised conditions." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Comment: groups were balanced with no significant baseline differences |
| Methods | Aims: to assess whether the health forecasting system can predict periods of higher risk and to assess the effect of the service on the frequency and severity of COPD exacerbations Study design: RCT; recruitment: primary care (*) Study duration: 4 months; study type: management; subtype: chronic obstructive pulmonary disease | |
| Participants | Inclusion criteria: all people aged > 40 with a diagnosis of chronic obstructive pulmonary disease confirmed with spirometry (forced expiratory volume in 1 second < 80% predicted, forced expiratory volume in 1 second/forced vital capacity ratio < 0.7) at 3 general practices in Devon, UK Sample size: 79; mean age: 69 years; sex: men ‐ 74%, women ‐ 26%; ethnicity:* Country: UK | |
| Interventions | Alert calls were made to the participant's normal telephone as occurs in the Healthy Outlook Service. The BlackBerry Smart Phones had their phone capabilities disabled and were only used for data collection and not to contact participants. The script for the alert call was successfully used in 2 pilot studies and as part of the routine health forecasting service since 2007. Automated calls were made on Tuesday evenings, with up to 2 repeat calls if the first was not answered Participants in the control group received no calls. | |
| Outcomes | Frequency of exacerbations and proportion of participants experiencing ≥ 1 exacerbations (primary); changes in health status (secondary) | |
| Funding | AstraZeneca | |
| Declaration of conflict of interest | "The authors (JMG, EMH, SWV, DN, EMH, SN, ABS, AB, MVR) report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article" | |
| Power calculations for sample size | "The study was powered to identify a 30% reduction in the proportion of patients experiencing an exacerbation, assuming (on the basis of previous studies) that 90% of patients in the control group would exacerbate over the winter." | |
| Notes | 75% of participants were on short‐acting β 2‐agonists | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent researcher who was not part of the study team used a list of binomial random numbers generated in block sizes of four to randomly allocate the participants" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The investigators were unaware of which patients were allocated to receive the forecast and patients were not informed of their allocation" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition (intervention n = 1, control n = 1). Missing outcome data balanced in numbers across groups. Quote: "Two patients did not complete the trial" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes of interest to the review have been reported |
| Other bias | Low risk | Groups were balanced at baseline. Quote: "The two groups were generally well matched; however, more patients in the group receiving alert calls had attended a chronic obstructive pulmonary disease education or exercise/rehabilitation programme and more controls were receiving inhaled corticosteroids/LABA therapy." |
| Methods | Aims: to evaluate the effectiveness of a telephonic outreach programme to improve blood pressure control among participants with hypertension Study design: RCT; recruitment: other ‐ health plan (organisational referral) Study duration: 4 weeks; study type: management; subtype: hypertension | |
| Participants | Inclusion criteria: Kaiser Permanente Southern California members > 18 years identified in a hypertension registry Sample size: 64,773; mean age: 61 years sex: men ‐ 46%, women ‐ 54%; ethnicity: white ‐ 41%, black ‐ 17%, Hispanic ‐ 25%, other/unknown – 9%, Asian ‐ 8% Country: USA | |
| Interventions | Outreach occurred 9‐16 August 2010, using an automated telephone messaging system. If the telephone call was answered by a live person or by a voicemail system, the automated message was delivered. Failed call attempts (i.e. busy signal or no answer) resulted in a maximum of 2 additional call attempts on the same day. Telephone calls were made between 10 am and 8 pm. The content of the automated message was developed by the KPSC outreach team. The message included a greeting stating the call was from Kaiser Permanente, an invitation to have a blood pressure measurement at a KPSC medical centre, and the hours of operation of the medical centre. The automated message was played by default in English with an option to listen to the message in Spanish Participants in the control group received usual care. | |
| Outcomes | Blood pressure (primary) | |
| Funding | Southern California Permanente Medical Group | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We randomised the eligible members on August 2, 2010, to a usual care arm (n=33,154) and an intervention arm (n=33,150) and subsequently excluded 1531 individuals (4.8%): 1528 did not have a valid telephone number and 3 were on a "do not call" list." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were balanced at baseline. Quote: "There were no statistically significant differences between patients in the intervention arm compared with those in the usual care arm" |
| Methods | Aims: to test the efficacy of motivational interviewing (MI) only and MI + HealthCall for drinking reduction among HIV primary care patients Study design: RCT; recruitment: primary care (health professional referral) Study duration:12 months; study type: management; study subtype: alcohol consumption | |
| Participants | Inclusion criteria: ≥ 4 US drinks of alcohol at least once, in the prior 30 days; HIV‐positive; English‐ or Spanish‐speaking; aged 18 years; and treated at the clinic Sample size: 254; mean age:46 years;sex: women ‐ 22%; men ‐ 78% ethnicity: African American ‐ 49%, Hispanic ‐ 45%, other ‐ 6% Country: USA | |
| Interventions | In MI + Health Call group, participants accessed the system via a toll‐free number for daily 1–3 min calls, answering pre‐recorded questions about 'yesterday' (morning, afternoon, evening) to ensure consistent reporting periods regardless of the hour called. Brief self‐monitoring questions covered alcohol consumption (e.g. 'How many beers did you drink yesterday?') and reasons for drinking or not drinking. Additional questions covered mood, medication adherence and well‐being. MI only. At baseline, counsellors administered a 20–25 min individual MI using standard techniques to motivate reduced drinking, encouraging participants to set a drinking‐reduction goal. Counsellors then provided the pamphlet and watch. At 30 and 60 days, counsellor and participant met for 10–15 min, discussed the participant's drinking during the past month, evaluated the drinking goal and set a new goal if participants wished. Participants in the control group received advice/education | |
| Outcomes | Number of drinks per drinking day in the last 30 days (primary) | |
| Funding | CDC: R01AA014323, K05AA014223 and the New York State Psychiatric Institute | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | N = 90 per group would provide 80% power at alpha = 0.05 to detect a moderate treatment effect on number of drinks per drinking day (d = 0.4) | |
| Notes | This is a comparison between MI + HealthCall versus advice/education | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In a parallel three‐arm individually randomised design (1:1:1 allocation ratio), 258 participants were assigned to advice/education control, MI‐only or MI+HealthCall between August 2007 and May 2010, with groups balanced on depression, drug abuse, unstable housing and hepatitis using urn randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Counselors and patients were not blinded to treatments after assignment" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition ‐ 94.5% of participants provided end‐of‐treatment data. Quote: "We conducted three sensitivity analyses to understand the robustness of our NumDD findings. Two involved multiple imputation" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "Treatment groups did not differ on these or other (e.g. demographic) variables." |
| Methods | Aims: to facilitate participant self‐monitoring and provide personalised feedback after a brief alcohol intervention by a primary care provider Study design: RCT; recruitment: primary care (health professional referral) Study duration: 6 months; study type: management; Study subtype: alcohol consumption | |
| Participants | Inclusion criteria: adults aged ≥ 21 who reported a pre‐BI average alcohol consumption exceeding NIAAA recommended guidelines of 7 and 14 standard drinks per week for women and men, respectively; who met the heavy drinking criterion of 4/5 drinks in a single day (NIAAA, 2005); or who endorsed ≥ 1 CAGE items Sample size: 338; mean age: 46 years;sex: men ‐ 64%, women ‐ 36% ethnicity: white ‐ 97% Country: USA | |
| Interventions | IVR + feedback: 6 months of daily calls plus monthly feedback in the form of a mailed, printed graph showing daily consumption reported to the IVR in comparison to participant's stated drinking goal, with each mailing including a personalised note from Dr Helzer to heighten the saliency of the graphs IVR + feedback + compensation: daily calls and monthly feedback (graph and personal note) as described above plus a financial incentive based on frequency of the participant's daily calls. The incentive amounted to about USD 13 per week for a perfect calling record IVR: daily phone calls for 6 months to the automated IVR system to report alcohol consumption and other items for the past 24 h No IVR: BI and standard follow‐up treatment only, no calls to the IVR system | |
| Outcomes | Weekly alcohol consumption (primary) | |
| Funding | National Institute on Alcohol Abuse and Alcoholism grants AA 11954 and AA 14270 | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between IVR + feedback and no IVR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "Patients who satisfied all inclusion/exclusion criteria and signed the informed consent were randomised to one of four study conditions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Follow‐up data were obtained for 284 subjects at 3 months (84%) and 273 at 6 months (81%). Of the 54 (16%) participants who did not complete a follow‐up assessment, 32 were lost to follow‐up and 22 declined to participate after randomisation." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no significant differences between participants in the four randomised groups on any of the measured subject characteristics" |
| Methods | Aim: to assess an intervention to increase cancer screening among participants in a safety‐net primary care practice Study design: RCT; recruitment: primary care (health professional referral) Study duration: 12 months; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: overdue for the targeted cancer screening and average‐risk for the cancer by EHR review. Age criteria were age 40–74 years for mammography (women) or 50–74 years for colorectal cancer (men and women) on the date of randomisation Sample size: 366; mean age: *; sex: *; ethnicity: non‐Hispanic white ‐ 50%, non‐Hispanic black ‐ 41%, other race including Hispanic ‐ 9% Country: USA | |
| Interventions | Multimodal intervention consisted of letters, automated telephone calls, a point‐of‐care prompt and mailing of a home testing kit to colorectal cancer screening participants. An automated telephone reminder system (Televox system) was utilised to deliver automated calls to the telephone number in the practice database for each intervention participant. The automated phone calls contained similar information to the letters, but in a brief form (approximately 25 s), with a phone number to call to arrange for screening. The automated calls were made on weeks 2 and 6 of the intervention period and repeated on weeks 14 and 25 for participants remaining unscreened on EHR review performed on week 11. Participants in the control group received usual care (blinded chart review). | |
| Outcomes | Breast cancer or colorectal cancer screening uptake at 12 months (primary) | |
| Funding | American Cancer Society (RSGT‐08‐077‐01‐CPHPS) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | The total cost for the automated calls was about USD 0.92, including the preparation of each list of call recipients from the database and the monitoring of post‐call status | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An offsite study statistician randomised participants to intervention or control groups using a random number algorithm stratified by the type of screening required (breast cancer, colorectal cancer or both)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Healthcare and data abstraction personnel were blinded to group assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "a research assistant blinded to treatment assignment abstracted data from the EHR" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "An intention‐to‐treat analysis was performed; that is, all patient originally assigned to a group were analysed." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐defined outcomes have been reported |
| Other bias | Unclear risk | Quote: "There were no significant differences in participants at baseline between those in the intervention and control groups in the colorectal cancer group". However, there were borderline significant differences in household income and age. |
| Methods | Aims: to measure the impact of an automated outbound telephone messaging system on herpes zoster (HZ) vaccinations among older adults in the community pharmacy setting Study design: cluster RCT; recruitment: primary care (organisational referral) Study duration: 3 months; study type: prevention; study subtype: immunisation | |
| Participants | Inclusion criteria: > 60 years of age, who had filled ≥ 1 prescription at a study pharmacy location during December 2006 Sample size: 16 pharmacies with a total of 11,982 participants; mean age: 72 years;sex: * ethnicity: * Country: USA | |
| Interventions | Automated outbound telephone messaging system in which the scripts were recorded and sent as an incoming automated telephone call to households using cNotify (Cintech, Mason, OH), which is an outbound messaging tool. Two 30‐second scripts were created to educate participants about their risk for developing HZ and invite them to speak to their pharmacist about vaccination opportunities Participants in the control group received no calls. | |
| Outcomes | The number of HZ vaccines administered (primary) | |
| Funding | APhA Foundation Incentive Grant | |
| Declaration of conflict of interest | Potential declared | |
| Power calculations for sample size | No | |
| Notes | The intervention was delivered to 9650 "households" due to duplicated phone numbers being deleted to rule out back to back messages being delivered to the same number for different people. Cluster RCT with 16 clusters randomised. Of these, 8 (5599 participants) were allocated to intervention and 8 (6383 participants) were allocated to control. Note clustering was unadjusted for in the paper: to calculate effective sample size in Hess 2013 study, we used the Fleiss‐Cuzick estimator (see Appendix 14 for calculations). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "16 pharmacies were randomised by a simple randomisation process into two cluster groups of 8 pharmacies each" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of key study personnel was ensured. Quote: "The results of the randomisation were not disclosed to pharmacists" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Because of the nature of the intervention, complete blinding was not possible" |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Participants in the intervention group were significantly older than control group participants (P < 0.001); not possible to judge selective recruitment of cluster participants based on the information reported. |
| Methods | Aims: to examine whether telephonic IVR or participant mailing could increase rates of bone mineral density testing in high risk, menopausal women Study design: quasi‐RCT; recruitment: other ‐ health plan (*) Study duration:12 months; study type: prevention; study subtype: osteoporosis | |
| Participants | Inclusion criteria: women between the ages of 50 and 64 years who, in addition to age, had ≥ 1 risk factor for osteoporosis as follows: recent discontinuation of hormone replacement therapy; exposure to oral corticosteroids, anti‐seizure medication, or tobacco use; history of fracture; or bilateral oophorectomy without evidence of hormone replacement therapy or oral contraceptive use. Sample limited to women who had no evidence of bone mineral density screening in the 2 years prior to the randomisation and who did not have a diagnosis of osteoporosis and were not known to be taking any FDA‐approved treatment for osteoporosis Sample size: 4685; mean age:57 years;sex: women ‐ 100%; ethnicity: * Country: USA | |
| Interventions | In addition to usual care, the IVR intervention was a single call lasting approximately 4–5 min. Each IVR call began with identification of the participant and proceeded if identification was correctly confirmed. A script was designed for the call that included a branching algorithm to calculate a fracture‐risk score, as well as the opportunity for women to indicate whether or not they had undergone bone mineral density testing, and whether or not they planned to follow up with their physician to discuss osteoporosis The participant mailing was a packet that included 5 illustrated pamphlets on osteoporosis, calcium and vitamin D, bone mineral density testing, osteoporosis risk assessment, and information about bone health and osteoporosis prevention + usual care Usual care group | |
| Outcomes | Bone mineral density screening within 12 months (primary) | |
| Funding | Merck, West Point, PA | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | No information | |
| Notes | This is a comparison of IVR (intervention) versus UC (control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Within each triplet, a pseudo‐random number generator assigned the patient panels of each primary care physician to a single treatment arm" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Because this study was non‐blinded, it is possible that patients in the usual care group became aware of the interventions to increase osteoporosis screening through communication with patients in the intervention groups, thus reducing the effect of the interventions" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were balanced at baseline. Quote: "Clinical and demographic characteristics of the study participants were similar across the three study groups at baseline" |
| Methods | Aims: to test a multifaceted intervention to improve adherence to cardiac medications Study design: RCT; recruitment: primary care (*) Study duration: 12 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: all patients who were admitted with acute coronary syndrome (ACS) as the primary reason for hospital admission and who used the VA for their usual source of care were screened for eligibility to participate Sample size: 241; mean age: 64 years; sex: men ‐ 98%, women ‐ 2%; ethnicity: white ‐ 78% Country: USA | |
| Interventions | Participants in the multimodal intervention group received: medication reconciliation and tailoring; patient education; collaborative care between pharmacists and providers (PCPs or cardiologists); and voice messaging reminders (educational and medication refill reminder calls). The voice messaging system contacts participants at regularly scheduled intervals. There are 2 types of calls: medication reminder and medication refill calls. The medication reminder calls occurred monthly. The medication refill calls were synchronised to when a medication refill was due. The calls occurred 14 days prior to the refill due date, 7 days prior to the refill due date, and on the due date. During months 2 through 6 of the intervention, participants received both medication reminder (monthly) and medication refill calls (timed to refill due dates) for the 4 medications of interest. During months 7 through 12 of the intervention, participants only received medication refill calls Participants in the control group received usual care (standard hospital discharge instructions e.g. numbers to call, follow‐up appointments, diet and exercise advice, a discharge medication list, and educational information about cardiac medications) | |
| Outcomes | The proportion of participants who are adherent with cardioprotective medications (β‐blockers, statins, clopidogrel, and ACE inhibitors) (primary); achievement of blood pressure and LDL cholesterol level targets (secondary) | |
| Funding | Veterans Health Administration Health Service Research & Development (HSR&D) Investigator Initiated Award (grant IIR 08‐302); Research Career Scientist Award VA HSR&D 08‐027 | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | We planned to recruit 280 participants over an 18‐month period and to follow participants for 12 months to have 80% power to detect a difference of 15% in the proportion of participants who were adherent to their cardioprotective medications | |
| Notes | ClinicalTrials.gov Identifier:NCT00520988. The annual incremental programme cost of the multifaceted intervention was USD 360 per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients with ACS were randomised using blocked randomisation stratified by study site in a 1:1 ratio to INT or UC" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed until a patient consented to participate and was generated centrally using the graphical user interface implemented for the study." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "At this visit, three blood pressure measurements were taken in standard fashion by someone blinded to study group assignment (eg, after 5 minutes of rest and 2 minutes apart between measurements)". Comment: insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We used an intent‐to‐treat approach for all analyses" |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Unclear risk | Quote: "Baseline characteristics of the patients were comparable . . . Usual care patients were more likely to undergo coronary artery bypass graft surgery (17.1% vs 6.7%; P = .02)." Insufficient evidence to judge that this imbalance has introduced bias |
| Methods | Aims: to examine the impact of an enhanced telemedicine system on glucose control and pregnancy outcomes in women with gestational diabetes mellitus Study design: RCT; recruitment: secondary care (organisational referral) Study duration: 26 months; study type: management; subtype: gestational diabetes | |
| Participants | Inclusion criteria: women aged 18‐45 years with a documented diagnosis of gestational diabetes mellitus on a 3‐h oral glucose tolerance test, using the criteria of Carpenter and Coustan. Women were required to be at ≤ 33 weeks of gestation at study entry Sample size: 80; mean age: 30 years; sex: men ‐ 0%; women ‐ 100%; ethnicity: white ‐ 41%, African American ‐ 34%, Latino/Hispanic ‐ 18 %, Asian and other – 7% Country: USA | |
| Interventions | ITSMyHealthrecord: the IVR system can be accessed from any phone over a dedicated toll‐free number and includes asynchronous phone messaging between clinicians and participants as well as automated reminders for participants to transmit data. Participants were prompted to input clinical data (i.e. blood glucose readings, changes in medication, and episodes of hypoglycaemia) and identify the day and time using the phone's keypad. They were provided feedback, emotional support, and reinforcement regarding diabetes self‐management with each transmission. In addition, women received a brief educational message/tip each time they accessed the system either by phone or Internet. Both systems allow women to append a message or ask a question (the IVR is set to accept 45 s of speaking, while the Internet‐based method allows virtually unlimited text input) after transmitting their health data. The data and messages are then queued for the clinician to respond to when he or she accesses the clinician portal of the system in which the participant data reside Participants in the control group received usual care. | |
| Outcomes | Maternal glucose control and infant birth weight (primary) | |
| Funding | National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health | |
| Declaration of conflict of interest | "CJH, LD, KR, WM, DM, and JG have nothing to disclose. WPS has stock ownership in Insight Telehealth Systems. A.A.B. is a consultant for Insight Telehealth Systems." | |
| Power calculations for sample size | NA | |
| Notes | Mean BMI: 34.1 kg/m2; participants in both groups monitor their blood glucose levels daily (before breakfast and 2 h after each meal), perform foetal movement counting 3 times a day, and also record insulin doses and episodes of hypoglycaemia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women were randomised into one of two groups: telemedicine or control (usual care)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition (intervention n = 4, control n = 2). Missing outcome data balanced in numbers, with similar reasons for missing data across groups. Quote: "Data were available for 38 women in the control group and 36 in the intervention group" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no significant differences at baseline between the two groups" |
| Methods | Aims: to evaluate the efficacy of a novel telehealth intervention, CareCall, on reducing pressure ulcers and depression and enhancing the use of appropriate health care in persons with spinal cord dysfunction Study design: RCT; recruitment: other ‐ community disability organisations, rehabilitation medicine outpatient clinics and inpatient services (*) Study duration: 6 months; study type: management; subtype: spinal cord dysfunction | |
| Participants | Inclusion criteria: wheelchair users ≥ 6 h/day during normal waking hours, more or equal to 18 years of age, report of physician confirmed diagnosis of multiple sclerosis or spinal cord injury, absence of cognitive impairment on the Telephone Interview of Cognitive Status‐Modified (TICS‐M), score more or equal to 20, able to give written, informed consent, able to speak and understand conversational English, health insurance or pending health insurance (any kind), available for the full 6 months of the study, able to complete CareCall Training Call, living in a private residence of any kind Sample size: 142; mean age: 48 years; sex: men ‐ 61%, women ‐ 39%; ethnicity: white ‐ 80% (inclusive of Hispanic or Latino – 7%), African American ‐ 11%, other ‐ 9% | |
| Interventions | Participants in the intervention group received weekly automated calls from the CareCall for 6 months and could call into CareCall any time. The CareCall scripts were organised into modules, integrating content relevant to: skin care, depression and wellness, and healthcare utilisation. The system also included relevant prerecorded vignettes from people with spinal cord dysfunction, and relevant recorded comments from healthcare professionals. These modules used branching logic based on personalised information and participants' responses during calls to tailor content throughout. Participants in the control group received usual care (current standard of care). They also received a CareCall resource book developed by clinical experts, containing information and local resources. | |
| Outcomes | Prevalence of pressure ulcers; depression severity; healthcare utilisation (all primary) | |
| Funding | CDC, Grant no. 5R01DD000155, the Department of Health and Human Services; and the National Institute of Disability and Rehabilitation Research, Grant nos. H133N060024, H133N110019, and H133N120002, the Department of Education | |
| Declaration of conflict of interest | Dr Friedman had stock ownership and a consulting agreement with Infomedics, the company that owns commercial rights to the TLC technology used in the computerised intervention. He is also a member of its board of directors. The remaining authors declared no conflict of interest. | |
| Power calculations for sample size | No | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We allocated participants to study groups using a stratified block randomization method to ensure balance by recruitment site" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "All study staff collecting data were masked as to study group assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition (intervention n = 4, control n = 3). Missing outcome data balanced in numbers. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "At baseline, there were no statistically significant study group differences in the prevalence of pressure ulcers, mean severity of depression or the percentage reporting issues with health‐care availability. However, the intervention group reported more emergency room visits and hospitalizations compared with control group subjects." |
| Methods | Aims: to evaluate the effectiveness of an automated telephone system as a relapse intervention in participants who completed a 4 week class based cholesterol lowering diet protocol Study design: RCT; recruitment: primary care (*) Study duration: 6 months; study type: management; subtype: hypercholesterolemia | |
| Participants | Inclusion criteria: participants who completed a 4‐week class based on cholesterol lowering diet protocol Sample size: 115; mean age: 48 years; sex: men ‐ 25%; women ‐ 75%; ethnicity: non‐Hispanic Caucasian ‐ 87%, other – 13% Country: USA | |
| Interventions | Computer‐phone system: asks participants 2‐4 prerecorded questions about recent eating behaviour, low‐fat nutrition knowledge, behavioural or maintenance skills, or expectations that may influence maintenance of cholesterol lowering behaviours. Participants responded by pressing the appropriate number on their touch‐tone phone. Based on this information, they received tailored feedback. Those failing to call the system in the first week received a reminder during the second week. Participants could leave a message for research staff who would then provide their response Participants in the control group received usual care. | |
| Outcomes | Total cholesterol reduction (primary); acceptability of the system (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | Complete case analysis. Quote: "A total of 16 participants dropped out and their measures were not used in the final analysis. Comparison of drop‐outs with completers showed no significant difference for age, BMI, baseline cholesterol, ethnicity, sex, smoking habits or education." |
| Selective reporting (reporting bias) | High risk | Weight outcomes at follow‐up were not provided |
| Other bias | Low risk | There were no significant differences at baseline between the 2 groups |
| Methods | Aims: to test the feasibility and effectiveness of a diet intervention (consisting of interactive mailings, computer‐generated phone calls, and classes) in hypercholesteraemic low‐income public clinic patients Study design: RCT; recruitment: primary care (telephone) Study duration: 6 months; study type: management; subtype: hypercholesterolemia | |
| Participants | Inclusion criteria: aged 18–65 years, have a past TC measurement, have a total cholesterol > 200 mg/dL, be English‐speaking, not require insulin, not be over 200% ideal body weight, not have cancer other than skin cancer, not have triglycerides over 400 mg/dL, plan to remain in the area ≥ 6 months, and not be on lipid‐lowering drugs Sample size: 123; mean age: 57 years; sex: men ‐25 %; women ‐ 75%; ethnicity: African American ‐ 77%, other – 23% Country: USA | |
| Interventions | IVR arm: participants continued to receive usual care but were offered and encouraged to use all 3 components of the system: mailed diet questionnaires with individualised mailed feedback, computer‐interactive phone calls, and a programme of 4 hour‐long classes. Intervention development was guided by social cognitive theory so that calls could provide opportunities for modelling, feedback and reinforcement, increasing self‐efficacy for change. The intervention also sought to increase practical skills such as reading labels, eating out, modifying recipes, and self‐monitoring. The intervention components were developed to reduce participant burden while utilising behavioural approaches to lifestyle change and to maintain sufficient contact, monitoring, and feedback, yet be practical for primary care Participants in the control group received usual care. Physicians in general provide very brief dietary counselling and prescribe lipid‐lowering drugs as deemed appropriate. Hypercholesterolaemic patients may be referred to clinic registered dietitians. After the trial the UC subjects were offered the series of classes | |
| Outcomes | Total cholesterol reduction (primary); self‐efficacy; dietary knowledge; fat intake scale (secondary) | |
| Funding | American Heart Association Texas Affiliate 91R‐172 | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation. Quote: "Allocated to treatment in a 1:1 ratio using a fixed randomisation scheme with blocks of size four." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate. Quote: "Of the 123 subjects, 80.5% (99) completed follow‐up cholesterol measurements." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "The 123 subjects randomised into the two study groups were generally comparable although the special intervention group had more African Americans (P = 0.04) and were younger at 54.6 versus 58.7 years of age (P = 0.03)". Comment: intervention group had significantly more African‐Americans and young participants compared to the control group. There is insufficient evidence that this imbalance has introduced bias |
| Methods | Aims: to evaluate the effectiveness of telecommunications technology to underpin an intervention that would be effective, easy to use, convenient, inexpensive, require little time commitment, and amenable to widespread distribution Study design: RCT; recruitment: primary care (mail and telephone) Study duration: 3 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: aged ≥ 60 years, English‐speaking, had to be sedentary (defined as participating in < 60 min of physical activity per week, with a minimum of 20 min of exercise per time and a minimum of 3 times per week), and also needed to have touch‐tone telephone service Sample size: 85; mean age: 67 years; sex: men ‐ 24%; women ‐ 76%; ethnicity: other ‐ 70%, African American ‐ 30% Country: USA | |
| Interventions | The Telephone‐Linked Communication (TLC) System is an interactive computer‐based telecommunication system that converses with participants in their homes over their telephone to motivate and improve health‐related behaviours. TLC 'speaks' to users over the telephone using computer‐controlled speech generation. Users communicate with TLC by using their telephone touch‐tone keypad. TLC functions as a monitor or 'counsellor' that provides positive feedback to reinforce or change the individual's health behaviour. TLC stores the user's response in a database. The information provided by the person controls the direction of the conservation. This information is also forwarded to the person's physician on a report, similar to a laboratory report, in which medical problems are highlighted. Participants in the control group received usual care. | |
| Outcomes | Minutes walked per week (primary); satisfaction (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Then subjects were randomised to use TLC‐ACT, or to a usual medical care control group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "'Research staff and subjects were blinded to the study assignment until the baseline questionnaire was completed." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | Complete case analysis. Quote: " The analysis was performed on the 68 subjects who completed the study" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes of interest to the review were reported |
| Other bias | Low risk | Quote: "There were no significant differences between the groups based on age, number of co‐morbidities, Stage of Adoption of Physical Activity, and minutes walked over the 4 recall days at baseline." |
| Methods | Aims: to assess the utility and cost‐effectiveness of an automated Diabetes Remote Monitoring and Management System (DRMS) in glycaemic control versus usual care Study design: RCT; recruitment: primary care (*) Study duration: 6 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: patients with an glycated haemoglobin between 7.0% and 9.0%, aged ≥ 18 years, and currently taking or starting insulin Sample size: 98; mean age: 59 years; sex: men ‐ 40%; women ‐ 60%; ethnicity: black ‐ 65%; white ‐ 30%; Hispanic ‐ 1%; Asian ‐ 1%; other ‐ 3% Country: USA | |
| Interventions | Participants in Diabetes Remote Monitoring and Management System (DRMS) were contacted daily, either through text messaging or automated voice. From these messages, participants could either respond by submitting their blood glucose levels or respond at a later time. If a participant did not submit his or her blood glucose level at the initial contact, the DRMS would text or call again that same day to remind the participant to check his or her blood glucose. However, if a participant submitted a reading before the reminder, the system would not contact the participant on that day. Providers could monitor the progress of their patients through a web‐based, secure portal, and information could also be downloaded directly into electronic medical records. Participants in the control group received usual care. | |
| Outcomes | Glycated haemoglobin; medication adherence; quality of life; cost‐effectiveness (all primary) | |
| Funding | Eli Lilly, National Institute of General Medical Sciences, National Institutes of Health | |
| Declaration of conflict of interest | Potential declared | |
| Power calculations for sample size | NA | |
| Notes | 60% of participants used phone calls to report into the system, and 40% used text messages exclusively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised, after informed consent was obtained, to either the intervention (DRMS) group or the control group by using a random‐number table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All statistical analysis used intent‐to‐treat methodology" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | There were some baseline differences between the groups in demographics; unclear whether those introduced bias. |
| Methods | Aims: to determine if automated telephone nutrition support counselling could help patients improve glycaemic control by duplicating a successful pilot in Mexico in a Spanish‐speaking population Study design: RCT; recruitment: primary care (telephone) Study duration: 3 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: ≥ 2 visits to the clinic in the last 1 year and a glycated haemoglobin level of > 8.0% on most recent visit, and any insulin status Sample size: 75; mean age: 52 years; sex: men ‐ 59%, women ‐ 41%; ethnicity: Hispanic (Spanish‐speaking) ‐ 100% | |
| Interventions | The system was designed and implemented using a Dialogic telephone card installed in a desktop computer and connected to a landline, programmed using Telesage software (Boston, MA). The system was designed to create a 'summary' estimate of high‐glycaemic index food consumption on survey conclusion that was then provided to participants at the conclusion of the call. If the sum of all high glycaemic index foods in the previous 24‐hour period was 2 or fewer servings, the message was one of congratulations and positive feedback; if 3‐4 servings, the message was more cautious and provided some education about appropriate low‐glycaemic index foods; and if ≥ 5 servings, then it provided a more educational message regarding high and low‐glycaemic index foods Participants in the control group received usual care. | |
| Outcomes | Glycated haemoglobin (primary); systolic blood pressure; diastolic blood pressure; BMI; waist circumference; total cholesterol; triglycerides; serum high‐density lipoproteins; serum Low density lipoproteins (secondary) | |
| Funding | National Institute of Diabetes and Digestive and Kidney Diseases for Diabetes Translational Research (CDTR) at Kaiser Permanente and University of California, San Francisco (P30DK092924) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | An 80% power to detect a difference in glycated haemoglobin of approximately 1.2% ± 1.5% between groups, and assuming 15% loss to follow‐up, investigators aimed to enrol 80 total participants into the study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator. Quote: "Patients were selected into one arm or the other of the study using a random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "We conducted a prospective, randomised, open‐label trial (ClinicalTrials.gov #NCT01040676) with blinded endpoint assessment" |
| Incomplete outcome data (attrition bias) | High risk | High attrition. Quote: "There was significant loss to follow‐up despite several attempts to reach patients" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "Patients in the intervention arm were broadly similar to those in the control arm but trended toward being more likely to be men (P = 0.12), having a larger waist circumference (P = 0.053), and being on a different number of diabetes medications (P = .07)" |
| Methods | Aims: to enhance engagement in low‐income adults with poorly controlled diabetes (glycated haemoglobin > 9%) Study design: RCT; recruitment: primary care (*) Study duration: 12 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: English‐ and Spanish‐speaking patients with telephone access who receive primary care at San Francisco General Hospital; glycated haemoglobin > 9% Sample size: 100; mean age: *; sex: *; ethnicity: * | |
| Interventions | Participants in the intervention group received weekly, 10‐min, automated phone calls, which delivered educational vignettes and detected triggers such as diabetes‐related adverse events or requests for medical appointments, medication assistance or a callback from a healthcare provider. Triggers were addressed with a follow‐up call from a diabetes specialist (NP, MD or CDE) within 48 h. Participants in the control group received usual care | |
| Outcomes | Glycated haemoglobin (primary) | |
| Funding | McKesson Foundation | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "patients with telephone access who receive primary care at San Francisco General Hospital were randomly selected to receive calls." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to evaluate the effectiveness of telephone‐based physical activity guidance and support delivered via a trained health educator or an automated system across an extended period of 12 months Study design: RCT; recruitment: primary care and community (advert elsewhere ‐ promotion in local media outlets, flyers and brochures in health clinics, pharmacies, senior centres, and other community settings) study duration: 12 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: aged ≥ 55 years, English‐speaking, not currently engaging in more than 60 min/week of moderate or vigorous intensity physical activity, free of any medical condition, BMI ≤ 40 kg/m2, alcohol intake ≤ 3 drinks/day, able to speak and understand English, and access to a touch‐tone phone Sample size: 218; mean age: 61 years; sex: men ‐ 31%; women ‐ 69%; ethnicity: white ‐ 90 %, other – 10% Country: USA | |
| Interventions | Automated advice (IVR) arm: the system spoke to participants using computer‐controlled speech generation; participats communicated using the touch‐tone keypad of their telephones. The contents included physical activity assessment (type, frequency, duration, steps accumulated on the pedometer), progress evaluation, individualised problem‐solving, goal‐setting, feedback, and delivery of positive support and tailored advice. Each call lasted 10‐15 min and occurred bi‐weekly then weekly. Quality control was implemented through semi‐weekly evaluation of the technical performance of the automated system via TLC's automatic contact summarisation database, as well as daily monitoring of the automated system's telephone helpline that was used by participants to report any problems while using the TLC system Human advice arm: this arm consisted primarily of telephone‐assisted physical activity counselling by a trained health educator. Individuals received an initial in‐person 30‐40 min health educator‐led instructional session, including development of an individualised plan emphasising a gradual progression of activity frequency, duration, and intensity towards a goal of ≥ 30 min of moderate‐intensity endurance exercise (primarily brisk walking) on most days of the week. The remaining intervention contacts occurred via brief (i.e. 10‐15 min) structured counsellor‐initiated telephone calls that occurred on a bi‐weekly, then monthly basis. Each participant was scheduled to receive approximately 15 contacts during the study year during which they received individualised information, support, and problem‐solving around physical activity barriers. Attention‐control arm: individuals randomised to this arm were offered weekly health education classes that focused on a variety of non‐physical activity topics of interest to middle‐ and older aged adults such as nutrition and home safety, and they were asked not to change their usual physical activity patterns during the 12‐month study period. At the end of 12 months, people in this arm were offered a 6‐month health educator‐delivered telephone based exercise programme. | |
| Outcomes | Minutes of moderate to vigorous physical activity (primary); physical functioning and well‐being (secondary) | |
| Funding | National Institute on Aging | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "A sample size of approximately 61 participants completing per group was judged to be adequate for detecting a 30 minute per week across 7 days as measured by the physical activity recall in moderate or vigorous physical activity at 90% power with 2‐sided alpha set at 0.05." | |
| Notes | This is a comparison between the IVR arm and the health education (attention‐control) classes. This study had a follow‐up of 18 months reported in King 2014 but no comparisons were made between these arms at that point. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomly assigned using a computerized version of the Efron procedure." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All study assessment staff were blinded to participant study arm assignments." |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers, with similar reasons for missing data across groups. Quote: "Of the 218 individuals enrolled in CHAT, 189 (86.7%) had 6‐and 12‐month 7‐Day physical activity recall data.The retention rates were not significantly different across the three study arms." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "Participants were similar across the three study arms on the major baseline variables of interest." |
| Methods | Aims: to determine whether centralised telephone‐based care management coupled with automated symptom monitoring can improve depression and pain in cancer patients Study design: RCT; recruitment: primary care (healthcare professional referral) Study duration: 12 months; study type: management; subtype: cancer | |
| Participants | Inclusion criteria: patients presenting for oncology clinic visits; depression with PHQ‐8 score of 10 or greater, with depressed mood and/or anhedonia; pain ‐ at least moderate in severity, defined as a Brief Pain Inventory worst score in the past week of 6 or greater, persistent despite a patient's having tried ≥ 1 different analgesic medication and cancer related Sample size: 405; mean age: 59 years; sex: men ‐ 32%, women ‐ 68%; ethnicity: white ‐ 80%, black ‐ 18%, other ‐ 2% Country: USA | |
| Interventions | Multimodal intervention (automated symptom monitoring (ASM)) was performed using either interactive voice–recorded telephone calls or Web‐based surveys based on participant preference. The 21‐item survey included the PHQ‐9 depression scale, 8 pain items from the Brief Pain Inventory (3 severity and 5 interference), and a single question for each of the following: medication adherence, adverse effects, global improvement, and whether the participant wanted a nurse care manager call. The monitoring survey was administered twice a week for the first 3 weeks, then weekly during weeks 4 through 11, twice a month during months 3 through 6, and once a month during months 7 through 12. However, more frequent administration could be reinstituted for participants who underwent treatment changes. Those not completing their scheduled assessment were contacted by telephone by the nurse care manager. In addition to ASM, participants also received telephone care management (delivered by nurse) and medication management (delivered by oncologist) Participants in the control group received usual care. | |
| Outcomes | Depression severity; pain severity (primary); health‐related quality of life; disability; healthcare use (outpatient physician visits); and co‐interventions (depression treatments) (secondary) | |
| Funding | National Cancer Institute, National Institutes of Health | |
| Declaration of conflict of interest | Dr Kroenke reported receiving research funding from Eli Lilly and Pfizer, and honoraria as a speaker, consultant, or advisory board member from Eli Lilly, Pfizer, and Forest Laboratories. No other authors reported disclosures | |
| Power calculations for sample size | The study was powered to detect clinically significant improvement in depression (HSCL‐20) and pain (Brief Pain Inventory). It was determined that 97 participants per symptom group would provide 80% power to detect a 20% absolute difference in response rates with 2‐tailed alpha < 0.05 | |
| Notes | "Symptom‐specific disability was high, with participants reporting an average of 16.8 of the past 28 days (i.e. 60% of their days in the past 4 weeks) during which they either were confined to bed (5.6 days) or had to reduce their usual activities by 50% (11.2 days) due to pain or depression. Moreover, 176 (43%) reported being unable to work due to health‐related reasons." Correspondence with the author: "The majority of patients did the symptom monitoring by IVR (89.3% by IVR; only 10.7% by web)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated in randomly varying block sizes of 4, 8 and 12 and stratified by symptom type (pain only, depression only, or both pain and depression)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All five assessments (baseline, 1, 3, 6, and 12 months) were administered by telephone interview and conducted by research assistants blinded to treatment arm." |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers, with similar reasons for missing data across groups. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "Analyses were based on intention‐to‐treat in all randomised participants" |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study's pre‐specified outcomes of interest have been reported |
| Other bias | Unclear risk | There were no significant differences between the intervention and usual‐care groups except for marginally significant differences for sex (P = 0.0512) and marital status (P = 0.0527). There is insufficient evidence that this imbalance has introduced bias. |
| Methods | Aims: to determine the effectiveness of a telecare intervention for chronic pain Study design: RCT; recruitment: primary care (mail and telephone) Study duration: 36 months; study type: management; subtype: chronic pain | |
| Participants | Inclusion criteria: participants aged 18‐65 years were eligible if they had pain that was musculoskeletal, defined as regional (joints, limbs, back, neck) or more generalised (fibromyalgia or chronic widespread pain); moderately severe, defined as a pain intensity item score of 5 or higher for either 'average' or 'worst' pain in the past week; and persistent (i.e. 3 months) despite trying ≥ 1 analgesic medication Sample size: 250; mean age: 55 years; sex: men ‐ 83%, women ‐ 17%; ethnicity: white race ‐ 77 % Country: USA | |
| Interventions | Multimodal intervention (automated symptom monitoring (ASM)), either by interactive voice recorded telephone calls or by Internet, depending on their preferences. Reports from ASM were scheduled weekly for the first month, every other week for months 2 and 3, and monthly for months 4 through 12. The 15‐item ASM measure included 7 symptom items: 3 pain items from the PEG instrument, 2 anxiety items from the 2‐Item Generalized Anxiety Disorder Questionnaire, and 2 depression items from the Patient Health Questionnaire 2. The other 8 items asked about how difficult pain made it to carry out usual activities; degree of relief from pain medications; global change in pain (worse, same, better) and, if better, the degree of improvement; analgesic adverse effects, adherence, and whether a medication change was desired; and a request for the nurse to call. Participants in this group also received optimised analgesic management by a team consisting of a nurse care manager and physician pain specialist Participants randomised to usual care continued to receive care for their chronic musculoskeletal pain from their primary care physician | |
| Outcomes | Pain intensity (primary); difference in response rates: mean Brief Pain Inventory interference; and pain severity scale scores (secondary) | |
| Funding | Department of Veterans Affairs (VA) Health Services Research and Development (VA HSR&D) Merit Review award to Dr Kroenke (IIR 07‐119) and a VA Career Development Award to Dr Krebs (CDA 07‐215) | |
| Declaration of conflict of interest | Dr Kroenke reported receiving honoraria from Eli Lilly outside the submitted work. No other authors reported disclosures | |
| Power calculations for sample size | Investigators determined that 100 participants were needed per group to detect a between‐group treatment difference of 0.4 SD in the Brief Pain Inventory total score (representing a small to moderate treatment effect), presuming a 2‐sided alpha < 0.05 and 80% power. Allowing for up to 20% attrition, the enrolment target was set at 250 participants. | |
| Notes | Correspondence with the author: "In a second more recent trial of ours (SCOPE) that also used IVR vs. web, we found 51% used IVR and 49% used web. Although we did not report results differently, we did a multivariable model on the primary pain outcome, and found that mode of symptom monitoring (IVR vs. web) did NOT make a difference in the treatment effect." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by patient opioid medication use at baseline (yes or no). To maintain allocation concealment, assignment to treatment group was determined by a computer‐generated randomisation list with randomly varying block sizes of 4 and 8." |
| Allocation concealment (selection bias) | Low risk | Quote: "To maintain allocation concealment, assignment to treatment group was determined by a computer‐generated randomisation list with randomly varying block sizes of 4 and 8." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment was ensured. Quote: "Research assistants responsible for outcome assessments were blinded to treatment group assignment." |
| Incomplete outcome data (attrition bias) | Low risk | Low drop‐out rate. Missing data have been imputed using appropriate methods. Quote: "As a sensitivity analysis, multiple imputation analysis was also performed." |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐specified outcomes have been reported in the pre‐defined way. |
| Other bias | Low risk | Groups were balanced in terms of baseline characteristics |
| Methods | Aims: to determine whether an automated telephone support system would improve quality of life and reduce death and hospital admissions for rural and remote heart failure patients Study design: cluster RCT; recruitment: primary care (*) Study duration: 12 months; study type: management; study subtype: heart failure | |
| Participants | Inclusion criteria: New York Heart Association (NYHA) class II–IV heart failure, left ventricular ejection fraction < 40% on echocardiogram, or echocardiographic features of diastolic dysfunction with impaired ventricular relaxation reported with no other diagnostic explanation for chronic heart failure‐type symptoms such as chronic obstructive airways disease and bronchial asthma; a recent primary hospital discharge diagnosis of heart failure within the previous 5 years; touch‐tone telephone access and the ability to operate this system Sample size: 405; median age: 73 years;sex: women ‐ 37%; men ‐ 63%; ethnicity: * Country: Australia | |
| Interventions | The TeleWatchTM system is a telephone‐based automated telemedicine system developed by Johns Hopkins Biomedical Engineering in conjunction with their clinical heart failure group. This telemedicine system was required to be dialled into by the participant on an at least a monthly basis at which time questions were asked with regard to heart failure clinical status, medical management of their condition, and social questions relevant to their heart failure status. Participants in the control group received usual care (standard general practice management of heart failure) | |
| Outcomes | Packer clinical composite score (death, hospital admission for heart failure, withdrawal from study due to worsening heart failure, 7‐point global health assessment questionnaire) (primary); hospitalisation for any cause; death or hospitalisation; and heart failure hospitalisation (secondary) | |
| Funding | National Health and Medical Research Council, National Heart Foundation of Australia, and Medical Benefits Fund | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | Calculations indicated that a shift of approximately 11% (to 37%, 47%, 16% in the intervention arm) corresponding to an odds ratio of 1.78 was able to be detected with 80% power using this sample size | |
| Notes | Cluster RCT; analyses appropriately adjusted for clustering at practice level by using a robust variance estimator Cluster RCT with 143 GPs (127 GP clusters) GPs recruiting 434 patients, of whom 405 were enrolled into the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study involved cluster randomisation at the level of the general practitioner (1:1, usual care, usual care plus intervention, stratified by rural, remote and outer metropolitan area [RRMA] classification). This was to minimize contamination across the two interventions to which patients were randomised." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All patients regardless of treatment allocation were followed up by an independent reviewer, blinded to treatment allocation, and asked to complete a telephone survey at baseline and at 6 and 12 months." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Intention‐to‐treat analyses were performed for all endpoints." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "Patients were well matched at baseline for disease severity, co‐morbidities, haemodynamic parameters, and concomitant medications". Insufficient information reported to judge whether or not selective recruitment of clusters may have introduced bias. |
| Methods | Aims: to assess the effect on cardiovascular death or re‐hospitalisation for heart failure of 3 different clinical management strategies: standard heart failure care, management in a cardiology clinic and home telephone self‐monitoring Study design: quasi‐RCT; recruitment: * (*) Study duration: 12 months; study type: management; subtype: heart failure | |
| Participants | Inclusion criteria: patients with left ventricular systolic dysfunction (LVEF 45%), recently discharged from hospital or diagnosed with acute or worsening heart failure up to 3 months before the study, between January 2007 and January 2008 Sample size: 138; mean age: 68 years; sex: men ‐ 79%, women ‐ 21%; ethnicity: * Country: France | |
| Interventions | Automated home telephone self‐monitoring (Telecard): participants were asked to call an automated system once a week, to listen to the voice questions and to answer using the telephone keypad. Guide Vocal‐Web (Guide Vocal‐Web, France Telecom, Orange Business Service, France) is software for specifying interactive voice dialogues between human and telephone. Briefly, using a computer linked to an Orange business website service, 3 heart failure‐related questions displayed in a tree manner with nodes were edited. Questions were about weight change, dyspnoea and general health. The text was then converted into a synthetic voice message. Participants were able to listen to audio advice, inviting them to repeat their call after a week (stable), after 3 days (minor worsening heart failure), to proceed to a medical visit (suspected worsening heart failure), or they were directly connected to cardiology clinic care giver (high risk of hospitalisation according to the algorithm) Cardiology clinic. A multidisciplinary team approach during visits to the heart failure clinic. Usual care | |
| Outcomes | Cardiovascular deaths, hospitalisation for heart failure (classified together as adverse events) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Most of the participants received beta‐blockers, ACE/AT2 inhibitors and diuretics. Cardiac re‐synchronisation therapy was delivered in 27% of participants. This is a comparison between the Telecard arm and the UC arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated to three different groups for heart failure monitoring in a non‐randomised fashion" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "All groups were similar in their clinical characteristics at inclusion" |
| Methods | Aims: to evaluate the impact of large‐scale, registry‐based reminder‐recall interventions on low immunisation rates in an inner‐city population Study design: RCT; recruitment: other – public health clinics, community health centres, hospitals, outpatient departments, private practices (organisational referral) Study duration: 24 months; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: residing in Fulton County, receiving care through its health department clinics or the public hospital health system, and were born between 1 July 1995, and 6 August 1996 Sample size: 3050; median age: 9 months; sex: boys ‐ 49%, girls ‐ 51%; ethnicity: black, non‐Hispanic ‐ 76%, Hispanic ‐ 14%, white, non‐Hispanic ‐ 7%, other, non‐Hispanic – 3% Country: USA | |
| Interventions | Autodialer (automated telephone or mail reminder recall). 7 days before a dose was due, a computer connected to a telephone delivered a recorded message to the family. Content: child should be taken to his or her health care provider for the needed dose. If there was no answer or a busy signal, the call was repeated every 30‐60 min. If these efforts failed to reach a person or an answering machine or if the telephone number was non‐working or not present in the database, an automated postcard with the same message was mailed to the family no later than 5 days before the due date. If 6 days after the due date the needed dose was not present in the registry, a computerised telephone message (or postcard in the absence of a working telephone) was sent to the family indicating that the child was behind in his or her immunisations. Unless the registry recorded the immunisation, the telephone message was repeated on days 11, 17, and 23. If these efforts failed, a computerised postcard was sent on day 28. All telephone calls were made between 5:30 and 9:00 pm. At the start of each message, an option for a Spanish‐language version was presented, and postcards contained the message in both Spanish and English. Outreach (in‐person telephone, mail, or home visit recall). Within 7 days of a child failing to receive a dose by the due date, the outreach worker attempted to contact the family by telephone or postcard in the absence of a working telephone. If 7 days later the dose was still not in the registry, a postcard was sent. If 30 days later the dose was still missing, a home visit was attempted, with continued monthly efforts until contact was made. At the home visit, the outreach worker attempted to determine what was needed to assist the family in obtaining immunisation for the child. The principal outreach worker was a college‐educated, African American woman who had been raised in inner‐city Atlanta. For Hispanic families, outreach was provided by a bilingual, college‐educated, Hispanic worker. The outreach workers and other study functions were supervised by a person with a doctorate in community psychology and extensive experience in conducting inner‐city studies Combination (Autodialer with outreach backup) Usual care (no interventions beyond normal clinic procedure, which in certain cases involved non‐automated postcard recall systems) | |
| Outcomes | Completion by the age of 24 months of the 4‐3‐1‐3 vaccination series | |
| Funding | National Immunisation Program, CDC, and the Georgia Department of Human Resources, Atlanta | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | The study population of 3050 provided 80% power for detection of 5% differences in immunisation rates among groups | |
| Notes | This is a comparison between Autodialer arm and usual care arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator. Quote: "At study initiation, participants were assigned by computer generated random numbers to 1 of 4 groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Participants and study staff were not blinded. Quote: "We did not attempt blinding" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "All analyses were based on intention to treat" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | No significant difference between the intervention and control groups for any demographic or vaccination characteristic |
| Methods | Aims: to investigate whether inexpensive telephone voice mail technology be used to improve medication adherence Study design: RCT; recruitment: community centre (advert in clinic) Study duration: 2 weeks; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: elders with no debilitating illness, depression, significant cognitive impairment, or medication schedules involving ≥ 2 drugs Sample size: 16; mean age: 71 years; sex: men ‐ 31%; women ‐ 69%; ethnicity: * Country: USA | |
| Interventions | Intervention group received TeleMinder, a computer hardware and software system that makes it possible for health care providers to enter elders' names, addresses, phone numbers, medication schedules, and other relevant information into a database. The health care provider also speaks the elders' names and a set of voice message segments that can later be merged in different combinations to make personalised voice messages for all or any subset of elders in the data base. TeleMinder message included the following: it asked them to verify that it had reached the correct person, it reminded them which medications they were supposed to scan, and it gave them 6 choices. These choices were hearing: the medication reminder again, a joke of the day, a health care tip of the day, the famous birthdays of the day, the big band 'name‐that‐tune' quiz, or they could hang up the phone. If they listened to 1 of the 4 messages, it was followed by a repeat of the medication reminder message, a brief goodbye message, and finally the phone line would disconnect. Participants in the control group received no calls. | |
| Outcomes | Medication non‐adherence; cognitive assessment (primary) | |
| Funding | SBIR grants #1 R44 AC06957‐02 and #R44AC06753‐02 from the National Institute on Aging | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This study has a very small sample size. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After selecting 16 subjects for phase one of the experiment, eight subjects were randomly assigned to the voice mail condition and eight to the control condition." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | No baseline data provided. Insufficient evidence to judge whether this has introduced bias. |
| Methods | Aims: to evaluate the effectiveness and cost‐effectiveness of sending letters, automated telephone messages, or both to families of under‐immunised 20‐month‐olds in an HMO Study design: RCT; recruitment: other ‐ health plan (organisational referral) Study duration: 4 months; study type: prevention; subtype: immunisations | |
| Participants | Inclusion criteria: under‐immunised 20‐month‐olds living in the residence areas of 10 northern California medical centres of the Kaiser Permanente Medical Care Program of Northern California, a non‐profit, group‐model health maintenance organisation Sample size: 752; mean age: 20 m; sex: * ; ethnicity:* Country: USA | |
| Interventions | An automated telephone message (IVR) alone. A prerecorded message approximately 1‐min long was sent to each family, stating that the child was overdue for immunisations and providing the telephone numbers of the advice/appointment lines at the nearest Kaiser Permanente clinics. The message was personalised to the extent that the child's first name was spoken by software that generated the name from text. The system prompted the listener to choose the language in which the message was to be delivered (English, Spanish, or Cantonese), asked him or her to confirm that the correct family had been reached, and also enabled him to replay the message if desired. The system kept records of the results of each call. Messages were sent on Tuesdays between 5 pm and 9 pm by the Customer‐Activated Appointment Processing Services (CAAPS), an automated telephone message system. Telephone numbers that could not be reached because there was no answer either by a person or an answering machine were called again the following evening, up to 6 attempts. An automated telephone message followed by a letter 1 week later A letter followed by an automated telephone message 1 week later Letter alone. The letters were personalised; printed in English, Spanish, and Cantonese; and included a list of which immunisations were needed by 24 months of age *Quote: "The current study did not randomise patients to no intervention because a previous randomised controlled trial in our setting had found that letters increased immunisation relative to no intervention. However, to estimate the proportion of under‐immunised 20‐month‐olds who would receive immunisations with no intervention, we evaluated a comparison group of similar patients who turned 20 months old during January 1996." | |
| Outcomes | Immunisation status by 24 months of age (primary); costs; acceptability (secondary) | |
| Funding | Northern California Kaiser Permanente and CDC | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | The sample size of 160 children to each intervention group was expected to have 80% power to detect a 16% difference in the percentage of children receiving any immunisation during the 4 months after their families were sent the message | |
| Notes | This is a comparison between the IVR alone arm and the letter alone arm. Costs of using automated telephone messages alone were USD 9.80, and USD 10.50 using letters alone | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Families of under immunized children were equally randomised to receive one of four interventions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "The primary analysis classified patients on the basis of intention‐to‐treat, i.e., a family assigned to receive an automated telephone message or letter was analysed as part of the assigned group regardless of whether our record indicated they received a completed message or letter" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aim: to determine whether multiple interventions influence adherence to glaucoma medication and to study the relationship between personality type and adherence Study design: RCT; recruitment: tertiary care (*) Study duration: 5 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: controlled disease (intraocular pressure at target level) on monotherapy with a topical prostaglandin agent; 18 years or older Sample size: 80; mean age: 66;sex: men ‐ 49%, women ‐ 51%; ethnicity: white ‐ 62%, African American ‐ 10%, Hispanic/Latino ‐ 9%, Asian ‐ 9%, East Indian ‐ 6% Country: USA | |
| Interventions | Participants in the intervention group received a programmed automated telephone call (SmartTalk, Televox Inc., Mobile, AL) once per month reminding them to take their eye drop medication. At the 3‐month visit, they participated in a scripted, interactive educational session with the research coordinator. The educational session, which lasted approximately 20‐30 min, reviewed the definition of glaucoma and its ability to cause blindness; the importance of using eye medications to control glaucoma; tips on using eye drop medication; and demonstration of how to instil eye drops into the eye. The control group was seen at the baseline visit and received a standard 5‐month follow‐up visit at the time of study completion. Although this group was also instructed in and monitored using the medication event monitoring system (MEMS) system, they did not receive any additional patient education materials or telephone reminders regarding glaucoma. The control group did not receive an attention placebo | |
| Outcomes | Adherence rate; therapeutic coverage (both primary) | |
| Funding | Allergan Incorporated and Research to Prevent Blindness, New York, NY | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | A sample size of 127 per group to achieve a power of 80% was calculated. However, medication dosing is different in our study (once daily dosing) than in Kass' prior studies and this may greatly alter the true sample size. In addition, investigators were also looking for a difference in adherence rates based on physician intervention and automated monthly telephone reminders, which is different than in the Kass studies. Therefore, 1/3 of the calculated sample size was chosen as a pilot study. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were prospectively randomised to either an intervention or a nonintervention group using a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Informed consent, interviews collecting demographic data and medical history, and testing sessions were administered by trained research assistants who were not masked to diagnosis" |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data were balanced in numbers, with similar reasons for missing data across groups. However, insufficient information to judge if missing data have been imputed using appropriate methods. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "No statistically significant difference existed between patients in the intervention and nonintervention groups in terms of age, sex, self‐reported ethnicity, glaucoma diagnosis, average length of diagnosis, number of systemic medications, number of medical problems, vision, intraocular pressure, highest level of education reported, and highest level of income reported" (Table 2) |
| Methods | Aims: to assess the effectiveness of computer‐generated telephone reminder and recall messages in increasing preschool immunisation visits Study design: quasi‐RCT;recruitment: other ‐ county health departments (organisational referral) Study duration: 5 months; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: any child younger than 2 years if his or her computerised immunisation record contained a telephone number and if the child was due or late for immunisation(s) at any time during the 4‐month enrolment period Sample size: 8002; mean age: < 2 years; sex: boys ‐ 51%, girls ‐ 49%; ethnicity: black ‐ 50%, white ‐ 45%, other – 5% (including Hispanic, Asian, and unknown) Country: USA | |
| Interventions | Intervention: before each calling session, children whose households were scheduled to receive a message were identified by the computer; telephone numbers and immunisation categories of these children were then downloaded to the automated dialing machine (System 606, Telecorp Systems Ine, Roswell, GA). For each calling session, the automated dialing machine recorded household‐specific information on the number of attempted contacts made and whether a successful contact had occurred. Following each calling session, this information was uploaded and merged with the study file.The households of children in the intervention group were called by the automated dialling machine twice daily for 7 days until successful telephone contact was established. To maximize the probability of reaching a parent, all weekday telephone attempts were made during evening hours. For children who were due for an immunisation, attempts at telephone contact began the day before the child was due. For children who were late for an immunisation, attempts at telephone contact began immediately after randomisation. Immunisation visits were immediately recorded in each health department's immunisation database when any child arrived for an immunisation. All children randomised to receive a telephone message received a second message if no immunisation visit was made in the week following the first successful telephone contact Control group received no calls. | |
| Outcomes | Immunisation status at 1 month | |
| Funding | CDC | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated in a non‐random way (odd or even numbers). Quote: "Children were allocated to an intervention group if their telephone numbers were assigned to an odd number; all other children were assigned to the non‐intervention group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | High risk | Data on the differences between the groups by county, type of residence, ethnicity, sex, or age were not reported |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to explore whether an individualised assessment and treatment programme (via IVR) could be devised that would train adaptive coping skills to alcoholic patients more effectively than current manual‐based coping skills treatments Study design: RCT; recruitment: primary care (advert elsewhere ‐ newspaper and radio, other research programmes) Study duration: 16 weeks; study type: management; subtype: alcohol consumption | |
| Participants | Inclusion criteria: to be eligible individuals had to be ≥ 18 years old, meet DSM‐IV criteria for alcohol abuse or dependence, and be willing to accept random assignment to either of the 2 treatment conditions Sample size: 110; mean age: 49 years; sex: men ‐ 58%, women ‐ 42%; ethnicity: white ‐ 86%, black ‐ 9%, Hispanic ‐ 3%, other ‐ 2% Country: USA | |
| Interventions | The Individualized Assessment and Treatment Program (IATP) employed a functional analysis of participants' behaviour as assessed by the IVR system during the 2‐week pretreatment experience‐sampling period. The situations that each participant encountered during experience sampling monitoring were reconstructed from the monitoring data, along with accompanying mood states, cognitive appraisals and coping actions taken. A functional analysis chart with this information was prepared by a research assistant and delivered to the therapist prior to the first IATP treatment session. IATP sessions focused on training 4 basic coping skills sets in each situation: avoidance, escape, environmental modification, and personal coping. Sessions 1 to 3 were devoted to analysing the high‐risk situations shown in the personalised functional analysis chart. Coping skills training initially addressed identification and avoidance of the participant's specific high‐risk situations. For situations that could not be avoided, training included skills such as environmental modification, drink refusal and assertiveness specifically tailored for dealing with the identified high‐risk situations, escape from high‐risk situations, and 'personal coping'. Homework was individualised, and built on information revealed in the functional analysis chart, as well as other situations recalled by the participant. Packaged Cognitive‐Behavioural Therapy was based on cognitive‐behavioural principles and designed to remediate deficits in skills for coping with interpersonal and intrapersonal antecedents to drinking. The treatment, based on manuals developed for previous clinical research and for Project MATCH, provided a structured experience using didactic presentations, behavioural rehearsal, and homework practice exercises. Homework was prescribed after every session, and was relevant to the material covered in that session. | |
| Outcomes | Proportion of days abstinent; proportion of heavy drinking days; continuous abstinence; drinking problems; coping problems (all primary) | |
| Funding | R21‐AA014202 from the National Institute on Alcohol Abuse and Alcoholism, and General Clinical Research Center grant M01‐RR06192 from the National Institutes of Health | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | A sample size of 50 per cell was determined to be sufficient to test most hypotheses with a power of 0.80 and alpha set at 0.05 based on effect sizes derived from previous studies of coping skills measures and outcomes | |
| Notes | ClinicalTrials.gov Identifier:NCT00298792. This is a comparison of 2 different interventions delivered via IVR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were assigned to treatment using an urn randomisation procedure that balanced the two groups for sex, age, baseline readiness to change, self‐efficacy and Coping Strategies Scale Total score" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the failure to blind research assistants to treatment assignment must be considered a weakness" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review have been reported |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to determine whether Spanish Diabetes Self‐Management Program (SDSMP) participants receiving monthly automated telephone reinforcement would maintain improvements in health status, health behaviours, and self‐efficacy at 18 months better than those not receiving reinforcement Study design: RCT;recruitment: other – community (word of mouth, announcements in churches, clinics, and Spanish language mass media) Study duration:18 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: participants were ≥ 18 years, not pregnant or in care for cancer, and had type 2 diabetes. They were enrolled in the SDSMP trial. Also included the control participants who had subsequently taken the SDSMP Sample size: 417; mean age: 53 years; sex: men ‐ 38%, women ‐ 62%; ethnicity: Hispanic (Spanish‐speaking) ‐ 100% (73% born in Mexico) Country: USA | |
| Interventions | Participants received automated telephone reinforcement once a month. They were greeted and asked to rate their diabetes self‐efficacy in the next month; had option to listen to two, 90‐s vignettes about various aspects of diabetes, and each of 15 vignettes was offered twice over 15 months—participants might hear about how Alexandra solved problems eating with her family or how Jose talked to his doctor about impotence; participants could leave a message. If necessary, a staff member responded to these messages. Participants in the control group received usual care. | |
| Outcomes | Glycated haemoglobin; health distress; global health; hypoglycaemia; hyperglycaemia; activity limitation; fatigue; glucose monitoring; self‐efficacy; healthcare utilisation (all primary) | |
| Funding | National Institutes of Health/National Institutes of Nursing Research Grant, Michigan Diabetes Research and Training Center | |
| Declaration of conflict of interest | KL receives royalties from Bull Publications for Tomando Control de su Salud, the book used by course participants | |
| Power calculations for sample size | NA | |
| Notes | The SDSMP is a 6‐week programme offered 2.5 h weekly by 2 peer leaders. Programmes were held in community settings in 6 San Francisco Bay Area counties. Class sizes ranged from 10 to 15 including participants' family and friends. Spanish‐speaking peer leaders (N = 43) came from the same communities as the participants. Most had type 2 diabetes and were not health professionals. They received 4 days of training. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following baseline data collection, most study participants were randomised to three groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | Complete case analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Comment: groups were comparable across all baseline characteristics but sex (control group had significantly more women participants compared with intervention, i.e. 67.2% vs 57.1%); however it is unclear if this imbalance has introduced bias. |
| Methods | Aims: to determine if a multimodal intervention composed of patient education, home blood pressure monitoring, blood pressure measurement reporting to an IVR phone system, and clinical pharmacist follow‐up improves blood pressure control compared with usual care. Study design: RCT;recruitment: primary care (telephone) Study duration: 6 months; study type: management; subtype: hypertension | |
| Participants | Inclusion criteria: patients with hypertension who were taking ≤ 4 antihypertensive medications and who had elevations in 2 of the 3 most recent electronic blood pressure measurements Sample size: 283; mean age: 65 years; sex: men ‐ 65%, women ‐ 35%; ethnicity: white ‐ 65%, other – 18%, Hispanic ‐ 17% Country: USA | |
| Interventions | Multimodal intervention included the following components: patient education, home blood pressure monitoring, home blood pressure measurement reporting to an IVR phone system, and clinical pharmacist management of hypertension with physician oversight. Participants input their systolic and diastolic blood pressure reading using the touch‐tone keypad of their phone during the weekly IVR calls. IVR, after calculating the average, provided feedback on whether their blood pressure measurements were at goal. They also had an opportunity to listen to educational messages or to request a call from the clinical pharmacist to answer questions. The blood pressure measurements were also reviewed by clinical pharmacists and participants received appropriate counselling. Those who did not enter any blood pressure measurements into the IVR system after 10 days received an automated reminder call, followed by a call from the pharmacist 4 days later Participants in the control group received usual care. | |
| Outcomes | Change in systolic and diastolic blood pressure (primary); medication adherence (secondary) | |
| Funding | American Heart Association and Colorado Department of Public Health and Environment | |
| Declaration of conflict of interest | "Dr Ho reports serving as a consultant for Wellpoint, Inc. The other authors (DJM, KLO, DWB, LKW, KES, ACLK, MEP, EPH) report no relationship or financial interest with any entity that would pose a conflict of interest with the subject matter of this article." | |
| Power calculations for sample size | NA | |
| Notes | ClinicalTrials.gov Identifier: NCT01162759 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A block randomisation design was used to ensure balance within healthcare systems. A random allocation sequence was computer generated using stratified randomisation with an allocation ratio of 1:1" |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was concealed until the intervention and usual care groups were assigned at the baseline visit." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The research assistant who obtained the blood pressure measurements was blinded to patient study group assignment." |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Of 338 patients enrolled in the study, 283 (84%) completed the 6‐month visit . . . Our primary analyses applied intent‐to‐treat principles to patients who completed the end‐of‐study visit . . .Patients who did not complete the study had higher baseline systolic and diastolic blood pressure" |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol is available; and all of the study's pre‐specified outcomes that are relevant to the review have been reported |
| Other bias | High risk | Baseline imbalance. The intervention had significantly higher systolic blood pressure compared with the control group. Quote: "At baseline, the mean (SD) blood pressures were significantly higher for 138 intervention patients vs 145 usual care patients" |
| Methods | Aims: to assess the effects of computer‐mediated automated IVR intervention designed to assist family caregivers managing persons with disruptive behaviours related to Alzheimer's disease (AD) Study design: RCT; recruitment: primary care and community (organisational referral and media adverts) Study duration: 18 months; study type: management; subtype: stress management | |
| Participants | Inclusion criteria: over the age of 21, provided ≥ 4 h per day of assistance or supervision for a minimum of 6 months to a family member with AD who had ≥ 2 impairments of instrumental activities of daily living (e.g. driving, shopping, or managing money) or 1 activity of daily living (e.g. toileting, bathing, eating), and exhibited ≥ 1 AD‐related disturbing behaviour Sample size: 1100 dyads; mean age: 63 years; sex: men ‐ 22%, women ‐ 78%; ethnicity: white ‐ 79%, black or African American ‐ 16%, Hispanic ‐ 2%, other – 2% Country: USA | |
| Interventions | Telephone‐Linked Care (REACH for TLC): participants chose the type of component, frequency, duration, and timing of the usage. The automated IVR conversation monitored the caregiver's stress levels and provided information on how to manage the care recipient's behavioural problems. Personal mailbox allowed caregivers to anonymously send and receive confidential communications through voice mail among themselves or to communicate with a clinical nurse specialist who directly answered or triaged questions to a multidisciplinary professional panel of AD experts. Bulletin board users anonymously posted messages and received responses back from other users. Activity–respite conversation provided personalised pleasant conversation to engage the listener in a safe, comforting, and non‐demanding activity. Participants in the control group received usual care. | |
| Outcomes | Caregiver's appraisal of the bothersome nature of care‐giving; anxiety; depression (primary) | |
| Funding | National Institutes of Health (NIH) National Institute on Aging | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two separate computer‐generated random assignment lists, one for men and one for women, were generated for each recruitment site, ensuring that each intervention and control group was balanced by sex and site." |
| Allocation concealment (selection bias) | Low risk | Quote: "After the completion of the baseline data, the interviewer opened an envelope that contained the group assignment." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All participants were subsequently interviewed at time points of 6, 12, and 18 months by different telephone interviewers who were blind to the study assignments except for the user satisfaction survey at the completion of the intervention period" |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers, with similar reasons for missing data across groups. "There was no significant difference in the frequency of missing data between intervention and control groups for the outcome measures (p > 0.05)" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | Quote: "The intervention and control groups did not differ significantly for any of the outcome dimensions at baseline." |
| Methods | Aims: to determine the impact of reminder systems on appointment non‐adherence rates in an low‐income inner city clinic population Study design: RCT; recruitment: primary care (organisational referral) Study duration: 2 months; study type: either; subtype: appointment reminders | |
| Participants | Inclusion criteria: patients due for an initial/annual gynaecology visit or initial prenatal intake visit in the women's health department over a period of 2 months Sample size: 2304; mean age: 29 years; sex: women ‐ 100%; ethinicty: Hispanic ‐ 66%, black ‐ 19%, white ‐ 13%, other ‐ 2% Country: USA | |
| Interventions | Automated telephone reminder of their appointment the day prior to the actual appointment Postcard reminder No reminder (control group) | |
| Outcomes | Attendance rate | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | The criterion for significance (alpha) was set at 0.05 (2‐tailed). To determine whether the sample size was sufficient to test our hypothesis, power was calculated using historical Hartford Hospital data and data reported previously in the literature. With the given effect size, a sample size of 1140 would have a power of 90 percent to yield a statistically significant result using a 3 x 2 Chi2 contingency test. | |
| Notes | This is a comparison between automated telephone and no reminder. The other intervention included postcard reminder | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients who verbally consented to participate in the study were randomly assigned to receive a phone reminder, mailed reminder, or no reminder (control group)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of study personnel was ensured. Quote: "Group assignment was unknown to those administering health care" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no significant differences in characteristics between the control and intervention groups for either the caregivers or the care recipients enrolled in the study" |
| Methods | Aims: to test whether IVR telephony may decrease the relapse rate after smoking cessation Study design: RCT; recruitment: other ‐ community (newspaper advert) Study duration: 24 months; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: smoking ≥ 35 cigarettes per week or ≥ 5 cigarettes per day for ≥ 2 years with no period of abstinence longer than 3 months Sample size: 44; mean age: 53 years; sex: men ‐ 67%; women ‐ 33%; ethnicity: * Country: Canada | |
| Interventions | After 12 weeks, the intervention group continued to receive IVR calls every 2 weeks from weeks 13–52. The IVR intervention consisted of 2 parts: establishing it is speaking to the study participant and the main data collection section. As instructed at the beginning of the call, the participant answers 'yes' or 'no' to all questions except when asked about their level of confidence and their side effects. The IVR asks if they have had a cigarette since their quit date, if they have smoked a cigarette, even a puff, if they have used varenicline in the last 14 days, have they experienced any side effects, how confident they are that they will remain a non‐smoker, and would they like to have a study nurse call them to help prevent relapse or provide advice about varenicline. Finally, there is a positive reinforcing message thanking and congratulating them followed by "remaining smoke‐free is the single most important thing you can do for your health". The calls are 3–5 min long, depending on their answers and which part of the algorithm they are directed to. The IVR made a call on their quit day, then on day 3, 8, and 11, and every 2 weeks thereafter The control group received no further IVR calls (no intervention) | |
| Outcomes | Self‐reported abstinence; biochemically confirmed smoking abstinence (both primary) | |
| Funding | Pfizer (Canada) | |
| Declaration of conflict of interest | Jiri Frohlich was a member of Pfizer (Canada) Medical Advisory Board and received speaking honoraria. He also participated in several clinical trials and received grants for investigator initiated studies. | |
| Power calculations for sample size | NA | |
| Notes | In phase 1 of the study, participants also received a 12‐week supply of varenicline: 0.5 mg to be taken on days 1–3, 0.5 mg twice a day on days 4–7, and 1 mg twice a day until the end of week 12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants who had quit smoking at 12 weeks were randomised into 2 groups matched by their level of motivation and level of addiction as per psychometric questionnaire at baseline. This was a stratified randomisation whereby participants were categorized by motivation and addiction." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Participants in the intervention smoked mean (SD) no. of cigarettes per day at baseline: 18.5 (6.6) versus 17.3 (8.6) in control. Insufficient evidence to judge whether this has introduced bias. |
| Methods | Aims: to evaluate a culturally adapted, automated telephone system to help hypertensive, urban African American adults improve their adherence to their antihypertensive medication regimen and to evidence‐based guidelines for dietary behaviour and physical activity Study design: RCT; recruitment: primary care (mail and telephone) Study duration: 12 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: self‐identification as African American; a diagnosis of hypertension on the active problem list of the patient's medical chart; a current prescription for ≥ 1 antihypertensive medications; ≥ 1 primary care office visits in the previous 2 months; 2 elevated clinic blood pressure readings in the previous 6 months (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg among non‐diabetic patients, and ≥ 130/80 among diabetic patients); and age ≥ 35 years Sample size: 337; Mean age:57 years; sex: men ‐ 30 %; women ‐ 70 %; ethnicity: African American ‐ 100 % Country: USA | |
| Interventions | Telephone‐Linked Care for Hypertension: at the onset, participants received a 75‐page resource manual that described hypertension, listed dietary recommendations, heart healthy food recipes, and local resources for exercise, and provided information to support antihypertensive medication adherence. Based on the manual, they received a 20‐min education session, and were given a pedometer and a digital weight scale. Participants in the intervention group also received a digital home blood pressure monitor. The automated telephone intervention delivered 1 call per week for 32 weeks. The first 3 calls introduced the 3 targeted behaviours and their role in blood pressure control. Subsequent calls were arranged as modules on medication adherence, physical activity, and diet, and were delivered in the order chosen by the participant. Each call consisted of an introduction, a section for reporting health information collected on study‐issued home measurement devices (pedometers, sphygmomanometers, weight scales), and theory‐based interactive education and counselling on the targeted behaviour. Physical activity module consisted of 12 calls to increase levels of moderate‐or‐greater intensity physical activity. The diet module consisted of 9 calls ‐ 1 overview call and 2 calls for each of 4 topics: fruits and vegetables, fibre, sodium, and fat. The content of these calls was designed to promote the DASH diet. The medication adherence module consisted of 8 calls. Study staff monitored participant use of the system and contacted those who did not call to assist or re‐engage them with the system Participants in the control group received usual care (education‐only). | |
| Outcomes | Medication adherence (primary); diet; physical activity; blood pressure (secondary) | |
| Funding | National Heart, Lung, and Blood Institute | |
| Declaration of conflict of interest | "Dr. Friedman has stock ownership and a consulting agreement with InfoMedics, the company that owns commercial rights to the TLC technology used in the computerized intervention. He is also a member of its Board of Directors. None of the other authors has any potential conflicts of interest to disclose." | |
| Power calculations for sample size | "Based on power analyses and projected attrition, we sought to randomise 360 patients expecting 300 to complete the 8‐month study assessment thus providing sufficient power to analyse the three primary behavioral outcomes." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was accomplished using a random number generator to assign subjects to one of the two groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither participants nor research assistants knew the group assignment until after baseline assessments were complete." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Missing data were imputed using the last value carried forward. For cases where data were available at time points before and after the missing value, the mean of these two values was used." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Quote: "Intervention group participants reported more moderate‐or‐greater physical activity per week than controls (162.4 min. vs. 126.3 min., p=0.04), and a greater percentage of the intervention group met national moderate‐or‐greater physical activity recommendations (38.5% vs. 26.2%, p<0.02). In addition, more intervention group participants than controls reported a history of stroke (11.2% vs. 4.2%, p<0.02)." Comment: groups were not comparable with significant baseline differences in physical activity (one of the study's primary outcomes) and stroke history |
| Methods | Aims: to enable oncology providers to receive and act on alert reports from patients about unrelieved symptoms during chemotherapy treatment Study design: RCT; recruitment: community centres and clinics (in‐person at the clinic) Study duration: 45 days; study type: management; subtype: cancer | |
| Participants | Inclusion criteria: eligible patients were to receive ≥ 3 chemotherapy cycles, were ≥ 18 years, had daily access to a touch‐tone telephone, understood English or Spanish, were physically and mentally able to participate, and reported ≥ 1 symptom of moderate or greater intensity during their first chemotherapy cycle Sample size: 250; mean age: 55.5 years; sex: men ‐ 24%; women ‐ 76%; ethnicity: Caucasian ‐ 91%, other ‐ 9% Country: USA | |
| Interventions | Automated monitoring system to report daily on 10 symptoms—pain, fatigue, nausea/vomiting, fever, trouble sleeping, anxiety, depressed mood, sore mouth, diarrhoea, and constipation. The symptoms were selected from the literature and confirmed in our pilot study as most frequently reported by patients receiving chemotherapy. Participants were queried if symptoms were present in the past 24 h and, if present, they rated severity and distress on a 1–10 scale. The 1–10 numeric scale is commonly used clinically and is an accepted standard in the measurement of symptoms; questions could be stated easily on the phone and answered numerically with the touch‐tone keypad. If fever was reported, the highest temperature was entered numerically; in addition, distress but not severity was measured for fever. For the treatment group, at completion of the phone call, the system immediately faxed or emailed (based on provider preference) symptom alert reports to the participant's oncologist and oncology nurse. Alert thresholds varied by symptom; they were initially established by an expert panel and then revised based on pilot work. 2 thresholds were set: a simple alert when severity or distress was > 5 or 7 (depending on the symptom) on the 10‐point scale and trend alerts based on a pattern of moderate severity over several days. For example, pain generated an alert when pain was rated at 5 or greater, whereas fatigue generated an alert at 7 or a trend alert based on a pattern of 3 out of the past 7 days reported at moderate levels (4–6). The report included not only severity and distress but a symptom profile including answers to drill‐down questions such as the number of vomiting episodes, oral intake, dizziness, and use of antiemetics for nausea. Reports also included graphs of symptom patterns since the first day of chemotherapy. On every call, all participants, regardless of group, were advised to call their oncology providers if they had concerns about their symptoms. In all of the participating provider teams, normal usual care procedure for unrelieved symptoms was to instruct participants to call the clinic office for symptom concerns Attention control group received equivalent contact time with the automated system including identical voice and assessment questions. They understood that the data they submitted were for research purposes only and were not available for clinical action. On every call, all participants, regardless of group, were advised to call their oncology providers if they had concerns about their symptoms. In all of the participating provider teams, normal usual care procedure for unrelieved symptoms was to instruct participants to call the clinic office for symptom concerns | |
| Outcomes | Symptom severity, and distress (primary); system usability and acceptability (secondary) | |
| Funding | National Institutes of Health, National Cancer Institute (R01 CA89474) | |
| Declaration of conflict of interest | None reported | |
| Power calculations for sample size | A post hoc sensitivity analysis was conducted with G*Power to access available statistical power. With a sample size of 223 participants, investigators had sufficient power (1 −Β)=0.91 to detect a small effect size Cohen's d = 0.10 and alpha = 0.05 | |
| Notes | Similar ATCS interventions were compared with each other. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were stratified by provider team to ensure equivalency of the treatment and control groups within teams and then randomly assigned to treatment or attentional control. Random assignments in blocks of ten were generated for each provider stratification group." |
| Allocation concealment (selection bias) | Low risk | Quote: "Research staff and patients did not know assignment until after informed consent." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Providers were not informed of random assignment but could not be blinded as they would only receive alert reports about treatment group patients." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Twenty‐seven participants dropped from the treatment group (21%) and 31 from the control group (26%), a non‐significant difference (p>0.05)." Comment: insufficient information whether this drop‐out rate introduced bias. |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Unclear risk | The intervention had significantly more women and breast cancer diagnosed participants compared with the control, but there is insufficient evidence that this imbalance has introduced bias. Quote: "Comparisons of group equivalence at baseline indicated that the treatment group was over‐represented by women (chi‐square 4.89; p=0.027) and breast cancer diagnosis (chi‐square=9.56; p=0.023)." |
| Methods | Aims: to evaluate feasibility, acceptability, and initial efficacy of a therapeutic IVR system for opioid dependent patients receiving methadone maintenance who were continuing to use illicit drugs while enrolled in treatment Study design: RCT; recruitment: primary care (clinic posters and flyers, brochures provided to counsellors, and word‐of‐mouth) Study duration: 1 month; study type: management; subtype: illicit drugs addiction | |
| Participants | Inclusion criteria: no current suicide or homicide risk; lack of a DSM‐IV current psychotic or bipolar disorder; not involved in another treatment study; ability to read or understand English; and lack of a life‐threatening or unstable medical problem Sample size: 36; mean age: 41 years; sex: men ‐ 58%; women ‐ 42%; ethnicity: white ‐ 58%, black ‐ 28%, other – 14% Country: USA | |
| Interventions | The Recovery Line plus treatment‐as‐usual involved a therapeutic IVR orientation session, 4 weeks of 24‐h access to the system, a participant notebook with summary Recovery Line information, and a weekly reminder from staff to use the system. The Recovery Line system was developed for participants to use in their own environment and obtain immediate assistance, training, and support for improved coping. Modules were designed to be brief (< 15 min) and easy to understand. System components included self‐monitoring, coping with urges and cravings, identifying/avoiding risky situations, and managing moods and stress. For self‐monitoring, a daily questionnaire of 3 items was included immediately upon system log in ("How are you doing?" "Have you taken your methadone today?" "Have you used illicit drugs since your last call?") Participants in the control group received usual care. The proposed system was meant to serve as an enhancement of current services being delivered, which included the requirement to attend 1 individual session per month and encouragement to attend open access groups (with ≥ 10 typically available Monday–Friday) covering a range of topics, including introduction to methadone, weekend planning, overdose planning, and spirituality. These are the services provided in the standard care comparison condition. | |
| Outcomes | Patient interest; perceived efficacy; treatment satisfaction; drug consumption (self‐reported use); methadone counselling; ease of use; coping skills (all primary) | |
| Funding | National Institute on Drug Abuse Grants and through the State of Connecticut, Department of Mental Health and Addiction Services support of the Connecticut Mental Health Center | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | None | |
| Notes | ClinicalTrials.gov Identifier: NCT01315184 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised (N=36) to 4 weeks of treatment‐as‐usual (TAU) or Recovery Line plus TAU." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "There were no statistically significant differences between the two treatment conditions, but several trends were controlled through covariance adjustments." There is insufficient evidence that these covariates have introduced bias. |
| Methods | Aims: to determine the effects of multicomponent physical activity counselling (PAC) promoting physical activity guidelines on gait speed and related measures of physical activity and function in older veterans Study design: RCT; recruitment: primary care (mail and telephone) Study duration: 12 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: patients were eligible for the study if they could walk 30 feet without human assistance and did not engage in regular physical activity Sample size: 398; mean age: 78 years; sex: men ‐ 100%; ethnicity: white ‐ 77% Country: USA | |
| Interventions | Multimodal intervention group received: baseline in‐person and biweekly then monthly telephone counselling by a lifestyle counsellor, onetime clinical endorsement of physical activity and monthly automated telephone messaging by primary care provider, and quarterly tailored mailings of progress in physical activity Participants in the control group received usual care. | |
| Outcomes | Gait speed (usual and rapid); self‐reported physical activity; function and disability (all primary); change in min of moderate/vigorous physical activity per week (secondary) | |
| Funding | Veterans Affairs Rehabilitation Research and Development # E3386R and NIH grant AG028716 | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | Quote: "We powered the sample size for this study to be able to detect a between group difference of 0.10 m/sec in both usual and rapid gait speeds." | |
| Notes | 1 participant died in the intervention group; 6 died in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was computer generated by a statistician" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was . . . sealed envelopes stored in the Veterans LIFE Study office until randomisation." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study was unblinded and patients were aware of the study objectives." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All assessments were made at baseline, three, six, and 12 months by individuals blinded to randomisation status" |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Return for follow‐up was similar for both groups with slightly more withdrawals in the PAC group [16 (8%)] than in the UC group [11 (5.5%)]. There were no differences between dropouts and individuals completing the trial except for usual gait speed which was significantly lower in the drop outs (−0.9 m/sec, p = 0.016)." Insufficient evidence to judge whether this introduced bias. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐specified outcomes have been reported accordingly. |
| Other bias | Low risk | Baseline characteristics were similar between the 2 groups. |
| Methods | Aims: to determine whether a home‐based multi‐component physical activity counselling (PAC) intervention is effective in reducing glycaemic measures in older prediabetic outpatients Study design: RCT; recruitment: primary care (mail and telephone) Study duration: 12 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: impaired glucose tolerance defined as a fasting glucose between 100–125 mg/dL, free from a diagnosis of diabetes, have a glycated haemoglobin below 7%, and not be on diabetes medications. A BMI between 25 and 45 kg/m2 was required Sample size: 302; mean age: 67 years; sex: men ‐ 97%, women ‐ 3%; ethnicity: white race ‐ 70% Country: USA | |
| Interventions | Multimodal intervention group received: 1 in‐person baseline counselling session, regular telephone counselling, physician endorsement in clinic with monthly automated (telephone calls) encouragement, and tailored mailed materials, plus a consult to a Veterans Affairs (VA) weight management programme Participants in the control group received usual care plus MOVE programme | |
| Outcomes | Fasting insulin and glucose levels measured with homeostasis model assessment of insulin resistance (HOMA‐IR); glycated haemoglobin; anthropometric measures; self‐reported physical activity; health‐related QOL; physical function | |
| Funding | VA Health Services Research and Development grant IIR‐06‐252‐3; National Institute on Aging grant AG028716; VA Rehabilitation Research Service grants (RRD‐E2756R, RRD‐E3386R) and National Cancer Institute grant CA106919; Department of Veterans Affairs Health Services Research and Development Career Scientist Award (RCS 08‐027) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | Power estimates were calculated using data from the STRRIDE study in which a group receiving a low dose of moderate exercise, equivalent to the dose of moderate exercise advocated for the Enhanced Fitness Study, reduced fasting insulin by 1.3 units while the control group experienced an increase in fasting insulin of 0.92 units with a pooled standard deviation of 3.9. With correction for multiple comparisons between adaptive strategies and a projected 12.5% attrition rate based on our previous experience, our sample size was 80% powered to detect a standardised difference of 0.39 in fasting insulin for a 2‐tailed test | |
| Notes | 2 participants died in the intervention group; 1 died in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Adaptive randomisation will allow us to mimic primary care by altering treatment based upon patient compliance". Insufficient information to judge whether this introduced bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "A statistician with no participant contact delivered sealed randomisation assignments to the project coordinator. These were kept in a locked cabinet until randomisation occurred." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "All of the outcomes were assessed at baseline, three months and 12 months by individuals blinded to intervention status" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Analyses were performed under the intent‐to‐treat criteria" |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐specified outcomes have been reported accordingly |
| Other bias | Low risk | Quote: "Sensitivity analyses revealed no baseline differences between groups for age, race, number of symptoms, general health and physical function" |
| Methods | Aims: to determine the effect of an automated telephone intervention on completion of faecal occult blood testing Study design: RCT; recruitment: other ‐ health plan (organisational referral) Study duration: 6 months; study type: prevention; subtype: screening | |
| Participants | Inlcusion criteria: eligible participants were due for routine colorectal cancer screening (and in whom stool occult blood testing was a clinically appropriate option) and who met other criteria such as: those due for colorectal cancer screening who have not had any of the following: colonoscopy within 10 years, flexible sigmoidoscopy or double‐contrast barium enema within 5 years, faecal occult blood testing screening within past 12 months, or order for faecal occult blood testing/double‐contrast barium enema in past 3 months Sample size: 6000; mean age: 60 years; sex: men ‐ 50%, women ‐ 50%; ethnicity: white ‐ 92%, non‐white – 7%, unknown ‐ 1% Country: USA | |
| Interventions | Automated Telephone Contact Intervention group received up to three 1‐min automated telephone calls providing a brief overview, including information about the benefits of colorectal cancer screening, and encouraged faecal occult blood testing as a relatively simple and low‐risk method of cancer screening. Recipients could request faecal occult blood testing cards by pressing a number via touch‐tone telephone. If a live person did not answer, callers heard a detailed message with a telephone number they could call to request cards. Participants who did not complete faecal occult blood testing screening received up to 2 reminder calls, 6 weeks apart. Call content was identical to the first automated telephone call. 1 additional reminder call was targeted to intervention participants who had requested an faecal occult blood testing kit but did not return the completed faecal occult blood testing cards within 4 to 5 weeks from the date of request. The call to non‐returners (call type 2) emphasised the benefits of colorectal cancer screening and reminded participants to return completed faecal occult blood testing cards. Participants were given the opportunity to request additional faecal occult blood testing cards if needed. Participants in the control group received usual care. Participants randomised to UC did not receive the telephone contact intervention but may have been referred for colorectal cancer screening by their clinicians during normal care processes. | |
| Outcomes | Completion of faecal occult blood testing during the 6 months after call initiation; screening through any the US Preventive Services Task Force recommended colorectal cancer screening modality during the RCT and included receipt of faecal occult blood test, colonoscopy, flexible sigmoidoscopy, or double‐contrast barium enema. | |
| Funding | National Cancer Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | We had 80% power to detect an absolute difference of 2.8% (relative difference of 28.6%), assuming the faecal occult blood test return rate was 9.7% in UC versus 12.5% in the intervention group. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 6000 patients were randomly assigned either to receive usual care (UC; n=3000) or automated telephone contacts (n=3000), using a stratified randomisation approach, balancing on age, sex, and prior colorectal cancer screening." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Attritions were small (< 2% in both groups) and unlikely to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were similar at baseline. Quote: "No statistically significant differences were found between the 2 populations for any of the baseline characteristics." |
| Methods | Aims: to evaluate the impact of an automated telephone reminder system on patients' on‐time maintenance medications refills Study design: RCT; recruitment: population level Study duration: 1 month; study type: prevention; subtype: adherence to medication/laboratory tests | |
| Participants | Inlcusion criteria: participants due for medication refills Sample size: 4,237,821; mean age: 56 years; sex: men ‐ 38.5%; women ‐ 61.5%; ethnicity: * Country: USA | |
| Interventions | Participants on maintenance prescription received automated IVR calls, 3 days before their refill was due, as a reminder. If participants had multiple medications due on a single day, only 1 call for all medications was made. A maximum of 2 attempts was made for unanswered calls. If both attempts fail and a participant's voicemail was available, a message was left with phone number to call back. Messages did not identify the medication by name or any other form of protected health information. Upon answering a call, participants were required to authenticate with their date of birth. After the participants agreed to refill, their maintenance medications were automatically processed for pick‐up. Participants received calls every time they have medication to refill. Participants in the control group received no calls. | |
| Outcomes | Daily and cumulative refill rates (the percentage of prescriptions refilled on or by a specific date around the expected refill date) | |
| Funding | Correspondence with the authors: "Yes, the study was funded internally by Walgreens Co." | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with the authors: "Yes, this was a simple randomised study. On the first day that a patient qualified for the study, the campaign management system would assign the patient to a test or control group based on the random number. The random numbers were generated by the system based on random seed that was changed every month." |
| Allocation concealment (selection bias) | Low risk | Correspondence with the authors: "Yes, the randomisation was automated without researcher involvement" |
| Blinding of participants and personnel (performance bias) | High risk | Correspondence with the authors: "No." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to evaluate the feasibility of a computer automated IVR system to reduce relapse following discharge from residential treatment Study design: RCT; recruitment: primary care (health professional referral) Study duration: 6 months; study type: management; subtype: alcohol consumption | |
| Participants | Inclusion criteria: men and women, aged 20–61 years, treated for alcohol dependence at the Herrington Recovery Center, a residential treatment facility of the Rogers Memorial Hospital Sample size: 60; mean age: 42 years; sex: men ‐ 55%, women ‐ 45%; ethnicity: Caucasian ‐ 95%, African American ‐ 5% Country: USA | |
| Interventions | Daily IVR reporting with personal follow‐up on non‐compliant callers. The study coordinator/counsellor was instructed to make a personal telephone call to participants any time they failed to make a daily call to the IVR system for 2 consecutive days. If participants did not begin using the system thereafter, the coordinator/counsellor continued calling them daily for ≥ 10 days. After 10 consecutive days of prompting non‐compliant participants to use the system without success, the coordinator/counsellor continued to call the participants at least twice each week until system use began or they withdrew consent for study participation Daily IVR reporting without follow‐up; participants had access to the same daily IVR reporting system but were not contacted or prompted to use it if they did not make daily calls to the system No IVR reporting (control group) | |
| Outcomes | Self‐reported drinking days, heavy drinking days and total drinks | |
| Funding | 1R43AA12366 from the NIAAA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "The relatively small sample sizes would provide inadequate statistical power to support clinical efficacy of any treatment effect that was not extremely large and that even modest study dropout rates would diminish the limited statistical power even further." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Volunteers to participate in the study were randomly assigned to one of three treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | Attrition reduced the already small sample size by 20%. Missing data have not been imputed using appropriate methods. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were similar at baseline. Quote: "No significant difference was evident between the randomised groups regarding sex, age, and length of stay in residential treatment" |
| Methods | Aims: to test the efficacy of a new automated call‐monitoring system for second and third trimester predominantly Medicaid‐eligible pregnant women in an urban free standing birth centre to promptly detect symptoms of influenza and assure rapid treatment to prevent adverse outcomes from influenza Study design: RCT; recruitment: primary care (*) Study duration: 2 months; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: pregnant for ≥ 12 weeks but not yet 38 weeks pregnant, attending Family Health and Birth Center for prenatal care (FHBC is the urban free‐standing birth centre, within Developing Families Center), able to speak English, operate a cell phone and agree to attend prenatal care visits Sample size: 50; mean age: 24 years; sex: men ‐ 0%; women ‐ 100%; ethnicity: African American ‐ 86%, white ‐ 14 % Country: USA | |
| Interventions | Automated telephone system called the automated call group participants every day at the time selected by the participant and asked questions about whether she had developed ≥ 1 of the specific influenza symptoms mentioned in the call in the past 24 h. If the participant answered 'yes', then the recording stated that she should speak immediately to the nurse midwife on call Participants in the control group received health information | |
| Outcomes | Immunisation rate; satisfaction | |
| Funding | National Institute of Child Health and Human Development | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random number generator in Excel was used to generate random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Random numbers were put into sealed envelopes and were opened at time of enrolment" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "None of the differences between experimental and control group were statistically significant at alpha of 0.05" |
| Methods | Aims: to assess the effectiveness of therapeutic IVR intervention in increasing treatment compliance and adherence in chronic pain patients and improving outcome at follow‐up Study design: RCT; recruitment: primary care (organisational referral) Study duration: 4 months; study type: management; subtype: chronic pain | |
| Participants | Inclusion criteria: at least 6 months of musculoskeletal pain (such as back pain, osteoarthritis, or fibromyalgia); met study threshold for severity of pain "over the past four weeks'' of ≥ 4 on a 10‐point scale measured at baseline on the McGill Pain Questionnaire short form; able to perform usual self‐care; had ongoing health care from a physician; aged ≥ 18, owned a touch‐tone phone Sample size: 55; mean age: 46 years; sex: men ‐ 14%, women ‐ 86%; ethnicity: white/Caucasian ‐ 96%, other 4% Country: USA | |
| Interventions | The intervention group received IVR calls. The system included the following:
Participants in the control group received usual care | |
| Outcomes | Pain (total pain experience, pain intensity); Function/disability; Coping | |
| Funding | National Institute of Drug Addiction (NIDA), National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS), National Institute on Alcohol Abuse and Alcoholism (NIAAA) | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "The study was powered to detect an effect size of 0.5 using ANCOVA for the endpoint comparisons between the two groups." | |
| Notes | Only those participants who successfully completed 11 weeks of group CBT were recruited in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Consenting subjects were stratified by level of pain and by sex, and then randomised to one of the two study groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was done after group therapy was completed in order to avoid the risk of differential CBT exposure based on group assignment." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All participants who successfully completed CBT and who agreed to be randomised were retained for the primary analyses. For 3 cases with missing data at the second or third follow‐ups the average of the scores from the prior and following time points was used. Two participants from the TIVR group who were missing the final set of questionnaires were assumed to have regressed to the baseline." |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aim: to evaluate the effect of 2 distinct interventions on medication adherence in elders treated for memory problems while taking factors such as depression and cognitive status into account Study design: RCT; recruitment: primary care (health professional referral) Study duration: 24 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: clinically judged to have a memory problem and were being treated with 1 of the approved cholinesterase inhibitor medications (donepezil, rivastigmine, or galantamine) or memantine and judged to be able to give informed consent for their participation Sample size: 27; mean age: 80;sex: * ethnicity: * Country: USA | |
| Interventions | Automated reminding: participants in this condition participated in regular study visits and assessments, but also received automated daily phone calls consisting of a recorded message from the investigator reminding the participant to take their medication. The message consisted of a recording of the first author stating that he was calling the participant to remind them to take their medication, either in Spanish or English Tailored information: participants in this condition at the second study visit received a 20‐min tailored information intervention that consisted of completing a questionnaire about information they wanted to receive about memory disorders and their treatment Participants in the control group received no intervention | |
| Outcomes | Medication adherence | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | Quote: "Given the small sample size employed in this study, it is possible that we simply did not have adequate statistical power to detect a relation that may have been present." | |
| Notes | This is a comparison between automated reminding and control arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were recruited during routine clinical visits at the memory disorders clinic or from contact information available because they had participated in other research studies at the clinic and randomised to one of the three conditions after written informed consent was obtained." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to compare the no‐show rates of an automated appointment reminder system, clinic staff reminder, or no system at all Study deign: RCT; recruitment: tertiary care (health professional referral) Study duration: 4 months; study type: either; subtype: appointment reminder | |
| Participants | Inclusion criteria: patients in ≥ 1 of 10 specialty outpatient practices of the University of Medicine and Dentistry of New Jersey–Robert Wood Johnson Medical School: heart transplantation, rheumatology, pulmonary, nephrology, haematology, general internal medicine, gastroenterology, endocrinology, cardiology, and allergy/infectious disease Sample size: 12,092; mean age: 56 years; sex: men ‐ 43%, women ‐ 57%; ethnicity:* | |
| Interventions | Automated appointment reminder system attempted to reach the participant each night for 3 nights before the appointment. As determined by each specialty, a practice‐customised computerised or live voice recording was played after a phone call was answered. The recipient of the call had the option of confirming the appointment or cancelling the appointment. After 3 attempts if an appointment was not confirmed, the participant remained registered for the appointment Staff reminder No reminder | |
| Outcomes | Non‐attendance rate; satisfaction | |
| Funding | None | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | A sample size (per group) of 1059 was calculated to be sufficient to detect a change from 8% to 5% (638 for 9% to 5%) with a power of 80% (beta) | |
| Notes | This is a comparison between automated system and no reminder. Additional group consists of clinical staff reminder group (STAFF) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then randomised by a computer‐generated allocation sequence into 1 of the 3 groups" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was concealed from the investigators and clinic staff." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Clinic staff were not blinded to the patients they were instructed to call; however, they were unaware to which group (ie, AUTO or NONE) the remaining scheduled patients were assigned." Comment: insufficient information whether blinding was achieved |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers, with similar reasons for missing data across groups. Quote: "Analysis of the no‐shows was performed by intention to treat"" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "Baseline characteristics of the 4 groups were similar" |
| Methods | Aims: to evaluate the ability of interactive voice recognition (IVR) technology to improve statin adherence in a cohort of new start patients Study design: RCT; recruitment: * (organisational referral) Study duration: 6 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: adults continuously enrolled in the health plan for 2 years, and new users of statin therapy (no statin prescription for past 12 months) Sample size: 15,051; mean age: *; sex: * ethnicity:* Country: USA | |
| Interventions | Participants in the intervention group received 3 automated phone calls; call 1 provided disease state education, call 2 was a refill reminder, and call 3 addressed the importance of physician follow‐up. The programme provided customised interaction based on participant response, primary vs secondary cardiovascular disease prevention, and refill behaviour Participants in the control group received usual care (control) | |
| Outcomes | Medication adherence | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a total of 6833 members were randomised to the intervention group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to evaluate the effectiveness of a web‐phone intervention in changing smoking behaviour Study design: RCT; recruitment: other ‐ university (military officer referral) Study duration: 9 weeks; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: smoking university students Sample size: 116; mean age: 20 years; sex: men ‐ 92%; women ‐ 8%; ethnicity: Asian ‐ 100% Country: Taiwan | |
| Interventions | The automated web‐phone intervention (WPI) delivered phone calls that assessed participants' smoking status and based on their responses, delivered motivational and educational recorded messages. The messages covered themes that were most frequently covered in the Taiwan Smokers Helpline counselling sessions based on the participant's stage of change. The question "Have you quit smoking cigarettes?" with the time frame modified for the current week of the 9‐week WPI was asked via a WPI automated phone call. The answers and scoring were 'No, and I do not intend to quit in the next 3 months', 'No, but I intend to quit in the next 3 months', 'No, but I intend to quit in the next 30 days', 'Yes, I quit less than 3 months ago'; and 'Yes, I quit more than 3 months ago'. The control group received the observation call in weeks 1 and 9 along with 2 assessments per week for 3 weeks, 1 assessment for 3 weeks, and 1 assessment for the last 3 weeks | |
| Outcomes | Stage of change; self‐efficacy; decisional balance | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Similar ATCS components were evaluated Correspondence with the author: "The intervention was based on automated IVR system which was consisted of reminders and questions and options." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the recruiting procedure, the 116 participants were assigned a unique number and randomly assigned using a systematic numbering system into one of the three groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The double‐blind principle was applied so neither the researcher nor the participants knew which group participants were in" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | No description of drop‐outs; imbalance in numbers and reasons for missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to compare the effectiveness of personalised letters, automated telephone calls, and both on breast cancer and colorectal cancer screening Study design: RCT; recruitment: primary care (organisational referral) Study duration: 36 weeks; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: registered patient at the study clinic; having ≥ 1 visit to the practice in the past 2 years; 50‐74 years old; and past due for mammography or colorectal cancer screening based on medical record documentation Sample size: 685; mean age: 58 years; sex: men ‐ 38%, women ‐ 62%; ethnicity: non‐Hispanic white ‐ 78%, non‐Hispanic black ‐ 13%, other (e.g. Hispanic) ‐ 9% Country: USA | |
| Interventions | Automated telephone calls (IVR) in up to 3 waves through a commercial vendor. IVR calls were attempted at varying times (up to 5 times) until a person or an answering machine responded during the first wave (week 1). These calls were repeated during the second wave (week 5). Participants who remained unscreened following a reassessment of screening (week 10) received a third wave (weeks 12 to 14). The automated messages contained content similar to that in the letter, including a number to call if they wanted a faecal immunochemical test to be mailed Personalised letter, signed by the participant's physician, explaining that the participant was past due for cancer screening; the importance of cancer screening; how to schedule the screening; the name and telephone number of the outreach worker available to assist participants with arranging screening; and the availability of free mammography and colorectal cancer screening IVR + personalised letter. Women eligible for both interventions received 1 letter indicating they were past due for both screenings and/or 2 separate automated calls indicating they were past due for mammography and colorectal cancer screening | |
| Outcomes | Completed mammogram or colorectal cancer screening within 36 weeks of randomisation (documented)(primary) | |
| Funding | American Cancer Society (RSGT‐08‐077‐01‐CPHPS) and the Agency for Healthcare Research and Quality (1 K18 HS022440‐01) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between the IVR alone arm and arm personalised letter alone arm. Costs: IVR: USD 0.92 per participant; Letter: USD 7.17 per participant/mailing; IVR + letter: USD 3.28/participant for breast cancer screening; and USD 8.09/participant for colorectal cancer screening | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician, who was offsite and blinded to the patients' identities, assigned participants equally to 1 of the 3 intervention groups using a computer‐generated random number algorithm. Randomization was stratified by the type of screening(s) for which the participants were past due (breast cancer, colorectal cancer, or both)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After confirming eligibility through medical record abstraction, each participant was assigned a unique study identification number" |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of study personnel was ensured. Quote: "The office clinicians and study staff were blinded to group assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment was ensured. Quote: "Research assistants, who were blinded to the intervention, abstracted data (screening date and results available by week 36)." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All participants were analysed in the originally assigned study group based on intention to treat." |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review have been reported |
| Other bias | Low risk | Groups were balanced at baseline with no statistically significant differences |
| Methods | Aims: to assess the impact of automated telephone disease management (ATDM) calls with telephone nurse follow‐up as a strategy for improving outcomes such as mental health, self‐efficacy, satisfaction with care, and health‐related quality of life (HRQL) among low‐income patients with diabetes mellitus Study design: RCT; recruitment: primary care (organisational referral) Study duration: 12 months; study type: management; subtype: diabetes mellitus | |
| Participants | Inclusion criteria: adults with a diagnosis of diabetes or an active prescription for a hypoglycaemic agent Sample size: 248; mean age: 55 years; sex: men ‐ 41%, women ‐ 59%; ethnicity: Hispanic ‐ 50%, white ‐ 29%, other – 21% Country: USA | |
| Interventions | Automated telephone disease management calls consisted of hierarchically structured messages composed of statements and queries recorded in a human voice. Each message began with an introductory script in which the nature of the call was explained to whoever was the initial call recipient. Biweekly ATDM assessment calls ‐ to check for blood glucose testing in the prior week. Those who had, were asked to indicate the time of their last self‐ monitored blood glucose (SMBG) reading and report the SMBG test result in milligrams per decilitre. Each assessment also included questions about intervention participants' perceptions of their glycaemic control; symptoms of poor control, foot problems, chest pain, and breathing problems; and self‐care issues related to SMBG and foot care. At a later stage, they were offered additional automated self‐care education calls that focused on glucose self‐monitoring, foot care, and medication adherence. Here, participants reported specific barriers to self‐care and received tailored education and advice. Within the medication adherence segment of the calls, participants were asked about their adherence to insulin, oral hypoglycaemic medications, antihypertensive medications, and antilipaemic medications. Compliant received positive feedback and reinforcement while those reporting sub‐optimal adherence were asked about specific barriers and were given advice about overcoming each barrier. The calls also asked whether the participant had a retinal examination in the prior year. At the end of each call, participants were instructed to call the study nurse if they had health problems or questions not covered in the assessment. Participants also had periodic telephone contact with a registered nurse who addressed their ATDM‐reported problems. The nurse was located outside the medical centre and had neither face‐to‐face contact with participants or ready access to their records. Her information base was limited to medical record data abstracted during the enrolment process, ATDM problem reports, and her notes from prior telephone contacts. Each interaction takes between 5‐8 min. All calls were outbound and were placed at times that the participant indicated were convenient. A small number of contacts were initiated by the participants themselves using the study's toll‐free telephone number, which was provided at baseline and during each ATDM call Participants in the control group received usual care. They had no contact with the system for clinical assessments, participant education, appointment reminders, or follow‐up data collection. | |
| Outcomes | Depression; anxiety; days in bed because of illness; days cut down on activities because of illness (all primary); diabetes‐specific HRQL; self‐efficacy (secondary) | |
| Funding | American Diabetes Association, Department of Veterans Affairs | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | Target sample size for this study was defined to have sufficient statistical power to detect a 1% between‐group difference in glycated haemoglobin (i.e. 9% versus 8%) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was based on a table of randomly permuted numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Neither providers, research staff, nor prospective participants had knowledge of group assignment until the patient had consented to participate." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "Outcome analyses were conducted on an intent‐to‐treat basis" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | Quote: "Baseline characteristics of intervention and usual care patients were similar, although patients in the intervention group were slightly older (P=0.072) and more likely to use insulin (P=0.035). There were no significant differences between the 2 groups in any baseline measures of patient‐centred outcomes." |
| Methods | Aims: to evaluate automated telephone disease management (ATDM) with telephone nurse follow‐up as a strategy for improving diabetes treatment processes and outcomes in Department of Veterans Affairs (VA) clinics Study design: RCT; recruitment: primary care (organisational referral) Study duration: 12 months; study type: management; subtype: diabetes mellitus | |
| Participants | Inclusion criteria: adults with a diagnosis of diabetes or an active prescription for a hypoglycaemic agent Sample size: 272; mean age: 61 years; sex: men ‐ 97%, women ‐ 3%; ethnicity: white ‐ 60%, black ‐18%, Hispanic ‐ 12%, other – 10% Country: USA | |
| Interventions | Automated telephone calls. The automated calls consisted of hierarchically structured messages composed of statements and queries recorded in a human voice. All calls were outbound (i.e. participants received the calls), and each assessment lasted 5–8 min. During each ATDM assessment, participants used their touch‐tone keypad to report information about their self‐monitored blood glucose (SMBG) readings, other self‐care activities, perceived glycaemic control, symptoms, and use of guideline‐recommended medical care. At the end of each assessment, participants were given the option of listening to health promotion messages Participants in the control group received usual care. | |
| Outcomes | Glycated haemoglobin; self‐monitoring of blood glucose; self‐monitoring of feet; self‐monitoring of diet; medication use; diabetic symptoms (all); satisfaction with care (all primary); speciality outpatient services use (secondary) | |
| Funding | Health Services Research and Development Service, Mental Health Strategic Health Care Group, Quality Enhancement Research Initiative, American Diabetes Association, Department of Veterans Affairs | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised using sealed envelopes containing group assignments and a sequence generated using a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised using sealed envelopes containing group assignments and a sequence generated using a table of random numbers" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "HbA1c and serum glucose levels were measured at baseline and 12 months in one laboratory by staff who were blinded to patients' experimental condition." |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "All analyses of intervention effects were conducted on an intent‐to‐treat basis" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Unclear risk | Quote: "Intervention and control groups had similar characteristics at baseline. However, intervention patients were more likely than control patients to be white and have somewhat more complications." Unclear whether this has introduced bias. |
| Methods | Aims: to evaluate the feasibility of utilising an IVR system to supplement hypertension self‐management for patients in underdeveloped regions in Mexico and Honduras Study design: RCT; recruitment: primary care (organisational referral) Study duration: 6 weeks; study type: management; subtype: hypertension | |
| Participants | Inclusion criteria: participants having access and were able to use a telephone, and had a systolic blood pressure suggesting hypertension (i.e. systolic blood pressure ≥ 130 mmHg if diabetic or ≥ 140 mmHg if non‐diabetic) Sample size: 200; mean age: 58 years; sex: men ‐ 33%, women ‐ 67%; ethnicity:* Country: Honduras; Mexico | |
| Interventions | Participants in intervention group received a series of weekly automated monitoring and behaviour change calls, as a reminder to check their blood pressure regularly and were asked about: recent systolic values above and below the normal range, medication adherence, and intake of salty foods. Based on this information, participants received additional self‐care information during the call and prompts to seek medical attention or medication refills to address unacceptably high or low blood pressure. Structured email alerts for health workers were generated automatically when participants reported that at least half the time in the prior week they had a systolic blood pressure > 140 mm Hg (non‐diabetic participants), > 130 mm Hg (diabetic participants), or systolic blood pressure < 100 mmHg (all participants). Alerts also were generated if the participant reported rarely or never taking their blood pressure medication or less than a 2‐week supply. Participants also had the option of enrolling with a family member or friend, who received a brief automated telephone update regarding the participant's self‐reported health status each week, including information about the participant's hypertension self‐care and how that caregiver could help the participant self‐manage more effectively. The intervention focused mainly on providing information and self‐management education to participants. At the onset, participants were given an electronic home blood pressure monitor and were instructed how to measure their blood pressure and keep a written record of the results. Whenever possible, an automated phone call was placed during enrolment to familiarise the participant with the call content and how to respond using their touch‐tone phone.The telecommunications infrastructure for the automated calls was maintained on a US server and interfaced with local telephone systems via session initiation protocol (SIP) lines and VoIP technology Participants in the control group received usual care and information | |
| Outcomes | Blood pressure (primary); health status; depression, satisfaction, medication‐related problems (secondary) | |
| Funding | University of Michigan (UM), OMRON TM | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | ClinicalTrials.gov Identifier: NCT01484782 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completing informed consent, participants were randomised to the intervention or usual care group based on a computer‐generated series of numbers that ensured balance between experimental groups within each country" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Given the nature of the intervention, it was not possible to blind patients or their clinicians to their experimental assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "Baseline characteristics were similar for intervention and control patients in the analytic sample. However, intervention and control patients differed at baseline in the percentage reporting use of antihypertensive medication. This variable was included as an additional control for confounding in multivariate models." Unclear whether this has introduced bias. |
| Methods | Aims: to examine the effects of a totally automated physical activity counselling system on self‐reported physical activity among sedentary adults Study design: RCT; recruitment: primary care (mail and telephone) Study duration: 6 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: > 25 years; sedentary (otherwise healthy individuals) with a sub‐optimal diet; not engaged in regular moderate to vigorous intensity physical activity Sample size: 298; mean age: 46 years; sex: men ‐ 28%, women ‐ 72%; ethnicity: white ‐ 45%; black ‐ 45%; other ‐ 10% Country: USA | |
| Interventions | The telephone‐linked communication‐physical activity promoted moderate‐intensity physical activity based on the transtheoretical model of behaviour change and social cognitive theory. At the beginning of each conversation, the system inquired about the user's current level of moderate‐intensity‐physical activity, defined as the number of days during the previous week the person engaged in such activities and the average number of minutes per day. The system also asks users to enter the value of a daily pedometer reading taken the day before the call. For users not yet engaging in any moderate‐intensity physical activity, the system assesses their intention to do so, to determine their motivational readiness. For users who engage in moderate‐intensity‐physical activity, the system determines whether they are at the goal level, as defined by CDC and the American College of Sports Medicine guidelines Participants in the control group (TLC‐Eat) received an automated intervention promoting healthy eating, which was also delivered via telephone | |
| Outcomes | Energy expenditure; proportion of participants who met recommendations for moderate‐vigorous intensity physical activity; motivational readiness for physical activity (all primary) | |
| Funding | National Heart, Lung and Blood Institute (HL55664) and the Harvard Pilgrim Health Care Foundation | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison of 2 similar ATCS interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the home visit, we obtained informed consent, randomised participants to one of the study arms, and trained them to use the TLC system." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. Quote: "Secondary analyses were performed using multiple imputation to account for the potential impact of subject dropout" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were similar across all baseline characteristics |
| Methods | Aims: to test the effectiveness of different systems of reminding patients about their appointments in order to reduce the rate of failed attendance Study design: RCT; recruitment: primary care (organisational referral) Study duration: 6 weeks; study type: either; subtype: appointment reminder | |
| Participants | Inclusion criteria: participants with dental appointments Sample size: 1000; mean age:* ; sex: men ‐ 33%, women ‐ 67%; ethnicity:* Country: UK | |
| Interventions | Automated telephone call Automated telephone + postal reminders + manual telephone Manual telephone call Postal reminder No reminder (controls) | |
| Outcomes | Appointment non‐attendance (primary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | For a 5% difference in response rate between intervention and control, with a significant level of 0.05, 500 participants per group were required | |
| Notes | This is a comparison between the automated telephone call arm and control. All reminder methods provided a net cost saving to the practice during the operation of the study (4‐5 weeks). The savings were: postal, GBP 201; manual telephone, GBP 280; automated telephone, GBP 198; and automated telephone + postal reminders + manual telephone, GBP 296. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to assess the feasibility of replacing a live telephone follow‐up call to recently hospitalised smokers with an automated IVR system and test whether the system could be used to connect patients to postdischarge counselling Study design: RCT; recruitment: secondary care (in‐person at the end of inpatient counselling sessions) Study duration: 12 weeks; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: patients were eligible for enrolment if they were identified on admission as having smoked cigarettes in the past year, received bedside counselling from the Massachusetts General Hospital (MGH) Tobacco Treatment Service (TTS) during their hospital stay, were discharged to home, and had not been enrolled at a previous admission during the study period Sample size: 731; mean age: 52 years; sex: men ‐ 56%, women ‐ 44%; ethnicity:* Country: USA | |
| Interventions | IVR + call back (CB). Participants received a series of 4 calls from the IVR system, at 3, 7, 14, and 30 days after discharge. The day 7 and day 30 calls were cancelled if the participant had indicated in a previous call that he or she did not want to stop smoking but the day 14 call was always made to assess smoking status outcomes. In addition to the assessment made for the other groups, participants in this group were offered a CB from a counsellor ("Would you like to have your smoking cessation counsellor contact you to help create a quit plan or provide advice about medications?"). To focus counselling efforts on those most likely to benefit from them, CB offers were made only to those who either had not smoked in the past 7 days or wanted to quit within the next 2 weeks. CB was offered within 48 h, with counsellors making 3 attempts to call, and spent about 10 min addressing participant's concerns. Participants who did not respond to the IVR at Day 14 were called by staff. Participants in the control group received a call from the IVR system 14 days after discharge, at which smoking status ("Have you smoked a cigarette, even a puff, in the past 7 days?") and cessation medication use since discharge (nicotine replacement therapy, bupropion, and varenicline) were assessed. The IVR system made up to 8 attempts to reach a participant over 48 h. Participants who were not reached by the IVR system were called by a research assistant who attempted to complete the outcome assessment. | |
| Outcomes | Self‐reported abstinence rates; self‐reported cessation medication use (primary) | |
| Funding | National Heart Lung and Blood Institute | |
| Declaration of conflict of interest | "Dr. Rigotti has received research grant funding from Pfizer, Sanofi‐Aventis, and Nabi Biopharmaceuticals for the study of investigational and/or marketed smoking cessation products. She is an unpaid consultant for Pfizer and Free & Clear, Inc." | |
| Power calculations for sample size | NA | |
| Notes | This study compares IVR + call back, i.e. ATCS Plus with IVR only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised by the counsellor immediately after giving consent. Group assignment was stratified by tobacco counsellor in balanced blocks of 4 randomly ordered assignments." |
| Allocation concealment (selection bias) | Low risk | Quote: "Each counsellor carried a set of sealed, sequentially numbered manila envelopes, each containing an individual assignment, along with an information sheet for the patient describing the corresponding IVR call protocol." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "After obtaining consent, the counsellor randomised the patient by opening the next envelope and reviewing the information sheet with the patient. In this way, the counsellors remained blind to the group assignment until after the patient had been counselled and enrolled." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessment was conducted by the IVR system. Those who did not respond were contacted by the research assistant. However, it is unclear whether the research assistant was blinded. Quote: "Participants who were not reached by the IVR system were called by a research assistant who attempted to complete the outcome assessment" |
| Incomplete outcome data (attrition bias) | Low risk | Attrition was small (n=3 in each group). Missing outcome data balanced in numbers across groups. |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review have been reported |
| Other bias | Low risk | Quote: "There were no significant differences between the arms for age, sex, cigarettes/day before admission, intention to remain quit after discharge, or the percent admitted to a cardiac service." |
| Methods | Aims: to determine the feasibility and potential efficacy of an IVR monitoring and follow‐up system to support smoking cessation in smokers hospitalised with coronary heart disease Study design: RCT; recruitment: tertiary care (organisational referral) Study duration: 12 months; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: participants were current smokers (≥ 5 cigarettes per day) over the age of 18 years, hospitalised at UOHI (University of Ottawa Heart Institute) for acute coronary syndrome (ACS), elective PCI or diagnostic catheterisation related to coronary heart disease Sample size: 100; mean age: 54 years; sex: men ‐ 68%, women ‐ 32%; ethnicity:* Country: Canada | |
| Interventions | The IVR group received automated telephone follow‐up calls 3, 14, and 30 days after discharge inquiring about their smoking status and confidence in remaining smoke‐free. When deemed necessary, they were offered additional counselling. The IVR system posed a series of questions concerning current smoking status, confidence in staying smoke‐free over the time period until the next planned call, and the use of pharmacotherapy, self‐help materials and other forms of cessation support. If participants admitted that they had resumed smoking but wanted to make another quit attempt soon or indicated that their confidence in remaining smoke‐free was low (less than 7 on a 10‐point scale), the IVR system flagged the participant in the software interface in order to ensure that they would be contacted by the nurse‐specialist, who then provided additional assistance consisting of counsellor‐led telephone sessions. Telephone counselling consisted of up to three 20‐min telephone counselling sessions over an 8‐week period. For participants who had returned to smoking but wished to make another quit attempt, the nurse‐specialist provided encouragement, reviewed problems encountered during the initial quit attempt, and helped identify possible solutions. They also assisted participants to set a new quit date, make preparations for quitting, access pharmacotherapy (if necessary), and recruit social support. For participants who were not smoking but whose confidence in remaining smoke‐free was low, the nurse‐specialist provided encouragement and assisted them in identifying tempting situations that were undermining confidence. The nurse‐specialist and the participant then worked to develop strategies to deal with these situations using cue control, healthful alternatives, pharmacotherapy and/or social support. Participants in the control group received usual care. Usual care participants received no further treatment after discharge, but were free to avail themselves of the outpatient smoking cessation programme and any other community resources they chose to access. | |
| Outcomes | Self‐reported abstinence rate at 52 weeks (primary) | |
| Funding | Canadian Tobacco Control Research Initiative | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | Feasibility study; power calculation was not performed | |
| Notes | All participants received the same UOHI standard in‐hospital treatment, which consisted of: personalised advice to quit smoking; access to nicotine replacement therapy during hospitalisation (if necessary); brief bedside counselling with a nurse‐specialist; a self‐help guide; and the provision of information about the UOHI outpatient smoking cessation programme and other community programmes. This treatment is consistent with current clinical practice guidelines for hospitalised smokers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to either a usual care (UC) control group or an IVR experimental group. Group assignment was mediated through the Clinical Epidemiology Unit's data centre, using a computer generated randomisation list. The randomisation was made in blocks of six" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Quote: "Research staff were unaware of the treatment allocation prior to randomizations" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Loss to follow up was relatively low; it did not differ significantly between groups. There was no significant difference between the UC and IVR groups as to the proportion of participants completing follow‐up measures at 12 weeks (100% versus 96.0%) or 52 weeks (83.7% versus 86.0%). One patient in the UC group died during the follow‐up period and was not included in analysis". Comment: low attrition (n = 1), unlikely to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Comment: groups were similar across all baseline characteristics but education level (participants in the UC group were more likely to have completed some postsecondary education); however, it is unclear whether this has introduced bias |
| Methods | Aims: to determine if continuous abstinence from smoking would be higher 26 and 52 weeks after discharge in smokers who received interactive voice‐response (IVR) mediated telephone follow‐up and triage to nurse counselling compared to those receiving standard care Study design: RCT; recruitment: secondary care (*) Study duration: 12 months; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: smokers (≥ 5 cigarettes/day) aged ≥ 18, diagnosed with coronary heart disease, and recently hospitalised at the University of Ottawa Heart Institute (UOHI) Sample size: 440; mean age: * sex: * ethnicity: * Country: Canada | |
| Interventions | ATCS Plus: participants received automated telephone calls 3, 14, 30, 60, 90, 120, 150, and 180 days after discharge. The calls posed a series of questions concerning smoking status, confidence in staying smoke‐free, and use of cessation medications. If the participant identified that they had resumed smoking or indicated that their confidence in remaining smoke‐free was low, they were contacted by a nurse‐counsellor who provided additional assistance. Participants in the control group received usual care that included: in‐hospital nurse counselling; nicotine replacement therapy (NRT) during hospitalisation; and a recommendation for ongoing NRT following discharge. | |
| Outcomes | Self‐reported continuous abstinence, 26 and 52 weeks after hospital discharge (primary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "A total of 440 smokers (5 cigarettes/d) hospitalised with coronary heart disease at the University of Ottawa Heart Institute were randomised to either standard care (SC) or IVR" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to test the effectiveness of an automated telephone reminder intervention to improve adherence to medications to lower cholesterol among adults with cardiovascular disease in a large, diverse integrated healthcare system Study design: RCT; recruitment: other ‐ health system (organisational referral) Study duration: 3 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: participants aged 18 years and older identified from a cardiovascular disease case‐identification database. Participants had a prescription for a cholesterol‐lowering agent overdue for refill between 2 weeks and 6 weeks Sample size: 30,610; mean age: * sex: * ethnicity: * Country: USA | |
| Interventions | Automated telephone outreach: an automated telephone call instructs participants to order a refill for their overdue prescription by calling the number on their medication bottle or by using an online refill system Participants in the control group received usual care. | |
| Outcomes | Refill rate at 2 weeks | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Data extraction based on abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "participants were randomly assigned to either an automated telephone outreach or a control group (usual care)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to determine whether an intervention to sustain tobacco treatment after hospital discharge increases smoking cessation rates compared with standard care Study design: RCT; recruitment: secondary care (health professional referral) Study duration: 6 months; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: current smokers (smoked ≥1 cigarette/day during the month before admission), received smoking cessation counselling in the hospital, stated that they planned to try to quit smoking after discharge Sample size: 397; mean age: 53 years; sex: men ‐ 48%, women ‐ 52%; ethnicity: white, non‐Hispanic ‐ 81%, Hispanic ‐ 6%, black, non‐Hispanic ‐ 4%, other or unknown ‐ 4%, Native American ‐ 3%, Asian/Pacific Islander ‐ 2.5% Country: USA | |
| Interventions | Intervention group received extended care: provision of 3 months of free medication of the participant's choice at discharge (nicotine replacement, bupropion, or varenicline); 5 automated outbound IVR phone calls at 2, 14, 30, 60, and 90 days after discharge; advice and support messages that prompted smokers to stay quit, encouraged proper use and adherence to cessation medication, offered medication refills, and triaged smokers to a return telephone call from a live counsellor for additional support. The automated telephone script encouraged participants to request a callback from a counsellor if they had low confidence in their ability to stay quit, had resumed smoking but still wanted to quit, needed a medication refill, had problems with a medication, or had stopped using any medication. A trained counsellor made the return telephone calls using a standardised protocol. A fax sent to the primary care clinician of each participant informed him/her of the treatment programme Participants in the control group received usual care, which included advice to contact a free telephone quit line and use smoking cessation medication after discharge | |
| Outcomes | Biochemically confirmed tobacco abstinence at 6 months (primary); self‐reported tobacco abstinence; costs (secondary) | |
| Funding | RC1 HL099668 and K24 HL004440 from the National Institutes of Health/National Heart, Lung, and Blood Institute; the National Cancer Institute, the National Institute on Drug Abuse, and the National Institutes of Health Office of Behavioral and Social Science Research; 1IK2CX000918‐ 01A1 (Dr Japuntich) from the US Department of Veterans Affairs Clinical Sciences Research and Development Service | |
| Declaration of conflict of interest | Dr Rigotti reported being an unpaid consultant for Pfizer Inc and AlereWellbeing Inc regarding smoking cessation; receiving royalties from UpToDate for reviews on smoking cessation; and receiving reimbursement for travel expenses from Pfizer to attend a consultant meeting. Dr Levy reported being a paid consultant to CVS Inc to provide expertise on tobacco policy. Dr Park reported receiving a grant from Pfizer to provide free varenicline for use in a trial funded by the National Cancer Institute. Dr Singer reported being a paid consultant for Pfizer Inc on matters separate from smoking cessation. No other disclosures were reported | |
| Power calculations for sample size | A sample of 330 was planned to provide 83% power to detect a 15% difference (20% vs 35%) in the primary outcome. The sample was increased to 400 without interim analysis to add statistical power | |
| Notes | The incremental per‐participant costs in the intervention group were USD 540 (year 1) and USD 294 (subsequent years) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned (1:1) to sustained care or standard care in permuted blocks of 8, stratified by daily cigarette consumption (<10 vs .10) and admitting service (cardiac vs other)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment assignment was concealed in sequentially numbered sealed envelopes within each stratum. Research staff opened the next envelope corresponding to the participant's randomisation stratum." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The analyses were performed using an intent‐to‐treat approach" |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study's pre‐specified outcomes that are relevant to the review were reported in the pre‐specified way. |
| Other bias | Low risk | Comment: groups were similar across all baseline characteristics. |
| Methods | Aims: to test the efficacy of a novel, fully automated continuing care programme, Alcohol Therapeutic IVR Study design: RCT; recruitment: primary care and community (clinic referrals, public service announcements, and local advertising online and in print) Study duration: 12 months; study type: management; subtype: alcohol consumption | |
| Participants | Inclusion criteria: age 18 or older, diagnosis of current or lifetime DSM‐IV Alcohol Dependence, past 90 days' report of ≥ 1 drink and ≥ 1 symptom of Alcohol Abuse or Alcohol Dependence, and attendance at ≥ 8 outpatient CBT sessions Sample size: 158; mean age: 49 years; sex: men ‐ 53%, women ‐ 47%; ethnicity: * Country: USA | |
| Interventions | Alcohol Therapeutic IVR for 4 months. Participants were encouraged to call daily, but were not paid for calling. In the first month, participants who missed 2 consecutive Alcohol Therapeutic IVR calls received a single reminder phone call from an RA, who offered assistance with any technical difficulties and/or provided suggestions for remembering to call, as appropriate. In months 2–4, a reminder call was made if a participant missed 3 consecutive Alcohol Therapeutic IVR calls. There were 6 primary components to the Alcohol Therapeutic IVR: daily journal, targeted daily feedback, CBT skills encouragement, coping skills review, coping skills practice, and monthly personalised therapist message. Daily journal (compulsory) consisted of: 16 items that assessed mood states, craving, confidence in abstaining, number of risk situations, time with non‐users, sobriety support, substance free recreation, coping management, and use of coping skills. Participants were instructed to respond to items based on the previous calendar day. If a participant indicated alcohol or drug use, a follow‐up question for the current day's use was asked. If a participant reported current use and missed a previous day's call, they were asked to report on alcohol and drug use for that missed day and any previous missed days up to 1 week prior. If a participant's daily journal indicated alcohol or drug use, high craving, low confidence, and/or low coping levels, that report was 'red flagged' as indicating high risk. These participants received a feedback message Participants in the control group received usual care. | |
| Outcomes | Alcohol consumption (number of drinks per drinking day) (primary); participant perceptions of the system (secondary) | |
| Funding | National Institute on Alcohol Abuse and Alcoholism | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | The study was estimated to have power (1‐beta) = 0.80 using alpha = 0.05 to detect a moderate effect size (Cohen's d = 0.45) for primary analyses of all randomised participants. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the conclusion of CBT, participants returned to the research office for an assessment, and were randomised in a 1:1 allocation to either ATIVR or usual care. Randomization was stratified based on whether subjects had legal issues pending relating to their alcohol use. Within each stratum, a blocked randomisation was used to insure that an equal number of subjects were randomised to each of the two treatment conditions within each sequential block of 10 participants." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcomes were balanced in numbers across groups, but reasons for missing data were not provided. Quote: "There was no differential follow‐up rate across groups". |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Comment: groups were similar across all baseline characteristics but drinking days (the IVR group had nearly significantly more drinking days per week than control group at baseline (P = 0.08)); however, it is unclear whether this imbalance has introduced bias. |
| Methods | Aims: to provide an initial test of a totally automated, multi‐session treatment for problem drinkers in the community using a sophisticated IVR system with speech recognition Study design: RCT; recruitment: other (adverts in newspapers and on the Internet) Study duration: 6months; study type: management; subtype: alcohol consumption | |
| Participants | Inclusion criteria: problem drinkers Sample size: 47; mean age: 57 years; sex: men ‐ 60%, women ‐ 40%; ethnicity: Caucasian ‐ 83%, African‐American ‐ 13% Country: USA | |
| Interventions | The intervention group: Miller and Munoz's self‐help book, Controlling Your Drinking: Tools to Make Moderation Work for You (2005) was adapted into a computer‐controlled IVR system that incorporated Miller and Munoz's strategies while enhancing the motivational aspects of the programme; participants could receive up to 26 calls over 13 weeks Participants in the control group received an informational pamphlet in the mail. | |
| Outcomes | Number of heavy drinking days per month; percent days abstinent per month; drinks per drinking day | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no significant differences between groups at baseline on demographics or drinking variables." |
| Methods | Aims: to examine the effectiveness of 2 self management strategies (SMS) across outcomes corresponding to the chronic care model Study design: RCT; recruitment: primary care (organisational referral) Study duration: 12 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: adult with type 2 diabetes having suboptimal glycaemic control; a glycated haemoglobin value of 8% in the previous 12 months; ≥ 1 primary care visit in the previous 12 months; English‐, Spanish‐, or Cantonese‐speaking; did not have limited vision or were hearing‐impaired; and no diagnoses of psychotic illness or end‐stage renal disease Sample size: 339; mean age: 55 years; sex: men ‐ 41%, women ‐ 59%; ethnicity: white/Latino ‐ 47%, Asian ‐ 23%, African American ‐ 21%, white/non‐Latino ‐ 8%, other/unknown – 1% Country: USA | |
| Interventions | The IDEALL Automated Telephone Disease Management (ATDM): the ATDM system provides weekly calls with rotating queries in participants' native language for 9 months regarding: self‐care (e.g. symptoms, medication adherence, diet, physical activity, self‐monitoring of blood glucose, smoking), psychosocial issues (e.g. coping, depressive symptoms), referrals for preventive services (e.g. ophthalmologist). Each call took 6‐12 min to complete. Participants selected call times at enrolment and could alter preferred times or call the system toll free. Participants respond via touch‐tone commands. Depending on the response to an individual item, participants also receive automated health education messages in the form of narratives. Participants answering "out of range" on ≥ 1 item, based on predetermined clinical thresholds, receive a call back from a language concordant nurse care manager within 24 to 72 h. The care manager helps participants problem‐solve around the issue identified in the report or any other concerns, with a focus on collaborative goal setting with action plans. Support, education, and patient activation through monthly group medical visits with physician and health educator Usual care | |
| Outcomes | Self‐management behaviours (primary consisting of the 4 domains/sub‐scales: self‐monitoring of blood glucose and self‐monitoring of diabetic foot, diet and exercise); and behavioural, functional, and metabolic outcomes (secondary) | |
| Funding | The Commonwealth Fund, Agency for Healthcare Research and Quality, the California Endowment, the San Francisco Department of Public Health, the California Healthcare Foundation, National Institutes of Health | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | "We determined that 339 subjects would result in 100 subjects in each arm at the end of the study (n= 300), providing 80% power to detect a difference in diabetes self‐care of 0.49 days/week, using 2 tailed tests, of 0.05, and Bonferroni correction for three group comparisons. However, the study was not adequately powered to provide definitive answers regarding relative impacts across subgroups, such as those with limited English proficiency and limited literacy." | |
| Notes | This is a comparison between ATDM arm and UC. The annual cost of the ATSM intervention per QALY gained, relative to usual care, was USD 65,167 for start‐up and ongoing implementation costs combined, and USD 32,333 for ongoing implementation costs alone. In sensitivity analyses, costs per QALY ranged from USD 29,402 to USD 72,407. The per‐participant cost to achieve a 10% increase in the proportion of intervention participants meeting American Diabetes Association exercise guidelines was estimated to be USD 558 when all costs were considered and USD 277 when only ongoing costs were considered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated using stratified (on languages) blocked randomisation." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Because the study was not blinded and because the usual care group did not receive any additional SMS intervention, systematic inaccuracies in patient‐reported outcomes may have occurred due to recall bias or social desirability." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Analyses were conducted on an intent‐to‐treat basis." Comment: missing data have been imputed using appropriate methods. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "There were no statistically significant differences in baseline characteristics across arms" |
| Methods | Aims: to determine if IVR can improve medication adherence and reduce adverse events as patients transition from hospital to home among postoperative cardiac surgical patients Study design: RCT; recruitment: secondary care (organisational referral) Study duration: 6 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: adults who were discharged from the UOHI were considered for inclusion if they underwent coronary artery bypass grafts and/or valvular surgery, had telephone service to their home, and spoke either English or French Sample size: 331; mean age: 63 years; sex: * ethnicity: * Country: Canada | |
| Interventions | Automated telephone calls at a predetermined time for 6 months, with calls made at 1, 2, 3, 4, 6, 8, 10, 12, 16, 20 and 24 weeks after discharge. The IVR system recorded participants' voiced responses (yes or no) into a central database. Used an algorithm of 11 questions addressing medication adherence, reporting of adverse events, providing information on common medications, and offering general medication safety tips. The intent of the IVR algorithm was to provide early identification of issues permitting timely intervention, provide a mechanism for tracking medication adherence, and provide medication information at the time deemed most valuable by the participant at his or her request and to provide longer term follow‐up as the participant transitioned from hospital to home. If the participant responded "yes" to medicine adherence, the system provided a short description of the medication, including trade and generic names, desired effects and possible adverse effects. Participants could use the callback option from a nurse if they wish to discuss any concerns. Participants in the control group received usual care. | |
| Outcomes | Medication adherence and adverse events (composite primary outcome); emergency room visits and hospitalisations; medication adherence; patient satisfaction | |
| Funding | Canadian Patient Safety Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "A sample size of 166 patients per group was sufficient to detect the important difference of 16% in the primary outcome with an alpha‐value of 0.05 and power of 80% using the Fisher exact tests. A dropout rate of 10% was anticipated over the six‐month follow‐up period and, therefore, a sample size of 368 patients (184 per group) was needed to assess the important difference of 16% in the primary outcome." | |
| Notes | ClinicalTrials.gov Identifier: NCT01151800. All data were stored in the IVR system using a study identifier. The data were password protected and the drive was backed up daily for protection against data loss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "Randomization occurred once consent to participate was obtained." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to the treatment group was blinded by using a sealed envelope identified by study number and containing the random allocation." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment was not blinded. Quote: "The six month surveys were conducted by telephone interview by the research nurse coordinator who had intervened with the patients during the study" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Statistical analysis was conducted on an intention‐to‐treat basis." Comment: missing data have been imputed using appropriate methods. ITT analysis was used to include all participants who received the intervention or usual care in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol was available; and all of the study's pre‐specified outcomes that are relevant to the review have been reported |
| Other bias | Unclear risk | Quote: "There were no statistical differences in baseline characteristics between the 2 groups other than the variable of employment status, which showed a clinically insignificant yet statistically significant difference." Comment: unclear whether this has introduced bias. |
| Methods | Aims: to assess whether customised mobile phone reminders would improve adherence to therapy and thus decrease virological failure among HIV infected patients starting antiretroviral treatment Study design: RCT; recruitment: primary care (advert in clinic) Study duration: 24 months; study type: management; subtype: HIV | |
| Participants | Inclusion criteria: HIV infected individuals with adequate documentation of their HIV positive status, aged 18‐60 years, ART naive, and meeting the criteria for start of first‐line ART as per the 2007 Indian national guidelines Sample size: 631; mean age: inestimable; sex: men ‐ 57%, women ‐ 43%; ethnicity: Asian ‐100% Country: India | |
| Interventions | Multimodal intervention was a customised motivational voice call that went out once a week at a time selected by each participant. The participant also chose the sex and language of the pre‐recorded voice call. This automated call began with a greeting and the hope that the participant was feeling well, followed by an inquiry whether medications were taken as prescribed. The message was considered interactive or bidirectional, since it required the participant to respond to a question about the previous day's pill doses, by pressing '1' for yes or '2' for no. If the participant failed to respond to the call, a maximum of 3 more calls were made over the ensuing 24 h until a response was obtained. The second aspect of the intervention included a weekly non‐interactive neutral pictorial message sent out as a reminder 4 days after the automated call. Participants in this group also received usual care. Participants in the control group received usual care, which included up to 3 counselling sessions prior to initiation of ART, routine clinical and laboratory tests at baseline, and follow‐up assessments every 6 months. First line ART regimens included those based on zidovudine, stavudine, or tenofovir, along with lamivudine and either nevirapine or efavirenz, and were dispensed free of cost as generic fixed‐dose combination pills every 1‐3 months. | |
| Outcomes | Time to virological failure (primary); ART adherence measured by pill count; death rate; attrition rate (secondary) | |
| Funding | European Union, Framework Program 7 (No 222946) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | A total sample of 532 participants (266 in each arm) would provide 90% power to detect such a risk difference in a 2‐sided log‐rank test with significance level of 0.05. Expecting an attrition rate of 10%, the trial was planned to have a minimum of 600 participants | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed stratified for sex, in permuted blocks of four or six." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered opaque sealed envelopes were used as a method of allocation concealment." |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients and the randomisation team were aware of the intervention assignment; while research staff assessing patients, laboratory staff, statisticians, and authors were blind to the allocation." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients and the randomisation team were aware of the intervention assignment; while research staff assessing patients, laboratory staff, statisticians, and authors were blind to the allocation." |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. Quote: "Trial analysis was performed using an intention‐to‐treat principle that included all originally randomised patients" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review have been reported |
| Other bias | Low risk | Comment: groups were similar across all baseline characteristics |
| Methods | Aims: to evaluate the efficacy of automated telephone needs assessment coupled with social worker follow‐up in outpatients with advanced cancer who were receiving chemotherapy Study design: quasi‐RCT; recruitment: secondary care (in‐person at chemotherapy clinics or by letter with a follow‐up phone call) Study duration: 24 months; study type: management; subtype: cancer | |
| Participants | Inclusion criteria: had primary tumours of the breast, colon/rectum, or lung; had recurrent or metastatic disease or non‐resectable tumours; were receiving non‐adjuvant outpatient chemotherapy; were 21 years of age or older; and spoke English with sufficient fluency to validly respond to the automated surveys and the research interview Sample size: 239; mean age: 58 years; sex: men ‐ 50%, women ‐ 50%; ethnicity: white ‐ 89%, black‐ 6%, Hispanic ‐ 4%, other 1% Country: USA | |
| Interventions | Intervention group received 3 automated telephone surveys (surveys 1, 2, and 3), scheduled approximately 6 weeks apart. The system was configured to: call participants at times they designated as convenient; conduct needs assessment surveys with them in a high‐quality, natural sounding, digitally stored voice; reliably interpret, confirm, and register their verbal answers to 12 questions; and identify participants who reported unmet need(s) so that they could receive prompt follow‐up by a social worker. Outcome was to be assessed in a final comprehensive needs assessment through an interview held with a social worker 6 weeks after the participant's completion of the automated surveys + the approximately hour‐long research interview by an experienced clinician. Participants in the control group completed the research interview for the comprehensive needs assessment within 2 weeks + the approximately hour‐long research interview by the experienced clinician. | |
| Outcomes | The prevalence of unmet needs | |
| Funding | National Cancer Institute (CA 41012) | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "To simplify field operations, blocks of time were randomly assigned as periods of accrual for either the experimental or control group; each eligible patient was assigned to the experimental or control group based on the block of time during which the patient was identified." Comment: non‐random assignment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "In the experimental group, the interviewer was never the same social worker who worked with the patient during the intervention. This was done to avoid any bias that might be associated with interviewer's knowledge of the patient's intervention history" Comment: insufficient information to judge whether assessors were blinded |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate. Quote: "Of the 266 patients accrued into the experimental group, 109 (41%) completed both the series of automated surveys and the final assessment interview within the study period, and 157 (59%) did not". |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "The control and experimental groups did not differ significantly with respect to almost all sociodemographic characteristics. However, patients in the experimental group were somewhat older than patients in the control group (mean age 60 versus 57 years)." Comment: groups were similar across all baseline characteristics. It is unlikely that the small age difference has introduced bias. |
| Methods | Aims: to test 2 multimodal interventions for multiple symptoms experienced by patients with multiple cancer sites Study design: RCT; recruitment: primary care (organisational referral) Study duration: 10 weeks; study type: management; subtype: cancer | |
| Participants | Inclusion criteria: aged 21 years and above, having a diagnosis of a solid tumour cancer or non‐Hodgkin's lymphoma, undergoing a course of chemotherapy, speak and read English, and having a touch‐tone telephone Sample size: 437; mean age: 57 years; sex: men ‐ 26%, women ‐ 74%; ethnicity:* Country: USA | |
| Interventions | Automated telephone symptom management (ATSM): prerecorded pleasant female voice queried participants about severity of 17 symptoms: fatigue, pain, dyspnoea, insomnia, distress, nausea, fever, difficulty remembering, lack of appetite, dry mouth, vomiting, numbness and tingling, diarrhoea, cough, constipation, weakness, and alopecia. If they report severity in ≥ 4 symptoms, then the call directed them to the relevant part in the symptom management guide (SMG) for strategies to manage those symptoms. Participants advised to call the oncology office if they report severity of ≥ 7 symptoms or if there was no improvement. On subsequent calls, in participants with severity of ≥ 4 symptoms in the previous calls, ATSM enquired if the participants tried the strategies suggested in the SMG and whether it helped in lowering the severity. Numerical prompts were used so participants could respond using their telephone keypad. When all symptoms above threshold at the previous contact were evaluated, the system then reviewed the current severity of all symptoms. Calls by specially trained nurses | |
| Outcomes | Symptom severity | |
| Funding | National Cancer Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "The trial was powered to detect an effect size of 0.37 for group differences on symptom severity at 10 weeks." | |
| Notes | Both total fixed and variable costs were greater for the nurse arm; total costs per participant were USD 69 and USD 167 for the ATSM and nurse arm respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[Participants] were randomized into either the NASM or the ATSM using a computer minimisation program that balanced the arms with respect to recruitment location and site of cancer". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across groups. ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "A total of 13 patients (10 in the ATSM and 3 in the NASM) did not complete any of the intervention contacts, but had 10‐week interviews. These patients were included in the intention‐to‐treat analysis of interview data" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "Most measures including symptom severity were equivalent at baseline." Comment: groups were similar across all baseline characteristics |
| Methods | Aims: to test the effectiveness of automated telephone outreach with speech recognition to improve rates of screening for colorectal cancer. The hypothesis is that the intervention improves rates of screening overall and specifically rates of colonoscopy. Study design: RCT; recruitment: primary care (organisational referral) Study duration: 12 months; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: aged 50‐64 at baseline and continuous enrolment in health plan Sample size: 20,936; mean age: 57 years; sex: men ‐ 47%, women ‐ 53%; ethnicity: white ‐ 86%, other – 9%, black ‐ 5% Country: USA | |
| Interventions | Automated telephone outreach (ATO) calls followed a script and branching algorithm that was informed by a theoretical framework, with the aim to educate the participants about the risk of colorectal cancer and about the importance and methods of screening, and to encourage them to contact their primary care providers to arrange for colorectal cancer screening. The calls used speech recognition technology and delivered the message with prerecorded human conversation either to the participant directly, or to another member who would then convey it to the intended participant. When unreachable, the system leaves a message and asks participants to call back. Participants in the control group received usual care | |
| Outcomes | Colorectal cancer screening including faecal occult blood testing, double‐contrast barium enema, flexible sigmoidoscopy, or colonoscopy within 12 months following the intervention (primary); screening by colonoscopy during the 12‐month period following the intervention (secondary) | |
| Funding | Harvard Pilgrim Health Care Foundation | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | The ATO calls verified participants identify and only after securing their permission did it proceed with the interaction regarding colorectal cancer screening. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly allocated to intervention and usual care arms, using a computerized random‐number generator" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The study was not blinded" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Groups were similar at baseline. Quote: "There were no baseline differences between the two study groups on any of the measured variables." |
| Methods | Aims: to assess the effects of automated telephone outreach with speech recognition (ATO‐SR) on rates of testing for retinopathy, glycaemia, hyperlipidaemia, and nephropathy in a diverse population of privately insured patients with diabetes. Study design: RCT; recruitment: primary care (organisational referral) Study duration:12 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: individuals with no insurance claim for a dilated eye examination in the prior year and no claim for ≥ 1 of the following tests: glycated haemoglobin, low‐density lipoproteins, or microalbumin. Sample size: 1200; mean age: 51 years; sex: men ‐ 62%, women ‐ 38%; ethnicity: other – 95%, black ‐ 5% Country: USA | |
| Interventions | ATO‐SR: the computerised system placed 3 calls to the participants' home telephone numbers, encouraging the participants to fulfil recommended testing if it had not been performed in the preceding year. The system offered a live telephone call back to assist in scheduling tests and also offered to send participants the following items: a voucher that would allow the provider to waive the co‐payment for a dilated eye examination; an educational nutrition video; a cookbook; or a pill box. For each of the 3 intervention calls, the automated telephone system made up to 6 attempts to reach the participant, leaving up to 2 messages requesting a call back. The system used speech recognition to respond to participants with segments of recorded text spoken with a human voice Participants in the control group received usual care (no intervention). | |
| Outcomes | Retinopathy examination (primary); tests for glycaemia, hyperlipidaemia, and nephropathy (secondary) | |
| Funding | American Diabetes Association, Agency for Healthcare Research and Quality, National Institute of Diabetes and Digestive and Kidney Diseases | |
| Declaration of conflict of interest | "No potential conflicts of interest relevant to this article were reported" | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis was used to include all participants who received the intervention or usual care in the analysis. Quote: "The main analyses included all subjects in the groups to which they were randomised" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "Compared with the usual care group, the intervention group was younger (50 vs. 52 years, P 0.02) and had a greater proportion of men (64 vs.41%, P 0.04); the groups were comparable on other socio‐demographic measures and clinical indicators" Comment: groups were similar across all baseline characteristics but age and sex; however, it is unclear whether this imbalance has introduced bias. |
| Methods | Aims: to evaluate compliance with 2 IVR monitoring protocols, subjective experiences with monitoring, and change in symptoms associated with monitoring (i.e. measurement reactivity). Study design: RCT; recruitment: primary care (advert in clinic) Study duration: 4 weeks; study type: management; subtype: alcohol consumption | |
| Participants | Inclusion criteria: all participants who had consumed alcohol in the prior 28 days, met diagnostic criteria for an alcohol use disorder (APA, 1994), and indicated an intention to abstain from alcohol and other drug use over the coming month Sample size: 98; mean age: 46 years; sex: men ‐ 91%, women ‐ 9%; ethnicity: non‐Hispanic white ‐ 45%, African American ‐ 40%, Native American ‐ 7%, other ‐ 6%, Hispanic ‐ 2% Country: USA | |
| Interventions | Daily IVR. Participants called a pre‐recorded IVR system daily using a toll‐free telephone number. A monitoring protocol to assess participant's alcohol substance use behaviour was used and they responded using an 8‐point response option (0–7 on the telephone key pad) in order to use 9 as a skip option and to reduce confusion for participants (i.e. omitting 8 as an option and not requiring an extra key stroke after each entry to signal the end of an entry). IVR system automatically tracked compliance with the monitoring protocol. When participants failed to call the system as scheduled the study coordinator attempted to contact participants within 2 working days in order to reconstruct the data from missed calls verbally and to resolve any difficulties. If participants indicated clinical deterioration during follow‐up calls, they were encouraged by the study coordinator to contact their clinical provider and were given the appropriate phone numbers to facilitate this. Participants in the IVR monitoring conditions received instruction on how to call into the IVR system and completed a practice call to familiarise themselves with the procedures. Participants received a "cheat sheet" that included the toll free number, the study coordinator's telephone number, their study ID number, and a list of the monitoring questions and response options. They also received incentives for each call that they made. At the end of each call, the IVR system informed the participants of the amount of money accumulated in their accounts. They could use the # key to repeat a question and the * key to back up to the previous question Weekly IVR calls No calls (controls) | |
| Outcomes | Drinking habits; alcohol craving; PTSD symptoms (all primary) | |
| Funding | University of Washington Alcohol and Drug Abuse Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between daily IVR versus no call | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "At the end of the baseline assessment participants were randomly assigned to one of three conditions" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing data have been imputed using appropriate methods. Quote: "Missing data on multi‐item scales were handled in the following ways: mean scores for the PACS were imputed for the two cases where one item was missing, and scores for the PCL‐C were generated with no mean imputation when ≥ 16 of the 17 items were completed; scores were not produced for three participants who were missing more than 1 item. No other missing data imputation techniques were used." |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | Comment: groups were similar across all baseline characteristics |
| Methods | Aims: to test the effectiveness of an intervention to improve care in patients at‐risk of osteoporosis Study design: RCT; recruitment: primary care (organisational referral) Study duration: 10 months; study type: prevention; subtype: screening | |
| Participants | Inclusion criteria: women 65 years of age and over; women and men 45 and older with a prior fracture of the hip, spine, forearm, or humerus; and women and men 45 and older who had used oral glucocorticoids for ≥ 90 days Sample size: 1973 participants; mean age: 69 years; sex: men ‐ 8%, women ‐ 92%; ethnicity: * Country: USA | |
| Interventions | Participants in the multimodal intervention group received education + an introductory letter from Horizon Blue Cross Blue Shield of New Jersey and then an automated telephone call from the insurer inviting them to undergo bone mineral density testing. This call employed IVR technology that has been used for other screening tests. Such automated calling provides tailored education through a branching logic algorithm. For example, people who had never had a bone mineral density test but expressed an interest were offered specific encouragement, "It's great that you plan on having a bone density test; the best way to tell if a person is at risk for osteoporosis is to have a bone density test. The test only takes about 5 minutes, you don't have to take off your clothes, and it's painless." At the conclusion of the educational call, participants were able to transfer directly to a centralised radiology service to schedule a bone mineral density test. Participants in the control group received no intervention. | |
| Outcomes | Either undergoing a bone mineral density testing or filling a prescription for a bone active medication | |
| Funding | Merck and Co., Inc.; NIH (AR48616, AG027066), the Arthritis Foundation, and the Engalitcheff Arthritis Outcomes Initiative | |
| Declaration of conflict of interest | Drs Weiss and Chen are both employees of Merck and Co., Inc | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "We conducted a randomised controlled trial among primary care physicians and their at‐risk patients" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Comment: missing data have been imputed using appropriate methods. An ITT analysis was used to include all participants who received the intervention or no intervention in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Between‐group differences at baseline were adjusted for as covariates. There is insufficient evidence that these differences have introduced bias. |
| Methods | Aims: to investigate the effectiveness of an automated telemedicine intervention to improve adherence to continuous positive airway pressure (CPAP) Study design: RCT; recruitment: primary care (home visit) Study duration:12 months; study type: management; subtype: obstructive sleep apnoea syndrome (OSAS) | |
| Participants | Inclusion criteria: aged 18 to 80 years with a physician diagnosis of OSAS and with polysomnography demonstrating an apnoea‐hypopnoea index (AHI) >10 Sample size: 250; mean age: 55 years; sex: men ‐ 82%, women ‐ 18%; ethnicity: * Country: USA | |
| Interventions | Telephone‐linked communications for CPAP (TLC‐CPAP): content includes assessment of the participant's perceptions about and experiences with OSAS and CPAP therapy and the participant's reported CPAP use (h per night and nights per week) during the week preceding each call; assessment of the participant's goals with regard to OSAS therapy; and feedback and counselling to enhance motivation to use CPAP and address barriers and poor self‐efficacy. A side effect management module addressing mucocutaneous side effects, air leaks and mask discomfort was developed and incorporated in the dialogues as appropriate. Participants in the control group received attention placebo: general health education via a TLC system. This system provides general information about a variety of health topics via telephone calls delivered on the same schedule as the TLC‐CPAP calls made by the intervention group. At each call, participants selected a topic from a list of 61 content areas that included common symptoms, medical conditions and preventive medicine topics | |
| Outcomes | CPAP use (primary); sleep symptoms checklist; functional outcomes of sleep questionnaire; depression (secondary) | |
| Funding | Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service | |
| Declaration of conflict of interest | MA is a paid employee of Philips/Respironics Inc and is a stockholder of Philips stock | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was stratified by sex, age and AHI using a randomised block design to ensure balance of these factors in the treatment arms." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Study personnel was blinded. Quote: "All data were collected by research assistants blind to group assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data balanced in numbers across groups; however, reasons for missing data are not provided. Quote: "CPAP adherence data were available from either the 6‐ or 12‐month follow‐up visit in 93.6% of subjects (figure 1), who were therefore included in the primary analysis" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | Quote: "The baseline characteristics of the intervention and control groups were similar." |
| Methods | Aims: to investigate the effectiveness of an automated telemedicine intervention that provides real‐time guidance and monitoring of resistance training in the home Study design: RCT; recruitment: primary care (*) Study duration: 12 months; study type: prevention; subtype: physical activity | |
| Participants | Inclusion criteria: no angina pectoris (unless symptomatically resolved post‐revascularisation), no history of myocardial infarction within 6 months or remote (> 6 months) myocardial infarction with current myocardial ischaemia on exercise stress test, no history of ventricular dysrhythmia requiring therapy, baseline systolic blood pressure smaller than 165 mmHg and/or diastolic blood pressure smaller than 100 mmHg, and not currently participating in a regular exercise programme less than once a week for 20 min per session Sample size: 103; mean age: 71 years; sex: men ‐ 69 %; women ‐ 31 %; ethnicity: * Country: USA | |
| Interventions | Telephone‐Linked Computer‐based Long‐term Interactive Fitness Trainer (TLC‐LIFT) system called participants, with a target exercise schedule of 3 days per week. At the initiation visit, users indicated what their preferred time to exercise was, and this was the time that TLC‐LIFT was scheduled to call. The TLC‐LIFT system is security enabled, so at the beginning of a call, each participant was asked to enter a personal password (PIN) to ensure security and confidentiality. Following the identification confirmation, TLC‐LIFT asked the participant if he/she was ready to perform his/her exercises. If the participant was not ready, he/she was asked to call a toll‐free number when ready, which informed TLC‐LIFT to call the person shortly thereafter to begin the exercise session. If the person failed to call back within 4 h of TLC's call, calls were repeated periodically during a time period previously set by the user. After a 24‐hour period had elapsed without the user completing a scheduled exercise session, the TLC system administrator was notified automatically and informed a staff member so that he or she could contact the user. Participants in the control group received attention: general health education via a TLC system at weekly intervals. This system provides general information about a variety of health topics via telephone calls. At each call, participants selected a topic from a list of content areas that included common symptoms, medical conditions, and preventive medicine topics. The health information dialogues were adapted from Harvard Health Letter articles (http://www.health.harvard.edu). The dialogs were developed to allow users to identify subtopics about which they wanted more information, and to skip others, and avoided long stretches of uninterrupted talking by the system | |
| Outcomes | Muscle strength; balance; walk distance; mood (all primary) | |
| Funding | Rehabilitation Research and Development Service of the Department of Veterans Affairs, the Boston Claude D. Pepper Older Americans Independence Centre, and the US Department of Agriculture | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | Study sample of 100 evaluable participants, approximately equally divided between intervention and control groups, provided 99.9% power to detect the smaller of these effects at a (2) = 0.05, and 80% power to detect a more conservative effect of 0.57 SD | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After eligible participants gave written informed consent, we collected baseline study data and then randomised them to one of two groups using a computer‐based algorithm (randomize function in Visual Basic) to perform random assignment without blocking or stratification." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Data for analyses were collected during four clinic‐based examinations (baseline, 3, 6, and 12 months), conducted by research assistants blind to group assignment." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Analyses were performed by intention to treat, using all outcome data collected regardless of adherence to assigned treatment" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "The intervention and control groups were similar on baseline characteristics except for 6‐minute walk (p=.02; Table 2)." Comment: groups were similar across all baseline characteristics but 6‐minute walk; however, it is unclear whether this imbalance has introduced bias |
| Methods | Aims: to examine Automated Voice Response (AVR) to manage symptoms and adherence to oral agents Study design: RCT; recruitment: tertiary care (*) Study duration: 10 weeks; study type: management; subtype: cancer | |
| Participants | Inclusion criteria: 21 years or older, having a solid tumour cancer; diagnosis, and being on non‐hormonal oral agents; understood English; having a touch‐tone phone and no hearing deficits that interfered with using a telephone; having no cognitive deficits; willing to complete phone contacts; and not being diagnosed with an emotional or psychological disorder Sample size: 119; mean age: 60 years; sex: men ‐ 31 %; women ‐ 69 %; ethnicity: white ‐ 76%, black ‐ 7%, other ‐ 17 % Country: USA | |
| Interventions | AVR system + symptom management toolkit (SMT) + nurse strategies to manage unresolved symptoms and improve adherence. In addition to the AVR calls, participants with ≥ 1 symptoms rated at a 4 or greater or non‐adherence defined as less than 80% during the immediate past 7‐day period received a brief telephone call from the nurse to deliver strategies to assist participants to manage symptoms and/or improve their adherence. Participants were called weekly until symptom severity fell below 4 or until adherent SMT + AVR phone system alone. Participants in this arm received calls from the AVR system; symptoms were assessed, and those reporting severity at a 4 or higher on a 0‐10 scale for any symptom were referred to the SMT for self‐management of symptoms. Adherence to oral agents was identified via participant report (no nurse was involved) AVR + SMT + nurse strategies to improve adherence alone. In group 3, in addition to the AVR calls, participants received brief calls from a nurse when the adherence rate was less than 100% to improve their adherence. Participants were called weekly until adherent. | |
| Outcomes | Adherence to medications; symptom severity (both primary) | |
| Funding | GlaxoSmithKline; Mary Margaret Walther Behavioural Oncology Group and the State of Michigan Nurse Corp | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | Study was powered to detect a medium effect size of 0.50 for pairwise differences between groups on symptom severity and adherence | |
| Notes | This is a comparison between AVR + SMT + nurse strategies to manage unresolved symptoms and improve adherence and SMT + AVR alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "After completion of the baseline interview, patients were randomised into the groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates with reasons were provided; attrition was balanced across the groups. |
| Selective reporting (reporting bias) | High risk | No outcomes reported on depression scores at the study's completion |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to assess the impact of a behaviour change programme to increase statin adherence using IVR technology Study design: RCT; recruitment: other ‐ health benefit company (organisational referral) Study duration: 6 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: continuously enrolled in the plan with a pharmacy benefit for a minimum of 12 months prior to the date of the index statin; no pharmacy claims evidence of any lipid‐lowering agent in the 6‐month period prior to the index statin; 21 years of age or older; and a statin prescription with a 30‐day supply. Sample size: 497; mean age: 54 years; sex: men ‐ 38%, women ‐ 62%; ethnicity:* Country: USA | |
| Interventions | Intervention group: automated calls were generated by a computerised voice activated technology (VAT) that provided highly tailored messages that specifically reinforced adherence, persistence with statin medication by using a combination of behavioural science theories and techniques in a personalised or tailored manner dependent on the participant's previous response characteristics. 6 calls were attempted over a period of 10 days. If an answering machine or another member of the household was reached, the participant was asked to call back at a toll‐free number. If the targeted participant was reached, and the calls went ahead, then a verbal informed consent was read. The subsequent calls referred respondents to the health plan website for additional information regarding dyslipidaemia, risk reduction, and lipid‐lowering medication. These calls were coupled with a print guide (mailed at the conclusion of the first call) that provided tailored messages designed to enhance commitment, improve communication with the health care team, and address specific barriers to adherence. Participants in the control group received enhanced care, which included non‐tailored behavioural advice from a single IVR call, coupled with a non‐tailored, generic, self‐help cholesterol management guide received through the mail. This guide provided educational material on cholesterol and lipid values, a brief knowledge quiz, and a non‐tailored action plan. | |
| Outcomes | Medication (statins) adherence (measured with 6‐month point prevalence persistency) | |
| Funding | NA | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | "it was anticipated that control group would have a 6‐month point prevalence rate of 65%, and that exposure to the experimental intervention would increase this rate to 75%.With power set at 0.80 and alpha at <0.05 (1‐sided test), it was necessary to impanel 260 participants per group. To account for 10% disenrollment over the 6‐month follow‐up period, approximately 290 participants per group were enrolled." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "the IVR system randomly assigned subjects to either the experimental or the enhanced care control group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "With the exception of the item assessing the number of chronic medications in the 3‐month period prior to the index statin (participants assigned to the experimental group had a lower number of concomitant medications), no statistically significant group differences were detected between the groups." Comment: groups were similar across all baseline characteristics but the number of chronic medications; however, it is unclear whether this has introduced bias. |
| Methods | Aims: to evaluate the effectiveness of computer‐generated telephoned reminders used to raise the rates of on‐time immunisation among preschool‐age children in 2 public clinics in Atlanta, GA Study design: RCT; recruitment: primary care (health professional referral) Study duration: 1 month; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: children due to receive diphtheria‐tetanus‐pertussis, poliovirus, or measles, mumps and rubella vaccines during the study's 6‐week enrolment period in February and March 1990 Sample size: 229; mean age: 9 months; sex: boys ‐ 52%, girls ‐ 48%;ethnicity: black ‐ 91%, other – 6%, Hispanic ‐ 3% Country: USA | |
| Interventions | Automated telephone reminder from the Fulton County Health Department. The text of the standard message, which was delivered in a normal human voice, was: "This is the Fulton County Health Department calling to remind you that your child is due for an immunisation or 'shot' this month. Please call the health centre for an appointment or bring your child in to the health centre any day this week, Monday through Friday, between 8:30 am and 4 pm. Immunisations are important to protect your child from certain diseases, such as whooping cough, measles, and polio. They are also required for day care or school attendance." Calls were made during 5 days, beginning the day before the child became due for his or her immunisation. A maximum of 9 attempts (not counting wrong numbers, non‐working numbers, or mis‐dials) were made to each child's home, until an answer was obtained; ≥ 5 of the calls were made between 6 and 9 pm. Calls not answered, responses by an answering machine (for which no reminder message was left), hang‐ups within 10 seconds, and busy signals were classified as missed attempts. Participants in the control group received no calls. | |
| Outcomes | Immunisation status | |
| Funding | CDC, Atlanta, Ga, and Cooperative Agreement TS‐622 from the Association for Teachers of Preventive Medicine, Washington, DC | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "Of the 229 children who met the eligibility criteria for entry into the study, 6 were lost to follow‐up (that is, clinic records could not be located after their follow‐up period), and 1 was deferred from receiving further vaccinations, pending medical evaluation." Comment: attrition was small (n = 7) and reasons for attrition were provided; however, it is unclear whether the attrition was similar across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Comment: groups were similar across all baseline characteristics |
| Methods | Aims: to explore the use of an innovative IVR system to increase participant adherence with antidepressant medication prescribed in primary care settings Study design: cluster RCT; recruitment: primary care practices (*) Study duration: 12 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: ≥ 18 years old, able to read English, not currently taking an antidepressant medication; newly prescribed an antidepressant medication by their primary care provider; access to a touch‐tone telephone; and willingness to participate in the study. Sample size: 647; mean age: *; sex: *; ethnicity:* Country: USA | |
| Interventions | Education: treatment team education and participant self‐care education Education + call: as above + 1 office nurse telephone call within 2 days of the visit when the antidepressant medication was prescribed. Education + call + IVR: as above + an IVR programme lasting for 3 months. A script was written for each of the IVR calls. In addition, the answer to each question generated a set of choices for the participant to respond to using a touch‐tone phone. | |
| Outcomes | Adherence to (antidepressant) medication (primary); satisfaction (secondary) | |
| Funding | Eli Lilly & Company | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between education + call versus education + call +IVR. Cluster RCT with 30 primary care study sites as the unit of randomisation. Note that analysis did not appear to adjust for clustering, therefore a unit of analysis error exists that may result in overly precise effect estimates for this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was randomised controlled clinical trial of 647 patients" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "all patients a given site received 1 of 3 randomly assigned treatment strategies" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | High attrition in the intervention group. No description of drop‐outs in the control group. Quote: "Of the 232 assigned to the IVR, 116 (50%) either never used the system or stopped using it before the 12‐week IVR program was completed" |
| Selective reporting (reporting bias) | High risk | The authors mentioned that there were no significant differences in medication adherence among the 3 groups. However, the analysis was restricted to 1 sub‐group of participants who completed the IVR calls. |
| Other bias | High risk | No baseline characteristics were provided. It was not possible to assess the possibility of selective recruitment of cluster participants based on the information reported. |
| Methods | Aims: to measure the effect of telephone‐based reminder/recall on immunisation and well‐child care (WCC) visit rates among adolescents in urban practices Study design: RCT; recruitment: primary care (organisational referral) Study duration: 18 months; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: subjects with a birth date between 1 June 1983, and 31 May 1987 (aged 11‐14 years at the start of the intervention) Sample size: 3006; mean age:* sex: boys ‐ 50%, girls ‐ 50%; ethnicity: other or unknown – 41%, black non‐Hispanic ‐ 35%, white non‐Hispanic ‐ 17%, Hispanic ‐ 7% Country: USA | |
| Interventions | Automated telephone message reminder system (Autodialer). The intervention mimicked an appointment‐scheduling module that is linked to a telephone‐reminder system. Adolescents were called if they were due for an annual WCC visit, a tetanus booster (5 years since diphtheria and tetanus toxoids and pertussis vaccination), or a hepatitis B vaccination according to Advisory Committee on Immunisation Practices guidelines. A variable number of calls was placed depending on the need for immunisations or WCC visits and prior response to reminder calls. The calls were voice recordings in English to request a vaccination appointment or WCC visit or to remind families of upcoming scheduled appointments. Calls were made 6 days per week during the day or early evening. During the initial 11 months of the 18‐month clinical trial, telephone calls were stopped if recipients indicated from a telephone menu option that the telephone number was incorrect, the adolescent had left the practice, the parent requested calls to be stopped, or no appointment was scheduled despite 5 calls placed within 30 days ('unresponsive numbers'). After 11 months, the Autodialer telephone reminder calls were restarted for those participants with 'unresponsive numbers' to give families a second opportunity to respond to subsequent reminders. Participants in the control group received usual care. | |
| Outcomes | Immunisation status | |
| Funding | CDC and Association for Teachers of Preventive Medicine, Washington, DC | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "To detect a 10% improvement in baseline immunisation rates of 50% (power of 0.80; =.05) within each practice required more than 750 adolescents per practice." | |
| Notes | The study design stratified for age group (11‐12 years and 13‐14 years) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[Participants were] randomly allocated into a study group (n=1496) or control group (n=1510) using a random‐number generator with the child as the unit of randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "Health care professionals were unaware of group allocation for specific subjects because the intervention used research personnel and reminders from a central office." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Health care professionals were unaware of group allocation for specific participants because the intervention used research personnel and reminders from a central office" |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Blinded medical record reviews at the end of the study using a standardized medical record review form." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Intention‐to‐treat analyses were performed for the 1496 study and 1510 control subjects" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "Study and control groups were similar with respect to age group, sex, practice, insurance, and race/ethnicity" |
| Methods | Aims: to assess the impact of a managed care‐based patient reminder/recall system on immunisation rates and preventive care visits among low‐income adolescents Study design: RCT; recruitment: primary care (organisational referral) Study duration: 12 months; study type: prevention; subtype: immunisation | |
| Participants | Inclusion criteria: adolescents aged 10.5 through 17 years enrolled in Monroe Plan on 31 December 2009, with a primary care provider in a participating practice Sample size: 4115; mean age:* sex: boys ‐ 50%, girls ‐ 50%; ethnicity:* Country: USA | |
| Interventions | Telephone reminders were sent at the same frequency as letters by an Autodialer service in which a recorded human voice in English or Spanish was used, with a message that mirrored the information in the letter reminders Mail reminders. The letters provided the practice's telephone number. Letters were sent at 10‐week intervals for Tdap, MCV4, and preventive care visits (maximum of 5 reminders over 12 months) Participants in the control group received usual care | |
| Outcomes | Immunisation status; preventive visit rate (both primary); process evaluation; costs (both secondary) | |
| Funding | CDC | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | The study had > 90% power for a 5% improvement in immunisation rates at study end assuming 50% for controls (2‐sided alpha = 0.05), using survival analysis and an intention to‐treat analysis | |
| Notes | Among all adolescents who received a reminder, the cost averaged USD 18.78 or USD 16.68 per adolescent per year for mail reminder group and telephone reminder group, respectively. There were no cost‐effectiveness data available for usual care group. This is a comparison between Autodialer and no intervention. The other intervention included mailed reminders. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation by AB (using Stata 9.2) stratifying on practice, age in years, and sex" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. Quote: "Health care providers were unaware of group assignment." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | An intention to‐treat data analysis was used. |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | Quote: "The control and intervention groups had similar demographics (Table 2) and baseline immunisation and preventive visit rates." |
| Methods | Aims: to evaluate the effectiveness of automated telephone reminder on appointment reminder in patients undergoing tuberculosis care Study design: quasi‐RCT; recruitment: other ‐ county health department (organisational referral) Study duration: 6 months; study type: either; subtype: appointment reminder | |
| Participants | Inclusion criteria: participants with a scheduled appointments in the Tuberculosis Control Programme of Santa Clara County Health Department over a period of 6 months Sample size: 2008; median age: 19 years; sex: male ‐ 54 %; female ‐ 46 %; ethnicity: Spanish‐speaking ‐ 39%, Vietnamese‐speaking ‐ 28%, English‐speaking ‐14%, other – 13%, Tagalog‐speaking Filipino – 6% Country: USA | |
| Interventions | Teleminder: an automated telephone reminder call of their upcoming appointment in either English, Spanish, Tagalog, or Vietnamese was made 1 day prior to the appointment. Additional information about the clinic address and the time of appointment was also provided. Participants had the option to hear to the message again if they remained online. Participants receiving authoritative endorsement identified the source of message as coming from the Public Health Nurse at the Health Department while in the importance statement, the following statement was added ‐ "coming to this appointment is important so that you and your family will not become seriously ill." Message was sent between 6 pm and 9 pm, the evening before the scheduled appointment. Message was left on answering machine and if the line was busy, up to 5 attempts were made at half hour intervals Basic reminder + authority endorsement Basic reminder + importance statement Basic reminder + authority endorsement + importance statement No reminder (controls) | |
| Outcomes | Attendance rate (primary); satisfaction (attitude questionnaire) (secondary) | |
| Funding | National Institute of Allergy & Infectious Diseases; National Institute on Ageing | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between Teleminder arm and control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random sequence generation. Quote: "random assignment of patients to conditions and the delivery of multiple messages on the same day would have required substantially more experimenter time" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to assess the impact of automated telephone reminder on tuberculin skin test return Study design: RCT; recruitment: * (organisational referral) Study duration: 2 months; study type: either; subtype: appointment reminder | |
| Participants | Inclusion criteria: participants of Santa Clara County immunisation programme who received tuberculin skin test Sample size: 701; age: 55% < 12 years; sex: boys ‐ 45 %; girls ‐ 55 %; ethnicity: English‐speaking ‐ 59%, Spanish‐speaking ‐ 29%, Vietnamese‐speaking ‐ 3%, other – 9% Country: USA | |
| Interventions | Participants in the Teleminder group received an automated reminder in either English, Spanish, or Vietnamese between 6 pm and 9 pm of the evening before the scheduled day to have the tuberculin skin test read. The message was pre‐recorded by a female speaker that also provided the time and place of appointment. The message was repeated twice and if it reached an answering machine, the message was saved. If the line was busy, then up to 5 attempts were made, at half‐hour intervals Participants in the control group received no calls | |
| Outcomes | Return of tuberculin test (primary); satisfaction (perceptions about reminders) (secondary) | |
| Funding | National Institute of Allergy & Infectious Diseases; National Institute on Ageing | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A research assistant randomly assigned the participants to either a control or an experimental group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to evaluate the effectiveness of IVR self‐monitoring to support natural resolutions among community‐dwelling problem drinkers who had recently stopped high‐risk drinking without treatment and who were abstaining or engaging in low‐risk drinking. Study design: RCT; recruitment: community (media adverts) Study duration: 6 months; study type: prevention; subtype: alcohol | |
| Participants | Inclusion criteria: participants aged ≥ 21 years, problem drinking history more than 2 years, currently not taking any drugs except nicotine, and cessation of high‐risk drinking in the past 3–16 weeks without alcohol‐focused interventions Sample size: 187; age: 45 years; sex: men ‐ 63 %; women ‐ 37 %; ethnicity: white ‐ 54%, other race/ethnicity ‐ 46% Country: USA | |
| Interventions | Participants in the intervention group received IVR: the system was programmed using commercial software (SmartQ Version 5 [5.0.141], Telesage, Inc., Chapel Hill, NC). A daily survey assessed ounces of beer, wine, and distilled spirits consumed; use of other drugs to 'get high'; and dollars spent on alcohol and other drugs during the preceding day (defined as the 24‐hour period midnight‐to‐midnight yesterday). When no substance use was reported, participants answered questions about other prior‐day activities to balance call duration. 4 once‐a‐week surveys on Mondays through Thursdays assessed other relevant domains (e.g. strategies used to avoid/limit drinking, activities paired with drinking) Participants in the control group received an assessment‐only. | |
| Outcomes | Drinking practices; spending on alcohol (both primary) | |
| Funding | National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (NIH/NIAAA) | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | No significant IVR main effects were found in any analysis either before or after adjusting for covariates (all ps > .20). Significant effects by compiler average causal effect (CACE) models examined IVR self‐monitoring effects. The other report from this trial had different aims: "to assess IVR in community‐dwelling HIV/AIDS patients in rural Alabama self‐monitored for enhancing daily HIV risk behaviours reporting.". Inclusion criteria: age ≥ 19 years (the age of majority in Alabama); reported use of alcohol or illicit drugs and sex with a partner within the past 3 months (in order to obtain sexually active substance users, the high risk target population for HIV risk reduction programmes); no health problems that precluded participation (e.g. dementia, psychosis); were not living in the HSC Hospice or other residential facility (e.g. inpatient substance abuse treatment programme) and were not taking any medication (e.g. disulfiram, methadone) that would substantially constrain opportunities for engaging in the risk behaviours of interest; and had daily phone access; Sample size: 54; mean age: 38 years; percentage of men ‐ 65 % and women ‐ 35 %; ethnicity: black ‐ 43%; and outcomes: changes in risk behaviours. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Urn randomisation used sex and race as balancing factors" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | High attrition rate. Data were not imputed using appropriate methods. Quote: "The follow‐up rate was about 70%. This suboptimal rate was partially addressed by including a 'missing' category as an outcome code along with the 3 resolution outcomes so that the analyses included all enrolled participants" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the study's pre‐specified outcomes that are relevant to the review were reported |
| Other bias | Low risk | Comment: groups were similar across all baseline characteristics |
| Methods | Aims: to determine the effectiveness of delivering 4 different interactive telephone technology programmes to reduce weight and improve blood glucose, insulin, high‐density lipoproteins, and triglycerides values Study design: RCT; recruitment: * (*) Study duration: 12 weeks; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: * Sample size: 140; mean age: * sex: * ethnicity: * Country: USA | |
| Interventions | Interactive telephone counselling (ITC) + control Online behaviour‐based incentives + control ITC + behaviour‐based incentives + control Control ‐ written materials and once monthly group meetings | |
| Outcomes | Weight change (primary); BMI; waist circumference; systolic blood pressure; blood glucose (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between ITC + control versus and control. Information derived from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to perform an effectiveness trial of nicotine replacement therapy (NRT) in combination with 3 low‐cost behavioural therapies (manuals, tailored expert system interventions, and an automated counselling intervention) Study design: RCT; recruitment: primary care (mail) Study duration: 30 months; study type: management; subtype: smoking | |
| Participants | Inclusion criteria: self‐identification as a smoker who regularly smoked ≥ 10 cigarettes per day and, therefore, met the requirements for using NRT Sample size: 2054; mean age: 51 years; sex: women ‐ 23%, men ‐ 77%; ethnicity: white ‐ 89%, black ‐ 5%, other ‐ 4%, Native American ‐ 2% Country: USA | |
| Interventions | Multimodal intervention automated counselling + NRT, manuals, and expert system (TEL + EXP + NRT + MAN). The interactive telecommunications system was developed for this study and employed a series of prerecorded voice files assembled in the form of a conversation that was tailored to the responses of the smoker. The telecommunications contacts served to both complete the assessment of progress on the 14 TTM variables and provide instant automated feedback. Material similar to that in the written paragraphs of the expert system progress reports was presented during the call and reproduced verbally Expert system + NRT and manuals (EXP + NRT + MAN) NRT + manuals (NRT + MAN) Stage‐matched manuals (MAN) | |
| Outcomes | Smoking abstinence | |
| Funding | National Cancer Institute Grant CA71356 | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | This is a comparison between the multimodal intervention and the stage‐matched manuals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completing the survey, all eligible smokers were randomised by computer‐based random number generator to one of four intervention conditions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Subjects were blinded to their treatment condition until they received the first intervention material; thus, awareness of the treatment condition could not influence the readiness for study participation. However, subjects were aware that several of the possible treatment conditions included NRT and that up to four follow‐up assessments by telephone were scheduled over the following 30 months." Insufficient information to judge whether this has introduced bias. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment ensured. Quote: "The survey centre staff was blind to treatment condition." |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The intention‐to‐treat analysis was conducted on the entire sample of 2,054 subjects identified as at risk for smoking" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "As a randomisation check, tests of significance ( p < .01) were performed to determine whether there were any differences between the four groups. All tests were non significant." |
| Methods | Aims: to test the ability of an automated telephone outreach intervention to reduce acute healthcare utilisation and improve quality of life among adult asthma patients in a large managed care organisation Study design: RCT; recruitment: other ‐ health plan (organisational referral) Study duration: 10 months; study type: management; subtype: asthma | |
| Participants | Inclusion criteria: aged ≥ 18 years and either on the Kaiser Permanente Northwest (KPNW) high‐risk asthma registry or had ≥ 180 days of antiasthma medication dispensing during the 2‐year period 2000‐2001 and ≥ 1 medical contact for asthma during the same 2 years Sample size: 6,948; mean age: 52 years; sex: men ‐ 35%, women ‐ 65%; ethnicity: white, non‐Hispanic ‐ 92%, other – 8% Country: USA | |
| Interventions | Automated telephone outreach system (ATOS): the calls consisted of a series of questions designed to assess recent emergency department or hospital care for which the member had not had a follow‐up visit, current level of asthma control, current patterns of asthma medication use, and whether the member could identify a primary care provider whom he or she usually saw for asthma care. Based on the responses to these initial questions, members were offered (optional) tailored feedback regarding their overall level of asthma control and their use of asthma medications. Feedback was designed to convey a positive message without being prescriptive. The calls lasted less than 10 min and were made using speech‐recognition technology. The telephone message were translated into text message that was continuously updated in the electronic medical record. Participants at high risk of a future exacerbation are flagged and an electronic alert via electronic surveillance system placed in the medical record prompting their provider to review the encounter and clear the alert from the record Live calls (the same script as above) Usual care (controls) | |
| Outcomes | Healthcare utilisation; asthma control; medication use; quality of life (all primary); satisfaction/acceptability to participants (secondary) | |
| Funding | CDC and the Kaiser Permanente Care Management Institute | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | Per protocol, the 2 intervention arms (automated and live‐person calling) were combined for the primary and post hoc analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible individuals were randomly assigned to either usual care (n = 3367) or telephone outreach (n = 3581)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The primary outcome analysis used an intention‐to‐treat design that included in the intervention group all randomised individuals, as well as persons who declined to participate in the intervention" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
| Methods | Aims: to evaluate the effectiveness of an intervention based on health information technology (HIT) that used speech recognition software to promote adherence to inhaled corticosteroids among individuals with asthma who were members of a large health maintenance organisation Study design: RCT; recruitment: other ‐ health plan (mail) Study duration: 18 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: treatment for asthma during the 12‐month period prior to randomisation; ≥ 1 dispensing of a respiratory medication at a Kaiser Permanente Northwest or Kaiser Permanente Hawaii outpatient pharmacy during the 12‐month period prior to randomisation; aged ≥ 18 years, continuous Kaiser Permanente membership from the start of the baseline year until the time of randomisation Sample size: 8517; mean age: 54 years; sex: men ‐ 34%; women ‐ 66%; ethnicity: white ‐ 50%, unknown – 26%, Asian ‐ 11%, mixed – 7%, Native Hawaiian/Pacific Islander ‐ 4%, African American ‐ 2%, American Indian/Alaskan Native – 1% Country: USA | |
| Interventions | Participants in the intervention group received IVR: 3 basic IVR call types, each of which typically lasted 2‐3 min: a refill reminder call, a tardy refill call, and an initiator/restart call. Each month, participants' electronic medical records were scanned to determine who was eligible for which type of call. The tardy refill call went to individuals who were more than 1 month past their projected refill date. It not only reminded participants that they were due for an inhaled corticosteroids refill, but also assessed asthma control, explored inhaled corticosteroids adherence barriers, and provided tailored educational messages. Poorly controlled participants who declined to be transferred to the automated pharmacy refill line were offered the option to speak to a live pharmacist. The initiator/restart call was designed to provide support to participants who were either starting inhaled corticosteroids for the first time (new users) or were lapsed users. These calls went to individuals with an inhaled corticosteroids order or dispensing in the previous month and no other inhaled corticosteroids dispensing in the previous 6 months, and were similar to the tardy refill calls in that they included probes for asthma control and adherence barriers and offered tailored educational messages Participants in the control group received usual care. | |
| Outcomes | Medication adherence (primary); asthma‐related healthcare utilisation (secondary) | |
| Funding | NA | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | "A priori power calculations showed near‐100% power to detect differences of 0.04 in adherence and 85% power to detect differences of 0.5 on the 7‐point mini‐AQLQ score." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "[Participants] were randomised to either the intervention or usual care arms, with randomisation stratified by region and the clinic facility to which each patient was paneled." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: missing data have been imputed using appropriate methods. An ITT analysis was used to include all participants who received the intervention or usual care in the analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "Baseline characteristics of the intervention and usual care groups were very similar." |
| Methods | Aims: to evaluate the utility of 2 electronic medical record‐linked, automated phone reminder interventions for improving adherence to cardiovascular disease medications Study design: RCT; recruitment: other ‐ health plan (organisational referral) Study duration: 12 months; study type: management; subtype: adherence to medication/laboratory tests | |
| Participants | Inclusion criteria: ≥ 40 years with diabetes mellitus and/or cardiovascular disease, suboptimally (< 90%) adherent to a statin or ACE inhibitor/angiotensin receptor blocker (ARB) during the previous 12 months, and due or overdue for a refill Sample size: 21,752; mean age: 64 years; sex: men ‐ 53%; women ‐ 47%; ethnicity: white ‐ 47%, Asian ‐ 17%, African American ‐15%, native Hawaiian/Pacific Islander ‐ 11%, unknown ‐ 9%, American Indian/Alaskan Native – 1% Country: USA | |
| Interventions | IVR calls. IVR participants received automated phone calls when they were due or overdue for a refill. The calls used speech‐recognition technology to educate participants about their medications and help them refill prescriptions (we created separate 'refill' and 'tardy' calls). The flow of each call was determined by participants' responses; each call lasted 2‐3 min. At randomisation, IVR participants received a pamphlet explaining these calls. Both call types offered a transfer to Kaiser Permanente's automated pharmacy refill line. The tardy call also offered a transfer to a live pharmacist. With permission, obtained at the first successful call contact, the programme left detailed messages on answering machines or with another household member Enhanced IVR (IVR Plus). In addition to IVR calls, participants in the IVR Plus arm received a personalised reminder letter if they were 60‐89 days overdue and a live outreach call if they were ≥ 90 days overdue, as well as electronic medical records‐based feedback to their primary care provider. IVR Plus participants received additional materials, including a personalised health report with their latest blood pressure and cholesterol levels, a pill organiser, and bimonthly mailings Usual care participants had access to the full range of usual services, including each region's normal education and care management outreach efforts to encourage statin and ACEI/ARB use | |
| Outcomes | Medication adherence (primary); blood pressure and lipid levels (secondary) | |
| Funding | R01HS019341 from the Agency for Healthcare Research and Quality | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | The study had roughly 90% power to detect effects of 0.032 (3.2 percentage points) in adherence for statins and 0.045 (4.5 percentage points) for ACEI/ARBs in sex‐specific subgroup analyses, and effects of 0.039 (statins) and 0.045 (ACEI/ARBs) in subgroups defined by terciles of some baseline factor | |
| Notes | The estimated costs were USD 9 to USD 17 per participant per year for IVR and USD 36 to USD 47 for IVR Plus. No costs for UC were provided. This is a comparison between IVR and control. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation assignments were stratified by region and blocked to assure balance across treatment arms." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Neither participants nor providers were blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We used an intention‐to‐treat analysis to compare primary and secondary outcomes between intervention and UC participants." |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and all of the study's pre‐specified outcomes that are relevant to the review have been reported. |
| Other bias | Low risk | Quote: "Baseline characteristics of the intervention and UC groups for the pooled statin and ACEI/ARB analysis samples were very similar" |
| Methods | Aims: to investigate the effects of the TLC Diabetes programme on health outcomes postintervention (time point 2) and at 12‐month follow‐up (time point 3) Study design: RCT; recruitment: primary care and the community (adverts in newspapers, flyers, newsletters and through diabetic clinics) Study duration: 12 months; study type: management; subtype: diabetes | |
| Participants | Inclusion criteria: participants with type 2 diabetes diagnosis of ≥ 3 months; aged 18‐70 years; residing in the greater Brisbane area (Australia); a glycated haemoglobin level of ≥ 7.5%; stable diabetes pharmacotherapy type for ≥ 3 months; stable pharmacotherapy dosage for ≥ 4 weeks; ability to clearly speak and understand English via the telephone, and weekly access to a telephone Sample size: 120 ; mean age: 57 years; sex: men ‐ 62.5%; women ‐ 37.5%; ethnicity: * Country: Australia | |
| Interventions | Telephone‐Linked Care (TLC) Diabetes system: participants receive TLC Diabetes kit containing the TLC Handbook, an ACCU‐CHEK Advantage glucose meter, test strips, and a Bluetooth device with which to upload their blood glucose results to the TLC Diabetes system. They call the system weekly using a landline or mobile phone. TLC's responses, including feedback and encouragement, were tailored according to information entered in the TLC database at the start and the answers that it received from participants during all calls. TLC stressed on the following self‐management behaviours: blood glucose testing (covered in all calls), nutrition (calls 9‐12; 21‐24), physical activity (calls 5‐8; 17‐20) and medication‐taking (calls 1‐4; 13‐16). Participants in the control group received usual care. | |
| Outcomes | Glycated haemoglobin; health‐related quality of life (physical and mental components of the Short‐Form‐26 (SF‐26) scale) (all primary) | |
| Funding | National Health Medical Research Council project grant, HCF Health and Medical Research Foundation, and Queensland Health | |
| Declaration of conflict of interest | Dr Friedman has stock ownership and a consulting agreement with Infomedics, the company that owns commercial rights to the TLC technology used in the computerised intervention. He is also a member of its Board of Directors. The other authors declare that they have no competing interests | |
| Power calculations for sample size | With 80% power and a type 1 error of 5% (2‐tailed), it was possible to detect a difference in the primary outcome, glycated haemoglobin, of 0.61% between the intervention and control arms (based on a standard deviation change of 1.0% between the randomised arms). | |
| Notes | 43% of total participants were on insulin (injected). Mean BMI: 33 kg/m2. The TLC coordinator phones intervention participants after their first 2 calls to the TLC system and at weeks 6, 12 and 20, to identify and resolve any issues faced during their use of the TLC Diabetes system or to identify reasons for not calling regularly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The arm allocation was conducted using a 4 x 4 block randomised block design with the participant as the unit of randomisation." |
| Allocation concealment (selection bias) | Low risk | Correspondence with the author: "We used opaque envelopes, so all envelopes were prepared at the start of the trial, contained allocation to intervention or control according to randomisation schedule.' |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The treating physicians were not blinded to the allocation." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data have been imputed using appropriate methods. Quote: "To account for subjects lost to follow‐up in intention‐to‐treat analyses, multiple imputation was performed using ten imputed datasets" |
| Selective reporting (reporting bias) | High risk | The protocol lists about 16 secondary outcome measures that were not reported in the 6‐month report. Correspondence with the author: "these data have been collected but I'm afraid no analyses have been performed yet. We could not fit the 6‐month secondary outcomes into this paper unfortunately." |
| Other bias | High risk | Quote: "Comparison of the baseline characteristics across usual care and intervention arms revealed important differences in e‐GFR which showed a significantly greater impairment in renal function in the intervention compared with usual care arm, and creatinine. Other differences observed were in age, education, and self‐care behaviours (adherence to blood glucose testing recommendations and daily insulin/diabetes medications, and foot inspections)." |
| Methods | Aims: to evaluate the acceptability and feasibility of a scalable obesity treatment programme integrated with paediatric primary care and delivered using IVR to families from underserved populations Study design: RCT; recruitment: primary care (advert in clinic) Study duration: 3 months; study type: prevention; subtype: weight management | |
| Participants | Inclusion criteria: 9‐12 years old, a BMI 0‐5 BMI points above the 95th percentile for age and sex, attended a paediatric visit within the last year, and due for an annual well‐child exam in 4 months Sample size: 50 dyads; mean age: 10 years; sex: boys ‐ 58%, girls ‐ 42%; ethnicity: white ‐ 6%, African American ‐ 72%, other ‐ 22% Country: USA | |
| Interventions | Intervention: both parents and children received a 12‐week telephone counselling delivered by an automated IVR system. The intervention also included an EHR behavioural counselling tool used by the PC clinician during well‐child follow‐up visits. Similar but separate interventions were developed for parents and children. The IVR was designed to monitor, educate, and counsel parents and children on healthy weight management and television time through weekly IVR telephone conversations. During these conversations, the system spoke to participants using computerised voice by means of text‐to‐speech technology. Participants communicated by speaking into the telephone receiver or by pressing keys on the telephone keypad. The conversation is tailored to the individual user of the IVR such that the IVR asked questions and provides tailored feedback based on the user's response. Questions are asked to monitor the user's behaviour and provided education and theory‐based behaviour change strategies for the targeted behaviours as well as generate a conversation that is more human‐like. The HEAT system stores responses that are used to tailor the questions asked during the same conversation or inform subsequent calls Participants in the control group received no calls (wait‐list). | |
| Outcomes | BMI z‐score; calorie intake; fat intake; fruit intake; vegetable intake; television‐viewing time (all primary) | |
| Funding | National Institute of Child and Human Development (NICHD R21 HD050939‐02) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Fifty parent‐child dyads were randomised in blocks of six to either the intervention condition (HEAT) or WLC condition. The blocks were generated by an investigator who did not have contact with the participants." |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignments to condition were placed in sealed envelopes and opened after all baseline measures were completed." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Intention‐to‐treat analyses with baseline values carried forward for those missing at follow‐up were also conducted" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Comment: groups were similar across all baseline characteristics but weight and height; however, it is unclear whether this has introduced bias |
| Methods | Aims: to evaluate the effects of an automated IVR system and specialist nurse support to reduce health care utilisation and improve health‐related quality of life in children with asthma Study design: RCT; recruitment: secondary care (*) Study duration: 6 months; study type: management; subtype: asthma | |
| Participants | Inclusion criteria: children and adolescents aged 3‐16 years with doctor‐diagnosed asthma who had either had an admission to hospital in the previous 12 months or had presented at least once to an emergency department or to their general practitioner or specialist with acute asthma requiring oral steroid rescue in the previous 12 months Sample size: 121; mean age: 7 years; sex: men ‐ 53%, women ‐ 47%; ethnicity:* Country: Australia | |
| Interventions | IVR: participants received an automated telephone call twice a week on their home phone or mobile phone. Children over 12 years old were encouraged to answer calls themselves. Parents answered calls for children younger than 12 years old. The IVR system asked questions about asthma symptoms and medication use and participants entered clinical data using the keypad on the phone. Educational messages, appropriate information from the asthma management plan, and medication reminders were given. Reports generated from the electronic system were sent to the primary physician electronically or by fax Nurse support group Usual care (control group) | |
| Outcomes | Healthcare utilisation (primary); use of oral steroid rescue; health‐related quality of life; costs (secondary) | |
| Funding | Asthma Foundations of Australia and Royal Children's Hospital Foundation Brisbane Australia | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | NA | |
| Notes | IVR was more cost‐effective than usual care in reducing the total health care costs (mean AUD −451 (95% CI −1075, 172); but less cost‐effective than nurse support group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation was used with random block sizes of three or six to create an allocation to one of the three groups for all study subjects" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "One child in the control group was lost to follow‐up during the study." Comment: low attrition rate and unlikely to have introduced bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Quote: "The groups were reasonably well matched at baseline, although the control group had fewer hospital admissions and ED presentations over the previous 12 months compared with Nurse Support and IVR groups at baseline." Comment: groups were similar across all baseline characteristics but hospital admissions and ED presentation; however, it is unclear whether this has introduced bias. |
| Methods | Aims: to evaluate the efficacy of technology‐based symptom monitoring and reporting in reducing symptom burden in patients with advanced lung cancer Study design: RCT; recruitment: primary care (*) Study duration: 12 weeks; study type: management; subtype: cancer | |
| Participants | Inclusion criteria: ≥ 18 years old, English‐speaking, having advanced non‐small cell lung cancer or small cell lung cancer, receiving active treatment with traditional chemotherapy no later than day 1 of cycle 2 or receiving oral therapy, having access to a telephone, and life expectancy of ≥ 6 months Sample size: 253; mean age: 61 years; sex: men ‐ 49%, women ‐ 51%; ethnicity: white ‐ 58%, black or African American ‐ 36%, other ‐ 6% Country: USA | |
| Interventions | Participants in the intervention group received monitoring and reporting (MR group) via IVR. The participants delivered reports of clinically significant symptoms to their clinical team for further assessment and/or management; and had paper copies of longitudinal, graphical displays of symptom scores available Participants in the control group received monitoring alone (MA) via IVR | |
| Outcomes | Symptom burden (primary); quality of life; treatment satisfaction; symptom management barriers; self‐efficacy (secondary) | |
| Funding | National Cancer Institute (R01‐CA115361) | |
| Declaration of conflict of interest | None declared | |
| Power calculations for sample size | "The study was powered to detect a difference between the two study groups in SDS total score. For this endpoint, a standardized effect size (mean group difference/common standard deviation) of 0.33 has been suggested to be meaningful in the measurement of PROs in several different cancer populations" | |
| Notes | Both groups received ATCS interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After providing informed consent, participants completed baseline measures and were randomly assigned by computer in a 1:1 ratio to the MR or the MA group. Randomization was blocked, stratified by institution, with a goal of enrolling 100 participants from each of the three sites (total N = 300), 150 in each group." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "This was a non‐blinded, randomised, controlled trial of technology‐based symptom monitoring with reporting (MR group) to the clinical team compared with symptom monitoring alone (MA group)" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "A blinded interim analysis of symptom severity and study burden data was planned after half of the randomised patients (N = 150) had reached the week 12 assessment, and this analysis was reviewed by the institutional cancer centre data and safety monitoring board." Insufficient information to judge whether blinded assessments were performed. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Analyses were based on intention‐to‐treat in all randomised participants and were not adjusted for multiple comparisons." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Low risk | Quote: "The study groups were equivalent in baseline characteristics" |
| Methods | Aims: to examine the effects of a brief, daily intervention targeting either personal control/mastery (MC) or mindful awareness/acceptance (MA) compared with a placebo treatment that consisted of tips to a healthy life‐style (HT) Study design: RCT; recruitment: community (phone and home visits) Study duration: 1 month; study type: management; subtype: depression | |
| Participants | Inclusion criteria: individuals with mild to moderate symptoms of depression Sample size: 73; mean age: * sex: * ethnicity: other – 74%, Hispanic – 26% Country: USA | |
| Interventions | Personal control/mastery. Intervention was delivered in pre‐recorded messages via phone each morning. Each evening, participants completed an on‐line daily diary that included the outcome measures Mindful awareness/acceptance (delivered as above) Healthy lifestyle (controls) | |
| Outcomes | Stress; depression | |
| Funding | NIA Grant RO1–AG‐6026006 | |
| Declaration of conflict of interest | NA | |
| Power calculations for sample size | NA | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information. Quote: "Seventy‐three adults recruited to participate in the trial, and randomly assigned to MC, MA, or HT conditions" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of study personnel was ensured. Quote: "The research assistants were blinded to the hypotheses of the study and did not have access to the daily diary data of the participants at any time during the study." |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition rate. Missing outcome data balanced in numbers, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Insufficient information |
ACE: angiotensin‐converting‐enzyme; ART: antiretroviral therapy; AT2: angiotensin 2; ATCS: automated telephone communication system; BI: brief intervention; BMI; body mass index; CBT: cognitive behavioural therapy; CDC: Centers for Disease Control and Prevention; COPD: chronic obstructive pulmonary disease; CPAP: continuous positive airway pressure; DSM: Diagnostic and Statistical Manual of Mental Disorders; EHR: electronic health record; EMR: electronic medical record; FDA: Food and Drug Administration; HMO: health maintenance organisation; ITT: intention‐to‐treat; IVR: interactive voice recognition; MI: motivational interviewing; NA: not available; NIAAA: National Institute on Alcohol Abuse and Alcoholism; OSAS: obstructive sleep apnoea syndrome; QALY: quality‐adjusted life year; PCI: percutaneous coronary intervention; PCP: primary care provider; PHQ‐8/9: personal health questionnaire, version 8/9; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; UC: usual care; UOHI: University of Ottawa Heart Institute.
aPlease note that for reporting of participants' ethnicity, the terms used by authors of the included studies have been used in each case and are cited directly from each of the included studies.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| No preventive healthcare or management of long‐term condition | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| No preventive healthcare or management of long‐term condition | |
| No preventive healthcare or management of long‐term condition | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| No preventive healthcare or management of long‐term condition | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Data for ATCS group unavailable. Contact with author: "I apologize for not being helpful and regret that the value of the data cannot be extended by inclusion in the systematic review. Apparently, the back‐up files for this project were scored on 3.5 inch floppy disks (!!) that were discarded during an office move". | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| No preventive healthcare or management of long‐term condition | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| No preventive healthcare or management of long‐term condition | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Inappropriate study design | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS | |
| Intervention does not use an ATCS |
ATCS: automated telephone communication system.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | diaBEAT‐it! |
| Methods | Aims: to determine the reach of each active intervention, the effectiveness of the strategies in supporting patients to lose and maintain a 5% weight loss, and the cost‐effectiveness of the interventions in achieving standard weight loss Study design: RCT; recruitment: primary care (mail and telephone) Study duration: ongoing; study type: management; subtype: diabetes |
| Participants | Inclusion criteria: age > 18; BMI > 25; and indicates high risk for developing diabetes, based on the diabetes risk test calculator Sample size: 360; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: small group intervention + 12 months of interactive voice response telephone follow‐up (SG‐IVR) Arm b: DVD version of the small group intervention with the same IVR follow‐up (DVD‐IVR) Arm c: standard care |
| Outcomes | Weight loss; reach; cost; physical activity; dietary intake |
| Starting date | 2014 |
| Contact information | |
| Notes | Clinicaltrials.gov identifier: NCT02162901 |
| Trial name or title | COPD‐SMART |
| Methods | Aims: to determine if a self‐management lifestyle physical activity intervention would improve physical functioning and dyspnoea Study design: RCT; recruitment: primary care (mail and telephone) Study duration: ongoing study type: management; subtype: chronic obstructive pulmonary disease |
| Participants | Inclusion criteria: age ≥ 45 years; physician diagnosis of chronic obstructive pulmonary disease; forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio < 70% and FEV1 < 70%; modified Medical Research Council dyspnoea score ≥ 2 Sample size: 305; mean age: 69; sex: women ‐ 50%, men ‐ 50%; ethnicity: white ‐ 92%, black ‐ 6%, Hispanic ‐ 1%, other ‐ 1% Country: USA |
| Interventions | Intervention group received self‐management needs assessment; chronic obstructive pulmonary disease self‐management education (Weeks 1‐6); physical activity self‐management (weeks 7‐36) ‐ the program is delivered using a structured workbook supported by one‐on‐one telephone counselling every other week by the health coach with computer assisted telephone calls on alternating weeks Usual care continued regular follow‐up with their physician and to call the health coach using a toll‐free number if they have any questions. Study‐related contact occurs through monthly automated telephone calls, which collect health care utilisation data, and follow‐up visits for data collection at 6, 12, and 18 months |
| Outcomes | Chronic Respiratory Questionnaire (CRQ) dyspnoea domain and 6‐minute walk distance; other CRQ domains (fatigue, emotion, and mastery); Quality of Life (SF‐12); Health care utilisation; Process outcomes |
| Starting date | 2010 |
| Contact information | |
| Notes | Clinicaltrials.gov identifier: NCT1108991 |
| Trial name or title | Boston Osteoarthritis Strengthening telephone linked‐communication (BOOST TLC) |
| Methods | Aims: to empower and motivate people with knee OA to adhere to strengthening exercise after participating in a class Study design: RCT; Recruitment: community (*) Study duration: ongoing Study type: management; Sub ‐ type: osteoarthritis |
| Participants | Inclusion criteria: subjects with painful knee osteoarthritis (OA) Sample size: 100; Mean age: * sex: * Ethnicity: * Country: USA |
| Interventions | Arm a: TLC is an automated, interactive conversation system that speaks with a recorded human voice. During the conversation the system asks questions, comments on the users' responses and educates and counsels them. TLC stores the users' answers in a database used to direct current and future TLC conversations. The system is run by a scheduling protocol with the ability to receive and make calls Arm b: the control group receives an automated message once per month, reminding them to strength train and record their progress in their log. |
| Outcomes | Pain and physical function; timed physical function tasks; isokinetic muscle strength |
| Starting date | 2013 |
| Contact information | |
| Notes | — |
| Trial name or title | ICT‐supported cardiovascular disease prevention through phone‐based automated lifestyle coaching |
| Methods | Aims: to support cardiovascular disease patients in performing appropriate behaviour changes in order to minimise their individual risk factors Study design: RCT; recruitment: * Study duration: ongoing study type: prevention; subtype: cardiovascular disease |
| Participants | Inclusion criteria: already suffered a stroke or transient ischaemic attack (TIA) or ≥ 2 risk factors for stroke: high blood pressure, overweight; low physical activity; smoking; unhealthy diet Sample size: 94; mean age: * sex: * ethnicity: * Country: Luxemburg |
| Interventions | Arm a: computer‐based lifestyle coaching system via IVR Arm b: no details of control group |
| Outcomes | Change in systolic blood pressure; serum high‐density lipoproteins, low‐density lipoproteins and triglycerides levels; glycated haemoglobin; glycaemia; BMI; acceptance; efficacy |
| Starting date | January 2013 |
| Contact information | Department of Neurology, Centre Hospitalier de Luxembourg, Luxembourg |
| Notes | ClinicalTrials.gov identifier: NCT02444715 |
| Trial name or title | A sustainable approach to increasing cancer screening (CATCH) |
| Methods | Aims: to compare the efficacy of two intervention arms intended to increase breast, cervical, and colon cancer screening rates among patients served by community health centres. Study design: RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: screening |
| Participants | Inclusion criteria: all eligible patients, using centre guidelines, in need of: breast, cervical or colorectal cancer screenings. Sample size: 13,675; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: consistent, but spaced‐out calls generated by an IVR system reminding them of breast, cervical and colon cancer screenings needed, as applicable. Arm b: IVR calls followed up by prevention care coordinator calls for those who do not respond to IVR |
| Outcomes | Change in population level cancer screening level at the health clinics involved |
| Starting date | September 2008 |
| Contact information | Karen Emmons, Harvard School of Public Health |
| Notes | ClinicalTrials.gov identifier: NCT01395459 |
| Trial name or title | CardiACTION! |
| Methods | Aims: to assess whether physical activity behaviour change is more likely when the participants' social‐cognitive beliefs are intervened upon (individual intervention), when access is provided to environmental resources for physical activity (environmental intervention), or when both social‐cognitive beliefs and access to environmental physical activity resources are manipulated (combination intervention including individual and environmental intervention components) Study design: randomised 2 × 2 factorial trial; recruitment: primary care (health professional referral) Study duration: ongoing; study type: prevention; subtype: physical activity |
| Participants | Inclusion criteria: patients who did not report meeting the recommended guidelines for physical activity (i.e. < 150 min of moderate physical activity per week), spoke English, did not currently have a fitness facility membership, and had a telephone Sample size: *; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: interactive computer session Arm b: automated telephone counselling. Over 6 months participants received frequent contacts delivered via IVR automated telephone calls and mailings, each providing intervention‐specific information to encourage and facilitate physical activity or healthful eating behaviour change Arm c: tailored mailings Arm d: combination intervention |
| Outcomes | Changes in physical activity levels |
| Starting date | — |
| Contact information | |
| Notes | — |
| Trial name or title | Health and economic effects from linking bedside and outpatient tobacco cessation services for hospitalised smokers in two large hospitals: study protocol for a RCT |
| Methods | Aims: the study assesses the effectiveness and cost‐effectiveness of linking a practical inpatient assisted referral to outpatient cessation services plus interactive voice recognition (AR + IVR) follow‐up calls, compared to usual care inpatient counselling (UC) Study design: RCT; recruitment: secondary care (*) Study duration: ongoing; study type: management; subtype: smoking |
| Participants | Inclusion criteria: aged ≥ 18 years who smoked ≥ 1 cigarettes in the past 30 days, willing to remain abstinent postdischarge, have a working phone, live within 50 miles of the hospital, speak English, and have no health‐related barriers to participation Sample size: 900; mean age: *; sex: men (KPNW ‐ 51.7%, OHSU ‐ 51.7%); women (KPNW ‐ 48.3%, OHSU ‐ 48.3%); Ethnicity: KPNW: white ‐ 79.4%, Hispanics ‐ 2.2%, black ‐ 4.1%; OHSU: white ‐ 89.0%, Hispanics ‐ 3.5%, Black ‐ 5.3% Country: USA |
| Interventions | Participants in the AR + IVR arm will receive a brief inpatient cessation consult plus a referral to available outpatient cessation programs and medications, and 4 IVR follow‐up calls over 7 weeks postdischarge. Control group will receive usual care. |
| Outcomes | Self‐reported 3‐day smoking abstinence at 6 months postrandomisation for outpatient cessation services plus interactive voice recognition (AR + IVR) participants compared to usual care |
| Starting date | — |
| Contact information | — |
| Notes | ClinicalTrials.gov identifier: NCT01236079 |
| Trial name or title | Information systems‐enabled outreach for preventing adverse drug events (ISTOP‐ADE) |
| Methods | Aims: to determine whether the ISTOP‐ADE system, compared to routine care, will reduce: the probability of discontinuing the use of prognosis‐altering medications; the probability of a patient experiencing a severe ADE; the proportion of patients experiencing ADEs, preventable ADEs and ameliorable ADEs; and health services utilisation Study design: RCT; recruitment: primary care (health professional referral) Study duration: ongoing; study type: management; subtype: adherence to medication/laboratory tests |
| Participants | Inclusion criteria: French‐ and English‐speaking adult patients (age >18) who receive a high‐risk incident prescription, use Régie de l’assurance maladie du Québec insurance to pay for medications and are followed by a physician who has consented to be in the Medical Office of the 21st Century research network Sample size: 2200; mean age: * sex: * ethnicity: * Country: Canada |
| Interventions | Arm a: IVR system paired with pharmacist support Arm b: routine care |
| Outcomes | Medication persistence; healthcare utilisation |
| Starting date | Date registered: 10 January 2014 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT02059044 |
| Trial name or title | Linking self‐management and primary care for diabetes 2 (LB2) |
| Methods | Aims: to evaluate the impact of 2 different interactive, multimedia self‐management programs, relative to 'enhanced' usual care Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: diabetes |
| Participants | Inclusion criteria: being 25‐75 years of age, live independently, have a telephone, are able to read in either English or Spanish, able to access the Internet at least twice per week are capable of providing informed consent, have been diagnosed with type 2 diabetes for at least 1 year are overweight (BMI ≥ 25), and have at least one additional UKPDS equation risk factor (i.e. high lipids, hypertension, glycated haemoglobin, or smoking) Sample size: 463; mean age: 60 years; sex: men ‐ 52%; women ‐ 48%;ethnicity: Latino ‐ 23% Country: USA |
| Interventions | Arm a: computer‐assisted self‐management plus social support. An interactive, automated self‐management (ASM) programme that uses web and interactive voice recognition (IVR) media combined with enhanced support in the form of group Diabetes Care Management visits and live follow‐up phone calls from Diabetes Care Managers Arm b: computer‐assisted self‐management (CASM). An interactive ASM programme that uses web and interactive voice recognition (IVR) media Arm c: usual care |
| Outcomes | Improvement in health behaviours (e.g. dietary patterns, physical activity, medication taking); and biologic outcomes (glycated haemoglobin, lipid ratio, blood pressure, and smoking status) |
| Starting date | January 2007 |
| Contact information | Russell E Glasgow, PhD, Kaiser Permanente |
| Notes | ClinicalTrials.gov identifier: NCT00987285 |
| Trial name or title | Interactive voice response (IVR)‐based treatment for chronic low back pain |
| Methods | Aims: the proposed study will test how well an innovative IVR method can be used for delivering treatment for chronic low back pain Study design: RCT; recruitment: secondary care Study duration: ongoing; study type: management; subtype: pain |
| Participants | Inclusion criteria: presence of at least a moderate level of pain (i.e. pain scores of ≥ 4) and presence of pain for a period of ≥ 3 months; ability to participate safely in the walking portion of the intervention as evidenced by ability to walk at least one block; availability of a touch‐tone telephone and computer with Internet access in the participant's residence; veteran receiving care at VA Connecticut Healthcare System Sample size: 230; mean age: 58.6 years sex: men ‐ 83%; women ‐ 17%; ethnicity: white ‐ 56.5% Country: USA |
| Interventions | Arm a: interactive CBT. IVR treatment consisted of a patient workbook supplemented by 10 weeks of daily IVR calls that provided pre‐recorded didactic information and weekly, pre‐recorded personalised therapist feedback. It also included daily IVR calls to collect pain‐related symptoms, adherence to pain coping skill practice and pedometer‐measured step counts Arm b: CBT |
| Outcomes | Numeric Rating Scale of Pain Intensity |
| Starting date | May 2011 |
| Contact information | — |
| Notes | ClinicalTrials.gov identifier: NCT01025752 |
| Trial name or title | Diabetes telephone‐linked care system for self‐management support in Thailand |
| Methods | Aims: to develop the diabetes telephone‐linked care system for self‐management support and test acceptability in terms of system uses, satisfaction and perception of easiness, helpfulness, and emotion with the system Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: diabetes |
| Participants | Inclusion criteria: * Sample size: 112; mean age: * sex: * ethnicity: * Country: Thailand |
| Interventions | The intervention group received the automated telephone system with diabetes knowledge IVR subsystem as the telephone‐linked care (TLC) No details of the control group reported |
| Outcomes | Glycemic control; patient satisfaction; system usability |
| Starting date | 2011 |
| Contact information | |
| Notes | — |
| Trial name or title | Technology‐enhanced quitline services to prevent smoking relapse (TEQ) |
| Methods | Aims: to see if automated telephone monitoring will enhance existing quit line services, such as Alere Wellbeing's Quit For Life programme, and help people quit smoking. Study design: RCT; recruitment: * Study duration: ongoing study type: management; subtype: smoking |
| Participants | Inclusion criteria: ≥ 18 years of age; enrolled in Free & Clear, Inc. services; self‐reported abstinence for at least 24 h at the quit date call; able to read and speak English; personal access to a touch‐tone telephone or cellular telephone Sample size: 1785; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: quit line service + 20 automated monitoring calls Arm b: quit line Service + 10 automated monitoring calls Arm c: usual care |
| Outcomes | Participant smoking status |
| Starting date | April 2010 |
| Contact information | Anna M McDaniel, PhD RN FAAN, Indiana University |
| Notes | ClinicalTrials.gov identifier: NCT00888992 |
| Trial name or title | Hospice and end‐of‐life symptom monitoring & support using an automated system designed for family caregivers (SCP) |
| Methods | Aims: to test an automated monitoring and coaching system for family caregivers during home hospice Study design: RCT; recruitment: secondary care (*) Study duration: ongoing study type: management; subtype: cancer |
| Participants | Inclusion criteria for patient/caregiver dyad: both patient and caregiver are adults aged ≥ 18 years; patient has a limited life expectancy and has histological diagnosis of cancer; caregiver is caring for a family member with a limited life expectancy and admitted to one of the participating home care hospice or palliative care programmes; caregiver is English‐speaking and writing; caregiver has access to a telephone on a daily basis; caregiver is cognitively and physically able to use the phone unassisted and complete questionnaire; patient is assigned to a nurse case manager who has consented to participate in the research project; caregiver and patient intend to reside in the local area until the time of the patient's death Sample size: 450; mean age: 73 years; sex: men ‐ 52 %; women ‐ 48 %; ethnicity: white/Caucasian ‐ 95% Country: USA |
| Interventions | Arm a: intervention group will receive a computer‐based telecommunication system to monitor symptoms as perceived and reported by the family caregiver; tailored care management messages that SCP provides directly to the caregivers to promote care management based on the individualised patient symptom profile and caregiver distress; and an automated alerting function that notifies the hospice nurse of unrelieved symptoms that have exceeded a pre‐set threshold Arm b: control group will receive usual care. |
| Outcomes | Family caregiver's assessment of dying patient's symptom severity level at end‐of‐life; caregiver's report their assessment of the severity of patient's symptoms daily |
| Starting date | May 2010 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT02112461 |
| Trial name or title | Telerehabilitation intervention to promote exercise for diabetes |
| Methods | Aims: to develop an innovative strategy to address the problems of obesity and diabetes by promoting exercise adoption Study design: RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: physical activity |
| Participants | Inclusion criteria: clinical diagnosis of type 2 diabetes mellitus; receive a medical clearance from physician; be sedentary; be interested in exercising; have a BMI > 25 kg/m2; have glycated haemoglobin of 7%‐10%; be on medication for diabetes Sample size: 89; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: Telephone‐Linked Care ‐ Promoting Exercise for Diabetes (TLC‐PED), a method that uses IVR and speech recognition technologies, will be developed to provide individualised and personalised motivational messages using automated telephone calls for veterans with type 2 diabetes who participate in a home‐based walking programme. Arm b: usual care |
| Outcomes | 7‐day physical activity recall; a self‐report measure of minutes of physical activity over the previous 7 days |
| Starting date | January 2009 |
| Contact information | Deanna L Mori, PhD, VA Medical Center, Jamaica Plain Campus |
| Notes | ClinicalTrials.gov identifier: NCT00334113 |
| Trial name or title | Interactive voice response system (IVRS) for managing symptoms of patients following thoracic surgery |
| Methods | Aims: to study the effectiveness of the IVR system (IVRS), which is designed to send a report to a patient's doctor about severe symptoms they are experiencing Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cancer |
| Participants | Inclusion criteria: patients scheduled for thoracic surgery for non‐small cell lung cancer, esophageal cancer and lung metastasis; aged ≥ 18, of any sex, who were English‐speaking and residing in the United States. Sample size: 100; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: IVR system + symptoms report (twice weekly) Arm b: IVR system only |
| Outcomes | NA |
| Starting date | 2006 |
| Contact information | Xin Shelley Wang, MD; Anderson Cancer Center |
| Notes | Clinicaltrials.gov: NCT00505024 |
| Trial name or title | Interactive voice response system in advanced cancer patients |
| Methods | Aims: to determine whether the IVR system, supplemented by nursing telephone intervention (NTI), results in better symptom management and quality of life than standard care for individuals with advanced cancer as evidenced by reduced scores on symptom measures. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cancer |
| Participants | Inclusion criteria: individuals with advanced cancer (incurable disease) who are seen in the supportive care centre at MD Anderson Cancer Center, who have a pain score of ≥ 4 or higher on the average pain scale item of the brief pain inventory for ≥ 2 weeks and at least 1 other symptom on the ESAS (fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, sleep), who are able to identify a primary caregiver who also agrees to participate in the study, who have no clinical evidence of cognitive failure in the opinion of the referring MD. Caregivers must be able to understand the instructions for the study, be ≥ 18 years of age, have access and utilise a touch‐tone telephone, be willing to engage in a telephone follow‐up with the IVR system and nurses every Monday, Wednesday and Friday, be willing to follow up by phone or in person on day 8 (+/‐ 3 days) and return for a follow‐up visit on day 15 (+/‐5 days), be willing and able to provide written informed consent; be a partner, parent, sibling, or child of the individual with advanced cancer; reside with the individual with advanced cancer and be responsible for most of the individual with advanced cancer's care Sample size: 136; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: IVR system phone calls made once daily, each taking about 3‐5 min to complete Arm b: standard care |
| Outcomes | Better symptom management and improved quality of life for participants |
| Starting date | January 2008 |
| Contact information | Sriram Yennurajalingam, MD, MD Anderson Cancer Center |
| Notes | ClinicalTrials.gov identifier: NCT00625638 |
| Trial name or title | Improving antihypertensive and lipid‐lowering therapy (CERT2) |
| Methods | Aims: to evaluate the impact of electronic health record clinical decision support and automated telephone outreach on antihypertensive and lipid‐lowering therapy in ambulatory care Study design: RCT; recruitment:* Study duration: ongoing; study type: management; subtype: cardiovascular disease |
| Participants | Inclusion criteria: Medical doctors, nurse practitioners, physician assistants, or doctors of osteopathic medicine practicing in primary care or medical subspecialties and using eClinical Works EHR .Patients of eligible physicians who have hypertension or hyperlipidaemia Sample size: 6000; mean age: * sex: * ethnicity: * Country:USA |
| Interventions | Arm a: hypertension and hyperlipidemia intervention with automated telephone outreach Arm b: hypertension and hyperlipidemia intervention using clinical decision support |
| Outcomes | The main outcome measure will be the proportion of participants at treatment goal |
| Starting date | May 2009 |
| Contact information | Steven Simon, VA Boston Healthcare System |
| Notes | ClinicalTrials.gov identifier: NCT00876330 |
| Trial name or title | Initiation of colon cancer screening in veterans or 'Start Screening Now' (SSN) |
| Methods | Aims: to increase first time colorectal cancer screening colorectal cancer among veterans aged ≥ 50 Study design: factorial RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: screening |
| Participants | Inclusion criteria: veterans aged 50‐64. Sample size: 1504; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: in step 1, investigators will evaluate a theory‐based minimal cue delivered by a letter, telephone call, or automated telephone call. People who do not complete colorectal cancer screening in step 1 will be randomised to step 2 using principles of motivational interviewing. Step 2 also will determine whether an automated approach, telephone‐linked communication (TLC), is as effective as a telephone counsellor in promoting initiation of colorectal cancer screening. Steps 1 and 2 together will address the important issue of the 'dose' needed to encourage completion of colorectal cancer screening. Arm b: a survey‐only control arm will be compared to the experimental arm to determine whether the 3 different delivery channels are equally efficacious and cost‐effective |
| Outcomes | Colorectal cancer screening |
| Starting date | July 2008 |
| Contact information | Sally Vernon, the University of Texas Health Science Center, Houston |
| Notes | ClinicalTrials.gov identifier: NCT01079533 |
| Trial name or title | Evaluation of treatments to improve smoking cessation medication adherence |
| Methods | Aims: to identify treatments that improve the use of cessation medications and to determine whether an increase in medication use results in increased cessation success. Study design: factorial RCT; recruitment: * Study duration: ongoing; study type: management; subtype: smoking |
| Participants | Inclusion criteria: ≥ 18 years of age or older; report smoking ≥ 5 cigarettes/day for the previous 6 months; able to read and write English; agree to attend visits, to respond to coaching calls, and to respond to IVR phone prompts; plans to remain in the intervention catchment area for at least 12 months; currently interested in quitting smoking (defined as would like to try to quit in the next 30 days). Sample size: 544; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: automated adherence prompting phone calls. Participants in this condition will receive fully automated prompts with messages designed to encourage participants to take their medication. Adherence prompting calls will occur twice in the first week of the quit attempt, and then once a week in weeks 2, 3, 4, 5, and 7. Those in the 26‐Week medication condition who are assigned to the active adherence prompting calls intervention, will receive one prompting call a week during Weeks 11, 15, 19 and 23. Arm b: electronic medication monitoring device (the helping hand) + feedback Arm c: cognitive medication adherence counselling (CAM) Arm d: intensive maintenance counselling Arm e: long‐term combination nicotine replacement therapy (patch + gum) Arm f: short‐term combination nicotine replacement therapy (patch + gum) |
| Outcomes | Latency to relapse |
| Starting date | June 2010 |
| Contact information | Michael C Fiore, MD, MPH, MBA, University of Wisconsin School of Medicine and Public Health, Center for Tobacco Research and Intervention |
| Notes | ClinicalTrials.gov identifier: NCT01120704 |
| Trial name or title | Computerized brief alcohol intervention (BI) for binge drinking HIV at‐risk and infected women |
| Methods | Aims: to examine two novel brief alcohol intervention delivery strategies specifically tailored to be culturally/socially relevant to this minority population Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: alcohol use |
| Participants | Inclusion criteria: 18 years of age or older; HIV infected or HIV negative and attending the Baltimore City Health Department sexually transmitted infection clinic for STI‐related services; consumes an average of 8 or more drinks per week OR has had two binge drinking episodes (4 drinks/occasion) in the last 3 months; sexually active; cognitively able to understand proposed research design (10 min screening, followed by random assignment to one of three study groups (if individual fulfills criteria for RCT enrollment); able to speak and understand English; able and willing to receive text messages Sample size: 450; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: computerised brief alcohol intervention + IVR booster calls: clinic‐based computerised brief alcohol intervention (delivered once) followed by 3 booster phone calls using interactive voice response technology + text messages Arm b: computerised brief alcohol intervention: clinic‐based computer‐delivered brief alcohol intervention delivered one time Arm c: attention control |
| Outcomes | Reduction in alcohol use |
| Starting date | Geetanjali Chander, MD |
| Contact information | Geetanjali Chander, MD, Johns Hopkins University |
| Notes | ClinicalTrials.gov identifier: NCT01125371 |
| Trial name or title | Trial of provider‐to‐patient interactive voice response (IVR) calls to improve weight management in community health centers (CHCs) |
| Methods | Aims: to test the effect of provider to patient interactive voice response (IVR) calls in local Community Health Centers within a weight management program. Study design: RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: weight management |
| Participants | Inclusion criteria: adult patients who have screened positive for overweight or obesity Sample size: 1228; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: a phone call with the pre‐recorded doctor's voice will be made to their patients who have been pre‐screened for obesity before the participant's appointments, prompting the participants to ask about physical activity, nutrition, and weight loss. Arm b: a phone call with a pre‐recorded neutral voice will be made to the doctor's patients who have been prescreened for obesity before their patient's appointments. The call will prompt them to ask their doctor about physical activity, nutrition, and weight loss. |
| Outcomes | Weight loss |
| Starting date | June 2009 |
| Contact information | Daniel O Clark, PhD, Indiana School of Medicine |
| Notes | ClinicalTrials.gov identifier: NCT01131143 |
| Trial name or title | Antidepressant adherence via telephonic interactive voice recognition (IVR) |
| Methods | Aims: to carry out a trial of a low‐cost, IT‐enabled antidepressants adherence program, specifically a direct‐to‐patient, automated telephone interactive voice recognition (IVR) intervention to boost patient antidepressants persistence Study design: RCT; recruitment: secondary care (*) Study duration: ongoing; study type: management; subtype: mental health |
| Participants | Inclusion criteria: Kaiser Permanente NW Region health plan members aged 21‐75 and be members for at least 6 months prior to the initial antidepressive medications dispense; with an EMR chart diagnosis or presenting complaint of a unipolar mood diagnosis, anxiety disorder, or any subclinical or 'not otherwise categorised' (NOC) variant of these. Sample size: 6000; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: no contact control arm Arm b: usual care (UC) control condition Arm c: UC plus the IVR automated telephone programme Arm d: UC plus the IVR automated telephone programme plus receipt of psycho‐education materials about antidepression medication use |
| Outcomes | Medication adherence (based on prescription refill data); cost‐effectiveness |
| Starting date | August 23, 2010 |
| Contact information | Clarke, Gregory; Kaiser Foundation Research Institute, Oakland, CA, United States |
| Notes | ClinicalTrials.gov identifier: NCT01188135 |
| Trial name or title | Text message reminder‐recalls for early childhood vaccination |
| Methods | Aims: to demonstrate the effectiveness of tailored text message appointment and immunisation reminders linked to a well‐established and functional immunisation registry to increase coverage rates and timeliness of the sentinel vaccines of measles, mumps and rubella and hepatitis A. Study design: RCT; recruitment:* Study duration: ongoing; study type: prevention; subtype: immunisations |
| Participants | Inclusion criteria: parents of child aged 9‐25 months; child with ≥ 1 visit to one of the participating clinical sites in the previous 12 months; parental cell phone number recorded in the registration system Sample size: 2586; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: automated phone call appointment reminder hep A: recall letter, automated phone call appointment reminder Arm b: text message reminders |
| Outcomes | Immunisation uptake (receipt of measles, mumps and rubella) |
| Starting date | June 2011 |
| Contact information | Melissa Stockwell, MD, MPH, Columbia University |
| Notes | ClinicalTrials.gov identifier: NCT01199666 |
| Trial name or title | ARemind: a personalized system to remind for adherence |
| Methods | Aims: to continue and complete development of a cellular phone‐based system that assists patients with their medication adherence Study design: RCT; recruitment: * Study duration: ongoing study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: stable ART (no change of ART for 3 months), ≥ 18 years of age self‐report adherence < 85% Sample size: 70; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: aRemind will personalise reminder messages based on adherence levels and facilitate patient phone calls with social workers/adherence counsellors when appropriate. It will also consist of a text‐messaging, IVR, or phone‐based pill count remote adherence assessment module Arm b: beepers are handheld portable devices which can be attached to a belt. At regular intervals corresponding to the participant's preferred reminder time, they buzz for a few minutes or until the participant presses a button to stop the buzzing. |
| Outcomes | Adherence to anti‐retroviral therapy |
| Starting date | October 2011 |
| Contact information | Vikram Sheel Kumar, Dimagi Inc. |
| Notes | ClinicalTrials.gov identifier: NCT01229722 |
| Trial name or title | Using IVR to maintain ACS patients on best practice guidelines (IVR‐ACS BPG) |
| Methods | Aims: to determine whether IVR technology can be used to bring postdischarge care for acute coronary syndrome (ACS) closer to best practice guidelines (BPGs) Study design: RCT; recruitment:* Study duration: ongoing; study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: patients discharged from London Health Science Centre with ACS (acute myocardial infarction, STEMI, NSTEMI or unstable angina); patients who have a land line telephone service at home; patients who speak English Sample size: *; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: participants in this arm will receive IVR follow‐up telephone calls at 1, 3, 6, 9, and 12 months postdischarge consisting of predetermined questions related to medication management, smoking cessation, diet, exercise and education as recommended by the ACC/AHA BPG for ACS. Upon completion of the IVR follow‐up, all participants will be called by a member of the clinical research staff and asked to complete a follow‐up survey. Arm b: usual care |
| Outcomes | Adherence with best practice guidelines |
| Starting date | January 2010 |
| Contact information | Neville Suskin, Lawson Health Research Institute |
| Notes | ClinicalTrials.gov identifier: NCT01260207 |
| Trial name or title | Interactive voice response technology to mobilize contingency management for smoking cessation |
| Methods | Aims: to examine the effectiveness of using interactive voice response technology (IVR) to implement contingency management in smokers who want to quit Study design: randomised controlled trial; recruitment: * Study duration: ongoing study type: management; subtype: smoking |
| Participants | Inclusion criteria: regular cigarette smoker, age ≥ 18, mailing address and valid photo I.D, wants transdermal nicotine Sample size: 90; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: contingency management for abstinence from cigarettes. Telephone counselling and nicotine patch plus contingency management (contingency management for smoking abstinence + transdermal nicotine + telephone counselling) Arm b: transdermal nicotine+ telephone counselling |
| Outcomes | Longest duration of abstinence |
| Starting date | January 2012 |
| Contact information | Sheila Alessi, PhD, University of Connecticut Health Center |
| Notes | ClinicalTrials.gov Identifier: NCT01484717 |
| Trial name or title | Kidney awareness registry and education (KARE) |
| Methods | Aims: to evaluate the feasibility and acceptability of two different interventions aimed at improving health outcomes among patients with chronic kidney disease, who are at high risk of chronic kidney disease progression Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: hypertension |
| Participants | Inclusion criteria: patients with chronic kidney disease (defined as estimated Glomerular Filtration Rate < 60 mL/min/1.73m2 or proteinuria consistently over 3 months) who speak English, Spanish or Cantonese and have a primary care provider Sample size: 100; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: automated telephone self‐management (ATSM) + health coach. Participants with chronic kidney disease will participate in an ATSM programme, which blends automated phone calls with live targeted call‐backs from a health coach. Participants will receive bi‐weekly automated calls for 52 weeks in their native language, consisting of pre‐recorded queries pertaining to the disease management, preventive services, and lifestyle changes. Participants will interact with the system using a touch‐tone keypad; out‐of‐range values or invalid responses will prompt a live call‐back within 24‐48 h by a health coach. Arm b: usual care |
| Outcomes | Change in blood pressure |
| Starting date | April 2013 |
| Contact information | Neil Powe, MD, University of California, San Francisco |
| Notes | ClinicalTrials.gov identifier: NCT01530958 |
| Trial name or title | Hybrid effectiveness‐implementation study to improve clopidogrel adherence |
| Methods | Aims: to test the effectiveness of a successfully piloted, evidence‐based, multifaceted intervention to improve patient adherence to clopidogrel following percutaneous coronary intervention (PCI) Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: all patients undergoing PCI with either a bare‐metal (BMS) or drug‐eluting stent (DES) and are prescribed clopidogrel regardless of the intended treatment duration; other potential antiplatelet medications (thienopyridines) used following PCI to accommodate changes in practice (e.g. prasugrel, ticagrelor, or ticlopidine); all patients undergoing PCI and receiving clopidogrel at the randomised sites, regardless of gender, ethnicity or race. Based on data from the national Clinical Assessment, Reporting and Tracking (CART) system, we anticipate ˜23% minorities (African American 16.8%, Hispanic 4.4%, Asian/American Indian 1.4%) and 3.1% women will be included in the study. Sample size: 2500; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: phone reminders and pharmacist. An alerted inpatient pharmacist or a designated study team member will bring the clopidogrel medication to the participant who has received a coronary stent. The participant will return home and receive IVR refill reminder calls. Arm b: usual care |
| Outcomes | Medication adherence |
| Starting date | January 2014 |
| Contact information | Michael Ho, MD PhD, VA Eastern Colorado Health Care System, Denver, CO |
| Notes | ClinicalTrials.gov identifier: NCT01609842 |
| Trial name or title | Improving transition outcomes through accessible health IT and caregiver support |
| Methods | Aims: to determine the extent to which the CarePartner model for supporting effective transitions from hospital to home improves outcomes of care, including lower readmission rates, emergency department visits, and improved patient functional status. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cardiovascular disease |
| Participants | Inclusion criteria: being discharged from study site with any diagnoses that indicate a chronic condition with a high risk of short‐term readmission, for example: stroke, heart failure, coronary artery disease, cardiac arrhythmias, COPD, peripheral vascular disease, deep venous thrombosis, pulmonary embolism, pneumonia, diabetes, urinary tract infection, cellulitis, gastroenteritis, fevers, and other infections; at least 50 years of age Sample size: 1692; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: telemonitoring plus self‐management support; automated telephone calls that ask about their health and self‐care along with tailored health‐related feedback. The participant's CarePartner receives health update reports about the participant and how they can help via e‐mail. Urgent health problems are reported to the participant's health care team via fax or e‐mail. Arm b: usual care |
| Outcomes | Short‐term readmission rates, emergency department visits, and participants' functional status |
| Starting date | August 2012 |
| Contact information | John Piette, University of Michigan |
| Notes | ClinicalTrials.gov identifier: NCT01672385 |
| Trial name or title | Trial of the CarePartner program for improving the quality of transition support |
| Methods | Aims: to determine the extent to which the CarePartner model for supporting effective transitions from hospital to home improves outcomes of care, including short‐term readmission rates, emergency department visits, and patients' functional status. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cardiovascular disease |
| Participants | Inclusion criteria: being discharged from study site with any diagnoses that indicate a chronic condition with a high risk of short‐term readmission, for example: stroke, heart failure, coronary artery disease, cardiac arrhythmias, COPD, peripheral vascular disease, deep venous thrombosis, pulmonary embolism, pneumonia, diabetes, urinary tract infection, cellulitis, gastroenteritis, fevers, and other infections; at least 21 years of age Sample size: 844; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: telemonitoring plus self‐management support; automated telephone calls that ask about their health and self‐care along with tailored health‐related feedback. The participant's CarePartner receives health update reports about the participant and how they can help via e‐mail. Urgent health problems are reported to the participant's health care team via fax or e‐mail. Arm b: usual care |
| Outcomes | Short‐term readmission rates, emergency department visits, and patients' functional status |
| Starting date | August 2012 |
| Contact information | John Piette, University of Michigan |
| Notes | ClinicalTrials.gov identifier: NCT01672398 |
| Trial name or title | Women's Walking Program (WWP3) |
| Methods | Aims: to compare the effects at 24 weeks and 48 weeks of the WWP plus three telephone conditions on increasing adherence to lifestyle physical activity over baseline physical activity. Study design: RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: physical activity |
| Participants | Inclusion criteria: Afican American women; sedentary, defined as no participation in regular planned (3 or more times a week) moderate (e.g. walking) or vigorous (e.g. jogging, speed walking) in the past 6 months; aged 40‐65 years; able to commit to attending the study group visits and have a telephone; without disabilities that would prevent regular participation in physical activity such as walking as determined by the physical activity readiness questionnaire (PAR‐Q) and baseline screening. Sample size: 288; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: walking programme + motivational interviewing calls Arm b: walking programme + automated calls Arm c: walking programme |
| Outcomes | Adherence to physical activity prescription |
| Starting date | March 2010 |
| Contact information | JoEllen Wilbur, PhD, APN, FAAN, Rush University Medical Center |
| Notes | ClinicalTrials.gov identifier: NCT01700894 |
| Trial name or title | Telemedicine for depression in primary care |
| Methods | Aims: to evaluate the feasibility and effectiveness of a care support programme developed in conjunction with the PC‐based assessment for patients suffering from depression, as based on two main objectives: to support GP decisions with treatment algorithms and improve the quality of GP and mental health service collaboration; and to improve patient adherence and treatment adherence by using appropriate telecommunication tools and technologically advanced tools to conduct systematic routine assessment. Study design: cluster randomised trial; recruitment: * Study duration: ongoing; study type: management; subtype: adherence to medication/laboratory tests |
| Participants | Inclusion criteria: patients aged 18‐65 years; PHQ‐9 score of ≥ 14 at baseline; IDS‐SR score of ≥ 26 at baseline; no filling of antidepressant medication; prescription for 270 prior days; illiteracy or the lack of working telephone to receive reminders. Sample size: 400; mean age: * sex: * ethnicity: * Country: Italy |
| Interventions | Arm a: GPs will use a CDSS with treatment algorithms, supervision from a consultant psychiatrist, and dispatch to participants of reminders via mobile texting or automatic mobile (or landline) phone calls to improve adherence to the treatment prescribed Arm b: treatment as usual |
| Outcomes | Proportion of participants reaching remission |
| Starting date | January 2013 |
| Contact information | Matteo Balestrieri, MD, IRCCS Centro San Giovanni di Dio Fatebenefratelli ([email protected]) |
| Notes | ClinicalTrials.gov Identifier: NCT01701791 |
| Trial name or title | 3M Study ‐ Maria Malmö mobile telephone study |
| Methods | Aims: to examine the effect on treatment retention of a mobile telephone follow‐up technique (interactive voice response), with or without personal feedback. Study design: RCT; recruitment: outpatient clinics Study duration: ongoing; study type: management; subtype: substance use |
| Participants | Inclusion criteria: patient applying for substance use disorder treatment at outpatient facility Maria Malmö, Malmö, Sweden, who are < 25 years old and who provide written informed consent to participate in the study Sample size: 120; mean age: * sex: * ethnicity: * Country: Sweden |
| Interventions | Arm a: IVR with personal feedback; twice weekly for 3 months with respect to symptoms and substance use, in both arms. This group also receives a personalised and automated feedback describing whether the symptom status of the participant is better, worse or equal, compared to the preceding follow‐up. Arm b: IVR without personal feedback |
| Outcomes | Retention in substance use disorder treatment at 3 months |
| Starting date | October 8, 2012 |
| Contact information | Anders C Håkansson, Region Skane |
| Notes | ClinicalTrials.gov identifier: NCT01706380 |
| Trial name or title | Comprehensive opioid management in patient aligned care teams (COMPACT) |
| Methods | Aims: to test the effectiveness of COMPACT for improving pain‐relevant outcomes including physical functioning and pain intensity; to determine whether opioid monitoring promotes guideline concordant care; and to examine key components of the intervention process to inform future implementation. Study design: factorial RCT; recruitment: * Study duration: ongoing; study type: management; subtype: pain |
| Participants | Inclusion criteria: presence of at least moderate non‐cancer, non‐headache pain (i.e. pain scores of ≥ 4 as measured by the Numeric Rating Scale) for a period of ≥ 3 months; receipt of chronic opioid therapy as defined by ≥ 90 continuous days out of any 104 day period in the prior 12 months; ability to participate safely in the walking portion of the intervention as evidenced by ability to walk at least one block; availability of a landline or cellular telephone Sample size: 308; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: the IVR system will be used to deliver a 12‐week course of opioid education and self‐management support followed by 24 weeks of skill maintenance training. Self‐management skills will include walking, stretching, pleasant activities, pacing, relaxation, cognitive restructuring, opioid education and sleep. Arm b: monitoring will include: proactive, IVR‐collected monthly information regarding opioid risk; and based on participants' IVR reports, automated output of electronic medical record documentation regarding participants' status for use by the primary care team |
| Outcomes | Pain‐related physical functioning; 7‐item interference sub‐scale of the brief pain inventory; providers' concordance with chronic opioid treatment practice guidelines |
| Starting date | October 2015 |
| Contact information | Alicia A Heapy, PhD, VA Connecticut Healthcare System |
| Notes | ClinicalTrials.gov identifier: NCT02508285; and NCT01737073 |
| Trial name or title | GlowCaps adherence randomized control trial |
| Methods | Aims: to study simple "behavioral economics" interventions that rely on consumer engagement to overcome cognitive and motivational barriers to medication adherence. Study design: RCT; recruitment: * Study duration: ongoing study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: patients diagnosed with chronic disease aged 16‐64 Sample size: 600; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: incentives and reminders (email reminders, text message reminders, or daily phone call reminders) Arm b: reminders only Arm c: no intervention |
| Outcomes | Medication adherence (number of doses taken) |
| Starting date | February 2015 |
| Contact information | Judd Kessler, University of Pennsylvania |
| Notes | ClinicalTrials.gov identifier: NCT01756001 |
| Trial name or title | Advanced comprehensive diabetes care for veterans with poorly‐controlled diabetes (ACDC) |
| Methods | Aims: to determine whether home telehealth‐based implementation of an evidence‐based intervention targeting veterans with persistent poorly controlled diabetes (PPDM) can improve glycated haemoglobin, patient self‐management, and comorbid depressive symptoms in this high‐risk, high‐cost population. Study design: RCT; recruitment: * Study duration: ongoing study type: management; subtype: diabetes |
| Participants | Inclusion criteria: veterans with type 2 diabetes managed for > 1 year at an eligible site (Durham, Raleigh, Greenville, or Morehead City) will be eligible for enrolment. Veterans with PPDM (defined as the presence of at least 2 glycated haemoglobin values of > 9.0% during the past year with no readings of < 9.0% despite ongoing medical care) by reviewing electronic medical records and soliciting referrals from primary physicians. Sample size: 50; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: health technology (HT) programme, provided with standard tele‐monitoring equipment by HT nursing staff (current HT practice at DVAMC is use of the Health Buddy 3 device for participants with landline phones and the Cardiocom IVR system for participants with cell phones), and will receive the study intervention for 6 months. Veterans without depressive symptoms on baseline PHQ‐9 assessment (PHQ‐9 < 10) will not initially be entered into the depression symptom management component of the intervention, but will be monitored for new symptoms throughout the intervention. Arm b: diabetes educational materials and management per their primary provider |
| Outcomes | Diabetes control; change in glycated haemoglobin from baseline to 6 months |
| Starting date | December 2013 |
| Contact information | Matthew Crowley, MD, VA Office of Research and Development |
| Notes | ClinicalTrials.gov identifier: NCT01778751 |
| Trial name or title | Can therapy alter CNS processing of chronic pain? A longitudinal study |
| Methods | Aims: to investigate whether a psycho‐therapeutic approach, group CBT + relapse prevention programme, Therapeutic Interactive Voice Response (TIVR), modifies the dysfunctional sensory, emotional, and cognitive neural circuitry associated with chronic pain Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: pain |
| Participants | Inclusion criteria: at least 12 months of muscular‐skeletal, non‐neuropathic pain Sample size: 120; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: 4 months of TIVR Arm b: group CBT Arm c: pain education Arm d: no intervention |
| Outcomes | Pain |
| Starting date | July 2010 |
| Contact information | Magdalena Naylor, MD, PhD, University of Vermont |
| Notes | ClinicalTrials.gov identifier: NCT01794988 |
| Trial name or title | Effectiveness of influenza vaccine reminder systems |
| Methods | Aims: to test the effectiveness and cost of different methods of reminders for annual influenza immunisation among adults with asthma and chronic obstructive pulmonary disease Study design: RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: immunisations |
| Participants | Inclusion criteria: aged 19‐64 years; enrolled in Kaiser Permanente Colorado health plan; diagnosis of asthma and/or COPD Sample size: 12,255; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: IVR only reminder group Arm b: postcard and IVR reminder group Arm c: postcard only reminder group |
| Outcomes | Receipt of influenza vaccine |
| Starting date | September 2012 |
| Contact information | Matthew F. Daley, MD, Kaiser Permanente |
| Notes | ClinicalTrials.gov identifier: NCT01852656 |
| Trial name or title | Optimizing veteran‐centered prostate cancer survivorship care |
| Methods | Aims: to conduct an RCT to compare a personally tailored automated telephone symptom management intervention for improving symptoms and symptom self‐management versus usual care Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cancer |
| Participants | Inclusion criteria: veteran patient at one of the three study sites, history of treatment for prostate cancer treated by surgery, radiation or androgen deprivation therapy between 1‐5 years prior to identification Sample size: 650; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: the intervention will consist of two components: automated telephone monitoring of prostate cancer survivor symptoms and goals for symptom reduction, based on a patient empowerment approach, and personally tailored newsletters that incorporate elements of CBT to improve survivors' identification with the material, confidence/self‐efficacy in symptom management, and to reduce common cognitive distortions related to successful implementation of behaviour change. Intervention‐group participants will receive 4 automated assessment and self‐management support calls over a 3‐month period (at baseline, 1 month, 2 months, 3 months) Arm b: enhanced usual care |
| Outcomes | The Expanded Prostate Cancer Index |
| Starting date | April 2015 |
| Contact information | Sarah T Hawley, PhD MPH BA, VA Office of Research and Development |
| Notes | ClinicalTrials.gov identifier: NCT01900561 |
| Trial name or title | Communication & peer support effects on physical activity in overweight postmenopausal women (BePHIT) |
| Methods | Aims: to design, develop and test the feasibility of implementing a physical activity intervention using tailored communication and IVR technology Study design: RCT; recruitment: * Study duration: ongoing; study type: prevention; subtype: weight management |
| Participants | Inclusion criteria: present a letter/documentation from a primary physician stating that they can participate in a physical activity programme that will require walking up to 10,000 steps per day, have a BMI of 25‐40 kg/m2 (inclusive), be postmenopausal, defined as no period for 12 months if over age 55, or no period for 12 months; also, women who have had their ovaries removed will be considered as postmenopausal, willing to participate in a wellness programme that lasts 12 weeks and involves walking for at least 30 min a day on most days, access to a cell phone during the 12‐week intervention, functional knowledge of English Sample size: 71; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: 12‐week physical activity intervention (walking programme) and receive health mail messages via IVR system and from a health coach. Participants in this arm of the study interacted with the IVR system and had the option of interacting with the health coach. Arm b: 12‐week physical activity intervention (walking programme) and receive health mail messages via IVR system. Participants in this arm of the study only interacted with the IVR system. |
| Outcomes | Change in time taken to complete a one mile walk |
| Starting date | April 2007 |
| Contact information | Electra Paskett, Ohio State University Comprehensive Cancer Center |
| Notes | ClinicalTrials.gov identifier: NCT01940016 |
| Trial name or title | Feasibility of using a structured daily diary |
| Methods | Aims: to implement a 66‐day structured daily diary with 90 HIV‐positive young men who have sex with men (MSM) to explore relationships among daily mood, stressful events, social support, substance use, sexual behavior, and adherence to ART among youth who are currently prescribed to take medication Study design: randomised cross‐over trial; recruitment: * Study duration: ongoing; study type: prevention; subtype: HIV |
| Participants | Inclusion criteria: receives services at one of the selected Adolescent Medicine Trial Unit (AMTU) sites; HIV‐1 infection as documented in the participant's medical record by at least one of the following criteria: reactive HIV screening test result with an antibody‐based, FDA‐licensed assay followed by a positive supplemental assay (e.g. HIV‐1 Western Blot, HIV‐1 indirect immunofluorescence); positive HIV‐1 DNA polymerase chain reaction (PCR) assay; plasma HIV‐1 quantitative RNA assay > 1000 copies/mL; or positive plasma HIV‐1 RNA qualitative assay; aged 16‐24 years, inclusive, at the time of screening; born biologically men and self‐identifies as man at the time of screening; HIV‐infected through sexual behavior; at least one self‐reported sexual encounter with another man involving oral or anal sex in the past 12 months prior to screening; at least one self‐reported episode of unprotected vaginal or anal intercourse within the past 90 days prior to screening and/or substance use, defined as at least 1 occasion in which ≥ 4 alcoholic beverages were consumed and/or ≥ 2 occasions of illicit drug use, in the past 90 days, as assessed by the assessment of substance use and sexual behavior questionnaire; has active cell phone service; is able to access his cell phone 7 days a week between 6:00 pm and 6:00 am the next morning; and is willing and able to use approximately 10 min of talk time and receive 2 text messages per day; consistent Internet access 7 days a week between 6:00 pm and 6:00 am the next morning; ability to understand, read, and speak English; ability to read at a fifth grade level, as assessed by the rapid estimate of adolescent literacy in medicine (REALM)‐TEEN; and willingness to provide signed informed consent for study participation. Sample size: 67; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: IVR system Arm b: interactive web response (IWR) system |
| Outcomes | Number of participants who complete the 66‐day structured daily diary; participant responses to how diary can provide personalised feedback on triggers to risk behaviors |
| Starting date | February 2013 |
| Contact information | Patrick Wilson, PhD, Columbia University |
| Notes | ClinicalTrials.gov identifier: NCT01953653 |
| Trial name or title | Screening and brief intervention via IVR for problematic use of alcohol: a randomized controlled trial |
| Methods | Aims: the study evaluates the efficacy of two interactive voice recognition (IVR) interventions, short IVR and therapeutic IVR Study design: RCT; recruitment: * Study duration: ongoing Study type: management; Sub ‐ type: alcohol |
| Participants | Inclusion criteria: alcohol Use Disorders Identification Test (AUDIT) >7 for men or AUDIT >5 for women. Sample size: 260; mean age: * sex: * ethnicity: * Country: Sweden |
| Interventions | Arm a: therapeutic IVR‐based conversation offering a menu of exercises and vignettes Arm b: IVR‐based alcohol diary with feedback Arm c: untreated control group |
| Outcomes | Change in total AUDIT score, as a summarised measure of alcohol use (including alcohol consumption and alcohol‐related problems |
| Starting date | February 2011 |
| Contact information | Anne H Berman, Karolinska Institutet |
| Notes | ClinicalTrials.gov Identifier: NCT01958359 |
| Trial name or title | Cancer symptom monitoring telephone system with nurse practitioner (NP) follow up |
| Methods | Aims: to test a daily telephone‐based automated symptom monitoring and response system to track and further treat unrelieved symptoms for patients living at home during chemotherapy treatment as compared with usual care which consists of participants calling their oncology provider for symptom concerns. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cancer |
| Participants | Inclusion criteria: adult (age ≥ 18); histological diagnosis of cancer; life expectancy of at least 3 months and cognitively able to participate; beginning a new course of chemotherapy that is planned for a minimum of 3 cycles; care is under the direction of one of the 8 designated provider teams; English‐speaking; has access to a telephone on a daily basis and is able to use the phone unassisted as verified by the study staff during participant orientation Sample size: 358; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: participants call the automated monitoring system daily to report presence, severity, and distress on 11 symptoms. The system provided automated self‐care coaching based on the symptoms reported and automatically generated alerts to the study NP if symptoms exceeded preset thresholds. 2 thresholds were set: a simple alert when severity or distress was ≥ 4 on a 10‐point scale and trend alerts based on a pattern of moderate severity over several days. The alerts went into a case management site. The study NP logged into the system daily and responded to the alerts within 24 h by calling participants to further assess the symptoms and to intensify symptom treatment using evidence based guidelines. Arm b: control group will receive usual care (via IVR). |
| Outcomes | Medical encounters telephone interview; symptom‐related interference with daily activities; SF‐36 functional status; work interference; work limitations questionnaire |
| Starting date | September 2007 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01973946 |
| Trial name or title | Improving follow‐up adherence in a primary eye care setting |
| Methods | Aims: to examine the effectiveness of three different ways of helping patients attend their recommended eye care appointments. Study design: RCT; recruitment: * Study duration: ongoing; study type: either; subtype: appointment reminders |
| Participants | Inclusion criteria: aged ≥ 18 years; primary eye care patients who were recommended for a 6‐, 12‐, or 24‐month follow‐up appointment in September 2013 to November 2013; access to a telephone Sample size: 1000; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: automated telephone call Arm b: personalised telephone call Arm c: usual care |
| Outcomes | Appointment adherence |
| Starting date | August 2013 |
| Contact information | Julia Haller, Wills Eye |
| Notes | ClinicalTrials.gov identifier: NCT02001129 |
| Trial name or title | Improving adherence to oral cancer agents and self care of symptoms using an IVR |
| Methods | Aims: to test and compare 2 strategies for improving adherence to their oral cancer medication prescriptions to standard care. Study design: factorial RCT; recruitment: * Study duration: ongoing; study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: ≥ 21 years, newly prescribed one of the designated oral cancer medications for treatment of cancer, ECOG score of 0,1, or 2, or Karnofsky score of 50 or higher, patient of one of the participating National Cancer Institute comprehensive cancer centres, able to speak, read, and understand English, able and willing to receive phone calls Sample size: 274; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: standard care for 12 weeks Arm b: standard care for 8 weeks + daily IVR for 4 weeks Arm c: daily IVR 8 weeks Arm d: daily IVR 4 weeks, every other day IVR 4 weeks |
| Outcomes | Medication adherence using pill count and self report |
| Starting date | March 2013 |
| Contact information | Barbara Given, Michigan State University ([email protected]) |
| Notes | ClinicalTrials.gov identifier: NCT02043184 |
| Trial name or title | Peer‐driven intervention for sleep apnea (PCORI) |
| Methods | Aims: to test whether participants in the peer‐driven intervention with IVR (PDI‐IVR) group will experience a greater participant satisfaction (measured by Likert scale and PACIC) and perception of care coordination (measured by CPCQ) than participants in the usual care (control) group. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: OSAS |
| Participants | Inclusion criteria: obstructive sleep apnea; 18‐85 years of age; availabilityaof cell or other reliable phone line (for subjects) Sample size: 257; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: IVR. Once a week for the first month followed by 4 phone conversations over the subsequent 2‐month period (8 scheduled telephone interactions) and as needed in the subsequent 3 months. There will be no more than 10 such 'as‐needed' phone calls in the latter 3 months between participant and peer‐buddy. Therefore, over the 6 months, there will not be in excess of 18 phone calls per subject assigned to peer‐buddy. Each phone conversation will last a maximum of 30 min. The PDI‐IVR system will be programmed to recognise the peer‐buddy's phones (cell or home) and be programmed to link this with the participant's phones (cell or home) and thereby protect the privacy of both participants Arm b: usual care |
| Outcomes | Patient rating of sleep‐specific services |
| Starting date | January 2014 |
| Contact information | Sairam Parthasarathy, MD, University of Arizona |
| Notes | ClinicalTrials.gov identifier: NCT02056002 |
| Trial name or title | Antiretroviral adherence and quality‐of‐life support for HIV+ patients in India with twice‐daily IVR calls with health and mental health messaging compared to weekly IVR survey only control condition: the mobile‐messaging adherence and support for health study, India. (MASHIndia) |
| Methods | Aims: to test whether twice‐daily IVR calls made at the estimated times of patients' antiretroviral (ART) medication dosing and 3 reminder calls for monthly clinic appointments, will result in improvements in ART adherence, appointment attendance, health indicators (CD4 cell counts), coping skills, social support, depressive symptoms, and other quality of life indicators, compared to a control group receiving one IVR assessment call each week, over 6 months. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: age ≥ 18; HIV +; taking first‐line ART 6 months or longer; missed taking any ART dose in the previous 6 months Able to speak and understand Bengali, Hindi, or English; willing to receive health‐related IVR messages on mobile phones; able to provide informed consent. Phase 2A ‐ client at Mamata Care and Treatment Center (MCTC) or member of Mamata Network of Positive Women (MNPW), or peer referral of MCTC client or MNPW member; received a CD4 count result in the prior 2 months. Phase 2B ‐ patient at Calcutta School of Tropical Medicine ART Centre, or peer referral of a patient Sample size: 400; mean age: * sex: * ethnicity: * Country: India |
| Interventions | Arm a: daily IVR calls intervention: consisting of 2 automated voice calls ('intervention messages') each day for 6 months, + 1 IVR assessment call (consisting of 4 questions) every week for 6 months Arm b: weekly IVR survey only control condition: consisting of standard care, + 1 IVR assessment call (consisting of 4 questions) every week for 6 months |
| Outcomes | Change in antiretroviral medication adherence measured by AIDS Clinical Trials Group (ACTG) self‐report measure |
| Starting date | April 2014 |
| Contact information | NA |
| Notes | ClinicalTrials.gov identifier: NCT02118454 |
| Trial name or title | Automated recovery line for medication assisted treatment |
| Methods | Aims: to test the effectiveness of Recovery Line in substance abuse Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: addiction |
| Participants | Inclusion criteria: ≥ 18 years old; currently receiving methadone maintenance treatment; illicit drug use in the past 14 days or a positive urine screen for any tested illicit drug Sample size: 60; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: Recovery Line plus usual care (RL + UC). Recovery Line is an automated computer‐based IVR system that provides CBT‐based modules. The RL + UC condition will include the customised therapeutic recommendations developed in Phase 1, and the contact reminders messages and time frame that maximised system use in Phase 2. Participants will receive an orientation, 24‐hour access, encouragement to use the system from clinic staff reminder, and technical assistance line for system problems. Participants will receive 12 weeks of system access. Arm b: usual care |
| Outcomes | Bi‐weekly urine screens negative for illicit drugs; self‐reported drug use; monthly days of self reported illicit drug abstinence |
| Starting date | October 2015 |
| Contact information | Brent A. Moore, Yale University |
| Notes | ClinicalTrials.gov identifier: NCT02124980 |
| Trial name or title | Smoking cessation following psychiatric hospitalisation |
| Methods | Aims: to adapt an Extended Care (ExC) model to smokers with severe mental illness (SMI) engaged in a psychiatric hospitalisation and to conduct a randomised, pragmatic effectiveness trial designed to assess the benefit of this adapted ExC in real‐world practice Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: smoking |
| Participants | Inclusion criteria: ≥ 18 years of age, current smoker (i.e.≥ 5 cigarettes/day when not hospitalised) Sample size: 422; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: extended care. A 40‐min, in‐hospital motivational counselling session about smoking cessation, 8 IVR phone calls over 90 days, including the possibility of a warm transfer to a telephone tobacco quit line and prescriptions for combination (2 types of) nicotine replacement medications. Arm b: brief education. A brief 5‐10 min education session with a hospital staff member, during which they will be provided with: a brochure describing the services of their local tobacco quit line and the services provided, and a brochure describing FDA‐approved smoking cessation medications, their usage and side effects. |
| Outcomes | Biochemically verified smoking abstinence via saliva cotinine |
| Starting date | April 2015 |
| Contact information | Nancy A Rigotti, MD Massachusetts General Hospital |
| Notes | ClinicalTrials.gov identifier: NCT02204956 |
| Trial name or title | Diabetes prevention among post‐partum women with history of gestational diabetes (Star‐Mama) |
| Methods | Aims: to develop a patient‐tailored telephone‐base counselling intervention for young Latino women who are at high risk of diabetes. Study design: RCT; recruitment: * Study duration: ongoing study type: management; subtype: weight management |
| Participants | Inclusion criteria: postpartum Latino women (English or Spanish speakers) with history of gestational diabetes; aged ≥ 18 Sample size: 180; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: 6 months weekly automated phone calls with queries and narratives about health habits. The participant's answers will be sent to a health coach who will follow up with the participant, and develop a plan with the participant to address her needs. Arm b: educational resource support |
| Outcomes | Weight loss |
| Starting date | December 2014 |
| Contact information | Margaret A Handley, MPH, PhD, University of California, San Francisco |
| Notes | ClinicalTrials.gov identifier: NCT02240420 |
| Trial name or title | System Alignment for VaccinE Delivery (SAVED): improving rates of influenza and pneumococcal vaccination through patient outreach, improved medical record accuracy and targeted physician alerts |
| Methods | Aims: to improve the capture of vaccinations administered to Reliant Medical Group (RMG) patients in the community, hospitals and nursing facilities via system‐level health information exchange (HIE) Study design: factorial RCT; recruitment: by invitation Study duration: ongoing; study type: prevention; subtype: immunisations |
| Participants | Inclusion criteria: RMG patients ≥ 18 years of age. Overdue for vaccination against influenza and/or not up‐to‐date on vaccination for pneumococcal vaccine per RMG EHR data. No documented allergy to the vaccination in question. Sample size: 30,000; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: e‐portal message with IVR call Arm b: e‐portal message with no IVR call Arm c: no e‐portal message with IVR call Arm d: no e‐portal message with no IVR call (control, e‐portal users) Arm e: IVR call Arm f: no IVR call (control, non e‐portal users) |
| Outcomes | Percent of intervention participants with self‐reported influenza vaccinations documented in electronic health record (EHR) |
| Starting date | October 10, 2014 |
| Contact information | Sarah Cutrona, University of Massachusetts, Worcester |
| Notes | ClinicalTrials.gov identifier: NCT02266277 |
| Trial name or title | Caring Others Increasing EngageMent in PACT (CO‐IMPACT) |
| Methods | Aims: to compare 2 methods of increasing engagement in care and success in diabetes management, among patients with diabetes with high‐risk features, who also have family members involved in their care. Study design: RCT; recruitment:* Study duration: ongoing; study type: management; subtype: diabetes |
| Participants | Inclusion criteria: provide signed and dated informed consent form; willing to comply with all study procedures and plan to be be available for the duration of the study; men or women, aged 30‐70 years old; plan to get most diabetes care at Ann Arbor VA over the subsequent 12 months; able to use telephone to respond to bi‐weekly automated IVR calls; be able to identify an adult family member or friend who is regularly involved in their health management or health care (involved with medications, managing sugars, coming to appointments, etc); have a diagnosis of diabetes and be at high‐risk for diabetes complications, defined as: a diagnosis of diabetes based on encounter diagnoses from 1 inpatient or 2 outpatient encounters (ICD9 code of 250.xx, 357.2x, 362.xx, 366.41, 962.3 or E932.3) OR a diabetes medication (at least one > 3 month prescription from VA drug classes HS501 (insulin) or HS502, other than metformin), have an assigned VAAAHS primary care provider and at least 2 visits to VAAHS primary care in the previous 12 months, poor glycaemic control (last glycated haemoglobin > 9% or glycated haemoglobin > 8% among participants < 55 years old) OR poor blood pressure control (last blood pressure 160/100 or mean 6 month blood pressure > 150/90); active AAVA primary care patients ‐ at least 2 visits in last 12 months (for patients) Sample size: 480; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: patient and supporter (dyad) receive one coaching session on action planning, communicating with providers, navigation skills and support skills; preparation by phone before patients primary care visits; after‐visit summaries by mail; and biweekly automated phone calls to prompt action on new patient health concerns Arm b: patient and their health supporter (dyad) will receive PACT care for high‐risk diabetes, which includes (at primary care team discretion): nurse care manager visits, diabetes education classes, chronic disease self‐management groups, telehealth, clinical pharmacist visits |
| Outcomes | Patient activation; cardiac event 5‐year risk score |
| Starting date | January 2016 |
| Contact information | VA Ann Arbor Healthcare System, Ann Arbor, MI, Ann‐[email protected] |
| Notes | ClinicalTrials.gov identifier: NCT02328326 |
| Trial name or title | Health literacy interventions to overcome disparities in colorectal cancer screening |
| Methods | Aims: to compare the effectiveness of 2 distinct follow‐up strategies to promote colorectal cancer screening: a prevention coordinator (PC) approach vs an automated telephone reminder (ATR) system Study design: randomised controlled trial; recruitment: * Study duration: ongoing; study type: prevention; subtype: screening |
| Participants | Inclusion criteria: a patient of the identified clinics, age 50‐75 (based on ACS guidelines) and can speak and understand English Sample size: 800; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: automated telephone reminder Arm b: prevention coordinator Arm c: health literacy appropriate education and demonstration |
| Outcomes | Colorectal cancer screening rate |
| Starting date | February 2015 |
| Contact information | Connie L Arnold, PhD ([email protected]) |
| Notes | ClinicalTrials.gov Identifier: NCT02360605 |
| Trial name or title | Interventions to support long‐term adherence and decrease cardiovascular events post‐myocardial infarction (ISLAND) |
| Methods | Aims: to evaluate whether and in what format to sustain and/or scale‐up post‐MI educational reminder interventions. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: adherence to medications/laboratory tests |
| Participants | Inclusion criteria: patients aged 18 years and older having a coronary angiography following a myocardial infarction (ST‐elevation myocardial infarction or non‐ST‐elevation myocardial infarction), with evidence of coronary artery disease (> 50% blockage of left main or > 70% blockage of either other main cardiac arteries); discharged from the catheterisation centre alive, either home or to a local (non‐cardiac) hospital; and Ontario residents Sample size: 2571; mean age: * sex: * ethnicity: * Country: Canada |
| Interventions | Arm a: usual care + letters + automated calls (IVR phone calls to the participant delivered approximately 2 weeks after the letters, as well as personalised telephone follow‐up by trained peer health workers for participants identified by the IVR system as non‐adherent. The automated algorithm is designed to identify patients who are non‐adherent and who may benefit from personalised educational phone call and/or system navigation support by the peer health worker. Peer health workers will not provide clinical advice. Arm b: usual care + letters |
| Outcomes | Medication adherence |
| Starting date | September 2015 |
| Contact information | Noah Ivers, Women's College Hospital |
| Notes | ClinicalTrials.gov identifier: NCT02382731 |
| Trial name or title | Developing accessible telehealth programs for diabetes and hypertension management in bolivia |
| Methods | Aims: to evaluate the feasibility and impact of an automated phone system in monitoring and improving self‐care and health outcomes among patients with diabetes and/or hypertension in Bolivia, in addition to assessing the additional benefit of support from a family member or friend. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: diabetes/hypertension |
| Participants | Inclusion criteria: 21‐80 years of age; diagnosis of hypertension, a systolic blood pressure > 140 mmHg, and/or diagnosis of diabetes; access to a functional cell phone; able to respond to automated telephone calls Sample size: 100; mean age: * sex: * ethnicity: * Country: Bolivia |
| Interventions | Arm a: experimental: participant only ‐ health information technology/care manager (HITCM)‐only participants enrolling without a CarePartner receive weekly HITCM automated assessment and self‐care support calls with feedback to the clinical team. Arm b: experimental: participant and CarePartner ‐ HITCM‐only participants enrolling with a CarePartner receive weekly HITCM automated assessment and self‐care support calls with feedback to the clinical team. Arm c: experimental: participant and CarePartner ‐ HITCM + CP participants enrolling with a CarePartner receive weekly HITCM automated assessment and self‐care support calls with feedback to the clinical team plus updates to their CarePartner via phone or email. |
| Outcomes | Change from baseline on self‐care behaviours and health at 16 weeks |
| Starting date | June 2014 |
| Contact information | John Piette, University of Michigan |
| Notes | ClinicalTrials.gov identifier: NCT02429297 |
| Trial name or title | Impact of automated calls on pediatric patient attendance in Chile (Health Call) |
| Methods | Aims: evaluate whether a patient reminder system, Health Call, can decrease the overall failure to attend appointment rate as a percentage of overall appointments. Study design: RCT; recruitment: * Study duration: ongoing; study type: either; subtype: appointment reminder |
| Participants | Inclusion criteria: guardian with a phone number (landline or mobile) who is able to receive and answer voice calls, is willing to take part in the study and complete the consent form, is sufficiently proficient in Spanish so as to complete the questionnaire, has a referral appointment at Hospital Luis Calvo Mackenna who is ≤ 18 years of age Sample size: 564; mean age: * sex: * ethnicity: * Country: Chile |
| Interventions | Arm a: Health Call is an automated interactive voice reminder system that can contact guardians of patients ahead of their child's appointment, asks then confirms a security question about the participant, then, if the call recipient passes the security screen, provides a reminder about upcoming appointment. Arm b: no calls |
| Outcomes | 'Do not attend' (DNA) |
| Starting date | December 2013 |
| Contact information | William Weiss, DrPH, MA ([email protected]), Johns Hopkins Bloomberg School of Public Health |
| Notes | Clinicaltrials.gov: NCT02442089 |
| Trial name or title | Walk On! Physical activity coaching |
| Methods | Aims: to determine the effectiveness of a 12‐month physical activity coaching intervention (Walk On!) compared to standard care for 1650 COPD patients from a large integrated health care system Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: COPD |
| Participants | Inclusion criteria: patients with any COPD‐related hospitalisation, emergency department visit or observational stay in the previous 12 months; COPD‐related encounters are defined according to the Centers for Medicare and Medicaid Services (CMS) and National Quality Forum (NQF) criteria for the Hospital Readmission Reduction Program. The following principal discharge diagnoses of COPD (ICD‐9 codes: 491.21, 491.22, 491.8, 491.9, 492.8, 493.20, 493.21, 493.22, and 496) or respiratory failure (ICD‐9 codes: 518.81, 518.82, 518.84, 799.1) with a secondary diagnosis of COPD exacerbation (ICD‐9 codes: 491.21, 491.22, 493.21, 493.22) will be used; age > 40 years; on at least a bronchodilator or steroid inhaler prior to the encounter or if not on an inhaler, had a previous disease diagnosis; continuous health plan membership in the 12 months prior to the encounter Sample size: 1650; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: the 12‐month Walk On! intervention includes a baseline in‐person assessment, collaborative monitoring of steps using 2 types of activity sensors, semi‐automated step goal recommendations using an IVR system or web application, ongoing individualised reinforcement from a physical activity coach, and peer/family support. Arm b: usual care |
| Outcomes | Composite: all‐cause hospitalisations, emergency department (ED) visits, observational stays, and mortality |
| Starting date | June 2015 |
| Contact information | Huong Q Nguyen, PhD, RN, Kaiser Permanente |
| Notes | ClinicalTrials.gov identifier: NCT02478359 |
| Trial name or title | SelfManagement Automated and Real‐Time telephonic support (SMARTSteps) |
| Methods | Aims: to investigate differences in 6‐month changes in patient‐centred outcomes including quality of life and functional status (SF‐12 and number of days spent in bed due to illness), comparing participants exposed to ATSM with wait‐list controls and comparing participants exposed to ATSM (SMARTSteps‐ONLY) with ATSM augmented by medication adherence and intensification (SMARTSteps‐PLUS) Study design: stepped wedge; recruitment: primary care (mail and telephone) Study duration: ongoing; study type: management; subtype: diabetes |
| Participants | Inclusion criteria: San Francisco Health Plan (SFHP) membership; ≥ 1 primary care clinic visit in the preceding 24 months at one of our designated clinics; age ≥ 18 years; a diagnosis of diabetes (type 1 or 2); English‐, Cantonese‐, or Spanish‐speaking; access to a touch‐tone phone; and plans to remain in the region during the evaluation period (12 months) Sample size: 362; mean age: 55 years; sex: women ‐ 71%, men ‐ 29%; ethnicity: Asian ‐ 58.6%, black ‐ 6.9%, white ‐ 9.4%, Hispanic ‐ 22.4% Native American/Eskimo ‐ 0.3%, Hawaiian/Pacific Islander ‐ 0.8%, other ‐1.4%, Unknown ‐ 0.3% Country: USA |
| Interventions | Arm a: SMARTSteps‐ONLY received the ATSM intervention within 2 weeks. Developed with extensive input from participants to be sensitive to literacy, language, and culture in the target populations, this ATSM system provided 27 weeks of 8‐12 min weekly calls in English, Cantonese, or Spanish. Participants specified the weekday and time convenient for their schedules or called toll‐free into the system if they missed their scheduled call. The content consisted of rotating sets of queries about self‐care (such as diet, exercise, and medication adherence), psychosocial issues (such as depressive symptoms), and access to preventive services (such as eye care). Participants responded via touch‐tone commands, and based on their answers, participants heard automated health education messages in the form of narratives Arm b: SMARTSteps‐PLUS intervention to detect and intervene for participants whose medication treatment was sub‐optimal Arm c: wait‐list (controls) continued to receive usual care through their clinics, as well as all existing SFHP benefits (reminders and incentives for receipt of recommended health services, including laboratory testing, eye and foot examination, and influenza vaccination). At the end of the 6‐month wait‐list period, each participant "crossed‐over" to begin SMARTSteps‐ONLY or SMARTSteps‐PLUS, depending on initial randomisation arm. |
| Outcomes | Quality of life and functional status; diabetes self‐efficacy and self‐management behaviour; medication adherence in the preceding 7 days; participant perspectives on the structure of their care; glycated haemoglobin; blood pressure; low‐density lipoproteins |
| Starting date | April 2009 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT00683020 |
| Trial name or title | The Helping HAND 2 |
| Methods | Aims: to test the hypothesis that a multi‐component sustained care intervention is more effective than standard care in helping hospitalised cigarette smokers stop smoking after hospital discharge Study design: RCT; recruitment: secondary care (health professional referral) Study duration: ongoing; study type: management; subtype: smoking |
| Participants | Inclusion criteria: admission to a participating hospital; received tobacco cessation counselling for > 5 min in hospital; age ≥18 years; current daily smoker (defined as having smoked ≥ 1 cigarette/day in the past month when smoking as usual); plan to sustain or initiate a quit attempt immediately after hospital discharge Sample size: 1350; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: multi‐component sustained care: the IVR calls at 2, 12, 28, 58, and 88 days after discharge. For each call, the IVR system makes up to 8 attempts to reach participants for each scheduled call, beginning on the scheduled call day and proceeding with 2 attempts per day for 4 days or until the call is completed; access to smoking cessation telephone counselling support; pharmacotherapy Arm b: standard care (control) group receive the same bedside counselling session in the hospital as the intervention group. The counsellor informs smokers about postdischarge counselling resources, provides specific advice to call the state telephone quit line, makes a specific recommendation to the hospital physician for postdischarge medication, and completes a consultation note in the participant's hospital record. No additional resources are provided to the participant after discharge from the hospital |
| Outcomes | Tobacco abstinence (biochemically validated); self‐reported tobacco abstinence; duration of tobacco abstinence after discharge; proportion of participants who make a 24‐h quit attempt after discharge |
| Starting date | December 2012 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01714323 |
| Trial name or title | The E‐Coach |
| Methods | Aims: to test the E‐Coach intervention in congestive heart failure and COPD patients admitted to a large tertiary hospital Study design: RCT; recruitment: tertiary care (health professional referral) Study duration: ongoing; study type: management; subtype: heart failure |
| Participants | Inclusion criteria: patients are considered for inclusion if they were admitted from home with chronic heart failure or COPD, have an estimated prognosis of greater than 6 months, are English‐speaking, have a telephone, and are expected to be discharged to home Sample size: 478; mean age: 63 years; sex: women ‐ 47%; men ‐53%; ethnicity: * Country: USA |
| Interventions | Arm a: E‐Coach intervention is delivered through an IVR monitoring system that is based on Coleman's 4 pillars of care transition support and a web‐based 'dashboard' for care transition nurses, with alerts of patient/caregiver concerns after discharge. After discharge, Ida is programmed to call participants daily for 7 days and for an additional 21 sessions thereafter (either daily or every 3 days, depending on participant preference). In a stepped‐care approach, the IVR is then supported by the care transition nurse, who monitors participant issues through the E‐Coach IVR secure web‐based dashboard. Support for participant self‐management is provided through personal telephone‐based interactions when needed, up to 2 months (60 days) after discharge. Clients are advised to use condoms as dual protection from HIV and sexually transmitted infections as appropriate. Follow‐up calls to clients are made during preferred times indicated by the client on her registration form. Clients in the intervention arm are also able to call the MOTIF service at any time to request to speak with a counsellor. Clients who opt to receive the OC or injectable can opt in to receive additional reminder messages appropriate to their method (that is, to start a new packet of pills or when to receive a new injection). The sixth and final voice message provides similar information to the first five, but also reminds the client that this will be the last message they will receive. Arm b: control group received usual care (no intervention). |
| Outcomes | Rehospitalisations; rehospitalisations at 90 days; community tenure |
| Starting date | 1 June 2010 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01135381 |
| Trial name or title | Care partners: web‐based support for caregivers of veterans undergoing chemotherapy |
| Methods | Aims: to determine if VA patients undergoing chemotherapy who receive automated telephonic assessment and symptom management advice plus web‐based feedback to inform and engage a CarePartner report significant improvement in the number and severity of symptoms compared to patients receiving monitoring only. Study design: RCT; recruitment: * Study duration: ongoing; study type: management; subtype: cancer |
| Participants | Inclusion criteria: all participants must be ≥ 18 years, cognitively intact, English‐speaking, able to hear, and own a telephone. Patients can have any solid tumour; must be initiating IV cytotoxic chemotherapy and, if recurrent, have experienced a 1 month treatment free interval. Caregivers must have a computer with high speed Internet access. Sample size: 214; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: participants receive automated telephone symptom assessment and symptom management advice; caregivers receive access to a website that updates them on participant's symptoms and provides tailored problem solving advice. Arm b: participants receive automated telephone symptom assessment and symptom management advice; caregivers receive nothing. |
| Outcomes | Symptom severity |
| Starting date | October 2010 |
| Contact information | Maria J Silveira, MD MA MPH, VA Ann Arbor Healthcare System, Ann Arbor, MI |
| Notes | ClinicalTrials.gov identifier: NCT00983892 |
| Trial name or title | MObile Technology for Improved Family Planning (MOTIF) |
| Methods | Aims: to evaluate a mobile phone‐based intervention using voice messages to support postabortion family planning (PAFP) in Cambodia by testing whether additional regular, structured, interactive mobile phone‐based support improves use of PAFP Study design: RCT; recruitment: primary care (health professional referral) Study duration: ongoing; study type: prevention; subtype: sexual health |
| Participants | Inclusion criteria: participants are eligible for the trial if they are attending for induced abortion, aged ≥ 18 years, own a mobile phone, do not want to have a child at the present time and are willing to receive simple voice messages from Marie Stopes International Cambodia related to contraception Sample size: 500; mean age: * sex: * ethnicity: Cambodian Country: the Netherlands |
| Interventions | Arm a: six automated voice messages to remind clients about available family planning methods and provide a conduit for additional support. Clients can respond to message prompts to request a phone call from a counsellor, or alternatively state they have no problems. Clients requesting to talk to a counsellor, or who do not respond to the message prompts, receive a call from a Marie Stopes International Cambodia counsellor who provides individualised advice and support regarding family planning. Arm b: standard of care without the additional mobile phone‐based support |
| Outcomes | Use of an effective modern method of contraception at 4 months; repeat abortion; contraceptive discontinuation |
| Starting date | 30 March 2013 |
| Contact information | |
| Notes | ClinicalTrials.gov identifier: NCT01823861 |
| Trial name or title | Rationale, design, and implementation protocol of the Dutch clinical practice guideline pain in patients with cancer: a cluster RCT with Short Message Service (SMS) and IVR |
| Methods | Aims: to evaluate the implementation of the Dutch guideline Pain in Patients with Cancer to improve pain reporting, pain measurement, and hence pain control in patients with cancer and pain Study design: cluster RCT; recruitment: secondary care (health professional referral) Study duration: ongoing; study type: management; subtype: cancer pain |
| Participants | Inclusion criteria: diagnosed with cancer; aged ≥ 18 years; pain intensity of 3 or more on a numeric rating scale for the worst pain experienced in the last 24 h; and having and being familiar with the use of a mobile phone Sample size: 210; mean age: * sex: * ethnicity: * Country: the Netherlands |
| Interventions | Arm a: SMS‐IVR + personal advice by phone on how to reduce pain if rating is ≥ 5 or higher on a numeric rating scale (NRS) of 0‐10. The research nurse of the hospital, specialised in pain treatment and trained for this project, will provide the personal advice Arm b: control group will receive a leaflet on cancer pain |
| Outcomes | The first primary outcome is the percentage of all participants that visit the medical oncology outpatient clinic with adequate pain therapy/medication. Pain treatment adequacy will be calculated with both the Cleeland's Pain Management Index (PMI) and Ward's variation of the PMI |
| Starting date | November 2009 |
| Contact information | |
| Notes | Netherlands Trial Register (NTR): NTR2739 |
| Trial name or title | The study of automated telephone programs for the maintenance of dietary change |
| Methods | Aims: to compare two theory‐based interventions (social cognitive theory (SCT) vs goal systems theory (GST)) designed to maintain previously achieved improvements in fruit and vegetableconsumption Study design: RCT; recruitment: other ‐ voter registration list (mail and telephone) Study duration: ongoing; study type: prevention; subtype: cancer |
| Participants | Inclusion criteria: participants were adults ≥ 18 years old who consumed less than the recommended level of fruits and vegetables (i.e. ≤ 5 servings/day), lived in the Boston area, had access to a touch‐tone telephone, and were generally healthy Sample size: 1049; mean age: * sex: * ethnicity: * Country: USA |
| Interventions | Arm a: TLC maintenance intervention based on SCT used a skills‐based approach to build self‐efficacy. It assessed confidence in and barriers to eating fruit and vegetables, provided feedback on how to overcome barriers, plan ahead, and set goals Arm b: control group received assessment only |
| Outcomes | Fruit and vegetable intake; self‐efficacy; costs |
| Starting date | July 2006 |
| Contact information | |
| Notes | Clinicaltrials.gov: NCT00148525 |
ACS: acute coronary syndrome; ART: antiretroviral therapy; ATCS: automated telephone communication system; ATSM: automated telephone self‐management; BMI; body mass index; CBT: cognitive behavioural therapy; CDSS: clinical decision support system; CPCQ: client perceptions of coordination questionnaire; COPD: chronic obstructive pulmonary disease; ECOG: Eastern Cooperative Oncology Group; EHR: electronic health record; EMR: electronic medical record; ESAS: Edmonton symptom assessment system; FDA: Food and Drug Administration; GP: general practitioner; IDS‐SR: inventory of depressive symptomatology (self‐report); IVR: interactive voice response; MI: motivational interviewing; NA: not available; NSTEMI: non‐ST‐elevation myocardial infarction; OSAS: obstructive sleep apnoea syndrome; PACIC: patient assessment of chronic illness care; PCI: percutaneous coronary intervention; PHQ‐9: personal health questionnaire, version 9; RCT: randomised controlled trial; SF‐36: Short Form‐36‐Health Survey; STEMI: ST‐elevation myocardial infarction; UC: usual care; UKPDS: UK Prospective Diabetes Study; VA: Veteran's Administration.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immunisation in children Show forest plot | 5 | 10454 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.18, 1.32] |
| Analysis 1.1  Comparison 1 ATCS vs control for improving health services uptake (immunisations), Outcome 1 Immunisation in children. | ||||
| 2 Immunisation in adolescents Show forest plot | 2 | 5725 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.02, 1.11] |
| Analysis 1.2  Comparison 1 ATCS vs control for improving health services uptake (immunisations), Outcome 2 Immunisation in adolescents. | ||||
| 3 Immunisation in adults Show forest plot | 2 | 1743 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.53, 9.02] |
| Analysis 1.3  Comparison 1 ATCS vs control for improving health services uptake (immunisations), Outcome 3 Immunisation in adults. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer screening Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 ATCS vs control for improving health services uptake (screening rates), Outcome 1 Breast cancer screening. | ||||
| 1.1 Multimodal/complex interventions | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.55, 3.04] |
| 1.2 IVR | 2 | 2599 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.11] |
| 2 Colorectal cancer screening Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2 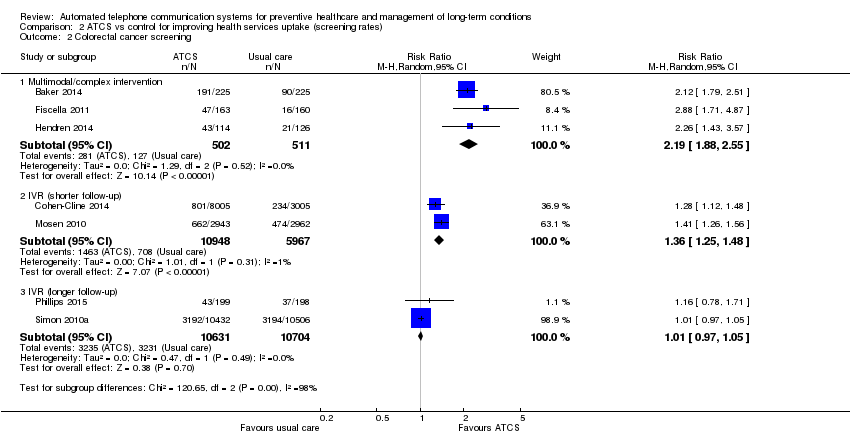 Comparison 2 ATCS vs control for improving health services uptake (screening rates), Outcome 2 Colorectal cancer screening. | ||||
| 2.1 Multimodal/complex intervention | 3 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.88, 2.55] |
| 2.2 IVR (shorter follow‐up) | 2 | 16915 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.25, 1.48] |
| 2.3 IVR (longer follow‐up) | 2 | 21335 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.97, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI adults Show forest plot | 3 | 672 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.38, 0.11] |
| Analysis 3.1 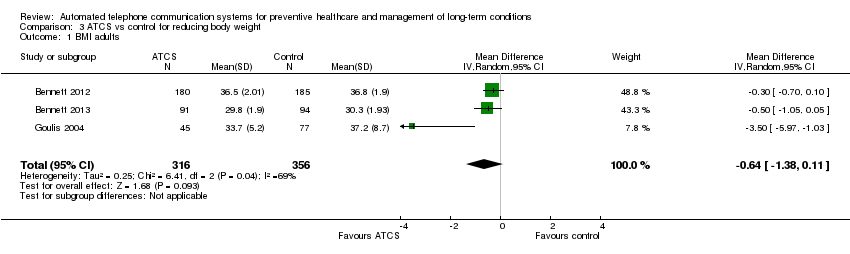 Comparison 3 ATCS vs control for reducing body weight, Outcome 1 BMI adults. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glycated haemoglobin Show forest plot | 7 | 1216 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.50, ‐0.01] |
| Analysis 4.1 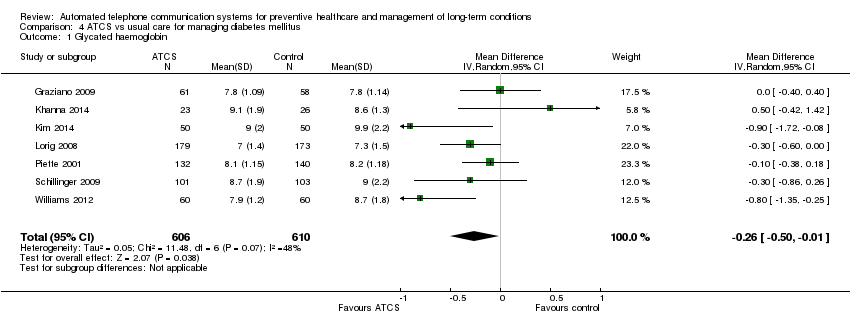 Comparison 4 ATCS vs usual care for managing diabetes mellitus, Outcome 1 Glycated haemoglobin. | ||||
| 2 Self‐monitoring of diabetic foot Show forest plot | 2 | 498 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.42] |
| Analysis 4.2 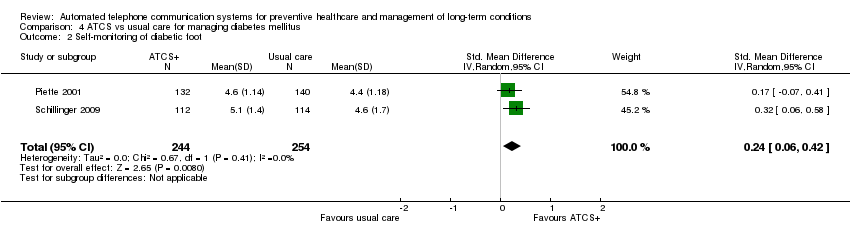 Comparison 4 ATCS vs usual care for managing diabetes mellitus, Outcome 2 Self‐monitoring of diabetic foot. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiac mortality Show forest plot | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.21, 1.67] |
| Analysis 5.1  Comparison 5 ATCS vs usual care for reducing healthcare utilisation in patients with heart failure, Outcome 1 Cardiac mortality. | ||||
| 2 All‐cause mortality Show forest plot | 3 | 2165 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.28] |
| Analysis 5.2 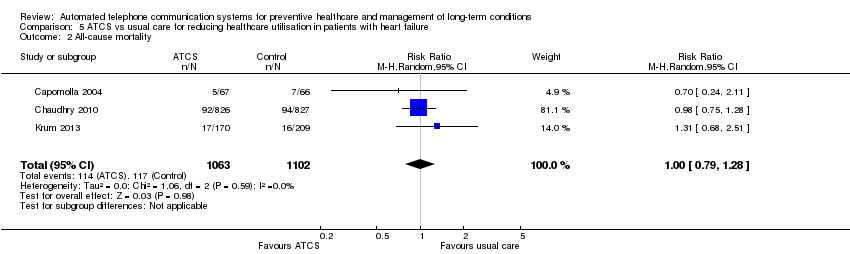 Comparison 5 ATCS vs usual care for reducing healthcare utilisation in patients with heart failure, Outcome 2 All‐cause mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic blood pressure Show forest plot | 3 | 65256 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.12, ‐1.66] |
| Analysis 6.1  Comparison 6 ATCS vs usual primary care and education or usual care for managing hypertension, Outcome 1 Systolic blood pressure. | ||||
| 2 Diastolic blood pressure Show forest plot | 2 | 65056 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐2.62, 2.66] |
| Analysis 6.2 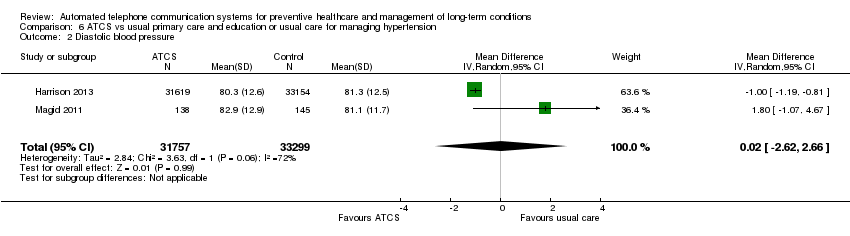 Comparison 6 ATCS vs usual primary care and education or usual care for managing hypertension, Outcome 2 Diastolic blood pressure. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking abstinence Show forest plot | 7 | 2915 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.98, 1.46] |
| Analysis 7.1  Comparison 7 ATCS for smoking cessation, Outcome 1 Smoking abstinence. | ||||

Primary preventive healthcare

Influencing factors and preventive strategies in type 2 diabetes

Conceptual framework of ATCS in preventive healthcare
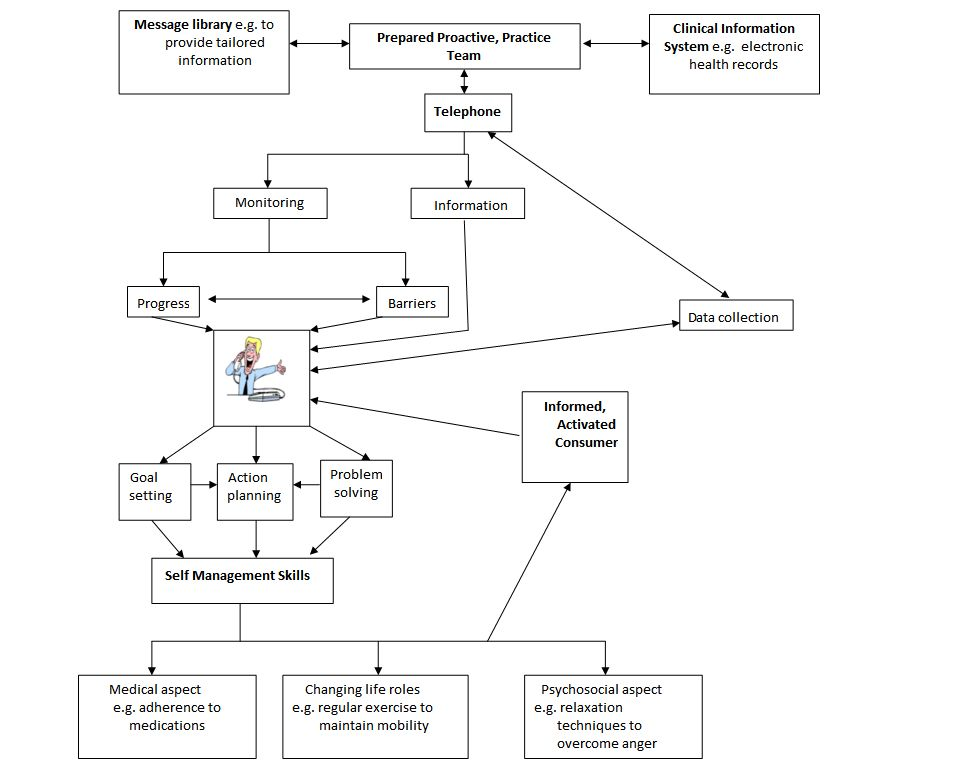
Conceptual framework of ATCS in the management of long‐term conditions

Management of long‐term conditions
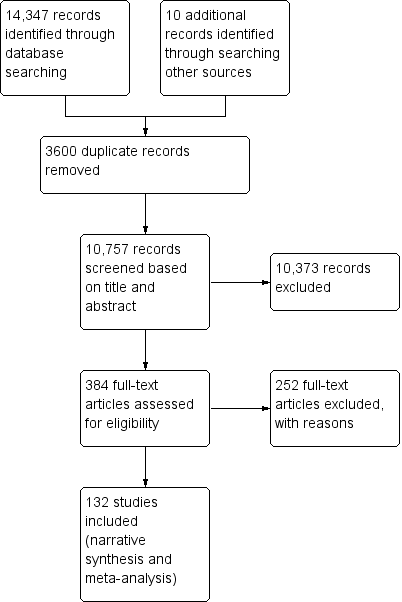
Study flow diagram

Subgroups for preventive health and/or management of long term conditions in this review
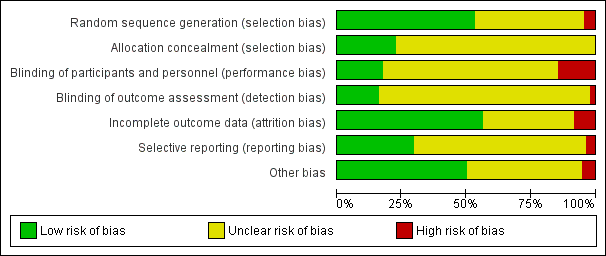
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
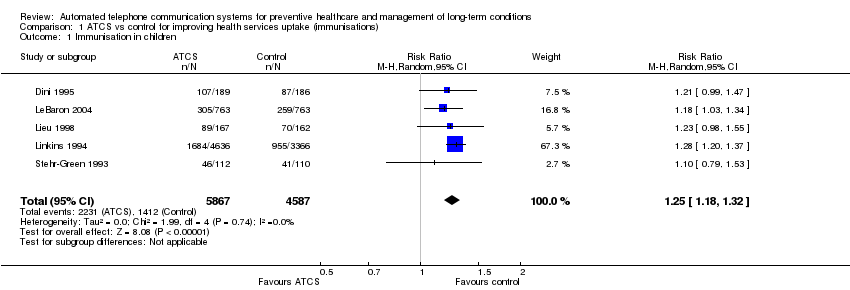
Comparison 1 ATCS vs control for improving health services uptake (immunisations), Outcome 1 Immunisation in children.

Comparison 1 ATCS vs control for improving health services uptake (immunisations), Outcome 2 Immunisation in adolescents.

Comparison 1 ATCS vs control for improving health services uptake (immunisations), Outcome 3 Immunisation in adults.

Comparison 2 ATCS vs control for improving health services uptake (screening rates), Outcome 1 Breast cancer screening.
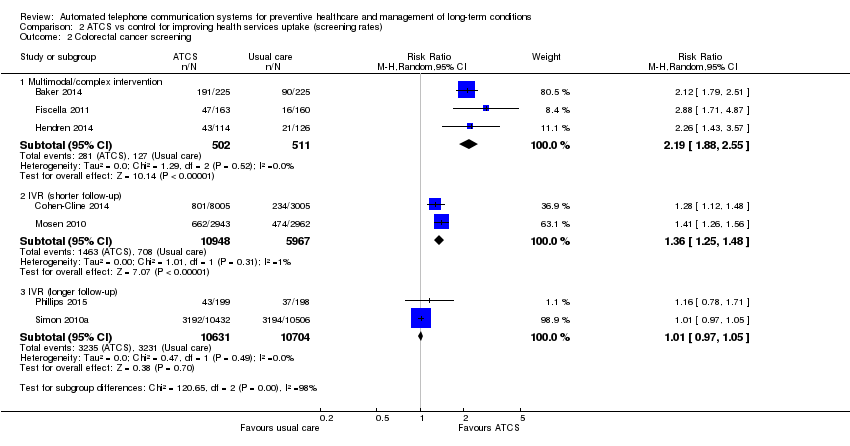
Comparison 2 ATCS vs control for improving health services uptake (screening rates), Outcome 2 Colorectal cancer screening.
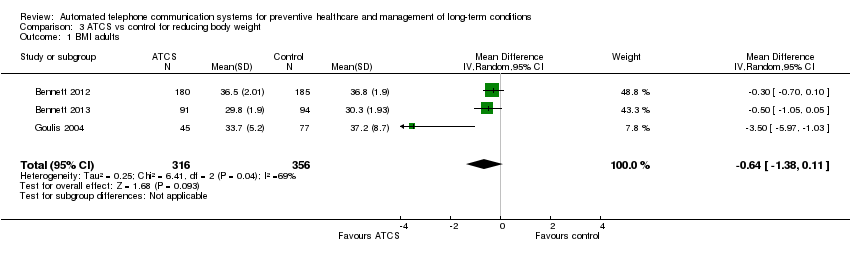
Comparison 3 ATCS vs control for reducing body weight, Outcome 1 BMI adults.
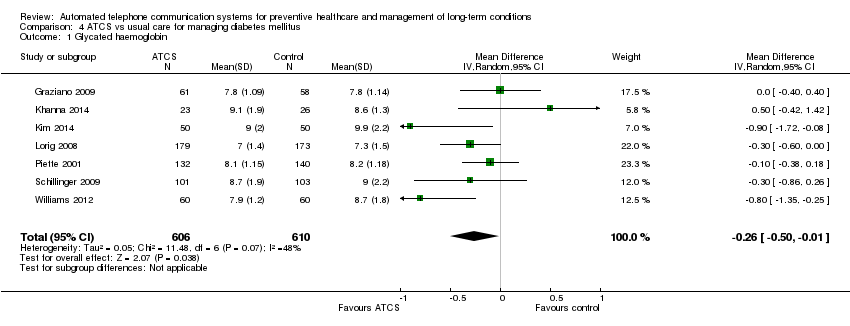
Comparison 4 ATCS vs usual care for managing diabetes mellitus, Outcome 1 Glycated haemoglobin.

Comparison 4 ATCS vs usual care for managing diabetes mellitus, Outcome 2 Self‐monitoring of diabetic foot.

Comparison 5 ATCS vs usual care for reducing healthcare utilisation in patients with heart failure, Outcome 1 Cardiac mortality.
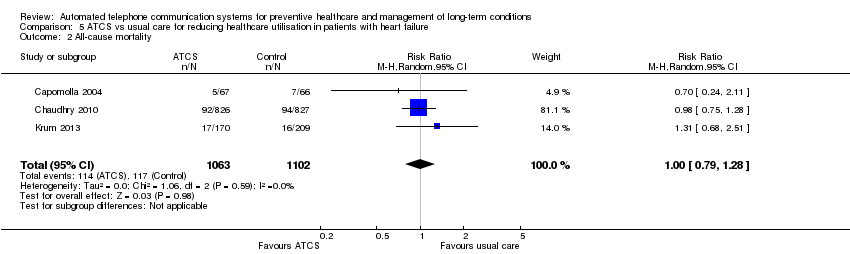
Comparison 5 ATCS vs usual care for reducing healthcare utilisation in patients with heart failure, Outcome 2 All‐cause mortality.

Comparison 6 ATCS vs usual primary care and education or usual care for managing hypertension, Outcome 1 Systolic blood pressure.

Comparison 6 ATCS vs usual primary care and education or usual care for managing hypertension, Outcome 2 Diastolic blood pressure.
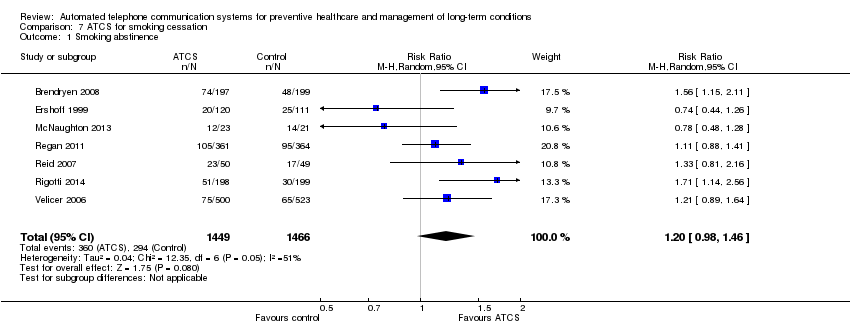
Comparison 7 ATCS for smoking cessation, Outcome 1 Smoking abstinence.
| ATCS versus control on immunisation rates | ||||||
| Patient or population: participants at risk of under‐immunisation (children, adolescents and adults) Comparison: no intervention, usual care or health information (letter) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | ATCS | |||||
| Behavioural outcome: immunisation rate ATCS Plus, IVR, unidirectional versus no calls, letters, usual care at median follow‐up of 4 months | Study populationa: children Comparator: no intervention | RR 1.25 (1.18 to 1.32) | 10,454 | ⊕⊕⊕⊝ | Franzini 2000 (N = 1138) reported that compared with controls (no calls), unidirectional ATCS (autodialer) may increase immunisation rates in children (86% versus 64%, low certainty).d | |
| 308 per 1000 | 385 per 1000 | |||||
| Moderateb | ||||||
| 373 per 1000 | 466 per 1000 | |||||
| Behavioural outcome: immunisation rate Unidirectional ATCS versus usual care at median follow‐up of 15 months | Study populationa: adolescents Comparator: usual care | RR 1.06 | 5725 | ⊕⊕⊕⊝ | Szilagyi 2013 (N = 4115) also reported that unidirectional ATCS probably slightly improves the uptake of preventive care visits, compared with usual care (63% ATCS versus 59% usual care; moderate certainty evidencef). | |
| 543 per 1000 | 576 per 1000 | |||||
| Moderateb | ||||||
| 540 per 1000 | 572 per 1000 | |||||
| Behavioural outcome: immunisation rate Unidirectional ATCS versus no calls or health information at median follow‐up of 2.5 months | Study populationa: adults Comparator: no calls or health information | RR 2.18 (0.53 to 9.02) | 1743 | ⊕⊝⊝⊝ | — | |
| 10 per 1000 | 21 per 1000 | |||||
| Moderateb | ||||||
| 66 per 1000 | 144 per 1000 | |||||
| Adverse outcome: unintended adverse events attributable to the intervention ATCS+, IVR, unidirectional versus various controls | No studies reported adverse events. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATCS Plus: automated telephone communication systems with additional functions; ATCS: automated telephone communication systems; CI: confidence interval; IVR: interactive voice response; RR: risk ratio; unidirectional ATCS enable non‐interactive voice communication and use one‐way transmission of information or reminders. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThe assumed risk represents the mean control group risk across studies (calculated by GRADEPro). | ||||||
| ATCS versus control on physical activity levels | |||
| Patient or population: participants at risk of developing long‐term conditions Settings: various settings Intervention: ATCS (multimodal/complex intervention, ATCS+, IVR) Comparison: no intervention, usual care, or IVR | |||
| Outcomes | Effect of intervention a | No of participants | Quality of the evidence |
| Behavioural outcome: physical activity Multimodal/complex interventionb versus no calls | The intervention may slightly improve the frequency of walks. | 181 (1 study) | ⊕⊕⊝⊝ Lowc |
| Behavioural outcome: physical activity, 12 months Multimodal/complex interventiond versus usual care | The intervention probably has mixed effects on gait speeds, little effect on functional outcomes (moderate certaintye) and may slightly increase physical activity levels (low certaintyf). | 700 (2 studies) | — |
| Behavioural outcome: physical activity ATCS Plus versus IVR control | 2 studies reported that ATCS Plus intervention may have little or no effect on different indices of physical activity. | 369 (2 studies) | ⊕⊕⊝⊝ Lowc |
| Behavioural outcome: physical activity IVR versus usual care, control or health education | 3 studies reported that IVR interventions may slightly improve several indices of physical activity (muscle strength, balance, moderate to vigorous physical activity) but may have little or no effect on others (physical activity levels, walking distance). | 216 (3 studies) | ⊕⊕⊝⊝ Lowg |
| Clinical outcome: metabolic markers, 12 months Multimodal/complex interventiond versus usual care | The intervention may have little or no effect on glycated haemoglobin, fasting insulin and glucose levels. | 302 (1 study) | ⊕⊕⊝⊝ Lowf |
| Clinical outcome: body weight measures Multimodal/complex interventiond ATCS Plus versus usual care or control | ATCS Plus intervention may have little or no effect on BMI, weight, waist or waist‐hip ratio, compared with control (71 participants; low certainty evidencec). Multimodal/complex intervention may have little or no effect on BMI, waist circumference or physical function, compared with usual care (302 participants; low certainty evidencef). | 373 (2 studies) | ⊕⊕⊝⊝ Low |
| Adverse outcome: unintended adverse events attributable to the intervention Multimodal/complex intervention, ATCS Plus, IVR versus various controls | No studies reported adverse events. | — | — |
| GRADE Working Group grades of evidence | |||
| ATCS: automated telephone communication systems; ATCS Plus: automated telephone communication systems with additional functions; IVR: interactive voice response. | |||
| aThe findings presented are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | |||
| ATCS versus control on screening rates | ||||||
| Patient or population: participants at risk for breast, colorectal or cervical cancer; or osteoporosis Settings: primary, secondary and tertiary care Intervention: ATCS (multimodal/complex intervention, ATCS Plus, IVR, unidirectional) Comparison: usual care, enhanced usual care or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care or enhanced usual care or no intervention | ATCS | |||||
| Behavioural outcome: breast cancer screening Multimodal/complex intervention versus usual care at 12 months follow‐up | Study populationa | RR 2.17 | 462 | ⊕⊕⊕⊕ | — | |
| 167 per 1000 | 363 per 1000 | |||||
| Moderateb | ||||||
| 167 per 1000 | 363 per 1000 | |||||
| Behavioural outcome: breast cancer screening IVR versus enhanced usual care at median follow‐up of 12 months | Study populationa | RR 1.05 | 2599 | ⊕⊕⊕⊝ | Unidirectional ATCS versus letter 1 further study (Fortuna 2014) (N = 1008) found that unidirectional ATCS (plus letter) probably has little or no effect on breast cancer screening rates at 12 months, adjusted OR 1.3 (95% CI 0.7 to 2.4; moderate certaintyd). | |
| 585 per 1000 | 614 per 1000 | |||||
| Moderateb | ||||||
| 432 per 1000 | 454 per 1000 | |||||
| Behavioural outcome: colorectal cancer screening Multimodal/complex intervention versus usual care at median follow‐up of 12 months | Study populationa | RR 2.19 | 1013 | ⊕⊕⊕⊕ | — | |
| 249 per 1000 | 545 per 1000 | |||||
| Moderateb | ||||||
| 167 per 1000 | 366 per 1000 | |||||
| Behavioural outcome: colorectal cancer screening IVR versus usual care at 6‐month follow‐up | Study populationa | RR 1.36 | 16915 | ⊕⊕⊕⊝ | IVR versus control 1 other study (Durant 2014) (N = 47,097) reported that IVR probably increases screening, with 1773 participants from the IVR group and 100 from the no‐call control group completing colorectal cancer screening within 3 months (moderate certaintyf). IVR versus usual care 1 study (Mosen 2010) (N = 6000) also reported that IVR probably increases completion of any colorectal cancer screening (moderate certaintyg). | |
| 119 per 1000 | 161 per 1000 | |||||
| Moderateb | ||||||
| 119 per 1000 | 162 per 1000 | |||||
| Behavioural outcome: colorectal cancer screening IVR, unidirectional ATCS versus usual care or letter at longer (9‐12 months) follow‐up | Study populationa | RR 1.01 | 21,335 | ⊕⊕⊕⊝ | IVR versus usual care 1 study (Simon 2010a) (N = 20,000) also reported that IVR probably increases slightly colorectal cancer screening via colonoscopy (moderate certaintyi). Unidirectional ATCS versus letter 1 further study (Fortuna 2014) (N = 1008) at 12 months found that unidirectional ATCS (plus letter) has probably little or no effect on colorectal cancer screening rates at 12 months (15.3% versus 12.2%; adjusted OR 1.2; 95% CI 0.6 to 2.4; moderate certaintyd). | |
| 302 per 1000 | 305 per 1000 | |||||
| Moderateb | ||||||
| 245 per 1000 | 247 per 1000 | |||||
| Behavioural outcome: cervical cancer screening ATCS Plus versus control (no calls) at 3 month follow‐up | See comment | See comment | Not estimable | 75,532 (1 study) | ⊕⊕⊕⊝ | Corkrey 2005 found that ATCS Plus intervention probably slightly improves cervical cancer screening rates at 3 months. |
| Adverse outcome: unintended adverse events attributable to the intervention Multimodal/complex intervention, ATCS Plus, IVR, unidirectional versus various controls | No studies reported adverse events. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThe assumed risk represents the mean control group risk across studies (calculated by GRADEPro). | ||||||
| ATCS versus control for body weight | ||||||
| Patient or population: overweight or obese individuals (both children and adults) Comparison: usual care, no intervention or control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Commentsa | |
| Assumed risk | Corresponding risk | |||||
| Controls | ATCS | |||||
| Clinical and behavioural outcome: BMI score in adults Multimodal/complex intervention, ATCS Plus or IVR versus usual care at median follow‐up of 18 months | The mean BMI in the control groups was 34.7 kg/m2 | The mean BMI of adults in the intervention groups was 0.64 kg/m2lower | Not estimable | 672 | ⊕⊕⊝⊝ | ATCS Plus versus control Vance 2011 (N = 140) found that ATCS Plus may reduce slightly BMI (low certainty evidencec). |
| Clinical and behavioural outcome: body weight in adults, 12 weeks | See comment | See comment | Not estimable | See comment | See comment | ATCS Plus versus control Vance 2011 (N = 140) found that ATCS Plus may reduce slightly body weight and waist circumference (low certainty evidencec). IVR versus control Estabrooks 2008 (N = 77) reported that IVR may have little or no effect on body weight (percent lost or change in) (low certainty evidenced). |
| Clinical and behavioural outcome: body weight in adults, at median follow‐up of 18 months | See comment | See comment | Not estimable | See comment | See comment | ATCS (multimodal/complex intervention, ATCS Plus, IVR) versus usual care Bennett 2012 (N = 365) found that ATCS Plus probably slightly reduces body weight at 18 months (moderate certainty evidence).eBennett 2013 (N = 194) found that multimodal/complex intervention may reduce body weight at 18 months (low certainty evidence).f IVR versus usual care Goulis 2004 (N = 122) found that IVR probably reduces slightly body weight but probably has little or no effect on obesity assessment scores at 6 months (moderate certainty evidence).f |
| Clinical and behavioural outcome: blood pressure, blood glucose, cholesterol levels | See comment | See comment | Not estimable | See comment | See comment | ATCS (ATCS Plus, IVR) versus usual care/control Bennett 2012 (N = 365) found that ATCS Plus probably has little or no effect on systolic or diastolic blood pressure at 18 months (moderate certainty evidencee). ATCS Plus versus control Vance 2011 found that ATCS Plus may slightly improve slightly systolic blood pressure and blood glucose levels at 12 weeks (low certainty evidencec). IVR versus usual care Goulis 2004 (N = 122) found that IVR probably has little or no effect on systolic or diastolic blood pressure, plasma glucose levels, or high‐density lipoprotein cholesterol, but it probably slightly reduces total cholesterol and triglyceride levels at 6 months (moderate certainty evidence).e |
| Clinical outcome: BMI z‐score in children at median follow‐up of 7.5 months | See comment | See comment | Not estimable | See comment | ⊕⊕⊕⊝ | ATCS Plus versus control Estabrooks 2009 (N = 220) found that ATCS Plus has probably little or no effect on BMI z‐scores in children at 12 months. IVR versus control Wright 2013 (N = 100) found that IVR has probably little or no effect on BMI z‐scores in children at 3 months. |
| Behavioural outcome: physical activity, dietary habits in children at median follow‐up of 7.5 months | See comment | See comment | Not estimable | See comment | ⊕⊕⊕⊝ | ATCS Plus versus control Estabrooks 2009 (N = 220) found that ATCS Plus has probably little or no effect on self‐reported physical activity, sedentary behaviours or dietary habits at 12 months. IVR versus control (no calls) Wright 2013 (N = 100) found that IVR has probably little or no effect on total caloric intake, fruit intake, or sedentary behaviours at 3 months. |
| Adverse outcome: unintended adverse events attributable to the intervention IVR versus usual care | See comment | See comment | See comment | 559 (2 studies) | See comment | Bennett 2012 (N = 365) reported 1 serious musculoskeletal injury in the intervention group and 3 events (1 cardiovascular and 2 cases of gallbladder disease) in the usual care group (moderate certainty evidence).e,g Bennett 2013 (N = 194) reported 6 serious adverse events in the intervention arm, including gynaecological surgery in 2 participants and knee replacement, breast abscess, musculoskeletal injury, and cancer diagnosis in 1 participant each; all participants except the one with the cancer diagnosis required hospitalisation (low certainty evidence).f,g |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATCS: automated telephone communication systems; ATCS Plus: automated telephone communication systems with additional functions; BMI: body Mass Index; CI: confidence interval; IVR: interactive voice response; SMD: Standardised mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAdditional findings presented are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | ||||||
| ATCS versus control as appointment reminders (reducing non‐attendance rates) | |||
| Patient or population: patients/healthcare consumers Comparison: no intervention (calls) or nurse‐delivered calls | |||
| Outcomes | Effect of interventiona | No of participants (studies) | Quality of the evidence |
| Health behaviour: attendance rates, 6 weeks ATCS Plus versus nurse‐delivered calls | ATCS Plus calls delivered 3 or 7 days prior to flexible sigmoidoscopy or/and colonoscopy examinations probably have little or no effect on appointment non‐attendance or preparation non‐adherence. | 3610 (1 study) | ⊕⊕⊕⊝ |
| Health behaviour: attendance rates, 4 months IVR versus no calls | IVR improves attendance rates: OR 1.52 (95% CI 1.34 to 1.71). | 12,092 (1 study) | ⊕⊕⊕⊕ High |
| Health behaviour: return tuberculin test rate, 3 days Unidirectional ATCS versus no calls | Unidirectional ATCS may improve test return rates. | 701 (1 study) | ⊕⊕⊝⊝ |
| Health behaviour: attendance rates, 1 month Unidirectional ATCS versus no calls | Undirectional ATCS may improve attendance rates RR 1.60 (95% CI 1.29 to 1.98). | 517 (1 study) | ⊕⊕⊝⊝ |
| Health behaviour: attendance rates, 6‐8 weeks Unidirectional ATCS versus no calls | 2 studies reported conflicting results: Reekie 1998 (N = 1000) reported that unidirectional ATCS probably decrease non‐attendance rates at 6 weeks; while Maxwell 2001 (N = 2304) reported the interventions probably have little or no effect at 2 months. | 3304 (2 studies) | ⊕⊕⊕⊝ |
| Health behaviour: attendance rates, 6 months Unidirectional ATCS versus no calls | Unidirectional ATCS may improve attendance: OR 1.50 (P < 0.01). | 2008 (1 study) | ⊕⊕⊝⊝ |
| Adverse outcome: unintended adverse events attributable to the intervention ATCS Plus, IVR, unidirectional ATCS versus various controls | No studies reported adverse events. | ||
| ATCS: automated telephone communication systems; ATCS Plus: automated telephone communication systems with additional functions; CI: confidence interval; IVR: interactive voice response; OR: odds ratio; RR: risk ratio. | |||
| GRADE Working Group grades of evidence | |||
| aThe findings presented are based on a narrative summary and synthesis of results, many of which were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | |||
| ATCS versus control for adherence to medication or laboratory tests | ||||
| Patient or population: patients with various conditions or at risk of low adherence to medication or laboratory tests Settings: various settings Intervention: ATCS (multimodal/complex intervention, ATCS Plus, IVR, unidirectional ATCS) Comparison: usual care, no calls, controls (other ATCS) | ||||
| Outcomes | Effect of interventionsa | No of participants | Quality of the evidence | Comments |
| Behavioural outcome: adherence to medication Multimodal/complex interventionsb versus usual care or control | The effects of multimodal/complex interventions are inconclusive. | 888 (2 studies) | See comment | Ho 2014 (N = 241) reported that the multimodal/complex intervention probably improves adherence to cardioprotective medications at 12 months (moderate certaintyc). Stuart 2003 (N = 647) found uncertain effects of the intervention on adherence to antidepressant medications (very low certaintyc,d). |
| Behavioural outcome: adherence to medication ATCS Plus versus control or single IVR call | Results suggest that ATCS Plus probably slightly improve measures of adherence. | 2340 (2 studies) | See comment | Cvietusa 2012 (N = 1393) reported that ATCS Plus, compared with control, probably improves time to first inhaled corticosteroid refill and probably slightly improves the proportion of days with medication on hand in children (moderate certaintye). Stacy 2009 (N = 947) reported that ATCS Plus probably slightly improves statin adherence at 6 months, compared with a single IVR call (moderate certaintyf). |
| Behavioural outcome: adherence to laboratory tests ATCS Plus or IVR versus no intervention or usual care | Results suggest that ATCS Plus probably has little or no effect on adherence to testing, while IVR probably improves test completion. | 15,218 (3 studies) | See comment | ATCS Plus versus no intervention Derose 2009 (N = 13,057) found that ATCS Plus probably has little or no effect on adherence to testing (completion of all 3 recommended laboratory tests for diabetes patients) at 12 weeks (moderate certaintyg). Simon 2010b (N = 1200) found that these interventions probably have little or no effect on retinopathy examination rates or tests for glycaemia, hyperlipidaemia or nephropathy in diabetic patients at 12 months (moderate certaintyh). IVR versus usual care Feldstein 2006 (N = 961) found that IVR probably improves patients' completion of all recommended laboratory tests at 25 days follow‐up (moderate certaintyi). |
| Behavioural outcome: adherence to medication or composite outcome (medication adherence and rate of adverse events) ATCS Plus versus usual care | Results indicate that ATCS Plus probably improves medication adherence and may slightly improve a composite measure. | 35,816 (4 studies) | See comment | 2 studies (Derose 2013 (N = 5216) and Vollmer 2014 (N = 21,752)) reported that ATCS Plus probably improves adherence to statins to some extent. Vollmer 2011 (N = 8517) found that ATCS Plus probably slightly improves adherence to inhaled corticosteroids (moderate certaintyj). Sherrard 2009 (N = 331) found that ATCS Plus may slightly improve a composite measure of medication adherence and adverse events at 6 months follow‐up (low certaintyc,k). |
| Behavioural outcome: adherence to medication or laboratory tests IVR versus control | Results suggest that IVR probably improves slightly medication adherence. | 4,238,362 (4 studies) | See comment | Adams 2014 (N = 475) found that IVR may slightly improve comprehensiveness of screening and counselling (low certaintyc,l). Bender 2010 (N = 50) reported that IVR may improve adherence to anti‐asthmatic medications at 2.5 months follow‐up (low certaintyc,e). Leirer 1991 (N = 16) reported that IVR may slightly reduce medication non‐adherence (low certaintym). Mu 2013 (N = 4,237,821) found that IVR probably slightly improves medication refill rates at 1 month (moderate certaintyn). |
| Behavioural outcome: adherence to medication IVR versus usual care | Results indicate that IVR probably slightly improves some measures of medication adherence. | 56,140 (8 studies) | See comment | 2 studies (Boland 2014 (N = 70); Friedman 1996 (N = 267)) reported that IVR probably slightly improves adherence to glaucoma and anti‐hypertensive medications at 3 and 6 months respectively (moderate certainty).o 2 further studies (Glanz 2012 (N = 312); Migneault 2012 (N = 337)) reported that IVR has probably little or no effect on medication adherence at 8 and 12 months, respectively (moderate certainty).p 2 studies (Green 2011 (N = 8306); Reynolds 2011 (N = 30,610)) assessed adherence via refill rates, reporting that IVR probably slightly improves medication refill rates at 2 weeks (moderate certainty).q 2 further studies reported medication adherence assessed by medication possession ratio (MPR) at different time points. Patel 2007 (N = 15,051) found that IVR probably slightly improves MPR at 3 to 6 months, while Bender 2014 (N = 1187) reported that IVR probably improves MPR at 24 months (both studies of moderate certaintyr). |
| Behavioural outcome: adherence to medication Unidirectional ATCS versus control | Results suggest that unidirectional ATCS may have little effect, or improve medication adherence to a small degree. | 107 (2 studies) | See comment | 2 studies (Lim 2013 (N = 80); Ownby 2012 (N = 27)) reported that the intervention may have little effect or slightly improve medication adherence (low certaintys). |
| Clinical outcome: blood pressure Multimodal/complex, ATCS Plus, IVR versus usual care | Results suggest that ATCS Plus probably slightly reduces blood pressure, while multimodal/complex or IVR interventions probably have little or no effect on blood pressure. | 22,597 (3 studies) | See comment | Multimodal/complex intervention versus usual care Ho 2014 (N = 241) reported that multimodal intervention probably has little or no effect on achieving reduced blood pressure targets (moderate certaintyc). ATCS Plus versus usual care Vollmer 2014 (N = 21,752) reported that ATCS Plus probably slightly reduces systolic blood pressure (moderate certaintyt). IVR versus usual care Migneault 2012 (N = 337) reported that IVR probably has little or no effect on systolic or diastolic blood pressure (moderate certaintyc), while Friedman 1996 (N = 267) found that IVR may have little or no effect on systolic blood pressure but may slightly decrease diastolic blood pressure (low certaintyc,f). |
| Adverse outcome: unintended adverse events attributable to the intervention Multimodal/complex intervention, ATCS Plus, IVR, unidirectional versus various controls | No studies reported adverse events. | |||
| ATCS Plus: automated telephone communication systems with additional functions; CI: confidence interval; HR: hazard ratio; IVR: interactive voice response; MPR: medication possession ratio; OR: odds ratio; RR: risk ratio; SD: standard deviation | ||||
| GRADE Working Group grades of evidence | ||||
| aMultimodal intervention included ATCS Plus, medication reconciliation and tailoring, patient education and collaborative care in Ho 2014; and education, nurse‐delivered call and IVR in Stuart 2003. | ||||
| ATCS versus control on alcohol consumption | |||
| Patient or population: participants addicted to alcohol Settings: various settings Intervention: ATCS (ATCS Plus, IVR) Comparison: no intervention, usual care, advice/education or packaged CBT | |||
| Outcomes | Effect of interventiona | No of participants | Quality of the evidence |
| Behavioural outcomes: number of drinks per drinking day ATCS Plus, IVR versus usual care, (various) controls at median follow‐up of 2 months | ATCS Plus versus usual care Rose 2015 (N = 158) reported that ATCS Plus may have little or no effect on the number of drinks per drinking day at 2 months (low certaintyb,c). ATCS Plus versus control (advice/education) Hasin 2013 (N = 254) found that ATCS Plus may reduce the number of drinks per drinking day in the last 30 days at 2 months (low certaintyb,c), but it may have little effect at 12 months. IVR versus control (information) Rubin 2012 (N = 47) reported that IVR may slightly reduce the number of drinks per drinking day at 6 months (low certaintyc,e). | 459 (3 studies) | ⊕⊕⊝⊝ |
| Behavioural outcomes: drinking days, heavy drinking days, or total number of drinks consumed ATCS Plus, IVR versus (various) controls | ATCS Plus versus no intervention Mundt 2006 (N = 60) found that ATCS Plus may have little or no effect on drinking days, heavy drinking days, or total number of drinks consumed (low certaintyc,f). ATCS Plus versus control (packaged CBT) Litt 2009 (N = 110) found that ATCS Plus may have little or no effect on the number of heavy drinking days at 12 weeks posttreatment (low certaintyc,g). IVR versus control (information) Rubin 2012 (N = 47) reported that IVR may slightly reduce the number of heavy drinking days per month at 6 months (low certaintyc,e). | 217 (3 studies) | ⊕⊕⊝⊝ |
| Behavioural outcomes: proportion of days abstinent, other alcohol consumption indices, 12 weeks ATCS Plus versus control (packaged CBT) | ATCS Plus may slightly reduce the proportion of days abstinent but have little or no effect on coping or drinking problems or continuity of abstinence (Litt 2009). | 110 (1 study) | ⊕⊕⊝⊝ |
| Behavioural outcomes: weekly alcohol consumption, 6 months ATCS Plus versus usual care | ATCS Plus may have little or no effect on weekly alcohol consumption (Helzer 2008). | 338 (1 study) | ⊕⊕⊝⊝ |
| Behavioural outcomes: AUDIT score, 6 weeks IVR versus control (no intervention) | IVR probably improve slightly AUDIT scores (Andersson 2012). | 1423 (1 study) | ⊕⊕⊕⊝ |
| Behavioural outcomes: other alcohol consumption indices, 4 weeks IVR versus control (no intervention) | IVR may have little or no effect on drinking habits, alcohol craving, or PTSD symptoms (Simpson 2005). | 98 (1 study) | ⊕⊕⊝⊝ |
| Adverse outcome: unintended adverse events attributable to the intervention ATCS Plus, IVR versus various controls | No studies reported adverse events. | ||
| ATCS Plus: automated telephone communication systems with additional functions; AUDIT: Alcohol Use Disorders Identification Test; CBT: cognitive behavioural therapy; IVR: interactive voice response; PTSD: post‐traumatic stress disorder. | |||
| GRADE Working Group grades of evidence | |||
| aThe findings presented in this table are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | |||
| ATCS versus control on severity of cancer symptoms | ||||
| Patient or population: cancer patients Settings: various settings Intervention: ATCS (multimodal/complex intervention, ATCS Plus, IVR) Comparison: usual care, control (other ATCS, nurse‐delivered calls) | ||||
| Outcomes | Effects of interventiona | No of participants | Quality of the evidence | Comments |
| Clinical outcomes: symptoms (severity or burden) ATCS Plus versus usual care (via ATCS) or control, 4‐12 weeks | Results suggest that ATCS Plus may have little or no effect on symptom severity, distress or burden. | 701 (4 studies) | See comment | Cleeland 2011 (N = 79) found that ATCS Plus may slightly reduce symptom threshold events and cumulative distribution of symptom threshold events; and it may have little or no effect on mean symptom severity between discharge and 4 week follow‐up (low certaintyb,c). Mooney 2014 (N = 250) found that ATCS Plus probably has little or no effect on symptom severity scores at 6 week follow‐up (moderate certaintyc). Spoelstra 2013 (N = 119) found that ATCS Plus may have little or no effect on symptom severity at 10 week follow‐up (low certaintyc,d). Yount 2014 (N = 253) reported that ATCS Plus may have little or no effect on symptom burden at 12 weeks (low certaintyc,e). |
| Clinical outcomes: symptom severity, 10 weeks IVR versus nurse delivered calls | Results suggest that IVR may have little or no effect on symptom severity. | 437 (1 study) | ⊕⊕⊝⊝ | — |
| Clinical outcomes: pain Multimodal/complex interventiong versus usual care | Results indicate that multimodal intervention probably reduces pain at 3 months and probably slightly reduces pain at 12 months. | 405 (1 study) | ⊕⊕⊕⊝ | — |
| Clinical outcomes: depression Multimodal/complex interventiong versus usual care | Results indicate that multimodal intervention probably slightly reduces depression at 3 and 12 months. | 405 (1 study) | ⊕⊕⊕⊝ | — |
| Clinical outcomes:distress, 6 weeks ATCS Plus versus usual care (via IVR) | Results indicate that ATCS Plus probably has little or no effect on distress. | 250 (1 study) | ⊕⊕⊕⊝ | — |
| Behavioural outcome: medication adherence ATCS Plus versus usual care | Results indicate that ATCS Plus may have little or no effect on medication non‐adherence. | 119 (1 study) | ⊕⊕⊝⊝ | — |
| Adverse outcome: unintended adverse events attributable to the intervention Multimodal/complex intervention, ATCS Plus, IVR versus various controls | No studies reported adverse events. | |||
| ATCS: automated telephone communication systems; ATCS Plus: automated telephone communication systems with additional functions; IVR: interactive voice response. | ||||
| GRADE Working Group grades of evidence | ||||
| aThe findings presented in this table are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | ||||
| ATCS versus usual care for managing diabetes mellitus | ||||||
| Patient or population: patients with diabetes mellitus Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Commentsa | |
| Assumed risk | Corresponding risk | |||||
| Usual care | ATCS | |||||
| Clinical outcome: glycated haemoglobin or blood glucose ATCS Plus, IVR versus usual care at median follow‐up of 6 months | The mean glycated haemoglobin in the control groups was 8.41% | The mean glycated haemoglobin in the intervention groups was | Not estimable | 1216 | ⊕⊕⊝⊝ | ATCS Plus versus usual care 1 further study, Katalenich 2015 (N = 98), found that ATCS Plus may have little or no effect on median glycated haemoglobin levels compared with usual care at 6 months follow‐up (low certaintyc). IVR versus usual care 1 additional study, Homko 2012 (N = 80), found that IVR may have little or no effect on fasting blood glucose levels in pregnancy or infant birth weight at 26 months (low certaintyc). |
| Behavioural outcome: self‐monitoring of diabetic foot (various scales) ATCS Plus versus usual care at 12 months follow‐up | The mean self‐monitoring of diabetic foot in the control groups was 4.5 (range from 0 to 7, with higher scores indicating better foot care) | The mean self‐monitoring of diabetic foot in the intervention groups was | Not estimable | 498 | ⊕⊕⊕⊝ | — |
| Behavioural outcome: self‐monitoring of blood glucose ATCS Plus, IVR versus usual care, 6‐12 months | See comment | See comment | Not estimable | See comment | See comment | ATCS Plus versus usual care Lorig 2008 (N = 417) found that ATCS Plus may have little no effect on self‐monitoring of blood glucose at 6 months (low certainty evidencef). At 12 months, 2 studies (Piette 2001 (N = 272); Schillinger 2009 (N = 339)) reported that ATCS Plus probably slightly improves self‐monitoring of blood glucose (moderate certaintye). IVR versus usual care Graziano 2009 (N = 112) found that IVR probably slightly increases the mean change in frequency of self‐monitoring of blood glucose (moderate certainty evidenceg). |
| Behavioural outcome: medication adherence or use ATCS Plus versus usual care, 6‐12 months | See comment | See comment | Not estimable | 370 (2 studies) | See comment | Katalenich 2015 (N = 98) reported that ATCS Plus may have little or no effect on adherence rates at 6 months (low certaintyc), and Piette 2001 (N = 272) found that ATCS Plus has probably little or no effect on medication use at 12 months (moderate certaintyg. |
| Behavioural outcome: physical activity, diet, weight monitoring ATCS Plus versus usual care, 6‐12 months | See comment | See comment | Not estimable | 1028 (3 studies) | See comment | Lorig 2008 (N = 417) found that ATCS Plus may have little or no effect on aerobic exercise at 6 months (low certaintyf). Schillinger 2009 (N = 339) found that ATCS Plus may slightly improve diet and exercise and moderate intensity physical activity levels, but it may have little or no effect on vigorous intensity physical activity levels at 12 months (low certaintyc). Piette 2001 (N = 272) reported that ATCS Plus probably has little or no effect on weight monitoring (moderate certaintyg). |
| Adverse outcome: unintended adverse events attributable to the intervention ATCS Plus, IVR versus usual care | No studies were found that reported adverse events. | |||||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATCS Plus: automated telephone communication systems with additional functions; CI: confidence interval; HRQoL: health‐related quality of life; IVR: interactive voice response; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAdditional results are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | ||||||
| ATCS versus usual care for patients with heart failure | ||||||
| Patient or population: patients with heart failure Comparison: usual care or usual community care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Commentsa | |
| Assumed risk | Corresponding risk | |||||
| Usual care or usual community care | ATCS | |||||
| Clinical outcome: cardiac mortality ATCS Plus, IVR versus usual care or usual community care at median follow‐up of 11.5 months | Study populationb | RR 0.60 | 215 | ⊕⊝⊝⊝ | — | |
| 95 per 1000 | 57 per 1000 | |||||
| Moderatec | ||||||
| 96 per 1000 | 58 per 1000 | |||||
| Clinical outcome: all‐cause mortality ATCS Plus versus usual care or usual community care at median follow‐up of 11 months | Study populationb | RR 1 (0.79 to 1.28) | 2165 | ⊕⊕⊕⊝ | — | |
| 106 per 1000 | 106 per 1000 | |||||
| Moderatec | ||||||
| 106 per 1000 | 106 per 1000 | |||||
| Clinical outcome: heart failure hospitalisation ATCS Plus, IVR versus usual care or usual community care at median follow‐up of 11.5 months | See comment | See comment | Not estimable | 2329 (4 studies) | See comment | ATCS Plus versus usual care or usual community care Chaudhry 2010 (N = 1653) found that the intervention had little or no effect on hospitalisation for heart failure (high certainty). Krum 2013 (N = 405) also reported that there was probably little or no effect of the intervention for this same outcome (moderate certaintyg), while Capomolla 2004 (N = 133) reported that ATCS Plus may decrease hospitalisation rates for heart failure (low certaintyh). IVR versus usual care Kurtz 2011 (N = 138) reported that IVR intervention has uncertain effects on hospitalisation for heart failure (very low certaintyi). |
| Clinical outcome: all‐cause hospitalisation ATCS Plus versus usual care or usual community care | See comment | See comment | Not estimable | 2191 participants (3 studies) | See comment | ATCS Plus versus usual care Capomolla 2004 (N = 133) found that ATCS Plus may reduce all‐cause hospitalisation (for chronic heart failure, cardiac cause and other cause; low certaintyh), and Krum 2013 (N = 405) similarly reported that the intervention probably slightly decreased all‐cause hospitalisation (moderate certaintyg).fChaudhry 2010 (N = 1653) found that ATCS Plus has little or no effect on readmission for any reason (high certainty). |
| Clinical outcome: global health (well‐being) rating (7‐item questionnaire) ATCS Plus versus usual care 12 months | See comment | See comment | Not estimable | 405 participants (1 study) | ⊕⊕⊕⊝ | Krum 2013 (N = 405) reported that ATCS Plus probably increases slightly the proportion of patients with improved global health questionnaire ratings at 12 months. |
| Clinical outcome: emergency room and other health service use outcomes ATCS Plus versus usual care or usual community care | See comment | See comment | Not estimable | 1786 participants (2 studies) | See comment | Emergency room use Capomolla 2004 (N = 133) found that ATCS Plus may reduce emergency room use at (median) 11 months (low certaintyh). Other service use Chaudhry 2010 (N = 1653) found that ATCS Plus had little or no effect on number of days in hospital or number of hospitalisations (readmissions)(high certainty). |
| Adverse outcome: unintended adverse events attributable to the intervention ATCS Plus, IVR versus usual care | See comment | See comment | See comment | 1791 (2 studies) | See comment | ATCS Plus versus usual care Chaudhry 2010 (N = 1653) reported that no adverse events had occurred during the study (high certainty). IVR versus usual care Kurtz 2011 (N = 138) classified adverse events as cardiac mortality plus rehospitalisation for heart failure, reporting uncertain effects upon this composite outcome (very low certaintyi). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATCS Plus: automated telephone communication systems with additional functions; CI: confidence interval; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAdditional results are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | ||||||
| ATCS versus usual care for management of hypertension | |||||
| Patient or population: patients with hypertension Comparison: usual care, with and without education | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants | Quality of the evidence | Commentsa | |
| Assumed risk | Corresponding risk | ||||
| Usual care | ATCS | ||||
| Clinical outcome: systolic blood pressure (automated sphygmomanometer or electronic pressure monitor) ATCS Plus or IVR versus usual care at median follow‐up of 6 weeks | The mean systolic blood pressure in the control group was 141.1 mmHg | The mean systolic blood pressure in the intervention groups was (2.12 to 1.66 lower) | 65,256 | ⊕⊕⊕⊝ | 1 additional study (Dedier 2014) (N = 253) reported that compared with usual care plus education, IVR may have little or no effect on systolic blood pressure at 3 months (low certaintyc). |
| Clinical outcome: diastolic blood pressure (automated sphygmomanometer and electronic cuff) ATCS Plus, unidirectional versus usual care at median follow‐up of 14 weeks | The mean diastolic blood pressure in the control group was 81.2 mmHg | The mean diastolic blood pressure in the intervention groups was 0.02 mmHg higher (2.62 lower to 2.66 higher) | 65,056 | ⊕⊕⊝⊝ | — |
| Clinical outcome: blood pressure control, 26 weeks Multimodal/complex interventionf versus usual care | See comment | See comment | 166 (1 study) | ⊕⊕⊕⊝ | Bove 2013 (N = 241) found that a multimodal/complex intervention probably has little or no effect on blood pressure control. |
| Clinical outcome: Health statush, depressioni, 6 weeks ATCS Plus versus enhanced usual care (plus information) | See comment | See comment | 200 (1 study) | ⊕⊕⊝⊝ | Piette 2012 (N = 200) found that ATCS Plus may slightly improve health status and may decrease depressive symptoms. |
| Behavioural outcome: medication use Multimodal/complexk, ATCS Plus versus usual care or enhanced usual care (plus information) | See comment | See comment | 483 (2 studies) | ⊕⊕⊝⊝ | Multimodal/complex versus usual care Magid 2011 (N = 283) found that multimodal/complex intervention may have little or no effect on medication adherence assessed by Medication Possession Ratio or proportion adherent (low certaintyl). ATCS Plus versus enhanced usual care Piette 2012 (N = 200) found that ATCS Plus may reduce the number of medication‐related problemsm (low certaintyj). |
| Behavioural outcome: physical activity levels, 12 weeks IVR versus enhanced usual care | See comment | See comment | 253 (1 study) | ⊕⊕⊝⊝ | IVR versus enhanced usual care Dedier 2014 (N = 253) reported that IVR may slightly increase physical activity levels. |
| Adverse outcome: unintended adverse events attributable to the intervention Multimodal/complex intervention, ATCS Plus, IVR, unidirectional ATCS versus various controls | No studies reported adverse events. | ||||
| ATCS Plus: automated telephone communication systems with additional functions; CI: confidence interval; IVR: interactive voice response; MD: mean difference; SD: standard deviation. | |||||
| GRADE Working Group grades of evidence | |||||
| aAdditional results are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | |||||
| ATCS versus control for smoking cessation | ||||||
| Patient or population: patients with tobacco dependence Comparison: usual care, control (no calls, 'placebo' (inactive) ATCS, self‐help intervention, stage‐matched manuals) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Commentsa | |
| Assumed risk | Corresponding risk | |||||
| Control | ATCS | |||||
| Behavioural outcome: smoking abstinence Multimodal/complex intervention, ATCS Plus, IVR versus (various) controls or usual care at median follow‐up of 12 months | Study populationb | RR 1.2 | 2915 | ⊕⊕⊝⊝ | ATCS Plus versus usual care 1 further study, Reid 2011 (N = 440), reported that ATCS Plus may improve smoking abstinence rates at 26 weeks, and this may be maintained at 52 weeks (low certainty evidencef). | |
| 201 per 1000 | 241 per 1000 | |||||
| Moderatec | ||||||
| 241 per 1000 | 289 per 1000 | |||||
| Behavioural outcome: medication use Multimodal/complex, ATCS Plus versus control (inactive IVR or self‐help booklet) | See comment | See comment | See comment | 1127 (2 studies) | ⊕⊕⊕⊝ Moderateg | Multimodal/complex intervention versus control (self‐help booklet) Brendryen 2008 (N = 396) found that a multimodal/complex intervention probably has little or no effect on adherence to NRT (moderate certainty evidence). ATCS Plus versus control (inactive IVR) Regan 2011 (N = 731) found that ATCS Plus probably has little or no effect on medication use (moderate certainty evidence). |
| Behavioural outcome: support programme enrolment ATCS Plus versus control (inactive IVR) | See comment | See comment | See comment | 521 (1 study) | ⊕⊕⊝⊝ | Carlini 2012 found that ATCS Plus may improve re‐enrolment into a quit line support programme. |
| Adverse outcome: unintended adverse events attributable to the intervention Multimodal/complex intervention, ATCS Plus, IVR versus various controls | No studies were found that reported adverse events. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ATCS Plus: automated telephone communication systems with additional functions; CI: confidence interval; IVR: interactive voice response; NRT: nicotine replacement therapy; OR: odds ratio; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAdditional results are based on a narrative summary and synthesis of results that were not amenable to statistical analysis; please see Effects of interventions for detailed findings. | ||||||
| Dichotomous outcomes | ||||||||||
| Primary outcome | Study ID | Timing of outcome assessment (months) | Intervention group | Comparator group | Between group difference | Notes | ||||
| Observed (n) | Total (N) | Observed (n) | Total (N) | P value | Effect estimate (OR/RR/HR) | 95% CI | ||||
| IMMUNISATIONS | ||||||||||
| Immunisation uptake | 24 | 107 | 189 | 87 | 186 | — | 1.21 | 0.99 to 1.47 | — | |
| * | 270 | 314 | 273 | 429 | — | — | — | — | ||
| 3 | 146 | 5599 | 46 | 6383 | < 0.001 | 3.69 | 2.64 to 5.15 | Cluster RCT unadjusted for clustering. Approximate sample size calculations gave the following adjusted values: intervention 20/791; control 6/902; see Appendix 14 for calculations | ||
| 4 | 89 | 167 | 70 | 162 | 0.11 | — | 29.0 to 43.8 | CIs for % values and for IVR alone; P value from Chi2 | ||
| 1 | 1684 | 4636 | 955 | 3366 | < 0.01 | 1.28 | 1.20 to 1.37 | — | ||
| 13 | 305 | 763 | 259 | 763 | < 0.05 | — | — | — | ||
| 2 | 3 | 26 | 3 | 24 | — | — | — | — | ||
| 1 | 46 | 112 | 41 | 110 | — | 1.07 | 0.78 to 1.46 | — | ||
| 18 | 928 | 1496 | 873 | 1510 | 0.02 | — | — | — | ||
| 12 | 748 | 1423 | 651 | 1296 | < 0.05 | 1.3 | 1.0 to 1.7 | — | ||
| SCREENING | ||||||||||
| Screening rate | 6 | 191 | 225 | 90 | 225 | < 0.001 | — | — | — | |
| 6 | 801 | 8005 | 234 | 3005 | 0.0012 | 1.32 | 1.14 to 1.52 | |||
| 3 | — | 45,303 | — | 30,229 | — | — | 1.28 to 1.42a 0.11 to 0.17b | aWomen aged 50‐69 years bWomen aged 20‐49 years | ||
| 10 to 14 | 960 | 1355 | 574 | 847 | 0.014 | 1.32 | 1.06 to 1.64 | — | ||
| 12 | 55a 47b | 134a 163b | 23a 16b | 137a 160b | — | 3.44a 3.70b | 1.91 to 6.19a 1.93 to 7.09b | aBreast cancer screening; bColorectal cancer screening | ||
| 12 | 36a 24b | 158a 157b | 28a 19b | 157a 156b | > 0.05 | 1.4a 1.3b | 0.8 to 2.4a 0.7 to 2.5b | aBreast cancer screening; bColorectal cancer screening (both crude estimates) | ||
| 12 | 30a 43b | 101a 114b | 15a 21b | 90a 126b | 0.034a 0.0002b | 1.96a 3.22b | 0.87 to 4.39a 1.65 to 6.30b | aBreast cancer screening; bColorectal cancer screening | ||
| 12 | 385 | 1565 | 290 | 1558 | < 0.001 | — | — | |||
| 6 | 662 | 2943 | 474 | 2962 | < 0.001 | 1.31 | 1.10 to 1.56 | — | ||
| 9 | 19a 33b | 90a 198b | 17a 27b | 88a 199b | — | — | — | aBreast cancer screening; bColorectal cancer screening | ||
| 12 | 3192 | 10,432 | 3194 | 10,506 | 0.76 | 1.01 | 0.94 to 1.07 | In the adjusted model | ||
| 10 | 144 | 997 | 97 | 976 | 0.006 | 1.52 | 1.13 to 2.05 | In the adjusted model | ||
| APPOINTMENT REMINDERS | ||||||||||
| Reducing non‐attendance rates | 1 | 144 | 277 | 78 | 240 | < 0.05 | 1.60 | 1.29 to 1.98 | — | |
| 1.5 | 333a 169b | 794a 411b | 324a 164b | 790a 409b | > 0.05 | — | −6 to 5a −8 to 7b | aColonoscopy bFlexible sigmoidoscopy | ||
| 2 | 347 | 700 | 322 | 670 | > 0.05 | — | — | — | ||
| 4 | 2662 | 3219 | 2576 | 3350 | < 0.001 | 1.52 | 1.34 to 1.71 | — | ||
| 1.5 | 473 | 500 | 453 | 500 | < 0.001 | 3.41 | 1.87 to 6.20 | — | ||
| 6 | 257 | 407 | 235 | 456 | < 0.01 | 1.50 | — | — | ||
| 3 days | 652 | 701 | 617 | 701 | < 0.05 | 1.71 | — | — | ||
| ADHERENCE | ||||||||||
| Adherence to medications/laboratory tests | 2.5 | 16 | 25 | 12 | 25 | 0.003 | — | — | — | |
| 3 | 453 | 2199 | 298 | 1550 | 0.31 | 1.09 | 0.92 to 1.28 | At 8 weeks differences were not significant (P = 0.23) | ||
| 25 days | 177 | 267 | 53 | 237 | < 0.001 | 4.1 | 3.0 to 5.6 | — | ||
| 6 | 24 | 133 | 16 | 134 | 0.03 | — | — | — | ||
| 12 | 47 | 157 | 42 | 155 | > 0.05 | — | — | — | ||
| 2 weeks | 1180 | 4124 | 958 | 4182 | < 0.001 | — | — | — | ||
| 12 | 109 | 122 | 88 | 119 | 0.003 | — | — | — | ||
| 5 | 29 | 38 | 34 | 42 | 0.233 | — | — | After the mid‐study visit | ||
| 12 | — | 169 | — | 168 | 0.19 | — | — | — | ||
| 1 | 1096975 | 4153634 | 18395 | 84187 | < 0.001 | — | — | — | ||
| 3 to 6 | 3362 | 6833 | 1865 | 4172 | — | — | — | |||
| 2 weeks | 4318 | 15,356 | 3309 | 15,254 | < 0.001 | — | — | — | ||
| 6 | 70 | 137 | 55 | 143 | 0.041 | 0.60 | 0.37 to 0.96 | Primary composite outcome of adherence and adverse effects (emergency room visit and hospitalisation) | ||
| 12 | — | 600 | — | 600 | — | 0.93 | 0.71 to 1.22 | — | ||
| 6 | 178 | 253 | 148 | 244 | < 0.05 | 1.54 | 1.13 to 2.10 | — | ||
| 18 | — | 3171 | — | 3260 | 0.002 | — | 0.01 to 0.03 | P value and CIs for Δ change | ||
| 12 | — | 7247 | — | 7255 | 0.022 | — | 0.011 to 0.034 | P value and CIs for Δ change | ||
| HEART FAILURE | ||||||||||
| Heart failure hospitalisation | 10 ± 6 (median 11) | 17 | 67 | 58 | 66 | < 0.05 | — | — | — | |
| 12 | 4 | 32 | 17 | 50 | < 0.05 | — | — | This was a cluster outcome: "cardiovascular deaths and hospitalisations‐ which ever event occurred first" | ||
| All‐cause mortality | 10 ± 6 (median 11 ) | 5 | 67 | 7 | 66 | > 0.05 | — | — | — | |
| 6 | 92 | 826 | 94 | 827 | 0.86 | 0.97 | 0.73 to 1.30 | Death or readmission | ||
| 12 | 17 | 170 | 16 | 209 | 0.439 | 1.36 | 0.63 to 2.93 | — | ||
| Cardiac mortality | 10 ± 6 (median 11) | 2 | 67 | 6 | 66 | > 0.05 | — | — | — | |
| 12 | 3 | 32 | 5 | 50 | > 0.05 | — | — | Cluster outcome: "cardiovascular deaths and hospitalisations‐ which ever event occurred first" | ||
| SMOKING | ||||||||||
| Smoking abstinence | 12 | 74 | 197 | 48 | 199 | 0.02 | 1.91 | 1.12 to 3.26 | — | |
| 9 | 20 | 120 | 25 | 111 | — | — | — | — | ||
| 12 | 12 | 23 | 14 | 21 | 0.33 | — | — | — | ||
| 3 | 105 | 361 | 95 | 364 | 1.13 | 0.90 to 1.41 | — | |||
| 12 | 23 | 50 | 17 | 49 | 0.25 | 1.60 | 0.71 to 3.60 | — | ||
| 6 | 51 | 198 | 30 | 199 | 0.009 | 1.71 | 1.14 to 2.56 | — | ||
| 30 | 75 | 500 | 65 | 523 | — | — | — | For 6 month prolonged abstinence | ||
| Continous outcomes | ||||||||||
| Primary outcome | study ID | Timing of outcome assessment (months) | Intervention group | Comparator group | Between‐group difference | Notes | ||||
| Mean | Standard deviation of change (SD) or SD | Mean | Standard deviation of change (SD) or SD | Change | Confidence intervals | P values | ||||
| ALCOHOL CONSUMPTION | ||||||||||
| Drinks per drinking day | 2 | 3.5 | 1.8 | 4.7 | 3.2 | 1.38 | 1.12 to 1.70 | < 0.01 | CIs are for the effect size | |
| 2 | 4 | 0.4 | 4.3 | 0.4 | — | — | 0.45 | — | ||
| CANCER | ||||||||||
| Symptom severity | 1 | — | — | — | — | — | — | — | Effect size: intervention = 0.75; control = 0.68 | |
| 1.5 | 5.76 to 7.36 (range) | — | 5.55 to 7.44 (range) | — | 0.06 | — | 0.58 | — | ||
| 2.5 | 20.73 | — | 20.80 | — | — | — | > 0.05 | Effect size: intervention = 0.59; control = 0.56 | ||
| 2.5 | 11.6 | — | 11.0 | — | — | — | 0.02 | — | ||
| DIABETES | ||||||||||
| Glycated haemoglobin (%) | 3 | 7.87 | 1.09 | 7.82 | 1.14 | — | — | 0.89 | — | |
| 6 | 8.10 | 7.90 | — | — | — | > 0.05 | Median values | |||
| 3 | 9.1 | 1.9 | 8.6 | 1.3 | — | — | 0.41 | — | ||
| 12 | 9.0 | 2 | 9.9 | 2.2 | — | — | 0.02 | — | ||
| 6 | 7.0 | 1.4 | 7.3 | 1.5 | — | — | 0.04 | — | ||
| 12 | 8.1 | 1.15 | 8.2 | 1.18 | — | — | 0.3 | — | ||
| 12 | 8.7 | 1.9 | 9.0 | 2.2 | 0.1 | 0.5 to 0.4 | 0.8 | — | ||
| 6 | 7.9 | 1.2 | 8.7 | 1.8 | 0.91 | 0.86 to 0.93 | 0.002 | — | ||
| Serum blood glucose (mg/dL) | 12 | 180 | 9 | 172 | 10 | — | — | 0.6 | — | |
| 26 | 107.4 | 12.9 | 109.7 | 16.5 | — | — | 0.44 | — | ||
| Self‐monitoring of blood glucose | 3 | 1.9 | 1.07 | 1.3 | 0.75 | — | — | < 0.001 | — | |
| 6 | 0.05 | 0.387 | 0.08 | 0.365 | — | — | 0.457 | — | ||
| 12 | 4.6 | 0.1 | 4.4 | 0.1 | — | — | 0.05 | — | ||
| 12 | 4.3 | 2.6 | 3.3 | 2.9 | 0.8 | 0.1 to 1.5 | 0.03 | — | ||
| Self‐monitoring of diabetic foot | 12 | 4.6 | 0.1 | 4.4 | 0.1 | — | — | — | — | |
| 12 | 5.1 | 1.4 | 4.6 | 1.7 | 0.6 | 0.2 to1.0 | 0.002 | — | ||
| HYPERTENSION | ||||||||||
| Systolic Blood Pressure (mm Hg) | 3 | 136.4 | 83.5 | 138.9 | 81.5 | — | — | > 0.05 | — | |
| 6 | 123.8 | 14.2 | 128.6 | 19.4 | — | — | > 0.05 | — | ||
| 6 | 158 | * | 160.2 | * | −1.8 | — | 0.20 | — | ||
| 1 | 141.2 | 15.1 | 143.1 | 14.6 | — | < 0.001 | — | |||
| 6 | 137.4 | 19.4 | 136.7 | 17.0 | −0.7 | — | 0.006 | — | ||
| 6 weeks | 142.5 | 2.3 | 143.6 | 2.4 | −4.2 | −9.1 to 0.7 | 0.09 | — | ||
| Diastolic Blood Pressure (mm Hg) | 6 | 80.9 | — | 83.2 | — | — | — | 0.02 | — | |
| 6 | 74.6 | 8.5 | 79.5 | 14.0 | — | — | > 0.05 | — | ||
| 1 | 80.3 | 12.6 | 81.3 | 12.5 | — | — | < 0.001 | — | ||
| 6 | 82.9 | 12.9 | 81.1 | 11.7 | −2.3 | −4.9 to −0.2 | 0.07 | — | ||
| OBSTRUCTIVE SLEEP APNOEA SYNDROME (OSAS) | ||||||||||
| Continuous positive airway pressure (CPAP) use | 2 | 4.4 | — | 2.9 | — | — | — | 0.076 | — | |
| 12 | — | — | — | — | — | 1.18 to 2.48 | 0.004 | — | ||
| WEIGHT MANAGEMENT | ||||||||||
| BMI in adults | 18 | 36.54 | 2.01 | 36.84 | 1.90 | −0.35 | −0.75 to 0.06 | — | — | |
| 18 | 29.8 | 1.90 | 30.3 | 1.93 | −0.6 | −1.2 to −0.1 | 0.03 | — | ||
| 6 | 33.7 | 5.2 | 37.2 | 8.7 | — | — | 0.06 | — | ||
| BMI‐z scores in children | 12 | 1.95 | 0.04 | 1.98 | 0.03 | — | — | > 0.05 | — | |
| 3 | 1.9 | 0.28 | 1.9 | 0.3 | −0.03 | — | 0.48 | — | ||
| Analyses limited to primary outcomes from at least 2 studies from the same category. | ||||||||||
| Study ID | Study typea | Study subtypeb | Country | Sample size | Mean age (years unless stated otherwise) | Male (%) | Female (%) | Ethnicityc | Duration of condition | Comorbidities, medication | Incentives for participation | Incentives |
| P | Alcohol misuse | USA | 187 | 45 | 63 | 37 | White ‐ 54% Other ‐ 46% | — | — | Yes | Visa gift cards or checks (USD 50 per in‐person interview, USD 15 per phone interview). IG participants received USD 0.50 minimum for each daily call and USD 1.00 after seven consecutive calls | |
| P | I | USA | 1138 | — | — | — | — | — | — | — | — | |
| P | I | USA | 11,982 | 72 | — | — | — | — | — | — | — | |
| P | I | USA | 1227 | 2‐3 months | — | — | — | — | — | — | — | |
| P | I | USA | 3050 | 9 monthsd | 49 | 51 | Black ‐ 76% Hispanic ‐ 14% White ‐ 7% Other – 3% | — | — | — | — | |
| P | I | USA | 752 | 20 months | — | — | — | — | — | — | — | |
| P | I | USA | 8002 | — | 51 | 49 | Black ‐ 50% White ‐ 45% Other – 5% | — | — | — | — | |
| P | I | USA | 50 | 24 | 0 | 100 | Black ‐ 86% White ‐ 14% | — | — | Yes | USD 35 per month to help pay for cell phone service or a free, unlimited minutes cell phone until 6 weeks postpartum | |
| P | I | USA | 229 | 9 months | 52 | 48 | Black ‐ 90% Other – 7% Hispanic ‐ 3% | — | — | — | — | |
| P | I | USA | 3006 | — | 51 | 49 | Other – 41% Black ‐ 35% White ‐ 17% Hispanic ‐ 7% | — | — | — | — | |
| P | I | USA | 4115 | 14 | 50 | 50 | — | — | — | — | — | |
| P | Physical activity | USA | 71 | 57 | 0 | 100 | White ‐ 93% Other ‐ 7% | — | — | — | — | |
| P | Physical activity | USA | 181 | 69 | 99 | 1 | — | — | Mean (SD) number of comorbidities IG: 3.8 (1.5) CG: 3.9 (1.4) | Yes | USD 15 for completing each | |
| P | Physical activity | USA | 85 | 67 | 24 | 76 | Other ‐ 70% Black ‐ 30% | — | Mean co‐morbidities: 3 | — | — | |
| P | Physical activity | USA | 218 | 61 | 31 | 69 | White ‐ 90% Other ‐ 10% | — | — | — | — | |
| P | Physical activity | USA | 398 | 78 | 100 | 0 | White ‐ 77% Black ‐ 23% | — | Mean (SD) number of diseases IG: 5.2 (2.5) CG: 5.5 (2.7) | — | — | |
| P | Physical activity | USA | 302 | 67 | 97 | 3 | White ‐ 70% | — | Mean (SD) number of comorbidities IG: 4.2 (2.4) CG: 3.9 (2.4) | — | — | |
| P | Physical activity | USA | 298 | 46 | 28 | 72 | White ‐ 45% Black ‐ 45% Other ‐ 10% | — | — | — | — | |
| P | Physical activity | USA | 103 | 71 | 69 | 31 | — | — | Depression (unclear %) | — | — | |
| P | Screening | USA | 450 | 60 | 28 | 72 | Hispanic 87% Other‐ 13% | — | ≥ 1 long‐term conditions ‐ 68% | No | — | |
| P | Screening | USA | 11,010 | 61 | 54 | 46 | White ‐ 86% Other ‐ 14% | — | — | — | — | |
| P | Screening | Australia | 75,532 | — | 0 | 100 | — | — | — | — | — | |
| P | Screening | USA | 3547 | — | 0 | 100 | White ‐ 88% Black ‐ 11% Asian or Other ‐ 1% | — | — | — | — | |
| P | Screening | USA | 47,097 | 58 | 47 | 53 | — | — | — | — | — | |
| P | Screening | USA | 469 | — | 56 (for colorectal cancer) | 44 (for colorectal cancer) | White ‐ 61% Black ‐ 28% Latinos ‐ 5% Asian ‐ 5% | — | — | — | — | |
| P | Screening | USA | 1008 | — | 45 | 55 | White ‐ 48% Black ‐ 37% Other ‐ 15% | — | — | No | — | |
| P | Screening | USA | 366 | — | — | — | White ‐ 50% Black ‐ 41% Other (including Hispanic) ‐ 9% | — | — | — | — | |
| P | Screening | USA | 4685 | 57 | 0 | 100 | — | — | Anticonvulsants ‐ 6% Corticosteroids ‐ 4% COPD (unclear %) Oophorectomy ‐ 3% | — | — | |
| P | Screening | USA | 6000 | 60 | 50 | 50 | White ‐ 93% Other ‐ 7% | — | Obesity ‐ 40% | — | — | |
| P | Screening | USA | 685 | 58 | 38 | 62 | Non‐Hispanic white ‐ 78% Black ‐ 13% Other ‐ 9% | — | — | — | — | |
| P | Screening | USA | 20,936 | 57 | 47 | 53 | White ‐ 86% Other ‐ 9% Black ‐ 5% | — | — | — | — | |
| P | Screening | USA | 1973 | 69 | 8 | 92 | — | — | Use of oral glucocorticoids ‐ 22% Fractures ‐ 12% | — | — | |
| P | Stress management | USA | 100 (caregiver) | 63 (caregiver) | 22 (caregiver) | 78 (caregiver) | Black ‐ 64% Hispanic ‐ 21% White ‐ 15% Other ‐ 1% | — | — | — | — | |
| P | Substance use | USA | 33 | 46 | 76 | 24 | White ‐ 64% White ‐ 15% Hispanic ‐ 21% | — | HIV medication ‐ 64% Hepatitis A, B, or C ‐ 49% | Yes | USD 20 gift certificates for each assessment | |
| P | Weight management | USA | 365 | 55 | 31 | 69 | Black ‐ 71% Hispanic ‐ 13% White ‐ 4% Other ‐ 2% | — | Cholesterol medication ‐ 36% Diabetes medication ‐ 30% Mean BMI ‐ 37 kg/m2 | Yes | USD 50 reimbursement at the first 3 follow‐up visits and USD 75 at 24 months | |
| P | Weight management | USA | 194 | 35 | 0 | 100 | Black ‐ 100% | — | Hypertension ‐ 36% Metabolic syndrome ‐ 31% Depression ‐ 22% Diabetes mellitus ‐ 7% | Yes | Reimbursements of USD 50 each at baseline and at all follow‐up study visits | |
| P | Weight management | USA | 77 | 59 | 29 | 71 | White ‐ 68% Hispanic ‐ 18% Other ‐ 7% Black ‐ 4% Asian ‐ 3% | — | — | — | — | |
| P | Weight management | USA | 220 | 11 | 54 | 46 | White‐ 63% Hispanic ‐ 26% Other ‐ 11% | — | — | — | — | |
| P | Weight management | Greece | 122 | 44 | 12 | 88 | — | — | Hypertension ‐ 13% Diabetes mellitus ‐ 2% | — | — | |
| P | Weight management | USA | 140 | — | — | — | — | — | — | — | — | |
| P | Weight management | USA | 50 (child) | 10 (child) 40 (parent) | 58 (child) 4 (parent) | 42 (child) 96 (parent) | Black ‐ 72% Other ‐ 22% White ‐ 6% (parent) | — | BMI (child) ‐ 25.7 kg/m2 BMI (parent) ‐ 34 kg/m2 | Yes | For completing assessments (USD 40 parents; USD 10 child) | |
| E | Appointment reminder | USA | 517 | — | — | — | — | — | — | — | — | |
| E | Appointment reminder | USA | 3610 | 63 | 95 | 5 | White ‐ 83% Other ‐ 16% Hispanic ‐ 1% | — | — | — | — | |
| E | Appointment reminder | USA | 2304 | 29 | — | 100 | Hispanic ‐ 66% Black ‐ 19% White ‐ 13% Other ‐ 2% | — | — | — | — | |
| E | Appointment reminder | USA | 12,092 | 56 | 43 | 57 | — | — | — | — | — | |
| E | Appointment reminder | UK | 1000 | — | 33 | 67 | — | — | — | — | — | |
| E | Appointment reminder | USA | 2008 | 19d | 54 | 46 | Spanish‐speaking ‐ 39% Vietnamese‐speaking ‐ 28% English‐speaking ‐14% Other – 13% Tagalog‐speaking Filipino – 6% | — | — | — | — | |
| E | Appointment reminder | USA | 701 | < 12e | 45 | 55 | English‐speaking ‐ 59% Spanish‐speaking ‐ 29% Vietnamese‐speaking ‐ 3% Other – 9% | — | — | — | — | |
| M | Illicit drugs addiction | USA | 36 | 41 | 58 | 42 | White ‐ 58% Black ‐ 28% Other – 14% | On methadone treatment mean = 21.7 | — | Yes | USD 20 per week for completing weekly assessments and providing a urine sample | |
| M | Alcohol consumption | Sweden | 1423 | — | — | — | — | — | — | — | — | |
| M | Alcohol consumption | USA | 254 | 46 | 78 | 22 | Black ‐ 49% Hispanic ‐ 45% Other – 6% | 12.8y | HIV/AIDS (unclear %) | Yes | USD 20; USD 40 at last 2 post‐treatment follow‐ups | |
| M | Alcohol consumption | USA | 338 | 46 | 64 | 36 | White ‐ 97% | Currently dependent ‐ 67% | — | Yes | USD 30 for the | |
| M | Alcohol consumption | USA | 110 | 49 | 58 | 42 | White ‐ 86% Black ‐9%, Hispanic‐3% Other‐2% | Mean of 1.2 (SD 2.4) | — | Yes | The possible total incentive was USD 50.00 per week | |
| M | Alcohol consumption | USA | 60 | 42 | 55 | 45 | White ‐ 95% Black ‐5% | 52.3 heavy drinking days within past 3 months | — | Yes | Patients were paid USD 75 for the 30‐day follow‐up, USD 125 for the 90‐day follow‐up, and USD 200 for the 180‐day follow‐up | |
| M | Alcohol consumption | USA | 158 | 49 | 53 | 47 | — | Regular alcohol use mean = 17.94 years | — | Yes | USD 25 for each interview | |
| M | Alcohol consumption | USA | 47 | 57 | 60 | 40 | Caucasian ‐ 83% African‐American ‐ 13% | — | — | — | — | |
| M | Alcohol consumption | USA | 98 | 46 | 91 | 9 | White ‐ 45% Black ‐ 40% Native American ‐ 7% Other ‐ 6% Hispanic ‐ 2% | — | — | Yes | USD 25.00 each for the baseline and for the follow‐up assessments | |
| M | Asthma | USA | 6948 | 52 | 35 | 65 | White ‐ 92% Other ‐ 8% | — | Beta agonist ‐ 55% Oral steroids ‐ 46% COPD ‐ 33% | — | — | |
| M | Asthma | Australia | 121 | 7 | 53 | 47 | — | — | — | — | — | |
| M | Cancer | USA | 79 | 60 | 53 | 47 | White ‐ 85% Black ‐ 15% | — | — | — | — | |
| M | Cancer | USA | 405 | 59 | 32 | 68 | White ‐ 80% Black‐ 18% Other ‐ 2% | — | Depression ‐ 76% Pain ‐ 68% | — | — | |
| M | Cancer | USA | 250 | 55 | 24 | 76 | White/Caucasian ‐ 91% Other ‐ 9% | — | — | — | — | |
| M | Cancer | USA | 239 | 58 | 50 | 50 | White ‐ 89% Black‐ 6% Hispanic ‐ 4% Other 1% | Mean (SD) time since cancer diagnosis‐ IG: 36 months (35) CG: 26 months (32) | Mean (SD) symptoms (out of 13) IG: 3.4 (2.2) CG: 4.0 (2.4) | — | — | |
| M | Cancer | USA | 437 | 57 | 25 | 75 | — | — | Mean comorbidities: 2 | — | — | |
| M | Cancer | USA | 119 | 60 | 31 | 69 | White ‐ 76% Asian ‐ 17 % Black ‐ 7% | — | Capecitabine ‐ 35% Erlotinib ‐ 24% Lapatinib ‐ 9% Imatinibf ‐ 8% Temozolomide ‐ 6% Sunitinib ‐ 5% | — | — | |
| M | Cancer | USA | 253 | 61 | 49 | 51 | White ‐ 58% Black ‐ 36% Other ‐ 6% | — | Planned single chemotherapy ‐ 9% Planned combination chemotherapy ‐ 90% | — | — | |
| M | Chronic pain | USA | 250 | 55 | 83 | 17 | White ‐ 77 % | ≤ 5 years = 29% 6‐10 years = 19% > 10 years = 52% | Major depression‐ 24% Post‐traumatic stress disorder‐ 17% | — | — | |
| M | Chronic pain | USA | 55 | 46 | 14 | 86 | White ‐ 96% Other ‐ 4% | — | — | — | — | |
| M | COPD | UK | 79 | 69 | 74 | 26 | — | — | SABA ‐ 75% LAMA ‐ 43% LABA/ICS ‐ 43% ICS ‐ 33% SAMA ‐ 32% Oral steroids ‐ 25% LABA ‐ 18% | — | — | |
| M | Adherence | USA | 475 | 5 (child) 35 (parent) | 52 (child) 7 (parent) | 48 (child) 93 (parent) | Black ‐ 67% (child) 47% (parent); Other – 33% (child) 53% (parent) | — | — | Yes | Gift cards | |
| M | Adherence | USA | 50 | 42 | 36 | 64 | White ‐ 58% Black ‐ 20% Hispanic ‐ 18% Asian ‐ 4% | — | — | Yes | USD 25 for each completed visit | |
| M | Adherence | USA | 1187 | — | — | — | — | — | — | — | — | |
| M | Adherence | USA | 70 | 66 | 49 | 51 | African American ‐ 58% European ‐ 32% Asian ‐ 6% Hispanic ‐ 3% Middle Eastern ‐ 1% | Median 5 years in IG; 4.5 years in CG | Bimatoprost ‐ 11.5% Travoprost ‐ 17.5% Latanoprost ‐ 71.5% Bilateral medication ‐ 70% | — | — | |
| M | Adherence | USA | 1393 | — | — | — | — | — | — | — | — | |
| M | Adherence | USA | 13,057 | 51 | 54 | 46 | Other ‐ 48 % White ‐ 23% Hispanic ‐ 14% Black ‐ 10% Asian ‐ 5% | — | — | — | — | |
| M | Adherence | USA | 5216 | 56 | 49 | 51 | Hispanic ‐ 30% White ‐ 28% Unknown ‐ 23% Black ‐ 10% Asian and Pacific Islander ‐ 7.1% Other ‐ 1.7% Native American‐ 0.2% | — | Mean low‐density lipoproteins = 146 mg/dL | — | — | |
| M | Adherence | USA | 961 | 59 | 47 | 53 | — | — | Statins ‐ 32% Depression ‐ 11% | — | — | |
| M | Adherence | USA | 267 | 77 | 23 | 77 | Other ‐ 89 % Black: 11% | — | Other – 81% Heart disease ‐ 32% Diabetes mellitus ‐ 18% Stroke ‐ 7% | — | — | |
| M | Adherence | USA | 312 | 63 | 62 | 38 | Black ‐ 91% White ‐ 9% | — | — | Yes | USD 25 gift card | |
| M | Adherence | USA | 8306 | — | — | — | — | — | — | — | — | |
| M | Adherence | USA | 241 | 64 | 98 | 2 | White ‐ 78% | — | Hypertension ‐ 91% Hyperlipidaemia ‐ 85% Diabetes mellitus ‐ 45% Chronic kidney disease ‐ 23% Chronic lung disease ‐ 20% Prior heart failure ‐ 12% Peripheral arterial disease ‐ 10% Cerebrovascular disease ‐ 7% | — | — | |
| M | Adherence | USA | 16 | 71 | 31 | 69 | — | — | — | Yes | USD 25 for participating | |
| M | Adherence | USA | 80 | 66 | 51 | 49 | White ‐ 62% African‐American ‐ 10% Hispanic/Latino ‐ 9% Asian ‐ 9% East Indian ‐ 6% | Mean IG: 25.79 months; CG: 22.1 months | Number of medical problems: IG: 3.43 CG: 3.32 | — | — | |
| M | Adherence | USA | 337 | 57 | 30 | 70 | Black ‐ 100% | — | BMI ‐ 34.4 kg/m2 Diabetes ‐ 38% Stroke ‐ 7.5% | — | — | |
| M | Adherence | USA | 4,237,821 | 56 | 38.5 | 61.5 | — | — | Correspondence with the author: "All participants were on maintenance medications" | — | — | |
| M | Adherence | USA | 27 | 80 | — | — | — | — | Participants had cognitive (memory) impairment and were on donepezil, rivastigmine, or galantamine | — | — | |
| M | Adherence | USA | 15,051 | 57 | 53 | 47 | — | — | — | — | — | |
| M | Adherence | USA | 30,610 | — | — | — | — | — | — | — | — | |
| M | Adherence | Canada | 331 | 63 | — | — | — | — | — | — | — | |
| M | Adherence | USA | 1200 | 51 | 62 | 38 | Other ‐ 95% Black ‐ 5% | — | Insulin ‐ 19.4% (participants) | — | — | |
| M | Adherence | USA | 497 | 54 | 38 | 62 | — | — | Lipitor – 54% Zocor – 16% Other statin – 16% | — | — | |
| M | Adherence | USA | 647 | — | — | — | — | — | — | — | — | |
| M | Adherence | USA | 8517 | 54 | 34 | 66 | White ‐ 50% Other – 26% Asian ‐ 11% Mixed – 7% Native Hawaiian/Pacific Islander ‐ 4% Black ‐ 2% American Indian/Alaskan Native – 1% | — | COPD ‐ 33% | — | — | |
| M | Adherence | USA | 21,752 | 64 | 53 | 47 | White ‐ 47% Asian ‐ 17% Black ‐15% Native Hawaiian/Pacific Islander ‐ 11% Black ‐ 2% American Indian/Alaskan Native – 1% | — | Diabetes mellitus ‐ 78% CVD ‐ 36% Statin only ‐ 40% ACEI/ARB only ‐ 25% Statin and ACEI/ARB ‐ 35% low‐density lipoproteins among statin users (mean) = 93.4 mg/dL | — | — | |
| M | Diabetes mellitus | USA | 119 | 62 | 55 | 45 | White ‐ 77% Other – 23% | — | — | Yes | USD 25 | |
| M | Diabetes mellitus | USA | 80 | 30 | 0 | 100 | White ‐ 41% Black ‐ 34% Hispanic ‐ 18% Asians or others ‐ 7% | — | BMI ‐ 34.1 kg/m2 | — | — | |
| M | Diabetes mellitus | USA | 98 | 59 | 40 | 60 | Black ‐ 65% White ‐ 30% Other ‐ 3% Hispanic ‐ 1% Asian ‐ 1% | — | Other antidiabetic medications + insulin ‐ 80% Basal‐bolus regimen ‐ 40% Long‐acting insulin only ‐ 33% Mixed insulin only ‐ 17% Short‐acting insulin only ‐ 10% | — | — | |
| M | Diabetes mellitus | USA | 75 | 52 | 59 | 41 | Hispanic ‐ 100% | — | — | Yes | USD 10 for initial visit and USD 20 incentive card at the follow‐up | |
| M | Diabetes mellitus | USA | 100 | — | — | — | — | — | Psychiatric illness ‐ 46% Had been hospitalised over the past year ‐ 28% | — | — | |
| M | Diabetes mellitus | USA | 417 | 53 | 38 | 62 | Hispanic ‐ 100% | — | — | — | — | |
| M | Diabetes mellitus | USA | 248 | 55 | 41 | 59 | Hispanic ‐ 50% White ‐ 29% Other – 21% | — | BMI ‐ 33.7 kg/m2 Mean comorbidities: 1 | — | — | |
| M | Diabetes mellitus | USA | 272 | 61 | 97 | 3 | White ‐ 60% Black ‐ 18% Hispanic ‐ 12% Other ‐ 10% | — | BMI ‐ 31 kg/m2 Mean comorbidities: 2 | — | — | |
| M | Diabetes mellitus | USA | 339 | 56 | 43 | 57 | Hispanic ‐ 47% Asian ‐ 22% Black ‐ 20% White ‐ 8% Other ‐ 2% | — | BMI: 31 kg/m2 | Yes | USD 15 and USD 25 for the baseline and 1‐year follow‐up visits, respectively | |
| M | Diabetes mellitus | Australia | 120 | 57 | 63 | 37 | — | — | Low depression – 73% Intermediate depression – 23% High depression – 4% Low anxiety – 89% Intermediate anxiety – 8% High anxiety – 3% BMI ‐ 33 kg/m2 Insulin – 43% | — | — | |
| M | Heart failure | Italy | 133 | 57 | 88 | 12 | — | — | Diuretics ‐ 89% ACE inhibitors ‐ 84% Carvedilol ‐ 50% Nitrates ‐ 40% Digitalis ‐ 33% K + saver ‐ 21% | — | — | |
| M | Heart failure | USA | 1653 | 61d | 58 | 42 | White ‐ 49% Black ‐ 39% Other – 12% (inclusive of Hispanic or Latino – 3%) | — | Beta‐blocker – 79% Loop diuretic – 78% Hypertension ‐ 77% ACE inhibitor or ARB – 70% Coronary artery disease ‐ 50% Diabetes mellitus ‐ 47% Chronic kidney disease ‐ 46% Aldosterone‐receptor antagonist – 33% Digoxin – 25% COPD ‐ 21% | No | — | |
| M | Heart failure | Australia | 405 | 73 | 63 | 37 | — | — | Diuretics ‐ 80% Heart failure specific beta‐blockers ‐ 61% Systolic heart failure ‐ 60% ACE inhibitors ‐ 57.5% Diabetes mellitus ‐ 30.5% Aldosterone antagonist ‐ 26% ARB ‐ 25% Diastolic heart Internal cardio defibrillator ‐ 4.5% | — | — | |
| M | Heart failure | France | 138 | 68 | 79 | 21 | — | — | Loop diuretic – 92% ACE/AT2– 79% Beta‐blocker – 79% Spironolactone – 29% Digoxin – 9% (mean values) | — | — | |
| M | HIV | India | 631 | — | 57 | 43 | Asian ‐100% | — | Zidovudine‐based antiretroviral treatment – 44% Tenofovir‐based antiretroviral treatment – 44% Stavudine‐based antiretroviral treatment – 12% | Yes | Mobile phone plus wage compensation | |
| M | Hypercholestorolaemia | USA | 115 | 48 | 25 | 75 | White ‐ 87% Other – 13% | — | — | — | — | |
| M | Hypercholesterolaemia | USA | 123 | 57 | 25 | 75 | Black ‐ 77% Other – 23% | — | Mean BMI ‐ 31 kg/m2 | — | — | |
| M | Hypertension | USA | 241 | 60 | 21 | 79 | Black ‐ 81% White‐ 15% Hispanic ‐ 3% Other ‐ 1% | — | Hyperlipidaemia ‐ 46% Diabetes ‐ 32% | — | — | |
| M | Hypertension | USA | 253 | 58 | 27 | 73 | Black ‐ 100% | — | — | — | — | |
| M | Hypertension | USA | 64,773 | 61 | 46 | 54 | White ‐ 41%, Hispanic ‐ 25% Black ‐ 17% Other – 9% Asian ‐ 8% | — | Cardiovascular disease ‐ 38% Diabetes mellitus ‐ 27% Chronic kidney disease ‐ 10% | — | — | |
| M | Hypertension | USA | 283 | 66 | 65 | 35 | White ‐ 65% Other – 18% Hispanic ‐ 17% | — | Diabetes mellitus or chronic kidney disease – 55% | Yes | Clinically validated electronic blood pressure cuffs were provided at no cost to those who did not own one | |
| M | Hypertension | Honduras; Mexico | 200 | 58 | 33 | 67 | — | — | Blood pressure medication – 83% Diabetes mellitus ‐ 23% BMI ‐ 30.7 kg/m2 | — | — | |
| M | Mental health | USA | 164 | 39 | 24 | 76 | White ‐ 56% Black ‐ 32% Other – 12% | — | — | — | — | |
| M | Mental health | USA | 218 | 39 | 58 | 42 | White ‐ 93% Other – 7% | — | Social phobia ‐ 9%, Generalised anxiety disorder ‐8% Simple phobia – 6% Major depression – 2% Dysthymia – 2% | — | — | |
| M | Mental health | USA | 73 | 54 | 18 | 82 | Other – 74% Hispanic – 26% | — | — | — | — | |
| M | OSAS | USA | 30 | 46 | — | — | — | — | BMI ‐ 38 kg/m2 | — | — | |
| M | OSAS | USA | 250 | 55d | 82 | 18 | — | — | BMI ‐ 35.1 kg/m2 | — | — | |
| M | Smoking | Norway | 396 | 36 | 47 | 53 | — | — | — | Yes | All participants in both groups received a sample packet of nicotine replacement therapy products. | |
| M | Smoking | USA | 521 | 36 | 36 | 64 | White ‐ 81% Black ‐ 6% Other – 5% Hispanic/Latino ‐ 4% Native American – 3% Asian ‐ 1% | — | ≥ 1 long‐term conditions ‐ 47% | No | — | |
| M | Smoking | USA | 332 | 30 | 0 | 100 | White ‐ 61% Black ‐ 16% Hispanic ‐ 15% Other – 8% | — | — | — | — | |
| M | Smoking | Canada | 44 | 53 | 67 | 33 | — | Mean cigarettes/d: intervention ‐ 18.5 control ‐ 17.3 | — | — | — | |
| M | Smoking | Taiwan | 116 | 20 | 92 | 8 | Asian ‐ 100% | — | — | Yes | Equivalent of USD 6 | |
| M | Smoking | USA | 731 | 52 | 56 | 44 | — | — | — | — | — | |
| M | Smoking | Canada | 100 | 54 | 68 | 32 | — | — | Acute coronary syndrome ‐ 83% | — | — | |
| M | Smoking | Canada | 440 | — | — | — | — | — | — | — | — | |
| M | Smoking | USA | 397 | 53 | 48 | 52 | White ‐ 81% Hispanic ‐ 6% Black ‐ 4% Other/unknown ‐ 4% Native American ‐ 3% Asian/Pacific Islander ‐2.5% | Mean cigarettes/d: IG = 17.1 CG = 16.3 | Depressive symptoms (mean) IG = 9.3 CG = 10.3 | Yes | USD 50 for a saliva sample | |
| M | Smoking | USA | 2054 | 51 | 77 | 23 | White ‐ 89%, Black ‐ 5%, Other ‐ 4% Native American ‐ 2% | Mean cigarettes/d IG = 23.85 CG = 25.18 | — | — | — | |
| M | Spinal cord dysfunction | USA | 142 | 48 | 61 | 39 | White ‐ 80% (inclusive of Hispanic or Latino – 7%) Black ‐ 11% Other ‐ 9% | 11.7 y | Depression – 39% Pressure ulcers ‐ 7% | — | — | |
| aStudy type: P: prevention; M: management; E: either. cPlease note that for reporting of participants' ethnicity, the terms used by authors of the included studies have been used in each case and are cited directly from each of the included studies. | ||||||||||||
| Study ID | ATCSa | Contentb | Theoryc | BCTsd | Received instructions? | Callere | Telephone keypadf | Toll free | Study duration | Call durationg | Frequencyh | Intensity i | Speaker | Security arrangementj |
| IVR | AF | — | 1 | — | P | Other | — | 25 months | 29.3 min | — | — | Synthetic speech | — | |
| ATCS Plus | AF,SF | MI | 9, 25, 40 | Yes | E | Yes | Yes | 2 months | 1‐3 min | Daily | — | — | — | |
| IVR | AF | — | 25 | — | — | — | — | 1.5 months | — | — | < 500 words | — | — | |
| Unidirectional¹ | AF | TCD | 1, 42 | No | H | — | — | 6 months | — | 2 | 92 words | — | — | |
| IVR | AF | HBM | 20, 21, 42 | — | H | Yes | Yes | 2.5 months | < 5 min | 2 | — | — | — | |
| IVR | AF | — | 20,21 | — | — | — | — | 24 months | — | — | — | — | — | |
| ATCS Plus | AF, CF | SCT, TRA, TTM | 3, 9, 12, 13, 17, 20, 21, 25, 33, 39, 40, 42 | — | — | — | — | 24 months | — | — | — | — | — | |
| ATCS Plus¹ | AF, CF | SCT, TRA, TTM, MI | 3, 9, 12, 13, 17, 20, 21, 25, 33, 39, 40, 42 | — | H | — | — | 12 months | 2–4 min | Weekly | — | — | — | |
| IVR | AF | — | 29,42 | — | H | Yes | — | 3 months | — | Daily | 51 words | — | Reminder information was sent securely to Memotext¹ | |
| ATCS Plus¹ | AF, CF, SF | — | 9,10,42 | Yes | P | Yes¹ | Yes | 6 months | — | Biweekly | — | — | Password and log‐in | |
| ATCS Plus¹ | AF, SF | CBT, SCT, MI, SRT, SCL, RP | 3,12, 20,27, 34, 39 | Yes | P | Yes | — | 24 months | — | Twice daily then biweekly¹ for 6 weeks | — | Personal pronouns and active voice | — | |
| ATCS Plus | AF, CF | — | 20, 42 | Yes | P | Yes | Yes | 12 months | — | Daily | — | — | PIN² | |
| ATCS Plus | AF, CF | — | 3 | — | H | Yes | Yes | 4 months | — | — | — | — | — | |
| ATCS Plus | AF, CF | — | 20, 42 | Yes | P | Yes | Yes | 6 months | — | Daily | — | — | — | |
| ATCS Plus | AF, SF | — | 21 | Yes | H | Yes | — | 1 month | 4 weeks | Biweekly | — | — | — | |
| IVR | AF | — | 1,42 | — | H | — | — | 12 months | 5 min¹ | 1 | — | — | — | |
| ATCS Plus | AF, CF | — | 3, 27, 39 | Yes | H | Yes | — | 6 months | — | — | — | — | — | |
| ATCS Plus | AF, CF | — | 29,42 | — | H | Other | — | 12 months | — | ≤ 3 | — | — | — | |
| ATCS Plus | AF, CF | TTM, SCT, PST | 3,10 | Yes | E | — | — | 3 months | 15‐30 s² | Twice daily | — | — | — | |
| IVR | AF | TTM, SCT | 1,9 | — | H | — | — | 3 months | 10 min | Weekly | — | Pre‐recorded human speech | — | |
| IVR | AF | HBM¹ | 27, 42 | — | H | Yes | — | 24 months | 69 s | Average ‐ 3 | 224 words | Female voice | Verification³ | |
| IVR | AF | — | 3, 18, 20, 40, 42 | Yes | P | Yes | — | 2 months | — | 3‐day call², weekly | — | — | Password protected⁴ | |
| ATCS Plus | AF, SF | — | 40, 42 | — | H | Yes | Yes | 6 months | 40 s | 1 | 100 words | — | PIN⁵ | |
| ATCS Plus | AF, SF | — | 40, 42 | — | H | — | Yes | 3 months | 40 s | 1 | — | — | — | |
| Unidirectional | AF | — | 40 | No | H | — | — | 1 months | — | 1 | — | — | — | |
| Unidirectional | AF | — | 42 | No | H | — | — | 36 months | — | 1³ | — | — | — | |
| Unidirectional¹ | AF | TTM | 9,10,40 | — | H | — | — | 10 months | — | — | Approx 30 words | Nurse | — | |
| IVR | AF | — | 42 | — | H | — | — | 2 weeks | — | 1‐2 attempts | — | — | — | |
| IVR | AF | TTM | 20, 39 | Yes | P | Yes | Yes | 34 weeks | 5 min | — | — | Professional | Password protected⁴, PIN² | |
| IVR | AF | — | 3, 9 | Yes | — | — | — | 3 months | 1‐10 min³ | Weekly | — | — | — | |
| ATCS Plus | AF, CF | GM | 2, 3, 9, 15, 20 | — | E | — | — | 12 months | — | 10 | — | — | — | |
| IVR | AF | — | 3, 6, 20, 30, 39, 42 | Yes | E | Yes | Yes | 6 months | 30‐90 min² | Monthly | — | Female voice actor | Password protected⁴ | |
| IVR | AF | — | 39, 42 | — | — | — | — | 25 days | — | — | — | — | PIN⁶ | |
| ATCS Plus¹ | AF, CF | — | 20,42 | — | H | — | — | 26 weeks | — | Up to 4 | — | — | — | |
| Unidirectional | AF | — | 29,30 | — | H | — | — | 12 months | — | 2 | — | — | — | |
| Unidirectional | AF | — | 40,42 | No | H | — | — | — | — | — | — | — | — | |
| IVR | AF | SCT | 21, 32, 35, 39 | — | E | Yes | Yes | 6 months | 4 min | Weekly | — | — | Password protected⁴ | |
| IVR | AF | — | 3,16 | — | E | Other | — | 9 months | — | 12 | — | — | — | |
| IVR | AF | — | 1, 10, 20, 21, | Yes | — | — | — | 6 months | 15 min | Weekly | — | — | — | |
| IVR | AF | HBM | 3, 7, 20, 21, 42 | — | H | — | — | 12 months | > 1 min | Daily | — | Trained actor | PIN⁷ | |
| IVR | AF | — | 16 | — | H | Yes | — | — | — | — | — | — | — | |
| ATCS Plus | AF, SF | BT | 34 | — | E | Yes | — | 3 months | 8.6 min¹ | 12 | — | — | — | |
| ATCS Plus | AF, CF | HBM, SMP | 3, 7, 27, 30, 40 | — | H | — | — | 6W | — | 1 | — | — | — | |
| ATCS Plus | AF, CF, SF | — | 21, 40, 42 | Yes | H | — | — | 4 months | — | Daily, 4⁴ | — | — | — | |
| Unidirectional | AF | — | 20,42 | — | H | — | — | 1 month | — | — | 80 words | — | — | |
| ATCS Plus | AF, CF, SF | MI | 9,12 | Yes | P | — | Yes | 12 months | 60 days | Daily | 1‐3 min | — | — | |
| ATCS Plus | AF,CF | — | 20,34 | Yes | P | Yes | Yes | 6 months | 2 min | Daily | — | — | — | |
| ATCS Plus¹ | AF, CF | — | 20,42 | — | H | — | — | 25 weeks | 25 s | Up to 4 | — | — | — | |
| Unidirectional | AF | — | 16 | — | H | — | — | 3 months | 1 min | Monthly | 2 30‐s scripts | — | — | |
| ATCS Plus | AF, CF | — | 16 | — | H | — | — | 3 months | 4‐5 min | 1 | — | — | — | |
| ATCS Plus¹ | AF, CF | — | 20,42 | — | H | — | — | 12 months | — | Monthly⁵ | — | — | — | |
| IVR | AF | — | 21 | Yes | P | Yes | Yes | 26 months | — | Weekly | 45 s of speaking | — | PIN² | |
| IVR | AF | TTM, SCT | 4,20,21 | Yes | H | — | — | 6 months | 4.12 min | Weekly | 12.4 calls¹ | Digitised | — | |
| IVR | AF | — | 20, 39, 42 | — | E | Yes | — | 6 months | — | Daily | — | — | — | |
| ATCS Plus | AF, CF | SCT | 20, 39, 42 | — | E | Yes | — | 6 months | 2‐3 min | Biweekly | — | — | — | |
| IVR | AF | TTM | 7, 9, 20, 39 | Yes | P | Yes | — | 3 months | — | Weekly | — | — | Password protected⁴ | |
| ATCS Plus | AF,CF, SF | — | 16,21,30 | — | E | — | — | 6 months | — | Daily | — | — | — | |
| ATCS Plus | AF, SF | — | 20 | Yes | H | Yes | — | 3 months | — | 2.2/week | 26 calls¹ | Female voice | — | |
| ATCS Plus | AF, CF | — | 20,29,30 | — | H | — | — | 12 months | ≤ 10 min | Weekly | — | — | — | |
| IVR | AF | SCT, TTM | 3, 9, 20, 39 | Yes | H | Yes | — | 12 months | 10‐15 min | Weekly⁶ | — | — | — | |
| ATCS Plus¹ | AF, CF | TCM | 39 | — | E | — | — | 12 months | — | Biweekly, weekly, bimonthly, monthly⁷ | — | — | — | |
| ATCS Plus¹ | AF, CF | — | 21,39 | — | H | — | — | 12 months | — | Weekly⁸ | — | — | — | |
| ATCS Plus | AF, CF, SF | — | 20,21,42 | — | E | Yes | — | 12 months | — | Monthly | 18 questions | — | — | |
| IVR | AF | — | 39 | No | P | Yes | — | 24 months | 48 s¹ | Weekly | — | — | — | |
| ATCS Plus | AF, SF | — | 40, 42 | — | H | — | — | 24 months | — | — | — | — | — | |
| IVR | AF | — | 20,42 | Yes | H | Yes | — | 2 weeks | — | — | 3 segments | Personalised voice messages | — | |
| IVR | AF | — | 42 | — | H | — | — | 4 months | 96.8 s | — | — | — | — | |
| Unidirectional | AF | — | 42 | — | H | — | — | 5 months | — | Monthly | — | — | — | |
| Unidirectional | AF | — | 42 | — | H | — | — | 5 months | — | 2/d⁹ | — | — | — | |
| ATCS Plus | AF,SF | CBT | 3,20,34,38 | — | H | Yes | — | 12 weeks | 2.5 min | 8/d¹⁰ | — | — | — | |
| ATCS Plus | AF, CF | — | 21 | — | — | — | — | 15 months | 90 s | Monthly | — | — | — | |
| ATCS Plus¹ | AF, CF | — | 20, 21, 25, 42 | Yes | P | Yes | — | 6 months | 5‐10 min | Weekly | — | — | — | |
| ATCS Plus | AF, SF | PT, PM | 3, 13, 25, 39, 42 | Yes | P | Yes | Yes | 18 months | 18 min | — | 22 h/d² | Professional radio announcer | Password protected⁴ | |
| Unidirectional | AF | — | 40 | No | H | — | — | 2 months | — | — | — | — | — | |
| IVR | AF | — | 20, 21, 25, 34, 42 | — | H | Other | — | 24 months | 3‐5 min | Biweekly | — | — | — | |
| IVR | AF | SCT, TTM, MI | 25, 39 | Yes | — | Yes | Yes | 8 months | — | Weekly | — | African – American voice professionals | — | |
| ATCS Plus | AF, SF, CF | — | 21 | — | P | Yes | Yes | 1.5 months | 5.18 min¹ | Daily | — | — | Personalised password | |
| ATCS Plus | AF, SF | CBT | 17,34,38 | Yes | H | Yes | — | 1 month | 9.3 min¹ | Daily | — | — | — | |
| Unidirectional¹ | AF | SCT, TTM | 9, 10, 20, 25, 39 | — | H | — | — | 12 months | — | Monthly | Approx 60 words | Primary care provider | — | |
| Unidirectional¹ | AF | SCT, TTM | 9, 10, 20, 25, 39 | — | H | — | — | 12 months | — | Monthly | Approx 60 words | Primary care provider | — | |
| IVR | AF | — | 27, 42 | — | H | Yes | Yes | 6 months | 1 min | 3 | — | — | — | |
| IVR | AF | — | 25, 39 | — | H | — | — | 1 month | — | — | — | — | Correspondence with the author: "Upon answering a call, patients are required to authenticate with their date of birth." | |
| ATCS Plus | AF, CF, SF | CBT | 4,20,34,38 | Yes | E | — | Yes | 6 months | 9.2 min | Daily | — | — | Valid ID number and a personally | |
| Unidirectional | AF | — | 29 | Yes | H | — | — | 2 months | — | At least every 3 days | Every hour for 2 consecutive hours | Community health worker | — | |
| IVR | AF | CBT | 3, 16, 20, 22, 25, 39, 42 | Yes | P | — | Yes | 4 months | 3‐16 min | Daily | — | Experienced therapist | — | |
| Unidirectional | AF | — | 42 | — | H | — | — | 24 months | — | Daily | — | First author | — | |
| IVR | AF | — | 40 | — | H | — | — | 4 months | — | — | — | — | — | |
| IVR | AF | — | 39, 42 | — | — | — | — | 6 months | — | 3 | — | — | — | |
| ATCS Plus | AF, SF | CBT, TTM, MI | 9, 0, 12, 20, 21 | — | H | Yes | — | 2 months | 18.9 min^^ | Biweekly weeks 1‐3, weekly weeks 4‐6, no call week 7, weekly week 8‐9 | 8.61²,³ | — | — | |
| IVR | AF | — | 29, 30 | — | H | — | — | 3.5 months | — | up to 5 times | — | — | — | |
| ATCS Plus | AF, CF | SCT | 3, 18, 21, 25, 39 | No | E | Yes | Yes | 24 months | 1‐8 min² | Biweekly¹¹ | — | Human voice | PIN² | |
| ATCS Plus | AF, CF | SCT | 3, 18, 21, 25, 39 | — | H | Yes | — | 12 months | 1‐8 min² | Biweekly¹¹ | — | — | — | |
| ATCS Plus | AF, CF | — | 13, 20, 21, 39 | Yes | — | Yes | Yes | 1.5 months | ≤ 9 min | Weekly | — | Native speaker | — | |
| ATCS Plus | AF, SF | DMT, SCT, TTM | 3, 19, 20, 32 | Yes | P | Yes | — | 6 months | 10 min | Weekly (first 3 months), and at least biweekly thereafter | — | Digitised | — | |
| Unidirectional | AF | — | 39, 40 | No | H | — | — | — | — | — | — | Receptionist | — | |
| ATCS Plus | AF, CF | — | 20, 42 | — | H | — | — | 3 months | — | — | — | — | — | |
| ATCS Plus | AF, CF | — | 3, 9, 13, 39, 40, 42 | — | H | — | — | 12 months | 1‐20 min⁵ | 3 | — | — | — | |
| ATCS Plus | AF, CF | — | 20, 42 | — | — | — | — | 12 months | — | 8 | — | — | — | |
| IVR | AF | — | 42 | — | P | — | — | — | — | — | — | — | — | |
| ATCS Plus | AF, CF, SF | — | 13, 34 | — | H | — | Yes | 6 months | — | 5 times¹² | — | — | — | |
| ATCS Plus | AF, CF, SF | CBT | 4, 20, 34, 38 | Yes | P | — | — | 4 months | — | Daily | — | — | — | |
| IVR | AF | MI | 9, 12 | — | H | Other | — | 6 months | — | ≤ 26 calls over 13 weeks | — | — | — | |
| ATCS Plus | AF, CF | CCM, SCT | 1, 3, 9, 10, 13, 20, 21, 35, 39 | — | E | Yes | Yes | 12 months | 6‐12 min | Weekly | — | — | — | |
| ATCS Plus | AF, CF | — | 27, 39, 42 | — | H | — | — | 6 months | — | 11 | — | — | Password protected⁴ | |
| IVR¹ | AF | TPB | 16,20 | — | H | — | — | 24 months | — | Weekly | — | — | — | |
| IVR | AF | — | 17, 20, 42 | — | H | Other | — | 6 weeks | — | 3 calls 6 weeks apart | 12 questions; 397 words | Digitally | — | |
| IVR | AF | — | 17, 20, 30, 39, 40 | — | H | Yes | — | 2 months | — | Weekly¹³ | — | Female voice | — | |
| IVR | AF | GMDBC, Others² | 3, 27, 39 | Yes | E | — | — | 3 months | 2‐6 min | — | — | — | Verification | |
| ATCS Plus | AF, CF | — | 30, 42 | No | H | No | — | 12 months | — | 3¹⁴ | — | Human voice | — | |
| IVR | AF | — | 20 | Yes | P_H | Yes | Yes | 1 months | — | Daily | — | — | — | |
| ATCS Plus¹ | AF, CF | — | 16 | — | H | — | Yes | — | — | — | — | Female voice | — | |
| IVR | AF | SCT, MI | 3, 12, 17, 20, 25, 39, 40, 42 | — | E | Yes | — | 12 months | — | Weekly then monthly¹⁵ | — | — | Password protected⁴ | |
| IVR | AF | SCT | 3, 12, 17, 20, 25, 39, 40, 42 | Yes | E | Yes | — | 12 months | — | Weekly then monthly¹⁵ | — | — | Password protected⁴ | |
| ATCS Plus | AF, CF | CBT | 2, 20 | Yes | H | Yes | — | 2 months | — | Weekly | — | — | — | |
| ATCS Plus | AF, SF | HBM, CCM, SCT, TTM, MI, SRT, RL | 2, 3, 20, 32, 39 | — | H | — | Yes | 6 months | — | 3 | — | — | — | |
| Unidirectional | AF | — | 42 | — | H | — | — | 1 month | — | 1³ | — | Human voice | — | |
| IVR¹ | AF | — | 9, 10, 42 | Yes | P | Yes | — | 3 months | — | Daily/2 weeks; weekly/10 weeks | 25 calls | Female voice | — | |
| Unidirectional | AF | — | 42 | — | H | — | — | 18 months | — | Weekly | — | — | — | |
| Unidirectional | AF | — | 42 | — | H | — | — | 12 months | — | — | — | — | — | |
| Unidirectional | AF | HBM | 7*, 39, 40, 42 | No | H | — | — | — | — | 1¹⁶ | — | Female using native languages | — | |
| Unidirectional | AF | HBM | 39, 40, 42 | No | H | — | — | — | — | 1¹⁶ | — | Female using native languages | — | |
| IVR | AF | BET | 9, 20, 25, 34 | Yes | — | — | — | 6 months | ≤ 5 min | Daily | — | — | PIN⁶ | |
| ATCS Plus | AF,SF | — | 10 | — | — | — | — | 3 months | — | 3/week | — | — | — | |
| IVR¹ | AF | TTM | 4, 13, 20, 34, 38 | — | E | Yes | — | 6 months | 15‐20 min | Weekly¹⁷ | — | — | — | |
| ATCS Plus | AF, CF | — | 39 | — | H | Other | — | 10 months | < 10 min | 3¹⁸ | — | — | — | |
| ATCS Plus | AF, CF | — | 20, 42 | — | H | Other | Yes | 18 months | 2‐3 min | — | — | — | — | |
| ATCS Plus | AF, CF, SF | — | 20, 42 | Yes | H | Other | Yes | 12 months | 2‐3 min | Monthly | — | — | — | |
| IVR | AF | — | 3, 21, 39 | Yes | P | Yes | Yes | 6 months | 5‐20 min | Weekly | — | — | PIN⁶ | |
| IVR | AF | SCT | 2, 9, 10 | No | P | Other | — | 3 months | — | Biweekly | — | Synthetic speech | — | |
| IVR | AF | — | 21, 40 | — | H | Yes | — | 6 months | — | Biweekly | — | — | — | |
| ATCS Plus | AF, SF | — | 21, 40 | — | P | Yes | — | 3 months | — | Weekly | — | — | PIN | |
| Unidirectional | AF | SCT | 4,13 | — | — | — | — | — | — | — | — | — | — | |
| a ATCS ¹For more detailed evaluation of multimodal/complex interventions, please refer to Table 4. b Content delivery: AF: automated functions; CF: communicative functions; SF: supplementary functions. d Behaviour change techniques: 1 ‐ action planning; 2 ‐ agree behavioural contract; 3 ‐ barrier identification/problem solving; 4 ‐ emotional control training; 5 ‐ environmental restructuring; 6 ‐ facilitate social comparison; 7 ‐ fear arousal; 8 ‐ general communication skills training; 9 ‐ goal setting (behaviour); 10 ‐ goal setting (outcome); 11 ‐ model/demonstrate the behaviour; 12 ‐ motivational interviewing; 13 ‐ plan social support/social change; 14 ‐ prompt anticipated regret; 15 ‐ prompt identification as a role model/position advocate; 16 ‐ prompt practice; 17 ‐ prompt review of behavioural goals; 18 ‐ prompt review of outcome goals; 19 ‐ prompt self talk; 20 ‐ prompt self‐monitoring of behaviour; 21 ‐ prompt self‐monitoring of behavioural outcome; 22 ‐ prompt use of imagery; 23 ‐ prompting focus on past success; 24 ‐ prompting generalisation of a target behaviour; 25 ‐ provide feedback on performance; 26 ‐ provide information about other's approval; 27 ‐ provide information on consequences of behaviour in general; 28 ‐ provide information on consequences of behaviour to the individual; 29 ‐ provide information on where and when to perform the behaviour; 30 ‐ provide instruction on how to the perform the behaviour; 31 ‐ provide normative information about others' behaviour; 32 ‐ provide rewards contingent on effort or progress towards behaviour; 33 ‐ provide rewards contingent on successful behaviour; 34 ‐ relapse prevention/coping planning; 35 ‐ set graded tasks; 36 ‐ shaping; 37 ‐ stimulate anticipation of future rewards; 38 ‐ stress management; 39 ‐ tailoring; 40 ‐ teach to use prompts/cues; 41 ‐ time management; 42 ‐ use of follow up prompts. e Caller: E ‐ either participant or healthcare provider/researcher; H ‐ healthcare provider/researcher; P ‐ participant; P_H ‐ If P fails to call then H calls. f Telephone keypad for response: the calls were made using speech recognition (or speech‐enabled) technology; ¹‐ both data entry via Web screen or voice or telephone keypad. g Duration of calls h Frequency i Intensity j Security arrangement | ||||||||||||||
| Assessment levels and criteria for each dimension | Study | Assessment of the intervention (a‐d)/description of the intervention/justification | Control (or usual care) |
| Core dimension 1: active components included in the intervention compared with the control | |||
| a. More than 1 component and delivered as a bundle b. More than 1 component c. 1 component | (a.) A mailed reminder letter, a free faecal immunochemical test, an automated telephone and text message reminding them that they were due for screening and that a faecal immunochemical test was being mailed to them, an automated telephone and text reminder 2 weeks later for those who did not return the faecal immunochemical test, and personal telephone outreach by a colorectal cancer screening navigator after 3 months | (b.) Usual care included computerised reminders, | |
| (a.) Behaviour change goals, self‐monitoring via IVR phone calls, tailored skills training materials, monthly interpersonal counselling calls, and a 12‐month gym membership. | (b.) Control group received + newsletters that covered general wellness topics but did not discuss weight, nutrition, or physical activity | ||
| (a.) Internet‐ and telephone‐based telemedicine system + automatically generated emails or telephone calls as reminders + sphygmomanometer, a weighting scale (if needed), a pedometer and instructions on their use | (c.) Control group received usual care | ||
| (a.) Email, webpages, IVR and short message service (SMS) + craving helpline | (c.) Control group received self‐help (booklet) | ||
| (a.) 10 personal phone calls from the nurse interspersed randomly with 10 automated phone calls + clinic‐based activity counselling | (b.) Clinic‐based activity counselling + no calls | ||
| (b.) Clinician prompt, patient prompts, patient outreach consisting of 2 personalised letters which also include testing kits for colorectal cancer and up to 4 ATCS calls over 26 weeks | (c.) The clinician was responsible for discussing cancer screening with the patients and for initiating any referral or for handing out faecal occult blood testing cards over 12 months | ||
| (b.) Letters, ATCS calls, a point‐of‐care prompt and mailing of a home colorectal cancer testing kit; and medical record reviews at week 12 | (c.) Usual care received blinded chart review | ||
| (b.) Medication reconciliation and tailoring, patient education (provided through automated voice messages and pharmacist telephone calls when requested by the patient), collaborative care between pharmacists and providers (primary care providers or cardiologists), and voice messaging reminders (educational and medication refill reminder calls). | (c.) Usual care received standard hospital discharge instructions e.g., numbers to call, follow‐up appointments, diet and exercise advice, a discharge medication list, and educational information about cardiac medications | ||
| (a.) Symptom monitoring by a nurse + automated monitoring either via IVR or by Internet + medications (analgesics, antidepressants) | (c.) Usual care from oncologist | ||
| (a.) Symptom monitoring, either via IVR or by Internet + nurse care + stepped care with analgesics | (c.) Usual care from primary care physician | ||
| (b.) Patient education, home blood pressure monitoring, home blood pressure measurement reporting to an ATCS, and clinical pharmacist management of hypertension with physician oversight + usual care | (c.) The control group received usual care | ||
| (b.) Baseline in‐person and biweekly then monthly telephone counselling by a lifestyle counsellor, one‐time clinical endorsement of physical activity and monthly automated telephone messaging by primary care provider, and quarterly tailored mailings of progress in physical activity. | (c.) Patients in the control group received usual care | ||
| (b.) 1 in‐person baseline counselling session, regular telephone counselling, physician endorsement in clinic with monthly ATCS calls encouragement, and tailored mailed materials, plus a consult to a Veterans Affairs (VA) weight management program. | (b.) Patients in the control group received usual care + MOVE | ||
| (b.) An IVR call once a week + a weekly non‐interactive neutral pictorial message sent out as a reminder 4 days after the IVR call + usual care | (b.) Usual care included up to 3 counselling sessions + antiretroviral treatment | ||
| (b.) Education and reminders delivered to primary care physicians + mailings and ATCS | (c.) The control group received no education | ||
| (b.) Treatment team education and patient self‐care education, nurse telephone call and IVR program | (c.) Treatment team education and patient self‐care education | ||
| (a.) Automated counselling plus nicotine replacement therapy, manuals, and expert system (TEL + EXP + NRT + MAN) | (c.) The control group received stage (of change) matched manuals | ||
| Core dimension 2: behaviours or actions of intervention recipients or participants to which the intervention is directed | |||
| a. Single target b. Dual target c. Multitarget d. Variesa | (a.) Single target: colorectal cancer screening | (a.) Single target: colorectal cancer screening | |
| (a.) Single target: weight management | (a.) Single target: weight management | ||
| (c.) Single target: diet, exercise, smoking and blood pressure control | (a.) Single target: blood pressure control | ||
| (a.) Single target: smoking abstinence | (a.) Single target: smoking abstinence | ||
| (c.) Multiple target: physical activity; BMI; mobility; quality of life | (c.) Multiple target: physical activity; BMI; mobility; quality of life | ||
| (b.) Dual target: breast cancer and colorectal cancer screening | (b.) Dual target: breast cancer and colorectal cancer screening | ||
| (b.) Dual target: breast cancer and colorectal cancer screening | (b.) Dual target: breast cancer and colorectal cancer screening | ||
| (c.) Medication adherence, blood pressure, blood lipid levels | (c.) Medication adherence, blood pressure, blood lipid levels | ||
| (b.) Dual target: pain and depression management | (b.) Dual target: pain and depression management | ||
| (a.) Single target: musculoskeletal pain management | (a.) Single target: musculoskeletal pain management | ||
| (b.) Dual target: blood pressure monitoring and blood pressure measuring | (b.) Dual target: blood pressure monitoring and blood pressure measuring | ||
| (c.) Multitarget: improving gait speed, self‐reported physical activity, function and disability | (c.) Multitarget: improving gait speed, self‐reported physical activity, function and disability | ||
| (c.) Multitarget: improving blood sugar indices, anthropometric measures, and self‐reported physical activity, health‐related quality of life, and physical function | (c.) Multitarget: improving blood sugar indices, anthropometric measures, and self‐reported physical activity, health‐related quality of life, and physical function | ||
| (a.) Single target: antiretroviral treatment medication adherence | (a.) Single target: antiretroviral treatment medication adherence | ||
| (a.) Single target: osteoporosis screening | (d.) Varies | ||
| (a.) Single target: antidepressant medication adherence | (a.) Single target: antidepressant medication adherence | ||
| (a.) Single target: smoking abstinence | (a.) Single target: smoking abstinence | ||
| Core dimension 3: organisational levels and categories targeted by the intervention | |||
| a. Multilevel b. Multicategory c. Single category | (c.) Intervention directed only at single category of individuals within the individual level: patients past due for colorectal cancer screening | (c.) Intervention directed only at single category of individuals within the individual level: patients past due colorectal cancer screening | |
| (c.) Intervention directed only at single category of individuals within the individual level: obese females of Black ethnic origin | (c.) Obese females of black ethnic origin | ||
| (c.) Intervention directed only at single category of individuals within the individual level: subjects with elevated blood pressure | (c.) Intervention directed only at single category of individuals within the individual level: subjects with elevated blood pressure | ||
| (c.) Intervention directed only at single category of individuals within the individual level: tobacco smokers | (c.) Tobacco smokers | ||
| (c.) Sedentary primary care patients | (c.) Sedentary primary care patients | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients past due recommended screening | (c.) Patients past due recommended screening | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients past due recommended screening | (c.) Patients past due recommended screening | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients after hospitalisation for acute coronary syndrome | (c.) Intervention directed only at single category of individuals within the individual level: patients after hospitalisation for acute coronary syndrome | ||
| (c.) Intervention directed only at single category of individuals within the individual level: cancer patients with depression and pain | (c.) Cancer patients with depression and pain | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients with musculoskeletal pain | (c.) Patients with musculoskeletal pain | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients with hypertension | (c.) Patients with hypertension | ||
| (c.) Intervention directed only at single category of individuals within the individual level: sedentary (otherwise healthy) older adults | (c.) Intervention directed only at single category of individuals within the individual level: sedentary (otherwise healthy) older adults | ||
| (c.) Intervention directed only at single category of individuals within the individual level: older adults at risk of diabetes mellitus | (c.) Intervention directed only at single category of individuals within the individual level: older adults at risk of diabetes mellitus | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients with HIV | (c.) Intervention directed only at single category of individuals within the individual level: patients with HIV | ||
| (b.) Intervention directed at 2 or more categories of individuals within the individual level: primary care physicians and their patients at‐risk of osteoporosis | (b.) Control intervention directed at 2 or more categories of individuals within the individual level: primary care physicians and their patients at‐risk of osteoporosis | ||
| (c.) Intervention directed only at single category of individuals within the individual level: patients with depression | (c.) Intervention directed only at single category of individuals within the individual level: patients with depression | ||
| (c.) Intervention directed only at single category of individuals within the individual level: tobacco smokers | (c.) Tobacco smokers | ||
| Core dimension 4: the degree of tailoring intended or flexibility permitted across sites or individuals in intervention implementation/application | |||
| a. Fully tailored/flexible b. Moderately tailored/flexible c. Inflexible d. Variesa | (b.) Moderately tailored/flexible: a mailed reminder letter, a free faecal immunochemical test, an automated telephone and text message reminder, an automated telephone and text reminder 2 weeks later (inflexible); personal telephone outreach (flexible) | (b.) Moderately tailored/flexible: computerised reminders, standing orders to give patients home faecal immunochemical test (inflexible); clinician feedback on colorectal cancer screening rates (flexible) | |
| (b.) Moderately tailored/flexible: self‐monitoring via IVR phone calls (inflexible); behaviour change goals, tailored skills training materials, monthly interpersonal counselling calls, and a 12‐month gym membership (flexible) | (c.) Newsletters sent were inflexible | ||
| (c.) Telemedicine system, reminders, sphygmomanometer, weighting scale and pedometer have all been standardised | (d.) Varies across interventions included in the review | ||
| (c.) E‐mail, web‐pages, IVR and SMS inflexible; quote: "Early in the morning, the user receives an e‐mail with instructions to open the day's web page. Each day for 6 weeks, the client opens a web page that is unique to that particular programme day." | (c.) Quote: "The booklet contains general cessation information, a 48‐day quit calendar, a 10‐day quit log, the telephone number of the national quit‐line and links to relevant and open on‐line tobacco cessation | ||
| (b.) Moderately tailored/flexible: nurse used a semi‐standardised protocol. | (d.) Varies across interventions included in the review | ||
| (c.) Clinician prompt, patient prompts, patient outreach all have been highly standardised | (b.) Discussions with patients were moderately tailored/flexible | ||
| (c.) Letters, ATCS calls, point‐of‐care prompts and blinded chart reviews all have been highly standardised | (c.) Blinded chart reviews have been highly standardised | ||
| (b.) Moderately tailored/flexible: medication reconciliation and tailoring, patient education, collaborative care between pharmacists and providers (flexible); and voice messaging reminders (inflexible) | (b.) Usual care was moderately tailored/flexible | ||
| (c.) Nurse care (using evidence‐based guidelines) and IVR monitoring and medications (all not flexible) | (d.) Varies across interventions included in the review | ||
| (c.) Symptom monitoring, either via IVR or by Internet, nurse care and stepped care with analgesics (not flexible) | (d.) Varies across interventions included in the review | ||
| (b.) Patient education, home blood pressure monitoring, home blood pressure measurement reporting to an ATCS (not flexible); and clinical pharmacist management of hypertension with physician oversight (flexible) | (d.) Varies across interventions included in the review | ||
| (b.) Moderately tailored/flexible: quote: "Providers were encouraged to modify the script to suit their personal style." | (d.) Varies across interventions included in the review | ||
| (b.) Moderately tailored/flexible: baseline counselling, regular telephone counselling, physician endorsement in clinic with monthly ATCS calls encouragement, and tailored mailed materials, plus a consult to a Veterans Affairs (VA) weight management programme | (d.) Varies across interventions included in the review | ||
| (c.) An IVR call once a week + a weekly non‐interactive neutral pictorial message sent out as a reminder 4 days after the IVR call + usual care (inflexible) | (c.) Usual care counselling sessions + antiretroviral treatment (inflexible) | ||
| (b.) An advance letter, an ATCS call, and the opportunity to schedule a bone mineral density test (not flexible); specially trained pharmacists educated physicians (flexible). | (d.) Varies across interventions included in the review | ||
| (b.) Moderately tailored/flexible: treatment team education and patient self‐care education, nurse telephone call and IVR programme | (b.) Moderately tailored/flexible: treatment team education and patient self‐care education, nurse telephone call | ||
| (b.) Automated counselling plus nicotine replacement therapy, manuals, and expert system were all moderately tailored/flexible | (c.) Stage‐based self‐help manuals were inflexible | ||
| Core dimension 5: the level of skill required by those delivering the intervention | |||
| a. High level skills b. Intermediate level skills c. Basic skills d. Variesa | (b.) Intermediate level skills required in setting up the IVR phone calls and text reminders, providing outreach calls | (b.) Intermediate level skills required in providing feedback on colorectal cancer screening rates | |
| (b.) Intermediate level skills required in delivering behaviour change goals, setting up the IVR phone calls, producing tailored skills materials and monthly interpersonal counselling calls | (c.) No specialised skills required in writing the newsletter | ||
| (b.) Intermediate level skills required in reviewing patients' reports (physicians), motivating patients (nurses), setting up the reminder calls, emails | (c.) No specialised skills required in providing usual care | ||
| (a.) Creating/writing emails, web‐pages, IVR and SMS requires high level skills | (c.) No specialised skills required in writing the booklet | ||
| (b.) Intermediate level skills required in prerecording automated telephone messages | (d.) Varies across interventions included in the review | ||
| (b.) Intermediate level skills required in e.g. prerecording automated telephone reminders | (c.) No specialised skills required in discussing breast cancer/colorectal cancer screening | ||
| (b.) Intermediate level skills required in e.g. prerecording automated telephone reminders | (c.) No specialised skills required in reviewing charts | ||
| (b.) Intermediate level skills required in e.g. educating patients and/or prerecording telephone reminders | (c.) No specialised skills required in providing usual care | ||
| (b.) Intermediate level skills required from nurses to manage pain and depression | (b.) Intermediate level skills required from nurses to manage pain and depression | ||
| (b.) Intermediate level skills required from nurses to manage musculoskeletal pain | (c.) Basic skills required from primary care physicians | ||
| (b.) Intermediate level skills required from pharmacists and physicians to manage hypertension | (d.) Varies | ||
| (b.) Intermediate level skills required from primary care providers in e.g., recording automated messages, counselling and consulting patients | (c.) Basc level skills required from primary care providers | ||
| (b.) Intermediate level skills required from primary care providers in recording automated messages, counselling and consulting patients | (b.) Intermediate level skills required from primary care providers in providing MOVE | ||
| (b.) Intermediate level skills required in setting up the IVR phone calls + pictorial messages | (c.) Basic skills required | ||
| (a.) Extensive specialised skills required: "The visits were conducted by specially trained pharmacists . . . These pharmacists also underwent a 1‐ day training program focused on osteoporosis and conducted by 2 of the study authors. This program included lectures on the epidemiology, diagnosis, and treatment of osteoporosis. Also, it reviewed principles of academic detailing and the specific goals of this intervention." | (d.) Varies | ||
| (b.) Intermediate level skills required in e.g., prerecording IVR calls | (c.) Basic skills required in delivering education and nurse calls | ||
| (b.) Intermediate level skills required in recording automated messages, providing feedback reports | (c.) Basic skills required | ||
| Core dimension 6: the level of skill required for the targeted behaviour when entering the study by those receiving the intervention in order to meet the intervention's objectives | |||
| a. High level skills b. Intermediate level skills c. Basic skills d. Variesa | (c.) No specialised skills required | (c.) No specialised skills required | |
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (b.) Intermediate level skills required. Quote: "subjects were trained on use of a computer and the Internet and were introduced to the Web site at the research centre. Telemedicine subjects received instructions on use of an optional telephone communication system". They also received instructions on the use of sphygmomanometer, weighting scale and pedometer | (c.) No specialised skills required | ||
| (b.) Access to the Internet, email and a cell phone on a daily basis was required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (b.) Patients were instructed about use of the ATCS, and trained on using an electronic blood pressure cuff | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (c.) No specialised skills required | ||
| (d.) Varies across interventions included in the review | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| (c.) No specialised skills required | (c.) No specialised skills required | ||
| Core dimension 7: the degree of interaction between intervention components/the independence/interdependence of intervention components | |||
| a. High level interaction d. Variesa e. Unclear or unable to assess | (a.) High level interaction; quote: "The majority of faecal occult blood testing completions were accomplished with | (d.) Varies across interventions included in the review | |
| (b.) Moderate level of interaction; quote: "comprised 5 mutually reinforcing components" | (e.) Unclear or unable to assess in individuals receiving usual care | ||
| (b.) Moderate level of interaction of the intervention components: the automated calls and emails and the use of sphygmomanometer to measure blood pressure, weighting scale and pedometer to promote physical activity | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (a.) High level interaction; quote: "further research is necessary to detect the active intervention | (c.) Independent | ||
| (a.) High level interaction; a synergistic effect has been observed | (e.) Unclear or unable to assess in patients receiving no calls | ||
| (a.) High level interaction; quote: "combined interventions are superior to simpler interventions such as reminders" | (c.) Independent | ||
| (a.) There is substantial interaction or inter‐dependency between ATCS calls, a point‐of‐care prompt and mailing of a home colorectal cancer testing kits | (c.) Independent | ||
| (b.) Moderate level of interaction: the intervention components are considered to be mutually reinforcing | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (a.) High level interaction; triggered telephone calls occurred when ATCS monitoring indicated inadequate symptom improvement, non‐adherence to medication, adverse effects, suicidal ideation | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (a.) High level interaction; IVR and nurse calls prompted adjustments in type or dose of analgesics delivered | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (a.) There was a high level interaction between the intervention components: Quote: "This difference was likely due to greater therapy intensification (number and intensity of hypertension medications) in the intervention group" | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (b.) Moderate level of interaction: personal and automatic calls are considered to be mutually reinforcing | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (b.) Moderate level of interaction: personal and automatic calls are considered to be mutually reinforcing | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (c.) The IVR calls and pictorial messages were independent of each other | (e.) Unclear or unable to assess in patients receiving usual care | ||
| (e.) Unable to assess the degree of interaction between physician education and ATCS calls | (e.) Unclear or unable to assess in patients receiving no intervention | ||
| (e.) Unable to assess the degree of interaction between education, nurse calls and IVR calls | (d.) Varies across interventions included in the review | ||
| (c.) Automated calls, nicotine replacement therapy, manuals, and expert system were independent of each other | (e.) Unclear or unable to assess in patients receiving stage‐matched manuals only | ||
| Core dimension 8: the interaction between the intervention and the context or setting | |||
| a. Highly context dependent d. Variesa e. Unclear or unable to assess | (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | |
| (e.) Unable to assess the level of interaction between the intervention and the context | (e.) Unable to assess the level of interaction between the intervention and the context | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (d.) Varies across interventions included in the review | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (e.) Unable to assess the level of interaction between the intervention and the context | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (e.) Unable to assess the level of interaction between the intervention and the context | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (d.) Varies across interventions included in the review | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (e.) Unable to assess the level of interaction between the intervention and the context | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (d.) Varies across interventions included in the review | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (d.) Varies across interventions included in the review | ||
| (e.) Unable to assess the level of interaction between the intervention and the context | (e.) Unable to assess the level of interaction between the intervention and the context | ||
| Core dimension 9: the degree to which the effects of an intervention are modified by factors relating to recipient, provider, or implementation factors | |||
| a. Highly dependent on individual‐level factors d. Variesa e. Unclear or unable to assess | (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | |
| (b.) Moderately dependent on individual‐level factor, i.e. registered dietitians or personalized progress reports | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (c.) Largely independent of individual‐level factors | (c.) Largely independent of individual‐level factors | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (c.) The effects of the blinded chart reviews are not modified substantially by recipient or provider factors | ||
| (d.) Varies across interventions included in the review | (d.) Varies across interventions included in the review | ||
| (b.) Moderately dependent on individual‐level factor, i.e. nurse and pain‐psychiatrist specialist interaction | (e.) Unclear or unable to assess | ||
| (b.) Moderately dependent on individual‐level factor, i.e. nurse management | (e.) Unclear or unable to assess | ||
| (b.) Moderately dependent on individual‐level factor, i.e. clinical pharmacist management with physician oversight | (e.) Unclear or unable to assess | ||
| (e.) Unclear or unable to assess | (e.) Unclear or unable to assess | ||
| (e.) Unclear or unable to assess | (e.) Unclear or unable to assess | ||
| (e.) Unclear or unable to assess | (e.) Unclear or unable to assess | ||
| (b.) The effects of the intervention are modified by one of recipient or factors, e.g. pharmacists' knowledge | (e.) Unclear or unable to assess | ||
| (e.) Unclear or unable to assess | (e.) Unclear or unable to assess | ||
| (e.) Unclear or unable to assess | (e.) Unclear or unable to assess | ||
| Core dimension 10: the length of the causal pathway between the intervention and the outcome it is intended to affect | |||
| a. Pathway variable, long d. Variesa e. Unclear or unable to assess | (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | |
| (c.) Pathway linear, short. Quote: "The high rates of IVR call engagement and their correlation with greater weight losses" | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| (d.) Varies across interventions included in the review | (e.) Unclear or unable to assess | ||
| aVaries across interventions to be considered for/included in the review. | |||
| Study ID | Typea | Subtype | Participant age (years) | Sex | Ethnicityb | Primary outcome measures | Effectc |
| P | Alcohol misuse | 41‐70 | > 50% M | W | Drinking practices Spending on alcohol | 2 2 (median effect = 2) | |
| P | Immunisation | — | — | — | Immunisation status Cost‐effectiveness | 1 1 (median effect = 1) | |
| P | Immunisation | ≥ 71 | — | — | Herpes zoster immunisations | 1 | |
| P | Immunisation | 0‐21 | — | — | Immunisation status | 2 | |
| P | Immunisation | 0‐21 | M = F (± 3%) | W,B,H | Completion by the age of 24 months of the 4‐3‐1‐3 immunisations series | 2 | |
| P | Immunisation | 0‐21 | — | — | Immunisation status | 2 | |
| P | Immunisation | — | M = F (± 3%) | W,B | Immunisation status | 1 | |
| P | Immunisation | 22‐40 | > 50% F | W,B | Immunisation rate | 2 | |
| P | Immunisation | 0‐21 | M = F (± 3%) | B,H | Immunisation status | 2 | |
| P | Immunisation | 0‐21 | M = F (± 3%) | W,B,H | Immunisation status | 2 | |
| P | Immunisation | 0‐21 | M = F (± 3%) | — | Immunisation status Preventive visit rate | 1 1 | |
| P | Physical activity | 41‐70 | > 50% F | W | 1‐mile walk after the intervention | 5 | |
| P | Physical activity | 41‐70 | > 50% M | — | Self‐reported (diary) walking adherence | 1 | |
| P | Physical activity | 41‐70 | > 50% F | B | Minutes walked per week | 1 | |
| P | Physical activity | 41‐70 | > 50% F | W | Minutes of moderate to vigorous physical activity | 1 | |
| P | Physical activity | ≥ 71 | > 50% M | W,B | Gait speed (usual and rapid) Self‐reported physical activity Function and disability | 2, 1 1 2 (median effect = 2) | |
| P | Physical activity | 41‐70 | > 50% M | W | Homeostasis model assessment of insulin resistance | 2 | |
| P | Physical activity | 41‐70 | > 50% F | W,B | Energy expenditure in moderate‐intensity‐physical activity % meeting recommendations for moderate‐intensity‐physical activity Motivational readiness for physical activity | 2 2 2 (median effect = 2) | |
| P | Physical activity | ≥ 71 | > 50% M | — | Muscle strength Balance Walk distance Mood | 1 1 2 1 (median effect = 1) | |
| P | Screening | 41‐70 | > 50% F | H | Colorectal cancer screening adherence (faecal occult blood testing) | 1 | |
| P | Screening | 41‐70 | > 50% M | W | Receipt of any recommended colorectal cancer screening | 1 | |
| P | Screening | — | > 50% F | — | Screening rate | 2 | |
| P | Screening | — | > 50% F | W,B,A | Mammography adherence | 1 | |
| P | Screening | 41‐70 | M = F (± 3%) | — | Receipt of colorectal cancer screening after 3 months | — | |
| P | Screening | — | > 50% F | W,B,H,A | Chart documentation of breast cancer screening, colorectal cancer screening, or both | 1 | |
| P | Screening | — | > 50% F | W,B | Breast cancer and colorectal cancer screening | 2 | |
| P | Screening | — | > 50% F | W,B,H | Documentation of breast cancer screening, colorectal cancer screening, or both | 1 | |
| P | Screening | 41‐70 | > 50% F | — | Bone mineral density screening after 12 months | 1 | |
| P | Screening | 41‐70 | M = F (± 3%) | W | Completion of faecal occult blood testing at 6 months | 1 | |
| P | Screening | 41‐70 | > 50% F | W,B | Completed mammogram or colorectal cancer screening within 36 weeks of randomisation | 2 | |
| P | Screening | 41‐70 | M = F (± 3%) | W | Colorectal cancer screening | 2 | |
| P | Screening | 41‐70 | > 50% F | — | Bone mineral density testing and/or filling a prescription for a bone active medication | 1 | |
| P | Stress management among caregivers | 41‐70 | > 50% F | W,B | Caregiver's appraisal of the bothersome nature of caregiving Anxiety Depression | 2 2 2 (median effect = 2) | |
| P | Substance use | 41‐70 | > 50% M | W,H | Days using primary drug | 2 | |
| P | Weight management | 41‐70 | > 50% F | B,H | Change in body weight and BMI | 1 | |
| P | Weight management | 22‐40 | > 50% F | B | Change in body weight and BMI | 1 | |
| P | Weight management | 41‐70 | > 50% F | W,B,H,A | Physical activity Dietary habits Weight loss | 2 2 2 (median effect = 2) | |
| P | Weight management | 0‐21 | > 50% M | W,H | BMI z‐score Physical activity and sedentary behaviour Dietary habits Eating disorder symptoms | 2 2 2 2 (median effect = 2) | |
| P | Weight management | 41‐70 | > 50% F | — | Body weight BMI Systolic blood pressure Diastolic blood pressure Plasma glucose Serum triglycerides Serum serum high‐density lipoprotein‐cholesterol Total serum cholesterol SF‐36 EQ‐5D Obesity Assessment Survey | 1 2 2 2 2 1 1 2 2 2 2 (median effect = 2) | |
| P | Weight management | — | — | — | Weight change | 2 | |
| P | Weight management | 0‐21 | > 50% M | W,B | BMI Calorie intake Fat intake Fruit intake Vegetable intake Television‐viewing time | 2 2 2 2 2 2 (median effect = 2) | |
| E | Appointment reminder | — | — | — | Appointment adherence | 1 | |
| E | Appointment reminder | 41‐70 | > 50% M | W | Appointment non‐attendance Preparation non‐adherence | 2 2 (median effect = 2) | |
| E | Appointment reminder | 22‐40 | > 50% F | W,B,H | Attendance rate | 2 | |
| E | Appointment reminder | — | M = F (± 3%) | — | Appointment adherence | 1 | |
| E | Appointment reminder | — | — | — | Appointment adherence | 2 | |
| E | Appointment reminder | 0‐21 | > 50% M | H | Appointment adherence | 1 | |
| E | Appointment reminder | 0‐21 | > 50% F | W,H | Appointment adherence (3‐day interval) | 1 | |
| M | Illicit drugs addiction | 41‐70 | > 50% M | W,B | Patient interest Perceived efficacy Ease of use Treatment satisfaction Retention rate Drug consumption Methadone counselling Coping | 5 5 5 4 4 2 2 2 (median effect = 4) | |
| M | Alcohol consumption | — | — | — | AUDIT score | 1 | |
| M | Alcohol consumption | 41‐70 | > 50% M | B,H | Number of drinks per drinking day | 1 | |
| M | Alcohol consumption | 41‐70 | > 50% M | W | Weekly alcohol consumption | 3 | |
| M | Alcohol consumption | 41‐70 | > 50% M | W,B,H | Proportion of days abstinent Proportion of heavy drinking days Continuous abstinence Drinking problems Coping problems | 1 2 2 2 2 (median effect = 2) | |
| M | Alcohol consumption | 41‐70 | > 50% M | W,B | Self‐reported drinking patterns Blood alcohol content Work and social adjustment scale Obsessive–compulsive drinking scale SF‐36 health survey Drinker inventory of consequences | 2 2 2 2 2 2 (median effect = 2) | |
| M | Alcohol consumption | 41‐70 | M = F (± 3%) | — | Alcohol consumption | 2 | |
| M | Alcohol consumption | 41‐70 | > 50% M | W,B | Number of heavy drinking days per month % days abstinent per month Drinks per drinking day | 2 2 2 (median effect = 2) | |
| M | Alcohol consumption | 41‐70 | > 50% M | W,B | Drinking habits Alcohol craving Post‐traumatic stress disorder symptoms | 2 2 2 (median effect = 2) | |
| M | Asthma | 41‐70 | > 50% F | W | Healthcare utilisation Medication use QoL | 2 2 2 (median effect = 2) | |
| M | Asthma | 0‐21 | M = F (± 3%) | — | Healthcare utilisation | 4 | |
| M | Cancer | 41‐70 | > 50% M | W | Number of symptom threshold events Cumulative distribution of symptom threshold events Differences in mean symptom severity between discharge and follow‐up | 1 2 2 (median effect = 2) | |
| M | Cancer | 41‐70 | > 50% F | W,B | Depression severity Pain severity | 1 1 (median effect = 1) | |
| M | Cancer | 41‐70 | > 50% F | W | Symptom presence, severity, and distress data | 2 | |
| M | Cancer | 41‐70 | M = F (± 3%) | W,B,H | Prevalence of unmet needs | 2 | |
| M | Cancer | 41‐70 | > 50% F | — | Symptom severity | 2 | |
| M | Cancer | 41‐70 | > 50% F | W,B,A | Adherence to medications Symptom severity | 2 1 (median effect = 2) | |
| M | Cancer | 41‐70 | M = F (± 3%) | W,B,H | Symptom burden | 2 | |
| M | Chronic Pain | 41‐70 | > 50% F | W | Pain Function/disability Coping | 1 1 1 (median effect = 1) | |
| M | Chronic Pain | 41‐70 | > 50% M | W | Pain intensity | 1 | |
| M | Chronic obstructive pulmonary disease | 41‐70 | > 50% M | — | Frequency of exacerbations Proportion of patients experiencing 1 or more exacerbations | 2 2 (median effect = 2) | |
| M | Adherence | 0‐21 | > 50% M | B | Comprehensiveness of screening and counselling | 1 | |
| M | Adherence | 41‐70 | > 50% F | W,B,H,A | Medication adherence | 1 | |
| M | Adherence | 0‐21 | — | — | Medication adherence | 1 | |
| M | Adherence | 41‐70 | M = F (± 3%) | W,B,H,A | Medication adherence | 1 | |
| M | Adherence | 0‐21 | — | — | Medication adherence | 1 | |
| M | Adherence | 41‐70 | > 50% M | W,B,H,A | Adherence (completion of all 3 recommended laboratory tests) | 2 | |
| M | Adherence | 41‐70 | M = F (± 3%) | W,B,H,A | Medication adherence | 1 | |
| M | Adherence | 41‐70 | M = F (± 3%) | — | Completion of all recommended laboratory tests | 1 | |
| M | Adherence | ≥ 71 | > 50% F | B | Medication adherence Systolic blood pressure Diastolic blood pressure | 1 2 1 (median effect = 1) | |
| M | Adherence | 41‐70 | > 50% M | W,B | Medication adherence Refill adherence Appointment adherence | 2 2 2 (median effect = 2) | |
| M | Adherence | — | — | — | Medication refill rate | 1 | |
| M | Adherence | 41‐70 | > 50% M | W | Medication adherence | 1 | |
| M | Adherence | ≥ 71 | > 50% F | — | Medication non‐adherence Cognitive assessment | 1 2 (median effect = 2) | |
| M | Adherence | 41‐70 | M = F (± 3%) | W,B,H,A | Adherence rate Therapeutic coverage | 2 2 (median effect = 2) | |
| M | Adherence | 41‐70 | > 50% F | B | Medication adherence Diet Moderate or greater intensity physical activity | 2 1 4 (median effect = 2) | |
| M | Adherence | — | — | — | Medication adherence | 1 | |
| M | Adherence | ≥ 71 | — | — | Medication adherence | 1 | |
| M | Adherence | 41‐70 | M = F (± 3%) | — | HRQLAdherence to statins | 1 | |
| M | Adherence | — | — | — | Medication adherence (refill rate) | 1 | |
| M | Adherence | 41‐70 | — | — | Adherence and adverse events | 1 | |
| M | Adherence | 41‐70 | > 50% M | B | Retinopathy examination | 4 | |
| M | Adherence | 41‐70 | > 50% F | — | 6‐month point prevalence | 1 | |
| M | Adherence | — | — | — | Adherence to medications | 2 | |
| M | Adherence | 41‐70 | > 50% F | W,B,A | Medication adherence | 1 | |
| M | Adherence | 41‐70 | M = F (± 3%) | W,B,A | Medication adherence | 1 | |
| M | Diabetes mellitus | 41‐70 | > 50% M | W | Glycated haemoglobin | 2 | |
| M | Diabetes mellitus | 41‐70 | > 50% F | W,B,H | Maternal blood glucose level Infant birth weight | 2 2 (median effect = 2) | |
| M | Diabetes mellitus | 41‐70 | > 50% F | W,B,H,A | Glycated haemoglobin Medication adherence Quality of life Cost‐effectiveness | 2 2 2 1 (median effect = 2) | |
| M | Diabetes mellitus | 41‐70 | > 50% M | H | Glycated haemoglobin | 4 | |
| M | Diabetes mellitus | — | — | — | Glycated haemoglobin | 1 | |
| M | Diabetes mellitus | 41‐70 | > 50% F | H | Glycated haemoglobin Health distress Global health Hypoglycaemia Hyperglycaemia Activity limitation Fatigue Physical activity levels Communication with physician Glucose monitoring Self‐efficacy Healthcare utilisation | 4 4 4 2 4 4 4 2 4 1 4 2 (median effect = 4) | |
| M | Diabetes mellitus | 41‐70 | > 50% F | W,H | Depression Days cut down on activities because of illness Diabetes‐specific health‐related quality of life Satisfaction with care (English speakers only) General health‐related quality of life (English speakers only) | 1 2 1 1 2 6 1 1 (median effect = 1) | |
| M | Diabetes mellitus | 41‐70 | > 50% M | W,B,H | Glucose monitoring Foot inspection Weight monitoring Medication adherence Glycated haemoglobin Serum glucose levels Diabetic symptoms (all) Hyperglycaemic symptoms Hypoglycaemic symptoms Vascular symptoms Other symptoms Satisfaction with care (summary score) | 1 1 2 4 2 2 1 2 2 2 1 1 (median effect = 2) | |
| M | Diabetes mellitus | 41‐70 | > 50% F | W,B,H,A | Change in self management behaviour (self‐monitoring of blood glucose and self‐monitoring of diabetic foot) | 1 | |
| M | Diabetes mellitus | 41‐70 | > 50% M | — | Glycated haemoglobin Health‐related quality of life (mental) Health‐related quality of life (physical) | 1 1 2 (median effect = 1) | |
| M | Heart failure | 41‐70 | > 50% M | — | All‐cause mortality Re‐hospitalisation Emergency room use (composite outcome) | 1 | |
| M | Heart failure | 41‐70 | > 50% M | W,B | Readmission for any reason or death from any cause | 4 | |
| M | Heart failure | ≥ 71 | > 50% M | — | Packer clinical composite score: death, hospital admission for heart failure, withdrawal from study due to worsening heart failure, 7‐point global health assessment questionnaire | 2 | |
| M | Heart failure | 41‐70 | > 50% M | — | Cardiovascular deaths and hospitalisations (outcomes in isolation included cardiovascular deaths, hospitalisations for heart failure, and time to primary endpoint) | 1 | |
| M | HIV | — | > 50% M | A | Time to virological failure (viral load > 400 copies/mL on 2 consecutive measurements) | 2 | |
| M | Hypercholestorolemia | 41‐70 | > 50% F | W | Total cholesterol reduction | 2 | |
| M | Hypercholesterolemia | 41‐70 | > 50% F | B | Total cholesterol reduction | 2 | |
| M | Hypertension | 41‐70 | > 50% F | W,B,H | Blood pressure control at 6 months | 2 | |
| M | Hypertension | 41‐70 | > 50% F | H | Change in minutes of moderate or greater physical activity Change in systolic blood pressure | 1 2 (median effect = 2) | |
| M | Hypertension | — | — | — | Blood pressure | 1 | |
| M | Hypertension | 41‐70 | > 50% M | W,H | Proportion to achieve guideline‐recommended blood pressure goals Systolic blood pressure Diastolic blood pressure | 2 1 2 (median effect = 2) | |
| M | Hypertension | 41‐70 | > 50% F | — | Systolic blood pressure | 2 | |
| M | Mental health | 22‐40 | > 50% F | W,B | Quality of life (physical scale score and mental scale score) Depression Stress levels Total well‐being | 2, 2 2 2 2 (median effect = 2) | |
| M | Mental health | 22‐40 | > 50% M | W | Yale‐Brown obsessive compulsive scale (YBOCS) score | 1 | |
| M | Mental health | — | — | — | Emotional health Physical health Stress | 1 1 1 (median effect = 1) | |
| M | Obstructive sleep apnoea syndrome | 41‐70 | — | — | Continuous positive airway pressure use | 2 | |
| M | Obstructive sleep apnoea syndrome | 41‐70 | > 50% M | — | Continuous positive airway pressure use | 1 | |
| M | Smoking | 22‐40 | M = F (± 3%) | — | Repeated point abstinence | 1 | |
| M | Smoking | 22‐40 | > 50% F | W,B,H,A | Re‐enrollment into quit line support | 1 | |
| M | Smoking | 22‐40 | > 50% F | W,B | Smoking abstinence | 2 | |
| M | Smoking | 41‐70 | > 50% M | — | Self‐reported abstinence Biochemically confirmed smoking abstinence | 2 4 (median effect = 3) | |
| M | Smoking | 0‐21 | > 50% M | A | Stage of change Self‐efficacy Decisional balance | 5 5 5 (median effect = 5) | |
| M | Smoking | 41‐70 | > 50% M | — | Smoking abstinence | 4 | |
| M | Smoking | 41‐70 | > 50% M | — | Smoking abstinence | 2 | |
| M | Smoking | — | — | — | Smoking abstinence | 2 | |
| M | Smoking | 41‐70 | M = F (± 3%) | W,B,H,A | Biochemically confirmed tobacco abstinence at 6 months | 1 | |
| M | Smoking | 41‐70 | > 50% M | W,B,A | 24‐h point prevalence 7‐d point prevalence 6‐month prolonged abstinence | 2 2 2 (median effect = 2) | |
| M | Spinal cord dysfunction | 41‐70 | > 50% M | W,B,H | Prevalence of pressure ulcers Depression severity Healthcare utilisation | 2 1 2 (median effect = 2) | |
| aStudy type: E: either prevention or management; M: management of long‐term condition; P: prevention. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Immunisation in children Show forest plot | 5 | 10454 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.18, 1.32] |
| 2 Immunisation in adolescents Show forest plot | 2 | 5725 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [1.02, 1.11] |
| 3 Immunisation in adults Show forest plot | 2 | 1743 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.53, 9.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer screening Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Multimodal/complex interventions | 2 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.55, 3.04] |
| 1.2 IVR | 2 | 2599 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.11] |
| 2 Colorectal cancer screening Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Multimodal/complex intervention | 3 | 1013 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.88, 2.55] |
| 2.2 IVR (shorter follow‐up) | 2 | 16915 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.25, 1.48] |
| 2.3 IVR (longer follow‐up) | 2 | 21335 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.97, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 BMI adults Show forest plot | 3 | 672 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.38, 0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Glycated haemoglobin Show forest plot | 7 | 1216 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.50, ‐0.01] |
| 2 Self‐monitoring of diabetic foot Show forest plot | 2 | 498 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.42] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cardiac mortality Show forest plot | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.21, 1.67] |
| 2 All‐cause mortality Show forest plot | 3 | 2165 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systolic blood pressure Show forest plot | 3 | 65256 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐2.12, ‐1.66] |
| 2 Diastolic blood pressure Show forest plot | 2 | 65056 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐2.62, 2.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Smoking abstinence Show forest plot | 7 | 2915 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.98, 1.46] |

