Pharmakologische Therapien zur Prävention von Epilepsie nach traumatischer Kopfverletzung
Abstract
Background
Head injury is a common event and can cause a spectrum of motor and cognition disabilities. A frequent complication is seizures. Antiepileptic drugs (AED) such as phenytoin are often used in clinical practice with the hopes of preventing post‐traumatic epilepsy. Whether immediate medical intervention following head trauma with either AEDs or neuroprotective drugs can alter the process of epileptogenesis and lead to a more favorable outcome is currently unknown. This review attempted to address the effectiveness of these treatment interventions. This review updates and expands on the earlier Cochrane review.
Objectives
To compare the efficacy of antiepileptic drugs and neuroprotective agents with placebo, usual care or other pharmacologic agents for the prevention of post‐traumatic epilepsy in people diagnosed with any severity of traumatic brain injury.
Search methods
We searched The Cochrane Epilepsy Group's specialized register, CENTRAL, MEDLINE, ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (ICTRP) in January 2015. We searched EMBASE, Biological Abstracts and National Research Register in September 2014 and SCOPUS in December 2013. The Cochrane Epilepsy Group performed handsearches of relevant journals.
Selection criteria
We included randomized controlled trials (RCTs) that include AEDs or neuroprotective agents compared with placebo, another pharmacologic agent or a usual care group. The outcomes measured included a seizure occurring within one week of trauma (early seizure), seizure occurring later than one week post‐trauma (late seizure), mortality and any adverse events.
Data collection and analysis
Two review authors independently assessed study quality and extracted the data. We calculated risk ratios (RR) and 95% confidence intervals (CI) for each outcome. We used random‐effects models in the meta‐analyses and performed pre‐defined subgroup and sensitivity analyses.
Main results
This review included 10 RCTs (reported in 12 articles) consisting of 2326 participants The methodological quality of the studies varied. The type of intervention was separated into three categories; AED versus placebo or standard care, alternative neuroprotective agent versus placebo or standard care and AED versus other AED. Treatment with an AED (phenytoin or carbamazepine) decreased the risk of early seizure compared with placebo or standard care (RR 0.42, 95% CI 0.23 to 0.73; very low quality evidence). There was no evidence of a difference in the risk of late seizure occurrence between AEDs and placebo or standard care (RR 0.91, 95% CI 0.57 to 1.46; very low quality evidence). There was no evidence of a significant difference in all‐cause mortality between AEDs and placebo or standard care (RR 1.08 95% CI 0.79 to 1.46,very low quality of evidence). Only one study looked at other potentially neuroprotective agents (magnesium sulfate) compared with placebo. The risk ratios were: late seizure 1.07 (95% CI 0.53 to 2.17) and all‐cause mortality 1.20 (95% CI 0.80 to 1.81). The risk ratio for occurrence of early seizure was not estimable.
Two studies looked at comparison of two AEDs (levetiracetam, valproate) with phenytoin used as the main comparator in each study. The risk ratio for all‐cause mortality was 0.53 (95% CI 0.30 to 0.94). There was no evidence of treatment benefit of phenytoin compared with another AED for early seizures (RR 0.66, 95% 0.20 to 2.12) or late seizures(RR 0.77, 95% CI 0.46 to 1.30).
Only two studies reported adverse events. The RR of any adverse event with AED compared with placebo was 1.65 (95% CI 0.73 to 3.66; low quality evidence). There were insufficient data on adverse events in the other treatment comparisons.
Authors' conclusions
This review found low‐quality evidence that early treatment with an AED compared with placebo or standard care reduced the risk of early post‐traumatic seizures. There was no evidence to support a reduction in the risk of late seizures or mortality. There was insufficient evidence to make any conclusions regarding the effectiveness or safety of other neuroprotective agents compared with placebo or for the comparison of phenytoin, a traditional AED, with another AED.
PICOs
Laienverständliche Zusammenfassung
Medikamente zur Verhinderung von Epilepsie nach traumatischen Kopfverletzungen
Hintergrund
Traumatische Kopfverletzungen treten häufig auf und können Verletzungen des Gehirns nach sich ziehen. Solche schweren Verletzungen lösen in weiterer Folge häufig Krampfanfälle aus, die den Schaden vergrößern und zu chronischer Epilepsie führen können. Dies ist eine neurologische Erkrankung, die sich durch häufig wiederkehrende Krampfanfälle auszeichnet. In der Regel werden antiepileptische Medikamente (Antiepileptika) verschrieben, um bereits diagnostizierte Krampfanfälle zu behandeln. Ihre Rolle bei der Heilung der Erkrankung und der Verhinderung einer Epilepsie bei Menschen, die nach einer Gehirnverletzung einschließlich einer Kopfverletzung als anfallgefährdet eingestuft werden, ist bisher nicht ausreichend geklärt.
Studienmerkmale
Ziel des systematischen Reviews war es, Studien zu identifizieren, die die Wirkung einer frühen Gabe von Antiepileptika oder anderen möglicherweise neuroprotektiven (die Struktur oder Funktion von Nerven schützenden) Wirkstoffen auf posttraumatische Epilepsie untersuchten. Die relevanten primären Endpunkte waren frühzeitig auftretende posttraumatische Krampfanfälle (innerhalb einer Woche nach dem Trauma) und spät auftretende Krampfanfälle (später als eine Woche nach dem Trauma). Weitere Endpunkte waren auftretende Todesfälle, Zeit bis zum späten Krampfanfall und Nebenwirkungen. Die Übersichtsarbeit berücksichtigte Studien bis zum Januar 2015.
Hauptergebnisse
Die Evidenz beruht auf 10 klinischen Studien (berichtet in 12 Publikationen) mit 2326 Teilnehmern. Die verfügbare Evidenz zeigte, dass eine frühe Behandlung mit herkömmlichen Antiepileptika (Phenytoin oder Carbamazepin) das Risiko von frühen posttraumatischen Krampfanfällen verringern kann. Herkömmliche Antiepileptika sind nicht wirksamer als Placebo (Scheinmedikament) oder die Regelversorgung, wenn es um die Verringerung später Krampfanfälle oder Sterblichkeit geht. Es standen kaum Daten für den Vergleich eines Antiepileptikums mit einem anderen Antiepileptikum sowie für den Vergleich anderer potenziell neuroprotektiver Wirkstoffe mit einem Placebo zur Verfügung. Die meisten Studien berichteten nicht über schwerwiegende und andere Nebenwirkungen.
Qualität der Evidenz
Die Qualität der Evidenz variierte zwischen den Endpunkten. Die Ergebnisse sollten mit Vorsicht interpretiert werden.
Authors' conclusions
Summary of findings
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
Background
Description of the condition
Head injury is a common event and can cause a spectrum of motor and cognition disabilities. A frequent complication is seizures. While 'early seizures' are frequently considered to be nonspecific diffuse reactions as a result of an acute encephalopathy and are self limited, seizures following several weeks or months after head trauma seem to reflect an underlying process of post‐traumatic scar formation and epileptogenesis. However, there is evidence from epidemiologic studies that early seizures can be predictors for late seizures (Wyllie 2010). This suggests that these definitions reflect simplifications of the underlying ongoing tissue transformation over time. The risk for late 'unprovoked' seizure recurrence increases with the severity of the injury, involvement of the cerebral cortex, presence of dura penetration, skull fracture and intracerebral hematoma, and the occurrence of early seizures (Jennett 1981; Annegers 1998). Timing and the interplay of potentially involved factors in the development of this epileptogenic process are unclear.
Description of the intervention
Behind the concept of preventing post‐traumatic epilepsy stands the hope that the silent period of weeks and months after the trauma, before seizure occurrence, is a window of opportunity to stop the process using appropriate interventional treatment strategies (Temkin 2009). Antiepileptic drugs (AEDs) can suppress seizures; however, it is the subject of a controversial debate if they are also able to interfere positively with the process leading to epilepsy. Experimental studies looking at neuroprotective agents, such as antioxidants and free radicals, have also been promising but historically have not translated well into the clinical environment (Slemmer 2008). Therefore, this Cochrane review will carefully evaluate the impact of either early or late use of AEDs and neuroprotective agents on the occurrence of unprovoked seizures following the trauma.
How the intervention might work
Current experimental epilepsy research using animal models, such as kindling and post‐status epileptic condition, suggests that some new AEDs may have the potential to alter the underlying epileptogenic process and act as disease‐modifying agents (Löscher 2002; Brandt 2006). There is also some evidence that neuroprotective agents may alter the epileptogenic process. For example, antioxidants may be able to suppress this process by interfering with free radical reactions initiated by hemorrhage associated with brain injuries (Willmore 2009).
Why it is important to do this review
Post‐traumatic seizures are quite prevalent. Most of these people undergo a careful functional and structural diagnostic algorithm including electroencephalography (EEG) and magnetic resonance imaging (MRI) or at least computed tomography (CT). Therefore, post‐traumatic seizures can be considered an ideal model to study tissue changes and regional hyperexcitability as part of the evolving epileptogenic scar. It is not yet known whether immediate medical intervention following head trauma with either AEDs or neuroprotective drugs can alter the process of epileptogenesis and lead to a more favorable outcome. There are limited data on traditional AEDs such as phenytoin, phenobarbital, valproate and carbamazepine.
With the advent since the mid‐2000s of many new AEDs and research into alternative treatments such as neuroprotective agents, it seems critical and timely to review the human experience carefully and evaluate how these experimental findings might translate into the prevention of post‐traumatic epilepsy in clinical practice. Therefore, this review will conduct a systematic, up‐to‐date review of randomized controlled trials (RCTs) examining the effectiveness and safety of both AEDs and neuroprotective agents with special focus on recently licensed products.
Objectives
To compare the efficacy of antiepileptic drugs and neuroprotective agents with placebo, usual care or other pharmacologic agents for the prevention of post‐traumatic epilepsy in people diagnosed with any severity of traumatic brain injury.
Methods
Criteria for considering studies for this review
Types of studies
RCTs that included AEDs or neuroprotective agents compared with placebo, another pharmacologic agent or a usual care group. We included studies published in any languages. We excluded quasi‐randomized studies, dose‐finding studies and cluster randomized or cross‐over trials.
Types of participants
People of all ages diagnosed with acute traumatic brain injury (TBI) who received prophylactic treatment with AEDs or neuroprotective agents. Administration was post‐injury and prior to the occurrence of a first post‐traumatic seizure (FPS). We excluded people with previously documented unprovoked seizures.
Types of interventions
Treatment
-
Any conventional AED post‐injury and prior to the occurrence of an FPS. Traditional AEDs included, but were not limited to, carbamazepine, phenytoin and valproate, and examples of new AEDs included but were not limited to oxcarbazepine, lamotrigine, levetiracetam and topiramate.
-
Any alternative neuroprotective pharmacologic treatments, including administration of distinct neurotrophic factors, hormones or antioxidants post‐injury and prior to the occurrence of an FPS.
Comparison
Other pharmacologic agent, placebo or usual care.
-
Pharmacologic agents (AED) versus placebo or usual care.
-
Neuroprotective agent versus placebo or usual care.
-
Pharmacologic agent A (AED) versus pharmacologic agent B (AED).
We analyzed each comparison separately.
Types of outcome measures
Primary outcomes
-
Proportion of participants who experience an early seizure post‐trauma, defined as occurring within one week of trauma.
-
Proportion of participants who experience a late seizure post‐trauma, defined as occurring later than one week post‐trauma.
Secondary outcomes
-
Mortality from any cause during follow‐up period.
-
Time to first seizure from randomization.
-
Proportion of participants experiencing serious treatment‐related adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
Cochrane Epilepsy Group Specialized Register (latest search date: 13 January 2015) using the search strategy given in Appendix 1;
-
Cochrane Central Register of Controlled Trials (CENTRAL) via Cochrane Register of Studies Online (CRSO), latest search date: 13 January 2015, using the search strategy given in Appendix 2;
-
MEDLINE (OVID; 1946 to 13 January 2015) using the search strategy given in Appendix 3;
-
EMBASE (Elsevier; latest search date: 5 September 2014) using the search strategy given in Appendix 4;
-
ClinicalTrials.gov and World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) searched on 13 January 2015 for 'traumatic head injury AND epilepsy';
-
SCOPUS (1823 to 17 December 2013) using the search strategy given in Appendix 5;
-
Biological Abstracts (latest search date: 5 September 2014) using the search strategy given in Appendix 6.
The electronic search strategy used in the review by Schierhout 2001 was expanded upon.
It is no longer necessary to search SCOPUS or EMBASE, because RCTs listed in EMBASE are now included in CENTRAL, and SCOPUS is a substitute for EMBASE.
Searching other resources
In addition to searching electronic databases, we consulted the following sources.
-
Bibliographies of related Cochrane reviews.
-
Reference sections of included papers and key systematic reviews (Temkin 2001; Beghi 2003).
-
Authors of relevant reports regarding any further published or unpublished work.
-
National Research Register.
-
Handsearching of content related journals as conducted by The Cochrane Epilepsy Group.
Data collection and analysis
Selection of studies
Two review authors independently reviewed titles and abstracts obtained from the literature search. Review authors then ranked each as follows.
-
Include: study met criteria.
-
Unclear: insufficient to determine if study met criteria.
-
Exclude: study did not meet criteria.
The review authors compared and discussed results; a third review author arbitrated any disagreements. We excluded papers where insufficient evidence was available in a study. If necessary, we contacted authors for further clarification. When multiple publications of the same study were found, we included the original study that met the inclusion criteria; we included the second study if it contained different outcomes. We placed no language or time restriction on included studies.
Data extraction and management
We used the full text to include or exclude studies where it was unclear from reading the abstracts. Two review authors independently extracted data from included RCTs in the following categories.
-
Participant characteristics:
-
-
inclusion and exclusion criteria;
-
number of participants per group;
-
age;
-
sex;
-
trauma characteristics such as severity, MRI or CT documented pattern of injury,
-
EEG findings.
-
-
Methods:
-
-
study design;
-
duration of study;
-
randomization method;
-
treatment allocation;
-
completeness of follow‐up;
-
presence of blinding;
-
intention‐to‐treat (ITT) analysis.
-
-
Intervention:
-
-
type of agent, treatment 1, treatment 2 or control;
-
method of administration;
-
dosage and duration of treatment;
-
time post‐trauma treatment was delivered;
-
control or usual care intervention.
-
-
Outcome measures and clinical findings:
-
-
seizure occurrence;
-
mortality;
-
number of seizures;
-
time to first seizure;
-
adverse events;
-
neurologic findings.
-
-
Possible sources of heterogeneity:
-
-
median age of participant at time of injury;
-
severity of trauma;
-
pharmacologic agent;
-
duration of treatment;
-
timing of treatment.
-
-
Other:
-
-
country and setting of study;
-
year of publication;
-
title;
-
authors.
-
We used a predefined form for this task (Appendix 7).
The form was developed and pilot tested on three trials prior to use on all studies.
Assessment of risk of bias in included studies
Two review authors independently assessed the quality of studies. Methods used for summary assessment are provided in the Cochrane Handbook for Systematic Reviews of Interventions 5.1, Section 8.4 (Higgins 2011). We scored each of the following domains as at 'high', 'low' or 'unclear' risk and reported the scores in the 'Risk of bias' table.
-
Selection bias:
-
sequence generation;
-
allocation of concealment.
-
-
Performance bias:
-
blinding of participants and personnel;
-
other potential threats to validity.
-
-
Detection bias:
-
blinding of outcome assessment;
-
other potential threats to validity.
-
-
Attrition bias:
-
incomplete outcome data.
-
-
Reporting bias:
-
selective outcome reporting.
-
-
Other bias
Measures of treatment effect
We performed statistical analyses and produced a summary of the data using Review Manager 5 (RevMan 2012). We presented dichotomous outcomes as risk ratios (RR) with corresponding 95% confidence intervals (CI). We planned to present time‐to‐event outcomes as hazard ratios with 95% CI. If hazard ratios were not given, we planned to use indirect estimation methods (Parmar 1998; Williamson 2002). None of the included studies presented time‐to‐event data; we therefore did not summarize results using hazard ratios. For individual listed adverse effects, we quoted 99% CIs, making an allowance for multiple testing. We performed separate analyses for each control group. We used an ITT analysis on outcomes from all randomized participants where possible for primary analyses.
Unit of analysis issues
The unit of analysis for this review was the individual participant
Dealing with missing data
We contacted authors where substantial outcomes of interest were not reported or to clarify uncertainty about study characteristics. We waited one month for a response from authors, after which time we formally considered data to be missing.
ITT analyses were performed.
Assessment of heterogeneity
We used forest plots to assess the statistical heterogeneity of studies visually and the Chi2 test to assess evidence of heterogeneity. We used a P value < 0.1 to determine statistical significance (Whitehead 1991). We calculated the I2 statistic with I2 values greater than 50% indicating substantial to considerable heterogeneity (Higgins 2011). If we found values of heterogeneity greater than 50%, we attempted to explain the heterogeneity based on the differences in study characteristics and participant profiles (such as severity of trauma, age).
Assessment of reporting biases
We planned to assess publication bias using funnel plots if more than 10 studies were included. Reasons for funnel plot asymmetry include publication bias, outcome reporting bias, language bias, citation bias, poor methodologic design and heterogeneity. We assessed these for each trial, where possible. We planned to include an ORBIT table to explore the impact of selective outcome reporting further (Kirkham 2010). We found few studies assessing each of the pre‐specified outcomes; we therefore did not prepare funnel plots or orbit tables
Data synthesis
To pool the data for each outcome, we used the random‐effects method (DerSimonian 1986) based on the inverse variance method , rather than using an fixed effects method. A random‐effects model meta‐analysis involves an assumption that the effects being estimated in the different studies are not identical but follow the same distribution (Higgins 2011). Summary intervention estimates are a weighted mean of the estimate from each individual study. A fixed‐effect model was considered as a sensitivity analysis.
'GRADEing' the evidence
We followed the recommended GRADE approach to assess the quality of the evidence for each outcome. We produced 'Summary of findings' tables for each treatment comparison for the primary outcomes (early seizure and late seizure) and secondary outcomes (all cause mortality, time to first seizure from randomisation and proportion of participants experiencing serious treatment‐related adverse events) based on established recommendations (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We intended to evaluate clinical and methodologic heterogeneity across studies by comparing the characteristics of participants, interventions and study designs.
We performed the following clinically relevant subgroup analyses to investigate possible sources of clinical heterogeneity.
-
Mean age of participants in study at time of injury: adults (ages over 17 years), school age children (ages six to 17 years) and children (ages under six years).
-
Pharmacologic agent: recently licensed AEDs and traditional AEDs.
-
Severity of trauma: minor, moderate and severe TBI. This review followed the paper by Teasell 2007 for classification of head trauma (Appendix 8).
-
Duration of treatment: short‐term treatment (treatment less than three months post‐injury), mid‐term treatment (more than three months and less than 12 months post‐injury) and long‐term treatment (any duration longer than one year post‐injury).
Tests of Interaction (Cochran's Q and Higgins I2) for subgroup differences were performed. Data were not available for all the pre‐planned subgroup analysis (see Differences between protocol and review).
Sensitivity analysis
We performed sensitivity analysis to evaluate the robustness of decisions made in the review methodology.
-
Study quality: excluding studies which were high risk of bias
-
Age range of participants: analysis repeated excluding those studies where it was not possible to separate participants that did not meet the inclusion criteria for age.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Of the 1929 initial citations identified, we screened 75 reports (See Figure 1).

Study flow diagram.
Included studies
Ten RCTs described in 12 published articles met review inclusion criteria and included 2326 randomized participants ages five years and older. All trials included participants with moderate and severe TBI and excluded people with pre‐existing epilepsy.
Five trials included children (McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992; Young 2004). Young 2004 included only children under the age of 10 years.
Six trials reported on short‐term treatments (five to seven days to one month) (Young 1983; Pechadre 1991; Temkin 1999; Young 2004; Temkin 2007; Szaflarski 2010), three reported on mid‐term treatments (six to 12 months) (McQueen 1983; Temkin 1990; Temkin 1999), and three trials reported on long‐term treatments (18 months to two years) (Glotzner 1983; Young 1983; Manaka 1992). Most studied traditional AEDs versus placebo or usual care: phenytoin (McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Young 2004), phenobarbital (Manaka 1992), carbamazepine (Glotzner 1983), and valproate (Temkin 1999); one studied a newly licensed agent: levetiracetam versus phenytoin (Szaflarski 2010), and one studied an 'other' agent, magnesium sulfate (MgSO4) versus placebo (Temkin 2007).
Six trials were conducted in the USA (Young 1983; Temkin 1990; Temkin 1999; Young 2004; Temkin 2007; Szaflarski 2010), three in Europe (Glotzner 1983; McQueen 1983; Pechadre 1991), and one in Japan (Manaka 1992).
We included nine trials in the meta‐analysis and assessed the primary and secondary outcomes of early seizures, late seizures, all‐cause mortality and adverse events (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Manaka 1992; Temkin 1999; Young 2004; Szaflarski 2010). All but two trials reported incidence of early and late seizures; Manaka 1992 and McQueen 1983 reported only late seizures. All trials but Manaka 1992 and Pechadre 1991 reported mortality. The majority of trials primarily investigated whether AEDs (traditional or newly licensed) prevented early or late (or both) seizure occurrence in people with TBI. One trial primarily investigated safety and reported on adverse events (Szaflarski 2010). McQueen 1983 and Temkin 1990 also reported the occurrence of skin rashes. Temkin 2007 was not included in a meta‐analysis as it was the only study included in the review that studied an 'other' agent. See Characteristics of included studies table for details.
Excluded studies
We excluded 63 studies from the review. Forty were not RCTs or quasi‐randomized trials, in seven the data were unavailable (i.e. trial cancelled due to lack of enrolment or unable to acquire details from author), four studies were secondary publications of studies already included, which contained no further relevant information. Five studies did not report treatment of interest, seven did not include the population of interest. See Characteristics of excluded studies table for details.
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risk of bias of included studies. We deemed no study to be at low risk of bias in all bias types. The majority of studies had a mix of low and unclear bias to varying degrees. Five trials had a number of bias types classified as high risk of bias (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992).
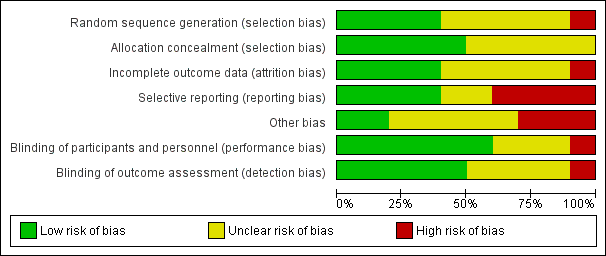
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
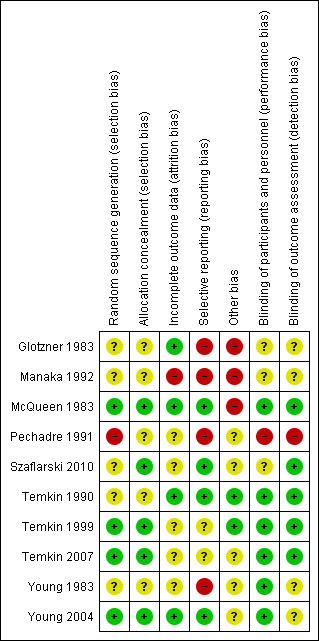
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Only three trials adequately described the sequence generation and allocation process (Temkin 1999; Young 2004; Temkin 2007). Szaflarski 2010 indicated the participants were randomized by the pharmacy but did not describe the sequence generation and, therefore, the risk of selection bias was unclear. Risk of selection bias was unclear in McQueen 1983; Young 1983; Temkin 1990; Manaka 1992 , due to lack of clear description of the sequence generation and allocation process. The sequence generation in Glotzner 1983 and Pechadre 1991 was based on odd/even birthday or days of admission and, therefore, at high risk of predicting group allocation.
Blinding
Six of the 10 trials were low risk for performance bias as they adequately described blinding of participants and personnel (McQueen 1983; Young 1983; Temkin 1990; Temkin 1999; Young 2004; Temkin 2007). In Glotzner 1983; Manaka 1992; and Szaflarski 2010, risk of bias for performance bias was unclear as these trials did not report on blinding of participants and personnel. Risk of detection bias was low in five trials (McQueen 1983; Temkin 1990; Temkin 1999; Temkin 2007; Szaflarski 2010), and unclear in four publications as they did not describe blinding of the outcome assessment (Glotzner 1983; Young 1983; Manaka 1992; Young 2004). Pechadre 1991 was at high risk for performance and detection bias as it did not describe blinding of the participants, personnel or outcome assessment and the predictable randomization process suggested that assessors could easily determine which participants were allocated to treatment and control groups.
Incomplete outcome data
Three trials were low risk for attrition bias as they clearly described outcome data and attrition patterns (McQueen 1983; Temkin 1990; Young 2004). Six trials had unclear risk for attrition bias due to poor descriptions of reasons for attrition (Glotzner 1983; Young 1983; Pechadre 1991; Temkin 1999; Temkin 2007; Szaflarski 2010), and Manaka 1992 was high risk of bias for lack of description or details on 52 people who were excluded or dropped out from study.
Selective reporting
Only four of the 10 trials were low risk for reporting bias (McQueen 1983; Temkin 1990; Young 2004; Szaflarski 2010). Four trials were at high risk of selective reporting as they did not report adverse events (Glotzner 1983; Young 1983; Pechadre 1991; Manaka 1992). In addition, Manaka 1992 and Pechadre 1991 did not report mortality and Young 1983 reported mortality inconsistently across age groups. Young 1983 reported mortality as a count of events for all ages in the short‐term treatment; however, mortality in adults on long‐term treatment were not reported as a count of events and, therefore, overall deaths for the entire trial were underestimated. Manaka 1992 was high risk for reporting bias as the trial did not report baseline characteristics. The remaining two trials had unclear risk (Temkin 1999; Temkin 2007).
Other potential sources of bias
Three publications were at high risk for other types of bias (Glotzner 1983; McQueen 1983; Manaka 1992). Glotzner 1983 reported that the majority of participants received an AED in the first week regardless of allocation group resulting in potential contamination of controls. McQueen 1983 reported potential significant baseline differences between the control and treatment groups with more five to 15 year olds in the treatment group and below therapeutic levels of the phenytoin were reported. Manaka 1992 did not report baseline characteristics, so it was impossible to compare baseline characteristics between treatment groups. Several studies reported difficulties with compliance (McQueen 1983; Young 1983; Temkin 1990; Temkin 1999), and maintaining therapeutic levels particularly when evaluating late seizure outcome.
Effects of interventions
See: Summary of findings for the main comparison Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury; Summary of findings 2 Neuroprotective agent versus placebo for people at risk of epilepsy following traumatic head injury; Summary of findings 3 Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury
1. Antiepileptic drug versus placebo or usual care
1.1 Occurrence of early seizure
Five trials involving 987 participants examined the occurrence of early seizures (Glotzner 1983; Pechadre 1991; Temkin 1990; Young 2004; Young 1983). All trials compared a traditional AED (carbamazepine or phenytoin) with a placebo or usual care. The trials included a range of ages from children to adult. Duration of treatment for this outcome varied from five to seven days. The proportion of participants experiencing an early seizure in the treatment group was 5.0% (25/499) compared with 13.9% (68/488) in the placebo group/usual care group. The pooled results favored the traditional AED treatment compared with the control group (RR 0.42, 95% CI 0.23 to 0.73; Analysis 1.1). Heterogeneity was low among the studies (I2 = 29%). We reanalyzed the outcome using fixed‐effect methods; results were consistent with those obtained using random‐effects models. We rated the quality of the evidence as low due to high selective reporting bias in 3 of the studies and inconsistency in RR results in the two studies with low risk of bias (Analysis 1.7).
1.2 Occurrence of late seizure
Six trials reported on late seizures in 1029 participants (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991; Manaka 1992). Manaka 1992 compared phenobarbital with usual care, Glotzner 1983 compared carbamazepine with placebo, while the other four trials compared phenytoin with placebo. Duration of treatment varied from three months to two years. Five of the six trials included adults and children; Temkin 1999 was the only trial to assess adults exclusively. About 15.6% (81/518) of participants receiving AED treatment experienced a late seizure compared with 17.8% (91/511) receiving placebo/usual care. The six‐pooled studies showed no statistically significant effect for traditional AEDs compared with placebo or usual care on late seizure occurrence (RR 0.91, 95% CI 0.57 to 1.467; Analysis 1.2). There was evidence of heterogeneity among the studies (I2 = 54%). This result was rated as very low quality due to high risk of bias in 2 or more categories for several studies, imprecision of pooled RR estimate and moderate level of heterogeneity.
1.3 All‐cause mortality
Five trials reported mortality in 1065 participants (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Young 2004). They compared a traditional AED (carbamazepine or phenytoin) with placebo. Duration of treatments varied from five days to 24 months. About 18.4% (101/549) of participants in the AED group died compared with 17.4% (90/516) in the placebo group. The five pooled trials showed no statistically significant difference in the RR of death between participants treated with traditional AEDs compared with placebo (RR 1.08, 95% CI 0.79 to 1.46; Analysis 1.3). Heterogeneity was low among the studies (I2 = 19%). This result was rated as very low quality due to high risk of bias in 2 or more categories for several studies, imprecision of pooled RR estimate with confidence interval ranging from benefit to harm.
1.4 Any serious event
McQueen 1983 and Temkin 1990 looked at any serious events comparing phenytoin with placebo. About 14.4% (42/292) of participants in treatment group experienced an adverse event compared with 9.4% (26/276) in the placebo group. The pooled RR of an adverse event in treatment group compared with placebo was 1.63 (95% CI 0.73 to 3.66; 568 participants; Analysis 1.4). Heterogeneity was low among the studies (I2 = 18%). We performed no subgroup analysis due to too few studies.The result was rated as low based on imprecision of RR estimate with confidence intervals covering both benefit and harm and serious risk of bias in one study.
1.5 Skin rash
McQueen 1983 and Temkin 1990 reported skin rash comparing phenytoin with placebo. About 10.3% (30/292) of participants in the phenytoin group experienced skin rash compared with 6.5% (18/276) in the placebo group. The RR of skin rash in the phenytoin group compared with placebo was 1.65 (99% CI 0.54 to 5.04; 568 participants; Analysis 1.5). Heterogeneity was low among the studies (I2 = 17%). We performed no subgroup analysis due to too few studies. The result was rated as low based on imprecision of RR estimate with confidence intervals covering both benefit and harm and serious risk of bias in one study.
1.6 Sensitivity analysis
Occurrence of early seizure: age of population
Four of the five trials that reported on early seizures had a mean or median age that was greater than 18 years (Glotzner 1983; Young 1983; Temkin 1990; Pechadre 1991). Young 2004 consisted solely of children. We ran the analysis excluding Young 2004. About 4.9% (22/453) of participants treated with an AED experienced an early seizure compared with 15% (65/432) receiving placebo. The result still favored AED treatments compared with placebo; producing a marginally lower RR compared with the original analysis in Section 1.1 (RR 0.36, 95% CI 0.21 to 0.60, I2 = 12%; Analysis 1.6) (compare with Analysis 1.1).
1.7 Sensitivity analysis
Occurrence of early seizure: study quality
Three of the five trials that examined early seizures had high risk of bias in one or more category (Glotzner 1983; Young 1983; Pechadre 1991). We reran the analysis excluding Glotzner 1983; Young 1983; and Pechadre 1991. The pooled results of the two remaining studies no longer showed a benefit of AED treatment compared with placebo (RR 0.48, 95% CI 0.11 to 2.18, 506 participants; Analysis 1.7) (Temkin 1990; Young 2004). Heterogeneity (I2 = 68%) increased compared with the original analysis (see Analysis 1.1). Differences in participant populations likely contributed to the increase in heterogeneity; participants in the Temkin 1990 trial were adults, whereas Young 2004 studied exclusively children.
1.8 Subgroup analysis
Occurrence of late seizure: type of antiepileptic drug
Four of the six trials compared a traditional AED treatment, phenytoin, with placebo for late seizures (McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991). The two remaining studies treated participants with other AEDs; carbamazepine compared with placebo (Glotzner 1983), and phenobarbital compared with usual care (Manaka 1992). In subgroup analysis, 15% (59/393) of participants treated with phenytoin experienced a late seizure compared with 17.5% (63/359) receiving placebo (RR 0.83, 95% CI 0.40 to 1.70; 752 participants; Analysis 1.8). About 17.6% (22/125) of participants receiving carbamazepine or phenobarbital experienced a late seizure compared with 18.4% (28/152) in the placebo or usual care group (RR 0.96, 95% CI 0.46 to 1.99, 277 participants; Analysis 1.8). There was no statistically significant subgroup difference between the types of antiepileptic drug (P=0.78, I2 =0.0%). The subgroup RRs did not differ substantially from the primary analysis results (see Analysis 1.2).
1.9 Subgroup analysis
Occurrence of late seizure: treatment duration
Five of the six trials that examined the occurrence of late seizures had a treatment duration ranging from 12 to 24 months (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Manaka 1992). One trial had a treatment duration less than one year (Pechadre 1991). In subgroup analysis, for treatment duration of 12 to 24 months, 16.3% (79/484) of participants in the AED group experienced late seizures compared with 15.1% (69/459) of participants in the control groups. In the Pechadre 1991 trial, 5.88% (2/34) of participants in the AED treatment group experienced late seizures compared with 42.3% (22/52) in the control group. Although there was no statistically significant subgroup difference between different treatment durations (P=0.87, I2 =0.004%) the results show greater risk in the AED treatment group for studies with longer treatment duration (12 to 24 months) (RR 1.08, 95% CI 0.81 to 1.46, 943 participants; Analysis 1.9) while the one study with treatment duration of less than one year showed reduced risk (RR 0.14, 95% CI 0.03 to 0.55, 86 participants; Analysis 1.9) (Pechadre 1991).
1.10 Sensitivity analysis
Occurrence of late seizure: age of population
Five of the six trials that examined occurrence of late seizures included both adults and children (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992). Removing Temkin 1990, the only study that excluded children, from the analysis did not alter the results substantially. The pooled effect remained statistically non‐significant as per the original results (RR 0.81, 95% CI 0.44 to 1.48, 706 participants; Analysis 1.10) as per the original results (see Analysis 1.2).
1.11 Sensitivity analysis
Occurrence of late seizure: comparison group
Five trials of the six trials that examined the occurrence of late seizures compared an AED with placebo (Glotzner 1983; McQueen 1983; Young 1983; Temkin 1990; Pechadre 1991). Manaka 1992 compared an AED treatment with usual care. The pooled RR, excluding Manaka 1992, remained not statistically significant (RR 0.83, 95% CI 0.48 to 1.41, 903 participants; Analysis 1.11) (see Analysis 1.2).
1.12 Sensitivity analysis
Occurrence of late seizure: study quality
Five of the six trials that examined the occurrence of late seizures had a high risk of bias in one or more bias categories (Glotzner 1983; McQueen 1983; Young 1983; Pechadre 1991; Manaka 1992). Temkin 1990 was the only trial that did not have a high risk of bias in any category. The Temkin 1990 trial favored the placebo group (RR 1.25, 95% CI 0.79 to 1.96, 323 participants; Analysis 1.18), which differs from the original analysis, which favored the AED treatment (see Analysis 1.2). However, neither comparison was statistically significant.
1.13 Subgroup analysis
All‐cause mortality: age of population
Two studies examined all‐cause mortality in children only (ages under 17 years) (Young 1983; Young 2004). The pooled proportion of children that died in the AED treatment group was 8.5% (8/71) and 22.2% (16/72) died in the placebo group (RR 0.54, 95% CI 0.25 to 1.19, 143 participants; Analysis 1.13). Temkin 1990 was the only study that exclusively enrolled participants over 17 years of age. About 23.5% (49/208) of adults treated with AED died compared with 20.1% (41/196) of adults receiving placebo. The pooled RR was 1.13 (95% CI 0.78 to 1.62, 404 participants; Analysis 1.13). The remaining two studies examined all‐cause mortality in a predominantly adult population; although children were included (Glotzner 1983;McQueen 1983). The pooled proportion that died in the AED treatment group was 20.1% (32/159) and 14.1% (22/156) died in the placebo group (RR 1.43, 95% CI 0.90 to 2.27, 315 participants; Analysis 1.13). The studies including exclusively or predominately adults showed an increased risk of mortality in the AED group compared with placebo (Glotzner 1983; McQueen 1983; Temkin 1990), while the studies including only children showed a decreased risk with treatment. The test for subgroup differences showed moderate heterogeneity (P=0.11, I2 =53.9%).
1.14 Subgroup analysis
All‐cause mortality: treatment duration
Two of the five trials examining all‐cause mortality had a short‐term treatment duration of less than one week (Young 1983; Young 2004). 9.9% (18/182) of participants treated with an AED for one week or less died compared with 15.2% (25/164) of participants in the control groups. The pooled RR for these short‐term treatments was non‐significant and favored AED treatment (RR 0.69, 95% CI 0.39 to 1.24, 346 participants; Analysis 1.14). In comparison, the three trials that used a treatment duration of a 12 months or longer had a non‐significant pooled RR that favored the control group; 22.1% (81/367) of participants in the AED group died compared with 17.9% (63/352) of participants in the control groups (RR 1.24, (95% CI 0.93 to 1.65, 719 participants; Analysis 1.14) (Glotzner 1983; McQueen 1983; Temkin 1990). The test for subgroup differences between studies of different treatment duration was statistically significant and suggested moderate heterogeneity (P=0.08, I2 =67.4%). Duration of treatment was not further divided into mid‐term and long‐term duration due to low number of studies.
1.15 Sensitivity analysis
All‐cause mortality: type of antiepileptic drug
In four of the five trials that examined mortality, participants received phenytoin in the AED group (McQueen 1983; Young 1983; Temkin 1990; Young 2004). Glotzner 1983 compared carbamazepine with placebo. Excluding Glotzner 1983, 15.6% (74/474) of participants treated with phenytoin died compared with 15.9% (70/440) of participants who received placebo (RR 0.97, 95% CI 0.65 to 1.43, 914 participants; Analysis 1.15). The results remain consistent with the original analysis (see Analysis 1.3).
1.16 Sensitivity analysis
All‐cause mortality: study quality
Three of the five trials that examined mortality had a high risk of bias in one or more bias categories (Glotzner 1983; McQueen 1983; Young 1983). We reran the analysis excluding these studies. The pooled results for the remaining studies with low/unclear risk of bias showed no statistically significant difference between treatment groups ((RR 1.00, 95% CI 0.72 to 1.41) 506 participants; Analysis 1.16) (Temkin 1990; Young 2004). The original results were also statistically non‐significant, but favored the placebo group (see Analysis 1.3).
2. Neuroprotective agent versus placebo
Only one study compared a pharmacologic agent (magnesium sulfate; MgSO4) other than an AED with a placebo (Temkin 2007).
2.1 Occurrence of early seizure
Temkin 2007 reported on the occurrence of early seizures. About 0.4% (1/250) of participants in the neuroprotective agent group experienced an early seizure compared with 0% (0/249) in the placebo group (Analysis 2.1). However, as reported in the results section of their paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate.
2.2 Occurrence of late seizure
Temkin 2007 reported on the occurrence of late seizures. About 6% (15/250) of participants treated with neuroprotective agent experienced late seizures compared with 5.6% (14/249) of participants treated with a placebo (RR 1.07, 95% CI 0.53 to 2.17, 498 participants; Analysis 2.2). There was no evidence of effect of neuroprotective agents compared with placebo on late seizures.
2.3 All‐cause mortality
Only Temkin 2007 reported mortality. About 21% (52/250) of participants died in the neuroprotective agent group compared with 14% (35/240) of participants in the control group (RR 1.20, 95% CI 0.80 to 1.81, 466 participants; Analysis 2.3).
3. Antiepileptic drugs versus other antiepileptic drugs
Two trials compared phenytoin with another AED (Temkin 1999; Szaflarski 2010). Szaflarski 2010 compared phenytoin with levetiracetam, a newly licensed AED while Temkin 1999 compared phenytoin with valproate. Treatment duration was one week in the Szaflarski 2010 trial compared with up to six months in the valproate arm of the Temkin 1999 study. The age ranges were similar in both studies and neither study showed high bias in any category. We performed no subgroup analysis due to too few studies and low evidence of heterogeneity between the two studies.
3.1 Occurrence of early seizure
Szaflarski 2010 and Temkin 1999 reported on the occurrence of early seizures and compared phenytoin with another AED. About 3.3% (5/150) of participants treated with phenytoin had an early seizure compared with 5.7% (16/281) of participants treated with another AED. The pooled results of Szaflarski 2010 and Temkin 1999 showed no statistically significant effect of phenytoin compared with another AED (RR 0.66, 95% CI 0.20 to 2.12, 558 participants; Analysis 3.1). Heterogeneity between the two studies was low (I2= 29%).
3.2 Occurrence of late seizure
Szaflarski 2010 and Temkin 1999 reported on the occurrence of late seizures and compared phenytoin with another AED drug. About 12.4% (17/137) of participants treated with phenytoin experienced late seizures compared with 16.6% (40/241) of participants treated with another AED. The pooled RR of experiencing late seizures on phenytoin compared with another AED was not statistically significant (RR 0.77, 95% CI 0.46 to 1.30, 378 participants; Analysis 3.2). Heterogeneity between the two studies was low (I2 = 0%).
3.3 All‐cause mortality
Szaflarski 2010 and Temkin 1999 reported all‐cause mortality. About 8.7% (13/150) of participants in the phenytoin group died compared with 16.4% (46/281) of participants in the other AED group. The pooled RR for mortality in the phenytoin group compared with the other AED group was 0.53 (95% CI 0.30 to 0.94; Analysis 3.3). Heterogeneity between the two studies was low (I2 = 0%). We performed no subgroup analysis due to too few studies and low evidence of heterogeneity.
Discussion
Summary of main results
The review included 10 RCTs described in 12 reports, involving 2326 participants. Interventions were reported in three categories; traditional AED versus placebo or usual care, phenytoin versus other AED treatment, and alternative neuroprotective agent versus placebo or usual care.
Five studies with 987 participants studied early seizure in participants treated with a traditional AED compared with placebo or usual care. There was low quality evidence that treatment with a traditional AED (phenytoin or carbamazepine) decreased the risk of early seizure compared with placebo or usual care (RR 0.42, 95% CI 0.23 to 0.73, P value = 0.003).
The risk of late seizure occurrence was reduced by AED treatment compared with placebo or usual care, although the benefit was not statistically significant (RR 0.91, 95% CI 0.57 to 1.46, 1029 participants). The risk of late seizure favored placebo in the only trial that did not have a high risk of bias in any category (RR 1.25, 95% 0.79 to 1.96, 323 participants), although evidence of effect remained non‐significant (Temkin 1990). Caution should be taking when considering this sensitivity analysis as it was based on only one study.
There was no significant difference in mortality between participants in the AED drug and participants in the placebo or usual care group (RR 1.08, 95% CI 0.79 to 1.46, P value = 0.64) although the results were based on very low quality of evidence due to imprecision and inconsistency in results.
The review included only one study that examined other potentially neuroprotective agents compared with placebo or usual care (Temkin 2007). There was no evidence of treatment effect on late seizures (RR 1.07, 95% CI 0.53 to 2.17) or all‐cause mortality (RR 1.20, 95% CI 0.80 to 1.81) for this comparison. There were no events in the placebo arm for the outcome of early seizure and a rate of 0.4% (1/250) early seizures in the treatment group. However, almost all participants (96%) in this study also received phenytoin for the first week following injury. No doses or details on phenytoin treatment levels were provided in the paper.
There was evidence of treatment benefit of phenytoin in comparison to another AED (levetiracetam or valproate). Phenytoin significantly reduced the risk of mortality compared with another AED (RR 0.53, 95% CI 0.30 to 0.94). Caution must be taken as this result was based on only two studies. No treatment benefit of phenytoin was observed compared with another AED for early seizure (RR 0.66, 95% CI 0.20 to 2.12) or late seizure (RR 0.77, 95% CI 0.46 to 1.3).
Only two of the included trials reported any serious treatment‐related adverse event. Both trials compared a traditional AED with placebo. There was no evidence of increased risk of adverse effects for the AED group (RR 1.63, 95% CI 0.73 to 3.66). Similarly there was no evidence of increased risk of skin rash (RR 1.65, 99% CI 0.54 to 5.04).
Overall completeness and applicability of evidence
All participants included in the review had a diagnosis of moderate‐to‐severe TBI. The methods of measurement of severity of TBI varied between studies. The majority of participants were admitted to trauma centers or emergency departments. Participants were randomized and received treatment within 24 hours of admission; however, one study reported that the majority of participants were treated within 14 days post‐injury. Three studies allowed the inclusion of participants with an immediate post‐injury seizure, while other studies listed this as an exclusion criteria. One study included participants if they had a pre‐injury seizure; however, these participants were excluded from outcomes of early and late seizure outcomes. Reporting of outcomes was not consistent across the studies with only two studies reporting any serious adverse event and skin rashes. Two studies also did not document mortality and the majority of studies did not consider time to first seizure or time to second seizure from first seizure. Maintaining therapeutic levels of AED was a challenge in many of the trials with reports of only 40% to 80% of participants maintaining therapeutic levels at follow‐up visits. Several studies did not follow the participants beyond the treatment duration, thereby limiting the ability to determine if the treatment effect is sustained once mediation is stopped.
Quality of the evidence
Overall quality of the evidence was varied. All included trials were RCTs yet the majority did not adequately describe the randomization and allocation processes clearly. The majority adequately blinded participants and personnel, but blinding of outcome assessment was considered either unclear or high in five of the 10 trials. Risk of bias of selective reporting was also unclear or high in six trials. Potential sources of bias in this review were: the inability to assess if treatment effects differed between children and adults adequately as children were included in many of the trials and not analyzed separately; and differing treatment protocols regarding timing and duration of treatment, AED dose, maintaining therapeutic levels of AEDs, differences in severity of trauma and different methods of evaluating seizure occurrence. Due to the high proportion of studies in this review that we categorized as unclear and high risk of bias, the findings in this review should be interpreted with caution.The evidence was graded as low to very low for all outcome comparisons based on the high risk of bias previously discussed as wells uncertainty in the estimates with many confidence intervals showing both harm and benefit.
Potential biases in the review process
The Cochrane Epilepsy Review Group conducted a comprehensive search of all published data as well as handsearching the bibliography of selected studies and reviews. We reviewed the full‐text reports and two review authors (KT and HA) extracted data and resolved disagreements by discussion to minimize bias. We were unable to obtain further information from some of the trials because they were published many years ago or the authors could not be contacted. We are unable to comment on the potential for publication bias in the review due to the insufficient number of studies to analyze publication bias in funnel plots.
Agreements and disagreements with other studies or reviews
This review differed from the original review by Schierhout 2001 in that we included a study looking at other potentially neuroprotective agents and studies with dual treatment groups. The comparison of traditional AEDs with placebo now includes a study carried out exclusively in children (Young 2004), which was not in the previous review. The results were consistent in both reviews, treatment with traditional AED reduced the risk of early seizure compared with placebo or usual care. When those studies with high bias in at least one category were removed, the evidence of treatment effect was no longer significant. However, heterogeneity among this subset of studies increased, potentially due to differences in treatment duration and median age of participants.
There was no evidence of treatment effect on late seizure occurrence or mortality. This result was consistent with the results for late seizure occurrence and mortality published in the previous review (Schierhout 2001).

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
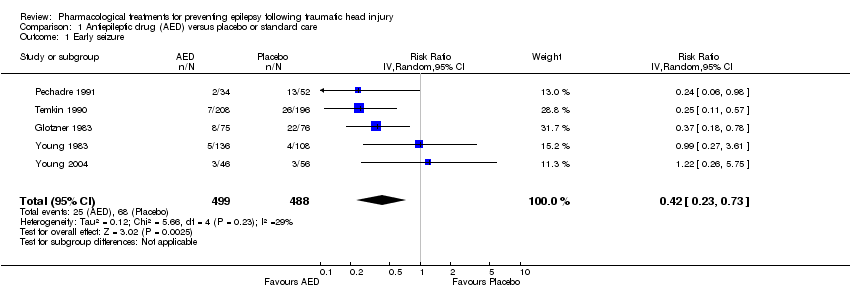
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 1 Early seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 2 Late seizure.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 3 All‐cause mortality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 4 Any serious event.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 5 Skin rash.
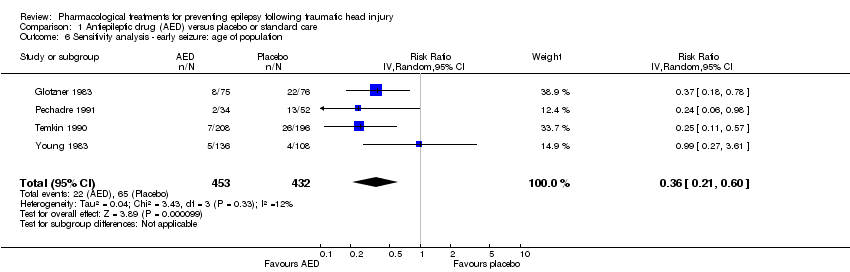
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 6 Sensitivity analysis ‐ early seizure: age of population.
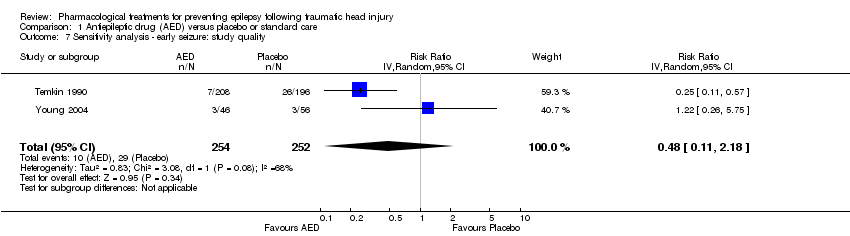
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 7 Sensitivity analysis ‐ early seizure: study quality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 8 Subgroup: late seizure: type of AED.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 9 Subgroup ‐ late seizure: treatment duration.
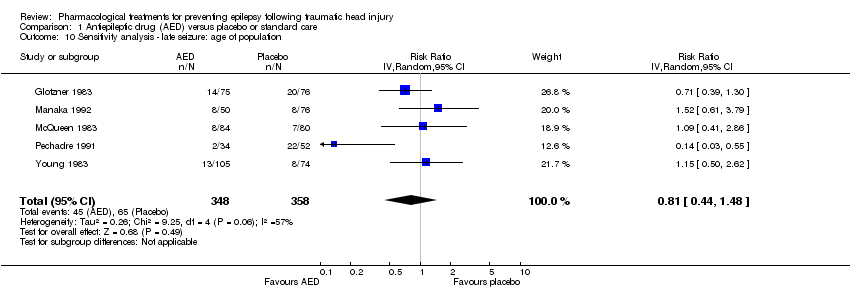
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 10 Sensitivity analysis ‐ late seizure: age of population.
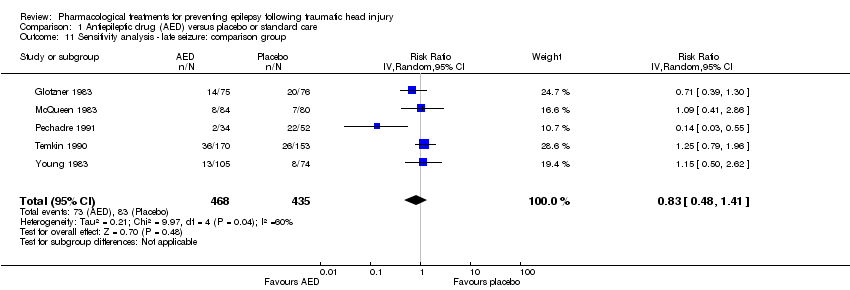
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 11 Sensitivity analysis ‐ late seizure: comparison group.
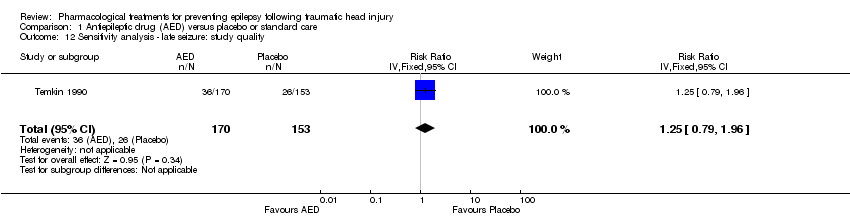
Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 12 Sensitivity analysis ‐ late seizure: study quality.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 13 Subgroup Analysis ‐ all‐cause mortality: age of population.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 14 Subgroup analysis ‐ all‐cause mortality: treatment duration.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 15 Sensitivity analysis ‐ all‐cause mortality: type of AED.

Comparison 1 Antiepileptic drug (AED) versus placebo or standard care, Outcome 16 Sensitivity analysis ‐ all‐cause mortality: study quality.

Comparison 2 Neuroprotective agent versus placebo, Outcome 1 Early seizure.
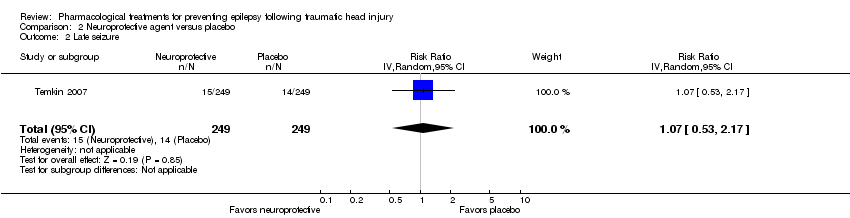
Comparison 2 Neuroprotective agent versus placebo, Outcome 2 Late seizure.
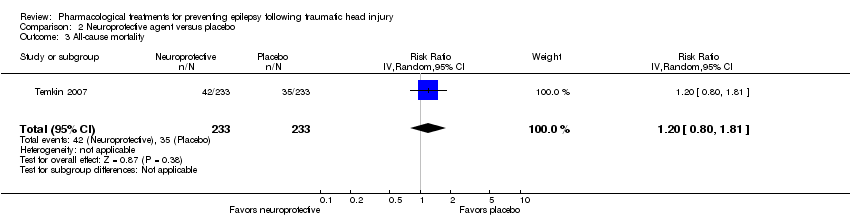
Comparison 2 Neuroprotective agent versus placebo, Outcome 3 All‐cause mortality.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 1 Early seizure.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 2 Late seizure.

Comparison 3 Antiepileptic drug (AED) versus other AED, Outcome 3 All‐cause mortality.
| Antiepileptic drugs compared with placebo or standard care for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or standard care | Antiepilepticdrugs | |||||
| Early seizures | 139 per 1000 | 59 per 1000 | RR 0.42 | 987 | ⊕⊕⊝⊝ | Sensitivity analysis by quality of the study shows that RR for early seizures in low/unclear risk studies was no longer significant (RR 0.59, 95% CI 0.20 and 1.73) |
| Late seizures | 178 per 1000 | 162 per 1000 | RR 0.91 | 1029 | ⊕⊝⊝⊝ | RR of late seizures remained insignificant regardless of type of antiepileptic drug, treatment duration, age of population or quality of the study |
| All‐cause mortality | 174 per 1000 | 188 per 1000 | RR 1.08 | 1065 | ⊕⊝⊝⊝ | RR for all‐cause mortality remained insignificant regardless of treatment duration, age of population or quality of the study |
| Any serious adverse event of treatment count of events Follow up: 12 months | 94 per 1000 | 154 per 1000 (69 to 345) | RR 1.63 (0.73 to 3.66) | 568 (2 studies) | ⊕⊕⊝⊝ | |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: Two studies included in this outcome had instances of high risk of bias assessment. The remaining studies had a mix of low and unclear risk of bias. 5 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. 6 Downgraded one level due to serious risk of bias: selection bias was likely in both trials | ||||||
| Neuroprotective agents compared with placebo for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Neuroprotective agents | |||||
| Early seizure Count of events Follow‐up: 7 days | 0 per 1000 | 0 per 1000 | RR 2.99 (0.12 to 73.00) | 499 | ⊕⊕⊝⊝ | No events occurred in the control group therefore corresponding risk is also zero |
| Late seizure Count of events Follow‐up: 6 months | 56 per 1000 | 60 per 1000 | RR 1.07 (0.53 to 2.17) | 498 | ⊕⊕⊕⊕ | |
| All‐cause mortality Follow‐up: 6 months | 150 per 1000 | 180 per 1000 | RR 1.20 (0.80 to 1.81) | 466 | ⊕⊕⊕⊕ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias: As reported in the study paper, 96% of participants received phenytoin for the first week in both treatment groups. This may have resulted in a very low early seizure rate 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Anti‐epileptic drugs compared to other anti‐epileptic drugs for people at risk of epilepsy following traumatic head injury | ||||||
| Patient or population: people with traumatic head injuries | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other AEDs | Phenytoin | |||||
| Early seizure Counts of events Follow up: 7 days | 57 per 1000 | 38 per 1000 | RR 0.66 (0.20 to 2.12) | 431 | ⊕⊕⊝⊝ | |
| Late seizure Counts of events Follow up: 6 months to 2 years | 166 per 1000 | 128 per 1000 | RR 0.77 (0.46 to 1.30) | 378 | ⊕⊕⊕⊝ | |
| All‐cause mortality Follow up: 6 months to 2 years | 164 per 1000 | 87 per 1000 | RR 0.53 (0.30 to 94) | 431 | ⊕⊕⊕⊝ | |
| Any serious adverse event of treatment | See comment | See comment | Not estimable | 0 | See comment | No study reported adverse event data |
| Time to first seizure from randomization | See comment | See comment | Not estimable | 0 | See comment | No study reported time to first seizure in an interpretable way |
| *The basis for the assumed risk is the event rate in the control (placebo or standard care) group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to risk of bias; unclear information reported in one study regarding study design (randomisation and blinding) and loss to follow up from the study 2 Downgraded one level due to imprecision of results: wide 95% CI that includes both considerable harm and benefit. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 5 | 987 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.23, 0.73] |
| 2 Late seizure Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 3 All‐cause mortality Show forest plot | 5 | 1065 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.79, 1.46] |
| 4 Any serious event Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.73, 3.66] |
| 5 Skin rash Show forest plot | 2 | 568 | Risk Ratio (IV, Random, 99% CI) | 1.65 [0.54, 5.04] |
| 6 Sensitivity analysis ‐ early seizure: age of population Show forest plot | 4 | 885 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.21, 0.60] |
| 7 Sensitivity analysis ‐ early seizure: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.11, 2.18] |
| 8 Subgroup: late seizure: type of AED Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 8.1 Late seizure ‐ phenytoin | 4 | 752 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.40, 1.70] |
| 8.2 Late seizure ‐ other AED | 2 | 277 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.46, 1.99] |
| 9 Subgroup ‐ late seizure: treatment duration Show forest plot | 6 | 1029 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.57, 1.46] |
| 9.1 Long treatment duration | 5 | 943 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.81, 1.46] |
| 9.2 Short treatment duration | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.03, 0.55] |
| 10 Sensitivity analysis ‐ late seizure: age of population Show forest plot | 5 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.44, 1.48] |
| 11 Sensitivity analysis ‐ late seizure: comparison group Show forest plot | 5 | 903 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.48, 1.41] |
| 12 Sensitivity analysis ‐ late seizure: study quality Show forest plot | 1 | 323 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.79, 1.96] |
| 13 Subgroup Analysis ‐ all‐cause mortality: age of population Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13.1 All‐cause mortality ‐ children only | 2 | 143 | Risk Ratio (IV, Random, 95% CI) | 0.54 [0.25, 1.19] |
| 13.2 All‐cause mortality ‐ adults and children | 2 | 315 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.90, 2.27] |
| 13.3 All‐cause mortality ‐ adults only | 1 | 404 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.78, 1.62] |
| 14 Subgroup analysis ‐ all‐cause mortality: treatment duration Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 14.1 All‐cause mortality ‐ short‐term treatment duration | 2 | 346 | Risk Ratio (IV, Random, 95% CI) | 0.69 [0.39, 1.24] |
| 14.2 All‐cause mortality ‐ long‐term treatment duration | 3 | 719 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.93, 1.65] |
| 15 Sensitivity analysis ‐ all‐cause mortality: type of AED Show forest plot | 4 | 914 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.65, 1.43] |
| 16 Sensitivity analysis ‐ all‐cause mortality: study quality Show forest plot | 2 | 506 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.72, 1.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Late seizure Show forest plot | 1 | 498 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.53, 2.17] |
| 3 All‐cause mortality Show forest plot | 1 | 466 | Risk Ratio (IV, Random, 95% CI) | 1.2 [0.80, 1.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early seizure Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.20, 2.12] |
| 2 Late seizure Show forest plot | 2 | 378 | Risk Ratio (IV, Random, 95% CI) | 0.77 [0.46, 1.30] |
| 3 All‐cause mortality Show forest plot | 2 | 431 | Risk Ratio (IV, Random, 95% CI) | 0.53 [0.30, 0.94] |

