Fotoféresis extracorpórea versus tratamiento alternativo para la enfermedad injerto contra huésped crónica posterior al trasplante de células madre hematopoyéticas en niños y adolescentes
Resumen
Antecedentes
La enfermedad injerto contra huésped (EIcH) crónica es una causa importante de morbilidad y mortalidad tras el trasplante de células madre hematopoyéticas, que se da en entre el 6% y el 65% de los receptores. Actualmente, la base terapéutica para la EIcH es el tratamiento con corticosteroides, combinados con frecuencia con otros agentes inmunosupresores en pacientes con manifestaciones de que no responden a los corticoides. No existe un tratamiento estándar establecido para la EIcH que no responde a los corticosteroides. Las opciones terapéuticas para estos pacientes incluyen la fotoféresis extracorpórea (FEC), un tratamiento inmunorregulador que incluye la obtención “ex vivo” de células mononucleares de la sangre periférica, la exposición al agente fotoactivo 8‐metoxipsoraleno, la radiación ultravioleta y la readministración del producto celular procesado. Los mecanismos de acción de la FEC no se comprenden por completo. Esta es la segunda actualización de una revisión Cochrane publicada por primera vez en 2014 y actualizada por primera vez en 2015.
Objetivos
Evaluar la efectividad y la seguridad de la FEC para el tratamiento de la EIcH en niños y adolescentes después del trasplante de células madre hematopoyéticas.
Métodos de búsqueda
Se hicieron búsquedas en las bases de datos Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials; CENTRAL) (2021), MEDLINE (PubMed) y Embase desde su inicio hasta el 25 de enero de 2021. Se hicieron búsquedas en las listas de referencias de los estudios potencialmente relevantes, sin limitaciones de idioma. Se realizaron búsquedas en cinco resúmenes de congresos y en nueve registros de ensayos clínicos el 9 de noviembre de 2020 y el 12 de noviembre de 2020, respectivamente.
Criterios de selección
El objetivo fue incluir ensayos controlados aleatorizados (ECA) que compararan FEC con o sin tratamiento alternativo versus tratamiento alternativo solo en niños y adolescentes con EIcH después del trasplante de células madre hematopoyéticas.
Obtención y análisis de los datos
Dos autores de la revisión realizaron de forma independiente la selección de los estudios. Las discrepancias en la selección de los ensayos se resolvieron mediante consulta con un tercer autor de la revisión.
Resultados principales
No se encontraron estudios que cumplieran los criterios de inclusión para la actualización de la revisión de 2021.
Conclusiones de los autores
No se pudo evaluar la eficacia de la FEC en el tratamiento de la EICH en niños y adolescentes después de un trasplante de células madre hematopoyéticas, ya que la segunda actualización de la revisión tampoco encontró ningún ECA. Las recomendaciones actuales se basan solamente en estudios retrospectivos u observacionales. Por lo tanto, idóneamente la FEC se debe aplicar solamente en el contexto de ensayos controlados. Sin embargo, llevar a cabo ECA en esta población será un desafío debido al número limitado de participantes aptos, el cuadro clínico variable de la enfermedad y la falta de criterios de respuesta definidos. Se precisará colaboración internacional, ensayos multicéntricos y financiación apropiada para dichos ensayos. Si se toman decisiones terapéuticas de acuerdo con datos clínicos a favor de la FEC, se deberá monitorizar con cautela a los receptores con respecto a los posibles efectos beneficiosos y perjudiciales. Además, se deben hacer esfuerzos para compartir esta información con otros médicos, por ejemplo, creando registros para los niños y adolescentes tratados con FEC.
PICO
Resumen en términos sencillos
Fotoféresis extracorpórea para la enfermedad injerto contra huésped crónica posterior al trasplante de células madre hematopoyéticas en niños y adolescentes
Antecedentes
La enfermedad de injerto contra huésped crónica (EIcHc) es una complicación frecuente después del trasplante de células madre hematopoyéticas (TCMH; trasplante de células madre que forman sangre). Las células inmunitarias (glóbulos blancos) del donante reconocen a las células del receptor como extrañas ("no propias"). Por lo tanto, las células inmunitarias trasplantadas atacan a las células del receptor. Los principales órganos afectados son la piel, el hígado y los intestinos, entre otros. Estas reacciones inmunitarias pueden causar una inflamación aguda (hinchazón repentina) seguida de cambios crónicos (a largo plazo) en órganos (p.ej. fibrosis, cicatrización de los pulmones). El tratamiento de primera línea suele consistir en medicamentos inmunosupresores (que reducen la potencia del sistema inmunitario del cuerpo) en forma de corticoides, en combinación con otros agentes inmunosupresores en los casos refractarios (en los que la enfermedad es resistente al tratamiento). Estos fármacos se supone que suprimen el ataque mediado por la reacción inmunitaria de las células del paciente. La efectividad limitada y los efectos secundarios graves de estos medicamentos han dado lugar a varios enfoques alternativos.
La fotoféresis extracorpórea (FEC) es un tratamiento inmunorregulador (un tratamiento que realiza cambios en la función inmunitaria) que incluye la extracción de células inmunitarias de sangre periférica del cuerpo del paciente. Estas células inmunitarias se exponen a un agente fotoactivo (una sustancia química que responde a la exposición a la luz; p.ej. el 8‐metoxipsoraleno), con la subsiguiente radiación ultravioleta‐A, y luego se reinyectan. No existe seguridad acerca de cómo afecta esto a la función inmunitaria. Varias recomendaciones actuales para la práctica clínica indican que se debe considerar la FEC en niños y adolescentes con EIcHc.
Características de los estudios
Se buscaron ensayos controlados aleatorizados (ECA) (estudios clínicos en los que los pacientes se asignan al azar a uno de dos o más grupos de tratamiento) en las bases de datos científicas diseñados para evaluar la efectividad y la seguridad de la FEC en el tratamiento de la enfermedad injerto contra huésped crónica en niños y adolescentes (menos de 18 años de edad) después del TCMH.
Resultados
No se encontró ningún ECA que analizara la eficacia de la FEC para los niños y adolescentes con EIcHc después del TCMH. Las recomendaciones actuales solo se basan en estudios retrospectivos (cuyos desenlaces se produjeron en los pacientes antes del comienzo del estudio) o estudios observacionales (en los que los investigadores no intervienen y sencillamente observan el desarrollo de los eventos). Lo idóneo es que la FEC solo se administre a niños y adolescentes en el contexto de ECA. La FEC podría tenerse en cuenta en personas con EIcHc refractaria a los corticoides, sin olvidar que este tratamiento no está fundamentado en evidencia de nivel alto. Si se administra FEC a niños y adolescentes, los médicos deben recopilar información sobre los efectos beneficiosos y perjudiciales y crear registros de las personas tratadas con FEC.
Authors' conclusions
Summary of findings
| Extracorporeal photopheresis (ECP) compared with alternative treatment for chronic graft‐versus‐host disease | ||||||
| Patient or population: children and adolescents with chronic graft‐versus‐host disease after haematopoietic stem cell transplantation Settings: paediatric hospitals worldwide Intervention: ECP alone or in combination with standard treatment Comparison: alternative treatment alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk without ECP | Risk with ECP | |||||
| Response | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Overall survival | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Failure‐free survival | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Adverse events | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Quality of life | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Cost of intervention per month | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% confidence interval). | ||||||
| GRADE Working Group grades of evidence | ||||||
Background
Description of the condition
Haematopoietic stem cell transplantation (HSCT) is a curative treatment option for children with haematological malignancies, haemoglobinopathies, immune deficiencies, inborn errors of metabolism and autoimmune diseases (Diaconescu 2005; Gaziev 2010; Greco 2019; Kennedy‐Nasser 2006; Peters 2003; Rappeport 2011; Walters 2000; Walters 2005). Chronic graft‐versus‐host disease (cGvHD) is considered one of the major complications following HSCT, limiting its wider application (Billingham 1966; Ferrara 2004; Sullivan 2004). cGvHD was traditionally defined by manifestation after 100 days following HSCT (Gilman 2000). Several advances in the practice of HSCT (including different haematopoietic stem cell sources, intensity of conditioning regimen, immunosuppression and donor lymphocyte infusions) resulted in a more time‐variable presentation of cGvHD (Bunin 2008; Eapen 2004). Therefore, cGvHD is described according to its pattern of presentation: following HSCT as a progression from acute graft‐versus‐host disease (aGvHD) (progressive form); as a recurrence after a disease‐free interval (quiescent form); and without a history of aGvHD (de novo form) (Shulman 1988). The US National Institutes of Health (NIH) developed a diagnosis and scoring system defining cGvHD based on specific clinical signs and histopathology rather than time of onset after HSCT (Filipovich 2005; Jagasia 2009). The NIH global severity score uses a numerical scoring system for individual organs to calculate a summary scale according to the number and severity of organs involved. Consequently, cGvHD is categorised as mild, moderate or severe (Filipovich 2005). This provides a more clinically informative and discriminating severity measure for use in clinical trials, and is an indicator for the necessity of systemic immunosuppressive treatment (Arai 2010). The NIH criteria for diagnosis of cGvHD were refined in 2014 and are still recommended to be used in clinical trials (Jagasia 2015; Schoemans 2018).
The incidence of cGvHD in the paediatric population varies between 6% and 65%, depending on the transplant procedure and disease‐specific variables (Baird 2010; Meisel 2007; Rocha 2000; Zecca 2002). Well‐known risk factors for developing cGvHD are precedent aGvHD, stem cell donor, the preparative regimen, prophylactic procedures and the underlying disease (Flowers 2011; Rocha 2000; Zecca 2002; Zeiser 2017). The exact pathogenesis of cGvHD remains unclear. Several studies have demonstrated the role of T cells in the development of cGvHD (De Bueger 1993; Higman 2004; Mutis 1999). Increased levels of non‐specific antibodies in people with cGvHD and response to B‐cell‐depleting antibodies suggest that B cells also play a role (Allan 2007; Zhang 2006). Moreover, a co‐ordinated T‐cell B‐cell interaction to minor histocompatibility alloantigens seems to account for the manifestation of cGvHD (Miklos 2005). Soluble inflammation‐associated factors are also involved in the pathogenesis of cGvHD (Fujii 2008; Lin 2003). Other pathogenetic suggestions include a defective negative selection of autoreactive T cells due to thymic damage, aberrant production of transforming growth factor‐beta and activation of the platelet‐derived growth factor receptor, as well as deficiency of CD8+ cells (Martin 2008; Toubai 2008; Zeiser 2017). Clinical manifestations of cGvHD are separated into rather inflammatory, acute type features (erythematous rash, mucositis, diarrhoea, transaminitis and pulmonary infiltrates) as opposed to more fibrotic, chronic characteristics (sclerotic and lichen planus‐like skin changes, fasciitis, sicca syndrome, oesophageal strictures and bronchiolitis obliterans) (Flowers 2002; Higman 2004). The most commonly affected organs, isolated or in combination, are the skin and oral cavity, with over 70% involvement for both, followed by eyes, liver, lungs, gastrointestinal tract and musculoskeletal system (Atkinson 1990; Beredjiklian 1998; Chosidow 1992; Cooke 2009; Dudek 2003; Filipovich 2005; Janin 1994; Lee 2003; Melin‐Aldana 2007; Pavletic 2005; Sullivan 1981; Treister 2005). While aGvHD mainly affects the skin, liver and gastrointestinal tract, cGvHD may involve every organ system (Baird 2010; Filipovich 2005; Zeiser 2017).
Both cGvHD and treatment of cGvHD lead to significant morbidity and mortality in children. The leading cause of death in affected children is life‐threatening infection due to long‐term immunosuppressive treatment (Baird 2010; Inagaki 2015; Vogelsang 2001). Only a few studies have evaluated the long‐term outcome in the paediatric population with cGvHD (Inagaki 2015; Jacobsohn 2011; Zecca 2002). Among these, a larger study involving children and adolescents with cGvHD reported a cumulative non‐relapse mortality of 15.8%, 18.1% and 24.4% at 5, 10 and 15 years following diagnosis of cGvHD. In the presence of severe cGvHD, the 10‐year cumulative non‐relapse mortality was significantly higher (35%) compared to mild (3.8%) or moderate cGvHD (4.4%) (Inagaki 2015). In order to control cGvHD, more than 50% of children and adolescents require systemic immunosuppressive therapy for more than one year (Jacobsohn 2011).
Management options
Since aGvHD also has a significant impact on the pathogenesis of cGvHD, prophylaxis to prevent graft‐versus‐host disease (GvHD) plays a major role (Ram 2009). Prevention is based on T‐cell inhibition including the following strategies: inhibition of T‐cell activation and function (calcineurin inhibitor), inhibition of T‐cell proliferation (methotrexate, mycophenolate mofetil) and elimination of T cells (alemtuzumab, anti‐thymoglobulin) (Shah 2007; Storb 1986). The majority of children undergoing HSCT receive GvHD prophylaxis, starting before transplantation and typically continuing for up to six months after transplantation (Barrett 2008).
The clinical management of people with cGvHD is complicated due to the variability of disease manifestation, clinical course, infectious complications and treatment‐related toxicity (Flowers 2002). Therefore, treatment of cGvHD in children is highly variable, and, due to lack of paediatric studies, mostly based on the experience in adults. In people with mild cGvHD and high‐risk malignancies where a graft‐versus‐leukaemia effect gains importance, topical therapy should be considered (Filipovich 2005). Drug therapy for mild cGvHD mostly includes locally applied immunosuppressive agents (steroids and calcineurin inhibitors) for the affected organ (skin, eyes, oral cavity and lungs) (Jacobsohn 2010). In contrast, moderate‐to‐severe cGvHD is mostly treated with systemic steroids (Filipovich 2005; Salmasian 2010). Prednisone is usually tapered (alternate day) for the next two to three months (Sullivan 1988a). Some reports suggest better response rates and fewer corticosteroid complications when adding a calcineurin inhibitor (ciclosporin, tacrolimus) to systemic steroids (Koc 2002; Sullivan 1988b; Vogelsang 2001). Regarding the effect of corticosteroids on the growth and development of children and the mean duration of therapy of three years for people with cGvHD, the management of a daily calcineurin inhibitor with alternate day prednisone is considered standard for the treatment of cGvHD (Koc 2002; Sullivan 1988a). This therapeutic regimen yielded an objective response in more than 50% of people (Sullivan 1988a; Sullivan 1988b).
In cases where there is no response to therapy within four weeks, second‐line treatment is mostly considered (Jacobsohn 2010). There are various salvage therapies, including general immunosuppressants (mycophenolate mofetil, azathioprine, methotrexate, sirolimus, thalidomide, pentostatin and hydroxychloroquine), monoclonal antibodies (rituximab, tocilizumab), kinase inhibitors (ibratunib, imatinib, ruxolitinib) and proteasome inhibitors (bortezomib) (Akpek 2001; Bolanos‐Meade 2008; Busca 2000; Cutler 2006; Dignan 2012; Fraser 2007; Gilman 2006; Goldberg 2003; Jacobsohn 2009; Johnston 2005; Lopez 2005; Martin 2009; Mookerjee 1999; Vogelsang 1992; Zeiser 2017).
Description of the intervention
Extracorporeal photopheresis (ECP), an immunomodulatory therapy, may play a role in the treatment of cGvHD. During ECP, peripheral mononuclear cells are collected ex vivo by leukapheresis, incubated with the photoactive and photosensitising drug 8‐methoxypsoralen (8‐MOP), exposed to ultraviolet‐A (UV‐A) light and then re‐infused into the patient without any adverse effects to other organs (Bethea 1999; Girardi 2002; Heald 1989). Psoralen occurs naturally in the seeds of the furocoumarin family of plants and its exposure to UV‐A light (wavelength 200 to 350 nm) facilitates the intercalation of psoralen with deoxyribonucleic acid (DNA), leading to the formation of both monofunctional and bifunctional adducts, which results in programmed cell death (apoptosis) of the majority of cells (Yoo 1996). Initially, the patients received psoralen orally prior to the leukapheresis (Bethea 1999). However, oral application results in an inconstant absorption of the drug and considerable gastrointestinal adverse effects (Brickl 1984). The now generally used ex vivo method, with the incubation of the collected cells in a bag, significantly reduces the exposure of the patient to 8‐MOP (Schooneman 2003).
ECP has been successfully applied in the treatment of cutaneous T‐cell lymphoma since the 1980s (Edelson 1987). Following this observation, the method has been implemented for a wider spectrum of immunologically mediated diseases such as systemic scleroderma, autoimmune disorders, solid organ rejection, aGvHD and cGvHD (Szodoray 2010). In the paediatric setting, many authors report a response rate of 33% to 93% to ECP as second‐line treatment in steroid‐resistant cGvHD (Foss 2005; Messina 2003; Smith 1998; Sniecinski 1994). Overall, the response rates differ with regard to the organs affected by cGvHD, with most favourable results in skin (74%), oral mucosal (72%) and liver (68%) involvement (Malik 2014). People with steroid‐refractory cGvHD who respond to ECP have a higher five‐year survival rate compared with non‐responders (96% with responders versus 85% with non‐responders) (Perotti 2010). Adverse reactions are uncommon (less than 0.003%), transient and mild (nausea, hypotension, dizziness, cytopenia, skin infection at site of venous access and abnormal clotting to heparin) (Kanold 2003). Moreover, ECP is not associated with increased risk of systemic infections and relapse of malignant disease (Dall'Amico 2002; Hackstein 2009; Scarisbrick 2008).
How the intervention might work
The mechanisms of action of ECP are not completely understood. It has been shown that the procedure induces apoptosis in mononuclear white blood cells (Voss 2010). However, only a small percentage of the peripheral mononuclear cells is treated and, therefore, an immunomodulatory effect of the apoptotic cells is hypothesised (Heshmati 2003). One suspected mechanism is that apoptotic T‐cell fragments presented by dendritic cells induce an anti‐idiotypic T‐suppressor activity, or down‐regulate a pre‐existing T‐cell response, and in this way generate a tolerogenic response and modulate cytokine production (Bladon 2006; Legitimo 2007; Xia 2009). In summary, the postulated mechanisms involved include: reduced stimulation of effector T cells, deletion of effector T cells, induction of regulatory T cells, increase of anti‐inflammatory cytokines (i.e. tumour necrosis factor‐beta, interleukin‐10), and reduction of proinflammatory cytokines (interleukin‐1beta, interleukin‐6, and tumour necrosis factor‐alpha) (Fimiani 2004). On the basis of this hypothesis, photopheresis seems to down‐regulate the T‐cell alloreactivity that plays the central role in the pathogenesis of GvHD after HSCT (Lamioni 2005; Maeda 2005).
Why it is important to do this review
cGvHD remains one of the major challenges for transplant‐related morbidity and mortality after stem cell transplantation in children and adolescents. All conventional therapies, including established first‐line therapy, have considerable adverse effects and probably increase the risk of infections and relapse of malignant disease. Therefore, it is essential to develop new therapeutic approaches for the selective immune control of cGvHD without generalised immunosuppression‐related complications (infections and pharmacological toxicity issues) (Wolff 2011). ECP seems to be an effective immunomodulatory therapy with very mild, if any, adverse effects and, therefore, may be a promising alternative for improving morbidity and mortality in children with cGvHD.
The aim of the second update of this systematic review, including its protocol (Weitz 2012), its initial review (Weitz 2014) and its first update (Weitz 2015), was to identify new RCTs evaluating the efficacy and safety of ECP in children and adolescents with cGvHD.
Objectives
To evaluate the effectiveness and safety of ECP for the management of cGvHD in children and adolescents after haematopoietic stem cell transplantation.
Methods
Criteria for considering studies for this review
Types of studies
We intended to consider randomised controlled trials (RCTs) for this review if they assessed any clinical outcome as described in the Types of outcome measures section. For medical reasons (e.g. we did not consider cGvHD a stable condition), we aimed to include only trials with a parallel group design. We excluded studies restricted to adults (≥ 18 years of age). For studies including both children and adults, we planned to include studies if children represented more than 50% of participants in the study.
Types of participants
We planned to include children and adolescents under 18 years of age who underwent HSCT therapy with presence of cGvHD, independent of the underlying disease and donor source. We planned to consider all stages and grades of cGvHD, independent of the type of organ involvement.
Types of interventions
For the purpose of this review, we considered systemic steroids with or without calcineurin inhibitors as standard treatment as first‐line therapy and considered all other treatment as second‐line therapy of cGvHD.
The following comparisons for ECP for cGvHD after HSCT were conceivable:
-
ECP versus standard treatment in children and adolescents with cGvHD as first‐line treatment.
-
ECP plus standard treatment versus standard treatment alone in children and adolescents with cGvHD as first‐line treatment.
-
ECP versus standard treatment in children and adolescents with steroid‐/calcineurin‐inhibitor‐refractory cGvHD (second‐line treatment).
-
ECP plus standard treatment versus standard treatment alone in children and adolescents with steroid‐/calcineurin‐inhibitor‐refractory cGvHD (second‐line treatment).
These comparisons constituted four separate groups, and we anticipated analysing them separately.
Types of outcome measures
Primary outcomes
-
Response to ECP treatment (defined as either classical response rates (i.e. number of people in complete or partial remission) or percentage of achieved reduction in either NIH score (Filipovich 2005; Jagasia 2009; Jagasia 2015; Schoemans 2018), scales of Akpek and Lee (Akpek 2001; Lee 2003), or steroid‐tapering under therapy with ECP (defined as number of people with at least 25% reduction in steroid dose).
Secondary outcomes
-
Overall survival (defined as the time to death from any cause, starting at the day of HSCT).
-
Failure‐free survival (defined as progression of GvHD, expressed as change in NIH score or intensification of treatment, or both).
-
Adverse events.
-
Quality of life.
-
Cost of intervention per month including length of hospital stay in days, number of outpatient attendances, direct medical resource use, direct medical costs, indirect medical resource use or costs and patient out‐of‐pocket expenses.
Search methods for identification of studies
Electronic searches
For the original review, we carried out an electronic search for the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 9, 2012), MEDLINE/PubMed (from 1945 to 12 September 2012) and Embase (Ovid) (from 1980 to 12 September 2012). The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are displayed in the appendices (Appendix 1; Appendix 2; Appendix 3).
For the 2015 search update, we applied the same strategy without modifications to identify studies from the date of the last search to 23 September 2015.
Due to the extended time period of more than five years between the first and the second update we decided to repeat the full search for the 2021 update in CENTRAL (Issue 1, 2021), MEDLINE/PubMed (from 1945 to 25 January 2021) and Embase database (www.embase.com) (from 1980 to 25 January 2021). We only modified the search strategies for the Embase search according to the use of the embase.com platform for the second update instead of the Ovid platform used previously (Appendix 4).
Searching other resources
Conference proceedings:
For the original review, we also searched the conference proceedings mentioned below using the regular search with keyword terms ("extracorporeal"; "photopheresis"; "photochemotherapy"; "psoralen"; "graft‐versus‐host") on 12 September 2012.
To cover conference proceedings from after the latest search date, for the 2015 and 2021 update, we re‐ran the searches without modifications on 29 September 2015 and 9 November 2020, respectively. Overall, these searches comprised the conference proceedings of the following societies and time spans:
-
International Society for Paediatric Oncology (SIOP) (from 2007 to 2020);
-
American Society of Clinical Oncology (ASCO): Journal of Clinical Oncology (1995 to 2020);
-
American Society of Hematology (ASH): Blood (2001 to 2020);
-
American Society for Transplantation and Cellular Therapy, formerly American Society of Blood and Marrow Transplantation: Biology of Blood and Marrow Transplantation (1990 to 2020)
-
European Society for Blood and Marrow Transplantation, formerly European Group for Blood and Marrow Transplantation (EBMT): Bone Marrow Transplantation (2000 to 2020).
Clinical trials registries:
In addition, for the initial review, we searched the following clinical trials registries for ongoing or recently completed trials, and for locating potential links to other related databases and resources for the initial review on 12 September 2012.
For the 2015 update of this review, we repeated the search for clinical trials on 29 September 2015, and for the 2021 update, on 12 November 2020.
We performed a regular search with keywords ("extracorporeal"; "photopheresis"; "photochemotherapy"; "psoralen"; "graft‐versus‐host") where applicable, applied no time restrictions, and did not limit the search to trials involving children.
We carried out all searches within the following clinical trials registries:
-
International Standard Randomized Controlled Trial Number (ISRCTN) registry (controlled-trials.com/);
-
ClinicalTrials.gov (clinicaltrials.gov/);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/);
-
Trials Central (www.trialscentral.org/) (for the previous review versions: via 'condition': 'graft vs host disease'; for the second update: via 'condition': 'Hematologic Diseases');
-
Internet Portal of the German Clinical Trials Register (DRKS) (www.drks.de/);
-
NCIC Clinical Trials Group (www.ctg.queensu.ca/);
-
National Cancer Institute (www.cancer.gov/) (under heading 'childhood cancers' and 'cancers in childhood and adolescents' using the 'research' button in both the initial review (2014) and the first update (2015); searching with the keywords mentioned above in the 2021 update);
-
Australian New Zealand Clinical Trials Registry (ANZCTR) (www.anzctr.org.au/trialSearch.aspx).
Furthermore, for the 2021 review update we added a search in the following trials' registry:
-
European Union (EU) Clinical Trials Register (www.clinicaltrialsregister.eu).
Further resources:
We reviewed the reference lists of relevant articles and review articles in order to identify additional studies. For the original review and its update we also contacted two selected experts in the field to request information on unpublished trials evaluating ECP in cGvHD following HSCT.
Additional information regarding the searches:
We applied no language restrictions.
In the initial review and its first update, we listed educational reviews, non‐randomised controlled studies, case reports and case series as 'excluded studies' (Appendix 5 and Appendix 6, respectively). For this second update, we decided to update both the full search and the study selection process. Consequently, we removed all studies not meeting the inclusion criteria in the study selection process.
Data collection and analysis
Selection of studies
The original review was undertaken by four review authors (MW, BS, FM, DB). One review author (MW) screened all titles and abstracts of the references identified by the search strategies for relevance. We only excluded citations that were clearly irrelevant at this stage. We considered citations as irrelevant that included only adults, were animal studies, did not describe cGvHD or used stem cell sources other than haematopoietic. Two review authors (MW, DB) independently screened the remaining titles, excluded all irrelevant publications and recorded details of the studies together with the reasons for exclusion. We resolved any disagreement on the eligibility of studies through discussion and consensus. We obtained full‐text versions of all potentially relevant papers. Two review authors (MW, DB) independently screened these manuscripts, identified potentially relevant studies and assessed eligibility of studies for inclusion. We resolved any disagreements on the eligibility of studies through discussion and consensus. For the 2015 update, an additional author (MS) screened titles, abstracts and potentially relevant studies. For the 2021 update, three additional authors (MZ, SB, KB) screened titles and abstracts (SB, KB) and potentially relevant studies (MZ, KB).
Data extraction and management
We planned to extract data using a data extraction form developed by the review authors, and one of the review authors (MW) would transcribe the data into Review Manager 5.4.1 (previous versions in the initial review and its first update; RevMan 2020 for the second update in 2021). Another review author (JM for the initial review and its first update, KB for the second update) was to verify all data entry for discrepancies. We planned to resolve any disagreements on data extraction and management issues through discussion and consensus, or if necessary through a third review author (DB or BS). We intended to request missing data from the original investigators.
Two review authors (MW and JM for the original review and its first update, MZ and KB for this second update) would have completed the characteristics of included studies table. Study characteristics would have included place of publication, date of publication, population characteristics, setting, detailed nature of intervention, detailed nature of comparator and detailed nature of outcomes. A key purpose of these data was to define unexpected clinical heterogeneity in included studies independently of analysis of results.
Two review authors (MW and JM for the original review and its first update, MZ and KB for this second update) intended to carefully record reasons why an included study did not contribute data on a particular outcome and to consider the possibility of selective reporting of results on particular outcomes.
Assessment of risk of bias in included studies
Two review authors (MW and DB for the initial review and its first update, MZ and KB for its second update) planned to assess independently each included study for risk of bias using the definitions for the different risk of bias items as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019): random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other potential threats to validity (Higgins 2019; Kjaergard 2001; Moher 1998; Schulz 1995). We also planned to assess the risk of bias for blinding of outcome assessors and incomplete outcome data separately for each outcome. We aimed to consider a trial as having a low risk of bias if we assessed all domains as adequate. We planned to consider a trial as having a high risk of bias if we assessed one or more domain as inadequate or unclear. We wanted to report the risk of bias table as part of the characteristics of included studies table, and present a risk of bias summary figure that would detail all the judgements made for all included studies in the review (Higgins 2019). For each included study, we planned to assess selective reporting bias by comparing the methods and results section of the individual studies. We intended to resolve any disagreements on the assessment of risk of bias through discussion and consensus, or if necessary through a third review author (JM or BS) and to explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
We planned to analyse extracted data using Review Manager 5 (RevMan 2020).
We planned to extract hazard ratios (HR) with their 95% confidence intervals (CI) for time‐to‐event outcomes, such as mortality. If HRs were not provided, we intended to use the indirect estimation methods described in Parmar 1998 and Williamson 2002 to calculate them. As an alternative, we intended to use the proportions of participants with the respective outcomes measured at certain time points to calculate risk ratios (RR).
We planned to express results for binary outcomes as RRs with 95% CIs as measures of uncertainty. For continuous outcomes, we planned to express the results as mean differences (MD), with 95% CIs as measures of uncertainty.
Unit of analysis issues
Since, for medical reasons, we only aimed to include parallel group randomised trials, unit of analysis issues related to cross‐over and cluster randomised trials were not relevant for this systematic review. In the context of ECP, 'body‐part randomisation' and 'body part analyses' did not make sense, so related issues do not need to be discussed here. In cases of parallel group designs with three or more treatment groups, we planned to divide the control group into several parts, so that the total number added up to the original size of the group.
Dealing with missing data
We planned to contact original investigators for missing data regarding study selection, data extraction and risk of bias assessment. To optimise the strategy for dealing with missing data, we intended to conduct an intention‐to‐treat analysis, which would have included all participants who did not receive the assigned intervention according to the protocol, as well as those who were lost to follow‐up. If unsuccessful, we wanted to address the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using the I2 statistic (Higgins 2002; Higgins 2003). This measure describes the percentage of total variation across studies that is caused by heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%. We planned to use a simplified categorisation of heterogeneity with the following categories: low (I2 less than 30%), moderate (I2 between 30% and 60%) and high (I2 more than 60%) (Deeks 2011).
If moderate or high heterogeneity had been detected, we intended to explore clinical heterogeneity by examining differences between groups as detailed below (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We minimised the likelihood of publication bias by using a comprehensive search strategy without language restrictions, and we also searched trial registries. In addition to the evaluation of reporting bias, as described in the Assessment of risk of bias in included studies section, we planned to assess reporting bias by constructing a funnel plot where a sufficient number of studies had been identified (i.e. at least 10 studies included in a meta‐analysis). If there were fewer studies, the power of these tests would be too low to distinguish chance from real asymmetry (Sterne 2011).
Data synthesis
For future updates, we will conduct meta‐analyses of pooled data from all contributing studies using Review Manager 5 (RevMan 2020). We intended to use a fixed‐effect model as primary analysis. If we had found high clinical, methodological or statistical heterogeneity (I2 more than 50%), we would, as a secondary analysis, have used a random‐effects model and reported results from both models. We planned to summarise studies in cases where pooling of results was not possible.
Subgroup analysis and investigation of heterogeneity
We planned to assess clinical heterogeneity by examining differences due to:
-
underlying disease;
-
type of haematopoietic stem cell source;
-
age of children at HSCT;
-
age at start of ECP;
-
type of conditioning regimen;
-
type of GvHD prophylaxis regimen; and
-
degree of human leukocyte antigen (HLA) compatibility (matched sibling donor versus matched unrelated donor versus mismatched unrelated donor).
Sensitivity analysis
We planned to investigate the robustness of our results through a sensitivity analysis on the basis of risk of bias of the included studies by defining the following groups:
-
low risk of bias (adequate sequence generation and allocation concealment; successful blinding of all participants, care providers and outcome assessors; incomplete outcome data for less than 20% of participants; no selective reporting or other sources of bias);
-
high risk of bias (no adequate sequence generation and allocation concealment; no adequate blinding of all participants, care providers and outcome assessors; incomplete outcome data for more than 20% of participants; selective reporting or other sources of bias);
-
unclear risk of bias (rating of unclear risk of bias in at least one of these seven categories).
We intended to perform sensitivity analyses for each risk of bias item separately.
Summary of findings and assessment of the certainty of the evidence
We planned to prepare summary of findings tables based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021) and using GRADEpro software (GRADEproGDT). For each comparison we would have presented the following outcomes: response, overall survival, failure‐free survival, adverse events, quality of life and costs of intervention per month. Two review authors would have independently assessed the certainty of the evidence (i.e. very low, low, moderate or high certainty) for each outcome according to the GRADE approach, which takes into account study limitations (risk of bias), inconsistency, indirectness, imprecision and publication bias.
Results
Description of studies
We found no RCTs meeting the inclusion criteria for the original version of the review, its first update in 2015 or this second update in 2021 (Figure 1; Figure 2; Figure 3) (see: Characteristics of excluded studies table).
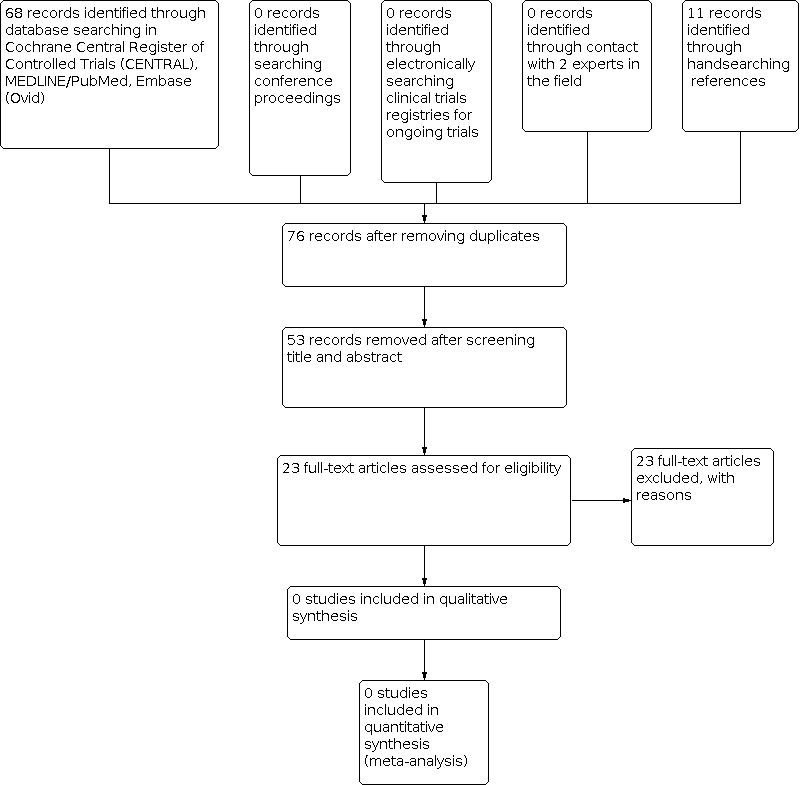
Identification of potentially eligible reports for the original review in 2014.
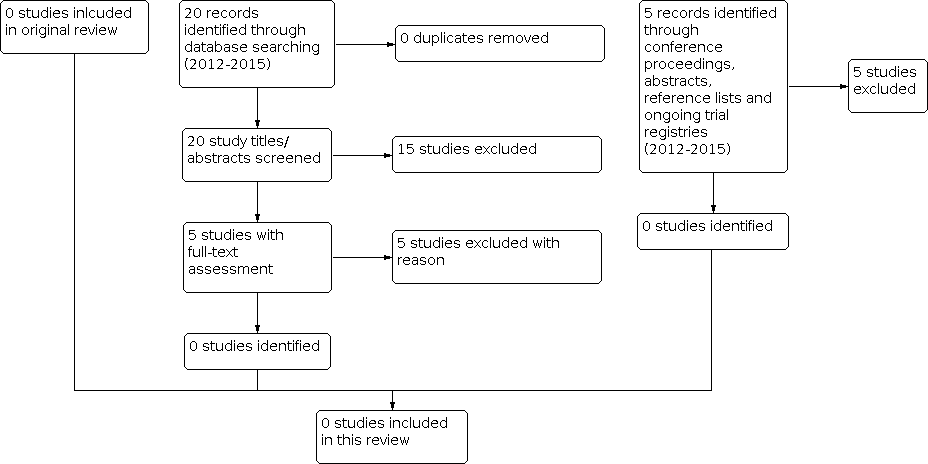
Identification of potentially eligible reports for the first review update in 2015.
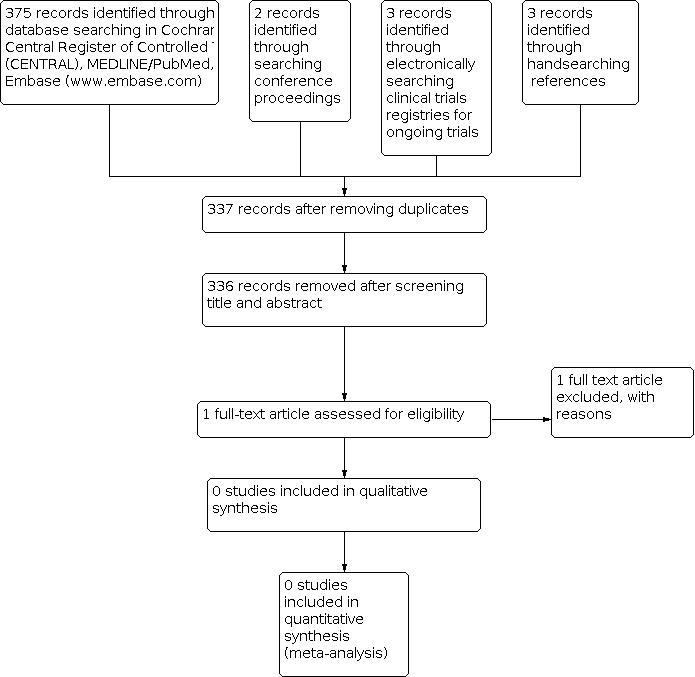
Identification of potentially eligible reports for the second review update in 2021.
Results of the search
The latest full search was carried out in the electronic literature databases on 25 January 2021, in the clinical trials registries on 12 November 2020, and in the conference proceedings on 9 November 2020.
These searches yielded 383 records including 46 duplicates or quadruplets in CENTRAL, MEDLINE, Embase, conference proceedings and clinical trials registries. Figure 3 depicts the result of the latest search strategy. After title and abstract screening of 337 unique references, we retrieved one article for full text review. This RCT was already mentioned in the previous versions of this review (Flowers 2008; see Excluded studies), but was excluded due to paediatric participants presenting less than 50% of the total study population.
Included studies
We found no RCTs meeting the inclusion criteria for this 2021 review update.
Excluded studies
For this 2021 review update, we excluded additional data from one RCT, which was already mentioned in the previous review versions.
See Characteristics of excluded studies table.
Risk of bias in included studies
We found no studies meeting the inclusion criteria for the original version of this review, its first update in 2015 or this second update in 2021. Therefore, the assessment of risk of bias was not applicable.
Effects of interventions
See: Summary of findings 1 Summary of findings
We found no studies meeting the inclusion criteria for the initial review, the first update in 2015 or this second update in 2021 (summary of findings Table 1). For that reason, the effectiveness and safety of ECP therapy in children and adolescents with cGvHD following HSCT remain unclear.
Discussion
Summary of main results
General findings
Chronic GvHD represents one of the major challenges for transplant‐related morbidity and mortality after HSCT in children and adolescents. In these patients, ECP is an alternative second‐ or third‐line treatment option. However, as in the previous review versions, this second update in 2021 identified no RCTs involving a study population of at least 50% paediatric participants aged ≤ 18 years. In the absence of reliable data from RCTs, this systematic review is still not able to ascertain if ECP treatment in children and adolescents with cGvHD after HSCT is an effective and safe option.
Paediatric studies evaluating ECP in cGvHD after HSCT
The available body of evidence on efficacy and safety of ECP in the paediatric population with cGvHD following HSCT suggests a benefit of ECP treatment in these patients. However, this evidence is limited to case reports, case series and mainly retrospective observational studies. As a consequence of methodological limitations, such as the non‐randomised study designs as well as inhomogeneous disease and response definitions, the results of these studies are difficult to compare and to transfer into clinical practice.
There was one earlier prospective trial designed as phase 2 study, which intended to examine the efficacy and safety of ECP added in adult and paediatric participants with cGvHD aged ≥ 4 years, weighting ≥ 30 kg, and not responding to steroids and ciclosporin A within 14 days. However, despite the enrolment of 25 participants in the study, the results have not yet been published (NCT00048789).
RCTs evaluating ECP in cGvHD after HSCT
During the initial review in 2014, we identified only one RCT examining the efficacy of ECP in cGvHD that aimed to include children weighing at least 40 kg among the participants (Flowers 2008). This study finally enrolled mainly adults (greater than 50%; median age of the ECP and control arm 41 years (range 16 to 67) and 43 years (range 13 to 67), respectively). This randomised controlled, single‐blinded, multicentre trial investigated the effect of ECP as second‐line therapy for skin manifestation of cGvHD in people with failed corticosteroid treatment following HSCT. People were eligible in case of: corticosteroid refractoriness, defined as a lack of response or disease progression after administration of at least 1 mg/kg of methylprednisolone equivalent, corticosteroid‐dependency after more than 10 mg of methylprednisolone equivalent to control skin manifestation, and corticosteroid intolerance due to intolerable adverse effects. Steroids had to be applied in a stable dose for at least two weeks prior to randomisation and had to be maintained on the same dose level for the first six study weeks (with the exception of reduction due to adverse effects). Immunosuppressants such as tacrolimus, mycophenolate and ciclosporin A were accepted as concomitant medication if they had been introduced at least four weeks before randomisation. Discontinuation of these agents during the study period was only permitted for safety reasons. The primary objective of the study was the median percentage change of total skin score (TSS) at week 12 compared with the pretreatment value. Secondary objectives included: the proportion of participants with at least 25% improvement in TSS, 50% or greater reduction in daily steroid dose compared with the baseline dose, or improvement of at least 25% in TSS in conjunction with steroid‐sparing at weeks 12 and 24. Skin assessments were performed on alternate weeks up to study week 12 and afterwards on alternate weeks up to study week 24. Participants were randomised with a block scheme in a 1:1 ratio to the conventional therapy arm (n = 49) or to the conventional therapy arm combined with ECP (n = 50). Conventional therapy consisted of corticosteroids and other immunosuppressive agents in refractory participants. ECP was administered three times during week one, and then twice weekly on consecutive days during weeks two to 12. Responding participants could continue ECP treatment until week 24 following the schedule of two treatments every week. Ten participants (four in the study group, six in the control group) withdrew consent prior to study week 12. Four participants died due to multiple organ failure, cardiac failure and infection. The changes in TSS from baseline until week 12 between the ECP group (‐14.5%) and the control group (‐8.5%) were not statistically different (P = 0.48; 95% confidence interval (CI) not reported). However, ECP‐treated participants had a 50% or greater reduction in the total daily dose of corticosteroids. At week 12, the percentage of participants experiencing both a 50% or greater reduction in daily corticosteroid dose and a 25% or greater improvement in the TSS was higher in the ECP group (8%) than the control group (0%) (P = 0.04; 95% CI not reported). Regarding TSS, 40% of the participants in the ECP group had a complete or partial skin response compared with 10% in the control group (P = 0.002; 95% CI not reported).
The aforementioned trial was continued as cross‐over randomised open‐label ECP study (Greinix 2011). In total, 29 participants were enrolled who had initially been randomised to the conventional study arm and presented with progression of cutaneous cGvHD, TSS improvement < 15% or steroid dose reduction ≤ 25% at the end of the previous study period. Of these, 25 participants completed the 24‐week ECP course. The prolonged duration of ECP resulted in an improvement of cutaneous and extracutaneous cGvHD, suggesting the need for prolonged ECP treatment in cGvHD to achieve sufficient treatment effects (Greinix 2011).
During the second review update in 2021, we found a further RCT, designed as a pilot phase 1 study and evaluating ECP as first‐line therapy in adults with HSCT‐related cGvHD (Jagasia 2019). This multicentre RCT used the revised 2014 NIH consensus criteria for diagnosis and response assessment of cGvHD (Jagasia 2015; Lee 2015). Overall, 60 participants with new‐onset moderate or severe cGvHD (≤ 3 years from HSCT) participated in the study and were randomised 1:1 either into the experimental (standard therapy and ECP; n = 29) or control arm (standard therapy alone; n = 31). The standard therapy consisted of corticosteroids (with a prednisone (or equivalent) starting dose of 1 mg/kg per day, which was tapered per protocol by week 8) and a calcineurin inhibitor (ciclosporin A or tacrolimus, with drug‐monitoring according to centre‐specific therapeutic blood levels). In the experimental group, ECP was carried out three times during the first week, twice per week until week 10, followed by two treatments biweekly until week 18 and two treatments four‐weekly until week 26. The outcome parameters comprised efficacy endpoint(s) at week 28, safety evaluation throughout the trial and long‐term follow‐up endpoint(s) after two years. The primary efficacy endpoint was defined as overall response rate (ORR) considering either complete or partial response with reference to the 2015 NIH criteria (Lee 2015). TSS change, cGvHD, cumulative steroid dose and quality of life were considered as secondary efficacy endpoints. The primary and secondary long‐term follow‐up endpoint was defined as overall and failure‐free survival, respectively. Most of the efficacy parameters were assessed bi‐ or four‐weekly until week 28, long‐term follow‐up was evaluated only in participants completing the 28‐week study period. Finally, due to missing baseline measurements, the intention‐to‐treat population comprised 53/60 individuals, including 29 in the experimental and 24 in the control arm. Of these, 20/29 versus 13/24 completed the 28‐week study‐period, and 15/29 versus 11/24 the two‐year‐follow‐up. At baseline assessment, 12/29 (41.1%) participants in the ECP group and 13/24 (54.2%) in the control arm were diagnosed with severe cGvHD, while progressive cGvHD onset was indicated for 6/29 (20.7%) and 1/24 (4.2%). For both study arms, organ involvement was as following: skin 15/29 (51.7%) versus 12/24 (50.0%); liver 4/29 (13.8%) versus 1/24 (4.2%); gastrointestinal tract 7/29 (24.1%) versus 3/29 (12.5%); and other organs 5/29 (17.2%) versus 0/24. The ORR estimated by blinded assessors at week 28 was 74.1% (95% CI: 53.7 to 88.9) in the ECP group and 60.9% (95% CI: 38.5 to 80.3) in the control arm. The investigator‐assessed ORR estimated at this time point was 56.0% (95% CI: 34.9 to 75.6) in the ECP and 66.7% (95% CI: 43.0 to 85.4) in the control group. Both groups presented with a decline in TSS from baseline to week 28 (experimental group versus control group: ‐22% ± 44% versus ‐34% ± 53%; P = 0.549, for blinded assessment; and ‐27% ± 29% versus ‐37% ± 43%; P = 0.856, for non‐blinded, investigator assessment). In both arms, the mean cumulative steroid dose at week 28 was similar. In contrast to participants additionally treated with ECP who did not show any quality of life changes over time, people treated with standard therapy alone indicated a decline in quality of life in several domains. In the entire study sample (n = 60), 17 participants accounted for 35 treatment‐related serious adverse events, including 8/27 (27.6%) participants in the ECP arm and 9/31 (29%) in the control arm. These adverse events led to the exclusion of six participants from each arm. Four participants died, all assigned to the ECP arm; however, death was not considered to be associated with ECP. Failure‐free survival was reported for 19/32 participants entering the two‐year follow‐up; the median failure‐free survival time was 12.5 months (95% CI: 4.8 to 22.4) in the ECP group and 7.8 months (95% CI: 1.8 to 19.5) in the control arm.
In summary, these trials assumed beneficial effects of ECP in adults with aGvHD following HSCT. Although these two RCTs were well‐designed, the results should be interpreted with caution due to the small number of individuals included in the trials and the high number of withdrawals throughout the study periods in both studies. Moreover, neither study was able to show a clearly superior treatment effect of ECP when added to first‐line therapy in cGvHD or applied as second‐line therapy for people with inadequate response to first‐line therapy (Flowers 2008; Jagasia 2019). However, these studies could serve as a basis for future trials which may answer the question about the utility of ECP in the treatment of people with cGvHD more precisely.
Overall completeness and applicability of evidence
The available paediatric studies were not designed as RCTs and provided only low quality evidence for the efficacy of ECP in children with cGvHD after HSCT. Hence, they should not be used to establish recommendations in children and adolescents after HSCT.
The presented adult RCTs showed several beneficial effects when ECP was either implemented as second‐line therapy or added to first‐line therapy (Flowers 2008; Greinix 2011; Jagasia 2019). However, until these results are confirmed by larger studies covering longer follow‐up periods, the real benefit of ECP as first‐ or second‐line therapy in people with cGvHD after HSCT remains undetermined.
Furthermore, insights gained from data of RCTs performed in the adult population are difficult to transfer into the paediatric population due to varying indications for HSCT, different comorbidities, long‐life expectancy, discrepancies in pharmacokinetics and the critical role of safety issues in children and adolescents, amongst others.
Quality of the evidence
We did not include any RCTs in this review, so were unable to assess the quality of the evidence.
Potential biases in the review process
For this second update in 2021, we chose to perform an updated full search. This latest and thorough search yielded significantly more records than the previous searches performed for the initial review and its first update (Weitz 2014; Weitz 2015). This may be attributed to several technical changes within the electronic literature databases, especially in MEDLINE (PubMed) in May 2020. Conversely, based on a less inclusive study selection process with strict elimination of studies not meeting the inclusion criteria, the number of records retrieved for full text assessment was significantly lower than in the previous review versions.
In order to detect all available information, we used a comprehensive search strategy that combined automated and manual search techniques. Nevertheless, missing information could not be completely ruled out.
Agreements and disagreements with other studies or reviews
Given the heterogeneous organ manifestations in cGvHD and the limited evidence for most treatment options, there is still no standardised treatment for children and adults with cGvHD following HSCT. This applies especially for those people who failed first‐line therapy with steroids and concomitant immunosuppressants. Several clinical practice guidelines on the treatment of adult and paediatric cGvHD advise consideration of ECP as secondary treatment for cGvHD (Dignan 2012; Flowers 2015; Wolff 2011). These guidelines were based on the limited number of RCTs and mainly resulted from evidence provided by non‐RCTs, including literature reviews and expert opinions, suggesting possible benefits from ECP treatment. Within this context, the Haemato‐oncology subgroup of the British Committee for Standards in Haematology and the British Society for Bone Marrow Transplantation recommended consideration of ECP as second‐line treatment for people with cGvHD with skin, oral or liver involvement (graded as a 1B recommendation) and as third‐line treatment in other organ manifestations (graded as a 2C recommendation) (Dignan 2012). Overall, the American Association for Apheresis graded the use of ECP in people with cGvHD following HSCT as a 1B recommendation (Padmanabhan 2019).
Previous systematic reviews and meta‐analyses evaluating ECP in cGvHD were based on different study selection criteria, did not focus particularly on the paediatric population and addressed different research questions (Abu‐Dalle 2014; Malik 2014; Yalniz 2018). However, these also emphasised the critical issues of the available body of evidence and the urgent need for high‐quality studies, notably RCTs, in order to better define the role of ECP in cGvHD.

Identification of potentially eligible reports for the original review in 2014.

Identification of potentially eligible reports for the first review update in 2015.

Identification of potentially eligible reports for the second review update in 2021.
| Extracorporeal photopheresis (ECP) compared with alternative treatment for chronic graft‐versus‐host disease | ||||||
| Patient or population: children and adolescents with chronic graft‐versus‐host disease after haematopoietic stem cell transplantation Settings: paediatric hospitals worldwide Intervention: ECP alone or in combination with standard treatment Comparison: alternative treatment alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk without ECP | Risk with ECP | |||||
| Response | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Overall survival | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Failure‐free survival | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Adverse events | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Quality of life | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| Cost of intervention per month | not estimable | not estimable | not estimable | 0 participants (0 studies) | not applicable |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% confidence interval). | ||||||
| GRADE Working Group grades of evidence | ||||||

