تیتر کردن دوز عوامل مهار کننده آنزیم مبدل آنژیوتانسین، عوامل مسدود کننده بتا‐آدرنرژیک، و مسدود کنندههای گیرنده آنژیوتانسین توسط پرستار در افراد مبتلا به نارسایی قلبی با کسر جهشی کاهشیافته
Referencias
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial | |
| Participants | 74 health professionals recruited 169 patients diagnosed with heart failure that met the Framingham criteria and a LVEF ≤ 45% or moderate or severe left ventricular systolic dysfunction on their "latest evaluation" | |
| Interventions | Health professionals were randomised to 1 of 3 groups Group 1: Health professionals were provided with education on the initiation and up‐titration of beta‐adrenergic blocking agents Group 2: Nurse facilitator group: The study nurse practitioner, supervised by 2 cardiologists, was responsible for initiating, titration, and stabilising heart failure patients on beta‐adrenergic blocking agents. Once the patient reached maximum tolerated dose of beta‐adrenergic blocking agents, they were referred back to the primary care physician Group 3: Provider and patient notification: Health professionals were given a list of their patients who were potential candidates for beta‐adrenergic blocking agents. Computer alerts were activated when the provider accessed their patient's electronic medical record for the first 2 visits post‐randomisation. All patients in this group were mailed a letter about beta‐adrenergic blocking agents for them to discuss with their health professional at their next visit | |
| Outcomes | Primary outcome: Number of patients initiated, up‐titrated, and maintained on beta‐adrenergic blocking agents Secondary outcome: Proportion of patients reaching target doses of beta‐adrenergic blocking agents | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A stratified randomisation using computer‐generated, random numbers" Comment: Randomisation occurred at the health professional level |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) | High risk | Comment: All patients and health professionals were aware of the group allocation. There was no blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An independent research assistant assessed the use of beta‐blocker therapy" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: There was no report about incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
| Methods | Parallel‐group randomised controlled trial | |
| Participants | 240 people diagnosed with heart failure and NYHA class lll‐lV. Diagnosis was based on symptoms and echocardiographic or radionuclide ventriculography tests. LVEF ≤ 45% | |
| Interventions | Group 1: Control group comprised of usual care and follow‐up with a cardiologist Group 2: The intervention was comprised of an intensive follow‐up for 12 months at an outpatient clinic led by a cardiologist and cardiovascular nurse. Participants' first visit was in week 1 postdischarge or referral from an outpatient clinic. At the first and second visit to the heart failure clinic, the participant was provided with education about heart failure, fluid management, early warning signs of heart failure and when to call for medical assistance, exercise, medication, importance of adherence, and possible adverse events. All participants saw a dietician who provided information about a low‐salt diet, fluid restriction, and weight reduction. At each clinic visit, the nurse performed a physical assessment, reviewed laboratory results, and proposed a treatment plan to the physician. The physician then reviewed the participant in conjunction with the nurse's assessment. At subsequent follow‐up visits at weeks 5 and 7 and months 3, 6, 9, and 12, participants were assessed by the nurse and education was reinforced. At 6 of the 9 visits the physician also assessed the participant and optimised their medical management in conjunction with the nurse | |
| Outcomes | Primary endpoint: composite of incidence of hospitalisation for worsening heart failure and/or all‐cause mortality Secondary endpoints: effect on LVEF, NYHA class, quality of life, NT‐proBNP, time to death, utilisation of heart failure medications and self care behaviour | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised by computer‐generated allocation" Comment: Randomisation occurred at the level of the participant |
| Allocation concealment (selection bias) | Unclear risk | Comment: The concealment of group allocation was not reported |
| Blinding of participants and personnel (performance bias) | High risk | Comment: There was no blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "An external clinical endpoint committee consisting of three experienced cardiologists and blinded to the allocation status of the patient, judged all causes of hospitalisation and death" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Incomplete outcome data was not reported |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
| Methods | Randomised controlled trial | |
| Participants | 28 stable CHF patients either with beta‐adrenergic blocking agent therapy newly initiated or with current beta‐adrenergic blocking agent therapy at less than half the recommended target dose Impaired left ventricular systolic dysfunction as documented by gated blood pool scanning or echocardiography within 6 months of enrolment into the study | |
| Interventions | Group 1: Usual care: Participants were referred to their primary physician for titration of beta‐adrenergic blocking agents. Participants randomised to the usual‐care group underwent assessment by a cardiologist at the heart failure clinic. Information outlining beta‐adrenergic blocking agent up‐titration was communicated in writing to both the participant and the primary care physician. The participants were not reviewed again in the heart failure clinic until their scheduled cardiologist visits at both 3 and 6 months after randomisation Group 2: Nurse‐led titration: Participants in the intervention group were reviewed by the heart failure nurse in the clinic weekly, fortnightly, or monthly until they reached the maximum‐possible dose of beta‐adrenergic blocking agents and had attended for the 6‐month intervention period. At each visit the heart failure nurse undertook a clinical examination of the participant; determined appropriate medication changes, tests and referrals; and educated the participant concerning medication changes. The referring cardiologist also reviewed the participant and approved proposed changes and completed medication prescriptions and referral forms. Each participant received a printed list of current medications including the new titrated dose of medications. It is important to note that, whilst the titration clinic was run by the heart failure nurse, a cardiologist was available to briefly see each participant and, especially in participants who had significant comorbidities and up‐titration difficulties, guide the nurse in the up‐titration process | |
| Outcomes | Primary endpoint was the difference in time taken to reach the optimal tolerated dose of beta‐adrenergic blocking agent Secondary endpoints were the likelihood of reaching maximal dose of beta‐adrenergic blocking agents by 6 months and the mean dose of beta‐adrenergic blocking agent at 6 months after entering the study. Tertiary endpoints of interest were all‐cause and heart failure hospital admissions, all‐cause and heart failure emergency department attendances, changes in general quality of life, and depression score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was according to computer generated random numbers held in opaque, sealed envelopes by a third party" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...random numbers held in opaque, sealed envelopes by a third party" |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Participants and nurses were aware of group allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Blindng of outcomes assessment was not reported |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Incomplete outcome data were not reported |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes were reported |
| Methods | Randomised controlled trial | |
| Participants | 706 people with systolic heart failure | |
| Interventions | Group 1: Telephone and nurse‐led intervention (HeartNetCare) (343 participants). As inpatients, participants were educated by a heart failure specialist nurse in self management of blood pressure, heart rate and rhythm, weight, and recognition of worsening signs and symptoms of heart failure. Telephone follow‐up calls commenced in week 1 postdischarge and occurred weekly for the first month Group 2: Usual care (363 participants). Standard follow‐up by primary care physican All participants were followed up for 18 months | |
| Outcomes | Type and dosage of heart failure medication (beta‐adrenergic blocking agent, ACEI, ARB, and MRA), LVEF as determined on echocardiography, NYHA class, and quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) | High risk | Comment: All participants and health professionals were aware of the group allocation. There was no blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Blinding of outcomes was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: There was no report about incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
| Methods | Randomised controlled trial | |
| Participants | A total of 28 residents from 33 long‐term aged care facilities and diagnosed with left ventricular systolic dysfunction | |
| Interventions | Group 1: Usual‐care group were referred to their primary care physician. The team cardiologist sent a letter to the primary care physician outlining the participant's management plan Group 2: Intervention group consisted of an initial visit with a cardiologist who implemented a management plan. The heart failure nurse then followed up the participant at the aged care facility once or twice a week. The heart failure nurse implemented the management plan including blood tests, clinical assessment, patient and carer education, and titration of medication All participants were followed up for 6 months | |
| Outcomes | Primary outcome: proportion of participants receiving optimum dose of ACEIs and beta‐adrenergic blocking agents at 6 months Secondary outcomes: percentage of participants prescribed ACEI or beta‐adrenergic blocking agents or both, heart failure‐related mortality, heart failure‐related hospitalisation, and changes in functional capacity and quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomisation used stratified blocks according to NYHA classification" Quote: "Randomisation occurred patient level." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was concealed" |
| Blinding of participants and personnel (performance bias) | High risk | Comment: All participants and health professionals were aware of the group allocation. There was no blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...a blinded assessor reviewed medical notes for changes in prescribing and heart failure events" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: There was no report about incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes were reported |
| Methods | Randomised controlled trial | |
| Participants | 406 people with documented left ventricular systolic dysfunction | |
| Interventions | Group 1: Usual care: Participants received information about how to manage their heart failure Group 2: Intervention group: The first visit with the heart failure nurse consisted of education about heart failure, self management strategies, lifestyle modifications, and medication adherence. All participants received printed information about heart failure. Nurses organised initiation and titration of medications. Subsequent visits were comprised of telephone follow‐up every 3 months for 12 months | |
| Outcomes | Primary endpoint: all‐cause hospitalisation Secondary endpoints: emergency department presentations, heart failure hospitalisations, and medications prescribed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated, random number sequence without blocking or stratification to centrally determine randomisation assignments" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...concealed treatment group assignments in sealed, opaque envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Comment: Participants and nurses were aware of group allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "...interviewers who were blinded to treatment assignments asked patients about hospitalisations at nonparticipating hospitals" |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "...we conducted tests for missing data bias suggested by Hogan and colleagues and these tests gave little evidence of informative missingness. We also used linear mixed models, which are robust to data missing at random, to estimate treatment effectiveness" |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
| Methods | Randomised controlled trial | |
| Participants | 106 people diagnosed with heart failure based on symptoms or diagnostic tests | |
| Interventions | Group 1: Usual care: Participants were followed up by their primary physician. Group 2: Intervention group: All participants in this group were followed up in a nurse‐led heart failure outpatient clinic 2 to 3 weeks postdischarge from a heart failure hospital admission. The clinic was staffed by heart failure nurse specialists who were responsible for making protocol‐led changes in medication. All visits consisted of: education about heart failure, advanced patient assessment, titration of medication according to a predetermined protocol, lifestyle modifications, and self management strategies | |
| Outcomes | Primary endpoint: composite of all‐cause mortality and/or all‐cause hospital admission at 12 months Secondary endpoint: mortality, number of all‐cause hospital readmissions, and self care behaviour | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was blinded with the use of a computer‐generated list of random numbers" Comment: Randomisation was at the participant level |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...random numbers and sealed envelopes" |
| Blinding of participants and personnel (performance bias) | High risk | Comment: There was no blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Blinding of outcome assessment was not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Incomplete outcome data were not reported |
| Selective reporting (reporting bias) | High risk | Comment: All outcomes except adverse events associated with the intervention were reported |
ACEI: angiotensin converting enzyme inhibitor
ARB: angiotensin receptor blocker
CHF: congestive heart failure
LVEF: left ventricular ejection fraction
MRA: mineralocorticoid receptor antagonist
NT‐proBNP: N‐terminal pro‐brain natriuretic peptide
NYHA: New York Heart Association
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| There was no outcome data about the titration of medications by the heart failure nurses | |
| We were unable to obtain a copy of the dissertation, and the results had not been published in an article | |
| There was no outcome data about the titration of medications by the heart failure nurses | |
| Quasi‐experimental design | |
| Both arms titrated medications | |
| There was no outcome data about the titration of medications by the heart failure nurses |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause hospital admissions Show forest plot | 4 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.72, 0.88] |
| Analysis 1.1  Comparison 1 Nurse‐led titration versus usual care, Outcome 1 All‐cause hospital admissions. | ||||
| 2 Heart failure‐related hospital admissions Show forest plot | 4 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.72] |
| Analysis 1.2  Comparison 1 Nurse‐led titration versus usual care, Outcome 2 Heart failure‐related hospital admissions. | ||||
| 3 All‐cause mortality Show forest plot | 6 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.92] |
| Analysis 1.3 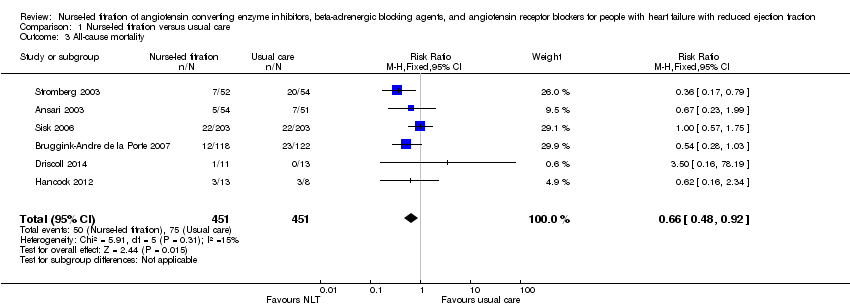 Comparison 1 Nurse‐led titration versus usual care, Outcome 3 All‐cause mortality. | ||||
| 4 All‐cause event free survival Show forest plot | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.77] |
| Analysis 1.4  Comparison 1 Nurse‐led titration versus usual care, Outcome 4 All‐cause event free survival. | ||||
| 5 Proportion reaching target dose of medications Show forest plot | 5 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.61, 2.47] |
| Analysis 1.5  Comparison 1 Nurse‐led titration versus usual care, Outcome 5 Proportion reaching target dose of medications. | ||||

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Nurse‐led titration versus usual care, Outcome 1 All‐cause hospital admissions.

Comparison 1 Nurse‐led titration versus usual care, Outcome 2 Heart failure‐related hospital admissions.

Comparison 1 Nurse‐led titration versus usual care, Outcome 3 All‐cause mortality.

Comparison 1 Nurse‐led titration versus usual care, Outcome 4 All‐cause event free survival.

Comparison 1 Nurse‐led titration versus usual care, Outcome 5 Proportion reaching target dose of medications.
| Nurse‐led titration versus usual care for people with heart failure with reduced ejection fraction | ||||||
| Patient or population: people with heart failure with reduced ejection fraction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nurse‐led titration versus usual care | |||||
| All‐cause hospital admissions | Study population | RR 0.80 | 560 | ⊕⊕⊕⊕ | ||
| 763 per 1000 | 610 per 1000 | |||||
| Moderate | ||||||
| 437 per 1000 | 350 per 1000 | |||||
| Heart failure‐related hospital admissions | Study population | RR 0.51 | 642 | ⊕⊕⊕⊝ | ||
| 248 per 1000 | 126 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 93 per 1000 | |||||
| All‐cause mortality | Study population | RR 0.66 | 902 | ⊕⊕⊕⊝ | ||
| 166 per 1000 | 110 per 1000 | |||||
| Moderate | ||||||
| 163 per 1000 | 108 per 1000 | |||||
| All‐cause event‐free survival | Study population | RR 0.60 | 370 | ⊕⊕⊕⊝ | ||
| 487 per 1000 | 292 per 1000 | |||||
| Moderate | ||||||
| 385 per 1000 | 231 per 1000 | |||||
| Proportion reaching target dose of medications | Study population | RR 1.99 | 966 | ⊕⊕⊝⊝ | ||
| 171 per 1000 | 340 per 1000 | |||||
| Moderate | ||||||
| 182 per 1000 | 362 per 1000 | |||||
| *The assumed risk is based on the observed incidence across the pooled control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1,2 I = 68% and P = 0.03 with a high Chi2 in relation to degrees of freedom. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause hospital admissions Show forest plot | 4 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.72, 0.88] |
| 2 Heart failure‐related hospital admissions Show forest plot | 4 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.72] |
| 3 All‐cause mortality Show forest plot | 6 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.92] |
| 4 All‐cause event free survival Show forest plot | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.46, 0.77] |
| 5 Proportion reaching target dose of medications Show forest plot | 5 | 966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.61, 2.47] |

