Tratamiento complementario con estiripentol para la epilepsia refractaria focal
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Controlled trial using a 'responder enriched' design First 2 study periods adopted a non‐randomised before‐after design Second portion of the trial adopted a randomised, placebo‐controlled, double‐blind, parallel design Only the second portion of this ’responder enriched’ trial was included | |
| Participants | Individuals who were responders when taking add‐on stiripentol during a pre‐randomisation baseline period were randomly assigned to continue add‐on stiripentol or to add‐on placebo. All participants who entered the preceding study were children with focal epilepsy. 32 participants were randomly assigned: 17 to add‐on stiripentol and 15 to add‐on placebo Add‐on stiripentol group: 7 male, 10 female (total 17 participants); age: 8 ± 3 years (mean ± standard deviation) Add‐on placebo group: 11 male, 4 female (total 15 participants); age: 10.4 ± 3.4 years Inclusion criteria for baseline period
Inclusion criteria for randomised, placebo‐controlled, double‐blind, trial: participants had to be responders (i.e. ≥ 50% decrease in seizure frequency during third month of open period vs baseline) to be eligible for randomisation Exclusion criteria for baseline period: participants receiving other drugs and those whose parents were unable to comply regularly with drug delivery and daily seizure diary Exclusion criteria during double‐blind period
| |
| Interventions |
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by a computer‐generated list |
| Allocation concealment (selection bias) | Low risk | Central allocation (pharmacy‐controlled randomisation). "Each patient received tablets of both stiripentol and "placebo of stiripentol" and tablets of both carbamazepine and "placebo of carbamazepine". "Individual tablets were prepared by the pharmacist" |
| Blinding of participants and personnel (performance bias) | Low risk | Second part of the trial was defined as “double blind”. "Each patient received tablets of both stiripentol and "placebo of stiripentol" and tablets of both carbamazepine and "placebo of carbamazepine". "Individual tablets were prepared by the pharmacist" |
| Blinding of outcome assessment (detection bias) | Low risk | "Each patient received tablets of both stiripentol and "placebo of stiripentol" and tablets of both carbamazepine and "placebo of carbamazepine". "Individual tablets were prepared by the pharmacist" |
| Incomplete outcome data (attrition bias) | High risk | Numbers of dropouts from each group were reported, along with reasons for dropout. However, number of dropouts in both arms was high (add‐on stiripentol and add‐on placebo) (53.3% vs 35.3%) |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes |
| Other bias | High risk | Through its 'responder‐enriched' design, this study resulted in a primary efficacy evaluation of an enriched population of participants, as a result of random assignment only of those who responded to open‐label treatment (high risk of selection bias). High risk of carry‐over and withdrawal effects |
Characteristics of excluded studies [ordered by year of study]
Ir a:
| Study | Reason for exclusion |
| Not randomised. Uncontrolled before‐after design | |
| Not randomised. Uncontrolled before‐after design | |
| Not specified whether study was conducted in individuals with focal epilepsy. Not conducted in those with refractory epilepsy | |
| Not randomised. Uncontrolled before‐after design | |
| This study was published as a conference proceeding and provided preliminary results (interim analyses) of the study of Chiron 2006, which was published a few years later and is included in the review |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ≥ 50% seizure reduction Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.81, 2.82] |
| Analysis 1.1  Comparison 1 Add‐on stiripentol versus placebo, Outcome 1 ≥ 50% seizure reduction. | ||||
| 2 Seizure freedom Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.31, 4.43] |
| Analysis 1.2  Comparison 1 Add‐on stiripentol versus placebo, Outcome 2 Seizure freedom. | ||||
| 3 ≥ 1 adverse effect Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.08, 6.47] |
| Analysis 1.3  Comparison 1 Add‐on stiripentol versus placebo, Outcome 3 ≥ 1 adverse effect. | ||||
| 4 Neurological adverse effects Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.88, 8.01] |
| Analysis 1.4 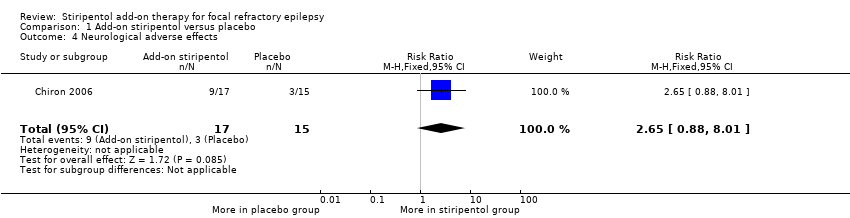 Comparison 1 Add‐on stiripentol versus placebo, Outcome 4 Neurological adverse effects. | ||||
| 5 Gastrointestinal adverse effects Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.56 [0.71, 189.36] |
| Analysis 1.5 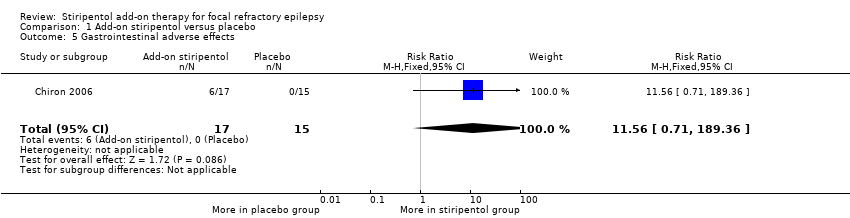 Comparison 1 Add‐on stiripentol versus placebo, Outcome 5 Gastrointestinal adverse effects. | ||||
| 6 Dropouts Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.47] |
| Analysis 1.6 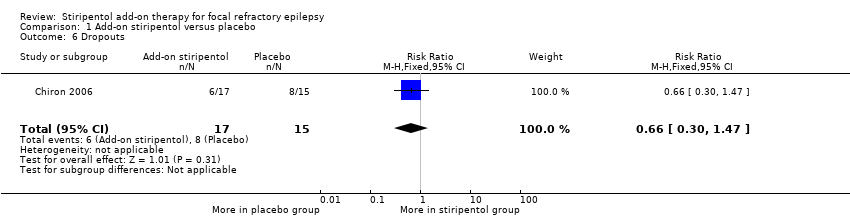 Comparison 1 Add‐on stiripentol versus placebo, Outcome 6 Dropouts. | ||||

Study flow diagram. The results shown in this figure refer both to the searches conducted in the present version of the review and in its previous versions (Brigo 2014; Brigo 2015).

Comparison 1 Add‐on stiripentol versus placebo, Outcome 1 ≥ 50% seizure reduction.

Comparison 1 Add‐on stiripentol versus placebo, Outcome 2 Seizure freedom.

Comparison 1 Add‐on stiripentol versus placebo, Outcome 3 ≥ 1 adverse effect.
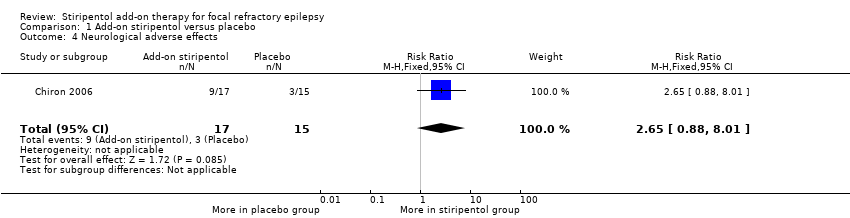
Comparison 1 Add‐on stiripentol versus placebo, Outcome 4 Neurological adverse effects.

Comparison 1 Add‐on stiripentol versus placebo, Outcome 5 Gastrointestinal adverse effects.

Comparison 1 Add‐on stiripentol versus placebo, Outcome 6 Dropouts.
| Stiripentol compared with placebo for focal refractory epilepsy | ||||||
| Patient or population: people with focal refractory epilepsy Settings: community Intervention: stiripentol Comparison: placebo | ||||||
| Outcomes* | Illustrative comparative risks** (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Stiripentol | |||||
| ≥ 50% seizure reduction | 467 per 1000 | 705 per 1000 | RR 1.51 (0.81 to 2.82) | 32 | ⊕⊕⊖⊖ lowa,b | |
| Seizure freedom | 200 per 1000 | 236 per 1000 | RR 1.18 (0.31 to 4.43) | 32 | ⊕⊕⊖⊖ lowa,b | |
| ≥ 1 adverse effect | 267 per 1000 | 707 per 1000 | RR 2.65 (1.08 to 6.47) | 32 | ⊕⊕⊖⊖ | |
| Neurological adverse effects | 200 per 1000 | 530 per 1000 | RR 2.65 (0.88 to 8.01) | 32 | ⊕⊕⊖⊖ | |
| Gastrointestinal adverse effects | 0 events occurred in the placebo group | 0 events occurred in the stiripentol group | RR 11.56 (0.71 to 189.36) | 32 | ⊕⊕⊖⊖ | |
| Dropouts | 533 per 1000 | 352 per 1000 | RR 0.66 (0.30 to 1.47) | 32 | ⊕⊕⊖⊖ | |
| * Quality of life was not assessed in this study. **The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) and is calculated according to the following formula: corresponding intervention risk, per 1000 = 1000 X ACR X RR. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once for risk of bias and once for imprecision (small sample size which is made even smaller with dropouts). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 ≥ 50% seizure reduction Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.81, 2.82] |
| 2 Seizure freedom Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.31, 4.43] |
| 3 ≥ 1 adverse effect Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.08, 6.47] |
| 4 Neurological adverse effects Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.88, 8.01] |
| 5 Gastrointestinal adverse effects Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.56 [0.71, 189.36] |
| 6 Dropouts Show forest plot | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.47] |

