Methylphenidat für Kinder und Jugendliche mit Aufmerksamkeitsdefizit‐Hyperaktivitäts‐Syndrom (ADHS)
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Eight‐week double‐blind, randomised, placebo‐controlled, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participant included: 19 (15 boys, 4 girls). Participants randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 19 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (combined (42%), hyperactive‐impulsive (0%), inattentive (58%)) Age: mean 10.05 years (SD 1.62, range 8 to 13) IQ: mean 107.1 (SD 14.3) Methylphenidate naive: 100% Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: ODD (26.3%), anxiety disorder (10.5%), dysthymic disorder (5.3%), conduct disorder (5.3%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible orders of OROS methylphenidate and placebo Mean methylphenidate dosage: 48.3 mg (range 18 ± 54 mg); weight‐based final OROS methylphenidate dose was 1.3 mg/kg Administration schedule: not stated Duration of each medication condition: 4 weeks: 2 weeks titration and 2 weeks optimal dose Washout before study initiation: 2 days between interventions Titration period: 2 weeks after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: Study protocol was reviewed and approved by the University’s institutional review board Comment from study authors
Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: December 2013. No supplemental information provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Post‐treatment scores for all 19 study children were obtained from parents. Because 1 child’s treatment was delayed and ran beyond the end of the school year, teacher data on 18 youngsters were analysed Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No protocol published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Investigator‐initiated trial funded by a grant from Ortho‐McNeil Janssen Scientific Affairs to Dr. Abikoff Conflicts of interest: Drs. Abikoff and Gallagher have a contract with Multi‐Health Systems to further develop the Children’s Organizational Skills Scale (COSS) used in this study. Dr. Abikoff has served on the ADHD Advisory Board of Shire Pharmaceuticals and of Novartis Pharmaceuticals. Dr. Boorady has served on the ADHD Advisory Board and Speakers’ Bureau of Shire Pharmaceuticals. Other study authors report no conflicts of interest |
| Methods | Three‐week double‐blind, placebo‐controlled, cross‐over trial, in which participants were randomly assigned to
| |
| Participants | Number of participants screened: not stated Number of participants included: 234. Participants were randomly assigned to low‐dose methylphenidate (0.3 mg/kg), high‐dose methylphenidate (0.5 mg/kg) or placebo Number of participants followed up: 206 Number of withdrawals: not stated, but it is described in the text that 4 children experienced severe side effects while taking Ritalin and could not complete the protocol Regarding the 206 participants Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: 5 to 15 years IQ: > 70 Sex: 161 boys, 45 girls Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
In addition, ≥ 3 of the following criteria had to be met
Children were divided into responders and non‐responders based on the following criteria
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to different orders of low‐dose Ritalin (0.3 mg/kg), high‐dose Ritalin (0.5 mg/kg) or placebo Administration schedule: 3 times a day Duration of each medication condition: 7 consecutive days Washout before study initiation: not stated Titration period: not stated Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation:no Ethics approval: yes; protocol was approved by the Institutional Review Board at the Marshfield Medical Center Key conclusion of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, but not described how and/or by whom |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind trial, identical appearing pills |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind trial, identical appearing pills |
| Incomplete outcome data (attrition bias) | Low risk | 206 had sufficient data for analyses Selection bias: Participants were divided into responders and non‐responders. However, data from this article pertain to side effects only and include both groups |
| Selective reporting (reporting bias) | Unclear risk | Not able to obtain protocol or other information |
| Vested interest bias | Low risk | Study was funded by Marshfield Clinic grants |
| Methods | Two identical, concurrently conducted, phase 4, double‐blind, randomised, cross‐over, analogue classroom trials with 2 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 78 (55 boys, 23 girls) Number of participants followed up: 71 Number of withdrawals before randomisation: 7 Number of withdrawals after randomisation: 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (81%), hyperactive‐impulsive (0%), inattentive (19%)) Age: mean 10.1 years (range 9 to 12 years) IQ: > 80 Methylphenidate naive: not stated Ethnicity: Caucasian (58%), African American (28%), other (14%) Country: USA Setting: out‐patient clinic Comorbidity: anxiety (0%), depressive disorders (0%), learning disability (32%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of OROS methylphenidate and placebo Mean OROS methylphenidate daily dosage: 40.5 mg Administration schedule: once daily (morning) Average duration of OROS methylphenidate treatment: 40 days Duration of placebo intervention: 1 day Washout before study initiation: up to 28 days Medication‐free period between interventions: no Titration period: before randomisation, up to 6 weeks Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; a history of failed response to methylphenidate was an exclusion criterion. Only children demonstrating the required decrease in ADHD symptoms with MPH‐OROS within the labelled dosing range were included in the randomised phase of the study. Children who may have required a dose > 54 mg to achieve full therapeutic effect may also have been excluded Any withdrawals due to adverse events: yes (n = 2) Email correspondence with study authors: June 2013 to June 2014. Obtained supplemental efficacy data (Swanson, Kotkit, Agler, M‐Flynn and Pelham Scale) and safety data. Awaiting data through the Yale Open Data Access Project | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Wigal 2011; computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Wigal 2011; blinding of investigators and participants maintained throughout the study |
| Blinding of outcome assessment (detection bias) | Low risk | Wigal 2011; blinding of investigators and participants maintained throughout the study |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes reported according to protocol |
| Vested interest bias | High risk | Supported by Ortho‐McNeil‐Janssen Scientific Affairs, LLC. Phase IV study Conflicts of interest: Several study authors had affiliations with pharmaceutical companies producing methylphenidate |
| Methods | Seven‐centre US study consisting of a 6‐week, open‐label, dose‐titration phase (Part A) and a 2‐week, double‐blind, randomised, parallel‐group, placebo‐controlled withdrawal study (Part B) with 2 arms
| |
| Participants | Number of patients screened: 116 Regarding Part A
Regarding Part B
No significant differences in baseline demographics were noted between the 2 groups. Thus, slightly more treatment‐naive participants were receiving d‐MPH than placebo Inclusion criteria
Exclusion criteria
| |
| Interventions | Part A Dexmethylphenidate dosage: 2.5 to 10 mg, twice daily depending on individual participants' prior medication experience. Children who had received d,l‐MPH began with half their total daily d,l‐MPH dose administered as dexmethylphenidate but not more than 20 mg/d; those who had not previously received d,l‐MPH started d‐MPH at 2.5 mg twice daily Duration of intervention: 6 weeks Treatment compliance: not stated Part B Participants were randomly assigned to dexmethylphenidate or placebo Mean methylphenidate dosage: 68.6% of dexmethylphenidate continuers and 79.5% of placebo participants were receiving 20 mg at end of Part B, mean (SD) not stated Administration schedule: 10 mg twice daily. Time points 7 AM to 8 AM and 11:30 AM to 12 PM Duration of intervention: 2 weeks Titration period: 6 weeks, initiated before randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms (Part B)
Non‐serious adverse events (Parts A and B)
| |
| Notes | Comments from study authors
Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: October 2013. Supplemental information regarding additional information was received. However, study authors advised us to contact the sponsoring drug company for additional information. This process has been difficult, and no further communication was attempted to request additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was central, irrespective of whether the drug was pre‐packaged and pre‐randomised, or if it was bottled and labelled by an unblinded dispenser who had no contact with participants and kept the other staff blind |
| Allocation concealment (selection bias) | Low risk | Randomisation was central. "In all industry studies I have been involved with, either the drug was pre‐packaged and pre‐randomized or it was bottled and labeled by an unblinded dispenser who had no contact with patients and kept the other staff blind" |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. In Part B, participants/guardians and medical personnel were blinded to the drug. Also, d‐methylphenidate was available in tablets, each identical in appearance to a matching placebo. Study drug (or placebo) was dispensed in bottles containing a weekly supply, labelled for use at “Home” and “School”, with the strength designated “A,” “B” or “C” |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind. In Part B, participants/guardians and medical personnel were blinded to the drug |
| Incomplete outcome data (attrition bias) | Low risk | ITT sample was used in analysis of efficacy parameters: Participants who received dexmethylphenidate and had a Part B baseline efficacy evaluation and ≥ 1 post‐baseline assessment |
| Selective reporting (reporting bias) | Low risk | No protocol published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Study was supported by the Celgene Corporation Conflicts of interest: Drs. Arnold, Wigal and Bohan received research funding from Celgene for the study reported. Dr. Wigal and Dr. West are on the Advisory Panel and Speakers' Bureau for Novartis. Dr. Arnold and Dr. Bohan are on the Speakers' Bureau for Novartis. Dr. Zeldis is Chief Medical Officer and Vice President of Medical Affairs at the Celgene Corporation |
| Methods | Three‐day, double‐blind, randomised, placebo‐controlled medication assessment (cross‐over) | |
| Participants | Number of patients screened: not stated Number of participants included: 50. Participants were randomly assigned to methylphenidate (low dose, high dose) or placebo Number of participants followed up: 36 (28 boys, 8 girls) Number of withdrawals: 14 Diagnosis of ADHD: DSM‐IV (combined (61%), hyperactive‐impulsive (8%), inattentive (31%)) Age: mean 10.5 years (range 9 to 12) IQ: mean 102 (SD 13) Methylphenidate naive: 7 participants Ethnicity: Caucasian (80%), African American (17%), mixed race (3%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (47%), conduct disorder (17%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 2 doses of long‐acting methylphenidate (OROS methylphenidate; Concerta) once a day and placebo
Methylphenidate dosage: low dose: mean 40 mg (SD 9.2); high dose: mean 76.5 mg (SD 13.2) Administration schedule: once daily, morning, 90 minutes before trial Duration of trial: 3 days Washout before study initiation: 24 hours before participation. No washout between interventions. To promote reasonable tolerability of the medication in participants who were stimulant naive or were previously prescribed only very low doses (0.4 mg/kg/d), 13 children were order‐restricted, so they received the 0.3 mg/kg t.i.d.‐equivalent dose before receiving the 0.6 mg/kg t.i.d.‐equivalent dose. Among those who were not order‐restricted, 6 possible drug orders were counterbalanced across participants Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: yes Comment from study authors
Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: wrote to study authors in July 2014 to ask for data on side effects, pulse and blood pressure; have received no response as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further information |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Low risk | Supported by grants from the National Institute of Mental Health (NIMH) and from the National Institute on Drug Abuse (NIDA) |
| Methods | Triple‐blind, randomised, cross‐over trial with 3 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 83 (71 boys, 12 girls). Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 80 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III‐R (subtype distribution not stated) Age: mean 8.2 years (range 5 to 13) IQ: mean 105.1 Methylphenidate naive: 85% Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: mothers, married (n = 48), divorced (n = 13), unmarried or widowed (n = 13) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 0.3 mg/kg methylphenidate, 0.5 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: morning and noon Duration of each medication condition: 7 to 10 days Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: none Treatment compliance: unused capsules returned to the clinic each week for adherence check No family was discontinued from the study because of non‐compliance with the drug regimen, defined as more than 1 day of failure to take medication, or 2 missed capsules per week | |
| Outcomes | ADHD symptoms
General behaviour
Adverse effects
| |
| Notes | Sample calculation: not stated Ethics approval: Study was approved by the Human Subjects Committee at the medical centre Comments from study authors
Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: yes; 3. One child discontinued because of nervous facial tics, headache and dizziness; a second as the result of excessive thinking and disjointed thinking (during high‐dose methylphenidate); and a third because of headache, dizziness and increased hyperactivity Email correspondence with study authors: July 2013. We obtained additional information regarding funding and ethics approval. Unfortunately, it was not possible to receive from the study authors supplemental data on ADHD symptoms and general behaviour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A completely counterbalanced design was used, with participants randomly assigned in relatively equal numbers to 1 of 6 possible drug conditions |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Both medication and placebo were crushed and placed within orange opaque gelatin capsules to disguise distinctive differences in flavour between medication and placebo and dose differences across conditions. Children and their parents and teachers, as well as the research assistant evaluating the children, were blinded to medication conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Children and their parents and teachers, as well as the research assistant evaluating the children, were blinded to medication conditions |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no information |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Vested interest bias | Low risk | Study was internally funded by the medical school |
| Methods | Triple‐blind, placebo‐controlled, cross‐over design with participants randomly assigned to the following conditions
Each intervention period lasted 1 week | |
| Participants | Number of participants screened: not stated Number of participants included: 40. Participants were randomly assigned to 1 of 6 possible drug condition orders (only 6 drug orders, so the highest dose was never given unless preceded by the moderate dose) Number of participants followed up: 40 (36 boys, 4 girls) Number of withdrawals: 0 Diagnosis of ADD: DSM‐III‐R (+H: 58%, ‐H: 42%) Age: mean 8.6 years (range 6 to 12) IQ: 103.5 Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: borderline and low internalising symptoms. No others stated Comedication: not stated Sociodemographics: 54.8 on Hollingheads 2 Factor Index Participants were divided into different categories
Inclusion criteria
Additional criteria for children with combined ADHD
Additional criteria for children with ADD‐H
Differences regarding the 2 ADHD groups
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of methylphenidate (5 mg, 10 mg and 15 mg) and placebo Administration schedule: b.i.d., morning and noon Duration of each medication condition: 1 week Washout before study initiation: no Titration period: none, but highest dose was never given unless preceded by moderate dose Compliance: Children were permitted to miss 1 day of medication over 7 days and still remain in the study. No families were removed from this study because of non‐compliance as defined in this way | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: Study was approved by the Institutional Review Board at the medical centre Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; excluded those with history of adverse reactions, but included both naive and prior users of antipsychotics Email correspondence with study author: 18 January 2013. Dr. Barkley informed us that data from the study on side effects, for example, are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to 1 of 6 possible drug conditions |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy prepared placebo (lactose powder) and methylphenidate by crushing and placing them into 6 orange opaque gelatin capsules |
| Blinding of participants and personnel (performance bias) | Low risk | Triple‐blind design |
| Blinding of outcome assessment (detection bias) | Low risk | Triple‐blind design |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data Selection bias: no |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Vested interest bias | Low risk | Research was supported by the National Institute of Mental Health (NIMH) Conflicts of interest: no information |
| Methods | Double‐blind, placebo‐controlled, within‐participant, cross‐over trial with 3 interventions
Phases: 5, but high doses of each stimulant always followed lower dose of the same stimulant | |
| Participants | Number of participants screened: 46 Number of participants included: 38. Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 35 (30 boys, 5 girls) Number of withdrawals: 2. One was a post hoc exclusion Diagnosis of ADHD: DSM‐IV (subtype not described) Age: mean 14 years (range 12 to 17) IQ: mean 103.9 (range 80 to 141) Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of 5 mg methylphenidate followed by 10 mg methylphenidate, 5 mg Adderall followed by 10 mg Adderall and placebo
Mean methylphenidate dosage: low dose 10 mg/d; high dose 20 mg/d Administration schedule: AM and midday Duration of each medication condition: 1 week Washout before study initiation: none Medication‐free period between interventions: none Titration period: none, although 5 mg dose was given before 10 mg, initiated after randomisation Treatment compliance: Parents were required to return all unused capsules, but nothing further was said about this | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not described Ethics approval: yes Comments from study authors
Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; excluded patients who had a history of adverse events to stimulants Email correspondence with study authors: January 2014. We received additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Email correspondence with study author: “Randomization was done by me as best as I can recall” (Krogh 2014a [pers comm]) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Opaque gelatin capsules were prepared by the pharmacist |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Completer analysis reported 11/46 teens LTFU, 15 parents LTFU, 33 English teachers and 31 Maths teachers LTFU Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Email correspondence with study author: "All planned analyses were done and all measures we collected as treatment endpoints were analyzed" (Krogh 2014a [pers comm]) |
| Vested interest bias | Low risk | University of Massachusetts Medical School |
| Methods | Four‐day randomised, double‐blind, placebo‐controlled, cross‐over trial with 2 interventions in 2 groups Interventions
Groups
| |
| Participants | Number of participants screened: not stated Number of participants included: 130. Participants were randomly assigned to 1 of 11 possible drug condition orders Number of participants followed up: 130 (110 boys, 20 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (63%), hyperactive‐impulsive (30%), inattentive (6%)) Age: mean 9 years (SD 1.46, range 6 to 12) IQ: mean 104.11 Methylphenidate naive: 70% Ethnicity: Caucasian (90%) Country: Canada Setting: out‐patient clinic Comorbidity: specific learning disorder (34%), conduct disorder (24%), oppositional defiant disorder (26%), generalised anxiety disorder (17%), separation anxiety disorder (11%) Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 11 possible drug condition orders of methylphenidate and placebo. Children weighing < 25 kg received 5 mg, 10 mg and 15 mg of methylphenidate; children weighing ≥ 25 kg received 10 mg, 15 mg and 20 mg of methylphenidate Mean methylphenidate dosage: 0.28 mg/kg, 0.45 mg/kg, 0.61 mg/kg Administration schedule: not stated Duration of each medication condition: 1 day Washout before study initiation: none Medication‐free period between interventions: not stated Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: approved by the institutional ethics review board Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: December 2013. Not able to get supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Master randomisation tables were prepared by the research support pharmacist at the hospital by using simple randomisation with restrictions (high dose not to be given on the first possible drug day nor immediately following placebo; no directly ascending or descending dose order). Therefore, a balanced block 22 design was used |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Examiner, psychiatrist, participant and participant’s family were not informed about participant’s randomisation order or daily medication status until completion. Placebo and active medication were prepared by the hospital pharmacist and were powdered and packaged in an opaque capsule to prevent identification of contents by colour, taste or volume |
| Blinding of outcome assessment (detection bias) | Low risk | Trained clinicians, blinded to other aspects of the participant’s assessment, conducted interviews independently |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Low risk | Funding and operating grant from the Canadian Institute of Health Research and funding from the Canada Research Chairs Programme Conflicts of interest: none |
| Methods | Two‐week, double‐blind, placebo‐controlled, cross‐over trial with 1 intervention
The purpose of the study was to investigate the relationship between SLC6A3 3’ end variable number tandem repeat (VNTR) polymorphism and behavioural/therapeutic response to methylphenidate Phases: not stated | |
| Participants | Number of participants screened: not stated Number of participants included: 42 (all boys). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.2 ± 1.8 years (range not stated) IQ: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: Canada Setting: not stated Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate (0.5 mg/kg/d) and placebo Mean methylphenidate dosage: not stated Administration schedule: not stated Time points: not stated Duration of each medication condition: not stated Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated. No information Treatment compliance: not stated | |
| Outcomes | General behaviour
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated Any withdrawals due to adverse events: not stated Email correspondence with study authors: October 2013 and April 2014. We wrote to the study authors twice to request supplementary information on data. No reply was received. Therefore we have no useable data from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Inadequate information contained in the abstract Selection bias: not stated |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Vested interest bias | Unclear risk | Not stated |
| Methods | Fifteen‐site, multi‐centre, double‐blind, randomised, 2‐week parallel trial with 2 arms
Phases: 3. Pre‐randomisation (4 weeks titration plus 1 week washout), randomisation, double‐blind treatment and open‐label extension | |
| Participants | Number of participants screened: unknown Number of participants included: 164 Number of participants titrated: 161 (122 boys, 39 girls) Number of participants randomly assigned: methylphenidate 66, placebo 71 Number of participants followed up: methylphenidate 63, placebo 71 Number of withdrawals: methylphenidate 3, placebo 0 Age: mean 8.81 years (range 6 to 14) IQ: not stated Methylphenidate naive: 94 (58.4%) Ethnicity: Caucasian (85.7%), African American (3.7%), Asian (1.2%), other (9.3%) Country: USA and Canada Setting: out‐patient clinic (naturalistic school setting) Comorbidity: not stated Comedication: not stated Sociodemographics: not stated. No significant difference in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to extended‐release methylphenidate or placebo Mean methylphenidate dosage: not stated Administration schedule: once daily in the morning Duration of intervention: 2 weeks (mean: methylphenidate 13.91, placebo 13.96) Titration period: 1 week before randomisation | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes. A total of 128 participants (n = 64 per treatment group) were required for analysis of the primary efficacy variable, based on an effect size of 0.5 with a power of 80% and a 2‐tailed α‐level of 0.05 Ethics approval: yes. An institutional review board approved this study at each participating site Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Any withdrawals due to adverse events: yes Comment from study authors
Key conclusion of study authors
Comments from review authors
Email correspondence with study authors. April 2014. Emailed study authors for additional information/data but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by Novartis Drug Supply Management, which used a validated system that automates random assignment of treatment groups to randomisation numbers |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not clear whether investigator was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear whether investigator was blinded |
| Incomplete outcome data (attrition bias) | Low risk | LOCF analysis of ITT population. ITT population included all participants who received the double‐blind study drug, and from whom ≥ 1 Conners’ ADHD/DSM‐IV Scale, Teachers, was obtained |
| Selective reporting (reporting bias) | Low risk | No protocol was published. Outcomes of interest have been reported |
| Vested interest bias | High risk | Funding was received from Novartis |
| Methods | Eleven‐day N‐of‐1 randomised, double‐blind, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: 1 boy Number included: 1. The participant was randomly assigned to methylphenidate and placebo across the 11 days Number of participants followed up: 1 Number of withdrawals: 1 Diagnosis of ADHD: ICD‐10 (predominantly hyperactive type) Age: 15 years IQ: not stated Methylphenidate naive: no Ethnicity: not stated Country: Germany Setting: not stated Comorbidity: not stated Comedication: not stated Sociodemographics: 2 parents | |
| Interventions | The participant was randomly assigned to methylphenidate and placebo across 11 days Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of each medication condition: The condition was changed daily, but placebo was given for 6 days and methylphenidate for 5 days Washout before study initiation: not relevant Medication‐free period between interventions: no Titration period: none Treatment compliance: 100% according to Table 6 | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: not stated Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: December 2013. Emailed study author to request information about missing data but received no response. Not possible to use data from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: not stated |
| Methods | This double‐blind, placebo‐controlled, cross‐over trial with the child's clinically most effective dose as identified by a systematic open‐label titration procedure investigated whether components of attention and executive functioning improve when children with ADHD are treated with OROS methylphenidate Two‐week, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: 41 Number of participants included: 34. Participants were assigned to OROS methylphenidate and placebo in random order Number of participants followed up: 30 (24 boys, 6 girls) Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV TR (combined (100%)) Age: mean 8 years 6 months (range 6 years 5 months to 12 years 6 months) IQ: mean 97.8 (range 77 to 132) Methylphenidate naive: number not stated Ethnicity: Caucasian (80%), African American (13.3%), other (6.7%) Country: USA Setting: out‐patient clinic Comorbidity type: oppositional defiant disorder (40%), specific learning difficulty (33.3%), anxiety (6.67%), dysthymia (3.3%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to OROS methylphenidate and placebo Methylphenidate dosage: 9 children treated with 18 mg, 13 with 36 mg and eight with 54 mg of OROS methylphenidate Administration schedule: not stated Duration of each medication condition: 1 week Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: 2‐ to 3‐week open‐label, multi‐dose‐titration protocol to determine the child’s optimal dose as recommended by practice guidelines Treatment compliance: 30 children completed the study; however, compliance regarding study medication is not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes; approved by the Committee for the Protection of Human Subjects at The Children’s Hospital of Philadelphia Comment from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Email correspondence with study authors: January 2014: Emailed study authors twice to request additional information but received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned and counterbalanced across participants |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Referred to as double‐blind but no information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Referred to as double‐blind but no information provided |
| Incomplete outcome data (attrition bias) | High risk | Completer analysis reported |
| Selective reporting (reporting bias) | Low risk | Clinical trial ID: NCT00530257 |
| Vested interest bias | High risk | Study was supported by an investigator‐initiated grant from Ortho McNeil Janssen Scientific Affairs, the manufacturer of OROS methylphenidate (Concerta) |
| Methods | Eleven‐week, double‐blind, placebo‐controlled, cross‐over study with 3 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 46 (all boys). Participants were randomly assigned to 1 of the possible drug condition orders Number of participants followed up: 45 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.6 years (range 6 to 12) IQ: mean 106.1 (range > 80) Methylphenidate naive: 13 have not received past stimulant treatment Ethnicity: Caucasian (72%), African American (22%), Asian/Hispanic (6%) Country: USA Setting: out‐patient clinic Comorbidity: medically healthy Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of methylphenidate, dextroamphetamine and placebo Mean methylphenidate dosage: 1.3 mg/kg Administration schedule: twice daily, 9:00 AM and 1:00 PM Duration of each medication condition: 3 weeks Washout before study initiation: 2 weeks Medication‐free period between interventions: none Titration period: During the 3 weeks, low dose was given week 1, intermediate dose week 2 and high dose week 3 Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double‐blind random fashion |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Oral medication in identical capsules was administered at 9:00 AM and 1:00 PM |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | All participants except 1 (who experienced adverse events) completed the study Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol; however, all outcomes stated in the Methods have been reported |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: not stated |
| Methods | Randomised, double‐blind, cross‐over, multi‐centre study evaluating the efficacy of the following over an 8‐hour laboratory classroom day in children with ADHD
Phases
| |
| Participants | Number of participants screened: 92 Number of participants included: 86 (53 boys, 33 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 86 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (87.2%), hyperactive‐impulsive (0%), inattentive (12.8%)) Age: mean 9.5 years (range 6 to 12) IQ: > 70 Methylphenidate naive: 0% Ethnicity: Caucasian (48.8%), African American (24.4%), Asian (2.3%), Hispanic (23.3%) Country: USA Setting: multi‐centre, out‐patient clinic Comorbidity: not stated Comedication: no antidepressant or other antipsychotic medication Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of once‐daily, extended‐release dexmethylphenidate 20 mg (Focalin XR (Novartis Pharmaceuticals Corporation) and placebo Mean methylphenidate dosage: 20 mg Administration schedule: once daily in the morning Duration of each medication condition: 7 days Washout before study initiation: 1 week before the study Titration period: Before study participation, all participants were stabilised on a total daily dose or nearest equivalent dose of methylphenidate 40 mg to 60 mg or dexmethylphenidate 20 mg to 30 mg for ≥ 2 weeks before screening Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; children with poor response or known sensitivity to methylphenidate or dexmethylphenidate were excluded Comments from study authors
Key conclusions of study authors
Email correspondence with study authors: September 2013. We received an email from Dr. Brams, in which we were told that Novartis had control and ownership of study data. Consequently, we had to contact the Public Affairs Department at Novartis to request the information (e.g. protocols) (Krogh 2013a [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by the study sponsor, who used an automated random assignment of treatment sequences to randomisation numbers in the specified ratio |
| Allocation concealment (selection bias) | Low risk | All study medications and packaging were identical in appearance for blinding purposes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, parents, study centre personnel and those who assessed outcomes were blinded to study treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, parents, study centre personnel and those who assessed outcomes were blinded to study treatment |
| Incomplete outcome data (attrition bias) | Low risk | The safety population consisted of all participants who took ≥ 1 dose of study medication. The efficacy population included all randomly assigned participants who provided valid efficacy measurements for both treatment periods Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | High risk | Sponsored by Novartis Pharmaceuticals Corporation Conflicts of interest: First study author has been a speaker, consultant and advisory board member for Novartis and Shire |
| Methods | Randomised, double‐blind, 3‐period × 3‐treatment cross‐over study in a 12‐hour laboratory classroom setting with 3 interventions
Each period lasted 7 days | |
| Participants | Number of participants screened: not stated Number of participants included: 165. Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 157 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐IV (combined or predominantly hyperactive‐impulsive subtype) Age: mean 9.6 years (range 9.3 to 10.0) IQ: above normal Sex: 57% boys, 43% girls Methylphenidate naive: 0% Ethnicity: Caucasian (38.2%), African American (31.5%), Hispanic (22.4%), other (7.9%) Country: USA Setting: out‐patient clinic (laboratory classroom) Comorbidity: no significant medical illness Comedication: no information Sociodemographics: no information Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 20 mg extended‐release dexmethylphenidate, 30 mg extended‐release dexmethylphenidate and placebo Administration schedule: once daily, morning Duration of each medication condition: 7 days Washout before study initiation: 1 week Medication‐free period between interventions: no Titration period: none (fixed doses) Treatment compliance: no information | |
| Outcomes | ADHD symptoms
Serious adverse events
One participant experienced 2 serious adverse events (peritonsillar abscess and oral bullae) while receiving 20 mg extended‐release dexmethylphenidate and was hospitalised for 6 days for the peritonsillar abscess. Serious adverse events were considered not related to study drug. Participant discontinued the study for missed study drug during hospitalisation Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; children with a poor prior response, or known sensitivity, to all methylphenidate or dexmethylphenidate products based on medical history were excluded Comments from study authors (limitations)
Key conclusions of study authors
Email correspondence with study authors: September 2013. Not possible to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 6 treatment sequences. All participants were given the lowest available number from the randomisation numbers provided at each site. A randomisation list was produced by using a validated system that automated the random assignment of treatment sequences to randomisation numbers in the specified ratio |
| Allocation concealment (selection bias) | Low risk | Randomisation data were kept strictly confidential until the time of unblinding |
| Blinding of participants and personnel (performance bias) | Low risk | All study medications and packaging were identical in appearance for blinding purposes. Participants, parents, study centre personnel and those who assessed outcomes were blinded to study treatment |
| Blinding of outcome assessment (detection bias) | Low risk | All study medications and packaging were identical in appearance for blinding purposes. Participants, parents, study centre personnel and those who assessed outcomes were blinded to study treatment |
| Incomplete outcome data (attrition bias) | Low risk | Eight drop‐outs from the methylphenidate group. The ITT population included all randomly assigned participants who took ≥ 1 dose of study medication and had ≥ 1 post‐dose efficacy measurement. The safety population consisted of all participants who took ≥ 1 dose of study medication Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | High risk | Funding by Novartis Pharmaceuticals Corporation with the following involvement reported: design and conduct of the study; collection, management, analysis and interpretation of data; and preparation, review and approval of the manuscript. All study authors are employees or consultants or have received research grants from pharmaceutical companies Conflicts of interest: All study authors are employees or consultants or have received research grants from pharmaceutical companies |
| Methods | Four‐week cross‐over trial with 2 interventions
Phases: 2 weeks of placebo and 2 weeks of methylphenidate treatment with sequence according to randomisation | |
| Participants | Number of participants screened: not stated Number of participants included: 11 (all boys). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 11 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III Age: mean 10 years, 5 months (range 9 years 1 month to 12 years 1 month) IQ: > 80 Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: b.i.d. Duration of each medication condition: 2 weeks Washout before study initiation: not stated Medication‐free period between interventions: time of day the pills were taken not stated Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusions of study authors
Email correspondence with study authors: November 2013. We received additional information regarding ethics approval, sample calculation, etc., from study authors. However, it was not possible to receive all requested data, as the study author no longer possessed raw data from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence of the 2 medication conditions was randomly assigned, but no information was provided on methods |
| Allocation concealment (selection bias) | Low risk | Triple blinding; dosage was administered twice daily in the form of opaque capsules packaged by hospital pharmacists to conceal the contents |
| Blinding of participants and personnel (performance bias) | Low risk | Child and parent, teacher and the physician were blinded to the child’s medication condition |
| Blinding of outcome assessment (detection bias) | Low risk | Physician was blinded to the child’s medication concealment |
| Incomplete outcome data (attrition bias) | Low risk | Data provided on all 11 participants Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | Unclear risk | Funded by National institute of Mental Health and National institutes of Health. Placebo and methylphenidate were supplied by CIBA‐GEIGY Corporation, Summit, New Jersey Conflicts of interest: not stated |
| Methods | Twelve‐week, randomised, parallel trial with 4 arms
| |
| Participants | Number of participants included: methylphenidate + cognitive training 10, cognitive training 10 Number of participants followed up: methylphenidate + cognitive training 10, cognitive training 10 Number of withdrawals: methylphenidate + cognitive training 0, cognitive training 0 Diagnosis of ADHD: DSM‐III (types not stated) Age: mean 11.36 years (range 6.4 to 11.9) IQ: 101.92 (range 91 to 136) Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: not stated Comorbidity: not stated Comedication: no. No child was receiving any psychopharmacological treatment Sociodemographics: not stated. No significant differences in baseline demographics between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate + cognitive training or to cognitive training only Mean methylphenidate dosage: 0.3 mg/kg (range 5 mg/d to 15 mg/d) Administration schedule: twice daily (morning and lunch) Duration of intervention: 12 weeks + 3 months (only with medication) Titration period: none Treatment compliance: not stated Cognitive training programme: individual, twice‐weekly, 1‐hour sessions for a total of 24 sessions spanning a 3‐month period | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label methylphenidate |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | All participants followed up |
| Selective reporting (reporting bias) | Low risk | No protocol/design published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | Low risk | Funding: research supported by US Public Health Services Grant from the National Institute of Mental Health (NIMH), and by the Biomedical Research Award from the National Institutes of Health (NIH). Methylphenidate provided by CIBA‐GEIGY Corporation, Summit, New Jersey Conflicts of interest: not stated |
| Methods | Eight‐week, double‐blind, randomised, cross‐over trial with 4 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 11 (all boys). Participants were randomly assigned to possible drug condition orders Number of participants followed up: 11 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 13 years, 7 months (range 12 years and 10 months to 14 years and 10 months) IQ: full‐scale mean 92.91 (range 86 to 104) Methylphenidate naive: not stated, but none of the participants had been treated with stimulants during the year preceding the study Ethnicity: African American (100%) Country: USA Setting: out‐patient clinic Comorbidity: conduct disorder, socialised aggressive (45%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criterion
| |
| Interventions | Interventions (mean dosage)
Mean methylphenidate dosage: methylphenidate low (4.38 mg), medium (12.55 mg), high (21.28 mg) Administration schedule: twice daily; morning and noon Duration of each medication condition: 2 weeks Washout before study initiation: none (but no stimulant treatment for the past year) Titration period: none Treatment compliance: Compliance was determined to be satisfactory | |
| Outcomes | ADHD symptoms At the end of each 2‐week trial, parents and teachers completed the following rating scales
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes; Institutional Review Board (IRB) at Emory University Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Comment from study authors
Email correspondence with study authors: October 2013. We received from study authors additional information about ethics approval, planned outcomes and participants followed up. Unfortunately, it was not possible for study authors to provide other data that we needed because the study was conducted many years ago, and study authors no longer had the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Drug order was randomly assigned across participants; no further description |
| Allocation concealment (selection bias) | Low risk | All medication was prepared in identical capsules by hospital pharmacists. Medication was dispensed in dated envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | All medication was prepared in identical capsules by hospital pharmacists. Medication was dispensed in dated envelopes. Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Researchers administered all measures that were proposed and reported these data in the published report |
| Vested interest bias | Low risk | Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health and Emory University Research Conflicts of interest: none reported |
| Methods | Double‐blind, randomised, cross‐over trial with 2 interventions
Phases: methylphenidate 10 mg, methylphenidate 15 mg, methylphenidate 20 mg, placebo | |
| Participants | Study consisted of 22 participants, but only 7 had ADD. As outcomes were reported separately for these 7 participants, we were able to include the study Number of participants screened: 25 Number of participants included: 22 (all boys). Participants were randomly assigned to 1 of 4 possible drug condition orders (in counterbalanced order) Number of participants followed up: 22 Number of withdrawals: 0 Diagnosis: DSM‐III (conduct disorder, with 7 of 22 also diagnosed with ADD) Age: mean:15.8 years (range 12.9 to18.9) IQ: 96.22 (SD 15.12, range 80 to 123) Methylphenidate naive: not stated Ethnicity: Caucasian (100%) Country: USA Setting: hospital/out‐patient clinic Comorbidity: conduct disorder (100%) Comedication: no Sociodemographics: middle and upper‐middle class Inclusion criterion
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of methylphenidate (10 mg, 15 mg, 20 mg) and placebo Mean methylphenidate dosage: 0.15 mg/kg, 0.22 mg/kg and 0.31 mg/kg Administration schedule: twice daily, 8:00 AM and 12:00 PM Duration of each medication condition: 4 days Washout before study initiation: not stated Titration period: 1 day before first phase Treatment compliance: 100% | |
| Outcomes | General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; initially participants received a 1‐day open trial of methylphenidate. Three participants were excluded because of intolerability Comments from study authors
Key conclusions of study authors
Comment from review authors
Email correspondence with study authors: unable to locate contact details for study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | All participants received each of the 4 doses in 1 of 24 possible randomly assigned sequences |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Methylphenidate and placebo were packaged in coloured gelatin capsules by the hospital pharmacist to avoid detection of dose, visually or by taste |
| Blinding of outcome assessment (detection bias) | Low risk | Under double‐blinded conditions |
| Incomplete outcome data (attrition bias) | Low risk | All participants followed up |
| Selective reporting (reporting bias) | Unclear risk | No protocol published. Not all pre‐specified outcomes of interest have been reported (e.g. scores on Conners' Teacher Rating Scale) |
| Vested interest bias | Unclear risk | Conflicts of interest: not stated |
| Methods | Randomised, cross‐over trial with 3 interventions
Phases
First 32 participants were randomly assigned to interventions 1 to 3 in the first treatment block, and to intervention 1 or 2 in the second treatment block. Next 20 participants were randomly assigned to intervention 2 or 3 in the first treatment block, and to intervention 2 or 3 in the second treatment block | |
| Participants | Number of participants screened: not stated Number of participants included: 52 (46 boys, 6 girls); however, because of an incomplete block design, only 46 were treated with methylphenidate and 31 were treated with placebo in first or second treatment block Number of participants followed up: 52 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 9.3 years (range 6 to 13) IQ: mean 94.2 Methylphenidate naive: 100% Ethnicity: not stated Country: the Netherlands Setting: out‐patient clinic Comorbidity: conduct disorder (38%); depressive disorder, dysthymia or major depressive disorder (15%); anxiety disorder, overanxious disorder or avoidant disorder (42%); psychomotor epilepsy (2%) Comedication: antiepileptic medication (carbamazepine) at a fixed dosage (2%) Sociodemographics: 20% were from families of high socioeconomic status, 50% of middle socioeconomic status and 30% of low socioeconomic status (on the Hollingshead Index). No significant difference in baseline characteristics were noted between groups of children treated with methylphenidate, pindolol or placebo Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to possible drug condition orders of 40 mg pindolol, 20 mg methylphenidate and placebo Fixed dosage: 10 mg methylphenidate, twice daily (approximately 0.6 mg/kg/d) Administration schedule: morning and noon Duration of each medication condition: 4 weeks Washout before study initiation: no (medication naive) Medication‐free period between interventions: 2 weeks Titration period: yes. After randomisation, during the first 3 days of a treatment period, participants received 1 morning dose (10 mg methylphenidate, 20 mg pindolol or placebo). After completion of endpoint assessment, medication was tapered off (3 days with 1 morning dose) Treatment compliance: good to very good in 96% of children. Two children had poor compliance under methylphenidate treatment as the result of side effects | |
| Outcomes | ADHD symptoms
Parents and teachers completed ratings at baseline, at week 2 and at endpoint of each treatment period. The psychologist completed ratings at baseline and at endpoint of each treatment period. Furthermore, ACRS was rated 30 minutes after drug administration Non‐serious adverse events
| |
| Notes | Sample calculation: yes; for comparison of pindolol with methylphenidate (50 participants) Ethics approval: yes; approved by the Committee for Research on Human Subjects of Utrecht University Hospital Comments from study authors
Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no; only medication‐naive participants Any withdrawals due to adverse events: no; however, in 4 participants, dosages of methylphenidate had to be adjusted in the first 2 weeks of the study because of increased agitation, restlessness and insomnia. Two participants remained on 5 mg of methylphenidate for 4 weeks, whereas dosage for the other 2 participants could be gradually increased to 10 mg methylphenidate in the last 2 weeks Email correspondence with study authors: January to March 2014. Requested but did not receive from study authors supplemental efficacy and safety data and information regarding randomisation, allocation concealment and blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, not further described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, not further described. Methylphenidate and placebo were administered in identical‐looking tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Two participants from the methylphenidate group had bad compliance but were included in the analyses, as an ITT analysis was planned Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no, but design of study changed during the course of the study because of adverse events |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: no affiliations with pharmaceutical companies stated |
| Methods | Cross‐over trial with 3 interventions
Phases: Study included 2 phases: a baseline phase and the medication trial itself. The baseline phase occurred during the first 2 weeks (9 days) of the programme, when the children were medication‐free. Each medication condition was administered for 7 days of the programme during the 21‐day trial | |
| Participants | Number of participants screened: not stated Number of participants included: 18. Participants were randomly assigned to 1 of 3 possible drug condition orders Number of participants followed up: 18 (14 boys, 4 girls) Number of withdrawals: 0 DSM‐III‐R criteria for ADHD and oppositional defiant disorder or conduct disorder Age: mean 9.4 years (range 6.1 to 12.2) IQ: not stated Methylphenidate naive: not stated Ethnicity: Caucasian (17%), African American (83%) Country: USA Setting: out‐patient clinic (summer school at clinic) Comorbidity: oppositional defiant disorder (56%) and conduct disorder (44%) Comedication: no Sociodemographics: Participants were predominantly from lower socioeconomic classes, with an average Hollingshead Index of Social Status of 3.83 (SD 1.65, range 1 to 5). Thirteen (72%) of the participants' families were receiving public assistance. Only 4 of the children lived with both biological parents; 12 (67%) lived with their biological mother only. Inner city environment characterised by higher than average rates of poverty and community violence. No significant differences in baseline demographics between groups Inclusion criterion
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of methylphenidate (0.3 mg/kg or 0.6 mg/kg) and placebo Mean methylphenidate dosage: not stated Administration schedule: 8:30 AM, 11:45 AM and 3:00 PM Duration of each medication condition: 7 days Washout before study initiation: 9 days Medication‐free period between interventions: none Titration period: none Treatment compliance: poor compliance with the weekend medication condition; most families missed ≥ 1 dose each weekend of the trial. Poor compliance with the 3‐dose regimen was so widespread that trialists omitted from the trial all data on weekend doses | |
| Outcomes | ADHD symptoms
Non‐serious adverse event
| |
| Notes | Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Schedule for each condition was randomly assigned across the 5 weekdays to minimise programme effects; the only qualifying condition was that approximately one‐half of the 7 days of each medication condition would occur during each half of the 21‐day trial |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Nurse and other Summer Treatment and Enrichment Program (STEP) staff, children and parents were blinded to dosages and schedules |
| Blinding of outcome assessment (detection bias) | Low risk | Each child's daily data were collected and entered by trained research associates, who were unaware of medication status |
| Incomplete outcome data (attrition bias) | Low risk | All included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Low risk | None |
| Methods | One‐week, double‐blind, parallel trial with 3 arms
| |
| Participants | Number of participants screened: not stated Number of participants included: 30 (all boys) Number of participants randomly assigned: methylphenidate 10, placebo 10 Number of participants followed up: methylphenidate 10, placebo 10 Diagnosis of ADHD: DSM‐III Age: mean in years (range 6 to 12) IQ: above 85 Methylphenidate naive: not stated Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated. No information about significant differences in baseline demographics between groups Inclusion criteria
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to adrenocorticotropic hormone, methylphenidate or placebo Mean methylphenidate dosage: 0.5 mg/kg Administration schedule: once daily, 7.30 AM Duration of intervention: 1 week. One week drug‐free followed by 1 week of placebo treatment. After placebo washout, randomly assigned to adrenocorticotropic hormone, methylphenidate or placebo Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse event
EEG, haematology, blood chemistry and urinalysis were within normal limits before treatment and remained so after treatment | |
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After placebo washout, treatment was assigned in a double‐blind and random manner |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Neurologist assessing EEG blinded. Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Low risk | Funded by the Scientific Development Group, Organon International B.V., Oss, the Netherlands Conflicts of interest: none |
| Methods | Double‐blind, placebo‐controlled, repeat‐measures (across drug and dosage), cross‐over trial with 6 interventions
Phases: Each child received placebo, desipramine, each of the 3 doses of methylphenidate (10 mg, 15 mg, 20 mg) and combined desipramine and methylphenidate (at the same 3 doses) | |
| Participants | Number of participants screened: not stated Number of participants included: 16 (all boys). Participants were randomly assigned to different drug condition orders and placebo Number of participants followed up: 16 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (62.5%), inattentive (12.5%), “in partial remission” (25%)) Age: mean: not reported (range 7.9 to 12.10 years) IQ: mean not stated (range overall 81 to 121; range verbal 74 to 113, range performance 73 to 126) Methylphenidate naive: 4 (25%) Ethnicity: Caucasian (87.5%), African American (12.5%) Country: USA Setting: in‐patient ward Comorbidity: yes; major depressive disorder (68.75%), dysthymic disorder (31.25%). “All had ODD, CD or both” Comedication: yes Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of methylphenidate (10 mg, 15 mg and 20 mg), twice daily at 7:30 AM and 11:30 AM × 6 days, then 1‐day washout; the titrated therapeutic level (125 ng/mL to 225 ng/mL) of desipramine twice daily at 3:30 PM and 7: 30 PM × 3 weeks minimum before final measures taken; and placebo Mean methylphenidate dosage: not stated Duration of each medication condition: 1 week Washout before study initiation: 14 days Medication‐free period between intervention: 1 day Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: maybe; hence exclusion criterion number 1: medical contraindication to medications being investigated Email correspondence with study authors. Emailed first study author to ask for outcome data in mean and SD format. Study authors replied in July 2014 to say that they were unable to help us | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not stated |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Medications were packaged in identical grey capsules (size 00) that were administered 4 times per day |
| Blinding of outcome assessment (detection bias) | Low risk | For this protocol, all raters (including teachers, nurses, psychologist and physicians, except for the attending child psychiatrist, who controlled the desipramine dosage but did not rate the children), children and parents were blinded to all medication conditions |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Unclear risk | Not stated |
| Methods | Six‐week, multi‐centre, double‐blind, parallel, randomised controlled trial (RCT) with 2 arms
RCT was preceded by a 4‐week, open‐label, atomoxetine and placebo phase, during which participants who had adequate response were removed | |
| Participants | Number of participants screened: not stated Number of participants included: phase 1 (4‐week open‐label atomoxetine and placebo phase) 25 (1 boy, 20 girls); phase 2 (6‐week, double‐blind RCT) 17 Number of participants randomly assigned: methylphenidate 9, placebo 8 Number of participants followed up: methylphenidate 8, placebo 7 Number of withdrawals: methylphenidate 1, placebo 1 Diagnosis of ADHD: DSM‐IV (combined (79% in phase 1)) Age: phase 1 mean 9.6 years (range 6 to 12) IQ: > 70 Stimulant‐naive: 4% Ethnicity: Caucasian (83%), other (17%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (50% in phase 1) Comedication: not stated Sociodemographics: not stated Inclusion criteria, including assessment details
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to extended‐release OROS methylphenidate or placebo Cointervention: atomoxetine Methylphenidate mean dosage: 1.02 mg/kg Administration schedule: once daily Duration of intervention: 6 weeks Titration period for methylphenidate: after randomisation to target dose of 1.08 mg/kg/d (max 1.2 mg/kg/d) Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: Included sample (25 participants) is too small in relation to the sample calculation (85 participants) Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no, but exclusion of atomoxetine/placebo responders before study phase 2 Comment from study authors
Key conclusion of study authors
Comment from review authors
Email correspondence with study authors. June to November 2013. We received from study authors and sponsoring pharmaceutical company, supplemental information regarding IQ and blinding procedures, as well as data on weight, treatment‐emergent adverse events and ECG | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned via an interactive voice response system to receive extended‐release methylphenidate or placebo |
| Allocation concealment (selection bias) | Low risk | Investigators and participants were blinded to the precise visit at which randomisation to blinded placebo or Concerta occurred, as this visit is not identified in the investigator’s copy of the protocol, and as use of blinded study drug begins at visit 2 and continues up to visit 8 |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Efficacy outcome measures were conducted on the ITT sample by using an LOCF method Selection bias: no |
| Selective reporting (reporting bias) | Low risk | Protocol identified |
| Vested interest bias | High risk | Funding: Research was funded by Eli Lilly and Company, Indianapolis, Indiana Conflicts of interest: Dr. Carlson has received research support or has consulted with the following companies: Abbott Laboratories, Cephalon, Eli Lilly and Company, Janssen, McNeil, Otsuka and Shire Pharmaceuticals. Dr. Dunn has received research support or has served on Speakers' Bureaus of the following companies: AstraZeneca, Eli Lilly and Company, NIH, Otsuka and Pfizer Pharmaceuticals. Drs. Kelsey, Ruff, Ball and Allen and Ms. Ahrbecker are employees and/or shareholders of Eli Lilly and Company |
| Methods | Nine‐week, double‐blind, cross‐over trial with 3 interventions for 3 weeks each
Study was followed up by an open clinical follow‐up of 22 months | |
| Participants | Number of participants screened: 64 Number of participants included: 22 (all boys). Participants were randomly assigned to different possible drug condition orders Number of participants followed up: 20 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐III‐R Age: mean 9.4 years (range 6 to 13) IQ: mean 98.8 Methylphenidate naive: 0 Ethnicity: Caucasian (80%), African American (10%), Asian (5%), Hispanic (5%) Country: USA Setting: out‐patient clinic Comorbidity: Tourette’s disorder (95%), chronic motor tics (5%), conduct disorder (5%), oppositional defiant disorder (30%), reading disorder (5%), overanxious disorder (5%), obsessive‐compulsive disorder (10%), enuresis (20%) Comedication: 4 received haloperidol Sociodemographics: no information Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of methylphenidate and placebo. Methylphenidate was increased weekly. For body weight > 30 kg, weekly methylphenidate doses were 15 mg/dose, 25 mg/dose and 45 mg/dose b.i.d. (for weight ≤30 kg, 12.5: 25 mg/dose and 45 mg/dose, b.i.d.) Mean methylphenidate dosage: main cohort 1.20 mg/kg, second cohort 0.69 mg/kg, third cohort 1.22 mg/kg Administration schedule: twice a day: breakfast and lunch Duration of each medication condition: 3 weeks. First cohort underwent weekly increases in stimulant doses described as low, medium and high. Second cohort underwent increase described as low, medium and medium, and the third cohort as low, high and high Washout before study initiation: minimum 4 weeks Medication‐free period between interventions: 20 hours Titration period: during the first 3 weeks of intervention Treatment compliance: no information | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: approved by the National Institute of Mental Health (NIMH) Institutional Review Board Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusion of study authors
Comment from review authors
Email correspondence with study authors: January 2014. Corresponding author was not able to supply us with further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Capsule formulation prepared by a pharmacy |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Two drop‐outs; their data are not included in subsequent analyses. One of these was dropped from the study because of acute exacerbation of tics, and 1 because of excessively disruptive behaviour |
| Selective reporting (reporting bias) | High risk | Yes; they seem to have reported only hyperactivity scores from Conners’ Teacher Rating Scale |
| Vested interest bias | Low risk | None declared Conflicts of interest: not stated |
| Methods | Six‐week within‐participant, randomised, placebo‐controlled, double‐blind, daily cross‐over trial with 3 interventions
Phases: daily cross‐over between medication conditions. Two weeks of baseline and adjustment period before 6‐week medication trial. Data from this paper pertain to 5‐ and 6‐year‐old children attending a summer treatment programme between 1987 and 1997 | |
| Participants | Number of participants screened: not stated Number of participants included: 36 (32 boys, 4 girls). Participants were randomly assigned to 1 of 3 possible groups Number of participants followed up: 36 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (n = 35, subtype not stated); DSM‐IV (n = 1, combined type) Age: mean 6.13 years (range 5 to 6) IQ: mean 102 (SD 15.50) Methylphenidate naive: not reported Ethnicity: Caucasian (86%), other (14%) Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: oppositional defiant disorder (50%), conduct disorder (27.7%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned, daily, to 1 of 3 possible interventions: 0.3 mg/kg methylphenidate, 0.6 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: b.i.d. 7:45 AM and 11:45 AM from Monday through Thursday Duration of each medication condition: daily shifted Washout before study initiation: not stated Medication‐free period between interventions: maximum 18‐hour gap between doses (11:45 AM to 7:45 AM the following day) and no medication Thursday to Sunday Titration period: Before the 6‐week trial began, participants underwent a 2‐week baseline and adjustment period Treatment compliance: not stated | |
| Outcomes | Serious adverse events
Non‐serious adverse events
Side effects are reported as occurring: "none" (i.e. symptom was assessed and was found absent), "mild" (i.e. symptom was present but was not sufficient to cause concern among child, peers or adults), "moderate" (i.e. symptom caused impairment of functioning or social embarrassment to the degree that benefits of medication must be considerable to justify risks of continuing medication) or "severe" (i.e. symptom caused significant impairment of functioning or social embarrassment to the degree that the child should not continue to receive medication). Rating of "moderate" or "severe" signifies clinically significant side effects. Side effects are based on staff/parent subjective report on severity of the side effect. Children were considered to have significant side effects in a particular area if clinically significant side effects were reported within that area for ≥ 50% of observations in a given condition | |
| Notes | Sample calculation: no information Ethics approval: no information Comments from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: unclear; 2 weeks of adjustment period before the medication condition ‐ no information on how many were screened and how many participated during baseline weeks Comments from review authors
Email correspondence with study authors: December 2013. We emailed study authors to request additional information about study sample and side effects. Study authors replied to say that the data are no longer available. Data from this study could not be used in meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Active medication and placebo were disguised in opaque capsules and were dispensed in daily pill reminders. Each condition occurred one or two times per week, with the order of the conditions randomized on a daily basis. Data were averaged across days within conditions for each child" |
| Allocation concealment (selection bias) | Unclear risk | “Each condition occurred one or two times per week, with the order of the conditions randomized on a daily basis” |
| Blinding of participants and personnel (performance bias) | Low risk | “Active medication and placebo were disguised in opaque capsules and were dispensed in daily pill reminders” Double‐blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blinding |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Side effects were measured for 30 participants |
| Selective reporting (reporting bias) | High risk | Unclear how the population sample was selected from the group of attendees at these programmes over a 10‐year period |
| Vested interest bias | High risk | During the conduct of this research, Dr. Pelham was supported by grants from the National Institute of Mental Health (NIMH) (MH48157, MH47390, MH45576, MH50467, MH53554, MH62946), NIAAA (AA06267, AA11873), National Institute on Drug Abuse (NIDA) (DA05605, DA12414), National Institute of Neurological Disorders and Stroke (NINDS) (NS39087), National Institute for Environmental Studies (NIES) (ES05015) and National Institute of Child Health and Human Development (NICHHD) (HD42080) Conflicts of interest: Several study authors have affiliations with medical companies |
| Methods | Five‐week, multi‐centre, multiple‐setting, phase III, double‐blind, randomised, placebo‐controlled, parallel trial with 4 arms
One to 3 weeks titration and 2 to 4 weeks maintenance period dependent on the allocated active drug group | |
| Participants | Number of participants screened: 332 Number of participants included: 253 (163 boys, 90 girls) Number of participants randomly assigned: extended‐release dexmethylphenidate hydrochloride 188 (10 mg, n = 66; 20 mg, n = 62; 30 mg, n = 60), placebo 65 Number of participants followed up: extended‐release dexmethylphenidate hydrochloride 161 (10 mg, n = 56; 20 mg, n = 54; 30 mg, n = 51), placebo 57 Number of withdrawals: extended‐release dexmethylphenidate hydrochloride 27 (10 mg, n = 10; 20 mg, n = 8; 30 mg, n = 9), placebo 8 Diagnosis of ADHD: DSM‐IV (combined (73.9%), hyperactive‐impulsive (2.8%), inattentive (21.7%), missing data (1.6 %)) Age: mean 8.7 years (SD 1.84, range not reported) IQ: not stated Methylphenidate naive: 69.2% Ethnicity: Caucasian (57.7%), African American (28.9%), Asian (0.8%), other (12.6%) Country: 34 centres in the United States Setting: out‐patient clinic Comorbidity: respiratory, thoracic and mediastinal disorders (methylphenidate 26.9%, placebo 38.1%), immune system disorders (methylphenidate 22.5%, placebo 14.3%), nervous system disorders (methylphenidate 18.7%, placebo 19.0%), surgical and medical procedures (methylphenidate 15.4%, placebo 11.0%), infections and infestations (methylphenidate 14.3%, placebo 17.5%) and skin and subcutaneous tissue disorders (methylphenidate 11.0%, placebo 9.5%) Comedication: ≥ 1 concomitant medication or non‐drug therapy after start of study (methylphenidate 39.0%, placebo 44.4%). The most common concomitant medications were analgesics, antihistamines and allergy medications Sociodemographics: not stated. Treatment groups were well balanced in relation to participant background characteristics, and baseline demographics were comparable among treatment groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to extended‐release dexmethylphenidate hydrochloride or placebo Fixed methylphenidate dosage: 10 mg, 20 mg or 30 mg Administration schedule: once daily, morning Duration of intervention: 5 weeks Washout before study initiation: up to 28 days (duration dependent on the half‐life of any previous psychotropic medication) Titration period: 1 to 3 weeks, initiated after randomisation Treatment compliance: 86% completed | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes; 252 participants (63 per treatment group) Ethics approval: Institutional review boards or ethical review committees at each centre approved the study Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from study authors
Key conclusions of study authors
Comment from review authors
Email correspondence with study authors in December 2013. We have contacted study authors twice in an attempt to obtain supplemental information regarding blinding, allocation concealment and weight loss. We have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by using a validated system that automated the assignment of treatment arms to randomisation numbers in the specified ratio |
| Allocation concealment (selection bias) | Low risk | Allocation concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, but no detailed description |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, but no detailed description |
| Incomplete outcome data (attrition bias) | Low risk | ITT population. LOCF Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | High risk | This study was funded by Novartis Pharmaceuticals Corporation. Novartis Pharma has been helping with development of the manuscript Conflicts of interest: Several study authors have received research support from, are speakers for, are consultants of, are on the Advisory Board, have served on the Speakers' Bureaus of or are employees of several pharmaceutical companies |
| Methods | Eight‐week within‐participant, placebo‐controlled, randomised, cross‐over trial (Summer Treatment Camp (STP) with 3 interventions)
Phases: 2‐week baseline assessment followed by 6‐week medication trial | |
| Participants | Number of participants screened: 48 Number of participants included: 21 (19 boys, 2 girls). Participants were randomly assigned to 1 of 7 drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Age: mean 10.26 years (±1.9, range 6 to 12) IQ: mean 109.9 (±18.8) Methylphenidate naive: 14 children had received methylphenidate before start of the study Ethnicity: White (100%) Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: learning problems (42.9%), oppositional defiant disorder (66.7%), conduct disorder (23.8%) Comedication: not stated Sociodemographics (median family income = $35,000/y (< $15,000/y to > $100,000/y); 66.7% of parents married Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 7 possible drug condition orders of immediate‐release methylphenidate, Adderall and placebo
Mean methylphenidate dosage: not stated Administration schedule: methylphenidate once, twice or 3 times daily according to the randomisation procedure Duration of each medication condition: All participants received medication each Monday through Friday throughout a period of 6 weeks for a 24‐day clinical assessment period. Assessment period was divided into three 8‐day segments. Within each segment, placebo occurred twice and each other condition occurred once, with the order of conditions randomly assigned on a daily basis Washout before study initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Any withdrawals due to adverse events: none during the trial Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusions of study authors
Comments from review authors
Email correspondence with study authors: July 2014. Emailed study authors twice to request additional information. Study author was not able to provide us with additional data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomized by day”, no description of how randomisation took place |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | To ensure blinding, placebo capsules were given at 11:30 AM in the Adderall conditions and for applicable doses in the other conditions. Active medication and placebo were disguised in opaque gelation capsules by a local pharmacy and were dispensed in daily pill reminders by the study doctor. Furthermore, children were informed that they would be receiving 2 different kinds of medication to see how well they worked, and that some days they would receive inactive pills, but that they, their counsellors or teachers and their parents would not know what kind of pill they would get each day |
| Blinding of outcome assessment (detection bias) | Low risk | Children were informed that they would be receiving 2 different kinds of medication to see how well they worked, and that some days they would receive inactive pills, but that they, their counsellors or teachers and their parents would not know what kind of pill they would get each day |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of drop‐out Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Vested interest bias | High risk | Supported by a grant from Shire‐Richwood Pharmaceuticals, Incorporated ‐ manufacturer of Adderall ‐ and from the National Institute of Mental Health (NIMH) |
| Methods | Twelve‐week randomised, placebo‐controlled, double‐blind cross‐over trial with 3 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 75 (all boys). Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined type); ICD‐10 (hyperkinetic disorder) Age: mean not reported (range 7 to 15 years) IQ: > 80 Methylphenidate naive: 100% Ethnicity: not stated Country: United Kingdom Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (41.3%), conduct disorder (28%), depressive disorder (4%), generalised anxiety disorder (2.7%), separation anxiety disorder (4%), tic disorder (2.7%), social phobia (1.3%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders: 3 mg/kg/dose methylphenidate or 6 mg/kg/dose methylphenidate and placebo Administration schedule: twice daily Duration of each medication condition: 4 weeks Washout before study initiation: no Titration period: none Treatment compliance: assessed by pill count and clinical enquiry but not further described | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of study authors
Comment from study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors in 2013. Emailed study authors twice with a request for additional information regarding protocol and number of participants on which analyses were based. Have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by an independent clinical trials pharmacist (using a computer‐generated random number sequence with block design to ensure equal numbers in each treatment arm) |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned by an independent clinical trials pharmacist |
| Blinding of participants and personnel (performance bias) | Unclear risk | Placebo‐controlled, double‐blind, no further information |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical status was assessed by interview conducted by an experienced, blinded child and adolescent psychiatrist. Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | No description about how many participants were included in analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Unclear risk | This work was supported by a local trust through a Tenovus Scotland initiative Conflicts of interest: Some study authors have affiliations with different pharmaceutical companies |
| Methods | Seven‐week, multi‐centre, double‐blind, parallel, dose‐optimised trial with 3 arms
| |
| Participants | Number of participants screened: not stated Number of participants included: 221 (181 boys, 40 girls) Number of participants randomly assigned: methylphenidate 112, placebo 111 Number of participants followed up: methylphenidate 74, placebo 42 Number of withdrawals: methylphenidate 38, placebo 68 Diagnosis of ADHD: DSM‐IV‐TR (combined (methylphenidate 86.4%, placebo 79.1%), hyperactive‐impulsive (methylphenidate 0.9%, placebo 6.4%), inattentive (methylphenidate 12.7%, placebo 14.5%) Age: mean 10.9 years (range 6 to 17) IQ: normal Methylphenidate naive: methylphenidate 60 (54.1%), placebo 58 (52.7%) Ethnicity: Caucasian (methylphenidate 96.4%, placebo 98.2%), African American (0%), Asian (0%), Hispanic/Latino (methylphenidate 1.8%, placebo 0%) Countries: Germany, Sweden, Spain, Hungary, France, the UK, Italy, Belgium, Poland, the Netherlands Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (methylphenidate 9.0%, placebo 7.3%), concomitant psychiatric diagnosis (methylphenidate 26.1%, placebo 18.2%) Comedication: not stated Sociodemographics: not stated. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to OROS methylphenidate or placebo Mean methylphenidate dosage: 45.4 ± 12.7 mg/d (9.9% 18 mg, 19.8% 36 mg, 53.2% 54 mg) Administration schedule: once a day, 7:00 AM Duration of intervention: 7 weeks Titration period: 4‐week stepwise dose‐optimisation period after randomisation. Three‐week dose‐maintenance period followed by 1‐week washout and safety follow‐up Treatment compliance: 2 discontinued in placebo group because of non‐compliance, 3 in methylphenidate group | |
| Outcomes | ADHD symptoms
Quality of life
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: approved by an independent ethics committee/institutional review board and regulatory agency at each centre (as appropriate) before study initiation Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Comments from study authors
Key conclusions of study authors
Comment from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive voice/web response system was used to allocate a unique randomisation number to each participant. Randomisation was stratified by country and age group (6 to 12 or 13 to 17 years of age) |
| Allocation concealment (selection bias) | Low risk | Interactive voice/web response system |
| Blinding of participants and personnel (performance bias) | Low risk | Study drugs were overencapsulated and appeared identical |
| Blinding of outcome assessment (detection bias) | Low risk | Masking: double‐blinded (participant, caregiver, investigator, outcomes assessor) |
| Incomplete outcome data (attrition bias) | Unclear risk | LOCF approach was used when efficacy assessments were incomplete for a participant owing to early withdrawal from the study or for missing data. However, as the review authors cannot understand the relationship between the n of the full analysis set and the endpoint data, risk of bias was assessed as unclear Selection bias (e.g. titration before randomisation → exclusion): none |
| Selective reporting (reporting bias) | Low risk | Outcomes according to protocol |
| Vested interest bias | High risk | Funded by Shire Development LLC Conflicts of interest: C. Anderson, R. Civil, N. Higgins, A. Lyne and L. Squires are employees of Shire and own stock/stock options. Some study authors have received compensation for serving as consultants or speakers, or they or the institutions they work for have received research support or royalties from different companies or organisations |
| Methods | Three‐month, randomised, blind, parallel‐group study with 3 arms
| |
| Participants | Number of participants screened: 24 Number of participants included: 24 (methylphenidate + clonidine 8 boys, 0 girls; placebo + clonidine 8 boys, 0 girls) Number of participants randomly assigned: methylphenidate + clonidine 8, placebo + clonidine 8, placebo + methylphenidate 8 Number of participants followed up: methylphenidate + clonidine 8, placebo + clonidine 6 Number of withdrawals: methylphenidate + clonidine 0, placebo + clonidine 2 Diagnosis of ADHD: DSM‐III‐R (combined (100%)) Mean age: methylphenidate + clonidine 10.1 years, placebo + clonidine 9.3 years (range 6 to 16) IQ: > 70 Methylphenidate naive: 54% Ethnicity: Caucasian (11%), African American (1%), Asian (0%), Hispanic (0%), other (0%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder or conduct disorder (100%) Comedication: not reported Sociodemographics: No significant differences in baseline demographics were noted between groups Inclusion criteria
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to methylphenidate + clonidine or placebo + clonidine Methylphenidate dose: 35.0 (5.67) mg/d Administration schedule: b.i.d. Duration of intervention: 12 weeks Titration period: 4 weeks after randomisation Treatment compliance: “All subjects were acceptably compliant with the protocol” | |
| Outcomes | General behaviour
Non‐serious adverse events
| |
| Notes | Intervention group: methylphenidate + clonidine; control group: placebo + clonidine Ethics approval: yes Sample calculation: no Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: June 2013. We obtained additional information from study authors, but it was not possible to receive supplemental data. We could not perform a meta‐analysis on any of the outcomes, as we did not have relevant data and transformation was not possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Eight participants were randomly assigned to each group. Three participants were previous methylphenidate treatment failures and refused randomisation to the methylphenidate‐alone study arm. These 3 participants were partially randomly assigned to methylphenidate and clonidine or to clonidine alone. All other children were fully randomly assigned |
| Allocation concealment (selection bias) | Low risk | After study completion, the medication blind was broken. All medication capsules and placebo capsules were prepared by the UMMS (University of Massachusetts Medical School) Pharmacy in identical capsules to disguise taste and smell. All participants in all study groups received an equal number of capsules per day |
| Blinding of participants and personnel (performance bias) | Low risk | All teachers, school nurses, parents, children and research assistants completing dependent measures were blinded to the child's treatment group for the study duration. Completion of electrocardiograms (ECGs) for only 2 clonidine treatment groups may have broken blinding for parent raters and for the child (but not for teacher raters nor for research assistants administering the GDS, who remained blinded as to whether the child had received an ECG). This is not relevant to us, as we are using data only on the 2 groups completing ECGs |
| Blinding of outcome assessment (detection bias) | Low risk | As stated above |
| Incomplete outcome data (attrition bias) | Low risk | LOCF Selection bias: no |
| Selective reporting (reporting bias) | Low risk | Reply from study author on our request for the protocol: protocol described in the study |
| Vested interest bias | Low risk | Supported by a UMMS (University of Massachusetts Medical School) Small Grants Project Award Conflicts of interest: not stated |
| Methods | Six‐week, double‐blind, single cross‐over, placebo‐controlled study conducted on a group of 15 participants with ADD and a comparison group of 10 age‐matched participants who did not have ADD ADD group was randomly assigned to 1 of 2 experimental groups
| |
| Participants | Regarding the ADD group Number of participants screened: not stated Number of participants included: 15. Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 15 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (100% met DSM‐III criteria for ADD with hyperactivity) Age: mean 104.5 months (range 6 to 10 years) IQ: mean 110.5 (SD 7.15) Methylphenidate naive: 100% Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: central auditory processing disorder (80%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria Participants were excluded if they had
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 0.30 mg/kg (0.057) Administration schedule: not stated Duration of each medication condition: 3 weeks Washout before study initiation: not relevant, 100% treatment naive Medication‐free period between interventions: minimum of 48 hours between the 2 interventions Titration period: Dose was titrated up to a maximum (methylphenidate tablets or placebo tablets) over the first 3 weeks of the experimental period Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no information Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from study authors
Comment from review authors
Key conclusion of study authors
Email correspondence with study authors: September 2013. Emailed study author requesting additional information about the study and data. Dr. Cook referred to his Ph.D. dissertation, which we managed to get | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to groups by a table of random numbers known only to the pharmacist |
| Allocation concealment (selection bias) | Low risk | Drugs were coded and administered by a pharmacist, and clinical titration of dosage was done by the participant’s paediatrician; neither practitioner was involved in data collection |
| Blinding of participants and personnel (performance bias) | Low risk | Physician, audiologist, teachers and parents involved in behaviour ratings and participants themselves were blinded to assigned treatment |
| Blinding of outcome assessment (detection bias) | Low risk | Physician, audiologist, teachers and parents involved in behaviour ratings and participants themselves were blinded to assigned treatment |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | Low risk | Supported by the Medical Center Rehabilitation Hospital Foundation and the School of Medicine, University North Dakota; the Veterans Hospital; the Dakota Clinic; and The Neuropsychiatric Institute, Fargo, North Dakota Conflicts of interest: not stated |
| Methods | Three‐week, blind medication trial with cross‐over design, in which children were randomly assigned to the following
| |
| Participants | Number of participants recruited: 28 Number of participants included: 21 (15 boys, 6 girls) Number of withdrawals: 7 children excluded from final data analyses Diagnosis of ADHD: DSM‐IV (combined type (52%), hyperactive‐impulsive type (10%), inattentive type (38%)) Age: mean not reported (range 6 years 1 month to 12 years 1 month) IQ: no intellectual disability Methylphenidate naive: All participants were stimulant medication‐naive Comorbidity: learning disabilities (29%), oppositional defiant disorder (10%), conduct disorder (0%), generalised anxiety disorder (0%), depression (0%) Setting: out‐patient clinic Country: Canada Ethnicity: All participants were Caucasian Sociodemographics: predominantly middle‐class families Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 medication‐dosing schedules, including 1 week of baseline, placebo and low and moderate immediate‐release methylphenidate (ritalin) dose condition. Children weighing ≤ 25 kg received 5‐mg and 10‐mg doses; children weighing > 25 kg received 10‐mg and 15‐mg doses. Children received medication Treatment compliance: no information | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: August to October 2013. We received supplemental information regarding additional data from study authors, but we never received first period data from the cross‐over trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Baseline data from 1 week were collected, followed by a 3‐week medication trial with random assignment of children to 1 of 3 medication‐dosing schedules. The original, fully randomised schedule was modified after a pilot study, conducted before the current study, indicated that children receiving a moderate dose before the low dose of methylphenidate reported increased side effects and were more likely to stop taking the third dose (4:00 PM) of medication |
| Allocation concealment (selection bias) | Low risk | Pharmacy local to the ADHD clinic prepared both placebo and active medication, which were packaged into identical gelatin capsules |
| Blinding of participants and personnel (performance bias) | Low risk | Child, family, school personnel and study investigators were unaware of the randomisation schedule. This information was made available only to the paediatrician and the pharmacist |
| Blinding of outcome assessment (detection bias) | Low risk | Child, family, school personnel and study investigators were unaware of the randomisation schedule. This information was made available only to the paediatrician and the pharmacist |
| Incomplete outcome data (attrition bias) | Unclear risk | 7 children excluded from final data analyses for the following reasons: actigraphic problems (i.e. data failure/loss of actigraph/refusal to wear actigraph) (n = 5), withdrawal of consent due to marital discord (n = 1) and decision to try alternative medication immediately before the start of the medication trial (n = 1) |
| Selective reporting (reporting bias) | Low risk | No subsequent correspondence with first study author regarding additional information on reporting |
| Vested interest bias | Low risk | Research was supported by a grant from the Izaak Walton Killam IWK Health Centre in Halifax, Nova Scotia Conflicts of interest: "none declared" |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial of adolescent drivers with ADHD who were assessed on a driving simulator after taking
Phases: 3 (2 relevant phases) | |
| Participants | Number of participants screened: not stated Number of participants included: 35 (19 boys, 16 girls). Participants were randomly assigned to 1 of 2 relevant (n = 3) possible drug condition orders Number of participants followed up: 35 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined 21 (60%), hyperactive‐impulsive 2 (6%), inattentive 12 (34%)) Age: mean 17.8 years (range 16 to 19) IQ: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: agoraphobia (2.9%), conduct disorder and marijuana abuse (2.9%), obsessive‐compulsive disorder (2.9%), obsessive‐compulsive disorder and hypomania (2.9%), nicotine dependence (5.7%) Comedication: 2 were taking no medication, 21 were taking methylphenidate and 12 were taking amphetamine formulations at the start of the study Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 relevant drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 72 mg/d Administration schedule: 2 overlaid capsules/tablets in blister packs each day on awakening Duration of each medication condition: 17 days Washout before study initiation: not stated Medication‐free period between interventions: 4 to 21 days between OROS methylphenidate and mixed amphetamine salts Titration period: half dose (36 mg/d OROS methylphenidate) days 1 to 5, full dose (72 mg/d OROS methylphenidate) days 6 to 17 initiated after randomisation Treatment compliance: not stated. Pill counts were completed at each study visit | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Comments from study authors
Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; excluded if any history of adverse events to stimulant medications Email correspondence with study authors: February 2014. We received additional information from study authors. Data from the Self‐Reported Stimulant Drug Side Effects Rating Scale are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random‐numbers table, each participant was assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Treatments were provided in different forms: "Participants took 2 overlaid capsules/tablets in blister packs each day on awakening". Participants and research assistants were blinded to medication conditions |
| Blinding of outcome assessment (detection bias) | High risk | "Participants were either tested on placebo or were not required to come in for testing". Participants and research assistants were blinded to medication condition |
| Incomplete outcome data (attrition bias) | Low risk | Email correspondence with study author: no drop‐outs and all were followed‐up (Krogh 2013b [pers comm]) Selection bias (e.g. titration after randomisation → exclusion): none described |
| Selective reporting (reporting bias) | Low risk | Email correspondence with study author, who stated that all planned outcome measures and analyses are described in the papers (Krogh 2013b [pers comm]) |
| Vested interest bias | High risk | Study was supported by funding from McNeil Pediatrics, a division of McNeil‐PPC Incorporated Conflicts of interest: none described |
| Methods | Five‐day cross‐over trial with 2 interventions
Phases
Before the study was undertaken, 16 drug and test order combinations for the morning test battery were ordered randomly. As participants entered the study, they were assigned to these drug‐plus‐test order combinations until all 16 participants had been assigned. The order of the 2 tests in the afternoon battery (arithmetic and word discovery) was alternated over children, and each child received tests in the same order over 5 days of testing | |
| Participants | Number of participants screened: not stated Number of participants included: 16 (15 boys, 1 girl). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 16 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtypes not stated) Age: mean 9.2 years (range 6 to 11.6) IQ: mean103.19 (range 89 to 125) Methylphenidate naive: 13 (81%) Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: Children’s families varied from I to V on the Hollingshead and Redlich Index (with most families falling within level III to IV (V being the poorest) Inclusion criteria
Participants were referred to the Hyperactivity Project at Montreal Children’s Hospital by paediatricians or school personnel. Referral was based on presence of the following symptoms
Symptoms were of sufficient severity to prompt referring physicians to consider a trial on stimulant medication Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible orders of drug (D) or placebo (P): (1) PDDPP; (2) DPPDD; (3) DDPPD; (4) PPDDP. On days when participants were assigned to active medication, they received a capsule containing the quantity of medication closest to a calculated dose of 0.3 mg/kg for each dose (morning and afternoon) Mean methylphenidate dosage: not stated Administration schedule: morning capsule administered approximately 1 hour after breakfast and 45 minutes before morning test battery was administered. Second capsule, identical to the morning capsule, administered before child left for lunch and returned to school. Time between morning and afternoon capsule was approximately 3½ hours Duration of each medication condition: 1 day each; in all 2 or 3 days, depending on order of drugs assigned Washout before study initiation: 24 hours before screening, 48 hours before first testing day Titration period: none Treatment compliance: capsule administered by examiner | |
| Outcomes | ADHD symptoms
Score for the Index is based on the mean of item ratings on a 4‐point scale (0 to 3) | |
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: none stated Email correspondence with study authors: July 2014. Emailed study authors to ask for additional information. Received information on ethics approval, but first study author was not able to provide additional data or information on the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Capsule was administered by examiner |
| Incomplete outcome data (attrition bias) | Unclear risk | In a few cases in which data were missing, scores from 1 or 2 days were used to compute means for drug and placebo conditions. No information on drop‐out rate |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Vested interest bias | Low risk | Research was supported by Grant Number MA 6913, from the Medical Research Council of Canada Conflicts of interest: no information |
| Methods | Eight‐day, double‐blind, cross‐over trial in which participants were randomly assigned to different doses of methylphenidate and placebo
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 17 (16 boys, 1 girl). Participants were randomly assigned to 1 of 24 possible drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R. Also had to meet DSM‐III criteria for ADD with hyperactivity Age: mean 9 years 5 months (range 6 years 3 months to 11 years 9 months) IQ: mean 104.3 (range 89.0 to 127) Methylphenidate naive: 53% Ethnicity: not stated Country: Canada Setting: treatment centre (hospital) or out‐patient clinic Comorbidity: oppositional defiant disorder (71%), conduct disorder (18%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of methylphenidate (0.3 mg/kg, 0.6 mg/kg, 0.9 mg/kg) and placebo Mean methylphenidate dosage: 0.3 mg/kg: 9.71; 0.6 mg/kg: 19.42; 0.9 mg/kg: 29.14 Administration schedule: once daily Duration of each medication condition: 1 day; each child received each dosage twice during the study (i.e. during first and second assessment weeks) Washout before study initiation: In the case of children currently receiving stimulants, medication was discontinued ≥ 48 hours before screening Titration period: none Treatment compliance: To ensure compliance, medications were administered at the laboratory | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: no information. Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Email correspondence with study authors: October 2013. Emailed last study author twice to get supplemental information (protocol, ethics approval, data on side effects, etc.) but received no response. Therefore we included no data from this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Drug order was determined by consecutive assignment to a randomly ordered list of 24 possible combinations of 4 medication levels for each of the 2 testing weeks |
| Allocation concealment (selection bias) | Low risk | Drug order was determined by consecutive assignment to a randomly ordered list of 24 possible combinations of 4 medication levels for each of the 2 testing weeks. To maximise blindness of examiners, all participants received a different drug order for assessment weeks 1 and 2 |
| Blinding of participants and personnel (performance bias) | Low risk | Medications containing active drug and placebo were prepared in identical opaque gelatin capsules and were administered in a double‐blind fashion |
| Blinding of outcome assessment (detection bias) | Low risk | To maximise blindness of examiners, all participants received a different drug order during assessment weeks 1 and 2 |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated whether any participants were LTFU |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Low risk | Funded by grants from the Medical Research Council of Canada and by William T. Grant Foundation Faculty Scholar Program Conflicts of interest: none |
| Methods | Four‐week, double‐blind, placebo‐controlled, cross‐over trial in which participants were randomly assigned to 3 doses of methylphenidate
| |
| Participants | Number of participants screened: not stated Number of participants included: 24 (19 boys, 5 girls). Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 24 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 11.09 years (range 9 to 15) IQ: > 70 Methylphenidate naive: not stated Ethnicity: Caucasian (100%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (21%), conduct disorder (% not reported) Comedication: not stated Sociodemographics: Children were primarily from lower middle class and middle class families Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of low‐ (0.16 mg/kg; SD 0.08), moderate‐ (0.29 mg/kg; SD 0.11 kg) and high‐dose (0.42 mg/kg; SD 0.14) methylphenidate and placebo Administration schedule: b.i.d., morning, noon Duration of each medication condition: 1 week Washout before study initiation: not stated Titration period: none Treatment compliance: No participant was removed from the investigation for non‐compliance (e.g. > 1 day of failure to administer medication as scheduled) | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: Human Subjects Research Board at the University of Massachusetts Medical Center Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Email correspondence with study authors: January 2013 to August 2013. Emailed first study author regarding additional information. Not able to get all data requested, as study author no longer has these data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomly assigned to 1 of 6 possible orders of methylphenidate dosage |
| Allocation concealment (selection bias) | Low risk | Medication was prepared by the hospital pharmacy in increments of 5 mg and packaged within opaque gelatin capsules |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, their parents and teachers and the research assistant in charge of collecting data were blinded to the order of medication |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, their parents and teachers and the research assistant in charge of collecting data were blinded to the order of medication |
| Incomplete outcome data (attrition bias) | Low risk | No Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Analyses on all dependent measures in the study are reported in the article |
| Vested interest bias | Low risk | No conflicts of interest |
| Methods | Randomised, controlled trial, parallel with 3 arms
| |
| Participants | Number of participants screened: 628 Number of participants included: 130 Number of participants randomly assigned: methylphenidate 44, control 42 Number of participants followed up: methylphenidate 30 (23 boys, 7 girls), control 30 (22 boys, 8 girls) Number of withdrawals: methylphenidate 14, control 12 Diagnosis of ADHD: ICD‐10 (subtype not stated) Age: methylphenidate 11.2 (SD 2.8), control 11.4 (SD 3.1) IQ: mean 87 Methyphenidate‐naive: not stated Ethnicity: not stated Country: Norway Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate and neurofeedback (intervention group) or to neurofeedback (control group) Methylphenidate dosage: 20 mg daily to 60 mg daily. No placebo pill Administration schedule: twice per day Duration of intervention: 11 to 13 weeks Titration period: not stated Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Ethics approval: yes Comments from study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: March to June 2013. Emailed first study author. All questions were answered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children with ADHD were randomly placed into 3 groups |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | No blinding, as it is not a “pure” placebo group |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of parents |
| Incomplete outcome data (attrition bias) | Low risk | No |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
| Vested interest bias | Low risk | No Conflicts of interest: Study authors declare no potential conflicts of interests with regard to authorship or publication of this article |
| Methods | Randomised, double‐dummy, double‐blind, cross‐over, multi‐centre trial with 3 interventions
Phases: Trial was subdivided into 5 stages: pre‐screening, run‐in phase (duration: 1 workday), trial phases 1 and 2 (duration in each case: 4 workdays plus weekend) and trial phase 3 (duration: 4 workdays). Participants also received a behavioural therapy intervention and social skills training at school | |
| Participants | Number of participants screened: not stated Number of participants included: 82. Participants were randomly assigned to different orders of immediate‐release methylphenidate, extended‐release methylphenidate and placebo Number of participants followed up: 79 (71 boys, 8 girls) Number of withdrawals: 3 Regarding the group followed up Diagnosis of ADHD: DSM‐IV or ICD‐10 (combined (92.4%), hyperactive‐impulsive (0%), inattentive (7.6%)) Age: mean 10 years (range 6 to 16) IQ: 103 ± 10.4 Methylphenidate naive: 0% Ethnicity: not stated Country: Germany Setting: out‐patient clinic Comorbidity: oppositional defiant disorder/conduct disorder (44%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned Mean methylphenidate dosage: 22 mg ± 6 mg. Dosage was identical in methylphenidate groups but did not exceed 1 mg/kg body weight. Thus, 9 (11%) participants received a daily dose of 10 mg, 54 (68%) received 20 mg, 14 (17%) received 30 mg and 2 (3 %) received 40 mg. Administration schedule: 9:00 AM and 1:00 PM Duration of each medication condition: 4 days, and for trial phase 1 + 2 (also weekends) Washout before study initiation: none Medication‐free period between interventions: none Titration period: had to be oriented to the optimum individual dosage previously determined in clinical treatment trials initiated before randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: approved by local university ethics committees Comment from study authors
Carry‐over effect: As no evidence for possible carry‐over effects was noted, no secondary analyses for carry‐over effects were performed Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate: yes; included only methylphenidate responders Email correspondence with study authors. We received some data from study authors in July 2013. We sent 2 additional emails to different study authors to request data in July 2014 but received no answer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order in which participants were allocated to respective treatment arms was randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Order in which participants were allocated to respective treatment arms was randomly assigned |
| Blinding of participants and personnel (performance bias) | Low risk | To guarantee a double‐blind trial, the double‐dummy method was used (i.e. participants took the capsule once a day and the tablets twice daily). Only 1 of the 2 galenical forms contained the active substance; the other form contained placebo. In the placebo group, participants took both placebo capsules and placebo tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol obtained |
| Vested interest bias | High risk | Study was conducted and sponsored by MEDICE Arzneimittel Pütter GmbH & Co. KG as part of the drug approval process for Medikinet‐RetardTM Conflicts of interest: Some study authors have affiliations with medical companies |
| Methods | Cross‐over trial with 2 interventions Three preliminary phases to determine optimal dose followed by 2‐phase trial of the following
| |
| Participants | Number of participants screened: not stated Number of participants included: not clear Number of participants followed up: 93 (numbers of boys and girls: not stated) Number of withdrawals: not clear Diagnosis of ADHD: DSM‐IV (combined (n = 45), hyperactive‐impulsive (n = 48), inattentive (n = 0)) Age: mean 8.11 years (range 7 to 11) IQ: mean 105.58 Methylphenidate naive: 100% Ethnicity: Caucasian (75%), African American (22%), other (3%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (n = 34), conduct disorder (n = 4), anxiety disorders (n = 31), mood disorders (n = 2) Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to an optimal dose of methylphenidate and placebo Mean methylphenidate dosage: 1.13 mg/kg Administration schedule: testing 1 to 4 hours after medication ingestion Duration of each medication condition: 1 week Washout before study initiation: no apparent washout Titration period: Each of the 3 doses was trialled for 1 week before random assignment to identify an optimal dose Treatment compliance: not reported | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Funding: National Institute of Health (NIH) and National Institute of Mental Health (NIMH) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusion of study authors
Email correspondence with study authors: April 2014. We were able to obtain supplemental information regarding data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind"; capsules were "identical" (p 2, p 1063) |
| Blinding of outcome assessment (detection bias) | Low risk | "Double‐blind", capsules were "identical" (p 2, p 1063) |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear whether any participants were LTFU |
| Selective reporting (reporting bias) | Low risk | No selective reporting |
| Vested interest bias | Low risk | No evidence of vested interests No evidence of conflicts of interest |
| Methods | Randomised, double‐blind, within‐participant design, cross‐over trial with 2 interventions
Phases: 0.15 mg/kg, 0.30 mg/kg and 0.60 mg/kg. Medication was randomly assigned for each child and varied daily during a 9‐week summer treatment programme | |
| Participants | Number of participants screened: not stated Number of participants included: 48 (44 boys, 4 girls). Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 47 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (not stated), hyperactive‐impulsive (not stated), inattentive (not stated)) Age: mean 9.35 years (SD 1.98, range 5 to 12) IQ: 106.33 (SD 14.61) Methylphenidate naive: not stated Ethnicity: Caucasian (79%); African American (12.5%); Hispanic, Native American or mixed race (8.5%) Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of immediate‐release methylphenidate (0.15 mg/kg, 0.30 mg/kg and 0.60 mg/kg) and placebo Mean methylphenidate dosage: 5.03 mg (range 2.5 to 10), 10.8 mg (range 5 to 20) and 21 mg (range 12.5 to 30) Administration schedule: 3 times daily (7:45 AM, 11:45 PM and 3:45 PM) Duration of each medication condition: varied daily Washout before study initiation: none Titration period: none/duration initiated before/after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Comments from study authors
Comments of review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Email correspondence with study authors: April 2014. We obtained supplemental information regarding risk of bias. No further information was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and allocation concealment were completed with a random number generator by a researcher not involved in treatment |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation concealment were completed with a random number generator by a researcher not involved in treatment |
| Blinding of participants and personnel (performance bias) | Low risk | "Medication assessment procedure was double‐blinded" (p 201) "children, their parents, and all clinical staff members were blinded to medication condition" (p 202) Medication was prepared in opaque capsules by a pharmacist not otherwise involved in the study. It was administered to children by research staff who were not involved in administration of behavioural treatment nor in daily activities |
| Blinding of outcome assessment (detection bias) | Low risk | Observers and raters were blinded to medication conditions |
| Incomplete outcome data (attrition bias) | High risk | One child's parents elected to stop medication for the child after 2 days because of their concerns about possible side effects of the medication. This child was not included in the analyses |
| Selective reporting (reporting bias) | Low risk | No protocol/design was published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | Low risk | This study was funded by the National Institute of Mental Health (NIMH) grant MH62946 Conflicts of interest: supported only by National Institutes |
| Methods | Three‐week, randomised, double‐blind, parallel trial with 3 arms
| |
| Participants | Number of participants screened: 346 Number of participants randomly assigned: 327 Number of participants included: 318; immediate‐release methylphenidate 133, modified‐release methylphenidate (EqXL) 139, placebo 46 Number of participants followed up: immediate‐release methylphenidate 120, modified‐release methylphenidate (EqXL) 120, placebo 39 (number in each arm is only per protocol population) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (71%), hyperactive‐impulsive (6%), inattentive (23%)) Age: mean 9.5 years (range not reported) IQ: above 80 Sex: 252 boys, 66 girls Methylphenidate naive: 0% Ethnicity: Caucasian (86%), African Caribbean (5.2%), Asian (0.3%), Hispanic (1.6%), other (6.9%) Country: Australia, Canada, USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to immediate‐release methylphenidate, extended‐release methylphenidate or placebo Mean methylphenidate dosage: not stated Administration schedule: twice daily, morning and lunch Duration of intervention: 3 weeks Titration period: All participants were stable while taking methylphenidate medication before randomisation Treatment compliance: not stated. Children with a previous total daily dose of 10 mg to 20 mg immediate‐release methylphenidate or 20 mg extended‐release methylphenidate were randomly assigned to receive 10 mg immediate‐release methylphenidate twice daily, 20 mg modified‐release methylphenidate (EqXL) once daily or placebo; children given a previous total daily dose of 25 mg to 40 mg immediate‐release methylphenidate of > 20 mg to < 40 mg extended‐release methylphenidate were randomly assigned to receive 20 mg immediate‐release methylphenidate twice daily, 40 mg modified‐release methylphenidate (EqXL) once daily or placebo; children given a previous total daily dose > 40 mg immediate‐release methylphenidate or < 40 mg extended‐release methylphenidate were randomly assigned to receive 20 mg immediate‐release methylphenidate twice daily, 60 mg modified‐release methylphenidate (EqXL) once daily or placebo | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes; independent ethics committee at each clinical site before study initiation Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Key conclusion of study authors
Comment from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but did not state how |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Both EqXL (modified‐release methylphenidate) capsules and immediate‐release methylphenidate tablets were overencapsulated in hard gelatin capsules identical to the placebo capsule |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Stated LOCF but primary efficacy population was the per protocol (PP) population, defined as participants who received study treatment and had ≥ 1 efficacy measurement after the first dose |
| Selective reporting (reporting bias) | Low risk | No protocol/design was published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Funding for this study was provided by Celltech Americas Incorporated, currently part of UCB (Union Chimique Belge) Conflicts of interest: Drs. Hatch and DeCory and Miss Cameron were employees of Celltech at the time of this study. Dr. Findling received research support, acted as a consultant and/or served on a Speakers' Bureau for Abbott, AstraZeneca, Bristol‐Myers Squibb, Celltech‐Medeva, Forest, GlaxoSmithKline, Johnson & Johnson, Lilly, New River, Novartis, Otsuka, Pfizer, Sanofi‐Synthelabo, Shire, Solvay and Wyeth. Dr. Quinn claims no competitive interests. Dr. McDowell has consulted for Janssen‐Cilag and Lilly |
| Methods | Four‐week, double‐blind, randomised, placebo‐controlled, cross‐over trial with 4 interventions Methylphenidate (at 3 different doses, in the morning and at midday)
Phases Participants were assigned to receive, at random, 1 of 6 possible dosing orders that included the following
Schedules were designed in such a way that participants did not receive the 15 mg dose before the 10 mg dose, so in the event that a participant experienced adverse events while taking a lower dose, the 15 mg dose was not administered | |
| Participants | Number of participants screened: not stated Number of participants included: 20. Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 16 (12 boys, 4 girls) Number of withdrawals: 4 Demographic data regarding the 16 who completed the study Diagnosis of ADHD: DSM‐IV (combined (94%), inattentive (6%)) Age: mean 10.43 years (range 5 to 17) IQ: > 70 Methylphenidate naive: not stated Ethnicity: White (75%), African American (6%), Hispanic (19%) Country: USA Setting: out‐patient clinic Comorbidity: bipolar (100%), oppositional defiant disorder (50%), conduct disorder (25%), enuresis (12.5%), encopresis (12.5%) Comedication: 100% divalproex sodium. Some received clonidine for sleep at night Sociodemographics: not stated. No statistically significant differences in distribution based on sex, ethnicity, age group, rate/proportion of comorbid oppositional defiant disorder or comorbid conduct disorder were found between 6 six dosing order groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of methylphenidate (5 mg b.i.d., 10 mg b.i.d., 15 mg b.i.d.) and placebo Mean methylphenidate dosage: not provided, as the trial compares methylphenidate at different doses Administration schedule: morning and midday Duration of each medication condition: 1 week Washout before study initiation: not stated Medication‐free period between interventions: between lunchtime dose and morning dose the following day Titration period: none. Dosing schedules were designed in such a way that patients did not receive the 15 mg dose before the 10 mg dose, so in the event that a participant experienced adverse events while on a lower dose, the 15 mg dose was not administered Treatment compliance: One of 20 screened participants was withdrawn from the study because of poor compliance. Among the 16 who participated, no compliance issues were reported | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes Comments from study authors (limitations)
Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: yes; 2 Email correspondence with study authors: November 2013. We wrote to the study author twice to request a copy of the protocol and information about sample size and allocation concealment but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was generated and was used by a pharmacist to assign dose orders to participants. Counterbalancing was applied in such a way that as each dose order was used, its number was eliminated from the next dose order assignment |
| Allocation concealment (selection bias) | Low risk | Placebo and methylphenidate in identical capsules. No participants were discontinued from this trial because of broken integrity of the blind |
| Blinding of participants and personnel (performance bias) | Low risk | At the conclusion of the 4‐week study, study physician, participant and participant's guardian determined a "best dose week", taking into consideration behaviour ratings and reports of any adverse events. After all assessments had been completed, the study blind was broken to reveal the dose that had been prescribed during the previously identified 'best dose week'. No participants were discontinued from this trial because of broken integrity of the blind |
| Blinding of outcome assessment (detection bias) | Low risk | "After all of the assessments had been completed, the study blind was then broken to reveal the dose that had been prescribed during the previously identified 'best dose week'" |
| Incomplete outcome data (attrition bias) | Low risk | All data present |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Unclear risk | Stanley Medical Research Institute Conflicts of interest: Some study authors have affiliations with pharmaceutical companies |
| Methods | Seven‐week randomised, phase III, double‐blind, multi‐centre, parallel‐group, placebo‐controlled, naturalistic home and school trial with 3 arms
Five‐week titration phase, 2‐week maintenance phase | |
| Participants | Number of participants screened: not stated Number of participants included: 282 (187 boys, 95 girls) Number of participants randomly assigned (and administered ≥ 1 dose of study medication): methylphenidate transdermal system 100, OROS methylphenidate 94, placebo 88 Number of participants followed up: methylphenidate transdermal system 71, OROS methylphenidate 66, placebo 32 Number of withdrawals: methylphenidate transdermal system 27, OROS methylphenidate 25, placebo 53 Diagnosis of ADHD: DSM‐IV‐TR (combined (80.5%), hyperactive‐impulsive (1.4%), inattentive (17.0%), unclassified (1.1%)) Age: mean 8.8 years (range 6 to 12). Methylphenidate transdermal system 8.9, OROS methylphenidate 8.8, placebo 8.5 IQ: ≥ 80 Methylphenidate naive: 86% Ethnicity: Caucasian (77.3%), African American (14.5%), Asian (0.7%), Hispanic (not stated), other (7.4%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated. No significant differences in baseline demographics (age, sex, ethnicity, ADHD subtype, prior ADHD medication use) among the 3 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate patch, OROS methylphenidate or placebo Titration period: 5 weeks (after randomisation) of optimisation to 1 of 4 total daily dosage strength. OROS methylphenidate: 18 mg, 27 mg, 36 mg and 54 mg. Methylphenidate transdermal system and placebo transdermal system: 10 mg, 15 mg, 20 mg and 30 mg over a 9‐hour period in patches of 12.5 cm², 18.75 cm² , 25 cm² and 37.5 cm², respectively Administration schedule: Treatments were administered at approximately 7:00 AM each morning; patches were applied to the hip area and were worn for approximately 9 hours daily, different hip each day Mean patch wear time: 8.70 (0.51) to 9.46 (0.53) hours Duration of intervention: 7 weeks Washout before study initiation: up to 28 days if applicable Treatment compliance: mean compliance 97% to 99% during both study phases | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes (258 participants) Ethics approval: yes Comments from study authors
Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes. An inclusion criterion is that participants are stimulant‐naive or stimulant‐responsive Any withdrawals due to adverse events: yes; 11 Email correspondence with study authors. June to September 2013. We attempted to obtain supplemental efficacy and safety data from study authors but without success | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers schedule |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers schedule |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy: Participants received both a patch and a capsule to be administered each day. Methylphenidate and placebo capsules were overencapsulated to blind the identity of the capsule’s content |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | ITT, LOCF Selection bias: yes; non‐responders excluded during the study. Participants who did not reach an acceptable condition by the final dose‐optimisation visit (week 5) were withdrawn from the study |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
| Vested interest bias | High risk | This study was funded by Shire Development Incorporated, Wayne, Pennsylvania Conflicts of interest: Some study authors received research support, acted as consultants and/or served on a Speakers' Bureau for several pharmaceutical companies |
| Methods | Phase IIIB, randomised, double‐blind, parallel‐group, placebo‐controlled, multi‐centre, dose‐optimisation study evaluating the efficacy and safety of the following in adolescents 13 to 17 years of age with ADHD
Study consisted of 4 experimental periods
Follow‐up consisted of an open‐label extension study conducted to evaluate the safety and efficacy of the methylphenidate transdermal system (10‐, 15‐, 20‐ or 30‐mg/9‐hour patches) for participants who completed all required study visits; consisted of 3 experimental periods | |
| Participants | By using 85% power to detect an effect size of 0.5 between active treatment and placebo at the significance level of 5%, it was estimated that 112 participants were needed for methylphenidate transdermal system groups and 56 for placebo transdermal system groups. Assuming a 20% drop‐out rate, ˜ 210 participants (methylphenidate transdermal system 140, placebo transdermal system 70) were required for the study For the double‐blind, randomised, controlled trial Number of participants screened: not stated Number of participants included: 217 (162 boys, 55 girls) Number of participants randomly assigned: methylphenidate 145, placebo 72 Number of participants followed up (ITT population): methylphenidate 143, placebo 72 Number of withdrawals: methylphenidate 2, placebo 0 Number that completed 7‐week dose‐optimisation/dose‐maintenance phase: methylphenidate 95, placebo 72 Diagnosis of ADHD: DSM‐IV (types not stated) Age: mean 14.6 years (SD 1.3, range 13 to 17) IQ: ≥ 80 Methylphenidate naive: 122 (56%) Ethnicity: Caucasian (77%), African American (18%), Asian (0.5%), other (4.5%) Country: USA Setting: out‐patient clinic Comorbidity: 0% Comedication: not stated Sociodemographics: not stated. No significant difference in baseline demographics were noted between the 2 groups For the open‐label extension (regarding safety measures) Number of participants included: 163 (previously taking methylphenidate 110, placebo 53) Number of participants followed up: 162 (121 boys, 41 girls) Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (types not stated) Age: 14.5 years (SD 1.24, range 13 to 17) IQ: ≥ 80 Methylphenidate naive: 122 (56%) Ethnicity: Caucasian (78%), African American (17%), Asian (0.6%), other (4.4%) Country: USA Comorbidity: 0 % Comedication: not stated Sociodemographics: not stated Inclusion criteria
Participant and parent of legally authorised representative (LAR) are able, willing and likely to fully comply with study procedures and restrictions
Exclusion criteria
Regarding the 6‐month open‐label study
| |
| Interventions | Participants were randomly assigned to methylphenidate transdermal (patches) or placebo Mean methylphenidate dosage at week 7: 10 mg (4.2%), 15 mg (16.7%), 20 mg (24.0%), 30 mg (55.2%); median exposure time: 48 days (range 4 to 57) Administration schedule: single patch in the morning, once daily for 9 hours Duration of intervention: 7 weeks Titration period: 5 weeks, initiated after randomisation Treatment compliance: 124 fulfilled the protocol. However, it is not stated in the article how compliance regarding the medication had to be assessed to fulfil the protocol Mean methylphenidate dosage at month 6: 10 mg (5.6%), 15 mg (7.9%), 20 mg (32.6%), 30 mg (53.9%); median exposure time: 168 days (range 3 to 200) Administration schedule: single patch in the morning, once daily for 9 hours Duration of intervention: 6 months Titration period: 5 weeks of the 6 months Treatment compliance: not stated. 88 fulfilled the protocol. However, it is not stated in the article how compliance regarding the medication had to be assessed to fulfil the protocol | |
| Outcomes | Regarding the 7‐week parallel study ADHD symptoms
Serious adverse events
Non‐serious adverse events
The investigator determined the clinical significance of any physical examination, height or vital sign measurement that was outside the normal range. Clinically significant deviations from measurements recorded at screening were reported as AEs. A 12‐lead ECG evaluation was obtained at screening, at baseline, at week 5 and at the final double‐blind study visit.Dermal skin reaction: Dermal Scale (DRS) was used to evaluate observed skin findings: range 0 to 7, where 0 shows no evidence of irritation Regarding 6‐month open‐label study
Changes noted between evaluation data at study entry and data obtained at schedule visits deemed to be clinically significant by the investigator were considered an adverse event | |
| Notes | Sample calculation: yes Ethics approval: yes Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusions of study authors
Comments from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated the next sequential randomisation number and received treatment that corresponded with that randomised number as given by an interactive voice response system |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule was produced by computer software that incorporated a standard procedure for generating random numbers |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, but no information on methylphenidate and placebo blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | Efficacy analyses were performed on the ITT population, defined as all randomly assigned participants who received ≥ 1 dose of study medicine and ≥ 1 post‐baseline primary efficacy assessment. Safety population was defined as all randomly assigned participants who received ≥ 1 dose of study medicine. Selection bias (e.g. titration after randomisation → exclusion): yes. No further dose titration was permitted for any participant after week 5, and participants who had not reached an acceptable response by the end of week 5 were withdrawn from the study |
| Selective reporting (reporting bias) | Low risk | Protocol registered 12 July 2007. First participant consent was obtained 29 August 2007 for the open‐label study |
| Vested interest bias | High risk | This study was funded by Shire Development Incorporated, which was involved in study design, conduct and data analysis. The open‐label study was industry‐sponsored Conflicts of interest: Dr. Findling has acted as consultant to, has served on Speakers' Bureaus of and/or has received research support from Abbott, Addrenex, AstraZeneca, Biovail, Bristol‐Myers Squibb, Eli Lilly, Forest Pharmaceuticals, GlaxoSmithKline, KemPharm, Johnson & Johnson, Lundbeck, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, sanofi‐aventis, Sepracor, Shire, Solvay, Supernus, Validus and Wyeth. Dr. Turnbow receives or has received research support, acted as a consultant and/or served on Speakers' Bureaus for Eli Lilly, Novartis US, sanofi‐aventis, Shire and UCB (Union Chimique Belge). Dr. Burnside has acted as consultant to, has served on Speakers’ Bureaus of and/or has received research support from Eli Lilly, Johnson & Johnson, Shire and Wyeth. Dr. Melmed has acted as consultant to, has served on Speakers' Bureaus of and/or has received research support from Bristol‐Myers, Eli Lilly, McNeil, Novartis and Shire. Drs. Civil and Li are full‐time employees of Shire Development Incorporated |
| Methods | Three‐week double‐blind, cross‐over trial with 2 interventions
Phases: medical trial or typical clinical procedure, concluded with recommendation for treatment, follow‐up (6 weeks and 3 months) | |
| Participants | Number of participants screened: 24 Number of participants randomly assigned: 12 "typical clinical procedure", 12 "medical trial" Number of participants included: 12 (sex not stated). Participants in the medical trial group were randomly assigned to different possible drug condition orders Number of participants followed up: 12 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 101.58 months (range 6 to 10 years) IQ: not stated Methylphenidate naive: 12 Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics (socioeconomic status calculated using Blishen Index; lower score indicates higher SES): medical trial: mean 3.42 (SD 0.90); typical clinical procedure: mean 3.50 (SD 1.88) Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants in the medical trial group were randomly assigned to different possible drug condition orders of 0.3 mg/kg and 0.6 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: b.i.d. Duration of each medication condition: "the different interventions were randomly assigned across days" Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: missed pills mean 1.92 (SD 1.44). Furthermore, compliance was assessed by urine test. By 6‐week follow‐up, parent‐reported compliance was 83%; by 3 months, it was 73% | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Comments from study authors
Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: June 2014. Emailed study authors for supplemental information and received the following response: "I did look at the questions, but unfortunately, given how long ago the study was conducted, I do not still have the data necessary to answer" (Krogh 2014b [pers comm] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 24 children were matched in pairs on sex and age and then were randomly assigned to the medication trial or to the typical clinical procedure. Higher and lower doses of methylphenidate and placebo were randomly assigned across days by the hospital pharmacy |
| Allocation concealment (selection bias) | Low risk | Higher and lower doses of methylphenidate and placebo were randomly assigned across days by the hospital pharmacy. Active medication and placebo were packaged in capsule form to disguise taste and visual differences, and were dispensed in envelopes for daily use |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, teacher, child and resident were blinded to the daily medication status of the child. "Active medication and placebo were packaged in capsule form to disguise taste and visual differences, and were dispensed in envelopes for daily use". All evaluations were conducted by psychiatric residents blinded to the hypotheses of the study |
| Blinding of outcome assessment (detection bias) | Low risk | Parents, teacher, child and resident were blinded to the daily medication status of the child. All evaluations were conducted by psychiatric residents blinded to the hypotheses of the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Across 12 children, ratings were available for an average of 2.58 placebo days, 1.33 low‐dose methylphenidate days and 1.75 high‐dose methylphenidate days. Ratings for each child were averaged across each of the 3 treatment conditions. Data for all participants were available for measures of missed pills and appointments. One participant was missing on measures of parent‐reported compliance at 6‐week follow‐up, and 2 participants at 3‐month follow‐up. Three participants lacked data for the number of missing envelopes. Four participants did not provide urine for analysis, and 4 were missing physician reports of compliance (6‐week and 3‐month follow‐ups). As the result of intervening school holidays, teacher reports of compliance are available for only 16 children (8 in each condition) at each follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All analyses mentioned are described, but no protocol was identified |
| Vested interest bias | High risk | Funding from CIBA‐GEIGY Canada |
| Methods | Three‐month, randomised, double‐blind, placebo‐controlled, parallel study, wherein participants were randomly assigned to 3 arms
| |
| Participants | Number of participants screened: 91 Number of participants included: not stated (includes boys and girls) Number of participants randomly assigned: not stated Number of participants followed up: intervention 18, control 13 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III Age: mean 7.32 years (range 5 to 9) Methylphenidate naive: not stated Ethnicity: not stated Country: not stated Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated IQ: 116 Sociodemographics: all children living at home with ≥ 1 parent. No significant differences in age and IQ between treatment groups. No data on remaining parameters Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate + parent training (intervention group) or to placebo + parent training (control group). First 3 to 4 weeks: Methylphenidate was titrated (after randomisation), starting with 5 mg b.i.d. (morning, noon), 7 days a week. After the titration period: Methylphenidate was given only on school days Average methylphenidate dosage: 22 mg/d (range 10 mg/d to 30 mg/d) Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Ethics approval: no information Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusions of study authors
Comment from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly assigned; no description of how |
| Allocation concealment (selection bias) | Unclear risk | No data |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, teachers, therapists and those testing the children were unaware of medication conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Parents, teachers, therapists and those testing the children were unaware of medication conditions |
| Incomplete outcome data (attrition bias) | Unclear risk | No data Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): none |
| Selective reporting (reporting bias) | Unclear risk | No data |
| Vested interest bias | Low risk | Ministry of Health Conflicts of interest: not stated |
| Methods | Cross‐over trial with 4 interventions
Phases: 4 pharmaceutical conditions, each lasting 2 weeks (including weekends), with different dosage schedules based on weight of child and type of intervention | |
| Participants | Number of participants screened: not stated Number of participants included: 19 (17 boys, 2 girls). Participants were randomly assigned to 1 of 24 possible drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADD: DSM‐III during Diagnostic Instrument for Childhood and Adolescence (ADD with hyperactivity 16/19) (ADD without hyperactivity 3/19) Age: mean 8.71 years (SD 1.33, range 6.9 to 11.5) IQ: 114.11 (SD 13.34) Methylphenidate naive: 18 Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: oppositional disorder (n = 12), oppositional + conduct disorder (n = 1), enuresis (n = 2), encopresis (n = 2), phobia (n = 1), overanxious (n = 1), adjustment disorder (n = 1) Comedication: not stated Sociodemographics: middle class (Hollingshead 4‐factor mean 38.11, SD 13.18) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 24 (3 methylphenidate and 1 placebo) possible drug condition orders of sustained‐release methylphenidate (SR), standard methylphenidate (SA), methylphenidate combination and placebo Mean methylphenidate dosage: SRSA‐MPH 17.1 mg (0.56 mg/kg), SASR‐MPH 20 mg (0.67 mg/kg), combination 11.8 mg SA MPH + 20 mg SR MPH (0.38 mg/kg SA, 0.67 mg/kg SR) Administration schedule: sustained‐release methylphenidate, mornings (8:00 AM) daily, SA MPH morning (8:00 AM) and noon daily Duration of each medication condition: 2 weeks Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: parents phoned weekly to encourage compliance. School nurse contacted at the beginning of each individual's participation to promote co‐operation and to check on compliance | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Double‐blind trial consisting of 4 pharmacological conditions, each lasting 2 weeks and ordered according to a Latin square (i.e. not randomly assigned) |
| Allocation concealment (selection bias) | High risk | Not randomised |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Blindness was maintained by administering placebo tablets consisting of the vehicle for the SA and SR preparations, respectively, as appropriate for each condition |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Data for 6 participants on Paired Associate Learning Test excluded, as they could not read well, but this was not 1 of our outcomes Selection bias (e.g. titration after randomisation): no, but because of emergent side effects, reductions of 2.5 mg SA MPH per dose were performed blindly for 1 participant in the combined condition. Similarly, blinded (but sham) adjustments of SA placebo were made for 2 participants in the placebo phase and for 1 in the SR condition |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Low risk | Research was supported by National Institute of Mental Health (NIMH) grant MH38118 Conflicts of interest: not stated |
| Methods | Four‐week, double‐blind, randomised, placebo‐controlled, cross‐over study of methylphenidate and placebo with weekly switches of 4 dosage levels; capsules of
Methylphenidate‐sensitive children continued in an open‐label study for 4 weeks | |
| Participants | Number of participants screened: 80 Number of participants included: 30 (22 boys, 8 girls). Participants were randomly assigned to 1 of the possible drug orders of low‐dose, moderate‐dose and high‐dose methylphenidate and placebo Number of participants followed up: 30 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (37%), inattentive (57%), hyperactive‐impulsive (7%)) Age: mean 105.2 months (SD 25.1, range 7 to 12 years) IQ: > 70 Methylphenidate naive: 100% Ethnicity: not stated Country: the Netherlands Setting: out‐patient clinic Comorbidity: developmental co‐ordination disorder (100%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to possible drug orders of the 3 daily doses of methylphenidate (0.5 mg/kg, 0.75 mg/kg, 1 mg/kg) and placebo Mean optimal dosage: 0.66 mg/kg/d (SD 0.22) Administration schedule: b.i.d. Duration of each medication condition: 1 week Washout before study initiation: none. All were medication naive Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: yes Ethics approval: Procedures were performed in accordance with the ethical standards of the University Medical Centre, University of Groningen Comment from study authors
Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: August 2013 to January 2014. We obtained supplemental information regarding participant demographics and efficacy data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random order by pharmacist |
| Allocation concealment (selection bias) | Low risk | Medication codes were broken at time 2 (endpoint) |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, teachers, children and paediatrician were kept blinded to the child’s drug condition and dosage level |
| Blinding of outcome assessment (detection bias) | Low risk | Parents, teachers, children and paediatrician were kept blinded to the child’s drug condition and dosage level |
| Incomplete outcome data (attrition bias) | Low risk | No participant withdrew; no ITT was needed. Seven children whose ADHD symptoms were not sensitive enough to methylphenidate (effect < 25%) were excluded from the 4‐week open‐label period. However, as we are using in this review only data from the preceding cross‐over period, exclusion of these children does not cause high risk of bias Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Vested interest bias | Low risk | For this study, no funding was available. This double‐blind placebo‐controlled (DBPC) trial of methylphenidate was performed as a clinical treatment programme as best clinical practice to determine the effects of methylphenidate and optimal dose compared with placebo Conflicts of interest: no affiliations with pharmaceutical companies or similar stated |
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 2 interventions
Phases: 4 | |
| Participants | Number of participants screened: 82 Number of participants included: 71 (all boys). Participants were randomly assigned to different possible drug condition orders of placebo and 3 doses of methylphenidate Number of participants followed up: Conners' Teacher (n = 47), Conners' Parent (n = 69) Number of withdrawals: Conners' Teacher 33.8%, Conners' Parent 12.6% Diagnosis of ADHD: DSM‐III‐R (hyperactive‐impulsive (100%)) Age: mean 9.3 years (range 7 to 11) IQ: mean 106.2 (range 67 to 137) Methylphenidate naive: 100% Ethnicity: ethnic minority (11.3%) Country: USA Setting: out‐patient clinic Comorbidity: conduct disorder (30/71) Comedication: no; had no psychotropics for a month before Sociodemographics: Alll participants were middle to upper class Inclusion criteria
Exclusion criterion
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.3, 0.6 and 1.0 mg/kg MPH (unless dosages would exceed 20 mg, so the low dose would be 0.15 mg/kg) 3 times a day and placebo Mean methylphenidate dosage: not stated Administration schedule: 3 times a day – 7:30 AM, 11:30 AM, 3:30 PM Duration of each medication condition: 7 days Washout before study initiation: not stated Titration period: nil Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Email correspondence with study authors. Emailed study authors to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Reported as double‐blind but not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Reported as double‐blind but not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no, but high loss to follow‐up for teachers' scores on Conners' Teacher Rating Scale Washout period between medication conditions: unclear |
| Selective reporting (reporting bias) | Unclear risk | Not clear |
| Vested interest bias | Low risk | Study supported by National Institute of Mental Health (NIMH) grant MH38686 Conflicts of interest: no affiliations described |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial of multiple methylphenidate doses in stimulant‐naive school‐aged children Phases: 3 active dosage weeks and 1 week of placebo | |
| Participants | Number of participants screened: 162 Number of participants included: 105. Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 89 (65 boys, 24 girls) Number of withdrawals: 16 Diagnosis of ADHD: DSM‐IV (combined (48%), hyperactive‐impulsive (0%), inattentive (52%)) Age: mean 8.13 years (range 7 to 11) IQ: mean: 105.34 ± 12.65 Methylphenidate naive: 100% Ethnicity: Caucasian (79%), African American (18%), Hispanic (2%), other (1%) Country: USA. Setting: out‐patient clinic Comorbidity: anxiety disorder (17%), mood disorder (2%), disruptive behaviour disorder (36%) Comedication: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of OROS methylphenidate (18 mg, 27 mg, 36 mg for children ≤ 25 kg; 18 mg, 36 mg or 54 mg for children > 25 kg; sample mean maximum dose 1.57 mg/kg/d) and placebo Administration schedule: once daily Duration of each medication condition: 1 week Washout before study initiation: drug naive Medication‐free period between interventions: not stated Titration period: none initiated before/after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: yes Ethics approval: yes; Cincinnati Children’s Hospital Institutional Review Board Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study author: October/November 2013. Contacted study author to obtain mean and SD for ADHD symptoms. Never received additional data. Therefore no further email correspondence regarding allocation concealment, randomisation, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to different drug orders. No description about how |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study medication consisted of identical capsules filled with an inert white powder (placebo) or prescribed dose of Concerta overencapsulated to preserve double‐blind Double‐blind, but not specified who was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study medication consisted of identical capsules filled with an inert white powder (placebo) or prescribed dose of Concerta overencapsulated to preserve double‐blind Double‐blind, but not specified who was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Data provided on 89 participants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | Not able to find protocol or trial identifier |
| Vested interest bias | Low risk | National Institute of Mental Health (NIMH) and Cincinnati Children’s Hospital Center for Education and Research Therapeutics Award Conflicts of interest: Dr. Epstein receives funding from Eli Lilly and Co. Dr. Stein has received research support from Eli Lilly and Co., McNeil Pharmaceuticals, Novartis and Shire. He has served on a Speakers' Bureau for Novartis and has served as consultant to Novartis, Shire and Shinogi Pharmaceuticals |
| Methods | Six‐week double‐blind, cross‐over trial in which participants received the following in random order
Furthermore, a single case report from the study is described | |
| Participants | Number of participants screened: not stated Number of participants included: 11 (11 boys, 0 girls). Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: between 9 and 10, depending on ratings Number of withdrawals: 1 to 2 Diagnosis of ADHD: DSM‐III (type not stated) Age: mean not reported (range 5.9 to 11.9 years) Case report: 10 years of age IQ: > 75 Stimulant‐naive: 6 Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: disruptive behaviour disorder Comedication: not reported Sociodemographics: Children represented a full range of socioeconomic backgrounds Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of low‐dose (0.3 mg/kg) and moderate‐dose (0.6 mg/kg) (upper limit 25 mg) methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: b.i.d., morning, noon, 3.5 hours apart, 7 days a week Duration of each medication condition: 2 weeks Washout before study initiation: yes Titration period: When moderate dose was not preceded by low‐dose condition, the child was gradually built up to the moderate dose. Data from these titration days were excluded from the analyses Treatment compliance: pill count, no further information | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from study authors
Key conclusion of study authors
Email correspondence with study authors: July 2013. Emailed first study author twice to get additional information (funding, ethics approval, etc.) and data from the study. Study authors not able to provide us with additional data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dose schedules were assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Medication and placebo pills were identical and were dispensed to parents and school nurses in dated, sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, teachers, observers, treating physicians and children were blinded to dose and order |
| Blinding of outcome assessment (detection bias) | Low risk | Parents, teachers, observers, treating physicians and children were blinded to dose and order |
| Incomplete outcome data (attrition bias) | Low risk | For classroom analyses, data on 10 boys were analysed, and for lunch room analyses, data on 9 boys were analysed Selection bias (e.g. titration before randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Email sent to first study author. No answer; therefore not able to get information |
| Vested interest bias | Unclear risk | Email sent to first study author in July 2013. No answer Ciba Pharmaceutical Company supplied methylphenidate placebo Conflicts of interest: not stated |
| Methods | Randomised, placebo‐controlled, double‐blind, cross‐over trial with 2 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 34 (31 boys, 3 girls). Participants were randomly assigned to different orders of the 3 dosages Number of participants followed up: RCT 34, MED (minimal effective dose, after RCT) 27; 12‐month follow‐up 30; 18‐month follow up 26; 24‐month follow‐up 26 Number of withdrawals: RCT: 0 Diagnosis of ADHD: DSM‐III‐R (subtypes not stated) Age: mean 8 years and 10 months (range 6.1 years to 11.9 years) IQ: mean 105.9 (SD 13.7, range no information) Methylphenidate naive: 24 (71%) Ethnicity: Caucasian (85%), African American (3%), Asian (3%), Hispanic (9%), other (0%) Country: USA Setting: out‐patient clinic Comorbidity: Data from only 21 children from Gadow et al. (1995): tics (100%); anxiety or depressive disorder, or both (8/21; 38%); obsessive‐compulsive disorder (3/2; 14%); most of the children also had oppositional defiant disorder or conduct disorder and academic problems Comedication: not during RCT. Four children were treated with an anti‐tic medication in combination with methylphenidate at some time during the course of follow‐up (neuroleptic 3, clonidine 1) Sociodemographics: no information Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of doses: 0.1 mg/kg (mean 4.4 mg), 0.3 mg/kg (mean 9.0 mg) and 0.5 mg/kg (mean 14.0 mg) of methylphenidate and placebo Upper dosage limit was 20 mg. When the 0.5‐mg/kg dose was not preceded by a low‐dose condition, the child was gradually built up to the moderate dose. Build‐up days occasionally fell on scheduled school observation days. Observers were unaware of these days and observations were conducted as usual, but these data were excluded from the analyses Administration schedule: b.i.d. (or t.i.d.) at morning and noon, approximately 3.5 hours apart, 7 days a week Duration of each medication condition: 2 weeks Washout before study initiation: methylphenidate 1 week, antipsychotic 3 weeks, clonidine 2 weeks Medication‐free period between interventions: none Titration period: none Treatment compliance: Parents and nurses were asked to return unused medication envelopes, which allowed researchers to assess compliance. No further information was provided in the paper Regarding 24‐month follow‐up: Total daily dose of methylphenidate, MED (minimal effective dose ‐ after RCT) mean 16.5 mg (range 5 mg to 40 mg); second visit mean 28.5 mg (range 15 mg to 60 mg); third visit mean 29.2 mg (range 10 mg to 90 mg); and fourth visit mean 34.5 mg (range 15 mg to 92 mg) | |
| Outcomes | During 8‐week RCT ADHD symptoms
General behaviour
Non‐serious adverse events
Physician evaluations
During 24‐month follow‐up Physician evaluations
Parent ratings
| |
| Notes | Sample calculation: yes Ethics approval: no information Comments from study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no; children were not excluded from participation in the study if they had prior experience with stimulant drug therapy, or if such therapy purportedly had exacerbated their tics Any withdrawals due to adverse events: no Key conclusions of study authors
Comments from study authors (limitations)
Comments from review authors
Email correspondence with study authors: April 2013. We emailed study authors for supplemental information regarding cross‐over data. Data were not available. Also no further data for the interventions were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dose schedules were counterbalanced and assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Medication and identically matching placebos were dispensed to parents and school nurses in dated, sealed envelopes at 2‐week intervals |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, teachers, participants, observers and physicians were blinded to the identity of those conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Medication was administered under double‐blind conditions (i.e. no one involved in clinical management of the participant, data collection or interaction with the school knew the identity of treatment conditions) |
| Incomplete outcome data (attrition bias) | Low risk | Study describes how many people were included in the different analyses Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified or received |
| Vested interest bias | Low risk | Research grants from the Tourette Syndrome Association and the National Institute of Mental Health (NIMH) Conflicts of interest: not stated |
| Methods | Double‐blind, randomised, cross‐over trial with interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 71 (39 + 32, cohorts 1 and 2, respectively). Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 71 (57 boys, 14 girls) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R or DSM‐IV (subtype not stated) Age: mean: 8.9 ± 1.9 years (range 6 to 12) IQ: mean 103.8 Methylphenidate naive: not stated Ethnicity: Caucasian (87%), African American (6%), Asian (1%), Hispanic (6%) Country: USA Setting: out‐patient clinic Comorbidity: Tourette’s syndrome (96%), chronic motor tic disorder (4%), oppositional defiant disorder (56%), conduct disorder (7%), overanxious or generalized anxiety (30%), simple phobia (7%), obsessive‐compulsive disorder (11%) Comedication: yes Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to placebo or to 1 of 3 possible drug condition orders of immediate‐release methylphenidate (0.1 mg/kg (mean 4.5 mg; SD 1.6), 0.3 mg/kg (mean 9.3 mg; SD 3.0) and 0.5 mg/kg (mean 14.3 mg; SD 3.3)), twice a day for 2 weeks, each under double‐blind conditions. Upper limit was 20 mg/d Administration schedule: twice daily, 3.5 hours apart. Most days, a morning dose and a noon dose, 7 days a week Duration of each medication condition: 2 weeks Washout before study initiation: Minimum washout periods for children receiving medication at referral were as follows: 1 week for stimulants (n = 10), 3 weeks for neuroleptic or selective serotonin reuptake inhibitor (n = 2) and 2 weeks for clonidine (n = 1) Medication‐free period between interventions: none Titration period: not stated initiated before/after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Comment from study authors
Key conclusion of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: April 2014. We obtained supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dose schedules were counterbalanced and assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Medication was administered twice daily, approximately 3.5 hours apart, 7 days per week, and was dispensed in dated, sealed envelopes at 2‐week intervals |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind conditions |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | None required withdrawal of medication |
| Selective reporting (reporting bias) | Unclear risk | No information to assess this |
| Vested interest bias | Low risk | This study was supported in part by a research grant from the Tourette Syndrome Association Incorporated, and by Public Health Service (PHS) grant number MH45358 from the National Institute of Mental Health (NIMH) Conflicts of interest: Study authors have no financial relationships to disclose |
| Methods | Eight‐week, placebo‐controlled, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 54 (42 boys, 12 girls). Participants were randomly assigned to the different possible drug condition orders: ADHD without anxiety 37, ADHD with anxiety 17 Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III or DSM‐IV (subtype not stated) Age: ADHD without anxiety 8.9 years, ADHD with anxiety 9.1 years (range 7 to 12) IQ: ADHD without anxiety mean 103.5, ADHD with anxiety mean 103.1 Methylphenidate naive: 48 (89%) Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: Tourette's (n = 52), chronic multiple tic disorder (n = 2), anxiety (n = 17), major depressive episode or dysthymia (n = 11), overanxious disorder or generalised anxiety disorder (n = 12), separation disorder (n = 6), social phobia (n = 1), oppositional defiant disorder (n = 36), conduct disorder (n = 4) Comedication: not stated Sociodemographics: ADHD without anxiety 37.1, ADHD with anxiety 35.4 (according to Hollingshead) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.1 mg/kg, 0.3 mg/kg and 0.5 mg/kg methylphenidate and placebo Mean methylphenidate dosage: 4.7 mg, 9.5 mg, 14.5 mg Administration schedule: "administered twice daily, approximately 3.5 hours apart, 7 days a week" Duration of each medication condition: 2 weeks Washout before study initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes; approved by a university Institutional Review Board (IRB) Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: May 2015. Emailed study authors requesting additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dose schedules were counterbalanced and were assigned on a random basis |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "...dispensed in dated, sealed envelopes at two‐week intervals" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | "In order to increase sample homogeneity, an additional three children with diagnosed specific phobia (n = 2) or major depressive episode without comorbid anxiety disorder (n = 1) were excluded from data analyses" |
| Vested interest bias | Low risk | Study was funded by the National Institute of Mental Health (NIMH) and the Tourette Syndrome Association Incorporated. CIBA Pharmaceutical Company supplied methylphenidate placebos. Novartis supplied immediate‐release methylphenidate Conflicts of interest: "Kenneth D. Gadow is a shareholder in Checkmate Plus, publisher of the Child Symptom Inventory‐4" |
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over experiment with 4 arms
Study lasted 20 weeks; first and last 2 weeks were baseline periods during which no medication was given. Different interventions lasted 3 weeks each (Monday to Friday) with a 7‐day washout between changes in drugs | |
| Participants | Number of participants screened: not stated Number of participants included: 12 (all boys). Participants were randomly assigned to the different possible drug condition orders Number of participants followed up: 12 Number of withdrawals: 0 Diagnosis: DSM‐III of ADD. Eight participants were day hospital patients and 4 were in‐patients Age: mean 7.3 years (range 5.9 to 11.6) IQ: > 70 Methylphenidate naive: 50% Ethnicity: not stated Country: USA Setting: out‐patient clinic and patient ward Comorbidity: no Comedication: no comedication during study period Sociodemographics: not stated. Children presented with remarkably similar clinical, family and educational histories. None of the children had localising neurological signs or met criteria for other psychiatric diagnoses Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to the different possible drug condition orders of methylphenidate, clomipramine, desipramine and placebo Mean methylphenidate dosage: 18 mg/d Administration schedule: b.i.d., morning and lunch Duration of each medication condition: 3 weeks Washout before study initiation: 2 weeks before study entry and 7 days between drug conditions Titration period: first week of the drug condition after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusions of study authors
Comment from review authors
Email correspondence with study authors. October 2013. We obtained supplemental information regarding number of drop‐outs and SD for Conners' Rating Scale. We also wanted other data but were not able to obtain these, as the study took place several years ago and the data are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly given methylphenidate, desipramine, clomipramine, placebo A Latin square was followed to control for the order of presentation of drugs |
| Allocation concealment (selection bias) | Low risk | Children were randomly given MPH, DMI, CMI, placebo. A Latin square was followed to control for the order of presentation of drugs |
| Blinding of participants and personnel (performance bias) | Low risk | Participants, parents, attending physicians, teachers, nursing staff and child care workers did not know which medication the child received, ensuring the double‐blind procedure. Medication was added to lactose powder and was placed in identical appearing gelatin capsules |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, parents, attending physicians, teachers, nursing staff and child care workers did not know which medication the child received, ensuring the double‐blind procedure. Medication was added to lactose powder and was placed in identical appearing gelatin capsules |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Vested interest bias | Low risk | Study was funded by Ontario Mental Health Foundation Conflicts of interest: none |
| Methods | Cross‐over trial with 2 interventions
Phases: Number of phases varied by dose group. For all dose groups, after completing first phase, participants were on a 1‐week washout period before cross‐over. Participants assigned to 1 of 3 maximum OROS methylphenidate dose groups in randomly assigned order
Participants assigned to next group after 3 participants have successfully completed preceding group with no side effects | |
| Participants | Number of participants screened: 40 Number of participants included: 33 (19 boys, 14 girls). Participants were randomly assigned to different possible drug condition orders Number of participants followed up: 33 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐R (combined (51.1%), inattentive (48.5%)) Age: mean 10.5 years (SD 3.0, range 6.4 to 17.5) IQ: mean 89.7 (SD 16.9, range 59 to 123) Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: hospital ward Comorbidity: epilepsy (100%), others (not stated) Comedication: yes Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of methylphenidate and placebo. Each patient was given 5 mg of immediate‐release methylphenidate in the morning and at noon for 1 day. If this dose was tolerated, 18 mg of OROS methylphenidate was administered in the morning for the remaining 6 days of the first week. For group 1, maximum dose remained 18 mg, and methylphenidate and placebo arms lasted 1 week. For group 2, a further 1 week of OROS methylphenidate at a dose of 36 mg was given in the morning for 1 week; this group was also administered placebo for 2 weeks. For group 3, maximum dose was 54 mg in the morning, and each arm of the cross‐over lasted 3 weeks Mean methylphenidate dosage: 11 participants < 1 mg/kg/d, 13 participants 1 to 1.5 mg/kg/d, 9 participants 1.5 to 2 mg/kg/d Administration schedule: 1 dose in the morning Duration of each medication condition: 1 week Washout before study initiation: not stated Medication‐free period between interventions: yes; 1 week Titration period: no Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Email correspondence with study authors: April 2014. We obtained supplemental efficacy data from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists for each maximum dose group were prepared by a statistician and maintained by the research pharmacist |
| Allocation concealment (selection bias) | Low risk | Randomisation lists for each maximum dose group were prepared by a statistician and maintained by the research pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were randomly assigned to take OROS methylphenidate or placebo. Principal investigator was blind to medication status. In cases of seizure worsening... Data and Safety Monitoring Board (DSMB) and Institutional Review Board (IRB) were informed of these seizures. Study personnel, the DSMB and the IRB were not unblinded through this process, as the participant would not be exposed again to the same condition |
| Blinding of outcome assessment (detection bias) | Low risk | Principal investigator was blinded to medication status |
| Incomplete outcome data (attrition bias) | Unclear risk | Five participants discontinued treatment while taking placebo and 14 while taking OROS methylphenidate; however, all were included in all analyses Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | All outcomes reported referred to parent‐ and teacher‐rated versions of ADHD Rating Scale, Fourth Edition, and to clinician‐rated ADHD Severity measured weeks 1 to 4 in the protocol. Barkley Total scores (although significantly different) were not reported, although individual effects were reported |
| Vested interest bias | Unclear risk | Supported by National Institute of Mental Health (NIMH) Grant, Number K23 MH066835 Conflicts of interest: Four study authors are involved in the pharmaceutical sector |
| Methods | Participants with ADHD took part in a randomly ordered, double‐blind, cross‐over clinical drug trial comprising 21 consecutive days of
| |
| Participants | Number of participants screened: not stated Number of participants included: 43 Number of participants followed up: 41 (21 boys, 20 girls) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined (n = 22), hyperactive‐impulsive (n = 0), inattentive (n = 19)) Age: mean 9.08 years (range 6.26 to 12.55) IQ: mean 107.66 Methylphenidate naive: 37 (out of 41) Ethnicity: Caucasian (92.67%) Country: USA Setting: out‐patient clinic Comorbidity: > 2 anxiety disorders (12.16%), lifetime affective disorder (2.46%), oppositional defiant disorder or conduct disorder (49.28%) Comedication: 0% Sociodemographics: mean 50.43 (range 22 to 66) (Hollingsworth SES) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 dose of methylphenidate and placebo Mean methylphenidate dosage: final daily dose 33.12 mg ± 1.36 (SE), range 25 to 50 mg/d (0.94 ± 0.02 mg/kg) Administration schedule: b.i.d. Duration of each medication condition: 21 days Washout before study initiation: not stated Medication‐free period between interventions: from lunch to the following morning Titration period: 0.25 mg/kg b.i.d. (breakfast and lunch) on days 1 to 2; 0.25 mg/kg b.i.d. plus 0.125 mg/kg at 4:00 PM on days 3 to 7; 0.3 mg/kg b.i.d. and 0.15 mg/kg at 4:00 PM on days 8 to 14; and 0.4 mg/kg b.i.d. and 0.2 mg/kg at 4:00 PM on days 15 to 21. All dosages were administered to the nearest 2.5 mg Titration: took place after randomisation Treatment compliance: not stated. Four participants with ADHD had undergone previous trials of stimulant therapy ranging from 2 weeks to 7 months | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: not described. Ethics approval: yes Comment from study authors
Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: January 2014. We obtained supplemental information from study authors. Study authors do not have the files anymore; therefore we are not able to obtain all of the data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Answer from study author: "Table of random numbers were used to make the allocation process, such that numbers ending in an even digit corresponded to one order and those ending in an odd digit to another" (Krogh 2014c [pers comm] |
| Allocation concealment (selection bias) | Low risk | Answer from study author: "Table of random numbers were used to make the allocation process, such that numbers ending in an even digit corresponded to one order and those ending in an odd digit to another" (Krogh 2014c [pers comm] |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo capsules, identical in appearance and taste to those containing methylphenidate, and administered on the same schedule |
| Blinding of outcome assessment (detection bias) | Low risk | Second author blinded. Placebo capsules, identical in appearance and taste to those containing methylphenidate, and administered on the same schedule |
| Incomplete outcome data (attrition bias) | Low risk | When parents reported serious side effects (n = 5), their children’s dosages were reduced, or planned increments were omitted. However, study authors noted "eliminating participants who could not tolerate their assigned dosage might potentially skew the sample" Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No Answer from study author: "Event related potentials and performance measures on a cognitive task were not reported (I didn't attempt to publish the ERP data because the analyses I conducted did not yield significant results) ‐ And since we do not look at this outcome, we see it as low" (Krogh 2014c [pers comm]) |
| Vested interest bias | Low risk | National Institute of Mental Health (NIMH) Conflicts of interest: Study authors have no financial relationships to declare |
| Methods | One‐day, randomised, parallel trial with 2 arms
Six‐month follow‐up of study participants continuing methylphenidate treatment | |
| Participants | Number of participants screened: not stated Number of participants included: 34 (20 boys, 14 girls) Number of participants randomly assigned: methylphenidate 22, placebo 12 Number of participants followed up: methylphenidate 22, placebo 12 Number of patients continuing methylphenidate treatment beyond the 1‐day trial: 16 Number of participants followed up: 15 Number of withdrawals from extension trial: 1 Regarding the group of patients participating in the 1‐day trial Diagnosis of ADHD: DSM‐IV‐TR (combined (33.3%), hyperactive‐impulsive (not stated), inattentive (50%), not otherwise specified (17.7%)) Age: mean 11.1 years (range 5 to 20) IQ: mean: 81.4 Methylphenidate naive: 61.8% Ethnicity: not stated Country: Israel Setting: hospital/out‐patient clinic Comorbidity in total sample: velocardiofacial syndrome (100%), oppositional defiant disorder (23.5%), specific phobia (26.5%), generalised anxiety disorder (11.8%), social phobia(11.8%), dysthymic disorder (8.8%) and separation anxiety (5.9%) Comorbidity in methylphenidate group: congenital anomalies of the heart and great vessels (54.5%) Comedication: no Sociodemographics: not stated. The 2 groups had similar baseline demographics Inclusion criteria
Exclusion criteria
| |
| Interventions | RCT: Participants were randomly assigned to methylphenidate or placebo Mean methylphenidate dosage: 15.7 ± 5.6 mg (0.5 mg/kg) Administration schedule: once Duration of intervention: 1 day Titration period: no mention, none Washout before study initiation: 3 days Treatment compliance: not stated Follow‐up: Some participants continued methylphenidate treatment beyond the RCT Mean MPH dosage: not stated Administration schedule: not stated Duration of treatment: 6 months Treatment compliance: 1 withdrew because of poor compliance | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: Study protocol was approved by the Institutional Review Board of the Rabin Medical Center Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Comments from study authors
Key conclusions of study authors
Email correspondence with study authors: November 2013. We obtained supplemental information regarding ADHD diagnostic criteria from study authors. Furthermore, we received safety data from the study sample, excluding participants older than 18 years or with IQ < 70 (or both), but we decided not to use these data in our analyses, as data were missing for 60% of the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly assigned to drug or placebo in a 2:1 ratio, according to their order of recruitment |
| Allocation concealment (selection bias) | High risk | Participants were randomly assigned to drug or placebo in a 2:1 ratio, according to their order of recruitment |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and their parents were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Study authors have confirmed that planned outcomes for the 1‐day (randomised controlled) trial were measured and reported |
| Vested interest bias | Low risk | This work was funded by the Basil O’Connor Starter Scholar Research Award of the March of Dimes, NARSAD (National Alliance for Research in Schizophrenia and Affective Disorders) Young Investigator Award, the Marguerite Stolz Award from the Sackler Faculty of Medicine and the National Institute on Drug Abuse (NIDA) Conflicts of interest: Study authors have had no institutional or corporate/commercial relationships for the past 36 months that might pose a conflict of interest&& |
| Methods | Three‐week, randomised, double‐blind, 32‐site, parallel trial with 2 arms
| |
| Participants | Number of participants screened: 507 Number of participants included: 321 Number of participants randomly assigned: methylphenidate 158, placebo 163 Number of participants followed up: methylphenidate 141, placebo 135 Number of withdrawals: methylphenidate 17, placebo 28 Diagnosis of ADHD: DSM‐IV (combined subtype or predominantly hyperactive‐impulsive subtype) Sex: 257 boys, 57 girls Age: mean 9 years (range 6 to 15) IQ: > 80 ADHD treatment naive: 36% Ethnicity: Caucasian (71%), African American (15%), Hispanic (10%), other (4%) Country: USA Setting: out‐patient clinic Comorbidity: none Comedication: concomitant use of clonidine, anticonvulsant drugs and medications known to affect blood pressure and heart rate was not allowed Sociodemographics: not stated. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to modified‐release methylphenidate or placebo Mean methylphenidate dosage: 40.7 mg/d (1.28 mg/kg/d) Administration schedule: once daily Duration of intervention: 3 weeks Titration period: none Treatment compliance: Medication counts showed satisfactory adherence in both groups | |
| Outcomes | General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes; probably approved by an institutional review board Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; participants with a documented allergy or intolerance to methylphenidate were excluded Comments from study authors (limitations)
Key conclusion of study authors
Email correspondence with study authors: November and December 2013: not possible to get supplemental information regarding the study through personal email correspondence with study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomised". Stratification based on previous treatment before randomisation ensured equal distribution across the 2 treatment groups |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Identical appearing modified‐release methylphenidate and placebo capsules were packaged in blister cards |
| Blinding of outcome assessment (detection bias) | Unclear risk | Stated "double‐blinded" |
| Incomplete outcome data (attrition bias) | Low risk | 316 were included in the safety population, and 314 in the ITT efficacy population. All statistical summaries and analyses were conducted for the ITT population using the LOCF approach for children who withdrew prematurely Selection bias (e.g. titration after randomisation → exclusion): yes; exclusion of placebo responders |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | This study was funded by Celltech Pharmaceuticals Incorporated Conflicts of interest: Dr. Greenhill is a consultant for Celltech‐Medeva and a member of its medical advisory board. Drs. Findling and Swanson are consultants for Celltech‐Medeva |
| Methods | Seven‐week multi‐centre, randomised, double‐blind, placebo‐controlled, parallel trial with 2 arms
To compare the efficacy and safety of extended‐release dexmethylphenidate vs placebo in paediatric patients with ADHD Pre‐randomisation phase of up to 2 weeks followed by double‐blind treatment phase for 7 weeks (5 weeks of dose titration followed by 1 week of optimal constant dose) | |
| Participants | Number of participants screened: not stated Number of participants included: 103 Number of participants randomly assigned: methylphenidate 53, placebo 50 Number of participants followed up: methylphenidate 48, placebo 37 (ITT analyses: methylphenidate 52, placebo 45) Number of withdrawals: methylphenidate 5, placebo 13 Age: mean methylphenidate 9.6 years, placebo 10.4 years (range 6 to 17) IQ: > 70 (age‐appropriate functioning levels academically) Sex: 66 boys, 37 girls Methylphenidate naive: 40 Ethnicity: Caucasian (60%), African American (23.3%), other (16.5%) Country: USA Setting: classroom setting Comorbidity: no psychiatric comorbidity Comedication: not stated Sociodemographics: not stated. No significant difference in baseline demographics was noted between the 2 groups. See Tables 1 and 2 Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to an extended‐release formulation of dexmethylphenidate or placebo. Permitted doses were 5 mg/d for the first week; 5 or 10 mg/d for the second week; 5 mg/d, 10 mg/d or 15 mg/d for the third week; 5 mg/d, 10 mg/d, 15 mg/d or 20 mg/d for the fourth week; and 5 mg/d, 10 mg/d, 15 mg/d, 20 mg/d or 30 mg/d for the fifth through seventh weeks Mean final methylphenidate dosage: 24.0 ± 7.1 mg/d Administration schedule: once daily in the mornings Duration of intervention: 5‐week titration period plus 2 weeks at a constant dose Titration period: initiated after randomisation Treatment compliance: At each study visit, compliance was assessed by investigator and study staff on the basis of pill count and participant report | |
| Outcomes | ADHD symptoms
Quality of life
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: Assumptions for sample size and power included treatment difference of 9.0 and and SD of 13.5 Ethics approval: no information provided Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; participants with history of poor response or intolerance to methylphenidate were excluded Withdrawals due to adverse events: methylphenidate 0, placebo 1 Comments from study authors
Key conclusions of study authors
Comments from review authors
Email correspondence: July 2014. Wrote to Novartis to request additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further description provided |
| Allocation concealment (selection bias) | Unclear risk | No description provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, no further description provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind, no further description provided |
| Incomplete outcome data (attrition bias) | Low risk | Study used an ITT analysis. LOCF analysis was used to impute missing values for all final visit analyses Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | High risk | Study authors defined baseline scores on Conners' ADHD/DSM‐IV Scales ‐ teacher‐rated, as inclusion criteria (different scores for age groups), but they informed efficacy as the difference from baseline to endpoint for all medicated individuals |
| Vested interest bias | High risk | Study funded by Novartis Conflicts of interest: Two study authors are employed by Novartis. Only Roberta R. Ball has no conflicts of interest |
| Methods | Double‐blind, cross‐over, placebo‐controlled, 2‐week trial of methylphenidate in children diagnosed with ADHD
Phases: 2 | |
| Participants | Number of participants screened: not stated Number of participants included: 430 (average IQ group). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 430 (334 boys, 96 girls) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined 96 (48.73%), hyperactive‐impulsive not stated for average IQ group alone, inattentive not stated for average IQ group alone) Age: mean 9.45 years (range 6 to 12) IQ: mean 96.68 ± 9.98 (range 80 to 120) Methylphenidate naive: not stated for average IQ group alone Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: income group 4.62 (SD 1.56). Income groups set at 1 (< Can$ 6000), 2 (Can$ 6000 to 10,000), 3 (Can$ 10,000 to 20,000), 4 (Can$ 20,000 to 30,000), 5 (Can$ 30,000 to 40,000) and 6 (> Can$ 40,000) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 0.5 mg/kg/d Administration schedule: b.i.d. morning and noon Duration of each medication condition: methylphenidate 1 week, placebo 1 week (including weekends) Washout before study initiation: 1 week before the start of the trial Medication‐free period between interventions: not stated, appears to have been none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes (see summary of participants) Any withdrawals due to adverse events: not stated Email correspondence with study authors: October 2013. We received some data from study authors. We sent another email to ask for additional information but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed by a research psychologist who had no contact with participants |
| Allocation concealment (selection bias) | Low risk | "The sealed envelope containing the information about the order of MPH and placebo administration for the participant was opened after the CCR rating was assigned and recorded" |
| Blinding of participants and personnel (performance bias) | Low risk | "Both drug and placebo were prepared by a pharmacist in identical coloured gelatine capsules" |
| Blinding of outcome assessment (detection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Estimated enrolment: 700 Study start date: November 1999 Estimated primary completion date: March 2018 (final data collection date for primary outcome measure) |
| Vested interest bias | Unclear risk | Study was supported by the Canadian Institutes of Health Conflicts of interest: Dr. Joober has received consultation honoraria from Pfizer Canada and Janssen Ortho. Dr. Zappitelli receives research salary support and grant funding from the Kidney Research Scientist Core Education and National Training Programme, the Fondation de Recherche en Sante du Quebec and the Research Institute of the McGill University Health Centre, for research not related to this manuscript |
| Methods | Fourteen‐day, double‐blind, placebo‐controlled, cross‐over study with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 37 (31 boys, 6 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 37 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (70%), hyperactive‐impulsive (11%), inattentive (19%)) Age: mean 9.2 years (range 6 to 12) IQ: mean 96.7 Methylphenidate naive: not stated Ethnicity: Caucasian (94%), other (6%) Country: Canada Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (30%), conduct disorder (46%), major depressive disorder (5%), general anxiety disorder (3%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 0.5 mg/kg/d Administration schedule: twice daily, morning and noon Duration of each medication condition: 7 days Washout before study initiation: no Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated | |
| Outcomes | General behaviour
Non‐serious adverse events
| |
| Notes | Ethics approval: Study was approved by the Research Ethics Board of Douglas Mental Health University Institute Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes (see exclusion criteria) Any withdrawals due to adverse events: no Email correspondence with study authors: February and March 2014. Emailed study author twice but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of administration (methylphenidate or placebo) was determined by random assignment |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "MPH and placebo were prepared in identical coloured gelatin capsules by the hospital’s clinical pharmacist, who was not involved in the study in any other way. Capsules were sealed in individual, daily‐dose envelopes to help control accurate administration" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): yes |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Low risk | This was not an industry‐supported study Conflicts of interest: Study authors have indicated no financial conflicts of interest |
| Methods | Cross‐over trial with 2 interventions
Phases: baseline, placebo, low‐dose methylphenidate and high‐dose methylphenidate for 4 weeks per medication phase | |
| Participants | Number of participants screened: 65 Number of participants included: 56 (39 boys, 17 girls). Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV‐TR (combined (58.9%), hyperactive‐impulsive (7.1%), inattentive (33.9%)) Age: mean 120.84 months (SD 30.85 months, range 6 to 16 years) IQ: mean 99.56 (SD 6.84, n = 41) Methylphenidate naive: number not stated ("All participants were either medication naive or received and appropriate wash‐out period") Ethnicity: European American (82%), African American (18%) Country: USA Setting: out‐patient clinic Comorbidity: specific learning disability (n = 13), oppositional defiant disorder/conduct disorder (n = 11), anxiety/depression (n = 6) Comedication: not stated Sociodemographics: middle class (n = 44), lower class (n = 12); urban (n = 36), suburban (n = 13), rural (n = 7) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of methylphenidate (0.15 mg/kg, 0.30 mg/kg) and placebo Mean methylphenidate dosage: not stated Administration schedule: twice daily Duration of each medication condition: 4 weeks Washout before study initiation: 2 days Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Comment from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All medications and placebos were prepared by the study pharmacist, who randomly assigned children to 1 of 6 trial orders in placebo (P), low‐dose (L) and high‐dose (H) conditions (P‐L‐H, P‐H‐L, L‐P‐H, L‐H‐P, H‐L‐P, H‐P‐L) |
| Allocation concealment (selection bias) | Low risk | Research assistants, teacher, parents and participants were blinded to the order of conditions. Ground methylphenidate tablet was placed in lactose‐filled opaque capsules for active drug conditions, with lactose included only for the placebo condition, and was administered twice per day |
| Blinding of participants and personnel (performance bias) | Low risk | Research assistants, teacher, parents and participants were blinded to the order of conditions. All medications and placebos were prepared by the study pharmacist. Ground methylphenidate tablet was placed in lactose‐filled opaque capsules for the active drug condition, with lactose included only for the placebo condition |
| Blinding of outcome assessment (detection bias) | Low risk | After results were analysed, the order of conditions was revealed |
| Incomplete outcome data (attrition bias) | High risk | Participants for whom data were missing or different instruments were used for methylphenidate response were excluded |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Low risk | Research part funded by the Neuropsychiatric Research Institute, Fargo, North Dakota, USA Conflicts of interest: Study authors disclose no conflicts of interest |
| Methods | Three‐month, parallel trial with 4 arms
| |
| Participants | Number of participants screened: 93 Number of participants included: 20 to 26 (NB: 6 withdrew, but unclear whether before or after random assignment) Number of participants randomly assigned: not stated Number of participants followed up: methylphenidate + parent training 4, methylphenidate + no training 4, placebo + training 4, placebo + no training 4 Number of withdrawals: not stated Sex: 13 boys, 3 girls Diagnosis of ADHD: DSM‐IV (combined (25%), hyperactive‐impulsive (56%), inattentive (19%)) Age: mean 4.78 years (range 3 to 6) IQ: mean 97 (range 80 to 123) Methylphenidate naive: 100% Ethnicity: no information Country: New Zealand Setting: out‐patient clinic Comorbidity (type: ODD 5/31%) Comedication: no Sociodemographics: SES group 1: 1, group 2: 0, group 3: 3, group 4: 4, group 5: 3, group 6: 5. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate + parent training, methylphenidate + no parent training, placebo + parent training or placebo + no parent training Mean methylphenidate dosage: 0.3 mg/kg Administration schedule: twice a day ‐ morning and lunchtime Duration of intervention: 3 months Titration period: Dosage was built up over the first week Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes; obtained from the University of Waikato, Department of Psychology Ethical Review Committee and the Waikato Ethics Committee for Waikato Hospital Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusion of study authors
Email correspondence with study authors: January, February and May 2014. Emailed study author but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated to conditions sequentially, using RAND function (SPSS) to generate the sequence. Each parent was randomly assigned to attend the training programme group or the support group. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Throughout the period of data collection, participants and therapist were blinded to medication status |
| Blinding of outcome assessment (detection bias) | Low risk | Throughout the period of data collection, participants and therapist were blinded to medication status. All parents and teachers were blinded to the intervention |
| Incomplete outcome data (attrition bias) | High risk | Only data for completing participants were reported Selection bias (e.g. titration after randomisation → exclusion): unclear. Four were excluded after randomisation because of "treatment integrity problems" |
| Selective reporting (reporting bias) | Unclear risk | We were unable to identify a protocol |
| Vested interest bias | Low risk | No funding to conduct the study was received from any party Conflicts of interest: None of the study authors are affiliated with pharmaceutical companies |
| Methods | Double‐blind, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 44. Participants were randomly assigned to different low and high doses of methylphenidate and to placebo Number of participants followed up: 44 (36 boys, 8 girls; 20 in‐patients, 24 out‐patients) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.4 years (SD 1.75, range not reported) IQ: mean 98.31 (SD 12.96) Methylphenidate naive: not stated Ethnicity: Caucasian (68.2%), African American (31.8%) Country: not stated Setting: out‐patient clinic and patient ward Comorbidity: not stated Comedication: not stated Sociodemographics: mean 3.59 (SD 1.29) (Hollingshead 2‐factor SES index) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different orders of methylphenidate (low‐dose 0.3 mg/kg, high‐dose 0.6 mg/kg) and placebo Mean methylphenidate dosage: not stated Administration schedule: b.i.d. morning and noon Duration of each medication condition: 12 for in‐patients, 19 for out‐patients Washout before study initiation: not stated Medication‐free period between interventions: 68 hours Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: March 2014. We obtained from study authors supplemental information regarding funding and ethics. Not able to obtain additional data as they are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Low risk | Funded by the National Institutes of Health (NIH) Conflicts of interest: not stated |
| Methods | Double‐blind, cross‐over trial with 2 interventions
Four orders
| |
| Participants | Number of participants screened: 95 Number of participants included: 50. Participants were randomly assigned to different orders of low‐dose and high‐dose methylphenidate and placebo Number of participants followed up: 50 (39 boys, 11 girls) Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R Age: mean 9.6 years (range 6 to 18.1) IQ: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible orders of high‐dose methylphenidate (0.3 mg/kg), low‐dose methylphenidate (0.15 mg/kg) and placebo (doses rounded up to the nearest 2.5 mg) Mean methylphenidate dosage: no information Administration schedule: twice a day ‐ 8:00 AM and 12:00 PM Duration of each medication condition: 1 week Washout before study initiation: "received an appropriate wash‐out period" Medication‐free period between interventions: from lunchtime until next morning ‐ 20 hours Titration period: none Treatment compliance: no information | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusion of study authors
Email correspondence with study authors: March 2014. Emailed study authors to request additional information but have received no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Pharmacists randomly assigned participants to 1 of 4 trial sequences |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo consisted of an opaque capsule filled with lactose; active drug consisted of the same lactose‐filled capsule, to which methylphenidate was added |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo consisted of an opaque capsule filled with lactose; active drug consisted of the same lactose‐filled capsule, to which methylphenidate was added |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Vested interest bias | Unclear risk | No information |
| Methods | Twelve‐week, double‐blind, randomised, parallel trial with 6 arms
Nine‐month follow‐up | |
| Participants | Number of participants screened: 117 Number of participants included: 107 (83 boys, 24 girls) Number of participants randomly assigned: 16 to each arm Number of withdrawals after randomisation: 18 Number followed up: at end of study (12 weeks) 78, 9 months after termination of study 71 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 8.27 years (range 7 to 11) IQ: > 70 Methylphenidate naive: not stated Ethnicity: Caucasian (84.9%), African American (9.4%), Hispanic (3.8%), Asian American (1.9%) Country: USA Setting: out‐patient clinic Comorbidity: conduct disorder (7.5%), disruptive oppositional disorder (15%), oppositional defiant disorder (43%) Comedication: not stated Sociodemographics: mean yearly family income USD $25,019. No significant differences in baseline demographics were noted between treatment groups or between treatment groups and treatment drop‐outs in any of the following parameters: child's age, grade, sex, IQ; parental marital status, annual family income and maternal education and age. However, a significantly greater number of non‐white children were included in the placebo alone condition than in remaining treatment conditions or among treatments drop‐outs Inclusion criteria
Exclusion criteria
| |
| Interventions | Washout before study initiation: 2 weeks before initial diagnostic evaluation Participants were randomly assigned to 1 of 6 treatment conditions, including placebo, low‐dose methylphenidate (0.4 mg/kg) and high‐dose methylphenidate (0.8 mg/kg) Administration schedule: daily Duration of intervention: 12 weeks Titration period: no Treatment compliance: 87.39% of parents in the non‐placebo medication conditions group reported anonymously that their child took the medications almost every day, whereas the remainder of families reported usage an average of 3 to 4 days a week. Stimulant medication was withdrawn immediately after post‐test assessments Follow‐up: 9 months | |
| Outcomes | ADHD symptoms All dependent measures were administered at pre‐test, post‐test (within 1 week or 2 weeks of the end of the treatment phase of the study) and follow‐up
Adverse events
| |
| Notes | Sample calculation: no information Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two of 12 families were randomly assigned to each of the 6 treatment conditions. This procedure was repeated 8 times, so that by the end of the study, 16 families were randomly assigned to each of the 6 treatment conditions, for a total of 96 families |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind. Note that assessors were blinded to treatment status at all times |
| Incomplete outcome data (attrition bias) | Low risk | Analyses were conducted on 4 samples: (1) participants completing the entire study and followed up at 9 months (n = 71), (2) participants completing the entire study (n = 78), (3) participants completing the entire study + participants completing ≥ 6 weeks (n = 90) and (4) all of the above + those who dropped out immediately after initiating treatment (n = 96). When no post‐test and/or follow‐up data were available for drop‐outs, pre‐test values or post‐test values, or both, were substituted. Given that analyses produced essentially the same results, only the analyses that include completers are reported Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Vested interest bias | Unclear risk | No information |
| Methods | Fourteen‐week, parallel trial with 3 arms
| |
| Participants | Number of participants screened: not stated Number of participants included: 48 (35 boys, 13 girls). 16 children randomly assigned to each of the following 3 treatment conditions: (1) medication placebo alone, (2) low‐dose (0.4 mg/kg) stimulant therapy, or (3) high‐dose (0.8 mg/kg) stimulant therapy. A sample of 21 non‐clinical controls (13 boys and 8 girls) was also included in the study Number of withdrawals: Seven families dropped out from pre‐test to post‐test: high‐dose methylphenidate 3, placebo 4 Numper of participants followed up: low‐dose methylphenidate 16, high‐dose methylphenidate 13, placebo 12 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 7.97 years (SD 1.4, range 7 to 11) IQ: ≤ 70 Methylphenidate naive: 98% Ethnicity: Caucasian (78.0%), African American (12.2%), Hispanic (9.8%) Country: USA Setting: out‐patient clinic Comorbidity: conduct disorder (n = 5), oppositional defiant disorder (n = 12) Comedication: no Sociodemographics: middle‐income families. No significant differences between treatment groups or between treatment groups and drop‐outs in any participant and/or demographic characteristics at pre‐test, with the exception of ethnicity. A significantly greater proportion of African American children were included in the placebo condition than in the high‐dose condition Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 treatment conditions: (1) medication placebo alone, (2) low‐dose (0.4 mg/kg) stimulant therapy or (3) high‐dose (0.8 mg/kg) stimulant therapy Duration of intervention: 14 weeks Titration period: no Treatment compliance: One check on medication compliance consisted of periodic dispensing of medication over the course of the study during follow‐up visits to staff paediatricians. In addition, a medical compliance questionnaire was completed anonymously by parents 1 month after post‐test assessments. 91.9% of parents in the medication conditions indicated that their child took the stimulant medication almost every day throughout the study, whereas nearly all remaining families reported average usage of 3 to 4 days a week | |
| Outcomes | ADHD symptoms
General behaviour
| |
| Notes | Sample calculation: no information Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Email correspondence with study authors: August 2013 and January 2014. We emailed study authors to request a copy of the protocol and additional information on, for example, the randomisation procedure and funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | All medication was dispensed in a double‐blinded fashion |
| Blinding of outcome assessment (detection bias) | Low risk | All medication was dispensed in a double‐blinded fashion |
| Incomplete outcome data (attrition bias) | High risk | No description of imputation method |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Unclear risk | No information |
| Methods | Single‐day, randomised, controlled, parallel, double‐blind study with 2 arms investigating postural stability in 24 children with ADHD
| |
| Participants | Number of participants screened: 80 Number of participants included: 24 Number randomly assigned: methylphenidate 12 (11 boys, 1 girl), placebo 12 (11 boys, 1 girl) Number of participants followed up in each arm: methylphenidate 12, placebo 12 Number of withdrawals in each arm: methylphenidate 0, placebo 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: methylphenidate mean 10.06 years (range 7 to 16), placebo mean 10.88 years (range 7 to 16) Methylphenidate naive: 0% Ethnicity: Israeli Country: Israel Setting: out‐patient clinic Comorbidity: not stated Comedication: no IQ: > 70 Sociodemographics: not stated Differences between groups
Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 5 mg of short‐acting methylphenidate or 5 mg placebo. They had been drug‐free for 24 hours before the start of the trial Administration schedule: 5 mg × 1 (single dose) Duration of intervention: 1 day Titration period: none Treatment compliance: 100% | |
| Outcomes | Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: no information Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; see 'Inclusion criteria' Any withdrawals due to adverse events: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Placebo pill was identical in appearance to the methylphenidate pill |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and tester administering the examination were blinded to group assignments |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and tester administering the examination were blinded to group assignments |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs in either group |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
| Vested interest bias | Unclear risk | No information |
| Methods | Multimodal Treatment Study of Children With ADHD (MTA) (Jensen 1999 (MTA)): 14‐month multi‐centre, randomised, parallel clinical trial with 4 arms
Phases: For the 2 groups receiving medication, an initial 28‐day titration period was provided. This titration phase was carried out as a randomised, double‐blind, placebo‐controlled, cross‐over trial with daily switching of methylphenidate doses (placebo, low, middle and high). Once delivery of randomly assigned treatments by MTA staff stopped at 14 months, the MTA became an observational study in which participants and families were free to choose their own treatment, but in the context of availability and barriers to care existing in their communities. The following follow‐up assessments took place after completion of the RCT at 10 months' follow‐up (24 months after randomisation), 3‐year follow‐up, 8‐year follow‐up and 10‐year follow‐up | |
| Participants | Titration period (Greenhill 2001) (Jensen 1999 (MTA)) 28‐day RCT (combined treatment + medication management group) Number of participants included: 289 (medication management 144, combined treatment 145) Number of participants completed: 256 Number of participants not finishing titration: 33. Of the completers, 198 were assigned to an individually best dose of methylphenidate for the 14‐month trial, the rest to other medication or no medication Main study (Jensen 1999) (Jensen 1999 (MTA)) Number of participants screened: 4541 Number of participants included: 579 Number of participants randomly assigned to methylphenidate + behavioural treatment (combined treatment): 145, MPH 144, behavioural treatment (BEH) 144 Number of participants followed up: combined treatment 142; medication 136; behavioural treatment 141 Number of withdrawals/drop‐outs: combined treatment 3; medication 8; behavioural treatment 3 Demographic data for combined treatment, behavioural treatment and medication management Diagnosis of ADHD: DSM‐IV (combined (100%)) Age: mean 8.4 years (range 7 to 9.9) IQ: mean 100.4 Sex: 346 boys, 87 girls Methylphenidate naive: 177 Ethnicity: White (60.3%), African American (20.6%), Hispanic (8.8%), other (10.4%) Country: USA and Canada Setting: out‐patient clinic Comorbidity: anxiety disorder (35.1%), conduct disorder (14.1%), oppositional defiant disorder (38.8%), affective disorder (3.5%), tic disorder (10.2%), mania/hypomania (3%) Comedication: not stated Sociodemographics: 130 families on welfare. Population ranges widely in socioeconomic status. No significant differences in baseline demographics were noted between the 3 groups 10‐Month follow‐up (MTA Group 2004) (Jensen 1999 (MTA)) Number of participants followed up in each arm: MGT 128, COMB 138, BEH 139; age (mean 8.4 years); sex (322 boys, 83 girls). No differences between demographic characteristics of the originally randomly assigned 579 MTA participants and participants assessed during 10‐month follow‐up. The only statistically significant difference among treatment groups was a trivial difference in age (MHT was 0.3 years older than BEH) 3‐Year follow‐up (Jensen 2007) (Jensen 1999 (MTA)) Number of participants followed up: medication 115, combined treatment 127 Age: mean 11.7 years (range 10 to 13) Sex: 291 boys, 78 girls No significant differences in baseline characteristics were noted between participants in the 36‐month assessment and those unable to be followed 8‐Year follow‐up (Molina 2008) (Jensen 1999 (MTA)) Number of participants followed up: medication management 101, combined treatment 119. 32.5% of the MTA sample was medicated more than 50% of days the previous year Age: mean 16.8 years (range 13 to 16). At 8‐year assessment, 55 MTA participants had turned 18 years of age. Only 30% of the sample still fulfilled an ADHD diagnosis. Participants lost to 8‐year follow‐up, compared with those retained, more often were male; had younger mothers, less educated parents and lower parent income; and were more likely to have been on welfare at baseline 10‐Year follow‐up, blood pressure (Vitiello 2012) (Jensen 1999 (MTA)) Number of participants followed up: medication management 77, combined treatment 93. A comparison of participants retained through the year 10 (n = 346) vs those who were not (n = 233) showed a smaller proportion of males in the retained group. Furthermore, participants were divided into groups on the basis of the following criteria: "never medicated", currently medicated, previously medicated. For the currently medicated group, numbers of participants were, respectively, 184, 184, 108, 50 and 12 for 24 months, 36 months, 72 months, 96 months and 120 months of follow‐up, respectively. Medication use during the previous 30 days was the criterion for positive medication status For growth outcomes, data from both the originally randomly assigned treatment groups and naturalistic subgroups were collected. Naturalistic subgroups consisted of those who had been medicated at all assessment points up to 24 months (medication use during previous 30 days was the criterion for positive medication status) and those who had not been medicated at assessment points Participants with consistent use of medication (med/med): 255 Participants with no use of medication (NoMed/NoMed) 139; combined treatment 135, medication management 120 Growth studies, 36‐month assessment (Swanson 2007) (Jensen 1999 (MTA)) Naturalistic subgroups were established on the basis of patterns of treatment with stimulant medication. If medication was used within a 30‐day period before assessment, medication status was positive (Med); otherwise it was negative (NoMed). If an individual’s medication status changed at any assessment point, he or she was placed in the inconsistently medicated group Participants with Med (n = 70). At baseline and at 14‐, 24‐ and 36‐month assessment points, respectively, percentages of medicated children taking methylphenidate were as follows: 85.4%, 79.7%, 76.8%, 73.5%. Naturalistic subgroups did not differ in initial size at birth (birth weight), age, parent or teacher ratings of ADHD symptoms, sex, expected adult size (mid‐parent size), welfare status or maternal smoking Inclusion criteria
Exclusion criteria
| |
| Interventions | Titration period (Greenhill 2001) (Jensen 1999 (MTA)) Mean methylphenidate dose during titration period: combined treatment 32.1 mg/d, medication management 28.9 mg/d. Medication management started with a 4‐day single‐blind, safety lead‐in period, during which participants were exposed to 3 progressively higher daily methylphenidate doses given 3 times daily. This was followed by a 28‐day, double‐blind, daily, switch titration of methylphenidate hydrochloride, with 5 randomly ordered repeats each of placebo, 5 mg, 10 mg and 15 mg or 20 mg. Cross‐site teams of experienced clinicians blindly reviewed graphs portraying parent and teacher ratings of responses to each of the 4 doses and by consensus selected each child’s best dose Compliance: not stated. 29 of 32 placebo responders had to go back to taking methylphenidate during the maintenance period Main study (Jensen 1999) (Jensen 1999 (MTA)) Participants were randomly assigned to medication management, behavioural treatment or combined treatment Mean methylphenidate dosage during main study: combined treatment 31.2 mg/d, medication management: 37.8 mg/d Administration schedule: 3 times a day ‐ breakfast, lunch and in the afternoon Duration of intervention: 14 months Treatment compliance: monthly pill counts, intermittent saliva measurements to monitor intake of methylphenidate and encouragement for families to make up missed visits. "The study achieved a high degree of adherence to protocol." NB! For participants not attaining an adequate response to methylphenidate during titration, alternate medications were titrated openly in the following order until a satisfactory choice was found: dextroamphetamine, pemoline, imipramine and others, if necessary approved by a cross‐site panel. Thus, 256 participants successfully completed titration; of these, 198 of 289 participants were assigned to an individually titrated best dose of methylphenidate, and 26 were titrated to dextroamphetamine. 32 were given no medication because of a robust placebo response 10‐Month follow‐up (MTA Group 2004) (Jensen 1999 (MTA)) Duration of intervention: 24 months (10‐month follow‐up from 14‐month RCT) Treatment compliance: not stated. From end of treatment to first follow‐up, percentage of participants taking medication decreased for combined treatment (87% vs 70%) and methylphenidate (93% vs 72%) but increased for behavioural intervention (23% to 38%) 3‐Year follow‐up (Jensen 2007) (Jensen 1999 (MTA)) Duration of intervention: 3 years (36‐month follow‐up from 14‐month RCT) Treatment compliance: not stated. At 36 months, percentage of participants taking medication was 70% for the combined group, 72% for the methylphenidate group and 45% for the behavioural intervention group 8‐Year follow‐up (Molina 2009) (Jensen 1999 (MTA)) Duration of intervention: 8 years Mean methylphenidate dosage: 44.93 mg 10‐Year follow‐up (Vitello 2012) (Jensen 1999 (MTA)) Duration of intervention: 10 years Treatment compliance: not stated Mean methylphenidate dosage: 54.3 mg | |
| Outcomes | Titration period (Greenhill 2001) (Jensen 1999 (MTA))
Main study (Jensen 1999)
Serious adverse events Main study (Jensen 1999)
Non‐serious adverse events Main study (Jensen 1999) Participants were provided up to 8 additional sessions when needed to address clinical emergencies or instances of possible study attrition
Titration period (Greenhill 2001) (Jensen 1999 (MTA))
3‐Year follow‐up (Molina 2007) (Jensen 1999 (MTA))
8‐Year follow‐up (Molina 2009) (Jensen 1999 (MTA))
8‐Year follow‐up, substance abuse (Molina 2009) (Jensen 1999 (MTA))
For analysis of stimulant treatment duration in relation to substance use at 8‐year follow‐up, the primary outcome was the number of substances used in the past 6 months, to ensure that most stimulant treatment received would have preceded substance use. Component variables included the following: "drunk" once or more or drank alcohol 3 to 4 or more times; 1 or more cigarettes per day in the past month (time frame exception specific to tobacco); marijuana 2 or more times; and any other illicit drug use or prescription medication misuse. Secondary analyses explored each class of substances separately. For analysis of stimulant treatment exposure over time in relation to substance use at 8‐year follow‐up, the primary outcome variable was substance use disorder in the past year for any substance (excluding tobacco). Secondary analyses explored alcohol and marijuana/other drug use disorders separately 10‐Year follow‐up (Vitello 2012) (Jensen 1999 (MTA))
Growth (Jensen 2004, Swanson 2007) (Jensen 1999 (MTA))
| |
| Notes | Sample calculation: yes; power analysis, with 576 participants required Ethics approval: yes; approved by both local institutional review boards and National Institutes of Health Office for Protection From Research Risk Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any drop‐outs due to adverse events: Four participants were removed during the lead‐in (titration period) because of prohibitive side effects: 1 child with buccal movements; another with skin picking; a third with depression, crying, sleep delay and appetite loss; and a fourth who was anorexic, listless and emotionally constricted Comments from study authors
Key conclusions of study authors
Comments from review authors
Email correspondence with study authors: January 2014 to June 2014. We sent several emails to the MTA group to request additional information. However, we were not able to obtain additional data. We did receive an email from Dr. Hinshaw confirming that the data on ADHD symptoms, parent‐rated, were wrong ‐ instead of a mean of 1.85, the correct mean was 0.85 for combined treatment after 14 months. We also received an email from Dr. Swanson in June 2014 stating that he would help with data collection. We wanted to conduct a reanalysis of data excluding those few participants not receiving methylphenidate. Dr. Swanson proved several helpful comments on this and enclosed published articles, but we did not receive additional data, in part because of the time frame of this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done centrally by the National Institute of Mental Health (NIMH) Data Center, stratified by site in blocks of 16 (4 to each group). Stratified by 6 sites. Sealed, ordered envelopes were sent to sites for successive entries |
| Allocation concealment (selection bias) | Low risk | Sealed, ordered envelopes were sent to sites for successive entries. Treatment assignment was concealed until the family confirmed agreement to accept randomisation |
| Blinding of participants and personnel (performance bias) | High risk | Treatment assignment was concealed until the family confirmed agreement to accept randomisation. After agreement on best dose, the blind was broken, and the agreed on dose (if not placebo) became the participant’s initial maintenance dose |
| Blinding of outcome assessment (detection bias) | High risk | After agreement on best dose, the blind was broken, and the agreed on dose (if not placebo) became the participant's initial maintenance dose. However, for some outcome measures, 3 strategies were devised to enlist blinded raters and objective observations |
| Incomplete outcome data (attrition bias) | Low risk | ITT analyses. Despite high compliance, we checked whether compliance with assessments (i.e. missing data) could have changed our findings. Random‐effects regression analyses were completed 2 ways: once with inclusion of all participants, and then including only participants who provided data over multiple time points during the study. No differences emerged from these 2 sets of analyses |
| Selective reporting (reporting bias) | Low risk | This study was supported by several grants from the National Institute of Mental Health (NIMH), Bethesda, Maryland |
| Vested interest bias | Low risk | This study was supported by several grants from the National Institute of Mental Health, Bethesda, Maryland Conflicts of interest: Several study authors have affiliations with medical companies |
| Methods | Two‐week cross‐over trial with 3 interventions
Phases: to define rebound effects in 21 boys 4 to 10 years of age, with a DSM‐III diagnosis of ADD and treated with methylphenidate some days, and placebo other days | |
| Participants | Number of participants screened: not stated Number of participants included: 21. Participants were assigned to different orders of methylphenidate and placebo Number of participants followed up: 21 (21 boys, 0 girls) Number of withdrawals: 0 Diagnosis of ADD: DSM‐III‐R (subtype not stated) Age: mean 7 years 7 months (range 4 to 10 years) IQ: mean 101 (range 79 to 120) Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: conduct disorder (n = 2), oppositional defiant disorder (n = 17), learning disability (n = 9) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Random sequence allocation to
Within‐participant random sequence, condition varied daily Mean methylphenidate dosage: not stated Duration of intervention: 2 weeks. Note: It is not clear for how many days each boy received either of the methylphenidate doses or placebo Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: none Ethics approval: no information Funding/vested interest: no information Any withdrawals due to adverse events: no Author affiliations: University. No conflicting interests stated Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated Email correspondence with study authors: Emailed study authors to request additional information. Also asked whether this study includes the Pelham 1989 reference. No answer from study author, so we extracted data as from 2 different studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of drug condition for each child was randomly assigned over days |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Child, parent, teacher and programme counsellors were blinded to the condition. Active medication and placebo were disguised in gelatin capsules and pre‐packaged in individually dated envelopes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Low risk | Conflicts of interest: During the writing of this report, C. Johnston was supported by a Doctoral Fellowship from the Social Sciences and Humanities Research Council of Canada |
| Methods | Three open trials, then a placebo‐controlled, double‐blind, cross‐over study with 2 interventions
| |
| Participants | Number of participants screened: 6. Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 6 (all boys) Number of withdrawals: 0. Three out‐patients were studied in an open‐label design Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 14.4 years (range 13 to 16) IQ: mean 86 (range 76 to 97) Methylphenidate naive: 5 (55%) Ethnicity: not stated Country: USA Setting: out‐patient clinic and in‐patient ward Comorbidity: aggressive conduct disorder (100%) Comedication: yes; diphenhydramine 50 mg Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.6 mg/kg, with 30 mg as a ceiling for methylphenidate and placebo Mean methylphenidate dosage: 0.47 mg/kg Administration schedule: twice a day, 8:00 AM and noon Duration of each medication condition: 3 weeks Washout before study initiation: 1 week Medication‐free period between interventions: 20 hours Titration period: 1 week after randomisation Treatment compliance: no information | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: no information Comment from study authors
Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: March 2014. Emailed study author twice to request additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | As the result of an oversight, assignment to methylphenidate or placebo as the first condition was made in the absence of a specific randomisation formula. Five of the 6 participants received placebo for the first condition and methylphenidate for the second condition |
| Allocation concealment (selection bias) | Low risk | Assignment to order condition was determined with no knowledge of the particular participants involved; thus participants did not receive 1 condition or the other based on symptoms or any other systematic bias |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | In 2 cases, school vacation at the in‐patient setting prevented teacher ratings from being obtained during 1 condition of the study. In those 2 instances, the decision was made to use Conners' ratings obtained from the unit nurse for both the condition for which no teacher ratings were provided and the condition for which teacher ratings were given; thus comparisons were consistent in terms of rater Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Unclear risk | No information |
| Methods | Double‐blind, cross‐over trial with 2 interventions
Followed by long‐term follow‐up of 12 children out of 21 who continued to receive methylphenidate. Follow‐up for an average of 16 months Phases
| |
| Participants | Regarding the cross‐over trial Number of participants screened: not stated Number of participants included: 26, but 5 children dropped out, 3 were removed by parents and 2 were disqualified because of a death in the family in 1 and a protocol procedural error in the other Number of participants randomly assigned: 21 (18 boys, 3 girls) Number of participants followed up: 21, plus 2 participants who had been withdrawn but returned later for follow‐up Number of withdrawals to follow‐up: 0, but data for a few variables were not obtained for all participants Diagnosis of ADHD: DSM‐III Age: mean 9.3 years (range 8 to 12) IQ: mean 100.7 Methylphenidate naive: 100% Ethnicity: White (62%), Hispanic/Oriental/Black (14%), mixed race (24%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional disorder (14%), enuresis (38%) Comedication: not stated Sociodemographics: 2‐parent household (95%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate or placebo Methylphenidate dosage: between 0.3 and 0.6 mg/kg/d in the short‐term phase Administration schedule: 2 time points a day Duration of each medication condition: not stated Washout before study initiation: from noon to the following morning (i.e. the next day) Titration period: no | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: January 2014. Study authors not able to provide us with further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Child assigned in a double‐blind, cross‐over format to methylphenidate followed by placebo or placebo followed by methylphenidate |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Because data for a few variables were not obtained for all participants, a procedure for unbalanced analysis of variance was required and was accomplished by using the general linear model (GLM) procedure in the Statistical Analysis System (SAS) Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Unclear risk | CIBA Geigy Pharmaceuticals provided placebos Conflicts of interest: no information |
| Methods | Double‐blind, cross‐over trial with 3 interventions
Phases: The first 2 doses (7:00 AM and noon) were unchanged from the open trial, whereas the 4:00 PM dose was 10 mg of methylphenidate, 15 mg of methylphenidate or placebo. Each of these 3 4:00 PM medication conditions was administered in random order during the 12‐day period. Each medication condition was administered for a total of 4 days | |
| Participants | Number of participants screened: not stated Number of participants included: 12 (11 boys, 1 girl). Participants were randomly assigned to different possible drug condition orders of methylphenidate and placebo Number of participants followed up: not stated (but all 12 seem to appear in the results) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 9.0 years (SD 2, range 5.5 to 11.25) IQ: not stated Methylphenidate naive: 60% Ethnicity: not stated Country: USA Setting: in‐patient ward Comorbidity: oppositional defiant disorder (25%), learning disability and oppositional defiant disorder (50%), conduct disorder (8%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 10 mg or 15 mg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 7:00 AM, noon and 4:00 PM Duration of each medication condition: 4 days Washout before study initiation: none stated Medication‐free period between interventions: none Titration period: Open titration of methylphenidate was accomplished within 14 days of the child’s admission Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Ethics approval: Informed consent for study participation was obtained from each child's parent or guardian. All procedures were approved by the study site's Human Subjects Research Review Committee Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; patients were excluded from consideration for study participation if they had known hypersensitivity to methylphenidate Any withdrawals due to adverse events: none Email correspondence with study authors: March 2014. Sent an email to study authors to request additional information but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each of the 3 4:00 PM medication conditions was administered in random order during the 12‐day double‐blind cross‐over period |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | The identity of each day’s 4:00 PM dose was known only to the hospital pharmacist, until the child completed the 12‐day protocol |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Results are provided for all 12 children Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Low risk | This work was supported by the John and Maxine Bendheim Fellowship and by the Leon Lowenstein Foundation Conflicts of interest: not stated |
| Methods | Cross‐over trial with 3 interventions
Phases: 3‐week, double‐blind, 2‐way cross‐over, long‐term (≥ 12 months) follow‐up | |
| Participants | Number of participants screened: not stated Number of participants included: 50 (38 boys, 12 girls). Participants were randomly assigned to different possible drug condition orders of methylphenidate and placebo Number of participants followed up: 43 Number of withdrawals: 7 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean not reported (range 4 to 14 years) IQ: "overall normal intelligence" Methylphenidate naive: not stated Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: depression (37%), anxiety (37%), learning disability (51%), conduct disorder (5%), "psychiatric disorder" (2%), Tourette’s disorder (12%), "other" (23%) Comedication: not stated Sociodemographics: "Fifteen (30%) live in households that have a family income below the Canadian poverty line ($20,000/y)", 26 "rural", 9 "suburban", 14 "urban". 21 live with 1 biological parent, 24 live with both parents, 4 live with adoptive parents or "guardians" Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.3 mg/kg methylphenidate and 0.6 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: Each new condition started on a Saturday morning (to allow parents’ observation/evaluation on weekend). Capsules given at 8:00 AM and 12:00 PM. Conners' administered at baseline and on the last day of each week. A 30‐minute semi structured follow‐up interview was conducted ≥ 12 months after completion of the trial Duration of each medication condition: 1 week Washout before study initiation: not stated Medication‐free period between interventions: 12:00 PM to 8:00 AM the following day Titration period: none initiated before/after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes; the Research Ethics Board of the IWK Grace Health Centre approved the protocol Comments from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Once enrolled in the MPT, the non‐blinded hospital pharmacist randomly assigned each child to a particular dosing schedule". "The capsules contained, in random order: placebo of the prescribed dose of methylphenidate (Ritalin) hydrochloride (0.3 mg/kg or 0.6 mg/kg)" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "Families (n = 50) with a child eligible for MPT were given 3 bottles of identical capsules". "The family, teacher, and physician were blinded for the order of medication" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "At the end of each trial the code was broken. The physician evaluated this information and made a clinical inference about the degree of response each week" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | High risk | Not stated Conflicts of interest: Study authors sponsored by Pharmaceutical Manufacturers’ Association of Canada Studentship |
| Methods | Cross‐over trial with 2 interventions
Phases: 2 | |
| Participants | Number of participants screened: not clear Number of participants included: 48. Participants were randomly assigned to 1 of 1 dose of methylphenidate and placebo Number of participants followed up: appears to have been 48 Number of withdrawals: not clear Diagnosis of ADHD: DSM‐III Age: mean not stated (range 12 to 18 years) IQ: mean 108.62 Sex: 42 boys, 6 girls Methylphenidate naive: 46/48; 2 had brief trials in childhood Ethnicity: Caucasian (n = 46) Country: USA Setting: out‐patient clinic Comorbidity: oppositional and conduct disorder (n = 24), oppositional not conduct disorder (n = 12), anxiety (n = 5), drug or alcohol abuse (n = 2), depression (n = 1) Comedication: no Sociodemographics: double‐ or single‐parent family, predominantly middle class (mean Hollingshead socioeconomic status score of 48.8 (i.e. social class II)) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 weeks of methylphenidate hydrochloride and placebo Mean methylphenidate dosage: 35.21 mg (± 5.94 (SD)). Range 15 mg (2 daily doses) to 40 mg (3 daily doses) Administration schedule: Doses were gradually increased at the end of the first and second weeks Washout before study initiation: not described Titration period: after randomisation Treatment compliance: not described | |
| Outcomes | ADHD symptoms
Mean parent‐ and teacher‐reported Conners' hyperactivity and inattention scores were graphed but SD values were not. Also, the paper did not refer to measures of impulsivity or total scores. We could not use these data in our meta‐analyses because values were missing and study authors were not able to provide supplemental data on this outcome Non‐serious adverse events
| |
| Notes | Sample calculation: not described Ethics approval: not described Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: April 2014. We obtained supplemental information/data from study authors (Magnusson 2014a [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Received information from study author (Magnusson 2014a [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Received information from study author (Magnusson 2014a [pers comm]) |
| Blinding of participants and personnel (performance bias) | Low risk | "Substances were dispensed in capsules of identical appearance" (p 703) |
| Blinding of outcome assessment (detection bias) | Low risk | Parent and teacher ratings were used and participants were blinded to which capsule they were receiving |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Low risk | National Institute of Mental Health (NIMH) grant MH38118 Conflicts of interest: no corporate affiliations declared |
| Methods | Randomised, placebo‐controlled, cross‐over trial with 2 possible drug interventions and placebo, as well as 2 possible psychological interventions
and
| |
| Participants | Number of participants screened: 70 Number of participants included: 22 (all boys). Participants were randomly assigned to different possible drug condition orders Number of participants followed up: 16 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐III‐R (subtypes not stated) Age: mean 9.6 years (range 6.9 to 12.9) IQ: not stated Methylphenidate naive: not stated Ethnicity: African American (75%) Country: USA Setting: "partial hospitalisation" summer treatment programme Comorbidity: conduct disorder (44%), oppositional defiant disorder (56%), anxiety disorder (18.8%), major depressive disorder (11.5%), dysthymia (6%), intermittent explosive disorder (6%), developmental articulation disorder (6%), asthma (12.5%) Comedication: not stated Sociodemographics: Three lived with 1 or both parents, 6 lived with grandparents, 1 lived with an aunt, 4 lived with non‐relatives, 2 lived with foster mother. 44% of families received welfare Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.3 mg/kg and 0.6 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 8:00 AM and 11:30 AM to 12.00 PM Duration of each medication condition: 1 day. Each medication condition was administered once per week for a total of 6 days during the trial Washout before study initiation: 2 weeks Medication‐free period between interventions: no Titration period: none Treatment compliance: not stated Behavioural intervention: behaviour modification and no behaviour modification were alternated on a weekly basis for a total of 3 weeks per condition | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study author: January 2014. We were unable to obtain additional data (Nilausen 2014 [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each methylphenidate condition was administered once per week for a total of 6 days during the trial, and behaviour modification and no behaviour modification were alternated on a weekly basis, for a total of 3 weeks per condition. Thus, daily methylphenidate conditions were crossed with 2 weekly behavioural intervention conditions |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | It is stated: "MPH or placebo was placed in identical opaque capsules" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Not stated Selection bias: exclusion of 2 participants with challenging behaviour |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: no information |
| Methods | Eight‐phase, 70‐week, multi‐centre trial (phase III study) including
If parents requested and clinicians agreed that participants were severely symptomatic, children could be moved directly into the medication phase that was concurrent with parent training. If participants did not tolerate the dosing in phase 4, they could enter the open‐label maintenance phase if they tolerated lower doses (e.g. 1.25 mg, 2.5 mg). If they tolerated all doses except 7.5 mg, they were eligible to enter phase 5, the cross‐over titration, with the planned week on a 7.5‐mg dose replaced by an additional 5‐mg week. After phase 5, cross‐over: (1) If the child showed the greatest clinical benefit during 1 of the 5 weeks of the cross‐over trial, with no room for improvement, the blind was broken and the child was randomly assigned to that methylphenidate dose or placebo in phase 6; (2) if the child was a placebo responder, phase 6 was skipped and the child was allowed to enter phase 7 while taking no medication and with monthly monitoring by the treating physician; (3) if a particular week was deemed best, with ongoing room for improvement, a 2‐week double‐blind trial of 7.5 mg and 10 mg t.i.d., each for 1 week, was implemented, and teacher and parent ratings and side effects data were subsequently blindly reviewed to determine the best dose; or (4) participants with no clinical benefit any week were not eligible to continue in phase 6 or 7 but were given 1‐month follow‐up and then were referred for community treatment | |
| Participants | Number of participants screened: 1915 Number of participants included in the study: 303 (229 boys, 74 girls) Number of participants who completed the last phase of the study: 8 Number of withdrawals during the study: 295 Diagnosis of ADHD: DSM‐IV (combined (75%), hyperactive‐impulsive (25%), inattentive (0%)) Age: mean 4.41 years (range not reported) IQ: > 70 (mean 99.06) Methylphenidate naive: 100% Ethnicity: Caucasian (63%), African American (19%), Asian (2%), Hispanic or Latino (16%), American Indian or Alaskan Native (0.7%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (52%), communication disorder (22%), elimination disorder (i.e. encopresis, enuresis; 8%), specific phobia (8%), anxiety disorder (8%), developmental co‐ordination disorder (3%), conduct disorder (2%), pica (2%), adjustment disorder (1%), reactive attachment disorder (1%), obsessive‐compulsive disorder (0.7%), sleepwalking disorder (0.3%) Comedication: no Sociodemographics: double‐parent family (76%), single‐parent family (18%), mean Hollingshead socioeconomic status 47.20 (SD 9.56) Phase 4, open‐label, safety lead‐in Number of participants included: 183 Numberof participants followed up: 169 Number of withdrawals: 12 Number of participants leaving the study phase and entering maintenance (phase 7): 2 Phase 5, cross‐over Number of participants included: 165 (122 boys, 43 girls) Number of participants followed up: 147 Number of withdrawals: 14 Number of participants leaving the study phase and entering maintenance (phase 7): 4 Age: mean 4.74 years (range 3 to 5.5) IQ: > 70 (mean 97.93) Methylphenidate naive: 100% Ethnicity: Caucasian (63%), African American (18%), Asian (1%), Hispanic or Latino (18%), American Indian or Alaskan Native (0.6%) Country: USA Comorbidity: oppositional defiant disorder (55%), communication disorder (20%), elimination disorder (i.e. encopresis, enuresis; 8%), specific phobia (7%), anxiety disorder (10%), developmental co‐ordination disorder (4%), conduct disorder (3%), pica (2%), adjustment disorder (0.6%), reactive attachment disorder (2%), obsessive‐compulsive disorder (0.6%), sleepwalking disorder (0.6%) Comedication: no Sociodemographics: double‐parent family (79%), single‐parent family (21%), mean Hollingshead socioeconomic status 47.01 (SD 9.58) Phase 6, parallel Number of participants included: 114 (85 boys, 29 girls) Number of participants randomly assigned: methylphenidate 61, placebo 53 Number of participants followed up: 77 Number of withdrawals: 1 Number of participants leaving the study phase and entering maintenance (phase 7): 36 Diagnosis of ADHD: DSM‐IV (combined (75%), hyperactive‐impulsive (25%), inattentive (0%)) Age: mean 4.76 years (range not reported) IQ: > 70 (mean 97.45) Methylphenidate naive: 100% Ethnicity: Caucasian (65%), African American (17%), Asian (0.9%), Hispanic or Latino (17%), American Indian or Alaskan Native (0.9%) Country: USA Comorbidity: oppositional defiant disorder (53%), communication disorder (22%), elimination disorder (i.e. encopresis, enuresis; 7%), specific phobia (7%), anxiety disorder (11%), developmental co‐ordination disorder (5%), conduct disorder (3%), pica (0.9%), adjustment disorder (0.9%), reactive attachment disorder (2%), obsessive‐compulsive disorder (0.9%), sleepwalking disorder (0.9%) Comedication: no Sociodemographics: double‐parent family (80%), single‐parent family (19%), mean Hollingshead socioeconomic status 47.61 (SD 9.45) Phase 7, open‐label maintenance Number of participants included: 140 (104 boys, 36 girls) Number of participants followed up: 95 Number of withdrawals: 45 Diagnosis of ADHD: DSM‐IV (combined (76.4%), hyperactive‐impulsive (23.6%), inattentive (0%)) Age: mean 4.4 years IQ: > 70 Methylphenidate naive: 100% Ethnicity: Caucasian (65.0%), African American (17.1%), Asian (1.4%), Hispanic (15.7%), American Indian (0.7%) Country: USA Comorbidity: oppositional defiant disorder (52.9%), communication disorder (19.3%), anxiety disorder (11.4%) Comedication: no Sociodemographics: double‐parent family (81.4%), single‐parent family (18.6%), mean Hollingshead socioeconomic status 47.2 (SD 9.5)
Additional inclusion criteria for phase 4, open‐label lead‐in
Additional inclusion criteria for phase 5, cross‐over
Exclusion criteria
‐‐‐‐‐‐‐ All cases were presented to a cross‐site panel of clinicians, and only patients for whom consensus indicated that all inclusion (and no exclusion) criteria were met could be enrolled | |
| Interventions | Phases 1 to 3 Enrolment, parent training; baseline: no medical intervention Phases 4 to 8 Medical intervention (short‐acting, immediate‐release methylphenidate) Phase 4, open‐label, safety lead‐in Titration: starting dose of 1.25 mg twice daily; increased to 7.5 mg 3 times daily Duration: 1 week Treatment compliance: not stated Phase 5, cross‐over Randomly assigned sequence of doses of 1.25 mg, 2.5 mg, 5.0 mg or 7.5 mg immediate‐release methylphenidate and placebo administered 3 times daily for a week Medication‐free period between interventions: none Mean methylphenidate dose: not stated Duration: 5 weeks Phase 6, parallel After a 24‐hour medication washout, 4 weeks of randomly assigned treatment with a participant's optimal methylphenidate dose as determined in phase 5, or placebo Mean methylphenidate dose: 14.22 mg/d, 0.7 mg/kg/d Duration: 4 weeks Treatment compliance: not stated Phase 7, open‐label, maintenance Maintenance starting doses were based on the best dose decision from cross‐over titration. Phase 5 placebo responders were maintained without medication for ≥ 4 weeks, unless their condition deteriorated, in which case open‐label treatment could be initiated. For any participant whose condition deteriorated, the methylphenidate dose was gradually titrated for optimal response. The dosing regimen was adjusted to minimise some adverse events to 3 times daily (at breakfast, around noon after lunch and at 3:30 PM), 7 days a week Mean methylphenidate dose: increased from 14.04 mg/d (0.71 mg/kg/d) at month 1 to 19.94 mg/d (0.92 mg/kg/d) at month 10 Duration: 10 months Treatment compliance: not stated Phase 8, discontinuation Randomised, double‐blind, placebo discontinuation trial, in which an abrupt medication replacement consisted of placebo for half of the children, while others continued on their best methylphenidate dose from the end of phase 7. Children returned to active medication if they met relapse criteria, in other words, Conners' Parent Rating Scale or Conners' Teacher Rating Scale scores on the Hyperactivity/Impulsivity Index > 1.5 SD above age‐ and sex‐adjusted norms, or a clinician rating of 4 on the Clinical Global Impressions ‐ Severity Scale Duration: 6 weeks Treatment compliance: not stated. Those who opted out of the double‐blind phases would be allowed to continue on open‐label maintenance therapy. This greatly reduced incentive for families to remain in the double‐blind phases, especially if there was reason to suspect that a child had been randomly assigned to placebo | |
| Outcomes | ADHD symptoms
General behaviour
Quality of life
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes. Target sample size stated in the online protocol 165. Sample to be randomly assigned 120 Ethics approval: yes; by institutional review boards at each study site. The study was monitored by the Data and Safety Monitoring Board of the National Institute of Mental Health Comments from study authors (limitations)
Key conclusions of study authors
Withdrawals due to adverse events: number of withdrawals due to adverse events during medication phases: 21 (i.e. 11%) Number of participants leaving study phase and entering maintenance (phase 7): 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
|
| Allocation concealment (selection bias) | Low risk |
|
| Blinding of participants and personnel (performance bias) | Low risk |
|
| Blinding of outcome assessment (detection bias) | Low risk |
|
| Incomplete outcome data (attrition bias) | Low risk |
|
| Selective reporting (reporting bias) | Low risk |
|
| Vested interest bias | High risk |
Conflicts of interest
|
| Methods | Double‐blind, placebo‐controlled, within‐participant trial of cross‐over design, lasting 6 days with 2 possible drug interventions and placebo
The order of drug conditions was randomly assigned, with the restriction that higher doses should never be administered after placebo | |
| Participants | Number of participants screened: not stated Number of participants included: 60 (44 boys, 16 girls) Number of participants randomly assigned: low‐dose methylphenidate 60; high‐dose methylphenidate 60; placebo 60 Number of participants followed up: 60 in each arm Number of withdrawals: not stated for each arm Diagnosis of ADHD: DSM‐IV (combined 47 (78%), inattentive 13 (22%)) Age: mean 10.8 years (SD 1.6, range 8 to 12) IQ: mean 97.4 (SD 10.7, range not stated) Methylphenidate naive: 100% Ethnicity: not stated Country: Germany Setting: out‐patient clinic and in‐patient ward Comorbidity: oppositional defiant disorder (n = 6; 10%), conduct disorder (n = 18; 30%), anxiety (n = 12; 18%), dyslexia (n = 19; 32%) Comedication: no Sociodemographics: not stated. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to "low‐dose" methylphenidate (0.25 mg/kg), "high‐dose" methylphenidate (0.5 mg/kg) and placebo Mean methylphenidate dosage: 9.2 mg (SD 2.2) for low‐dose group (0.25 mg/kg), 18.4 mg (SD 5.4) for high‐dose group (0.5 mg/kg) Administration schedule: Medication was given between 7:00 AM and 8:00 AM for 6 days. "Cognitive testing began 60 minutes after medication ingestion and lasted 80 minutes". The order of drug conditions was randomly assigned, with the restriction that higher doses should never be administered after placebo. Thus, 11 orders were possible for the 6‐day procedure as a whole, and 6 orders were possible for the sequence of the 3 neuropsychological assessments. Children were assigned in equal numbers to the 6 orders Duration of intervention: 6‐day intervention Titration period: 1 week of 0.3 mg/kg methylphenidate for each participant "to ascertain tolerance" Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: not stated Ethics approval: yes; "the study was approved by the Medical Ethical Committee of the University Hospital of Aachen" Comments from study authors
Key conclusions of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: unclear; only children without a prior history of stimulant treatment were included in the study protocol Any withdrawals due to adverse events: not stated Email correspondence with study authors: January 2014. We sent an email to the study author to request additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Within the 6‐day protocol, the order of drug conditions was randomly assigned, with the restriction that higher doses should never be administered after placebo |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "The active medication and placebo were prepared by a study protocol physician who was not involved in the assessment. All capsules were identical opaque gelatin capsules and were administered in a double‐blinded manner. Capsules containing placebo (lactose) or 0.25 mg/kg or 0.5 mg/kg doses of methylphenidate were prepared for each participant" |
| Blinding of outcome assessment (detection bias) | Low risk | As above |
| Incomplete outcome data (attrition bias) | High risk | "Due to computer problems, data for four children in one task in one condition were missing. As recommended by Tabachnik and Fidell (1996), these data were replaced by the average of the group per condition" Selection bias: yes; before testing, all children were given 0.3 mg/kg methylphenidate each day for ≥ 1 week to ascertain tolerance |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Low risk | This research was funded by the German Society for the Advancement of Scientific Research (DFG grant KFO112) Conflicts of interest: none declared |
| Methods | Cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 44 (37 boys (84%), 7 girls (16%)) Number of participants followed up: 44 Number of withdrawals: not stated Age: mean 10.3 years (SD 1.9, range 8 to 12) IQ: mean 98.1 Methylphenidate naive: not stated Ethnicity: not stated Country: Germany Setting: in‐patient ward Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 0.25 mg/kg (low dose) or 0.5 mg/kg (high dose) methylphenidate and placebo Mean methylphenidate dosage: 9.4 mg (SD 2.3) for the 0.25‐mg/kg dose; 18.6 mg (SD 5.3) for the 0.50‐mg/kg dose Administration schedule: not stated Time points: not stated Duration of each medication condition: 2 days Washout before study initiation: not stated Medication‐free period between interventions: no Titration period: Before testing, all children were given 0.30 mg/kg methylphenidate each day for ≥ 1 week to ascertain tolerance Treatment compliance: not stated. Within the 6‐day protocol, the order of drug conditions was randomly assigned, with the restriction that the high dose never occurred right after placebo | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: not stated Ethics approval: yes; informed parental consent was obtained for all participants, and the study was approved by the Medical Ethical Committee of the University Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; before testing, all children were given 0.30 mg/kg methylphenidate each day for ≥ 1 week to ascertain tolerance Any withdrawals due to adverse events: not stated Email correspondence with study author: July 2015. Email sent to study author to ask for additional information, but we have received no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within the 6‐day protocol, the order of drug conditions was randomly assigned, with the restriction that the high dose never was given immediately after placebo |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Active medication and placebo were prepared by a study protocol physician who was not involved in the assessment. All capsules were identical opaque gelatin capsules and were administered in a double‐blind manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Low risk | Funding for this research was provided through a grant from the German Research Foundation (DFG grant: KFO112–TP5) Conflicts of interest: none declared |
| Methods | Nine‐week, randomised, controlled trial (RCT), cross‐over design, with 2 interventions
Phases: 1 | |
| Participants | Number of participants screened: 154 Number of participants included: 58 (sex not stated). Participants were randomly assigned to 1 of 3 possible drug condition orders Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (95%), hyperactive‐impulsive (2%), inattentive (3%)) Age: mean not stated (range 6 to 12 years) IQ: > 80 Methylphenidate naive: 19% Ethnicity: not stated Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: oppositional defiant disorder (52%), conduct disorder (10.5%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.15 mg/kg (t.i.d.) methylphenidate, 0.3 mg/kg (t.i.d.) methylphenidate, 0.6 mg/kg (t.i.d.) methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 3 time points Duration of each medication condition: 12 days for placebo, methylphenidate 0.15 mg and methylphenidate 0.3 mg; 9 days for methylphenidate 0.6 mg Washout before study initiation: not stated Medication‐free period between interventions: no Titration period: not stated Treatment compliance: not stated | |
| Outcomes | General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated Any withdrawals due to adverse events: not stated Email correspondence with study author: June 2014. Emailed the study author to ask for raw data regarding side effects, data from the Disruptive Behavior Disorders Rating Scale and other data. Study author responded to say he did not have them (Holmskov 2014 [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Drug dose varied daily on a randomized basis and included four conditions (...)" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Double‐blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | High risk | Conflicts of interest: Dr. Waxmonsky has served on the Speakers' Board for Novartis, received an honorarium from Shire and received research support from Shire and Eli Lilly. Dr. Erbe has received educational and research support from Genzyme Corporation. Dr. Pelham was paid an honorarium by Shire Pharmaceuticals |
| Methods | Four‐week, randomised, double‐blind, parallel trial with 2 arms
| |
| Participants | Number of participants screened: 102 Number of participants included: 85 (75 boys, 10 girls) Number of participants randomly assigned: methylphenidate 43, placebo 42 Number of participants followed up: methylphenidate 40, placebo 38 Number of withdrawals: methylphenidate 3, placebo 4 Diagnosis of ADHD: DSM‐IV (combined (74.1%), hyperactive‐impulsive (0%), inattentive (24.7%), unspecified (1.2%)) Age: mean 9.8 years (range 6 to 15) Mean IQ: methylphenidate 104.8, placebo 102.7 (range 85 to 146) Methylphenidate naive: 25 (29.4%) Ethnicity: German (82), other (3) Country: Germany Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (51.8%), conduct disorder (9.4%), unspecified conduct disorder (3.5%), dysthymia (1.2%) Comedication: yes (n = 4) Sociodemographics: not stated. The 2 groups were homogeneous in terms of sex distribution, school type, school class and nationality (At baseline, SERS‐D ratings for disturbed sleep, nightmares, sadness, weepiness, anxiety, drowsiness and nervous twitching were far more pronounced in group 1 than in group 2. These initial differences – except for sadness – levelled out during the 4‐week trial period) Additional conduct disorders are less frequent in the group treated with medication (25 vs 30 participants), but this slight difference is not significant (Fisher's exact test, P value = 0.26) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to sustained‐release methylphenidate or placebo Mean methylphenidate dosage: not stated Administration schedule: once daily after breakfast Duration of intervention: 4 weeks Titration period: weekly dose titration initiated after randomisation. Initially, two 5 mg methylphenidate/placebo tablets/d for 2 days, then 20 mg (1 sustained‐release methylphenidate capsule/placebo) dosage increased to 40 mg and 60 mg, depending on weight and course of symptoms. Titration up to 40 mg and 60 mg modified‐release methylphenidate was possible in the second and third weeks of treatment, respectively (20 kg to 30 kg, maximum 20 mg modified‐release methylphenidate; 31 kg to 50 kg, maximum 40 mg modified‐release methylphenidate; < 50 kg, maximum 60 mg modified‐release methylphenidate) Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation:yes Ethics approval: approved by local university ethics committees Comments from study authors
Key conclusions of study authors
Comments from review authors
Any withdrawals due to adverse events: yes; 2 (appendicitis and aggression) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: April to May 2014. We emailed study authors twice but received no further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to methylphenidate or placebo by the following 4 strata: age (6 to 8 years; 9 to 11 years; 12 to 16 years), sex, severity of the problem according to the teachers’ evaluation (Fremdbeurteilungsbogen für Hyper kinetische Störungen; sum‐score > 40 and < 40) and study centre attended. Central randomisation was performed |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Study drug (or placebo) was dispensed in packages containing a weekly supply that was blinded from medical personnel and parents |
| Blinding of outcome assessment (detection bias) | Low risk | Study drug (or placebo) was dispensed in packages containing a weekly supply that was blinded from medical personnel and parents |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | High risk | Financial support for this study was provided by Medice Arzneimittel Pütter GmbH & Co. KG, Kuhloweg 37, D‐58638 Iserlohn Conflicts of interest: Dr. Doepfner is a consultant for Lilly, Medice, Novartis and Union Chimique Belge; serves on the Advisory Boards of Lilly, Medice, Shire, Novartis and Union Chimique Belge; participates as a member of the Speakers' Bureaus of Lilly, Medice, Janssen‐Cilag and Union Chimique Belge; and has research contracts with Lilly, Medice, Novartis, Union Chimique Belge, the German Research Foundation and the Federal Ministry of Health. Dr. Lehmkuhl is on the Advisory Boards of Lilly and Medice. Dr. Sinzig has no financial relationships to disclose |
| Methods | Three‐week, randomised, double‐blind, cross‐over, within‐participant study conducted to test methylphenidate/placebo to assess correlations between measures of attention and inhibition with dopamine and norepinephrine blood levels
| |
| Participants | Number of participants screened: not stated Number of participants included: 15 (13 boys, 2 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 15 Number of withdrawals: 0 Age: mean 10.74 years (range 7 to 13) IQ: mean 97.60 Methylphenidate naive: 0% Ethnicity: not stated Country: the Netherlands Setting: out‐patient clinic Comorbidity: anxiety (n = 6), oppositional defiant disorder (n = 5) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 0.5 mg/kg or 1.0 mg/kg methylphenidate and placebo Mean methylphenidate dosage: 22.67 mg Administration schedule: not stated Duration of each medication condition: 1 day Washout before study initiation: 24 hours before testing Medication‐free period between interventions: no Titration period: none/duration Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes; "The study was approved by the national medical ethical committee (CCMO)" Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: "four participants were too fatigued after placebo or the 1.0 mg/kg dose to continue with the change task after they first completed the stop task" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs. "All participants were familiar with the intake of MPH for at least 1 year" Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: none declared |
| Methods | Eight‐week, multi‐centre (31 sites in 5 countries), double‐blind, placebo‐controlled, comparator (MPH‐OROS), parallel study with 3 interventions
Phases
| |
| Participants | Number of participants screened: 448 Number of participants included: 340 (70.6% boys, 29.4% girls). Participants were randomly assigned to 1 of 5 possible drug condition orders Number of participants followed up: 210 Number of withdrawals: 60 Age: mean 11.6 years (range 6 to 17) IQ: not stated Methylphenidate naive: All participants treated with methylphenidate were medication‐naive. 44% of placebo‐treated participants, 47% of edivoxetine‐treated participants in the 0.1 mg/kg/d arm and 49% of edivoxetine‐treated participants in each of the 0.2 mg/kg/d and 0.3 mg/kg/d arms had used stimulants previously Ethnicity: Caucasian (72.6%), African American (not stated), Asian (not stated), Hispanic (not stated), other (not stated) Country: USA, Canada, Taiwan, Mexico and Puerto Rico Setting: out‐patient clinic Comorbidity: oppositional defiant disorder ("less than 20%") Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of 18 mg, 36 mg or 54 mg OROS methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 1 a day Duration of each medication condition: 8 weeks Washout before study initiation: all methylphenidate naive Medication‐free period between interventions: no Titration period: none, initiated after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: yes (3 participants) Email correspondence with study authors: April 2015. We emailed study authors to ask for supplemental information/data but have received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Interactive voice response system was used for randomisation and to determine which study drug should be dispensed |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Exclusion of methylphenidate non‐responders (after randomisation) |
| Selective reporting (reporting bias) | High risk | Yes |
| Vested interest bias | High risk | Study sponsored by Ely Lilly Conflicts of interest: Five authors work for Lilly |
| Methods | Cross‐over trial with 3 interventions
Phases: 4
Evaluated on day 0, randomly assigned to drug condition on days 7, 14, 21 and 28. One practice visit for a study duration of 5 weeks in total | |
| Participants | Number of participants screened: not stated Number of participants included: 36 (29 boys, 7 girls). Participants were randomly assigned to 1 of 24 (4 × 3 × 2 × 1) possible drug condition orders Number of participants followed up: 36 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9 years (range 6 to 12) IQ: not stated Methylphenidate‐naive: no Ethnicity: Caucasian (36%), African American (27%), Hispanic or other (36%) Country: USA Setting: out‐patient clinic Co‐morbidity: not stated Co‐medication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of methylphenidate (20 mg), (18 mg) Concerta, (36 mg) Concerta and placebo Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of each medication condition: 1 day Washout before study initiation: not stated Medication‐free period between interventions: On the morning following each study period, participants resumed their regularly prescribed medication up to Thursday evening before the next study period day on Saturday Titration period: All participants had been stabilised on an equivalent dose of 10 mg twice daily of methylphenidate before study entry Treatment compliance: No participants discontinued the study prematurely | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes Comment from study authors
Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: April 2014. We received supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of medication assignment was determined by random assignment by a computer programme |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned to 4 treatment periods. For purposes of this study, with the exception of the medicating nurse, all study personnel were blinded to the medication administered to the child |
| Blinding of participants and personnel (performance bias) | High risk | Single‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | For purposes of this study, with the exception of the medicating nurse, all study personnel were blinded to the medication administered to the child |
| Incomplete outcome data (attrition bias) | Low risk | None reported. No participants discontinued the study prematurely Selection bias: no, but all patients were stabilised previously on methylphenidate |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
| Vested interest bias | High risk | The study was funded by Novartis Conflicts of interest: Dr. Silva is a consultant and a member of the Speakers' Bureau for Novartis. Dr. Lopez is a consultant for Eli Lilly, Novartis and Shire. He is also a member of the Speakers' Bureaus for Novartis and Shire |
| Methods | Cross‐over trial with 2 interventions
Phases: Participants randomly assigned to 3 weeks of methylphenidate, 3 weeks of placebo. No washout. Assessment at baseline and by the end of each phase | |
| Participants | Number of participants screened: not stated Number of participants included: 20 (18 boys, 2 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 20 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.23 years (± 1.62, range 7.17 to 12.42) IQ: > 70 Methylphenidate naive: 100% Ethnicity: not stated Country: Israel Setting: out‐patient clinic Comorbidity: none Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 10 mg methylphenidate and placebo Mean methylphenidate dosage: 10 mg/d Administration schedule: mornings Duration of each medication condition: 3 weeks Washout before study initiation: All participants were treatment‐naive Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "everyone involved in the assessment phase ...was blind to the type of medication..." |
| Blinding of outcome assessment (detection bias) | Low risk | "...the parents, the teacher, the child and the psychologist who tested the child did not know what kind of medication the child was taking..." |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Unclear risk | No information |
| Methods | Six‐week, randomised, double‐blind, placebo‐controlled, cross‐over study conducted to detect the effects of methylphenidate on co‐ordination and handwriting
| |
| Participants | Number of participants screened: 19 Number of participants included: 19 (12 boys, 7 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 19 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.51 years (range 7.08 to 13.83) IQ: > 70 Methylphenidate naive: 0% Ethnicity: not stated Country: Israel Setting: out‐patient clinic Comorbidity: not stated Comedication: no Sociodemographics: "Participants were from a medium level social‐economic status" Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 0.4 mg/kg Administration schedule: not stated Duration of each medication condition: 3 weeks Washout before study initiation: no Medication‐free period between interventions: yes Titration period: not stated Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Comments from study authors (limitations)
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no drop‐outs Email correspondence with study authors: October 2013. We obtained additional information on IQ from the first study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" |
| Allocation concealment (selection bias) | Unclear risk | "The study was designed as a double‐blind, randomized, crossover, placebo‐control procedure" |
| Blinding of participants and personnel (performance bias) | Low risk | "Each participant was given exactly 0.4 mg/kg of MPH in special capsules of MPH and a placebo to avoid recognition of the medication" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: not stated |
| Methods | Four‐week, double‐blind titration, placebo‐controlled protocol. Cross‐over trial with 2 interventions in 4 doses
Phases
Manos 1999: Seven youths given methylphenidate and 4 given Adderall did not receive the 15 mg dose of medication. The decision to forego the 15 mg condition was based on the paediatrician’s assessment that the child was too young or underweight for this high dose and our assessment that the best dose had already been achieved at a lower dose | |
| Participants | Number of participants screened: 195 Number of participants included: 177. Participants were randomly assigned to different possible drug condition orders Number of participants followed up: 134 Number of withdrawals: 43 Diagnosis of ADHD: DSM‐IV (combined (55%), inattentive (45%)) Age: mean 10.1 years (SD not stated, range 5 to 17) IQ: > 70 Sex: 33 boys, 9 girls Methylphenidate naive: not stated Ethnicity: Caucasian (93%), African American (7%), Asian (0%), Hispanic (0%), others (0%) Country: USA Setting: out‐patient clinic Comorbidity: no significant comorbid disorders. Although no formal comorbidity data are available, it appears that psychiatric comorbidity was modest in this cohort Comedication: not stated Sociodemographics: predominantly well educated Manos 1999 and Faraone 2002: 42 were participants matched to the Adderall group in order of diagnostic category, age and sex. Only these 42 of 117 receiving methylphenidate were compared with the Adderall group Findling 2001a: 195 youths entered the study. Data for a best dose were provided for 177 participants: 111 in the methylphenidate group, 66 in the mixed amphetamine salts group Diagnosis of ADHD: inattentive (47%), combined (53%). Inattentive subtype is overrepresented in the older age group Age group: 4 to 8 years (mean 6.35) 69 (57 boys); 8 to 11 years (mean 9.47) 56 (45 boys); 11 to 17.59 years (mean 13.64) 52 (41 boys) Sociodemographics: No significant differences in baseline demographics were noted between groups Findling 2001b Number of participants included: 195 Number of participants completed: 137: methylphenidate 82, Adderall 55 Diagnosis of ADHD: DSM‐IV (combined (57%), inattentive (43%)) Age: mean 10 years (SD not stated, range 4 to 17) IQ: > 70 Sex: 66 boys, 16 girls Ethnicity: Caucasian (84%), African American (6%), other (10%) Country: USA Comorbidity: without significant comorbid disorders Comedication: possible, but not recorded Sociodemographics: predominantly well educated. No differences in sex, ethnicity or ADHD subtype were found between methylphenidate and Adderall groups. No participants had a history of hypertension, hypotension or clinically significant cardiovascular disease Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different orders of 5 mg, 10 mg or 15 mg methylphenidate and placebo Mean methylphenidate dosage: best dose 9.1 mg to 10.4 mg Administration schedule: morning (at 8.00 AM) and noon Duration of each medication condition: 1 week Washout before study initiation: none Titration period: none Treatment compliance: 11 terminated because of adverse events | |
| Outcomes | ADHD symptoms Findling 2001: compared treatment for children and adolescents and weight‐adjusted dosing of methylphenidate. Measurement instruments include the following
Best dose based only on Abbreviated Symptoms Questionnaire ‐ Teacher. Does not separate methylphenidate and mixed amphetamine salts in tables Non‐serious adverse events
Findling 2001b
| |
| Notes | Sample calculation: Not stated Ethics approval: Not stated Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; 15 youths in the Adderall sample had been tried on methylphenidate before enrolment in this medication trial. Because of lack of response or serious side effects, these children discontinued use of methylphenidate. A total of 37% of the Adderall sample subsequently was composed of children who had unsuccessfully used methylphenidate but successfully responded to Adderall Any withdrawals due to adverse events: yes; Findling 2001a (11/195), due to multiple adverse events Email correspondence with study authors: April 2014. Emailed study authors twice to request additional data but have received no answer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First phase not blinded. Methylphenidate is a selected group |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Clinician, teacher and parent were blinded only to dose, not to medication |
| Blinding of outcome assessment (detection bias) | High risk | Clinician, teacher and parent were blinded only to dose, not to medication |
| Incomplete outcome data (attrition bias) | Unclear risk | Only best dose compared with placebo. Findling (Abbreviated Symptoms Questionnaire) + 30 participants. Of 43 participants with < 4 weeks of data, 30 had only 3 weeks of data because physicians considered them too young or too small to receive 15 mg before initiating protocol |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | High risk | Study was supported in part by funding from Shire Pharmaceutical Development Incorporated to Dr. Faraone Conflicts of interest: Study authors acknowledge partial support to the second author from the National Institute on Drug Abuse (NIDA) (grants R01‐DA07957 and MCJ‐390592) and from the Maternal and Child Health Program, Health Resources and Service Administration, Department of Health and Human Services (grant 390715), and to the third author from the Stanley Foundation |
| Methods | Four‐week‐long, double‐blind, randomised, parallel trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 40 (all boys) Number of participants randomly assigned: methylphenidate 21, placebo 19 Number of participants followed up: methylphenidate 19, placebo 19 Number of withdrawals: methylphenidate 2, placebo 0 Age: mean methylphenidate 9 years (SD 2.2, range 6 to 14), placebo 9.6 years (SD 2.8, range 6 to 14) IQ: mean, methylphenidate 97.3, placebo 93.5 Methylphenidate naive: not stated Ethnicity: European‐Brazilian: methylphenidate 16 (76.2%), placebo 15 (78.9%) Country: Brazil Setting: out‐patient clinic Comorbidity: yes; conduct or oppositional defiant disorder (methylphenidate 57.2%, placebo 57.9%) Comedication: not stated, no psychiatric medication Sociodemographics: Monthly family income was calculated according to the following formula: total monthly income received by all members of the family (expressed in number of minimum wages) divided by the number of persons in the family. A value lower than 0.7 (approximately US$ = 68 per family member per month) is usually an indicator of poverty in Brazil. Methylphenidate 3.3, placebo 2.4. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate 7 days a week or to methylphenidate on weekdays and placebo on weekends Mean methylphenidate dosage: Initial dose of methylphenidate was 0.3 mg/kg/d the first week. Dose was raised to 0.5 mg/kg/d the second week and to 0.70 mg/kg/d the third and fourth weeks Administration schedule: twice a day: breakfast and lunch Duration of intervention: 4 weeks Titration period: none Treatment compliance: Only 7 of 160 blister packs were returned with unused pills | |
| Outcomes | ADHD symptoms
Serious adverse events
(Completed only by parents for assessment of side effects on weekends) | |
| Notes | Sample calculation: no Ethics approval: approved by the Ethical Committee of the Hospital de Clínicas de Porto Alegre (HCPA) (approved as an International Review Board (IRB) by the Office for Human Research Protections, United States of America) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Comments from study authors
Key conclusion of study authors
Email correspondence with study authors: We have contacted study authors several times to ask for additional information about data, but we have received no data from this study that we can use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, double‐blind, parallel‐group design was used. Participants were randomly assigned to 1 of 2 groups, according to a computer‐derived algorithm (EPIINFO.06) |
| Allocation concealment (selection bias) | Low risk | Computer‐derived algorithm |
| Blinding of participants and personnel (performance bias) | Low risk | Methylphenidate and placebo pills were of the same shape and colour |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind. No other information |
| Incomplete outcome data (attrition bias) | Low risk | Measured adherence to protocol by assessing returned, unopened blister packs. None of the findings in the analyses was significantly affected by the 2 participants (they did not follow the protocol as stated by researchers) from the methylphenidate group who did not receive a few doses appropriately on weekends or on Monday. Some teacher ratings (8.5%) were missed because of the child’s absence from school on a specific day of evaluation or because of a school holiday Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Vested interest bias | Unclear risk | Methylphenidate and placebo pills were supplied by Novartis Pharmaceuticals (São Paulo, Brazil) at no cost and without restrictions. No additional funding was requested or received from Novartis or any other commercial entity Conflicts of interest: Study authors have reported no conflicts of interest |
| Methods | Individual, double‐blind, cross‐over trial for 4 weeks with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 73. Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 70 (53 boys, 17 girls) Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III (77% met the criteria for ADD with hyperactivity) Mean age: methylphenidate responders 8.5 (SD not stated), methylphenidate non‐responders 9.5 (SD not stated). Range 6 to 17 years Mean IQ: methylphenidate responders 102 (SD 21), methylphenidate non‐responders 89 (SD 23) Methylphenidate naive: 71 (97%) Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: carbamazepine or phenytoin or valproic acid or mephobarbital (6.4 receiving a combination of these drugs). Clonidine (n = 1) Sociodemographics: no information Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg methylphenidate and placebo. Methylphenidate was rounded to the nearest 1.25 mg Mean methylphenidate dosage: no information, but mean dose during follow‐up was 0.36 mg/kg/dose Administration schedule: morning and 4 hours later Duration of each medication condition: 2 weeks Washout before study initiation: none Medication‐free period between interventions: none Titration period: none Treatment compliance: no information | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
None of the parents of responders who had experienced side effects during the trial thought the effects were significant enough that they should not treat their child with methylphenidate, and no side effects other than appetite suppression continued during regular therapy after the trial Follow‐up 6 months after: n = 33. 15 had no change in weight curves. 1 gained 7 kg beyond his original percentile | |
| Notes | Sample calculation: no information Ethics approval: no information Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The pharmacist labelled the 2 sets of capsules as “Medicine A” and “Medicine B” in either order by coin flip for the first 22 trials, and then by using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | The manner of labelling was sealed by the pharmacist in an envelope that was not opened until the trial had ended and findings had been discussed with the parents |
| Blinding of outcome assessment (detection bias) | Unclear risk | Objectivity was lessened for a few parents because the decreased appetite associated with methylphenidate led them to suspect which capsules contained the drug |
| Incomplete outcome data (attrition bias) | Low risk | In the few trials for which 1 to 3 of 10 items on Conners' questionnaire had not been scores, the score was prorated based on 30 points maximum Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | None |
| Vested interest bias | Unclear risk | No information |
| Methods | Phase II, randomised, double‐blind, placebo‐controlled, laboratory‐classroom, cross‐over study with a lead‐in open‐label dose‐optimisation phase
| |
| Participants | Number of participants screened: 93 Number of participants included: 80 Number of participants randomly assigned: methylphenidate 80, placebo 80 (cross‐over) Number of withdrawals: 7 "adverse events", 3 consent withdrawn, 2 lost to follow‐up, 1 because of "lack of efficacy". 1 participant discontinued because of "protocol violation" Diagnosis of ADHD: DSM‐IV‐R (combined 62 (79%), hyperactive‐impulsive 4 (5%), inattentive 13 (17%)) Age: mean 9.1 years (SD 1.7, range 6 to 12) IQ: > 70 Sex of baseline participants included in ITT: 57 boys (72%), 22 girls (28%) Methylphenidate naive: 37% Ethnicity: Caucasian 55 (70%), African American 8 (10%), Asian 2 (3%), other 14 (18%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: "At the end of the dose optimization phase of the study, the majority of patients (78%) were optimized to either the 16 mg or 20 mg dosage strengths" Administration schedule: once daily Duration of each medication condition: 1 week Washout before study initiation: "up to 28 days" Medication‐free period between interventions: 4:00 PM to 7:00 AM the next day (15 hours) Titration period: 5‐week dose‐optimisation phase Treatment compliance: "During the laboratory classroom period, 97% and 96% of participants were compliant with [methylphenidate transdermal system] MTS and placebo treatments respectively" | |
| Outcomes | ADHD symptoms
"The mean values of the CPRS‐R over the 11:00 AM and 3:00 PM time points were used in the analysis" General behaviour
Non‐serious adverse outcomes
| |
| Notes | Sample calculation: yes Ethics approval: yes Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; "Participants were either known to be responsive to stimulants or naive to stimulant treatment" Any withdrawals due to adverse events: yes; 7/93 participants were withdrawn because of adverse effects during the dose‐optimisation period (i.e. before randomisation) Email correspondence with study authors: June 2014. We obtained supplemental information regarding risk of bias. Additional data were not available (Ramstad 2013a [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized into either 1 week of [methylphenidate transdermal system] MTS or 1 week of [placebo transdermal system] PTS (in their individually optimized dose) and were crossed over to the opposite treatment the following week" From correspondence: "Participants were randomized centrally for each of the study conditions. Randomization codes were not available to site study staffs, but were provided to research pharmacies at each site which corresponded to a particular dose pack" (Ramstad 2014 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) | Low risk | "Phase‐II randomized, double‐blind, placebo‐controlled laboratory classroom, crossover study with a lead‐in open‐label dose optimization phase" From correspondence: "Active and inactive patches were identical in appearance" (Ramstad 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) | High risk | Safety population according to Table Two is n = 93. Table Six lists safety data for n = 80 participants only. Safety data for 13 participants appear to be "missing" ‐ including those from participants withdrawn before randomisation as the result of adverse events |
| Selective reporting (reporting bias) | High risk | Safety population according to Table Two is n = 93. Table Six lists safety data for n = 80 participants only. Safety data for 13 participants appear to be "missing" ‐ including those from participants withdrawn before randomisation as the result of adverse events |
| Vested interest bias | High risk | "This study was supported by funding from Shire US Inc" Conflicts of interest: Two medical writers acknowledged (Amy M. Horton & Michelle Roberts) but were unclear about where they came from or what their role was in the publication |
| Methods | Cross‐over trial with 2 interventions
Phases: 4 | |
| Participants | Number of participants screened: 17 Number of participants included: 16 (12 boys, 4 girls). Participants were randomly assigned to 1 of 12 possible drug condition orders Number of participants followed up: 16 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (63%), hyperactive‐impulsive (6%), inattentive (31%)) Age: mean 9.2 years (range 7 to 12) IQ: mean 107.7 (range not reported) Methylphenidate naive: ˜ 80% Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (19%), conduct disorder (25%), generalised anxiety disorder (31%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 12 possible drug condition orders of low, medium and high methylphenidate and placebo Mean methylphenidate dosage: low (mean 0.21 mg/kg to 0.33 mg/kg, SD 0.07 to 0.02); medium (mean 0.31 mg/kg to 0.43 mg/kg, SD 0.09 to 0.03); and high (mean 0.42 mg/kg to 0.65 mg/kg, SD 0.13 to 0.15) methylphenidate Administration schedule: 1 per day at 9:00 AM Duration of each medication condition: 1 day Washout before study initiation: 48 hours before study Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusions of study authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | 'Multiple blind procedures', capsules identically packaged by pharmacists |
| Blinding of outcome assessment (detection bias) | Low risk | 'Examiner, who was kept blind to child’s medication status' |
| Incomplete outcome data (attrition bias) | Low risk | No LTFU Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | High risk | Symptom data not reported |
| Vested interest bias | High risk | Supported by funds from The Psychiatric Endowment Fund Conflicts of interest: Study authors had received funding from Eli Lilly, Shire Pharmaceuticals, Janssen‐Ortho and McNeil Pharmaceuticals |
| Methods | Two‐week, randomised, double‐blind, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: 78 Number of participants included: 57 (all boys). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 57 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined and hyperactive‐impulsive (53%), inattentive (47%)) Age: mean 9.5 years (range 7 to 12) IQ: normal Methylphenidate naive: 100% Ethnicity: not stated Country: Israel Setting: out‐patient clinic Comorbidity: no Comedication: no Sociodemographics: representing all socioeconomic strata Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg immediate‐release methylphenidate and placebo Methylphenidate dose range: 6 mg to 12 mg Administration schedule: once daily Duration of each medication condition: 1 week Washout before study initiation: no (drug‐naive) Titration period: no Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: yes Ethics approval: yes Comments from study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: April to October 2013. We received supplemental data from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned with a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Placebo (prepared as look‐alike capsules by the hospital pharmacy) |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | According to study authors, all planned outcomes were assessed and analysed |
| Vested interest bias | Low risk | Not funded Conflicts of interest: none declared |
| Methods | Randomised, multi‐centre, double‐blind, 5‐period, cross‐over trial in a laboratory classroom setting with 3 interventions
Phases: extended‐release dexmethylphenidate 20 mg/d, extended‐release dexmethylphenidate 30 mg/d, extended‐release racemic methylphenidate hydrochloride 36 mg/d, extended‐release racemic methylphenidate hydrochloride 54 mg/d and placebo | |
| Participants | Number of participants screened: 84 Number of participants included: 84 (55 boys, 29 girls). Participants were randomly assigned to 1 of 5 possible drug condition orders Number of participants followed up: 81 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (combined (89.3%), inattentive (10.7%)) Age: mean 9.5 years (SD 1.7, range 6 to 12) IQ: not stated Methylphenidate naive: 0% Ethnicity: Caucasian (42.9%), African American (27.4%), Hispanic (28.6%), other (1.2%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 5 possible drug condition orders of extended‐release dexmethylphenidate 20 mg/d, 30 mg/d, 36 mg/d, 54 mg/d and placebo Mean methylphenidate dosage: not stated Administration schedule: once daily. Morning dosing as 2 capsules Sunday to Saturday. 6 doses were administered at home (Sunday to Friday), and the Saturday dose was administered by research staff. This was repeated until all 5 treatments had been administered. Mean duration of exposure to study medication was 7 days for all 5 treatments Washout before study initiation: 6 days medication‐free Titration period: "On stabilized total daily dose or the nearest equivalent dose of 40 to 60 mg of d,l‐MPH or 20 to 30 mg d‐MPH for at least 2 weeks prior to the screening visit" initiated before randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes; "It was determined that approximately 90 patients were required to detect a 0.05‐level treatment difference at 84% power assuming a difference and standard deviation of 3.5 and 11 for SKAMP‐Combined score, at 2 hours post‐dose using a paired t‐test" Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; "Children recruited for this study had been stabilized on a total daily dose or the nearest equivalent dose of 40 to 60 mg of d,l‐MPH or 20 to 30 mg d‐MPH for at least 2 weeks prior to the screening visit" ‐ so presumably non‐responders to methylphenidate and those experiencing intolerable adverse events while taking methylphenidate were not included Email correspondence with study authors: July 2014. Emailed study authors to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A total of 84 subjects were randomized to receive treatment and were included in the efficacy and safety analyses" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "After the Practice Day, the first assigned treatment was dispensed to the parents as blinded capsules according to their child’s randomized sequence. To maintain blinding, all treatments were over‐encapsulated and the same number of capsules were given once daily for each sequence" |
| Blinding of outcome assessment (detection bias) | Low risk | "The ratings were based on the frequency and quality of behaviours as observed by three independent, blinded raters in each class. To maintain consistency throughout the study, the blinded observers were responsible for observing and rating the same 6 children at specified intervals throughout the 12‐hour testing period at each center" |
| Incomplete outcome data (attrition bias) | Low risk | "The intent‐to‐treat population included all randomized patients who took at least 1 dose of study medication and had at least 1 post‐dose efficacy measurement" Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting |
| Vested interest bias | High risk | "This study was funded by Novartis Pharmaceuticals Corporation and reports the following involvement: design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, and approval of the manuscript" Conflicts of interest: Dr. Muniz is an employee of Novartis Pharmaceuticals Corporation. He has no other relationships to disclose. Dr. Brams reports the following relationships: serves as speaker, consultant and Advisory Board member for Novartis and Shire; receives grant research support from Novartis, Shire and Eli Lilly. Dr. Mao reports the following relationships: speaker for Novartis, Eli Lilly, Bristol‐Myers Squibb, AstraZeneca and Shire; consultant for Eli Lilly, Novartis and Shire; receives grant research support from Novartis. Mr. McCague is an employee of Novartis Pharmaceuticals Corporation. He has no other relationships to disclose. Ms. Pestreich is an employee of Novartis Pharmaceuticals Corporation. She has no other relationships to disclose. Dr. Silva reports the following relationships: none since 15 December 2006; before that, she was a speaker for Novartis, AstraZeneca and Janssen; received grant/research support from Novartis and Celgene |
| Methods | Phase IV, double‐blind, randomised, cross‐over, analogue classroom trials with 2 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 89. Participants randomly assigned to 1 of 2 possible drug condition orders: 68 Number of withdrawals before randomisation: 21 Number of withdrawals after randomisation: 2 Diagnosis of ADHD: DSM‐IV‐TR (combined (59%), hyperactive‐impulsive (4%), inattentive (37%)) Age: mean 10.75 years (range 5 to 15) IQ: > 80 Sex: 45 boys, 23 girls Methylphenidate naive: 65% Ethnicity: Caucasian (62%), African American (28%), Asian (not stated), Hispanic (not stated), other (10%) Country: USA Setting: out‐patient clinic Comorbidity: anxiety (9%), depressive disorders (1%), learning disability (38%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of OROS methylphenidate and placebo Mean OROS methylphenidate daily dosage: 47.6 mg Administration schedule: once daily, morning Average duration of OROS methylphenidate treatment: not stated Duration of placebo intervention: 1 day Washout before study initiation: up to 28 days Medication‐free period between interventions: no Titration period: before randomisation, up to 6 weeks Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; a history of failed response to methylphenidate was an exclusion criterion Any withdrawals due to adverse events: 2 Email correspondence with study authors: June 2013 to June 2014. We have attempted to obtain supplemental efficacy data (Swanson, Kotkit, Agler, M‐Flynn and Pelham Scale) and safety data from study authors. We are awaiting data from the Yale Open Data Access Project | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Placebo and OROS methylphenidate were matched in appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | 3 participants received OROS methylphenidate on both laboratory school days in error; therefore, only data for their first laboratory school assessment were included in the analyses Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
| Vested interest bias | High risk | Supported by Ortho‐McNeil Janssen Scientific Affairs, LLC Conflicts of interest: Several study authors had affiliations with pharmaceutical companies producing methylphenidate |
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over trial with 3 interventions
| |
| Participants | Number of participants screened: 109 Number of participants included: 54 met inclusion criteria; of these, the parents of 13 children refused methylphenidate treatment. In the final sample, 41 children were included. Participants were randomly assigned to 1 of the possible drug condition orders Number of participants followed up: 31 Number of withdrawals: 10 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Regarding participants completing the study Age: mean 58.07 months (range 48 to 70) IQ: mean 99.26 Sex: 26 boys, 5 girls Methylphenidate naive: 93.5% Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (84%), conduct disorder (19%), mood disorder (0%), obsessive‐compulsive disorder (0%), overanxious disorder (0%), somatisation disorder (0%), psychotic symptoms (0%) Comedication: not stated Sociodemographics: combined parental income Canadian $42,000: 2‐parent home (74%), single‐parent home (26%). Significant differences were observed between the treatment refused group (n = 13) and the treatment completed group (n = 31) on baseline symptoms assessed by Diagnostic Interview for Children and Adults‐Parents; Swanson, Nolan and Pelham scale; and Conners' hyperactivity ratings Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of low‐dose (0.3 mg/kg) and higher‐dose (0.5 mg/kg) methylphenidate and placebo Administration schedule: twice daily, morning and lunch Duration of each medication condition: 7 to 10 days Washout before study initiation: 48 hours before screening assessment Titration period: none Treatment compliance: Treatment compliance was determined by counting the number of pills returned to the researcher at the end of each assessment week Data on compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: no information Comment from study authors
Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: June 2013. Personal email correspondence with study author did not provide supplemental data and information as requested because of author's retirement, and because he has no access to the data because the study took place 15 years ago | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was presented in a fully randomised order as prepared by the hospital's pharmacy department |
| Allocation concealment (selection bias) | Low risk | Methylphenidate and placebo were placed in orange gelatin capsules to disguise taste differences |
| Blinding of participants and personnel (performance bias) | Low risk | All participants, research personnel and medical personnel were unaware of the order of medication conditions |
| Blinding of outcome assessment (detection bias) | Low risk | All participants, research personnel and medical personnel were unaware of the order of medication conditions |
| Incomplete outcome data (attrition bias) | High risk | Only data for children completing the entire study were analysed |
| Selective reporting (reporting bias) | Unclear risk | Not possible to get a copy of the protocol |
| Vested interest bias | Low risk | Supported by Health Canada grant Conflicts of interest: none declared |
| Methods | Twenty‐site, randomised, placebo‐controlled, double‐blind, 6‐week, parallel trial with 3 arms
| |
| Participants | Number of participants screened: 635 Number of participants included: 516 Number of participants randomly assigned: methylphenidate 220, placebo 74 Number of participants followed up: methylphenidate 180, placebo 57 Number of withdrawals: methylphenidate 40, placebo 17 DSM‐IV diagnosis of ADHD (combined (67%), hyperactive‐impulsive (1%), inattentive (32%)) Age: MPH mean 10.2, P mean 10.1 (range 6 to 16) IQ: not stated Sex: 211 boys, 83 girls MPH‐naive: 41%/121 Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (36%) Comedication: not stated Sociodemographics: not stated. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to OROS methylphenidate or placebo Mean methylphenidate dosage: 39.9 mg/d (SD 14.6) or 1.26 mg/kg/d (SD 0.55) Administration schedule: single morning dose Duration of intervention: 6 weeks Titration period: none Treatment compliance: not stated. Patients were required to discontinue any psychoactive medication for ≥ 5 days before entering the study | |
| Outcomes | ADHD symptoms
Quality of life
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes; approved by each site’s ethical review board Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Comments from study authors (limitations)
Key conclusion of study authors
Email correspondence with study authors: November 2013 and January 2014. We requested additional data from study authors but never received them | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Study drugs were administered according to a double‐dummy design. Identically appearing capsules were used |
| Blinding of outcome assessment (detection bias) | Low risk | Study drugs were administered according to a double‐dummy design. Identically appearing capsules were used |
| Incomplete outcome data (attrition bias) | Low risk | NNTB was calculated for each treatment in relation to placebo and for atomoxetine in relation to methylphenidate. The number of participants was chosen to have 90% power to declare non‐inferiority on the basis of a comparison of response rates, with a non‐inferiority margin of 15% Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Supported by Eli Lilly and Company Conflicts of interest: Dr. Newcorn receives grant support from Eli Lilly and McNeil; is a consultant and/or advisor for Eli Lilly, McNeil, Shire, Novartis and Sanofi‐Aventis; and is a member of Speakers' Bureaus for Eli Lilly and Novartis. Dr. Kratochvil receives grant support from Abbott, Cephalon, Eli Lilly, McNeil, Pfizer, Shire and Somerset; receives from Eli Lilly study medication for an NIMH (National Institute of Mental Health)‐funded study; is a consultant for Abbott, AstraZeneca, Eli Lilly and Pfizer; and is a member of the Eli Lilly Speakers' Bureau. Dr. Casat receives research funding from Eli Lilly, Novartis and Abbott, and serves on an advisory board for Eli Lilly. Dr. Allen and Dr. Ruff are employees and shareholders of Eli Lilly. Dr. Michelson and Dr. Moore are former employees of Eli Lilly |
| Methods | Cross‐over trial with 2 interventions
Phases: Each trial included 3 pairs of treatment periods (n‐of‐1). Each pair contained the stimulant and the comparator stimulant or placebo. The child’s doctor individualised the dosing, and the order of drugs was randomly assigned within each pair. Two treatment periods were included in a school week, and 2 days per treatment period (not including Wednesdays and weekends) | |
| Participants | Number of participants screened: 108 (85 boys, 21 girls). Two boys repeated n‐of‐1 trials; 22 (20%) trials were not completed Number of participants included: 86. Participants were randomly assigned to 1 of 3 possible drug condition orders Number of participants followed up: 86 Number of withdrawals: 10 (12%) trials were not completed Age: median 10 years (range 5 to 16) IQ: not stated Sex: 66 boys, 18 girls Methylphendaite‐naive: 36 were taking methylphenidate, 47 dexamphetamine, 3 unknown pre‐trial medication Ethnicity: All were Caucasian (Nikles 2007 in Nikles 2006) Country: Australia Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: caregiver's occupation: full‐time work 21%, part‐time or casual work 33%, unemployed or retired 5%, unpaid homemakers 25%, other 10%, unknown 5% Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of individual doses of methylphenidate, dexamphetamine and placebo Mean methylphenidate dosage: not stated Duration of each medication condition: 2 days each of methylphenidate and placebo per week Washout before study initiation: 40 hours (from 4:00 PM Tuesday to 8:00 AM Thursday) and 64 hours (4:00 PM Friday to 8:00 AM Monday) Titration period: not stated, but all were stabilised on an "optimal" dose of stimulant, initiated before randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms First 41 participants used at the end of each treatment period
From 42nd participant onwards used at the end of each treatment period
Non‐serious adverse events
| |
| Notes | Sample calculation: irrelevant Ethics approval: yes Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: yes (n = 1, insomnia and depression) Comments from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of drugs was randomly assigned within each pair"; "There were three pairs of treatment periods, with the order of drugs randomly assigned by a computer‐generated randomisation schedule within each" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) | Low risk | "Patients, parents, doctors, and the research assistant were all blinded to medication order" |
| Blinding of outcome assessment (detection bias) | Low risk | "A hospital pharmacy encapsulated the medication (crushing of tablets and production of identical capsules containing either medication or placebo)"; "Active medication was encapsulated and identical placebo capsules were produced by a hospital pharmacy" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Vested interest bias | Unclear risk | No information Conflicts of interest: Study authors have indicated that they have no financial relationships relevant to this article to disclose |
| Methods | Cross‐over trial with 3 interventions
Phases: Five‐day trial lasting for 3 consecutive weeks, with 2 medication‐free days between phases | |
| Participants | Number of participants screened: 30 Number of participants included: 4. Participants were randomly assigned to different possible drug condition orders of methylphenidate, lactose placebo and vitamin C placebo Number followed up: 4 (2 boys, 2 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (66.7%), hyperactive‐impulsive (0%), inattentive (33.3%)) Age: mean 8.25 years (range 5 to 11) IQ: mean 72.5 (range 63 to 79) Methylphenidate naive: 100% Ethnicity: Native American (100%) Country: USA Setting: out‐patient clinic (residential school) Comorbidity: not stated Comedication: no Sociodemographics: not living with family Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of methylphenidate (0.6 mg/kg), lactose placebo and vitamin C placebo Mean methylphenidate dosage: not stated (range 10 mg/d to 17.5 mg/d) Administration schedule: 7:30 AM, 11:00 AM and 2:00 PM Duration of each medication condition: 5 days for 3 consecutive weeks Washout before study initiation: not stated Medication‐free period between interventions: 2 days Titration period: none. Fixed dose. Initiated before/after randomisation Treatment compliance: medication given by nurse (directly observed treatment) | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes; Human Subjects Committee of the University of South Dakota's School of Medicine and the Research Committee of the Black Hills Children's Home Society Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from review authors
Email correspondence with study authors: not able to find email addresses for study authors because information is missing from the paper. Not possible to find on the Internet, etc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The order of the trials was randomly determined |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | School caretakers, nurses, teachers and researchers were blinded as to whether treatment consisted of placebo or active agent. All agents were placed in identical capsules |
| Blinding of outcome assessment (detection bias) | Low risk | On each day of each trial, a teacher, blinded to evaluation data, rated the child's behaviour in school using Conners' Teacher Rating Scale (39 items). The caregiver, blinded to evaluation data, completed Conners' Parent Rating Scale (48 items) on a daily basis. School caretakers, nurses, teachers and researchers were blinded as to whether treatment consisted of placebo or active agent. All agents were placed in identical capsules |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Low risk | Research was supported by a University of South Dakota/USF‐Mini Grant Conflicts of interest: none declared |
| Methods | Double‐blind, randomised, cross‐over trial with 4 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 16. Participants were randomly assigned to 1 of the possible drug condition orders Number of participants followed up: 16 (all boys) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (combined (100%)) Age: mean 10.4 years (range 7 to 12) IQ: 95.4 Methylphenidate naive: 0 (0%) Ethnicity: not stated Country: the Netherlands Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (n = 6), comorbid anxiety disorder (n = 1), specific developmental disorders (n = 3) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of methylphenidate, desipramine, L‐dopa and placebo Mean methylphenidate dosage: 15 mg Administration schedule: once, in the afternoon Duration of each medication condition: 1 day Washout before study initiation: 3 days before for methylphenidate users Medication‐free period between interventions: not stated Titration period: none Treatment compliance: 100% | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; all participants were already stable on methylphenidate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) | Unclear risk | "A double‐blind randomised design was used" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Low risk | Research supported by Netherlands Organisation for Scientific Research (NWO) Grant 575‐63‐082 Conflicts of interest: not declared |
| Methods | Multi‐centre, randomised, double‐blind, 16‐week, parallel trial with 2 × 2 factorial design
Two successive 4‐week titration periods followed by an 8‐week maintenance period | |
| Participants | Number of participants screened: 205 Number of participants included: 122 (98 boys, 24 girls) Number randomly assigned: methylphenidate 29, placebo 30, methylphenidate + clonidine 32, clonidine 31 Number followed up: methylphenidate 18, placebo 10, methylphenidate + clonidine 24, clonidine 31 Number of withdrawals: methylphenidate 11, placebo 20, methylphenidate + clonidine 8, clonidine 5 DSM‐IV diagnosis of ADHD (combined (76%), hyperactive‐impulsive (4%), inattentive (20%)) Age: mean 9.5 (range not reported) IQ: > 70 Methylphenidate‐naive: 57 (46.7%) Ethnicity: Caucasian (77.9%), African American (10.7%), Hispanic (6.6%), other (4.9%) Country: USA Setting: out‐patient clinic Co‐morbidity: oppositional defiant disorder (47%), conduct disorder (9%) Co‐medication: no. Protocol‐based behavioural interventions Sociodemographics: not stated. Participant groups were similar except for a higher percentage of whites in the clonidine group and some minor differences with regard to family history of ADHD and tics Inclusion criteria
Exclusion criteria
Previous use of methylphenidate or clonidine was permitted | |
| Interventions | Participants were randomly assigned to immediate‐release methylphenidate or placebo Mean methylphenidate dosage: 0.76 ± 0.54 mg/kg/d (30.2 ± 18.9 mg/d (methylphenidate‐only group) and 25.4 ± 18.2 mg/d (methylphenidate + clonidine)) Administration schedule: 1 to 3 times daily (morning, noon and afternoon) Duration of intervention: 12 to 16 weeks Washout before study initiation: 2 weeks Titration period: 8‐week flexible‐dose titration period (4 weeks for clonidine, then 4 weeks for methylphenidate) initiated after randomisation Maintenance period of optimal dose: 8 weeks Treatment compliance: monitored using pill counts (results of monitoring not stated) | |
| Outcomes | ADHD symptoms
Quality of life
Non‐serious adverse events
| |
| Notes | Sample calculation: yes; sample size of 140 participants (35 per treatment group) was determined to provide between 80% and 90% power to detect a group difference (effects of clonidine) Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: Moderate to severe adverse events were cited as a reason for withdrawal in 8 participants: methylphenidate + clonidine 5, clonidine 2, methylphenidate 1 Methylphenidate + clonidine: irritability (n = 1), tearfulness and irritability (n = 1), headaches (n = 1), itching (n = 1), asymptomatic ECG abnormalities (prolonged QTc) (n = 1) Methylphenidate: tachycardia and palpitations (n = 1) Email correspondence with study authors: March 204 to June 2014. We attempted to obtain supplemental efficacy data from the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation plan included stratification by centre (investigator) and by sexual maturity status (Prepubertal: Tanner I‐II, Pubertal: Tanner III‐V) |
| Allocation concealment (selection bias) | Low risk | Only the programmer in the Biostatistics Centre and the pharmacist knew the allocation |
| Blinding of participants and personnel (performance bias) | Low risk | Methylphenidate (or matching placebo) powder packaged in gelatin capsules |
| Blinding of outcome assessment (detection bias) | Low risk | All investigators, study co‐ordinators, teachers, parents and children were blinded to treatment assignments |
| Incomplete outcome data (attrition bias) | Unclear risk | Statistical analyses were performed according to the ITT principle, and last available observations were carried forward and imputed when needed for both efficacy and safety measures. However, only data from the ADHD Rating Scale for children who completed the titration period were analysed |
| Selective reporting (reporting bias) | Low risk | Protocol published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | This project was supported by NIH (National Institutes of Health) and NINDS (National Institute of Neurological Disorders and Stroke) Conflicts of interest: Some study authors are on the ADHD Advisory Board and the Speakers' Bureau of; are scientific consultants or principal or site investigators for; and/or have received educational or funding support from several pharmaceutical companies |
| Methods | Four‐week, cross‐over trial with 2 interventions
Phases
| |
| Participants | Number of participants screened: 94 Number of participants included: 24 (19 boys, 5 girls). Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 24 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined 19, inattentive 5) Age: mean 8.8 years (range 7.1 to 12.7) IQ: mean 85 (range 46 to 112) Methylphenidate naive: 13 Ethnicity: Caucasian (n = 13), African American (n = 4), Asian (n = 1), Hispanic (n = 5), other (n = 1) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (n = 5), obsessive‐compulsive disorder (n = 2), separation anxiety disorder (n = 1) Comedication: yes Sociodemographics: Hollingshead 4 Factor Social Class 1.7 (0.9); Hollingshead 4 Factor SES Score 52.3 (10.8) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of (low, medium and high) methylphenidate and placebo Mean methylphenidate dosage: low = 0.21 mg/kg; medium = 0.35 mg/kg; high = 0.48 mg/kg Administration schedule: b.i.d. Duration of each medication condition: 1 week Washout before study initiation: yes Medication‐free period between interventions: not stated Titration period: 1 week before randomisation Treatment compliance: "Parents completed a medication administration form, were asked about missing or late doses at weekly interviews and teachers were asked as well. Forms were verified by number of pills in returned vials. Families were asked again in case of discrepancy. Parents and teachers were also asked about unanswered items om questionnaires" | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | We used the Digram balanced randomisation method that Dr. David Lane (a professor of both psychology and statistics at nearby Rice University) used to create the 8 balanced drug dose orders to which our 24 participants were assigned |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) | High risk | One‐week, single‐blind, "lead‐in‐dosing"; all study personnel with participant contact were blind with respect to dosages given during the drug trial |
| Blinding of outcome assessment (detection bias) | Low risk | "The study medication was prepared by the University of Texas Psychiatry Research Pharmacy: the Ritalin LA beads were mixed with (inert) placebo beads and placed in two opaque gelatin capsules, and the white generic IR‐MPH was crushed and mixed with cornstarch and placed in two size 1 gelatin capsules" |
| Incomplete outcome data (attrition bias) | High risk | Blank was left for missing data Selection bias: yes; exclusion of placebo responder during single‐blind placebo washout week |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Vested interest bias | Low risk | Funded by grant number MH072263 from National Institute of Mental Health (NIMH) Conflicts of interest: none declared&& |
| Methods | Cross‐over trial with 2 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 24. Participants were randomly assigned to 1 of 2 possible drug condition orders; condition varied daily: placebo b.i.d. and 0.3 mg MPH/kg b.i.d. Number followed up: not stated Number of withdrawals: not stated Age: mean not reported (range: boys 5 years 6 months to 11 years; girls 5 years 8 months to 11 years 3 months) IQ: boys 100.8 (SD 14.23), girls 104.0 (SD16.52) Sex: 12 boys, 12 girls Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: attention deficit disorder (1/24), conduct disorder (5/24), oppositional defiant disorder (15/24), learning disability (6/25) Comedication: yes; other doses of stimulants reported but not on study days Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg methylphenidate and placebo. "Each child received, in random order with condition varied daily, placebo b.i.d. and 0.3 mg MPH/kg b.i.d." Administration schedule: at breakfast and at lunchtime Duration of each medication condition: not stated Washout before study initiation: not stated Medication‐free period between interventions: no Titration period: none Treatment compliance: no information | |
| Outcomes | ADHD symptoms
General behaviour
| |
| Notes | Sample calculation: no Comment from study authors
Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no information Email correspondence with study authors: October 2014. We received no supplemental information/data from study authors. We asked authors whether this article was part of the Johnston 1988 study but received no response, so we extracted the data as from 2 separate studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each child received, in random order with condition varied daily, placebo and methylphenidate |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Active medication and placebo were disguised in gelatin capsules and were pre‐packaged in individual, dated envelopes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Unclear risk | Not stated |
| Methods | Double‐blind, placebo‐controlled, cross‐over trial
| |
| Participants | Number of participants screened: not stated Number of participants included: 22 (all boys). Participants were randomly assigned to 1 of the possible drug condition orders Number of participants followed up: 22 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 10.39 years (range 8.08 to 13.17) IQ: mean 105.68 Methlyphenidate‐naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic (summer treatment programme) Comorbidity: oppositional/defiant disorder (n = 9), conduct disorder (n = 4), "suggesting the presence of a learning disability", but IQ > 80 (n = 13) Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned different possible drug condition orders of methylphenidate (10 mg b.i.d., SR‐20 q.a.m.) and placebo Mean methylphenidate dosage: immediate‐release methylphenidate 10 mg = 0.29 mg/kg Administration schedule: twice daily: immediate‐release methylphenidate twice daily: morning and lunchtime; sustained‐release methylphenidate once daily: morning Duration of each medication condition: 1 day, but in total 3 to 6 days Washout before study initiation: none Medication‐free period between interventions: not stated Titration period: no Treatment compliance: none All completed study: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Comments from study authors
Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: January 2014. No contact made through author correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Active medication and placebo were disguised in gelatin capsules and were pre‐packaged in individual daily pill reminders |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: not stated |
| Methods | Cross‐over trial with 2 interventions
Phases: 3 | |
| Participants | Number of participants screened: not stated Number of participants included: 31. Participants were randomly assigned to 1 of 3 possible drug condition orders Number of participants followed up: 31 (all boys) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (therefore no subtype) Age: mean 98.8 months (range 5.42 to 9.92) IQ: mean 110.7 (range not stated) Methylphenidate naive: not stated Ethnicity: Caucasian (93.5%), African American (6.5%) Country: USA Setting: hospital (Summer Treatment Program) Comorbidity: oppositional defiant disorder (32%), conduct disorder (48%) Comedication: not stated Sociodemographics: not stated Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 (of not stated) possible drug condition order of 0.3 mg/kg and 0.6 mg/kg methylphenidate and placebo Mean methylphenidate dosage (SD): low 8.1 mg (range 5 to 15); high 16.0 mg (range 10 to 22.5) Administration schedule: twice a day morning and midday; conditions were changed daily over 6 weeks Duration of each medication condition: 2 weeks overall (individual treatment condition 1 day) Washout before study initiation: not described Titration period: none Treatment compliance: not reported | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: not stated Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Email correspondence with study author: June 2014. Emailed study author to ask for additional information but have received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Referred to as a randomised study but sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Capsules were identically packaged |
| Blinding of outcome assessment (detection bias) | Unclear risk | Referred to as double‐blind but not described |
| Incomplete outcome data (attrition bias) | Low risk | Appears to be 100% follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
| Vested interest bias | Unclear risk | None reported Conflicts of interest: no information |
| Methods | Six‐week, within‐participant, double‐blind, placebo‐controlled, cross‐over design
Phases: 5 | |
| Participants | Number of participants screened: 26 Number of participants included: 25 (21 boys, 4 girls). Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 23 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.6 years (range 5.8 to 12.7) IQ: average intelligence Methylphenidate naive: not stated Ethnicity: Caucasian (88%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (n = 13), conduct disorder (n = 8) Comedication: not stated Sociodemographics: "Median family income was US $40 000, with incomes ranging widely (from US $10 000 per year to US $100 000 per year)" Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 10 mg or 17.5 mg methylphenidate and placebo Administration schedule: 2 time points Duration of each medication condition: 5‐day period Washout before study initiation: not stated Medication‐free period between interventions: no Titration period: none initiated before/after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: yes; 1 (exacerbation of tic disorder) Email correspondence with study authors: April 2014. We requested additional information from study authors but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | "The clinical team was not blind to medication condition when making their recommendations. Therefore, as a reliability check of the clinical team’s recommendations, one of the authors (J.W.) who was not involved in the clinical team meetings made independent recommendations based on the same data given to the clinical team. This rater was blind to drug condition except placebo" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "The clinical team was not blind to medication condition when making their recommendations. Therefore, as a reliability check of the clinical team’s recommendations, one of the authors (J.W.) who was not involved in the clinical team meetings made independent recommendations based on the same data given to the clinical team. This rater was blind to drug condition except placebo" |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | High risk | Grants from the Shire Richwood Pharmaceutical Company and National Institute of Mental Health (Grants MH53554, MH45576 and MH50467) |
| Methods | Cross‐over trial with 3 interventions
Phases: 3 | |
| Participants | Number of participants screened: 70 Number of participants included: 68. Participants were randomly assigned to 1 possible drug condition order Number of participants followed up: 68 (66 for ADHD symptoms) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.1 years (range 6 to 12) IQ: mean 104.8 Sex: 89% boys, 11% girls Methylphenidate naive: 0% Ethnicity: Caucasian (94%), other (6%) Country: USA Setting: out‐patient clinic (Summer Treatment Program) Comorbidity: oppositional defiant disorder (43%), conduct disorder (37%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of immediate‐release methylphenidate 3 times a day (t.i.d.); Concerta methylphenidate once a day (qd); and placebo Mean methylphenidate dosage: 0.75 mg/kg (SD 0.34) (Three dosing levels were used: 5 mg immediate‐release methylphenidate t.i.d./18 mg Concerta q.d.; 10 mg immediate‐release methylphenidate t.i.d./36 mg Concerta q.d.; and 15 mg immediate‐release methylphenidate t.i.d./54 mg Concerta q.d. The dose level used for each child was based on that child’s methylphenidate dosing before the start of the study Administration schedule: immediate‐release methylphenidate 3 times a day; Concerta once daily Duration of each medication condition: 7 days Washout before study initiation: not described Titration period: none Treatment compliance: "virtually 100%" | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Comment from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; all participants were required to be medicated with methylphenidate and were receiving a stable dose for ≥ 4 weeks before the start of the study Email correspondence with study author: June 2014. Emailed study author to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Referred to as double‐blind; capsules were identical |
| Blinding of outcome assessment (detection bias) | Low risk | Referred to as double‐blind; capsules were identical |
| Incomplete outcome data (attrition bias) | Unclear risk | Data reported for 66/70 for ADHD and behaviour |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | High risk | Research was supported by ALZA Corporation, the manufacturers of Concerta Conflicts of interest: Dr. Pelham is a member of the ALZA advisory committee on Concerta and its development. Drs. Hoffman and Lock are members of the ALZA paediatric advisory board |
| Methods | Cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 136 (all boys) (110 for 30‐day follow‐up). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 136 (106 for 30‐day follow‐up) Number of withdrawals: 0 (4 for 30‐day follow‐up) Diagnosis of ADHD: DSM‐III‐R (therefore no subtype) Age: mean 9.7 years (range 7.6 to 12.7) IQ: mean 104.5 Methylphenidate naive: not stated Ethnicity: Caucasian (81%), African American (15%) Country: USA Setting: Summer Treatment Program Comorbidity: oppositional defiant disorder (53%), conduct disorder (24%) Comedication: not stated Sociodemographics: median family income: US $25,000, (range US $10,000 to > $100,000) Inclusion criterion
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 possible drug condition order of 0.3 mg/kg methylphenidate and placebo Mean methylphenidate dosage: 10 mg (SD 2.7) Administration schedule: twice daily at 7:45 AM and 11:45 AM Duration of each medication condition programme: 12 days (order randomly assigned daily over 6 weeks, doses administered over weekdays, except on Fridays); follow‐up: 30 days Washout before study initiation: 2 weeks medication free baseline in programme, unclear for follow‐up Titration period: none Treatment compliance: not reported | |
| Outcomes | ADHD symptoms
General behaviour
| |
| Notes | Sample calculation: no Ethics approval: yes Comment from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: June 2014. Emailed study authors to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Capsules were identical |
| Blinding of outcome assessment (detection bias) | Low risk | Raters were blinded to medication condition |
| Incomplete outcome data (attrition bias) | Unclear risk | No loss to follow‐up apparent during programme but data reported for 106/110 followed up in the community |
| Selective reporting (reporting bias) | Unclear risk | Although measures were rated daily, how data were aggregated/reported as a single result per phase is not clear |
| Vested interest bias | High risk | Supported by NIMH (Grant MH48157) Conflicts of interest: Pelham served as an advisor for ALZA Corporation (see Pelham 2001a) |
| Methods | Eight‐day, multi‐centre, double‐blind, randomised, dose‐ranging, cross‐over trial with 2 interventions
From a Summer Treatment Program | |
| Participants | Number of participants screened: 36 Number of participants included: 36 (33 boys, 3 girls). Participants were randomly assigned to 1 of 8 possible drug conditions Number of participants followed up: 36 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.6 years (range 6 to 13) IQ: mean 105.3 (SD 18) Stimulant‐naive: 15 (42%) Ethnicity: Caucasian (75%), African American (19%), Hispanic (3%), mixed African American/White (3%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: no; antidepressants were withdrawn from 2 participants before study enrolment Sociodemographics: Parents were manual workers (6%), clerical/sales workers (32%), technicians/semi professionals (23%) and executives/major professionals (32%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 8 possible drug conditions of methylphenidate transdermal system and placebo Methylphenidate transdermal system dosage: 6.25 cm² (0.45 mg/h), 12.5 cm² (0.9 mg/h), 25 cm² (1.8 mg/h) Administration schedule: 1×/d (at 6:00 AM and 7:00 AM). Application sites were alternated each day between left and right hips Duration of each medication condition (crossed with time of application): 1‐day; patch worn for ≥ 12 hours/d Duration of study: 8 days Washout before study initiation: no Medication‐free period between interventions: no Titration period: none Treatment compliance: 100%. Parents returned patches and dosing records to the study site | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes (36 to 48) Ethics approval: yes; institutional review board at each site approved the study Comments from study authors
Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: February to March 2014. We attempted to obtain supplemental information regarding randomisation, allocation concealment, washout period and efficacy and safety data from study authors but without success | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study sponsor produced random orders and prepared medication kits in numbered containers for each participant |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Treatment sequences were concealed until completion of the study |
| Blinding of outcome assessment (detection bias) | Low risk | Treatment sequences were concealed until completion of the study |
| Incomplete outcome data (attrition bias) | High risk | Evaluable participant data were analysed. As the result of record‐keeping difficulties at 1 site, efficacy data were excluded for 5 participants Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | High risk | Study was supported by Noven Pharmaceuticals. Furthermore, Dr. Pelham was supported by grants from NIAAA, NIDA, NIMH and NINDS Conflicts of interest: Several study authors have received consulting fees and research funding and have been consultants and/or served on the Speakers' Bureaus of several pharmaceutical companies in the past year |
| Methods | Three‐week, randomised, double‐blind, double‐dummy, cross‐over trial with 3 interventions
Parents and children spent Friday evening to Sunday morning in a laboratory setting, where data collection took place | |
| Participants | Number of participants screened: not stated Number of participants included: 10 (all boys). Participants were randomly assigned to different drug orders of immediate‐release methylphenidate, methylphenidate transdermal system and placebo Number of participants followed up: 9 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (80%), hyperactive‐impulsive (0%), inattentive (20%)) Age: mean 8.6 years (range 6.4 to 9.7) IQ: mean 95.3 (range 83 to 109) Methylphenidate naive: none; all receiving a stable dose of immediate‐release methylphenidate before enrolment Ethnicity: Caucasian (50%), African American (20%), Asian (0%), Hispanic (0%), Native American (10%), other (20%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder/conduct disorder (80%) Comedication: no other psychotropics Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to immediate‐release methylphenidate, methylphenidate transdermal system or placebo Immediate‐release methylphenidate Mean dosage: 30 mg/24 h Administration schedule: 10 mg t.i.d. morning, lunch, afternoon Methylphenidate transdermal system Mean dosage: 33 mg/24 h Administration schedule: two 10 cm² methylphenidate transdermal system patches worn for 24 hours at the buttock, with sides alternated daily Time point: applied in the morning Duration of each medication condition: 1 week Washout before study initiation: 48 hours before study (no washout between treatment periods) Titration period: none Treatment compliance: Dosing and adhesion records were collected each week, but no data were available in the article | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Key conclusion of study authors
Comments from study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no; however, all participants had been receiving methylphenidate previously, and this may explain the small number of side effects Email correspondence with study authors: July 2014. Emailed study authors to request additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment; no information about how |
| Allocation concealment (selection bias) | Low risk | Double‐dummy procedure: All children received patches and capsules each day, with applicable placebos |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | One participant discontinued the study during the first week because of worsening behaviour while taking placebo and was not included in the analyses: all other participants completed the study Adverse events: All participants receiving ≥ 1 dose of medication were included in the safety analysis Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | Not possible to find the protocol |
| Vested interest bias | High risk | Sponsored by a grant from Noven Pharmaceuticals (manufacturer of the MTS) Conflicts of interest: Dr. Pelham has served as a consultant for Shire, McNeil, Noven, Celltech/Medeva, Novartis and Abbott Laboratories; has received honoraria from Shire and Janssen and research support from Shire, Alza, Eli Lilly, Noven and Cephalon; and holds common stock in Abbott Laboratories. Dr. Waxmonsky has served on the Speakers' Bureau for Novartis and has received research support from Eli Lilly and Shire Incorporated. Dr. Hoffman has served on the advisory board and Speakers’ Bureau for Shire Pharmaceuticals and on the Speakers' Bureau for McNeil. Dr. Ballow has received research support from GlaxoSmithKline, Panacos, Boehringer Ingelheim, Pharmasset, Jacobus and Pharmena. Dr. Schentag has served as a consultant for or received support from Noven, Wyeth, Daiichi, Targanta Therapeutics and Astellas. Dr. Gonzalez is a full‐time employee of P’Kinetics International Incorporated. No other conflicts of interest are known |
| Methods | Three‐week, randomised, controlled, cross‐over trial with 2 factors ‐ medication and behavioural intervention ‐ with daily medication changes
| |
| Participants | Number of participants screened: not stated Number of participants included: 48 (44 boys, 4 girls). Participants were randomly assigned to 1 of 3 different doses of drug condition orders Number of participants followed up: 47 Number of withdrawals: 1 Age: mean 9.35 years (range 5 to 12) IQ: mean 106.33 (SD 14.61; range not stated) Methylphenidate naive: not stated Ethnicity: Caucasian (79%), African American (12.5%) Country: USA Setting: Summer Treatment Program Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 0.15 mg/kg/dose methylphenidate t.i.d.; 0.3 mg/kg/dose methylphenidate t.i.d.; 0.6 mg/kg/dose methylphenidate t.i.d. and placebo Mean methylphenidate dosage: not clearly stated: "average doses were 5.4 mg (range = 2.5 – 10), 11 mg (range = 6.25 – 20), and 21 mg (range = 11.25 – 30), respectively" Administration schedule: 3 time points Duration of each medication condition: 0.15 mg dose for 4 days, 0.30 mg dose for 4 days and 0.6 mg dose for 3 days, switched daily Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated. Treatment compliance: "One child’s parents withdrew from the study after 2 days because of their concerns about possible side effects of the medication" | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Comments from study authors
Key conclusion of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; only participants not having any documented adverse response to methylphenidate were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | "The children, their parents, and clinical staff members were uninformed of medication condition and only the research coordinator, pharmacist and medical director had access to the medication order. The medical director could reveal medication conditions in cases of severe side‐effect reports" |
| Blinding of outcome assessment (detection bias) | Low risk | "Observers were independent staff members who were not involved in the children’s treatment" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol. No description in clinicaltrials.gov |
| Vested interest bias | Low risk | Funded by a grant from the National Institute of Mental Health (MH62946). Dr. Pelham was funded by grants from the National Institutes of Health (MH62946, MH69614, MH53554, MH69434, MH65899, MH78051, MH062946, NS39087, AA11873, DA12414, HD42080) and the Institute of Education Sciences (L03000665A). Dr. Fabiano was supported in part by a Ruth S. Kirschstein National Research Service Award Predoctoral Fellowship (1F31MH064243‐01A1) and by the Department of Education, Institute of Education Sciences (R324J06024, R324B06045) Conflicts of interest: not declared |
| Methods | Twelve‐month, randomised, controlled, parallel study with 2 different treatment strategies A) Participants with a score ≧ 2.5/1.8 on the Swanson, Nolan and Pelham Scale, Fourth Edition (teacher/parents), were randomly assigned to
B) Participants with score < 2.5/1.8 on the Swanson, Nolan and Pelham Scale, Fourth Edition, were randomly assigned to
| |
| Participants | Number of participants screened: not stated Number of participants included: 150 Number randomly assigned: Concerta + psychology 59, humanistic psychology 59 Number followed up: Concerta + psychology 59, humanistic psychology 59 Number of withdrawals: Concerta + psychology 0, humanistic psychology 0 Diagnosis of ADHD: DSM‐IV‐TR (combined (67%), hyperactive‐impulsive (0%), inattentive 33%)) Age: mean 10 years (range 7 to 14) Sex: boy:girl 4:1 for those with combined type; 1:3 for those with inattentive type Methylphenidate naive: 100% Ethnicity: not stated Country: Spain Setting: out‐patient clinic Comorbidity: no Comedication: no IQ: > 70 Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to Concerta + psychology or humanistic psychology OROS methylphenidate dose: not stated Administration schedule: not stated Duration of intervention: 12 months Titration period: not stated Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Key conclusion of study authors
Comment from review authors
Email correspondence with study authors: June 2014. We received supplemental information regarding funding, ethics approval and protocol from the study authors, although they were not able to send data from the Swanson, Nolan and Pelham Scale, Fourth Edition | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, but no information was given to explain how assignment was done |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No; all planned analyses are described in the paper |
| Vested interest bias | Low risk | Study was not funded. Research was part of the workday, participants were voluntary and no funding was needed to implement the study Conflicts of interest: none. Investigators are staff members at institutions (affiliations) reported in the paper |
| Methods | Four‐week, double‐blind, cross‐over trial, in which children were randomly assigned to the following
| |
| Participants | Number of participants screened: 79 Number of participants included: 46 Number of participants followed up: 43 (30 without anxiety, 13 with anxiety) Number of withdrawals: 3. Participants were randomly assigned to different orders of methylphenidate low dose, methylphenidate high dose or placebo Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 9 years (range not reported) IQ: > 70 Sex: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: anxiety (30%), oppositional defiant disorder (56.0%), conduct disorder (23.3%) (Participants who expressed transient anxiety or depression about consequences of punishment for misbehaviour were considered to not meet the criteria for an overanxious disorder) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
No patients met the criteria for any psychotic or depressive disorder. Participants were free of medication, as well as any other medical disorder | |
| Interventions | A total of 4 weeks of medication. First week always placebo; remaining 3 weeks, participant were randomly assigned to different orders of placebo and low‐ (0.25 mg/kg to 0.40 mg/kg) and high‐dose (0.45 mg/kg to 0.70 mg/kg) methylphenidate. Doses were as follows: 5 mg and 10 mg for weight < 25 kg; for those overweight, the 2 dose conditions were 10 mg and 20 mg Administration schedule: b.i.d., 7:00 AM and 12 noon. Saturday, the dose was adjusted so that it was given 90 minutes before measurements were taken Duration of each medication condition: 1 week Washout before study initiation: not relevant; did not take methylphenidate before Medication‐free period between interventions: 17 hours Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no information Ethics approval: no information Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: November 2013. We received from the study authors supplemental information regarding participants' intellectual function and funding and additional data. The raw data no longer exist; therefore we cannot analyse data from different periods in the cross‐over trial, only endpoint data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to placebo and low‐dose and high‐dose methylphenidate |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned to placebo and low‐dose and high‐dose methylphenidate. Not stated how investigators allocated participants to the intervention |
| Blinding of participants and personnel (performance bias) | Low risk | The child, his or her parents, teachers and research assistants were all blinded to drug status |
| Blinding of outcome assessment (detection bias) | Low risk | The child, his or her parents, teachers and research assistants were all blinded to drug status |
| Incomplete outcome data (attrition bias) | Unclear risk | Only 3 drop‐outs. All other participants were included in the final analysis Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | The entire outcome as stated in the methods was reported in the results |
| Vested interest bias | Low risk | National Institute of Mental Health (NIMH) Conflicts of interest: not declared |
| Methods | Three‐week, randomised, double‐blind, placebo‐controlled, parallel trial with 3 arms
| |
| Participants | Number of participants screened: 73 Number of participants included: 58 Number randomly assigned: methylphenidate 20, placebo 18 Number followed up: methylphenidate 19, placebo 16 Number of withdrawals: methylphenidate 1, placebo 2 Diagnosis of ADHD: DSM‐IV. Diagnostic Interview Schedule for Children diagnosis of ADHD (subtype not stated) Age: mean 9 years (range not reported) IQ: > 75 Sex: not stated Methylphenidate naive: methylphenidate 5 (25%), placebo 1 (6%) Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (methylphenidate 14%, placebo 10%), conduct disorder (methylphenidate 1%, placebo 2%), anxiety disorder (methylphenidate 20%, placebo 5%) Comedication: not stated Sociodemographics: not stated. No significant difference in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to immediate‐release methylphenidate or placebo Mean methylphenidate dosage: total dose 25.2 mg/d Administration schedule: 17 (85%) received 2 or more doses Time points: 1 to 3 times a day: morning, after school and additional noon if needed Duration of intervention: 3 weeks Titration period: none Treatment compliance: not stated. Dosage was adjusted at the end of weeks 1 and 2 via an algorithm based on teacher and parent ratings | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: no Comment from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: January 2014. Supplemental information received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned: "We used a random number generator to determine which of the 3 groups the child was assigned to" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, children, teachers and treating physicians were blind to medication status. Medication was crushed, mixed with a blue food powder and placed in opaque capsules |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo participants were randomly assigned to follow methylphenidate or Adderall treatment algorithm. The blinded psychiatrist could not determine the child’s medication status simply by knowing which algorithm was being followed. Principal Investigator knew the medication status of participants |
| Incomplete outcome data (attrition bias) | High risk | Morning and afternoon IOWA scores were averaged at the end of the week Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were reported |
| Vested interest bias | High risk | Research funded by Shire Richwood Incorporated Conflicts of interest: Dr. Browne is currently with Watson Pharmaceuticals, Corona, California |
| Methods | Five‐week double‐blind, cross‐over, placebo‐controlled trial, conducted to examine electrophysiological effects of methylphenidate on inhibitory control in children with ADHD
| |
| Participants | Number of participants screened: 12 Number of participants included: 12 (8 boys, 4 girls). Participants were randomly assigned to 1 of (not stated) possible drug condition orders Number of participants followed up: 12 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (100%)) Age: mean 12.3 years (range 9 to 15) IQ: not stated Methylphenidate naive: 10 Ethnicity: Caucasian (67%), African American (8%), Hispanic (25%) Country: USA Setting: not stated Comorbidity: oppositional defiant disorder (45%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of 5 mg, 10 mg, 15 mg, 20 mg methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 3 time points Duration of each medication condition: 7 days Washout before study initiation: not stated Medication‐free period between interventions: 0 Titration period: none initiated before/after randomisation | |
| Outcomes | General behaviour
| |
| Notes | Sample calculation: no Ethics approval: yes; study approved by the Institutional Review Board of the University Comments from study authors
Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: May 2014. Study authors could not give us the necessary data (e.g. separate data for each intervention period); therefore we could not use Conners' Global Index data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Double‐blind, crossover, placebo‐controlled" |
| Allocation concealment (selection bias) | Low risk | From email: One investigator was assigned on the basis of chance; the other remained blinded |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind. One investigator assigned on the basis of chance; the other remained blinded |
| Blinding of outcome assessment (detection bias) | Low risk | One investigator assigned on the basis of chance; the other remained blinded |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | Low risk | Funded by the National Institute of Mental Health Grant R01 MH63986 Conflicts of interest: Pliszka received honoraria and research support from Shire and MacNeil and research support from Ely Lilly and Cephalon |
| Methods | Cross‐over trial with 3 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 32. Participants were randomly assigned to possible drug condition orders Number of participants followed up: 31 (all boys) Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (87.1%), hyperactive‐impulsive (9.7%), inattentive (3.2%)) Age: not stated (range 9 to 12 years) IQ: > 80 Methylphenidate naive: 0% Ethnicity: not stated Country: USA and Canada Setting: out‐patient clinic Comorbidity: (0%) Comedication: 0% for other medications for ADHD Sociodemographics: not stated
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of dex,MPH, dex,I‐MPH and placebo. Both the order of drugs and the dose sequence were randomly assigned Mean methylphenidate dosage: d‐MPH 2.5 mg, 5 mg or 10 mg; dex,l‐MPH 5 mg, 10 mg or 20 mg Administration schedule: once at 8.30 AM Duration of each medication condition: 1 day. Interventions were separated by ≥ 6 days Washout before study initiation: 24 hours Medication‐free period between interventions: ≥ 24 hours Titration period: none Treatment compliance: administered at the laboratory | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: approved by each centre’s institutional review board Comments from study authors (limitations)
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; included only children stable on methylphenidate Email correspondence with study authors: July to August 2014. We contacted study authors to obtain safety data and supplemental information regarding randomisation, allocation concealment, blinding, handling of incomplete outcome data and outcomes, but we were not successful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. Both the order of drugs and the dose sequence were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Except for first practice day, on which only participants were blinded, administration of doses was double‐blinded throughout the study |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind. Blinded observers rated behaviour |
| Incomplete outcome data (attrition bias) | Unclear risk | Study authors report only 1 respondent (LTFU), not related to side effects Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. No protocol available |
| Vested interest bias | High risk | Study was funded by Celgene Conflicts of interest: All study authors disclosed that they have past and present affiliations with the pharmaceutical industry |
| Methods | Six‐week, cross‐over trial with 3 interventions
Phases: 3 | |
| Participants | Number of participants screened: not stated Number of participants included: 36. Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 36 (29 boys, 7 girls) (n = 34 for adverse event data) Number of withdrawals: 0 Age: mean 11.4 years (range 9 to 14) IQ: mean 90.9 Methylphenidate naive: 100% Ethnicity: not stated Country: Norway Setting: out‐patient clinic Comorbidity: anxiety/depressive disorder (n = 9, 25%), oppositional defiant disorder (n = 20, 55%), learning disability (n = 22, 61%) and Asperger syndrome (n = 1, 3%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of methylphenidate (30 mg/d to 40 mg/d), dextroamphetamine (10 mg/d to 20 mg/d) and placebo Mean methylphenidate dosage: not stated Administration schedule: methylphenidate, 3 time points (morning, lunch and afternoon); dextroamphetamine, 2 time points (morning and afternoon) Duration of each medication condition: 2 weeks (first week: low dose (30 mg/d), second week: high dose (40 mg/d)) Washout before study initiation: no; all were stimulant‐naive Medication‐free period between interventions: yes; Saturday and Sunday (48 hours) Titration period: 2 weeks initiated after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Comments from study authors
Key conclusions of study authors
Comments from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: April 2015. We obtained supplemental information or data regarding comedication, randomisation and blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly and evenly assigned to each of 6 possible drug orders. "The children were assigned to the six possible drug condition orders by drawing numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) | High risk | Participants, parents, teachers and test administrators were blinded to drug order. One parent identified the drug order. Tablets of similar colours, shapes and textures were administered, but the drugs were not camouflaged in identical capsules |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Vested interest bias | High risk | First phase was conducted as part of ordinary clinical practice at Neuropsychiatric Unit, Østfold Hospital Trust. Second and third phases, data analysis and preparation of manuscript were sponsored by South‐Eastern Norway Regional Health Authority, and also by Østfold Hospital Trust and National Resource Centre for ADHD, both under the umbrella of South‐Eastern Norway Regional Health Authority Conflicts of interest: Henning Aabech is a member of the Strattera Advisory Board, Eli Lilly Norway |
| Methods | Triple‐blind, randomised, cross‐over trial with 4 interventions
Phases
| |
| Participants | Number of participants screened: 22 Number of participants included: 14 (12 boys, 2 girls) Number of participants followed up: 11 Number of withdrawals: 1 Number of participants excluded during the study: 2 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: 8.3/7.75 years (range 6 to 10) IQ: > 100 Methylphenidate naive: not stated Ethnicity: Caucasian (86%), not stated (14%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: no Sociodemographics: Low to middle socioeconomic status Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of fixed doses of methylphenidate (5 mg, 10 mg, 15 mg) and placebo. As a result of the small sample size, not all drug orders were used. Post hoc comparison showed that doses were distributed approximately equally across different positions in the sequence Administration schedule: once daily, in the morning Duration of each medication condition: 7 days Washout before study initiation: none, but all weekly dosage changes occurred on Saturdays to control for potential rebound effects Titration period: none Treatment compliance: Both used and unused envelopes with capsules were returned to control for medication compliance. No results regarding compliance were reported | |
| Outcomes | ADHD symptoms
No useable data | |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; included only methylphenidate‐favourable responders Any withdrawals due to adverse events:no Email correspondence with study authors: July 2013. Study authors informed us that original records were shredded after 20 years, and that they could not provide the requested data (Ramstad 2013c [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of drug administration was determined by randomly assigning each child to 1 of 24 possible drug disorders |
| Allocation concealment (selection bias) | Low risk | Methylphenidate was packaged by the pharmacist in coloured gelatin capsules to avoid detection of dose and taste. Capsules were placed in individually dated envelopes to ensure accurate dose administration |
| Blinding of participants and personnel (performance bias) | Low risk | Triple‐blind design |
| Blinding of outcome assessment (detection bias) | Low risk | Teachers and observers were blind to when medication was administered and what specific doses were given |
| Incomplete outcome data (attrition bias) | High risk | Data from 2 of 12 children in the present investigation were not included in the statistical analyses because they were classified as non‐responders according to results of the Paired Associates Learning test |
| Selective reporting (reporting bias) | Unclear risk | Not possible to receive a copy of the protocol |
| Vested interest bias | Unclear risk | No information regarding funding Conflicts of interest: not declared |
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 2 interventions
Phases: Five methylphenidate doses were given in a randomly assigned, counterbalanced sequence. Children received each dose for 6 consecutive days | |
| Participants | Number of participants screened: 134. Children were screened for inclusion after referrals from paediatricians, psychiatrists and school personnel over a 5‐year period. Different publications reported different numbers of included children, ranging from 22 to 76 children. Study authors stated: "this is a sample of children who were recruited across several years with publications presenting findings over that time period. Thus, there were slight differences in sample composition as more participants were added. But essentially these studies included the same sample". Participants were randomly assigned to 1 of 4 possible drug condition orders. Different publications reported different figures for missing data, number of withdrawals and other important information. This was included in our 'Risk of bias' assessment and is reported in the 'Risk of bias' table. One‐day washout (Saturdays) was described in each publication Age: 10 to 12 years. Reported ages varied across publications but, in most publications, ranged from 6 to 11 years DSM‐III diagnosis of ADHD (subtype not stated) IQ: mean 101. DuPaul 1993 stated that children were of average or above average intelligence Sex: 37 boys and 5 girls (Rapport 1987) Methylphenidate naive: 100% (in both Rapport 1987 + DuPaul 1993). In Rapport 1987, 8 had experienced brief trials of stimulants within the previous 4 years Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: No children were taking medication before participating in the study (DuPaul 1993 in Rapport 1987) Sociodemographics: low to middle socioeconomic status (Hollingshead) (Rapport 1987 and DuPaul 1993 in Rapport 1987) Inclusion criteria
We believe that this study consists of 13 articles. When study authors were asked about that, Dr. DuPaul answered that many of the publications based on the Rhode Island Construction Leadership Council (CLC) Clinic "were fairly the same study". Even though some publications describe 6 inclusion criteria and others describe 5, we believe that the publications are based on the same study (Ramstad 2013b [pers comm]) | |
| Interventions | Participants were randomly assigned to 1 of 5 possible drug condition orders of methylphenidate and placebo. Methylphenidate was described by each child's paediatrician in the following doses: placebo, 5 mg, 10 mg, 15 mg and 20 mg. Fixed doses were prescribed (rather than mg/kg) Administration schedule: Children were seen once a week at the Rhode Island Construction Leadership Council (CLC) Clinic. Baseline measures were obtained during the child's second clinic visit to allow familiarisation with clinic personnel and testing procedures. On subsequent testing days, children were administered a capsule of the active agent (methylphenidate) or placebo. This procedure continued until each child received each dose for 6 consecutive days. All weekly dosage changes occurred on Sundays, and no medication was administrated on Saturdays, to allow for needed washout due to inter‐individual variation in serum and blood plasma levels following acute administration of methylphenidate. All children described in the present study were classified as favourable responders, whereas those whose performance did not improve to this extent were classified as non‐responders. These criteria were established a priori. All children described in the present study were favourable responders based on the given criteria. (Children showing drug‐induced facilitation of performance ≥ 25% (i.e. a 25% drop in error rate) compared with baseline or placebo were classified as favourable responders, whereas those whose performance did not improve to this extent were classified as non‐responders) Treatment compliance: Rapport 1987 and DuPaul 1993 (in Rapport 1987): Both used and unused envelopes were returned to the Rhode Island Construction Leadership Council (CLC) on a weekly basis to assess medication compliance. DuPaul 1993 and Denney 1999 (in Rapport 1987): Medication was properly administered nearly 100% of the time, with "make‐up" observation days scheduled after rare occasions when compliance was not obtained | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated. From Kelly 1988 in Rapport 1987: believe the study was approved by the Institutional Review Board IRB at the University of Rhode Island (the site of the study), but Dr. Rapport could confirm this (Ramstad 2013c [pers comm]) Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; excluded methylphenidate non‐responders Any withdrawals due to adverse events: not stated Email correspondence with study author: We contacted Dr. Rapport by email (Ramstad 2013c [pers comm]). He has stated that he does not have any supplementary data except those provided in these articles. We also contacted Dr. DuPaul (Ramstad 2013b [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Rapport 1987: All children received each of the 5 methylphenidate doses in a randomly assigned counterbalanced sequence |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | All methylphenidate and placebo doses were packaged in coloured gelatin capsules by the clinic's pharmacist. Capsules were sealed in individual daily‐dated envelopes. All teachers were blinded as to time of administration and specific dose |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): yes (4 participants) |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Unclear risk | Study was not supported by any funding, either external or internal. This project was supported in part by a Biomedical Research Support Grant (no. S07 RR05712), which was awarded to the first study author by the Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health Conflicts of interest: no information |
| Methods | Double‐blind, placebo‐controlled, 5‐week, cross‐over trial with 2 interventions
Phases: baseline (1 week) and 5 cross‐over phases (1 week placebo and 1 week methylphenidate each) | |
| Participants | Number of participants screened: not stated Number of participants included: 65. Participants were randomly assigned to 1 (of not stated) possible drug condition order Number of participants followed up: 65 (58 boys, 7 girls) Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (all were of the combined subtype) Age: mean 8.56 years (SD 1.25; range 6 to 11) IQ: mean 102.8 (SD 10.0; range not stated) Methylphenidate naive: Eight had experienced brief trials of stimulant therapy within the previous 4 years. None were prescribed psychostimulants immediately before the start of the current study Ethnicity: Caucasian (100%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: low to middle Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of immediate‐release methylphenidate (5 mg/d, 10 mg/d, 15 mg/d, 20 mg/d) and placebo Mean methylphenidate dosage: not stated Administration schedule: once daily, in the morning Duration of each medication condition: 1 week Washout before study initiation: None were prescribed psychostimulants immediately before the start of the current study Medication‐free period between interventions: 1 day Titration period: none Treatment compliance: "nearly 100%; envelopes were returned on a weekly basis" | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Comments from study authors
Key conclusion of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: April 2014. Obtained supplemental information regarding data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By email: We used a computer‐generated random assignment (without replacement) procedure with an additional stipulation that a nearly equal number of children had to be assigned to each of the possible drug condition orders. Children were entered onto the list as they entered the study and followed that particular order (of which coders, parents, teachers and evaluators were unaware throughout the study) (Magnusson 2014b [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Same as above |
| Blinding of participants and personnel (performance bias) | Low risk | Methylphenidate and placebo dosages were packaged in coloured gelatin capsules by the clinic's pharmacist to avoid detection of dose and taste |
| Blinding of outcome assessment (detection bias) | Low risk | Rater was blinded |
| Incomplete outcome data (attrition bias) | Low risk | Information received by email: no drop‐outs (Magnusson 2014b [pers comm]) Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Vested interest bias | Low risk | None Conflicts of interest: no financial, corporate or commercial relationships to disclose |
| Methods | Sixteen‐week, randomised, 11‐centre, parallel‐group trial with 2 arms
Participants in both medication groups received manual‐standardised, individual cognitive‐behavioural therapy through motivational enhancement approaches throughout the 16‐week medication trial | |
| Participants | Number of participants screened: 1334 Number of participants included: 303 (239 boys, 64 girls) Number of participants randomly assigned: methylphenidate 151, placebo 152 Number of participants followed up in each arm: methylphenidate 151, placebo 152 Number of withdrawals in each arm: methylphenidate 33, placebo 43 Diagnosis of ADHD: DSM‐IV (combined (68.6%), hyperactive‐impulsive (2.6%), inattentive (28.1%)) Age: mean 16.5 years (SD 1.3; range 13 to 18) IQ: All participants were cognitively normal Methylphenidate naive: not stated Ethnicity: Hispanic (15.2%) Race: Caucasian (61.7%), African American (23.2%), Asian (1.3%), Native American/Alaskan (1.0%), other (12.7%) Country: USA Setting: out‐patient clinic Comorbidity: major depressive disorder (12.5%), conduct disorder (32.3%), non‐nicotine substance use disorder (100%) Comedication: drug/alcohol use Sociodemographics: not stated Differences between groups
Inclusion criteria
Exclusion criteria
Opiate dependence
Exploratory analyses of treatment response (ADHD‐RS)
| |
| Interventions | Participants were randomly assigned to OROS methylphenidate or placebo Mean methylphenidate dosage at the end of treatment: 68 mg Administration schedule: single dose in the morning Duration of intervention: 16 weeks Titration period: 2 weeks post randomisation Treatment compliance: 79% (pill counts), 82.3% (self reports) | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: yes; sample size of 300 participants was calculated for ADHD Rating Scale Ethics approval: yes; Institutional Review Boards approved the protocol before participant enrolment Comments from study authors
Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from review authors
Email correspondence with study authors: December 2013. We obtained supplemental information (IQ, allocation concealment and detailed description of 1 of the serious adverse events) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to OROS methylphenidate or matching placebo in a 1:1 ratio, stratified by site and completed by computer at a centralised location |
| Allocation concealment (selection bias) | Low risk | Product was supplied in pre‐randomly assigned kits containing individual bottles. Kits and bottles were labelled with the protocol number and treatment/randomisation number. Labeling protected the study, ensuring that it remained blinded, and indicated that the medication was investigational |
| Blinding of participants and personnel (performance bias) | Low risk | Sites were provided blinded medication bottles for each randomly assigned participant Masking: double‐blinded (participant, caregiver, investigator, outcomes assessor) |
| Blinding of outcome assessment (detection bias) | Low risk | Masking: double‐blinded (participant, caregiver, investigator, outcomes assessor) |
| Incomplete outcome data (attrition bias) | Low risk | Primary analyses were ITT, including all randomly assigned study participants Completers (N = 227) were not different from non‐completers (N = 76) in baseline demographic or clinical characteristics, and the proportion of completers did not differ by treatment assignment |
| Selective reporting (reporting bias) | Low risk | No major violation compared with CT.gov All pre‐specified outcomes of interest have been reported |
| Vested interest bias | Unclear risk | OROS methylphenidate and matching placebo were supplied to the Clinical Trials Network contract pharmacy (EMINENT Services Corporation) by McNeil Consumer and Specialty Pharmaceuticals (distributor for Concerta), at no cost Principal investigators are NOT employed by the organisation sponsoring the study. NO agreement between principal investigators and study sponsor (or its agents) restricts the principal investigator's rights to discuss or publish trial results after the trial is complete Conflicts of interest: Some study authors have received research support from, served on Speakers' Bureaus of or acted as consultants for pharmaceutical companies |
| Methods | Five‐day, randomised, double‐blind, placebo‐controlled, cross‐over trial with 4 interventions and 11 possible orders
| |
| Participants | Number of participants screened: 170 Number of participants included: 18. Participants were randomly assigned to 1 of 11 possible drug condition orders Number of participants followed up: 18 (15 boys, 3 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐IV (combined (28%), hyperactive‐impulsive (0%), inattentive (72%)) Age: mean 9.73 years (range not reported) IQ: mean 104.44 Methylphenidate naive: 0% Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: not stated Comedication: no Sociodemographics: no information Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 11 possible drug condition orders of methylphenidate (10 mg, 15 mg and 20 mg) and placebo Mean methylphenidate dosage: 0.27 mg/kg, 0.42 mg/kg and 0.58 mg/kg, respectively Administration schedule: not stated Duration of each medication condition: 1 day Washout before study initiation: 24 hours Medication‐free period between interventions: not stated Titration period: none Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Comment from study authors
Key conclusion of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Master randomisation tables were prepared by the research support pharmacist at the hospital using simple randomisation with restrictions. A balanced block 22 design was used |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Examiner, psychiatrist, child and child’s family were not informed about the child’s randomisation order or daily medication status until trial completion. Placebo and active medication were prepared by the hospital pharmacist as powdered and packaged in an opaque gelatin capsule to prevent identification of content by colour, taste or volume. Each child’s medication was placed in an individually named and dated envelope to ensure accurate administration |
| Blinding of outcome assessment (detection bias) | Low risk | Examiner, psychiatrist, child and child’s family were not informed about the child’s randomisation order or daily medication status until trial completion. |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Low risk | Research was completed while Dr. Rubinsten was a post‐doctoral fellow at the Hospital for Sick Children (HSC), in Toronto, Canada, and was supported by the Rothschild Fellowship from Israel. It was undertaken, in part, through funding received from the Canadian Institutes of Health (CIHR: Grant #MOP 64312), a CIHR post‐doctoral fellowship (A‐CB), and the Canada Research Chairs Program (RT) |
| Methods | Double‐blind, randomised, cross‐over trial with 2 interventions
Phases: 2 | |
| Participants | Number of participants screened: not stated Number of participants included: 6. Participants were randomly assigned to 1 (not stated) possible drug condition order Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean (not stated) IQ: mean (not stated) Sex: not stated Methylphenidate naive: not stated Ethnicity: Caucasian (100%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 (not stated) possible drug condition order of (not stated) methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: Each child was kept on his or her previous regimen of treatment and a corresponding placebo Duration of each medication condition: 24 hours Washout before study initiation: 3‐day run‐in Titration period: Children were kept on stable medication doses and schedules Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
No separate data were available for methylphenidate | |
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: July 2014. Emailed study authors to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described; "randomized in double‐blind fashion" |
| Allocation concealment (selection bias) | Unclear risk | "Randomized in double‐blind fashion" |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "double‐blind". All medications and placebo were identically packaged by the research pharmacy |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Only participants who successfully underwent an ambulatory blood pressure monitoring study at the end of each phase were included in the primary analyses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: not declared |
| Methods | Twelve‐month, parallel RCT with 4 arms
Follow‐up at baseline (before treatment), at end of titration (3 to 4 weeks) and after 4, 8 and 12 months of treatment Follow‐up study of cohort completing the 12‐month RCT: Participants were evaluated annually for 5 years | |
| Participants | Number of participants screened: 302 Number of participants included: 91 included in the RCT (methylphenidate: 37 boys, 9 girls; placebo: 37 boys, 8 girls) Number of participants randomly assigned: methylphenidate 46, placebo 45 Number of participants followed up: methylphenidate 45, placebo 18 Number of withdrawals: methylphenidate 1, placebo 27 Diagnosis of ADHD: DSM‐III‐R diagnosis of ADHD (subtype not stated) Mean age: methylphenidate 8.4 years, placebo 8.3 years (range 6 to 12) IQ: mean (methylphenidate 110.3; placebo 107.6) Methylphenidate naive: 100% Ethnicity: not stated Country: Canada Setting: research unit at hospital. Comorbidity: oppositional defiant disorder (methylphenidate 56.5%, placebo 44.4%), conduct disorder (methylphenidate 6.5%, placebo 20.0%), anxiety (methylphenidate 21.7%, placebo 24.4%) Comedication: no Sociodemographics: Placebo group had a higher score at baseline for psychosocial adversity than those assigned to the methylphenidate group Psychosocial risk index: methylphenidate 1.5%, placebo 2.7% Inclusion criteria Screening
Diagnostic evaluation
Final inclusion criteria
Exclusion criteria
| |
| Interventions | Randomly assigned to 1 of the 4 treatment groups after stratification based on comorbid, conduct or oppositional disorder: methylphenidate + parent training/parent support, placebo + parent training/parent support Titration period: 3 to 4 weeks (depending on child's weight) after randomisation Mean methylphenidate dosage: target dose 0.7 mg/kg body weight, twice daily Administration schedule: breakfast and lunch Duration of intervention: 12 months Treatment compliance: methylphenidate 80%, placebo 64% | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse effects
| |
| Notes | Sample calculation: yes Ethics approval: yes Comments from study authors
Key conclusions of study authors RCT
Follow‐up study
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: September to October 2013. Unfortunately, they were not able to provide supplemental data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned after stratification based on the presence of comorbid conduct or oppositional disorder and assigned to 1 of the 4 treatment groups. Randomly assigned based on a random number table |
| Allocation concealment (selection bias) | Low risk | Concealed from the physician and participants before randomisation and maintained throughout the study |
| Blinding of participants and personnel (performance bias) | Low risk | All participants (child, research staff, parents and teachers) were blinded to the medication assignment |
| Blinding of outcome assessment (detection bias) | Low risk | All participants (child, research staff, parents and teachers) were blinded to the medication assignment |
| Incomplete outcome data (attrition bias) | High risk | No imputation method: The effect of treatment was analysed among participants who adhered to their original medication assignment. At the 4‐month point, children not taking any medication were grouped with those taking placebo. When a participant switched from placebo to methylphenidate treatment, they were counted within the methylphenidate group Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not possible to find protocol |
| Vested interest bias | Unclear risk | Medical Research Council of Canada, National Health Research Development Program of Canada and the Department of Psychiatry, The Hospital for Sick Children, Toronto. Placebo pills were provided by Ciba Geigy, Canada, Ltd Conflicts of interest: Two study authors have reported working as consultants for pharmaceutical companies, and 1 has furthermore received industry‐sponsored research grants |
| Methods | Single‐centre, randomised, double‐blind, 3‐way cross‐over trial with 2 interventions
Phases: immediate‐release and multi‐layer‐release | |
| Participants | Number of participants screened: not stated Number of participants included: 18 Number of participants followed up: 17 (15 boys, 2 girls) Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV diagnosis of ADHD (combined (100%)) Age: mean 11.3 years (range 6.8 to 15.3) IQ: ≥ 85 Methylphenidate naive: 5 (29.4) Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: no Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of immediate‐release and extended‐release methylphenidate and placebo Mean methylphenidate dosage: 31.2 mg/d (SD 11.7 mg/d; range 20 to 60 mg/d), 1.2 mg/kg Administration schedule: twice daily, in the morning and at noon (4 hours apart) Duration of each medication condition: 6 days Washout before study initiation: 1 day Titration period: none. Participants who were receiving methylphenidate at the time of study entry received the dose of methylphenidate that they were taking before entry into the study/duration initiated before/after randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Email correspondence with study authors: May 2014. Emailed study authors to ask for supplementary information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to a treatment sequence according to a Latin square |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind; not further described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind; not further described |
| Incomplete outcome data (attrition bias) | High risk | All enrolled participants with data from all 3 treatment phases who did not have major protocol violations were considered evaluable for efficacy; all participants were evaluated for safety |
| Selective reporting (reporting bias) | Low risk | No protocol was published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Study was funded by Purdue Pharma (Canada) Conflicts of interest: Some study authors are working for Purdue Pharma |
| Methods | Double‐blind, randomised, multi‐centre, triple cross‐over design with 3 interventions
| |
| Participants | Number of patients screened: 147 Number of participants included: 147 Number of participants randomly assigned: not stated Number of participants followed up: 139 Number of withdrawals: 1 Age: mean 10.2 years (range 6 to 14) IQ: not stated Sex: 119 boys, 28 girls Methylphenidate naive: 0% Ethnicity: not stated Country: Germany Setting: out‐patient clinic Comorbidity: 6.8% (disturbance in social behaviour (2.7%), initial insomnia (0.7%), oppositional defiant disorder (1.36%), dysphemia (0.68%), encopresis (0.68%)) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Pre‐randomisation phase and 3 treatment periods of 7 days each. Participants were randomly assigned to 1 of 6 possible drug condition orders of 20 mg Ritalin LA methylphenidate, 20 mg Medikinet Retard methylphenidate and placebo Mean methylphenidate dosage: 20 mg daily Administration schedule: once daily, morning Time points: 9:00 AM Duration of each medication condition: 7 days Washout before study initiation: none Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events
Non‐serious adverse events
Clinically notable increased values for SBP were recorded under all 3 treatments: 15% on placebo, 17% on Ritalin LA and 18% on Medikinet. Abnormal increases in DBP were noted in 5% of patients taking placebo and Ritalin and in 3% of patients given Medikinet. Abnormal values in SBP among participants who had normal values at screening and at baseline were recorded in only 7 participants (3 given placebo, 3 taking Medikinet and 2 taking Ritalin LA ‐ as reported in the article). Changes in vital signs were attributed to sympathomimetic effects of methylphenidate and were not considered clinically relevant | |
| Notes | Sample calculation: yes Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; efficacy trial to prove non‐inferiority of Ritalin LA vs Medikinet Retard. Highly restrictive inclusion criterion ‐ responders only. Study authors acknowledge "it might overestimate the treatment effect and the tolerability in the overall population, as no treatment naive patients have been included in this trial" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centrally generated with a validated system that automated the random assignment of treatment sequences to randomization numbers in a specified ratio" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "All treating physicians, other site staff, and patients, as well as data analysts and Novartis in‐house personnel, remained blinded from the time of randomization until database lock. The raters also did not have access to information about adverse events to maintain the blind"; "To ensure blinding of the study, all capsules were overencapsulated in an identical optical design" |
| Blinding of outcome assessment (detection bias) | Low risk | "The scale was rated by three treatment‐blinded observers in the classroom who were specifically trained on the SKAMP scale" |
| Incomplete outcome data (attrition bias) | Low risk | All randomly assigned participants were included in the intention‐to‐treat (ITT) population. Missing values for the primary endpoint were replaced by the worst value observed in another participant under the same treatment at the same assessment time. For non‐inferiority comparisons, the per‐protocol (PP) population consisting of participants without major protocol violations was considered primary Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | High risk | Trial aimed at showing efficacy of Ritalin LA with purpose of obtaining marketing authorisation Conflicts of interest: Almost all study authors have received grants, research support or other kinds of financial support from the medical industry |
| Methods | Two‐week, double‐blind, placebo‐controlled, cross‐over, randomised clinical trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 44 (37 boys, 7 girls) Number of participants followed up: 44 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.2 years (range 6 to 12) IQ: > 70 Methylphenidate naive: 16 Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: conduct disorder (39%), oppositional defiant disorder (32%), separation anxiety disorder (16%), major depression (7%) Comedication: no Sociodemographics: income level category, mean 3.7 Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of (0.5 mg/kg) methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: twice per day: morning/noon time points Duration of each medication condition: 7 days Washout before study initiation: yes (2 weeks) Medication‐free period between interventions: not stated Titration period: none stated Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: post hoc power calculation Ethics approval: yes Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; exclusion of children with history of intolerance Email correspondence with study authors: March to April 2014. We emailed study authors twice to ask for supplemental information regarding data on ADHD symptoms but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After baseline assessments, children randomly received either placebo or (...)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | High risk | Grants from Le Fonds de la Recherche en Santé du Québec and the Canadian Institutes of Health Research Conflicts of interest: Yes. Dr. Joober is a principal investigator on a clinical trial not related to this study that is sponsored by AstraZeneca Canada Incorporated, and receives no direct compensation for this trial. Dr. Boivin has the following industry financial ties: The Litebook Company Ltd., Medicine Hat, Alberta, Canada; and Pulsar Informatics Inc., Vancouver, British Columbia, Canada |
| Methods | Cross‐over trial with 3 interventions
Phases
| |
| Participants | Number of participants screened: 150 (girls) Number of participants included: 42 girls and 56 comparison boys Number of participants followed up: 42 Number of withdrawals: 1 girl given placebo at each phase Diagnosis of ADHD: DSM‐IV (subtype not stated) Mean age: 9.1 years (range 6.0 to 12.7) Mean IQ: boys 109.3, girls 105.2 Sex: 56 boys, 42 girls Methylphenidate naive: Elia 1991: 18 out of 48 had no previous stimulant drug treatment; Castellanos 1996: 80% had been previously treated with stimulants Ethnicity: girls: Caucasian (67%), African American (19%), Hispanic (14%); boys: Caucasian (73%), African American (21%), Hispanic (4%), Asian (2%) Country: USA Setting: out‐patient clinic Comorbidity: 71% boys, 69% girls; oppositional defiant disorder (33% boys, 50% girls), conduct disorder (7% boys, 2% girls), major depression (0% boys, 7% girls), separation anxiety (0% boys, 2% girls), specific phobias (0% boys, 7% girls), trichotillomania (2% boys, 0% girls), tic disorders not otherwise specified (13% boys, 2% girls), enuresis (18% boys, 12% girls), reading disorder (5% boys, 8% girls) Comedication: not stated Sociodemographics: SES mean score 48.0 ± 25.8 (girls), 52.4 ± 26.9 (boys) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of methylphenidate, dextroamphetamine and placebo Sharp 1999 Mean methylphenidate dosage: week 1 mean dose 0.45 mg/kg, week 2 mean dose 0.85 mg/kg and week 3 mean dose 1.28 mg/kg Administration schedule: breakfast and lunch ‐ 5 days per week administered by nurse; administered by parent on weekends Elia 1991 Mean methylphenidate dosage: week one 0.9 mg/kg, week two 1.5 mg/kg and week three 2.5 mg/kg Administration schedule: 9:00 AM and 1:00 PM throughout the entire week (7 days) Duration of each medication condition: 3 weeks Washout before study initiation: 3‐week medication‐free period before study Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events Mean beneficial and adverse effects of dextroamphetamine and methylphenidate were nearly identical for all ratings, including ratings of appetite problems. However, objectively verified significant decreases in body weight (drug main effect, F 10.27; P value = 0.0002) were significantly greater for dextroamphetamine (mean change ‐1.1 ± 1.0 kg from baseline; P value = 0.02) than for methylphenidate (‐0.4 ± 1.1 kg; not significant) Stimulant‐related adverse effects and body weight Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: no information available Key conclusion of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study authors: June 2014. We received supplemental information from Dr. Castellanos | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of medication (methylphenidate, placebo or dextroamphetamine) was randomly assigned on the basis of a table administered by the research pharmacy. Dose escalation was fixed, with 2 ranges, depending on body weight (> or < 30 kg). Three weeks (1 dose per week) in each phase. For most children, this was followed by random assignment to pemoline vs placebo after another washout period |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Methylphenidate, dextroamphetamine and placebo were packaged in identical capsules by the NIH pharmacy and were administered by NH nurses in double‐blinded randomly assigned order |
| Blinding of outcome assessment (detection bias) | Low risk | All ratings were performed blind to treatment assignment |
| Incomplete outcome data (attrition bias) | Unclear risk | Did not describe what they did with the missing data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol published. All outcomes discussed were reported |
| Vested interest bias | Unclear risk | None mentioned Conflicts of interest: not declared |
| Methods | "3‐day, double‐blind, placebo‐controlled medication assessment" | |
| Participants | Number of participants screened: not stated Number of participants included: 49 Number of participants randomly assigned: not stated Number of participants followed up: not stated Number of withdrawals: methylphenidate 0, placebo 0 Diagnosis of ADHD: DSM‐IV (combined 35 (71%), hyperactive‐impulsive 3 (6%), inattentive 11 (23%)) Age: mean 10.5 years (range 9 to 12) IQ: mean 104 (range not stated) Sex: 80% boys, 20% girls Methylphenidate naive: not stated Ethnicity: Caucasian (76%), African American (14%), other (10%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (43%), conduct disorder (22%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were assigned to extended‐release methylphenidate in "high" or "low" dose (range 18 to 90 mg/d) Mean methylphenidate: low dose 39 mg Concerta, high dose 73 mg Concerta Administration schedule: once daily Time points: in the morning, 90 minutes before cognitive task Duration of intervention: 7:30 AM to 5:00 PM, Monday through Thursday Titration period: not stated Treatment compliance: not stated Washout before study initiation: methylphenidate 1 week if taking atomoxetine, 24 hours if taking methylphenidate | |
| Outcomes | General behaviour Impairment Rating Scale (IRS; Fabiano 2006) Non‐serious adverse events Pittsburgh Side Effects Rating Scale, blood pressure and heart rate assessed daily during times of peak medication effects | |
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: none Key conclusions of study authors
Comment from review authors
Email correspondence with study authors: July 2014. Emailed study authors to request additional information but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | "To maintain blinding, participants were given the same number of opaque capsules per day regardless of actual MPH dose" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Vested interest bias | High risk | Not described Conflicts of interest: "In the past 3 years, James G. Waxmonsky has served on the Speakers Bureau for Novartis, received honoraria from Scepter, and received research support from Eli Lilly" |
| Methods | Six‐week, randomised, single‐blind, placebo‐controlled, cross‐over trial with 5 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 54 (34 boys, 20 girls) Number of participants followed up: 53 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (70.4%), hyperactive‐impulsive (1.9%), inattentive (27.8%)) Age: mean 9.4 years (range 6 to 12) IQ: > 80 Methylphenidate naive: 0% Ethnicity: Caucasian (63.0%), African American (14.8%), Asian (0%), other (22.2%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 10 possible drug condition orders of 20 mg extended‐release methylphenidate, 40 mg extended‐release methylphenidate, 18 mg OROS methylphenidate, 36 mg OROS methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: once Duration of each medication condition: 1 day Washout before study initiation: 1 day Medication‐free period between interventions: Participants were instructed to continue to take their regularly prescribed medication Sundays through Thursdays between study days; no medication was administered on Fridays to avoid possible carry‐over effects during the Saturday treatment period Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
Only 1 adverse event was considered to be drug‐related | |
| Notes | Sample calculation: yes; 46 Ethics approval: approved by an independent investigational review board Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Email correspondence with study authors: April 2014. Sent an email to study authors to ask for additional data but have not receive a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | All study personnel, with the exception of the nurse, pharmacist or physician dispensing medication, were blinded Study medications were administered in an opaque container with a small aperture, so participants could not see them during administration |
| Blinding of outcome assessment (detection bias) | Low risk | The Swanson, Kotkin, Agler, M‐Flynn and Pelham Scale was completed by blinded raters |
| Incomplete outcome data (attrition bias) | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Vested interest bias | High risk | Funding from Novartis Pharmaceuticals Corporation Conflicts of interest: All study authors have been consultants, have received honoraria or have worked for Novartis |
| Methods | Cross‐over trial with 2 interventions
Phases: 2 cross‐over phases | |
| Participants | Number of participants screened: not stated Number of participants included: 54 (38 boys, 16 girls) Number of participants followed up: 53 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (combined (90.7%), hyperactive‐impulsive (0%), inattentive (9.3%)) Age: mean 9.4 years (range 6 to 12) IQ: not stated Methylphenidate naive: none Ethnicity: predominantly Caucasian Country: USA Setting: laboratory classroom Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 20 mg/d d‐MPH‐ER and placebo Mean methylphenidate dosage: 20 mg/d Administration schedule: once daily; morning Duration of each medication condition: 1 week Washout before study initiation: 1 day Medication‐free period between interventions: 1 day Titration period: none Treatment compliance: 100% in laboratory classroom sessions | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: not stated Comments from study authors
Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Any withdrawals due to adverse events: yes; 1 (during placebo) Email correspondence with study authors: June 2014. Sent twice to study author to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computerised random number assignment |
| Allocation concealment (selection bias) | Low risk | Study medication comprised 1 bottle of 5 capsules of blinded study medication or 1 bottle of 5 matching placebo capsules |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Study medication comprised 1 bottle of 5 capsules of blinded study medication or 1 bottle of 5 matching placebo capsules |
| Blinding of outcome assessment (detection bias) | Low risk | Three independent blinded raters |
| Incomplete outcome data (attrition bias) | Low risk | Safety population consisted of all participants who received ≥ 1 dose of study medication. Efficacy population comprised all randomly assigned participants who provided valid efficacy measurements for both treatment periods Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | High risk | Financed by Novartis Conflicts of interest: Some study authors have affiliations with medical companies |
| Methods | Randomised, multi‐centre, double‐blind, placebo‐controlled, cross‐over design with 2 interventions
| |
| Participants | Number of participants screened: 68 (45 boys, 23 girls) Number of participants included: 68 Number of participants followed up: 67 Number of withdrawals: 1 (from the placebo group) Diagnosis of ADHD: DSM‐IV (combined (82.4%), hyperactive‐impulsive (0%), inattentive (17.6 %)) Age: mean 9.5 years (range 6 to 12) IQ: > 70 Methylphenidate naive: none Ethnicity: Caucasian (50%), African American (22.1%), Asian (0%), Hispanic (19.1%), other (8.8%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to d‐ER‐MPH or placebo Mean methylphenidate dosage: 20 mg/d Administration schedule: once daily, in the morning Duration of each medication condition: 7 days Washout before study initiation: 2 days Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Comment from study authors
Limitations
Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of MPH non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Email correspondence with study authors: October 2013. We received supplemental information from study authors regarding ethics approval and data. We were not able to obtain first period data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | All study medications were identical in appearance for blinding purposes; double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | Safety population consisted of all participants who took ≥ 1 dose of study medication. Efficacy population included all randomly assigned participants who provided valid efficacy measurements for both treatment periods Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes according to protocol |
| Vested interest bias | High risk | Funded by Novartis Pharmaceuticals Corporation Conflicts of interest: Some study authors have affiliations with medical companies |
| Methods | Main study Eight‐week intensive Summer Treatment Program, including a 6‐week, double‐blind, cross‐over trial with 4 interventions
Follow‐up study Retrospective follow‐up study of 16 individuals who completed double‐blind, placebo‐controlled, cross‐over studies during 2 separate Summer Treatment Programs | |
| Participants | Main study Number of participants screened: not stated Number of participants included: 49 Number of participants followed up: 46 (41 boys, 5 girls) Number of withdrawals: 3 Age: mean 13.8 years (range 12 to 17) IQ: mean 101 (range 65 to 129) Methylphenidate naive: 28% Ethnicity: Caucasian (85%), African American (15%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (50%), conduct disorder (15%) Comedication: no Sociodemographics: median family income US $38,500 Inclusion criteria
Exclusion criteria
Follow‐up study Number of participants included: 16 (all boys) Number of participants followed up: 16 Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R Age: mean (children) 10.2 years (range 8 to 11); mean (adolescents) 12.7 years (range 12 to 14.5) IQ: mean (children) 109; mean (adolescents) 107 Methylphenidate naive: 4 (25%) Ethnicity: Caucasian (100%) Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: family income (children US $37,943; adolescents US $49,650) Inclusion criteria
Exclusion criteria
| |
| Interventions | Main study Participants were randomly assigned to 1 of the possible drug condition orders of 25 mg/d, 50 mg/d or 75 mg/d methylphenidate and placebo Mean methylphenidate dosage: 0.17 mg/kg Administration schedule: 3 times a day: 7:45 AM, 11:45 AM, 3:45 PM Duration of each medication condition: 6 days Washout before study initiation: 2 weeks Medication‐free period between interventions: 16 hours Titration period: none Treatment compliance: not stated Follow‐up study Participants were randomly assigned to 1 of the possible drug condition orders of 0.3 mg/kg methylphenidate and placebo Mean methylphenidate dosage: 0.3 mg/kg Administration schedule: twice a day: 8:00 AM and 12:00 PM Duration of each medication condition: 1 day Washout before study initiation: 2 weeks Medication‐free period between interventions: 20 hours Titration period: none Treatment compliance: not stated. Adolescents were evaluated on a protocol that included placebo and 3 doses of methylphenidate. To facilitate comparison, doses for adolescents were converted to milligrams per kilogram, and the dose closest to 0.3 mg/kg was used in this study | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Comments from study authors Main study
Follow‐up study
Key conclusions of study authors Main study
Follow‐up study
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Medication conditions were randomly assigned daily, with each condition occurring once a week. Thus, adolescents received a mode of 6 replications of each medication condition |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind; no further information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind; no further information |
| Incomplete outcome data (attrition bias) | Unclear risk | "Some missing data was caused by holidays or absences from the program" Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Vested interest bias | Low risk | Supported by grants from the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Child Health and Human Development Conflicts of interest: not declared |
| Methods | n‐of‐1 randomised, double‐blind, cross‐over trial investigating the effects of Ritalin on the disruptive behaviour of a child diagnosed with ADHD. One‐day antecedent analysis in the clinic followed by an extended school‐based trial, during which participants received methylphenidate or placebo before evaluation Duration: 15 days | |
| Participants | Number of participants screened: not stated Number of participants included: 1 boy Number of participants followed up: 1 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (subtype: not stated) Age: 11 years IQ: 124 Methylphenidate naive: no Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participant was randomly assigned to 1 of 2 possible drug condition orders of (20 mg/d) immediate‐release methylphenidate and placebo. Out‐patient clinic setting, duration 1 day: ingestion of 20 mg methylphenidate or placebo 45 minutes before evaluation (randomised) Cross‐over 4 hours later followed by second evaluation School setting: 15 days (3 school weeks) Administration schedule: once daily, mornings, 45 minutes before first evaluation Medication‐free periods: on weekends; a total of 9 days with medication and 6 days without, randomly assigned throughout the trial period Titration period: no Washout period before study initiation: none other than weekends Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval:not stated Comments from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: October 2013. We received from study authors supplemental information regarding diagnostic criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A third party, who was not involved in the clinical procedure, determined the selection of actual medication packs |
| Allocation concealment (selection bias) | Low risk | A third party, who was not involved in the clinical procedure, determined the selection of actual medication packs |
| Blinding of participants and personnel (performance bias) | Unclear risk | A third party, not involved in the clinical procedures, determined the selection of actual medication packs. Thus, persons conducting the direct evaluation were blinded to all medication manipulations (i.e. the child, parents, therapists and data collectors). However, no description was provided about whether medication and placebo pills were identical. "Prior to clinical assessment, two packets of medication were provided to the participant's parent. One package contained 20 mg of Ritalin and the other package contained a placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | A third party, who was not involved in the clinical procedure, determined the selection of actual medication packs. Thus, persons conducting the direct evaluation were blinded to all medication manipulations (i.e. the child, parents, therapists and data collectors) |
| Incomplete outcome data (attrition bias) | Low risk | No Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified and no email from study author |
| Vested interest bias | Unclear risk | Not stated |
| Methods | Cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 26 (20 boys, 6 girls) Number of participants followed up: not stated Number of withdrawals: not stated Diagnosis of ADHD: DSM‐IV (combined (77% ), inattentive (23%)) Age: mean 9.63 years (range 6.5 to 12) IQ: mean 99.71 Methylphenidate naive: 24 Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant/conduct disorder (n = 13a), anxiety disorders (n = 13), nocturnal enuresis (n = 4), vocal tics (n = 4), motor tics (n = 3) Comedication: not stated Sociodemographics: middle class status; mean social class of 2.12 Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 0.78 mg/kg/d Administration schedule: 3 times daily for 14 days per medication conditions Time points: morning, noon and 4:00 PM Duration of each medication condition: 14 days Washout before study initiation: not stated Medication‐free period between interventions: no Titration period: on days 1 to 3, participants received a dose 2.5 mg below their target dose of 0.3 mg/kg b.i.d. On days 4 to 7, dose was raised to 0.3 mg/kg b.i.d., and on day 8, the 4:00 PM dose was added Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
Relatively few side effects were reported, probably in part because of adjustment in dosages in response to emergent symptoms | |
| Notes | Sample calculation: no Ethics approval: not stated Comment from study authors
Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with trial authors: March 2014. We received supplemental data from trial authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Medications were administered in random order |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Medications were administered in random order, under double‐blind conditions; capsules of identical appearance and taste |
| Blinding of outcome assessment (detection bias) | Low risk | At the end of each phase, an investigator blind to pharmacological conditions administered a structured interview to the child’s parent concerning emergent side effects in the preceding period |
| Incomplete outcome data (attrition bias) | Unclear risk | Reports of side effects led to bind reduction of dosages or omission of planned increments for 3 participants under methylphenidate Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Table with numbers of reported somatic effects not given |
| Vested interest bias | Low risk | Research supported by National Institute of Mental Health (NIMH) Grant MH 38228; Rafael Klorman Conflicts of interest: not declared |
| Methods | Cross‐over trial with 2 interventions
Phases: week‐long exposure to placebo and to each of 3 different dosage regimens of immediate‐release methylphenidate | |
| Participants | Number of participants screened: not stated Number of participants included: 30 Number of participants followed up: 25 Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (combined (60%), inattentive (40%)) Age: not stated IQ: not stated Sex: not stated Methylphenidate naive: All but 1 were stimulant‐naive Ethnicity: not stated Country: USA Setting: hospital Comorbidity: oppositional defiant disorder (combined 13%, predominantly inattentive 20%); learning disability (combined 40%, predominantly inattentive 20%); anxiety (combined 0%, predominantly inattentive 10%) Comedication: no Sociodemographics: minority representation (combined 53%, predominantly inattentive 20%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 24 possible drug condition orders of low‐dose, medium‐dose and high‐dose methylphenidate and placebo Mean methylphenidate dosage (Combined vs predominantly Inattentive): low dose 0.50 ± 0.12 vs 0.44 ± 0.13; medium dose 0.83 ± 0.20 vs 0.73 ±‐0.21; high dose 1.54 ± 0.31 vs 1.40 ± 0.38 Administration schedule: 3 times daily: morning, midday and 3:00 PM Duration of each medication condition: 1 week Washout before study initiation: not required Medication‐free period between interventions: no Titration period: open‐label lead‐in week, not specified whether before or after randomisation of treatment Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: April 2014. We received supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Double‐blind, cross‐over with week‐long exposure to placebo and each of 3 different dosage regimens of immediate‐release methylphenidate in randomly assigned order |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, cross‐over with week‐long exposure to placebo and each of 3 different dosage regimens of immediate‐release methylphenidate in randomly assigned order. Study medications were prepared and coded by the hospital pharmacy using identical gelatin capsules for active medication and placebo |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Across the 4 placebo and drug conditions, data points were missing as follows: Parent Conners' 1%, Teacher Conners' 6%, Parent SKAMP 1%, Teacher SKAMP 4%, Side Effects 0%. Missing scores were replaced by the mean of scores for that group in that condition Selection bias: titration conducted but unclear whether before or after randomisation. No participants were excluded after the 1‐week titration period |
| Selective reporting (reporting bias) | Low risk | Protocol identified; all outcomes reported |
| Vested interest bias | High risk | No information Conflicts of interest: Three study authors have served or received grants from pharmaceutical companies in the past |
| Methods | Five‐week, triple‐blind, placebo‐controlled, cross‐over trial with 4 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 25 (all boys) Number of participants followed up: 24 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III‐R (combined (88%), inattentive (12 %)) Age: mean 8.0 years (range 6 to 12) IQ: not stated Methylphenidate naive: 44% Ethnicity: Caucasian (96%), Hispanic (4%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (28%), conduct disorder (12%) Comedication: no Sociodemographics: Hollingshead SES category I: 8 (33%), II: 8 (33%), III: 3 (12.5%), IV: 5 (20.8%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 8.8 mg, 0.3 mg/kg/dose Administration schedule: twice/3 times daily: 8:00 AM, 12:00 PM (and 2:00 PM) Duration of each medication condition: 1 week Washout before study initiation: ≥ 5 days Medication‐free period between interventions: none Titration period: initiated after randomisation Treatment compliance: "there was generally good compliance, with a mean of 1.5 missed doses per child over the 5‐week course of the study" | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Board of the University of Chicago Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 4 different orders of drug administration. The research pharmacist used a random numbers table to assign participants to different orders of administration |
| Allocation concealment (selection bias) | Low risk | "All medication and placebos were prepared by the study pharmacist and placed in opaque gelatin capsules" In addition to the pharmacist, 1 investigator had access to the dosage code in the event of a medical emergency that necessitated breaking the blind |
| Blinding of participants and personnel (performance bias) | Low risk | Triple‐blinded: Participants always took 3 capsules daily during the study. "Active drug phase (b.i.d. or three t.i.d.) was always preceded by a titration phase. The purpose of the titration phase was to introduce a typical dose of MPH gradually, so that any observation of side effects during the b.i.d. and t.i.d. dosing phases could not be attributed to rapid introduction of MPH"; "All medication and placebos were prepared by the study pharmacist and placed in opaque gelatin capsules"; "In addition to the pharmacist, one investigator had access to the dosage code in the event of a medical emergency that necessitated breaking the blind" |
| Blinding of outcome assessment (detection bias) | Low risk | Triple‐blinded: In addition to the pharmacist, one investigator had access to the dosage code in the event of a medical emergency that necessitated breaking the blinding |
| Incomplete outcome data (attrition bias) | Low risk | One drop‐out. Data collected on this participant were used in the descriptive information for baseline, t.i.d. and titration phases Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All planned outcomes were measured and reported |
| Vested interest bias | Low risk | None declared. The work was supported by the Smart Family Foundation Conflicts of interest: no affiliations with pharmaceutical companies stated |
| Methods | Four‐week, double‐blind, placebo‐controlled, cross‐over trial in which participants were randomly assigned to
| |
| Participants | Number of participants screened: not stated Number of participants included: 47 (33 boys, 14 girls) Number of participants followed up: 39 Number of withdrawals: 8 Diagnosis of ADHD: DSM‐IV (combined (68%), hyperactive‐impulsive (0%), inattentive (32%)) Age: mean 9.02 years (range 5 years 11 months to 16 years) IQ: mean 106.8 Stimulant‐naive: 70% Ethnicity: Caucasian (89.4%), African American (4.3%), Hispanic (2.1%), other (4.3%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (17%), encopresis/enuresis (10.6%), tic disorder (2.1%) Comedication: no Sociodemographics: predominantly mid to upper socioeconomic status referral base of the clinic Inclusion criteria
Exclusion criteria:
| |
| Interventions | Participants were randomly assigned to different orders of OROS methylphenidate (18 mg, 36 mg, 54 mg) and placebo. No child could start with the 54‐mg dose, and 1 child who weighed < 40 kg did not receive the 54‐mg dose to minimise potential side effects in smaller children Administration schedule: once daily Duration of each medication condition: 7 days Washout before study initiation: 2‐week washout period for children who took stimulant medication before study start Medication‐free period between interventions: no Titration period: none Treatment compliance: 92% of all study medications were given | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Boards of The University of Chicago, Children’s National Medical Center and the General Clinical Research Center Advisory Council Comments from study authors
In interpreting the results, several limitations should be kept in mind
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Correspondence with study authors: August 2014. We contacted study authors to request additional data. Study authors no longer have access to the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dosing schedules were assigned from a randomly ordered list of all dosing schedules |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Each medication differed in appearance, size and colour and, in the case of the 54‐mg condition, number of capsules. The placebo capsule was slightly larger than the methylphenidate preparations. Study authors conducted several analyses to investigate the impact of these differences in appearance on the findings of the study (almost no impact on the results) but still considered risk of bias to be high. Parents and clinicians who were blinded to genotype and medication status rated ADHD symptoms, impairment and stimulant side effects each week |
| Blinding of outcome assessment (detection bias) | Low risk | Parents and clinicians who were blinded to genotype and medication status rated ADHD symptoms, impairment and stimulant side effects each week |
| Incomplete outcome data (attrition bias) | Unclear risk | Any child who could not complete a treatment phase was classified as a premature discontinuation. Their data were included for all phases that were completed. No description on how many participants the analyses were based on |
| Selective reporting (reporting bias) | High risk | Data were included for all phases that were completed. There is no description that reveals how many people the analyses were based on. In the abstract belonging to the study (Stein et al. 2004), researchers write that they will "prospectively evaluate the effects of different doses of stimulant medication on these and other sleep problems". This is not provided in any of the articles included in the Stein 2003 study |
| Vested interest bias | High risk | This study was supported by the National Institute of Mental Health, the General Clinical Research Center Program of the National Center for Research Resources and the National Institutes of Health, Department of Health and Human Services Conflicts of interest: Drs. Stein, Robb, Conlon and Newcorn participate in the Speakers' Bureau for McNeil Consumer and Specialty Pharmaceuticals, and Drs. Stein and Newcorn are members of the Concerta National Advisory Committee |
| Methods | Eight‐week, double‐blind, cross‐over trial comparing the following
with a week of randomly assigned placebo within each drug period | |
| Participants | Number of participants screened: 77 Number of participants included: 65 Number of participants followed up: 56 Number of withdrawals: 9 Demographic data on the 56 participants Diagnosis of ADHD: DSM‐IV (combined (67%), hyperactive‐impulsive (0%), inattentive (33%)) Age: mean 11.78 years (range 9 to 17) IQ: > 70 Sex: 41 boys, 15 girls MPH‐naive: 35.7% Ethnicity: Caucasian (41.0%), African American (41.0%), Asian (2%), Hispanic (7%), Bi‐racial/mixed (9%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 dose conditions of ER d‐MPH and ER MAS administered sequentially from lowest to highest dose with a randomly assigned week of placebo during each period Methylphenidate dosage: 10 mg, 20 mg and 25 to 30 mg. Maximum dose was 25 mg in smaller children (i.e. < 35 kg) to minimise potential side effects Administration schedule: once daily Duration of each medication condition: 1 week Washout before study initiation: 2‐day washout period before beginning of the trial. No washout period between treatment periods Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: approved by the Institutional Review Board of University of Illinois at Chicago Comment from study authors
Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; see comments above Email correspondence with study authors: November and December 2013. We received supplemental data from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Order of drug was randomly assigned so that 50% started with ER d‐MPH and 50% started with ER MAS; also a randomly assigned week of placebo during each period |
| Allocation concealment (selection bias) | Low risk | Weekly blister packs containing capsules of study drug, which were indistinguishable from each other |
| Blinding of participants and personnel (performance bias) | Low risk | Participant, caregivers, outcome assessors |
| Blinding of outcome assessment (detection bias) | Low risk | Participant, caregivers, outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | All study participants who received ≥ 2 weeks of study drug to ensure that all participants had been exposed to ≥ 1 week of active drug were analysed Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No. Regarding the Wiebe et al. abstract, a paper is almost ready for submission |
| Vested interest bias | High risk | Investigator‐initiated study sponsored by Novartis Pharmaceuticals, with additional support provided by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) Conflicts of interest: Some study authors are affiliated with pharmaceutical companies |
| Methods | Two n‐of‐1, double‐blind, placebo‐controlled, cross‐over, randomised controlled trial. Both cases received 4 levels of the following
After the double‐blind trial, the code was broken, and the best dose was administered to the participant. Follow‐up data on the best dose were also measured. Follow‐up data were recorded anecdotally for the first case (Dan) and systematically for the second case (Bill) | |
| Participants | Number of participants screened: 2 boys Number of participants included: 2 Number of participants followed up: 2 Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: Dan 9 years, Bill 13 years IQ: not stated Methylphenidate naive: 100% Ethnicity: not stated Country: USA Setting: not stated Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different orders of 3 doses of methylphenidate and placebo Mean methylphenidate dosage: not relevant Administration schedule: once daily at breakfast Duration of each medication condition: Dan received placebo for 3 days, and 5 days of treatment with 5 mg, 10 mg or 15 mg methylphenidate consecutively. Bill received placebo for 3 days, and 3 days of treatment with 5 mg, 10 mg or 15 mg methylphenidate consecutively Washout before study initiation: treatment‐naive Medication‐free period between interventions: 24 hours between doses Titration period: none Treatment compliance: Parents were instructed to initial a monitoring form each time they gave a dose to their child after breakfast. No further description of this was provided Regarding the follow‐up trial: Dan 15 mg; Bill 10 mg (5 mg twice daily) | |
| Outcomes | General behaviour
Non‐serious adverse events
No relevant outcomes measured in the follow‐up phase | |
| Notes | Sample calculation: not relevant Ethics approval: not stated Comments from study authors
Key conclusion of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: no Email correspondence with study author: January 2014. Wrote to study author to request additional information regarding ethics approval, intellectual disability, etc., but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The order of medication levels was determined randomly. In packing the envelopes, 1 of the study authors arranged the coded envelopes according to the randomly determined order of trials |
| Allocation concealment (selection bias) | Low risk | Each medication level was prepared and packed in separate envelopes by a pharmacist |
| Blinding of participants and personnel (performance bias) | Low risk | Each dose of methylphenidate and placebo was grounded into a powder, mixed with an inert compound, and was sealed in a small coloured drug capsule, so that doses were identical in appearance and taste. Thus, the pharmacist, the data collectors, the participants and their families did not know the order of drug administration |
| Blinding of outcome assessment (detection bias) | Low risk | Each dose of methylphenidate and placebo was grounded into a powder, mixed with an inert compound, and was sealed in a small coloured drug capsule, so that doses were identical in appearance and taste. Thus, the pharmacist, the data collectors, the participants and their families did not know the order of drug administration |
| Incomplete outcome data (attrition bias) | Low risk | All data were provided Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol was identified, and no answer was received from the study author |
| Vested interest bias | Low risk | National Association of School Psychologists Conflicts of interest: not declared |
| Methods | Three‐week, triple‐blind, randomised, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 31 Number of participants followed up: not stated Number of withdrawals: 1 Age: mean not stated (range 6 to 14 years) IQ: not stated Sex: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders: placebo, low, medium doses of OROS methylphenidate; or low, medium doses of OROS methylphenidate, placebo Doses: low (18 mg), medium (participants < 50 kg: 27 mg; participants > 50 kg: 36 mg) Administration schedule: not stated Duration of each medication condition: 1 week Washout before study initiation: 1 week Titration period: no Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Comments from study authors (limitations)
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; exclusion of participants who have exhibited significant treatment‐limiting adverse effects while treated with methylphenidate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to 1 of 2 treatment sequence groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Triple‐blinded: Study participants, their parents/guardians and clinicians were blinded to study assignment in terms of medications and dosing sequences. An unblinded prescribing physician and an unblinded study co‐ordinator did not participate in clinical ratings. Placebo was matched to blinded medication, so that the 2 were indistinguishable |
| Blinding of outcome assessment (detection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) | Unclear risk | One participant withdrew before the placebo visit and was not included in these analyses |
| Selective reporting (reporting bias) | Unclear risk | No protocol was available |
| Vested interest bias | Unclear risk | It was not clear who sponsored the study, but someone did (see authors' affiliations) Conflicts of interest: Calvin R. Sumner is an employee of and an equity holder for the study sponsor. Virginia S. Haynes, PhD, is an employee of 3i Global (Basking Ridge, NJ) and a paid consultant for the study sponsor. Martin H. Teicher, MD, PhD, served as paid consultant and clinical investigator for the sponsor. Jeffrey H. Newcorn, MD, serves as advisor and consultant for Lilly, Ortho‐McNeil Janssen, Schering‐Plough and Shire. He receives research support from Lilly, Ortho‐McNeil Janssen and Shire |
| Methods | Cross‐over trial with 3 arms
Phases: 3 | |
| Participants | Number of participants screened: not stated Number of participants included: 20 (16 boys, 4 girls) Number of participants followed up: 20 Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 10.5 years (range 10 to 12) IQ: < 80 Methylphenidate naive: none Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (n = 4), learning disability (n = 8) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to low‐ (mean 0.28 mg/kg) and high‐dose (mean 0.56 mg/kg) methylphenidate and placebo in the morning and afternoon Duration of each medication condition: 2 days Washout before study initiation: no Titration period: no Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation:not stated Ethics approval:no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: unclear Key conclusion of study authors
Email correspondence with study authors: April 2014. Wrote to study authors to ask for additional data but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | Not clear |
| Vested interest bias | High risk | Research was supported by a RESTRACOM graduate studentship for The Hospital for Sick Children Research Institute and Novartis Pharmaceuticals |
| Methods | Randomized, double‐blind, cross‐over study with 6 interventions
Adderall in 4 different doses
| |
| Participants | Number of participants screened: 36. Number of participants included: 33 Number of participants followed up: 29 Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10.58 years (range 7 to 14) IQ: > 80 Sex: 26 boys, 7 girls Methylphenidate naive: 0% Ethnicity: not stated Country: USA Setting: out‐patient clinic (laboratory classroom) Comorbidity: none Comedication: none Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 36 possible drug condition orders of unknown dose of methylphenidate, placebo and 4 doses of Adderall Mean methylphenidate dosage: not stated Administration schedule: once, morning Duration of each medication condition: 7 days Washout before study initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: 30 of 33 participants completed the 7‐week trial | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; only known methylphenidate responders were included Comments from study authors
Key conclusions of study authors
Email correspondence with study authors: January 2014. No reply has been received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Latin‐square design (with provisions for 36 participants) was used to determine the within‐participant order of administration of the 6 medication conditions, so that for each of the first 6 weeks, approximately one sixth of participants would be assigned to each of the 6 conditions. Separate randomisation was used to determine assignment of conditions across participants for week 7, to provide an opportunity to make up missed weeks |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | For each condition, a pharmacist prepared a set of 7 identical capsules, all containing placebo (lactose), 1 of the 4 doses of Adderall or methylphenidate |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | 30 of 33 participants completed the 7‐week trial; 98% of data were collected as planned. For each individual, missing data (1.1% for SKAMP, 0% for Stimulant Side Effects) were replaced by the average of values from adjacent time points (or adjacent values for session 1 or 6) |
| Selective reporting (reporting bias) | Low risk | No information |
| Vested interest bias | High risk | Supported by a grant from Richwood Pharmaceutical Company |
| Methods | Four‐period, double‐blind, randomised, cross‐over trial with 2 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 38 (33 boys, 5 girls) Number of participants randomly assigned: 34. Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 31 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 9.2 years (range 7 to 12) IQ: not stated Methylphenidate naive: 0% Ethnicity: not stated Country: USA Setting: out‐patient clinic (laboratory classroom) Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of methylphenidate and placebo Mean MPH dosage: not stated. Nominal dose: 20 mg/d Administration schedule: 30‐minute intervals from 7:30 AM to 3:00 PM Duration of each medication condition: 1 day Washout before study initiation: none Titration period: none (participants were kept on current standard treatment in‐between trial days) Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from study authors
Key conclusion of study authors
Comment from review authors
Email correspondence with study authors: May 2014. Emailed study authors to ask for additional information. No reply has been received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | On subsequent Saturdays, each child received (in random order) 1 of 3 possible drug condition orders of methylphenidate and placebo |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | All treatments were administered in identical capsules |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Only completers were followed up |
| Selective reporting (reporting bias) | Low risk | No protocol was published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Supported by ALZA Corporation, Palo Alto, California |
| Methods | Randomised, double‐blind, cross‐over design
| |
| Participants | Number of participants screened: not stated Number of participants included: 32 (28 boys, 4 girls) Number of participants followed up: 30 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined (93.8%), hyperactive‐impulsive (6.2%), inattentive (0%)) Age: mean 9.9 years (range 7 to 13) IQ: not stated Methylphenidate naive: 0% Ethnicity: not stated Country: USA Setting: out‐patient clinic (laboratory classroom) Comorbidity: 0% Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 5 mg, 10 mg or 15 mg t.i.d., or 18 mg/d, 36 mg/d or 54 mg/d administered in bolus at 7:30 AM and once every 30 minutes for 8 hours of methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 3 time points Duration of each medication condition: 1 day Washout before study initiation: not stated Medication‐free period between interventions: none Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes Comments from study authors (limitations)
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: All children in the trial were undergoing treatment with methylphenidate when they were enrolled Email correspondence with study authors: Study authors were contacted twice by email and were asked for much supplemental information regarding data, but we have received no data; therefore, most of the data from this study cannot be used in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized, 3‐way, crossover trial in which a double‐blind, double‐dummy procedure was used" |
| Allocation concealment (selection bias) | Unclear risk | Not enough information |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not enough information |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not enough information |
| Incomplete outcome data (attrition bias) | Unclear risk | Not enough information Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no, but all children in the trial were undergoing treatment with methylphenidate when they were enrolled |
| Selective reporting (reporting bias) | Low risk | "Two additional items developed (from SKAMP) for the NIMH Collaborative Multisite Multimodal Treatment Study of Children With ADHD (MTA) (Greenhill et al., 2001) were included in the classroom ratings but were not included in the analyses of this study" |
| Vested interest bias | High risk | "Supported by the ALZA Corporation" |
| Methods | Randomized, 3‐way, cross‐over trial with 2 interventions
Phases: 3 | |
| Participants | Number of participants screened: not stated Number of participants included: 64 Number of participants followed up: 59 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐IV (combined (82.8%), hyperactive‐impulsive (not stated), inattentive (not stated)) Age: mean 9.2 years (range 6 to 12) IQ: not stated Sex: 81.3% boys Methylphenidate naive: none Ethnicity: Caucasian (82.8%), African American (not stated), Asian (not stated), Hispanic (not stated) Country: USA Setting: out‐patient clinic (laboratory classroom) Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of 5 mg, 10 mg or 15 mg t.i.d., or 18 mg, 36 mg or 54 mg OROS methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: t.i.d. 7:30 AM, 11:30 AM and 3:30 PM; OROS 7:30 AM Duration of each medication condition: 1 week Washout before study initiation: not stated Medication‐free period between interventions: not stated Titration period: none Treatment compliance: no measure | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; "The children with ADHD in the proof‐of‐concept and proof‐of‐product studies were selected based on a history of clinical response to stimulant medication, and this limits the extrapolation of the findings to the drug‐naive population" Any withdrawals due to adverse events: no Email correspondence with study authors: March 2014. We contacted study authors twice by email and asked for supplemental information regarding data, but we have received no data; therefore most of the data from this study cannot be used in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind procedures were implemented by administration of methylphenidate or placebo in capsules |
| Blinding of participants and personnel (performance bias) | Unclear risk | Double‐blind, double‐dummy procedure was used to disguise the 3 treatments |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Children with ADHD in proof‐of‐concept and proof‐of‐product studies were selected on the basis of a history of clinical response to stimulant medication; this limits extrapolation of findings to the drug‐naive population |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Vested interest bias | High risk | Supported by ALZA Corporation |
| Methods | Multi‐centre, double‐blind, double‐dummy, placebo‐controlled, cross‐over study in an analogue classroom setting comparing 3 treatment conditions
Each treatment intervention lasted a week | |
| Participants | Number of participants screened: 214 Number of participants included: 184 Number of participants followed up: 157/184 ITT Number of withdrawals: 27 Diagnosis of ADHD: DSM‐IV (combined (82.2%), hyperactive‐impulsive (4.8%), inattentive (13.0 %)) Age: mean 9.6 years (range 6 to 12) IQ: > 80 Sex: 131 boys, 48 girls Methylphenidate naive: none Ethnicity: Caucasian (70%), African American (11.5%), Asian (1.7%), Hispanic (12.5%), other (5.3%) Country: USA Setting: out‐patient clinic Comorbidity: Approximately 25% had a comorbid condition; anxiety and oppositional defiant disorder were most frequent. Females had a greater rate of comorbid anxiety disorder (from Sonuga‐Barke 2007). Anxiety: 20.8 girls, 5.9 boys. Oppositional defiant disorder: 12.5 girls, 8.8 boys. Insomnia: 2.1 girls, 5.1 boys. Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of Metadate CD, Concerta and placebo Dose: Children treated with low doses (20 mg/d) of methylphenidate were randomly assigned to receive Metadate CD 20, Concerta 18 or placebo; those treated with medium doses (20 to 40 mg/d) were randomly assigned to receive Metadate CD 40, Concerta 36 or placebo; children treated with high doses (40 mg/d) were randomly assigned to receive Metadate CD 60, Concerta 54 or placebo Administration schedule: once daily in the morning Duration of each medication condition: 7 days Washout before study initiation: no Medication‐free period between interventions: not stated Titration period: none Treatment compliance: Regarding the medicine, not stated. 157 received all 3 levels of treatment and participated in all 7 classroom sessions | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes Comments from study authors
Limitations
Key conclusions of study authors
Regarding sex differences
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation:yes Any withdrawals due to adverse events: Three study participants discontinued because of adverse events that were judged to be unrelated to medications (gastroenteritis: Concerta (n = 1); fever: placebo (n = 1); sunburn: placebo (n = 1)) Email correspondence with study authors: June 2014. We emailed study authors to obtain supplemental data. Unfortunately, data in the correct format are no longer accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomised |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, double‐dummy. Treatments were packaged according to a double‐dummy design. Each treatment pack contained a 1‐week supply of study treatment, with each day’s supply consisting of 1 large capsule to accommodate the size of any dose level of Concerta (containing Concerta or placebo) and, depending on dose level, between 1 and 3 smaller Metadate CD‐sized capsules (containing Metadate CD or placebo) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Data from participants finishing the whole study (n = 157) and from the ITT population (n = 184) Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | High risk | Study was funded by Celltech Pharmaceuticals Incorporated Conflicts of interest: Some study authors are consultants for pharmaceutical companies |
| Methods | Prospective, randomised, double‐blind, placebo‐controlled, cross‐over, single‐case designs were used to evaluate methylphenidate administration for 3 school‐aged children with cerebral palsy and comorbid ADHD symptoms
Phases: 4 (including baseline) | |
| Participants | Number of participants screened: not stated Number of participants included: 2 (1 boy, 1 girl) Number of participants followed up: 2 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV (combined (100%), hyperactive‐impulsive (0%), inattentive (0%)) Age: mean 10.4 years (range 9 to 11) IQ: > 70. Participant 2 = 92 non‐verbal and 96 performance non‐verbal (range not stated) Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: cerebral palsy (100%), mild cognitive impairment (100%), autism spectrum disorder (50%), physical impairment (50%), learning disability (50%), other health impairment (50%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of methylphenidate (low or high dose) and placebo Mean methylphenidate dosage: 2 doses of methylphenidate administered: low (0.3 mg/kg) and high (0.5 mg/kg) Administration schedule: once daily, mornings, before the child’s school arrival Duration of each medication condition: Order of drug/placebo administration was randomly assigned, and administration was provided for 5 consecutive school days in each condition. Drug treatment was not administered on weekends For participant 2, the order of administration was as follows: baseline, low dose, placebo, high dose For participant 3, the order of administration was as follows: baseline, placebo, low dose, high dose Washout before study initiation: not stated Medication‐free period between interventions: weekends Titration period: not stated Treatment compliance: not stated | |
| Outcomes | General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Order of drug/placebo administration was randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Parents, school personnel (i.e. teachers, teacher assistants) and research assistants responsible for observational data collection and coding were kept blind to drug/placebo conditions |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Vested interest bias | Unclear risk | Not stated Conflicts of interest: This work was supported, in part, by a McKnight Land‐Grant Professorship to Frank Symons |
| Methods | Four‐day, double‐blind, placebo‐controlled, randomised, fixed dose‐escalating of methylphenidate, parallel‐group trial | |
| Participants | Number of participants screened: not stated Number of participants included: 36 (all boys) Number of participants randomly assigned: methylphenidate 19, placebo 17 Number of participants followed up: methylphenidate 19, placebo 17 Number of withdrawals: none Diagnosis of ADHD: DSM‐IV, combined (methylphenidate 84.6%, placebo 76.5%) Age: mean 11.6 years IQ: mean 94.7 Methylphenidate naive: no psychiatric medication for the past 6 months Ethnicity: European‐Brazilian (89%) Country: Brazil Setting: out‐patient clinic Comorbidity: Conduct disorder and oppositional defiant disorder (methylphenidate 58.8%, placebo 68.4%), depressive disorder (methylphenidate 5.2%, placebo 5.9%), multiple anxiety disorder (methylphenidate 5.2%, placebo 0%), tic disorder (methylphenidate 0%, placebo 5.9%) Comedication: not stated Sociodemographics: low‐ to middle‐income families Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate or placebo Mean methylphenidate dosage: 0.72 mg/kg/d Administration schedule: twice a day: morning and lunchtime Duration of intervention: 4 days Titration period: first day 0.35 mg/kg Treatment compliance: good | |
| Outcomes | ADHD symptoms
Quality of life
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: approved by the Ethical Committee of the HCPA (approved as an IRB by the Office for Human Research Protections, USA) Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusion of study authors
Email correspondence with study authors: October 2013. We received from the study authors supplemental information regarding blinding and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned on the basis of a computer‐derived algorithm (EPIINFO6) |
| Allocation concealment (selection bias) | Low risk | Only the doctor performing the randomisation knew the allocation, and he had nothing to do with data collection |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind: Participants, parents and all research team members who had contact with participants. Both methylphenidate and placebo pills were manufactured by a single pharmaceutical company; they had the same format and colour and were given to participants in 4 different blisters |
| Blinding of outcome assessment (detection bias) | Low risk | All research team members who had contact with participants |
| Incomplete outcome data (attrition bias) | Low risk | None Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo‐responders): no |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | High risk | This work was supported by research funds from Hospital de Clínicas de Porto Alegre, FAPERGS and NOVARTIS |
| Methods | Cross‐over trial with 2 interventions
Phases: 2‐ or 3‐week phases, with each group receiving placebo or SODAS methylphenidate | |
| Participants | Number of participants screened: 25 from a previous ADHD/substance misuse study and 15 through advertising Number of participants included: 16 Number of participants followed up: 16 Number of withdrawals: 2 (both from group A; withdrawal rate 12.5%) Diagnosis of ADHD: DSM‐IV (combined 12 (75%), inattentive 3 (18.75%), hyperactive/impulsive (n = 1)) Mean age: group A 17.5 years (SD 2.33), group B 17.38 (SD 2.2) IQ: group A 79.43 (SD 16.66), group B 84.75 (SD 21.16) Sex: all boys (100%) Methylphenidate naive: 100% Ethnicity: European‐Brazilian (group A 3 (37.5%), group B 7 (87.5%)) Country: Brazil Setting: out‐patient clinic Comorbidity: conduct disorder (group A 100%, group B 75%); oppositional defiant disorder (group A 25%, group B 37.5%); depression (group A 12.5%, group B 25%) Comedication: yes (marijuana and cocaine); group A: marijuana (100%) and cocaine (50%); group B: marijuana (87.5%) and cocaine (37.5%) Sociodemographics: divorced parents (group A 37.5%, group B 50%); socioeconomic group A + B + C (group A 50%, group B 87.5%); group D + E (group A 50%, group B 12.5%) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean SODAS methylphenidate dosage: 0.3, 0.7 and 1.2 mg/kg/d for weeks 1, 2 and 3 for group A, and for weeks 4, 5 and 6 for group B Administration schedule: morning dose time points Duration of each medication condition: 3 weeks Washout before study initiation: no Medication‐free period between interventions: 24 hours Titration period: none Treatment compliance: "Study compliance was assessed by self‐report, mother’s report and pill counting" | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval:"The project was approved by the Institutional Review Board (IRB) of Hospital de Clinicas de Porto Alegre (approved as an IRB by the Office for Human Research Protections, United States of America, IRB 00000921" Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: One withdrawal resulted from a participant feeling "worse", "more restless" Email correspondence with study authors: May 2014. We received supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "One of the investigators (LAR) randomized the 16 subjects into groups A or B and prepared weekly blisters of medications for each participant" |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "A pharmacist packaged mPH‐SODAS and matching placebo in capsules so that the MPH‐SODAS and placebo could not be visually differentiated" |
| Blinding of outcome assessment (detection bias) | Low risk | From author correspondence: All persons who evaluated outcome measures were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): unclear |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | High risk | "The ADHD outpatient program receives research support from Bristol‐Myers Squibb, Eli‐Lilly, Janssen‐Cilag and Novartis" Conflicts of interest: Study authors are consultants and speakers for various companies |
| Methods | Within‐participant, cross‐over trial with 3 interventions
Two different drug conditions each day | |
| Participants | Number of participants screened: 16 Number of participants included: 19 Number of participants followed up: 12 (10 boys, 2 girls) Number of withdrawals: none Diagnosis of ADHD: DSM‐III ADD‐H (equivalent to 'combined' in later classifications) Age: mean 8.4 years (range 6 to 11) IQ: mean 105 Methylphenidate naive: Five received methylphenidate previously. Three were taking methylphenidate at the time of referral and had a 48‐hour pre‐trial washout Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (n = 4), learning difficulties (n = 8; according to school record and defined as < 25th percentile on 1 or more subtests within the Wide Range Achievement Test (WRAT‐R)) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of 0.3 mg/kg or 0.6 mg/kg methylphenidate and placebo Mean methylphenidate dosage: not stated. Administration schedule: Interval of 4 hours separated morning and afternoon doses Duration of each medication condition: not stated Washout before study initiation: 4 hours Titration period: none Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated Key conclusions of study authors
Comment from review authors
Email correspondence with study authors: sent email to study authors to ask for additional information but have not received a reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of medication condition was randomized with the restrictions that each child receive two different medication conditions each day (e.g. high, low) occur with equal frequency in the morning and afternoon" "The order of these six combinations was randomized for each child" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "The methylphenidate and placebo were packaged in coloured gelatin capsules by the hospital pharmacist to avoid detection of dose and taste, packaged in individual envelopes and dispensed by project staff 1 hour before testing" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Low risk | Jointly funded by Ontario Mental Health Foundation (Grant No. 963‐86/88) and Health and Welfare Canada (Grant No. 6606‐3166‐42) Conflict of interest: Study authors' affiliations were not declared |
| Methods | Cross‐over trial with 2 interventions
Phases: 3 separate drug conditions: placebo, low‐dose (0.3 mg/kg) and high‐dose (1.0 mg/kg) medication, with 2 test sessions each. Drug conditions changed on a daily basis: 6 test sessions plus baseline practice session | |
| Participants | Number of participants included: 26 Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 23 Number of withdrawals: 3 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 9.2 years (range not stated) IQ: mean 105.9 (SD 10.5) Sex: 24 boys, 2 girls Methylphenidate naive (not clear; "9 children receiving stimulant medication prior to the present study") Ethnicity: not stated Country: Canada Setting: hospital/out‐patient department Comorbidity: 12 Comedication: not clear Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: N/A (modal dose at 0.3 mg/kg = 7.5 mg (range 5.0 mg to 15 mg), modal dose at 1.0 mg/kg = 27.5 mg (range 17.5 mg to 47.5 mg)) Administration schedule: daily single dose Duration of each medication condition: 1 day Washout before study initiation: 48 hours before study initiation Medication‐free periods between interventions: no Titration period: none Treatment compliance: capsules administered by project staff | |
| Outcomes | General behaviour
Non‐serious adverse events
Three children exhibited unusual side effects leading to trial termination. One exhibited hypersensitivity (skin rash, urticaria and throat clearing), and 2 became somewhat disoriented and confused and complained of odd sensations in their limbs | |
| Notes | Sample calculation: no Ethics approval: not stated Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Any withdrawals due to adverse events: yes; 3 Email correspondence with study authors: April 2014. We emailed study authors twice to ask for supplemental information/data but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear |
| Allocation concealment (selection bias) | Low risk | Double‐blind, placebo‐controlled design. Drug order for the first 3 test sessions was randomly assigned, and each child retained the same order for the remaining 3 sessions |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled design. Medication packaged in coloured gelatin capsules to avoid detection of dose and taste. Each child followed the same routine for every test session |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear |
| Incomplete outcome data (attrition bias) | High risk | Incomplete outcome data for 3 respondents with unusual side effects |
| Selective reporting (reporting bias) | Unclear risk | No information presented on numbers of participants with side effects or outcomes for data on nausea, blood pressure, etc. |
| Vested interest bias | Low risk | Research supported in part by a grant from the Canadian Psychiatric Research Foundation and a Post‐Doctoral Fellowship by the Ontario Mental Health Foundation Conflicts of interest: not declared |
| Methods | Cross‐over trial with 2/3 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: methylphenidate 22, control 16. Participants were randomly assigned to 1 of 6 possible drug condition orders Number of participants followed up: 22 (21 boys, 1 girl) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 9.4 years (range not stated) IQ: mean 105.5 (SD 11.3) Methylphenidate naive: not clear: "Thus all these children would have received a trial with psychostimulants independently of this study"; "8 children had been receiving stimulant medication prior to this study") Ethnicity: not stated Country: Canada Setting: out‐patient clinic Comorbidity: 55%; oppositional disorder (n = 5), conduct disorder (n = 3), emotional disorder as well as oppositional or conduct disorder (n = 4), major depression (n = 2), avoidant disorder (n = 1), separation anxiety disorder (n = 1) and learning disorder (n = 6) Comedication: not clear Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 6 possible drug condition orders of (dose not stated) methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: 6 separate medication administrations: 2 placebo, 2 low dose (0.3 mg/kg) and 2 high dose (1.0 mg/kg). First 3 test sessions were ordered randomly, than were ordered repeatedly for last 3 sessions: daily for 6 days Duration of each medication condition: 3 hours Washout before study initiation: 48 hours Medication‐free period between interventions: not clear Titration period: none Treatment compliance: "Medication was administered by project staff" | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Double‐blind, placebo‐controlled, within‐participant design was used ...randomly assigned, counterbalanced sequence. Medications were prepared individually for each child and were packaged in coloured gelatin capsules to avoid detection of dose and taste |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled, within‐participant design was used ...randomly assigned, counterbalanced sequence. Medications were prepared individually for each child and were packaged in coloured gelatin capsules to avoid detection of dose and taste |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals were noted Selection bias (e.g. titration before randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Vested interest bias | Low risk | Study funded by the Canadian Psychiatric Research Foundation and the Medical Research Council of Canada Conflicts of interest: not declared |
| Methods | Four‐day, randomised, double‐blind, placebo‐controlled, cross‐over trial with
With initial 1‐day open‐label trial | |
| Participants | Number of participants screened: 28 Number of participants included: 28 (25 boys, 3 girls). Participants were randomly assigned to 3 doses of methylphenidate and placebo Number of participants followed up: 28 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 8.9 years (range 7 to 11) IQ: mean 106.5 (15.6) Methylphenidate naive: 80% Ethnicity: Caucasian (90%), other (10%) Country: Canada Setting: out‐patient clinic Comorbidity: oppositional defiant disorder or conduct disorder (35%), learning disabilities (39%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 doses of methylphenidate (0.3 mg/kg, 0.6 mg/kg, 0.9 mg/kg) and placebo Mean methylphenidate dosage: not stated Administration schedule: once daily Duration of each medication condition: 1 day Washout before study initiation: For children receiving methylphenidate before trial, washout period ≥ 48 hours before trial, with no washout between periods Titration period: All children participated in an initial 1‐day open trial with a 0.3 mg/kg dose of methylphenidate to ascertain tolerance, before proceeding with the double‐blind, placebo‐controlled trial Treatment compliance: not stated | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes; Institutional Research Ethics Board Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; 1‐day open‐label trial to ascertain tolerance towards medication, but all participants were analysed Key conclusions of study authors
Email correspondence with study authors: August 2013. We received from the first study authors supplemental information regarding drop‐outs, screening and ethics approval | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised |
| Allocation concealment (selection bias) | Low risk | All medication was prepared by the hospital pharmacy and was packaged in opaque gelatin capsules to avoid detection of dose and taste |
| Blinding of participants and personnel (performance bias) | Low risk | Children, parents, teachers and research staff were all blinded to medication conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Children, parents, teachers and research staff were all blinded to medication conditions |
| Incomplete outcome data (attrition bias) | Low risk | Data from all 28 participants were analysed Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | Low risk | Medical Research Council of Canada and Health and Welfare Canada Conflicts of interest: nothing to declare |
| Methods | Cross‐over trial with 1 interventions
Phases: washout (if applicable), baseline, trial | |
| Participants | Number of participants screened: 50 Number of participants included: 40 (34 boys, 6 girls). Participants were randomly assigned to 1 of 12 possible drug condition orders Number of participants followed up: 40 Number of withdrawals: none Diagnosis of ADHD: DSM‐III‐R (no subtype) Age: mean not stated (range 7 to 11 years); ADHD mean 9.4; ADHD + anxiety mean 9.1 IQ: > 80 Methylphenidate naive: not stated Ethnicity: Caucasian (90%), African American or Asian (10%) Country: Canada Setting: out‐patient clinic Comorbidity (type: overanxious 27.5%, separation anxiety 5%, overanxiety and separation anxiety 10%, avoidant disorder with overanxious traits 2.5%, ODD 55%, conduct disorder 10%) Comedication: not stated Sociodemographics: predominantly from middle‐class families Inclusion criteria
Exclusion criteria
Inclusion criteria for the ADHD + anxiety group One or more of the following
Children in the ADHD group did not meet any of these criteria for anxiety | |
| Interventions | Participants were randomly assigned to 1 of 12 possible drug condition orders of 0.3 mg/kg, 0.6 mg/kg or 0.9 mg/kg methylphenidate and placebo Mean methylphenidate dosage: 0.3 mg/kg = 8.75 (± 2.2); 0.6 mg/kg = 17.75 (± 4.2); 0.9 mg/kg = 27.25 (± 5.6) Administration schedule: once per day Duration of each medication condition: 1 day Washout before study initiation: 48 hours when applicable Medication‐free period between interventions: not stated Titration period: 1‐day open trial on 0.3 mg/kg before proceeding to ascertain tolerance. Not stated whether initiated before/after randomisation Treatment compliance: Medication was administered at the laboratory | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Key conclusion of study authors
Comment from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; see above Any withdrawals due to adverse events: no Email correspondence with study authors: June 2014. Emailed study authors twice to request supplemental information/data but have received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug order was counterbalanced and determined by random assignment, such that an approximately equal numbers of children were assigned to each of 12 possible orders |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Active medication and placebo were prepared by the hospital pharmacy, packaged in identical opaque gelatin capsules and administered in a double‐blind manner |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): 1‐day open trial to assess tolerance before main trial, but no children were excluded |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Vested interest bias | Low risk | Research was supported in part by the Ontario Mental Health Foundation and the National Health Research and Development Program, Health Canada |
| Methods | Double‐blind, randomised, placebo‐controlled, cross‐over trial with 2 arms
Each treatment period lasted for 3 weeks with a washout period of 1 week planned between treatments and up‐titration to optimum dosage during the 3 weeks | |
| Participants | Number of participants screened: 64 Number of participants included: 39. Of these, 26 had an ADHD diagnosis according to DSM‐III. Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 38 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (n = 26; type not stated) The following data reflect the whole trial (n = 38) Age: mean 8.6 years (range 6 to 10) IQ: all > 65; mean 93.4 Sex: all boys Methylphenidate naive: 100% Ethnicity: not stated Country: England Setting: out‐patient clinic Comorbidity: no attentive or restless behaviour, but antisocial, disruptive or aggressive in conduct (n = 6), hyperactive but not antisocial or aggressive (n = 9), with both hyperactive and disruptive behaviour (n = 23), conduct disorder (n = 7), relationship problems (n = 2) and disturbance of emotions specific to childhood (n = 3) Comedication: not stated Sociodemographics: 40% from broken homes Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Mean methylphenidate dosage: 9 received doses from 0.2 mg/kg to 0.5 mg/kg, 21 from 0.5 mg/kg to 0.9 mg/kg and 8 from 0.9 mg/kg to 1.4 mg/kg Administration schedule: time points not stated Duration of each medication condition: 3 weeks Washout before study initiation: All were stimulant‐naive Medication‐free period between interventions: 1 week Titration period: A flexible dosage regimen was used after randomisation. Each child began with 5 mg daily, with dosage adjustments made at 2‐ to 3‐day intervals. The optimum dosage was assessed for each child, in the light of clinical response and the occurrence of side effects, to a maximum of 30 mg daily Treatment compliance: Compliance with medication was rated as good or very good in 89% | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no; all methylphenidate treatment‐naive Comment from study authors (limitation)
Key conclusion of study authors
Comments from review authors
Email correspondence with trial authors: October 2013. We received from trial authors no supplemental information regarding data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Children were randomly allocated to receive drug first or placebo first. This was carried out by pharmacy staff, who knew only the name and identifying number of each case |
| Allocation concealment (selection bias) | Low risk | Allocation was carried out by pharmacy staff, who knew only the name and identifying number of each case |
| Blinding of participants and personnel (performance bias) | Low risk | Tablets were dispensed to a trial member, who did not know what they contained and handed them to parents |
| Blinding of outcome assessment (detection bias) | Low risk | Psychiatrist supervising treatment also assessed and recorded side effects, physical findings and parents’ general impressions at the end of each treatment; other assessors of outcomes were blinded to the treatment given but also to possible clues arising from physical effects of the drug. Behavioural measures were carried out independently of one another by investigators blind to results of the other tests and to teacher ratings |
| Incomplete outcome data (attrition bias) | Low risk | Data were missing for 3 children: One child had missing data on the Parental Account of Childhood Symptoms because he had been taken into care, and two children had missing data on the Conners’ Teacher Rating Scale hyperactivity factor because they had been excluded from school. Method of imputation not described |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | High risk | Partially funded by grant from CIBA Ltd., which provided medicine and placebo Conflicts of interest: Dr. Schachar was supported during this period by a fellowship from the Medical Research Council of Canada&& |
| Methods | Four‐week, double‐blind, cross‐over trial with 3 interventions
Each medication dose/placebo was given for 1 week | |
| Participants | Number of participants screened: 57 Number of participants included: 32 (27 boys, 5 girls) were randomly assigned to possible drug condition orders Number of participants followed up: 32 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R Age: mean 10.26 years (range 7.0 to 12.9) IQ: mean 107 (SD 16.2; range 78 to 139) Methylphenidate naive: 12 (37.5%) Ethnicity: no information Country: Canada Setting: out‐patient clinic Comorbidity: no information Comedication: no information Sociodemographics: no information Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to possible drug condition orders of, respectively, mean 6.72 mg (5 mg to 10 mg) and mean 11.88 mg (10 mg to 15 mg) methylphenidate and placebo Administration schedule: morning and noon Duration of each medication condition: 1 week Washout before study initiation: none Titration period: none Treatment compliance: no information | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: no information Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Key conclusion of study authors
Email correspondence with study authors: January 2014. No supplemental information was received. We therefore have no more information on numbers of participants with non‐serious adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. All medication and placebo testing was conducted under double‐blind conditions with randomly assigned testing order |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Active medication and placebo substances were placed in identical red and white capsules in powder form. Matched on both taste and appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | All medication and placebo testing was conducted under double‐blind conditions with randomly assigned testing order, but how blinding was done was not described |
| Incomplete outcome data (attrition bias) | High risk | Selection bias: This series of 32 children with ADHD does not include an additional group of 12 children with ADHD, who after drug trials were deemed "non‐responders" |
| Selective reporting (reporting bias) | High risk | Did not report adverse event data |
| Vested interest bias | Unclear risk | Not stated |
| Methods | Triple‐blind, 3‐way, cross‐over trial with 2 interventions
Phases
| |
| Participants | Number of participants screened: not stated Number of participants included: 63 (49 boys, 14 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 49 Number of withdrawals: 14 Diagnosis of ADHD: DSM‐IV (combined (70%), hyperactive‐impulsive (16%), inattentive (5%), other (9%)) Age: mean 9 years 10 months (SD 2 years 10 months; range not stated) IQ: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: motor dysfunction (35%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible dose levels of immediate‐release methylphenidate and placebo Mean methylphenidate dosage: not stated Administration schedule: twice daily; time points not stated Duration of each medication condition: 6 days Washout before study initiation: 1 day Titration period: not stated Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: no Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusions of study authors
Comments from review authors
Email correspondence with study authors: April 2013. We received from study authors the full dataset in SPSS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Low risk | Only the clinical pharmacist knew the sequence of phases |
| Blinding of participants and personnel (performance bias) | Low risk | Capsules of medication and placebo were made and dispensed as described by Barkley (1988). Tablets were crushed and placed within orange opaque gelatin capsules. Capsules disguised the taste of methylphenidate and lactose placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Triple‐blind |
| Incomplete outcome data (attrition bias) | High risk | 14 of the 63 trials were excluded from the analysis because of inadequately completed outcome measures. Four of the 14 children did not complete the trial because of adverse reactions to medication (e.g. irritability, headache, stomachache). These children received high‐dose medication in the first trial interval |
| Selective reporting (reporting bias) | Low risk | No protocol was published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | Unclear risk | No conflicts of interest have been disclosed |
| Methods | 16‐day, double‐blind, cross‐over, counterbalanced study with 2 interventions
| |
| Participants | Number of participants screened: not stated Number of participants included: 20 (16 boys, 4 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 20 Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (ADD 30%, ADHD 70%) Age: mean 9.3 years (range 7 to 12) IQ: mean 102 (SD 11) Methylphenidate naive: 100% Ethnicity: not stated Country: Israel Setting: out‐patient clinic Comorbidity: no Comedication: no Sociodemographics: middle (12), upper‐middle (5) and low (3) socioeconomic status of parents Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo Methylphenidate dose range: 0.3 mg/kg to 0.5 mg/kg Administration schedule: twice daily Duration of each medication condition: 8 days Washout before study initiation: no (participants were methylphenidate naive) Titration period: no Treatment compliance: Parents were asked to bring their packages of tablets back for pill count; no data | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation: no Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Funding: no funding Key conclusions of study authors
Email correspondence with study authors: August 2013. Study author stated that data were discarded (Ramstad 2013d [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Look‐alike placebo tablets were supplied by the hospital pharmacy and were similarly administered |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Unclear risk | No information |
| Methods | Double‐blind, controlled, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: unknown Number of participants included: 11 (8 boys, 3 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 10 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: median 9 years 8 months (range 6.9 to 12.3 years) IQ: median 106 (range 92 to 118) Methylphenidate naive: 100% Ethnicity: not stated Country: Israel Setting: out‐patient clinic Comorbidity: none Comedication: none Sociodemographics: low middle class (n = 2), middle class (n = 8) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of 0.3 mg/kg to 0.4 mg/kg methylphenidate hydrochloride (total dose 10 mg to 15 mg) and placebo Mean methylphenidate dosage: 0.3 mg/kg to 0.4 mg/kg Administration schedule: once daily at 7:30 AM on 6 of the 8 days. During the other 2 days, a second dose was administered at 2:00 PM Duration of each medication condition: 8 days Washout before study initiation: not stated Medication‐free period between interventions: 3 days Titration period: none Treatment compliance: As measured by returned package pill counts, this was rated as full compliance for the 10 children remaining in the study | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: December 2013. We were unable to receive supplemental data from study authors because the study is 20 years old (Ramstad 2013d [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A drug‐placebo sequence was used for children assigned odd numbers in the study and vice versa |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Look‐alike placebo tablets were supplied |
| Blinding of outcome assessment (detection bias) | Low risk | Investigator who analysed the data was unaware of the drug‐placebo sequence |
| Incomplete outcome data (attrition bias) | Unclear risk | No information Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Vested interest bias | Unclear risk | No information |
| Methods | Sixteen‐week, multi‐centre, randomised, double‐blind, placebo‐controlled, parallel trial to assess the efficacy of clonidine and methylphenidate for children and adolescents with ADHD and chronic tic disorder Weeks 1 to 4: clonidine/placebo dose titration; week 5 to 8: addition of methylphenidate/placebo dose titration; week 9 to 16: maintenance therapy | |
| Participants | Number of participants screened: not stated Number of participants included: 136 Number of participants randomly assigned: methylphenidate 37, placebo 32, clonidine 34, methylphenidate + clonidine 33 Number of participants followed up: methylphenidate 33, placebo 25, clonidine 30, methylphenidate + clonidine 33 Number of withdrawals: methylphenidate 4, placebo 7, clonidine 4, clonidine + methylphenidate 4 Diagnosis of ADHD: DSM‐IV: methylphenidate: combined 32%, hyperactive‐impulsive 3%, inattentive 65%; placebo: combined 19%, hyperactive‐impulsive 0%, inattentive 81%; clonidine: combined 21%, hyperactive‐impulsive 3%, inattentive 76%; clonidine + methylphenidate: combined 33%, hyperactive‐impulsive 3%, inattentive 64% Mean age: methylphenidate 10.7 years (SD 2.0); placebo 9.7 years (SD 1.8); clonidine 9.7 years (SD 1.8); clonidine + methylphenidate 10.6 years (SD 1.9). Total age range 7 to 14 Sex: methylphenidate 34 boys, 3 girls; placebo 29 boys, 3 girls; clonidine 29 boys, 5 girls; clonidine + methylphenidate 24 boys, 9 girls Stimulant‐naive: 42% Ethnicity: methylphenidate 81% Caucasian; placebo 94% Caucasian; clonidine 94% Caucasian; clonidine + methylphenidate 85% Caucasian Country: USA Setting: out‐patient clinic Comorbidity: tic disorder diagnosis 100%; primarily obsessive‐compulsive disorder (OCD) and oppositional defiant disorder (ODD). Furthermore, conduct disorder, generalised anxiety disorder and major depressive disorder. Methylphenidate: obsessive‐compulsive disorder (11%), oppositional defiant disorder (33%). Placebo: obsessive‐compulsive disorder (22%), oppositional defiant disorder (41%). Clonidine: obsessive‐compulsive disorder (15%), oppositional defiant disorder (48%). Clonidine + methylphenidate: obsessive‐compulsive disorder (16%), oppositional defiant disorder (31%) Comedication: not stated IQ: > 70 Sociodemographics: not stated. Participant groups were similar, except that participants assigned to methylphenidate (alone or in combination with clonidine) are approximately 1 year older, present a higher proportion of pubertal cases, show underrepresentation of the inattentive subtype of ADHD (and overrepresentation of the combined subtype) and have lower baseline Conners' Abbreviated Symptom Questionnaire (ASQ)‐Teacher scores. A higher proportion of girls was found in the combined treatment group Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate (Ritalin; Novartis) alone, clonidine alone, clonidine + methylphenidate or placebo Methylphenidate dosage: methylphenidate alone 25.7 mg/d, methylphenidate + clonidine 26.1 mg/d Administration schedule: 2 to 3 times daily Duration of intervention: 12 weeks (4‐week titration of methylphenidate, 8‐week maintenance phase) Titration period: 4‐week initial dose titration period for clonidine, after which came 4‐week dose titration for methylphenidate. Both titration periods took place after randomisation Treatment compliance: Pill count monitored compliance | |
| Outcomes | ADHD symptoms
General behaviour
Quality of life
Non‐serious adverse events
| |
| Notes | Sample calculation: yes; 120 participants Ethics approval: yes; the protocol was approved by the institutional review board at each site Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from study authors
Key conclusions of study authors
Comments from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. Stratification by centre (investigator) and sexual maturity status (prepubertal: Tanner stage I to II; pubertal: Tanner stage III to V) Blocking was used to ensure approximate balance among treatment groups within each stratum |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes that contained participants' treatment assignments |
| Blinding of participants and personnel (performance bias) | Low risk | Blinded: participants, clinicians, data collectors, outcome assessors, data analysts, data safety and monitoring committee, manuscript writers |
| Blinding of outcome assessment (detection bias) | Low risk | Only the programmer in the Biostatistics Center who generated the plan and the pharmacist in the Pharmacy Center who packaged and labeled the drug were aware of treatment assignments |
| Incomplete outcome data (attrition bias) | Low risk | Primary statistical analyses were performed according to the ITT principle and were based on all randomly assigned participants, as randomised. For analysis of outcome variables for efficacy, if a participant was missing a response at a particular visit, the last available observation for that participant was carried forward and imputed for that visit Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): no |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | Unclear risk | Vested interest: the National Institute of Neurological Disorders and Stroke, the General Clinical Research Center, the National Center for Research Resources, the Tourette Syndrome Association Boeringer Ingelheim Inc. (particularly Dr. Virgil Dias), for supplying clonidine and matching placebo; Bausch and Lomb, Inc., for supplying small gifts for our study participants Conflicts of interest: none declared |
| Methods | Multi‐centre, open‐label RCT including behaviour treatment as cointervention with 2 arms
| |
| Participants | Number of participants screened: 142 Number of participants included: 109 Number of participants followed up: 104 (66 boys, 38 girls) Number of withdrawals: 5 Diagnosis of ADHD: DSM‐IV (subtypes not described) Age: mean 8.4 years (range 6 to 12) IQ: not stated; all age‐appropriate cognitive functioning Methylphenidate naive: 100% Ethnicity: Caucasian (73.1%), African American (24.0%), other (2.9%) Country: USA Setting: out‐patient clinic Comorbidity: none Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to extended‐release methylphenidate plus behavioural treatment or to behavioural treatment alone Mean methylphenidate dosage: not stated Administration schedule: once daily Duration of intervention: 3 months Titration period: initiated after randomisation. Methylphenidate was started at 10 mg/d and could be increased weekly in intervals of 10 mg/d to a maximum of 60 mg/d. Methylphenidate was administered to achieve the desired clinical effect with minimum or no side effects Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes; approved by the ethics committees for all 17 study centres Key conclusion of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no; all participants were Methylphenidate naive Email correspondence with study authors: December 2013 and January 2014. Not able to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further description |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was stratified by age group (6 to 8 years and 9 to 12 years) and by centre |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Evaluation of cytogenetic damage by blinded slide readers |
| Incomplete outcome data (attrition bias) | High risk | Outcome data reported for 68 of 109 randomly assigned participants |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified |
| Vested interest bias | High risk | This work was funded by Novartis Pharmaceuticals Corporation Conflicts of interest: Some study authors were employed by Novartis (5 of 8 had a Novartis email address) |
| Methods | Eight‐week, cross‐over trial
Two phases
The trial was conducted over 3 years | |
| Participants | Number of participants screened: not stated Number of participants included: 86. Participants were randomly assigned to 3 doses of methylphenidate and placebo Number of participants followed up: 86 (67 boys, 19 girls) Number of withdrawals: 0 Age: mean 8.6 years (SD 1.8; range not stated) IQ: > 70 Methylphenidate naive: not stated Ethnicity: African American (17.4%), other (82.6%) Country: USA Setting: not stated Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different doses of methylphenidate (0.3 mg/kg, 0.5 mg/kg and 0.8 mg/kg) and placebo Administration schedule: once daily, before school Duration of each medication condition: 1 week Washout before study initiation: dose taken in the morning to the next day's dose Titration period: 4 weeks Treatment compliance: "compliance were probably high" | |
| Outcomes | ADHD symptoms
| |
| Notes | Sample calculation:no information Ethics approval:no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comment from study authors
Key conclusion of study authors
Email correspondence with study authors: not able to find first or second study author's contact information; therefore not able to request supplemental data necessary for meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment |
| Allocation concealment (selection bias) | High risk | No description; only "methylphenidate in opaque capsules" |
| Blinding of participants and personnel (performance bias) | High risk | No description |
| Blinding of outcome assessment (detection bias) | Low risk | Teacher rating under blinded conditions |
| Incomplete outcome data (attrition bias) | Low risk | Data were pooled for a total of 86 participants Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Unable to obtain protocol |
| Vested interest bias | Unclear risk | National Institutes of Mental Health (NIMH). Ciba‐Geigy provided medication and placebo Conflicts of interest: no information |
| Methods | Double‐blind, cross‐over trial in which participants were randomly assigned to the following conditions
| |
| Participants | Number of participants screened: not stated Number of participants included: 118. Participants were randomly assigned to 3 different doses of methylphenidate and placebo Number of participants followed up: 118 (92 boys, 26 girls) Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.6 years (range 6 to 14) IQ: > 70 Methylphenidate naive: not stated Ethnicity: Caucasian (81.4%), African American (18.6%) Country: USA Setting: school setting and out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 3 different doses of methylphenidate (0.3 mg/kg, 0.5 mg/kg and 0.8 mg/kg) and placebo Mean methylphenidate dosage: not stated Administration schedule: not stated Duration of each medication condition: 1 week Washout before study initiation: no information Titration period: none Treatment compliance: excellent compliance in all but a few children | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
Unable to obtain data on adverse events, as could not locate contact details of the first or second study author (see notes) | |
| Notes | Sample calculation: no Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Comment from study authors (on side effects)
Comment from review authors
Email correspondence with study authors: not able to find first or second study author's contact information; therefore not able to obtain additional data (e.g. data (table) on adverse effects) from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Daily doses of methylphenidate or placebo were placed in gelatin capsules to disguise taste and dose differences; medication for each week was packaged in a dated envelope |
| Blinding of participants and personnel (performance bias) | Low risk | Children, parents and teachers were blinded to dose order, as was the assistant who recorded data sent in by the teachers |
| Blinding of outcome assessment (detection bias) | Low risk | Children, parents and teachers were blinded to dose order, as was the assistant who recorded data sent in by the teachers |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Vested interest bias | Unclear risk | Supported in part by a National Institute of Mental Health (NIMH) grant. Ciba‐Geigy provided medication and placebo Conflicts of interest: not declared |
| Methods | Double‐blind, placebo‐controlled, within‐participant (cross‐over) trial looking at cardiovascular effects of methylphenidate (0.3 mg/kg, 0.6 mg/kg and 0.9 mg/kg) doses at baseline and after 60 and 120 minutes post methylphenidate challenge in 2 groups of participants with ADHD
| |
| Participants | Number of participants screened: not stated Number of participants included: 63 (58 boys, 5 girls) Number of participants followed up: 63 (ADHD without anxiety 34, ADHD with anxiety 29) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (but "82% of the ADHD and 93% of the ADHD/ANX group" would meet the DSM‐IV criteria for a diagnosis of ADHD combined type) Age: ADHD without anxiety, mean 9.1 years (SD 1.3; range 6 to 12); ADHD with anxiety, mean 8.7 years (SD 1.4) (overall range 6 to 12) IQ: not stated Methylphenidate naive: 63 (100%) Ethnicity: Caucasian (90%), "African or Asian descent" (10%) Country: Canada Setting: not stated Comorbidity: ADHD group: oppositional defiant disorder (14; 42%), conduct disorder (5; 15%); ADHD with anxiety group: oppositional defiant disorder (11; 38%), conduct disorder (8; 28%). Also, in the ADHD with anxiety group, 3 met the DSM‐III criteria for overanxious disorder, 2 met criteria for separation anxiety disorder and 5 met criteria for both overanxious and separation anxiety disorders) Sociodemographics: "The children tended to come from middle‐class families" Inclusion criteria ADHD with anxiety group
ADHD without anxiety group
Exclusion criteria ADHD without anxiety group
| |
| Interventions | Participants were randomly assigned to 1 of 4 possible drug condition orders of placebo and methylphenidate (0.3 mg/kg, 0.6 mg/kg or 0.9 mg/kg), given once daily over a 4‐day period. Dosage was based on a child’s body weight to the nearest 2.5 mg. Children followed the same routine for every test session with pre‐methylphenidate measurements and with measurements at 60 minutes and again at 120 minutes after taking methylphenidate Duration of each medication condition: once daily, administered over a 4‐day period ‐ each medication condition given once to each child Washout before study initiation: All were methylphenidate naive Medication‐free period between interventions: not stated Treatment compliance: "Any child with missing data for a particular measure was excluded from the analysis of that measure", but study authors did not indicate the overall quantity of missing data. "Medication was administered by a research nurse" | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: no information Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not stated Comment from study authors
Key conclusions of study authors
Comments from review authors
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The order of medications was counterbalanced and determined by random assignment such that an approximately equal number of children received each dose on a given day in the trial" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "All medication was prepared by the hospital pharmacy and packaged in opaque gelatin capsules, to avoid detection of dose and taste"; "Children, parents and research staff were blind to the medication conditions" |
| Blinding of outcome assessment (detection bias) | Low risk | "Medication was administered by a research nurse who was blind to children’s medication condition and classification of anxiety" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Vested interest bias | Low risk | This work was supported in part by funds from the Medical Research Council of Canada and the Research Institute of the Hospital for Sick Children Conflicts of interest: not declared |
| Methods | Seven‐week, randomised, double‐blind, placebo‐controlled, parallel‐group trial with 3 arms
| |
| Participants | Number of participants screened: not stated Number of participants included: 72 (62 boys, 10 girls) Number of participants randomly assigned: methylphenidate 24, placebo 24, clonidine 24 Number of participants followed up in each arm: methylphenidate 23, placebo 24 Number of withdrawals in each arm: methylphenidate 1, placebo 0 Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 8.8 years (range 7 to 12) IQ: > 70 Methylphenidate naive: 100% Ethnicity: mainly Caucasian Country: the Netherlands Setting: out‐patient clinic Comorbidity: conduct disorder (11%), oppositional defiant disorder (33%), depressive disorder (3%), overanxious disorder (1%), dysthymia (3%), generalized tonic‐clonic seizures (1%), ventricular septal defect (1%), congenital hypothyroidism (1%), precocious puberty (1%), deaf in right ear (1%), atresia in 1 ear (1%) Comedication: yes; for participants who had diseases that required it, for example, hypothyroidism Sociodemographics: 44% from lower socioeconomic families Differences between groups
Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate, placebo or clonidine Total oral daily methylphenidate dosage: 0.06 mg/kg Mean of the absolute methylphenidate dose: 9.85 mg (2.26) Administration schedule: breakfast and lunchtime Duration of intervention: 7 weeks Titration: no, but dosage adjustments were made/allowed during first weeks Titration period: none Treatment compliance: maintained and checked by instructions (both oral and written) to both parents and child, and by counting tablets remaining at the end of treatment. Only 1 participant in the methylphenidate group showed poor compliance | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: yes (≥ 15 participants in each group) Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Email correspondence with study authors: September 2013. We obtained supplemental information regarding a publication with information about randomisation and supplemental data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The statistician sent the randomisation list to the pharmacist. Participants were then randomly assigned by a research pharmacist. To ensure blinding, pharmacists applied randomisation blocks at random at a length of 2 or 4 participants. Methylphenidate and matching placebos were used |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Teacher, parent, clinician, child and experimenter were blind to treatment conditions |
| Blinding of outcome assessment (detection bias) | Low risk | Teacher, parent, clinician, child and experimenter were blind to treatment conditions |
| Incomplete outcome data (attrition bias) | Low risk | ITT Selection bias: no, but dosage adjustments were made for several participants because of annoying adverse events |
| Selective reporting (reporting bias) | Low risk | No |
| Vested interest bias | High risk | This study was supported by grants from the Sophia Foundation for Medical Research and Boehringer Ingelheim BV, the Netherlands |
| Methods | Double‐blind, single‐participant, randomised, cross‐over trial
Conducted in 11 hospitalised children with ADHD | |
| Participants | Number of participants screened: not stated Number of participants included: 11. Participants were randomly assigned to 1 of 2 conditions: methylphenidate and placebo Number followed up: 11 Sex: not reported Number of withdrawals: 0 Diagnosis of ADHD: DSM‐III‐R (subtype not described) Age: mean 9 years 5 months (range 4 to 13 years) IQ: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Setting: hospital/out‐patient clinic Comorbidity type: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to methylphenidate and placebo on a daily basis Mean methylphenidate dosage: not stated Administration schedule: daily Duration of each medication condition: methylphenidate 2 to 7 days, placebo 3 to 6 days Washout before study initiation: not described Medication‐free period between interventions: none Titration period: Optimal dose was based on maximal effectiveness and minimal side effects, but this appears to reflect daily response to methylphenidate Treatment compliance: not described | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Comment from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Comment from review authors
Email correspondence with study authors: We could find no contact information; therefore, we could not get additional data through personal email correspondence with study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was determined by "coin toss method" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | "After completion of each trial, the treatment code was broken", but it was not clear whether the psychiatry physician and the fellow who made the decision to cease methylphenidate were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | All data appear to have been reported, but methylphenidate was discontinued on the basis of results; therefore, data collection did not continue for some participants |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
| Vested interest bias | Unclear risk | Veterans Administration Medical Center, Vermont Conflicts of interest: not declared |
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 3 interventions
| |
| Participants | Number of participants screened: 28 (20 boys, 8 girls) Number of participants included: not clear. Participants were randomly assigned to 1 of possible drug condition orders Number of participants followed up: unclear Number of withdrawals: unclear Diagnosis of ADHD: DSM‐III (subtype not stated) Age: mean 8.4 years (range 6 to 12) IQ: mean 79.64 Methylphenidate naive: 28 (100%) Ethnicity: not stated Country: USA Setting: out‐patient clinic and in‐patient ward Comorbidity: not stated Comedication: not stated Sociodemographics: 3.52 (Hollingshead‐Redlich Index) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of methylphenidate (0.3 mg/kg and 0.6 mg/kg) and placebo Mean methylphenidate dosage: no information Administration schedule: twice a day, 8:30 AM and noon Duration of each medication condition: 12 days for in‐patients and 19 days for out‐patients (mean 15.25 days) Washout before study initiation: none Medication‐free period between interventions: 68 hours Titration period: none Treatment compliance: no information | |
| Outcomes | General behaviour
| |
| Notes | Sample calculation: none Ethics approval: no information Key conclusions of study authors
Comment from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Email correspondence with study authors: December 2013. No supplemental information available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Receiving interventions in counterbalanced orders |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Agreement of 90% across all categories had to be reached with this validity observer Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Protocol not identified |
| Vested interest bias | Low risk | Funded in part by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grants and the University of Southern California Faculty Research and Innovation Fund Conflicts of interest: not declared |
| Methods | Nine‐week therapeutic summer camp consisting of a cross‐over trial with 4 interventions
Varied daily within a cross‐over design of 3 intensities of behaviour modification therapy
Each lasted 3 weeks | |
| Participants | Number of participants screened: 106 participants in the 2003 and 2004 Summer Treatment Program (University of Buffalo) Number of participants included: 101. ADHD subgroup 33, ADHD + severe mood dysregulation (SMD) subgroup 68 Number of participants followed up: 99 Number of withdrawals: 2 Diagnosis of ADHD: DSM‐IV (combined (92%), hyperactive‐impulsive (not stated), inattentive (not stated)) Age: mean 8.5 years (range 5 to 12) IQ: mean105 Sex: 82 boys, 19 girls Methylphenidate naive: not stated Ethnicity: predominantly Caucasian Country: USA Setting: out‐patient clinic (Summer Treatment Program) Comorbidity: oppositional defiant disorder (54%), conduct disorder (12%) Comedication: not stated Sociodemographics: predominantly middle class Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants attended a Summer Treatment Program each Monday through Friday for 9 weeks. Participants were randomly assigned possible drug condition orders of 0.15 mg/kg t.i.d., 0.3 mg/kg t.i.d. and 0.6 mg/kg t.i.d. and placebo Average methylphenidate dosages: 5 mg, 10 mg and 18 mg for 0.15 mg/kg, 0.3 mg/kg and 0.6 mg/kg doses, respectively Administration schedule: 7:45 AM, 11:45 AM and 3:45 PM Duration of each medication condition: Each dose varied daily and was repeated 3 or 4 times within each behavioural treatment condition Study duration: Monday through Friday for 9 weeks, totalling 45 days Washout before study initiation: 1 week Medication‐free period between interventions: 0 to 2 days Titration period: none Treatment compliance: not stated Cointervention: Three behavioural conditions (no behaviour modification, low‐intensity behaviour modification and high‐intensity behaviour modification) are delivered in random order, with each condition lasting 3 weeks. Parents attended training sessions and implemented behaviour programmes at home | |
| Outcomes | Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes; by the Health Sciences Institutional Review Board (IRB) of the University of Buffalo Comment from study authors
Key conclusion of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking MPH before randomisation: yes; excluded patients with prior serious reactions to methylphenidate Email correspondence with study authors: August 2014. Obtained supplemental information regarding randomisation, allocation concealment and handling of missing data. Not possible to retrieve safety data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random orders were generated by computer with the restrictions that each condition occurred at least once in each week. Children were assigned to previously generated codes at enrolment |
| Allocation concealment (selection bias) | Low risk | Because this was a cross‐over study in which all children received all conditions multiple times, each child's entire 9‐week schedule was assigned at once. Treatment orders were concealed in an opaque envelope and were stored in a locked cabinet in the medication lab. Only authorised staff members had access to this cabinet |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind. Children, parents and staff were blinded to medication conditions. Placebo and methylphenidate were packaged in identical opaque capsules to maintain blinding |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind. Parents and staff were blinded to medication conditions |
| Incomplete outcome data (attrition bias) | Low risk | ITT Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
| Vested interest bias | High risk | This study was funded by National Institute of Mental Health (NIMH) Grant MH62946 and a Klingenstein Third Generation Foundation Fellowship in Child and Adolescent Depression Research Conflicts of interest: Several authors have affiliations with pharmaceutical companies |
| Methods | Two cross‐over trials with 3 interventions conducted 3 years apart
| |
| Participants | Number of participants screened: unknown Number of participants included: study one 24, study two 25. Participants were randomly assigned to 1 of the possible drug condition orders Number of participants followed up: study one 24 (22 boys, 2 girls), study two 25 (25 boys) Number of withdrawals: study one 0, study two 0 ADHD diagnosis: study 1 DSM‐III‐R, study 2 DSM‐III Age: study one mean 9 years 8 months (range 6.4 to 13.2 years), study two mean 9 years 1 month (range 6.4 to 12.5 years) IQ: no mental retardation (both studies) Methylphenidate naive: 0 % (both studies) Ethnicity: study one: Caucasian (92%), African American (4%), Hispanic (4%); study 2: Caucasian (72%), African American (12%), Asian (8%), Hispanic (8%) Country: USA Setting: out‐patient clinic (Summer Treatment Program) Comorbidity: study one not stated, study two no gross neurological dysfunction Comedication: not stated Sociodemographics: All were from middle‐ or low middle‐income backgrounds (both studies) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of the possible drug condition orders of 0.3 mg/kg (study one) or 0.6 mg/kg (study two) of methylphenidate and placebo. As regards study two, a single dose of 25 mg was set as the upper limit Mean methylphenidate dosage: 8.75 mg Administration schedule: twice a day, morning and lunchtime Duration of each medication condition: 1 week Washout before study initiation: unknown Medication‐free period between interventions: 20 hours Titration period: none Treatment compliance: good (2 staff dispensed the medication for ingestion) | |
| Outcomes | General behaviour
| |
| Notes | Sample calculation: no Ethics approval: no information Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; only participants taking maintenance dosage of methylphenidate or well titrated Comment from study authors
Key conclusions of study authors
Email correspondence with study authors: January 2014. No supplemental information has been received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study one: no information (unclear risk of bias) Study two: Two thirds of the boys received medication first, and the remaining third received placebo first. (This unequal split resulted from the boys’ simultaneous participation in a randomised 3‐week dose‐response assessment that was not pertinent to the present study) (high risk of bias) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | High risk | Both active medication and placebo were placed in opaque gelatin capsules. Two staff, blinded to medication status, dispensed medication (low risk of bias) Study 2: All staff were familiar with the boys, and some knew which boys had been diagnosed with ADHD, but none knew medication dosage or status (high risk of bias) |
| Blinding of outcome assessment (detection bias) | High risk | Study 2: All staff were familiar with the boys, and some knew which boys had been diagnosed with ADHD, but none knew medication dosage or status |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | None |
| Vested interest bias | Unclear risk | No information |
| Methods | Double‐blind, 2‐stage, cross‐over, pharmacokinetic and pharmacodynamic trial with 4 interventions
Phases: initial screening week, stage 1 cross‐over of Ritalin vs placebo, stage 2 cross‐over of treatment C and treatment D. Four weeks, with each study period lasting 1 week | |
| Participants | Number of participants screened: not stated Number of participants included: 27. Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 25 (21 boys, 4 girls) Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 10 ± 1.4 years (range 7 to 12) IQ: not stated Methylphenidate naive: not stated Ethnicity: Caucasian (88%), African American (8%), Asian (4%), Hispanic (0%), other (0%) Country: USA Setting: out‐patient clinic (laboratory and community) Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate and placebo in each of the 2 stages, 1 and 2, with each treatment period lasting 1 week Stage 1
Stage 2 Half of the participants in each treatment group were randomly assigned to 20 mg/d dosage of methylphenidate, the other half to 40 mg/d dosage
Mean methylphenidate dosage: 20 mg/d or 40 mg/d Administration schedule: once daily or twice daily Time points: mornings and after lunch Duration of each medication condition: 1 week Washout before study initiation: not stated Medication‐free period between interventions: 1 day (Sundays) Titration period: none Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: yes; study protocol and participant's informed consent form were approved by the Institutional Review Board (Office of the Vice Chancellor for Research, University of California at Irvine) Key conclusion of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; methylphenidate tolerability was an inclusion criterion. Serious adverse events were an exclusion criterion Email correspondence with study authors: July 2014. Emailed study authors twice for supplemental information but have received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Qualified participants were randomly assigned to a 2‐stage, double‐blind study sequence consisting of 4 study treatments, each lasting for a period of 1 week |
| Allocation concealment (selection bias) | Low risk | Ritalin (10 mg) tablets were placed into capsule shells by Eurand Americas Incorporated. Resulting capsules were tested according to US Pharmacopeial Convention (USP) dissolution conditions for methylphenidate tablets and showed a dissolution profile comparable with intact Ritalin tablets. All medications were supplied in white, opaque, size 3, hard gelatin capsule shells, and were packaged in blister cards to enhance compliance |
| Blinding of participants and personnel (performance bias) | Low risk | Ritalin (10 mg) tablets were placed into capsule shells by Eurand Americas Incorporated. Resulting capsules were tested according to US Pharmacopeial Convention (USP) dissolution conditions for methylphenidate tablets and showed a dissolution profile comparable with intact Ritalin tablets. All medications were supplied in white, opaque, size 3, hard gelatin capsule shells, and were packaged in blister cards to enhance compliance |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | High risk | Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): yes |
| Selective reporting (reporting bias) | Unclear risk | "Because the administration of the Side Effects Rating Form involved queries about specific adverse events, the frequency of adverse events reported in the parents’ and teachers’ Side Effect Rating Form was higher than that obtained from the reports elicited by general inquire (data not shown)" |
| Vested interest bias | High risk | Funding for this study was provided by Celltech Americas Incorporated Conflicts of interest: Some study authors are working for Celltech Americas Incorporated |
| Methods | Four‐week, randomised, double‐blind, placebo‐controlled, ITT, parallel trial conducted at 12 US centres with 3 arms
| |
| Participants | Number of participants screened: 174 Number of participants included: 132 (116 boys, 16 girls) Number of participants randomly assigned: dexmethylphenidate 44, dex,l‐methylphenidate 46, placebo 42 Number of participants followed up: dexmethylphenidate 42, dex,l‐methylphenidate 40, placebo 37 Number of withdrawals: dexmethylphenidate 2, dex,l‐methylphenidate 6, placebo 5 Diagnosis of ADHD: DSM‐IV (combined (64%), hyperactive‐impulsive (1%), inattentive (35%)) Age: mean 9.8 years (range 6 to 17) IQ: not stated. No mental retardation Methylphenidate naive: 95 (72%) Ethnicity: Caucasian (78%), African American (14%), other (8%) Country: USA Setting: out‐patient clinic Comorbidity: no Comedication: no Sociodemographics: not stated. No significant differences in baseline demographics were noted between the 2 groups Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to immediate‐release methylphenidate (dex‐ or d,l‐ racemats) or placebo Mean methylphenidate dosage: dexmethylphenidate 18.25 mg/d, dex,i‐methylphenidate 32.14 mg/d Administration schedule: twice daily in the morning (7:00 AM to 8:00 AM) and at noon (11:30 AM to 2:30 PM) Duration of intervention: 4 weeks Titration period: maximum 3 weeks initiated after randomisation, included in the 4 weeks of intervention Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes; approved by the institutional review board at each centre Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Comments from study authors
Key conclusions of study authors
Comment from review authors
Email correspondence with study authors: January 2014. Not possible to obtain data from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Drug (dexmethylphenidate and dex,l‐methylphenidate) and placebo were identical in appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | Sample size calculations were based on a clinically meaningful effect size of 0.75 in the change in score over 4 weeks on the teacher‐rated Swanson, Nolan and Pelham Scale between dexmethylphenidate and placebo groups. Efficacy parameters were performed on the ITT sample, which included participants who received medication, had a baseline efficacy evaluation and had ≥ 1 post‐baseline efficacy evaluation Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No protocol published All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | This study was supported by Celgene Corporation Conflicts of interest: Dr. Wigal reports extensive disclosure |
| Methods | Four‐ to six‐week, open‐label treatment (dose optimisation), cross‐over, 2‐week double‐blind trial with 2 interventions
Phases: 2 | |
| Participants | Number of participants screened: 45 (32 boys, 12 girls) Number of participants included: 44. Participants were randomly assigned to 1 of the possible drug condition orders Number of participants followed up: 39 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV (combined (70.5%), hyperactive‐impulsive (2.3%), inattentive (27.3%)) Age: mean 8.8 years (range 6 to 12) IQ: not stated Methylphenidate naive: 0 (0%) Ethnicity: White 35 (79.5%), Black/African American 4 (9.1%), Asian 3 (6.8%), other 2 (4.5%) Country: USA Setting: out‐patient clinic Comorbidity: elimination disorder (4; 9.1%), oppositional defiant disorder (8; 18.2%), specific phobias (2; 4.5%) Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different sequences of methylphenidate and placebo Mean methylphenidate dosage: 32.8 mg/d Administration schedule: q.i.d. Duration of each medication condition: 1 week Washout before study initiation: yes (1 day for stimulants) Medication‐free period between interventions: no Titration period: 3 weeks before randomisation Treatment compliance: 2 withdrawals of assent/consent, 2 adverse events, 1 lack of efficacy, 1 LTFU | |
| Outcomes | ADHD symptoms
Non‐serious adverse events 42 participants (93.3%) experienced a treatment‐emergent adverse event. Three (6.7%) participants experienced severe adverse effects (affect lability, aggression and initial insomnia), and 2 (4.4%) participants had to discontinue medication (affect lability and aggression)
| |
| Notes | Sample calculation: no Ethics approval: yes Comments from study authors
Key conclusion of study authors
Comments from review authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: not clear Any withdrawals due to adverse events: yes (n = 2) Email correspondence with study authors: emailed study authors to request additional information but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Results from open‐label phase |
| Blinding of outcome assessment (detection bias) | High risk | Results from open‐label phase |
| Incomplete outcome data (attrition bias) | High risk | Participants had to be in treatment with suboptimal efficacy (no inclusion of placebo responders) Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): Six participants were LTFU during the open‐label titration phase, 2 as a result of adverse events. These participants are not included in our analyses |
| Selective reporting (reporting bias) | Low risk | ADHD Rating Scale not reported (used only in the open phase) |
| Vested interest bias | High risk | Study received funds from NextWave Pharmaceutics (Belden and Berry are with NextWave) Conflicts of interest: All study authors are affiliated with NextWave Pharmaceuticals |
| Methods | Cross‐over trial with 2 interventions
Phases: 4
| |
| Participants | Number of participants screened: 32 Number of participants included: 26 (in open‐label phase). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 20 Number of withdrawals: 6 Diagnosis of ADHD: DSM‐IV‐TR (combined (n = 11), hyperactive‐impulsive (n = 3), inattentive (n = 12)) Age: mean 8.7 years (range 6 to 12) IQ: mean not stated (range 86 to 133) Sex: 12 boys, 10 girls Methylphenidate naive: none Ethnicity: White (82%), Black (9%), Asian (5%), Hispanic or Latino (23%), other (5%) Country: USA Setting: out‐patient clinic Comorbidity: 11; generalised anxiety disorder, enuresis, oppositional defiant disorder, chronic motor or vocal tic disorder, transient tic disorder Comedication: no Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate (15 mg, 20 mg, 30 mg or 40 mg) and placebo Mean methylphenidate dosage: 32 mg Administration schedule: once a day Time points: in the morning Duration of each medication condition: 1 week Washout before study initiation: 2 days Medication‐free period between interventions: not stated Titration period: yes; 2 to 4 weeks before randomisation Treatment compliance: yes; verified at scheduled study visits by study personnel who examined documentation of drug dispensed, drug consumed and remaining drug, and recorded the information on the drug reconciliation form Compliance was calculated to be > 82% throughout the study | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: yes Ethics approval: yes Comment from study authors
Key conclusions of study authors
Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; 2 withdrew during the open‐label phase as the result of lack of efficacy Any withdrawals due to adverse events: yes (n = 1) Email correspondence with study authors: April 2015. Obtained supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was determined by using a table of random numbers. Randomisation tables were provided to the randomisation monitor, who created unblinding envelopes and packaged the drug with blinded labels |
| Allocation concealment (selection bias) | High risk | One participant received placebo at 2 periods ‐ the method was not efficacious |
| Blinding of participants and personnel (performance bias) | Low risk | All sponsor representatives, investigators, participants and independent raters remained blinded until after data were locked |
| Blinding of outcome assessment (detection bias) | Low risk | Yes; this has been done |
| Incomplete outcome data (attrition bias) | Low risk | ITT were used Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo‐responders): no; no exclusion of methylphenidate non‐responders after randomisation |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias |
| Vested interest bias | High risk | Study was funded by Rhodes Pharmaceuticals L.P. Conflicts of interest: Several study authors work for, or have received grant and research support or both from pharmaceutical companies |
| Methods | Two‐week, randomised, double‐blind, 15‐centre, parallel trial with 2 arms
Phases: preceded by a 4‐week, open‐label, dose‐titration phase, and followed by an 8‐week, open‐label, follow‐up phase | |
| Participants | Number of participants screened: not stated Number of participants included: 220 in the 4‐week dose‐titration phase Number of participants randomly assigned: 177 (142 boys, 35 girls); methylphenidate 87, placebo 90 Number of withdrawals: methylphenidate 16, placebo 28 Number of participants followed up: 171; number completing follow‐up: 135 Diagnosis of ADHD: DSM‐IV (subtype not stated) Age: mean 14.6 years (range 13 to 18) IQ: not stated Methylphenidate naive: not stated ADHD treatment‐naive: 24 Ethnicity: Caucasian (75.1%), African American (13.6%), other (11.3%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated. The 2 groups were similar demographically, but the placebo group had a greater ratio of males (P value < 0.04) Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to OROS methylphenidate or placebo Mean methylphenidate dosage: 0.84 mg/kg Administration schedule: once daily Duration of intervention: 2 weeks Titration period: 4 weeks initiated before randomisation. All participants initiated therapy at 18 mg/d, and clinical response was measured after 1 week. If response to treatment was inadequate, as per the a priori study definition, the dose was titrated upward (in 18‐mg increments) at 1‐week intervals for up to 4 weeks, with maximum dose of 72 mg/d Treatment compliance: not stated; 8‐week, open‐label follow‐up on individualised dosage | |
| Outcomes | ADHD symptoms
Serious adverse events
Non‐serious adverse events
No participants experienced clinically important effects on ECG indexes, heart rate or blood pressure during the study | |
| Notes | Sample calculation: yes Ethics approval: yes; study was approved by the institutional review boards for all participating centres before the start of the study Inclusion of methylphenidate responders only/exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; criteria of response, defined as ≥ 30% improvement from baseline on the investigator‐scored ADHD Rating Scale. Participants who successfully completed the open‐label dose‐titration phase were assigned a randomisation number Comments from study authors
The short duration of the double‐blind phase may have decreased the likelihood of detecting potential rare adverse events. Rates of adverse events reported for OROS methylphenidate have been underestimated because participants entering this study phase were already stabilised on an affective tolerated dosage of medication Key conclusions of study authors
Email correspondence with study authors: December 2013 and Janurary 2014. Not able to make contact with study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but method was not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | Investigators were supplied with packages containing medication for each participant, as identified by randomisation number. Therefore, investigators and participants were blinded to whether a participant was receiving active medication or placebo |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators were supplied with packages containing medication for each participant, as identified by randomisation number. Therefore, investigators and participants were blinded to whether a participant was receiving active medication or placebo |
| Incomplete outcome data (attrition bias) | High risk | Non‐responders not included. LOCF technique was used for all assessments in the double‐blind phase. Of 182 (83%) participants who successfully achieved the criteria for improvement at the dose‐titration phase, only 177 were randomly assigned to the double‐blind phase, because 5 reached the criteria after the double‐blind phase was closed. Of 177 randomly assigned participants, 1 did not enter the double‐blind phase, and efficacy data were not collected for another participant. Therefore, 175 participants were included in the efficacy analysis of the double‐blind phase, but 177 were included in the dosage and safety analysis |
| Selective reporting (reporting bias) | Low risk | No major violation compared with what is reported in ClinicalTrials.gov All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | Funded by McNeil Consumer and Specialty Pharmaceuticals Conflicts of interest: Several study authors have had commitments (e.g. speakers, consultants, advisors) with various pharmaceutical companies |
| Methods | Open‐label, 5‐week, dose‐optimisation period. This dose was maintained during the subsequent 3 weeks of the trial, except for 1 day per week of 3‐way cross‐over assessment Randomised, double‐blind, 8‐centre, 3‐way, 3‐week, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: 148 Number of participants included: 128 in open‐label, dose‐titration; 120 randomly assigned to 1 of 3 possible drug orders Number followed up: 127 for safety and 117 for efficacy Number of withdrawals: 2 Characteristics of the 127 followed up for safety Sex: 84 boys, 42 girls Diagnosis of ADHD: DSM‐IV (combined or hyperactive‐impulsive (92.1%) inattentive (not stated)) Age: mean 8.8 years (SD 1.84; range 6 to 12) IQ: > 80 Methylphenidate naive: not stated Ethnicity: Caucasian (63.2%), African American (15.4%), Asian (not stated) Country: USA Comorbidity: not allowed Comedication: not stated Sociodemographics: not stated Characteristics of the 117 followed up for efficacy Sex: 75 boys, 42 girls Diagnosis of ADHD: DSM‐IV (type not stated) Age: mean 8.8 years (range 6 to 12) IQ: > 80 MPH‐naive: not stated Ethnicity: Caucasian (63.2%), African American (15.4%), Asian (0%), other (21.4%) Country: USA Setting: out‐patient clinic Comorbidity: not allowed Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
11 participants discontinued before the double‐blind randomisation phase, resulting in an ITT population of 117 participants Seven participants discontinued before the double‐blind randomisation phase, 3 were randomly assigned but did not undergo methylphenidate transdermal system (MTS) treatment Two participants did not complete the analogue classroom phase of the study: 1 because of an application site reaction, and 1 because of an adverse event (conjunctivitis), resulting in a total of 115 study completers | |
| Interventions | Participants were randomly assigned to 1 of 3 possible drug orders of methylphenidate (4 and 6 hours) and placebo Mean methylphenidate dosage: 10 mg patch (n = 15), 15 mg patch (n = 34), 20 mg patch (n = 32) and 30 mg patch (n = 36) Administration schedule: once daily in the morning; patch worn for 9 hours daily, and for 4 or 6 hours for cross‐over assessments Duration of each medication condition: 1 day Washout before study initiation: none; in‐between treatment with optimal dose of methylphenidate Titration period: 5 weeks before randomisation Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Quality of life
Non‐serious adverse events
No clinically meaningful changes from baseline were observed in vital signs, ECG, urinalysis and haematological results or physical examinations. Adverse events were recorded from the time informed consent was signed until 30 days (week 12) after the last drug treatment | |
| Notes | Sample calculation: yes; assuming a mean difference in Swanson, Kotkit, Agler, M‐Flynn and Pelham deportment score of 2.0 between active treatment and placebo, an SD of 5.0, a between correlation of 0.2, 90% power and a probability level of .05 (2‐sided), it was estimated that approximately 102 participants were needed to complete the double‐blind, cross‐over phase of the study Ethics approval: yes; institutional review board at each site approved the study Comments from study authors
Key conclusion of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; participants with a history of failing to respond to psychostimulant treatment were also excluded Any withdrawals due to adverse events: yes; 1 (conjunctivitis) Email correspondence with study authors in April to June 2014. Emailed study authors twice to ask for additional data but never received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Single randomisation schedule prepared by an independent statistician using computer software that generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | Each participant wore 2 patches prepared by an unblinded pharmacist to maintain treatment blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | ITT population (more than 1 application of a study drug and pre‐dose efficacy assessment at week 6). Participants who did not reach an optimal dose by week 5 were discontinued from the study Selection bias (e.g. titration after randomisation → exclusion of methylphenidate non‐responders or placebo responders): yes; participants who did not reach an optimal dose by week 5 were discontinued from the study |
| Selective reporting (reporting bias) | Unclear risk | Protocol published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | This study was funded by Shire Development Incorporated Conflicts of interest: Several study authors have affiliations with medical companies |
| Methods | Blind, randomised, 4‐week, cross‐over trial with 2 interventions
| |
| Participants | Number of participants screened: unknown Number of participants included: 36. Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 30 (25 boys, 5 girls) Number of withdrawals: 4 Diagnosis of ADHD: DSM‐IV (combined (53%), hyperactive‐impulsive (3%), inattentive (43%)) Age: mean 9.17 years (SD 1.84; range 6 to 12) IQ: > 70 Methylphenidate naive: 14 (47%) Ethnicity: Caucasian (90%), African American (not stated), Asian (3%), Hispanic (not stated), other (7%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional disorder (70%), conduct disorder (7%), major depressive disorder (3%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate (20 mg) and placebo Mean methylphenidate dosage: not stated Administration schedule: patch applied in morning and worn for 9 hours Duration of each medication condition: 2 weeks Washout before study initiation: none Titration period: 1 week after randomisation Treatment compliance: > 80% for all participants | |
| Outcomes | ADHD symptoms
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; participants with a history of no response or intolerability to methylphenidate were excluded for ethical reasons Comment from study authors
Key conclusion of study authors
Email correspondence with study authors: March/April 2014. We contacted the first study author twice to ask for supplemental data but have not received a response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned |
| Allocation concealment (selection bias) | Low risk | Prescriptions filled by hospital pharmacy |
| Blinding of participants and personnel (performance bias) | Unclear risk | No additional information from study author |
| Blinding of outcome assessment (detection bias) | Unclear risk | Double‐blind (participant, caregiver, investigator) |
| Incomplete outcome data (attrition bias) | High risk | ITT: participants who completed 1 week of treatment; LOCF Selection bias: yes. Excluded participants not following 1 week of treatment |
| Selective reporting (reporting bias) | Low risk | Protocol published. All pre‐specified outcomes of interest have been reported |
| Vested interest bias | High risk | This study and medication/placebo were funded by a grant through Shire Pharmaceuticals. Shire had no role in design, collection, analysis, interpretation, writing or decision to submit Conflicts of interest: Some study authors have received research support from medical companies |
| Methods | Double‐blind, placebo‐controlled, cross‐over trial with 2 interventions
Phases: 2 | |
| Participants | Number of participants screened: not stated Number of participants included: 16. Participants were randomly assigned to 1 possible drug condition order Number of participants followed up: 16 implied (16 boys, 0 girls) Number of withdrawals: not stated Diagnosis of ADHD: DSM‐III‐R (subtype not stated) Age: mean 10.2 years (range 8 to 13) IQ: mean 112.6 Methylphenidate naive: 0% Ethnicity: not stated Country: USA Setting: out‐patient clinic Comorbidity: no Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate (normal dose) and placebo Mean methylphenidate dosage: 0.030 mg/kg (range 0.08 to 1.10 mg/kg/d) Administration schedule: not stated Duration of each medication condition: 36 hours Washout before study initiation: not stated Titration period: not stated Treatment compliance: not stated | |
| Outcomes | General behaviour
| |
| Notes | Sample calculation: not described Ethics approval: not described Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no Key conclusion of study authors
Comment from review authors
Email correspondence with study authors: May 2014. We obtained supplemental information from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From correspondence: generated by pharmacist (Magnusson 2014c [pers comm]) |
| Allocation concealment (selection bias) | Low risk | From correspondence: Recruiter had no involvement in and was blinded to allocation sequence (Magnusson 2014c [pers comm]) |
| Blinding of participants and personnel (performance bias) | Low risk | From correspondence: Pharmacist instructed parents (via instructions on pill bottle) regarding which pills should be administered and at what time (Magnusson 2014c [pers comm]) |
| Blinding of outcome assessment (detection bias) | Low risk | See above |
| Incomplete outcome data (attrition bias) | Low risk | From correspondence: Approximately 2% of skin conductance and heart rate data were affected by participant movement and were replaced with interpolated values Physiological data were lost for 2 participants with ADHD and for 2 participants without ADHD. For 3 of the remaining 28 participants, 1 of the 4 repeated measurements of interbeat intervals was replaced with the mean of the participant's diagnostic group because his ECG recordings were inadequate (Magnusson 2014c [pers comm]) Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | No reporting bias |
| Vested interest bias | Low risk | Research was supported by University of Utah Biomedical Sciences Research Grant and a grant from the University Research Committee Conflicts of interest: no corporate affiliations described |
| Methods | Three‐week cross‐over trial with 3 interventions
Phases
| |
| Participants | Number of participants screened: 123 ("treated in the clinic") Number of participants included: 57 (47 boys, 10 girls) Number of participants followed up: 57 Number of withdrawals: 66. Away on summer vacation (n = 30), teacher failed to complete all rating forms (n = 29), teacher marked 2 values indicated on a single dimension (n = 3), adverse medication side effect (n = 2), dropped out before child’s medication trial was completed (n = 2) Diagnosis of ADHD: DSM‐III‐R Age: mean 8.5 years (SD 2.1; range 6 to 14) IQ: not stated Methylphenidate naive: not stated Ethnicity: Caucasian "Anglo" 54 (95%), Hispanic 3 (5%) Country: USA Setting: out‐patient clinic Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders (5 mg methylphenidate or 15 mg methylphenidate) and placebo, given at 8:00 AM and 12:00 PM Mean methylphenidate dosage: 10 mg/d, 30 mg/d and placebo Administration schedule: 3‐week, triple‐blind, cross‐over medication trial consisting of placebo, 5 mg of methylphenidate and 15 mg of methylphenidate, twice a day. Each drug condition lasted 7 days (Thursday through Wednesday) Duration of each medication condition: 3 weeks Washout before study initiation: approximately 20 hours (lunchtime until "immediately before school" the next day) Titration period: not stated Treatment compliance: not stated Number of withdrawals: 2. Parents dropped out of the study before their child’s medication trial was completed | |
| Outcomes | ADHD symptoms
General behaviour
Serious adverse events
Non‐serious adverse events
| |
| Notes | Sample calculation: not stated Ethics approval: not stated Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes Key conclusions of study authors
Email correspondence with study authors: June 2014. We obtained supplemental information from study authors. We contacted study author to ask for missing data, but s/he was not able to supply the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects then underwent a three‐week, triple‐blinded, cross‐over medication trial consisting of placebo, 5 mg, and 15 mg of MPH dose twice a day (immediately before school and at lunchtime)" (p 83); "Order of administration was counterbalanced so that equivalent numbers of children received each sequence" (p 83) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | "Subjects then underwent a three‐week, triple‐blinded, cross‐over medication trial consisting of placebo, 5 mg, and 15 mg of MPH dose twice a day (immediately before school and at lunchtime). Each drug condition lasted seven days (Thursday through Wednesday)" (p 83); "Doses were prepared and packaged for the three week trial by a licensed pharmacist who split MPH tablets and placed them with lactose into re‐sealable capsules. The placebo dose consisted of lactose‐filled capsules only. Equivalent numbers of capsules (three) were dispensed at each dosing time to maintain uniformity and disguise presence/amount of medication, as three capsules were required to encompass the largest dose (15 mg)" (p 83) |
| Blinding of outcome assessment (detection bias) | High risk | "This procedure was followed for three weeks, with the medication code being broken the final week and determination about medication efficacy and decision to continue addressed at that time" (p 84) |
| Incomplete outcome data (attrition bias) | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Vested interest bias | Unclear risk | No information |
| Methods | Twenty‐eight‐day, randomised, parallel, double‐blind clinical trial with 3 arms
| |
| Participants | Number of participants screened: 405 Number of participants included: 312; OROS methylphenidate 94, immediate‐release methylphenidate 94, placebo 89 Number of participants followed up: OROS methylphenidate 79, immediate‐release methylphenidate 81, placebo 46 Number of withdrawals: OROS methylphenidate 15, immediate‐release methylphenidate 13, placebo 43 Diagnosis of ADHD: DSM‐IV (combined (73.4%), hyperactive‐impulsive (7.1%), inattentive (19.5%)) Age: mean 9.0 years (range 6 to 12) IQ: > 70 Sex: 233 boys, 49 girls Methylphenidate naive: 20.2% Ethnicity: Caucasian (84.4%), African American (7.4%), Asian (0.4%), Hispanic (3.5%), other (4.3%) Country: USA Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (41.8%), conduct disorder (11.3%), tic disorder (5.3%), anxiety disorder (1.4%), depression (0.7%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
During the course of the study, participants were allowed to receive behavioural interventions as long as the interventions had been initiated before the start of the study and did not change during the study. New behavioural therapy was not allowed during the course of the study | |
| Interventions | Average total daily dose: immediate‐release methylphenidate 29.5 mg per day (0.90.4 mg/kg/d), OROS methylphenidate 34.3 mg per day (1.1 to 0.5 mg/kg/d) Administration schedule: 3 times a day Duration of intervention: 4 weeks Titration period: 4‐week titration period before randomisation (open‐label) for study participants who had not received methylphenidate for ADHD from their own practitioner in the 4 weeks before study entry. Participants who had taken methylphenidate during the 4 weeks before study entry were assigned to a dose level based on their pre‐study therapeutic dose and regimen Treatment compliance: not stated | |
| Outcomes | ADHD symptoms
Quality of life
| |
| Notes | Sample size calculation: yes Ethics approval: yes Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: yes; 111 who had not received methylphenidate before the study initially were enrolled into a dose‐titration study. One of these did not enrol in the randomised study because the 54‐mg dose was found to be ineffective Key conclusion of study authors
Comments from review authors in July 2013:corresponded with first study author, Wolraich, who answered all of our questions (Krogh 2013c [pers comm]) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Within each dose level, participants were randomly assigned equally to OROS methylphenidate qd, immediate‐release methylphenidate (overencapsulated Ritalin) t.i.d. or placebo in a 3‐group parallel design. Stratified randomisation was conducted centrally at ALZA Corporation |
| Allocation concealment (selection bias) | Low risk | Double‐dummy |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind; double‐dummy |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind; double‐dummy |
| Incomplete outcome data (attrition bias) | Low risk | LOCF Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Unclear risk | Not able to get study protocol |
| Vested interest bias | High risk | Funded by ALZA Corporation (medical company) Conflicts of interest: Study authors are part of the Concerta Study Group |
| Methods | Cross‐over trial with 2 interventions
Phases: 7 weeks (3 weeks of medication, 1 week washout, 3 weeks of placebo) Zeiner 1995 (in Zeiner 1999): extended treatment (mean duration 634 ± 130 days) | |
| Participants | Number of participants screened: 46 Number of participants included: 38. Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 36 (36 boys, 0 girls) Number of withdrawals: 2 Diagnosis of ADHD: DSM‐III‐R or DSM‐IV (combined type (> 75%), hyperactive‐impulsive and inattentive types not stated) Age: mean 8,7 years (range 7 to 11) IQ: mean 102 (range 79 to 139) Methylphenidate naive: 100% Ethnicity: not stated Country: Norway Setting: out‐patient clinic Comorbidity: oppositional defiant disorder (23; 64%) Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to 1 of 2 possible drug condition orders of methylphenidate (0.5 mg/kg) and placebo. Thirty‐one children received a morning dose of 10 mg or 15 mg (and 5 young boys received 7.5 mg) Mean methylphenidate dosage: not stated; 0.55 mg/kg to 0.56 mg/kg daily across the entire duration of the extended treatment period (Zeiner 1995 in Zeiner 1999) Administration schedule: Capsules were given in 2 doses (at 8 AM and 11.30 AM) Duration of each medication condition: 3 weeks Washout before study initiation: not relevant Methylphenidate naive: all Medication‐free period between interventions: 1 week Titration period: none Treatment compliance: no information | |
| Outcomes | ADHD symptoms
Assessments of the child were made during the last week of each trial period Non‐serious adverse events
| |
| Notes | Sample calculation: no Comment from study authors
Key conclusions of study authors
Comments from review authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: no | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Children were randomly allocated to receive methylphenidate first or placebo first |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) | Low risk | "A randomized crossover, double‐blind design with methylphenidate and placebo". Placebo and methylphenidate capsules that were used were identical, to ensure blindness to the drug condition |
| Blinding of outcome assessment (detection bias) | Low risk | "All raters were blind to the drug condition" |
| Incomplete outcome data (attrition bias) | High risk | "The report from a teacher during one of the periods was missing for one child. Owing to technical problems or unwillingness to participate data were missing on some of the test measures [which measured side effects] for a few children" (Zeiner 1995 in Zeiner 1999) Selection bias: yes; only responders from the 7‐week trial were included in the extended‐treatment methylphenidate group |
| Selective reporting (reporting bias) | Low risk | No selective outcome reporting |
| Vested interest bias | Low risk | This research was supported by the Norwegian Medical Research Council, the Norwegian Public Health Association and the Legacy of Haldis and Josef Andresen Conflicts of interest: not declared |
| Methods | Randomised, cross‐over trial with 2 interventions
Phases: 2; randomisation, cross‐over | |
| Participants | Number of participants screened: 30 Number of participants included: 16 (9 boys, 7 girls). Participants were randomly assigned to 1 of 2 possible drug condition orders Number of participants followed up: 14 Number of withdrawals: 1 Diagnosis of ADHD: DSM‐IV (inattentive (7.1%), hyperactive (14.3%), combined (78.6%), out of n = 14) Age: mean 10.71 years (SD 1.86; range 8 to 17) IQ: > 70 Methylphenidate‐naive: not stated Ethnicity: European‐Brazilian (71.4%), other (28.6%) Country: Brazil Setting: out‐patient clinic Co‐morbidity: bipolar disorder (71.4%), borderline personality disorder (28.6%), anxiety disorder (57.1%), conduct disorder (57.1%), oppositional defiant disorder (78.6%), psychosis (50%), out of n = 14 Co‐medication: aripiprazole (100%) Sociodemographics: divorced parents (57.1%); socioeconomic level A + B + C: 92.9%; D + E: 7.1% Inclusion criteria
Exclusion criteria
| |
| Interventions | Participants were randomly assigned to different possible drug condition orders of methylphenidate (0.3 mg/kg for the first week, 0.7 mg/kg for the second week) and placebo, both alongside aripiprazole Mean methylphenidate dosage: 15 mg in the first week (10 mg in the morning and 5 mg in the afternoon), 35 mg in the second week (20 mg in the morning and 15 mg in the afternoon) Administration schedule: b.i.d. (morning and afternoon) Duration of each medication condition: 2 weeks Washout before study initiation: none (but recruited from 6‐week aripiprazole study) Medication‐free period between interventions: not stated Titration period: none Treatment compliance: self report, mother’s report, pill counting in returned blister packs | |
| Outcomes | ADHD symptoms
General behaviour
Non‐serious adverse events
| |
| Notes | Sample calculation: no Ethics approval: yes Comments from study authors
Key conclusions of study authors
Exclusion of methylphenidate non‐responders/children who have previously experienced adverse events while taking methylphenidate before randomisation: Only participants who reported improved borderline personality disorder in the previous aripiprazole trial were included. However, participants who reported that ADHD improved too much (as indicated by a score < 1.5 on the Swanson, Nolan, and Pelham Scale, Fourth Edition) in the previous aripiprazole trial were excluded (see 'Exclusion criteria') Any withdrawals due to adverse events: 1; exacerbation of borderline personality disorder and ADHD requiring hospitalisation Email correspondence with study authors: May 2014. We obtained supplemental information regarding data. Study authors provided us with data from the first period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent third party randomly assigned participants to groups A and B |
| Allocation concealment (selection bias) | Low risk | A pharmacist packaged methylphenidate and matching placebo in capsules, so they could not be differentiated by shape, colour, smell, weight or taste |
| Blinding of participants and personnel (performance bias) | Low risk | A pharmacist packaged methylphenidate and matching placebo in capsules, so they could not be differentiated by shape, colour, smell, weight or taste |
| Blinding of outcome assessment (detection bias) | Low risk | After all 4 assessments were completed, study blinding was broken |
| Incomplete outcome data (attrition bias) | Low risk | Analyses of primary and secondary outcome measures were performed using a mixed‐effects model (MEM) approach, which provides a flexible framework for analysis of repeated measures, while accounting for missing data (i.e. lost to follow‐up) Selection bias (e.g. titration after randomisation → exclusion): no |
| Selective reporting (reporting bias) | Low risk | Outcomes reported according to protocol |
| Vested interest bias | Unclear risk | This work was fully supported by research grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, Brazil) (Grant 471761=03‐6) and Hospital de Clinicas de Porto Alegre (GPPG 03‐325). Aripiprazole was provided by Bristol‐Myers Squibb without restriction Conflicts of interest: stated, "this is an independent investigator trial". However some study authors have affiliations with medical companies |
ADD: attention deficit disorder.
ADD‐H: attention deficit disorder with hyperactivity.
ADHD: attention deficit hyperactivity disorder.
b.i.d.: twice a day.
CNS: central nervous system.
DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised.
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.
DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision.
ECG: electrocardiogram.
EEG: electroencephalogram.
EKG: electrocardiogram.
FDA: Food and Drug Administration.
ITT: intention‐to‐treat.
LLC: limited liability company.
LOCF: last observation carried forward.
LTFU: loss to follow‐up.
MedDRA: Medical Dictionary for Regulatory Activities.
MPH: methylphenidate.
MPT: methylphenidate trial.
MTA: Multimodal Treatment Study of Children With ADHD.
MTS: methylphenidate transdermal system.
NNTB: number needed to treat for an additional beneficial outcome.
OROS: Osmotic Release Oral System.
PTS: placebo transdermal system.
q.a.m.: every morning.
qd: once a day.
RCT: randomised controlled trial.
SD: standard deviation.
SODAS: spheroidal oral drug absorption system.
t.i.d.: three times a day.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| N = No information Likely no relevant outcome for our review Outcomes reported: Resting state brain function | |
| N = 10 boys Likely no relevant outcome for our review Outcomes reported: Functional magnetic resonance relaxometry | |
| N = 28 Likely no relevant outcome for our review Outcomes reported: Home Situations Questionnaire; Parenting Stress Index; Beck Depression Inventory | |
| N = No information Likely no relevant outcome for our review Outcomes reported: "2 questionnaires"; "Electronic apparatus" | |
| N = 24 Likely no relevant outcome for our review Outcomes reported: Movement Assessment Battery for Children – Second edition; Online Continuous Performance Test | |
| N = 31 Likely no relevant outcome for our review Outcomes reported: Response interference; Stroop Naming Speed | |
| N = 59 Likely no relevant outcome for our review Outcomes reported: Parent Interview for Child Symptoms; Teacher Telephone Interview–IV; Selective Stop‐Signal Task | |
| N = 26 Likely no relevant outcome for our review Outcomes reported: Parent Interview for Child Symptoms; Teacher Telephone Interview–IV; Reading subtest/Wide Range Achievement Test ‐ 3; Word Attack and Word Identification subtests of the Woodcock Reading Mastery; Test‐Revised Cambridge Neuropsychological Testing Automated Battery | |
| N = 40 Likely no relevant outcome for our review Outcomes reported: Working memory; Test of Word and Language Efficiency; Wide Range Achievement Test ‐ 3; Woodcock‐Johnson Tests of Achievement; Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Span | |
| N = 70 Likely no relevant outcome for our review Outcomes reported: Behavioural management, behavioural disinhibition | |
| N = 55 Likely no relevant outcome for our review Outcomes reported: Hastening phenomena | |
| N = 25 Likely no relevant outcome for our review Outcomes reported: Reading measures | |
| N = 20 Likely no relevant outcome for our review Outcomes reported: Children's Checking Task | |
| N = 19 Likely no relevant outcome for our review Outcomes reported: Prosocial behavior | |
| N = 30 Likely no relevant outcome for our review Outcomes reported: Reactions time on Tachistoscopic Task | |
| N = 13 Likely no relevant outcome for our review Outcomes reported: Reaction times; cognitive tasks | |
| N = 24 Likely no relevant outcome for our review Outcomes reported: On task and disruptive behaviour; academic work completion and accuracy | |
| N = 6 Likely no relevant outcome for our review Outcomes reported: Driving performance ‐ measured by computer | |
| N = 13 Likely no relevant outcome for our review Outcomes reported: Mirsky's proposed factors of attention: sustained attention, focus/execute, encode, and stability of attention | |
| N = 17 Likely no relevant outcome for our review Outcomes reported: Specific attention function: sustained attention, information processing, response organization | |
| N = 21 Likely no relevant outcome for our review Outcomes reported: Cambridge Gamble Task; measures of response inhibition and reflection‐impulsivity on the Information Sampling Task | |
| N = No information Likely no relevant outcome for our review Outcomes reported: Verbal memory and learning | |
| N = 14 Likely no relevant outcome for our review Outcomes reported: Memory tasks | |
| N = 50 Likely no relevant outcome for our review Outcomes reported: Story telling and story grammar analysis | |
| N = 20 Likely no relevant outcome for our review Outcomes reported: Performance on paired‐associate learning task | |
| N = 26 Likely no relevant outcome for our review Outcomes reported: Social behaviour | |
| N = 371 Likely no relevant outcome for our review Outcomes reported: Academic behaviour; sustained attention; impulse inhibition control | |
| N = 54 Likely no relevant outcome for our review Outcomes reported: Sustained attention measured by computer attention tests | |
| N = No information Likely no relevant outcome for our review Outcomes reported: Event‐related potentials | |
| N = 45 Likely no relevant outcome for our review Outcomes reported: Computerized attention tasks | |
| N = 19 Likely no relevant outcome for our review Outcomes reported: Paired Associates Learning Task; Tower of Hanoi | |
| N = 25 Likely no relevant outcome for our review Outcomes reported: Prosocial behavior | |
| N = 22 Likely no relevant outcome for our review Outcomes reported: Antisocial behaviour | |
| N = 24 Likely no relevant outcome for our review Outcomes reported: Maze‐tracking performance | |
| N = 75 Likely no relevant outcome for our review Outcomes reported: Social information processing | |
| N = 32 Likely no relevant outcome for our review Outcomes reported: Laboratory provocation task: measuring hostile, instrumental, reactive, and proactive aggression | |
| N = 58 Likely no relevant outcome for our review Outcomes reported: Reaction time; alertness; vigilance; divided attention | |
| N = 16 Likely no relevant outcome for our review Outcomes reported: Gait; stride to stride variability, memory, visual‐spatial, verbal, and attention domains | |
| N = 31 Likely no relevant outcome for our review Outcomes reported: Word processing; reaction time; cognitive decision task | |
| N = 26 Likely no relevant outcome for our review Outcomes reported: Impulsive responding | |
| N = 17 Likely no relevant outcome for our review Outcomes reported: Right hemisphere dysfunction | |
| N = 24 Likely no relevant outcome for our review Outcomes reported: Depression; addiction rate; Continuous Performance Task; heart rate and blood pressure | |
| N = 14 Likely no relevant outcome for our review Outcomes reported: Working memory | |
| N = 26 Likely no relevant outcome for our review Outcomes reported: Continuous Performance Task | |
| N = 21 Likely no relevant outcome for our review Outcomes reported: Task persistence | |
| N = 16 Likely no relevant outcome for our review Outcomes reported: Reaction time; visuospatial attention | |
| N = 23 Likely no relevant outcome for our review Outcomes reported: Non‐verbal learning | |
| N = 9 Likely no relevant outcome for our review Outcomes reported: Verbal information processing | |
| N = 29 Likely no relevant outcome for our review Outcomes reported: Classroom academic and social behaviour | |
| N = 17 Likely no relevant outcome for our review Outcomes reported: Attention during baseball game; on task behaviour; ability to answer question about the status of the game | |
| N = 28 Likely no relevant outcome for our review Outcomes reported: Self‐reported attribution and evaluation of behaviour | |
| N = 60 Likely no relevant outcome for our review Outcomes reported: Performance, self‐evaluation, persistence, and attributions on cognitive task | |
| N = 68 Likely no relevant outcome for our review Outcomes reported: A large number of different measures of behaviour | |
| N = 45 Likely no relevant outcome for our review Outcomes reported: Paired Associates Learning Task | |
| N = 42 Likely no relevant outcome for our review Outcomes reported: Reading achievement | |
| N = 13 Likely no relevant outcome for our review Outcomes reported: Motor timing | |
| N = 58 Likely no relevant outcome for our review Outcomes reported: Auditory amplitude | |
| N = 188 Likely no relevant outcome for our review Outcomes reported: Task oriented behaviour | |
| N = No information Likely no relevant outcome for our review Outcomes reported: Neural substrates | |
| N = 20 Likely no relevant outcome for our review Outcomes reported: Functional magnetic resonance imaging | |
| N = 12 Likely no relevant outcome for our review Outcomes reported: Attention during play measured by locomotor activity; Children's Checking Test; and fine motor control | |
| N = 22 Likely no relevant outcome for our review Outcomes reported: Continuous Performance Test | |
| N = 9 Likely no relevant outcome for our review Outcomes reported: Sustained attention measured by computer attention tests | |
| N = 17 Likely no relevant outcome for our review Outcomes reported: Working memory | |
| N = 25 Likely no relevant outcome for our review Outcomes reported: Motor functions | |
| N = 26 Likely no relevant outcome for our review Outcomes reported: Impulsive responding | |
| N = 36 Likely no relevant outcome for our review Outcomes reported: Cerebral blood flow | |
| N = 47 Likely no relevant outcome for our review Outcomes reported: Naming speed and academic measures | |
| N = 19 Likely no relevant outcome for our review Outcomes reported: Computerized Contiuous Performance Test and functional magnetic resonance imaging | |
| N = 14 Likely no relevant outcome for our review Outcomes reported: Rate‐dependent behavioural effects | |
| N = 11 Likely no relevant outcome for our review Outcomes reported: McLean Motion Attention Test | |
| N = 32 Likely no relevant outcome for our review Outcomes reported: Auditory performance | |
| N = 44 Likely no relevant outcome for our review Outcomes reported: Go‐No‐GO performance | |
| N = 134 Likely no relevant outcome for our review Outcomes reported: Cognitive tasks; emotional functions | |
| N = 58 Likely no relevant outcome for our review Outcomes reported: Reaction time tasks | |
| N = 12 Likely no relevant outcome for our review Outcomes reported: Continuous Performance Test | |
| N = 61 Likely no relevant outcome for our review Outcomes reported: Academic oriented tasks | |
| N = 24 Likely no relevant outcome for our review Outcomes reported: Social behaviour |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT 14 days Studied all aspects of methylphenidate, amphetaminil, mesocarb and placebo in a group of 118 children |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article is written in Czech. We have not yet been able to have the article translated |
| Methods | The purpose of this trial was to examine the efficacy of psychostimulant medication in a naturalistic sample of pre‐school children. Researchers examined the benefits and side effects of methylphenidate and mixed amphetamine salts (Adderall) |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | 16‐week, placebo‐controlled RCT with methylphenidate in 100 medication‐naive participants with ADHD |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | This article describes methods and design. We are awaiting additional publications |
| Trial name or title | |
| Methods | Placebo‐controlled trial of methylphenidate involving 30 medication‐naive children with ADHD |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Correspondence with trial authors in August 2013. Trial will be submitted for review over the next few months |
| Trial name or title | Clinical and pharmacogenetic study of attention deficit hyperactivity disorder (ADHD) |
| Methods | RCT |
| Participants | Children with ADHD, aged 6 to 12 years |
| Interventions | Ritalin and placebo |
| Outcomes | Conners' Global Index (CGI) |
| Starting date | |
| Contact information | Ridha Jobber, McGill University |
| Notes |
| Trial name or title | Examining tolerance to CNS stimulants in ADHD |
| Methods | RCT |
| Participants | Children with ADHD, aged 6 to 12 years |
| Interventions | Methylphenidate |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Pharmacogenetics of growth effects complicating ADHD treatment (Neuropsychopharmacology) |
| Methods | 8‐week RCT: double‐blind, randomised, placebo‐controlled trial with 4 interventions and 16‐month extension
Phases: 8‐week, double‐blind; 16 months open |
| Participants | Number of participants screened: not stated Number of participants included: 209 (sex not stated). Participants were randomly assigned to 1 of 4 possible drug condition orders Number of participants followed up: 99 Number of withdrawals: not stated Diagnosis of ADHD: not stated Age: not stated IQ: not stated Methylphenidate naive: not stated Ethnicity: not stated Country: USA Comorbidity: not stated Comedication: not stated Sociodemographics: not stated Inclusion criteria
Exclusion criteria
|
| Interventions | Participants were randomly assigned to 1 of 3 possible drug condition orders of dexmethylphenidate and placebo Administration schedule: not stated Duration of each medication condition: not stated Washout before trial initiation: not stated Medication‐free period between interventions: not stated Titration period: not stated Treatment compliance: not stated |
| Outcomes | Outcomes: adverse events Both dexmethylphenidate monotherapy (Z‐score decrease of ‐0.12 for height and ‐0.84 for BMI) and combination treatment (Z‐score decrease of ‐0.19 for height and ‐0.83 for BMI) were associated with slowing of growth. In the methylphenidate‐only condition, DRD3 rs3732790 minor allele homozygotes showed a 2.0 greater Z‐score weight loss than was seen with common allele carriers (P value = 1.09 × 10–8) |
| Starting date | 2012 (date on which conference abstract published). Study end date unknown |
| Contact information | Erika L Nurmi: [email protected] James T McCracken: [email protected]. Department of Psychiatry and Biobehavioral Sciences, UCLA NPI‐Semel Institute, 760 Westwood Plaza, Los Angeles, CA 90024‐1759, USA |
| Notes | Sample calculation: no Ethics approval: not stated Results of this trial have not yet been published. Trial authors expect to publish their findings within the year |
ADHD: Attention deficit hyperactivity disorder.
BMI: Body mass index.
CNS: Central nervous system.
RCT: randomised controlled trial.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| Analysis 1.1  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 1 All parallel‐group trials and first‐period cross‐over trials. | ||||
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 2 Subgroup analysis: types of scales Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2 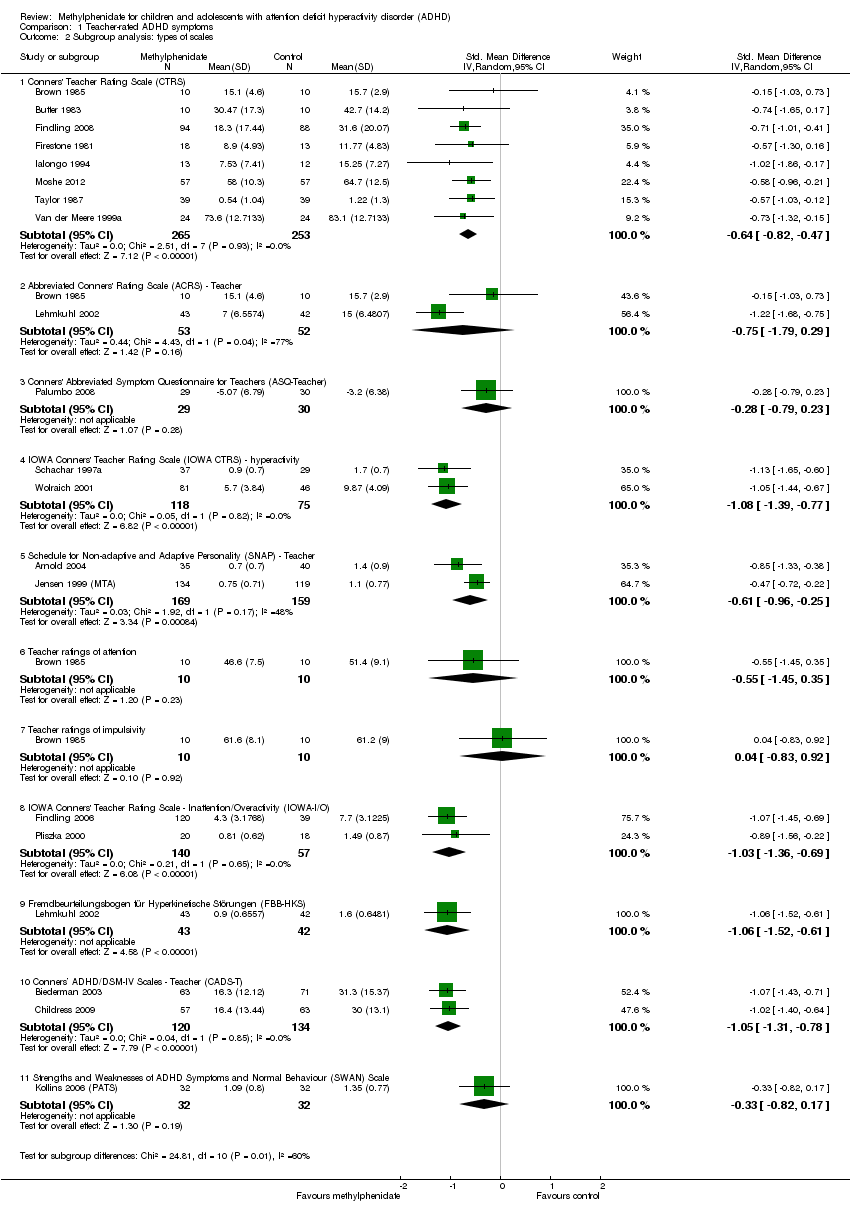 Comparison 1 Teacher‐rated ADHD symptoms, Outcome 2 Subgroup analysis: types of scales. | ||||
| 2.1 Conners' Teacher Rating Scale (CTRS) | 8 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.82, ‐0.47] |
| 2.2 Abbreviated Conners' Rating Scale (ACRS) ‐ Teacher | 2 | 105 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.79, 0.29] |
| 2.3 Conners' Abbreviated Symptom Questionnaire for Teachers (ASQ‐Teacher) | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.79, 0.23] |
| 2.4 IOWA Conners' Teacher Rating Scale (IOWA CTRS) ‐ hyperactivity | 2 | 193 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.39, ‐0.77] |
| 2.5 Schedule for Non‐adaptive and Adaptive Personality (SNAP) ‐ Teacher | 2 | 328 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.25] |
| 2.6 Teacher ratings of attention | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.45, 0.35] |
| 2.7 Teacher ratings of impulsivity | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.83, 0.92] |
| 2.8 IOWA Conners' Teacher Rating Scale ‐ Inattention/Overactivity (IOWA‐I/O) | 2 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.36, ‐0.69] |
| 2.9 Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.52, ‐0.61] |
| 2.10 Conners’ ADHD/DSM‐IV Scales ‐ Teacher (CADS‐T) | 2 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.31, ‐0.78] |
| 2.11 Strengths and Weaknesses of ADHD Symptoms and Normal Behaviour (SWAN) Scale | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.82, 0.17] |
| 3 Medication status: medication naive versus not medication naive Show forest plot | 6 | 717 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.08, ‐0.50] |
| Analysis 1.3 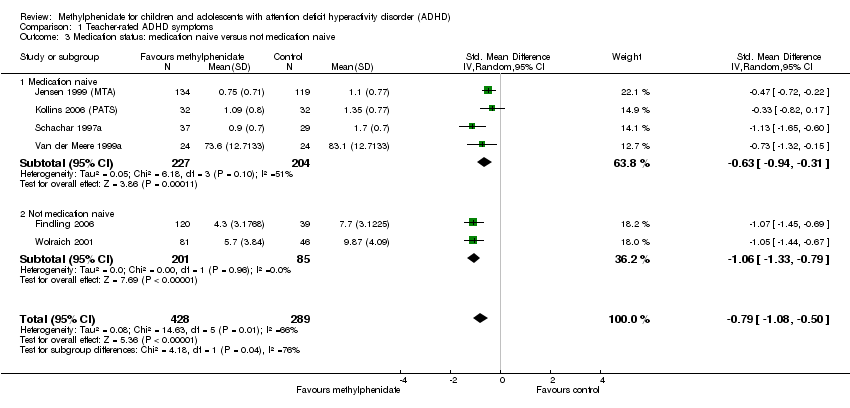 Comparison 1 Teacher‐rated ADHD symptoms, Outcome 3 Medication status: medication naive versus not medication naive. | ||||
| 3.1 Medication naive | 4 | 431 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.94, ‐0.31] |
| 3.2 Not medication naive | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.33, ‐0.79] |
| 4 Subgroup analysis: duration of treatment Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| Analysis 1.4  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 4 Subgroup analysis: duration of treatment. | ||||
| 4.1 Short term (up to 6 months) | 18 | 1445 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.94, ‐0.68] |
| 4.2 Long term (over 6 months) | 1 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.72, ‐0.22] |
| 5 Subgroup analysis: dose Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 5 Subgroup analysis: dose. | ||||
| 5.1 Low dose | 8 | 493 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.82, ‐0.46] |
| 5.2 High dose | 7 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.08, ‐0.54] |
| 5.3 Unknown dose | 6 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.06, ‐0.68] |
| 6 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| Analysis 1.6  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 6 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials. | ||||
| 6.1 Parallel‐group trials | 17 | 1506 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.95, ‐0.65] |
| 6.2 First‐period cross‐over trials | 2 | 192 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.87, ‐0.29] |
| 7 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| Analysis 1.7  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 7 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants. | ||||
| 7.1 Trials with cohort selection bias of all participants | 7 | 994 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.01, ‐0.57] |
| 7.2 Trials without cohort selection bias of all participants | 12 | 704 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.93, ‐0.59] |
| 8 ADHD symptoms, cross‐over trial (endpoint data) Show forest plot | 59 | 5145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.06, ‐0.80] |
| Analysis 1.8  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 8 ADHD symptoms, cross‐over trial (endpoint data). | ||||
| 8.1 Low risk of bias | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.97, ‐0.30] |
| 8.2 High risk of bias | 55 | 4941 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.09, ‐0.82] |
| 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose Show forest plot | 59 | 6821 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐0.96, ‐0.74] |
| Analysis 1.9  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose. | ||||
| 9.1 Low dose | 42 | 3408 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐0.89, ‐0.57] |
| 9.2 High dose | 36 | 3413 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.13, ‐0.84] |
| 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) Show forest plot | 75 | 6344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.01, ‐0.80] |
| Analysis 1.10  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data). | ||||
| 10.1 All parallel‐group trials and first‐period cross‐over trials | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 10.2 Cross‐over trials (endpoint data) | 56 | 4646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.09, ‐0.82] |
| 11 All parallel‐group trials and cross‐over trials: risk of bias Show forest plot | 75 | 6344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.01, ‐0.80] |
| Analysis 1.11  Comparison 1 Teacher‐rated ADHD symptoms, Outcome 11 All parallel‐group trials and cross‐over trials: risk of bias. | ||||
| 11.1 Low risk of bias | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.97, ‐0.30] |
| 11.2 High risk of bias | 71 | 6140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.03, ‐0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| Analysis 2.1 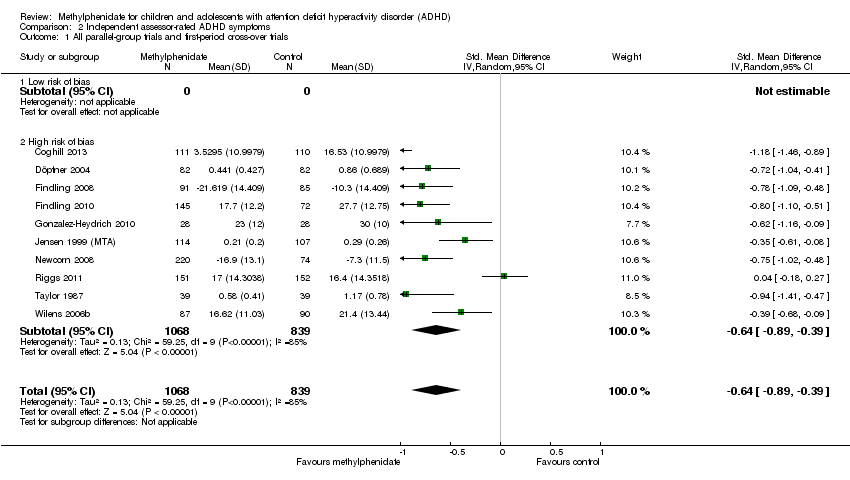 Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 1 All parallel‐group trials and first‐period cross‐over trials. | ||||
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 2 Subgroup analysis: types of scales Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 2 Subgroup analysis: types of scales. | ||||
| 2.1 Swanson, Kotkin, Agler, M‐Glynn and Pelham (SKAMP) Scale | 1 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.04, ‐0.41] |
| 2.2 ADHD Rating Scale (ADHD‐RS ) | 7 | 1442 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.09, ‐0.32] |
| 2.3 Swanson, Nolan and Pelham (SNAP) Scale | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.61, ‐0.08] |
| 2.4 Unknown | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.41, ‐0.47] |
| 3 Subgroup analysis: duration of treatment Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| Analysis 2.3  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 3 Subgroup analysis: duration of treatment. | ||||
| 3.1 Short term (up to 6 months) | 9 | 1686 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.95, ‐0.40] |
| 3.2 Long term (over 6 months) | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.61, ‐0.08] |
| 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| Analysis 2.4  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials. | ||||
| 4.1 Parallel‐group trials | 7 | 1609 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.91, ‐0.28] |
| 4.2 First‐period cross‐over trials | 3 | 298 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.99, ‐0.52] |
| 5 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| Analysis 2.5 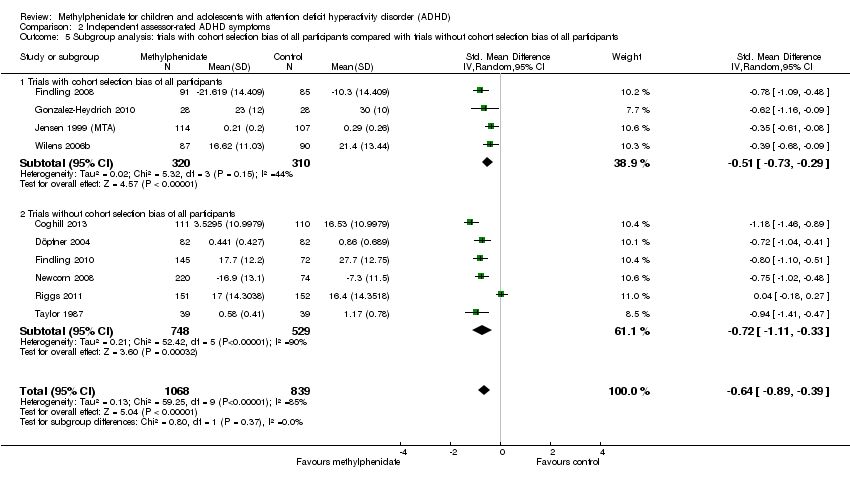 Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 5 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants. | ||||
| 5.1 Trials with cohort selection bias of all participants | 4 | 630 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.73, ‐0.29] |
| 5.2 Trials without cohort selection bias of all participants | 6 | 1277 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.11, ‐0.33] |
| 6 Subgroup analysis: dose Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| Analysis 2.6  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 6 Subgroup analysis: dose. | ||||
| 6.1 Low dose | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High dose | 6 | 1380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.91, ‐0.20] |
| 6.3 Unknown dose | 4 | 527 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.98, ‐0.61] |
| 7 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: risk of bias Show forest plot | 19 | 2471 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.16, ‐0.84] |
| Analysis 2.7  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 7 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: risk of bias. | ||||
| 7.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 High risk of bias | 19 | 2471 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.16, ‐0.84] |
| 8 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose Show forest plot | 19 | 3874 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.02, ‐0.75] |
| Analysis 2.8  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 8 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose. | ||||
| 8.1 Low dose | 16 | 2021 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.82, ‐0.55] |
| 8.2 High dose | 12 | 1853 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.31, ‐0.95] |
| 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) Show forest plot | 28 | 4215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐0.98, ‐0.67] |
| Analysis 2.9  Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data). | ||||
| 9.1 All parallel‐group trials and first‐period cross‐over trials | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 9.2 Cross‐over trials (endpoint data) | 18 | 2308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.13, ‐0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| Analysis 3.1 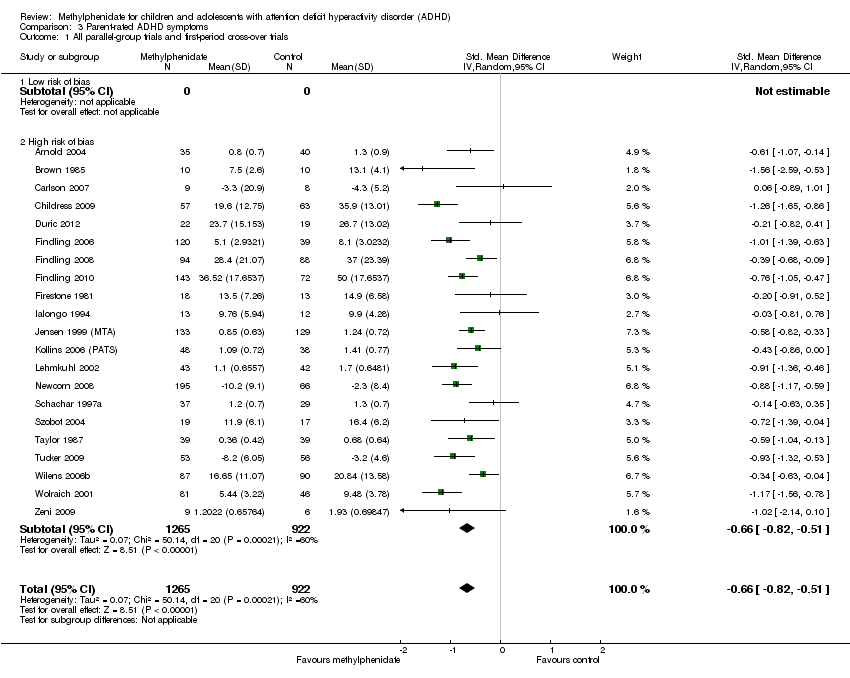 Comparison 3 Parent‐rated ADHD symptoms, Outcome 1 All parallel‐group trials and first‐period cross‐over trials. | ||||
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 2 Subgroup analysis: types of scales Show forest plot | 21 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.2 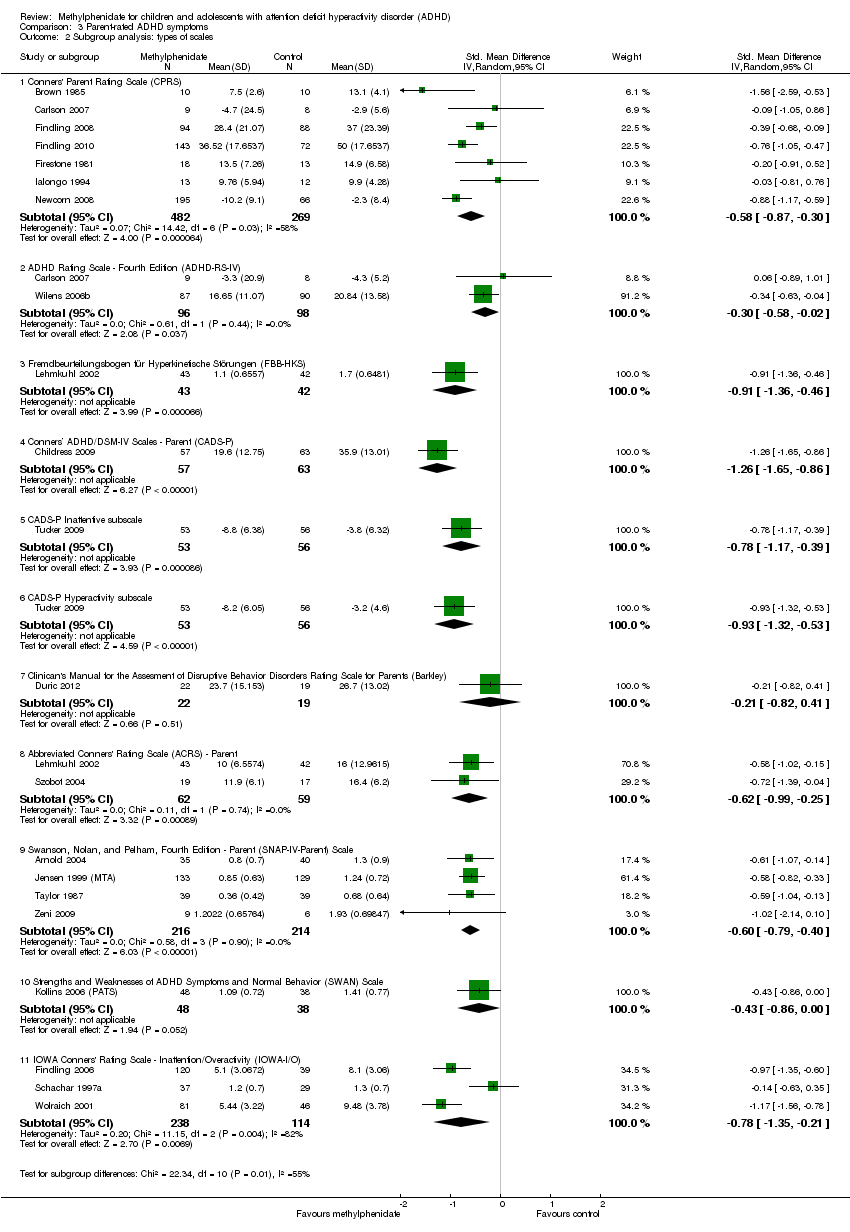 Comparison 3 Parent‐rated ADHD symptoms, Outcome 2 Subgroup analysis: types of scales. | ||||
| 2.1 Conners' Parent Rating Scale (CPRS) | 7 | 751 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.87, ‐0.30] |
| 2.2 ADHD Rating Scale ‐ Fourth Edition (ADHD‐RS‐IV) | 2 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.58, ‐0.02] |
| 2.3 Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.36, ‐0.46] |
| 2.4 Conners’ ADHD/DSM‐IV Scales ‐ Parent (CADS‐P) | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.65, ‐0.86] |
| 2.5 CADS‐P Inattentive subscale | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.17, ‐0.39] |
| 2.6 CADS‐P Hyperactivity subscale | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.32, ‐0.53] |
| 2.7 Clinican's Manual for the Assesment of Disruptive Behavior Disorders Rating Scale for Parents (Barkley) | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.82, 0.41] |
| 2.8 Abbreviated Conners' Rating Scale (ACRS) ‐ Parent | 2 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.99, ‐0.25] |
| 2.9 Swanson, Nolan, and Pelham, Fourth Edition ‐ Parent (SNAP‐IV‐Parent) Scale | 4 | 430 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.79, ‐0.40] |
| 2.10 Strengths and Weaknesses of ADHD Symptoms and Normal Behavior (SWAN) Scale | 1 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.86, 0.00] |
| 2.11 IOWA Conners' Rating Scale ‐ Inattention/Overactivity (IOWA‐I/O) | 3 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.35, ‐0.21] |
| 3 Subgroup analysis: duration of treatment Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| Analysis 3.3 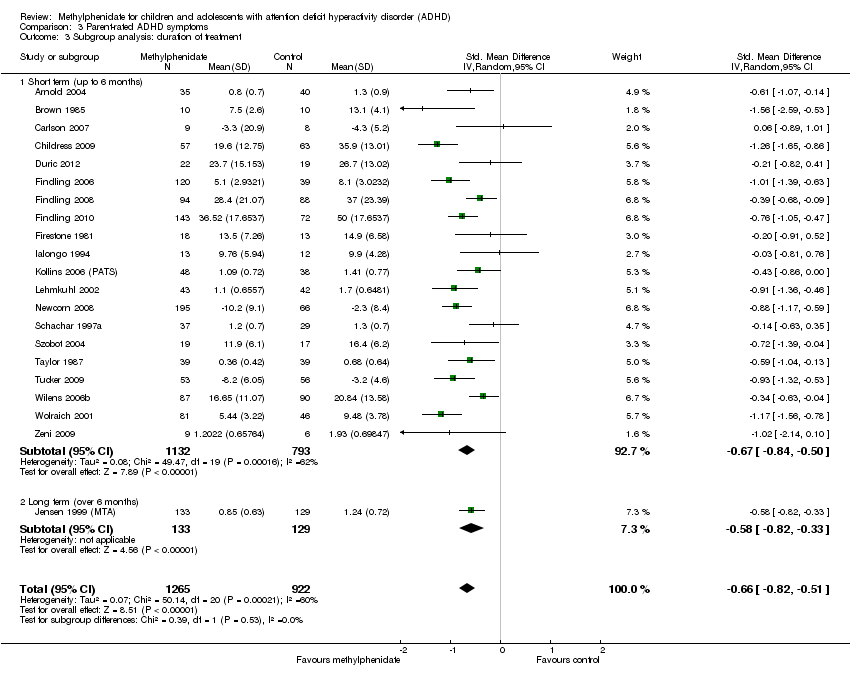 Comparison 3 Parent‐rated ADHD symptoms, Outcome 3 Subgroup analysis: duration of treatment. | ||||
| 3.1 Short term (up to 6 months) | 20 | 1925 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.84, ‐0.50] |
| 3.2 Long term (over 6 months) | 1 | 262 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.82, ‐0.33] |
| 4 Subgroup analysis: dose Show forest plot | 21 | 2335 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.79, ‐0.48] |
| Analysis 3.4  Comparison 3 Parent‐rated ADHD symptoms, Outcome 4 Subgroup analysis: dose. | ||||
| 4.1 Low dose | 5 | 329 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [1.00, ‐0.07] |
| 4.2 High dose | 10 | 1132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.86, ‐0.33] |
| 4.3 Unknown dose | 8 | 874 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.92, ‐0.52] |
| 5 Medication status: medication naive versus not medication naive Show forest plot | 7 | 795 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.05, ‐0.48] |
| Analysis 3.5  Comparison 3 Parent‐rated ADHD symptoms, Outcome 5 Medication status: medication naive versus not medication naive. | ||||
| 5.1 Medication naive | 4 | 492 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.03, ‐0.35] |
| 5.2 Not medication naive | 3 | 303 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.33, ‐0.42] |
| 6 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| Analysis 3.6  Comparison 3 Parent‐rated ADHD symptoms, Outcome 6 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants. | ||||
| 6.1 Trials with selection bias of all participants | 10 | 1559 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.86, ‐0.51] |
| 6.2 Trials without cohort selection bias of all participants | 11 | 628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.90, ‐0.33] |
| 7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| Analysis 3.7  Comparison 3 Parent‐rated ADHD symptoms, Outcome 7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials. | ||||
| 7.1 Parallel‐group trials | 19 | 2094 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.83, ‐0.50] |
| 7.2 First‐period cross‐over trials | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.07, ‐0.23] |
| 8 ADHD symptoms, cross‐over trials (endpoint data) Show forest plot | 41 | 3734 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.90, ‐0.67] |
| Analysis 3.8  Comparison 3 Parent‐rated ADHD symptoms, Outcome 8 ADHD symptoms, cross‐over trials (endpoint data). | ||||
| 8.1 Low risk of bias | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.96, ‐0.13] |
| 8.2 High risk of bias | 37 | 3530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.92, ‐0.69] |
| 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose Show forest plot | 41 | 4918 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.79, ‐0.58] |
| Analysis 3.9 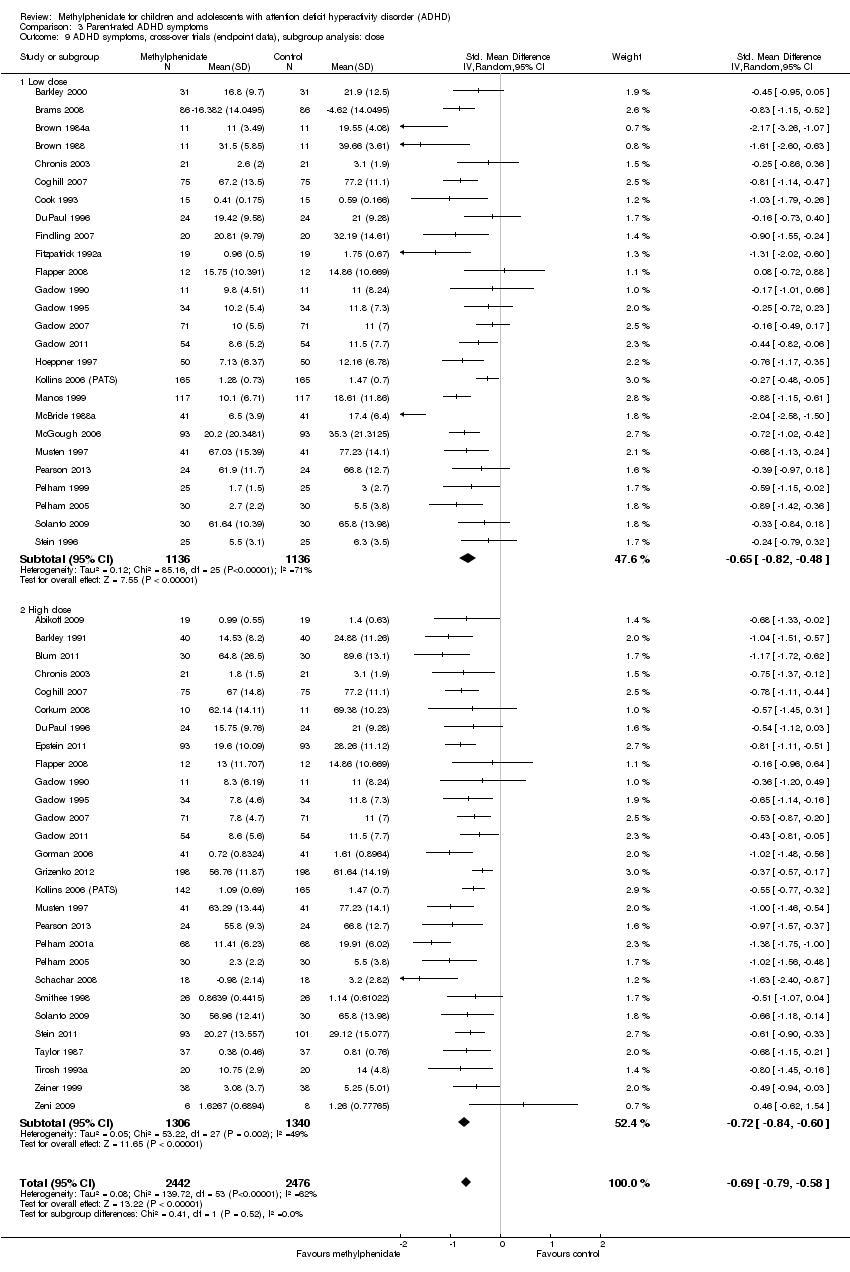 Comparison 3 Parent‐rated ADHD symptoms, Outcome 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose. | ||||
| 9.1 Low dose | 26 | 2272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.82, ‐0.48] |
| 9.2 High dose | 28 | 2646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.84, ‐0.60] |
| 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) Show forest plot | 59 | 5861 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.83, ‐0.65] |
| Analysis 3.10  Comparison 3 Parent‐rated ADHD symptoms, Outcome 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data). | ||||
| 10.1 All parallel‐group trials and first‐period cross‐over trials | 21 | 2215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 10.2 Cross‐over trials (endpoint data) | 39 | 3646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐0.90, ‐0.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparision of raters Show forest plot | 31 | 5697 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.79, ‐0.59] |
| Analysis 4.1  Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Comparision of raters. | ||||
| 1.1 Teacher‐rated | 19 | 1689 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.93, ‐0.63] |
| 1.2 Independent assessor‐rated | 9 | 1829 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.87, ‐0.35] |
| 1.3 Parent‐rated | 21 | 2179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.81, ‐0.50] |
| 2 Age Show forest plot | 6 | 1039 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.74, ‐0.14] |
| Analysis 4.2 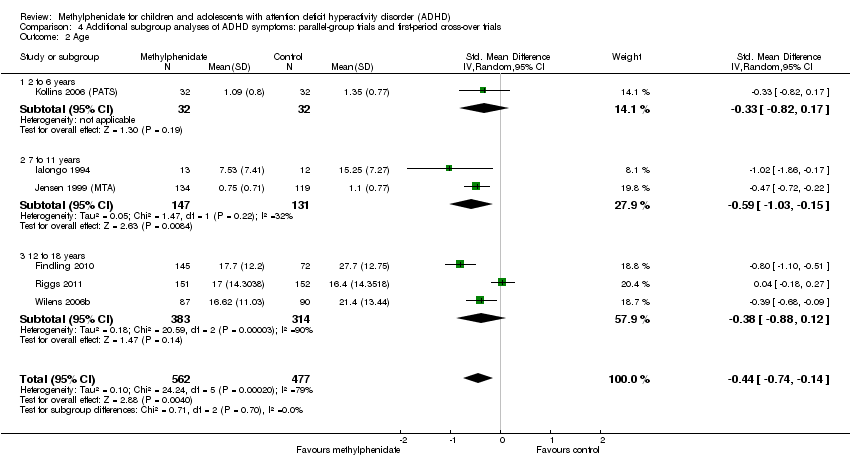 Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Age. | ||||
| 2.1 2 to 6 years | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.82, 0.17] |
| 2.2 7 to 11 years | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.03, ‐0.15] |
| 2.3 12 to 18 years | 3 | 697 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.88, 0.12] |
| 3 Comorbidity versus no comorbidity Show forest plot | 20 | 2310 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.91, ‐0.53] |
| Analysis 4.3  Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Comorbidity versus no comorbidity. | ||||
| 3.1 ADHD with comorbidity | 18 | 1981 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.94, ‐0.51] |
| 3.2 ADHD without comorbidity | 2 | 329 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.92, ‐0.46] |
| 4 Subtypes ADHD: ADHD Rating Scale (parent‐, teacher‐ or independent assessor‐rated) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 4 Subtypes ADHD: ADHD Rating Scale (parent‐, teacher‐ or independent assessor‐rated). | ||||
| 4.1 Combined ADHD | 2 | 559 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐1.30, 2.60] |
| 4.2 Inattentive ADHD | 1 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.61, ‐1.01] |
| 5 Cross‐over trials: first‐period data versus endpoint data (parent‐, independent assessor‐ and teacher‐rated) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 5 Cross‐over trials: first‐period data versus endpoint data (parent‐, independent assessor‐ and teacher‐rated). | ||||
| 5.1 First‐period data | 4 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.85, ‐0.44] |
| 5.2 Endpoint data | 4 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.18, ‐0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of serious adverse events (SAE) Show forest plot | 9 | 1532 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.44, 2.22] |
| Analysis 5.1  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Number of serious adverse events (SAE). | ||||
| 2 Nervous system Show forest plot | 6 | 2280 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.30, 2.53] |
| Analysis 5.2  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Nervous system. | ||||
| 2.1 Aggression | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.49] |
| 2.2 Concussion | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| 2.3 Loss of consciousness | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 2.4 Psychosis | 4 | 712 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.19, 16.96] |
| 2.5 Syncope | 3 | 741 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.23, 8.47] |
| 3 Digestive system: gastrointestinal disorders Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Digestive system: gastrointestinal disorders. | ||||
| 4 Urinary system: kidney infection Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 4 Urinary system: kidney infection. | ||||
| 5 Circulatory and respiratory systems: asthma Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 5 Circulatory and respiratory systems: asthma. | ||||
| 6 Immune system: cyst rupture Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.6  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 6 Immune system: cyst rupture. | ||||
| 7 Other: drug toxicity Show forest plot | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| Analysis 5.7  Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 7 Other: drug toxicity. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of serious adverse events (SAE) Show forest plot | 8 | 1721 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.34, 7.71] |
| Analysis 6.1 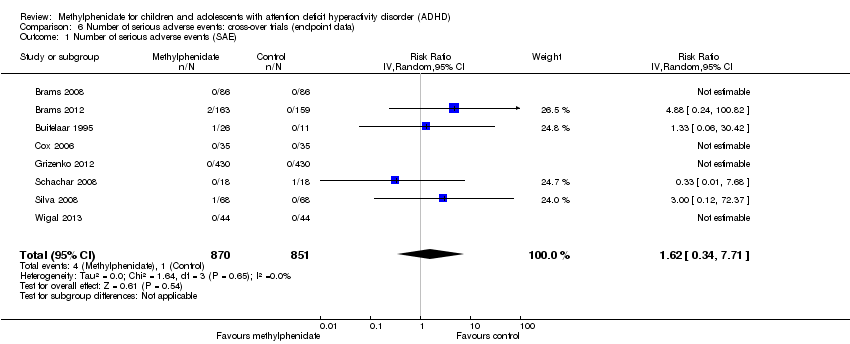 Comparison 6 Number of serious adverse events: cross‐over trials (endpoint data), Outcome 1 Number of serious adverse events (SAE). | ||||
| 2 Hallucinations/psychosis Show forest plot | 4 | 187 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.18, 6.72] |
| Analysis 6.2  Comparison 6 Number of serious adverse events: cross‐over trials (endpoint data), Outcome 2 Hallucinations/psychosis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total number of non‐serious adverse events Show forest plot | 21 | 3132 | Risk Ratio (IV, Random, 95% CI) | 1.29 [1.10, 1.51] |
| Analysis 7.1  Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Total number of non‐serious adverse events. | ||||
| 2 Subgroup analysis: total number of non‐serious adverse events according to dose Show forest plot | 21 | 3135 | Risk Ratio (IV, Random, 95% CI) | 1.28 [1.11, 1.49] |
| Analysis 7.2  Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Subgroup analysis: total number of non‐serious adverse events according to dose. | ||||
| 2.1 Low dose | 2 | 151 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.82, 1.46] |
| 2.2 High dose | 10 | 1761 | Risk Ratio (IV, Random, 95% CI) | 1.22 [1.10, 1.35] |
| 2.3 Unknown dose | 10 | 1223 | Risk Ratio (IV, Random, 95% CI) | 1.37 [1.01, 1.87] |
| 3 Nervous system Show forest plot | 21 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 7.3 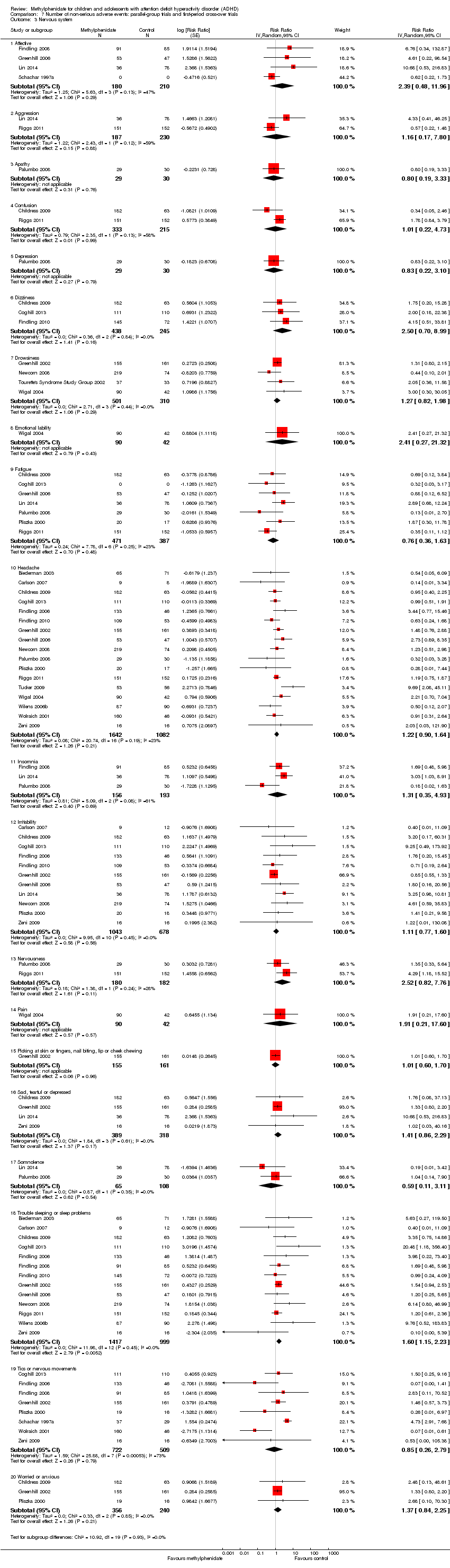 Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Nervous system. | ||||
| 3.1 Affective | 4 | 390 | Risk Ratio (Random, 95% CI) | 2.39 [0.48, 11.96] |
| 3.2 Aggression | 2 | 417 | Risk Ratio (Random, 95% CI) | 1.16 [0.17, 7.80] |
| 3.3 Apathy | 1 | 59 | Risk Ratio (Random, 95% CI) | 0.80 [0.19, 3.33] |
| 3.4 Confusion | 2 | 548 | Risk Ratio (Random, 95% CI) | 1.01 [0.22, 4.73] |
| 3.5 Depression | 1 | 59 | Risk Ratio (Random, 95% CI) | 0.83 [0.22, 3.10] |
| 3.6 Dizziness | 3 | 683 | Risk Ratio (Random, 95% CI) | 2.50 [0.70, 8.99] |
| 3.7 Drowsiness | 4 | 811 | Risk Ratio (Random, 95% CI) | 1.27 [0.82, 1.98] |
| 3.8 Emotional lability | 1 | 132 | Risk Ratio (Random, 95% CI) | 2.41 [0.27, 21.32] |
| 3.9 Fatigue | 7 | 858 | Risk Ratio (Random, 95% CI) | 0.76 [0.36, 1.63] |
| 3.10 Headache | 17 | 2724 | Risk Ratio (Random, 95% CI) | 1.22 [0.90, 1.64] |
| 3.11 Insomnia | 3 | 349 | Risk Ratio (Random, 95% CI) | 1.31 [0.35, 4.93] |
| 3.12 Irritability | 11 | 1721 | Risk Ratio (Random, 95% CI) | 1.11 [0.77, 1.60] |
| 3.13 Nervousness | 2 | 362 | Risk Ratio (Random, 95% CI) | 2.52 [0.82, 7.76] |
| 3.14 Pain | 1 | 132 | Risk Ratio (Random, 95% CI) | 1.91 [0.21, 17.60] |
| 3.15 Picking at skin or fingers, nail biting, lip or cheek chewing | 1 | 316 | Risk Ratio (Random, 95% CI) | 1.01 [0.60, 1.70] |
| 3.16 Sad, tearful or depressed | 4 | 707 | Risk Ratio (Random, 95% CI) | 1.41 [0.86, 2.29] |
| 3.17 Somnolence | 2 | 173 | Risk Ratio (Random, 95% CI) | 0.59 [0.11, 3.11] |
| 3.18 Trouble sleeping or sleep problems | 13 | 2416 | Risk Ratio (Random, 95% CI) | 1.60 [1.15, 2.23] |
| 3.19 Tics or nervous movements | 8 | 1231 | Risk Ratio (Random, 95% CI) | 0.85 [0.26, 2.79] |
| 3.20 Worried or anxious | 3 | 596 | Risk Ratio (Random, 95% CI) | 1.37 [0.84, 2.25] |
| 4 Digestive system Show forest plot | 18 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.4 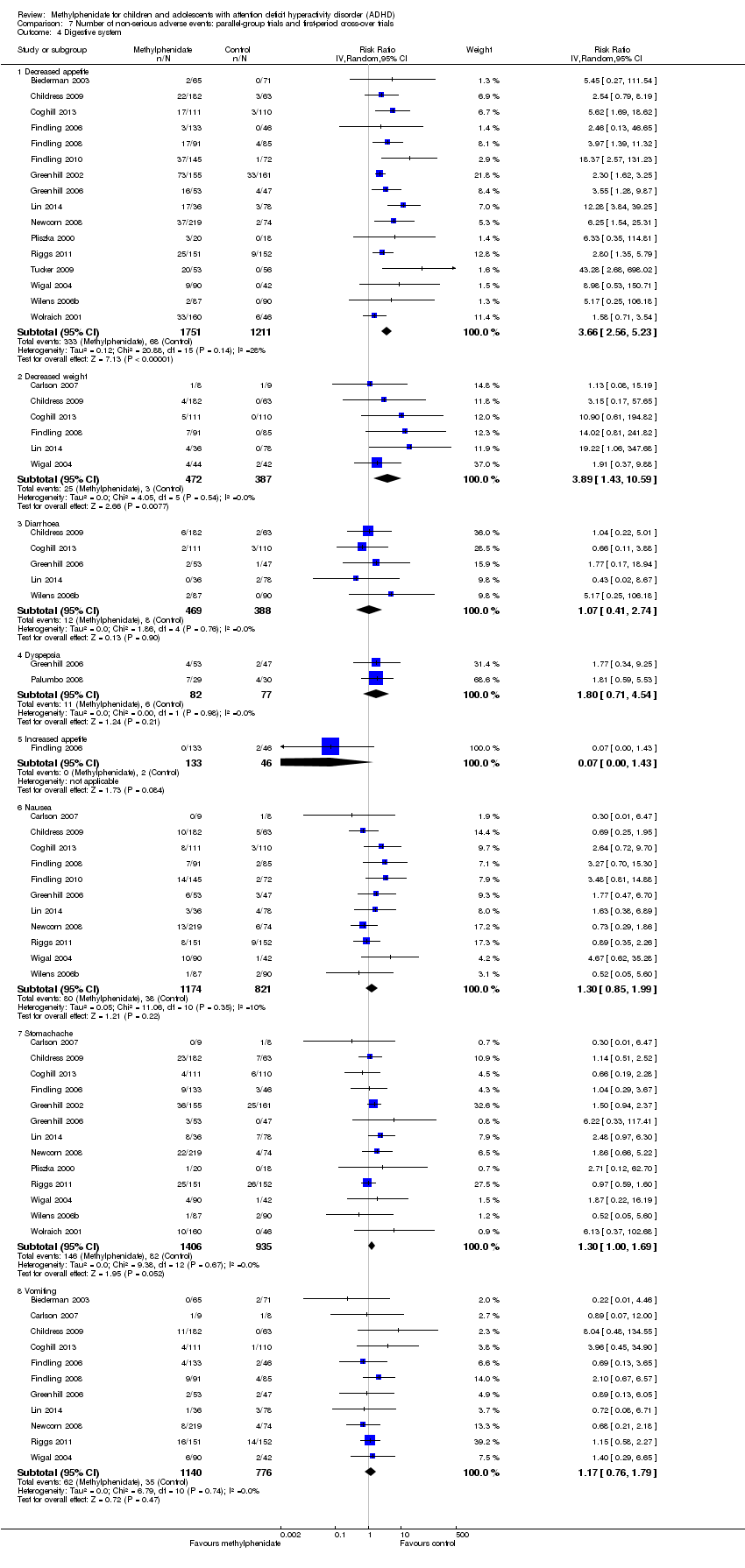 Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 4 Digestive system. | ||||
| 4.1 Decreased appetite | 16 | 2962 | Risk Ratio (IV, Random, 95% CI) | 3.66 [2.56, 5.23] |
| 4.2 Decreased weight | 6 | 859 | Risk Ratio (IV, Random, 95% CI) | 3.89 [1.43, 10.59] |
| 4.3 Diarrhoea | 5 | 857 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.41, 2.74] |
| 4.4 Dyspepsia | 2 | 159 | Risk Ratio (IV, Random, 95% CI) | 1.80 [0.71, 4.54] |
| 4.5 Increased appetite | 1 | 179 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.00, 1.43] |
| 4.6 Nausea | 11 | 1995 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.85, 1.99] |
| 4.7 Stomachache | 13 | 2341 | Risk Ratio (IV, Random, 95% CI) | 1.30 [1.00, 1.69] |
| 4.8 Vomiting | 11 | 1916 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.76, 1.79] |
| 5 Circulatory and respiratory systems Show forest plot | 8 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 7.5  Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 5 Circulatory and respiratory systems. | ||||
| 5.1 ECG: prolonged QT‐interval | 2 | 466 | Risk Ratio (Random, 95% CI) | 0.81 [0.13, 5.00] |
| 5.2 ECG: tachycardia | 1 | 245 | Risk Ratio (Random, 95% CI) | 1.04 [0.11, 10.18] |
| 5.3 Cough | 4 | 996 | Risk Ratio (Random, 95% CI) | 0.95 [0.41, 2.18] |
| 5.4 Nasal congestion | 2 | 479 | Risk Ratio (Random, 95% CI) | 1.19 [0.59, 2.41] |
| 5.5 Pharyngolaryngeal pain | 1 | 303 | Risk Ratio (Random, 95% CI) | 1.12 [0.59, 2.13] |
| 5.6 Supraventricular extrasystoles | 1 | 17 | Risk Ratio (Random, 95% CI) | 3.00 [0.11, 84.55] |
| 5.7 Upper respiratory tract infection | 1 | 217 | Risk Ratio (Random, 95% CI) | 1.07 [0.42, 2.76] |
| 6 Skeletal and muscular systems Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.6  Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 6 Skeletal and muscular systems. | ||||
| 6.1 Arthralgia | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.24, 1.84] |
| 6.2 Asthenia | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.01, 4.25] |
| 6.3 Back pain | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.39, 1.66] |
| 6.4 Myalgia | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.23, 1.62] |
| 6.5 Toothache | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.43, 2.35] |
| 7 Immune system Show forest plot | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.7 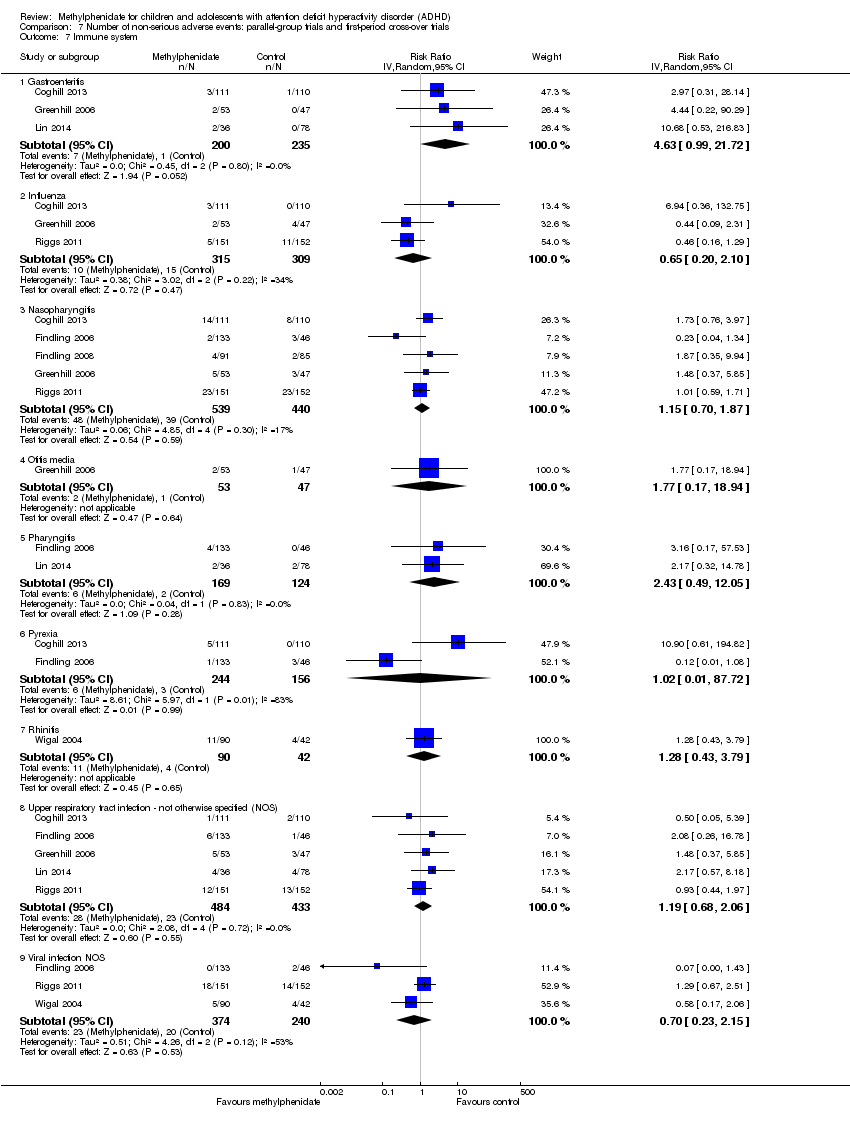 Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 7 Immune system. | ||||
| 7.1 Gastroenteritis | 3 | 435 | Risk Ratio (IV, Random, 95% CI) | 4.63 [0.99, 21.72] |
| 7.2 Influenza | 3 | 624 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.20, 2.10] |
| 7.3 Nasopharyngitis | 5 | 979 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.70, 1.87] |
| 7.4 Otitis media | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.17, 18.94] |
| 7.5 Pharyngitis | 2 | 293 | Risk Ratio (IV, Random, 95% CI) | 2.43 [0.49, 12.05] |
| 7.6 Pyrexia | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.01, 87.72] |
| 7.7 Rhinitis | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.43, 3.79] |
| 7.8 Upper respiratory tract infection ‐ not otherwise specified (NOS) | 5 | 917 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.68, 2.06] |
| 7.9 Viral infection NOS | 3 | 614 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.23, 2.15] |
| 8 Height Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.8  Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 8 Height. | ||||
| 9 Weight Show forest plot | 5 | 805 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.55, ‐0.70] |
| Analysis 7.9 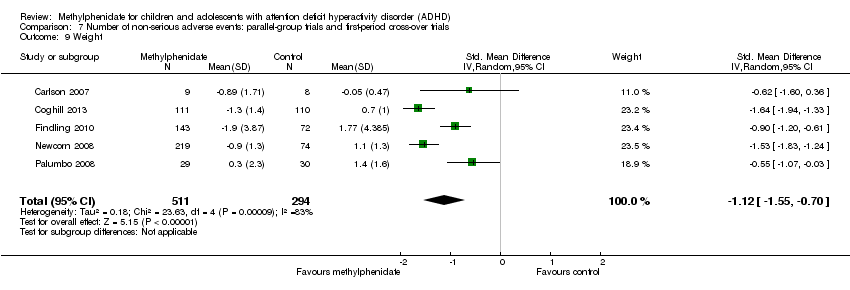 Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 9 Weight. | ||||
| 10 BMI Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.10  Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 10 BMI. | ||||
| 11 Vital signs Show forest plot | 9 | 3374 | Mean Difference (IV, Random, 95% CI) | 1.41 [0.30, 2.52] |
| Analysis 7.11 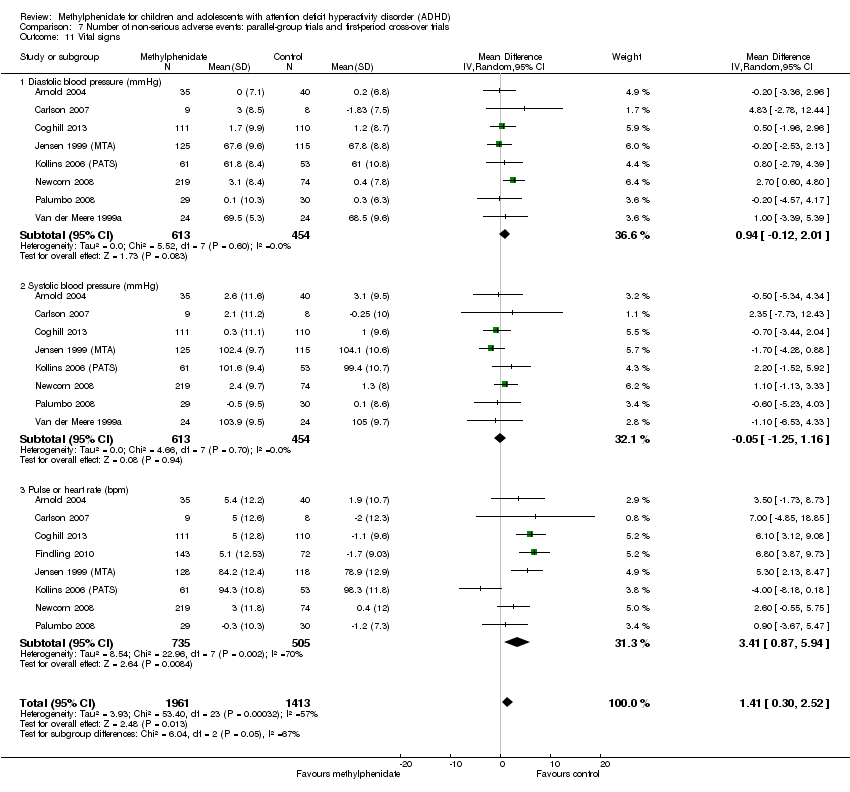 Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 11 Vital signs. | ||||
| 11.1 Diastolic blood pressure (mmHg) | 8 | 1067 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.12, 2.01] |
| 11.2 Systolic blood pressure (mmHg) | 8 | 1067 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐1.25, 1.16] |
| 11.3 Pulse or heart rate (bpm) | 8 | 1240 | Mean Difference (IV, Random, 95% CI) | 3.41 [0.87, 5.94] |
| 12 Other Show forest plot | 5 | 1815 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.56, 2.57] |
| Analysis 7.12 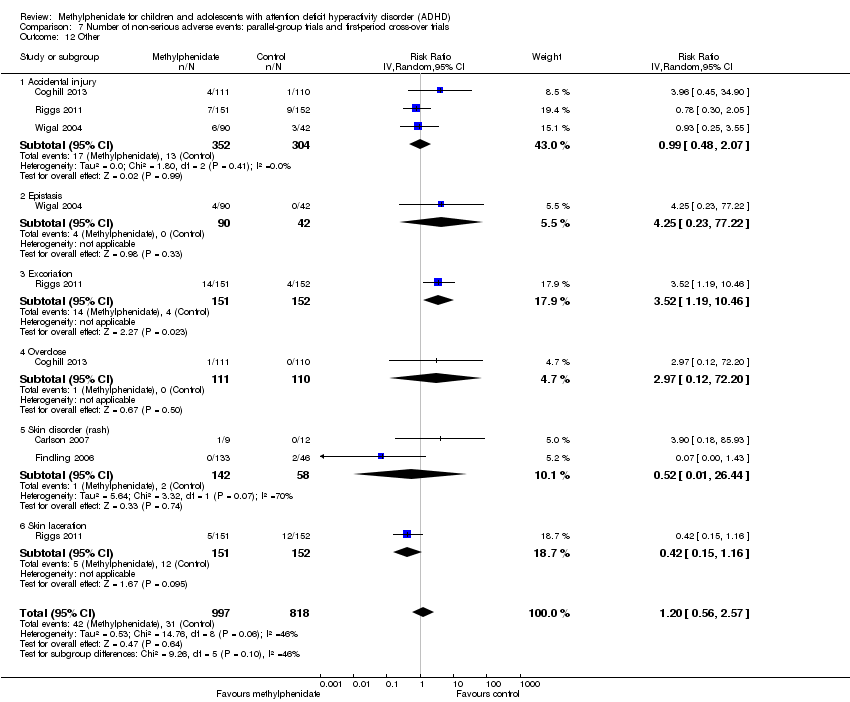 Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 12 Other. | ||||
| 12.1 Accidental injury | 3 | 656 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.48, 2.07] |
| 12.2 Epistasis | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 4.25 [0.23, 77.22] |
| 12.3 Excoriation | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 3.52 [1.19, 10.46] |
| 12.4 Overdose | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.20] |
| 12.5 Skin disorder (rash) | 2 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.01, 26.44] |
| 12.6 Skin laceration | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.15, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total number of non‐serious adverse events Show forest plot | 21 | 2072 | Risk Ratio (Random, 95% CI) | 1.33 [1.11, 1.58] |
| Analysis 8.1 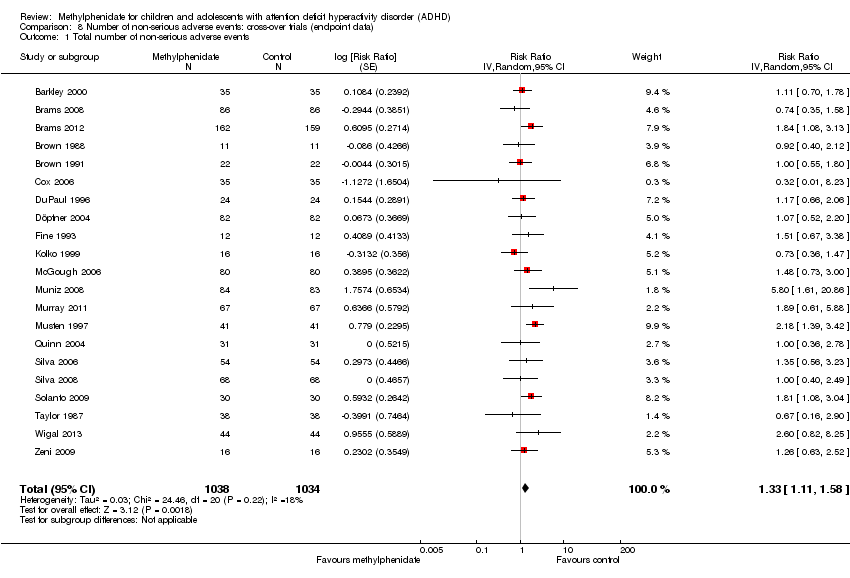 Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 1 Total number of non‐serious adverse events. | ||||
| 2 Subgroup analysis: total number of non‐serious adverse events according to dose Show forest plot | 21 | 2859 | Risk Ratio (Random, 95% CI) | 1.26 [1.11, 1.44] |
| Analysis 8.2  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 2 Subgroup analysis: total number of non‐serious adverse events according to dose. | ||||
| 2.1 Low dose | 16 | 1539 | Risk Ratio (Random, 95% CI) | 1.11 [0.94, 1.31] |
| 2.2 High dose | 12 | 1080 | Risk Ratio (Random, 95% CI) | 1.57 [1.22, 2.01] |
| 2.3 Unknown dose | 2 | 240 | Risk Ratio (Random, 95% CI) | 0.98 [0.51, 1.86] |
| 3 Nervous system Show forest plot | 50 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 8.3  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 3 Nervous system. | ||||
| 3.1 Aggression | 2 | 589 | Risk Ratio (Random, 95% CI) | 0.52 [0.17, 1.60] |
| 3.2 Agitation | 1 | 62 | Risk Ratio (Random, 95% CI) | 1.18 [0.38, 3.60] |
| 3.3 Anger | 3 | 264 | Risk Ratio (Random, 95% CI) | 0.45 [0.26, 0.77] |
| 3.4 Behavioural complaints | 1 | 82 | Risk Ratio (Random, 95% CI) | 0.55 [0.35, 0.86] |
| 3.5 Buccal or lingual movements | 4 | 302 | Risk Ratio (Random, 95% CI) | 1.06 [0.62, 1.79] |
| 3.6 Compulsive acts | 1 | 90 | Risk Ratio (Random, 95% CI) | 2.57 [1.45, 4.56] |
| 3.7 Daydreaming | 3 | 222 | Risk Ratio (Random, 95% CI) | 0.66 [0.44, 0.98] |
| 3.8 Dizziness | 9 | 746 | Risk Ratio (Random, 95% CI) | 1.17 [0.89, 1.55] |
| 3.9 Drowsiness: dull, tired, listless or sleepy | 21 | 1350 | Risk Ratio (Random, 95% CI) | 0.97 [0.73, 1.28] |
| 3.10 Euphoria | 6 | 405 | Risk Ratio (Random, 95% CI) | 1.06 [0.72, 1.57] |
| 3.11 Headache | 37 | 3752 | Risk Ratio (Random, 95% CI) | 1.21 [1.01, 1.45] |
| 3.12 Insomnia or sleep problems | 31 | 3270 | Risk Ratio (Random, 95% CI) | 1.57 [1.20, 2.06] |
| 3.13 Irritability | 23 | 2238 | Risk Ratio (Random, 95% CI) | 0.92 [0.66, 1.27] |
| 3.14 Nightmares | 10 | 686 | Risk Ratio (Random, 95% CI) | 0.97 [0.66, 1.42] |
| 3.15 Overly meticulous | 1 | 96 | Risk Ratio (Random, 95% CI) | 40.77 [2.35, 706.72] |
| 3.16 Obsessive thinking | 1 | 90 | Risk Ratio (Random, 95% CI) | 2.35 [1.53, 3.62] |
| 3.17 Picking at skin or fingers, nail biting, lip or cheek chewing | 15 | 888 | Risk Ratio (Random, 95% CI) | 1.12 [0.88, 1.41] |
| 3.18 Repetitive language | 1 | 48 | Risk Ratio (Random, 95% CI) | 1.0 [0.32, 3.10] |
| 3.19 Sad, tearful or depressed | 23 | 1849 | Risk Ratio (Random, 95% CI) | 1.15 [0.94, 1.41] |
| 3.20 Socially withdrawn ‐ decreased interaction with others | 12 | 771 | Risk Ratio (Random, 95% CI) | 1.24 [0.82, 1.87] |
| 3.21 Sleep efficiency (SEF) | 2 | 108 | Risk Ratio (Random, 95% CI) | 0.48 [0.02, 14.28] |
| 3.22 Stares a lot | 9 | 904 | Risk Ratio (Random, 95% CI) | 1.03 [0.75, 1.40] |
| 3.23 Tics or nervous movements | 19 | 1403 | Risk Ratio (Random, 95% CI) | 1.33 [1.03, 1.72] |
| 3.24 Unusual blinking | 1 | 48 | Risk Ratio (Random, 95% CI) | 3.13 [0.12, 80.68] |
| 3.25 Worried or anxious | 20 | 1673 | Risk Ratio (Random, 95% CI) | 0.82 [0.60, 1.12] |
| 4 Digestive system Show forest plot | 42 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| Analysis 8.4 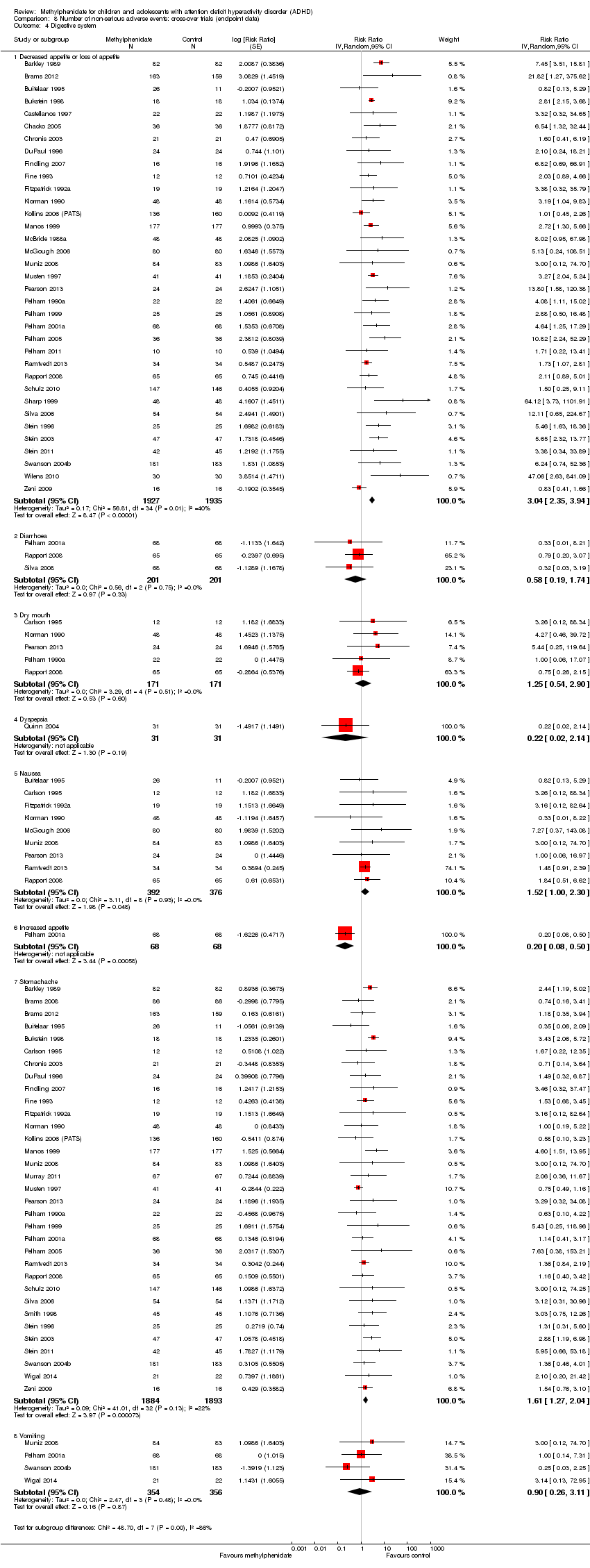 Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 4 Digestive system. | ||||
| 4.1 Decreased appetite or loss of appetite | 35 | 3862 | Risk Ratio (Random, 95% CI) | 3.04 [2.35, 3.94] |
| 4.2 Diarrhoea | 3 | 402 | Risk Ratio (Random, 95% CI) | 0.58 [0.19, 1.74] |
| 4.3 Dry mouth | 5 | 342 | Risk Ratio (Random, 95% CI) | 1.25 [0.54, 2.90] |
| 4.4 Dyspepsia | 1 | 62 | Risk Ratio (Random, 95% CI) | 0.22 [0.02, 2.14] |
| 4.5 Nausea | 9 | 768 | Risk Ratio (Random, 95% CI) | 1.52 [1.00, 2.30] |
| 4.6 Increased appetite | 1 | 136 | Risk Ratio (Random, 95% CI) | 0.20 [0.08, 0.50] |
| 4.7 Stomachache | 33 | 3777 | Risk Ratio (Random, 95% CI) | 1.61 [1.27, 2.04] |
| 4.8 Vomiting | 4 | 710 | Risk Ratio (Random, 95% CI) | 0.90 [0.26, 3.11] |
| 5 Urinary system: urinary incontinence Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.5  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 5 Urinary system: urinary incontinence. | ||||
| 6 Skeletal and muscular system: somatic complaints Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.6  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 6 Skeletal and muscular system: somatic complaints. | ||||
| 7 Immune system Show forest plot | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.7  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 7 Immune system. | ||||
| 7.1 Allergic rhinitis | 4 | 475 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.35, 5.51] |
| 7.2 Fever | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.09, 20.56] |
| 7.3 Lymphadenitis | 2 | 296 | Risk Ratio (IV, Random, 95% CI) | 3.93 [0.44, 35.11] |
| 7.4 Pharyngolaryngeal pain | 1 | 160 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.19, 21.62] |
| 7.5 Pharyngitis | 4 | 754 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.19, 2.62] |
| 7.6 Upper respiratory tract infection | 5 | 888 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.27, 1.37] |
| 8 Skin Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.8  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 8 Skin. | ||||
| 8.1 Rash | 2 | 208 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.14, 6.41] |
| 8.2 Skin laceration | 1 | 167 | Risk Ratio (IV, Random, 95% CI) | 2.96 [0.12, 71.75] |
| 9 Vital signs Show forest plot | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.9 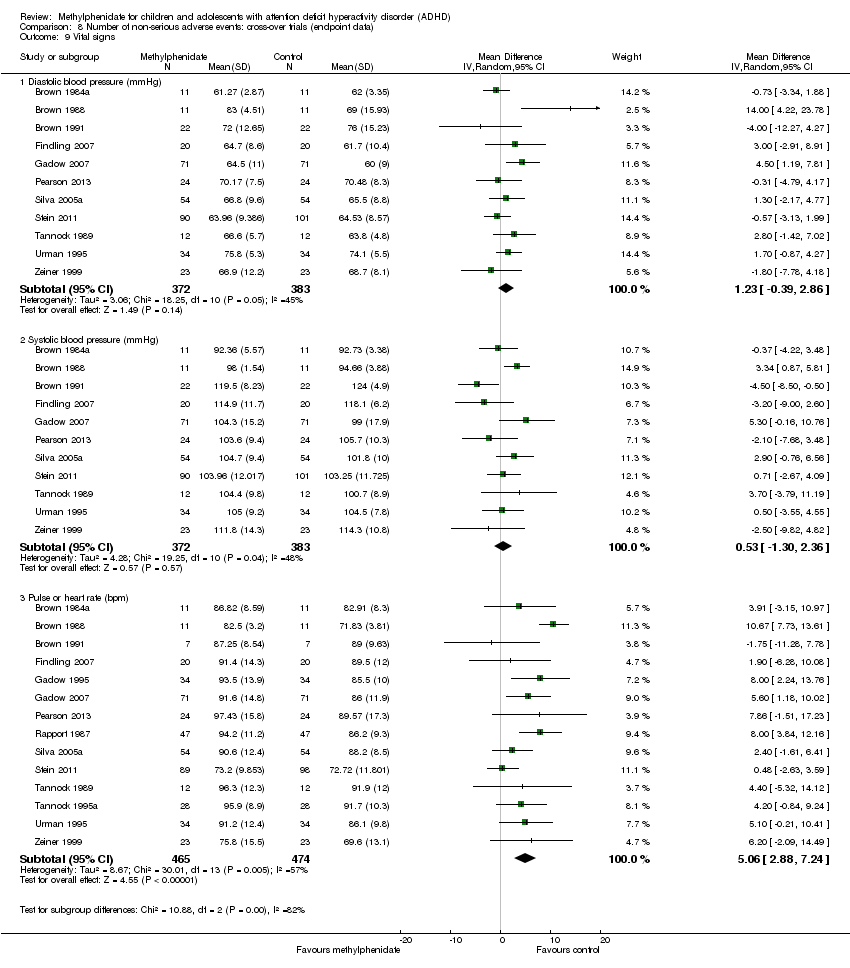 Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 9 Vital signs. | ||||
| 9.1 Diastolic blood pressure (mmHg) | 11 | 755 | Mean Difference (IV, Random, 95% CI) | 1.23 [‐0.39, 2.86] |
| 9.2 Systolic blood pressure (mmHg) | 11 | 755 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐1.30, 2.36] |
| 9.3 Pulse or heart rate (bpm) | 14 | 939 | Mean Difference (IV, Random, 95% CI) | 5.06 [2.88, 7.24] |
| 10 Height (cm) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.10  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 10 Height (cm). | ||||
| 11 Weight Show forest plot | 6 | 530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| Analysis 8.11  Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 11 Weight. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials: risk of bias Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| Analysis 9.1  Comparison 9 Teacher‐rated general behaviour, Outcome 1 All parallel‐group trials and first‐period cross‐over trials: risk of bias. | ||||
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 2 Subgroup analysis: types of scales Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| Analysis 9.2  Comparison 9 Teacher‐rated general behaviour, Outcome 2 Subgroup analysis: types of scales. | ||||
| 2.1 Conners' Global Index ‐ Teacher (CGI‐T) | 1 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.14, ‐0.68] |
| 2.2 Groninger Behaviour Observation Scale (GBOS) | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.46, ‐0.21] |
| 2.3 Conners' Teacher Rating Scale ‐ Conduct problems | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.48, 0.14] |
| 2.4 IOWA Conners' Rating Scale ‐ Oppositional/Defiant (IOWA‐O/D) | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.12, ‐0.59] |
| 3 Subgroup analysis: dose Show forest plot | 5 | 696 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.02, ‐0.69] |
| Analysis 9.3  Comparison 9 Teacher‐rated general behaviour, Outcome 3 Subgroup analysis: dose. | ||||
| 3.1 Low dose | 2 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.19] |
| 3.2 High dose | 3 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.08, ‐0.70] |
| 3.3 Unknown dose | 1 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.21, ‐0.46] |
| 4 Subgroup analysis: duration of treatment Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| Analysis 9.4  Comparison 9 Teacher‐rated general behaviour, Outcome 4 Subgroup analysis: duration of treatment. | ||||
| 4.1 Short term (up to 6 months) | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 4.2 Long term (over 6 months) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Subgroup analysis: parallel‐group trials versus first‐period cross‐over trials Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| Analysis 9.5 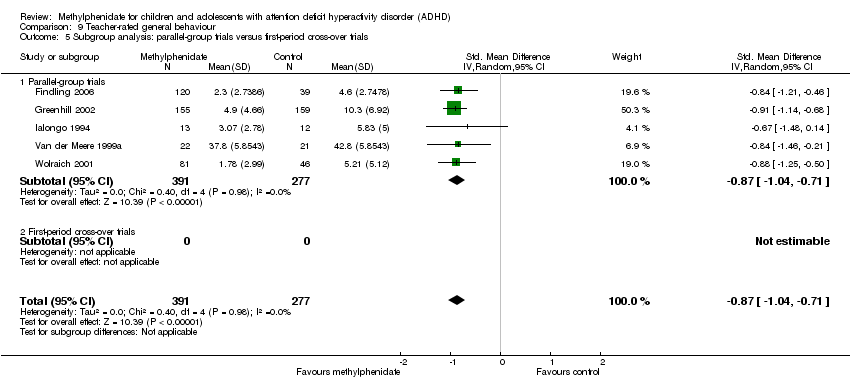 Comparison 9 Teacher‐rated general behaviour, Outcome 5 Subgroup analysis: parallel‐group trials versus first‐period cross‐over trials. | ||||
| 5.1 Parallel‐group trials | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 5.2 First‐period cross‐over trials | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 General behaviour, cross‐over trials (endpoint data) Show forest plot | 16 | 2014 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.78, ‐0.60] |
| Analysis 9.6 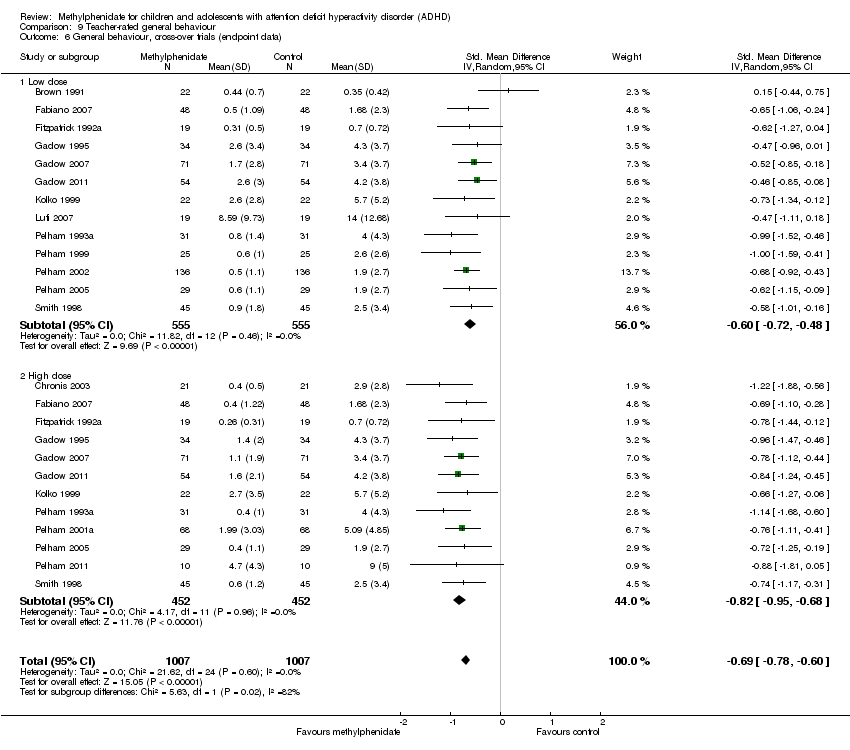 Comparison 9 Teacher‐rated general behaviour, Outcome 6 General behaviour, cross‐over trials (endpoint data). | ||||
| 6.1 Low dose | 13 | 1110 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.72, ‐0.48] |
| 6.2 High dose | 12 | 904 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐0.95, ‐0.68] |
| 7 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias Show forest plot | 16 | 1308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| Analysis 9.7  Comparison 9 Teacher‐rated general behaviour, Outcome 7 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias. | ||||
| 7.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 High risk of bias | 16 | 1308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| 8 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (teacher‐rated) versus cross‐over trials (endpoint data) Show forest plot | 21 | 1976 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐0.88, ‐0.70] |
| Analysis 9.8 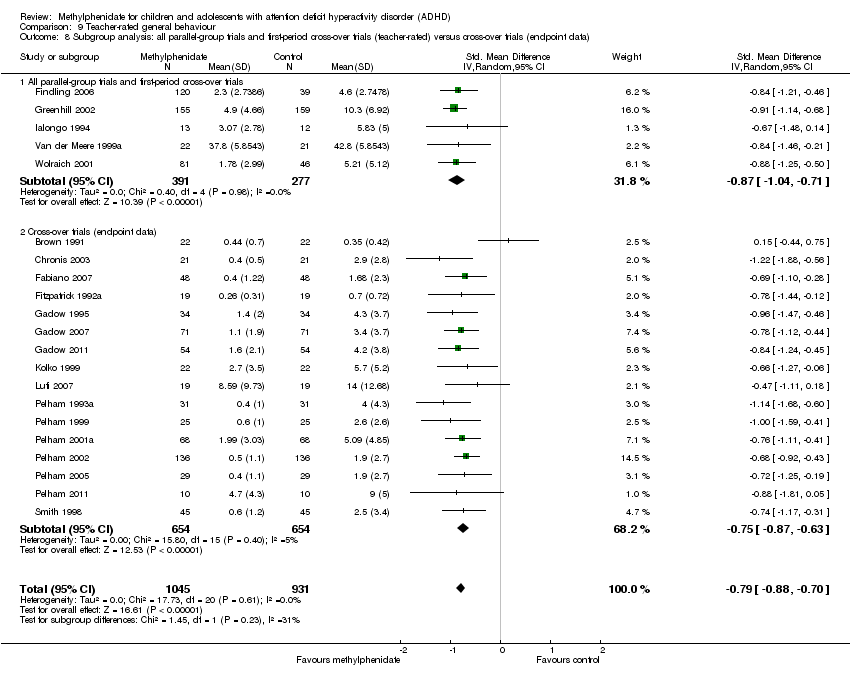 Comparison 9 Teacher‐rated general behaviour, Outcome 8 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (teacher‐rated) versus cross‐over trials (endpoint data). | ||||
| 8.1 All parallel‐group trials and first‐period cross‐over trials | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 8.2 Cross‐over trials (endpoint data) | 16 | 1308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General behaviour, cross‐over trials (endpoint data) Show forest plot | 8 | 1241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.75, ‐0.46] |
| Analysis 10.1  Comparison 10 Independent assessor‐rated general behaviour, Outcome 1 General behaviour, cross‐over trials (endpoint data). | ||||
| 1.1 Low dose | 7 | 903 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.76, ‐0.36] |
| 1.2 High dose | 5 | 338 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.93, ‐0.49] |
| 2 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias Show forest plot | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| Analysis 10.2  Comparison 10 Independent assessor‐rated general behaviour, Outcome 2 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias. | ||||
| 2.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 High risk of bias | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| 3 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (independent assessor‐rated) compared with cross‐over trials (endpoint data) Show forest plot | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| Analysis 10.3 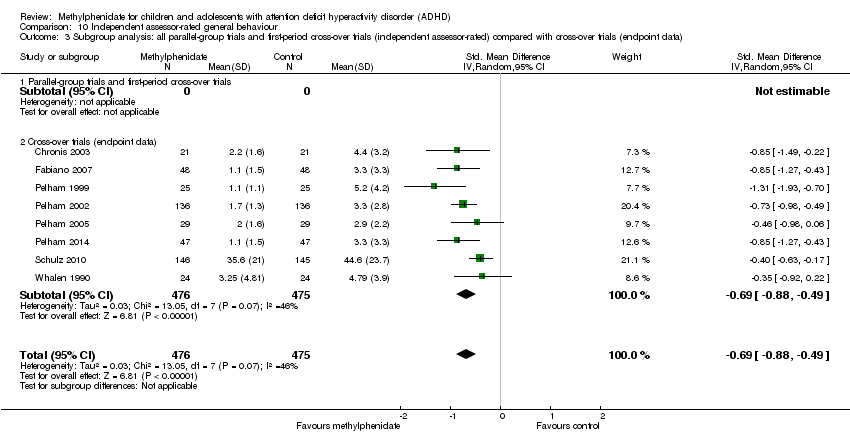 Comparison 10 Independent assessor‐rated general behaviour, Outcome 3 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (independent assessor‐rated) compared with cross‐over trials (endpoint data). | ||||
| 3.1 Parallel‐group trials and first‐period cross‐over trials | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Cross‐over trials (endpoint data) | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| Analysis 11.1 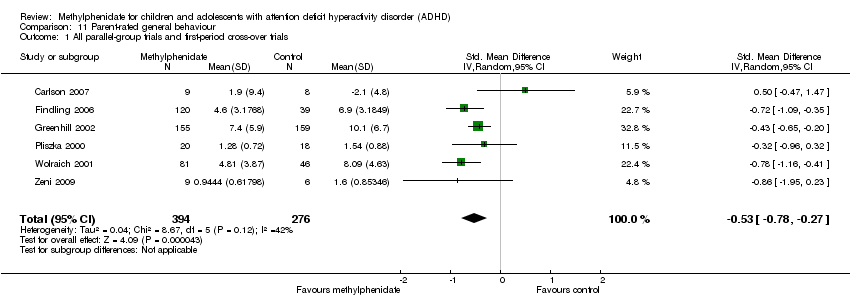 Comparison 11 Parent‐rated general behaviour, Outcome 1 All parallel‐group trials and first‐period cross‐over trials. | ||||
| 2 Subgroup analysis: risk of bias Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| Analysis 11.2  Comparison 11 Parent‐rated general behaviour, Outcome 2 Subgroup analysis: risk of bias. | ||||
| 2.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 High risk of bias | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 3 Subgroup analysis: types of scales Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| Analysis 11.3  Comparison 11 Parent‐rated general behaviour, Outcome 3 Subgroup analysis: types of scales. | ||||
| 3.1 The Weekly Parent Ratings of Evening and Morning Behaviour (WPREMB) ‐ Revised | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.47, 1.47] |
| 3.2 Conners' Global Index (CGI) ‐ Parent | 2 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.63, ‐0.20] |
| 3.3 Swanson, Nolan and Pelham, Fourth Edition ‐ Oppositional (SNAP‐IV‐Oppositional) | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.95, 0.23] |
| 3.4 IOWA Conners' Rating Scale ‐ Oppositional/Defiant (IOWA‐I/O) | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.01, ‐0.49] |
| 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| Analysis 11.4  Comparison 11 Parent‐rated general behaviour, Outcome 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials. | ||||
| 4.1 Parallel‐group trials | 5 | 655 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.78, ‐0.23] |
| 4.2 First‐period cross‐over trials | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.95, 0.23] |
| 5 Subgroup analysis: duration of treatment Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| Analysis 11.5  Comparison 11 Parent‐rated general behaviour, Outcome 5 Subgroup analysis: duration of treatment. | ||||
| 5.1 Short term (up to 6 months) | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 5.2 Long term (over 6 months) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Subgroup analysis: dose Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| Analysis 11.6 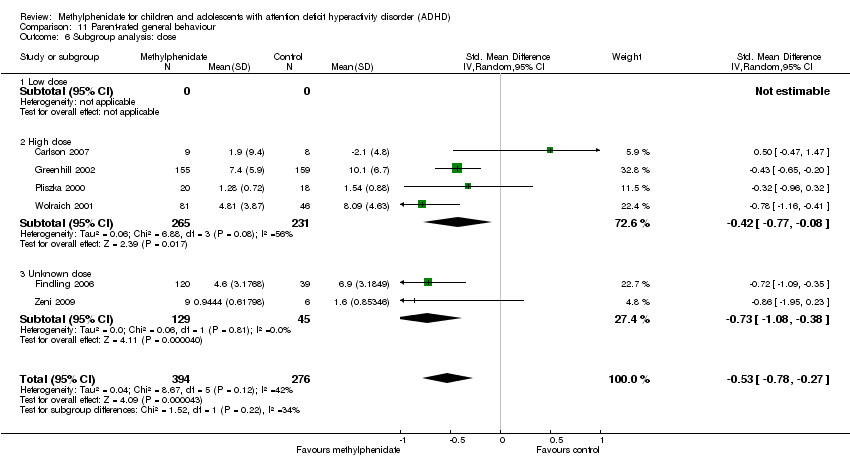 Comparison 11 Parent‐rated general behaviour, Outcome 6 Subgroup analysis: dose. | ||||
| 6.1 Low dose | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High dose | 4 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.77, ‐0.08] |
| 6.3 Unknown dose | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.08, ‐0.38] |
| 7 General behaviour, cross‐over trials (endpoint data) Show forest plot | 6 | 550 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.93, ‐0.56] |
| Analysis 11.7  Comparison 11 Parent‐rated general behaviour, Outcome 7 General behaviour, cross‐over trials (endpoint data). | ||||
| 7.1 Low dose | 5 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.93, ‐0.38] |
| 7.2 High dose | 4 | 302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.07, ‐0.60] |
| 8 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias Show forest plot | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| Analysis 11.8  Comparison 11 Parent‐rated general behaviour, Outcome 8 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias. | ||||
| 8.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 High risk of bias | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (parent‐rated) compared with cross‐over trials (endpoint data) Show forest plot | 12 | 1054 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.86, ‐0.50] |
| Analysis 11.9  Comparison 11 Parent‐rated general behaviour, Outcome 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (parent‐rated) compared with cross‐over trials (endpoint data). | ||||
| 9.1 All parallel‐group trials and first‐period cross‐over trials | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 9.2 Cross‐over trials (endpoint data) | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparisions of raters Show forest plot | 8 | 1338 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.86, ‐0.52] |
| Analysis 12.1 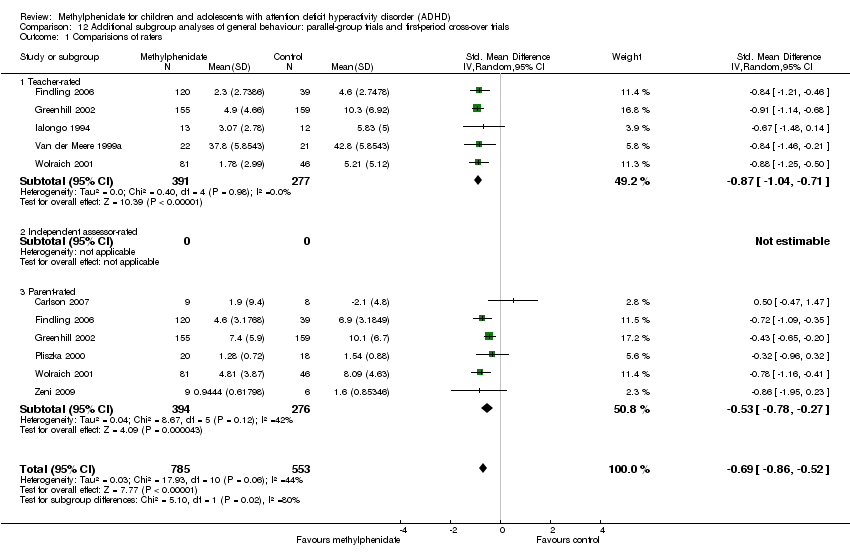 Comparison 12 Additional subgroup analyses of general behaviour: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Comparisions of raters. | ||||
| 1.1 Teacher‐rated | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 1.2 Independent assessor‐rated | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Parent‐rated | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 2 Comorbidity versus no comorbidity Show forest plot | 7 | 579 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.98, ‐0.43] |
| Analysis 12.2 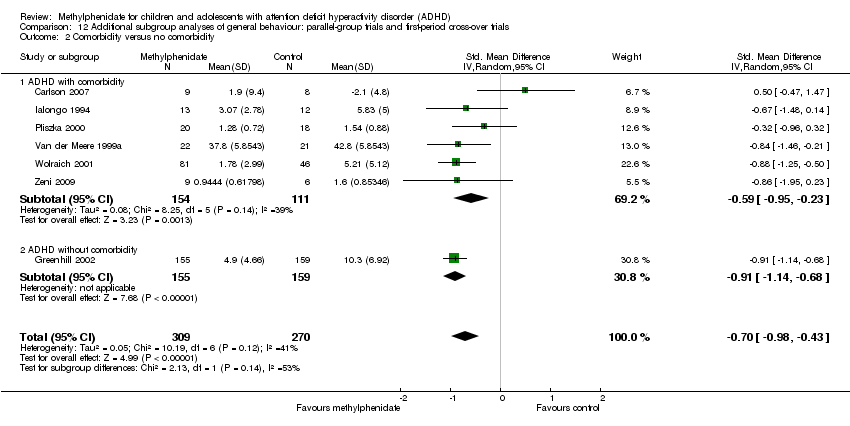 Comparison 12 Additional subgroup analyses of general behaviour: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Comorbidity versus no comorbidity. | ||||
| 2.1 ADHD with comorbidity | 6 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.95, ‐0.23] |
| 2.2 ADHD without comorbidity | 1 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.14, ‐0.68] |
| 3 Cross‐over trials: first‐period data versus endpoint data (teacher‐, parent‐, and independent assessor‐rated) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 12.3  Comparison 12 Additional subgroup analyses of general behaviour: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Cross‐over trials: first‐period data versus endpoint data (teacher‐, parent‐, and independent assessor‐rated). | ||||
| 3.1 First‐period data | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.75, 0.13] |
| 3.2 Endpoint data | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.71, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subgroup analysis: types of scales Show forest plot | 3 | 514 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.42, 0.80] |
| Analysis 13.1  Comparison 13 Quality of life: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Subgroup analysis: types of scales. | ||||
| 1.1 Child Health Questionnaire (CHQ) | 1 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.25, 0.83] |
| 1.2 Children´s Global Assessment Scale (CGAS) | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.10, 1.47] |
| 1.3 Child Health and Illness Profile, Child Edition: Parent Report Form (CHIP‐CE:PRF) | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.37, 0.91] |
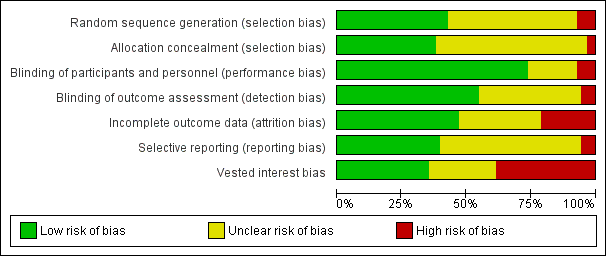
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Funnel plot of comparison: 1. Teacher‐rated ADHD symptoms, outcome: 1.8 All data at low and high risk of bias (parallel‐group and cross‐over trials).

Trial Sequential Analysis: serious adverse events.
Trial Sequential Analysis: non‐serious adverse events.

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 1 All parallel‐group trials and first‐period cross‐over trials.
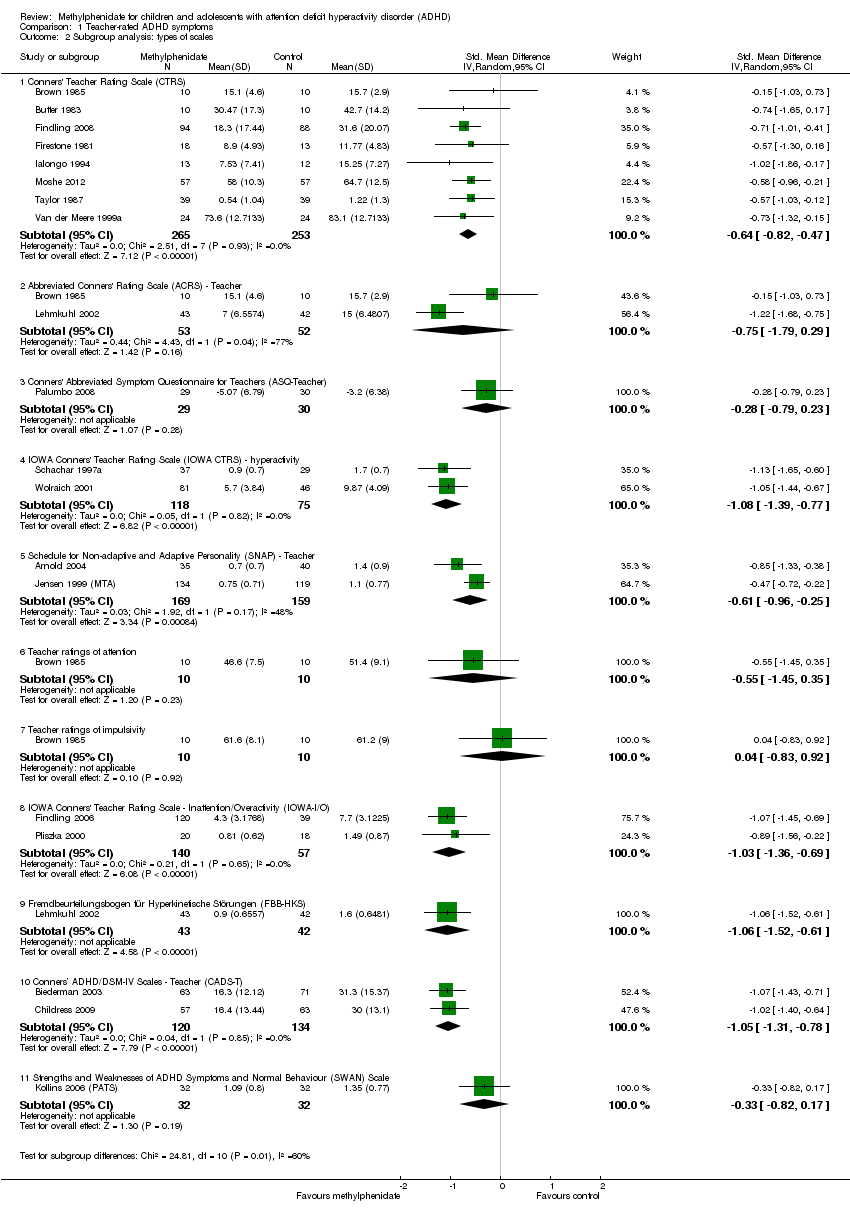
Comparison 1 Teacher‐rated ADHD symptoms, Outcome 2 Subgroup analysis: types of scales.

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 3 Medication status: medication naive versus not medication naive.

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 4 Subgroup analysis: duration of treatment.

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 5 Subgroup analysis: dose.
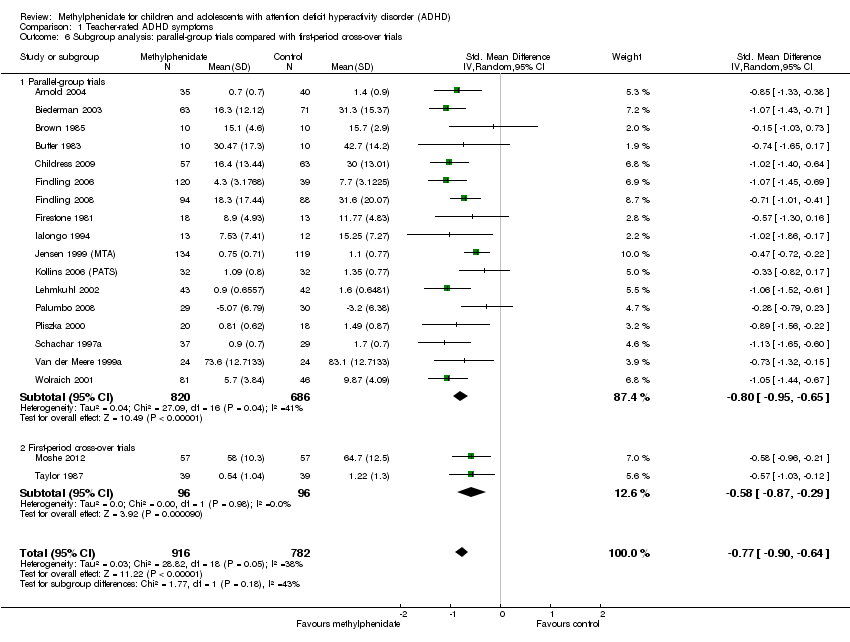
Comparison 1 Teacher‐rated ADHD symptoms, Outcome 6 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials.

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 7 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants.

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 8 ADHD symptoms, cross‐over trial (endpoint data).

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose.
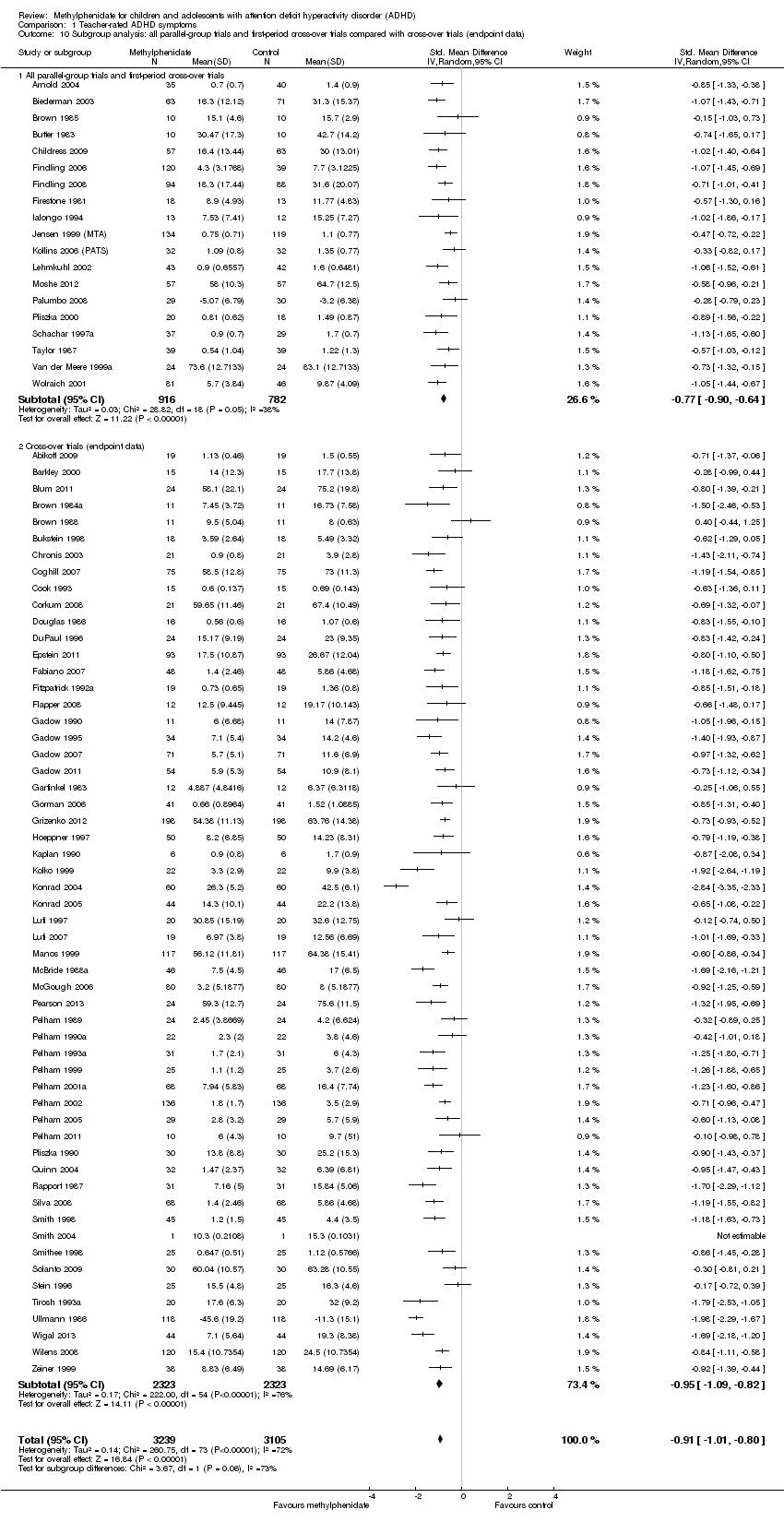
Comparison 1 Teacher‐rated ADHD symptoms, Outcome 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data).

Comparison 1 Teacher‐rated ADHD symptoms, Outcome 11 All parallel‐group trials and cross‐over trials: risk of bias.

Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 1 All parallel‐group trials and first‐period cross‐over trials.

Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 2 Subgroup analysis: types of scales.
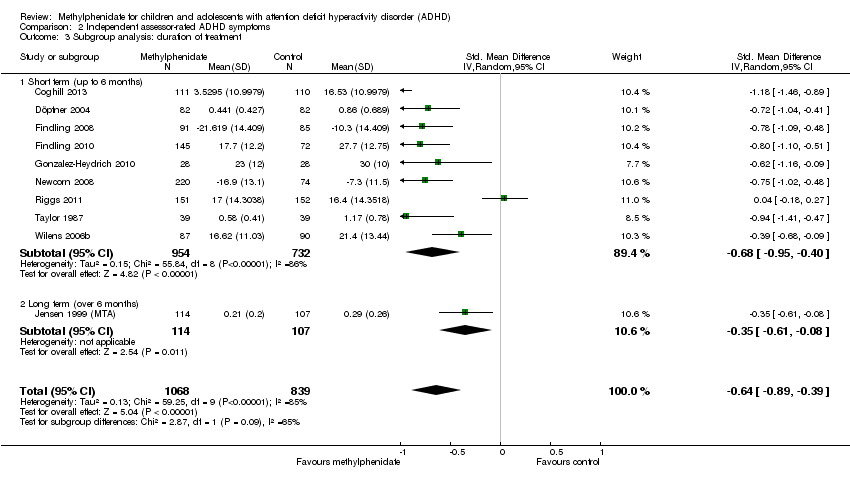
Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 3 Subgroup analysis: duration of treatment.
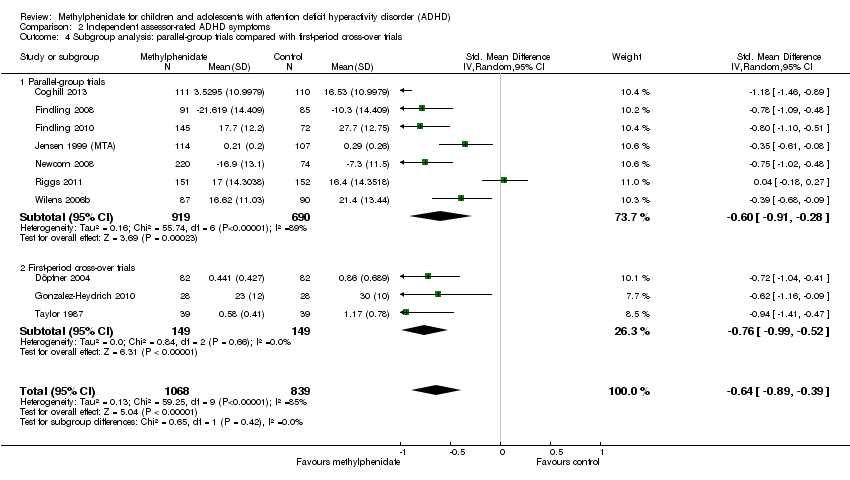
Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials.
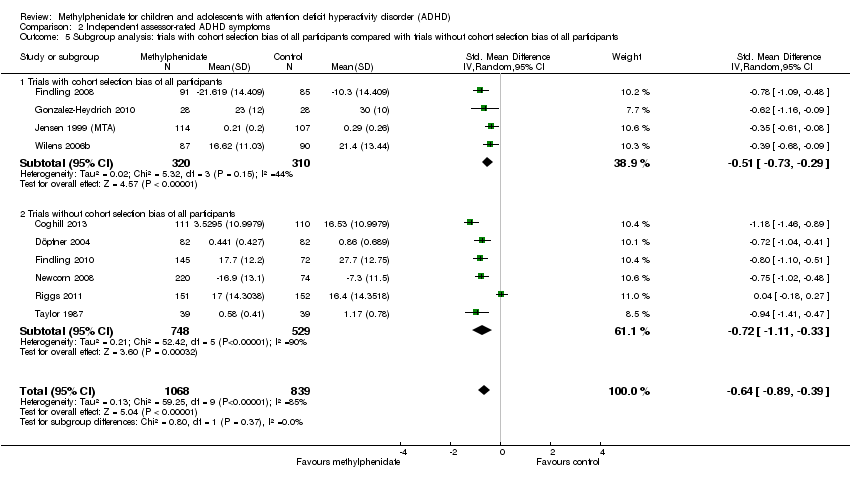
Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 5 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants.
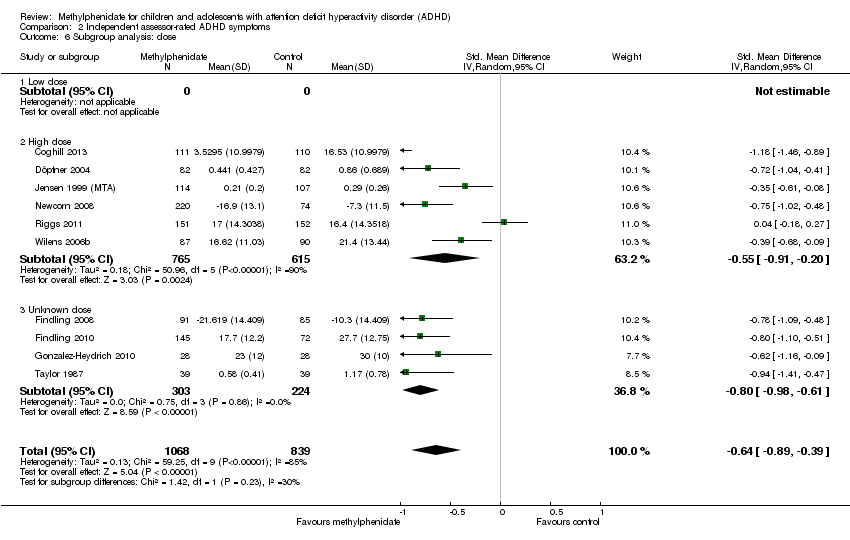
Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 6 Subgroup analysis: dose.

Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 7 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: risk of bias.
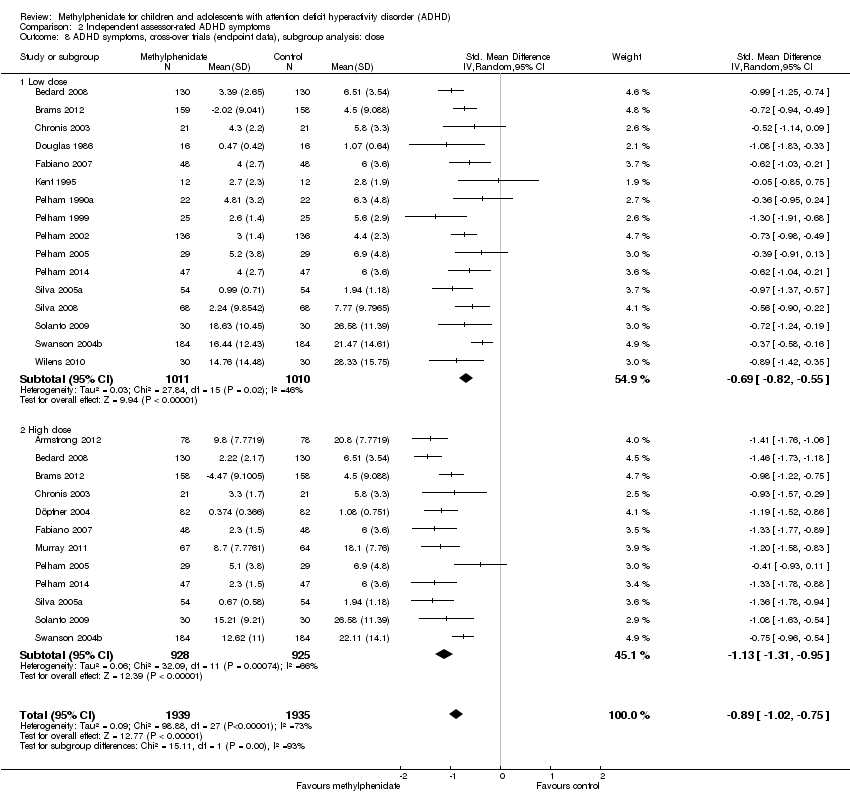
Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 8 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose.
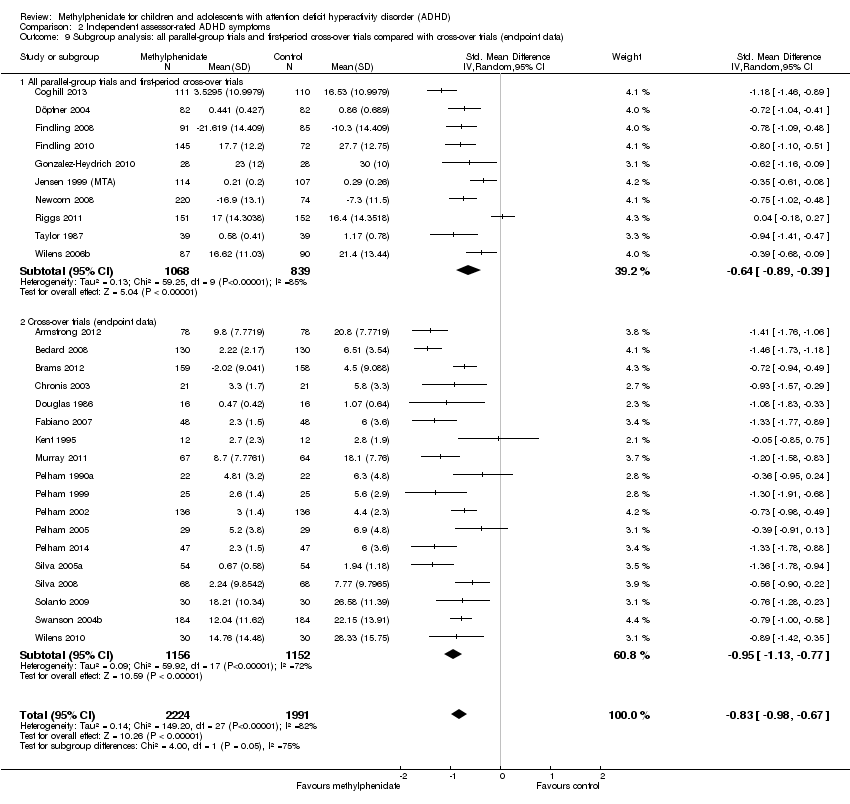
Comparison 2 Independent assessor‐rated ADHD symptoms, Outcome 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data).
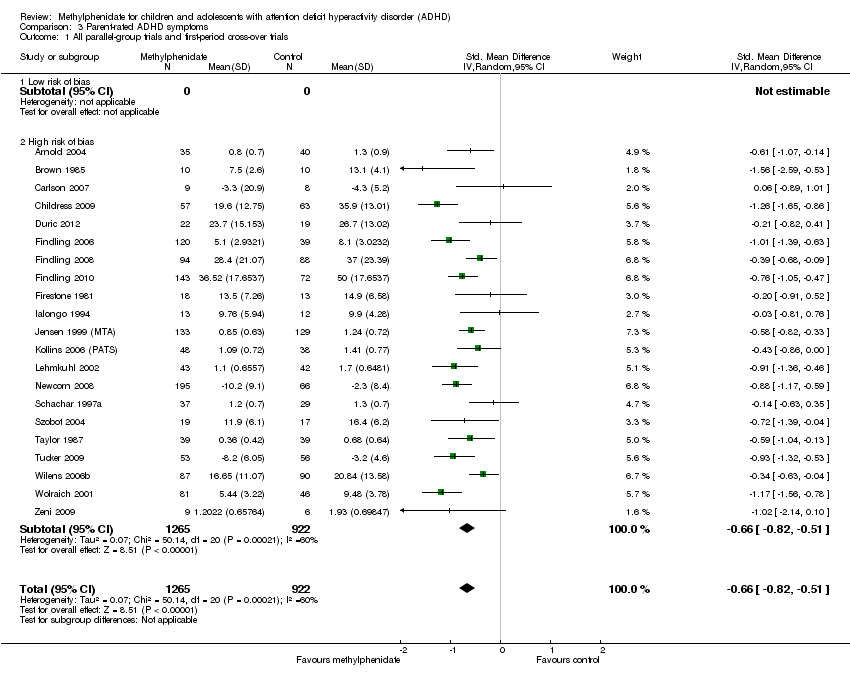
Comparison 3 Parent‐rated ADHD symptoms, Outcome 1 All parallel‐group trials and first‐period cross‐over trials.

Comparison 3 Parent‐rated ADHD symptoms, Outcome 2 Subgroup analysis: types of scales.

Comparison 3 Parent‐rated ADHD symptoms, Outcome 3 Subgroup analysis: duration of treatment.
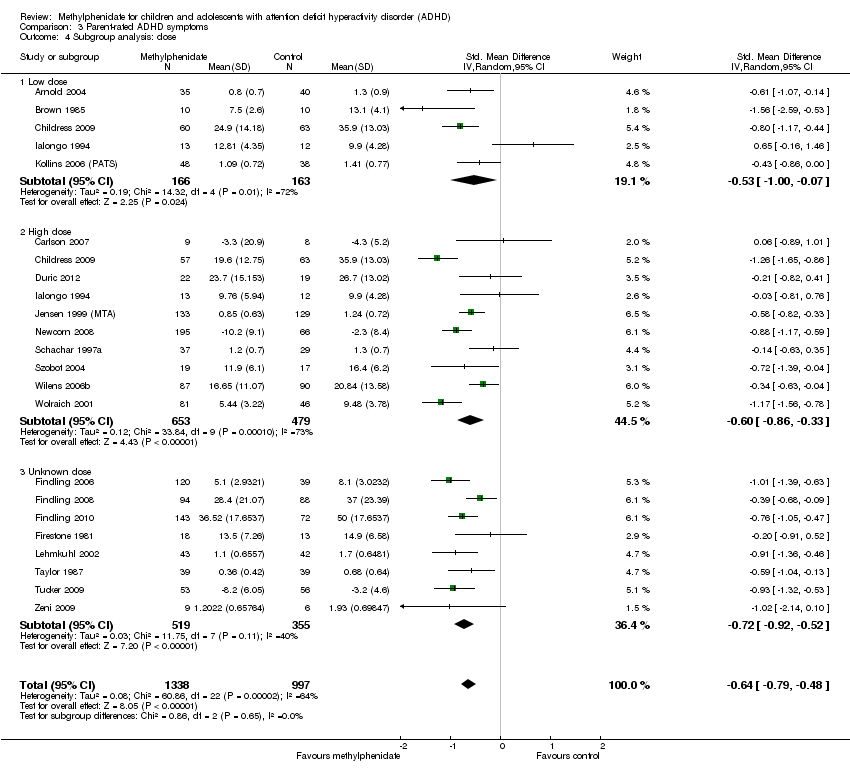
Comparison 3 Parent‐rated ADHD symptoms, Outcome 4 Subgroup analysis: dose.

Comparison 3 Parent‐rated ADHD symptoms, Outcome 5 Medication status: medication naive versus not medication naive.

Comparison 3 Parent‐rated ADHD symptoms, Outcome 6 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants.

Comparison 3 Parent‐rated ADHD symptoms, Outcome 7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials.

Comparison 3 Parent‐rated ADHD symptoms, Outcome 8 ADHD symptoms, cross‐over trials (endpoint data).
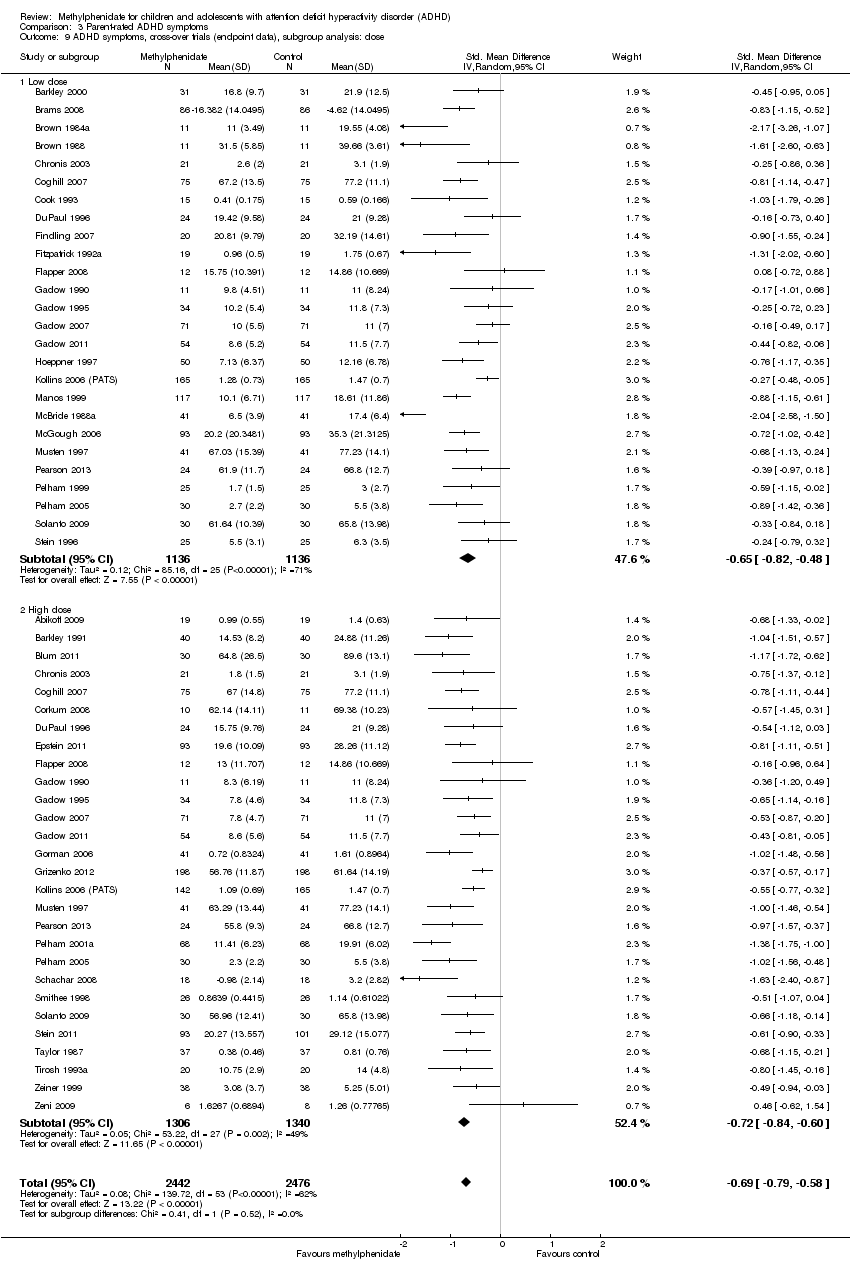
Comparison 3 Parent‐rated ADHD symptoms, Outcome 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose.
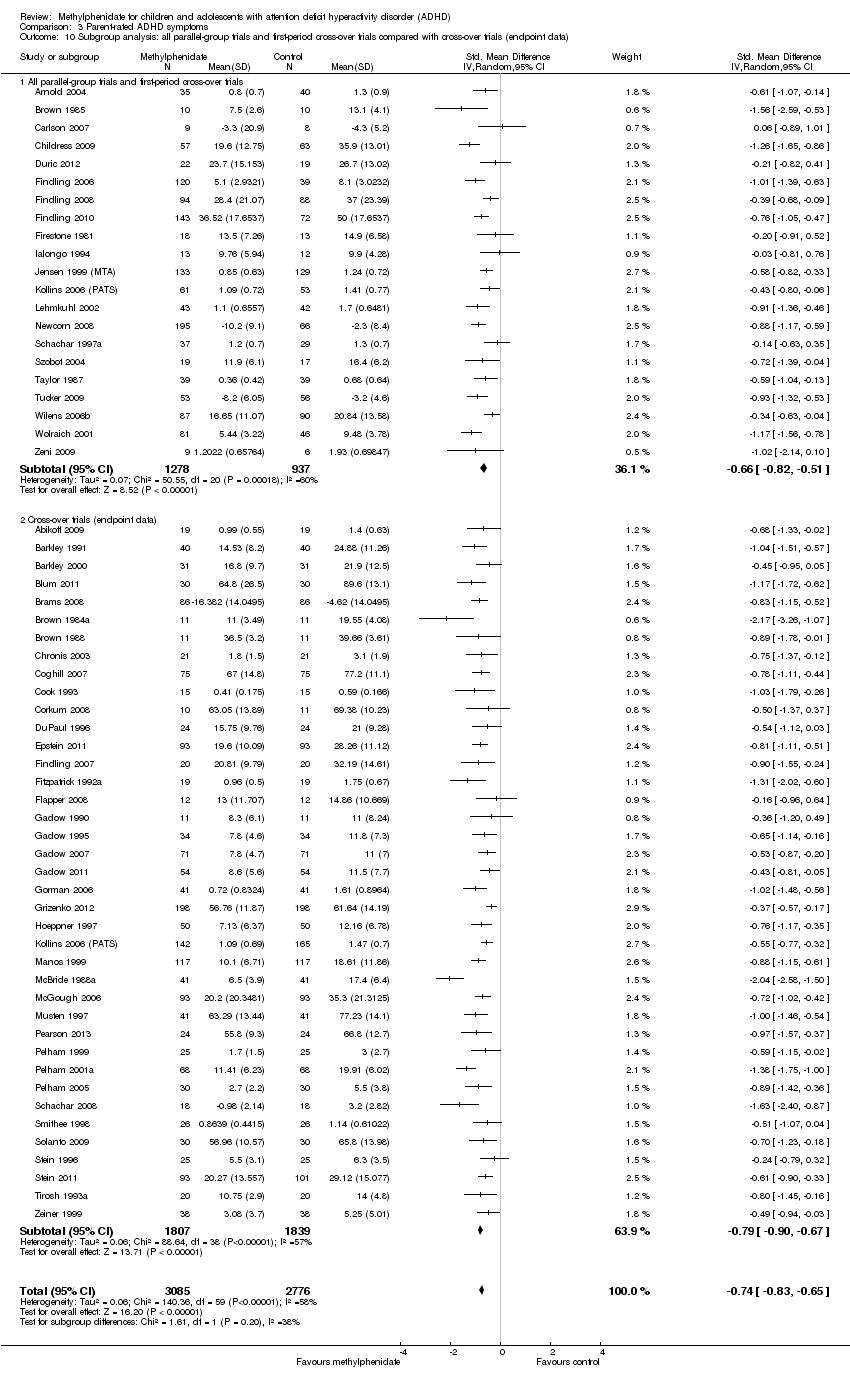
Comparison 3 Parent‐rated ADHD symptoms, Outcome 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data).

Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Comparision of raters.

Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Age.

Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Comorbidity versus no comorbidity.
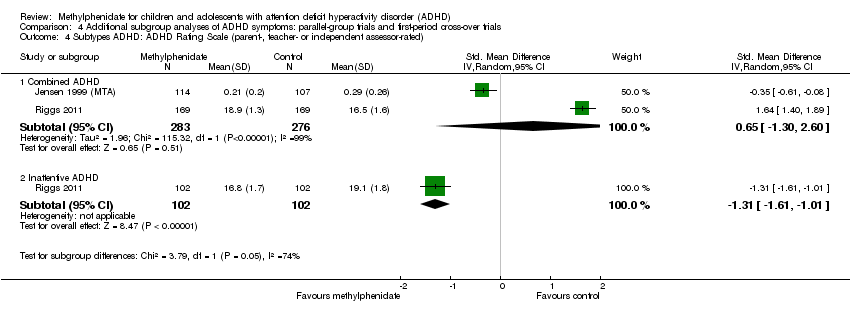
Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 4 Subtypes ADHD: ADHD Rating Scale (parent‐, teacher‐ or independent assessor‐rated).

Comparison 4 Additional subgroup analyses of ADHD symptoms: parallel‐group trials and first‐period cross‐over trials, Outcome 5 Cross‐over trials: first‐period data versus endpoint data (parent‐, independent assessor‐ and teacher‐rated).

Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Number of serious adverse events (SAE).
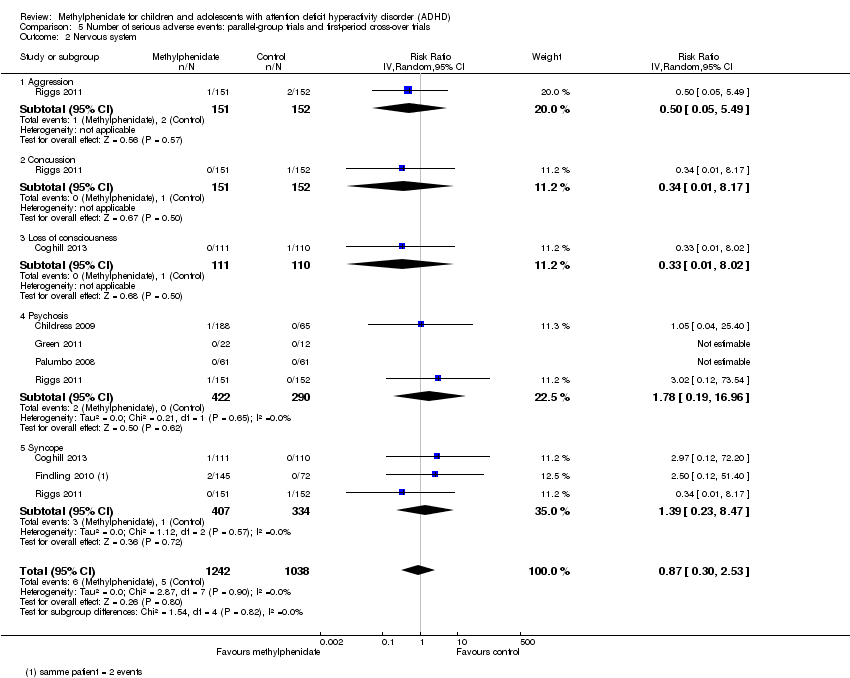
Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Nervous system.

Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Digestive system: gastrointestinal disorders.

Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 4 Urinary system: kidney infection.
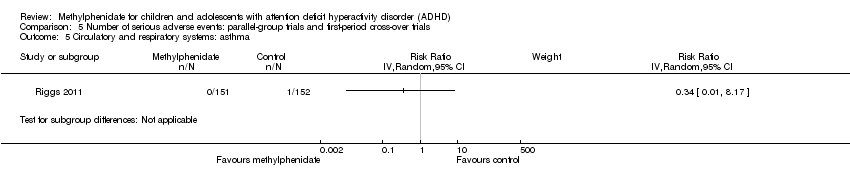
Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 5 Circulatory and respiratory systems: asthma.

Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 6 Immune system: cyst rupture.

Comparison 5 Number of serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 7 Other: drug toxicity.

Comparison 6 Number of serious adverse events: cross‐over trials (endpoint data), Outcome 1 Number of serious adverse events (SAE).

Comparison 6 Number of serious adverse events: cross‐over trials (endpoint data), Outcome 2 Hallucinations/psychosis.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Total number of non‐serious adverse events.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Subgroup analysis: total number of non‐serious adverse events according to dose.
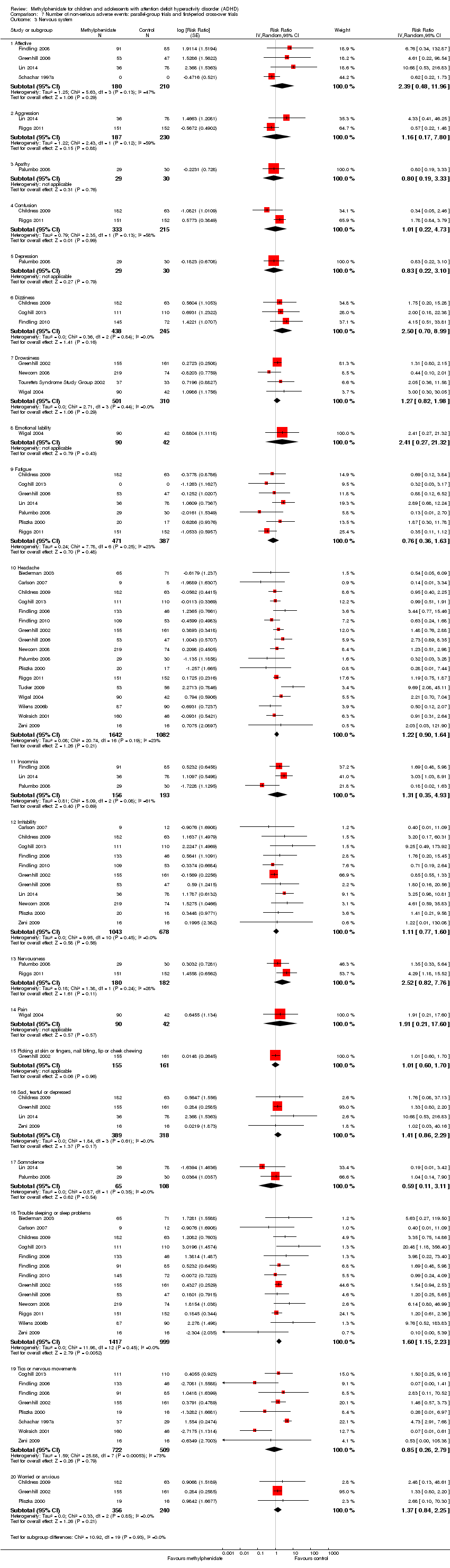
Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Nervous system.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 4 Digestive system.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 5 Circulatory and respiratory systems.
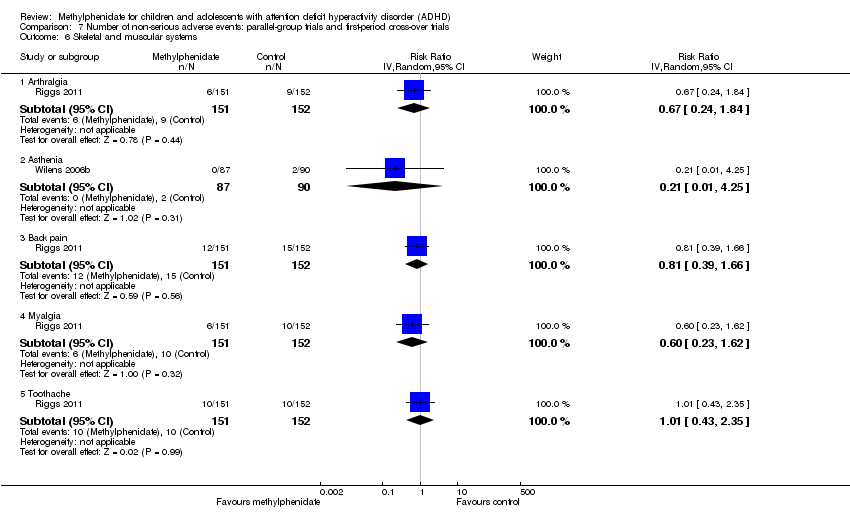
Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 6 Skeletal and muscular systems.
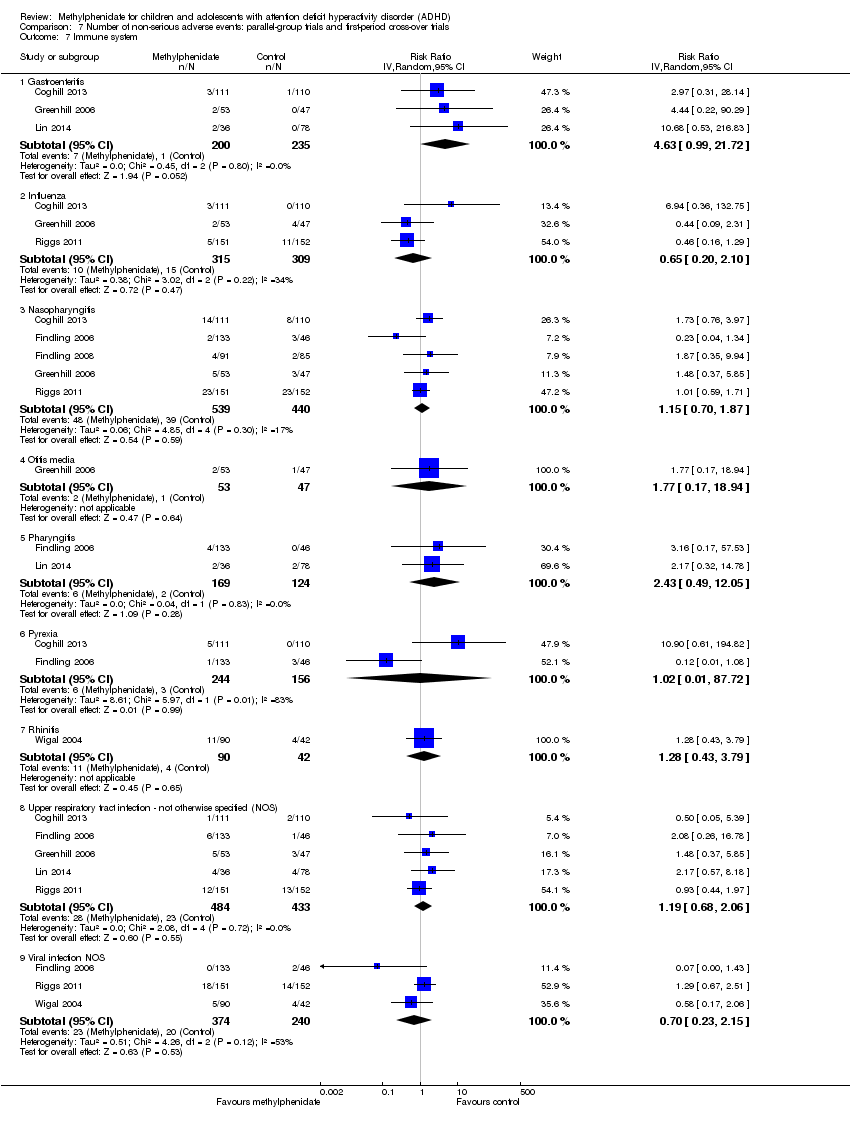
Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 7 Immune system.
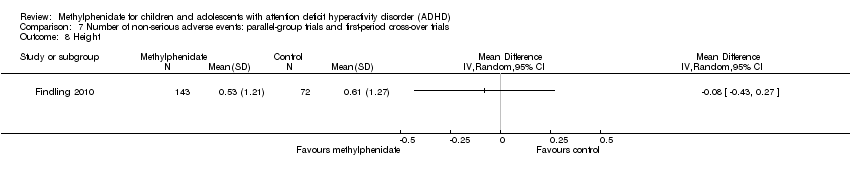
Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 8 Height.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 9 Weight.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 10 BMI.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 11 Vital signs.

Comparison 7 Number of non‐serious adverse events: parallel‐group trials and first‐period cross‐over trials, Outcome 12 Other.

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 1 Total number of non‐serious adverse events.

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 2 Subgroup analysis: total number of non‐serious adverse events according to dose.
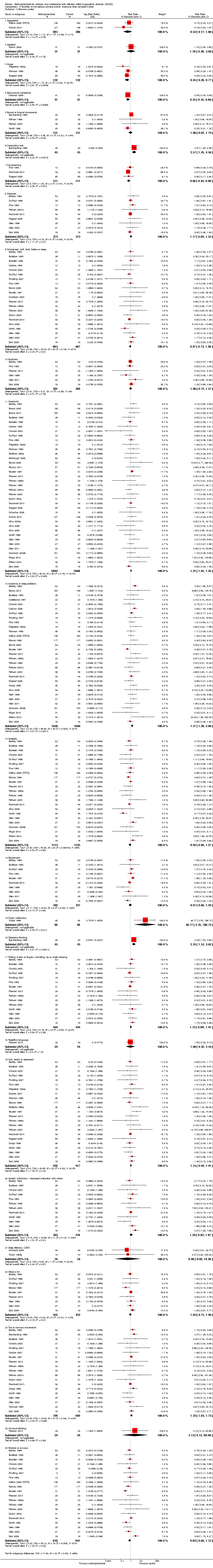
Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 3 Nervous system.

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 4 Digestive system.

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 5 Urinary system: urinary incontinence.
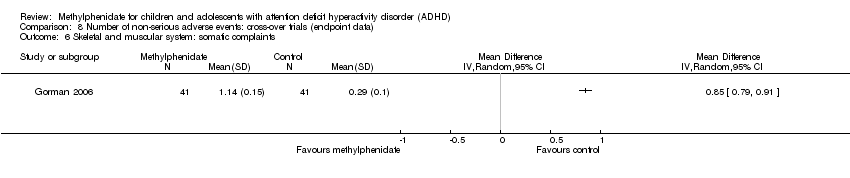
Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 6 Skeletal and muscular system: somatic complaints.

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 7 Immune system.
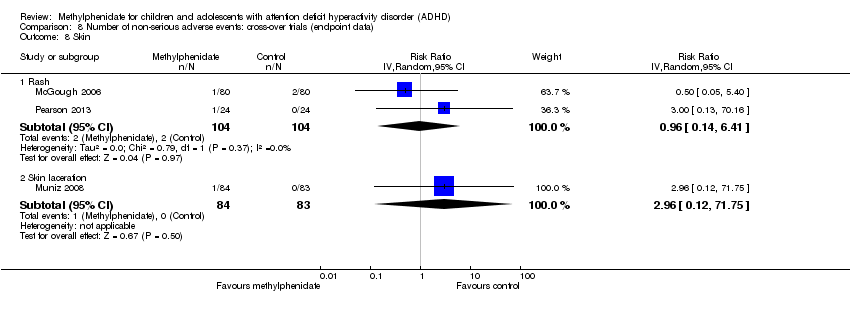
Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 8 Skin.

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 9 Vital signs.
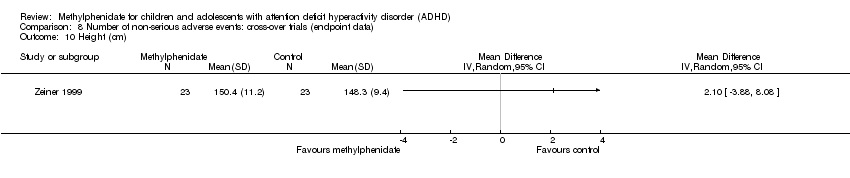
Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 10 Height (cm).

Comparison 8 Number of non‐serious adverse events: cross‐over trials (endpoint data), Outcome 11 Weight.

Comparison 9 Teacher‐rated general behaviour, Outcome 1 All parallel‐group trials and first‐period cross‐over trials: risk of bias.

Comparison 9 Teacher‐rated general behaviour, Outcome 2 Subgroup analysis: types of scales.

Comparison 9 Teacher‐rated general behaviour, Outcome 3 Subgroup analysis: dose.

Comparison 9 Teacher‐rated general behaviour, Outcome 4 Subgroup analysis: duration of treatment.
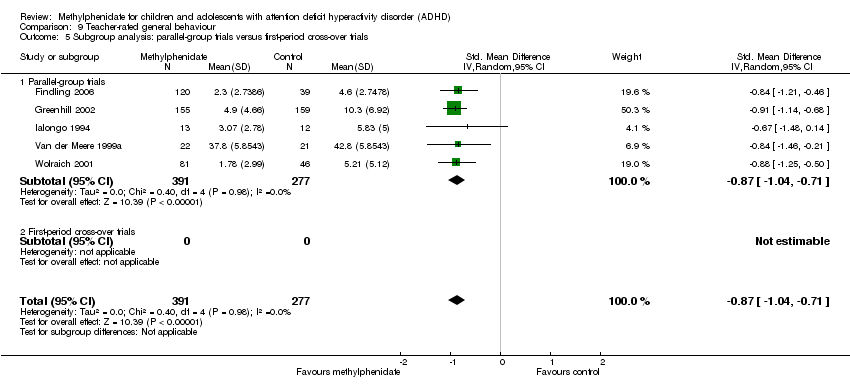
Comparison 9 Teacher‐rated general behaviour, Outcome 5 Subgroup analysis: parallel‐group trials versus first‐period cross‐over trials.

Comparison 9 Teacher‐rated general behaviour, Outcome 6 General behaviour, cross‐over trials (endpoint data).

Comparison 9 Teacher‐rated general behaviour, Outcome 7 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias.

Comparison 9 Teacher‐rated general behaviour, Outcome 8 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (teacher‐rated) versus cross‐over trials (endpoint data).

Comparison 10 Independent assessor‐rated general behaviour, Outcome 1 General behaviour, cross‐over trials (endpoint data).
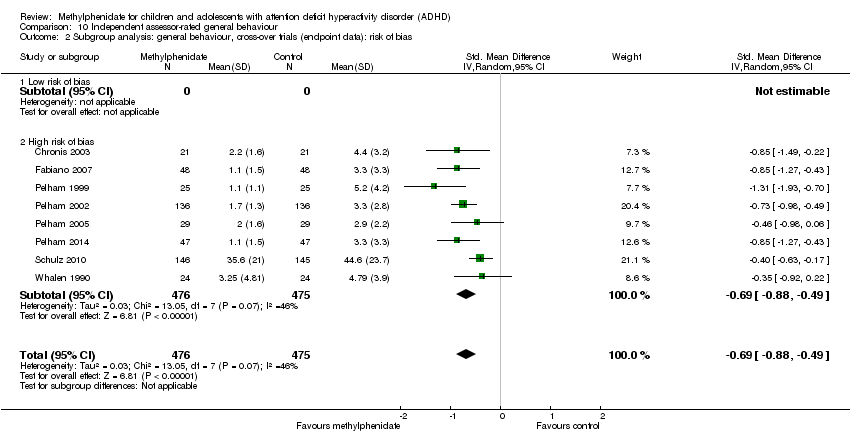
Comparison 10 Independent assessor‐rated general behaviour, Outcome 2 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias.
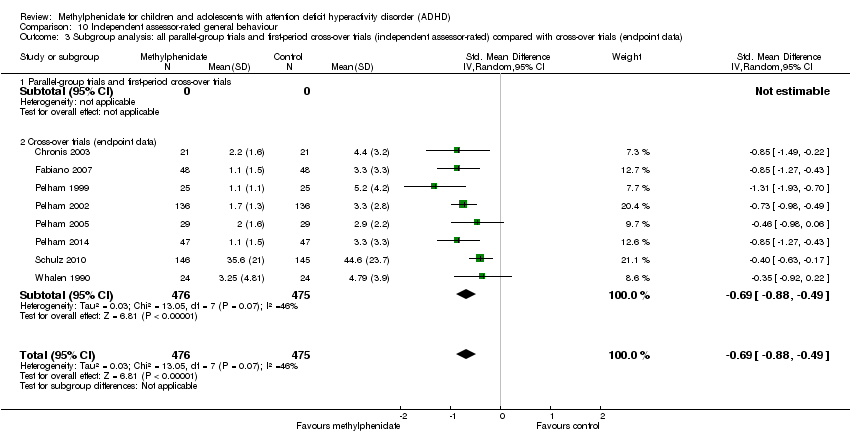
Comparison 10 Independent assessor‐rated general behaviour, Outcome 3 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (independent assessor‐rated) compared with cross‐over trials (endpoint data).

Comparison 11 Parent‐rated general behaviour, Outcome 1 All parallel‐group trials and first‐period cross‐over trials.
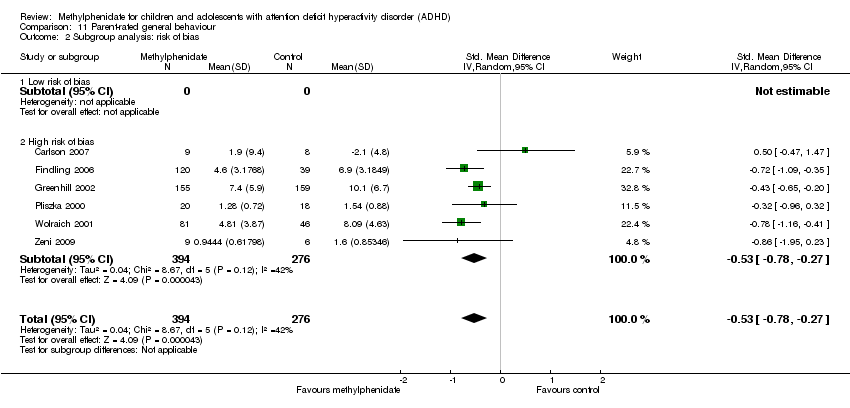
Comparison 11 Parent‐rated general behaviour, Outcome 2 Subgroup analysis: risk of bias.

Comparison 11 Parent‐rated general behaviour, Outcome 3 Subgroup analysis: types of scales.

Comparison 11 Parent‐rated general behaviour, Outcome 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials.

Comparison 11 Parent‐rated general behaviour, Outcome 5 Subgroup analysis: duration of treatment.

Comparison 11 Parent‐rated general behaviour, Outcome 6 Subgroup analysis: dose.

Comparison 11 Parent‐rated general behaviour, Outcome 7 General behaviour, cross‐over trials (endpoint data).
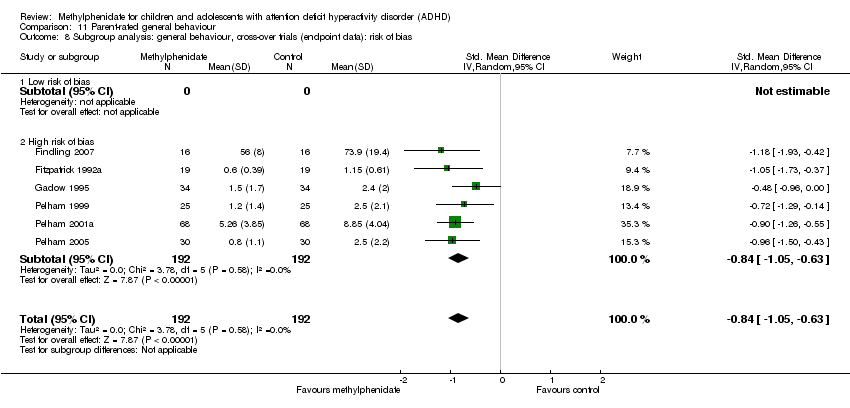
Comparison 11 Parent‐rated general behaviour, Outcome 8 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias.

Comparison 11 Parent‐rated general behaviour, Outcome 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (parent‐rated) compared with cross‐over trials (endpoint data).
Comparison 12 Additional subgroup analyses of general behaviour: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Comparisions of raters.

Comparison 12 Additional subgroup analyses of general behaviour: parallel‐group trials and first‐period cross‐over trials, Outcome 2 Comorbidity versus no comorbidity.

Comparison 12 Additional subgroup analyses of general behaviour: parallel‐group trials and first‐period cross‐over trials, Outcome 3 Cross‐over trials: first‐period data versus endpoint data (teacher‐, parent‐, and independent assessor‐rated).

Comparison 13 Quality of life: parallel‐group trials and first‐period cross‐over trials, Outcome 1 Subgroup analysis: types of scales.
| Methylphenidate compared with placebo or no intervention for ADHD | ||||||
| Patient or population: children and adolescents (up to and including 18 years of age) with ADHD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Methylphenidate | |||||
| ADHD symptoms: all parallel‐group trials and first‐period cross‐over trials Average study duration: 74.8 days | Mean ADHD symptom score in the intervention groups corresponds to a mean difference of 9.6 (95% CI 11.25 to 8.00) on ADHD Rating Scale | SMD ‐0.77 (‐0.90 to ‐0.64) | 1698 | ⊕⊝⊝⊝ | The analysis was conducted on a standardised scale with data from studies that used different teacher‐rated scales of symptoms (Conners' Teacher Rating Scale (CTRS), Strengths and Weaknesses of ADHD Symptoms and Normal Behaviour (SWAN) Scale, Schedule for Non‐adaptive and Adaptive Personality (SNAP) ‐ Teacher, Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS)). The effect size has been translated on to the ADHD Rating Scale from the SMD | |
| Total number of serious adverse events | Trial population | RR 0.98 | 1532 | ⊕⊝⊝⊝ | ‐ | |
| 16 per 1000 | 16 per 1000 | |||||
| Total number of non‐serious adverse events | Trial population | RR 1.29 | 3132 | ⊕⊝⊝⊝ | ‐ | |
| 408 per 1000 | 526 per 1000 | |||||
| General behaviour: all parallel‐group trials and first‐period cross‐over trials | Mean general behaviour score in the intervention groups was 0.87 standard mean deviations lower (95% CI 1.04 to 0.71 lower) | SMD ‐0.87 (‐0.71 to ‐1.04) | 668 | ⊕⊝⊝⊝ | ‐ | |
| Quality of life (Parent‐rated) | Mean quality of life score in the intervention groups corresponds to a mean difference of 8.0 (95% CI 5.49 to 10.46) on the Child Health Questionnaire | SMD 0.61 | 514 | ⊕⊝⊝⊝ | ‐ | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded two levels due to high risk of bias (systematic errors causing overestimation of benefits and underestimation of harms) in several risk of bias domains, including lack of sufficient blinding and selective outcome reporting (many of the included trials did not report on this outcome). | ||||||
| Name of scale | Abbreviation | Reference |
| Abbreviated Conners’ Rating Scales, Parent (ACPRS) and Teacher (ACTRS), including Abbreviated Parent Rating Scale (APRS) and Teacher Rating Scale, Hyperkinesis Index and ADHD and Emotional Lability subscales | ACRS | |
| Abbreviated Symptom Questionnaire, including ASQ Teacher and ASQ Parent | ASQ | |
| Academic Performance Rating Scale | APRS | |
| The ADD/H Comprehensive Teacher Rating Scale | ACTeRS | |
| ADHD/ODD Rating Scale, Parent‐ and Teacher‐Rated | ADHD‐RS | |
| ADHD Rating Scale, including ADHD Rating Scale Parent and Teacher Ratings | ADHD‐RS | |
| ADHD Rating Scale‐IV, including ADHD Rating Scale‐IV Parent and Teacher Versions | ADHD‐RS‐IV | |
| Brief Psychiatric Rating Scale for Children | BPRS | |
| Child Attention Problems Rating Scale | CAP | |
| Child Attention Profile | CAP | |
| Child Behavior Rating Form | NCBHF | |
| Child Symptom Inventory | CSI | |
| Children’s Psychiatric Rating Scale | CPRS | |
| Conners’ Abbreviated Hyperactivity Questionnaire | C‐HI | |
| Conners’ Abbreviated Questionnaire | ASQ | |
| Conners’ Abbreviated Parent Teacher Questionnaire | APTQ | |
| Conners’ Abbreviated Rating Scale | ABRS | |
| Conners’ Abbreviated Symptom Questionnaire | ASQ | |
| Conners Abbreviated Symptom Questionnaire for Parents | ASQ‐Parent | |
| Conners’ Abbreviated Symptom Questionnaire for Teachers | ASQ‐Teacher | |
| Conners’ Abbreviated Teacher Rating Scale | ABTRS | |
| Conners’ ADHD/DSM‐IV Scales Adolescent | CADS‐A | |
| Conners’ ADHD/DSM‐IV Scales Parent | CADS–P, CADS‐P DSM‐IV | |
| Conners’ ADHD/DSM‐IV Scale Teacher, including Inattentive and Hyperactive‐Impulsive subscales | CADS‐T, CADS‐T DSM‐IV | |
| Conners’ Rating Scale ‐ Revised, Parent and Teacher: Hyperactivity and Conduct Factors score | CPRS‐R and CTRS‐R | |
| Conners’ Hyperactivity Index, Parent and Teacher, including abbreviated versions | CPRS/CTRS‐Hyperactivity index | |
| Conners’ Hyperkinesis Index | ‐ | |
| Conners, Loney and Milich Scale | CLAM | |
| Conners’ Parent and Teacher Rating Scale ‐ Revised, Short Form | CRS‐R:S | |
| Conners’ Parent Rating Scale, including abbreviated versions | CPRS | |
| Conners’ Parent Rating Scale ‐ Revised | CPRS‐R | |
| Conners’ Parent Rating Scale ‐ Revised, Short Form | CPRS‐R:S | |
| Conners’ Parent Rating Scale ‐ Revised, Long Version | CPRS‐R:L | |
| Conners’ Rating Scale ‐ Revised | CRS‐R | |
| Conners’ Short Form Rating Scale, Parent and Teacher | ‐ | |
| Conners’ Teacher Rating Scale | CTRS | |
| Conners’ Teacher Rating Scale ‐ Revised, Long Version | CTRS‐R:L | |
| Diagnostic and Statistical Manual of Mental Disorders Total | DSM‐IV | |
| Diagnostiksystem für Psychische Störungen im Kindes ‐ und Jugendalter nach ICD‐10 und DSM‐IV, Parental Questionnaire of ADHD symptoms | DISYPS | |
| Fremdbeurteilungsbogen für Hyperkinetische Störungen | FBB‐HKS | |
| German Teacher’s report on ADHD symptoms | FBB‐HKS of the DISYPS | |
| Hyperactivity Index of the Revised Conners Parent and Teacher Rating Scales | ‐ | |
| IOWA Conners Parent Rating Scale, including abbreviated versions | IOWA CPRS | |
| IOWA Conners Teacher Rating Scale, including abbreviated versions | IOWA CTRS | |
| IOWA Conners Teacher Rating Scale, Inattention/Overactivity (I/O) and Oppositional/Defiant (O/D) subscales | IOWA‐I/O and O/D subscales | |
| IOWA Inattention/Overactivity and Aggression/Noncompliance scales ‐ Parent and Teacher rating | IOWA | |
| Lehrer‐Fragenbogen von Steinhausen | LF | |
| Loney’s Time on Task Scale, Hyperactivity, Attention and Aggression subscales | TOTS | |
| Modified Conner Scale Parent and Teacher | ACR | |
| Mothers’ Objective Method for Subgrouping | MOMS | |
| Parent Symptom Checklist | PSC ADHD | |
| Parental Account of Children’s Symptoms | PACS | |
| Restricted Academic Situation Scale | RASS | |
| Schedule for Affective Disorders and Schizophrenia | K‐SADS/ K‐SADS‐E for diagnosis | |
| Schedule for Non‐adaptive and Adaptive Personality | SNAP | |
| Swanson, Nolan, and Pelham ‐ IV SNAP‐ADHD Rating scale | SNAP‐ADHD | |
| Swanson, Nolan, and Pelham ‐ IV SNAP‐IV (Brazilian Version) | SNAP‐IV | |
| Swanson, Kotkin, Atkins, M‐Flynn, Pelham Scale (SKAMP combined, SKAMP attention, and SKAMP deportment) | SKAMP (SKAMP combined, SKAMP attention, and SKAMP deportment) | |
| Teacher Self‐control Rating Scale | SCRS | |
| Turgay ‐ DSM‐IV Scale, Parent | T‐DSM‐IV Scale, Parent | |
| Turgay ‐ DSM‐IV Scale, Teacher | T‐DSM‐IV Scale, Teacher | |
| Teacher Hyperactivity Index | THI | |
| Teacher Symptom Checklist | TSC | |
| Vanderbilt ADHD Rating Scale | VADP(T)RS | |
| Wender Utah Rating Scale | WURS | |
| Wide Range Achievement Test | WRAT‐4 | |
| Wide Range Achievement Test Revised | WRAT‐R | |
| ADD/H: Attention deficit disorder with hyperactivity. | ||
| Name of scale | Abbreviation | Reference |
| Achenbach Child Behaviour Checklist | CBCL | |
| Achenbach’s Teacher Report | ATRF | |
| ADHD Rating Scale | ADHD‐RS | |
| ADHD School Observation Code | ADHD‐SOC | |
| Barkley Scales, Disruptive Behavior Disorders Rating Scale | ‐ | |
| Child Attention Problems Scale | CAP | |
| Child Attention Profile | CAP | |
| Child Behavior Checklist | CBCL | |
| Child Health Questionnaire | CHQ | |
| Child and Adolescent Psychiatric Assessment, selected items | CAPA | |
| Children’s Psychiatric Rating Scale | CPRS | |
| Classroom Observation Code (Abikoff Classroom Observational System) | COC | |
| Code for Observing Social Activity | COSA | |
| Conners' Child Behavior Scale | UC‐CCBS | |
| Conners' Global Index Scale | CGI‐S | |
| Conners’ Global Index ‐ Parent | CGI‐P | |
| Conners' Global Index ‐ Teacher | CGI‐T | |
| Conners', Loney and Milich Scale | CLAM | |
| Conners’ Parent Questionnaire | CPQ | |
| Conners’ Parent Rating Scale | CPRS | |
| Conners’ Teacher Rating Scale | CTRS | |
| Conners’ Teacher Rating Conduct Problems | ‐ | |
| Disruptive Behavior Disorders Rating Scale, Parent‐ and Teacher‐Rated | DBS | |
| Disruptive Behavior Disorders Rating Scale | DBD | |
| Groninger Behaviour Observation Scale | GOO and GBO | |
| Groninger Behaviour Checklists, Parent and Teacher Versions of the abbreviated Groninger | GGGS and GGBS | |
| Hillside Behavior Rating Scale | HBRS | |
| Home Situations Questionnaire | HSQ | |
| Home Situations Questionnaire ‐ Revised | HSQ‐R | |
| Humphrey’s Teacher Self‐Control Rating Scale | TSCRS | |
| Hyperactivity Index from the Conners Revised Teacher Rating Scale | CTRS‐R‐Hyperactivity Index | |
| Impairment Rating Scale | IRS | |
| Inpatient Global Rating Scale, Revised | IGRS | |
| Inpatient Global Rating Scale, Somatic factor | IGRS‐S | |
| IOWA Conners' Rating Scale, Oppositional/Defiant (O/D) subscales | IOWA‐O/D subscales | |
| Nisonger Child Behavior Rating Form | NCBRF | |
| Paired Associates Learning | PAL | |
| Parent Global Assessment for Improvement | PGA | |
| Peer Conflict Scale | PCS | |
| Personality Inventory for Children | PIC | |
| School Situations Questionnaire | SSQ | |
| School Situations Questionnaire ‐ Revised | SSQ‐R | |
| Schedule for Nonadaptive and Adaptive Personality | SNAP | |
| Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Scale, Parent and Teacher | SWAN | |
| Subjective Treatment Emergent Symptom Scale | STESS‐R | |
| Swanson, Nolan and Pelham, Fourth Edition | SNAP‐IV | |
| Teachers Report Form | TRF | |
| Telephone Interview Probe (Parent and Teacher) | TIP | |
| Vanderbilt ADHD rating scales: Vanderbilt ADHD Diagnostic Parent Rating Scale and Vanderbilt ADHD Diagnostic Teacher Rating Scale | VADPRS and VADTRS | |
| Wahler, House and Stambaugh’s Ecobehavioral Assessment System | ECO | |
| The Weekly Parent Ratings of Evening and Morning Behaviour | WREMB‐R | |
| Werry‐Weiss‐Peters Activity Rating Scale | WWP | |
| Woodcock‐Johnson Achievement Battery | WJ‐III Ach | |
| ADHD: attention deficit hyperactivity disorder. | ||
| Name of scale | Abbreviation | Reference |
| ADHD Impact Module‐Child | AIM‐C | |
| Child Impact Scale and Home Impact Scale | CIS/HIS | |
| Child Health and Illness Profile, Child Edition: Parent Report Form | CHIP‐CE:PRF | |
| Child Health Questionnaire | CHQ‐P | |
| Children's Global Assessment Scale | CGAS | |
| Comprehensive Psychopathological Rating Scale | CPRS | |
| Health Utilities Index ‐ 2 | HUI‐2 | |
| ADHD: attention deficit hyperactivity disorder. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 2 Subgroup analysis: types of scales Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Conners' Teacher Rating Scale (CTRS) | 8 | 518 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.82, ‐0.47] |
| 2.2 Abbreviated Conners' Rating Scale (ACRS) ‐ Teacher | 2 | 105 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.79, 0.29] |
| 2.3 Conners' Abbreviated Symptom Questionnaire for Teachers (ASQ‐Teacher) | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.79, 0.23] |
| 2.4 IOWA Conners' Teacher Rating Scale (IOWA CTRS) ‐ hyperactivity | 2 | 193 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐1.39, ‐0.77] |
| 2.5 Schedule for Non‐adaptive and Adaptive Personality (SNAP) ‐ Teacher | 2 | 328 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.25] |
| 2.6 Teacher ratings of attention | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.45, 0.35] |
| 2.7 Teacher ratings of impulsivity | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.83, 0.92] |
| 2.8 IOWA Conners' Teacher Rating Scale ‐ Inattention/Overactivity (IOWA‐I/O) | 2 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.36, ‐0.69] |
| 2.9 Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.52, ‐0.61] |
| 2.10 Conners’ ADHD/DSM‐IV Scales ‐ Teacher (CADS‐T) | 2 | 254 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.31, ‐0.78] |
| 2.11 Strengths and Weaknesses of ADHD Symptoms and Normal Behaviour (SWAN) Scale | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.82, 0.17] |
| 3 Medication status: medication naive versus not medication naive Show forest plot | 6 | 717 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.08, ‐0.50] |
| 3.1 Medication naive | 4 | 431 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.94, ‐0.31] |
| 3.2 Not medication naive | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.33, ‐0.79] |
| 4 Subgroup analysis: duration of treatment Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 4.1 Short term (up to 6 months) | 18 | 1445 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.94, ‐0.68] |
| 4.2 Long term (over 6 months) | 1 | 253 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.72, ‐0.22] |
| 5 Subgroup analysis: dose Show forest plot | 19 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Low dose | 8 | 493 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.82, ‐0.46] |
| 5.2 High dose | 7 | 688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.08, ‐0.54] |
| 5.3 Unknown dose | 6 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.06, ‐0.68] |
| 6 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 6.1 Parallel‐group trials | 17 | 1506 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.95, ‐0.65] |
| 6.2 First‐period cross‐over trials | 2 | 192 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.87, ‐0.29] |
| 7 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants Show forest plot | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 7.1 Trials with cohort selection bias of all participants | 7 | 994 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.01, ‐0.57] |
| 7.2 Trials without cohort selection bias of all participants | 12 | 704 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.93, ‐0.59] |
| 8 ADHD symptoms, cross‐over trial (endpoint data) Show forest plot | 59 | 5145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.06, ‐0.80] |
| 8.1 Low risk of bias | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.97, ‐0.30] |
| 8.2 High risk of bias | 55 | 4941 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.09, ‐0.82] |
| 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose Show forest plot | 59 | 6821 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐0.96, ‐0.74] |
| 9.1 Low dose | 42 | 3408 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐0.89, ‐0.57] |
| 9.2 High dose | 36 | 3413 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.13, ‐0.84] |
| 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) Show forest plot | 75 | 6344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.01, ‐0.80] |
| 10.1 All parallel‐group trials and first‐period cross‐over trials | 19 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.90, ‐0.64] |
| 10.2 Cross‐over trials (endpoint data) | 56 | 4646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.09, ‐0.82] |
| 11 All parallel‐group trials and cross‐over trials: risk of bias Show forest plot | 75 | 6344 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.01, ‐0.80] |
| 11.1 Low risk of bias | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.97, ‐0.30] |
| 11.2 High risk of bias | 71 | 6140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.03, ‐0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 2 Subgroup analysis: types of scales Show forest plot | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Swanson, Kotkin, Agler, M‐Glynn and Pelham (SKAMP) Scale | 1 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.04, ‐0.41] |
| 2.2 ADHD Rating Scale (ADHD‐RS ) | 7 | 1442 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.09, ‐0.32] |
| 2.3 Swanson, Nolan and Pelham (SNAP) Scale | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.61, ‐0.08] |
| 2.4 Unknown | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐1.41, ‐0.47] |
| 3 Subgroup analysis: duration of treatment Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 3.1 Short term (up to 6 months) | 9 | 1686 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.95, ‐0.40] |
| 3.2 Long term (over 6 months) | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.61, ‐0.08] |
| 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 4.1 Parallel‐group trials | 7 | 1609 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.91, ‐0.28] |
| 4.2 First‐period cross‐over trials | 3 | 298 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐0.99, ‐0.52] |
| 5 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 5.1 Trials with cohort selection bias of all participants | 4 | 630 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.73, ‐0.29] |
| 5.2 Trials without cohort selection bias of all participants | 6 | 1277 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.11, ‐0.33] |
| 6 Subgroup analysis: dose Show forest plot | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 6.1 Low dose | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High dose | 6 | 1380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.91, ‐0.20] |
| 6.3 Unknown dose | 4 | 527 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐0.98, ‐0.61] |
| 7 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: risk of bias Show forest plot | 19 | 2471 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.16, ‐0.84] |
| 7.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 High risk of bias | 19 | 2471 | Std. Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.16, ‐0.84] |
| 8 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose Show forest plot | 19 | 3874 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.02, ‐0.75] |
| 8.1 Low dose | 16 | 2021 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.82, ‐0.55] |
| 8.2 High dose | 12 | 1853 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐1.31, ‐0.95] |
| 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) Show forest plot | 28 | 4215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐0.98, ‐0.67] |
| 9.1 All parallel‐group trials and first‐period cross‐over trials | 10 | 1907 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.89, ‐0.39] |
| 9.2 Cross‐over trials (endpoint data) | 18 | 2308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.13, ‐0.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 2 Subgroup analysis: types of scales Show forest plot | 21 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Conners' Parent Rating Scale (CPRS) | 7 | 751 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.87, ‐0.30] |
| 2.2 ADHD Rating Scale ‐ Fourth Edition (ADHD‐RS‐IV) | 2 | 194 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.58, ‐0.02] |
| 2.3 Fremdbeurteilungsbogen für Hyperkinetische Störungen (FBB‐HKS) | 1 | 85 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.36, ‐0.46] |
| 2.4 Conners’ ADHD/DSM‐IV Scales ‐ Parent (CADS‐P) | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.65, ‐0.86] |
| 2.5 CADS‐P Inattentive subscale | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.17, ‐0.39] |
| 2.6 CADS‐P Hyperactivity subscale | 1 | 109 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.32, ‐0.53] |
| 2.7 Clinican's Manual for the Assesment of Disruptive Behavior Disorders Rating Scale for Parents (Barkley) | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.82, 0.41] |
| 2.8 Abbreviated Conners' Rating Scale (ACRS) ‐ Parent | 2 | 121 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.99, ‐0.25] |
| 2.9 Swanson, Nolan, and Pelham, Fourth Edition ‐ Parent (SNAP‐IV‐Parent) Scale | 4 | 430 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.79, ‐0.40] |
| 2.10 Strengths and Weaknesses of ADHD Symptoms and Normal Behavior (SWAN) Scale | 1 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.86, 0.00] |
| 2.11 IOWA Conners' Rating Scale ‐ Inattention/Overactivity (IOWA‐I/O) | 3 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.35, ‐0.21] |
| 3 Subgroup analysis: duration of treatment Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 3.1 Short term (up to 6 months) | 20 | 1925 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐0.84, ‐0.50] |
| 3.2 Long term (over 6 months) | 1 | 262 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.82, ‐0.33] |
| 4 Subgroup analysis: dose Show forest plot | 21 | 2335 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.79, ‐0.48] |
| 4.1 Low dose | 5 | 329 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [1.00, ‐0.07] |
| 4.2 High dose | 10 | 1132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.86, ‐0.33] |
| 4.3 Unknown dose | 8 | 874 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.92, ‐0.52] |
| 5 Medication status: medication naive versus not medication naive Show forest plot | 7 | 795 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.05, ‐0.48] |
| 5.1 Medication naive | 4 | 492 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.03, ‐0.35] |
| 5.2 Not medication naive | 3 | 303 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.33, ‐0.42] |
| 6 Subgroup analysis: trials with cohort selection bias of all participants compared with trials without cohort selection bias of all participants Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 6.1 Trials with selection bias of all participants | 10 | 1559 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.86, ‐0.51] |
| 6.2 Trials without cohort selection bias of all participants | 11 | 628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐0.90, ‐0.33] |
| 7 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 21 | 2187 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 7.1 Parallel‐group trials | 19 | 2094 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.83, ‐0.50] |
| 7.2 First‐period cross‐over trials | 2 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.07, ‐0.23] |
| 8 ADHD symptoms, cross‐over trials (endpoint data) Show forest plot | 41 | 3734 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.90, ‐0.67] |
| 8.1 Low risk of bias | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.96, ‐0.13] |
| 8.2 High risk of bias | 37 | 3530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐0.92, ‐0.69] |
| 9 ADHD symptoms, cross‐over trials (endpoint data), subgroup analysis: dose Show forest plot | 41 | 4918 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.79, ‐0.58] |
| 9.1 Low dose | 26 | 2272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.82, ‐0.48] |
| 9.2 High dose | 28 | 2646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.84, ‐0.60] |
| 10 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials compared with cross‐over trials (endpoint data) Show forest plot | 59 | 5861 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐0.83, ‐0.65] |
| 10.1 All parallel‐group trials and first‐period cross‐over trials | 21 | 2215 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.82, ‐0.51] |
| 10.2 Cross‐over trials (endpoint data) | 39 | 3646 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐0.90, ‐0.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparision of raters Show forest plot | 31 | 5697 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.79, ‐0.59] |
| 1.1 Teacher‐rated | 19 | 1689 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐0.93, ‐0.63] |
| 1.2 Independent assessor‐rated | 9 | 1829 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.87, ‐0.35] |
| 1.3 Parent‐rated | 21 | 2179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.81, ‐0.50] |
| 2 Age Show forest plot | 6 | 1039 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.74, ‐0.14] |
| 2.1 2 to 6 years | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.82, 0.17] |
| 2.2 7 to 11 years | 2 | 278 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.03, ‐0.15] |
| 2.3 12 to 18 years | 3 | 697 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.88, 0.12] |
| 3 Comorbidity versus no comorbidity Show forest plot | 20 | 2310 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.91, ‐0.53] |
| 3.1 ADHD with comorbidity | 18 | 1981 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐0.94, ‐0.51] |
| 3.2 ADHD without comorbidity | 2 | 329 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.92, ‐0.46] |
| 4 Subtypes ADHD: ADHD Rating Scale (parent‐, teacher‐ or independent assessor‐rated) Show forest plot | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Combined ADHD | 2 | 559 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐1.30, 2.60] |
| 4.2 Inattentive ADHD | 1 | 204 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐1.61, ‐1.01] |
| 5 Cross‐over trials: first‐period data versus endpoint data (parent‐, independent assessor‐ and teacher‐rated) Show forest plot | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 First‐period data | 4 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐0.85, ‐0.44] |
| 5.2 Endpoint data | 4 | 372 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.18, ‐0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of serious adverse events (SAE) Show forest plot | 9 | 1532 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.44, 2.22] |
| 2 Nervous system Show forest plot | 6 | 2280 | Risk Ratio (IV, Random, 95% CI) | 0.87 [0.30, 2.53] |
| 2.1 Aggression | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.50 [0.05, 5.49] |
| 2.2 Concussion | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| 2.3 Loss of consciousness | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 8.02] |
| 2.4 Psychosis | 4 | 712 | Risk Ratio (IV, Random, 95% CI) | 1.78 [0.19, 16.96] |
| 2.5 Syncope | 3 | 741 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.23, 8.47] |
| 3 Digestive system: gastrointestinal disorders Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4 Urinary system: kidney infection Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Circulatory and respiratory systems: asthma Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Immune system: cyst rupture Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Other: drug toxicity Show forest plot | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.01, 8.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of serious adverse events (SAE) Show forest plot | 8 | 1721 | Risk Ratio (IV, Random, 95% CI) | 1.62 [0.34, 7.71] |
| 2 Hallucinations/psychosis Show forest plot | 4 | 187 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.18, 6.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total number of non‐serious adverse events Show forest plot | 21 | 3132 | Risk Ratio (IV, Random, 95% CI) | 1.29 [1.10, 1.51] |
| 2 Subgroup analysis: total number of non‐serious adverse events according to dose Show forest plot | 21 | 3135 | Risk Ratio (IV, Random, 95% CI) | 1.28 [1.11, 1.49] |
| 2.1 Low dose | 2 | 151 | Risk Ratio (IV, Random, 95% CI) | 1.09 [0.82, 1.46] |
| 2.2 High dose | 10 | 1761 | Risk Ratio (IV, Random, 95% CI) | 1.22 [1.10, 1.35] |
| 2.3 Unknown dose | 10 | 1223 | Risk Ratio (IV, Random, 95% CI) | 1.37 [1.01, 1.87] |
| 3 Nervous system Show forest plot | 21 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Affective | 4 | 390 | Risk Ratio (Random, 95% CI) | 2.39 [0.48, 11.96] |
| 3.2 Aggression | 2 | 417 | Risk Ratio (Random, 95% CI) | 1.16 [0.17, 7.80] |
| 3.3 Apathy | 1 | 59 | Risk Ratio (Random, 95% CI) | 0.80 [0.19, 3.33] |
| 3.4 Confusion | 2 | 548 | Risk Ratio (Random, 95% CI) | 1.01 [0.22, 4.73] |
| 3.5 Depression | 1 | 59 | Risk Ratio (Random, 95% CI) | 0.83 [0.22, 3.10] |
| 3.6 Dizziness | 3 | 683 | Risk Ratio (Random, 95% CI) | 2.50 [0.70, 8.99] |
| 3.7 Drowsiness | 4 | 811 | Risk Ratio (Random, 95% CI) | 1.27 [0.82, 1.98] |
| 3.8 Emotional lability | 1 | 132 | Risk Ratio (Random, 95% CI) | 2.41 [0.27, 21.32] |
| 3.9 Fatigue | 7 | 858 | Risk Ratio (Random, 95% CI) | 0.76 [0.36, 1.63] |
| 3.10 Headache | 17 | 2724 | Risk Ratio (Random, 95% CI) | 1.22 [0.90, 1.64] |
| 3.11 Insomnia | 3 | 349 | Risk Ratio (Random, 95% CI) | 1.31 [0.35, 4.93] |
| 3.12 Irritability | 11 | 1721 | Risk Ratio (Random, 95% CI) | 1.11 [0.77, 1.60] |
| 3.13 Nervousness | 2 | 362 | Risk Ratio (Random, 95% CI) | 2.52 [0.82, 7.76] |
| 3.14 Pain | 1 | 132 | Risk Ratio (Random, 95% CI) | 1.91 [0.21, 17.60] |
| 3.15 Picking at skin or fingers, nail biting, lip or cheek chewing | 1 | 316 | Risk Ratio (Random, 95% CI) | 1.01 [0.60, 1.70] |
| 3.16 Sad, tearful or depressed | 4 | 707 | Risk Ratio (Random, 95% CI) | 1.41 [0.86, 2.29] |
| 3.17 Somnolence | 2 | 173 | Risk Ratio (Random, 95% CI) | 0.59 [0.11, 3.11] |
| 3.18 Trouble sleeping or sleep problems | 13 | 2416 | Risk Ratio (Random, 95% CI) | 1.60 [1.15, 2.23] |
| 3.19 Tics or nervous movements | 8 | 1231 | Risk Ratio (Random, 95% CI) | 0.85 [0.26, 2.79] |
| 3.20 Worried or anxious | 3 | 596 | Risk Ratio (Random, 95% CI) | 1.37 [0.84, 2.25] |
| 4 Digestive system Show forest plot | 18 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Decreased appetite | 16 | 2962 | Risk Ratio (IV, Random, 95% CI) | 3.66 [2.56, 5.23] |
| 4.2 Decreased weight | 6 | 859 | Risk Ratio (IV, Random, 95% CI) | 3.89 [1.43, 10.59] |
| 4.3 Diarrhoea | 5 | 857 | Risk Ratio (IV, Random, 95% CI) | 1.07 [0.41, 2.74] |
| 4.4 Dyspepsia | 2 | 159 | Risk Ratio (IV, Random, 95% CI) | 1.80 [0.71, 4.54] |
| 4.5 Increased appetite | 1 | 179 | Risk Ratio (IV, Random, 95% CI) | 0.07 [0.00, 1.43] |
| 4.6 Nausea | 11 | 1995 | Risk Ratio (IV, Random, 95% CI) | 1.30 [0.85, 1.99] |
| 4.7 Stomachache | 13 | 2341 | Risk Ratio (IV, Random, 95% CI) | 1.30 [1.00, 1.69] |
| 4.8 Vomiting | 11 | 1916 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.76, 1.79] |
| 5 Circulatory and respiratory systems Show forest plot | 8 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 ECG: prolonged QT‐interval | 2 | 466 | Risk Ratio (Random, 95% CI) | 0.81 [0.13, 5.00] |
| 5.2 ECG: tachycardia | 1 | 245 | Risk Ratio (Random, 95% CI) | 1.04 [0.11, 10.18] |
| 5.3 Cough | 4 | 996 | Risk Ratio (Random, 95% CI) | 0.95 [0.41, 2.18] |
| 5.4 Nasal congestion | 2 | 479 | Risk Ratio (Random, 95% CI) | 1.19 [0.59, 2.41] |
| 5.5 Pharyngolaryngeal pain | 1 | 303 | Risk Ratio (Random, 95% CI) | 1.12 [0.59, 2.13] |
| 5.6 Supraventricular extrasystoles | 1 | 17 | Risk Ratio (Random, 95% CI) | 3.00 [0.11, 84.55] |
| 5.7 Upper respiratory tract infection | 1 | 217 | Risk Ratio (Random, 95% CI) | 1.07 [0.42, 2.76] |
| 6 Skeletal and muscular systems Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Arthralgia | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.24, 1.84] |
| 6.2 Asthenia | 1 | 177 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.01, 4.25] |
| 6.3 Back pain | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.39, 1.66] |
| 6.4 Myalgia | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.23, 1.62] |
| 6.5 Toothache | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 1.01 [0.43, 2.35] |
| 7 Immune system Show forest plot | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Gastroenteritis | 3 | 435 | Risk Ratio (IV, Random, 95% CI) | 4.63 [0.99, 21.72] |
| 7.2 Influenza | 3 | 624 | Risk Ratio (IV, Random, 95% CI) | 0.65 [0.20, 2.10] |
| 7.3 Nasopharyngitis | 5 | 979 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.70, 1.87] |
| 7.4 Otitis media | 1 | 100 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.17, 18.94] |
| 7.5 Pharyngitis | 2 | 293 | Risk Ratio (IV, Random, 95% CI) | 2.43 [0.49, 12.05] |
| 7.6 Pyrexia | 2 | 400 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.01, 87.72] |
| 7.7 Rhinitis | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 1.28 [0.43, 3.79] |
| 7.8 Upper respiratory tract infection ‐ not otherwise specified (NOS) | 5 | 917 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.68, 2.06] |
| 7.9 Viral infection NOS | 3 | 614 | Risk Ratio (IV, Random, 95% CI) | 0.70 [0.23, 2.15] |
| 8 Height Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Weight Show forest plot | 5 | 805 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.55, ‐0.70] |
| 10 BMI Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Vital signs Show forest plot | 9 | 3374 | Mean Difference (IV, Random, 95% CI) | 1.41 [0.30, 2.52] |
| 11.1 Diastolic blood pressure (mmHg) | 8 | 1067 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.12, 2.01] |
| 11.2 Systolic blood pressure (mmHg) | 8 | 1067 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐1.25, 1.16] |
| 11.3 Pulse or heart rate (bpm) | 8 | 1240 | Mean Difference (IV, Random, 95% CI) | 3.41 [0.87, 5.94] |
| 12 Other Show forest plot | 5 | 1815 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.56, 2.57] |
| 12.1 Accidental injury | 3 | 656 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.48, 2.07] |
| 12.2 Epistasis | 1 | 132 | Risk Ratio (IV, Random, 95% CI) | 4.25 [0.23, 77.22] |
| 12.3 Excoriation | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 3.52 [1.19, 10.46] |
| 12.4 Overdose | 1 | 221 | Risk Ratio (IV, Random, 95% CI) | 2.97 [0.12, 72.20] |
| 12.5 Skin disorder (rash) | 2 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.01, 26.44] |
| 12.6 Skin laceration | 1 | 303 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.15, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total number of non‐serious adverse events Show forest plot | 21 | 2072 | Risk Ratio (Random, 95% CI) | 1.33 [1.11, 1.58] |
| 2 Subgroup analysis: total number of non‐serious adverse events according to dose Show forest plot | 21 | 2859 | Risk Ratio (Random, 95% CI) | 1.26 [1.11, 1.44] |
| 2.1 Low dose | 16 | 1539 | Risk Ratio (Random, 95% CI) | 1.11 [0.94, 1.31] |
| 2.2 High dose | 12 | 1080 | Risk Ratio (Random, 95% CI) | 1.57 [1.22, 2.01] |
| 2.3 Unknown dose | 2 | 240 | Risk Ratio (Random, 95% CI) | 0.98 [0.51, 1.86] |
| 3 Nervous system Show forest plot | 50 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Aggression | 2 | 589 | Risk Ratio (Random, 95% CI) | 0.52 [0.17, 1.60] |
| 3.2 Agitation | 1 | 62 | Risk Ratio (Random, 95% CI) | 1.18 [0.38, 3.60] |
| 3.3 Anger | 3 | 264 | Risk Ratio (Random, 95% CI) | 0.45 [0.26, 0.77] |
| 3.4 Behavioural complaints | 1 | 82 | Risk Ratio (Random, 95% CI) | 0.55 [0.35, 0.86] |
| 3.5 Buccal or lingual movements | 4 | 302 | Risk Ratio (Random, 95% CI) | 1.06 [0.62, 1.79] |
| 3.6 Compulsive acts | 1 | 90 | Risk Ratio (Random, 95% CI) | 2.57 [1.45, 4.56] |
| 3.7 Daydreaming | 3 | 222 | Risk Ratio (Random, 95% CI) | 0.66 [0.44, 0.98] |
| 3.8 Dizziness | 9 | 746 | Risk Ratio (Random, 95% CI) | 1.17 [0.89, 1.55] |
| 3.9 Drowsiness: dull, tired, listless or sleepy | 21 | 1350 | Risk Ratio (Random, 95% CI) | 0.97 [0.73, 1.28] |
| 3.10 Euphoria | 6 | 405 | Risk Ratio (Random, 95% CI) | 1.06 [0.72, 1.57] |
| 3.11 Headache | 37 | 3752 | Risk Ratio (Random, 95% CI) | 1.21 [1.01, 1.45] |
| 3.12 Insomnia or sleep problems | 31 | 3270 | Risk Ratio (Random, 95% CI) | 1.57 [1.20, 2.06] |
| 3.13 Irritability | 23 | 2238 | Risk Ratio (Random, 95% CI) | 0.92 [0.66, 1.27] |
| 3.14 Nightmares | 10 | 686 | Risk Ratio (Random, 95% CI) | 0.97 [0.66, 1.42] |
| 3.15 Overly meticulous | 1 | 96 | Risk Ratio (Random, 95% CI) | 40.77 [2.35, 706.72] |
| 3.16 Obsessive thinking | 1 | 90 | Risk Ratio (Random, 95% CI) | 2.35 [1.53, 3.62] |
| 3.17 Picking at skin or fingers, nail biting, lip or cheek chewing | 15 | 888 | Risk Ratio (Random, 95% CI) | 1.12 [0.88, 1.41] |
| 3.18 Repetitive language | 1 | 48 | Risk Ratio (Random, 95% CI) | 1.0 [0.32, 3.10] |
| 3.19 Sad, tearful or depressed | 23 | 1849 | Risk Ratio (Random, 95% CI) | 1.15 [0.94, 1.41] |
| 3.20 Socially withdrawn ‐ decreased interaction with others | 12 | 771 | Risk Ratio (Random, 95% CI) | 1.24 [0.82, 1.87] |
| 3.21 Sleep efficiency (SEF) | 2 | 108 | Risk Ratio (Random, 95% CI) | 0.48 [0.02, 14.28] |
| 3.22 Stares a lot | 9 | 904 | Risk Ratio (Random, 95% CI) | 1.03 [0.75, 1.40] |
| 3.23 Tics or nervous movements | 19 | 1403 | Risk Ratio (Random, 95% CI) | 1.33 [1.03, 1.72] |
| 3.24 Unusual blinking | 1 | 48 | Risk Ratio (Random, 95% CI) | 3.13 [0.12, 80.68] |
| 3.25 Worried or anxious | 20 | 1673 | Risk Ratio (Random, 95% CI) | 0.82 [0.60, 1.12] |
| 4 Digestive system Show forest plot | 42 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 Decreased appetite or loss of appetite | 35 | 3862 | Risk Ratio (Random, 95% CI) | 3.04 [2.35, 3.94] |
| 4.2 Diarrhoea | 3 | 402 | Risk Ratio (Random, 95% CI) | 0.58 [0.19, 1.74] |
| 4.3 Dry mouth | 5 | 342 | Risk Ratio (Random, 95% CI) | 1.25 [0.54, 2.90] |
| 4.4 Dyspepsia | 1 | 62 | Risk Ratio (Random, 95% CI) | 0.22 [0.02, 2.14] |
| 4.5 Nausea | 9 | 768 | Risk Ratio (Random, 95% CI) | 1.52 [1.00, 2.30] |
| 4.6 Increased appetite | 1 | 136 | Risk Ratio (Random, 95% CI) | 0.20 [0.08, 0.50] |
| 4.7 Stomachache | 33 | 3777 | Risk Ratio (Random, 95% CI) | 1.61 [1.27, 2.04] |
| 4.8 Vomiting | 4 | 710 | Risk Ratio (Random, 95% CI) | 0.90 [0.26, 3.11] |
| 5 Urinary system: urinary incontinence Show forest plot | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6 Skeletal and muscular system: somatic complaints Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Immune system Show forest plot | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Allergic rhinitis | 4 | 475 | Risk Ratio (IV, Random, 95% CI) | 1.38 [0.35, 5.51] |
| 7.2 Fever | 2 | 91 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.09, 20.56] |
| 7.3 Lymphadenitis | 2 | 296 | Risk Ratio (IV, Random, 95% CI) | 3.93 [0.44, 35.11] |
| 7.4 Pharyngolaryngeal pain | 1 | 160 | Risk Ratio (IV, Random, 95% CI) | 2.0 [0.19, 21.62] |
| 7.5 Pharyngitis | 4 | 754 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.19, 2.62] |
| 7.6 Upper respiratory tract infection | 5 | 888 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.27, 1.37] |
| 8 Skin Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Rash | 2 | 208 | Risk Ratio (IV, Random, 95% CI) | 0.96 [0.14, 6.41] |
| 8.2 Skin laceration | 1 | 167 | Risk Ratio (IV, Random, 95% CI) | 2.96 [0.12, 71.75] |
| 9 Vital signs Show forest plot | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Diastolic blood pressure (mmHg) | 11 | 755 | Mean Difference (IV, Random, 95% CI) | 1.23 [‐0.39, 2.86] |
| 9.2 Systolic blood pressure (mmHg) | 11 | 755 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐1.30, 2.36] |
| 9.3 Pulse or heart rate (bpm) | 14 | 939 | Mean Difference (IV, Random, 95% CI) | 5.06 [2.88, 7.24] |
| 10 Height (cm) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Weight Show forest plot | 6 | 530 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.23, 0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials: risk of bias Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 1.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 High risk of bias | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 2 Subgroup analysis: types of scales Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 2.1 Conners' Global Index ‐ Teacher (CGI‐T) | 1 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.14, ‐0.68] |
| 2.2 Groninger Behaviour Observation Scale (GBOS) | 1 | 43 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.46, ‐0.21] |
| 2.3 Conners' Teacher Rating Scale ‐ Conduct problems | 1 | 25 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.48, 0.14] |
| 2.4 IOWA Conners' Rating Scale ‐ Oppositional/Defiant (IOWA‐O/D) | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.12, ‐0.59] |
| 3 Subgroup analysis: dose Show forest plot | 5 | 696 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.02, ‐0.69] |
| 3.1 Low dose | 2 | 71 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.16, ‐0.19] |
| 3.2 High dose | 3 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.08, ‐0.70] |
| 3.3 Unknown dose | 1 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.21, ‐0.46] |
| 4 Subgroup analysis: duration of treatment Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 4.1 Short term (up to 6 months) | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 4.2 Long term (over 6 months) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Subgroup analysis: parallel‐group trials versus first‐period cross‐over trials Show forest plot | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 5.1 Parallel‐group trials | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 5.2 First‐period cross‐over trials | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 General behaviour, cross‐over trials (endpoint data) Show forest plot | 16 | 2014 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.78, ‐0.60] |
| 6.1 Low dose | 13 | 1110 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.72, ‐0.48] |
| 6.2 High dose | 12 | 904 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐0.95, ‐0.68] |
| 7 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias Show forest plot | 16 | 1308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| 7.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 High risk of bias | 16 | 1308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| 8 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (teacher‐rated) versus cross‐over trials (endpoint data) Show forest plot | 21 | 1976 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐0.88, ‐0.70] |
| 8.1 All parallel‐group trials and first‐period cross‐over trials | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 8.2 Cross‐over trials (endpoint data) | 16 | 1308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.87, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General behaviour, cross‐over trials (endpoint data) Show forest plot | 8 | 1241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.75, ‐0.46] |
| 1.1 Low dose | 7 | 903 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.76, ‐0.36] |
| 1.2 High dose | 5 | 338 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐0.93, ‐0.49] |
| 2 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias Show forest plot | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| 2.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 High risk of bias | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| 3 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (independent assessor‐rated) compared with cross‐over trials (endpoint data) Show forest plot | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| 3.1 Parallel‐group trials and first‐period cross‐over trials | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Cross‐over trials (endpoint data) | 8 | 951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.88, ‐0.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All parallel‐group trials and first‐period cross‐over trials Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 2 Subgroup analysis: risk of bias Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 2.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 High risk of bias | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 3 Subgroup analysis: types of scales Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 3.1 The Weekly Parent Ratings of Evening and Morning Behaviour (WPREMB) ‐ Revised | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.47, 1.47] |
| 3.2 Conners' Global Index (CGI) ‐ Parent | 2 | 352 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.63, ‐0.20] |
| 3.3 Swanson, Nolan and Pelham, Fourth Edition ‐ Oppositional (SNAP‐IV‐Oppositional) | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.95, 0.23] |
| 3.4 IOWA Conners' Rating Scale ‐ Oppositional/Defiant (IOWA‐I/O) | 2 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.01, ‐0.49] |
| 4 Subgroup analysis: parallel‐group trials compared with first‐period cross‐over trials Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 4.1 Parallel‐group trials | 5 | 655 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.78, ‐0.23] |
| 4.2 First‐period cross‐over trials | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.95, 0.23] |
| 5 Subgroup analysis: duration of treatment Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 5.1 Short term (up to 6 months) | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 5.2 Long term (over 6 months) | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Subgroup analysis: dose Show forest plot | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 6.1 Low dose | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 High dose | 4 | 496 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.77, ‐0.08] |
| 6.3 Unknown dose | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.08, ‐0.38] |
| 7 General behaviour, cross‐over trials (endpoint data) Show forest plot | 6 | 550 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐0.93, ‐0.56] |
| 7.1 Low dose | 5 | 248 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.93, ‐0.38] |
| 7.2 High dose | 4 | 302 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.07, ‐0.60] |
| 8 Subgroup analysis: general behaviour, cross‐over trials (endpoint data): risk of bias Show forest plot | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| 8.1 Low risk of bias | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 High risk of bias | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| 9 Subgroup analysis: all parallel‐group trials and first‐period cross‐over trials (parent‐rated) compared with cross‐over trials (endpoint data) Show forest plot | 12 | 1054 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.86, ‐0.50] |
| 9.1 All parallel‐group trials and first‐period cross‐over trials | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 9.2 Cross‐over trials (endpoint data) | 6 | 384 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.05, ‐0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Comparisions of raters Show forest plot | 8 | 1338 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.86, ‐0.52] |
| 1.1 Teacher‐rated | 5 | 668 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.04, ‐0.71] |
| 1.2 Independent assessor‐rated | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Parent‐rated | 6 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.78, ‐0.27] |
| 2 Comorbidity versus no comorbidity Show forest plot | 7 | 579 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.98, ‐0.43] |
| 2.1 ADHD with comorbidity | 6 | 265 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.95, ‐0.23] |
| 2.2 ADHD without comorbidity | 1 | 314 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.14, ‐0.68] |
| 3 Cross‐over trials: first‐period data versus endpoint data (teacher‐, parent‐, and independent assessor‐rated) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 First‐period data | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.75, 0.13] |
| 3.2 Endpoint data | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.71, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Subgroup analysis: types of scales Show forest plot | 3 | 514 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.42, 0.80] |
| 1.1 Child Health Questionnaire (CHQ) | 1 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.25, 0.83] |
| 1.2 Children´s Global Assessment Scale (CGAS) | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [0.10, 1.47] |
| 1.3 Child Health and Illness Profile, Child Edition: Parent Report Form (CHIP‐CE:PRF) | 1 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.37, 0.91] |