Moduladores de los receptores de progesterona para la endometriosis
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised double‐blind multi‐centre study | |
| Participants | 269 British women aged 18 to 45 years | |
| Interventions | Gestrinone 2.5 mg twice weekly plus 'dummy' danazol for 6 months (132 women) | |
| Outcomes | AFS scores at laparoscopy after 6 months of treatment | |
| Notes | Repeat laparoscopy 23 days (median) after cessation of treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random, no other details |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind', 'double‐dummy'. Participants received 2 identical tablets |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | High risk | A total of 15 participants from the gestrinone group, including 4 with hirsutism, and 17 participants from the danazol group, including 6 with headache, withdrew because of adverse symptoms. An additional 22 participants, including 10 from the gestrinone group and 12 from the danazol group, withdrew because of lack of efficacy, pregnancy, elevated hepatic function tests, or reasons unrelated to the trial (19% attrition rate for efficacy outcomes) |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes and side effects |
| Other bias | Low risk | No other specific source of bias detected |
| Methods | Randomised double‐blind study | |
| Participants | 26 women | |
| Interventions | Group I received 1 tablet of 25 mg mifepristone daily Group II received 1 tablet of 5 mg mifepristone daily | |
| Outcomes | AFS scores at laparoscopy after 6 months of treatment Side effects | |
| Notes | Laparoscopy and endometrial biopsy were performed before and after treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random list |
| Allocation concealment (selection bias) | Low risk | Applied opaque sealed envelopes that contained a card indicating the treatment group to which the participant was assigned |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | Low risk | All women completed 6 months of treatment |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Unclear risk | No other specific source of bias detected |
| Methods | Randomised double‐blind study | |
| Participants | 360 women | |
| Interventions | Group I received 1 tablet of 2.5 mg mifepristone daily for 6 months (90 women) Group II received 1 tablet of 5 mg mifepristone daily for 6 months (90 women) Group III received 1 tablet of 10 mg mifepristone daily for 6 months (90 women) Group IV received 1 tablet of placebo daily for 3 months (90 women) | |
| Outcomes | AFS scores at laparoscopy after 6 months of treatment Side effects | |
| Notes | Laparoscopy and endometrial biopsy were performed before and after treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a random list obtained from the MEDSTAT 2.1 programme |
| Allocation concealment (selection bias) | Low risk | Applied opaque sealed envelopes that contained a card indicating the treatment group to which the participant was assigned |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | Low risk | All women in treatment group completed 6 months of treatment, and placebo group finished 3 months of treatment |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes |
| Other bias | Low risk | No other specific source of bias detected |
| Methods | Multi‐centre double‐blind placebo‐controlled parallel‐group study | |
| Participants | 130 women | |
| Interventions | Asoprisnil 5 mg (n = 31), 10 mg (n = 33), 25 mg (n = 32), or placebo (n = 34) was administered orally once daily for 12 weeks | |
| Outcomes | Pain scores during treatment and at 1 year follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised double‐blind study |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, no details |
| Other bias | Unclear risk | Unclear, insufficient details to permit a judgement |
| Methods | Phase II prospective randomised double‐blind study | |
| Participants | 11 patients | |
| Interventions | Gestrinone 1.25 mg (5 participants) or 2.5 mg (6 participants) orally twice a week for 24 weeks | |
| Outcomes | Revised AFS scores before and at the end of treatment Serum hormone, sex hormone binding globulin, and lipid concentrations were measured Quantitated computerised tomography of thoracic 12 through lumbar 4 vertebral bodies | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to 1 of 2 treatment groups according to a computer‐generated order |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear, no details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, insufficient details to allow a judgement |
| Other bias | Unclear risk | Unclear, insufficient details to allow a judgement |
| Methods | Open randomised trial | |
| Participants | 39 Italian women aged 23 to 35 years | |
| Interventions | Gestrinone 2.5 mg twice weekly (20 women) increasing to 3 times a week if no amenorrhoea by 1 month (7 of the 20) | |
| Outcomes | rAFS scores at laparoscopy 1 month after cessation of treatment | |
| Notes | Follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'patients were randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | All women accounted for |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes |
| Other bias | High risk | Only 7 participants given gestrinone and 9 participants taking danazol had repeat laparoscopy. If amenorrhoea was not obtained after 1 month of treatment, the gestrinone dose was increased to 2.5 mg 3 times a week (7 participants) and the danazol dose to 800 mg a day (2 participants) |
| Methods | Randomised double‐blind double‐dummy multi‐centre trial | |
| Participants | 55 Italian women aged 18 to 40 years | |
| Interventions | Gestrinone 2.5 mg twice weekly plus placebo injections (27 women) | |
| Outcomes | Pain symptoms | |
| Notes | Follow‐up: 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomized', 'allocating consecutively numbered anonymous packages' |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing randomization codes |
| Blinding of participants and personnel (performance bias) | Low risk | 'double blind, double dummy'. Each participant received an active drug and a dummy placebo. Participants and clinicians were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | 'double blind, double dummy'. Each participant received an active drug and a dummy placebo. Participants and clinicians were blinded |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up detailed, 6 withdrawals during treatment period, 7 lost to follow‐up (11%) |
| Selective reporting (reporting bias) | Low risk | Includes main outcomes |
| Other bias | Low risk | No other specific source of bias detected |
| Methods | Randomised double‐blind trial | |
| Participants | 12 American women | |
| Interventions | Gestrinone 1.25 mg twice weekly (6 women) | |
| Outcomes | rAFS scores for endometriosis at laparoscopy following treatment | |
| Notes | Second laparoscopy within 4 weeks of treatment completion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial, method not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up: 2 (17%) |
| Selective reporting (reporting bias) | Unclear risk | Reports main outcomes. No details on pelvic pain scores after treatment |
| Other bias | Low risk | No other specific source of bias detected |
| Methods | Randomised, double‐blind | |
| Participants | 38 women | |
| Interventions | 6 months of treatment with 3 doses of ulipristal, CDB‐4124 (12.5 mg, 25 mg, and 50 mg) compared with leuprolide acetate depot | |
| Outcomes | Pain scores during treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated at random, no other details |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear, no details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, insufficient details to allow a judgement |
| Other bias | Unclear risk | Unclear, insufficient details to allow a judgement |
| Methods | Randomised double‐blind | |
| Participants | 20 premenopausal women with mild to moderate endometriosis | |
| Interventions | 1.25 mg or 2.5 mg gestrinone 2 times per week for 6 months | |
| Outcomes | Metabolic effects of oral gestrinone on plasma lipoprotein risk markers for cardiovascular disease and on bone density Median total endometriosis scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | Unclear, no details |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear, no details |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear, no details |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear, no details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, insufficient details to allow a judgement |
| Other bias | Unclear risk | Unclear, insufficient details to allow a judgement |
AFS: American Fertility Society
GnRH: gonadotropin‐releasing hormone
rAFS: retrospective American Fertility Society score
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT; single‐arm study | |
| Not an RCT | |
| Not an RCT | |
| The “three step” therapy discussed in this study is a mixture of surgical and medical therapy | |
| 23/25 participants taking gestrinone and 18/18 participants given danazol had surgery before receiving medical treatment | |
| Comparison of danazol vs oral contraceptive pill | |
| This study concentrates on effects on asymptomatic endometriosis and fertility | |
| Conservative or half‐conservative surgery combined with drug therapy | |
| Using laparoscopic minimally invasive combined drug therapy |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dysmenorrhoea at 3 months Show forest plot | 1 | 352 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.17] |
| Analysis 1.1  Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Dysmenorrhoea at 3 months. | ||||
| 1.1 Mifepristone 2.5 mg | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.64] |
| 1.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.16] |
| 1.3 Mifepristone 10 mg | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.17] |
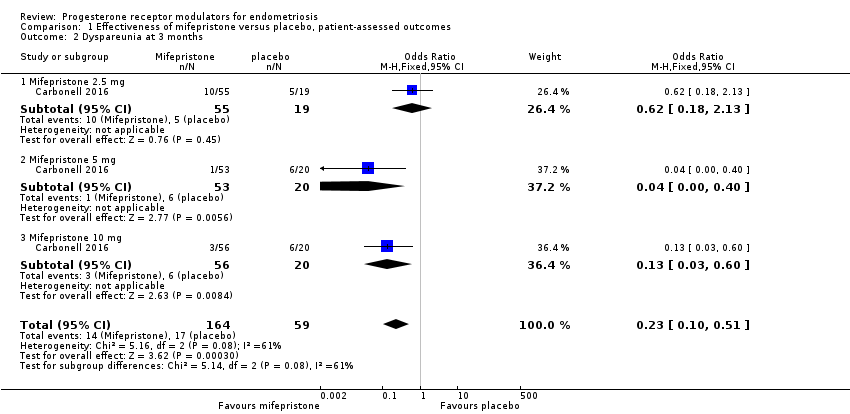
| 2 Dyspareunia at 3 months Show forest plot | 1 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.51] |
| Analysis 1.2  Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Dyspareunia at 3 months. | ||||
| 2.1 Mifepristone 2.5 mg | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.18, 2.13] |
| 2.2 Mifepristone 5 mg | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.40] |
| 2.3 Mifepristone 10 mg | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Amenorrhoea at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Amenorrhoea at 3 months. | ||||
| 1.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 686.16 [92.29, 5101.33] |
| 2 Amenorrhoea at 3 months: subgroup analysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 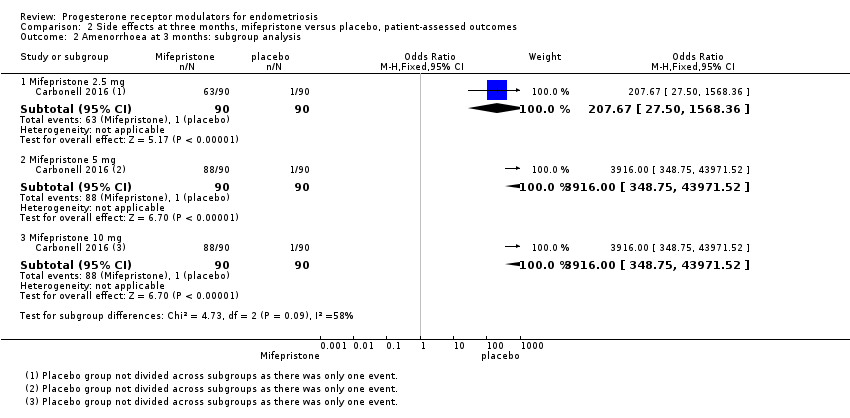 Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Amenorrhoea at 3 months: subgroup analysis. | ||||
| 2.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 207.67 [27.50, 1568.36] |
| 2.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 2.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
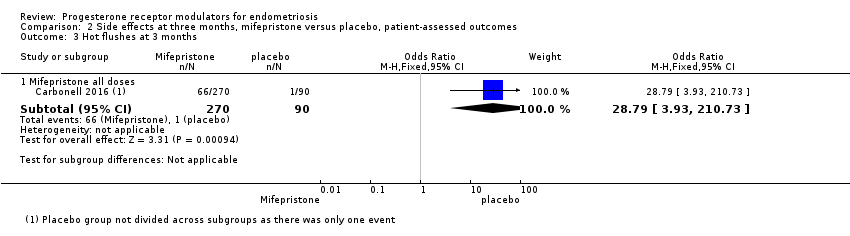
| 3 Hot flushes at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 3 Hot flushes at 3 months. | ||||
| 3.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.79 [3.93, 210.73] |
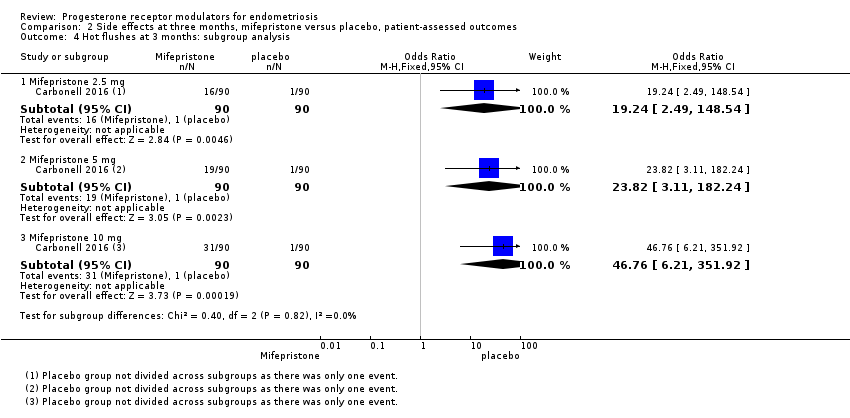
| 4 Hot flushes at 3 months: subgroup analysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 4 Hot flushes at 3 months: subgroup analysis. | ||||
| 4.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.24 [2.49, 148.54] |
| 4.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 23.82 [3.11, 182.24] |
| 4.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 46.76 [6.21, 351.92] |
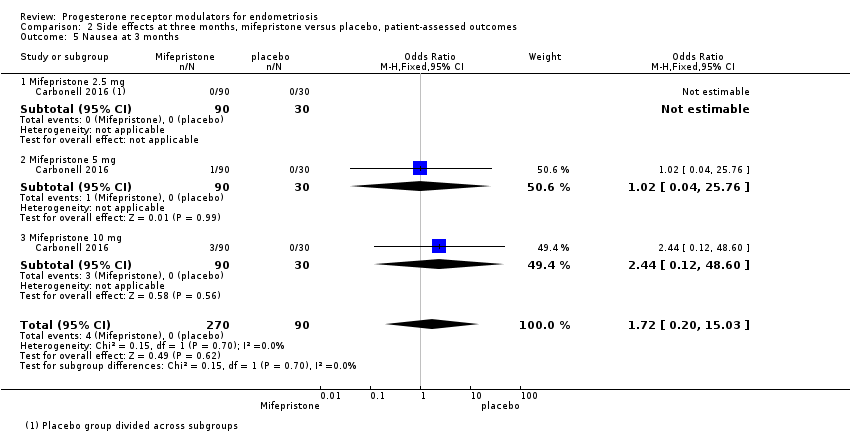
| 5 Nausea at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.20, 15.03] |
| Analysis 2.5 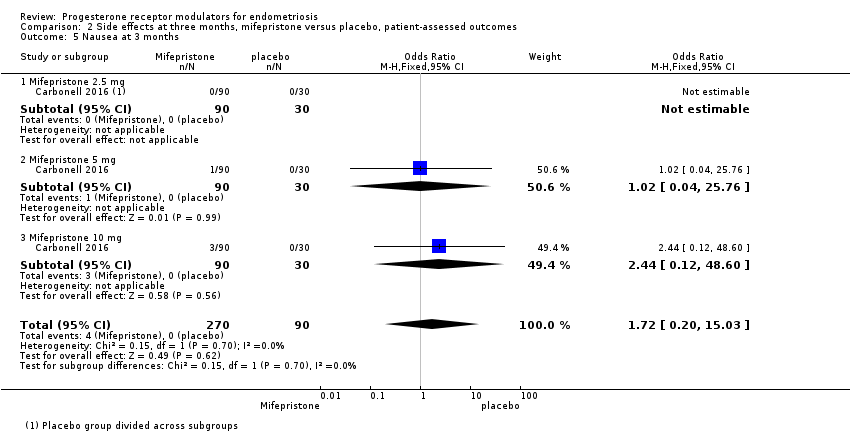 Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 5 Nausea at 3 months. | ||||
| 5.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 5.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.12, 48.60] |
| 6 Vomiting at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.01] |
| Analysis 2.6 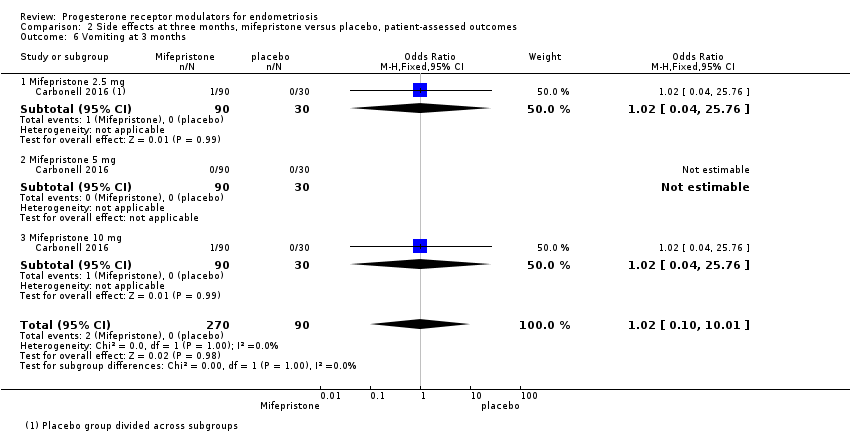 Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 6 Vomiting at 3 months. | ||||
| 6.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 6.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 7 Fatigue/Tiredness at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.48 [0.71, 42.27] |
| Analysis 2.7  Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 7 Fatigue/Tiredness at 3 months. | ||||
| 7.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.21, 73.08] |
| 7.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.40, 125.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevalence of dysmenorrhoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 1 Prevalence of dysmenorrhoea. | ||||
| 1.1 2.5 mg vs 5 mg | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.22, 3.29] |
| 1.2 5 mg vs 10 mg | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.32, 4.71] |
| 2 Dysmenorrhoea score 0‐10 VAS scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 2 Dysmenorrhoea score 0‐10 VAS scale. | ||||
| 2.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prevalence of dyspareunia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 3 Prevalence of dyspareunia. | ||||
| 3.1 2.5 mg vs 5 mg | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.37 [0.74, 54.81] |
| 3.2 5 mg vs 10 mg | 1 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.90] |
| 4 Dyspareunia score 0‐10 VAS scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4 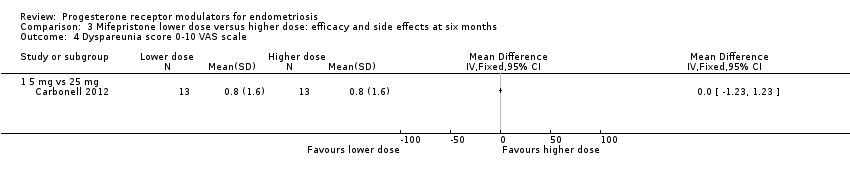 Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 4 Dyspareunia score 0‐10 VAS scale. | ||||
| 4.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Prevalence of pelvic pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 5 Prevalence of pelvic pain. | ||||
| 5.1 2.5 mg vs 5 mg | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.63, 5.17] |
| 5.2 5 mg vs 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.79, 19.97] |
| 6 Prevalence of amenorrhoea Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 6 Prevalence of amenorrhoea. | ||||
| 6.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 6.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.56] |
| 6.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.08, 4.41] |
| 7 Prevalence of hot flushes Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 7 Prevalence of hot flushes. | ||||
| 7.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.89] |
| 7.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.58] |
| 7.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.12, 87.13] |
| 8 Prevalence of nausea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 8 Prevalence of nausea. | ||||
| 8.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.71] |
| 8.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.24] |
| 9 Prevalence of vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.9 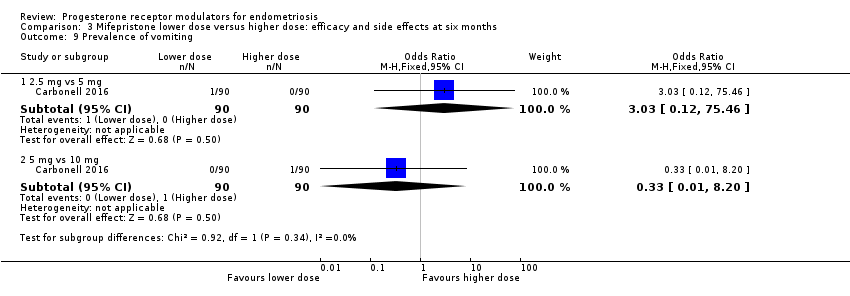 Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 9 Prevalence of vomiting. | ||||
| 9.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.46] |
| 9.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.20] |
| 10 Prevalence of fatigue/tiredness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.10  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 10 Prevalence of fatigue/tiredness. | ||||
| 10.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.92 [0.77, 250.94] |
| 10.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.54] |
| 11 Prevalence of endometrial thickness > 20 mm Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.11  Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 11 Prevalence of endometrial thickness > 20 mm. | ||||
| 11.1 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 None or mild pelvic pain Show forest plot | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.56] |
| Analysis 4.1  Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 1 None or mild pelvic pain. | ||||
| 2 None or mild dysmenorrhoea Show forest plot | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.33] |
| Analysis 4.2 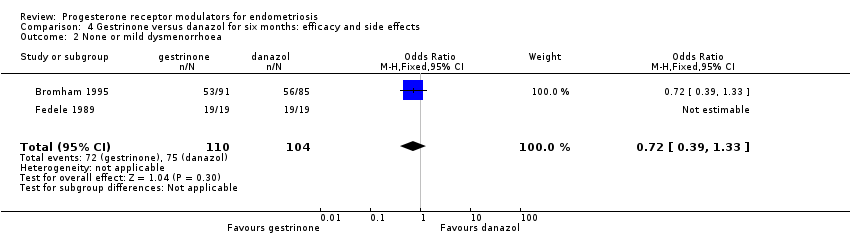 Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 2 None or mild dysmenorrhoea. | ||||
| 3 None or mild dyspareunia Show forest plot | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.86] |
| Analysis 4.3  Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 3 None or mild dyspareunia. | ||||
| 4 Adverse effects Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 4 Adverse effects. | ||||
| 4.1 Acne | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.33] |
| 4.2 Seborrhoea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.67, 4.46] |
| 4.3 Hirsutism | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.60, 4.32] |
| 4.4 Voice problems | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.43] |
| 4.5 Swelling hands/feet | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.82, 2.38] |
| 4.6 Hot flushes | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.26] |
| 4.7 Decreased breast size | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 0.98] |
| 4.8 Leg or muscle cramps | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.78] |
| 4.9 Headache | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.21] |
| 4.10 Nausea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.19] |
| 4.11 Vomiting | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.43] |
| 4.12 Loss of appetite | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.72, 2.37] |
| 4.13 Hunger | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.97] |
| 4.14 Dizziness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.75, 2.05] |
| 4.15 Tiredness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.84, 2.45] |
| 4.16 Faintness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.54, 2.76] |
| 4.17 Skin rash | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.20] |
| 4.18 Weight gain | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.27] |
| 4.19 Vaginal dryness | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| 4.20 Raised liver transaminases | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painful periods, visual analogue scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.15, 1.49] |
| Analysis 5.1 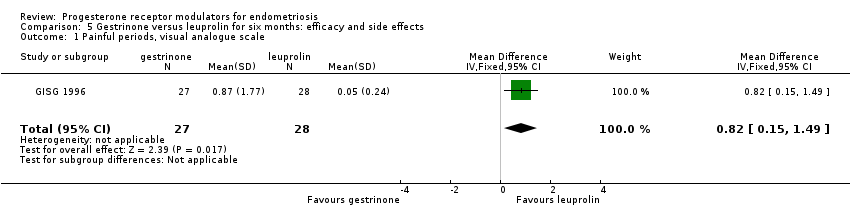 Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 1 Painful periods, visual analogue scale. | ||||
| 2 Painful periods, verbal rating scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.12, 0.58] |
| Analysis 5.2 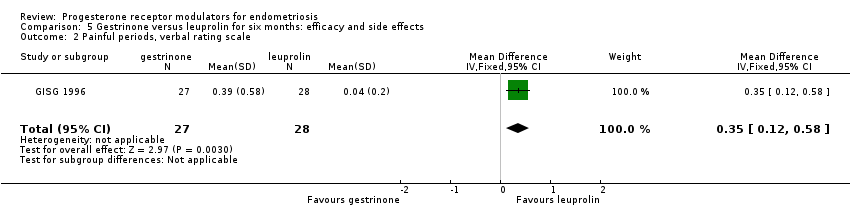 Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 2 Painful periods, verbal rating scale. | ||||
| 3 Pain on intercourse, visual analogue scale Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐2.08, ‐0.24] |
| Analysis 5.3  Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 3 Pain on intercourse, visual analogue scale. | ||||
| 4 Pain on intercourse, verbal rating scale Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.62, ‐0.04] |
| Analysis 5.4 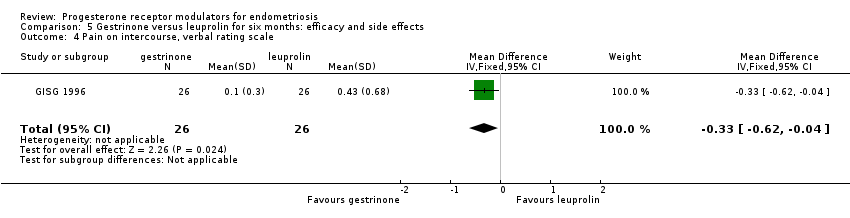 Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 4 Pain on intercourse, verbal rating scale. | ||||
| 5 Non‐menstrual pain, visual analogue scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.76, 0.94] |
| Analysis 5.5  Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 5 Non‐menstrual pain, visual analogue scale. | ||||
| 6 Side effects Show forest plot | 1 | 813 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| Analysis 5.6  Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 6 Side effects. | ||||
| 6.1 Seborrhoea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.2 Swelling hands/feet | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.26, 121.96] |
| 6.3 Hot flushes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.4 Leg or muscle cramps | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.55] |
| 6.5 Headache | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.6 Nausea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.7 Dizziness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.8 Skin rash | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.9 Vaginal dryness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.21] |
| 6.10 Mood changes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.10, 4.34] |
| 6.11 Joint pain | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.12 Drowsiness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.13 Tachycardia | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.14 Amenorrhoea | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.38] |
| 6.15 Spotting or bleeding | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.92 [2.64, 198.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 improvement in pain Show forest plot | 1 | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 8.46] |
| Analysis 6.1  Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 1 improvement in pain. | ||||
| 2 Side effect Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2 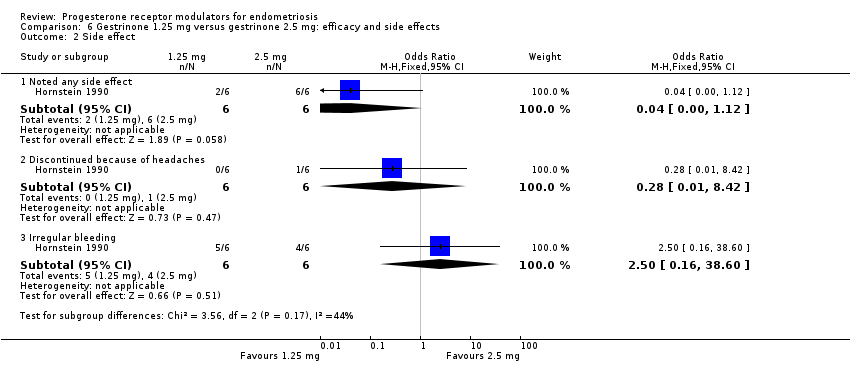 Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 2 Side effect. | ||||
| 2.1 Noted any side effect | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 1.12] |
| 2.2 Discontinued because of headaches | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 8.42] |
| 2.3 Irregular bleeding | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.16, 38.60] |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
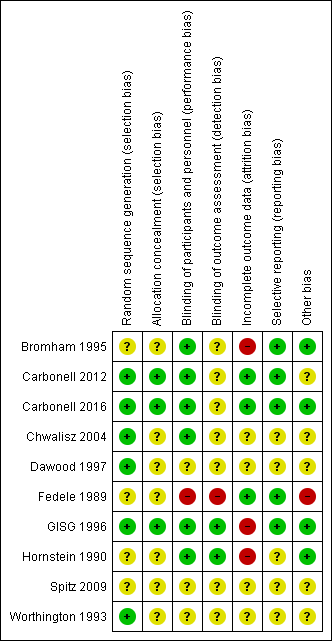
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, outcome: 1.1 Dysmenorrhoea at three months.

Forest plot of comparison.
2 Mifepristone versus placebo, patient‐assessed outcomes, outcome: 2.1 Amenorrhoea at three months.

Forest plot of comparison: side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, outcome: 2.2 Hot flushes at three months.

Forest plot of comparison: 7 Gestrinone versus leuprolin for six months: efficacy and side effects, outcome: 7.1 Painful periods, visual analogue scale.

Forest plot of comparison: 7 Gestrinone versus leuprolin for six months: efficacy and side effects, outcome: 7.3 Pain on intercourse, visual analogue scale.
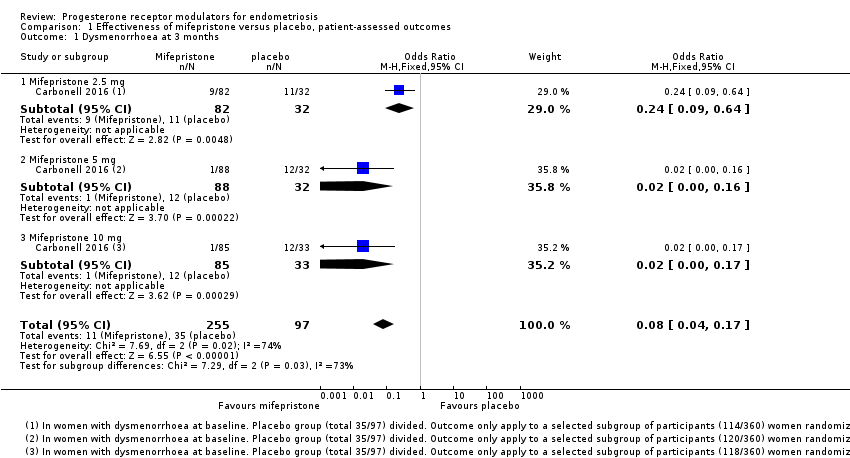
Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Dysmenorrhoea at 3 months.
Comparison 1 Effectiveness of mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Dyspareunia at 3 months.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 1 Amenorrhoea at 3 months.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 2 Amenorrhoea at 3 months: subgroup analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 3 Hot flushes at 3 months.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 4 Hot flushes at 3 months: subgroup analysis.
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 5 Nausea at 3 months.

Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 6 Vomiting at 3 months.
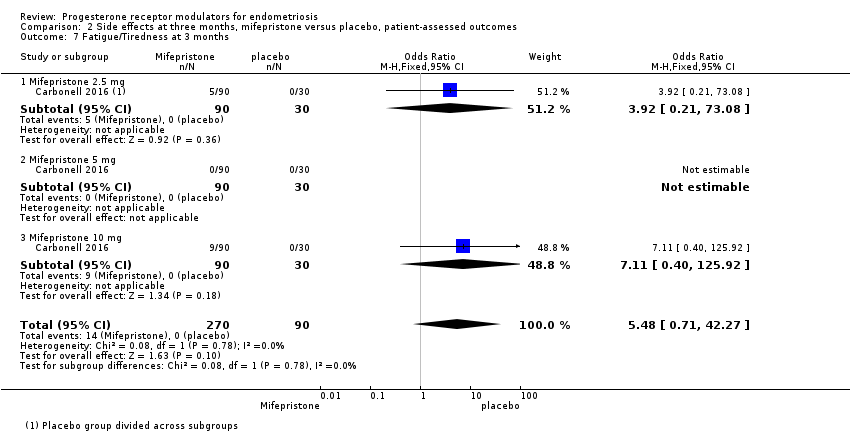
Comparison 2 Side effects at three months, mifepristone versus placebo, patient‐assessed outcomes, Outcome 7 Fatigue/Tiredness at 3 months.
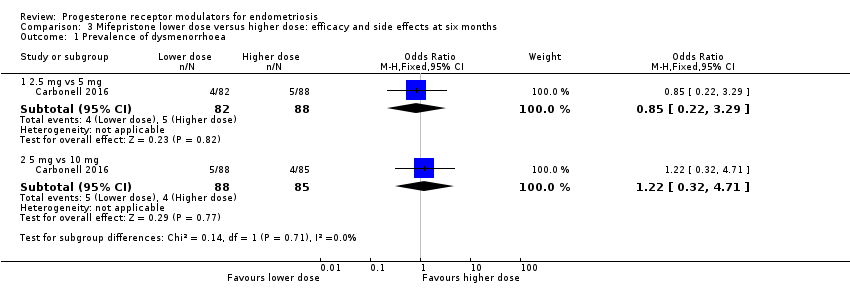
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 1 Prevalence of dysmenorrhoea.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 2 Dysmenorrhoea score 0‐10 VAS scale.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 3 Prevalence of dyspareunia.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 4 Dyspareunia score 0‐10 VAS scale.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 5 Prevalence of pelvic pain.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 6 Prevalence of amenorrhoea.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 7 Prevalence of hot flushes.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 8 Prevalence of nausea.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 9 Prevalence of vomiting.

Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 10 Prevalence of fatigue/tiredness.
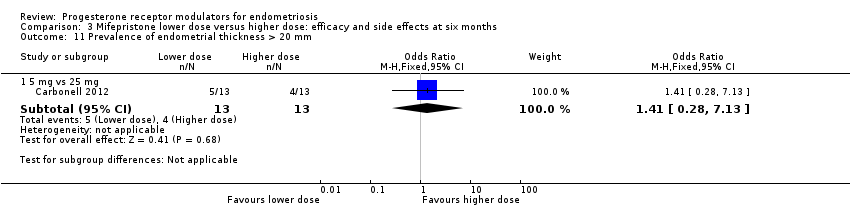
Comparison 3 Mifepristone lower dose versus higher dose: efficacy and side effects at six months, Outcome 11 Prevalence of endometrial thickness > 20 mm.
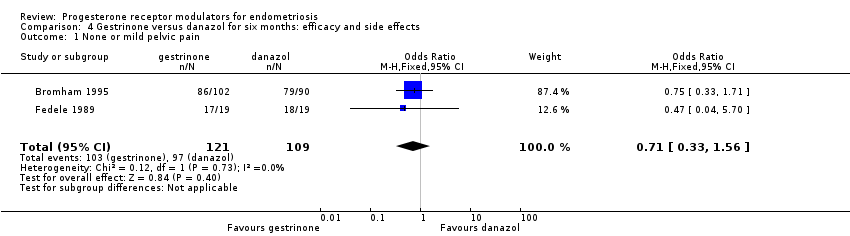
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 1 None or mild pelvic pain.
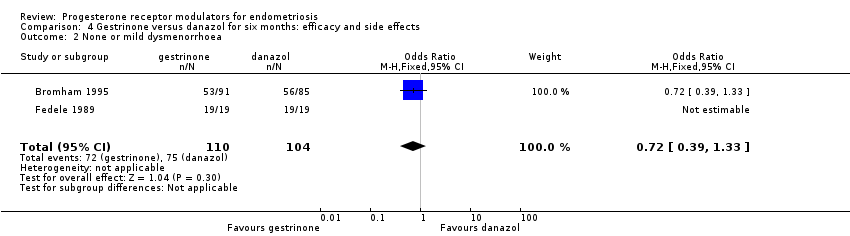
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 2 None or mild dysmenorrhoea.
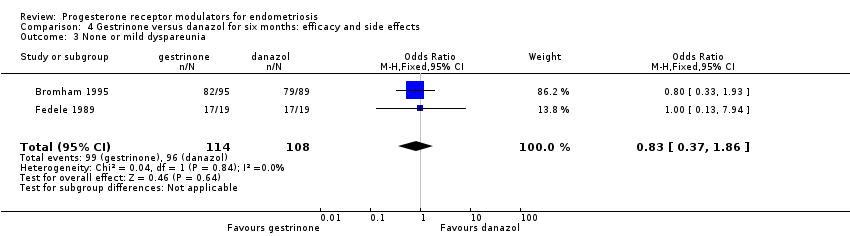
Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 3 None or mild dyspareunia.

Comparison 4 Gestrinone versus danazol for six months: efficacy and side effects, Outcome 4 Adverse effects.
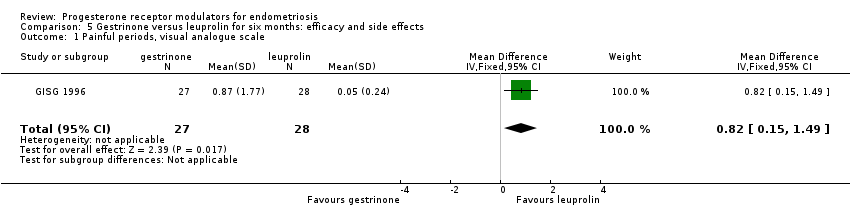
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 1 Painful periods, visual analogue scale.

Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 2 Painful periods, verbal rating scale.

Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 3 Pain on intercourse, visual analogue scale.
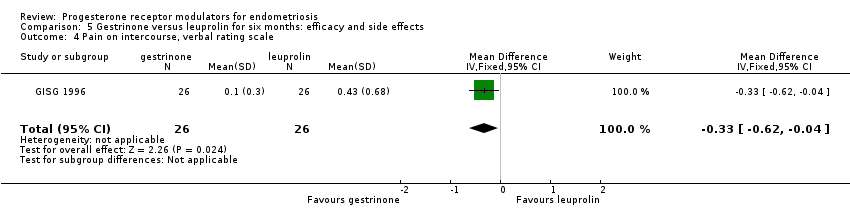
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 4 Pain on intercourse, verbal rating scale.
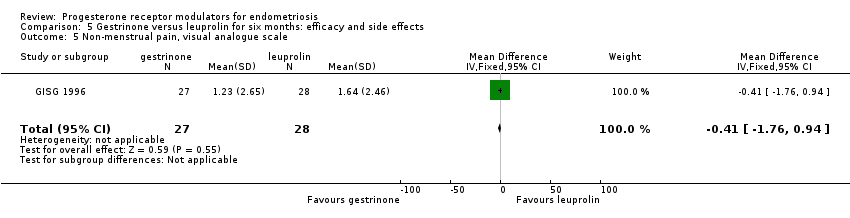
Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 5 Non‐menstrual pain, visual analogue scale.

Comparison 5 Gestrinone versus leuprolin for six months: efficacy and side effects, Outcome 6 Side effects.
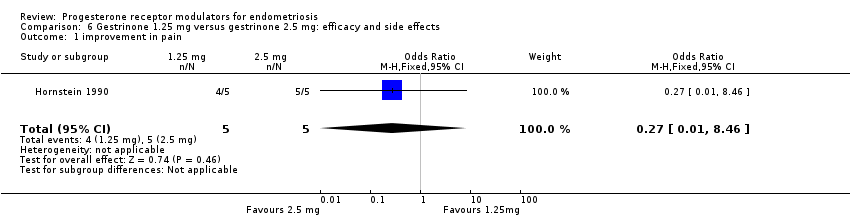
Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 1 improvement in pain.
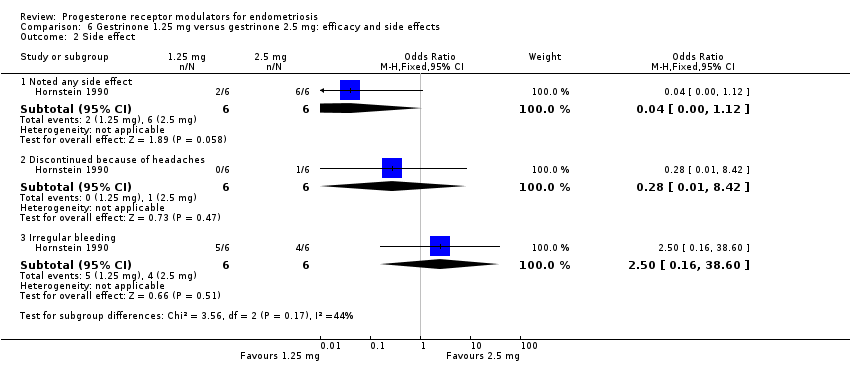
Comparison 6 Gestrinone 1.25 mg versus gestrinone 2.5 mg: efficacy and side effects, Outcome 2 Side effect.
| Mifepristone versus placebo for endometriosis | ||||||
| Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (mifepristone) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mifepristone | |||||
| Prevalence of dysmenorrhoea Follow‐up: 3 months | 402 per 1000 | 51 per 1000 | OR 0.08 (0.04 to 0.17) | 352 | ⊕⊕⊕⊝ | |
| Prevalence of dyspareunia Follow‐up: 3 months | 288 per 1000 | 85 per 1000 | OR 0.23 (0.10 to 0.51) | 223 | ⊕⊕⊝⊝ | |
| Side effects: amenorrhoea Follow‐up: 3 months | 11 per 1000 | 884 per 1000 | OR 686.16 (92.29 to 5101.33) | 360 | ⊕⊕⊕⊝ High | 239/270 events in the mifepristone group vs 1/90 in the placebo group |
| Side effects: hot flushes Follow‐up: 3 months | 11 per 1000 | 243 per 1000 | OR 28.79 (3.93 to 210.73) | 360 | ⊕⊕⊕⊝ High | 66/270 events in the mifepristone group vs 1/90 in the placebo group |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for serious imprecision (wide confidence intervals and/or very few events) bOutcome applied only to women with dysmenorrhoea at baseline, but this was 352/360 women randomised, so not downgraded for indirectness cOutcome applied only to women with dyspareunia at baseline, which was 223/360 women randomised. Downgraded one level for serious indirectness | ||||||
| Gestrinone versus danazol for endometriosis | ||||||
| Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (gestrinone) Comparison: danazol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Danazol | Gestrinone | |||||
| Pelvic pain: none or mild | 890 per 1000 | 852 per 1000 (727 to 927) | OR 0.71 (0.33 to 1.56) | 230 (2) | ⊕⊕⊝⊝ | |
| Dysmenorrhoea: none or mild Follow‐up: 6 months | 721 per 1000 | 650 per 1000 | OR 0.72 (0.39 to 1.33) | 214 | ⊕⊝⊝⊝ | |
| Dyspareunia: none or mild Follow‐up: 6 months | 889 per 1000 | 869 per 1000 | OR 0.83 (0.37 to 1.86) | 222 | ⊕⊝⊝⊝ | |
| Side effects: hirsutism Follow‐up: 6 months | 248 per 1000 | 464 per 1000 | OR 2.63 (1.60 to 4.32) | 302 | ⊕⊝⊝⊝ | I2 = 68% |
| Decreased breast size Follow‐up: 6 months | 477 per 1000 | 360 per 1000 | OR 0.62 (0.38 to 0.98) | 302 | ⊕⊕⊝⊝ | |
| Side effects: hot flushes Follow‐up: 6 months | 425 per 1000 | 368 per 1000 (270 to 482) | OR 0.79 (0.50 to 1.26) | 302 (2 studies) | ⊕⊝⊝⊝ | I2 = 72% |
| Side effects: acne Follow‐up: 6 months | 556 per 1000 | 644 per 1000 (529 to 744) | OR 1.45 (0.90 to 2.33) | 302 (2 studies) | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aAssessed in all randomised participants. Not all were symptomatic at baseline (although results show no significant differences in baseline symptoms between groups). Outcome therefore applies only to a select subgroup of participants: downgraded one level for serious indirectness bDowngraded one level for serious risk of bias associated with poor reporting of study methods, high attrition in one study, and high risk of other bias in both studies cImprecision of results (wide confidence intervals and/or few events), downgraded one level for serious imprecision dDowngraded one level for serious inconsistency | ||||||
| Gestrinone versus leuprolin for endometriosis | ||||||
| Patient or population: women with symptomatic endometriosis Settings: gynaecology clinic Intervention: progesterone receptor modulator (gestrinone) Comparison: leuprolin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Leuprolin | Gestrinone | |||||
| Dysmenorrhoea, verbal rating scale Follow‐up: 6 months | In control group, mean score for dysmenorrhoea on verbal rating scale was 0.04 points | Mean score in gestrinone group was 0.35 points higher (0.12 to 0.58 higher) | 55 | ⊕⊕⊝⊝ | Verbal rating scale defines dysmenorrhoea according to limitation of ability to work (mild = 1, moderate = 2, incapacitated = 3) | |
| Dyspareunia, verbal rating scale Follow‐up: 6 months | In control group, mean score for dyspareunia on verbal rating scale was 0.43 points | Mean score in gestrinone group was 0.33 points lower (0.62 to 0.04 lower) | 52 | ⊕⊕⊝⊝ | Verbal rating scale defines dyspareunia according to limitation of sexual activity (discomfort tolerated = 1; pain interrupts intercourse = 2, intercourse avoided owing to pain = 3) | |
| Amenorrhoea Follow‐up: 6 months | 962 per 1000 | 500 per 1000 | OR 0.04 (0.01 to 0.38) | 49 | ⊕⊕⊝⊝ | Only 55 events overall |
| Spotting or bleeding Follow‐up: 6 months | 38 per 1000 | 475 per 1000 | OR 22.92 (2.64 to 198.66) | 49 | ⊕⊕⊝⊝ | Only 12 events overall |
| Side effects: hot flushes Follow‐up: 6 months | 679 per 1000 | 297 per 1000 (112 to 571) | OR 0.20 (0.06 to 0.63) | 55 | ⊕⊕⊝⊝ | Only 27 events overall |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded two levels for very serious imprecision: confidence intervals were compatible with no clinically meaningful difference between groups, or with small benefit in one group bDowngraded two levels for very serious imprecision: small overall sample size (n = 55) and low event rates | ||||||
| Outcome | Study | Comparison | Measure | Int group | Control group | P value | |
| Combined non‐pelvic pain, dysmenorrhoea, and dyspareunia | Asoprisnil: 5 mg (n = 31), 10 mg (n = 33), 25 mg (n = 32) Placebo (n = 34) | Mean reduction at 3 months on 0‐4 pain scale | 0.5 points for each dose | < 0.1 points | < 0.05 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Dysmenorrhoea at 3 months Show forest plot | 1 | 352 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.04, 0.17] |
| 1.1 Mifepristone 2.5 mg | 1 | 114 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.09, 0.64] |
| 1.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.16] |
| 1.3 Mifepristone 10 mg | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.17] |
| 2 Dyspareunia at 3 months Show forest plot | 1 | 223 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.51] |
| 2.1 Mifepristone 2.5 mg | 1 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.18, 2.13] |
| 2.2 Mifepristone 5 mg | 1 | 73 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.40] |
| 2.3 Mifepristone 10 mg | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Amenorrhoea at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 686.16 [92.29, 5101.33] |
| 2 Amenorrhoea at 3 months: subgroup analysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 207.67 [27.50, 1568.36] |
| 2.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 2.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3916.0 [348.75, 43971.52] |
| 3 Hot flushes at 3 months Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mifepristone all doses | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 28.79 [3.93, 210.73] |
| 4 Hot flushes at 3 months: subgroup analysis Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Mifepristone 2.5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.24 [2.49, 148.54] |
| 4.2 Mifepristone 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 23.82 [3.11, 182.24] |
| 4.3 Mifepristone 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 46.76 [6.21, 351.92] |
| 5 Nausea at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.20, 15.03] |
| 5.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 5.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.12, 48.60] |
| 6 Vomiting at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.01] |
| 6.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 6.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.04, 25.76] |
| 7 Fatigue/Tiredness at 3 months Show forest plot | 1 | 360 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.48 [0.71, 42.27] |
| 7.1 Mifepristone 2.5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.92 [0.21, 73.08] |
| 7.2 Mifepristone 5 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Mifepristone 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.11 [0.40, 125.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prevalence of dysmenorrhoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 2.5 mg vs 5 mg | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.22, 3.29] |
| 1.2 5 mg vs 10 mg | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.32, 4.71] |
| 2 Dysmenorrhoea score 0‐10 VAS scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Prevalence of dyspareunia Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 2.5 mg vs 5 mg | 1 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.37 [0.74, 54.81] |
| 3.2 5 mg vs 10 mg | 1 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.90] |
| 4 Dyspareunia score 0‐10 VAS scale Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 5 mg vs 25 mg | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Prevalence of pelvic pain Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 2.5 mg vs 5 mg | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.63, 5.17] |
| 5.2 5 mg vs 10 mg | 1 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [0.79, 19.97] |
| 6 Prevalence of amenorrhoea Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 6.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.56] |
| 6.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.08, 4.41] |
| 7 Prevalence of hot flushes Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.38, 1.89] |
| 7.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.35, 1.58] |
| 7.3 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.12, 87.13] |
| 8 Prevalence of nausea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.71] |
| 8.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 16.24] |
| 9 Prevalence of vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 75.46] |
| 9.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.20] |
| 10 Prevalence of fatigue/tiredness Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 2.5 mg vs 5 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.92 [0.77, 250.94] |
| 10.2 5 mg vs 10 mg | 1 | 180 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.54] |
| 11 Prevalence of endometrial thickness > 20 mm Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 5 mg vs 25 mg | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 None or mild pelvic pain Show forest plot | 2 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.56] |
| 2 None or mild dysmenorrhoea Show forest plot | 2 | 214 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.39, 1.33] |
| 3 None or mild dyspareunia Show forest plot | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.86] |
| 4 Adverse effects Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Acne | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.90, 2.33] |
| 4.2 Seborrhoea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.67, 4.46] |
| 4.3 Hirsutism | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.60, 4.32] |
| 4.4 Voice problems | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.34, 1.43] |
| 4.5 Swelling hands/feet | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.82, 2.38] |
| 4.6 Hot flushes | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.50, 1.26] |
| 4.7 Decreased breast size | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 0.98] |
| 4.8 Leg or muscle cramps | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.78] |
| 4.9 Headache | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.21] |
| 4.10 Nausea | 2 | 302 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.84, 2.19] |
| 4.11 Vomiting | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.43] |
| 4.12 Loss of appetite | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.72, 2.37] |
| 4.13 Hunger | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.97] |
| 4.14 Dizziness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.75, 2.05] |
| 4.15 Tiredness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.84, 2.45] |
| 4.16 Faintness | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.54, 2.76] |
| 4.17 Skin rash | 1 | 264 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.20] |
| 4.18 Weight gain | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.27] |
| 4.19 Vaginal dryness | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| 4.20 Raised liver transaminases | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Painful periods, visual analogue scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.15, 1.49] |
| 2 Painful periods, verbal rating scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.12, 0.58] |
| 3 Pain on intercourse, visual analogue scale Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐2.08, ‐0.24] |
| 4 Pain on intercourse, verbal rating scale Show forest plot | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.62, ‐0.04] |
| 5 Non‐menstrual pain, visual analogue scale Show forest plot | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐1.76, 0.94] |
| 6 Side effects Show forest plot | 1 | 813 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 6.1 Seborrhoea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.2 Swelling hands/feet | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.59 [0.26, 121.96] |
| 6.3 Hot flushes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.4 Leg or muscle cramps | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.55] |
| 6.5 Headache | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.63] |
| 6.6 Nausea | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.7 Dizziness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.8 Skin rash | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.23 [0.13, 82.71] |
| 6.9 Vaginal dryness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 4.21] |
| 6.10 Mood changes | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.10, 4.34] |
| 6.11 Joint pain | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.12 Drowsiness | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.18, 25.32] |
| 6.13 Tachycardia | 1 | 55 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.06, 17.49] |
| 6.14 Amenorrhoea | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.38] |
| 6.15 Spotting or bleeding | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 22.92 [2.64, 198.66] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 improvement in pain Show forest plot | 1 | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.01, 8.46] |
| 2 Side effect Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Noted any side effect | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 1.12] |
| 2.2 Discontinued because of headaches | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 8.42] |
| 2.3 Irregular bleeding | 1 | 12 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.16, 38.60] |

