Operation für schnellenden Finger
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Study design: quasi‐randomised controlled trial. Duration of the study: from January 2011 to December 2013. Protocol was published before recruitment of patients: not reported. Details of trial registration: not registered. Funding sources: none known. | |
| Participants | Place of study: Mazandaran University of Medical Sciences, Iran. Number of participants assigned: 50 participants (50 fingers); 25 percutaneous surgery and 25 steroid injection. Number of participants assessed: 50 participants (50 fingers); 25 percutaneous surgery and 25 steroid injection. Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Side:
Digits:
Classification of injury: trigger fingers were classified according to the Quinnell 1980 criteria (graded I to V). | |
| Interventions | Timing of intervention: not reported. Duration of treatment: not reported. Type of intervention:
Rehabilitation: the authors did not clearly describe whether all study participants received physiotherapy. Any co‐interventions: analgesia was given for 3 days. | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: not reported. Primary outcomes: Symptomatic relief: the authors did not clearly define what they considered as "symptomatic relief" and reported incomplete data, stating only that both groups showed improved symptoms within 2 weeks of follow‐up, and after 2 weeks the response was better in the steroid injection group. Patient satisfaction.
Secondary outcomes: Pain:measured by Visual Analog Scale (VAS) Recurrence of triggering: the authors did not clearly define what they considered recurrence. Outcomes included in this review: Pain: measured by Visual Analog Scale (VAS). Patient satisfaction. Recurrence of triggering Adverse events:
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised controlled trial. Participants were randomised to either steroid injection or percutaneous surgery using their birth year. Those with even numbers were allocated to the steroid group and uneven numbers to the percutaneous group. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised controlled trial. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. The authors did not clearly described whether all study participants completed follow‐up. |
| Selective reporting (reporting bias) | High risk | There was no protocol published. Outcome of interest in the review (resolution of trigger finger and functional status of the hand) were not reported. Pain was reported incompletely, without numerical values. |
| Other bias | Unclear risk | The authors did not report data about baseline balance, and they did not clearly report about care providers and rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: 1 May 2007 to 31 December 2008. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none known. | |
| Participants | Place of study: Thailand. Number of participants assigned: 142 participants (160 fingers); 80 percutaneous surgery and 80 open surgery. Number of participants assessed: 142 participants (160 fingers); 80 percutaneous surgery and 80 open surgery. Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Side: not reported. Digits:
Classification of injury: Trigger fingers were graded according to Froimson’s modification of Quinnell’s classification (graded I to IV) (Froimson 1993). | |
| Interventions | Timing of intervention: at least 3 months. Duration of treatment
Type of intervention:
Rehabilitation: not reported. Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: none lost to follow‐up. Primary outcomes: Operative time. Range of motion of finger PIP or thumb IP. Patient satisfaction score. Patient pain score. Surgical complications (adverse events):
Neurovascular injury. Outcomes included in this review: Resolution of trigger finger: considered as the relief of pain and the cessation of finger locking after the procedure. Although reported in the study, it was not considered as primary outcome by the author. Pain (0 to 3 scale). Satisfaction scores (0 to 3 scale). Recurrence of triggering: the author did not clearly define what he considered recurrence. Although reported in the study, it was not considered as primary outcome by the author. Adverse events measured by:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Pain and satisfaction score were measured by non‐validated instruments and the results were exhibited in figures, with inaccurate values and no measure of variance, compromising the assessment. |
| Other bias | Low risk | There was no baseline imbalance, and no risk of bias was associated with care providers or differences in rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: January 2007 to May 2007. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none known. | |
| Participants | Place of study: Varese, Italy. Number of participants assigned: 30 participants (30 fingers); 15 open surgery and 15 steroid injection plus hyaluronic acid injection ultrasound‐guided. Number of participants assessed: 30 participants (30 fingers); 15 open surgery and 15 steroid injection plus hyaluronic acid injection ultrasound‐guided. Inclusion criteria:
Exclusion criteria: Trigger finger grade IV. Comorbidities:
Age:
Gender:
Side: not reported. Digits: 16 thumb, 7 ring and 7 long. Classification of injury: Trigger fingers were graded according to Froimson’s modification of Quinnell’s classification (graded I to IV) (Froimson 1993). | |
| Interventions | Timing of intervention: average period: 3.5 months of symptoms (range 1 to 6 months). Duration of treatment: the duration of the surgical procedure was not reported. Hyaluronic acid injection was injected 10 days after steroid injection (injection group). Type of intervention:
Rehabilitation:
Any co‐interventions: 10 patients in open surgery group needed physiotherapy and local or oral analgesics for complete resolution of symptoms, which was approximately 30 to 40 days post surgery. | |
| Outcomes | Length of follow‐up: 1 year. Participants were evaluated before intervention, at 6 weeks and at 3, 6, and 12 months. Loss of follow‐up: none lost to follow‐up. Primary outcomes: Resolution of trigger finger: considered as the remission of symptoms within 6 weeks, with no recurrence within 6 months. Recurrence of triggering: was considered the return of any degree of triggering after a full remission period of trigger finger. Secondary outcomes: Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale). Functional status of the hand: was used DASH (0 to 100%). Satisfaction scores: measured by Satisfaction Visual Analog Scale (SVAS: 0 to 10 scale). Outcomes included in this review: Resolution of trigger finger Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale). Functional status of the hand: measured by DASH (0 to 100%). Satisfaction scores: measured by Satisfaction Visual Analog Scale (SVAS: 0 to 10 scale). Recurrence of triggering: Adverse event (Although reported in the study, it was not considered as primary outcome by the authors):
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Although outcomes of interest in the review were reported, the authors failed to report any measure of variance for the validated instruments (VAS, DASH and SVAS). |
| Other bias | High risk | There were different rehabilitations in 2 groups. In both groups, at the first follow‐up visit, participants were advised to mobilise the finger, depending on the level of pain experienced, but 10 participants in open surgery group needed physiotherapy, and local and/or oral analgesics for complete resolution of symptoms. |
| Methods | Study design: randomised controlled trial. Duration of the study: January 2005 to February 2007. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none known. | |
| Participants | Place of study: China. Number of participants assigned: 86 participants (97 trigger thumbs); 42 participants (47 thumbs) in percutaneous surgery group and 44 patients (50 thumbs) in steroid injection group. Number of participants assessed: 83 participants (93 trigger thumbs); 41 participants (46 thumbs) in percutaneous surgery group and 42 patients (47 thumbs) in steroid injection group. Inclusion criteria: idiopathic adult trigger thumbs with grade III–V on the Quinnell classification. Exclusion criteria: patients who had rheumatoid arthritis, diabetes mellitus or chronic systemic disease. Age:
Gender:
Side:
Digits: 97 thumbs. Classification of injury: The trigger thumb was graded according to Quinnell classification (graded I to V) (Quinnell 1980). | |
| Interventions | Timing of intervention: 4 months' duration of the symptoms in both groups. Duration of treatment: not reported. Type of intervention:
Rehabilitation: did not have rehabilitation. Any co‐interventions: topical NSAIDs were administered for 3 days with the occasional use of paracetamol for pain control in both groups when necessary. | |
| Outcomes | Length of follow‐up: 1 year Loss of follow‐up: 1 patient (1 thumb) in percutaneous surgery group and 2 patients (3 thumbs) in steroid injection group were lost to follow‐up at 12 months and were excluded. Primary outcomes: Successful procedure or satisfaction (resolution of trigger finger): "satisfactory" was considered to be participants who progressed with pain score lower than or equal to 1 (VAS scale) and cessation of triggering. Pain:measured by Visual Analog Scale (VAS:0 to 10 scale). Adverse events measured:
Neurovascular injury. Outcomes included in this review: Resolution of trigger finger: "satisfactory" was considered to be participants who progressed with pain score lower than or equal to 1 (VAS scale) and cessation of triggering. Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale). Recurrence of triggering: the authors did not evaluate it as a study outcome, but they published indirect data on recurrence. Adverse events measured:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomly assigned by the selection of number 1 or 2 from sealed envelopes in the presence of a witness. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced in numbers across intervention groups; 1 thumb 1/47 (2%) in percutaneous release group and 3 thumbs 3/50 (6%) in steroid injection group were lost to follow up of 12 months and excluded; however they were reported. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Functional status (as primary outcome using validated instruments to measure hand function) was not evaluated by the authors. |
| Other bias | Unclear risk | There was no baseline imbalance, but the authors did not describe about care providers or rehabilitation. |
| Methods | Study design: quasi‐randomised controlled trial. Duration of the study: during the year 2003. Protocol was published before recruitment of patients: not reported. Details of trial registration: not registered. Funding sources: none known. | |
| Participants | Place of study: Oldenburg, Germany. Number of participants assigned: 36 participants (36 fingers); 20 percutaneous surgery and 16 open surgery. Number of participants assessed: 36 participants (36 fingers); 20 percutaneous surgery and 16 open surgery. Inclusion criteria: patients with primary trigger finger and age between 18 and 80 years. Exclusion criteria: patients with trigger thumb, more than 1 trigger finger, previous operations of the upper extremity, evidence of symptomatic synovitis, or diseases possibly influencing pain scores or hand function (e.g. nerve entrapments, neuropathy, diabetes, and rheumatoid arthritis), or patients with any joint extension lag. Age:
Gender:
Side: not reported. Digits: not reported. Classification of injury: not reported. | |
| Interventions | Timing of intervention:
Duration of treatment:
Type of intervention:
Rehabilitation: A direct postoperative mobilization protocol was used in both groups. The authors did not describe which protocol was used, nor for how long it was used. Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: 12 weeks. Loss of follow‐up: none. Primary outcomes: Range of motion (ROM) of the PIP joint. Grip strength. Pain: mean score assessed using a scale from 1 to 6; 1 = no pain and 6 = extreme pain. Time of surgery. Postoperative complications (adverse events and neurovascular injury). Costs of the surgical techniques. Outcomes included in this review: Resolution of trigger finger: considered as complete relief of symptoms (the authors did not evaluate it as a study primary outcome, but they published indirect data on resolution). Pain: mean score assessed using a scale from 1 to 6; 1 = no pain and 6 = extreme pain. Recurrence of triggering: the authors did not evaluate it as a study primary outcome, but they published indirect data on recurrence. Adverse events:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised controlled trial. Participants were randomised to either open or percutaneous surgery using their patient numbers. When their numbers started with an uneven number, they were treated percutaneously, but if their numbers started an even number, they were treated by open surgery. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised controlled trial. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Functional status (as primary outcome using validated instruments to measure hand function) was not evaluated by the authors and pain was measured by non‐validated instrument. |
| Other bias | Low risk | There was no baseline imbalance, and no risk of bias was associated with care providers or differences in rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: between February 1993 and October 1994. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none known. | |
| Participants | Place of study: Rotterdam, the Netherlands. Number of participants assigned: 96 participants (100 trigger digits); 54 percutaneous surgery and 46 open surgery. Number of participants assessed: 96 participants (100 trigger digits); 54 percutaneous surgery and 46 open surgery. Inclusion criteria: Patients had to be older than 18 years and have symptoms of a trigger digit for at least 1 month. Exclusion criteria:
Age:
Gender:
Side: not reported. Digits: although the authors assessed the results of 100 fingers, they reported data on 99 fingers.
Classification of injury: not reported. | |
| Interventions | Timing of intervention: Mean duration of symptoms:
Duration of treatment:
Type of intervention: Percutaneous surgery:
Open surgery:
Rehabilitation:
Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: Follow‐up was 12 weeks. Patients were examined 10 days, 6 weeks, and 12 weeks after surgery. Loss of follow‐up: not reported. Primary outcomes: Mean duration of surgery (minutes). Mean duration of postoperative pain (days). Recovery of motor function (days). Return to work (days). Success rate (resolution of trigger finger): considered as the cessation of triggering, with no recurrence during follow‐up (3 months). Complications. Outcomes included in this review: Resolution of trigger finger: considered as the cessation of triggering, with no recurrence during follow‐up (3 months). Pain: reported the average postoperative pain duration in days. Recurrence of triggering: was reported in the study although it was not considered as primary outcome by the authors; the authors did not clearly define what they considered recurrence. Adverse event:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Pain and functional status of the hand (as primary outcomes, measured by validated instruments) were not evaluated by the authors. |
| Other bias | Low risk | There was no baseline imbalance, and no risk of bias was associated with care providers or differences in rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: January 2012 to May 2015. Protocol was published before recruitment of patients: yes. Details of trial registration: ClinicalTrials.gov, www.clinicaltrials.gov NCT01486420 Funding sources: the authors have declared no conflicts of interest. | |
| Participants | Place of study: Center for Planned Surgery, Regional Hospital Silkeborg, Silkeborg, Denmark. Number of participants assigned: 165 participants (165 fingers); 84 open surgery and 81 steroid injection. Number of participants assessed: 153 participants (153 fingers); 76 open surgery and 77 steroid injection. Inclusion criteria:
Exclusion criteria: Patients were excluded if they had insulin‐dependent diabetes mellitus, rheumatoid arthritis, amyloidosis, mucopolysaccharidosis, previous treatment of trigger finger in the included digit, Dupuytren disease affecting the included digit, or medical contraindications to corticosteroid injection. Age:
Gender:
Side: 97 right and 68 left. Digits: the allocated digit was the thumb in 39%, index in 5%, middle in 25%, ring in 25%, and little in 6%. Classification of injury: The trigger finger was graded according to Quinnell' classification modified by adding a history of uneven movement with pain or discomfort at the A1 pulley (graded I to V) (Quinnell 1980). | |
| Interventions | Timing of intervention:
Duration of treatment: not reported. Type of intervention:
Rehabilitation: not reported. Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: 12 months. The patients were prospectively assessed after 3 and 12 months. Loss of follow‐up: 1 patient in both groups were lost to follow‐up at 3 months, and 1 participant in open surgery group and 2 participants in steroid injection group were lost to follow‐up at 12 months and were excluded. Six participants in open surgery group and one participant in steroid injection group did not receive allocated intervention. Primary outcomes: Resolution of trigger finger (cure): the authors considered normal movement with or without pain or discomfort after 12 months of follow‐up. Secondary outcomes: Topical pain: defined as patient‐reported pain when pressure was applied on the palmar side of the hand at the level of the A1 pulley, assessed by a numerical rating scale from 1 to 10 (1 = no pain, and 10 = worst imaginable pain). Complications (adverse events):
Outcomes included in this review: Resolution of trigger finger. Pain: assessed by a numerical rating scale from 1 to 10 (1 = no pain, and 10 = worst imaginable pain). Recurrence of triggering. Adverse event:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The schedule for randomization was generated by the randomization software Research Randomizer (http://www.randomizer.org). The authors used a block randomization with blocks of 5 patients. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced in numbers across intervention groups; eight participants 8/84 (9%) in open surgery group and four participants 4/81 (5%) in steroid injection group were allocated to treatment, but they did not complete to follow up of 12 months and they were excluded; however they were reported. |
| Selective reporting (reporting bias) | Low risk | The study protocol was previously published. No changes in methods were applied after the trial commenced. |
| Other bias | Unclear risk | The groups were similar at baseline except for lower alcohol consumption in the open surgery group.The authors did not describe about differences in care providers or rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: between January 2013 and February 2014. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: the authors have declared no conflicts of interest. | |
| Participants | Place of study: Nijmegen, the Netherlands. Number of participants assigned: 30 participants (32 trigger digits); Open surgery by transversal incision of the skin in the distal palmar crease: 11 trigger fingers. Open surgery by transversal incision of the skin about 2–3 mm distally from distal palmar crease: 10 trigger fingers. Open surgery by longitudinal incision of the skin at the level of the A1‐pulley without crossing the distal palmar crease proximal: 11 trigger fingers. Number of participants assessed: 30 participants (32 trigger digits). Open surgery by transversal incision of the skin in the distal palmar crease: 11 trigger fingers. Open surgery by transversal incision of the skin about 2–3 mm distally from distal palmar crease: 10 trigger fingers. Open surgery by longitudinal incision of the skin at the level of the A1‐pulley without crossing the distal palmar crease proximal: 11 trigger fingers. Inclusion criteria:
Exclusion criteria:
Age (mean): 61.77 years. Gender: 13 male and 17 female. Side:
Digits: 4 index, 17 long, 8 ring and 3 little finger. Classification of injury: The trigger finger was graded according to Quinnell classification (Quinnell 1980). | |
| Interventions | Timing of intervention: at least 3 months. Duration of treatment: not reported. Type of intervention: All participants were submitted to open surgery, and was randomised to one of three kinds of skin incision:
All surgeries were performed under local anesthesia. Tourniquet was placed at the Rehabilitation:
Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: Follow‐up was 12 months. Patients were examined 1, 3 and 12 months after surgery. Loss of follow‐up: not reported. Primary outcomes: Functional status of the hand: was used DASH score. Scar volume: was measured using an high‐resolution ultrasound. Outcomes included in this review: Functional status of the hand: was used DASH score. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | Unclear risk | The authors did not assess or report any objective outcome. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Outcomes of interest in the review (resolution of trigger finger and pain) were not reported. |
| Other bias | High risk | There was baseline imbalance. The authors reported there was a significant difference in baseline DASH scores between groups. |
| Methods | Study design: randomised controlled trial. Duration of the study: October 1998 to December 2001. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none known. | |
| Participants | Place of study: Bangkok, Thailand. Number of participants assigned:
Number of participants assessed:
Inclusion criteria: Idiopathic adult trigger thumb grade II (actively correctable) III (passively correctable) or IV (fixed in flexion) according to the Quinnell classification (Quinnell 1980). Exclusion criteria:
Age:
Gender:
Side: Percutaneous release with steroid injection:
Steroid injection alone:
Digits: 127 thumbs. Classification of injury: The trigger thumb was graded according to Quinnell classification (graded 0 to IV) (Quinnell 1980). | |
| Interventions | Timing of intervention: Percutaneous release with steroid injection (mean): 4 months (1 to 36). Steroid injection alone (mean): 4 months (1 to 20). Duration of treatment: not reported. Type of intervention: Percutaneous release with steroid injection:
Steroid injection alone:
Rehabilitation: not reported. Any co‐interventions:
| |
| Outcomes | Length of follow‐up: range 23 months (6 to 42 months). Participants were evaluated at 2 and 6 weeks, and 6 or more months. Loss of follow‐up: 1 participant (1 thumb) was lost to follow‐up at 6 months in both groups. Primary outcomes: Resolution of trigger finger: "satisfactory" was considered to be participants who progressed with pain score lower than or equal to 1 (VAS scale) and cessation of triggering. Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale) and paracetamol requirement in the first 2 weeks. Outcomes included in this review: Resolution of trigger finger Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale). Adverse events: were reported in the study although not considered as primary outcomes by the author. Superficial infection (cellulitis). Partial loss of movement. Neurovascular injury: was reported in the study although not considered as primary outcome by the author. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data were balanced in numbers across intervention groups. 1 thumb was lost in both groups at 6 months: in percutaneous surgery plus steroid injection group 1/66 (1.5%); and in steroid injection 1/61 (1.6%). |
| Selective reporting (reporting bias) | High risk | No protocol was published. The authors did not report standard deviations on VAS score, and the functional status of the hand (using validated instruments) was not evaluated. |
| Other bias | Unclear risk | There was no baseline imbalance and no risk of bias was associated with care providers, but the author did not report about rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: not reported. Protocol was published before recruitment of patients: yes. Details of trial registration: clinicaltrials.gov/ct2/show/study/NCT02830672. Funding sources: the authors have declared no conflicts of interest. | |
| Participants | Place of study: National and Kapodistrian University of Athens, Greece. Number of participants assigned: 32 participants (32 fingers); 16 ultrasound‐guided percutaneous surgery and 16 open surgery. Number of participants assessed: 32 participants (32 fingers); 16 ultrasound‐guided percutaneous surgery and 16 open surgery. Inclusion criteria:
Exclusion criteria:
Age: mean age of 32 patients: 45.5 years old.
Gender: 12 male; 20 female.
Side: not reported. Digits: not reported. Classification of injury: Trigger fingers were graded according to Froimson’s modification of Quinnell’s classification (graded I to IV) (Froimson 1993). | |
| Interventions | Timing of intervention: at least 3 months. Duration of treatment: not reported. Type of intervention:
Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: none lost to follow‐up. Primary outcomes: Resolution of triggering was expressed as the “success rate” per digit. The time for taking postoperative pain killers. QuickDASH score. Range of motion recovery. Return to normal activities (including work). Cosmetic results. Outcomes included in this review: Resolution of triggering was expressed as the “success rate” per digit. Pain: postoperative pain duration (measured by mean time in days for taking postoperative pain killers). Functional status of the hand: QuickDASH score. Adverse events measured by:
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Low risk | Closed envelopes were used. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | Low risk | The self‐reported subjective outcomes assessors were blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | Unclear risk | Although results were evaluated and explained according to protocol published in July 6, 2016 and the study was published in February 18, 2017, the paper was received in the World Journal of Orthopedics' editorial in July 4, 2016 before date of the protocol publication. Although the protocol published by authors estimated the enrollment of 60 patients in the study, the sample of the paper was only 32 participants. |
| Other bias | Unclear risk | The authors did not report data about baseline balance, and they did not clearly report about care providers and rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: October 2005 to March 2006. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none known. | |
| Participants | Place of study: University of Milan, Italy. Number of participants assigned: 200 participants (231 fingers); Endoscopic surgery: 114 trigger fingers in 100 participants. Open surgery: 117 trigger fingers in 100 participants. Number of participants assessed: 200 participants (231 fingers). Endoscopic surgery: 114 trigger fingers in 100 participants. Open surgery: 117 trigger fingers in 100 participants. Inclusion criteria: Participants affected by trigger finger, despite other concomitant disease. Exclusion criteria: not reported. Age:
Gender:
Side: The authors reported data incompletely; in the endoscopic surgery group they reported the affected side in relation to the fingers, while in the open surgery group they reported the affected side in relation to the participants. Endoscopic surgery: 71 (fingers) right hand and 43 (fingers) left hand. Open surgery: 69 (participants) right hand and 31 (participants) left hand. Digits:
Classification of injury: not reported. | |
| Interventions | Timing of intervention: not reported Duration of treatment: Endoscopic surgery (mean): 4 min and 30 seconds (range: 2 to 9 min). Open surgery (mean): 5 min (range: 2 to 7 min). Type of intervention: Endoscopic surgery:
Open surgery:
Rehabilitation:
Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: 90 days. Follow‐up at 7, 30 and 90 days. The first follow‐up was excluded because the dressing still present in open surgery group did not allow a proper evaluation. Loss of follow‐up: none. Primary outcome: Resolution of trigger finger: considered as the disappearance of triggering after the procedure. Pain: reported the average postoperative pain duration in days. Adverse event:
Neurovascular injury. Outcomes included in this review: Resolution of trigger finger. Pain: reported the average postoperative pain duration in days. Adverse event:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Pain and functional status of the hand (as primary outcomes, measured by validated instruments) were not evaluated by the authors. |
| Other bias | High risk | There was baseline imbalance. Several participants in both groups had associated pathologies in hand and underwent surgery. |
| Methods | Study design: randomised controlled trial. Duration of the study: November 2002 to March 2007. Protocol was published before recruitment of patients: yes. Details of trial registration: Current Controlled Trials, www.controlled‐trials.com/ ISRCTN19255926. Funding sources: the authors have declared no conflicts of interest. | |
| Participants | Place of study: Federal University of São Paulo, Brazil. Number of participants assigned: 137 participants (150 fingers); 45 percutaneous surgery, 56 open surgery and 49 steroid injection. Number of participants assessed: 137 participants (150 fingers); 45 percutaneous surgery, 56 open surgery and 49 steroid injection. Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Side: not reported. Digits: 31 thumb, 4 index, 77 long, 35 ring and 3 little. Classification of injury: The trigger finger was graded according to Quinnell classification (graded 0 to IV) (Quinnell 1980). | |
| Interventions | Timing of intervention:
Duration of treatment: not reported. Type of intervention:
Rehabilitation: not reported. Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: 6 months. The patients were prospectively assessed after 1, 2 weeks, 1, 2, 4 and 6 months. Loss of follow‐up: none. Primary outcomes: Resolution of trigger finger (cure): the authors considered the remission of symptoms with the cessation of blockage with no recurrence within 6 months. Recurrence of triggering: the authors defined recurrence (relapse) as the return of finger locking within 6 months of follow‐up. Secondary outcomes: Topical pain. Articular pain. Total active motion (TAM) of the fingers. Complications (adverse events):
Neurovascular injury. Outcomes included in this review: Resolution of trigger finger. Pain: assessed through presence or not presence of the pain (topical or articular) in the hand. Recurrence of triggering. Adverse event:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was done by means of a 6‐sided die with 2 sides representing 1 of the 3 treatments. |
| Allocation concealment (selection bias) | Low risk | The result of each draw was placed in an opaque envelope, which was then sealed; envelopes were numbered from 1 to 150. None of the project participants had prior access to the envelope contents. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | The authors did not report missing data. |
| Selective reporting (reporting bias) | Unclear risk | Although results were evaluated and explained according to previous protocol published in October 2010, the study started in November 2002 and finished in March 2007. |
| Other bias | Unclear risk | There was no baseline imbalance or differences in care providers, but the authors did not describe rehabilitation. |
| Methods | Study design: quasi‐randomised controlled trial. Duration of the study: from January 2005 to June 2005. Protocol was published before recruitment of patients: not reported. Details of trial registration: not registered. Funding sources: none known. | |
| Participants | Place of study: Penang General Hospital, Malaysia. Number of participants assigned: 26 participants (26 fingers); 14 percutaneous surgery and 12 steroid injection. Number of participants assessed: 26 participants (26 fingers); 14 percutaneous surgery and 12 steroid injection. Inclusion criteria:
Exclusion criteria:
Age:
Gender:
Side:
Digits:
Classification of injury: The trigger finger was graded according to Quinnell classification (graded 0 to IV) (Quinnell 1980). | |
| Interventions | Timing of intervention: not reported. Duration of treatment: not reported. Type of intervention:
Rehabilitation: the authors did not clearly describe if all study participants did physiotherapy. They reported that in percutaneous surgery group 2 participants developed stiffness of digit which responded to aggressive physiotherapy. Any co‐interventions: analgesia was given for 3 days in steroid injection group. | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: not reported. Primary outcomes: Pain. Patient satisfaction. Recurrence of triggering: the authors did not clearly define what they considered recurrence. Adverse events:
Outcomes included in this review: Pain. Patient satisfaction. Recurrence of triggering Adverse events:
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised controlled trial. Patients were randomised to either steroid injection or percutaneous surgery using their birth year. Those with even numbers were allocated to the steroid group and uneven numbers to the percutaneous group. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised controlled trial. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | High risk | The self‐reported subjective outcomes assessors were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | High risk | The objective outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Outcomes of interest in the review (resolution of trigger finger and functional status of the hand) were not reported. Pain was reported incompletely, without numerical values. |
| Other bias | Unclear risk | The authors did not report data about baseline balance, and they did not clearly report about care providers and rehabilitation. |
| Methods | Study design: randomised controlled trial. Duration of the study: January 2008 to May 2009. Protocol was published before recruitment of patients: not reported. Details of trial registration: not reported. Funding sources: none declared. | |
| Participants | Place of study: Pomeranian Medical University in Szczecin, Poland. Number of participants assigned: 115 participants. Number of participants assessed: 95 participants (105 digits); 43 participants (46 digits) percutaneous surgery and 52 participants (59 digits) steroid injection. Inclusion criteria: participants with trigger digits. Exclusion criteria: not reported. Age:
Gender:
Side:
Digits:
Classification of injury: Trigger fingers were graded according to Froimson’s modification of Quinnell’s classification (graded I to IV) (Froimson 1993). | |
| Interventions | Timing of intervention:
Duration of treatment: not reported. Type of intervention:
Rehabilitation: not reported. Any co‐interventions: not reported. | |
| Outcomes | Length of follow‐up: 6 months (at 1 and 6 months). Loss of follow‐up: 20 participants (12 in the percutaneous surgery group and 8 in the steroid injection group). Primary outcomes: Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale). Active range of motion (AROM) of the affected digit. Total grip strength: expressed as a proportion of the strength of the contralateral, healthy hand. Recurrence of triggering: considered as the return to the baseline grade, after a period of total or partial improvement of the trigger finger. Adverse event:
Outcomes included in this review: Pain: measured by Visual Analog Scale (VAS: 0 to 10 scale). Recurrence of triggering. Adverse event:
Neurovascular injury. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to the groups by drawing slips of paper marked 1 (percutaneous release) or 2 (steroid injection) from a sealed envelope in the presence of a witness. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) ‐ subjective outcomes (e.g. resolution, recurrence, pain, function, satisfaction) | Low risk | The self‐reported subjective outcomes assessors were blinded. |
| Blinding of outcome assessment (detection bias) ‐ objective outcomes (e.g. adverse events, neurovascular injury) | Low risk | The objective outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | High risk | 20 of 115 patients (17%) who were recruited did not attend follow‐up and were excluded (12 participants in the percutaneous surgery group and 8 in the steroid injection group). The authors did not describe how many fingers were affected in these 20 patients. |
| Selective reporting (reporting bias) | High risk | No protocol was published. Outcomes of interest in the review (resolution of trigger finger and functional status of the hand) were not reported. Pain was reported incompletely, without any measure of variance for the validated instruments (VAS). |
| Other bias | Unclear risk | There was no baseline imbalance and no risk of bias was associated with care providers, but the author did not report about rehabilitation. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. | |
| Design of study not relevant: retrospective comparative study. | |
| Design of study not relevant: narrative review. | |
| Design of study not relevant: retrospective comparative study. | |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. | |
| Design of study not relevant: retrospective comparative study. | |
| Intervention is steroid injection plus percutaneous surgery versus percutaneous surgery alone. | |
| Intervention is steroid injection plus percutaneous surgery versus percutaneous surgery alone. | |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. | |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. | |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. | |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. | |
| This study was not included because none of the outcomes of interest in this review (resolution of trigger finger, severity of pain or tenderness at the base of the digit on the palm of the hand, functional status of the hand, participant‐reported treatment success or satisfaction, frequency of recurrence of triggering, number of patients experiencing any adverse event or neurovascular injury) were assessed. The authors defined a surgical model as the combination of a procedure (sonographically guided, wide awake, or classic) and a setting (day surgery or office‐based), and they assessed the outcomes turnover analysis and economic analysis for each model. | |
| Although the author randomised 3 different surgical treatments in trigger finger initially (surgical release of the third proximal, middle and distal pulley A1), there was intraoperative change in all patients after it was observed that the partial release of one‐third pulley A1 was not curative treatment for trigger finger. So the authors chose to perform the complete open release of the A1 pulley in all cases. Thus, it was not characterized a randomised clinical trial. | |
| Design of study not relevant: not a randomised or quasi‐randomised controlled trial. |
Characteristics of ongoing studies [ordered by year of study]
| Trial name or title | The efficacy of Trigger Finger treatment: a randomised, controlled, prospective clinical multicentre trial. |
| Methods | Study design: randomized controlled trial. Random sequence generation: not reported. Allocation concealment: not reported. Masking: open label. |
| Participants | Location: plastic surgery outpatient clinic in the UMC Utrecht, The Hand Clinic Amsterdam, Diakonessenhuis Zeist, the Mesos Medical Center Utrecht, the St. Antonius Hospital Nieuwegein, the Zuwe Hofpoort Hospital Woerden and the Meander Medical Center Amersfoort, the Netherlands. Target sample size (N): 490 participants. Inclusion criteria: adults with trigger finger. Exclusion criteria:
|
| Interventions | Type of surgical intervention: open surgery. Type of conservative intervention: local corticosteroid injections (triamcinolone acetonide). |
| Outcomes | Primary outcomes:
Secondary outcomes:
Timing of outcomes measurement: not reported. |
| Starting date | Main ID: NTR1135 Date of registration: 18 November 2007. Last refreshed on: 30 April 2017. Date of first enrolment: 1 January 2008. Status: recruiting. Estimated Study Completion date: January 2011. |
| Contact information | Name: A.S.E. Esschendal. Address: Postbus 85500 , Secretariaat Plastische Chirurgie UMC Utrecht kamer G04.122 Utrecht, the Netherlands. Telephone: +31 30 250 6954 Email: [email protected] Affiliation: not reported. |
| Notes |
| Trial name or title | A1‐Pulley release using open conventional technique or percutaneously with a modified Kirschner wire: a prospective randomised‐controlled trial. |
| Methods | Study design: randomised controlled trial. Random sequence generation: not reported. Allocation concealment: not reported. Masking: open label. |
| Participants | Location: Department of Orthopedics, Faculty of Medicine, Khonkaen University, Thailand. Target sample size (N): 51 participants. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Type of surgical intervention: open surgery. Type of conservative intervention: percutaneous release by using a modified Kirschner wire. |
| Outcomes | Primary outcomes: time to return to work. Timing of outcomes measurement: 1 year. |
| Starting date | Main ID: TCTR20150416001. Date of registration: 16 April 2015. Last refreshed on: 11 September 2017. Date of first enrolment: 16 April 2015. Status: recruiting. Estimated Study Completion date: 31 December 2016. |
| Contact information | Name: Surut Jianmongkol, M.D. Address: Department of Orthopedics, Faculty of Medicine, Khonkaen University Khonkaen 40002 Thailand. Telephone: 6643348398 Email: [email protected] Affiliation: not reported. |
| Notes |
| Trial name or title | Percutaneous trigger finger release, probe knife compared with 18‐gauge needle : A randomized control trial. |
| Methods | Study design: randomised controlled trial. Random sequence generation: not reported. Allocation concealment: not reported. Masking: single blind (masked roles: outcomes assessor). |
| Participants | Location: Songklanakarin Hospital, Thailand. Target sample size (N): 128 participants. Inclusion criteria:
Exclusion criteria:
|
| Interventions | Type of surgical intervention: percutaneous release with needle. Type of surgical intervention: percutaneous release with probe scalpel. |
| Outcomes | Primary outcomes:
Secondary outcomes:
Timing of outcomes measurement: not reported. |
| Starting date | Main ID: TCTR20140529001 Date of registration: May 29, 2014. Last refreshed on: September 11, 2017. Date of first enrolment: May 30, 2014. Status: Active, not recruiting. Estimated Study Completion date: not reported. |
| Contact information | Name: Sittichoke Anuntaseree, M.D. Address: Songklanakarin Hospital, Hat Yai 90110 Thailand. Telephone: 66869691017 Email: [email protected] Affiliation: Faculty of Medicine, PSU. |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 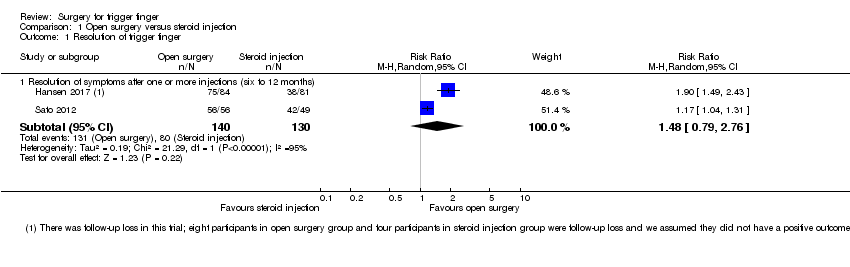 Comparison 1 Open surgery versus steroid injection, Outcome 1 Resolution of trigger finger. | ||||
| 1.1 Resolution of symptoms after one or more injections (six to 12 months) | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.79, 2.76] |
| 2 Pain on the palm of the hand Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Open surgery versus steroid injection, Outcome 2 Pain on the palm of the hand. | ||||
| 2.1 Pain short‐term (one week) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 3.69 [1.99, 6.85] |
| 2.2 Pain intermediate‐term (six months) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.77] |
| 3 Pain (1 to 10 scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Open surgery versus steroid injection, Outcome 3 Pain (1 to 10 scale). | ||||
| 3.1 Pain short‐term (three months) | 1 | 156 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 3.2 Pain long‐term (12 months) | 1 | 153 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐2.68, ‐1.32] |
| 4 Frequency of recurrence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Open surgery versus steroid injection, Outcome 4 Frequency of recurrence. | ||||
| 4.1 Recurrence (range six to 12 months) | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.33] |
| 5 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Open surgery versus steroid injection, Outcome 5 Adverse events. | ||||
| 5.1 Infection | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.88, 7.99] |
| 5.2 Tendon or pulley injury | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Flare around procedure site | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.15] |
| 5.4 Cutaneous discomfort around procedure site (after 12 months) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [1.25, 10.44] |
| 5.5 Fat necrosis at the procedure site | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.47, 3.54] |
| 5.6 Total adverse events | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.84] |
| 6 Neurovascular injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Open surgery versus steroid injection, Outcome 6 Neurovascular injury. | ||||
| 6.1 Neurovascular injury | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.70, 6.77] |
| 7 Subgroup analyses for resolution Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Open surgery versus steroid injection, Outcome 7 Subgroup analyses for resolution. | ||||
| 7.1 Resolution short‐term | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.21] |
| 7.2 Resolution intermediate‐term | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.04, 1.31] |
| 7.3 Resolution long‐term | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.49, 2.43] |
| 8 Subgroup analyses for recurrence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8 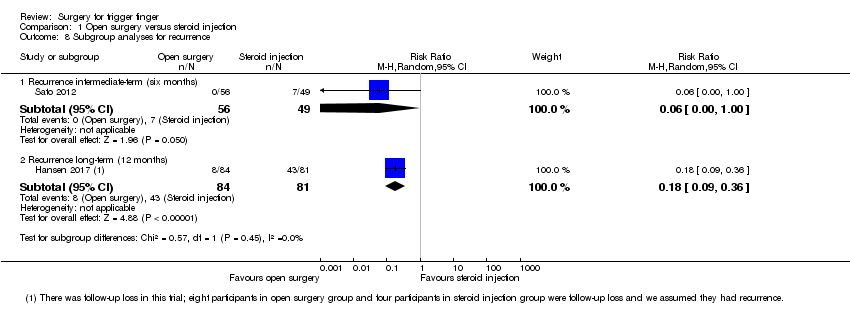 Comparison 1 Open surgery versus steroid injection, Outcome 8 Subgroup analyses for recurrence. | ||||
| 8.1 Recurrence intermediate‐term (six months) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.00] |
| 8.2 Recurrence long‐term (12 months) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.09, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 1 Resolution of trigger finger. | ||||
| 1.1 Resolution of symptoms after one or more injections (six to 12 months) | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.31, 14.51] |
| 2 Pain (VAS: 0 to 10 scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 2 Pain (VAS: 0 to 10 scale). | ||||
| 2.1 Pain short‐term (one month) | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐5.72, 2.12] |
| 2.2 Pain long‐term (12 months) | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐6.5 [‐7.25, ‐5.75] |
| 3 Pain on the palm of the hand Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 3 Pain on the palm of the hand. | ||||
| 3.1 Pain short‐term (one week) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.63 [1.94, 6.78] |
| 3.2 Pain intermediate‐term (six months) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.18] |
| 4 Frequency of recurrence Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 4 Frequency of recurrence. | ||||
| 4.1 Recurrence (range six to 12 months) | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.59] |
| 5 Adverse events Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 5 Adverse events. | ||||
| 5.1 Infection | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.29] |
| 5.2 Partial loss of movement | 3 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.87, 10.97] |
| 5.3 Tendon or pulley injury | 4 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.81] |
| 5.4 Dysaesthesia | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.67] |
| 5.5 Skin atrophy or hypopigmentation | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.63] |
| 5.6 Total adverse events | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.91, 2.75] |
| 6 Neurovascular injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 6 Neurovascular injury. | ||||
| 6.1 Neurovascular injury | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.29] |
| 7 Subgroup analyses for resolution Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 7 Subgroup analyses for resolution. | ||||
| 7.1 Resolution short‐term | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.55, 3.05] |
| 7.2 Resolution intermediate‐term | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.03, 1.31] |
| 7.3 Resolution long‐term | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 3.90 [2.37, 6.42] |
| 8 Subgroup analyses for recurrence Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Percutaneous surgery versus steroid injection, Outcome 8 Subgroup analyses for recurrence. | ||||
| 8.1 Recurrence short‐term (one month) | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.76, 3.94] |
| 8.2 Recurrence intermediate‐term (six months) | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 5.50] |
| 8.3 Recurrence long‐term (range nine to 12 months) | 3 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.10, 2.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound, Outcome 1 Resolution of trigger finger. | ||||
| 1.1 Resolution of symptoms long‐term (12 months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.98, 1.85] |
| 1.2 Resolution of symptoms intermediate‐term (six month) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.89, 1.28] |
| 2 Frequency of recurrence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound, Outcome 2 Frequency of recurrence. | ||||
| 2.1 Recurrence long‐term (12 months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.55] |
| 2.2 Recurrence intermediate‐term (six months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3 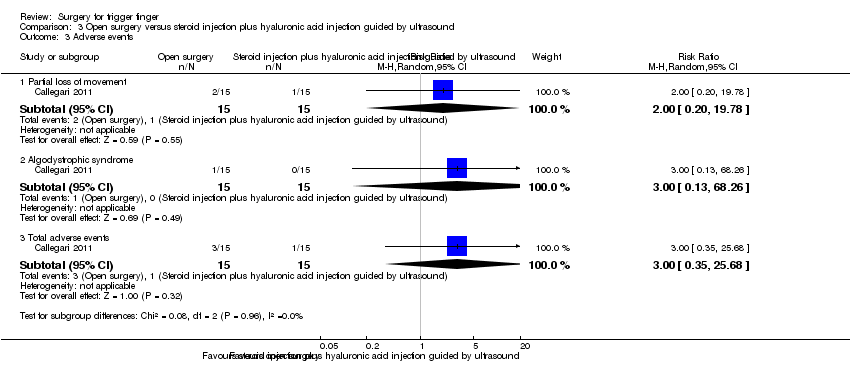 Comparison 3 Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound, Outcome 3 Adverse events. | ||||
| 3.1 Partial loss of movement | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 19.78] |
| 3.2 Algodystrophic syndrome | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 68.26] |
| 3.3 Total adverse events | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.35, 25.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Percutaneous surgery plus steroid injection versus steroid injection, Outcome 1 Resolution of trigger finger. | ||||
| 1.1 Resolution of symptoms after one or more injections (range 6 to 42 months) | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.21, 1.90] |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Percutaneous surgery plus steroid injection versus steroid injection, Outcome 2 Adverse events. | ||||
| 2.1 Infection | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.04, 4.97] |
| 2.2 Partial loss of movement | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.17, 19.87] |
| 2.3 Total adverse events | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.13, 6.36] |
| 3 Neurovascular injury Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Percutaneous surgery plus steroid injection versus steroid injection, Outcome 3 Neurovascular injury. | ||||
| 3.1 Neurovascular injury | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.06, 14.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1 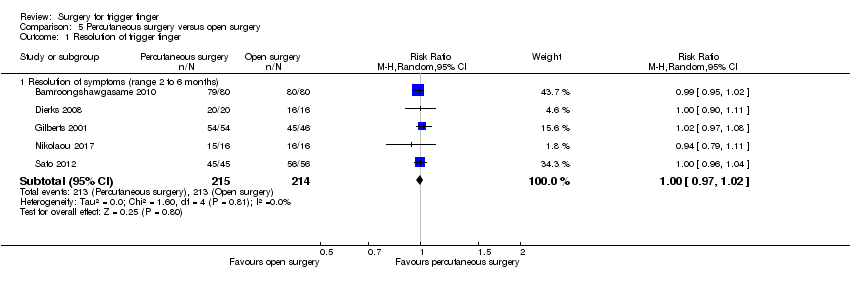 Comparison 5 Percutaneous surgery versus open surgery, Outcome 1 Resolution of trigger finger. | ||||
| 1.1 Resolution of symptoms (range 2 to 6 months) | 5 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 2 Pain (1 to 6 scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Percutaneous surgery versus open surgery, Outcome 2 Pain (1 to 6 scale). | ||||
| 2.1 Pain short‐term (1 week) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.34, 0.94] |
| 2.2 Pain short‐term (12 weeks) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.52, 0.52] |
| 3 Pain on the palm of the hand Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3 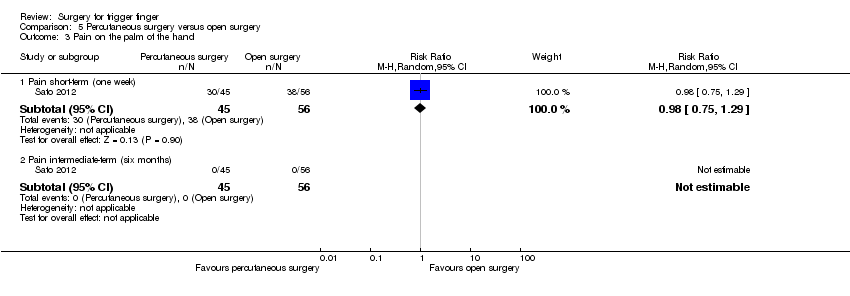 Comparison 5 Percutaneous surgery versus open surgery, Outcome 3 Pain on the palm of the hand. | ||||
| 3.1 Pain short‐term (one week) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.29] |
| 3.2 Pain intermediate‐term (six months) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Frequency of recurrence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Percutaneous surgery versus open surgery, Outcome 4 Frequency of recurrence. | ||||
| 4.1 Recurrence (range 2 to 6 months) | 4 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
| 5 Adverse events Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Percutaneous surgery versus open surgery, Outcome 5 Adverse events. | ||||
| 5.1 Infection | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Partial loss of movement | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Tendon or pulley injury | 2 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Edema or inflammation or hematoma | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.12, 5.30] |
| 5.5 Adherence | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
| 5.6 Others (it did not specified) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.11, 61.45] |
| 5.7 Total adverse events | 5 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.17, 3.68] |
| 6 Subgroup analyses for resolution Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.6 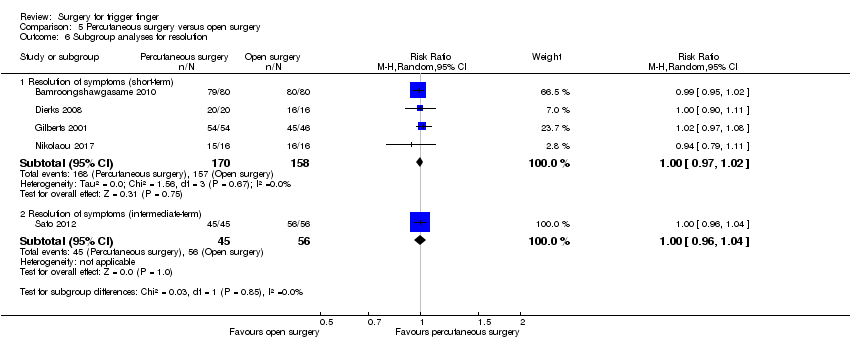 Comparison 5 Percutaneous surgery versus open surgery, Outcome 6 Subgroup analyses for resolution. | ||||
| 6.1 Resolution of symptoms (short‐term) | 4 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 6.2 Resolution of symptoms (intermediate‐term) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.96, 1.04] |
| 7 Subgroup analyses for recurrence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.7  Comparison 5 Percutaneous surgery versus open surgery, Outcome 7 Subgroup analyses for recurrence. | ||||
| 7.1 Recurrence short‐term (eight to 12 weeks) | 3 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
| 7.2 Recurrence intermediate‐term (six months) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.1 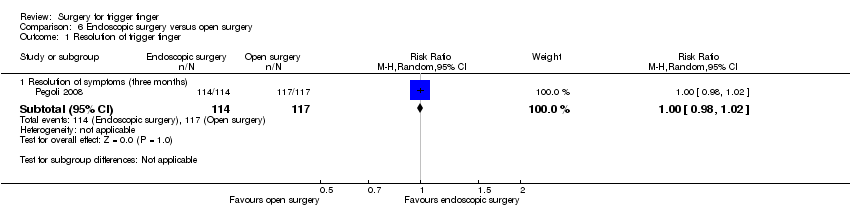 Comparison 6 Endoscopic surgery versus open surgery, Outcome 1 Resolution of trigger finger. | ||||
| 1.1 Resolution of symptoms (three months) | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.98, 1.02] |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.2 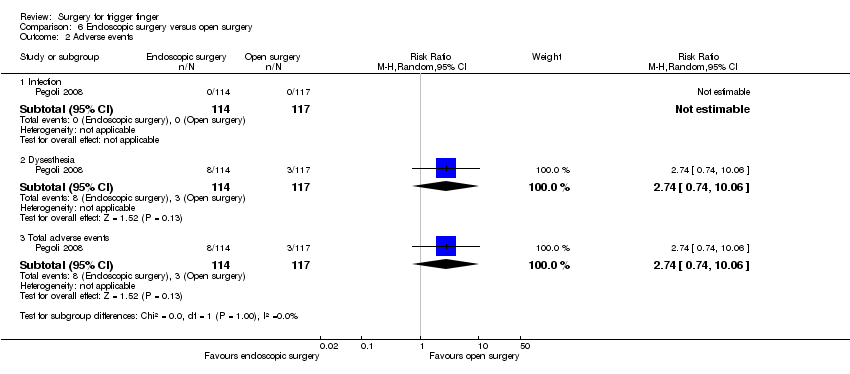 Comparison 6 Endoscopic surgery versus open surgery, Outcome 2 Adverse events. | ||||
| 2.1 Infection | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Dysesthesia | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [0.74, 10.06] |
| 2.3 Total adverse events | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [0.74, 10.06] |
| 3 Neurovascular injury Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 6.3  Comparison 6 Endoscopic surgery versus open surgery, Outcome 3 Neurovascular injury. | ||||
| 3.1 Neurovascular injury | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [0.13, 74.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 7.1 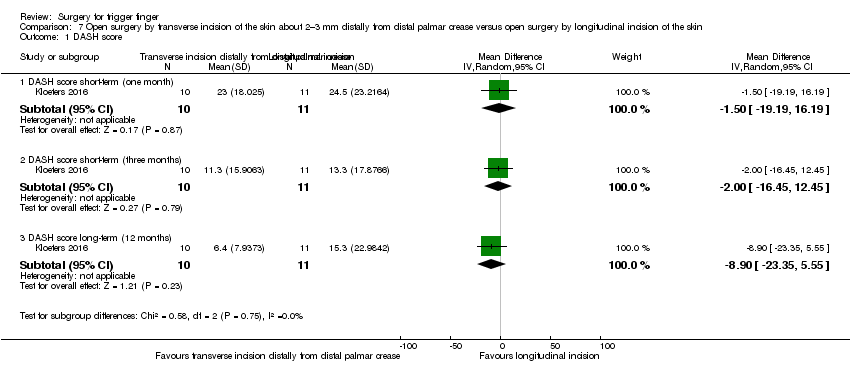 Comparison 7 Open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease versus open surgery by longitudinal incision of the skin, Outcome 1 DASH score. | ||||
| 1.1 DASH score short‐term (one month) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐19.19, 16.19] |
| 1.2 DASH score short‐term (three months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐16.45, 12.45] |
| 1.3 DASH score long‐term (12 months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐8.9 [‐23.35, 5.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 8.1 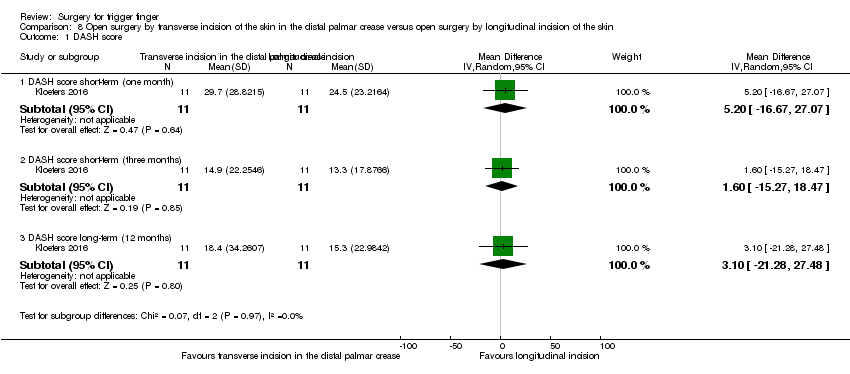 Comparison 8 Open surgery by transverse incision of the skin in the distal palmar crease versus open surgery by longitudinal incision of the skin, Outcome 1 DASH score. | ||||
| 1.1 DASH score short‐term (one month) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 5.20 [‐16.67, 27.07] |
| 1.2 DASH score short‐term (three months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐15.27, 18.47] |
| 1.3 DASH score long‐term (12 months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐21.28, 27.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Open surgery by transverse incision of the skin in the distal palmar crease versus open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease, Outcome 1 DASH score. | ||||
| 1.1 DASH score short‐term (one month) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 6.70 [‐13.67, 27.07] |
| 1.2 DASH score short‐term (three months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 3.60 [‐12.84, 20.04] |
| 1.3 DASH score long‐term (12 months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 12.00 [‐8.84, 32.84] |

Study flow diagram.
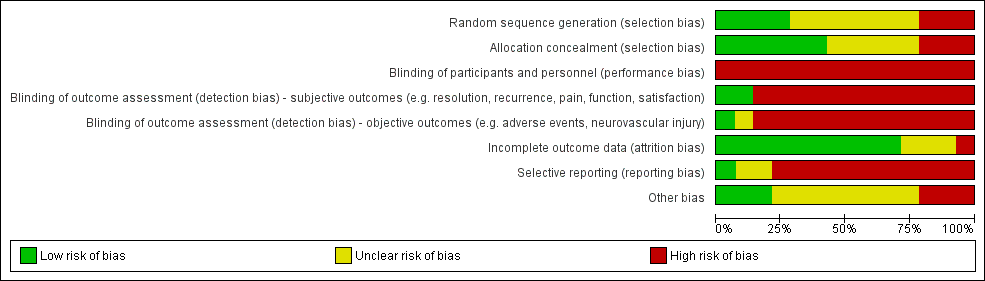
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
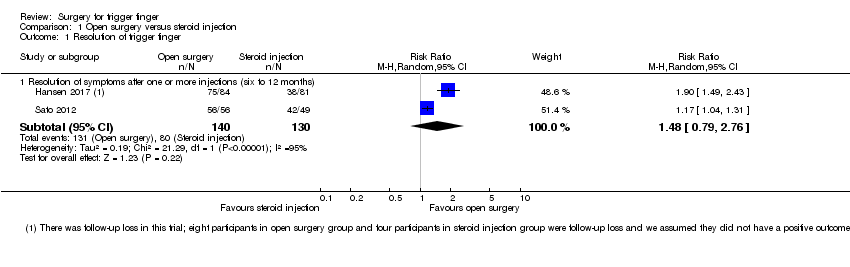
Comparison 1 Open surgery versus steroid injection, Outcome 1 Resolution of trigger finger.

Comparison 1 Open surgery versus steroid injection, Outcome 2 Pain on the palm of the hand.
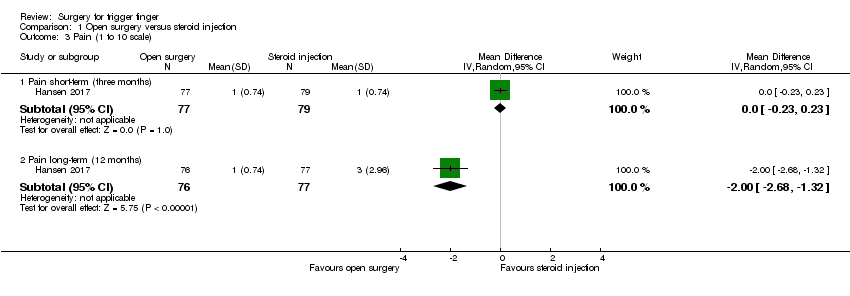
Comparison 1 Open surgery versus steroid injection, Outcome 3 Pain (1 to 10 scale).

Comparison 1 Open surgery versus steroid injection, Outcome 4 Frequency of recurrence.
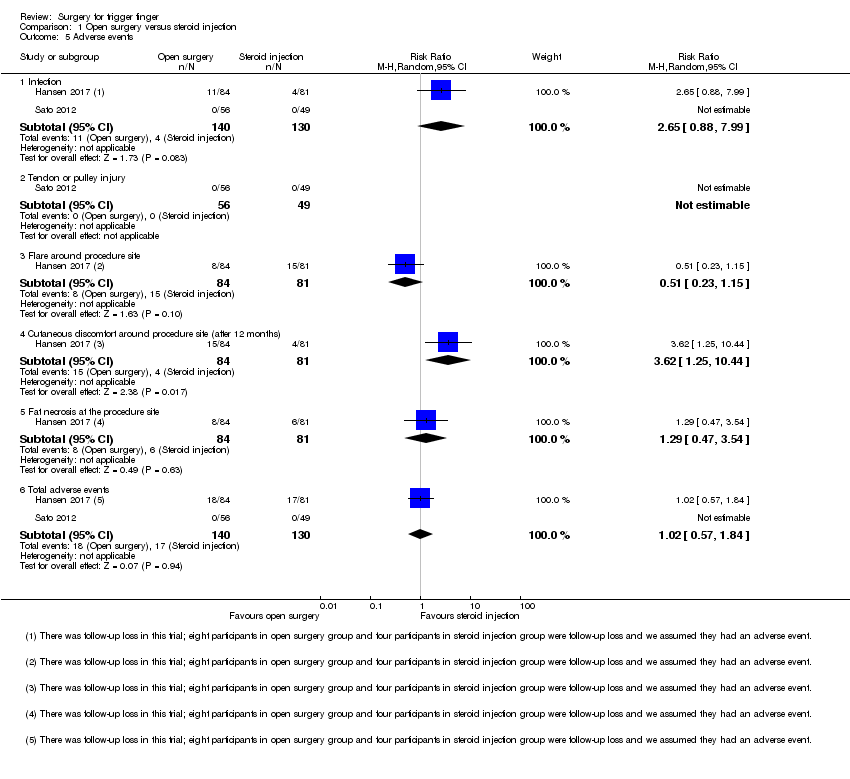
Comparison 1 Open surgery versus steroid injection, Outcome 5 Adverse events.
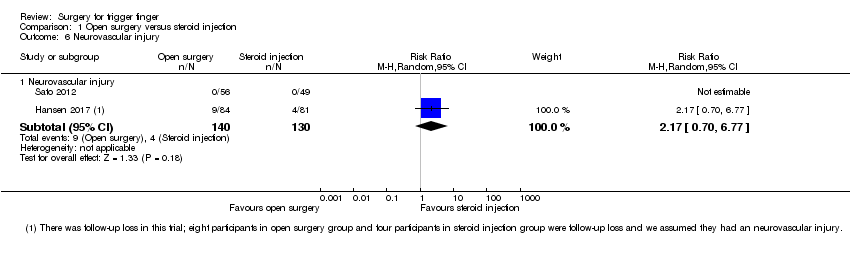
Comparison 1 Open surgery versus steroid injection, Outcome 6 Neurovascular injury.
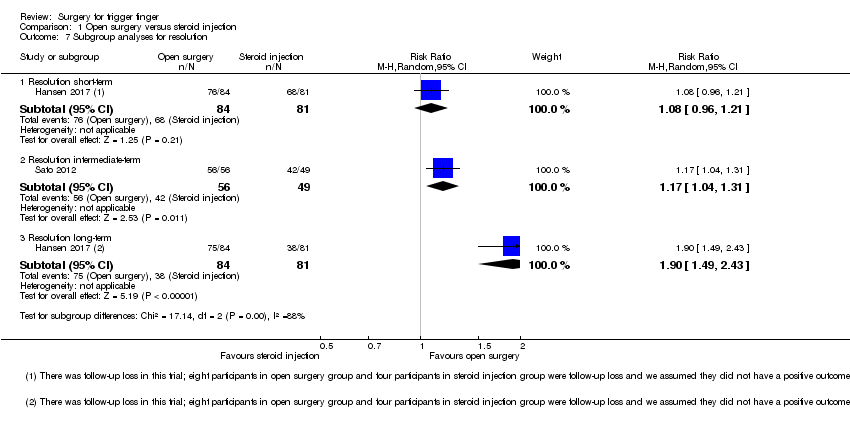
Comparison 1 Open surgery versus steroid injection, Outcome 7 Subgroup analyses for resolution.
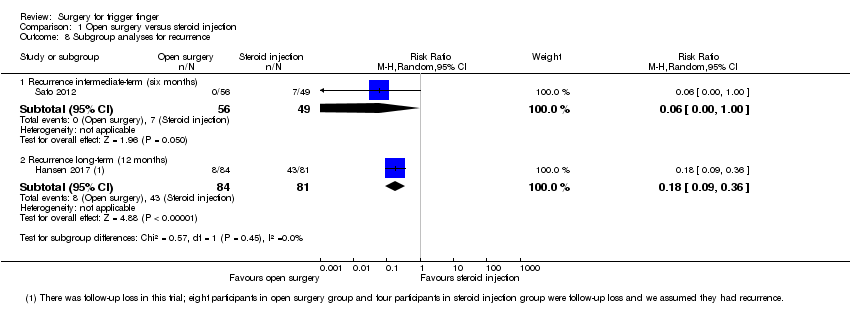
Comparison 1 Open surgery versus steroid injection, Outcome 8 Subgroup analyses for recurrence.
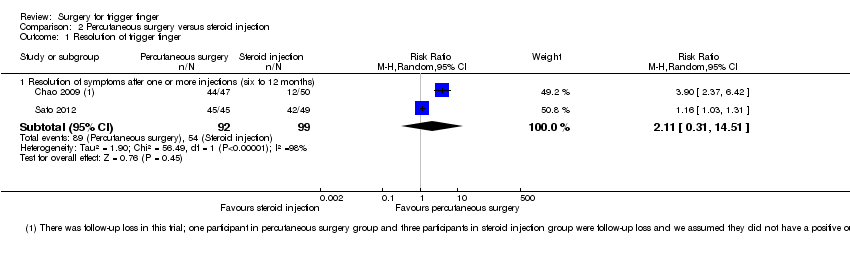
Comparison 2 Percutaneous surgery versus steroid injection, Outcome 1 Resolution of trigger finger.
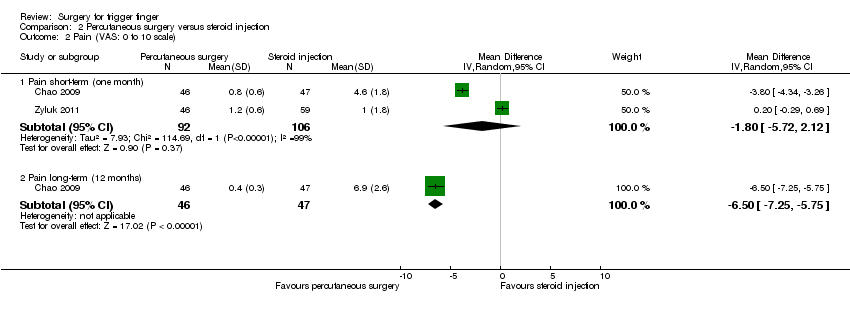
Comparison 2 Percutaneous surgery versus steroid injection, Outcome 2 Pain (VAS: 0 to 10 scale).
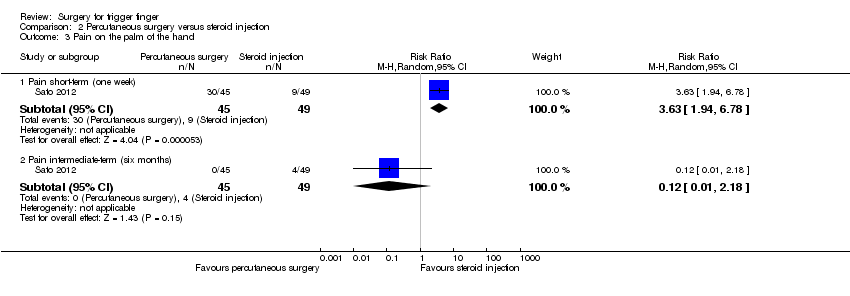
Comparison 2 Percutaneous surgery versus steroid injection, Outcome 3 Pain on the palm of the hand.

Comparison 2 Percutaneous surgery versus steroid injection, Outcome 4 Frequency of recurrence.
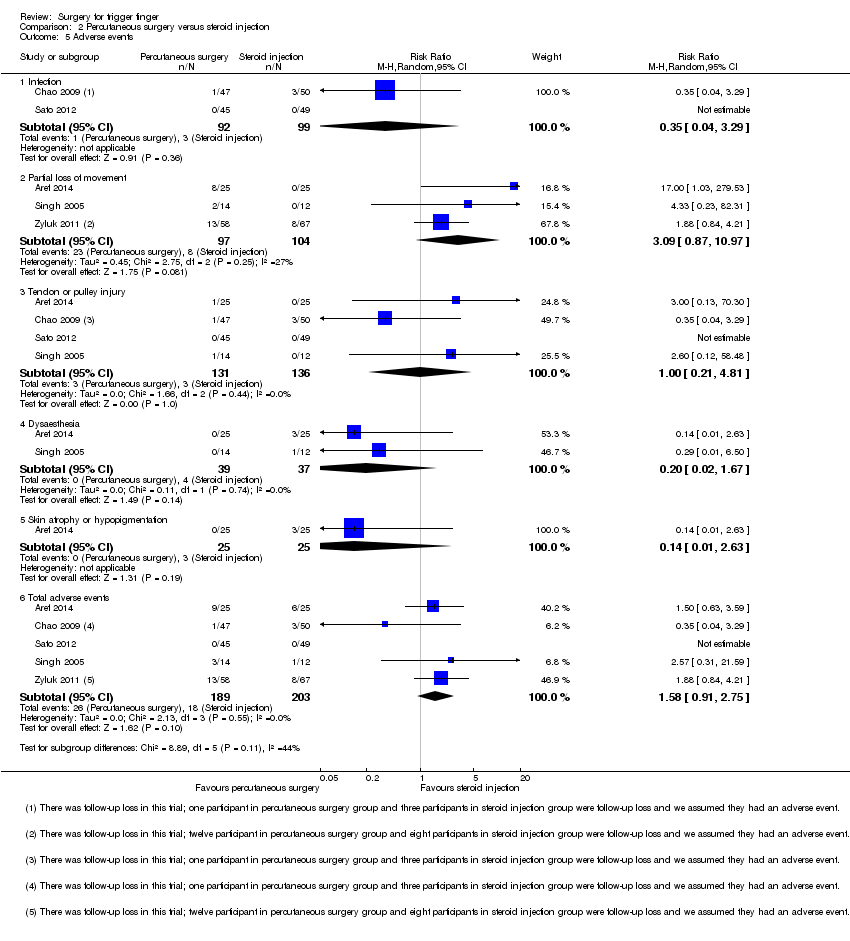
Comparison 2 Percutaneous surgery versus steroid injection, Outcome 5 Adverse events.

Comparison 2 Percutaneous surgery versus steroid injection, Outcome 6 Neurovascular injury.

Comparison 2 Percutaneous surgery versus steroid injection, Outcome 7 Subgroup analyses for resolution.

Comparison 2 Percutaneous surgery versus steroid injection, Outcome 8 Subgroup analyses for recurrence.

Comparison 3 Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound, Outcome 1 Resolution of trigger finger.
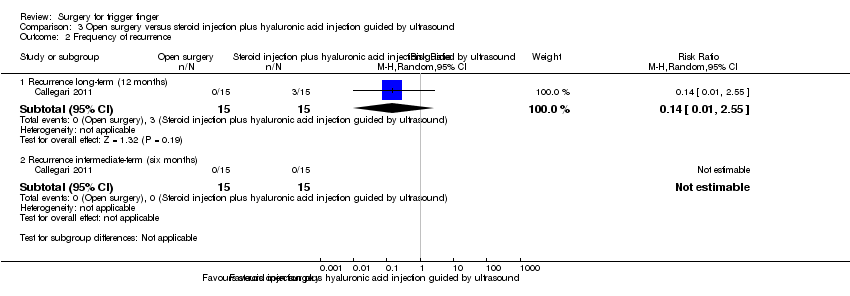
Comparison 3 Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound, Outcome 2 Frequency of recurrence.

Comparison 3 Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound, Outcome 3 Adverse events.

Comparison 4 Percutaneous surgery plus steroid injection versus steroid injection, Outcome 1 Resolution of trigger finger.

Comparison 4 Percutaneous surgery plus steroid injection versus steroid injection, Outcome 2 Adverse events.

Comparison 4 Percutaneous surgery plus steroid injection versus steroid injection, Outcome 3 Neurovascular injury.
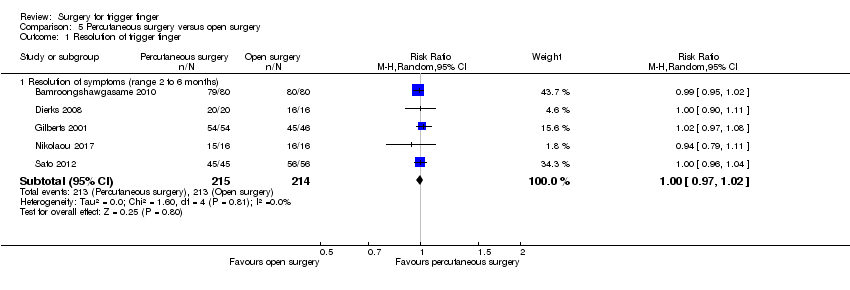
Comparison 5 Percutaneous surgery versus open surgery, Outcome 1 Resolution of trigger finger.

Comparison 5 Percutaneous surgery versus open surgery, Outcome 2 Pain (1 to 6 scale).

Comparison 5 Percutaneous surgery versus open surgery, Outcome 3 Pain on the palm of the hand.
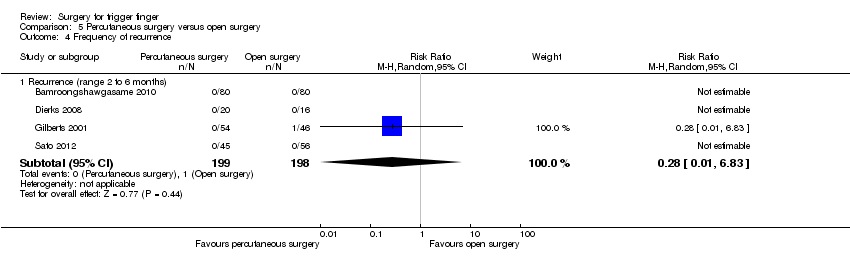
Comparison 5 Percutaneous surgery versus open surgery, Outcome 4 Frequency of recurrence.
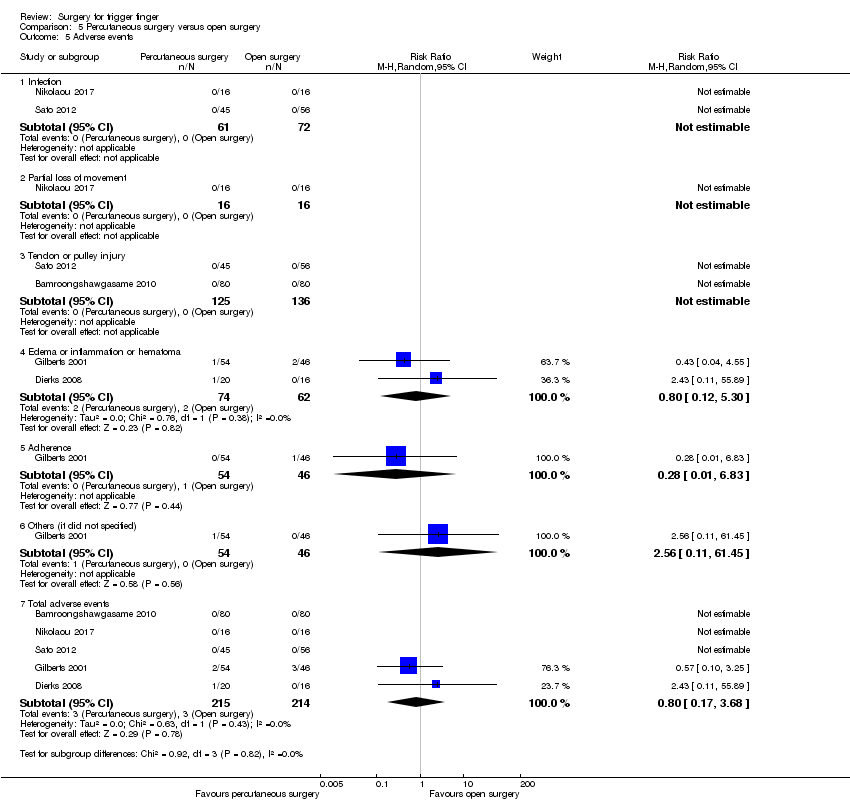
Comparison 5 Percutaneous surgery versus open surgery, Outcome 5 Adverse events.

Comparison 5 Percutaneous surgery versus open surgery, Outcome 6 Subgroup analyses for resolution.

Comparison 5 Percutaneous surgery versus open surgery, Outcome 7 Subgroup analyses for recurrence.

Comparison 6 Endoscopic surgery versus open surgery, Outcome 1 Resolution of trigger finger.

Comparison 6 Endoscopic surgery versus open surgery, Outcome 2 Adverse events.
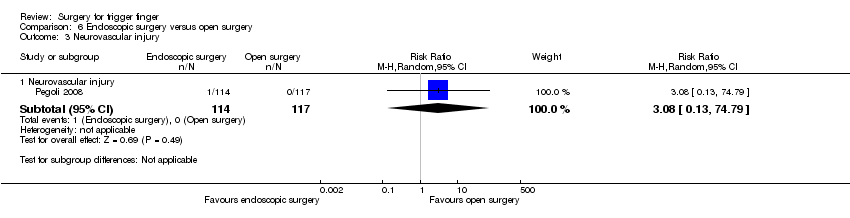
Comparison 6 Endoscopic surgery versus open surgery, Outcome 3 Neurovascular injury.

Comparison 7 Open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease versus open surgery by longitudinal incision of the skin, Outcome 1 DASH score.

Comparison 8 Open surgery by transverse incision of the skin in the distal palmar crease versus open surgery by longitudinal incision of the skin, Outcome 1 DASH score.
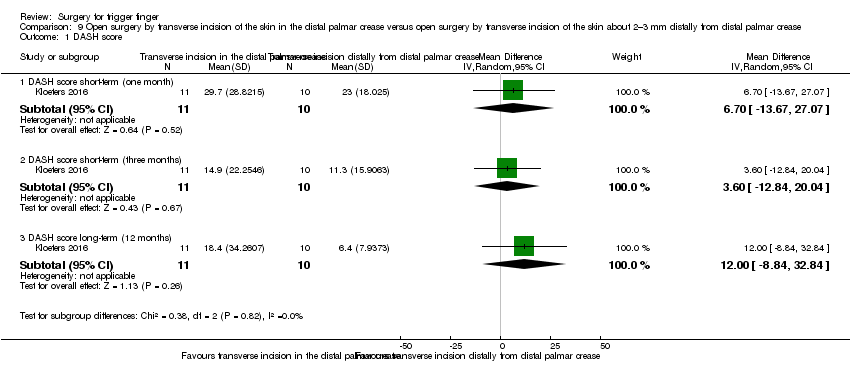
Comparison 9 Open surgery by transverse incision of the skin in the distal palmar crease versus open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease, Outcome 1 DASH score.
| Open surgery versus steroid injection for treating trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Steroid injection | Open surgery | |||||
| Resolution of symptoms (after one or more injections) Follow‐up: 6 to 12 months | 615 per 1000 | 911 per 1000 | RR 1.48 | 270 | ⊕⊝⊝⊝ LOW 1,2,3 | Absolute difference: 28% more had resolution of symptoms with open surgery (2% fewer to 58% more); relative change: 48% more (21% fewer to 176% more). The NNTH n/a4. |
| Pain Proportion with pain on the palm of the hand Follow‐up: 1 week | 184 per 1000 | 678 per 1000 | RR 3.69 | 105 | ⊕⊕⊝⊝ | Absolute risk difference: 49% more people had pain with surgery (33% to 66% more), and the relative percent change translates to worsening of 269% (585% to 99% worse). The NNTH was 3 (95% CI 1 to 5). |
| Function Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Participant global assessment of success Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Recurrence Follow‐up: range 6 to 12 months | 385 per 1000 | 65 per 1000 | RR 0.17 | 270 | ⊕⊕⊝⊝ | Absolute risk difference: 29% fewer people had recurrence with open surgery (60% fewer to 3% more), and the relative percent change translates to improvement of 83% (67% to 91% better). NNTB 4 (95% CI 3 to 4). |
| Adverse events (infection, tendon or pulley injury, flare, cutaneous discomfort, fat necrosis) Follow‐up: range 6 to 12 months | 131 per 1000 | 133 per 1000 | RR 1.02 (0.57 to 1.84) | 270 | ⊕⊕⊝⊝ | Absolute risk difference: 0% (3% fewer to 4% more), and the relative percent change translates to worsening of 2% (43% better to 84% worse). The NNTH n/a4. |
| Neurovascular injury Follow‐up: range 6 to 12 months | 31 per 1000 | 67 per 1000 | RR 2.17 (0.7 to 6.77) | 270 | ⊕⊕⊝⊝ | Absolute risk difference: 2% more people had neurovascular injury with open surgery (6% fewer to 11% more); relative change: 117% more (30% fewer to 577% more). The NNTB n/a4. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The trials had methodological flaws: risk of detection bias. 2Inconsistency: heterogeneity was high. 3Imprecision: the total number of events was small, or the 95% confidence interval includes both open surgery and steroid injection groups, or the 95% confidence interval includes both no clinical effect, and "appreciable benefit" in favour of the open surgery group. 4Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Percutaneous surgery versus steroid injection for treating trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Steroid injection | Percutaneous surgery | |||||
| Resolution of symptoms (after 1 or more injections) Follow‐up: range 6 to 12 months | 545 per 1000 | 1000 per 1000 | RR 2.11 | 191 | ⊕⊝⊝⊝ | Absolute difference: 42% more had resolution of symptoms with percutaneous surgery (15% fewer to 98% more); relative change: 111% more (69% fewer to 1351% more) NNTB n/a4 |
| Pain (Visual analogue scale: Follow‐up: 1 month | The mean pain in the control group was 2.7 | The mean pain in the intervention group was 1.8 lower (5.72 lower to 2.12 higher) | 222 | ⊕⊝⊝⊝ | Absolute reduction: 18% pain reduction with percutaneous surgery (57% reduction to 21% increase), and the relative percent change translates to improvement of 25% (29% worse to 78% better)5. NNTB n/a4 Although a decrease of 1.8 in the VAS score (VAS: 0 to 10 points; where 0 mean no pain and 10 severe pain) may correspond to clinical improvement, there was no statistical difference between the groups and some participants' pain worsened). | |
| Function Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Participant global assessment of success Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Recurrence Follow‐up: range 6 to 12 months | 197 per 1000 | 112 per 1000 | RR 0.57 | 392 | ⊕⊝⊝⊝ | Absolute risk difference: 9% fewer people had recurrence with percutaneous surgery (19% fewer to 2% more), and the relative percent change translates to improvement of 43% (59% worse to 79% better). The NNTB n/a4. |
| Adverse events (infection, partial loss of movement, tendon or −0 injury, dysaesthesia, and skin atrophy or hypopigmentation) Follow‐up: range 6 to 12 months | 89 per 1000 | 140 per 1000 | RR 1.58 | 392 | ⊕⊝⊝⊝ | Absolute risk difference: 3% more people had adverse events with percutaneous surgery (5% fewer to 11% more), and the relative percent change translates to worsening of 58% (9% better to 175% worse). The NNTH n/a4. |
| Neurovascular injury Follow‐up: range 6 to 12 months | 30 per 1000 | 11 per 1000 (1 to 100) | RR 0.35 | 191 | ⊕⊕⊝⊝ | Absolute difference: fewer than 1% had neurovascular injury with percutaneous surgery (5% fewer to 3% more); relative change: 65% fewer (229% fewer to 96% more). The NNTB n/a4. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The two trials had methodological flaws; they did not blind the outcome assessor, and they had selective reporting or unclear risk for incomplete outcome data. 2Inconsistency: heterogeneity was high. 3Imprecision: the total number of events was small, or the 95% confidence interval includes both percutaneous surgery and steroid injection groups, or the 95% confidence interval includes both no clinical effect, and "appreciable benefit" in favour of the steroid injection group. 4Number needed to treat for an additional beneficial outcome (NNTB), or for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). 5Basis for assumed risk was the mean baseline risk from the studies in the meta‐analysis. 6The five trials had methodological flaws; two were quasi‐randomised; four did not blind the outcome assessor and three had unclear risk for incomplete outcome data (one trial had follow‐up loss 17%, but 'intention to treat analysis' was not done; two trials did not report the follow‐up loss); four trials had selective reporting. We opted by double downgrade. | ||||||
| Open surgery versus steroid injection plus hyaluronic acid injection guided by ultrasound for treating trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Steroid injection plus hyaluronic acid injection guided by ultrasound | Open surgery | |||||
| Resolution of symptoms Follow up: 12 months | 733 per 1000 | 990 per 1000 | RR 1.35 | 30 | ⊕⊝⊝⊝ | Absolute difference: 27% more had resolution of symptoms with open surgery (3% to 50% more); relative change: 35% more (2% fewer to 85% more). The NNTB n/a3. |
| Pain (short‐term) Not measured | See comment | See comment | ‐ | See comment | Pain in 6‐month follow‐up was assessed by visual analogue scale (VAS: 0 to 10 points; where 0 means no pain and 10 severe pain), but the authors failed to report any measurement of variance: standard deviation, standard errors, P values, or confidence intervals. The VAS score was 1 point (range 0 to 2) in open surgery group, and 1 point (range 0 to 3) in steroid plus hyaluronic acid injection group. This is not clinically significant. | |
| Function Not measured | See comment | See comment | ‐ | See comment | Functional status of the hand in follow‐up of 6 months was assessed by DASH score, but the authors failed to report any measurement of variance. The DASH score was 11% (range 7 to 16) in open surgery group and 13% (range 7 to 20) in steroid plus hyaluronic acid injection group; it translates to absolute improvement of 2% (DASH score: 0 to 100%; where 0 means no disability and 100 the most severe disability) in the open surgery group. This is not a clinically significant difference. | |
| Participant global assessment of success Not measured | See comment | See comment | ‐ | See comment | Patient satisfaction in follow‐up was at 6 months assessed by satisfaction visual analogue scale (SVAS: 0 to 10 points; where 0 means totally unsatisfied and 10 completely satisfied), but the authors failed to report any measurement of variance: standard deviation, standard errors, P values, or confidence intervals. The SVAS score was 7.8 points (3 to 10 range) in the open surgery group, and 7.4 points (2 to 10 range) in steroid plus hyaluronic acid injection group; it translates to absolute improvement of 0.4 point in the open surgery group. This is not a clinically significant difference. | |
| Recurrence Follow‐up: 12 months | 200 per 1000 | 28 per 1000 | RR 0.14 | 30 | ⊕⊝⊝⊝ | Absolute risk difference: 20% fewer people had recurrence with open surgery (42% fewer to 2% more), and the relative percent change translates to improvement of 86% (99% worse to 155% better). The NNTB n/a3. |
| Adverse events (partial loss of movement, algodystrophic syndrome) Follow‐up: 12 months | 67 per 1000 | 200 per 1000 | RR 3.00 | 30 | ⊕⊝⊝⊝ | Absolute risk difference: 13% more people had adverse events with open surgery (11% fewer to 37% more), and the relative percent change translates to worsening of 200% (2468% worse to 65% better). The NNTH n/a3. |
| Neurovascular injury Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The trial had methodological flaws; it did not describe how random sequence generation was created and whether allocation concealment was done; the outcome assessor was not blinded, and it had selective reporting. We opted by double downgrade. 2Imprecision: the total number of events was small, or the 95% confidence interval includes both no clinical effect, and "appreciable benefit" in favour of the open surgery group, or the 95% confidence interval includes both open surgery and steroid injection plus hyaluronic acid injection groups. 3Number needed to treat for an additional beneficial outcome (NNTB), or for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Percutaneous surgery plus steroid injection versus steroid injection for treating trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Steroid injection | Percutaneous surgery plus steroid injection | |||||
| Resolution of symptoms (after 1 or more injections) Follow up: range 6 to 42 months | 590 per 1000 | 891 per 1000 | RR 1.51 | 127 | ⊕⊝⊝⊝ | Absolute difference: 30% more had resolution of symptoms with percutaneous surgery plus steroid injection (16% to 45% more); relative change: 51% more (21% to 90% more). NNTB 4 (95% CI 3 to 6). |
| Pain (short‐term) Not measured | See comment | See comment | See comment | ‐ | See comment | Pain in follow‐up of 2 weeks was assessed by visual analogue scale (VAS: 0 to 10 points; where 0 means no pain and 10 severe pain), but the authors failed to report any measurement of variance: standard deviation, standard errors, P values, or confidence intervals. The VAS score was 0.4 point in percutaneous surgery plus steroid injection group, and 0.3 point in steroid injection group; it translates to absolute worsening of 0.1 point in the percutaneous surgery plus steroid injection group. This is not a clinically significant difference. |
| Function Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Participant global assessment of success Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Recurrence Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Adverse events (infection, partial loss of movement) Follow‐up: range 6 to 42 months | 33 per 1000 | 30 per 1000 | RR 0.92 | 127 (1 RCT) | ⊕⊝⊝⊝ | Absolute risk difference: 0% (6% fewer to 6% more); relative percentage change: 8% fewer (536% fewer to 87% more). The NNTB n/a3. |
| Neurovascular injury Follow‐up: range 6 to 42 months | 16 per 1000 | 15 per 1000 | RR 0.92 | 127 | ⊕⊝⊝⊝ | Absolute risk difference: 0% (4% fewer to 4% more); relative percentage change: 8% fewer (1346% fewer to 94% more). The NNTB n/a3. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The trial had methodological flaws; it did not describe how random sequence generation was created and whether allocation concealment was done; the outcome assessor was not blinded and it had selective reporting. We opted by double downgrade. 2Imprecision: the total number of events was small, or the 95% confidence interval includes both percutaneous surgery plus steroid injection, and steroid injection groups. 3Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Percutaneous surgery versus open surgery for treating trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open surgery | Percutaneous surgery | |||||
| Resolution of symptoms Follow‐up: range 2 to 6 months | 995 per 1000 | 995 per 1000 | RR 1.00 | 429 | ⊕⊕⊝⊝ | Absolute risk difference: 0% (3% fewer to 2% more); relative percentage change: 0% (3% fewer to 2% more). NNTB n/a2. |
| Pain (1 to 6 scale) Follow‐up: 1 week | The mean pain short term (1 to 6 scale) in the control group was 2.5 | The mean pain short term (1 to 6 scale) in the intervention group was 0.3 higher (0.34 lower to 0.94 higher) | ‐ | 36 | ⊕⊝⊝⊝ | Absolute risk difference: 6% increase in pain with percutaneous surgery (7% reduction to 19% increase), and the relative percent change translates to worsening of 7% (22% worse to 8% better)5. NNTH n/a2. The difference of 0.3 points in the pain score (pain score: 1 to 6 points; where 1 means no pain and 6 extreme pain) is not clinically significant. |
| Function Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Participant global assessment of success Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Recurrence Follow‐up: range 2 to 6 months | 5 per 1000 | 1 per 1000 | RR 0.28 | 397 | ⊕⊕⊝⊝ | Absolute risk difference: 0% (2% fewer to 2% more); relative percentage change: 72% fewer (583% fewer to 99% more). The NNTB n/a2. |
| Adverse events (infection, partial loss of movement, tendon or pulley injury, oedema or inflammation or hematoma, adherence) Follow‐up: range 2 to 6 months | 14 per 1000 | 11 per 1000 | RR 0.80 | 429 | ⊕⊝⊝⊝ | Absolute risk difference: 0% (2% fewer to 2% more); relative percentage change: 20% fewer (268% fewer to 83% more). The NNTB n/a2. |
| Neurovascular injury Follow‐up: range 2 to 6 months | 0 per 1000 | 0 per 1000 | Could not be estimated | 397 | ⊕⊝⊝⊝ | There was no injury in both groups. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1All five trials had methodological flaws; one was quasi‐randomised; three had adequate concealed treatment allocation, and one did not describe how this allocation concealment was done; the subjective outcomes assessor was blinded in one, and no trial blinded the objective outcomes assessor; selective reporting was observed in three trials. We choe to double downgrade. 2Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). 3This quasi‐randomised trial had bias in the random sequence generation and allocation concealment; the outcome assessor was not blinded, and it had selective reporting. We opted by double downgrade. 4Imprecision: the total number of events was small, or the 95% confidence interval includes both no clinical effect and "appreciable benefit" in favour of the open surgery group, or the 95% confidence interval includes both percutaneous and open surgery. 5Basis for assumed risk was the mean baseline risk from the study in the meta‐analysis. 6 The four trials had methodological flaws; one was quasi‐randomised; only two had adequate concealed treatment allocation, and one did not describe how this allocation concealment was done; the outcome assessor was not blinded and three had selective reporting in the trial. We opted by double downgrade. | ||||||
| Endoscopic surgery versus open surgery for treating trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open surgery | Endoscopic surgery | |||||
| Resolution of symptoms Follow‐up: 3 months | 1000 per 1000 | 1000 per 1000 | RR 1.00 | 231 | ⊕⊝⊝⊝ | Absolute risk difference: 0% (2% fewer to 2% more); relative percentage change: 0% (2% fewer to 2% more).The NNTB n/a3. |
| Pain (short‐term) Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Function Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Participant global assessment of success Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Recurrence Not measured | See comment | See comment | See comment | ‐ | See comment | Not measured in any trial. |
| Adverse events (infection, dysaesthesia) Follow‐up: 3 months | 26 per 1000 | 70 per 1000 | RR 2.74 | 231 | ⊕⊝⊝⊝ | Absolute risk difference: 4% more people had adverse events with endoscopic surgery (1% fewer to 10% more), and the relative percent change translates to worsening of 174% (906% worse to 26% better). The NNTH n/a3. |
| Neurovascular injury Follow‐up: 3 months | 0 per 1000 | 0 per 1000 | RR 3.08 | 231 | ⊕⊝⊝⊝ | Absolute difference: 1% more had neurovascular injury with endoscopic surgery (2% fewer to 3% more); relative change: 208% more (87% fewer to 7379% more). The NNTH n/a3. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The trial had methodological flaws; the authors did not describe how randomisation sequence was created and whether allocation concealment was done; the outcome assessor was not blinded and the trial had selective reporting. We chose to double downgrade. 2Imprecision: the total number of events was small, or the 95% confidence interval includes both open surgery and endoscopic surgery groups. 3Number needed to treat for an additional beneficial outcome (NNTB), or for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease compared to open surgery by longitudinal incision of the skin for trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open surgery by longitudinal incision of the skin | Open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease | |||||
| Resolution of symptoms Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Pain (short‐term) Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Function (DASH score: 0 to 100 points) Follow‐up: 12 months | The mean DASH score in the control group was 15.3 | The mean DASH score in the intervention group was 8.9 lower (23.35 lower to 5.55 higher) | ‐ | 21 | ⊕⊝⊝⊝ | Absolute risk difference: 8.9% increase in hand function with open surgery by transverse incision of the skin about 2–3 mm distally from the distal palmar crease (23.35% increase to 5.55% reduction), and the relative percentage change translates to improvement of 21.7% (13.54% worse to 56.95% better)3. NNTH n/a4. The difference of 8.9 points in the DASH score (DASH: 0 to 100 points; where 0 means no disability and 100 means the most severe disability) is not clinically significant. |
| Participant global assessment of success Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Recurrence Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Adverse events Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Neurovascular injury Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial had methodological flaws; the authors did not describe how randomisation sequence was created and whether allocation concealment was done; the outcome assessor was not blinded and the trial had selective reporting. We opted by double downgrade. 2 Imprecision: the total number of events was small, or the 95% confidence interval includes both groups (transverse incision of the skin about 2–3 mm distally from distal palmar crease and longitudinal incision of the skin). 3Basis for assumed risk was the mean baseline risk from the study in the meta‐analysis. 4Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Open surgery by transverse incision of the skin in the distal palmar crease compared to open surgery by longitudinal incision of the skin for trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open surgery by longitudinal incision of the skin | Open surgery by transverse incision of the skin in the distal palmar crease | |||||
| Resolution of symptoms Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Pain (short‐term) Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Function (DASH score: 0 to 100 points) Follow‐up: 12 months | The mean DASH score in the control group was 15.3 | The mean DASH score in the intervention group was 3.1 higher (21.28 lower to 27.48 higher) | ‐ | 22 | ⊕⊝⊝⊝ | Absolute reduction: 3.1% function reduction with open surgery by transverse incision of the skin in the distal palmar crease (27.48% reduction to 21.28% increase), and the relative percent change translates to worsening of 7.56% (67.02% worse to 51.9% better)3. NNTB n/a4. The difference of 3.1 points in the DASH score (DASH: 0 to 100 points; where 0 means no disability and 100 means the most severe disability) is not clinically significant. |
| Participant global assessment of success Not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial. |
| Recurrence Not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial. |
| Adverse events Not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial. |
| Neurovascular injury Not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial had methodological flaws; the authors did not describe how randomisation sequence was created and whether allocation concealment was done; the outcome assessor was not blinded and the trial had selective reporting. We chose to double downgrade. 2 Imprecision: the total number of events was small, or the 95% confidence interval includes both groups (transverse incision of the skin in the distal palmar crease and longitudinal incision of the skin). 3Basis for assumed risk was the mean baseline risk from the study in the meta‐analysis. 4Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Open surgery by transverse incision of the skin in the distal palmar crease compared to open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease for trigger finger | ||||||
| Patient or population: patients with trigger finger | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Open surgery by transverse incision of the skin about 2–3 mm distally from distal palmar crease | Open surgery by transverse incision of the skin in the distal palmar crease | |||||
| Resolution of symptoms Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Pain (short‐term) Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Function (DASH score: 0 to 100 points) Follow‐up: 12 months | The mean DASH score in the control group was 6.4 | The mean DASH score in the intervention group was 12 higher | ‐ | 21 | ⊕⊝⊝⊝ | Absolute risk difference: 12% decrease in function with open surgery by transverse incision of the skin in the distal palmar crease (32.84% reduction to 8.84% increase), and the relative percentage change translates to worsening of 61.22% (167.55% worse to 45.1% better)3. NNTH n/a4. Although an increase of 12 in the DASH score (DASH: 0 to 100 points; where 0 means no disability and 100 means the most severe disability) may correspond to clinical worsening, there was no statistical difference between the groups and some participants' hand function improved). |
| Participant global assessment of success Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Recurrence Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Adverse events Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| Neurovascular injury Not measured | See comment | See comment | ‐ | See comment | Not measured in any trial. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The trial had methodological flaws; the authors did not describe how randomisation sequence was created and whether allocation concealment was done; the outcome assessor was not blinded and the trial had selective reporting. We chose to double downgrade. 2 Imprecision: the total number of events was small, or the 95% confidence interval includes both groups (transverse incision of the skin in the distal palmar crease and transverse incision of the skin about 2–3 mm distally from distal palmar crease). 3Basis for assumed risk was the mean baseline risk from the study in the meta‐analysis. 4Number needed to treat to benefit (NNTB), or harm (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated using Cates NNT calculator (www.nntonline.net/visualrx/). NNT for continuous outcomes calculated using Wells Calculator (CMSG editorial office). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Resolution of symptoms after one or more injections (six to 12 months) | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.79, 2.76] |
| 2 Pain on the palm of the hand Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Pain short‐term (one week) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 3.69 [1.99, 6.85] |
| 2.2 Pain intermediate‐term (six months) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.77] |
| 3 Pain (1 to 10 scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Pain short‐term (three months) | 1 | 156 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.23, 0.23] |
| 3.2 Pain long‐term (12 months) | 1 | 153 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐2.68, ‐1.32] |
| 4 Frequency of recurrence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Recurrence (range six to 12 months) | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.33] |
| 5 Adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Infection | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.65 [0.88, 7.99] |
| 5.2 Tendon or pulley injury | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Flare around procedure site | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.23, 1.15] |
| 5.4 Cutaneous discomfort around procedure site (after 12 months) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [1.25, 10.44] |
| 5.5 Fat necrosis at the procedure site | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.47, 3.54] |
| 5.6 Total adverse events | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.57, 1.84] |
| 6 Neurovascular injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Neurovascular injury | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.70, 6.77] |
| 7 Subgroup analyses for resolution Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Resolution short‐term | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.21] |
| 7.2 Resolution intermediate‐term | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.04, 1.31] |
| 7.3 Resolution long‐term | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [1.49, 2.43] |
| 8 Subgroup analyses for recurrence Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Recurrence intermediate‐term (six months) | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.00] |
| 8.2 Recurrence long‐term (12 months) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.09, 0.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Resolution of symptoms after one or more injections (six to 12 months) | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.31, 14.51] |
| 2 Pain (VAS: 0 to 10 scale) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Pain short‐term (one month) | 2 | 198 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐5.72, 2.12] |
| 2.2 Pain long‐term (12 months) | 1 | 93 | Mean Difference (IV, Random, 95% CI) | ‐6.5 [‐7.25, ‐5.75] |
| 3 Pain on the palm of the hand Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Pain short‐term (one week) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.63 [1.94, 6.78] |
| 3.2 Pain intermediate‐term (six months) | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.18] |
| 4 Frequency of recurrence Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Recurrence (range six to 12 months) | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.21, 1.59] |
| 5 Adverse events Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Infection | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.29] |
| 5.2 Partial loss of movement | 3 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.87, 10.97] |
| 5.3 Tendon or pulley injury | 4 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.81] |
| 5.4 Dysaesthesia | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.02, 1.67] |
| 5.5 Skin atrophy or hypopigmentation | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.63] |
| 5.6 Total adverse events | 5 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.91, 2.75] |
| 6 Neurovascular injury Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Neurovascular injury | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.29] |
| 7 Subgroup analyses for resolution Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Resolution short‐term | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.55, 3.05] |
| 7.2 Resolution intermediate‐term | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.03, 1.31] |
| 7.3 Resolution long‐term | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 3.90 [2.37, 6.42] |
| 8 Subgroup analyses for recurrence Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Recurrence short‐term (one month) | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.76, 3.94] |
| 8.2 Recurrence intermediate‐term (six months) | 2 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.02, 5.50] |
| 8.3 Recurrence long‐term (range nine to 12 months) | 3 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.10, 2.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Resolution of symptoms long‐term (12 months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.98, 1.85] |
| 1.2 Resolution of symptoms intermediate‐term (six month) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.89, 1.28] |
| 2 Frequency of recurrence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Recurrence long‐term (12 months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.55] |
| 2.2 Recurrence intermediate‐term (six months) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Partial loss of movement | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 19.78] |
| 3.2 Algodystrophic syndrome | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 68.26] |
| 3.3 Total adverse events | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.35, 25.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Resolution of symptoms after one or more injections (range 6 to 42 months) | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.21, 1.90] |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Infection | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.04, 4.97] |
| 2.2 Partial loss of movement | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.17, 19.87] |
| 2.3 Total adverse events | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.13, 6.36] |
| 3 Neurovascular injury Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Neurovascular injury | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.06, 14.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Resolution of symptoms (range 2 to 6 months) | 5 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 2 Pain (1 to 6 scale) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Pain short‐term (1 week) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.34, 0.94] |
| 2.2 Pain short‐term (12 weeks) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.52, 0.52] |
| 3 Pain on the palm of the hand Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Pain short‐term (one week) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.29] |
| 3.2 Pain intermediate‐term (six months) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Frequency of recurrence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Recurrence (range 2 to 6 months) | 4 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
| 5 Adverse events Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Infection | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Partial loss of movement | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Tendon or pulley injury | 2 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Edema or inflammation or hematoma | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.12, 5.30] |
| 5.5 Adherence | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
| 5.6 Others (it did not specified) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.11, 61.45] |
| 5.7 Total adverse events | 5 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.17, 3.68] |
| 6 Subgroup analyses for resolution Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Resolution of symptoms (short‐term) | 4 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.97, 1.02] |
| 6.2 Resolution of symptoms (intermediate‐term) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.96, 1.04] |
| 7 Subgroup analyses for recurrence Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Recurrence short‐term (eight to 12 weeks) | 3 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.83] |
| 7.2 Recurrence intermediate‐term (six months) | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Resolution of trigger finger Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Resolution of symptoms (three months) | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.98, 1.02] |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Infection | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Dysesthesia | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [0.74, 10.06] |
| 2.3 Total adverse events | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [0.74, 10.06] |
| 3 Neurovascular injury Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Neurovascular injury | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [0.13, 74.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 DASH score short‐term (one month) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐19.19, 16.19] |
| 1.2 DASH score short‐term (three months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐16.45, 12.45] |
| 1.3 DASH score long‐term (12 months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | ‐8.9 [‐23.35, 5.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 DASH score short‐term (one month) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 5.20 [‐16.67, 27.07] |
| 1.2 DASH score short‐term (three months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐15.27, 18.47] |
| 1.3 DASH score long‐term (12 months) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 3.10 [‐21.28, 27.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 DASH score Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 DASH score short‐term (one month) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 6.70 [‐13.67, 27.07] |
| 1.2 DASH score short‐term (three months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 3.60 [‐12.84, 20.04] |
| 1.3 DASH score long‐term (12 months) | 1 | 21 | Mean Difference (IV, Random, 95% CI) | 12.00 [‐8.84, 32.84] |

