Memberus antara gigi untuk mencegah dan mengawal penyakit gusi dan karies gigi di kalangan orang dewasa.
Abstract
Background
Effective oral hygiene is a crucial factor in maintaining good oral health, which is associated with overall health and health‐related quality of life. Dental floss has been used for many years in conjunction with toothbrushing for removing dental plaque in between teeth, however, interdental brushes have been developed which many people find easier to use than floss, providing there is sufficient space between the teeth.
Objectives
To evaluate the effects of interdental brushing in addition to toothbrushing, as compared with toothbrushing alone or toothbrushing and flossing for the prevention and control of periodontal diseases, dental plaque and dental caries.
Search methods
We searched the following electronic databases: the Cochrane Oral Health Group's Trials Register (to 7 March 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 2), MEDLINE via OVID (1946 to 7 March 2013), EMBASE via OVID (1980 to 7 March 2013), CINAHL via EBSCO (1980 to 7 March 2013), LILACS via BIREME (1982 to 7 March 2013), ZETOC Conference Proceedings (1980 to 7 March 2013) and Web of Science Conference Proceedings (1990 to 7 March 2013). We searched the US National Institutes of Health Trials Register (http://clinicaltrials.gov) and the metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/) for ongoing trials to 7 March 2013. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomised controlled trials (including split‐mouth design, cross‐over and cluster‐randomised trials) of dentate adult patients. The interventions were a combination of toothbrushing and any interdental brushing procedure compared with toothbrushing only or toothbrushing and flossing.
Data collection and analysis
At least two review authors assessed each of the included studies to confirm eligibility, assessed risk of bias and extracted data using a piloted data extraction form. We calculated standardised mean difference (SMD) and 95% confidence interval (CI) for continuous outcomes where different scales were used to assess an outcome. We attempted to extract data on adverse effects of interventions. Where data were missing or unclear we attempted to contact study authors to obtain further information.
Main results
There were seven studies (total 354 participants analysed) included in this review. We assessed one study as being low, three studies as being high and three studies as being at unclear risk of bias. Studies only reported the clinical outcome gingivitis and plaque data, with no studies providing data on many of the outcomes: periodontitis, caries, halitosis and quality of life. Three studies reported that no adverse events were observed or reported during the study. Two other studies provided some data on adverse events but we were unable to pool the data due to lack of detail. Two studies did not report whether adverse events occurred.
Interdental brushing in addition to toothbrushing, as compared with toothbrushing alone
Only one high risk of bias study (62 participants in analysis) looked at this comparison and there was very low‐quality evidence for a reduction in gingivitis (0 to 4 scale, mean in control): mean difference (MD) 0.53 (95% CI 0.23 to 0.83) and plaque (0 to 5 scale): MD 0.95 (95% CI 0.56 to 1.34) at one month, favouring of use of interdental brushes. This represents a 34% reduction in gingivitis and a 32% reduction in plaque.
Interdental brushing in addition to toothbrushing, as compared with toothbrushing and flossing
Seven studies provided data showing a reduction in gingivitis in favour of interdental brushing at one month: SMD ‐0.53 (95% CI ‐0.81 to ‐0.24, seven studies, 326 participants, low‐quality evidence). This translates to a 52% reduction in gingivitis (Eastman Bleeding Index). Although a high effect size in the same direction was observed at three months (SMD ‐1.98, 95% CI ‐5.42 to 1.47, two studies, 107 participants, very low quality), the confidence interval was wide and did not exclude the possibility of no difference. There was insufficient evidence to claim a benefit for either interdental brushing or flossing for reducing plaque (SMD at one month 0.10, 95% CI ‐0.13 to 0.33, seven studies, 326 participants, low‐quality evidence) and insufficient evidence at three months (SMD ‐2.14, 95% CI ‐5.25 to 0.97, two studies, 107 participants very low‐quality evidence).
Authors' conclusions
Only one study looked at whether toothbrushing with interdental brushing was better than toothbrushing alone, and there was very low‐quality evidence for a reduction in gingivitis and plaque at one month. There is also low‐quality evidence from seven studies that interdental brushing reduces gingivitis when compared with flossing, but these results were only found at one month. There was insufficient evidence to determine whether interdental brushing reduced or increased levels of plaque when compared to flossing.
Ringkasan bahasa mudah
Membersih di antara gigi dengan berus antara gigi untuk mencegah dan mengawal penyakit gusi dan kerosakan gigi di kalangan orang dewasa
Soalan ulasan
Ulasan yang dibuat oleh Kumpulan Kesihatan Oral Cochrane ini bertujuan menilai keberkesanan memberus antara gigi (celah gigi) sebagai tambahan kepada pemberusan gigi secara tunggal atau memberus gigi dan memflos untuk pencegahan dan kawalan penyakit gusi, plak gigi (filem melekit yang mengandungi bakteria) dan karies gigi (kerosakan gigi)
Latarbelakang
Penyakit gusi serta kerosakan gigi merupakan sebab utama kehilangan gigi. Jika tidak diberus, plak akan berkumpul atas permukaan gigi. Pengumpulan plak boleh menyebabkan keradangan gusi dan penyakit gusi dan merupakan faktor utama pembentukan kerosakan gigi.
Memberus gigi secara konvensional sahaja tidak cukup berkesan untuk membuang plak di antara gigi. Flos gigi telah digunakan sekian lama bersama pemberusan gigi untuk membuang plak di antara gigi. Namun, buat masa kini teknik memberus antara gigi telah dibangunkan dan orang ramaimendapati ia lebih mudah diguna berbanding flos. Sekiranya teknik pemberusan antara gigi digunakan, perlu ada cukup ruang antara gigi untuk membolehkannya.
Berus antara gigi adalah berus gigi berkepala kecil, didapati dalam pelbagai saiz kelebarannya untuk menepati ruang antara gigi. Ia boleh benbentuk tirus atau silinder. Berus yang diguna di sekeliling implan mempunyai dawai bersalut untuk menghindar cakaran atau kejutan.
Bersama denga flos gigi, berus antara gigi sering disyorkan, diiklankan dan sedia ada untuk membersih di antara gigi.
Ciri kajian
Bukti ulasan ini adalah berdasarkan yang terkini iaitu sehingga 7 Mac 2013. Tujuh kajian dengan jumlah keseluruhan 354 peserta terlibat dalam ulasan ini. Peserta berumur 16 tahun ke atas dan mempunyai gigi. Bagi pemilihan kajian‐kajian untuk dimasukkan dalam ulasan ini, tiada perbezaan dibuat atas dasar bangsa, jantina, pekerjaan (status sosioekonomi), tempat, latarbelakang pendedahan kepada fluorida, status kesihatan permulaan, persekitaran atau masa intervensi.Kajian‐kajian adalah dikecualikan daripada ulasan jika majoriti peserta mempunyai aplians ortodontik (pendakap), jika peserta dipilih atas dasar keadaan kesihatan khas atau jika majoriti peserta mempunyai penyakit gusi yang teruk.
Keputusan utama
Terdapat sedikit bukti berkualiti rendah menyokong bahawa penggunaan berus di antara gigi bersama memberus gigi lebih bermanfaat daripada memberus gigi sahaja untuk kawalan plak dan gingivitis pada tempoh satu bulan. Terdapat juga bukti berkualiti rendah bahawa penggunaan berus antara gigi mengurangkan gingivitis (keradangan gusi) sebanyak 52% berbanding memflos sahaja pada tempoh satu bulan. Tiada cukup bukti untuk menunjukkan manfaat bagi kedua‐dua pemberusan di antara gigi atau memflos untuk mengawal plak.
Tiada kajian yang melaporkan kerosakan gigi kerana tiada kajian yang menepati tempoh untuk mengesan perubahan kerosakan awal gigi. Tiga kajian melaporkan tiada kesan buruk dalam tempoh kajian. Dua kajian lain memberi data berkenaan kesan buruk/masalah seperti kesukaran untuk manipulasi flos, untuk sampai kepada gigi belakang, berus antara gigi menjadi herot atau melengkok (paling serius) dan flos membuat gusi sakit, namun kami tidak dapat menganalisa data secara formal kerana kajian‐kajian tidak memberi cukup maklumat. Dua kajian tidak melaporkan sama ada kesan buruk berlaku.
Kualiti bukti
Kualiti kajian tunggal membandingkan pemberusan di antara gigi dengan pemberusan gigi sahaja dinilai sebagai berkualiti rendah. Bukti kualiti untuk perbandingan pemberusan antara gigi dan memflos dengan tambahan kepada pemberusan gigi adalah rendah. Tiada kajian mealporkan pembentukan kerosakan gigi.
Authors' conclusions
Summary of findings
| Interdental brushing for periodontal diseases and dental caries in adults | ||||||
| Patient or population: Adults, 16 years and older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No IDB | IDB | |||||
| Gingivitis | The mean gingivitis in the control group was 1.56 | The mean gingivitis in the intervention groups was 0.53 lower (95% CI 0.23 to 0.83) | 62 | ⊕⊝⊝⊝ | 34% reduction in gingivitis | |
| Periodontitis | Not estimable | 0 | See comment | No included study assessed periodontitis as an outcome | ||
| Plaque | The mean plaque in the control groups was | The mean plaque in the intervention groups was 0.95 lower (95% CI 0.56 to 1.34) | 62 | ⊕⊝⊝⊝ | 32% reduction in plaque | |
| Interproximal caries | Not estimable | 0 | See comment | No included study assessed caries as an outcome | ||
| Harms and adverse outcomes | Not estimable | 0 | See comment | Only 1 study reported adverse outcomes in terms of problems with the use of the assigned interdental cleaning aids | ||
| Bad breath (halitosis) | Not estimable | 0 | See comment | No included study assessed bad breath as an outcome | ||
| Quality of life | Not estimable | 0 | See comment | No included study assessed quality of life as an outcome | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Single study at high risk of bias. | ||||||
| Interdental brushing compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Patient or population: Adults, 16 years and older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flossing | IDB | |||||
| Gingivitis | The mean gingivitis in the flossing groups was | The mean gingivitis in the intervention groups was | 390 | ⊕⊕⊝⊝ | The estimate is for the 1‐month (4 to 6 weeks) time point and converts back to 52% reduction (of control mean) for IDB (based on 1 study). Results (based on 2 studies, very low‐quality evidence) at 3 months show a large SMD but we are unable to draw conclusions on the effect due to the wide confidence interval including no effect | |
| Periodontitis | Not estimable | 0 | See comment | No included study assessed clinical attachment loss, a measure of progression of periodontitis | ||
| Plaque | The mean plaque in the flossing groups was | The mean plaque in the intervention groups was | 326 | ⊕⊕⊝⊝ | The estimate is for the 1‐month (4 to 6 weeks) time point, and converts back to 2% reduction (of control mean) for IDB (based on 1 study). The effect for the 3 months time point somewhat differs, with a large SMD but we are unable to draw conclusions on the effect due to the wide confidence interval including no effect (based on 2 studies, very low‐quality evidence) | |
| Interproximal caries | Not estimable | 0 | See comment | No included study assessed caries as an outcome | ||
| Harms and adverse outcomes | Not estimable | 0 | See comment | 2 studies reported adverse outcomes in terms of problems with the use of the assigned interdental cleaning aids. We were unable to pool data | ||
| Bad breath (halitosis) | Not estimable | 0 | See comment | No included study assessed bad breath as an outcome | ||
| Quality of life | Not estimable | 0 | See comment | No included study assessed quality of life as an outcome | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Re‐expressed from SMD into Eastman Interdental Bleeding Index score. The results should be interpreted with caution since back‐translation of the effect size is based on the results of only one study (Jackson 2006). The estimate is for the one‐month time point with a SMD of ‐0.53. A larger effect was observed for the three months time point with a SMD of ‐1.98. | ||||||
Background
Introduction
Effective oral hygiene is a crucial factor in maintaining good oral health, which is associated with overall health and health‐related quality of life (McGrath 2002; Sheiham 2005). Poor oral health may affect appearance in terms of stained or missing teeth (Exley 2009) and can contribute to bad breath (Morita 2001) thus negatively influencing confidence, self expression and communication (Exley 2009; McGrath 2002). Poor oral health is often accompanied by pain arising from carious lesions (Exley 2009) which can lead to discomfort when eating (Dahl 2011; McGrath 2002).
Individuals with high levels of dental plaque are more likely to experience dental caries and periodontal disease (Broadbent 2011).
Dental plaque‐induced gingivitis and incipient, non‐cavitated carious lesions are reversible (Mariotti 1999; Silverstone 1983). The progression in either disease may be attributed to a tip in the environmental equilibrium that favours disease conditions. For example, in periodontal disease the key is to treat gingivitis when inflammation is only in the gingival tissues and has not affected other parts of the periodontal system (Mariotti 1999). Early carious lesions can be arrested in the enamel and may or may not progress to the dentine depending on the dynamic equilibrium between demineralisation and remineralisation (Marinho 2002b; Marinho 2002a; Marinho 2003).
Periodontal diseases and dental caries are found in high, middle and low‐income countries. Although the incidence of periodontal disease and dental caries differs, based on regional, social and genetic factors, the prevention of these diseases has a significant healthcare and economic benefit, to both society as a whole and individual patients.
The effective removal of dental plaque is important in the prevention of these common diseases. In conjunction with professionally provided plaque removal services, for example as provided by a dental hygienist, daily mechanical self care disruption of dental plaque is considered important for oral health maintenance (Needleman 2005; Rösing 2006; Zaborskis 2010). Besides toothbrushing, which is the most common method for removing dental plaque (Addy 1986; Mak 2011; Richardson 1977), different interdental aids to plaque removal, such as dental floss and interdental brushes, are widely available and are recommended to be used in addition to toothbrushing (Bosma 2011; Särner 2010). Whilst floss can be used in all interdental spaces, the interdental brush requires sufficient interdental space to be used by patients. The choice of brush will depend on the size of the space.
This review will evaluate the evidence for interdental brushes and is similar to the Cochrane review on flossing (Sambunjak 2011). Together these two reviews will provide a comprehensive assessment of the evidence for the two most common means of interdental cleaning to aid clinical decision‐making and consumer understanding.
Description of the condition
Periodontal diseases
Periodontal diseases are multifactorial oral health conditions (Llorente 2006; Timmerman 2006), consisting of a diverse family of pathological conditions affecting the periodontium (a collective term that comprises gingival tissue, periodontal ligament, cementum and alveolar bone), that commonly affect the population (Adult Dental Health Survey 2009; Eke 2012). Periodontal diseases are comprised of two main conditions: gingivitis and periodontitis. Gingivitis is defined as the presence of gingival inflammation without loss of connective tissue attachment and appears as red, puffy, shiny gums that bleed easily (Mariotti 1999). Periodontitis is defined as inflammation and destruction of the supportive tissues of teeth and is, by its behaviour, characterised as aggressive or chronic (Armitage 1999). Susceptibility to periodontal disease is variable and depends upon the interaction of factors such as genetic predisposition, smoking, stress, immunocompromising diseases and drugs, and certain systemic diseases, for example diabetes (Mariotti 1999). Socioeconomic factors, for instance, educational and income levels have been found to be strongly associated with the prevalence and severity of periodontal diseases (Borrell 2012).
The prevalence of periodontitis is difficult to establish across studies because of non‐standardised criteria, different study population characteristics, different clinical measurements, and the use of partial versus full mouth examinations (Cobb 2009; Savage 2009).
Of particular concern is the differing definitions and clinical measurements being used (Cobb 2009; Savage 2009). Recent national studies have assessed oral cleanliness, periodontal disease and oral hygiene behaviour. In the UK only 17% of adults had healthy gums, 66% had visible plaque and, of those with plaque, 65% had bleeding gums compared with 33% with no plaque (Adult Dental Health Survey 2009). Whilst the most severe forms of periodontal disease, with alveolar bone loss, are much less common, gingivitis is prevalent at all ages and is the most common form of periodontal disease (Mariotti 1999). Some form of periodontitis affects the majority of the population (Adult Dental Health Survey 2009; Eke 2012). Periodontitis can influence quality of life through psychosocial impacts as a result of negative effects on comfort, function, appearance and socialisation (Durham 2013; Needleman 2004). It can also lead to tooth loss (Broadbent 2011), which negatively impacts on both aesthetics and function. Since periodontal diseases are inflammatory, bacterially mediated diseases that trigger the host's immune system, it is postulated that the individual's oral health status may influence their systemic health. Studies have demonstrated associations between periodontal diseases and coronary heart disease (Machuca 2011), hyperlipidaemia (Fentoğlu 2012), preterm births (Huck 2011) and lack of glycaemic control in people with diabetes mellitus (Columbo 2012; Simpson 2010).
Dental plaque is the primary aetiological factor in the development of periodontal diseases and dental caries (Dalwai 2006; Kuramitsu 2007; Marsh 2006; Periasamy 2009; Selwitz 2007). Dental plaque is a highly organised and specialised biofilm comprising of an intercellular matrix consisting of various micro‐organisms and their by‐products. The bacteria found within dental plaque mutually support each other, using chemical messengers, in a community that protects them from an individual's immune system and chemical agents such as antimicrobial mouth rinses. Bacteria in biofilm are 1000 to 1500 times more resistant to antibiotics than in their free‐floating state, reducing the effectiveness of chemical agents as a solo treatment option. Therefore disruption of the oral biofilm via mechanical methods remains one of the best treatment options (Chandki 2011). Calcified plaque (calculus) is not involved in the pathogenesis of periodontal disease but it provides an ideal surface to collect further dental plaque and acts as a 'retention web' for bacteria, protecting plaque from appropriate preventive and therapeutic periodontal measures (Ismail 1994; Lindhe 2003).
Dental caries
Dental caries is a multifactorial, bacteriologically mediated, chronic disease (Addy 1986; Richardson 1977; Rickard 2004). According to the World Oral Health Report 2003 (Petersen 2003), dental caries affects 60% to 90% of school children and the vast majority of adults, making it one of the most common diseases in the world population (WHO 1990). Although the prevalence and severity of dental caries in most industrialised countries has substantially decreased in the past two decades (Marthaler 1996), this preventable disease continues to be a common public health problem for other parts of the world (Burt 1998).
Deep pits and fissures, as well as interdental spaces, represent areas of increased risk for the collection and accumulation of dental plaque and are therefore regarded as susceptible tooth surfaces for the occurrence of carious lesions. The presence and growth of dental plaque is further encouraged by compromised host response factors, for example reduced salivary flow (hyposalivation) (Murray 1989). Fermentation of sugars by cariogenic bacteria within the plaque results in localised demineralisation of the tooth surface, which may ultimately result in cavity formation (Marsh 2006; Selwitz 2007).
Patients with carious teeth may experience pain and discomfort (Milsom 2002; Shepherd 1999) and, if left untreated, may lose their teeth. In the United Kingdom, tooth decay accounts for almost half of all dental extractions performed (NHS 1999).
Prevention of dental caries and periodontal disease is generally regarded as a priority for oral healthcare professionals because it is more cost‐effective than treating it (Brown 2002; Burt 1998). Effective plaque control by toothbrushing is a key self care strategy for oral health (Addy 1986; Richardson 1977). Patients routinely use toothbrushes to remove supragingival dental plaque, but toothbrushes are unable to penetrate the interdental area where periodontal disease first develops and is prevalent (Asadoorian 2006; Berchier 2008; Berglund 1990; Casey 1988). Interdental plaque is more prevalent (Lindhe 2003), forms more readily (Igarashi 1989) and is more acidogenic than plaque on other tooth surfaces in the mouth. Therefore interdental cleaning is often recommended as an adjunctive self care therapy.
Description of the intervention
Interdental brushes
Interdental brushes are small cylindrical or cone‐shaped bristles on a thin wire that may be inserted between the teeth. They have soft nylon filaments aligned at right angles to a central stiffened rod, often twisted stainless steel wire, very similar to a bottle brush. Interdental brushes used for cleaning around implants have coated wire to avoid scratching the implants or causing galvanic shock. They are available in a range of different widths to match the interdental space and their shape can be conical or cylindrical. Most are round in section, although interdental brushes with a more triangular cross‐section can also be found in the market. Originally, interdental brushes were recommended by dental professionals to patients with large embrasure spaces between the teeth (Slot 2008; Waerhaug 1976), caused by the loss of interdental papilla mainly due to periodontal destruction. Patients who had interdental papillae that filled the embrasure space were usually recommended to use dental floss as an interdental cleansing tool. However, with the greater range of interdental brush sizes and cross‐sectional diameters now available, they are considered a potentially suitable alternative to dental floss for patients who have interdental papillae that fill the interdental space (Imai 2011). Daily dental flossing adherence is low among patients because it requires a certain degree of dexterity and motivation (Asadoorian 2006), whereas interdental brushes have been shown as being easier to use and are therefore preferred by patients (Christou 1998; Imai 2010). Furthermore, when compared to dental floss, they are thought to be more effective in plaque removal because the bristles fill the embrasure and are able to deplaque the invaginated areas on the tooth and root surfaces (Bergenholtz 1984; Christou 1998; Imai 2011; Jackson 2006; Kiger 1991; Waerhaug 1976). However, there are conflicting study results regarding the efficacy of interdental brushes in the reduction of clinical parameters of gingival inflammation (Jackson 2006; Noorlin 2007) and whether they are only suitable for patients with moderate to severe attachment loss and open embrasures, or whether they are a suitable aid for healthy patients to prevent gingivitis who have sufficient interdental space to accommodate them (Gjermo 1970; Imai 2011).
Why it is important to do this review
Together with dental floss, interdental brushes are one of the most commonly recommended, advertised and available aids for interdental cleaning. It is unclear whether they are as good or better than dental floss as an adjunct to toothbrushing in reducing dental disease. A systematic review and meta‐analysis combining the results of randomised controlled trials will provide practitioners with evidence as to the effects of the use of interdental brushes on oral health.
Objectives
To evaluate the effects of interdental brushing in addition to toothbrushing, as compared with toothbrushing alone or toothbrushing and flossing for the prevention and control of:
-
periodontal diseases (gingivitis and periodontitis);
-
dental plaque;
-
dental caries.
This also includes assessing the safety of interdental brushing procedures, in terms of potential harms and adverse effects, balancing important benefits against important harms.
In this review we focused exclusively on interdental brushing, in addition to toothbrushing (with or without flossing). The effects of dental flossing in addition to toothbrushing compared to toothbrushing alone have been assessed in another Cochrane review (Sambunjak 2011).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (including split‐mouth design and cross‐over trials) and cluster‐randomised trials. Studies where random allocation was not used or indicated were excluded. The second part of cross‐over trials was included only if they had a minimum washout period of two weeks before the cross‐over. Studies were included regardless of their publication status and language.
Types of participants
The review included studies of dentate participants 16 years of age and older, regardless of race, gender, socioeconomic status, geographical location, background exposure to fluoride, initial dental health status, setting or time of the intervention. Studies were excluded if the majority of participants had any orthodontic appliances. Likewise, studies were excluded if participants were selected on the basis of special (general or oral) health conditions (for example, severely immunocompromised patients), or if the majority of participants had severe periodontal disease.
The minimum age of the participants was decided because by the age of 16 all permanent teeth, except third molars, should be fully evolved and erupted in the mouth (Proffit 2006). The exclusion of children with primary (or mixed) dentition was predefined because the primary dentition is characterised by generalised spacing between teeth, which is most visible in the anterior part of the dentition. Such spacing is essential for future alignment of permanent dentition and decreases with eruption of permanent teeth at the early mixed dentition when proximal contacts start to develop (Proffit 2006). The lack of interdental spacing is considered to be associated with the increased accumulation of plaque (Mathewson 1995) and higher susceptibility of interproximal surfaces to caries (Ben‐Basset 1997; Parfitt 1956; Warren 2003).
Moreover, the exclusion of primary dentition is based on varying morphological, chemical and physiological aspects between deciduous and permanent teeth enamel (Mortimer 1970; Sonju‐Clasen 1997). Primary tooth enamel has lower levels of mineralisation, 80.6% as opposed to permanent tooth enamel which is 89.7% mineralised (Mortimer 1970). Differences in enamel mineralisation are particularly observed in the outermost layers of the enamel (Wang 2006). Furthermore, permanent tooth enamel is up to two times thicker than primary tooth enamel (Araújo 1995; Mortimer 1970). Because of lower mineral density and lower thickness of the enamel, primary teeth are believed to respond differently to caries than permanent teeth (Hunter 2000; Marquezan 2008; Wang 2006) with possibly faster and higher rates of dental caries progression due to such differences (Amaechi 1999; Featherstone 1981; Johansson 2001; Wang 2006).
A subgroup analysis based on the participants' age or dental status could have been conducted, but the preliminary search found no eligible studies involving children.
Types of interventions
We included all studies that compared a combination of toothbrushing and any interdental brushing procedure with toothbrushing only or toothbrushing and flossing. Interventions could have been self performed, supervised or unsupervised. The primary comparison was self performed, unsupervised, interdental brushing plus toothbrushing versus toothbrushing alone.
We included studies exploring other comparative interventions (for example, mouthrinsing) if they contained study arms with interventions of interest to this review (i.e. interdental brushing plus toothbrushing and toothbrushing alone or toothbrushing and flossing).
We excluded studies where the intervention group alone or both the intervention and control groups received any additional active agent(s) (i.e. caries preventive agents) as part of the study (e.g. chlorhexidine mouthwash, additional fluoride‐based procedures, oral hygiene procedures, xylitol chewing gum) in addition to interdental brushing, flossing or toothbrushing. However, we included studies using floss impregnated with active agents such as chlorhexidine or fluoride. We included studies that included participants receiving additional measures as part of their routine oral care, such as oral hygiene advice, supervised brushing, fissure sealants, etc.
The minimum duration of the intervention was set at four weeks.
Based on what was found in the included studies, we made a decision on which time points to include in the analyses.
Types of outcome measures
Major outcomes
We considered the following outcomes to be most relevant and important to clinicians and patients.
-
Gingivitis ‐ assessed by gingival indices (both inflammatory and bleeding).
-
Periodontitis ‐ assessed by clinical attachment loss.
-
Interproximal caries ‐ assessed by (a) progression of caries into enamel or dentine, (b) change in decayed, missing and filled tooth surfaces (D(M)FS) index, (c) radiographic evidence. Studies had to contain explicit criteria for diagnosing dental caries. As caries increment could be reported differently in different trials, we used a set of a priori rules to choose the primary outcome data for analysis from each study (Marinho 2003).
-
Plaque indices.
-
Harms and adverse effects.
We analysed gingival and plaque indices according to the primary outcome data presented in the included studies.
When full‐mouth and interdental indices were presented, we considered interdental indices for the analyses.
For the studies that used both gingival and bleeding indices, we used gingivitis scores in the meta‐analyses because gingivitis indices assess clinical signs of inflammation in the gingivae and are based on visual (non‐invasive) and bleeding (invasive) components (Armitage 1996).
As for the bleeding indices, for studies in which both bleeding on probing (BOP) and Eastman Interdental Bleeding Index (EIBI) were used, we included EIBI in the meta‐analyses. The suitability of the EIBI is justified by its reproducibility and high inter‐examiner and intra‐examiner reliability (Blieden 1992).
Based on what was found in the included studies and to facilitate comparison between this review and the Cochrane reviews on flossing and toothbrushing (Deacon 2010; Robinson 2005; Sambunjak 2011), we planned to include the four to six‐week data (combined) and 12‐week (or nearest) time points in the analyses of gingivitis and plaque indices. From a clinical viewpoint one can see some tissue healing within four weeks (or one month) in patients with gingivitis and consequent reductions in the clinical indices used in the outcomes (bleeding, gingival, plaque). The three months mark is important because microbiologically, the periopathogens return in sufficient numbers to cause disease. Hence, patients with periodontal disease are recommended to be on three‐month periodontal maintenance recall visits (Haffajee 1997; Haffajee 2006).
For the outcome of clinical attachment loss, it was anticipated that this would be assessed after at least six months of follow‐up, and for assessment of interproximal caries the time of assessment should be at least one year.
Minor outcomes
-
Bad breath (halitosis).
-
Quality of life.
Search methods for identification of studies
For the identification of studies included or considered for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE (OVID) (Appendix 1) but revised appropriately for each database.
The search strategy used a combination of controlled vocabulary and free‐text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). The searches of EMBASE and CINAHL were linked to the Cochrane Oral Health Group filters for identifying RCTs, and the search of LILACS was linked to the Brazilian Cochrane Center filter (seeAppendix 2, Appendix 3 and Appendix 4 for details).
Electronic searches
The following databases were searched:
-
the Cochrane Oral Health Group's Trials Register (to 7 March 2013) (Appendix 5);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 2) (Appendix 6);
-
MEDLINE via OVID (1946 to 7 March 2013) (Appendix 1);
-
EMBASE via OVID (1980 to 7 March 2013) (Appendix 2);
-
CINAHL via EBSCO (1980 to 7 March 2013) (Appendix 3);
-
LILACS via BIREME (1982 to 7 March 2013) (Appendix 4).
We placed no restrictions on the language or date of publication when searching the electronic databases.
Searching other resources
We searched for conference proceedings and abstracts by using the following resources:
-
ZETOC Conference Proceedings (1980 to 7 March 2013) (Appendix 7);
-
ISI Web of Science Conference Proceedings (1990 to 7 March 2013) (Appendix 8).
We checked references of all the included studies, other reviews, guidelines and related articles for other relevant studies.
We searched for ongoing studies in the following trial registries:
-
the US National Institutes of Health Trials Register (www.clinicaltrials.gov) (to 7 March 2013) (Appendix 9);
-
the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (to 7 March 2013) (Appendix 10).
For abstracts whose results could not be confirmed in subsequent publications, we contacted trial authors to collect unpublished data. We contacted manufacturers of interdental cleaning devices and asked them about their knowledge of any unpublished or ongoing clinical trials.
Data collection and analysis
Selection of studies
Two review authors independently carried out the selection of studies and made decisions about eligibility; one of them a methodologist and the other a topic area specialist. If the relevance of a report was unclear, we reviewed the full text and resolved all disagreements by discussion.
In case of doubt regarding eligibility of studies, data extraction or data analysis, the third review author was consulted.
Data extraction and management
Three review authors independently extracted data; one of them a methodologist and other two topic area specialists. We compared extracted data against each other and identified disagreements, which we then resolved by consensus. The review authors were not be blinded to the authors, interventions or results obtained in the included studies.
We extracted and entered the following data into a customised collection form.
(1) Study characteristics: study design, including details of how the study differs from standard parallel‐group design (e.g. split‐mouth or cross‐over); date and duration of study; setting of the study.
(2) Participants:
-
sample size;
-
inclusion and exclusion criteria;
-
demographic characteristics of participants: age, gender, country of origin, ethnicity, gender, socioeconomic status, comorbidity, caries and periodontal disease risk status. We recorded demographic characteristics for the study as a whole and for each intervention group, if available.
(3) Intervention: we collected details of the experimental and the comparison interventions:
-
type of interdental brushing, type of floss (automated or manual, waxed or non‐waxed, with or without fluoride), type of toothbrush (powered or manual), type of toothpaste (with or without fluoride);
-
frequency of interdental brushing, duration of the intervention period and of the individual interdental brushing procedure;
-
were the participants trained/instructed how to brush interdentally, floss and/or toothbrush, and by whom?
-
length of follow‐up, loss to follow‐up;
-
assessment of adherence;
-
level of fluoride in the water supply.
(4) Outcomes:
-
detailed description of the outcomes of interest (both beneficial and adverse), including the definition and timing of measurement;
-
methods of assessment.
Furthermore, we made a list of other outcomes found in the included studies. We extracted results for prespecified outcomes of interest.
Other data that we extracted included:
-
ethical approval;
-
sample size calculation (yes/no);
-
funding sources;
-
key conclusions of the included studies as reported by their authors.
We designed the data extraction form for this review and piloted it before use. When needed, coding instructions accompanied the data extraction form. We extracted data from multiple reports of the same studies in a single form. In cases of studies reporting both preliminary and final results, only the final report (including full number of participants) was included. We consulted a statistician in cases of doubt about data extraction, as well as with regard to data analysis. We contacted authors for missing information.
Assessment of risk of bias in included studies
We carried out assessment of risk of bias by using The Cochrane Collaboration's 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The tool addresses the six following domains: sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting and other issues. Since blinding of the study participants for the interventions of interest was not possible, the primary consideration was given to the blinding of the outcome assessors. For split‐mouth and cross‐over designs, assessment of risk of bias included additional considerations, such as suitability of the design, risk of carry‐over or spill‐over effects, and appropriateness of the statistical analysis. We recorded each piece of information extracted for the 'Risk of bias' tool together with the precise source of this information and used this to assign a judgement of low, high or unclear risk of bias for each domain within each included study. We tested data collection forms and assessments of the risk of bias on a pilot sample of articles. The assessors were not blinded to the names of the authors, institutions, journal or results of a study. At least two review authors independently, and in duplicate, carried out the assessment of risk of bias; one of them a methodologist and the other a topic area specialist. If any piece of information important for the assessment of risk of bias was missing in the included reports, we made attempts to contact the study investigators and obtain the required information by use of open‐ended questions.
Summarising risk of bias for a study
After taking into account the additional information provided by the authors of the trials, we grouped studies into the following categories. We assumed that the risk of bias was the same for all outcomes and assessed each study as follows.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to alter the results seriously | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
For gingivitis and plaque outcomes, we expected the measures of treatment effect would mostly be continuous. In such cases, the mean difference (or difference in means) and standardised mean difference, when combining different clinical indices, were the effect measures used. We calculated the corresponding 95% confidence intervals for each study.
Clinical attachment loss can be a continuous measure, but the incidence is often so low that it can be dichotomised on a patient basis and considered a binary measure. We planned to use risk ratios together with 95% confidence intervals to combine dichotomous data. For completeness, raw values (mean, standard deviation (SD), N) were to be presented for clinical attachment loss.
For caries outcomes, we planned to calculate the prevented fraction (PF) where appropriate. The PF is expressed as the mean increment in the control group minus the mean increment in the intervention group divided by the mean increment in the control group, i.e. the caries increment in the treatment group expressed as a percentage of the control group.
We planned to enter data from cross‐over and split‐mouth studies, and for the prevented fraction, into RevMan using the generic inverse variance outcome type (Review Manager (RevMan)).
Unit of analysis issues
The unit of analysis was individual patients or groups of measuring sites within individual patients (e.g. interproximal sites: proportion of sites that have bleeding averaged over the number of patients). Contact with study authors was necessary to obtain data in the right form. We analysed split‐mouth studies taking the pairing into account as described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Appropriate methods would also have been applied to cross‐over and cluster trials had any been included.
Dealing with missing data
As described in Table 16.1.a in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), there are several types of missing data in a systematic review or meta‐analysis. The problems of missing studies and outcomes are addressed in the Assessment of reporting biases part of this review. A common problem is missing summary data, such as standard deviations for continuous outcomes, or separate sample sizes for each intervention group. Missing summary data was not a reason to exclude a study from the review and we used the methods outlined in section 16.1.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for imputing missing standard deviations. In some studies, data on individuals were missing from the reported results. When necessary, we made attempts to contact the study authors to ask them for more information.
We made assumptions about the reasons why the data were missing explicit. For the data judged to be 'missing at random', i.e. their being missing is unrelated to their actual values, analysis included only the available data and ignored the missing data. If data were judged to be 'not missing at random', we performed a sensitivity analysis to assess how the changes in assumptions might have affected the results. The potential impact of missing data on the findings of the review will be addressed in the 'Discussion' section of the review.
Assessment of heterogeneity
Prior to meta‐analysis, we first assessed studies for clinical homogeneity with respect to type of therapy, control group and the outcomes. Clinically heterogeneous studies were not combined in the analysis. For studies judged as clinically homogeneous, we tested statistical heterogeneity using the Chi2 test and I2 statistic. We interpreted a Chi2 test resulting in a P value less than 0.10 as indicating significant statistical heterogeneity. In order to assess and quantify the possible magnitude of inconsistency (i.e. heterogeneity) across studies, we used the I2 statistic with a rough guide for interpretation as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity.
Assessment of reporting biases
We assessed possible reporting biases on two levels: within‐study and between‐study. Within‐study selective outcome reporting was examined as a part of the overall 'Risk of bias' assessment (seeAssessment of risk of bias in included studies). We made attempts to find protocols of included studies and compare the outcomes stated in the protocols with those reported in the publications. If protocols were not found, we compared the outcomes listed in the methods sections on a publication against those whose results are reported. In case some indications of reporting bias were found, we contacted study authors for clarification. If there were at least 10 studies included in a meta‐analysis, we would have created a funnel plot of effect estimates against their standard errors to assess a possible between‐study reporting bias. If an asymmetry of the funnel plot was found either by inspection or statistical tests, we planned to consider possible explanations and take this into account in the interpretation of the overall estimate of treatment effects.
Data synthesis
Meta‐analysis included only the studies reporting the same outcomes. Since there are a number of different indices measuring what we consider the same basic concept (plaque or gingivitis), we used the standardised mean difference (SMD), along with the appropriate 95% confidence intervals (CI), to combine the results of different indices in meta‐analysis. Some studies measured plaque and gingivitis on selected sites and we used indices based on these data. Risk ratios were also combined for binary data. As considerable heterogeneity was expected in the included studies, we planned a random‐effects model to be used as a primary method of meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
-
Periodontal status.
-
Conical versus cylindrical interdental brushes.
-
Trained (instructed) versus untrained (uninstructed) interdental brushing.
Sensitivity analysis
Primary meta‐analysis included all eligible studies irrespective of their risk of bias. Sensitivity analysis excluded those studies at high risk of bias to assess how the results of meta‐analysis might be affected.
We performed sensitivity analysis taking into account the sources of funding of the included studies. We carried out primary analysis on all included studies, and compared the results against the results of analysis that included only non‐industry funded studies.
We also performed sensitivity analysis to evaluate the impact of restricting the analysis to studies where we did not need to estimate standard deviations.
Summary of findings
We adopted the GRADE system for evaluating the quality of the evidence of systematic reviews (Guyatt 2008a; Guyatt 2008b; Higgins 2011) and used it to construct 'Summary of findings' tables. We assessed the quality of the body of evidence with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates and the risk of publication bias. We classified the quality of the body of evidence into four categories: high, moderate, low and very low.
Results
Description of studies
Results of the search
Figure 1 shows the study selection flow chart with the search strategy that yielded 865 unique records, consisting of titles with or without abstracts. Of these 865 references, two authors independently judged 840 as irrelevant for the review. We obtained the 25 studies that both authors who screened the records could not confidently exclude, based on their titles and abstracts, in full‐text versions and two authors carefully reviewed them independently. As a result, 17 studies were found ineligible for inclusion and they are presented in the Characteristics of excluded studies table. The remaining seven studies (8 articles) were finally included in this review (Figure 1).

Study selection flow diagram.
Included studies
Design
Four studies had a parallel design (Jared 2005; Jackson 2006; Yankell 2002; Yost 2006) and three studies had a split‐mouth design (Christou 1998; Imai 2011; Ishak 2007). Regarding the number of study arms, five studies had two arms (Christou 1998; Imai 2011; Ishak 2007; Jackson 2006; Yankell 2002), one study had four arms (Yost 2006) and one study had five arms (Jared 2005).
Sample sizes
For the analyses of interdental brushing versus flossing the total number of study participants that provided data for the analyses was 326, 197 of which were enrolled in the interdental brushing plus toothbrushing study arms and 195 participants in the flossing plus toothbrushing control groups.
The median number of participants, calculated as the median of the sample sizes of the included studies, was 59 (range 10 to 77).
As for toothbrushing versus toothbrushing plus interdental brushing, only one study (Jared 2005) provided data. The total number of participants enrolled in these two study arms was 62, of which 30 participants were enrolled in the toothbrushing plus interdental brushing study arm and 32 participants in the toothbrushing only study arm.
Only one study reported a sample size calculation (Jackson 2006). Imai 2011 derived the sample size from the sample sizes of Jackson 2006 and Yost 2006.
Setting
Three trials were conducted in the United States of America (Jared 2005; Yankell 2002; Yost 2006), two in the United Kingdom (Ishak 2007; Jackson 2006), one in Canada (Imai 2011) and one in the Netherlands (Christou 1998).
Participants
Except for two studies which did not report the oral health status of the participants (Yankell 2002; Yost 2006), all other included studies selected participants based on their existing periodontal diseases. Three studies included patients with moderate to severe periodontitis (Christou 1998; Ishak 2007; Jackson 2006) and two studies included patients that showed clinical signs of gingivitis (Imai 2011; Jared 2005).
Details of the participants' oral health status included in these studies are as follows.
-
Participants had to have probing depth > 5 mm, assessed by a force‐controlled probe, radiographic evidence of alveolar bone loss and inflamed gingiva, that was assessed by the bleeding on probing (Christou 1998).
-
Measurements taken at 10 sites in each quadrant at the baseline visit were presented as mean and standard deviations and are as follows: bleeding on probing (BOP): 10.3 + 4.22 ‐ 11.3 + 4.16; % sites > 3 mm probing depth (PD): 26 + 20.38 ‐ 29.5 + 22.78 (Ishak 2007).
-
Participants had to have at least one shallow pocket of 4 to 5 mm or at least one deep pocket > 6 mm (Jackson 2006).
-
Participants were selected if they had plaque‐induced gingivitis, determined by red gingival tissue that bleeds upon stimulation with probing depths < 4 mm (Imai 2011).
-
Participants had to have at least one test site defined as an interproximal space of 1.0 mm that exhibited bleeding from the facial and lingual side (Jared 2005).
Smokers were excluded from three studies (Imai 2011; Ishak 2007; Jared 2005). Two studies included smokers (Jackson 2006; Yost 2006) and in two studies smoking as a criteria was not reported (Christou 1998; Yankell 2002).
None of the included studies reported the participants' socioeconomic status.
Intervention
Seven studies provided data for the comparison between toothbrushing and interdental brushing with toothbrushing and flossing. One study consisted of an interdental brushing study arm with a placebo gel (Jared 2005) but we extracted the data for the meta‐analyses for the interdental brushing group without gel. One study (Yost 2006) had flossers and regular floss study arms. Because they both refer to manual flossing, we combined data from the two intervention groups into a single intervention group using methods outlined in Chapter 7 of the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011).
Only one study (Jared 2005) included the interdental brushing plus toothbrushing study arm and compared it to the toothbrushing alone study arm.
Four studies reported frequency of interdental brushing and flossing, whereas the frequency in three studies was once daily (Imai 2011; Jared 2005; Yost 2006) and in one study the frequency was twice daily (Yankell 2002). Three studies did not report the frequency of the assigned interdental cleaning method (Christou 1998; Ishak 2007; Jackson 2006).
No specific instructions were given for the use of any of the distributed oral hygiene materials in one study (Yankell 2002), where only one brush size was used. In all remaining studies participants were provided with detailed instructions on the use of the assigned product. There was often detailed information on the size of the brushes to be used, and how this was determined for each individual patient (see Characteristics of included studies).
Baseline cleaning that was performed in order to facilitate application of the assigned interdental device was reported in all included studies except in one study (Yankell 2002).
Participants' adherence was assessed in five studies (Christou 1998; Imai 2011; Ishak 2007; Jared 2005; Yost 2006) but two studies did not report adherence assessments (Jackson 2006; Yankell 2002).
Outcomes
All data concerning the means and standard deviations extracted from the studies and used in the analyses are presented in Additional Table 1 and Table 2.
| Study | Time | Interdental brushing | Floss | ||||
| n | Mean | SD | n | Mean | SD | ||
| 6 weeks | 26 | 0.83 | 0.18 | 26 | 0.86 | 0.15 | |
| 6 weeks | 29 | 0.11 | 0.03 | 29 | 0.17 | 0.04 | |
| 12 weeks | 30 | 0.08 | 0.02 | 30 | 0.2 | 0.04 | |
| 4 weeks | 10 | 0.056 | 0.0479 | 10 | 0.081 | 0.0506 | |
| 6 weeks | 39 | 0.14 | 0.15 | 38 | 0.23 | 0.22 | |
| 12 weeks | 39 | 0.1 | 0.11 | 38 | 0.16 | 0.17 | |
| 4 weeks | 30 | 1.03 | 0.57 | 29 | 1.29 | 0.7 | |
| 4 weeks | 31 | 1.21 | 0.27 | 31 | 1.41 | 0.39 | |
| 6 weeks | 31 | 0.78 | 0.83** | 31 | 0.95 | 0.83* | |
| 28 | 0.91 | 0.79* | |||||
*Split‐mouth studies.
**The standard deviations in the Yost 2006 study were calculated from standard errors reported within graphs in the study report.
| Study | Time | Interdental brushing | Floss | ||||
| n | Mean | SD | n | Mean | SD | ||
| 6 weeks | 26 | 2.15 | 0.99 | 26 | 2.47 | 0.86 | |
| 6 weeks | 29 | 1.23 | 0.18 | 29 | 1.23 | 0.18 | |
| 12 weeks | 30 | 1.26 | 0.24 | 30 | 1.28 | 0.22 | |
| 4 weeks | 10 | 0.057 | 0.0221 | 10 | 0.053 | 0.0306 | |
| 6 weeks | 39 | 0.68 | 0.28 | 38 | 1 | 0.36 | |
| 12 weeks | 39 | 0.72 | 0.37 | 38 | 0.96 | 0.4 | |
| 4 weeks | 30 | 2.02 | 0.77 | 29 | 2.23 | 0.83 | |
| 4 weeks | 31 | 1.67 | 0.29 | 31 | 1.71 | 0.28 | |
| 6 weeks | 31 | 1.84 | 1.1** | 31 | 2.06 | 1.1* | |
| 28 | 1.98 | 1.05* | |||||
*Split‐mouth studies.
**The standard deviations in the Yost 2006 study were calculated from standard errors reported within graphs in the study report.
The indices reported for each trial (those included are indicated by an asterisk) are shown below.
| Study | Time points | Gingivitis index (scale) | Plaque index (scale) |
| 1 month* | Bleeding on probing (nr)* | Quigley & Hein Plaque Index (Volpe modification)* | |
| 1, 3 months | Eastman Interdental Bleeding Index (0/1)* | Silness & Löe Plaque Index (0 to 3)* | |
| 1 month | Bleeding on probing* (nr) | Supragingival plaque* and subgingival plaque using dental floss (+/‐) | |
| 1, 3 months | Eastman Interdental Bleeding Index (0/1)* Bleeding on probing (0/1) | Plaque Index (Silness & Löe) (0 to 3)* | |
| 1 month | Löe‐Silness Gingival Index (Lobene modification) (0 to 4)* Bleeding on probing (Van der Weijden modification) (+/‐) | Quigley & Hein Plaque Index (Turesky modification) (0 to 5)* | |
| 1 month | Eastman Interdental Bleeding Index (0/1) Löe‐Silness Gingival Index (Lobene modification)* (0 to 4) | Quigley & Hein Plaque Index (Turesky modification) (0 to 5)* | |
| 1 month | Eastman Interdental Bleeding Index Löe‐Silness Gingival Index* (0 to 3) | Quigley & Hein Plaque Index (Benson modification)* |
*One month includes four to six weeks data.
Gingivitis
Gingivitis as an outcome was assessed in all seven included studies. Four studies used only bleeding indices (Christou 1998; Imai 2011; Ishak 2007; Jackson 2006), whereas one study used two bleeding indices (Jackson 2006). The remaining three studies used bleeding and gingivitis indices to assess the progression of the disease (Jared 2005; Yankell 2002; Yost 2006).
Gingivitis data from the included studies were used in meta‐analysis.
-
Two studies used the Lobene modification of the Löe‐Silness gingival index (Lobene 1985) with one study (Yankell 2002) reporting the gingival index on facial and lingual margins of the Ramfjord teeth and the other study (Jared 2005) reporting scores on the interproximal surfaces of test teeth.
-
Yost 2006 used the Löe‐Silness gingival index in whole mouth.
The following bleeding indices were used in the included studies:
-
Bleeding on probing index (BOP) in Christou 1998; Ishak 2007 and Jackson 2006.
-
Modified bleeding on marginal probing (Van der Weijden 1994) was used in Jared 2005.
-
Eastman Interdental Bleeding Index (EIBI) in Imai 2011; Jackson 2006; Yankell 2002 and Yost 2006.
Two studies (Imai 2011; Jackson 2006) presented data using EIBI dichotomous bleeding scores, three studies scored bleeding using ordinal indices (Jared 2005; Yankell 2002; Yost 2006) and in two studies scoring of the bleeding indices used remained unclear (Christou 1998; Ishak 2007).
Examiner's reliability was assessed in three studies (Ishak 2007; Jackson 2006; Jared 2005), whereas in Ishak 2007 it was stated that an experienced examiner performed all the measurements and in the Jared 2005 examiner was trained and calibrated. As for the remaining four studies (Christou 1998; Imai 2011; Yankell 2002; Yost 2006) the intra‐examiner's training and reliability was not reported.
Two studies (Christou 1998; Ishak 2007) used a force‐controlled probe to assess gingival bleeding and Jared 2005 used a manual probe, while for two studies (Yankell 2002; Yost 2006) no information was given on the kind of probe that was used in the assessment of bleeding. In two studies (Imai 2011; Jackson 2006) bleeding was assessed by the EIBI in which a wooden interdental cleaner is used to elicit bleeding.
Yost 2006 used Löe‐Silness gingival index and reported it as means without standard deviations. The study's results were nevertheless included in meta‐analyses with standard deviations estimated from standard errors presented in a very poor graph.
Plaque
-
One study (Christou 1998) used the Volpe modification of the Quigley and Hein plaque index (Quigley 1962; Volpe 1993) on approximal sites.
-
Two studies (Yankell 2002; Jared 2005) used the Turesky modification of the Quigley and Hein plaque index (Turesky 1970), whereas one study (Jared 2005) assessed interproximal scores only and other (Yankell 2002) evaluated plaque levels on the gingival areas on the facial and lingual surfaces of the Ramfjord teeth.
-
Whole mouth plaque assessment was performed in Yost 2006 using the Benson modification of the Quigley and Hein index (Benson 1993).
-
Imai 2011 and Jackson 2006 used the Löe and Silness plaque index on interproximal surfaces (disto‐buccal, disto‐lingual, mesio‐buccal, mesio‐lingual).
-
Ishak 2007 assessed plaque on proximal surfaces as positive or negative.
Adverse events
Three studies reported adverse events. This was done through participants completing a questionnaire in two studies (Christou 1998; Ishak 2007), reporting problems with the use of the assigned products. In another study the participants completed a diary for adherence (Yost 2006). In a further study (Jared 2005) participants were requested to keep a log of any symptoms experienced during the study but no such results were reported.
Data considerations for exploration of heterogeneity
To further explore the clinical heterogeneity, we intended to perform a subgroup analysis on the periodontal status by categorising the studies based on the participants' baseline periodontal status. This categorisation of studies was undertaken according to the Periodontal Disease Classification System of the American Academy of Periodontology (Armitage 1999) in which slight periodontitis is described with 1 to 2 mm clinical attachment loss (CAL), moderate with 3 to 4 mm CAL and severe > 5 mm CAL, whether it was localised or generalised with > 30% of sites involved. Only three studies (Christou 1998; Ishak 2007; Jackson 2006) stated the degree of CAL among the participants, and we therefore decided that the data available were insufficient to conduct a subgroup analysis.
The subgroup analysis for conical versus cylindrical interdental brushes was also not possible since neither of the included studies provided information on the shape of the interdental brushes used.
Excluded studies
After having screened the full texts of the studies, we judged 17 studies as ineligible for inclusion in this review based on the following reasons: cross‐over study without sufficient washout period (two), intervention period less than four weeks (five), no interdental brush among treatment groups (five), article was a preliminary report/abstract (three), special toothbrush compared with a regular toothbrushing (one), results presented without standard errors and in a manner that cannot be used in the review (one). A list of the excluded studies with explanation of the decisions for exclusion is presented in Characteristics of excluded studies.
Risk of bias in included studies
Summaries of the risk of bias are presented for each study and domain in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was mentioned in all included studies. The allocation sequence generation was clearly described in three studies (Imai 2011; Jackson 2006; Jared 2005). One study (Ishak 2007) reported that a statistician generated the randomisation sequence so it was assumed that it was done properly despite the lack of further details about the randomisation process. However, in three studies (Christou 1998; Yankell 2002; Yost 2006) randomisation was only mentioned with no adequate description of the sequence generation method.
Allocation concealment is not as important in split‐mouth studies so these have all been assessed as at low risk of bias (Christou 1998; Imai 2011; Ishak 2007). There were no reports of allocation concealment in the other four studies (Jackson 2006; Jared 2005; Yankell 2002; Yost 2006) and we assessed these as unclear.
Blinding
Blinding of the examiner on clinical outcomes was clearly reported in four studies (Christou 1998; Imai 2011; Ishak 2007; Jackson 2006). The other studies were described as either double or single‐blind and so we also assessed this as at low risk of bias (Jared 2005; Yankell 2002; Yost 2006).
There was no blinding of the participants in any of the included studies, and this did not form part of the 'Risk of bias' assessment.
Incomplete outcome data
Withdrawals were adequately reported in all included studies. In one study (Yost 2006) the number of drop‐outs was reported but reasons and breakdown by study arms were not provided. Nevertheless, having considered the number of participants that were lost from the study, we judged attrition as unlikely to affect the results.
Selective reporting
We judged four studies (Christou 1998; Imai 2011; Jackson 2006; Yankell 2002) as having low risk of bias regarding selective outcome reporting. In Jared 2005 data on possible adverse effects were not reported although participants were asked to keep a log, so we assessed this as at high risk of bias. We also assessed a further two studies as at high risk of bias for selective reporting: in one study (Ishak 2007) the mean difference and standard deviations were not reported for the clinical outcomes taking into account the split‐mouth nature of the data, and in Yost 2006 standard deviations were not reported.
Other potential sources of bias
We judged the risk of other potential sources of bias as low in four studies (Christou 1998; Imai 2011; Ishak 2007; Jackson 2006) and unclear in the remaining three studies (Jared 2005; Yankell 2002; Yost 2006).
Two of these studies (Jared 2005; Yost 2006) were financially supported by industry.
Adherence was not assessed and intra‐examiner's reliability was not reported in two studies (Yankell 2002; Yost 2006). In one study (Jared 2005) compliance was not reported although participants were asked to keep log of their dental cleaning habits, but the examiner's reliability was tested.
Overall risk of bias
We assessed one study as at low risk of bias (Imai 2011) and three studies as high (Ishak 2007; Jared 2005; Yost 2006), with the remainder being assessed as unclear (Christou 1998; Jackson 2006; Yankell 2002).
Effects of interventions
See: Summary of findings for the main comparison Interdental brushing with toothbrushing compared to toothbrushing alone for periodontal diseases and dental caries in adults; Summary of findings 2 Interdental brushing compared to flossing for periodontal diseases and dental caries in adults
Comparison: Interdental brushing and toothbrushing versus toothbrushing alone
The first objective of this review was to examine whether or not interdental brushes are effective in reducing the progression of periodontal diseases or dental caries when used together with regular toothbrushing. Only one study, at high risk of bias, compared interdental brushing plus toothbrushing versus toothbrushing alone (Jared 2005), so a meta‐analysis was not possible.
Gingivitis
Interproximal gingival index was observed and measured at two and four weeks. The mean difference (MD) for gingivitis at four weeks between the two study groups was ‐0.53 (95% confidence interval (CI) ‐0.83 to ‐0.23; P value = 0.001) in favour of toothbrushing plus interdental brushing.
Periodontitis
Periodontitis was not reported as an outcome in this study.
Interproximal caries
Interproximal caries was not reported as an outcome in this study.
Plaque
Interproximal plaque index was observed and measured at two and four weeks. The mean difference for plaque at four weeks was ‐0.95 (95% CI ‐1.34 to ‐0.56; P value < 0.0001), again in favour of interdental brushing.
Harms and adverse effects
No adverse effects were reported in this study.
Comparison: Interdental brushing plus toothbrushing versus flossing plus toothbrushing
All seven studies contributed data for gingivitis and plaque at one month and two studies provided data on both conditions at three months.
Gingivitis
Gingivitis at one month
All seven studies were included in the meta‐analysis for gingivitis at the one‐month time point (Christou 1998; Imai 2011; Ishak 2007; Jackson 2006; Jared 2005; Yankell 2002; Yost 2006). One was assessed as low risk of bias, three as unclear risk of bias and three as at high risk of bias.
The standardised mean difference (SMD) was ‐0.53 (95% CI ‐0.81 to ‐0.24) with a P value of 0.0003, indicating that there is evidence of benefit for using interdental brushing plus toothbrushing for the reduction of gingivitis at one month when compared to flossing plus toothbrushing.
Statistical analysis of the I2 statistic (79%), Chi2 (28.09 (degrees of freedom (df) = 6)) and the corresponding P value (P < 0.0001) indicates substantial statistical heterogeneity among studies.
We examined the pre‐specified subgroups where appropriate to determine possible reasons for any heterogeneity. For trained versus untrained interdental brushing we undertook a subgroup analysis and the results for gingivitis at one month for the two subgroups are presented: trained (six trials) and untrained interdental brushing (one trial). There was no evidence of a difference between subgroups (P value = 0.74) (Analysis 1.1).
Gingivitis at three months
Two studies were included in the meta‐analysis assessing gingivitis at the three‐month time point (Imai 2011; Jackson 2006). We judged one as low and one as unclear risk of bias. The SMD was ‐1.98 (95% CI ‐5.42 to 1.47; P value = 0.26). We therefore conclude that there is insufficient evidence to support or refute the claim that interdental brushing is effective in reducing gingivitis at three months when compared to flossing. Considerable heterogeneity was observed (Chi2 23.14 (df = 1); P value < 0.00001; I2 = 96%). It is, however, difficult to interpret such heterogeneity since there are only two studies available for the analysis, both differing methodologically in terms of study design. Clinical heterogeneity among the two studies refers to the participants' baseline periodontal status and the inclusion of smokers in the Jackson 2006 study (Analysis 1.2).
Overall, there is some evidence that interdental brushing plus toothbrushing reduces gingivitis at one month when compared with flossing plus toothbrushing. More evidence is needed to support the conclusions about the effect at three months.
Periodontitis
Periodontitis was not reported as an outcome in any included study.
Interproximal caries
Interproximal caries was not reported as an outcome in any included study.
Plaque
Plaque at one month
The meta‐analysis of plaque at one month included all seven studies. We assessed one as at low risk of bias, three as at unclear risk of bias and three as at high risk of bias. The pooled estimate resulted in a SMD of 0.10 (95% CI ‐0.13 to 0.33; P value = 0.39), which showed low‐quality evidence of no difference in the effectiveness of interdental brushing plus toothbrushing compared to flossing plus toothbrushing in the reduction of plaque parameters at one month. Considerable heterogeneity among studies was observed (I2 = 85%; Chi2 = 39.36 (df = 6); P value < 0.00001). We carried out subgroup analysis of trained versus untrained interdental brushing but this was not significant (P value = 0.34) (Analysis 1.3).
Plaque at three months
Two studies (Imai 2011; Jackson 2006) assessed the plaque outcome at the three‐month time point. The studies were at low and unclear risk of bias. The resulting effect estimate was SMD ‐2.14 (95% CI ‐5.25 to 0.97; P value = 0.18). We conclude that there is insufficient evidence to determine whether there is a difference between the two interventions. Considerable heterogeneity between studies was observed (Chi2 = 18.84 (df = 1); I2 = 95%; P value < 0.0001), but it is difficult to interpret such heterogeneity because only two studies were included in the analysis, as for gingivitis at three months (Analysis 1.4).
Harms and adverse effects
Adverse effects assessed in this review are regarded in terms of potential harms or damages of to oral soft tissue caused by interdental brushing or flossing.
One study (Christou 1998) reported 14 patients experiencing problems with the use of dental floss, two patients experiencing problems with the use of interdental brushes and two patients reported problems with the use of both interdental brushes and dental floss. However, no detailed information was given on the nature of these problems, but they were most commonly associated with difficulty in manipulating the dental floss. In one study (Ishak 2007) participants reported that interdental brushes tended to buckle or distort while for dental floss they reported that it sometimes stuck between teeth or caused soreness. Considering the ways in which the adverse outcomes were presented, a meta‐analysis was not appropriate.
Three studies reported that no adverse effects were observed or reported during the study (Imai 2011; Jackson 2006; Yankell 2002).
Two studies did not report data on adverse effects. In one of these studies (Jared 2005) participants were asked to keep a log with details of any symptoms that might be experienced during the trial but no data on adverse effects were reported in the trial. Another study (Yost 2006) reported that examination of the oral soft tissue was performed at six weeks (i.e. at the final visit), but provided no data on adverse effects.
Other outcomes
Economic cost and halitosis were not reported in any of the included studies.
Sensitivity analysis for gingivitis and plaque
We conducted sensitivity analyses omitting Yost 2006 (which did not report standard deviations and was judged as at high risk of bias) at the one‐month time point; this led to similar effect estimates. For gingivitis the resulting SMD was ‐0.59 (95% CI ‐0.95 to ‐0.23) and for plaque the SMD was 0.09 (95% CI ‐0.16 to 0.34). Sensitivity analysis omitting the three studies at high risk of bias (Jared 2005; Yankell 2002; Yost 2006) also led to similar estimates for gingivitis and plaque at one month, the SMD values being ‐0.69 (95% CI ‐1.25 to ‐0.12) and SMD 0.06 (95% CI ‐0.28 to 0.40), respectively. We performed sensitivity analysis based on funding, excluding the two industry‐sponsored studies (Jared 2005; Yost 2006) from the analysis at the one‐month time point, resulting in a SMD of ‐0.63 (95% CI ‐1.06 to ‐0.21) for gingivitis and SMD 0.07 (95% CI ‐0.20 to 0.34) for plaque.
Converting SMDs back to original indices
As the results for plaque and gingivitis in all of the included studies were presented as continuous data, but with different instruments (i.e. indices to measure the gingivitis and plaque), we used the standardised mean difference as the statistic for the meta‐analysis. As standardised mean differences are unitless and difficult to interpret, we have re‐expressed them in the original scales and presented them as the indices used in these studies.
Jackson 2006 was selected for the gingivitis outcome at one month because the study was representative of the population and intervention, was judged to have unclear risk of bias and used the Eastman Interdental Bleeding Index. However, for the plaque outcome at one month, we selected Imai 2011 instead because it used the most common plaque index, the Silness & Löe Plaque Index. We judged it to have low risk of bias.
We calculated the mean difference by multiplying the standard deviation of the control group (flossing) by the pooled SMD. We calculated the reduction of control mean by dividing the reduction in mean scores by the control mean and multiplying it by 100 in order to present the data as percentages.
The tables below represent these calculations for the gingivitis index and plaque indices at one month. The differences are expressed as percentage reductions of the control (flossing) mean.
| Gingivitis index | Study | Time | Reduction in mean scores (95% CI) | Control mean (SD) | Reduction as % of control mean |
| Eastman Interdental Bleeding Index | 1 month | ‐ 0.12 (95% CI ‐0.18 to ‐0.05) | 0.23 (0.22) | 52 |
| Plaque index | Study | Time | Difference in mean scores | Control mean | Difference as % of control mean |
| Silness & Löe Plaque Index | 1 month | 0.02 (95% CI ‐0.02 to 0.06) | 1.23 (0.18) | 2 |
All data concerning the means and standard deviations extracted from the studies and used in the analyses are presented in Additional Table 1 and Table 2.
Discussion
Summary of main results
This review found very low‐quality evidence, based on a single study, of the effectiveness of interdental brushing plus toothbrushing when compared to toothbrushing alone for gingivitis and plaque.
This review also found low‐quality evidence of the benefit of interdental brushing plus toothbrushing when compared to flossing plus toothbrushing for the outcome of gingivitis at one month. This result is based on seven studies and translates to a 52% reduction in bleeding. There is low‐quality evidence of no difference in plaque at one month and insufficient evidence to determine whether there is a difference when using interdental brushes or flossing for plaque reduction at three months.
No studies were identified that reported dental caries as an outcome, although the presence of a plaque biofilm is implicit in the development of caries. Therefore it is not possible to demonstrate the effectiveness, or not, of interdental brushing plus toothbrushing for managing dental caries. The studies also did not report clinical attachment loss, halitosis or quality of life.
Harms and adverse effects were reported in five studies and unreported in two. In one of these five studies participants reported problems with the use of interdental brushing and flossing. This was more frequent with dental floss and was most commonly associated with difficulty in manipulating the dental floss in the posterior areas of the mouth. In another study, problems listed for the interdental brushes were that they tended to buckle or distort. With flossing, participants again reported difficulty in manipulating the dental floss in the posterior areas of the mouth and that it sometimes stuck between teeth. However, the most important harm identified for dental floss was that it may cause soreness of the soft tissue.
summary of findings Table 2 (summary of findings for the main comparison) shows the seven main outcomes and the quality of evidence associated with them, using the GRADE approach (Atkins 2004).
Overall completeness and applicability of evidence
Only one study reported on one of our objectives, comparing toothbrushing with and without interdental brushing. All seven included studies addressed our second objective in comparing toothbrushing with interdental brushing with toothbrushing and flossing.
We did not find any studies fitting the inclusion criteria that reported on the effects of interdental brushing on the development of dental caries.
A total of 326 participants from seven studies aged between 18 and 75 provided data for analysis for our second objective. There were more female than male participants, the percentages being 63% female and 37% male, although one study did not report on gender proportions. It is possible that the greater number of female participants in the studies included in this review may have influenced the gingivitis outcomes, as gingivitis is more prevalent in males than females. The observed epidemiological differences between males and females are explained by females' greater knowledge and a more positive approach to oral health compared to their male counterparts (Furuta 2011).
None of the studies provided data about periodontitis, as assessed by clinical attachment loss (CAL). This is not surprising as the maximum study length was only 12 weeks, which is not long enough for changes in CAL to be detected. Also, none of the studies provided data about bad breath (halitosis) or quality of life: it would be very useful for future studies to report on halitosis, as bad breath is frequently seen by patients to be a possible consequence of failure to adequately clean their teeth and supporting tissues.
The participants in three of the studies had periodontal disease, which was described as moderate to severe (two studies) or chronic periodontitis (one study). It is important that studies are conducted on participants who have periodontitis, so that the effects of interdental brushing can be analysed in participants who are representative of the whole population, some of whom will have periodontal disease, rather than those with only gingivitis.
Inclusion of smokers in two studies (Jackson 2006; Yost 2006) may also contribute to the overall representativeness of the sample. Smokers experience less gingival bleeding on probing which may cause problems in the diagnosis of gingivitis (Axelsson 1998). Decreased gingival bleeding in smokers has been explained by a vasoconstricting effect of nicotine on the peripheral blood vessels (Axelsson 1998; Newbrun 1996). However, we are not able to conclude to what extent smoking has affected the results of this review as we are not confident about the total number of smokers included, since in two studies (Christou 1998; Yankell 2002) smoking as a criteria was not taken into account.
For plaque, there is almost no difference between smokers and non‐smokers (Axelsson 1998), so smoking is unlikely to influence the results.
There is also evidence (Christou 1998; Imai 2010; Ishak 2007) that people find interdental brushing easier to perform than flossing and are thus more likely to continue doing so. One of these studies (Christou 1998) provided evidence that patients regarded interdental brushes as more effective in cleaning their teeth than dental floss.
Quality of the evidence
We assessed the quality of the body of evidence for the comparison between toothbrushing with interdental brushing with toothbrushing alone as very low for both plaque and gingivitis (summary of findings Table for the main comparison).
We assessed the quality of the body of evidence for the comparison between interdental brushing and flossing as low for both plaque and gingivitis at one month and very low at three months (summary of findings Table 2).
There is a possibility of publication bias, as studies are more likely to be published if the results are positive. However, the number of studies was insufficient for us to undertake a funnel plot analysis to check for the existence of publication bias.
We assessed three of the studies included in this review as being at high risk of bias (Jared 2005; Yankell 2002; Yost 2006). We assessed three as being at unclear risk of bias and the remaining one as being at low risk. Allocation concealment was unclear in four studies. The outcome assessor was blinded in all studies. Attrition bias due to participants dropping out of the studies was low and was not thought to affect the results. We tried to obtain further information about missing data, particularly if the data were 'not missing at random'. We undertook a sensitivity analysis omitting the studies assessed as at high risk of bias; thisled to similar effect estimates at one month for gingivitis and plaque and thus did not affect the results overall.
Heterogeneity was substantial for both the gingivitis and plaque analyses at the one‐month interval and considerable for both at the three‐month interval. We have been unable to account for this degree of heterogeneity but is probably due to methodological and clinical variability between the studies.
Potential biases in the review process
The search strategy used was sensitive and did not exclude studies based on language nor unpublished studies. We contacted manufacturers of interdental brushes to identify any unpublished studies or studies in progress. It is possible that studies in non‐indexed journals, particularly from developing countries, may not have been identified.
Agreements and disagreements with other studies or reviews
The findings of this review are generally in keeping with those of other reviews (Imai 2012; Slot 2008). The systematic review by Slot 2008 found nine randomised controlled trials (RCTs) and reported that there was a statistically significant reduction in plaque scores in five out of the eight studies that compared interdental brushing to floss. However, we only found weak and unreliable evidence to support or deny any difference in effectiveness. The Slot 2008 systematic review did not find a statistically significant difference in gingivitis between interdental brushing and floss, but we did. The systematic review by Imai 2012 identified seven RCTs, six being the same that we identified, but included one that we had excluded and excluded one that we had included. The overall findings of Imai 2012 are similar to our review, that interdental brushing is more effective than floss in reducing gingivitis, but are dissimilar in that we found no evidence for a reduction in plaque scores, when comparing interdental brushing to flossing.

Study selection flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Interdental brushing versus flossing, Outcome 1 Gingivitis at 1 month.

Comparison 1 Interdental brushing versus flossing, Outcome 2 Gingivitis at 3 months.
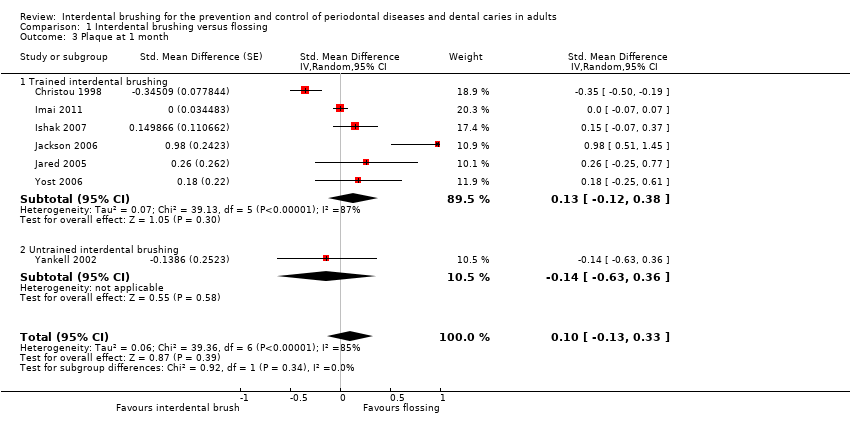
Comparison 1 Interdental brushing versus flossing, Outcome 3 Plaque at 1 month.
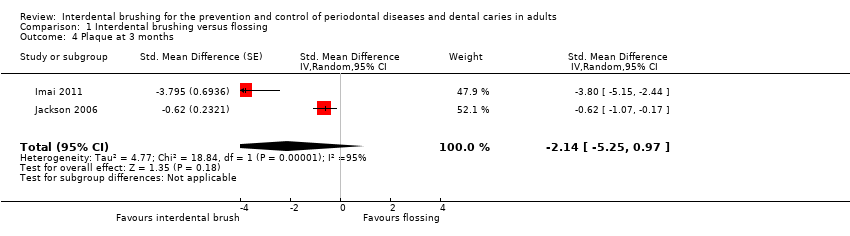
Comparison 1 Interdental brushing versus flossing, Outcome 4 Plaque at 3 months.
| Interdental brushing for periodontal diseases and dental caries in adults | ||||||
| Patient or population: Adults, 16 years and older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No IDB | IDB | |||||
| Gingivitis | The mean gingivitis in the control group was 1.56 | The mean gingivitis in the intervention groups was 0.53 lower (95% CI 0.23 to 0.83) | 62 | ⊕⊝⊝⊝ | 34% reduction in gingivitis | |
| Periodontitis | Not estimable | 0 | See comment | No included study assessed periodontitis as an outcome | ||
| Plaque | The mean plaque in the control groups was | The mean plaque in the intervention groups was 0.95 lower (95% CI 0.56 to 1.34) | 62 | ⊕⊝⊝⊝ | 32% reduction in plaque | |
| Interproximal caries | Not estimable | 0 | See comment | No included study assessed caries as an outcome | ||
| Harms and adverse outcomes | Not estimable | 0 | See comment | Only 1 study reported adverse outcomes in terms of problems with the use of the assigned interdental cleaning aids | ||
| Bad breath (halitosis) | Not estimable | 0 | See comment | No included study assessed bad breath as an outcome | ||
| Quality of life | Not estimable | 0 | See comment | No included study assessed quality of life as an outcome | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Single study at high risk of bias. | ||||||
| Interdental brushing compared to flossing for periodontal diseases and dental caries in adults | ||||||
| Patient or population: Adults, 16 years and older | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Flossing | IDB | |||||
| Gingivitis | The mean gingivitis in the flossing groups was | The mean gingivitis in the intervention groups was | 390 | ⊕⊕⊝⊝ | The estimate is for the 1‐month (4 to 6 weeks) time point and converts back to 52% reduction (of control mean) for IDB (based on 1 study). Results (based on 2 studies, very low‐quality evidence) at 3 months show a large SMD but we are unable to draw conclusions on the effect due to the wide confidence interval including no effect | |
| Periodontitis | Not estimable | 0 | See comment | No included study assessed clinical attachment loss, a measure of progression of periodontitis | ||
| Plaque | The mean plaque in the flossing groups was | The mean plaque in the intervention groups was | 326 | ⊕⊕⊝⊝ | The estimate is for the 1‐month (4 to 6 weeks) time point, and converts back to 2% reduction (of control mean) for IDB (based on 1 study). The effect for the 3 months time point somewhat differs, with a large SMD but we are unable to draw conclusions on the effect due to the wide confidence interval including no effect (based on 2 studies, very low‐quality evidence) | |
| Interproximal caries | Not estimable | 0 | See comment | No included study assessed caries as an outcome | ||
| Harms and adverse outcomes | Not estimable | 0 | See comment | 2 studies reported adverse outcomes in terms of problems with the use of the assigned interdental cleaning aids. We were unable to pool data | ||
| Bad breath (halitosis) | Not estimable | 0 | See comment | No included study assessed bad breath as an outcome | ||
| Quality of life | Not estimable | 0 | See comment | No included study assessed quality of life as an outcome | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Re‐expressed from SMD into Eastman Interdental Bleeding Index score. The results should be interpreted with caution since back‐translation of the effect size is based on the results of only one study (Jackson 2006). The estimate is for the one‐month time point with a SMD of ‐0.53. A larger effect was observed for the three months time point with a SMD of ‐1.98. | ||||||
| Study | Time | Interdental brushing | Floss | ||||
| n | Mean | SD | n | Mean | SD | ||
| 6 weeks | 26 | 0.83 | 0.18 | 26 | 0.86 | 0.15 | |
| 6 weeks | 29 | 0.11 | 0.03 | 29 | 0.17 | 0.04 | |
| 12 weeks | 30 | 0.08 | 0.02 | 30 | 0.2 | 0.04 | |
| 4 weeks | 10 | 0.056 | 0.0479 | 10 | 0.081 | 0.0506 | |
| 6 weeks | 39 | 0.14 | 0.15 | 38 | 0.23 | 0.22 | |
| 12 weeks | 39 | 0.1 | 0.11 | 38 | 0.16 | 0.17 | |
| 4 weeks | 30 | 1.03 | 0.57 | 29 | 1.29 | 0.7 | |
| 4 weeks | 31 | 1.21 | 0.27 | 31 | 1.41 | 0.39 | |
| 6 weeks | 31 | 0.78 | 0.83** | 31 | 0.95 | 0.83* | |
| 28 | 0.91 | 0.79* | |||||
| *Split‐mouth studies. **The standard deviations in the Yost 2006 study were calculated from standard errors reported within graphs in the study report. | |||||||
| Study | Time | Interdental brushing | Floss | ||||
| n | Mean | SD | n | Mean | SD | ||
| 6 weeks | 26 | 2.15 | 0.99 | 26 | 2.47 | 0.86 | |
| 6 weeks | 29 | 1.23 | 0.18 | 29 | 1.23 | 0.18 | |
| 12 weeks | 30 | 1.26 | 0.24 | 30 | 1.28 | 0.22 | |
| 4 weeks | 10 | 0.057 | 0.0221 | 10 | 0.053 | 0.0306 | |
| 6 weeks | 39 | 0.68 | 0.28 | 38 | 1 | 0.36 | |
| 12 weeks | 39 | 0.72 | 0.37 | 38 | 0.96 | 0.4 | |
| 4 weeks | 30 | 2.02 | 0.77 | 29 | 2.23 | 0.83 | |
| 4 weeks | 31 | 1.67 | 0.29 | 31 | 1.71 | 0.28 | |
| 6 weeks | 31 | 1.84 | 1.1** | 31 | 2.06 | 1.1* | |
| 28 | 1.98 | 1.05* | |||||
| *Split‐mouth studies. **The standard deviations in the Yost 2006 study were calculated from standard errors reported within graphs in the study report. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gingivitis at 1 month Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐0.81, ‐0.24] | |
| 1.1 Trained interdental brushing | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.52 [‐0.83, ‐0.21] | |
| 1.2 Untrained interdental brushing | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.13, ‐0.11] | |
| 2 Gingivitis at 3 months Show forest plot | 2 | Std. Mean Difference (Random, 95% CI) | ‐1.98 [‐5.42, 1.47] | |
| 3 Plaque at 1 month Show forest plot | 7 | Std. Mean Difference (Random, 95% CI) | 0.10 [‐0.13, 0.33] | |
| 3.1 Trained interdental brushing | 6 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.12, 0.38] | |
| 3.2 Untrained interdental brushing | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.63, 0.36] | |
| 4 Plaque at 3 months Show forest plot | 2 | Std. Mean Difference (Random, 95% CI) | ‐2.14 [‐5.25, 0.97] | |

