ارجاع فوری به کولپوسکوپی در برابر نظارت با سیتولوژی برای اختلالات خفیف سیتولوژیکی سرویکس در غیاب تست HPV
چکیده
پیشینه
تعداد قابل توجهی از زنان در غربالگری سرویکس، مبتلا به اختلالات خفیف سیتولوژیکی تشخیص داده میشوند. بسیاری از صاحبنظران، مراقبت و نظارت (surveillance) را توصیه میکنند، زیرا ممکن است پسرفت (regression) خودبهخودی رخ دهد. با این حال، حضور بیمار برای پیگیری سیتولوژی در طول زمان کاهش یافته و ممکن است برخی از زنان را در معرض خطر ابتلا به بیماری تهاجمی قرار دهد.
اهداف
ارزیابی استراتژی بهینه مدیریتی برای زنان مبتلا به اختلالات سیتولوژیکی خفیف سرویکس (سلولهای سنگفرشی غیرمعمول با اهمیت نامعین ‐ atypical squamous cells of undetermined significance; ASCUS یا ضایعات سنگفرشی داخل اپیتلیالی با درجه پائین ‐ low‐grade squamous intra‐epithelial lesion; LSIL) در غربالگری اولیه در صورت نبود تست HPV (ویروس پاپیلومای انسانی) DNA.
روشهای جستوجو
ما بانکهای اطلاعاتی الکترونیکی زیر را جستوجو کردیم: پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL شماره 4، 2016)، MEDLINE (1946 تا هفته دوم اپریل 2016) و Embase (1980 تا هفته شانزدهم 2016).
معیارهای انتخاب
ما کارآزماییهای تصادفیسازی و کنترل شدهای (randomised controlled trials; RCTs) را وارد کردیم که کولپوسکوپی فوری را با نظارت سیتولوژیکی در زنان مبتلا به سلولهای سنگفرشی غیرمعمول با اهمیت نامعین (ASCUS/borderline) یا ضایعات سنگفرشی داخل اپیتلیالی با درجه پائین (LSIL/دیسکاریوز خفیف (mild dyskaryosis)) مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
معیار پیامد اولیه که مورد مطالعه قرار گرفت، وقوع نئوپلازی داخل اپیتلیالی سرویکس (cervical intra‐epithelial neoplasia; CIN) بود. معیارهای پیامد ثانویه مورد مطالعه، عبارت بودند از میزان شکست، اضطراب و افسردگی معنیدار از نظر بالینی، و سایر عوارض جانبی گزارش شده توسط فرد.
مطالعات را بر اساس دوره مراقبت و نظارت طبقهبندی کردیم: 6؛12؛ 24 یا 36 ماه، همچنین در 18 ماه، که از معاینات خروج احتمالی مستثنی شدند. خطرات نسبی (RR) تجمعی و 95% فواصل اطمینان (CI) را با استفاده از مدل اثرات تصادفی با وزندهی معکوس واریانس محاسبه کردیم. ناهمگونی داخل مطالعات از طریق آماره I2 ارزیابی شد.
نتایج اصلی
پنج RCT را با 11,466 شرکتکننده شناسایی کردیم که واجد معیارهای ورود به مطالعه بودند. هجده مورد سرطان تهاجمی سرویکس وجود داشت، بهترتیب، هفت مورد در گروه کولپوسکوپی فوری و 11 مورد در گروه مراقبت سیتولوژیکی. اگرچه کولپوسکوپی فوری سریعتر از سیتولوژی موارد +CIN2 و +CIN3 را شناسایی کرد، این تفاوتها طی 24 ماه دیگر قابل مشاهده نبودند (+CIN2: 3 مطالعه؛ 4331 زن؛ 17.9% در برابر 18.3%؛ RR: 1.14؛ CI؛ 0.66 تا 1.97؛ +CIN3: 3 مطالعه، 4331 زن؛ 10.3% در برابر 11.9%؛ RR: 1.02؛ CI؛ 0.53 تا 1.97). ناهمگونی داخل مطالعات قابل توجه بود (I2 بزرگتر از 90%). علاوه بر این، وارد کردن نتایج معاینات خروج در طی 24 ماه که میتوانست میزان تشخیص CIN را در مراقبت سیتولوژیکی بالا ببرد، ممکن است منجر به سوگیری (bias) ناشی از طراحی مطالعه شده باشد؛ بنابراین کیفیت این شواهد را پائین در نظر گرفتیم.
هنگامی که معاینات زمان خروج (exit examination) را خارج کردیم، میزان تشخیص ضایعات با درجه بالا در مدت 18 ماه پیگیری پس از کولپوسکوپی فوری بالاتر بود (+CIN2: 2 مطالعه؛ 4028 زن؛ 14.3% در برابر 10.1%؛ RR: 1.50؛ CI؛ 1.12 تا 2.01؛ +CIN3: 2 مطالعه؛ 4028 زن؛ 7.8% در برابر 6.9%؛ RR: 1.24؛ CI؛ 0.77 تا 1.98) هر دو آنها ناهمگونی داخل مطالعاتی اساسی داشتند (I2 بزرگتر از 60%) و کیفیت این شواهد را در سطح متوسط در نظر گرفتیم).
متاآنالیز نشان داد که ارجاع فوری به کولپوسکوپی برخلاف تکرار سیتولوژی بعد از 24 ماه نظارت بیمار، تشخیص اختلالات بدون اهمیت بالینی سرویکس را بهطور معناداری افزایش میدهد (وقوع کویلوسیتوز (koilocytosis): 2 مطالعه؛ 656 زن؛ 32% در برابر 21%؛ RR: 1.49؛ 95% CI؛ 1.17 تا 1.90؛ شواهد با کیفیت متوسط) بروز هرگونه CIN: 2 مطالعه؛ 656 زن؛ 64% در برابر 32%؛ RR: 2.02؛ 95% CI؛ 1.33 تا 3.08؛ شواهد با کیفیت پائین؛ بروز هرگونه CIN1: 2 مطالعه؛ 656 زن؛ 21% در برابر 8%؛ RR: 2.58؛ 95% CI؛ 1.69 تا 3.94؛ شواهد با کیفیت متوسط).
به دلیل تفاوتهای موجود در طراحی و شرایط کارآزماییها، تنوع زیادی در میزان قصور میان مطالعات وارد شده وجود داشت. خطر قصور در گروه سیتولوژی مجدد، بیشتر بود، 4 برابر افزایش طی 6 ماه، شش برابر طی 12 ماه و 19 برابر در مدت 24 ماه (6 ماه: 3 مطالعه؛ 5117 زن؛ 6.3% در برابر 13.3%؛ RR: 3.85؛ 95% CI؛ 1.27 تا 11.63؛ شواهد با کیفیت متوسط؛ 12 ماه: 3 مطالعه؛ 5115 زن؛ 6.3% در برابر 14.8%؛ RR: 6.39؛ 95% CI؛ 1.49 تا 29.29؛ شواهد با کیفیت متوسط؛ 24 ماه: 3 مطالعه؛ 4331 زن؛ 0.9% در برابر 16.1%؛ RR: 19.1؛ 95% CI؛ 9.02 تا 40.43؛ شواهد با کیفیت متوسط).
نتیجهگیریهای نویسندگان
بر اساس شواهدی با کیفیت پائین یا متوسط، با استفاده از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) و خطر سوگیری کلی پائین، به نظر نمیرسد تفاوتی در میزان تشخیص +CIN2 یا +CIN3 پس از دو سال بین کولپوسکوپی فوری و نظارت سیتولوژیکی در نبود تست HPV وجود داشته باشد، اگرچه ممکن است زنان از پیگیری غیبت کرده باشند. احتمالا کولپوسکوپی فوری، باعث تشخیص سریعتر ضایعات با درجه بالا میشود، اما ضایعات با درجه پائین بیشتری نیز که به لحاظ بالینی بدون اهمیت هستند، شناسایی میشوند. بنابراین در زمان عدم اطمینان از همکاری خوب بیمار، کولپوسکوپی میتواند انتخاب اول باشد. این نتایج، نیاز را به یک تست دقیق تریاژ HPV رفلکسی برای جداسازی زنانی که نیاز به پیگیری تشخیصی دارند، از زنانی که میتوانند با خیال راحت به ویزیت معمول بازگردند، نشان میدهند.
PICO
خلاصه به زبان ساده
مدیریت اختلالات خفیف سیتولوژیک شناسایی شده در غربالگری سرویکس
موضوع
برنامههای غربالگری سرویکس، از طریق بهکارگیری سیتولوژی سرویکس (تستهای اسمیر) خطر سرطان دهانه رحم را کاهش میدهند، که هدف این تستها، تشخیص و درمان هر نوع تغییرات پیشسرطانی است که میتوانند برخی زنان را در معرض خطر ابتلا به بیماری تهاجمی (سرطان تهاجمی سرویکس) در آینده قرار دهند. معمولا فقط تغییرات پیشسرطانی شدید نیازمند درمان هستند، با این حال، در صورت موجود نبودن آزمایش HPV (ویروس پاپیلومای انسانی) بهطور معمول، تفاوتهایی در نحوه مدیریت زنان مبتلا به تغییرات سیتولوژیک خفیف (سلولهای سنگفرشی غیرمعمول با اهمیت نامعین (atypical squamous cells of undetermined significance; ASCUS/borderline) یا ضایعات سنگفرشی داخل اپیتلیالی با درجه پائین (low‐grade squamous intra‐epithelial lesion; LSIL/دیسکاریوز (dyskaryosis) خفیف) وجود دارد.
هدف مطالعه مروری
هدف ما، ارزیابی این نکته بود که برای زنان مبتلا به اختلالات خفیف سیتولوژیکی سرویکس، کولپوسکوپی فوری بهتر است یا «انتظار همراه با مشاهده» همراه با تکرار سیتولوژی سرویکس.
یافتههای اصلی چه هستند؟
ما 5 کارآزمایی تصادفیسازی و کنترل شده را با 11,466 شرکتکننده مبتلا به اختلالات خفیف در سیتولوژی سرویکس وارد کردیم، که تحت درمان با کولپوسکوپی فوری یا سیتولوژی مکرر قرار گرفتند. مطالعات وارد شده، تفاوتهای موجود را بین این دو درمان از نظر وقوع ضایعات پیشسرطانی سرویکس ارزیابی کردند.
نتایج نشان دادند که احتمال شناسایی یافتههای بدون اهمیت بالینی در زنانی که در کولپوسکوپی فوری پس از یک آزمایش سیتولوژی غیرطبیعی سرویکس با درجه پائین شرکت داشتند، نسبت به زنانی که با «انتظار همراه با مشاهده» مدیریت شدند، بیشتر بود.
هجده مورد ابتلا به سرطان تهاجمی دهانه رحم وجود داشت، هفت بیمار در گروه کولپوسکوپی فوری و 11 مورد در گروههای نظارت بیمار با سیتولوژی. میزان تشخیص ضایعات بدون اهمیت از نظر بالینی با درجه پائین، همچنین میزان تشخیص ضایعات پیشسرطانی با اهمیتتر از نظر بالینی با درجه بالا (CIN2 یا CIN3 یا بدتر) طی 18 ماه، اما نه در 24 ماه، در گروه کولپوسکوپی فوری بیشتر بود.
خطر عدم همکاری، بهطور معناداری در بازوی سیتولوژی مکرر بالاتر بوده و با طول مدت پیگیری، افزایش یافت.
کیفیت شواهد چگونه است؟
ما شواهد را با کیفیت پائین تا متوسط رتبهبندی کردیم.
نتیجهگیریها چه هستند؟
نشان داده شده که تست HPV DNA، ابزار تریاژ موثری برای زنان مبتلا به اختلالات خفیف سیتولوژی سرویکس است. با این حال، این تست در حال حاضر در سراسر جهان بهصورت معمول در دسترس نیست. بنابراین، اگر امکان انجام تست HPV DNA نباشد، احتمال تشخیص سریعتر ضایعات پیشسرطانی بیشتر در کولپوسکوپی فوری نسبت به نظارت بیمار با سیتولوژی بالاتر است، اما پس از دو سال، بهنظر نمیرسد که تفاوتی میان این دو رویکرد وجود داشته باشد. اگر همکاری بیمار در روش نظارت با سیتولوژی ضعیف تلقی شود، میتوان زنان را بعد از یک آزمایش سیتولوژی غیرطبیعی سرویکس با درجه پائین یا حد مرزی، برای کولپوسکوپی فوری ارجاع داد. زمانی که همکاری بیمار در پیگیری خوب تصور شود، میتوان تکرار سیتولوژی سرویکس را توصیه کرد، زیرا میتواند خطر تشخیص و درمان بیش از حد را کاهش دهد.
Authors' conclusions
Summary of findings
| Immediate colposcopy compared with cytological surveillance for minor cervical cytological abnormalities: occurrence of different grades CIN in histology according to follow‐up time and default rates | ||||||
| Patient or population: women with ASCUS or LSIL Settings: colposcopy clinic Intervention: immediate colposcopy Comparison: cytological surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Risk with cytological surveillance | Risk with immediate colposcopy | |||||
| Occurrence of CIN2+ in histology at 18 months | 101 per 1000 | 151 per 1000 | RR 1.50 (1.12 to 2.01) | 4028 | ⊕⊕⊕⊝ | |
| Occurrence of CIN2+ in histology at 24 months | 183 per 1000 | 209 per 1000 | RR 1.14 (0.66 to 1.97) | 4331 | ⊕⊕⊝⊝ | |
| Occurrence of CIN3+ in histology at 18 months | 69 per 1000 | 86 per 1000 | RR 1.24 (0.77 to 1.98) | 4028 | ⊕⊕⊕⊝ | |
| Occurrence of CIN3+ in histology at 24 months | 119 per 1000 | 121 per 1000 | RR 1.02 (0.53 to 1.97) | 4331 | ⊕⊕⊝⊝ | |
| Occurrence of any CIN in histology at 24 months | 316 per 1000 | 639 per 1000 | RR 2.02 (1.33 to 3.08) | 656 | ⊕⊕⊝⊝ | |
| Default rates at 6 months | 63 per 1000 | 241 per 1000 | RR 3.85 | 5117 | ⊕⊕⊕⊝ moderate6 | |
| Default rates at 12 months | 63 per 1000 | 413 per 1000 | RR 6.60 | 5115 | ⊕⊕⊕⊝ moderate7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For default rates the relative effect is calculated between cytological surveillance versus immediate colposcopy. For histology the relative effect is calculated between immediate colposcopy versus cytological surveillance. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded to moderate due to substantial inter‐study heterogeneity (P = 0.08, I2= 61%). | ||||||
Background
Cervical cancer is largely preventable through screening and treatment of screen‐detected cervical lesions. Despite this, cervical cancer remains the most common female malignancy in virtually all low‐ and middle‐income countries, and the third most common in women worldwide (GLOBOCAN 2013). Of all cervical cancer, 83% occurs in low‐income countries. Cervical cancer still remains an important public health issue in Europe with more than 66,000 new cases and 29,000 deaths annually. The majority of these cases are diagnosed in Eastern European countries, which reflects the absence of a screening programme (Arbyn 2007). A woman's risk of developing cervical cancer by age 75 years ranges from 0.9% in high‐income countries to 1.9% in low‐ and middle‐income countries (Arbyn 2011). In Europe, about 60% of women with cervical cancer are alive five years after diagnosis (EUROCARE 2003). The disease primarily affects younger women and therefore, the total years‐of‐life lost is proportionately higher than that for most other cancers, which often have a later onset.
The purpose of cervical cytology screening programmes using cytology (also known as Pap smear, named after Dr Papanicolaou (Papanicolaou 1941), is the early detection and treatment of pre‐invasive lesions and, ultimately, reduction in both the incidence and mortality from cervical cancer. Screening programmes have proven their value and efficacy in reducing both the incidence and mortality from cervical cancer in countries where they have been widely applied, including the UK. In countries with an established screening programme, there are different challenges: improving coverage and accuracy of screening, as well as the selection and better management of women with lesions of true malignant potential that require intervention. Without doubt, the most significant advance globally has been the realisation that persistent infection with oncogenic human papillomavirus (HPV) is causally associated with cervical cancer, as well as the development of prophylactic vaccines. The HPV DNA test that aims to detect the viral genome has also been developed and has potential clinical applications in primary screening, in the triage of minor cytological abnormalities, and in follow‐up after treatment (Arbyn 2004; Bulkmans 2007; Koliopoulos 2007; Naucler 2007; Paraskevaidis 2004).
Description of the condition
Cervical cytology may be classified according to the Bethesda system (Solomon 2002), or the British Society of Cervical Cytology (BSCC) terminology (NHSCSP 2000), and can be reported in order of severity as: (1) normal; (2) atypical squamous cells of undetermined significance (ASCUS) (Bethesda)/borderline (BSCC); (3) low‐grade squamous intra‐epithelial lesion (LSIL) (Bethesda)/mild dyskaryosis (BSCC); (4) high‐grade squamous intraepithelial lesion (HSIL) (Bethesda)/high‐grade dyskaryosis (either favours moderate or severe) (BSCC). Cervical cytology classified as high‐grade (moderate and severe dyskaryosis in the UK) occurs in roughly 1% to 3% of the screened population. A high‐grade lesion (cervical intra‐epithelial neoplasia 2+ (CIN2+)) is confirmed by histology in greater than 50% to 60% of these cases. The risk of subsequent progression to malignancy is approximately 30%, although a significant proportion (20% to 30%) have regressive CIN.
Although it is widely accepted that women with high grade cytological abnormalities should be referred immediately for a colposcopic examination or subsequent treatment, or both, uncertainty exists regarding the optimum way of managing those with low‐grade findings.
Women with cytology classified as ASCUS or LSIL (Bethesda classification) (Solomon 2002), or their British Society of Cervical Cytology (BSCC) terminology (NHSCSP 2000) equivalents of borderline and mild dyskaryosis, comprise approximately 7% of all the smears performed in the UK every year (Department of Health 2006). These minor abnormalities, with unknown or low malignant potential, are more common in younger women and present a difficult problem with regards to their management; the implications are important as they consume a disproportionate amount of clinical resources, with their significance still debatable. However, despite a low‐grade cytological smear, a considerable proportion of these women (15% to 20%) still have an underlying histological high‐grade lesion (CIN2+) (Bolger 1988; Contreras‐Melendez 1992; Flannelly 1997; Giles 1989; Paraskevaidis 2002; Soutter 1986; Walker 1986) and therefore, are at risk of developing invasive disease.
The HPV test appears to have a role in the triage of those women who need referral to colposcopy. Evidence in the literature reports a significantly better sensitivity and similar specificity for the HPV test in comparison to repeat cytology for the detection of high‐grade lesions for initial ASCUS/borderline cytology (55% positivity). The introduction of the HPV DNA test in cases with ASCUS cytologic findings could enhance the detection of those women with underlying high‐grade CIN who should be referred to colposcopy or returned to routine recall instead of repeat cytology (Arbyn 2004; Arbyn 2012; Kelly 2011). A survey amongst 43 European countries in 2013 showed that the majority (90%) of the countries have introduced HPV testing for ASCUS triage, and 62% use the test in the triage of LSIL as well (Arbyn 2015).
However, this does not appear to be true for LSIL/mild dyskaryosis lesions or women under 30 to 40 years, as the high‐positivity rate of HPV in this group (85%), does not support its use as a triage tool (Arbyn 2005; Arbyn 2013a; TOMBOLA 2009). Furthermore the HPV test is not yet widely available, reinforcing the need for clear recommendations in settings without access to these triage tests (Cuzick 2008).
Description of the intervention
Until new, markedly reliable triage markers develop, the management options of LSIL cytology remain either immediate referral to colposcopy (examination of the cervix and vagina using magnifying light microscope, colposcope, and acetic acid) or cytological surveillance with repeat smears.
A repeat cytology sample at six months may identify women with persistent lesions who require referral to colposcopy.
The management after immediate referral to colposcopy will depend on the colposcopic findings. If they are suggestive of a high‐grade lesion, multiple punch biopsies may be appropriate, particularly in young women of reproductive age. If the colposcopic findings are consistent with a low‐grade lesion, surveillance every six months and treatment, only if the abnormality persists beyond two years, may be justified, particularly in young women, as a significant proportion of these lesions may regress. The rate of regression decreases significantly with increasing age, and in the presence of a high‐risk HPV subtype and increasing duration of presence of the lesion (Kyrgiou 2010).
How the intervention might work
Optimising management of women with LSIL cervical cytology is difficult.
A policy of immediate referral to colposcopy could potentially result, not only in increased numbers of referrals, thus overloading colposcopy clinics and increasing costs, but also in over‐intervention or over‐treatment, or both, due to subtle colposcopic findings. Many young women of reproductive age might be exposed to the physical and psychological sequelae of unnecessary interventions and treatment, which can also be associated with long‐term, pregnancy‐related, morbidity (Arbyn 2008; Founta 2010; Kyrgiou 2006; Kyrgiou 2012a; Kyrgiou 2014; Kyrgiou 2016a; Paraskevaidis 2007).
On the other hand, triage with repeat cytology, and referral to colposcopy, only if the abnormality persists, may result in a potential reduction of the number of unnecessary referrals, but carries risks of missing high‐grade lesions and increased non‐attendance rates (Shafi 1997). Default from screening is known to put women with equivocal smears and occult high‐grade disease at risk of developing invasive cancer.
The TOMBOLA study in the UK showed that, compared with cytological surveillance, a policy of immediate colposcopy does detect more high‐grade lesions, but might lead to over‐treatment. To reduce this, the trial authors suggested that a policy of targeted punch biopsies with subsequent treatment for CIN2 and CIN3, and cytological surveillance for CIN1 or less, provides the best balance between benefit and harm for the management of these women. Immediate loop excision resulted in over‐treatment and more adverse effects, and was therefore not be recommended (TOMBOLA 2009).
Why it is important to do this review
This review aimed to appraise the current evidence from RCTs on cytological surveillance versus immediate colposcopy in the absence of HPV‐testing in order to make a decision on optimum evidence based practice, as currently this is an area of uncertainty.
A list of abbreviations used in the text can be found in Appendix 1.
Objectives
To assess the optimum management strategy for women with minor cervical cytological abnormalities (atypical squamous cells of undetermined significance ‐ ASCUS or low‐grade squamous intra‐epithelial lesions ‐ LSIL) at primary screening in the absence of HPV (human papillomavirus) DNA test.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs comparing immediate referral to colposcopy versus cytological surveillance in women with ASCUS or LSIL cytological abnormalities at primary cervical screening in the absence of HPV DNA testing. We only included studies reporting combined data for minor abnormalities with more severe grades (high‐grade SIL, moderate and severe dyskaryosis), if separate data according to the grade were available. In case of overlap or duplicate reports, we extracted all relevant outcomes from all the publications of each trial. We included trials with multiple arms if at least two arms addressed an eligible comparison; non‐eligible arms were excluded. We excluded non‐randomised studies and pseudo‐randomised trials with alternate allocation of subjects; meeting and conference abstracts; and studies comparing HPV DNA test to colposcopy or repeat cytology.
Types of participants
We included studies with adult women (greater than 18 years old) with minor cytological cervical abnormalities (ASCUS/borderline dyskaryosis and LSIL/mild dyskaryosis). From included studies we excluded women with high‐grade squamous intra‐epithelial lesions (HSIL)/moderate and severe dyskaryosis, when separate data for LSIL abnormalities were available.
Types of interventions
Surveillance with repeat cytology in any setting versus immediate colposcopy.
The gold standard was histological diagnosis at colposcopy or at the end of the surveillance period in the form of punch biopsies or excisional treatment. Excisional treatment included large loop excision of the transformation zone (LLETZ or LEEP), laser conization (LC), cold knife conization (CKC) and needle or straight wire excision of the transformation zone (NETZ/SWETZ). We excluded trials that did not report on histological results (gold standard) (for example, in the form of punch biopsies or excisional treatment).
Types of outcome measures
Primary outcomes
The primary outcomes were the cumulative incidence of cervical intra‐epithelial neoplasia grade 2 or worse (CIN2+) and grade 3 or worse (CIN3+) from histology assessment.
Secondary outcomes
The secondary outcomes included:
-
cumulative incidence at other histological thresholds, including HPV‐associated morphological findings and CIN1;
-
default rates from repeat cytology or colposcopy clinic appointments;
-
anxiety and depression scores (based on validated questionnaires);
-
short‐term adverse effects of management (pain, bleeding, and discharge, together with the duration (in days) and severity; and
-
treatment rates in both groups.
Search methods for identification of studies
We searched for papers in all languages.
We searched the literature from 1946, when the conservative methods of treatment for CIN were first introduced into clinical practice, and included references published up to the present day.
We identified the RCTs comparing these alternative strategies for the management of low‐grade cervical abnormalities by a computerised literature search, by tracing references listed in relevant articles and by a manual search of appropriate journals.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews.
We searched the following electronic databases.
-
The Cochrane Central Register of Controlled Trials (CENTRAL, 2016, Issue 4)
-
MEDLINE (Ovid) (1946 to April week 2, 2016)
-
Embase (Ovid) (1980 to 2016, week 16)
We used the following main keywords: ‘randomised controlled trials’ ‘cervical intraepithelial neoplasia (CIN)’, ‘cervical cancer’, ‘ASCUS’, ‘borderline’, ‘LSIL’, ‘low‐grade squamous intra‐epithelial lesion’, ‘mild dyskaryosis’, ‘colposcopy’, ‘smear’, ‘cytology’.
We used the 'related articles' feature in MEDLINE to retrieve additional references. For databases other than MEDLINE, we adapted the search strategy accordingly. The search strategies for all the databases are available in Appendix 2, Appendix 3 and Appendix 4.
Searching other resources
We searched the metaRegister of controlled trials (www.isrctn.com/page/mrct), the National Cancer Institute's database (www.cancer.gov/clinicaltrials), Physicians Data Query (www.cancer.gov/publications/pdq), Current Controlled Trials (www.controlled-trials.com), and ClinicalTrials.gov (www.clinicaltrials.gov) for ongoing studies.
We searched conference proceedings and abstracts through Zetoc (http://zetoc.mimas.ac.uk) and WorldCat Dissertations (www.oclc.org/support/documentation/firstsearch/databases/dbdetails/details/WorldCatDissertations.htm). We also searched reports of conferences in the following sources.
-
Annual Meetings of the British Society of Colposcopy and Cervical Pathology.
-
Triannual Meetings of the International Federation of Cervical Pathology and Colposcopy.
-
Triannual Meetings of the European Federation of Colposcopy.
-
Annual Meetings of the American Society of Colposcopy and Cervical Pathology.
We checked the citation lists of included studies and contacted experts in the field, Presidents of the British, European, American and International Societies of Colposcopy and Cervical Pathology to identify further reports of studies.
We intended to include both published and unpublished studies, if they met the inclusion criteria for the review.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching into the reference management database Endnote. We removed duplicates and two review authors (MK, IK) independently examined the remaining references. We excluded those trials which clearly did not meet the inclusion criteria, and obtained copies of the full text of potentially relevant references. We (MK, IK) independently assessed the eligibility of the retrieved papers. We then compared the results and resolved any disagreements by discussion. If necessary, we involved a third review author (MA) to reach consensus. We documented the reasons for exclusion.
Data extraction and management
We (MK, IK) extracted the relevant data from the trials identified. This included study population, population characteristics, sample size, study methods, method of randomisation, methodological quality, follow‐up and dropout, assessment of outcomes, and results).
We classified trials according to the length of cytological surveillance (6, 12, 18, 24, 30, and 36 months) and analysed them in different groups.
We extracted or computed from all included studies, the total number of women included and incidence of all grades of histological diagnoses, default rates, depression, anxiety scores, after‐effects and other patient‐reported outcomes in both groups, whenever available. We contacted trial authors to obtain separate data when data on LSILs were merged with HSIL lesions.
In addition, we collected data on the length of period of surveillance and the type of histology used at the end of surveillance or at colposcopy (gold standard: punch biopsies or excisional treatment).
We also documented the diagnostic criteria used for the interpretation of cytology and histology in each study, where these were read, and whether there was a central review in each study in order to assess if the above had significant variation that may have impacted on the value of the review.
For included trials, we extracted the following data.
-
Author, year of publication, journal and language
-
Country
-
Setting where the trial was conducted
-
Inclusion and exclusion criteria
-
Trial design, methodology
-
Trial population
-
total number enrolled and number included in each group
-
participant characteristics (such as age and other demographic and socioeconomic characteristics ‐ risk factors for cervical cancer)
-
index cytology, history of previous cytological lesions
-
type of cytology (conventional or liquid‐based)
-
-
Intervention details
-
surveillance with repeat cytology in primary care and number of follow‐up smears
-
immediate colposcopy and related interventions (i.e. punch biopsies and/or treatment and type)
-
the type of gold standard used (histology at colposcopy or at the end of the surveillance period): punch biopsies or excisional treatment. We planned to report the type of excisional treatment: LLETZ or LEEP, LC, CKC and NETZ or SWETZ.
-
-
Risk of bias (See Assessment of risk of bias in included studies)
-
Outcomes reported in each trial
-
Primary outcomes:
-
-
the cumulative incidence of CIN2+ and CIN3+ at histology
-
-
-
Secondary outcomes:
-
cumulative incidence at other histological thresholds HPV‐associated morphological findings and CIN1
-
default rates from repeat cytology or colposcopy clinic appointments
-
treatment rates in both groups
-
anxiety scores
-
depression scores
-
short‐term after‐effects of management (rates and types), specifically pain, bleeding, and discharge, together with the duration (in number of days) and severity
-
other self‐reported outcomes
-
-
-
-
Details of outcomes reported:
-
for each outcome: outcome definition (with diagnostic criteria if relevant)
-
unit of measurement (if relevant)
-
for scales: upper and lower limits, and whether high or low score is good
-
results: number of participants allocated to each intervention group
-
for each outcome of interest: sample size; missing participants
-
the time points at which outcomes were collected and reported
-
-
We extracted data on outcomes as below.
-
For dichotomous outcomes (all outcomes were reported as dichotomous), we extracted the number of women in each treatment arm who experienced the outcome of interest and the number of women assessed at endpoint, in order to estimate a risk ratio (RR).
We (MK, IK) independently extracted data in a data extraction form specially designed for the review. We resolved differences between review authors by discussion or by appeal to a third review author (MA), if necessary.
Assessment of risk of bias in included studies
We used the Cochrane tool for assessing risk of bias (Higgins 2011). We (MK, IK) independently assessed the risk of bias within each included study based on the following six domains, with our judgements presented as 'low risk of bias'; 'high risk of bias', and 'unclear'.
For RCTs, if identified, we included assessment of:
-
sequence generation;
-
allocation concealment;
-
blinding (of participants, healthcare providers and outcome assessors);
-
incomplete outcome data:
-
we recorded the proportion of participants whose outcomes were not reported at the end of the study; we noted if loss to follow‐up was not reported. We coded the satisfactory level of loss to follow‐up for each outcome as:
-
'low risk of bias', if fewer than 20% of participants were lost to follow‐up, and reasons for loss to follow‐up were similar in both treatment arms
-
'high risk of bias', if more than 20% of participants were lost to follow‐up, or reasons for loss to follow‐up differed between treatment arms
-
'unclear' if loss to follow‐up was not reported;
-
-
-
selective reporting of outcomes; and
-
other possible sources of bias.
Measures of treatment effect
For all (dichotomous) outcomes, we used the risk ratio (RR).
Dealing with missing data
We contacted one study author to obtain data stratified by grade of index cytology (Flannelly 1994). Otherwise, all relevant data were available from the original publications. We did not impute any missing outcome data.
Assessment of heterogeneity
We assessed inter‐study heterogeneity with the Cochran Q test (Cochran 1954), by visual inspection of forest plots (Deeks 2011), by estimation of the percentage of heterogeneity between studies which cannot be ascribed to sampling variation (I2 statistic) (Higgins 2003), and by a formal test of the significance for heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for it.
Assessment of reporting biases
We explored potential publication bias graphically in the funnel plot (Sterne 2011).
Data synthesis
When sufficient clinically similar studies were available, we pooled their results in meta‐analyses. We calculated the risk ratio (RR) and 95% confidence intervals (95% CI) for each reported outcome in the immediate colposcopy versus repeat cytology arm for dichotomous outcomes using the Cochrane Review Manager 5 software (RevMan 2014). We used random‐effects models with inverse variance weighting for all meta‐analyses (Dersimonian 1986). If data were not of suitable quality for meta‐analysis, we presented the data in tables and discussed the data in the text of the review. We analysed data according to different lengths of surveillance: 6, 12, 18, 24, 30, 36 or more months.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis and analysed the data separately for ASCUS/borderline and LSIL/mild dyskaryosis smears, wherever possible.
Sensitivity analysis
We performed a sensitivity analysis excluding one trial (ALTS 2003) that showed opposite evidence compared to other included studies. No additional sensitivity or subgroup analyses were possible due to the small number of studies in each meta‐analysis.
Quality of evidence
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results (Langendam 2013). We created a 'Summary of findings' table based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and using GRADEpro GDT. We used the GRADE checklist and GRADE Working Group quality of evidence definitions (Meader 2014).
-
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low quality: We are very uncertain about the estimate.
Results
Description of studies
The characteristics of the included and excluded studies and the outcomes examined are described in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The searches identified 5176 records (4715 after de‐duplication). We screened the abstracts and excluded 4699 records. We identified 16 potentially eligible full‐text articles for further assessment. We did not identify any unpublished studies. Altogether eight of the 16 identified full‐text articles were supporting reports of the same large trial (TOMBOLA 2009) and two full‐text articles reported the same outcomes for one trial (ALTS 2003), but separately, depending on the initial histology of ASCUS or LSIL. We excluded a further two studies after full text review: one that was not a RCT (De Bie 2011), and one study that did not report required outcomes (Elit 2011). After combinations and exclusions, five unique studies fulfilled the inclusion criteria of this review (ALTS 2003; Flannelly 1994; Kitchener 2004; Shafi 1997; TOMBOLA 2009). More details of the literature search and the reasons for exclusion are presented in the PRISMA flowchart (Moher 2009) (Figure 1).
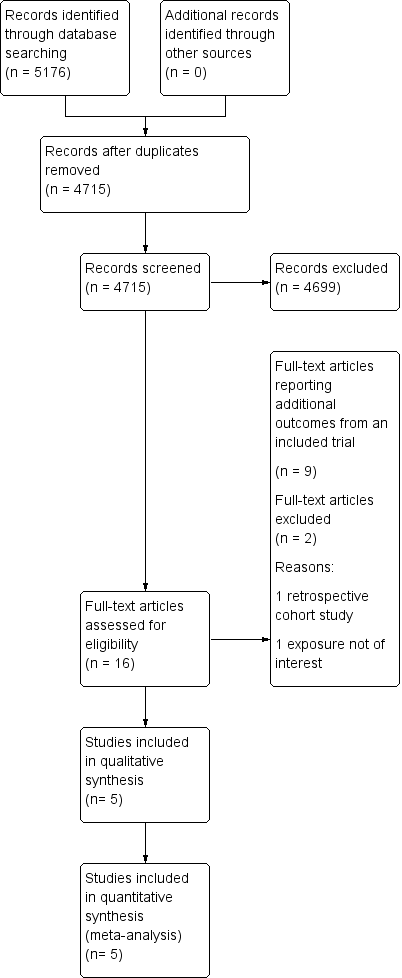
Study flow diagram
Included studies
The detailed characteristics of the included studies are shown in Characteristics of included studies and individual outcomes in Table 1. The five studies included 11,466 participants. All studies were, one conducted in the USA (ALTS 2003) and four in the UK (Flannelly 1994; Kitchener 2004; Shafi 1997; TOMBOLA 2009). Four studies explored the two management strategies in women with either ASCUS (borderline) or LSIL (mild dyskaryosis) (ALTS 2003; Kitchener 2004; Shafi 1997; TOMBOLA 2009), and one study looked at women with mild or moderate dyskaryosis (Flannelly 1994). We obtained separate data for women referred with mild or moderate dyskaryosis from the study authors (Flannelly 1994) and only included women with mild dyskaryosis in the analysis. All studies assessed non‐attendance and the rates for different CIN grades at histology for the immediate colposcopy group, as compared to the histological diagnosis at the end of the surveillance period. Two studies (Kitchener 2004; TOMBOLA 2009) assessed the psychological morbidity of the different approaches (i.e. anxiety, depression and other self‐reported outcomes). The length of the surveillance period varied between studies. Three of the included RCTs followed up women for up to 24 months (ALTS 2003; Flannelly 1994; Shafi 1997), one for 12 months (Kitchener 2004) and another for 36 months (TOMBOLA 2009). One study had four arms with different lengths of follow‐up (immediate colposcopy versus surveillance for 6, 12, or 24 months) (Flannelly 1994). Three studies provided interim histological results (ALTS 2003; Shafi 1997; TOMBOLA 2009), while two of the studies provided results only at the end of the surveillance period (Flannelly 1994; Kitchener 2004). The largest trial randomised 5060 women (ALTS 2003) and the smallest 353 (Shafi 1997).
| Study | Outcomes | Immediate colposcopy n/N (%) | Cytological surveillance n/N (%) | RR + 95% CI |
| ALTS 2003(ASCUS) | Histology at 18 monthsa | |||
| CIN 2 | 61/1163 (5.2) | 26/1164 (2.2) | 2.35 [1.49, 3.69] | |
| CIN 2+ | 119/1163 (10.2) | 92/1164 (7.9) | 1.29 [1.00, 1.68] | |
| CIN 3+ | 58/1163 (5.0) | 66/1164 (5.7) | 0.88 [0.62, 1.24] | |
| Histology at 24 months | ||||
| CIN 2 | 61/1163 (5.2) | 60/1164 (5.2) | 1.02 [0.72,1.44] | |
| CIN 2+ | 119/1163 (10.2) | 168/1164 (14.4) | 0.71 [0.57, 0.88] | |
| CIN 3+ | 58/1163 (5.0) | 108/1164 (9.3) | 0.54 [0.39, 0.73] | |
| Default rates: | ||||
| Default rate at 24 months | 15/1163 (1.3) | 165/1164 (14.2) | 10.99 [6.52, 18.53] | |
| ALTS 2003(LSIL) | Histology at 18 monthsa | |||
| CIN 2 | 63/673 (9.4) | 36/675 (5.3) | 1.76 [1.18, 2.61] | |
| CIN 2+ | 127/673 (18.9) | 95/675 (14.1) | 1.34 [1.05, 1.71] | |
| CIN 3+ | 64/673 (9.5) | 59/675 (8.7) | 1.09 [0.78, 1.52] | |
| Histology at 24 months | ||||
| CIN 2 | 63/673 (9.4) | 58/675 (8.6) | 1.09 [0.78,1.53] | |
| CIN 2+ | 127/673 (18.9) | 151/675 (22.4) | 0.84 [0.68, 1.04] | |
| CIN 3+ | 64/673 (9.5) | 93/675 (13.8) | 0.69 [0.51, 0.93] | |
| Default rates: | ||||
| Default rate at 24 months | 4/673 (0.6) | 110/675 (16.3) | 27.42 [10.17, 73.93] | |
| Histology at 6 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 28/160 (17.5) | 0.52 [0.28, 0.95] | |
| Any CIN | 121/145 (83.4) | 86/160 (53.8) | 1.55 [1.32, 1.82] | |
| CIN 1 | 23/145 (15.9) | 27/160 (16.9) | 0.94 [0.57, 1.56] | |
| CIN 2 | 32/145 (22.1) | 26/160 (16.3) | 1.36 [0.85, 2.16] | |
| CIN 2+ | 98/145 (67.6) | 59/160 (36.9) | 1.83 [1.45, 2.31] | |
| CIN 3+ | 66/145 (45.5) | 33/160 (20.6) | 2.21 [1.55, 3.14] | |
| Histology at 12 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 18/158 (11.4) | 0.79 [0.40, 1.55] | |
| Any CIN | 121/145 (83.4) | 96/158 (60.8) | 1.37 [1.19, 1.59] | |
| CIN 1 | 23/145 (15.9) | 25/158 (15.8) | 1.00 [0.60, 1.69] | |
| CIN 2 | 32/145 (22.1) | 26/158 (16.5) | 1.34 [0.84, 2.14] | |
| CIN 1 / 2 | 55/145 (37.9) | 51/158 (32.3) | 1.18 [0.86, 1.60] | |
| CIN 2+ | 98/145 (67.6) | 71/158 (44.9) | 1.50 [1.22, 1.85] | |
| CIN 3+ | 66/145 (45.5) | 45/158 (28.5) | 1.60 [1.18, 2.17] | |
| Histology at 24 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 11/158 (7.0) | 1.29 [0.60, 2.78] | |
| Any CIN | 121/145 (83.4) | 53/158 (33.5) | 2.49 [1.97, 3.13] | |
| CIN 1 | 23/145 (15.9) | 9/158 (5.7) | 2.78 [1.33, 5.82] | |
| CIN 2 | 32/145 (22.1) | 12/158 (7.6) | 2.91 [1.56, 5.42] | |
| CIN 2+ | 98/145 (67.6) | 44/158 (27.8) | 2.43 [1.84, 3.20] | |
| CIN 3+ | 66/145 (45.5) | 32/158 (20.3) | 2.25 [1.57, 3.21] | |
| Default rates: | ||||
| Default rate at 6 months | 0/145 (0) | 19/160 (11.9) | 35.37 [2.15, 580.52] | |
| Default rate at 12 months | 0/145 (0) | 23/158 (14.6) | 43.16 [2.65, 704.13] | |
| Default rate at 24 months | 0/145 (0) | 38/158 (24.1) | 70.70 [4.38, 1140.47] | |
| Histology at 12 months | ||||
| Any CIN | 83/130 (63.8) | 71/243 (29.2) | 2.19 [1.73, 2.76] | |
| CIN 1 / 2 | 61/130 (46.9) | 47/243 (19.3) | 2.43 [1.77, 3.32] | |
| CIN 3+ | 22/130 (16.9) | 24/243 (9.9) | 1.71 [1.00, 2.93] | |
| Default rates: | ||||
| Default rate at 6 months | 5/130 (3.8) | 46/243 (18.9) | 4.92 [2.01, 12.08] | |
| Default rate at 12 months | 5/130 (3.8) | 95/243 (39.1) | 10.16 [4.24, 24.35] | |
| GHQ casenessb | Choicec | No choicec | ||
| Baseline | 134/233 (58) | 119/241 (49) | 1.16 [0.98, 1.38] | |
| 6 months (pre visit) | 71/183 (39) | 77/190 (41) | 0.96 [0.75, 1.23] | |
| 6 months (post visit) | 59/175 (34) | 66/177 (37) | 0.90 [0.68, 1.20] | |
| 12 months | 40/135 (29) | 35/127 (28) | 1.08 [0.73, 1.58] | |
| Histology at 18 monthsa | ||||
| CIN 2 | 8/182(4.4) | 2/171 (1.1) | 3.76 [0.81, 17.45] | |
| CIN 2+ | 43/182 (23.6) | 16/171 (9.4) | 2.53 [1.48, 4.31] | |
| CIN 3+ | 35/182 (19.2) | 14/171(8.2) | 2.35 [1.31, 2.45] | |
| Histology at 24 months | ||||
| HPV / Koilocytic atypia | 92/182 (50.5) | 57/171 (33.3) | 1.52 [1.17, 1.96] | |
| Any CIN | 88/182 (48.4) | 51/171 (29.8) | 1.62 [1.23, 2.13] | |
| CIN 1 | 45/182 (24.7) | 17/171 (9.9) | 2.49 [1.48, 4.17] | |
| CIN 2 | 8/182 (4.4) | 10/171 (5.8) | 0.75 [0.30, 1.86] | |
| CIN 2+ | 43/182 (23.6) | 34/171 (19.9) | 1.19 [0.80, 1.77] | |
| CIN 3+ | 35/182 (19.2) | 24/171 (14.0) | 1.37 [0.85, 2.20] | |
| Default rates: | ||||
| Default rate at 24 months | 1/182 (0.5) | 36/171 (21.1) | 38.32 [5.31, 276.40] | |
| Tombola 2009 | Histology at 30 monthsa | |||
| CIN 2 | 181/2216 (8.2) | 101/2223 (4.5) | 1.80 [1.42, 2.28] | |
| CIN 2+ | 369/2216 (16.7) | 269/2223 (12.1) | 1.38 [1.19, 1.59] | |
| CIN 3+ | 188/2216 (8.5) | 168/2223 (7.6) | 1.12 [0.92, 1.37] | |
| Histology at 36 months | ||||
| CIN 2 | 181/2216 (8.2) | 157/2223 (7.1) | 1.16 [0.94, 1.42] | |
| CIN 2+ | 369/2216 (16.7) | 350/2223 (15.7) | 1.06 [0.93, 1.21] | |
| CIN 3+ | 188/2216 (8.5) | 193/2223 (8.7) | 0.98 [0.81, 1.18] | |
| Default rates: | ||||
| Default rate at 6 months | 151/2216 (6.8) | 285/2223 (12.8) | 1.88 [1.56, 2.27] | |
| Default rate at 12 months | 151/2216 (6.8) | 327/2223 (14.7) | 2.16 [1.80, 2.59] | |
| Paind | ||||
| Any pain | 304/782 (38.9) | 145/968 (15.0) | 2.60 [2.18, 3.09] | |
| Moderate or more severe | 144/774 (18.6) | 56/965 (5.8) | 3.21 [2.39, 4.30] | |
| Bleedingd | ||||
| Any bleeding | 366/781 (46.9) | 166/967 (17.2) | 2.73 [2.33, 3.19] | |
| Moderate or more severe | 144/772 (18.6) | 16/961 (1.7) | 11.20 [6.74, 18.61] | |
| Discharged | ||||
| Any discharge | 267/780 (34.2) | 83/964 (8.6) | 3.98 [3.17, 4.99] | |
| Moderate or more severe | 133/777 (17.1) | 36/962 (3.7) | 4.57 [3.20, 6.53] | |
| Anxietye | ||||
| 6 weeks | 59/751 (7.9) | 121/900 (13.4) | 0.58 [0.43, 0.79] | |
| 12 months | 190/1161 (16.4) | 218/1130 (19.3) | 0.85 [0.71, 1.01] | |
| 18 months | 162/1050 (15.4) | 177/1008 (17.6) | 0.88 [0.72, 1.07] | |
| 24 months | 179/1001 (17.9) | 177/962 (18.4) | 0.97 [0.81, 1.17] | |
| 30 months | 146/949 (15.4) | 143/887 (16.1) | 0.95 [0.77, 1.18] | |
| Depressionf | ||||
| 6 weeks | 50/757 (6.6) | 68/902 (7.5) | 0.88 [0.62, 1.25] | |
| 12 months | 110/1162 (9.5) | 132/1136 (11.6) | 0.81 [0.64, 1.04] | |
| 18 months | 106/1052 (10.1) | 114/1016 (11.2) | 0.90 [0.70, 1.15] | |
| 24 months | 111/1001 (11.1) | 104/964 (10.8) | 1.03 [0.80, 1.32] | |
| 30 months | 101/948 (10.7) | 108/887 (12.2) | 0.88 [0.68, 1.13] |
For Immediate colposcopy, n = n at immediate colposcopy visit, possible follow‐up excluded.
a Cumulative incidence during follow‐up, excluding the exit examination or deferred treatment.
b GHQ caseness = GHQ (General Health Questionnaire) score ≥ 4.
c Analysis for this outcome between the original randomization groups.
d Based on Questionnaire 6 weeks after immediate colposcopy or first cytological surveillance visit.
e ≥ 11 on hospital anxiety and depression anxiety subscale
f ≥ 8 on hospital anxiety and depression subscale
The design, management protocol and exit examination somewhat varied for different studies. Flannelly 1994 randomly allocated women into four groups: immediate colposcopy, or 6, 12 and 24 months' surveillance. All recruited women underwent LLETZ at the conclusion of their study arm that was preceded by a directed punch biopsy, if a distinct lesion was seen. Women were withdrawn if there were severe cytological abnormalities or colposcopic impression of possible microinvasion. Shafi 1997 had a similar design and treated all women immediately on the day of recruitment or at the completion of 24 months of surveillance. Women were treated during follow‐up, if the follow‐up smear showed severe dyskaryosis or worse.
Kitchener 2004 had a different design and randomised women into two principal groups: cytological surveillance for 12 months (with colposcopy and treatment when necessary at 6 or 12 months, if either follow‐up smear was abnormal) versus the choice to have surveillance or immediate colposcopy and treatment when necessary. Results on attendance rates were presented for 6 and 12 months' surveillance. In TOMBOLA 2009 (the Trial Of Management of Borderline and Other Low grade Abnormal smears) women were followed up at 6‐month intervals for 36 months in the cytological surveillance arm. The follow‐up, as well as the immediate colposcopy, were done within a routine nationwide healthcare practice. Moderate dyskaryosis or worse, or three consecutive inadequate results led to referral to colposcopy, whereas women who reverted to normal were discharged to "routine 3‐yearly recall" after three consecutive normal smears. Women allocated to immediate colposcopy were further randomised to either immediate treatment (LLETZ) or directed biopsies with selective recall for treatment, if the colposcopy was adequate and showed abnormalities (no additional procedures were carried out if the colposcopy was normal). Women from both arms were recalled for exit examination at 36 months that included colposcopy and LLETZ if the transformation zone was abnormal.
In the ALTS 2003 (ASCUS‐LSIL Triage Study) trial, all women after randomisation had a repeat liquid‐based cytology sample (LBC) for cytology and HPV testing at enrolment. Women in the cytological surveillance arm were followed up for 24 months at 6‐month intervals with pelvic examination, cervical smear, HPV‐test and cervicography taken at each visit. If the cytology suggested HSIL or worse at enrolment or during surveillance, women were referred to colposcopy with treatment if CIN2 or worse was detected on a biopsy. The immediate‐colposcopy‐arm women underwent a colposcopic examination on the same day or within 3 weeks from recruitment and received treatment if CIN2 or 3 was detected at biopsy. Women from both arms were recalled for exit colposcopy at 24 months; treatment was performed if CIN2 or 3 was detected on biopsy and for women with CIN1 with history of LSIL or HPV+ ASCUS during at least one of the previous two visits.
Excluded studies
Studies excluded during full‐text review are described in the Characteristics of excluded studies table. The reasons for exclusion were non‐randomised design in one (De Bie 2011) and in one study the exposure of interest was low‐grade histological changes (CIN1 to 2) instead of cytological changes (Elit 2011).
Risk of bias in included studies
Generally we considered the risk of selection and reporting biases across included trials to be low. The risk of performance bias and other possible sources of bias we considered high in all included trials. Risk of detection bias (TOMBOLA 2009), as well as attrition bias (ALTS 2003) were each considered low in only one trial. We have presented the quality assessment of the included RCTs with the Cochrane tool in Risk of bias in included studies and in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We considered the random sequence generation appropriate in all studies and the allocation concealment in all but one study (Flannelly 1994), which did not describe the allocation concealment process in full.
Blinding
Blinding of participants and personnel was not possible given the nature of the intervention. There was no blinding of the outcome in two studies (Kitchener 2004; Shafi 1997), while the risk of detection bias was low in TOMBOLA 2009 and unclear in the remaining studies (ALTS 2003; Flannelly 1994). Kitchener 2004 and Shafi 1997 stated that the outcome assessors were not blinded. Flannelly 1994 described no details of the blinding. ALTS 2003 reported that all clinical information were unmasked during the exit examination, but included no description of the blinding of the assessors during the follow‐up period. TOMBOLA 2009 described that the colposcopists performing the exit examination were blinded to the initial cytology results, the randomisation arm and the clinical events that occurred after randomisation, although it is unclear whether the personnel were blinded during surveillance.
Incomplete outcome data
Loss to follow‐up rates were high overall. The highest rates were seen in Kitchener 2004 and TOMBOLA 2009 (23% and 42% respectively), while the impact was judged as unclear in another two (Flannelly 1994; Shafi 1997). ALTS 2003 had the lowest rates of losses to follow‐up (14% to 17%).
Selective reporting
We considered the risk of selective reporting low in all trials, although only TOMBOLA 2009 and ALTS 2003 had pre published protocols.
Other potential sources of bias
Other potential sources of bias were considered to be high in all trials. One trial used Zelen randomisation, where participants themselves in one randomisation group (choice‐group) chose whether they preferred an immediate colposcopy or cytological surveillance, which might have created bias towards either direction in terms of later incidence of CIN2+ or CIN3+ lesions (Kitchener 2004). All other trials included either a deferred treatment (Shafi 1997), a colposcopy in addition to cytological surveillance on all follow‐up visits (Flannelly 1994) or had an exit examination with colposcopy regardless of the results of the cytological surveillance at the end of trial (ALTS 2003; TOMBOLA 2009). This could well have introduced detection bias and over inflated the CIN detection rate in the cytological surveillance arms. We further accounted for this possible source of bias by presenting and meta‐analysing the results without the exit‐examination when possible (18‐month follow‐up window).
Effects of interventions
See: Summary of findings 1 Summary of findings: occurrence of CIN and default rates
Primary outcomes
Occurrence of CIN2+
In the majority of the included studies that presented data on more than one time point (ALTS 2003; Flannelly 1994; Shafi 1997; TOMBOLA 2009), the incidence of CIN2+ was higher for the immediate colposcopy group at the first time point (before the exit examination at the time of completion), but showed no difference with time (at the time of the trial’s completion) (Table 1). Flannelly 1994 revealed differences between the intervention groups for all time points assessed.
The meta‐analysis at 18 months (without the exit examination) revealed a difference in the detection of CIN2+ between the two groups (14.3% versus 10.0%, RR 1.50, CI 1.12 to 2.01) with some heterogeneity (P = 0.08, I2 = 61%), (moderate‐quality evidence). There was no difference at 24 months (17.9% versus 18.3%, RR 1.14, 95% CI 0.66 to 1.97), but there was high inter‐study heterogeneity (P < 0.00001, I2 = 94%) and presence of other possible bias, resulting in a falsely high CIN detection rate in the cytological surveillance arm, and we considered this to be low‐quality evidence (Analysis 1.1; Figure 3; summary of findings Table 1).

Forest plot of comparison: occurrence of CIN2+ at different lengths of follow‐up
Occurrence of CIN3+
All the studies assessed the detection rate of CIN3 or worse (CIN3+), although the time point assessed varied. The studies showed conflicting results, as some studies showed an increase in the CIN3+ detection with immediate colposcopy (Flannelly 1994; Kitchener 2004), one found improved detection at 18 months (without the exit examination) but not at 24 months (Shafi 1997), TOMBOLA 2009 found no difference, while ALTS 2003 found no difference at 18 months (without the exit examination), and a higher CIN3+ detection rate for cytological surveillance at 24 months (Table 1).
The cumulative incidence of CIN3+ was increased after immediate colposcopy when compared to cytological surveillance at 12 months (32% versus 14.0%, RR 2.07, 95% CI 1.54 to 2.79, P = 0.41, I2 = 0%), but due to other possible bias resulted in a falsely high CIN detection rate in the cytological surveillance arm, although at 18 months (without the exit examination) (7.8% versus 6.9%, (moderate‐quality evidence), 95% CI 0.77 to 1.98) and 24 months (10.3% versus 11.9%, RR 1.02, CI 0.53 to 1.97), this difference was no longer observed. This was mainly driven by the opposing results of a large trial (ALTS 2003). There was high inter‐study heterogeneity for the comparison at 18 months (P < 0.02, I2 = 75%) (moderate‐quality evidence), and at 24 months (P < 0.00001, I2 = 93%). In addition to high heterogeneity the presence of other possible bias probably resulting in a falsely high CIN detection rate in the cytological surveillance arm, meant that we regarded this as low‐quality evidence (Analysis 2.1; Figure 4; summary of findings Table 1).

Forest plot of comparison: occurence of CIN3+ at different lengths of follow‐up
Secondary outcomes
Incidence of other CIN grades at histology
Occurrence of histologically proven HPV or koilocytic atypia
One study assessed this at 6, 12 and 24 months (Flannelly 1994) and another at 24 months of surveillance (Shafi 1997). Flannelly 1994 reported a lower rate of HPV‐associated histological changes for immediate colposcopy as compared to six‐month cytological surveillance (9% versus 17%, RR 0.52, 95% CI 0.28 to 0.95) but no difference was observed at 12 or 24 months. Interestingly, Shafi 1997 identified a higher rate for women attending for immediate colposcopy, as opposed to repeat cytology (50.5% versus 33.3%, RR 1.52, 95% CI 1.17 to 1.96) (Table 1).
The meta‐analysis revealed that immediate referral to colposcopy increased the detection of clinically non‐significant infections as opposed to repeat cytology at 24 months (32% versus 21%, RR 1.49, 95% CI 1.17 to 1.90) (Analysis 4.1). There was no evident inter‐study heterogeneity (P = 0.69, I2 = 0%), but due to other possible bias resulting in a falsely high CIN detection rate in the cytological surveillance arm, we regarded this as moderate‐quality evidence.
Occurrence of any CIN
Three studies provided data on the rates of any CIN grade (Flannelly 1994; Kitchener 2004; Shafi 1997). Flannelly 1994 provided data for cytological surveillance at 6, 12 and 24 months, Kitchener 2004 at 12 months and Shafi 1997 at 24 months. All individual studies demonstrated that women undergoing immediate colposcopy had a higher rate of detected CIN of any grade (Table 1).
The meta‐analysis confirmed that the cumulative incidence of any grade of CIN was higher among women referred to immediate colposcopy (12 months: RR 1.72, 95% CI 1.09 to 2.70; 24 months: RR 2.02, 95% CI 1.33 to 3.08). Considerable heterogeneity was present at both time points (P = 0.001, I2 = 91% and P = 0.02, I2 = 82%, respectively) and due to other possible bias resulting in falsely high CIN detection rates in the cytological surveillance arm, we regarded this as low‐quality evidence (Analysis 3.1; Analysis 4.1; summary of findings Table 1).
Occurrence of CIN1
One study that assessed the rate of CIN1 at six and 12 months suggested no difference between immediate colposcopy and cytological surveillance (Flannelly 1994) (Table 1). Two studies assessed the occurrence of CIN1 rate at 24 months (Flannelly 1994; Shafi 1997), both of which suggested a higher rate of detection of low‐grade disease in the immediate colposcopy group (Table 1).
The meta‐analysis revealed at least double the rate of CIN1 detection when women underwent colposcopy immediately as opposed to repeat cytology (21% versus 8%, RR 2.58, 95% CI 1.69 to 3.94), suggesting that immediate colposcopy may have increased the detection of clinically insignificant lesions (Analysis 4.1). There was no evident inter‐study heterogeneity (P = 0.81, I2 = 0%), but due to other possible bias resulting in a falsely high CIN detection rate in the cytological surveillance arm we regarded this as moderate‐quality evidence.
Occurrence of CIN2
Four studies assessed the incidence of CIN2 (ALTS 2003; Flannelly 1994; Shafi 1997; TOMBOLA 2009); one reported results at 6 and 12 months (Flannelly 1994), two (ALTS 2003; Shafi 1997) at 18 months (without the exit examination), three (ALTS 2003; Flannelly 1994; Shafi 1997) at 24 months and one (TOMBOLA 2009) at 30 and 36 months. The rate of CIN2 was higher in the immediate colposcopy group in the majority of the studies, apart from one (Shafi 1997). The difference in CIN2 rate detection was higher at 18 months rather than at 24 months in ALTS 2003 and similarly at 30 months as opposed to 36 months in TOMBOLA 2009 (Table 1).
The overall rate of CIN2 in the meta‐analysis was higher for the immediate colposcopy group at 18 months (6.5% versus 3.2%, RR 2.04, CI 1.52 to 2.73) with low heterogeneity (P = 0.46, I2= 0%); high‐quality evidence (Analysis 5.1; Figure 5). There was no evident difference at 24 months of surveillance (7.6% versus 5.4%, RR 1.45, 95% CI 0.87 to 2.40); there was considerable inter‐study heterogeneity (P = 0.009, I2= 74%), and additionally, due to the presence of other possible bias resulting in a falsely high CIN detection rate in the cytological surveillance arm, this was regarded as low‐quality evidence.

Forest plot of comparison: occurence of CIN2 at different lengths of follow‐up
In total there were 18 cases of invasive cervical cancer, seven in immediate colposcopy and 11 in cytological surveillance groups: one case of stage 1A1 cervical cancer in the deferred treatment group detected by cytological smear in Shafi 1997, three cases of invasive cervical cancer in both immediate colposcopy and cytological surveillance groups in ALTS 2003 and four cases of invasive disease in immediate colposcopy and seven in the cytological surveillance group in TOMBOLA 2009. There were no cases of invasive cancer in the two other included trials (Flannelly 1994; Kitchener 2004).
Default rates
All five included trials reported on the non‐compliance rates for immediate colposcopy versus cytological surveillance (Table 1). Two reported non‐attendance rates at six and 12 months (Kitchener 2004; TOMBOLA 2009), two at 24 months (ALTS 2003; Shafi 1997) and one for all the time points (6, 12 and 24 months) (Flannelly 1994). The risk of non‐compliance was significantly greater for the repeat cytology arm and increased with the length of the follow‐up. TOMBOLA 2009, the largest study and one with high methodological quality, reported an almost double risk of default for women followed up in the community when compared to those referred to immediate colposcopy (6 months: RR 1.88, 95% CI 1.56 to 2.27; 12 months: RR 2.16, 95% CI 1.80 to 2.59) (Table 1). The magnitude of effect was much greater in the remaining trials. In ALTS 2003 the RR for cumulative risk of default at 24 months was up to 27.42 (95% CI 10.17 to 73.93) for the surveillance arm at the exit exam, as opposed to the immediate colposcopy group (Table 1). The study setting and protocols varied greatly between the included trials, which could well explain the observed differences in default rates between studies.
The meta‐analysis suggested that the risk for non‐compliance was higher for the repeat cytology group with a four‐fold increase at 6 months, a six‐fold increase at 12 months and a 19‐fold increase at 24 months (6 months: RR 3.85, 95% CI 1.27 to 11.63; 12 months: RR 6.60, 95% CI 1.49 to 29.29; 24 months: RR 19.1 95% CI 9.02 to 40.43). The inter‐study heterogeneity was considerable for the analysis at six and 12 months (P = 0.02, I2 = 76% and P < 0.0004, I2 = 87%, respectively), and we therefore regarded this as moderate‐quality evidence, whereas we considered the evidence to be of high quality at 24 months (Analysis 6.1; Figure 6; summary of findings Table 1).

Forest plot of comparison: default rates at different lengths of follow‐up
Presence of after‐effects
TOMBOLA 2009 was the only study that reported on the incidence of after‐effects based on a questionnaire completed six weeks after immediate colposcopy or the first cytological follow‐up smear. Pain, bleeding, or vaginal discharge were all more common after immediate colposcopy than after cytology (Table 1).
Presence of anxiety, distress or depression
Both TOMBOLA 2009 and Kitchener 2004 reported incidence of psychosocial morbidity in the randomisation groups (Table 1). In TOMBOLA 2009, the authors reported the incidence of anxiety and depression at six weeks, and thereafter at six‐month intervals until 30 months of surveillance. Anxiety, measured as score of 11 or more on the Hospital Anxiety and Depression anxiety sub scale, was less common in the immediate colposcopy group compared to the cytological surveillance group at six weeks (RR 0.58, 95% CI 0.43 to 0.79). There was no difference between the randomisation groups thereafter. Incidence of depression, defined as a score of 8 or more on the Hospital Anxiety and Depression Scale (HADS) (Zigmond 1983), did not differ between the two groups throughout the follow‐up period. Kitchener 2004 used the General Health Questionnaire (GHQ)‐caseness (Bridges 1986) (defined as a score of 4 or above) to measure the presence of anxiety or distress. There were no differences between choice and no‐choice arms at baseline or after six and 12 months of surveillance. Given the differences in the comparison groups and the assessment methods, a meta‐analysis was not possible.
Subgroup analyses
The optimum management option may be different for women found to have ASCUS (borderline) or LSIL cytology (mild dyskaryosis) in the baseline smear. We were unable to perform a subgroup analysis for women with ASCUS cytology as only one study (ALTS 2003) presented this separately. We could extract separate data for women referred with LSIL cytology only from two studies (ALTS 2003; Flannelly 1994). These studies reported on the incidence of CIN2, CIN2+ and CIN3+ or worse at 24 months.
The incidence of the above histological outcomes was higher for immediate colposcopy as opposed to cytology, although based on small sample size (CIN2: 8.4% versus 11.6%, RR 1.72, 95% CI 0.66 to 4.48; CIN2+: 23.4% versus 27.5%, RR 1.43, 95% CI 0.51 to 4.01: CIN3+: 15% versus 15.9%, RR 1.24, 95% CI 0.39 to 3.94); all analyses demonstrated inter‐study heterogeneity (P < 0.01, I2 > 75%) and presented moderate‐quality evidence (Analysis 7.1).
ALTS 2003 revealed opposing direction of effect to the other included studies in some of the outcomes assessed. We performed a subgroup analysis that excluded this trial after 24 months of surveillance and found little or no difference in the rate of CIN2 and CIN2+ between the immediate colposcopy and cytological surveillance groups: CIN2 (RR 1.54, 95% CI 0.41 to 5.78, P = 0.02, I2= 83%) and for CIN2+ (RR 1.72, 95% CI 0.86 to 3.47, P = 0.004, I2 = 88%; moderate‐quality evidence). Immediate colposcopy increased the detection of CIN3+ as opposed to cytological surveillance (30.9% versus 17%, RR 1.80, 95% CI 1.11 to 2.92, heterogeneity: P = 0.10, I2= 62%; high‐quality evidence) (Analysis 8.1; Analysis 8.2; Analysis 8.3).
Discussion
Summary of main results
The results of this meta‐analysis suggest that the difference in the CIN2 or CIN2+ rate detection was higher for the immediate colposcopy group at 18 months (without the exit examination), but this was no longer the case at 24 months. Although this suggests that colposcopy may allow earlier diagnosis of CIN2 lesions that cytology would require longer to detect, it may also be explained by the trials’ design. All participants in the cytological surveillance arms underwent exit examination, a colposcopy with or without treatment, irrespective of their cytological status in all trials. This ‘artificial’ detection of cases of high‐grade disease at completion would plausibly be lower in a ‘real‐life’ setting in the cytological surveillance arm; this may over inflate the reliability of repeat cytology to detect high‐grade disease.
Although the evidence suggests that there may be little or no difference in the rate of CIN3+ detection at 24 months between the two arms, this result was mainly driven by a large RCT (ALTS 2003) with almost opposing results to the remaining RCTs (Flannelly 1994; Kitchener 2004; Shafi 1997; TOMBOLA 2009). The ALTS study had a different design in that all women randomised and analysed in the cytological surveillance arm had a repeat smear that was assessed by an expert cytopathologist at recruitment. Almost 40% of CIN3+ lesions in the cytology arm were detected during this repeat cytology smear and this may have inflated the ability of cytology to detect disease when compared to immediate colposcopy (ALTS 2003). After exclusion of this trial, the difference of CIN3+ detection favoured immediate colposcopy.
Women attending for immediate colposcopy after a single LSIL or ASCUS smear were 50% more likely to have clinically insignificant HPV infections detected. The rates of histologically detected lesions associated with HPV infection were lower for the repeat cytology group, possibly explained by spontaneous regression of clinically insignificant lesions during cytological surveillance. Similarly, there was an increase in the detection of CIN1 lesions, supporting the perception that immediate colposcopy may increase the risk of over‐intervention and over‐treatment, by detection of insignificant lesions that would otherwise spontaneously revert to normal over time.
The evidence suggests that the risk for default to follow‐up was probably higher for the repeat cytology group at 6, 12 and 24 months. The difference between the two groups changed from four‐fold at 6 months and six‐fold at 12 months up to 19‐fold at 24 months. The point estimate at 24 months could well be biased and over‐inflated due to selection of studies at that follow‐up window, that is, lack of data from one study (TOMBOLA 2009) and inclusion of another with a very different trial setting (ALTS 2003). Compliance with follow‐up still appeared to decline with time and women may be at increased risk of invasion due to underlying occult high‐grade disease. The inter‐study heterogeneity was significant, possibly due to differences in the magnitude of the default rates in the immediate colposcopy group between different trials. These differences may be explained by differences in the design and randomisation process that could increase (TOMBOLA 2009: randomisation and admittance to either colposcopy or cytological surveillance after HPV test results) or decrease (ALTS 2003: immediate colposcopy preferably on the same day) the risk of non‐compliance in the immediate colposcopy arm. Only one study reported on the after‐effects ‐ pain, bleeding or vaginal discharge ‐ and found that these were significantly more common after immediate colposcopy (TOMBOLA 2009). Meta‐analysis of the after‐effects and anxiety and depression was not possible.
Overall completeness and applicability of evidence
This is the first meta‐analysis that comprehensively investigates the value of immediate colposcopy versus cytological surveillance in women with lesions of equivocal significance in the absence of HPV test. A previous systematic review only included three small RCTs (Kyrgiou 2007a). Two large trials, one from the US (ALTS 2003) and the other from the UK (TOMBOLA 2009) addressed this comparison comprehensively with large sample sizes. Because all included studies were conducted in high‐income setting, the applicability of the evidence to lower income settings is not clear. This systematic review still provides a comprehensive review of the existing literature and meta‐analytical pooling that will allow clinicians to evaluate the trade‐off between the risk of detection of insignificant lesions versus the risk of poor compliance and non‐detection of occult high‐grade disease.
Quality of the evidence
All of the included studies were RCTs, however, our analysis has several limitations and the results should be interpreted with caution. There are only a small number of included studies, two of which included a comparatively large number of participants (ALTS 2003; TOMBOLA 2009), which may account for some of the heterogeneity that was observed and which was considerable or substantial in many of the comparisons. It was this high heterogeneity, as well as the presence of bias from study design possibly resulting in falsely high CIN detection rate in the cytological surveillance arm in the 24‐month follow‐up window, that accounted for the down‐grading of the evidence to 'moderate' or 'low' in several cases following the GRADE assessment. Several of the included studies varied in their design and comparison groups, with the presence of exit examination and the length of follow‐up. Kitchener 2004, for example, had a choice and non‐choice arm, ALTS 2003 performed a repeat cytology at recruitment in the cytology surveillance arm, while one of the older studies (Shafi 1997) performed a treatment for all participants at completion. For many of the explored outcomes and comparisons, there was substantial heterogeneity, possibly explained by the difference in the studies’ design.
The results presented in the individual trials are those derived from women who completed the given period of surveillance as the presence or absence of disease cannot be verified in those women who defaulted from surveillance. Furthermore, all the included studies, apart from Kitchener 2004, performed exit examinations at the time of the study completion for both arms, some of which included an excision of the transformation zone for all subjects (Shafi 1997). The comparison of the rates of different grades of disease at the time of the study completion may represent an ‘artificial’ detection of lesions that would not have been detected in the cytology arm in a ‘real‐life’ setting, with the risk to inflate the ability of repeat cytology to detect disease. We therefore considered the quality of evidence in the 24‐month follow‐up window to be low.
Most of the included studies did not assess the impact of age of the population in the preferred management approach. Only TOMBOLA 2009 assessed the effect of age on histological outcomes and concluded that the difference in cumulative incidence of CIN2+ between the trial arms was more pronounced amongst younger women (20 to 39 years), as opposed to older women (40 to 59 years). Furthermore, in younger women (age 20 to 39 years) the risk ratios for CIN2 or worse were markedly higher than CIN3 or worse.
Potential biases in the review process
All of the included studies were RCTs. It was not possible to blind participants and personnel given the nature of the intervention. The random sequence generation was considered appropriate in all studies and the allocation concealment in all but one study (Flannelly 1994), where the allocation concealment process was not described in full. Only one study (TOMBOLA 2009) described blinding of assessors; the colposcopist was blinded at exit examination only with regards to the women's cytology status, randomisation category and any clinical outcomes. The lack of assessor blinding in the other included studies (ALTS 2003; Flannelly 1994; Kitchener 2004; Shafi 1997) may have introduced a degree of detection bias. Loss to follow‐up rates were high overall ranging from 14% to 42%, which may have introduced a degree of attrition bias. The risk of selective reporting was considered low in all trials.
In order to minimise bias whilst undertaking the review, two review authors (MK and IK) independently reviewed the retrieved citations and the extracted data. There was no discrepancy in the included studies; some minor discrepancies in the data extraction were resolved with discussion and the involvement of a third reviewer (MA) if necessary.
Agreements and disagreements with other studies or reviews
Almost one in ten women screened in the UK will have a minor lesion detected in her smear. Several authorities recommend conservative surveillance in the primary care setting, as spontaneous regression of most of these lesions will occur (Kyrgiou 2007a). Referral to colposcopy may increase psychological morbidity for these women who experience similar levels of anxiety to those referred with high‐grade disease (TOMBOLA 2009). This increase in anxiety appears to be transient and is no different after six weeks. Furthermore, further investigation of lesions that were deemed to regress without intervention increases the risk of over‐intervention and over‐treatment with subsequent possible adverse reproductive sequale (Arbyn 2008; Founta 2010; Kyrgiou 2006;Kyrgiou 2012a; Kyrgiou 2014;Kyrgiou 2015; Kyrgiou 2016a). The opponents supporting immediate assessment with colposcopy emphasise that protracted attendance for repeat cytology decreases with time (Soutter 2012) and this was also confirmed in this analysis. The evidence suggests that approximately one third of women referred with borderline or low‐grade cytological abnormalities have an occult high‐grade disease and non‐compliance with surveillance may put some women at risk of developing invasive disease (Bolger 1988; Contreras‐Melendez 1992; Giles 1989; Paraskevaidis 2002; Soutter 1986; Walker 1986). The participants lost to follow‐up due to poor compliance in these trials could result in an underestimation of the true number of high‐grade lesions that were missed in the cytology group.
The financial implications of the two approaches to health services should also be considered. Cost‐effectiveness analysis in some of the earlier studies suggested that referral to colposcopy is a more cost‐effective alternative to repeat cytology (Flannelly 1997; Johnson 1993). The cost‐effectiveness analysis of the TOMBOLA trial suggested no difference amongst the two approaches although cytological surveillance was less costly (TOMBOLA 2009). The ALTS trial suggested that HPV‐triage or repeat cytology of a single repeat smear was cost‐effective, although there was no difference in cost‐effectiveness if two or more smears were performed (ALTS 2003). The cost‐effectiveness of the two approaches largely depends on the policy of subsequent surveillance in the case of negative colposcopy; this was not addressed in any of the included studies.
Strong evidence supports the preferential use of HPV test particularly in the triage of women with ASCUS (borderline) cytology (Arbyn 2004; Arbyn 2012; Arbyn 2013a) that would benefit from colposcopy as a more accurate and cost‐effective modality. Its value is less pronounced in younger women below the age of 30 and women with LSIL (mild dyskaryosis) (Arbyn 2009; Arbyn 2013a). However, this test is not always readily available, particularly in low‐resource settings. In Europe alone, one in ten women with ASCUS cytology and four in ten women with LSIL will not have a triage HPV test and clear recommendations are required to manage such cases. Newer markers such as mRNA or p16/Ki67 immunostaining have more recently shown promising results claiming an equal sensitivity to that of the DNA test but with improved specificity in this setting (Arbyn 2013b; Nasioutziki 2011; Tsoumpou 2009; Tsoumpou 2011). Further combinations of novel biomarkers and Clinical Decision Support Scoring Systems (DSSS) based on mathematical modelling that will create user‐friendly tools have the potential to further improve the management of this population (Bountris 2014; Karakitsos 2011; Karakitsos 2012; Kyrgiou 2016b).
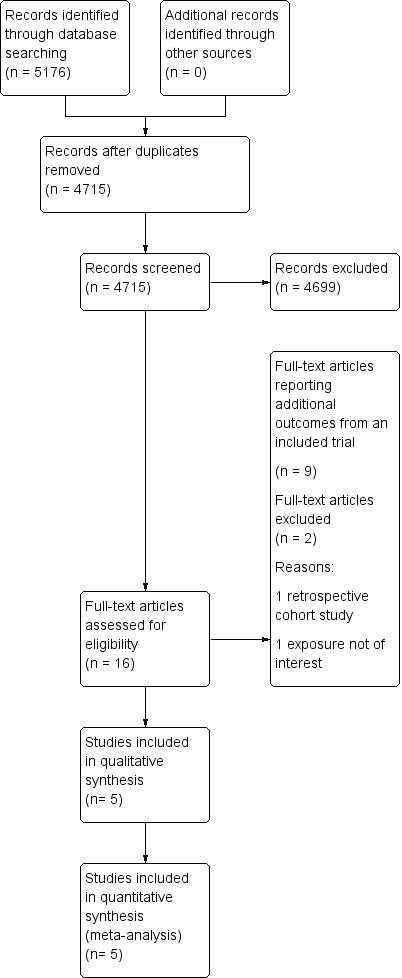
Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: occurrence of CIN2+ at different lengths of follow‐up

Forest plot of comparison: occurence of CIN3+ at different lengths of follow‐up

Forest plot of comparison: occurence of CIN2 at different lengths of follow‐up

Forest plot of comparison: default rates at different lengths of follow‐up

Comparison 1: Occurence of CIN2+ at different lengths of follow‐up: immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN2+

Comparison 2: Occurence of CIN3+ at different lengths of follow‐up: immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN3+

Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 1: Presence of any CIN in histology at 12 months

Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 2: Presence of CIN1/2 in histology at 12 months

Comparison 3: Histology at 12 months: immediate colposcopy versus cytological surveillance, Outcome 3: Presence of CIN3+ in histology at 12 months

Comparison 4: Histology at 24 months: immediate colposcopy versus cytological surveillance, Outcome 1: Histology at 24 months

Comparison 5: Occurence of CIN2 at different lengths of follow‐up: Immediate colposcopy versus cytological surveillance, Outcome 1: Occurrence of CIN2

Comparison 6: Default rates: immediate colposcopy versus cytological surveillance, Outcome 1: Default rates

Comparison 7: Histology at 24 months: immediate colposcopy versus cytological surveillance, LSIL/mild dyskaryosis only, Outcome 1: CIN incidence at 24 months, after LSIL/mild dyskaryosis at baseline

Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 1: Presence of CIN2 in histology at 24 months

Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 2: Presence of CIN2+ in histology at 24 months

Comparison 8: Histology at 24 months: immediate colposcopy versus cytological surveillance, excluding 1 trial, Outcome 3: Presence of CIN3+ in histology at 24 months
| Immediate colposcopy compared with cytological surveillance for minor cervical cytological abnormalities: occurrence of different grades CIN in histology according to follow‐up time and default rates | ||||||
| Patient or population: women with ASCUS or LSIL Settings: colposcopy clinic Intervention: immediate colposcopy Comparison: cytological surveillance | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Risk with cytological surveillance | Risk with immediate colposcopy | |||||
| Occurrence of CIN2+ in histology at 18 months | 101 per 1000 | 151 per 1000 | RR 1.50 (1.12 to 2.01) | 4028 | ⊕⊕⊕⊝ | |
| Occurrence of CIN2+ in histology at 24 months | 183 per 1000 | 209 per 1000 | RR 1.14 (0.66 to 1.97) | 4331 | ⊕⊕⊝⊝ | |
| Occurrence of CIN3+ in histology at 18 months | 69 per 1000 | 86 per 1000 | RR 1.24 (0.77 to 1.98) | 4028 | ⊕⊕⊕⊝ | |
| Occurrence of CIN3+ in histology at 24 months | 119 per 1000 | 121 per 1000 | RR 1.02 (0.53 to 1.97) | 4331 | ⊕⊕⊝⊝ | |
| Occurrence of any CIN in histology at 24 months | 316 per 1000 | 639 per 1000 | RR 2.02 (1.33 to 3.08) | 656 | ⊕⊕⊝⊝ | |
| Default rates at 6 months | 63 per 1000 | 241 per 1000 | RR 3.85 | 5117 | ⊕⊕⊕⊝ moderate6 | |
| Default rates at 12 months | 63 per 1000 | 413 per 1000 | RR 6.60 | 5115 | ⊕⊕⊕⊝ moderate7 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For default rates the relative effect is calculated between cytological surveillance versus immediate colposcopy. For histology the relative effect is calculated between immediate colposcopy versus cytological surveillance. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded to moderate due to substantial inter‐study heterogeneity (P = 0.08, I2= 61%). | ||||||
| Study | Outcomes | Immediate colposcopy n/N (%) | Cytological surveillance n/N (%) | RR + 95% CI |
| ALTS 2003(ASCUS) | Histology at 18 monthsa | |||
| CIN 2 | 61/1163 (5.2) | 26/1164 (2.2) | 2.35 [1.49, 3.69] | |
| CIN 2+ | 119/1163 (10.2) | 92/1164 (7.9) | 1.29 [1.00, 1.68] | |
| CIN 3+ | 58/1163 (5.0) | 66/1164 (5.7) | 0.88 [0.62, 1.24] | |
| Histology at 24 months | ||||
| CIN 2 | 61/1163 (5.2) | 60/1164 (5.2) | 1.02 [0.72,1.44] | |
| CIN 2+ | 119/1163 (10.2) | 168/1164 (14.4) | 0.71 [0.57, 0.88] | |
| CIN 3+ | 58/1163 (5.0) | 108/1164 (9.3) | 0.54 [0.39, 0.73] | |
| Default rates: | ||||
| Default rate at 24 months | 15/1163 (1.3) | 165/1164 (14.2) | 10.99 [6.52, 18.53] | |
| ALTS 2003(LSIL) | Histology at 18 monthsa | |||
| CIN 2 | 63/673 (9.4) | 36/675 (5.3) | 1.76 [1.18, 2.61] | |
| CIN 2+ | 127/673 (18.9) | 95/675 (14.1) | 1.34 [1.05, 1.71] | |
| CIN 3+ | 64/673 (9.5) | 59/675 (8.7) | 1.09 [0.78, 1.52] | |
| Histology at 24 months | ||||
| CIN 2 | 63/673 (9.4) | 58/675 (8.6) | 1.09 [0.78,1.53] | |
| CIN 2+ | 127/673 (18.9) | 151/675 (22.4) | 0.84 [0.68, 1.04] | |
| CIN 3+ | 64/673 (9.5) | 93/675 (13.8) | 0.69 [0.51, 0.93] | |
| Default rates: | ||||
| Default rate at 24 months | 4/673 (0.6) | 110/675 (16.3) | 27.42 [10.17, 73.93] | |
| Histology at 6 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 28/160 (17.5) | 0.52 [0.28, 0.95] | |
| Any CIN | 121/145 (83.4) | 86/160 (53.8) | 1.55 [1.32, 1.82] | |
| CIN 1 | 23/145 (15.9) | 27/160 (16.9) | 0.94 [0.57, 1.56] | |
| CIN 2 | 32/145 (22.1) | 26/160 (16.3) | 1.36 [0.85, 2.16] | |
| CIN 2+ | 98/145 (67.6) | 59/160 (36.9) | 1.83 [1.45, 2.31] | |
| CIN 3+ | 66/145 (45.5) | 33/160 (20.6) | 2.21 [1.55, 3.14] | |
| Histology at 12 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 18/158 (11.4) | 0.79 [0.40, 1.55] | |
| Any CIN | 121/145 (83.4) | 96/158 (60.8) | 1.37 [1.19, 1.59] | |
| CIN 1 | 23/145 (15.9) | 25/158 (15.8) | 1.00 [0.60, 1.69] | |
| CIN 2 | 32/145 (22.1) | 26/158 (16.5) | 1.34 [0.84, 2.14] | |
| CIN 1 / 2 | 55/145 (37.9) | 51/158 (32.3) | 1.18 [0.86, 1.60] | |
| CIN 2+ | 98/145 (67.6) | 71/158 (44.9) | 1.50 [1.22, 1.85] | |
| CIN 3+ | 66/145 (45.5) | 45/158 (28.5) | 1.60 [1.18, 2.17] | |
| Histology at 24 months | ||||
| HPV / Koilocytic atypia | 13/145 (9.0) | 11/158 (7.0) | 1.29 [0.60, 2.78] | |
| Any CIN | 121/145 (83.4) | 53/158 (33.5) | 2.49 [1.97, 3.13] | |
| CIN 1 | 23/145 (15.9) | 9/158 (5.7) | 2.78 [1.33, 5.82] | |
| CIN 2 | 32/145 (22.1) | 12/158 (7.6) | 2.91 [1.56, 5.42] | |
| CIN 2+ | 98/145 (67.6) | 44/158 (27.8) | 2.43 [1.84, 3.20] | |
| CIN 3+ | 66/145 (45.5) | 32/158 (20.3) | 2.25 [1.57, 3.21] | |
| Default rates: | ||||
| Default rate at 6 months | 0/145 (0) | 19/160 (11.9) | 35.37 [2.15, 580.52] | |
| Default rate at 12 months | 0/145 (0) | 23/158 (14.6) | 43.16 [2.65, 704.13] | |
| Default rate at 24 months | 0/145 (0) | 38/158 (24.1) | 70.70 [4.38, 1140.47] | |
| Histology at 12 months | ||||
| Any CIN | 83/130 (63.8) | 71/243 (29.2) | 2.19 [1.73, 2.76] | |
| CIN 1 / 2 | 61/130 (46.9) | 47/243 (19.3) | 2.43 [1.77, 3.32] | |
| CIN 3+ | 22/130 (16.9) | 24/243 (9.9) | 1.71 [1.00, 2.93] | |
| Default rates: | ||||
| Default rate at 6 months | 5/130 (3.8) | 46/243 (18.9) | 4.92 [2.01, 12.08] | |
| Default rate at 12 months | 5/130 (3.8) | 95/243 (39.1) | 10.16 [4.24, 24.35] | |
| GHQ casenessb | Choicec | No choicec | ||
| Baseline | 134/233 (58) | 119/241 (49) | 1.16 [0.98, 1.38] | |
| 6 months (pre visit) | 71/183 (39) | 77/190 (41) | 0.96 [0.75, 1.23] | |
| 6 months (post visit) | 59/175 (34) | 66/177 (37) | 0.90 [0.68, 1.20] | |
| 12 months | 40/135 (29) | 35/127 (28) | 1.08 [0.73, 1.58] | |
| Histology at 18 monthsa | ||||
| CIN 2 | 8/182(4.4) | 2/171 (1.1) | 3.76 [0.81, 17.45] | |
| CIN 2+ | 43/182 (23.6) | 16/171 (9.4) | 2.53 [1.48, 4.31] | |
| CIN 3+ | 35/182 (19.2) | 14/171(8.2) | 2.35 [1.31, 2.45] | |
| Histology at 24 months | ||||
| HPV / Koilocytic atypia | 92/182 (50.5) | 57/171 (33.3) | 1.52 [1.17, 1.96] | |
| Any CIN | 88/182 (48.4) | 51/171 (29.8) | 1.62 [1.23, 2.13] | |
| CIN 1 | 45/182 (24.7) | 17/171 (9.9) | 2.49 [1.48, 4.17] | |
| CIN 2 | 8/182 (4.4) | 10/171 (5.8) | 0.75 [0.30, 1.86] | |
| CIN 2+ | 43/182 (23.6) | 34/171 (19.9) | 1.19 [0.80, 1.77] | |
| CIN 3+ | 35/182 (19.2) | 24/171 (14.0) | 1.37 [0.85, 2.20] | |
| Default rates: | ||||
| Default rate at 24 months | 1/182 (0.5) | 36/171 (21.1) | 38.32 [5.31, 276.40] | |
| Tombola 2009 | Histology at 30 monthsa | |||
| CIN 2 | 181/2216 (8.2) | 101/2223 (4.5) | 1.80 [1.42, 2.28] | |
| CIN 2+ | 369/2216 (16.7) | 269/2223 (12.1) | 1.38 [1.19, 1.59] | |
| CIN 3+ | 188/2216 (8.5) | 168/2223 (7.6) | 1.12 [0.92, 1.37] | |
| Histology at 36 months | ||||
| CIN 2 | 181/2216 (8.2) | 157/2223 (7.1) | 1.16 [0.94, 1.42] | |
| CIN 2+ | 369/2216 (16.7) | 350/2223 (15.7) | 1.06 [0.93, 1.21] | |
| CIN 3+ | 188/2216 (8.5) | 193/2223 (8.7) | 0.98 [0.81, 1.18] | |
| Default rates: | ||||
| Default rate at 6 months | 151/2216 (6.8) | 285/2223 (12.8) | 1.88 [1.56, 2.27] | |
| Default rate at 12 months | 151/2216 (6.8) | 327/2223 (14.7) | 2.16 [1.80, 2.59] | |
| Paind | ||||
| Any pain | 304/782 (38.9) | 145/968 (15.0) | 2.60 [2.18, 3.09] | |
| Moderate or more severe | 144/774 (18.6) | 56/965 (5.8) | 3.21 [2.39, 4.30] | |
| Bleedingd | ||||
| Any bleeding | 366/781 (46.9) | 166/967 (17.2) | 2.73 [2.33, 3.19] | |
| Moderate or more severe | 144/772 (18.6) | 16/961 (1.7) | 11.20 [6.74, 18.61] | |
| Discharged | ||||
| Any discharge | 267/780 (34.2) | 83/964 (8.6) | 3.98 [3.17, 4.99] | |
| Moderate or more severe | 133/777 (17.1) | 36/962 (3.7) | 4.57 [3.20, 6.53] | |
| Anxietye | ||||
| 6 weeks | 59/751 (7.9) | 121/900 (13.4) | 0.58 [0.43, 0.79] | |
| 12 months | 190/1161 (16.4) | 218/1130 (19.3) | 0.85 [0.71, 1.01] | |
| 18 months | 162/1050 (15.4) | 177/1008 (17.6) | 0.88 [0.72, 1.07] | |
| 24 months | 179/1001 (17.9) | 177/962 (18.4) | 0.97 [0.81, 1.17] | |
| 30 months | 146/949 (15.4) | 143/887 (16.1) | 0.95 [0.77, 1.18] | |
| Depressionf | ||||
| 6 weeks | 50/757 (6.6) | 68/902 (7.5) | 0.88 [0.62, 1.25] | |
| 12 months | 110/1162 (9.5) | 132/1136 (11.6) | 0.81 [0.64, 1.04] | |
| 18 months | 106/1052 (10.1) | 114/1016 (11.2) | 0.90 [0.70, 1.15] | |
| 24 months | 111/1001 (11.1) | 104/964 (10.8) | 1.03 [0.80, 1.32] | |
| 30 months | 101/948 (10.7) | 108/887 (12.2) | 0.88 [0.68, 1.13] | |
| For Immediate colposcopy, n = n at immediate colposcopy visit, possible follow‐up excluded. a Cumulative incidence during follow‐up, excluding the exit examination or deferred treatment. b GHQ caseness = GHQ (General Health Questionnaire) score ≥ 4. c Analysis for this outcome between the original randomization groups. d Based on Questionnaire 6 weeks after immediate colposcopy or first cytological surveillance visit. e ≥ 11 on hospital anxiety and depression anxiety subscale f ≥ 8 on hospital anxiety and depression subscale | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Occurrence of CIN2+ Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 1.50 [1.12, 2.01] |
| 1.1.2 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Occurrence of CIN3+ Show forest plot | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 12 months' surveillance | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 2.07 [1.54, 2.79] |
| 2.1.2 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.77, 1.98] |
| 2.1.3 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Presence of any CIN in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.72 [1.09, 2.70] |
| 3.2 Presence of CIN1/2 in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.69 [0.83, 3.43] |
| 3.3 Presence of CIN3+ in histology at 12 months Show forest plot | 2 | 676 | Risk Ratio (IV, Random, 95% CI) | 1.63 [1.25, 2.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Histology at 24 months Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1.1 HPV/Koilocytic atypia | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.49 [1.17, 1.90] |
| 4.1.2 Any CIN | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 2.02 [1.33, 3.08] |
| 4.1.3 CIN1 | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 2.58 [1.69, 3.94] |
| 4.1.4 CIN2 | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.25 [0.80, 1.96] |
| 4.1.5 CIN2+ | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.14 [0.66, 1.97] |
| 4.1.6 CIN3+ | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.02 [0.53, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Occurrence of CIN2 Show forest plot | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1.1 18 months' surveillance | 2 | 4028 | Risk Ratio (IV, Random, 95% CI) | 2.04 [1.52, 2.73] |
| 5.1.2 24 months' surveillance | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 1.45 [0.87, 2.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Default rates Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 Default rates at 6 months | 3 | 5117 | Risk Ratio (IV, Random, 95% CI) | 3.85 [1.27, 11.63] |
| 6.1.2 Default rates at 12 months | 3 | 5115 | Risk Ratio (IV, Random, 95% CI) | 6.60 [1.49, 29.29] |
| 6.1.3 Default rates at 24 months | 3 | 4331 | Risk Ratio (IV, Random, 95% CI) | 19.10 [9.02, 40.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 CIN incidence at 24 months, after LSIL/mild dyskaryosis at baseline Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.1.1 CIN2 incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.66, 4.48] |
| 7.1.2 CIN2+ incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.43 [0.51, 4.01] |
| 7.1.3 CIN3+ incidence at 24 months | 2 | 1651 | Risk Ratio (IV, Random, 95% CI) | 1.24 [0.39, 3.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Presence of CIN2 in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.54 [0.41, 5.78] |
| 8.2 Presence of CIN2+ in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.72 [0.86, 3.47] |
| 8.3 Presence of CIN3+ in histology at 24 months Show forest plot | 2 | 656 | Risk Ratio (IV, Random, 95% CI) | 1.80 [1.11, 2.92] |

