استفاده از آنتیبیوتیکها برای سرفه یا خسخس سینه پایدار به دنبال ابتلا به برونشیولیت حاد در کودکان
Appendices
Appendix 1. Database search strategies
CENTRAL (the Cochrane Library)
#1 MeSH descriptor Bronchiolitis explode all trees
#2 MeSH descriptor Respiratory Syncytial Virus Infections explode all trees
#3 bronchioliti*:ti,ab,kw
#4 RSV*:ti,ab,kw
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Cough, this term only
#7 MeSH descriptor Respiratory Sounds explode all trees
#8 cough*:ti,ab,kw
#9 wheez*:ti,ab,kw
#10 post‐viral*:ti,ab,kw
#11 post‐acute*:ti,ab,kw
#12 Any MeSH descriptor with qualifier: CO
#13 MeSH descriptor Recurrence explode all trees
#14 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13)
#15 (#5 AND #14)
#16 post‐RSV*:ti,ab,kw
#17 post‐bronchiolit*:ti,ab,kw
#18 (#15 OR #16 OR #17)
#19 MeSH descriptor Anti‐Bacterial Agents explode all trees
#20 antibiotic*:ti,ab,kw
#21 MeSH descriptor Macrolides explode all trees
#22 (macrolide* or azithromycin or clarithromycin or erythromycin or roxithromycin or spiramycin):ti,ab,kw
#23 (penicillin or amoxicillin or amoxycillin or ampicillin or benzylpenicillin or cloxacillin or dicloxacillin or flucloxacillin or piperacillin or ticarcillin or sulbactam):ti,ab,kw
#24 (cephalosporin* or cephalexin or cephaclor or cefaclor or cefepime or cefotaxime or cephamycin* or cefotetan or cefoxitin or cefmetazole or cefpirome or cefpodoxime or ceftazidime or ceftriaxone or cephamandole or cephazolin):ti,ab,kw
#25 (fluoroquinolone* or ciprofloxacin or enoxacin or norfloxacin or ofloxacin or pefloxacin or fleroxacin or levofloxacin or moxifloxacin):ti,ab,kw
#26 (tetracycline* or doxycycline or methacycline or minocycline):ti,ab,kw
#27 (amikacin or gentamicin or neomycin or netilmicin):ti,ab,kw
#28 (clindamycin or lincomycin):ti,ab,kw
#29 (chloramphenicol or amantadine or cotrimoxazole or trimethoprim):ti,ab,kw
#30 (#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29)
#31 (#18 AND #30)
#32 MeSH descriptor Child explode all trees
#33 MeSH descriptor Pediatrics explode all trees
#34 MeSH descriptor Infant explode all trees
#35 MeSH descriptor Adolescent, this term only
#36 (paediatric* or paediatric* or child* or adolescen* or infant* or young* or preschool* or pre‐school* or newborn* or new‐born* or neonat* or neo‐nat*):ti,ab,kw
#37 (#32 OR #33 OR #34 OR #35 OR #36)
#38 (#31 AND #37)
MEDLINE (Ovid)
1. exp Bronchiolitis/
2. Respiratory Syncytial Virus Infections/
3. bronchioliti$.tw.
4. RSV$.tw.
5. or/1‐4
6. Cough/
7. Respiratory Sounds/
8. cough$.tw.
9. wheez$.tw.
10. post‐viral$.tw.
11. post‐acute$.tw.
12. co.fs.
13. Recurrence/
14. or/6‐13
15. 5 and 14
16. post‐RSV$.tw.
17. post‐bronchiolit$.tw.
18. 15 or 16 or 17
19. exp Anti‐Bacterial Agents/
20. antibiotic$.tw.
21. exp Macrolides/
22. (macrolide$ or azithromycin or clarithromycin or erythromycin or roxithromycin or spiramycin).tw.
23. (penicillin$ or amoxicillin or amoxycillin or ampicillin or benzylpenicillin or cloxacillin or dicloxacillin or flucloxacillin or piperacillin or ticarcillin or sulbactam).tw.
24. (cephalosporin$ or cephalexin or cephaclor or cefaclor or cefepime or cefotaxime or cephamycin$ or cefotetan or cefoxitin or cefmetazole or cefpirome or cefpodoxime or ceftazidime or ceftriaxone or cephamandole or cephazolin).tw.
25. (fluoroquinolone$ or ciprofloxacin or enoxacin or norfloxacin or ofloxacin or pefloxacin or fleroxacin or levofloxacin or moxifloxacin).tw.
26. (tetracycline$ or doxycycline or methacycline or minocycline).tw.
27. (amikacin or gentamicin or neomycin or netilmicin).tw.
28. (clindamycin or lincomycin).tw.
29. (chloramphenicol or amantadine or cotrimoxazole or trimethoprim).tw.
30. or/19‐29
31. 18 and 30
32. exp Child/
33. exp Pediatrics/
34. exp infant/
35. exp adolescent/
36. (paediatric$ or paediatric$ or child$ or adolescen$ or infant$ or young$ or preschool$ or pre‐school$ or newborn$ or new‐born$ or neonat$ or neo‐nat$).tw.
37. or/32‐36
38. 31 and 37
39. (clinical trial or controlled clinical trial or randomised controlled trial).pt.
40. (randomised or randomised).ab,ti.
41. placebo.ab,ti.
42. dt.fs.
43. randomly.ab,ti.
44. trial.ab,ti.
45. groups.ab,ti.
46. or/39‐45
47. Animals/
48. Humans/
49. 47 not (47 and 48)
50. 46 not 49
51. 38 and 50
Embase (Ovid)
1. exp bronchiolitis/
2. respiratory syncytial virus infection/
3. RSV$.tw.
4. bronchioliti$.tw.
5. or/1‐4
6. exp coughing/
7. wheezing/
8. cough$.tw.
9. wheez$.tw.
10. post‐viral$.tw.
11. post‐acute$.tw.
12. co.fs.
13. recurrent disease/
14. or/6‐13
15. 5 and 14
16. post‐RSV$.tw.
17. post‐bronchiolit$.tw.
18. 15 or 16 or 17
19. exp antibiotic agent/
20. antibiotic$.tw.
21. exp macrolide/
22. (macrolide$ or azithromycin or clarithromycin or erythromycin or roxithromycin or spiramycin).tw.
23. (penicillin$ or amoxicillin or amoxycillin or ampicillin or benzylpenicillin or cloxacillin or dicloxacillin or flucloxacillin or piperacillin or ticarcillin or sulbactam).tw.
24. (cephalosporin$ or cephalexin or cephaclor or cefaclor or cefepime or cefotaxime or cephamycin$ or cefotetan or cefoxitin or cefmetazole or cefpirome or cefpodoxime or ceftazidime or ceftriaxone or cephamandole or cephazolin).tw.
25. (fluoroquinolone$ or ciprofloxacin or enoxacin or norfloxacin or ofloxacin or pefloxacin or fleroxacin or levofloxacin or moxifloxacin).tw.
26. (tetracycline$ or doxycycline or methacycline or minocycline).tw.
27. (amikacin or gentamicin or neomycin or netilmicin).tw.
28. (clindamycin or lincomycin).tw.
29. (chloramphenicol or amantadine or cotrimoxazole or trimethoprim).tw.
30. or/19‐29
31. 18 and 30
32. child/
33. exp pediatrics/
34. infant/
35. adolescent/
36. (paediatric$ or paediatric$ or child$ or adolescen$ or infant$ or young$ or preschool$ or pre‐school$ or newborn$ or new‐born$ or neonat$ or neo‐nat$).tw.
37. or/32‐36
38. 31 and 37
39. Randomized Controlled Trial/
40. randomisation/
41. Controlled Study/
42. Clinical Trial/
43. controlled clinical trial/
44. Double Blind Procedure/
45. Single Blind Procedure/
46. Crossover Procedure/
47. or/39‐46
48. (clinica$ adj3 trial$).mp.
49. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).mp.
50. exp Placebo/
51. placebo$.mp.
52. random$.mp.
53. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).mp.
54. (crossover$ or cross‐over$).mp.
55. or/48‐54
56. 47 or 55
57. exp ANIMAL/
58. Nonhuman/
59. Human/
60. 57 or 58
61. 60 not 59
62. 56 not 61
63. 38 and 62
Clinicaltrials.gov
Search terms=bronchiolitis
Study type=interventional studies
Interventions=antibiotics

Study flow diagram.
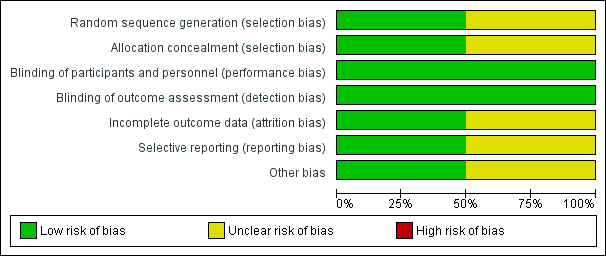
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.1 Number of participants who were not cured at follow‐up (up to 6 months).

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.2 Number of participants who were rehospitalised within 6 months.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.3 Proportion of participants with wheeze (within 6 months of intervention).
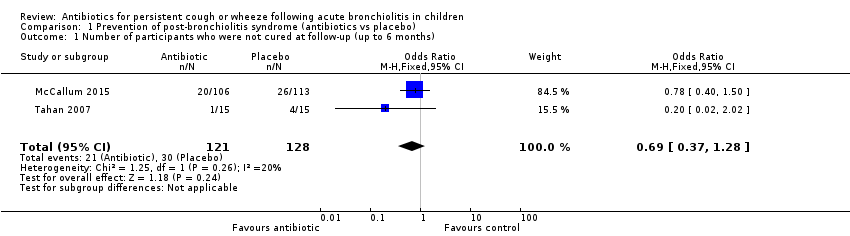
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 1 Number of participants who were not cured at follow‐up (up to 6 months).
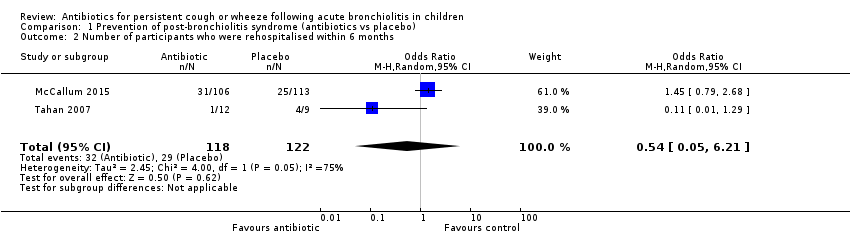
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 2 Number of participants who were rehospitalised within 6 months.

Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 3 Proportion of participants with wheeze (within 6 months of intervention).
| Antibiotics compared with placebo or no treatment for persistent respiratory symptoms following acute bronchiolitis | ||||||
| Patient or population: children < 24 months with persistent respiratory symptoms following acute bronchiolitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Number of participants who were not cured at follow‐up | 234 per 1000 | 174 per 1000 | OR 0.69 | 249 | ⊕⊕⊝⊝ | |
| Number of participants rehospitalised for a respiratory illness | 238 per 1000 | 271 per 1000 | OR 1.19 | 240 | ⊕⊕⊝⊝ | |
| Proportion of participants with recurrent wheeze | 123 per 1000 | 99 per 1000 | OR 0.47 (0.06 to 3.95) | 240 | ⊕⊕⊝⊝ | |
| ^Intervention/Comparison group: treatment initiated during child's hospitalisation for acute bronchiolitis *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| We used GRADEPro software to create this table (GRADEpro GDT 2015) GRADE Working Group grades of evidence | ||||||
| aQuality downgraded because of small numbers of studies and participants and high attrition in the Tahan study. Hence, we cannot be confident of the effect estimate | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who were not cured at follow‐up (up to 6 months) Show forest plot | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.37, 1.28] |
| 2 Number of participants who were rehospitalised within 6 months Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.21] |
| 3 Proportion of participants with wheeze (within 6 months of intervention) Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.95] |

