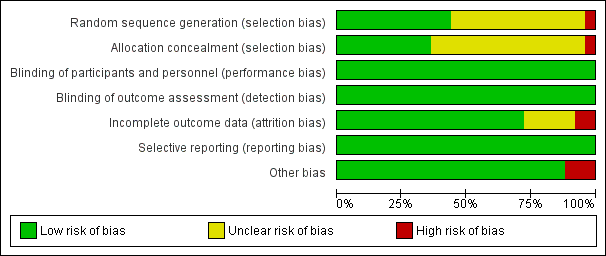
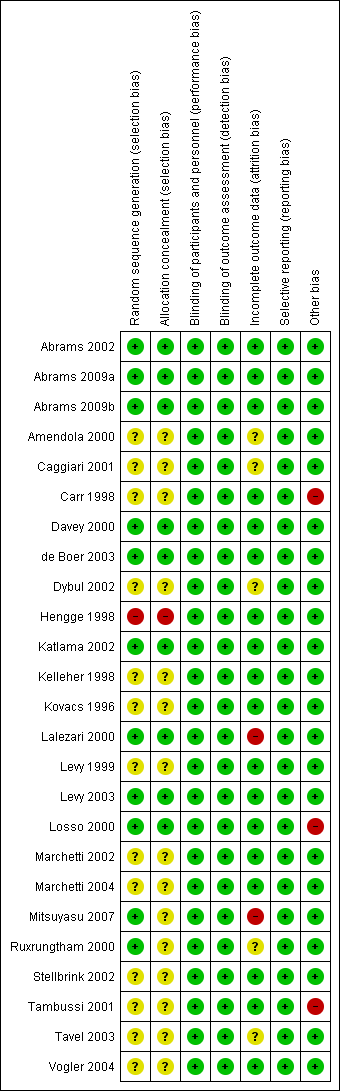
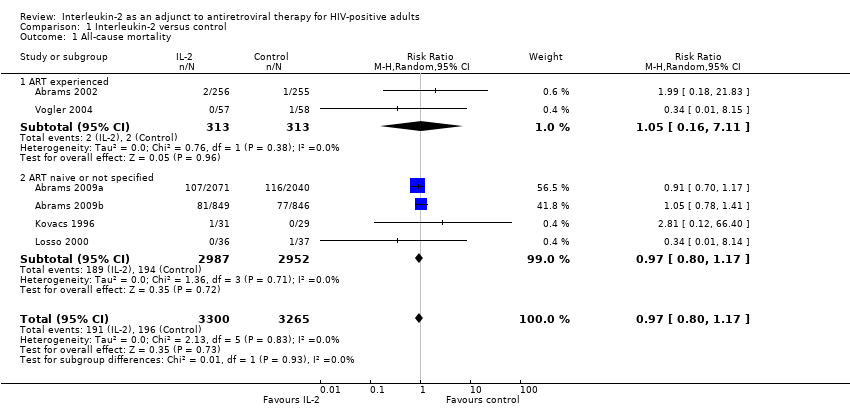
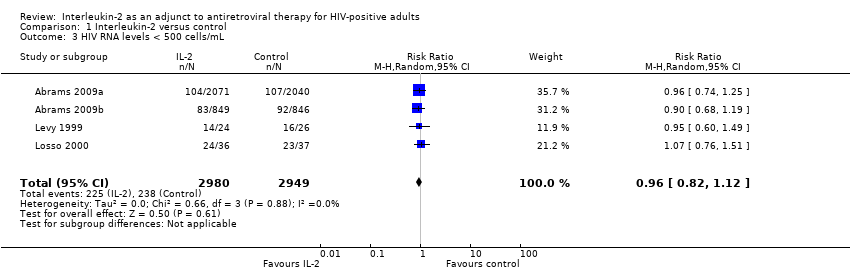
| Number | Trial ID | Follow‐up duration | Dosing regimen for interleukin‐2 (IL‐2) | Comparisons | Outcomes | ART experienced or naive |
| 1 | Abrams 2002 | 16 months | Dose: 2 doses (4.5 and 7.5 miu) Route: subcutaneous Duration: twice daily for 5 days every 8 weeks | ART not specified | Viral load CD4 cell count | ART experienced |
| 2 | Abrams 2009b | 7 years | Dose: 4.5 miu Route: subcutaneous Duration: twice daily, 6 cycles | ART not specified | Opportunistic infections Death from any cause Adverse events | Not specified |
| 3 | Abrams 2009a | 7 years | Dose: 7.5 miu Route: subcutaneous Duration: twice daily, 3 cycles | ART not specified | Opportunistic infections Death from any cause Adverse events | Not specified |
| 4 | Amendola 2000 | 28 weeks | Dose: 1 miu Route: subcutaneous Duration: daily for 5 days/week every alternate week for 3 months | Indinavir, stavudine, and lamivudine | CD4 cell count Viral load | ART naive |
| 5 | de Boer 2003 | 12 months | Dose: 12 miu Route: intravenous Duration: for 3, 4, or 5 days every 8 weeks for 6 cycles | ART not specified | CD4 cell count Viral load Adverse events and serious adverse events AIDS defining complex | ART experienced |
| 6 | Caggiari 2001 | 12 months | Dose: 6 miu Route: subcutaneous Duration: from days 1 to 5 and days 8 to 12 of a 28‐day cycle for 6 cycles | 2 nucleoside reverse transcriptase inhibitors (NRTIs) or 2 NRTIs and indinavir | CD4 cell count Viral load | ART naive |
| 7 | Carr 1998 | 12 months | Dose: 1 miu Route: subcutaneous and intravenous Duration: Group A: 12 miu daily for 5 days every 8 weeks (27 participants) Group B: 1 miu per cycle in equal divided doses in day 1 and 3 every 8 weeks (58 participants) | Zidovudine + didanosine + zalcitabine | CD4 cell count Adverse events Viral load Opportunistic infections | ART experienced |
| 8 | Davey 2000 | 48 weeks | Dose: 7.5 miu Route: subcutaneous Duration: 6 cycles every 12 hours for 5 days every 8 weeks | ART not specified | CD4 cell count Viral load Adverse events | ART experienced |
| 9 | Dybul 2002 | 12 months | Dose: 7.5 miu Route: subcutaneous Duration: 3 cycles for 5 days every 8 week | ART not specified | CD4 cell count Viral load | Not specified |
| 10 | Hengge 1998 | 12 months | Dose: 9.6 miu Route: subcutaneous Duration: 5 cycles were given. One cycle was given every 6 weeks over a period of 52 weeks. Treatment group A : subcutaneous administered daily in cycles consisting of 5 days. Treatment group B: subcutaneous administered at a dose of 9.6 miu daily whenever CD4 counts dropped to below 1.25 fold of individual's baseline value | Saquinavir, lamivudine, and zidovudine | CD4 cell count Viral load Opportunistic infections | ART experienced |
| 11 | Katlama 2002 | 24 weeks with outcomes measured at weeks 1, 6, 12, 18, and 24 | Dose: 4.5 miu Route: subcutaneous Duration: every 6 weeks for 4 cycles, every 12 hours for 5 days | 2 nucleoside analogues and one PI | CD4 cell count Viral load Adverse events ART experienced | Not specified |
| 12 | Kelleher 1998 | 48 weeks | Dose: 12 miu Route: intravenous Duration: Group A: 12.6 miu as continuous intravenous infusions for 5 days every 8 weeks for 6 cycles. Group B (IL‐2 linked to polyethylene glycol plus ART): subcutaneous injections on days 1 and 3 of each 8‐week cycles | ART included nucleoside analogues such as lamivudine | CD4 cell count Viral load | ART experienced |
| 13 | Kovacs 1996 | 14 months | Dose: 18 miu Route: intravenous Duration: daily for 5 days every other month for 6 cycles from month 0 to 10 | ART included didanosine, zidovudine, zalcitabine, or stavudine | CD4 cell count Plasma HIV RNA | Not specified |
| 14 | Lalezari 2000 | 6 months | Dose: 1.2 miu, and then increased by 0.3 miu every 2 weeks for 6 months until a participant experienced grade 2 or greater toxicity. Route: subcutaneous Duration: once daily for 2 weeks | ART not specified | CD4 cell count Viral load Adverse events | ART experienced |
| 15 | Levy 1999 | 14 months | Dose: 12 miu and 3 miu Route: 12 miu intravenous and 3 miu subcutaneous intravenously (12 miu/day, N = 22) or subcutaneously (3 miu/m² twice daily, N = 24) for 5 days, or 2 miu/m² intravenous bolus, N = 22) administered every 2 months from week 2 to week 50 (7 cycles). | Zidovudine (600 mg/day) plus didanosine (400 mg/day) | CD4 cell count Viral load Adverse events | ART naive |
| 16 | Levy 2003 | 18 months | Dose: 5 miu Route: subcutaneous Duration: twice daily for a 5 day cycle given every 4 weeks for the first 3 cycles and then subsequently every 8 weeks for the next 7 cycles | ART included lamivudine (300 mg/day), stavudine (60 to 80mg/day) and indinavir (2400 mg/day) | CD4 cell counts Viral load AIDS defining events | ART naive or naive to PIs alone |
| 17 | Losso 2000 | 24 weeks | Dose: escalating doses of 1.5 miu, 4.5 miu, 7.5 miu Route: subcutaneous Duration: twice daily for 5 consecutive days every 8 weeks | ART not specified | CD4 cell counts . Viral load | Both naive and experienced participants were included in the study. |
| 18 | Marchetti 2002 | 48 weeks | Dose: 3 miu Route: subcutaneous Duration: administered as a single subcutaneous injection at days 1 to 5 and 8 to 12 of a 4‐week cycle, for a total of 3 cycles | ART was either 2 nucleoside reverse transcriptase inhibitor
and one PI or at least one non nucleoside reverse transcriptase inhibitor | CD4 cell count Viral load Adverse events | ART experienced |
| 19 | Marchetti 2004 | 48 weeks | Dose: 3 miu Route: subcutaneous Duration: administered at day 1 to 5 and 8 to 12 for 10 weeks | ART not specified | CD4 cell count | ART experienced |
| 20 | Mitsuyasu 2007 | 84 weeks | Dose: Group A 9 miu and Group B 7.5miu Route: intravenous and subcutaneous Duration: Group A: intravenous infusions 5 days every 8 weeks. Group B: subcutaneous injections 7.5 miu twice daily for 5 days every 8 weeks | Received ART alone, 2 nucleosides and a PI | CD4 cell count Viral load | Not specified |
| 21 | Ruxrungtham 2000 | 24 weeks | Dose: Group A 1.5 miu, Group B 4.5 miu, and Group C 7.5 miu Route: subcutaneous Duration: twice daily for 5 days, every 8 weeks for three cycles 8‐weekly | ART not specified | CD4 cell count Viral load | ART experienced |
| 22 | Stellbrink 2002 | 601 days | Dose: 9 miu Route: subcutaneous Duration: once daily (with an option to switch to 4.5 miu twice daily) for 5 consecutive days per cycle administered at 6‐weekly intervals | ART consisting of stavudine 30 to 40 mg twice daily, and lamivudine 150 mg twice daily, nelfinavir 750 mg 3 times daily and saquinavir 600 mg 3 times daily. | CD4 cell count Viral load | ART naive |
| 23 | Tambussi 2001 | 12 months | Dose: 3 regimens of IL‐2 Route: intravenous and subcutaneous Duration:differed by group see details below Group A: 12 miu by continuous intravenous infusion followed by subcutaneous 7.5 miu twice a day for 5 days every 8 weeks for the remaining 4 cycles. Group B: subcutaneous 7.5 miu twice a day for 5 days every 8 weeks for 6 cycles. Group C: subcutaneous 3 miu twice a day every 4 weeks | 2 NRTIs and saquinavir | CD4 cell count Viral load | ART experienced |
| 24 | Tavel 2003 | 12 months | Dose: 7.5 miu Route: subcutaneous Duration: Group A: 7.5 miu twice daily for 5 days versus placebo plus ART. Group B: 7.5 miu twice a day for 5 days | Nucleosides analogue reverse transcriptase inhibitor and either a non‐nucleosides analogue reverse transcriptase inhibitor or PI | CD4 cell count Viral load | ART experienced |
| 25 | Vogler 2004 | 24 weeks | Dose: 1 miu Route: subcutaneous Duration: once daily | 2 nucleoside reverse transcriptase inhibitors | CD4 cell count Viral load | ART experienced |