Interleukin‐2 kao dodatak antiretroviralnoj terapiji za HIV‐pozitivne odrasle osobe
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Open‐label randomized controlled trial (RCT) | |
| Participants | Eligibility criteria
Exclusion criteria
The trial included a total of 511 (256 in the interleukin group and 255 in the control group) HIV‐1 infected adults
| |
| Interventions | Intervention group: intermittent administration of 2 doses (4.5 and 7.5 miu) of subcutaneous plus antiretroviral treatment (ART) Control group: ART alone. | |
| Outcomes |
| |
| Notes | The trial was conducted in the USA. Duration of follow‐up: minimum of 12 months. Median duration of follow‐up was 16.2 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used permuted block randomization with stratification by the CPCRA unit. |
| Allocation concealment (selection bias) | Low risk | The trial obtained random allocation of participants by calling the CPCRA Statistical Centre. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Less than 15% of the participants were excluded from the final analysis or lost to follow‐up, and it was by intention‐to‐treat (ITT) analysis. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of bias from other sources. |
| Methods | Open‐label RCT | |
| Participants | Eligibility criteria: HIV‐infected adult Exclusion criteria: not specified | |
| Interventions | Intervention group: 3 cycles and a dose of 7.5 miu of IL‐2 twice daily plus ART Control group: ART alone . | |
| Outcomes |
| |
| Notes | The trial was conducted in the USA. The median duration of follow‐up was 7.0 years. Ths trial was funded and sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial stratified randomization by individual clinical site. |
| Allocation concealment (selection bias) | Low risk | The central coordinating facility prepared all randomizations schedules. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The analysis was based on an ITT principle and less than 15% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other forms of bias. |
| Methods | Open‐label RCT | |
| Participants | Eligibility criteria: HIV‐positive adults with CD4 cell count between 50 and 299 cells/mm³ Exclusion criteria: not specified | |
| Interventions | Intervention group: 1 cycle of a dose of 4.5 miu twice daily for 5 consecutive days Control group: ART alone | |
| Outcomes |
| |
| Notes | The trial was conducted in the USA. The median duration of follow‐up was 7.6 years. The NIAID provided regulatory sponsorship, and Chiron, and subsequently Novartis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial stratified randomization by individual clinical site. |
| Allocation concealment (selection bias) | Low risk | The central co‐ordinating facility prepares all randomization schedules. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The analysis was based on an ITT principle and less than 15% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other forms of bias. |
| Methods | Open‐label RCT | |
| Participants | 22 HIV‐infected adults (12 males and 10 females) Inclusion criteria
Exclusion criteria
| |
| Interventions | The participants were enrolled in 3 randomized groups.
All participants were treated with ART for 1 month before receiving differentiated therapies (ART; ART 1rIL‐2; (G‐CSF) ART 1rIL‐2) for an additional 12/24 weeks. | |
| Outcomes |
| |
| Notes | The trial was conducted in Italy. Duration of follow‐up: 24 weeks. Outcomes were analysed at baseline, 12, and 24 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe whether this was done or not. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe whether this was done or not. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The study was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | We do not have enough information from the trial to make a judgement. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | Paralell single centred RCT | |
| Participants | 14 HIV‐infected adults Inclusion criteria
Exclusion criteria
| |
| Interventions | Intervention group: 6 miu of IL‐2 from days 1 to 5 and 8 to 12 of a 28 day cycle for 6 cycles plus ART(2 reverse transcriptase inhibitors and indinavir) Control group: ART alone | |
| Outcomes |
| |
| Notes | This trial was conducted in Italy. Duration of follow‐up: 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no mention of the specific method of sequence generation or randomization but in the discussion section it was stated that randomization was done to ensure comparability of both groups |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of the specific method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and not likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | We do not have enough information from the study to make a judgement. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | RCT | |
| Participants | 115 HIV‐infected adults Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | There were 3 trial arms
ART consisted of zidovudine + didanosine + zalcitabine | |
| Outcomes |
| |
| Notes | The trial was conducted in Australia. Duration of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial not describe the method for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not described the method for allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial authors analysed all participants included in the trial. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | There was a high risk of selection bias and detection bias. Participants were randomized on a 1:2:1 basis for the continuous intravenous IL‐2, PEG‐IL‐2 ( Polyethylene glycol ) modified IL‐2, and control groups respectively. The trial authors rationalized that the unequal randomization allowed for determination of the maximally tolerated dose significance levels of PEG IL‐2 as well as its efficacy. This was bound to cause selection bias. Secondly, IL‐2 participants were hospitalized the first 5 to 6 days of the cycle causing possible detection bias |
| Methods | Multicentred RCT | |
| Participants | Inclusion criteria: HIV‐infected adults Exclusion criteria: not specified | |
| Interventions | Intervention: 6 cycles of IL‐2, 7.5 miu + ART Control: ART alone | |
| Outcomes | CD4 cell count Viral load Adverse events | |
| Notes | The trial was conducted in the USA. Duration of follow‐up was 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial randomly assigned participants to treatment groups by a computer generated block randomization with block sizes of 4 for the first 2 blocks and subsequently block sizes of 2. |
| Allocation concealment (selection bias) | Low risk | Central randomization by a biostatistician who was not part of the data analysis. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | Biostatisticians were blinded from knowing which participants were in which treatment groups. |
| Incomplete outcome data (attrition bias) | Low risk | It is unlikely that there was attrition bias since < 15% withdrew or were lost to follow‐up. There was no differential loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective outcome reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
| Methods | Multi‐centred RCT | |
| Participants | Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group: participants received intravenous recombinant IL‐2 12 miu/day for 3, 4, or 5 days + ART every 8 weeks for 6 cycles Control group: ART alone | |
| Outcomes |
| |
| Notes | The trial was conducted in USA. Duration of follow‐up was 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There is likely to be a low risk of selection bias as participants were assigned in equal proportions of 1 to 4 treatments and participants were stratified by treatment centre. |
| Allocation concealment (selection bias) | Low risk | Randomization was done centrally. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial addressed loss to follow‐up and was less than 15%. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of potential reporting bias from selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
| Methods | RCT | |
| Participants | 9 participants Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group: intermittent IL‐2 administered subcutaneously in 3 cycles for 5 days every 8 week plus ART Control group: ART alone. regimen: stavudine 30 mg to 40 mg twice daily, lamivudine 150 mg twice daily, indinavir 800 mg twice daily | |
| Outcomes |
| |
| Notes | This was a pilot study conducted in the USA. Duration of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not provide any details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any details. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | We do not have enough information from the trial to make a judgement. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | This was a prospective RCT | |
| Participants | There was a total of 64 participants. Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group A (22): ART plus subcutaneous IL‐2 administered at a dose of 9.6 miu daily in cycles consisting of 5 days. A total of 5 cycles were given. One cycle was given every 6 weeks over a period of 52 weeks. Treatment group B (22): ART plus subcutaneous IL‐2 administered at a dose of 9.6 miu daily whenever CD4 counts dropped to below 1.25 fold of individual's baseline value. Control group: ART alone participants were followed up for a duration of 12 months | |
| Outcomes |
| |
| Notes | The trial was conducted in Germany. Duration of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was no true randomization as controls were chosen from participants fulfilling the inclusion criteria who did not wish to experience the potentially adverse effects of lL‐2. Secondly, the trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | High risk | There is unlikely to be allocation concealment based on the support for judgement for sequence generation above. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | There is unlikely to have been attrition bias as the trial excluded less than 15% of the participants. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting of outcomes. |
| Other bias | Low risk | There were no other potential sources of bias. |
| Methods | Multicentred open label RCT | |
| Participants | A total of 72 participants. Inclusion criteria
Exclusion criteria: participants on cytotoxic chemotherapy or corticosteroids within 3 months prior to the trial | |
| Interventions | Intervention: 4.5 miu of IL‐2 administered subcutaneously plus ART every 6 weeks for 4 cycles, every 12 hours for 5 days Control: ART alone | |
| Outcomes |
| |
| Notes | The trial was conducted in 18 clinical centres in France. Duration of follow‐up: 24 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial performed randomization of participants by using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | The trial performed allocation centrally. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial authors used ITT for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | This was a single centred pilot study which was planned as a pilot study of a multi‐centred RCT to be conducted. | |
| Participants | 18 participants consecutively enrolled into 3 groups Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group A (IL‐2 plus ART only)
as continuous intravenous infusions for 5 days every 8 weeks for 6 cycles Treatment group B (IL‐2 linked to polyethylene glycol plus ART)
Control group
Each group had 150 mg of lamivudine twice daily added to their regimen at 30 weeks. | |
| Outcomes |
| |
| Notes | This was a multicentred trial conducted in Australia. Duration of follow‐up: 48 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial excluded less than 15% of the participants. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | This was a single‐centred RCT | |
| Participants | A total of 60 participants were randomized Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group: intravenous IL‐2 given at intermittent infusions of 18 miu plus ART Control group: ART alone consisting of zidovudine, zalcitabine, or stavudine with didanosine | |
| Outcomes |
| |
| Notes | Study was conducted in the USA. The duration of follow‐up was 14 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Less than 15% were lost to follow‐up and the trial authors described withdrawals. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | Multicentred phase 2 RCT | |
| Participants | A total of 115 participants Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group (IL‐2 plus ART): 51 participants
Control group: ART alone
| |
| Outcomes |
| |
| Notes | This trial was conducted in the USA. Duration of follow‐up: 26 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was by block randomization stratified by study site. |
| Allocation concealment (selection bias) | Low risk | The trial authors used a central randomization process. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | High risk | More than 15% withdrew from the trial due to adverse effects and the trial authors did not analyse their results. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective outcome reporting. |
| Other bias | Low risk | There was no evidence of any other sources of bias. |
| Methods | Multicentred an open label RCT | |
| Participants | A total of 94 participants Inclusion criteria
Exclusion criteria: not specified | |
| Interventions |
| |
| Outcomes |
| |
| Notes | This study was conducted in 14 university clinics in France. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | There was no evidence of incomplete outcome data. Less than 15% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting bias. |
| Other bias | Low risk | There was no evidence of other sources of bias. |
| Methods | This was a prospective RCT. | |
| Participants | There were a total of 118 participants Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment group: ART started 4 weeks before plus subcutaneous IL‐2 administered at a dose of 5 miu twice daily for 5 days given every 4 weeks for the first 3 cycles and then subsequently every 8 weeks for the next 7 cycles. Control group: ART alone consisting of lamivudine (300 mg/day), stavudine (60 to 80mg/day), and indinavir (2400 mg/day). | |
| Outcomes |
| |
| Notes | This was a multicentred study conducted in 14 university clinics in France. Duration of follow‐up was 18 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors did not describe the method of sequence generation. However, the trial authors mentioned that randomization was centralized and stratified according to ART status. |
| Allocation concealment (selection bias) | Low risk | The trial authors used a centralized method of randomization. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial excluded less than 15% (2 out of 118 participants) from the analysis. There was no differential loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other sources of bias. |
| Methods | Study design was a prospective open‐labelled RCT | |
| Participants | There were a total of 73 participants Inclusion criteria
Exclusion criteria
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | This was a multi‐centred trial conducted in 6 clinical centres in Buenos Aires, Argentina. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a stratified block randomization method, using blocks of 24 stratified according to ART history (naive or experienced) and clinical centres. |
| Allocation concealment (selection bias) | Low risk | The trial used a centralized method of randomization. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial excluded less than 15% (2 out of 73 participants) from the analysis. There was no differential loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | There was more monitoring in the treatment group compared to the control group. |
| Methods | Open labelled parallel RCT | |
| Participants | 22 participants were randomized: Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group (12): they were commenced on IL‐2 for a 4 week cycle for 3 cycles plus ART Control group (10): ART consisting of 2 nucleoside reverse transcriptase inhibitors (NRTI) and 1 PI | |
| Outcomes |
| |
| Notes | This trial was an explanatory trial conducted at the University of Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial followed up all participants to the end of the trial, and even included those who were lost to follow‐up afterwards in the results. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting bias. |
| Other bias | Low risk | There was no evidence of other potential sources bias. |
| Methods | This was a RCT | |
| Participants | There were a total of 15 participants Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group (8): participants received 3 cycles of low dose subcutaneous IL‐2 over a 48‐week period. Each cycle consisted of 3 miu IL‐2 administered at days of 1 to 5 and days 8 to 12 of a 10‐week duration plus ART Control group (7): ART alone | |
| Outcomes |
| |
| Notes | This was a small immunological trial conducted to investigate the long‐term kinetics of CD4 and CD8 cells when low dose IL‐2 is administered in Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting of outcomes. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
| Methods | This was a multi‐arm parallel RCT | |
| Participants | There were a total of 159 participants Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group A (intravenous IL‐2 plus ART): received continuous infusions of IL‐2 at doses of 9 miu for 5 days every 8 weeks plus ART Treatment group B (subcutaneous IL‐2 plus ART): received subcutaneous injections of IL‐2 7.5 miu twice daily for 5 days every 8 weeks plus ART Control group: ART alone, 2 NRTIs, and a PI | |
| Outcomes |
| |
| Notes | This was a multicentred trial conducted at 26 AIDS Clinical Trials Group (ACTG) sites in the USA. Duration of the trial was 84 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There is likely to be a low risk of selection bias because although the trial authors did not describe the method of sequence generation, participants were randomized in proportions of 1:1:1 and stratified by participation in a previous trial ACTG 928 and nucleosides. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | High risk | The trial excluded more than 15% of participants from the analysis. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
| Methods | Multicentred parallel RCT | |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment groups (A, B, and C): received 1.5 miu, 4.5 miu, and 7.5 miu of IL‐2 administered twice daily for 5 days, every 8 weeks for three cycles. Control groups: ART alone. | |
| Outcomes |
| |
| Notes | This was a multi‐arm pragmatic trial conducted in Thailand. Duration of follow‐up was 24 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors did not clearly specify the method of random sequence generation but it appeared to be low risk. Randomization was stratified by clinical centre and ART treatment history, that is naive or pretreated. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | The trial authors did not describe withdrawals or missing data. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
| Methods | Prospective RCT | |
| Participants | There were 56 participants. Inclusion criteria
Exclusion criteria
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | This was an open label RCT conducted in Germany. Mean follow‐up duration ranged from 582 ‐ 601 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial authors performed analysis was ITT and there were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
| Methods | This was a phase 2 RCT. | |
| Participants | There was a total of 61 participants. Inclusion criteria
Exclusion criteria: not specified | |
| Interventions | Treatment arm A (high dose intravenous arm): ART plus 12 miu of IL‐2 by continuous intravenous infusion followed by subcutaneous 7.5 miu IL‐2 twice a day for 5 days every 8 weeks for the remaining 4 cycles. Treatment arm B (high dose subcutaneous arm): ART+subcutaneous 7.5 miu IL‐2 twice a day for 5 days every 8 weeks for 6 cycles. Treatment arm C (low dose arm): ART plus subcutaneous IL‐2 +3 miu twice a day every 4 weeks Control: ART alone | |
| Outcomes |
| |
| Notes | This was an phase 2 RCT conducted in Milan Italy. The duration of follow‐up was a 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial authors performed analysis by ITT principle and less than 15% of participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | There were differential treatment participants in the 2 groups. Participants were followed up as outpatients in 1 group and as inpatients in the other group. |
| Methods | This was a double blinded randomized placebo controlled trial | |
| Participants | There were a total of 19 participants. Inclusion criteria
Exclusion criteria
| |
| Interventions | Group A: subcutaneous IL‐2 (7.5 miu IL‐2 twice daily for 5 days) and placebo) plus ART Group B: IL‐2 (7.5 miu IL‐2 twice daily for 5 days) and 0.5 mg prednisone/kg/day for 7 days every 8 weeks plus ART Group C: 0.5 mg prednisone/ kg/day for 7 days every 8 weeks plus ART Group D: placebo orally for 7 days (1 cycle ) every 8 weeks plus ART | |
| Outcomes |
| |
| Notes | This study was conducted in the USA. Duration of follow‐up was 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | There was no loss to follow‐up and ITT. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
| Methods | This was a phase 2 multi‐centred randomized open label trial | |
| Participants | There was a total of 115 participants Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group: self administered subcutaneous IL‐2 1 miu daily in 0.2 mL volume at rotating skin sites in combination with continued ART Control: ART alone. | |
| Outcomes |
| |
| Notes | This trial was conducted in the USA. The duration of follow‐up was 24 weeks. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The trial excluded less than 15% from the analysis. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Abbreviations: AIDS: acquired immunodeficiency virus; ART: antiretroviral therapy; CNS: central nervous system; PEG: polyethylene glycol; HIV: human immunodeficiency virus; ITT: intention to treat; IL‐2: interleukin‐2; NRTI: nucleoside reverse transcriptase inhibitors; PI: protease inhibitor; RCT: randomized controlled trial; USA: United States of America.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Both the intervention and control group received interleukin‐2 (IL‐2) | |
| This was a cross‐sectional study | |
| This was a cohort study | |
| This was a study of interleukin‐12 (IL‐12) and not IL‐2 | |
| Participants in the intervention group received both IL‐2 and a vaccine | |
| Participants in the intervention group received both IL‐2 and interferon‐gamma | |
| The study did not report any outcomes relevant to this review | |
| This was a review of randomized controlled trials | |
| The study compared participants receiving no treatment, IL‐2 alone, or IL‐2 with antiretroviral treatment (ART) | |
| Here the comparison was between subcutaneous IL‐2 and ART and intravenous IL‐2 and ART. The control group was not relevant to this review |
Abbreviations: IL‐2: interleukin‐2.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 6 | 6565 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| Analysis 1.1  Comparison 1 Interleukin‐2 versus control, Outcome 1 All‐cause mortality. | ||||
| 1.1 ART experienced | 2 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 7.11] |
| 1.2 ART naive or not specified | 4 | 5939 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 2 HIV RNA levels < 50 cells/mL Show forest plot | 5 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.15] |
| Analysis 1.2  Comparison 1 Interleukin‐2 versus control, Outcome 2 HIV RNA levels < 50 cells/mL. | ||||
| 3 HIV RNA levels < 500 cells/mL Show forest plot | 4 | 5929 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.12] |
| Analysis 1.3  Comparison 1 Interleukin‐2 versus control, Outcome 3 HIV RNA levels < 500 cells/mL. | ||||
| 4 Opportunistic infections Show forest plot | 7 | 6141 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.13] |
| Analysis 1.4  Comparison 1 Interleukin‐2 versus control, Outcome 4 Opportunistic infections. | ||||
| 4.1 ART experienced | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.86] |
| 4.2 ART naive or not specified | 5 | 6044 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.56, 1.19] |
| 5 Adverse events (grade 3 or 4) Show forest plot | 6 | 6291 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.10, 1.96] |
| Analysis 1.5  Comparison 1 Interleukin‐2 versus control, Outcome 5 Adverse events (grade 3 or 4). | ||||

Study flow diagram
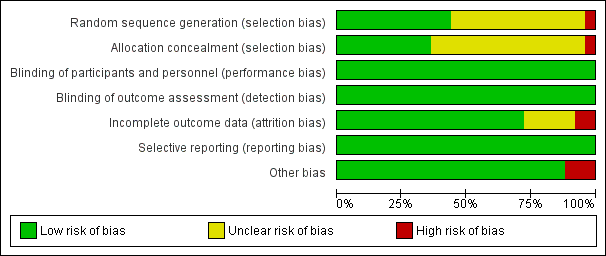
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials
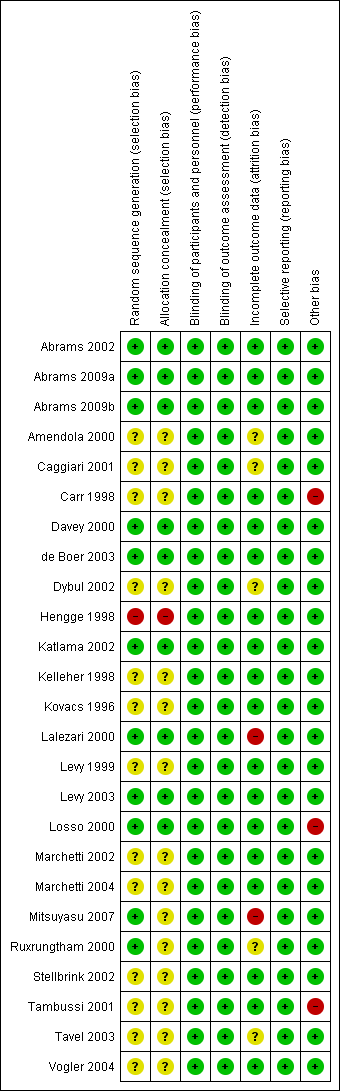
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial
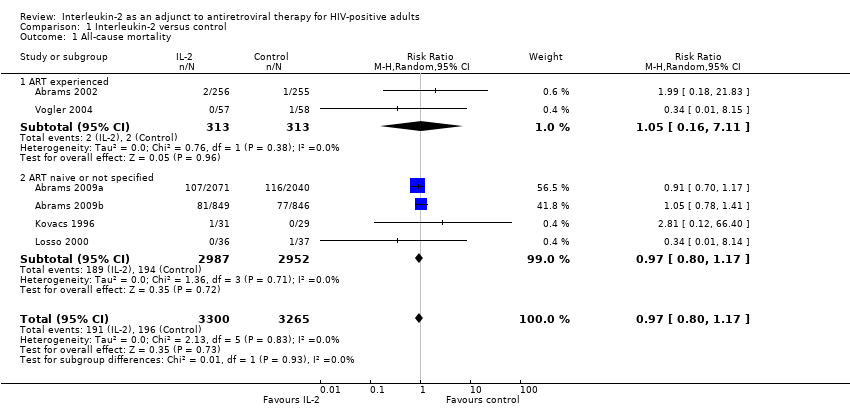
Comparison 1 Interleukin‐2 versus control, Outcome 1 All‐cause mortality.

Comparison 1 Interleukin‐2 versus control, Outcome 2 HIV RNA levels < 50 cells/mL.
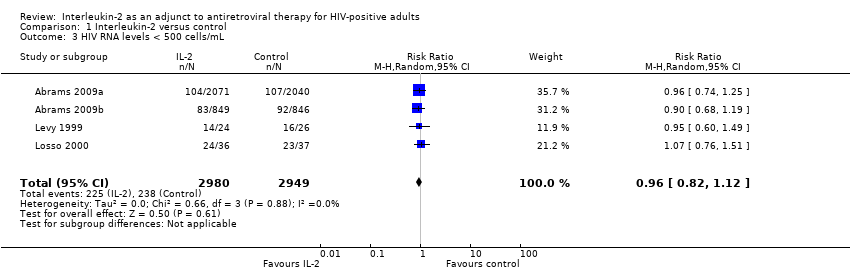
Comparison 1 Interleukin‐2 versus control, Outcome 3 HIV RNA levels < 500 cells/mL.

Comparison 1 Interleukin‐2 versus control, Outcome 4 Opportunistic infections.

Comparison 1 Interleukin‐2 versus control, Outcome 5 Adverse events (grade 3 or 4).
| Interleukin‐2 compared to control for HIV‐positive adults | ||||||
| Patient or population: HIV‐positive adults | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | IL‐2 | |||||
| All‐cause mortality | 60 per 1000 | 58 per 1000 | RR 0.97 | 6565 | ⊕⊕⊕⊕ | There is little or no effect on all cause mortality |
| CD4 cell count | Tended to increase in all but one study | 7600 (21 trials) | — | Tended to increase in all but one study | ||
| HIV RNA levels less than 50 cells/mL | 636 per 1000 | 617 per 1000 | RR 0.97 | 805 | ⊕⊕⊕⊕ | There is little or no effect on viral suppression |
| HIV RNA levels less than 500 cells/mL | 81 per 1000 | 77 per 1000 | RR 0.96 | 5929 | ⊕⊕⊕⊕ | |
| Opportunistic infections | 46 per 1000 | 39 per 1000 | RR 0.79 | 6141 | ⊕⊕⊝⊝ | There may be little or no effect on opportunistic infections |
| Adverse events (grade 3 or 4) | 197 per 1000 | 242 per 1000 | RR 1.47 | 6291 | ⊕⊕⊕⊝ | There is probably an increase in adverse events |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by 2 for imprecision due to low event rate resulting in a wide 95% CI is wide. The overall meta‐analysis remains underpowered to confidently exclude effects. | ||||||
| Number | Trial ID | Follow‐up duration | Dosing regimen for interleukin‐2 (IL‐2) | Comparisons | Outcomes | ART experienced or naive |
| 1 | 16 months | Dose: 2 doses (4.5 and 7.5 miu) Route: subcutaneous Duration: twice daily for 5 days every 8 weeks | ART not specified | Viral load CD4 cell count | ART experienced | |
| 2 | 7 years | Dose: 4.5 miu Route: subcutaneous Duration: twice daily, 6 cycles | ART not specified | Opportunistic infections Death from any cause Adverse events | Not specified | |
| 3 | 7 years | Dose: 7.5 miu Route: subcutaneous Duration: twice daily, 3 cycles | ART not specified | Opportunistic infections Death from any cause Adverse events | Not specified | |
| 4 | 28 weeks | Dose: 1 miu Route: subcutaneous Duration: daily for 5 days/week every alternate week for 3 months | Indinavir, stavudine, and lamivudine | CD4 cell count Viral load | ART naive | |
| 5 | 12 months | Dose: 12 miu Route: intravenous Duration: for 3, 4, or 5 days every 8 weeks for 6 cycles | ART not specified | CD4 cell count Viral load Adverse events and serious adverse events AIDS defining complex | ART experienced | |
| 6 | 12 months | Dose: 6 miu Route: subcutaneous Duration: from days 1 to 5 and days 8 to 12 of a 28‐day cycle for 6 cycles | 2 nucleoside reverse transcriptase inhibitors (NRTIs) or 2 NRTIs and indinavir | CD4 cell count Viral load | ART naive | |
| 7 | 12 months | Dose: 1 miu Route: subcutaneous and intravenous Duration: Group A: 12 miu daily for 5 days every 8 weeks (27 participants) Group B: 1 miu per cycle in equal divided doses in day 1 and 3 every 8 weeks (58 participants) | Zidovudine + didanosine + zalcitabine | CD4 cell count Adverse events Viral load Opportunistic infections | ART experienced | |
| 8 | 48 weeks | Dose: 7.5 miu Route: subcutaneous Duration: 6 cycles every 12 hours for 5 days every 8 weeks | ART not specified | CD4 cell count Viral load Adverse events | ART experienced | |
| 9 | 12 months | Dose: 7.5 miu Route: subcutaneous Duration: 3 cycles for 5 days every 8 week | ART not specified | CD4 cell count Viral load | Not specified | |
| 10 | 12 months | Dose: 9.6 miu Route: subcutaneous Duration: 5 cycles were given. One cycle was given every 6 weeks over a period of 52 weeks. Treatment group A : subcutaneous administered daily in cycles consisting of 5 days. Treatment group B: subcutaneous administered at a dose of 9.6 miu daily whenever CD4 counts dropped to below 1.25 fold of individual's baseline value | Saquinavir, lamivudine, and zidovudine | CD4 cell count Viral load Opportunistic infections | ART experienced | |
| 11 | 24 weeks with outcomes measured at weeks 1, 6, 12, 18, and 24 | Dose: 4.5 miu Route: subcutaneous Duration: every 6 weeks for 4 cycles, every 12 hours for 5 days | 2 nucleoside analogues and one PI | CD4 cell count Viral load Adverse events ART experienced | Not specified | |
| 12 | 48 weeks | Dose: 12 miu Route: intravenous Duration: Group A: 12.6 miu as continuous intravenous infusions for 5 days every 8 weeks for 6 cycles. Group B (IL‐2 linked to polyethylene glycol plus ART): subcutaneous injections on days 1 and 3 of each 8‐week cycles | ART included nucleoside analogues such as lamivudine | CD4 cell count Viral load | ART experienced | |
| 13 | 14 months | Dose: 18 miu Route: intravenous Duration: daily for 5 days every other month for 6 cycles from month 0 to 10 | ART included didanosine, zidovudine, zalcitabine, or stavudine | CD4 cell count Plasma HIV RNA | Not specified | |
| 14 | 6 months | Dose: 1.2 miu, and then increased by 0.3 miu every 2 weeks for 6 months until a participant experienced grade 2 or greater toxicity. Route: subcutaneous Duration: once daily for 2 weeks | ART not specified | CD4 cell count Viral load Adverse events | ART experienced | |
| 15 | 14 months | Dose: 12 miu and 3 miu Route: 12 miu intravenous and 3 miu subcutaneous intravenously (12 miu/day, N = 22) or subcutaneously (3 miu/m² twice daily, N = 24) for 5 days, or 2 miu/m² intravenous bolus, N = 22) administered every 2 months from week 2 to week 50 (7 cycles). | Zidovudine (600 mg/day) plus didanosine (400 mg/day) | CD4 cell count Viral load Adverse events | ART naive | |
| 16 | 18 months | Dose: 5 miu Route: subcutaneous Duration: twice daily for a 5 day cycle given every 4 weeks for the first 3 cycles and then subsequently every 8 weeks for the next 7 cycles | ART included lamivudine (300 mg/day), stavudine (60 to 80mg/day) and indinavir (2400 mg/day) | CD4 cell counts Viral load AIDS defining events | ART naive or naive to PIs alone | |
| 17 | 24 weeks | Dose: escalating doses of 1.5 miu, 4.5 miu, 7.5 miu Route: subcutaneous Duration: twice daily for 5 consecutive days every 8 weeks | ART not specified | CD4 cell counts . Viral load | Both naive and experienced participants were included in the study. | |
| 18 | 48 weeks | Dose: 3 miu Route: subcutaneous Duration: administered as a single subcutaneous injection at days 1 to 5 and 8 to 12 of a 4‐week cycle, for a total of 3 cycles | ART was either 2 nucleoside reverse transcriptase inhibitor | CD4 cell count Viral load Adverse events | ART experienced | |
| 19 | 48 weeks | Dose: 3 miu Route: subcutaneous Duration: administered at day 1 to 5 and 8 to 12 for 10 weeks | ART not specified | CD4 cell count | ART experienced | |
| 20 | 84 weeks | Dose: Group A 9 miu and Group B 7.5miu Route: intravenous and subcutaneous Duration: Group A: intravenous infusions 5 days every 8 weeks. Group B: subcutaneous injections 7.5 miu twice daily for 5 days every 8 weeks | Received ART alone, 2 nucleosides and a PI | CD4 cell count Viral load | Not specified | |
| 21 | 24 weeks | Dose: Group A 1.5 miu, Group B 4.5 miu, and Group C 7.5 miu Route: subcutaneous Duration: twice daily for 5 days, every 8 weeks for three cycles 8‐weekly | ART not specified | CD4 cell count Viral load | ART experienced | |
| 22 | 601 days | Dose: 9 miu Route: subcutaneous Duration: once daily (with an option to switch to 4.5 miu twice daily) for 5 consecutive days per cycle administered at 6‐weekly intervals | ART consisting of stavudine 30 to 40 mg twice daily, and lamivudine 150 mg twice daily, nelfinavir 750 mg 3 times daily and saquinavir 600 mg 3 times daily. | CD4 cell count Viral load | ART naive | |
| 23 | 12 months | Dose: 3 regimens of IL‐2 Route: intravenous and subcutaneous Duration:differed by group see details below Group A: 12 miu by continuous intravenous infusion followed by subcutaneous 7.5 miu twice a day for 5 days every 8 weeks for the remaining 4 cycles. Group B: subcutaneous 7.5 miu twice a day for 5 days every 8 weeks for 6 cycles. Group C: subcutaneous 3 miu twice a day every 4 weeks | 2 NRTIs and saquinavir | CD4 cell count Viral load | ART experienced | |
| 24 | 12 months | Dose: 7.5 miu Route: subcutaneous Duration: Group A: 7.5 miu twice daily for 5 days versus placebo plus ART. Group B: 7.5 miu twice a day for 5 days | Nucleosides analogue reverse transcriptase inhibitor and either a non‐nucleosides analogue reverse transcriptase inhibitor or PI | CD4 cell count Viral load | ART experienced | |
| 25 | 24 weeks | Dose: 1 miu Route: subcutaneous Duration: once daily | 2 nucleoside reverse transcriptase inhibitors | CD4 cell count Viral load | ART experienced | |
| Abbreviations: ART antiretroviral therapy; IL‐2 Interleukin 2; NRTI nucleoside reverse transcriptase inhibitors; PI protease inhibitor | ||||||
| Increase in CD4 cell count with statistically significant difference | |
| (at 12 months follow‐up) n = 511 | The average difference change in CD4 cell count between the IL‐2 group and control group was 217.1 cells/mm³ (95% CI 188.6 to 245.5; P < 0.001) measured at 12 months. |
| (at 12 months follow‐up) n = 115 | Median CD4 cell count increases of 359 and 44 cells/mm³ and a decline of 46 cells/mm³ in the cyclical continuous intravenous IL‐2, subcutaneous IL‐2, and ART alone group, respectively, over 12 months (P < 0.0001 for each intergroup comparison). |
| (at 12 months follow‐up) n = 82 | The median increase in CD4 count at 12 months was 279 cells/mm³ in the IL‐2 group compared with 50 cells/mm³ in the control (P < 0.001). |
| n = 81 | The mean per cent increase in CD4 cell counts was 24.5% for IL‐2 recipients compared to a mean per cent decrease of 30.5% for control participants (P < 0.005). |
| (at 12 months follow‐up) n = 64 | The median CD4 cell counts increased from 363 to 485 (+ 33.6% standard deviation) in the IL‐2 group Group A (P < 0.01) and from 358 to 462 (+ 29.1%) in Group B (P < 0.01) and from 350 to 375 (+ 6.9% in the control group (not significant), respectively. |
| (at 24 weeks, that is 6 months follow‐up) n = 72 | The median increase in CD4 cells at week 24 was significantly higher in the IL‐2 group than in the control group (65 versus 18 cells/mm³; P < 0.0001). |
| (at 12 months follow‐up) n = 60 | There was an increase in the mean (± SE) CD4 count from 428 ± 25 cells/mm³ at baseline to 916 ± 128 in the IL‐2 group, compared to a decreased from 406 ± 29 cells/mm3 to 349 ± 41 cells/mm³ in the control group (P < 0.001). |
| (at 26 weeks follow‐up) n = 115 | The percentage increase in CD4 count from baseline of 3.59% in the IL‐2 group compared to 1.33% in the control group (P < 0.001). |
| (at 56 weeks follow‐up) n = 94 | The median increase in CD4 count from baseline at 56 weeks was 564 cells/mm³ (P > 0.0001), 105 cells/mm³ (P = 0.58), and 676 cells/mm³ (P = 0.0002) in the subcutaneous (SC), polyethylene glycol modified, and intravenous (IV) IL‐2 group respectively, compared to 55 cells/mm³ in the control group |
| (at 74 weeks follow‐up) n = 118 | The median increase in CD4 count from baseline at week was 865 cells/mm³ in the IL‐2 group compared to 262 cells/mm³ in the control group (P < 0.00001). |
| (at 24 weeks follow‐up) n = 73 | The mean increase in CD4 count from baseline at week 24 of 27 cells/mm³ (P = 0.105), 105 cells/mm³ (P = 0.006), and 492 cells/mm³ (P < 0.001) in the 1.5, 4.5, and 7.5 miu dose groups of IL‐2. Overall 14 out of 36 (41%) of the IL‐2 group and 3 out of 37 (8%) of the controls had a magnitude increase of ≥ 1000 cells/mm³. |
| (48 weeks follow‐up) n = 22 | IL‐2 treated participants had mean absolute CD4 T cell counts (S.E) significantly increase at the end of the IL‐2 treatment (week 48) from 147 (18) cells/mm³ at baseline to 298 (43.3) cells/mm³ (P=0001). The control participants also had a significant increase was observed 16 weeks 228 (29) cells/mm³ (P = 0.002). |
| (at 48 weeks follow‐up) n = 159 | Reported median increases of CD4 cell count were 459, 312, and 102 cells/mm³ in the intravenous, SC Il‐2, and control groups respectively at 48 weeks (P < 0.001 for both). |
| (at 12 months follow‐up) n = 19 | Reported a mean increase in CD4 count from baseline of 452 cells/mm3 in the IL‐2 group compared to 135 cells/mm3 in the control group (P < 0.05). |
| n = 82 | Reported an increase in the time weighted mean CD4 cell count 252 x 106 cells/mm³ over 24 weeks for the overall scIL‐2 group compared with 42 x 106 cell/mm³. |
| Increase in CD4 cell count but statistical significance not reported in the trials | |
| (at 12 months follow‐up) (at 6 years follow‐up) n = 4111 | Six trials measured at 1 year; median CD4 increase of 206 versus 21 cells/mm³ in the IL‐2 group versus the control group and reported an average median increase of 109 more in the IL‐2 group more than the control group over the entire 7 years (95% CI 40 to 60 over 6 years) |
| (at 12 months follow‐up) n = 1695 | Six trials measured at 1 year; median CD4 increase of 131 versus 32 cells/mm³ in the IL‐2 group versus the control group over 12 months and reported an average median increase of 53 more in the IL‐2 group more than the control group over the entire 7 years (95% CI 40 to 60 over 6 years) |
| Dybul 2002 (at 12 months follow‐up) n = 9 | Four participants treated with HAART plus 3 cycles of intermittent IL‐2 had an increase in median absolute CD4+ T cell count from 529 cells/mm³ (range: 502 to 738 cells/mm³) at enrolment to 1995 cells/mm³ (range: 1112 to 3064 cells/mm³; 268% increase) after 12 months of treatment (Figure 1A). Five participants treated with HAART alone had an increase in median CD4+ T cell count from 580 cells/mm³ (range: 416 to 662 cells) at enrolment to 712 cells/mm³ (range: 667 to 1160 cells/mm³; 52% increase) after 12 months of treatment |
| No significant increase in CD4 cell count | |
| (at 24 weeks) n = 115 | Mean change in CD4 count in the IL‐2 group and the control group was 40 and −1 respectively |
| n = 61 | Reports that there was a progressive increase in circulating CD4 cells, determined at the beginning of each IL‐2 cycle, was observed in all participants receiving ART plus IL‐2, in comparison with those receiving ART alone but gave the values for the within subgroup variation |
| (at 6 months follow‐up) n = 22 | No significant difference between changes in CD4 counts in both groups |
| Abbreviations: ART: antiretroviral therapy; ESPIRIT: Evaluation of Subcutaneous Proleukin in a Randomised International Trial; IL‐2: interleukin‐2; SILICAAT: subcutaneous recombinant human interleukin‐2 in HIV‐infected patients low CD4 counts under active antiretroviral therapy | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 6 | 6565 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 1.1 ART experienced | 2 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 7.11] |
| 1.2 ART naive or not specified | 4 | 5939 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 2 HIV RNA levels < 50 cells/mL Show forest plot | 5 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.15] |
| 3 HIV RNA levels < 500 cells/mL Show forest plot | 4 | 5929 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.12] |
| 4 Opportunistic infections Show forest plot | 7 | 6141 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.13] |
| 4.1 ART experienced | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.86] |
| 4.2 ART naive or not specified | 5 | 6044 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.56, 1.19] |
| 5 Adverse events (grade 3 or 4) Show forest plot | 6 | 6291 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.10, 1.96] |






