Tipos de trócar en la laparoscopia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, 16 surgeons, randomised, double‐blinded study Study duration: undefined | |
| Participants | 250 adults were enrolled, age not reported, sex not reported, BMI or weight not reported 119 participants included in the radially expanding (STEP) trocar group and 125 in the conventional cutting trocar group Type of procedure/setting: elective laparoscopic: cholecystectomy (86 participants), hernia (59), fundoplication (57), colectomy (13), other (29). At tertiary care centres and community hospitals in San Francisco, US Exclusion criteria: acute inflammatory conditions and conversion to laparotomy not related to a trocar‐related complication | |
| Interventions | Intervention group: primary (and secondary) entry with radially expanding trocars (Step, Innerdyne, Inc., Sunnyvale, USA), diameter not reported Control group: primary (and secondary) entry with conventional disposable cutting trocars (US Surgical Corp. Norwalk, Connecticut USA and Ethicon Endo‐Surgery, Cincinnati, USA or Origin Inc., Sunnyvale USA), diameter not reported Technique/type of surgeons: insertion of the first port after establishing a pneumoperitoneum with the use of a standard Veress needle and inserting the device using the blind technique. 16 different general surgeons Closure of fascial defects: defects created by conventional cutting trocars ≥ 10 mm were closed unless they were too small to be found. Any defect large enough to accommodate the tip of the surgeon's little finger was closed. Defects created by the radially expanding trocars were not closed unless they met this criterion Analgesics: not recorded | |
| Outcomes | Visceral injury, intraoperative Vascular injury, intraoperative Trocar site bleeding, intraoperative Trocar site herniation, 6‐18 months' follow‐up Wound haematoma, 4 and 24 hours postoperative Continued wound bleeding, 4 and 24 hours postoperative Incisional pain, 4, 8, 12 and 24 hours postoperative | |
| Length of follow up | 6‐18 months, not further specified | |
| Funding source | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Low risk | Assignment of participants to either the radially expanding trocar group or the conventional cutting trocar group was carried out at the time of surgery by drawing consecutive sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to the choice of trocar used in the operations |
| Blinding of outcome assessment (detection bias) | Low risk | Postoperative observers were blinded to the choice of trocar used in the operations |
| Incomplete outcome data (attrition bias) | High risk | In the outcome table for intraoperative complications in the STEP group approximately 6% and in the conventional group approximately 3% of data were missing For the outcome, pain, completeness of data was unclear. For the outcome, wound complications (haematoma and continued bleeding) at follow‐up, 4‐hour data of approximately 22% of participants were missing and 24‐hour data approximately 33% |
| Intention‐to‐treat analysis | Low risk | 6 excluded participants were randomised into the radially expanding trocar group. These participants are excluded for reasons other than trocar‐related complications. The data were analysed as randomised |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. No protocol was published. Endpoints in results section are according to those in methods section |
| Group similarity at baseline (selection bias) | Low risk | The groups were similar with regard to age, sex and type of procedure. Adequate randomisation and allocation concealment, no exclusion of participants leading to imbalance |
| Co‐interventions (performance bias) | High risk | Fascial defects created by conventional trocars ≥ 10 mm were closed unless they were too small to be found. Any defect large enough to accommodate the tip of the surgeon's little finger was closed. Defects created by the Step trocars were not closed unless they met this criterion. In the Step group 3% of participants had fascial defects that needed to be closed, compared to 93% in the conventional group Port manipulation was unclear (material extraction or morcellation) The different type of procedures and 16 participating surgeons Different analgesia protocols were used, specification not reported |
| Timing of outcome assessment (detection bias) | Low risk | The outcome assessment for intraoperative complications, postoperative haematoma, persistent bleeding and pain was identical for both intervention groups until 24 hours postoperative. The timing, method of assessment and completeness for trocar site herniation is unclear and varies between 6 and 18 months |
| Methods | Single centre, randomised, double‐blind study. Number of surgeons unclear Study duration: from April 2003 to May 2004 | |
| Participants | 77 adults; median age: study group 47 years, control group 48 years; median BMI: study group 24, control group 25; male : female ratio: study group 35 : 3, control group 28 : 11 38 participants were included in the radially expanding trocar group and 39 participants were included in the conventional cutting trocar group Type of procedure/setting: elective laparoscopic cholecystectomy in a semi‐ambulatory unit in an university hospital Gentofte Hospital, Denmark Exclusion criteria: ASA 4, aged < 18 and > 75 years, pregnancy, chronic pain diseases other than gallstone, use of opioids or tranquillisers (for > 1 week before surgery), foreign language, mental disorder, history of alcoholism or drug abuse and conversion to laparotomy | |
| Interventions | Study group: primary and secondary entry with radially expanding trocars (VersaStep system, Tyco Healthcare, Copenhagen, Denmark) Control group: primary and secondary entry with conventional cutting trocars (Endopath II, Ethicon Endosurgery, Inc, Cincinnati, USA). Disposable Technique/type of surgeons: laparoscopic cholecystectomy was performed using 2 x 10‐mm and 2 x 5‐mm trocars. Gallbladder was retracted via the umbilical 10‐mm trocar port incision. The closed entry technique was used. Operations were conducted or supervised by experienced laparoscopic surgeons, equally distributed between the 2 surgical groups Closure of fascial defects: fascia at the umbilical port incision was closed using a resorbable suture Analgesics: all participants received a similar general anaesthesia, incisional local aesthetics were given and postoperative standard analgesics were given | |
| Outcomes | Trocar site bleeding, intraoperative Trocar site herniation, up to 3 year Postoperative wound haematoma, postoperative day 2 Incisional pain during mobilisation (overall, not at individual ports) 6, 24 and 48 hours postoperatively (primary); VAS and VRS | |
| Length of follow up | 30 days' and 3 years' follow‐up via the electronic national hospital data register and manual check of hospital files. Median follow‐up was 39 months (range 33‐46 months) | |
| Funding source | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Based on block‐randomised computer‐generated list. After introduction of anaesthesia, the surgeon randomised the participants to laparoscopic cholecystectomy using radially expanding trocar or conventional cutting trocars |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method. The randomisation code was kept separate from the investigators in a lockup, and the randomisation sequence was concealed until data analysis was completed |
| Blinding of participants and personnel (performance bias) | Low risk | The participants were blinded to the type of trocar used. At the end of the operation, the incisions were covered with non‐transparent standard dressing and the participants were instructed to keep the dressings on for the first 2 postoperative days |
| Blinding of outcome assessment (detection bias) | Low risk | The surgical staff, including the nurses, was blinded to the type of trocar used. The operating surgeon and the anaesthesiologist in charge did not participate in the postoperative assessment and did not attend to the participants |
| Incomplete outcome data (attrition bias) | Low risk | 3 exclusions from trial. "One of the three excluded patients underwent conversion to an open procedure (radial group) and the remaining two patients (one from each group) had no study data available due to loss of their study diary." The number of 3 out of 77 participants loss for short‐term follow‐up would not lead to a substantial bias. It remains unclear what the numbers were for follow‐up at 30 days for complications, and for the 3 years of evaluation |
| Intention‐to‐treat analysis | Low risk | All randomised participants are analysed in the group they were allocated to |
| Selective reporting (reporting bias) | Unclear risk | Not referred to. No protocol was published. Endpoints in results section were according to those in methods section |
| Group similarity at baseline (selection bias) | Low risk | Groups did not significantly differ for age, BMI, ASA classification and the regimen of general anaesthesia. The sex ratio was significantly different: more women in the study group. Preoperative pain scores were equal for both groups |
| Co‐interventions (performance bias) | High risk | In the radially expanding trocar group, significantly more participants (23/38) needed an additional incision to retract the gallbladder compared to the cutting group (11/39). All participants received standardised anaesthetic and analgesic treatment. There were no significant differences in total opioid requirements |
| Timing of outcome assessment (detection bias) | Low risk | The outcome assessment was identical for both groups until 48 hours postoperative. The timing of assessment for trocar site herniation was unclear and varied between 33 and 46 months. Participants were not clinically examined for trocar herniation during the follow‐up |
| Methods | Multicentre, 7 surgeons, randomised, double‐blind study Study duration: April 1996 to January 1997 | |
| Participants | 87 women, aged 18‐54 years, BMI or weight not reported 45 participants were randomised in the radially expanding (REA) trocar group and 42 participants were randomised in the conventional cutting trocar group Type of procedures/setting: various (22 participants) operative and diagnostic laparoscopic procedures in 2 hospitals (Germany and the US) Exclusion criteria were not reported | |
| Interventions | Intervention group: primary and secondary entry with radially expanding trocars (Step, InnerDyne, Inc., Sunnyvale, USA), diameter not specified Control group: primary and secondary entry with either disposable or non‐disposable conventional cutting trocars, diameter not specified Technique/type of surgeons: primary and secondary ports were created with REA or conventional cutting trocars. Probably the closed technique was used. 7 different surgeons well trained, and 1 year' experience with REA Closure of fascial defects: defects created by conventional cutting trocars ≥ 10 mm were closed. Defects created by the radially expanding trocars were intended not to be closed: it was up to the surgeon Analgesics: type and amount of postoperative analgesics were not recorded | |
| Outcomes | Visceral injury, intraoperative Vascular injury, intraoperative Trocar site bleeding, intraoperative, 4 and 24 hours postoperative Incisional pain, 4, 8, 12 and 24 hours postoperative | |
| Length of follow up | 24 hours | |
| Funding source | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not referred to means of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not referred to allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded as to which type of instrument was used |
| Blinding of outcome assessment (detection bias) | Low risk | A blinded observer assessed the operative wounds at 4 and 24 hours postoperatively |
| Incomplete outcome data (attrition bias) | Unclear risk | The number of participants who were included in the study was unclear. The number of evaluated participants was recorded. But unclear if all observations are complete |
| Intention‐to‐treat analysis | Low risk | Participants' outcomes were analysed as randomised |
| Selective reporting (reporting bias) | Unclear risk | Not referred to. Unknown if all the results from all pre‐specified outcomes were adequately reported. Insufficient information |
| Group similarity at baseline (selection bias) | High risk | Unclear method of randomisation and allocation concealment. There was a significant difference in the BMI and in the mean weights for the 2 groups, with participants in the radially expanding trocar group having lower weights. Pain scores were not measured before surgery |
| Co‐interventions (performance bias) | High risk | All conventional trocar sites ≥ 10 mm or larger were closed (100% sutured), full or partial thickness as required. In contrast, all but 2 of the ≥ 10 mm Step device sites were left unsutured (4, 17% sutured) Type and frequency of port manipulation (material extraction or morcellation) was unclear. "One of the two Step defects requiring closure resulted from the enlargement of the defect to allow passage of a bag containing a dermoid cyst." This was not clearly reported for both groups Type and amount of postoperative analgesics given were not recorded |
| Timing of outcome assessment (detection bias) | Low risk | Outcome assessment identical for both intervention groups |
| Methods | Single centre, randomised, non‐blinded study. Number of surgeons unclear Study duration: undefined | |
| Participants | 30 adults, median age: cutting group 45 years, blunt group 42 years; median BMI: cutting group 27 kg/m2, blunt group 29 kg/m2, male : female ratio: cutting group 2 : 3, blunt group 1 : 2 15 participants were randomised in the cutting trocar group and 15 participants were randomised in the blunt‐tipped trocar group Type of procedure/setting: laparoscopic procedures including cholecystectomy (14 participants), Nissen fundoplication (5), staging laparoscopy (4), gastrojejunostomy (3), others (4). Setting not clearly described Exclusion criteria: not reported | |
| Interventions | Intervention group: secondary port entry using 5 and 10 mm reusable conical blunt‐tipped metal trocars (Mantis Surgical Ltd, Newbury, UK) Control group: secondary port entry using 5 and 10 mm reusable cutting metal trocars with 3 sharp fixed blades (Mantis Surgical Ltd, Newbury, UK) Technique/type of surgeons: primary port insertion was accomplished using direct a blunt‐tipped trocar at a site other than the umbilicus and without insufflation. Experience of surgeon(s) unclear. A device, to apply traction force to displace the port, was attached to the port Closure of fascial defects: not reported Analgesics: general anaesthesia, postoperative analgesia not recorded | |
| Outcomes | Trocar site bleeding, intraoperative | |
| Length of follow up | No follow‐up, only intraoperative measures | |
| Funding source | Not reported | |
| Notes | Primary outcomes of this study were port fixity, friction forces and port dislodgement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list, produced by independent statistician. Entry of a participant into the randomisation process was initiated after consent had been obtained and the participant had been given a general anaesthetic and brought into the theatre for surgery |
| Allocation concealment (selection bias) | Low risk | An independent person produced envelopes containing the number of the study participant and a card labelled 'blunt' or 'sharp'. The envelopes were sealed and placed in the operating theatre |
| Blinding of participants and personnel (performance bias) | Low risk | Not applicable. The surgeon was not blinded to the type of trocar. This will probably not have had influence on trocar site bleeding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not referred to. Unclear if the surgeon's assessment for port site bleeding could have been influenced by being unblinded |
| Incomplete outcome data (attrition bias) | Low risk | None. No per‐operative drop‐out |
| Intention‐to‐treat analysis | Low risk | All randomised participants were analysed in the group they were allocated to by randomisation |
| Selective reporting (reporting bias) | Unclear risk | Protocol was published online on 29 September 2006, after the projected finish of the study. Protocol described inclusion of 2 x 25 participants, while 2 x 15 participants were randomised. No explanation was given in the article |
| Group similarity at baseline (selection bias) | Low risk | The 2 groups were comparable for age, gender, BMI and operating time |
| Co‐interventions (performance bias) | Unclear risk | In the blunt trocar group, more trocars were applied: 63 ports in the blunt group and 51 in the cutting group. In all participants, generous skin incisions were made to ensure a loose fit of the skin around the port so that the anchorage of the port to the abdominal wall could be solely attributed to the fascial and muscular layers of the anterior abdominal wall. The anaesthetist ensured full muscle relaxation during the procedure Type and frequency of port manipulation (material extraction or morcellation) is unclear |
| Timing of outcome assessment (detection bias) | Low risk | Trocar site bleeding was assessed intraoperative, no postoperative follow‐up |
| Methods | Single centre, randomised, single‐blind study. Number of surgeons unclear Study duration: July 1997 to September 1998 | |
| Participants | 54 adults, mean age: study group 55.1 years, control group 57.8 years; male : female ratio 35 : 19; BMI or weight not reported 23 participants in the REA group and 31 participants in the conventional cutting trocar group had their data entry completed and returned for analysis Type of procedure/setting: laparoscopic cholecystectomy at the Department of Surgery, United Christian Hospital, Hong Kong Exclusion criteria: acute cholecystitis, known gallbladder malignancy | |
| Interventions | Intervention group: secondary epigastric port entry with a 10 mm epigastric transverse skin incision followed by introduction of a 10 mm radially expanding trocar (Step, Innerdyne, Inc., Sunnyvale, USA) Control group: secondary epigastric port entry with a 10 mm epigastric transverse skin incision followed by introduction of a 10 mm conventional metal cutting trocar Technique: Hasson technique entry, the operation was performed with a standardised technique with 4 trocars. Gallbladder recovered through the epigastric port. Experience of surgeons unclear Closure of fascial defects: not recorded Analgesia: oral dologesic (paracetamol/phenyltoloxamine) on demand up to 4 times, intake not recorded | |
| Outcomes | Conversion rate, unspecified whether trocar related or not, intraoperative Trocar site bleeding, timing and method of assessment unspecified Trocar site infection, timing and method of assessment unclear Incisional pain, 24, 48 and 72 hours postoperative | |
| Length of follow up | Up to 72 hours for the outcome, pain. Undefined for other outcomes | |
| Funding source | All STEP™ trocars used in this study were supplied free of charge by Kojima Healthcare Asia Ltd | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | An erroneous duplication of a randomisation envelope led to the inclusion of 1 more case in the control group. Not reported how assignment was generated |
| Blinding of participants and personnel (performance bias) | Low risk | The participants were blinded to the type of epigastric trocar used for their surgery |
| Blinding of outcome assessment (detection bias) | Low risk | Physicians not performing the surgery served as independent observers to measure pain experience by the participants. Participants were blinded |
| Incomplete outcome data (attrition bias) | High risk | In the study group, 23/30 (77%) participants had their data entry completed and returned for analysis. All 31 participants of the control group had their results available for analysis. Unclear how missing values were dealt with and the conversion rates (10% conversion rate in the control group and 9% in the study group) |
| Intention‐to‐treat analysis | Low risk | Intention‐to‐treat analysis was applied |
| Selective reporting (reporting bias) | Unclear risk | Unclear if all the results from all pre‐specified outcomes were adequately reported. Insufficient information. No protocol was published. Endpoints in results section were according to those in methods section |
| Group similarity at baseline (selection bias) | Low risk | Variables used to check for balanced randomisation included the participant's age, sex, diagnosis, operation time, conversion rate and subumbilical wound pain. Subumbilical wound pain was measured in addition to epigastric wound pain as a test for case randomisation |
| Co‐interventions (performance bias) | Unclear risk | Operation was performed with a standardised technique. The gallbladder recovered through the epigastric port. Fascial closure management was unclear |
| Timing of outcome assessment (detection bias) | Low risk | Outcome assessment for pain was identical for both intervention groups |
| Methods | Multicentre (2 centres), randomised, double‐blind study. 3 surgeons Study duration: July 2005 to March 2006 | |
| Participants | 100 women; age, BMI and weight not reported 49 participants were randomised into treatment with radially expanding trocars, 51 participants were randomised into treatment with conventional cutting trocars Type of procedure/setting: elective laparoscopic benign gynaecological surgical procedures in 2 centres: University Hospital of Kiel, Germany and Mercy Hospital for Women, Victoria, Australia Exclusion criteria: acute inflammatory conditions | |
| Interventions | Intervention group: primary and secondary entry with radially expanding trocars (Step, Innerdyne, Inc., Sunnyvale USA), diameter not specified Control group: primary and secondary entry with reusable conventional cutting trocars, diameter not specified Closed entry technique Technique/type of surgeons: conventional conic trocars were introduced in a Z‐wise fashion not further specified. Experience of the surgeons unclear Closure of fascial defects: participants for whom regular trocars were used had 10 mm trocar sites closed, as opposed to the Step defects, which were 50% smaller and therefore did not require closure Analgesics: neither group was any more likely than the other to use analgesics at any time postoperatively | |
| Outcomes | Visceral injury, intraoperative Vascular injury, intraoperative Trocar site bleeding, intraoperative Trocar site herniation, up to 12 months Pain at 4, 8, 12, 24, 48 and 72 hours postoperative | |
| Length of follow up | 12 months, completeness not specified | |
| Funding source | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not referred to allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | All participants were blinded as to which type of access instrument was used |
| Blinding of outcome assessment (detection bias) | Low risk | A trained observer was blinded as to which type of access instrument was used |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up until 72 hours postoperative of exclusions stated. For long‐term outcome (trocar site herniation, the completeness of follow‐up was unclear |
| Intention‐to‐treat analysis | Low risk | Data are analysed as randomised. |
| Selective reporting (reporting bias) | Unclear risk | Remains unknown if all the results from all pre‐specified outcomes were adequately reported. Insufficient information. No protocol was published. Endpoints in results section were according to those in methods section |
| Group similarity at baseline (selection bias) | Unclear risk | Not referred to |
| Co‐interventions (performance bias) | High risk | All participants for whom regular trocars were used had > 10 mm trocar sites closed, as opposed to the Step sites, which were 50% smaller and did therefore not require closure. Type and frequency of port manipulation (material extraction or morcellation) is unclear. Analgesics use was unclear |
| Timing of outcome assessment (detection bias) | Low risk | Outcome assessment identical for both intervention groups. The timing, method of assessment and completeness for trocar site herniation is unclear |
| Methods | Single centre, randomised, 4 arm, double‐blind study. Number of surgeons unclear Study duration: undefined | |
| Participants | 56 adults, 30 men and 26 women, mean age: 58 years, mean BMI: 31.3 14 participants randomised in each arm (total 56 participants) to receive 1 of the 4 types of 12‐mm study trocars a pyramidal‐bladed, single‐bladed, axially dilating and radially dilating trocar. 165 trocar sites for evaluation in the study including 43 pyramidal‐bladed, 41 single‐bladed, 38 axially dilating and 43 radially dilating trocar sites Setting: US Type of surgery: laparoscopic transperitoneal renal procedures, flank approach. The procedures included radical or total nephrectomy (36 participants), nephron‐sparing surgery (9), pyeloplasty (8) and renal cyst decortication (3) Exclusion criteria: not reported | |
| Interventions | Pyramidal‐bladed group: disposable pyramidal‐bladed trocars (Ethicon Inc., Cincinnati, OH) Single‐bladed group: disposable single‐bladed trocars (Ethicon Inc., Cincinnati, OH) Axially dilating group: disposable axially dilating trocars (Ethicon Inc., Cincinnati, OH) Radially dilating group: disposable radially dilating trocars (US Surgicals Inc., CA) Technique/type of surgeons: all trocars were inserted after pneumoperitoneum was established with a Veress needle. A standardised lateral 5 mm, non‐cutting, metal trocar was placed in each participant. Trocars were placed in a standard 'diamond' configuration: 3 x 12‐mm study trocars and 1 lateral 5‐mm trocar that served as a reference point for normalising participant's pain scores. Experience of surgeons unclear The morcellation site, specimen extraction and hand‐assist device site location when used were documented Closure of fascial defects: performed for single‐bladed and pyramidal‐bladed trocar sites by using a Carter‐Thomason closure device. Radially dilating and axially dilating trocar sites were not routinely closed unless frequent dislodgment of the trocar occurred Analgesics: not reported | |
| Outcomes | Visceral injury, intraoperative Vascular injury, intraoperative Trocar site herniation, 1 week; 3, 6, 12 and 18 months; during clinic visit or telephone interview Trocar site bleeding, intraoperative, 3 and 24 hours postoperative Wound infection, postoperative but unclear which time intervals Postoperative wound haematoma, 3 and 24 hour postoperative Pain at 3 hours, 24 hours, 1 week and 3 months postoperatively | |
| Length of follow up | Follow‐up for trocar site hernias was 18 months (range 14‐36) | |
| Funding source | The manufacturing companies supplied all the trocars used in this study. There was no financial assistance provided by any company | |
| Notes | Normalisation of pain scores was performed by calculating the mean pain score for lumbar trocar site and normalising it to the 5 mm lateral port pain score for each individual participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not referred to |
| Allocation concealment (selection bias) | Unclear risk | Not referred to |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | A physician who did not perform or assist the operation assessed the trocar sites for pain, bleeding and ecchymoses at 3 and 24 hours postoperative |
| Incomplete outcome data (attrition bias) | Unclear risk | No exclusions or loss to follow‐up stated |
| Intention‐to‐treat analysis | Low risk | Ports were analysed as randomised. There are issues on 'unit‐of‐analysis': the number of observations in the analysis (= number of ports) did not match the number of 'units' that were randomised (number of participants) |
| Selective reporting (reporting bias) | Unclear risk | Not referred to. No protocol was published. Endpoints in results section were according to those pre‐specified in methods section. |
| Group similarity at baseline (selection bias) | Unclear risk | "Mean body mass index was 31.3 (range 20 to 62) and was similar among all 4 trocar study groups." Other baseline characteristics were not compared |
| Co‐interventions (performance bias) | High risk | The location of the primary entry port was not standard. Closure of the fascial layer was not performed with the expanding trocars on 82% of occasions. The fascial layer of the expanding trocar sites was closed on 6 occasions, and all the cutting trocar sites were closed. It was unclear how they dealt with the morcellation, hand‐assistance and specimen extraction sites. Analgesics use was not reported |
| Timing of outcome assessment (detection bias) | Unclear risk | Follow‐up evaluations were performed at 1 week and 3 months for pain and trocar site hernia. Physical examination specifically evaluating for the presence of trocar site hernia was carried out during the clinic visit by the attending physician or by a telephone interview at 6, 12 and 18 months. The range in follow‐up was 14‐36 months. Therefore, detection bias in the long term was possible. Low risk for short‐term endpoints |
ASA: American Society of Anaesthesiologists; BMI: body mass index; REA: radially expanding access; VAS: visual analogue scale; VRS: visual rating scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Non‐randomised study | |
| Non‐randomised study | |
| Randomisation of different sites of the body/abdomen to different trocars. 'Split‐mouth' design | |
| Study tried both trocar type and trocar entry method at same time | |
| Study evaluated intervention time and the duration of interruption of the intervention for correction of trocars. Trocar‐related complications or postoperative pain were not studied | |
| Randomisation of different sites of the body/abdomen to different trocars. 'Split‐mouth' design |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 1 Visceral injury. | ||||
| 2 Vascular injury Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 2 Vascular injury. | ||||
| 3 Trocar site herniation Show forest plot | 4 | 463 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.3 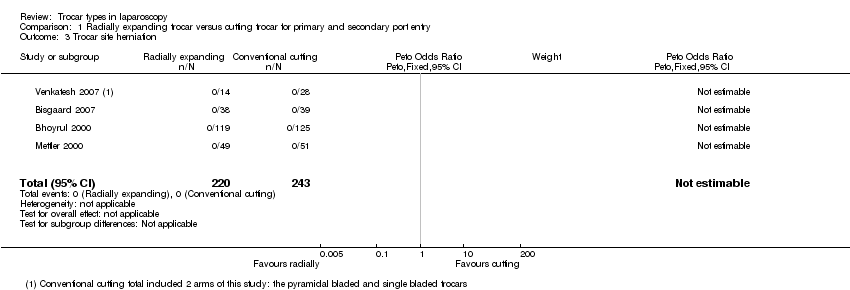 Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 3 Trocar site herniation. | ||||
| 4 Trocar site bleeding Show forest plot | 5 | 553 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.14, 0.54] |
| Analysis 1.4  Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 4 Trocar site bleeding. | ||||
| 5 Trocar site haematoma Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 5 Trocar site haematoma. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 1 Visceral injury. | ||||
| 2 Vascular injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 2 Vascular injury. | ||||
| 3 Trocar site herniation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 3 Trocar site herniation. | ||||
| 4 Trocar site bleeding, intraoperative Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 4 Trocar site bleeding, intraoperative. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Radially expanding trocar versus conical blunt‐tipped trocar for secondary port entry, Outcome 1 Visceral injury. | ||||
| 2 Vascular injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2 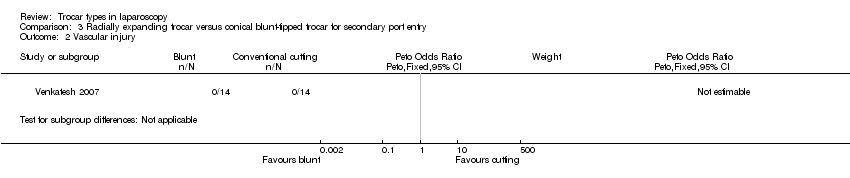 Comparison 3 Radially expanding trocar versus conical blunt‐tipped trocar for secondary port entry, Outcome 2 Vascular injury. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1 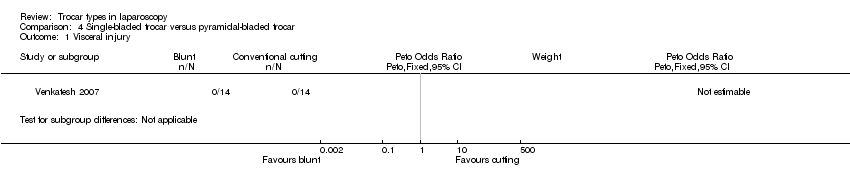 Comparison 4 Single‐bladed trocar versus pyramidal‐bladed trocar, Outcome 1 Visceral injury. | ||||
| 2 Vascular injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2 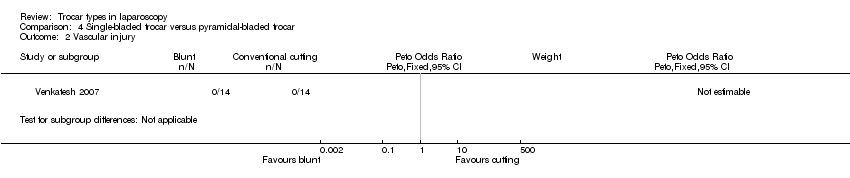 Comparison 4 Single‐bladed trocar versus pyramidal‐bladed trocar, Outcome 2 Vascular injury. | ||||
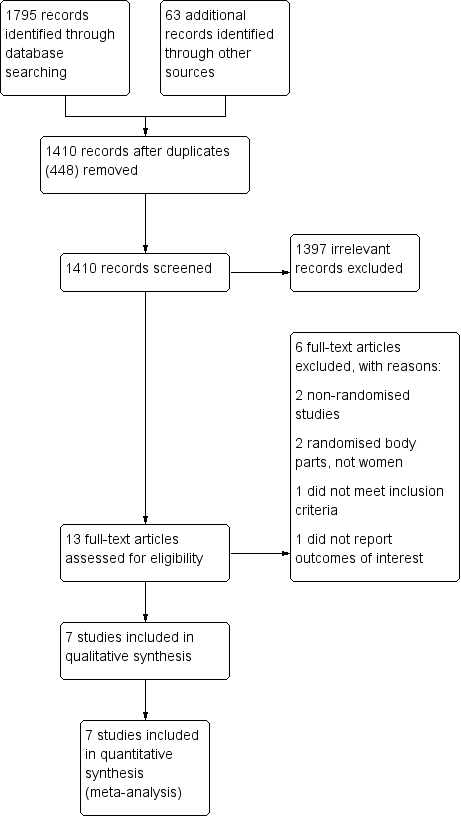
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, outcome: 1.3 Trocar site herniation.
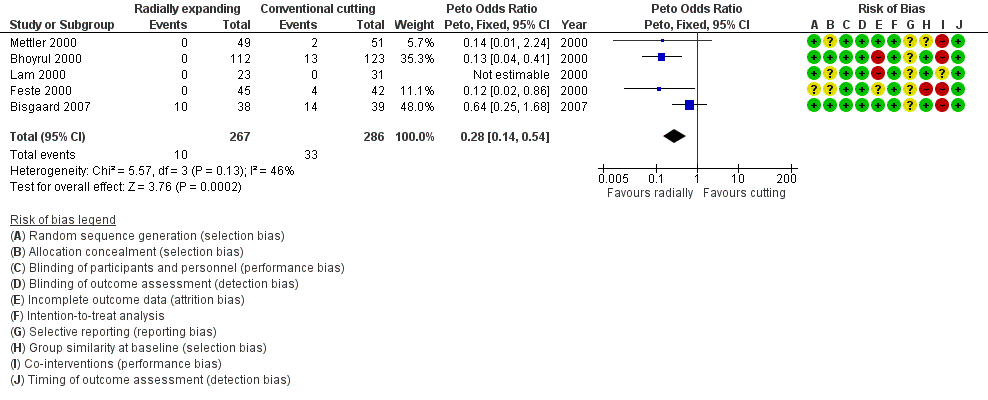
Forest plot of comparison: 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, outcome: 1.4 Trocar site bleeding.

Forest plot of comparison: 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, outcome: 1.5 Trocar site haematoma.

Forest plot of comparison: 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, outcome: 2.1 Visceral injury.

Forest plot of comparison: 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, outcome: 2.2 Vascular injury.
Forest plot of comparison: 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, outcome: 2.3 Trocar site herniation.
Forest plot of comparison: 3 Radially expanding trocar versus conical blunt‐tipped trocar for secondary port entry, outcome: 3.1 Visceral injury.

Forest plot of comparison: 3 Radially expanding trocar versus conical blunt‐tipped trocar for secondary port entry, outcome: 3.2 Vascular injury.
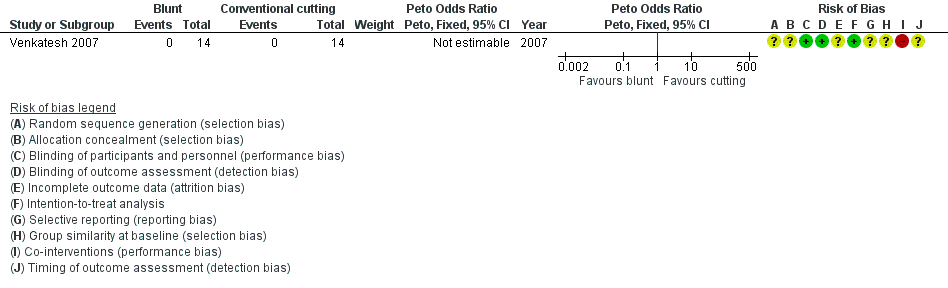
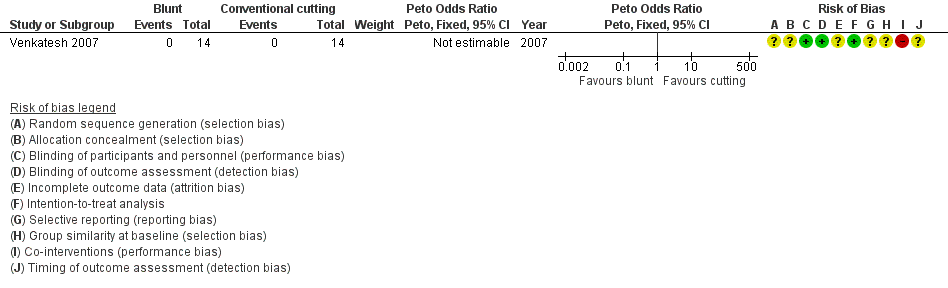
Forest plot of comparison: 4 Single‐bladed trocar versus pyramidal‐bladed trocar, outcome: 4.1 Visceral injury.
Forest plot of comparison: 4 Single‐bladed trocar versus pyramidal‐bladed trocar, outcome: 4.2 Vascular injury.

Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 1 Visceral injury.

Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 2 Vascular injury.

Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 3 Trocar site herniation.

Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 4 Trocar site bleeding.
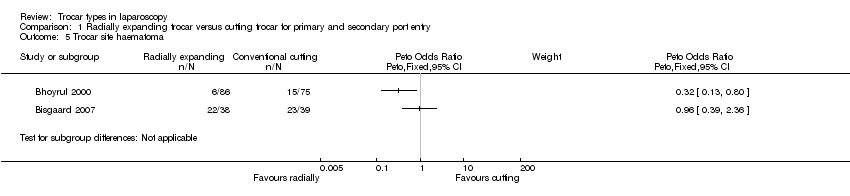
Comparison 1 Radially expanding trocar versus cutting trocar for primary and secondary port entry, Outcome 5 Trocar site haematoma.

Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 1 Visceral injury.
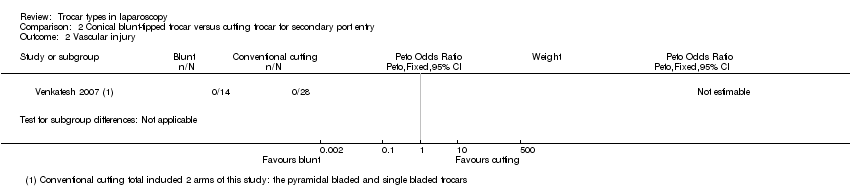
Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 2 Vascular injury.
Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 3 Trocar site herniation.
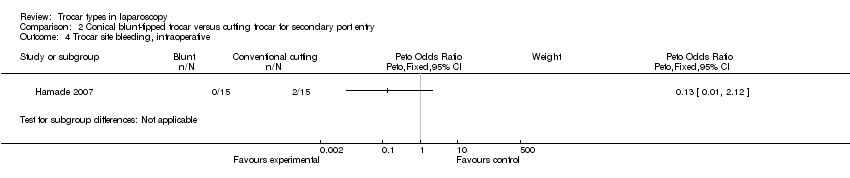
Comparison 2 Conical blunt‐tipped trocar versus cutting trocar for secondary port entry, Outcome 4 Trocar site bleeding, intraoperative.

Comparison 3 Radially expanding trocar versus conical blunt‐tipped trocar for secondary port entry, Outcome 1 Visceral injury.

Comparison 3 Radially expanding trocar versus conical blunt‐tipped trocar for secondary port entry, Outcome 2 Vascular injury.
Comparison 4 Single‐bladed trocar versus pyramidal‐bladed trocar, Outcome 1 Visceral injury.
Comparison 4 Single‐bladed trocar versus pyramidal‐bladed trocar, Outcome 2 Vascular injury.
| Radially expanding trocars compared to cutting trocars for laparoscopy | ||||||
| Patient or population: people undergoing laparoscopy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cutting trocars | Radially expanding trocars | |||||
| Visceral injury | 4 per 1000 | 4 per 1000 | OR 0.95 | 473 | ⊕⊝⊝⊝ | ‐ |
| Vascular injury | 4 per 1000 | 1 per 1000 | OR 0.14 | 473 | ⊕⊝⊝⊝ | ‐ |
| Trocar site herniation | No events reported | No events reported | Not estimable3 | 463 | ⊕⊝⊝⊝ | ‐ |
| Trocar site bleeding | 115 per 1000 | 35 per 1000 | OR 0.28 | 553 | ⊕⊝⊝⊝ | ‐ |
| Trocar site haematoma5 | See comment5 | See comment5 | Not estimable5 | 238 | ⊕⊝⊝⊝ | ‐ |
| Postoperative pain6 | See comment6 | See comment6 | Not estimable6 | 306 | See comment6 | ‐ |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to high risk of attrition bias. | ||||||
| Conical blunt‐tipped trocar compared to cutting trocar for laparoscopy | ||||||
| Patient or population: people undergoing laparoscopy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cutting trocar | Conical blunt‐tipped trocar | |||||
| Visceral injury | No events reported | No events reported | Not estimable1 | 42 | ⊕⊝⊝⊝ | ‐ |
| Vascular injury | No events reported | No events reported | Not estimable1 | 42 | ⊕⊝⊝⊝ | ‐ |
| Trocar site bleeding | 133 per 1000 | 20 per 1000 | OR 0.13 | 30 | ⊕⊝⊝⊝ | ‐ |
| Postoperative pain5 | See comment5 | See comment5 | Not estimable5 | 42 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No events reported. | ||||||
| Radially expanding trocar compared to conical blunt‐tipped trocar for laparoscopy | ||||||
| Patient or population: people undergoing laparoscopy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conical blunt‐tipped trocar | Radially expanding trocar | |||||
| Visceral injury | No events reported | No events reported | Not estimable1 | 28 | ⊕⊝⊝⊝ | ‐ |
| Vascular injury | No events reported | No events reported | Not estimable1 | 28 | ⊕⊝⊝⊝ | ‐ |
| Trocar site herniation | No events reported | No events reported | Not estimable1 | 28 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No events reported. | ||||||
| Single‐bladed trocar compared to pyramidal‐bladed trocar for laparoscopy | ||||||
| Patient or population: people undergoing laparoscopy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Pyramidal‐bladed trocar | Single‐bladed trocar | |||||
| Visceral injury | No events reported | No events reported | Not estimable1 | 28 | ⊕⊝⊝⊝ | ‐ |
| Vascular injury | No events reported | No events reported | Not estimable1 | 28 | ⊕⊝⊝⊝ | ‐ |
| Trocar site herniation | No events reported | No events reported | Not estimable1 | 28 | ⊕⊝⊝⊝ | ‐ |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No events reported. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2 Vascular injury Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3 Trocar site herniation Show forest plot | 4 | 463 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Trocar site bleeding Show forest plot | 5 | 553 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.28 [0.14, 0.54] |
| 5 Trocar site haematoma Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2 Vascular injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3 Trocar site herniation Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4 Trocar site bleeding, intraoperative Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2 Vascular injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Visceral injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2 Vascular injury Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |

