Лазерная субэпителиальная кератэктомия в сравнении с фоторефракционной кератэктомией в коррекции миопии
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009799.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 febrero 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Conceiving of and designing the review: Reza Yousefi‐Nooraie (original author), SML, SYZ, NLW
Data collection for the review
‐ Designing search strategies: Iris Gordon, CEV Trials Search Co‐ordinator
‐ Undertaking manual searches: SML, SYL
‐ Screening search results: SML, SYL, JH, HAL
‐ Organising retrieval of papers: Iris Gordon
‐ Screening retrieved papers against inclusion criteria: SML, JH
‐ Appraising risk of bias: SML, JH, HAL
‐ Abstracting data from papers: SML, HAL
‐ Writing to authors of papers for additional information: SML, HAL
‐ Managing data for the review: SML, HAL
‐ Entering data into RevMan: SML, HAL
Analysis of data: SML, HAL
Interpretation of data
‐ Providing a methodological perspective: HAL, SML
‐ Providing a clinical perspective: SML
Writing of the review: SML, HAL
Sources of support
Internal sources
-
Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing, China.
External sources
-
Grant from the Major State Basic Research Development Program of China (“973” Program, 2011CB504601) from the Ministry of Science and Technology, Beijing, China.
-
Grant from the Major International (Regional) Joint Research Project (81120108807) of the National Natural Science Foundation of China, from the Major State Basic Research Development Program, China.
-
2012 Beijing Nova Program (Z121107002512055), China.
-
Grant from China Postdoctoral Science Foundation (20110490247), China.
-
Research Foundation of Beijing Tongren Hospital Affiliated to Capital Medical University (2012‐YJJ‐019), China.
-
Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
-
Declarations of interest
The review authors declare no conflict of interest.
Acknowledgements
-
We would like to acknowledge the original review team that started this protocol: Reza Yousefi‐Nooraie, Hassan Hashemi, Hamid Foudazi, Mahammad Miraftab, and Farhad Rezvan.
-
We acknowledge Iris Gordon, Cochrane Eyes and Vision (CEV) Trials Search Co‐ordinator, for creating and executing electronic searches for this review.
-
We thank Marie Diener‐West, Colm McAlinden, and George Settas for comments to earlier versions of this review.
-
We thank Alex Shortt and Gerry Clare, the CEV Contact Editors for this review, for comments provided, and Anupa Shah, Managing Editor for CEV, for assistance provided throughout the review process.
-
We thank Jennifer Evans and Kristina Lindsley for comments and methodological support provided during the review process.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Feb 22 | Laser‐assisted subepithelial keratectomy (LASEK) versus photorefractive keratectomy (PRK) for correction of myopia | Review | Shi‐Ming Li, Siyan Zhan, Si‐Yuan Li, Xiao‐Xia Peng, Jing Hu, Hua Andrew Law, Ning‐Li Wang | |
| 2012 Apr 18 | Photorefractive keratectomy (PRK) versus laser‐assisted subepithelial keratectomy (LASEK) for myopia correction | Protocol | Shi‐Ming Li, Si‐Yan Zhan, Si‐Yuan Li, Xiao‐Xia Peng, Jing Hu, Ning‐Li Wang | |
Differences between protocol and review
In the protocol (Li 2012), we framed the comparison of interest as photorefractive keratectomy (PRK) versus laser epithelial keratomileusis (LASEK). Because PRK can be considered as the control intervention and LASEK as the experimental intervention, we analyzed data for this review as LASEK versus PRK.
We did not set out to assess other potential bias. However in the review, we assessed trials with industry funding and conflicts of interest as having high risk of other potential bias; conversely, we judged trials with no conflict of interest as having low risk of bias.
We did not reduce the effective sample size, as most included trials used a paired‐eye design. We stated in the protocol "if the unit of analysis was different from the unit of randomization (for example, analysis by eyes but randomization by individual), the trials were reduced to their effective sample size according to the formula 1+(M‐1) ICC, used to incorporate a cluster‐randomized trial."
None of the studies enrolled participants 60 years of age or older; therefore we did not exclude studies on the basis of age as specified in the protocol. However, we think the review is applicable to people 60 years of age or older. Therefore, this version and future updates of this review should include studies with participants 60 years of age or older.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 LASEK versus PRK, Outcome 1 Proportion of eyes within ± 0.50 D of target refraction at 12 months post treatment.

Comparison 1 LASEK versus PRK, Outcome 2 Postoperative mean spherical equivalent (D) at 1 week.

Comparison 1 LASEK versus PRK, Outcome 3 Postoperative mean spherical equivalent (D) at 1 month.

Comparison 1 LASEK versus PRK, Outcome 4 Postoperative mean spherical equivalent (D) at 3 months.
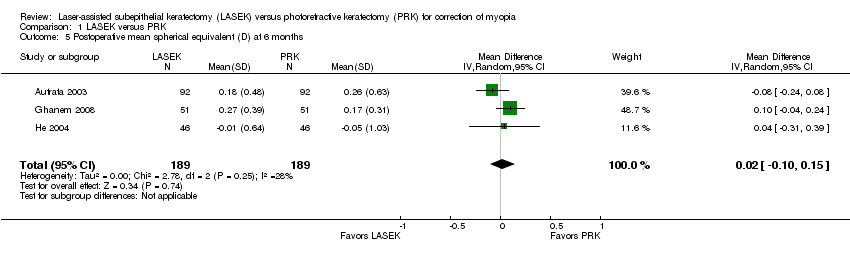
Comparison 1 LASEK versus PRK, Outcome 5 Postoperative mean spherical equivalent (D) at 6 months.

Comparison 1 LASEK versus PRK, Outcome 6 Postoperative mean spherical equivalent (D) at 12 months.

Comparison 1 LASEK versus PRK, Outcome 7 Proportion of eyes with uncorrected visual acuity (UCVA) of 20/20 or better at 1 week.

Comparison 1 LASEK versus PRK, Outcome 8 Proportion of eyes with uncorrected visual acuity (UCVA) of 20/20 or better at 1 month.
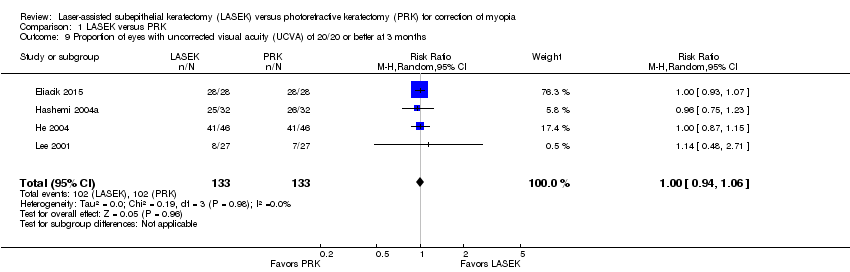
Comparison 1 LASEK versus PRK, Outcome 9 Proportion of eyes with uncorrected visual acuity (UCVA) of 20/20 or better at 3 months.

Comparison 1 LASEK versus PRK, Outcome 10 Proportion of eyes within ± 0.50 D of target refraction at 1 month.

Comparison 1 LASEK versus PRK, Outcome 11 Proportion of eyes within ± 0.50 D of target refraction at 3 months.

Comparison 1 LASEK versus PRK, Outcome 12 Postoperative epithelial healing time (days) at 1 week.

Comparison 1 LASEK versus PRK, Outcome 13 Postoperative pain scores at 1 day.

Comparison 1 LASEK versus PRK, Outcome 14 Postoperative pain scores at 2 days.

Comparison 1 LASEK versus PRK, Outcome 15 Postoperative pain scores at 3 days.

Comparison 1 LASEK versus PRK, Outcome 16 Postoperative corneal haze score at 1 month.

Comparison 1 LASEK versus PRK, Outcome 17 Postoperative corneal haze score at 3 months.

Comparison 1 LASEK versus PRK, Outcome 18 Postoperative corneal haze score at 6 months.

Comparison 1 LASEK versus PRK, Outcome 19 Postoperative corneal haze score at 12 months.
| Laser epithelial keratomileusis (LASEK) compared with photorefractive keratectomy (PRK) for myopia | ||||||
| Population: adult participants (≥ 18 years) with myopia Settings: surgical procedure Intervention: LASEK Comparison: PRK | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of eyes** | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| PRK | LASEK | |||||
| UCVA of 20/20 or better | 980 per 1000 | 961 per 1000 (902 to 1000) | RR 0.98 | 102 | ⊕⊕⊝⊝ | RR < 1 favors PRK |
| Within ± 0.50 D of target refraction at 12 months post treatment | 934 per 1000 | 869 per 1000 (785 to 962) | RR 0.93 (0.84 to 1.03) | 152 | ⊕⊕⊝⊝ | RR < 1 favors PRK |
| Loss of 1 or more lines of BSCVA | See comment | See comment | RR 3.00 (0.13 to 71.96) | 102 | ⊕⊝⊝⊝ | No event in the PRK group compared with one event in the LASEK group observed; RR > 1 favors PRK |
| Mean spherical equivalent | Mean spherical equivalent ranged across control groups from ‐0.27 to 0.17 D | Mean spherical equivalent in intervention group was 0.06 D higher (0.02 lower to 0.14 higher) | 386 | ⊕⊕⊕⊝ | ||
| Adverse outcomes ‐ corneal haze score at 12 months (grade 1.0 ‐ more prominent haze that does not interfere with visibility of fine iris details; grade 2.0 ‐ mild obscuration of iris details; grade 3.0 ‐ moderate obscuration of iris and lens; and grade 4.0 ‐ completely opaque stroma in the area of ablation) | See comment | See comment | 284 eyes | ⊕⊝⊝⊝ | The 3 included studies showed inconsistent results, so no pooled analysis was done. One study showed little or no difference in corneal haze score between LASEK and PRK, one study reported that corneal haze scores were lower in the LASEK group than in the PRK group, and one study did not report analyzable data for the PRK group, so we could not estimate the treatment effect for this trial. | |
| Adverse outcomes ‐ glare and halo scores at 3 months (grade 0 ‐ none; grade 1 ‐ very low; grade 2 ‐ low; grade 3 ‐ moderate; grade 4 ‐ high; and grade 5 ‐ very high) | See comment | See comment | 64 eyes | ⊕⊝⊝⊝ | Only 1 included study reported these adverse effects; below are the reported estimates For glare score: MD ‐0.04, 95% CI ‐0.61 to 0.53 For halo score: MD ‐0.09, 95% CI ‐0.72 to 0.54 | |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) **Both eyes of participants were included for all studies (i.e. all studies used a paired‐eye design in which 1 eye received PRK and the fellow eye received LASEK) | ||||||
| GRADE (Grades of Recommendation, Assessment, Development and Evaluation) Working Group grades of evidence | ||||||
| Assumed control risk (ACR) is calculated using this algorithm; in the control group (total number of events/total number of eyes) × 100 = ACR aDowngraded for potential reporting bias (‐2): core outcome reported by only 1 study. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of eyes within ± 0.50 D of target refraction at 12 months post treatment Show forest plot | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 2 Postoperative mean spherical equivalent (D) at 1 week Show forest plot | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.07, 0.18] |
| 3 Postoperative mean spherical equivalent (D) at 1 month Show forest plot | 7 | 672 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.05, 0.25] |
| 4 Postoperative mean spherical equivalent (D) at 3 months Show forest plot | 7 | 652 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.03, 0.17] |
| 5 Postoperative mean spherical equivalent (D) at 6 months Show forest plot | 3 | 378 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.10, 0.15] |
| 6 Postoperative mean spherical equivalent (D) at 12 months Show forest plot | 3 | 386 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.02, 0.14] |
| 7 Proportion of eyes with uncorrected visual acuity (UCVA) of 20/20 or better at 1 week Show forest plot | 4 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.92, 1.81] |
| 8 Proportion of eyes with uncorrected visual acuity (UCVA) of 20/20 or better at 1 month Show forest plot | 5 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.84, 1.08] |
| 9 Proportion of eyes with uncorrected visual acuity (UCVA) of 20/20 or better at 3 months Show forest plot | 4 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 10 Proportion of eyes within ± 0.50 D of target refraction at 1 month Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.77, 1.19] |
| 11 Proportion of eyes within ± 0.50 D of target refraction at 3 months Show forest plot | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.22] |
| 12 Postoperative epithelial healing time (days) at 1 week Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Postoperative pain scores at 1 day Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14 Postoperative pain scores at 2 days Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Postoperative pain scores at 3 days Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16 Postoperative corneal haze score at 1 month Show forest plot | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Postoperative corneal haze score at 3 months Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18 Postoperative corneal haze score at 6 months Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19 Postoperative corneal haze score at 12 months Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

