Вмешательства для профилактики рецидивов рожи и целлюлита
Appendices
Appendix 1. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Cellulitis] explode all trees
#2 MeSH descriptor: [Erysipelas] explode all trees
#3 MeSH descriptor: [Soft Tissue Infections] explode all trees
#4 MeSH descriptor: [Impetigo] explode all trees
#5 MeSH descriptor: [Staphylococcus] explode all trees
#6 MeSH descriptor: [Streptococcus] explode all trees
#7 MeSH descriptor: [Skin] explode all trees
#8 (cellulitis or erysipelas or impetigo or "soft tissue infection" or "soft tissue infections"):ti,ab,kw
#9 staphylococc* or streptococc*:ti,ab,kw AND skin:ti,ab,kw
#10 #5 or #6
#11 #7 and #10
#12 #1 or #2 or #3 or #4 or #8 or #9 or #11
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. (animals not (humans and animals)).sh.
10. 8 not 9
11. exp Erysipelas/ or erysipelas.ti,ab.
12. exp Cellulitis/ or cellulitis.ti,ab.
13. impetigo.ti,ab. or exp *Impetigo/
14. exp *Soft Tissue Infections/
15. Ignis sacer.ti,ab.
16. holy fire.ti,ab.
17. st anthony's fire.ti,ab.
18. exp *Staphylococcus/ or staphylococc$.ti,ab.
19. exp *Streptococcus/ or streptococc$.ti,ab.
20. 18 or 19
21. exp *Skin/
22. 20 and 21
23. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 22
24. 10 and 23
[Lines 1‐10: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 3. EMBASE (Ovid) search strategy
1. exp Erysipelas/ or erysipelas.ti,ab.
2. exp Cellulitis/ or cellulitis.ti,ab.
3. impetigo.ti,ab. or exp *Impetigo/
4. Ignis sacer.ti,ab.
5. holy fire.ti,ab.
6. st anthony's fire.ti,ab.
7. exp *Staphylococcus/ or staphylococc$.ti,ab.
8. exp *Streptococcus/ or streptococc$.ti,ab.
9. 7 or 8
10. exp *Skin/
11. 9 and 10
12. exp *soft tissue infection/
13. 1 or 2 or 3 or 4 or 5 or 6 or 11 or 12
14. random$.mp.
15. factorial$.mp.
16. (crossover$ or cross‐over$).mp.
17. placebo$.mp. or PLACEBO/
18. (doubl$ adj blind$).mp.
19. (singl$ adj blind$).mp.
20. (assign$ or allocat$).mp.
21. volunteer$.mp. or VOLUNTEER/
22. Crossover Procedure/
23. Double Blind Procedure/
24. Randomized Controlled Trial/
25. Single Blind Procedure/
26. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27. 13 and 26
Appendix 4. LILACS search strategy
(cellulitis or erysipelas or celulitis or "flemon difuso" or erisipela)
In LILACS we searched using the above terms and the Controlled clinical trials topic‐specific query filter.

Study selection flow diagram.
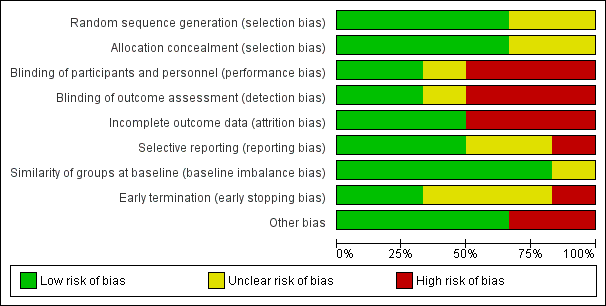
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 1 Recurrence of cellulitis.
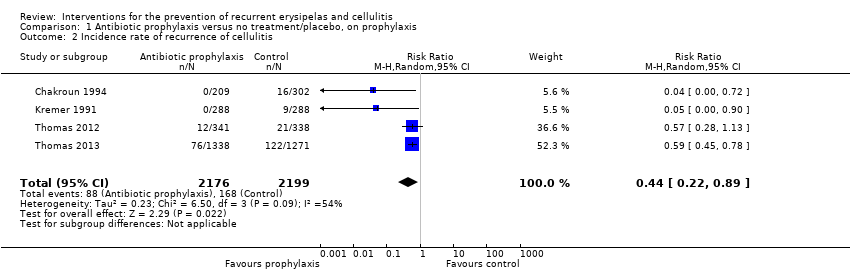
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 3 Time to next episode of cellulitis.
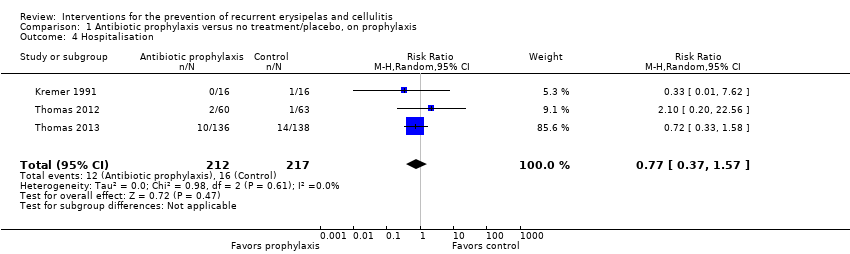
Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 4 Hospitalisation.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 5 Any adverse reactions.

Comparison 1 Antibiotic prophylaxis versus no treatment/placebo, on prophylaxis, Outcome 6 Mortality.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 1 Recurrence of cellulitis.

Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 2 Incidence rate of recurrence of cellulitis.
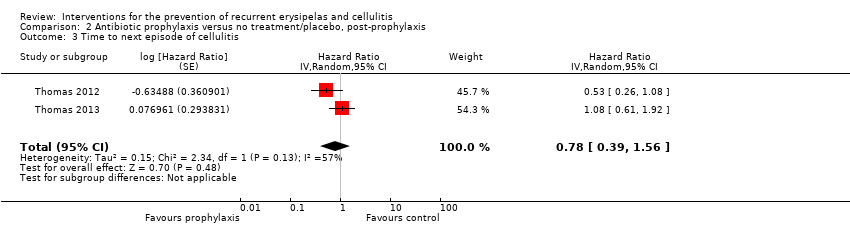
Comparison 2 Antibiotic prophylaxis versus no treatment/placebo, post‐prophylaxis, Outcome 3 Time to next episode of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 1 Recurrence of cellulitis.

Comparison 3 Antibiotic prophylaxis versus no treatment/placebo, overall, Outcome 2 Incidence rate of recurrence of cellulitis.
| Antibiotic prophylaxis compared to no treatment/placebo for the prevention of recurrent erysipelas and cellulitis ‐ on prophylaxis | ||||||
| Patient or population: People with recurrent erysipelas or cellulitis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment/placebo | Antibiotic prophylaxis | |||||
| Recurrence of cellulitis | Study population | RR 0.31 | 513 | ⊕⊕⊕⊝ | Number needed to treat for 1 additional beneficial outcome (NNTB) is 6 | |
| 316 per 1000 | 98 per 1000 | |||||
| Incidence rate of cellulitis | Study population | RR 0.44 (0.22 to 0.89) | 473 (4 RCTs) | ⊕⊕⊕⊝ | ‐ | |
| 43 fewer episodes of cellulitis per 1000 person‐months in treatment group (from 8 fewer to 60 fewer) | ||||||
| Time to next episode of cellulitis | Not estimable | HR 0.51 | 437 | ⊕⊕⊕⊝ | ‐ | |
| Hospitalisation | Study population | RR 0.77 | 429 | ⊕⊕⊝⊝ | ‐ | |
| 74 per 1000 | 57 per 1000 | |||||
| Any adverse reactions | Study population | RR 0.87 | 469 | ⊕⊕⊝⊝ | ‐ | |
| 287 per 1000 | 250 per 1000 | |||||
| Quality of life | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by one level because of imprecision due to the low number of events and the small sample size (optimal information size ‐ OIS). 2 We downgraded by two levels because of imprecision due to the low number of events and the small sample size (OIS) and the 95% confidence interval overlapping the line of no effect and ranging from benefit to harm. 3We downgraded by two levels because of imprecision due to the considerable low number of events and the small sample size (OIS). We decided not to downgrade any of the outcomes for risk of bias as we decided it was not serious enough to affect the overall quality of the outcome. | ||||||
| Medical term | Explanation |
| Ambulatory | Ambulatory is when the patient can walk and is not bedridden. When referring to medical care it means that it is being provided to patients that are not hospitalised (outpatients) |
| Block randomisation | A method of randomisation that ensures allocation of participants into roughly equal sizes of comparison groups |
| Clostridium difficile | A bacterium that causes inflammation of the colon (colitis), typically resulting in diarrhoea, and is strongly associated with the use of antibiotics |
| Comorbidity | The presence of one or more diseases or conditions other than those of primary interest |
| Contralateral | On the opposite side of the body (e.g. a repeat episode of leg cellulitis can recur in the same leg [see 'ipsilateral'] or the other, contralateral leg |
| Control event rate (CER) | The rate at which events of interest (i.e. episodes of cellulitis in our review) occur in the control group of the study |
| Diuretics | Commonly known as "water pills" these are drugs that help the body to eliminate unneeded water and salt through the urine |
| Epidemiology | The study of the health of populations and communities, not just particular individuals |
| Erythema | Redness of the skin caused by increased blood flow. Often a sign of inflammation or infection |
| Filariasis | A disease caused by infection with worms, usually in tropical and subtropical areas of the world. The worms reside in the lymphatic system of the affected person and interfere with the drainage of the lymph, subsequently causing a significant swelling of the involved limb |
| Filarial lymphoedema | see Filiariasis |
| Folliculitis | Inflammation of hair follicles |
| Furunculosis | Deep form of inflammation of the hair follicles resulting in lumps caused by the accumulation of pus (boils) |
| Gastrointestinal | Relating to the stomach and the intestines |
| Incidence rate/Incidence rate ratio | The number of new occurrences of events in a population divided by its time period at risk; Incidence rate ratio is the ratio of two incidence rates |
| Ipsilateral | On the same side of the body; as opposed to 'contralateral' |
| Mastectomy | Surgical removal of one or both breasts |
| Outpatient/Inpatient | Outpatient is a person that is being treated without being hospitalised overnight and visits the physician in the clinic, hospital or other facility; compared with an inpatient who requires an overnight stay in hospital for medical treatment |
| Person‐months | The sum of the number of months each participant in the trial has been under observation (treated/followed) |
| 'Per protocol'/Intention‐to‐treat (ITT) analyses | 'Per protocol' analysis compares participants in a study based on the treatment they actually took and includes only those patients who completed the treatment originally allocated, as opposed to intention‐to‐treat analysis that compares participants on the basis of their random assignment to groups (treatment or placebo), regardless of adherence to treatment |
| Prophylaxis | Preventive treatment for disease |
| Retrospective cohort study | An observational study in which a defined group of people (the cohort) is followed over time. A retrospective cohort study identifies persons from past medical records and follows them from the time of those records to the present |
| Sensitivity analysis | An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done |
| Tinea pedis | Fungal infection of the foot (athlete's foot) |
| Tonsillectomy | Surgical removal of tonsils |
| Study | Way of communication | Date | Information provided | Notes |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ Criteria for diagnosis ‐ Adherence ‐ Adverse reactions ‐ Informed consent ‐ Ethical committee approval ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| airmail, email, website | 2013 ‐ 2014 | ‐ | Investigator did not reply to our queries We also contacted a potential sponsor, not reported by the author, who confirmed their financial support for the conduct of this study (email correspondence with the head of medical‐scientific department of 'biosyn Arzneimittel GmbH' from January 2015) | |
| email and telephone | 12/2013 and 1/2014 | ‐ | Data were not available and the investigator did not remember any details | |
| | 12/2013 | ‐ Allocation concealment ‐ Participants follow‐up ‐ By whom cellulitis was diagnosed ‐ Adherence ‐ Source of funding | Full information was not available for all queries, but investigators responded to all of them | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐Time to next episode ‐ Adverse events by study arm ‐ Duration of hospitalisation ‐ Quality of life | ‐ | |
| | 1/2014 | ‐ Episodes of recurrent cellulitis per person‐months (incidence rate) ‐ Quality of life | Hospitalisation and quality of life were not evaluated directly in this trial but were reported by indirect evaluation in Mason 2014 |
| Guideline | Organisation | Recommended antibiotic | Duration of Px | No of episodes to initiate Px | Adjunctive Tx | Quality of evidence † |
| BLS | penicillin by mouth; alternatives: cephalexin, erythromycin, clarithromycin, clindamycin, doxycycline | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx, alcohol wipes; SIT | NS | |
| ALA | penicillin by mouth; alternatives: cephalexin, erythromycin, clindamycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, bacterial decolonisation Tx; SIT | NS | |
| IDSA | penicillin by mouth/IM; alternatives:erythromycin | as long as RF persist | 3 – 4 /y | skin care, Tx of oedema, weight reduction | antibiotic Px ‐weak, moderate ‡ duration of Px ‐ strong, moderate ‡ skin care ‐ strong, moderate ‡ | |
| penicillin by mouth/IM; alternatives:erythromycin | NS | frequent | skin care, Tx of oedema compression stockings, diuretics; SIT | grade IIB § | ||
| ISL | penicillin; alternatives: broad spectrum antibiotic | NS | repeat despite physical Tx | skin care, antifungal Tx | NS | |
| SIMIT and ISC | penicillin or macrolide | NS | recurrent | skin hygiene and compression stockings | NS | |
| NHG | penicillin by mouth/IM | 1 ‐ 2 y | 2 ≥/y | skin care, compression stockings; SIT | NS | |
| ILF | penicillin by mouth; alternatives:erythromycin, clindamycin, clarithromycin | 2 y; life‐long Px if recurrence after Px stopped | 2 ≥/y | skin care, decongestive Tx, antifungal Tx; SIT | NS | |
| CREST | penicillin or erythromycin by mouth | 2 y | 2 ≥/y | SIT may be preferable | weak and inconclusive | |
| NICE | a trial should be considered | NS | > 2/y | skin care, Tx of oedema, compression stockings, weight reduction | weak and inconclusive | |
| Other* | antibiotic may be needed; type of antibiotic NS | long term | NS | skin care, Tx of oedema, antifungal Tx; SIT | NS | |
| SFD | penicillin by mouth/IM; alternatives: macrolide | prolonged, probably indefinitely | several/poorly controlled RF | skin care, Tx of oedema | NS | |
| FMSD | antibiotic should be considered; type of antibiotic NS | long term | frequent | skin care | NS | |
| † Assessement of quality of evidence as defined and graded by the authors of the document. ‡ Strong recommendation, moderate quality ‐ desirable effects clearly outweigh undesirable effects; evidence from RCTs with important limitations or exceptionally strong evidence from unbiased observational studies; recommendation can apply to most patients in most circumstances and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. Weak recommendation, moderate quality ‐ desirable effects closely balanced with undesirable effects; evidence from RCTs with important limitations;recommendation may change when higher‐quality evidence becomes available; and further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. § Moderate evidence ‐ should generally be offered; II ‐ evidence from one well‐designed clinical trial. Abbreviations IM ‐ injections into the muscle (intramuscular) Medical organisations BLS ‐ British Lymphology Society * 5 of 6 experts in this consensus paper were from North America; published in the Journal of the British Society for Antimicrobial Chemotherapy | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 5 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.72] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 4 | 4375 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| 3 Time to next episode of cellulitis Show forest plot | 3 | Hazard Ratio (Random, 95% CI) | 0.51 [0.34, 0.78] | |
| 4 Hospitalisation Show forest plot | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.37, 1.57] |
| 5 Any adverse reactions Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.58, 1.30] |
| 5.1 Penicillin | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.10] |
| 5.2 Erythromycin | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 125.44] |
| 6 Mortality Show forest plot | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.32, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.31] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 4566 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.36] |
| 3 Time to next episode of cellulitis Show forest plot | 2 | Hazard Ratio (Random, 95% CI) | 0.78 [0.39, 1.56] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrence of cellulitis Show forest plot | 2 | 397 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 2 Incidence rate of recurrence of cellulitis Show forest plot | 2 | 7854 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.85] |

