Leukozytendepletion zur Vorbeugung unerwünschter Reaktionen auf körperfremde Bluttransfusionen
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Parallel group RCT Country: The Netherlands Multicentre study: Yes (two university hospitals: Academic Medical Center and Leiden University Medical Center) Setting: Hospital Follow‐up: 90 days Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Adults > 18 years undergoing valve surgery Exclusion criteria: Those with a medical indication for leukocyte‐depleted erythrocytes or who had received blood transfusions within the previous three months Patients enrolled: 496 Patients randomised: 474 Buffy coat‐depleted packed cells: 237 Leukocyte‐depleted erythrocytes (LDs): 237 Patients transfused: not clearly reported Patients considered for the analysis: 474 Main characteristics of patients: Age: Buffy coat‐depleted packed cells group = 66.6 ± 12.5 years; LD group = 65.3 ± 14.7 years Percentage of men: Buffy coat‐depleted packed cells group = 57%; LD group = 52% Percentage of erythrocyte transfusions more or equal to four: Buffy coat‐depleted packed cells group= 55.3%; LD group= 61.2% Storage time of the units: Buffy coat‐depleted packed cells group = 19.7 ± 5.4 ; LD group = 17.4 ± 5 Percentage of use of aprotinin: Buffy coat‐depleted packed cells group = 37.1; LD group = 36.3 | |
| Interventions |
Co‐intervention:
| |
| Outcomes | Primary:
Secondary:
Quote: "An independent safety committee monitored the interim results of the primary end point." (Page 2757). | |
| Notes | Trial registration: Not reported Funding: This study was financially supported by the Netherlands Heart Foundation (grant 98.183) (Page 2760) Rol of sponsor: Not reported A priori sample size estimation: Yes Conducted: Between June 1999 and May 2001 Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a straightforward randomizations was performed by using a fixed block size (n=24) to ensure a balance between the randomizations groups" (Page 2756). |
| Allocation concealment (selection bias) | Low risk | Quote: "...the technicians randomly assigned the patients by opening a sealed and numbered envelope." "In the hospital electronic information system, a code was used during the study period to hide the random assignment" (Page 2756). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The patients, surgeons, anaesthesiologists, and the trial coordinators were blinded to the random assignment, as the technicians placed uniform study labels on the description on the erythrocyte bags" (Page 2756). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The patients, surgeons, anesthesiologists, and the trial coordinators were blinded to the random assignment, as the technicians placed uniform study labels on the description on the erythrocyte bags. In the hospital electronic information system, a code was used during the study period to hide the random assignment" (Page 2756). |
| Incomplete outcome data (attrition bias) | Low risk | Loss after transfusion: 0.4% (2/474) Loss after transfusion in prestorage by filtration of LDs (LDs): 0.8% (2/237) Loss after transfusion in Buffy coat–depleted packed cells (PCs): 0% (0/237) Loss after transfusion. Imbalance between comparison groups: 0.8% |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Methods | Design: Parallel group RCT Country: USA Multicentre study: Yes (three centre trial) Setting: Hospital Follow‐up: 2 to 12 months Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria:
Exclusion criteria: Not reported in the abstract
Prestorage leukoreduced RBC (LR‐RBCs): The number of patients is not clearly reported in the abstract. Standard RBCs (S‐RBCs): The number of patients is not clearly reported in the abstract.
Main characteristics of patients: "Groups were statistically equivalent demographically and by all Society of Thoracic Surgery risk criteria". | |
| Interventions |
Co‐intervention: not reported in the abstract | |
| Outcomes | Primary:
Secondary: Not reported in the abstract. | |
| Notes | Trial registration: Not reported in the abstract Funding: Not reported in the abstract Role of sponsor: Not reported in the abstract A priori sample size estimation: Not reported in the abstract Conducted: Not reported in the abstract Declared conflicts of interest: "No relevant conflicts of interest to declare" (reported in the abstract) Other relevant information: All study characteristics were obtained from the abstract. We tried to contact two trial authors by email, but no response has not yet been obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients (undergoing coronary artery bypass grafting, cardiac valve replacement, or a combination of the two) were pre‐operatively randomised to receive either LR‐ or S‐RBCs (Abstract). Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Patients and clinicians were blinded as to product type" (Abstract). The blinding methods are not clearly reported. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Patients and clinicians were blinded as to product type" (Abstract). Comments: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | "562 patients (45.8%) were transfused: 304 received LR‐RBCs and 258 S‐RBCs" (Abstract) Loss after transfusion: Not reported in the abstract Loss after transfusion LR group: Not reported in the abstract Loss after transfusion Control group: Not reported in the abstract Loss after transfusion. Imbalance between comparison groups: Not reported in the abstract. Comment: Insufficient reporting of attrition/exclusions to permit judgement of ‘low risk’ or ‘high risk’ (e.g. number randomised not stated, no reasons for missing data provided). |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’ (Abstract data). |
| Other bias | High risk | Design bias: 1226 patients randomised; only 562 (45.8) were transfused. |
| Methods | Design: Parallel group RCT Country: USA Multicentre study: Yes (11 academic medical centres) Setting: Hospital Follow‐up: 12 months Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Those 14 years of older, with confirmed HIV infection and documented CMV infection (by chart or antibody testing), Karnofsky performance score of ≥ 40, expected survival of more than 1 month, symptomatic anaemia requiring red blood concentrates transfusion and no received transfusions within 72 hours of enrolment. Exclusion criteria: Those with a surgical reason for transfusion, a priori history for transfusion, renal failure requiring dialysis, thrombocytopenic purpura, used intravenous immunoglobulin within 6 weeks of entry or those that had started new antiretroviral drugs or systematic immunomodulators within 2 weeks of entry.
Leukoreduced red blood concentrates transfusion: 265 Unmodified red blood concentrates transfusion: 266
Main characteristics of patients: Age: Leukoreduced group = 38.3 ± 8.2 years; Unmodified group = 38.4 ± 8.3 years Percentage of men: Leukoreduced group = 79%; Unmodified group = 79% Percentage of antiretroviral therapy‐potent combination: Leukoreduced group = 27%; Unmodified group = 22% Karnofsky score: Leukoreduced group = 71.4 ± 13.2; Unmodified group = 70.9 ± 12.8 CD4 cells/μL, median: Leukoreduced group = 16 (3 to 71.5); Unmodified group = 12.5(4 to 76) | |
| Interventions |
Cointervention: Leukoreduced platelets if was required. | |
| Outcomes |
| |
| Notes | Trial registration: Not reported Funding: This study was financially supported by Roche Molecular systems and National Heart, Lung and Blood Institute (contract RR00046) (Page 1600). Role of sponsor: Reagents: Funding/Support: Reagents for detection and quantitation of CMVDNA by polymerase chain reaction were contributed by Roche Molecular Systems (Alameda,Calif). Support provided by National Heart was not detailed A priori sample size estimation: Yes Conducted: July 1995 through June 1999 Declared conflicts of interest: Trial authors received research funding from Roche | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was a randomised, double blind, comparative study" (Page 1593). Quote: "Treatment allocation was made centrally by the study coordinating center, using stratified permuted blocks with dynamic balancing within each center" (Page 1593). |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was made centrally by the study coordinating center, using stratified permuted blocks with dynamic balancing within each center" (Page 1593). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The participants, investigators, study coordinators, and persons having any contact with the patients were blinded to study treatment assignments. Blood bank technical staff who prepared the blood products were aware of the treatment assignments" (Page 1593). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The participants, investigators, study coordinators, and persons having any contact with the patients were blinded to study treatment assignments. Blood bank technical staff who prepared the blood products were aware of the treatment assignments" (Page 1593). |
| Incomplete outcome data (attrition bias) | Low risk | Loss after transfusion: 9.8% (51/521) Loss after transfusion LR group: 10.8 (28/259) Loss after transfusion Control group: 8.8% (23/262) Imbalance between comparison groups: 2% |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias. |
| Methods | Design: Parallel group RCT Country: Italy Multicentre study: No Setting: Hospital Follow‐up: Not clearly reported, outcomes were measured up to 1 hour after blood transfusion Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Adult patients admitted to ICU of the AOU Ospedali Riuniti of Ancona with sepsis, severe sepsis or septic shock as diagnosed according to standard criteria and requiring blood transfusion for haemoglobin (Hb) levels of less than 8 g/dL or as indicated by the attending physician in accordance with the local hospital guidelines. Exclusion criteria: Aged < 18 years, previous blood transfusions during ICU stay, previous history of coagulation disorders, cardiogenic or hemorrhagic shock, pregnancy and factors impeding the sublingual microcirculation evaluation (oral surgery and maxillofacial trauma)
Leukodepleted RBC transfusion: 10 Non‐leukodepleted RBC transfusion: 10
Main characteristics of patients: Age: Non‐leukodepleted group = 70 (65 to 72) years; leukodepleted group = 74 (64 to 79) years Percentage of men: Non‐leukodepleted group = 50%; leukodepleted group = 70% | |
| Interventions |
Cointervention: Related to sepsis treatment (fluid therapy, vasopressors and inotropic agents, antibiotics, etc.) | |
| Outcomes |
| |
| Notes | Trial Registration: NCT01584999 Funding: Not reported Role of sponsor: Not reported A priori sample size estimation: Yes Conducted: February 2011 and 2012 Declared conflicts of interest: One trial author "CI is the inventor of Sidestream Dark Field imaging technology" technique used to measure the outcomes, this author "holds shares in MicroVision Medical and was a consultant for this company more than four years ago but has had no further contact with the company since then. He declares that he has no other competing interests in this field other than his commitment to promoting the importance of the microcirculation during patient care and no other relationships or activities that could appear to have influenced the submitted work. HV holds the position of chief scientific officer in GlycoCheck BV. The other authors declare that they have no competing interests". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | "Blood product randomization was performed through sealed envelopes by a physician at the blood bank" (Page 2). |
| Blinding of participants and personnel (performance bias) | Low risk | "a physician at the blood bank blindly provided the blood bags to the ICU; neither the attending physician nor the investigators nor the patients were aware of the type of RBCs transfused" (Page 2). |
| Blinding of outcome assessment (detection bias) | Low risk | "a physician at the blood bank blindly provided the blood bags to the ICU; neither the attending physician nor the investigators nor the patients were aware of the type of RBCs transfused" (Page 2). |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All patients transfused were analysed. Patients lost to follow‐up: None. |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | — |
| Methods | Design: Parallel group RCT Country: Denmark Multicentre study: Yes (2 university departments of colorectal surgery; page 844). Setting: Hospital Follow‐up: 30 days after surgery Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Patients admitted for elective colorectal surgery Exclusion criteria: < 18 years of age, need for emergency surgery, or immunosuppressive treatment, except steroids
Leucocyte‐depleted red cells group: 290 Buffy‐coat‐poor red cells group: 299
Main characteristics of patients: Age = Median (range): Leucocyte‐depleted red cells group = 69 (35 to 89) years; Buffy‐coat‐poor red cells group = 68 (29 to 92) years Percentage of men: Leucocyte‐depleted red cells group = 50%; buffy‐coat‐poor red cells group = 48% Number of procedures: Sigmoid resection: Leucocyte‐depleted red cells group = 11; Buffy‐coat‐poor red cells group = 19 Blood loss = Median (range): Leucocyte‐depleted red cells group = 715 (50 to 3500) mL; Buffy‐coat‐poor red cells group = 805 (10 to 4300) mL. | |
| Interventions |
Cointervention: Quote: "All patients received an intravenous dose of cefuroxime 3 g and metronidazole 1.5 g after induction of anaesthesia" (Page 842). | |
| Outcomes | This RCT did not specify by primary or secondary outcomes.
| |
| Notes | Trial Registration: Not reported. Funding: Not reported. Role of sponsor: Not stated. A priori sample size estimation: Yes. Conducted: Between January 1992 and January 1995. Declared conflicts of interest: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly allocated using sealed envelopes to receive either..." (Page 841). Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were randomly allocated using sealed envelopes to receive either..." (Page 841) Use of opaque envelopes is not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Physicians who were blinded to the transfusion protocol performed follow‐up examinations..." (Page 842). |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All patients transfused were analysed. (Page 842). |
| Selective reporting (reporting bias) | Low risk | Comment: The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Unclear risk | Comment: Sample size estimation took into account loss to follow‐up due to no transfusion. |
| Methods | Design: Parallel group RCT Country: France Multicentre study: No Setting: Hospital Follow‐up: 6 months Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: "Patients included in the trial were more than 18 years of age and of both sexes and were to undergo surgery for cancer...with a transfusion‐during‐surgery (TdS) risk greater than 30 percent". Exclusion criteria: "Patients were not eligible for inclusion if they presented contraindications to the use of UN‐RBCs or presented any RBC antibodies and/or anti‐HLA and/or antigranulocyte alloimmunization" (page 1692).
Unmodified RBCs (UN‐RBC): 19 Leukoreduced RBCs (LR‐RBC): 18
Main characteristics of patients: Age = Median (range): Unmodified RBCs group = 56 (40 to 73) years; Leukoreduced RBCs group= 54 (37 to 65) years Percentage of men: Unmodified RBCs group = 33%; Leukoreduced RBCs group = 47% Tumour location: Pelvis: Unmodified RBCs group = 11; Leukoreduced RBCs group = 8. Head and neck: Unmodified RBCs group = 6; Leukoreduced RBCs group = 7. Miscellaneous: Unmodified RBCs group = 1; Leukoreduced RBCs group = 2 Blood loss = Median (range): Unmodified RBCs group = 1500 (300 to 3650) mL; Leukoreduced RBCs group = 1325 (300 to 6800) mL | |
| Interventions |
Cointervention: Two patients (11%) in the unmodified RBCs group and three patients (18%) received fresh‐frozen plasma. None of the patients received platelet concentrate. | |
| Outcomes | "The main endpoint of the study was to compare immune status in both treatment groups" (page 1692). This was measured through surrogate variables (i.e. microchimerism, peripheral blood cell counts and cytokine production capacity). However, other outcomes were described: "In addition to immune status, other factors compared between the two groups were survival and incidence of nosocomial infection" (page 1694). | |
| Notes | Trial registration: ClinicalTrials database NCT00180869 Funding: "grants from the French Association for Cancer Research (L'Association pour la Recherche sur le Cancer [9653]) and the French Association for Blood Transfusion Research (L'Association pour la Recherche enTransfusion [081997])" (page 1691) Role of sponsor: Not stated A priori sample size estimation: Yes Conducted: between 15 September 1997 and 28 February 1998 Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was performed centrally by computer in the Gustave Roussy Cancer Institute Biostatistics Unit after the investigator had sent a fax indicating the minimization factors of the patient" (page 1693). |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "Patients and surgeons were not blinded of the treatment allocation" (page 1693). |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Patients and surgeons were not blinded of the treatment allocation" (page 1693). |
| Incomplete outcome data (attrition bias) | Low risk | 36 patients were transfused: 18 received unmodified RBCs (UN‐RBC) and 18 received Leukoreduced RBCs (LR‐RBC). Loss after transfusion: 2.77% (1/36). Loss after transfusion LR group: 1 died during surgery. Loss after transfusion Control group: None. Loss after transfusion. Imbalance between comparison groups: 5%. |
| Selective reporting (reporting bias) | Unclear risk | Survival after perioperative transfusion is reported in Figure 2. However, number of death is not clearly reported. |
| Other bias | Unclear risk | Comment: Sample size estimate was calculated based on Th2 polarization. It is unclear what dropout rate was considered in the sample size estimation of 75 participants. |
| Methods | Design: Parallel group RCT Country: USA Multicentre study: No Setting: Hospital Follow‐up: 28 days after randomisation Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Age of above 17 years and red cell transfusion within 24 hours of injury Exclusion criteria: Those with an anticipated survival of less than 48 hours, active infection at presentation, receipt of blood products for the current injury before randomisation, individuals with blood group AB Rh negative or B Rh negative, patients with clinically significant red cell alloantibodies requiring an antiglobulin crossmatch, recipients with prior requirements for irradiation, leukoreduction, od CMV protection, subjects enrolled in a concurrent study of pre‐hospital hypertonic saline resuscitation or incarcerated subjects.
Standard transfusion: 935 Leukoreduced transfusion: 929
Main characteristics of patients included in full analysis. Age: Standard group = 42.1 ± 18 years; Leukoreduced group = 42.3 ± 19 years Percentage of men: Standard group = 69%; Leukoreduced group = 66% Percentage of penetrating injury mechanism: Standard group = 18%; Leukoreduced group = 19% Injury Severity Score: Standard group = 25.5 ± 11; Leukoreduced group = 23.9 ± 11 | |
| Interventions |
Cointerventions: "All patients received apheresis platelets when platelets were required" (Page 343). | |
| Outcomes |
Note: TRALI was assessed by Watkins 2008 as a secondary analysis. | |
| Notes | Trial registration: www.clinicaltrials.gov, August 23, 2005. Registration No.: NCT00135291 Funding: National Institutes of Health (NIH) Role of sponsor: The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review or approval of the manuscript. A priori sample size estimation: Yes Conducted: Between 3 February 2003 and 30 August 2004. Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The hospital‐based transfusion support service performed the randomization in a 1:1 ratio, using a permuted block scheme (block size of six)" (Page 344). |
| Allocation concealment (selection bias) | Low risk | Quote: "Using preprinted sealed opaque envelopes containing the study identification number and randomization arm (listed as arm 1 or arm 2) to conceal allocation" (Page 344). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Before unit issue, the transfusion service added a Food and Drug Admnistration approved study label to blind the leukoreduction process; the transfusion report accompanying the red cell unit was also blinded" (Page 343). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Before unit issue, the transfusion service added a Food and Drug Admnistration approved study label to blind the leukoreduction process; the transfusion report accompanying the red cell unit was also blinded" (Page 343). |
| Incomplete outcome data (attrition bias) | Low risk | Loss after transfusion: 5% (16/324). Loss after transfusion LR group: 7% (11/156). Loss after transfusion Control group: 3% (5/168). Imbalance between comparison groups: 4%. |
| Selective reporting (reporting bias) | Low risk | Comments: The study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias. |
| Methods | Design: Parallel group RCT Country: USA Multicentre study: No Setting: Hospital Follow‐up: 1 year Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: All potential cadaver renal allograft recipients Exclusion criteria: Not clearly reported
PRBCs group: 42 Leukocyte poor red cells group: 45 Mixed group: 20
Main characteristics of patients included in full analysis were not fully stated: Quote: "there were no significant differences between transfusion groups in terms of demographic factors, including number of transfusions, age, race, sex, degree of HLA match, number of diabetics or the use of ATG posttransplant" (Page 117). | |
| Interventions |
Cointerventions: "All transplant recipients received conventional maintenance immunosuppressive therapy with azathioprine and prednisone, and antithymocyte globulin (ATG) and/or bolus methylprednisolone was used for the treatment of rejection episodes" (Page 117). | |
| Outcomes | This RCT did not specify by primary or secondary outcomes.
| |
| Notes | Trial registration: Not reported. Funding: National Kidney Foundation of North Carolina Role of sponsor: Not stated A priori sample size estimation: No Conducted: Between September 1980 and June 1982 Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...after obtaining informed consent, patients were randomly assigned to receive" (Page 116). Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...after obtaining informed consent, patients were randomly assigned to receive..." (Page 116). Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Selective reporting (reporting bias) | High risk | Comment: The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Other bias | High risk | Design bias. |
| Methods | Design: Parallel group RCT Country: USA Multicentre study: No Setting: Hospital Follow‐up: Unclear. Quote: "Patients were followed up daily in the hospital until discharge (...) and contacted by phone following discharge to elicit possible symptoms of infection". Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Those scheduled for inpatient gastrointestinal surgery under general anaesthesia Exclusion criteria: Not clearly reported
Filtered group: 106 Unfiltered group: 118
Main characteristics of patients: Age (Unclear if median or mean): Filtered group = 54 years; Unfiltered group = 50 years Percentage of men: Filtered group = 49%; Unfiltered group = 50% Hematocrit (Unclear if median or mean): Filtered group = 38; Unfiltered group = 37 Percentage of hypertension: Filtered group = 14%; Unfiltered group = 10% Percentage of diagnosis of malignancy: Filtered group = 38%; Unfiltered group = 38% | |
| Interventions |
Cointerventions: "Preoperative preparation included intravenous antibiotics for all patients and bowel cleansing with Golytely for patients in whom transection of the colon or rectum was anticipated" (Page 463) | |
| Outcomes | This RCT did not specify by primary or secondary outcomes.
| |
| Notes | Trial Registration: Not reported. Funding: Pall Corporation, Gel Cove, NY Role of sponsor: Not stated. A priori sample size estimation: Unclear. Trial authors reported the criteria for sample size calculation, including a 60% of dropout rate ("40% of gastrointestinal surgery patients at our institution receive blood transfusions"). The software or method for sample size calculation is not reported. Using the ARCSINUS approximation, and using the same criteria reported by authors, 174 subjects are necessary in first group and 174 in the second to find as statistically significant a proportion difference, expected to be of 0.2 in group 1 and 0.05 in group 2, with an anticipated drop‐out rate of 60%. The study included 224 participants (118 and 106 patients in each group), 59 were transfused (26%) and 50 participants received allogeneic RBC transfusions. Conducted: Between 1 August 1993 and 31 January 1994 Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated by the study personnel in the blood bank" (Page 463). Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all patients transfused were analysed (Page 842). |
| Selective reporting (reporting bias) | High risk | This RCT did not report mortality. Comment: The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Other bias | High risk | Design bias. Sample size bias. Trial authors reported the criteria for sample size calculation, including a 60% of dropout rate ("40% of gastrointestinal surgery patients at our institution receive blood transfusions"). The software or method for sample size calculation is not reported. Using the ARCSINUS approximation, and using the same criteria reported by the study authors, 174 subjects are necessary in first group and 174 in the second to find as statistically significant a proportion difference, expected to be of 0.2 in group 1 and 0.05 in group 2, with an anticipated drop‐out rate of 60%. The study included 224 participants (118 and 106 patients respectively), 59 were transfused (26%) and 50 participants received allogeneic RBC transfusions. |
| Methods | Design: Parallel group RCT Country: Denmark Multicentre study: No Setting: Hospital Follow‐up: 30 days Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Patients admitted —acute and elective— to the Department of Colorectal surgery, undergoing open colorectal surgery Exclusion criteria: Those receiving blood transfusions within the final 3 months prior to surgery and those below 18 years
Leukocyte‐depleted erythrocyte suspensions group (LD‐SAGM): 139 patients Buffycoat‐depleted group: 140 patients
Main characteristics of patients: Age: Median/range: Leukocyte‐depleted erythrocyte group = 71 (66 to 77) years; Leukocyte‐depleted erythrocyte = 73 (62 to 79) years Percentage of men: LD‐SAGM group = 51%; Non‐leukocyte‐depleted erythrocyte suspensions (SAGM) group = 60% Number of malignant colorectal disease: LD‐SAGM group = 37; SAGM group = 56 Hemoglobin: Median/range: LD‐SAGM group = 12.6 (10.6 to 14.2) g/dL; SAGM group = 12.4 (11.1 to 13.9) g/dL | |
| Interventions |
Cointervention: "all patients received perioperative prophylactic antibiotics intravenously (3 g ampicillin or 3 g cefuroxim and 1.5 g metronidazol)" (Page 149). Platelet transfusion as co‐intervention was not reported | |
| Outcomes | This RCT did not specify by primary or secondary outcomes.
| |
| Notes | Trial registration: Not reported Funding: Not reported Role of sponsor: Not reported A priori sample size estimation: Yes (but not fulfilled) Conducted: Between May 1997 and November 1999 Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were allocated in the blood bank to receive either..." Page 148. Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The blood units were supplied by the blood bank, and all units were blinded. White labels were placed on the unit product code numbers, but bar code labels were intact, ensuring safe handling" (Page 149). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Surgeons who were blinded to the transfusion protocol performed the follow‐up examinations" (Page 149). |
| Incomplete outcome data (attrition bias) | Low risk | Loss after transfusion: 10.4% (13/125). Loss after transfusion LR group: 12.7 % (7/55). Loss after transfusion Control group: 8.5% (6/70). Imbalance between comparison groups: 4.2%. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk | Sample size bias. "Discontinued study due to large exclusions than expected, as well as higher rates of infection, insufficient sample size" (Page 149). The estimation of sample size bias considered 10% of random error and did not reported it. Design bias. Industry bias: Unclear. |
| Methods | Design: Parallel group RCT Country: The Netherlands Multicentre study: No Setting: Hospital Follow‐up: 60 days after surgery Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Adults patients undergoing Coronary Artery Bypass grafting (CAGB) surgery, cardiac valve surgery or a combination of both, who had not received blood within the last 6 months. Exclusion criteria: None clearly reported
Packed cells without buffy coat: 306 Fresh‐filtered units: 305 Stored‐filtered units: 303
Main characteristics of patients: Age: Standard packed cells without buffy coat group = 64.4 ± 9.5 years; Fresh‐filtered units group = 62.9 ± 9.8 years. Stored‐filtered units group = 63.3 ± 9.1 years Percentage of men: Standard packed cells without buffy coat group = 72%; Fresh‐filtered units group = 74%. SF group = 68% Percentage of history of myocardial infarction: Standard packed cells without buffy coat group = 50.3%; Fresh‐filtered units group = 44.6%. Stored‐filtered units group = 46.4% Preoperative Hb: Standard packed cells without buffy coat group = 8.8 ± 0.9 mmol/L; Fresh‐filtered units group = 8.9 ± 0.9 mmol/L. Stored‐filtered units group = 8.8 ± 0.9 mmol/L Postoperative Hb: Standard packed cells without buffy coat group = 6.6 ± 0.7 mmol/L; Fresh‐filtered units group = 6.6 ± 0.7 mmol/L. Stored‐filtered units group = 6.5 ± 0.7 mmol/L | |
| Interventions |
Cointervention: Quote: "Antibiotic prophylaxis was given for 24 hours with CABG and for 48 hours with valve or combined surgery" (Page 563). | |
| Outcomes |
| |
| Notes | Trial registration: Not reported Funding: NPBI bv, Emmer‐Compascuum, The Netherlands Role of sponsor: Not reported A priori sample size estimation: unclear. Trial authors reported the only two criteria for sample size calculation (the proportion expected in each group). The dropout rate expected is not reported. Conducted: Between March 1992 and August 1994 Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were, by means of a randomizations list at the hospital transfusion service, randomly allocated..." (Page 563). |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "The surgeons and anesthetists were blind to the randomizations result" (Page 563). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "The surgeons and anesthetists were blind to the randomization result" (Page 563). |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All patients transfused were analysed (Page 567). |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | Low risk | |
| Methods | Design: Parallel group RCT Country: The Netherlands Multicentre: Yes, 19 hospitals (3 university, 10 clinical, 6 general) Setting: Hospital Follow‐up: 15 months Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Patients with ruptured aortic aneurysm elective non‐ruptured AA surgery or gastrointestinal oncology) Exclusion criteria: Those aged under 18 years, had received transfusions in the three month before the date of randomisation, or had a previous adverse reaction to blood transfusions or had a specific indications for filtered products.
Non‐filtered products: 605 Filtered products: 595
Main characteristics of patients allocated to groups (transfused+non‐transfused): Age: Non‐filtered group = 67 ± 11 years; Filtered group = 66 ± 11.5 years Percentage of men: Non‐filtered group = 69%; Filtered group = 68% Percentage of patients transfused: Non‐filtered group = 53%; Filtered group = 51% Duration of surgery: Non‐filtered group = 210 min; Filtered group = 205 min | |
| Interventions |
| |
| Outcomes | Primary:
Secondary:
| |
| Notes | Trial registration: www.clinicaltrials.gov, 23 August 2005. Registration No.: NCT00135291 Funding: Health insurance Board, the Netherlands, The National Sanquin Bllod banks. Role of sponsor: Not reported A priori sample size estimation: Yes Conducted: Since June 2000 until December 2001 Declared conflicts of interest: Yes Note: 22 patients because of administrative and logistic errors. The intake of patients in the study had to be stopped at the end of 2001 because of the implementation of universal leucocyte depletion of RBCs in The Netherlands. This measure was taken by the Dutch Ministry of Health in an effort to reduce the risk of possible transmission of variant Creutzfeldt‐Jacob disease in non‐filtered transfusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed either by telephone (central registration of randomisation) or by opening numbered and sealed envelopes at the hospital blood transfusion services. The transfusion service ensured that the released RBCs appeared identical" (Page 2). |
| Allocation concealment (selection bias) | Low risk | Quote: "randomisation was performed either by telephone (central registration of randomisation) or by opening numbered and sealed envelopes at the hospital blood transfusion services. The transfusion service ensured that the released RBCs appeared identical" (Page 2). |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Neither the identity of the patient nor the randomisation group was stored in the main database. The actual randomisation was provided to the statistician only at the final analysis. (Page 2). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "During the study, protocol violations were reported monthly to the national trial office. Patients who received products in violation of randomisation remained in the assigned arm for intention to treat analysis." (Page 2). |
| Incomplete outcome data (attrition bias) | High risk | Loss after transfusion: 9.35% (51/545). Loss after transfusion filtered RBC transfusions: 11% (30/267). Loss after transfusion non‐filtered RBC transfusions: 7.5% (21/278). Imbalance between comparison groups: 3.5%. Comment: Missing outcome data are balanced in numbers across study groups. However, reasons for missing outcome data are likely to be related to true outcome (Protocol violations). |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Other bias | High risk | Design bias. |
| Methods | Design: Parallel group RCT Country: UK Multicentre study: No Setting: Hospital Follow‐up: 3 months after discharge Unit of allocation: Patients Unit of analysis: Patients | |
| Participants | Inclusion criteria: Patients admitted for elective coronary artery bypass grafting or aortic or mitral valve replacement, either singly or in combination Exclusion criteria: Those with a history of recurrent infections, had a current blood disorder, were taking steroid or other immunosuppressive drugs or received transfusions within the past 12 months
Plasma reduced group: 198 patients Buffy coat‐depleted group: 204 patients WBC filtered group: 195 patients
Main characteristics of patients (included non‐transfused): Age: Plasma reduced group = 62.2 ± 9.1 years; Buffy coat‐depleted group = 62.4 ± 8.1 years. WBC filtered group = 61.7 ± 8.6 years Gender ratio (men to women): Plasma reduced group = 2.9; Buffy coat‐depleted group = 2.6; WBC filtered group= 2.9 Preoperative Hb in g/dL: Plasma reduced group = 14.2 ± 1.2; Buffy coat‐depleted group = 14.1 ± 1.2. WBC filtered group = 14.2 ± 1.2 Discharge Hb in g/dL: Plasma reduced group = 11.3 ± 0.9; Buffy coat‐depleted group = 11.3 ± 1. WBC filtered group = 11.1 ± 0.9 | |
| Interventions |
| |
| Outcomes | Primary
Secondary:
| |
| Notes | Trial registration: Not reported Funding: This study was supported in part by a grant from the Northern and Yorkshire R & D directorate of the National Health Service Role of sponsor: Not reported A priori sample size estimation: Yes Conducted: Not reported Declared conflicts of interest: Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to receive, in the event of a transfusion, PR, BCD, or WCF blood" (Page 1128). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization by sealed envelopes was carried out in the hospital blood transfusion department at the time of the admission clinic" (Page 1128). Use of opaque envelopes is not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "The surgical staff were not blinded as to the blood component given, but were unaware of the randomization when the first decision to transfuse was made" (Page 1128). |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: Collection and review of data to determine postoperative infections and other variables were carried out without knowledge of the randomization or type of blood given" (Page 1128). |
| Incomplete outcome data (attrition bias) | Low risk | Comment: All patients transfused were analysed. |
| Selective reporting (reporting bias) | Low risk | The study protocol is unavailable but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The study population received other blood components along with or different from PRBC transfusion. | |
| Non‐randomised clinical trial. | |
| Study protocol included patients with no indication of RBCs. Intervention poorly specified. | |
| No control group. | |
| No transfusion intervention. | |
| Study protocol included patients with no indication of RBCs. | |
| Autologous transfusion as intervention. | |
| The study population received other blood components along with or different from PRBC transfusion. | |
| The study population received other blood components along with or different from PRBC transfusion. | |
| Autologous transfusion as intervention. | |
| Autologous transfusion as intervention. | |
| Study protocol included patients with no indication of RBCs. | |
| The study population received other blood components along with or different from PRBC transfusion. | |
| The study population received other blood components along with or different from PRBC transfusion (platelets intervention). | |
| No transfusion intervention. | |
| No transfusion intervention. | |
| Non‐randomised clinical trial. | |
| Study protocol included patients with no indication of RBCs. | |
| Control group did not receive transfusion. | |
| No transfusion intervention. | |
| Non‐randomised clinical trial. | |
| Did not report a control group. | |
| Meta‐analysis. | |
| Narrative review. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Study design: Parallel group RCT Allocation: Randomized Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Single blind (Investigator) Primary purpose: Prevention |
| Participants | Inclusion criteria:
Exclusion criteria:
Condition requiring high volume transfusion therapy. |
| Interventions | Active comparator 1: Standard blood components Transfusion, if ordered by physician, with unfiltered RBCs and apheresis platelets Experimental 2: Leukoreduced blood components Transfusion, if ordered by a physician, of leukoreduced RBCs and apheresis platelets Experimental: 3 Leukoreduced and irradiated Transfusion, if ordered by physician, of gamma irradiated leukoreduced RBCs and gamma irradiated apheresis platelets |
| Outcomes | Primary outcomes:
Secondary outcomes:
(Time frame: 0 to 5 weeks after surgery) |
| Notes | This study has been completed, but we did not find any publication related to it in the search. Inclusion or exclusion decision cannot be made because sufficient information is not currently available. |
| Methods | Study Design: RCT Allocation: Randomized Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Open labeled Primary purpose: Prevention |
| Participants | Sixty‐four consecutive ICU. All had severe falciparum malaria and required blood transfusion. Pregnant women and patients with previous blood transfusion were excluded. |
| Interventions | Filtered group: Leukodepleted blood transfusion using bedside leukodepletion filter versus regular Control group: non‐leukodepleted blood transfusion |
| Outcomes | Death from all cases at 28 days, incidence of Acute Respiratory Distress Syndrome and sepsis, severity of multiple organ dysfunction, and length of ICU stay in the two treatment groups. Patients were studied over 16 months. |
| Notes | Registration number: Unknown Inclusion or exclusion decision cannot be made because sufficient information is not currently available. |
| Methods | Study design: RCT Allocation: Randomized Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Unknown Primary purpose: Prevention |
| Participants | One hundred patients with cirrhosis of liver, gastric ulcer and cancer were selected to receive RBC concentrates with leukocyte filtration. Another group of 50 patients with liver necrosis, gastric ulcer and cancer were selected to receive non‐filtered RBC concentrates. Two hundred and forty patients with acute or chronic leukaemia, aplastic anaemia, multiple myeloma, thrombocytopenia purpura, diabetes mellitus, cirrhosis of liver, upper gastrointestinal haemorrhage, severe hepatitis, burn and cancer post radioactive or chemical treatment were divided into 2 groups with 120 patients in each one and selected randomly to receive platelet concentrates. |
| Interventions | RBC concentrates with leukocyte filtration versus non‐filtered RBC concentrates. |
| Outcomes | incidence rates of febrile nonhaemolytic transfusion reactions (FNHTR). |
| Notes | Registration number: Unknown. Inclusion or exclusion decision cannot be made because sufficient information is not currently available. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TRALI. Number of events of the total of randomised patients reported Show forest plot | 1 | 1864 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.36] |
| Analysis 1.1 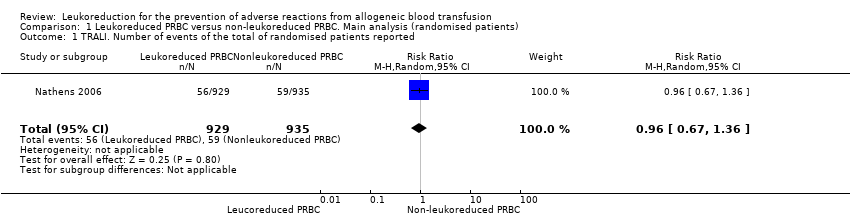 Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 1 TRALI. Number of events of the total of randomised patients reported. | ||||
| 2 Death. Number of events of the total of randomised patients reported Show forest plot | 9 | 6485 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| Analysis 1.2 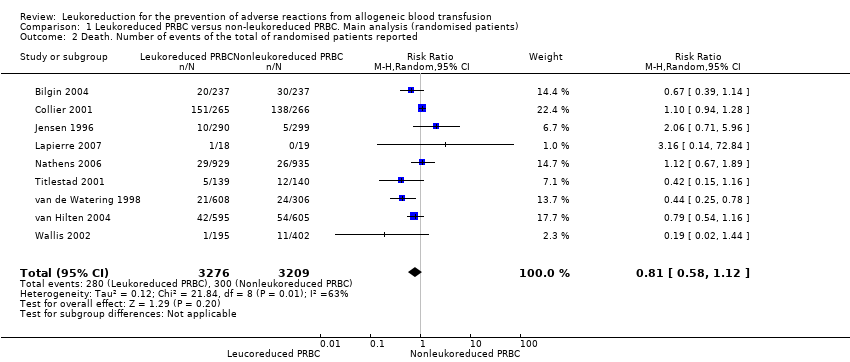 Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 2 Death. Number of events of the total of randomised patients reported. | ||||
| 3 Infection. Number of events of the total of randomised patients reported Show forest plot | 10 | 6709 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| Analysis 1.3  Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 3 Infection. Number of events of the total of randomised patients reported. | ||||
| 4 Adverse events. Number of events of the total of randomised patients reported Show forest plot | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| Analysis 1.4  Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 4 Adverse events. Number of events of the total of randomised patients reported. | ||||
| 4.1 Fever | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TRALI. Number of events of the total of transfused patients reported Show forest plot | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.29] |
| Analysis 2.1  Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 1 TRALI. Number of events of the total of transfused patients reported. | ||||
| 2 Death. Number of events of the total of transfused patients reported Show forest plot | 10 | 4060 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.07] |
| Analysis 2.2 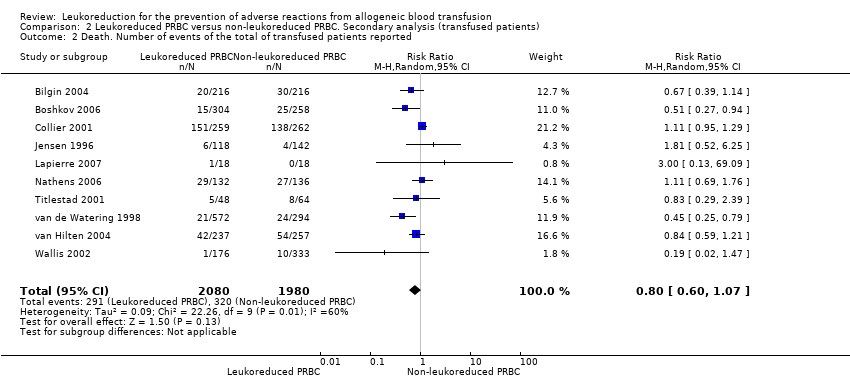 Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 2 Death. Number of events of the total of transfused patients reported. | ||||
| 3 Infection. Number of events of the total of transfused patients reported Show forest plot | 10 | 3557 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| Analysis 2.3  Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 3 Infection. Number of events of the total of transfused patients reported. | ||||
| 4 Adverse events. Number of events of the total of transfused patients reported Show forest plot | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| Analysis 2.4 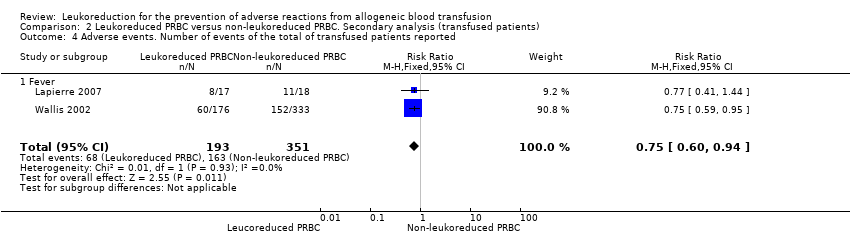 Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 4 Adverse events. Number of events of the total of transfused patients reported. | ||||
| 4.1 Fever | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
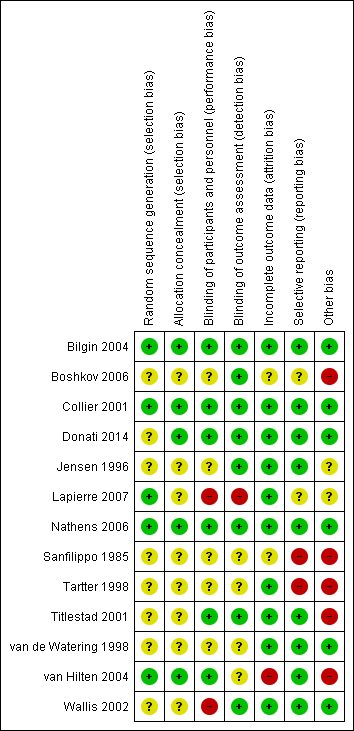
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
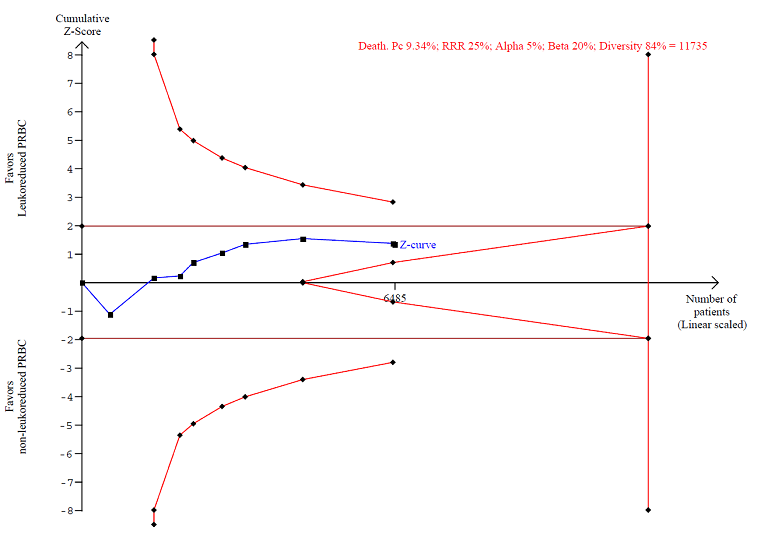
TSA calculated to reliably detect a 25% relative change in the incidence of death from any cause, assuming a control group event rate of 9.34% with a power of 80% at an alpha of 5%

Funnel plot of comparison: 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main Analysis (Randomised patients), outcome: 1.3 Infection. Number of events of the total of randomised patients reported.

TSA calculated to reliably detect a 25% relative change in the incidence of infection from any cause, assuming a control group event rate of 20.4% with a power of 80% at an alpha of 5%.

TSA calculated to reliably detect a 25% relative change in the incidence of fever, assuming a control group event rate of 38.7% with a power of 80% at an alpha of 5%.

Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 1 TRALI. Number of events of the total of randomised patients reported.

Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 2 Death. Number of events of the total of randomised patients reported.

Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 3 Infection. Number of events of the total of randomised patients reported.
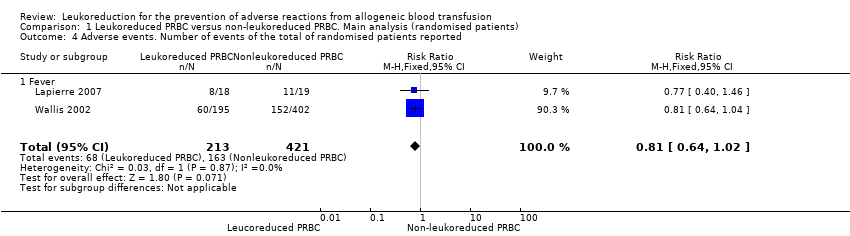
Comparison 1 Leukoreduced PRBC versus non‐leukoreduced PRBC. Main analysis (randomised patients), Outcome 4 Adverse events. Number of events of the total of randomised patients reported.
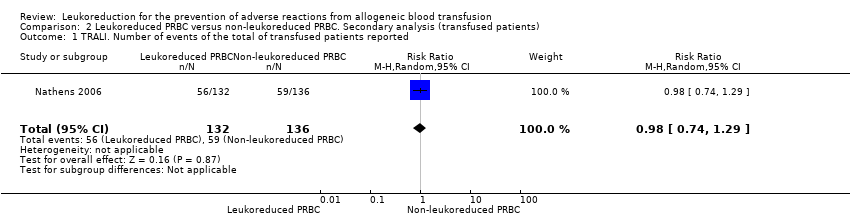
Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 1 TRALI. Number of events of the total of transfused patients reported.
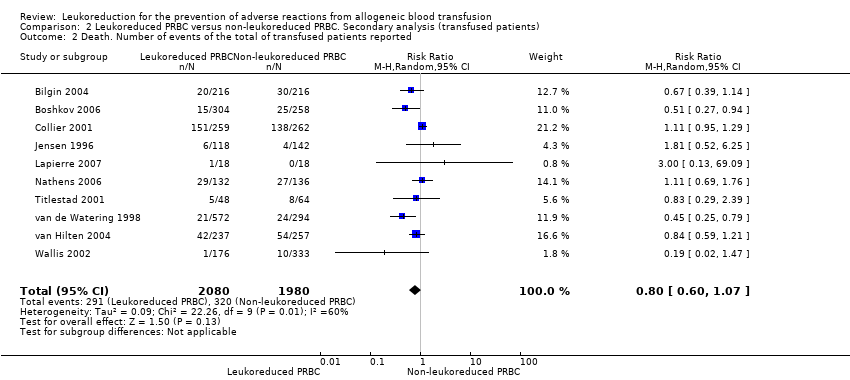
Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 2 Death. Number of events of the total of transfused patients reported.

Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 3 Infection. Number of events of the total of transfused patients reported.

Comparison 2 Leukoreduced PRBC versus non‐leukoreduced PRBC. Secondary analysis (transfused patients), Outcome 4 Adverse events. Number of events of the total of transfused patients reported.
| Leukoreduced PRBCs versus non‐leukoreduced PRBCs for preventing adverse reaction from allogeneic blood transfusion | ||||||
| Patient or population: Patients receiving RBC transfusion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐leukoreduced packed RBCs | Leukoreduced packed RBCs | |||||
| TRALI | Study population | RR 0.96 | 1864 | ⊕⊕⊝⊝ | TSA yielded an inconclusive result. | |
| 63 per 1000 | 61 per 1000 | |||||
| Death due to any cause | Study population | RR 0.81 | 6485 | ⊕⊝⊝⊝ | TSA yielded an inconclusive | |
| 93 per 1000 | 76 per 1000 | |||||
| Infection from any cause | Study population | RR 0.80 | 6709 | ⊕⊝⊝⊝ | TSA yielded an inconclusive | |
| 204 per 1000 | 163 per 1000 | |||||
| Adverse events | Study population | RR 0.81 | 634 | ⊕⊕⊝⊝ | TSA yielded an inconclusive | |
| 387 per 1000 | 314 per 1000 | |||||
| Non‐infectious complication | Study population | Not estimable | — | — | No trial assessed this outcome. | |
| Not estimable | Not estimable | |||||
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by two due to imprecision: small sample size as compared with the calculated DARIS and the wide CI overlapping zones of no effect, as well as potential harm or benefit, or both. Few events reported. 3Downgraded due to: high risk of bias (Seven of 10 included studies were at high or unclear risk of bias, ‐1); important heterogeneity (I² statistic: 84%, ‐2); and imprecision due to the CI crossing the threshold of meaningful effect and an insufficient sample size as compared with the DARIS (‐1) 4Downgraded due to: high risk of bias (All included studies evaluated were at high risk of bias, ‐1) and imprecision due to the CI crossing the threshold of meaningful effect and an insufficient sample size as compared with the DARIS (‐1). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TRALI. Number of events of the total of randomised patients reported Show forest plot | 1 | 1864 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.36] |
| 2 Death. Number of events of the total of randomised patients reported Show forest plot | 9 | 6485 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 3 Infection. Number of events of the total of randomised patients reported Show forest plot | 10 | 6709 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 4 Adverse events. Number of events of the total of randomised patients reported Show forest plot | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| 4.1 Fever | 2 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TRALI. Number of events of the total of transfused patients reported Show forest plot | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.29] |
| 2 Death. Number of events of the total of transfused patients reported Show forest plot | 10 | 4060 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.60, 1.07] |
| 3 Infection. Number of events of the total of transfused patients reported Show forest plot | 10 | 3557 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.58, 1.00] |
| 4 Adverse events. Number of events of the total of transfused patients reported Show forest plot | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 4.1 Fever | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |

