Niacina para la prevención primaria y secundaria de eventos cardiovasculares
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: parallel‐group, factorial (niacin x antioxidant x warfarin), pilot trial Recruitment: 468 participants from 1993‐1994 in 6 study centres in the USA Setting: primary, secondary, and tertiary care Funding: Bristol Myers Squibb supplied pravastatin, Hoffman LaRoche supplied antioxidants, Merck Dupont supplied warfarin, and Upsher Smith supplied niacin | |
| Participants | Inclusion criteria: 30 years or older, ankle‐brachial index < 0.85, documented surgery or angioplasty for peripheral arterial disease, average LDL‐C level < 190 mg/dL. Able to tolerate niacin and warfarin (see run‐in) Exclusion criteria: baseline fasting TG 500 mg/dL or averaged 400 mg/dL; overt complications of peripheral arterial disease, cardiovascular events within 6 months, unstable angina, history of congestive heart failure NYHA class III or IV, atrial fibrillation, poorly controlled diabetes, uncontrolled hypertension, active peptic ulcer, history of bleeding, history of repeated venous thromboembolic disease, cancer within last 10 years, renal insufficiency, liver disease, thrombocytopenia, anaemia, history of gout, history of myositis/rhabdomyolysis, hypothyroidism, therapy with warfarin, heparin or ticlopidine, lipid‐lowering drug, cyclosporine, corticosteroids, alcohol consumption > 14 drinks/week, Women with child‐bearing potential, contraindications to study medications, non‐compliance during run‐in Run‐in/enrichment: 3‐4 months, niacin 1 mg/day (eligibility criteria), warfarin 1 mg/day, and placebos Baseline characteristics Age: 65 years, SD 9 Men: 81% (379/468) Diabetes: 24% (110/468) Current smoker: 39% (183/468) Prior MI/established CHD: 40% (187) Hypertension: 61% (287/486) Statin therapy: 100% | |
| Interventions | Arm 1: Niacin 3000 mg/day or maximally tolerated dosage (randomised = 237, complete cases = 213) Arm 2: Placebo (randomised = 231, complete cases = 209) Duration of treatment: 11 months, “follow‐up at 48 weeks was approximately 85% in each treatment group.” Measure to prevent flushing/unblinding due to flushing: 15% of placebo tables contained low dose niacin (50 mg, no lipid effect expected). Participants therefore experienced intermittent flushing in order to minimise unmasking of niacin therapy Background therapy: All participants received open‐label pravastatin titrated to achieve LDL‐C < 130 mg/dL. Factorial trial: participants were randomly assigned either to active or placebo antioxidant (beta‐carotene, vitamin E, and vitamin C antioxidants). Participants were randomly assigned to active or placebo warfarin. All participants were encouraged to stop smoking and/or maintain abstinence from smoking. All participants received aspirin | |
| Outcomes | Multiple primary outcomes: (1) assessment of the ability to treat and follow symptomatic and asymptomatic participants with peripheral arterial disease in a multifactorial, doubly‐masked trial; (2) determination of the feasibility of recruiting women and minorities, asymptomatic people with peripheral arterial disease, and people without overt coronary vascular disease; (3) assessment of the ability to maintain therapy masking; (4) success in treatment during follow‐up measured in terms of the proportion of values within target range at the 3‐month follow‐up for biochemical parameters (LDL‐C, 70 mg/dL‐130 mg/dL; HDL‐C, increased 20% to 25%; international normalised ratio, 1.5 to 2.0; additionally, antioxidant levels were obtained to measure the effect of the antioxidant therapy); (5) safety maintained by close monitoring of side effects, alanine aminotransferase, haemoglobin A1c, and international normalised ratio; and (6) adherence to therapy measured by pill count and proportion of scheduled follow‐up visits completed and by dropout rate Secondary outcomes: Not reported | |
| Notes | Compliance: based on pill count, 90% in the niacin group and 87% in the placebo group Registration: Not reported Not completed as planned: Original sample size was 600 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not explicitly reported but likely computer‐generated. "Randomization assignments at each clinical centre were made in blocks of random size where the block size was a multiple of 8" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind", placebo‐controlled, specific measures to blind investigators and prevent unblinding of participants, "assessment of the ability to maintain therapy masking" mentioned as outcome |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Outcome mortality not reported. Outcome "discontinuation of treatment due to side effects": proportion of missing data 10% in both groups; events/missing: 19/43 in intervention, 9/31 in control |
| Selective reporting (reporting bias) | Unclear risk | Only retrospectively published protocol available |
| Other bias | Low risk | None |
| Methods | Design: 2 parallel‐groups Recruitment: 3414 participants from 2006‐2010 at 92 centres in USA and Canada Setting: Not reported Funding: National Heart, Lung, and Blood Institute, unrestricted grant from Abbott Laboratories. Abbott Laboratories donated the extended‐release niacin, the matching placebo, and ezetimibe; Merck donated simvastatin. Neither of these companies had any role in the oversight or design of the study or in the analysis or interpretation of the data | |
| Participants | Inclusion criteria: 45 years or older, established cardiovascular disease (documented stable CHD, cerebrovascular or carotid disease, or peripheral arterial disease), low baseline levels of HDL cholesterol (< 40 mg/dL for men; < 50 mg/dL for women), elevated triglyceride levels (150 mg/dL‐400 mg/dL), LDL‐C levels lower than 180 mg/dL. Exclusion criteria: hospitalised for an acute coronary syndrome or had undergone a planned revascularisation within 4 weeks, stroke within 8 weeks, fasting glucose > 180 mg/dL or haemoglobin A1C > 9.0%, BP > 200/100 mm Hg unresponsive to medical therapy, active peptic ulcer, active liver disease, recent history of acute gout, chronic renal insufficiency, risk of pregnancy, significant comorbidity likely to cause death in the 3‐ to 5‐year follow‐up, AIDS/active HIV infection, history of substance abuse within 5 years Run‐in/enrichment: open‐label simvastatin 40 mg/day + extended‐release niacin increasing to 2000 mg/day. Run‐in phase could be extended to 8 weeks to demonstrate tolerance of at least 1500 mg/day of niacin Baseline characteristics Age: Mean 63.7, SD 8.7 Men. 85% Diabetes: 33% Current smoker: not reported Prior MI/established CHD: 56% Hypertension: 71% Statin therapy: 94% | |
| Interventions | Arm 1: niacin extended‐release at a dose of 1500 mg/day‐2000 mg/day plus simvastatin 40 mg/day. For those limited to a niacin dose of 1500 mg/day during the run‐in, there was a subsequent attempt to increase dosage to 2000 mg/day over the first year (randomised = 1718, complete cases = 1693) Arm 2: simvastatin + a matching placebo (randomised = 1696, complete cases = 1672) Duration of treatment: mean 36 months Measure to prevent flushing/unblinding due to flushing: medication at bedtime with a low‐fat snack and, if allowed by private physician, taking 325 mg aspirin up to 30 min before taking blinded study medication, avoid hot or spicy food/drink around the time of dosing. Each placebo tablet included a sub‐therapeutic dose of immediate‐release niacin 50 mg. Background therapy: simvastatin 40 mg/day titrated to LDL‐C level in the range of 40 mg/dL‐80 mg dL. Participants in both groups could receive ezetimibe, at a dose of 10 mg/day, to achieve the target LDL‐C level | |
| Outcomes | Primary outcome: composite, first occurrence of CHD death, non‐fatal MI, ischaemic stroke, hospitalisation for acute coronary syndrome, or symptom‐driven coronary or cerebral revascularisation Secondary outcomes: composite end points of (1) CHD death, non‐fatal MI, ischaemic stroke, or high‐risk acute coronary syndrome; or (2) CHD death, non‐fatal MI, or ischaemic stroke; or (3) any cardiovascular death Tertiary outcomes: all‐cause death, composite of all‐cause death, admission for acute coronary syndrome, ischaemic stroke or any arterial revascularisation, and the individual components of the end points | |
| Notes | Compliance: the study drug was discontinued in 25.4% of the participants in the niacin group and in 20.1% of the participants in the placebo group. The overall rate of adherence among the participants who continued treatment was at least 75% Registration: NCT00120289 Not completed as planned: “As a result of the much lower than expected overall event rate, the primary endpoint was redefined.” In addition, the follow‐up was stopped for futility and harm: “the data and safety monitoring board recommended that the blinded intervention be stopped because the boundary for lack of efficacy had been crossed and an unexpected higher rate of ischaemic stroke had been observed among patients who were being treated with niacin” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not explicitly reported but likely computer‐generated: "Randomization was performed with the use of a secure Internet application" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed with the use of a secure Internet application" |
| Blinding of participants and personnel (performance bias) | Low risk | "Blinded treatment to patients and study personnel" |
| Blinding of outcome assessment (detection bias) | Low risk | "A clinical events committee reviewed suspected primary end points (including silent myocardial infarction) with supporting documentation that did not reveal the treatment assignments" |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of missing data: 1.5% in both groups; event/missing: 96/25 in intervention and 82/24 in control |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the prospectively published trial registry record were subsequently reported |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 38 participants from 2011‐2012 in the USA, number of study centres not reported, veterans Setting: tertiary care Funding: North Texas Veterans Healthcare System | |
| Participants | Inclusion criteria: ≥ 18 years, coronary saphenous vein graft, graft stenosis 30%‐60% of angiographic diameter, undergoing clinically‐indicated coronary angiography Exclusion criteria: known intolerance to niacin or statin, life expectancy less than 12 months, a history of liver disease, TG > 500 mg/dL, LDL‐C > 200 mg/dL, HDL‐C > 60 mg/dL, poorly controlled diabetes or hypertension, congestive heart failure NYHA class III or IV Run‐in/enrichment: 4 weeks Baseline characteristics Age: 65 years, SD 6 Men: not reported Diabetes: 63% Current smoker: not reported Prior MI/established CHD: 67% Hypertension: 95% Statin therapy: 100% | |
| Interventions | Arm 1: extended‐release niacin (Niaspan), 1500 mg/day‐2000 mg/day (randomised = 19, complete cases = 19) Arm 2: placebo (randomised = 19, complete cases = 19) Duration of treatment: 12 months Measure to prevent flushing/unblinding due to flushing: 4 week run‐in, matching placebo contained 50 mg of crystalline niacin that causes flushing but has no effect on lipid levels Background therapy: all participants received statin drugs | |
| Outcomes | Primary outcome: change in percent atheroma volume at intravascular ultrasonography Secondary outcomes: a number of radiographic measures for Intermediate saphenous vein graft lesions, exercise capacity and ischaemia assessed by exercise stress testing, carotid intima‐media thickness, reactive hyperemia index, endothelial progenitor cells‐colony forming units/mL of peripheral blood, major adverse cardiac events | |
| Notes | Compliance: 89% in the intervention, and 95% in the control arm Registration: NCT01221402 ALPINE‐SVG was stopped early after publication of AIM‐HIGH 2011 and HPS2‐THRIVE 2014 (planned: 138 participants, enrolled: 38 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment (detection bias) | Low risk | "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)" |
| Incomplete outcome data (attrition bias) | Low risk | "All patients entering the trial prior to early termination of enrolment completed the trial" |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the prospectively published trial protocol were subsequently reported |
| Other bias | Low risk | None |
| Methods | Design: Parallel‐group Recruitment: 167 participants from 2001‐2003 at 1 study centre in the USA Setting: tertiary care military medical centre Funding: partial funding for this study was provided by Kos Pharmaceuticals in the form of an unrestricted research grant administered by the Henry M. Jackson Foundation for the Advancement of Military Medicine | |
| Participants | Inclusion criteria: 30 years or older, coronary vascular disease, currently treated with a statin, LDL‐C < 130 mg/dL and HDL‐C < 45 mg/dL Exclusion criteria: known intolerance to niacin, a history of liver disease, or abnormal liver associated enzymes Run‐in/enrichment: not reported Baseline characteristics Age: 67 years, SD 10 Men: 91% Diabetes: 28% Current smoker: 10% Prior MI/established CHD: 50% Hypertension: 75% Statin therapy: 100% | |
| Interventions | Arm 1: extended‐release niacin (Niaspan), dose increased from 500 mg‐1000 mg within 30 days (randomised = 87, complete cases = 78) Arm 2: placebo (randomised = 80, complete cases = 71) Duration of treatment: maximum 12 months Measure to prevent flushing/unblinding due to flushing: medication taken at night, taken with the participant’s usual daily dose of aspirin Background therapy: all participants received statin drugs | |
| Outcomes | Primary outcome: common carotid intima‐media thickness Secondary outcomes: changes in serum lipid concentrations, liver‐associated enzyme elevations, composite of clinical cardiovascular events including any hospitalisation for an acute coronary syndrome, stroke, an arterial revascularisation procedure, or sudden cardiac death | |
| Notes | Compliance: adherence to study medication based on pill counts at 90, 180, 270, and 365 days ranged from 90.3% to 94.5% and was not statistically different between the placebo and niacin groups. Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated sequence" |
| Allocation concealment (selection bias) | Low risk | "Central research pharmacy to dispense the study medication" |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind", "Only the research pharmacist was aware of the study drug assignment." |
| Blinding of outcome assessment (detection bias) | Low risk | "Only the research pharmacist was aware of the study drug assignment." |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 10% in intervention and 11% in control; event/missing: 1/9 in intervention and 2/9 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 270 participants in 39 centres in the USA (time not reported) Setting: tertiary care Funding: AstraZeneca Pharmaceuticals, LP, Wilmington, DE. The primary study site at Thomas Jefferson University also received support from the Sidney Kimmel Laboratory for Preventive Cardiology | |
| Participants | Inclusion criteria: aged ≥ 18 years, combined dyslipidaemia, fasting levels of cholesterol ≥ 200 mg/dL, TG ≥ 200 mg/dL and ≤ 800 mg/dL, apolipoprotein B ≥ 110 mg/dL, and HDL‐C < 45 mg/dL Exclusion criteria: active arterial disease within 3 months, major organ dysfunction, taking other medications that posed potential study concerns, women at risk of pregnancy, uncontrolled hypertension, hypothyroidism; creatine kinase > 3 times the upper limit of normal; serum creatinine concentrations > 1.8 mg/dL, use of concomitant medications known to affect serum lipid levels or present safety concerns Run‐in/enrichment: 6‐week, instruction to discontinue all lipid‐modifying medications, dietary supplements, and food additives, and to adhere to the American Heart Association Step I diet Baseline characteristics Age: 56.8, SD 10.5 Men: 74% Diabetes: 15% Current smoker: not reported Prior MI/established CHD: 0% Hypertension: not reported (uncontrolled hypertension was an exclusion criterion) Statin therapy: 100% (part of interventions) | |
| Interventions | Arm 1: rosuvastatin 40 mg monotherapy: rosuvastatin 10 mg for 12 weeks, 20 mg for 6 weeks, and 40 mg for 6 weeks (randomised = 72, complete cases = 60) Arm 2: niacin extended‐release 0.5 g for 4 weeks, 1.0 g for 8 weeks, 1.5 g for 6 weeks, and 2.0 g for 6 weeks Arm 3: rosuvastatin 40 mg/niacin extended‐release 1 g: niacin 0.5 g for 4 weeks, 1.0 g for 2 weeks, 1.0 g plus rosuvastatin 10 mg for 6 weeks, 1.0 g plus rosuvastatin 20 mg for 6 weeks, and 1.0 g plus rosuvastatin 40 mg for 6 weeks (randomised = 46, complete cases = 43) Arm 4: rosuvastatin 10‐mg/niacin extended‐release 2‐g group: niacin 0.5 g for 4 weeks, 1.0 g for 2 weeks, 1.0 g plus rosuvastatin 10 mg for 6 weeks, 1.5 g plus rosuvastatin We included the comparison arm 1 vs. arm 3 Duration of treatment: maximum 12 months Measure to prevent flushing/unblinding due to flushing: extended‐release, niacin taken with water at bedtime after a low‐fat snack Background therapy: not reported | |
| Outcomes | Primary outcome: fasting plasma LDL‐C levels Secondary outcomes: Fasting plasma levels of TC, non‐HDL cholesterol, TG, VLDL cholesterol, apolipoprotein B, HDL cholesterol, apolipoprotein A‐1, and lipoprotein(a) (Lp[a]) | |
| Notes | Compliance: intervention: 67%, control: 47% Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | "Open‐label"; low risk of bias for mortality, high for subjective outcomes |
| Blinding of outcome assessment (detection bias) | High risk | "Open‐label" |
| Incomplete outcome data (attrition bias) | Low risk | Outcome overall mortality not reported. Outcome discontinuation of treatment due to side effects: proportion of missing data: 7% in intervention and 4% in control; events/missing: 7/5 in intervention, 1/2 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 432 participants from 2006‐2008 worldwide (countries not reported) Setting: not reported Funding: Merck Sharp & Dohme Corp | |
| Participants | Inclusion criteria: 18‐70 years, heterozygous familial hypercholesterolaemia, LDL‐C > 100 mg/dL, TG < 400 mg/dL, stable dose of intensive LDL‐C‐lowering therapy Exclusion criteria: < 80% drug study compliance, medical conditions known to influence serum lipids, lipoproteins, or ultrasound acoustic window, medication at unstable dose, premenopausal women, poorly controlled or new onset diabetes mellitus, stenosis of the carotid artery, chronic heart failure, uncontrolled cardiac arrhythmias, unstable hypertension, active or chronic hepatobiliary or hepatic disease, HIV positive, episode of gout Run‐in/enrichment: niacin for 8 weeks. Baseline characteristics Age: 54 years, SD 9 Men: 63% Diabetes: not reported Current smoker: bot reported Prior MI/established CHD: not reported Hypertension: not reported Statin therapy: 100% (inclusion criterion) | |
| Interventions | Arm 1: niacin 2000 mg/day + laropiprant (dose not reported) (randomised = 214, complete cases = 180) Arm 2: placebo (randomised = 218, complete cases = 204) Duration of treatment: maximum 96 weeks Measure to prevent flushing/unblinding due to flushing: laropiprant Background therapy: not reported | |
| Outcomes | Primary outcome: carotid intima media thickness Secondary outcomes: lipid profile | |
| Notes | Compliance: not reported Registration: NCT00384293 Not completed as planned: no reason provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Outcome overall mortality not reported. Outcome fatal or non‐fatal MI: proportion of missing data: 16% in intervention and 6% in control; events/missing ratio: 0/34 in intervention, 1/14 control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, clinical outcomes not specified in registry |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 8341 participants from 1966‐1969 in 53 study centres in the USA Setting: not reported Funding: National Heart and Lung Institute | |
| Participants | Inclusion criteria: men; aged 30‐64 years; proved previous MI (class I or II of the functional classification of the NYHA and free from a specified list of diseases and conditions), at least 3 months beyond their most recent MI, free of evidence of recent worsening of their coronary disease or of other major illnesses Exclusion criteria: not reported Run‐in/enrichment: 2‐month control period Baseline characteristics Age: ≥ 55 years Men: 44% Diabetes: 5% oral hypoglycaemic drug Current smoker: 38% Prior MI/established CHD: 100% Hypertension: 52% Statin therapy: 0% (not available at the time) | |
| Interventions | Arm 1: conjugated estrogens, 2.5 mg/day Arm 2: conjugated estrogens, 5.0 mg/day Arm 3: clofibrate, 1.8 g/day Arm 4: dextrothyroxine sodium, 6.0 mg/day Arm 5: niacin, 3.0 g/day (randomised = 1119, complete cases = 1116) Arm 6: placebo (randomised = 2798, complete cases = 2797) We included the comparison arm 5 vs arm 6 Duration of treatment: maximum 96 weeks Measure to prevent flushing/unblinding due to flushing: not reported Background therapy: not reported | |
| Outcomes | Primary outcome: overall mortality Secondary outcomes: other major end points included cause‐specific mortality, particularly coronary mortality and sudden death, and non‐fatal cardiovascular events such as recurrent MI, acute coronary insufficiency, development of angina pectoris, congestive heart failure, stroke, pulmonary embolism, and arrhythmias | |
| Notes | Compliance: median compliance 85% over 5 years Registration: NCT00000482 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Neither the participant nor the clinic staff was informed of participant drug allocation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | "Only four dropout patients (three in niacin, and one in placebo) have been lost to follow‐up such that their vital status at the five year follow‐up was not known." Events/missing: 237/3 in intervention and 583/1 in control |
| Selective reporting (reporting bias) | Unclear risk | Protocol published after end of recruitment, registered retrospectively |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 131 participants in 8 study centres in the USA (time period not reported) Setting: not reported Funding: this study was supported by Kos Pharmaceuticals, Inc., Miami, Florida | |
| Participants | Inclusion criteria: either average LDL‐C ≥ 190 mg/dL and no CHD risk factors, or average LDL > 160 and < 190 mg/dL and a minimum of 2 CHD risk factors Exclusion criteria: secondary hyperlipoproteinaemia, type I or uncontrolled type II diabetes mellitus, baseline alanine aminotransferase levels > 1.3 times the upper limit of normal, active peptic ulcer disease, gout, and hyperuricaemia. Run‐in/enrichment: 6‐week, diet run‐in followed by a 2‐week phase to determine LDL‐C stability Baseline characteristics: Age: mean 54 years, range 21‐75 Men: 59% Diabetes: not reported (but part of exclusion criteria) Current smoker: not reported Prior MI/established CHD: not reported Hypertension: not reported Statin therapy: not reported | |
| Interventions | Arm 1: niacin extended‐release 3000 mg/day 1 dose at bedtime. Initial dosing with extended‐release placebo was 375 mg/day, raised to 500 mg/day, and further increased in 500‐mg increments at 4‐week intervals to a maximum of 3000 mg/day (randomised = 87, complete cases = 46) Arm 2: placebo (randomised = 44, complete cases = 34) Duration of treatment: 25 weeks maximum Measure to prevent flushing/unblinding due to flushing: extended‐release, medication at bedtime, 325 mg aspirin 30 min before medication Background therapy: not reported | |
| Outcomes | Primary outcome: LDL‐C and apolipoprotein B levels Secondary outcome: TC, HDL‐C, VLDL, plasma TG, HDL subfractions, apolipoprotein A‐1, and lipoprotein(a) | |
| Notes | Compliance: not reported Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 47% in intervention and 23% in control; events/missing: 0/41 in intervention and 1/10 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 1220 participants from 2005‐2008 in 106 study centres in the USA Setting: not reported Funding: Merck/Schering‐Plough Pharmaceuticals | |
| Participants | Inclusion criteria: aged 18‐79 years, LDL‐C levels (130 mg/dL‐190 mg/dL), triglyceride levels (≤ 500 mg/dL), and metabolic and clinical stability (e.g. euthyroid, creatinine < 2 mg/dL, creatinine kinase ≤ 2 x ULN, transaminases ≤ 1.5 x ULN) were eligible for inclusion in the study Exclusion criteria: not reported Run‐in/enrichment: 4‐week washout period Baseline characteristics Age: mean 57 years, SD 10.5 Men: 50% Diabetes: 16% Current smoker: not reported Prior MI/established CHD: 9% Hypertension: 65% Statin therapy: 100% (part of interventions) | |
| Interventions | Arm 1: ezetimibe/simvastatin (10/20 mg/day) + niacin (titrated to 2 g/day) (randomised = 676, complete cases = 391) Arm 2: niacin (titrated to 2 g/day) Arm 3: ezetimibe/simvastatin (10/20 mg/day) (randomised = 272, complete cases = 213) We included the comparison arm 1 vs arm 3 Duration of treatment: maximum 24 weeks (first part of a 64‐week study) Measure to prevent flushing/unblinding due to flushing: participants were consulted to take niacin at bedtime with a low–fat snack, aspirin (325 mg), or ibuprofen (200 mg) 30 min before taking niacin, and to avoid alcoholic and hot beverages near the time of taking niacin Background therapy: not reported | |
| Outcomes | Primary outcome: LDL‐C Secondary outcomes: non–HDL‐C, HDL‐C, TG, LDL‐C, non–HDL‐C, TC, apolipoprotein B, ApoA‐I, lipid/lipoprotein ratio, and high‐ sensitivity C‐reactive protein | |
| Notes | Compliance: not reported Registration: NCT00271817 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported, probably low risk of bias |
| Allocation concealment (selection bias) | Low risk | "Central allocation" |
| Blinding of participants and personnel (performance bias) | Low risk | "All study personnel remained blinded to treatment allocation" |
| Blinding of outcome assessment (detection bias) | Low risk | "All study personnel remained blinded to treatment allocation" |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 42% in intervention and 22% in control; events/missing: 0/285 in intervention and 0/59 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, prospectively registered but clinical outcomes not pre‐specified |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 210 from 1 centre in India Setting: tertiary care Funding: Reagent kits sponsored by Reddys laboratories, a Pharma company | |
| Participants | Inclusion criteria: aged 30‐70 years, at least 6 months on statin therapy, at least 2 months on atorvastatin therapy, HDL ≤ 35 mg/dL, adhering to NYHA step II diet Exclusion criteria: triglyceride > 300 mg/dL, hepatobiliary and renal disease, type I diabetes or poorly‐controlled diabetes, secondary forms of hyperlipidaemia, acute MI or unstable angina, hypothyroidism, gout and hyperuricaemia, left ventricular dysfunction Run‐in/enrichment: 8 weeks of atorvastatin if participants were taking an other statin Baseline characteristics (based on comparison of interest) Age: mean 52.5 years, range 22‐70 Men: 97% Diabetes: not reported Current smoker: not reported Prior MI/established CHD: 65% Hypertension: not reported Statin therapy: 100% (part of intervention) | |
| Interventions | Arm 1: niacin 1.5 g/day + atorvastatin (randomised = 104, complete cases = 102) Arm 2: atorvastatin (randomised = 106, complete cases = 102) Duration of treatment: 9 months, SD 1.8 months Measure to prevent flushing/unblinding due to flushing: aspirin along with niaci (dose not reported) Background therapy: for uniformity in interpreting data, only participants on atorvastatin were included. Those participants who were taking a statin other than atorvastatin entered the trial after a run‐in period of 8 weeks of atorvastatin after stopping the other statin. Atorvastatin was used in conventional dosages as would be required for target LDL‐C levels | |
| Outcomes | Primary outcome: not defined Outcomes: completion 8 months' follow‐up, intolerance attributable to study drug which participant feels unable to continue, rise in liver enzymes, rise in creatin kinase asymptomatic, generalised muscle pain/tenderness, worsening glucose intolerance/diabetes | |
| Notes | Compliance: not reported Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi randomised, alternating weekly according to authors |
| Allocation concealment (selection bias) | High risk | Quasi randomised, alternating weekly according to authors |
| Blinding of participants and personnel (performance bias) | High risk | "Open label" |
| Blinding of outcome assessment (detection bias) | High risk | "Open label" |
| Incomplete outcome data (attrition bias) | Low risk | Outcome mortality not reported. Outcome "discontinuation of treatment due to side effects": proportion of missing data, 2% in intervention and 4% in control; events/missing: 4/2 in intervention, 1/4 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 221 from > 3 centres in the USA (time span and exact number of centres not reported) Setting: primary and secondary care Funding: National Institutes of Health, Baylor College of Medicine General Clinical Research Center. Study drugs provided by Abbott Laboratories, Neither the NIH nor Abbott had any role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. Abbott asked to read a draft of the manuscript before its submission for publication | |
| Participants | Inclusion criteria: HIV, 21‐65 years, stable highly active antiretroviral therapy (HAART) regimen for a minimum of 6 months, fasting serum triglyceride level 1.7 mmol/L, body mass index ≥ 18.5 and ≤ 30 Exclusion criteria: fasting serum triglyceride level ≥ 11.3 mmol/L, diabetes, use of any medications known to affect lipid or lipoprotein metabolism including, nutritional supplements (including but not limited to fish oils, creatine), steroidal compounds or anabolic agents, inability to perform the prescribed graded exercise regimen, CD4 cell count less than 200 x 106 cells/L, or presence of an opportunistic infection or conditions likely to prevent the subject from completing the required exercise regimen through the course of the study, history of symptomatic coronary artery disease (MI, angina) or peripheral vascular disease (claudication). Conditions that could affect drug safety including known adverse reactions to niacin or fibrates, serum alanine or aspartate aminotransferase level greater than two‐fold the ULN adult range, renal insufficiency, treatment with warfarin anticoagulants, pregnancy, history of myositis or rhabdomyolysis, past or present alcohol abuse, peptic ulcer disease, cholelithiasis, and gout or hyperuricaemia Run‐in/enrichment: not reported Baseline characteristics (based on comparison of interest) Age: mean 43 years, SD 1.4 Men: 88% Diabetes: 0% Current smoker: not reported (58% had history of smoking) Prior MI/established CHD: 0% (exclusion criterion) Hypertension: not reported Statin therapy: 0% (exclusion criterion) | |
| Interventions | Arm 1: usual care + guideline for nutrition and health Arm 2: low‐saturated‐fat diet and exercise Arm 3: low‐saturated‐fat diet and exercise + fenofibrate 145 Arm 4: low‐saturated‐fat diet and exercise + niacin 2 g /day Arm 5: low‐saturated‐fat diet and exercise + fenofibrate 145 mg + niacin 2 g/day We included the comparison pooled arms 4 + 5 (randomised = 92, complete cases = 49) vs pooled arms 2 + 3 (randomised = 88, complete cases = 53) Duration of treatment: 6 months maximum Diet: education in weight‐maintaining diet with 50% of calories from carbohydrates, 30% of calories from fat, cholesterol no greater than 200 mg/d, and fibre 20–30 g/d Exercise: exercise programme at a study gymnasium, following guidelines of the American College of Sports Medicine. The sessions were supervised by certified trainers 3/weekly for 75–90 min, with aerobic and resistance components We compared pooled arms 4 + 5 vs pooled arms 2 + 3 Measure to prevent flushing/unblinding due to flushing: placebo contained 50 mg niacin Background therapy: not reported | |
| Outcomes | Primary outcomes: fasting triglyceride levels, HDL‐C, and non‐HDL‐C Secondary outcomes: insulin sensitivity, glycaemia, adiponectin, C‐reactive protein, energy expenditure, body composition | |
| Notes | Compliance: not reported Registration: NCT00246376 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number table" |
| Allocation concealment (selection bias) | High risk | "Study personnel were blinded to group allocations except for the person who performed the randomisation and acted as liaison between the pharmacy and the clinical coordinator" |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind", "placebo‐controlled" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Outcome 'mortality' not reported. Outcome 'flushing': proportion of missing data, 47% in intervention and 40% in control; events/missing: 16/26 in intervention, 2/19 in control |
| Selective reporting (reporting bias) | Unclear risk | Protocol published and registered, clinical outcomes not pre‐specified |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 25,673 participants from 2007‐2010 in 245 centres in China, UK, Denmark, Finland, Norway, and Sweden Setting: secondary and tertiary care Funding: Merck | |
| Participants | Inclusion criteria: history of MI, cerebrovascular atherosclerotic disease; or peripheral arterial disease, diabetes mellitus with any of the above or with other evidence of symptomatic CHD Exclusion criteria: < 50 or > 80 years, acute MI, coronary syndrome or stroke within 3 months; planned revascularisation procedure, history of chronic liver disease, or abnormal liver function, breathlessness at rest for any reason, renal insufficiency, active inflammatory muscle disease, adverse reaction to a statin, ezetimibe, niacin or laropiprant, active peptic ulcer, concurrent treatment with fibrate, niacin, ezetimibe, statin, potent CYP3A4 inhibitor, ciclosporin, amiodarone, verapamil, danazol, known to be poorly compliant with clinic visits or prescribed medication; medical history that might limit the individual’s ability to take trial treatments for the duration of the study Run‐in/enrichment: 4 weeks to standardised simvastatin 40 mg daily or, if not sufficient to achieve a TC < 3.5 mmol/L when measured after 4 weeks, simvastatin 40 mg plus ezetimibe 10 mg daily Baseline characteristics Age: mean 64.9 years, SD 7.5 Men: 83% Diabetes: 32% Current smoker: 18% Prior MI/established CHD: 78% Hypertension: 62% (treated hypertension) Statin therapy: 100% (background therapy) | |
| Interventions | Arm 1: niacin extended‐release 2 g plus laropiprant 40 mg daily (randomised = 12,838, complete cases = 12,730) Arm 2: matching placebo (randomised = 12,835, complete cases = 12,745) Duration of treatment: median of 3.9 years Measure to prevent flushing/unblinding due to flushing: extended‐release Background therapy: statin‐based LDL‐C–lowering therapy | |
| Outcomes | Primary outcome: composite of first non‐fatal MI, coronary death, stroke, or arterial revascularisation Secondary outcome: major coronary events, non‐fatal MI or coronary death | |
| Notes | Compliance: 75% in intervention, 83% in control Registration: NCT00461630 and ISRCTN29503772 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization... was provided by the study clinic computer which was synchronized frequently with the study database at the coordinating centre in the Clinical Trial Service Unit, Oxford via secure Internet connection." |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Low risk | "Blind to treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of missing data: 1% in both arms; events/missing: 798/108 in intervention and 732/90 in control |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the prospectively published trial registry record were subsequently reported |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 237 in 1999 from 23 centres in the USA Setting: not reported Funding: Kos Pharmaceuticals, Inc | |
| Participants | Inclusion criteria: ≥ 18 years, elevated LDL‐C levels or elevated LDL‐C and TG levels. Exclusion criteria: TG > 800 mg/dL, hepatic dysfunction, renal disease, biliary disease, severe hypertension, recent major vascular event, peptic ulcer, gout, type 1 or uncontrolled type 2 diabetes mellitus, cancer, risk of pregnancy, statin within 4 weeks Run‐in/enrichment: 6 weeks' wash out and baseline evaluation Baseline characteristics (based on comparison of interest) Age: mean 59 years, SD 12 Men: 51% Diabetes: not reported Current smoker: not reported Prior MI/established CHD: not reported Hypertension: not reported Statin therapy: 100% (part of the intervention) | |
| Interventions | Arm 1: niacin extended‐release 1000 mg/day + lovastatin 20 mg/day Arm 2: niacin extended‐release 2000 mg/day + lovastatin 40 mg/day (randomised = 57, complete cases = 57) Arm 3: niacin extended‐release 2000 mg/day Arm 4: lovastatin 40 mg/day (randomised = 61, complete cases = 61) We included comparison arm 2 vs arm 4 Duration of treatment: maximum 28 weeks Measure to prevent flushing/unblinding due to flushing: medication at bedtime along with a low‐fat snack and were allowed to take aspirin 325 mg Background therapy: not reported | |
| Outcomes | Primary outcome: LDL‐C Secondary outcomes: TC, HDL‐C, TG, lipoprotein(a), and apolipoprotein B, non‐HDL‐C | |
| Notes | Compliance: not reported for each arm Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind", "Several measures were undertaken to ensure blinding. First, all study medications were identical in shape, size, and colour. Second, equal numbers of active treatment and matched placebo tablets were administered to all four treatment groups during each phase of the study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Loss to follow‐up per group not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol published retrospectively, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel groups Recruitment: 71 participants from a single centre in the UK (time period not reported) Setting: not reported Funding: investigator‐initiated study funded by Merck KGaA | |
| Participants | Inclusion criteria: HDL‐C < 40 mg/dL in previous 12 months and carotid atherosclerosis or peripheral arterial disease Exclusion criteria: contraindications to MRI or to niacin; severe carotid stenosis (> 70%); treatment with fibrates, nicorandil, or oral nitrates, recent acute coronary syndrome; uncontrolled diabetes; fasting triglyceride level > 500 mg/dL; peptic ulcer; cardiac failure requiring diuretic treatment Run‐in/enrichment: not reported Baseline characteristics Total randomised: 71 Age: mean 65, SD 9 Men: 94% Diabetes: 65% Current smoker: 83% Prior MI/established CHD: 48% Hypertension: 78% Statin therapy: 100% | |
| Interventions | Arm 1: nicotinic acid was increased on a weekly basis from 375 mg to 500 mg, and then to 750 mg daily. Participants subsequently received 1000 mg for 4 weeks, 1500 mg for a further 4 weeks, and then 2000 mg daily for the remainder of the study (randomised = 35, complete cases = 25) Arm 2: placebo (randomised = 36, complete cases = 30) Duration of treatment: maximum 12 months Measure to prevent flushing/unblinding due to flushing: medication at night, together with aspirin Background therapy: not reported | |
| Outcomes | Primary outcome: carotid artery wall area Secondary outcomes: other MRI outcomes | |
| Notes | Compliance: niacin (93%) and placebo (92%) based on pill count Registration: NCT00232531 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Outcome "mortality" not reported. Outcome "discontinuation of treatment due to side effects": proportion of missing data, 17% in intervention and 14% in control; events/missing: 7/6 in intervention, 2/5 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, clinical outcomes not specified in registry |
| Other bias | Low risk | None |
| Methods | Design: pilot, parallel Recruitment: 28 participants from 1987‐1989 in 6 centres in Korea Setting: not reported Funding: Korean Society of Circulation (Industrial‐Educational Cooperation 2006) | |
| Participants | Inclusion criteria: 20‐70 years, coronary stenosis in angiogram, and who had not been taking, hormone therapy or anti‐oxidant vitamins within the previous 2 months. Exclusion criteria: cholesterol lowering, anti‐oxidants, or hormones within 2 months, premenopausal women, hypercholesterolaemia, cyclosporine or antifungal agents (azole), severe left ventricular dysfunction, liver disease, renal dysfunction, hypothyroidism, ileal bypass. Run‐in/enrichment: not reported Baseline characteristics Age: mean 60, SD 7 Men: 50% Diabetes: 46% Current smoker: 29% Prior MI/established CHD: 57% Hypertension: 32% Statin therapy: 100% (part of intervention) | |
| Interventions | Arm 1: niacin 1,000 mg + simvastatin 40 mg (randomised = 14, complete cases = 14) Arm 2: simvastatin 40 mg (randomised = 14, complete cases = 14) Duration of treatment: maximum 9 months Measure to prevent flushing/unblinding due to flushing: medication at night Background therapy: not reported | |
| Outcomes | Primary outcomes: normalised total atheroma volume, percent atheroma volume, C‐reactive protein, matrix metalloproteinase‐9, soluble CD40 ligand Secondary outcome: secondary end points were changes in high sensitivity C‐reactive protein, matrix metalloproteinase‐9 and soluble CD40 ligand | |
| Notes | Compliance: not reported Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | "Open‐label" |
| Blinding of outcome assessment (detection bias) | High risk | "Open‐label" |
| Incomplete outcome data (attrition bias) | Low risk | No missing data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel‐group Recruitment: 60 participants in 6 centres in Germany (timeframe not reported) Setting: not reported Funding: Merck (not involved in either the study design or the data analysis) and Leipzig University, Germany | |
| Participants | Inclusion criteria: between 35 and 65 years HDL‐C < 1.0 mmol/L. Impaired glucose tolerance, absence inflammatory disease, undetectable antiGAD antibodies, (3) systolic BP < 140 mmHg, diastolic BP < 90 mmHg Exclusion: cardiovascular or peripheral artery disease, thyroid dysfunction, concomitant medication intake, alcohol or drug abuse, pregnancy, impaired liver function, impaired renal function Run‐in/enrichment: not reported Baseline characteristics Age: mean 45 years, SD 4 Men: 70% Diabetes: 0% (exclusion criterion) Current smoker: not reported Prior MI/established CHD: 0% (exclusion criterion) Hypertension: 0% (exclusion criterion) Statin therapy: 0% (exclusion criterion) | |
| Interventions | Arm 1: extended‐release niacin 1000 mg /day (randomised = 30, complete cases = 30) Arm 2: Usual care, any medication or lifestyle intervention (randomised = 30, complete cases = 30) Duration of treatment: maximum 6 months Measure to prevent flushing/unblinding due to flushing: extended‐release, aspirin 300 mg Background therapy: not reported | |
| Outcomes | Primary outcome: not reported Secondary outcome: not reported | |
| Notes | Compliance: 100% Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | "Open‐label" |
| Blinding of outcome assessment (detection bias) | High risk | "Open‐label" |
| Incomplete outcome data (attrition bias) | Low risk | No missing data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 1613 participants multiple centres worldwide (countries and timeframe not reported) Setting: not reported Funding: Merck | |
| Participants | Inclusion criteria: age 18–85, primary hypercholesterolaemia or mixed dyslipidaemia, ongoing statin, be at or below their National Cholesterol Education Program, LDL‐C < 100 mg⁄ dL for high‐risk participants, < 130 mg⁄ dL (3.37 mmol⁄ L) for participants with multiple risk factors. 130‐190 mg/dL for low‐risk participants, TG < 350 mg⁄ dL Exclusion criteria: impaired renal function, impaired liver function, creatine kinase > 2 x ULN or thyroid stimulating hormone outside the central laboratory’s normal reference range. Experiencing menopausal flashes, poorly controlled, unstable, or new onset diabetes, various concomitant drugs Run‐in/enrichment: 4 weeks' placebo Baseline characteristics (based on all randomised participants) Total randomised: 1613 (813 in comparison of interest. Other arms: 800 in arm 1) Age: mean 58, SD 11 Men: 61% Diabetes: 16% Current smoker: not reported Prior MI/established CHD: not reported Hypertension: not reported Statin therapy: 67% | |
| Interventions | Arm 1: niacin extended‐release 2000 mg/day + laropiprant 40 mg/day Arm 2: niacin extended‐release 2000 mg/day Arm 3: placebo We included the comparison combined arms 1 and 2 (randomised = 1343, complete cases = 917) vs arm 3 (randomised = 270, complete cases = 239) Duration of treatment: Max 26 weeks Measure to prevent flushing/unblinding due to flushing: extended‐release, laropiprant, medication at bedtime after snack, aspirin 100 mg permitted Background therapy: Not reported | |
| Outcomes | Primary outcome: LDL‐C levels, flushing Secondary outcomes: additional lipid end‐points, additional flushing end‐points including discontinuation of treatment due to flushing | |
| Notes | Compliance: not reported Registration: NCT00269204 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported, but probably low |
| Allocation concealment (selection bias) | Low risk | "Randomisation of study drug was achieved via an Interactive Voice Response System" |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 32% in intervention group, 12% in control group; event/missing: 2/230 in intervention and 0/31 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, clinical outcomes not specified in registry |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 796 from 2007‐2008 in 32 centres in the USA and 62 international centres Setting: not reported Funding: Merck | |
| Participants | Inclusion criteria: 18‐80 years, type 2 diabetes mellitus, stable dose of anti‐diabetes mellitus medication, LDL‐C between 1.55 and 2.97 mmol/L, TG ≤ 5.65 mmol/L Exclusion criteria: type 1 diabetes mellitus, renal dysfunction, liver dysfunction, elevated thyroid‐stimulating hormones, poorly‐controlled type 2 diabetes mellitus (within 3 months of randomisation), various concomitant drugs Run‐in/enrichment: 4 weeks lipid‐modifying run‐in period to attain LDL‐C < 2.97 mmol/L if necessary Baseline characteristics (based on all randomised participants) Age: 62 years, SD 9.4 Men: 314/796, 39% Diabetes: 796/796, 100% Current smoker: not reported Prior MI/established CHD: not reported Hypertension: not reported Statin therapy: 78% | |
| Interventions | Arm 1: extended‐release niacin + laropiprant. Starting dose 1 g/20 mg, doubled after 4 weeks of double‐blind treatment to 2 g/40 mg (randomised = 454, complete cases = 298) Arm 2: placebo (randomised = 342, complete cases = 277) Duration of treatment: maximum 36 weeks Measure to prevent flushing/unblinding due to flushing: extended‐release, laropiprant Background therapy: permitted lipid‐altering therapies included fish oils, statins, fibrates, ezetimibe, ezetimibe/simvastatin combination tablet, and bile acid sequestrants | |
| Outcomes | Primary outcome: LDL‐C levels Secondary outcomes: other lipid endpoints and C‐reactive protein | |
| Notes | Compliance: not reported Registration: NCT00485758 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 34% in intervention and 19% in control; events/missing ratio: 0/156 for intervention and 1/65 for control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, clinical outcomes not specified in registry |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 97 participants in 3 centres in the USA Setting: not reported Funding: National Institute on Disability and Rehabilitation Research, US Department of Education; and Kos Pharmaceuticals, Inc | |
| Participants | Inclusion criteria: 18‐65 years, chronic tetraplegia for longer than 1 year, in good health and without evidence of acute illness Exclusion criteria: recurrent acute infection or illness, trauma, or surgery within 6 months; pregnancy; previous MI or cardiac surgery; lipid‐lowering therapy within 6 months; daily alcohol consumption; abnormal menstruation; lifestyle modifications within 6 months of study enrolment; various concomitant medication Run‐in/enrichment: none Baseline characteristics (based on all randomised participants) Age: Mean 33.0, SD 8.7 Men: not reported Diabetes: mot reported Current smoker: 0% Prior MI/established CHD: 0% (exclusion criterion) Hypertension: not reported Statin therapy: not reported | |
| Interventions | Arm 1: placebo (randomised = 23, complete cases = 23) Arm 2: extended‐release niacin 2000 mg/day (randomised = 31, complete cases = 31) Duration of treatment: maximum 48 weeks Measure to prevent flushing/unblinding due to flushing: extended‐release, 325‐mg aspirin, niacin before bedtime after snack, avoidance of alcohol and hot drinks Background therapy: not reported | |
| Outcomes | Primary outcome: fasting HDL‐C level and plasma TC/HDL‐C ratio Secondary outcomes: other lipid outcomes | |
| Notes | Compliance: not reported Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not reported but likely computer‐generated, "permuted block design" |
| Allocation concealment (selection bias) | Low risk | Central allocation, "Study drug and placebo were dispensed, at the beginning of each study month, by the research pharmacies located at each study site." |
| Blinding of participants and personnel (performance bias) | High risk | "Single‐blind design", "Subjects were masked from their group assignment until after the study was completed or they withdrew from the trial" |
| Blinding of outcome assessment (detection bias) | High risk | "Single‐blind design", "Subjects were masked from their group assignment until after the study was completed or they withdrew from the trial" |
| Incomplete outcome data (attrition bias) | Low risk | No missing data reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 145 participants in a single centre in the USA (timeframe not reported) Setting: secondary care Funding: National Institute on Aging. Kos Pharmaceuticals, later acquired by Abbott Pharmaceuticals, provided study drug at no cost and funding to complete data analysis | |
| Participants | Inclusion criteria: ≥ 65 years, history of cardio‐ vascular events or evidence of atherosclerosis, with baseline LDL < 3.24 mmol/L if already on statin therapy and < 3.89 mmol/L if untreated. Exclusion criteria: current use or intolerance of niacin, contraindication to MRI or gadolinium contrast, liver dysfunction, renal failure Run‐in/enrichment: none Baseline characteristics (based on all randomised participants) Age: 73, interquartile range 69–77 Men: 81% Diabetes: 26% Current smoker: 39% Prior MI/established CHD: 31% Hypertension: 78% Statin therapy: 100% | |
| Interventions | Arm 1: placebo (randomised = 73, complete cases = 58) Arm 2: extended‐release niacin 1500 mg/day (randomised = 72, complete cases = 59) Duration of treatment: maximum 18 months Measure to prevent flushing/unblinding due to flushing: extended‐release Background therapy: not reported | |
| Outcomes | Primary outcome: internal carotid artery wall volume Secondary outcomes: HDL, LDL, volumes of internal carotid artery lumen, internal carotid artery lipid core, common carotid artery wall, common carotid artery lumen and common carotid artery lipid core Specified in trial registry but not reported: cardiovascular events | |
| Notes | Compliance: "A minimum pill count compliance of 80% was required to maintain enrolment" Registration: NCT00127218 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Likely computer‐generated, "using a random number schema stratified to ensure equal numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and investigators were blinded to treatment group assignments |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 18% in intervention and 21% in control; events/missing 0/13 in intervention and 1/15 in control |
| Selective reporting (reporting bias) | High risk | Cardiovascular events specified in registry record but subsequently not reported |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 85 participants from 1986‐1987 in Italy (number of centres not reported) Setting: not reported Funding: not reported | |
| Participants | Inclusion criteria: 45‐55 years, ischaemic heart disease Exclusion criteria: presence of symptoms of carotid and/or femoral artery disease Run‐in / enrichment: not reported Baseline characteristics Age: 51 years, SD 3 Men: 95% Diabetes: 24% Current smoker: 31% Prior MI/established CHD: 89% Hypertension: 62% Statin therapy: not reported | |
| Interventions | Arm 1: hypolipidaemic diet (randomised = 45, complete cases = 34) Arm 2: hypolipidaemic diet + acipimox 500 mg/day‐750 mg/day (nicotinic compound) (randomised = 40, complete cases = 30) Duration of treatment: maximum 3 years Measure to prevent flushing/unblinding due to flushing: not reported Background therapy: not reported | |
| Outcomes | Primary outcome: stenosis level of carotid and femoral artery Secondary outcome: not reported | |
| Notes | Compliance: "The compliance with drug treatment was good" Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed by utilizing a table of casual numbers; its sequence was applied to the patients' list." |
| Allocation concealment (selection bias) | Unclear risk | "Randomization was performed by utilizing a table of casual numbers; its sequence was applied to the patients' list." |
| Blinding of participants and personnel (performance bias) | High risk | "Cardiologists and patients were aware of the distribution into groups" |
| Blinding of outcome assessment (detection bias) | High risk | "Cardiologists and patients were aware of the distribution into groups" |
| Incomplete outcome data (attrition bias) | High risk | Proportion of missing data: 25% in intervention and 24% in control; events/missing ratio: 3/10 in intervention, 4/11 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
| Methods | Design: parallel Recruitment: 108 participants from 2006‐2007 in a single centre in China Setting: not reported Funding: not reported | |
| Participants | Inclusion criteria: at least 50% stenosis of one coronary artery Exclusion criteria: serious hepatic or kidney diseases; haemodynamic instability; cancer with expected survival < 1 year; administration of lipid‐lowering drugs within the month before inclusion Run‐in/enrichment: not reported Baseline characteristics: Age: 71 years, SD 9 Men: 61% Diabetes: 65% Current smoker: not reported Prior MI/established CHD: imbalance between groups: 36% control, 10% intervention Hypertension: 67% Statin therapy: 100% (part of intervention) | |
| Interventions | Arm 1: atorvastatin 10 mg/day (randomised = 56, complete cases = 56) Arm 2: atorvastatin 10 mg/day + extended‐release niacin 1 g/day (randomised = 52, complete cases = 52) Duration of treatment: maximum 12 months Measure to prevent flushing/unblinding due to flushing: extended‐release Background therapy: all participants were given advice on lifestyle modification and smoking cessation as well as professional training in moderate exercise. They were permitted no lipid‐modifying therapy other than the study drug | |
| Outcomes | Primary outcome: not defined Outcomes: LDL‐C, HDL‐C, TC, TG, apolipoprotein A, apolipoprotein B, lipoprotein a, and fasting glucose, haemoglobin A1c, creatine kinase, creatine kinase MB isoenzyme, aspartate aminotransferase, alanine aminotransferase, adverse events, death from any cause, MI, rehospitalisation, revascularisation | |
| Notes | Compliance: not reported Registration: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | High risk | High risk of bias due to insufficient reporting of methods and substantial imbalance of prognostic factors between groups |
| Methods | Design: parallel‐groups; modified factorial (niacin x estrogen x thyroxin) Recruitment: 570 US veterans between February 1963 and August 1966, number of centres not reported Setting: not reported Funding: drugs supplied by the Ayerst Laboratories, the National Drug Company and Travenol Laboratories, Inc | |
| Participants | Inclusion criteria: only men; documented evidence of a transmural MI within 12 months prior to randomisation Exclusion criteria: major medical diseases (other than atherosclerosis) which might lead to death in < 5 years; presence of any medical condition in which the use of 1 of the 3 active therapeutic agents might be contraindicated Run‐in/enrichment: 1 month prior to randomisation; all participants received placebo. Baseline characteristics (based on all randomised participants) Age: ≤ 45 years: 35%; 46‐65 years: 47%; ≥ 66 years: 18% Men: 100% (570/570) Diabetes: 9% (54/570) Current smoker: not reported Prior MI/established CHD: 100% (inclusion criterion) Hypertension: 19% (106/570) Statin therapy: 0% (not available at the time) | |
| Interventions | Each participant received 3 medications: estrogen (1.25 mg daily), dextrothyroxine (increasing from 1.0 mg to 4.0 mg daily over 4 months), and nicotinic acid (increasing from 1.0 to 4.0 mg daily over 1 month) – or identical placebo: Arm 1: placebo/placebo/placebo, n = 143 Arm 2: estrogen/placebo/placebo, n = 141 Arm 3: placebo/niacin/placebo, n = 77 Arm 4: estrogen/niacin/placebo, n = 68 Arm 5: placebo/placebo/thyroxin, n = 74 Arm 6: estrogen/placebo/thyroxin, n = 67 Duration of treatment: median 36 months We compared pooled arms 3 + 4 (niacin, randomised = 141, complete cases = 140) to pooled arms 1 + 2 (control, randomised = 284, complete cases = 283) Measure to prevent flushing/unblinding due to flushing: none Background therapy: 50% received estrogen (due to factorial design) | |
| Outcomes | Primary outcome: serum cholesterol Outcomes 'flushing' and 'diarrhoea' were only reported for all groups receiving niacin vs. and groups without niacin. Therefore, 33% (141/425) of participants in the placebo group received thyroxin but no participants in the niacin group Secondary outcome: not reported | |
| Notes | Compliance: "Nicotinic acid caused the most troublesome side‐effects, leading to frequent reduction in dosage. Some 28% of participants were maintained at full dose, another 32% had the drug discontinued altogether and the remaining 40% were at intermediate doses." Registration: not available at the time Conflicting information about number of participants lost to follow‐up proportions; proportions range between 8% and 50% for outcome 'overall mortality' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Medications were dispensed in the hospital pharmacy from bottles bearing coded numbers |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported, low risk of bias for participant‐reported outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Proportion of missing data: 0.5% in both groups; events/missing for overall mortality: 31/1 in intervention, 54/1 in control |
| Selective reporting (reporting bias) | Unclear risk | No protocol published, not registered |
| Other bias | Low risk | None |
BP: blood pressure
CHD: coronary heart disease
HDL‐C: high‐density lipoprotein cholesterol
LDL‐C: low‐density lipoprotein cholesterol
MI: myocardial infarction
MRI: magnetic resonance imaging
NYHA: New York Heart Association
TC: total cholesterol
TG: triglycerides
ULN: upper limit of normal
VLDL: very low‐density lipoprotein
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No comparison of interest | |
| No outcome of interest | |
| No comparison of interest | |
| No comparison of interest | |
| No outcome of interest | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No comparison of interest | |
| No comparison of interest | |
| No comparison of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| Follow‐up shorter than 6 months | |
| No outcome of interest | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No outcome of interest | |
| No comparison of interest | |
| No clinical outcome | |
| No comparison of interest | |
| No comparison of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months | |
| No comparison of interest | |
| No comparison of interest | |
| No outcome of interest | |
| No comparison of interest | |
| Follow‐up shorter than 6 months |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Carotid plaque composition study |
| Methods | Randomised parallel groups , double‐blind, follow‐up: 5 years |
| Participants | Inclusion criteria: Aged 21‐70, clinically established coronary artery disease or carotid artery disease, family history of cardiovascular disease, apolipoprotein B level ≥ 120 mg/dL, LDL 100 mgdL‐190 mg/dL without medication, lipid therapy for no more than 12 months before study entry, medically stable, able to undergo MRI procedure Exclusion criteria: immediate plans for carotid endarterectomy, alcohol or drug abuse, liver disease, elevated serum creatine kinase, elevated serum creatinine, diabetes, uncontrolled high BP Run‐in/enrichment: not reported |
| Interventions | Arm 1: atorvastatin, placebo niacin, and placebo colesevelam. Target for LDL: ≤ 80 mg/dL Arm 2: atorvastatin, niacin, and placebo colesevelam. Target for LDL: ≤ 80 mg/dL Arm 3: atorvastatin, niacin, and colesevelam. Target for LDL‐C: ≤ 60 mg/dL Measure to prevent flushing/unblinding due to flushing: not reported |
| Outcomes | Primary outcome: carotid plaque composition, as assessed by MRI Secondary outcomes: composite of cardiovascular disease death, non‐fatal heart attack, stroke, and worsening ischaemia requiring medical interventions |
| Starting date | June 2001 |
| Contact information | See NCT00715273 |
| Notes |
| Trial name or title | Early aortic valve lipoprotein(a) lowering trial (EAVaLL) |
| Methods | Randomised parallel groups, double‐blind, pilot trial, follow‐up: 2 years |
| Participants | Inclusion criteria: aged > 50 and < 85 years, aortic sclerosis, elevated lipoprotein A Exclusion criteria: current use or documented indication for niacin therapy, niacin intolerance, bicuspid valve, unicuspid valve or other congenital cardiac anomaly, renal disease, comorbidity limiting life expectancy to < 2 years, liver disease, newly diagnosed or poorly controlled diabetes, gout or use of anti‐hyperuricaemic medications Run‐in/enrichment: low‐dose niacin (500 mg/d) for 6 weeks to randomisation to assess tolerability and compliance to the intervention. The niacin dose will be increased by 500 mg increments weekly, as tolerated, to a maximum of 1500 mg/day |
| Interventions | Arm 1: extended‐release niacin 1500 mg/day‐2000 mg/day Arm 2: placebo Measure to prevent flushing/unblinding due to flushing: extended‐release |
| Outcomes | Primary outcome: calcium score by cardiac CT Secondary outcome: lipoprotein A, disease progression by echocardiography, peak velocity, mean gradient, aortic valve area, drug compliance, side effects and adverse events |
| Starting date | May 2014 |
| Contact information | See NCT02109614 |
| Notes |
| Trial name or title | The CKD optimal management with bInders and nicotinamide (COMBINE) study |
| Methods | Randomised parallel groups, double‐blind, pilot study |
| Participants | Inclusion criteria: eGFR between 20 and 45 mL/min/1.73 m2, aged 18‐85 years, serum phosphate ≥ 2.8 mg/dL, platelet count ≥ 125,000/mm3 Exclusion criteria: intolerance to study drugs, liver disease, elevated creatine kinase, major haemorrhagic event within the past 6 months, blood transfusion within the past 6 months, secondary hyperparathyroidism, malabsorption, anaemia, decreased serum albumin, dialysis or kidney transplantation, immunosuppressive medications, abuse of alcohol or drugs, vitamin D, phosphate binder, niacin/nicotinamide > 100 mg/day, malignancy Run‐in/enrichment: not reported |
| Interventions | Arm 1: lanthanum carbonate 3000 mg/day + nicotinamide 1500 mg/day Arm 2: lanthanum carbonate 3000 mg/day + nicotinamide placebo Arm 3: lanthanum carbonate placebo and nicotinamide 1500 mg/day Arm 4: lanthanum carbonate placebo and nicotinamide placebo Measure to prevent flushing/unblinding due to flushing: not reported |
| Outcomes | Primary outcome: feasibility, serum phosphate, FGF23 Secondary outcomes: cardiovascular disease, left ventricular mass index, left ventricular end diastolic volume, and left atrial volume, intra‐renal oxygenation and fibrosis, brain natriuretic peptide, troponin T, cholesterol, asymmetric dimethylarginine, parathyroid hormone, calcitriol, klotho, N terminal propeptide of type 1 procollagen, tartrate‐resistant acid phosphatase, glomerular filtration, albuminuria, C reactive protein, interleukin 6 |
| Starting date | March 2015 |
| Contact information | See NCT02258074 |
| Notes |
| Trial name or title | Anticancer activity of nicotinamide on lung cancer |
| Methods | Randomised, parallel, double‐blind, 2 years' follow‐up |
| Participants | Inclusion criteria: Aged 19‐80 years, non‐small‐cell lung carcinoma, EGFR mutated, life expectation > 3 months, > 1 measurable lesion by RECIST 1.1 which were not exposed to radiation previously, Eastern Cooperative Oncology Group performance status grade 0˜2 Exclusion criteria: metastasised brain lesion needing operation or radiation, above grade 2 Common Toxicity Criteria for Adverse Effects criteria for blood, liver and kidney, no contraception, allergy to nicotinamide Run‐in/enrichment: not reported |
| Interventions | Arm 1: nicotinamide 1000 mg/day + gefitinib 250 mg/day or erlotinib 150 mg/day Arm 2: placebo + gefitinib 250 mg/day or erlotinib 150 mg/day Measure to prevent flushing/unblinding due to flushing: not reported |
| Outcomes | Primary: progression‐free survival Secondary: response rate, quality of life, overall survival |
| Starting date | March 2015 |
| Contact information | See NCT02416739 |
| Notes |
| Trial name or title | NIAC‐PKD2 |
| Methods | Randomised, parallel, double‐blind, pilot study, 12 months' follow‐up |
| Participants | Inclusion criteria: aged 18‐60 years, confirmed diagnosis of autosomal dominant polycystic kidney disease, EGFR > 50 mL/min/1.73 m2 Exclusion criteria: liver disease, alcohol intake, malabsorption, thrombocytopenia, hypophosphataemia, pregnancy or lactation, anti‐epileptic drugs, tolvaptan, not able to undergo MRI Run‐in/enrichment: not reported |
| Interventions | Arm 1: niacinamide 30 mg/kg/day Arm 2: placebo |
| Outcomes | Primary outcome: acetylated/total p53 ratio Secondary: kidney volume, pain, MCP‐1, EGFR |
| Starting date | September 2015 |
| Contact information | See NCT02558595 |
| Notes |
BP: blood pressure
CT: computed tomography
EGFR: estimated glomerular filtration rate
MRI: magnetic resonance imaging
RECIST: response evaluation criteria in solid tumours
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall mortality Show forest plot | 12 | 35543 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.12] |
| Analysis 1.1  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 1 Overall mortality. | ||||
| 2 Overall mortality, sensitivity analysis with stratification by risk of bias trials only Show forest plot | 12 | 35543 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.12] |
| Analysis 1.2  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 2 Overall mortality, sensitivity analysis with stratification by risk of bias trials only. | ||||
| 2.1 High risk of bias | 10 | 6703 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.09] |
| 2.2 Low risk of bias | 2 | 28840 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.00, 1.20] |
| 3 Fatal myocardial infarction Show forest plot | 6 | 33336 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| Analysis 1.3  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 3 Fatal myocardial infarction. | ||||
| 4 Cardiovascular mortality Show forest plot | 5 | 32966 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.93, 1.12] |
| Analysis 1.4  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 4 Cardiovascular mortality. | ||||
| 5 Non‐cardiovascular mortality Show forest plot | 5 | 32966 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.98, 1.28] |
| Analysis 1.5 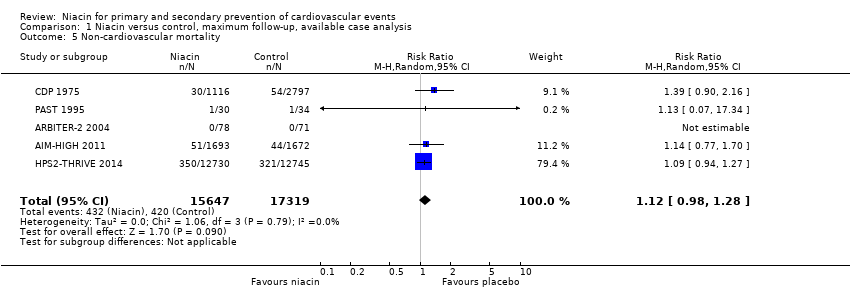 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 5 Non‐cardiovascular mortality. | ||||
| 6 Non‐fatal myocardial infarction Show forest plot | 4 | 33164 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.77, 1.07] |
| Analysis 1.6  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 6 Non‐fatal myocardial infarction. | ||||
| 7 Fatal or non‐fatal myocardial infarction Show forest plot | 9 | 34829 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 1.00] |
| Analysis 1.7 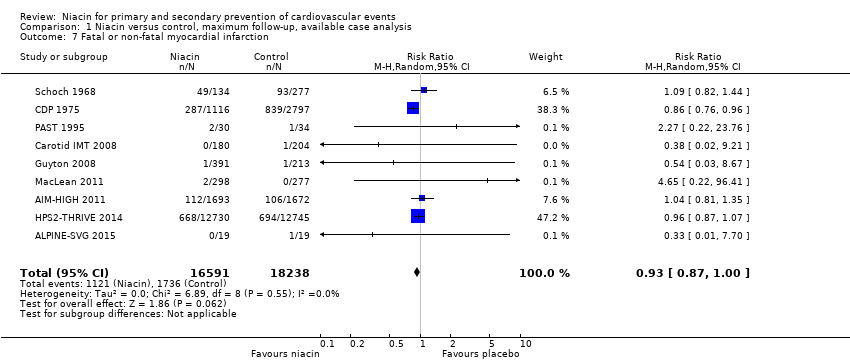 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 7 Fatal or non‐fatal myocardial infarction. | ||||
| 8 Fatal and non‐fatal stroke Show forest plot | 7 | 33661 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.22] |
| Analysis 1.8  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 8 Fatal and non‐fatal stroke. | ||||
| 9 Revascularisation procedures Show forest plot | 8 | 33130 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.06] |
| Analysis 1.9 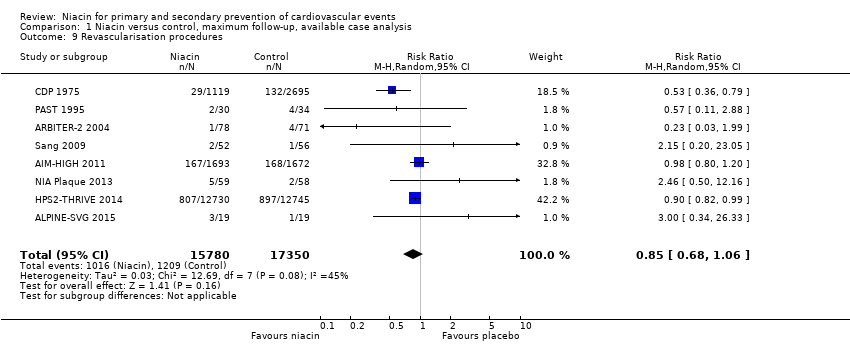 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 9 Revascularisation procedures. | ||||
| 10 Flushing Show forest plot | 15 | 11038 | Risk Ratio (M‐H, Random, 95% CI) | 7.69 [4.14, 14.28] |
| Analysis 1.10 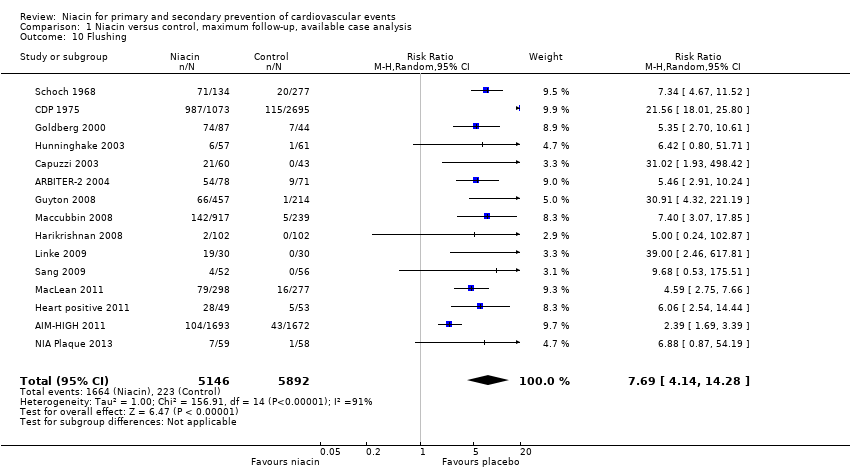 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 10 Flushing. | ||||
| 11 Pruritus Show forest plot | 6 | 5800 | Risk Ratio (M‐H, Random, 95% CI) | 5.26 [2.68, 10.32] |
| Analysis 1.11  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 11 Pruritus. | ||||
| 12 Rash Show forest plot | 9 | 31485 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [1.94, 5.13] |
| Analysis 1.12 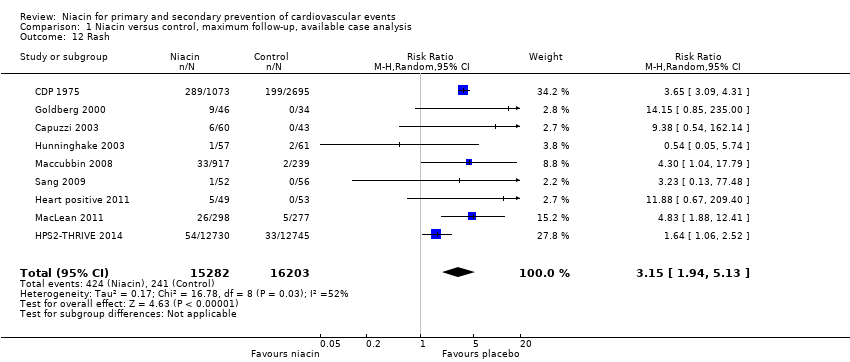 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 12 Rash. | ||||
| 13 Headache Show forest plot | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.86, 2.28] |
| Analysis 1.13 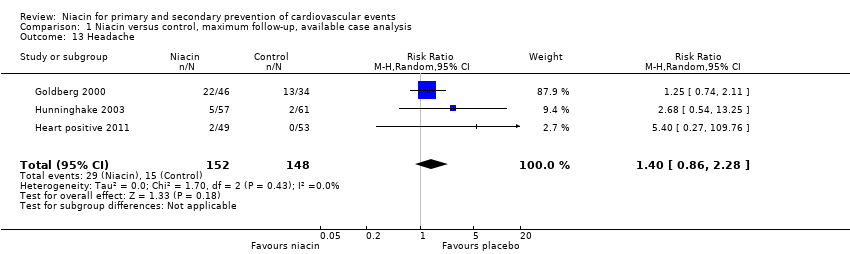 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 13 Headache. | ||||
| 14 Gastrointestinal symptoms Show forest plot | 12 | 35353 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.37, 2.07] |
| Analysis 1.14  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 14 Gastrointestinal symptoms. | ||||
| 15 Discontinuation of treatment due to side effects Show forest plot | 17 | 33539 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.70, 2.77] |
| Analysis 1.15 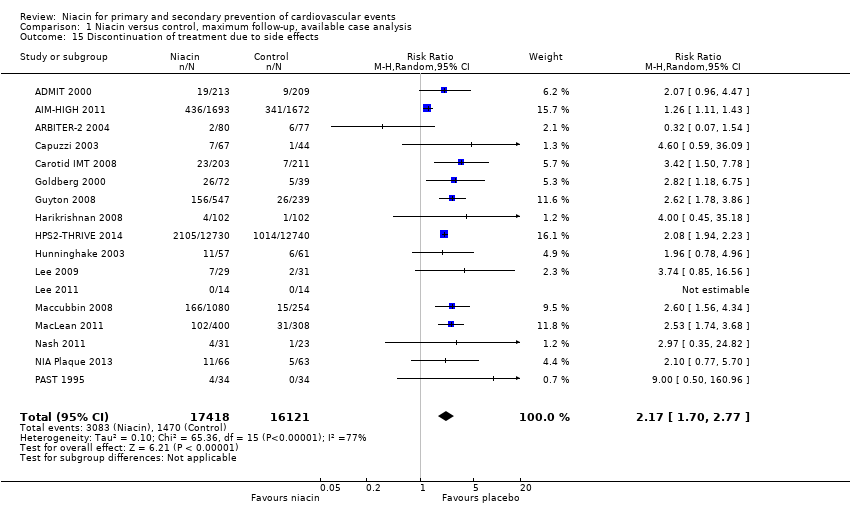 Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 15 Discontinuation of treatment due to side effects. | ||||
| 16 New onset diabetes) Show forest plot | 3 | 27982 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.16, 1.51] |
| Analysis 1.16  Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 16 New onset diabetes). | ||||

Study flow diagram

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Meta‐regression by duration of treatment using the 'matreg' command in Stata version 13 (stata.com) (Number of observations: 12, P = 0.15)
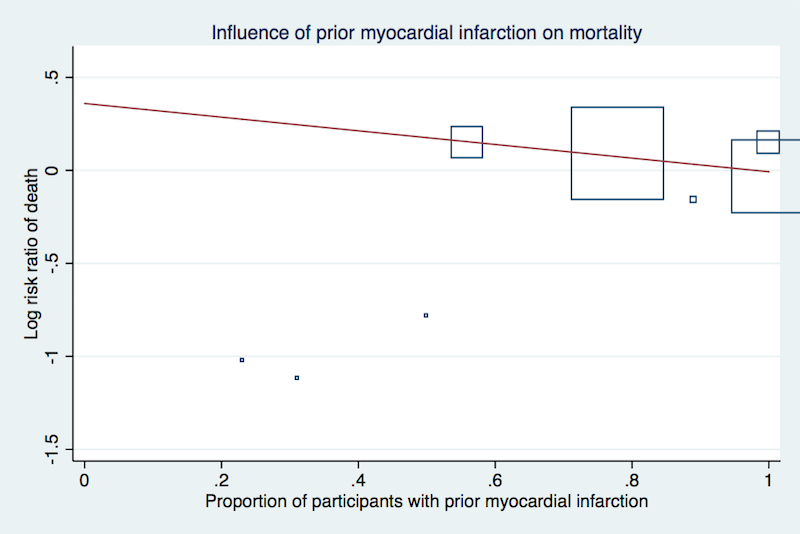
Meta‐regression by proportion of participants with prior myocardial infarction using the 'matreg' command in Stata version 13 (stata.com) (Number of observations:8, P = 0.19)

Meta‐regression by proportion of participants receiving background statin therapy using the 'matreg' command in Stata version 13 (stata.com) (Number of observations: 10, P = 0.15)
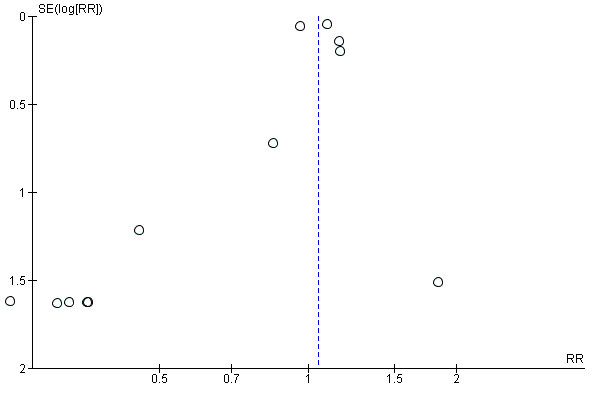
Funnel plot of comparison: 1 niacin over placebo, maximum follow‐up, available case analysis, outcome: 1.1 overall mortality

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 1 Overall mortality.
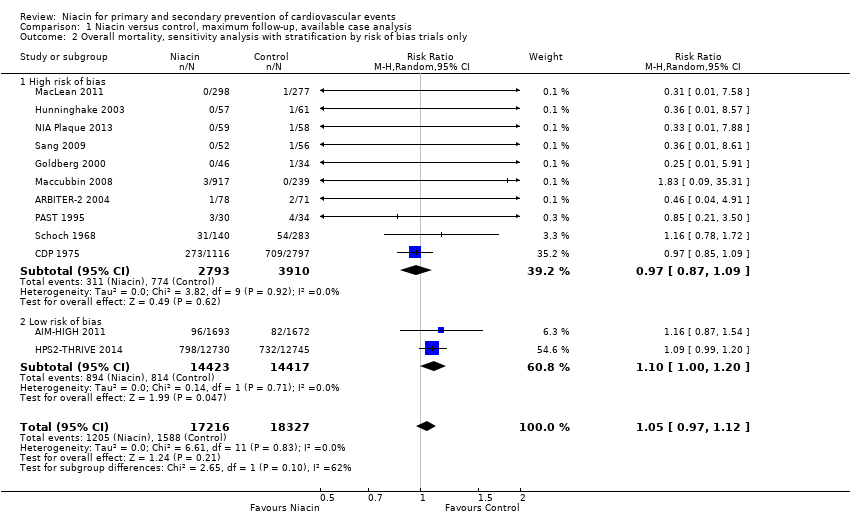
Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 2 Overall mortality, sensitivity analysis with stratification by risk of bias trials only.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 3 Fatal myocardial infarction.
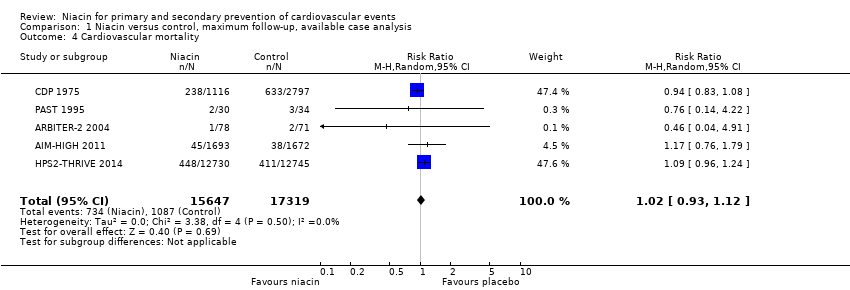
Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 4 Cardiovascular mortality.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 5 Non‐cardiovascular mortality.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 6 Non‐fatal myocardial infarction.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 7 Fatal or non‐fatal myocardial infarction.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 8 Fatal and non‐fatal stroke.
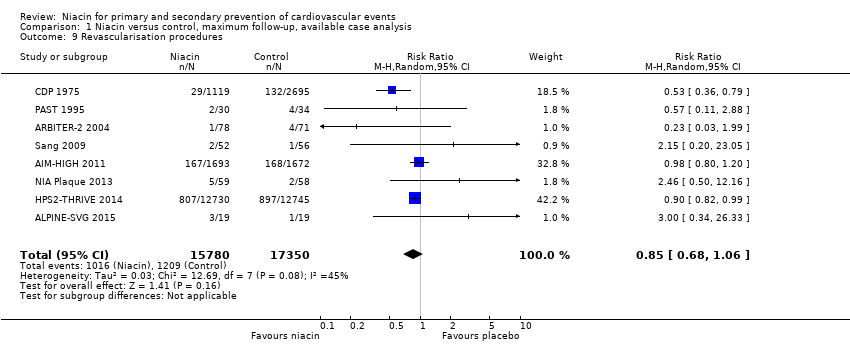
Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 9 Revascularisation procedures.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 10 Flushing.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 11 Pruritus.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 12 Rash.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 13 Headache.
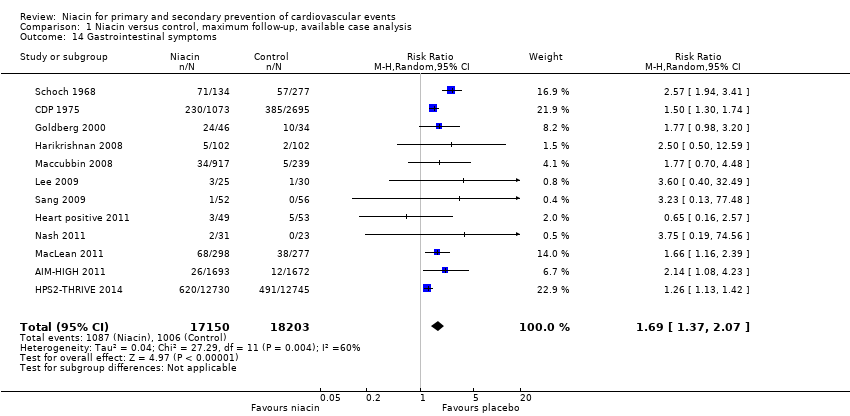
Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 14 Gastrointestinal symptoms.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 15 Discontinuation of treatment due to side effects.

Comparison 1 Niacin versus control, maximum follow‐up, available case analysis, Outcome 16 New onset diabetes).
| Niacin for primary and secondary prevention of cardiovascular events | ||||||
| Patient or population: people with or at risk of cardiovascular disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with niacin | |||||
| Overall mortality | Study population | RR 1.05 | 35,543 | ⊕⊕⊕⊕ | High‐quality evidence that niacin does not reduce overall mortality (CI excludes clinically important benefit) | |
| 86 per 1000 | 90 per 1000 | |||||
| Cardiovascular mortality (follow‐up: 1 year to 5 years) | Study population | RR 1.02 | 32,966 | ⊕⊕⊕⊝ | Moderate‐quality evidence that niacin does not reduce cardiovascular mortality | |
| 63 per 1000 | 64 per 1000 | |||||
| Non‐cardiovascular mortality (follow‐up: 1 year to 5 years) | Study population | RR 1.12 | 32,966 | ⊕⊕⊕⊕ | High‐quality evidence that niacin does not reduce non‐cardiovascular mortality (CI excludes clinically important benefit) | |
| 24 per 1000 | 27 per 1000 | |||||
| Fatal or non‐fatal myocardial infarction (follow up: 0.5 years to 5 years) | Study population | RR 0.93 | 34,829 | ⊕⊕⊕⊝ | Moderate‐quality evidence that niacin does not reduce the number of fatal and non‐fatal myocardial infarctions | |
| 95 per 1000 | 90 per 1000 | |||||
| Fatal and non‐fatal stroke (follow‐up: 0.5 years to 5 years) | Study population | RR 0.95 | 33,661 | ⊕⊕⊝⊝ | Low‐quality evidence that niacin does not reduce the number of strokes | |
| 47 per 1000 | 45 per 1000 | |||||
| Discontinuation of treatment due to side effects (follow‐up: 0.5 years to 4 years) | Study population | RR 2.17 | 33,539 | ⊕⊕⊕⊝ | Moderate‐quality evidence that niacin does increase the number of participants discontinuing treatment due to side effects | |
| 91 per 1000 | 210 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Confidence interval includes clinically relevant benefit and no benefit. We downgraded by one level due to imprecision. | ||||||
| Outcome | Available case analysis | IMOR 1.0, 1.0* | IMOR 0.5, 2.0* | IMOR 2.0, 0.5* | ||||
| RR (95% CI) | I2 | RR (95% CI) | I2 | RR (95% CI) | I2 | RR (95% CI) | I2 | |
| Overall mortality | 1.05 (0.97 to 1.12) | 0% | 1.05 (0.97 to 1.12) | 0% | 1.04 (0.96 to 1.11) | 0% | 1.06 (0.98 to 1.14) | 0% |
| Cardiovascular mortality | 1.02 (0.93 to 1.12) | 0% | 1.02 (0.93 to 1.12) | 0% | 1.01 (0.92 to 1.11) | 0% | 1.03 (0.94 to 1.13) | 0% |
| Non‐cardiovascular mortality | 1.12 (0.98 to 1.28) | 0% | 1.12 (0.98 to 1.28) | 0% | 1.11 (0.97 to 1.27) | 0% | 1.14 (1.00 to 1.30) | 0% |
| Fatal or non‐fatal myocardial infarction | 0.93 (0.87 to 1.00) | 0% | 0.93 (0.87 to 1.00) | 0% | 0.92 (0.86 to 0.99) | 0% | 0.96 (0.87 to 1.05) | 14% |
| Fatal myocardial infarction | 1.01 (0.91 to 1.11) | 0% | 1.01 (0.91 to 1.11) | 0% | 1.00 (0.90 to 1.10) | 0% | 1.02 (0.92 to 1.12) | 0% |
| Non‐fatal myocardial infarction | 0.91 (0.77 to 1.07) | 53% | 0.91 (0.77 to 1.07) | 53% | 0.89 (0.76 to 1.05) | 47% | 0.92 (0.77 to 1.10) | 57% |
| Fatal or non‐fatal stroke | 0.95 (0.74 to 1.22) | 42% | 0.95 (0.74 to 1.22) | 42% | 0.94 (0.73 to 1.21) | 42% | 0.97 (0.75 to 1.26) | 42% |
| Revascularisation | 0.85 (0.68 to 1.06) | 45% | 0.85 (0.68 to 1.06) | 45% | 0.83 (0.66 to 1.04) | 48% | 0.88 (0.69 to 1.09) | 47% |
| Discontinuation of treatment due to side effects | 2.16 (1.70 to 2.76) | 77% | 2.15 (1.68 to 2.74) | 75% | 1.96 (1.55 to 2.49) | 73% | 2.35 (1.82 to 3.03) | 77% |
| Flushing | 7.69 (4.15 to 14.26) | 91% | 7.66 (4.11 to 14.29) | 91% | 6.68 (3.54 to 12.58) | 91% | 8.61 (4.67 to 15.87) | 90% |
| Rash | 3.16 (1.96 to 5.12) | 52% | 3.14 (1.93 to 5.10) | 51% | 2.74 (1.80 to 4.19) | 40% | 3.69 (2.13 to 6.40) | 60% |
| Pruritus | 5.15 (2.62 to 10.13) | 67% | 5.21 (2.68 to 10.13) | 62% | 4.23 (1.94 to 9.23) | 72% | 6.48 (3.78 to 11.10) | 46% |
| Gastrointestinal symptoms | 1.69 (1.37 to 2.09) | 62% | 1.69 (1.36 to 2.11) | 60% | 1.53 (1.23 to 1.91) | 59% | 1.88 (1.48 to 2.39) | 66% |
| Headache | 1.41 (0.86 to 2.30) | 0% | 1.43 (0.83 to 2.46) | 0% | 1.14 (0.64 to 2.03) | 0% | 1.76 (1.05 to 2.97) | 0% |
| CI: confidence interval; IMOR: informative missingness odds ratio; RR: risk ratio Sensitivity analysis for random‐effects meta‐analysis assuming different relationship between the outcomes from observed and missing participants and accounting for the uncertainty introduced by the proportion of missing data and assumed relationship (informative missingness odds ratio, IMOR = odds of event in missing data/odds of event in observed data, SD(logIMOR) = 0.5). We used the “metamiss”‐command in Stata (version 13) (stata.com). *The two numbers represent the assumed IMORs for the niacin and the control arm, respectively: 1.0, 1.0: missing at random; 0.5, 2.0: assumption favours niacin, 2.0, 0.5: assumption favours control. We could not conduct sensitivity analysis for the outcome 'new onset diabetes' because the proportion of missing data was not reported. | ||||||||
| Study | Niacin dose g/day | Follow‐up in months | Total cholesterol | LDL‐cholesterol | HDL‐cholesterol | Triglycerides |
| Baseline mean, | ||||||
| 3 | 11 | 214 (‐4) | 138 (‐6) | 41 (+11) | 176 (‐34) | |
| 2 | 38 | NA (NA) | 74 (‐3) | 35 (+10) | 165 (‐21) | |
| 2 | 12 | 136 (+1) | 69 (+2) | 38 (+3) | 158 (‐19) | |
| 1 | 12 | 158 (+6) | 89 (+3) | 40 (+8) | 163 (‐12) | |
| 2 | 6 | 262 (+3) | 146 (+6) | 36 (+6) | 377 (‐6) | |
| 2 | 18 | 237 (‐6) | 154 (‐9) | 42 (+6) | 201 (‐16) | |
| 3 | 72 | 249 (‐20) | NA (NA) | NA (NA) | NA (NA) | |
| 3 | 6 | 300 (‐31) | 216 (‐48) | 45 (+8) | 191 (NA) | |
| 2 | 6 | 241 (‐4) | 156 (‐9) | 51 (+11) | 159 (‐30) | |
| 1.5 | 9 | 178 (‐9) | 112 (‐11) | 35 (+5) | 157 (‐5) | |
| 2 | 6 | 211 (‐7) | NA (NA) | 39 (+5) | 306 (‐25) | |
| 2 | 23 | 128 (‐5) | 63 (‐10) | 43 (+6) | 124 (‐33) | |
| 2 | 6 | NA (NA) | 188 (‐10) | 44 (+24) | 197 (‐23) | |
| 2 | 12 | 157 (+1) | 85 (‐15) | 38 (+22) | 180 (‐7) | |
| 1 | 9 | 198 (NA) | 122 (NA) | 49 (NA) | 160 (NA) | |
| 1 | 6 | 218 (+4) | 133 (‐9) | 33 (+5) | 154 (‐29) | |
| 2 | 6 | 192 (‐9) | 223 (‐20) | 52 (+22) | 122 (‐57) | |
| 2 | 8 | 127 (NA) | 164 (‐33) | 86 (+21) | 50 (‐15) | |
| 2 | 12 | 178 (‐15) | 118 (‐22) | 33 (+8) | 141 (‐21) | |
| 1.5 | 18 | 172 (0) | 90 (‐4) | 60 (+8) | 130 (‐26) | |
| 0.5 | 36 | 243 (‐8) | 169 (‐13) | 42 (+1) | 162 (‐25) | |
| 1 | 12 | 183 (NA) | 105 (NA) | 50 (NA) | 147 (NA) | |
| 4 | 38 | 242 (‐34) | NA (NA) | NA (NA) | NA (NA) | |
| NA: not available | ||||||
| Study | Outcome | Niacin group | Control group | ||||||
| Randomised | Complete | Missing | Events | Randomised | Complete | Missing | Events | ||
| Discontinuation of treatment due to side effects | 237 | 213 | 24 | 19 | 231 | 209 | 22 | 9 | |
| Fatal myocardial infarction | 1718 | 1693 | 25 | 38 | 1696 | 1672 | 24 | 34 | |
| Non‐cardiovascular mortality | 1718 | 1693 | 25 | 51 | 1696 | 1672 | 24 | 44 | |
| Fatal or non‐fatal myocardial infarction | 1718 | 1693 | 25 | 112 | 1696 | 1672 | 24 | 106 | |
| Cardiovascular mortality | 1718 | 1693 | 25 | 45 | 1696 | 1672 | 24 | 38 | |
| Overall mortality | 1718 | 1693 | 25 | 96 | 1696 | 1672 | 24 | 82 | |
| Non‐fatal myocardial infarction | 1718 | 1693 | 25 | 104 | 1696 | 1672 | 24 | 93 | |
| Revascularisation procedures | 1718 | 1693 | 25 | 167 | 1696 | 1672 | 24 | 168 | |
| Fatal or non‐fatal stroke | 1718 | 1693 | 25 | 30 | 1696 | 1672 | 24 | 18 | |
| Flushing | 1718 | 1693 | 25 | 104 | 1696 | 1672 | 24 | 43 | |
| Gastrointestinal symptoms | 1718 | 1693 | 25 | 26 | 1696 | 1672 | 24 | 12 | |
| Discontinuation of treatment due to side effects | 1718 | 1693 | 25 | 436 | 1696 | 1672 | 24 | 341 | |
| Flushing | 87 | 78 | 9 | 54 | 80 | 71 | 9 | 9 | |
| Overall mortality | 87 | 78 | 9 | 1 | 80 | 71 | 9 | 2 | |
| Cardiovascular mortality | 87 | 78 | 9 | 1 | 80 | 71 | 9 | 2 | |
| Non‐cardiovascular mortality | 87 | 78 | 9 | 0 | 80 | 71 | 9 | 0 | |
| Revascularisation procedures | 87 | 78 | 9 | 1 | 80 | 71 | 9 | 4 | |
| Fatal or non‐fatal stroke | 87 | 78 | 9 | 0 | 80 | 71 | 9 | 1 | |
| Discontinuation of treatment due to side effects | 87 | 80 | 7 | 2 | 80 | 77 | 3 | 6 | |
| Fatal or non‐fatal myocardial infarction | 19 | 19 | 0 | 0 | 19 | 19 | 0 | 1 | |
| Fatal and non‐fatal stroke | 19 | 19 | 0 | 0 | 19 | 19 | 0 | 1 | |
| Revascularisation procedures | 19 | 19 | 0 | 3 | 19 | 19 | 0 | 1 | |
| Flushing | 72 | 60 | 12 | 21 | 46 | 43 | 3 | 0 | |
| Pruritus | 72 | 60 | 12 | 5 | 46 | 43 | 3 | 0 | |
| Rash | 72 | 60 | 12 | 6 | 46 | 43 | 3 | 0 | |
| Discontinuation of treatment due to side effects | 72 | 67 | 5 | 7 | 46 | 44 | 2 | 1 | |
| Fatal or non‐fatal myocardial infarction | 214 | 180 | 34 | 0 | 218 | 204 | 14 | 1 | |
| Discontinuation of treatment due to side effects | 214 | 203 | 11 | 23 | 218 | 211 | 7 | 7 | |
| Overall mortality | 1119 | 1116 | 3 | 273 | 2798 | 2797 | 1 | 709 | |
| Cardiovascular mortality | 1119 | 1116 | 3 | 238 | 2798 | 2797 | 1 | 633 | |
| Non‐cardiovascular mortality | 1119 | 1116 | 3 | 30 | 2798 | 2797 | 1 | 54 | |
| Fatal myocardial infarction | 1119 | 1116 | 3 | 203 | 2798 | 2797 | 1 | 535 | |
| Non‐fatal myocardial infarction | 1119 | 1116 | 3 | 114 | 2798 | 2797 | 1 | 386 | |
| Fatal or non‐fatal myocardial infarction | 1119 | 1116 | 3 | 287 | 2798 | 2797 | 1 | 839 | |
| Fatal or non‐fatal stroke | 1119 | 1116 | 3 | 95 | 2798 | 2797 | 1 | 311 | |
| Revascularisation procedures | 1119 | 1116 | 3 | 29 | 2798 | 2695 | 103 | 132 | |
| Gastrointestinal symptoms | 1119 | 1073 | 46 | 230 | 2798 | 2695 | 103 | 385 | |
| Flushing | 1119 | 1073 | 46 | 987 | 2798 | 2695 | 103 | 115 | |
| Pruritus | 1119 | 1073 | 46 | 525 | 2798 | 2695 | 103 | 167 | |
| Rash | 1119 | 1073 | 46 | 289 | 2798 | 2695 | 103 | 199 | |
| Flushing | 87 | 87 | 0 | 74 | 44 | 44 | 0 | 7 | |
| Headache | 87 | 46 | 41 | 22 | 44 | 34 | 10 | 13 | |
| Gastrointestinal symptoms | 87 | 46 | 41 | 24 | 44 | 34 | 10 | 10 | |
| Pruritus | 87 | 46 | 41 | 10 | 44 | 34 | 10 | 0 | |
| Rash | 87 | 46 | 41 | 9 | 44 | 34 | 10 | 0 | |
| Overall mortality | 87 | 46 | 41 | 0 | 44 | 34 | 10 | 1 | |
| Discontinuation of treatment due to side effects | 87 | 72 | 15 | 26 | 44 | 39 | 5 | 5 | |
| Overall mortality | 676 | 391 | 285 | 0 | 272 | 213 | 59 | 0 | |
| Fatal or non‐fatal myocardial infarction | 676 | 391 | 285 | 1 | 272 | 213 | 59 | 1 | |
| Fatal or non‐fatal stroke | 676 | 391 | 285 | 0 | 272 | 213 | 59 | 1 | |
| Flushing | 676 | 457 | 219 | 66 | 272 | 214 | 58 | 1 | |
| New onset diabetes | 569 | NR | NR | 25 | 229 | NR | NR | 2 | |
| Discontinuation of treatment due to side effects | 676 | 547 | 129 | 156 | 272 | NR | 33 | 26 | |
| Flushing | 104 | 102 | 2 | 2 | 106 | NR | 4 | 0 | |
| Gastrointestinal symptoms | 104 | 102 | 2 | 5 | 106 | 102 | 4 | 2 | |
| Discontinuation of treatment due to side effects | 104 | 102 | 2 | 4 | 106 | 102 | 4 | 1 | |
| Gastrointestinal symptoms | 92 | 49 | 43 | 1 | 88 | 53 | 35 | 2 | |
| Rash | 723 | 412 | 311 | 1 | 315 | 237 | 78 | 2 | |
| Headache | 780 | 493 | 287 | 2 | 378 | 315 | 63 | 0 | |
| Flushing | 92 | 49 | 43 | 28 | 88 | 53 | 35 | 5 | |
| Fatal or non‐fatal myocardial infarction | 12838 | 12730 | 108 | 668 | 12835 | 12745 | 90 | 694 | |
| Non‐fatal myocardial infarction | 12838 | 12730 | 108 | 402 | 12835 | 12745 | 90 | 431 | |
| Non‐cardiovascular mortality | 12838 | 12730 | 108 | 350 | 12835 | 12745 | 90 | 321 | |
| Fatal myocardial infarction | 12838 | 12730 | 108 | 302 | 12835 | 12745 | 90 | 291 | |
| Cardiovascular mortality | 12838 | 12730 | 108 | 448 | 12835 | 12745 | 90 | 411 | |
| Fatal or non‐fatal stroke | 12838 | 12730 | 108 | 498 | 12835 | 12745 | 90 | 499 | |
| Revascularisation procedures | 12838 | 12730 | 108 | 807 | 12835 | 12745 | 90 | 897 | |
| Overall mortality | 12838 | 12730 | 108 | 798 | 12835 | 12745 | 90 | 732 | |
| New onset diabetes | 8704 | NR | NR | 494 | 8670 | NR | NR | 376 | |
| Gastrointestinal symptoms | 12838 | 12730 | 108 | 620 | 12835 | 12745 | 90 | 491 | |
| Rash | 12838 | 12730 | 108 | 54 | 12835 | 12745 | 90 | 33 | |
| Discontinuation of treatment due to side effects | 12838 | 12730 | 108 | 2105 | 12835 | 12740 | 95 | 1014 | |
| Flushing | 57 | 57 | 0 | 6 | 61 | 61 | 0 | 1 | |
| Overall mortality | 57 | 57 | 0 | 0 | 61 | 61 | 0 | 1 | |
| Headache | 57 | 57 | 0 | 5 | 61 | 61 | 0 | 2 | |
| Pruritus | 57 | 57 | 0 | 4 | 61 | 61 | 0 | 1 | |
| rash | 57 | 57 | 0 | 1 | 61 | 61 | 0 | 2 | |
| Discontinuation of treatment due to side effects | 57 | 57 | 0 | 11 | 61 | 61 | 0 | 6 | |
| Gastrointestinal symptoms | 35 | 25 | 10 | 3 | 36 | 30 | 6 | 1 | |
| Discontinuation of treatment due to side effects | 35 | 29 | 6 | 7 | 36 | 31 | 5 | 2 | |
| Discontinuation of treatment due to side effects | 14 | 14 | 0 | 0 | 14 | 14 | 0 | 0 | |
| flushing | 30 | 30 | 0 | 19 | 30 | 30 | 0 | 0 | |
| Overall mortality | 30 | 30 | 0 | 0 | 30 | 30 | 0 | 0 | |
| Rash | 1343 | 917 | 426 | 33 | 270 | 239 | 31 | 2 | |
| Discontinuation of treatment due to side effects | 1339 | 1080 | 259 | 166 | 270 | 254 | 16 | 15 | |
| Overall mortality | 1343 | 917 | 426 | 3 | 270 | 239 | 31 | 0 | |
| Pruritus | 1343 | 917 | 426 | 34 | 270 | 239 | 31 | 6 | |
| Flushing | 1343 | 917 | 426 | 142 | 270 | 239 | 31 | 5 | |
| Gastrointestinal symptoms | 1343 | 917 | 426 | 34 | 270 | 239 | 31 | 5 | |
| New onset diabetes | 1129 | NR | NR | 7 | 232 | NR | NR | 2 | |
| Discontinuation of treatment due to side effects | 454 | 400 | 54 | 102 | 342 | 308 | 34 | 31 | |
| Overall mortality | 454 | 298 | 156 | 0 | 342 | 277 | 65 | 1 | |
| Fatal or non‐fatal myocardial infarction | 454 | 298 | 156 | 2 | 342 | 277 | 65 | 0 | |
| Gastrointestinal symptoms | 454 | 298 | 156 | 68 | 342 | 277 | 65 | 38 | |
| Pruritus | 454 | 298 | 156 | 71 | 342 | 277 | 65 | 9 | |
| Rash | 454 | 298 | 156 | 26 | 342 | 277 | 65 | 5 | |
| Flushing | 454 | 298 | 156 | 79 | 342 | 277 | 65 | 16 | |
| Gastrointestinal symptoms | 31 | 31 | 0 | 2 | 23 | 23 | 0 | 0 | |
| Discontinuation of treatment due to side effects | 31 | 31 | 0 | 4 | 23 | 23 | 0 | 1 | |
| Revascularisation procedures | 72 | 59 | 13 | 5 | 73 | 58 | 15 | 2 | |
| Fatal or non‐fatal stroke | 72 | 59 | 13 | 1 | 73 | 58 | 15 | 0 | |
| Overall mortality | 72 | 59 | 13 | 0 | 73 | 58 | 15 | 1 | |
| Flushing | 72 | 59 | 13 | 7 | 73 | 58 | 15 | 1 | |
| Discontinuation of treatment due to side effects | 72 | 66 | 6 | 11 | 73 | 63 | 10 | 5 | |
| Overall mortality | 40 | 30 | 10 | 3 | 45 | 34 | 11 | 4 | |
| Fatal myocardial infarction | 40 | 30 | 10 | 2 | 45 | 34 | 11 | 3 | |
| Cardiovascular mortality | 40 | 30 | 10 | 2 | 45 | 34 | 11 | 3 | |
| Non‐cardiovascular mortality | 40 | 30 | 10 | 1 | 45 | 34 | 11 | 1 | |
| Fatal or non‐fatal myocardial infarction | 40 | 30 | 10 | 2 | 45 | 34 | 11 | 1 | |
| Revascularisation procedures | 40 | 30 | 10 | 2 | 45 | 34 | 11 | 4 | |
| Discontinuation of treatment due to side effects | 40 | 34 | 6 | 4 | 45 | 34 | 11 | 0 | |
| Rash | 52 | 52 | 0 | 1 | 56 | 56 | 0 | 0 | |
| Flushing | 52 | 52 | 0 | 4 | 56 | 56 | 0 | 0 | |
| Gastrointestinal symptoms | 52 | 52 | 0 | 1 | 56 | 56 | 0 | 0 | |
| Revascularisation procedures | 52 | 52 | 0 | 2 | 56 | 56 | 0 | 1 | |
| Overall mortality | 52 | 52 | 0 | 0 | 56 | 56 | 0 | 1 | |
| Fatal myocardial infarction | 52 | 52 | 0 | 0 | 56 | 56 | 0 | 1 | |
| Gastrointestinal symptoms | 141 | 134 | 7 | 71 | 284 | 277 | 7 | 57 | |
| Flushing | 141 | 134 | 7 | 71 | 284 | 277 | 7 | 20 | |
| Overall mortality | 141 | 140 | 1 | 31 | 284 | 283 | 1 | 54 | |
| Fatal myocardial infarction | 141 | 134 | 7 | 28 | 284 | 277 | 7 | 48 | |
| Non‐fatal myocardial infarction | 141 | 134 | 7 | 21 | 284 | 277 | 7 | 45 | |
| Fatal or non‐fatal myocardial infarction | 141 | 134 | 7 | 49 | 284 | 277 | 7 | 93 | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Overall mortality Show forest plot | 12 | 35543 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.12] |
| 2 Overall mortality, sensitivity analysis with stratification by risk of bias trials only Show forest plot | 12 | 35543 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.97, 1.12] |
| 2.1 High risk of bias | 10 | 6703 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.09] |
| 2.2 Low risk of bias | 2 | 28840 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [1.00, 1.20] |
| 3 Fatal myocardial infarction Show forest plot | 6 | 33336 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.11] |
| 4 Cardiovascular mortality Show forest plot | 5 | 32966 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.93, 1.12] |
| 5 Non‐cardiovascular mortality Show forest plot | 5 | 32966 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.98, 1.28] |
| 6 Non‐fatal myocardial infarction Show forest plot | 4 | 33164 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.77, 1.07] |
| 7 Fatal or non‐fatal myocardial infarction Show forest plot | 9 | 34829 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 1.00] |
| 8 Fatal and non‐fatal stroke Show forest plot | 7 | 33661 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.22] |
| 9 Revascularisation procedures Show forest plot | 8 | 33130 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.06] |
| 10 Flushing Show forest plot | 15 | 11038 | Risk Ratio (M‐H, Random, 95% CI) | 7.69 [4.14, 14.28] |
| 11 Pruritus Show forest plot | 6 | 5800 | Risk Ratio (M‐H, Random, 95% CI) | 5.26 [2.68, 10.32] |
| 12 Rash Show forest plot | 9 | 31485 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [1.94, 5.13] |
| 13 Headache Show forest plot | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.86, 2.28] |
| 14 Gastrointestinal symptoms Show forest plot | 12 | 35353 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.37, 2.07] |
| 15 Discontinuation of treatment due to side effects Show forest plot | 17 | 33539 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.70, 2.77] |
| 16 New onset diabetes) Show forest plot | 3 | 27982 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.16, 1.51] |

