نقش داروهای ضد‐فاکتور رشد اندوتلیال عروقی در درمان رتینوپاتی پرهماچوریتی
چکیده
پیشینه
فاکتور رشد اندوتلیال عروقی (vascular endothelial growth factor; VEGF) در فرایند آنژیوژنز دوران جنینی، نقشی کلیدی دارد. اخیرا محققین تلاش کردهاند، تا از عوامل آنتی‐VEGF در درمان رتینوپاتی پرهماچوریتی (retinopathy of prematurity; ROP) که ناشی از اختلالات تکثیر عروقی (vasoproliferative disorders) است، استفاده کنند. در حال حاضر، ایمنی و اثربخشی استفاده از این عوامل در نوزادان پرهترم مبتلا به ROP نامطمئن است.
اهداف
ارزیابی ایمنی و اثربخشی استفاده از داروهای آنتی‐VEGF، در درمان ROP تیپ یک در نوزادان پرهترم، به صورت مونوتراپی ‐ بدون همراهی با سرمادرمانی یا درمان با لیزر ‐ یا در همراهی با سرمادرمانی/لیزردرمانی با برنامه مشخص، (ROP تیپ 1 به این شکل تعریف میشود: درگیری منطقه (zone) یک با هر مرحله (stage) همراه با بیماری مثبت، درگیری منطقه یک مرحله 3 با یا بدون بیماری مثبت، درگیری منطقه دو با مرحله 2 یا 3، با بیماری مثبت).
روشهای جستوجو
پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL؛ 2016، شماره 11)؛ MEDLINE (از 1966 تا 11 دسامبر 2016)؛ Embase (از 1980 تا 11 دسامبر 2016)؛ CINAHL (از 1982 تا 11 دسامبر 2016) و خلاصه مقالات کنفرانسها را جستوجو کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی یا شبه‐تصادفیسازی و کنترل شدهای وارد شدند که به ارزیابی اثربخشی یا ایمنی (یا هر دو) استفاده از عوامل آنتی‐VEGF در مقایسه با روشهای مرسوم قبلی، در درمان نوزادان پرهترم مبتلا به ROP میپرداختند.
گردآوری و تجزیهوتحلیل دادهها
از روشهای استاندارد کاکرین و گروه نوزادان در کاکرین برای جمعآوری و تجزیهوتحلیل دادهها استفاده کردیم. از رویکرد درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) برای ارزیابی کیفیت شواهد استفاده کردیم.
نتایج اصلی
شش کارآزمایی که در مجموع 383 نوزاد در آنها شرکت کرده بودند، معیارهای ورود به این مرور را داشتند. پنج کارآزمایی به مقایسه تزریق داخل زجاجیه بواسیزوماب (bevacizumab) (n = 4) یا رانیبیزوماب (ranibizumab) (n = 1) با درمان مرسوم لیزر (مونوتراپی) پرداختند، درحالی که در ششمین مطالعه، تزریق داخل زجاجیه پگاپتانیب (pegaptanib) همراه با لیزردرمانی مرسوم با لیزردرمانی/سرمادرمانی (درمان ترکیبی) مقایسه شد.
زمانی که به صورت مونوتراپی استفاده شد، بواسیزوماب/رانیبیزوماب نتوانست خطر جدا شدگی کامل یا ناقص شبکیه چشم (3 مطالعه؛ 272 نوزاد؛ خطر نسبی (RR): 1.04؛ 95% فاصله اطمینان (CI): 0.21 تا 5.13؛ تفاوت خطر (RD): 0.00؛ 95% CI؛ 0.04‐ تا 0.04؛ شواهد با کیفیت بسیار پائین)، مرگومیر پیش از ترخیص (2 مطالعه؛ 229 نوزاد؛ RR: 1.50؛ 95% CI؛ 0.26 تا 8.75)، کدورت قرنیه که نیاز به پیوند قرنیه داشته باشد (1 مطالعه؛ 286 چشم؛ RR: 0.34؛ 95% CI؛ 0.01 تا 8.26)، یا کدورت لنز که نیاز به برداشتن کاتاراکت داشته باشد (3 مطالعه؛ 544 چشم؛ RR: 0.15؛ 95% CI؛ 0.01 تا 2.79) را کاهش دهد. خطر عود ROP که نیاز به درمان داشته باشد نیز بین گروهها تفاوتی نداشت (2 مطالعه؛ 193 نوزاد؛ RR: 0.88؛ 95% CI؛ 0.47 تا 1.63؛ RD: ‐0.02؛ 95% CI؛ 0.12‐ تا 0.07؛ شواهد با کیفیت بسیار پائین). تجزیهوتحلیل زیر‐گروه کاهش معنیداری را در خطر عود در نوزادان مبتلا به zone I ROP نشان دادند (RR: 0.15؛ 95% CI؛ 0.04 تا 0.62)، اما افزایش خطر عود در نوزادان مبتلا به zone II ROP دیده شد (RR: 2.53؛ 95% CI؛ 1.01 تا 6.32). تجزیهوتحلیل تجمعی از مطالعاتی که پیامدها را در سطح چشم گزارش کرده بودند نیز افزایش قابل توجهی را در خطر عود ROP در چشمهایی نشان دادند که بواسیزوماب دریافت کرده بودند (RR: 5.36؛ 95% CI؛ 1.22 تا 23.50؛ RD: 0.10؛ 95% CI؛ 0.03 تا 0.17). نوزادانی که بواسیزوماب داخل زجاجیه دریافت کردند، بهطور قابل توجهی با خطر کمتر خطاهای انکساری (میوپی بسیار بالا) در سن 30 ماهگی روبهرو شدند (1 مطالعه؛ 211 چشم؛ RR: 0.06؛ 95% CI؛ 0.02 تا 0.20؛ RD: ‐0.40؛ 95% CI؛ 0.50‐ تا 0.30‐؛ شواهد با کیفیت پائین).
داروی پگاپتانیب داخل زجاجیه، زمانی که در ترکیب با لیزردرمانی استفاده شد، در مقایسه با لیزردرمانی/سرمادرمانی منجر به کاهش خطر جدا شدگی شبکیه چشم شد (152 چشم؛ RR: 0.26؛ 95% CI؛ 0.12 تا 0.55؛ RD: ‐0.29؛ 95% CI؛ 0.42‐ تا 0.16‐؛ شواهد با کیفیت پائین). بروز عود ROP تا سن هفته 55 پس از قاعدگی در گروه پگاپتانیب به اضافه لیزردرمانی نیز کمتر بود (76 نوزاد؛ RR: 0.29؛ 95% CI؛ 0.12 تا 0.7؛ RD: ‐0.35؛ 95% CI؛ 0.55‐ تا 0.16‐؛ شواهد با کیفیت پائین). تفاوتی در خطر هموراژهای حولوحوش زمان انجام جراحی در شبکیه چشم بین دو گروه دیده نشد (152 چشم؛ RR: 0.62؛ 95% CI؛ 0.24 تا 1.56؛ RD: ‐0.05؛ 95% CI؛ 0.16‐ تا 0.05؛ شواهد با کیفیت بسیار پائین). با این حال، خطر عوارض جانبی سیستمیک تاخیری با هر نوعی از سه داروی آنتی‐VEGF نامشخص است.
نتیجهگیریهای نویسندگان
کاربردهای عملی: بواسیزوماب/رانیبیزوماب داخل زجاجیه، زمانی که به صورت مونوتراپی استفاده میشود، خطر عیوب انکساری را در دوره کودکی کاهش میدهد، اما خطر جدا شدگی شبکیه چشم یا عود ROP را در نوزادان مبتلا به نوع 1 ROP کاهش نمیدهد. در حالی که مداخله ممکن است خطر عود ROP را در نوزادان مبتلا به zone I ROP کاهش دهد، میتواند بهطور بالقوه منجر به خطرات عود بیشتری شود که نیاز به درمان در نوزادان مبتلا به zone II ROP دارد. داروی پگاپتانیب داخل زجاجیه، زمانی که در ترکیب با لیزردرمانی استفاده شود، خطر جدا شدگی شبکیه چشم و همچنین عود ROP را در نوزادان مبتلا به نوع 1 ROP کاهش میدهد. با این حال، کیفیت شواهد برای بسیاری از پیامدها بسیار پائین تا پائین بود که علت آن هم، خطر سوگیری (bias) تشخیص و دیگر سوگیریها بود. تاثیرات بر دیگر پیامدهای مهم و از آن مهمتر، عوارض جانبی سیستمیک طولانیمدت داروها شناخته نشدهاند. دادههای ناکافی مانع از نتیجهگیری قوی در زمینه استفاده روتین از عوامل آنتی‐VEGF میشود، چه به صورت مونوتراپی یا در ترکیب با لیزردرمانی، در نوزادان پرهترم مبتلا به ROP نوع 1.
کابردهای تحقیقاتی: مطالعات بیشتری برای ارزیابی تاثیر عوامل آنتی‐VEGF بر پیامدهای ساختاری و عملکردی در دوران کودکی و عوارض جانبی سیستمیک تاخیری مانند پیامدهای جانبی تکامل سیستم عصبی مورد نیاز است.
PICO
خلاصه به زبان ساده
داروهای ضد‐فاکتور رشد اندوتلیال عروقی در درمان رتینوپاتی پرهماچوریتی
پیشینه
رتینوپاتی پرهماچوریتی (retinopathy of prematurity; ROP)، اختلال عروقی در شبکیه چشم تکامل نیافته است که میتواند باعث اختلال دید و حتی کوری در نوزادان پرهترم شود. این اختلال غالبا با برداشن قسمت بیعروق شبکیه چشم درمان میشود، قسمتی از شبکیه چشم که عروق خونی ندارد به کمک لیزردرمانی یا سرمادرمانی برداشته میشود. گرچه این درمانها موجب بهبودی قابل ملاحظه در پیامدهای طولانیمدت میشوند، ثمره آنها با نتایج دلخواه فاصله زیادی دارد. به خصوص که موجب کاهش همیشگی میدان دید جانبی (peripheral visual field) میشوند. اخیرا مطالعاتی پیرامون ارزیابی استفاده از عوامل آنتی‐VEGF برای درمان ROP صورت گرفته است. این عوامل از طریق مهار کار VEGF، که تنظیم کننده کلیدی تشکیل عروق جدید در دوران جنینی است، عمل میکنند. پیشتر در تحقیقات روی نمونههای حیوانی، کاهش قبل ملاحظهای در ایجاد عروق جدید به دنبال تزریق آنتیبادیهای آنتی‐VEGF درون زجاجیه چشم (intravitreal therapy) ایجاد شده بود.
ویژگیهای مطالعه
در دسامبر 2016، بانکهای اطلاعاتی علمی را برای یافتن مطالعاتی که به ارزیابی ایمنی و اثربخشی تزریق عوامل آنتی‐VEGF درون زجاجیه در درمان نوزادان پرهترم مبتلا به ROP میپرداختند، جستوجو کردیم. شش کارآزمایی تصادفیسازی و کنترل شده با مجموع 383 نوزاد شرکتکننده شناسایی کردیم. در پنج کارآزمایی، درمان با تزریق داخل زجاجیه بواسیزوماب (bevacizumab) یا رانیبیزوماب (ranibizumab) با لیزردرمانی مرسوم قیاس شده بود. یک کارآزمایی درمان با تزریق پگاپتانیب (pegaptanib) در زجاجیه را در همراهی با لیزردرمانی، با سرمادرمانی/لیزردرمانی تنها مقایسه کرده بود.
نتایج کلیدی
نتایچ مطالعات نشان میداد که تزریق داخل زجاجیه عوامل آنتی‐VEGF، خطر عیوب انکساری (نزدیکبینی شدید) را در دوران کودکی کاهش میدهد، اما زمانی که به تنهایی استفاده میشوند، خطر جدا شدگی شبکیه چشم یا عود ROP را کاهش نمیدهند. درمان با تزریق پگاپتانیب در زجاجیه در همراهی با لیزردرمانی، خطر جدا شدگی شبکیه چشم را کاهش میدهد. تاثیرات این روشها بر سایر پیامدهای مهم شامل عوارض جانبی تاخیری نظیر سکته مغزی (stroke) مشخص نیست. مطالعات بیشتری برای ارزیابی این پیامدها نیاز است.
کیفیت شواهد
کیفیت شواهد برای اغلب پیامدهای کلیدی، بسیار پائین یا پائین ارزیابی شد.
مراکز انجام
واحدهای نوزادان در چین، جمهوری چک، ایتالیا، ایران، ایرلند و آمریکا.
Authors' conclusions
Summary of findings
| Anti‐vascular endothelial growth factor (anti‐VEGF) therapy compared to conventional laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity (ROP) | ||||||
| Patient or population: preterm infants with type 1 ROP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with conventional laser/cryotherapy | Risk with anti‐VEGF therapy | |||||
| Structural outcome ‐ retinal detachment | Study population | RR 1.04 | 272 | ⊕⊝⊝⊝ | ||
| 15 per 1000 | 16 per 1000 | |||||
| Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) | Study population | RR 0.33 | 26 | ⊕⊝⊝⊝ | ||
| 77 per 1000 | 25 per 1000 | |||||
| Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) | Study population | RR 0.06 | 211 | ⊕⊕⊝⊝ | ||
| 416 per 1000 | 25 per 1000 | |||||
| Mortality before discharge from primary hospital | Study population | RR 1.50 | 229 | ⊕⊕⊝⊝ | ||
| 18 per 1000 | 27 per 1000 | |||||
| Mortality at 30 months of age | Study population | RR 0.86 | 150 | ⊕⊕⊝⊝ | ||
| 93 per 1000 | 80 per 1000 | |||||
| Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) | Study population | RR 0.34 | 286 | ⊕⊝⊝⊝ | ||
| 7 per 1000 | 2 per 1000 | |||||
| Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) | Study population | RR 0.15 | 544 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 2 per 1000 | |||||
| Recurrence of ROP (up to 6 months of age) | Study population | RR 0.88 | 193 | ⊕⊝⊝⊝ | ||
| 204 per 1000 | 180 per 1000 | |||||
| Recurrence of ROP (unit of analysis: eyes) | Study population | RR 5.36 | 188 | ⊕⊝⊝⊝ | ||
| 23 per 1000 | 123 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Outcome assessment not masked. | ||||||
| Anti‐vascular endothelial growth factor (anti‐VEGF) therapy combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity (ROP) | ||||||
| Patient or population: preterm infants with type 1 ROP | ||||||
| Outcomes* | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with conventional laser/cryotherapy | Risk with anti‐VEGF therapy | |||||
| Structural outcome ‐ retinal detachment (unit of analysis: eyes) | Study population | RR 0.26 | 152 | ⊕⊕⊝⊝ | ||
| 393 per 1000 | 102 per 1000 | |||||
| Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) | Study population | RR 0.62 | 152 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 89 per 1000 | |||||
| Recurrence of ROP by 55 weeks' postmenstrual age | Study population | RR 0.29 | 76 | ⊕⊕⊝⊝ | ||
| 500 per 1000 | 145 per 1000 | |||||
| *Only the outcomes for which data are available are reported here. #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Outcome assessment not masked. | ||||||
Background
Description of the condition
Retinopathy of prematurity (ROP) is one of the major avoidable causes of childhood blindness in high‐, middle‐, and low‐income countries (Gilbert 2008). Essentially a neovascularising disease of the retina, ROP occurs mostly in preterm very low birth weight (VLBW) infants. With improved survival of VLBW infants, the absolute number of children with visual impairment secondary to ROP has increased in recent years (Gilbert 2005; Mantagos 2009).
The incidence of ROP varies inversely with gestation and weight at birth. A multicentre study of infants born between 1986 and 1987 reported that 81.6% of infants weighing less than 1000 g developed ROP, while only 46.9% of those weighing 1000 g to 1250 g had ROP (Palmer 1997).
Other risk factors of ROP include exposure to varying oxygen concentrations, hypercapnia, anaemia, acidosis, chronic lung disease, and intraventricular haemorrhage (Ashton 1953; Smith 2003; Tasman 2006).
The predisposition of preterm infants to the development of ROP relates to their immature retinal vasculature. In humans, retinal vascularisation begins at about 12 weeks and is completed by 36 to 40 weeks of gestation. Normally, the blood vessels develop from the optic disc and then progress outwards towards the ora serrata. Infants born before this period will, therefore, have an immature retina with a peripheral avascular zone. Retinopathy of prematurity develops if there is a disruption in the new vessel formation (angiogenesis) in this zone. The disruption of angiogenesis has been found to occur in two sequential phases: a vaso‐obliterative phase followed by a vaso‐proliferative phase (Ashton 1954). In the vaso‐obliterative phase (phase 1), the normally high arterial oxygen saturation in the postnatal life coupled with hyperoxia secondary to oxygen supplementation leads to involution and loss of formed blood vessels. In the vaso‐proliferative phase (phase 2), the relatively hypoxic environment due to ischaemia caused by vessel loss coupled with the high metabolic demands of the avascular retina leads to upregulation of various angiogenic factors, resulting in abnormal neovascularisation. In most infants, the newly formed vessels regress without leaving any sequelae. However, in some infants the neovascularisation goes unchecked leading to retinal scarring, traction, and finally detachment.
The extent and severity of ROP are traditionally described in terms of location (zones; I to III), severity (stages; 1 to 5), extent (clock hours; 1 to 12), and vascular dilatation and tortuosity (plus disease) according to the International Classification of ROP definitions (Committee for Classification of ROP 1984). In addition to defining the progression of the disease, this classification also serves as a guide for surgical intervention. In 2005, the classification was revised to include aggressive posterior ROP (AP‐ROP), pre‐plus disease, and a practical way to estimate the extent of zone I with the indirect ophthalmoscope. Concomitantly, the recommendations for treatment were also revised based on the results of the Early Treatment for Retinopathy of Prematurity trial (ETROP Group 2003). The new recommendations place more emphasis on the presence of plus disease, rather than the number of clock hours, to decide upon the need for treatment (American Academy of Pediatrics 2006). Accordingly, treatment is initiated for the following retinal findings (type 1 ROP).
-
Zone I ROP: any stage with plus disease
-
Zone I ROP: stage 3 ‐ no plus disease
-
Zone II ROP: stage 2 or stage 3 with plus disease
The current treatment strategy for infants with type 1 ROP involves peripheral retinal ablation by either cryotherapy or laser therapy. Both techniques result in significant improvement in the structural and functional outcomes; a Cochrane Review reported significant reduction in the risk of early unfavourable retinal structure from 47.9% to 28.1% and unfavourable visual acuity in early childhood from 63% to 50.6% following peripheral retinal ablation (Andersen 1999). However, in a small but significant proportion of preterm infants, the disease progresses despite treatment. Also, visual fields are slightly smaller in eyes subjected to peripheral retinal ablation as compared to 'control' eyes (Andersen 1999). Moreover, the ablation techniques are cumbersome and require sedation, general anaesthesia, or both. This has led to a quest for simpler and more effective treatment strategies.
Description of the intervention
Vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis in foetal life. In the normally developing retina, VEGF is released in response to the higher oxygen demand of the retinal tissue, which leads to the development of blood vessels from the optic nerve to the periphery. In preterm infants with disrupted angiogenesis, however, the expression and levels of VEGF differ markedly in the two different phases. While the levels are suppressed in the vaso‐obliterative phase, there is an overproduction/expression of VEGF, leading to abnormal vascular proliferation in the vaso‐proliferative phase. Hugely elevated levels of VEGF have been documented in the vitreous cavity of eyes with stage 4 ROP (Lashkari 2000).
The key role of VEGF in inducing retinal neovascularisation prompted researchers to explore the role of anti‐VEGF drugs in the management of ROP. Intravitreal injection of neutralising anti‐VEGF antibodies had demonstrated a significant reduction in the neovascular response in animal studies (Aiello 1995). Two anti‐VEGF drugs, namely pegaptanib sodium (a pegylated anti‐VEGF aptamer) and ranibizumab (an anti‐VEGF monoclonal antibody) had been approved by the United States Food and Drug Administration (FDA) for intraocular use in adults in neovascular and age‐related macular degeneration. The third inhibitor, bevacizumab (a humanised anti‐VEGF monoclonal antibody), is being used off‐label for intraocular injection in adults with similar results. Recently, a fourth drug ‐ Aflibercept ‐ has been approved for the treatment of wet macular degeneration in adults. The drug is a recombinant fusion protein comprising the VEGF binding portions from the extracellular domains of human VEGF receptors 1 and 2 and the Fc portion of human IgG1. Unfortunately, none of these drugs have been approved for intraocular use in children to date (Mantagos 2009). However, given the limitations of existing treatment strategies (see above), many investigators have evaluated the off‐label use of these agents in infants with ROP (Shah 2007; Kong 2008; Mintz‐Hittner 2008). These studies ‐ predominantly case series and retrospective studies ‐ used one or more of the following approaches to evaluate the efficacy of VEGF inhibitors:
-
monotherapy: using an anti‐VEGF drug instead of cryo/laser therapy;
-
combination therapy: using anti‐VEGF simultaneously with cryo/laser therapy;
-
rescue therapy: using anti‐VEGF in infants with progression of the disease despite adequate treatment and in the rare instances where the infant presents with advanced ROP (stage 4 or more).
While most of the studies demonstrated the efficacy of anti‐VEGF drugs in ROP, the safety of the drugs is yet to be established. Though no significant adverse events have been reported so far, concerns still remain regarding their potential local and systemic adverse effects. By inhibiting VEGF, a key factor in regulation of angiogenesis in the developing retina as well as the central nervous system, these drugs could result in significant local and systemic adverse effects. Indeed, there have been concerns regarding the risk of cerebrovascular accidents following intravitreal ranibizumab injections in adults with age‐related macular degeneration (Ueta 2009). Though bevacizumab, the most frequently tested drug in ROP, has a lower risk of systemic absorption following intravitreal injections, the distinct possibility of its systemic effects cannot be ruled out in preterm infants with immature, and often impaired, blood‐retinal barrier (Law 2010).
The risk of systemic absorption, though small, brings its own complexity in the methods of randomisation and analysis in trials involving a locally administered drug. For a drug with truly local action, one can randomise either the study participant or a local body part of the participant to intervention and control groups. In the former, infants would be randomised ‐ both eyes of the infants (if needed) would receive the intervention or control therapy as per the group allocation. In the latter, eyes of the infants would be randomised ‐ one eye would receive the intervention, while the other eye would receive 'control' therapy. However, if the drug is likely to have systemic effects (like most anti‐VEGF agents), randomising the body part is not an ideal method of randomisation.
Why it is important to do this review
Treatment of ROP, to date, is largely by cryotherapy or laser therapy. Although these treatments result in a significant improvement in long‐term visual outcomes, the results are far from perfect. Despite appropriate treatment, progression to tractional retinal detachment occurs in 10% to 15% of infants with high‐risk prethreshold disease (ETROP Group 2003). In addition, the ablation procedures invariably cause permanent loss of the peripheral visual field. Simple, effective, and less destructive treatment strategies would be preferable to these procedures.
The recent reports of success following bevacizumab use have prompted various investigators to conduct clinical studies on the efficacy of this intervention. However, many of these studies are not powered to detect any serious adverse events ‐ local or systemic ‐ in the enrolled infants. Also, most have not systematically documented the risk of recurrence of ROP at a later age or examined the duration of follow‐up required to detect recurrence following intravitreal therapy. Given the protracted course of the disease and the short half‐life of anti‐VEGF drugs, the potential risk of recurrence is high (Wong 2015).
An earlier systematic review on the use of bevacizumab for severe ROP that included only case reports/case series and retrospective studies found considerable variability in how bevacizumab is used for the treatment of ROP (Micieli 2009). It concluded that "further randomized control trials are warranted". The purpose of this review was to identify all available randomised controlled trials on intravitreal anti‐VEGF therapy and to systematically analyse their results.
Objectives
To evaluate the efficacy and safety of anti‐VEGF drugs when used either as monotherapy, that is without concomitant cryotherapy or laser therapy, or in combination with planned cryo/laser therapy in preterm infants with type 1 ROP (defined as zone I any stage with plus disease, zone I stage 3 with or without plus disease, or zone II stage 2 or 3 with plus disease).
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised or quasi‐randomised controlled trials that evaluated the efficacy or safety of administration, or both, of anti‐VEGF agents in human preterm infants for inclusion in this review. We included only those trials that used VEGF inhibitors either alone (i.e. monotherapy) or in combination with cryo/laser therapy (i.e. combination therapy). We excluded those studies that used these drugs when other treatments such as cryo/laser therapy or vitrectomy had failed ('rescue therapy').
Types of participants
We considered studies that enrolled preterm (< 37 weeks' gestation at birth) infants with type 1 ROP at enrolment for inclusion. Type 1 ROP was defined as zone I any stage with plus disease, zone I stage 3 ROP with or without plus disease, or zone II stage 2 or 3 ROP with plus disease (ETROP Group 2003). For the purpose of this review, we excluded those studies that enrolled infants with more advanced ROP, that is stage 4 or more, at the time of enrolment (irrespective of the treatment strategy employed).
Types of interventions
Objective 1
-
Intervention: administration of any anti‐VEGF agent by intravitreal route
-
Control: cryotherapy/laser therapy
Objective 2
-
Intervention: intravitreal administration of VEGF inhibitors within seven days (before or after) of planned laser or cryotherapy
-
Control: cryotherapy/laser therapy alone
Types of outcome measures
Primary outcomes
-
Functional outcome: blindness or severe visual impairment (acuity ≤ 20/200) at 6 months to 12 months of corrected age.
-
Structural outcome: progression to retinal detachment involving the macula.
Secondary outcomes
-
Functional outcome(s) at 6 months to 12 months of corrected age:
-
amblyopia;
-
nystagmus; and/or
-
refractive error in either eye.
-
-
Unfavourable structural outcomes, assessed at 6 months to 12 months of corrected age, and defined as:
-
retinal fold involving the macula;
-
retinal detachment involving zone I of the posterior pole; and/or
-
retrolental tissue or 'mass' obscuring the view of the posterior pole.
-
-
Childhood unfavourable visual acuity, assessed at four years to six years, and defined as absence of vision or Snellen visual acuity of 20/200 or worse.
-
Mortality measured as:
-
death before discharge from the primary hospital;
-
death before two years corrected age.
-
-
Adverse neurodevelopmental outcomes at 18 months to 24 months of corrected age:
-
cerebral palsy; and/or
-
moderate to severe developmental delay as assessed on performance in formal neurodevelopmental testing such as Bayley scale.
-
-
Local adverse effects such as conjunctival haemorrhage, vitreous haemorrhage, and endophthalmitis after the procedure.
-
Acute systemic effects such as apnoea requiring respiratory support and cardiorespiratory arrest during or immediately after the treatment procedure.
-
Delayed systemic effects such as cerebrovascular accidents (stroke) and myocardial dysfunction (based on echocardiographic parameters such as ejection fraction and fractional shortening) diagnosed in the first 24 months of life.
-
Parental satisfaction regarding the treatment procedure employed (in Likert or other such scales).
-
Recurrence of ROP requiring retreatment up to 6 months of age.
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register). We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 11, 2016), MEDLINE (1966 to 11 December 2016) via PubMed, Embase (1980 to 11 December 2016), and CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1982 to 11 December 2016) using the following search terms: (bevacizumab OR Avastin OR ranibizumab OR pegaptanib sodium OR anti‐angio* OR angiogenesis inhibitors), plus database‐specific limiters for randomised controlled trials and neonates (see Appendix 1 for the full search strategies for each database). We limited the searches to human studies. We did not apply any language restrictions. We searched clinical trials registries for ongoing or recently completed trials (ClinicalTrials.gov (clinicaltrials.gov), the World Health Organization International Clinical Trials Registry Platform (www.whoint/ictrp/search/en/), and the ISRCTN registry (www.isrctn.com/)).
Searching other resources
We searched for unpublished studies by handsearching the conference proceedings of the Society for Pediatric Research (2002 to 2013) and American Academy of Ophthalmology Annual Meeting (1999 to 2016). We also searched the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
For this updated review, two review authors (MJS and JS) independently searched and identified eligible trials based on the following characteristics: study population (preterm infants with type 1 ROP), study intervention (administration of anti‐VEGF drugs with or without laser/cryotherapy), and study design (randomised controlled trials).
The review authors screened the titles and abstracts to identify potentially relevant citations. We retrieved and reviewed the full text of the article if we could not ascertain relevance by screening the title and the abstract. The review authors independently assessed the eligibility of the studies by filling out eligibility forms designed in accordance with the specified inclusion criteria. Any disagreements were resolved by discussion.
We contacted the study investigators for additional information or for clarification of the method of randomisation, participant characteristics, details of interventions, definitions of events, additional relevant outcomes, and losses to follow‐up, as necessary.
Data extraction and management
We performed data extraction using a data extraction form designed and pilot tested by the review authors. We extracted information regarding:
-
study setting (e.g. country and settings);
-
study intervention;
-
sample size;
-
length of follow‐up;
-
randomisation procedure;
-
risk of different biases (see Assessment of risk of bias in included studies);
-
outcomes as listed above.
For dichotomous outcomes, we extracted the total number of participants for each group and the number of participants experiencing an event. For continuous outcomes, we extracted mean, standard deviation (or data required to calculate this), and the total number of participants for each group.
Assessment of risk of bias in included studies
Two review authors (MJS and JS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool for the following domains (Higgins 2011).
-
Sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Any other bias
Any disagreements were resolved by discussion or by a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used the standard methods of the Cochrane Neonatal Review Group to synthesise the data. We expressed effects as risk ratio (RR), risk difference (RD), and 95% confidence intervals (CI) for categorical data, and mean difference (MD) and 95% CI for continuous data. For significant differences, we also calculated the number needed to treat for an additional beneficial outcome (NNTB) based on 1/RD. We used the fixed‐effect model for pooling the results of individual studies.
Unit of analysis issues
We anticipated that the units of randomisation and analysis in the included trials would be individual infants and not eyes (it is difficult to randomise eyes because intravitreal anti‐VEGF can be absorbed into the systemic circulation). However, had a given study randomised eyes and not infants, we intended to use the study data but refrained from pooling these data with data of studies that had randomised infants. We decided a priori to use the eye‐level data (and not infant‐level data) in these studies, that is incidence of outcomes in eyes randomised to anti‐VEGF versus incidence of outcomes in eyes randomised to the control group; consequently, we did not consider individual‐level outcomes such as mortality or long‐term neurodevelopment in these studies. We a priori assumed that the beneficial effect, if any, would be diluted in these studies, that is the effect size would be closer to the null effect, if systemic absorption of anti‐VEGF agents were to occur (because the eye randomised to control group would be exposed to both anti‐VEGF agents and 'control' treatment).
Had a given study randomised infants but provided the outcome data for eyes, we planned to contact the authors to obtain infant‐level data so as to avoid unit of analysis error; using eyes as the denominator without adjusting for non‐independence between the eyes can result in spuriously precise results, that is narrow confidence intervals similar to those seen in cluster randomised trials when the clusters are randomised but the outcomes are analysed at the individual level without adjusting for 'cluster' effect (Higgins 2011a). If we could not obtain that information, we used the data for eyes but mentioned up‐front that the analysis referred to eyes and not infants.
Dealing with missing data
At the outcome level, if the data were measured but not reported, we planned to request such data from the study authors. If there was a discrepancy in the number randomised and the number analysed in each treatment group, we calculated and reported the percentage lost to follow‐up in each group.
Assessment of heterogeneity
We intended to assess heterogeneity between trial results by inspecting the forest plots and quantifying the impact of heterogeneity in any meta‐analysis using a measure of the degree of inconsistency in the studies’ results (Deeks 2011).
We estimated the proportion of total statistical heterogeneity not explained by chance using the I2 statistic (Higgins 2003). I2 (calculated as I2 = 100% x (Q ‐ df )/Q; where Q is Cochran’s heterogeneity statistic and df is the degrees of freedom) lies between 0% and 100%.
Data synthesis
We entered quantitative data into Review Manager 5 and analysed the data using the standard methods of Cochrane and Cochrane Neonatal (Review Manager 2014). We used the Mantel‐Haenszel method for estimates of typical RR and RD. We analysed continuous outcomes using the inverse variance method.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
-
Structural outcome: retinal detachment
-
Structural outcome: complete retinal detachment
-
Refractive error: very high myopia at 30 months of age
-
Mortality before discharge
-
Mortality at 30 months of age
-
Local adverse effects: corneal opacity requiring corneal transplant
-
Local adverse effects: lens opacity requiring cataract removal
-
Local adverse effects: perioperative retinal haemorrhages
-
Recurrence of ROP
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality, but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used GRADEpro GDT to create a ‘Summary of findings’ table to report the quality of the evidence (GRADEpro GDT).
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
-
High: We are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We used GRADEpro GDT to import data from Review Manager 5 to create the 'Summary of findings' tables (GRADEpro GDT; Review Manager 2014). These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered.
Subgroup analysis and investigation of heterogeneity
Considerations of clinical diversity included assessment of differences in the nature of the surgical intervention, type and extent of disease, and the number and route of administration of VEGF inhibitors. Accordingly, we planned to analyse the studies based on differences in the following pre‐planned subgroups.
-
Nature of retinal ablation procedure (cryotherapy versus laser therapy)
-
Location of ROP at enrolment (zone I versus zone II)
-
Severity of ROP at enrolment (aggressive posterior retinopathy of prematurity versus others)
-
Specific anti‐VEGF agent administered
-
Number of doses of anti‐VEGF drug (single versus multiple)
-
Birth weights of the enrolled infants (< 1250 g versus ≥ 1250 g)
Sensitivity analysis
We intended to conduct a sensitivity analysis to investigate the robustness of the results for the primary outcome by excluding trials at high risk of bias or with dropout rates of more than 10% (overall).
Results
Description of studies
See Characteristics of included studies
Results of the search
Upon updating the search, we retrieved a total of 71 unique records, of which 58 were excluded after we scanned the title or abstract, or both (Figure 1).

Study flow diagram: review update.
Six randomised trials fulfilled the eligibility criteria and were included in the review (BEAT‐ROP Trial 2011; Autrata 2012; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016). Three articles remain in 'Studies awaiting classification'.
Included studies
Of the six included studies, four compared intravitreal bevacizumab monotherapy with conventional laser therapy (BEAT‐ROP Trial 2011; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016); one compared ranibizumab monotherapy with laser therapy (Zhang 2016); and one study compared intravitreal pegaptanib plus conventional laser therapy with laser and cryotherapy (Autrata 2012). Four trials randomised the infants (BEAT‐ROP Trial 2011; Autrata 2012; Karkhaneh 2016; Zhang 2016), while the other two trials randomised the eyes of the infants to the two groups (Lepore 2014; O'Keeffe 2016). Two studies enrolled infants with only zone II ROP (Karkhaneh 2016; Zhang 2016), while one study included those with zone I ROP only (Lepore 2014); the remaining three studies enrolled infants with either zone I or zone II ROP (BEAT‐ROP Trial 2011; Autrata 2012; O'Keeffe 2016). Two trials allowed cross‐over treatment for infants with recurrence/reactivation of ROP (i.e. laser therapy for bevacizumab group and vice versa) (O'Keeffe 2016; Zhang 2016).
BEAT‐ROP Trial 2011 was a multicentre randomised trial conducted at 15 hospitals in the USA (BEAT‐ROP Trial 2011). It enrolled 150 preterm infants with zone I or zone II posterior stage 3+ ROP and randomly assigned them to receive intravitreal bevacizumab (0.625 mg in 0.025 mL of solution) or conventional laser therapy, bilaterally. The primary outcome was treatment failure, defined as the recurrence of neovascularisation in one or both eyes requiring retreatment, by 54 weeks’ postmenstrual age. RetCam (retinal imaging) photographs taken at different time points were evaluated to document recurrence of ROP. The study investigators reported the refractive outcomes at 30 months of age in a separate publication in 2014 by Geloneck (see BEAT‐ROP Trial 2011). Of the originally enrolled 150 infants, 131 infants underwent cycloplegic retinoscopic refraction to assess refractive outcomes at this age.
Autrata 2012 enrolled 76 preterm infants with zone I or zone II posterior stage 3+ ROP, admitted in a university hospital in the Czech Republic. Enrolled infants were randomly assigned to receive intravitreal pegaptanib (0.3 mg in 0.02 mL of solution) plus conventional diode laser therapy or laser therapy combined with cryotherapy, bilaterally. The primary outcome was treatment success, defined as absence of recurrence of stage 3+ ROP in one or both eyes by 55 weeks' postmenstrual age. RetCam photographs taken at different time points were evaluated to document recurrence of ROP. Infants who were randomised to the intervention group and had recurrence were given an additional intravitreal pegaptanib injection; those in the laser‐plus‐cryotherapy group did not receive pegaptanib for recurrence.
Karkhaneh 2016 enrolled 79 preterm infants (158 eyes) with zone II/stage 2 or 3 ROP and plus disease in a tertiary referral hospital in Tehran, Iran. Of them, 43 infants (86 eyes) were assigned to receive intravitreal bevacizumab injections (0.625 mg/0.025 mL) and 36 infants (72 eyes) were assigned to receive conventional indirect laser therapy. The primary outcome was defined as treatment failure, that is ROP persistence or recurrence by 90 weeks' postmenstrual age. Three experienced retina specialists (who were not involved in the initial treatment) performed follow‐up visits.
O'Keeffe 2016 conducted a prospective randomised study in 15 preterm infants with zone I or posterior zone II ROP admitted in a hospital in Dublin, Ireland. One eye of each infant was randomised to intravitreal bevacizumab, while the other eye was allocated to diode laser therapy. The investigators followed complications, regression/reactivation of ROP, visual outcome, refractive error, and systemic complications until five years of age.
Zhang 2016 conducted a randomised controlled trial of 50 infants with zone II treatment‐requiring ROP (i.e. stage 2 or 3 ROP with plus disease) admitted in the participating hospitals of 'Shenzhen Screening for ROP Cooperative Group', Shenzen, China.
Infants were randomly assigned to receive intravitreal injection of ranibizumab monotherapy or laser therapy. Follow‐up interval was at least six months. Any eyes that developed recurrence of ROP underwent cross‐over retreatment.
Lepore 2014 conducted a single‐centre randomised trial and enrolled 13 infants with type 1 ROP in zone I in both eyes who required treatment according to Early Treatment for Retinopathy of Prematurity (ETROP) criteria (ETROP Group 2003). One eye of the enrolled infants was randomised to receive an intravitreal injection of 0.5 mg bevacizumab, while the fellow eye received conventional laser photoablation. The eye assigned to conventional laser peripheral ablation was treated first. The primary outcome was presence of retinal and choroidal abnormalities on fluorescein angiography at nine months of age. After treatment, binocular indirect ophthalmoscopy and RetCam imaging were performed every three days, and fluorescein angiography was performed every two weeks until discharge. Fluorescein angiography was done again at nine months of age under general anaesthesia.
Further details of the six studies are provided in the Characteristics of included studies table.
Excluded studies
We identified a large number of publications that were retrospective studies/case series of treatment with anti‐VEGF. For brevity, only the studies that provided data hitherto unreported in the randomised trials included in the review ‐ such as those comparing bevacizumab with ranibizumab, evaluating long‐term neurodevelopmental outcomes, etc. ‐ are mentioned here.
Alyamac 2016 reported a two‐centre retrospective study to compare the effects on the process of retinal vascularisation of intravitreal ranibizumab (IVR) and intravitreal bevacizumab (IVB) in the treatment of severe ROP. Forty‐four eyes of 22 participants in group 1 were applied 0.625 mg bevacizumab, and 46 eyes of 23 participants in group 2 were applied 0.25 mg ranibizumab. Retinal vascularisation was evaluated clinically.
Araz‐Ersan 2015 conducted a longitudinal follow‐up study of preterm infants who received 0.625 mg IVB therapy in addition to standard laser photocoagulation therapy. For comparison of the ophthalmological and neurological assessment outcomes of these infants, a control group was formed with 13 birth weight‐ and gestational age‐matched infants who were treated with laser therapy alone for type 1 ROP. The neurological status of the study group and the control group was examined systematically, and neurodevelopmental evaluation was assessed by the Bayley Scales of Infant Development (BSID‐III). The study included a total of 18 eyes of 13 infants.
Erol 2015 reported a retrospective evaluation of 36 eyes of 20 participants with type 1 ROP who received anti‐VEGF intravitreal injections between August 2011 and February 2013. Fifteen eyes of 8 participants received 0.25 mg ranibizumab (group 1), and 21 eyes of 12 participants received 0.625 mg bevacizumab (group 2). Eyes were examined by indirect ophthalmoscopy on the first day, third day, first week, and first month and as required after injections. Laser photocoagulation was performed in cases with progression of ROP.
Gunay 2016 conducted a retrospective interventional case series study including the data of 134 infants (264 eyes) who were treated with IVB, IVR, or laser photocoagulation for ROP. The data were collected from two major ROP treatment centres in Turkey without any randomisation or masking. Regression of ROP, recurrence profile, complications after each treatment modality, and indications for retreatment were evaluated. The main outcome measures included the total inactivation of ROP with anatomic and refractive outcomes at 1.5 years of adjusted age. There were 55 infants (41.1%) in the IVB group, 22 infants (16.4%) in the IVR group, and 57 infants (42.5%) in the laser photocoagulation group.
Han 2016 reported a case series addressing the clinical outcomes of IVB injection, with different dosing (0.25 mg/0.01 mL versus 0.625 mg/0.025 mL) in each eye of the same participant with ROP. Intravitreal bevacizumab was injected into 8 participants with stage 3+ in zone I or posterior zone II ROP (16 eyes). Different doses of bevacizumab (0.25 mg/0.01 mL and 0.625 mg/0.025 mL) were injected into the vitreous cavity of each eye.
Kabatas 2017 conducted a review of infants treated for ROP to evaluate the effectiveness of treatment modalities, major complications, and refractive errors in children who were treated with IVB, IVR, or laser photocoagulation for type 1 ROP. Preterm infants who underwent IVB monotherapy (group 1, 24 eyes of 12 infants), IVR monotherapy (group 2, 12 eyes of 6 infants), or laser photocoagulation (group 3, 72 eyes of 36 infants) for type 1 ROP and infants with spontaneously regressed ROP (group 4, 148 eyes of 74 infants) were included in the study. The study evaluated major complications, recurrence rate, recurrence time, total retinal vascularisation time, and refractive errors at 18 months of corrected age.
Lien 2016 conducted a retrospective observational case series at an institutional referral centre. The purpose of the study was to investigate the neurodevelopment of preterm infants after IVB for the treatment of ROP up to the two years of age. Infants with type 1 ROP were classified into three groups: laser only, IVB only, and a combination of IVB and laser treatment. Main outcome measures were neurodevelopmental outcomes of the infants after treatment assessed by BSID. Sixty‐one infants for whom the neurodevelopmental survey was finished were included.
Lin 2016 conducted a comparative, consecutive case study reporting on the axial length, refraction, and retinal vascularisation one year after ranibizumab or bevacizumab treatment for threshold ROP. Twenty‐five eyes of 13 participants with threshold ROP received one IVR treatment, and 15 eyes of eight participants received one IVB treatment.
Morin 2016 retrospectively reviewed data from the Canadian Neonatal Network and the Canadian Neonatal Follow‐Up Network databases on infants born at less than 29 weeks' gestation in 2010 to 2011 and treated for ROP. Neurodevelopmental outcome at 18 months was assessed by neurologic examination and the Bayley Scales of Infant and Toddler Development, Third Edition.
Wong 2015 reported a retrospective chart review on consecutive infants screened for ROP. Infants treated with peripheral retinal ablation, bevacizumab 0.625 mg/0.025 mL, or ranibizumab 0.25 mg/0.025 mL were specifically identified for review of their clinical outcomes. All treated infants had at least six months of follow‐up with the treating team and were examined until total regression of ROP. One hundred and forty‐two infants were screened over a two‐year period. Six infants with a mean gestational age of 23.48 weeks and mean birth weight of 620 g received anti‐VEGF agents. Ten eyes from the six infants received anti‐VEGF treatment.
Awaiting further assessment
The studies of Autrata 2012a, Kong 2015, and Moran 2014 are awaiting further assessment.
Risk of bias in included studies
Risk of bias assessments are detailed in the Characteristics of included studies table and are summarised in Figure 2.
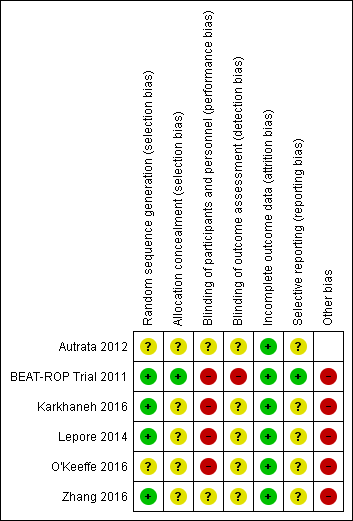
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one trial reported an adequate allocation concealment method (BEAT‐ROP Trial 2011); details of allocation concealment were not reported in the remaining studies (Autrata 2012; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016).
Blinding
Given the nature of the intervention, masking (blinding) of the intervention was not possible in any of the included studies.
BEAT‐ROP Trial 2011 formed a panel of six independent experts to examine the photographs taken at 54 weeks' postmenstrual age, and masked them to the treatment assignments by cropping the photographs to include only the optic disk and macula without laser marks. This enabled the study investigators to perform masked assessment of some of the secondary outcomes (e.g. macular dragging) but not the primary outcome of the study. Paediatric ophthalmologists who performed the cycloplegic retinoscopic refractions to assess the refractive errors at 30 months of age were not masked to the treatment assignments. In Karkhaneh 2016, all follow‐up visits were performed by three experienced retina specialists who were not involved in the initial treatment. However it remains unclear if the outcome assessors were truly masked to the groups, as experienced ophthalmologists could potentially identify the spots left by laser therapy in the control group.
It is not clear if the outcome assessors were masked to the group allocation in the other trials (Autrata 2012; Lepore 2014; O'Keeffe 2016; Zhang 2016).
Incomplete outcome data
Three trials reported no loss to follow‐up until 54 weeks' postmenstrual age (BEAT‐ROP Trial 2011; Autrata 2012; Karkhaneh 2016). Two trials reported no loss to follow‐up at six to nine months of age (Lepore 2014; Zhang 2016), while one trial reported no loss until five years of age (O'Keeffe 2016). In one trial (BEAT‐ROP Trial 2011), about 17% of eligible infants were lost to follow‐up at 30 months of age.
Selective reporting
BEAT‐ROP Trial 2011 reported all outcomes listed in the protocol (see NCT00622726 in BEAT‐ROP Trial 2011). We assessed the other trials as being at unclear risk of reporting bias. We could not refer to the study protocol of Autrata 2012, Karkhaneh 2016, O'Keeffe 2016, and Zhang 2016, and secondary outcomes were not provided in the study protocol of Lepore 2014.
Other potential sources of bias
Lepore 2014 and O'Keeffe 2016 randomised the eyes of enrolled infants. If there was significant systemic absorption of bevacizumab, the eye randomised to the control group would have been exposed to both the anti‐VEGF agent and control treatment, resulting in better outcomes in that eye.
We intended to assess the likelihood of potential publication bias using funnel plots, provided there were at least 8 to 10 trials (Sterne 2011).
Effects of interventions
See: Summary of findings for the main comparison Anti‐vascular endothelial growth factor therapy compared to conventional laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity; Summary of findings 2 Anti‐vascular endothelial growth factor therapy combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity
Comparison 1: anti‐VEGF versus cryo/laser therapy ('monotherapy')
Four trials compared IVB with conventional laser therapy (BEAT‐ROP Trial 2011; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016), while one trial compared IVR with laser therapy (Zhang 2016).
Primary outcomes
Functional outcome: blindness or severe visual impairment at 6 to 12 months of corrected age
None of the studies reported this outcome.
Structural outcome: progression to retinal detachment involving the macula (Outcomes 1.1 to 1.2)
Of the four trials that reported this outcome (BEAT‐ROP Trial 2011; Lepore 2014; Karkhaneh 2016; Zhang 2016), two reported no cases of retinal detachment in either of the two groups (Karkhaneh 2016; Zhang 2016). BEAT‐ROP Trial 2011 did not report any difference in the incidence of complete or partial retinal detachment between the two groups (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.21 to 5.13; risk difference (RD) 0.00, 95% CI ‐0.06 to 0.07; Analysis 1.1). Only a small number of infants (two infants each in the two groups) had retinal detachment. We graded the quality of evidence as very low due to the small number of events and the potential risk of detection bias (summary of findings Table for the main comparison). No separate data were available for the risk of retinal detachment involving only the macula.
The fourth trial (Lepore 2014), which randomised eyes of the enrolled infants, reported progression to complete retinal detachment four weeks after treatment in one eye (8.5%) treated with conventional laser therapy; none of the eyes randomised to IVB had retinal detachment (RR 0.33, 95% CI 0.01 to 7.50; RD ‐0.08, 95% CI ‐0.27 to 0.11) (Analysis 1.2).
Secondary outcomes
Functional outcome(s): refractive error at or after 12 months of age (Outcomes 1.3 to 1.4)
One study reported the outcome using eyes as the denominator (BEAT‐ROP Trial 2011). The risk of very high myopia, defined as ‐8 dioptres (D) or more, at 30 months of age was significantly lower in the eyes of infants randomised to IVB (RR 0.06, 95% CI 0.02 to 0.20; RD ‐0.40, 95% CI ‐0.50 to ‐0.30) (Analysis 1.3). The magnitude of benefit was almost the same in both zone I and zone II posterior ROP. We graded the quality of evidence as low due to the unit of analysis error and risk of detection bias (summary of findings Table for the main comparison). The mean spherical equivalent refractive error at 30 months of age was also significantly less in the eyes of infants who received IVB (mean difference (MD) 5.68 D, 95% CI 4.33 to 7.02) (BEAT‐ROP Trial 2011). The magnitude of difference was almost the same in both zone I (MD 6.93 D, 95% CI 4.26 to 9.60) and zone II posterior ROP (MD 5.25 D, 95% CI 3.69 to 6.81) (Analysis 1.4).
Another study reported no difference in refractive error between the bevacizumab‐treated and laser‐treated eyes at one‐year follow‐up (O'Keeffe 2016). However, the study allowed cross‐over retreatment (i.e. laser therapy for eyes randomised to bevacizumab and vice versa) in infants with recurrence of ROP, which makes it difficult to ascertain the true effect of the initial intervention. Moreover, the study did not provide relevant data to include the results in the pooled analysis.
Unfavourable structural outcomes, assessed at 6 to 12 months of corrected age
None of the studies reported this outcome.
Childhood unfavourable visual acuity, assessed at four to six years
One study reported significant myopic shift in the eyes treated with diode laser compared to the eyes that received IVB at five‐year follow‐up (O'Keeffe 2016). However, the study did not provide relevant data to estimate the risk ratio and its 95% confidence interval. Again, the study allowed cross‐over retreatment in infants with recurrent ROP.
Mortality before discharge from the primary hospital and at 30 months of age (Outcomes 1.5 to 1.6)
BEAT‐ROP Trial 2011 reported no difference in the risk of mortality between the two groups, either before discharge from the primary hospital (RR 1.50, 95% CI 0.26 to 8.75; Analysis 1.5) or at a mean age of 30 months (RR 0.86, 95% CI 0.30 to 2.45; Analysis 1.6). However, the number of events was very small. Another study reported no deaths in either group until discharge from the hospital or until 90 weeks' postmenstrual age (Karkhaneh 2016).
Adverse neurodevelopmental outcomes at 18 to 24 months of corrected age
None of the four studies that randomised infants to the two groups reported this outcome. One study that randomised eyes of the infants to the two groups reported no adverse changes attributable to bevacizumab therapy in magnetic resonance imaging (MRI) at one year of age (O'Keeffe 2016).
Local adverse effects (Outcomes 1.7 to 1.10)
Corneal opacity
One study reported this outcome using eyes as the denominator (BEAT‐ROP Trial 2011). There was no significant difference in the incidence of corneal opacity requiring corneal transplant between the two groups (RR 0.34, 95% CI 0.01 to 8.26) (Analysis 1.7).
Lens opacity
Three studies reported this outcome using eyes as the denominator (BEAT‐ROP Trial 2011; Karkhaneh 2016; Zhang 2016). Of these, two studies did not report any case of cataract in either of the groups (Karkhaneh 2016; Zhang 2016). The third study did not find any significant difference in the incidence of lens opacity requiring cataract removal between the two groups (RR 0.15, 95% CI 0.01 to 2.79) (Analysis 1.8) (BEAT‐ROP Trial 2011).
Endophthalmitis and vitreous haemorrhage
The two studies that reported this outcome did not find any case of endophthalmitis (Analysis 1.9) or vitreous haemorrhage (Analysis 1.10) in infants randomised to bevacizumab/ranibizumab or in those randomised to laser therapy (Karkhaneh 2016; Zhang 2016).
Choroidal ischaemia/rupture
None of the studies reported this outcome.
Acute systemic effects during or immediately after the treatment procedure
None of the studies reported this outcome.
Delayed systemic effects
One study that randomised eyes of the infants to the two groups reported no evidence of systemic adverse effects associated with bevacizumab at five‐year follow‐up (O'Keeffe 2016). None of the other studies reported this outcome.
Parental satisfaction regarding the treatment (in Likert or other such scales)
None of the studies reported this outcome.
Recurrence of ROP (Outcomes 1.11 to 1.12)
A total of four studies reported this outcome (BEAT‐ROP Trial 2011; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016). Pooled analysis revealed no significant difference in the risk of recurrence of ROP requiring retreatment up to six months of age in infants randomised to IVB or IVR and those who received conventional laser therapy (RR 0.88, 95% CI 0.47 to 1.63; RD ‐0.02, 95% CI ‐0.12 to 0.07; 2 studies; fixed‐effect model) (Analysis 1.11). We graded the quality of evidence for this outcome as very low (summary of findings Table for the main comparison). There was large heterogeneity (I2 = 86%), which was essentially due to the differences in the direction of effect between infants with zone I and those with zone II ROP (subgroup analysis); while there was a significant reduction in the risk of recurrence in infants with zone I ROP (RR 0.15, 95% CI 0.04 to 0.62), the risk of recurrence was significantly higher in infants with zone II ROP who received bevacizumab/ranibizumab monotherapy (RR 2.53, 95% CI 1.01 to 6.32) (Analysis 1.11).
Pooled analysis of the other two studies, which reported eye‐level outcomes (not infant‐level outcomes), revealed significant increase in the risk of recurrence of ROP in the eyes that received bevacizumab therapy (RR 5.36, 95% CI 1.22 to 23.50) (Karkhaneh 2016; O'Keeffe 2016). Of these two studies, O'Keeffe 2016 enrolled infants with zone I or II ROP, while Karkhaneh 2016 enrolled infants with only zone II ROP (Analysis 1.12).
Comparison 2: anti‐VEGF plus cryo/laser therapy ('combination therapy') versus cryo/laser therapy alone
Autrata 2012 compared intravitreal pegaptanib plus conventional laser therapy with laser and cryotherapy in preterm infants with stage 3+ ROP.
Primary outcomes
Functional outcome: blindness or severe visual impairment at 6 to 12 months of corrected age
The study did not report this outcome.
Structural outcome: progression to retinal detachment involving the macula (Outcome 2.1)
The study reported the outcome using eyes as the denominator. The risk of complete or partial retinal detachment was significantly lower in the eyes of infants randomised to intravitreal pegaptanib plus laser therapy (RR 0.26, 95% CI 0.12 to 0.55; RD ‐0.29, 95% CI ‐0.42 to ‐0.16; 1 study; 152 infants) (Analysis 2.1). We graded the quality of evidence as low due to the unit of analysis error and risk of detection bias (summary of findings Table 2).
No separate data were available for the risk of retinal detachment involving only the macula.
Secondary outcomes
Functional outcome(s): refractive error at 6 to 12 months of age or later
The study did not report this outcome.
Unfavourable structural outcomes at 6 to 12 months of age
The study did not report this outcome.
Childhood unfavourable visual acuity
The study did not report this outcome.
Mortality before discharge from the primary hospital and at 30 months of age
The study did not report this outcome.
Adverse neurodevelopmental outcomes at 18 to 24 months of corrected age
The study did not report this outcome.
Local adverse effects (Outcomes 2.2)
The study reported the outcome using eyes as the denominator. There was no significant difference in the risk of perioperative retinal haemorrhages after laser therapy between the two groups (RR 0.62, 95% CI 0.24 to 1.56; 1 study; 152 infants) (Analysis 2.2). The study did not report the risk of conjunctival haemorrhage or vitreous haemorrhage in the two groups.
The study reported that "no systemic or significant ocular complications of intravitreal pegaptanib injections, such as endophthalmitis or RD were found during the follow‐up period after treatment", but did not provide the corresponding data for the other group.
Acute systemic effects during or immediately after the treatment procedure
The study did not report this outcome (see 'Local adverse effects' above).
Delayed systemic effects
The study did not report this outcome.
Parental satisfaction regarding the treatment (in Likert or other such scales)
The study did not report this outcome.
Recurrence of ROP (Outcome 2.3)
Infants randomised to intravitreal pegaptanib plus laser therapy had a significantly lower risk of recurrence of ROP by 55 weeks' postmenstrual age compared to those randomised to laser therapy with cryotherapy (RR 0.29, 95% CI 0.12 to 0.70; RD ‐0.35, 95% CI ‐0.55 to ‐0.16; number needed to treat for an additional beneficial outcome 3, 95% CI 2 to 6; 1 study; 76 infants) (Analysis 2.3). We graded the quality of evidence for this outcome as low due to the risk of detection bias and unclear risk of selection bias (summary of findings Table 2).
Discussion
Summary of main results
The systematic review included six randomised trials, of which five evaluated the effects of bevacizumab/ranibizumab monotherapy, while the sixth one examined the effects of intravitreal pegaptanib plus laser therapy. When used as monotherapy, IVB/IVR did not improve short‐term structural outcomes (partial or complete retinal detachment and recurrence of ROP) but significantly reduced the risk of refractive errors at 30 months of age. When used in conjunction with laser therapy, intravitreal pegaptanib reduced the risk of retinal detachment as well as recurrence of ROP in infants with stage 3+ ROP. We noted no significant difference in the incidence of local adverse events with any of the drugs. However, the quality of evidence was very low to low for most outcomes due to the risk of detection bias and other biases.
On subgroup analysis, we found the risk of recurrence of ROP requiring retreatment to be different in infants with zone I ROP and those with zone II ROP receiving IVB/IVR monotherapy: while the risk was reduced in infants with zone I ROP, we found it to be significantly higher in those with zone II ROP. However, the numbers were too small to draw any meaningful conclusion on this subgroup analysis.
Overall completeness and applicability of evidence
The updated evidence remains incomplete for three major reasons. The first reason is the limited number of studies included in the review. Despite the well‐established pathophysiological rationale for using anti‐VEGF agents and the short‐term benefits observed in numerous case reports, case series, and non‐randomised studies (Shah 2007; Kong 2008; Mintz‐Hittner 2008; Wu 2013; Nicoara 2016), only six randomised controlled trials enrolling 383 infants have been published so far. Consequently, the short‐term benefits observed with anti‐VEGF agents in the observational studies could be neither confirmed nor refuted with enough confidence in the current review. Secondly, the long‐term beneficial effects, if any, in terms of favourable structural and functional outcomes are not yet known. Though IVB monotherapy has been shown to reduce the risk of refractive errors (very high myopia) at 30 months of age, the effects of the intervention on other long‐term outcomes are largely unknown. Thirdly, the safety concerns of anti‐VEGF drugs have yet to be addressed. One trial reported no difference in the risk of mortality between intervention and control groups at a mean age of 30 months (BEAT‐ROP Trial 2011), but the number of events was very small. Another trial that randomised eyes of the infants to the two groups reported no evidence of abnormal MRI findings or systemic adverse effects attributable to bevacizumab therapy at one and five years of follow‐up, respectively (O'Keeffe 2016). Given the potential risk of systemic absorption and consequent adverse effects like cerebrovascular accidents following intravitreal anti‐VEGF therapy, the lack of evidence on safety outcomes is a major concern. A recently published study that used the data from the Canadian Neonatal Network demonstrated 3.1 times higher odds (95% CI 1.2 to 8.4) of severe neurodevelopmental disabilities in preterm infants born before 29 weeks' gestation and treated with bevacizumab, after adjusting for key confounders like gestation, gender, maternal education, Score for Neonatal Acute Physiology‐II (SNAP‐II) score, bronchopulmonary dysplasia, sepsis, and severe brain injury (Morin 2016). These findings further underscore the importance of evaluating long‐term safety outcomes of anti‐VEGF therapy .
The incomplete evidence indeed limits our ability to identify a simple, safe, and effective therapy for ROP. Unlike the current standard of treatment, laser therapy, anti‐VEGF administration is technically simple and does not require general anaesthesia or the services of a skilled retinal surgeon. This could be a great boon, particularly in settings with limited resources in low‐ and middle‐income countries. There is an urgent need to generate more evidence on the long‐term structural outcomes as well as the adverse effects following intravitreal therapy with anti‐VEGF agents before they can be considered for routine clinical use in infants with ROP. Future studies should also examine how these drugs affect the natural history of the disease, the focus being late recurrence that might warrant repeat doses of the drug, long‐term follow‐up, and the risk of local complications like infections following therapy, especially in resource‐restricted settings.
Quality of the evidence
We intended to include all primary and secondary outcomes of the review in the 'Summary of findings' tables. However, many of the outcomes were not reported in the included studies. We therefore reported only nine outcomes for the comparison of 'anti‐VEGF versus cryo/laser therapy' and three outcomes for the comparison of 'anti‐VEGF plus cryo/laser therapy versus cryo/laser therapy alone' in the 'Summary of findings' tables.
We graded the quality of evidence as very low to low for almost all outcomes (summary of findings Table for the main comparison; summary of findings Table 2). The risk of detection bias was high in all of the studies because the outcome assessors were not masked to the group allocation. The risk of other biases, including selection and reporting bias, was low in BEAT‐ROP Trial 2011, and unclear in the other studies (Autrata 2012; Lepore 2014; Karkhaneh 2016; O'Keeffe 2016; Zhang 2016).
Potential biases in the review process
Most outcomes of the review were not reported in the included studies. We are contacting the authors of the studies to collect additional information on these outcomes. Also, we did not perform a subgroup analysis based on the specific anti‐VEGF agent used because of the small number of studies (and the small number of infants enrolled in them).
Agreements and disagreements with other studies or reviews
An earlier systematic review, 'Off‐label use of bevacizumab for severe retinopathy of prematurity', was published in 2009 (Micieli 2009). It included nine articles, of which six were case reports, two were retrospective studies, and one was a prospective case series, and found considerable variability in how bevacizumab is used for the treatment of ROP, concluding that "further randomized control trials are warranted". Another systematic review, published in 2015, included 24 studies that evaluated anti‐VEGF therapy in 1457 eyes (Pertl 2015). Almost all the studies were observational except for one randomized and two case‐control studies. The review estimated a 6‐month risk of retreatment of 2.8% per eye, and a 6‐month risk of ocular complication without the need of retreatment of 1.6% per eye. Only isolated incidents of systemic complications were reported. The study concluded that "VEGF inhibitors seem to be associated with low recurrence rates and ocular complication rates".
The current review included six randomised trials, five on intravitreal bevacizumab/ranibizumab monotherapy and one on intravitreal pegaptanib combination therapy.

Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 1 Structural outcome ‐ partial or complete retinal detachment.

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes).

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 3 Refractive error ‐ very high myopia ‐ at or after 12 months of age (unit of analysis: eyes).

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes).

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 5 Mortality before discharge from primary hospital.
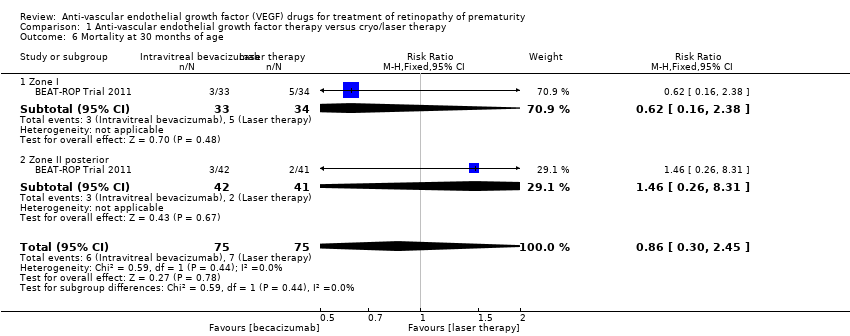
Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 6 Mortality at 30 months of age.

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes).

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes).

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 9 Local adverse effects ‐ endophthalmitis.

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 10 Local adverse effects ‐ vitreous haemorrhage.

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 11 Recurrence of ROP.

Comparison 1 Anti‐vascular endothelial growth factor therapy versus cryo/laser therapy, Outcome 12 Recurrence of ROP (unit of analysis: eyes).
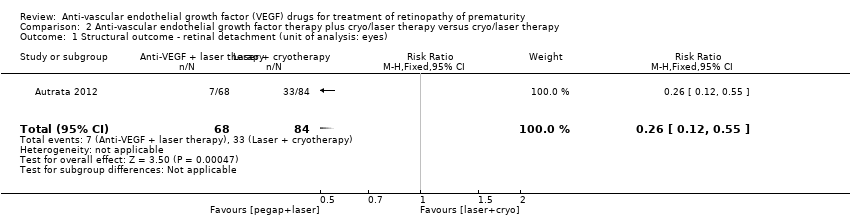
Comparison 2 Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy, Outcome 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes).

Comparison 2 Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy, Outcome 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes).

Comparison 2 Anti‐vascular endothelial growth factor therapy plus cryo/laser therapy versus cryo/laser therapy, Outcome 3 Recurrence of ROP by 55 weeks' postmenstrual age.
| Anti‐vascular endothelial growth factor (anti‐VEGF) therapy compared to conventional laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity (ROP) | ||||||
| Patient or population: preterm infants with type 1 ROP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with conventional laser/cryotherapy | Risk with anti‐VEGF therapy | |||||
| Structural outcome ‐ retinal detachment | Study population | RR 1.04 | 272 | ⊕⊝⊝⊝ | ||
| 15 per 1000 | 16 per 1000 | |||||
| Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) | Study population | RR 0.33 | 26 | ⊕⊝⊝⊝ | ||
| 77 per 1000 | 25 per 1000 | |||||
| Refractive error ‐ very high myopia ‐ at 30 months of age (unit of analysis: eyes) | Study population | RR 0.06 | 211 | ⊕⊕⊝⊝ | ||
| 416 per 1000 | 25 per 1000 | |||||
| Mortality before discharge from primary hospital | Study population | RR 1.50 | 229 | ⊕⊕⊝⊝ | ||
| 18 per 1000 | 27 per 1000 | |||||
| Mortality at 30 months of age | Study population | RR 0.86 | 150 | ⊕⊕⊝⊝ | ||
| 93 per 1000 | 80 per 1000 | |||||
| Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) | Study population | RR 0.34 | 286 | ⊕⊝⊝⊝ | ||
| 7 per 1000 | 2 per 1000 | |||||
| Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) | Study population | RR 0.15 | 544 | ⊕⊝⊝⊝ | ||
| 11 per 1000 | 2 per 1000 | |||||
| Recurrence of ROP (up to 6 months of age) | Study population | RR 0.88 | 193 | ⊕⊝⊝⊝ | ||
| 204 per 1000 | 180 per 1000 | |||||
| Recurrence of ROP (unit of analysis: eyes) | Study population | RR 5.36 | 188 | ⊕⊝⊝⊝ | ||
| 23 per 1000 | 123 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Outcome assessment not masked. | ||||||
| Anti‐vascular endothelial growth factor (anti‐VEGF) therapy combined with laser/cryotherapy compared to laser/cryotherapy in preterm infants with type 1 retinopathy of prematurity (ROP) | ||||||
| Patient or population: preterm infants with type 1 ROP | ||||||
| Outcomes* | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with conventional laser/cryotherapy | Risk with anti‐VEGF therapy | |||||
| Structural outcome ‐ retinal detachment (unit of analysis: eyes) | Study population | RR 0.26 | 152 | ⊕⊕⊝⊝ | ||
| 393 per 1000 | 102 per 1000 | |||||
| Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) | Study population | RR 0.62 | 152 | ⊕⊝⊝⊝ | ||
| 143 per 1000 | 89 per 1000 | |||||
| Recurrence of ROP by 55 weeks' postmenstrual age | Study population | RR 0.29 | 76 | ⊕⊕⊝⊝ | ||
| 500 per 1000 | 145 per 1000 | |||||
| *Only the outcomes for which data are available are reported here. #The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Outcome assessment not masked. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ partial or complete retinal detachment Show forest plot | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.13] |
| 1.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.26] |
| 1.2 Zone II | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.45] |
| 2 Structural outcome ‐ complete retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.50] |
| 3 Refractive error ‐ very high myopia ‐ at or after 12 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.20] |
| 3.1 Zone I | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 3.2 Zone II posterior | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.34] |
| 4 Refractive error ‐ spherical equivalent refractions ‐ at 30 months of age (unit of analysis: eyes) Show forest plot | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 5.68 [4.33, 7.02] |
| 4.1 Zone I | 1 | 87 | Mean Difference (IV, Fixed, 95% CI) | 6.93 [4.26, 9.60] |
| 4.2 Zone II posterior | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [3.69, 6.81] |
| 5 Mortality before discharge from primary hospital Show forest plot | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.26, 8.75] |
| 5.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.80] |
| 5.2 Zone II | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 20.71] |
| 6 Mortality at 30 months of age Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
| 6.1 Zone I | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.16, 2.38] |
| 6.2 Zone II posterior | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.26, 8.31] |
| 7 Local adverse effects ‐ corneal opacity requiring corneal transplant (unit of analysis: eyes) Show forest plot | 1 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 7.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Zone II posterior | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.26] |
| 8 Local adverse effects ‐ lens opacity requiring cataract removal (unit of analysis: eyes) Show forest plot | 3 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 8.1 Zone I | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Zone II | 3 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 9 Local adverse effects ‐ endophthalmitis Show forest plot | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Local adverse effects ‐ vitreous haemorrhage Show forest plot | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Recurrence of ROP Show forest plot | 2 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.63] |
| 11.1 Zone I | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 11.2 Zone II | 2 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.01, 6.32] |
| 12 Recurrence of ROP (unit of analysis: eyes) Show forest plot | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.36 [1.22, 23.50] |
| 12.1 Zone I or zone II | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.35, 25.68] |
| 12.2 Zone II | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.53 [0.98, 58.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Structural outcome ‐ retinal detachment (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.55] |
| 2 Local adverse effects ‐ perioperative retinal haemorrhages (unit of analysis: eyes) Show forest plot | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.24, 1.56] |
| 3 Recurrence of ROP by 55 weeks' postmenstrual age Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.70] |

