L'immunothérapie pour le traitement de la plexopathie lombo‐sacrée idiopathique
Résumé scientifique
Contexte
La plexopathie lombo‐sacrée idiopathique (PLSI), également connue sous le nom de plexitis ou de neuropathie non‐diabétique du plexus lombosacré (radiculo) est une entité clinique rare. Les caractéristiques principales correspondent à des douleurs dans les jambes qui sont (sub) aiguës, sévères, asymétriques, suivie d'une faiblesse multifocale et d’une atrophie dans les semaines ou les mois qui suivent. les symptômes sensoriels comprennent des troubles de paresthésie, d’hypoesthésie d’allodynie et d’autonomie. La PLSI est généralement monophasique et disparait spontanément. La récupération commence lentement pendant plusieurs mois voire plusieurs années et est presque toujours incomplète. Certaines études suggèrent que cette condition possède une étiologie à médiation immunitaire. Des biopsies de segments distaux du nerf cutané ont montré des caractéristiques suggérant un microvasculitis inflammatoire provoquant des lésions ischémiques des nerfs. Les résultats cliniques et pathologiques sont similaires à ceux observés dans la neuropathie diabétique du plexus lombosacré et suggèrent que l'inflammation peut constituer une partie du chemin commun final dans les deux pathologies.
Objectifs
Évaluer les effets d'une quelconque forme d'immunothérapie pour le traitement de la PLSI.
Stratégie de recherche documentaire
Le 15 octobre 2013, nous avons effectué des recherches dans le registre spécialisé du groupe Cochrane sur les affections neuromusculaires, CENTRAL, MEDLINE, EMBASE, LILACS et l’Indice de Thèses. Nous avons vérifié des résumés de conférences et consulté les bases de données d'essais pour les essais en cours. Nous avons examiné toutes les références bibliographiques dans les essais identifiés et contacté les auteurs afin d'identifier d'autres données publiées ou non publiées.
Critères de sélection
Notre objectif était d'inclure tous les essais contrôlés randomisés (ECR) ou quasi‐ECR de toute immunothérapie administrée dans les six semaines suivant l'apparition de la maladie, chez les participants présentant des maladies remplissant les conditions suivantes : l'apparition de douleur aiguë ou subaiguë et une faiblesse des motoneurones inférieurs impliquant principalement les muscles proximaux des membres inférieurs, une faiblesse qui n'est pas confinée à un nerf ou à la distribution d’une racine nerveuse, des tests électrophysiologiques montrant principalement des neuropathies axonales, l'exclusion d'autres causes de radiculopathie et de plexopathie lombo‐sacrée ainsi que les patients diabétiques atteints de glycémie plasmatique(à jeun supérieur à 7,0 mmol/l, aléatoire supérieur à 11,1 mmol/L).
Recueil et analyse des données
Deux auteurs ont indépendamment examiné toutes les références trouvées par la recherche pour sélectionner celles répondant aux critères d'inclusion, selon la méthodologie Cochrane standard.
Résultats principaux
Nous n’avons identifié aucun ECR de toute immunothérapie pour le traitement de la PLSI.
Conclusions des auteurs
Il n'existe actuellement aucune preuve issue d'essais randomisés pour recommander un quelconque traitement à l’immunothérapie pour la PLSI.
PICO
Résumé simplifié
Traitement immunitaire pour la plexopathie lombo‐sacrée d'origine inconnue (une maladie qui affecte le réseau des nerfs à la base de la colonne vertébrale)
Question de la revue
Les traitements qui agissent sur le système immunitaire ont‐ils des effets sur de la plexopathie lombo‐sacrée idiopathique(PLSI)?
Contexte
La PLSI est un trouble rare des nerfs périphériques et du plexus lombosacré qui provoque une douleur asymétrique et affaiblit les membres inférieurs. Le plexus lombosacré est un réseau nerveux à proximité de la base de la colonne vertébrale, là où les fibres nerveuses émergent de la colonne vertébrale, les racines nerveuses se réorganisant dans les nerfs périphériques qui s’étendent dans les jambes. Lors d’une PLSI, un processus endommage ce réseau nerveux et des changements de sensation peuvent survenir, tels que des picotements et une hypersensibilité au toucher. Des effets peuvent également survenir au niveau du système nerveux qui contrôle les fonctions physiologiques (le système nerveux autonome). La cause ou les causes de PLSI ne sont pas claires, mais on pense que les vaisseaux sanguins deviennent enflammés pour des raisons que l’on ignore toujours et cela réduit le débit sanguin vers le réseau nerveux. Certains experts pensent que la réduction du flux sanguin endommage les nerfs. Il est possible que les médicaments qui réduisent l’inflammation puissent être bénéfiques.
Résultats
Après une vaste recherche d'études de haute qualité du traitement (essais contrôlés randomisés, ou équivalents), nous n'avons trouvé aucun essai de PLSI avec une quelconque forme de thérapie qui agit sur le système immunitaire ou réduit l'inflammation. Il n'existe actuellement aucune preuve issue d'essai pour déterminer si les immunothérapies aident les patients atteints de PLSI.
Les recherches dans les bases de données pour cette revue ont été effectuées le 15 octobre 2013.
Authors' conclusions
Background
Description of the condition
Idiopathic lumbosacral plexopathy (ILSP), also called lumbosacral plexitis or non‐diabetic lumbosacral plexus neuropathy, is a rare clinical entity. The core features are (sub)acute severe asymmetrical leg pain, followed by asymmetrical multifocal weakness, and muscle atrophy in the following weeks or months. The proximal and distal lower limb segments may both be involved. Sensory symptoms include paresthesias (tingling), hypesthesia (reduced sensation), allodynia (pain on touch) and autonomic dysfunction (damage to nerves that control unconscious functions). ILSP is generally a monophasic illness and the course is self limiting. Recovery starts slowly over months to several years and is nearly always incomplete, resulting in long‐term deficits in most patients (Dyck 2001b). The condition shares similar clinical features with diabetic amyotrophy but patients typically do not go on to develop diabetes (Dyck 2001b).
The exact incidence of ILSP is not known, but the prevalence is low. Men and women are equally affected. Evidence about racial or geographic predilection is currently lacking. ILSP is predominantly a disorder of the elderly with a median age of almost 70 years, but can occur at any age (Evans 1981; Sander 1981; Dyck 2001b). Nerve conduction studies mainly demonstrate decreased amplitudes of lower limb motor and sensory action potentials. Electromyography usually shows evidence of denervation in affected muscles. Sometimes paraspinal muscles are also involved. Associated findings are weight loss, upper limb neuropathy (mononeuropathies, cervical radiculopathies, and brachial plexus involvement), thoracic radiculopathies, and an acellular cerebrospinal fluid with an elevated protein content. Lumbosacral plexus magnetic resonance imaging (MRI) and erythrocyte sedimentation rate are usually normal. By definition, serum glucose levels are normal.
The etiology and pathogenesis of ILSP is uncertain, but the disorder is considered to be immune‐mediated in origin. Biopsies of distal cutaneous nerve segments have shown the following: focal or multifocal fibre degeneration or loss, focal degeneration or scarring of the perineurium, epineurial neovascularization, abortive nerve fiber regeneration (injury neuroma), and changes in myelinated fibres typical of ischemic changes (Dyck 2000a). In addition to epineurial perivascular inflammatory collections and fibrinoid degeneration, the ischemic changes have been attributed to microvasculitis (Dyck 2000a). The clinical and pathological findings are similar to those found in diabetic lumbosacral plexus neuropathy and suggest inflammation may form part of the final common pathway in both conditions (Dyck 2002).
Several diseases are in the differential diagnosis and have to be excluded: diabetic amyotrophy, other plexopathies (neoplastic infiltration, trauma, surgical complications, retroperitoneal hemorrhage, radiation, sarcoidosis, abscess, and neuroborreliosis), lumbosacral radiculopathies, and lower limb mononeuropathies.
Description of the intervention
Anecdotal evidence suggests that immunotherapies, such as intravenous immunoglobulin (IVIg), plasma exchange (PE), or corticosteroids might be useful to treat the severe pain of ILSP and improve functional recovery from the disorder. We will also include any other form of immunotherapy (for example cyclophosphamide, azathioprine or mycophenolate, ciclosporin, and methotrexate).
How the intervention might work
Immunotherapy is a rational intervention assuming an immune‐mediated microvasculitis as the cause of ILSP. Although the exact mode of action of most forms of immunotherapy is unknown, these treatments are known to have anti‐inflammatory and immunosuppressive effects. Prednisone in high doses suppresses cellular immunity. IVIg has a passive immunizing effect and probably an immunomodulating effect. Cyclophosphamide alkylates DNA and is an immunosuppressive drug that decreases B cell function, CD4+ T cell function, and to a lesser extent CD8+ T cell function. Ciclosporin is an immunosuppressant and decreases T cell function and cytokine production. Methotrexate is a folic acid antagonist with an immunosuppressive and anti‐inflammatory effect mainly as a result of its inhibitory effect on cell division.
Why it is important to do this review
ILSP is a disorder that causes chronic pain and weakness. Most patients have long‐term deficits and recovery is nearly always incomplete, with long‐term disability. The current standard treatment is insufficient to either relieve the pain or to prevent (ongoing) nerve damage. Additionally, information about treatment of ILSP is scarce (Bradley 1984; Awerbuch 1989; Verma 1994; Triggs 1997; Dyck 2000b; Dyck 2001a). As has been attempted for diabetic amyotrophy, there is a need for proper information on the effects of immunotherapies (corticosteroids, PE, IVIg, or any other immunomodulatory pharmacological treatments sometimes used) in the acute phase of ILSP (Chan 2009).
Objectives
To assess the effects of any form of immunotherapy for ILSP.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) or quasi‐RCTs of immunotherapy with IVIg, corticosteroids, PE or any other form of immunotherapy for idiopathic lumbosacral plexopathy within six weeks of disease onset were eligible for inclusion. We intended to review any dose and duration of treatment. (Quasi‐RCTs are trials where the method for allocating participants to treatment groups is partly systematic, for example, using date of birth or hospital record number.)
We also assessed non‐RCTs of more than 30 participants and consecutive case series of more than 10 patients because no RCTs were available. We have summarized observational studies separately in the Discussion.
Types of participants
Participants of any age or sex with ILSP according to the following criteria.
-
Acute or subacute onset of pain and lower motor neuron weakness involving predominantly the proximal muscles of the lower limbs.
-
Weakness that is not confined to one nerve or nerve root distribution.
-
Electrophysiological tests showing predominantly axonal neuropathies.
-
Exclusion of other causes of lumbosacral radiculopathy and plexopathy. We excluded all patients with plasma sugar in the diabetic range (glycosylated hemoglobin greater than 6.5 %, fasting blood glucose greater than 7.0 mmol/L, random blood glucose greater than 11.1 mmol/L).
Types of interventions
Treatment with IVIg, corticosteroids, PE or any other form of immunotherapy for ILSP compared with no treatment, placebo, or other treatment.
Types of outcome measures
Primary outcomes
Our primary outcome was change in disability from baseline as measured by an appropriate, validated scale such as the lower limb scale of the Overall Disability Sum Score (ODSS) (Merkies 2002), at 12 months. We planned to scale results from publications using different follow‐up periods to the same follow‐up period before pooling in a meta‐analysis and to investigate the implications of the necessary assumptions.
Secondary outcomes
1. Reduction from baseline in pain measured with a validated scale at four weeks. A dichotomized version of this outcome could also be used, for example a decrease of three points or more on the Numerical Rating Scale or a decrease of 30% on the Visual Analogue Scale score from the McGill Pain Questionnaire (Melzack 1975).
2. Change in grades of a disability scale, such as the Modified Rankin Scale (van Swieten 1988) and the ODSS, three months and six months after treatment onset.
3. Change in sum score of an impairment scale, such as the Mayo Neuropathy Impairment Scale (Dyck 1980) or the Medical Research Council (MRC) sum score (Kleyweg 1991), at least 12 weeks after treatment onset.
4. Change in summated distal compound muscle action potential (Dyck 1994) of lower limb nerves at least 12 weeks after treatment onset.
5. Adverse events encountered during the treatment period and until 30 days after treatment.
We would have included a 'Summary of findings' table if data had allowed. We would have included primary and secondary outcome measures where possible.
Search methods for identification of studies
Electronic searches
On 15 October 2013 we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2013, Issue 9 in The Cochrane Library ), MEDLINE (1966 to September 2013), EMBASE (1980 to October 2013), LILACS (1982 to October 2013), and Index to Theses (October 2013).
Searching other resources
We scanned conference abstracts for RCTs involving any immunotherapy in ILSP by searching the Open Grey database (www.opengrey.eu/) on 15 October 2013, the British Library Inside database from 2000 to 2012 and ISI Web of Science (WoS) meetings (on 15 October 2013). We planned to check all references in any identified trials and contact authors to identify any additional published or unpublished data. For ongoing studies we additionally searched trial registries: ClinicalTrials.gov (www.clinicaltrials.gov/) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
The detailed search strategies are in the appendices: CENTRAL Appendix 1 , MEDLINE Appendix 2, EMBASE Appendix 3, LILACS Appendix 4, Open Grey Appendix 5, Index to Theses Appendix 6, WoS Appendix 7, and clinical trials registries Appendix 8.
Data collection and analysis
Selection of studies
Two review authors (JvE, YCC) independently examined all references retrieved by the search. We selected potentially relevant studies from the titles and abstracts. We carried out study selection for the review without considering whether the studies reported our prespecified outcomes. We retrieved the full text versions and would have translated reports into English if necessary. There were no disagreements.
Data extraction and management
Two review authors would have independently extracted data using a newly prepared data extraction form if randomized trials had been available. The form includes: type of study, quality criteria described below, number, age and sex of participants, diagnostic criteria, type of intervention used (including details of dosage, duration and administration route), outcome measures, results, adverse events, and risk of bias. We planned to include in the review all studies fulfilling the inclusion criteria and where possible include them in a meta‐analysis. We would have performed sensitivity and subgroup analyses as described below. A third author would have resolved any disagreements. We intended that one review author would enter data into the Cochrane authoring and statistical software Review Manager 5 (Revman) (RevMan 2012) and a second author would check the data entry.
Assessment of risk of bias in included studies
If there had been data available, two review authors would have independently assessed the risk of bias in included studies according to the methods described in the Cochrane Handbook for Systematic Reviews of InterventionsHiggins 2011a. We would have assessed sequence generation; allocation concealment, blinding of participants, treaters and assessors, completeness of outcome data, selective outcome reporting, and other sources of bias and would have made a judgement about whether the study is at high, low or unclear risk of bias for each domain. The authors would have resolved any disagreements by discussion.
Measures of treatment effect
The review authors planned to use RevMan to compute the following comparative statistics and their 95% confidence intervals (CIs): risk ratios (RRs) for dichotomous data and mean differences (MDs) for continuous data.
Unit of analysis issues
In case of non‐standard designs of included studies we intended to follow Chapters 16.3, 16.4 and 16.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
In the event of missing data we would have contacted the trial investigators. When we obtained data we would have inputted them into the analysis. If data had been unavailable we would have investigated why they were missing (whether at random or from specific groups) and would have assessed the impact.
Assessment of heterogeneity
If data had been available, we would have used a fixed‐effect model for meta‐analysis when the results of different studies were homogeneous. If the results were heterogeneous, we intended to inspect trial characteristics to identify which studies were responsible for heterogeneity and the reasons for it. Unexplained heterogeneity could have lead to the decision not to pool the studies or to pool using a random‐effects model. We planned to use a Q‐test to quantify heterogeneity and would have chosen either not to pool or to use a random‐effects model when I2 ≥ 50%.
Assessment of reporting biases
We have searched trials registries to detect unpublished trial resuIts and planned to include such studies in the analysis. Where there was any suspicion of outcome reporting bias we would have asked authors for unpublished outcomes and would have tried to determine why those outcomes were not published.
Data synthesis
As RCTs are often of shorter duration, we intended to use evidence from non‐randomized studies to capture longer term evidence about adverse events and, if data had been available, we would have considered this evidence in the Discussion.
Subgroup analysis and investigation of heterogeneity
If data had sufficed, we would have undertaken subgroup analyses to test the effect of interventions in participants presenting with asymmetrical weakness versus symmetrical weakness and of patients with minimal or no pain versus patients with severe pain.
Sensitivity analysis
We planned to perform sensitivity analyses to omit trials with a higher risk of bias to discover whether our conclusions from all the trials were robust, if there had been sufficient trials addressing the same intervention.
We have listed any deviations from the protocol (van Eijk 2012) in Differences between protocol and review.
Results
Description of studies
Results of the search
From a total of 153 references (Cochrane Neuromuscular Disease Group Specialized Register 1, MEDLINE 45, EMBASE 62, LILACS 1, Index to Theses 2, and ISI WoS 42) we identified 21 publications concerning therapy of ILSP. We found no RCTs or quasi‐RCTs so there are none included in this review. Our searches of trials registries did not reveal any ongoing trials. See Figure 1 for a flow chart showing the study selection process.
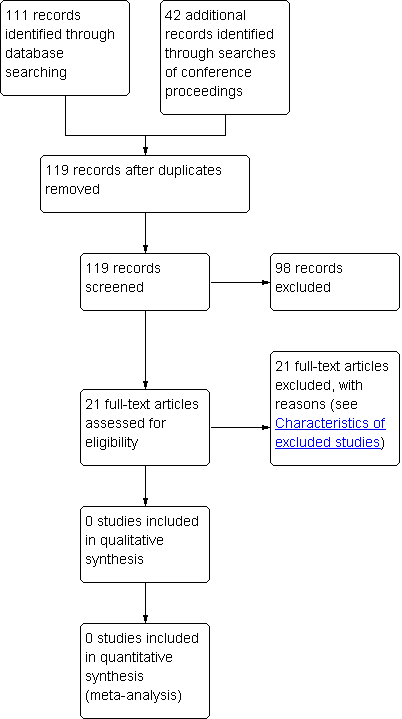
Study flow diagram.
We have described excluded studies in Characteristics of excluded studies.
Risk of bias in included studies
Not applicable
Effects of interventions
Not applicable
Discussion
Summary of main results
The review authors identified no RCTs of any immunotherapy for idiopathic lumbosacral plexopathy. They found anecdotal evidence on immunotherapy for ILSP from 20 publications. They did not find any non‐randomized trials containing more than 30 participants. They found one case series of over 10 patients treated with intravenous methylprednisolone (Dyck 2001a). The publication did not contain sufficient details to assess the primary or secondary outcome measures of this review. Of 11 participants (median age 67 years, seven women) with idiopathic lumbosacral plexopathy, 10 received weekly infusions of intravenous methylprednisolone (1 g/week) for 8 to 16 weeks. The other participant received an equivalent dosage of oral prednisone. The Neuropathy Impairment Score (NIS) significantly improved from a median of 42 points (range 9 to 106) before treatment to 20 points (range 5 to 57) after treatment (P = 0.005) (range 2 to 14 months). The severity subscore of the Neuropathy Symptoms and Change (NSC) score showed significant improvement from before treatment (median 26.5, range 9 to 40) to after treatment (median 19, range 7 to 30) (P = 0.04). All severity scores except one showed significant improvement. Weakness markedly improved in 9 of the 11 patients. Ambulation improved as well: six participants were wheelchair dependent before treatment and one was wheelchair dependent after treatment. Three participants suffered relapses, but two improved again after retreatment with intravenous methylprednisolone (in the third, treatment was ongoing). No data on side effects were available. Natural history data from 42 patients from the same neuromuscular centre showed recovery to baseline in three patients at a median of 35.5 months. The fact that improvement started with initiation of therapy after worsening of symptoms over months to years and the large magnitude of improvement suggest a treatment effect. However, interpretation of the data is hampered by lack of a control group and because the spontaneous recovery in ILSP cannot be fully excluded in an open label study. Since ILSP is similar to diabetic amyotrophy with respect to its pathophysiology, clinical features, and prognosis (Dyck 2001b; Dyck 2002), it may be relevant to note that an RCT found pulse methylprednisolone therapy not superior to placebo for functional improvement in diabetic amyotrophy (Dyck 2006).
For intravenous immunoglobulins (IVIg) the largest series consists of five patients (Triggs 1997). This (descriptive) publication did not contain sufficient patients and details to provide data for the primary or secondary outcome measures of this review. Three of the five patients had complete pain relief and all had marked improvement of strength soon after IVIg, either with or without (methyl)prednisolone or azathioprine. Two of the five patients required long‐term IVIg therapy because of relapses; one patient received prednisolone for three months and azathioprine for six months after the initial IVIg treatment. One patient suffered congestive heart failure and discontinued IVIg after three days. Again the fast and clinically clear improvement after initiation of therapy suggests a treatment effect, but the evidence is of the lowest level due to the lack of a control group.
Overall completeness and applicability of evidence
As our review includes no RCTs, the evidence cannot be regarded as complete and the applicability is questionable as the total number of patients in non‐RCTs is very low.
Quality of the evidence
Only anecdotal evidence from a non‐randomized case series is available. This can be classified as very low quality evidence (level C; non‐comparative trials and level D; expert opinion or case series).
Potential biases in the review process
None
Agreements and disagreements with other studies or reviews
Our results are in line with other published reviews (Dyck 2001b; Thaisetthawatkul 2010).

Study flow diagram.

