Tratamientos tópicos para la psoriasis del cuero cabelludo
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | This was a single‐centre, parallel‐group, active‐controlled, randomised trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% shampoo, once daily for 4 weeks on dry scalp (N = 14 participants) B: clobetasol propionate 0.05% gel, once daily for 4 weeks on dry scalp and rinsed off after 15 minutes (N = 12 participants) | |
| Outcomes |
Definition: HPA axis suppression: pre‐stimulation values of serum‐cortisol below 7 µg/dl, and/or post‐stimulation values lower than 20 µg/dl after 60 minutes of intravenous injection of 0.25 mg cosyntropin Ocular tolerability: intraocular pressure, results of slit lamp examination, visual acuity assessment, patient rating of burning or sting sensation on a scale from 0 (absent) to 3 (severe) Overall safety: adverse events probably related to treatment DSS (0‐9): sum of erythema, adherent desquamation and plaque thickening on score from 0 (none) to 3 (severe) Visits: baseline, week 1, 2, 3 and 4 | |
| Notes | Galderma Laboratories supported the study and employed all authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 328): "computer generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 330): "Twenty‐five of the 26 enrolled subjects completed the study." |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | High risk | Quote (page 330): "more females in the clobetasol propionate shampoo group [...] symptom severity and extend of involvement at baseline were worse in the clobetasol propionate shampoo group [...]" Comment: imbalance of baseline characteristics likely to bias outcome |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 328): "due to the different formulations and ways of administration, it was not possible to mask the identity of the treatment from the subjects" Comment: no blinding of participants performed |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 328): "Investigators did not know the treatment provided to the subjects." |
| Methods | This was a multicentre, open‐label, parallel‐group, randomised trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics This was not stated | |
| Interventions | A: calcipotriol solution + tar‐based shampoo, for 8 weeks B: calcipotriol + non‐medicated shampoo, for 8 weeks | |
| Outcomes |
Definition: TSS: composite score for redness, thickness and scaliness Visits: baseline, week 8 | |
| Notes | This study was published as a letter Number of participants not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 175): "randomised" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | This was not stated |
| Selective reporting (reporting bias) | High risk | Quote: "A greater reduction in the ‘mean total sign score’ (...) was found for calcipotriol + tar‐based shampoo than calcipotriol + non‐medicated shampoo (p=0.040)." Quote: "A significant difference in patient assessment of itching in favour of calcipotriol + tar‐based shampoo was found (p=0.032)." Comment: not all results were reported in sufficient detail. No baseline data for each specific group reported. Study published as letter only. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 175): "open‐label" Comment: no blinding performed |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 175): "open‐label" Comment: no blinding performed |
| Methods | This was a randomised, single‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol foam, for 2 weeks (N of participants unknown) B: clobetasol cream (0.05%) plus clobetasol solution (0.05%) regimen, for 2 weeks (N of participants unknown) Application frequency varied individually according to patients' decision The study size was 32 participants | |
| Outcomes |
Definition: SAPASI: Assessment according to the PASI performed by the patient QOL: assessment according to 2 established tools: DLQI and EQ‐5D Cost of treatment: to find the cost of treating 1% BSA: amount of medication used (in grams) divided by the total percentage BSA treated Visits: baseline and week 2 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 408): "patients were randomized into 2 treatment groups" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 408): "3 were excluded because of noncompliance: 1 did not return for a second visit, 1 misapplied the foam, and 1 applied the medication intermittently over a 3‐week period." Comment: per‐protocol analysis performed. Unclear if all drop‐outs belonged to one group. In this case drop‐out rate may be > 10% per group and would introduce relevant bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 409): "The cost per change of one unit in PASI score was $21.60 for patients using foam and $16.42 for those using cream/solution; the difference was not statistically significant" Comment: this outcome was not pre‐specified in the method section |
| Other bias | Low risk | No other potential source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 408): "single‐blind design" Comment: unclear which side was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 408): "single‐blind design" Comment: unclear which side was blinded |
| Methods | This was a multicentre, randomised, double‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: augmented betamethasone dipropionate 0.05% propylene glycol lotion twice daily for 3 weeks (N = 84 participants) B: fluocinonide 0.05% solution twice daily for 3 weeks (N = 85 participants) | |
| Outcomes |
Definition: TSS (0 to 12): sum of the scores of the 4 disease parameters Disease parameter: induration (0 to 3), scaling (0 to 3), pruritus (0 to 3), erythema (0 to 3) Global Improvement Score: 1 (cleared), 2 (marked improvement), 3 (moderate improvement), 4 (slight improvement), 5 (no change), 6 (exacerbation) Visits: baseline, day 4, 8, 15 and 22 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 19): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 19‐20): "12 patients [...] were excluded from efficacy analysis because baseline entrance criteria for disease severity were not met" Comment: no intention‐to‐treat analysis performed. Reason for exclusion considered to introduce bias. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | High risk | Quote (page 19‐20): "An additional 12 patients [...] were excluded from efficacy analysis because baseline entrance criteria for disease severity were not met." Comment: these 12 patients with low disease severity were excluded for efficacy evaluations, but included in safety evaluations |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 19): "double‐blind" Comment: insufficient detail was reported about the method used to blind study participants or personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 19): "double‐blind" Comment: insufficient detail was reported about the method used to blind the outcome assessor from the intervention a participant received |
| Methods | This was a multicentre, prospective, randomised, active‐controlled, double‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g scalp formulation, once daily for 8 weeks (N = 108 participants) B: betamethasone dipropionate 0.5 mg/g (same vehicle), once daily for 8 weeks (N = 110 participants) Patients having 'absence of disease' according to the IGA of disease severity at visits 2 to 5 were allowed to withdraw from the study | |
| Outcomes | Primary outcomes of the trial
Secondary outcomes of the trial
Definition: TSS: sum of the score for each disease parameters ranging from 0 to 12 Disease parameter (0 (no symptoms) ‐ 4 (very severe symptoms)): redness, thickness, scaliness Patient assessment: 7‐category scale: 0 = worse to 6 = cleared, treatment success: patient rated as 'marked improvement', 'almost clear' or 'cleared' IGA (6‐point scale): 'absence of', 'very mild', 'mild', 'moderate', 'severe', 'very severe' Extend of scalp psoriasis: score of 3 = 30% to 49% involvement of the scalp Visits: baseline, week 1, 2, 4, 6 and 8 | |
| Notes | The study was sponsored by LEO Pharma A/S, Ballerup, Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 108): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 109): "All patients who had received any trial medication and from whom the presence or confirmed absence of adverse events was available were included in the safety analysis set.[...] The primary efficacy criterion was analysed for the intention‐to‐treat (ITT) population [...] a last observation carried forward approach was used." Comment: missing data have been imputed using appropriate methods. Incomplete outcome data sufficiently reported. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 108): "double blind [...] same vehicle" Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 108): "double blind [...] same vehicle" Comment: insufficient information about method used to ensure blinding of outcome assessor throughout the study |
| Methods | This was a double‐blind, randomised, active‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: betamethasone dipropionate 0.05% with salicylic acid 2% lotion, twice daily for 3 weeks (N = 22 participants) B: betamethasone valerate 0.1% lotion, twice daily for 3 weeks (N = 17 participants) | |
| Outcomes |
Definition: Outcome 1 to 9: severity graded on a 4‐point scale 'none', 'slight', 'moderate', 'severe' Overall evaluation: graded on a 5‐point scale from 'cured' to 'worse' Percentage area of involvement: estimated approximately Outcome 16 to 17 : assessed by patient Visits: baseline, day 7, 14 and 21 | |
| Notes | This study was supported by a grant from Schering‐Plough Ltd. 56 participants: 39 psoriasis, 17 eczema | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 203): "Patients were allocated at random" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 203): "Of the 59 patients who entered the trial, 3 were excluded from the analysis because of protocol violations." Quote (page 205): "Of the 56 patients included in the study, 47 completed the 3‐week treatment regime, and nine were regarded as treatment failures." Comment: no ITT analysis performed. Unclear if 'treatment failures' (N = 9 participants) were included in overall evaluation or if any imputation method was used |
| Selective reporting (reporting bias) | High risk | Quote (page 203): "The other symptoms of crusting, induration, lichenification and exudation were not widespread enough at baseline to warrant evaluation." Comment: not all pre‐specified outcomes reported |
| Other bias | High risk | Number of participants listed in diagram of overall evaluation (Fig. 3) not consistent with number of participants reported in the text |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 203): "treatments were applied in a double‐blind manner" Comment: insufficient detail was reported about the method used to blind study participants or personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 203): "treatments were applied in a double‐blind manner" Comment: insufficient detail was reported about the method used to blind the outcome assessor from the intervention a participant received |
| Methods | This was a randomised, single‐blind, active‐controlled trial | |
| Participants | Inclusion criteria of the trial This was not stated Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics This was not stated | |
| Interventions | A: hydrocortisone 17‐butyrate 0.1% emulsion, twice daily for 4 weeks (N = 74 participants) B: betamethasone 17,21 dipropionate 0.05% lotion, twice daily for 4 weeks (N = 76 participants) Patients could withdraw if healing occurred prior to week 4 | |
| Outcomes |
Visits: baseline, week 2 and 4 | |
| Notes | This study was published as a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page S104): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | No ITT analyses for safety data used: 71 out of 76 (betamethasone group) and 70 out 74 (hydrocortisone group) reported. However, number of missing data not considered as likely to introduce bias significantly. |
| Selective reporting (reporting bias) | High risk | Only data about clearance reported Data available for only 1 adverse event Insufficient reporting about other outcomes assessed |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page S104): "single‐blind study" Comment: insufficient information about which side was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page S104): "single‐blind study" Comment: insufficient information about which side was blinded |
| Methods | This was a randomised, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/ml solution, twice daily for 6 weeks (N = 24 participants) B: betamethasone valerate 1% lotion, twice daily for 6 weeks (N = 18 participants) | |
| Outcomes |
Definition: TSS (0 to 12): sum of erythema, thickness and scaling score Erythema/scaling/thickness score (0 to 4): 0 = absent to 4 = severe Percentage of response to treatment: percentage of patients categorised into 'worse', 'no change', 'mild', 'marked' (IGA = 'very mild'), 'cleared' (IGA = 'clear') Visits: baseline, week 2, 4 and 6 | |
| Notes | TSS was calculated with sign scores reported in figures 2 to 4 by review author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 65): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Data for all randomised patients reported |
| Selective reporting (reporting bias) | High risk | The pre‐specified outcome of recurrences was not reported in the results section |
| Other bias | Low risk | No other potential source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | This was not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | This was not stated |
| Methods | This was a randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: amcinonide lotion 0.1%, twice daily for 3 weeks (N = 83 participants) B: placebo (vehicle), twice daily for 3 weeks (N = 82 participants) Patients were allowed to withdraw, if complete clearance occurred prior to week 3 | |
| Outcomes |
Definition: Overall therapeutic efficacy assessed by investigator (1 to 7): 1 = cleared (complete clearing), 2 = excellent (> 75% improvement), 3 = good (> 50% improvement), 4 = fair (> 25% improvement), 5 = poor (< 25% improvement), 6 = no effect, 7 = exacerbation (clinical signs and symptoms worse) Outcomes 2 to 6.: rated by investigator on a scale from 0 to 3, 0 = absent, 0.5 to 1.0 = mild, 1.5 to 2.0 = moderate, 2.5 to 3.0 = severe Patient's overall evaluation (0 to 3): 0 = poor, 1 = fair, 2 = good and 3 = excellent Patient acceptability evaluation: form with 11 questions Compliance: count of the returned medication bottles Visits: baseline, week 1, 2 and 3 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 316): "computer‐generated randomization list designed to produce approximately equal numbers of patients in each study arm" Comment: probably sufficient |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 317): "Those patients who met the protocol requirements and had at least one follow‐up visit were included in the analyses of efficacy." Quote (page 323): "For the evaluable patients, the endpoint evaluation was defined as the patient's last valid evaluation." Comment: no ITT analysis, but per protocol analysis performed |
| Selective reporting (reporting bias) | High risk | Quote (page 323): "Both test formulations were cosmetically acceptable to patients." Quote (page 317): "no formal analysis was performed because there were few adverse events" Comment: insufficient data about prespecified outcome (patient acceptability, compliance) reported |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 314): "double‐blind" Comment: vehicle‐controlled trial. Blinding probably sufficient. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 314): "double‐blind" Comment: insufficient information about how blinding of assessor was ensured throughout the study |
| Methods | This was a randomised, blinded, active‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: amcinonide lotion 0.1%, twice daily for 3 weeks (N = 29 participants) B: fluocinonide solution 0.05%, twice daily for 3 weeks (N = 30 participants) | |
| Outcomes |
Definition: Overall therapeutic efficacy (1 to 7): 1 = cleared (complete clearing), 2 = excellent (> 75% improvement), 3 = good (> 50% improvement), 4 = fair (> 25% improvement), 5 = poor (25% improvement), 6 = no effect, 7 = exacerbation (clinical signs and symptoms worse) Outcomes 2 to 6: rated by investigator on a scale from 0 to 3, 0 = absent, 0.5 to 1.0 = mild, 1.5 to 2.0 = moderate, 2.5 to 3.0 = severe Disease symptoms score (0 to 12): sum of erythema, induration, excoriation, scaling Patient's overall evaluation (0 to 3): 0 = poor, 1 = fair, 2 = good and 3 = excellent Patient cosmetic acceptability evaluation: form with 11 questions completed by patient Visits: baseline, week 1, 2 and 3 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 472): "patients were sequentially assigned to one of the two treatment groups by means of a computer‐generated randomization list" Comment: probably sufficient |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | No intention‐to‐treat analysis Withdrawal due to adverse events: A: 0, B: 1 Patients excluded from efficacy analysis: A: 0, B: 3 |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 471): "blinded comparison" Comment: insufficient detail was reported about the method used to blind study participants and personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 471): "blinded comparison" Comment: insufficient detail was reported about the method used to blind the outcome assessors from the intervention a participant received |
| Methods | This was a multicentre, randomised, single‐blind trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: betamethasone valerate 0.12%, once daily for 4 weeks (N = 46 participants) B: betamethasone valerate 0.12%, twice daily for 4 weeks (N = 33 participants) | |
| Outcomes |
Definition: Investigator’s and patients’ global assessment of response (0 to 6): 0 = completely clear, 1= almost clear, 2 = marked improvement, 3 = moderate improvement, 4 = slight improvement, 5 = no change, 6 = worse GSS (0 to 12): sum of scores of erythema, thickness and scaling Erythema (0 to 4): 0 = no erythema, 1 = faint erythema, pink to very light red, 2 = definite light red erythema, 3 = dark red erythema, 4 = very dark red “beefy” erythema Thickness (0 to 4): 0 = no plaque elevation, 1 = slight, barely perceptible elevation, 2 = definite elevation but not thick, 3 = definite elevation, thick plaque with sharp edge, 4 = very thick plaque with sharp edge Scaling (0 to 4): 0 = no scaling, 1 = sparse fine scale lesions, 2 = coarser scales, most of lesions covered, 3 = entire lesion covered with coarse scales, 4 = very thick coarse scales, possibly fissured Visits: baseline and week 4 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 387): "Subjects were randomly assigned to either the qd or the bid dosing group by the study coordinator at each site [...]" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 388): "The results were analysed on the basis of intention‐to‐treat [...]" Comment: no information about imputation method used (6 drop‐outs). No information about the distribution of the drop‐outs across groups. However, all randomised patients included for analysis. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | High risk | Slightly different values for erythema and plaque thickness scores reported in text and table 3 (i.e. erythema score: initial mean: 2.7 ± 0.8 (text) versus 2.7 ± 0.7 (table 3)) Inclusion criteria: at least 18 years, but age at baseline ranged from 17 to 90 years Comment: these findings are not considered to have a significant impact on outcomes Quote (page 388): "The subject and the investigator graded the global response at the follow up visit." Comment: no baseline IGA and PGA assessed, thus change in these scores not evaluable |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 387): "single‐blind study" Quote (page 388): "The subjects self‐administered the treatment to the entire scalp under the instruction of the study personnel [...]" Comment: insufficient information about which side was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 387): "single‐blind study" Quote (page 387): "The physician‐grader performing the evaluations was blinded to the assignment." Comment: insufficient information about method used to ensure blinding of outcome assessor |
| Methods | This was a multicentre, randomised, double‐blind, vehicle‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: calcipotriene foam 0.005%, twice daily for 8 weeks (N = 181 participants) B: placebo vehicle foam, twice daily for 8 weeks (N = 182 participants) | |
| Outcomes | Primary outcome
Secondary outcomes
Definition: Scalp ISGA (0 to 5): 0 = clear, 5 = very severe Erythema, thickness, scaling score (0 to 5, respectively): 0 = clear, 5 = very severe Target lesion score: sum of erythema, thickness, scaling scores Visits: baseline, week 1, 2, 4 and 8 | |
| Notes | This trial was supported by Stiefel, a GSK company, Research Triangle Park, NC NCT01139580 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 301): " Subjects were allocated [...] using a 1:1 randomization schedule generated before the study." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 301): "All analysis were on the ITT population [...] a last‐observation‐carried‐forward (LOCF) method was used" Comment: missing data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 301): "Canister were labelled identically", "Study group allocation was unblinded to study personnel after all data were collected and validated [...]" Comment: probably sufficient |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 301): "Canister were labelled identically", "Study group allocation was unblinded to study personnel after all data were collected and validated [...]" Comment: probably sufficient |
| Methods | This was a multicentre, randomised, double‐blind, active‐ and vehicle‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: betamethasone valerate foam 0.1%, twice daily for 4 weeks (N = 57 participants) B: betamethasone valerate lotion 0.1%, twice daily for 4 weeks (N = 58 participants) C: placebo foam, twice daily for 4 weeks (N = 28 participants) D: placebo lotion, twice daily for 4 weeks (N = 29 participants) | |
| Outcomes |
Definition: IGA /PGA (7‐point scale): lesions rated as completely clear, almost clear (= 'very mild'), marked improvement, moderate improvement, slight improvement, no change, worse Erythema, thickness, pruritus and scaling score (0 to 4, respectively): 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe Visits: baseline, week 2 and 4 | |
| Notes | This trial was funded by Connetics Corporation, Palo Alto, California | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 629): "[...] randomly assigned to one of four treatment groups in a 2 : 1 : 2 : 1 ratio [...]" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 631): "One hundred and seventy‐two patients (85 men, 87 women), from a total of 190 enrolled, completed the safety and efficacy study." Comment: insufficient reporting of reasons for attrition or exclusions |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 629): "double‐blind study" Comment: insufficient information about how participants and personnel were blinded regarding difference between the 2 vehicles used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 629): "double‐blind study" Comment: insufficient information about how blinding of outcome assessors was ensured throughout the study |
| Methods | This was a multicentre, randomised, double‐blind, active‐ and vehicle‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: clobetasol propionate (CP) foam 0.05%, twice daily for 14 days (N = 62 participants) B: CP solution 0.05%, twice daily for 14 days (N = 63 participants) C: placebo foam, twice daily for 14 days (N = 31 participants) D: placebo solution, twice daily for 14 days (N = 32 participants) Assignment in a 2:1:2:1 ratio: CP foam:placebo foam:CP solution:placebo solution | |
| Outcomes |
Definition: IGA/PGA (7‐point scale): lesions rated as completely clear, almost clear (= 'very mild'), marked improvement, moderate improvement, slight improvement, no change, or worse Erythema, thickness, pruritus and scaling score (0 to 4, respectively): 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe Visits: baseline, week 1, 2 and 4 (follow‐up) | |
| Notes | Funded by Connetics Corporation, Palo Alto, CA, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 536): "All enrolled subjects [...] successfully completed the study." Comment: no missing outcome data |
| Selective reporting (reporting bias) | High risk | Quote (page 537): "The percentage of patients reporting adverse events and the incidence of adverse events judged as being related to study medication did not differ significantly among the treatment groups." Comment: insufficient data regarding the incidence or characteristics of adverse events reported |
| Other bias | High risk | Baseline characteristics (TSS, calculated by review authors) not balanced between groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 535): "double‐blind" Comment: insufficient information about how participants and personnel were blinded regarding difference between the 2 vehicles used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 535): "double‐blind" Comment: insufficient information about how blinding of outcome assessors was ensured throughout the study |
| Methods | This was a randomised, double‐blind, active‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: betamethasone 17,21‐dipropionate 0.05% in alcoholic solution, twice daily for 4 weeks (N = 29 participants) B: betamethasone 17,21‐dipropionate 0.05% plus salicylic acid 2.0% in alcoholic solution, twice daily for 4 weeks (N = 30 participants) C: triamcinolone acetonide 0.2% plus salicylic acid 2.0% in alcoholic solution, twice daily for 4 weeks (N = 31 participants) | |
| Outcomes |
Definition: Outcomes 1 to 8: each rated on a score from 0 to 3: 0 = absence, 1 = mild, 2 = moderate, 3 = severe Physician's overall evaluation: clinical cure = complete remission, clinical improvement = marked (> 70% but < 100%), moderate (>30% but < 70%), slight (< 30%), treatment failure = no change or worsening Visits: baseline, week 2 and 4 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 253): "patients were allocated one of the preparations at random" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 253): "All patients completed the trial period." Comment: data for each outcome completely addressed |
| Selective reporting (reporting bias) | High risk | Quote (page 253): "There was no statistically significant difference regarding the variables lichenification, excoriation, crusting, scaling, pruritus and pain [...] were present to too small a degree to make an analysis meaningful" Comment: some results were not reported. No baseline data for each specific group reported. No baseline severity as inclusion criteria defined. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 253): "identical plastic bottles bearing only a patient number [...] choice remaining unknown to the investigator and to the patient until the code was broken at the end of the trial" Comment: probably sufficient |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 253): "identical plastic bottles bearing only a patient number [...] choice remaining unknown to the investigator and to the patient until the code was broken at the end of the trial" Comment: probably sufficient |
| Methods | This was a randomised, double‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: hydrocortisone 17‐butyrate 0.1% cream, once daily for 4 weeks (N = 20 participants) B: fluocinolone acetonide 0.025% cream, once daily for 4 weeks (N = 20 participants) | |
| Outcomes |
Definition: Desquamation, excoriation score (1 to 5, respectively): 1 = none, 5 = very severe Infiltration score: 5 = severe, 4 = moderate, 3 = slight, 2 = erythema only, 1 = normal skin alone Visits: baseline, week 1, 2, 3 and 4 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 198): "[...] 40 patients with psoriasis of the scalp were randomly allocated to one of 2 groups." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data complete |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 198): "double‐blind study" Comment: insufficient detail was reported about the method used to blind study participants or personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 198): "double‐blind study" Comment: insufficient detail was reported about the method used to blind assessor from the intervention a participant received |
| Methods | This was a multicentre, prospective, randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol solution 50 µg/ml, twice daily for 4 weeks (N = 25 participants) B: placebo vehicle, twice daily for 4 weeks (N = 24 participants) | |
| Outcomes |
Definition: Redness, scaliness and thickness, pruritus, flaking score (0 to 4): 0 = absent, 1 = slight, 2 = moderate, 3 = severe, 4 = severest possible Extent of scalp psoriasis (0 to 5): 0 = no involvement, 1 = < 20%, 2 = 20% to 39%, 3 = 40% to 59%, 4 = 60% to 79%, 5 = 80% to 100% IOA/POA (‐1 to 3): ‐1 = worse, 0 = no change, 1 = slight improvement, 2 = marked improvement, 3 = cleared (matched with IGA/PGA = clear) Blood work: haematology and biochemistry (including serum total calcium, hepatic and renal parameters) TSS (0 to 12): sum of the 3 parameters thickness, redness and scaliness Visits: baseline, week 1, 2 and 4 | |
| Notes | This study was sponsored by Leo Pharmaceutical Products, Ballerup, Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 483): "[...] patients were randomly allocated to receive either calcipotriol solution (50 /ig/ml) or placebo [...]" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote (page 486): "Forty‐six of the 49 patients completed the study‐one in the active group withdrew because of local side‐effects [...] two in the placebo group withdrew because of an inadequate treatment response." Comment: no ITT analysis used, but all outcomes except for TSS with ITT population reported. No imputation method reported. |
| Selective reporting (reporting bias) | Low risk | All results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 483‐4): "The active and placebo preparations [...] were similar in appearance, smell, and texture, and supplied in identical packaging." Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 483‐4): "The active and placebo preparations [...] were similar in appearance, smell, and texture, and supplied in identical packaging." Comment: blinding probably sufficient |
| Methods | This was a 4‐week, multicentre, randomised, parallel‐group, investigator‐masked trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% shampoo, once daily for 4 weeks (N = 121 participants) B: tar blend 1% shampoo, twice daily for 4 weeks (N = 41 participants) Allocation was done in a 3:1 (A:B) ratio | |
| Outcomes | Primary outcomes of the trial
Secondary outcomes of the trial
Definition: TSS (0 to 9): sum of scores for erythema, desquamation and plaque thickening GSS (0 to 5): 0 = none ‐ 5 = very severe Erythema, desquamation and thickness, pruritus score (0 to 3): 0 = absent ‐ 3 = severe Subject's global assessment of improvement: (‐1 to 5): ‐1 = worse to 5 = clear Cutaneous safety: 0 = none to 3 = severe for telangiectasia, skin atrophy and burning, respectively Ocular safety: 0 = none to 3 = severe Cosmetic acceptability: questionnaire at the end of the trial with 12 items Visits: baseline, week 2 and 4 | |
| Notes | Galderma Medical Affairs assisted in writing the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 91): "Subjects were randomized at baseline to either clobetasol propionate 0.05% shampoo or to tar blend 1% shampoo in a ratio of 3:1." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | "All efficacy parameters, with the exception of the subject’s assessment of global improvement, were analysed at the last visit (ITT analysis) or at the week visit (PP analysis) using an analysis of covariance (ANCOVA), using the baseline variable as a covariate and centre and treatment as factors." Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (91): "[...] investigator‐masked [...]" Comment: no blinding of participants or personnel done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 91): "The investigator was masked to the treatment allocation." Comment: blinding of outcome assessment insufficiently reported |
| Methods | This was a multicentre, randomised, parallel‐group, double‐blind trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: micronized betamethasone 17‐valerate 0.1% in isopropyl alcohol lotion, twice daily for 2 weeks (N = 45 participants) B: vehicle, twice daily for 2 weeks (N = 33 participants) | |
| Outcomes |
Definition: Patient's clinical response: rated on a score from 1 to 6: 1 = no evaluation, 2 = exacerbation, 3 = poor or no effect, 4 = fair, partial clinical control of condition (less than 50%), 5 = good, moderate clinical control of condition (50% to 75%), 6 = excellent, complete clinical control of condition (75% or more) (matched with IGA = responder) Outcomes 2 to 14: graded on a score from 1 to 4: 1 = none, 2 = slight, 3 = moderate, 4 = severe Visits: baseline, day 5, 8 and 14 | |
| Notes | The study investigated 2 groups separately: patients with seborrhoeic dermatitis and patients with psoriasis of the scalp | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 640): "[...] according to a randomized code." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote (page 393): "The test preparations ware supplied to each investigator in identical packages, code labelled for blind randomized assignment to patients. [...] Master codes for each study were maintained separately from the investigators" Comment: probably sufficient |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 642): "Of the 95 psoriasis patients entered into the eleven studies, 5 did not return for further follow‐up after the initial visit, and 12 were excluded for the following reasons: 9 used a concomitant medicated shampoo and 3 used a concomitant corticosteroid." Comment: no ITT, but per‐protocol analysis performed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | High risk | Quote (page 340): "The majority of the patients were placed on a bid basis." Comment: insufficient information about how many and why participants were placed on bid or opd basis. Performance bias possible. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 639): "They compared a betamethasone valerate lotion and a placebo (the lotion vehicle) [...]" Quote (page 640): "Neither patient nor physician was aware of which of the two was being used." Comment: probably sufficient blinding performed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 639‐40): "The test preparations ware supplied to each investigator in identical packages, code labelled for blind randomized assignment to patients [...] " Quote (page 639): "They compared a betamethasone valerate lotion and a placebo (the lotion vehicle) [...]" Quote (page 640): "Neither patient nor physician was aware of which of the two was being used." Comment: probably sufficient blinding performed |
| Methods | This was a randomised, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: tacrolimus 0.1% ointment, twice daily for 8 weeks (N = 20 participants) B: pine tar 5% ointment, twice daily for 8 weeks (N = 20 participants) | |
| Outcomes |
Definition: Cure rate: percent of patients with the change ratio of an area score of 100% Response rate: percent of patients with the change ratio of an area score of 75% Area score: 5‐point score: 0 = psoriasis completely improved, 1 = 75% to 90% improvement, 2 = 50% to 74% improvement, 3 = 25% to 49% improvement, 4 = < 25% improvement Severity score of sign symptoms (0 to 16): sum of dander, erythema, pruritus, thickness/infiltration scores Dander, erythema, pruritus, thickness/infiltration scores: each rated on a scale from 0 = none to 4 = very severe Visits: baseline, week 2, 4, 6 and 8 | |
| Notes | This article was translated by Mrs. Sai Zhao of Systematic Review Solutions Ltd, China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 254, abstract): "patients were randomly assigned" Comment: insufficient detail was reported about the method used to generate the allocation sequence. No further information in the article found by the translator. |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | No ITT analysis reported, but data for all randomised participants provided |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | This was not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | This was not stated |
| Methods | This was a randomised, double‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics This was not stated | |
| Interventions | A: betamethasone 17,21‐dipropionate solution plus salicylic acid in 2% alcoholic solution, twice daily for 3 weeks (N = 39 participants) B: betamethasone valerate solution, twice daily for 3 weeks (N = 39 participants) | |
| Outcomes |
Definition: Overall treatment response (6‐point scale): graded as 'cure', 'marked improvement' (> 70%), 'moderate improvement' (30% to 70%), 'slight improvement' (≤ 30%), 'no change' or 'worse' Visits: baseline, week 1, 2 and 3 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 48): "The patients were randomly allocated to one of the two groups [...]" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 49, Table III): "*patient No. 8 not evaluated" (for week 2), "**patient No. 72 not evaluated" (for week 3) No ITT analysis performed, but influence on outcome data not considered as significant |
| Selective reporting (reporting bias) | Low risk | All results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 48): "The bottles looked identical. Neither staff nor patients knew who received which treatment until the trial was finished." Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 48): "The bottles looked identical. Neither staff nor patients knew who received which treatment until the trial was finished." Comment: blinding probably sufficient |
| Methods | This was a randomised, double‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: betamethasone 17, 21‐dipropionate plus salicylic acid 2% alcoholic solution, twice daily for 3 weeks (N = 25 participants) B: clobetasol propionate lotion, twice daily for 3 weeks (N = 25 participants) Participants could withdraw prior to week 3 if healing occurred | |
| Outcomes |
Definition: Investigator's overall evaluation: patient's response from initial state rated as 'cure' = complete remission of signs and symptoms, 'marked improvement' = ≥ 70%, 'moderate improvement' = 30% to 70%, 'slight improvement' = ≤ 30%, 'failure' = no change or worse Visits: baseline, week 1, 2 and 3 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 419): "patients were randomly allocated." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 419): "Two of the patients did not return at the follow‐up visits. The remaining fifty‐one patients [...] did not show any treatment group differences" Quote: (page 420): "One patient [...] has been excluded from the evaluation of efficacy due to concomitant therapy with methotrexate." Quote (page 420): "In the Dermovate group [...] three were treatment failure and two drop‐outs" Comment: no ITT analysis and no adequate imputation method performed. Insufficient reporting about reasons for drop‐out. However, attrition considered too small (< 10%) to have a relevant impact on outcome. |
| Selective reporting (reporting bias) | High risk | Quote (page 420): "Pruritus [...] was the only symptom showing a statistically significant difference" Comment: insufficient reporting of this outcome. In addition, no data for other pre‐specified outcomes (induration, lichenification, crusting, pain, scaling) reported. |
| Other bias | Unclear risk | Selection criteria for patients of which the blood cortisol level was assessed unclear. Selection bias possible. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 419): "using a double‐blind technique" Comment: insufficient detail was reported about the method used to blind study participants or personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 419): "using a double‐blind technique" Comment: insufficient detail was reported about the method used to blind the outcome assessor from the intervention a participant received |
| Methods | This was a randomised, double‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: desoximethasone solution 0.25% plus salicylic acid 1%, twice daily for 2 weeks (N = 20 participants) B: betamethasone valerate solution 0.1%, twice daily for 2 weeks (N = 20 participants) | |
| Outcomes |
Definition: Outcomes 1 to 4.: each graded on a score from 0 to 3: 0 = none, 1 = slight, 2 = moderate, 3 = severe Degree of severity (0 to 12): sum of erythema, scaling, infiltration and pruritus Patient's and investigator's overall evaluation: results rated as 'much better', 'slightly better', 'no change', 'slightly worse', 'much worse' Cosmetic acceptability: results rated by the patient as 'very good', 'good', 'moderate', 'poor', 'no answer' Visits: baseline, week 1, 2 and 3 (follow‐up) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 171): "The randomization was done in advance by the manufacturer with the aid of a table with random digits and kept secret from the doctor during the study." Comment: probably sufficient sequent generation |
| Allocation concealment (selection bias) | Low risk | Quote (page 171): "The randomization was done in advance by the manufacturer with the aid of a table with random digits and kept secret from the doctor during the study." Comment: probably sufficient |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 172, Table 1): "Two patients are not included because they were treatment failures after the first week of treatment." Comment: no ITT analysis and no adequate imputation method performed |
| Selective reporting (reporting bias) | Low risk | All results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 171): "50ml bottle with identical appearance. [...] the two solutions differed in odour [...]" Comment: unclear if odour could affect outcome |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 171): "50ml bottle with identical appearance. [...] The doctor was informed that the two solutions differed in odour and that he was not allowed to sniff them." Comment: blinding probably insufficiently ensured |
| Methods | This was a randomised, single‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: betamethasone valerate 0.12% foam, twice daily for 2 weeks (N = 13 participants) B: fluocinolone acetonide 0.01% oil, once daily for 2 weeks (N = 12 participants) Cross‐over took place after 2 weeks | |
| Outcomes |
Definition: TSS: sum of erythema, scaling and thickness score Outcomes 4 to 7.: each graded on a score from 0 to 4, higher scores indicating worse severity Patient's and investigator's global response: results rated on a scale from 0 to 6, higher scores indicating worse disease severity Treatment preference measure (‐21 to +21): for both intervention each characteristic "ease of application", "application time", "absorption", "how it feels to odour", "how it feels on skin" and "how much it stains" were rated by the patient on a scale from ‐3 to 3: ‐3 = extremely unappealing, ‐2 = moderately unappealing, ‐1 = slightly unappealing, 0 = neutral, +1 = slightly appealing, +2 = moderately appealing and +3 = extremely appealing QOL score: patients graded each intervention on a scale from ‐3 to 3: ‐3 = will greatly worsen quality, ‐2 = will moderately worsen the quality, ‐1 = slightly worsen the quality, 0 = will have no effect, +1 = will slightly improve the quality, +2 = will moderately improve the quality and +3 = will greatly improve the quality Visits: baseline, day 14 and 28 | |
| Notes | This study was supported by Connetics Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 28): "[...] each patient was randomized [...]" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 28): "The physician‐grader was blinded to treatment‐group assignment." Comment: insufficient information about how allocation concealment was performed |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 29): "Twenty‐four of 25 patients completed the trial (1 patient was lost to follow‐up)." Comment: no ITT analysis performed, but attrition not considered to have a significant impact on outcome |
| Selective reporting (reporting bias) | High risk | Quote (page 29): "total severity scores [...] were not significantly different for the medications" Comment: insufficient reporting of relevant outcomes: TSS, erythema, scaling, thickness, pruritus, investigator's/patient's global assessment of response and QOL score Quote (page 29): "As a final indication of preference, 18 patients requested prescriptions for particular medications at the conclusion of the study: 11 requested foam, 4 requested oil, and 1 requested both" Comment: this outcome was not pre‐specified in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 27): "[...] single‐blind trial [...]" Comment: blinding of patients not possible due to physical difference of vehicles (foam versus oil). This might have an impact on subjective outcomes (pruritus, quality of life). |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 27): "[...] single‐blind trial [...]" Comment: insufficient reporting about method of blinding |
| Methods | This was a randomised, double‐blind, bilateral‐paired comparison (split‐face) trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: augmented betamethasone dipropionate 0.05% lotion, once daily for 3 weeks (N = 55 participants) B: clobetasol propionate 0.05% solution, once daily for 3 weeks (N = 55 participants) This was a split‐face comparison | |
| Outcomes |
Definition: Degree of change: difference between TSS during treatment and pre‐treatment divided by the TSS at pre‐treatment, multiplied by 100 TSS (0 to 3): sum of erythema, thickness, scaling and pruritus scores Pruritus score (0 to 3): 0 = none, 1 = mild, 2 = moderate, 3 = severe Global evaluation score: comparison of the patients' overall disease status at each return visit to their initial baseline condition Visits: baseline, day 4, 8, 15 and 22 | |
| Notes | This was a split‐face comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 105): "Treatment assignment to the right or left side was determined by a random code." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 107, Table 2): "**Endpoint=the last valid visit, independent of the visit day on which it occurred." Comment: only for endpoint (day 22) data for the ITT population for efficacy analysis reported using the last observation carried forward (LOCF) imputation method. Other visits: per‐protocol population. |
| Selective reporting (reporting bias) | Low risk | All results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 105): "double‐blind" Comment: insufficient detail was reported about the method used to blind study participants or personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 105): "double‐blind" Comment: insufficient detail was reported about the method used to blind the outcome assessor |
| Methods | This was a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% shampoo, once daily for 4 weeks (N = 95 participants) B: vehicle shampoo, once daily for 4 weeks (N = 47 participants) Assignment in 2:1 (A:B) ratio | |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: GSS (0 to 5): 0 = clear, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe TSS (0 to 9): sum of erythema, thickness, scaling scores Outcomes 2 to 5.: each rated on score from 0to 3: 0 = none, 3 = severe IAGI/PAGI (0to 5): scale from 0 = clear to 5 = very severe (matched with IGA/PGA = responder) Visits: baseline, week 2, 4 and 6 (follow‐up) | |
| Notes | This study was supported by Galderma R&D Inc. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 368): "computer‐generated randomization list" Comment: probably adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 368‐369): "The intent‐to‐treat (ITT) population was primary for the efficacy analysis. [...] with missing data imputed using the last observation carried forward (LOCF) [...]" Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | This was a double‐blind trial with vehicle used as control |
| Blinding of outcome assessment (detection bias) | Unclear risk | This was a double‐blind trial with vehicle used as control, but insufficient reporting about how blinding of the investigator was performed and maintained |
| Methods | This was a multicentre, randomised, double‐blind, active‐ and vehicle‐controlled, 4‐arm, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriene 50 µg/g plus betamethasone dipropionate 0.5 mg/g gel, once daily for 8 weeks (N = 541 participants) B: betamethasone dipropionate 0.5 mg/g gel, once daily for 8 weeks (N = 556 participants) C: calcipotriene 50 µg/g gel, once daily for 8 weeks (N = 272 participants) D: placebo‐vehicle, once daily for 8 weeks (N = 136 participants) Assignment in 4:4:2:1 ratio Quote (page 457): "Patients graded to have "absence of disease" [...] could stop treatment with study medication at the investigator's discretion, but after implementation of a protocol amendment were required to remain in the study and attend all clinic visits." | |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: IGA (6‐point scale): disease severity rated as'absence of disease', 'very mild disease', 'mild disease', 'moderate disease', 'severe disease' and 'very severe disease' Patient's assessment of overall response (7‐point scale): extent and severity of scalp lesions rated as 'worse', 'unchanged', 'slight improvement', 'moderate improvement', 'marked improvement', 'almost clear' and 'cleared' AE: including severe AEs Blood samples: serum calcium and serum albumin Erythema, thickness, scaling score: 0 = no signs, 1 = slight signs, 2 = moderate signs, 3 = severe signs, 4 = very severe signs TSS (0‐12): sum of erythema, thickness, scaling scores Visits: baseline, week 1, 2, 4, 6 and 8 | |
| Notes | This trial was supported by LEO Pharma A/S, Ballerup, Denmark. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 456): "[...] according to a preplanned computer‐ Comment: probably adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 457‐8): "All randomized patients were included in the full analysis set and were analysed for efficacy. [...] using last observation carried forward [...]" Comment: ITT analysis and appropriate imputation method performed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 457): "Packaging and labelling of the investigational products or placebo contained no evidence of their identity. [...] No effects of the Investigational Products revealed the identity of the individual treatment allocations" Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 457): "Packaging and labelling of the investigational products or placebo contained no evidence of their identity. [...] No effects of the Investigational Products revealed the identity of the individual treatment allocations" Comment: blinding probably sufficient |
| Methods | This was a mono‐centre, randomised, open‐labelled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: betamethasone dipropionate lotion plus RV3423A shampoo alternated with extra gentle shampoo, every day alternation for 4 weeks (N = 40 participants) B: betamethasone dipropionate lotion plus extra gentle shampoo, once daily for 4 weeks (N = 39 participants) | |
| Outcomes |
Definition: LS (0 to 9): sum of desquamation, erythema and induration scores Outcomes 2 to 4: rated on scale from 0 to 3: 0 = absence, 3 = very severe IGA: not defined in this abstract ('healing' matched with 'clear') Visits: baseline, weeks 2, 4 and 8 (not relevant) | |
| Notes | Total sign score (TSS) in data analysis calculated from LS by the review author After 4 weeks, participants were treated until week 8 in a manner that was not relevant for this review This was a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 391): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | This was not stated |
| Selective reporting (reporting bias) | Unclear risk | Not all pre‐specified outcomes reported (e.g. pruritus). Only abstract available. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 391): "open‐labeled" Comment: no blinding performed |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 391): "open‐labeled" Comment: no blinding performed |
| Methods | This was a 2‐week, randomised, multicentre, investigator‐blinded, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: augmented betamethasone dipropionate 0.05% lotion, twice daily for 2 weeks (N = 98 participants) B: clobetasol propionate 0.05% solution, twice daily for 2 weeks (N = 99 participants) | |
| Outcomes |
Definition: Investigators'evaluation of global clinical response (0 to 6): 1 = clear, 2 = marked improvement (matched with IGA = responder), 3 = moderate improvement, 4 = slight improvement, 5 = no change, 6 = exacerbation TSS (0 to 12): sum of erythema, thickness, pruritus, scaling scores Outcomes 2 to 5 (0 to 3): each rated as 0 = none, 1 = mild, 2 = moderate or 3 = severe Skin atrophy score (0 to 3): rated as 0 = none, 1 = mild, 2 = moderate or 3 = severe Treatment failure: no response to treatment or exacerbation Visits: baseline, days 4, 8 and 15 | |
| Notes | This study was supported by the Medical and Scientific Affairs Department of Schering Laboratories, Kenilworth, New Jersey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 391): "Patients were randomised" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 393): "One hundred ninety‐seven patients [...] were enrolled; 196 patients [...] were included in the safety analysis and 193 (96 betamethasone dipropionate, 97 clobetasol propionate) in the efficacy analysis." Comment: number and reason for attrition and exclusion adequately reported for each group. They are balanced between groups and considered as too small to have a significant impact on outcomes. |
| Selective reporting (reporting bias) | Unclear risk | No data for pre‐specified outcome of skin atrophy reported. This outcome is not relevant for this review. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 390): "investigator‐blinded" Comment: no blinding of participants |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 392): "Study medications were supplied in commercially available 50‐mL bottles that were wrapped in an occlusive vinyl covering to prevent product identification." Comment: blinding of investigator probably sufficient |
| Methods | This publication reported 2 randomised, double‐blind, parallel‐group trials | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period
Baseline characteristics This was not stated | |
| Interventions | A: calcipotriol 0.005% solution, twice daily for 8 weeks (N per group unclear) B: vehicle solution, twice daily for 8 weeks (N per group unclear) N = 235 participants | |
| Outcomes |
Definition: None of the outcomes was further defined. Visits: baseline, day 1, 4 and week 1, 2, 4, 6 and 8 | |
| Notes | This publication was published as a poster abstract and reported data about 2 parallel‐group trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 301): "randomized" Comment: insufficient information provided on how sequence allocation was performed |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote (page 301): "The total subjects enrolled for the first trial was 235 with 204 subjects completing the study." Comment: no ITT analysis performed. Number of drop‐outs per group not reported. Number of drop‐outs probably too low to introduce significant bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 301): "the mean score for all the psoriasis characteristics evaluated was statistically lower for the calcipotriene solution 0.005% group than its vehicle (p<0.009)" Comment: results insufficiently reported Quote (page 301): "mean serum calcium levels across treatments remained within the normal range" Comment: this outcome was not pre‐specified |
| Other bias | Low risk | No other potential source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 301): "double‐blind" Comment: this study was vehicle‐controlled. Blinding of participants therefore considered as probably sufficient. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 301): "double‐blind" Comment: insufficient information on how blinding of investigators was performed and maintained |
| Methods | This publication reported 2 randomised, double‐blind, parallel‐group trials | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period
Baseline characteristics This was not stated | |
| Interventions | A: calcipotriol 0.005% solution, twice daily for 8 weeks (N per group unclear) B: vehicle solution, twice daily for 8 weeks (N per group unclear) N = 239 participants | |
| Outcomes |
Definition: None of the outcomes was further defined Visits: baseline, day 1, 4 and week 1, 2, 4, 6 and 8 | |
| Notes | This publication was published as a poster abstract and reported data about 2 parallel‐group trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 301): "randomized" Comment: insufficient information provided on how sequence allocation was performed |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote (page 301): "The second trial enrolled 239 subjects with 210 completing the study" Comment: no ITT analysis performed. Number of drop‐outs per group not reported. Number of drop‐outs probably too low to introduce significant bias. |
| Selective reporting (reporting bias) | High risk | Quote (page 301): "the mean score for all the psoriasis characteristics evaluated was statistically lower for the calcipotriene solution 0.005% group than its vehicle (p<0.009)" Comment: results insufficiently reported Quote (page 301): "mean serum calcium levels across treatments remained within the normal range" Comment: this outcome was not pre‐specified |
| Other bias | Low risk | No other potential source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 301): "double‐blind" Comment: this study was vehicle‐controlled. Blinding of participants therefore considered as probably sufficient. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 301): "double‐blind" Comment: insufficient information on how blinding of investigators was performed and maintained |
| Methods | This was a multicentre, randomised, double‐blind, prospective, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/ml solution, twice daily for 4 weeks (N = 240 participants) B: betamethasone 17‐valerate 1 mg/ml solution, twice daily for 4 weeks (N = 234 participants) | |
| Outcomes |
Definition: Investigator's/patient's overall assessment of treatment response (1 to 5): 1 = worse, 2 = no change, 3 = slight improvement, 4 = marked improvement, 5 = cleared TSS (0 to 12): sum of erythema, thickness, scaling scores Extend of scalp psoriasis: 0 = no involvement, 1 = < 20%, 2 = 20% to 39%, 3 = 40% to 59%, 4 = 60% to 79%, 5 = 80% to 100% Pruritus/flaking score: rated on a scale from 0 to 3 by patient Outcomes 3 to 5 (0 to 4): each rated as 0 = absent, 1 = slight, 2 = moderate, 3 = severe and 4 = severest possible Patient's assessment of acceptability: greasiness, ease of application, whether treatment caused dryness of the scalp and odour were rated on as 1 = very poor, 2 = poor, 3 = acceptable, 4 = good, 5 = excellent Blood sample analysis: including haematology and biochemistry (with serum total calcium) Relapse rate: percentage of patients with satisfactory results after double‐blind period, who experience relapse (increase in TSS to at least 50% of baseline at start of study) within 4 weeks after end of treatment Visits: baseline, week 1, 2, 4 and 8 (optional follow‐up) | |
| Notes | This study was sponsored and supported by Leo Pharmaceutical Products, Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 679): "patients were randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 680): "474 were randomized [...] Twenty‐nine patients [...] withdrew from the study, leaving 445 patients [...]" Comment: no ITT analysis performed: data for 236/240 (calcipotriol group) and data for 232/234 (BTM group) reported in efficacy analysis. Missing outcome data balanced between groups and reasons for drop‐outs similar. Proportion of missing outcomes considered as not enough to introduce a clinically relevant impact on the intervention effect estimate. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 679): "double‐blind treatment" Comment: insufficient detail was reported about the method used to blind study participants or personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 679): "double‐blind treatment" Comment: blinding method of outcome assessment insufficiently reported |
| Methods | This was a multicentre, prospective, randomised, open‐label trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/ml solution, twice daily for 8 weeks (N = 238 participants) B: coal tar 1% plus coconut oil 1% plus salicylic acid 0.5% shampoo, once daily for 8 weeks (N = 237 participants) | |
| Outcomes |
Definition: Investigators' overall assessment (0 to 5): 0 = worse, 1 = no change, 2 = slight improvement, 3 = moderate improvement, 4 = marked improvement, 5 = cleared Patients' self assessment: severity of scalp psoriasis, flaking, pruritus and effectiveness of treatment measured on 100 mm visual analogue scales with left limit (0 mm) representing worst and right limit (100 mm) representing best assessment TSS (0 to 12): sum of erythema, thickness, scaling scores Extend of scalp psoriasis: 0 = no involvement, 1 = < 20%, 2 = 20% to 39%, 3 = 40% to 59%, 4 = 60% to 79%, 5 = 80% to 100% Outcomes 3 to 5 (0 to 4): each rated as 0 = absent, 1 = slight, 2 = moderate, 3 = severe, 4 = severest possible Blood sample analyses: including haematology and biochemistry (serum total calcium, phosphate, albumin, bilirubin, alkaline, phosphatase, alanine aminotransferase, creatinine) Urine sample analysis: including urinary calcium and urinary creatinine Visits: baseline, week 4 and 8 | |
| Notes | This trial consisted of a second uncontrolled, non‐randomised, open‐labelled phase after 8 weeks (until week 24) The study was sponsored by Leo Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 22): "Treatment was allocated at random." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote (page 23): "Fifty‐two patients were excluded from analysis because of loss to follow‐up [...] or stopping study medication [...] The efficacy population therefore consisted of 423 patients" Comment: no ITT analysis performed. More than 10% drop‐outs in each group. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 22): "open‐label design" Comment: no blinding performed |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 22): "open‐label design" Comment: no blinding performed |
| Methods | This was an international, multicentre, randomised, investigator‐blind, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g gel, once daily for 8 weeks (N = 207 participants) B: calcipotriol 50 µg/g solution, twice daily for 8 weeks (N = 105 participants) Randomized in a 2:1 ratio Patients whose scalp psoriasis cleared before the end of the 8‐week treatment period stopped treatment, but remained in the study | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: TSS (0 to 12): sum of erythema, thickness, scaling scores Outcomes 2 to 4 (0 to 4): each rated as 0 = absent, 1 = slight, 2 = moderate, 3 = severe, 4 = very severe IGA (6‐point scale): 0 = absent, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe PGA (5‐point scale): clear, very mild, mild, moderate, severe Pruritus: rated by the patient as "none", "mild", "moderate", "severe" Relapse: recurrence of at least "moderate" disease according to IGA Time to relapse: number of days from the last application of medication to relapse Compliance: self reported use of study medication Rebound: increase in one category in IGA from baseline Blood sample analyses: including haematology and biochemistry (serum calcium) QOL (published fromOrtonne 2009):
Product acceptability: rated on a 7‐point scale ranging from "very unacceptable" to "very acceptable" Visits: baseline, week 1, 2, 4, 6, 8 and 12, 16 (both: follow‐up) | |
| Notes | The study was sponsored by LEO Pharma A/S, Ballerup, Denmark Additional data about quality of life provided by Ortonne 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 160): "a computer‐generated schedule randomized patients 2 : 1 to treatment [...]" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote (page 160): "Each patient was assigned an exclusive randomization code in ascending order." Comment: probably sufficient |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 161): "The intent‐to‐treat (ITT) population [...] was used for analysis of all efficacy endpoints. [...] The safety population comprised all patients who received any treatment with study medication and for whom the presence or confirmed absence of AEs was available. A last observation carried forward (LOCF) approach accounted for missing data." Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 160): "Due to differences in the dosing frequency and formulation between the investigational products, it was not possible to undertake a double‐blind study." Comment: lack of blinding not considered as likely to influence the primary outcomes (TSS, IGA). Subjective outcomes (i.e. PGA and QOL scores) likely to be biased. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 160): "The packaging and labels for the medications were identical [...] dispensed by a designated independent non assessor, to maintain blinding of investigators performing the assessments. Patients were instructed not to reveal the formulation or dosing frequency of their medication to their assessor." Comment: blinding of outcome assessment probably sufficient |
| Methods | This was a randomised, parallel‐group, active‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/g ointment (occlusive bandaging over night), once daily for 5 days (N = 21 participants) B: clobetasol 17‐propionate scalp solution, twice daily for 10 days (N = 22 participants) | |
| Outcomes |
Definition: TSS (0 to 9): sum of erythema, thickness, scaling scores Outcomes 2 to 4 (0 to 3): each rated as 0 = absent, 1 = slight, 2 = moderate, 3 = severe Blood sample analyses: including haematology and biochemistry (serum calcium) Visits: before and after each treatment and additionally on day 10 and 20 (follow‐up) | |
| Notes | This trial was presented as a correspondence letter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 287): "43 patients were randomly allocated" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 287): "All patients completed the study." Comment: no use of ITT analysis reported, but no loss to follow‐up. Incomplete outcome data sufficiently addressed. |
| Selective reporting (reporting bias) | High risk | Quote (page 287): "85% of patients in the calcipotriol group and 91% of patients in the clobetasol group showed clearance or marked improvement of their scalp psoriasis" Comment: this outcome was not pre‐specified in the methods section. Insufficient reporting of TSS and its subscores. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported and highly unlikely due to difference in treatment application and duration between the 2 intervention groups |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported and highly unlikely due to difference in treatment application and duration between the 2 intervention groups |
| Methods | This was a prospective, double‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% solution, twice daily for 2 weeks (N = 20 participants) B: betamethasone‐17,21‐dipropionate 0.05% solution, twice daily for 2 weeks (N = 20 participants) | |
| Outcomes |
Definition: Outcomes 1 to 4 (0 to 3): each rated as 3 = severe, 2 = moderate, 1 = mild or 0 = absent TSS (0 to 9): calculated with given data: sum of erythema, scaling, thickness Visits: baseline, week 1 and 2 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 366): "patients were non‐selectively divided into two groups" Comment: considering the year of publication, this was interpreted as a randomised study, but insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. Data for all participants provided. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote (page 365): "double‐blind evaluation" Comment: insufficient detail was reported about the method used to blind study participants or personnel from the intervention a participant received |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 365): "double‐blind evaluation" Comment: insufficient detail was reported about the method used to blind the outcome assessor |
| Methods | This was a double‐blind, placebo‐controlled, within‐patient trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: halcinonide solution 0.1%, 3 times daily for 2 weeks (N = 29 participants) B: placebo‐vehicle, 3 times daily for 2 weeks (N = 29 participants) This was a split‐face comparison | |
| Outcomes |
Definition: Overall evaluation of therapeutic response: rapidity and completeness of the therapeutic response rated as excellent (matched with IGA = responder), good, fair or poor Comparative evaluation: comparison of preparation judged as 'markedly superior' if the difference was easily discernible and 'slightly superior' if the difference was only barely discernible Visits: baseline, week 1 and 2 | |
| Notes | This was a split‐face comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 572): "The designation of right or left treatment site was predetermined for the respective drugs by a randomised assignment schedule." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 572): "Results in two other patients were excluded from the evaluation because each had received, and responded well to, other topical steroids within a week prior to entering the present study. [...] therapeutic benefits [...] may possibly have biased the results of therapy with halcinonide." Comment: reason for missing outcome not related to true outcome. In addition, the number of excluded participants was considered as too small (< 10%) to have a significant impact on outcomes. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | High risk | Quote (page 572): "patients were instructed to apply the formulation [...] on one side of the scalp and then, after thoroughly cleansing the hands, apply the solution designated for the contralateral lesions [...] three times a day" Comment: the treatment procedure appears difficult. The authors did not state how they ensured that the patient applied the medication correctly. This may have introduced bias. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 572): "Treatment was administered in a double‐blind fashion, [...] supplied in identical bottles labeled only with the patient number and the side of the body to which the contents were to be applied." Comment: this blinding method was probably sufficient. In addition, this trial was vehicle‐controlled. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 572): "Treatment was administered in a double‐blind fashion, [...] supplied in identical bottles labeled only with the patient number and the side of the body to which the contents were to be applied." Comment: this blinding method was probably sufficient. In addition, this trial was vehicle‐controlled. |
| Methods | This was a 52‐week, international, prospective, randomised, double‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
These are baseline data for the safety analysis set, A: 419, B: 431 | |
| Interventions | A: calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g gel, once daily for 52 weeks (N = 429 participants) B: calcipotriol 50 µg/mg (same vehicle), once daily for 52 weeks (N = 440 participants) If clearance occurred during study, patients were allowed to stop treatment, but remained in the study. If recurrence occurred, they restarted the same treatment. | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: IGA (6‐point scale): severity of disease rated as 'absent', 'very mild', 'mild', 'moderate', 'severe', or 'very severe'; 'absent' to 'mild' = 'satisfactory' Patient ratings of treatment efficacy: rated as 'satisfactory' or 'not satisfactory' Compliance: non‐compliance was defined as failure to use the treatment for any reason other than no treatment being required Visits: baseline, every 4 weeks until week 52 (14 visits) | |
| Notes | The study was sponsored by LEO Pharma, Ballerup, Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 322): "patients were randomized in a 1:1 ratio" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 323): "Safety analyses comprised all randomized patients who received any trial medication and for whom information was available. [...] Efficacy analyses were performed on the full analysis set, which included all randomized patients." Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 322): "double‐blind" Quote (page 323): "same vehicle" Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 322): "double‐blind" Quote (page 323): "same vehicle" Comment: insufficient detail reported about method used to ensure blinding of outcome assessor |
| Methods | This was a multicentre, randomised, double‐blind, vehicle‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics This was not stated | |
| Interventions | A: betamethasone valerate 0.1% lotion, for 2 weeks (N = 117 participants) B: placebo vehicle, for 2 weeks (N = 102 participants) Frequency of application per day unknown | |
| Outcomes |
Definition: Investigator's evaluation of efficacy: treatment results rated as 'excellent' = complete clinical control of the condition (75% or more), 'good' = moderate control (50% to 75%), 'fair' = partial control (less than 50%), 'poor' = no effect or 'exacerbation' Outcomes 4 to 8: evaluated by investigator for treatment differences Visits: baseline, day 3, 7 and 14 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 503): "Each patient was assigned a sequential admission number corresponding to a treatment unit outlined on the accompanying randomization schedule." Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Data for all randomised patients reported |
| Selective reporting (reporting bias) | High risk | Data for the following pre‐specified outcomes insufficiently reported: patient's evaluation of efficacy, physician evaluation as compared to his standard therapy, physician evaluation of efficacy at each visit, physician's evaluation of excoriation, inflammation, scaling, crusting, pruritus |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 503): "double‐blind technique" Comment: this was a vehicle‐controlled trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 503): "double‐blind technique" Comment: insufficient detail reported about method used to ensure blinding of outcome assessor |
| Methods | This was a multicentre, randomised, single‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: ung cocois ointment (12% coal tar, 4% precipitated sulphur and 2% salicylic acid in a coconut oil base), overnight for 2 weeks (N = 18 participants) B: coal tar shampoo, once daily for 2 weeks (N = 16 participants) Participants whose TSS had fallen by less than 50% by week 2 were crossed over to the alternative treatment and re‐assessed at week 4 | |
| Outcomes |
Definition: "Total score"/TSS (0 to 9): sum of sign scores (0 to 3) of erythema, scaling and thickness Outcomes 4 to 6 (0 to 3): each rated as 0 = none, 1 = mild, 2 = moderate, 3 = severe Treatment failure: participants whose TSS had fallen by less than 50% by week 2 Visits: baseline, week 2 and 4 | |
| Notes | 'Total score' of the trial was interpreted as TSS by the review author This study was sponsored by Bioglan Laboratories Ltd, Hitchin, UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 159): "patients were randomly allocated to treatment" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 160): "Two patients in the Cocois group stopped treatment" Comment: insufficient information at which time point participants stopped treatment and if the authors included all data in the outcome assessment. However, number of drop‐outs considered as too small to have a relevant impact on outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 160): "Investigator global assessments at 2 weeks indicated an improvement of 73% in the Cocois group and 42% in the Polytar group. Patient global assessments indicated a 73% improvement in the Cocois group and 33% in the Polytar group." Comment: detailed data for IGA and PGA insufficiently reported |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 161): "the highly recognizable physical properties of Cocois made it impossible to 'blind' the patients" Comment: no blinding of participants performed |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 160): "The doctor assessing the patient was 'blind' to the treatment allocated, and detailed instructions were given to the patient by the hospital pharmacist." Comment: blinding probably sufficient |
| Methods | This was a multicentre, randomised, active‐controlled, investigator‐blinded, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g gel, once daily for 4 weeks (N = 120 participants) B: calcipotriol 50 µg/ml solution, twice daily for 4 weeks (N = 124 participants) | |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: IGA (6‐point scale): disease severity rated as 'clear', 'minimal', 'mild', 'moderate', 'severe' and 'very severe' IGA 'controlled disease': patients rated as 'clear' or 'minimal' PGA: scalp psoriasis rated on a 5‐point scale by the patient as 'clear', 'very mild', 'mild', 'moderate', 'severe'. This assessment was made prior to the investigator's assessments. PGA 'controlled disease': 'clear' or 'very mild' according to Patient's Global Assessment of Disease Severity Patients with success: according to total sign score (TSS ≤ 1). TSS: redness, thickness and scaliness, each rated by the investigator on a 5‐point scale ranging from 0 to 4 (0 = best; 4 = worst). The sum of the 3 individual scores ranged from 0 to 12 (0 = best; 12 = worst). Percentage of patients with success for each clinical sign: TSS ≤ 1 for each sign Patient's itching score: no score definition provided Quality of life: no definition provided Visits: week 2 and 4 | |
| Notes | This trial was sponsored by LEO Pharma Data and all information were extracted from clinicaltrials.gov (NCT01195831, http://www.clinicaltrials.gov/ct2/show/NCT01195831) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (study results): "Allocation: Randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (results, serious and other adverse events): "The number of participants at risk is based on the number of participants included in the safety analysis set, i.e. all randomised patients who had at least one dose of medication, and had at least one post‐baseline safety assessment (118 out of 120 patients in Xamiol® gel group and all (124) patients in Calcipotriol scalp solution group)." Comment: safety outcome data sufficiently addressed and efficacy outcomes for all randomised participants provided even though it remained unclear whether LOCF was performed |
| Selective reporting (reporting bias) | High risk | Results for the prespecified outcomes of quality of life and patient's itching score were not addressed |
| Other bias | Unclear risk | Comparability between study groups limited, since no data on baseline severity provided |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "investigator‐blind" Comment: no blinding of participants. However, this may only affect subjective outcomes (e.g. PGA, itching) |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "investigator‐blind" Comment: vehicles were not identical (gel versus solution). Method on how blinding was performed remained unclear. |
| Methods | This was a double‐blind, randomised, vehicle‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% solution, twice daily for 2 weeks (N = 188 participants) B: placebo vehicle, twice daily for 2 weeks (N = 189 participants) Participants whose condition cleared after 1 week discontinued treatment at that time | |
| Outcomes |
Definition: IGA: rated on 6‐point scale as "clear" = 100% cleared or residual discolouration only, "excellent" = 75% to 99% improvement, "good" = 50% to 74% improvement, "fair" = 25% to 49% improvement, "poor" = < 25% improvement, or "worse" "Total score"/TSS (0 to 9): sum of sign scores (0 to 3) of erythema, scaling and thickness Outcomes 3 to 6 (0 to 3) with 0.5 increments: each rated as 0 = none, 1 = mild, 2 = moderate, 3 = severe Patients evaluation of treatment response: rated as "excellent" (matched with PGA = responder), "good", "fair" or "poor" Visits: baseline, day 4, 8, 14 and 21 (follow‐up) | |
| Notes | "Total score" of the trial was interpreted as TSS by the review author This study was supported in part by a grant from Glaxo, Inc., Research Triangle Park, North Carolina | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 444): "Patients were randomly assigned" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 444): "A full course of therapy was completed by 350 patients [...] all returned for the posttreatment visit." Comment: no ITT analysis performed. According to the graph for efficacy outcome at week 2 all evaluated patients were assessed and data reported. This is not consistent with the cited text. However, attrition considered as too small (< 10%) to have a relevant impact on outcome. |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 444): "double‐blind, vehicle‐controlled" Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 444): "double‐blind, vehicle‐controlled" Comment: insufficient reporting about how blinding of assessor was ensured throughout the study |
| Methods | This was a randomised, double‐blind, vehicle‐controlled, multicentre trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: fluocinolone acetonide 0.01% oil formulation, once daily for 3 weeks (N = 43 participants) B: placebo‐vehicle, once daily for 3 weeks (N = 46 participants) After application, the participants covered their scalp with a shower cap either overnight, or for a minimum of 4 hours | |
| Outcomes |
Definition: Physician global evaluations: improvement from baseline rated as 1 = cleared, 100% clearance of signs/symptoms monitored, residual discolouration excluded, 2 = excellent, 75% but less than 100% improvement of signs/symptoms, 3 = good, 50% but less than 75% improvement of signs/symptoms, 4 = moderate, 25% but less than 50% improvement of signs/symptoms, 5 = slight, less than 25% improvement of signs/symptoms, 6 = no change, no detectable improvement, 7 = exacerbation, flare‐up of study site Physician global assessment: rated as 'good or better', 'moderate', 'fair or less' 'Total score'/TSS (0 to 9): sum of sign scores (0 to 3) of erythema, scaling and thickness Outcomes 3 to 6 (0 to 3): each rated as 0 = none, 1 = slight or mild, 2 = moderate or average, easily discernable, 3 = severe or extensive, markedly evident Visits: baseline, day 11, 21 and 28 (follow‐up) | |
| Notes | 'Total score' of the trial was interpreted as TSS by the review author No sponsorship reported, but one author works for Hill Dermaceuticals, Inc., Sanford, FL 32773, USA Patients could deviate from the treatment plan without being eliminated provided that discontinuation/deviation from the study medication was not on more than 2 consecutive days, nor for more than a total of 4 days out of 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 361): "Patients were randomized to treatment" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | No ITT analysis performed, but attrition considered as too small (< 10%) to have a relevant impact on outcome |
| Selective reporting (reporting bias) | High risk | The reported outcome "Patient Global Assessment (PGA)" was not pre‐specified in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 360): "double‐blind, vehicle‐controlled" Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 360): "double‐blind, vehicle‐controlled" Comment: insufficient detail was reported about the method used to ensure blinding of outcome assessor |
| Methods | This was an open‐labelled, randomised, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial This was not stated Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: shampoo including urea, salicylic acid, glycolic acid, ichthyol pale and polidocanol, once every second day for 8 weeks (N = 27 participants) B: coal tar shampoo, once every second day for 8 weeks (N = 10 participants) | |
| Outcomes |
Definition: Overall efficacy rated by doctor/patient: rated on a 4‐point scale (0 = poor, 1 = moderate, 2 = good and 3 = excellent) Tolerability: rated on a 4‐point scale (0 = poor, 1 = moderate, 2 = good and 3 = excellent) Cosmetic properties: smell, ease of combing wet hair, ease of combing dry hair, softness of dry hair, shine of dry hair, static electricity and overall cosmetic effect rated on a 4‐point scale (0 = poor, 1 = moderate, 2 = good and 3 = excellent) Clinical signs: including erythema, desquamation, infiltration, pruritus and stinging sensation rated on a visual analogue scale from 0 to 10 Visits: baseline, week 4 and 8 | |
| Notes | The study was only available as a conference abstract This study was sponsored by Isdin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: the abstract did not provide sufficient detail about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated in this conference abstract |
| Incomplete outcome data (attrition bias) | Unclear risk | The abstract does not provide sufficient information whether the findings are based on the ITT population or if any drop‐outs occurred |
| Selective reporting (reporting bias) | Unclear risk | This was a conference abstract. Evaluation therefore limited. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label trial |
| Blinding of outcome assessment (detection bias) | High risk | This was an open‐label trial |
| Methods | This was a multicentre, randomised, investigator‐blind, 3‐arm, active‐ and vehicle‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% shampoo, for 4 weeks (N = 63 participants) B: clobetasol propionate 0.05% gel, for 4 weeks (N = 61 participants) C: placebo vehicle shampoo, for 4 weeks (N = 20 participants) Frequency of application per day not reported | |
| Outcomes |
Definition: TSS (0 to 9): sum of sign scores (0 to 3) of erythema, scaling and thickness GSS (0 to 5): 0 = none to 5 = severe Visits: baseline and week 4 | |
| Notes | This trial was presented as a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 283): "144 subjects were randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 283): "The results in the intent‐to‐treat population (all randomized subjects) were similar." Comment: data for the ITT population not reported. Insufficient reporting of attrition or exclusions, but attrition considered as too small (< 10%) to have a relevant impact on outcome. |
| Selective reporting (reporting bias) | High risk | Data for the following pre‐specified outcomes were not reported:
|
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 283): "investigator‐blind" Comment: subjective outcomes (subject's global assessment) likely to be biased. These data are not reported and the outcome provided (TSS) is not considered to be influenced by lack of blinding of the participants. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 283): "investigator‐blind" Comment: insufficient detail was reported about the method used to blind the outcome assessor |
| Methods | This was a multicentre, randomised, investigator‐masked, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% shampoo, once daily for 4 weeks (N = 76 participants) B: calcipotriol 0.005% solution, twice daily for 4 weeks (N = 75 participants) | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: TSS (0 to 9): sum of sign scores of erythema, scaling/desquamation and thickness Erythema, scaling/desquamation, pruritus and thickness score (0 to 3): each rated as 0 = none, 1 = mild, 2 = moderate, 3 = severe GSS (0 to 5): 0 = none to 5 = very severe Scalp surface area: expressed percentage of total scalp surface area IGA/PGA: scale from ‐1 = worse to 5 = cleared Skin atrophy and burning sensation on the scalp/neck/face: rated on scale from 0 = none to 3 = severe Visits: baseline, week 2 and 4 | |
| Notes | This study was funded by Galderma R&D, Sophia Antipolis, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 32): "Subjects were randomized, using a computer‐generated randomization list" Comment: method of randomisation probably appropriate |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 33): "The superiority analysis was done, using the ITT population [...] (LOCF)." Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 33): "The joint primary outcome measures of GSS and TSS improved in both groups during the study [...] The percentage of scalp surface area affected also showed a significant difference in favour of clobetasol propionate (P = 0.02)." Data for GSS and change in percentage of scalp area affected insufficiently reported |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 32): "Because the appearance of the two treatments was very different, masking of the treatments' identity to the subjects was not possible." Comment: no blinding of participants done |
| Blinding of outcome assessment (detection bias) | Low risk | Quote (page 32): "Blinding to investigators was maintained by using independent study personnel to dispense medication and collect returned medication. Subjects were advised not to discuss the study medication with the investigator." Comment: blinding of outcome assessor probably sufficient |
| Methods | This was a multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: tacalcitol emulsion (4 μg/g), once daily for 8 weeks (N of participants unclear) B: placebo, once daily for 8 weeks (N of participants unclear) N = 273 participants | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: TSS (0 to 12): sum of sign scores of erythema, scaling and thickness Erythema, scaling, pruritus and thickness score (0 to 4): each rated as 0 = none to 4 = very severe Participants assessed degree of scaling and pruritus: according to the sign score Blood work and urine samples: to assess calcium homeostasis (serum and urine calcium, parathormone, calcitonin, urine creatinine, urine phosphate, calcium/creatinine ratio) Visits: baseline, week 1, 2, 4, 6 and 8 | |
| Notes | This study was sponsored by Hermal/BHI, Reinbek, Germany The product name of the tacalcitol emulsion was Curatoderm® | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 165): "randomised" Comment: insufficient information on how sequence allocation was performed |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote (page 166): "237 patients were treated" Comment: unclear how many participants were randomised. According to the legends of figures 2‐5: results for ITT population provided. |
| Selective reporting (reporting bias) | High risk | Quote (page 166): "21.1 % of the patients of the tacalcitol group achieved complete clearance compared to 4.5% treated with placebo." Quote (page 168): "At the end of treatment patients were asked for an overall evaluation of the study medication. Most participants rated the application of emulsion as very good and good. Furthermore, the ease of use of the emulsion was assessed." Comment: these outcomes were not pre‐specified in the methods section. Not clear if 'emulsion' included the placebo as well. Quote (page 169): "Withdrawal rate were similar between both groups: 1.5% in the tacalcitol group versus 2.9 in the vehicle group. [...] None of the participants withdrew because of safety concerns of the application" Comment: reasons for withdrawal not provided. It is therefore not evaluable if participants withdrew due to adverse events that the investigators might not have rated as drug‐related. |
| Other bias | Unclear risk | Quote (page 166): "In 39 patients vitamin D metabolites were additionally assessed" Comment: unclear according to which criteria this group was selected. Selection bias possible. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 165): "double‐blind" Comment: vehicle used as control. Participants probably sufficiently blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 165): "double‐blind" Comment: insufficient information on how blinding of investigators was performed and maintained |
| Methods | This was a randomised, double‐blind, single‐centre, parallel‐group, vehicle‐controlled trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: ciclopirox olamine 1.5% shampoo, 3 times per week for 4 weeks (N = 29 participants) B: placebo‐vehicle, 3 times per week for 4 weeks (N = 11 participants) | |
| Outcomes |
Definition: Overall extent of scalp psoriasis: 0 = normal scalp, 1 = residual scaling without plaque, 2 = < 25% scalp covered by plaque without thick scaling, 3 = < 25% scalp covered by thick plaque, 4 = 25% to 50% scalp covered by plaque without thick scaling, 5 = 25% to 50% scalp covered by thick scaly plaque, 6 = 50% to 75% scalp covered by plaque without thick scaling, 7 = 50% to 75% scalp covered by thick scaly plaque, 8 = > 75%, scalp covered by plaque without thick scaling, 9 = > 75% scalp covered by thick scaly plaque Overall clinical change: 0 = completely cleared, 1 = very much improved, 2 = much improved, 3 = minimally improved, 4 = no change, 5 = minimally worse, 6 = much worse, 7 = very much worse Scaling: rated on a scale from 0 to 0.5 = none, 1 to 1.5 = slight, 2 to 2.5 = moderate, 3 to 3.5 = severe, 4 to 4.5 = very severe Patient's self assessment: following questions were rated on a scale from 0 = none, 1 = slight, 2 = moderate, 3 =severe, to 4 = very severe: "Which best describes yours scalp psoriasis today?" and "Which best describes your itching today?" Patient's overall opinion: following question was rated on a scale from 1 = poor, 2 = fair, 3 = good, 4 = very good, to 5 = excellent: "Overall, how do you rate the treatment you've been using over the last four weeks with respect to your scalp psoriasis?" Assessment of acceptability: following questions were rated on a scale from 0 = very dry, 1 = dry, 2 = normal, 3 = greasy, to 4 = very greasy: "Which best describes the dryness/greasiness of your hair today? Visits: baseline, days 8, 15 and 29 | |
| Notes | This study was sponsored by Stiefel Laboratories and conducted by Globecrown International Ltd (Maldon, UK) Quote (page 166): "The lower‐than‐planned recruitment resulted in a reduction in the analytical power of the study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 164): "according to a predetermined randomization schedule [...] The randomization was prepared in blocks of ten with seven patients per block to receive ciclopirox olamine shampoo and three per block to receive matching placebo." Comment: sequence generation probably adequate |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 165): "Three patients failed to complete the study (one on ciclopirox olamine was withdrawn after using a proscribed medication; two on placebo withdrew owing to severe pruritus in one and scalp irritation and increased scaling in the other)" Comment: data about the ITT population not reported, but attrition (3/40) considered as too small (< 10%) to have a relevant impact on outcome |
| Selective reporting (reporting bias) | Unclear risk | Data for "patient's overall opinion of treatment" insufficiently reported |
| Other bias | Unclear risk | Inconsistent wording of definition of "clinical assessment of scalp psoriasis" and "extent of scalp psoriasis" |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 163‐4): "double‐blind [...] matching placebo shampoo base" Comment: vehicle‐controlled, blinding of participants and personnel probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 163‐4): "double‐blind [...] matching placebo shampoo base" Comment: insufficient reporting about how blinding of investigator was ensured throughout the study |
| Methods | This was a multicentre, randomised, double‐blind, vehicle‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: clobetasol propionate 0.05% spray, twice daily for 4 weeks (N = 41 participants) B: placebo‐vehicle, twice daily for 4 weeks (N = 40 participants) Participants with a GSS = 0 at week 2 completed the study at that time | |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: GSS (0 to 5): 0 = clear, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe TSS (0 to 12): sum of erythema, thickness, scaling scores Outcomes 4 to 6 (0 to 4): each point of 5‐point scale exact defined for each outcome, that can be translated as: 0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = very severe Extend of scalp surface involvement: 0 = none, 1 = < 20%, 2 = 20% to 39%, 3 = 40% to 59%, 4 = 60% to 79% and 5 = 80% to 100% Pruritus score (0 to 3): 0 = no itching, 1 = slight itching, 2 = itching as somewhat bothersome, without loss of sleep, 3 = intense itching, night rest interrupted Local tolerability: skin atrophy, teleangiectasia, stinging/burning rated on a scale (0 to 3), 0 = none and folliculitis, assessed as absent or present QOL: 'Scalpdex score' used as dermatitis specific instrument with symptom, function and emotion subscales containing 23 questions with the individual question score ranging between 0 (never) and 100 (all the time) Subject satisfaction: questionnaire at the end of treatment Compliance: participants considered as compliant if they used at least 80% and not more as 120% of expected applications based on self reporting AE: in particular absence or presence of Cushing syndrome based on clinical judgement Visits: baseline, week 2 and 4 | |
| Notes | Scalpdex data provided by Cook Bolden 2012, poster abstract This study was supported by Galderma Laboratories, L.P., Fort Worth, Texas | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 886): "subjects were randomly assigned" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Low risk | Quote (page 886): "Study product [...] was assembled into treatment kits based on the randomisation scheme and contained two bottles of identical product [...] and were numbered sequentially" Comment: probably sufficient |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 887): "safety population included all subjects randomized with documented use of at least one application of study medication [...] missing data for the intend‐to‐treat population were handled using the last observation carried forward method" Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 886): "Packaging for the active treatment and vehicle spray was similar in appearance" Comment: this was a vehicle‐controlled trial. Blinding probably sufficient. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 886): "Packaging for the active treatment and vehicle spray was similar in appearance" Comment: insufficient reporting about how blinding of investigator was ensured throughout the study |
| Methods | This was a multicentre, randomised, double‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics This was not stated | |
| Interventions | A: mometasone furoate lotion 0.1%, once daily for 3 weeks (N = 103 participants) B: triamcinolone acetonide lotion 0.1%, twice daily for 3 weeks (N = 99 participants) | |
| Outcomes |
Definition: TSS (0 to 12): sum of erythema, thickness, scaling and pruritus scores Outcomes 2 to 5 (0 to 3): rated on a 4‐point scale 0 = none, 1 = mild, 2 = moderate, 3 = severe Global evaluation: scale including marked improvement and complete clearing Patient's evaluation of efficacy: scale including excellent and good Visits: baseline, day 8, 15 and 22 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 680): "randomly assigned" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Efficacy analysis (marked improvement or clearing) only reported for 99/103 (mometasone group) and 93/99 (triamcinolone group). Insufficient reporting of attrition or exclusions. No ITT analysis performed. Comment: attrition (< 10%) not considered as sufficient to have significant impact on outcomes |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcome of local safety evaluation (skin atrophy) not reported, but this outcome is not considered as relevant for this review |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote page 680): "third‐party blind" Comment: this was a opd versus bid comparison. Thus, blinding of participants and personnel probably not done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote page 680): "third‐party blind" Comment: unclear if outcome assessors were adequately blinded throughout the study |
| Methods | This was a multicentre, randomised, double‐blind, vehicle‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriene 50 µg/g plus betamethasone dipropionate 0.5 mg/g, once daily for 8 weeks (N = 135 participants) B: placebo vehicle, once daily for 8 weeks (N = 42 participants) Assignment in a 3:1 ratio Treatment was stopped if clearance occurred and restarted when scalp psoriasis relapsed | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: IGA: disease severity rated as 'clear', 'minimal', 'mild', 'moderate', 'severe' and 'very severe disease' Patient's global assessment of severity: rated as 'clear', 'very mild', 'mild', 'moderate', 'severe' Erythema, thickness, scaling score: rated from 0 = none to 4 = very severe TSS (0‐12): sum of erythema, thickness, scaling scores Compliance: returned medication was weighted Visits: baseline, weeks 2, 4, 6 and 8 | |
| Notes | This study was sponsored by LEO Pharma A/S | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1329): "Randomization was pre‐planned according to a computer‐generated randomization schedule, and was stratified by ethnicity" Comment: probably appropriate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 1330): "All randomized patients were included in the full analysis set on which all efficacy analyses were performed, unless indicated otherwise [...] A total of 27 (15.3%) randomized patients withdrew during the double‐blind phase, 19 (14.1%) in the two‐compound group and 8 (19.0%) in the vehicle group." Comment: for efficacy analysis, data for the ITT population was reported, but not the imputation method. Quote (page 1330): "Of the randomized patients, seven in the two‐compound group and four in the vehicle group provided no data on the presence or absence of adverse events, and so were excluded from the safety analysis set." Comment: reason to exclude these patients from safety set appears reasonable |
| Selective reporting (reporting bias) | Low risk | All reported results are pre‐specified outcomes in the methods section |
| Other bias | Unclear risk | Baseline IGA (Table 3) of both groups not well matched. IGA = moderate: 81.5% (2‐compound) versus 76.2% (vehicle): IGA = severe: 18.5% versus 23.8% Comment: unclear if baseline imbalance may have introduced bias |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 1329): "The packaging and labelling of the two‐compound scalp formulation and its vehicle were identical, so it was not possible to distinguish between them by examination." Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 1329): "The packaging and labelling of the two‐compound scalp formulation and its vehicle were identical, so it was not possible to distinguish between them by examination." Comment: unclear if outcome assessors were adequately blinded throughout the study |
| Methods | This was a multicentre, randomised, open‐labelled, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol (50 µg/g 30 to 50 ml/week) solution, for 4 weeks (N = 41 participants) B: dithranol/tar regimen, for 4 weeks (N = 47 participants)
| |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: Investigator's/patient's assessment of overall response: rated as 1 = worse, 2 = unchanged, 3 = slight improvement, 4 = moderate improvement, 5 = marked improvement and 6 = clearance Erythema, thickness, scaling score: rated as 0 = complete lack of cutaneous involvement, 1 = slight, 2 = moderate, 3 = severe and 4 = severest possible involvement TSS (0‐12): sum of erythema, thickness, scaling scores Extend: assessed by investigator 0 = no involvement, 1 = < 10%, 2 = 10% to 29%, 3 = 30% to 49%, 4 = 50% to 69%, 5 = 70% to 89% and 6 = 90% to 100% involvement 24‐hour urinary excretion: expressed as calcium/creatinine ratio Blood/urine analysis: assessment of calcium metabolic parameters (e.g. serum calcium), indices of bone turnover (osteocalcin, alkaline phosphatase and 1‐collagen telopeptide (1‐CTP)) Visits: baseline, weeks 1, 2, 4 and 1 week post‐treatment (follow‐up) | |
| Notes | This study was sponsored by Leo Pharmaceutical Products Quote (page 216): "At most centres, a short‐contact dithranol regimen (concentrations varying from 0.125 to 8%) was applied in combination with or without a tar preparation (concentrations varying from 1 to 25%)." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 216): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 217): "Except for 1 patient (randomised to dithranol/tar) who left the study after randomisation but before treatment was initiated, all randomised patients were included in the analyses of efficacy and safety" Comment: no ITT analysis performed, but drop‐out not considered to introduce bias |
| Selective reporting (reporting bias) | High risk | Outcome data at 1 week of follow‐up not reported |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 216): "Blinding of the study was not considered possible due to the staining properties of tar and dithranol." Comment: no blinding of participants or personnel was done |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page 216): "Blinding of the study was not considered possible due to the staining properties of tar and dithranol." Comment: no blinding of outcome assessors was done |
| Methods | This was a multicentre, prospective, randomised, double‐blind, active‐controlled, 3‐arm, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g gel, once daily for 8 weeks (N = 568 participants) B: betamethasone dipropionate 0.5 mg/g (same vehicle as A), once daily for 8 weeks (N = 563 participants) C: calcipotriol 50 µg/g (same vehicle as A), once daily for 8 weeks (N = 286 participants) Patients with "absence of disease" according to the IGA at weeks 1 to 8 could stop treatment with study medication | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: IGA (6‐point scale): rated as 'absence of disease', 'very mild disease', 'mild disease', 'moderate disease', 'severe disease', 'very severe disease' PGA (7‐point scale): rated as 'worse', 'unchanged', 'slight improvement', 'moderate improvement', 'marked improvement', 'almost clear', 'clear' Erythema, thickness, scaling score: rated as 0 = complete lack of cutaneous involvement, 1 = slight, 2 = moderate, 3 = severe and 4 = severest possible involvement TSS (0‐12): sum of erythema, thickness, scaling scores Compliance: self reported and weighting of returned medication Blood analysis: assessment of serum calcium and albumin Visits: baseline, weeks 1, 2, 4, 6 and 8 | |
| Notes | This study was sponsored by LEO Pharma A S, Ballerup, Denmark NCT00216840 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 171): "randomized according to a computer‐generated schedule" Comment: sequence generation considered as probably adequate. |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 172): "All randomized patients were included in the full analysis set [...] for efficacy parameters, and all patients who had received any trial medication and from whom the presence or confirmed absence of adverse events was available were included in the safety analysis set [...] using last observation carried forward (LOCF)" Comment: incomplete outcome data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 170): "double‐blind [...] same vehicle" Comment: blinding of participants and personnel probably sufficient |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 170): "double‐blind [...] same vehicle" Comment: insufficient information about how blinding of outcome assessor was ensured throughout the study |
| Methods | This was a multicentre, randomised, third‐party‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: mometasone furoate 0.1% lotion, once daily for 3 weeks (N = 101 participants analysed) B: betamethasone valerate 0.1% lotion, twice daily for 3 weeks (N = 102 participants analysed) | |
| Outcomes |
Definition: Target area evaluation: based on 1) changes in individual disease sign/symptom scores erythema, scaling, induration and pruritus rated on a scale from 0 to 3; 2) percent improvement in total sign/symptom score Outcomes 2 to 5 (0 to 3): rated on a 4‐point scale 0 = none, 1 = mild, 2 = moderate, 3 = severe Global evaluation of overall change: rated as 'clear' = 100% clearance of disease signs/symptoms, 'marked' = 75% to < 100% improvement, 'moderate' = 50% to < 75% improvement, 'slight' = < 50% improvement, 'no change' = no detectable improvement, or 'exacerbation' = flare of signs/symptoms Visits: baseline, day 8, 15 and 22 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 146): "randomized" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 146): "study conducted in 207 patients [...] In order to compensate for any bias that may have been caused by the loss of patients due to missed visits, all analyses were also performed using the last valid visit (i.e., the Endpoint of treatment) for each patient, irrespective of visit timing." Comment: last observation carried forward (LOCF) method used, but only for 203 participants (ITT = 207) data reported, but amount of missing data considered as too small to introduce bias |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 146): "third‐party‐blind" Comment: no blinding of participants or personnel was done. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 146): "third‐party‐blind" Comment: insufficient information on how blinding of outcome assessor was ensured throughout the study |
| Methods | This was an 8‐week, multicentre, open‐label, randomised, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial This was not stated Washout period This was not stated Baseline characteristics
| |
| Interventions | A: calcipotriol 50 µg/ml solution bid and coal tar twice weekly, for 8 weeks (N = 236 participants) B: calcipotriol 50 µg/ml solution bid plus non‐medicated shampoo twice weekly, for 8 weeks (N = 225 participants) | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: TSS (0 to 12): sum of erythema, thickness and scaling scores Erythema, thickness and scaling scores (0 to 4): each rated as 0 = absent to 4 = severest possible Visits: baseline and week 8 (reported) | |
| Notes | This study was sponsored by Leo Pharmaceutical This study was published as a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page S337): "randomised" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition or exclusions |
| Selective reporting (reporting bias) | High risk | Quote (page S337): "There were no statistically significant differences in investigator assessments of time to achieve treatment success or extent of scalp psoriasis" Comment: no data reported. This abstract does not pre‐specify any outcomes. |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page S337): "open‐label" Comment: no blinding performed |
| Blinding of outcome assessment (detection bias) | High risk | Quote (page S337): "open‐label" Comment: no blinding performed |
| Methods | This was a multicentre, randomised, active‐controlled, phase 2 trial | |
| Participants | Unknown | |
| Interventions | A: mometasone emulsion (LAS41002), for 3 weeks B: mometasone solution, for 3 weeks N = 70 participants | |
| Outcomes | Primary outcome of the trial
Secondary outcome of the trial
Definition: TSS: compound evaluation of erythema, thickness and scaliness Visits: baseline and week 3 | |
| Notes | Full‐text of this study was not available. Information was retrieved from a power‐point presentation and an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised " Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Only abstract and power‐point presentation available, insufficient details |
| Incomplete outcome data (attrition bias) | Unclear risk | Only abstract and power‐point presentation available, insufficient details |
| Selective reporting (reporting bias) | Unclear risk | Only abstract and power‐point presentation available, insufficient details |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "observer‐blind" Comment: participants were not blinded. Patient‐assessed outcomes possibly biased. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "observer‐blind" Comment: insufficient information about how blinding of outcome assessors was ensured throughout the study |
| Methods | This was a multicentre, double‐blind, randomised, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: desoximetasone 0.05% gel, twice daily for 2 weeks (N = 62 participants) B: fluocinonide 0.05% gel, twice daily for 2 weeks (N = 61 participants) | |
| Outcomes |
Definition: Outcomes 1 to 4 (0 to 3): rated on a 5‐point scale 1 = none, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe Investigator's overall evaluation: rated as 1 = 'excellent' (76% to 100% improvement), 2 = 'good' (51% to 75% improvement), 3 = 'fair' (26% to 50% improvement), 4 = 'poor' (≤ 25% improvement), or 5 = 'exacerbation' AE: severity of burning, stinging and itching were rated by the patient from 1 = none to 4 = severe Mean disease status: 1 = exacerbating rapidly, 2 = exacerbating slowly, 3 = stable Visits: baseline, day 4, 7 and 14 | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 274): "patients were randomly assigned" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote (page 277): "Evaluation scores for patients with missing visits out of range were estimated by an averaging procedure" Quote (page 278, Table II): "Patients with signs/symptom clear at baseline and throughout study are excluded. Two patients did not return past the visit on day 4 (one in each group)" Comment: inconsistency between intended method of imputation and procedure/reported results. Data for 123/125 randomised participants reported. Not clear to which group the missing 2 participants were randomised. |
| Selective reporting (reporting bias) | Low risk | All reported results are pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote (page 276): "double‐blind" Comment: probably the same vehicle used |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 276): "double‐blind" Comment: insufficient reporting about how blinding of outcome assessor was ensured throughout the study |
| Methods | This was a randomised, single‐blind, active‐controlled, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period This was not stated Baseline characteristics
| |
| Interventions | A: dithranol 0.1% in urea base 17%, once daily for 3 weeks (N = 18 participants) B: pomade of coal tar 6% solution plus salicylic acid 2% and Tween 20 1% and emulsifying ointment, once daily for 3 weeks (N = 20 participants) After each application, participants of both groups washed their hair with 17.5% cetrimide shampoo and covered the hair with a perforated shower cap until the next application | |
| Outcomes |
Definition: Clearance: sum of sign scores ≤ 3 Erythema, thickness/scaling, pruritus score: sign scores rated as 0 = none to 3 = severe TSS (0 to 9): sum of erythema, thickness/scaling, pruritus scores Area of involvement: assessed as 0 = none, 1 = 1% to 25%, 2 = 26% to 50%, 3 = 51% to 75% or 4 = 76% to 100% Patient's opinion about acceptability and effectiveness: at the end of treatment patients were asked whether they would be happy to use the treatment at home or not AE: assessment of stinging, burning and staining of the hair (4‐point scale) Visits: baseline, day 4, 7, 11 and 14 | |
| Notes | For this study patients were hospitalised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 375): "allocated at random" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | Quote (page 375): "Forty patients [...] were recruited to the study [...] and allocated at random" Quote (page 376): "Results were available from 38 patients [...] There were insufficient patients left in the study at days 18 and 21 make comparison meaningful." Comment: no ITT analysis performed, but number of drop‐outs per group (< 10%) considered as too small to introduce significant bias |
| Selective reporting (reporting bias) | High risk | Quote (page 376): "There were insufficient patients left in the study at days 18 and 21 make comparison meaningful." Comment: outcomes for the pre‐specified endpoint at week 3 not reported. Results for assessment of area of involvement not reported. |
| Other bias | High risk | Quote (page 376): "Results were available from 38 patients [...] There were insufficient patients left in the study at days 18 and 21 make comparison meaningful." Comment: study stopped earlier than scheduled. Thus, overestimation of effect possible. |
| Blinding of participants and personnel (performance bias) | High risk | Quote (page 375): "single blind comparison" Comment: no blinding of participants or personnel was done |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote (page 375): "single blind comparison" Comment: insufficient reporting of method used to blind the outcome assessor |
| Methods | This was a randomised, active‐controlled, 3‐arm, parallel‐group trial | |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics
| |
| Interventions | A: calcipotriol lotion, twice daily for 4 weeks (N = 15 participants) B: mometasone furoate lotion, once daily for 4 weeks (N = 15 participants) C: calcipotriol lotion (morning) plus mometasone furoate lotion (evening), once daily for 4 weeks (N = 15 participants) | |
| Outcomes |
Definition: Extend (1 to 5): rated on a scale from 1 = < 20%, 2 = 20% to 39%, 3 = 40% to 59%, 4 = 60% to 79%, to 5 = 80% to 100% IGA/ PGA (1 to 5): 1 = worse, 2 = none, 3 = slight improvement, 4 = marked improvement, 5 = clear PSSI: = extend x TSS Percent improvement of PSSI: PSSI (pre‐treatment) ‐ PSSI (post‐treatment)/PSSI (pre‐treatment) x 100 Erythema, thickness, scaling score: rated as 0 = absent, 1 = slight, 2 = moderate, 3 = severe and 4 = severest possible TSS (0 to 12): sum of erythema, thickness, scaling scores Pruritus score: rated as 0 = absent, 1 = slight, 2 = moderate, 3 = severe and 4 = severest possible Blood/urine analysis: blood count, renal/hepatic function, glucose, calcium, phosphate Visits: baseline, week 1 and 4 | |
| Notes | This Turkish article was translated for the review author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 110): "randomized into three groups of equal number" Comment: insufficient detail was reported about the method used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | This was not stated |
| Incomplete outcome data (attrition bias) | Low risk | No ITT analysis mentioned, but results for all randomised participants reported (no attrition or exclusions) |
| Selective reporting (reporting bias) | Low risk | All reported results were pre‐specified outcomes in the methods section |
| Other bias | Low risk | No other source of bias identified |
| Blinding of participants and personnel (performance bias) | High risk | Due to difference in application, no blinding of participants considered as possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | No blinding of outcome assessors reported |
ADR: adverse drug reaction
AE: adverse event
bid: twice daily
BTM: betamethasone
BSA: body surface area
DLQI: Dermatology Life Quality Index
DSS: dermatologic sum score
GSS: global severity score
HPA: hypothalamic‐pituitary‐adrenal
IGA: investigator's global assessment
IGSA: Investigator's Static Global Assessment
ITT: intention‐to‐treat
LOCF: last observation carried forward
LS: lesion score
NSAID: nonsteroidal anti‐inflammatory drug
opd: once per day
PASI: Psoriasis Area and Severity Index
PGA: patient global assessment
PSSI: Psoriasis Scalp Severity Index
PUVA: psoralen and ultraviolet A
QOL: quality of life
SAPASI: self administered PASI
SD: standard deviation
TSS: total sign score
UV: ultraviolet
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Treatment of control group not specified | |
| Study assessed different scalp disorders and did not assess scalp psoriasis separately | |
| Not a RCT | |
| Not a RCT | |
| This study assessed erythematous squamous dermatoses | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Both groups were treated with concomitant PUVA therapy | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Study investigated body psoriasis and did not assess the scalp separately | |
| Not a RCT | |
| Not a RCT | |
| Study investigated body psoriasis and did not assess the scalp separately | |
| Study investigated body psoriasis and did not assess the scalp separately | |
| Not a RCT | |
| Study assesses maintenance treatment. Participants received the same medication during induction therapy. | |
| Not a RCT | |
| No topical treatment | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Systemic concomitant treatment was allowed during the study |
PUVA: psoralen and ultraviolet A
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unknown |
| Participants | Unknown |
| Interventions | A: clobetasol propionate 0.05% shampoo (number of participants unclear) |
| Outcomes | Hypothalamic‐pituitary‐adrenal axis suppression, atrophogenicity and ocular safety |
| Notes | The text of this poster abstract was not available |
| Methods | Single‐centre, randomised, active‐controlled, investigator‐blinded, parallel‐group trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics This was not yet stated |
| Interventions | A: dimethicone formulation, once daily for 2 weeks (number of participants unclear) B: 10% salicylic acid gel, once daily for 2 weeks (number of participants unclear) Total estimated number of participants enrolled: 90 |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: Detailed definition of the outcomes insufficiently addressed Visits: baseline, day 3, 7 and 14 |
| Notes | This trial was identified on clinicaltrials.gov (NCT01914627, http://www.clinicaltrials.gov/ct2/show/NCT01914627 This study was supported by G. Pohl‐Boskamp GmbH & Co. KG |
| Methods | Multicentre, randomised, double‐blinded, vehicle‐controlled, parallel‐group trial |
| Participants | Age between 18 and 80 years Diagnosis of mild to moderate scalp psoriasis |
| Interventions | A: tacalcitol lotion 4 mg/g, once daily B: vehicle N = 250 participants |
| Outcomes | Assessment of efficacy and safety. The abstract did not address the outcomes in detail |
| Notes | Full‐text of this study was not available |
| Methods | Multicentre, randomised, controlled, phase 3 study |
| Participants | Diagnosis of mild to moderate scalp psoriasis |
| Interventions | A: clobetasol propionate 0.05% shampoo B: Polytar Liquid N = 160 participants |
| Outcomes | Assessment of efficacy and safety. The abstract did not address the outcomes in detail. |
| Notes | Full text of this study was not available |
| Methods | International, multicentre, randomised, vehicle‐controlled, phase 3 trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
Baseline characteristics This was not stated |
| Interventions | A: calcipotriol 50 µg/g and betamethasone dipropionate 0.5 mg/g gel (Xamiol®), for 8 weeks B: calcipotriol 50 µg/g gel, for 8 weeks C: betamethasone dipropionate 0.5 mg/g gel, for 8 weeks D: vehicle, for 8 weeks N = 1485 participants |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: Detailed definition of the outcomes insufficiently addressed |
| Notes | Full text of this study was not available Study ID: MBL 0405 INT ClinicalTrials.gov‐ID: NCT00216827 This study is sponsored by LEO Pharma |
| Methods | Unknown |
| Participants | Unknown |
| Interventions | A: clobetasol propionate 0.5% shampoo B: Polytar Liquid |
| Outcomes | Efficacy and safety. The abstract did not address the outcomes in detail. |
| Notes | Full text of this study was not available |
| Methods | Randomised controlled trial |
| Participants | Diagnosis of moderate to severe scalp psoriasis |
| Interventions | A: agent A 0.05% shampoo B: agent B shampoo/liquid N = 8 participants |
| Outcomes | Efficacy and safety. The abstract did not address the outcomes in detail. |
| Notes | Full text of this study was not available |
| Methods | Unknown |
| Participants | Unknown |
| Interventions | A: calcitriol solution B: Capasal (tar preparation) |
| Outcomes | Efficacy. The abstract did not address the outcomes in detail. |
| Notes | Full text of this study was not available |
| Methods | Multicentre, double‐blinded, randomised, vehicle‐controlled, parallel‐group trial |
| Participants | Unknown |
| Interventions | A: Tacalcitol lotion (4 µg/g) B: Vehicle |
| Outcomes | Efficacy and safety. The abstract did not address the outcomes in detail. |
| Notes | Full text of this study was not available |
| Methods | Multicentre, randomised, active‐controlled, double‐blinded, parallel‐group, phase 2 trial |
| Participants | Adult patients (= 18 years) with plaque psoriasis (psoriasis vulgaris) of at least mild severity by Physician's Global Assessment (PGA) |
| Interventions | A: calcipotriene 0.005% plus betamethasone dipropionate 0.064% foam, once daily for up to 4 weeks B: betamethasone dipropionate 0.064% foam, once daily for up to 4 weeks C: calcipotriene 0.005% foam, once daily for up to 4 weeks |
| Outcomes | Treatment success of the involved scalp according to modified Psoriasis Area Severity Index (mPASI) and safety |
| Notes | The information was derived from a conference abstract. It was presented at the 73rd Annual Meeting of the American Academy of Dermatology San Francisco, CA United States. Conference start: 20 March 2015, Conference end: 24 March 2015. The full text of this study was not available. |
| Methods | Multicentre, randomised, active‐controlled, investigator‐blinded, parallel‐group, phase 3 trial |
| Participants | Diagnosis of moderate to severe scalp psoriasis |
| Interventions | A: clobetasol propionate 0.05% shampoo B: Plytar liquid |
| Outcomes | Efficacy (global severity score and total severity score) and safety. The abstract did not address the outcomes in more detail. |
| Notes | Full‐text of this study was not available |
| Methods | Unknown |
| Participants | Unknown |
| Interventions | A: first month: camellia oil‐containing shampoo alone, second month: camellia oil spray plus camellia oil‐containing shampoo (2‐step care) B: first month: camellia oil spray plus camellia oil‐containing shampoo (2‐step care), second month: camellia oil‐containing shampoo alone |
| Outcomes | Scalp PASI, scaling, itching, number of adverse events. The abstract did not address the outcomes in more detail. |
| Notes | Full text of this study was not available. The abstract does not provide sufficient information on study design. |
| Methods | Multicentre, randomised, prospective, double‐blinded, parallel‐group trial |
| Participants | Unknown |
| Interventions | A: calcipotriol cream, twice daily, for 6 weeks B: vehicle, twice daily, for 6 weeks |
| Outcomes | Efficacy and safety. The abstract did not address the outcomes in detail. |
| Notes | Full text of this study was not available |
| Methods | Multicentre, prospective, open‐label, randomised, parallel‐group trial |
| Participants | Unknown |
| Interventions | A: calcipotriol solution, for 8 weeks B: Capasal shampoo, for 8 weeks |
| Outcomes | Assessment of treatment response until week 24 of patients that cleared or responded to calcipotriol after 8 weeks of treatment. The abstract did not address the outcomes in more detail. |
| Notes | Full text of this study was not available |
AE: adverse event
BSA: body surface area
DLQI: Dermatology Life Quality Index
IGA: investigator's global assessment
mPASI: modified Psoriasis Area Severity Index
PASI: Psoriasis Area and Severity Index
PBI: Patient Benefit Index
PGA: Physician Global Assessment
PSSI: Psoriasis Scalp Severity Index
PUVA: psoralen and ultraviolet A
sPGA: Scalp Physician Global Assessment
WHO: World Health Organization
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | 'Double‐blind, randomised, clinical study to compare the efficacy and safety of betamethasone 0.05% salicylic acid 2% vs. Diprosalic solution vs. vehicle for the treatment of psoriasis capitis' |
| Methods | This is a randomised, active and vehicle‐controlled, double‐blinded, 3‐arm trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
|
| Interventions | A: betamethasone dipropionate 0.05% plus salicylic acid 2% solution for 3 weeks (number of participants unclear) B: betamethasone dipropionate plus salicylic acid solution (diprosalic acid) for 3 weeks (number of participants unclear) C: vehicle for 3 weeks (number of participants unclear) Total estimated number of participants enrolled: 225 |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: Sum score (0 to 12): consists of the activity parameters (clinical signs) erythema, desquamation, thickening of the skin and pruritus each rated on a scale from 0 to 3 Visits: baseline, day 7, 14, 21 and 35 |
| Starting date | 31/05/2011 (first enrolment) |
| Contact information | Lil‐Dagover‐Ring 7, 82031 Grünwald, Germany, Telephone: 004908964186121, Email: [email protected] |
| Notes | This study is sponsored by Dermapharm AG http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2010‐024033‐24‐DE |
| Trial name or title | 'Phase II study of a non‐steroidal novel treatment for scalp psoriasis' |
| Methods | This is a randomised, active and vehicle‐controlled, double‐blind, 4‐arm trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
|
| Interventions | A: calcipotriol plus nicotinamide (DermiPsor's DPS‐102), twice daily for 12 weeks (number of participants unclear) B: vehicle, twice daily for 12 weeks (number of participants unclear) C: calcipotriol, twice daily for 12 weeks (number of participants unclear) D: nicotinamide, twice daily for 12 weeks (number of participants unclear) Total estimated number of participants to be enrolled: 160 |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: PGA: investigator (physician) rating scalp psoriasis on a 6‐point scale (clear, minimal, mild, moderate, severe and very severe) Visits: baseline, week 2, 4, 8 and 12 |
| Starting date | April 2013 |
| Contact information | Shay Marcus, VP Business Development & Marketing, DermiPsor Ltd. ClinicalTrials.gov‐ID: NCT01368887 |
| Notes | Recruitment is currently suspended (April 2014) This study is sponsored by DermiPsor, Ltd. |
| Trial name or title | 'A multicenter, randomised, double‐blind, phase 3 study of the safety, efficacy, systemic exposure, and pharmacodynamics of calcipotriene foam, 0.005%, versus vehicle foam in paediatric subjects (ages 2 to 11 years) with plaque psoriasis' |
| Methods | This is a multicentre, randomised, active‐controlled, double‐blind, parallel‐group trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
|
| Interventions | A: calcipotriene 0.005% foam (STF 115469), twice daily for 8 weeks (number of participants unclear) B: vehicle foam, twice daily for 8 weeks (number of participants unclear) Total estimated number of participants to be enrolled: 180 Participants will be randomly assigned in a 2:1 ratio |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Definition: Treatment success: treatment success = ISGA score 0 or 1, and a minimum improvement in the ISGA score of 2 grades from baseline to week 8 Improvement of clinical signs: erythema, scaling and plaque thickness. Target lesion score of 0 or 1 for all 3 signs and at least to 2‐grade improvement from baseline for erythema and scaling. Visits: baseline, week 2 and 8 |
| Starting date | April 2013 |
| Contact information | US GSK Clinical Trials Call Center 877‐379‐3718 ClinicalTrials.gov‐ID: NCT01582932 |
| Notes | This trial may only be eligible for inclusion, if the authors report outcomes for the scalp separately This study is sponsored by GlaxoSmithKline |
| Trial name or title | 'Patient preference of Taclonex ointment to Taclonex scalp suspension in adult subjects with psoriasis vulgaris' |
| Methods | This is a monocentre, open‐label, randomised, investigator‐blinded, cross‐over trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period
|
| Interventions | A: calcipotriene 0.005% and betamethasone dipropionate 0.064% within an ointment (Taclonex®), once daily for 3 days B: calcipotriene 0.005% and betamethasone dipropionate 0.064% within a suspension (Taclonex Scalp®), once daily for 3 days Participants will be randomised to use either the ointment or the scalp suspension for 3 days, then cross over to use the other product for 3 days Estimated enrolment: 20 participants |
| Outcomes | Primary outcome of the trial
Definition: Patient preference: participants will complete a questionnaire about their psoriasis treatment preferences Visits: baseline, day 3 and 6 |
| Starting date | October 2012 |
| Contact information | Steven R. Feldman, Professor of Dermatology, Wake Forest University |
| Notes | Sponsor: Wake Forest University, Collaborator: LEO Pharma Other study ID number: 21361 This study will only be eligible for inclusion if the authors report one of the safety outcomes of this review: the number of withdrawals due to adverse events or the number of patients with at least one adverse event |
| Trial name or title | 'A randomised double‐blind vehicle controlled dose ranging multiple site phase 2 clinical study to evaluate the efficacy and safety of desoximetasone 0.25% shampoo in patients with moderate to severe scalp psoriasis' |
| Methods | This is a randomised, vehicle‐controlled, double‐blind, parallel‐group trial |
| Participants | Inclusion criteria of the trial
Exclusion criteria of the trial
Washout period of the trial
|
| Interventions | A: desoximetasone 0.25% shampoo, once daily for a total of 28 days B: vehicle shampoo, once daily for a total of 28 days Estimated enrolment: 180 participants |
| Outcomes | Primary outcome of the trial
Definition: Clinical success: IGA of 0 or 1 Visits: number of visits not reported |
| Starting date | March 2015 |
| Contact information | Taro Pharmaceuticals USA, Inc. Hawthorne, New York, United States, 10532 Tel.: 914‐345‐9001 Study director: Natalie Yantovskiy |
| Notes | Sponsor: Taro Pharmaceuticals USA, Inc. |
| Trial name or title | 'Long‐term treatment of scalp psoriasis with Xamiol® gel in a large adult Chinese population' |
| Methods | This is a randomised, investigator‐blinded, active‐controlled, parallel‐group trial |
| Participants | Inclusion criteria :
Exclusion criteria :
Washout period
|
| Interventions | A: calcipotriol 50 µg/g plus betamethasone dipropionate 0.5 mg/g gel (Xamiol®), once daily as required for 28 weeks B: calcipotriol 50 µg/g solution (Daivonex®), twice daily as required for 28 weeks Estimated enrolment: 900 participants |
| Outcomes | Primary outcome of the trial
Secondary outcomes of the trial
Visits: number of visits not reported |
| Starting date | September 2015 |
| Contact information | Annette Trotz, BSc, +61 7 3250 1200, annette.trotz@leo‐pharma.com ClinicalTrials.gov‐ID: NCT02533973 |
| Notes | Sponsor: LEO Pharma, Collaborator: Hangzhou Tigermed Consulting Co. Ld Principle Investigator: Min Zheng, MD, PHD |
BSA: body surface area
ISGA: Investigator's Static Global Assessment
IUD: intrauterine device
PGA: patient global assessment
PUVA: psoralen and ultraviolet A
TSS: total sign score
UV: ultraviolet
WHO: World Health Organization
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean score of the IGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Steroid: once versus twice daily, Outcome 1 Mean score of the IGA. | ||||
| 1.1 Betamethasone valerate 0.12% (hydrophilic leave‐on) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean score of the PGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Steroid: once versus twice daily, Outcome 2 Mean score of the PGA. | ||||
| 2.1 Betamethasone valerate 0.12% (hydrophilic leave‐on) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 14.58 [7.28, 29.17] | ||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 Steroid versus the vehicle, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||||||||||||||||||
| 1.1 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 16.80 [2.29, 123.30] | ||||||||||||||||||||||||||||||||||||
| 1.2 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 10.15 [3.83, 26.88] | ||||||||||||||||||||||||||||||||||||
| 1.3 Clobetasol propionate (hydrophilic leave‐on) | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 22.83 [7.31, 71.30] | ||||||||||||||||||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 9 | 2114 | Risk Ratio (M‐H, Random, 95% CI) | 5.24 [3.83, 7.17] | ||||||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 Steroid versus the vehicle, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||||||||||||||||||
| 2.1 Betamethasone dipropionate (hydrophilic leave‐on) | 2 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 4.06 [2.85, 5.79] | ||||||||||||||||||||||||||||||||||||
| 2.2 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 5.19 [2.60, 10.36] | ||||||||||||||||||||||||||||||||||||
| 2.3 Betamethasone valerate 0.1% (hydrophilic leave‐on) | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [1.81, 4.85] | ||||||||||||||||||||||||||||||||||||
| 2.4 Clobetasol propionate (hydrophilic leave‐on) | 3 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 7.93 [5.46, 11.51] | ||||||||||||||||||||||||||||||||||||
| 2.5 Clobetasol propionate (rinse‐off) | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 15.83 [2.23, 112.33] | ||||||||||||||||||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| Analysis 2.3
Comparison 2 Steroid versus the vehicle, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||||||||||||||||||
| 4 Improvement in quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 2.4  Comparison 2 Steroid versus the vehicle, Outcome 4 Improvement in quality of life. | ||||||||||||||||||||||||||||||||||||||||
| 4.1 Scalpdex | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 5 Number of participants withdrawing due to adverse events Show forest plot | 4 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.67] | ||||||||||||||||||||||||||||||||||||
| Analysis 2.5  Comparison 2 Steroid versus the vehicle, Outcome 5 Number of participants withdrawing due to adverse events. | ||||||||||||||||||||||||||||||||||||||||
| 5.1 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] | ||||||||||||||||||||||||||||||||||||
| 5.2 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.06, 15.53] | ||||||||||||||||||||||||||||||||||||
| 5.3 Clobetasol propionate (hydrophilic leave‐on) | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.13] | ||||||||||||||||||||||||||||||||||||
| 6 Number of participants achieving 'response' by PGA Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||
| Analysis 2.6  Comparison 2 Steroid versus the vehicle, Outcome 6 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||||||||||||||||||
| 6.1 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [2.17, 4.26] | ||||||||||||||||||||||||||||||||||||
| 6.2 Betamethasone valerate 0.1% (hydrophilic leave‐on) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 3.52 [1.97, 6.29] | ||||||||||||||||||||||||||||||||||||
| 6.3 Clobetasol propionate (hydrophilic leave‐on) | 2 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 6.92 [4.42, 10.83] | ||||||||||||||||||||||||||||||||||||
| 6.4 Clobetasol propionate (rinse‐off) | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 14.35 [2.02, 102.12] | ||||||||||||||||||||||||||||||||||||
| 7 Mean score of the PGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 2.7 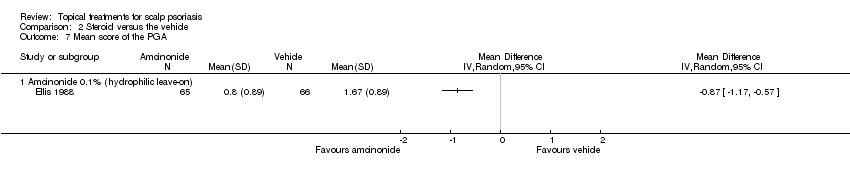 Comparison 2 Steroid versus the vehicle, Outcome 7 Mean score of the PGA. | ||||||||||||||||||||||||||||||||||||||||
| 7.1 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 8 Number of participants with at least one adverse event Show forest plot | 7 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.08] | ||||||||||||||||||||||||||||||||||||
| Analysis 2.8  Comparison 2 Steroid versus the vehicle, Outcome 8 Number of participants with at least one adverse event. | ||||||||||||||||||||||||||||||||||||||||
| 8.1 Betamethasone valerate 0.1% (hydrophilic leave‐on) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.01, 5.86] | ||||||||||||||||||||||||||||||||||||
| 8.2 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.32, 5.99] | ||||||||||||||||||||||||||||||||||||
| 8.3 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.10] | ||||||||||||||||||||||||||||||||||||
| 8.4 Fluocinolone acetonide 0.01% (lipophilic leave‐on) | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.61] | ||||||||||||||||||||||||||||||||||||
| 8.5 Clobetasol propionate (hydrophilic leave‐on) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.56, 4.37] | ||||||||||||||||||||||||||||||||||||
| 8.6 Clobetasol propionate (rinse‐off) | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.18] | ||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 3.1  Comparison 3 Vitamin D versus the vehicle, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||
| 1.1 Vitamin D (hydrophilic leave‐on) | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [1.49, 10.11] | ||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 3.2  Comparison 3 Vitamin D versus the vehicle, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||
| 2.1 Calcipotriene (hydrophilic leave‐on) | 2 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.20, 3.69] | ||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 3.3
Comparison 3 Vitamin D versus the vehicle, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 3.4 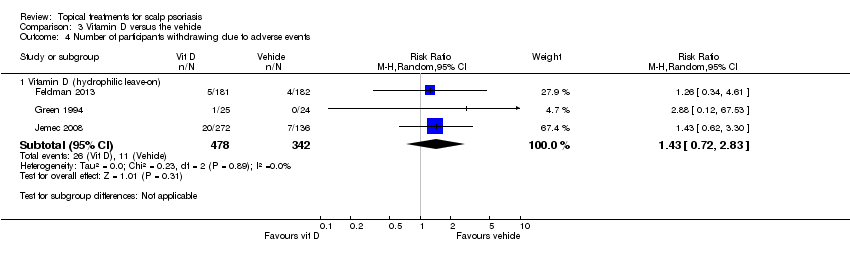 Comparison 3 Vitamin D versus the vehicle, Outcome 4 Number of participants withdrawing due to adverse events. | ||||||||||||||||||||||||
| 4.1 Vitamin D (hydrophilic leave‐on) | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.72, 2.83] | ||||||||||||||||||||
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 3.5  Comparison 3 Vitamin D versus the vehicle, Outcome 5 Number of participants achieving 'clearance' by PGA. | ||||||||||||||||||||||||
| 5.1 Calcipotriol (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||
| 6 Number of participants achieving 'response' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||
| Analysis 3.6  Comparison 3 Vitamin D versus the vehicle, Outcome 6 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||
| 6.1 Calcipotriene (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||
| 7 Number of participants with at least one adverse event Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||
| Analysis 3.7  Comparison 3 Vitamin D versus the vehicle, Outcome 7 Number of participants with at least one adverse event. | ||||||||||||||||||||||||
| 7.1 Vitamin D (hydrophilic leave‐on) | 3 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.36] | ||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | ||||||||||
| Analysis 4.1  Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 1 Number of participants achieving 'clearance' by IGA. | |||||||||||||
| 1.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | ||||||||||
| Analysis 4.2  Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 2 Number of participants achieving 'response' by IGA. | |||||||||||||
| 2.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | |||||||||||
| Analysis 4.3
Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 3 Mean of the TSS. | |||||||||||||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||
| Analysis 4.4 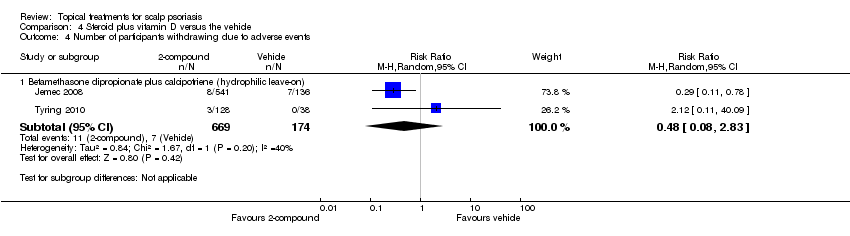 Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 4 Number of participants withdrawing due to adverse events. | |||||||||||||
| 4.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.08, 2.83] | |||||||||
| 5 Number of participants achieving 'response' by PGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | ||||||||||
| Analysis 4.5  Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 5 Number of participants achieving 'response' by PGA. | |||||||||||||
| 5.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||
| 6 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | ||||||||||
| Analysis 4.6 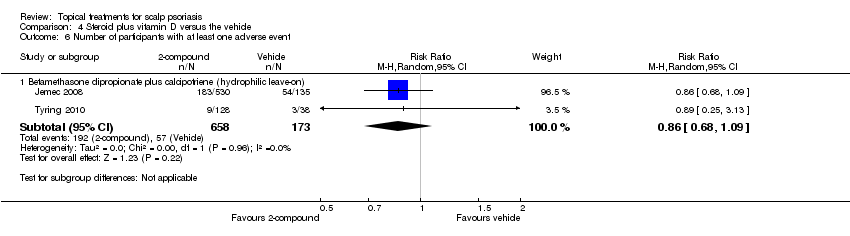 Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 6 Number of participants with at least one adverse event. | |||||||||||||
| 6.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.68, 1.09] | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||
| Analysis 5.1 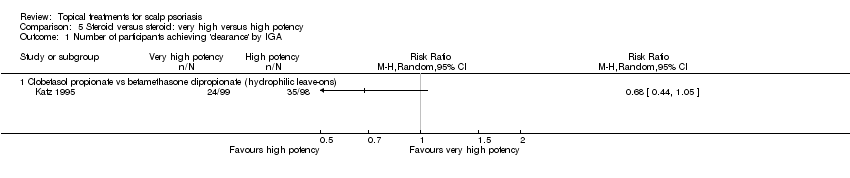 Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||
| 1.1 Clobetasol propionate vs betamethasone dipropionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||
| Analysis 5.2  Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||
| 2.1 Clobetasol propionate vs betamethasone dipropionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 5.3
Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 3 Mean of the TSS. | ||||||||||||||||
| 4 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||
| Analysis 5.4  Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 4 Number of participants with at least one adverse event. | ||||||||||||||||
| 4.1 Clobetasol propionate vs betamethasone dipropionate (hydrophilic leave‐ons) | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.32, 2.48] | ||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | |||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.56, 2.39] | |||||||||
| Analysis 6.1  Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 1 Number of participants achieving 'clearance' by IGA. | |||||||||||||
| 1.1 Betamethasone dipropionate vs hydrocortisone 17‐butyrate 0.1% (hydrophilic leave‐on) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.93] | |||||||||
| 1.2 Fluocinolone acetonide 0.025% vs hydrocortisone 17‐butyrate 0.1% (lipophilic leave‐on) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.77, 3.22] | |||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | ||||||||||
| Analysis 6.2  Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 2 Number of participants achieving 'response' by IGA. | |||||||||||||
| 2.1 Fluocinonide 0.05% vs desoximetasone 0.05% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | |||||||||||
| Analysis 6.3
Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 3 Mean of the TSS. | |||||||||||||
| 4 Number of participants with at least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | ||||||||||
| Analysis 6.4 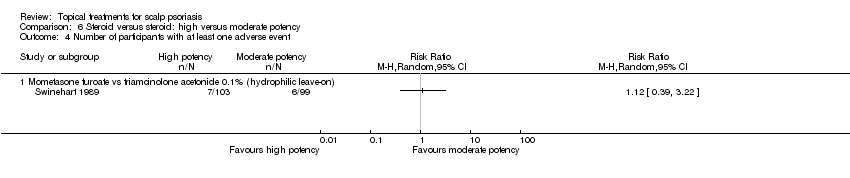 Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 4 Number of participants with at least one adverse event. | |||||||||||||
| 4.1 Mometasone furoate vs triamcinolone acetonide 0.1% (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||
| Analysis 7.1  Comparison 7 Steroids versus steroid: both of high potency, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||
| 1.1 Mometasone furoate vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||
| Analysis 7.2  Comparison 7 Steroids versus steroid: both of high potency, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||
| 2.1 Mometasone furoate vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||
| Analysis 7.3
Comparison 7 Steroids versus steroid: both of high potency, Outcome 3 Mean of the TSS. | ||||||||||||||||||||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||
| Analysis 7.4 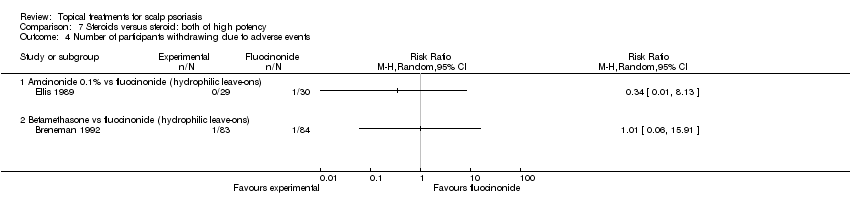 Comparison 7 Steroids versus steroid: both of high potency, Outcome 4 Number of participants withdrawing due to adverse events. | ||||||||||||||||||||
| 4.1 Amcinonide 0.1% vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 4.2 Betamethasone vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 5 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||
| Analysis 7.5  Comparison 7 Steroids versus steroid: both of high potency, Outcome 5 Number of participants with at least one adverse event. | ||||||||||||||||||||
| 5.1 Amcinonide 0.1% vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| 5.2 Betamethasone dipropionate vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.52, 2.18] | ||||||||||||||||||||||||||||
| Analysis 8.1  Comparison 8 Steroid versus vitamin D, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||||||||||
| 1.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.31, 2.60] | ||||||||||||||||||||||||||||
| 1.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.46, 2.24] | ||||||||||||||||||||||||||||
| 1.3 Mometasone furoate vs calcipotriol (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.43, 9.32] | ||||||||||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 3 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.80, 2.41] | ||||||||||||||||||||||||||||
| Analysis 8.2 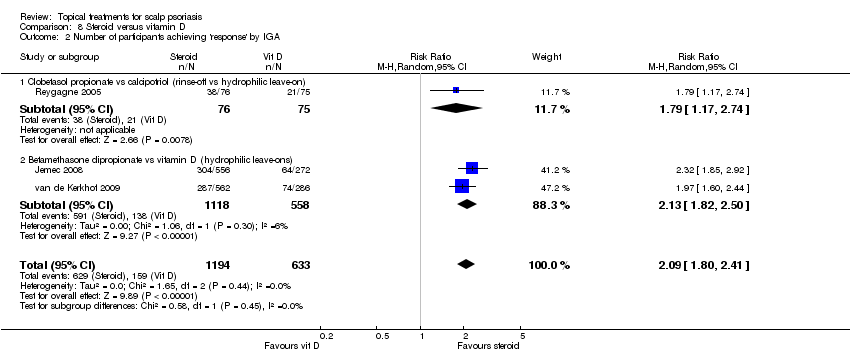 Comparison 8 Steroid versus vitamin D, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||||||||||
| 2.1 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.17, 2.74] | ||||||||||||||||||||||||||||
| 2.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.82, 2.50] | ||||||||||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||
| Analysis 8.3
Comparison 8 Steroid versus vitamin D, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||||||||||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 4 | 2291 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.42] | ||||||||||||||||||||||||||||
| Analysis 8.4 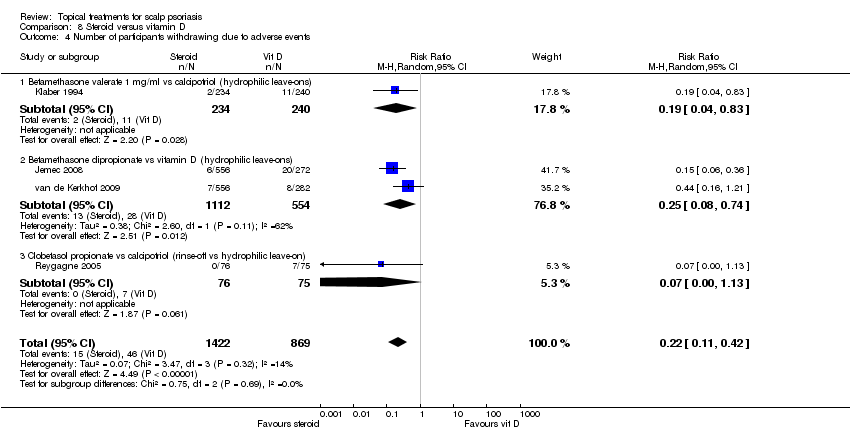 Comparison 8 Steroid versus vitamin D, Outcome 4 Number of participants withdrawing due to adverse events. | ||||||||||||||||||||||||||||||||
| 4.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.83] | ||||||||||||||||||||||||||||
| 4.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.74] | ||||||||||||||||||||||||||||
| 4.3 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] | ||||||||||||||||||||||||||||
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 2 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.47, 3.35] | ||||||||||||||||||||||||||||
| Analysis 8.5 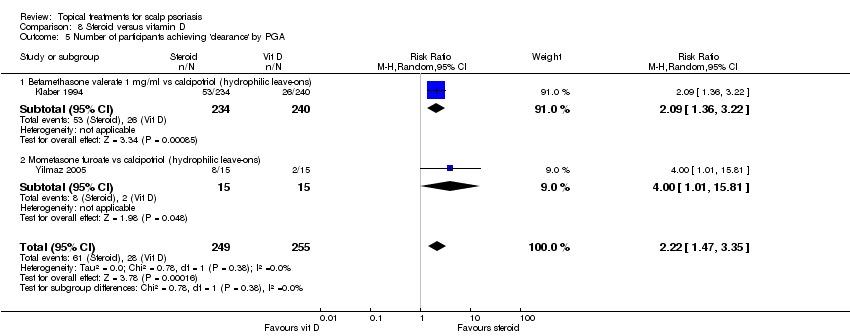 Comparison 8 Steroid versus vitamin D, Outcome 5 Number of participants achieving 'clearance' by PGA. | ||||||||||||||||||||||||||||||||
| 5.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.36, 3.22] | ||||||||||||||||||||||||||||
| 5.2 Mometasone furoate vs calcipotriol (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [1.01, 15.81] | ||||||||||||||||||||||||||||
| 6 Number of participants achieving 'response' by PGA Show forest plot | 3 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.28, 1.72] | ||||||||||||||||||||||||||||
| Analysis 8.6 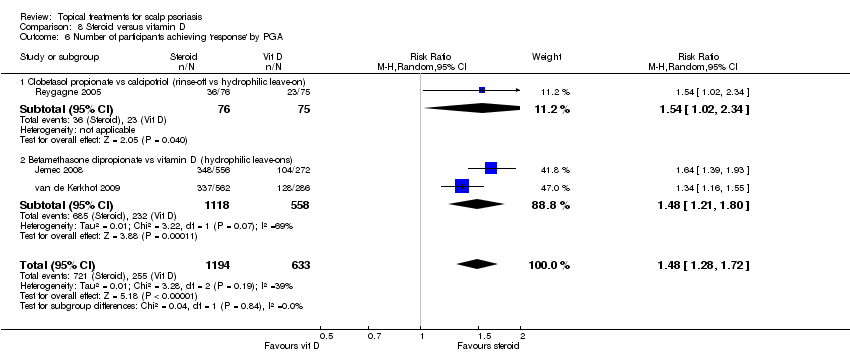 Comparison 8 Steroid versus vitamin D, Outcome 6 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||||||||||
| 6.1 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.02, 2.34] | ||||||||||||||||||||||||||||
| 6.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.21, 1.80] | ||||||||||||||||||||||||||||
| 7 Number of participants with at least one adverse event Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||
| Analysis 8.7  Comparison 8 Steroid versus vitamin D, Outcome 7 Number of participants with at least one adverse event. | ||||||||||||||||||||||||||||||||
| 7.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.25, 0.53] | ||||||||||||||||||||||||||||
| 7.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1652 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.97] | ||||||||||||||||||||||||||||
| 7.3 Clobetasol propionate vs calcipotriol (hydrophilic leave‐on vs occlusive lipophilic leave‐on) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.34] | ||||||||||||||||||||||||||||
| 7.4 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.16, 0.72] | ||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.1 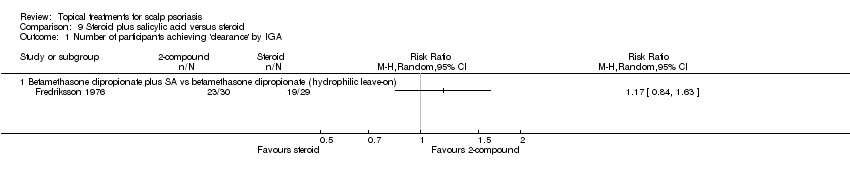 Comparison 9 Steroid plus salicylic acid versus steroid, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||
| 1.1 Betamethasone dipropionate plus SA vs betamethasone dipropionate (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 9.2  Comparison 9 Steroid plus salicylic acid versus steroid, Outcome 2 Number of participants achieving 'response' by IGA. | ||||
| 2.1 Betamethasone dipropionate plus SA vs betamethasone dipropionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 2474 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.36] | ||||||||||||||||||||||||
| Analysis 10.1 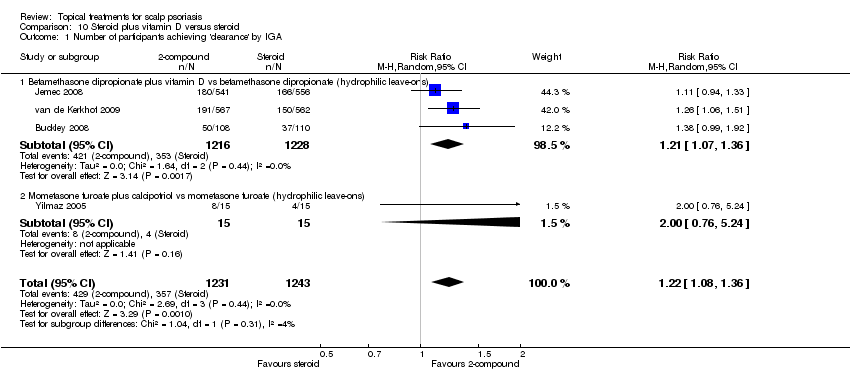 Comparison 10 Steroid plus vitamin D versus steroid, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||||||
| 1.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2444 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.07, 1.36] | ||||||||||||||||||||||||
| 1.2 Mometasone furoate plus calcipotriol vs mometasone furoate (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.76, 5.24] | ||||||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 10.2  Comparison 10 Steroid plus vitamin D versus steroid, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||||||
| 2.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2444 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.25] | ||||||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 10.3
Comparison 10 Steroid plus vitamin D versus steroid, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||||||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 10.4 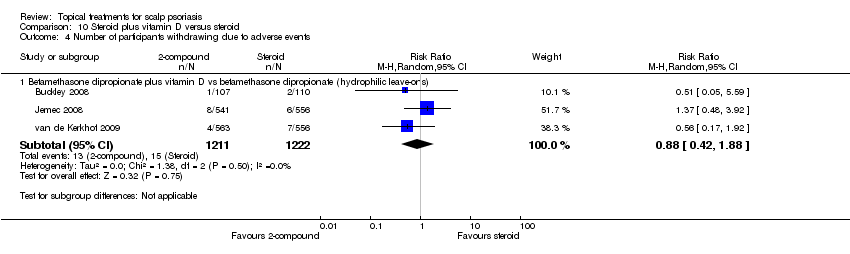 Comparison 10 Steroid plus vitamin D versus steroid, Outcome 4 Number of participants withdrawing due to adverse events. | ||||||||||||||||||||||||||||
| 4.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2433 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.88] | ||||||||||||||||||||||||
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 10.5 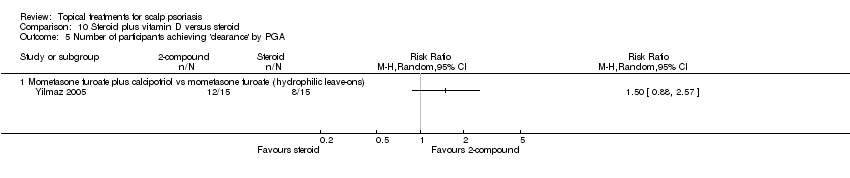 Comparison 10 Steroid plus vitamin D versus steroid, Outcome 5 Number of participants achieving 'clearance' by PGA. | ||||||||||||||||||||||||||||
| 5.1 Mometasone furoate plus calcipotriol vs mometasone furoate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 6 Number of participants achieving 'response' by PGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 10.6  Comparison 10 Steroid plus vitamin D versus steroid, Outcome 6 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||||||
| 6.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 2 | 2226 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.06, 1.20] | ||||||||||||||||||||||||
| 7 Number of participants with at least one adverse event Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 10.7  Comparison 10 Steroid plus vitamin D versus steroid, Outcome 7 Number of participants with at least one adverse event. | ||||||||||||||||||||||||||||
| 7.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2414 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.07] | ||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 2008 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.87, 2.78] | ||||||||||||||||||||||||
| Analysis 11.1 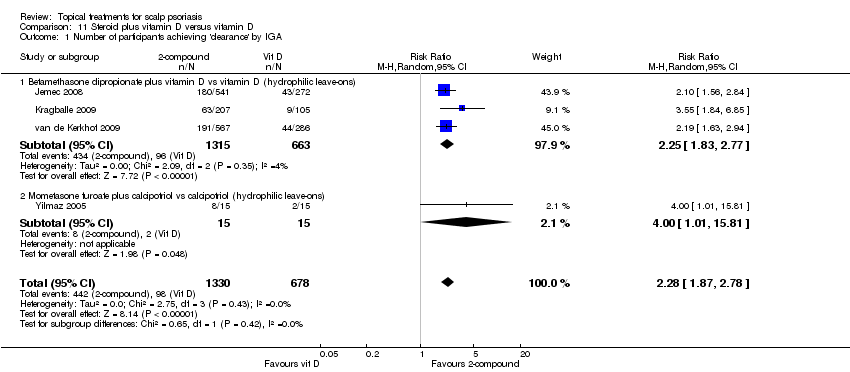 Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||||||
| 1.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐ons) | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.83, 2.77] | ||||||||||||||||||||||||
| 1.2 Mometasone furoate plus calcipotriol vs calcipotriol (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [1.01, 15.81] | ||||||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 4 | 2222 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.75, 3.04] | ||||||||||||||||||||||||
| Analysis 11.2  Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||||||
| 2.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐on) | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [2.03, 3.22] | ||||||||||||||||||||||||
| 2.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) ‐ trial register study | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.43, 2.07] | ||||||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||
| Analysis 11.3
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||||||
| 4 Number of participants withdrawing due to adverse events (short‐term) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||
| Analysis 11.4 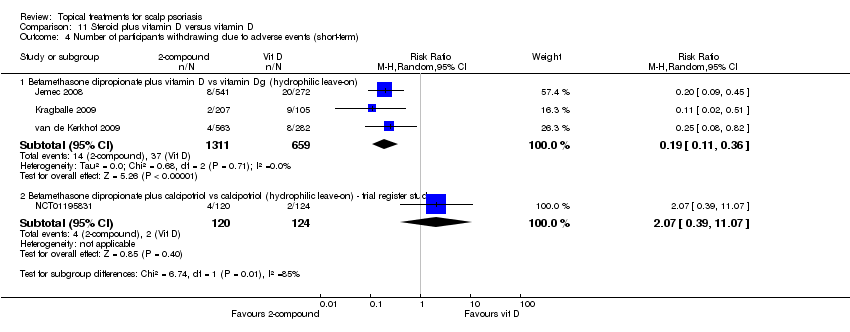 Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 4 Number of participants withdrawing due to adverse events (short‐term). | ||||||||||||||||||||||||||||
| 4.1 Betamethasone dipropionate plus vitamin D vs vitamin Dg (hydrophilic leave‐on) | 3 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.11, 0.36] | ||||||||||||||||||||||||
| 4.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) ‐ trial register study | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.39, 11.07] | ||||||||||||||||||||||||
| 5 Number of participants withdrawing due to adverse events (long‐term) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 11.5  Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 5 Number of participants withdrawing due to adverse events (long‐term). | ||||||||||||||||||||||||||||
| 5.1 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 6 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 11.6  Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 6 Number of participants achieving 'clearance' by PGA. | ||||||||||||||||||||||||||||
| 6.1 Mometasone furoate plus calcipotriol vs calcipotriol (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| 7 Number of participants achieving 'response' by PGA Show forest plot | 4 | 2222 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.46, 2.12] | ||||||||||||||||||||||||
| Analysis 11.7 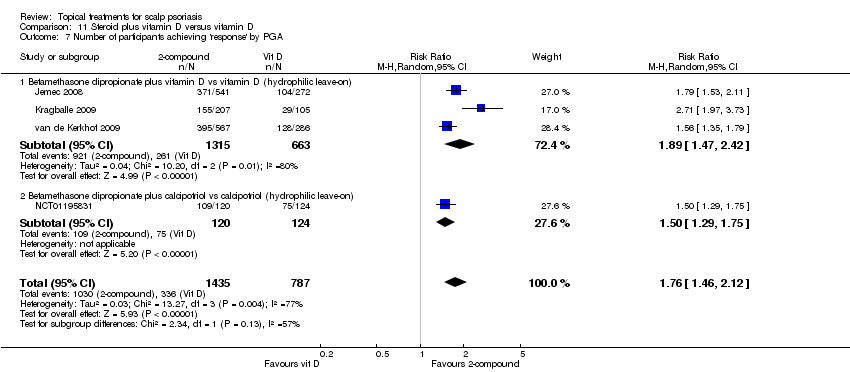 Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 7 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||||||
| 7.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐on) | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.47, 2.42] | ||||||||||||||||||||||||
| 7.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.29, 1.75] | ||||||||||||||||||||||||
| 8 Number of participants with at least one adverse event (short‐term) Show forest plot | 4 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.85] | ||||||||||||||||||||||||
| Analysis 11.8  Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 8 Number of participants with at least one adverse event (short‐term). | ||||||||||||||||||||||||||||
| 8.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐on) | 3 | 1951 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.88] | ||||||||||||||||||||||||
| 8.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) ‐ trial register study | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.25, 0.76] | ||||||||||||||||||||||||
| 9 Number of participants with at least one adverse event (long‐term) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||
| Analysis 11.9  Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 9 Number of participants with at least one adverse event (long‐term). | ||||||||||||||||||||||||||||
| 9.1 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 12.1  Comparison 12 Tar and dithranol, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||||||||||||||||||
| 1.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 1.2 Tacrolimus vs pine tar (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 1.3 Dithranol/urea combination vs coal tar plus salicylic acid combination (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 12.2 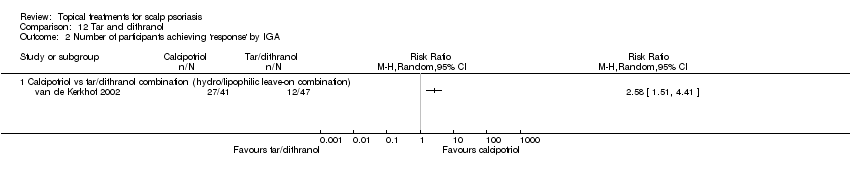 Comparison 12 Tar and dithranol, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||||||||||||||||||
| 2.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| Analysis 12.3
Comparison 12 Tar and dithranol, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||||||||||||||||||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 12.4  Comparison 12 Tar and dithranol, Outcome 4 Number of participants withdrawing due to adverse events. | ||||||||||||||||||||||||||||||||||||||||
| 4.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 4.2 Calcipotriol vs coal tar/coconut oil/salicylic acid (hydrophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 4.3 Clobetasol propionate vs tar blend (rinse‐offs) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 4.4 Cocois vs coal tar (lipophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 12.5  Comparison 12 Tar and dithranol, Outcome 5 Number of participants achieving 'clearance' by PGA. | ||||||||||||||||||||||||||||||||||||||||
| 5.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 6 Number of participants achieving 'response' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 12.6  Comparison 12 Tar and dithranol, Outcome 6 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||||||||||||||||||
| 6.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 7 Number of participants with at least one adverse event Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||||||
| Analysis 12.7  Comparison 12 Tar and dithranol, Outcome 7 Number of participants with at least one adverse event. | ||||||||||||||||||||||||||||||||||||||||
| 7.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 7.2 Calcipotriol vs coal tar/coconut oil/salicylic acid (hydrophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 7.3 Clobetasol propionate vs tar blend (rinse‐offs) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 7.4 Tacrolimus vs pine tar (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 7.5 Dithranol/urea combination vs coal tar plus salicylic acid combination (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| 7.6 Cocois vs coal tar (lipophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||
| Analysis 13.1  Comparison 13 Steroid: vehicle comparisons, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||||||||||||||||||||||||||||||||||
| 1.1 Betameth diprop (BP) + RV3423A shampoo/shampoo vs BP + shampoo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||
| 2 Number of participants achieving 'response' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||
| Analysis 13.2  Comparison 13 Steroid: vehicle comparisons, Outcome 2 Number of participants achieving 'response' by IGA. | ||||||||||||||||||||||||||||||||||||
| 2.1 Betamethasone valerate 0.1%: foam vs lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||
| 2.2 Clobetasol propionate: foam vs solution | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| Analysis 13.3
Comparison 13 Steroid: vehicle comparisons, Outcome 3 Mean of the TSS. | ||||||||||||||||||||||||||||||||||||
| 4 Number of participants achieving 'response' by PGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||
| Analysis 13.4  Comparison 13 Steroid: vehicle comparisons, Outcome 4 Number of participants achieving 'response' by PGA. | ||||||||||||||||||||||||||||||||||||
| 4.1 Betamethasone valerate 0.1%: foam vs lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||
| 4.2 Clobetasol propionate: foam vs solution | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||
| 5 Number of participants with at least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |||||||||||||||||||||||||||||||||
| Analysis 13.5 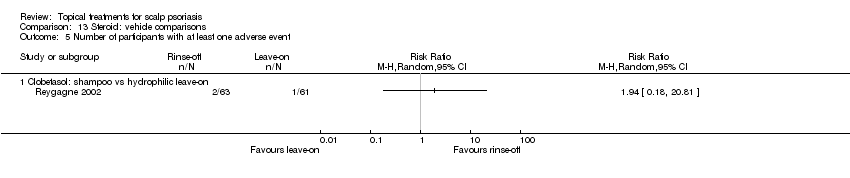 Comparison 13 Steroid: vehicle comparisons, Outcome 5 Number of participants with at least one adverse event. | ||||||||||||||||||||||||||||||||||||
| 5.1 Clobetasol: shampoo vs hydrophilic leave‐on | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 14.1  Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 1 Number of participants achieving 'clearance' by IGA. | ||||
| 1.1 Betamethasone dipropionate (BDP) plus SA vs triamcinolone acetonide 0.2% plus SA (hydrophilic leave‐ons) | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.47, 4.74] |
| 1.2 Betamethasone dipropionate (BDP) plus SA vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.59, 3.32] |
| 1.3 Betamethasone dipropionate (BDP) plus SA vs clobetasol propionate (hydrophilic leave‐ons) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.97, 2.01] |
| 1.4 Betamethasone dipropionate (BDP) vs triamcinolone acetonide 0.2% plus SA (hydrophilic leave‐on) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.23, 4.16] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 14.2  Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 2 Number of participants achieving 'response' by IGA. | ||||
| 2.1 Betameth diprop plus SA vs triamcin acet 0.2% plus SA (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Betameth diprop vs triamcin acet 0.2% plus SA (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants withdrawing due to adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 14.3  Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 3 Number of participants withdrawing due to adverse events. | ||||
| 3.1 Betamethasone dipropionate plus SA vs clobetasol propionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 14.4 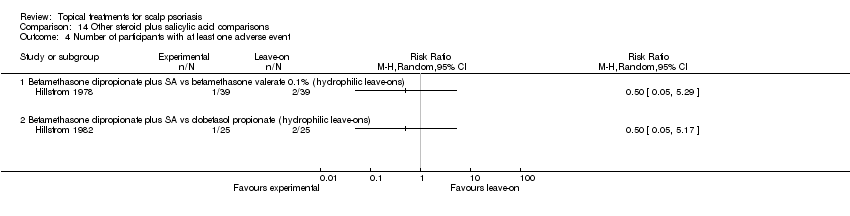 Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 4 Number of participants with at least one adverse event. | ||||
| 4.1 Betamethasone dipropionate plus SA vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Betamethasone dipropionate plus SA vs clobetasol propionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean score of the IGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 15.1  Comparison 15 Antifungals versus vehicle, Outcome 1 Mean score of the IGA. | ||||
| 2 Number of participants withdrawing due to adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 15.2  Comparison 15 Antifungals versus vehicle, Outcome 2 Number of participants withdrawing due to adverse events. | ||||
| 2.1 Ciclopirox olamine (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean score of the PGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 15.3  Comparison 15 Antifungals versus vehicle, Outcome 3 Mean score of the PGA. | ||||
| 4 Number of participants with at least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 15.4 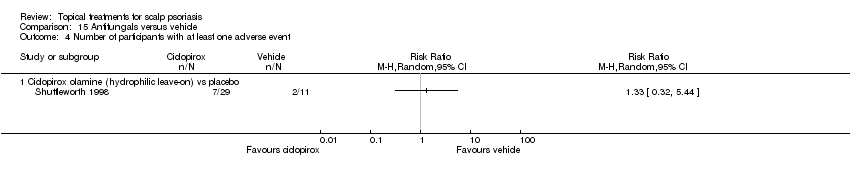 Comparison 15 Antifungals versus vehicle, Outcome 4 Number of participants with at least one adverse event. | ||||
| 4.1 Ciclopirox olamine (hydrophilic leave‐on) vs placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Legend: "?" = unclear risk of bias; "+" = low risk of bias; "‐" = high risk of bias

Forest plot of comparison: 10 Steroid plus vitamin D vs steroid, outcome: 10.2 IGA: response.

Forest plot of comparison: 10 Steroid plus vitamin D vs steroid, outcome: 10.7 Number of participants with at least one AE.
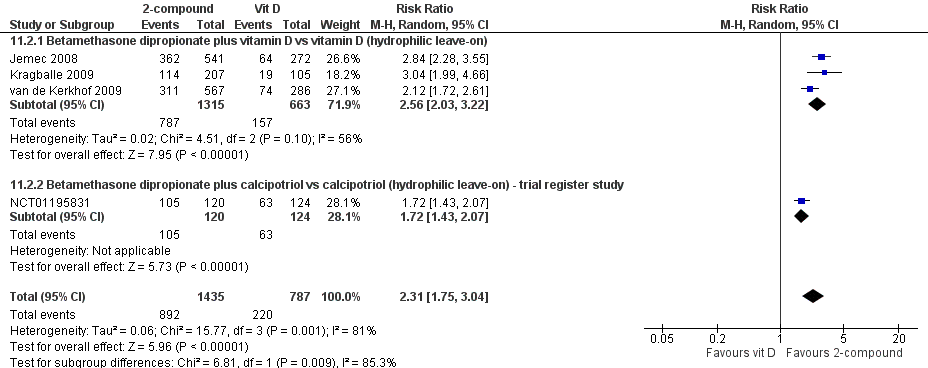
Forest plot of comparison: 11 Steroid plus vitamin D vs vitamin D, outcome: 11.2 IGA: response.

Forest plot of comparison: 11 Steroid plus vitamin D vs vitamin D, outcome: 11.8 Number of participants with at least one AE (short term).

Comparison 1 Steroid: once versus twice daily, Outcome 1 Mean score of the IGA.

Comparison 1 Steroid: once versus twice daily, Outcome 2 Mean score of the PGA.

Comparison 2 Steroid versus the vehicle, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 2 Steroid versus the vehicle, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Comparison: Steroid vs vehicle | Steroid | Placebo |
| Franz 1999 | Betamethasone valerate (hydrophilic leave‐on) vs placebo | ‐57% | ‐24% |
| Franz 2000 | Clobetasol propionate (hydrophilic leave‐on) vs placebo | ‐72% | ‐37% |
| Jarratt 2004 | Clobetasol propionate (rinse‐off) vs placebo vs placebo | ‐49% | ‐18% |
| Jemec 2008 | Betamethasone dipropionate (hydrophilic leave‐on) vs placebo | ‐64% | ‐36% |
| Olsen 1991 | Clobetasol propionate (hydrophilic leave‐on) vs placebo | ‐72% | ‐30% |
| Pauporte 2004 | Fluocinolone acetonide (hydrophilic leave‐on) vs placebo | ‐59% | ‐32% |
| Sofen 2011 | Clobetasol propionate (hydrophilic leave‐on) vs placebo | ‐79% | ‐25% |
Comparison 2 Steroid versus the vehicle, Outcome 3 Mean of the TSS.

Comparison 2 Steroid versus the vehicle, Outcome 4 Improvement in quality of life.

Comparison 2 Steroid versus the vehicle, Outcome 5 Number of participants withdrawing due to adverse events.

Comparison 2 Steroid versus the vehicle, Outcome 6 Number of participants achieving 'response' by PGA.

Comparison 2 Steroid versus the vehicle, Outcome 7 Mean score of the PGA.

Comparison 2 Steroid versus the vehicle, Outcome 8 Number of participants with at least one adverse event.

Comparison 3 Vitamin D versus the vehicle, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 3 Vitamin D versus the vehicle, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Vitamin D vs vehicle | Vitamin D | Vehicle |
| Green 1994 | Calcipotriol vs vehicle | ‐52% | ‐24% |
| Jemec 2008 | Calcipotriene vs vehicle | ‐54% | ‐36% |
| Ruzicka 2004 | Tacalcitol vs vehicle | ‐53% | ‐30% |
Comparison 3 Vitamin D versus the vehicle, Outcome 3 Mean of the TSS.

Comparison 3 Vitamin D versus the vehicle, Outcome 4 Number of participants withdrawing due to adverse events.

Comparison 3 Vitamin D versus the vehicle, Outcome 5 Number of participants achieving 'clearance' by PGA.

Comparison 3 Vitamin D versus the vehicle, Outcome 6 Number of participants achieving 'response' by PGA.

Comparison 3 Vitamin D versus the vehicle, Outcome 7 Number of participants with at least one adverse event.

Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 1 Number of participants achieving 'clearance' by IGA.
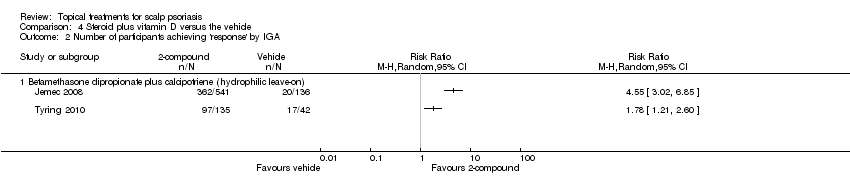
Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Betamethasone dipropionate/calcipotriene combination | Placebo (vehicle) |
| Jemec 2008 | ‐70% | ‐36% |
Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 3 Mean of the TSS.

Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 4 Number of participants withdrawing due to adverse events.

Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 5 Number of participants achieving 'response' by PGA.
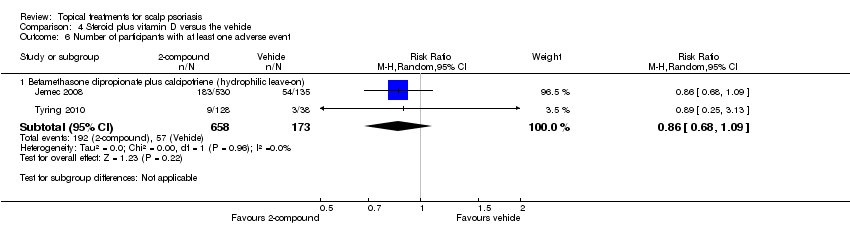
Comparison 4 Steroid plus vitamin D versus the vehicle, Outcome 6 Number of participants with at least one adverse event.

Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 1 Number of participants achieving 'clearance' by IGA.
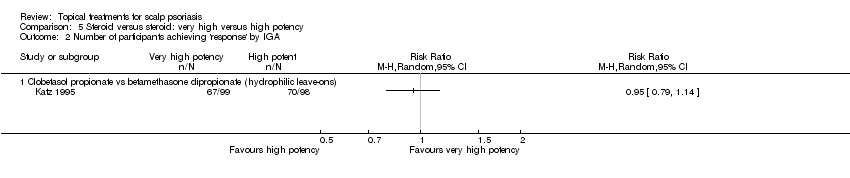
Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Clobetasol propionate | Betamethasone dipropionate |
| Katz 1995 | ‐75% | ‐83% |
| Lassus 1976 | ‐81% | ‐58% |
Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 3 Mean of the TSS.
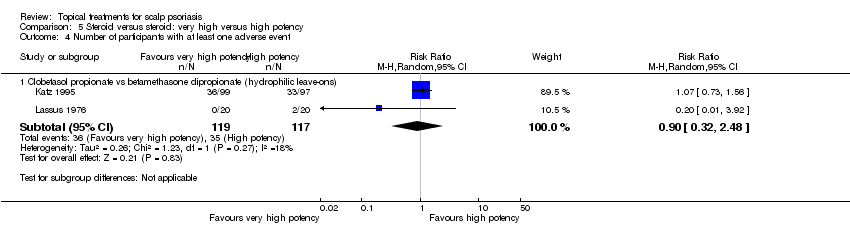
Comparison 5 Steroid versus steroid: very high versus high potency, Outcome 4 Number of participants with at least one adverse event.
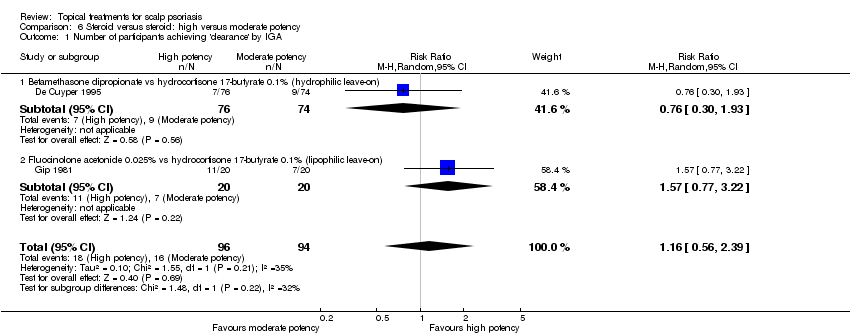
Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Mometasone furoate | Triamcinolone acetonide |
| Swinehart 1989 | ‐79% | ‐70% |
Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 3 Mean of the TSS.

Comparison 6 Steroid versus steroid: high versus moderate potency, Outcome 4 Number of participants with at least one adverse event.

Comparison 7 Steroids versus steroid: both of high potency, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 7 Steroids versus steroid: both of high potency, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Steroid vs steroid of high potency | Steroid (1) | Steroid (2) |
| Breneman 1992 | Fluocinonide (1) vs BTM dipropionate (2) | ‐84% | ‐85% |
| Van der Ploeg 1989 | Mometasone furoate (1) vs BTM valerate (2) | ‐85% | ‐70% |
Comparison 7 Steroids versus steroid: both of high potency, Outcome 3 Mean of the TSS.
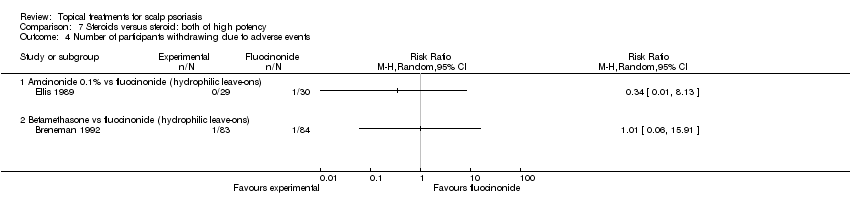
Comparison 7 Steroids versus steroid: both of high potency, Outcome 4 Number of participants withdrawing due to adverse events.

Comparison 7 Steroids versus steroid: both of high potency, Outcome 5 Number of participants with at least one adverse event.

Comparison 8 Steroid versus vitamin D, Outcome 1 Number of participants achieving 'clearance' by IGA.
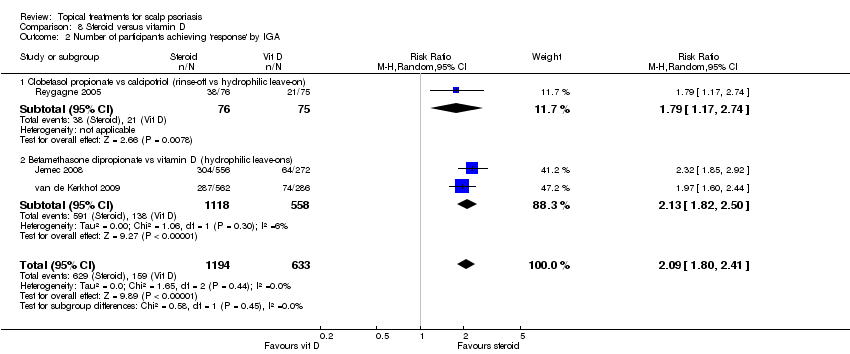
Comparison 8 Steroid versus vitamin D, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Corticosteroid vs vitamin D | Corticosteroid | Calcipotriol |
| Jemec 2008 | Betamethasone dipropionate vs calcipotriene | ‐62% | ‐44% |
| Klaber 1994 | Betamethasone valerate vs calcipotriol | ‐62% | ‐48% |
| Reygagne 2005 | Clobetasol vs calcipotriol | ‐64% | ‐52% |
| van de Kerkhof 2009 | Betamethasone dipropionate vs calcipotriol | ‐57% | ‐43% |
| Yilmaz 2005 | Mometasone vs calcipotriol | ‐52% | ‐49% |
Comparison 8 Steroid versus vitamin D, Outcome 3 Mean of the TSS.
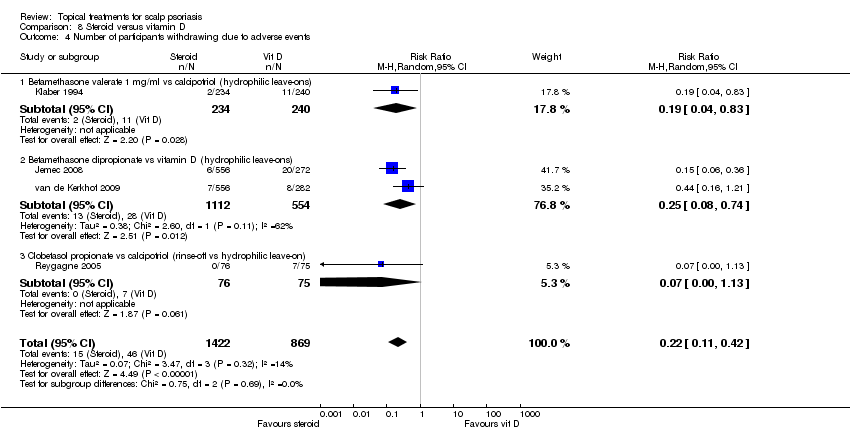
Comparison 8 Steroid versus vitamin D, Outcome 4 Number of participants withdrawing due to adverse events.

Comparison 8 Steroid versus vitamin D, Outcome 5 Number of participants achieving 'clearance' by PGA.

Comparison 8 Steroid versus vitamin D, Outcome 6 Number of participants achieving 'response' by PGA.

Comparison 8 Steroid versus vitamin D, Outcome 7 Number of participants with at least one adverse event.

Comparison 9 Steroid plus salicylic acid versus steroid, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 9 Steroid plus salicylic acid versus steroid, Outcome 2 Number of participants achieving 'response' by IGA.
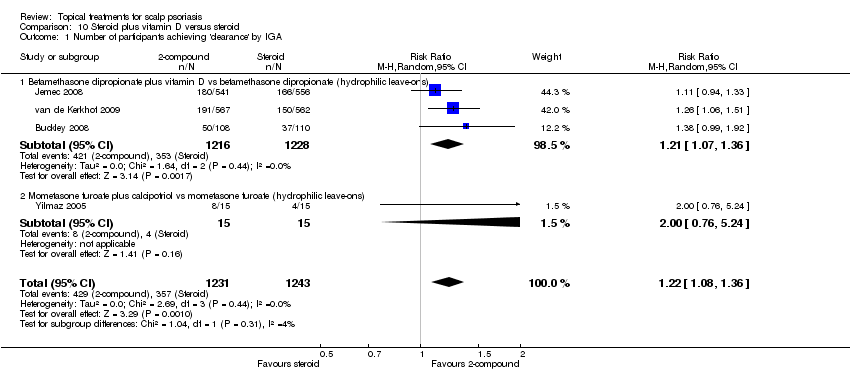
Comparison 10 Steroid plus vitamin D versus steroid, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 10 Steroid plus vitamin D versus steroid, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Steroid/ vitamin D combination vs steroid | Steroid/calcipotriol | Steroid |
| Buckley 2008 | Betamethasone dipropionate/calcipotriol vs betamethasone dipropionate | ‐69% | ‐62% |
| Jemec 2008 | Betamethasone dipropionate/calcipotriene vs betamethasone dipropionate | ‐70% | ‐64% |
| van de Kerkhof 2009 | Betamethasone dipropionate/calcipotriol vs betamethasone dipropionate | ‐62% | ‐57% |
| Yilmaz 2005 | Mometasone/calcipotriol vs mometasone | ‐81% | ‐52% |
Comparison 10 Steroid plus vitamin D versus steroid, Outcome 3 Mean of the TSS.

Comparison 10 Steroid plus vitamin D versus steroid, Outcome 4 Number of participants withdrawing due to adverse events.

Comparison 10 Steroid plus vitamin D versus steroid, Outcome 5 Number of participants achieving 'clearance' by PGA.
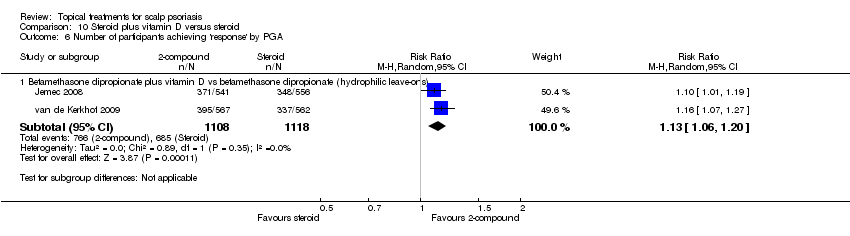
Comparison 10 Steroid plus vitamin D versus steroid, Outcome 6 Number of participants achieving 'response' by PGA.
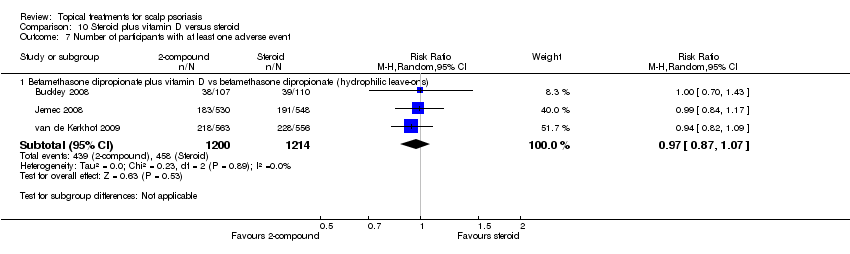
Comparison 10 Steroid plus vitamin D versus steroid, Outcome 7 Number of participants with at least one adverse event.
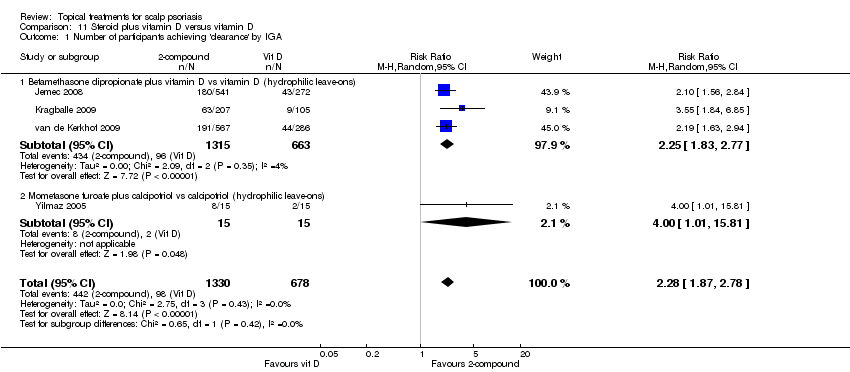
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 1 Number of participants achieving 'clearance' by IGA.
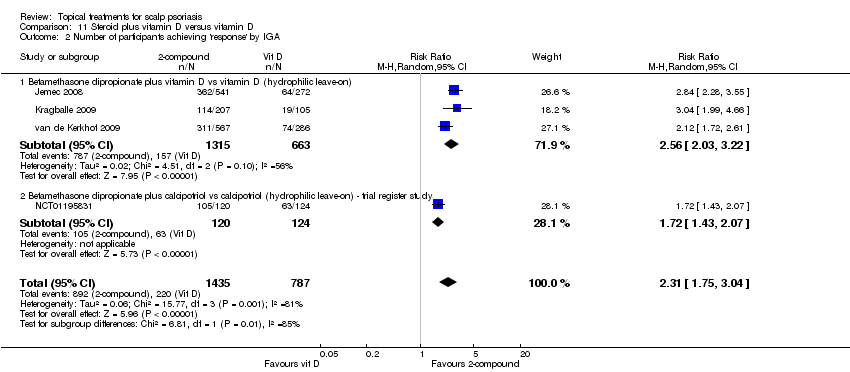
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Steroid/vitamin D combination vs vitamin D | Steroid/calcipotriol | Calcipotriol |
| Jemec 2008 | Betamethasone dipropionate/calcipotriol vs calcipotriol | ‐70% | ‐54% |
| Kragballe 2009 | Betamethasone dipropionate/calcipotriol vs calcipotriol | ‐64% | ‐38% |
| van de Kerkhof 2009 | Betamethasone dipropionate/calcipotriol vs calcipotriol | ‐62% | ‐43% |
| Yilmaz 2005 | Mometasone/calcipotriol vs calcipotriol | ‐81% | ‐49% |
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 3 Mean of the TSS.
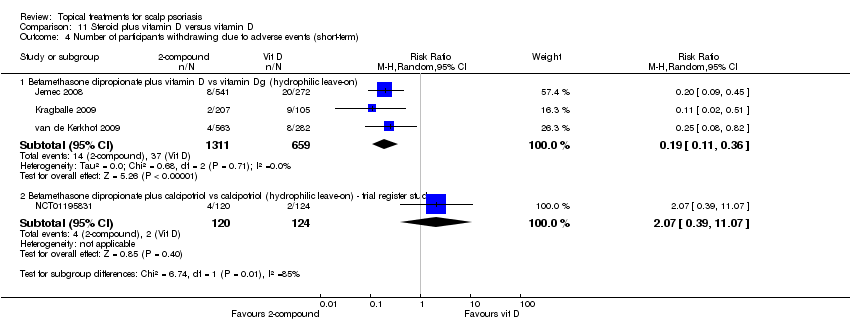
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 4 Number of participants withdrawing due to adverse events (short‐term).

Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 5 Number of participants withdrawing due to adverse events (long‐term).
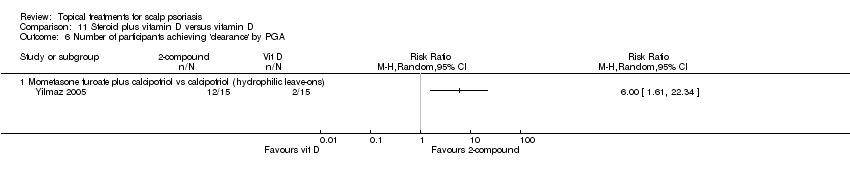
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 6 Number of participants achieving 'clearance' by PGA.

Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 7 Number of participants achieving 'response' by PGA.
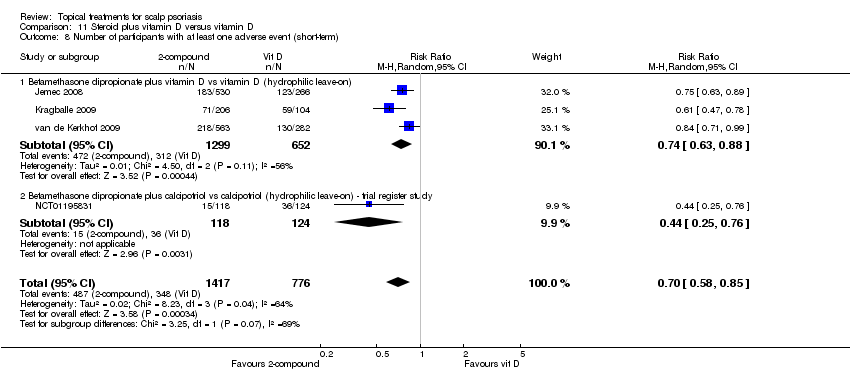
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 8 Number of participants with at least one adverse event (short‐term).
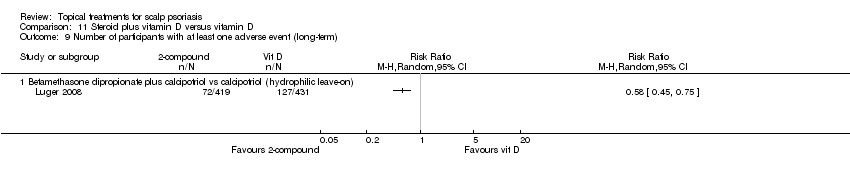
Comparison 11 Steroid plus vitamin D versus vitamin D, Outcome 9 Number of participants with at least one adverse event (long‐term).
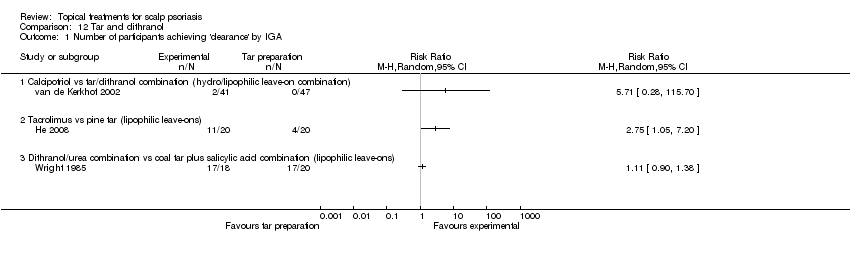
Comparison 12 Tar and dithranol, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 12 Tar and dithranol, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Comparison | Other therapy | Tar containing product |
| Griffiths 2006 | Clobetasol propionate vs tar blend | ‐48% | ‐16% |
| He 2008 | Tacrolimus vs pine tar | ‐80% | ‐55% |
| Klaber 2000 | Calcipotriol vs coal tar/coconut oil/salicylic acid combination | ‐28% | ‐23% |
| Monk 1995 | Cocois vs coal tar | ‐67% | ‐14% |
| van de Kerkhof 2002 | Calcipotriol vs tar/dithranol‐combination | ‐51% | ‐17% |
| Wall 1999 | Calcipotriol vs calcipotriol/coal tar combination | ‐41% | ‐48% |
| Wright 1985 | Dithranol/urea vs coal tar | ‐51% | ‐47% |
Comparison 12 Tar and dithranol, Outcome 3 Mean of the TSS.

Comparison 12 Tar and dithranol, Outcome 4 Number of participants withdrawing due to adverse events.

Comparison 12 Tar and dithranol, Outcome 5 Number of participants achieving 'clearance' by PGA.
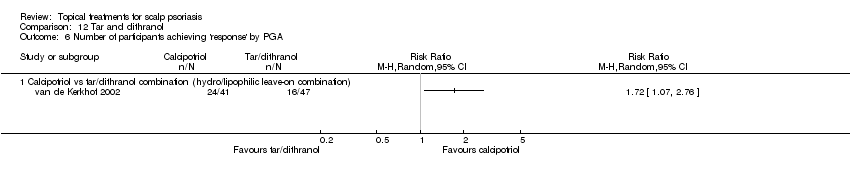
Comparison 12 Tar and dithranol, Outcome 6 Number of participants achieving 'response' by PGA.

Comparison 12 Tar and dithranol, Outcome 7 Number of participants with at least one adverse event.
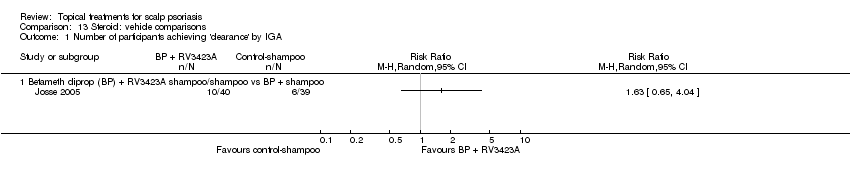
Comparison 13 Steroid: vehicle comparisons, Outcome 1 Number of participants achieving 'clearance' by IGA.
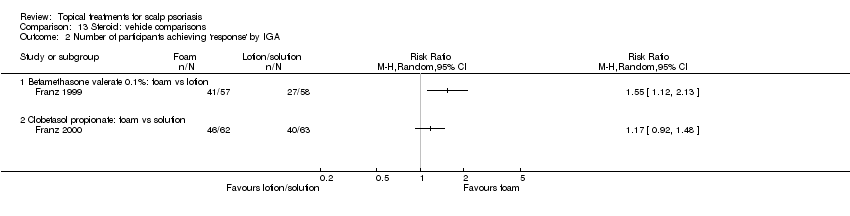
Comparison 13 Steroid: vehicle comparisons, Outcome 2 Number of participants achieving 'response' by IGA.
| Study | Comparison: vehicle (1) vs (2) | Vehicle 1 | Vehicle 2 |
| Andres 2006 | Clobetasol propionate: rinse‐off (1) vs hydrophilic leave‐on (2) | ‐70% | ‐75% |
| Franz 1999 | Betamethasone valerate: foam (1) vs lotion (2) | ‐65% | ‐49% |
| Franz 2000 | Clobetasol propionate: foam (1) vs solution (2) | ‐75% | ‐68% |
| Josse 2005 | Betamethasone dipropionate: RV3423A/extra gentle shampoo (1) vs extra gentle shampoo (2) | ‐63% | ‐53% |
| Reygagne 2002 | Clobetasol propionate: rinse‐off (1) vs hydrophilic leave‐on (2) | ‐67% | ‐80% |
| Wilhelm 2013 | Mometasone: emulsion (LAS41002) (1) vs solution (2) | ‐81.82% | ‐84.55% |
Comparison 13 Steroid: vehicle comparisons, Outcome 3 Mean of the TSS.
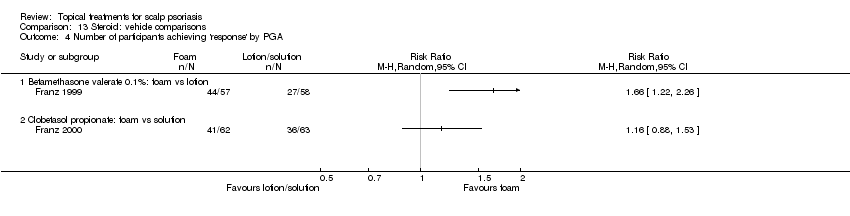
Comparison 13 Steroid: vehicle comparisons, Outcome 4 Number of participants achieving 'response' by PGA.

Comparison 13 Steroid: vehicle comparisons, Outcome 5 Number of participants with at least one adverse event.
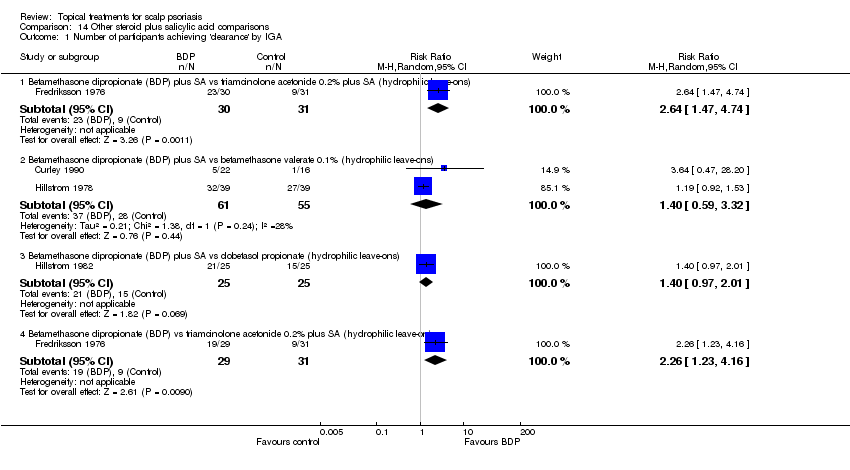
Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 1 Number of participants achieving 'clearance' by IGA.

Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 2 Number of participants achieving 'response' by IGA.

Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 3 Number of participants withdrawing due to adverse events.
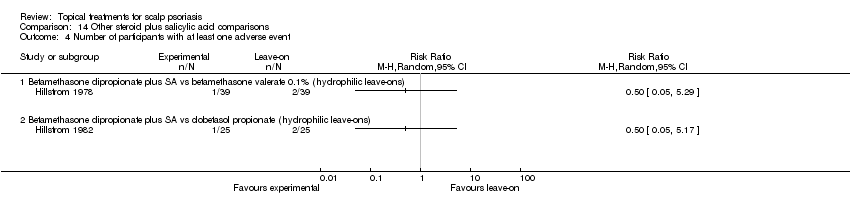
Comparison 14 Other steroid plus salicylic acid comparisons, Outcome 4 Number of participants with at least one adverse event.
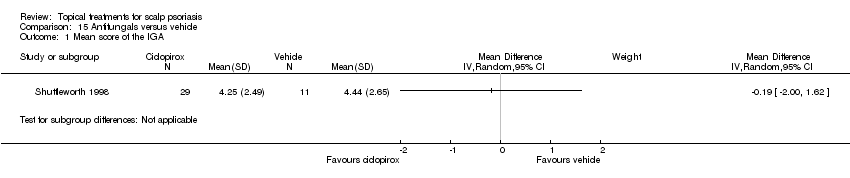
Comparison 15 Antifungals versus vehicle, Outcome 1 Mean score of the IGA.

Comparison 15 Antifungals versus vehicle, Outcome 2 Number of participants withdrawing due to adverse events.
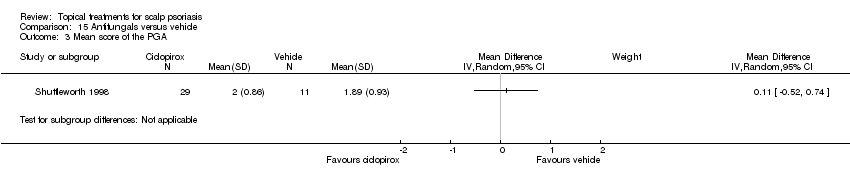
Comparison 15 Antifungals versus vehicle, Outcome 3 Mean score of the PGA.
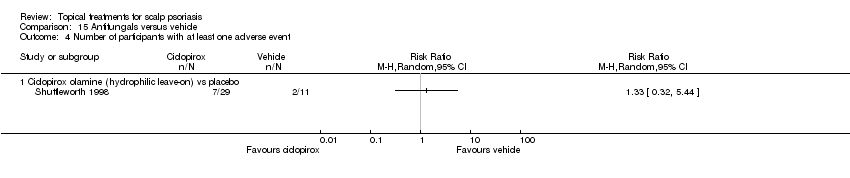
Comparison 15 Antifungals versus vehicle, Outcome 4 Number of participants with at least one adverse event.
| Steroid compared to vitamin D for scalp psoriasis | ||||||
| Patient or population: scalp psoriasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with vitamin D | Risk with steroid | |||||
| Number of participants achieving 'clearance' by IGA | Study population | RR 1.82 | 2180 | ⊕⊕⊕⊝ | — | |
| 159 per 1000 | 289 per 1000 | |||||
| Number of participants achieving 'response' by IGA | Study population | RR 2.09 | 1827 | ⊕⊕⊕⊕ | — | |
| 251 per 1000 | 525 per 1000 | |||||
| Quality of life | Study population | Not estimable | (0 studies) | — | No study addressed this outcome. | |
| 0 per 1000 | 0 per 1000 | |||||
| Number of participants withdrawing due to adverse events (AE) | Study population | RR 0.22 | 2291 | ⊕⊕⊕⊝ | No study reported the sort of AE that caused withdrawal. In 2 small studies with high risk of bias (Köse 1997, N = 43 participants; Yilmaz 2005, N = 30 participants) no withdrawals occurred. | |
| 53 per 1000 | 12 per 1000 | |||||
| Number of participants achieving 'response' by PGA | Study population | RR 1.48 | 1827 | ⊕⊕⊕⊝ | — | |
| 403 per 1000 | 596 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias: three out of four studies with unclear blinding of outcome assessment (Klaber 1994; van de Kerkhof 2009; Yilmaz 2005); all studies with unclear allocation concealment. 2 Downgraded by one level for risk of bias: two out of four studies with unclear blinding of outcome assessment (Klaber 1994; van de Kerkhof 2009); all studies with unclear allocation concealment. 3 Downgraded by one level for risk of bias: all three studies with unclear allocation concealment; one study with unclear risk of bias in selective reporting (Reygagne 2005); one study with unclear blinding of outcome assessment (van de Kerkhof 2009). | ||||||
| Steroid plus vitamin D compared to steroid for scalp psoriasis | ||||||
| Patient or population: scalp psoriasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with steroid | Risk with steroid plus vitamin D | |||||
| Number of participants achieving 'clearance' by IGA | Study population | RR 1.22 | 2474 | ⊕⊕⊕⊝ | — | |
| 287 per 1000 | 350 per 1000 | |||||
| Number of participants achieving 'response' by IGA | Study population | RR 1.15 | 2444 | ⊕⊕⊕⊝ | — | |
| 546 per 1000 | 628 per 1000 | |||||
| Quality of life | Study population | Not estimable | (0 studies) | — | No study addressed this outcome | |
| 0 per 1000 | 0 per 1000 | |||||
| Number of participants withdrawing due to adverse events (AE) | Study population | RR 0.88 | 2433 | ⊕⊕⊕⊝ | No study reported the sort of AE that caused withdrawal. In one small study with high risk of bias (Yilmaz 2005, N = 30 participants) no withdrawals occurred. | |
| 12 per 1000 | 11 per 1000 | |||||
| Number of participants achieving 'response' by PGA | Study population | RR 1.13 | 2226 | ⊕⊕⊕⊕ | — | |
| 613 per 1000 | 692 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for imprecision because CI crosses line of minimal important difference (MID) threshold: statistically significant difference of uncertain clinical importance. 2 Downgraded by one level for inconsistency due to moderate heterogeneity (I² = 35%). 3 Downgraded by one level for imprecision because CI crosses line of MID threshold: uncertain whether there is any difference. | ||||||
| Steroid plus vitamin D compared to vitamin D for scalp psoriasis | ||||||
| Patient or population: scalp psoriasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with vitamin D | Risk with steroid plus vitamin D | |||||
| Number of participants achieving 'clearance' by IGA | Study population | RR 2.28 | 2008 | ⊕⊕⊕⊕ | — | |
| 145 per 1000 | 330 per 1000 | |||||
| Number of participants achieving 'response' by IGA | Study population | RR 2.31 | 2222 | ⊕⊕⊕⊝ | — | |
| 280 per 1000 | 646 per 1000 | |||||
| Quality of life | Study population | Not estimable | (0 studies) | — | Only one study (Ortonne 2009) addressed this outcome, but did not provide sufficient information to allow assessment of the quality of evidence. | |
| 0 per 1000 | 0 per 1000 | |||||
| Number of participants withdrawing due to adverse events | Study population | RR 0.19 | 1970 | ⊕⊕⊕⊕ | No study reported the sort of AE that caused withdrawal. In one small study with high risk of bias (Yilmaz 2005, N = 30 participants) no withdrawals occurred. | |
| 56 per 1000 | 11 per 1000 | |||||
| Number of participants achieving 'response' by PGA | Study population | RR 1.76 | 2222 | ⊕⊕⊕⊝ | — | |
| 427 per 1000 | 751 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for inconsistency due to substantial heterogeneity (I² = 81%), may be due to different application frequency in studies (once versus twice daily). 2 Downgraded by one level for inconsistency due to substantial heterogeneity (I² = 77%), may be due to different application frequency in studies (once versus twice daily). | ||||||
| Medical terms | Explanation |
| Apoptosis | The process of programmed cell death, which is the natural developmental and maintenance process of the normal removal of cells |
| Immuno‐suppressive | Specific property of certain medications (e.g. corticosteroids) that diminish the response or activation of the immune system |
| Hyperproliferation | An abnormality associated with a high rate of growth of cells by rapid division |
| Differentiation | The process by which a less specialised cell becomes a more specialised cell type |
| Erythematous | An abnormal redness of the skin caused by various agents, such as sunlight or drugs etc, which irritate and congest the blood capillaries |
| Plaque psoriasis | Patches with the well‐defined characteristic of red, raised skin. They can appear on any skin surface, although the knees, elbows, scalp, trunk and nails are the most common locations. |
| Keratolytic agents | A chemical or physical agent that causes peeling by the softening and shedding of the horny outer layer of the skin |
| Hypervascularisation | A local increase in the formation of blood vessels leading to a higher blood supply of the tissue mainly as a response to the action of the vascular endothelial growth factor (VEGF) |
| Pro‐inflammatory | Provoking inflammation |
| Anti‐inflammatory | Inhibiting or reducing inflammation |
| Keratinocytes | Skin cells forming the superficial layer of the skin (epidermis) |
| Epigenetics | The interactions of specific proteins with the genome that influence the expression of certain genes without changing the genetic sequence |
| PUVA | A photochemotherapy of certain dermatological conditions with psoralen and ultra violet A light (UVA). Usually, psoralen is applied topically on the affected area to increase the sensibility to the UVA light. |
| Rosacea | A chronic skin disease affecting mainly the cheeks, nose, forehead, scalp and ears. On the involved ruddy skin thin superficial blood vessels (teleangiectasia), papules and pustules may appear. In a certain type of rosacea, some facial areas (e.g. nose) enlarge and become bulbous (e.g. rhinophyma). |
| Cytoplasm | The watery fluid (cytosol) inside the cell in which the organelles and the skeleton of the cell are embedded |
| Comparison | Outcome | Sensitivity analysis: ITT population¹ | Sensitivity analysis: allocation concealment² | Meta‐analysis³ |
| Steroid versus the vehicle | IGA: clearance | RR 11.67; 95% CI 4.88 to 27.90 | RR 20.49; 95% CI 2.89 to 145.19 | RR 14.58; 95% CI 7.28 to 29.17 |
| IGA: response | RR 7.67; 95% CI 4.24 to 13.89 | RR 6.11; 95% CI 2.98 to 12.52 | RR 5.24; 95% CI 3.83 to 7.17 | |
| Withdrawal due to AE | RR 0.22; 95% CI 0.08 to 0.61 | RR 0.33; 95% CI 0.01 to 7.76 | RR 0.27; 95% CI 0.11 to 0.67 | |
| Number of participants with at least 1 AE | RR 0.87; 95% CI 0.70 to 1.09 | RR 1.56; 95% CI 0.56 to 4.37 | RR 0.87; 95% CI 0.70 to 1.08 | |
| Steroid versus vitamin D | IGA: clearance | RR 1.81; 95% CI 1.47 to 2.24 | Not applicable | RR 1.82; 95% CI 1.52 to 2.18 |
| Withdrawal due to AE | RR 0.22; 95% CI 0.08 to 0.57 | Not applicable | RR 0.22; 95% CI 0.11 to 0.42 | |
| PGA: clearance | RR 4.00; 95% CI 1.01 to 15.81 | Not applicable | RR 2.22; 95% CI 1.47 to 3.35 | |
| Steroid plus vitamin D versus vitamin D | IGA: clearance | Not applicable | RR 3.55; 95% CI 1.84 to 6.85 | RR 2.28; 95% CI 1.87 to 2.78 |
| IGA: response | Not applicable | RR 3.04; 95% CI 1.99 to 4.66 | RR 2.31; 95% CI 1.75 to 3.04 | |
| Withdrawal due to AE (short‐term) | Not applicable | RR 0.11; 95% CI 0.02 to 0.51 | RR 0.19; 95% CI 0.11 to 0.36 | |
| Number of participants with at least 1 AE (short‐term) | Not applicable | RR 0.61; 95% CI 0.47 to 0.78 | RR 0.70; 95% CI 0.58 to 0.85 | |
| Bold text indicates a meaningful difference between effect estimates. 1Total effect estimate of all studies that provided sufficient information to ensure that ITT analysis was performed. 2Total effect estimate of all studies that provided sufficient information to ensure that allocation concealment was adequately performed. 3Total effect estimate of meta‐analysis that included all relevant studies, regardless if ITT analysis or allocation concealment was performed or not. AE: adverse event | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean score of the IGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Betamethasone valerate 0.12% (hydrophilic leave‐on) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean score of the PGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Betamethasone valerate 0.12% (hydrophilic leave‐on) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 14.58 [7.28, 29.17] |
| 1.1 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 16.80 [2.29, 123.30] |
| 1.2 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 10.15 [3.83, 26.88] |
| 1.3 Clobetasol propionate (hydrophilic leave‐on) | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 22.83 [7.31, 71.30] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 9 | 2114 | Risk Ratio (M‐H, Random, 95% CI) | 5.24 [3.83, 7.17] |
| 2.1 Betamethasone dipropionate (hydrophilic leave‐on) | 2 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 4.06 [2.85, 5.79] |
| 2.2 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 5.19 [2.60, 10.36] |
| 2.3 Betamethasone valerate 0.1% (hydrophilic leave‐on) | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [1.81, 4.85] |
| 2.4 Clobetasol propionate (hydrophilic leave‐on) | 3 | 646 | Risk Ratio (M‐H, Random, 95% CI) | 7.93 [5.46, 11.51] |
| 2.5 Clobetasol propionate (rinse‐off) | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 15.83 [2.23, 112.33] |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Improvement in quality of life Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Scalpdex | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants withdrawing due to adverse events Show forest plot | 4 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.67] |
| 5.1 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.61] |
| 5.2 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.06, 15.53] |
| 5.3 Clobetasol propionate (hydrophilic leave‐on) | 2 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.13] |
| 6 Number of participants achieving 'response' by PGA Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 692 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [2.17, 4.26] |
| 6.2 Betamethasone valerate 0.1% (hydrophilic leave‐on) | 1 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 3.52 [1.97, 6.29] |
| 6.3 Clobetasol propionate (hydrophilic leave‐on) | 2 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 6.92 [4.42, 10.83] |
| 6.4 Clobetasol propionate (rinse‐off) | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 14.35 [2.02, 102.12] |
| 7 Mean score of the PGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of participants with at least one adverse event Show forest plot | 7 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.08] |
| 8.1 Betamethasone valerate 0.1% (hydrophilic leave‐on) | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.01, 5.86] |
| 8.2 Amcinonide 0.1% (hydrophilic leave‐on) | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.32, 5.99] |
| 8.3 Betamethasone dipropionate (hydrophilic leave‐on) | 1 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.69, 1.10] |
| 8.4 Fluocinolone acetonide 0.01% (lipophilic leave‐on) | 1 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.61] |
| 8.5 Clobetasol propionate (hydrophilic leave‐on) | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.56, 4.37] |
| 8.6 Clobetasol propionate (rinse‐off) | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.29, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Vitamin D (hydrophilic leave‐on) | 2 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [1.49, 10.11] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Calcipotriene (hydrophilic leave‐on) | 2 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.20, 3.69] |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Vitamin D (hydrophilic leave‐on) | 3 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.72, 2.83] |
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Calcipotriol (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of participants achieving 'response' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Calcipotriene (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of participants with at least one adverse event Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Vitamin D (hydrophilic leave‐on) | 3 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.08, 2.83] |
| 5 Number of participants achieving 'response' by PGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Betamethasone dipropionate plus calcipotriene (hydrophilic leave‐on) | 2 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.68, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Clobetasol propionate vs betamethasone dipropionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Clobetasol propionate vs betamethasone dipropionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Clobetasol propionate vs betamethasone dipropionate (hydrophilic leave‐ons) | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.32, 2.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 2 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.56, 2.39] |
| 1.1 Betamethasone dipropionate vs hydrocortisone 17‐butyrate 0.1% (hydrophilic leave‐on) | 1 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.93] |
| 1.2 Fluocinolone acetonide 0.025% vs hydrocortisone 17‐butyrate 0.1% (lipophilic leave‐on) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.77, 3.22] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Fluocinonide 0.05% vs desoximetasone 0.05% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants with at least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Mometasone furoate vs triamcinolone acetonide 0.1% (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Mometasone furoate vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Mometasone furoate vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Amcinonide 0.1% vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Betamethasone vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Amcinonide 0.1% vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Betamethasone dipropionate vs fluocinonide (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.52, 2.18] |
| 1.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.31, 2.60] |
| 1.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.46, 2.24] |
| 1.3 Mometasone furoate vs calcipotriol (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.43, 9.32] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 3 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.80, 2.41] |
| 2.1 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.17, 2.74] |
| 2.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.82, 2.50] |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 4 | 2291 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.42] |
| 4.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.04, 0.83] |
| 4.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1666 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.74] |
| 4.3 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] |
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 2 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.47, 3.35] |
| 5.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.36, 3.22] |
| 5.2 Mometasone furoate vs calcipotriol (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [1.01, 15.81] |
| 6 Number of participants achieving 'response' by PGA Show forest plot | 3 | 1827 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.28, 1.72] |
| 6.1 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.02, 2.34] |
| 6.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1676 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.21, 1.80] |
| 7 Number of participants with at least one adverse event Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Betamethasone valerate 1 mg/ml vs calcipotriol (hydrophilic leave‐ons) | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.25, 0.53] |
| 7.2 Betamethasone dipropionate vs vitamin D (hydrophilic leave‐ons) | 2 | 1652 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.97] |
| 7.3 Clobetasol propionate vs calcipotriol (hydrophilic leave‐on vs occlusive lipophilic leave‐on) | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.10, 2.34] |
| 7.4 Clobetasol propionate vs calcipotriol (rinse‐off vs hydrophilic leave‐on) | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.16, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Betamethasone dipropionate plus SA vs betamethasone dipropionate (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Betamethasone dipropionate plus SA vs betamethasone dipropionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 2474 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.08, 1.36] |
| 1.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2444 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.07, 1.36] |
| 1.2 Mometasone furoate plus calcipotriol vs mometasone furoate (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.76, 5.24] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2444 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.25] |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2433 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.42, 1.88] |
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Mometasone furoate plus calcipotriol vs mometasone furoate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of participants achieving 'response' by PGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 2 | 2226 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.06, 1.20] |
| 7 Number of participants with at least one adverse event Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Betamethasone dipropionate plus vitamin D vs betamethasone dipropionate (hydrophilic leave‐ons) | 3 | 2414 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.87, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | 2008 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.87, 2.78] |
| 1.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐ons) | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.83, 2.77] |
| 1.2 Mometasone furoate plus calcipotriol vs calcipotriol (hydrophilic leave‐ons) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [1.01, 15.81] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 4 | 2222 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.75, 3.04] |
| 2.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐on) | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [2.03, 3.22] |
| 2.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) ‐ trial register study | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.43, 2.07] |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events (short‐term) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Betamethasone dipropionate plus vitamin D vs vitamin Dg (hydrophilic leave‐on) | 3 | 1970 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.11, 0.36] |
| 4.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) ‐ trial register study | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.39, 11.07] |
| 5 Number of participants withdrawing due to adverse events (long‐term) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Mometasone furoate plus calcipotriol vs calcipotriol (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of participants achieving 'response' by PGA Show forest plot | 4 | 2222 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.46, 2.12] |
| 7.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐on) | 3 | 1978 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.47, 2.42] |
| 7.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) | 1 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.29, 1.75] |
| 8 Number of participants with at least one adverse event (short‐term) Show forest plot | 4 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.58, 0.85] |
| 8.1 Betamethasone dipropionate plus vitamin D vs vitamin D (hydrophilic leave‐on) | 3 | 1951 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.88] |
| 8.2 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) ‐ trial register study | 1 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.25, 0.76] |
| 9 Number of participants with at least one adverse event (long‐term) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.1 Betamethasone dipropionate plus calcipotriol vs calcipotriol (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tacrolimus vs pine tar (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Dithranol/urea combination vs coal tar plus salicylic acid combination (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants withdrawing due to adverse events Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Calcipotriol vs coal tar/coconut oil/salicylic acid (hydrophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Clobetasol propionate vs tar blend (rinse‐offs) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Cocois vs coal tar (lipophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants achieving 'clearance' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of participants achieving 'response' by PGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of participants with at least one adverse event Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 Calcipotriol vs tar/dithranol combination (hydro/lipophilic leave‐on combination) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Calcipotriol vs coal tar/coconut oil/salicylic acid (hydrophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Clobetasol propionate vs tar blend (rinse‐offs) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Tacrolimus vs pine tar (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Dithranol/urea combination vs coal tar plus salicylic acid combination (lipophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Cocois vs coal tar (lipophilic leave‐on vs rinse‐off) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Betameth diprop (BP) + RV3423A shampoo/shampoo vs BP + shampoo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Betamethasone valerate 0.1%: foam vs lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clobetasol propionate: foam vs solution | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean of the TSS Show forest plot | Other data | No numeric data | ||
| 4 Number of participants achieving 'response' by PGA Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Betamethasone valerate 0.1%: foam vs lotion | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Clobetasol propionate: foam vs solution | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of participants with at least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Clobetasol: shampoo vs hydrophilic leave‐on | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants achieving 'clearance' by IGA Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Betamethasone dipropionate (BDP) plus SA vs triamcinolone acetonide 0.2% plus SA (hydrophilic leave‐ons) | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.47, 4.74] |
| 1.2 Betamethasone dipropionate (BDP) plus SA vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.59, 3.32] |
| 1.3 Betamethasone dipropionate (BDP) plus SA vs clobetasol propionate (hydrophilic leave‐ons) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.97, 2.01] |
| 1.4 Betamethasone dipropionate (BDP) vs triamcinolone acetonide 0.2% plus SA (hydrophilic leave‐on) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [1.23, 4.16] |
| 2 Number of participants achieving 'response' by IGA Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Betameth diprop plus SA vs triamcin acet 0.2% plus SA (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Betameth diprop vs triamcin acet 0.2% plus SA (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of participants withdrawing due to adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Betamethasone dipropionate plus SA vs clobetasol propionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of participants with at least one adverse event Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Betamethasone dipropionate plus SA vs betamethasone valerate 0.1% (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Betamethasone dipropionate plus SA vs clobetasol propionate (hydrophilic leave‐ons) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean score of the IGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Number of participants withdrawing due to adverse events Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Ciclopirox olamine (hydrophilic leave‐on) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mean score of the PGA Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Number of participants with at least one adverse event Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Ciclopirox olamine (hydrophilic leave‐on) vs placebo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

