Le topiramate pour les tremblements essentiels
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised parallel, double‐blind, placebo‐controlled study. | |
| Participants | 24 participants randomised (12 topiramate; 12 placebo). Topiramate group: baseline TRS 30.2 (SD 10.2). Placebo group: baseline TRS 24.0 (SD 11.6). | |
| Interventions | Topiramate 50 mg/day to 200 mg/day; titration 25 mg/week. Placebo. Follow‐up: 2 weeks. | |
| Outcomes | TRS total score. | |
| Notes | General and clinical characteristics of participants not reported, mean dose of treatment not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not used. |
| Allocation concealment (selection bias) | High risk | No information regarding how the treatment was supplied and how participants were allocated. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Personnel administering the treatment unaware of the content of the containers. No information detailing blinding methods for participants. |
| Incomplete outcome data (attrition bias) | Unclear risk | No information regarding treatment discontinuation. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | High risk | The study did not provide information about the general characteristics of the population and there was uncertainty regarding the comparability of the 2 groups. The short duration of the follow‐up may have influenced the outcomes assessments. |
| Methods | 3 pooled, double‐blind, placebo‐controlled cross‐over studies. | |
| Participants | 62 participants randomised (30 topiramate; 32 placebo). Topiramate group: mean age 67 (SD 12) years; men 50%; co‐therapy 83%; baseline TRS 36.8 (SD 17.8). Placebo group: mean age 57 (SD 15) years; men 44%; co‐therapy 72%; baseline TRS 40.0 (SD 18.9). | |
| Interventions | Topiramate 25 mg to 400 mg (titration 8 weeks); mean dose: 292 mg/day. Placebo. Follow‐up: 25 weeks. | |
| Outcomes | TRS total score. | |
| Notes | Duration of disease was not reported; 77% of participants received concomitant anti‐tremor therapy (mainly beta‐blockers). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) | Low risk | "All clinical assessments were made by a rater blinded to the treatment assignment". |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial was described as a "double blind" study but the blinding of participants and personnel was not specified. |
| Incomplete outcome data (attrition bias) | High risk | Outcome data missed for 13 (43%) participants in the topiramate group and 8 (25%) participants in the placebo group and reasons for missing outcomes differed (more participants with adverse events in the topiramate group). Authors imputed missing outcome data with the use of baseline observation carried forward. |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | High risk | The study was sponsored by, and had at least 1 author affiliated to, the pharmaceutical company of topiramate. |
| Methods | Randomised parallel, multicentre, double‐blind, placebo‐controlled study. | |
| Participants | 223 participants randomised (117 topiramate; 106 placebo). Topiramate group: mean age 61 years (SD 13); men 54%; duration of tremor 24 years (SD 17); co‐therapy 46%; baseline TRS 38.7 (SD 12.4). Placebo group: mean age 64 years (SD 13); men 57%; duration of tremor 22 years (SD 18); co‐therapy 55%; baseline TRS 37.3 (SD 12.0). | |
| Interventions | Topiramate 25 mg to 400 mg (titration 3 months); mean dose: 215 mg/day. Placebo. Follow‐up 6 months. | |
| Outcomes | TRS total score and subscales (severity, motor tasks and functional disability). | |
| Notes | Exclusion criteria: history of discontinuing topiramate due to adverse events. 43.5% of participants in the topiramate group and 41% in the placebo group received co‐therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized random number generator"; "Randomisations was one to one to topiramate or placebo with permuted block size of four". |
| Allocation concealment (selection bias) | Unclear risk | Study medication supplied in identical containers marked with unique code numbers. |
| Blinding of outcome assessment (detection bias) | Low risk | "Investigators who were performing TRS assessment were blinded to all other clinical assessments including medication adverse events". |
| Blinding of participants and personnel (performance bias) | Low risk | "Study medication was supplied as identically appearing 25 or 100 mg topiramate tablets or matching placebo in identical containers marked with unique code numbers prepared by the study sponsor". "The randomisation code was not revealed to patients, investigators, clinical staff, study monitor or study sponsor, personnel until the database was finalized". |
| Incomplete outcome data (attrition bias) | High risk | 45 (38.5%) participants in the topiramate group and 23 (22%) in the placebo group discontinued before study completion. Reasons for missing data different across groups (more participants with adverse events in the topiramate group). The intention‐to‐treat population defined as "all randomised patients who took at least one dose of study medication and had at least one on‐treatment efficacy assessment". |
| Selective reporting (reporting bias) | Unclear risk | Not specified. |
| Other bias | High risk | The study was sponsored by, and had at least 1 author affiliated to, the pharmaceutical company of topiramate. |
SD: standard deviation; TRS: Tremor Rating Scale.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Case series. | |
| Case series. | |
| Case series. | |
| Case series. | |
| Case report of combined therapy with topiramate and declorazepam. | |
| Case series. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional disability component related to tremor Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 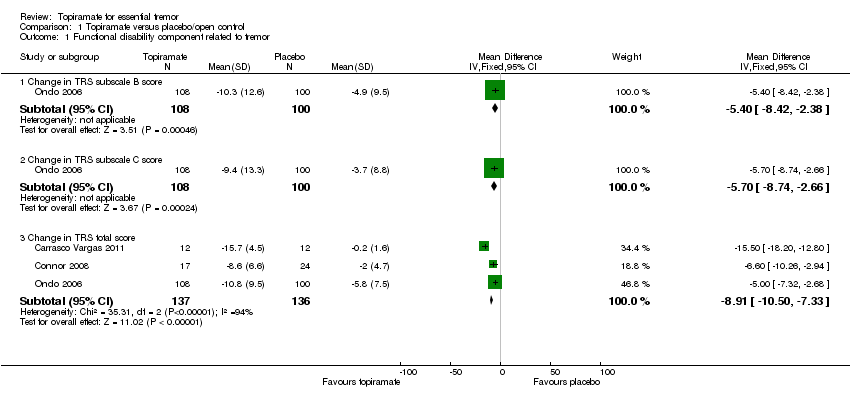 Comparison 1 Topiramate versus placebo/open control, Outcome 1 Functional disability component related to tremor. | ||||
| 1.1 Change in TRS subscale B score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.4 [‐8.42, ‐2.38] |
| 1.2 Change in TRS subscale C score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐8.74, ‐2.66] |
| 1.3 Change in TRS total score | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐8.91 [‐10.50, ‐7.33] |
| 2 Withdrawals Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Topiramate versus placebo/open control, Outcome 2 Withdrawals. | ||||
| 2.1 Withdrawals: lack of efficacy | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.53] |
| 2.2 Withdrawals: AEs | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.79, 5.63] |
| 2.3 Withdrawals: other reasons | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.45, 2.05] |
| 2.4 Withdrawals: total | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.60] |

Study flow diagram.
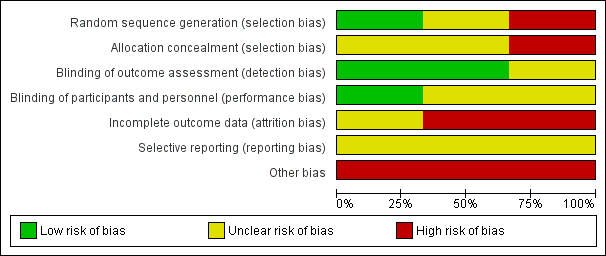
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.1 Functional disability component related to tremor.

Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.2 Withdrawals.

Comparison 1 Topiramate versus placebo/open control, Outcome 1 Functional disability component related to tremor.

Comparison 1 Topiramate versus placebo/open control, Outcome 2 Withdrawals.
| Topiramate for essential tremor | ||||||
| Patient or population: people with essential tremor Settings: outpatients Intervention: topiramate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topiramate | |||||
| Functional disability (follow‐up duration 24 weeks) TRS subscale B score (motor tasks) TRS subscale C score (functional disability) | The mean improvement in the control group was | The mean improvement in the intervention groups was | ‐ | 223 | ⊕⊝⊝⊝ | ‐ |
| Study withdrawal (follow‐up duration 10 to 24 weeks) Number of participants withdrawn from the study | Study population | RR 1.78 | 285 | ⊕⊕⊝⊝ | ‐ | |
| 217 per 1000 | 347 per 1000 | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Adverse events (follow‐up duration 24 weeks) Number of AEs | Study population | ‐ | 221 | ⊕⊕⊝⊝ | ‐ | |
| 71 AEs per 105 participants | 195 AEs per 116 participants | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; RR: risk ratio; TRS: Tremor Rating Scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to serious risk of bias: high number of study withdrawals (attrition bias); blinding of outcome assessment probably revealed by the presence of serious AEs in the topiramate group: trials should be regarded as single blind (detection bias); potential conflicts of interest due to the presence of authors sponsored by pharmaceutical companies. 3 Downgraded due to imprecision: small sample size (< 300 participants) and small number of included studies (three). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional disability component related to tremor Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Change in TRS subscale B score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.4 [‐8.42, ‐2.38] |
| 1.2 Change in TRS subscale C score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐8.74, ‐2.66] |
| 1.3 Change in TRS total score | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐8.91 [‐10.50, ‐7.33] |
| 2 Withdrawals Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Withdrawals: lack of efficacy | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.53] |
| 2.2 Withdrawals: AEs | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.79, 5.63] |
| 2.3 Withdrawals: other reasons | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.45, 2.05] |
| 2.4 Withdrawals: total | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.60] |

