Topiramato para el temblor esencial
Información
- DOI:
- https://doi.org/10.1002/14651858.CD009683.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Trastornos del movimiento
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
EB: protocol and review editing, literature searching, study selection, quality assessment, data extraction.
AN: protocol and review editing.
GQ: literature searching, quality assessment, data extraction.
RA: protocol editing, literature searching, study selection, quality assessment, data extraction.
GF: protocol editing, editing and revising the review.
CC: protocol editing, quality assessment, study selection.
MZ: protocol editing, revising review.
Sources of support
Internal sources
-
None, Not specified.
External sources
-
None, Not specified.
Declarations of interest
EB: none.
AN: none.
GQ: none.
RA: none.
GF: none.
CC: none.
MZ: none.
Acknowledgements
The authors would like to express their gratitude to the Editors of the Movement disorders Group for the comments and guidance provided during the development of the final version of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 14 | Topiramate for essential tremor | Review | Elisa Bruno, Alessandra Nicoletti, Graziella Quattrocchi, Roberta Allegra, Graziella Filippini, Carlo Colosimo, Mario Zappia | |
| 2012 Mar 14 | Topiramate for essential tremor | Protocol | Elisa Bruno, Alessandra Nicoletti, Graziella Quattrocchi, Roberta Allegra, Carlo Colosimo, Graziella Filippini, Mario Zappia | |
Differences between protocol and review
In an attempt to provide a standardised and reliable assessment of the quality of the evidence of the study outcomes, we decided to use the GRADE evidence profile, a systematic and explicit system for grading the evidence into four quality categories. We reported the results obtained through this approach in a 'Summary of findings' table”.
Methods for future updates
Two pre‐planned analysis were not performed due to insufficient data. These will be implemented, if possible, in future updates of the review.
Methods for analysing continuous data: the scales used to assess tremor in the majority of the studies are continuous. We will transform ordinal scales with enough categories to continuous scales by assigning a score to each grade so that we can express the intervention effect as a difference in means or as a standardised mean difference. In the case of an ordinal scale with few categories, we will combine data from adjacent categories into two categories, and use methods for binary data as risk ratios (RR) or risk differences (RD) to evaluate the intervention effect.
Sensitivity analysis: we will undertake sensitivity analyses to assess the robustness of results to fixed‐effect versus random‐effects assumptions, and the inclusion or exclusion of studies at high risk of bias (i.e. inadequate allocation concealment and lack of blinded outcome assessor). We will use best‐ and worst‐case scenarios for taking into account missing data.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Activities of Daily Living;
- Anticonvulsants [adverse effects, *therapeutic use];
- Essential Tremor [*drug therapy];
- Fructose [adverse effects, *analogs & derivatives, therapeutic use];
- Patient Dropouts [statistics & numerical data];
- Publication Bias;
- Randomized Controlled Trials as Topic;
- Topiramate;
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
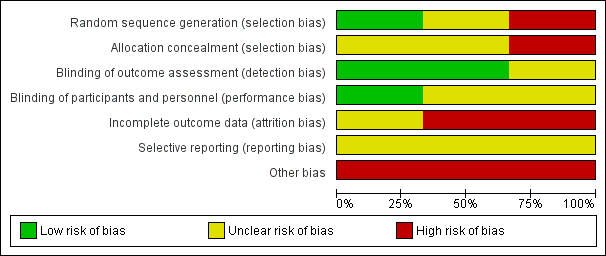
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.1 Functional disability component related to tremor.

Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.2 Withdrawals.

Comparison 1 Topiramate versus placebo/open control, Outcome 1 Functional disability component related to tremor.

Comparison 1 Topiramate versus placebo/open control, Outcome 2 Withdrawals.
| Topiramate for essential tremor | ||||||
| Patient or population: people with essential tremor Settings: outpatients Intervention: topiramate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topiramate | |||||
| Functional disability (follow‐up duration 24 weeks) TRS subscale B score (motor tasks) TRS subscale C score (functional disability) | The mean improvement in the control group was | The mean improvement in the intervention groups was | ‐ | 223 | ⊕⊝⊝⊝ | ‐ |
| Study withdrawal (follow‐up duration 10 to 24 weeks) Number of participants withdrawn from the study | Study population | RR 1.78 | 285 | ⊕⊕⊝⊝ | ‐ | |
| 217 per 1000 | 347 per 1000 | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Adverse events (follow‐up duration 24 weeks) Number of AEs | Study population | ‐ | 221 | ⊕⊕⊝⊝ | ‐ | |
| 71 AEs per 105 participants | 195 AEs per 116 participants | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; RR: risk ratio; TRS: Tremor Rating Scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to serious risk of bias: high number of study withdrawals (attrition bias); blinding of outcome assessment probably revealed by the presence of serious AEs in the topiramate group: trials should be regarded as single blind (detection bias); potential conflicts of interest due to the presence of authors sponsored by pharmaceutical companies. 3 Downgraded due to imprecision: small sample size (< 300 participants) and small number of included studies (three). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional disability component related to tremor Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Change in TRS subscale B score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.4 [‐8.42, ‐2.38] |
| 1.2 Change in TRS subscale C score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐8.74, ‐2.66] |
| 1.3 Change in TRS total score | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐8.91 [‐10.50, ‐7.33] |
| 2 Withdrawals Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Withdrawals: lack of efficacy | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.53] |
| 2.2 Withdrawals: AEs | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.79, 5.63] |
| 2.3 Withdrawals: other reasons | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.45, 2.05] |
| 2.4 Withdrawals: total | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.60] |

