نقش توپیرامات در درمان ترمور اساسی
چکیده
پیشینه
ترمور اساسی (essential tremor; ET) یکی از شایعترین اختلالات حرکتی است. مدیریت درمانی آن عمدتا بر عوامل دارویی بنا شده و در بالین، پروپرانولول (propranolol) و پریمیدون (primidone) به عنوان درمان خط اول در نظر گرفته میشوند. با این حال، این درمانها در 25% تا 55% افراد غیر‐موثر بوده و اغلب با عوارض جانبی (AE) جدی همراه هستند. به همین دلایل، ارزیابی دیگر روشهای درمانی ET ارزشمند است. توپیرامات (topiramate) به عنوان یک عامل بالقوه مفید در درمان ET شناخته شده، اما عدم‐قطعیت در مورد اثربخشی و بیخطری (safety) آن وجود دارد.
اهداف
ارزیابی اثربخشی و بیخطری توپیرامات در درمان ET.
روشهای جستوجو
جستوجوی سیستماتیک و بدون محدودیت زبانی را برای شناسایی تمام کارآزماییهای مرتبط در پایگاه مرکزی ثبت کارآزماییهای کنترل شده کاکرین (CENTRAL)، MEDLINE (ژانویه 1966 تا ژانویه 2017)، Embase (ژانویه 1988 تا ژانویه 2017)، موسسه ملی سلامت و تعالی مراقبت (National Institute for Health and Care Excellence; NICE) (1999 تا ژانویه 2017)، ClinicalTrials.gov (1997 تا ژانویه 2017) و پلتفرم بینالمللی پایگاه ثبت کارآزماییهای بالینی سازمان جهانی بهداشت (ICTRP؛ 2004 تا ژانویه 2017) انجام دادیم. BIOSIS Citation Index (2000 تا ژانویه 2017) را برای یافتن خلاصه مقالات کنفرانسها جستوجو کردیم. منابع علمی خاکستری و فهرست منابع مطالعات و مرورهای شناسایی شده را به صورت دستی جستوجو کردیم.
معیارهای انتخاب
همه کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) را از مقایسه توپیرامات در مقابل دارونما (placebo)/ کنترل باز یا هر نوع درمان دیگری وارد کردیم. مطالعاتی را وارد کردیم که تشخیص ET بر اساس معیارهای تشخیصی پذیرفته شده و معتبر گذاشته شد. مطالعاتی را خارج کردیم که در افراد مبتلا به فرمهای ثانویه ترمور انجام شده یا فقط از پارامترهای نوروفیزیولوژیکی برای ارزیابی پیامدها استفاده کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم با استفاده از فرم جمعآوری دادهها، آنها را جمعآوری و استخراج کردند. خطر سوگیری (bias) و کیفیت شواهد را ارزیابی کردیم. از یک متاآنالیز اثر‐ثابت برای تجمیع دادهها استفاده کردیم.
نتایج اصلی
این مرور شامل سه کارآزمایی بود که به مقایسه توپیرامات با دارونما (309 شرکتکننده) پرداختند. همه آنها در معرض خطر بالای سوگیری قرار داشتند. کیفیت شواهد در محدوده بسیار پائین و پائین قرار داشت. شرکتکنندگان تحت درمان با توپیرامات در مقایسه با دارونما، بهبودی قابلتوجهی را در ناتوانی عملکردی و افزایش خطر خروج از مطالعه نشان دادند (خطر نسبی (RR): 1.78؛ %95 فاصله اطمینان (CI): 1.23 تا 2.60). بروز عوارض جانبی بیشتری در شرکتکنندگان درمان شده با توپیرامات گزارش شد، بهخصوص پاراستزی، کاهش وزن، کاهش اشتها و مشکل حافظه.
نتیجهگیریهای نویسندگان
این مرور سیستماتیک وجود دادههای محدود و شواهدی را با کیفیت بسیار پائین تا پائین برای حمایت از اثربخشی واضح مداخله و وقوع عوارض جانبی محدودکننده درمان را در افراد مبتلا به ET درمان شده با توپیرامات برجسته کرد. انجام تحقیقات بیشتری برای ارزیابی اثربخشی و بیخطری توپیرامات در درمان ET نیاز است.
PICOs
خلاصه به زبان ساده
استفاده از توپیرامات در درمان ترمور اساسی
سوال مطالعه مروری
نویسندگان این مرور تلاش کردند تا اثربخشی و بیخطری (safety) توپیرامات را در افراد مبتلا به ترمور اساسی ارزیابی کنند.
پیشینه
ترمور اساسی نوعی تکان خوردن یا لرزش غیر‐قابل کنترل قسمتهای بدن است. اگرچه این وضعیت تاثیر منفی بر طول عمر ندارد، بهطور کلی پیشرونده بوده و ممکن است ناتوانکننده شود. درمان معمولا با دارو (پروپرانولول و پریمیدون) صورت میگیرد، که ممکن است در یک‐چهارم تا نیمی از افراد مبتلا به ترمور اساسی تاثیری نداشته باشد. برخی از متخصصان پیشنهاد کردهاند که داروی دیگر، توپیرامات، ممکن است برای درمان این وضعیت مفید باشد.
ویژگیهای مطالعه
بانکهای اطلاعاتی پزشکی را برای یافتن مطالعاتی جستوجو کردیم که توپیرامات را با دارونما (placebo) (قرص ساختگی) یا هر داروی دیگری در بزرگسالان مبتلا به ترمور اساسی مقایسه کردند. سه مطالعه را برای مقایسه توپیرامات و دارونما یافتیم که شامل 309 شرکت کننده مبتلا به ترمور اساسی متوسط تا شدید بودند.
نتایج کلیدی
به دلیل کیفیت شواهد بسیار پائین، تاثیر توپیرامات بر فعالیتهای روزانه، خطر توقف درمان و عوارض جانبی نامشخص بود.
کیفیت شواهد
فقط چند مطالعه را یافتیم که روشهای انجام آنها مشکل داشتند، بنابراین نمیتوانیم نتیجهگیری دقیقی در این مورد داشته باشیم که توپیرامات چه عملکردی داشته و آیا عوارض جانبی ایجاد میکند یا خیر.
Authors' conclusions
Summary of findings
| Topiramate for essential tremor | ||||||
| Patient or population: people with essential tremor Settings: outpatients Intervention: topiramate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topiramate | |||||
| Functional disability (follow‐up duration 24 weeks) TRS subscale B score (motor tasks) TRS subscale C score (functional disability) | The mean improvement in the control group was | The mean improvement in the intervention groups was | ‐ | 223 | ⊕⊝⊝⊝ | ‐ |
| Study withdrawal (follow‐up duration 10 to 24 weeks) Number of participants withdrawn from the study | Study population | RR 1.78 | 285 | ⊕⊕⊝⊝ | ‐ | |
| 217 per 1000 | 347 per 1000 | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Adverse events (follow‐up duration 24 weeks) Number of AEs | Study population | ‐ | 221 | ⊕⊕⊝⊝ | ‐ | |
| 71 AEs per 105 participants | 195 AEs per 116 participants | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; RR: risk ratio; TRS: Tremor Rating Scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to serious risk of bias: high number of study withdrawals (attrition bias); blinding of outcome assessment probably revealed by the presence of serious AEs in the topiramate group: trials should be regarded as single blind (detection bias); potential conflicts of interest due to the presence of authors sponsored by pharmaceutical companies. 3 Downgraded due to imprecision: small sample size (< 300 participants) and small number of included studies (three). | ||||||
Background
Description of the condition
Essential tremor (ET) is one of the most common movement disorders, with an overall estimated prevalence ranging from 0.9% to 2.2%, even higher among people over 65 years of age (4.6%) (Louis 2010).
It is characterised by postural and kinetic tremor involving the arms and less commonly the head, lower limbs and voice (Louis 2005), frequently accompanied by a family history of a similar tremor. However, ET is a heterogeneous disorder and there is little agreement among specialists regarding both clinical definition and diagnostic criteria (Jankovic 2002). Although benign in term of its effect on life expectancy, it often causes embarrassment and, in a small percentage of people, also serious disability (Busenbark 1991; Koller 1986). Moreover, symptoms are typically progressive and potentially disabling, sometimes forcing people to change job or seek early retirement (Deuschl 2000). The treatment is primarily based on pharmacological agents, although surgical intervention may be an option in the most disabling cases. Pharmacotherapy may be used to improve function or reduce the embarrassment associated with ET, but treatment should be tailored to the person's level of disability. Although propranolol and primidone are well‐established agents used in clinical practice for the treatment of ET, additional medications may be useful in reducing tremor (Sullivan 2004). In fact, even though it has been reported that both propranolol and primidone improve tremor in about two‐thirds of people (Koller 1989; Wasielewski 1998), these agents tend to lose efficacy over time (Louis 2001a). In addition, their use is limited, particularly among elderly people (aged over 70 years) (Zesiewicz 2002), due to adverse effects (AEs) and their interactions with medications commonly used in these people (e.g. digoxin, calcium channel blockers and antiarrhythmics) (Hansten 2004). Anticonvulsants have been suggested as potentially useful agents for the treatment of ET and they are usually well tolerated (Ondo 2000; Ondo 2006; Pahwa 1998; Zesiewicz 2007a).
Description of the intervention
Topiramate is a sulphamate substituted monosaccharide, derived from D‐fructose. The pharmacological potential of topiramate is the result of multiple mechanisms of action: it inhibits sodium conductance in neural membranes, enhances the α1‐subunit of the gamma‐aminobutyric acid (GABA‐A) receptor activity, increases GABA‐mediated chloride influx into neurons, and reduces the activity of L‐type high voltage‐activated calcium channels and of the glutamate receptor amino‐3‐hydroxy‐5‐methylisoxazole‐4‐proprionic acid (AMPA) subtype. Topiramate is rapidly absorbed orally, with a bioavailability approaching 100%. The time to peak blood levels is about two hours and it is widely distributed in tissues, including the brain. It is metabolised in the liver by the P450 microsomal enzymes, and eliminated via renal mechanism, with a plasma elimination half‐life between 19 and 25 hours, independently of dose. Despite being a mild inhibitor of P450 isoenzyme CYP2C19, in practice topiramate does not affect the steady state of other drugs (Shorvon 2005).
How the intervention might work
ET may be caused by a deficiency in the GABA‐A receptor, as demonstrated in a knockout model in mice (Kralic 2005). This mechanism suggests that the GABAergic system could be a potential target for pharmacotherapy, and that GABA‐A receptor agonists may be effective in ET (Kralic 2005; Pahwa 2003). In fact, considering their mechanisms of action, all anticonvulsants that enhance GABAergic neurotransmission could be effective in the treatment of ET. A positive effect of alprazolam (Huber 1988), clonazepam (Thompson 1984), and zonisamide (Morita 2005) on ET, due to enhancement of GABA release, downregulation of the number of GABA reuptake transporter proteins and upregulation of the glutamate transporter EAAC‐1, has been reported (Bialer 2004; Ueda 2003; Yoshida 2005). Gabapentin, which may also facilitate GABAergic function, has also been used for ET (Ondo 2000). With regards to topiramate, several studies showed that its multiple pharmacological activities may contribute to a neurostabilising effect (White 2003), including enhancement of GABA activity, modulation of GABA‐A receptors (White 2005), carbonic anhydrase inhibition, antagonism of AMPA receptor activity and blockage of voltage‐dependent calcium and sodium channels (Shank 2000).
Why it is important to do this review
In 2005, the American Academy of Neurology published the practice parameter for ET (Zesiewicz 2005), basing the recommendation on an arbitrary four‐tiered level of evidence scheme and concluding that propranolol and primidone should be used as first‐line therapy. Topiramate was considered probably effective in reducing tremor, receiving a level B recommendation. In one update, the conclusion on these treatments appeared unchanged (Zesiewicz 2011). Another publication based on the use of GRADE system for assessing the quality of evidence and the strength of recommendations, concluded that the potential role of topiramate among first‐line treatments should be considered, assigning strong recommendation with moderate quality of evidence (Zappia 2013). As primidone or propranolol administration may be limited due to the risk of serious AEs, and as these agents could lose their efficacy in long‐term therapy, it may be worthwhile evaluating treatment alternatives for ET. As there is uncertainty about the efficacy of topiramate, a systematic review aimed at evaluating whether this agent could be an effective alternative for people with ET may generate clinically useful information.
Objectives
To assess the efficacy and safety of topiramate in the treatment of ET.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), both parallel group and cross‐over designs.
Types of participants
Adults (aged 16 years or older) with ET diagnosed according to the criteria proposed by the Tremor Investigation Group (Bain 2000a), the Consensus Statement of the Movement Disorder Society on Tremor (Deuschl 1998), or previous accepted and validated clinical criteria (Chouinard 1997; Haerer 1992; Louis 1998; Rajput 1984; Salemi 1994; Snow 1989).
We excluded from our review studies considering participants with a secondary form of tremor (e.g. thyroid disease).
Types of interventions
Topiramate for ET compared to: placebo/open control; any other pharmacological treatment, by any dose or route of administration.
Types of outcome measures
We excluded studies that reported only neurophysiological parameters (e.g. electromyographic recordings, accelerometry, spirography, digitising tablets) to assess outcomes. These instrumental tests have important limitations since their accuracy and reproducibility are not well established. Moreover, neurophysiological measures are only weakly correlated with a person's functional disability (Bain 1997; Bain 2000b).
Primary outcomes
-
Functional disability component related to tremor, measured by the Fahn‐Tolosa‐Marin Tremor Rating Scale (TRS) subscales B and C (Fahn 1993) at the end of follow‐up.
The TRS assesses rest, postural and action tremor. TRS total score is derived from three TRS subscales:
-
-
examiner‐reported upper limb postural and action tremor severity (amplitude), four elements;
-
examiner‐reported ability to perform specific motor tasks (writing, drawing and pouring with dominant and non‐dominant hands), nine elements;
-
participant‐reported functional disabilities due to tremor (eating, speaking, drinking, hygiene, dressing, writing, working and social activities), eight elements.
-
Each subscale element is rated from 0 to 4 (none to severe tremor) giving a maximum score of 16, 36 and 32 for each subscale. The overall TRS score is the sum of individual elements calculated as a fraction of the subscale's maximum score and converted to a 100‐point scale (0 to 100).
We considered other validated scales to assess and measure tremor severity, such as the Unified Tremor Rating Scale (UTRA) (Findley 1995; Jankovic 1996), the Bain scale (Bain 1998), and the Washington Heights‐Inwood Genetic Study of Essential Tremor (WHIGET) rating scale (Louis 2001b).
-
Withdrawal, defined in a standard manner, and number of AEs associated with treatment.
To analyse the main causes of study withdrawal, we grouped participant's reason of discontinuation using the following categories: lack of efficacy, AEs, other reasons (including participant choice, lost to follow‐up, non‐compliance and unknown reasons). We also grouped AEs considering the symptom presented: paraesthesia, weight loss, nausea, fatigue, appetite decrease, memory difficulty, dizziness, diarrhoea and headache.
Secondary outcomes
-
Tremor severity, measured by:
-
-
Fahn‐Tolosa‐Marin TRS subscale A and total score;
-
participant self‐rated severity score: Patient Global Impression (PGI);
-
clinician‐rated global score: Clinical Global Impression (CGI).
-
-
Quality of life (QoL) measured by:
-
-
a validated QoL scale or questionnaire: 36‐item Short Form (SF‐36), EuroQol.
-
Search methods for identification of studies
We carried out a systematic search with no language restrictions to identify all relevant published and unpublished RCTs.
Electronic searches
We searched the following databases for relevant trials.
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 1).
-
MEDLINE (January 1966 to January 2017).
-
Embase (January 1988 to January 2017).
-
National Institute for Health and Care Excellence (NICE) (1999 to January 2017).
-
ClinicalTrials.gov (1997 to January 2017).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (2004 to January 2017).
We additionally searched BIOSIS Citation Index (2000 to January 2017) for conference proceedings.
We based the search strategies for each database on the strategy developed for MEDLINE, revising it appropriately for each database to take into account the differences in controlled vocabulary and syntax rules. See Appendix 1 and Appendix 2.
Searching other resources
In addition to the electronic searches, we:
-
handsearched reference lists of all available review articles and primary studies;
-
handsearched the references quoted in the most recent abstract books of European Federation of Neurological Societies (2005 to 2016), American Academy of Neurology (2003 to 2016), American Neurological Association (2006 to 2016), World Federation of Neurology (2008 to 2016) and of The Movement Disorder Society (2003 to 2016);
-
contacted the corresponding authors of relevant trials;
-
contacted drug manufacturers for information on ongoing trials.
Data collection and analysis
Two review authors (EB and RA) independently assessed the titles and abstracts of all the studies identified by the electronic searching or handsearching. We obtained the full text of potentially relevant trials.
Selection of studies
After reading the abstracts, two review authors (EB and RA) independently selected the eligible articles and scrutinised the full texts of the selected studies and decided which trials met the inclusion criteria for this review. We resolved any disagreements concerning inclusion and exclusion of trials by discussion.
Data extraction and management
Two review authors (EB and RA) independently extracted the following data, using a data collecting form:
-
trial design;
-
randomisation methods;
-
allocation concealment;
-
blinding of treatments and assessments;
-
comparability of treatment groups in terms of demographic and clinical characteristics;
-
inclusion and exclusion criteria;
-
duration of treatment;
-
length of follow‐up;
-
outcome measures (use of validated scales);
-
number of withdrawals and respective causes;
-
description of AEs.
We resolved disagreements on extracted data by discussion.
Assessment of risk of bias in included studies
The review authors independently judged trial quality according to the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We considered seven specific domains:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessment;
-
incomplete outcome data;
-
selective reporting;
-
other sources of bias.
Two review authors (EB and RA) independently assessed the risk of bias of each included study and resolved any disagreements by discussion to reach consensus. The overall assessment of risk of bias was based on recommendations reported in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If one or more domains were assessed at high risk of bias, we rated the overall score as high. If all domains were rated as having a low risk of bias, the overall score was considered low. All the other combinations were rated as unclear overall risk of bias.
We considered the risk of bias in included studies in the interpretation of primary outcome results using the GRADE approach. We examined consistency, directness and precision to grade the quality of evidence according to GRADE guidelines (GRADEpro). We rated overall quality of evidence as 'high', 'moderate', 'low' and 'very low'. Through the GRADE approach, RCTs were assigned an initial high rating that was subsequently modified by the sequential judgement of limitations, inconsistency of the results, indirectness of the evidence, imprecision of data and presence of publication bias. The primary outcomes considered were functional disability related to tremor, withdrawals and number of AEs. Two review authors (EB and RA) independently graded the body of evidence using GRADE guidance and resolving discrepancies through discussion aimed at achieving consensus.
Measures of treatment effect
We analysed measurement scales to assess ET as continuous variables. We calculated and expressed the intervention effect as mean differences (MD) and standard deviations (SD) for individual studies and for the pooled estimates with 95% confidence intervals (CI). We used changes from baseline for all continuous variables.
We expressed categorical variables (number of withdrawals and number of AEs) as frequencies and percentages.
Unit of analysis issues
To avoid the 'carry‐over' effect that can induce alteration of the response to subsequent treatment in cross‐over studies (Sibbald 1998), we used only data from the first treatment phase after randomisation.
Dealing with missing data
To estimate the effect of participant withdrawals or loss to follow‐up on primary outcomes, we extracted available information about incomplete data and about the intention‐to‐treat analysis performed. We only included data for participants whose results were reported. We calculated the frequency of withdrawals for each treatment group for separate and pooled studies. We considered the impact of missing data during the assessment of risk of bias.
Assessment of reporting biases
We assessed reporting biases concerning both primary and secondary outcomes. We planned to assess reporting biases comparing the outcomes reported in the study protocol with the outcomes in the published study. If the study protocol was not available, we compared the outcomes listed or mentioned in the methods section of the article with those whose results were reported.
Data synthesis
Within the different comparisons, we calculated MD and SD to assess efficacy and frequencies and percentages for withdrawals and AEs with 95% CI.
Provided that, for each comparison, an outcome of interest was reported by at least two included studies, we combined data in a meta‐analysis. There were no restrictions based on risk of bias. We used a fixed‐effect model to combine data.
We used inverse variance methods for continuous outcomes and measurement scales. Difference between treatment groups was compared as MD. For dichotomous outcomes (withdrawals, AEs), we expressed the effect of intervention as risk ratio (RR). We also used risk differences (RDs) to compare treatment groups. We estimated pooled RR using the Mantel‐Haenszel method. We used Review Manager 5 software for data management and analysis (RevMan 2014). We reported 95% CI.
Subgroup analysis and investigation of heterogeneity
We planned to investigate heterogeneity by conducting subgroup analyses based on prespecified study characteristics. We planned to investigate potential positive or negative interactions between topiramate and other anti‐tremor medications on primary outcomes, performing a subgroup analysis of trials in which only the experimental anti‐tremor medication was allowed (topiramate/placebo/other treatment) and of trials including participants using other concomitant anti‐tremor medications during the study period. For trials in which treatment effects were reported for more than one dose, we planned to investigate the effect of the different doses reported separately. We assessed heterogeneity using the I2 statistic (Higgins 2003).
Sensitivity analysis
We planned to perform sensitivity analyses to investigate the effect of inclusion or exclusion of studies at high risk of bias, by removing single trials at high risk of bias. We planned to use best‐ and worst‐case scenarios for taking into account missing binary data.
Results
Description of studies
Results of the search
The search of electronic databases retrieved 37 references. We identified one additional study as a result of screening reference lists of review articles. We excluded 26 studies after reading titles and abstracts: 16 were published as review articles, six references were duplicates, three were observational studies and one included people with neuropsychiatric problems. We selected 12 studies aimed at evaluating topiramate treatment for ET and obtained the full text.
Figure 1 shows a flowchart of the results of the electronic search.

Study flow diagram.
Included studies
Three studies were eligible for this review (Carrasco Vargas 2011; Connor 2008; Ondo 2006) (see Characteristics of included studies table).
Trial design
Ondo 2006 and Carrasco Vargas 2011 were double‐blind parallel RCTs; Connor 2008 reported pooled data from three cross‐over studies (Connor 2002, and Edwards K and Tarsy D, unpublished data) that "featured the same design and inclusion/exclusion criteria". The statistical analysis used to pool the data from the three trials was not reported in the manuscript. The authors adjusted the primary analysis (analysis of covariance for a two‐period, two‐treatment cross‐over study) for baseline characteristics and source centre of each participant. Considering that, even after contact with the authors, additional data were not available and that Connor 2008 did not provide sufficient information for identifying and extracting data obtained from the three different trials (including Connor 2002), we retained only the pooled study (Connor 2008). Additionally, Hulihan 2003 and Ondo 2004 reported preliminary results further presented and detailed in other studies included in this review (Connor 2008; Ondo 2006) and were not retained for the same reasons.
All the studies compared topiramate with placebo. The duration of the study was two weeks in Carrasco Vargas 2011, 10 weeks in Connor 2008, and 24 weeks in Ondo 2006.
Participants
The studies included people with definite upper‐limb ET according to criteria proposed by Tremor Investigation Group (Bain 2000a). Connor 2008 included also 12 participants with head tremor. Studies included 223 participants (Ondo 2006), 62 participants (Connor 2008), and 24 participants (Carrasco Vargas 2011). Baseline TRS total scores were 38.7 (SD 12.4) (Ondo 2006), 36.8 (SD 17.8) (Connor 2008), and 27.1 (SD 10.9) (Carrasco Vargas 2011). Ondo 2006 reported a disease duration of 24 (SD 17) years. Age ranged between 18 and 80 years. Moreover, the studies included people treated with a stable anti‐tremor medication for at least two to four weeks before randomisation. The co‐therapy (mainly beta‐blockers and benzodiazepine in Connor 2008 and beta‐blockers and primidone in Ondo 2006) was maintained throughout the study period. Both Connor 2008 and Ondo 2006 excluded people with other neurological causes of tremor (degenerative disease, nervous system trauma, epilepsy, psychogenic tremor, alcohol or drugs abuse) and concomitant systemic disease (liver disease, endocrinological disease, renal stones, renal insufficiency, nephrolithiasis), occurrence of AEs during previous treatment with topiramate or psychiatric disorders. Carrasco Vargas 2011 included participant between 18 and 80 years. Participants' general characteristics and disease duration were not reported.
Intervention
Connor 2008 included a group of 30 participants receiving topiramate for 10 weeks, followed by 10 weeks of placebo, with a washout period of two weeks between the two treatments. A second group of 32 participants received placebo followed by topiramate. Dosage ranged between 25 mg/day and 400 mg/day divided into two daily doses.
Ondo 2006 compared a group of 117 participants receiving topiramate for 24 weeks to a group of 106 participants receiving placebo for 24 weeks. Dosage ranged between 25 mg/day and 400 mg/day divided into two daily doses.
Carrasco Vargas 2011 compared a group of 12 participants receiving topiramate for two weeks with a group of 12 participants receiving placebo for 12 weeks. Treatment was initiated at the dose of 50 mg divided into two daily doses and increased up to 200 mg/day. The mean treatment dose was not reported.
Outcome measures
The three studies used TRS score as the primary efficacy outcome measure. All studies reported TRS total score at baseline and at the study endpoint, while only Ondo 2006 reported TRS subscale A, B and C scores separately. Ondo 2006 also reported a qualitative global rating scale (very good, good, no change, poor, very poor), completed by both investigators and participants.
Adverse events
The three studies reported the number of participants experiencing AEs, while only two studies reported the number of participants who withdrew/dropped out (Ondo 2006; Connor 2008). Moreover, Connor 2008 did not report the number of AEs by period (i.e. before and after the cross‐over) and Carrasco Vargas 2011 did not report the total number of AEs and the number of withdrawals.
Excluded studies
We excluded six studies after reading the full texts (see Characteristics of excluded studies table). Five were case series (Bermejo 2007; Gálvez‐Jimenez 2000; Gatto 2003; Siniscalchi 2007; Zesiewicz 2007b). We excluded one study as it reported only electrophysiological data (Frima 2006).
Risk of bias in included studies
The results are reported in Figure 2 and Figure 3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
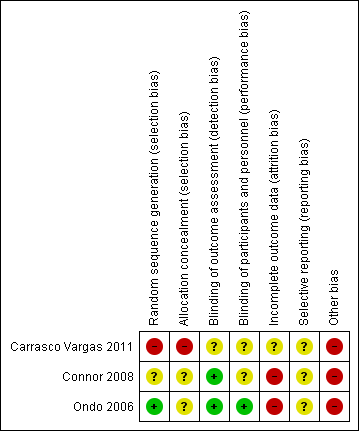
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ondo 2006 reported methods for sequence generation and allocation concealment and was considered at low risk of bias. Connor 2008 did not provide enough information to assess if order of receiving treatments was randomised and the risk of bias was unclear. Carrasco Vargas 2011 did not provide any information regarding randomisation techniques and allocation concealment and reported that participants were randomly assigned either to the treatment or placebo group. This was at high risk of bias.
Blinding
Ondo 2006 reported adequate methods for blinding personnel, participants and outcome assessors to treatment allocation. Connor 2008 reported adequate method for blinding of outcome assessors but the authors did not provide enough information to assess the blinding of personnel and participants and therefore risk of performance bias was unclear. Carrasco Vargas 2011 did not provide information to assess the blinding of outcome assessment and participants and the risk was unclear.
Incomplete outcome data
In Ondo 2006, 45/117 (38.5%) randomised participants in the topiramate group and 23/106 (22%) participants in the placebo group discontinued before study completion. Reasons for missing data were different across groups since more participants withdrew for AEs in the topiramate group. The authors defined the intention‐to‐treat population (108 participants in the topiramate group and 100 participants in the placebo group) as "all randomised patients who took at least one dose of study medication and had at least one on‐treatment efficacy assessment". This definition implies that an imputation method with a carried forward procedure was used for dealing with missing outcome data and this can lead to serious bias (Higgins 2011). In Connor 2008, more than 40% of participants in the topiramate group and 25% of participants in the placebo group dropped out, indicating a high risk of bias. Moreover, these authors also imputed missing outcome data with the use of baseline observation carried forward. Both studies were at high risk of attrition bias because neither reported times at which outcomes were genuinely measured in participants for whom outcomes were carried forward.
Carrasco Vargas 2011 provided no information regarding treatment discontinuation and it was at unclear risk of bias.
Selective reporting
There was no identified reporting bias.
Other potential sources of bias
Two studies excluded people with already known hypersensibility to topiramate (Connor 2008; Ondo 2006. Moreover, both the studies were sponsored by and had at least one author affiliated to the pharmaceutical company that marketed topiramate.
The findings reported by these two studies should be interpreted with caution in light of the limitations described, in particular the high risk of attrition bias. Moreover, since Ondo 2006 was a multicentre study including among co‐authors and investigators the same authors of Connor 2008, we contacted, unsuccessfully, Dr Connor asking for information about the possible inclusion of some participants in both the studies.
Carrasco Vargas 2011 did not provide information on the general and clinical characteristics of the study participants, making homogeneity and comparability of the two groups uncertain. Moreover, the limited follow‐up of only two weeks may have influenced the outcome (efficacy and safety) measurements. For these reasons, the study was judged at high risk of other potential bias.
Effects of interventions
See: Summary of findings for the main comparison Topiramate for essential tremor
See: summary of findings Table for the main comparison reporting the comparison topiramate versus placebo and GRADE assessment.
The studies compared topiramate and placebo, involving a total of 309 participants (159 participants in the topiramate group and 150 participants in the placebo group).
We extracted only data from the first treatment phase from Connor 2008. These included TRS total score and number of withdrawals. We excluded TRS subscale (A, B and C) scores and number of AEs from the analysis because they were reported only for the second treatment phase.
The overall risk of bias was high for all the studies. The quality of evidence in the outcomes ranged from very low to low.
Primary outcomes
Functional disability component related to tremor
At the study end (24 weeks), Ondo 2006 reported a mean specific motor tasks/function (TRS subscale B) reduction of 10.3 (SD 12.6) with topiramate and 4.9 (SD 9.5) with placebo (P = 0.001). The MD between the two groups was 5.4 (SD 3.0) (Analysis 1.1; Figure 4). The mean functional disability (TRS subscale C) reduction reported was 9.4 (SD 13.3) with topiramate and 3.7 (SD 8.8) with placebo (P = 0.001). The MD between the two groups was 5.7 (SD 3.0) (Analysis 1.1).
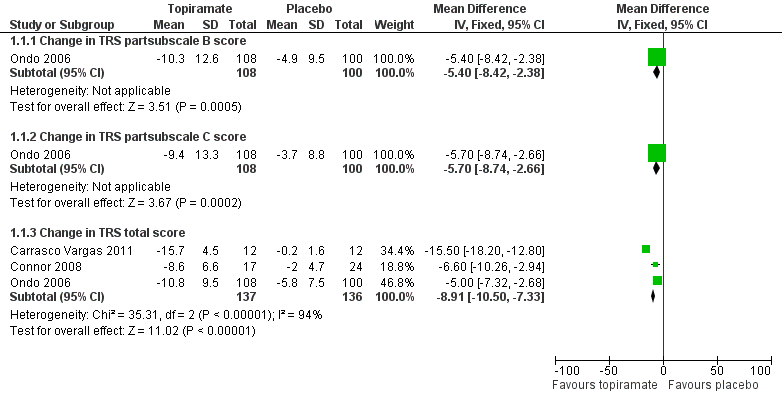
Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.1 Functional disability component related to tremor.
Withdrawal
Two studies reported the number of withdrawals as a primary outcome (Connor 2008; Ondo 2006). Forty‐five participants in the topiramate group and 23 participants in the placebo group withdrew in Ondo 2006, while 12 participants in the topiramate group and seven participants in placebo group withdrew in Connor 2008. We combined data concerning total withdrawals (Analysis 1.2; Figure 5). There was an increased risk of withdrawals for topiramate, with a Mantel‐Haenszel RR of 1.78 (95% CI 1.23 to 2.60) and a RD of 0.17 (95% CI 0.07 to 0.28). Analysing the causes of study discontinuation, there was evidence to suggest that participants receiving topiramate were more likely to withdraw due to AEs compared to participants receiving placebo, with a Mantel‐Haenszel RR of 3.17 (95% CI 1.79 to 5.63), while there was no evidence of a difference between groups for other reasons for withdrawal (including lack of efficacy, participant choice, lost to follow‐up, non‐compliance and unknown reasons).
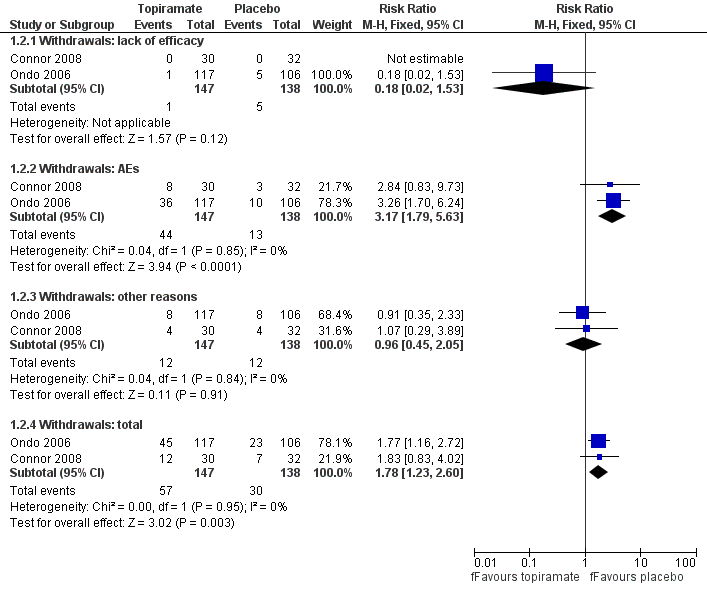
Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.2 Withdrawals.
Number of adverse events associated with treatment
Ondo 2006 reported the occurrence of AEs, without specifically categorising severity. Overall, 116 participants reported 195 AEs with topiramate, giving a mean of 1.7 AEs per participant. The most frequently reported were: paraesthesia (28 participants), weight loss (22), taste perversion (22), upper respiratory tract infections (19), fatigue (16), nausea (16), appetite decrease (14), memory difficulty (13), dizziness (13), somnolence (12), diarrhoea (12) and headache (11). Participants on placebo treatment reported mainly upper respiratory tract infections (14 participants), dizziness (11), diarrhoea (eight), headache (eight) and nausea (seven), resulting in 71 AEs per 105 participants and a mean of 0.7 AEs per participant. Carrasco Vargas 2011 reported that two participants treated with topiramate presented with AEs, including nausea and weight loss. There were no AEs in the placebo group.
Subgroup analyses
We performed no subgroup analyses of studies categorised according to the combination of anti‐tremor treatments since there were not enough trials or adequate information available.
Sensitivity analyses
We performed no sensitivity analyses excluding studies at high risk of bias were due to the small number of trials included in the review and to the absence of trials at low risk of bias.
Secondary outcomes
Tremor severity
At the study end (24 weeks), Ondo 2006 reported a mean reduction from baseline of the overall TRS score of 10.8 (SD 9.5) with topiramate and of 5.8 (SD 7.5) with placebo (P < 0.001) and a mean upper‐limb tremor severity reduction of 12.7 (SD 14.8) with topiramate and of 8.9 (SD 13.2) with placebo (P = 0.06).
Ondo 2006 reported a post‐hoc analysis to assess the potential clinical significance of TRS score change. The authors grouped the participants in categories according to the self‐evaluation scale (very good, good, no change) and calculated the mean TRS score change observed in each category. Based on this analysis, topiramate produced a moderate improvement while placebo produced a mild improvement with unreported statistical and clinical significance.
At the end of period one (10 weeks), Connor 2008 reported a mean overall TRS change from baseline of 8.56 (SD 6.6) with topiramate and of 1.94 (SD 4.7) with placebo (P = 0.0004).
At the study end (two weeks), Carrasco Vargas 2011 reported a mean overall TRS change from baseline of 15.7 (SD 4.5) with topiramate and of 0.2 (SD 1.6) with placebo (P < 0.05).
A fixed‐effect meta‐analysis pooled data on efficacy. There was a statistically significant difference in terms of efficacy for TRS total score, which favoured topiramate (MD ‐8.91, 95% CI ‐10.50 to ‐7.33) (Analysis 1.1; Figure 4).
Scores of a non‐validated participant self‐evaluation rating scale and of an investigator‐rated global assessment scale were reported in Ondo 2006. The participant‐rated global assessment indicated that 69% of participants in the topiramate group and 15% of participants in the placebo group reported a "good" or "very good" improvement; similarly, investigators attributed a "good" or "very good" improvement to 67% of participants in the topiramate group and to 20% of participants in the placebo group.
Quality of life
None of the studies reported QoL.
Discussion
Summary of main results
The review included three RCTs comparing topiramate with placebo for the treatment of ET (Carrasco Vargas 2011; Connor 2008; Ondo 2006; ). The studies involved a population of 309 participants presenting with moderate to severe ET, mostly of long duration (about 20 years). One study assessed treatment efficacy in terms of function and ability improvement, showing a statistically significant difference favouring topiramate versus placebo (Ondo 2006). However, the quality of the evidence in this outcome was very low. However, there was an almost two‐fold risk of study withdrawal, mainly due to the occurrence of AEs, for participants treated with topiramate. The quality of the evidence for these outcomes was low. Thus, these effect estimates should be interpreted cautiously due to the scarce number of trials included, the high risk of bias and the low to very low quality of evidence provided.
Overall completeness and applicability of evidence
In this review, efficacy outcomes were measured with the TRS scale. Only one study adequately assessed one of the primary outcomes (functional disability component related to tremor), which was measured as a reduction of the TRS subscales B and C scores (Ondo 2006). Given the lack of studies reporting what TRS score differences should be considered clinically relevant, the definition of improvement was taken from the original articles. Ondo 2006 assessed the potential clinical significance of TRS score changes in a post‐hoc analysis. However, we believe the definition of improvement provided was inadequate because it was based on a retrospective assessment of the level of improvement, because it included only the TRS total score disregarding the TRS subscales scores, it contained an overlap in attributing the class of improvement (the number 10 was included in two consecutive categories) and it was tailored on an unvalidated global assessment scale (completed by both participants and raters), without specifying if the analysis was based on participant‐rated or on investigator‐rated scores. Connor 2008 and Carrasco Vargas 2011 did not report TRS subscale B and C scores, or provide a definition of improvement, making the interpretation of the results difficult from a clinical perspective.
Despite being considered a recommended scale in the assessment of tremor severity (Elble 2013), the TRS scale has demonstrated limited inter‐rater reliability, unless the raters have been rigorously trained (Stacy 2007). This could influence results and should be taken into account especially in multicentre trials. Moreover, since TRS sensitivity in detecting relevant clinical changes in studies assessing ET therapies has not been evaluated, the clinical relevance of statistically significant changes in TRS scores is unclear.
The number of AEs was not adequately reported in Connor 2008 and Carrasco Vargas 2011 and it was not measured using standardised questionnaires in Ondo 2006. Unreported symptoms could have been neglected. It should be also underlined that the risk of AEs could have been further underestimated due to the exclusion of people with previously reported AEs or hypersensitivity to topiramate.
An additional remark regarding topiramate therapeutic scheme should be made. Given the lack of studies assessing the minimum efficacious dose of topiramate for the treatment of ET, the dose used in these trials (ranging between 25 mg/day and 400 mg/day) was arbitrarily chosen and thus, could have been inadequate (excessive or insufficient), as well as the duration of the follow‐up.
Finally, the effects of treatment on participants' functional ability, QoL and validated participant self‐evaluating rating scales were not reported, representing a limitation in the overall completeness of the assessment and the ability to balance the overall risk‐benefit ratio linked to treatment.
Quality of the evidence
Review of bias evaluation and strength of evidence assessment disclosed several major limitations in the evidence‐base. Several important factors limited the validity of the reported results:
-
the treatment effect was measured using a carried forward procedure to deal with a significant amount of missing outcome data;
-
there was a high risk of attrition bias given the number of withdrawals due to AEs, especially among participants treated with topiramate;
-
the presence of a large proportion of participants receiving other anti‐tremor medications during the study period (50% in Ondo 2006 and 89% in Connor 2008) might have influence the efficacy measures;
-
there were potential conflicts of interest due to the presence of authors sponsored by or affiliated to the pharmaceutical company of topiramate.
Different major points seriously limited the global quality of the evidence produced by these studies.
Blinding of outcome assessment was described in two studies (Connor 2008; Ondo 2006) and not reported in one (Carrasco Vargas 2011); however, even in the first, the well documented AEs of topiramate make it likely that treated participants had become aware of the treatment they were receiving during the course of the trial, and these trials should be regarded as single‐blind.
In addition to this, the assessment of the quality of the evidence highlighted the presence of potential indirectness related to the uncertain relevance of the reported TRS‐score changes as indicators of a clinical improvement (which is meaningful to people with ET), and the presence of imprecision of the evidence due to the small sample size and small number of included studies.
Potential biases in the review process
To identify all relevant studies minimising the risk of bias, we performed a comprehensive systematic review, searching different databases without language restrictions. In addition to all the limitations previously highlighted, there are a number of other potential biases. First, we included a trial reporting pooled data. Additional information from authors and the original reports were not available, making it impossible to clarify the method used to combine data and to exclude the possibility that some participants were included in both trials. Furthermore, since people undergoing additional anti‐tremor therapies were not separately assessed in the original studies, a potential combined effect of different treatments was included in the results presented.
Agreements and disagreements with other studies or reviews
There are two major reviews of the literature analysing topiramate treatment for ET (Zappia 2013; Zesiewicz 2005). These reviews produced different conclusions compared to the present work, due to the use of different methodology and criteria for rating the quality of the studies and of the evidence.
-
The Practice Parameter for Essential Tremor concluded that topiramate should be used as second‐line therapy (Zesiewicz 2005). The review was developed on a four‐tiered classification scheme based mainly on study design and including uncontrolled studies, case series and case reports. Neurophysiological parameters (electromyographic recordings, accelerometry, spirography, digitising tablets) were considered among outcome measures. Recommendation on topiramate were formulated on the basis of three class II studies (Connor 2002; Hulihan 2003; Ondo 2004) and one class IV study (Gálvez‐Jimenez 2000).
-
The systematic review of evidence and recommendations from the Italian Movement Disorders Association (DISMOV‐SIN) considered the potential role of topiramate among first‐line treatments (Zappia 2013). The review used GRADE to assign the level of evidence. Besides RCTs, the review also included case series, case reports and studies using neurophysiological assessment of tremor. The conclusions on topiramate were based on the analysis of five studies that were assigned a higher quality category compared to studies investigating propranolol and primidone, classically considered as first‐line therapy (Connor 2008; Frima 2006; Gálvez‐Jimenez 2000; Gatto 2003; Ondo 2006).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.1 Functional disability component related to tremor.

Forest plot of comparison: 1 Topiramate versus placebo/open control, outcome: 1.2 Withdrawals.
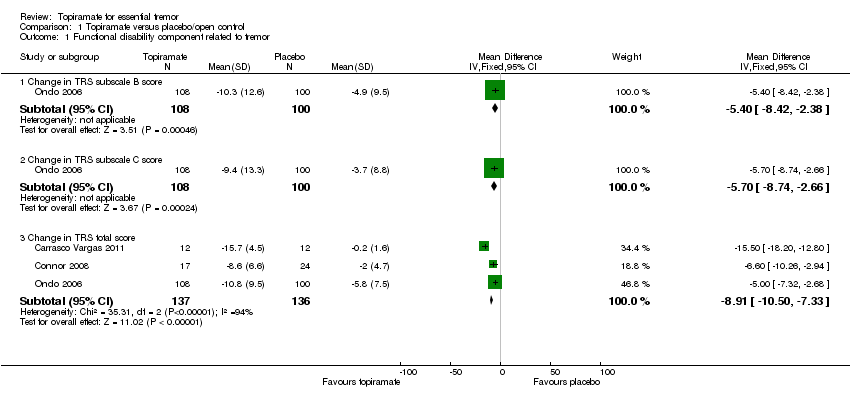
Comparison 1 Topiramate versus placebo/open control, Outcome 1 Functional disability component related to tremor.

Comparison 1 Topiramate versus placebo/open control, Outcome 2 Withdrawals.
| Topiramate for essential tremor | ||||||
| Patient or population: people with essential tremor Settings: outpatients Intervention: topiramate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topiramate | |||||
| Functional disability (follow‐up duration 24 weeks) TRS subscale B score (motor tasks) TRS subscale C score (functional disability) | The mean improvement in the control group was | The mean improvement in the intervention groups was | ‐ | 223 | ⊕⊝⊝⊝ | ‐ |
| Study withdrawal (follow‐up duration 10 to 24 weeks) Number of participants withdrawn from the study | Study population | RR 1.78 | 285 | ⊕⊕⊝⊝ | ‐ | |
| 217 per 1000 | 347 per 1000 | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| Adverse events (follow‐up duration 24 weeks) Number of AEs | Study population | ‐ | 221 | ⊕⊕⊝⊝ | ‐ | |
| 71 AEs per 105 participants | 195 AEs per 116 participants | |||||
| Moderate | ||||||
| ‐ | ‐ | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AE: adverse event; CI: confidence interval; RR: risk ratio; TRS: Tremor Rating Scale. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to serious risk of bias: high number of study withdrawals (attrition bias); blinding of outcome assessment probably revealed by the presence of serious AEs in the topiramate group: trials should be regarded as single blind (detection bias); potential conflicts of interest due to the presence of authors sponsored by pharmaceutical companies. 3 Downgraded due to imprecision: small sample size (< 300 participants) and small number of included studies (three). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Functional disability component related to tremor Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Change in TRS subscale B score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.4 [‐8.42, ‐2.38] |
| 1.2 Change in TRS subscale C score | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐5.7 [‐8.74, ‐2.66] |
| 1.3 Change in TRS total score | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐8.91 [‐10.50, ‐7.33] |
| 2 Withdrawals Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Withdrawals: lack of efficacy | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.53] |
| 2.2 Withdrawals: AEs | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.79, 5.63] |
| 2.3 Withdrawals: other reasons | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.45, 2.05] |
| 2.4 Withdrawals: total | 2 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.23, 2.60] |

